Note: Descriptions are shown in the official language in which they were submitted.
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
NITROGEN-CONTAINING TRICYCLIC COMPOUNDS AND USES THEREOF IN
MEDICINE
FIELD OF THE INVENTION
[0001] The present invention relates to nitrogen-containing tricyclic
compounds which can
bind to the FXR and act as FXR modulators, and pharmaceutical compositions
thereof, as well as
the uses of said compounds and pharmaceutical compositions in the preparation
of medicaments
for the treatment of diseases and/or conditions mediated by FXR.
BACKGROUND OF THE INVENTION
[0002] FXR is a member of the nuclear hormone receptor superfarnily, and is
mainly
expressed in the liver, kidneys and intestines (Seol etal. MoLEndocrinol
(1995), 9:72-85;
Forman Cell (1995), 81:687-693). It functions as a heterodimer with the RXR,
and regulates
gene transcription by binding to the response elements of the target gene
promoter. The
FXR-RXR heterodimer binds with highest affinity to an inverted repeat-1(IR-1)
response
element, wherein consensus receptor-binding hexamers are separated by a
nucleotide. FXR is
activated by bile acids (cholesterol metabolism end products) (Makishima et
al., Science (1999),
284:1362-1365; Parks et al., Science (1999), 284:1365-1368; Wang et al.,
Ma.Cell. (1999),
3:543-553), and the bile acid is used to inhibit cholesterol catabolism.
(Urizar et al., (2000)
J.Biol.Chem. 275:39313-393170).
[0003] FXR is a critical regulator of cholesterol homeostasis, triglyccride
synthesis and
adipogenesis (Crawley, Expert Opinion Ther. Patents (2010), 20:1047-1057). In
addition to
target for treatingdyslipidemia, obesity, vitamin D-related diseases,
intestinal diseases,
drug-induced side effects as well as hepatitis (Crawley, Expert Opinion Ther.
Patents (2010),
20:1047-1057), FXR is also the target of hepatic and gall disease, chronic
hepatitis, nonalcoholic
fatty liver disease (NAFLD), nonalcoholic steatohepatitis (NASH), cholestasis,
liver fibrosis,
cirrhosis of liver, hepatitis B, metabolic diseases, lipid metabolism
disorders, carbohydrate
-1-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
metabolic diseases, cardiovascular and metabolic diseases, atherosclerosis,
type II diabetes and
diabetic complications (Frank G. Schaap et al., Journal of Medicinal
Chemistry, (2005),
48:5383-5402).
[0004] Small molecule compounds which act as FXR modulators have been
disclosed in
the following patents: WO 2000/037077, WO 2003/015771, WO 2004/048349, WO
2007/076260, WO 2007/092751, WO 2007/140174, WO 2007/140183, WO 2008/051942,
WO
2008/157270, WO 2009/005998, WO 2009/012125, WO 2009/149795, WO 2008/025539,
WO
2008/025540, WO 2012/087520, WO 2012/087521, WO 2012/087519, W02013/007387 and
WO 2015/036442. R.C.Buijsman et al. also reviewed more-small molecule
modulators of FXR
(R.C.Buijsman et al., Curr. Med. Chem. 2005, 12, 1017-1075).
[0005] Although the development of FXR modulators has a certain progress,
the
development space is still enormous.
SUMMARY OF THE INVENTION
[0006] The object of the present invention is to provide novel nitrogen-
containing tricyclic
compounds which act as FXR modulators. The biological activity and
pharmacokinetic
properties of the compounds disclosed herein are superior to these of the
known FXR modulators.
The present invention provides a compound, or a pharmaceutical composition
thereof, which
binds to FXR and acts as modulator of FXR. The present invention further
relates to said
compound or the use of said compound in the preparation of a medicament for
the treatment of
diseases and/or conditions through said compound binding to the FXR nuclear
receptor. The
present invention further describes the synthetic method of the compound. The
compounds of the
invention exhibit improved biological activity and pharmacokinetic advantages.
[0007] Specifically,
[0008] in one aspect, provided herein is a compound having formula (I), or
a stereoisomer,
a geometric isomer, a tautomer, an N-oxide, a hydrate, a solvate, a
metabolite, a pharmaceutically
acceptable salt or a prodrug thereof,
-2-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
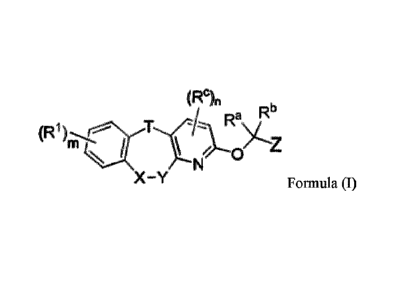
(RC)n
RI)
X ¨Y (I);
wherein:
[0009] T is -NH-, -0-, -S-, -C(=O)- or -CH2-;
[0010] each of X and Y is independently a bond, -0-, -S(=0)1-, -NR'-, -
CRYle- or -C(=0)-,
or -X-Y- is -CHRh-CHRk- or -CRY=Cle-;
[0011] each Rx is independently hydrogen, deuterium, alkyl, aminoalkyl,
haloalkyl,
cycloalkyl, heterocyclyl, aryl, halo-substituted aryl or arylalkyl;
[0012] each RY and Rz is independently hydrogen, deuterium, F, Cl, Br, I,
hydroxy, amino,
nitro, cyano, alkyl, haloalkyl, hydroxyalkyl, aminoalkyl, alkoxy, alkylamino,
alkenyl, alkynyl,
cycloalkyl, heterocyclyl, aryl, halo-substituted aryl or arylalkyl; or RY and
le, together with the
same carbon atom to which they are attached, independently and optionally form
a cycloalkane
ring or heterocyclic ring, and wherein the cycloalkane ring and heterocyclic
ring is independently
and optionally substituted with substituents selected from F, Cl, Br, I,
hydroxy, amino, nitro,
cyano, methyl, ethyl, isopropyl and trifluoromethyl;
[0013]
each of Rh and Rk is independently hydrogen, deuterium, F, Cl, Br, I, hydroxy,
amino, nitro, cyano, methyl, ethyl, isopropyl or trifluoromethyl; or Rh and Rk
together with the
carbon atoms to which they are attached, form a cycloalkane ring or a
heterocyclic ring, wherein
the cycloalkane ring and heterocyclic ring is independently and optionally
substituted with
substituents selected from F, Cl, Br, I, hydroxy, amino, nitro, cyano, methyl,
ethyl, isopropyl and
trifluoromethyl;
[0014] each of le and Rh is independently hydrogen, deuterium or Ci_3
alkyl;
[0015] each le is independently hydrogen, deuterium, F, Cl, Br, I, oxo,
hydroxy, amino,
nitro, cyano, methyl, ethyl, n-propyl, isopropyl, difluoromethyl,
trifluoromethyl,
difluoromethoxy, trifluoromethoxy, hydroxymethyl, aminomethyl, methylamino,
dimethylamino,
-3-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
methoxymethyl, isopropoxymethyl, vinyl, ethynyl, cyclopropyl, cyclobutyl,
cyclopentyl,
cyclohexyl, azetidinyl, tetrahydrofuranyl,
pyrrolidinyl, piperidinyl, piperazinyl,
tetrahydropyranyl, morpholinyl, thiomorpholinyl, phenyl, thiazolyl, thienyl,
oxazolyl, triazolyl,
tetrazolyl, pyridinyl, pyrimidinyl, -COOH, -C(=0)0-C1_3 alkyl, -C(=0)NHS(.0)2-
C1_3 alkyl,
-C(=0)NHS(=0)2-phenyl, -C(=0)NH-C1_3 alkylene-
S(=0)20H, -C(=0)NH-C1_3
alkylen e-C(= 0)0H, -S(=0)2NH2, -S(=0)20H, -S(=0)2-C1_3 alkyl, -C(=0)NH2 or
-C(=0)N(CH3)2;
[0016] each R1
is independently hydrogen, deuterium, F, Cl, Br, I, hydroxy, amino, nitro,
cyano, alkyl, haloalkyl, hydroxyalkyl, aminoalkyl, alkoxy, alkylamino,
haloalkoxy, alkoxyalkyl,
alkenyl, alkynyl, cycloalkyl, cycloalkoxy, cycloalkylalkyl, heterocyclyl,
heterocyclylalkyl, aryl,
halo-substituted aryl, arylalkyl, heteroaryl, -L1-C(=0)0R8, -L1-S(=0)1R9, -0-
L2-C(=0)0R8,
-0-L2-S(=0)tR9, -C(=0)NR10R11
, -C(=0)N(R10)S(=0)2R9, -C(=NR10)NR oRii,
-C(=0)N(R10)-L3-S(=0)20R8, -C(=0)N(R10)C(=0)0R8 or -C(=0)N(R10)-L3-C(=0)0R8;
or two
adjacent RI, together with the ring atoms to which they are attached
independently and
optionally form a carbocyclic ring, heterocyclic ring, aromatic ring or
heteroaromatic ring, and
wherein each R1 is independently and optionally substituted with one or more
R12;
[0017] each R8
is independently hydrogen, deuterium, alkyl, aminoalkyl, alkenyl, alkynyl,
haloalkyl, cycloalkyl, heterocyclyl or aryl;
[0018] each R9
is independently hydrogen, deuterium, hydroxy, amino, alkyl, aminoalkyl,
alkenyl, alkynyl, haloalkyl, cycloalkyl, heterocyclyl aryl or -NR10R11;
[0019] each R1 and is
independently hydrogen, deuterium, alkyl, aminoalkyl, alkenyl,
alkynyl, haloalkyl, cycloalkyl, heterocyclyl or aryl; or R1 and R11, together
with the nitrogen
atom to which they are attached, independently and optionally form a
heterocyclic ring or
heteroaromatic ring;
[0020] each R12
is independently hydrogen, deuterium, F, Cl, Br, I, oxo, hydroxy, amino,
nitro, cyano, alkyl, haloalkyl, alkoxy, alkylamino, alkenyl, alkynyl,
cycloalkyl, heterocyclyl, aryl
-4-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
or heteroaryl;
[0021] each L1 is independently a bond, -NH-, -C(=0)-, C1_3 alkylene, C24
alkenylene or
C24 alkynylene;
[0022] each L2 is independently C1_3 alkylene, C24 alkenylene or C24
alkynylene;
[0023] each L3 is independently a bond or C1_3 alkylene;
R3 R3 R3 R3
R2 R4 R2 R4 R2 ,,, R4 R2 R4
..,õ. 1
1.,....õ0=N R6
S µ S
Z is R7
R3 R3 R3 R3 R3
R2 air ,R4 112 R4
R2 R4 Rg ,,,, R4
1
ti R
x.T.
L 4111 R5 Xi.,<-I
L R.' L 91IF F1s L Sli R5
4f.,N1,4 R3 R
N N y C)
W , RI R7 Rr R7 Or
3 3 ,
R5
R2 ...x:1R4
I
L R'
R6
i"--µ-',=*I'N
N¨N
/
Ft7 =
,
[0024] each L is independently a bond, -0-, -S-, -NH-, -CH2-, -CH2-CH2-, -0-
CH2-,
-0-CH2-CH2- or -CH2-0-CH2-;
[0025] each R2, R3, R4, R5 and R6 is independently hydrogen, deuterium, F,
Cl, Br, I,
hydroxy, amino, nitro, cyano, C1_3 alkyl, C1_3 haloalkyl, C1_3 alkoxy or C1_3
haloalkoxy;
[0026] each R7 is independently hydrogen, deuterium, F, Cl, Br, I, hydroxy,
amino, nitro,
cyano, C1_6 alkyl, Ci_6 hydroxyalkyl, C1_6 haloalkyl, C1_6 alkoxy, C1_6
alkylamino, C1-6
alkoxy-C1_6 alkyl, C3_6 cycloalkyl or C2_6 heterocyclyl, and wherein the C3_6
cycloalkyl and C2-6
-5-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
heterocydyl is independently and optionally substituted with subsitituents
selected from F, Cl,
Br, I, hydroxy, amino, nitro and cyano;
[0027] m is 0, 1, 2, 3 or 4;
[0028] n is 0, 1 or 2; and
[0029] each t is independently 0, 1 or 2;
[0030] with the proviso that a compound having formula (I) is not:
O 411 ilit1 0
01 ci 'I
HO HO
ilk = N I* 'C,)õ, N
r
Ili 1
o o o 4
CI i
ci a
HO
* 0 \ , =
µ 6
N 0
11
o
.`1.8 a .1
=
d ti
/
lr
.4
--
H2N o 1 o
OP
. ,
Olt * 0 N
\ No" HO 0 CN 40, 0 It --;
071
N
0
4 I 0 41111 41
CI C CI CI
HO HO .
=-._=-._ .- '
N ...
TN * \=r 0 *I 6 C:, =.,4.4
=
N N = N-4 N = ' 0
<:( H.
-4
U o
i cs * 1 a 4101 0
HO 0 HO CI I
* r.).- 110 s'414 HO
\I
1-1112 N' \ -IN q..--J 1
6
0 N
/ / / 1
-6-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
0
"No-sstilt ct and HO rigL. 0
k N
1,1 o d
.4
[0031] In some embodiments, each Rx is independently hydrogen, deuterium,
C1.6 alkyl,
C1_6 aminoalkyl, C1-6 haloalkyl, C3_6 cycloalkyl, C2-6 heterocyclyl, phenyl,
halo-substituted
phenyl or benzyl;
[0032] each RY and Rz is independently hydrogen, deuterium, F, CI, Br, I,
hydroxy, amino,
nitro, cyano, C1_6 alkyl, C1_6 haloalkyl, C1_6 hydroxyalkyl, C1_6 aminoalkyl,
C1_6 alkoxy, C1-6
alkylamino, C2_6 alkenyl, C26 alkynyl, C3_6 cycloalkyl, C26 heterocyclyl,
phenyl, halo-substituted
phenyl or benzyl; or RY and Rz together with the same carbon atom to which
they are attached,
independently and optionally form a C3_6 cycloalkane ring or C26 heterocyclic
ring, and wherein
the C3_6 cycloalkane ring and C2-6 heterocyclic ring is independently and
optionally substituted
with substituents selected from F, Cl, Br, I, hydroxy, amino, nitro, cyano,
methyl, ethyl, isopropyl
and trifluoromethyl;
[0033] each of Rh and Rk is independently methyl, ethyl, isopropyl or
trifluoromethyl; or Rh
and Rk together with the carbon atoms to which they are attached, form a C3_6
cycloalkane ring or
C2.6 heterocyclic ring, and wherein the C3_6 cycloalkane ring and C2_6
heterocyclic ring is
independently and optionally substituted with substituents selected from F,
Cl, Br, I, hydroxy,
amino, nitro, cyano, methyl, ethyl, isopropyl and trifluoromethyl.
[0034] In other embodiments, each Rx is independently hydrogen, deuterium,
methyl,
ethyl, isopropyl, aminomethyl, difluoromethyl, trifluoromethyl, cyclopropyl,
cydohexyl,
tetrahydropyranyl, piperidinyl, phenyl, halo-substituted phenyl or benzyl;
[0035] each RY and re is independently hydrogen, deuterium, F, Cl, Br, I,
hydroxy, amino,
nitro, cyano, methyl, ethyl, n-propyl, isopropyl, difluoromethyl,
trifluoromethyl,
2,2-difluoroethyl, 2,2,2-trifluoroethyl, hydroxymethyl, 2-hydroxyethyl,
aminomethyl, methoxy,
ethoxy, isopropoxy, t-butoxy, methylamino, dimethylamino, vinyl, ethynyl,
cyclopropyl,
-7-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
cyclobutyl, cyclopentyl, cyclohexyl, azetidinyl, tetrahydrofuranyl,
pyrrolidinyl, piperidinyl,
piperazinyl, tetrahydropyranyl, morpholinyl, thiomorpholinyl, phenyl, halo-
substituted phenyl or
benzyl; or RY and Rz, together with the same carbon atom to which they are
attached,
independently and optionally form cyclopropane, cyclobutane, cyclopentane,
cyclohexane,
azetidine, tetrahydrofuran, pyrrolidine, piperidine, piperazine,
tetrahydropyran, morpholine or
thiomorpholine, and wherein the cyclopropane, cyclobutane, cyclopentane,
cyclohexane,
azetidine, tetrahydrofuran, pyrrolidine, piperidine, piperazine,
tetrahydropyran, morpholine and
thiomorpholine is independently and optionally substituted with substituents
selected from F, Cl,
Br, I, hydroxy, amino, nitro, cyano, methyl, ethyl, isopropyl and
trifluoromethyl;
[00361 each of
Rh and Rk is independently methyl, ethyl, isopropyl or trifluoromethyl; or Rh
and Rk, together with the carbon atoms to which they are attached, form
cyclopropylene,
cyclobutylene, cyclopentylene,
cyclohexylene, azetidinylene, tetrah ydrofu ran ylcnc,
pyrrolidinylene, piperidinylene, piperazinylene, tetrahydropyranylene,
morpholinylene or
thiomorpholinylene, and wherein the cyclopropylene, cyclobutylene,
cyclopcntylene,
cyclohexylene, azetidinylene, tetrahydrofuranylene, pyrrolidinylene,
piperidinylene,
piperazinylene, tetrahydropyranylene, morpholinylene and thiomorpholinylene is
independently
and optionally substituted with substituents selected from F, Cl, Br, I,
hydroxy, amino, nitro,
cyano, methyl, ethyl, isopropyl and trifluoromethyl.
[0037] In some
embodiments, each R1 is independently hydrogen, deuterium, F, Cl, Br, I,
oxo, hydroxy, amino, nitro, cyano, C1_6 alkyl, C1_6 haloalkyl, C1_6
hydroxyalkyl, C1_6 aminoalkyl,
C1,6 alkoxy, Ci_6 alkylamino, Ci_6 haloalkoxy, C1-6 alkoxy-C1_3 alkyl, C2-6
alkenyl, C2_6 alkynyl,
C3_6 cycloalkyl, C3_6 cycloalkox y, C3_6 oycloalkyl-C1_3 alkyl, C2_6
heterocycl yl, C2-6
heterocyclyl-C1_3 alkyl, phenyl, halo-substituted phenyl, benzyl, C1.5
heteroaryl, -1_,1-C(=0)0R8,
-L1-S(.0)tR9, -0-L2-C(=0)0R8, -0-L2-S(.0)tR9, -C(=0)NR10R11,
_c(=o)N(R10)s(=0)2R9
,
-C(=NR10)NR10R11,
-C(=0)N(R10)-L3-S(=0)20R8, -C(=0)N(R10)C(=0)0R8 or
-C(=0)N(R10)-L3-C(=0)0R8; or two adjacent R1, together with the ring atoms to
which they are
-8-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakcs Ref.: 75920/00027
attached, independently and optionally form a C3_6 carbocyclic ring, C2_6
heterocyclic ring,
benzene ring or C1_5 heteroaromatic ring, and wherein each the R1 is
independently and
optionally substituted with one or more R12;
[0038] s =
each R
independently hydrogen, deuterium, C1_6 alkyl, C1_6 aminoalkyl, C2-6
alkenyl, C2-6 alkynyl, C2_6 haloalkyl, C3.6 cycloalkyl, C2_6 heterocyclyl or
phenyl;
[0039] each R9
is independently hydrogen, deuterium, hydroxy, amino, C1_6 alkyl, C1-6
aminoalkyl, C2-6 alkenyl, C2_6 alkynyl, C2-6 haloalkyl, C3_6 cycloalkyl, C2_6
heterocyclyl, phenyl
or -NR10R11;
[0040] each 121
and R11 is independently hydrogen, deuterium, C1_6 alkyl, C1_6 aminoalkyl,
C2_6 alkenyl, C2_6 alkynyl, C1_6 haloalkyl, C3_6 cycloalkyl, C2_6 heterocyclyl
or phenyl, or R1 and
R11 together with the nitrogen atom to which they are attached, independently
and optionally
form a C2-6 heterocyclic ring or C1_5 heteroaromatic ring;
[0041] each R12
is independently hydrogen, deuterium, F, Cl, Br, I, oxo, hydroxy, amino,
nitro, cyano, Ci_6 alkyl, C1_6 haloalkyl, C1_6 alkoxy, C1_6 alkylamino, C2_6
alkenyl, C2_6 alkynyl,
C3_6 cycloalkyl, C2-6 heterocyclyl, phenyl or C1_5 heteroaryl.
[0042] In other
embodiments, each R1 is independently hydrogen, deuterium, F, Cl, Br, I,
hydroxy, amino, nitro, cyano, C1_3 alkyl, C1_3 haloalkyl, C1_3 hydroxyalkyl,
C1_3 aminoalkyl, C1_3
alkoxy, C1_3 alkylamino, C1_3 haloalkoxy, C1_3 alkoxy-C1_3 alkyl, C2_4
alkenyl, C2_4 alkynyl, C3_6
cycloalkyl, C36 CyClOalkeay, C36 cycloalkyl-C13 alkyl, C2_6 heterocyclyl, C2_6
heterocyclyl-C1-3
alkyl, phenyl, halo-substituted phenyl, benzyl, C1_5 heteroaryl, -L1-C(=0)0R8,
-1_,1-8(=0)9R9,
-0-L2-C(=0)0R8, -0-L2-S(=0),R9, -C(=0)NRI0R11,
-C(=0)N(R10)S(.0)2R9,
-C(=NR10)NR10R11, -C(.0)N(R10)-L3-S(.0)20R8, -
C(=0)N(R10)C(.0)0R8 or
-C(=0)N(R10)-L3-C(=0)0R8; or two adjacent R1, together with the ring atoms to
which they are
attached, independently and optionally form a C3_6 carbocyclic ring, C2_6
heterocyclic ring,
benzene ring or C1-5 heteroaromatic ring, and wherein each R1 is independently
and optionally
substituted with one or more R12;
-9-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
[0043] each R8
is independently hydrogen, deuterium, C1,3 alkyl, C1.3 aminoalkyl, C2-4
alkenyl, C2_4 alkynyl, C24 haloalkyl, C34 cycloalkyl, C2_6 heterocyclyl or
phenyl;
[0044] each R9
is independently hydrogen, deuterium, hydroxy, amino, C1-3 alkyl, C1-3
aminoalkyl, C24 alkenyl, C24 alkynyl, C24 haloalkyl, C34 cycloalkyl, C2-6
heterocyclyl, phenyl
or -NR10R11;
[0045] each R1
and R11 is independently hydrogen, deuterium, C1,3 alkyl, C1_3 aminoalkyl,
C24 alkenyl, C2_4 alkynyl, C1_3 haloalkyl, C3-6 cycloalkyl, C2-6 heterocyclyl
or phenyl; or R1 and
R11, together with the nitrogen atom to which they are attached, independently
and optionally
form a C2_6 heterocyclic ring or Ci_5 heteroaromatic ring;
[0046] each R12
is independently hydrogen, deuterium, F, Cl, Br, I, oxo, hydroxy, amino,
nitro, cyano, C1-3 alkyl, C1-3 haloalkyl, C1_3 alkoxy, C1_3 alkylamino, C24
alkenyl, C24 alkynyl,
C3_6 cycloalkyl, C2-6 heterocyclyl, phenyl or Ci_5 heteroaryl.
[0047] In other
embodiments, each R1 is independently hydrogen, deuterium, F, Cl, Br, I,
hydroxy, amino, nitro, cyano, methyl, ethyl, n-propyl, isopropyl,
difluoromethyl, trifluoromethyl,
difluoromethoxy, trifluoromethoxy, hydroxymethyl, aminomethyl, methylamino,
dimethylamino,
methoxylmethyl, isopropoxylmethyl, ethenyl, ethynyl, cyclopropyl, cyclobutyl,
cyclopentyl,
cyclohexyl, azetidinyl, tetrahydrofuranyl,
pyrrolidinyl, piperidinyl, piperazinyl,
tetrahydropyranyl, morpholinyl, thiomorpholinyl, phenyl, thiazolyl, thienyl,
oxazolyl, triazolyl,
tetrazolyl, pyridlnYI, pyrirnidinyl, -COOH, -C(=0)0-C1_3 alkyl, -C(.0)NHS(=0)2-
C1_3 alkyl,
-C(=0)NHS(=0)2-phenyl, -C(=0)NH-C1_3 alkylene-
S(=0)20H, -C(=0)NH-C1-3
alkylene-C(.0)0H, -S(=0)2NH2, -S(=0)20H, -S(=0)2-C1_3 alkyl, -C(=0)NH2 or
-C(=0)N(CH3)2; wherein each R1 is independently and optionally substituted
with one or more
R12;
[0048] each R12
is independently hydrogen, deuterium, F, Cl, Br, I, oxo, hydroxy, amino,
nitro, cyano, methyl, trifluoromethyl, methoxy, methylamino, vinyl, ethynyl,
cyclopropyl,
cyclohexyl, tetrahydrofuranyl, piperazinyl, phenyl or pyridinyl.
-10-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
[0049] In some
embodiments, each R2, R3, R4, R5 and R6 is independently hydrogen,
deuterium, F, Cl, Br, I, hydroxy, amino, nitro, cyano, methyl, isopropyl,
difluoromethyl,
trifluoromethyl, methoxy, isopropoxy, difluoromethoxy or trifluoromethoxy;
[0050] 7 i each
R s independently hydrogen, deuterium, F, Cl, Br, I, hydroxy, amino, nitro,
cyano, methyl, ethyl, isopropyl, t-butyl, hydroxymethyl, 2-hydroxyisopropyl,
difluoromethyl,
trifluoromethyl, 2-fluoroisopropyl, methoxy, isopropoxy, t-butoxy,
dimethylamino,
isopropoxymethyl, t-butoxymethyl, cyclopropyl, cyclobutyl, cyclopentyl,
cyclohexyl,
tetrahydrofuranyl, pyrrolidinyl, piperidinyl, piperazinyl, morpholinyl or
thiomorpholinyl, and
wherein the cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl,
tetrahydrofuranyl, pyrrolidinyl,
piperidinyl, piperazinyl, morpholinyl and thiomorpholinyl is independently and
optionally
substituted with subsitituents selected from F, Cl, Br, I, hydroxy, amino,
nitro and cyano.
[0051] In one
aspect, provided herein is a pharmaceutical composition comprisingthe
compound described above, the pharmaceutical composition optionally further
comprise a
pharmaceutically acceptable carrier, excipient, diluent, adjuvant, vehicle or
a combination
thereof.
[0052] In one
aspect, provided herein is use of the compound described above in the
manufacture of a medicament for preventing, managing, treating or lessening a
disease mediated
by FXR in a patient.
[0053] In some
embodiments, the disease mediated by FXR is a cardiovascular and
cerebrovascular disease, a disease related to dyslipidemia, metabolic
syndrome, a
hyperproliferative disease, fibrosis, an inflammatory disease or a disease
related to liver and
gallbladder.
[0054] In one
aspect, provided herein is the compound described above for use in
preventing, managing, treating or lessening a disease mediated by FXR in a
patient.
[0055] In other
aspect, provided herein is a method of preparing, separating or purifying
the compound described above.
-11-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
[0056] The foregoing merely summarizes certain aspects disclosed herein and
is not
intended to be limiting in nature. These aspects and other aspects and
embodiments are described
more fully below.
DETAILED DESCRIPTION OF THE INVENTION
DEFINITIONS AND GENERAL TERMINOLOGY
[0057] Reference will now be made in detail to certain embodiments
disclosed herein,
examples of which are illustrated in the accompanying structures and formulas.
The present
invention is expected to cover all alternatives, variants and equivalents,
which may be included
within the scope of the invention as defined in claim. Those skilled in the
art will recognize
many methods and materials similar or equivalent to those described herein,
which may be
applied to the practice of the present invention. The present invention is by
no means limited to
the methods and materials described herein. There are a lot of literature and
similar substances
distinguished or inconsistent with the present invention, including but not
limited to the
definitions of terms, the use of terminology, described techniques, or the
like, this application
controls.
[0058] The present invention will apply the following definitions unless
otherwise
specified. For purposes of this invention, the chemical elements are defined
according to the
Periodic Table, CAS version and chemical manuals, 75, thEd, 1994.
Additionally, general
principles of organic chemistry are described in "Organic Chemistry", Thomas
Sorrell,
University Science Books, Sausalito: 1999, and Smith et al., and "March's
Advanced Organic
Chemistry", John Wiley & Sons, New York: 2007.
[0059] The term "comprise" is an open expression, it includes the contents
disclosed
herein, but don't exclude other contents.
[0060] As described herein, compounds disclosed herein may optionally be
substituted
with one or more substituents, such as are illustrated generally formula in
the invention, or as
exemplified by particular classes, subclasses, and species of the invention.
It will be appreciated
-12-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakcs Ref.: 75920/00027
that the phrase "optionally substituted" is used interchangeably with the
phrase "substituted or
unsubstituted". In general, the term "optionally" whether or not located
before the term
"substituted" refers to the replacement of one or more hydrogen radicals in a
given structure with
the radical of a specified substituent. Unless otherwise indicated, an
optionally substituted group
may have a substituent at each substitutable position of the group. When more
than one position
in a given structure can be substituted with more than one substituent
selected from a specified
group, the substituent may be either the same or different at each position.
Wherein the
substituents may be, but are not limited to H, F, Cl, Br, I, nitro, cyano, oxo
(=0), hydroxy, alkyl,
hydroxyalkyl, alkylamino, aminoalkyl, haloalkoxy, cycloalkyl, amino, aryl,
heterocyclyl,
heteroaryl, alkenyl, alkynyl, cycloalkyloxy, alkoxy, alkoxyalkyl, haloalkyl, -
COOH,
-alkyl ene-C(= 0)0 -alkyl, -alkylene-S(=0)2-alkyl, -alkylene-S(=0)2-amino, -
S(=0)2-alkyl,
-S(0)2-amino, -S (= 0)20H, -0-
alkylene-C(=0)0-alkyl, -0-alkylene-S(=0)2-alkyl,
-0-alkylene-S(=0)2-amino, -0-alkylene-S (= 0)20H, -C(=
0)NH2, -C(=0)NH -alkyl,
-C(.0)N(alkyl)-alkyl, -C(=0)NHS(=0)2-alkyl, -C(=0)NHS(.0)2-amino, -
C(.0)NHS(.0)20H,
-N(R1 )C(.0)NRI0R11, -0C(=0)R9, -
N(haloalkyl)-alkyl, -N(alkyl)-S(=0)2-alkyl,
-NHS(=0)2-alkyl, -NHS(=0)2-
haloalkyl, -N(alkyl)S(=0)2-haloalkyl,
-N(alkyl)S(=0)2-alkylamino, -NHC(=0)-alkyl, -NHC(=0)-haloalkyl, -N(alkyl)C(=0)-
haloalkyl,
-N(al kyl)C(= 0)-alkyl am i no, -N(alkyl)C(=0)0-alkyl , -NHC(=0)0-alkyl, -
NHC(=0)0-hal o al kyl,
-N(alkyl)C(=0)0-haloalky1, -
N(alkyl)C(=0)0-aminoalkyl, -NHC(=0)-NH2,
-NHC(=0)NH-(alkyl), -NHC (=0)NH(h aloalk yl), -NHC(.0)N(alkyl)-alkyl, -0C(=0)-
alkyl,
-0C(.0)-amino, -0C(=0)-alkylamino, -0C(=0)-
aminoalkyl, -0C(.0)-alkoxy,
-C(=0)N(alkyl)S(=0)2-alkyl, -C(=
0)N(alkyl)S (=0)2-amino, -C(=0)NH-S(.0)20H,
-C(=NH)NH2, -C(=NH)NH-alkyl, -
C(=NH)N(alkyl)-alkyl, -C(=N-alkyl)-NH2,
-C(=0)NH-alkylene-S(=0)20H, -
C(=0)NHC(=0)0H, -C(=0)NHC(=0)0-alkyl,
-C(.0)N(alkyl)C(=0)0-alkyl, -C(=0)NH-alkylene-C(=0)0H and
-C(=0)NH-alkylene-C(=0)0-alkyl, and the like, and wherein R9, R1 and R11 are
as defined
-13-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakcs Ref.: 75920/00027
herein.
[0061] The term
"alkyl" refers to a saturated linear or branched-chain monovalent
hydrocarbon radical of 1 to 20 carbon atoms, or 1 to 10 carbon atoms, or 1 to
6 carbon atoms, or
1 to 4 carbon atoms, or 1 to 3 carbon atoms, or 1 to 2 carbon atoms, wherein
the alkyl radical
may be optionally and independently substituted with one or more substituents
described herein.
