Note: Descriptions are shown in the official language in which they were submitted.
CA 03030559 2019-01-10
WO 2018/013485 PCT/US2017/041382
SELF-CHARGING AND/OR SELF-CYCLING ELECTROCHEMICAL
CELLS
TECHNICAL FIELD
The present disclosure relates to self-charging and/or self-cycling
electrochemical cells containing a solid glass electrolyte.
BACKGROUND
An electrochemical cell has two electrodes, the anode and the cathode,
separated by an electrolyte. In a traditional electrochemical cell, materials
in these
electrodes are both electronically and chemically active. The anode is a
chemical
reductant and the cathode is a chemical oxidant. Both the anode and the
cathode are
able to gain and lose ions, typically the same ion, which is referred to as
the working
ion of the battery. The electrolyte is a conductor of the working ion, but
normally it is
not able to gain and lose ions. The electrolyte is an electronic insulator, it
does not
allow the movement of electrons within the battery. In a traditional
electrochemical
cell, both or at least one of the anode and the cathode contain the working
ion prior to
cycling of the electrochemical cell.
The electrochemical cell operates via a reaction between the two electrodes
that has an electronic and an ionic component. The electrolyte conducts the
working
ion inside the cell and forces electrons also involved in the reaction to pass
through an
external circuit.
A battery may be a simple electrochemical cell, or it may be a combination of
multiple electrochemical cells.
SUMMARY
The present disclosure provides an electrochemical cell including a solid
glass
electrolyte including an alkali metal working ion that is conducted by the
electrolyte,
and a dipole, an anode having an effective anode chemical potential A, and a
cathode
having an effective cathode chemical potential uc. One or both of the cathode
and
anode substantially lack the working ion prior to an initial charge or
discharge of the
electrochemical cell. At open-circuit prior to an initial charge or discharge,
an electric
1
CA 03030559 2019-01-10
WO 2018/013485 PCT/US2017/041382
double-layer capacitor is formed at one or both of an interface between the
solid glass
electrolyte and the anode and an interface between the solid glass electrolyte
and the
cathode due to a difference between [tA and p.c.
The electrochemical cell may have any or all combinations of the following
additional features, unless such features are clearly mutually exclusive: a)
at least one
or both of the cathode and the anode may include a metal; b) at least one or
both of
the cathode and anode may consist essentially of or consist of a metal; c)
both the
cathode and the anode may substantially lack the working ion prior to an
initial charge
or discharge of the electrochemical cell; d) one of the cathode and the anode
may
include, consists essentially of, or consist of a metal and the other may
include a
semiconductor; e) one or both of the cathode and the anode may include a
catalytic
molecular or particle relay that determines its effective chemical potential;
f) the
working ion may include lithium ion (Lit), sodium ion (Nat), potassium ion
(K+)
magnesium ion (Mg2+), Aluminum (A13+), or any combinations thereof; g) the
dipole
may have the general formula AX z or the general formula Ay_iXz-q, wherein A
is Li,
Na, K, Mg, and/or Al, X is S and/or 0 , 0<z<3, y is sufficient to ensure
charge
neutrality of dipoles of the general formula AyXz, or a charge of -q of
dipoles of the
general formula Ay_iXz-q, and 1<q<3; h) the dipole may include up to 50 wt% of
a
dipole additive; i) the dipole additive may include one or a combination of
compounds having the general formula AX z or the general formula Ay_iXz-q,
wherein
A is Li, Na, K, Mg, and/or Al, X is S, 0, Si, and/or OH, 0<z<3, y is
sufficient to
ensure charge neutrality of dipole additives of the general formula AyXz, or a
charge
of -q of dipole additives of the general formula Ay_iXz-q, and 1<q<3; j) the
electrochemical cell may have a cycle life of at least a thousand cycles; k)
the cell,
upon closing of an open-circuit thereof, may exhibit a discharge current
without ever
having received energy from an external source; 1) the electrochemical cell
may
exhibit a self-cycling component of a charge or discharge current and/or
voltage at a
fixed control current imposed by an external potentiostat; m) the
electrochemical cell
may plate the working ion reversibly and dendrite-free on the anode during
charge; n)
the electrochemical cell may exhibit a self-charge without a control charging
current;
o) the electrochemical cell may exhibit a self-charge current component with a
control
charging current component; p) the electrochemical cell may exhibit a self-
charge
2
CA 03030559 2019-01-10
WO 2018/013485 PCT/US2017/041382
component without a control discharging current; q) the electrochemical cell
may
exhibit self-charge without a control discharging current; r) the
electrochemical cell
may have a charge/discharge coulomb efficiency of greater than 100%; s) the
electrochemical cell may exhibit a measured charge or discharge current
smaller than
a control current; t) the electrochemical cell, upon charge, may have a
charging
current greater than a control current; u) the electrochemical cell may have a
measured current in the opposite direction of a control current; v) the
electrochemical
cell, on discharge, may have a measured discharge current larger than a
control
current and exhibit self-cycling of both the measured discharge current and
the
voltage; w) the electrochemical cell may exhibit an alternating current having
a period
of at least one minute; x) the electrochemical cell may exhibit an alternating
current
having a period of at least one day.
The present disclosure further includes a battery containing one
electrochemical cell as described above, or at least two such cells, which may
be in
.. series or in parallel.
Electrochemical cells and batteries disclosed above and elsewhere herein may
be rechargeable.
BRIEF DESCRIPTION OF THE DRAWINGS
For a more complete understanding of the present invention and its features
and advantages, reference is now made to the following description, taken in
conjunction with the accompanying drawings.
FIG. 1A is a schematic, cross-sectional diagram of an electrochemical cell
according to the present disclosure prior to the first charge at open circuit.
FIG. 1B is a schematic, cross-sectional diagram of the electrochemical cell of
FIG. 1A during a self-charge.
FIG. 1C is a schematic, cross-sectional diagram of the electrochemical cell of
FIG. 1A and FIG. 1B during discharge.
FIG. 2 is a schematic diagram of an equivalent circuit that may be used to
calculate the time dependence of the evolution of the measured voltage at open-
circuit
of the electrochemical cell of FIG. 1A.
