Note: Descriptions are shown in the official language in which they were submitted.
CA 03030354 2019-01-09
WO 2018/019842
PCT/EP2017/068784
1
Method for controlling PPO resistant weeds
Description
The invention relates to a method for controlling PPO resistant weeds, wherein
at least one or
more compounds of formula (I) are applied to the PPO inhibitor herbicide
resistant weed, parts
of it or its propagation material.
Herbicide resistant weeds present a serious problem for efficient weed control
because such
resistant weeds are increasingly widespread and thus weed control by the
application of
herbicides is no longer effective. In particular PPO resistant weeds are a
huge problem to
farmers.
Thus, there is a need for an effective and efficient method for the control of
herbicide resistant
weeds, in particular PPO resistant weeds.
In crop protection, it is desirable to increase the specificity and
reliability of the action of active
compounds. In particular, it is desirable for the crop protection product to
control the harmful
plants (weeds) effectively and, at the same time, to be tolerated by the
useful plants (crops) in
question.
Thus, there is a need for a novel method to effectively control herbicide
resistant weeds, in
particular PPO resistant weeds, which at the same time is tolerated by the
useful plants (crops)
in question.
Surprisingly it has been found that compounds of formula (I) provide an
efficient control against
PPO resistant weeds.
Some of the compounds of formula (I) and their herbicidal activities are
disclosed in
US 6,537,948 and WO 2011/137088.
WO 2017/007873 discloses inter alia the control of glyphosate-resistant weeds
by application of
compositions comprising some compounds of formula (I) and glyphosate.
However, acceptable efficacy of compounds of formula (I) against PPO resistant
weeds is
unknown.
Accordingly, the present invention provides a method for controlling the
growth of PPO resistant
weeds, which comprises contacting such weeds, parts of it, its propagation
material or its
habitat with compounds of formula (I)
CA 03030354 2019-01-09
WO 2018/019842
PCT/EP2017/068784
2
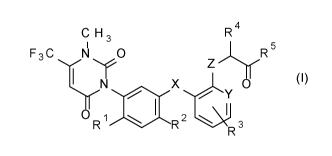
C H 3 R4
I 5
F 3 C N 0
R
1 I
x 0
0 R 1 2
R R 3
wherein
R1 is H, F or CI;
R2 is F, Cl, Br, ON, C(0)NH2 or C(S)NH;
R3 is H, F, CI, CH3 or 00H3;
R4 is H, ON, CH3, OF3, OCH3, 002H5, SCH3, SC2H5, (C0)002H5 or
CH2R6,
wherein R6 is F, Cl, OCH3, SCH3, SC2H5, CH2F, CH2Br or CH2OH;
R5 is (C1-06-alkyl)amino, (C1-06-dialkyl)amino, (NH)0R7, OH, OW or
SR8,
wherein R7 is CH3, 02H5 or phenyl; and
R8 is CI-Cs-alkyl, 02-06-alkenyl, 03-06-alkynyl, C1-06-haloalkyl, 01-06-
alkoxy-Ci-06-alkyl, C1-06-alkoxy-C1-06-alkoxy-C1-06-alkyl, 02-06-
cyanoalkyl, C1-04-alkoxy-carbonyl-C1-04-alkyl, 01-04-alkyl-carbonyl-
amino, C1-06-alkylsulfinyl-C1-06-alkyl, C1-06-alkyl-sulfonyl-C1-06-alkyl,
C1-06-dialkoxy-C1-06-alkyl, C1-06-alkyl-carbonyloxy-C1-06-alkyl,
phenyl-carbonyl-CI-Cs-alkyl, tri(C1-03-alkyl)-silyl-C1-06-alkyl, tri(C1-03-
alkyl)-sily1-02-06-alkenyl, tri(C1-03-alkyl)-sily1-02-06-alkynyl, tri(01-03-
alkyl)-silyl-C1-06-alkoxy-C1-06-alkyl, dimethylamino, tetrahydropyra-
nyl, tetrahydrofuranyl-Ci-03-alkyl, phenyl-C1-06-alkoxy-C1-06-alkyl,
phenyl-Ci-03-alkyl, pyridyl-Ci-03-alkyl, pyridyl or phenyl,
wherein the pyridyl and phenyl rings independently of one
another are substituted by one to five substituents selected
from the group consisting of halogen, Ci-03-alkyl or C1-02-
haloalkyl;
03-06-cycloalkyl or 03-06-cycloalkyl-Ci-04-alkyl,
wherein the cycloalkyl rings indenpently of one another are
unsubstituted or substituted by one to five substituents selected
from the group consisting of halogen, Ci-03-alkyl and C1-02-
haloalkyl,
X is 0, S, S(0) or S(0)2;
Y is N or CH; and
Z is 0, S, S(0) or S(0)2;
wherein the PPO resistant weeds are weeds, that are resistant to PPO-
inhibiting herbicides
except the compounds of formula (I).
The invention particularly relates to a method for controlling PPO resistant
weeds in crops which
comprises applying compounds of formula (I) according to the method of the
present invention
to crops, where said PPO herbicide resistant weeds occur or might occur.
CA 03030354 2019-01-09
WO 2018/019842
PCT/EP2017/068784
3
The invention furthermore relates to a method for controlling herbicide
resistant weeds, which
comprises allowing compounds of formula (I) according to the present invention
to act on plants,
their habitat or on seed.
The present invention also provides a method for controlling PPO resistant
weeds, wherein
herbicidal compositions comprising at least one compound of formula (I)
(component A) and at
least one further compound selected from the herbicidal compounds B (component
B) and/or
safeners C (component C) are applied to such PPO resistant weeds, parts of
them or their
propagation material.
The present invention also provides a method for controlling PPO resistant
weeds, wherein
agrochemical compositions comprising at least one compounds of formula (I) and
auxiliaries
customary for formulating crop protection agents are applied to the PPO
inhibitor herbicide
resistant weed, parts of it or its propagation material.
The invention furthermore relates to the use of compounds of formula (I) or
herbicidal
composition comprising them for controlling PPO resistant weeds.
Accordingly, in another aspect of the invention there is provided use of
compounds of formula
(I) for controlling herbicide resistant weeds, in particular PPO resistant
weeds.
The invention furthermore relates to a method for controlling undesirable
vegetation, the method
comprises applying compound of formula (I) according to the present invention
to the
undesirable plants. Application can be done before, during and/or after the
emergence of the
undesirable plants.
Further embodiments of the present invention can be found in the claims, the
description and
the examples. It is to be understood that the features mentioned above and
those still to be
illustrated below of the subject matter of the invention can be applied not
only in the respective
given combination but also in other combinations without leaving the scope of
the invention.
As used herein, the terms "controlling" and "combating" are synonyms.
As used herein, the terms "undesirable vegetation", "harmful plants" and
"weeds" are
synonyms.
As used herein, the terms "PPO inhibitor", "PPO inhibitor herbicide", "PPO-
inhibiting herbicide",
"protoporphyrinogen IX oxidase inhibitor herbicide", "protoporphyrinogen IX
oxidase-inhibiting
herbicide", "protoporphyrinogen oxidase inhibitor herbicide" and and
"protoporphyrinogen
oxidase-inhibiting herbicide" are synonyms and refers to herbicide that
inhibits enzyme
protoporphyrinogen oxidase of a plant.
CA 03030354 2019-01-09
WO 2018/019842
PCT/EP2017/068784
4
As used herein, the terms "PPO inhibitor herbicide resistant weed", "PPO-
inhibiting herbicide
resistant weed", "PPO inhibitor resistant weed", "PPO resistant weed",
"protoporphyrinogen IX
oxidase inhibitor herbicide resistant weed", "protoporphyrinogen IX oxidase
inhibiting herbicide
resistant weed", "protoporphyrinogen oxidase inhibitor herbicide resistant
weed" , and
"protoporphyrinogen oxidase inhibiting herbicide resistant weed" are synonyms
and refer to a
plant that, in relation to a treatment with an appropriate or over-appropriate
rate of PPO-
inhibiting herbicide application, has inherited, developed or acquired an
ability
(1) to survive that treatment, if it is one that is lethal to (i.e.
eradicates) the wild type weed;
or
(2) to exhibit significant vegetative growth or thrive after that treatment,
if it is one that
suppresses growth of the wild-type weed.
Effective weed control is defined as at least 70% weed suppression or
eradication from the
crop, or as at least 70% weed plant phototixicty, as determined 2 weeks after
treatment.
Thus, PPO resistant weeds are weeds, which are not controlled by the
application of PPO
inhibitors except the compound of formula (I), whereas the respective
sensitive biotype is
controlled at that use rate.
Here, "not controlled" means that in a visual rating the weed control
(herbicidal effect) is <70 %
of weed suppression or eradication as determined 2 weeks after treatment; and
"controlled"
means that in a visual rating the weed control is > 90 % of weed suppression
or eradication as
determined 2 weeks after treatment.
Preferably, PPO resistant weeds are weeds, which are not controlled (i.e. in a
visual rating the
weed control is < 70 % of weed suppression or eradication as determined 2
weeks after
treatment) by the application of PPO-inhibiting herbicides except the compound
of formula (I).
Also preferably, PPO resistant weeds are weeds, which are not controlled (i.e.
in a visual rating
the weed control is <70 % of weed suppression or eradication as determined 2
weeks after
treatment) by the application of PPO-inhibiting herbicides selected from
azafenidin and
flumioxazin.
Also preferably, PPO resistant weeds are weeds, which are not controlled (i.e.
in a visual rating
the weed control is <70 % of weed suppression or eradication as determined 2
weeks after
treatment) by the application of PPO-inhibiting herbicides selected from
fomesafen and lactofen.
Also preferably, PPO resistant weeds are weeds, which are not controlled (i.e.
in a visual rating
the weed control is <70 % of weed suppression or eradication as determined 2
weeks after
treatment) by the application of PPO-inhibiting herbicides selected from
azafenidin, flumioxazin,
fomesafen and lactofen.
CA 03030354 2019-01-09
WO 2018/019842
PCT/EP2017/068784
Also preferably, PPO resistant weeds are weeds, which are not controlled (i.e.
in a visual rating
the weed control is < 70 % of weed suppression or eradication as determined 2
weeks after
treatment) by the application of PPO-inhibiting herbicides selected from
flumioxazin, fomesafen,
lactofen, oxyfluorfen and sulfentrazone.
5
Also preferably, PPO resistant weeds are weeds, which are not controlled (i.e.
in a visual rating
the weed control is <70 % of weed suppression or eradication as determined 2
weeks after
treatment) by the application of PPO-inhibiting herbicides selected from
azafenidin, flumioxazin,
fomesafen, lactofen, oxyfluorfen and sulfentrazone.
Also preferably, PPO resistant weeds are weeds, which are not controlled (i.e.
in a visual rating
the weed control is <70 % of weed suppression or eradication as determined 2
weeks after
treatment) by the application of PPO-inhibiting herbicides selected from
acifluorfen,
carfentrazone, flumiclorac, flumioxazin, fomesafen, lactofen, oxadiazon,
oxyfluorfen, pyraflufen
and sulfentrazone.
Also preferably, PPO resistant weeds are weeds, which are not controlled (i.e.
in a visual rating
the weed control is <70 % of weed suppression or eradication as determined 2
weeks after
treatment) by the application of PPO-inhibiting herbicides selected from
acifluorfen, azafenidin,
carfentrazone, flumiclorac, flumioxazin, fomesafen, lactofen, oxadiazon,
oxyfluorfen, pyraflufen
and sulfentrazone.
Also preferably, PPO resistant weeds are weeds, which are not controlled (i.e.
in a visual rating
the weed control is < 70 % of weed suppression or eradication as determined 2
weeks after
treatment) by the application of at least one PPO-inhibiting herbicide except
the compound of
formula (I).
Also preferably, PPO resistant weeds are weeds, which are not controlled (i.e.
in a visual rating
the weed control is <70 % of weed suppression or eradication as determined 2
weeks after
treatment) by the application of at least one PPO-inhibiting herbicide
selected from azafenidin
and flumioxazin.
Also preferably, PPO resistant weeds are weeds, which are not controlled (i.e.
in a visual rating
the weed control is <70 % of weed suppression or eradication as determined 2
weeks after
treatment) by the application of at least one PPO-inhibiting herbicide
selected from fomesafen
and lactofen.
Also preferably, PPO resistant weeds are weeds, which are not controlled (i.e.
in a visual rating
the weed control is <70 % of weed suppression or eradication as determined 2
weeks after
treatment) by the application of at least one PPO-inhibiting herbicide
selected from azafenidin,
flumioxazin, fomesafen and lactofen.
Also preferably, PPO resistant weeds are weeds, which are not controlled (i.e.
in a visual rating
the weed control is <70 % of weed suppression or eradication as determined 2
weeks after
CA 03030354 2019-01-09
WO 2018/019842
PCT/EP2017/068784
6
treatment) by the application of at least one PPO-inhibiting herbicide
selected from flumioxazin,
fomesafen, lactofen, oxyfluorfen and sulfentrazone.
Also preferably, PPO resistant weeds are weeds, which are not controlled (i.e.
in a visual rating
the weed control is <70 % of weed suppression or eradication as determined 2
weeks after
treatment) by the application of at least one PPO-inhibiting herbicide
selected from azafenidin,
flumioxazin, fomesafen, lactofen, oxyfluorfen and sulfentrazone.
Also preferably, PPO resistant weeds are weeds, which are not controlled (i.e.
in a visual rating
the weed control is < 70 % of weed suppression or eradication as determined 2
weeks after
treatment) by the application of at least one PPO-inhibiting herbicide
selected from acifluorfen,
carfentrazone, flumiclorac, flumioxazin, fomesafen, lactofen, oxadiazon,
oxyfluorfen, pyraflufen
and sulfentrazone.
Also preferably, PPO resistant weeds are weeds, which are not controlled (i.e.
in a visual rating
the weed control is <70 % of weed suppression or eradication as determined 2
weeks after
treatment) by the application of at least one PPO-inhibiting herbicide
selected from acifluorfen,
azafenidin, carfentrazone, flumiclorac, flumioxazin, fomesafen, lactofen,
oxadiazon, oxyfluorfen,
pyraflufen and sulfentrazone.
Also preferably, PPO resistant weeds are weeds, which are not controlled (i.e.
in a visual rating
the weed control is <70 % of weed suppression or eradication as determined 2
weeks after
treatment) by the application of PPO-inhibiting herbicides except the compound
of formula (I),
whereas the respective sensitive biotype is controlled (i.e. in a visual
rating the weed control is >
90 % of weed suppression or eradication as determined 2 weeks after
treatment).
Also preferably, PPO resistant weeds are weeds, which are not controlled (i.e.
in a visual rating
the weed control is <70 % of weed suppression or eradication as determined 2
weeks after
treatment) by the application of PPO-inhibiting herbicides selected from
azafenidin and
flumioxazin, whereas the respective sensitive biotype is controlled (i.e. in a
visual rating the
weed control is > 90 % of weed suppression or eradication as determined 2
weeks after
treatment).
Also preferably, PPO resistant weeds are weeds, which are not controlled (i.e.
in a visual rating
the weed control is <70 % of weed suppression or eradication as determined 2
weeks after
treatment) by the application of PPO-inhibiting herbicides selected from
fomesafen and lactofen,
whereas the respective sensitive biotype is controlled (i.e. in a visual
rating the weed control is >
90 % of weed suppression or eradication as determined 2 weeks after
treatment).
Also preferably, PPO resistant weeds are weeds, which are not controlled (i.e.
in a visual rating
the weed control is <70 % of weed suppression or eradication as determined 2
weeks after
treatment) by the application of PPO-inhibiting herbicides selected from
azafenidin, flumioxazin,
fomesafen and lactofen, whereas the respective sensitive biotype is controlled
(i.e. in a visual
CA 03030354 2019-01-09
WO 2018/019842
PCT/EP2017/068784
7
rating the weed control is > 90 % of weed suppression or eradication as
determined 2 weeks
after treatment).
Also preferably, PPO resistant weeds are weeds, which are not controlled (i.e.
in a visual rating
the weed control is <70 % of weed suppression or eradication as determined 2
weeks after
treatment) by the application of PPO-inhibiting herbicides selected from
flumioxazin, fomesafen,
lactofen, oxyfluorfen and sulfentrazone, whereas the respective sensitive
biotype is controlled
(i.e. in a visual rating the weed control is > 90 % of weed suppression or
eradication as
determined 2 weeks after treatment).
Also preferably, PPO resistant weeds are weeds, which are not controlled (i.e.
in a visual rating
the weed control is < 70 % of weed suppression or eradication as determined 2
weeks after
treatment) by the application of PPO-inhibiting herbicides selected from
azafenidin, flumioxazin,
fomesafen, lactofen, oxyfluorfen and sulfentrazone, whereas the respective
sensitive biotype is
.. controlled (i.e. in a visual rating the weed control is > 90 % of weed
suppression or eradication
as determined 2 weeks after treatment).
Also preferably, PPO resistant weeds are weeds, which are not controlled (i.e.
in a visual rating
the weed control is <70 % of weed suppression or eradication as determined 2
weeks after
treatment) by the application of PPO-inhibiting herbicides selected from
acifluorfen,
carfentrazone, flumiclorac, flumioxazin, fomesafen, lactofen, oxadiazon,
oxyfluorfen, pyraflufen
and sulfentrazone, whereas the respective sensitive biotype is controlled
(i.e. in a visual rating
the weed control is > 90 % of weed suppression or eradication as determined 2
weeks after
treatment).
Also preferably, PPO resistant weeds are weeds, which are not controlled (i.e.
in a visual rating
the weed control is <70 % of weed suppression or eradication as determined 2
weeks after
treatment) by the application of PPO-inhibiting herbicides selected from
acifluorfen, azafenidin,
carfentrazone, flumiclorac, flumioxazin, fomesafen, lactofen, oxadiazon,
oxyfluorfen, pyraflufen
and sulfentrazone, whereas the respective sensitive biotype is controlled
(i.e. in a visual rating
the weed control is > 90 % of weed suppression or eradication as determined 2
weeks after
treatment).
Also preferably, PPO resistant weeds are weeds, which are not controlled (i.e.
in a visual rating
the weed control is <70 % of weed suppression or eradication as determined 2
weeks after
treatment) by the application of at least one PPO-inhibiting herbicide except
the compound of
formula (I), whereas the respective sensitive biotype is controlled (i.e. in a
visual rating the weed
control is > 90 % of weed suppression or eradication as determined 2 weeks
after treatment).
Also preferably, PPO resistant weeds are weeds, which are not controlled (i.e.
in a visual rating
the weed control is <70 % of weed suppression or eradication as determined 2
weeks after
treatment) by the application of at least one PPO-inhibiting herbicide
selected from azafenidin
and flumioxazin, whereas the respective sensitive biotype is controlled (i.e.
in a visual rating the
CA 03030354 2019-01-09
WO 2018/019842
PCT/EP2017/068784
8
weed control is > 90 % of weed suppression or eradication as determined 2
weeks after
treatment).
Also preferably, PPO resistant weeds are weeds, which are not controlled (i.e.
in a visual rating
the weed control is <70 % of weed suppression or eradication as determined 2
weeks after
treatment) by the application of at least one PPO-inhibiting herbicide
selected from fomesafen
and lactofen, whereas the respective sensitive biotype is controlled (i.e. in
a visual rating the
weed control is > 90 % of weed suppression or eradication as determined 2
weeks after
treatment).
Also preferably, PPO resistant weeds are weeds, which are not controlled (i.e.
in a visual rating
the weed control is <70 % of weed suppression or eradication as determined 2
weeks after
treatment) by the application of at least one PPO-inhibiting herbicide
selected from azafenidin,
flumioxazin, fomesafen and lactofen, whereas the respective sensitive biotype
is controlled (i.e.
in a visual rating the weed control is > 90 % of weed suppression or
eradication as determined 2
weeks after treatment).
Also preferably, PPO resistant weeds are weeds, which are not controlled (i.e.
in a visual rating
the weed control is <70 % of weed suppression or eradication as determined 2
weeks after
treatment) by the application of at least one PPO-inhibiting herbicide
selected from flumioxazin,
fomesafen, lactofen, oxyfluorfen and sulfentrazone, whereas the respective
sensitive biotype is
controlled (i.e. in a visual rating the weed control is > 90 % of weed
suppression or eradication
as determined 2 weeks after treatment).
Also preferably, PPO resistant weeds are weeds, which are not controlled (i.e.
in a visual rating
the weed control is <70 % of weed suppression or eradication as determined 2
weeks after
treatment) by the application of at least one PPO-inhibiting herbicide
selected from azafenidin,
flumioxazin, fomesafen, lactofen, oxyfluorfen and sulfentrazone, whereas the
respective
sensitive biotype is controlled (i.e. in a visual rating the weed control is >
90 % of weed
suppression or eradication as determined 2 weeks after treatment).
Also preferably, PPO resistant weeds are weeds, which are not controlled (i.e.
in a visual rating
the weed control is <70 % of weed suppression or eradication as determined 2
weeks after
treatment) by the application of at least one PPO-inhibiting herbicide
selected from acifluorfen,
carfentrazone, flumiclorac, flumioxazin, fomesafen, lactofen, oxadiazon,
oxyfluorfen, pyraflufen
and sulfentrazone, whereas the respective sensitive biotype is controlled
(i.e. in a visual rating
the weed control is > 90 % of weed suppression or eradication as determined 2
weeks after
treatment).
Also preferably, PPO resistant weeds are weeds, which are not controlled (i.e.
in a visual rating
the weed control is <70 % of weed suppression or eradication as determined 2
weeks after
treatment) by the application of at least one PPO-inhibiting herbicide
selected from acifluorfen,
azafenidin, carfentrazone, flumiclorac, flumioxazin, fomesafen, lactofen,
oxadiazon, oxyfluorfen,
pyraflufen and sulfentrazone, whereas the respective sensitive biotype is
controlled (i.e. in a
CA 03030354 2019-01-09
WO 2018/019842
PCT/EP2017/068784
9
visual rating the weed control is > 90 % of weed suppression or eradication as
determined 2
weeks after treatment).
According to a specific embodiment the present invention provides a method for
controlling the
growth of PPO resistant weeds, which comprises contacting such weeds, parts of
it, its
propagation material or its habitat with compounds of formula (I), wherein the
PPO resistant
weeds are weeds, that are resistant to flumioxazin;
i.e. a method for controlling the growth of flumioxazin resistant weeds, which
comprises
contacting such weeds, parts of it, its propagation material or its habitat
with compounds of
formula (I).
According to another specific embodiment the present invention provides a
method for
controlling the growth of PPO resistant weeds, which comprises contacting such
weeds, parts of
it, its propagation material or its habitat with compounds of formula (I),
wherein the PPO
resistant weeds are weeds, that are resistant to fomesafen;
i.e. a method for controlling the growth of fomesafen resistant weeds, which
comprises
contacting such weeds, parts of it, its propagation material or its habitat
with compounds of
formula (I).
According to another specific embodiment the present invention provides a
method for
controlling the growth of PPO resistant weeds, which comprises contacting such
weeds, parts of
it, its propagation material or its habitat with compounds of formula (I),
wherein the PPO
resistant weeds are weeds, that are resistant to lactofen;
i.e. a method for controlling the growth of lactofen resistant weeds, which
comprises contacting
such weeds, parts of it, its propagation material or its habitat with
compounds of formula (I).
According to another specific embodiment the present invention provides a
method for
controlling the growth of PPO resistant weeds, which comprises contacting such
weeds, parts of
it, its propagation material or its habitat with compounds of formula (I),
wherein the PPO
resistant weeds are weeds, that are resistant to oxyfluorfen;
i.e. a method for controlling the growth of oxyfluorfen resistant weeds, which
comprises
contacting such weeds, parts of it, its propagation material or its habitat
with compounds of
formula (I).
According to another specific embodiment the present invention provides a
method for
controlling the growth of PPO resistant weeds, which comprises contacting such
weeds, parts of
it, its propagation material or its habitat with compounds of formula (I),
wherein the PPO
resistant weeds are weeds, that are resistant to sulfentrazone;
i.e. a method for controlling the growth of sulfentrazone resistant weeds,
which comprises
contacting such weeds, parts of it, its propagation material or its habitat
with compounds of
formula (I).
CA 03030354 2019-01-09
WO 2018/019842
PCT/EP2017/068784
According to another specific embodiment the present invention provides a
method for
controlling the growth of PPO resistant weeds, which comprises contacting such
weeds, parts of
it, its propagation material or its habitat with compounds of formula (I),
wherein the PPO
resistant weeds are weeds, that are resistant to at least one PPO selected
from azafenidin and
5 flumioxazin;
i.e. a method for controlling the growth of azafenidin and/or flumioxazin
resistant weeds, which
comprises contacting such weeds, parts of it, its propagation material or its
habitat with
compounds of formula (I).
10 According to another specific embodiment the present invention provides
a method for
controlling the growth of PPO resistant weeds, which comprises contacting such
weeds, parts of
it, its propagation material or its habitat with compounds of formula (I),
wherein the PPO
resistant weeds are weeds, that are resistant to at least one PPO selected
from fomesafen and
lactofen;
i.e. a method for controlling the growth of fomesafen and/or lactofen
resistant weeds, which
comprises contacting such weeds, parts of it, its propagation material or its
habitat with
compounds of formula (I).
According to another specific embodiment the present invention provides a
method for
controlling the growth of PPO resistant weeds, which comprises contacting such
weeds, parts of
it, its propagation material or its habitat with compounds of formula (I),
wherein the PPO
resistant weeds are weeds, that are resistant to at least one PPO selected
from azafenidin,
flumioxazin and fomesafen;
i.e. a method for controlling the growth of azafenidin, flumioxazin and/or
fomesafen resistant
weeds, which comprises contacting such weeds, parts of it, its propagation
material or its
habitat with compounds of formula (I).
According to another specific embodiment the present invention provides a
method for
controlling the growth of PPO resistant weeds, which comprises contacting such
weeds, parts of
it, its propagation material or its habitat with compounds of formula (I),
wherein the PPO
resistant weeds are weeds, that are resistant to at least one PPO selected
from azafenidin,
flumioxazin and lactofen;
i.e. a method for controlling the growth of azafenidin, flumioxazin and/or
lactofen resistant
weeds, which comprises contacting such weeds, parts of it, its propagation
material or its
habitat with compounds of formula (I).
According to another specific embodiment the present invention provides a
method for
controlling the growth of PPO resistant weeds, which comprises contacting such
weeds, parts of
it, its propagation material or its habitat with compounds of formula (I),
wherein the PPO
resistant weeds are weeds, that are resistant to at least one PPO selected
from azafenidin,
flumioxazin, fomesafen and lactofen;
i.e. a method for controlling the growth of azafenidin, flumioxazin fomesafen
and/or lactofen
resistant weeds, which comprises contacting such weeds, parts of it, its
propagation material or
its habitat with compounds of formula (I).
CA 03030354 2019-01-09
WO 2018/019842
PCT/EP2017/068784
11
According to another specific embodiment the present invention provides a
method for
controlling the growth of PPO resistant weeds, which comprises contacting such
weeds, parts of
it, its propagation material or its habitat with compounds of formula (I),
wherein the PPO
.. resistant weeds are weeds, that are resistant to at least one PPO selected
from flumioxazin,
fomesafen, lactofen, oxyfluorfen and sulfentrazone;
i.e. a method for controlling the growth of flumioxazin, fomesafen, lactofen,
oxyfluorfen and/or
sulfentrazone resistant weeds, which comprises contacting such weeds, parts of
it, its
propagation material or its habitat with compounds of formula (I).
According to another specific embodiment the present invention provides a
method for
controlling the growth of PPO resistant weeds, which comprises contacting such
weeds, parts of
it, its propagation material or its habitat with compounds of formula (I),
wherein the PPO
resistant weeds are weeds, that are resistant to at least one PPO selected
from azafenidin,
fflumioxazin, fomesafen, lactofen, oxyfluorfen and sulfentrazone;
i.e. a method for controlling the growth of azafenidin, flumioxazin,
fomesafen, lactofen,
oxyfluorfen and/or sulfentrazone resistant weeds, which comprises contacting
such weeds,
parts of it, its propagation material or its habitat with compounds of formula
(I).
According to another specific embodiment the present invention provides a
method for
controlling the growth of PPO resistant weeds, which comprises contacting such
weeds, parts of
it, its propagation material or its habitat with compounds of formula (I),
wherein the PPO
resistant weeds are weeds, that are resistant to at least one PPO selected
from acifluorfen,
carfentrazone, flumiclorac, flumioxazin, fomesafen, lactofen, oxadiazon,
oxyfluorfen, pyraflufen
and sulfentrazone;
i.e. a method for controlling the growth of acifluorfen, carfentrazone,
flumiclorac, flumioxazin,
fomesafen, lactofen, oxadiazon, oxyfluorfen, pyraflufen and/or sulfentrazone
resistant weeds,
which comprises contacting such weeds, parts of it, its propagation material
or its habitat with
compounds of formula (I).
