Note: Descriptions are shown in the official language in which they were submitted.
PHARMACEUTICAL COMPOSITIONS FOR STAINING MEMBRANES AND OTHER
BIOLOGICAL STRUCTURES
Field of the Invention
The present invention refers to pharmaceutical compositions
comprising delphinidin, alone or combined with other dyes, namely
brilliant blue (G or FCF) and trypan blue, to a process for
producing those pharmaceutical compositions and to a method for
staining biological membranes and structures using those
pharmaceutical compositions.
The present invention facilitates the identification of
biomembranes and their structures, including different types of
ocular membranes and tissues, during surgical procedures such as
chromovitrectomy. It also protects cells and tissues from damage
induced by surgical light.
Therefore, this invention is in the technical domain of medical
and pharmaceutical industry and related ones.
Background of the Invention
Surgery frequently requires the visualization, manipulation and
removal of fragile biological membranes. These structures are
often semi-transparent and thin and, therefore, their adequate
identification and manipulation is difficult, which makes the
surgical procedure complex and impacts on the recovery time.
1
CA 3028294 2018-12-21
The use of vital dyes for staining human tissues during surgery
has been established for more than 30 years. However, in
ophthalmology, the use of this tool dates back to the thirties.
Indeed, Sorsby in 1939 and Gifford in 1940 began the injection
of Kiton-fast-green V, Xylene-Fast-green B and fluorescein
intravenously. Kutschera, in 1969, began injecting patent blue
intra-vitreally to stain retinal tissue and assess retinal breaks
in retinal detachments.
The onset of the pars plana vitrectomy surgical procedure allowed
the treatment of serious retinal diseases such as diabetic
retinopathy, macular hole and retinal detachment. The procedure
consists in removing the intraocular vitreous gel and pre-retinal
membranes, followed by the restoration of the ocular volume, for
instance, with balanced salt solution (BSS).
The removal of pre-retinal membranes, specifically, constitutes
a technically difficult surgical step because of the thin and
semi-transparent nature of the tissues. In fact, several studies
have shown retinal complications caused by the inaccurate removal
of these structures.
The use of stains in chromovitrectomy was first described by Burk
et al. in 2000 and it was a major breakthrough in vitreoretinal
surgery: the vital dye indocyanine green (ICG) was shown to
exhibit high affinity for the internal limiting membrane (ILM)
and the previously complex task of removing the ILM and
epiretinal membranes became much easier. This highly improved
anatomic and functional surgery outcomes.
2
CA 3028294 2018-12-21
Nevertheless, clinical trials soon raised safety concerns
demonstrating postoperative complications such as visual field
and retinal pigment epithelium changes related to the use of ICG.
In vitro and in vivo studies have shown a dose-dependent toxicity
of ICG in retinal cells. Such toxicity has resulted into an
active search for less toxic compounds. Two candidate compounds
are trypan blue and patent blue.
However, the acceptance of these dyes has been limited due to
low chemical affinity for retinal membranes and no precise safety
indicators.
The ideal dye would then exhibit high affinity to retinal
membranes and reduced or no retinal toxicity. In the search for
these putative pharmaceutical compositions, the present
invention proposes the use of a natural dye, delphinidin, as a
new alternative for use in chromovitrectomy or other procedures
where it is necessary or helpful to guide a health care
professional by defining layers or boundaries.
Delphinidin or 2-(3,4,5-Trihydroxyphenyl)chromenylium-3,5,7-
triol is a natural anthocyanin, produced by plants such as
cranberries and blueberries (Vaccinium), blackcurrants (Ribes),
violets (Viola), delphinium (Delphinium), some types of grapes
and eggplant (Solanum) being responsible for the blue and blue
red colour of flowers and fruits. This plant pigment also shows
strong antioxidant activity and due to its pH sensitivity can
additionally be used as a natural pH indicator, changing from
red in acidic solution to blue in basic solution.
3
CA 3028294 2018-12-21
Delphinidin has a diphenylpropane-based polyphenolic ring
structure that carries a positive charge in its central ring.