3
CA 03030559 2019-01-10
WO 2018/013485 PCT/US2017/041382
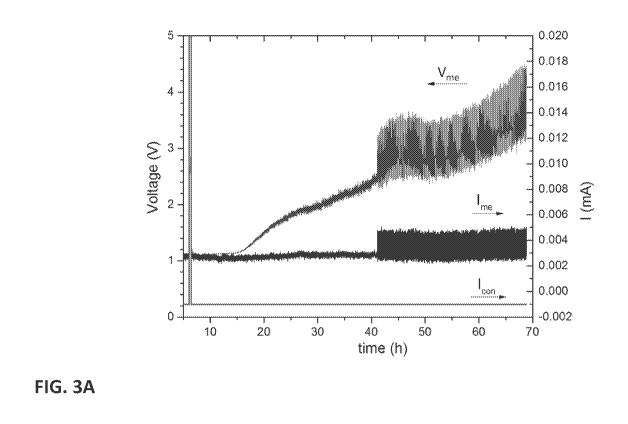
FIGs. 3A-3D are a series of graphs of test results for an electrochemical cell
having an Al-C anode, a S-C-Cu cathode and a Li+ solid glass electrolyte
containing
10% Li2S. Measured voltage is designated Vme(t). Measured current is
designated
Ime(t). Control current is designated Icon(t) and is -1 [LA versus time (t)
after a short
charge (vertical line on left in FIG. 3A). FIG. 3A presents results for an
electrochemical cell allowed to self-charge without cycling for 41 hours
followed by
self-cycling for 27 hours with a cycling period of about 8 min. FIG. 3B
presents an
expanded time-scale graph of the period of the graph of FIG. 3A between 40 and
42
hours. FIG. 3C presents results from a second cycle of the electrochemical
cell of
FIGs. 3A and 3B after a second short charge while the electrochemical cell is
heated,
beginning at 20.5 hours from 12 C to 22 C over a two-hour period, followed
by slow
cooling. FIG. 3D presents an expanded time-scale graph of the period of the
graph of
FIG. 3C during which the electrochemical cell is heated.
FIG. 4 is graph of measured discharge voltage versus time after closing the
circuit of an electrochemical cell with a Li anode, a C-Ni mesh cathode, and a
Li+
solid glass electrolyte. The cycling period of self-cycling is 24 hours.
FIG. 5A is a graph of test results for an electrochemical cell having an an Al-
C
anode, a S-C-Cu cathode and a Li + solid glass electrolyte containing 10%
Li2S.
Measured voltage is designated Vine(t). Measured current is designated Lne(t).
Control current is designated I0(t) and is 0.2 mA during charge and -1 [LA
during
self-charge. FIG. 5B presents an expanded current axis for a portion of the
graph of
FIG. 5A.
FIG. 6 is a graph of the measured first discharge current versus time after
closing the circuit of a jelly roll electrochemical cell with an Al-C anode, a
Cu
cathode, and a Li+ solid glass electrolyte. Discharge was with a low load
resistance.
FIG. 7 is a graph of the measured voltage versus time over a 5-hour charge/5-
hour discharge cycle for an electrochemical cell with a Li anode, a yMn02-C-Li
glass-
Cu cathode, and a Li+ solid glass electrolyte. The electrochemical cell was
cycled for
190 days. Peak voltages are indicated by arrows.
4
CA 03030559 2019-01-10
WO 2018/013485 PCT/US2017/041382
DETAILED DESCRIPTION
The present disclosure relates to an electrochemical cell including a solid
glass
electrolyte that contains electric dipoles as well as the working ion and that
is able to
reversibly plate the working ion on either electrode without being resupplied
by the
other electrode, which causes the electrochemical cell to exhibit a self-
charge and a
self-cycling behavior. The cell has a solid electrolyte containing, typically,
not only
mobile alkali metal working cations that can be plated dendrite free on a
metallic
current collector, but also slower moving molecular electric dipoles. A
difference in
the electrochemical potentials of the two electrodes is a driving force to
create, at
.. open-circuit, an electric-double-layer capacitor at each
electrode/electrolyte interface
as in a normal electrochemical cell. However, the difference in the
translational
mobilities of the working cations and of the orientational and translational
mobilities
of the electric dipoles creates a slower formation of any excess charge in the
electrolyte at the interface. The dipole contribution to the interface charge
may be
large enough to induce plating of the working cation as an alkali-metal layer
on the
anode, which represents a self-charge. If the counter electrode is unable to
resupply
the working cation last from the electrolyte via this self-plating or in an
applied
charging power, the electrolyte becomes charged negatively until an
equilibrium
between plating and stripping is reached. The charge in the electrolyte is
represented
in an equivalent circuit as an inductor in parallel with a capacitor, which
can create a
self-cycling component of a charge or discharge current and/or voltage at a
fixed
control current imposed by an external potentiostat.
The electrolyte is referred to as glass because it is amorphous, as may be
confirmed through X-ray diffraction. The working ion may be an alkali-metal
cation,
such as Lit, Nat, Kt, or a metal cation, such as Mg2t, and/or Art.
Self-charging refers to a phenomenon, as described further herein, in which,
an electrochemical cell contains electrodes that, on fabrication, do not
contain the
working ion of the cell and yet delivers a discharge current on closing the
external
circuit without ever having received a charging current from an external
source. This
phenomenon is the result of an alignment and displacement of electric dipoles
within
the electrolyte after cell assembly as a result of dipole-dipole interactions
and an
internal electric field. The internal electric field provided by the dipole
alignment is
5
CA 03030559 2019-01-10
WO 2018/013485
PCT/US2017/041382
large enough to plate the working ion as a metal from the electrolyte on one
of the
electrodes without resupply to the electrolyte from the other electrode,
thereby
charging the electrolyte negative. On closing the external circuit, the
working ions
are returned to the electrolyte and electrons are sent to the external circuit
where they
provide a discharge current.