Some non-limiting examples of the alkyl group include, methyl (Me, -CH3),
ethyl (Et,
-CH2CH3), n-propyl (n-Pr, -CH2CH2CH3), isopropyl (i-Pr, -CH(CH3)2), n-butyl (n-
Bu,
-CH2CH2CH2CH3), isobutyl (i-Bu, -CH2CH(CH3)2), sec-butyl (s-Bu, -
CH(CH3)CH2CH3),
tert-butyl (t-Bu, -C(CH3)3), n-pentyl (-CH2CH2CH2CH2CH3), 2-pentyl (-
CH(CH3)CH2CH2CF13),
3-pen tyl (-CH(CH2CH3)2), 2-methyl-2-butyl (-
C(CH3)2CH2CH3), 3-meth y1-2-but yl
(-CH(CH3)CH(CH3)2), 3-methyl-1-butyl (-
CH2CH2CH(CH3)2), 2-methyl-1-butyl
(-CH2CH(CH3)CH2CH3), n-hexyl (-CH2CH2CH2CH2CH2CH3), 2-hex yl
(-CH(CH3)CH2CH2CH2CH3), 3-hex yl (-CH(CH2CH3)(CH2CH2CH3)), 2-meth y1-2-pentyl
(-C(CH3)2CH2CH2CH3), 3-methyl-2-pentyl (-CH(CH3)CH(CH3)CH2CH3), 4-methyl-2-
pentyl
(-CH(CH3)CH2CH(CH3)2), 3-meth y1-3-pentyl (-
C(CH3)(CH2CH3)2), 2-meth y1-3-pentyl
(-CH(CH2CH3)CH(CH3)2), 2,3-dimethy1-2-butyl (-C(CH3)2CH(CH3)2), 3,3-dimethy1-2-
butyl
(-CH(CH3)C(CH3)3, n-heptyl and n-octyl, etc. The term "alkyl" or the prefix
"alk-" is inclusive
of both straight chain and branched saturated carbon chain. The term
"alkylene" used herein
refers to a saturated divalent hydrocarbon group derived from a straight or
branched chain
saturated hydrocarbon by the removal of two hydrogen atoms, such exaples
include, but are not
limited to methylene, ethylidene and isopropylidene, and the like.
[0062] The term
"alkenyl" refers to a linear or branched chain monovalent hydrocarbon
radical of 2 to 12 carbon atoms, or 2 to 8 carbon atoms, or 2 to 6 carbon
atoms, or 2 to 4 carbon
atoms, with at least one site of unsaturation, i.e., a carbon-carbon, sp2
double bond, wherein the
alkenyl radical may be independently and optionally substituted with one or
more substituents
described herein, and includes radicals having "cis" and "trans" orientations,
or alternatively, "E"
-14-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
and "Z" orientations. Examples include, but are not limited to, ethenyl or
vinyl (-CH=CH2), allyl
(-CH2CH=CH2), but-3-enyl (-CH2CH2CH=CH2), and the like.
[0063] The term "alkynyl" refers to a linear or branched chain monovalent
hydrocarbon
radical of 2 to 12 carbon atoms, or 2 to 8 carbon atoms, or 2 to 6 carbon
atoms, or 2 to 4 carbon
atoms, with at least one site of unsaturation, i.e., a carbon-carbon, sp
triple bond, wherein the
alkynyl radical is independently and optionally substituted with one or more
substituents
described herein. Examples include, but are not limited to, ethynyl (-CE-CH),
propargyl
(-CH2CECH), and the like.
[0064] The term "heteroatom" refers to one or more of 0, S, N, P and Si,
including any
oxidized form of C, N, S, or P; the quaternized form of any basic N; or a
substitutable nitrogen of
a heterocyclic ring, for example, N (as in 3,4-dihydro-2H-pyrroly1), NH (as in
pyrrolidinyl) or
NR (as in N-substituted pyrrolidinyl); or -CH2- of a heterocyclic ring is
oxidized to form -C(=0)-
form.
[0065] The term "halogen" or "halo" refers to fluoro (F), chloro (Cl),
bromo (Br), or iodo
(I).
[0066] The term "unsaturated" refers to a moiety having one or more units
of unsaturation.
[0067] The term "alkoxy" refers to an alkyl group, as defined herein,
attached to the
remaining part of the molecule through an oxygen atom. In some embodiments,
alkoxy is C1-4
alkoxy. Examples include, but are not limited to, methoxy, ethoxy, propoxy,
butoxy, and the like.
The alkoxy group may be optionally substituted with one or more substituents
disclosed herein.
[0068] The term "alkoxyalkyl" refers to an alkyl group substituted with one
or more
alkoxy, and alkoxy and alkyl are as defined herein. In some embodiments,
alkoxyalkyl is C1-6
alkoxy-C1_6 alkyl. In other embodiments, alkoxyalkyl is C1_3 alkoxy-C1_3
alkyl. And each said
alkoxyalkyl can be independently and optionally substituted with one or more
substituents
described herein.
[0069] The terms "haloalkyl", "haloalkenyl" or "haloalkoxy" refer to alkyl,
alkenyl, or
-15-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
alkoxy, as the case may be, substituted with one Of more halogen atoms. In
some embodiments,
haloalkyl is C1-6 haloalkyl. In other embodiments, haloalkyl is C1,3
haloalkyl. In some
embodiments, haloalkoxy is Ci_6 haloalkoxy. In other embodiments, haloalkoxy
is C1_3
haloalkoxy. Some non-limiting examples of "haloalkyl", "haloalkenyl" or
"haloalkoxy" groups
include trifluoromethyl, 2-chloro-vinyl, 2,2-difluoroethyl, trifluoromethoxy,
and the like. And
wherein optionally each of the haloalkyl, haloalkenyl or haloalkoxy may be
optionally
substituted with one or more substituents described herein.
[0070] The term "alkylamino" refers to "N-alkylamino" and "N,N-
dialkylamino", wherein
amino groups are independently substituted with one alkyl radical or two alkyl
radicals,
respectively. In some embodiments, the alkylamino group is C1_6 alkylamino or
a (C1,6 alkyl)
amino group. In other embodiments, the alkylamino group is C1,3 alkylamino or
a (C1,3 alkyl)
amino group. Some non-limiting examples of such group include, N-methylamino,
N-ethylamino, N,N-dimethylamino, N,N-diethylamino, and the like. And wherein
the alkylamino
radical is optionally substituted with one or more substituents described
herein.
[0071] The term "cycloalkyl" or "cycloalkane" refers to a monovalent or
multivalent
saturated ring having 3 to 12 carbon atoms as a monocyclic, bicyclic, or
tricyclic carbon ring
system, but not an aromatic ring. In some embodiments, the cycloalkyl group
contains 3 to 12
carbon atoms. In other embodiments, the cycloalkyl group contains 3 to 8
carbon atoms. In still
other embodiments, the cycloalkyl group contains 3 to 6 carbon atoms. Examples
include, but
are not limited to, cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, and the
like. The cycloalkyl
group may be optionally substituted with one or more substituents disclosed
herein.
[0072] The term "cycloalkyloxy" refers to a cycloalkyl attached to the rest
of the compound
molecule through an oxygen atom, and cycloalkyl is as defined herein.
[0073] The term "cycloalkylalkyl" refers to a cycloalkyl attached to the
rest of the compound
molecule through an alkyl, and cycloalkyl and alkyl are as defined herein.
[0074] The term "carbocycle", "carbocyclyl", or "carbocyclic ring" refers
to a monovalent
-16-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
or multivalent non-aromatic, saturated or partially unsaturated ring having 3
to 12 carbon atoms
as a monocyclic, bicyclic or tricyclic ring system. A carbobicyclic ring
system includes a Spiro
carbobicyclyl or a fused carbobicyclyl. In some embodiments, suitable
carbocyclyl groups
include 3-8 carbon atoms; in other embodiments, carbocyclyl groups include 3-6
carbon atoms.
Suitable carbocyclyl groups include, but are not limited to, cycloalkyl,
cycloalkenyl, and
cycloalkynyl. Further examples of carbocyclyl groups include cyclopropyl,
cyclobutyl,
cyclopentyl, 1 -cyclop ent-l-enyl, 1-
cyclopent-2-enyl, 1-cyclopent-3-enyl, cycloh exyl,
1-cyclohex-1-enyl, 1-cyclohex-2-enyl, 1-cyclohex-3-enyl, cyclohexadienyl,
cycloheptyl,
cyclooctyl, cyclononyl, cyclodecyl, cyclohendecyl, cyclododecyl, and the like.
The said
carbocyclyl groups may be independently unsubstituted or substituted by one or
more groups
disclosed herein.
[0075] The term
"heterocycle", "heterocyclyl", or "heterocyclic ring" as used
interchangeably herein refers to a saturated or partially unsaturated
monocyclic, bicyclic or
tricyclic ring containing 3-12 ring atoms, but not an aromatic ring, of which
at least one ring
atom is a heteroatom. Unless otherwise specified, the heterocyclyl group may
be carbon or
nitrogen linked, and the heteroatom is as defined herein. Some non-limiting
examples of the
heterocyclyl group include oxiranyl, azetidinyl, oxetanyl, thietanyl,
pyrrolidinyl, 2-pyrrolinyl,
3-pyrrolinyl, pyrazolinyl, pyrazolidinyl, imidazolinyl, imidazolidinyl,
tetrahydrofuranyl,
dihydrofuranyl, tetrahydrothienyl, dihydrothienyl, 1,3-dioxolanyl,
dithiolanyl, tetrahydropyranyl,
dihydropyranyl, 2H-pyranyl, 4H-pyranyl, tetrahydrothiopyranyl, piperidinyl,
morpholinyl,
thiomorpholinyl, piperazinyl, dioxanyl, dithianyl, thioxanyl, homopiperazinyl,
homopiperidinyl,
oxepanyl, thiepanyl, oxazepinyl, diazepinyl, thiazepinyl and 2-oxa-5-
azabicyclo[2.2.1]hept-5-yl.
Some non-limiting examples of heterocyclyl wherein -CH2- group is replaced by -
C(...0)- moiety
include 2-oxopyrrolidinyl, oxo-1,3-thiazolidinyl, 2-piperidinonyl, 3,5-
dioxopiperidinyl and
pyrimidinedionyl. Some non-limited examples of heterocyclyl wherein the ring
sulfur atom is
oxidized include sulfolanyl, 1,1-dioxo-thiomorpholinyl. The heterocyclyl group
may be
-17-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
optionally substituted with one or more substituents disclosed herein.
[0076] The term "heterocyclylalkyl" refers to a heterocyclyl attached to
the rest of the
compound molecule through an alkyl, and heterocyclyl and alkyl is as defined
herein.
[0077] The term "aryl" refers to monocyclic, bicyclic and tricyclic
carbocyclic ring systems
having a total of six to fourteen ring members, or six to twelve ring members,
or six to ten ring
members, wherein at least one ring in the system is aromatic, wherein each
ring in the system
contains 3 to 7 ring members, and that has a single point or multipoint of
attachment to the rest
of the molecule. The term "aryl" and "aromatic ring" can be used
interchangeably herein.
Examples of aryl ring may include phenyl, naphthyl and anthracene. The aryl
group may be
optionally and independently substituted with one or more substituents
disclosed herein.
[0078] The term "arylalkyl" refers to an alkyl group subustituted with one
or more aryl
groups, wherein the alkyl group and aryl group are as defined herein. Some non-
limiting
examples of the arylalkyl group include phenylmethyl, phenylethyl, and the
like.
[0079] The term "heteroaryl" refers to monocyclic, bicyclic and tricyclic
carbocyclic ring
systems having a total of five to twelve ring members, or five to ten ring
members, or five to six
ring members, wherein at least one ring system is an aromatic ring, and at
least one ring system
contains one or more hetero atoms, and wherein each ring in the system
contains 5 to 7 ring
members and that has a single point or multipoint of attachment to the rest of
the molecule. The
term "hetreroaryl" and "heteroaromatic ring" or "heteroaromatie compound" can
be used
interchangeably herein. The heteroaryl group is optionally substituted with
one or more
substituents disclosed herein. In one embodiment, a 5-10 membered heteroaryl
group comprises
1, 2, 3 or 4 heteroatoms independently selected from 0, S and N, wherein the
nitrogen atom can
be further oxidized.
[0080] Some non-limiting examples of heteroaryl rings include furanyl,
imidazolyl (e.g.,
N-imidazolyl, 2-imidazolyl, 4-imidazolyl, 5-imidazoly1), isoxazolyl, oxazolyl
(e.g. 2-oxazolyl,
4-oxazolyl, 5-oxazoly1), pyrrolyl (e.g., N-pyrrolyl, 2-pyrrolyl, 3-pyrroly1),
pyridyl, pyrimidinyl
-18-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
(e.g. 2-pyrimidinyl, 4-pyrimidinyl, 5-pyrimidinyl), pyridazinyl, thiazolyl
(e.g., 2-thiazolyl,
4-thiazolyl, 5-thiazoly1), tetrazolyl (e.g., 5-tetrazoly1), triazolyl, thienyl
(e.g., 2-thienyl, 3-
thienyl), pyrazolyl isothiazolyl, 1,2,3-oxadiazolyl, 1,2,5-oxadiazolyl, 1,2,4-
oxadiazolyl,
1,2,3-triazolyl, 1,2,3-thiadiazolyl, 1,3,4-thiadiazolyl, 1,2,5-
thiadiazolyl, pyrazinyl,
1,3,5-triazinyl, and the following bicycles: benzimidazolyl, benzofuryl,
benzothiophenyl, indolyl
(e.g., 2-indoly1), purinyl, quinolinyl (e.g., 2-quinolinyl, 3-quinolinyl, 4-
quinolinyl),
1,2,3,4-tetrahydroisoquinolinyl, 1,3-benzodioxolyl,
indolinyl, isoquinolinyl (e.g.,
1-isoquinolinyl, 3-isoquinolinyl or 4-
isoquinolinyl), imidazo[1,2-a]pyridyl,
pyrazolo[1,5-a]pyridyl, pyrazolo[1,5-a]pyrimidyl,
imidazo[1,2-b]pyridazinyl,
[1,2,4]triazolo[4,3-b]pyridazinyl,
[1,2,4]triazolo [1,5-a]pyrimidinyl, and
[1,2,4]triazolo[1,5-a]pyridyl, and the like.
[0081] The term
"aminoalkyl" refers to a C1_10 linear or branched-chain alkyl group
substituted with one or more amino groups. In some embodiments, the aminoalkyl
is a C1_6 alkyl
substituted with one or more amino groups. Some non-limiting examples of the
aminoalkyl
group include aminomethyl, aminoethyl, aminopropyl, aminobutyl and aminohexyl.
The
aminoalkyl group is optionally substituted with one or more substituents
described herein.
[0082] The term
"hydroxyalkyl" refers to an alkyl group substituted with one or more
hydroxy groups, wherein the alkyl group is as defined herein. Some non-
limiting examples of the
hydroxyalkyl group include hydroxymethyl, hydroxyethyl, and 1,2-dihydroxy-
ethyl, and the like.
[0083] The term
"halo-substituted aryl" refers to an aryl group substituted with one or more
identical or different halogen atoms, wherein halogen and aryl are as
described herein. Such
examples include, but are not limited to, fluorophenyl, difluorophenyl,
trifluorophenyl,
chlorophenyl, dichlorophenyl, trichlorophenyl, bromophenyl, tribromophenyl,
dibromophenyl,
chlorofluorophenyl, fluorobromophenyl, chlorobromophenyl, and the like. The
halogen-substituted aryl group is optionally substituted with one or more
substituents disclosed
herein.
-19-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
[0084] The term "alkylene" refers to a saturated divalent hydrocarbon group
derived from a
straight or branched chain saturated hydrocarbon by the removal of two
hydrogen atoms. Unless
otherwise specified, the alkylene group contains 1-12 carbon atoms. Wherein
the alkylene group
is optionally substituted with one or more substituents described herein. In
some embodiments,
the alkylene group contains 1-6 carbon atoms. In other embodiments, the
alkylene group
contains 1-4 carbon atoms. In still other embodiments, the alkylene group
contains 1-3 carbon
atoms. In yet other embodiments, the alkylene group contains 1-2 carbon atoms.
And alkylene
group is exemplified by methylene (-CH2-), ethylene (-CH2CH2-), isopropylene
(-CH(CH3)CH2-), and the like.
[0085] The term "alkenylene" refers to an unsaturated divalent hydrocarbon
group derived
from an alkylene group by the removal of two hydrogen atoms. Unless otherwise
specified, the
alkenylene group contains 1-12 carbon atoms. Wherein the alkenylene group is
optionally
substituted with one or more substituents described herein. In some
embodiments, the alkenylene
group contains 1-6 carbon atoms. In other embodiments, the alkenylene group
contains 1-4
carbon atoms. In still other embodiments, the alkenylene group contains 1-3
carbon atoms. In yet
other embodiments, the alkenylene group contains 1-2 carbon atoms. Some non-
limiting
examples of the alkenylene group include ethenylene (-CH=CH-), propenylene (-
CH2CH=CH-),
and the like.
[0086] The term "alkynylene" refers to a linear or branched divalent
hydrocarbon radical of
2 to 12 carbon atoms with at least one site of unsaturation, i.e., a carbon-
carbon, sp triple bond,
wherein the alkynylene radical may be optionally substituted with one or more
substituents
described herein. In some embodiments, the alkynylen contains 2 to 8 carbon
atoms. In other
embodiments, the alkynylene contains 2 to 6 carbon atoms. In still other
embodiments, the
alkynylene contains 2 to 4 carbon atoms. Examples of such groups include, but
are not limited
to, ethynylene (-C-C-), and the like.
[0087] As described herein, substituents connected to the center position
of the ring through
-20-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
a bond represent that the ring to which substituents connected may be
substituted in any
reasonable and connectable position. For example, formula (a) represents ring
E can be
substituted with one or more R substituents in any reasonable and
substitutable position.
(R 0)_I)
P
A (a)
[0088] Furthermore, unless otherwise stated, the phrase "each... is
independently" is used
interchangeably with the phrase "each (of)...and...is independently". It
should be understood
broadly that the specific options expressed by the same symbol are independent
of each other in
different radicals; or the specific options expressed by the same symbol are
independent of each
other in same radicals.
[0089] Unless otherwise stated, structures depicted herein are also meant
to include all
isomeric (e.g., enantiomeric, diastereomeric, geometric or conformational)
forms of the
structure; for example, the R and S configurations for each asymmetric center,
(Z) and (E) double
bond isomers, and (Z) and (E) conformational isomers. Therefore, single
stereochemical isomers
as well as enantiomeric, diastereomeric, geometric, or conformational mixtures
of the present
compounds are within the scope disclosed herein.
[0090] Unless otherwise stated, structures and compounds depicted herein
are also meant
to include all isomeric (e.g., enantiomeric, diastereomeric, geometric or
conformational) forms,
N-oxide, hydrate, solvate, metabolite, pharmaceutically acceptable salt or a
prodrug thereof.
Therefore, single stereochemical isomers as well as enantiomer, diastereomer,
geometric isomer,
conformer, N-oxide, anhydrate, solvate, metabolite, pharmaceutically
acceptable salt or prodrug
of the compounds disclosed herein are within the scope of the present
invention. Additionally,
unless otherwise stated, structures depicted herein are also meant to include
compounds that
differ only in the presence of one or more isotopically enriched atoms.
[0091] A "metabolite" refers to a product produced through metabolism in
the body of a
specified compound, a pharmaceutically acceptable salt, analog or derivative,
which exhibits
-21-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
similar activity of the compound of Formula (I) in vivo or in vitro. The
metabolites of a
compound may be identified using routine techniques known in the art and their
activities can be
determined using tests such as those described herein. Such products may
result for example
from oxidation, reduction, hydrolysis, amidation, deamidation, esterification,
deesterification, or
enzyme cleavage, and the like, of the administered compound. Accordingly, the
invention
includes metabolites of the compounds disclosed herein, including metabolites
produced by
contacting a compound disclosed herein with a mammal for a sufficient time
period.
[0092]
Stereochemical definitions and conventions used herein generally follow S. P.
Parker, Ed., McGraw-Hill Dictionary of Chemical Terms (1984) McGraw-Hill Book
Company,
New York; and Eliel, E. and Wilen, S., "Stereochemistry of Organic Compounds",
John
Wiley&Sons, Inc., New York, 1994. The compounds disclosed herein may contain
asymmetric
or chiral centers, and therefore exist in different stereoisomeric forms. It
is intended that all
stereoisomeric forms of the compounds disclosed herein, including, but not
limited to,
diastereomers, enantiomers and atropisomers, as well as mixtures thereof such
as racemic
mixtures, form part of the present invention. Many organic compounds exist in
optically active
forms, i.e., they have the ability to rotate the plane of plane-polarized
light. In describing an
optically active compound, the prefixes D and L, or R and S, are used to
denote the absolute
configuration of the molecule about its chiral center(s). The prefixes d and 1
or (+) and (-) are
employed to designate the sign of rotation of plane-polarized light by the
compound, with (-) or 1
meaning that the compound is levorotatory. A compound prefixed with (+) or d
is dextrorotatory.
For a given chemical structure, these stereoisomers are identical except that
they are mirror
images of one another. A specific stereoisomer may also be referred to as an
enantiomer, and a
mixture of such isomers is often called an enantiomeric mixture. A 50:50
mixture of enantiomers
is referred to as a racemic mixture or a racemate, which may occur where there
has been no
stereoselection or stereospecificity in a chemical reaction or process. The
term "racemic mixture"
or "racemate" refers to an equimolar mixture of two enantiomeric species,
devoid of optical
-22-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
activity.
[0093] The term "tautomer" or "tautomeric form" refers to structural
isomers of different
energies which are interconvertible via a low energy barrier. Some non-
limiting examples of
proton tautomers (also known as prototropic tautomers) include
interconversions via migration
of a proton, such as keto-enol and imine-enamine isomerizations. Valence
tautomers include
interconversions by reorganization of some of the bonding electrons.
[0094] A "pharmaceutically acceptable salts" refers to organic or inorganic
salts of a
compound disclosed herein. Pharmaceutically acceptable salts are well known in
the art. For
example, Berge et al., describe pharmaceutically acceptable salts in detail in
J. Pharmacol Sci,
1977, 66:1-19. Some non-limiting examples of pharmaceutically acceptable and
nontoxic salts
include salts of an amino group formed with inorganic acids such as
hydrochloric acid,
hydrobromic acid, phosphoric acid, sulfuric acid and perchloric acid or with
organic acids such
as acetic acid, oxalic acid, maleic acid, tartaric acid, citric acid, succinic
acid and malonic acid or
by using other methods used in the art such as ion exchange. Other
pharmaceutically acceptable
salts include adip ate, malate, 2-hydroxy propionate, alginate, ascorb ate,
aspartate,
benzenesulfonate, benzoate, bisulfate, borate, butyrate, camphorate,
camphorsulfonate,
cyclopentanepropionate, digluconate, dodecylsulfate, ethanesulfonate, formate,
fumarate,
glucoheptonate, glycerophosphate, gluconate, hemisulfate, heptanoate,
hexanoate, hydroiodide,
2-hydroxy-ethanesulfonate, lactobionate, lactate, laurate, laurylsulfate,
malate, methanesulfonate,
2-naphthalenesulfonate, nicotinate, nitrate, oleate, palmitate, pamoate,
pectinate, persulfate,
3-phenylpropionate, picrate, pivalate, propionate, stearate, thiocyanate, p-
toluenesulfonate,
undecanoate, valerate salts, and the like. Salts derived from appropriate
bases include alkali
metal, alkaline earth metal, ammonium and N+(C1_4 alky1)4 salts. This
invention also envisions
the quaternization of any basic nitrogen-containing groups of the compounds
disclosed herein.
Water or oil soluble or dispersable products may be obtained by such
quaternization.
Representative alkali or alkaline earth metal (formed) salts include sodium,
lithium, potassium,
-23-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
calcium, magnesium, and the like. Further pharmaceutically acceptable salts
include, when
appropriate, nontoxic ammonium, quaternary ammonium, and amine cations formed
using
counterions such as halide, hydroxide, carboxylate, sulfate, phosphate,
nitrate, C1_8 sulfonate or
aryl sulfonate.
[0095] The term "hydrate" refers to the complex where the solvent molecule
is water.
[0096] The term "solvate" refers to an association or complex of one or
more solvent
molecules and a compound disclosed herein. Some non-limiting examples of the
solvent that
form solvates include water, isopropanol, ethanol, methanol, dimethylsulfoxide
(DMSO), ethyl
acetate, acetic acid and ethanolamine.
[0097] An "ester" refers to an in vivo hydrolysable ester of a compound of
the Formula (1)
containing hydroxy group, for example, a pharmaceutically acceptable ester
which is hydrolysed
in the human or animal body to produce the parent alcohol. Some non-limiting
examples of in
vivo hydrolysable ester forming groups of a compound of the Formula (I)
containing hydroxy
group include phosphate, acetoxymethoxy, 2,2-dimethylpropionyloxymethoxy,
alkanoyl,
benzoyl, phenylacetyl, alkoxycarbonyl,
dialkylcarbamoyl,
N-(dialkylaminoethyl)-N-alkylcarbamoyl, and the like.
[0098] An "N-oxide" refers to one or more than one nitrogen atoms oxidised
to form
N-oxides, where a compound contains several amine functions. Particular
examples of N-oxides
are the N-oxides of a tertiary amine or a nitrogen atom of a nitrogen-
containing heterocycle.
N-oxides can be formed by treatment of the corresponding amine with an
oxidizing agent such as
hydrogen peroxide or a per-acid (e.g. a peroxycarboxylic acid) (See, Advanced
Organic
Chemistiy, by Jerry March, 4th Edition, Wiley Interscience, pages). More
particularly, N-oxides
can be made by the procedure of L. W. Deady (Syn. Comm. 1977, 7, 509-514), in
which the
amine compound is reacted with m-chloroperoxybenzoic acid (MCPBA), for
example, in an inert
solvent such as dichloromethane.
[0099] The term "prodrug" refers to a compound that is transformed in vivo
into a
-24-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
compound of Formula (I). Such a transformation can be affected, for example,
by hydrolysis of
the prodrug form in blood or enzymatic transformation to the parent form in
blood or tissue.
Prodrugs of the compounds disclosed herein may be, for example, esters. Some
common esters
which have been utilized as prodrugs are phenyl esters, aliphatic (C1-C24)
esters, acyloxymethyl
esters, carbonates, carbamates and amino acid esters. For example, a compound
disclosed herein
that contains a hydroxy group may be acylated at this position in its prodrug
form. Other prodrug
forms include phosphates, such as, those phosphate compounds derived from the
phosphonation
of a hydroxy group on the parent compound. A thorough discussion of prodrugs
is provided in T.
Higuchi and V. Stella, Pro-drugs as Novel Delivery Systems, Vol. 14 of the
A.C.S. Symposium
Series, Edward B. Roche, ed., Bioreversible Carriers in Drug Design, American
Pharmaceutical
Association and Pergamon Press, 1987, J. Rautio et al., Prodrugs: Design and
Clinical
Applications, Nature Review Drug Discovery, 2008, 7, 255-270, and S. J. Hecker
et al, Prodrugs
of Phosphates and Phosphonates, Journal of Medicinal Chemistry, 2008, 51, 2328-
2345.
[001001 The term
"protecting group" or "Pg" refers to a substituent that is commonly
employed to block or protect a particular functionality while reacting with
other functional
groups on the compound. For example, an "amino-protecting group" is a
substituent attached to
an amino group that blocks or protects the amino functionality in the
compound. Suitable
amino-protecting groups include acetyl, trifluoroacetyl, t-butoxy-carbonyl
(BOC),
benzyloxycarbonyl (CBZ) and 9- fluorenylmethylenoxy-carbonyl (Fmoc), and the
like.
Similarly, a "hydroxy-protecting group" refers to a substituent of a hydroxy
group that blocks or
protects the hydroxy functionality. Suitable protecting groups include methyl,
methoxymethyl,
acetyl and shy!, and the like. A "carboxy-protecting group" refers to a
substituent of the carboxy
group that blocks or protects the carboxy functionality. Common carboxy-
protecting groups
include -CH2CH2S02Ph, cyanoethyl, 2-(trimethylsilyl)ethyl, 2-
(trimethylsilyl)ethoxy-methyl,
2-(p-toluenesulfonyl)ethyl, 2-(p-
nitrophenylsulfonyl)ethyl, 2-(diphenylphosphino)ethyl,
nitroethyl, and the like. For a general description of protecting groups and
their use, see: T W.
-25-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
Greene, Protective Groups in Organic Synthesis, John Wiley&Sons, New York,
1991; and P. J.
Kocienski, Protecting Groups, Thieme, Stuttgart, 2005.
[00101] As used herein, the term "therapeutically effective amount" refers
to an amount of a
compound of the Formula (1) which is sufficient to obtain the desired
therapeutic effect. Thus, a
therapeutically effective amount of a compound of the Formula (I) for the
treatment of
conditions mediated by FXR is sufficient to treat conditions mediated by FXR.
[00102] As used herein, the term "dyslipidemia" refers to an abnormality of
lipids and
lipoproteins, or abnormal amounts of lipids and lipoproteins in the blood, as
well as these
diseases generated, caused, exacerbated or accompanied by such abnormalities
(See Dorland's
Illutrated Medical Dictionary, 29th edition, W.B. Saunders Publishing Company,
New York,
NY). These diseases encompassed within the definition of dyslipidemia include
hyperlipidemia,
hypertriglyceridemia, low plasma HDL, high plasma LDL, high plasma VLDL, liver
cholestasis,
and hypercholesterolemi a.
[00103] As used herein, the term "diseases related to dyslipidemia"
include, but are not
limited to, atherosclerosis, thrombosis, coronary artery disease, stroke and
hypertension disease.
And diseases related to dyslipidemia also include metabolic diseases such as
obesity, diabetes,
insulin resistance and complications thereof. The term "cholestasis" refers to
any condition
caused by that the flow of bile from the liver is blocked, which may be
intrahepatic (i.e.,
occurring inside the liver) or extrahepatic (i.e., occurring outside the
liver).
[00104] As used herein, the term "liver fibrosis" includes liver fibrosis
caused by any
reason, including but not limited to virus-induced liver fibrosis, such as
liver fibrosis caused by
the hepatitis B and hepatitis C; liver fibrosis caused by contacting with
alcohol (alcoholic liver
disease), pharmaceutical compounds, oxidative stress, cancer radiation or
industrial chemicals;
and liver fibrosis caused by primary biliary cirrhosis, fatty liver, obesity,
non-alcoholic
steatohepatitis, cystic fibrosis, hemochromatosis, and autoimmune hepatitis
and other diseases.
[00105] As used herein, the term "non-alcoholic fatty liver disease
(NAFLD)" refers to a
-26-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
metabolic disease associated with insulin resistance, including simple fatty
liver (SFL),
non-alcoholic steatohepatitis (NASH), steatohepatic fibrosis and liver
cirrhosis.
[00106] As used herein, "FXR modulators" refers to a substance that
directly binds to the
FXR and regulates activity of the FXR, including FXR agonists, FXR partial
agonists and FXR
antagonists.
[00107] As used herein, the term "FXR agonist" refers to a substance that
directly binds to
the FXR and upregulates activity of the FXR.
[00108] Unless otherwise indicated herein or clearly contradicted by the
context, the terms
"a", "an", "the4 and similar terms used in the context of the present
invention (particularly in the
context of the claims) are to be construed to cover both the singular and the
plural.
DESCRIPTION OF COMPOUNDS OF THE INVENTION
[00109] The present invention relates to compounds, or pharmaceutical
compositions
thereof, which bind to FXRand act as modulators of the FXR). The present
invention further
relates to said compounds or the use thereof in the manufacture of a
medicament for the
treatment of diseases and/or conditions through said compounds binding to the
FXR nuclear
receptor disclosed herein. The present invention further describes a method
for the synthesis of
the compounds. The compounds of the invention exhibit improved biological
activity and
pharmacokinetic advantages.
