Note: Descriptions are shown in the official language in which they were submitted.
PRODRUGS OF NH-ACIDIC COMPOUNDS
RELA _________ LLD APPLICATIONS
This application claims the benefit of U.S. Provisional Application No.'s.
61/220,480, filed on June 25, 2009; 61/293,087, filed on January 7, 2010; and
61/293,133, filed on January 7, 2010.
BACKGROUND OF THE INVENTION
(i) Field of the Invention.
The present invention relates to prodrugs of lactam, amide, imide,
sulfonamide,
carbamate, urea, benzamide, and acylaniline containing pharmacophores.
(ii) Background of the Invention.
Drug delivery systems are often critical for the safe and effective
administration
of a biologically active agent. Perhaps the importance of these systems is
best realized
when patient compliance and consistent dosing are taken under consideration.
For
instance, reducing the dosing requirement for a drug from four-times-a-day to
a single
dose per day would have significant value in terms of ensuring patient
compliance and
optimizing therapy.
Optimization of a drug's bioavailability has many potential benefits. For
patient
convenience and enhanced compliance it is generally recognized that less
frequent
dosing is desirable. By extending the period through which the drug is
released, a longer
duration of action per dose is expected. This will then lead to an overall
improvement of
dosing parameters such as taking a drug once a day where it has previously
required four
doses per day or dosing once a week or even less frequently when daily dosing
was
previously required. Many drugs are presently dosed once per day, but not all
of these
drugs have pharmacokinetic properties that are suitable for dosing intervals
of exactly
twenty-four hours. Extending the period through which these drugs are released
would
also be beneficial.
One of the fundamental considerations in drug therapy involves the
relationship
between blood levels and therapeutic activity. For most drugs, it is of
primary
1
CA 3035442 2019-03-01
importance that serum levels remain between a minimally effective
concentration and a
potentially toxic level. In pharmacokinetic terms, the peaks and troughs of a
drug's blood
levels ideally fit well within the therapeutic window of serum concentrations.
For certain
therapeutic agents, this window is so narrow that dosage formulation becomes
critical.
In an attempt to address the need for improved bioavai lability, several drug
release modulation technologies have been developed. For example, poorly
soluble 5,5
diphenylimidazolidine-2,4-diones have been derivatized into phosphate ester
prodrugs to
improve solubility. (Stella et. al., U.S. Patent No. 4,260,769, 1981). Enteric
coatings
have been used as a protector of pharmaceuticals in the stomach and
microcncapsulating
active agents using proteinaceous microspheres, liposomes or polysaccharides
have been
effective in abating enzymatic degradation of the active agent. Enzyme
inhibiting
adjuvants have also been used to prevent enzymatic degradation.
A wide range of pharmaceutical formulations provide sustained release through
microencapsulation of the active agent in amides of dicarboxylic acids,
modified amino
acids or thermally condensed amino acids. Slow release rendering additives can
also be
intermixed with a large array of active agents in tablet formulations.
While microencapsulation and enteric coating technologies impart enhanced
stability and time-release properties to active agent substances these
technologies suffer
from several shortcomings. Incorporation of the active agent is often
dependent on
diffusion into the mieroencapsulating matrix, which may not be quantitative
and may
complicate dosage reproducibility. In addition, encapsulated drugs rely on
diffusion out
of the matrix or degradation of the matrix, or both, which is highly dependent
on the
chemical properties and water solubility of the active agent. Conversely,
water-soluble
mierospheres swell by an infinite degree and, unfortunately, may release the
active agent
in bursts with limited active agent available for sustained release.
Furthermore, in some
technologies, control of the degradation process required for active agent
release is
unreliable. For example, because an enterically coated active agent depends on
pH to
release the active agent and pH and residence time varies, the release rate is
difficult to
control.
Several implantable drug delivery systems have utilized polypeptide attachment
to drugs. Additionally, other large polymeric carriers incorporating drugs
into their
matrices are used as implants for the gradual release of drug. Yet another
technology
combines the advantages of covalent drug attachment with liposome formation
where the
active ingredient is attached to highly ordered lipid films.
2
CA 3035442 2019-03-01
However there is still a need for an active agent delivery system that is able
to
deliver certain active agents which have been heretofore not formulated or
difficult to
formulate in a sustained release formulation for release over a sustained
period of time
and which is convenient for patient dosing.
There is a generally recognized need for sustained delivery of drugs that
reduces
the daily dosing requirement and allows for controlled and sustained release
of the parent
drug and also avoids irregularities of release and cumbersome formulations
encountered
with typical dissolution controlled sustained release methods.
SUMMARY OF THE INVENTION
The present invention accomplishes this by extending the period during which a
lactam, amide, imide, sulfonamide, carbamate, urea, benzamide, acylaniline,
and cyclic
amide containing parent drug is released and absorbed after administration to
the patient
and providing a longer duration of action per dose than the parent drug
itself. In one
embodiment, the compounds suitable for use in the methods of the invention are
derivatives of lactam-, amide-, imide-, sulfonamide-, carbamate-, urea-,
benzamidc-,
acylaniline-, and cyclic amide -containing parent drugs that are substituted
at the amide
nitrogen or oxygen atom with labile aldehyde-linked prodrug moieties.
Preferably, the
prodrug moieties are hydrophobic and reduce the polarity and solubility of the
parent
drug under physiological conditions.
In one embodiment, the invention provides a prodrug compound of Formula I, II
or III:
A
A
A ___________________________________________ 0ISI __ /R1
X
(B
7
1
Formula I Formula II Formula III
and the geometric isomers, enantiomers, diastereomers, racemates,
pharmaceutically
acceptable salts and solvates thereof;
wherein A and B together with the -N(C=X)- or -N=C-X- or -S(0)2-N- group to
which
they are attached form a parent drug;
Xis -S- or -0-;
3
CA 3035442 2019-03-01
=
RI is selected from ¨C(RA)(Ra)-0R20, -C(RA)(R8)-0C(0)0R20, -C(RA)(RE0-
0C(0)R20,
-C(RA)(Rn)-0C(0)NR2oR21, -(C(RA)(Rn))-0P03MY, -(C(RA)(Rn))-0P(0)(0R2o)(0R21).
-[C(RA)(Rn)0],-R20, -[C(RA)(R00],.-C(0)0R20, -[C(R,
0(Rn)0]z-C(0)R20, -
[C(RA)(RH)01,-C(0)NR20R2i, -[C(RA)(RB)01,-OPO3MY, ¨[C(RA)(RB)0],-
P(0)2(0R20)M and [C(RA)(RH)01,-P(0)(0R20)(0R21);
wherein z is 2 or 3;
wherein each RA and RE3 is independently selected from hydrogen, halogen,
aliphatic, substituted aliphatic, aryl or substituted aryl;
each R20 and R21 is independently selected from hydrogen, aliphatic,
substituted
aliphatic, aryl or substituted aryl;
Y and M are the same or different and each is a monovalent cation; or M and Y
together is a divalent cation, and,
wherein when said parent drug contains a 5,5 diphenylimidazolidine-2,4-dione
moiety of formula I, R1 is other than -CH(RA)0P03IvIY, CH(RA)0P(0)(OH)2, or
¨CH(RA)0C(0)R20.
The invention further provides a method for sustained delivery of a parent
drug
by the administration of a conjugate of the parent drug with a labile moiety,
wherein the
conjugate is represented by formula I, II or III.
BRIEF DESCRIPTION OF THE DRAWINGS
The foregoing and other objects, features and advantages of the invention will
be
apparent from the following more particular description of preferred
embodiments of the
invention, as illustrated in the accompanying drawings in which like reference
characters
refer to the same parts throughout the different views. The drawings are not
necessarily
to scale, emphasis instead being placed upon illustrating the principles of
the invention.
Figure 1: PXRD spectrum of Compound-7
Figure 2: IR Spectrum of Compound-7
Figure 3: Raman spectrum of Compound-7
Figure 4: TGA thermogram of Compound-7
Figure 5: DSC thermogram of Compound-7
Figure 6: Pharrnacodynamic (PD) study of compound-4 in AMPH induced
locomotion model.
Figure 7: Pharmacodynamic (PD) study of compound-7 in AMPH induced
locomotion model.
4
CA 3035442 2019-03-01
Figure 8: Plasma concentration of aripiprazole after intravenous
administration
of (0.5 mg/Kg) compound 7 to rats.
Figure 9: Plasma concentration of aripiprazole, dehydroaripiprazole and
compound 7 after intramuscular administration of 30 mg/kg of compound 7 to
dogs.
Figure 10: Pharmacokinetic profile of pioglitazone, Compound-1002 and
Compound-1008 after intravenous administration (20 mg pioglitazone equivalent)
to
rats.
DETAILED DESCRIPTION OF THE INVENTION
One aspect of the present invention provides a compound having the general
formula I, II or III:
A
A 0 RI
X _______________ (
X
A¨S¨N
R/ \B
1 0
Formula I Formula II Formula III
or its geometric isomers, enantiomers, diastereomers, racemates,
pharmaceutically
acceptable salts and solvates thereof;
wherein A and B together with the ¨N(C=X)- or ¨N=C-X- or ¨S(0)2-N- they are
attached forms a parent drug;
X is ¨S- or --0-;
R1 is selected from ¨C(RA)(Ra)-0R20, -C(RA)(Ra)-0C(0)0R20, -C(Rx)(Ris)-
0C(0)R2o,
-C(RA)(143)-0C(0)NR20R21, -(C(RA)(R3))-OPO3MY, -(C(RA)(RB))-
0P(0)(0R2.0)(0R21),
-[C(RA)(ROO1z-R20, -{C(RA)(Ra)012-C(0)0R20, 1C(RA)(RFOOk-C(0)R2o, -
[C(RA)(RB)0],-C(0)NR2oR21, -{C(RA)(RB)Oh-OPO3W, ¨[C(RA)(RB)0],-
P(0)2(0R20)1\4 and ¨{C(RA)(Re)01z-P(0)(0R2o)(0R21);
wherein each RA and R5 is independently selected from hydrogen, halogen,
aliphatic, substituted aliphatic, aryl or substituted aryl;
each R20 and R21 is independently selected from hydrogen, aliphatic,
substituted
aliphatic, aryl or substituted aryl;
Y and M are the same or different and each is a monovalent cation; or M and Y
together is a divalent cation; and,
5
CA 3035442 2019-03-01
wherein when said parent drug contains a 5,5 diphenylimidazolidine-2,4-dione
moiety of formula I, RI is other than -CH(RA)0P03MY, CFI(RA)0P(0)(OH)2, or ¨
CH(RA)0C(0)R20.
In one embodiment, the compounds of the invention having Formulas I, II and
III
are less soluble, and are preferably at least an order of magnitude less
soluble, as
compared to the parent drug from which they were derived. In one embodiment,
the
prodrugs of Formulas I, II and III have an aqueous solubility of less than
about 0.5
mg/ml, preferably less than about 0.1 mg/mL, preferably less than about 0.01
mg/mL,
preferably less than about 0.001 mg/mL, preferably less than about 0.0001
mg/mL and
even more preferably less than about 0.00001 mg/m1 when solubility is measured
in a
phosphate buffer (pH 7.4) at room temperature.
In a preferred embodiment, a compound of the invention provides sustained
delivery of the parent drug over hours, days, weeks or months when
administered, for
example, orally or parenterally, to a subject. For example, the compounds can
provide
sustained delivery of the parent drug for at least 8, 12, 24, 36 or 48 hours
or at least 4, 7,
15, 30, 60, 75 or 90 days or longer. Without being bound by a theory, it is
believed that
the compounds of the invention form an insoluble depot upon parenteral
administration,
for example subcutaneous, intramuscular or intraperitoneal injection. In one
embodiment a prodrug of the invention may further comprise a sustained release
delivery
system for providing additional protection of the prodrug from enzymatic or
chemical
degradation.
In another embodiment, the invention provides a method for sustained delivery
of
a parent lactam, amide, imide, sulfonamide, carbamate, urea, benzamide, or
acylaniline
containing drug to a subject in need thereof. Each of these groups comprises
an amidic
N-H group. The method comprises administering to the subject an effective
amount of a
prodrug formed by substituting on the NH group a labile, hydrophobic aldehyde-
linked
prodrug moiety wherein the prodrug has reduced solubility under physiological
conditions compared to the parent drug and provides for longer sustained
therapeutic
levels of the parent drug following administration than observed levels
following
administration of the parent drug. In a preferred embodiment, the amidic N-H
group has
a pKa of about 5 to about 22, preferably about 5 to about 21, and preferably
about 5 to
about 20.
In a preferred embodiment, RI is selected from Table-1.
6
CA 3035442 2019-03-01
Table 1
V
o o
o
.,...\....1r0\
0 0 0
,
.,...õ,-...
......A. 0,,,,z,, ,z0..... yc,
0
0
.
0
o
o o
0....y0\ 0<ir.0\
0 0 0
0
,,,r..0\ ao-\
0
. .
0,....),4
,
0 0 0
0 0 0
0 0 0
,......,,
0
, 0 , 0 1
7
CA 3035442 2019-03-01
....4...)...,..ro.....s.õ,\ ______________________________________
0 0 0
_
,
0 1 5
0
0.õ..õ...A
i 1 3
0
0 0
0 0
.<.....),..õ<õ..õ,0,,,,.,õ,,,\
1 5
7 0\ 7 0
7 5
0
1 I
0 1 0 0
_
3 7 0\ 7 0 44 0
/
\ \ \
-
' ( -/--- \ /--\
- 6 4
0 0
\---j i i
0 0 '
____________________________ e----, 0" 0..---1
...õ.===-\........Ø...,_,A /4-_,.. 0 ,,-;\ j.y0,),
0 0 0
,,,),y0...y....\ =.,.......-õL.,(0.õ:\
1
0 0
0
8
CA 3035442 2019-03-01
0;\ 0 '2,
'.--' ,,
o o o
c . I ay,?2,
0
0 0 0
ozz, --i-o----'\
0
0 .
0 0
0
.....õ....õ...ni3Oõ......-\ 0:\
..õ.......õ,.õ.......sr,,o.,,,..-q.1,........,..õ.õ,.....,(,..õ
0 0 0 1
..,........,.....õ....,.....yØ,õ..-,q, ...,_,,...õ,...1r.0T.:\
,,,(4.L3,1(017-,
0 0
8 I
o o
0,,,
, 0 0 0
,
,
0
/7
: I 0
9
CA 3035442 2019-03-01
N..)\ 0,5z4 ,,<,7o,N.,,õ=\
9 ii 13
o
0 o
0 0
V\O 15 0
\07 5 7 1
)----
0
cl< \
0 0 0
_ ________________________________________________________________
0 0
0 0
\-------C i
o 1
0/ ,._/ 0
0
0'---,
,
41(
0 , o___, 0 ,
0
0
0
0,, 74yõ:\
0 , 0 ,
0 ,
CA 3035442 2019-03-01
!
o 1X,24
0
0
_ _________________________________________________________________
0
0 ,N
0 OH
=
/
0 ,,,,,.,
0 õ,..",.,., 0
__________________________________________________________________ I
, O' c\
o__-'-
alro;\ ff/(j
0 ,,/N.,,, I
I
I
I
0
I 0 1
I
0 ,,../ "'N ..õ 0 ..,,,',,, I
1
I
A-p_irox-\ ________________________________________________________
õ..k..pziõ..0,.....,A )0;\
0 ,.........,
0 õõ....,, 0 ,......,,
,
1
0
)4Z2....ro...A
I I 0
I
11
CA 3035442 2019-03-01
o
o .,,..-,,,
,
o
I
,
=.0Ot7z4
0
___
o 4 7 6,, \ (i/=1,,(4(,,
0
-
7 osys5 5 1,..L.7_,
7
__________________________________________________________________ I
0 0
I 0
¨ \
- . __________________________
_ r--- \ \-----
4 0
I 0 1
I
i
o _________________________________________________________________ y
oc/'
0 0 .,.,., ,, 0 .,..,_.
0 0 0 )
12
CA 3035442 2019-03-01
\
0
.)
0 0
Ws,
.00
õ;=õ
)
oo
0
Oyx\ Ckro
Or
0
0
13
CA 3035442 2019-03-01
0 0 0 c),0
0
o
0
0 _________________________ \
o- 00
0 0o 0
o
o o
o
0
cpc,
7
11 o
0
oJ
14
CA 3035442 2019-03-01
15
13 -
o 00
0 0
)
7 0 0
0 0 s.
4 7 0"- \ 0
0
\ 0 (r17'----
\(=1<l0./
_
( [ / 6
\:-.---
---1 0 i 0
0 \-----Cr
1
i !
. ,
0 ,
,
'1
o 0''=0
e--ei
a.--1
0
,
e e a
o 0 1 o 0 o
a , 1
013-0.õ,)-1, 1 0-P-0.õ--\, 0-ri-ox\
8 MY 8 MY 0
MY
a a a
a? 1 a? a? 0-P-0...,..)\ 0-P-0., II
8 Ca 2' 0
Ca2' 8
-"" Ca2+
,
CA 3035442 2019-03-01
8 8 0
, ,,----... --I
,
,
\ e \ \ -
0-
\ , \ , \ ,
0--1-0..õ=\, 0-p-0õ.--\ 0--pi-0,,,,\
8 8 8
...,........õ
/ /
'10 \ .1
04-0 NI,
8 0
0-P-0-11/, 8 *Nr \ 0+0\
!