Self cycling occurs where the working ion of the electrolyte is plated on an
electrode, which charges the electrolyte negative. The negative charge in the
electrolyte, when large enough, strips the plated metal back to the
electrolyte as
cations and releases electrons to the external circuit. The different rates of
response
of the dipoles and ions in the electrolyte and the electrons to the external
circuit result
in a cycling of the currents in the external circuit and/or the cell voltage.
Although both self-charging and self-cycling behaviors may occur without an
external energy input, both phenomena may also occur as a component of the
cell
charge/discharge performance with an external charge/discharge input. For
example,
a self-charging electrochemical cell may be provided with a charging current
as an
external energy input, in which case it will exhibit a greater charge than is
dictated by
the charging current to give a coulomb efficiency greater than 100%. As
another
example, the discharge current and/or voltage may have a self-cycling
component of
frequency that is different from the charge/discharge cycling frequency.
The solid glass electrolyte of this disclosure is non-flammable and is capable
of plating dendrite-free alkali metals on an electrode current collector
and/or on itself;
the atoms of the plated metal come from the working ion of the electrolyte;
the plated
working ions may or may not be resupplied to the electrolyte from the other
electrode.
In particular, the solid electrolyte of this disclosure may be a glass
containing as the
working ion an alkali-metal cation, such as Lit, Nat, K+, a metal cation, such
as Mg2+,
Al3+ as well as electric dipoles such as A2X or AX-, or MgX or Al2X3 where A =
Li,
Na, or K and X = 0 or S or another element or dipole molecule. Suitable A+-
glass
electrolytes and methods of making them have been previously described in
W02015
128834 (A Solid Electrolyte Glass for Lithium or Sodium Ion Conduction) and in
W02016205064 (Water-Solvated Glass/Amorphous Solid Ionic Conductors), where
the alkali-metal-ion disclosures of both are incorporated by references
therein.
6
CA 03030559 2019-01-10
WO 2018/013485 PCT/US2017/041382
In general, the metal working ion in the solid glass electrolyte used in the
electrochemical cells of this disclosure may be and alkali-metal ion, such as
Lit, Nat,
K+, or Mg2+ or Al3+; some of these mobile working ions may also be attached to
an
anion to form a less mobile electric dipole such as A2X, AX-, or condensates
of these
into larger ferroelectric molecules in which A = Li, Na, K, Mg, Al and x = 0,
S, or
another anion atom. The glass may also contain as additives up to 50 w% of
other
electric-dipole molecules than those formed form the precursors used in the
glass
synthesis without dipole additives. The presence of the electric dipoles gives
the glass
a high dielectric constant; the dipoles are also active in promoting the self-
charge and
self-cycling phenomenon. In addition, the solid glass electrolytes are not
reduced on
contact with metallic lithium, sodium, or potassium and they are not oxidized
on
contact with high-voltage cathodes such as the spinel Li[Nio5Mni dal or the
olivines
LiCo(PO4) and LiNi(PO4). Therefore, there is no formation of a passivating
solid-
electrolyte interphase (SET). Also, the surfaces of the solid-glass
electrolytes are wet
by an alkali metal, which allows plating from the glass electrolyte dendrite-
free alkali
metals that provide a low resistance to transfer of ions across an
electrode/electrolyte
interface over at least a thousand, at least two thousand, or at least five
thousand
charge/discharge cycles.
The solid glass electrolyte may be applied as a slurry over a large surface
area;
the slurry may also be incorporated into paper or other flexible cellulose or
polymer
membranes; on drying, the slurry forms a glass. The membrane framework may
have
attached electric dipoles or, on contact with the glass, forms electric
dipoles that have
only rotational mobility. The electric dipoles within the glass may have
translational
as well as rotational mobility at 25 C. Reactions between the dipoles with
translational mobility may form dipole-rich regions within the glass
electrolyte with
some dipole condensation into ferroelectric molecules; the coalescence of the
dipoles,
which is referred to as aging of the electrolyte, may take days at 25 C, but
can be
accomplished in minutes at 100 C.
One or more of the dipoles may have some mobility even at 25 C. An
electrode consists of a current collector and/or a material having an active
redox
reaction. An electrode current collector is a good metal such as Al or Cu; it
may also
be a form of carbon, an alloy, or a compound such as TiN or a transition-metal
oxide.
7
CA 03030559 2019-01-10
WO 2018/013485 PCT/US2017/041382
The current collector may be an electrode without an active material on it or
it may
transport electrons to/from an active material on it; the active material may
be an
alkali metal, an alloy of the alkali metal, or a compound containing an atom
of the
working ion of the electrolyte. The current collector transports electrons to
or from the
external circuit and to or from my active material of an electrode reacts with
the
working ion of the electrolyte by having electronic contact with the current
collector
and ionic contact with the electrolyte. The ionic contact with the electrolyte
may
involve only excess or deficient working-ion concentration at the
electrode/electrolyte
interface, which creates an electric-double-layer capacitor (EDLC), or it may
also
involve formation of a chemical phase at the electrode surface. In a
rechargeable cell,
any chemical formation on an electrode surface as well as the EDLC across the
electrode/electrolyte interface is reversible.
According to the present invention, one or both electrodes in the
electrochemical cell are, on fabrication, only current collectors containing
no
detectable atom of the working ion of the electrolyte down to 7000 ppm by, for
example, atomic absorption spectroscopy. However, after cell assembly, atoms
of the
working ion of the electrolyte may be detected on the electrode by atomic
absorption
spectroscopy or by other means.
In addition, one or both electrodes of the cell may contain an additional
.. electronically conductive material such as carbon that aids plating of the
working
cation on the current collector without changing significantly the effective
Fermi level
of the composite current collector.
The solid glass electrolyte may have a large dielectric constant, such as a
relative permittivity (GR) of 102 or higher. Solid glass electrolytes are non-
flammable
and may have an ionic conductivity GA for the working ion A+, of at least 10-2
S/cm at
25 C. This conductivity is comparable to the ionic conductivity of the
flammable
conventional organic-liquid electrolytes used in Li-ion batteries, which makes
the
cells safe.