[00110] The invention relates to a compound having formula (I), or a
stereoisomer, a
geometric isomer, a tautomer, an N-oxide, a hydrate, a solvate, a metabolite,
a pharmaceutically
acceptable salt or a prodrug thereof,
OR%
Ra
(RI
/ 0 Z
¨ (I),
wherein:
-27-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
[00111] T is -NH-, -0-, -S-, -C(=0)- or -CH2-;
[00112] each of X and Y is independently a bond, -0-, -S(=0)t-, -NR'-, -
CRYRz- or
or -X-Y- is -CHRh-CHRk- or -CRY=CRz-;
[00113] each Rx is independently hydrogen, deuterium, alkyl, aminoalkyl,
haloalkyl,
cycloalkyl, heterocyclyl, aryl, halo-substituted aryl or arylalkyl;
[00114] each RY and le is independently hydrogen, deuterium, F, Cl, Br, I,
hydroxy, amino,
nitro, cyano, alkyl, haloalkyl, hydroxyalkyl, aminoalkyl, alkoxy, alkylamino,
alkenyl, alkynyl,
cycloalkyl, heterocyclyl, aryl, halo-substituted aryl or arylalkyl; or RY and
le together with the
same carbon atom to which they are attached, independently and optionally form
a cycloalkane
ring or heterocyclic ring, and wherein the cycloalkane ring and heterocyclic
ring is independently
and optionally substituted with substituents selected from F, Cl, Br, I,
hydroxy, amino, nitro,
cyano, methyl, ethyl, isopropyl and trifluoromethyl;
[00115] each of Rh and Rk is independently hydrogen, deuterium, F, Cl, Br,
I, hydroxy,
amino, nitro, cyano, methyl, ethyl, isopropyl or trifluoromethyl; or Rh and
Rk, together with the
carbon atoms to which they are attached, form a cycloalkane ring or
heterocyclic ring, wherein
the cycloalkane ring and heterocyclic ring is independently and optionally
substituted with
substituents selected from F, Cl, Br, I, hydroxy, amino, nitro, cyano, methyl,
ethyl, isopropyl and
trifluoromethyl;
[00116] each of le and Rb is independently hydrogen, deuterium or C1_3
alkyl;
[00117] each Re is independently hydrogen, deuterium, F, Cl, Br, I, oxo,
hydroxy, amino,
nitro, cyano, methyl, ethyl, n-propyl, isopropyl, difluoromethyl,
trifluoromethyl,
difluoromethoxy, trifluoromethoxy, hydroxymethyl, aminomethyl, methylamino,
dimethylamino,
methoxymethyl, isopropoxymethyl, vinyl, ethynyl, cyclopropyl, cyclobutyl,
cyclopentyl,
cyclohexyl, azetidinyl, tetrahydrofuranyl,
pyrrolidinyl, piperidinyl, piperazinyl,
tetrahydropyranyl, morpholinyl, thiomorpholinyl, phenyl, thiazolyl, thienyl,
oxazolyl, triazolyl,
tetrazolyl, pyridinyl, pyrimidinyl, -COOH, -C(.0)0-C1_3 alkyl, -C(=0)NHS(=-0)2-
C1_3 alkyl,
-28-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
-C(=0)NHS(=0)2-phenyl, -C(=0)NH-C1_3 alkylene-
S(.0)20H, -C(=0)NH-C1_3
alky1ene-C(.0)0H, -S(=0)2NH2, -S(=0)20H, -S(=0)2-C1_3 alkyl, -C(=0)NH2 or
[00118] each R1
is independently hydrogen, deuterium, F, Cl, Br, I, hydroxy, amino, nitro,
cyano, alkyl, haloalkyl, hydroxyalkyl, aminoalkyl, alkoxy, alkylamino,
haloalkoxy, alkoxyalkyl,
alkenyl, alkynyl, cycloalkyl, cydoalkoxy, cycloalkylalkyl, heterocyclyl,
heterocyclylalkyl, aryl,
halo-substituted aryl, arylalkyl, heteroaryl, -L1-C(=0)0R8, -L1-S(=0),R9, -0-
L2-C(=0)0R8,
-0-L2-S(=.0)tR9, -C(.0)NR10R11,
-C(.0)N(R10)S(.0)2R9, -
C(=NR10)NR10R11,
-C(=0)N(R10)-L3-S(=0)20R8, -C(=0)N(R1 )C(=0)0R8 or -C(=0)N(R1 )-L3-C(=0)0R8;
or two
adjacent R1, together with the ring atoms to which they are attached,
independently and
optionally form a carbocyclic ring, heterocyclic ring, aromatic ring or
heteroaromatic ring, and
wherein each R1 is independently and optionally substituted with one or more
R12;
[00119] each R8
is independently hydrogen, deuterium, alkyl, aminoalkyl, alkenyl, alkynyl,
haloalkyl, cycloalkyl, heterocyclyl or aryl;
[00120] each R9
is independently hydrogen, deuterium, hydroxy, amino, alkyl, aminoalkyl,
alkenyl, alkynyl, haloalkyl, cycloalkyl, heterocyclyl, aryl or -NR10R11;
[00121] each R1
and R11 is independently hydrogen, deuterium, alkyl, aminoalkyl, alkenyl,
alkynyl, haloalkyl, cycloalkyl, heterocyclyl or aryl; or R1 and R11, together
with the nitrogen
atom to which they are attached, independently and optionally form a
heterocyclic ring or
heteroaromatic ring;
[00122] each R12
is independently hydrogen, deuterium, F, Cl, Br, I, oxo, hydroxy, amino,
nitro, cyano, alkyl, haloalkyl, alkoxy, alkylamino, alkenyl, alkynyl,
cycloalkyl, heterocyclyl, aryl
or heteroaryl;
[00123] each L1
is independently a bond, -NH-, -C(=0)-, C1_3 alkylene, C2-4 alkenylene or
C2_4 alkynylene;
[00124] each 1,2 is independently C1_3 alkylene, C2_4 alkenylene or C2_4
alkynylene;
-29-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
[00125] each L3 is independently a bond or C1_3 alkylene;
R3 R3 Ra R3
R2 R4 R2 ail R4 Fe iiii R4 R2 R4
101 11111 * .
L Rs L Rs L µ1111P1 R5 L IR'
Re R6 R6 s_ R6
0 µ s
Z is R' , R7 , R7 RI
R3 R3 R-1- R3 R3
R2 R4 R7 irb 1R4 R7x1,-.õ, R4 R2 R"' R2 Frt
I .V SI RI' L MP RS L ---K-R-5 L
L R'
S----(N'N Rti , rZi ..õ, R6 NII-N R6 isil\N R8 /
1+j R4
N i 0
R7 R7 R7 , R/ R7 or
, , ,
W
R2 R4
R6
1"--c-}\¨N
INI¨ti
/
R7
'
/
[00126] each L is independently a bond, -0-, -S-, -NH-, -CH2-, -CH2-CH2-, -
0-CH2-,
-0-CH2-CH2- or -CH2-0-CH2-;
[00127] each R2, R3, R4, R5 and R6 is independently hydrogen, deuterium, F,
Cl, Br, I,
hydroxy, amino, nitro, cyano, C1_3 alkyl, C1_3 haloalkyl, Ci_3 alkoxy or C1_3
haloalkoxy;
[00128] each R Is independently hydrogen, deuterium, F, CI, Br, I, hydroxy,
amino, nitro,
cyano, C1-6 alkyl, C1_6 hydroxyalkyl, Ci_6 haloalkyl, C1_6 alkoxy, C1_6
alkylamino, C1-6
alkoxy-C1_6 alkyl, C3_6 cycloalkyl or C2_6 heterocyclyl, and wherein the C3_6
cycloalkyl and C2-6
heterocyclyl is independently and optionally substituted with subsitituents
selected from F, Cl,
Br, I, hydroxy, amino, nitro and cyano;
[00129] m is 0, 1, 2, 3 or 4;
[00130] n is 0, 1 or 2; and
-30-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
[00131] each t is independently 0, 1 or 2;
[00132] with the proviso that a compound having formula (I) is not:
0 = .
HO HO =
N
11
PO
1 0
o
ci a y
HO 01 1
N \ Nr
N 0
7 7
0 \s4P
110
Ntl CI I cP µ1.1 CI CI
N a µ ..' f 0 \ N
N 0
'V
<1
1-0 0 õ r?
,, 0
ss." 01 c4
0* lio-kijors1411
N ' 0
' 7
0 0
CI 4. CI 1 CI
HO HO
al? 4 N11
N * µ I \ /
NA 0
. ,
N 771,4
4 HO
0 * 0
Ci * 1 a 1
HO HO
...ib C...... C/1.:1'01
+7. HO
oqi e ti"
6
0 <
, ' ,
o
ei CI
HO
.7.
/ \
_ N
and .
-31-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
[00133] In some embodiments, each le is independently hydrogen, deuterium,
Ci_6 alkyl,
Ci_6 aminoalkyl, C1_6 haloalkyl, C3-6 cycloalkyl, C2_6 heterocyclyl, phenyl,
halo-substituted
phenyl or benzyl.
[00134] In other embodiments, each le is independently hydrogen, deuterium,
methyl,
ethyl, isopropyl, aminomethyl, difluoromethyl, trifluoromethyl, cyclopropyl,
cyclohexyl,
tetrahydropyranyl, piperidinyl, phenyl, halo-substituted phenyl or benzyl.
[00135] In some embodiments, each Rand le is independently hydrogen,
deuterium, F, Cl,
Br, I, hydroxy, amino, nitro, cyano, C1_6 alkyl, C1_6 haloalkyl, C1_6
hydroxyalkyl, Ci_6 aminoalkyl,
C1_6alkoxy, C1_6 alkylamino, C2_6 alkenyl, C2_6 alkynyl, C3_6 cycloalkyl, C2_6
heterocyclyl, phenyl,
halo-substituted phenyl or benzyl; or RY and le together with the same carbon
atom to which
they are attached, independently and optionally form a C3_6cycloalkane ring or
C2_6 heterocyclic
ring, and wherein the C3_6 cycloalkane ring and C2_6 heterocyclic ring is
independently and
optionally substituted with substituents selected from F, Cl, Br, I, hydroxy,
amino, nitro, cyano,
methyl, ethyl, isopropyl and trifluoromethyl.
[00136] In other embodiments, each RY and le is independently hydrogen,
deuterium, F, Cl,
Br, I, hydroxy, amino, nitro, cyano, C1_3 alkyl, C1.3 haloalkyl, C1_3
hydroxyalkyl, C1_3 aminoalkyl,
C1_3 alkoxy, C1_3 alkylamino, C2-4 alkenyl, C2_4 alkynyl, C3_6 cycloalkyl,
C2_6 heterocyclyl,
phenyl, halo-substituted phenyl or benzyl; or RY and le together with the same
carbon atom to
which they are attached, independently and optionally form a C3_6 cycloalkane
ring or C2-6
heterocyclic ring, and wherein the C3_6 cycloalkane ring and C2-6 heterocyclic
ring is
independently and optionally substituted with substituents selected from F,
Cl, Br, I, hydroxy,
amino, nitro, cyano, methyl, ethyl, isopropyl and trifluoromethyl.
[00137] In other embodiments, each RY and le is independently hydrogen,
deuterium, F, Cl,
Br, I, hydroxy, amino, nitro, cyano, methyl, ethyl, n-propyl, isopropyl,
difluoromethyl,
trifluoromethyl, 2,2-difluoroethyl, 2,2,2-trifluoroethyl, hydroxymethyl, 2 -h
ydrox yethyl,
aminomethyl, methoxy, ethoxy, isopropoxy, t-butoxy, methylamino,
dimethylamino, vinyl,
-32-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
ethynyl, cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, azetidinyl,
tetrahydrofuranyl,
pyrrolidinyl, piperidinyl, piperazinyl, tetrahydropyranyl, morpholinyl,
thiomorpholinyl, phenyl,
halo-substituted phenyl or benzyl; or RY and Rz, together with the same carbon
atom to which
they are attached, independently and optionally form cyclopropane,
cyclobutane, cyclopentane,
cyclohexane, azetidine, tetrahydrofuran, pyrrolidine, piperidine, piperazine,
tetrahydropyran,
morpholine or thiomorpholine, and wherein the cyclopropane, cyclobutane,
cyclopentane,
cyclohexane, azetidine, tetrahydrofuran, pyrrolidine, piperidine, piperazine,
tetrahydropyran,
morpholine and thiomorpholine is independently and optionally substituted with
substituents
selected from F, Cl, Br, I, hydroxy, amino, nitro, cyano, methyl, ethyl,
isopropyl and
trifluoromethyl.
[00138] In some
embodiments, each of Rh and Rk is independently methyl, ethyl, isopropyl
or trifluoromethyl; or Rh and Rk together with the carbon atoms to which they
are attached, form
a C3_6 cycloalkane ring or C2_6 heterocyclic ring, and wherein the C3_6
cycloalkane ring and C2_6
heterocyclic ring is independently and optionally substituted with
substituents selected from F,
Cl, Br, I, hydroxy, amino, nitro, cyano, methyl, ethyl, isopropyl and
trifluoromethyl.
[00139] In other
embodiments, each of Rh and Rk is independently methyl, ethyl, isopropyl
or trifluoromethyl; or Rh and Rk together with the carbon atoms to which they
are attached, form
cyclopropylene, cyclobutylene, cyclopentylene,
cyclohexylene, azetidin ylene,
tetrahydrofuranylene, pyrrolidinylene, piperidinylene, piperazinylene,
tetrahydropyranylene,
morpholinylene or thiomorpholinylene, and wherein the cyclopropylene,
cyclobutylene,
cyclopentylene, cyclo hex ylene, azetidinylene,
tetrahydrofuranylene, pyrrolidinylene,
piperidinylene, piperazinylene, tetrahydropyranylene, morpholinylene and
thiomorpholinylene is
independently and optionally substituted with substituents selected from F,
Cl, Br, I, hydroxy,
amino, nitro, cyano, methyl, ethyl, isopropyl and trifluoromethyl.
[00140] In some
embodiments, each R1 is independently hydrogen, deuterium, F, Cl, Br, I,
hydroxy, amino, nitro, cyano, C1_6 alkyl, C1_6 haloalkyl, C1_6 hydroxyalkyl,
C1_6 aminoalkyl, C1_6
-33-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
alkoxy, C1-6 alkylamino, C1_6 haloalkoxy, C1_6 alkoxy-Ci_3 alkyl, C2_6
alkenyl, C2-6 alkynyl, C3-6
cycloalkyl, C3_6 cycloalkoxy, C3_6 cycloalkyl-C1_3 alkyl, C2_6 heterocyclyl,
C2-6 heterocyclyl-C1_3
alkyl, phenyl, halo-substituted phenyl, benzyl, C1_5 heteroaryl, -L1-C(=0)0R8,
-L1-S(=0)1R9,
-0-L2-C(=0)0R8, -0-L2-S(=0)tle, -C(=0)NR10R11,
-C(=0)N(R10)S(=0)2R9,
_c(=NRio)NR
-C(=0)N(R10)-L3-S(.0)20R8, -C(.0)N(R10)C(.0)0R8 or
-C(=0)N(R10)-L3-C(=0)0R8; or two adjacent R1, together with the ring atoms to
which they are
attached, independently and optionally form a C36 carbocyclic ring, C2_6
heterocyclic ring,
benzene ring or C1-5 heteroaromatic ring, and wherein each R1 is independently
and optionally
substituted with one or more R12;
[00141] wherein, each L1, L2, L3, t, R8, R9, ¨10, R1--1 and R12 is as
defined herein.
[00142] In other
embodiments, each R1 is independently hydrogen, deuterium, F, Cl, Br, I,
hydroxy, amino, nitro, cyano, C1_3 alkyl, C1_3 haloalkyl, C1_3 hydroxyalkyl,
C1_3 aminoalkyl, C1_3
alkoxy, C1_3 alkylamino, C1_3 haloalkoxy, Ci_3 alkoxy-C1_3 alkyl, C2_4
alkenyl, C2_4 alkynyl, C3-6
cycloalkyl, C3-6 cycloalkoxy, C3-6 cycloalkyl-C1_3 alkyl, C2-6 heterocyclyl,
C2-6 heterocyclyl-Ci-3
alkyl, phenyl, halo-substituted phenyl, benzyl, Cis heteroaryl, -L1-C(=0)0R8, -
L1-S(.0),119,
-0-L2-C(=0)0R8, -C(=0)NR
1 OR11, -C(=0)N(R10)S(=0)2R9,
_q_NR10)NR10Ri1
,
-C(=0)N(R10)-L3-S(=0)20R8, -C(=0)N(R10)C(=0)0R8 or
-C(=0)N(R10)-L3-C(=0)0R8; or two adjacent R1, together with the ring atoms to
which they are
attached, independently and optionally form a C3_6 carbocyclic ring, C2_6
heterocyclic ring,
benzene ring or C1_5 heteroaromatic ring, and wherein each said R1 is
independently and
optionally substituted with one or more R12;
[00143] wherein, each L1, L2, L3, t, R8, R9, Rio, Rti and R'2
is as defined herein.
[00144] In other
embodiments, each R1 is independently hydrogen, deuterium, F, Cl, Br, I,
hydroxy, amino, nitro, cyano, methyl, ethyl, n-propyl, isopropyl,
difluoromethyl, trifluoromethyl,
difluoromethoxy, trifluoromethoxy, hydroxymethyl, aminomethyl, methylamino,
dimethylamino,
methoxylmethyl, isopropoxylmethyl, ethenyl, ethynyl, cyclopropyl, cyclobutyl,
cyclopentyl,
-34-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakcs Ref.: 75920/00027
cyclohexyl, azetidinyl, tetrahydrofuranyl,
pyrrolidinyl, piperidinyl, piper azinyl,
tetrahydropyranyl, morpholinyl, thiomorpholinyl, phenyl, thiazolyl, thienyl,
oxazolyl, triazolyl,
tetrazolyl, pyridinyl, pyrimidinyl, -COOH, -C(=0)0-C1_3 alkyl, -C(.0)NHS(.0)2-
C1_3 alkyl,
-C(=0)NHS(=0)2-phenyl, -C(=0)NH-C1_3 alkylene-
S(=0)20H, -C(=0)NH-C1_3
alkylene-C(=0)0H, -S(=0)2NH2, -S(=0)20H, -S(=0)2-C1_3 alkyl, -C(=0)NH2 or
-C(=0)N(CH3)2; wherein each R1 is independently and optionally substituted
with one or more
R12;
[00145] wherein, each R12 is as defined herein.
[00146] In some embodiments, each R8 is independently hydrogen, deuterium,
C1_6 alkyl,
C1..6 aminoalkyl, C2_6 alkenyl, C2_6 alkynyl, C2_6 haloalkyl, C3_6 cycloalkyl,
C2_6 heterocyclyl or
phenyl.
[00147] In other embodiments, each R8 is independently hydrogen, deuterium,
C1_3 alkyl,
C1.3 aminoalkyl, C24 alkenyl, C24 alkynyl, C24 haloalkyl, C3_6 cycloalkyl,
C2_6 heterocyclyl or
phenyl.
[00148] In some embodiments, each R9 is independently hydrogen, deuterium,
hydroxy,
amino, C1_6 alkyl, C1-6 aminoalkyl, C2-6 alkenyl, C2_6 alkynyl, C2 haloalkyl,
C3_6 cycloalkyl, C2-6
heterocyclyl, phenyl or -NR10R11;
[00149] wherein, each R1 and le is as defined herein.
[00150] In other embodiments, each R9 is independently hydrogen, deuterium,
hydroxy,
amino, C1_3 alkyl, C1_3 aminoalkyl, C24 alkenyl, C24 alkynyl, C24 haloalkyl,
C3_6 cycloalkyl, C2-6
heterocyclyl, phenyl or -NR10R11;
[00151] wherein, each R1 and R11 is as defined herein.
[00152] In some embodiments, each R1 is independently hydrogen, deuterium,
Ci_6 alkyl,
C1_6 aminoalkyl, C2_6 alkenyl, C2_6 alkynyl, C haloalkyl, C3_6 cycloalkyl,
C2_6 heterocyclyl or
phenyl.
[00153] In other embodiments, each R1 is independently hydrogen,
deuterium, C1_3 alkyl,
-35-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.. 75920/00027
C1_3 aminoalkyl, C24 alkenyl, C24 alkynyl, C3 haloalkyl, C3-6 cycloalkyl, C2_6
heterocyclyl or
phenyl.
[00154] In some embodiments, each R11 is independently hydrogen, deuterium,
C1_6 alkyl,
C16 aminoalkyl, C2_6 alkenyl, C2_6 alkynyl, C1_6 haloalkyl, C3_6 cycloalkyl,
C2_6 heterocyclyl or
phenyl.
[00155] In other embodiments, each R11 is independently hydrogen,
deuterium, C1_3 alkyl,
C1-3 aminoalkyl, C24 alkenyl, C2.4 alkynyl, C1_3 haloalkyl, C3_6 cycloalkyl,
C2-6 heterocyclyl or
phenyl.
[00156] In some embodiments, R1 and R11 together with the nitrogen atom to
which they
are attached, independently and optionally form a C2_6 heterocyclic ring or
C1_5 heteroaromatic
ring.
[00157] In some embodiments, each R2 is independently hydrogen, deuterium,
F, Cl, Br, I,
hydroxy, amino, nitro, cyano, methyl, isopropyl, difluoromethyl,
trifluoromethyl, methoxy,
isopropoxy, difluoromethoxy or trifluoromethoxy.
[00158] In some embodiments, each R3 is independently hydrogen, deuterium,
F, Cl, Br, I,
hydroxy, amino, nitro, cyano, methyl, isopropyl, difluoromethyl,
trifluoromethyl, methoxy,
isopropoxy, difluoromethoxy or trifluoromethoxy.
[00159] In some embodiments, each R4 is independently hydrogen, deuterium,
F, Cl, Br, I,
hydroxy, amino, nitro, cyano, methyl, isopropyl, difluoromethyl,
trifluoromethyl, methoxy,
isopropoxy, difluoromethoxy or trifluoromethoxy.
[00160] In some embodiments, each R5 is independently hydrogen, deuterium,
F, Cl, Br, I,
hydroxy, amino, nitro, cyano, methyl, isopropyl, difluoromethyl,
trifluoromethyl, methoxy,
isopropoxy, difluoromethoxy or trifluoromethoxy.
[00161] In some embodiments, each R6 is independently hydrogen, deuterium,
F, Cl, Br, I,
hydroxy, amino, nitro, cyano, methyl, isopropyl, difluoromethyl,
trifluoromethyl, methoxy,
isopropoxy, difluoromethoxy or trifluoromethoxy.
-36-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
[00162] In some
embodiments, each R7 independently is hydrogen, deuterium, F, Cl, Br, I,
hydroxy, amino, nitro, cyano, C1_3 alkyl, C1_3 hydroxyalkyl, C3 haloalkyl,
C1_3 alkoxy, C1-3
alkylamino, C1-3 alkoxy-C3 alkyl, C3_6 cycloalkyl or C2_6 heterocyclyl, and
wherein the C3_6
cycloalkyl and C2_6 heterocyclyl is independently and optionally substituted
with subsitituents
selected from F, Cl, Br, I, hydroxy, amino, nitro and cyano.
[00163] In other
embodiments, each R7 is independently hydrogen, deuterium, F, Cl, Br, I,
hydroxy, amino, nitro, cyano, methyl, ethyl, isopropyl, t-butyl,
hydroxymethyl,
2-hydroxyisopropyl, difluoromethyl, trifluoromethyl, 2-fluoroisopropyl,
methoxy, isopropoxy,
t-butoxy, dimethylamino, isopropoxymethyl, t-butoxymethyl, cyclopropyl,
cyclobutyl,
cyclopentyl, cyclohexyl, tetrahydrofuranyl, pyrrolidinyl, piperidinyl,
piperazinyl, morpholinyl or
thiomorpholinyl, and wherein the cyclopropyl, cyclobutyl, cyclopentyl,
cyclohexyl,
tetrahydrofuranyl, pyrrolidinyl, piperidinyl, piperazinyl, morpholinyl and
thiomorpholinyl is
independently and optionally substituted with subsitituents selected from F,
Cl, Br, I, hydroxy,
amino, nitro and cyano.
[00164] In one
aspect, provided herein is the compound having one of the following
structures, or a stereoisomer, a geometric isomer, a tautomer, an N-oxide, a
solvate, a hydrate, a
metabolite, ester, a pharmaceutically acceptable salt or a prodrug thereof,
but are not limited to:
141 ei ay=ci
HO Ilf _ 1-10Acc.p._
\
N = d N 6
11" 4
(1), (2),
0
of
Ho
H.
(3), F 3
(4), (5),
-37-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
a 0 H
4
et a 6.
HO HO 0
0 0
0
F 0 -4
(6), (7), (8),
a
cr-L: 1 õ1 co o
Fio
o
N ' * ,
= N
d I F y(9), (10), 4
0 0
HO 0 HO
N
N/ 6 Iv
Ho H
(11), 41 (12), (13),
OH
0 0
0
* Cla
Hoitrt\:ps4 cl Ho Ho4
NI
o -C:CP-13r:
H = (14), F (15), 1
N.. 0
0 i
/- CA I
Oi I HO
HO 0
N
nt 14/
N/ d
F
H
(16), (17), (18),
0
a
Ho-itcco.Theel Ho rk., N * H0
N / N V d
=
F
(19), GO),
0
4 o a
a =
o
1:1
11 )C:ZP0 '
H
(21), 4 (22), (23),
, 0
o 0
a HO
Gi 01/ycl
HO HO
N N
6 V 'Oh IP=0
6 N 0 d
a
3 (24), (25), ft
-38-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Makes Ref.: 75920/00027
OH
4 a A
a 1 N
0 0 * ' a
War
(26), r'r (27), It,
v (28),
OH I 0 OH
1 1 CI 4
= -- Ookscc
--..
-..v
HO igi i
N N
-71:11)
(29), H 4111 (30),
o o
a C/
HO HO 0
N
NI i d
cy, r F F
(31), (32),
0 o
0
GI
HO 110 0 a *
HO , * 0 -)
_, -"--- 1)
,,. ... ist
'N
N - N' \ d
*
F
(35), r -4
G 0
4
110Acrcip.o, a 41, = kC 0 a 4.
bt 4 411
(36), (37), (38),
0 4 ci
CI =
0 CI 0 ci
0 , '011# 0 *rip N 0 114*ry
_ ' 11, = , .
. HO
(39), il (40),
o o
et `IP' t C.1,:11%Cl
HO HC 0
_
(41), <1 (42), (43),
o o 0
4
N
HO--11441- 3 SI 1
N CI *
.14 a
d
't. -4 (44), (45), 1/2...._
V 4
-39-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
0 0
NO
NO riL\ 0 --
1110 4 111k N kW. I , r N
N
.F.,a
(46), F,1 (47), (48),
o o I
o . a
140 AL\ = 0
N
N N
N N s
4
(49), (50),
a
ci I 1 0
HO
N a
a; 4111
N
i
(51), 4 (52), F 1 (53),
,..-
a
-la 0 cl --kccp.o
ri HO fifil = ¨ CI
N ht. I
F3C4 4
(54) , (55) ,
o 4 a
"I
HO ci CI I
-- HO
N
No' N
4
(56) and (57).
[00165] In one
aspect, provided herein is a pharmaceutical composition comprising the
compound described above, and the pharmaceutical composition optionally
further comprise a
pharmaceutically acceptable carrier, excipient, diluent, adjuvant, vehicle or
a combination
thereof.
[00166] In one
aspect, provided herein is use of the compound described above in the
manufacture of a medicament for preventing, managing, treating or lessening a
disease mediated
by FXR in a patient.
[00167] In some
embodiments, the disease mediated by FXR is a cardiovascular and
cerebrovascular disease, a disease related to dyslipidemia, metabolic
syndrome, a
-40-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
hyperproliferative disease, fibrosis, an inflammatory disease or a
hepatobiliary related disease.
[00168] In some embodiments, the cardiovascular and cerebrovascular disease
is
atherosclerosis, acute myocardial infarction, veno-occlusive disease, portal
hypertension,
pulmonary hypertension, heart failure, peripheral arterial occlusive disease
(PAOD), sexual
dysfunction, stroke or thrombosis.
[00169] In some embodiments, the metabolic syndrome is insulin resistance,
hyperglycemia,
hyperinsulinemia, elevated blood level of fatty acids or glycerol,
hyperlipidemia, obesity,
hypertriglyceridemia, hypercholesterolemia, X syndrome, diabetic
complications,
atherosclerosis, hypertension, acute anemia, neutropenia, dyslipidemia, type
II diabetes, diabetic
nephropathy, diabetic neuropathy, diabetic retinopathy or the merger disorders
of diabetes and
abnormally high body mass index (BMI).
[00170] In some embodiments, the hyperproliferative diseaseis
hepatocellular carcinoma,
colonic adenoma, polyposis, colonic adenocarcinoma, breast cancer, membrane
cancer, Barrctt's
esophageal cancer and other forms of gastrointestinal tract disease or liver
tumor.
[00171] In some embodiments, the fibrosis, inflammatory disease and
hepatobiliary related
disease is nonalcoholic fatty liver disease (NAFLD), nonalcoholic
steatohepatitis (NASH),
cholestasis, liver fibrosis, primary biliary cirrhosis (PBC), primary
sclerosing cholangitis (PSC),
progressive familial intrahepatic cholestasis (PFIC), cystic fibrosis, drug-
induced bile duct
injury, cirrhosis of the liver, hepatitis B, sebaceous disease, cirrhosis of
the liver caused by
alcohol, biliary obstruction, cholelithiasis, colitis, newborn yellow disease,
riboflavin disease
prevention or intestinal bacterial overgrowth.
[00172] In one aspect, provided herein is a method of preventing, managing,
treating or
lessening a disease mediated by FXR comprising administering to the patient a
therapeutic
effective amount of the compound or the pharmaceutical composition of the
present invention.
[00173] In some embodiments, the disease mediated by FXR is a
cardiovascular and
cerebrovascular disease, a disease related to dyslipidemia, metabolic
syndrome, a
-41-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
hyperproliferative disease, fibrosis, an inflammatory disease or a disease
related to liver and
gallbladder.
[00174] In some embodiments, the cardiovascular and cerebrovascular disease
is
atherosclerosis, acute myocardial infarction, veno-occlusive disease, portal
hypertension,
pulmonary hypertension, heart failure, peripheral arterial occlusive disease
(PAOD), sexual
dysfunction, stroke or thrombosis.
[00175] In some embodiments, the metabolic syndrome is insulin resistance,
hyperglycemia,
hyperinsulinemia, elevated blood level of fatty acids or glycerol,
hyperlipidemia, obesity,
hypertriglyceridemia, hypercholesterolemia, X syndrome, diabetic
complications,
atherosclerosis, hypertension, acute anemia, neutropenia, dyslipidemia, type
II diabetes, diabetic
nephropathy, diabetic neuropathy, diabetic retinopathy or the merger disorders
of diabetes and
abnormally high BMI.
[00176] In some embodiments, the hyperproliferative disease is
hepatocellular carcinoma,
adenomatous, polyposis, colon cancer, breast cancer, membrane cancer,
Barrett's esophageal
cancer and other forms of gastrointestinal tract disease or liver tumor.
[00177] In some embodiments, inflammatory disease and disease related to
liver and
gallbladder is nonalcoholic fatty liver disease (NAFLD), nonalcoholic
steatohepatitis (NASH),
cholestasis, liver fibrosis, primary biliary cirrhosis (PBC), primary
sclerosing cholangitis (PSC),
progressive familial intrahepatic cholestasis (PFIC), cystic fibrosis, drug-
induced bile duct
injury, gallstones, cirrhosis of liver, hepatitis B, sebaceous disease,
cirrhosis of the liver caused
by alcohol, biliary obstruction, cholelithiasis, colitis, newborn yellow
disease, riboflavin disease
prevention or intestinal bacterial overgrowth.
[00178] In one aspect, provided herein is the compound or the
pharmaceutical composition
of the present invention for use in preventing, managing, treating or
lessening a disease mediated
by FXR in a patient.
[00179] In some embodiments, the disease mediated by FXR is a
cardiovascular and
-42-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
cerebrovascular disease, a disease related to dyslipidemia, metabolic
syndrome, a
hyperproliferative disease, fibrosis, an inflammatory disease or a disease
related to liver and
gallbladder.
[00180] In some embodiments, the cardiovascular and cerebrovascular disease
is
atherosclerosis, acute myocardial infarction, veno-occlusive disease, portal
hypertension,
pulmonary hypertension, heart failure, peripheral arterial occlusive disease
(PAOD), sexual
dysfunction, stroke or thrombosis.
[00181] In some embodiments, the metabolic syndrome is insulin resistance,
hyperglycemia,
hyperinsulinemia, elevated blood level of fatty acids or glycerol,
hyperlipidemia, obesity,
hypertriglyceridemia, hypercholesterolemia, X syndrome, diabetic
complications,
atherosclerosis, hypertension, acute anemia, neutropenia, dyslipidemia, type
II diabetes, diabetic
nephropathy, diabetic neuropathy, diabetic retinopathy or the merger disorders
of diabetes and
abnormally high BMI.
[00182] In some embodiments, the hyperproliferative disease is
hepatocellular carcinoma,
adenomatous, polyposis, colon cancer, breast cancer, membrane cancer,
Barrett's esophageal
cancer and other forms of gastrointestinal tract disease or liver tumor;
[00183] In some embodiments, inflammatory disease and disease related to
liver and
gallbladder is nonalcoholic fatty liver disease (NAFLD), nonalcoholic
steatohepatitis (NASH),
cholestasis, liver fibrosis, primary biliary cirrhosis (PBC), primary
sclerosing cholangitis (PSC),
progressive familial intrahepatic cholestasis (PFIC), cystic fibrosis, drug-
induced bile duct
injury, gallstones, cirrhosis of liver, hepatitis B, sebaceous disease,
cirrhosis of the liver caused
by alcohol, biliary obstruction, cholelithiasis, colitis, newborn yellow
disease, riboflavin disease
prevention or intestinal bacterial overgrowth.