Anti-angiogenic and anticancer properties have been attributed
to this pigment. It has also been shown to protect keratocytes
and mouse skin from UVB-Mediated oxidative stress and apoptosis
and to have anti-inflammatory properties in an in vitro model of
psoriasis.
Document EP0410749 A2 refers to the use of delphinidin to treat
injury or harmful agent-induced hyperpermeability of ciliary body
blood vessels, known regulators of ocular pressure and of the
blood-water barrier, as well as of the production of aqueous
humour. However, said document does not disclose compositions,
or methods for staining biological membranes and related
structures nor the protection of tissues and cells exposed to
surgical light during surgery.
Document W02013167632 Al discloses a viscoelastic device
comprising a viscoelastic polymer covalently bound to at least
one dye, being the said dye for example an anthocyanin, that is
used to fill the anterior chamber after phacoemulsification, a
cataract surgery in which the lens is emulsified with ultrasounds
and aspirated from the eye. Aspirated fluids are then replaced
with BSS irrigation to maintain the anterior chamber. This
viscoelastic device is used in order to protect the endothelium
and to easily detect or indicate normal or abnormal pH values in
the ocular tissue. This document does not disclose compositions,
methods for staining biological membranes and related structures
to facilitate their identification, manipulation and removal
during surgical procedures nor does it disclose the protection
of tissues and cells exposed to light during surgical procedures.
4
CA 3028294 2018-12-21
A recent scientific paper (Chuang L., Wu A., Wang N., Chen K.,
Liu, L., Hwang, Y., Yeung, L., Wu W., Lai C. The intraocular
staining potential of anthocyanins and their retinal
biocompatibility: a preclinical study. Cutaneous and Optical
Toxicology 2018, 4:1-8) has shown preclinically the potential of
delphinidin as an intraocular stain. Several staining suspensions
comprising anthocyanins, namely delphinidin, are herein
disclosed. Each anthocyanin was suspended in 10 ml of 5% glucose
in water solvent with resulting anthocyanin concentration of 1
mg/ml for improving the contrast of the ILM and remaining retinal
tissues during chromovitrectomy procedures. Results show that
delphinidin was used with success to stain pig eye lenses and
intravitreal and subretinal injection in rats promoted no
morphologic nor functional toxicity. A clinical trial has shown
that oral delphinidin improves glucose metabolism in pre-diabetic
individuals, is well tolerated and generates no adverse effects.
However, the concentration of the staining solutions based on
the studied anthocyanins had to be substantially higher, since
each formulation comprises only one dye. Moreover, despite
staining solutions comprising other known dyes are mentioned,
such as ICG, these dyes are referred to as non-usable mainly due
to their toxicity. There is no mention or hint of staining
solutions comprising a combination of at least one anthocyanin
and another dye to reduce the toxicity of said solutions, to
increase the contrast between the different tissues of the eye
during intraocular interventions or to protect bio structures
from damage caused by exposure to surgical light.
Therefore, there is a need to develop staining pharmaceutical
compositions that are not only able to stain biological tissues
but are also able to do it selectively in order to produce a
5
CA 3028294 2018-12-21
contrast between close related tissues and allow accurate
identification, manipulation and removal of biological membranes
and structures. This improves the safety of surgical procedures
and promotes better recovery periods. There is also a need to
protect bio structures from damage caused by exposure to surgical
light.
In this sense, the present invention proposes pharmaceutical
compositions that can be used not only for staining biological
membranes, in particular ocular membranes, but also for
protecting intraocular structures from light exposure by topical
application or injection into the eye.
Summary of the Invention
The present invention refers to pharmaceutical compositions
comprising delphinidin, alone or combined with other dyes, such
as brilliant blue (G or FCF) and/ or trypan blue, to a process
for producing the said compositions and to a method for staining
biological membranes and structures to improve their
identification and processing during surgical procedures, such
as chromovitrectomy. It also discloses the protection of tissues
from damage induced by surgical light.
Therefore, it is a first object of the present invention to
provide pharmaceutical compositions comprising delphinidin alone
or in combination with brilliant blue (G or FCF) and/ or trypan
blue for staining biological membranes and cell structures,
according to claim 1.