The solid glass electrolyte may be formed by transforming a crystalline
electronic insulator containing the working ion or its constituent precursors
(typically
containing the working ion bonded to 0, OH, and/or a halide) into a working-
ion-
conducting glass/amorphous solid. This process can take place in the presence
of
8
CA 03030559 2019-01-10
WO 2018/013485 PCT/US2017/041382
dipole additives as well. The working ion-containing crystalline, electronic
insulator
or its constituent precursors may be a material with the general formula
A3,Hx0X,
wherein 0 < x < 1, A is at least one alkali metal, and X is the at least one
halide.
Water may exit the solid glass electrolyte during its formation.
An electrochemical cell containing a solid glass electrolyte as disclosed
herein
may have a large energy gap Eg, in which Eg = Ee ¨ E. Ec is the bottom of the
conduction band and Ec > A, where [tA is the anode chemical potential. Ev is
the top
of the valence band and E.,õ < i.tc, where i.tc is the cathode chemical
potential. The
energy difference pA ¨ [Lc may drive the self-charging and self-cycling
behaviors. In
addition, the dipoles contribution causes the electrochemical cell to have a
capacitance at open or closed circuit that is higher than in an otherwise
identical
electrochemical cell with an electrolyte other than a solid glass electrolyte
as
disclosed herein.
An electrochemical cell containing the solid glass electrolyte as disclosed
herein may also be able to plate and strip the working ion from one or both
electrodes
such that an electrolyte-electrode bond between the electrolyte and at least
one
electrode is sufficiently strong for electrolyte volume changes during cycling
to be
substantially perpendicular to the interface between the electrolyte and the
electrode.
Typically the bond will be sufficiently strong between the electrolyte and
both
electrodes for the electrolyte volume changes to be substantially
perpendicular to both
interfaces between the electrolyte and both electrodes.
In what follows, a control current Lõ is the current specified by the
potential
difference between the two electrodes controlled by a load in a potentiostat,
whereas
the measured current /õ,, is the actual measured current, which includes the
current
specified by the potentiostat and the current resulting from the self-charge.
The
current resulting from the self-charge may be in the same or the opposite
direction of
Lõ on discharge. The subscript dis and ch are added to specify whether we
refer to
discharge or charge currents and voltages.
An electrochemical cell as disclosed herein may have a measured discharge
current 'me-dis and/or a measured charging current Ime_ch less than the
control current
Icon.
9
CA 03030559 2019-01-10
WO 2018/013485 PCT/US2017/041382
During charge, an electrochemical cell as disclosed herein may have a
charging current Ich that is greater than the control current Icon. During
discharge, an
electrochemical cell as disclosed herein may have a measured discharge current
Irne-dis
that is larger than the discharge control current Ichs_õn. Such an
electrochemical cell,
in addition to normal discharge, may exhibits self-cycling of both the
measured
discharge current Inze-dis and voltage Vrne-dis
An electrochemical cell as disclosed herein may, at open-circuit, develop a
voltage that is less than or equal to the theoretical voltage as a result of
the difference
in the electrode electrochemical potentials.
An electrochemical cell as disclosed herein may have a self-voltage
sufficiently large to cause a working ion in the electrolyte to plate onto the
anode at
open-circuit. Moreover, the electric power delivered by the self-charge may be
sufficient to light a red LED for over a year.
An electrochemical cell as disclosed herein may exhibit plating of the ion on
either electrode current collector when subjected to a constant /con or Vcon.
Plating of
the working cation from the electrolyte without being resupplied by the
counter
electrode may result in a self-cycling component of the measured current /me
and
measured voltage 17.e.
In what follows, a control current /cm is the current specified by the
potential
difference between the two electrodes controlled by a load in a potentiostat,
whereas
the measured current /me is the actual measured current, which includes the
current
specified by the potentiostat and the current resulting from the self-charge.
The
current resulting from the self-charge may be in the same or the opposite
direction of
/con on discharge. The subscripts dis and ch are added to specify whether we
refer to
discharge or charge currents and voltages.
An electrochemical cell as disclosed herein may exhibit self-cycling at a
given
cycle period. Due to self-charging, the period of the self-cycling is
independent of
charge/discharge period.
The measured current /me of an electrochemical cell as described herein
contains both direct current and alternating current components. In some
applications, only the alternating-current portion may be used, for example in
CA 03030559 2019-01-10
WO 2018/013485 PCT/US2017/041382
signaling. The alternating current period is the self-cycling period which may
have a
period of between minutes and days.
The principles by which an electrochemical cell as disclosed herein may
operate are better understood through reference to FIG. 1, which includes FIG.
1A to
illustrate the electrochemical cell 100 prior to cycling, FIG. 1B to
illustrate the
electrochemical cell 100 during self-charge, and FIG. 1C to illustrate the
electrochemical cell 100 during discharge.
In FIG. 1A, FIG. 1B, and FIG. 1C, electrochemical cell 100 contains anode 10
with contact 15, cathode 20 with contact 25, and solid alkali-metal-ion glass
electrolyte 30. Excess working ions in electrolyte 30 are represented by +
circles.
Depletion of working ions in electrolyte 30 are represented by - circles.
Electric
dipoles in electrolyte 30 are represented by +/- ellipses. Electronic charges
in the
electrodes are represented by + and - circles. In FIG. 1B and FIG. 1C, arrows
attached to circles represent directions of motion of the charges associated
excess
mobile cations or deficiency of mobile cations in the electrolyte, electrons
in the
electrode.
FIG. 1A illustrates schematically electrochemical cell 100 at open-circuit.
The
chemical potential ( A) of anode 10 is higher than the chemical potential
(i.tc) of
cathode 20. To balance the energy between the anode and the cathode chemical
potentials, electric double-layer capacitors 40a and 40b are formed at the
interfaces
between the electrodes and the electrolyte. Energy for the formation of these
electric
double-layer capacitors 40a and 40b is supplied by the differences in chemical
potentials of anode 10 and cathode 20.
One such electrochemical cell 100 may have an alkali metal anode 10 and a
Cu cathode 20, with an alkali-metal working ion (A+) in the solid glass
electrolyte 30.
