Note: Descriptions are shown in the official language in which they were submitted.
LIQUID NALOXONE SPRAY
Field of the Invention
[0001] The invention is directed to liquid spray formulations containing
naloxone, a
pharmaceutically acceptable salt thereof, or a derivative thereof. The
invention is further directed
to methods of treating opioid dependence, opioid overdose, and congenital
insensitivity to pain
with anhidrosis by administering liquid spray formulations containing
naloxone, pharmaceutically
acceptable salts thereof, or derivatives thereof to a patient in need thereof.
Background of the Invention
[0002] Naloxone has the following structure and is synthesized from
thebaine:
H 401,
0
[0003] Naloxone is most commonly used to treat patients suffering from
opioid
dependence or overdose because it is a competitive -opioid antagonist that
blocks the effects of
opioids. Naloxone is currently available in Suboxone (Suboxone is a registered
trademark of
Reckitt Benckiser Healthcare (UK) Limited) as tablet or sublingual film strip
formulations.
Suboxone contains buprenorphine and naloxone in a 4:1 ratio. Naloxone is also
available as an
aqueous nasal spray under the trademark Narcan (Narcan is a registered
trademark of Adapt
Pharma Operations Limited LLC, "Adapt Pharma"), which contains 4.42% w/w
naloxone
hydrochloride dihydrate, 0.01% w/w benzalkonium chloride ("BKC") as a
preservative, 0.74%
w/w sodium chloride as an isotonicity agent and 0.2 % w/w edetate disodium
dihydrate ("EDTA")
as a stabilizing agent. Adapt Pharma has U.S. Patent Nos. 9,211,253, 9,468,747
and 9,561,117
listed in the U.S. Food and Drug Administration's Orange Book for Narcan 4
milligram nasal
spray. Each of these patents discloses and claims naloxone formulations
containing an isotonicity
agent Additional Adapt Pharma also has U.S. Patent No. 9,480,644 listed in the
Orange Book for
a 2-milligram naloxone nasal spray, which discloses and claims naloxone
formulations that also
contain an isotonicity agent. U.S. Patent Nos. 9,192,570 and 9,289,425
assigned to Indivior, Inc
1
Date recue/Date Received 202401-22
disclose and claim naloxone nasal sprays that contain both citric acid as a
buffer and benzyl alcohol
as an anti-microbial agent.
[0004] One issue with other opioid dependence treatments is that they can
become
addictive. Naloxone, however, does not appear to be addictive and patients do
not build up a
tolerance.
[0005] Naloxone has also been used as a treatment for cognitive
insensitivity to pain with
anhidrosis. Insensitivity to pain with cognitive anhidrosis is a disorder in
which the patient cannot
feel pain.
[0006] Naloxone may be administered orally, intravenously, by injection or
via the nasal
mucosa. Naloxone has a low mean serum half-life when administered parentally.
The quick
metabolism may require repeat dosing or cause patient discomfort between
doses. Enteral
administration has low bioavailability due to hepatic first pass metabolism.
[0007] Accordingly, while there are some naloxone formulations currently
available, there
is a need for safe and effective liquid spray formulations that are stable
including physically and
chemically stable and contain naloxone, pharmaceutically acceptable salts or a
derivative thereof.
Summary of the Invention
[0008] The liquid spray formulations of the present invention are for
intranasal and/or
sublingual administration.
[0009] In one aspect, the invention is directed to liquid spray
formulations comprising an
effective amount of naloxone, a pharmaceutically acceptable salt thereof, or a
derivative thereof,
water, and a chelating agent, wherein the formulation does not contain an
isotonicity agent or a
buffer.
[00010] In another aspect, the stable liquid spray formulations of the
present invention are
suitable for intranasal administration.
[00011] In another aspect, the liquid spray formulations of the present
invention do not
contain an isotonicity agent.
[00012] In another aspect, the liquid spray formulations of the present
invention do not
contain sodium chloride.
[00013] In another aspect, the liquid spray formulations of the present
invention do not
contain benzalkonium chloride.
2
Date recue/Date Received 202401-22
[00014] In another aspect, the liquid spray formulations of the present
invention do not
contain a buffer.
[00015] In another aspect, the liquid spray formulations of the present
invention do not
contain citric acid.
[00016] In another aspect, the liquid spray formulations of the present
invention do not
contain an alcohol.
[00017] In yet another aspect, the invention is directed to methods for
treating opioid
dependence comprising administering the liquid spray formulations of the
present invention to a
patient in need of opioid dependence treatment, wherein administration occurs
either intranasally,
sublingually or intranasally and sublingually, wherein if administration
occurs intranasally and
sublingually administration occurs simultaneously, sequentially or
concomitantly.
[00018] In a further aspect, the invention is directed to methods for
treating opioid overdose
comprising administering the liquid spray formulations of the present
invention to a patient in need
of opioid overdose treatment, wherein administration occurs either
intranasally, sublingually or
intranasally and sublingually, wherein if administration occurs intranasally
and sublingually
administration occurs simultaneously, sequentially or concomitantly.
[00019] In an additional aspect, the invention is directed to methods for
treating congenital
insensitivity to pain with anhidrosis comprising administering the liquid
spray formulations of the
present invention to a patient in need of treatment for congenital
insensitivity to pain with
anhidrosis, wherein administration occurs either intranasally, sublingually or
intranasally and
sublingually, wherein if administration occurs intranasally and sublingually
administration occurs
simultaneously, sequentially or concomitantly.
Brief Description of the Figures
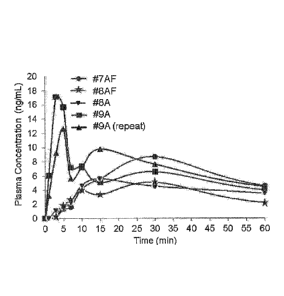
[00020] Figure 1. Mean plasma concentration of Formulations #9A, #9A
repeat #8A, #8AF
and #7AF normalized to a 4-mg dosage. Values based on a geometric mean.
Detailed Description of the Invention
[00021] Applicants have created new liquid naloxone formulations that are
stable and
comfortable to the user despite containing no buffer or isotonicity agent. The
formulations that do
not contain an alcohol are especially suitable for administration to children.
Further, the alcohol-
free formulations may be suitable for patients in recovery from alcohol
addiction.
3
Date recue/Date Received 202401-22
[00022] In a preferred embodiment, the liquid naloxone formulation is a
spray. In yet a
more preferred embodiment, the liquid naloxone formulation is in a simple
solution form. As used
herein, the term "simple solution" refers to a solution in which the solute(s)
has fully dissolved in
the solvent.
[00023] As used herein, the term "stable" includes but is not limited
physical and chemical
stability.
[00024] In one embodiment, the present invention is directed to liquid
spray formulations
comprising an effective amount of naloxone, a pharmaceutically acceptable salt
thereof, or a
derivative thereof, water, and a chelating agent, wherein the formulation does
not contain an
isotonicity agent or a buffer.
[00025] In another embodiment, the liquid spray formulations of the
present invention is for
intranasal administration.
[00026] In another embodiment, the liquid spray formulations of the
present invention do
not contain sodium chloride, citric acid, benzyl alcohol, or benzalkonium
chloride.
1000271 In another embodiment, the present invention is directed to liquid
spray
formulations comprising an effective amount of naloxone, a pharmaceutically
acceptable salt
thereof, or a derivative thereof, a co-solvent selected from the group
consisting of an alcohol, a
glycol, and a combination thereof water, and edetate disodium dihydrate as a
chelating agent,
wherein the formulation does not contain an isotonicity agent or a buffer.
[00028] In another embodiment, the present invention is directed to a
liquid spray
formulation comprising an effective amount of naloxone, a pharmaceutically
acceptable salt
thereof, or a derivative thereof, water, and a chelating agent, wherein the
formulation does not
contain an isotonicity agent or a buffer and wherein the formulation further
comprises a co-solvent
selected from the group consisting of ethanol, propylene glycol, and a
combination thereof. In
another embodiment, the liquid spray formulations of the present invention
have a pH from about
3.0 to about 6.0, more preferably about 4.5.
1000291 In another embodiment, the present invention is directed to liquid
spray
formulations comprising an effective amount of naloxone, a pharmaceutically
acceptable salt
thereof, or a derivative thereof, water, a chelating agent, and an
antioxidant, preferably sodium
ascorbate, wherein the formulation does not contain an isotonicity agent or a
buffer.
4
Date recue/Date Received 202401-22
[00030] In another embodiment, the present invention is directed to liquid
spray
formulations comprising:
from about 1% to about 16% w/w naloxone, a pharmaceutically acceptable salt or
a
derivative thereof, preferably from about 2% to about 10% w/w;
from about 10% to about 99% w/w water;
from about 0.0001% to 0.05% w/w of a chelating agent, preferably edetate
disodium
dehydrate,
wherein the formulation does not contain an isotonicity agent or a buffer.
[00031] In another embodiment, the liquid spray formulations of the
present invention do
not contain an alcohol.
[00032] In another embodiment, the present invention is directed to liquid
spray
formulations comprising:
from about 1% to about 16% w/w naloxone, a pharmaceutically acceptable salt or
a
derivative thereof, preferably from about 2% to about 10% w/w;
from about 80% to about 98% w/w water;
from about 0.0001% to 0.05% w/w of a chelating agent, preferably edetate
disodium
dihydrate,
wherein the formulation does not contain an isotonicity agent, a buffer or a
co-solvent.
[00033] In another embodiment, the present invention is directed to liquid
spray
formulations comprising:
from about 1% to about 16% w/w naloxone, a pharmaceutically acceptable salt or
a
derivative thereof, preferably from about 2% to about 10% w/w;
from about 35% to about 85% w/w water;
from about 0.0001% to 0.05% w/w of a chelating agent, preferably edetate
disodium
dihydrate; and
from about 2% to about 90% w/w of a co-solvent selected from the group
consisting of
ethanol, propylene glycol and a combination thereof, preferably ethanol at a
concentration
from about 2% to about 50% w/w, or a combination of propylene glycol at a
concentration
from about 5% to about 10% w/w and ethanol at a concentration from about 2% to
about
50% w/w or a combination of ethanol at about 20 % w/w and propylene glycol at
about 5
Date recue/Date Received 202401-22
% w/w or a combination of ethanol at about 50 % w/w and propylene glycol at
about 5 %
w/w,
wherein the formulation does not contain an isotonicity agent or a buffer.
[00034] In another embodiment, the present invention is directed to liquid
spray
formulations comprising
from about 1% to about 16% w/w naloxone, a pharmaceutically acceptable salt or
a
derivative thereof, preferably from about 2% to about 10% w/w;
from about 35% to about 85% w/w water;
from about 0.0001% to 0.05% w/w of a chelating agent, preferably edetate
disodium
dihydrate; and
propylene glycol as a co-solvent at a concentration from about 5% to about 10%
w/w,
wherein the formulation does not contain an isotonicity agent, a buffer or an
alcohol.
[00035] In another embodiment, the liquid spray formulations of the
present invention
comprise a preservative selected from the group consisting of butyl paraben,
methyl paraben, ethyl
paraben, propyl paraben, sodium benzoate, benzoic acid and a combination
thereof, preferably
from about 0.005% to about 0.2% w/w methyl paraben and more preferably 0.1%
w/w methyl
paraben.
[00036] In another embodiment, the liquid spray formulations of the
present invention do
not contain a preservative.
[00037] In another embodiment, the liquid spray formulations of the
present invention are
administered in a nasal spray device.
[00038] In another embodiment, the liquid spray formulations of the
present invention are
administered in a nasal spray device that is capable of producing a droplet
size distribution wherein
greater than 90% of the composition particles are greater than 10 microns in
diameter during
administration or a droplet size distribution wherein:
the mean Dv(10) is from about 5 to about 40 microns during administration;
the mean Dv(50) is from about 20 to about 80 microns during administration;
and
the mean Dv(90) is from about 50 to about 700 microns during administration,
or
6
Date recue/Date Received 202401-22
a spray plume that has an ovality ratio of from about 1.0 to 2.5, or a spray
plume width from about
25 to about 70 millimeters during administration and a spray plume angle from
about 15 to about
70 degrees during administration.
[00039] In another embodiment, the liquid spray formulations of the
present invention are
administered in a nasal spray device that has a single reservoir comprising
about 125 p.1 to 127 pit
of the formulation.
[00040] In another embodiment, the liquid spray formulations of the
present invention are
administered in a nasal spray device that delivers about 100 pd., of the
formulation by a single
actuation.
Formulations with An Alcohol
[00041] In one embodiment, the invention is directed to liquid spray
formulations
comprising an effective amount of naloxone, a pharmaceutically acceptable salt
or a derivative
thereof, water as a solvent, a co-solvent and an antioxidant or chelating
agent. In a preferred
embodiment, naloxone is in salt form.
[00042] In another embodiment, the invention is directed to liquid spray
formulations
comprising an effective amount of naloxone, a pharmaceutically acceptable salt
or a derivative
thereof, water as a solvent, a co-solvent and a permeation enhancer or
chelating agent. In a
preferred embodiment, naloxone is in salt form.
[00043] The co-solvent may be an alcohol, a glycol, or a mixture thereof.
The formulations
preferably contain from about 5 to about 90% w/w co-solvent. More preferably
the formulations
contain from about 10 % to about 70 % w/w from about 10 % to about 55% w/w or
from about
40% to about 65% w/w or from about 45% to about 60 % w/w or from about 45 % to
about 55 %
w/w co-solvent. In a preferred embodiment, the formulations contain about 10%
w/w, about 12%
w/w, about 25% w/w or about 55 % w/w co-solvent. In a more preferred
embodiment, the
formulations contain about 10 % w/w ethanol as a co-solvent or about 2% to
about 45% ethanol
as a co-solvent, or about 10% to about 20% ethanol as a co-solvent, or about
10 % w/w propylene
glycol and about 2% w/w ethanol as a co-solvent or about 20% w/w ethanol and
about 5% w/w
propylene glycol as a co-solvent or about 50% w/w ethanol and 5 % w/w
propylene glycol as co-
solvent.