0
i,...õ...
1
1
,
o-------------- 0w. '-µ\
0+-0,\
0
0 0 ,----,
...__Z-N
\
, .
0 / i 0
---N
)
Or-I
---N ---N --N
13
1
16
CA 3035442 2019-03-01
0
)13
i
_ _________________________________________________________________
0 7-1 0 i i 0 T-1
0 )LO )L0
-----N "N ----N
,,i=J )15 ,-Sei )17 )) . )19
0
0 /---i
\---0
"N
"N
) 23 3) )9
- 0
---N ---11
\
õ-('') 11 1
0 ) ?
I
,-0 0
? 0 >
7-N
/C)
o 0
/ N )¨o
/......sz...___Zy
4:15-
/4 i7 N
))13
. _________________________________________________________________
0 0 _____________ 0 ___
)L0
}i)5 )) ) 7
17
CA 3035442 2019-03-01
, ________________________________________________________________
0 0 0 )
)13
0
-----N _______________________________________________ 0
,..0 )15 ).' ) 17 )) )19
"N
21 23
o _______________ 3
----N -__Z-N
e
0
)-0 3
/....,y....._/-11 0
)\--0 0---- ________________
=
)-0
fr7--N "N
(- )13
18
CA 3035442 2019-03-01
0 ? o
q o ?
1
,--o ' )\---o
---N)\--o
----N
----N
0 ? o __
)\---0
----N ----N
)13 )h15
)) ) 11
0 c-4 0--- ____________ 0
----N ---N
----N
õA-7 ),I)19
õJ.-1)21
0 1
)-0
-----N
,
õJ-1 )23
i
0
s,..,01
0.,õ.\ ,õ1--0-1r0--A
0 0
0.,,,,,0A, ,..,--,,---0--,,--0---;\
II II
0 0 o
-...õ...õ.....õ..,.....õ.,..oyo\ crOO...õ--7-41, dr-) n
-y.-..,...A.,
o
0 0
19
CA 3035442 2019-03-01
\ ,
y0 0 0 -..--;\
o 0 0
0
,,,,----=õõ,..0y0,,,,---1/41, 1 -2_ _,-",,,_õ,----,,_,õ0-õre...0,,,,\,
0 0
0
0 0
y --..õ--
ltz, ,--..õØ.õ,,0õ,..\ .N,,o,,,c).ji
H II
0 0
0
,z, 'N.Y(1{"' '''-z2t'
0 % , i
i 0
i 0
I
1
--.Z.,),0=,,,,...,..0õ,.....õX
Li k..) 0
15 17 I 19
0 0 0 1
,
-i.....y0.õ,..,Ø..,....7\
210 1 23
0
0 oyoõ); ,---...,,o 0 \ 0
ri \-- y -----
0 f-1--- 0 \ 9 I
!
CA 3035442 2019-03-01
,
- 0
\
7 , 8
/o
o
,
----\\¨/)8
0
0\
..erfri 7 N
\ & __ 0 _______________________________ 0
\ -/ " '
0 ,
1
I
7 . 5
1 0 I
0----k
0
o 0-----'-L
,
\---j ,
,
,
,-0..\?1,.0õ,.,..;\ *N.,...õ,0-,,,i,,,OT:\
1
ii
0 0 -NI 0
0õ....-......L. __________________________________________________ yoA,
..,......õ___ 0 y 0,...,2\
0 I
0 0 '
,
i _________________________________________________________________
,.._.õ.,0y 0722,
0 i 0 0 I
`,..,....õ," ,........õ...., ....,,........0y0,..A 0 0A 0,õõ......,
0 0 0 ________
1
21
CA 3035442 2019-03-01
cr,(:),.,0,,\ c _,A ,(:) y 0
=,,\
Y
110 0 0
Y., o, ,.Ø, A
0 .,.,.õ.o..r.,o.õ,:z, Tof T
.---,...--1<-0.,õ0.,),,
8 I X Y
o
-,------,----,---0,0,\ _______________________________________ 00,\
1( o,o ,
,),, `9.-
1 7 I
0
0
1.4.0,,,,0_,..\, =,(._).,0.,y0,õ,\,
.9,.0,,,ON,..,,\
1 9 I 11 II 13 II
0 0 0
0 \---y--0
15 II 17 I 19 I
0 0
..9,..0,õ\
21 I 23 II0 I Oya,y).4
0 0 ' 0 I
0 0,,,,,o,,\ 0 olio,),, / )9 0\
g I /L0
0
'\)-----
___________________________________________________________________ _
22
CA 3035442 2019-03-01
------0 ' ( 7
\ 7 8
0 /0
/0
0
µ1-
-
12 )4
1 7
)4 \28
/68 }----0
0
0
'µI.--- "----
-
0 - 0
O'Lc) O'L
0
'µr
'`,,....õ, 0 ,,,,.(0 ...,,22.4 1 .õ.."-
",,,...,,O.r.O.,,A =,,..s.õ,0%,,,,,O.õ,.7777,
0
',,,, 0 ...,/\ ', 0
õ..,.."'µ,,,,,
,,.._00=N,\
11 C10 \IA _,/,,,,,,,a,,r0
g
,,,,^=,,,,.,,,0,r0.,.....-\ ,0,,,,,0õ:õ __________________________
0
,
0 , ! 0 ,
,
,
8 .----.. o ,........õ 0
n 7-....õ
0,... o .v-zzz,
vy =,,,.,,,A4 õAy
0 ,,''N. 0 ,'NN, 0
23
CA 3035442 2019-03-01
1, ZN/Nr4
O.,,..,-0 ()Nr
,:z,
0
I 0 zN
0 ..õ.----,,,,
______________________________________________________________ ¨
0,,,, 0
II
I
,
\ 1 0,(0,-%, ______ 14,00,,,
7
0,fõ0õ,,,, -..0,0,0..-.122,
9 11 I 13 rõIl
o____-
Li..õ, Li õõ--,=õ,,
j,1 19 ,I,
...õ---õõ Li ..,õ..
21 I 23 õ
0 ,..õ---,, k.) ----=,., 0
= 0y0,_;\ 0 olry,
0 ____________________________________________________________
0
)--0
(CHA-0
I ________________________________________________ /
I
24
CA 3035442 2019-03-01
,
--___ 0
7 8 0 ( 7 '''''''-- 8 0
\
0
)4 ) 4 o ¨ 0
\J)8 ___________________ c:ko o 0¨(
1....¨ 0
(
0 1
0
o------c
C) 0 --.----L
__________________________________________________________________ ,
In a more preferred embodiment, R1 is selected from Table 2.
Table 2
aro 112s
0 0
0 _
,/\\y-0.,,,,*-422, =,õ...J.,_õi_Ø.. ),,(0,,,,,,,,
1
0 0 0 !
--..............-.,-..
0 0
1
I 0
CA 3035442 2019-03-01
1 _________________________________________________________________
,
0 i
(0,\
0
0 0
0 0 0
` \r= a
0A,
o\
\
0
0 0
0 I\
0 1 0
1 0
0 0 0
__________________________________________________________________ I
!
0N A&I.,,,0,..,..-\ 0.,...;\
0 0 0
8
0 0
Agy0,-,, 70.130,-N,
o 0 I 0
A3.2_12 ro,A,
0 5 0
0
9
I1 ill % /13
0
26
CA 3035442 2019-03-01
0 0
\)/A0
15 117 V
0 7 )
'..? \ .
0 0 0
,
,
-
V4 V¨
7 1 1
( / \ __ /
6
&D 1
0
\---) !
1
1 I i
0
1 o _______________ o
1
eThe I
1 '=-= o----1
!
_________________________________________________________________ 1
In a more preferred embodiment, R1 is selected from Table 3.
Table 3
0 0
--- N
1
0
0/ 0
0
¨ 0
/-1
Z.--
----N
)
_
0
/----
1 0 0 /-1
0 1 )L 0 )L0
------N ---N ----N
)j3
i
27
CA 3035442 2019-03-01
I µ )L0 0
7_....../...___/-i
)13
I
I
t 1 0
---N ; ----N ----N1
)15 117 )j ) 19
!
0
7-1 0 r¨i 0
----N ------NI --NI
)J )21
)" ) 23
0
0
kk r¨i
-----N
I
In a more preferred embodiment, RI is selected from Table 4.
Table 4
...õ--",......õ-00,..,...-\
0 =-=,.,. 0 .- 0
CLNI.f=-' /".Zzt, " y ---;\ .--4Dyc-Liz2,
II 0
o o
0y0õ),,,
i
0 0
0
0 o
i 0
cyoyo,,:z, doyo,,,,,\ , 0 y Ck..; \
0
0
___________________________________________________ 0
28
CA 3035442 2019-03-01
___________________________________________________________________ _
o ,c)..,oõ,\
H
0 0
õ..-...õ..õ<õ,0y0,\ N7N,,--'' 0y0N)2,,
0
0 0
...x...,
0 I 01'2, C).)-raj''''
0
7 II
0
0
9 I 11 I 13
15 17 I "19 ll
0 0 0
*-$3.-0,,,õ0,_,122, 1 =9,0,-0,,,,\ 0
21 I 23
0 0
i
0 0y0.\, r-01.0), 0) 0,7-1
0 fl,,,,..--
,
, /cH2),-0
0 \
0 µ 7
8
0
\ / ) 8 0
0
0
1
'''..
I i ____________________________________
29
CA 3035442 2019-03-01
7
______________________ 0 0
0
8 (0
0
\4)
0
0
PRODRUGS OF LACTAM, CYCLIC UREA, IMIDE, CARBAMATE
CONTAINING PHARNIACOPHORES
In one embodiment, compounds of the present invention are represented by
formula IV or V as illustrated below, or its geometric isomers, enantiomers,
diastercomers, raccmates, pharmaceutically acceptable salts co-crystals and
solvates
thereof:
/RI
X Ri X
) )-1µ1\
,rx, x,, __ õ
x3
.x, t 1xt
Formula IV Formula V
wherein --- represents a single or double bond;
X and R1 are as defined above;
each X1, X2, and X3 is independently selected from absent, -S-, -0-, -S(0)-, -
S(0)2-, -
N(Rio)-, -C(0)-, -C(01240)(R11)-, -[C(Rio)(R11)1v-, -C(Rio)=C(Rin)-; wherein v
is 0, 1,2,
3, 4, 5, 6, 7, 8, 9 or 10;
CA 3035442 2019-03-01
wherein each Rio and Rii is independently absent, hydrogen, halogen,
aliphatic,
substituted aliphatic, aryl or substituted aryl; alternatively two R10 and R11
together with the atoms to which they are attached may form an additional
optionally substituted, 3, 4, 5, 6 or 7 membered ring; and
t is 0, 1, 2 or 3.
In one embodiment, compounds of the present invention are represented by
formula VI or VII as illustrated below, and the geometric isomers,
enantiomers,
diastereomers, racematcs, pharmaceutically acceptable salts and solvates
thereof:
F2 , -
II
(R3)q )ci
F (R3 Tr
i-CY ____ (R4)m FiCdr (Um
Xi = N
Gi Rt GI
Formula VI Formula VII
wherein ----- represents a single or double bond;
X, XI, X2 and Ri are as defined above;
ring Y is an optionally substituted cycloalkyl, cycloalkenyl, heterocycly1 or
aryl
containing one, two or three rings;
each Fi and F2 is independently selected from absent and Rs-A-Cyi-B-D-;
wherein, A is selected from absent, optionally substituted alkyl, optionally
substituted alkenyl, optionally substituted alkynyl, -S-, -0-, -S(0)-, -S(0)2-
, -
S[C(R30)(R30]u-, -S(0)[C(R30)(R3i)b-, -S(0)2(C(R30)(1131)].-, -0[C(R3o)(R3i)].-
,
-N(R30)-, -N(R30)[C(R31)(R32)1u-,1C(R3o)(1131)].,-C(0)[C(R3a)(R31)].-;
wherein each u is independently 1, 2, 3, 4, 5, 6 or 7;
Cy i is absent or an optionally substituted cycloalkyl, optionally substituted
cycloalkenyl, optionally substituted heterocyclyl, optionally substituted aryl
or
optionally substituted heteroaryl;
B is absent, or a linker;
D is selected from absent, -0-, -NR33, -C(R34)(R33)-, -S-, -5(0)-, -S(0)2-,
and -
C(0)-;
31
CA 3035442 2019-03-01
each GI and G2 is independently selected from absent, -S-, -0-, ¨S(0)-, ¨S(0)2-
, -
SC(R40)(R41)-, ¨S(0) C(R40)(R41)-, ¨S(0)2C(R40)(R41)-, -C(0)-, -C(01140)(R4:)-
, -
0C(R4AR41)-, -N(R40)-, -C(R40)=C(1141)-, -N(R40)-C(RII)(R42)-, and -
[C(R4o)(R41)1,-;
each R3, R4, R5, R30, R31, R32 R33, R34, R35, R40, R41, and R42 is
independently selected
from absent, hydrogen, halogen, -0R1 0, -SRio, -NRI012.11-, -C(0)R10,
optionally
substituted aliphatic, optionally substituted aryl or optionally substituted
heterocycly1;
= alternatively, two R3 groups or two R4 groups or one R3 group with one
R.4 group
together with the atoms to which they are attached and any intervening atoms
form an
optionally substituted ring;
m and q are independently selected from 0, 1, and 2.
In a preferred embodiment, G2 is selected from ¨N- or ¨C(12.10)-.
In a preferred embodiment. the R5 moiety is an aryl or hetcroaryl group
selected
from:
R100 4 )
c ____ 5
CI a a a
R101.< \ ____________________________________________ Riol,EN\
?
GI
CI
FR,004, _______________________ \ ci 5
A, 1
R ________________________________________________________
Rioo ..100k /
Rioo, : \
Ric).'µ / Rioo*%, \ ?
N
)
R _________________________________________________ Rioo,g \
R101 _____________________________________________________ /
Rloi ( 5 10N1).,µ /
R1001 \ $ R1004 \ ,
\ _____________________________________________________________
Rio- li Riot) /7-
11 ),,-
Rioi
32
CA 3035442 2019-03-01
R100 \ ,)/1,. __ \ i õ..N ,s
N-=- .\
R100 R101 ..--- S -- R100
\ Rloo // ) 5
,
I7,101 R100
R101
7". . ,
H ¨ ¨
VW". VVVV, JVVV,,
./.' N, X' ...¨
N` S N.":7 0 N's NR 103
----R
we R100 R100
wherein R100 and Rio. each represent 1 to 4 substituents independently
selected from
hydrogen, halogen, optionally substituted C1-C8 alkyl, optionally substituted
C2-C8
alkenyl, optionally substituted C2-C8 alkynyl, optionally substituted C3-C8
cycloalkyl,
optionally substituted C1-C8 alkoxy, optionally substituted C1-Cs alkylamino
and
optionally substituted Ci-Cg arõ,l; and, R103 is selected from hydrogen,
halogen,
optionally substituted C1-Cg alkyl, optionally substituted C2-Cs alkenyl,
optionally
substituted C2-C8 alkynyl, optionally substituted C3-Cs cycloalkyl, optionally
substituted
CI CS alkoxy, optionally substituted C1-C8 alkylamino and optionally
substituted CI-Cs
aryl.
In a preferred embodiment, Cyi is selected from:
Rwo
V-----N
Rio() Rioi
R100
_N() r\1\
\
N-1 ¨N\____ 1--1 NJ
I ---
¨N\ )
33
CA 3035442 2019-03-01
vN, -Rioo
¨0 ¨N\ /0
r-N7\
= N vN 111 tv
Rioo
/-1¨\
Rioo
\..õ.7).5.5
\-N
ssC R
N loo
,c\ Rioo
-1=1
n
R101 r\ioi N
In a preferred embodiment, the bivalent B is a direct bond, a straight chain
CI-Cio
alkyl, C1-Cio alkenyk alkynyl, C1-C10 alkoxy, alkoxyCi-Cioalkoxy, C1-C10
alkylarnino, alkoxyCi-Cioalkylarnino, alkylcarbonylamino, CI-C10
alkylaminocarbonyl, aryloxyCI-Cioalkoxy, aryloxyCi-Cioalkylamino, aryloxyCi-
Cioalkylamino carbonyl, C1-C10-aLkylaminoalkylaminocarbonyl, CI-Cio alkyl(N-
alkyl)aminoatIcyl-aminocarbonyl, alkylaminoalkylamino,
alk-ylcarbonylaminoalkylamino, alkyl(N-alkyl)aminoalkylamino. (N-
alkyl)alicylearbonylaminoalkylamino, alkylaminoalkyl,
alkylaminoallcylaminoalkyl,
alkylpiperazinoalkyl, piperazinoalkyl, alkylpiperazino, alkenylaryloxyCl-
ClOalkoxy,
alkenylarylaminoCI-Cioalkoxy, alkenylaryllalkylaminoCI-Cioalkoxy,
alkenylaryloxyC1-
34
CA 3035442 2019-03-01
C; oalkylamino, alkenylaryloxyCj-Cloalkylaminecarbonyl, piperazinoalkylaryl,
heteroarylCi -Cloalkyl, heteroaryIC2-C1oalkenyl, heteroary1C2-Cioalkynyl,
heteroarylCI-
C10alkylamino, heteroarylCi-Cioalkoxy, heteroaryloxyCi-Cioalkyl,
heteroaryloxyC2-
C10alkenyl, heteroaryloxyC2-C10a1kyny1, heteroaryloxyCi-Cloalkylamino or
heteroaryloxyC1-Cloalkoxy.