[00184] In one aspect, provided herein is a method of preventing, managing,
treating or
lessening a disease mediated by FXR comprising administering a therapeutic
effective amount of
the compound or the pharmaceutical composition of the present invention to the
patient.
-43-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
[00185] In one aspect, provided herein is a method of preventing, managing,
treating or
lessening a disease mediated by FXR comprising administering a
pharmaceutically acceptable
effective dosage of the compounds of the present invention to the patient.
[00186] In other aspect, provided herein is a method of preparing,
separating or purifying
the compound of Formula (I).
PHARMACEUTICAL COMPOSITIONS, PREPARATIONS, ADMINISTRATION,
AND USES OF THE COMPOUNDS AND PHARMACEUTICAL COMPOSITIONS
[00187] In other aspect, the characteristics of the pharmaceutical
composition of the present
invention include compound of formula (I), compound listed in the present
invention, or one or
more compounds of examples 1-19, and a pharmaceutically acceptable carrier,
adjuvant or
vehicle. The effective amount of the compound in the composition of the
present invention is
capable of treating or lessening disease mediated by FXR in the patients.
[00188] It will also be appreciated that the compounds disclosed herein can
exist in free
form, or where appropriate, as a pharmaceutically acceptable derivative
thereof. Some
non-limiting examples of the pharmaceutically acceptable derivative include
pharmaceutically
acceptable prodrugs, salts, esters, salts of such esters, or any other adducts
or derivatives which
upon administration to a patient in need is capable of providing, directly or
indirectly, a
compound as otherwise described herein, or a metabolite or residue thereof.
[00189] As described above, the pharmaceutically acceptable composition
disclosed herein
further comprises a pharmaceutically acceptable carrier, an adjuvant, or a
vehicle, which, as used
herein, includes any and all solvents, diluents, or other liquid vehicle,
dispersion or suspension
aids, surface active agents, isotonic agents, thickening or emulsifying
agents, preservatives, solid
binders, lubricants, and the like, as suited to the particular dosage form
desired. As described in
the following references: In Remington: The Science and Practice of Pharmacy,
21st ed., 2005,
ed. D.B. Troy, Lippincott Williams & Wilkins, Philadelphia, and Encyclopedia
of
Pharmaceutical Technology, eds. J. Swarbrick and J. C. Boylan, 1988-1999,
Marcel Dekker, New
-44-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
York, and discloses various carriers used in formulating pharmaceutically
acceptable
compositions and known techniques for the preparation thereof. Except insofar
as any
conventional carrier medium incompatible with the compounds disclosed herein,
such as by
producing any undesirable biological effect or otherwise interacting in a
deleterious manner with
any other components of the pharmaceutically acceptable composition, its use
is contemplated to
be within the scope of this invention.
[00190] The compound of the present invention can be combined as the active
ingredient in
intimate admixture with a pharmaceutical carrier according to conventional
pharmaceutical
compounding techniques. The carrier may take a wide variety of forms depending
on the form of
preparation desired for administration, e.g., oral or parenteral (including
intravenous). In
preparing the compositions for oral dosage form, any of the usual
pharmaceutical media may be
employed, such as, for example, water, glycol, oils, alcohols, fragrances,
preservative, coloring
agents and the like in the case of oral liquid preparations, such as, for
example, suspensions,
elixirs and solutions; or carriers such as starches, sugars, microcrystalline
cellulose, diluents,
granulating agents, lubricants, binders, disintegrating agents and the like in
the case of oral solid
preparations, such as, for example, powders, hard capsules and soft capsules
and tablets, with the
solid oral preparations being preferred over the liquid preparations.
[00191] Because of their ease of administration, tablets and capsules
represent the most
advantageous oral dosage unit form in which case solid pharmaceutical carriers
are obviously
employed. If desired, tablets may be coated by standard aqueous or nonaqueous
techniques. Such
compositions and preparations should contain at least 0.1 percent of active
compound. The
percentage of active compound in these compositions may, of course, be varied
and may
conveniently be between about 2 percent to about 60 percent of the weight of
the unit. The
amount of active compound in such therapeutically useful compositions is such
that an effective
dose will be obtained. The active compounds can also be administered
intranasally as, for
example, liquid drops or spray.
-45-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
[00192] The tablets, pills, capsules and the like may also contain a binder
(such as gum
tragacanth, acacia, corn starch or gelatin); excipients (such as dicalcium
phosphate); a
disintegrant agent (such as corn starch, potato starch, alginic acid); a
lubricant (such as
magnesium stearate); and a sweetening agent (such as sucrose, lactose or
saccharin). When a
dosage unit form is a capsule, it may contain, in addition to materials of the
above type, a liquid
carrier (such as a fatty oil).
[00193] Various other materials may be present as coatings or to modify the
physical form
of the dosage unit. For instance, tablets may be coated with shellac, sugar or
both. A syrup or
elixir may contain, in addition to the active ingredient, sucrose as a
sweetening agent, methyl or
propylparaben as preservatives, a dye and a flavoring (such as cherry or
orange flavor).
[00194] Ophthalmic formulations, eye ointments, powders, solutions and the
like, are also
contemplated as being within the scope of this invention.
[00195] The compounds of the present invention may also be administered
parenterally.
Solutions or suspensions of these active compounds can be prepared suitably
mixed with a
surfactant (e.g., hydroxyl-propylcellulose) in water. Dispersions can also be
prepared in glycerol,
liquid polyethylene glycols and mixtures thereof in oils. Under ordinary
conditions of storage
and use, these preparations contain a preservative to prevent the growth of
microorganisms.
[00196] The pharmaceutical forms suitable for injectable use include
sterile aqueous
solutions or dispersions and sterile powders for the extemporaneous
preparation. In all cases, the
form must be sterile and must be fluid to the extent easy syringability
exsists. It must be stable
under the conditions of manufacture and storage and must be preserved against
the
contaminating action of microorganisms such as bacteria and fungi. The carrier
can be a solvent
or dispersion medium containing, for example, water, ethanol, polyol (e.g.
glycerol, propylene
glycol and liquid polyethylene glycol), suitable mixtures thereof, and
vegetable oils.
[00197] Any suitable route of administration may be employed for providing
a mammal,
especially a human, with an effective dose of a compound of the present
invention. For example,
-46-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
oral, rectal, topical, parenteral, ocular, pulmonary, nasal, and the like may
be employed. Dosage
forms include tablets, troches, dispersions, suspensions, solutions, capsules,
creams, ointments,
aerosols and the like. Preferably compounds of the present invention are
administered orally.
[00198] The therapeutically effective dosage of a compound, the
pharmaceutical
composition, or the combinations thereof, is dependent on the species of the
subject, the body
weight, age and individual condition, the disorder or disease or the severity
thereof being treated.
A physician, clinician or veterinarian of ordinary skill can readily determine
the effective amount
of each of the active ingredients necessary to prevent, treat or inhibit the
progress of the disorder
or disease.
[00199] When treating or preventing of conditions mediated by FXR for which
compounds
of the present invention are indicated, generally satisfactory results are
obtained when the
compounds of the present invention are administered at a daily dose of from
about 0.1
milligrams to about 100 milligrams per kilogram of animal body weight,
preferably given as a
single daily dose or in divided doses two to six times a day, or in sustained
release form. For
most large mammals, the total daily dose is from about 1.0 milligrams to about
1000 milligrams,
preferably from about 1.0 milligrams to about 50 milligrams. In the case of a
70 kg human, the
total daily dose will generally from about 7.0 milligrams to about 350
milligrams. This dosage
regimen may be adjusted to provide the optimal therapeutic response.
[00200] The present invention relates to compounds, compositions, or a
pharmaceutically
acceptable salt or hydrate thereof for effective use in preventing, managing,
treating or
alleviating a disease mediated by the FXR, particularly effective in the
treatment of
non-alcoholic fatty liver (NAFLD), nonalcoholic steatohepatitis (NASH),
obesity,
hypertriglyceridemia, atherosclerosis, chronic intrahepatic cholestasis,
primary biliary cirrhosis
(PBC), primary sclerosing cholangitis (PSC), progressive familial intrahepatic
cholestasis
(PFIC), drug-induced bile duct injury, gallstones, cirrhosis, hepatitis B,
steatosis, cirrhosis of the
liver caused by alcohol, cystic fibrosis, biliary obstruction, gallstone
disease, liver fibrosis,
-47-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakcs Ref.: 75920/00027
dyslipidemia, atherosclerosis, type II diabetes, diabetic nephropathy,
diabetic neuropathy,
diabetic retinopathy, peripheral arterial occlusive disease (PAOD), colitis,
newborn yellow
disease, riboflavin disease prevention, vein occlusive disease, portal
hypertension, metabolic
syndrome, acute myocardial infarction, acute stroke, thrombosis,
hypercholesterolemia, intestinal
bacterial overgrowth, erectile dysfunction, gastrointestinal tumor and liver
tumor.
GENERAL SYNTHETIC PROCEDURES
[00201] Generally, the compounds disclosed herein may be prepared by
methods described
herein, wherein the substituents are as defined for Formula (I) above, except
where further noted.
The following non-limiting schemes and examples are presented to further
exemplify the
invention.
[00202] Persons skilled in the art will recognize that the chemical
reactions described may
be readily adapted to prepare a number of other compounds disclosed herein,
and alternative
methods for preparing the compounds disclosed herein are deemed to be within
the scope
disclosed herein. For example, the synthesis of non-exemplified compounds
according to the
invention may be successfully performed by modifications apparent to those
skilled in the art,
e.g., by appropriately protecting interfering groups, by utilizing other
suitable reagents known in
the art other than those described, and/or by making routine modifications of
reaction conditions.
Alternatively, the known reaction conditions or the reaction disclosed in the
present invention
will be recognized as having applicability for preparing other compounds
disclosed herein.
[00203] In the examples described below, unless otherwise indicated all
temperatures are set
forth in degrees Celsius. Reagents were purchased from commercial suppliers
such as Aldrich
Chemical Company, Arco Chemical Company and Alfa Chemical Company, and were
used
without further purification unless otherwise indicated. Common solvents were
purchased from
commercial suppliers such as Shantou XiLong Chemical Factory, Guangdong
Guanghua
Reagent Chemical Factory Co. Ltd., Guangzhou Reagent Chemical Factory, Tianjin
YuYu Fine
Chemical Ltd., Qingdao Tenglong Reagent Chemical Ltd., and Qingdao Ocean
Chemical
-48-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
Factory.
[00204] Anhydrous THF, dioxane, toluene, and ether were obtained by
refluxing the solvent
with sodium. Anhydrous CH2C12 and CHCl3 were obtained by refluxing the solvent
with Caf12.
Et0Ac, PE, hexane, DMAC and DMF were treated with anhydrous Na2SO4 prior to
use.
[00205] The reactions set forth below were done generally under a positive
pressure of
nitrogen or argon or with a drying tube (unless otherwise stated) in anhydrous
solvents, and the
reaction flasks were typically fitted with rubber septa for the introduction
of substrates and
reagents via syringe. Glassware was oven dried and/or heat dried.
[00206] Column chromatography was conducted using a silica gel column.
Silica gel
(300-400 mesh) was purchased from Qingdao Ocean Chemical Factory. 1H NMR
spectra were
recorded with a Bruker 400 MHz or 600 MHz spectrometer using CDC13, d6-DMSO,
CD3OD or
d6-acetone as solvents (reported in ppm), and using TMS (0 ppm) or chloroform
(7.25 ppm) as
the reference standard. For multiple peaks, the following abbreviations used:s
(singlet), d
(doublet), t (triplet), m (multiplet), hr (broadened), dd (doublet of
doublets), q (quartet),dt
(doublet of triplets), tt (triplet of triplets),dddd (doublet of doublet of
doublet of doublets), qd
(quartet of doublets), ddd (doublet of doublet of doublets), td (triplet of
doublets), dq (doublet of
quartets), ddt (doublet of doublet of triplets), tdd (triplet of doublet of
doublets), dtd (doublet of
triplet of doublets). Coupling constants, when given, were reported in Hertz
(Hz).
[00207] Low-resolution mass spectral (MS) data were determined by an
Agilent 6320 Series
LC-MS spectrometer equipped with a G1312A binary pump and a G1316A TCC (column
was
operated at 30 C). G1329A autosampler and G1315B DAD detector were applied in
the
analysis, and an ESI source was used in the LC-MS spectrometer.
[00208] Low-resolution mass spectral (MS) data were determined by an
Agilent 6120 Series
LC-MS spectrometer equipped with a G1311A quaternary pump and a G1316A TCC
(column
was operated at 30 C). G1329A autosampler and G1315D DAD detector were
applied in the
analysis, and an ESI source was used on the LC-MS spectrometer.
-49-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
[00209] Both LC-MS spectrometers were equipped with an Agilent Zorbax SB-
C18, 2.1 x
30 mm, 5 gm column. Injection volume was decided by the sample concentration.
The flow rate
was 0.6 mL/min. The HPLC peaks were recorded by UV-Vis wavelength at 210 nm
and 254 nm.
The mobile phase was 0.1% formic acid in acetonitrile (phase A) and 0.1%
formic acid in
ultrapure water (phase B). The gradient elution conditions were shown in Table
1:
Table 1: The gradient condition of the mobile phase in Low-resolution mass
spectrum analysis
A (CH3CN, 0.1% B (H20, 0.1%
Time (min)
HCOOH) HCOOH)
0 - 3 5 - 100 95-0
3 - 6 100 0
6 - 6.1 100 - 5 0-95
6.1 - 8 5 95
[00210] Purities of compounds were assessed by Agilent 1100 Series high
performance
liquid chromatography (HPLC) with UV detection at 210 nm and 254 nm (Zorbax SB-
C18, 2.1 x
30 mm, 4 micorn, 10 min, 0.6 mL/min flow rate, 5 to 95 % (0.1 % formic acid in
CH3CN) in (0.1
% formic acid in H20). Column was operated at 40 C.
[00211] The following abbreviations are used throughout the specification:
CDC13 chloroform-d
DMF N,N-dimethylformamide
DMSO dimethylsulfoxide
DMSO-d6 d i methyl sulfoxide-d6
CD3OD methyl alcohol-d4
Me0H methanol
THF tetrahydrofuran
DCM difluoromethane
Et0Ac, EA ethyl acetate
-50-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
PE petroleum ether
Pd/C, Pd-C Palladium on activated carbon
g gramme
mg milligramme
H20 water
M mole/liter
mol mole
mmol millimole
mL milliliter
uL microliter
MPa million Pascal
rt room temperature
[00212] Typical synthetic procedures for preparing the compounds of the
present invention
disclosed are shown in the following synthetic schemes. Unless otherwise
specified, RI, T, X, Y,
Z, Ra, Rb, Rc, K¨y,
Rz,m and n are as defined herein.
Schemes
Scheme 1
(R (R
R8 b
Ra R
(R
gl
X ¨Y
la lb (I)
[00213] W is a leaving group, including, but are not limited to, halogen,
methyl sulfonyloxy,
p-methyl phenylsulfonyloxy, and the like.
[00214] The substitution reaction of compound la with compound lb can
afford Compound
(I) under an alkaline condition. The bases include but are not limited to
potassium phosphate,
and the like. The reaction can be carried out in an insert solvent. The
solvents include but are not
-51-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
limited to N,N-dimethylformamide, and the like.
Scheme 2
(Rc),
M
icfr.--Dc.Trt. .....), R b
-11*Nal TII)., 1 `R; HO L / /14.2
" 0 ' A 0
N
2a (la)
[00215] M is alkyl, ml is 0, 1, 2 or 3.
[00216] By hydrolysis reaction, compound 2a can be converted to compound
(Ia). The
hydrolysis reaction can refer to "Protective Groups in Organic Synthesis".
Scheme 3
(RC)
(jayOP9 0Pg
(OEt)2 MO -10 ,Di I
PA 3b " N,,oro
(R1)rn Ry RY
w
38 3c \Al
(irn 3d CR%
(RC)?) (Re)n
/ 0 \
(RI) (RI jrn H
m RY RY
3e 31
[00217] M is alkyl, W is a leaving group, include but is not limited to
halogen, methyl
sulfonyloxy, p-methyl phenylsulfonyloxy, and the like. Pg is a protecting
group defined herein.
[00218] Horner-Wadsworth-Emmons reaction between compound 3a and compound
3b can
afford compound 3c. In Horner-Wadsworth-Emmons reaction, the reaction starting
materials can
be reacted in the presence of a base (e.g., sodium hydride, potassium t-
butoxide, etc.) in a
solvent. The reaction can be preferably carried out in an inert solvent. The
solvents include, but
are not limited to, tetrahydrofuran, and the like.
[00219] The hydroxy protecting group of compound 3c can be removed to
afford compound
-52-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
3d. The removal of hydroxy protecting group can refer to "Protective Groups in
Organic
Synthesis".
[00220] By coupling reaction in the presence of a catalyst and a ligand
under an alkaline
condition, compound 3d can be converted to compound 3e. The catalysts include,
but are not
limited to, cuprous iodide, and the like. The ligands include, but are not
limited to,
N,N-dimethylglycine, and the like. The bases include, but are not limited to,
cesium carbonate,
and the like. The reaction can be carried out in an inert solvent. The
solvents include, but are not
limited to, 1,4-dioxane, and the like.
[00221] The hydroxy protecting group of compound 3e can be removed to
afford compound
3f. The removal of hydroxy protecting group can refer to "Protective Groups in
Organic
Synthesis".
Scheme 4
(RC)n
ve
es<---r-
wi w, oci
/---
ao
H 4b m1 6 .
410
..-yisl (R1pr6--- 1
, ,
(Ri)m
õolv
da 4 4d VV2
c 1
( 511 al%
V'in (R9,
4================ .)1/ ..... 1 "OM ...".........
CCSO.,0
0):
1-1
do 4f
[00222] M is alkyl; each of W, Wl and W2 is independently a leaving group,
including but
not limited to halogen, methyl sulfonyloxy, p-methyl phenylsulfonyloxy, and
the like.
[00223] The substitution reaction of compound 4a with compound 4b can
afford Compound
4c under an alkaline condition. The bases include but are not limited to
potassium phosphate, and
the like. The reaction is carried out in an insert solvent. The solvents
include but are not limited
to N,N-dimethylformamide, and the like.
[00224] By coupling reaction in the presence of a catalyst and a ligand
under an alkaline
-53-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
condition, compound 4c can be converted to compound 4d. The catalysts include,
but are not
limited to, cuprous iodide, and the like. The ligands include, but are not
limited to,
1,10-phenanthroline, and the like. The bases include, but are not limited to,
potassium hydroxide,
and the like. The reaction can be carried out in an inert solvent. The
solvents include, but are not
limited to, dimethylsulfoxide, water or mixed solvent and the like.
[00225] By coupling reaction in the presence of a catalyst and a ligand
under an alkaline
condition, compound 4d can be converted to compound 4e. The catalysts include,
but are not
limited to, cuprous iodide, and the like. The ligands include, but are not
limited to,
N,N-dimethylglycine, and the like. The bases include, but are not limited to,
cesium carbonate,
and the like. The reaction can be carried out in an inert solvent. The
solvents include, but are not
limited to, 1,4-dioxane, and the like.
[00226] The hydroxy protecting group of compound 4e can be removed to
afford compound
4f. The removal of hydroxy protecting group can refer to "Protective Groups in
Organic
Synthesis".
Scheme 5
1R9,, onõ
\ D 0
40 g On_ thi (RI
OR% "1 5. (W)rn kli -ri
5k 9
i<e* yW 1
mNOA'N A`f 4 I
5b R4'
0Pg. I-1
(r41477 I Iti N 0, IR' - 1 Ivi 1.1 k
_...... --.... /-- N
I ki i M t
(111) ¨MI M
5d (Ii9, 5a N
I
(R`k,
H
rrt
Aa'k, w
5g irr)õ 5ii (RI, SI N
-54-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
[00227] M is alkyl; W is a leaving group, including but not limited to
halogen, methyl
sulfonyloxy, p-methyl phenylsulfonyloxy, and the like. M1 is hydrogen, alkyl,
haloalkyl or
cycloalkyl.
[00228] Horner-Wadsworth-Emmons reaction between compound 5a and compound
5b can
afford compound 5c. In Horner-Wadsworth-Emmons reaction, the reaction starting
materials can
be reacted in the presence of a base (e.g., sodium hydride, potassium t-
butoxide, etc.) in a
solvent. The reaction can be preferably carried out in an inert solvent. The
solvents include, but
are not limited to, tetrahydrofuran, and the like.
[00229] The hydroxy protecting group of compound Sc can be removed to
afford compound
5d. The removal of hydroxy protecting group can refer to "Protective Groups in
Organic
Synthesis".
[00230] By coupling reaction in the presence of a catalyst and a ligand
under an alkaline
condition, compound 5d can be converted to compound 5e. The catalysts include,
but are not
limited to, cuprous iodide, and the like. The ligands include, but are not
limited to,
N,N-dimethylglycine, and the like. The bases include, but are not limited to,
cesium carbonate,
and the like. The reaction can be carried out in an inert solvent. The
solvents include, but are not
limited to, 1,4-dioxane, and the like.
[00231] The hydroxy protecting group of compound 5e can be removed to
afford compound
5f. The removal of hydroxy protecting group can refer to "Protective Groups in
Organic
Synthesis".
[00232] Compound 5c can be reduced to afford compound 5g in the presence of
a reducing
agent under an alkaline condition. The reducing agents include, but are not
limited to, p-methyl
benzenesulfonohydrazide, and the like. The bases include, but are not limited
to, sodium acetate,
and the like. The reaction can be carried out in an inert solvent. The
solvents include, but are not
limited to, tetrahydrofuran, water or a mixed solvent, and the like.
[00233] The hydroxy protecting group of compound 5g can be removed to
afford compound
-55-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
5h). The removal of hydroxy protecting group can refer to "Protective Groups
in Organic
Synthesis".
[00234] By coupling reaction in the presence of a catalyst and a ligand
under an alkaline
condition, compound 5h can be converted to compound 51. The catalysts include,
but are not
limited to, cuprous iodide, and the like. The ligands include, but are not
limited to,
N,N-dimethylglycine, and the like. The bases include, but are not limited to,
cesium carbonate,
and the like. The reaction can be carried out in an inert solvent. The
solvents include, but are not
limited to, 1,4-dioxane, and the like.
[00235] The hydroxy protecting group of compound 5i can be removed to
afford compound
5j. The removal of hydroxy protecting group can refer to "Protective Groups in
Organic
Synthesis".
[00236] The cyclization reaction of compound 5e with a methylating agent
can afford
compound 5k. The methylating agents include, but are not limited to, trimethyl
iodide sulfoxide,
and the like. The reaction can be carried out in an inert solvent. The
solvents include, but are not
limited to, dimethyl sulfoxide, and the like.
[00237] Compound 5k can be converted to compound 51 by removing hydroxy
protecting
groups, and the removal of hydroxy protecting groups can refer to "Protective
Groups in Organic
Synthesis".
-56-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakcs Ref.: 75920/00027
Scheme 6
(Rcl,
OP9 C ) M i\Avvy9
iR a? b PODEt)2 ay"R.
6b m
,
(Ri)n,
60 w
6a 6ci W
03% 03%
.:\ \iO
/
hi C'el 0
N
(R/0I)mõ..S (RP H
6e 6f
[00238] M is alkyl; Pg is a protecting group defined herein; W is a leaving
group, including
but not limited to halogen, methyl sulfonyloxy, p-methyl phenylsulfonyloxy,
and the like.
[00239] Horner-Wadsworth-Emmons reaction betwwen compound 6a and compound
6b can
afford compound 6c. In Horner-Wadsworth-Emmons reaction, the reaction starting
materials can
be reacted in the presence of a base (e.g., sodium hydride, potassium t-
butoxide, etc.) in a
solvent. The reaction can be preferably carried out in an inert solvent. The
solvents include, but
are not limited to, tetrahydrofuran, and the like.
[00240] Compound 6c can be converted to compound 6d by removing hydroxy
protecting
groups, and the removal of hydroxy protecting groups can refer to "Protective
Groups in Organic
Synthesis".
[00241] By coupling reaction in the presence of a catalyst and a ligand
under an alkaline
condition, compound 6d can be converted to compound 6e. The catalysts include,
but are not
limited to, cuprous iodide, and the like. The ligands include, but are not
limited to,
NA-dimethylglycine, and the like. The bases include, but are not limited to,
cesium carbonate,
and the like. The reaction can be carried out in an inert solvent. The
solvents include, but are not
limited to, 1,4-dioxane, and the like.
-57-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
[00242] Compound 6e can be converted to compound 6f by removing hydroxy
protecting
groups, and the removal of hydroxy protecting groups can refer to "Protective
Groups in Organic
Synthesis".
Scheme 7
II*)n
0Pg M0--S7W' alOri......\Pg 0Pg
7:NAY''. R1)
,P1 . - õN =
*1
(Rs)m 0 0 I
7a 7c Mn Td (Ft%
(Friõ trich
\
N ¨*. 11
--
r ......=III.. Irj N
RYRI (RI)ni 2H
Te VI% 7-f (R4)= 7g 7h
i
(Rci= II%
,ti,)H (
z
----4" N N
HO R HO R
.I.2
Ti (RN
YRz (R 6 Fell
Y
71 7k
[00243] t i M is alkyl; Pg is a
protecting group defined herein; W s a leaving group, including
but not limited to halogen, methyl sulfonyloxy, p-methyl phenylsulfonyloxy,
and the like.
[00244] The reaction of compound 7a with compound 7b can afford Compound 7c
under an
alkaline condition. The bases include but are not limited to, sodium
bis(trimethylsilyl)amide, and
the like. The reaction is carried out in an insert solvent. The solvents
include but are not limited
to tetrahydrofuran, and the like.
[00245] The substitution reaction of compound 7c with haloalkanes or
halogenating agents
can afford Compound 7d under an alkaline condition. The haloalkanes include
but are not
limited to 1,2-dibromoethane. The halogenating agents include but are not
limited to
N-fluorobenzenesulfonimide. The bases include but are not limited to sodium
hydroxide, sodium
-58-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
bis(trimethylsilyl)amide, and the like. The reaction is carried out in an
insert solvent. The
solvents include but are not limited to toluene, tetrahydrofuran, and the
like.
[00246] Compound 7d can be converted to compound 7e by removing hydroxy
protecting
groups, and the removal of hydroxy protecting groups can refer to "Protective
Groups in Organic
Synthesis".
[00247] The reaction of compound 7e with methylchloroformate can afford
intermediate
active ester under an alkaline condition. Then the active ester can be further
reduced to obtain
compound 7f. The bases include but are not limited to triethylamine, and the
like. The reducing
agents include but are not limited to sodium borohydride, and the like. The
reaction is carried out
in an insert solvent. The solvents include but are not limited to
tetrahydrofuran, and the like.
[00248] By coupling reaction in the presence of a catalyst and a ligand
under an alkaline
condition, compound 7f can be converted to compound 7g. The catalysts include,
but are not
limited to, cuprous iodide, and the like. The ligands include, but are not
limited to,
N,N-dimethylglycine, and the like. The bases include, but are not limited to,
cesium carbonate,
and the like. The reaction can be carried out in an inert solvent. The
solvents include, but are not
limited to, 1,4-dioxane, and the like.
[00249] Compound 7g can be converted to compound 7h by removing hydroxy
protecting
groups, and the removal of hydroxy protecting groups can refer to "Protective
Groups in Organic
Synthesis".
[00250] Compound 7i can be obtained by a reaction of compound 7e with a
reducing agent.
The reducing agent includes, but is not limited to sodium borohydride, and the
like. The reaction
can be carried out in an inert solvent. The solvents include, but are not
limited to,
tetrahydrofuran, and the like.
[00251] By coupling reaction in the presence of a catalyst and a ligand
under an alkaline
condition, compound 7i can be converted to compound 7j. The catalysts include,
but are not
limited to, cuprous iodide, and the like. The ligands include, but are not
limited to,
-59-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
N,N-dimethylglycine, and the like. The bases include, but are not limited to,
cesium carbonate,
and the like. The reaction can be carried out in an inert solvent. The
solvents include, but are not
limited to, 1,4-dioxane, and the like.
[00252] Compound 7j can be converted to compound 7k by removing hydroxy
protecting
groups, and the removal of hydroxy protecting groups can refer to "Protective
Groups in Organic
Synthesis".
Scheme 8
WI
(Fe")n (R1)m W1
e\cõ,%0P9
CHO
111 ktN, 1.4
6 m
P90
8a ac PgC Ed (Rir,
(R9,
(R% OR%
N
(w),
HO
ae Of $9
[00253] M is alkyl; Pg is a protecting group defined herein; W1 is a
leaving group, including
but not limited to halogen, methyl sulfonyloxy, p-methyl phenylsulfonyloxy,
and the like.
[00254] Horner-Wadsworth-Emmons reaction between compound 8a and compound
8b can
afford compound 8c. In Horner-Wadsworth-Emmons reaction, the reaction starting
materials can
be reacted in the presence of a base (e.g., sodium hydride, potassium t-
butoxide, etc.) in a
solvent. The reaction can be preferably carried out in an inert solvent. The
solvents include, but
are not limited to, tetrahydrofuran, and the like.
[00255] Compound 8c can be reduced to afford compound 8d in the presence of
a reducing
agent under an alkaline condition. The reducing agents include, but are not
limited to, p-methyl
benzenesulfonohydrazide, and the like. The bases include, but are not limited
to, sodium acetate,
and the like. The reaction can be carried out in an inert solvent. The
solvents include, but are not
limited to, tetrahydrofuran, water or a mixed solvent, and the like.
[00256] Compound 8d can be converted to compound 8e by removing hydroxy
protecting
-60-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakcs Ref.: 75920/00027
groups, and the removal of hydroxy protecting groups can refer to "Protective
Groups in Organic
Synthesis".
[00257] By coupling reaction in the presence of a catalyst and a ligand
under an alkaline
condition, compound 8e can be converted to compound 8f. The catalysts include,
but are not
limited to, cuprous iodide, and the like. The ligands include, but are not
limited to,
NA-dimethylglycine, and the like. The bases include, but are not limited to,
cesium carbonate,
and the like. The reaction can be carried out in an inert solvent. The
solvents include, but are not
limited to, 1,4-dioxane, and the like.
[00258] Compound 8f can be converted to compound 8g by removing hydroxy
protecting
groups, and the removal of hydroxy protecting groups can refer to "Protective
Groups in Organic
Synthesis".
Scheme 9
(gP16o
IR% w 9a
M.
JOE02i
O4 *
9b (Ftc)m (Fe)el
Pg
(Ftihn¨..õ, 021), Q-
Cli5i43k0
)1.1 114 (R16 N H
M1 w
ec 9t1 (Fe). 9a 91
JOH -
(Wha (R1)rn
¨ IN( R
14.4 0:21)M mi m
9g (R% 9h (174 9i 91
[00259] M is alkyl; W is a leaving group, including but not limited to
halogen, methyl
sulfonyloxy, p-methyl phenylsulfonyloxy, and the like. M1 is hydrogen, alkyl,
haloalkyl or
cycloalkyl.
[00260] Horner-Wadsworth-Emmons reaction betwwen compound 9a and compound
9b can
-61-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
afford compound 9c. In Horner-Wadsworth-Emmons reaction, the reaction starting
materials can
be reacted in the presence of a base (e.g., sodium hydride, potassium t-
butoxide, etc.) in a
solvent. The reaction can be preferably carried out in an inert solvent. The
solvents include, but
are not limited to, tetrahydrofuran, and the like.
[00261] Compound 9c can be converted to compound 9d by removing hydroxy
protecting
groups, and the removal of hydroxy protecting groups can refer to "Protective
Groups in Organic
Synthesis".
[00262] By coupling reaction in the presence of a catalyst and a ligand
under an alkaline
condition, compound 9d can be converted to compound 9e. The catalysts include,
but are not
limited to, cuprous iodide, and the like. The ligands include, but are not
limited to,
NA-dimethylglycine, and the like. The bases include, but are not limited to,
cesium carbonate,
and the like. The reaction can be carried out in an inert solvent. The
solvents include, but are not
limited to, 1,4-dioxane, and the like.
[00263] Compound 9e can be converted to compound 9f by removing hydroxy
protecting
groups, and the removal of hydroxy protecting groups can refer to "Protective
Groups in Organic
Synthesis".
[00264] Compound 9c can be reduced to afford compound 9g in the presence of
a reducing
agent under an alkaline condition. The reducing agents include, but are not
limited to, p-methyl
benzenesulfonohydrazide, and the like. The bases include, but are not limited
to, sodium acetate,
and the like. The reaction can be carried out in an inert solvent. The
solvents include, but are not
limited to, tetrahydrofuran, water or a mixed solvent, and the like.