6
CA 3028294 2018-12-21
These pharmaceutical compositions selectively dye the biological
structure of interest whereas the surrounding structures are not
dyed or are not dyed to the same extent. Furthermore, they also
stain biological structures at a concentration that is
physiologically and toxicologically acceptable and therefore,
the lowest amount of dye that provides a visible differentiation
between the selected biological membranes and the surrounding
tissues can be used. Despite these dyes show little or no
toxicity, any remaining pharmaceutical composition in the
surgical field is removed briefly after the procedure, further
reducing the possibility of adverse effects.
It is another object of the present invention to provide a process
for producing staining pharmaceutical compositions based on
delphinidin, according to claim 10.
It is yet another object of the present invention to provide a
method for staining biological membranes and structures by using
pharmaceutical compositions based on delphinidin, according to
claim 6.
This method allows dyeing the biological structure of interest
selectively whereas the surrounding structures are not dyed or
are not dyed to the same extent resulting in a more accurate
identification of the desired structure and its easier surgical
manipulation/ removal, reducing the risk of damaging the
surrounding tissues and consequently reducing the recovery time.
This process can be used during surgical procedures or in any
other procedures that require staining of biological material,
7
CA 3028294 2018-12-21
improving the contrast between the different types of biological
tissues.
By using the pharmaceutical compositions and methods of the
present invention it is possible to increase the protection of
tissues and cells from damage due to light present in the surgical
field during surgical procedures.
Furthermore, by applying the present invention it is also
possible to protect intra-ocular structures by topical
application or by injection into the eye of one or more of the
said pharmaceutical compositions that include the natural
substance delphinidin, with low toxicity risk for the patient.
Detailed Description of the Invention
The present invention refers to pharmaceutical compositions
comprising delphinidin, alone or combined with other dyes, namely
brilliant blue (G or FCF) and/ or trypan blue, to a process for
producing said pharmaceutical compositions and to a method for
staining biological membranes and their structures.
The present invention allows improved identification of
biological materials and their structures, including different
types of ocular membranes and tissues, during surgical procedures
such as chromovitrectomy. It also relates to the use of
delphinidin comprising pharmaceutical compositions that protect
tissues and cells from surgical light-induced damage.
In the scope of the present invention intraocular membranes
referred herein are particularly the anterior and posterior
8
CA 3028294 2018-12-21
capsule, epiretinal membrane, ILM, vitreous and posterior
hyaloid.
1. Dyes of the compositions of the invention
The first aspect of the present invention relates to
pharmaceutical compositions comprising delphinidin, alone or in
combination with brilliant blue (G or FCF) and/ or trypan blue
dyes and pharmaceutically acceptable vehicles.
Staining pharmaceutical compositions according to the present
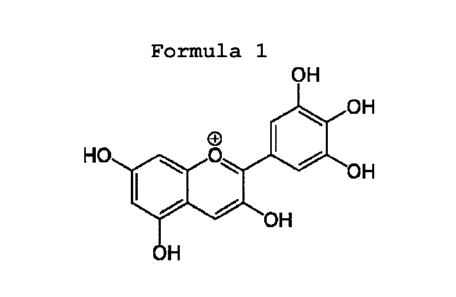
invention comprise delphinidin or 2-(3,4,5-trihydroxyphenyl)
chromenylium-3,5,7-triol (formula 1), as a main dyeing agent.
Delphinidin, is a safe and an effective natural dye for staining
biological tissues and structures that can be used in surgical
procedures, such as chromovitrectomy, allowing an easy
identification of biological membranes and structures by the
ophthalmic surgeon, an effective performance during surgery and,
remarkably, a decrease of iatrogenic retinal complications
derived from the surgical procedure.
Formula 1
OH
OH
HO 0
OH
OH
9
CA 3028294 2018-12-21
Comparing with other anthocyanins, delphinidin is the one
exhibiting the best staining properties with no toxicity. It has
also shown the ability to protect skin from UV radiation.