In one embodiment, compounds of the present invention are represented by
formula VIII or VIIIA as illustrated below, and the geometric isomers,
enantiomers,
diastereomers, racemates, pharmaceutically acceptable salts and solvates
thereof:
F2 F.,RI
X
q(R3) (R4)m q(R3)
b,
1
u1
Formula VIII Formula VIIIA
wherein Ring Y, RI, R3, R4, GI, G2, X. F2, m and q are as defined above.
In a more preferred embodiment, compounds of the present invention are
represented by formula IX or X as illustrated below, and the geometric
isomers,
enantiomers, diastereomers, racemates, pharmaceutically acceptable salts and
solvates
thereof:
F2
____________________________ 0 /F2
(R3)q __________
0 RI
\RI (R3)q __
Formula IX Formula X
wherein RI, R3, F2, and q are as defined above.
CA 3035442 2019-03-01
,
In a preferred embodiment a compound is selected from Table IX-X. A more
preferred embodiment is a compound from Table IX-X wherein 11, is selected
from
tables 1-4.
Table IX-X
Is. Structure No Structure
s
411 =-N 40, .!-N1
r?L) Nrk=-''-1 0
RI 0
2, 90
,1-----\N .
N---- "\---/ N----,---( 1 \---/ CF:
/ 0F3 0¨R1
Ri jc
-TT io
411 N
i Ni.-----( (N1
/N---k 0---Ri
Ri 0
4 0 õR , II R,
/
H r, .
N,....r, --N
H 0
\ ---1
N 0 0 N
N.,,,
I ..õ...____N...õ_0-cL
= N0.--
CI
CI
5 Ri 0 12 0
--,----f
N
0.-0---N 0
it N \\_.......,N.,õõra 0
CI
1 0
,
36
CA 3035442 2019-03-01
6 ________________________________ F
010 N
0 NO
Ri 0 ORi
7 F 14
N
= N,)
rN1
/N--k
R 0
In a more preferred embodiment prodrugs of domperidone are disclosed.
(Formula 4 and 11 from Table IX-X). A more preferred embodiment is a compound
of
Formula 4 from Table IX-X, wherein RI is selected from table 1. In a more
preferred
embodiment, a compound of Formula 4 from Table IX-X, wherein R1 is selected
from
tables 2-4 is disclosed.
In a more preferred embodiment, prodrugs of droperidol are disclosed. (Formula
6 and 13, from Table IX-X). In a more preferred embodiment, a compound of
Formula 6
from Table IX-X wherein RI is selected from table 1 is disclosed. A more
preferred
embodiment is a compound of Formula 6 from Table IX-X wherein R1 is selected
from
tables 2-4.
In a more preferred embodiment, prodrugs of pimozide are disclosed. (Formula 7
and 14 from Table IX-X). In a more preferred embodiment, a compound of Formula
7
from Table 1X-X wherein R1 is selected from table 1 is disclosed. In a more
preferred
embodiment, a compound of Formula 7 from Table 1X-X wherein R1 is selected
from
tables 2-4 is disclosed.
In another embodiment, compounds of the present invention are represented by
Formula XI or XII as illustrated below, and the geometric isomers,
enantiomers,
diastereomers, racemates, pharmaceutically acceptable salts and solvates
thereof:
37
CA 3035442 2019-03-01
R1
q(R3) G q(R3)
X
Fi /rn F
AN
b, R1 G1
Formula XI Formula XII
wherein Ring Y, R1, R3. R4, X. F1. GI. G2, m and q are as defined above.
In another embodiment, compounds of the present invention are represented by
Formula XIA or XIIA as illustrated below, and the geometric isomers,
enantiomers,
diastereomers, racemates, pharmaceutically acceptable salts and solvates
thereof:
/G4¨tr r I
(NI
R11 X20
R; x,,--N (R2)P
(R::/11
N
RI71\/ N,)\), (On
(R4)m
0
Formula XIA Formula XLIA
wherein R1, R3, R4, R5, R10, R. A. D, m, and q are as defined above;
R2 is selected from absent, hydrogen, halogen, -012.15, -SR -NRioRtl-,
optionally
substituted aliphatic, optionally substituted aryl or aryl or optionally
substituted
heterocycly1;
r is 0, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10 or 11;
each 03 and G4 is independently selected from ¨N-, and -C(Rio)-[C(Rio)(Ri Oh-,
wherein
a is 0, 1 or 2;
X20 is ¨C(R10)- or ¨N-; and,
pis 0, 1, 2 or 3.
In another embodiment, compounds of the present invention are represented by
Formula XIB or XIIB as illustrated below, and the geometric isomers,
enantiomers.
diastercomers, racemates, pharmaceutically acceptable salts and solvates
thereof
38
CA 3035442 2019-03-01
A¨N / ____________ \N¨ R
3
)
q
r 1 7
/ \t/
R5 R11
(R,lp
RI (R4)m
0
/ _______________ \ RioD ...73) q
A¨N N--'r 1 '''''.
/ \...1_/ R
R5 ¨11
(RDP
NI...... XI-
(R4)m
,,..0
RI
Formula X1B Formula XLIB
wherein 1R_1, R2, R3, R4, R5, R10, R11, A, D. m, p and q are as defined above.
In another embodiment, compounds of the present invention are represented by
Formula X1C or X1IC as illustrated below, and the geometric isomers,
enantiomers,
diastereomers, racemates. pharmaceutically acceptable salts and solvates
thereof:
CI a
ci a
0 . Nr¨\--(CH2*-0
N .1¨\N¨(CflOw -0 \_____/
-
\____/
I
,N 1
R;
0 RI
Formula XIC Formula XIIC
wherein RI, is as defined above; and,
w iS 1, 2, 3, 4, 5, 6, 7, 8, 9, 10 or 11.
In another embodiment, compounds of the present invention are represented by
Formula X1D or XIID as illustrated below, and the geometric isomers,
enantiomers,
diastereomers, racemates, pharmaceutically acceptable salts and solvates
thereof:
39
CA 3035442 2019-03-01
(R3)q (R3)q
/ \ "D-......,----",/,, / __ \
R5--A¨G3 G4-B
I R5--A."--G3 G4--B
1
Vti
1
---.J
N'i N
(R2)P (RA)
X--i X---/K
0 O¨R,
Formula XID Formula XI1D
wherein, Xi, RI, R2, RA, R5, A, B, D, G3, G4, p, q, R10 and R11 are as defined
above.
In another embodiment, compounds of the present invention are represented by
Formula X1E or X_IIE as illustrated below, and the geometric isomers,
enantiomers,
diastereomers, racemates, pharmaceutically acceptable salts and solvates
thereof:
RiµloRi: RiooRi 1
(R3)q (R3:
igh (ILI I
rG4 I
1...;-=-Ri
G3--/
X.I4/ X,, (R)nL7-i X, N / A./G3) (R4)11L-1-
\ 0 1
R./(i) R, ,
_
R
Formula XIE Formula XIIE
wherein, X, RI, R2, R3, R4, A, D, G3, G4, In, q, r, Rio and R11 are as defined
above.
In another embodiment, compounds of the present invention are represented by
Formula XlE or )U1E as illustrated below, and the geometric isomers,
enantiomers,
diastereomers, racemates, pharmaceutically acceptable salts and solvates
thereof:
:
Cl Cl
Rio Ri I R.10 \_)11
)Ar D 7FFD
0 1 N
N-RI J N 110 N io N ,
xAs .
. ,o
R2 R, RI
14)
CA 3035442 2019-03-01
Formula XIF Formula XliF
wherein, X, RI, R2, D, r, Rio and RI 2 are as defined above.
In another embodiment, compounds of the present invention are represented by
Formula XIG or X EIG as illustrated below, or its geometric isomers,
enantiomers,
diastereomers, racemates, pharmaceutically acceptable salts and solvates
thereof:
11110.
CI
N..N)
CI
S'N
¨N
Ns
0 RI RI
Formula XIG Formula XllG
wherein RI, is as defined above.
In another embodiment, compounds of the present invention are represented by
Formula XIH or XIIH as illustrated below, and the geometric isomers,
enantiomers,
diastereomers, racemates, pharmaceutically acceptable salts and solvates
thereof:
..--R
xK
0 I
0
R5-A¨G rD
D
R5-A¨G3 G4-B
3 10) N=RI
11011
(R) 15 p (R2)
Formula XIH Formula XIIH
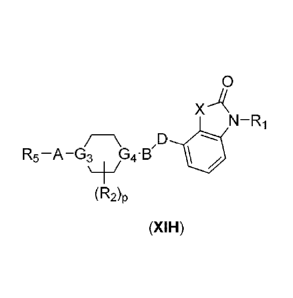
wherein, X, Ri, R2, R5, A, D, 63, G4 and p, are as defined above.
In another embodiment, compounds of the present invention are represented by
Formula XI-! or XIT-I as illustrated below, and the geometric isomers,
enantiomers,
diastereomers, racemates, pharmaceutically acceptable salts and solvates
thereof:
41
CA 3035442 2019-03-01
RI ==.,
o o
0 N-R, 0 Isl
N N N N
/
=
Formula XI-! Formula XII-I
wherein RI, is as defined above.
In another embodiment, compounds of the present invention are represented by
Formula XIJ or XID as illustrated below, and the geometric isomers,
enantiomers,
diastereomers, raccmates, pharmaceutically acceptable salts and solvates
thereof:
Rio
R5-A¨N/ q
N
R11 N-õ%s
(R=,)p
RI own,
0
Rio (R3) q
,
R5-A¨N r
RI] N.Nri"õõ,
(R2)I)
(R4)111
Formula XII Formula XIII
wherein, X, RI, R2, R3, Ra, R5, A, D, G3, G4, 1), Rio and RAI arc as defined
above.
In another embodiment, compounds of the present invention arc represented by
Formula XIK or XIII( as illustrated below, or its geometric isomers,
enantiomcrs,
diastereomers, racemates, pharmaceutically acceptable salts and solvates
thereof:
42
CA 3035442 2019-03-01
N --Py"--
1..õõ,.N,,,,----,,,,,---0,--v."-No N'i
Fi R'
Formula XIK Formula XI1K
wherein RI, is as defined above.
In a preferred embodiment a compound is selected from Table XI-XII. A more
preferred embodiment is a compound from Table XI-XII wherein R1 is selected
from
Table 1-4.
TABLE XI-XLI
5,...
1 :1 r--NN'N----\/ 7
1 a,----0
a I rµN---N...---Nz
N6r,
N...,,i 8
CI
2
io lq.õ_)
RiN / N
\
0
R1----0
Cl2Th ________________________________________ -, ___________
i ,
,-----
S'N --N
N, ¨N
0 RI 0µ
R1
-4 0 10 RI,
0\ N.,N
N N NN-40
41 *
43
CA 3035442 2019-03-01
11
N'Th
/L' N
6 12
N 0
In a more preferred embodiment, prodrugs of aripiprazole arc disclosed.
(Formula 1 and 7 from Table XI-XII). In a more preferred embodiment, a
compound of
Formula 1 wherein R1 is selected from table 1 is disclosed. In a more
preferred
5 embodiment, a compound of Formula 1 wherein R1 is selected from tables 2-
4 is
disclosed.
In a more preferred embodiment, prodrugs of dehydroaripiprazole are disclosed.
(Formula 2 and 8 from Table XI-XII). hi a more preferred embodiment, a
compound of
Formula 2 wherein R1 is selected from table l is disclosed. In a more
preferred
embodiment, a compound of Formula 2 wherein R1 is selected from tables 2-4 is
disclosed.
In a more preferred embodiment, prodrugs of ziprasidone are disclosed.
(Formula 3 and 9 from Table XI-XII). In a more preferred embodiment, a
compound of
Formula 3 wherein R1 is selected from table 1 is disclosed. In a more
preferred
embodiment, a compound of Formula 3 wherein R1 is selected from tables 2-4 is
disclosed.
In a more preferred embodiment, prodrugs of bifeprunox are disclosed. (Formula
4 and 11 from Table XI-XII). In a more preferred embodiment, a compound of
Formula
4 wherein R1 is selected from table 1 is disclosed. In a more preferred
embodiment, a
compound of Formula 4 wherein R1 is selected from tables 2-4 is disclosed.
Representative compounds according to the invention are those selected from
the
Tables A-I below and the geometric isomers, enantiomers, diastereomers,
racemates,
pharmaceutically acceptable salts and solvates thereof:
44
CA 3035442 2019-03-01
Table A
No 1Structure No Structure
1
CI r'N"-s"--"---"C) 60
CI
CI 0 N)CI so N.,)
0 N
O 0 1 0 0
2 ci N=\/`,..s0,, 61 c, r ,
1 1 N
CI 0 Ns,) y'', ' CI $ N,...)
O 0 0 0
3 CI 'N-'13 62
Cl 0 N,) CI 4.6. NI,...)
110 õ."..,,,,,,õ,.0,r,ON
O 0 0 0
4 CI (---NC) 63 C,
CI 0 N.,..) CI * N)
_fy80,11,-0,_,N
O 0 0 0
64 .
CI r-"N---N"-"--N," a [---"N"--------
CI 0 N) CI
0 0 N
....--
--ii--13y
O 0 0 0
,
6 0i r------N---,---,0 65 a ir\ N''.--7-N--'
CI Aim NõJ ci Oils)
CI, N)
0
N 0 /---N
11"0--/ )L0
0
1 I
CA 3035442 2019-03-01
________________________________________ - _____________________
7
CI i-----N---"------.-----" 66
CI r----Nr--'-----s"--".
CI 0 N ,...,) CI s N,)
0
N o/-1J
)L0
0
--)
8
CI r----N-----------0 67
CI
CI 0 N õ..õ....J CI . N,,)
0
N 0 T-N
)L0
0 r-----N
f \> 0
9 r-----N-----.....---õ0 68
a
c, 0 N.õ.õ....-1 CI s N,,..)
0
0 __./........;:r-j 0
*10
CI r'N"--"N"--"--C) 69
CI 0 N.õ....) 0 CI 0 N....)
, 0
----N 0
11
a r'N".....-."`"-- 70 a r-N.-------"--- D
CIN.,...õ...J CI 0 N.õ.-J
....r...õ
0
0
----N
0
..,LI 113
16
CA 3035442 2019-03-01
112 _______ '
a r-----N 71 a (--N-----------0 0
a fõ,... N,..J
ir 0
N CI 0 N)
0
N 0
.....,c, 113
13 ' 72 ________________________
ci f------N CI r¨N .-.'(:) 0
CI = N ,,-I CI 0 N.$)
0
Nsti-iict)'0--/N 0 A_ N)1-0
0
)) 8
14 73 a (---õN"---""\--.0
i,-.N,.0
. 0 N..õ.J a CI 0 N.õ)
0
0,T, N
0
0 ,-. 0 HO 0
15 a (....N,0 0 74 Oi
a 0 N...õ..,õ) CI 0 N,)
0
0 H0' 0
0
16 75 C
I r _________________ N......,......õ0
cl
CI 0 N) CI * N ,.õ,)
0
0 0 ,,.. 0
HO o
17
CI r-NN,0 0 76
CI 0 Nõ...) CI gilk IV ,J
0
WO
0 .,-. O N
0
0 HOy 0
47
CA 3035442 2019-03-01
= 18 I ' 77 I c,
a r---N"--.'-----'--"
ci, N,....) CIN.,..)
0
oõ..,....x
_
78 ci
CI (....-,N...--...õ..õ--õ,.õ0 r N`''''-'''-'()
CI N
19 ,)
0 I WI ,wi-Oy N
N
0 o
0 o o
20 CI (----N----'`="----'-'"0 79 , ci r---N--
--------
a, N,) a 0 N,,)
0 o,iro,_,N
0 0
0
21 rN--------"----0 80
CI a r---N--------- di
a 0 N,...) CI 0
0
N ----- y v
4-k0¨/ 0 0
0
22 rN--".....---,,,-0
CI 81 ci r---N--------- 0
ci 1. N.,,) a
0
IP CO0 N
ill H2N,..}.,N0 N
----- O o
H0 0
23 r,N,....,¨..õ-0 82
CI
CI dik N..i
0 CI
VP H2Nõ)..N---)(0.õN CI * N)
H 0 0
\ / 5 0 0
24 CI (--N-C) 83 ,--., ...-
....-...0
ci r N
CI diu. N.,)
0
WH2NN"--)r-0 CI 0 N.,õ)
`---jj"---N 0y0õ.N
H
...,..--,...., 0 0 I 1 1 0 0
I
,
48
CA 3035442 2019-03-01
25
c, (----N,.Ø....-,, !,,,,, I
CI rN --o
CI 0 N 0 I
-,..1õ-------' õ N CI 0 ,.) ,,J a
r
N 0Y N
H r Y
.....õ. 0 0 0 0
26 a (N' Y 85 0
CI 17-"N"N-----."---
CI Ai N ,)
WI CI 0 Nõ)
=-...,- 0y0,õ, N
0 0 0 0
27 I
CI (N y 86 ci r--N-,----
,o 0
c, 0 N ) CI ivi N .,J
0.......õ. N WI
HO'Thr -...ir 0....r,N
O 0 1 0
28 ci r---N--,,---0 87
ci 0 N,)
H a 0 N,)
N...õ,e..0 N =-.....õ--..r.0,y,N
II 0 I 0
0 0 0
29 '
CI r----- N '-"-- 88 a r---N---,----o
ci 0 NN) a 0 N)
0y0 N ==,,,,,,,,(0.,y,N
0 I 0
0 o 0
CI rN1...¨..õ..õ.õ¨..õ,0 89 GI r-----N,.0
ci,N,) ci * N)
II
=-.,õ,""' 0 N
Wir0,y,.N
O 0 0 I 0
31 CI r---N----.-0,,,, '90 ci
N) CI 0 , 0
-.....,..,-;.-1,- c 0 N,J
)cg,ir
O 0 0 0
I
49
CA 3035442 2019-03-01
. ..