[00265] The hydroxy protecting group of compound 9 can be removed to afford
compound
9h). The removal of hydroxy protecting group can refer to "Protective Groups
in Organic
Synthesis".
[00266] By coupling reaction in the presence of a catalyst and a ligand
under an alkaline
condition, compound 9h can be converted to compound 9i. The catalysts include,
but are not
-62-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
limited to, cuprous iodide, and the like. The ligands include, but are not
limited to,
N,N-dimethylglycine, and the like. The bases include, but are not limited to,
cesium carbonate,
and the like. The reaction can be carried out in an inert solvent. The
solvents include, but are not
limited to, 1,4-dioxane, and the like.
[00267] The hydroxy protecting group of compound 9i can be removed to
afford compound
9j. The removal of hydroxy protecting group can refer to "Protective Groups in
Organic
Synthesis".
[00268] The following examples disclosed herein are presented to further
describe the
invention. However, these examples should not be used to limit the scope of
the invention.
DESCRIPTION OF THE PREFERRED EMBODIMENTS
Examples
Example 1: 2-((5-cyclopropyl-3-(2,6-dichlorophenyl)isoxazol-4-yl)methoxy)-10-
fluorobenzo
[6,71oxepino[3,2-blpyridine-7-carboxylic acid
0
ci 0111
NO
0
N
n \ 4
N 0
<if
Step 1: methyl 3-acetoxy-4-methylbenzoate
[00269] To a mixture of methyl 3-hydroxy-4-methylbenzoate (4.3 g, 26 mmol)
and pyridine
(3.5 mL, 43 mmol) in dichloromethane (50 mL) was added acetic anhydride (4.0
mL, 43 mmol)
under an ice bath. Water (50 mL) was added to quench the reaction after the
reaction mixture was
stirred for 5 h at rt. The resulting mixture was extracted with
dichloromethane (50 mL x 2). The
combined organic layers were washed with hydrochloric acid (1 M), dried over
anhydrous
sodium sulfate, filtered and concentrated under reduced pressure. The residue
was purified by
column chromatography eluted with PE/EA (v/v = 10/1) to give the title
compound as colourless
oil (5.3 g, 98 %).
-63-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
Step 2: methyl 3-acetoxy-4-(bromomethypbenzoate
[00270] To a mixture of methyl 3-acetoxy-4-methylbenzoate (5.3 g, 25.5
mmol) and
N-bromosuccinimide (4.6 g, 25.7 mmol) in tetrachloromethane (100 mL) was added
azodiisobutyronitrile (210 mg, 1.3 mmol). Then the mixture was heated to
reflux for 5 h. The
reaction mixture was cooled to rt and concentrated under reduced pressure. The
residue was
purified by column chromatography eluted with PE/EA (v/v = 20/1) to give the
title compound
as colourless oil (5.7 g, 77 %).
Step 3: methyl 3-acetoxy-4-((diethoxyphosphoryl)methyl)benzoate
[00271] A mixture of methyl 3-acetoxy-4-(bromomethyl)benzoate (5.6 g, 20
mmol) in
triethyl phosphite (10 mL) was heated at 150 C and stirred overnight. Then
the reaction mixture
was cooled to rt and concentrated to remove triethyl phosphite. The residue
was purified by
column chromatography eluted with dichloromethane/methanol (v/v = 10/1) to
give the title
compound as colourless oil (6.7 g, 100 %).
Step 4: methyl 4-((diethoxyphosphoryl)methyl)-3-hydroxybenzoate
[00272] To a mixture of methyl 3-acetoxy-4-
((diethoxyphosphorypmethyl)benzoate (6.5 g,
19 mmol) in methanol (100 mL) was added aqueous potassium carbonate (30 mL, 30
mmol, 1.0
M). Then the reaction mixture was stirred for 1 h at rt and concentrated to
remove most of
methanol. The residue was diluted with water (20 mL), extracted with ethyl
acetate (50 mLx2).
The combined organic layers were combined, washed with saturated brine (10
mL), dried over
anhydrous sodium sulfate, filtered and concentrated. The residue was purified
by column
chromatography on a silica gel eluted with PE/EA (v/v = 2/1) to give the title
compound as a
white solid (5.2 g, 91 %).
[00273] MS (ESI, pos. ion) m/z: 303.2 [M+H].
Step 5: methyl 4-((diethoxyphosphorypmethyl)-3-(methoxymethoxy)benzoate
[00274] To a mixture of methyl 4-((diethoxyphosphoryl)methyl)-3-
hydroxybenzoate (5.2 g,
17 mmol) and diisopropylethylamine (6.0 mL, 34 mmol) in dichloromethane (60
mL) was added
-64-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
chloromethyl methyl ether (2.3 mL, 30 mmol) under an ice bath. The reaction
mixture was
stirred at rt overnight. The reaction was quenched with water (50 mL),
extracted with
dichloromethane (50 mLx2), washed with saturated brine (10 mL), dried over
anhydrous sodium
sulfate, filtered and concentrated in vacuo. The residue was purified by
column chromatography
eluted with PE/EA (v/v = 4/1) to give the title compound as colourless oil
(3.8 g, 64 %).
[00275] MS (ESI, pos. ion) m/z: 347.2 [M+H]+.
Step 6: methyl 4-((diethoxyphosphoryl)fluoromethyl)-3-(methoxymethoxy)benzoate
[00276] To a mixture of methyl
4-((dieth ox yph osphoryl)meth y1)-3-(methoxymethoxy)benzo ate
(3.0 g, 8.7 mmol) in anhydrous THF (15 mL) was added a solution of sodium
bis(trimethylsilypamide in anhydrous THF (6.5 mL, 13 mmol, 2 mol/L) at -78 C.
The reaction
mixture was stirred at this temperature for 1 h. Then a solution of N-
fluorobenzenesulfonimide
(1.0 g, 4.6 mmol) in anhydrous THF (10 mL) was added. The reaction mixture was
stirred for
another 1 h at this temperature and then heated to -30 C and stirred
overnight. The reaction
mixture was quenched with hydrochloric acid (0.01 M, 15 mL), extracted with
dichloromethane
(80 mLx2), washed with saturated brine (20 mL), dried over anhydrous sodium
sulfate, filtered
and concentrated. The residue was purified by column chromatography on a
silica gel eluted with
PE/EA (v/v = 2/1) to give the title compound as yellow oil (1.86 g, 59 %).
[00277] MS (ESI, pos. ion) m/z: 365.1 [M+H].
Step 7: methyl 4-(2-(3-bromo-6-methoxypyridin-2-yl)-1-fluorovinyl)-3-
(methoxymethoxy)
benzoate
[00278] To a mixture of methyl
4-((diethoxyphosphoryl)fluoromethyl)-3-(methoxymethoxy)benzoate (1.86 g, 5.0
mmol) in DMF
(30 mL) was added sodium hydride (0.26 g, 6.6 mmol, 60%) under an ice bath.
The reaction
mixture was stirred for 0.5 h and then 3-bromo-6-methoxypicolinaldehyde (0.95
g, 4.4 mmol)
was added (See the synthetic method described in Organic and Biomolecular
Chemistry, 2003,
-65-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
(1)16, 2865 ¨ 2876). The reaction mixture was then warmed to rt and stirred
for 0.5 h, quenched
with saturated aquous ammonium chloride (10 mL), extracted with EA (20 mLx2).
The
combined organic layers were washed with saturated brine (20 mL), dried over
anhydrous
sodium sulfate, filtered and concentrated in vacuo. The residue was purified
by column
chromatography eluted with PE/EA (v/v = 10/1) to give the title compound as
yellow oil (900
mg, 48 %).
[00279] MS (ESI, pos. ion) m/z: 426.0 [M+11]+;
Step 8: methyl 4-(2-(3-bromo-6-methoxypyridin-2-yl)-1-fluoroyiny1)-3-
hydroxybenzoate
[00280] To a mixture of methyl 4-(2-(3-bromo-6-methoxypyridin-2-y1)-1-
fluorovinyl)
-3-(methoxymethoxy)benzoate (770 mg, 1.8 mmol) in THF (20 mL) was added
hydrochloric
acid (3 mL, 18 mmol, 6 M). The reaction mixture was stirred at 50 C for 5 h,
then cooled to rt
and diluted with water (20 mL). The resulting mixture was adjusted to
alkalinity with solid
potassium carbonate. The mixture was extracted with EA (30 mLx2), washed with
saturated
brine (10 mL), dried over anhydrous sodium sulfate, filtered and concentrated
in vacuo. The
residue was purified by column chromatography eluted with PE/EA (v/v, 5/1) to
give the title
compound as light yellow oil (605 mg, 88 %).
[00281] MS (ESI, pos. ion) m/z: 382.2 [M+H].
Step 9: methyl 10-fluoro-2-methoxybenzo [6,7] oxepino[3,2-b]pyridine-7-
carboxylate
[00282] A mixture of cuprous iodide (120 mg, 0.63 mmol), /V,N-
dimethylglycine (330 mg,
3.2 mmol), cesium carbonate (1.02 g, 3.2 mmol) and methyl
4-(2-(3-bromo-6-methoxypyridin-2-y1) -1-fluoroviny1)-3-hydroxybenzoate (605
mg, 1.6 mmol)
in 1,4-dioxane (8 nth) was heated to reflux overnight under nitrogen
atmosphere. The reaction
mixture was cooled to rt, filtered, concentrated in vacuo to obtain the
residue which was purified
by column chromatography eluted with PE/EA (v/v = 8/1) to give the title
compound as a yellow
solid (200 mg, 42 %).
[00283] MS (ESI, pos. ion) m/z: 302.2 [M+H].
-66-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
Step 10: methyl
10-fluoro-2-oxo-1,2-dihydrobenzo [6,7]oxepino [3,2-b]pyridine-7-carboxylate
[00284] To a mixture of methyl
10-fluoro-2-methoxybenzo[6,7]oxepino[3,2-b]pyridine-7-carboxylate (250 mg,
0.84 mmol) and
sodium iodide (390 mg, 3.2 mmol) in acetonitrile (5 mL) was added
trimethylehlorosilane (0.25
mL, 3.0 mmol) at rt. The reaction mixture was stirred at 85 C for 3 h,
followed by addition of
saturated aquous sodium thiosulfate (50 mL) to quench the reaction.The mixture
was extracted
with EA (50 mLx2), and the combined organic layers were washed with saturated
brine (30 mL),
dried over anhydrous sodium sulfate, filtered and concentrated in vacuo. The
residue was
purified by column chromatography on a silica gel eluted with
dichloromethane/methanol (v/v =
30/1) to give the title compound as yellow oil (120 mg, 50 %).
[00285] MS (ESI, pos. ion) m/z: 288.2 [M+H]+.
Step 11: methyl 2-05-cyclopropy1-3-(2,6-dichlorophenyl)isoxazol-4-yl)methoxy)-
10
-fluorobenzo [6,7] oxepino P,2-blpyridine-7-carboxylate
[00286] A mixture of methyl 10-fluoro-2-oxo-1,2-
dihydrobenzo[6,7]oxepino[3,2-b]pyridine
-7-carboxylate (120 mg, 0.4 mmol), 4-(chloromethyl)-5-cyclopropy1-3-(2,6-
dichlorophenyl)
isoxazole (170 mg, 0.54 mmol) and potassium phosphate (180 mg, 0.8 mmol) in
DMF (8 mL)
was stirred at 60 C overnight, cooled to rt, diluted with water (30 mL). The
mixture was
extracted with EA (40 mLx2), and the combined organic layers were washed with
saturated brine
(20 mL), dried over anhydrous sodium sulfate, filtered and concentrated in
vacuo. The residue
was purified by column chromatography eluted with PE/EA (v/v = 8/1) to give
the title
compound as yellow oil (180 mg, 78 %).
[00287] MS (ESI, pos. ion) m/z: 553.1 [M+H]4.
Step 12:
2-05 -cyclopropy1-3-(2,6-dichlorop henyl) isoxazol-4-yOmethoxy)-10-fluoroben
zo [6,7]
oxepino [3,2-b]pyridin e-7-carboxylic acid
-67-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
[00288] To a mixture of methyl 2((5-cyclopropy1-3-(2,6-
dichlorophenypisoxazol-4-y1)
methoxy)-10-fluorobenzo[6,7]oxepino[3,2-b]pyridine-7-carboxylate (180 mg, 0.33
mmol) in a
mixed solvent of THF (8 mL) and water (8 mL) was added sodium hydroxide (100
mg, 2.5
mmol). The reaction mixture was stirred at rt overnight, concentrated to
remove most of the
solvent. The residue was diluted with water (10 mL), adjusted to acidity with
hydrochloric acid
(2 M, 8 mL), and the mixture wasextracted with EA (30 mLx2). The combined
organic layer
were washed with saturated brine (10 mL), dried over anhydrous sodium sulfate,
filtered and
concentrated. The residue was purified by column chromatography eluted with
dichloromethane/methanol (v/v = 10/1) to give the title compound as a yellow
solid (130 mg, 74
%).
[00289] MS (ESI, pos. ion) m/z: 539.0 [M+H];
[00290] 111 NMR (400 MHz, DMSO-d6) 8 13.4 (s, 1H), 7.89 ¨ 7.37 (m, 2H),
7.75 ¨7.68 (m,
2H), 7.52 (d, J = 8.2 Hz, 2H), 7.45 ¨7.37 (m, 1H), 6.65 (d, J -= 8.8 Hz, 1H),
6.59 (d, J = 18.0 Hz,
1H), 5.18 (s, 2H), 2.54 ¨2.46 (m, 1H), 1.21 ¨ 1.16 (m, 2H), 1.13 ¨ 1.10 (m,
2H).
Example 2:2-05-cyclopropy1-3-(2,6-dichlorophenypisoxazol-4-yInnethoxy)-11H-
benzo
[2,31 [1,4]dioxep in o [6,5-blpyridine-7-carboxylic acid
0
141
CI CI
C,.114
0
Step 1: 3-bromo-2-(bromomethyl)-6-methoxypyridine
[00291] To a mixture of (3-bromo-6-methoxypyridin-2-yl)methanol (1.9 g, 8.7
mmol) in
dichloromethane (100 mL) was added N-bromosuccinimide (1.8 g, 10 mmol) and
triphenylphosphine (2.6 g, 10 mmol) under an ice bath condition and nitrogen
atmosphere. The
reaction mixture was stirred at rt for 3 h and then concentrated in vacuo, the
residue was purified
by column chromatography on a silica gel eluted with PE/EA (v/v = 30/1) to
give the title
-68-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
compound as light yellow liquid (1.4 g, 57 %).
Step 2: methyl 4-((3-bromo-6-methoxypyridin-2-yl)methoxy)-3-iodobenzoate
[00292] A mixture of 3-bromo-2-(bromomethyl)-6-methoxypyridine (1.4 g, 5.0
mmol),
methyl 3-iodo-4-hydroxybenzoate (1.52 g, 5.5 mmol), potassium phosphate (1.6
mg, 7.5 mmol)
in DMF (10 mL) was stirred at 60 C for 3 h. Then the reaction mixture was
cooled to rt, diluted
with water (40 mL), extracted with EA (80 mLx2). The combined organic layers
were washed
with saturated brine (20 mL), dried over anhydrous sodium sulfate, filtered
and concentrated.
The residue was purified by column chromatography eluted with PE/EA (v/v =
20/1) to give the
title compound as a white solid (2.2 g, 92 %).
[00293] MS (ESI, pos. ion) m/z: 477.9 [M+H].
Step 3: 4-((3-bromo-6-methoxypyridin-2-yl)methoxy)-3-hydroxybenzoic acid
[00294] A mixture of methyl 4-((3-bromo-6-methoxypyridin-2-yl)methoxy)-3-
iodobenzoate
(478 mg, 1.0 mmol), cuprous iodide (19 mg, 0.1 mmol), 1,10-phenanthroline (36
mg, 0.2 mmol)
and potassium hydroxide (337 mg, 6.0 mmol) in a mixed solvent of DMSO (0.4 mL)
and water
(0.4 mL) was heated to 100 C and stirred for 24 h under nitrogen atomsphere.
The reaction
mixture was cooled to rt and diluted with water (5 mL), adjusted to acidity
with hydrochloric
acid (I M, 10 mL). The resulting mixture was extracted with EA (30 mLx2). The
combined
organic lalyers were washed with saturated brine (10 mL), dried over anhydrous
sodium sulfate,
filtered and concentrated in vacuo. The residue was purified by column
chromatography eluted
with PE/EA (v/v = 1/1) to give the title compound as a yellow solid (230 mg,
65 %).
[00295] MS (ESI, pos. ion) m/z: 354.0 [M+H].
Step 4: methyl 4-((3-bromo-6-methoxypyridin-2-yl)methoxy)-3-hydroxybenzoate
[00296] A mixture of 4((3-bromo-6-methoxypyridin-2-yl)methoxy)-3-
hydroxybenzoic acid
(230 mg, 0.65 mmol) in methanol (100 mL) was treated with 2 drops of
concentrated sulfuric
acid. The mixture was heated to reflux and stirred overnight. The reaction
mixture was cooled to
rt and concentrated in vacuo. The residue was purified by column
chromatography on a silica gel
-69-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
eluted with PE/EA (v/v = 10/1) to give the title compound as a light yellow
semisolid (150 mg,
63 %).
Step 5: methyl 2-methoxy-11H-benzo[2,3][1,4]dioxepino[6,5-b]pyridine-7-
carboxylate
[00297] A mixture of methyl
4-((3-bromo-6-methoxypyridin-2-yl)methoxy)-3-hydroxybenzoate (150 mg, 0.4
mmol), cuprous
iodide (30 mg, 0.16 mmol), N,N-dimethylglycine (17 mg, 0.16 mmol) and cesium
carbonate (270
mg, 0.8 mmol) in anhydrous 1,4-dioxane (5 mL) was heated to reflux and stirred
overnight under
nitrogen atmosphere. The reaction mixture was cooled to rt, filtered and
concentrated in vacuo.
The residue was purified by column chromatography eluted with PE/EA (v/v =
5/1) to give the
title compound as a white solid (57 mg, 49 %).
Step 6: methyl 2-oxo-
2,11-dihydro-1H-benzo[2,3][1,4]dioxepino[6,5-b]pyridine
-7-carboxylate
[00298] To a mixture of methyl 2-methoxy-11H-benzo[2,3][1,4]dioxepino[6,5-
b]pyridine-7-
carboxylate (57 mg, 0.2 mmol) and sodium iodide (100 mg, 0.66 mmol) in
acetonitrile (5 mL)
was added trimethylchlorosilane (0.1 mL, 1.0 mmol) at rt. Then the mixture was
heated to 85 C
and stirred for 3 h, cooled to rt, quenched with saturated aquous sodium
thiosulfate (50 mL). The
resulting mixture was extracted with EA (50 mLx2). The combined organic layers
were washed
with saturated brine (30 mL), dried over anhydrous sodium sulfate, filtered
and concentrated.
The residue was purified by column chromatography eluted with
dichloromethane/methanol (v/v
= 30/1) to give the title compound as a light yellow solid (54 mg, 100 %).
[00299] MS (ESI, neg. ion) m/z: 272.0 [M-H].
Step 7: methyl 2-((5-cyclopropy1-3-(2,6-dichlorophenyl)isoxazol-4-yl)methoxy)-
11H-benzo
[2,3][1,4]dioxepino[6,5-b]pyridine-7-carboxylate
[00300] A mixture of methyl
2-oxo-2,11-dihydro-1H-benzo[2,3][1,4]dioxepino[6,5-b]pyridine -7-carboxylate
(55 mg, 0.2
mmol), 4-(chloromethy1)-5-cyclopropy1-3-(2,6-dichlorophenyl)isoxazole (100 mg,
0.3 mmol)
-70-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
(See the synthetic method of compound A6e in W02011020615) and potassium
phosphate (100
mg, 0.5 mmol) in DMF (5 mL) was heated to 60 C and stirred for 3 h. The
mixture was cooled
to rt, diluted with water (40 mL), extracted with EA (80 mLx2). The combined
organic layers
were washed with saturated brine (20 mL), dried over anhydrous sodium sulfate,
filtered and
concentrated in vacuo. The residue was purified by column chromatography on a
silica gel eluted
with PE/EA (v/v = 20/1) to give the title compound as light yellow oil (90 mg,
80 %).
[00301] MS (ESI, pos. ion) m/z: 539.0 [M+H].
Step 8: 2-45-cyclopropy1-3-(2,6-dichlorophenyl)isoxazol-4-yl)methoxy)-11H-
benzo [2,31[1,4]
dioxepino[6,5-b]pyridine-7-carboxylic acid
[00302] To a mixture of methyl 2-((5-cyclopropy1-3-(2,6-
dichlorophenyl)isoxazol
-4-yl)methoxy)-11H-benzo[2,3][1,4]dioxepino[6,5-b]pyridine-7-carboxylate (90
mg, 0.17 mmol)
in a mixed solvent of THF (2 mL) and water (1.75 mL) was added sodium
hydroxide (70 mg,
1.75 mmol). The reaction mixture was heated to 60 C and stirred overnight.
Then the mixture
was concentrated to remove most of the solvent, diluted with water (20mL). The
mixture was
adjusted to acidity with hydrochloric acid (1 M, 5 mL), and the resulting
mixture was extracted
with EA (30 mLx2). The combined oraganic layers were washed with saturated
brine (10 mL),
dried over anhydrous sodium sulfate, filtered and concentrated in vacuo to
give the title
compound as a light yellow solid (60 mg, 70 %).
[00303] MS (ESI, neg. ion) m/z: 523.0 [M-Hr;
[00304] 1H NMR (400 MHz, CDC13) 5 7.92 (d, J = 1.7 Hz, 1H), 7.75 (dd, J =
8.5, 1.8 Hz, 1H), 7.50 -
7.35 (m, 3H), 7.34 - 7.29 (m, 1H), 7.03 (d, J = 8.5 Hz, 1H), 6.60 (d, J = 8.8
Hz, 1H), 5.24 (s, 2H), 5.19 (s, 2H),
2.40 - 2.28 (m, 1H), 1.32 - 1.29 (m, 2H), 1.21 -1.15 (m, 211).
Example 3: 34(5-cyclopropy1-3-(2,6-dichlorophenyl)isoxazol-4-Amethoxy)-1a,10b-
dihydro
4H-benzo[6,71cyclopropa[4,5]Rixepinol[3,2-b]pyridine-8-carboxylic acid
-71-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
0
ri 4111 ci
* =
N
[00305] To a mixture of methyl 3-((5-cyclopropy1-3-(2,6-
dichlorophenyl)isoxazol
-4-yl)methoxy)-1 a,1 Ob-dihydro-1H-benzo [6,7] cyclopropa[4,5] oxepino[3,2-
b]pyridine-8-
carboxylate (12 mg, 0.02 mmol) and potassium fluoride (50 mg, 0.86 mmol) in
N-methyl-2-pyrrolidone (2 mL) was added thiophenol (100 mg, 0.91 mmol). Then
the reaction
mixture was heated to 200 C and stirred for 10 mins. The mixture was cooled
to rt and diluted
with water (20 mL), extracted with EA (50 mLx2). The combined organic layers
were dried over
with anhydrous sodium sulfate, filtered and concentrated. The residue was
purified by column
chromatography eluted with dichloromethane/methanol (v/v = 10/1) to give the
title compound
as a white solid (5.6 mg, 48 %).
[00306] MS (ESI, pos. ion) m/z: 535.4 [M+Hr;
[00307] 1H NMR (400 MHz, CDC13) 6 7.81 (d, J = 7.9 Hz, 1H), 7.77 (s, 1H),
7.43 (d, J =
7.9 Hz, 1H), 7.39 ¨ 7.29 (m, 3H), 7.18 (t, J = 8.1 Hz, 1H), 6.39 (d, J = 8.6
Hz, 1H), 5.20 (d, J =
1.7 Hz, 2H), 3.41 (t, J = 7.0 Hz, 1H), 3.02 (t, J = 7.0 Hz, 1H), 2.36 ¨ 2.29
(m, 1H), 1.69 ¨ 1.61
(m, 1H), 1.55 ¨ 1.48 (in, 1H), 1.28¨ 1.25 (m, 2H), 1.17¨ 1.13 (m , 2H).
Example 4:2-05-cyclopropy1-3-(2,6-dichlorophenyl)isoxazol-4-yl)methoxy)-9-
fluoro
-10,11-dihydrobenzo[6,71oxepino[3,2-b]pyridine-7-carboxylic acid
0
Cr- 41:1 CI
= HO -4 0
= C iN
N 0
Step 1: 4-bromo-2-fluoro-6-hydroxybenzaldehyde
-72-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
[00308] To a mixture of 4-bromo-2,6-difluorobenzaldehyde (2.0 g, 9.0 mmol)
in DMF (10
mL) was added dropwise slowly a solution of potassium hydroxide (1.0 g, 18.0
mmol,) in water
(15 mL). The reaction mixture was heated to 60 C, stirred for 2 h and then
cooled to rt, diluted
with water (20 mL). The mixture was adjusted to acidity with hydrochloric acid
(2 M, 15 mL)
and the resulting mixture was extracted with EA (50 mLx2). The combined
organic layers were
washed with saturated brine (10 mL), dried over with anhydrous sodium sulfate,
filtered and
concentrated in vacuo. The residue was purified by column chromatography
eluted with PE/EA
(v/v = 20/1) to give the title compound as yellow oil (1.5 g, 76 %).
[00309] MS (ESI, neg. ion) m/z: 217.0 [M-1-1]-.;
Step 2: 4-bromo-2-fluoro-6-(methoxymethoxy)benzaldehyde
[00310] To a mixture of 4-bromo-2-fluoro-6-hydroxybenzaldehyde (7.5 g, 34
mmol) and
diisopropylethylamine (11.0 mL, 66 mmol) in dichloromethane (100 mL) was added
dropwise
chloromethyl methyl ether (4.1 mL, 51 mmol) under an ice bath condition. Then
the reaction
mixture was stirred at rt overnight. The mixture was quenched with water (200
mL), extracted
with dichloromethane (50 mLx2). The combined organic layers were washed with
saturated
brine (10 mL), dried over anhydrous sodium sulfate, filtered and concentrated
in vacuo. The
residue was purified by column chromatography on a silica gel eluted with
PE/EA (v/v = 20/1) to
give the title compound as a yellow solid (4.3 g, 48 %).
[00311] 1H NMR (400 MHz, CDC13) 8 10.39 (s, 1H), 7.24 (s, 1H), 7.01 (dd, J
= 9.9, 1.0 Hz,
1H), 5.31 (s, 2H), 3.54 (s, 3H).
Step 3: 3-bromo-2-(4-bromo-2-fluoro-6-(methoxymethoxy)styry1)-6-
methoxypyridine
[00312] To a mixture of diethyl ((3-bromo-6-methoxypyridin-2-
yl)methyl)phosphonate (2.5
g, 7.4 mmol) in THF (40 mL) was added sodium hydride (0.29 g, 7.3 mmol, 60%)
under an ice
bath condition. The mixture was stirred for 0.5 h at this temperature,
followed by addition of a
solution of 4-bromo-2-fluoro-6-(methoxymethoxy) benzaldehyde (1.5 g, 5.7 mmol)
in THF (20
mL). Then the reaction mixture was warmed to rt and stirred for further 5 h.
The reaction was
-73-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
quenched with saturated aquous ammonium chloride (50 mL). The resulting
mixture was
extracted with EA (60 mLx2). The combined organic layers were washed with
saturated brine
(20 mL), dried over anhydrous sodium sulfate, filtered and concentrated in
vacuo. The residue
was purified by column chromatography eluted with PE/EA (v/v = 10/1) to give
the title
compound as a white solid (1.5 g, 60 %).
Step 4: 3-bromo-2-(4-bromo-2-fluoro-6-(methoxymethoxy)phenethyl)-6-
methoxypyridine
[00313] A mixture of
3-bromo-2-(4-bromo-2-fluoro-6-(methoxymethoxy)styry1)-6-methoxypyridine (1.25
g, 2.8
mmol), sodium acetate (1.38 g, 16.8 mmol) and p-toluenesulfonyl hydrazide
(3.12 g, 16.8 mmol)
in a mixed solvent of THF (40 mL) and water (20 mL) was heated to reflux for 7
h. Then the
reaction mixture was cooled to rt, diluted with water (200 mL), extracted with
EA (100 mLx2).
The combined organic layers were washed with saturated brine (20 mL), dried
over anhydrous
sodium sulfate, filtered and concentrated in vacuo. The residue was purified
by column
chromatography on a silica gel eluted with PE/EA (v/v = 20/1) to give the
title compound as a
solid (1.2 g, 96 %).
[00314] MS (ESI, pos. ion) m/z: 447.9 [M+H].
Step 5: 5-bromo-2-(2-(3-bromo-6-methoxypyridin-2-yflethyl)-3-fluorophenol
[00315] To a mixture of 3-bromo-2-(4-bromo-2-fluoro-6-
(methoxymethoxy)phenethyl)
-6-methoxypyridine (1.5 g, 3.3 mmol) in THF (30 mL) was added hydrochloric
acid (6 mL, 36
mmol). Then the mixture was stirred at 50 C overnight, then cooled to rt,
diluted with water (20
mL) and adjusted to alkalinity with potassium carbonate. The resulting mixture
was extracted
with EA (30 mLx2). The combined organic layers were washed with saturated
brine (10 mL),
dried over anhydrous sodium sulfate, filtered and concentrated in vacuo. The
residue was
purified by column chromatography eluted with PE/EA (v/v = 20/1) to give the
title compound
as a white solid (1.2 g, 89 %).
Step 6: 7-bromo-9-fluoro-2-methoxy-10,11-dihydrobenzo[6,71oxepino[3,2-
b]pyridine
-74-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
[00316] A mixture of cuprous iodide (94 mg, 0.50 mmol), N,N-dimethylglycine
(120 mg,
1.16 mmol), cesium carbonate (1.3 g, 4.0 mmol) and 5-bromo-2-(2-(3-bromo-6-
methoxypyridin
-2-yl)ethyl)-3-fluorophenol (800 mg, 2.0 mmol) in 1,4-dioxane (40 mL) was
heated to reflux
overnight under nitrogen atomsphere. The mixture was cooled to rt, filtered
and concentrated in
vacuo. The residue was purified by column chromatography eluted with PE/EA
(v/v, 15/1) to
give the title compound as a white solid (400 mg, 60 %).
[00317] '1-1 NMR (400 MHz, CDC13) 6 7.37 (d, J = 8.7 Hz, 1H), 7.16 (s, 1H),
7.02 (dd, J
8.7, 1.7 Hz, 1H), 6.56 (d, J = 8.7 Hz, 1H), 3.90 (s, 3H), 3.25 ¨3.18 (m, 2H),
3.12 ¨ 3.06 (m, 2H).
Step 7: methyl 9-fluoro-2-methoxy-10,11-dihydrobenzo[6,7]oxepino[3,2-
1Apyridine-7-
carboxylate
[00318] A mixture of
7-bromo-9-fluoro-2-methoxy-10,11-dihydrobenzo[6,7]oxepino[3,2-b]pyridine (0.5
g, 1.5 mmol),
triethylamine (0.4 mL, 3.0 mmol) and 1,1'-
bis(diphenylphosphino)ferrocene]palladium(II)
chloride (10 mg, 0.015 mmol) in methanol (30 mL) was stirred at 100 C in an
autoclave under
carbon monoxide atmosphere (3.0 Mpa) for 2 days. Then the reaction mixture was
cooled to rt
and concentrated in vacuo to remove the solvent. The residue was purified by
column
chromatography eluted with PE/EA (v/v= 30/1) to give the title compound as a
white solid (300
mg, 60 %).
Step 8: methyl 9-fluoro-2-oxo-1,2,10,11-tetrahydrobenzo[6,71oxepino[3,2-
blpyridine-7-
carboxylate
[00319] To a mixture of methyl
9-fluoro-2-methoxy-10,11-dihydrobenzo[6,7]oxepino[3,2-b]pyridine -7-
carboxylate (310 mg, 1.0
mmol) and sodium iodide (460 mg, 3.1 mmol) in acetonitrile (9 mL) was added
trimethylchlorosilane (0.27 mL, 3.1 mmol) at rt. Then the reaction mixture was
heated to 85 C
and stirred for 1.5 h, then cooled to rt and quenched with saturated aquous
sodium thiosulfate (50
mL). The resulting mixture was extracted with EA (50 mLx2), washed with
saturated brine (30
-75-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
mL), dried over anhydrous sodium sulfate, filtered and concentrated in vacuo.
The residue was
purified by column chromatography eluted with dichloromethane/methanol (v/v =
15/1) to give
the title compound as a yellow solid (290 mg, 98 %).