[00044] Suitable antioxidants include butylated hydroxyanisole (BHA),
butylated
hydroxytoluene (BHT), methionine, sodium ascorbate, sodium thiosulfate,
thioglycerol, ascorbic
7
Date recue/Date Received 2024-01-22
acid, ascorbyl palmitate, propyl gallate, dL-alpha-tocopherol, sodium sulfite,
sodium
metabisulfite, sodium bisulfite cysteine hydrochloride, glutathione and a
combination thereof.
Presently preferred antioxidants include BHA, BHT, sodium thiosulfate, dL
alpha-tocopherol
(Vitamin E) and sodium ascorbate.
[00045] In a preferred embodiment, the amount of antioxidant included in
the formulation
is from about 0.001% to about 0.5% w/w.
[00046] In another preferred embodiment, the amount of antioxidant is
about 0.01% w/w of
BHA.
[00047] In an alternative embodiment, the antioxidant is a mixture of
about 0.01% w/w of
BHA and about 0.005% w/w of BHT.
[00048] In yet another embodiment, the antioxidant is about 0.01% w/w of
sodium
thi osul fate.
[00049] In a preferred embodiment, the antioxidant is about 0.3 % w/w dL
alpha-tocopherol.
[00050] In a most preferred embodiment, the antioxidant is about 0.02% w/w
of sodium
asc orb ate.
[00051] In the present formulations, water is used as the solvent.
Preferably, formulations
of the present invention contain from about 10% to about 99% w/w water, more
preferably, from
about 10% to about 98% w/w water, more preferably from about 35% to about 85%
w/w, more
preferably from about 35% to about 84% w/w and more preferably about 29.8%,
33.2%, 31.32 %,
34.5%, or 35.5%,37.5%, 65.2%, 71.1%, 79.3%, 81.1%, or 83.9% w/w water. Hydro-
alcohol
formulations of the present invention preferably contain from about 40 % to
about 90% w/w water,
more preferably, from about 50 % to about 90 % w/w water. In preferred
embodiments, hydro-
alcoholic formulations contain about from about 30 % to about 80 % w/w water.
[00052] In a preferred embodiment, the formulations of the present
invention have a pH of
from about 2 to about 7. In a more preferred embodiment, the formulations of
the present invention
have a pH of from about 3 to about 6, even more preferably from about 3 to
about 4.5.
[00053] In a most preferred embodiment, the formulations of the present
invention have a
pH of about 3.0 0.2 or 3.5 0.2 or 4.0 0.2 or 4.5 0.2.
[00054] In another preferred embodiment, the formulations contain ethanol
as the co-
solvent.
8
Date recue/Date Received 202401-22
1000551 In yet another preferred embodiment, the formulations contain
propylene glycol as
the co-solvent.
[00056] In a more preferred embodiment, the formulations contain a mixture
of ethanol and
propylene glycol as the co-solvent.
[00057] In another embodiment, the formulations of the present invention
contain a
chelating agent. In a preferred embodiment, the chelating agent is edetate
disodium dihydrate,
(also known as edetate disodium or ethylenediaminetetraacetic acid disodium
salt or EDTA)
preferably at a concentration from about 0.0001% to about 0.5% w/w and more
preferably from
about 0.001% to about 0.05% w/w and even more preferably from about 0.005% to
about 0.05%
w/w and even more preferably from about 0.001% to about 0.02% w/w.
[00058] In a preferred embodiment, the present invention is directed to
liquid spray
formulations comprising naloxone, a pharmaceutically acceptable salt or a
derivative thereof, in
an amount from about 1% to about 16 % w/w, water in an amount from about 10%
to about 95%
w/w, a co-solvent in an amount from about 2% to about 90% w/w, and a chelating
agent in an
amount from about 0.0001% to 0.05% w/w.
[00059] In a preferred embodiment, the present invention is directed to
liquid spray
formulations comprising naloxone, a pharmaceutically acceptable salt or a
derivative thereof, in
an amount from about 1% to about 20 % w/w, water in an amount from about 30 %
to about 99%
w/w, a co-solvent in an amount from about 2% to about 90% w/w, and a chelating
agent in an
amount from about 0.0005% to 0.05% w/w.
[00060] In a preferred embodiment, the liquid spray formulations of the
present invention
further comprise a permeation enhancer selected from the group consisting of
menthol in an
amount from about 0.001% to about 10.0% w/w, caprylic acid in an amount from
about 0.1% to
10% w/w, benzalkonium chloride ("BKC") in an amount from about 0.001% to 10 %
w/w and a
combination thereof.
[00061] In another preferred embodiment, the formulation contains edetate
disodium
dihydrate as the chelating agent at 0.001 % w/w or 0.05 % w/w.
[00062] In yet another embodiment, the present invention is directed to
naloxone, a
pharmaceutically acceptable salt or a derivative thereof, in an amount from
about 24% to about
16% w/w, water in an amount from about 20% to about 85% w/w, a co-solvent in
an amount from
about 5% to about 55% w/w, and a chelating agent in an amount from about
0.0001% to 0.05%.
9
Date recue/Date Received 202401-22
In a preferred embodiment of the formulation, naloxone is a salt. In yet
another preferred
embodiment, the formulation further comprises a permeation enhancer selected
from menthol in
an amount from about 0.01% to about 10 % w/w, caprylic acid in an amount from
about 0.1% to
10% w/w, BKC in an amount from about 0.001% to 10 % w/w, and a combination
thereof.
[00063] In another preferred embodiment, the chelating agent is edetate
disodium dihydrate,
preferably at a concentration from about 0.001% to about 0.5% w/w.
[00064] In yet another embodiment, the present invention is directed to
naloxone, a
pharmaceutically acceptable salt or a derivative thereof, in an amount from
about 1% to about 10%
w/w, water in an amount from about 30% to about 85% w/w, a co-solvent in an
amount from about
7% to about 55% w/w, and a chelating agent in an amount from about 0.0001% to
0.05%, In a
preferred embodiment of the formulation, naloxone is a salt. In another
preferred embodiment,
the formulation further comprises a preservative, preferably from about 0.01%
to about 0.5% w/w.
In a more preferred embodiment the chelating agent is edetate disodium
dihydrate. In another
preferred embodiment, the preservative is methyl paraben.
[00065] In another embodiment, formulations of the present invention do
not contain a
preservative.
[00066] In a further embodiment, the present invention is directed to
naloxone, a
pharmaceutically acceptable salt or a derivative thereof in an amount from
about 1% to about 10%
w/w, water in an amount from about 35% to about 85% w/w, a co-solvent in an
amount from about
7% to about 55% w/w, and a chelating agent in an amount from about 0.001% to
about 0.02%
w/w. In a preferred embodiment of this formulation, naloxone is a salt. In
another preferred
embodiment, the formulation also contains a preservative in an amount from
about 0.05% to about
0.2% w/w. In yet another preferred embodiment, the formulation contains
edetate disodium
dihydrate as the chelating agent.
[00067] In a further embodiment, the present invention is directed to
liquid spray
formulations comprising naloxone hydrochloride dihydrate from about 1% to
about 10% w/w,
water from about 35% to about 84% w/w, ethanol from about 2% to about 50% w/w,
EDTA from
about 0.001% to about 0.02% w/w and optionally propylene glycol from about 5%
to about 10%
w/w and optionally, methyl paraben at about 0.1% w/w.
[00068] In another embodiment, the liquid spray formulations of the
present invention do
not contain an isotonicity agent.
Date recue/Date Received 202401-22
[00069] In another embodiment, the liquid spray formulations of the
present invention do
not contain sodium chloride.
[00070] In another embodiment, the liquid spray formulations of the
present invention do
not contain benzalkonium chloride.
[00071] In another embodiment, the liquid spray formulations of the
present invention do
not contain a buffer.
[00072] In another embodiment, the liquid spray formulations of the
present invention do
not contain citric acid.
[00073] In some embodiments, the formulations of the present invention
contain citric acid
or sodium hydroxide or hydrochloric acid solution as a pH adjustor.
[00074] Pharmaceutically acceptable salts that can be used in accordance
with the current
invention include but are not limited to hydrochloride, hydrochloride
dihydrate, hydrobromide,
hydroiodide, nitrate, sulfate, bisulfate, phosphate, acid phosphate,
isonicotinate, acetate, lactate,
salicylate, citrate, tartrate, pantothenate, bitartrate, ascorbate, succinate,
maleate, gentisinate,
fumarate, gluconate, glucaronate, saccharate, formate, benzoate, glutamate,
methanesulfonate,
ethanesulfonate, benzensulfonate, p-toluenesulfonate and pamoate (i.e., 1,1'-
methylene-bis-(2-
hydroxy-3-n aphth o ate)) salts.
[00075] In preferred embodiments, the pharmaceutically acceptable salt is
hydrochloride.
[00076] Derivatives of naloxone that can be used in accordance with the
current invention
include but are not limited to 3-0-acyl, phenylhydrazone, and methiodide
derivatives.
[00077] The solvent used with the present invention is United States
Pharmacopeia ("USP")
purified water.
[00078] Co-solvents that can be used in accordance with the current
invention are alcohols,
and glycols or a mixture thereof.
[00079] Alcohols that can be used in accordance with the current invention
include but are
not limited to methanol, ethanol (also known as dehydrated alcohol), propyl
alcohol, butyl alcohol
and the like, but do not include benzyl alcohol.
[00080] In formulations of the current invention that do not contain an
alcohol, the term
"alcohol" includes all alcohols including benzyl alcohol.
11
Date recue/Date Received 202401-22
[00081] Glycols that can be used in accordance with the current invention
include but are
not limited to propylene glycol, polypropylene glycol, and butylene glycol and
polyethylene
glycols such as PEG 200, PEG 300, PEG 400 and PEG 600 and the like.
[00082] In preferred embodiments, the co-solvent is ethanol or propylene
glycol or a
mixture thereof.
[00083] In another preferred embodiment, the amount of co-solvent included
in the
formulation is from about 2% to about 90% w/w. In other more preferred
embodiments, the
amount of co-solvent included in the formulation is about 5% or about 10% w/w
propylene glycol.
In other more preferred embodiments, the amount of co-solvent included in the
formulation is
about 2%, about 10%, about 20% or about 50% w/w ethanol.
[00084] In other more preferred embodiments the co-solvent is a mixture of
propylene
glycol at about 5% w/w and ethanol at about 50% w/w, or a mixture of propylene
glycol at about
5% w/w and ethanol at about 20% w/w, or a mixture of propylene glycol at about
10% w/w and
ethanol at about 10% w/w, or propylene glycol at about 10% w/w and ethanol at
about 2% w/w or
10% w/w ethanol.
[00085] Solubilizers that can be used in accordance with the current
invention are
hydroxypropyl beta-cyclodextrin ("HPI3CD") and sulfobutylether cyclodextrin or
a mixture
thereof.
[00086] In preferred embodiments, the solubilizer is HPI3CD.
[00087] In more preferred embodiments the amount of HPI3CD is about 30%
w/w.
[00088] Permeation enhancers that can be used in accordance with the
current invention
include but are not limited to menthol, limonene, carvone, methyl chitosan,
polysorbates
including Tween 80 (polysorbate 80; Tween is a registered trademark of
Uniqema Americas,
LLC), sodium lauryl sulfate, glyceryl oleate, caprylic acid, pelargonic acid,
capric acid,
undecylenic acid, lauric acid, myristic acid, palmitic acid, oleic acid,
stearic acid, linolenic acid,
arachidonic acid, benzalkonium chloride (BKC), cetylpyridium chloride, edetate
disodium
dihydrate, sodium desoxycholate, sodium deoxyglycolate, sodium glycocholate,
sodium caprate,
sodium taurocholate, sodium hydroxybenzoyal amino caprylate, dodecyl dimethyl
aminopropionate, L-lysine, glycerol oleate, glyceryl monostearate, citric
acid, and peppermint oil.
Preferably the permeation enhancer is selected from the group consisting of
menthol,
benzalkonium chloride, edetate disodium dihydrate, caprylic acid, and a
combination thereof.
12
Date recue/Date Received 202401-22
[00089] In preferred embodiments, the amount of permeation enhancer is
from about
0.001% to about 10 % w/w. In a more preferred embodiment, the formulations
contain from about
0.01% to about 5.0% w/w permeation enhancer. In a preferred embodiment, the
formulations
contain from about 0.02% to about 2.0 % w/w permeation enhancer. In a most
preferred
embodiment, the formulations contain 2.0 % w/w permeation enhancer.
[00090] In preferred embodiment, the permeation enhancer is L-menthol,
caprylic acid,
BKC, edetate disodium dihydrate (EDTA) or combination thereof, the preferred
amount of L-
menthol is from about 0.001% to about 10.0 % w/w, caprylic acid is from about
0.1% to about
10% w/w, BKC is from about 0.001 to about 10 % w/w, and EDTA is from about
0.0005% to
0.1% w/w. In a more preferred embodiment, the formulations contain from about
0.01% to about
0.5% w/w L-menthol, about 0.5% to about 5% w/w caprylic acid, about 0.005 to
about 0.1% w/w
BKC, about 0.005% to about 0.05% w/w EDTA, or a combination thereof. In an
even more
preferred embodiment, the formulations contain from about 0.02% to about 0.5%
w/w L-menthol,
about 1% to about 2% w/w caprylic acid, about 0.01 to about 0.1% w/w BKC,
about 0.005 to about
0.05 % w/w EDTA or a combination thereof. In a most preferred embodiment, the
formulations
contain about 0.5 % w/w L-menthol, about 2% w/w caprylic acid, about 0.01 %
w/w BKC, about
0.005 % edetate disodium dihydrate, or a combination thereof.