. 32 I
CI r'N'-'-----"`---- '91
a (--N"----------.'"---0
CI 0 11.,,i CI 0 1,1,)
,,Thr-0.õN
o I o
O 0
33 ci r-----N-C) 92 CI
CI, I\1.) C CI Mr ai.6 1\1..õ-J
0 8
34
ci r--N--------,o 93
ci r"--'N'-'=-="----C) 0
CI, N,..)
CI =N)
O 0
CI
CI 0 N.,,..) air CI 0 N,_.)
0 N
=-.....- 0N
O 0
36 '
ci (----N--%'-'-`.--- 195 ci r"---nr"---"--" 0
CI 0 N,...õ) a
H
j.- y ......
HO 0 0
37 CI r-----N"-'s--/-s*---' __ 96 a
CI 0 N1')H0 a
f
Ni 0 N A-glirayN
1 ,
1 HO 0 0
38
c, r----N'-'"----'-' 97 a r----N----,----,---0
CI, N,)
0\\ CI dikb N,,I
IP )4.13.1r0,_,N
(-II
N-.) 0 , 0 õ..---...õ 0
/
I 1 _____________________
CA 3035442 2019-03-01
= . 139 CI r---N-----------o- CI r----
-N-----------0
a lath N.,,,)
>, __ 0 N CI 111P1- rat I \ I
ca e
o
e 1
0 0 .
I
O 0
C
CI r----N 99 ci r---,N-------0
*N 9
0
0 1
Ca2. 0-P-0,..õ..,N
0 8 1
0
\
41 100
CI r-rv,--,"() CI r---N--õ----s--o 0
CI 0 tµ1õ,õJ
0 CI riiik N ,..) e
0.) __ O N Mr 0
0 1
Ca2#
7---/
0---7- 0 8 0
/
42 101
CI r'''N'' a
a 0 N,.,.J a
Y-0
--N 0
O \
43 102
ci rN ci __ r---N----,-----õ0
cl * N)
O "" N 0
N
44 ___________________________________________________ -
Cl f---"N 103 Cl
CI 0 .N)
HN-,0N 0, ---N
Y-0
----N 0
0 0
0 )1)13
__________________________________________________
CI N''''- 1 ______________________ 104 ci r¨N"---
*'--"---*"--"
CI 0 N.,_) I
µ7 I CI iii6 N,)
HN -,====,..,---)(0.,....,N y, ' 14P O'N
0 0 ----N 0
0 )J)13
51
CA 3035442 2019-03-01
46 1 _______________________________ ----r __ .
CI r-- N--"\-----1)- 5 105
CI
0 N.,,..) a 0 N)
H o -----N
===(,.,)._N y0,.., N )L0
9 0 0
)8
47 CI r N,0 __ 106 c, rN-----*()
CI 0 N,,,) CI 0 N,_,-1
H
N 0 N 0 c-N
/k- )LN0
O 0 0_6 0
}.) 8
48 107
CI (Nõ.....0 CI ______________ 0
CI 0 N.,...) CI 0 N,....)
0
Ni0y0,,.N )1-0
O 0
49 CI 108 _______________________
(--NICI 0
CI 0 N,) 0,-) CI, N)
0¨c¨N
I I N)-0
0
O 0 /('-)71Y 1
,3J) 13
CI ,...-,N-----....---.-----0 109 cl
CI 0 N,.....) cl 0 N,)
0
, r-N
H2N-1 '=-'" N
O 0 0 0
51 CI r'N'..--'-- 110 a ____
r='''''N'''''''''''''-'. = ____
CI mith Nõ).,µ
ilir H2NThr0õ,,,N a fithi N õ....)
lir
O 0 0 I 0
52
CA 3035442 2019-03-01
52 1
CI (----'1N`'-'-''''',-- -"---"'"',, t 1 l
l I C,
a 0 N,....)
NH2 '''''r..'"' CI 0 N)
0 0 e''' 0 ,;-',-, 0
0 0
----1 C.
53 ( 112
CI(w-'-- CI (-N----,..----_-0 0
H2N
CI
CI 0 NN)
-Th( "---"N ----
0 0 -,, 0 0
I
C.----
I
54 113
c, ,----,N-------,--0 a r----N--0 0
a 0 N....) c, = N.,,)
NH2
===-=,-
õ..,-.,.. 0 0 0 0
1
..,-
\
55 a ct
CI (---"-N-"=,.."....-0 114
i--\
NH2
N , 0,,N
0 0 0
o
56 CI ,---N 115 CI
CI 0 NI) '
....--
N 1
H
01
I
I
53
CA 3035442 2019-03-01
=
57 I
CI r--N---,--,0 0 ,116 a
CI iiii N.,,..,) CI 0 N ...,...)
NH2
1111rH2N,..,,õ.,..--..,...,.1(0.,N
N---=
0 0 0 0
I
..-4:."-----.
'IN,,,-.
58 C i.--.N-""`,../`....- 117
I CI
CI Alb Ns,...) CI ilk N,)
0
lirN.--
Il 0 0 0 0
1
---
-,,
59 0 (---.N---.....--,....0 118
1 a r-N--,--0 =
CI , N,...õ--I ci iiih,, N.,..)
0
IP H2N,..,õ.1L,N ..,..õ0,,..N igr 0.. N
7
H 0 0 0 0
1
N.
Table B
No Structure No Structure
150 CI r'N'-'--7--'-'"C) 209 õ--",,. ,.............."--
,...õ.0
CI r N
ci * N.,....)
CI 0 NI,...)
I
.10r.oõN
1
o
II
0 0
151 Ci (---N-------. 0 ____ 210
CI ,---NC)-`.-'-
=
a ifik N,J
IIP ....õ.....ro,N I CI =N ,,)
o o
0 0
54
CA 3035442 2019-03-01
. .. , Cl r.N......0 to 211
CI
I CI 0 N.,.)
,....-.,,,...-..õ.õ.. 0 ,,,,0 ,....,.. N
II
O 0
153 212
a i----N-----------0 *
I CI 0 N.,...)
WI ...........-...,..õ.....(0.,,N
I
O 0
154
CI (---N----------0 . 213 ________________ ci r---N----,--..--
o
N CI 0 s...)
I
..õ-............ir-O-,N I
0 0 N
0 0 -'-er'13Y
O 0
155 a r---N.------,0 * 214
Cl * N,)
0 I a
N
'ThrilLO-/ 0 Cl *
0 N
o I
)-r-
.--11 0
1
156 215
a r--N------,0 0
CI 0 N.,...)
CI r-----N"'N"----0
0 N I
0 CI 0 N,)
0
0)L"
I)
157 Cl r-,---,o 0 216
Ciµ'air4,,.1
..,40_71,4
0
:
.......nN 0
CA 3035442 2019-03-01
158
217
01 r......N,..0 _____________________
c, . Nõ)
*
e, i N
0 I
I
0
....r...7:21 0
159 ' 218 ______________________
a r-----N
CI dui N.,....õ)
WO 0
N I 01 r---N---,------ 0
CI = N,_,....1
0
0
----N 0
160 219
CI = N,,..)
c, r..N..."...,,,-
...õ....õ0 0
0
N I CI 0 N..,...)
0
........N)L0
0
11 3
161 220 ________________________
, r,--..N,0
01 N,,,,...i
CI, will 0
N I ci r---N----,-------0
N,)
0
)475-N 0
113
162 221 ________________________
a i----N
CI( CI 0
k,zr, N ...,..)
ci r¨N------------0
0
Nõ)
N1`361(j0---/ 0
MT N
0
56
CA 3035442 2019-03-01
1 ________________________________________________________________
163 a r N ---s."-"--'`-r 222 CI r N"--------()
CI s N .õ..1 N CI 46 N.,)
o I
0
0 õ=-. 0
HO 0
164 223
CI r.N.¨.......¨......0 ____________________ ci r"N-------0 0
ci 0 N,J 0 ci * NN)
I I
o
I-100
165 224 ________________________
CI r-----N.".
ci 0 N....) ci . N.,)
0 I I
/ \ Ao_7 ,,w)r0N
0 9-100 0
166 225 '
CI (---N __________________________________ CI
a, ni.,) Cl 0 N,õ)
l I
o N
N.....-=
o 0 .,.. 0
HO 0
167 CI r---N---''''-"s'-' __ 226 CI
CI 0 N ,,,,,,i CI * N)0 I I
-1-) I0/No 0 0 N
9 I
.,.. 0
HO 0
57
CA 3035442 2019-03-01
. .
168 ' 227
CI N'-'-'. CI r-.N-'..*-=C)
CI.
0
N I CI 0 N.,,,,,I
4-11'0¨i
0 .---i 0 .-,-'-, 0
0 0
169 228
CI rN-..----s-"--".. . a r----N-"....--
",..,--
CI Ali N,)
WI 0
N I CI 0 N.,)
-..,..0y0,,,N I
0 0 0
170 229
a r'N^---,c) a r'N'----'"----
N.,..) *
46
lir 0
N I CI idils N,,)
Mr CO 0 N
CI I
..-...--
0 0 0
171 230
Cr (-----N-------------0 0 c, (--N----õ---,---0
.1 = N,J 0
c, s 0 N
....) ,
,
H2,..,}... N 1
N---ir ....
" 0 0 II
0 0
172 c, r-N---------,0 .
CI , 0 0 231 c, (N0W H2N \A 0 NyIJ CI 0 N.,)
N-y N-
H 1
0 0 1,1-^y 0,õ. N
0 0
I
58
CA 3035442 2019-03-01
173 -... = I,-z2 I -
CI r- NID
CI rN1-----------C
CI riki N a
j
I I
W H2NN 0roN,N 0.1(0 N
,..., 0 0 o
174 233 _______________________
Sr
a N...)r¨N-NrN-0 s
CI Cki5r,NON
0 H2NI.. II.....ive,0-..-- N I I NI- 01 N I
121
0 0 0
175 _____________________________________________________________
a (---N----.0 N 234ci 1,----N--N"----N-- 0
CI 0 N.,) 1 CI * N,...)
tc)0 N i
--ir ¨ y,õ...0y0N I
H2N,,A.0 0 0 o 0
176 235
CI, N,i CI ilk. Nj
1 I
HOir Mr --"N =)r-ON,,,N
0 0 0 I 0
177 ________________________________ 1236
CI (----N--N"----"---C) a r-----N
,
1 CI 0 N.) H 1 CI 0 N.,,)
I
=0 6
. 0 I 0
1
59
CA 3035442 2019-03-01
- 179
CI r'N'-'N-'(:) ' 237 I CI _____________
r11,......õ.....õ0
CI. N.õ--I CI * N.,..)
I
0.,,,0 N
II
,0 0 0 I 0
179 I 238
(-7µ111 0 CI r----N----,-----,--0
CI N.,) CI a 1µ1..)
I 1
0,,,A -.....,õ..."..,ii0.y.N
I
= _________________________ dik, =
1111P 0 I 0
180 239 _______________________
CIr-----N---'-----.""" ci rN"--'"----
---===' 0
Cl * N) 0
0 N I CI * IN,)
,,,A.ir 0,y, N I
,..---
I
0 0 0 0
181 CI 240 r--N--,,----0 ci r-----N---------
--
CI O N..,) Cl I Ali N.,..J
IPS I
I I I
0 0 0 ' 0
182 241
CI r-----N Cl
CI 0 NI. CI $
I I
0 0 0 0
1
CA 3035442 2019-03-01
. .
183 CI 242 '
r'-'-'N''''-"..----() _____________ ci (--14-0
CI, 14,..)
is 1\1,,i
I
0 CI N
I
0 0
0 .7N 0
184 243
CI r----N---,---,--0 _______________________ a r----N-------õ0 0
0 to N,_.)
1 1
0 N 0,T,N
.....,
0 0
185 244
a r---N----,_-----o = 0
CI * Isl.õ,) CI lab N)I
IV f 1 I 1 0 N 0 N Y wi y
HO 0 0 0 ,õ.õ 0
186 245
CI (--N-A 0 CI r---N----------0 so
ci iik NJ
VP- FICK-')
N 0 N I CI 0 Nõ,..)
.,{4-EtIrOyN I
f )or HO 0 0 )õ 0
187 246
CI (---14(3 CI r...-"'N0
CI 0 N.,..) 0\\ CI = N..,)
I I
0,..,...N r'N A<TrOyN
N-) 0 0 õ..-1õ.... 0
61
CA 3035442 2019-03-01
183 247
CI (-----NC. a r---- N"-------'"---C)
N CI 46 ,õ...,)
Mr IR\
CI gal N,_)
WI Ca e
o
o ,
24 O-P-0 N
it ====' I
01 0
0 0
01
=
189 CI rt\r-13 _____ 0 248 CI rN-------0
ci = Nõ) e
c=\ a 0 , 0
µ ________________________________ 0 N NI)I 9 i
Ca2+ O-P-0 N
õ--.....- 8 T
0 0 0
\
= .
190 ______________________________________________________________
CI (N--N 249 a 0
0 I
0 0 N,..) CI 0 ,,,i 9
0
INI
, 0 " Ca" O-P-0
N I
ti '"'-'
/-*---/ 0
0--/-s 0 ,".., 0
/
191 250
CI r''''NID CI r-N,-,,..õ----.õ...0
CI 0 Nõ,) CI icki N,µõ)
I 0.....0,...,N I
lir 0
--N)-0
0 0
\
i 92 CI r-NC)' 251 CI r---N-----------0
CI 0 I\1 0 ,) CI NJ
,I, Ili .1
, 7O-N.--I`I,õ--j- I 0 __ N 1
ii )L0
0 ----N 0
\
62
CA 3035442 2019-03-01
'
193 __________________________________ 252
CI r----N---N"-----."---- CI r---N0
CI s N,õJ CI, N.,,)
I
)L 0
0 0 ---'-'- N 0
)13
194 CI __________________ 253
r--N--,-----õo 0
CI, N.) ,,.. CI . N ........)
I
HN(0,...,.N 0 cN I
)-0
0 0 --N 0
õSi )13
195 0 _____ 254
CI 0 I (N'-" 0
CI s CI * N.,..,...J
H I
<õ,)9N,r.0,õ.N
0
0 0 A--N 0
)) 8
196 __________________________________ 255
CI r''''N
N,,,)
CI
giP 0 N
0 0 \)L0,1
197
CI r'N -''-'1C) 256
CI 0 Ni a r---NC) a
I CI 0 1.1õ)
II 0 ----N I
0 0 o-0
0
}-) 13
63
CA 3035442 2019-03-01
198 257
CI
CI 0 Nõ) cy-, CI r-N----- I.
ci 0 N,..)
I I 0-4)¨N I
0 0
0
õJr) 13
199
a 258
a
r-N .....0
,.......õ..,....õ, rN---,----,0
CI. N,_,,J , c, s N.,õ)
H2N,¨y0õ..N I 0 I
/---N
)-0
0 0 --ID 0
200 259'
CI r--,,1"-s.."----"--- ci 1----N"----
--N-- 0
CI 0 NI,...,,i A-1 CI . N,....)
H2N--y "---N
0 0 0 I 0
201 01 r,õ--,...--,-.0 260 Cl
CI N,$)
RP NH2
0 N
^...- I CI s N)
0 0
r 0 ..... 0
0 0
1--.
64
CA 3035442 2019-03-01
1261 _____________________________________________________________ .
...
, CI r-IV --'----'() CI r-----N-----
----,0 0
ci,....),, NiJ N ) '`,./ CI 0 ,,,)
õ...,;N,õ,-,-.1r0 N I
H2N Tr ---
0 0 -, o 0
I
-x---..,--
203 CI 262 ,-----N.---..,,,-,...õ,..0
CI r--,---,---,0 0
CI . N.,..) CI Isl..õ)
NH2
I I
,,ac ji0.,,N Ors,õ....x. N
====,..-=
0 0
I
204 263 CI CI __________
ar----N------------0
ci 0N....) NH2 \ ___i * r---
\N,0 0
Halr,s,õ,L11,0 N I 1
0 0 0 r .,
0 o
205 264
a r--- (:)
CI, N .,..) CI N.)
H 0 0 0
,
1
\----- ---.'.----
CA 3035442 2019-03-01
206 ! CI 1------N 265 CI r----N----------,0
. CI N.,) CI
. di NH2
s..--
0 0 0
I
..." ',.,
207 CI 266
rN---...'-r----'-'- 0 CI rN--,---.--0 0
N__õ,i 0 a * 0 N
Nõ)
Ur H2N,11,,0 N I I
.,.,,
0 0 0 0
1
...-
208 0 267
CI r---N * CI r-NN"-.----"-
's--". *
a Ail N ,...) a N,)
0
1111111 f * H2N,,IL.NThr.0 0- -=N
õ..,,N I I
H 0 0 ....is-NsIr 0
I
).õ...,.....:%."---",
Table C
No, Strucnire
400 0 415 0
1
0 N--\Cc-I
o---\ 0 N 0
N N 0,ty
NN . HO
1
66
CA 3035442 2019-03-01
. . _ .. . . .