Delphinidin as a dying agent can be used in combination with
other dyes. In the scope of the present invention, the
pharmaceutical compositions comprise delphinidin in combination
with a dye selected from the group including ingredients or
extracts of brilliant blue (G or FCF) and/ or trypan blue.
Brilliant Blue FCF is an azo and triarylmethane synthetic dye
(Formula 2) that is commonly used as food colorant due to its
poor absorption in the gastrointestinal tract. It is added to
drug formulations as an inactive substance.
Formula 2
0-
N
2Na 0 rji
\SC'
Brilliant Blue G is a triphenylmethane dye commonly used for
staining proteins (Formula 3) by interacting electrostatically
but non-covalently with the amino and carboxyl groups of
proteins. Its colour varies in function of the acidity of the
CA 3028294 2018-12-21
solution due to the different charged states of the dye molecule,
in particular at a value pH less than 0 all three nitrogen atoms
carry a positive charge conferring to the dye a red color, whilst
at pH values above 2, the two sulfonic acid groups have extremely
low pica and are negatively charged conferring to the dye a bright
blue color.
Formula 3
H03
SO3-
N
HN 41,
Trypan blue, also known as diamine blue and Niagara blue (Formula
4), is a blue azo synthetic dye that is used to selectively dye
dead tissues or cells. In ophthalmic cataract surgery it is used
to stain the anterior capsule in the presence of a mature
cataract, to aid in visualization, before creating the continuous
curvilinear capsulorhexis.
Formula 4
11
CA 3028294 2018-12-21
2. Formulations of the invention
Typically, the pharmaceutical compositions of the present
invention comprise delphinidin as the main dye in concentrations
of 0.01 to 0.1%, preferably of 0.05 to 0.5%, more preferably of
0.55 to 0.65%, even more preferably in a concentration of 0.06%.
Brilliant blue (G or FCF) can be present in the pharmaceutical
compositions of the present invention in an amount of 0.025 to
0.05% of the total composition, preferably 0.045 to 0.055%.
Trypan blue can be present in the pharmaceutical compositions of
the present invention in an amount of 0.001 to 0.2% of the total
composition, preferably of 0.04 to 0.15%.
These dyes can be advantageously combined with delphinidin to
produce pharmaceutical compositions for staining biological
material, namely biological membranes and/or structures,
particularly ocular membranes.
Therefore, in the scope of the present invention, pharmaceutical
compositions comprising delphinidin as main dye, for staining
biological material can be produced in one of the following
formulations:
a) Delphinidin + brilliant blue (G or FCF);
b) Delphinidin + brilliant blue (G or FCF) + trypan blue;
c) Delphinidin + trypan blue;
d) Delphinidin.
Acceptable pharmaceutical vehicles suitable to be included in
delphinidin pharmaceutical compositions of the present invention
12
CA 3028294 2018-12-21
can be, for example acetic acid, benzyl alcohol, borax, boric
acid, BSS, calcium chloride, carbomer 934, carbopol, chondroitin
sulphate, citric acid, dextran sodium polysorbate, glycocholic
acid, hyaluronic acid, magnesium chloride, metaphosphoric acid,
methylcellulose and derivatives, phenylphosphate, phosphate
buffer, polyethylene glycol, polyvinyl alcohol, potassium
chloride, potassium phosphate, propylene glycol, purified water,
sodium acetate, sodium chloride, sodium citrate, sodium edetate,
sodium phosphate, sodium phthalate and/or tweens.
Each one of the above-mentioned synthetic dyes has its staining
specificity and accordingly, when used in combination with
delphinidin, the biological structure of interest is selectively
stained and the surrounding structures are not or are not to the
same extent. This allows accurate identification of the structure
and surgical manipulation/ removal, reducing the risk of damaging
the surrounding tissues and consequently reducing the time of
recovery and iatrogenic complications.
Moreover, they show little or no toxicity and any remaining
residues that are left in the surgical field are removed briefly
after the procedure, further reducing the possibility of adverse
effects. In addition, they also stain biological structures at a
concentration that is physiologically and toxicologically
acceptable.