Displacement of the working ion (A+) and the electric dipoles in solid glass
electrolyte 30 allows the formation of electric double-layer capacitors 40a
and 40b.
As illustrated in FIG. 1A, at open-circuit, electric double-layer capacitor
40a at the
interface of anode 10 (which is the negative terminal of electrochemical cell
100) and
electrolyte 30 has an excess of working ions in electrolyte 30 (represented as
+),
which results from the shift of those working ions in electrolyte 30 towards
its anode
side. The portion of anode 10 at the interface will have an excess of negative
11
CA 03030559 2019-01-10
WO 2018/013485 PCT/US2017/041382
electronic charge, (represented as -), with a compensatory excess of positive
cation
charge on the electrolyte side of the interface between anode (1) and
electrolyte 30
that are separated from one another.
Electric double-layer capacitor 40b at the interface of cathode 20 (which is
the
.. positive terminal of electrochemical cell 100) and electrolyte 30 has a
depletion of
working cations in electrolyte 30 (represented as -), which results from the
shift of
working ions in the electrolyte 30 towards its anode side and away from its
cathode
side. The portion of cathode 20 at the interface will have a compensatory
excess of
positive electronic charge (represented as +), in cathode 20 distant from the
interface
with electrolyte 30.
Other than at the interfaces with the electrodes where charge varies as
described above, electrolyte 30 will have a neutral net charge.
The shifting of the working ion in electrolyte 30 and the resulting formation
of
electric double-layer capacitors 40a and 40b occurs fairly rapidly once
electrochemical cell 100 is assembled.
Due to the shift in working ions in electrolyte 30 and the resulting electric
field in a direction to equilibrate the difference in the electrochemical
potentials of the
two electrodes, electric dipoles in the solid glass electrolyte will tend to
orient
themselves with their negative ends towards the interface between electrolyte
30 and
anode 10 and with their positive ends towards the interface between
electrolyte 30 and
cathode 20, as illustrated by the + and - indicators in FIG. 1A.
This alignment of the electric dipoles and their translational motion does not
occur instantaneously and is a considerably slower process than formation of
the
electric double-layer capacitors 40a and 40b. As a result, the open circuit
voltage as
measured over time (Voc(t)) evolves as alignment and dipole translations
occur. This
evolution of Voc(t) may be modeled using an equivalent circuit, as shown in
the
schematic diagram of FIG. 2. The schematic diagram of FIG. 1A represents a
hypothetical electrochemical cell 100 in which the dipoles a in solid glass
electrolyte
are all aligned and displaced. In reality, complete alignment and displacement
are
30 not likely to be achieved and dipole translations and alignment will
increase as Voc(t)
evolves. Increased dipole alignment also strengthens the electric field across
electrolyte 30 and drives further dipole alignment and movement. Maximum
12
CA 03030559 2019-01-10
WO 2018/013485 PCT/US2017/041382
alignment and motion in a given electrochemical cell 100 may be assumed to
have
occurred when Võ ceases to change in a consistent direction over time, or
during a
given period of time, such as for at least one minute or at least five minutes
for
alignment and days or minutes for movement depending on the temperature.
In some instances, the additional electric field created by dipole alignment
and
motion in electrolyte 30 may be sufficient to even drive plating of the
working ion on
anode 10 when electrochemical cell 100 is at open-circuit. Eventually, the net
negative charge that electrolyte 30 develops as a result of this plating
process will be
sufficiently high that it cannot be overcome by the electric field and plating
on anode
10 will cease.
FIG. 1B illustrates electrochemical cell 100 when its circuit is first closed,
for
example by connecting an external circuit to contacts 15 and 25. The external
circuit
includes a potentiostat that imposes a charging control current Icon that may
be less
than some critical current lc. The critical current is the maximum Lon-ths at
which self-
charge phenomenon still exist as shown in Fig. 1B. When the circuit is closed,
the
energy stored in electric double-layer capacitors 40a and 40b and by the
aligned
dipoles in electrolyte 30 introduces a charging current Id,. This phenomena
self-
charges electrochemical cell 100.
During self-charge, while the discharging control current Icon is being
applied,
some electrons are transported from cathode 20 to anode 10 where they reduce
excess
working ions in electrolyte 30 near the interface with anode 10 reduced, to
plate then
on anode 10 forming plated metal 50.
Cathode 20 lacks the working ion, so it cannot resupply working ions as they
are depleted from electrolyte 30 to form plated metal 50. Accordingly, the
electrolyte
30 at the interface with anode 10 becomes increasingly negatively charged,
eventually
reaching the point where the working ion is no longer being plated on anode 10
as
plated metal 50 and the working ions begin to be stripped back to the
electrolytes.
Alternatively, the plated metal may become so thick that the electrode
chemical potential becomes that of the plated metal so rather than that of the
current
collector, 10, which makes it more difficult to plate the working cation from
the
electrolyte as the metal 50, thereby terminating the plating process. In
either case, as
13
CA 03030559 2019-01-10
WO 2018/013485 PCT/US2017/041382
plating of the working ion to anode 10 decreases, the measured charging
current Ime_ch,
decreases.
In addition the initial electrons removed from the cathode by the charging
current Id, retains electric double-layer capacitor 40b, resulting in a shift
of the dipoles
in direction 70, towards cathode 20. Working ions in electrolyte 30, however,
do not
shift towards cathode 20.
Eventually the depletion of the working ion from electrolyte 30 near anode 10
induces its stripping from plated metal 50 back to the electrolyte 30 in a
discharge
phase, Iine_ch further decreases and the electric double-layer capacitors 40a
and 40b are
restored (not shown). Eventually the energy stored in the electric double-
layer
capacitors is sufficiently high that further stripping of the working ion from
plated
metal 50 cannot contribute sufficient additional energy to still occur, and
plating of
the working ion on anode 10 as plated metal 50 resumes, causing an increase in
Ime_ch.
Although the above description presents plating and stripping in a simplified
manner, with one happening at any time, in the actual electrochemical cell
100,
typically both plating and stripping occur at the same time during at least
part of each
cycle, but one process predominates so that there is net plating or net
stripping.