401 0 410 0
0
0).LNj
r--s,
N N * 9
N N *
402 9 417
. C1
0 0
0 W-...\ //
0-- \
N * ON'3
N
µI13
\-1
* * Nr-NN =
\--J
403 0 418 0
--k. 0 \ 0 0-4\13
N N * 15
\--/
N N *
404 0 419 0
0AN o
U9
\-..._.= / Nr- \N 411111)( 6
\......-/
1
405 0 420 0
r0
0 N h 0 NJ-\N 0j4:41
0--\
0)!µij f
rTh
.
1
i ________________________________________
406 0 421 0
--1,.. 0 1 0
1 0 nr-N // 0
N / ,J , --\ 0--\N--I -Y ,----
0A N . 'a 9 N
\ 10
\____/ IMN =
\_...._/
67
CA 3035442 2019-03-01
407 0 422 0
)' 0 N_.. 0
ii 0 0
oXNj --11\4?
N N
µ 12
NN .\__/
408 0 423 0
.-k 0 0
0 147\ p o'll'N--\ .......
0-N
HN 14111 0
Ni¨\N * /TN #
= *
409 0 424 0
)c 0 ..k 0
0 N- \ A 0 N\ .....,
rTh 0
NN == W.') N N *
410 0 425 0
0)( -( N 0
...111? ---4. 0
0
Ni--isi * 0 )9
r¨\ 0\
N N *
411 0 426 0
0 --JIN 0 0
)(N-"\ f/ 0
0
11-\N . 0--)) 9 Ni---\N #
..._/
412
OH 427 0
0
0 N 0 1,7"-\ ....4so
/---\ 0
Nr-\1 . 1\ N
\___../ .
,
_
I I I
68
CA 3035442 2019-03-01
...
= 413 0 428 0
A
.-)Q 0
0 N-Acrico
C W--10 j\ /"--\ .
414 0 429 _______________________
0 0......_ Jt. 0
0AN_J 0 N--Ncric
N/- \ x *
Nr-NN * 4*
430 431
4,
= =
I 0 __
õ...\ A
I. . ,
,_,
o_d-N\ ii
/N *
432 ) 433
=
..... 0
. N . 0_6-Tr\N
0
/ \
I
#
1
/
434 0 435
0 N-A bN-Mb
0
N N II N --0
I _
\ - /
1 /
69
CA 3035442 2019-03-01
-- - - 436 0 437
Nr--\N
---0
, ____________________________
I
-
,
Table I)
No. Structure
501
517
IP
ONNO igir Cr,..
O
O,NN tslrl
IN.,..õ.--.)
<0:-0
502
AI. N
518
ailli
Mr N"i :-.--M....Q...
ON
N "=-=
NO
-Th ON;
) ctµ1,-,)
0)
503 519
14---') 0 N N.=-==0 Ni ,-...
0 NN 0
o)
0
0---Lo
CA 3035442 2019-03-01
...
504 . 520 !
--_,MN'Th 0 N N 0 IsiN) ONNO
0)
0
505 = 521 .
1101 tsr.- I
ONNO
ONNO
N".".)
.....v60-.{j
---k-(5.--µ0 0
506
it. . 522
4 if
1111=1 1' . = , - =',z,'.'''sµ'r -- . . . - i
- - . WI I X r" ' " I . . = . .
N'Th ONNO N.") ONNO
L,..,.N.........."......) c,N.,,,,,N.)
o)
0)
.s10
507
523 0
.
N 0 N N'...0 Isr'Ni ONNO
c..õ.N.,..........,..)
o) N
o)
---k-0 o
508
O 524
O N.'-')ONNO;Cr
N'ITh
ONNO
0) 0
1c)
1
509 525 !
..--%',,-----)
h !
,--1-n
1 N N .---, ..-'=
0 N 0 N'Th ONNO
0)
1
71
CA 3035442 2019-03-01
. . .
510
dif 526 5
WI N 'Th 0 N N ''.r 0 0
WM I
ON NO
1......,,. N ..õ..............õ...-I (1µ1,., )
0)
511
A ill 527 *
N 'Th ON N 0 N'Th ON NO
1=,,,. N .,,...,=,.) 1,,,,,.Nõ,,,,...)
0) 0)
...\4109-le
co 0
512
1660 528 5
11111r -. I
N'Th ONNO WM ON NO
0)
"142 1'1-o
0
513 529 5
.-.."-----N,
I
0 ....: 1
N ^) o'N ^ N''.0 NrTh ON NO
)
0)
514 ' 0 530
N)--1 0)N)'' N 0 N'Th
0)1
C:7)0 Tr(1
_I
72
CA 3035442 2019-03-01
515
AI. 531 .
11 I 1 r a
N'Th ON NO * N"---.) ONO
CrLO 0,1(
Cf 0
516
532
4=
tit 0
-- - x - - - - ...,
0
x:: '.== Cr I .e%
411" N "Th 0 N N 0 N'Th ONNO
(õ.N.,..õ.".......)do-1 )
CrLO
533
1 I 111 534 .
4 r a
0 1
N ' ) 0ri N N 0 N"....) ONNO
=
= 1...õ. N ..õ,...,..,..) ),y0
0)-f0y1.N.õ_,..-
0.,...õ--
r-N
Is)
/
535 536 1
NrTh 0ANN
0l N"..0
0)
0,----
0 - 0
/
\ ---
/
[
73
CA 3035442 2019-03-01
537 538
1101 I
. I
o N N 0
N-Th 0 N N 0 N-Th
o)
0
cIJL0
0
539 = 540
N'Th
ryTh
ONNO
isleTh
0
OANNO
0
In another embodiment, the invention relates to a compound of formula LI and
LIU
/¨
N 0
Ri
N
> _______________________ N
\Ri N
N 1101 N
N
Formula LI Formula LII
In another aspect of the invention, compounds of formula LI and LII are
selected
from Table E and F:
74
CA 3035442 2019-03-01
= - Table E
No Structure No Structure
700 F 759 F\sci,
. Nr"..0
1,(-0
0 N\_.1 \ 1-1,k 0 Ni,hiNaN)-:\ -0
\--0 6>---
e----"\ 7- \
>
0
. 701 F 760 F\c:i
/ aN
)--r\
0 )¨N\ ) N_\ c> ) = IshNN
i¨t:0(--(
0
702 F 761 F7)\)
*
rki-o
= Ni-ri--->_1-N\__0>__>__
iiii N\ ./-\\ .)--,N\_0 crp
lir r-- \ /--- \
\
o
703 F 762 'F
fi\--0 N(\--O
0-NX-N-0 0
4110 N>
ICNi/ \ /)---N,
- \ -N 0
\ \---j N.---14 \ Y 1'4
o
704 F 763 F
= e 111-0
N/---.0
0
)-N 0 / x N -0 0
NN.hr4\ _ \ y 13
0
CA 3035442 2019-03-01
_ .
.
705 F 764 F
=
0
N Ni_Nais= \ _cy)8 O Niti--)__
N\
N
T
706 F * 765 F)::
fs0=0
O Nii
No_Nf cy),0
\ 0 7
o
707 F 766 F\0)
d----,0
N)--/--)- -'1
* )-0-1\ \---y),2 0
o
708 F /67 F\( /
.
N/\----0 /rry
\-0 N
10Q--NO-<- T.
.
h3
N \
o
709 F
= 768 F *
/--- \ N --r---)=.0
N
0 N)--0--N \ N\---0,1)) 14 fial N/hisi(
IVI, N
N
0
710 F
* 769 F .
1\,_9=
0 Nn=.0
\ N iiii N/>_/---)._
\ y * N>.--Nr)--N \____cy/16 WI N ' \
N \ 0
0
76
CA 3035442 2019-03-01
711 F 770 F
. =
0
)-Nr-}-N\
la 18
o
712 F 771 a
. CI =N)
N/0 0 /---N
so
0
N
713 Ft) \772 F
*
NtrO N0
7--N\ =
* )-0-N\ A6 N>....N\D__
- --1))22 N
lir N
HCrtY
0
714 F 773 F
= *
11---.
N
* N>__N/-->)0 ,.,
NI
N \ .
0 HO
715 F 774 F
. *
0 N / \ ,--N N /
---N N \--0, 0 N)-----N\
N \ / - - - \
HO a 0
o
716 F 775 F
e .
N/----0 /----
N '0
)___N I
,--"'",=,..r-N>___ / N\ )___N\Y-N\___0
0
HO 6
0 0
77
CA 3035442 2019-03-01
_____________ .. . _ 717 F 776
F:::\ki
*
NI--o N o
\cr
* N> w 0 N
N. ___>Na_1-- y) e.<\ -0 14
H 0
718 Ft 777 F\o)
NO
\
-1\ ..._(),.ir,( 8
N
719 Ftz 778 Fz))
r,v7o iv --/---o
)--
rai Nx_No_r-ri N -\_
lir- N \ \-- --ew
T
720 F 779 = N
0 Ft),
.
Ntr'0 11:=)= 0
.
721 F 780 F
* .
N -/---0 0 /
N)____NO
= N\ \_0õ,,c
NH2 N II
0
722 rti: 7g1 F
N/": *0 o (-* ( N1/\
0 NN?___N _____________________ [sriy
N
NH2 *rN/>--11\
)---N>-- \---0,0
0 ,1)-- \
II
I 0
78
CA 3 0 354 42 2 0 1 9-0 3-01
... _ ... . .
723 F ' - 782 F
NH2
0
N li
0
724 F:::: 783
Ni=0
0
0 oi
Nri___Na:?-f--\
NHz
725 F 784
*
tr--0 Np=..... \......;-(1
Aril isl( )--N?-
\
0 ,N
II
N 0
H2N
I
726 F 785 IF
1 N 0 Ni\---0
jn=
N
N / )--N
\O.,..,0
0 1-1\ )--- 0 1-1}- ?-\ )--y
HO/
727 F 786 1 F
N --/ 0 0 C
1 -N7
1
0 5 )---N)---N\ --0)( 0 Nr
/ \ \ H N>
N o
728 F 787 F arrik
gr
Nr-----0 N/170
0
/ )___ N C).__N/--).)-N
N)--\ \-0)(9
N \ \ \
1 o
79
CA 3035442 2 0 1 9-03-0 1
.. _ .. .
. . . _..
- 729 F 788
* NI---0,.._ Nti=
0 N Nr)---NI N\ -0).P
N \ 0
o
730 F 789 F
. .
NT---0 N/=)=0
0 0
N \
731 F 790 Fz)
*
N/---0 NIJ .
)___N i--"
N__/ 0 N)._140_44
\
N
0 1 . \ ---N\ \---)--(
0
732 F * 791 F
lik
NO N0
)¨N
* >-/ \ y-N\_0
cK
N \ r \ I
ONN>___Nr)__N\ o,
11
o
733 F 792 F
=
Nt 0
N µ0
0 0
N
1 Nr)--N
>--N\ ___O
\ )---0
o
734 F 793 F
= _/-10 N/---0
ipr-Nµ / \ N)--\
\--0 / \ 1
\
\ ) Ni- \ I- \ Olr
0
1
CA 3035442 2019-03-01
. . ..¨.. 735 F):0: 794 F
jON
i¨NIN
0
736 795 F
9
N 14)=0
N Ckfc)_0_1\
o
737 F=z:::)\) N 796
r="- \µ),.,0 fc--0
0 / 0N
738 9 797 F F milk
W
. tqc,
o ),--N 0
* N N,o .
_NN\ 2.
0 N)õ,õ)....õ,___N
8
N 4o
739 F
798
=1\ F =1/---- \
N 0 ---- N \---/-0
)--N 0
N N
0
* >.__No¨ 0 NN--0¨N\ )¨o+8
Ca2'
N'-- C-C.
8
N \ 0
740 799
F F jak
*0
ti-0 --) >--N 1 1
k___ 0 NN,>-- NO-- u\,,.?--
N
r-r\--o--co
g
=
81
CA 3035442 2019-03-01
.. .
741 F 800 F
-
Ilik *
N=O \
N¨
*N>tsr--x_N \--- _ * N¨ND¨N \ Ni-0--
N \ A N 0
742 F 301 F
= *
Nr 0 "N¨
: --
N ¨N
0 ¨11/..-)¨N\,,,,(0--µ
\_0.--- N 0
. NNO¨Nr:
743 F 802 F
. ¨ ----? NNONH * /---
-\ tili\-- \p-a13
01
,4 N
N)--NO¨N)¨N\--06./y5 N 0
N \
744 \ 803 F
F
*
* (0 Nr:x_rt0
\N.4_,(13
N(-0 NH 0 NN--ND¨N\.,,,,(-0-io
* N) 0 N)¨N\--0)
N \
0
745 F 804 F
= * (Ka
N ,
8
N(0 d\----0 rµi.4._4
)--N 7(
1-4
al r,,._N \
, -N>--N\-D.i
* )-1---)-N \--0 ---N IIP- N \ / \ /
N \ \ o \\O
746 F 1805 - F
e\ = /--\
(c.8 ,
i <0\ Nµ C:31
N ( 48
, .. .___Z
* 1\1-Ni--)-N):1-0--i
* N\ NI/ \ _NI Nv_o
I N 0
e \ / \ 0
82
CA 3035442 2019-03-01
747 F 806 F .
7)--
*
O NhN o_N\
N f-\ N --/---0 414
_
,--N
W ---N\_}--N\-0--µN 13
N 0
_
748 F 807 F
. *
2=la o 14 /s1=- 0
4)(
. hi_No_i 13
Ai
VP N \
a IMP N %
749 Fzs. 808 F
*
0 N).__Nal-\_cric..õ
NH2 0 N,)_NaN)-N\_0_
0-
N N \ 0
750 F\r3 809 F
N7\c:)
0 Ni/ --N )---Ni \-0--4-2 ,NH 2 . rµj-NO-N 1-0
\ \ N \
0
751 F 810 F
/--
.
N ---/-0
)---N * N _41-7;
--N( ) )--N\ \-o 101 N--Nr-)-N )-0
N NH2 N 0
0 JO
\
752 F 811 F
* *
0
N(-\h krt....õ. Ni\---0
N )___. )---N,
)-N/ \ \-0 40 N/>N/ )=-N\_____0
NH2 N \
N \ )-N\ 0
-..,.
\ -...
----,
/
/
83
CA 3035442 2019-03-01
753 F 812 F
Na--Nr-toO
N112
0
0
754 FZ)f 813 F
N1-0¨N\ 1`1112 Nr"--, 0
755 F 1814 F
41.
Nr--O
)0 0 H
)¨N
IS_Ni \__N
N \ 0
756 F 815 F
H2N
0
= )¨N( )r0
N¨N1--)¨N /
NH2 N 0
0
84
CA 3035442 2019-03-01
757 F 816 F
=
0 NYII2=
N
1101
N 0
0
758 Fti) 817 F
tr)-0
CC-N/D-N\ Nr--=0
NI12 \
N \ 0
Table F
No Structure No Structure
900 F 927 F
=
NO N %
r-N
N
0
901 F 928 F
\ 0
N
,/\N) 0\ )
CA 3035442 2019-03-01
. . . ... ,
.... . .. .. _ .. ..
902 F 929
eri---).--o
N
. ).--NI\ j-
N-N)-- 0 >cd-- Cr--NO--N\)¨N (Ci_cfp
N
N \
903 F * 930 Fz)
0 N).__Na.)-\ --N <>5
\ yl=-rf
N
= o
904 F p31 F.r:21)
.
Ni=)--00--
14-11
* NiN( .....N\
905 932 F
Nl\ ),s( --0,
N
O NN/ Nr)--N).-- oy, 8 0
\ N \
6.1
906 F 933 F
. . N N > r->--0
/-- r
0 \ i \ N)-N 21)3 ) 10
01.N,,L,
Nr¨ \ 1¨ \ 101 .
o
907 F 934 F
. /- =
Pl---)--0
I 1--N0._ 0 h 2 .
0 NN>--NO-N\ 0II N
f \ ' ..õ---- s
0 ,
0
86
CA 3035442 2019-03-01
908 F 935 Fµr:))
..r,..N ...j"-
N "---N
0 ) 13 0
909 F
= 936 F 0
10-- Ocr
1-)---c,
. Ni_o___)_\ ____N oy
) 14 0 0--Nalr:
910 F 937
Nc)-0 Ny'fiv -Os,
iiii. N)NDN 2.1.4.\ ) 16 0
ckiN,4.6
lir N \
911 F Cl--o 938 F
* --
Ny_tir-=\/-0>,11)13
\,
)18
N /-- \ 0 Ni¨Na-N\
oiN-K3
N>r\ r-N \ . e
o
912 F 939 F
/-
N
\>--- 0
N (....}._ N \ ,-1,11
N)
N>---N N
\ 0>,1,c\ ) 20 0 N /
IN\ / N\ 0 I
)-0
8 ,
8
87
CA 3035442 2019-03-01
. ,
. , .., ,....., . ... ... .
- 913 F 940 F
* *
Nr-)--0 0 ti_N
N/D.27)--0 .,.,
N a,_N ze, )--N
* i?--N õ )22 N N.,,,,,0
\
OI
o
914 Ft) 941 F):::
rc.>-o
= )....."->)--N N
0 N-C\-hin
N\
%.,
915 F02)\? 942 F
.