According to another specific embodiment the present invention provides a
method for
controlling the growth of PPO resistant weeds, which comprises contacting such
weeds, parts of
it, its propagation material or its habitat with compounds of formula (I),
wherein the PPO
resistant weeds are weeds, that are resistant to at least one PPO selected
from acifluorfen,
azafenidin, carfentrazone, flumiclorac, flumioxazin, fomesafen, lactofen,
oxadiazon, oxyfluorfen,
pyraflufen and sulfentrazone;
i.e. a method for controlling the growth of acifluorfen, azafenidin,
carfentrazone, flumiclorac,
flumioxazin, fomesafen, lactofen, oxadiazon, oxyfluorfen, pyraflufen and/or
sulfentrazone
resistant weeds, which comprises contacting such weeds, parts of it, its
propagation material or
.. its habitat with compounds of formula (I).
CA 03030354 2019-01-09
WO 2018/019842
PCT/EP2017/068784
12
Also preferably, PPO resistant weeds are weeds, which are not controlled (i.e.
in a visual rating
the weed control is <70 % of weed suppression or eradication as determined 2
weeks after
treatment) by the application rate of
200 g/ha or lower,
particularly preferred 100 g/ha or lower,
especially preferred 50 to 200 g/ha,
more preferred 50 to 100 g/ha,
of PPO-inhibiting herbicides except the compound of formula (I), whereas the
respective
sensitive biotype is controlled (i.e. in a visual rating the weed control is >
90 % of weed
.. suppression or eradication as determined 2 weeks after treatment) at that
use rate.
Also preferably, PPO resistant weeds are weeds, which are not controlled (i.e.
in a visual rating
the weed control is <70 % of weed suppression or eradication as determined 2
weeks after
treatment) by the application rate of
200 g/ha or lower,
particularly preferred 100 g/ha or lower,
especially preferred 50 to 200 g/ha,
more preferred 50 to 100 g/ha,
of PPO-inhibiting herbicides selected from azafenidin and flumioxazin, whereas
the respective
sensitive biotype is controlled (i.e. in a visual rating the weed control is >
90 % of weed
suppression or eradication as determined 2 weeks after treatment) at that use
rate.
Also preferably, PPO resistant weeds are weeds, which are not controlled (i.e.
in a visual rating
the weed control is <70 % of weed suppression or eradication as determined 2
weeks after
treatment) by the application rate of
200 g/ha or lower,
particularly preferred 100 g/ha or lower,
especially preferred 50 to 200 g/ha,
more preferred 50 to 100 g/ha,
of PPO-inhibiting herbicides selected from fomesafen and lactofen, whereas the
respective
sensitive biotype is controlled (i.e. in a visual rating the weed control is >
90 % of weed
suppression or eradication as determined 2 weeks after treatment) at that use
rate.
Also preferably, PPO resistant weeds are weeds, which are not controlled (i.e.
in a visual rating
the weed control is <70 % of weed suppression or eradication as determined 2
weeks after
treatment) by the application rate of
200 g/ha or lower,
particularly preferred 100 g/ha or lower,
especially preferred 50 to 200 g/ha,
more preferred 50 to 100 g/ha,
CA 03030354 2019-01-09
WO 2018/019842
PCT/EP2017/068784
13
of PPO-inhibiting herbicides selected from azafenidin, flumioxazin, fomesafen
and lactofen,
whereas the respective sensitive biotype is controlled (i.e. in a visual
rating the weed control is >
90 % of weed suppression or eradication as determined 2 weeks after treatment)
at that use
rate.
Also preferably, PPO resistant weeds are weeds, which are not controlled (i.e.
in a visual rating
the weed control is <70 % of weed suppression or eradication as determined 2
weeks after
treatment) by the application rate of
200 g/ha or lower,
particularly preferred 100 g/ha or lower,
especially preferred 50 to 200 g/ha,
more preferred 50 to 100 g/ha,
of PPO-inhibiting herbicides selected from flumioxazin, fomesafen, lactofen,
oxyfluorfen and
sulfentrazone, whereas the respective sensitive biotype is controlled (i.e. in
a visual rating the
weed control is > 90 % of weed suppression or eradication as determined 2
weeks after
treatment) at that use rate.
Also preferably, PPO resistant weeds are weeds, which are not controlled (i.e.
in a visual rating
the weed control is <70 % of weed suppression or eradication as determined 2
weeks after
treatment) by the application rate of
200 g/ha or lower,
particularly preferred 100 g/ha or lower,
especially preferred 50 to 200 g/ha,
more preferred 50 to 100 g/ha,
of PPO-inhibiting herbicides selected from azafenidin, flumioxazin, fomesafen,
lactofen,
oxyfluorfen and sulfentrazone, whereas the respective sensitive biotype is
controlled (i.e. in a
visual rating the weed control is > 90 % of weed suppression or eradication as
determined 2
weeks after treatment) at that use rate.
Also preferably, PPO resistant weeds are weeds, which are not controlled (i.e.
in a visual rating
the weed control is <70 % of weed suppression or eradication as determined 2
weeks after
treatment) by the application rate of
200 g/ha or lower,
particularly preferred 100 g/ha or lower,
especially preferred 50 to 200 g/ha,
more preferred 50 to 100 g/ha,
of PPO-inhibiting herbicides selected from acifluorfen, carfentrazone,
flumiclorac, flumioxazin,
fomesafen, lactofen, oxadiazon, oxyfluorfen, pyraflufen and sulfentrazone,
whereas the
respective sensitive biotype is controlled (i.e. in a visual rating the weed
control is > 90 % of
weed suppression or eradication as determined 2 weeks after treatment) at that
use rate.
CA 03030354 2019-01-09
WO 2018/019842
PCT/EP2017/068784
14
Also preferably, PPO resistant weeds are weeds, which are not controlled (i.e.
in a visual rating
the weed control is <70 % of weed suppression or eradication as determined 2
weeks after
treatment) by the application rate of
200 g/ha or lower,
particularly preferred 100 g/ha or lower,
especially preferred 50 to 200 g/ha,
more preferred 50 to 100 g/ha,
of PPO-inhibiting herbicides selected from acifluorfen, azafenidin,
carfentrazone, flumiclorac,
flumioxazin, fomesafen, lactofen, oxadiazon, oxyfluorfen, pyraflufen and
sulfentrazone, whereas
the respective sensitive biotype is controlled (i.e. in a visual rating the
weed control is > 90 % of
weed suppression or eradication as determined 2 weeks after treatment) at that
use rate.
Also preferably, PPO resistant weeds are weeds, which are not controlled (i.e.
in a visual rating
the weed control is <70 % of weed suppression or eradication as determined 2
weeks after
treatment) by the application rate of
200 g/ha or lower,
particularly preferred 100 g/ha or lower,
especially preferred 50 to 200 g/ha,
more preferred 50 to 100 g/ha,
of at least one PPO-inhibiting herbicide except the compound of formula (I),
whereas the
respective sensitive biotype is controlled (i.e. in a visual rating the weed
control is > 90 % of
weed suppression or eradication as determined 2 weeks after treatment) at that
use rate.
Also preferably, PPO resistant weeds are weeds, which are not controlled (i.e.
in a visual rating
the weed control is <70 % of weed suppression or eradication as determined 2
weeks after
treatment) by the application rate of
200 g/ha or lower,
particularly preferred 100 g/ha or lower,
especially preferred 50 to 200 g/ha,
more preferred 50 to 100 g/ha,
of at least one PPO-inhibiting herbicide selected from azafenidin and
flumioxazin, whereas the
respective sensitive biotype is controlled (i.e. in a visual rating the weed
control is > 90 % of
weed suppression or eradication as determined 2 weeks after treatment) at that
use rate.
Also preferably, PPO resistant weeds are weeds, which are not controlled (i.e.
in a visual rating
the weed control is <70 % of weed suppression or eradication as determined 2
weeks after
treatment) by the application rate of
200 g/ha or lower,
particularly preferred 100 g/ha or lower,
especially preferred 50 to 200 g/ha,
more preferred 50 to 100 g/ha,
CA 03030354 2019-01-09
WO 2018/019842
PCT/EP2017/068784
of at least one PPO-inhibiting herbicide selected from fomesafen and lactofen,
whereas the
respective sensitive biotype is controlled (i.e. in a visual rating the weed
control is > 90 % of
weed suppression or eradication as determined 2 weeks after treatment) at that
use rate.
5 Also preferably, PPO resistant weeds are weeds, which are not controlled
(i.e. in a visual rating
the weed control is <70 % of weed suppression or eradication as determined 2
weeks after
treatment) by the application rate of
200 g/ha or lower,
particularly preferred 100 g/ha or lower,
10 especially preferred 50 to 200 g/ha,
more preferred 50 to 100 g/ha,
of at least one PPO-inhibiting herbicide selected from azafenidin,
flumioxazin, fomesafen and
lactofen, whereas the respective sensitive biotype is controlled (i.e. in a
visual rating the weed
control is > 90 % of weed suppression or eradication as determined 2 weeks
after treatment) at
15 that use rate.
Also preferably, PPO resistant weeds are weeds, which are not controlled (i.e.
in a visual rating
the weed control is <70 % of weed suppression or eradication as determined 2
weeks after
treatment) by the application rate of
200 g/ha or lower,
particularly preferred 100 g/ha or lower,
especially preferred 50 to 200 g/ha,
more preferred 50 to 100 g/ha,
of at least one PPO-inhibiting herbicide selected from flumioxazin, fomesafen,
lactofen,
oxyfluorfen and sulfentrazone, whereas the respective sensitive biotype is
controlled (i.e. in a
visual rating the weed control is > 90 % of weed suppression or eradication as
determined 2
weeks after treatment) at that use rate.
Also preferably, PPO resistant weeds are weeds, which are not controlled (i.e.
in a visual rating
the weed control is <70 % of weed suppression or eradication as determined 2
weeks after
treatment) by the application rate of
200 g/ha or lower,
particularly preferred 100 g/ha or lower,
especially preferred 50 to 200 g/ha,
more preferred 50 to 100 g/ha,
of at least one PPO-inhibiting herbicide selected from azafenidin,
flumioxazin, fomesafen,
lactofen, oxyfluorfen and sulfentrazone, whereas the respective sensitive
biotype is controlled
(i.e. in a visual rating the weed control is > 90 % of weed suppression or
eradication as
determined 2 weeks after treatment) at that use rate.
CA 03030354 2019-01-09
WO 2018/019842
PCT/EP2017/068784
16
Also preferably, PPO resistant weeds are weeds, which are not controlled (i.e.
in a visual rating
the weed control is <70 % of weed suppression or eradication as determined 2
weeks after
treatment) by the application rate of
200 g/ha or lower,
particularly preferred 100 g/ha or lower,
especially preferred 50 to 200 g/ha,
more preferred 50 to 100 g/ha,
of at least one PPO-inhibiting herbicide selected from acifluorfen,
carfentrazone, flumiclorac,
flumioxazin, fomesafen, lactofen, oxadiazon, oxyfluorfen, pyraflufen and
sulfentrazone, whereas
the respective sensitive biotype is controlled (i.e. in a visual rating the
weed control is > 90 % of
weed suppression or eradication as determined 2 weeks after treatment) at that
use rate.
Also preferably, PPO resistant weeds are weeds, which are not controlled (i.e.
in a visual rating
the weed control is <70 % of weed suppression or eradication as determined 2
weeks after
treatment) by the application rate of
200 g/ha or lower,
particularly preferred 100 g/ha or lower,
especially preferred 50 to 200 g/ha,
more preferred 50 to 100 g/ha,
of at least one PPO-inhibiting herbicide selected from acifluorfen,
azafenidin, carfentrazone,
flumiclorac, flumioxazin, fomesafen, lactofen, oxadiazon, oxyfluorfen,
pyraflufen and
sulfentrazone, whereas the respective sensitive biotype is controlled (i.e. in
a visual rating the
weed control is > 90 % of weed suppression or eradication as determined 2
weeks after
treatment) at that use rate.
Also preferably PPO-resistant weeds are those classified as being "PPO
resistant" and thus listed
according to Anonymous: List of herbicide resistant weeds by herbicide mode of
action - weeds
resistant to PPO-inhibitors (URL:
http://www.weedscience.org/summary/MOA.aspx).
Particularly preferred the PPO resistant weeds are selected from the group
consisting of
Acalypha ssp., Amaranthus ssp., Ambrosia ssp., Avena ssp., Conyza ssp.,
Descurainia ssp.,
Euphorbia ssp. and Senecio ssp.;
especially preferred Amaranthus ssp., Ambrosia ssp. and Euphorbia ssp.;
more preferred Amaranthus ssp. and Ambrosia ssp..
Also particularly preferred the PPO resistant weeds are selected from the
group consisting of
Asian copperleaf (Acalypha australis), smooth pigweed (Amaranthus hybridus),
Palmer
amaranth (Amaranthus Palmeri), redroot pigweed (Amaranthus retroflexus),
tall/common
waterhemp (Amaranthus tuberculatus, Amaranthus rudis or Amaranthus
tamariscinus), common
ragweed (Ambrosia artemisiifolia or Ambrosia eliator), wild oat (Avena fatua),
fleabane (Conyza
CA 03030354 2019-01-09
WO 2018/019842
PCT/EP2017/068784
17
ambigua), marestail (Conyza Canadensis), flixweed (Descurainia Sophia), wild
poinsettia
(Euphorbia heterophylla) and eastern groundsel (Senecio vemalis);
especially preferred smooth pigweed (Amaranthus hybridus), Palmer amaranth
(Amaranthus
Palmeri), redroot pigweed (Amaranthus retroflexus), tall/common waterhemp
(Amaranthus
tuberculatus, Amaranthus rudis or Amaranthus tamariscinus), common ragweed
(Ambrosia
artemisiifolia or Ambrosia eliator) and wild poinsettia (Euphorbia
heterophylla);
more preferred tall/common waterhemp (Amaranthus tuberculatus, Amaranthus
rudis or
Amaranthus tamariscinus) and common ragweed (Ambrosia artemisiifolia or
Ambrosia eliator);
Most PPO resistant weeds, in particular the biotypes of Amaranthus
tuberculatus, are resistant due
to a codon deletion on the nuclear-encoded gene PPX2L that codes for the PPO
enzyme which is
dual-targeted to the mitochondria and the chloroplasts. This results in a loss
of the glycine amino
acid in position 210 (see e.g. B. G. Young et al, Characterization of PPO-
Inhibitor-Resistant
Waterhemp (Amaranthus tuberculatus) Response to Soil-Applied PPO-Inhibiting
Herbicides, Weed
Science 2015, 63, 511-521).
A second type of mutation, in particular in a resistant biotype of Ambrosia
artemisiifolia, was identified
as a mutation that expressed a R98L change of the PPX2 enzyme (S. L.
Rousonelos, R. M. Lee, M.
S. Moreira, M. J. VanGessel, P. J. Tranel, Characterization of a Common
Ragweed (Ambrosia
artemisiifolia) Population Resistant to ALS- and PPO-Inhibiting Herbicides,
Weed Science 60, 2012,
335-344.).
Accordingly, preferably PPO-resistant weeds are weeds whose Protox enzyme is
resistant to the
application of PPO inhibitors due to a mutation that is expressed as a AG210
or R98L change of
said Protox enzyme or equivalents to the PPX2L or PPX2 respectively, in
particular that is
expressed as a AG210 or R98L change of said Protox enzyme.
If the compounds of formula (I), the herbicidal compounds B and/or the
safeners C as described
herein are capable of forming geometrical isomers, for example E/Z isomers, it
is possible to
use both, the pure isomers and composition thereof, in the method according to
the invention.
If the compounds of formula (I), the herbicidal compounds B and/or the
safeners C as described
herein have one or more centers of chirality and, as a consequence, are
present as
enantiomers or diastereomers, it is possible to use both, the pure enantiomers
and
diastereomers and their composition, in the method according to the invention.
If the compounds of formula (I), the herbicidal compounds B and/or the
safeners C as described
herein have ionizable functional groups, they can also be employed in the form
of their
CA 03030354 2019-01-09
WO 2018/019842
PCT/EP2017/068784
18
agriculturally acceptable salts. Suitable are, in general, the salts of those
cations and the acid
addition salts of those acids whose cations and anions, respectively, have no
adverse effect on
the activity of the active compounds.
Preferred cations are the ions of the alkali metals, preferably of lithium,
sodium and potassium,
of the alkaline earth metals, preferably of calcium and magnesium, and of the
transition metals,
preferably of manganese, copper, zinc and iron, further ammonium and
substituted ammonium
in which one to four hydrogen atoms are replaced by C1-04-alkyl, hydroxy-C1-04-
alkyl, 01-04-
alkoxy-C1-04-alkyl, hydroxy-C1-04-alkoxy-C1-04-alkyl, phenyl or benzyl,
preferably ammonium,
methylammonium, isopropylammonium, dimethylammonium, diethylammonium,
diisopropylammonium, trimethylammonium, triethylammonium,
tris(isopropyl)ammonium,
heptylammonium, dodecylammonium, tetradecylammonium, tetramethylammonium,
tetra-
ethylammonium, tetrabutylammonium, 2-hydroxyethylammonium (olamine salt), 2-(2-
hydroxyeth-1-oxy)eth-1-ylammonium (diglycolamine salt), di(2-hydroxyeth-1-
yl)ammonium
(diolamine salt), tris(2-hydroxyethyl)ammonium (trolamine salt), tris(2-
hydroxypropyl)ammonium, benzyltrimethylammonium, benzyltriethylammonium, N,N,N-
trimethylethanolammonium (choline salt), furthermore phosphonium ions,
sulfonium ions,
preferably tri(C1-04-alkyl)sulfonium, such as trimethylsulfonium, and
sulfoxonium ions,
preferably tri(C1-04-alkyl)sulfoxonium, and finally the salts of polybasic
amines such as N,N-bis-
(3-aminopropyl)methylamine and diethylenetriamine.
Anions of useful acid addition salts are primarily chloride, bromide,
fluoride, iodide,
hydrogensulfate, methylsulfate, sulfate, dihydrogenphosphate,
hydrogenphosphate, nitrate,
bicarbonate, carbonate, hexafluorosilicate, hexafluorophosphate, benzoate and
also the anions
of C1-04-alkanoic acids, preferably formate, acetate, propionate and butyrate.
Compounds of formula (I), herbicidal compounds B and/or safeners C as
described herein
having a carboxyl group can be employed in the form of the acid, in the form
of an agriculturally
suitable salt as mentioned above or else in the form of an agriculturally
acceptable derivative,
for example as amides, such as mono- and di-C1-06-alkylamides or arylamides,
as esters, for
example as allyl esters, propargyl esters, Ci-Cio-alkyl esters, alkoxyalkyl
esters, tefuryl
((tetrahydrofuran-2-yl)methyl) esters and also as thioesters, for example as
Ci-Cio-alkylthio
esters. Preferred mono- and di-C1-06-alkylamides are the methyl and the
dimethylamides.
Preferred arylamides are, for example, the anilides and the 2-chloroanilides.
Preferred alkyl
esters are, for example, the methyl, ethyl, propyl, isopropyl, butyl,
isobutyl, pentyl, mexyl
(1-methylhexyl), meptyl (1-methylheptyl), heptyl, octyl or isooctyl (2-
ethylhexyl) esters. Preferred
Ci-04-alkoxy-C1-04-alkyl esters are the straight-chain or branched Ci-04-
alkoxy ethyl esters, for
example the 2-methoxyethyl, 2-ethoxyethyl, 2-butoxyethyl (butotyl), 2-
butoxypropyl or 3-
butoxypropyl ester. An example of a straight-chain or branched Ci-Cio-
alkylthio ester is the
ethylthio ester.
CA 03030354 2019-01-09
WO 2018/019842
PCT/EP2017/068784
19
The preferred embodiments of the invention mentioned herein below have to be
understood as
being preferred either independently from each other or in combination with
one another.
The organic moieties mentioned in the definition of the variables R1 to R8,
are - like the term
halogen - collective terms for individual enumerations of the individual group
members. The
term halogen denotes in each case fluorine, chlorine, bromine or iodine. All
hydrocarbon chains,
i.e. all alkyl, can be straight-chain or branched, the prefix On-Cm denoting
in each case the
possible number of carbon atoms in the group. Examples of such meanings are:
- 01-02-alkyl: for example CH3 and 02H5;
- 01-03-alkyl and also the 01-03-alkyl moieties of tri(C1-03-alkyl)-silyl-
C1-06-alkyl, tri(C1-03-
alkyl)-silyl-C1-06-alkenyl, tri(C1-03-alkyl)-sily1-02-06-alkynyl, tri(C1-03-
alkyl)-silyl-C1-06-alkoxy-C1-
06-alkyl, tetrahydrofuranyl-C1-03-alkyl, phenyl-C1-03-alkyl, pyridyl-C1-03-
alkyl: for example CH3,
02H5, n-propyl and CH(0H3)2;
- 01-04-alkyl and also the 01-04-alkyl moieties of 03-06-cycloalkyl-C1-04-
alkyl, 01-04-
alkylcarbonylamino: for example CH3, 02H5, n-propyl, and CH(0H3)2 n-butyl,
CH(0H3)-02H5,
0H2-CH(0H3)2 and C(0H3)3;
- 01-06-alkyl and also the 01-06-alkyl moieties of C1-06-alkoxy-C1-06-
alkyl, C1-06-alkoxy-C1-
Cs-alkoxy-Ci-Cs-alkyl, Ci-Cs-alkoxycarbonyl-Ci-Cs-alkyl, Ci-Cs-alkylsulfinyl-
Ci-Cs-alkyl, 01-06-
alkylsulfonyl-C1-06-alkyl, 01-06-dialkoxy-C1-06-alkyl, Ci-Cs-alkyl-carbonyloxy-
Ci-Cs-alkyl,
phenyl-carbonyl-CI-Cs-alkyl, tri(C1-03-alkyl)-silyl-C1-06-alkyl, tri(C1-03-
alkyl)-silyl-C1-06-alkoxy-
01-06-alkyl, phenyl-01-06-alkoxy-01-06-alkyl: 01-04-alkyl as mentioned above,
and also, for
example, n-pentyl, 1-methylbutyl, 2-methylbutyl, 3-methylbutyl, 2,2-
dimethylpropyl, 1-
ethylpropyl, n-hexyl, 1,1-dimethylpropyl, 1,2-dimethylpropyl, 1-methylpentyl,
2-methylpentyl, 3-
methylpentyl, 4-methylpentyl, 1,1-dimethylbutyl, 1,2-dimethylbutyl, 1,3-
dimethylbutyl, 2,2-
dimethylbutyl, 2,3-dimethylbutyl, 3,3-dimethylbutyl, 1-ethylbutyl, 2-
ethylbutyl, 1,1,2-
trimethylpropyl, 1,2,2-trimethylpropyl, 1-ethyl-1-methylpropyl or 1-ethyl-2-
methylpropyl,
preferably methyl, ethyl, n-propyl, 1-methylethyl, n-butyl, 1,1-dimethylethyl,
n-pentyl or n-
hexyl;
- Ci-02-haloalkyl: 01-02-alkyl as mentioned above which is partially or
fully substituted by
fluorine, chlorine, bromine and/or iodine, for example, chloromethyl,
dichloromethyl,
trichloromethyl, fluoromethyl, difluoromethyl, trifluoromethyl,
chlorofluoromethyl,
dichlorofluoromethyl, chlorodifluoromethyl, bromomethyl, iodomethyl, 2-
fluoroethyl, 2-
chloroethyl, 2-bromoethyl, 2-iodoethyl, 2,2-difluoroethyl, 2,2,2-
trifluoroethyl, 2-chloro-2-
fluoroethyl, 2-chloro-2,2-difluoroethyl, 2,2-dichloro-2-fluoroethyl, 2,2,2-
trichloroethyl,
pentafluoroethyl;
- Ci-04-haloalkyl: 01-04-alkyl as mentioned above which is partially or
fully substituted by
fluorine, chlorine, bromine and/or iodine, for example, chloromethyl,
dichloromethyl,
trichloromethyl, fluoromethyl, difluoromethyl, trifluoromethyl,
chlorofluoromethyl,
dichlorofluoromethyl, chlorodifluoromethyl, bromomethyl, iodomethyl, 2-
fluoroethyl, 2-
chloroethyl, 2-bromoethyl, 2-iodoethyl, 2,2-difluoroethyl, 2,2,2-
trifluoroethyl, 2-chloro-2-
fluoroethyl, 2-chloro-2,2-difluoroethyl, 2,2-dichloro-2-fluoroethyl, 2,2,2-
trichloroethyl,
pentafluoroethyl, 2-fluoropropyl, 3-fluoropropyl, 2,2-difluoropropyl, 2,3-
difluoropropyl, 2-
CA 03030354 2019-01-09
WO 2018/019842
PCT/EP2017/068784
chloropropyl, 3-chloropropyl, 2,3-dichloropropyl, 2-bromopropyl, 3-
bromopropyl, 3,3,3-
trifluoropropyl, 3,3,3-trichloropropyl, 2,2,3,3,3-pentafluoropropyl,
heptafluoropropyla 01-03-
haloalkyl radical as mentioned above, and also, for example, 1-(fluoromethyl)-
2-fluoroethyl,
1-(chloromethyl)-2-chloroethyl, 1-(bromomethyl)-2-bromoethyl, 4-fluorobutyl, 4-
chlorobutyl, 4-
5 bromobutyl, nonafluorobutyl, 1,1,2,2,-tetrafluoroethyl and 1-
trifluoromethy1-1,2,2,2-
tetrafluoroethyl;
- C1-06-haloalkyl: C1-04-haloalkyl as mentioned above, and also, for
example,
5-fluoropentyl, 5-chloropentyl, 5-bromopentyl, 5-iodopentyl,
undecafluoropentyl, 6-fluorohexyl,
6-chlorohexyl, 6-bromohexyl, 6-iodohexyl and dodecafluorohexyl;
10 - 03-06-cycloalkyl and also the 03-06-cycloalkyl moieties of 03-06-
cycloalkyl-C1-04-alkyl: a
monocyclic saturated hydrocarbon having 3 to 6 ring members, such as
cyclopropyl, cyclobutyl,
cyclopentyl and cyclohexyl;
- 03-06-alkenyl: for example 1-propenyl, 2-propenyl, 1-methylethenyl, 1-
butenyl, 2-butenyl,
3-butenyl, 1-methyl-1-propenyl, 2-methyl-1-propenyl, 1-methyl-2-propenyl, 2-
methyl-2-propenyl,
15 1-pentenyl, 2-pentenyl, 3-pentenyl, 4-pentenyl, 1-methyl-1-butenyl, 2-
methyl-1-butenyl, 3-
methyl-1-butenyl, 1-methyl-2-butenyl, 2-methyl-2-butenyl, 3-methyl-2-butenyl,
1-methyl-3-
butenyl, 2-methyl-3-butenyl, 3-methyl-3-butenyl, 1,1-dimethy1-2-propenyl, 1,2-
dimethyl-
1-propenyl, 1,2-d imethy1-2-propenyl, 1-ethyl-1-propenyl, 1-ethyl-2-propenyl,
1-hexenyl, 2-
hexenyl, 3-hexenyl, 4-hexenyl, 5-hexenyl, 1-methyl-1-pentenyl, 2-methyl-1-
pentenyl, 3-methyl-
20 1-pentenyl, 4-methyl-1-pentenyl, 1-methyl-2-pentenyl, 2-methyl-2-
pentenyl, 3-methyl-2-
pentenyl, 4-methyl-2-pentenyl, 1-methyl-3-pentenyl, 2-methyl-3-pentenyl, 3-
methyl-3-pentenyl,
4-methyl-3-pentenyl, 1-methyl-4-pentenyl, 2-methyl-4-pentenyl, 3-methyl-4-
pentenyl, 4-methyl-
4-pentenyl, 1,1-dimethy1-2-butenyl, 1,1-dimethy1-3-butenyl, 1,2-dimethy1-1-
butenyl, 1,2-dimethyl-
2-butenyl, 1,2-dimethy1-3-butenyl, 1,3-dimethy1-1-butenyl, 1,3-dimethy1-2-
butenyl, 1,3-dimethyl-
3-butenyl, 2,2-dimethy1-3-butenyl, 2,3-dimethy1-1-butenyl, 2,3-dimethy1-2-
butenyl, 2,3-dimethy1-
3-butenyl, 3,3-dimethy1-1-butenyl, 3,3-dimethy1-2-butenyl, 1-ethyl-1-butenyl,
1-ethyl-2-butenyl, 1-
ethyl-3-butenyl, 2-ethyl-1-butenyl, 2-ethyl-2-butenyl, 2-ethyl-3-butenyl,
1,1,2-trimethy1-2-
propenyl, 1-ethyl-1-methyl-2-propenyl, 1-ethyl-2-methyl-1-propenyl and 1-ethyl-
2-methyl-2-
propenyl;
- 02-06-alkenyl and also the 02-06-alkenyl moieties of tri(C1-03-alkyl)-
sily1-02-06-alkenyl:
03-06-alkenyl as mentioned above, and also ethenyl;
- 03-06-alkynyl: for example 1-propynyl, 2-propynyl, 1-butynyl, 2-butynyl,
3-butynyl, 1-