[00091] In yet another embodiment, the permeation enhancer is about 0.5%
w/w of menthol.
[00092] In yet another preferred embodiment, the permeation enhancer is
about 2.0 % w/w
caprylic acid.
[00093] In a most preferred embodiment, the permeation enhancer is about
0.01% w/w of
benzalkonium chloride (BKC).
[00094] In a most preferred embodiment, the permeation enhancer is about
0.005%, 0.01%,
0.015% or 0.02% w/w of edetate disodium dihydrate (EDTA).
[00095] In a further most preferred embodiment, the permeation enhancer is
a combination
of 2.0 % w/w caprylic acid and 0.01% w/w of benzalkonium chloride.
[00096] Formulations of the present invention may have a pH range from
about 2.0 to about
7.0, preferably from about 3 to about 6 and more preferably from about 3 to
about 4.5 pH, most
preferably 3 or 4.5 01, pH adjustors that can be used in accordance with the
present invention
include but are not limited to citric acid and sodium hydroxide. In preferred
embodiments, the
amount of sodium hydroxide or citric acid is from about 0.002% to about 0.03%
w/w. In more
13
Date recue/Date Received 202401-22
preferred embodiments, the amount of sodium hydroxide is about 0.015% w/w. In
other more
preferred embodiments, the amount of sodium hydroxide is about 0.012% w/w.
[00097] In a further embodiment, the formulation contains a permeation
enhancer, a
sweetener, a sweetness enhancer, a pH modifier, a flavoring agent, a
preservative, or a combination
thereof.
[00098] In a preferred embodiment, the formulations contain a sweetener. In
a more
preferred embodiment, the sweetener is selected from the group consisting of
sucralose, aspartame,
saccharin, dextrose, mannitol, glycerin, and xylitol. In a preferred
embodiment, the formulations
contain from about 0.001% w/w to about 2% w/w of sweetener. In a more
preferred embodiment,
the formulations contain from about 0.05% w/w to about 1% w/w of the
sweetener. In a most
preferred embodiment, the formulations contain sucralose as sweetener at about
0.8% w/w.
[00099] In another embodiment, the formulations contain a flavoring agent.
In a preferred
embodiment, the formulations contain a flavoring agent selected from the group
consisting of
peppermint oil, menthol, spearmint oil, citrus oil, cinnamon oil, strawberry
flavor, cherry flavor,
raspberry flavor, orange oil, and a combination thereof. Other appropriate
flavoring agents known
by those of skill in art could also be added to formulations of the present
invention. In a preferred
embodiment, the formulations contain from about 0.001% w/w to about 1% w/w of
the flavoring
agent In a more preferred embodiment, the formulations contain from about
0.005% w/w to about
0.5% w/w of the flavoring agent. In a most preferred embodiment, the
formulations contain
strawberry as flavoring agent at about 0.08% w/w.
[000100] In yet another embodiment, the formulations of the present
invention are capable
of producing a droplet size distribution wherein the mean Dv(10) is from about
11 to about 35
microns during administration.
[000101] In a further embodiment, the formulations of the present invention
are capable of
producing a droplet size distribution wherein the mean Dv(50) is from about 25
to about 55
microns during administration.
[000102] In yet another embodiment, the formulations of the present
invention are capable
of producing a droplet size distribution wherein the mean Dv(90) is from about
75 to about 600
microns during administration. Preferably, the formulations of the present
invention are capable
of producing a droplet size distribution wherein the mean Dv(90) is from about
85 to about 500
microns during administration.
14
Date recue/Date Received 202401-22
Formulations Without An Alcohol
[000103] In a further embodiment, the invention is directed to stable
liquid spray
formulations comprising an effective amount of naloxone, a pharmaceutically
acceptable salt or a
derivative thereof, water, a chelating agent and optionally, a co-solvent and
the formulations do
not contain an alcohol.
[000104] In a further embodiment, the invention is directed to stable
liquid spray
formulations comprising an effective amount of naloxone, a pharmaceutically
acceptable salt or a
derivative thereof, water, and a permeation enhancer or a chelating agent, and
optionally, a co-
solvent and the formulations do not contain an alcohol.
[000105] In another embodiment, the stable liquid spray formulations of the
present
invention contain a preservative, preferably from about 0.01% to about 0.5%
w/w. In a more
preferred embodiment, the preservative is methyl paraben.
[000106] In another embodiment, the stable liquid spray formulations of the
present
invention do not contain a preservative.
[000107] In another embodiment, the stable liquid spray formulations of the
present
invention are suitable for nasal administration.
[000108] In another embodiment, the liquid spray formulations of the
present invention do
not contain an isotonicity agent.
[000109] In another embodiment, the liquid spray formulations of the
present invention do
not contain sodium chloride.
[000110] In another embodiment, the liquid spray formulations of the
present invention do
not contain benzalkonium chloride.
[000111] In another embodiment, the liquid spray formulations of the
present invention do
not contain a buffer.
[000112] In another embodiment, the liquid spray formulations of the
present invention do
not contain citric acid.
[000113] In a preferred embodiment, the liquid spray formulation comprises
from about
0.01% w/w to about 20 % w/w naloxone or a salt or derivative thereof. In a
more preferred
embodiment, the liquid spray foimulation comprises from about 1% w/w to about
12% w/w
naloxone or a salt or derivative thereof. In an even more preferred
embodiment, the formulations
contain from about 2% w/w to about 10% w/w naloxone or a salt or derivative
thereof.
Date recue/Date Received 202401-22
[000114] In another embodiment, the formulations contain from about 20% w/w
to about
99% water. In a preferred embodiment, the formulations contain from about 30%
w/w to about
98% w/w water. In a more preferred embodiment, the formulations contain from
about 80% w/w
to about 98% w/w water. In a most preferred embodiment, the formulations
contain from about
81% w/w to about 98% w/w water. Aqueous formulations of the present invention
preferably
contain from about 70% to about 99% w/w water, more preferably, from about 80%
to about 99%
w/w water. In most preferred embodiments, aqueous formulations contain about
from about 84%
to about 98% w/w water.
[000115] In an embodiment, the formulations contain from about 5% w/w to
about 50% w/w
glycerol. In a preferred embodiment, the formulations contain from about 10%
w/w to about 40%
w/w glycerol. In a more preferred embodiment, the formulations contain from
about 15% w/w to
about 35% w/w glycerol.
[000116] In another embodiment, the formulations may contain from about
0.1% w/w to
about 50% w/w polyethylene glycol 400. In a more preferred embodiment, the
formulations
contain from about 10% w/w to about 40% w/w polyethylene glycol 400.
[000117] In another embodiment, the formulations contain from about 0.1%
w/w to about
50% w/w propylene glycol. In a more preferred embodiment, the formulations
contain from about
10% w/w to about 40% w/w propylene glycol. In an even more preferred
embodiment, the present
invention contains from about 5% to about 10% w/w propylene glycol.
[000118] In another embodiment, the formulation contains a pharmaceutically
acceptable salt
of naloxone. In a preferred embodiment, the formulation contains a salt
selected from the group
consisting of hydrochloride, citrate, halide, phosphate, sulfate, acetate,
ascorbate, maleate,
succinate, carbonate, mesylate and lactate. One of skill in the art could use
other pharmaceutically
acceptable naloxone salts in the formulations of the present invention.
[000119] In a preferred embodiment, the antioxidant is selected from the
group consisting of
ascorbic acid, cysteine HC1 monohydrate, citric acid, ethylenediamine tetra
acetic acid (EDTA),
methionine, sodium citrate, sodium ascorbate, sodium thiosulfate, sodium
metabisulfite, sodium
bisulfite, glutathione and thioglycerol. Other appropriate antioxidants known
by those of skill in
the art could also be added to formulations of the present invention.
[000120] In a preferred embodiment, the formulations contain from about
0.0001% w/w to
about 0.5% w/w of the antioxidant. In a more preferred embodiment, the
formulations may contain
16
Date recue/Date Received 202401-22
from about 0.005% w/w to about 0.2% w/w of the antioxidant. In a most
preferred embodiment,
the formulations contain 0.05%w/w or 0.02% w/w of the antioxidant.
[000121] In another embodiment, the formulations of the present invention
contain a
chelating agent. In a preferred embodiment, the chelating agent is edetate
disodium dihydrate
[000122] In an embodiment, the formulations contain from about 0.0001% to
about 0.5%
w/w of the chelating agent. In a preferred embodiment, the formulations
contain from about
0.001% to about 0.50% w/w of the chelating agent. In a more preferred
embodiment, the
formulations contain from about 0.005% to about 0.05% w/w of the chelating
agent.
[000123] In a further embodiment, the formulation contains a permeation
enhancer, a
sweetener, a sweetness enhancer, a pH modifier, a flavoring agent, a
preservative, or a combination
thereof.
[000124] In another embodiment, the formulation contains a permeation
enhancer. In a
preferred embodiment, the permeation enhancer is selected from the group
consisting of menthol,
limonene, carvone, methyl chitosan, caprylic acid pelargonic acid, capric
acid, undecylenic acid,
lauric acid, myristic acid, palmitic acid, oleic acid, stearic acid, linolenic
acid, arachidonic acid,
polysorbates including Tween 80, sodium edetate, benzalkonium chloride (BKC),
cetylpyridinium chloride, sodium lauryl sulfate, citric acid, sodium
desoxycholate, sodium
deoxyglycolate, glyceryl oleate, glyceryl monostearate, Sodium hydroxybenzoyal
amino
caprylate, sodium caprate, dodecyl dimethyl aminopropionate, L-lysine, sodium
glycocholate,
citric acid, peppermint oil and a combination thereof. In a more preferred
embodiment, the
permeation enhancer is selected from the group consisting of polysorbates
including Tweenhi 80,
sodium edetate, benzalkonium chloride (BKC), cetylpyridiniurn chloride, sodium
lauryl sulfate,
citric acid, sodium desoxycholate, sodium deoxyglycolate, glyceryl oleate,
glyceryl monostearate,
L-lysine, sodium glycocholate, sodium taurocholate, citric acid, and a
combination thereof. In an
even more preferred embodiment, the permeation enhancer is selected from the
group consisting
of menthol, caprylic acid and BKC.
[000125] In preferred embodiments, the amount of permeation enhancer is
from about
0.001% to about 10 % w/w. In a more preferred embodiment, the formulations
contain from about
0.001% to about 2.5% w/w permeation enhancer. In a most preferred embodiment,
the
formulations contain from about 0.02% to about 2.0 % w/w permeation enhancer.
17
Date recue/Date Received 202401-22
[000126] In a preferred embodiment, the permeation enhancer is menthol,
caprylic acid, BKC
or a combination thereof, the preferred amount of L-menthol is from about
0.001% to about 10 %
w/w, caprylic acid is from about 0.1% to 10% w/w, BKC is from about 0.001 to
10% w/w. In a
more preferred embodiment, the formulations contain from about 0.01% to about
0.5% w/w L-
menthol, about 0.5% to 5% w/w caprylic acid, about 0.005 to 0.1% w/w BKC. In
an even more
preferred embodiment, the formulations contain from about 0.02% to about 0.5%
w/w L-menthol,
about 1% to 2% w/w caprylic acid, about 0.01 to 0.1% w/w BKC. In a most
preferred embodiment,
the formulations contain about 0.5 % w/w L-menthol, about 2% w/w caprylic acid
and about 0.005
w/w BKC.
[000127] In yet another embodiment, the permeation enhancer is about 0.5%
w/w of menthol.
[000128] In yet another preferred embodiment, the permeation enhancer is
about 2.0 % w/w
caprylic acid.
[000129] In a most preferred embodiment, the permeation enhancer is about
0.01% w/w of
benzaikonium chloride (BKC).
[000130] In a preferred embodiment, the formulations contain a sweetener.
In a more
preferred embodiment, the sweetener is selected from the group consisting of
sucralose, aspartame,
saccharin, dextrose, mannitol, glycerin, and xylitol. In a preferred
embodiment, the formulations
contain from about 0.001% w/w to about 2% w/w of sweetener. In a more
preferred embodiment,
the formulations contain from about 0.05% w/w to about 1% w/w of the
sweetener. In a most
preferred embodiment, the formulations contain sucralose as a sweetener at
about 0.8% w/w.
[000131] In a further embodiment, the formulation may contain a sweetness
enhancer, an
ammonium salt form of crude and refined Glycyrrhizic Acid, for example,
Magnasweet product
(available from Mafco Worldwide Corporation, Magnasweet is a registered
trademark of Mafco
Worldwide Corporation). Magnasweet products use the ammonium salt forms of
crude and
refined Glycyrrhizic Acid. Glycyrrhizic Acid is also available as a pure
derivative in the sodium
and potassium salt forms.
[000132] In another embodiment, the formulations contain a pH modifier. In
a preferred
embodiment, the pH modifier adjusts the pH of the formulation to from about 2
to about 7. In a
more preferred embodiment, the pH modifier adjusts the pH of the formulation
to from about 3 to
about 6, from about 4 to about 5 or from about 2 to about 4. In most preferred
embodiments, the
pH modifier adjusts the pH of the formulations to about 2.5, or 3, or 4.5
0.1.
18
Date recue/Date Received 202401-22
[000133] In another embodiment, the formulations contain a flavoring agent.