N()--0
c}->Nr....N )--N > r----
0 N)....0_,\ ril 0 N>...._Ni x...N
Nti2 N \ \ 0y0 r.
o
916 Ft 943 F
e
0 ")--d<
NH2
N \ 0
110
0
9 917 F 44 F .
. /-
OCNN)--tsia-N\ N0
0 NH2 00
\
ll
0
918 Fr.)) 945 F
NH2 1 N /
0 > N
-11 I
0
88
CA 3035442 2019-03-01
- 919 F . 946 F --
)._.N
N
$ r>-NNril ? 11
CN-1) la 1.--1\--)---N\ ?---0-f
H2N
920 F
. 947 F .
11--)-(k2 N
0-;P-8 Caz
0 Ni_NaN ox * 1-NO-N\
8
\
Ho
921 948 F
* *
N
p-0,N9 N )-N V...
*
\
\ H N 0
922 F 949 F
.
1---)-o 0 9
N 19
0 NN\ \ N,..,,, < ,iL --/-:---7
0 0 * Nr\...)._N}-N d
T- i \ 7- \
N 0
/
/
923 F 1950 F
. =1I\ 0 /,---
N N -0
40 N,____N, NX-N ) 0 N._t\i/ \_...NX-N >
N \ / \ 0
I \ /
,- =-=,
"N ----
89
CA 3035442 2019-03-01
924 ¨ F 951 F
)¨N N /
N¨ND¨N )--N)s¨N
\ 0 N 0 ¨0
0
925 F 952 F
N
)¨N
110 \ 0 N \ 0
= ¨0
0
926 F 953 F
N/=)-0
N/>___N/
N N 0
0
Lr
Compounds of formula DC, X, XI, XII and in particular compounds of tables A-D
are useful for the treatment of neurological and psychiatric disorders
including
schizophrenia, mania, anxiety and bipolar disease. These compounds provide
sustained
release of parent pharmacophores by cleavage of the labile moiety, R. As such,
the
compounds of formula IX, X, XI, XII and in particular compounds of tables A-D
are
CA 3035442 2019-03-01
useful for the treatment of neurological disorders by providing sustained
release of
parent drugs.
In another embodiment, compounds of the present invention are represented by
formula XIII or XIV as illustrated below, or its geometric isomers,
enantiomers,
diastereomers, racemates, pharmaceutically acceptable salts and solvates
thereof
0 RI
, K100
R103¨ I \
K-101 R101
R102 /
,X100 R102 '100
Formula XIII Formula XIV
wherein R100, Rol, R102, and R103 are independently selected from absent,
hydrogen,
halogen, -OR.10, -NRioRii-, optionally substituted aliphatic, optionally
substituted
aryl or aryl or optionally substituted heterocyclyl;
alternatively, two Rioo, and R101 together with the atoms they are attached
and any
intervening atoms form an optionally substituted ring; and,
X100 is ¨CH- or ¨N-.
A preferred embodiment is a compound selected from Table XII1-XIV. A more
preferred embodiment is a compound from Table XIII-XIV wherein R1 is selected
from
tables 1-4.
91
CA 3035442 2019-03-01
Table XIH-XIV
RI NI b 11 R1
I 1 0
dil
Br IP" ----N 0 11
B ----N
\ /4
\ 11
2
RI 0 12 R1
\
µ
iiii Ni 0
0
0 N---....,_.(0
CI 411Pj -----N OH
Cl ---N OH
*
R1 13 R
3
L_..i o
02N -----N
02N -----N
Cl
CI
Rj 14 RI
4 \ 0
Ni 10
CI N
0
CI
92
CA 3035442 2019-03-01
. _ ..
R - 15
V 0 ..._540"-R1_\
CI NH 0 Cl NH 0
F F
RI 16 R1
6 1- 0
N-- OH .._____
5_ 0 N_`0
OH
Cl
CI NH
F
0 F
R 17
7 µi 0 RI
Ni 0
CI N 1
0- CI N
Cl
R1 IS RI
8 \ o µ
NI 0
NI
02N ----N
02N --N
1 I
,
,
,
Ri 19
9 V 0 N----f RI
5
1 .
Cl''''''''''"=-=---- ' N CI 1%1....
0
0----
93
CA 3035442 2019-03-01
_
10 rxNi
BT
Br
0
PRODRUGS OF ACYLANILINES
In another embodiment, compounds of the present invention are represented by
formula XV or XVI as illustrated below, or its geometric isomers, enantiomers,
5 diastereomers, racemates, pharmaceutically acceptable salts and
solvates thereof:
R50 R1 R50
R51 NI yR55 R51 1\j/R55
0
R52 R54 R52 R54 RI
R53 R53
Formula XV Formula XVI
wherein R1 is as defined above;
each Rso, R51, R52, R53, R54 and R55 is independently selected from hydrogen,
halogen, -
10 0R10, -SR10, -NRioRil-, optionally substituted aliphatic, optionally
substituted aryl or
aryl or optionally substituted heterocyclyl;
alternatively, two or more R50, R51, R52, R53, R54 and R55 together with the
atoms to
which they are attached form an optionally substituted ring.
A preferred embodiment is a compound selected from Table XV-XV1. A more
15 preferred embodiment is a compound from Table XV-XVI wherein R1 is
selected from
tables 1-4.
94
CA 3035442 2019-03-01
_ _.. .. .
..
- - - ' ----- - - - - - - - Table XV-XVI
1 40
H H
I Ri.,0
./..\AN
1
RI
2
r 41
..1.
111,,,, N., r
,õ,
3.1-----N,.--
H
-)N iv
H
3 R
--IN 0 OH 42 OH
I VI:NI *
R1
4 43
0 0 0
)LS 0 OH 0 OH
NI N
RI
_
RI 0 44
NI
*NH NA
01H * N.'s OH
0
R,
6 OH 45
OH
0
N
lit1
7 . RI 46
1\tk..../
i
N
I
* 0 O.,
0 RI N 0 IN
0
CA 3035442 2019-03-01
.
. _
8 47
=-=õ,,,,,.-0%.,,
R1 1
1
0 O.,
R1
9 Iii 48
IT0-'C'' Ho'Cri
_ ________________________________________________________________
10ilt1 49
0/RI
N
NH )=--N
4t1,1--F NH F
I * F
CI
N I ,,, 0 I
NH N
/
/NH
11 HQH9 50
RI
C Cr14 OH
1
F F
12 ? RI 51 0
1
'''I'''' = . . N:-..õ,.. --'
I
0 1110--- 0 RI0
13 OH 52 OH 0
RI OH OH
U
1
NI
N \
-
0 R 1,"0
96
CA 3035442 2019-03-01
,
14 ii fitoe 53 0 1,,,,y,õ-
..,..1 oi:)..
Ø0
e3c 0 N F3
0 0
NC F N RI F
15 R1 54
I
Br Br RI
0
16 RI 55
I .I\IY
0 Ny--........õ....-
../'-= O 0., OH
R1
. 0 0 OH
17 ,........,.. 56
R1
I
INT..r. ..õ, = * I.=N
N
18 57
'N-f-N RI
NI
N 0 0 N
1
INT 58
9
---isit (----\
N
oc,I..15)^-NH "-OH
.1 CI 'Iti
I
---
R1
I
1 0
0
Rr,
1 1
97
CA 3035442 2019-03-01
. .
RI
= N
Ri
22 FyL 61
0,.
02N 02N RI
23 62 I
0_ jrNU Ri 0 NLN
NI
a .
= 0 0,RI
24 H RI 63 on
H I H
N N,
. N
0
6 = ' =H R,
m
25 64
.lit 0
0 N / 1\41,
0 0
F3C F3C R1
26 65
C..., a
R1 N N
NIIrk
0 o
RI
98
CA 3035442 2019-03-01
..
...., ..._..
27 R1 66
I
N¨ /--
N
0 )
* N
?
28 11 1 67 1
H ..),1 Hso
H N * NycH
HeY 0 N yLH
0
'Ili
CN CN
29 R1 .,..,^...,.. 68 õ....,--..,õ
I
N.)
Ny-,_N,.-
0 0 I *
RI
30 69
0 0.y113
RI
1
0 N 0 NTO1 N
S RI
31 ( ,0 70 &
R1 N N
I
Ny
LL Y-
0 LJ 'R1
_
99
CA 3035442 2019-03-01
_ .
... ._. . _ ._,......
32 CI 71 Cl
CI RI CI
02N
NJJ
02N RI
NN, 0
$ 0 OH 0 O. OH
R1 72
I
:1
R1
NO2 NO2
34
.N1 73
.N1
RI
I
N
* Nµir-2\11 0 .õN.1
0 0õ
. R1
35 RI 74
1
0 isLT.
0 Nr.
=Ii RI
ri .
1`,0
H
36 R1 75
1
0 r0
37 RI 76
1
iki Ny'N'-') OH
lir 0 l,_,N
II0
I
100
CA 3035442 2019-03-01
-
38 77
=.,s. =,...,
RI
I
Ri
39 Ri ilit 78
H SI NI I IV 0-
1
= H
a 0,
RI
C1
Thiazoildinones
In another embodiment, compounds of the present invention are represented by
formula XVll, XVIII or XIX as illustrated below, and the geometric isomers,
enantiomers, diastereomers, racemates, pharmaceutically acceptable salts and
solvates
thereof:
0
R,
0 \ FI ,N_____1(
0
F
S'''---\( S--õ_.<
/
0 0 RI
Formula XVII Formula XVIII Formula XIX
wherein F1 and R1 are as defined above.
A preferred embodiment is a compound of formula )0C, XXI or XXII as
illustrated below, and the geometric isomers, enantiomers, diastereomers,
racemates,
pharmaceutically acceptable salts and 1Øvates theieof:
101
CA 3035442 2019-03-01
Cy2,
X5
S
c
y
0 0
Formula XX Formula XXI
Cy2
X5
SN
0
Rr
Formula XXII
wherein Ri is as defined above;
Cy2 is an optionally substituted heterocyclic ring; and,
X5 is selected from absent, -S-, -0-, -S(0)-, -S(0)2-, -N(Rio)-, -C(0)-, -
C(012.10)(R11)-, -
[C(R1o)(R101,-, -0[C(RIARI tAv-, -0[C(R10)(R11)]0-,-S[C(R10)(RI -
Nit12[C(R10)(Ri -NR.12[C(R10)(Ri1)], S-, -S[C(Rio)(Rii)],-, -
C(0)[C(12.10)(R11)]v-,
and -C(Rio)=C(Rio)-; wherein v is 0, 1, 2, 3, 4, 5, 6, 7, 8, 9 or 10.
A preferred embodiment is a compound of formula XXIV as illustrated below, and
the geometric isomers, enantiomers, diastereomers, racemates, pharmaceutically
acceptable salts and solvates thereof:
)¨N1-1
SyN,
0
Formula XXIV
In a more preferred embodiment of formula XXIV, Ri is selected from tables 1-
4.
102
CA 3035442 2019-03-01
A preferred embodiment is a compound of formula )0(V as illustrated below, and
the geometric isomers, enantiomers, diastereomers, racemates, pharmaceutically
acceptable salts and solvates thereof:
0
0
Formula XX'V
In a more preferred embodiment of formula XXV, R1 is selected from tables 1-4.
A preferred embodiment is a compound of formula XXVI as illustrated below, and
the geometric isomers, enantiomers, diastereomers, racemates, pharmaceutically
acceptable salts and solvates thereof:
0
N
)¨N
SsrN
RI.'"0
Formula XXVI
In a more preferred embodiment of formula XXVI, R1 is selected from tables 1-
4.
A preferred embodiment is a compound of formula XXVII as illustrated below,
and the geometric isomers, enantiomers, diastereomers, racemates,
pharmaceutically
acceptable salts and solvates thereof.
< o
0
S\ =
r- RI
0
Formula XXVII -
In a more preferred embodiment of formula XXVII, RI is selected from tables 1-
4.
103
CA 3035442 2019-03-01
A preferred embodiment is a compound of formula XXVIII as illustrated below,
and the geometric isomers, enantiomers, diastereomers, racemates,
pharmaceutically
acceptable salts and solvates thereof:
s 11\:i
N
0
Formula XXVIII
In a more preferred embodiment of formula XXVIII, RI is selected from tables 1-
4.
A preferred embodiment is a compound of formula XXIX as illustrated below, and
the geometric isomers, enantiomers, diastereomers, racemates, pharmaceutically
acceptable salts and solvates thereof:
N
/ 0
Sy.-N
0,
RI
Formula XXIX
In a more preferred embodiment of formula XXIX, R1 is selected from tables 1-
4.
A preferred embodiment is a compound of formula XXX as illustrated below, and
the geometric isomers, enantiomers, diastereomers, racemates, pharmaceutically
acceptable salts and solvates thereof:
0
y
Ho \ 0
Formula XXX
104
CA 3035442 2019-03-01
In a more preferred embodiment of formula XXX, R1 is selected from Table 1.
A preferred embodiment is a compound of formula XXXi as illustrated below, and
the geometric isomers, enantiomers, diastereomers, rac,emates,
pharmaceutically
acceptable salts and solvates thereof:
O'RI
\N 0
HO 0
Formula MI
In a more preferred embodiment of formula XXXI, RI is selected from Table I.
A preferred embodiment is a compound of formula XXXII as illustrated below,
and the geometric isomers, enantiomers, diastereomers, racemates,
pharmaceutically
acceptable salts and solvates thereof:
0
0
0
HO
Formula XXXII
In a more preferred embodiment of formula XXXII, R1 is selected from Table 1.
In a preferred embodiment a compound of formula X.X-XXII is selected from
table XX-)0(11 below, wherein RI is as described above. A more preferred
embodiment
is a coinpound.of table X.X-XXII wherein R1 is selected from tables 1-4.
105
CA 3035442 2019-03-01
Table XX-XXII
1 2 RI
c )__
0-N\ SyN,
8 R1 1
3 4
i N
0,
S N
sr sl.,.N....
R,
ft.10--
N RI 6
/6
s r STN
Ri
7 JP 8
1\
H 0
HO
9 'Cr 10
S-....f
----i
H 0--R1 0
11 RI 12
F
\N N
S----_
.RI
106
CA 3035442 2019-03-01
13 14
N--R1
/N
N\
15 RI 160 =
\N
=
17 18
19 =
1 20 0
N
I
s
21
ON
04
/ s
Thiazolidinedione prodrugs of formula XVII to X)OCII are useful for the
treatment of type 2 diabetes mellitus. Herein provided is a method of treating
type 2
diabetes mellitus by the administration of a prodrug of formula XVII to XXXII,
in
particular a compound of table XX-XXII above wherein the prodrug provides
sustained
107
CA 3035442 2019-03-01
release of the parent drug: The parent drug results from the cleavage of the
labile R1
moiety.
hi some embodiments, a compound of formula XXVII is selected from table G:
Table G
No. Structure
1000. 1021.
1001
1001. 1022. to
(0.---'--. 1:::::L.4---\
o Thc)
1002. 1023.
- lo
o
1003. o 1024. 0
N
(0.---'s---. N---\
--f¨r
1004. S 1025. i
Nr o
S----\
or
N
N/ \ 0 0
s's14-6--
1005. S 1026. i
Nr 0 o
---\
0 \ NTh
\ i
N / O'kErg
0
0
N14.-
108
CA 3035442 2019-03-01
1006. SN....0 1027. io
o
--J\ N
N/ \
...4-1:
1007. 1028.
r 0
S---.\
,
\ N---\
\ 12 o-r0
N
1008.
S-Nr 0 1029.
0
N-Th
0---r
N
N/ \ 0
4-1-4.0
1009. S 1030. p
Nro
o
s-----\
N f
N/ \ 0¨,r0
0
1010. Sm
1 1031.
N 0
\ 0)1V8
N /
N/ \ ct.r0
0
0
109
CA 3035442 2019-03-01
. . .
1011. S...s.o 1032. 1/0
\ i 0
----jS,
N--......\
N
\ \C(1\H/20
1012. ' lir
1033.
s 416 ______________________________________ ,
0 r
,
1013. 1034.
(Cf 0
\
1014. 1035. 0
\
0
(5
, ____________________________________________________________________
1015. 1036. p
--it
0.:,.= 0 I N--\\0_(
0
0/
1 0 1037. 016.
4
0
.....N.-õ,
o i
N
g 0
I
/0/
110
CA 3035442 2019-03-01
...... .. _ ._ . _.. . .. .......
1017. 1038.1
---\
r1)-'-''.--- ---\
.¨
1018. 1039. ,0
I N---\ .L.,),,,
o 0
0
-...õ
/ .
/
,
1019. 1040. 0
=
1
, \ -''''....-
0 0
1 _0_ ......._ 0
,.....
..,
1020. 1041.
o
Table II
No Structure No Structure
1100. I r----N,----------A 1134 a r.-...N,-.
ci N.,,,,J 0
N W o N
I 1 0
o f
0a2+
8 N./
111
CA 3035442 2019-03-01
. ..._.,_ _......_.,..._
1101. ...,6frt4 , 1135
.1&/-----N
CIJ,...,..i a
N.....,...i
N N
1102. ..15,..r.--.N 1136 0 r----N
a N.....)---'..--""" ckN,,)
N
1103. 1137
CI r----N------------0 0
0.....6,1 Ni-s''''......