methyl-2-propynyl, 1-pentynyl, 2-pentynyl, 3-pentynyl, 4-pentynyl, 1-methyl-2-
butynyl, 1-methyl-
3-butynyl, 2-methyl-3-butynyl, 3-methyl-1-butynyl, 1,1-dimethy1-2-propynyl, 1-
ethyl-2-propynyl,
1-hexynyl, 2-hexynyl, 3-hexynyl, 4-hexynyl, 5-hexynyl, 1-methyl-2-pentynyl, 1-
methyl-3-
pentynyl, 1-methyl-4-pentynyl, 2-methyl-3-pentynyl, 2-methyl-4-pentynyl, 3-
methyl-1-pentynyl, 3-
methyl-4-pentynyl, 4-methyl-1-pentynyl, 4-methyl-2-pentynyl, 1,1-dimethy1-2-
butynyl, 1,1-
dimethy1-3-butynyl, 1,2-dimethy1-3-butynyl, 2,2-dimethy1-3-butynyl, 3,3-
dimethy1-1-butynyl, 1-
ethyl-2-butynyl, 1-ethyl-3-butynyl, 2-ethyl-3-butynyl and 1-ethyl-1-methyl-2-
propynyl;
- 02-06-alkyny and also the 02-06-alkynyl moieties of tri(C1-03-alkyl)-
sily1-02-06-alkynyl:
03-06-alkynyl as mentioned above and also ethynyl;
- Ci-06-alkoxy and also the Ci-06-alkoxy moieties of Ci-06-alkoxy-C1-06-
alkyl, 01-06-
alkoxy-C1-06-alkoxy-C1-06-alkyl, Ci-06-alkoxycarbonyl-C1-06-alkyl, tri(C1-03-
alkyl)-silyl-C1-06-
alkoxy-C1-06-alkyl, phenyl-C1-06-alkoxy-C1-06-alkyl, 01-06-dialkoxy-C1-06-
alkyl: Ci-04-alkoxy as
CA 03030354 2019-01-09
WO 2018/019842
PCT/EP2017/068784
21
mentioned above, and also, for example, pentoxy, 1-methylbutoxy, 2-
methylbutoxy, 3-
methoxylbutoxy, 1,1-dimethylpropoxy, 1,2-dimethylpropoxy, 2,2-dimethylpropoxy,
1-
ethylpropoxy, hexoxy, 1-methylpentoxy, 2-methylpentoxy, 3-methylpentoxy, 4-
methylpentoxy,
1,1-dimethylbutoxy, 1,2-dimethylbutoxy, 1,3-dimethylbutoxy, 2,2-
dimethylbutoxy, 2,3-
dimethylbutoxy, 3,3-dimethylbutoxy, 1-ethylbutoxy, 2-ethylbutoxy, 1,1,2-
trimethylpropoxy, 1,2,2-
trimethylpropoxy, 1-ethyl-1-methylpropoxy and 1-ethyl-2-methylpropoxy;
- (C1-06-alkyl)amino: (C1-04-alkylamino) as mentioned above, and also,
for example,
pentylamino, 1-methylbutylamino, 2-methylbutylamino, 3-methylbutylamino, 2,2-
dimethylpropylamino, 1-ethylpropylamino, hexylamino, 1,1-dimethylpropylamino,
1,2-
dimethylpropylamino, 1-methylpentylamino, 2-methylpentylamino, 3-
methylpentylamino, 4-
methylpentylamino, 1,1-dimethylbutylamino, 1,2-dimethylbutylamino, 1,3-
dimethylbutylamino,
2,2-dimethylbutylamino, 2,3-dimethylbutyl-amino 3,3-dimethylbutylamino, 1-
ethylbutylamino, 2-
ethylbutylamino, 1,1,2-trimethylpropylamino, 1,2,2-trimethyl-propylamino, 1-
ethyl-1-
methylpropylamino or 1-ethyl-2-methylpropylamino;
- di(C1-06-alkyl)amino: di(C1-04-alkyl)amino as mentioned above, and also,
for example,
N-methyl-N-pentylamino, N-methyl-N-(1-methylbutyl)amino, N-methyl-N-(2-
methylbutyl)amino,
N-methyl-N-(3-methylbutyl)amino, N-methyl-N-(2,2-dimethylpropyl)amino, N-
methyl-N-(1-
ethylpropyl)amino, N-methyl-N-hexylamino, N-methyl-N-(1,1-
dimethylpropyl)amino, N-methyl-N-
(1,2-dimethylpropyl)amino, N-methyl-N-(1-methylpentyl)amino, N-methyl-N-(2-
methylpentyl)amino, N-methyl-N-(3-methylpentyl)amino, N-methyl-N-(4-
methylpentyl)amino, N-
methyl-N-(1,1-dimethylbutyl)amino, N-methyl-N-(1,2-dimethylbutyl)amino, N-
methyl-N-(1,3-
dimethylbutyl)amino, N-methyl-N-(2,2-dimethylbutyl)amino, N-methyl-N-(2,3-
dimethylbutyl)amino, N-methyl-N-(3,3-dimethylbutyl)amino, N-methyl-N- (1-
ethylbutyl)amino, N-
methyl-N-(2-ethylbutyl)amino, N-methyl-N-(1,1,2-trimethylpropyl)amino, N-
methyl-N- (1,2,2-
trimethylpropyl)amino, N-methyl-N-(1-ethyl-1-methylpropyl)amino, N-methyl-N-
(1-ethyl-2-
methylpropyl)amino, N-ethyl-N-pentylamino, N-ethyl-N-(1-methylbutyl)amino, N-
ethyl-N-(2-
methylbutyl)amino, N-ethyl-N-(3-methylbutyl)amino, N-ethyl-N-(2,2-
dimethylpropyl)amino, N-
ethyl-N-(1-ethylpropyl)amino, N-ethyl-N-hexylamino, N-ethyl-N-(1,1-
dimethylpropyl)amino, N-
ethyl-N-(1,2-dimethylpropyl)amino, N-ethyl-N-(1-methylpentyl)amino, N-ethyl-N-
(2-methyl-
pentyl)amino, N-ethyl-N-(3-methylpentyl)amino, N-ethyl-N-(4-
methylpentyl)amino, N-ethyl-N-
(1,1-d imethylbutyl)amino, N-ethyl-N-(1,2-dimethylbutyl)amino, N-ethyl-N-(1,3-
dimethylbutyl)amino, N-ethyl-N-(2,2-dimethylbutyl)amino, N-ethyl-N-(2,3-
dimethylbutyl)amino,
N-ethyl-N-(3,3-dimethylbutyl)amino, N-ethyl-N-(1-ethylbutyl)amino, N-ethyl-N-
(2-
ethylbutyl)amino, N-ethyl-N-(1,1,2-trimethylpropyl)amino, N-ethyl-N-(1,2,2-
trimethylpropyl)amino, N-ethyl-N-(1-ethyl-1-methylpropyl)amino, N-ethyl-N-(1-
ethyl-2-
methylpropyl)amino, N-propyl-N-pentylamino, N-butyl-N-pentylamino, N,N-
dipentylamino, N-
propyl-N-hexylamino, N-butyl-N-hexylamino, N-pentyl-N-hexylamino or N,N-
dihexylamino;
- C1-06-alkylsulfinyl (C1-06-Alkyl-S(=0)-) and also the C1-06-
alkylsulfinyl moieties of 01-06-
alkylsulfinyl-C1-06-alkyl: for example methylsulfinyl, ethylsulfinyl,
propylsulfinyl, 1-me-
thylethylsulfinyl, butylsulfinyl, 1-methylpropylsulfinyl, 2-
methylpropylsulfinyl, 1,1-di-
methylethylsulfinyl, pentylsulfinyl, 1-methylbutylsulfinyl, 2-
methylbutylsulfinyl, 3-
methylbutylsulfinyl, 2,2-dimethylpropylsulfinyl, 1-ethylpropylsulfinyl, 1,1-
dimethylpropylsulfinyl,
1,2-dimethylpropylsulfinyl, hexylsulfinyl, 1-methylpentylsulfinyl, 2-
methylpentylsulfinyl, 3-
methylpentylsulfinyl, 4-methylpentyl-sulfinyl, 1,1-dimethylbutylsulfinyl, 1,2-
dimethylbutylsulfinyl,
CA 03030354 2019-01-09
WO 2018/019842
PCT/EP2017/068784
22
1,3-dimethylbutyl-sulfinyl, 2,2-dimethylbutylsulfinyl, 2,3-
dimethylbutylsulfinyl, 3,3-dimethylbutyl-
sulfinyl, 1-ethylbutylsulfinyl, 2-ethylbutylsulfinyl, 1,1,2-
trimethylpropylsulfinyl, 1,2,2-
trimethylpropylsulfinyl, 1-ethyl-1-methylpropylsulfinyl and 1-ethyl-2-
methylpropylsulfinyl;
- C1-06-alkylsulfonyl (C1-06-alkyl-S(0)2-) and also the C1-06-
alkylsulfonyl moieties of Ci-
Cs-alkylsulfonyl-Ci-Cs-alkyl: for example methylsulfonyl, ethylsulfonyl,
propylsulfonyl, 1-
methylethylsulfonyl, butylsulfonyl, 1-methylpropylsulfonyl, 2-methyl-
propylsulfonyl, 1,1-
dimethylethylsulfonyl, pentylsulfonyl, 1-methylbutylsulfonyl, 2-
methylbutylsulfonyl, 3-
methylbutylsulfonyl, 1,1-dimethylpropylsulfonyl, 1,2-dimethylpropylsulfonyl,
2,2-
dimethylpropylsulfonyl, 1-ethylpropylsulfonyl, hexylsulfonyl, 1-
methylpentylsulfonyl, 2-
methylpentylsulfonyl, 3-methylpentylsulfonyl, 4-methylpentylsulfonyl, 1,1-
dimethylbutylsulfonyl,
1,2-dimethylbutylsulfonyl, 1,3-dimethylbutylsulfonyl, 2,2-
dimethylbutylsulfonyl, 2,3-dimethyl-
butylsulfonyl, 3,3-dimethylbutylsulfonyl, 1-ethylbutylsulfonyl, 2-
ethylbutylsulfonyl, 1,1,2-trimethyl-
propylsulfonyl, 1,2,2-trimethylpropylsulfonyl, 1-ethyl-1-methylpropylsulfonyl
and 1-ethyl-2-
methylpropylsulfonyl.
According to a preferred embodiment of the invention preference is also given
to those
compounds of formula (I), wherein the variables, either independently of one
another or in
combination with one another, have the following meanings:
Preferred are the compounds of formula (I), wherein R1 is F.
Also preferred are the compounds of formula (I), wherein R2 is Cl.
Also preferred are the compounds of formula (I), wherein R3 is H.
Also preferred are the compounds of formula (I), wherein
R4 is H, CH3 or OCH3;
particularly preferred is H.
Also preferred are the compounds of formula (I), wherein
R4 is H, OCH3 or 002H5;
particularly preferred is H or OCH3;
especially preferred is H.
Also preferred are the compounds of formula (I), wherein
R5 is OR8, wherein R8 is Ci-Cs-alkyl;
particularly preferred OR8, wherein R8 is 02H5.
Also preferred are the compounds of formula (I), wherein
R5 is OH or OR8, wherein R8 is Ci-Cs-alkyl;
particularly preferred OH or OR8, wherein R8 is CH3 or 02H5;
especiylly preferred OR8, wherein R8 is 02H5.
CA 03030354 2019-01-09
WO 2018/019842
PCT/EP2017/068784
23
Also preferred are the compounds of formula (I), wherein X is 0.
Also preferred are the compounds of formula (I), wherein
Y is N;
also preferred CH.
Also preferred are the compounds of formula (I), wherein Z is 0.
Also preferred are the compounds of formula (I), wherein
R1 is F;
R2 is 01;
R3 is H;
R4 is H, 00H3 or 002H5;
R5 is OH or OR8, wherein R8 is 01-06-alkyl;
X is 0;
Y is N or CH; and
Z is 0;
particularly preferred
R1 is F;
R2 is 01;
R3 is H;
R4 is H or 00H3;
R5 is OH or OR8, wherein R8 is CH3 or 02H5;
X is 0;
Y is N or CH; and
Z is 0;
especially preferred
R1 is F;
R2 is 01;
R3 is H;
R4 is H;
R5 is OH or OR8, wherein R8 is CH3 or 02H5;
X is 0;
Y is N or CH; and
Z is 0;
more preferred
R1 is F;
R2 is 01;
R3 is H;
R4 is H;
R5 is OH or OR8, wherein R8 is 02H5;
CA 03030354 2019-01-09
WO 2018/019842
PCT/EP2017/068784
24
X is 0;
Y is N or CH; and
Z is 0.
Also preferred are the compounds of formula (1), wherein
R1 is F;
R2 is Cl;
R3 is H;
R4 is H, CH3 or OCH3;
R5 is OH or OR8, wherein R8 is C1-06-alkyl;
X is 0;
Y is N or CH; and
Z is 0;
particularly preferred
R1 is F;
R2 is 01;
R3 is H;
R4 is H, CH3 or 00H3;
R5 is OH or OR8, wherein R8 is CH3 or 02H5;
X is 0;
Y is N or CH; and
Z is 0.
In a particularly preferred embodiment, the compound of formula (1) is the
compound (1).1 (CAS
HO3
I
C H 3 0 0.k,õ....
I
F 3 C N 0
1 Y 0
DA .
N 0 (
N
0 401 1
F 01 \%
353292-31-6):
Accordingly, in a preferred embodiment of the present invention the method
according to the
present invention comprises the application of compound (1).1 to PPO resistant
weeds.
In another particularly preferred embodiment, the compound of formula (1) is
the
compound (1).2:
CA 03030354 2019-01-09
WO 2018/019842
PCT/EP2017/068784
C H 3
I
O 0
C H 3 ......--
I
F3C NI 0
0
i Y (I).2.
N 0
N
* 1
F Cl
Accordingly, in a preferred embodiment of the present invention the method
according to the
present invention comprises the application of compound (1).2 to PPO resistant
weeds.
5
In another particularly preferred embodiment, the compound of formula (1) is
the
compound (1).3:
O OH
C H 3 .....,......>,....
I
F3C N 0
0
i Y (1).3.
N 0
N
0 * 1
F Cl
Accordingly, in a preferred embodiment of the present invention the method
according to the
present invention comprises the application of compound (1).3 to PPO resistant
weeds.
In a particularly preferred embodiment, the compound of formula (1) is the
compound (1).4:
HO3
I
O 0
C H 3
i
F3C N 0
0
i Y (1).4.
N 0
0 *
F CI ill
Accordingly, in a preferred embodiment of the present invention the method
according to the
present invention comprises the application of compound (1).4 to PPO resistant
weeds.
CA 03030354 2019-01-09
WO 2018/019842
PCT/EP2017/068784
26
In another particularly preferred embodiment, the compound of formula (1) is
the
compound (1).5:
C H 3
I
0 0
C H 3 ...k....õ...
1
F3C N 0
1 Y 0
(I).5.
N 0
0 401
F CI 401
Accordingly, in a preferred embodiment of the present invention the method
according to the
present invention comprises the application of compound (1).5 to PPO resistant
weeds.
In another particularly preferred embodiment, the compound of formula (1) is
the
compound (1).6:
0 OH
C H 3 ...>õ........õ..-
I
F3C N 0
1 Y 0
(1).6.
N 0
0 401
F CI 110
Accordingly, in a preferred embodiment of the present invention the method
according to the
present invention comprises the application of compound (1).6 to PPO resistant
weeds.
In another preferred embodiment of the present invention the method according
to the present
invention comprises the application of at least one of the compounds selected
from (1).1 and
(1).4 to PPO resistant weeds [i.e. the compound of formula (1) is selected
from at least one of the
compounds of formulae (1).1 and (1.)4].
To broaden the spectrum of action and to achieve synergistic effects, the
compounds of formula
(1) may be mixed with a large number of representatives of other herbicidal or
growth-regulating
active ingredient groups and then applied concomitantly. Suitable components
for mixtures are,
for example,
herbicides from the classes of the acetamides, amides,
aryloxyphenoxypropionates,
benzamides, benzofuran, benzoic acids, benzothiadiazinones, bipyridylium,
carbamates,
chloroacetamides, chlorocarboxylic acids, cyclohexanediones, dinitroanilines,
dinitrophenol,
diphenyl ether, glycines, imidazolinones, isoxazoles, isoxazolidinones,
nitriles, N-
phenylphthalimides, oxadiazoles, oxazolidinediones, oxyacetam ides,
phenoxycarboxylic acids,
CA 03030354 2019-01-09
WO 2018/019842
PCT/EP2017/068784
27
phenylcarbamates, phenylpyrazoles, phenylpyrazolines, phenylpyridazines,
phosphinic acids,
phosphoroamidates, phosphorodithioates, phthalamates, pyrazoles,
pyridazinones, pyridines,
pyridinecarboxylic acids, pyridinecarboxamides, pyrimidinediones,
pyrimidinyl(thio)benzoates,
quinolinecarboxylic acids, semicarbazones, sulfonylaminocarbonyltriazolinones,
sulfonylureas,
tetrazolinones, thiadiazoles, thiocarbamates, triazines, triazinones,
triazoles, triazolinones,
triazolocarboxamides, triazolopyrimidines, triketones, uracils, ureas.
It may furthermore be beneficial to apply the compounds of formula (I) alone
or in combination
with other herbicides, or else in the form of a mixture with other crop
protection agents, for
example together with agents for controlling pests or phytopathogenic fungi or
bacteria.
Also of interest is the miscibility with mineral salt solutions, which are
employed for treating
nutritional and trace element deficiencies. Other additives such as non-
phytotoxic oils and oil
concentrates may also be added.
The present invention also relates to a method for controlling PPO herbicide
resistant weeds,
wherein a herbicidal composition of at least one compound of formula (I) and
one or more
further active compound as defined herein after is applied to the PPO
herbicide resistant weeds.
In one embodiment of the present invention the method according to the present
invention
comprises the application of at least one compounds of formula (I) (compound
A) and at least
one further active compound selected from herbicides B, preferably herbicides
B of class b1) to
b15), and safeners C (compound C) to PPO resistant weeds.
In another embodiment of the present invention the method according to the
present invention
comprises the application of at least one compounds of formula (I) and at
least one further
active compound B (herbicide B) to PPO resistant weeds.
In one embodiment of the invention, the method according to the present
invention comprises
the application of a herbicidal composition comprising at least one,
preferably exactly one
compound of formula (I) and at least one further active compound selected from
herbicides B,
preferably herbicides B of class b1) to b15), and safeners C (compound C) to
control PPO
resistant weeds.
Accordingly, in a preferred embodiment of the present invention the method
according to the
present invention comprises the application of a herbicidal composition
comprising at least one,
preferably exactly one compound of formula (I) and at least one further active
compound
selected from herbicides B, preferably herbicides B of class b1) to b15), and
safeners C
(compound C) to PPO resistant weeds.
CA 03030354 2019-01-09
WO 2018/019842
PCT/EP2017/068784
28
The further herbicidal compound B (component B) is preferably selected from
the herbicides of
class b1) to b15):
B) herbicides of class b1) to b15):
b1) lipid biosynthesis inhibitors;
b2) acetolactate synthase inhibitors (ALS inhibitors);
b3) photosynthesis inhibitors;
b4) protoporphyrinogen-IX oxidase inhibitors (PPO inhibitors) other than
the
compounds of formula (I);
b5) bleacher herbicides;
b6) enolpyruvyl shikimate 3-phosphate synthase inhibitors (EPSP
inhibitors);
b7) glutamine synthetase inhibitors;
b8) 7,8-dihydropteroate synthase inhibitors (DHP inhibitors);
b9) mitosis inhibitors;
b10) inhibitors of the synthesis of very long chain fatty acids (VLCFA
inhibitors);
b11) cellulose biosynthesis inhibitors;
b12) decoupler herbicides;
b13) auxinic herbicides;
b14) auxin transport inhibitors; and
b15) other herbicides selected from the group consisting of bromobutide,
chlorflurenol,
chlorflurenol-methyl, cinmethylin, cumyluron, dalapon, dazomet, difenzoquat,
difenzoquat-metilsulfate, dimethipin, DSMA, dymron, endothal and its salts,
etobenzanid, flamprop, flamprop-isopropyl, flamprop-methyl, flamprop-M-
isopropyl, flamprop-M-methyl, flurenol, flurenol-butyl, flurprimidol,
fosamine,
fosamine-ammonium, indanofan, indaziflam, maleic hydrazide, mefluidide, metam,
methiozolin (CAS 403640-27-7), methyl azide, methyl bromide, methyl-dymron,
methyl iodide, MS MA, oleic acid, oxaziclomefone, pelargonic acid,
pyributicarb,
quinoclamine, triaziflam, tridiphane and 6-chloro-3-(2-cyclopropy1-6-
methylphenoxy)-4-pyridazinol (CAS 499223-49-3) and its salts and esters;
including their agriculturally acceptable salts or derivatives.
In one embodiment of the invention, the method according to the present
invention comprises
the application of compositions containing at least one, preferably exactly
one compound of
formula (I) and as further active compound at least one inhibitor of the lipid
biosynthesis
(herbicide b1). These compounds inhibit lipid biosynthesis. Inhibition of the
lipid biosynthesis
can be affected either through inhibition of acetylCoA carboxylase
(hereinafter-termed ACCase
herbicides) or through a different mode of action (hereinafter termed non-
ACCase herbicides).
The ACCase herbicides belong to the group A of the HRAC classification system
whereas the
non-ACCase herbicides belong to the group N of the HRAC classification.
In another embodiment of the invention, the method according to the present
invention
comprises the application of compositions containing at least one, preferably
exactly one
compound of formula (I) and as further active compound at least one ALS
inhibitor (herbicide
CA 03030354 2019-01-09
WO 2018/019842
PCT/EP2017/068784
29
b2). The herbicidal activity of these compounds is based on the inhibition of
acetolactate
synthase and thus on the inhibition of the branched chain amino acid
biosynthesis. These
inhibitors belong to the group B of the HRAC classification system.
In another embodiment of the invention, the method according to the present
invention
comprises the application of compositions containing at least one, preferably
exactly one
compound of formula (I) and as further active compound at least one inhibitor
of photosynthesis
(herbicide b3). The herbicidal activity of these compounds is based either on
the inhibition of the
photosystem II in plants (so-called P511 inhibitors, groups Cl, 02 and 03 of
HRAC
classification) or on diverting the electron transfer in photosystem I in
plants (so-called PSI
inhibitors, group D of HRAC classification) and thus on an inhibition of
photosynthesis. Amongst
these, PSII inhibitors are preferred.
In another embodiment of the invention, the method according to the present
invention
comprises the application of compositions containing at least one, preferably
exactly one
compound of formula (I) and as further active compound at least one inhibitor
of
protoporphyrinogen-IX-oxidase (herbicide b4). The herbicidal activity of these
compounds is
based on the inhibition of the protoporphyrinogen-IX-oxidase. These inhibitors
belong to the
group E of the HRAC classification system.
In another embodiment of the invention, the method according to the present
invention
comprises the application of compositions containing at least one, preferably
exactly one
compound of formula (I) and as further active compound at least one bleacher-
herbicide
(herbicide b5). The herbicidal activity of these compounds is based on the
inhibition of the
carotenoid biosynthesis. These include compounds which inhibit carotenoid
biosynthesis by
inhibition of phytoene desaturase (so-called PDS inhibitors, group F1 of HRAC
classification),
compounds that inhibit the 4-hydroxyphenylpyruvate-dioxygenase (HPPD
inhibitors, group F2 of
HRAC classification), compounds that inhibit DOXsynthase (group F4 of HRAC
class) and
compounds which inhibit carotenoid biosynthesis by an unknown mode of action
(bleacher -
unknown target, group F3 of HRAC classification).
In another embodiment of the invention, the method according to the present
invention
comprises the application of compositions containing at least one, preferably
exactly one
compound of formula (I) and as further active compound at least one EPSP
synthase inhibitor
(herbicide b6). The herbicidal activity of these compounds is based on the
inhibition of
enolpyruvyl shikimate 3-phosphate synthase, and thus on the inhibition of the
amino acid
biosynthesis in plants. These inhibitors belong to the group G of the HRAC
classification
system.
In another embodiment of the invention, the method according to the present
invention
comprises the application of compositions containing at least one, preferably
exactly one
CA 03030354 2019-01-09
WO 2018/019842
PCT/EP2017/068784
compound of formula (I) and as further active compound at least one glutamine
synthetase
inhibitor (herbicide b7). The herbicidal activity of these compounds is based
on the inhibition of
glutamine synthetase, and thus on the inhibition of the aminoacid biosynthesis
in plants. These
inhibitors belong to the group H of the HRAC classification system.
5
In another embodiment of the invention, the method according to the present
invention
comprises the application of compositions containing at least one, preferably
exactly one
compound of formula (I) and as further active compound at least one DHP
synthase inhibitor
(herbicide b8). The herbicidal activity of these compounds is based on the
inhibition of 7, 8-
10 dihydropteroate synthase. These inhibitors belong to the group I of the
HRAC classification
system.
In another embodiment of the invention, the method according to the present
invention
comprises the application of compositions containing at least one, preferably
exactly one
15 compound of formula (I) and as further active compound at least one
mitosis inhibitor (herbicide
b9). The herbicidal activity of these compounds is based on the disturbance or
inhibition of
microtubule formation or organization, and thus on the inhibition of mitosis.
These inhibitors
belong to the groups K1 and K2 of the HRAC classification system. Among these,
compounds
of the group K1, in particular dinitroanilines, are preferred.
In another embodiment of the invention, the method according to the present
invention
comprises the application of compositions containing at least one, preferably
exactly one
compound of formula (I) and as further active compound at least one VLCFA
inhibitor (herbicide
b10). The herbicidal activity of these compounds is based on the inhibition of
the synthesis of
very long chain fatty acids and thus on the disturbance or inhibition of cell
division in plants.
These inhibitors belong to the group K3 of the HRAC classification system.
In another embodiment of the invention, the method according to the present
invention
comprises the application of compositions containing at least one, preferably
exactly one
compound of formula (I) and as further active compound at least one cellulose
biosynthesis
inhibitor (herbicide b11). The herbicidal activity of these compounds is based
on the inhibition of
the biosynthesis of cellulose and thus on the inhibition of the synthesis of
cell walls in plants.
These inhibitors belong to the group L of the HRAC classification system.
In another embodiment of the invention, the method according to the present
invention
comprises the application of compositions containing at least one, preferably
exactly one
compound of formula (I) and as further active compound at least one decoupler
herbicide
(herbicide b12). The herbicidal activity of these compounds is based on the
disruption of the cell
membrane. These inhibitors belong to the group M of the HRAC classification
system.
CA 03030354 2019-01-09
WO 2018/019842
PCT/EP2017/068784
31
In another embodiment of the invention, the method according to the present
invention
comprises the application of compositions containing at least one, preferably
exactly one
compound of formula (I) and as further active compound at least one auxinic
herbicide
(herbicide b13). These include compounds that mimic auxins, i.e. plant
hormones, and affect
the growth of the plants. These compounds belong to the group 0 of the H RAC
classification
system.
In another embodiment of the invention, the method according to the present
invention
comprises the application of compositions containing at least one, preferably
exactly one
compound of formula (I) and as further active compound at least one auxin
transport inhibitor
(herbicide b14). The herbicidal activity of these compounds is based on the
inhibition of the
auxin transport in plants. These compounds belong to the group P of the H RAC
classification
system.
As to the given mechanisms of action and classification of the active
substances, see e.g.
"HRAC, Classification of Herbicides According to Mode of Action",
http://www.plantprotection.org/hrac/M0A.html).
Preference is given to those methods according to the present invention
comprising the
application of composition comprising at least one herbicide B selected from
herbicides of class
b1, b2, b3, b4, b5, b6, b7, b10, b13, b14 and b15.
Specific preference is given to those methods according to the present
invention comprising the
application of compositions comprising at least one herbicide B selected from
the herbicides of
class b2, b4, b6, b7, b9, b10 and b13.
Particular preference is given to those methods according to the present
invention comprising
the application of compositions comprising at least one herbicide B selected
from the herbicides
of class b4, b6, b7 and b13.