This alteration between net plating and net stripping of the working ion from
plated metal 50 on anode 10 results in self-cycling of electrochemical cell
100, with
concurrent cycling of the voltage (V). This self-cycling occurs with a given
cycle
period, that tends to remain constant as long as cycling continues. The cycle
period
depends on the difference in the rates of electronic versus cation and dipole
translational motion.
In an electrochemical cell 100 such as that of FIG. 1B, where Icon is less
than
lc, the cycle period may be on the order of minutes, between one and ten
minutes.
FIG. 1C illustrates electrochemical cell 100 during discharge through an
external circuit that includes a potentiostat that imposes a control current
Iõõ that is
greater than the critical current lc, as in a low-load external circuit, such
as might
include a light-emitting diode (LED). As in the electrochemical cell of FIG.
1B, once
the circuit is closed, energy is stored in electric double-layer capacitors
40a and 40b
and by the aligned dipoles in electrolyte 30.
14
CA 03030559 2019-01-10
WO 2018/013485 PCT/US2017/041382
During discharge, a current Idis is created and may be controlled by Icon. The
load of the external circuit is sufficiently low for electrons to flow from
anode 10 to
cathode 20 to attract working ions from electrolyte 30 to cathode 20, where
they
combine with the electrons and form plated metal 60 on cathode 20. The
transfer of
electrons from anode 10 to cathode 20 reduces both electric double-layer
capacitors
40a and 40b, but it primarily reduces electric double-layer capacitor 40b, at
the
electrolyte-cathode interface. This lowers the electric field across
electrolyte 30,
allowing the working ion to be plated on cathode 20. Although electric double-
layer
capacitor 40b is depleted, it is typically not destroyed so long as the
majority of the
dipoles in electrolyte 30 remain oriented in the same way as when
electrochemical
cell 100 is at open circuit. The dipoles in electrolyte 30 are, however,
compressed in
direction 80.
Anode 10 substantially lacks the working ion, so it cannot resupply working
ions as they are depleted from electrolyte 30 to form plated metal 60. In
addition,
changes in the internal electric field of electrolyte 30 that are created by
electron
transfer that occurs much faster than the working ion and electric dipoles can
redistribute and align to accommodate them. Accordingly, the electrolyte 30 at
the
interface with cathode 20 becomes increasingly negatively charged, eventually
reaching the point where the working ion is also stripped from plated metal 60
and
returned to electrolyte 30, resulting in a measured discharge current Iine
that is
increasingly lower until it reaches /me minimum until plating and stripping
are in
equilibrium.
Alternatively, in some electrochemical cells 100, plated metal 60 may
eventually become so thick, electrolyte 30 is effectively screened from
cathode 20 and
working ions in electrolyte 30 are substantially all exposed to plated metal
60 which
does not have a sufficient difference in chemical potential as compared to
anode 10 to
cause additional plating based on the difference in chemical potential alone
or
combined with any remaining electric field in electrolyte 30. In such cases,
the
working ion may no longer be plated to cathode 20 as plated metal 60.
Iine increases as the working ion is plated metal 60. When Iine reaches
maximum, the process reverses and working ions are once again plated on
cathode 20
as metal plate 60.
CA 03030559 2019-01-10
WO 2018/013485 PCT/US2017/041382
This alteration between plating and stripping of the working ion from plated
on metal 60 on cathode 20 results in self-cycling of electrochemical cell 100,
with
concurrent cycling of the voltage and Lne. This self-cycling occurs with a
given cycle
period that tends to remain constant as long as cycling continues. The cycle
period
depends on the rate of compression in direction 80 of the dipoles in
electrolyte 30
during discharge and their expansion during charge. Typically increasing 'me
towards
maximum is faster than decreasing it to minimum.
In an electrochemical cell 100 such as that of FIG. 1C, where is
greater
than lc, the period may be on the order of days, such as one and seven days.
Electrons can only pass one way through an LED. Accordingly, in actual use
of electrochemical cell 100, anode 10 is increasingly positively charged and
cathode
is increasingly negatively charged until the electric field across electrolyte
30
reverses the orientation of the electric dipoles, which then switches off the
current that
flows through the load. For electrochemical cells with a small 'me, the time
required
15 for this
to occur may be lengthy, even more that a year. Thus, an electrical device
may be powered by electrochemical cell 100 for that length of time with no
external
energy input.
In an electrochemical cell 100 in which both electrodes substantially lack the
working ion prior to cell assembly, an external charging current Ich may
create large
20 electric
double layer capacitors 40a and 40b, which may cause the working ion to
plate from electrolyte 30 onto anode 10, as such plating occurs during
charging of a
conventional rechargeable electrochemical cell. However, depletion of the
working
ion from the electrolyte 30 with no resupply from cathode 20, in contrast to a
conventional rechargeable electrochemical cell in which such resupply does
occur,
causes increasing resistance to further plating on anode 10 as negative charge
in
electrolyte 30 near anode 10 increases. In addition, changes in the electric
field across
electrolyte 30 by molecular and atomic accommodations in the electrolyte that
are
slower than the electronic motions in the current collectors change the rates
of self-
charge and self-cycling. Accordingly, as in the case of the electrochemical
cell of
FIG. 1B above, stripping of working ions from anode 10 also occurs.
Eventually, at a
constant control external charging current Icon_ch, an equilibrium is reached
between
plating and stripping of the working ion at anode 10.
16
CA 03030559 2019-01-10
WO 2018/013485 PCT/US2017/041382
In such an electrochemical cell, the measured charging current Loe_ch is
larger
than the constant control external charging current Icoo_ch because the
electric double-
layer capacitors 40a and 40b are charged in addition to the working ion being
plated
and stripped. In addition, the magnitude of the measured charging voltage
Vme_ch and
the measured charging current Loe_ch change cyclically over time as plating
and
stripping alternate.
In such a cell, average Irne-ch may be greater than or equal to Icoo_ch while
the
working ion is being stripped from and plated to anode 10. As the plating/and
stripping at anode 10 continues, the electric double-layer capacitor 40a near
anode 10
is also being charged. Eventually, electric double-layer capacitor 40a is
charged
sufficiently for the negative charge on the anode side to block the return of
electrons
to anode 10, thereby preventing further stripping of the working ion from
anode 10.