In short, benefits of using pharmaceutical compositions of the
present invention comprising delphinidin, a natural dye, alone
or in combination with one or both of these synthetic dyes
include:
13
CA 3028294 2018-12-21
- clear and easy identification of the desired membrane and/
or biological structure to manipulate and/ or remove during
eye surgery;
- prevention of adverse effects caused by inaccurate
(insufficient or excessive) removal of the desired biological
structure;
- reduction of surgery time and reduction of the post-operative
period with consequent reduction of health care costs;
- increased safety during the procedure and later phases;
- possible antioxidant/ anti-inflammatory effects associated
with the use of delphinidin in the surgery or other medical
procedure;
- protection of bio structures from damage caused by exposure
to surgical light.
The compositions of the present invention can be presented in
the form of a solution, a dispersion, a suspension or an emulsion.
3. Process for producing the pharmaceutical compositions
The overall process of pharmaceutical composition preparation
includes diluting the selected dyes in a pharmaceutical vehicle
to achieve the final concentrations of:
- Delphinidin: 0.01 to 0.1%, preferably 0.05 to 0.5%; more
preferably 0.55 to 0.65%;
- Brilliant blue (G or FCF): 0.025 to 0.05%; preferably 0.045
to 0.055%, and/or
- Trypan blue: 0.001 to 0.2%, preferably 0.04 to 0.15%.
14
CA 3028294 2018-12-21
A pharmaceutical acceptable vehicle is added to the above
compositions. The said compounds are already listed in the
previous section.
In addition, the dye may be formulated as a solution, suspension,
dispersion or emulsion by the known techniques in the art.
All the steps are conducted under the required conditions for
manufacturing, handling and processing pharmaceutical quality
materials.
4. Method for staining biological material
The method for staining biological material, such as biological
tissue, membranes or other structures of the eye includes the
application of a pharmaceutical composition according to the
present invention, topically or by injection. This application
can be done during a surgical procedure or any other procedure
that requires staining of a biological material and/ or
structure.
Examples
Example 1. A pharmaceutical composition with delphinidin
The packaging material and raw materials are received and the
raw materials are weighed. Handling and/ or filtration takes
place in an ISO 8 class room. In-process quality control is
performed before septic or aseptic filling.
CA 3028294 2018-12-21
Should the filling be aseptic, it follows the flow: Filling in
the ISO 5 class room > Sealing in ISO 7 class room > Sterilization
in the final sterilization room. Should the filling be septic,
it follows the flow: Sterilizing Filtration in ISO 5 class room
> Filling in ISO 5 class room > Sealing in ISO 7 class room.
Packaging and quality control of the finished product then
follows.
Example 2. A pharmaceutical composition with delphinidin and
brilliant blue FCF
All the steps were performed as described in Example 1 with
exception of the amounts of dyes that were the following:
Delphinidin and brilliant blue were weighted in separate and
diluted together in a pharmaceutical vehicle to achieve amounts
of delphinidin 0.06% and brilliant blue of 0.025 to 0.05% of the
total composition, preferably 0.045 to 0.055% of the final
composition.
Example 3. A pharmaceutical composition with delphinidin and
trypan blue
Similarly, to the described in example 2, delphinidin and trypan
blue were weighted in separate and diluted together to achieve
amounts of delphinidin 0.06% and trypan blue of 0.001 to 0.2% of
the total composition, preferably of 0.04 to 0.15% of the final
composition.
16
CA 3028294 2018-12-21
Example 4. A pharmaceutical composition with delphinidin,
brilliant blue FCF and trypan blue
In this example, all the described steps of the previous examples
were followed and a pharmaceutical composition having delphinidin
0.06%, brilliant blue FCF of 0.025 to 0.05% of the total
composition, preferably 0.045 to 0.055% and trypan blue of 0.001
to 0.2% of the total composition, preferably of 0.04 to 0.15% of
the final composition was achieved.
Example 5. Pharmaceutical compositions with different
concentrations of delphinidin alone or in combination with
brilliant blue and/or trypan blue
Different pharmaceutical compositions were obtained, by the
process described in any of the previous examples, with different
concentrations of the 3 dyes of interest.