Examples of herbicides B which can be used in combination with the compound of
formula (I)
according to the present invention are:
b1) from the group of the lipid biosynthesis inhibitors:
ACC-herbicides such as alloxydim, alloxydim-sodium, butroxydim, clethodim,
clodinafop,
clodinafop-propargyl, cycloxydim, cyhalofop, cyhalofop-butyl, diclofop,
diclofop-methyl,
fenoxaprop, fenoxaprop-ethyl, fenoxaprop-P, fenoxaprop-P-ethyl, fluazifop,
fluazifop-butyl,
fluazifop-P, fluazifop-P-butyl, haloxyfop, haloxyfop-methyl, haloxyfop-P,
haloxyfop-P-methyl,
metamifop, pinoxaden, profoxydim, propaquizafop, quizalofop, quizalofop-ethyl,
quizalofop-
tefuryl, quizalofop-P, quizalofop-P-ethyl, quizalofop-P-tefuryl, sethoxydim,
tepraloxydim,
CA 03030354 2019-01-09
WO 2018/019842
PCT/EP2017/068784
32
tralkoxydim,
4-(4'-Chloro-4-cyclopropy1-2'-fluoro[1,11-bipheny1]-3-y1)-5-hydroxy-2,2,6,6-
tetramethyl-2H-pyran-
3(6H)-one (CAS 1312337-72-6); 4-(2',4'-Dichloro-4-cyclopropyl[1,11-bipheny1]-3-
y1)-5-hydroxy-
2,2,6,6-tetramethyl-2H-pyran-3(6H)-one (CAS 1312337-45-3); 4-(4'-Chloro-4-
ethy1-2'-fluoro[1,1'-
biphenyl]-3-y1)-5-hydroxy-2,2,6,6-tetramethy1-2H-pyran-3(6H)-one (CAS 1033757-
93-5); 4-(2',4'-
Dichloro-4-ethyl[1,11-bipheny1]-3-y1)-2,2,6,6-tetramethyl-2H-pyran-3,5(4H,6H)-
dione (CAS
1312340-84-3); 5-(Acetyloxy)-4-(4'-chloro-4-cyclopropy1-2'-fluoro[1,11-
bipheny1]-3-y1)-3,6-
dihydro-2,2,6,6-tetramethyl-2H-pyran-3-one (CAS 1312337-48-6); 5-(Acetyloxy)-4-
(2",4'-
dichloro-4-cyclopropyl- [1,11-bipheny1]-3-y1)-3,6-dihydro-2,2,6,6-tetramethy1-
2H-pyran-3-one; 5-
(Acetyloxy)-4-(4'-chloro-4-ethy1-2'-fluoro[1,11-bipheny1]-3-y1)-3,6-dihydro-
2,2,6,6-tetramethyl-2H-
pyran-3-one (CAS 1312340-82-1); 5-(Acetyloxy)-4-(2',4'-dichloro-4-ethyl[1,11-
bipheny1]-3-y1)-3,6-
dihydro-2,2,6,6-tetramethyl-2H-pyran-3-one (CAS 1033760-55-2); 4-(4'-Chloro-4-
cyclopropy1-2'-
fluoro[1,11-bipheny1]-3-y1)-5,6-dihydro-2,2,6,6-tetramethyl-5-oxo-2H-pyran-3-
ylcarbonic acid
methyl ester (CAS 1312337-51-1); 4-(2",4'-Dichloro -4-cyclopropyl- [1,11-
bipheny1]-3-y1)-5,6-
dihydro-2,2,6,6-tetramethy1-5-oxo-2H-pyran-3-ylcarbonic acid methyl ester; 4-
(4'-Chloro-4-
ethy1-2'-fluoro[1,11-bipheny1]-3-y1)-5,6-dihydro-2,2,6,6-tetramethyl-5-oxo-2H-
pyran-3-ylcarbonic
acid methyl ester (CAS 1312340-83-2); 4-(2',4'-Dichloro-4-ethyl[1,11-bipheny1]-
3-y1)-5,6-dihydro-
2,2,6,6-tetramethyl-5-oxo-2H-pyran-3-ylcarbonic acid methyl ester (CAS 1033760-
58-5); and
non ACC herbicides such as benfuresate, butylate, cycloate, dalapon,
dimepiperate, EPTC,
esprocarb, ethofumesate, flupropanate, molinate, orbencarb, pebulate,
prosulfocarb, TCA,
thiobencarb, tiocarbazil, triallate and vernolate;
b2) from the group of the ALS inhibitors:
sulfonylureas such as amidosulfuron, azimsulfuron, bensulfuron, bensulfuron-
methyl,
chlorimuron, chlorimuron-ethyl, chlorsulfuron, cinosulfuron, cyclosulfamuron,
ethametsulfuron,
ethametsulfuron-methyl, ethoxysulfuron, flazasulfuron, flucetosulfuron,
flupyrsulfuron,
flupyrsulfuron-methyl-sodium, foramsulfuron, halosulfuron, halosulfuron-
methyl, imazosulfuron,
iodosulfuron, iodosulfuron-methyl-sodium, iofensulfuron, iofensulfuron-sodium,
mesosulfuron,
metazosulfuron, metsulfuron, metsulfuron-methyl, nicosulfuron,
orthosulfamuron, oxasulfuron,
primisulfuron, primisulfuron-methyl, propyrisulfuron, prosulfuron,
pyrazosulfuron,
pyrazosulfuron-ethyl, rimsulfuron, sulfometuron, sulfometuron-methyl,
sulfosulfuron,
thifensulfuron, thifensulfuron-methyl, triasulfuron, tribenuron, tribenuron-
methyl, trifloxysulfuron,
triflusulfuron, triflusulfuron-methyl and tritosulfuron,
imidazolinones such as imazamethabenz, imazamethabenz-methyl, imazamox,
imazapic,
imazapyr, imazaquin and imazethapyr, triazolopyrimidine herbicides and
sulfonanilides such as
cloransulam, cloransulam-methyl, diclosulam, flumetsulam, florasulam,
metosulam,
penoxsulam, pyrimisulfan and pyroxsulam,
pyrimidinylbenzoates such as bispyribac, bispyribac-sodium, pyribenzoxim,
pyriftalid,
pyriminobac, pyriminobac-methyl, pyrithiobac, pyrithiobac-sodium, 4-[[[2-[(4,6-
dimethoxy-2-
pyrimidinyl)oxy]phenyl]methyl]amino]-benzoic acid-1-methylethyl ester (CAS
420138-41-6), 4-
[[[2-[(4,6-dimethoxy-2-pyrimidinyl)oxy]phenyl]methyl]amino]-benzoic acid
propyl ester (CAS
420138-40-5), N-(4-bromopheny1)-2-[(4,6-dimethoxy-2-
pyrimidinyl)oxy]benzenemethanamine
(CAS 420138-01-8),
CA 03030354 2019-01-09
WO 2018/019842
PCT/EP2017/068784
33
sulfonylaminocarbonyl-triazolinone herbicides such as flucarbazone,
flucarbazone-sodium,
propoxycarbazone, propoxycarbazone-sodium, thiencarbazone and thiencarbazone-
methyl;
and triafamone;
among these, a preferred embodiment of the invention relates to those
compositions comprising
at least one imidazolinone herbicide;
b3) from the group of the photosynthesis inhibitors:
amicarbazone, inhibitors of the photosystem II, e.g. 1-(6-tert-butylpyrimidin-
4-yI)-2-hydroxy-4-
methoxy-3-methyl-2H-pyrrol-5-one (CAS 1654744-66-7), 1-(5-tert-butylisoxazol-3-
y1)-2-hydroxy-
4-methoxy-3-methyl-2H-pyrrol-5-one (CAS 1637455-12-9), 1-(5-tert-butylisoxazol-
3-y1)-4-chloro-
2-hydroxy-3-methyl-2H-pyrrol-5-one (CAS 1637453-94-1), 1-(5-tert-butyl-1-
methyl-pyrazol-3-y1)-
4-chloro-2-hydroxy-3-methyl-2H-pyrrol-5-one (CAS 1654057-29-0), 1-(5-tert-
butyl-1-methyl-
pyrazol-3-y1)-3-chloro-2-hydroxy-4-methyl-2H-pyrrol-5-one (CAS 1654747-80-4),
4-hydroxy-1-
methoxy-5-methyl-344-(trifluoromethyl)-2-pyridyl]imidazolidin-2-one; (CAS
2023785-78-4), 4-
hydroxy-1,5-dimethy1-344-(trifluoromethyl)-2-pyridyl]imidazolidin-2-one (CAS
2023785-79-5), 5-
ethoxy-4-hydroxy-1-methyl-344-(trifluoromethyl)-2-pyridyl]imidazolidin-2-one
(CAS 1701416-69-
4), 4-hydroxy-1-methyl-344-(trifluoromethyl)-2-pyridyl]imidazolidin-2-one (CAS
1708087-22-2),
4-hydroxy-1,5-dimethy1-341-methyl-5-(trifluoromethyl)pyrazol-3-yl]imidazolidin-
2-one (CAS
2023785-80-8), 1-(5-tert-butylisoxazol-3-y1)-4-ethoxy-5-hydroxy-3-methyl-
imidazolidin-2-one
(CAS 1844836-64-1), triazine herbicides, including of chlorotriazine,
triazinones, triazindiones,
methylthiotriazines and pyridazinones such as ametryn, atrazine, chloridazone,
cyanazine,
desmetryn, dimethametryn,hexazinone, metribuzin, prometon, prometryn,
propazine, simazine,
simetryn, terbumeton, terbuthylazin, terbutryn and trietazin, aryl urea such
as chlorobromuron,
chlorotoluron, chloroxuron, dimefuron, diuron, fluometuron, isoproturon,
isouron, linuron,
metamitron, methabenzthiazuron, metobenzuron, metoxuron, monolinuron, neburon,
siduron,
tebuthiuron and thiadiazuron, phenyl carbamates such as desmedipham,
karbutilat,
phenmedipham, phenmedipham-ethyl, nitrile herbicides such as bromofenoxim,
bromoxynil and
its salts and esters, ioxynil and its salts and esters, uraciles such as
bromacil, lenacil and
terbacil, and bentazone and bentazone-sodium, pyridate, pyridafol,
pentanochlor and propanil
and inhibitors of the photosystem I such as diquat, diquat-dibromide,
paraquat, paraquat-
dichloride and paraquat-dimetilsulfate. Among these, a preferred embodiment of
the invention
relates to those compositions comprising at least one aryl urea herbicide.
Among these,
likewise a preferred embodiment of the invention relates to those compositions
comprising at
least one triazine herbicide. Among these, likewise a preferred embodiment of
the invention
relates to those compositions comprising at least one nitrile herbicide;
b4) from the group of the protoporphyrinogen-IX oxidase inhibitors:
acifluorfen, acifluorfen-sodium, azafenidin, bencarbazone, benzfendizone,
bifenox, butafenacil,
carfentrazone, carfentrazone-ethyl, chlorphtalim, chlomethoxyfen, cinidon-
ethyl, fluazolate,
flufenpyr, flufenpyr-ethyl, flumiclorac, flumiclorac-pentyl, flumioxazin,
fluoroglycofen,
fluoroglycofen-ethyl, fluthiacet, fluthiacet-methyl, fomesafen, halosafen,
lactofen, oxadiargyl,
oxadiazon, oxyfluorfen, pentoxazone, profluazol, pyraclonil, pyraflufen,
pyraflufen-ethyl,
saflufenacil, sulfentrazone, thidiazimin, tiafenacil, trifludimoxazin, ethyl
[342-chloro-4-fluoro-5-(1-
methyl-6-trifluoromethy1-2,4-dioxo-1,2,3,4-tetrahydropyrimidin-3-yl)phenoxy]-2-
CA 03030354 2019-01-09
WO 2018/019842
PCT/EP2017/068784
34
pyridyloxy]acetate (CAS 353292-31-6; S-3100), N-ethyl-3-(2,6-dichloro-4-
trifluoro-
methylphenoxy)-5-methyl-1H-pyrazole-1-carboxamide (CAS 452098-92-9), N-
tetrahydrofurfury1-
3-(2,6-dichloro-4-trifluoromethylphenoxy)-5-methyl-1H-pyrazole-1-carboxamide
(CAS 915396-
43-9), N-ethyl-3-(2-chloro-6-fluoro-4-trifluoromethylphenoxy)-5-methyl-1H-
pyrazole-1-
.. carboxamide (CAS 452099-05-7), N-tetrahydrofurfury1-3-(2-chloro-6-fluoro-4-
trifluoro-
methylphenoxy)-5-methyl-1H-pyrazole-1-carboxamide (CAS 452100-03-7), 347-
fluoro-3-oxo-4-
(prop-2-yny1)-3,4-dihydro-2H-benzo[1,4]oxazin-6-y1]-1,5-dimethy1-6-thioxo-
[1,3,5]triazinan-2,4-
dione (CAS 451484-50-7), 2-(2,2,7-trifluoro-3-oxo-4-prop-2-yny1-3,4-dihydro-2H-
benzo[1,4]oxazin-6-y1)-4,5,6,7-tetrahydro-isoindole-1,3-dione (CAS 1300118-96-
0), 1-methyl-6-
trifluoromethy1-3-(2,2,7-trifluoro-3-oxo-4-prop-2-yny1-3,4-dihydro-2H-
benzo[1,4]oxazin-6-y1)-1H-
pyrimidine-2,4-dione (CAS 1304113-05-0), methyl (E)-442-chloro-544-chloro-5-
(difluoromethoxy)-1H-methyl-pyrazol-3-y1]-4-fluoro-phenoxy]-3-methoxy-but-2-
enoate (CAS
948893-00-3), and 347-chloro-5-fluoro-2-(trifluoromethyl)-1H-benzimidazol-4-
y1]-1-methyl-6-
(trifluoromethyl)-1H-pyrimidine-2,4-dione (CAS 212754-02-4);
b5) from the group of the bleacher herbicides:
PDS inhibitors: beflubutamid, diflufenican, fluridone, flurochloridone,
flurtamone, norflurazon,
picolinafen, and 4-(3-trifluoromethylphenoxy)-2-(4-
trifluoromethylphenyl)pyrimidine (CAS
180608-33-7), HPPD inhibitors: benzobicyclon, benzofenap, bicyclopyrone,
clomazone,
fenquinotrione, isoxaflutole, mesotrione, oxotrione (CAS 1486617-21-3),
pyrasulfotole,
pyrazolynate, pyrazoxyfen, sulcotrione, tefuryltrione, tembotrione,
tolpyralate, topramezone ,
bleacher, unknown target: aclonifen, amitrole flumeturon and 2-chloro-3-
methylsulfanyl-N-(1-
methyltetrazol-5-y1)-4-(trifluoromethypenzamide (CAS 1361139-71-0), 2-(2,4-
dichlorophenyl)methyl-4,4-dimethy1-3-isoxazolidone (CAS 81777-95-9) and 2-(2,5-
dichlorophenyl)methy1-4,4-dimethy1-3-isoxazolidinone (CAS 81778-66-7);
preferably PDS inhibitors: beflubutamid, diflufenican, fluridone,
flurochloridone, flurtamone,
norflurazon, picolinafen, and 4-(3-trifluoromethylphenoxy)-2-(4-
trifluoromethylphenyl)pyrimidine
(CAS 180608-33-7), HPPD inhibitors: benzobicyclon, benzofenap, bicyclopyrone,
clomazone,
fenquinotrione, isoxaflutole, mesotrione, oxotrione (CAS 1486617-21-3),
pyrasulfotole,
pyrazolynate, pyrazoxyfen, sulcotrione, tefuryltrione, tembotrione,
tolpyralate, topramezone ,
bleacher, unknown target: aclonifen, amitrole flumeturon and 2-chloro-3-
methylsulfanyl-N-(1-
methyltetrazol-5-y1)-4-(trifluoromethypenzamide (CAS 1361139-71-0);
b6) from the group of the EPSP synthase inhibitors:
glyphosate, glyphosate-isopropylammonium, glyposate-potassium and glyphosate-
trimesium
(sulfosate);
b7) from the group of the glutamine synthase inhibitors:
bilanaphos (bialaphos), bilanaphos-sodium, glufosinate, glufosinate-P and
glufosinate-
ammonium;
b8) from the group of the DHP synthase inhibitors:
asulam;
CA 03030354 2019-01-09
WO 2018/019842
PCT/EP2017/068784
b9) from the group of the mitosis inhibitors:
compounds of group K1: dinitroanilines such as benfluralin, butralin,
dinitramine, ethalfluralin,
fluchloralin, oryzalin, pendimethalin, prodiamine and trifluralin,
phosphoramidates such as
amiprophos, amiprophos-methyl, and butamiphos, benzoic acid herbicides such as
chlorthal,
5 chlorthal-dimethyl, pyridines such as dithiopyr and thiazopyr, benzamides
such as propyzamide
and tebutam; compounds of group K2: carbetamide, chlorpropham, flamprop,
flamprop-
isopropyl, flamprop-methyl, flamprop-M-isopropyl, flamprop-M-methyl and
propham ; among
these, compounds of group K1 , in particular dinitroanilines are preferred;
10 b1 0) from the group of the VLCFA inhibitors:
chloroacetamides such as acetochlor, alachlor, amidochlor, butachlor,
dimethachlor,
dimethenamid, dimethenamid-P, metazachlor, metolachlor, metolachlor-S,
pethoxamid,
pretilachlor, propachlor, propisochlor and thenylchlor, oxyacetanilides such
as flufenacet and
mefenacet, acetanilides such as diphenamid, naproanilide, napropamide and
napropamide-M,
15 tetrazolinones such fentrazamide, and other herbicides such as anilofos,
cafenstrole,
fenoxasulfone, ipfencarbazone, piperophos, pyroxasulfone and isoxazoline
compounds of the
formulae 11.1, 11.2, 11.3, 11.4, 11.5, 11.6, 11.7, 11.8 and 11.9
F C F3C
F N-CH 0 0 0 0
\\ /, \\ /, N-CH3
H C>hr S
H3C>c,S
3 OCHF OCHF2
H3C 0-N 2 H3C 0-N F
11.1
11.2
F3C\,...õ' \ N F3C N F3C N
R
F . /p N-CH 3 0 0
\\ // \-- \
N-CH 0 0
\\ //
N-CH
H C,h, s 7N' (N'
H3C>CV S H 3C >Cr S
3
H3C 0-N H3C 0- N F H3C 0-N
11.3 11.4 11.5
F3C\NI, F3C\ _...N
0 0
\\ /, N-OH3 \\ // N-CH3
H 3 C >c, S V/
H3C >CV S)\N
F F OCHF2 H3C 0-N F F
H3C 0-N
11.6 11.7
F3C\ F3C\N\
____N
F 0 P '-
\ \NcH3
F 0õ p N-CH3
S (---- H3C>&SN'
H3C>& A \
H3C 0 _ N' F F OCH F2 H3C 0-N F F
1
11.8 1.9
CA 03030354 2019-01-09
WO 2018/019842
PCT/EP2017/068784
36
the isoxazoline compounds of the formula (1)1 are known in the art, e.g. from
WO
2006/024820, WO 2006/037945, WO 2007/071900 and WO 2007/096576;
among the VLCFA inhibitors, preference is given to chloroacetamides and
oxyacetamides;
b11) from the group of the cellulose biosynthesis inhibitors:
chlorthiamid, dichlobenil, flupoxam, indaziflam, isoxaben, triaziflam and 1-
cyclohexy1-5-
pentafluorphenyloxy-1441,2,4,6]thiatriazin-3-ylamine (CAS 175899-01-1);
b12) from the group of the decoupler herbicides:
dinoseb, dinoterb and DNOC and its salts;
b13) from the group of the auxinic herbicides:
2,4-D and its salts and esters such as clacyfos, 2,4-DB and its salts and
esters,
aminocyclopyrachlor and its salts and esters, aminopyralid and its salts such
as aminopyralid-
dimethylammonium, aminopyralid-tris(2-hydroxypropyl)ammonium and its esters,
benazolin,
benazolin-ethyl, chloramben and its salts and esters, clomeprop, clopyralid
and its salts and
esters, dicamba and its salts and esters, dichlorprop and its salts and
esters, dichlorprop-P and
its salts and esters, flopyrauxifen, fluroxypyr, fluroxypyr-butometyl,
fluroxypyr-meptyl, halauxifen
and its salts and esters (CAS 943832-60-8); MCPA and its salts and esters,
MCPA-thioethyl,
MCPB and its salts and esters, mecoprop and its salts and esters, mecoprop-P
and its salts and
esters, picloram and its salts and esters, quinclorac, quinmerac, TBA (2,3,6)
and its salts and
esters, triclopyr and its salts and esters, florpyrauxifen, florpyrauxifen-
benzyl (CAS 1390661-72-
9) and 4-amino-3-chloro-5-fluoro-6-(7-fluoro-1H-indo1-6-Apicolinic acid (CAS
1629965-65-6);
b14) from the group of the auxin transport inhibitors: diflufenzopyr,
diflufenzopyr-sodium,
naptalam and naptalam-sodium;
b15) from the group of the other herbicides: bromobutide, chlorflurenol,
chlorflurenol-methyl,
cinmethylin, cumyluron, cyclopyrimorate (CAS 499223-49-3) and its salts and
esters, dalapon,
dazomet, difenzoquat, difenzoquat-metilsulfate, dimethipin, DSMA, dymron,
endothal and its
salts, etobenzanid, flurenol, flurenol-butyl, flurprimidol, fosamine, fosamine-
ammonium,
indanofan, maleic hydrazide, mefluidide, metam, methiozolin (CAS 403640-27-7),
methyl azide,
methyl bromide, methyl-dymron, methyl iodide, MSMA, oleic acid,
oxaziclomefone, pelargonic
acid, pyributicarb, quinoclamine and tridiphane.
Particularly preferred herbicides B are the herbicides B as defined above; in
particular the
herbicides B.1 - B.86 listed below in table B:
Table B
Herbicide(s) B Herbicide(s) B
B.1 clethodim B.3 quizalofop
B.2 sethoxydim B.4 fluazifop
CA 03030354 2019-01-09
WO 2018/019842
PCT/EP2017/068784
37
Herbicide(s) B Herbicide(s) B
B.5 imazamox B.46 topramezone
B.6 imazamox-ammonium B.47 topramezone-sodium
B.7 imazaquin B.48 glyphosate
B.8 imazaquin-ammonium B.49 glyphosate-ammonium
B.9 imazethapyr B.50 glyphosate-
B.10 imazethapyr-ammonium dimethylammonium
B.11 imazethapyr- B.51 glyphosate-
isopropylammonium isopropylammonium
B.12 cloransulam B.52 glyphosate-trimesium
B.13 diclosulam (sulfosate)
B.14 flumetsulam B.53 glyphosate-potassium
B.15 chlorimuron B.54 glufosinate
B.16 pyrithiobac B.55 glufosinate-ammonium
B.17 prosulfuron B.56 glufosinate-P
B.18 nicosulfuron B.57 glufosinate-P-ammonium
B.19 primisulfuron B.58 pendimethalin
B.20 foramsulfuron B.59 acetochlor
B.21 halosulfuron B.60 flufenacet
B.22 iodosulfuron B.61 metolachlor
B.23 trifloxysulfuron B.62 S-metolachlor
B.24 rimsulfuron B.63 dimethenamid-P
B.25 thifensulfuron B.64 pyroxasulfone
B.26 thifensulfuron-methyl B.65 2,4-D
B.27 ametryne B.66 2,4-D-isobutyl
B.28 atrazine B.67 2,4-D-dimethylammonium
B.29 bentazone B.68 2,4-D-N,N,N-
B.30 bentazone-sodium trimethylethanolammonium
B.31 bromoxynil B.69 dicamba
B.32 bromoxynil-octanoate B.70 dicamba-butotyl
B.33 bromoxynil-heptanoate B.71 dicamba-diglycolamine
B.34 bromoxynil-potassium B.72 dicamba-
B.35 fluometuron dimethylammonium
B.36 simazin B.73 dicamba-diolamine
B.37 sulfentrazone B.74 dicamba-
B.38 carfentrazone-ethyl isopropylammonium
B.39 flumioxazin B.75 dicamba-potassium
B.40 saflufenacil B.76 dicamba-sodium
B.41 trifludimoxazin B.77 dicamba-trolamine
B.42 bicyclopyrone B.78 dicamba-N,N-bis-(3-
B.43 isoxaflutole aminopropyl)methylamine
B.44 mesotrione B.79 dicamba-
B.45 tembotrione diethylenetriamine
CA 03030354 2019-01-09
WO 2018/019842
PCT/EP2017/068784
38
Herbicide(s) B Herbicide(s) B
B.80 diflufenzopyr B.85 dicamba-N,N-bis-(3-
B.81 diflufenzopyr-sodium aminopropyl)methylamine
B.82 cinmethylin + glyphosate-
B.83 dicamba-diglycolamine + isopropylammonium
glyphosate- B.86 dicamba-N,N-bis-(3-
isopropylammonium aminopropyl)methylamine
B.84 dicamba-diglycolamine + + glyphosate-potassium
glyphosate-potassium
Particularly preferred herbicides B are selected from the group consisting of
glyphosate,
glyphosate-ammonium, glyphosate-dimethylammonium, glyphosate-
isopropylammonium,
glyphosate-trimesium (sulfosate), glyphosate-potassium, glufosinate,
glufosinate-ammonium,
glufosinate-P, glufosinate-P-ammonium, 2,4-D, 2,4-D-isobutyl, 2,4-D-
dimethylammonium, 2,4-
D-N,N,N-trimethylethanolammonium, dicamba, dicamba-butotyl, dicamba-
diglycolamine,
dicamba-dimethylammonium, dicamba-diolamine, dicamba-isopropylammonium,
dicamba-
potassium, dicamba-sodium, dicamba-trolamine, dicamba-N,N-bis-(3-
aminopropyl)methylamine
.. and dicamba-diethylenetriamine.
Accordingly, in one embodiment of the invention, the method according to the
present invention
comprises the application of a herbicidal composition comprising at least one,
preferably exactly
one compound of formula (I), at least one further active compound selected
from herbicides B,
.. preferably herbicides B of class b1) to b15), and, in addition, a further
active compound selected
from the group consisting of glyphosate, glyphosate-ammonium, glyphosate-
dimethyl-
ammonium, glyphosate-isopropylammonium, glyphosate-trimesium (sulfosate),
glyphosate-
potassium, glufosinate, glufosinate-ammonium, glufosinate-P, glufosinate-P-
ammonium, 2,4-D,
2,4-D-isobutyl, 2,4-D-dimethylammonium, 2,4-D-N,N,N-trimethylethanolammonium,
dicamba,
dicamba-butotyl, dicamba-diglycolamine, dicamba-dimethylammonium, dicamba-
diolamine,
dicamba-isopropylammonium, dicamba-potassium, dicamba-sodium, dicamba-
trolamine,
dicamba-N,N-bis-(3-aminopropyl)methylamine and dicamba-diethylenetriamine to
control PPO
resistant weeds.
The present invention also relates to a method for controlling PPO resistant
weeds in crops
which comprises applying compositions, comprising at least one compound of
formula (I) and at
least one safener C.
Safeners are chemical compounds which prevent or reduce damage on useful
plants without
having a major impact on the herbicidal action of the herbicidal active
components of the
present compositions towards unwanted plants. They can be applied either
before sowings (e.g.
on seed treatments, shoots or seedlings) or in the pre-emergence application
or post-
emergence application of the useful plant. The safeners and the compound of
formula (I) and/or
the herbicides B can be applied simultaneously or in succession.
CA 03030354 2019-01-09
WO 2018/019842
PCT/EP2017/068784
39
Examples of preferred safeners are benoxacor, cloquintocet, cyometrinil,
cyprosulfamide,
dichlormid, dicyclonon, dietholate, fenchlorazole, fenclorim, flurazole,
fluxofenim, furilazole,
isoxadifen, mefenpyr, mephenate, naphthalic anhydride, oxabetrinil, 4-
(dichloroacetyI)-1-oxa-4-
azaspiro[4.5]decane (CAS 71526-07-3), 2,2,5-trimethy1-3-(dichloroacety1)-1,3-
oxazolidine (CAS
52836-31-4), metcamifen, MG191 (2-dichloromethy1-2-methyl-1,3-dioxolane) or
their salts and
esters.
Especially preferred safeners are benoxacor, cloquintocet, cyprosulfamide,
dichlormid,
fenchlorazole, fenclorim, flurazole, fluxofenim, furilazole, isoxadifen,
mefenpyr, naphthalic
anhydride, oxabetrinil, 4-(dichloroacetyI)-1-oxa-4-azaspiro[4.5]decane (CAS
71526-07-3), 2,2,5-
trimethy1-3-(dichloroacety1)-1,3-oxazolidine (CAS 52836-31-4) and metcamifen
or their salts and
esters.
Particularly preferred safeners are benoxacor, cloquintocet, cyprosulfamide,
dichlormid,
fenchlorazole, fenclorim, furilazole, isoxadifen, mefenpyr, naphtalic
anhydride, 4-(dichloro-
acety1)-1-oxa-4-azaspiro[4.5]decane (CAS 71526-07-3), 2,2,5-trimethy1-3-
(dichloroacety1)-1,3-
oxazolidine (CAS 52836-31-4) and metcamifen or their salts and esters.