At this point, continued charging can only charge the electric double-layer
capacitor
40a. As a result, self-cycling of the voltage stops and the voltage increases
linearly
with time at a rate that depends on Icoo_ch.
In a variation, if the working ion has been plated on cathode 20 before the
external charging current is applied, then the measured discharge current Lne-
dis self-
cycles until the working ion has been substantially stripped from cathode 20.
The
measured discharge voltage Vine, however, does not cycle.
In FIG. 2, an inductance is introduced into the equivalent circuit to
represent
the role of the negative charge introduced into electrolyte 30 by plating of
the working
cation from the electrolyte without a resupply to the electrolyte from a
counter-cation.
In FIG.. 2, C represents the capacitance of the association in series of 40a
and 40b
EDLCs, ItL the plating resistance, and Vo =
" e-1.1C the driving force.
Electrochemical cells of the present disclosure may be used in batteries. Such
batteries may be simple batteries containing few components other than an
electrochemical cell and a casing or other features. Such batteries may be in
standard
battery formats, such as coin cell, standard jelly roll, pouch, or prismatic
cell formats.
They may also be in more tailored formats, such as tailored prismatic cells.
Electrochemical cells of the present disclosure may also be used in more
complex batteries, such as batteries containing complex circuitry and a
processor and
memory computer-implemented monitoring and regulation.
17
CA 03030559 2019-01-10
WO 2018/013485 PCT/US2017/041382
Regardless of simplicity, complexity, or format, all batteries using
electrochemical cells of the present disclosure may exhibit improved safety,
particularly a lower tendency to catch fire when damaged, as compared to
batteries
with organic-liquid electrolytes.
A battery may contain a single electrochemical cell as disclosed herein, or
two
or more such cells, which may be connected in series or in parallel.
Electrochemical cells as disclosed herein and batteries containing them may be
rechargeable.
Electrochemical cells of the present disclosure may also be used in devices
that take advantage of the electric double-layer capacitor, such as in
capacitors. They
may also be used in devices that take advantage of the cycle period,
particularly the
AC period, such as signaling devices.
By way of example, electrochemical cells of the present disclosure may be
used as a dielectric gate of a field-effect transistor; in portable, hand-held
and/or
wearable electronic device, such as a phone, watch, or laptop computer; in a
stationary electronic device, such as a desktop or mainframe computer; in an
electric
tool, such as a power drill; in an electric or hybrid air, land or water
vehicle, such as a
boat, submarine, bus, train, truck, car, motorcycle, moped, powered bicycle,
aircraft,
drone, other flying vehicle, and toy versions thereof; for other toys; for
energy
storage, such as in storing electricity from wind, solar, hydropower, wave, or
nuclear
energy and/or in grid storage or as a stationary power store for small-scale
use, such
as for a home, business, or hospital; for a sensor, such as a portable medical
or
environmental sensor; to generate a low frequency electromagnetic wave, such
as for
underwater communication; as a capacitor, such as in a supercapacitor or a
coaxial
cable; or as a transducer.
EXAMPLES
The following examples are provided to further illustrate the principles and
specific aspects of the invention. They are not intended to and should not be
interpreted to encompass the entire breath of all aspects of the invention.
Example 1 ¨ Icon <I in an Electrochemical Cell
18
CA 03030559 2019-01-10
WO 2018/013485 PCT/US2017/041382
FIG. 3 presents results of an experiment demonstrating self-charge and self-
cycling of an electrochemical cell with an Al-C anode, a S-C-Cu cathode and a
Li+
solid glass electrolyte containing 10 wt% Li2S (an Al-C/Li+ -glass/S-C-Cu
cell).
The Al anode had a carbon layer (Al-C) contacting the Li-glass electrolyte.
The cathode was a Cu current collector with a carbon layer containing a sulfur
relay
contacting the Li-glass electrolyte.
In FIG. 3A, the measured voltage of the electrochemical cell versus time
showed, after 6 h 26 min, a charge of 0.5 mA for 25 min that was followed by
an
abrupt discharge of the electric double-layer capacitors. The voltage at a
constant Icon
increased little for the next 8 h 22 min as the electric double-layer
capacitors were
recharged, before increasing to 2.4 V with small-amplitude noise over the next
35 h
29 min. The measured voltage, V., of 2.4 V was the maximum voltage for self-
charge
plating of lithium metal Li on the anode. A brief break in the rate of
voltage increase
occurred when V., is 2.2 V before oscillations begin. The theoretical V., for
(11A-
[tc)/e was 2.2 V. Once the thickness of the plated Li on the Al-C anode
reached a
critical thickness, plating/stripping of Li oscillated between plating at
[tA(Li) ¨
lic(Cu) and [tA(A1) - [tc(Cu).
FIG. 3B shows an enlarged image of the onset of self-cycling between [ A(Li)
R(Cu)]/e and [ A(A1) ¨ [tc(Cu)]/e in the measurement of FIG. 3A, allowing the
oscillations to be more clearly seen.
FIG. 3C shows the V., of the cell of FIG. 3A in a second cycle at a constant
Icon after an initial charge of Co2 mA for 25 min. During self-charge, Li +
from the
glass electrolyte was plated on the Al anode after charging of the electric
double-layer
capacitors, and the Li + working ions were not resupplied to the electrolyte
from the
Cu cathode. On reaching V., of 1.65 V, as further visible in enlarged FIG. 3D,
a self-
cycling with an average V., of approximately 1. 7 V began.