[00320] MS (ESI, pos. ion) m/z: 290.0 [M+H]+.
Step 9: methyl
2-45-cyclopropy1-3-(2,6-dichlorophenyflisoxazol-4-yflmethoxy)-9-fluoro-10,11
-dihydrobenzo[6,7]oxepino[3,2-b]pyridine-7-carboxylate
[00321] A mixture of methyl
9-fluoro-2-oxo-1,2,10,11-tetrahydrobenzo[6,7]oxepino[3,2-b]pyridine -7-
carboxylate (310 mg,
1.1 mmol), 4-(chloromethyl)-5-cyclopropy1-3-(2,6-dichlorophenyl) isoxazole
(420 mg, 1.4
mmol) and potassium phosphate (450 mg, 2.1 mmol) in DMF (20 mL) was stirred at
50 C for 4
h. Then the reaction mixture was cooled to rt and diluted with water (30 mL).
The resulting
mixture was extracted with EA (40 mLx2). The combined organic layers were
washed with
saturated brine (20 mL), dried over anhydrous sodium sulfate, filtered and
concentrated. The
residue was purified by column chromatography on a silica gel eluted with
PE/EA (v/v = 4/1) to
give the title compound as yellow oil (290 mg, 49 %).
Step 10: 2- ((5-cycl op ropy1-3 -(2,6-dichlo rophenyl)isoxazol-4-yl)m eth oxy)-
9-fluoro-1 0,11
-dihydrobenzo[6,7]oxepino[3,2-b]pyridine-7-carboxylic acid
[00322] To a mixture of methyl
24(5 -cyclopropy1-3-(2,6-dichlorophenyl)isoxazol-4-yl)methoxy)
-9-fluoro-10,11-dihydrobenzo[6,7]oxepino[3,2-b]pyridine-7-carboxylate (290 mg,
0.52 mmol) in
a mixed solvent of THF (9 mL) and water (9 mL) was added sodium hydroxide (72
mg, 1.8
mmol). Then the reaction mixture was stirred at 60 C overnight. The reaction
mixture was
cooled to rt, concentrated to remove most of the solvent under reduced
pressure. The residue was
diluted with water (10 mL), adjusted to acidity with hydrochloric acid (2 M, 2
mL). The resulting
mixture was extracted with EA (30 mL x2). The combined organic layers were
washed with
-76-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
saturated brine (10 mL), dried over anhydrous sodium sulfate, filtered and
concentrated in vacuo.
The residue was purified by column chromatography eluted with PE/EA (v/v, 1/1)
to give the
title compound as a light yellow solid (270 mg, 96 %).
[00323] MS (ESI, pos. ion) m/z: 541.0 [M+H]+;
[00324] 1H NMR (400 MHz, CDC13) 8 7.70 (s, 1H), 7.57 (d, J = 9.2 Hz, 1H),
7.41 ¨ 7.34
(m, 3H), 7.28 ¨7.25 (m, 1H), 6.44 (d, J = 8.7 Hz, 1H), 5.16 (s, 2H), 3.24
¨3.10 (m, 4H), 2.31 ¨
2.27 (m, 1H), 1.32¨ 1.29 (m, 2H), 1.20 ¨ 1.12 (m, 2H).
Example 5 : 24(5 -cyclop ropy1-3 - (2,6-dichl orophenypisoxazol -4-yl)methoxy)-
11 oroben zo
[6,7] oxepino [3,2-b] pyridine-7-carboxylic acid
0
011
CI CI
0
411
Step 1: 4-bromo-2-(methoxymethoxy)benzaldehyde
[00325] Using 4-bromo-2-hydroxybenzaldehyde (15.0 g, 74.6 mmol) as a
starting material,
the title compound was prepared according to the procedure described in step 5
of example 1 as a
white solid (6.0 g, 98 %).
Step 2: methyl 4-formy1-3-(methoxymethoxy)benzoate
[00326] Using 4-bromo-2-(methoxymethoxy)benzaldehyde (2.9 g, 11.8 mmol) as
a starting
material, the title compound was prepared according to the procedure described
in step 7 of
example 4 as a white solid (2.6 g, 98 %).
Step 3: 3-bromo-2-(bromomethyl)-6-methoxypyridine
[00327] Using 3-bromo-6-methoxy-2-methylpyridine (20.0 g, 99 mmol) as a
starting
material, the title compound was prepared according to the procedure described
in step 2 of
example 1 as colourless oil (26.5 g, 95 %).
Step 4: diethyl ((3-bromo-6-methoxypyridin-2-yl)methyl)phosphonate
-77-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
[00328] Using 3-bromo-2-(bromomethyl)-6-methoxypyridine (26.5 g, 94 mmol)
as a
starting material, the title compound was prepared according to the procedure
described in step 3
of example 1 as light yellow oil (20.7 g, 65 %).
[00329] MS (ESI, pos. ion) m/z: 338.0 [M+H]+.
Step 5: diethyl ((3-bromo-6-methoxypyridin-2-y0fluoromethyl)phosphonate
[00330] Using diethyl ((3-bromo-6-methoxypyridin-2-yl)methyl)phosphonate
(3.0 g, 8.9
mmol) as a starting material, the title compound was prepared according to the
procedure
described in step 6 of example 1 as light yellow oil (2.8 g, 87 %).
[00331] MS (ESI, pos. ion) m/z: 356.3 [M+H]4.
Step 6: methyl 4-(2-(3-bromo-6-methoxypyridin-2-y1)-2-fluoroviny1)-3-
(methoxymethoxy)
benzoate
[00332] Using diethyl ((3-bromo-6-methoxypyridin-2-
yl)fluoromethyl)phosphonate (2.0 g,
5.8 mmol) and methyl 4-formy1-3-(methoxymethoxy)benzoate (1.0 g, 4.5 mmol) as
a starting
material, the title compound was prepared according to the procedure described
in step 7 of
example 1 as yellow oil (1.26 g, 66 %).
Step 7: methyl 4-(2-(3-bromo-6-methoxypyridin-2-yl)-2-fluoroviny1)-3-
hydroxybenzoate
[00333] Using methyl
4-(2-(3-bromo-6-methoxypyridin-2-y1)-2-fluoroviny1)-3-(methoxymethoxy)
benzoate (1.26 g,
3.0 mmol) as a starting material, the title compound was prepared according to
the procedure
described in step 8 of example 1 as a yellow solid (1.0 g, 88 %).
[00334] MS (ESI, pos. ion) m/z: 382.0 {M+H}.
Step 8: methyl 11-fluoro-2-methoxybenzo[6,7]oxepino[3,2-b]pyridine-7-
carboxylate
[00335] Using methyl
4-(2-(3-bromo-6-methoxypyridin-2-y1)-2-fluoroviny1)-3-hydroxybenzoate (0.89 g,
2.3 mmol) as
a starting material, the title compound was prepared according to the
procedure described in step
9 of example 1 as a yellow solid (0.31 g, 44 %).
-78-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
[00336] MS (ESI, pos. ion) m/z: 302.1 [M+H]4;
Step 9: methyl 11-fluoro-2-oxo-1,2-dihydrobenzo[6,7]oxepino[3,2-b]pyridine-7-
carboxylate
[00337] Using methyl 11-fluoro-2-methoxybenzo[6,7]oxepino[3,2-b]pyridine-7-
carboxylate
(310 mg, 1.0 mmol) as a starting material, the title compound was prepared
according to the
procedure described in step 10 of example 1 as a grey solid (200 mg, 68 %).
[00338] MS (ESI, pos. ion) m/z: 288.1 [M+H].
Step 10: methyl 2-((5-cyclopropy1-3-(2,6-dichlorophenyl)isoxazol-4-yOmethoxy)-
11
-fluorobenzo [6,7] oxepino [3,2-b]pyridine-7-carboxylate
[00339] Using methyl
11 -fl u oro-2-ox o-1,2-di h ydrobenzo [6,7] ox epin o [3,2-b]pyridine-7-
carboxyl at e (200 mg, 0.7
mmol) and 4-(chloromethyl)-5-cyclopropy1-3-(2,6-dichlorophenyl)isoxazole (280
mg, 0.9 mmol)
as a starting material, the title compound was prepared according to the
procedure described in
step 11 of example 1 as a grey solid (260 mg, 67 %).
Step 11:
24(5 -cyclop ropyl-3-(2,6-d ichlorophenyl) isoxazol-4-y1) methoxy)- 11 -
fluorobenzo [6,7]
oxepino[3,2-b]pyridine-7-carboxylic acid
[00340] Using methyl 24(5-cyclopropy1-3-(2,6-dichlorophenyl)isoxazol-4-
yl)methoxy)-11-
fluorobenzo[6,7]oxepino[3,2-b]pyridine-7-carboxylate (260 mg, 0.47 mmol) as a
starting
material, the title compound was prepared according to the procedure described
in step 12 of
example 1 as a grey solid (190 mg, 74 %).
[00341] MS (ESI, pos. ion) m/z: 539.0 [M+H]t;
[00342] 1H NMR (400 MHz, CDC13) S 7.92 ¨ 7.89 (m, 2H), 7.45 (d, J = 8.7 Hz,
1H), 7.37 ¨
7.35 (m, 2H), 7.33 ¨ 7.21 (m, 2H), 6.80 ¨ 6.65 (m, 2H), 5.24 (s, 2H), 2.48
¨2.42 (m, 114), 1.36 ¨
1.30 (m, 2H), 1.18 ¨ 1.13 (m, 2H).
Example 6: 24(5-
cyclopropy1-3-(2,6-dichlorophenyl)isoxazol-4-yl)methoxy)-8-fluoro
-10,11-dihydrobenzo[6,7]oxepino[3,2-b}pyridine-7-carboxylic acid
-79-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakcs Rcf.: 75920/00027
0
14,
CI CI
HO
0 ---
/ 0 \
[00343] Using 4-bromo-5-fluoro-2-hydroxybenzaldehyde as a starting
material, the title
compound was prepared according to the procedure described in example 4 as a
yellow solid
(320 mg).
[00344] MS (ESI, pos. ion) m/z: 541.0 [M+H];
[00345] 1H NMR (400 MHz, CDC13) 7.77 (d, J = 6.3 Hz, 1H), 7.40 ¨ 7.33 (m,
3H), 7.28 ¨
7.25 (m, 1H), 7.04 (d, J = 10.5 Hz, 1H), 6.42 (d, J = 8.7 Hz, 1H), 5.12 (s,
2H), 3.22 ¨ 3.06 (m,
4H), 2.37 ¨ 2.28 (m, 1H), 1.31 ¨ 1.27 (m, 2H), 1.18 ¨1.11 (m, 2H).
Example 7: 2-05-cyclopropy1-3-(2,6-dichlorophenypisoxazol-4-ypmethoxy)-
11-
(trifluoromethybbenzo [6,7] oxepino[3,2-b]pyridin e-7-carboxylic acid
0
CI 111/111 CI
HO
* 0 ---
0 C:01:1
CF3
Step 1: 1-(3-bromo-6-methoxypyridin-2-y1)-2,2,2-trifluoroethanol
[00346] To a mixture of 3-bromo-6-methoxypicolinaldehyde (5.0 g, 23 mmol)
in THF (10
mL) were added trimethyl(trifluoromethyl)silane (5.2 mL, 35 mmol) and a
solution of
tetrabutylammonium fluoride in THF (1.2 mL, 1.2 mmol, 1 mol/L). Then the
reaction mixture
was stirred at rt overnight. The mixture was concentrated in vacuo to remove
most of the
solvent.The residue was diluted with water (50 mL) and the resulting mixture
was extracted with
EA (50 mLx2). The combined organic layers were washed with saturated brine (30
mL), dried
over anhydrous sodium sulfate, filtered and concentrated in vacuo. The residue
was purified by
-80-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
column chromatography eluted with PE/EA (v/v = 30/1) to give the title
compound as a light
yellow solid (4.1 g, 62 %).
Step 2: 1-(3-bromo-6-methoxypyridin-2-y1)-2,2,2-trifluoroethanone
[00347] To a mixture of 1-(3-bromo-6-methoxypyridin-2-y1)-2,2,2-
trifluoroethanol (500 mg,
1.75 mmol) in anhydrous dichloromethane (4.5 mL) were added 2,6-lutidine (0.46
mL, 3.9
mmol) and 4-acetamido-2,2,6,6-tetramethyl-1-oxopiperidinium tetrafluoroborate
(1.31 g, 4.37
mmol) at rt. The reaction mixture was stirred overnight, then concentrated
under reduced
pressure. The residue was diluted with ethyl ether (50 mL) followed by
stirring for further 0.5 h,
filtered to remove the insoluble substance. The filtrate was concentrated in
vacuo. The residue
was purified by column chromatography eluted with PE to give the title
compound as yellow oil
(360 mg, 73 %).
Step 3: methyl 4-(2-(3-bromo-6-methoxypyridin-2-y1)-3,3,3-trifluoroprop-1-en-1-
3,0-3-
(methoxymethoxy)ben zoate
[00348] Using 1-(3-bromo-6-methoxypyridin-2-y1)-2,2,2-trifluoroethanone
(2.0 g, 7.0
mmol) and methyl 4-((diethoxyphosphoryl)methyl)-3-(methoxymethoxy)benzoate
(2.8 g, 8.1
mmol) as a starting material, the title compound was prepared according to the
procedure
described in step 7 of example 1 as yellow oil (1.1 g, 33 %).
[00349] MS (ESI, pos. ion) m/z: 476.4 [M+H].
Step 4: methyl 4-(2-(3-bromo-6-methoxypyridin-2-y1)-3,3,3-trifluoroprop-1-en-l-
y1)
-3-hydroxybenzoate
[00350] Using methyl
44243 -bromo-6-methox ypyridin-2-y1)-3,3,3-trifluoroprop-1-en-1-y1)-3-(methox
ymethoxy)benz
oate (1.1 g, 2.3 mmol) as a starting material, the title compound was prepared
according to the
procedure described in step 8 of example 1 as yellow oil (0.96 g, 96 %).
[00351] MS (EST, pos. ion) m/z: 431.9 [M+H]+.
Step 5: methyl
-81-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
2-methoxy -11-(tri fluoromethyl)ben zo [6,7] oxepino [3,2-b]pyridine-7-
carboxylate
[00352] Using methyl
4 -(2-(3-bromo-6-methox ypyridin-2-y1)-3,3,3 -trifluoroprop-1 -en-l-y1)-3 -
hydroxybenzoate (960
mg, 2.2 mmol) as a starting material, the title compound was prepared
according to the procedure
described in step 9 of example 1 as a yellow solid (530 mg, 68 %).
[00353] MS (ESI, pos. ion) m/z: 352.3 [M+H].
Step 6: methyl 2-oxo-11-(trifluoromethyl)-1,2-dihydrobenzo[6,71oxepino[3,2-
b]pyridine
-7-carboxylate
[00354] Using methyl
2-methoxy-11-(trifluoromethyl)benzo[6,7]oxepino[3,2-b]pyridine-7-carboxylate
(250 mg, 0.72
mmol) as a starting material, the title compound was prepared according to the
procedure
described in step 10 of example 1 as a yellow solid (220 mg, 92 %).
[00355] MS (ESI, pos. ion) m/z: 338.0 [M+H].
Step 7: methyl 2-((5-cyclopropy1-3-(2,6-dichlorophenyl)isoxazol-4-yl)methoxy)-
11
-(trifluoromethyl)benzo [6,7] oxepino [3,2 -b]pyrid in e-7-carboxylate
[00356] Using methyl
2-oxo-11-(trifluoromethyl)-1,2-dihydrobenzo[6,7]oxepino[3,2-b]pyridine -7-
carboxylate (220
mg, 0.65 mmol) and 4-(chloromethyl)-5-cyclopropy1-3-(2,6-dichlorophenyl)
isoxazole (280 mg,
0.9 mmol) as a starting material, the title compound was prepared according to
the procedure
described in step 11 of example 1 as a yellow solid (368 mg, 93 %).
[00357] MS (ESI, pos. ion) m/z: 603.0 [M+H].
Step 8: 2-((5-cyclopropy1-3-(2,6-dichlorophenyl)isoxazol-4-yl)methoxy)-11-
(trifluoromethyl)
benzo [6,7]oxepino [3,2-b]pyridin e-7-carboxylic acid
[00358] Using methyl 2-45-cyclopropy1-3-(2,6-dichlorophenyl)isoxazol-4-
y1)methoxy)-11
-(trifluoromethyl)benzo[6,7]oxepino[3,2-b]pyridine-7-carboxylate (368 mg, 0.61
mmol) as a
starting material, the title compound was prepared according to the procedure
described in step
-82-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
12 of example 1 as a yellow solid (240 mg, 95 %).
[00359] MS (ESI, pos. ion) m/z: 589.4 [M+H]+;
[00360] 1H NMR (400 MHz, DMSO-do) 8 13.42 (s, 1H), 7.89 - 7.86 (m, 3H),
7.83 - 7.77
(m, 2H), 7.31 (d, J = 8.1 Hz, 2H), 7.10 (t, J = 8.1 Hz, 1H), 6.76 (d, J = 8.8
Hz, 1H), 5.25 (s, 2H),
2.46 - 2.37 (m, 1H), 1.17 - 1.11 (m, 4H).
Example 8: 24(5-
cyclopropy1-3-(2,6-dichlorophenypisoxazol-4-yl)methoxy)-11
-(trifluoromethyl)-10,11-dihydrobenzo [6, 7]oxepino [3,2-b]pyridine-7-
carboxylic acid
,
0 1
-` CI
NO 0
* 'N
CF3
Step 1: methyl 2-methoxy-11-(trifluoromethyl)-10,11-
dihydrobenzo[6,7]oxepino[3,2-b]
pyridine-7-carboxylate
[00361] To a mixture of methyl 2-methoxy-11-
(trifluoromethyl)benzo[6,7]oxepino[3,2-b]
pyridine-7-carboxylate (120 mg, 0.34 mmol) in EA (12 mL) was added 10% Pd/C
(56 mg) under
hydrogen atmosphere and stirred at rt overnight. The mixture was filtered and
concentrated in
vacuo. The residue was purified by column chromatography eluted with PE/EA
(v/v = 10/1) to
give the title compound as colourless oil (116 mg, 96 %).
[00362] MS (ESI, pos. ion) m/z: 354.0 [MA-Hr.
Step 2: methyl 2-oxo-11-(trifluoromethyl)-1,2,10,11-
tetrahydrobenzo[6,7]oxepino[3,2-b]
pyridine-7-carboxylate
[00363] Using methyl
2-methox y-11 -(trifluoromethyl)-10,11-dihydrobenzo [ 6,7] oxepino[3,2-
b]pyridine -7-carboxyl ate
(116 mg, 0.33 mmol) as a starting material, the title compound was prepared
according to the
procedure described in step 10 of example 1 as a yellow solid (110 mg, 99 %).
[00364] MS (ESI, pos. ion) m/z: 340.0 [M+H].
-83-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
Step 3: methyl 2-05-cyclopropy1-3-(2,6-dichlorophenyl)isoxazol-4-yl)methoxy)-
11
-(trifluoromethyl)-10,11 -dihydrobenzo [6,7] oxepino [3,2-b]pyridine-7-
carboxylate
[00365] Using methyl
2-oxo-11-(triflu orometh y1)-1,2,10,11 -tetrahyd robenzo [ 6,7] oxepino [3,2-
b] pyridine-7-carbox ylate
(90 mg, 0.26 mmol) and 4-(ehloromethyl)-5-cyclopropyl-3- (2,6-
dichlorophenyl)isoxazole (110
mg, 0.36 mmol) as a starting material, the title compound was prepared
according to the
procedure described in step 11 of example 1 as colourless oil (110 mg, 69 %).
[00366] MS (ESI, pos. ion) m/z: 605.0 [M+H].
Step 4: 2-05-cyclopropyl-3-(2,6-dichlorophenybisoxazol-4-yl)methoxy)-11-
(trifluoromethyl)
-10,11-dihydrobenzo[6,7]oxepino [3,2-blpyridine- 7-carboxylic acid
[00367] Using methyl 24(5-cyclopropy1-3-(2,6-dichlorophenyl)isoxazol-4-
yl)methoxy)-11-
(trifluoromethyl)-10,11-dihydrobenzo[6,7]oxepino[3,2-b]pyridine-7-carboxylate
(110 mg, 0.18
mmol) as a starting material, the title compound was prepared according to the
procedure
described in step 12 of example 1 as a white solid (78 mg, 73 %).
[00368] MS (ESI, pos. ion) m/z: 591.0 [M+H]+;
[00369] 1H NMR (600 MHz, DMSO-d6) ö 13.15 (s, 1H), 7.74 ¨ 7.69 (m, 3H),
7.55 (d, J =
8.1 Hz, 1H), 7.50 (d, J = 7.8 Hz, 1H), 7.46 (d, J = 8.0 Hz, 1H), 7.38 (t, J =
8.1 Hz, 1H), 6.63 (d,
= 8.8 Hz, 1H), 5.28 (d, J = 13.0 Hz, 1H), 5.06 (d, J = 13.0 Hz, 1H), 4.15 ¨
4.09 (m, 1H), 3.51 ¨
3.48 (m, 111), 3.30 ¨ 3.27 (m, 1H), 2.45 ¨2.41 (m, 1H), 1.18 ¨ 1.12 (m, 4H).
Example 9: 2-05-cyclopropy1-3-(2,6-dichlorophenypisoxazol-4-yl)methoxy)-10H-
spiro
Lbenzo[6,7)oxepino[3,2-b)pyridine-11,1'-cyclopropanel-7-carboxylic acid
0
HOJt\
1011
CI
CI
0
0 1,
0
Step 1: methyl 4-bromo-2-(methoxymethoxy)benzoate
-84-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
[00370] To a
mixture of methyl 4-bromo-2-hydroxybenzoate (10 g, 43 mmol) and
diisopropylethylamine (11.0 mL, 65 mmol) in dichloromethane (100 mL) was added
dropwise
chloromethyl methyl ether (4.9 mL, 65 mmol) under an ice bath condition. Then
the reaction
mixture was warmed to rt and stirred overnight. The reaction was quenched with
water (200
mL), and the resulting mixture was extracted with dichloromethane (150 mLx2).
The combined
organic layers were washed with saturated brine (10 mL), dried over anhydrous
sodium sulfate,
filtered and concentrated in vacuo. The residue was purified by column
chromatography eluted
with PE/EA (v/v = 20/1) to give the title compound as colourless oil (10 g, 84
%).
Step 2:
1-(4-bromo-2-(methoxymethoxy)pheny1)-2-(3-bromo-6-methoxypyridin-2-yl)ethanone
[00371] To a
mixture of methyl 4-bromo-2-(methoxymethoxy)benzoate (11 g, 40.0 mmol)
and 3-bromo-6-methoxy-2-methyl-pyridine (8.08 g, 40.0 mmol) in THF (100 mL)
was added
dropwise a solution of sodium bis(trimethylsilyl)amide in THF (40 mL, 80 mmol,
2.0 M) under
an ice bath condition and nitrogen atmosphere. The reaction mixture was warmed
to rt and
stirred for 3 h. The reaction was quenched with saturated aquous ammonium
chloride (50 mL),
then the resulting mixture was diluted with water (100 mL) and extracted with
EA (150 mLx2).
The combined organic layers were washed with saturated brine (20 mL), dried
over anhydrous
sodium sulfate, filtered and concentrated. The residue was purified by column
chromatography
on a silica gel eluted with PE/EA (v/v = 20/1) to give the title compound as a
yellow solid (11 g,
62 %).
Step 3: (4-bromo-
2-(methoxymethoxy)phenyl)(1-(3-bromo-6-methoxypyridin-2-y1)
cyclopropyl)methanone
[00372] Under nitrogen atmosphere, to a mixture of
1-(4-bromo-2-(methoxymethoxy)phenyl) -2-(3-bromo-6-methoxypyridin-2-
yl)ethanone (2.0 g,
4.5 mmol) in toluene (60 mL) was added tetrabutylammonium hydrogen sulfate
(0.15 g, 0.45
mmol) and a solution of sodium hydroxide (6.24 g, 156 mmol) in water (6 mL) at
rt. The
-85-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
reaction mixture was stirred at rt for 10 mins, followed by addition of 1,2-
dibromoethane (1.0
mL, 11.7 mmol) and then the mixture was stirred at rt overnight. The reaction
mixture was
diluted with water (50 mL) and extracted with EA (60 mLx2). The combined
organic layers were
washed with saturated brine (20 mL), dried over anhydrous sodium sulfate,
filtered and
concentrated in vacuo. The residue was purified by column chromatography
eluted with PE/EA
(v/v = 30/1) to give the title compound as yellow oil (1.9 g, 90 %).
[00373] 1H NMR (400 MHz, CDC13) 6 7.52 (d, J = 8.6 Hz, 1H), 7.33 - 7.25 (m,
2H), 6.98
(d, J = 8.2 Hz, 1H), 6.48 (d, J = 8.6 Hz, 1H), 5.13 (s, 2H), 3.94 (s, 3H),
3.49 (s, 3H), 1.92 - 1.87
(m, 2H), 1.65 - 1.61 (m, 2H).
Step 4: (4-bromo-2-hydroxyphenyl)(1-(3-bromo-6-methoxypyridin-2-ypcyclopropyl)
methanone
[00374] To a mixture of
(4-bromo-2-(methoxymethoxy)phenyl)(1-(3-bromo-6-methox ypyridin
-2-yl)cyclopropyl)methanone (2.24 g, 4.8 mmol) in THF (30 mL) was added
hydrochloric acid
(8 mL, 48 mmol, 6 M). The reaction mixture was heated to 50 C and stirred for
3 h. The
reaction mixture was cooled to rt, diluted with water (20 mL), and adjusted to
alkalinity with
potassium carbonate. The resulting mixture was extracted with EA (30 mLx2).
The combined
organic layers were washed with saturated brine (10 mL), dried over anhydrous
sodium sulfate,
filtered and the filtrate was concentrated in vacuo. The residue was purified
by column
chromatography eluted with PE/EA (v/v = 20/1) to give the title compound as
yellow oil (1.56 g,
77 %).
Step 5: 5-bromo-2-(1-(3-bromo-6-methoxypyridin-2-
yl)cyclopropanecarbonyl)phenyl
methyl carbonate
[00375] To a solution of (4-bromo-2-hydroxyphenyl)(1-(3-bromo-6-
methoxypyridin-2-y1)
cyclopropyl)methanone (1.56 g, 3.7 mmol) in anhydrous THF (40 mL) was added
dropwise
triethylamine (0.77 mL, 5.5 mmol) and chloromethyl formate (0.33 mL, 4.2 mmol)
under an ice
-86-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
bath condition and nitrogen atmosphere. The reaction mixture was stirred for
1.5 h under the ice
bath condition. The mixture was filtered to give a light yellow solution of
the title compound (1.7
g, 96 %) in THF and used in next step directly without concentration.
Step 6: 5-bromo-2-((1-(3-bromo-6-methoxypyridin-2-yl)cyclopropyl)methyl)phenol
[00376] To a sodium borohydride (0.552 g, 14.6 mmol) in water (20 mL) was
added a
solution of 5-bromo-2-(1-(3-bromo -6-methoxypyridin-2-yl)cyclopropanecarb
onyl)ph en yl methyl
carbonate (1.7 g, 3.7 mmol) in THF (40 mL) under an ice bath. The reaction
mixture was stirred
at rt overnight, diluted with water (100 mL) and extracted with EA (100 mLx2).
The combined
organic layers were washed with saturated brine (20 mL), dried over anhydrous
sodium sulfate,
filtered and concentrated in vacuo. The residue was purified by column
chromatography eluted
with PE/EA (v/v = 20/1) to give the title compound as yellow oil (1.3 g, 86
%).
[00377] MS (ESI, pos. ion) m/z: 411.9 [M+H].
Step 7:
7-bromo-2-methoxy-10H-spiro [ben zo [6,7]oxepino [3,2-b] pyridine- 11 , 1 -
cydopropane]
[00378] A mixture of cuprous iodide (28 mg, 0.15 mmol), N,N-dimethylglycine
(30 mg,
0.30 mmol), cesium carbonate (470 mg, 1.4 mmol)
and
5-bromo-241-(3-bromo-6-methoxypyridin-2-y1) cyclopropyl)methyl)phenol (300 mg,
0.70
mmol) in 1,4-dioxane (20 mL) was heated to reflux and stirred for 2.5 h under
nitrogen
atmosphere. The reaction mixture was cooled to rt, filtered and the filtrate
was concentrated
under reduced pressure. The residue was purified by column chromatography
eluted with PE/EA
(v/v = 20/1) to give the title compound as colourless oil (92 mg, 40 %).
[00379] MS (ESI, pos. ion) m/z: 332.0 [M+H].
Step 8: methyl 2-methoxy-10H-spiro[benzo[6,7]oxepino[3,2-b]pyridine-11,1'-
cyclopropane]
-7-carboxylate
[00380] A mixture of
7-bromo -2-methox y-10H-spiro [benzo [6,7] oxepino [3,2-b]pyri dine-11,1 '-
cyclopropane] (0.44 g,
-87-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakcs Ref.: 75920/00027
1.3 mmol), triethylamine (0.4 mL, 3.0 mmol) and [1,1'-
bis(diphenylphosphino)ferrocene]
dichloropalladium (10 mg, 0.015 mmol) in methanol (30 mL) was heated to 100 C
and stirred
for 2 days in an autoclave under carbon monoxide (3.0 MPa). The reaction
mixture was cooled to
rt, concentrated to remove the solvent under reduced pressure. The residue was
purified by
column chromatography eluted with PE/EA (v/v = 30/1) to give the title
compound as a white
solid (280 mg, 68 %).
[00381] 1H NMR
(400 MHz, CDC13) 6 7.86 ¨ 7.80 (m, 2H), 7.41 (d, J = 8.6 Hz, 1H), 7.25
(d, J = 7.7 Hz, 1H), 6.45 (d, J = 8.6 Hz, 1H), 3.92 (s, 3H), 3.78 (s, 3H),
3.10 (s, 2H), 1.43 ¨ 1.40
(m, 2H), 0.86 ¨0.82 (m, 2H).
Step 9: methyl 2-oxo-2,10-dihydro-1H-spiro[benzo[6,71oxepino[3,2-b]pyridine-
11,1'
-cyclopropane]-7-earboxylate
[00382] To a mixture of methyl
2-methox y-10H-spiro [b en zo [6,7]oxepino [3,2-b] pyridine-11,1 ' -
cyclopropane]-7-carbox ylate
(280 mg, 0.9 mmol) and sodium iodide (270 mg, 1.8 mmol) in acetonitrile (9 mL)
was added
trimethyl chlorosilane (0.15 mL, 1.7 mmol) at rt. The reaction mixture was
heated to 85 C and
stirred for 3 h, and then cooled to rt, quenched with saturated aquous sodium
thiosulfate (50 mL).
The resulting mixture was extracted with EA (50 mLx2). The combined organic
layers were
washed with saturated brine (30 mL), dried over anhydrous sodium sulfate,
filtered, and the
filtrate was concentrated in vacuo. The residue was purified by column
chromatography eluted
with dichloromethane/methanol (v/v = 15/1) to give the title compound as a
yellow solid (230
mg, 82 %).
Step 10: methyl 2-05-cyclopropy1-3-(2,6-diehlorophenypisoxazol-4-y1)methoxy)-
10H
-spiro[benzo[6,7]oxepino[3,2-b]pyridine-11,1'-cyclopropane]-7-carboxylate
[00383] A mixture
of 4-(chloromethyl)-5-cyclopropy1-3-(2,6-dichlorophenyl)isoxazole (350
mg, 1.2 mmol), methyl 2-oxo-2,10-dihydro-1H-spiro[benzo[6,7]oxepino[3,2-
b]pyridine-11,1'
-cyclopropane]-7-carboxylate (230 mg, 0.77 mmol) and potassium phosphate (330
mg, 1.6
-88-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
mmol) in DMF (10 mL) was heated to 50 C and stirred for 4 h. The mixture was
then cooled to
rt, diluted with water (10 mL) and extracted with EA (20 mLx2). The combined
organic layers
were washed with saturated brine (20 mL), dried over anhydrous sodium sulfate,
filtered, and the
filtrate was concentrated in vacuo. The residue was purified by column
chromatography eluted
with PE/EA (v/v = 7/1) to give the title compound as a white solid (230 mg, 53
%).
[00384] 1H NMR (400 MHz, CDC13) 6 7.85-7.79 (m, 2H), 7.36-7.30 (m, 3H),
7.26-7.17
(m, 2H), 6.28 (d, J = 8.6 Hz, 1H), 5.01 (s, 2H), 3.92 (s, 3H), 3.06 (s, 2H),
2.17-2.09 (m, 1H),
1.31-1.28 (m, 4H), 1.17-1.10 (m, 211), 0.83-0.78 (m, 2H).