Particularly preferred safeners C, which, as component C, can be used in the
method according
to the invention are the safeners C as defined above; in particular the
safeners 0.1 - 0.17 listed
below in table C:
Table C
Safener C
0.1 benoxacor
0.2 cloquintocet
0.3 cloquintocet-mexyl
0.4 cyprosulfamide
0.5 dichlormid
0.6 fenchlorazole
0.7 fenchlorazole-ethyl
0.8 fenclorim
0.9 furilazole
0.10 isoxadifen
0.11 isoxadifen-ethyl
0.12 mefenpyr
0.13 mefenpyr-diethyl
0.14 naphtalic acid anhydride
0.15 4-(dichloroacetyI)-1-oxa-4-azaspiro[4.5]decane (M0N4660, CAS 71526-07-3)
0.16 2,2,5-trimethy1-3-(dichloroacety1)-1,3-oxazolidine (R-29148, CAS 52836-31-
4)
0.17 metcamifen
CA 03030354 2019-01-09
WO 2018/019842
PCT/EP2017/068784
In another preferred embodiment of the invention, the method according to the
present
invention comprises the application of a composition comprising, in addition
to a compound of
formula (I), at least one, especially exactly one safener C, in particular
selected from the group
5 consisting of benoxacor, cloquintocet, cyprosulfamide, dichlormid,
fenchlorazole, fenclorim,
furilazole, isoxadifen, mefenpyr, 4-(dichloroacetyI)-1-oxa-4-
azaspiro[4.5]decane (M0N4660,
CAS 71526-07-3) and 2,2,5-trimethy1-3-(dichloroacety1)-1,3-oxazolidine (R-
29148, CAS 52836-
31-4).
10 The active compounds B of groups b1) to b15) and the active compounds
Care known
herbicides and safeners, see, for example, The Compendium of Pesticide Common
Names
(http://www.alanwood.net/pesticides/); Farm Chemicals Handbook 2000 volume 86,
Meister
Publishing Company, 2000; B. Hock, C. Fedtke, R. R. Schmidt, Herbizide
[Herbicides], Georg
Thieme Verlag, Stuttgart 1995; W. H. Ahrens, Herbicide Handbook, 7th edition,
Weed Science
15 Society of America, 1994; and K. K. Hatzios, Herbicide Handbook,
Supplement for the 7th
edition, Weed Science Society of America, 1998. 2,2,5-Trimethy1-3-
(dichloroacety1)-1,3-
oxazolidine [CAS No. 52836-31-4] is also referred to as R-29148. 4-
(DichloroacetyI)-1-oxa-4-
azaspiro[4.5]decane [CAS No. 71526-07-3] is also referred to as AD-67 and MON
4660.
The piperazine compounds of formula (111) as defined above (hereinafter also
referred to as
20 "compound Ill") as well as its pesticidal action and methods for
preparation are described in WO
2010/049369, WO 2010/037727 und WO 2010/012649.
The assignment of the active compounds to the respective mechanisms of action
is based on
current knowledge. If several mechanisms of action apply to one active
compound, this
25 substance was only assigned to one mechanism of action.
Herbicide compounds B and safeners C having a carboxyl group can be employed
in the form
of the acid, in the form of an agriculturally suitable salt as mentioned above
or else in the form
of an agriculturally acceptable derivative in the compositions according to
the invention.
In the case of dicamba, suitable salts include those, where the counterion is
an agriculturally
acceptable cation. For example, suitable salts of dicamba are dicamba-sodium,
dicamba-
potassium, dicamba-methylammonium, dicamba-dimethylammonium, dicamba-
isopropylammonium, dicamba-diglycolamine, dicamba-olamine, dicamba-diolamine,
dicamba-
trolamine, dicamba-N,N-bis-(3-aminopropyl)methylamine and dicamba-
diethylenetriamine.
Examples of a suitable ester are dicamba-methyl and dicamba-butotyl.
Suitable salts of 2,4-D are 2,4-D-ammonium, 2,4-D-dimethylammonium, 2,4-D-
diethyl-
ammonium, 2,4-D-diethanolammonium (2,4-D-diolamine), 2,4-D-triethanolammonium,
2,4-D-
isopropylammonium, 2,4-D-triisopropanolammonium, 2,4-D-heptylammonium, 2,4-D-
dodecyl-
ammonium, 2,4-D-tetradecylammonium, 2,4-D-triethylammonium, 2,4-D-tris(2-
hydroxypro-
pyl)ammonium, 2,4-D-tris(isopropyl)ammonium, 2,4-D-trolamine, 2,4-D-lithium,
2,4-D-sodium.
CA 03030354 2019-01-09
WO 2018/019842
PCT/EP2017/068784
41
Examples of suitable esters of 2,4-D are 2,4-D-butotyl, 2,4-D-2-butoxypropyl,
2,4-D-3-butoxy-
propyl, 2,4-D-butyl, 2,4-D-ethyl, 2,4-D-ethylhexyl, 2,4-D-isobutyl, 2,4-D-
isooctyl, 2,4-D-isopropyl,
2,4-D-meptyl, 2,4-D-methyl, 2,4-D-octyl, 2,4-D-pentyl, 2,4-D-propyl, 2,4-D-
tefuryl and clacyfos.
Suitable salts of 2,4-DB are for example 2,4-DB-sodium, 2,4-DB-potassium and
2,4-DB-di-
methylammonium. Suitable esters of 2,4-DB are for example 2,4-DB-butyl and 2,4-
DB-isoctyl.
Suitable salts of dichlorprop are for example dichlorprop-sodium, dichlorprop-
potassium and
dichlorprop-dimethylammonium. Examples of suitable esters of dichlorprop are
dichlorprop-
butotyl and dichlorprop-isoctyl.
Suitable salts and esters of MCPA include MCPA-butotyl, MCPA-butyl, MCPA-
dimethyl-
ammonium, MCPA-diolamine, MCPA-ethyl, MCPA-thioethyl, MCPA-2-ethylhexyl, MCPA-
isobutyl, MCPA-isoctyl, MCPA-isopropyl, MCPA-isopropylammonium, MCPA-methyl,
MCPA-
olamine, MCPA-potassium, MCPA-sodium and MCPA-trolamine.
A suitable salt of MCPB is MCPB sodium. A suitable ester of MCPB is MCPB-
ethyl.
Suitable salts of clopyralid are clopyralid-potassium, clopyralid-olamine and
clopyralid-tris-(2-
hydroxypropyl)ammonium. Example of suitable esters of clopyralid is clopyralid-
methyl.
Examples of a suitable ester of fluroxypyr are fluroxypyr-meptyl and
fluroxypyr-2-butoxy-1-
methylethyl, wherein fluroxypyr-meptyl is preferred.
Suitable salts of picloram are picloram-dimethylammonium, picloram-potassium,
picloram-
triisopropanolammonium, picloram-triisopropylammonium and picloram-trolamine.
A suitable
ester of picloram is picloram-isoctyl.
A suitable salt of triclopyr is triclopyr-triethylammonium. Suitable esters of
triclopyr are for
example triclopyr-ethyl and triclopyr-butotyl.
Suitable salts and esters of chloramben include chloramben-ammonium,
chloramben-diolamine,
chloramben-methyl, chloramben-methylammonium and chloramben-sodium. Suitable
salts and
esters of 2,3,6-TBA include 2,3,6-TBA-dimethylammonium, 2,3,6-TBA-lithium,
2,3,6-TBA-
potassium and 2,3,6-TBA-sodium.
Suitable salts and esters of aminopyralid include aminopyralid-potassium and
aminopyralid-
tris(2-hydroxypropyl)ammonium.
Suitable salts of glyphosate are for example glyphosate-ammonium, glyphosate-
diammonium,
glyphoste-dimethylammonium, glyphosate-isopropylammonium, glyphosate-
potassium,
glyphosate-sodium, glyphosate-trimesium as well as the ethanolamine and
diethanolamine
salts, preferably glyphosate-diammonium, glyphosate-isopropylammonium and
glyphosate-
trimesium (sulfosate).
CA 03030354 2019-01-09
WO 2018/019842
PCT/EP2017/068784
42
A suitable salt of glufosinate is for example glufosinate-ammonium.
A suitable salt of glufosinate-P is for example glufosinate-P-ammonium.
A suitable salt of bentazone is for example bentazone sodium.
Suitable salts and esters of bromoxynil are for example bromoxynil-butyrate,
bromoxynil-
heptanoate, bromoxynil-octanoate, bromoxynil-potassium and bromoxynil-sodium.
Suitable salts and esters of ioxonil are for example ioxonil-octanoate,
ioxonil-potassium and
ioxonil-sodium.
Suitable salts and esters of mecoprop include mecoprop-butotyl, mecoprop-
dimethylammonium,
mecoprop-diolamine, mecoprop-ethadyl, mecoprop-2-ethylhexyl, mecoprop-isoctyl,
mecoprop-
methyl, mecoprop-potassium, mecoprop-sodium and mecoprop-trolamine.
Suitable salts of mecoprop-P are for example mecoprop-P-butotyl, mecoprop-P-
dimethyl-
ammonium, mecoprop-P-2-ethylhexyl, mecoprop-P-isobutyl, mecoprop-P-potassium
and
mecoprop-P-sodium.
A suitable salt of diflufenzopyr is for example diflufenzopyr-sodium.
A suitable salt of naptalam is for example naptalam-sodium.
Suitable salts and esters of aminocyclopyrachlor are for example
aminocyclopyrachlor-
dimethylammonium, aminocyclopyrachlor-methyl, aminocyclopyrachlor-
triisopropanolammonium, aminocyclopyrachlor-sodium and aminocyclopyrachlor-
potassium.
A suitable salt of quinclorac is for example quinclorac-dimethylammonium.
A suitable salt of quinmerac is for example quinclorac-dimethylammonium.
A suitable salt of imazamox is for example imazamox-ammonium.
Suitable salts of imazapic are for example imazapic-ammonium and imazapic-
isopropylammonium.
Suitable salts of imazapyr are for example imazapyr-ammonium and imazapyr-
isopropylammonium.
A suitable salt of imazaquin is for example imazaquin-ammonium.
Suitable salts of imazethapyr are for example imazethapyr-ammonium and
imazethapyr-
isopropylammonium.
CA 03030354 2019-01-09
WO 2018/019842
PCT/EP2017/068784
43
A suitable salt of topramezone is for example topramezone-sodium.
In one preferred embodiment of the invention, the method according to the
present invention
comprises the application of a composition comprising at least one, preferably
exactly one,
compound of formula (I) and at least one, preferably exactly one herbicide B.
In another preferred embodiment of the invention, the method according to the
present
invention comprises the application of a composition comprising at least one,
preferably exactly
one, compound of formula (I), and at least two, preferably exactly two
herbicides B different
from each other.
In another preferred embodiment of the invention, the method according to the
present
invention comprises the application of a composition comprising at least one,
preferably exactly
one, compound of formula (I), and at least three, preferably exactly three
herbicides B different
from each other.
According to a further preferred embodiment, the method according to the
present invention
comprises the application of a composition comprising ternary compositions
which correspond
to the binary compositions mentioned above and additionally comprise a safener
C, in particular
selected from the group consisting of benoxacor, cloquintocet, cyprosulfamide,
dichlormid,
fenchlorazole, fenclorim, furilazole, isoxadifen, mefenpyr, 4-(dichloroacetyI)-
1-oxa-4-
azaspiro[4.5]decane (M0N4660, CAS 71526-07-3) and 2,2,5-trimethy1-3-
(dichloroacety1)-1,3-
oxazolidine (R-29148, CAS 52836-31-4).
Here and below, the term "binary compositions" includes compositions
comprising one or more,
for example 1, 2 or 3, active compounds of the formula (I) and either one or
more, for example
1, 2 or 3, herbicides B or one or more safeners.
Correspondingly, the term "ternary compositions" includes compositions
comprising one or
more, for example 1, 2 or 3, active compounds of the formula (I), one or more,
for example 1, 2
or 3, herbicides B and one or more, for example 1, 2 or 3, safeners C.
In another preferred embodiment, the method according to the present invention
comprises the
application of a composition comprising at least one, preferably exactly one
compound of
formula (I), and at least one, preferably exactly one safener C.
In another preferred embodiment, the method according to the present invention
comprises the
application of a composition comprising at least one, preferably exactly one
compound of
formula (I), at least one, preferably exactly one herbicide B, and at least
one, preferably exactly
one, safener C.
CA 03030354 2019-01-09
WO 2018/019842
PCT/EP2017/068784
44
In another preferred embodiment, the method according to the present invention
comprises the
application of a composition comprising at least one, preferably exactly one
compound of
formula (I), preferably exactly two herbicides B different from each other,
and at least one,
preferably exactly one, safener C.
In another preferred embodiment, the method according to the present invention
comprises the
application of a composition comprising at least one, preferably exactly one
compound of
formula (I), at least three, preferably exactly three herbicides B different
from each other, and at
least one, preferably exactly one, safener C.
In binary compositions comprising at least one compound of formula (I) as
component A and at
least one herbicide B, the weight ratio of the active compounds A:B is
generally in the range of
from 1:1000 to 1000:1, preferably in the range of from 1:500 to 500:1, in
particular in the range
of from 1:250 to 250:1 and particularly preferably in the range of from 1:75
to 75:1.
In binary compositions comprising at least one compound of formula (I) as
component A and at
least one safener C, the weight ratio of the active compounds A:C is generally
in the range of
from 1:1000 to 1000:1, preferably in the range of from 1:500 to 500:1, in
particular in the range
of from 1:250 to 250:1 and particularly preferably in the range of from 1:75
to 75:1.
In ternary compositions comprising both at least one compound of formula (I)
as component A,
at least one herbicide B and at least one safener C, the relative proportions
by weight of the
components A:B are generally in the range of from 1:1000 to 1000:1, preferably
in the range of
from 1:500 to 500:1, in particular in the range of from 1:250 to 250:1 and
particularly preferably
in the range of from 1:75 to 75:1, the weight ratio of the components A:C is
generally in the
range of from 1:1000 to 1000:1, preferably in the range of from 1:500 to
500:1, in particular in
the range of from 1:250 to 250:1 and particularly preferably in the range of
from 1:75 to 75:1,
and the weight ratio of the components B:C is generally in the range of from
1:1000 to 1000:1,
preferably in the range of from 1:500 to 500:1, in particular in the range of
from 1:250 to 250:1
and particularly preferably in the range of from 1:75 to 75:1. The weight
ratio of components A +
B to component C is preferably in the range of from 1:500 to 500:1, in
particular in the range of
from 1:250 to 250:1 and particularly preferably in the range of from 1:75 to
75:1.
The method according to the invention can be employed in a further number of
crop plants for
eliminating the PPO inhibitor herbicide resistant weeds. Examples of suitable
crops are the
following:
Allium cepa, Ananas comosus, Arachis hypogaea, Asparagus officinalis, Avena
sativa, Beta
vulgaris spec. altissima, Beta vulgaris spec. rapa, Brassica napus var. napus,
Brassica napus
var. napobrassica, Brassica rapa var. silvestris, Brassica oleracea, Brassica
nigra, Camellia
sinensis, Carthamus tinctorius, Carya illinoinensis, Citrus limon, Citrus
sinensis, Coffee arabica
(Coffee canephora, Coffee liberica), Cucumis sativus, Cynodon dactylon, Daucus
carota, Elaeis
guineensis, Fragaria vesca, Glycine max, Gossypium hirsutum, (Gossypium
arboreum,
Gossypium herbaceum, Gossypium vitifolium), Helianthus annuus, Hevea
brasiliensis, Hordeum
CA 03030354 2019-01-09
WO 2018/019842
PCT/EP2017/068784
vulgare, Humulus lupulus, 1pomoea batatas, Juglans regia, Lens culinaris,
Linum usitatissimum,
Lycopersicon lycopersicum, Malus spec., Manihot esculenta, Medicago sativa,
Musa spec.,
Nicotiana tabacum (N.rustica), Olea europaea, Oryza sativa, Phaseolus lunatus,
Phaseolus
vulgaris, Picea abies, Pinus spec., Pistacia vera, Pisum sativum, Prunus
avium, Prunus persica,
5 Pyrus communis, Prunus armeniaca, Prunus cerasus, Prunus dulcis and
prunus domestica,
Ribes sylvestre, Ricinus communis, Saccharum officinarum, Secale cereale,
Sinapis alba,
Solanum tuberosum, Sorghum bicolor (s. vulgare), Theobroma cacao, Trifolium
pratense,
Triticum aestivum, Triticale, Triticum durum, Vicia faba, Vitis vinifera, Zea
mays.
10 Preferred crops are Arachis hypogaea, Beta vulgaris spec. altissima,
Brassica napus var.
napus, Brassica oleracea, Citrus limon, Citrus sinensis, Coffea arabica
(Coffea canephora,
Coffea liberica), Cynodon dactylon, Glycine max, Gossypium hirsutum,
(Gossypium arboreum,
Gossypium herbaceum, Gossypium vitifolium), Helianthus annuus, Hordeum
vulgare, Juglans
regia, Lens culinaris, Linum usitatissimum, Lycopersicon lycopersicum, Malus
spec., Medicago
15 sativa, Nicotiana tabacum (N.rustica), Olea europaea, Oryza sativa,
Phaseolus lunatus,
Phaseolus vulgaris, Pistacia vera, Pisum sativum, Prunus dulcis, Saccharum
officinarum,
Secale cereale, Solanum tuberosum, Sorghum bicolor (s. vulgare), Triticale,
Triticum aestivum,
Triticum durum, Vicia faba, Vitis vinifera and Zea mays.
20 Especially preferred crops are crops of cereals, corn, soybeans, rice,
oilseed rape / canola,
sunflowers, cotton, potatoes, peanuts or plantation crops.
Particularly preferred are crops of corn, soybeans, oilseed rape / canola and
cotton.
In another embodiment, the invention refers to a plant cell transformed by a
nucleic acid
encoding a herbicide tolerant PPO polypeptide disclosed herein or to a plant
cell which has
been mutated to obtain a plant expressing a nucleic acid encoding a mutated
PPO polypeptide
according to the present invention, wherein expression of the nucleic acid in
the plant cell
results in increased resistance or tolerance to PPO inhibitor herbicides,
preferably the
compounds of formula (I), as compared to a wild type variety of the plant
cell.
The term "expression/expressing" or "gene expression" means the transcription
of a specific
gene or specific genes or specific genetic construct. The term "expression" or
"gene expression"
in particular means the transcription of a gene or genes or genetic construct
into structural RNA
(rRNA, tRNA) or mRNA with or without subsequent translation of the latter into
a protein. The
process includes transcription of DNA and processing of the resulting mRNA
product.
To obtain the desired effect, i.e. plants that are tolerant or resistant to
PPO inhibitor herbicides,
preferably the compounds of formula (I) of the present invention, it will be
understood that the at
least one nucleic acid is "over-expressed" by methods and means known to the
person skilled in
the art.
The term "increased expression" or "overexpression" as used herein means any
form of
CA 03030354 2019-01-09
WO 2018/019842
PCT/EP2017/068784
46
expression that is additional to the original wild-type expression level.
Methods for increasing
expression of genes or gene products are well documented in the art and
include, for example,
overexpression driven by appropriate promoters, the use of transcription
enhancers or
translation enhancers. Isolated nucleic acids which serve as promoter or
enhancer elements
may be introduced in an appropriate position (typically upstream) of a non-
heterologous form of
a polynucleotide so as to upregulate expression of a nucleic acid encoding the
polypeptide of
interest. For example, endogenous promoters may be altered in vivo by
mutation, deletion,
and/or substitution (see, Kmiec, US 5,565,350; Zarling et al., W09322443), or
isolated
promoters may be introduced into a plant cell in the proper orientation and
distance from a gene
of the present invention so as to control the expression of the gene.
If polypeptide expression is desired, it is generally desirable to include a
polyadenylation region
at the 3'-end of a polynucleotide coding region. The polyadenylation region
can be derived from
the natural gene, from a variety of other plant genes, or from T-DNA. The 3'
end sequence to be
added may be derived from, for example, the nopaline synthase or octopine
synthase genes, or
alternatively from another plant gene, or less preferably from any other
eukaryotic gene.
An intron sequence may also be added to the 5' untranslated region (UTR) or
the coding
sequence of the partial coding sequence to increase the amount of the mature
message that
accumulates in the cytosol. Inclusion of a spliceable intron in the
transcription unit in both plant
and animal expression constructs has been shown to increase gene expression at
both the
mRNA and protein levels up to 1000-fold (Buchman and Berg (1988) Mol. Cell
biol. 8: 4395-
4405; Callis et al. (1987) Genes Dev 1:1183-1200). Such intron enhancement of
gene
expression is typically greatest when placed near the 5' end of the
transcription unit. Use of the
maize introns Adh1-5 intron 1, 2, and 6, the Bronze-1 intron are known in the
art. For general
information see: The Maize Handbook, Chapter 116, Freeling and Walbot, Eds.,
Springer, N.Y.
(1994)
The term "introduction" or "transformation" as referred to herein encompasses
the transfer of an
exogenous polynucleotide into a host cell, irrespective of the method used for
transfer. Plant
tissue capable of subsequent clonal propagation, whether by organogenesis or
embryogenesis,
may be transformed with a genetic construct of the present invention and a
whole plant
regenerated there from. The particular tissue chosen will vary depending on
the clonal
propagation systems available for, and best suited to, the particular species
being transformed.
Exemplary tissue targets include leaf disks, pollen, embryos, cotyledons,
hypocotyls,
megagametophytes, callus tissue, existing meristematic tissue (e.g., apical
meristem, axillary
buds, and root meristems), and induced meristem tissue (e.g., cotyledon
meristem and
hypocotyl meristem). The polynucleotide may be transiently or stably
introduced into a host cell
and may be maintained non-integrated, for example, as a plasmid.
Alternatively, it may be
integrated into the host genome. The resulting transformed plant cell may then
be used to
regenerate a transformed plant in a manner known to persons skilled in the
art.
The transfer of foreign genes into the genome of a plant is called
transformation.
Transformation of plant species is now a fairly routine technique.
Advantageously, any of
CA 03030354 2019-01-09
WO 2018/019842
PCT/EP2017/068784
47
several transformation methods may be used to introduce the gene of interest
into a suitable
ancestor cell. The methods described for the transformation and regeneration
of plants from
plant tissues or plant cells may be utilized for transient or for stable
transformation.
Transformation methods include the use of liposomes, electroporation,
chemicals that increase
free DNA uptake, injection of the DNA directly into the plant, particle gun
bombardment,
transforrmation using viruses or pollen and microprojection. Methods may be
selected from the
calcium/polyethylene glycol method for protoplasts (Krens, F.A. et al., (1982)
Nature 296, 72-74;
Negrutiu I et al. (1987) Plant Mol Biol 8: 363-373); electroporation of
protoplasts (Shillito R.D. et
al. (1985) Bio/Technol 3, 1099-1102); microinjection into plant material
(Crossway A et al.,
(1986) Mol. Gen Genet 202: 179-185); DNA or RNA-coated particle bombardment
(Klein TM et
al., (1987) Nature 327: 70) infection with (non-integrative) viruses and the
like. Transgenic
plants, including transgenic crop plants, are preferably produced via
Agrobacterium-mediated
transformation. An advantageous transformation method is the transformation in
planta. To this
end, it is possible, for example, to allow the agrobacteria to act on plant
seeds or to inoculate
the plant meristem with agrobacteria. It has proved particularly expedient in
accordance with the
invention to allow a suspension of transformed agrobacteria to act on the
intact plant or at least
on the flower primordia. The plant is subsequently grown on until the seeds of
the treated plant
are obtained (Clough and Bent, Plant J. (1998) 16, 735-743). Methods for
Agrobacterium-
mediated transformation of rice include well known methods for rice
transformation, such as
those described in any of the following: European patent application EP
1198985 Al, Aldemita
and Hodges (Planta 199: 612-617, 1996); Chan et al. (Plant Mol Biol 22 (3):
491-506, 1993),
Hiei et al. (Plant J 6 (2): 271-282, 1994), which disclosures are incorporated
by reference herein
as if fully set forth. In the case of corn transformation, the preferred
method is as described in
either lshida et al. (Nat. Biotechnol 14(6): 745-50, 1996) or Frame et al.
(Plant Physiol 129(1):
13-22, 2002), which disclosures are incorporated by reference herein as if
fully set forth. Said
methods are further described by way of example in B. Jenes et al., Techniques
for Gene
Transfer, in: Transgenic Plants, Vol. 1, Engineering and Utilization, eds.
S.D. Kung and R. Wu,
Academic Press (1993) 128-143 and in Potrykus Annu. Rev. Plant Physiol. Plant
Molec. Biol. 42
(1991) 205-225). The nucleic acids or the construct to be expressed is
preferably cloned into a
vector, which is suitable for transforming Agrobacterium tumefaciens, for
example pBin19
(Bevan et al., Nucl. Acids Res. 12 (1984) 8711). Agrobacteria transformed by
such a vector can
then be used in known manner for the transformation of plants, such as plants
used as a model,
like Arabidopsis (Arabidopsis thaliana is within the scope of the present
invention not
considered as a crop plant), or crop plants such as, by way of example,
tobacco plants, for
example by immersing bruised leaves or chopped leaves in an agrobacterial
solution and then
culturing them in suitable media. The transformation of plants by means of
Agrobacterium
tumefaciens is described, for example, by Hofgen and Willmitzer in Nucl. Acid
Res. (1988) 16,
9877 or is known inter alia from F.F. White, Vectors for Gene Transfer in
Higher Plants; in
Transgenic Plants, Vol. 1, Engineering and Utilization, eds. S.D. Kung and R.
Wu, Academic
Press, 1993, pp. 15-38.
In addition to the transformation of somatic cells, which then have to be
regenerated into intact
plants, it is also possible to transform the cells of plant meristems and in
particular those cells
which develop into gametes. In this case, the transformed gametes follow the
natural plant
CA 03030354 2019-01-09
WO 2018/019842
PCT/EP2017/068784
48
development, giving rise to transgenic plants. Thus, for example, seeds of
Arabidopsis are
treated with agrobacteria and seeds are obtained from the developing plants of
which a certain
proportion is transformed and thus transgenic [Feldman, KA and Marks MD
(1987). Mol Gen
Genet 208:274-289; Feldmann K (1992). In: C Koncz, N-H Chua and J Shell, eds,
Methods in
Arabidopsis Research. Word Scientific, Singapore, pp. 274-289]. Alternative
methods are based
on the repeated removal of the inflorescences and incubation of the excision
site in the center of
the rosette with transformed agrobacteria, whereby transformed seeds can
likewise be obtained
at a later point in time (Chang (1994). Plant J. 5: 551-558; Katavic (1994).
Mol Gen Genet, 245:
363-370). However, an especially effective method is the vacuum infiltration
method with its
modifications such as the "floral dip" method. In the case of vacuum
infiltration of Arabidopsis,
intact plants under reduced pressure are treated with an agrobacterial
suspension [Bechthold, N
(1993). C R Aced Sci Paris Life Sci, 316: 1194-1199], while in the case of the
"floral dip" method
the developing floral tissue is incubated briefly with a surfactant-treated
agrobacterial
suspension [Clough, SJ and Bent AF (1998) The Plant J. 16, 735-743]. A certain
proportion of
transgenic seeds are harvested in both cases, and these seeds can be
distinguished from non-
transgenic seeds by growing under the above-described selective conditions. In
addition the
stable transformation of plastids is of advantages because plastids are
inherited maternally is
most crops reducing or eliminating the risk of transgene flow through pollen.
The transformation
of the chloroplast genome is generally achieved by a process which has been
schematically
displayed in Klaus et al., 2004 [Nature Biotechnology 22 (2), 225-229].
Briefly the sequences to
be transformed are cloned together with a selectable marker gene between
flanking sequences
homologous to the chloroplast genome. These homologous flanking sequences
direct site
specific integration into the plastome. Plastidal transformation has been
described for many
different plant species and an overview is given in Bock (2001) Transgenic
plastids in basic
research and plant biotechnology. J Mol Biol. 2001 Sep 21; 312 (3):425-38 or
Maliga, P (2003)
Progress towards commercialization of plastid transformation technology.
Trends Biotechnol.
21, 20-28. Further biotechnological progress has recently been reported in
form of marker free
plastid transformants, which can be produced by a transient co-integrated
maker gene (Klaus et
al., 2004, Nature Biotechnology 22(2), 225-229). The genetically modified
plant cells can be
regenerated via all methods with which the skilled worker is familiar.
Suitable methods can be
found in the abovementioned publications by S.D. Kung and R. Wu, Potrykus or
Hofgen and
Willmitzer.