N N
,.,õµõ,õ,icx.,./ =
1104. I r---N-------= . 1138 0
46 ci
a Nj
N . so N.,.....,..)
VP N
,./ = /
1105. I rThµl'. 1139
Cl...6,N.õ) . CI
N Ur 14
1106. a r----N--------------0 0 1140 a
r..,Nõ..-......õ,...õ.õ0 0
0 =-...--J
N CI CI,
0 N.'-') N
0 0
I.
112
CA 3035442 2019-03-01
---
, _________________________________________________________________
1107. a (------N 1141 1 (----N------
------= Ai-
a....õaõ..NJ
0
0
.tlfilL 0--/
\IC'.
1108. CI r....N...\,,...õ--0 1142
a * NJ
N 8 criCk&N
0
-I11-10L ¨"j
1109. 1143, _____________________
CI
up I re...A 1144 _______________ r...-
...w...,,,,,,,....õ.0
NJ a
IIIP N N
0 0
I 0
r1j4 (.. i 12
1111. ci r.õ-..,N,...õ.........õ,o ___________________ 1145
CI 46 NJ CI iiiki Nõ)
MP N
WI Ns,
1112. 1146
CI
r'N---"-----"--"- 0 N N
0
1.... _.,..0
I
113
CA 3035442 2019-03-01
.
..,_
1113. 1147
,&0---------.0-
CI iii hl,....)
IIPII N CI
N
0. 0
...),.c___/ .õ----,-1-=
...----....--....--......--.....,_, 1-...
1114. r---,w--,0 1148,
I a r----,
. ...., Nj a Ali Nj
WI -11:? WI N
0
ylc_f0
1115.
CI r.......N.,...õ........õ.0 1149
a a (----,N
ci....6.N....) c., Nõ......,..i
o''.. =
7 t'91
0_,P -------NA0--/
L-,...-
1116. r"-re\, 15
CI 10
015..N,,,,J r-----N.------------0-
0......6,N.....)
0
1117. ci r--.N,¨....õ--....= 0 1151. a r-----N-----
------0
CI N...J .,...b...m,..]
111.1 N ,
0
-t-r4
r
1118. (---N---''------""c) 1152.
cc a r-----N------
------o isi,
0, 0 N,J d * N.,..)
NICI:
0
cyko_20
'I<
114
CA 3035442 2019-03-01
1119. a (--.N.---.-,,----....,-= irk - 1153
a (----N
. 0 N.......) vP c.,6,..,..)-'4I,
N.
010_/0 0
L'Vir
1120. a
a iiki Nj CI Nj
N
VP
OCI rs1
N
LerrO
1121. 0 1155
CI, Nj Cl...itrN.....)'
N ,
0
* 0--Z
(ffr2
1122.
c, r.--.N.,0 0 1156 a r----N------------0
cti..Nõ) c, iiii. N....,)
N.
WI N
0
0
= ."1-/-14'it ,.--1L0¨/o
1123. 1157
ci a
o
i,NN,Ko_zo
zyl-c-i
a
1124.
CI 0 1158
N..õ_,...i ci N..,,....] ...-
0 N
. 0
ri
OH
115
CA 3035442 2019-03-01
__________________________________ 01
' - - -- = = = = . 1125. 1 r"-N 1159 .,,Tri.----
Nt,r-",,...----....- 0
a CI N1J
0 Nj N N
0
.11.... ,
-11N v-
-....t.C.'H - =
1126. i r'''-ri 1160
CI
CI. ..,..,,,..1 N 0 CI 5N)
0.
'
0
HNT10_/0 )L
/C)
H
1127. 1161
a
r...N.------.'*--):: T....õ-,0 _____ 0
., Ai, NJ CI NJ
IP * N
0
------/N11N10-/ 'Nt-r-N.A= =
H
1128. r...-...N..---.õ---........= 0 1162 0
1
CI = N.,......) Cl...br.N.,,,,)
N
0 0
õ-----------N-1-0-, "1-r"--NA-0
H 12 H
1129. Ts...Nõ _________ 1163
CI. N, J c, . NJ
NN N
0
1130. 1164 _____________________ rN,¨..,--.......0 0
r---,--------,04 c,
c 0 Nõ....i c, 0 N..s.õ,,J
N
4 H
116 =
CA 3035442 2019-03-01
. ... _
1131. 1165 r..--õN.---...--,..,-0
iii
, f-----N--------,------0 a ci
c, 0 N.,.....) CI N,..) W.=IH-
N
IP N
0 =
N,...,.õ-=
1132. 1166
CI
CI rihi,
MP
I
1133. a r---.N.-----.....-.,-= 0 1167
CI N..õ..) ci gaii, N., j
IIPP- N
Ur N
I A \ /N=01,0--/
Table I
No Structure No Structure
1200. I r'ts1------"- 1234
Cl N,,) CI, Ali Nj
0 N e N. I
0
8 I
Y/ Ca2" O¨P-0 0
8 N/
0
117
CA 3035442 2019-03-01
........
, .õ.. . , ...... . . .. .., .
1201. I N 12351 ci rs-'1.1-. =
CI 0 N, r'',..) Clicar.N..,....)
N),,,L I
N õ
0
¨A= =
1202. I rs"Is, 1236
CI, N) Cl-beN.,......õ.J
N I N .. I
0
7----())C-2
1203. (---..N.,. 0 1237 ________ r.N..õ. .
, c,
c, * 14)
N I N
( al .
0
1204. 1 (--,,,,...= 0 123
CI N
CI 0 N,.) Ck.sor.Nõ,õõ)
I o_jo
=-=.õ..,.,--.õ,..-....(0,../0
0
1205. I rN. 0 123 a (.'"N"---"-. 0
CI 0 N.,...) N CI AN, N,i
1 111 N. I
L0 0 )0
120. CI r--, 1240 __ , (--,---,,,- 0
Ckb/Nj CI,..6r.N,õ)
N I
0 0
=
Nti-i,IL0_-_/ =
\\*V4r¨eilN
=-= ¨
118
CA 3035442 2019-03-01
..
.. ,
. =
1207. a r'r," 1241. CI
CI 0 N,) CI 46,, N.,,.,,J
N Mr N I
0
1208. a r N-,-----,..-1 0 1242 ci
a 46, Nõ...) CI 0 Nj
N
1209. (""N"'''...."-",...- . 1243
r'''tsle CI
0
CI
CI . N.õ,...) CI 0 11..õ....)
1
N. I N
4
`(..r'',05(0..__Ja
N't/l'a 10
1210. 0 r-N-----,--\.-- 1244 c, 0
CI N I 0, N.õ.õ.õ..i CI N.,...)
0 N., I
0
1211. 0 re.............,, 1245
a r-----N-------------
a ,,,,,,, Nj c, 0 Nj
w N N I
o
'str=j11.' --/ '11"-->DA0--/
14
1212. (-^- 1246
CI t,
0 PI4J
I: ,1µ2:
o
_ zo
4 ---- -.N
1 . . .
.
... ...
119
CA 3035442 2019-03-01
,_. ..._ .
... .
1247
CI (--õN
1213. "....'-`-',A 0 C I 1------141.-----,,,-----
,...-o
CI Ai, N....s.) a "...../..-1
UPI N N
0
7
1--..
- 1214. ci rs-N---'',-"-'"-,-. 0 1248- Ci r'e"..-------",-
--- =
CI
lwNJ CI . Nj
411' N I
N '
O 0
L..'"
1215. 1249:
a1
CI laIPw N2 ........
N I N
O 0
'N'.10--/
12162 a r' N-'''. 0 1250
ci 0
ci.,6..N,) N N,..,)
I
go" 1
N
O 0
,,V.s_/0 A =
'..1.1"2.-N11,71.,=
1217. ,...---.N.----........---.....õ- 1251 , r----N---,---,---
o ,,,/
c,...6õN,õ)
1
10- N
O 0
N)(0 ?
Ltr4--
1218. (--..,,,, 0 1252
a 0 Cti.N.,,,.....J
N , I
N I
.. . 0
0
__fa
N't"'CN 0¨
Ler:
120
CA 3035442 2019-03-01
. " = = ¨ - 1219. __ , rw,, 1253 .15....r..,N,0 0
CI 0 Nõ...) CI Nõ,......,J
N N ,
0
LK
1220. . r.N..."....f"........., 1254 i r-1,1. 0
CI Nõ,) CI NJ WI N 0 .. N õ I
0
N3,0_,0
---K-1-0.---,õ.0
1221.
(--- 1255 a 0
N
N I 0
T ___/ 0
*0
(
1222. ____ .N
---.,....õ--. ibi r,.......N,.
1 i
.. c, . NJ õ 1256
wr c, N , NN..)I
, IP N
0 . 0
)1L.,
-NO14
1223. ci i¨,.-..--,-- 1257
CI NJ CIN&O.......''All
IIIP N
0 0
r,,elso_
.-N.,......)
----,,)
0
121 '
CA 3 0 354 42 2 0 1 9-0 3-0 1
.. ...
1224. =
.
a 3. 1258
CI, Nj
1 0,6,niji
o
HO,,,,,,N,c_zo
rni
1225.
I rN 1259 a (----N 0
a ihiN, N.,,,)
WI N CI O Nj *
N
0
--TiAO¨/ 10_2
H
1226.
a r----N---,..----i __________________ 1 260
a 14J 1 r.--..N.--......-..õ-
1 0 Nj
0
8 13
1227. __ r.N,.. 0 261.
a Nj , r-----N-----,---,--0 ,--Ic
CI
du. Nj
N _ up
0 0
,o H
'1228. Cl (--.,.. 0 262 a r---N----------.---= 46
alrar,õõ)
a 0 Nj VP ,
I
N N _ '
0 0
."-N...-"=Ao_..../.
if 12 H
229. a r----14--------....-- 0 1263.
C iiihm,, N,,....)
Ur N 1 C ,N
0 N
''11-2-'isirli.
10----/.
Ei
122
CA 3035442 2019-03-01
. ,.õ, ...,. ... , .
= - -- = ¨ = = -1230. a r,Nr---.. 0
1264 I rN"-. thi
CI 0 CI N.,, i fah, Nj lir ,
0
1 =
=
'N1-{N)L0----7 01 =
4 H
1231. ts r-r"--. & N''
CI to
a '" 1265 1
a 0 Nõ) ilw a ra, Nj
N I WIP N ,.
0
i-----N 0
0 N......)
1232. 1 r..N.,........,,,.. Ai 1266
cidli
N , .. I
0 =
0
1233. r.,--.N.---.......,-,......... Ali--
1267 a 0 r------N-------------
LIP qr
= N . I
1,,6..N.,....õ.J
:110 l
=
BARBITURATES
In another embodiment, compounds of the present invention are represented by
formula XXXIII-XXX'VII as illustrated below, and the geometric isomers,
enantiomers,
diastereomers, racemates, pharmaceutically acceptable salts and solvates
thereof:
123
CA 3035442 2019-03-01
RI oo RI -
NX
X f Xi
x1P
2
Ii Ii
Rr R100 Rioo
Xio XI Xio
Formula XXXLII Formula XXX1V Formula XXXV
Rloo
Rio
NI
X r
Xi
R100
Rt
X10
Formula XXXV1 Formula )(XXVII
wherein, X, X,X2, R100, Run, and R1 are as defined above;
Xio is -S or -O.
In a preferred embodiment a compound from Table XXXIII-XXXVII is
provided. A more preferred embodiment is a compound of table XXXIII-XXXVII
wherein R1 is selected from tables 1-4.
TABLE 100:11.1-XXXIV
1 9 0
TIN ___________________________________________________
NH 0 N-Ri
0
0
124
CA 3035442 2019-03-01
2 10
\N--(
0
N-R,
N-R1
0
3
\N- 11 \N¨
N-121
0 N¨R1
o 0
4 0 12 0
NH
HN <
N¨R1
0 0
13 HN¨\
,N¨R1
1
0
0
6 0 14
NN
\N <
ONN
NH
0
125
CA 3035442 2019-03-01
s
15 IS
HN HN <
N¨Ri N¨R1
0 0
8 Rix M 16
(N NH
0
0
Pyridone pyrimidone and pyrimidione prodru2s
In another embodiment, compounds of the present invention are represented by
formula )(XXVIII or XXXIX as illustrated below, and the geometric isomers,
enantiomers, diastereomers, racemates, pharmaceutically acceptable salts and
solvates
thereof:
RI
X
X13 13
(R3)(1 _________________________________ (113)cl
()m ><X
(R.4)nl XI
Formula XYJCVIII Formula XXXIX
wherein X, RI, R3, R4, m and q are as defined above;
XII is ¨N- or ¨C(R10)-;
X12 is -C(0)-, -C(S)-, -C(R)0)(R11)- or ¨C(Rio)(0Rii)-; and,
X13 is¨O, -S, -N(Z10)(R11))
126
CA 3035442 2019-03-01
.. . .,. ... ._ _.
A preferred embodiment is a compound selected from table XXXVIII-XXXIX.
A more preferred embodiment is a compound from table XXXVIII- XXXVIX wherein
R1 is selected from tables 1-4.
TABLE XXXVIII
1 9 o
H2N ....ØR1 NC .....õ-Ri
N N
0
I
...."' N
2 o 10 0
R
..................õ--,,, _Ai ,--""
R..õ.......õ.õ....--.......... 1
N"- N
1 1
HO.,.........Ø........ j
OH
3 11 0
F3C,,,,(1,... ,.,Ri 1,....õ............".....õ, õ.....R1
N N
'N. ...,..o
rr o N
HO H =
A H H
H = H
127
CA 3035442 2019-03-01
4 o 12
F3c,,,L1,R1
N
Cc,O.,111
HO,,,.,
OH
N3
13 ' 0
N¨ N.
N
H
HO
0 S N
I 0
6 NH 14 ' NH
N N N
( I
N/N
H)õ..,õ0,,,_ j HO.
OH F
OH
_ .
*
7 15
,,,e.,,,N,,,.R1 -.,%s...,/=1-.,,N,,.-Ri
F N 0 N
H S
H
128
CA 3035442 2019-03-01
= 8 ______________ 0 ________________________ I(;
(-LV'Rj
NO
NH
0 HO
11111111p1 7.
IN
PRODRUGS OF BENZAMEIDE PHARIVIACOPHORES
In another embodiment, compounds of the present invention are represented by
formula XL or XL1 as illustrated below, and the geometric isomers,
enantiomers,
diastereomers, racemates, pharmaceutically acceptable salts and solvates
thereof:
R50 0 R50
R51 R51
R52 R54 R52 R54
Rs3 R53
Formula XL Formula KLI
wherein RI, Rso, R51, R52, R53, R54 and R55 are as defined above.
Table XL-XLI
1 r---% 22
ND
R
ND
\N
(i)
129
CA 3035442 2019-03-01
_ _... ...
- 00 _______ 0 r- 23 _______ ,.-R-1
I N
Cc1)1
N
H2N 0
I H2N 0
I
3 24 õal #,.õ.....,...
o
I
N,
I
RI
I N
I 1 N
I
4 0 25 .,..Ri
H 0
r.õ...0 N H
1
N \
CF3 R, ....,........õ...-
0 ' CF3
, 0) 0CF3 ...µ,N.,./.-
.,3%_,
26
011CL R1-,,
HO...õ.."..0
N**N'==e---/- \ 0
N
I
RI OH OH
HN r....--N.
7 _____________________________________________________________ (N!\___NH
N N
0 0
130
CA 3035442 2019-03-01
- - 6 H
0 C).,,,,.,:,õ,,roo 27 IZI.õ0H00
0 114 0 "-.1,4 ----
,_,..^ .-,,,r
RI 0
OH OH
..,
C
r.----N____3:7
N L----1/4 /
--N N
--N
H2N
H2N
7
ol-10 CI 28 R114
H0
N 0OH
I
RI
. HN BN
H H I H H
'---CNNy-NH2 L...-NN,,vNH2
II
N
0
8
= HO0 29 RI.,oHO 0
0 IsTr
I
* RI OH
IN OH
r0 FNI 0 0 r 0
Nii:II)LN
I A I
N N NH2
H H N NH2
H H
131
CA 3035442 2019-03-01
9 30 R1 .
._
RI ....,
CI I
OCH3
1.1 H OCH3
aNyNi
H H
0 0 aNN
0 I
31 =
0 0 R.I.,
0....,y/ 0 .s p 0
H2N
RI1 112N
i N
Ct Cl
, _____________________________________________________________
11 0 OH = 32 RI
N , H
0 (R N
H
- OH
H
12 0 33
s) el
CI
H2N 0
RI
I H21."
?
13 Cl 34 CI
RI
I
N 0 NO2 .).4 0 NO2
L:rJLiOH 0 OH 0
Cl R( Ci
- 14 Ok
02N
RN, 0.2N..s
,L ) 1 )
0 N N =====.N N
I
RI
132
CA 3035442 2019-03-01
15
0 r 36 RIN,0
r
....--..,,,,..N....õ......
0 T *
RI
H2N H2N
16 H 37 H
N 0 N
=,.., =.JLJ
,..
RI
NI
N
'...