Step 11: 2-05-cyclopropy1-3-(2,6-dichlorophenypisoxazol-4-yOmethoxy)-10H-
spiro[benzo
[6,7]oxepinol3,2-b]pyridine-11,1'-cyclopropanel-7-carboxylic acid
[00385] To a mixture of methyl 2((5-cyclopropy1-3-(2,6-
dichlorophenyl)isoxazol-4-y1)
methox y)-10H-spiro [benzo [6,7] ox epino [3,2-b]pyridine-11,1 '-cyclopropane]-
7-carbox ylate (230
mg, 0.41 mmol) in a mixed solvent of THF (12 mL) and water (12 mL) was added
sodium
hydroxide (163 mg, 4.0 mmol). The reaction mixture was heated to 80 C and
stirred for 3 h.
Then the mixture was cooled to rt, concentrated to remove most of the solvent
under reduced
pressure. The residue was diluted with water (10 mL), adjusted to acidity with
hydrochloric acid
(3 mL, 2 M) and extracted with EA (20 mLx2). The combined organic layers were
washed with
saturated brine (10 mL), dried over with anhydrous sodium sulfate, filtered
and concentrated
under reduced pressure to give the title compound as a white solid (190 mg, 85
%).
[00386] MS (ESI, pos. ion) m/z: 549.0 [M+H]+;
[00387] 1H NMR (600 MHz, CDC13) 6 7.91 ¨ 7.87 (m, 2H), 7.37 ¨ 7.31 (m,
311), 7.27 (s,
1H), 7.24 ¨ 7.19 (m, 1H), 6.29 (d, J = 8.6 Hz, 1H), 5.02 (s, 211), 3.09 (s,
2H), 2.16 ¨ 2.11 (m,
1H), 1.33 ¨ 1.30 (m, 211), 1.30¨ 1.27 (m, 2H), 1.16 ¨ 1.11 (m, 2H), 0.84 ¨
0.81 (m, 2H).
Example 10: 2-45-cyclopropy1-3-(2,6-dichlorophenypisoxazol-4-yl)methoxy)-11,11-
difluoro
-10,11-dihydrobenzo[6,7]oxepino[3,2-b]pyridine-7-carboxylic acid
-89-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
OH
CI
0
* N
Step 1: 1-(4-bromo-2-(methoxymethoxy)pheny1)-2-(3-bromo-6-methoxypyridin-2-y1)
-2-fluoroethanone
[00388] To a mixture of 1-(4-bromo-2-(methoxymethoxy)pheny1)-2-(3-bromo
-6-methoxypyridin-2-yl)ethanone (3.00 g, 6.7 mmol) in DMF (40 mL) was added
1-chloromethy1-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane
bis(tetrafluoroborate) (4.50 g, 12.7
mmol) at rt. The reaction mixture was stirred at rt for 4 h and then diluted
with water (200 mL).
The resulting mixture was extracted with EA (150 mLx3). The combined organic
layers were
washed with saturated brine (20 mL), dried over with anhydrous sodium sulfate,
filtered and the
filtrate was concentrated in vacuo. The residue was purified by column
chromatography eluted
with PE/EA (v/v = 10/1) to give the title compound as a white solid (2.91 g,
93 %).
Step 2: 1-(4-bromo-2-(methoxymethoxy)pheny1)-2-(3-bromo-6-rnethoxypyridin-2-
y1)
-2,2-difluoroethanone
[00389] To a mixture of
1-(4-bromo-2-(methoxymethoxy)pheny1)-2-(3-bromo-6-methoxypyridin -2-y1)-2-
fluoroethanone
(2.91 g, 6.3 mmol) in THF (60 mL) was added dropwise a solution of sodium
bis(trimethylsilyl)amide in THF (9.4 mmol, 4.7 mL, 2.0 mol/L) at -78 C under
nitrogen
atmosphere. The reaction mixture was stirred at this temperature for 30 mins,
followed by
addition of a solution of N-fluorobenzenesulfonimide (3.96 g, 12.6 mmol) in
THF (20 mL). The
mixture was stirred at this temperature for another 1 h and warmed to rt and
stirred for 30 mins.
The reaction mixture was quenched with saturated aquous ammonium chloride (40
mL) and
extracted with EA (30 mLx2). The combined organic layers were washed with
saturated brine
(20 mL), dried over anhydrous sodium sulfate, filtered and concentrated in
vacuo. The residue
-90-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
was purified by column chromatography eluted with PE/EA (v/v = 20/1) to give
the title
compound as yellow oil (2.6 g, 86 %).
[00390] MS (ESI, pos. ion) m/z: 479.8 [M+H];
Step 3: 1-(4-
bromo-2-hydroxypheny1)-2-(3-bromo-6-methoxypyridin-2-y1)-2,2
-difluoroethanone
[00391] Using
1-(4-bromo -2-(methoxymethoxy)pheny1)-2-(3-bromo -6-methoxypyridin-2-y1)-2,2-
difluoroethanone (2.6 g, 5.4 mmol) as a starting material, the title compound
was prepared
according to the procedure described in step 4 of example 9 as yellow oil (2.1
g, 89 %).
[00392] MS (ES!, neg. ion) m/z: 433.8 [M-1-1]-.
Step 4: 5-bromo-2-(2-(3-bromo-6-methoxypyridin-2-y1)-2,2-difluoroacetyl)phenyl
methyl
carbonate
[00393] Using 1 -(4-
bromo-2-hydroxyphen y1)-2-(3-bromo-6-methoxypyridin-2-y1)-2,2-
difluoroethanone (2.3 g, 5.3 mmol) as a starting material, the title compound
was prepared
according to the procedure described in step 5 of example 9 as yellow oil (2.5
g, 95 %).
Step 5: 5-bromo-2-(2-(3-bromo-6-methoxypyridin-2-y1)-2,2-difluoroethyl)phenol
[00394] Using 5-bromo -2-(2-(3-bromo-6-methoxypyridin-2-y1)-2,2-
difluoroacetyl)phenyl
methyl carbonate (2.5 g, 5.0 mmol) as a starting material, the title compound
was prepared
according to the procedure described in step 6 of example 9 as yellow oil (0.3
g, 10 %).
[00395] MS (ES!, neg. ion) m/z: 419.8
Step 6: 7-bromo-11,11-difluoro-2-methoxy-10,11-dihydrobenzo[6,71oxepino[3,2-
b]pyridine
[00396] Using 5 -bromo-2-(2-(3-bromo-6-methoxypyridin-2-y1)-2,2-
difluoroethyl)phenol)
(1.3 g, 3.1 mmol) as a starting material, the title compound was prepared
according to the
procedure described in step 7 of example 9 as a white solid (0.25 g, 24 %).
[00397] MS (ES!, pos. ion) m/z: 342.0 [M+H];
Step 7: methyl 11,11-difluoro-2-methoxy-10,11-dihydrobenzo[6,7]oxepino[3,2-
b]pyridine
-91-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
-7-carboxylate
[00398] Using
7-bromo-11,11-difluoro-2-methoxy-10,11-dihydrobenzo[6,7]oxepino[3,2-b]pyridine
(250 mg,
0.73 mmol) as a starting material, the title compound was prepared according
to the procedure
described in step 8 of example 9 as a yellow solid (40 mg, 20 %).
[00399] 1H NMR (400 MHz, CDC13) 8 7.90 (d, J = 7.9 Hz, 2H), 7.59 (d, J =
8.9 Hz, 1H),
7.48 (d, J = 7.6 Hz, 1H), 6.85 (d, J = 8.8 Hz, 1H), 3.97 (s, 3H), 3.94 (s,
3H), 3.73 (t, J = 14.0 Hz,
2H).
Step 8: methyl 11,11-difluoro-2-oxo-1,2,10,11-tetrahydrobenzo[6,7]oxepino[3,2-
b]pyridine
-7-carboxylate
[00400] Using methyl
11,11-difluoro-2-methox y-10,11-dihydrobenzo [6,7] oxepino [3,2-b]pyridine-7-
carboxylate (70
mg, 0.22 mmol) as a starting material, the title compound was prepared
according to the
procedure described in step 9 of example 9 as a white solid (50 mg, 75 %).
Step 9: methyl 24(5-cyclopropyl-3-(2,6-dichlorophenypisoxazol-4-yl)methoxy)-
11,11-
difluoro-10,11-dihydrobenzo[6,7]oxepino[3,2-b]pyridine-7-carboxylate
[00401] Using methyl
11,11-difluoro-2-oxo-1,2,10,11-tetrahydrobenzo[6,7]oxepino[3,2-b]pyridine -7-
carboxylate (40
mg, 0.13 mmol) and 4-(chloromethyl)-5-cyclopropy1-3-(2,6-dichlorophenyl)
isoxazole (59 mg,
0.2 mmol) as a starting material, the title compound was prepared according to
the procedure
described in step 10 of example 9 as colourless oil (57 mg, 76 %).
Step 10: 2-05-cyclopropy1-3-(2,6-dichlorophenypisoxazol-4-yl)methoxy)-11,11-
difluoro
-10,11 -dihydrobenzo [6,7] oxepino[3,2-b] pyridine-7-carboxylic acid
[00402] Using methyl
24(5 -cycloprop y1-3 -(2,6-dichloroph en yl)isoxazol-4-yl)methoxy)-11,11-
difluoro
-10,11-dihydrobenzo[6,7]oxepino[3,2-b]pyridine-7-carboxylate (50 mg, 0.087
mmol) as a
-92-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
starting material, the title compound was prepared according to the procedure
described in step
11 of example 9 as a light yellow solid (40 mg, 82 %).
[00403] MS (ESI, pos. ion) m/z: 559.0 [M+H]+;
[00404] 1H NMR (400 MHz, CDC13) 6 8.00 -7.89 (m, 2H), 7.53 (dd, J = 19.1,
8.3 Hz, 2H),
7.38 (d, J = 8.0 Hz, 2H), 7.27 - 7.24 (m, 1H), 6.71 (d, J = 8.8 Hz, 1H), 5.23
(s, 2H), 3.72 (t, J =
13.9 Hz, 2H), 2.45 -2.38 (m, 1H), 1.25 - 1.22 (m, 2H), 1.16 - 1.10 (m, 2H).
Example 11: 2-45-cyclopropy1-3-(2,6-dichlorophenybisoxazol-4-yOmethoxy)-8-
hydroxy
-10,11-dihydrobenzo[6,7]oxepino[3,2-b]pyridine-7-carboxylic acid
0
401
CI CI
HO 0
HO * C-)2"-0
Step 1: 6-methoxy-2-methy1-3-(4,4,5,5-tetramethy1-1,3,2-dioxaborolan-2-
yl)pyridine
[00405] A mixture of 3-bromo-6-methoxy-2-methylpyridine (10 g, 49.5 mmol),
bis(pinacolato)diboron (16.3 g, 64.4 mmol), potassium acetate (14.6 g, 148.5
mmol) and
[1,11-bis(diphenylphosphino)ferrocene]dichloropalladium(H) (1.8 g, 2.5 mmol)
in DMF (100
mL) was heated to 80 oC and stirred for 2.5 h under nitrogen atmosphere. Then
the reaction
mixture was cooled to rt and diluted with water (700 mL), extracted with EA
(300 mLx2). The
combined organic layers were washed with saturated brine (20 mL), dried over
anhydrous
sodium sulfate, filtered and the filtrate was concentrated in vacuo. The
residue was purified by
column chromatography on a silica gel eluted with PE/EA (v/v = 40/1) to give
the title
compound as colourless oil (11 g, 89 %).
[00406] 1H NMR (400 MHz, CDC13) 6 7.92 (d, J = 8.2 Hz, 1H), 6.53 (d, J =
8.2 Hz, 1H),
3.95 (s, 3H), 2.67 (s, 3H), 1.35 (s, 12H).
Step 2: 6-methoxy-2-methylpyridin-3-ol
[00407] To a mixture of
-93-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
6-methox y-2-meth y1-3-(4,4,5,5-tetrameth y1-1,3,2-dioxaborolan-2-yl)pyridine
(12 g, 48.17 mmol)
in THF (50 mL) was added aquous sodium hydroxide (80 mL, 120 mmol, 1.5 M) and
30%
hydrogen peroxide (16 mL, 144 mmol) under an ice bath condition. The reaction
mixture was
stirred at rt for 3 h, then diluted with water (20 mL) and extracted with EA
(100 mLx2). The
combined organic layers were washed with saturated brine (20 mL), dried over
anhydrous
sodium sulfate, filtered and the filtrate was concentrated under reduced
pressure. The residue was
purified by column chromatography eluted with PE/EA (v/v = 10/1) to give the
title compound
as a white solid (2.3 g, 34 %).
[00408] MS (ESI, pos. ion) m/z: 140.0 [M+H].
Step 3: 3-((tert-butyldiphenylsilyl)oxy)-6-methoxy-2-methylpyridine
[00409] To a mixture of 6-methoxy-2-methylpyridin-3-ol (6.0 g, 43 mmol) in
DMF (70 mL)
were added imidazole (7.3 g, 108 mmol) and t-butydiphenylchlorosilane (17 mL,
65 mmol)
under an ice bath condition. The reaction mixture was stirred at rt for 3 h,
then diluted with water
(200 mL). The resulting mixture was extracted with EA (100 mLx 2). The
combined organic
layers were washed with saturated brine (20 mL), dried over anhydrous sodium
sulfate, filtered
and the filtrate was concentrated under reduced pressure. The residue was
purified by column
chromatography eluted with PE/EA (v/v = 20/1) to give the title compound as a
white solid (15
g, 92 %).
[00410] MS (ESI, pos. ion) m/z: 378.1 [M+H]t
Step 4: 2-(bromomethyl)-3-((tert-butyldiphenylsilyl)oxy)-6-methoxypyridine
[00411] Using 3-((tert-butyldiphenylsilyl)oxy)-6-methoxy-2-methylpyridine
(15 g, 40
mmol) as a starting material, the title compound was prepared according to the
procedure
described in step 2 of example 1 as a white solid (17 g, 94 %).
[00412] MS (ESI, pos. ion) m/z: 456.0 [M+H].
Step 5: diethyl
((3-((tert-butyldiphenylsilyl)oxy)-6-methoxypyridin-2-yl)methyl)phosphonate
-94-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
[00413] Using 2-(bromomethyl)-3-((tert-butyldiphenylsilypoxy)-6-
methoxypyridine (6.0 g,
13 mmol) as a starting material, the title compound was prepared according to
the procedure
described in step 3 of example 1 as colourless oil (5.8 g, 86 %).
Step 6: 5-bromo-2-hydroxy-4-methylbenzoic acid
[00414] To a mixture of 2-hydroxy-4-methylbenzoic acid (24.5 g, 161 mmol)
in methanol
(300 mL) was added dropwise a solution of bromine (28 g, 180 mmol) in methanol
(50 mL) at
-20 C and the reaction mixture was stirred for 0.5 h, then warmed to rt and
stirred for another 1
h. The reaction was quenched with saturated aquous sodium sulfite (100 mL).
The reaction
mixture was concentrated to remove methanol and the residue was diluted with
water (50
mL).The mixture was filtered and the filtrate was dried to give a red-brown
solid (33 g, 89 %).
[00415] MS (ESI, pos. ion) m/z: 231.1 [M+H]+.
Step 7: methyl 5-bromo-2-hydroxy-4-methylbenzoate
[00416] To a solution of 5-bromo-2-hydroxy-4-methylbenzoic acid (33 g, 143
mmol) in
methanol (120 mL) was added concentrated sulfuric acid (3 mL, 58 mmol) under
an ice bath.
The mixture was heated to 75 C and stirred overnight. The reaction mixture
was cooled to rt and
concentrated under reduced pressure to remove the solvent. The residue was
diluted with water
(500 mL), dried to give the title compound as a light yellow solid (33 g, 94
%).
Step 8: methyl 2-acetoxy-5-bromo-4-methylbenzoate
[00417] Using methyl 5-bromo-2-hydroxy-4-methylbenzoate (10 g, 41 mmol) as
a starting
material, the title compound was prepared according to the procedure described
in step 1 of
example 1 as a white solid (9.5 g, 81 %).
[00418] 1H NMR (600 MHz, CDC13) 8 8.19 (s, IH), 7.01 (s, 11-1), 3.88 (s,
3H), 2.44 (s, 3H),
2.36 (s, 3H).
Step 9: methyl 2-acetoxy-5-bromo-4-(dibromomethyl)benzoate
[00419] Under nitrogen atmosphere, to a
mixture of methyl
2-acetoxy-5-bromo-4-methylbenzoate (8.0 g, 28 mmol) in tetrachloromethane (150
mL) were
-95-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
added N-bromosuccinimide (11.0 g, 62 mmol) and benzoyl peroxide (0.7 g, 2.8
mmol). Then the
reaction mixture was heated to 80 C and stirred overnight. The reaction was
cooled to rt and
quenched with saturated aquous sodium bicarbonate (80 mL). The reaction
mixture was
extracted with EA (100 mLx2). The combined organic layers were washed with
saturated brine
(20 mL), dried over anhydrous sodium sulfate, filtered and the filtrate was
concentrated under
reduced pressure. The residue was purified by column chromatography eluted
with PE/EA (v/v =-
10/1) to give the title compound as a light yellow solid (12.0 g, 97 %).
[00420] 1H NMR (400 MHz, CDC13) 8 8.18 (s, 111), 7.78 (s, IH), 6.99 (s,
111), 3.91 (s, 3H),
2.39 (s, 3H).
Step 10: methyl 2-acetoxy-5-bromo-4-formylbenzoate
[00421] To a mixture of methyl 2-acetoxy-5-bromo-4-(dibromomethyl)benzoate
(12 g, 27
mmol) in acetonitrile (80 mL) was added a solution of silver nitrate (11.0 g,
66 mmol) in water
(30 mL). The mixture was heated to 90 C and stirred for 2 h, then cooled to
rt and stirred
overnight. The reaction mixture was diluted with water (200 mL) and extracted
with EA (100
mLx2). The combined organic layers were washed with saturated brine (20 mL),
dried over
anhydrous sodium sulfate, filtered, and the filtrate was concentrated under
reduced pressure. The
residue was purified by column chromatography eluted with PE/EA (v/v = 20/1)
to give the title
compound as a yellow solid (7.8 g, 96 %).
[00422] MS (ESI, pos. ion) m/z: 300.9 [M+H].
Step 11: methyl 5-bromo-4-formy1-2-hydroxybenzoate
[00423] Using methyl 2-acetoxy-5-bromo-4-formylbenzoate (8.0 g, 27 mmol) as
a starting
material, the title compound was prepared according to the procedure described
in step 4 of
example 1 as a light yellow solid (6.8 g, 98 %).
Step 12: methyl 5-bromo-4-formy1-2-(methoxymethoxy)benzoate
[00424] Using methyl 5-bromo-4-formy1-2-hydroxybenzoate (6.8 g, 26 mmol) as
a starting
material, the title compound was prepared according to the procedure described
in step 5 of
-96-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
example 1 as yellow oil (6.7 g, 84 %).
Step 13: methyl
5-bromo-4-(2-(3-((tert-butyldiphenylsilyl)oxy)-6-methoxypyridin-2-yl)vinyl)
-2-(methoxymethoxy)benzoate
[00425] Using
methyl 5-bromo-4-formy1-2-(methoxymethoxy)benzoate (2.8 g, 9.2 mmol)
and diethyl 03-((tert-butyldiphenylsilypoxy)-6-methoxypyridin-2-
yl)methyl)phosphonate (5.9 g,
11 mmol) as a starting material, the title compound was prepared according to
the procedure
described in step 7 of example 1 as a yellow solid (4.4 g, 71 %).
Step 14: methyl
5-bromo-4-(2-(3-((tert-butyldiphenylsilyl)oxy)-6-methoxypyridin-2-yl)ethyl)
-2-(methoxymethoxy)benzoate
[00426] Using methyl
5-bromo-4-(2-(3-((tert-butyldiphenylsilyl)oxy)-6-methox ypyridin-2-yl)vinyl)
-2-(methoxymethoxy)benzoate (4.4 g, 6.6 mmol) as a starting material, the
title compound was
prepared according to the procedure described in step 4 of example 4 as yellow
oil (3.3 g, 75 %).
Step 15: methyl
5-bromo-4-(2-(3-hydroxy-6-methoxypyridin-2-ypethyl)-2-(methoxymethoxy)
benzoate
[00427] To a mixture of methyl
-bromo-4-(2-(3-((tert-b utyl diphen yl sil yl)oxy)-6-methox ypyridin
-2-yl)ethyl)-2-(methoxymethoxy)benzoate (3.3 g, 5.0 mmol) in THF (80 mL) was
added a
solution of tetrabutylammonium fluoride in THF (10 mL, 10 mmol, 1 mol/L) at
rt. The reaction
mixture was stirred at rt overnight, and then diluted with water (80 mL),
extracted with EA (100
mLx2). The combined organic layers were washed with saturated brine (20 mL),
dried over
anhydrous sodium sulfate, filtered, and the filtrate was concentrated under
reduced pressure. The
residue was purified by column chromatography eluted with PE/EA (v/v = 10/1)
to give the title
compound as a yellow solid (1.9 g, 90 %).
-97-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
Step 16: methyl 2-methoxy-8-(methoxymethoxy)-10,11-
dihydrobenzo[6,7]oxepino[3,2-10]
pyridine-7-carboxylate
[00428] Using methyl
5-bromo-4-(2-(3-hydroxy-6-methoxypyridin-2-yDethyl)-2-(methoxymethoxy)
benzoate (300 mg,
0.7 mmol) as a starting material, the title compound was prepared according to
the procedure
described in step 9 of example 1 as yellow oil (120 mg, 50 %).
[00429] MS (ESI, pos. ion) m/z: 346.0 [M+Hr.
Step 17: methyl 8-hydroxy-2-oxo-1,2,10,11-tetrahydrobenzo[6,7]oxepino[3,2-
b]pyridine-
7-carboxylate
[00430] Using methyl
2-methox y-8-(methoxymethoxy)-10,11-dihydrobenzo [6,7] oxepino [3,2-b]
pyridine-7-carbox ylate
(1.0 g, 2.9 mmol) as a starting material, the title compound was prepared
according to the
procedure described in step 10 of example 1 as yellow oil (720 mg, 87 %).
Step 18: methyl 2-05-cyclopropy1-3-(2,6-dichlorophenypisoxazol-4-yl)methoxy)-8-
hydroxy
-10,11-dihydrobenzo[6,7]oxepino[3,2-b]pyridine-7-carboxylate
[00431] Using methyl
8-hydroxy-2-oxo-1,2,10,11-tetrahydrobenzo[6,7]oxepino[3,2-b]pyridine -7-
carboxylate (400 mg,
1.4 mmol) and 4-(chloromethyl)-5-cyclopropy1-3-(2,6-dichlorophenyl) isoxazole
(420 mg, 1.4
mmol) as a starting material, the title compound was prepared according to the
procedure
described in step 11 of example 1 as colourless oil (105 mg, 14 %).
[00432] 1H NMR (400 MHz, CDC13) 6 10.56 (s, 11-1), 7.58 (s, 1H), 7.40 ¨7.38
(m, 1H), 7.35
¨ 7.33 (m, 2H), 7.27 ¨ 7.22 (m, 1H), 6.86 (s, 1H), 6.39 (d, J = 8.7 Hz, 1H),
5.12 (s, 2H), 3.95 (s,
3H), 3.17 ¨ 3.11 (m, 2H), 3.10 ¨ 3.03 (m, 2H), 2.38 ¨ 2.28 (m, 1H), 1.30 ¨
1.27 (m, 2H), 1.17-
1.11 (m, 2H).
Step 19: 2-45-cyclopropy1-3-(2,6-dichlorophenypisoxazol-4-yOmethoxy)-8-hydroxy-
10,11-
dihydrobenzo[6,7]oxepino[3,2-b]pyridine-7-carboxylic acid
-98-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
[00433] Using methyl
245-cycloprop y1-3 -(2,6-dichlorophe nyl)isoxazol-4-yOmethoxy)-8-hydroxy-
10,11-dihydrobenzo[6,7]oxepino[3,2-b]pyridine-7-carboxylate (105 mg, 0.19
mmol) as a starting
material, the title compound was prepared according to the procedure described
in step 12 of
example 1 as a white solid (50 mg, 49 %).
[00434] MS (ESI, neg. ion) m/z: 536.8 [M-Hr;
[00435] 1H NMR (400 MHz, CDC13) 8 7.62 (s, 1H), 7.38 ¨ 7.30 (m, 3H), 7.27 ¨
7.20 (m,
1H), 6.84 (s, 1H), 6.39 (d, J = 8.5 Hz, 1H), 5.12 (s, 2H), 3.21 ¨ 3.00 (m,
4H), 2.37 ¨ 2.25 (m,
1H), 1.30 ¨ 1.27 (m, 2H), 1.17 ¨ 1.10 (m, 2H).
Example 12: 2-05-cyclopropy1-3-(2,6-dichlorophenyl)isoxazol-4-yl)methoxy)-
11,11-difluoro
-10-hydroxy-10,11-dihydrobenzo[6,7]oxepino[3,2-b]pyridine-7-carboxylic acid
0 I
Cl "`CI
HO
\ 0
F
Step 1: 5-bromo-2-(2-(3-bromo-6-methoxypyridin-2-yl)-2,2-difluoro-1-
hydroxyethyl)phenol
[00436] To a mixture of 1-(4-bromo-2-hydroxypheny1)-2-(3-bromo-6-
methoxypyridin-2-y1)
-2,2-difluoroethanone (0.1 g, 0.23 mmol) in ethanol (5 mL) was added sodium
borohydride
(0.040 g, 1.1 mmol) at rt. The reaction mixture was stirred at rt for 3 h, and
then diluted with
water (50 mL), extracted with EA (50 mLx3). The combined organic layers were
washed with
saturated brine (20 mL), dried over anhydrous sodium sulfate, filtered, and
the filtrate was
concentrated under reduced pressure. The residue was purified by column
chromatography on a
silica gel eluted with PE/EA (v/v = 10/1) to give the title compound as
colourless oil (0.090 g, 89
%).
Step 2: 7-bromo-11,11-difluoro-2-methoxy-10,11-dihydrobenzo[6,7]oxepino[3,2-
b]pyridin
-10-ol
-99-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN201 7/095724
Blakes Ref.: 75920/00027
[00437] Using
5-bromo-2-(2-(3-bromo-6-methoxypyridin-2-y1)-2,2-difluoro-1-
hydroxyethyl)phenol (2.5 g, 5.7
mmol) as a starting material, the title compound was prepared according to the
procedure
described in step 7 of example 9 as a yellow solid (0.660 g, 32 %).
[00438] MS (ESI, pos. ion) m/z: 358.0 [M+H].
Step 3: methyl
11,11-difluoro-10-hydroxy-2-methoxy-10,11-dihydrobenzo[6,7]oxepino[3,2-b]
pyridine-7-carboxylate
[00439] Using
7-bromo-11,11-difluoro-2-methoxy-10,11 -dih ydrobenzo [6,7] ox epin o [3,2-
b]pyridin-10 -ol (660
mg, 1.8 mmol) as a starting material, the title compound was prepared
according to the procedure
described in step 8 of example 9 as a yellow solid (450 mg, 72 %).
[00440] 1H NMR (400 MHz, CDC13) 8 7.98 (d, J = 8.0 Hz, 1H), 7.90 (s, 1H),
7.79 (d, J =
8.0 Hz, 1H), 7.60 (d, J = 8.9 Hz, 1H), 6.88 (d, J = 8.9 Hz, 1H), 5.56 (d, J =
18.6 Hz, 1H), 3.98 (s,
3H), 3.95 (s, 3H), 3.05 (d, J = 3.2 Hz, 1H).
Step 4: methyl 11,11-difluoro-10-hydroxy-2-oxo-1,2,10,11-
tetrahydrobenzo[6,7]oxepino
[3,2-b]pyridine-7-carboxylate
[00441] Using methyl
11,11 -difluoro-10-hydrox y-2-methoxy-10,11-dihydrobenzo [6,7] oxepino
[3,2-b]pyridine-7-carboxylate (150 mg, 0.44 mmol) as a starting material, the
title compound
was prepared according to the procedure described in step 9 of example 9 as a
white solid (120
mg, 83 %).
Step 5: methyl
2-05-cyclopropy1-3-(2,6-dichlorophenypisoxazol-4-yOmethoxy)-11,11-difluoro
-10-hydroxy-10,11-dihydrobenzo[6,7]oxepino[3,2-b]pyridine-7-carboxylate
[00442] Using methyl
-100-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
11,11-difluoro-10 -hydroxy-2-oxo-1,2,10,11 -tetrahy drobenzo [6,7] oxepino
[3,2-b]pyridine-7-carboxylate (120 mg, 0.37 mmol) and 4-(chloromethyl)-5-
cyclopropyl
-3-(2,6-dichlorophenyl)isoxazole (168 mg, 0.56 mmol) as a starting material,
the title compound
was prepared according to the procedure described in step 10 of example 9 as a
white solid (160
mg, 73 %).
Step 6: 2-45-cyclopropyl-3-(2,6-dichlorophenyl)isoxazol-4-yllmethoxy)-11,11-
difluoro
-10-hydroxy-1O,11 -dihydrobenzo [6,7] oxepino [3,2-b]pyridine-7-carboxylic
acid
[00443] Using methyl
2-45-cyclopropy1-3-(2,6-dichlorophenyl)isoxazol-4-yOmethoxy)-11,11-difluoro
-10-hydroxy-10,11-dihydrobenzo[6,7]oxepino[3,2-b]pyridine-7-carboxylate (160
mg, 0.27
mmol) as a starting material, the title compound was prepared according to the
procedure
described in step 11 of example 9 as a light yellow solid (120 mg, 77 %).
[00444] MS (EST, pos. ion) m/z: 575.1 [M+H]+;
[00445] 1H NMR (400 MHz, CDC13) 8 8.03 (d, J = 6.1 Hz, 1H), 7.92 (s, 1H),
7.80 (d, J =
6.3 Hz, 1H), 7.55 (d, J = 7.8 Hz, 1H), 7.44 ¨ 7.33 (m, 2H), 7.28 (s, 1H), 6.73
(d, J = 7.6 Hz, 1H),
5.54 (d, J = 17.7 Hz, 1H), 5.24 (s, 2H), 3.25 (s, 1H), 2.46 ¨ 2.34 (m, 111),
1.34 ¨ 1.26 (m, 2H),
1.16 ¨ 1.08 (m, 2H).
Example 13:
24(5-cyclopropyl-3-(2,6-dichlorophenypisoxazol-4-yl)methoxy)-11-methyl-10,11
-dihydrobenzo [6,7] oxepin o [3,2-6] pyridin e-7 -carboxylic acid
0
4111
CI a
HO =O_ 0 CA,N
[00446] Using 1-(3-bromo-6-methoxypyridin-2-yl)ethanone as a starting
material, the title
compound was prepared according to the procedure described in example 8 as a
white solid (80
-101-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
mg).
[00447] MS (ESI, pos. ion) m/z: 537.0 [M+1-11+;
[00448] 1H NMR (400 MHz, CDC13) 7.93 - 7.74 (m, 2H), 7.38 - 7.12 (m, 5H),
6.38 (d, J
= 8.6 Hz, 1H), 5.23 (d, J = 12.8 Hz, 1H), 5.08 (d, J = 12.8 Hz, 1H), 3.48 -
3.32 (m, 1H), 3.31 -
3.18 (m, 1H), 3.03 - 2.92 (m, 1H), 2.36 - 2.17 (m, 1H), 1.28 - 1.07 (m, 5H),
0.96 - 0.77 (m,
2H).
Example 14: 2-((5-cyclopropy1-3-(2,6-dichlorophenyl)isoxazol-4-yl)methoxy)-11-
methyl
benzo[6,7]oxepino[3,2-b]pyridine-7-carboxylic acid
0
CI CI
HO fikõ 0
1111F rt-C/
4111
[00449] Using 1-(3-bromo-6-methoxypyridin-2-yl)ethanone as a starting
material, the title
compound was prepared according to the procedure described in example 7 as a
white solid (60
mg).
[00450] MS (ESI, pos. ion) m/z: 535.0 [M+H]+;
[00451] 1H NMR (400 MHz, CDC13) .5 7.97 -7.79 (m, 2H), 7.38 (d, J = 8.5 Hz,
1H), 7.27 -
7.18 (m, 3H), 7.08 (t, J = 8.0 Hz, 1H), 6.84 (s, 111), 6.57 (d, J = 8.6 Hz,
1H), 5.23 (s, 2H), 2.37 -
2.19 (m, 4I1), 1.19 - 1.08 (m, 2H), 0.93 -0.83 (m, 2H).