Generally after transformation, plant cells or cell groupings are selected for
the presence of one
or more markers which are encoded by plant-expressible genes co-transferred
with the gene of
interest, following which the transformed material is regenerated into a whole
plant. To select
transformed plants, the plant material obtained in the transformation is, as a
rule, subjected to
selective conditions so that transformed plants can be distinguished from
untransformed plants.
For example, the seeds obtained in the above-described manner can be planted
and, after an
initial growing period, subjected to a suitable selection by spraying. A
further possibility consists
in growing the seeds, if appropriate after sterilization, on agar plates using
a suitable selection
agent so that only the transformed seeds can grow into plants. Alternatively,
the transformed
plants are screened for the presence of a selectable marker such as the ones
described above.
CA 03030354 2019-01-09
WO 2018/019842
PCT/EP2017/068784
49
Following DNA transfer and regeneration, putatively transformed plants may
also be evaluated,
for instance using Southern analysis, for the presence of the gene of
interest, copy number
and/or genomic organisation. Alternatively or additionally, expression levels
of the newly
introduced DNA may be monitored using Northern and/or Western analysis, both
techniques
being well known to persons having ordinary skill in the art.
The generated transformed plants may be propagated by a variety of means, such
as by clonal
propagation or classical breeding techniques. For example, a first generation
(or Ti)
transformed plant may be selfed and homozygous second-generation (or T2)
transformants
selected, and the T2 plants may then further be propagated through classical
breeding
techniques. The generated transformed organisms may take a variety of forms.
For example,
they may be chimeras of transformed cells and non-transformed cells; clonal
transformants
(e.g., all cells transformed to contain the expression cassette); grafts of
transformed and
untransformed tissues (e.g., in plants, a transformed rootstock grafted to an
untransformed
scion).
Preferably, the wild-type or mutated PPO nucleic acid comprises a
polynucleotide sequence
selected from the group consisting of: a) a polynucleotide encoding a
polypeptide of interest; b)
a polynucleotide comprising at least 60 consecutive nucleotides of any of a);
and c) a
polynucleotide complementary to the polynucleotide of any of a) through b).
Preferably, the expression of the nucleic acid in the plant results in the
plant's increased
resistance to PPO inhibitor herbicides, preferably the compounds of formula
(I), as compared to
a wild type variety of the plant.
In another embodiment, the invention refers to a plant, comprising a plant
cell according to the
present invention, wherein expression of the nucleic acid in the plant results
in the plant's
increased resistance to PPO inhibitor herbicides, preferably the compounds of
formula (I), as
compared to a wild type variety of the plant.
The plants described herein can be either transgenic crop plants or non-
transgenic plants.
For the purposes of the invention, "transgenic", "transgene" or "recombinant"
means with regard
to, for example, a nucleic acid sequence, an expression cassette, gene
construct or a vector
comprising the nucleic acid sequence or an organism transformed with the
nucleic acid
sequences, expression cassettes or vectors according to the invention, all
those constructions
brought about by recombinant methods in which either
(a) the nucleic acid sequences encoding proteins useful in the methods of
the invention, or
(b) genetic control sequence(s) which is operably linked with the nucleic
acid sequence
according to the invention, for example a promoter, or
(c) a) and b)
are not located in their natural genetic environment or have been modified by
recombinant
methods, it being possible for the modification to take the form of, for
example, a substitution,
addition, deletion, inversion or insertion of one or more nucleotide residues
in order to allow for
CA 03030354 2019-01-09
WO 2018/019842
PCT/EP2017/068784
the expression of the mutated PPO of the present invention. The natural
genetic environment is
understood as meaning the natural genomic or chromosomal locus in the original
plant or the
presence in a genomic library. In the case of a genomic library, the natural
genetic environment
of the nucleic acid sequence is preferably retained, at least in part. The
environment flanks the
5 nucleic acid sequence at least on one side and has a sequence length of
at least 50 bp,
preferably at least 500 bp, especially preferably at least 1000 bp, most
preferably at least 5000
bp. A naturally occurring expression cassette ¨ for example the naturally
occurring combination
of the natural promoter of the nucleic acid sequences with the corresponding
nucleic acid
sequence encoding a polypeptide useful in the methods of the present
invention, as defined
10 above ¨ becomes a transgenic expression cassette when this expression
cassette is modified
by non-natural, synthetic ("artificial") methods such as, for example,
mutagenic treatment.
Suitable methods are described, for example, in US 5,565,350 or WO 00/15815.
A transgenic plant for the purposes of the invention is thus understood as
meaning, as above,
15 that the nucleic acids of the invention are not at their natural locus
in the genome of said plant, it
being possible for the nucleic acids to be expressed homologously or
heterologously. However,
as mentioned, transgenic also means that, while the nucleic acids according to
the invention or
used in the inventive method are at their natural position in the genome of a
plant, the sequence
has been modified with regard to the natural sequence, and/or that the
regulatory sequences of
20 the natural sequences have been modified. Transgenic is preferably
understood as meaning the
expression of the nucleic acids according to the invention at an unnatural
locus in the genome,
i.e. homologous or, preferably, heterologous expression of the nucleic acids
takes place.
Preferred transgenic plants are mentioned herein. Furthermore, the term
"transgenic" refers to
any plant, plant cell, callus, plant tissue, or plant part, that contains all
or part of at least one
25 recombinant polynucleotide. In many cases, all or part of the
recombinant polynucleotide is
stably integrated into a chromosome or stable extra-chromosomal element, so
that it is passed
on to successive generations. For the purposes of the invention, the term
"recombinant
polynucleotide" refers to a polynucleotide that has been altered, rearranged,
or modified by
genetic engineering. Examples include any cloned polynucleotide, or
polynucleotides, that are
30 linked or joined to heterologous sequences. The term "recombinant" does
not refer to alterations
of polynucleotides that result from naturally occurring events, such as
spontaneous mutations,
or from non-spontaneous mutagenesis followed by selective breeding.
Plants containing mutations arising due to non-spontaneous mutagenesis and
selective
35 breeding are referred to herein as non-transgenic plants and are
included in the present
invention. In embodiments wherein the plant is transgenic and comprises
multiple mutated PPO
nucleic acids, the nucleic acids can be derived from different genomes or from
the same
genome. Alternatively, in embodiments wherein the plant is non-transgenic and
comprises
multiple mutated PPO nucleic acids, the nucleic acids are located on different
genomes or on
40 the same genome. As used herein, "mutagenized" refers to an organism or
DNA thereof having
alteration(s) in the biomolecular sequence of its native genetic material as
compared to the
sequence of the genetic material of a corresponding wild-type organism or DNA,
wherein the
alteration(s) in genetic material were induce and/or selected by human action.
Methods of
inducing mutations can induce mutations in random positions in the genetic
material or can
CA 03030354 2019-01-09
WO 2018/019842
PCT/EP2017/068784
51
induce mutations in specific locations in the genetic material (i.e., can be
directed mutagenesis
techniques), such as by use of a genoplasty technique.
In certain embodiments, the present invention involves herbidicide-resistant
plants that are
produced by mutation breeding. Such plants comprise a polynucleotide encoding
a mutated
PPO and are tolerant to one or more PPO inhibitor herbicides, preferably
compounds of formula
(I). Such methods can involve, for example, exposing the plants or seeds to a
mutagen,
particularly a chemical mutagen such as, for example, ethyl methanesulfonate
(EMS) and
selecting for plants that have enhanced tolerance to at least one or more PPO
inhibitor
herbicides, preferably compounds of formula (I).
However, the present invention is not limited to herbicide-tolerant plants
that are produced by a
mutagenesis method involving the chemical mutagen EMS. Any mutagenesis method
known in
the art may be used to produce the herbicide-resistant plants of the present
invention. Such
mutagenesis methods can involve, for example, the use of any one or more of
the following
mutagens: radiation, such as X-rays, Gamma rays (e.g., cobalt 60 or cesium
137), neutrons,
(e.g., product of nuclear fission by uranium 235 in an atomic reactor), Beta
radiation (e.g.,
emitted from radioisotopes such as phosphorus 32 or carbon 14), and
ultraviolet radiation
(preferably from 2500 to 2900 nm), and chemical mutagens such as base
analogues (e.g., 5-
bromo-uracil), related compounds (e.g., 8-ethoxy caffeine), antibiotics (e.g.,
streptonigrin),
alkylating agents (e.g., sulfur mustards, nitrogen mustards, epoxides,
ethylenamines, sulfates,
sulfonates, sulfones, lactones), azide, hydroxylamine, nitrous acid, or
acridines. Herbicide-
resistant plants can also be produced by using tissue culture methods to
select for plant cells
comprising herbicide-resistance mutations and then regenerating herbicide-
resistant plants
therefrom. See, for example, U.S. Patent Nos. 5,773,702 and 5,859,348, both of
which are
herein incorporated in their entirety by reference. Further details of
mutation breeding can be
found in "Principals of Cultivar Development" Fehr, 1993 Macmillan Publishing
Company the
disclosure of which is incorporated herein by reference
In addition to the definition above, the term "plant" is intended to encompass
crop plants at any
stage of maturity or development, as well as any tissues or organs (plant
parts) taken or derived
from any such plant unless otherwise clearly indicated by context. Plant parts
include, but are
not limited to, stems, roots, flowers, ovules, stamens, leaves, embryos,
meristematic regions,
callus tissue, anther cultures, gametophytes, sporophytes, pollen,
microspores, protoplasts, and
the like.
The plant of the present invention comprises at least one mutated PPO nucleic
acid or over-
expressed wild-type PPO nucleic acid, and has increased tolerance to PPO
inhibitor herbicides,
preferably the compounds of formula (I), as compared to a wild-type variety of
the plant. It is
possible for the plants of the present invention to have multiple wild-type or
mutated PPO
nucleic acids from different genomes since these plants can contain more than
one genome.
For example, a plant contains two genomes, usually referred to as the A and B
genomes.
Because PPO is a required metabolic enzyme, it is assumed that each genome has
at least one
gene coding for the PPO enzyme (i.e. at least one PPO gene). As used herein,
the term "PPO
CA 03030354 2019-01-09
WO 2018/019842
PCT/EP2017/068784
52
gene locus" refers to the position of an PPO gene on a genome, and the terms
"PPO gene" and
"PPO nucleic acid" refer to a nucleic acid encoding the PPO enzyme. The PPO
nucleic acid on
each genome differs in its nucleotide sequence from an PPO nucleic acid on
another genome.
One of skill in the art can determine the genome of origin of each PPO nucleic
acid through
.. genetic crossing and/or either sequencing methods or exonuclease digestion
methods known to
those of skill in the art.
The present invention includes plants comprising one, two, three, or more
mutated PPO alleles,
wherein the plant has increased tolerance to PPO inhibitor herbicides,
preferably the
.. compounds of formula (I), as compared to a wild-type variety of the plant.
The mutated PPO
alleles can comprise a nucleotide sequence selected from the group consisting
of a
polynucleotide encoding a polypeptide of interest, a polynucleotide comprising
at least 60
consecutive nucleotides of any of the aforementioned polynucleotides; and a
polynucleotide
complementary to any of the aforementioned polynucleotides.
"Alleles" or "allelic variants" are alternative forms of a given gene, located
at the same
chromosomal position. Allelic variants encompass Single Nucleotide
Polymorphisms (SNPs), as
well as Small Insertion/Deletion Polymorphisms (I NDELs). The size of I NDELs
is usually less
than 100 bp. SNPs and I NDELs form the largest set of sequence variants in
naturally occurring
.. polymorphic strains of most organisms.
The term "variety" refers to a group of plants within a species defined by the
sharing of a
common set of characteristics or traits accepted by those skilled in the art
as sufficient to
distinguish one cultivar or variety from another cultivar or variety. There is
no implication in
.. either term that all plants of any given cultivar or variety will be
genetically identical at either the
whole gene or molecular level or that any given plant will be homozygous at
all loci. A cultivar or
variety is considered "true breeding" for a particular trait if, when the true-
breeding cultivar or
variety is self-pollinated, all of the progeny contain the trait. The terms
"breeding line" or "line"
refer to a group of plants within a cultivar defined by the sharing of a
common set of
.. characteristics or traits accepted by those skilled in the art as
sufficient to distinguish one
breeding line or line from another breeding line or line. There is no
implication in either term that
all plants of any given breeding line or line will be genetically identical at
either the whole gene
or molecular level or that any given plant will be homozygous at all loci. A
breeding line or line is
considered "true breeding" for a particular trait if, when the true-breeding
line or breeding line is
self-pollinated, all of the progeny contain the trait. In the present
invention, the trait arises from a
mutation in a PPO gene of the plant or seed.
In some embodiments, traditional plant breeding is employed whereby the PPO
inhibitor
herbicides-tolerant, preferably the compounds of formula (I)-tolerant, trait
is introduced in the
.. progeny plant resulting therefrom. In one embodiment, the present invention
provides a method
for producing a PPO inhibitor herbicide-tolerant, preferably a compound of
formula (I)-tolerant,
progeny plant, the method comprising: crossing a parent plant with a PPO
inhibitor herbicide-
tolerant, preferably a compound of formula (I)-tolerant, plant to introduce
the PPO inhibitor
herbicide-tolerance, preferably the compound of formula (I)-tolerance,
characteristics of the
CA 03030354 2019-01-09
WO 2018/019842
PCT/EP2017/068784
53
PPO inhibitor herbicide-tolerant, preferably the compound of formula (I)-
tolerant, plant into the
germplasm of the progeny plant, wherein the progeny plant has increased
tolerance to the PPO
inhibitor herbicide, preferably the compound of formula (I), relative to the
parent plant. In other
embodiments, the method further comprises the step of introgressing the PPO
inhibitor
herbicide-tolerance, preferably the compound of formula (I)-tolerance,
characteristics through
traditional plant breeding techniques to obtain a descendent plant having the
PPO inhibitor
herbicide-tolerance, preferably the compound of formula (I)-tolerance,
characteristics.
The herbicide-resistant plants of the invention that comprise polynucleotides
encoding mutated
PPO polypeptides also find use in methods for increasing the herbicide-
resistance of a plant
through conventional plant breeding involving sexual reproduction. The methods
comprise
crossing a first plant that is a herbicide-resistant plant of the invention to
a second plant that
may or may not be resistant to the same herbicide or herbicides as the first
plant or may be
resistant to different herbicide or herbicides than the first plant. The
second plant can be any
plant that is capable of producing viable progeny plants (i.e., seeds) when
crossed with the first
plant. Typically, but not necessarily, the first and second plants are of the
same species. The
methods can optionally involve selecting for progeny plants that comprise the
mutated PPO
polypeptides of the first plant and the herbicide resistance characteristics
of the second plant.
The progeny plants produced by this method of the present invention have
increased resistance
to a herbicide when compared to either the first or second plant or both. When
the first and
second plants are resistant to different herbicides, the progeny plants will
have the combined
herbicide tolerance characteristics of the first and second plants. The
methods of the invention
can further involve one or more generations of backcrossing the progeny plants
of the first cross
to a plant of the same line or genotype as either the first or second plant.
Alternatively, the
progeny of the first cross or any subsequent cross can be crossed to a third
plant that is of a
different line or genotype than either the first or second plant. The present
invention also
provides plants, plant organs, plant tissues, plant cells, seeds, and non-
human host cells that
are transformed with the at least one polynucleotide molecule, expression
cassette, or
transformation vector of the invention. Such transformed plants, plant organs,
plant tissues,
plant cells, seeds, and non-human host cells have enhanced tolerance or
resistance to at least
one herbicide, at levels of the herbicide that kill or inhibit the growth of
an untransformed plant,
plant tissue, plant cell, or non-human host cell, respectively. Preferably,
the transformed plants,
plant tissues, plant cells, and seeds of the invention are Arabidopsis
thaliana and crop plants.
In other aspects, plants of the invention include those plants which, in
addition to being tolerant
to PPO inhibitor herbicides, preferably the compounds of formula (I), have
been subjected to
further genetic modifications by breeding, mutagenesis or genetic engineering,
e.g. have been
rendered tolerant to applications of specific other classes of herbicides,
such as AHAS
inhibitors; auxinic herbicides; bleaching herbicides such as
hydroxyphenylpyruvate dioxygenase
(HPPD) inhibitors or phytoene desaturase (PDS) inhibitors; EPSPS inhibitors
such as
glyphosate; glutamine synthetase (GS) inhibitors such as glufosinate; lipid
biosynthesis
inhibitors such as acetyl CoA carboxylase (ACCase) inhibitors; or oxynil {i.e.
bromoxynil or
ioxynil) herbicides as a result of conventional methods of breeding or genetic
engineering, Thus,
PPO inhibitor herbicides-tolerant, preferably compounds of formula (I)-
tolerant, plants of the
CA 03030354 2019-01-09
WO 2018/019842
PCT/EP2017/068784
54
invention can be made resistant to multiple classes of herbicides through
multiple genetic
modifications, such as resistance to both glyphosate and glufosinate or to
both glyphosate and
a herbicide from another class such as HPPD inhibitors, AHAS inhibitors, or
ACCase inhibitors.
These herbicide resistance technologies are, for example, described in Pest
Management
Science (at volume, year, page): 61, 2005, 246; 61, 2005, 258; 61, 2005, 277;
61, 2005, 269;
61, 2005, 286; 64, 2008, 326; 64, 2008, 332; Weed Science 57, 2009, 108;
Australian Journal
of Agricultural Research 58, 2007, 708; Science 316, 2007, 1185; and
references quoted
therein. For example, PPO inhibitor herbicides, preferably compounds of
formula (I)-tolerant,
plants of the invention, in some embodiments, may be tolerant to ACCase
inhibitors, such as
"dims" {e.g., cycloxydim, sethoxydim, clethodim, or tepraloxydim), "fops"
{e.g. , clodinafop,
diclofop, fluazifop, haloxyfop, or quizalofop), and "dens" (such as
pinoxaden); to auxinic
herbicides, such as dicamba; to EPSPS inhibitors, such as glyphosate; to other
PPO inhibitors;
and to GS inhibitors, such as glufosinate.
In addition to these classes of inhibitors, PPO inhibitor herbicides-tolerant,
preferably
compounds of formula (I)-tolerant, plants of the invention may also be
tolerant to herbicides
having other modes of action, for example, chlorophyll/carotenoid pigment
inhibitors, cell
membrane disrupters, photosynthesis inhibitors, cell division inhibitors, root
inhibitors, shoot
inhibitors, and combinations thereof.
Such tolerance traits may be expressed, e.g. : as mutant or wildtype PPO
proteins, as mutant
AHASL proteins, mutant ACCase proteins, mutant EPSPS proteins, or mutant
glutamine
synthetase proteins; or as mutant native, inbred, or transgenic
aryloxyalkanoate dioxygenase
(AAD or DHT), haloarylnitrilase (BXN), 2,2-dichloropropionic acid dehalogenase
(DEH),
glyphosate-N- acetyltransferase (GAT), glyphosate decarboxylase (GDC),
glyphosate
oxidoreductase (GOX), glutathione-S-transferase (GST), phosphinothricin
acetyltransferase
(PAT or bar), or CYP450s proteins having an herbicide-degrading activity.
PPO inhibitor herbicides-tolerant, preferably compounds of formula (1)-
tolerant, plants hereof
can also be stacked with other traits including, but not limited to,
pesticidal traits such as Bt Cry
and other proteins having pesticidal activity toward coleopteran,
lepidopteran, nematode, or
other pests; nutrition or nutraceutical traits such as modified oil content or
oil profile traits, high
protein or high amino acid concentration traits, and other trait types known
in the art.
Furthermore, in other embodiments, PPO inhibitor herbicides-tolerant,
preferably compounds of
formula (I)-tolerant, plants are also covered which are, by the use of
recombinant DNA
techniques and/or by breeding and/or otherwise selected for such
characteristics, rendered able
to synthesize one or more insecticidal proteins, especially those known from
the bacterial genus
Bacillus, particularly from Bacillus thuringiensis, such as [delta]-
endotoxins, e.g. CryIA(b),
CryIA(c), CryIF, Cryl F(a2), CryllA(b), CryllIA, CryIIIB(b1) or Cry9c;
vegetative insecticidal proteins
(VIP), e.g. VIP1, VIP2, VIP3 or VIP3A; insecticidal proteins of bacteria
colonizing nematodes,
e.g. Photorhabdus spp. or Xenorhabdus spp.; toxins produced by animals, such
as scorpion
toxins, arachnid toxins, wasp toxins, or other insect-specific neurotoxins;
toxins produced by
fungi, such streptomycete toxins; plant lectins, such as pea or barley
lectins; agglutinins;
CA 03030354 2019-01-09
WO 2018/019842
PCT/EP2017/068784
proteinase inhibitors, such as trypsin inhibitors, serine protease inhibitors,
patatin, cystatin or
papain inhibitors; ribosome-inactivating proteins (RIP), such as ricin, maize-
RIP, abrin, luffin,
saporin or bryodin; steroid metabolism enzymes, such as 3-hydroxy-steroid
oxidase,
ecdysteroid-IDP-glycosyl-transferase, cholesterol oxidases, ecdysone
inhibitors or HMG- CoA-
5 reductase; ion channel blockers, such as blockers of sodium or calcium
channels; juvenile
hormone esterase; diuretic hormone receptors (helicokinin receptors); stilben
synthase,
bibenzyl synthase, chitinases or glucanases. In the context of the present
invention these
insecticidal proteins or toxins are to be understood expressly also as pre-
toxins, hybrid proteins,
truncated or otherwise modified proteins. Hybrid proteins are characterized by
a new
10 combination of protein domains, (see, e.g. WO 02/015701). Further
examples of such toxins or
genetically modified plants capable of synthesizing such toxins are disclosed,
e.g., in EP-A 374
753, WO 93/007278, WO 95/34656, EP-A427 529, EP-A 451 878, WO 03/18810 und WO
03/52073. The methods for producing such genetically modified plants are
generally known to
the person skilled in the art and are described, e.g. in the publications
mentioned above. These
15 .. insecticidal proteins contained in the genetically modified plants
impart to the plants producing
these proteins tolerance to harmful pests from all taxonomic groups of
arthropods, especially to
beetles (Coeloptera), two-winged insects (Diptera), and moths (Lepidoptera)
and to nematodes
(Nematoda).
20 .. In some embodiments, expression of one or more protein toxins (e.g.,
insecticidal proteins) in
the PPO inhibitor herbicides-tolerant, preferably compounds of formula (I)-
tolerant, plants is
effective for controlling organisms that include, for example, members of the
classes and
orders: Coleoptera such as the American bean weevil Acanthoscelides obtectus;
the leaf beetle
Agelastica alni; click beetles (Agriotes lineatus, Agriotes obscurus, Agriotes
bicolor); the grain
25 .. beetle Ahasverus advena; the summer schafer Amphimallon solstitialis;
the furniture beetle
Anobium punctatum; Anthonomus spp. (weevils); the Pygmy mangold beetle
Atomaria linearis;
carpet beetles (Anthrenus spp., Attagenus spp.); the cowpea weevil
Callosobruchus maculates;
the fried fruit beetle Carpophilus hemipterus; the cabbage seedpod weevil
Ceutorhynchus
assimilis; the rape winter stem weevil Ceutorhynchus picitarsis; the wireworms
Conoderus
30 vespertinus and Conoderus falli; the banana weevil Cosmopolites
sordidus; the New Zealand
grass grub Costelytra zealandica; the June beetle Cotinis nitida; the
sunflower stem weevil
Cylindrocopturus adspersus; the larder beetle Dermestes lardarius; the corn
rootworms
Diabrotica virgifera, Diabrotica virgifera virgifera, and Diabrotica barberi;
the Mexican bean
beetle Epilachna varivestis; the old house borer Hylotropes bajulus; the
lucerne weevil Hypera
35 postica; the shiny spider beetle Gibbium psylloides; the cigarette
beetle Lasioderma serricorne;
the Colorado potato beetle Leptinotarsa decemlineata; Lyctus beetles {Lyctus
spp. , the pollen
beetle Meligethes aeneus; the common cockshafer Melolontha melolontha; the
American spider
beetle Mezium americanum; the golden spider beetle Niptus hololeuc s; the
grain beetles
Oryzaephilus surinamensis and Oryzaephilus Mercator; the black vine weevil
Otiorhynchus
40 sulcatus; the mustard beetle Phaedon cochleariae, the crucifer flea
beetle Phyllotreta
cruciferae; the striped flea beetle Phyllotreta striolata; the cabbage steam
flea beetle Psylliodes
chrysocephala; Ptinus spp. (spider beetles); the lesser grain borer
Rhizopertha dominica; the
pea and been weevil Sitona lineatus; the rice and granary beetles Sitophilus
oryzae and
Sitophilus granaries; the red sunflower seed weevil Smicronyx fulvus; the
drugstore beetle
CA 03030354 2019-01-09
WO 2018/019842
PCT/EP2017/068784
56
Stegobium paniceum; the yellow mealworm beetle Tenebrio molitor, the flour
beetles Tribolium
castaneum and Tribolium confusum; warehouse and cabinet beetles {Trogoderma
spp.); the
sunflower beetle Zygogramma exclamationis; Dermaptera (earwigs) such as the
European
earwig Forficula auricularia and the striped earwig Labidura riparia;
Dictyoptera such as the
oriental cockroach Blatta orientalis; the greenhouse millipede Oxidus
gracilis; the beet fly
Pegomyia betae; the frit fly OscineIla frit; fruitflies (Dacus spp.,
Drosophila spp.); lsoptera
(termites) including species from the familes Hodotermitidae, Kalotermitidae,
Mastotermitidae,
Rhinotermitidae, Serritermitidae, Termitidae, Termopsidae; the tarnished plant
bug Lygus
lineolaris; the black bean aphid Aphis fabae; the cotton or melon aphid Aphis
gossypii; the
.. green apple aphid Aphis pomi; the citrus spiny whitefly Aleurocanthus
spiniferus; the sweet
potato whitefly Bemesia tabaci; the cabbage aphid Brevicoryne brassicae; the
pear psylla
Cacopsylla pyricola; the currant aphid Cryptomyzus ribis; the grape phylloxera
Daktulosphaira
vitifoliae; the citrus psylla Diaphorina citri; the potato leafhopper Empoasca
fabae; the bean
leafhopper Empoasca Solana; the vine leafhopper Empoasca vitis; the woolly
aphid Eriosoma
lanigerum; the European fruit scale Eulecanium corni; the mealy plum aphid
Hyalopterus
arundinis; the small brown planthopper Laodelphax striatellus; the potato
aphid Macrosiphum
euphorbiae; the green peach aphid Myzus persicae; the green rice leafhopper
Nephotettix
cinticeps; the brown planthopper Nilaparvata lugens; the hop aphid Phorodon
humuli; the bird-
cherry aphid Rhopalosiphum padi; the grain aphid Sitobion avenae; Lepidoptera
such as
Adoxophyes orana (summer fruit tortrix moth); Archips podana (fruit tree
tortrix moth);
Bucculatrix pyrivorella (pear leafminer); Bucculatrix thurberiella (cotton
leaf perforator); Bupalus
piniarius (pine looper); Carpocapsa pomonella (codling moth); Chilo
suppressalis (striped rice
borer); Choristoneura fumiferana (eastern spruce budworm); Cochylis hospes
(banded
sunflower moth); Diatraea grandiosella (southwestern corn borer); Eupoecilia
ambiguella
(European grape berry moth); Helicoverpa armigera (cotton bollworm);
Helicoverpa zea (cotton
bollworm); Heliothis vires cens (tobacco budworm), Homeosoma electellum
(sunflower moth);
Homona magnanima (oriental tea tree tortrix moth); Lithocolletis blancardella
(spotted tentiform
leafminer); Lymantria dispar (gypsy moth); Malacosoma neustria (tent
caterpillar); Mamestra
brassicae (cabbage armyworm); Mamestra configurata (Bertha armyworm);
Operophtera
brumata (winter moth); Ostrinia nubilalis (European corn borer), Panolis
flammea (pine beauty
moth), Phyllocnistis citrella (citrus leafminer); Pieris brassicae (cabbage
white butterfly);
Rachiplusia ni (soybean looper); Spodoptera exigua (beet armywonn); Spodoptera
littoralis
(cotton leafworm); Sylepta derogata (cotton leaf roller); Trichoplusia ni
(cabbage looper);
Orthoptera such as the common cricket Acheta domesticus, tree locusts
(Anacridium spp.), the
.. migratory locust Locusta migratoria, the twostriped grasshopper Melanoplus
bivittatus, the
differential grasshopper Melanoplus differ entialis, the redlegged grasshopper
Melanoplus
femurrubrum, the migratory grasshopper Melanoplus sanguinipes, the northern
mole cricket
Neocurtilla hexadectyla, the red locust Nomadacris septemfasciata, the
shortwinged mole
cricket Scapteriscus abbreviatus, the southern mole cricket Scapteriscus
borellii, the tawny
mole cricket Scapteriscus vicinus, and the desert locust Schistocerca
gregaria; Symphyla such
as the garden symphylan Scutigerella immaculata; Thysanoptera such as the
tobacco thrips
Frankliniella fusca, the flower thrips Frankliniella intonsa, the western
flower thrips Frankliniella
occidentalism the cotton bud thrips Frankliniella schultzei, the banded
greenhouse thrips
Hercinothrips femoralis, the soybean thrips Neohydatothrips variabilis,
Kelly's citrus thrips
CA 03030354 2019-01-09
WO 2018/019842
PCT/EP2017/068784
57
Pezothrips kellyanus, the avocado thrips Scirtothrips perseae, the melon
thrips Thrips palmi,
and the onion thrips Thrips tabaci; and the like, and combinations comprising
one or more of the
foregoing organisms.