Example 6. Efficacy of delphinidin in staining the lens capsule
and ILM - Comparative example (COMP)
Lens capsule and ILM were stained with a delphinidin solution
according to the method described by Chuang et al.
Lenses and retinas were obtained from fresh pig eyes purchased
from a slaughterhouse, thirty minutes before staining at room
temperature. A delphinidin solution was prepared at a
concentration of 1 mg/ml in a 5% glucose solution in water and
twenty-four lenses were soaked in the dye solution for 1 minute.
17
CA 3028294 2018-12-21
The lenses were rinsed with BSS to remove the excess dye and
examined under a surgical microscope by the same cataract
surgeon. Regarding ILM staining, the vitreous of the eye globe
was removed from the surface of retina, stained and examined
similarly to the lenses. Both the lens capsule and ILM were
stained, exhibiting a blue color.
Example 7. Delphinidin safety and neuroprotective effects in
vitro - Comparative example (COMP)
The viability ARPE19 and RGC5 cell cultures was determined by
the method described by Chuang et al. after incubation with
delphinidin. Cell viability was determined using the reagent WST-
1 (Roche Diagnostics GmbH, Penzberg, Germany), a tetrazolium salt
that is cleaved by mitochondrial dehydrogenase, present in
metabolically active cells. Such reaction generates a coloured
formazan dye, detected in a microplate reader that measures
absorbance at 450nm. At a concentration of 1 mg/ml, delphinidin
was non-toxic with similar survival rates as controls treated
with 5% glucose. Additionally, after H202 treatment, delphinidin
significantly increased the survival rate of the ARPE19 cells
compared with that of the control cells, indicating a
neuroprotective effect.
Example 8. Delphinidin injection in rats, histology and retinal
function evaluation - Comparative example (COMP)
Anesthetized rats were injected with a solution of 0.15 ml/kg of
an equal volume of 2% lidocaine (Xylocaine; Astra, Sweden) and
18
CA 3028294 2018-12-21
50 mg/ml ketamine (Ketalar; Parke-Davis, USA), according to the
method described in Chuang et al. After anaesthesia, the pupils
were dilated and the eyes were protruded. A 1.5-cm/33-gauge
Hamilton blunt-tip syringe needle was inserted into the vitreous
under a surgical microscope, injecting 5 pl of dye solution. The
control eye was injected with BSS. Regarding the subretinal
injection, a 1.5-cm/33-gauge blunt-tip syringe (Hamilton, Reno,
NV) was inserted tangentially toward the posterior pole of the
eye, and delphinidin was injected. The control eye was injected
with BSS.
For histologic examination, the eyeballs were collected from six
rats, 1 and 3 months after delphinidin injection, and fixed with
4% paraformaldehyde at 4 C for 24 hours. The fixed tissues were
embedded in paraffin, sectioned at 5 pm and stained with
haematoxylin and eosin. The histology of the delphinidin-treated
and control eyes did not show major anatomical signs of toxicity.
Apoptotic cell death evaluation by TUNEL essay performed 1 month
after intravitreal injection showed similar staining among the
groups, with no differences in cell death compared with the
control group.
Corneal electroretinograms (ERGs) were obtained from the injected
rats a month after the intravitreal injections of delphinidin or
BSS. ERGs were recorded with an ERG instrument (UTAS-E 300; LKC
Technologies, Gaithersburg, MD, USA). Briefly, rats were
maintained in the dark for 1 hour before recording the ERGs and
all procedures were performed in a dark room subsequently.
Animals were placed on a heating pad at 35-36 C and, after
anaesthesia, the eyes were dilated and protruded. The Ag/AgC1
recording electrode was attached to the cornea, the reference
19
CA 3028294 2018-12-21
electrode to the shaven skin of the head and the ground electrode
to the rat's ear. The light stimulus was a light flash of 100 ms
at a distance of 30 cm from both eyes. Responses were then
recorded. ERG data from the rats were normal, showing no
reductions in function at baseline or one month after the
intravitreal injection of delphinidin.
CA 3028294 2018-12-21