Example 15:
2-04-cyclopropy1-1-(2,6-dichloropheny1)-1H-pyrazol-5-yl)methoxy)-11-
methylbenzo[6,7]ox
epino[3,2-b]pyridine-7-carboxylic acid
-102-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
0
CI CI
HO 0
* N,
IN
Step 1: methyl
2-((4-cyclop ropy1-1-(2,6-d ichloropheny1)-1H-pyrazol-5-yl)methoxy)-11-
methylbenzo [6,7] ox
epino[3,2-b]pyridine-7-carboxylate
[00452] Using methyl
11-methy1-2-oxo-1,2-dihydrobenzo[6,7]oxepino[3,2-b]pyridine-7-carboxylate (281
mg, 0.99
mmol) and 5-(chloromethyl)-4-cyclopropyl-1-(2,6-dichloropheny1)-1H-pyrazole
(200 mg, 0.66
mmol) (See synthetic method of compound Int-2-7 described in W02016096115) as
starting
materials, the title compound was prepared according to the procedure
described in step 10 of
example 9 as a white solid (320 mg, 88 %).
Step 2:
2- 04-cyclopro pyl-1- (2,6-dichloroph enyl)-1H-pyrazol-5-yOmethoxy)-11-
methylbenzo [6,7] ox
epinol3,2-blpyridine-7-carboxylic acid
[00453] Using methyl
24(4-cyclopropy1-1-(2,6-dichloropheny1)-1H-pyrazol-5-yOmethoxy)-11-methylbenzo
[6,7] oxepi
no[3,2-b]pyridine-7-carboxylate (320 mg, 0.58 mmol) as a starting material,
the title compound
was prepared according to the procedure described in step 11 of example 9 as a
light yellow solid
(130 mg, 42 %).
[00454] MS (ESI, pos. ion) m/z: 534.0 [M+H]+;
[00455] 1H NMR
(400 MHz, CDC11) 7.87 (d, J = 8.1 Hz, 2H), 7.48 (s, 1H), 7.37 (d, J =
8.7 Hz, 1H), 7.26-7.24 (m, 3H), 7.02 (t, J = 8.1 Hz, 1H), 6.85 (s, 1H), 6.56
(d, J = 8.7 Hz, 1H),
5.41 (s, 2H), 2.35 (s, 3H), 1.89 ¨ 1.77 (m, 1H), 0.99 ¨0.90 (m, 2H), 0.72-0.68
(m, 2H).
Example 16:
-103-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakcs Ref.: 75920/00027
11-cyclopropy1-24(5-cyclopropy1-3-(2,6-dichlorophenyl)isoxazol-4-
yl)methoxy)benzo [6,7] ox
epino[3,2-b]pyridine-7-carboxylic acid
0 le CI
CI
HO
\ ts1/ 0CrtN
Step 1: methyl
4-(2-(3-bromo-6-methoxypyridin-2-y1)-2-cyclopropylvinyI)-3-
(methoxymethoxy)benzoate
[00456] Using (3-
bromo-6-methoxypyridin-2-y1)(cyclopropyl)methanone (3.5 g, 13.7
mmol) and methyl 4-(diethoxyphosphorylmethyl)-3- (methoxymethoxyl) benzoate
(5.68 g, 16
mmol) as starting materials, the title compound was prepared according to the
procedure
described in step 3 of example 7 as a yellow solid (1.8 g, 29 %).
Step 2: methyl
4-(2-(3-bromo-6-methoxypyridin-2-y1)-2-cyclopropylviny1)-3-hydroxybenzoate
[00457] Using methyl
4-(2-(3-bromo-6-methoxypyridin-2-y1)-2-cyclopropylviny1)-3-
(methoxymethoxy)benzoate (1.0
g, 2.23 mmol) as a starting material, the title compound was prepared
according to the procedure
described in step 4 of example 7 as colourless oil (510 mg, 57 %).
[00458] MS (ESI, pos. ion) m/z: 404.1 [M-1-H];
Step 3: methyl 11-cyclopropy1-2-methoxybenzo [6,7]oxepino[3,2-b]pyridin e-7-
carboxylate
[00459] Using methyl
4-(2-(3-bromo-6-methoxypyridin-2-y1)-2-cyclopropylviny1)-3-hydroxybenzoate
(510 mg, 1.3
mmol) as a starting material, the title compound was prepared according to the
procedure
described in step 5 of example 7 as yellow oil (235 mg, 58 %).
Step 4: methyl
11-cyclopropy1-2-oxo-1,2-dihydrobenzo [6,7] oxep ino [3,2-b] pyridin e-7-carbo
xylate
-104-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
[00460] Using methyl
11-cyclopropy1-2-methoxybenzo[6,7]oxepino[3,2-b]pyridine-7-carboxylate (240
mg, 0.73 mmol)
as a starting material, the title compound was prepared according to the
procedure described in
step 6 of example 7 as a yellow solid (220 mg, 98 %).
[00461] MS (ESI, pos. ion) m/z: 310.1 [M+H];
Step 5: methyl
11-cyclopropy1-24(5-cyclopropy1-3-(2,6-dichlorophenypisoxazol-4-
y1)methoxy)benzo[6,71ox
epino[3,2-b]pyridine-7-carboxylate
[00462] Using methyl
11 -cycloprop y1-2-oxo-1,2-dihydrob enzo [6,7] oxepino [3,2-b]pyridi ne-7-ca
rboxylate (220 mg,
0.71 mmol) and 4-(chloromethyl)-5-cyclopropy1-3-(2,6-dichlorophenyl)isooxazole
(320 mg, 1.1
mmol) (See synthetic method of compound A6e described in W02011020615) as
starting
materials, the title compound was prepared according to the procedure
described in step 7 of
example 7 as a white solid (310 mg, 76 %).
Step 6:
11-cyclopropy1-24(5-cyclopropy1-3-(2,6-dichlorophenypisoxazol-4-
yl)methoxy)benzo[6,7]ox
epino[3,2-b]pyridine-7-carboxylic acid
[00463] Using methyl
11-cyclopropy1-2-((5 -cyclopropy1-3-(2,6-dichlorophenyl)isoxazol-4-yl)methox
y)b enzo [6,7] oxepi
no[3,2-b]pyridine-7-carboxylate (310 mg, 0.54 mmol) as a starting material,
the title compound
was prepared according to the procedure described in step 7 of example 7 as a
light yellow solid
(110 mg, 36 %).
[00464] MS (ESI, pos. ion) m/z: 561.2 [M+H]+;
[00465] 1H NMR (400 MHz, CDC13) ö 7.88-7.83 (m, 2H), 7.40 (d, J = 8.7 Hz,
1H),
7.28-7.24 (m, 3H), 7.11 (t, J = 8.1 Hz, 1H), 6.59 (d, J = 8.7 Hz, 1H), 6.52
(s, 1H), 5.41 (s, 2H),
2.38 -2.33 (m, 1H), 2.27-2.22 (m, 1H), 1.29-1.27 (m, 2H), 1.15-1.10 (m, 2H),
0.99-0.94 (m, 2H),
-105-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakcs Ref.: 75920/00027
0.82-0.77 (m, 2H).
Example 17:
11-cyclopropy1-2-45-cyclopropy1-3-(2,6-dichlorophenypisoxazol-4-yl)methoxy)-
10,11-dihyd
robenzo[6,7]oxepino[3,2-b]pyridine-7-carboxylic acid
0
101)
Cl Cl
HO
111'" 114
Step 1: methyl
11-cyclopropy1-24(5-cyclopropy1-3-(2,6-dichlorophenypisoxazol-4-yl)methoxy)-
10,11-dihyd
robenzo [6,7]oxepino [3,2-bl pyridin e-7-carbo xylate
[00466] Using methyl
11 -cycloprop y1-2-((5 -cyclopropy1-3-(2,6-dichlorophen yl)isox azol-4-
yl)methox y)benzo [6,7] oxepi
no[3,2-b]pyridine-7-carboxylate (200 mg, 0.35 mmol) as a starting material,
the title compound
was prepared according to the procedure described in step 4 of example 4 as a
white solid (120
mg, 61 %).
Step 2:
11-cyclopropy1-24(5-cyclopropy1-3-(2,6-dichlorophenypisoxazol-4-yl)methoxy)-
10,11-dihyd
robenzo [6,7]oxepino[3,2-b]pyridine-7-carboxylic acid
[00467] Using methyl
11-cycloprop y1-2-45 -cyclopropy1-3-(2,6-dichloro phenyl)isox azol-4-
yl)methoxy)-10,11-dih ydrob
enzo[6,7]oxepino[3,2-b]pyridine-7-carboxylate (200 mg, 0.35 mmol) as a
starting material, the
title compound was prepared according to the procedure described in step 4 of
example 4 as a
white solid (40 mg, 20 %).
[00468] MS (ESI, pos. ion) m/z: 563.0 [M+H];
[00469] 1H NMR
(400 MHz, CDC13) 6 7.93-7.78 (m, 2H), 7.42-7.31 (m, 4H), 7.27-7.19 (m,
-106-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
1H), 6.44 (d, J = 8.4 Hz, 1H), 5.24 (d, J = 12.7 Hz, 1H), 5.07 (d, J = 12.7
Hz, 1H), 3.51-3.40 (m,
111), 3.14 -3.04 (m, 1H), 2.40-2.31 (m, 111), 2.28-2.18 (m, 1H), 1.20-1.07 (m,
311), 0.95-0.67 (m,
3H), 0.61-0.41 (m, 3H).
Example 18:
2-45-cyclop ropy1-3- (2,6-dichloroph enyl) isoxazol-4-yl)methoxy)-10-
methylbenzo [6,7] oxepin
o[3,2-b]pyridine-7-carboxylic acid
0
CI CI
HO
0
Step 1: methyl 4-acetyl-3-(methoxymethoxy)benzoate
[00470] To a
solution of methyl 4-acetyl-3-hydroxybenzoate (5.0 g, 26 mmol) and
/V,N-diisopropylethylamine (14 mL, 79 mmol) in DCM (80 mL) was added dropwise
chloromethyl methyl ether (3 mL, 39.5 mmol) under ice bath and N2 atmosphere.
The mixture
was then warmed to rt and stirred overnight. The reaction was quenched by
adding water (100
mL) and extracted with DCM (80 mLx2). The organic phase was combined, washed
with
saturated saline (20 mL), dried over anhydrous Na2SO4, filtered and
concentrated under reduced
pressure. The residue was purified by a column chromatography using (PE/EA
(v/v) = 5/1) as
eluent to give the title compound as light yellow oil (5.0 g, 82 %).
Step 2: methyl
4-(1-(3-bromo-6-methoxypyridin-2-yl)prop-1-en-2-y1)-3-(methoxymethoxy)benzoate
[00471] To a
solution of diethyl ((3-bromo-6-methoxypyridine-2-y1) methyl) phosphate
(5.96 g, 17.6 mmol) in DMF (20 mL) under ice bath was added 60% NaH (0.77 g,
19 mmol).
The mixture was stirred for 0.5 h under ice bath and methyl
4-acetyl-3-(methoxymethoxy)benzoate (3.5 g, 15 mmol) was added. And then the
mixture was
warmed to rt and stirred overnight, quenched with saturated ammonium chloride
aqueous
-107-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
solution (10 mL) and extracted with EA (20 mLx2). The orgnic phase was
combined, washed
with saturated saline (20 mL), dried over anhydrous sodium sulfate, filtered
and concentrated
under reduced pressure. The residue was purified by a column chromatography
using (PE/EA
(v/v) = 10/1) as eluent to give the title compound as yellow oil (2.9 g, 47
%).
[00472] MS (ESI, pos. ion) m/z: 422.0 [M+H]+;
Step 3: methyl 4-(1-(3-bromo-6-methoxypyridin-2-yl)prop-1-en-2-y1)-3-
hydroxybenzoate
[00473] To a solution of methyl
4-(1-(3-bromo-6-methoxypyridin-2-yl)prop-1-en-2-y1)-3-(methoxymethoxy)benzoate
(2.9 g, 6.8
mmol) in THF (10 mL) was added hydrochloric acid (10 mL, 60 mmol, 6 M). The
mixture was
heated to 50 C and stirred for 5 h. Then the reaction mixture was cooled to
rt, diluted with water
(20 mL), adjusted to alkaline by using potassium carbonate and extracted with
EA (30 mLx2).
The organic phase was combined, washed with saturated saline (10 mL), dried
over anhydrous
sodium sulfate, filtered and concentrated under reduced pressure. The residue
was purified by a
column chromatography using (PE/EA (v/v) = 10/1) as eluent to give the title
compound as
yellow oil (2.3 g, 88 %).
[00474] MS (ESI, pos. ion) m/z: 378.1 [M+H]+,
Step 4: methyl 2-metho xy-10-methylben zo [6,7] oxepino [3,2-b]pyridine-7-
carboxylate
[00475] A mixture of cuprous iodide (150 mg, 0.79 mmol), N,N-
dimethylglycine (330 mg,
3.2 mmol), cesium carbonate (3.1 g, 9.5
mmol) and methyl
4-(1-(3-bromo-6-methoxypyridin-2-yl)prop-1-en-2-y1)-3-hydroxybenzoate (2.0 g,
5.3 mmol) in
1,4-dioxane (20 mL) was heated to reflux and stirred overnight. The reaction
mixture was cooled
to rt, filtered and concentrated under reduced pressure. The residue was
purified by a column
chromatography using (PE/EA (v/v) = 10/1) as eluent to give the title compound
as a yellow
solid (1.0 g, 64 %).
Step 5: methyl 10-methyl-2-oxo-1,2-dihydrobenzo [6,7] oxepin o [3,2-b] pyridin
e-7-carbo xylate
[00476] To a solution of methyl
-108-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
2-methoxy-10-methylbenzo[6,7]oxepino[3,2-b]pyridine-7-carboxylate (1.0 g, 3.4
mmol) and
sodium iodide (1.5 g, 10 mmol) in MeCN (20 mL) was added trimethylchlorosilane
(0.9 mL, 10
mmol). Then the reaction mixture was heated to 85 C and stirred for 3 h,
cooled to rt, quenched
with saturated sodium thiosulfate aqueous solution (50 mL) and extracted with
EA (50 mLx2).
The organic phase was combined, washed with saturated saline (30 mL), dried
over anhydrous
sodium sulfate, filtered and concentrated under reduced pressure. The residue
was purified by a
column chromatography using (DCM/methanol (v/v) = 30/1) as eluent to give the
title compound
as a yellow solid (950 mg, 100 %).
[00477] MS (ESI, pos. ion) m/z: 284.0 [M+H];
Step 6: methyl
2-05-cyclopropyl-3-(2,6-dichlorophenyl)isoxazol-4-ylimethoxy)-10-
methylbenzo[6,7]oxepin
o[3,2-b]pyridine-7-carboxylate
[00478] A mixture of methyl
10-meth y1-2-ox o-1,2-dihydrobenzo [6,7]oxepino [3,2-b]pyridine-7-carboxylate
(950 mg, 3.4
mmol), 4-(chloromethyl)-5-cyclopropy1-3-(2,6-dichlorophenypisoxazole (2.0 g,
6.6 mmol) (See
synthetic method of compound A6e in W02011020615) and potassium phosphate (1.8
g, 8.4
mmol) in DMF (20 mL) was heated to 60 C and stirred for 3 h. Then the
reaction mixture was
cooled to rt, diluted with water (40 mL) and extracted with EA (80 mLx2). The
organic phase
was combined, washed with saturated saline (20 mL), dried over anhydrous
sodium sulfate,
filtered and concentrated under reduced pressure. The residue was purified by
a column
chromatography using (PE/EA (v/v) = 10/1) as eluent to give the title compound
as a yellow
solid (800 mg, 40 %).
[00479] MS (ESI, pos. ion) m/z: 549.2 [M+H];
Step 7:
2- 05-cyclopropy1-3- (2,6-dichloroph enyl) isoxazol-4-y I) methoxy)-10-
methylben zo [6,7] oxepin
o[3,2-b]pyridine-7-carboxylic acid
-109-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
[00480] To a mixed solution of methyl
245-cycloprop y1-3-(2,6-dichlorophenyl)isoxazol-4-yl)methoxy)-10-meth ylbenzo
[6,7]oxepino [3
,2-b]pyridine-7-carboxylate (800 mg, 1.5 mmol) in THF (15 mL) and water (12
mL) was added
sodium hydroxide (600 mg, 15 mmol). The mixture was heated to 60 C and
stirred overnight,
concentrated under reduced pressure to remove most of the solvent, diluted
with water (20mL),
adjusted to acidic with hydrochloric acid (25 rriL, 1 M) and extracted with EA
(30 mLx2). The
organic phase was combined, washed with saturated saline (10 mL), dried over
anhydrous
sodium sulfate, filtered and concentrated under reduced pressure to give the
title compound as a
yellow solid (700 mg, 90 %).
[00481] MS (ESI, pos. ion) m/z: 535.1 [M+H]+;
[00482] 1H NMR
(400 MHz, CDC13) 8 7.99 ¨ 7.86 (m, 2H), 7.47 (d, J = 8.1 Hz, 1H), 7.41 ¨
7.31 (m, 311), 7.27 ¨ 7.19 (m, 111), 6.74 (s, 1H), 6.53 (d, J = 8.7 Hz, 1H),
5.14 (s, 211), 2.42 (s,
3H), 2.37 ¨ 2.26 (m, 1H), 1.30¨ 1.27 (m, 2H), 1.18 ¨ 1.11 (m, 2H).
Example 19:
24(5-cyclopropy1-3-(2,6-dichlorophenyl)isoxazol-4-yl)methoxy)-10-methyl-10,11-
dihydrobe
nzo[6,7]oxepino[3,2-b]pyridine-7-carboxylic acid
0
CI
HO ma\ 0
Cl
11, CoV
111111
Step 1: methyl
4-(1-(3-bromo-6-methoxypyridin-2-yl)propan-2-y1)-3-(methoxymethoxy)benzoate
[00483] A mixed solution of methyl
4-(1-(3-bromo-6-methoxypyridin-2-yl)prop-1-en-2-y1)-3-(methoxymethoxy)benzoate
(2.9 g, 6.9
mmol), sodium acetate (3.4 g, 41 mmol) and 4-methylbenzenesulfonohydrazide
(7.7 g, 41 mmol)
in THF (20 mL) and water (20 mL) was heated to reflux and stirred overnight.
Then the reaction
-110-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
mixture was cooled to rt, diluted with water (20mL) and extracted with EA (100
mLx2). The
organic phase was combined, washed with saturated saline (20 mL), dried over
anhydrous
sodium sulfate, filtered and concentrated under reduced pressure. The residue
was purified by a
column chromatography using (PE/EA (v/v) = 4/1) as eluent to give the title
compound as
colourless oil (2.7 g, 93 %).
[00484] MS (ESI, pos. ion) m/z: 424.0 [M+H]
Step 2: methyl 4-(1-(3-bromo-6-methoxypyridin-2-yl)propan-2-yl)-3-
hydroxybenzoate
[00485] To a solution of methyl
4-(1-(3-bromo-6-methoxypyridin-2-yl)propan-2-y1)-3-(methoxymethoxy)benzoate
(2.7 g, 6.4
mmol) in TI-IF (10 mL) was added hydrochloride acid (10 mL, 60 mmol, 6 M). The
reaction
mixture was heated to 50 C and stirred overnight, cooled to rt, diluted with
water (20 mL),
adjusted to alkaline with potassium carbonate and extracted with EA (30 mLx2).
The organic
phase was combined, washed with saturated saline (10 mL), dried over anhydrous
sodium
sulfate, filtered and concentrated under reduced pressure. The residue was
purified by a column
chromatography using (PE/EA (v/v) = 10/1) as eluent to give the title compound
as colourless oil
(2.3 g, 95 %).
[00486] MS (ESI, pos. ion) m/z: 380.1 [M+H]
Step 3: methyl
2-methoxy-10-methyl-10,11-dihydrobenzo [6,7]oxepino [3,2-b]pyridine-7-
carboxylate
[00487] A solution of cuprous iodide (170 mg, 0.89 mmol), N,N-Dimethyl
glycine (370 mg,
3.6 mmol), cesium carbonate (3.5 g, 11 mmol)
and methyl
4-(1-(3-bromo-6-methoxypyridin-2-yl)propan-2-y1)-3-hydroxybenzoate (2.3 g, 6.0
mmol) in
1,4-dioxane (20 mL) was heated to reflux and stirred overnight under N2
atmosphere. The
reaction mixture was cooled to rt, filtered and concentrated under reduced
pressure. The residue
was purified by a column chromatography using (PE/EA (v/v) = 10/1) as eluent
to give the title
compound as a yellow solid (1.1 g, 63 %).
-111-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
[00488] MS (ES!, pos. ion) m/z: 300.2 [M+H]
Step 4: methyl
10-methyl-2-oxo-1,2,10,11-tetrahydrobenzo[6,7]oxepino[3,2-b]pyridine-7-
carboxylate
[00489] To a solution of methyl
2-methoxy-10-methy1-10,11-dihydrobenzo[6,71oxepino [3,2-b]pyridine-7-
carboxylate
(1.1 g, 3.7 mmol) and sodium iodide (1.7 g, 11 mmol) in MeCN (20 mL) was added
trimethylchlorosilane (1.0 mL, 11.5 mmol). Then the reaction mixture was
heated to 85 C and
stirred for 3 h, cooled to rt, quenched with saturated sodium thiosulfate
aqueous solution (50 mL)
and extracted with EA (50 mLx2). The organic phase was combined, washed with
saturated
saline (30 mL), dried over anhydrous sodium sulfate, filtered and concentrated
under reduced
pressure. The residue was purified by a column chromatography using
(DCM/methanol (v/v) =
30/1) as eluent to give the title compound as a yellow solid (1.0 g, 95 %).
Step 5: methyl
2-05-cyclopropyl-3-(2,6-dichlorophenyl)isoxazol-4-yl)methoxy)-10-methyl-10,11-
dihydrobe
nzo[6,7]oxepino[3,2-b]pyridine-7-carboxylate
[00490] A mixture of methyl
10-methy1-2-oxo-1,2,10,11-tetrahydrobenzo[6,7]oxepino[3,2-b]pyridine-7-
carboxylate (1.0 g,
3.5 mmol), 4-(chloromethyl)-5-cyclopropy1-3-(2,6-dichlorophenypisoxazole (1.1
g, 3.6 mmol)
(See synthetic method of compound A6e in W02011020615) and potassium phosphate
(1.5 g,
7.1 mmol) in DMF (10 mL) was heated to 60 C and stirred for 3 h. Then the
reaction mixture
was cooled to rt, diluted with water (40 mL) and extracted with EA (80 mLx2).
The organic
phase was combined, washed with saturated saline (20 mL), dried over anhydrous
sodium
sulfate, filtered and concentrated under reduced pressure. The residue was
purified by a column
chromatography using (PE/EA (v/v) = 50/1) as eluent to give the title compound
as a light
yellow solid (800 mg, 40 %).
[00491] MS (ESI, pos. ion) m/z: 551.2 [M-i-H]+;
-112-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
Step 6:
2-45-cyclopropy1-3-(2,6-dichlorophenyDisoxazol-4-yllmethoxy)-10-methyl-10,11-
dihydrobe
nzo[6,7]oxepino[3,2-b]pyridine-7-carboxylic add
[00492] To a mixed solution of methyl
24(5 -cycloprop y1-3 -(2,6-d ichlorophenypi sox azol-4-yl)m ethoxy)-10-meth y1-
1 0,11-dihydrobenzo
[6,7]oxepino[3,2-b]pyridine-7-carboxylate (1.0 g, 1.8 mmol) in THF (5 mL) and
water (5 mL)
was added lithium hydroxide monohydrate (230 mg, 5.5 mmol). The mixture was
heated to 60
C and stirred for 4 h, concentrated under reduced pressure to remove most of
the solvent,
diluted with water (20mL), adjusted to acidic with hydrochloric acid (10 mL, 1
M) and extracted
with EA (30 mLx2). The organic phase was combined, washed with saturated
saline (10 mL),
dried over anhydrous sodium sulfate, filtered and concentrated under reduced
pressure to give
the title compound as a white solid (870 mg, 89 %).
[00493] MS (ESI, pos. ion) m/z: 537.2 [M+H]+;
[00494] 1H NMR (400 MHz, CDC13) 6 7.95 ¨ 7.71 (m, 2H), 7.42 ¨ 7.29 (m, 4H),
7.26 ¨
7.17 (m, 1H), 6.39 (d, J = 8.6 Hz, 1H), 5.12 (s, 2H), 3.77 ¨ 3.54 (m, 1H),
3.29 ¨ 3.16 (m, 1H),
2.85 ¨2.74 (m, 1H), 2.35 ¨2.29 (m, 1H), 1.45 ¨ 1.37 (m, 211), 1.34 ¨ 1.23 (m,
3H), 1.19 ¨ 1.06
(m, 2H).
Example 20: TR-FRET farnesoid X receptor coactivator assay
[00495] Purchasing invitrogen PV4833 kits. The procedure refers to
LanthaScreenTM
TR-FRET Farnesoid X Receptor Coactivator Assay
[00496] First, the required amount of the compound was weighed and
dissolved in 100%
DMSO at the maximum concentration of 3000 M. The solution at the maximum
concentration
was diluted by 3-fold serial dilution in DMSO to get 10 concentrations;
[00497] Second, the above prepared solutions of different concentrations
were diluted to
50-fold with a buffer supplied with the kit, to 2x compound solution, followed
by mixing and
then 10 L of the diluted solution was added to a 384 well plate;
-113-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
[00498] Third, FXR-LBD was diluted with a buffer to 4x solution, and 5 uL
of the diluent
was added to the 384 well plate of second step;
[00499] Fourth, Fluorescein-SRC2-2 and Tb anti-GST antibody were diluted to
4x
Fluorescein-SRC2-2 and Tb anti-GST antibody solution. Then two reagents were
mixed together,
and 5 u1_, of the mixture was added to the 384 well plate of third step;
[00500] Finally, the solution of the 384 well above was mixed uniformly by
centrifuging,
and then incubated at room temperature for 1 h. Then the TR-FRET Endpoint
method was used
for measuring the solution at wavelengths of 520 nm, 495 nm and 337 nm. EC50
values were
calculated according to the measured value of ER = 520 nm / 495 nm.
[00501] The TR-FRET farnesoid X receptor coactivator assay results of the
partial
compounds of the invention are shown in Table 2
Table 2: Test results of TR-FRET farnesoid X receptor protein coactivator
activity
Compound No. EC50 (nM)
Example 1 49.3
Example 2 5.1
Example 3 76.1
Example 4 60.4
Example 5 25.7
Example 6 31.0
Example 11 17.0
Example 13 19.5
Example 14 15.5
[00502] 3. Conclusion:
[00503] EC50 values of the partial example compounds in Table 2 indicated
that
thecompounds of the present invention showed good farnesoid X receptor
activation activity.
Example 21 Mammalian one hybrid (M1H) assay
-114-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
[00504] Mammalian one hybrid technology is also called chimeric receptor
gene assay.
Based on the GALA chimera receptor assay, the activity of compounds which
mediated
activation of FXR was detected by dual luciferase reporter assay. The methods
were as follows:
cDNA encoding the FXR ligand binding domain (LBD), the yeast GALA DNA binding
domain
(DBD) and the Renilla luciferase DNA were fused to construct a chimera
expression plasmid
pBIND-FXR (Promega). The GeneBank number of cDNA of is 096RI1.2, and the
corresponding amino acid sequence is 261-481. The plasmid pG5Luc (Promega) was
used as the
reporter plasmid, containing the GAL4 upstream activation sequence (UAS) and
the Firefly
luciferase receptor gene. All GALA reporter gene assays were done in HEK293
cells. Cells were
cultured at 37 C, 5% CO2 under humidified conditions. The transfection
mixture contains
pBIND-FXR (25 ng/well), pG5Luc (25 ng/well), transfection reagent FuGENE HD
(0.15
1/well) and no FBS media (1.85 l/well). The contents were mixed completely
and incubated
the mixtures for 15 minutes at room temperature. The volume of the
transfection mixture was 2.5
Trypsinized and diluted the cell slurry at a density of 600,000 cells/well
(100 I/well for
96-well plate). Then added required volume of transfection mixture prepared
previously into the
cell slurry, and dispensed 100 1/well of the cell slurry into the 96-well
plate and incubated the
plate for 24 hours at 37 C, 5% CO2 under humidified conditions. Compounds
prediluted in
complete medium were added into each well and then incubated at 37 C, 5% CO2
under
humidified conditions for 18 hours. The firefly and renilla luciferase signal
was assayed by
Promega's Dual Luciferase Reporter Assay System.
[00505] The %Activation value was calculated by the following equation:
Activation% =
[(X-Min)/ (Max-Min)] x 100%. "X" is the "F/R" value from compounds. "F/R"
means
"Firefly/Renilla". "MM" is the mean "F/R" value from no compound control.
"Max" is the mean
"FIR" value from reference compound control. Finally, the EC50 value of each
compounds were
calculated by GraphPrism 5Ø
[00506] The results of GAL4 reporter gene test of the partial compounds of
the invention
-115-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
are shown in Table 3.
Table 3 :Result of GAL4 reporter gene test
Example ECso (nM)
example 5 104
example 7 11
example 8 26
example 9 2
example10 12
example11 105
_
ex ample12 136
example13 9
example14 12
example 15 13
example 16 5
example 17 65
example 18 93
[00507] EC50 values of the partial example compounds in Table 3 indicated
that the
compounds of the present invention showed good FXR agonistic potency and then
regulated
expressions of downstream genes.
Example 22 Pharmacokinetics test
[00508] Experimental animals: six healthy adult male SD rats (purchased
from Hunan SJA
Laboratory Animal Co. Ltd.) were divided into two groups, three rats in each
group, and the two
grops were given intravenous injection and oral administration respectively.
[00509] Drug preparation: a quantity of a compound of the present invention
was weighed,
and 5% DMSO, 10% Kolliphor HS15 and 85% saline (0.9%) were added to give
target
concentrations of the compound solution.
-116-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
[00510]
Administration and sample collection: animals were fasted for 12 h before
administration, and fed at the time point of 3 hours after the administration.
SD rats of one group
were given intravenous injection (IV, 1 mg/kg) via hind legs and SD rats of
the other group were
given oral administration (PO, 5 mg/kg) respectively. Then blood was collected
in the rat tail
vein at the time point of 0, 0.083, 0.25, 0.5, 1, 2, 4, 6, 8, 24 h
respectively, and the blood volume
was about 200-400pL/time point. After the collection of whole blood at each
time point, the
colleted blood was set in K2EDTA anticoagulant tube, and the tube was placed
in the incubator
with ice packs. All samples were centrifuged for 5 min at 4 C at 4600 r/min
within 15 min.
Plasma was separated and collected. The concentrations of different compounds
in the plasma of
the rats after adminstration were measured using LC/MS/MS method, and the
pharmacokinetic
parameters were calculated according to the curve of the drug concentration ¨
time.
[00511]
Pharmacokinetic properties of the compounds of the present invention were
tested
by the experiment above, and the pharmacokinetic parameters of the partial
example compounds
were shown in Table 4.
Table 4: Pharmacokinetic activity of the compound of the invention
Route
of Dosage Cl MRTIN
Compou AUCINF AUClast Cmax T112 Tmax
Vss
admi (mg/kg F(%) (ml/mi n/k
nd No. (h*ng/m1) (h*ng/m1) (ng/ml) (h) (h)
(1/kg)
nstrat ) (h)
ion
example iv 1 1540 1540 11.2 1890
0.792 0.723 0.083 0.522
________________ 55
1 po 5 4210 4180 N/A 2760 1.32
0.914 0.500 N/A
example iv 1 1150 1150 14.8 2060
0.549 0.884 0.083 0.479
________________ 72
2 po 5 4110 4110 N/A 3640
0.931 0.71 0.500 N/A
example iv 1 1580 1580 10.6 3040
0.548 0.682 0.083 0.344
________________ 95
po 5 7480 7450 N/A 6430 1.07 0.839
0.500 N/A
-117-
23544251.1
CA 03030377 2019-01-09
CA Application
National Entry of PCT/CN2017/095724
Blakes Ref.: 75920/00027
example iv 1 1330 1330 12.6 3020
0.429 0.794 0.083 0.322
________________ 88
po 5 5840 5790 N/A 3760 1.38 0.957
0.500 N/A
[00512] The
results of the partial example showed in Table 4 indicated that blood
concentrations and exposure levels of the rats were high after oral
administration of the
compounds of the present invention, clear rate of the compound was low, and
bioavailability of
the compound was high. So the compounds of the present invention had good
pharmacokinetic
activity.
[00513] Finally,
it should be noted that there are other ways to practice the invention.
Accordingly, embodiments of the present invention is to be described as
examples, but the
present invention is not limited to the contents described herein, and further
modifications within
the scope of the present invention or the equivalents added in the claims are
all examples within
the scope of the invention.
-118-
23544251.1