In a preferred
embodiment, the formulations contain a flavoring agent selected from the group
consisting of
peppermint oil, menthol, spearmint oil, citrus oil, cinnamon oil, strawberry
flavor, cherry flavor,
raspberry flavor, orange oil, and a combination thereof. Other appropriate
flavoring agents known
by those of skill in the art could also be added to formulations of the
present invention. In a
preferred embodiment, the formulations contain from about 0.001% w/w to about
1% w/w of the
flavoring agent. In a more preferred embodiment, the formulations contain from
about 0.005%
w/w to about 0.5% w/w of the flavoring agent. In a most preferred embodiment,
the formulations
contain strawberry as the flavoring agent at about 0.08% w/w.
[000134] In yet another embodiment, the formulations may contain a
preservative. In a
preferred embodiment, the preservative is selected from the group consisting
of butyl paraben,
methyl paraben, ethyl paraben, propyl paraben, sodium benzoate, and benzoic
acid. In a preferred
embodiment, the formulations contain from about 0.001% w/w to about 1% w/w of
the
preservative. In a more preferred embodiment, the formulations contain from
about 0.005% w/w
to about 0.2% w/w of the preservative. In a most preferred embodiment, the
formulations contain
methyl paraben as a preservative at about 0.1% w/w.
[000135] In a further embodiment, the invention is directed to stable
liquid spray
formulations comprising from about 1% to about 16% w/w naloxone, a
pharmaceutically
acceptable salt or a derivative thereof, about 10% to about 98% w/w water,
about 0.005% to about
0.05% w/w of a chelating agent, preferably edetate disodium dihych-ate and
optionally, about 2%
to about 90% w/w of a co-solvent, preferably propylene glycol and the
formulations do not contain
an alcohol.
[000136] In a further embodiment, the invention is directed to stable
liquid spray
formulations comprising from about 1% to about 16% w/w naloxone, a
pharmaceutically
acceptable salt or a derivative thereof, about 30% to about 98% w/w water,
about 0.005% to about
0.05% w/w of a chelating agent, preferably edetate disodium dihydrate and
optionally, about 5%
to about 55% w/w of a co-solvent, preferably propylene glycol and the
formulations do not contain
an alcohol.
[000137] In a further embodiment, the invention is directed to stable
liquid spray
formulations comprising from about 1% to about 10% w/w naloxone, a
pharmaceutically
acceptable salt or a derivative thereof, about 80% to about 98% w/w water,
about 0.005% to about
19
Date recue/Date Received 202401-22
0.05% w/w of a chelating agent, preferably edetate disodium dihydrate and
optionally, about 5%
to about 10% w/w of a co-solvent, preferably propylene glycol, and optionally
about 0.1% w/w of
a preservative, preferably methyl paraben and the formulations do not contain
an alcohol.
[000138] In yet another embodiment, the formulations of the present
invention are capable
of producing a droplet size distribution wherein the mean Dv(10) is from about
12 to about 20
microns during administration.
[000139] In a further embodiment, the formulations of the present invention
are capable of
producing a droplet size distribution wherein the mean Dv(50) is from about 25
to about 35
microns during administration.
[000140] In yet another embodiment, the formulations of the present
invention are capable
of producing a droplet size distribution wherein the mean Dv(90) is from about
40 to about 150
microns during administration. Preferably, the formulations of the present
invention are capable
of producing a droplet size distribution wherein the mean Dv(90) is from about
60 to about 110
microns during administration.
[000141] All claims, aspects and embodiments of the invention, and specific
examples
thereof, are intended to encompass equivalents thereof.
[000142] In a further embodiment, the invention is directed to treating
patients by
administering the formulations (with or without an alcohol) of the present
invention to the patient.
In a preferred embodiment, the formulations are administered in order to treat
opioid dependence,
opioid overdose, and/or congenital insensitivity to pain with anhidrosis.
Definitions
[000143] As used herein, all numerical values relating to amounts, weights,
and the like, that
are defined as "about" each particular value is plus or minus 10%. For
example, the phrase "about
10% w/w" is to be understood as "9% to 11% w/w." Therefore, amounts within 10%
of the claimed
value are encompassed by the scope of the claims.
[000144] As used herein "% w/w" refers to the percent weight of the total
formulation.
[000145] As used herein the term "effective amount" refers to the amount
necessary to treat
a patient in need thereof.
Date recue/Date Received 202401-22
[000146]
As used herein the term "patient" refers but is not limited to a person that
is being
treated for opioid dependence, opioid overdose, insensitivity to pain with
anhidrosis, or another
affliction or disease that can be treated with naloxone.
[000147]
As used herein the phrase "pharmaceutically acceptable" refers to ingredients
that
are not biologically or otherwise undesirable in a sublingual or intranasal
dosage form.
[000148]
As used herein, "stable" refers to formulations which maintain greater than
95%
purity following at least four weeks at about 40 C.
[000149]
Preferably, the (alcohol and alcohol-free) formulations of the present
invention are
propellant free. As used herein, "propellant free" refers to a formulation
that is not administered
using compressed gas.
[000150]
As used herein, the term "isotonicity agent" refers to any compound used to
alter
or regulate the osmotic pressure of a formulation.
[000151]
As used herein, the twin "buffer" refers to any compound used to maintain the
pH
of a formulation.
[000152]
The following examples are intended to illustrate the present invention and to
teach
one of ordinary skill in the art how to make and use the invention. They are
not intended to be
limiting in any way.
Examples
Example 1: Preparation of Naloxone Formulations Containing Ethanol
[000153]
Liquid spray formulations were created by first degassing ethanol and USP
purified
water, separately. Next, the ethanol and purified water were each purged with
nitrogen. Soluble
excipients were then dissolved in either the ethanol or the purified water
based on their solubility.
Next, the solutions were combined. Naloxone was added to the final solution
and mixed until
dissolved.
[000154] Strawberry
flavor was used as the source of the flavoring agent.
Table 1. Stable Liquid Naloxone Spray Formulations
Formulation Control #1A #2A #3A #4A #5A #6A #7A
Naloxone
Hydrochloride 2.44 2.44 2.44 2.44 2.44 4M0 6.7
10.1
Dihydrate
Water (USP) 37.56 37.55 37.55 37.54 37.54 34.45
33.23 29.83
Ethanol 55 55 55 55 55 55 55
55
Propylene Glycol 5 5 5 5 5 5 5 5
21
Date recue/Date Received 202401-22
L-menthol 0.05
Sodium Thiosulfate 0.01 0.01
Citric Acid 0.0025
Flavoring agent 0.08
MIN
Edetate disodiiim
0.005 0.005 0.005 0.005 0.005 0.005
dihydrate
BHA 0.01
BHT 0.005
Waif Bir,
Sodium Ascorbate 0.02 0.02 0.02
0.02
values = % w/w
Example 2: Stability Testing of Naloxone Formulations
10001551
The formulations listed in Table 1 were subjected to stability testing at 40 C
and
55 C 2 C under 75% 5% relative humidity for eight weeks. The stability
data was collected
at zero, one, two, three, four, and eight weeks at 55 C and at zero, four, and
eight weeks at 40 C.
Assay and impurities were detected using high performance liquid
chromatography with an
ultraviolet detector. The assay was performed at 288 nm and indicated as a %
of initial
concentration. For all impurities, analysis was performed at 240 nm and
expressed as a % area.
Amounts of particular impurities are listed in Tables 2A to 2F and 3A to 3H as
a percentage of the
area of each formulation along with amount of total impurities. "BQL" refers
to "Below
Quantifiable Limit" and "ND" refers to "Not Detected."
Tables 2A to 2F. Stability Data for Liquid Naloxone Spray Formulations stored
at 40 C 2 C
under 75% 5% relative humidity
2A. Stability of Control Stored at 40 C
Naloxone RRT T=0 4 Weeks 8 Weeks
Impurity C 0.66 ND 0.81% 0.92%
Impurity A 0.83 ND 0.37% 0.51%
Impurity F 0.93 ND ND ND
Impurity D 1.14 ND ND ND
Impurity E 2.85 ND 5.59% 5.53%
Impurity B 3.21 ND ND ND
0.30 ND 0.13% 0.18%
Unknown Impurities
0.50 ND 0.28% 0.46%
Total Impurities 0.00% 7.18% 7.60%
22
Date recue/Date Received 202401-22
2B. Stability of Form. #1A (with Sod. Thiosulphate & Citric Acid)
Stored at 40 C
Naloxone RRT T=0 4 Weeks 8 Weeks
Impurity C 0.66 _ ND BQL BQL
Impurity A 0.83 ND BQL BQL
Impurity F 0.93 ND ND ND
Impurity D 1.14 ND ND ND
Impurity E 2.85 ND ND ND
Impurity B 3.21 ND ND ND
Total Impurities 0.00% 0.00% 0.00%
2C. Stability of Form. #2A (with Sod. Thiosulphate & Edetate
Disodium Dihydrate) Stored at 40 C
Naloxone RRT T=0 4 Weeks 8 Weeks
Impurity C 0.66 ND BQL BQL
Impurity A 0.83 ND BQL BQL
Impurity F 0.93 ND ND ND
Impurity D 1.14 ND ND ND
Impurity E 2.85 ND ND ND
Impurity B 3.21 ND ND ND
Total Impurities 0.00% 0.00% 0.00%
2D. Stability of Form. #3A (with BHA & BHT) Stored at 40 C
Naloxone RRT T=0 4 Weeks 8 Weeks
Impurity C 0.66 _ ND BQL BQL
Impurity A 0.83 ND BQL BQL
Impurity F 0.93 ND ND ND
Impurity D 1.14 ND ND ND
Impurity E 2.85 ND ND ND
Impurity B 3.21 ND ND ND
Total Impurities 0.00% 0.00% 0.00%
2E. Stability of Form. #4A (with Sod. Ascorbate and Edetate
Disodium Dihydrate) Stored at 40 C
Naloxone RRT T=0 4 Weeks 8 Weeks
Impurity C 0.66 ND BQL BQL
Impurity A 0.83 ND 0.15% 0.19%
Impurity F 0.93 ND ND ND
23
Date recue/Date Received 202401-22
Impurity D 1.14 ND ND ND
Impurity E 2.85 ND ND ND
Impurity B 3.21 ND ND ND
Total Impurities 0.00% 0.15% 0.19%
2F. Stability of Form. #5A (with Sod. Ascorbate and Edetate Disodium
Dihydrate) Stored at
40 C
Naloxone RRT T=0 4 Weeks 3 Months
_
Assay (%) 100 97.77 97.6
Impurity C 0.66 ND ND 0.03
Impurity A 0.83 0.11 0.12 0.15
Impurity F 0.93 ND ND ND
Impurity D 1.14 ND ND ND
Impurity E 2.85 ND 0.13 0.13
Impurity B 3.21 ND ND ND
Total Impurities 0.11% 0.25% 0.29%
[000156] Liquid naloxone formulations of the present invention contained
less than one
percent total impurities after eight weeks at 40 C. This is a stark contrast
to the control formulation
which contained 7.6% impurities at the same time. Specifically, the
formulations which contained
sodium thiosulfate or BHA and BHT resulted in 0% detected impurities after
eight weeks. Also,
formulations which contain sodium ascorbate (0.02% wt/wt) and edetate disodium
dihydrate
(0.005% wt/wt) resulted in only 0.29% total impurities after 3 months.