In some embodiments, expression of one or more protein toxins (e.g.,
insecticidal proteins) in
the PPO inhibitor herbicides-tolerant, preferably compounds of formula (I)-
tolerant, plants is
effective for controlling flea beetles, i.e. members of the flea beetle tribe
of family
Chrysomelidae, preferably against Phyllotreta spp., such as Phyllotreta
cruciferae and/or
Phyllotreta triolata. In other embodiments, expression of one or more protein
toxins {e.g.,
insecticidal proteins) in the PPO inhibitor herbicides-tolerant, preferably
compounds of
formula (I)-tolerant, plants is effective for controlling cabbage seedpod
weevil, the Bertha
armyworm, Lygus bugs, or the diamondback moth.
Furthermore, in one embodiment, PPO inhibitor herbicides-tolerant, preferably
compounds of
formula (I)-tolerant, plants are also covered which are, e.g. by the use of
recombinant DNA
techniques and/or by breeding and/or otherwise selected for such traits,
rendered able to
synthesize one or more proteins to increase the resistance or tolerance of
those plants to
bacterial, viral or fungal pathogens. The methods for producing such
genetically modified plants
are generally known to the person skilled in the art.
Furthermore, in another embodiment, PPO inhibitor herbicides-tolerant,
preferably compounds
of formula (I)-tolerant, plants are also covered which are, e.g. by the use of
recombinant DNA
techniques and/or by breeding and/or otherwise selected for such traits,
rendered able to
synthesize one or more proteins to increase the productivity (e.g. oil
content), tolerance to
drought, salinity or other growth- limiting environmental factors or tolerance
to pests and fungal,
bacterial or viral pathogens of those plants.
Furthermore, in other embodiments, PPO inhibitor herbicides-tolerant,
preferably compounds of
formula (I)-tolerant, plants are also covered which are, e.g. by the use of
recombinant DNA
techniques and/or by breeding and/or otherwise selected for such traits,
altered to contain a
modified amount of one or more substances or new substances, for example, to
improve human
or animal nutrition, e.g. oil crops that produce health-promoting long-chain
omega-3 fatty acids
or unsaturated omega-9 fatty acids (e.g. Nexera(R) rape, Dow Agro Sciences,
Canada).
Furthermore, in some embodiments, PPO inhibitor herbicides-tolerant,
preferably compounds of
formula (I)-tolerant, plants are also covered which are, e.g. by the use of
recombinant DNA
techniques and/or by breeding and/or otherwise selected for such traits,
altered to contain
increased amounts of vitamins and/or minerals, and/or improved profiles of
nutraceutical
compounds.
In one embodiment, PPO inhibitor herbicides-tolerant, preferably compounds of
formula (1)-
tolerant, plants of the present invention, relative to a wild-type plant,
comprise an increased
amount of, or an improved profile of, a compound selected from the group
consisting of:
glucosinolates (e.g., glucoraphanin (4-methylsulfinylbutyl-glucosinolate),
sulforaphane, 3-
indolylmethyl-glucosinolate(glucobrassicin),1-methoxy-3-indolylmethyl-
glucosinolate
(neoglucobrassicin)); phenolics (e.g., flavonoids (e.g., quercetin,
kaempferol), hydroxycinnamoyl
CA 03030354 2019-01-09
WO 2018/019842
PCT/EP2017/068784
58
derivatives (e.g., 1 ,2,2'- trisinapoylgentiobiose, 1 ,2-
diferuloylgentiobiose, I ,2'-disinapoy1-2-
feruloylgentiobiose, 3-0- caffeoyl-quinic (neochlorogenic acid)); and vitamins
and minerals (e.g.,
vitamin C, vitamin E, carotene, folic acid, niacin, riboflavin, thiamine,
calcium, iron, magnesium,
potassium, selenium, and zinc).
In another embodiment, PPO inhibitor herbicides-tolerant, preferably compounds
of formula (1)-
tolerant, plants of the present invention, relative to a wild-type plant,
comprise an increased
amount of, or an improved profile of, a compound selected from the group
consisting of:
progoitrin; isothiocyanates; indoles (products of glucosinolate hydrolysis);
glutathione;
carotenoids such as beta-carotene, lycopene, and the xanthophyll carotenoids
such as lutein
and zeaxanthin; phenolics comprising the flavonoids such as the flavonols
(e.g. quercetin,
rutin), the flavans/tannins (such as the procyanidins comprising coumarin,
proanthocyanidins,
catechins, and anthocyanins); flavones; phytoestrogens such as coumestans,
lignans,
resveratrol, isoflavones e.g. genistein, daidzein, and glycitein; resorcyclic
acid lactones;
organosulphur compounds; phytosterols; terpenoids such as carnosol, rosmarinic
acid,
glycyrrhizin and saponins; chlorophyll; chlorphyllin, sugars, anthocyanins,
and vanilla. In other
embodiments, PPO inhibitor herbicides-tolerant, preferably compounds of
formula (I)-tolerant,
plants of the present invention, relative to a wild-type plant, comprise an
increased amount of,
or an improved profile of, a compound selected from the group consisting of:
vincristine,
vinblastine, taxanes (e.g., taxol (paclitaxel), baccatin III, 10-
desacetylbaccatin III, 10-desacetyl
taxol, xylosyl taxol, 7- epitaxol, 7-epibaccatin III, 10-
desacetylcephalomannine, 7-
epicephalomannine, taxotere, cephalomannine, xylosyl cephalomannine,
taxagifine, 8-
benxoyloxy taxagifine, 9-acetyloxy taxusin, 9-hydroxy taxusin, taiwanxam,
taxane la, taxane lb,
taxane lc, taxane Id, GMP paclitaxel, 9-dihydro 13-acetylbaccatin III, 10-
desacety1-7-epitaxol,
tetrahydrocannabinol (THC), cannabidiol (CBD), genistein, diadzein, codeine,
morphine,
quinine, shikonin, ajmalacine, serpentine, and the like.
It is to be understood that the plant of the present invention can comprise a
wild type PPO
nucleic acid in addition to a mutated PPO nucleic acid. It is contemplated
that the PPO inhibitor
herbicides-tolerant, preferably compounds of formula (I)-tolerant, lines may
contain a mutation
in only one of multiple PPO isoenzymes. Therefore, the present invention
includes a plant
comprising one or more mutated PPO nucleic acids in addition to one or more
wild type PPO
nucleic acids.
Examples of PPO inhibitor herbicide resistant weed species are Asian
copperleaf (Acalypha
australis), smooth pigweed (Amaranthus hybridus), Palmer amaranth (Amaranthus
Palmeri),
red root pigweed (Amaranthus retroflexus), tall/common waterhemp (Amaranthus
tuberculatus
or Amaranthus rudis), common ragweed (Ambrosia artemisiifolia), wild oat
(Avena fatua),
fleabane (Conyza ambigua), marestail (Conyza Canadensis), flixweed
(Descurainia Sophia),
wild poinsettia (Euphorbia heterophylla) and eastern groundsel (Senecio
vemalis).
Preferred is the method according to the invention, wherein the PPO resistant
weeds to be
controlled are selected from the group consisting of Asian copperleaf, smooth
pigweed, Palmer
CA 03030354 2019-01-09
WO 2018/019842
PCT/EP2017/068784
59
amaranth, redroot pigweed, tall/common waterhemp, common ragweed, wild oat,
fleabane,
marestail, flixweed, wild poinsettia and Eastern groundsel;
preferably are selected from Asian copperleaf, smooth pigweed, Palmer
amaranth, redroot
pigweed, tall/common waterhemp, common ragweed, wild oat, flixweed, wild
poinsettia and
Eastern groundsel;
particularly preferably are selected from the group consisting of waterhemp,
Palmer amaranth
and common ragweed.
In a particularly preferred embodiment of the invention, the PPO resistant
weed to be controlled
is Asian copperleaf.
In another particularly preferred embodiment of the invention, the PPO
resistant weed to be
controlled is smooth pigweed.
In another particularly preferred embodiment of the invention, the PPO
resistant weed to be
controlled is Palmer amaranth.
In another particularly preferred embodiment of the invention, the PPO
resistant weed to be
controlled is redroot pigweed.
In another particularly preferred embodiment of the invention, the PPO
resistant weed to be
controlled is tall/common waterhemp.
In another particularly preferred embodiment of the invention, the PPO
resistant weed to be
controlled is common ragweed.
In another particularly preferred embodiment of the invention, the PPO
resistant weed to be
controlled is wild oat.
In a particularly preferred embodiment of the invention, the PPO resistant
weed to be controlled
is fleabane.
In a particularly preferred embodiment of the invention, the PPO resistant
weed to be controlled
is marestail.
In another particularly preferred embodiment of the invention, the PPO
resistant weed to be
controlled is flixweed.
In another particularly preferred embodiment of the invention, the PPO
resistant weed to be
controlled is wild poinsettia.
In another particularly preferred embodiment of the invention, the PPO
resistant weed to be
controlled is Eastern groundsel.
CA 03030354 2019-01-09
WO 2018/019842
PCT/EP2017/068784
In another preferred embodiment of the invention the method according to the
present invention
comprises the application of a herbicidal composition comprising at least one,
preferably exactly
one compound (I) and at least one further active compound selected from
herbicides B,
preferably herbicides B of class b1) to b15), and safeners C (compound C) to
PPO resistant
5 weeds, such as Asian copperleaf, smooth pigweed, Palmer amaranth, redroot
pigweed,
tall/common waterhemp, common ragweed, wild oat, fleabane, marestail,
flixweed, wild
poinsettia and eastern groundsel.
In another preferred embodiment of the invention the method according to the
present invention
10 comprises the application of a herbicidal composition comprising at
least one, preferably exactly
one compound (I) and at least one further active compound selected from
herbicides B,
preferably herbicides B of class b1) to b15), and safeners C (compound C) to
PPO resistant
weeds, such as Asian copperleaf, smooth pigweed, Palmer amaranth, redroot
pigweed,
tall/common waterhemp, common ragweed, wild oat, flixweed, wild poinsettia and
eastern
15 groundsel.
In another preferred embodiment of the invention, the method according to the
present
invention comprises the application of a herbicidal composition comprising at
least one,
preferably exactly one compound (I) and at least one further active compound
selected from
20 herbicides B, preferably herbicides B of class b1) to b15), and safeners
C (compound C) to
PPO resistant weeds selected from common waterhemp, Palmer amaranth and common
ragweed.
In a particularly preferred embodiment of the invention, the method comprises
the application of
25 a herbicidal composition comprising at least one, preferably exactly one
compound of formula
(I) and at least one further active compound selected from herbicides B,
preferably herbicides B
of class b1) to b15), and safeners C (compound C) to control Asian copperleaf.
In another particularly preferred embodiment of the invention, the method
comprises the
30 application of a herbicidal composition comprising at least one,
preferably exactly one
compound of formula (I) and at least one further active compound selected from
herbicides B,
preferably herbicides B of class b1) to b15), and safeners C (compound C) to
control smooth
pigweed.
35 In another particularly preferred embodiment of the invention, the
method comprises the
application of a herbicidal composition comprising at least one, preferably
exactly one
compound of formula (I) and at least one further active compound selected from
herbicides B,
preferably herbicides B of class b1) to b15), and safeners C (compound C) to
control Palmer
amaranth.
In another particularly preferred embodiment of the invention, the method
comprises the
application of a herbicidal composition comprising at least one, preferably
exactly one
compound of formula (I) and at least one further active compound selected from
herbicides B,
preferably herbicides B of class b1) to b15), and safeners C (compound C) to
control redroot
CA 03030354 2019-01-09
WO 2018/019842
PCT/EP2017/068784
61
pigweed.
In another particularly preferred embodiment of the invention, the method
comprises the
application of a herbicidal composition comprising at least one, preferably
exactly one
.. compound of formula (I) and at least one further active compound selected
from herbicides B,
preferably herbicides B of class b1) to b15), and safeners C (compound C) to
control
tall/common waterhemp.
In another particularly preferred embodiment of the invention, the method
comprises the
.. application of a herbicidal composition comprising at least one, preferably
exactly one
compound of formula (I) and at least one further active compound selected from
herbicides B,
preferably herbicides B of class b1) to b15), and safeners C (compound C) to
control common
ragweed.
In another particularly preferred embodiment of the invention, the method
comprises the
application of a herbicidal composition comprising at least one, preferably
exactly one
compound of formula (I) and at least one further active compound selected from
herbicides B,
preferably herbicides B of class b1) to b15), and safeners C (compound C) to
control wild oat.
In another particularly preferred embodiment of the invention, the method
comprises the
application of a herbicidal composition comprising at least one, preferably
exactly one
compound of formula (I) and at least one further active compound selected from
herbicides B,
preferably herbicides B of class b1) to b15), and safeners C (compound C) to
control fleabane.
In another particularly preferred embodiment of the invention, the method
comprises the
application of a herbicidal composition comprising at least one, preferably
exactly one
compound of formula (I) and at least one further active compound selected from
herbicides B,
preferably herbicides B of class b1) to b15), and safeners C (compound C) to
control marestail.
In another particularly preferred embodiment of the invention, the method
comprises the
application of a herbicidal composition comprising at least one, preferably
exactly one
compound of formula (I) and at least one further active compound selected from
herbicides B,
preferably herbicides B of class b1) to b15), and safeners C (compound C) to
control flixweed.
In another particularly preferred embodiment of the invention, the method
comprises the
application of a herbicidal composition comprising at least one, preferably
exactly one
compound of formula (I) and at least one further active compound selected from
herbicides B,
preferably herbicides B of class b1) to b15), and safeners C (compound C) to
control wild
poinsettia.
In another particularly preferred embodiment of the invention, the method
comprises the
application of a herbicidal composition comprising at least one, preferably
exactly one
compound of formula (I) and at least one further active compound selected from
herbicides B,
preferably herbicides B of class b1) to b15), and safeners C (compound C) to
control Eastern
CA 03030354 2019-01-09
WO 2018/019842
PCT/EP2017/068784
62
groundsel.
Particularly preferred are the methods 1.1 to 1.87, especially 1.1 to 1.82,
wherein the
substance(s) as defined in the respective row of table 1 is/are applied to
Asian copperleaf:
Table 1 (methods 1.1 to 1.87)
meth. cpd herbi- meth. cpd herbi- meth. cpd herbi-
no (1) cide B no (1) cide B no (1)
cide B
1.1 (1).1 -- 1.30 (1).1 B.29 1.59
(1).1 B.58
1.2 (1).1 B.1 1.31 (1).1 B.30 1.60
(1).1 B.59
1.3 (1).1 B.2 1.32 (1).1 B.31 1.61
(1).1 B.60
1.4 (1).1 B.3 1.33 (1).1 B.32 1.62
(1).1 B.61
1.5 (1).1 B.4 1.34 (1).1 B.33 1.63
(1).1 B.62
1.6 (1).1 B.5 1.35 (1).1 B.34 1.64
(1).1 B.63
1.7 (1).1 B.6 1.36 (1).1 B.35 1.65
(1).1 B.64
1.8 (1).1 B.7 1.37 (1).1 B.36 1.66
(1).1 B.65
1.9 (1).1 B.8 1.38 (1).1 B.37 1.67
(1).1 B.66
1.10 (1).1 B.9 1.39 (1).1 B.38 1.68
(1).1 B.67
1.11 (1).1 B.10 1.40 (1).1 B.39 1.69
(1).1 B.68
1.12 (1).1 B.11 1.41 (1).1 B.40 1.70
(1).1 B.69
1.13 (1).1 B.12 1.42 (1).1 B.41 1.71
(1).1 B.70
1.14 (1).1 B.13 1.43 (1).1 B.42 1.72
(1).1 B.71
1.15 (1).1 B.14 1.44 (1).1 B.43 1.73
(1).1 B.72
1.16 (1).1 B.15 1.45 (1).1 B.44 1.74
(1).1 B.73
1.17 (1).1 B.16 1.46 (1).1 B.45 1.75
(1).1 B.74
1.18 (1).1 B.17 1.47 (1).1 B.46 1.76
(1).1 B.75
1.19 (1).1 B.18 1.48 (1).1 B.47 1.77
(1).1 B.76
1.20 (1).1 B.19 1.49 (1).1 B.48 1.78
(1).1 B.77
1.21 (1).1 B.20 1.50 (1).1 B.49 1.79
(1).1 B.78
1.22 (1).1 B.21 1.51 (1).1 B.50 1.80
(1).1 B.79
1.23 (1).1 B.22 1.52 (1).1 B.51 1.81
(1).1 B.80
1.24 (1).1 B.23 1.53 (1).1 B.52 1.82
(1).1 B.81
1.25 (1).1 B.24 1.54 (1).1 B.53 1.83
(1).1 B.82
1.26 (1).1 B.25 1.55 (1).1 B.54 1.84
(1).1 B.83
1.27 (1).1 B.26 1.56 (1).1 B.55 1.85
(1).1 B.84
1.28 (1).1 B.27 1.57 (1).1 B.56 1.86
(1).1 B.85
1.29 (1).1 B.28 1.58 (1).1 B.57 1.87
(1).1 B.86
The specific number for each single method is deductible as follows:
CA 03030354 2019-01-09
WO 2018/019842
PCT/EP2017/068784
63
Method 1.20 for example comprises the application of the compound (1).1 and
foramsulfuron
(B.20) (see above as well as table B, entry B.20) to Asian copperleaf.
Method 2.20 for example comprises the application of the compound (1).1 and
foramsulfuron
(B.20) (see above as well as table B, entry B.20) to smooth pigweed.
Also especially preferred are the methods 2.1. to 2.87 which differ from the
corresponding
methods 1.1 to 1.87 only in that the substance(s) as defined in the respective
row of table 1
is/are applied to smooth pigweed.
Also especially preferred are the methods 3.1. to 3.87 which differ from the
corresponding
methods 1.1 to 1.87 only in that the substance(s) as defined in the respective
row of table 1
is/are applied to Palmer amaranth.
Also especially preferred are the methods 4.1. to 4.87 which differ from the
corresponding
methods 1.1 to 1.87 only in that the substance(s) as defined in the respective
row of table 1
is/are applied to redroot pigweed.
Also especially preferred are the methods 5.1. to 5.87 which differ from the
corresponding
methods 1.1 to 1.87 only in that the substance(s) as defined in the respective
row of table 1
is/are applied to tall/common waterhemp.
Also especially preferred are the methods 6.1. to 6.87 which differ from the
corresponding
methods 1.1 to 1.87 only in that the substance(s) as defined in the respective
row of table 1
is/are applied to common ragweed.
Also especially preferred are the methods 7.1. to 7.87 which differ from the
corresponding
methods 1.1 to 1.87 only in that the substance(s) as defined in the respective
row of table 1
is/are applied to wild oat.
Also especially preferred are the methods 8.1. to 8.87 which differ from the
corresponding
methods 1.1 to 1.87 only in that the substance(s) as defined in the respective
row of table 1
is/are applied to fleabane.
Also especially preferred are the methods 9.1. to 9.87 which differ from the
corresponding
methods 1.1 to 1.87 only in that the substance(s) as defined in the respective
row of table 1
is/are applied to marestail.
Also especially preferred are the methods 10.1. to 10.87 which differ from the
corresponding
methods 1.1 to 1.87 only in that the substance(s) as defined in the respective
row of table 1
is/are applied to flixweed.
CA 03030354 2019-01-09
WO 2018/019842
PCT/EP2017/068784
64
Also especially preferred are the methods 11.1. to 11.87 which differ from the
corresponding
methods 1.1 to 1.87 only in that the substance(s) as defined in the respective
row of table 1
is/are applied to wild poinsettia.
Also especially preferred are the methods 12.1. to 12.87 which differ from the
corresponding
methods 1.1 to 1.87 only in that the substance(s) as defined in the respective
row of table 1
is/are applied to eastern groundsel.
Also especially preferred are the methods 13.1. to 13.87 which differ from the
corresponding
methods 1.1 to 1.87 only in that within the substance(s) as defined in the
respective row of table
1, compound (1).1 is replaced by compound (1).2.
Also especially preferred are the methods 14.1. to 14.87 which differ from the
corresponding
methods 1.1 to 1.87 only in that within the substance(s) as defined in the
respective row of table
1, compound (1).1 is replaced by compound (1).2, and such defined substance(s)
is/are applied
to smooth pigweed.
Also especially preferred are the methods 15.1. to 15.87 which differ from the
corresponding
methods 1.1 to 1.87 only in that within the substance(s) as defined in the
respective row of table
1, compound (1).1 is replaced by compound (1).2, and such defined substance(s)
is/are applied
to Palmer amaranth.
Also especially preferred are the methods 16.1. to 16.87 which differ from the
corresponding
methods 1.1 to 1.87 only in that within the substance(s) as defined in the
respective row of table
1, compound (1).1 is replaced by compound (1).2, and such defined substance(s)
is/are applied
to redroot pigweed.
Also especially preferred are the methods 17.1. to 17.87 which differ from the
corresponding
methods 1.1 to 1.87 only in that within the substance(s) as defined in the
respective row of table
1, compound (1).1 is replaced by compound (1).2, and such defined substance(s)
is/are applied
to tall/common waterhemp.
Also especially preferred are the methods 18.1. to 18.87 which differ from the
corresponding
methods 1.1 to 1.87 only in that within the substance(s) as defined in the
respective row of table
1, compound (1).1 is replaced by compound (1).2, and such defined substance(s)
is/are applied
to common ragweed.
Also especially preferred are the methods 19.1. to 19.87 which differ from the
corresponding
methods 1.1 to 1.87 only in that within the substance(s) as defined in the
respective row of table
1, compound (1).1 is replaced by compound (1).2, and such defined substance(s)
is/are applied
to wild oat.
CA 03030354 2019-01-09
WO 2018/019842
PCT/EP2017/068784
Also especially preferred are the methods 20.1. to 20.87 which differ from the
corresponding
methods 1.1 to 1.87 only in that within the substance(s) as defined in the
respective row of table
1, compound (1).1 is replaced by compound (1).2, and such defined substance(s)
is/are applied
to fleabane.
5
Also especially preferred are the methods 21.1. to 21.87 which differ from the
corresponding
methods 1.1 to 1.87 only in that within the substance(s) as defined in the
respective row of table
1, compound (1).1 is replaced by compound (1).2, and such defined substance(s)
is/are applied
to marestail.
Also especially preferred are the methods 22.1. to 22.87 which differ from the
corresponding
methods 1.1 to 1.87 only in that within the substance(s) as defined in the
respective row of table
1, compound (1).1 is replaced by compound (1).2, and such defined substance(s)
is/are applied
to flixweed.
Also especially preferred are the methods 23.1. to 23.87 which differ from the
corresponding
methods 1.1 to 1.87 only in that within the substance(s) as defined in the
respective row of table
1, compound (1).1 is replaced by compound (1).2, and such defined substance(s)
is/are applied
to wild poinsettia.
Also especially preferred are the methods 24.1. to 24.87 which differ from the
corresponding
methods 1.1 to 1.87 only in that within the substance(s) as defined in the
respective row of table
1, compound (1).1 is replaced by compound (1).2, and such defined substance(s)
is/are applied
to eastern groundsel.
Also especially preferred are the methods 25.1. to 25.87 which differ from the
corresponding
methods 1.1 to 1.87 only in that within the substance(s) as defined in the
respective row of table
1, compound (1).1 is replaced by compound (1).3.
Also especially preferred are the methods 26.1. to 26.87 which differ from the
corresponding
methods 1.1 to 1.87 only in that within the substance(s) as defined in the
respective row of table
1, compound (1).1 is replaced by compound (1).3, and such defined substance(s)
is/are applied
to smooth pigweed.
Also especially preferred are the methods 27.1. to 27.87 which differ from the
corresponding
methods 1.1 to 1.87 only in that within the substance(s) as defined in the
respective row of table
1, compound (1).1 is replaced by compound (1).3, and such defined substance(s)
is/are applied
to Palmer amaranth.
Also especially preferred are the methods 28.1. to 28.87 which differ from the
corresponding
methods 1.1 to 1.87 only in that within the substance(s) as defined in the
respective row of table
1, compound (1).1 is replaced by compound (1).3, and such defined substance(s)
is/are applied
to redroot pigweed.
CA 03030354 2019-01-09
WO 2018/019842
PCT/EP2017/068784
66
Also especially preferred are the methods 29.1. to 29.87 which differ from the
corresponding
methods 1.1 to 1.87 only in that within the substance(s) as defined in the
respective row of table
1, compound (1).1 is replaced by compound (1).3, and such defined substance(s)
is/are applied
to tall/common waterhemp.
Also especially preferred are the methods 30.1. to 30.87 which differ from the
corresponding
methods 1.1 to 1.87 only in that within the substance(s) as defined in the
respective row of table
1, compound (1).1 is replaced by compound (1).3, and such defined substance(s)
is/are applied
to common ragweed.
Also especially preferred are the methods 31.1. to 31.87 which differ from the
corresponding
methods 1.1 to 1.87 only in that within the substance(s) as defined in the
respective row of table
1, compound (1).1 is replaced by compound (1).3, and such defined substance(s)
is/are applied
to wild oat.
Also especially preferred are the methods 32.1. to 32.87 which differ from the
corresponding
methods 1.1 to 1.87 only in that within the substance(s) as defined in the
respective row of table
1, compound (1).1 is replaced by compound (1).3, and such defined substance(s)
is/are applied
to fleabane.
Also especially preferred are the methods 33.1. to 33.87 which differ from the
corresponding
methods 1.1 to 1.87 only in that within the substance(s) as defined in the
respective row of table
1, compound (1).1 is replaced by compound (1).3, and such defined substance(s)
is/are applied
to marestail.
Also especially preferred are the methods 34.1. to 34.87 which differ from the
corresponding
methods 1.1 to 1.87 only in that within the substance(s) as defined in the
respective row of table
1, compound (1).1 is replaced by compound (1).3, and such defined substance(s)
is/are applied
to flixweed.
Also especially preferred are the methods 35.1. to 35.87 which differ from the
corresponding
methods 1.1 to 1.87 only in that within the substance(s) as defined in the
respective row of table
1, compound (1).1 is replaced by compound (1).3, and such defined substance(s)
is/are applied
to wild poinsettia.
Also especially preferred are the methods 36.1. to 36.87 which differ from the
corresponding
methods 1.1 to 1.87 only in that within the substance(s) as defined in the
respective row of table
1, compound (1).1 is replaced by compound (1).3, and such defined substance(s)
is/are applied
to eastern groundsel.
CA 03030354 2019-01-09
WO 2018/019842
PCT/EP2017/068784
67
Also especially preferred are the methods 37.1. to 37.87 which differ from the
corresponding
methods 1.1 to 1.87 only in that within the substance(s) as defined in the
respective row of table
1, compound (1).1 is replaced by compound (1).4.
Also especially preferred are the methods 38.1. to 38.87 which differ from the
corresponding
methods 1.1 to 1.87 only in that within the substance(s) as defined in the
respective row of table
1, compound (1).1 is replaced by compound (1).4, and such defined substance(s)
is/are applied
to smooth pigweed.
Also especially preferred are the methods 39.1. to 39.87 which differ from the
corresponding
methods 1.1 to 1.87 only in that within the substance(s) as defined in the
respective row of table
1, compound (1).1 is replaced by compound (1).4, and such defined substance(s)
is/are applied
to Palmer amaranth.
Also especially preferred are the methods 40.1. to 40.87 which differ from the
corresponding
methods 1.1 to 1.87 only in that within the substance(s) as defined in the
respective row of table
1, compound (1).1 is replaced by compound (1).4, and such defined substance(s)
is/are applied
to redroot pigweed.
Also especially preferred are the methods 41.1. to 41.87 which differ from the
corresponding
methods 1.1 to 1.87 only in that within the substance(s) as defined in the
respective row of table
1, compound (1).1 is replaced by compound (1).4, and such defined substance(s)
is/are applied
to tall/common waterhemp.
Also especially preferred are the methods 42.1. to 42.87 which differ from the
corresponding
methods 1.1 to 1.87 only in that within the substance(s) as defined in the
respective row of table
1, compound (1).1 is replaced by compound (1).4, and such defined substance(s)
is/are applied
to common ragweed.