A sharp drop in V., occurred when the temperature of the electrochemical cell
was increased by heating from 12 C to 26 C. The electrochemical cell was
then
removed from the heater and allowed to return slowly to 12 . During cooling,
V.,
increased to 2.1 V, the difference between the chemical potentials of Al and
Cu. At
that voltage, the electrochemical cell began to plate the working ion again,
but
without periodic cycling, until a small discharge occurred, followed by longer
period
19
CA 03030559 2019-01-10
WO 2018/013485 PCT/US2017/041382
cycling; a discharge of the electric double-layer capacitor to a V., of
approximately
1.4 V occurred. At that point, plating resumed. Throughout these voltage
changes,
the measured charging current 'me remained nearly constant, with only a small
decrease during the final plating that was terminated abruptly at the final
recorded
discharge. The changes with temperature reflect the ionic and molecular
motions in
the electrolyte.
Example 2 -I > lc in a LED-Containing External Circuit
Electrochemical cells with an electrochemical cell with an Al anode, a Cu
cathode and a Li+ solid glass electrolyte (Al/LIP-glass/Cu cells) were
constructed and
then connected in series to light a red LED in an external circuit. These
cells
exhibited self-charging with a discharge current, Idis that is greater than a
critical
current L. The electrochemical cells had been previously cycled, but were not
charged or otherwise supplied with an external energy input. The cells have
powered
the LED for approximately two years. Data per cell for the first year is
presented in
Table 1. The total density of energy delivered over the first year was 373.8
Wh/g.
Table 1 - Al/Lit-glass/Cu Cells Over One Year with an LED-Containing
External Circuit
Days 365
I(A) = Imes-des> 1.6 x 10-4
Capacity, Q(Coulombs) 5046
Voltage (V) average cell 0.53
Energy (J or Ws) per cell 2691
Energy (Wh) per cell 0.748
CA 03030559 2019-01-10
WO 2018/013485 PCT/US2017/041382
Mass per cell (kg) 2 x 10-3
Energy (Wh/kg) per cell 373.8
Example 3 ¨ Icon> Ic in an Electrochemical Cell with a Li Anode
FIG. 4 presents the measured voltage Vine(t) of a discharging electrochemical
cell with a Li metal anode, a Li + glass electrolyte and a C-Ni cathode, with
carbon
located in a Ni mesh (Li-metal/Li+-glass/C-Ni cell). The load resistance was 1
mega
ohm and the discharge current of approximately 2.5 [LA. The periodic hillocks
in the
voltage were the result of a periodic self-cycling of a self-charge component
in the
cell discharge compound. -Plating on the cathode had a longer period than
plating on
the anode (as shown in FIG. 3B). Due to the excess energy supplied by self-
charging,
the electrochemical cell exhibited a charge/discharge coulomb efficiency
greater than
100% on cycling.
Example 4- Constant Applied Charging Current a con-ch) >
FIG. 5A presents data showing self-cycling of a measured charging current
Lne_ch with a constant charging control current Icon_ch in an Al-C/Li+-glass/S-
C-Cu cell
such as that used in Example 1 on a second charge/discharge cycle. The cells
were
recharged by an external power source after the first discharge. The measured
voltage
(Vine) was 2.1 V, corresponding to 4tA(A1) - 1.tc(Cu)]/e. Irne-ch, which was
greater than
/con, exhibited self-cycling with an amplitude such that Irne-ch remained
greater than
Icon, as seen in FIG. 5B.
Lne_ch remained greater than Icc,õ_ch, indicating that stripping of lithium
metal
from the cathode contributed to Lne_ch, with plating/stripping cycling that
must result
from an electrolyte charge localized near the interface of the electrolyte and
the
cathode because any possible resupply of Li + from the anode would take longer
than
the short self-cycling period. The average Lne_ch remained constant until
most, if not
all of the lithium metal was stripped from the cathode and charging of the
cathode
21
CA 03030559 2019-01-10
WO 2018/013485 PCT/US2017/041382
electric double-layer capacitor commenced. There was no corresponding cycling
of
V., because the cycling phenomena was localized to the cathode/electrolyte
interface.
Example 5 - Id,s>lc in a Jelly-Roll Electrochemical Cell
FIG. 6 presents the measured discharge current I., of a jelly-roll Al-
C/Li+glass/Cu cell, otherwise similar to that of Example 2, with a small load
resistance RL of 0.1 Q. At open-circuit, the cathode electric double-layer
capacitor
depleted Li + in the electrolyte at its interface with the cathode. When the
circuit was
closed, electrons from the anode imparted a negative charge to the Cu cathode,
which
attracted Li + back to the cathode interface. However, ion movement is slower
than
electron movement, so I., increased at the rate of Li + movement back to the
cathode/electrolyte interface. At a critical electrolyte/cathode electric
double-layer
capacitor voltage, the Li + from the electrolyte were plated on the cathode as
lithium
metal and I., abruptly dropped. Subsequent rebuilding of the
cathode/electrolyte
electric double-layer capacitor took more time, as the negative electrolyte
charge
impeded Li + migration to the cathode/electrolyte interface, where plating
once again
occurred.
Example 6- Charge/Discharge Cycling of an All-Solid-State Lithium
Electrochemical
Cell
FIG. 7 shows the variation with time of the charge and discharge voltage on
cycling an electrochemical cell with a Li anode, a Li + glass electrolyte, and
a Mn02-
C-Li-glass-Cu cathode with a yMn02 catalytic relay in a carbon layer
contacting a Cu
current collector (a Li/Li+-glass/Mn02-C-Cu cell). Each cycle was 10 h 30 min
for
444 cycles at a control current Icon of 70 p.A and a measured discharge
current I., of
53 p,A.
I., being less than Icon indicates the presence of a self-charge current that
opposed the discharge Icon. The profile of V., showed cycling typical of self-
charge
via plating on the anode in excess of the Li + resupplied to the electrolyte
via stripping
from the cathode. The discharge voltage profile also showed a long cycle
period of
34 days.
22
CA 03030559 2019-01-10
WO 2018/013485 PCT/US2017/041382
The above disclosed subject matter is to be considered illustrative, and not
restrictive, and the appended claims are intended to cover all such
modifications,
enhancements, and other embodiments which fall within the true spirit and
scope of
the present disclosure. Thus, to the maximum extent allowed by law, the scope
of the
present disclosure is to be determined by the broadest permissible
interpretation of the
following claims and their equivalents and shall not be restricted or limited
by the
foregoing detailed description.
23