Tables 3A to 3H. Stability Data for Liquid Naloxone Spray Formulations stored
at 55 C 2 C
3A. Stability of Control Stored at 55 C
Naloxone RRT T=0 1 Week 2 Weeks 3 Weeks 4 Weeks 8 Weeks
Impurity C 0.66 ND ND ND 0.54% 0.33% 0.35%
Impurity A 0.83 ND ND ND 1.31% 1.39% 1.59%
Impurity F 0.93 _ ND ND ND ND ND ND
Impurity D 1.14 ND ND ND ND ND ND
Impurity E 2.85 ND ND ND ND ND ND
Impurity B 3.21 ND ND ND ND ND ND
0.30 - - - 0.1% 0.32%
Unknown 0.35 - - - 0.15% 0.16% 0.08%
Impurities 0.50 - - - 0.83% 0.81% 0.67%
______________ 2.85 - - 4% 7.50% 6.65%
24
Date recue/Date Received 202401-22
Total Impurities 0.00% 0.00% 0.00% 6.83%
10.29% 9.66%
3B. Stability of Form. #1A (with Sod. Thiosulphate & Citric Acid) Stored at 55
C
Naloxone RRT T=0 . 1 Week 2 Weeks 3 Weeks 4 Weeks . 8 Weeks
Impurity C 0.66 ND ND ND 0.12% 0.37% 0.29%
Impurity A 0.83 ND ND ND 0.14% 0.67% 1.01%
Impurity F 0.93 ND ND _ ND ND ND ND
Impurity D 1.14 ND ND ND ND ND ND
Impurity E 2.85 ND ND ND 0.55% 1.88% 1.52%
Impurity B 3.21 ND _ ND ND ND ND ND
Unknown 0.32 0.09% 0.25%
Impurities 0.52 - 0.06% 0.51% 0.59%
Total Impurities _ 0.00% 0.00% 0.00% 0.87% 3.52%
3.66%
3C. Stability of Form. #2A (with Sod. Thiosulphate & Edetate Disodium
Dihydrate) Stored at 55 C
Naloxone RRT T=0 1 Week 2 Weeks 3 Weeks 4 Weeks 8 Weeks
Impurity C 0.66 ND ND ND ND BQL BQL
Impurity A 0.83 , ND ND ND BQL 0.07% 0.11%
Impurity F 0.93 ND ND ND ND ND ND
Impurity D 1.14 ND ND ND ND ND ND
Impurity E 2.85 _ ND ND ND ND ND ND
Impurity B 3.21 ND ND ND ND ND ND
Unknown
0.52 0.08%
Impurities
Total
0.00% 0.00% 0.00% 0.00% 0.07% 0.19%
Impurities
3D. Stability of Form. #3A (with BHA & BHT) Stored at 55 C
Naloxoni s 8 Weeks
Impurity C 0.66 ND ND ND ND ND BQL
Impurity A 0.83 ND ND ND BQL 0.07% 0.13%
Impurity F 0.93 ND ND ND ND ND ND
Impurity D 1.14 ND ND ND ND ND ND
Impurity E 2.85 ND ND ND ND ND ,
ND
Impurity B 3.21 ND ND ND ND ND ND
Unknown
0.50 - - - 0.08%
Impurities
Date recue/Date Received 202401-22
Total
0.00% 0.00% 1 0.00% 0.00% 0.07%
0.21%
impurities
3E. Stability of Form. #4A (with Sod. Ascorbate and Edetate Disodium
Dihydrate) Stored at
55 C
Naloxone RRT T=0 1 Week 2 Weeks 3 Weeks 4 Weeks 8 Weeks
Impurity C 0.66 ND ND ND ND ND
0.06%
Impurity A 0.83 ND ND NI) 0.11% 0.19%
0.19%
Impurity F 0.93 ND ND ND ND ND ND
Impurity D 1.14 ND ND ND ND ND ND
Impurity E 2.85 ND ND ND ND ND ND
Impurity B 3.21 ND ND ND ND ND ND
Total
0.00% 0.00% 0.00% 0.11% 0.19% 0.25%
Impurities
3F. Stability of Form. # 5A (with Sod. Ascorbate and Edetate Disodium
Dihydrate) Stored at
55 C
Naloxone RRT T=0 2 Weeks 4 Weeks 6 Weeks 8 Weeks
Assay (%) 100 102.37 98.75 98.51 100.76
Impurity C 0.66 ND ND ND ND 0.05%
Impurity A 0.83 0.11 0.14 0.15 0.19 0.17%
Impurity F 0.93 ND ND ND ND ND
Impurity D 1.14 ND ND ND ND ND
Impurity E 2.85 ND 0.13 0.11 0.12 0.12%
Impurity B 3.21 ND ND ND ND ND
0.49 - - - 0.06% 0.05%
Unknown
0.79 - - - 0.03%
Impurities
3.90 - - 0.05% 0.07% 0.05%
Total
0.11% 0.27% 0.31% 0.47% 0.44%
Impurities
3H. Stability of Form. #6A (with Sod. Ascorbate and Edetate Disodium
Dihydrate) Stored at
55 C
Naloxone RRT T=0 2 Weeks 4 Weeks 8 Weeks
Assay (%) 100.00 101.35 102.69 102.99
Impurity C 0.66 ND BQL BQL 0.08%
Impurity A 0.81 BQL 0.08% 0.19% 0.18%
Impurity F 0.93 ND ND ND ND
26
Date recue/Date Received 202401-22
Impurity D _ 1.14 ND ND _ ND ND
Impurity E 2.85 0.04% 0.07% 0.06% 0.10%
Impurity B 3.21 ND ND ND 0.12%
Unknown
0.50 - 0.06%
Impurities
Total Impurities 0.04% 0.15% 0.25% 0.54%
3G. Stability of Form. #7A (with Sod. Ascorbate and Edetate Disodium
Dihydrate) Stored at
55 C
RRT T=0 2 Weeks 4 Weeks 8 Weeks
Assay (%) 100.00 100.91 100.92 102.05
Impurity C 0.66 ND 0.06% 0.05% 0.11%
Impurity A 0.81 BQL 0.11% 0.22% 0.17%
Impurity F 0.93 ND ND ND ND
Impurity D 1.14 ND ND ND ND
Impurity E 2.85 0.06% 0.07% 0.06% 0.11%
Impurity B 3.21 ND ND ND 0.13%
Unknown 0.50 - - 0.05%
Impurities 2.41 - 0.05%
Total Impurities 0.06% 0.24% 0.33% 0.62%
[000157] Similar to the stability study at 40 C, all of the formulations of
the present invention
had significantly fewer impurities at eight weeks compared to the control. The
superior stability
characteristics of the formulations of the present invention will allow the
formulations to be
effective when used by patients.
Example 3: Droplet Testing
[000158] In order to determine the spray profile of Formulation #5A, it was
subjected to
standardized droplet testing. A challenge of creating a Naloxone sublingual
and/or intranasal spray
formulation is that it must be capable of producing spray droplets that are
over 10 microns in
diameter. Spray droplets 10 microns or smaller could be inhaled into the
lungs. The optimal
particle size for sublingual and intranasal spray droplets is from 20 to about
200 microns in
diameter. It is desirable for the formulation to have droplet sizes near 20
because this increases
the surface area and increased surface area exposure is one factor that
contributes to a high
27
Date recue/Date Received 202401-22
bioavailability. Sublingual and intranasal formulations should be able to
maintain a consistent
droplet size throughout its shelf life.
[000159] Droplet analysis was conducted using standard laser analysis
procedures known by
those of skill in the art. Droplet size distribution (Dv10, Dv50, Dv90, and
Span were tested at two
distances, 3 cm and 6 cm). Dv10 refers to droplet size for which 10% of the
total volume is
obtained; Dv50 refers to droplet size for which 50% of the total volume is
obtained; Dv90 refers
to droplet size for which 90% of the total volume is obtained; Span refers to
distribution span
(Dv90-Dv10)/Dv50; %RSD refers to the percent relative standard deviation. The
results of these
tests can be seen below in Tables 4 to 9. Applicant found during testing that
formulations of the
present invention yielded desirable droplet sizes for sublingual and
intranasal administration. The
testing also revealed that the formulation dose remains consistent when
administered with a spray
pump.
Table 4. Spray Profile of Naloxone Spray Formulation #5A, Particle Size at 3
cm
Particle Size
Formulation #5A
DV(10) DV(50) DV(90) %<10 pi Span
Actuation 1 14.79 28.92 389.9 1.225 .. 12.97
Actuation 2 17.98 32.05 455.6 0.001 .. 13.65
3 cm
Actuation 3 13.46 36.92 584.8 4.747 15.48
Average 15.41 32.63 476.8 1.991 14.03
Table 5. Spray Profile of Naloxone Spray Formulation #5A, Particle Size at 6
cm
Particle Size
Formulation #5A
DV(10) DV(50) DV(90) %<10 Span
Actuation 1 20.58 38.64 498.6 1.918 .. 12.37
Actuation 2 18.67 37.59 529.4 1.537 .. 13.59
6 cm
Actuation 3 21.26 36.44 452.3 1.767 11.83
Average 20.17 37.56 493.4 1.741 12.60
Table 6. Spray Profile of Naloxone Spray Formulation #5A, Spray Pattern at 3
cm
Spray Pattern
Formulation #5A
Dmin (mm) Dmax (mm) Ovality Ratio
Actuation 1 21.2 33.4 1.577
3 cm
Actuation 2 23.5 31.5 1.342
28
Date recue/Date Received 202401-22
Actuation 3 17.6 30.9 1.755
Average 20.8 31.9 1.558
Table 7. Spray Profile of Naloxone Spray Formulation #SA, Spray Pattern at 6
cm
Spray Pattern
Formulation #5A
Dmin (mm) Dmax (mm) Ovality Ratio
Actuation 1 24.5 55.6 2.268
Actuation 2 34.3 49.7 1.447
6 cm
Actuation 3 33.9 52 1.535
Average 30.9 52.4 1.750
Table 8. Spray Profile of Naloxone Spray Formulation #5A, Plume geometry data
at 3 cm
Plume Geometry
Formulation #5A Angle
Width (mm) (0)
Actuation 1 28.7 51.1
Actuation 2 25.5 45.9
3 cm
Actuation 3 35.4 60.4
Average 29.9 52.5
Table 9. Spray Profile of Naloxone Spray Foimulation #5A,
Plume geometry data at 6 cm
Plume Geometry
Formulation #5A Angle
Width (mm) (0)
Actuation 1 54.3 48.4
Actuation 2 52.6 47.3
6 cm
Actuation 3
Average 53.5 47.9
10001601 As can be seen in Tables 4 to 9, Formulation #5A of the present
invention provided
excellent plume geometry and spray patterns.
Example 4: Preparation of Naloxone Formulations that are Alcohol-Free
10001611 In order to prepare a naloxone liquid formulation, the components
as indicated in
"Table 10. The Components ofFormulation MP' below were weighed. The components
were
mixed until a clear solution was formed.
29
Date recue/Date Received 202401-22
[000162] Naloxone HCL dihydrate base U.S.P. was used as the source of
naloxone in the
formulations that follow. Methyl paraben, U.S.P., (available from Spectrum)
was used as the
preservative source. Strawberry flavor, Nat&Art 915.0543 U, (available from
FONA) was used
as the source of flavoring agent. Edetate Disodium Dihydrate, U.S.P.,
(available from Spectrum)
was used as the source of chelating agent or as antioxidant. Water, U.S.P.,
purified, (available
from RICCA) was used as the source of solvent.
Table 10. The Components of Formulation #1AF
Ingredients % w/w
Naloxone HC1 Dihydrate 4.82
Sucralose 0.80
Methyl Paraben 0.10
0.08
Flavoring agent
0.05
Edetate Disodium Dihydrate
Water USP 94.15
100.0
Example 5. Preparation of Additional Naloxone Liquid Formulations
[000163] In order to prepare naloxone liquid formulations, the components
as indicated in
"Table 11. The Components of Control and Formulations #1AF to #6AF" below were
weighed.
The components were mixed until a clear solution was formed.
Strawberry flavoring was used as the source of flavoring agent.
Table 11. The Components of Control and Formulations #1AF to #6AF
Formulation Control #1AF #2AF #3AF #4AF #5AF #6AF
Naloxone HCL Dihydrate 4.83 4.82 4.89 4.89 4.89 4.83
4.82
Water (USP) 94.19 94.15 95.01 94.08 93.98
94.16 94.15
Sucralose 0.8 0.8 0.8 0.8 0.8 0.8
Methyl Paraben 0.1 0.1 0.1 0.1 0.1 0.1
Flavoring 0.08 0.08 0.08 0.08 0.08 0.08
0.08
Edetate Disodium Dihydrate iL 0.05 0.005 0.05 0.05 0.005
0.05
L-cysteine Hydrochloride 11111 0.1
Monohydrate
Sodium Ascorbate 0.02 0.02
pH 3.03 2.5 4.46 4.16 2.56 3.02 3
Date recue/Date Received 202401-22
Example 6: Stability Testing of Naloxone Formulations
10001641 The formulations listed in Table 11 were subjected to stability
testing at 40 C and
55 C 2 C under 75% 5% relative humidity for eight weeks. The stability
data was collected
at zero, one, two, three, four, at 55 C and at zero, four weeks at 40 C. Assay
and impurities were
detected using high performance liquid chromatography with an ultraviolet
detector. The assay
was performed at 288 nm and indicated as a % of initial concentration. For all
impurities, analysis
was performed at 240 nm and expressed as a % area. Amounts of particular
impurities are listed
in Tables 12Ato 12G and 13A to 13C as a percentage of the area of each
formulation along with
amount of total impurities. "BQL" refers to "Below Quantifiable Limit" and
"ND" refers to "Not
Detected." "Ppm" refers to parts per million.
Tables 12A to 12G. Stability Data for Liquid Naloxone Spray Formulations
stored at 55 C
12A. Stability of Control Stored at 55 C
Naloxone RRT T=0 1 Week 55 C
Assay (%) 100 101.48
Impurity C 0.66 ND ND
Impurity A 0.83 0.15 0.15
Impurity F 0.93 ND ND
Impurity D 1.14 ND ND
Impurity E 3.20 0.07 0.62
Impurity B 3.40 ND ND
Unknown 0.49 0.07
Impurities 0.59 0.12
Total
0.22% 0.96%
Impurities
12B. Stability of Form. #2AF (with Sod. ascorbate & Edetate Disodium
Dihydrate) Stored at
55 C
Naloxone RRT T=0 1 Week 2 Weeks 3 Weeks 4 Weeks
pH 4.469 4.21 4.239 4.02 4.224
Assay (%) 100 99.6 101.48 98.07 98.00
Impurity C 0.66 ND BQL BQL BQL BQL
Impurity A 0.83 BQL 0.09 0.28 0.27 0.18%
Impurity F 0.93 ND ND ND ND ND
Impurity D 1.14 ND ND ND ND ND
31
Date recue/Date Received 202401-22
Impurity E 3.20 ND ND ND ND ND
Impurity B 3.40 ND ND ND ND ND
0.33 - - 0.07 0.1 0.13
0.49 - 0.05 0.07 0.07%
Unknown
0.56 - 0.08 0.08 0.09 0.08
Impurities
0.59 - 0.07 0.12 0.14 0.14
3.90 - 0.09 0.15 0.13 0.14
_
Total Impurities 0.00% 0.33% 0.82% 0.80%
0.74%
12C. Stability of Form. #3AF (Edetate Disodium Dihydrate) Stored at 55 C
Naloxone RRT T=0 1 Weeks 2 Weeks 3 Weeks 4 Weeks
pH 4.16 4.23 4.168 3.94 4.33
Assay (%) 100 100.5 100.7 98.03 98.63
Impurity C 0.66 ND ND ND ND ND
Impurity A 0.83 BQL BQL 0.21 0.18 0.07
Impurity F 0.93 ND ND ND ND ND
Impurity D 1.14 ND ND ND ND ND
Impurity E 3.20 0.13 0.11 0.09 0.09 0.08
Impurity B 3.40 ND ND ND ND ND
0.33 0.11 0.12 0.18
0.49 0.09 0.1 0.11%
Unknown
0.59 ND 0.12 0.11 0.14 0.15
Impurities
3.67 - , - - 0.07
, 3.90 - 0.08 0.14 0.13 0.13
Total Impurities 0.13% 0.31% 0.72% 0.76% 0.79%
12D. Stability of Form. #4AF (Edetate Disodium Dihydrate and L-Cysteine
hydrochloride)
Stored at 55 C
Naloxone RRT T=0 1 Week 2 Weeks 3 Weeks 4
Weeks
pH 2.56 2.5 2.44 2.38 2.413
_
Assay (%) 100 98.5 100.03 97.87
98.59
Impurity C 0.66 ND ND 0.13 0.11 0.09%
Impurity A 0.83 BQL ND 0.22 0.29 0.17%
Impurity F 0.93 ND ND ND ND ND
32
Date recue/Date Received 202401-22
Impurity D L14 ND ND ND ND ND
Impurity E 3.20 ND ND ND ND ND
Impurity B 3.40 ND ND ND ND ND
Unknown
0.56 NI) ND 0.05 0.07 0.06
Impurities
Total Impurities 0.00% 0.00% 0.40% 0.47% 0.32%
12E. Stability of Form. #5AF (Edetate Di sodium dihydrate and Sodium
ascorbate) Stored at
55 C
Naloxone RRT T=0 1 Week 2 Weeks
pH NP NP 3.185
Assay (%) _ 100 98.37 98.12
Impurity C 0.66 ND BQL BQL
Impurity A 0.83 0.15 0.18 0.11
Impurity F 0.93 ND ND ND
Impurity D 1.14 ND ND ND
Impurity E 3.20 0.07 0.16 0.12
Impurity B 3.40 ND ND ND
Total Impurities 0.22% 0.34% 0.23%
12F. Stability of Form. #6AF (Edetate Disodium Dihydrate) Stored at 55 C
Naloxone RRT T=0 1 Week 2 Weeks 3 Weeks 4 Weeks
pH 3.013 3.443 3.132 3.241 3.21
Assay (%) 100.00% 98.34% 98.36% 98.34% 100.07%
Impurity C 0.66 0.10% 0.10% 0.21% 0.13% ..