Also especially preferred are the methods 43.1. to 43.87 which differ from the
corresponding
methods 1.1 to 1.87 only in that within the substance(s) as defined in the
respective row of table
1, compound (1).1 is replaced by compound (1).4, and such defined substance(s)
is/are applied
to wild oat.
Also especially preferred are the methods 44.1. to 44.87 which differ from the
corresponding
methods 1.1 to 1.87 only in that within the substance(s) as defined in the
respective row of table
1, compound (1).1 is replaced by compound (1).4, and such defined substance(s)
is/are applied
to fleabane.
Also especially preferred are the methods 45.1. to 45.87 which differ from the
corresponding
methods 1.1 to 1.87 only in that within the substance(s) as defined in the
respective row of table
1, compound (1).1 is replaced by compound (1).4, and such defined substance(s)
is/are applied
to marestail.
CA 03030354 2019-01-09
WO 2018/019842
PCT/EP2017/068784
68
Also especially preferred are the methods 46.1. to 46.87 which differ from the
corresponding
methods 1.1 to 1.87 only in that within the substance(s) as defined in the
respective row of table
1, compound (1).1 is replaced by compound (1).4, and such defined substance(s)
is/are applied
to flixweed.
Also especially preferred are the methods 47.1. to 47.87 which differ from the
corresponding
methods 1.1 to 1.87 only in that within the substance(s) as defined in the
respective row of table
1, compound (1).1 is replaced by compound (1).4, and such defined substance(s)
is/are applied
to wild poinsettia.
Also especially preferred are the methods 48.1. to 48.87 which differ from the
corresponding
methods 1.1 to 1.87 only in that within the substance(s) as defined in the
respective row of table
1, compound (1).1 is replaced by compound (1).4, and such defined substance(s)
is/are applied
to eastern groundsel.
Also especially preferred are the methods 49.1. to 49.87 which differ from the
corresponding
methods 1.1 to 1.87 only in that within the substance(s) as defined in the
respective row of table
1, compound (1).1 is replaced by compound (1).5.
Also especially preferred are the methods 50.1. to 50.87 which differ from the
corresponding
methods 1.1 to 1.87 only in that within the substance(s) as defined in the
respective row of table
1, compound (1).1 is replaced by compound (1).5, and such defined substance(s)
is/are applied
to smooth pigweed.
Also especially preferred are the methods 51.1. to 51.87 which differ from the
corresponding
methods 1.1 to 1.87 only in that within the substance(s) as defined in the
respective row of table
1, compound (1).1 is replaced by compound (1).5, and such defined substance(s)
is/are applied
to Palmer amaranth.
Also especially preferred are the methods 52.1. to 52.87 which differ from the
corresponding
methods 1.1 to 1.87 only in that within the substance(s) as defined in the
respective row of table
1, compound (1).1 is replaced by compound (1).5, and such defined substance(s)
is/are applied
to redroot pigweed.
Also especially preferred are the methods 53.1. to 53.87 which differ from the
corresponding
methods 1.1 to 1.87 only in that within the substance(s) as defined in the
respective row of table
1, compound (1).1 is replaced by compound (1).5, and such defined substance(s)
is/are applied
to tall/common waterhemp.
Also especially preferred are the methods 54.1. to 54.87 which differ from the
corresponding
methods 1.1 to 1.87 only in that within the substance(s) as defined in the
respective row of table
1, compound (1).1 is replaced by compound (1).5, and such defined substance(s)
is/are applied
to common ragweed.
CA 03030354 2019-01-09
WO 2018/019842
PCT/EP2017/068784
69
Also especially preferred are the methods 55.1. to 55.87 which differ from the
corresponding
methods 1.1 to 1.87 only in that within the substance(s) as defined in the
respective row of table
1, compound (1).1 is replaced by compound (1).5, and such defined substance(s)
is/are applied
to wild oat.
Also especially preferred are the methods 56.1. to 56.87 which differ from the
corresponding
methods 1.1 to 1.87 only in that within the substance(s) as defined in the
respective row of table
1, compound (1).1 is replaced by compound (1).5, and such defined substance(s)
is/are applied
to fleabane.
Also especially preferred are the methods 57.1. to 57.87 which differ from the
corresponding
methods 1.1 to 1.87 only in that within the substance(s) as defined in the
respective row of table
1, compound (1).1 is replaced by compound (1).5, and such defined substance(s)
is/are applied
to marestail.
Also especially preferred are the methods 58.1. to 58.87 which differ from the
corresponding
methods 1.1 to 1.87 only in that within the substance(s) as defined in the
respective row of table
1, compound (1).1 is replaced by compound (1).5, and such defined substance(s)
is/are applied
to flixweed.
Also especially preferred are the methods 59.1. to 59.87 which differ from the
corresponding
methods 1.1 to 1.87 only in that within the substance(s) as defined in the
respective row of table
1, compound (1).1 is replaced by compound (1).5, and such defined substance(s)
is/are applied
to wild poinsettia.
Also especially preferred are the methods 60.1. to 60.87 which differ from the
corresponding
methods 1.1 to 1.87 only in that within the substance(s) as defined in the
respective row of table
1, compound (1).1 is replaced by compound (1).5, and such defined substance(s)
is/are applied
to eastern groundsel.
Also especially preferred are the methods 61.1. to 61.87 which differ from the
corresponding
methods 1.1 to 1.87 only in that within the substance(s) as defined in the
respective row of table
.. 1, compound (1).1 is replaced by compound (1).6.
Also especially preferred are the methods 62.1. to 62.87 which differ from the
corresponding
methods 1.1 to 1.87 only in that within the substance(s) as defined in the
respective row of table
1, compound (1).1 is replaced by compound (1).6, and such defined substance(s)
is/are applied
to smooth pigweed.
Also especially preferred are the methods 63.1. to 63.87 which differ from the
corresponding
methods 1.1 to 1.87 only in that within the substance(s) as defined in the
respective row of table
CA 03030354 2019-01-09
WO 2018/019842
PCT/EP2017/068784
1, compound (1).1 is replaced by compound (1).6, and such defined substance(s)
is/are applied
to Palmer amaranth.
Also especially preferred are the methods 64.1. to 64.87 which differ from the
corresponding
5 methods 1.1 to 1.87 only in that within the substance(s) as defined in
the respective row of table
1, compound (1).1 is replaced by compound (1).6, and such defined substance(s)
is/are applied
to redroot pigweed.
Also especially preferred are the methods 65.1. to 65.87 which differ from the
corresponding
10 methods 1.1 to 1.87 only in that within the substance(s) as defined in
the respective row of table
1, compound (1).1 is replaced by compound (1).6, and such defined substance(s)
is/are applied
to tall/common waterhemp.
Also especially preferred are the methods 66.1. to 66.87 which differ from the
corresponding
15 methods 1.1 to 1.87 only in that within the substance(s) as defined in
the respective row of table
1, compound (1).1 is replaced by compound (1).6, and such defined substance(s)
is/are applied
to common ragweed.
Also especially preferred are the methods 67.1. to 67.87 which differ from the
corresponding
20 methods 1.1 to 1.87 only in that within the substance(s) as defined in
the respective row of table
1, compound (1).1 is replaced by compound (1).6, and such defined substance(s)
is/are applied
to wild oat.
Also especially preferred are the methods 68.1. to 68.87 which differ from the
corresponding
25 methods 1.1 to 1.87 only in that within the substance(s) as defined in
the respective row of table
1, compound (1).1 is replaced by compound (1).6, and such defined substance(s)
is/are applied
to fleabane.
Also especially preferred are the methods 69.1. to 69.87 which differ from the
corresponding
30 methods 1.1 to 1.87 only in that within the substance(s) as defined in
the respective row of table
1, compound (1).1 is replaced by compound (1).6, and such defined substance(s)
is/are applied
to marestail.
Also especially preferred are the methods 70.1. to 70.87 which differ from the
corresponding
35 methods 1.1 to 1.87 only in that within the substance(s) as defined in
the respective row of table
1, compound (1).1 is replaced by compound (1).6, and such defined substance(s)
is/are applied
to flixweed.
Also especially preferred are the methods 71.1. to 71.87 which differ from the
corresponding
40 methods 1.1 to 1.87 only in that within the substance(s) as defined in
the respective row of table
1, compound (1).1 is replaced by compound (1).6, and such defined substance(s)
is/are applied
to wild poinsettia.
CA 03030354 2019-01-09
WO 2018/019842
PCT/EP2017/068784
71
Also especially preferred are the methods 72.1. to 72.87 which differ from the
corresponding
methods 1.1 to 1.87 only in that within the substance(s) as defined in the
respective row of table
1, compound (1).1 is replaced by compound (1).6, and such defined substance(s)
is/are applied
to eastern groundsel.
The agrochemical compositions which can be used for the method according to
the invention
comprise an herbicidal effective amount of at least one compound of formula
(1), optionally at
least one further active compound selected from herbicides B and safeners C,
and auxiliaries
which are customary for the formulation of crop protection agents.
The compounds of formula (1), or herbicidal compositions comprising the
compounds of formula
(1), can be used, for example, in the form of ready-to-spray aqueous
solutions, powders,
suspensions, also highly concentrated aqueous, oily or other suspensions or
dispersions,
emulsions, oil dispersions, pastes, dusts, materials for broadcasting, or
granules, by means of
spraying, atomizing, dusting, spreading, watering or treatment of the seed or
mixing with the
seed. The use forms depend on the intended purpose; in any case, they should
ensure the
finest possible distribution of the active ingredients according to the
invention.
Examples of auxiliaries customary for the formulation of crop protection
agents are inert
auxiliaries, solid carriers, surfactants (such as dispersants, protective
colloids, emulsifiers,
wetting agents and tackifiers), organic and inorganic thickeners,
bactericides, antifreeze agents,
antifoams, optionally colorants and, for seed formulations, adhesives.
The person skilled in the art is sufficiently familiar with the recipes for
such formulations.
Examples of thickeners (i.e. compounds which impart to the formulation
modified flow
properties, i.e. high viscosity in the state of rest and low viscosity in
motion) are
polysaccharides, such as xanthan gum (Kelzan from Kelco), Rhodopol 23 (Rhone
Poulenc)
or Veegum (from R.T. Vanderbilt), and also organic and inorganic sheet
minerals, such as
Attaclay (from Engelhard).
Examples of antifoams are silicone emulsions (such as, for example, Si!ikon
SRE, Wacker or
Rhodorsil from Rhodia), long-chain alcohols, fatty acids, salts of fatty
acids, organofluorine
compounds and mixtures thereof.
Bactericides can be added for stabilizing the aqueous herbicidal formulations.
Examples of
bactericides are bactericides based on diclorophen and benzyl alcohol
hemiformal (Proxel
from ICI or Acticide RS from Thor Chemie and Kathon MK from Rohm & Haas),
and also
isothiazolinone derivates, such as alkylisothiazolinones and
benzisothiazolinones (Acticide MBS
from Thor Chemie).
CA 03030354 2019-01-09
WO 2018/019842
PCT/EP2017/068784
72
Examples of antifreeze agents are ethylene glycol, propylene glycol, urea or
glycerol.
Examples of colorants are both sparingly water-soluble pigments and water-
soluble dyes.
Examples which may be mentioned are the dyes known under the names Rhodamin B,
CI
Pigment Red 112 and CA. Solvent Red 1, and also pigment blue 15:4, pigment
blue 15:3,
pigment blue 15:2, pigment blue 15:1, pigment blue 80, pigment yellow 1,
pigment yellow 13,
pigment red 112, pigment red 48:2, pigment red 48:1, pigment red 57:1, pigment
red 53:1,
pigment orange 43, pigment orange 34, pigment orange 5, pigment green 36,
pigment green 7,
pigment white 6, pigment brown 25, basic violet 10, basic violet 49, acid red
51, acid red 52,
acid red 14, acid blue 9, acid yellow 23, basic red 10, basic red 108.
Examples of adhesives are polyvinylpyrrolidone, polyvinyl acetate, polyvinyl
alcohol and tylose.
Suitable inert auxiliaries are, for example, the following: mineral oil
fractions of medium to high
boiling point, such as kerosene and diesel oil, furthermore coal tar oils and
oils of vegetable or
animal origin, aliphatic, cyclic and aromatic hydrocarbons, for example
paraffin,
tetrahydronaphthalene, alkylated naphthalenes and their derivatives, alkylated
benzenes and
their derivatives, alcohols such as methanol, ethanol, propanol, butanol and
cyclohexanol,
ketones such as cyclohexanone or strongly polar solvents, for example amines
such as N-
methylpyrrolidone, and water.
Suitable carriers include liquid and solid carriers.
Liquid carriers include e.g. non-aqueous solvents such as cyclic and aromatic
hydrocarbons,
e.g. paraffins, tetrahydronaphthalene, alkylated naphthalenes and their
derivatives, alkylated
benzenes and their derivatives, alcohols such as methanol, ethanol, propanol,
butanol and
cyclohexanol, ketones such as cyclohexanone, strongly polar solvents, e.g.
amines such as N-
methylpyrrolidone, and water as well as mixtures thereof.
Solid carriers include e.g. mineral earths such as silicas, silica gels,
silicates, talc, kaolin,
limestone, lime, chalk, bole, loess, clay, dolomite, diatomaceous earth,
calcium sulfate,
magnesium sulfate and magnesium oxide, ground synthetic materials, fertilizers
such as
ammonium sulfate, ammonium phosphate, ammonium nitrate and ureas, and products
of
vegetable origin, such as cereal meal, tree bark meal, wood meal and nutshell
meal, cellulose
powders, or other solid carriers.
Suitable surfactants (adjuvants, wetting agents, tackifiers, dispersants and
also emulsifiers) are
the alkylated seed oil, alkali metal salts, alkaline earth metal salts and
ammonium salts of
aromatic sulfonic acids, for example lignosulfonic acids (e.g. Borrespers-
types, Borregaard),
phenolsulfonic acids, naphthalenesulfonic acids (Morwet types, Akzo Nobel) and
dibutylnaphthalenesulfonic acid (Nekal types, BASF SE), and of fatty acids,
alkyl- and
alkylarylsulfonates, alkyl sulfates, lauryl ether sulfates and fatty alcohol
sulfates, and salts of
sulfated hexa-, hepta- and octadecanols, and also of fatty alcohol glycol
ethers, condensates of
sulfonated naphthalene and its derivatives with formaldehyde, condensates of
naphthalene or of
the naphthalenesulfonic acids with phenol and formaldehyde, polyoxyethylene
octylphenol
ether, ethoxylated isooctyl-, octyl- or nonylphenol, alkylphenyl or
tributylphenyl polyglycol ether,
alkylaryl polyether alcohols, isotridecyl alcohol, fatty alcohol/ethylene
oxide condensates,
ethoxylated castor oil, polyoxyethylene alkyl ethers or polyoxypropylene alkyl
ethers, lauryl
CA 03030354 2019-01-09
WO 2018/019842
PCT/EP2017/068784
73
alcohol polyglycol ether acetate, sorbitol esters, lignosulfite waste liquors
and proteins,
denaturated proteins, polysaccharides (e.g. methylcellulose), hydrophobically
modified
starches, polyvinyl alcohol (Mowiol types Clariant), polycarboxylates (BASF
SE, Sokalan types),
polyalkoxylates, polyvinylamine (BASF SE, Lupamine types), polyethyleneimine
(BASF SE,
Lupasol types), polyvinylpyrrolidone and copolymers thereof.
Powders, materials for broadcasting and dusts can be prepared by mixing or
concomitant
grinding the active ingredients together with a solid carrier.
Granules, for example coated granules, impregnated granules and homogeneous
granules, can
be prepared by binding the active ingredients to solid carriers.
Aqueous use forms can be prepared from emulsion concentrates, suspensions,
pastes,
wettable powders or water-dispersible granules by adding water.
To prepare emulsions, pastes or oil dispersions, the compounds of formula (I),
or herbicidal
compositions comprising the compounds of formula (I), either as such or
dissolved in an oil or
solvent, can be homogenized in water by means of a wetting agent, tackifier,
dispersant or
emulsifier. Alternatively, it is also possible to prepare concentrates
comprising active compound,
wetting agent, tackifier, dispersant or emulsifier and, if desired, solvent or
oil, which are suitable
for dilution with water.
The concentrations of the active compounds, especially of the compounds of
formula (I), or
herbicidal compositions comprising the compounds of formula (I), in the ready-
to-use
preparations (formulations) can be varied within wide ranges. In general, the
formulations
comprise approximately from 0.001 to 98% by weight, preferably 0.01 to 95% by
weight of at
least one active ingredient. The active ingredients are employed in a purity
of from 90% to
100%, preferably 95% to 100% (according to NMR spectrum).
In the formulation of the compounds of formula (I) according to the present
invention the active
ingredients, e.g. the compounds of formula (I), or herbicidal compositions
comprising the
compounds of formula (I), are present in suspended, emulsified or dissolved
form. The
formulation according to the invention can be in the form of aqueous
solutions, powders,
suspensions, also highly concentrated aqueous, oily or other suspensions or
dispersions,
aqueous emulsions, aqueous microemulsions, aqueous suspo-emulsions, oil
dispersions,
pastes, dusts, materials for spreading or granules.
The compounds of formula (I) according to the present invention, or herbicidal
compositions
comprising the compounds of formula (I), can, for example, be formulated as
follows:
1. Products for dilution with water
A) Water-soluble concentrates
10 parts by weight of active compound are dissolved in 90 parts by weight of
water or a water-
soluble solvent. As an alternative, wetters or other adjuvants are added. The
active compound
dissolves upon dilution with water. This gives a formulation with an active
compound content of
CA 03030354 2019-01-09
WO 2018/019842
PCT/EP2017/068784
74
10% by weight.
B) Dispersible concentrates
20 parts by weight of active compound are dissolved in 70 parts by weight of
cyclohexanone
with addition of 10 parts by weight of a dispersant, for example
polyvinylpyrrolidone. Dilution
with water gives a dispersion. The active compound content is 20% by weight.
C) Emulsifiable concentrates
parts by weight of active compound are dissolved in 75 parts by weight of an
organic
solvent (e.g. alkylaromatics) with addition of calcium dodecylbenzenesulfonate
and castor oil
ethoxylate (in each case 5 parts by weight). Dilution with water gives an
emulsion. The
10 formulation has an active compound content of 15% by weight.
D) Emulsions
parts by weight of active compound are dissolved in 35 parts by weight of an
organic
solvent (eg. alkylaromatics) with addition of calcium dodecylbenzenesulfonate
and castor oil
ethoxylate (in each case 5 parts by weight). This mixture is introduced into
30 parts by weight of
15 water by means of an emulsifier (Ultraturrax) and made into a
homogeneous emulsion. Dilution
with water gives an emulsion. The formulation has an active compound content
of 25% by
weight.
E) Suspensions
In an agitated ball mill, 20 parts by weight of active compound are comminuted
with addition of
20 10 parts by weight of dispersants and wetters and 70 parts by weight of
water or an organic
solvent to give a fine active compound suspension. Dilution with water gives a
stable
suspension of the active compound. The active compound content in the
formulation is 20% by
weight.
F) Water-dispersible granules and water-soluble granules
25 50 parts by weight of active compound are ground finely with addition of
50 parts by weight of
dispersants and wetters and made into water-dispersible or water-soluble
granules by means of
technical appliances (for example extrusion, spray tower, fluidized bed).
Dilution with water
gives a stable dispersion or solution of the active compound. The formulation
has an active
compound content of 50% by weight.
G) Water-dispersible powders and water-soluble powders
75 parts by weight of active compound are ground in a rotor-stator mill with
addition of 25 parts
by weight of dispersants, wetters and silica gel. Dilution with water gives a
stable dispersion or
solution of the active compound. The active compound content of the
formulation is 75% by
weight.
H) Gel formulations
In a ball mill, 20 parts by weight of active compound, 10 parts by weight of
dispersant, 1 part
by weight of gelling agent and 70 parts by weight of water or of an organic
solvent are mixed to
give a fine suspension. Dilution with water gives a stable suspension with
active compound
content of 20% by weight.
2. Products to be applied undiluted
I) Dusts
5 parts by weight of active compound are ground finely and mixed intimately
with 95 parts by
weight of finely divided kaolin. This gives a dusting powder with an active
compound content of
CA 03030354 2019-01-09
WO 2018/019842
PCT/EP2017/068784
5% by weight.
J) Granules (GR, FG, GG, MG)
0.5 parts by weight of active compound are ground finely and associated with
99.5 parts by
weight of carriers. Current methods here are extrusion, spray-drying or the
fluidized bed. This
5 gives granules to be applied undiluted with an active compound content of
0.5% by weight.
K) ULV solutions (UL)
10 parts by weight of active compound are dissolved in 90 parts by weight of
an organic
solvent, for example xylene. This gives a product to be applied undiluted with
an active
compound content of 10% by weight.
10 Aqueous use forms can be prepared from emulsion concentrates,
suspensions, pastes,
wettable powders or water-dispersible granules by adding water.
Application can be done before, during and/or after, preferably during and/or
after, the
15 emergence of the PPO resistant weeds.
The compounds of formula (I) or the herbicidal compositions comprising them
can be applied
pre- or post-emergence, pre-plant or together with the seed of a crop plant.
It is also possible to
apply the method by applying seed pretreated with the compound of formula (I),
or herbicidal
20 compositions comprising them, of a crop plant.
If the active ingredients are less well tolerated by certain crop plants,
application techniques
may be used in which the herbicidal compositions are sprayed, with the aid of
the spraying
equipment, in such a way that as far as possible they do not come into contact
with the leaves
of the sensitive crop plants, while the active ingredients reach the leaves of
undesirable plants
25 growing underneath, or the bare soil surface (post-directed, lay-by).
In a further embodiment, the method, i.e. thecompounds of formula (I) or the
herbicidal
compositions comprising them, can be applied by treating plant propagation
material,
particularly seed. The treatment of seeds comprises essentially all procedures
familiar to the
30 person skilled in the art (seed dressing, seed coating, seed dusting,
seed soaking, seed film
coating, seed multilayer coating, seed encrusting, seed dripping and seed
pelleting) based on
the compounds of formula (I) according to the invention or the compositions
prepared
therefrom. Here, the herbicidal compositions can be applied diluted or
undiluted.
35 The term "seed" comprises plant reproductive material of all types, such
as, for example, corms,
grains, seeds, fruits, tubers, bulbs, nuts, seedlings and similar forms. Here,
preferably, the term
seed describes grains and seeds. The seed used can be seed of the useful
plants mentioned
above, but also the seed of transgenic plants or plants obtained by customary
breeding
methods.
The compound of formula (I) or composition comprising the compound of formula
(I) according
to the present invention may be applied prior to planting, at planting, after
planting and prior to
emergence of, and over the top of or as a directed spray to or near crops,
preferably herbicide
resistant crops, to control PPO herbicide resistant weeds near the crops
without injury to the
CA 03030354 2019-01-09
WO 2018/019842
PCT/EP2017/068784
76
crops. If the compounds of formula (I) or composition comprising the compound
of formula (I)
according to the present invention are applied prior to planting of a crop,
they may preferably be
applied to control not only PPO resistant weeds but any vegetation including
weeds (such as
PPO resistant weeds), volunteer crop plants and other vegetation (so-called
burn-down'
application).
The compound of formula (I) or composition comprising the compound of formula
(I) according
to the present invention may furthermore be applied to non-crop areas such as
e. g. industrial
sites, railroads, powerlines or the vicinity thereof, as well as for forestry
uses.
The rates of application of the active compound of formula (I) according to
the present invention
(total amount of compound of formula (I)) are from 0,1 g/ha to 3000 g/ha,
preferably 10 g/ha to
1000 g/ha of active substance (a.s.), depending on the control target, the
season, the target
plants and the growth stage.
In another preferred embodiment of the invention, the application rates of the
compounds of
formula (I) are in the range from 0.1 g/ha to 5000 g/ha and preferably in the
range from 1 g/ha
to 2500 g/ha or from 5 g/ha to 2000 g/ha of active substance (a.s.).
In another preferred embodiment of the invention, the application rate of the
compounds of
formula (I) is 0.1 to 1000 g/ha, preferably1 to 750 g/ha, more preferably 5 to
500 g/ha, of active
substance.
To treat the seed, the compounds I are generally employed in amounts of from
0.001 to 10 kg
per 100 kg of seed.
Examples: - The herbicidal activity of the compound of formula (I) was
demonstrated by the
following experiments:
The culture containers used were plastic flowerpots containing loamy sand with
approximately
3.0% of humus as the substrate. The seeds of the test plants were sown
separately for each
species and/or resistant biotype. For the pre-emergence treatment, the active
ingredients, which
had been suspended or emulsified in water, were applied directly after sowing
by means of
finely distributing nozzles. The containers were irrigated gently to promote
germination and
growth and subsequently covered with transparent plastic hoods until the
plants had rooted.
This cover caused uniform germination of the test plants, unless this had been
impaired by the
active ingredients. For the post-emergence treatment, the test plants were
first grown to a
.. height of 3 to 15 cm, depending on the plant habit, and only then treated
with the active
ingredients which had been suspended or emulsified in water. For this purpose,
the test plants
were either sown directly and grown in the same containers, or they were first
grown separately
as seedlings and transplanted into the test containers a few days prior to
treatment. Depending
on the species, the plants were kept at 10 ¨ 25 C or 20 ¨ 35 C, respectively.
The test period
CA 03030354 2019-01-09
WO 2018/019842
PCT/EP2017/068784
77
extended over 2 to 4 weeks. During this time, the plants were tended, and
their response to the
individual treatments was evaluated.
The evaluation was carried out by using a scale from 0 to 100. 100 means no
emergence of the
plants or complete destruction of at least the above-ground parts, and 0 means
no damage, or
normal course of growth.
The plants used in a first greenhouse experiment were of the following species
and biotype
(ambrosia eliator mentioned below is to be understood being a variety of
Ambrosia
artemisiifolia):
Weed Bayer Scientific name Common name Biotype
no. code
w.1 AMATA Amaranthus Common Sensitive
tamariscinus waterhemp
w.2 AMATA Amaranthus Common PPO resistant
biotype 1
tamariscinus waterhemp
w.3 AMATA Amaranthus Common PPO resistant
biotype 2
tamariscinus waterhemp
w.4 AMBEL Ambrosia elatior Common Sensitive
ragweed
w.5 AMBEL Ambrosia elatior Common
PPO resistant biotype 3 that
ragweed
contains the R98L mutation
w.6 AMBEL Ambrosia elatior Common
PPO resistant biotype 4 that
ragweed
contains the R98L mutation
The results shown in the following table 2 demonstrate that compound (1).1 has
very good
activity on both sensitive (w.1, w.4) and resistant weeds (w.2, w.3, w.5, w.6)
whereas the known
PPO inhibitor flumioxazin shows much weaker control of resistant in comparison
to sensitive
biotypes.
Table 2
Herbicide Use rate Weed control (%)
compound [g/ha] w.1 w.2 w.3 w.4 w.5
w.6
(1).1 2 100 100 100 100 100
100
(1).1 1 100 100 100 100 100
100
flumioxazin 2 95 30 70 65 10
20
flumioxazin 1 90 30 0 50 0
0
In another greenhouse experiment, plants of the following species and biotype
were tested:
CA 03030354 2019-01-09
WO 2018/019842
PCT/EP2017/068784
78
Weed Bayer Scientific name Common name
Biotype
no. code
w.7 AMATU Amaranthus Tall waterhemp
Sensitive
tuberculatus
w.8 AMATU Amaranthus Tall waterhemp PPO
resistant biotype 5 that
tuberculatus contains the AG210
mutation
w.9 AMATU Amaranthus Tall waterhemp PPO
resistant biotype 6 that does
tuberculatus not contain the AG210
mutation
The results shown in the following table 3 demonstrate that compound (1).1 has
very good
activity on both sensitive (w.7) and resistant weeds contain the AG210
mutation (w.8) as well as
those that don't (w.9).
Table 3
Herbicide Use rate Weed control (%)
compound [g/ha] 7 8 9
(1).1 2 100 100 100
(1).1 1 100 100 95
The plants used in a third greenhouse experiment were of the following species
and biotype:
Weed Bayer Scientific name Common name
Biotype
no. code
w.10 AMATA Amaranthus Common Sensitive
tamariscinus waterhemp
w.11 AMATA Amaranthus Common PPO resistant biotype 7
tamariscinus waterhemp
w.12 AMATA Amaranthus Common PPO resistant biotype 8
tamariscinus waterhemp
The results shown in the following table 4 demonstrate that compound (1).4 has
very good
activity on both sensitive (w.10) and resistant weeds (w.11, w.12) whereas the
known PPO
inhibitor azafenidin shows much weaker control of resistant in comparison to
sensitive biotypes.
Table 4
Herbicide Use rate Weed control (%)
compound [g/ha] w.10 w.11 w.12
(1).4 4 100 100 99
(1).4 2 100 89 100
azafenidin 4 98 73 72
azafenidin 2 95 67 70