0.13%
Impurity A _ 0.83 BQL BQL BQL BQL BQL
Impurity F _ 0.93 ND ND ND ND ND
7479 1.
Impurity D 1.14 ND 0.83 ppm 1. ND
PPm PPm
Impurity E 3.20 0.15% 0.15% 0.15% 0.17%
0.17%
Impurity B 3.40 ND ND ND ND ND
Unknown 0.49 0.06% 0.06% 0.12%
Impurities 0.77 - - BQL BQL 0.05%
Total Impurities 0.25% 0.25% 0.42% 0.36% 0.47%
33
Date recue/Date Received 202401-22
12G. Stability of Form. #1AF (Edetate Disodium Dihydrate) Stored at 55 C
Naloxone RRT T=0 1 Week 2 Weeks 3 Weeks 4 Weeks
pH 2.505 2.907 2.581 2.616 2.62
Assay (%) 100.00% 107.80% 100.51% 100.17% 102.39%
Impurity C 0.66 0.10% 0.10% 0.10% 0.10% 0.09%
Impurity A 0.83 BQL BQL BQL BQL BQL
Impurity F 0.93 ND ND ND ND ND
Impurity D 1.14 NP 0.95 ppm 1.25 ppm 1.59 ppm ND
Impurity E 3.20 0.15% 0.14% 0.15% 0.16% 0.18%
Impurity B 3.40 ND ND ND ND ND
0.06 0.13% 0.13% 0.13% 0.13% ND
Unknown
0.49 0.08% 0.06% 0.05%
Impurities
4.63 ND ND ND BQL ND
Total Impurities 0.38% 0.37% 0.46% 0.45% 0.32%
[000165] Liquid naloxone formulations of the present invention contained
less than 0.8% of
total impurities after four weeks at 55 C. This is a stark contrast to the
control formulation which
contained 0.96% impurities after 1 week at 55 C. Specifically, the
formulations which contained
sodium ascorbate or edetate disodium dihydrate exhibited lower impurities
after four weeks.
Additionally, the formulations which contained edetate disoditun dihydrate
were very stable.
Tables 13A to 13C. Stability Data for Liquid Naloxone Spray Formulations
stored at 40 C under
75% Relative Humidity
13A. Stability of Form. #2AF (with Sod. ascorbate & Edetate Disodium
Dihydrate) Stored at
40 C under 75% Relative Humidity
Naloxone RRT T=0 4 Weeks
pH 4.469 4.394
Assay (%) 100 98.12
Impurity C 0.66 ND 0.06
Impurity A 0.83 BQL 0.12
Impurity F 0.93 ND ND
Impurity D 1.14 ND ND
Impurity E 3.20 ND ND
34
Date recue/Date Received 202401-22
Impurity B 3.40 ND ND
0.49 ND 0.06
Unknown
0.59 ND 0.06
Impurities
3.90 ND 0.14
Total Impurities 0.00% 0.44%
13B. Stability of Form. #3AF (Edetate Disodium Dihydrate) Stored at 40 C under
75% Relative
Humidity
Naloxone RRT T=0 4 Weeks
pH 4.16 4.596
Assay (%) 100 99.69
Impurity C 0.66 ND ND
Impurity A 0.83 BQL BQL
Impurity F 0.93 ND ND
Impurity D 1.14 ND ND
Impurity E 3.20 0.13 ND
Impurity B 3.40 ND ND
Unknown
0.59 ND 0.11
Impurities
3.90 ND 0.11
Total Impurities 0.13% 0.22%
13C. Stability of Form. 111AF (Edetate Disodium Dihydrate and L-Cysteine
hydrochloride)
Stored at 40 C under 75% Relative Humidity
4
Naloxone RRT T=0
Weeks
pH 2.56 2.502
Assay (%) 100 97.08
Impurity C 0.66 ND ND
Impurity A 0.83 BQL BQL
Impurity F 0.93 ND ND
Impurity D 1.14 ND ND
Impurity E 3.20 ND ND
Impurity B 3.40 ND ND
Total Impurities 0.00% 0.00%
Date recue/Date Received 202401-22
10001661 The naloxone formulations of the present invention contained less
than 0.45% of
total impurities after four weeks at 40 C.
Example 7: Freeze/Thaw Testing
10001671 In order to further determine the stability of Formulations #1AF
and #6AF, the
formulations were subjected to standard freeze/thaw stability testing. The
results are below in
"Table 14. Stability of Formulations filAF and it-6AF to Freeze/Thaw Testing."
Table 14. Stability of Formulations #1AF and #6AF to Freeze/Thaw Testing
Formulation
#1AF to Drug Cycle 1, - Cycle 1, Cycle 2, - Cycle 2, Cycle
3, - Cycle 3,
#6AF Substance t=0 20 C 25 C 20 C 25 C 20 C
25 C
Date
Observed:
3/12/2015 3/16/2015 3/18/2015 3/20/2015 3/22/2015 3/24/2015 3/26/2015
Physical
appearance clear clear clear clear clear Clear clear
clear
Color colorless colorless colorless colorless colorless colorless colorless
Colorless
[000168] The naloxone formulations #1AF to #6AF were clear and colorless
after several
cycles of freezing and thawing. This study further demonstrates the stability
of the formulations.
Example 8: Droplet Testing
[000169] In order to determine the spray profile of Formulation #1AF, it
was subjected to
standardized droplet testing. As previously explained, the optimal particle
size for sublingual and
intranasal spray droplets is from 20 to about 200 microns in diameter. It is
desirable for the
formulation to have droplet sizes near 20 because this increases the surface
area and increased
surface area exposure is one factor that contributes to a high
bioavailability. Sublingual and
intranasal formulations should be able to maintain a consistent droplet size
throughout its shelf
life.
10001701 Droplet analysis was conducted using standard laser analysis
procedures known by
those of skill in the art. Droplet size distribution (Dv10, Dv50, Dv90, and
Span were tested at two
distances, 3 cm and 6 cm). Dv10 refers to droplet size for which 10% of the
total volume is
obtained; Dv50 refers to droplet size for which 50% of the total volume is
obtained; Dv90 refers
to droplet size for which 90% of the total volume is obtained; Span refers to
distribution span
(Dv90-Dv10)/Dv50; %RSD refers to the percent relative standard deviation. The
results of these
tests can be seen below in Tables 15 to 20. Applicant found during testing
that formulations of
the present invention yielded desirable droplet sizes for sublingual and
intranasal administration.
36
Date recue/Date Received 202401-22
The testing also revealed that the formulation dose remains consistent when
administered with a
spray pump.
Table 15. Spray Profile of Naloxone Spray Formulation #1AF, Particle Size at 3
cm
Particle Size
Fonnulati on #1AF
DV(10) DV(50) DV(90) %<10 II Span
Actuation 1 13.16 26.23 63.21 2.792 1.908
Actuation 2 11.52 27 90.85 6.547 2.939
3 cm
Actuation 3 12.95 28.39 144 3.505 4.615
Average 12.54 27.21 99.4 4.281 3.15
Table 16. Spray Profile of Naloxone Spray Formulation #1AF, Particle Size at 6
cm
Particle Size
Formulation #1AF
DV(10) DV(50) DV(90) %<l0j.t Span
Actuation 1 20.18 32.51 53.9 1.198 1.037
Actuation 2 18.02 31.45 58.48 0.024 1.286
6 cm
Actuation 3 16.81 33.44 77.92 1.799 1.828
Average 18.34 32.47 63.4 1.007 1.38
Table 17. Spray Profile of Naloxone Spray Formulation #1AF, Spray Pattern at 3
cm
Spray Pattern
Faimulation #1AF
Dmin (mm) Dmax (mm) Ovality Ratio
Actuation 1 26.5 41.3 1.557
Actuation 2 24.8 43.5 1.751
3 cm
Actuation 3 29 40.6 1.402
Average 26.8 41.8 1.570
Table 18. Spray Profile of Naloxone Spray Formulation #1AF, Spray Pattern at 6
cm
Spray Pattern
Formulation #1AF
Dmin (mm) Dmax (mm) Ovality Ratio
Actuation 1 52.6 68.6 1.304
Actuation 2 40.3 61.4 1.524
6 cm
Actuation 3 47.5 59.7 1.256
Average 46.8 63.2 1.361
Table 19. Spray Profile of Naloxone Spray Formulation #1AF, Plume geometry
data at 3 cm
37
Date recue/Date Received 202401-22
Plume Geometry
Formulation #1AF
Width (mm) Angle ( )
Actuation 1 39.7 66.7
Actuation 2 37.7 64.3
3 cm
Actuation 3 33.5 58
Average 37.0 63.0
Table 20. Spray Profile of Naloxone Spray Formulation #1AF, Plume geometry
data at 6 cm
Plume Geometry
Formulation #1AF
Width (mm) Angle ( )
Actuation 1 63 54.9
Actuation 2 67.1 58.3
6 cm
Actuation 3 68 59
Average 66.0 57.4
10001711 As can be seen in Tables 15 to 20, Formulation #1AF of the present
invention
provided excellent plume geometry and spray patterns.
Example 9. Preparation of Additional Naloxone Liquid Formulations
10001721 In order to prepare naloxone liquid formulations, the components
as indicated in
"Table 21. The Components of Formulations #8A, #9A, #7AF and #8AF" below were
weighed.
The components were mixed until a clear solution was formed.
Strawberry flavoring was used as the source of flavoring agent.
Table 21. The Components of Formulations #8A, #9A, #7AF and #8AF
Formulation Control #8A #9A #7AF #8AF
Naloxone 2.44 10.419 10.265 4.4196 4.4196
Water (USP) 37.56 29.506 31.324 94.769 94.779
Ethanol 55 55 50 111111111 -
Propylene Glycol 5 5 5
111E1
L-menthol 0.05 0.5 Egg
BKC 0.01 O.() I
Sodium Chloride
Flavoring agent 0.08 I
aletate disodium
dihydrate 111 0.005 F 0.001 0.001
0.001
Sucralose 0.8
Caprylic Acid 2
38
Date recue/Date Received 202401-22
Sodium Ascorbate 0.02 0.02 DID
pH- 4.5 0.2
Example 10. Preparation of Additional Naloxone Nasal Spray Formulations
[000173] In order to prepare naloxone liquid formulations, the components
as indicated in
Tables 22, 23 and 24 below were weighed. The components were mixed until a
clear solution was
formed.
Table 22. Stable Naloxone Nasal Spray Formulations
#10A #11A #12A #13A #14A #15A #16A
Naloxone 9.49 8.85 8.75 8.57 4.0 8.75 8.75
Water (USP) 35.505 66.14 71.135 79.31 83.88 81.135 81.135
Ethanol 50.0 20.0 10.0 2.0 2.0 10.0 10.0
Propylene Glycol 5.0 5.0 10.0 10.0 10.0 -
EDTA 0.005 0.01 0.015 0.02 0.02 0.015 0.015
Methyl Paraben - 0.1 0.1 0.1 0.1 0.1
pH 4.5 0.1 4.5 0.1 4.5 0.1 4.5 0.1 4.5 0.1 4.5 0.1 3.0 0.1
Table 23. Stable Naloxone Nasal Spray Formulations
#17A #18A #19A #20A
Naloxone 2.405 2.52 4.788 4.477
Water (USP) 42.594 72.738 40.207 40.513
Ethanol 50.0 20.0 50.0 20.0
Propylene Glycol 5.0 5.0 5.0 5.0
EDTA 0.005 0.01 0.005 0.01
pH 4.5 0.1 4.5 0.1 4.5 0.1 4.5 0.1
values = % w/w
Table 24. Stable Non-Alcoholic Naloxone Nasal Spray Formulations
#9AF #10AF #11AF #12AF
Naloxone 4.83 4.4 8.55 4.0
Water (USP) 95.02 90.495 81.33 85.88
Propylene Glycol - 5.0 10.0 10.0
39
Date recue/Date Received 2024-01-22
EDTA 0.05 0.005 0.02 0.02
Methyl Paraben 0.1 0.1 0.1 0.1
pH 3.0+0.1 4.5+0.1 4.5+0.1 4.5+0.1
values = % w/w
10001741 All formulations of Tables 22, 23 and 24 were stable upon mixing.
Formulations
of Tables 22, 23 and 24 differ from prior art naloxone nasal spray
formulations because the
formulations of Tables 22, 23 and 24 do not contain an isotonicity agent,
specifically sodium
chloride, a buffer, specifically citric acid, an anti-microbial agent,
specifically benzyl alcohol or
benzalkonium chloride. Further, formulations of Tables 22, 23 and 24 contain
EDTA at a
concentration of no more than 0.05% w/w.
Table 25. Stability of Formulations # 9A and # 8A to Freeze/Thaw Testing
Drug Cycle 1, Cycle 1, Cycle 2, Cycle
2, Cycle 3, Cycle 3,
Formulation Substance t4I 20 C 40 C 20 C 40 C 20 C
40 C
Date
3/
Observed:
1/12/2016 1/14/2016 1/16/2016 1/18/2016 1/20/2016 1/22/2016 24/2015
Physical
appearance clear clear clear clear clear Clear clear
clear
Color colorless colorless colorless colorless colorless colorless
colorless Colorless
[000175] The naloxone formulations #8A and #9A were clear and colorless
after several
cycles of freezing and thawing. This study further demonstrates the stability
of the formulations.
Example 11: Droplet Testing
[000176] In order to determine the spray profile of Formulation #9A, it was
subjected to
standardized droplet testing. As previously explained, the optimal particle
size for sublingual and
intranasal spray droplets is from 20 to about 200 microns in diameter. It is
desirable for the
formulation to have droplet sizes near 20 because this increases the surface
area and increased
surface area exposure is one factor that contributes to a high
bioavailability. Sublingual and
intranasal formulations should be able to maintain a consistent droplet size
throughout its shelf
life.
[000177] Droplet analysis was conducted using standard laser analysis
procedures known
by those of skill in the art. Droplet size distribution (Dv10, Dv50, Dv90, and
Span were tested at
Date recue/Date Received 202401-22
two distances, 3 cm and 6 cm). Dv10 refers to droplet size for which 10% of
the total volume is
obtained; Dv50 refers to droplet size for which 50% of the total volume is
obtained; Dv90 refers
to droplet size for which 90% of the total volume is obtained; Span refers to
distribution span
(Dv90-Dv10)/Dv50; %RSD refers to the percent relative standard deviation. The
results of these
tests can be seen below in Tables 26 to 41. Applicant found during testing
that formulations of
the present invention yielded desirable droplet sizes for sublingual and
intranasal administration.
The testing also revealed that the formulation dose remains consistent when
administered with a
spray pump.
Table 26. Spray Profile of naloxone Spray Formulation #9A, Particle Size at 3
cm
Particle Size
Formulation #9A
DV(10) DV(50) DV(90) %<10p, Span
Actuation 1 23.06 44.28 99.7 0 1.731
Actuation 2 22.08 44.34 104.4 0.661 1.856
3 cm
Actuation 3 22.27 55.05 107.5 1.012 2.82
Average 22.47 47.89 103.9 0.558 2.14
Table 27. Spray Profile of Naloxone Spray Formulation #9A, Particle Size at 6
cm
Particle Size
Formulation #9A
DV(10) DV(50) DV(90) %<10p Span
Actuation 1 28.54 51.29 91.87 2.113 1.235
Actuation 2 25.75 50.01 103.7 1.594 1.56
6 cm
Actuation 3 31.99 51.77 85 0 1.024
Average 28.76 51.02 93.5 1.236 1.27
Table 28. Spray Profile of Naloxone Spray Formulation #9A, Spray Pattern at 3
cm
Spray Pattern
Formulation #9A Dmin Dmax Ovality
(mm) (mm) Ratio
Actuation 1 14.7 22.7 1.544
Actuation 2 14.4 21.8 1.517
3 cm
Actuation 3 15.2 20.9 1.372
Average 14.8 21.8 1.478
Table 29. Spray Profile of Naloxone Spray Formulation #9A, Spray Pattern at 6
cm
41
Date recue/Date Received 202401-22
Spray Pattern
Formulation #9A Dmin Dmax Ovality
(mm) (mm) Ratio
Actuation 1 23.5 30.4 1.291
Actuation 2 24.5 41.8 1.707
6 cm
Actuation 3 20.5 32.7 1.597
Average 22.8 35.0 1.532
Table 30. Spray Profile of Naloxone Spray Formulation #9A, Plume geometry data
at 3 cm
Plume Geometry
Formulation #9A
Width (mm) Angle ( )
Actuation 1 22.5 39.9
Actuation 2 15.5 28.6
3 cm
Actuation 3 25.7 44.3
_ Average 21.2 37.6
Table 31. Spray Profile of Naloxone Spray Formulation #9A,
Plume geometry data at 6 cm
Plume Geometry
Formulation #9A
Width (mm) Angle ( )
Actuation 1 26.4 24.3
Actuation 2 25 23.3
6 cm
Actuation 3 37.6 33.2
Average 29.7 26.9
Table 32. Spray Profile of Naloxone Spray Formulation # 10A, Particle Size at
3 cm
Particle Size
Formulation # 10A
DV(10) DV(50) DV(90) %<10g Span
Actuation 1 19.84 46.86 112.10 3.744 2.011
3 cm Actuation 2 21.21 48.69 110.70 2.503 1.837
Average 20.53 47.77 111.40 1.924 3.124
Table 33. Spray Profile of Naloxone Spray Formulation # 10A, Spray Pattern at
3 cm
Formulation # 10A Spray Pattern
42
Date recue/Date Received 202401-22
Dmin Dmax Ovality
________________________________ (mm) (mm) Ratio
14.6 18.4 14.6 1.261
14.1 17.9 14.1 1.265
3 cm
15.1 17.9 15.1 1.182
14.6 18.1 14.6 1.2
Table 34. Spray Profile of Naloxone Spray Formulation # 10A, Spray Pattern at
6 cm
Spray Pattern
Formulation # 10A Dmin Dmax Ovality
(mm) (mm) Ratio
Actuation 1 23.7 29.8 1.259
Actuation 2 20.2 31.6 1.566
6 cm
Actuation 3 22.0 32.0 1.453
Average 22.0 31.20 1.40
Table 35. Spray Profile of Naloxone Spray Formulation #10A, Plume geometry
data at 3 cm
Plume Geometry
Formulation # 10A
Width (mm) Angle ( )
Actuation 1 36.7 19.97
3 cm Actuation 2 36.82 19.97
Average 36.76 19.97
Table 36. Spray Profile of Naloxone Spray Formulation # 10A,
Plume geometry data at 6 cm
Plume Geometry
Formulation #10A
Width (mm) Angle ( )
Actuation 1 27.23 29.29
6 cm Actuation 2 21.96 23.3
Average 24.60 26.30
Table 37. Spray Profile of Naloxone Spray Formulation # 11A, Particle Size at
3 cm
Particle Size
Formulation # 11A
DV(10) _ DV(50) DV(90) %<101.1 Span
Actuation 1 15.7 38.14 90.81 4.56 1.969
3 cm Actuation 2 15.11 37.9 86.75 5.09 1.89
Average 15.405 38.02 88.78 4.83 1.93
43
Date recue/Date Received 202401-22
Table 38. Spray Profile of Naloxone Spray Formulation # 11A, Spray Pattern at
3 cm
Spray Pattern
Formulation # 11A Dmin Dmax Ovality
(mm) (mm) Ratio
Actuation 1 15.9 22.4 1.410
Actuation 2 18.8 20.4 1.086
3 cm
Actuation 3 16.2 22.5 1.392
Average 16.9 21.8 1.30
Table 39. Spray Profile of Naloxone Spray Formulation # 11A, Spray Pattern at
6 cm
Spray Pattern
Formulation # 11A Dmin Dmax Ovality
(mm) (mm) Ratio
Actuation 1 20.8 29.4 1.411
Actuation 2 20.8 31.1 1.495
6 cm
Actuation 3 23.1 31.8 1.376
Average 21.6 30.8 1.40
Table 40. Spray Profile of Naloxone Spray Formulation #11A, Plume geometry
data at 3 cm
Plume Geometry
Formulation # 11A
Width (mm) Angle ( )
Actuation 1 31.30 19.98
3 cm Actuation 2 36.63 19.97
Average 33.97 19.98
Table 41. Spray Profile of Naloxone Spray Formulation # 11A,
Plume geometry data at 6 cm
Plume Geometry
Formulation #11A
Width (mm) Angle ( )
Actuation 1 21.1 16.98
6 cm Actuation 2 21.22 22.64
Average 21.16 19.81
10001781 As can be seen in Tables 26 to 41, Formulation #9A, # 10A, and 11A of
the present
invention provided excellent plume geometry and spray patterns.
44
Date recue/Date Received 202401-22
Example 12. Pharmacokinetic Analysis
[000179] The naloxone formulations described in Example 9, Table 21 of the
instant
specification were used. For formulations #7AF and #8AF a 4-mg dose was
administered. For
formulations #8A and #9A a 16-mg dose was administered.
Pharmacokinetic and Bioavailability Analysis
[000180] Protocol was a single dose crossover study. Five healthy male
Yucatan minipigs
weighing approximately forty kilograms each were sublingually administered the
formulations of
Table 21. The minipigs were fasted overnight and through four hours' post
administration.
Administration was followed by a one week washout period. Blood samples were
taken prior to
administration and 1, 3, 5, 7, 10, 15, 30 min, 1, 2, 4, 8 and 24 hours' post
dose. Blood samples
were measured for naloxone concentrations via liquid chromatography-tandem
mass
spectrometry.
[000181] The following pharmacokinetic parameters were calculated: peak
concentration
in plasma (Cmax) and area under the concentration-time curve from time-zero to
the time of the
last quantifiable concentration (AUCo-t).
Results and Conclusions
[000182] Results of the pharmacokinetic and statistical analysis for the
naloxone
formulations in Table 21 of the present invention are shown in Table 42.
Table 42. Summary of pharmacokinetic parameters for naloxone after sublingual
administration
of single doses of 4 mg and 16 mg of naloxone formulations to Yucatan minipigs
under fasted
conditions.
Parameter* #7AF #8AF #8A #9A
#9A (repeat)
C. (ng/mL) 8.9 2.2 6.8 2.2 26.3 1.8
86.4 2.4 58.6 2.8
Conc. @ 1 min
NA 0.1 NA 24.2 NA
(ng/mL)
Conc. @ 3 min
0.2 0.3 5 68.5 12.9
(ng/mL)
Conc. @ 5 min
1.4 1.9 6.3 62.7 36.9
(ng/mL)
Conc. @ 7 min
1.6 2.6 9.1 28.8 50.5
(ng/mL)
AUC0-0
746.2 2.2 432.3 2.0 2382.5 2.0 3108.5 2.2 3564.8 2.8
(ng*min/mL)
AUC @ 15 min
41.6 38 188.2 615.8 515.6
(ng*min/mL)
Date recue/Date Received 202401-22
AUC @ 30 min
151.8 106.8 504.4 987.4 1063.3
(ng*min/mL)
*Geometric mean geometric standard deviation. Sample size is 5.
10001831 The peak mean naloxone concentration was significantly higher for
formulation
#9A and #8A over #8AF and #7AF. Additionally, the area under the concentration-
time curve
from time-zero to the time of the last quantifiable concentration was
significantly higher for
formulations #9A and #8A over #8AF and #7AF. To determine if this result was
based on the
four-fold increase in the dose of naloxone in formulation #9A and #8A over
#8AF and #7AF the
geometric mean was normalized to 4 mg dose. See Figure 1. A similar pattern
remains even
after normalization. Further, the peak mean naloxone concentration was
significantly higher for
formulation #9A, over #8A, which cannot be explained by the dosage as
formulations #9A and
#8A were each administered at 16 mg doses.
[000184] Additionally, formulation #9A reached about 80% of its peak mean
naloxone
concentration within 3 minutes of administration. In comparison, formulation
#8A had reached
only 35% of its peak mean naloxone concentration within 7 minutes, #8AF 38% in
7 minutes
and #7AF 19% in 7 minutes. In a similar comparison formulation #9A reached 19%
of its
AUC(0_0 within 15 minutes of administration, #8A reached 7.9% in 15 minutes,
#8AF reached
8.8% in 15 minutes and #7AF reached 5.6% in 15 minutes.
[000185] Administration of naloxone in formulations with co-solvents
resulted in superior
bioavailability. Compare formulation #9A and #8A to #8AF and #7AF. Further,
the addition of
permeation enhancers such as caprylic acid and BKC resulted in further
increase in
bioavailability. Compare formulations #9A to #8A and #7AF to #8AF.
Example 13. Stability testing of additional Naloxone Formulations
[000186] Formulation #9A, # 10A, and 11A from Table 21 above was subjected
to stability
testing at 25 C/60% RH 5%, 40 C/75% 5% relative humidity and 55 C 2 C.
The stability
data was collected at predetermined time points. Assay and impurities were
detected using high
performance liquid chromatography with an ultraviolet detector. The assay was
performed at 288
nm and indicated as a % of initial concentration. For all impurities, analysis
was performed at 240
nm and expressed as a % area. Amounts of particular impurities are listed in
Tables 43 A to I as
a percentage of the area of each formulation along with amount of total
impurities.
Table 43A. Stability Data for the Formulation #9A Stored at 25 C 2 C /60%
+5% RH
46
Date recue/Date Received 202401-22
Naloxone RRT T=0 4 Weeks 3 Months 6 months
Clear,
Physical appearance (Clarity, Clear, Clear, Clear,
Pale yellow
Color) Colorless Colorless Colorless
Assay (%) 100 98.7 99.01 98.89
Impurity C 0.53 ND 0.01 ND ND
Impurity A 0.63 0.02 0.03 0.02 0.04
Impurity F 0.93 ND ND ND ND
Impurity D 1.14 , ND ND ND , ND
Impurity E 1.42 0.05 0.02 0.02 0.03
Impurity B 1.87 ND ND ND ND
Unknown Impurities 0.90 ND ND 0.07
0.04
1.48 ND 0.01 0.05 0.1
Total Impurities 0.07% 0.07% 0.16%
0.21%
ND = Not Detected
ppm = parts per million
Table 43B. Stability Data for the Formulation #9A Stored at 40 C 2 C / 75 %
5% RH
Naloxone RRT T=0 4 Weeks 3 Months 6 months
Clear,
Physical appearance Clear, Clear, Clear, light
light
brownish
(Clarity, Color) Colorless light yellow
yellow brownish
yellow
Assay (%) 100 99.09 98.63 98.08
Impurity C 0.53 ND 0.01 0.01 0.02
Impurity A 0.63 0.02 0.04 0.06 0.16
Impurity F 0.93 ND ND ND ND
Impurity D 1.14 ND ND ND ND
Impurity E 1.42 0.05 _ 0.01 0.01 0.02
Impurity B 1.87 ND ND ND ND
0.56 ND 0.05 0.04 0.10
0.71 ND _ 0.04 _ 0.03
0.06
0.79 ND ND 0.01 0.05
0.90 ND ND 0.05 ND
Unknown Impurities 1.23 ND ND ND 0.05
1.27 ND _ ND 0.02 0.05
1.48 ND 0.07 0.14 0.21
1.56 ND ND ND 0.07
1.84 ND ND 0.05 0.02
47
Date recue/Date Received 202401-22
Total Impurities 0.07% 0.22% 0.42% 0.81%
ND = Not Detected
ppm = parts per million
Table 43C. Stability Data for the Formulation # 9A Stored at 55 C 2 C
Naloxone RRT T=0 4 Weeks 6 Weeks 8 Weeks
Physical appearance Clear, Clear, Clear,
Clear,
(Clarity, Color) Colorless yellow yellow yellow
Assay (%) 100 97.28 97.28 94.28
Impurity C 0.53 ND 0.03 0.03 0.04
Impurity A 0.63 0.02 0.21 0.28 0.39
Impurity F 0.93 ND ND ND ND
36 9.
Impurity D 1.14 ND ND ND
, PPm
Impurity E 1.42 0.05 0.05 0.05 0.05
Impurity B 1.87 ND ND ND ND
0.56 ND 0.13 0.14 0.19
0.71 ND 0.06 0.07 0.06
0.79 ND 0.08 0.11 0.19
1.13 ND 0.01 0.05 0.05
1.23 ND 0.04 0.06 0.08
Unknown Impurities
1.30 ND ND ND 0.06
1.38 ND 0.02 0.06 0.12
1.48 _ ND 0.11 0.12 0.13
1.54 ND 0.06 0.07 0.15
1.66 ND 0.03 0.05 ND
Total Impurities 0.07% 0.83% 1.09% 1.51%
Table 43D. Stability Data for the Formulation # 10A Stored at 25 C 2 C / 60%
5% RH
Naloxone RRT T=0 4 Weeks 8 Weeks
Physical appearance Clear, Clear, Clear,
(Clarity, Color) Colorless Colorless Colorless
Assay (%) 100 98.9 101.55
Naloxone N-oxide ND NT) NT)
Impurity C 0.53 ND ND ND
Impurity A 0.63 ND ND ND
48
Date recue/Date Received 202401-22
Impurity F 0.93 ND ND ND
Impurity D 1.14 ND ND ND
Impurity E 1.42 0.05 0.04 0.04
Impurity B 1.87 ND ND ND
1.07 0.07 0.08 0.1
1.50 ND 0.07 0.11
Total Impurities 0.12% 0.19% 0.25%
Table 43E. Stability Data for the Formulation # 10A Stored at 40 C 2 C /75
% 5% RH
Naloxone RRT T-0 4 Weeks 8 Weeks
Physical appearance Clear, Clear, Clear,
(Clarity, Color) Colorless Colorless Colorless
Assay (%) 100 100.65 99.4
Naloxone n-oxide ND 0.02 NP
Impurity C 0.53 ND ND ND
Impurity A 0.63 ND 0.01 ND
Impurity F 0.93 ND ND ND
Impurity D 1.14 ND ND NP
Impurity E 1.42 0.05 0.04 0.14
Impurity B 1.87 ND ND ND
0.71 0.01 0.05 0.03
Unknown Impurities 1.07 0.07 0.05 0.08
1.50 ND 0.14 0.21
Total Impurities 0.13% 0.31% 0.46%
Table 43F. Stability Data for the Formulation # 10A Stored at 55 C 2 C
Naloxone RRT T=0 1 Weeks , 2 Weeks 4 Weeks 8 Weeks
Physical Clear,
Clear, Clear, Clear, Clear,
appearance Light
Colorless Colorless yellow
yellow
(Clarity, Color) yellow
-
Assay (%) 100 98.11 98.89 99.96 100.34
Naloxone n-
ND ND 0.05 0.03 NP
oxide
Impurity C 0.53 ND 0.01 0.01 0.01 0.01
49
Date recue/Date Received 202401-22
Impurity A 0.63 ND 0.02 0.02 0.03 0.03
Impurity F 0.93 ND ND ND ND ND
Impurity D 1.14 ND ND 2.31 ppm NP NP
Impurity E 1.42 0.05 0.04 0.02 0.01 0.02
_
Impurity B 1.87 ND ND ND ND ND
0.56 ND 0.02 0.02 0.02 0.06
0.71 0.01 0.05 0.03 0.02 0.03
0.75 ND ND 0.03 0.03 0.05
Unknown 1.07 0.07 0.07 0.07 0.07 0.07
Impurities 1.27 ND ND ND 0.04 0.08
1.30 ND ND 0.01 0.04 0.05
1.39 ND ND 0.02 0.03 0.05
1.50 ND 0.1 0.14 0.17 0.18
Total Impurities 0.13% 0.31% 0.42% 0.50% 0.63%
Table 43G. Stability Data for the Formulation # 11A Stored at 25 C 2 C / 60%
5% RH
Naloxone RRT T=0 4 Weeks 8 Weeks
Physical appearance (Clarity, Clear, Clear, Clear,
Color) Colorless Colorless
Colorless
Assay (%) 100 101.44 104.95
Naloxone n-oxide ND ND NP
Impurity C 0.53 ND ND ND
Impurity A 0.61 ND ND 0.01
Impurity F 0.93 ND ND ND
Impurity D 1.14 ND ND ND
Impurity E 1.42 0.03 0.09 0.03
Impurity B 1.87 ND ND ND
0.71 0.02 0.05 0.06
Unknown Impurities 1.07 0.07 0.1 0.1
1.50 ND 0.02 0.04
Total Impurities 0.12% 0.17% 0.24%
Table 43H. Stability Data for the Formulation # 11A Stored at 40 C 2 C /75
% 5% RH
Naloxone RRT T=0 4 Weeks 8
Weeks
Date recue/Date Received 202401-22
Physical appearance Clear, Clear, Clear,
(Clarity, Color) Colorless Colorless Colorless
Assay (%) 100 100.39 101.31
Naloxone n-oxide ND 0.02 ND
Impurity C 0.53 ND 0.01 ND
_
Impurity A 0.61 ND 0.02 0.03
_
Impurity F 0.93 ND ND ND
Impurity D 1.14 ND ND ND
_
Impurity E 1.42 0.03 0.05 0.04
Impurity B 1.87 ND ND ND
0.71 0.02 0.06 0.04
1.07 0.07 0.05 0.06
Unknown Impurities
1.27 ND ND 0.07
1.50 ND 0.05 0.08
Total Impurities 0.12% 0.26% 0.32%
Table 431. Stability Data for the Formulation if 11A Stored at 55 C 2 C
Naloxone RRT T=.0 1 Weeks 2 Weeks 4 Weeks 8 Weeks
Physical Clear, Clear,
Clear, Clear, Clear,
appearance Colorless Colorless Light Light
(Clarity, Color) yellow yellow yellow
Assay (%) 100 98.48 102.74 101.14 103.18
Naloxone n-
ND ND 0.04 0.02 NP
oxide (%)
Impurity C 0.53 ND ND 0.01 0.02 0.01
Impurity A 0.61 ND 0.03 0.04 0.05 0.07
Impurity F 0.93 ND ND ND ND ND
Impurity D 1.14 ND ND 3 .18ppm ND
Impurity E 1.42 0.03 0.04 0.02 0.02 0.04
Impurity B 1.87 ND ND ND ND ND
0.71 0.02 0.07 0.04 0.02 0.02
1.07 0.07 0.09 0.07 0.06 0.09
1.16 ND ND 0.01 0.02 0.05
Unknown
1.30 ND ND 0.02 0.05 0.03
Impurities
1.32 ND ND ND 0.01 0.06
1.50 ND 0.01 0.05 0.10 0.13
1.59 ND ND ND 0.02 0.05
51
Date recue/Date Received 202401-22
1.62 ND ND ND 0.01 0.07
1.63 ND ND ND ND 0.07
1.73 ND ND 0.02 0.03 0.05
Total Impurities 0.12% 0.24% 0.32% 0.43%
0.74%
ND = Not Detected
ppm = parts per million
[000187] The data suggest that formulation #9A, #10A, and #1 lA
demonstrates satisfactory
stability with no significant increase in individual or total impurities.
Based upon these results, the
formulation containing 0.02 % w/w of sodium ascorbate as an antioxidant and
0.001 % edetate
disodium dihydrate as a chelating agent is chemically stable. Additionally,
formulations containing
0.01 % and 0.005% edetate disodium dihydrate as a chelating agent are
chemically stable.
Example 14. Intranasal and Sublingual Administration of Naloxone Spray
Formulations
Method
[000188] Protocol design was a Phase I, open-label, randomized, single-
dose, five-way
crossover study. The study assessed the bioavailability of a single 8
milligrams and 16 milligrams
dose of naloxone in a formulation of the present invention either intranasally
or sublingually to a
single 0.4 milligram intramuscular dose of naloxone under fasted conditions.
145 subjects were
randomly assigned to one of five groups including 8 milligrams sublingual
dose, 16 milligrams
sublingual dose, 8 milligrams intranasal dose, 16 milligrams intranasal dose
and 0.4 milligram
naloxone dose. Plasma concentrations were taken at pre-dose, 0.03, 0.07, 0.1,
0.13, 0.17, 0.25,
0.5, 1, 2 4, 8 and 12 hours' post-dose.
Results
[000189] As seen in Table 31 below each of intranasal administration and
sublingual
administration resulted in significantly greater plasma concentrations than
intramuscular
administration at all time points tested up to 1 hour after administration.
Further intranasal
administration of naloxone resulted in significantly greater plasma
concentration than sublingual
administration at all times points tested up to 1 hour after administration.
[000190] However, at 2 and 4 hours post-dose the mean plasma concentration
of naloxone in
subjects that were intranasally administered 16 milligrams of naloxone was
significantly lower
52
Date recue/Date Received 202401-22
than that for those subject that were sublingually administered 16 milligrams
of naloxone. The
same pattern was found with those subjects administered 8 milligram doses of
naloxone.
[000191] Further, peak concentration in plasma (C.) and area under the
concentration-time
curve from time-zero to 1 hour post dose (AUC) followed the exact same pattern
as described
above for mean plasma concentrations.
Table 44. Mean Plasma Concentration for 8 mg and 16 mg Intranasal and
Sublingual
Administration
Parameters* 8 mg SL 8 mg IN 16 mg SL
16 mg IN 0.4 mg IM
29 28 29 29 30
Conc. @ 0.03 h
0.18 0.29 6.19 14.47 0.52 0.8 9.29 14.31 0.15
0.29
(ng/mL)
Conc. @ 0.07 h
0.62 0.96 13.95 19.06 1.69 1.85 29.69 28.01 0.36
0.38
(ng/mL)
Conc. @ 0.1 h
0.88 0.94 18.75 15.61 2.31 2.08 46.34 36.64 0.51
0.41
(ng/mL)
Conc. @ 0.13 h
1.17 1.13 20.48 13.11 3.16 3.39 49.31 33.98 0.58
0.39
(ng/mL)
Conc. @ 0.17 h
1.54 1.1 19.96 10.73 3.63 3.83
45.77 24.58 0.63 0.36
(ng/mL)
Conc. @ 0.25 h
1.98 1.29 16.49 6.71 4.39 4.24 35.04 13.23 0.69
0.4
(ng/mL)
Conc. @ 0.5 h
2.03 1.28 9.88 4.42 3.94 2.96 22.35
8.19 0.62 0.22
(ng/mL)
Conc. @ 1 h
1.61 0.85 6.29 2.7 2.96 1.66 14.07 5.61 0.51
0.16
(ng/mL)
C.,, (ng/mL) 2.03 20.48 4.39 49.31 0.69
AUC @ lh
1.68 11.18 3.42 24.91 0.57
(ng*h/mL)
*Mean Standard Deviation
SL denotes sublingual administration
IN denotes intranasal administration
IM denotes intramuscular administration
N denotes number of subjects tested
h denotes hours
53
Date recue/Date Received 202401-22