0 0 OH ...-'
R0I 0 OH
17 ''''.0 0
) 38 s., R1...õ
0 0
)
N
N
0 0
Br I I
Br
18 0 0 ) 39 R1....,
v o
)
H2N" 0 N
II/N..0
H2N---
N
(i) 0
I
19 40
Ri.._
I N
HO Ri HO
0 0
133
CA 3035442 2019-03-01
20 41
NI
OH OH
Cl Cl
21 42
01
e,N
,
0 0
PRODRUGS OF 1MIDE PRARMACOPHORES
In another embodiment, compounds of the present invention are represented by
formula XL,II, XLII1 or XLIV as illustrated below, and the geometric isomers,
enantiomers, diastereomers, racemates, pharmaceutically acceptable salts and
solvates
thereof:
R1..õ
X X X
Rims R100 \
Rio; _4 /jt, RW17'7
..r.z Xsz
X2 0 X2 0 -
Formula XIII Formula XLIII Formula XLIV
wherein R1 Rioo, R101, X, X1 and X2 are as defined above; alternatively R100
and R101
together with the atoms to which they are attached form an optionally
substituted 3, 4, 5,
6, or 7 membered ring.
A preferred embodiment is a compound selected from table XLII-XLIV. A more
preferred embodiment is a compound from table XLII. XLIV wherein R1 is
selected
from tables 1-4.
134
CA 3035442 2019-03-01
. _ .
..
. . . . , . .... . .. .. ... ..
....... ..... . -
Table XLII-idAV .
. .
XLII- XLM- XLIV-
1 1 0 1
R.] '
N0
N "---N
0 0 R 0 0
NH 1 NH2 N/42 Ck
12.[
2 2 .-R 1 2
1
H2N H2N 142N
3 3 R 3
.TRI
0 , ,..=RI
1
,
0
/ . N
* --
* N
N N
0Ao 0 111 1
,...---..., _.,..a 1
0 0
6 0 6 Ri 6 0 =
--.1(
>?.........(
>I...?
0
135
CA 3035442 2019-03-01
7 0
1
0 0
8 0 8 --R 8 0
0 1
0 0
i
NH2 2 N112 R1
NH
_ .
9
N-Nri RI 1) L--) j-Nr-fiprN
N-Nrsr
N../
02N 0 02N 0"."---0 0?
RI
0
10 R 10
,...121
N N
N I
o'RI
0
0 -I1
NJ
N---/
N
c
c <
ii _ õ...y.0 11 * N-N/y R1ON 11
C--=N N* - 1,1-Nrifj
= =
02N,> 1.--Nõ 1 02N
02N tN
R
R,
12 (I, ' 12 Rr., 12
NRi
(IT NNO
IINTi
1-1Ny
0
13 13 Ri,, 13
rIN,R1
riN
f')./=1
/L0 RI
0--N.,--,....õ,N,N.7L0
HNõr)
FIN,,..)
0
0
_
136
CA 3035442 2019-03-01
14 R, 14 0 ___ .
RI
%
N 6 .
i
N
N \ N .
\\ , NI
/ / \
I\ N
\
15 . 15 ' 15
ift1
rJ4NO
N
0 0 12, 0 0
RI
¨ ______________
16 p 16 0 16 I
Hir--1 11N-4
-Ri N-R.1 N-R1
0 0 0
In another embodiment, compounds of the present invention having the formula
IV-VII is selected from table IV-V.
Table IV-VII
1 0 26 0
-'-iL0 )LO
0\,...... 0\_.....
110 #
o 0
101 .... 11
=
0
N 0 N 0
1 1
R1 R1
/ Ri 2'7 Ri
1 0 O
I
OH ..,,,,,,,..--
OH
)..µ'IL'j _________ .=1:1,0
137
CA 3035442 2019-03-01
=
¨3 Ri 28 1 Ri
1
N
N 0 0
..-- 0 ----
OH OH
4 Ri 29 Ri1
P i
N 0
Cr) N 0
--..
,N N
N,, i7--\_,õõ...s.,0
'N¨N 'N¨N
,
Ri 4 30
N 0
..... P N o
---
,N NN
t,
1N¨N ;NI¨N
6 Ri 31 N 0,
N.,..,0
i CI 0
0
CI // CF3
CF3
7
32
0 )1--
NH NH
---" -,'
--,
0 N 9 N
141 R1
8 f=I 33 N
Nii
0
)0-0 0
0 0
¨0
---"µ 0
0
138
CA 3035442 2019-03-01
9 01-I R1 - . ' .. - 34 ' OH
1
N 0 N 0,
--- Ri
HO
I HO
I
1:21 35 N Ri
0
N.1
''''l ..õ,=-=õ_õ..N
,..,,-..,õ...N
11 ( 36
( /
0
0 N.---/ N--/
N
N
/ \ H
/ \
F / N F
0 1 0\
N N `RI
ii,
12----
Iti.,,)*-...
0 N 0 N
I
R1
13 ------,N'0 .38
I I
0 0\
N = / RI
N
\
R,
14
NC__()..
NC
N N
N 0 N 0
I
R1
______________________________________________________________ i
139
CA 3035442 2019-03-01
---
.,..._. . _
15 / \ 40 k
/
NC NC/
N N
N S N S
R1 .
16-0 41 __ 0
0\
(:\
¨0 ¨0
0
/ d
N N
1
RI
17 H _______ 42
0 N) N
N IR.,
)---0
0 \ g'sNi :
HOf e?-1=1 lit1
N N
18 43 ,0*0 0 Oct
C1 N
\
R1
R1
19 R1 44
I
N..s.,,..0
ID2)L'N)11 41111
0 I
CI RI
H3C
20 45 ' NO2 R1
I
-.., N..-N12 CI N0
I I
N ,--- RI
Cl N0
H
140
CA 3035442 2019-03-01
.. .
, ....
21 NO2 a 46
H
CI N 0 Y=
Cl 0 X
N 0 CON *
F
411;
I
R1
22 RI 47 Fy0
RI a
1
F
N
77) I
a
H
23 H 48 R1
N
I N I
Fr- \ /N
_
/
r, N I-12N
.., \
RI
24 N
. 1 R,Iro
/ 49 !
1
0
i
H2N O NH2
25 ! I 50 It!
N
c`'=<N--1.-----N cr-l-cr-cF3
- A
= Ri
H
N112
,
CF,
51 55 52
no,
N
0.2(MILN
Rr-----1 r
RI
CF
F
_ ______________________________________________
141
CA 3035442 2019-03-01
- 53
no, NRN
N 0 F3
R,
F3
54 __________________________________________________________
RkoAni-Lr
f,
PRODRUGS OF SULFONAMIDE PHARMACOPHORES
In another embodiment, compounds of the present invention are represented by
formula III as illustrated below, or its geometric isomers, enantiorners,
diastereomers,
racemates, pharmaceutically acceptable salts and solvates thereof.
0
A¨S¨N
II
0
Formula III
A preferred embodiment is a compound selected from table III. A more preferred
embodiment is a compound from table III wherein RI is selected from tables 1-
4.
Table III
1 37
H2N<Y
s3
H2N
0
/SN)
\RI
142
CA 3035442 2019-03-01
. . _ .... . .
2 0)...õ,\......_ s 38 a
o
_ > (---=o \ NR
/
)
ZN-----N }IN¨R1 12,---NH N
0$
3 39 0 sH
cO
C)'-sSo,
01
0
R H
0
0>
NH I
Rf--
=
4 xIc SV 40
a H
N'N-C
F3
1
-.11\,
0
0
IIIIIxiJ
N 0
R-" N
H 41 0
CN...,.,,.,....,,,
04
N" N
) 0 NH H
= 0
./NH
Rr
6 0 42 112N04j
02) . N--=-Nll>¨NH2
N
R,¨NH
Ho
=
_ =
143
CA 3035442 2019-03-01
-
.
....
. . .,..
0 43 %
0=-N 41 NH2 0, * NH2
R1---N/ RI¨N
N...,,,,...Øõ.......
\ (...,... õ...1...õ. ,......
N 0
. ---0
_
8 44 H2N
(3(-. =
NH2
4 = N=N--6-NH2
R1¨N/
Ri-NH _____________________________________________
N)--)---ot
)---=---N \
-9
(1--... 45t __________________ 0
$1 S \
it I
0 "
, \ /
0
RI
,
= 10
46
oi . Nii 0
/
fr-0
R1-NH __________________________________ 0
=
Itr".
_11 ______________________ 47
.0 ......,
er.)<- 1
,..õ..-N
0 = 0___ -,
T.-- l4---R,
0 N
R NH.
1 N.
'1:::),µõ......õ....N
1 %/N
144
CA 3035442 2019-03-01
, . .
....... ..... . ..............õ
. ... .. . .
_ . . ... .. ...... .. . Ti1 0=-\ H 48
H...1........Lri
H
/ a N
Ri¨NH
FIN CI ,,,NII
0-7:1 No
1.---(C1 õNR 0
Rr
13 11N¨R11 49 \0
o...õ....,.<0 ,
Ir:CN¨NH
CI
0
0 i
Cii,.01,-µ1
\
0 N\
14 0 50 H __________
R( IL. ii._ s CI
S
1 -1 Nri'lr 0....
......
N¨N
NH 6m4
0
R.rr-
....
15 H 5 1 ct
rks>
li I
/ C
\----
OH c
7
Hi RI
N\se-N
16 a N...,,,,,..õ...../......õs 52 Cl O N
"NH
. o NH 0
EIN-R, HN¨R1
17 53 _________ NI)
...,
ori I / 7
ir Ri----NH
1/N N/',''''N.. ...1411
.
o
145
CA 3035442 2019-03-01
.. 18
54 i a
NH e. \Ri O60
IiNI¨
t
H .
1-P
\
NH
/
R,
19 55
F \ 1
S¨NH2
0
\ II
N
R,/ 1 NH2
. CI
0
-;/,,,,,..,
--S,_,,,,
'-'0
. IN
! \
! R.,
20 i
i V 56 V
¨NH2 ¨NH2
I)
RI 0
R/
_.1 0
CI
21 57
V NH2 V NH2
ir Cl HN-1
/
WI g R,
CI 01 I
22 58 x o
RrA
( /
0 N-0NH
RI 0
,
a CI
146
CA 3035442 2019-03-01
23 H 59 Cl
CI iii...6 N 0
II
0=---6 * NH
A. 1 __
0c- \ 0 0
R1
HN¨R1
0
24 59 Cl
H
00. \ .........s,..
0 0
1 piN¨R,
7, 1
c,
25 60 H
¨
I /
HN N
/ J = - N
k N ()Y \ )1\Ds --
F
26 6 1)
1 ' ',õ.....",..-0
s
/ ) 70
(--=.--o N
/
RI
/
RI
NH
NB
I
27 '
H 0 62 H
F3C N....,,
CI N II
0 ,N
0 ,
--;s -P 0 0
_ HN -R1 0
147
CA 3035442 2019-03-01
H 63 ___________ H
Cl N.,...1 F3C N,....,
..,:b
e \ 0.'
HN¨R1 0 HN¨R1
29 H,f) 64 Cl
CI N H
0
0õ...-,.., OH 0- 1
I-I1¨R1 0
0 \
HN¨R1 0
_
30 65 -õ
0 0
7 1 ...---N H
..., 0
->
0 \
HN¨R1 0 CN
31 66 0 CI
\
,N C1/4j
/ Ri-., ,....b is
N
H
µ o
s/7 ,
NH
\
RI \ -.......0
32 67
CI
H,
o, ?
1\ s
qi rr--
H-40 H
- 0
148
CA 3035442 2019-03-01
t
.,.
= 33 68
\4
Ri\NN/ H---4\0
34 0 69 RN (Ssfi 41 I
\--/
0
70 r, R CV7 =
N. ......,s 1
N---i µ-----=N\ _4
HN
0 0
N--
H
0
,
36 71
R,, ....õ..-..õ,,
'N
f--.INIII\I
I
ip o
N"---
/
N----
H 0
0 a H2N
Chlorothiazide and hydrochlorothiazide compounds of formula III and in
particular table III are useful for the treatment of hypertension, congestive
heart failure,
osteoporosis, symptomatic edema peripheral edema, kidney stones, diabetes,
nephrogenic diabetes irtsipidus, hypercalcaernia, Dent's disease and Meniere's
disease.
Compounds of formula III and table III provide sustained release of parent
drugs by
149
CA 3035442 2019-03-01
, .
- cleavage of the labile R1 moiety. ..Compounds of formula HE, for
example 111-63 to 111-71
are useful as prodrugs for the treatment of diabetes.
In another aspect of the invention a general method to convert compounds of
formula XLV with secondary amides to substituted tertiary amides is provided
(Scheme
1).
Scheme 1
A
\NH 0\
+
R103
Formula XLV
R103
OOH
Formula XLVI
1
R103 0 R103 0 B 111 oa 9
0 Rio ONOLNH 0
A Rioo A Rioo
Formula XLVII Formula XLVIII Formula XLIX
R103
Rum
A
Formula L
150
CA 3035442 2019-03-01
In addition to the reaction of aldehyde or ketone to compounds of formula XLV,
other process for converting secondary lactam groups can be used. For example,
alicylation followed by addition of sodium in inert solvents, or addition of
potassium
hydroxide or sodium hydroxide followed by alkyl halide addition can be used.
Microwave based synthetic procedures can also be used to convert secondary
lactams to
substituted tertiary lactam compounds of the instant application. (For a
general review
see March J. Advanced Organic Chemistry, Wiley, 1992; Inoue et at., Bull.
Chem. Soc.
Jpn., 58, 2721-2722, 1985; Mijin et al., J.Serb. Chem. Soc., 73(10) 945-950,
2008;
Bogdal et at. Molecules, 1999, 4, 333-337; U.S. Patent No. 5,041,659).
The invention further relates the sustained delivery of a compound of formula
XLV by the administration of a compound of formula Upon
administration of a
compound of formula 1411, the labile R1 moiety may be cleaved off
enzymatically,
chemically or through first phase metabolism giving a compound of formula
)a,v.
Without being bound to any theory, it is postulated that for some of the
compounds of
formula 1411, the release of a compound of formula XLV upon cleavage of the R1
moiety
results in a therapeutically active agent. For example such active ingredient
can be
aripiprazole, ziprasidone or bifeprunox. In one embodiment, the sustained
release
comprises a therapeutically effective amount of a compound of formula XLV in
the
blood stream of the patient for a period of at least about 8, preferably at
least about 12,
more preferably at least about 24 and even more preferably at least about 36
hours after
administration of a compound of formula 1-Ill. In one embodiment, the compound
of
formula XLV is present in the blood stream of the patient for a period
selected from: at
least 48 hours, at least 4 days, at least one week, and at least one month. In
one
embodiment, a compound of formula is administered by injection.
Compounds of formula IX, X, XI, XII, XIII, XIV, XXVII, XXXIV, XDOCV,
XXXVI, and XXXVII are useful for the treatment of neurological and
psychological
disorders. Neurological and psychiatric disorders include, but are not limited
to,
disorders such as cerebral deficit subsequent to cardiac bypass surgery and
grafting,
stroke, cerebral ischernia, spinal cord trauma, head trauma, perinatal
hypoxia, cardiac
arrest, hypoglycemic neuronal damage, dementia (including AIDS-induced
dementia),
Alzheimer's disease, Huntington's Chorea, amyotrophic lateral sclerosis,
ocular damage,
retinopathy, cognitive disorders, idiopathic and drug-induced Parkinson's
disease,
muscular spasms and disorders associated with muscular spasticity including
tremors,
epilepsy, convulsions, cerebral deficits secondary to prolonged status
epilepticus,
151
CA 3035442 2019-03-01
migraine (including migraine headache), urinary incontinence, substance
tolerance,
substance withdrawal (including, substances such as opiates, nicotine, tobacco
products,
alcohol, benzodiazepines, cocaine, sedatives, hypnotics, etc.), psychosis,
schizophrenia,
anxiety (including generalized anxiety disorder, panic disorder, social
phobia, obsessive
compulsive disorder, and post-traumatic stress disorder (PTSD)), mood
disorders
(including depression, mania, bipolar disorders), circadian rhythm disorders
(including
jet lag and shift work), trigeminal neuralgia, hearing loss, tinnitus, macular
degeneration
of the eye, emesis, brain edema, pain (including acute and chronic pain
states, severe
pain, intractable pain, neuropathic pain, inflammatory pain, and post-
traumatic pain),
tardive dyskinesia, sleep disorders (including narcolepsy), attention
deficit/hyperactivity
disorder, eating disorders and conduct disorder.
Definitions
Listed below are definitions of various terms used to describe this invention.
These definitions apply to the terms as they are used throughout this
specification and
claims, unless otherwise limited in specific instances, either individually or
as part of a
larger group.
The term "aliphatic group" or "aliphatic" refers to a non-aromatic moiety that
may be saturated (e.g. single bond) or contain one or more units of
unsaturation, e.g.,
double and/or triple bonds. An aliphatic group may be straight chained,
branched or
cyclic, contain carbon, hydrogen or, optionally, one or more heteroatoms and
may be
substituted or unsubstituted. In addition to aliphatic hydrocarbon groups,
aliphatic
groups include, for example, polyalkoxyalkyls, such as polyalkylene glycols,
polyamines, and polyimines, for example. Such aliphatic groups may be further
substituted. It is understood that aliphatic groups may include alkyl,
substituted alkyl,
alkenyl, substituted alkenyl, alkynyl, substituted alkynyl, and substituted or
onsubstituted
cycloallcyl groups as described herein.
The term "acyl" refers to a carbonyl substituted with hydrogen, alkyl,
partially
saturated or fully saturated cycloalkyl, partially saturated or fully
saturated heterocycle,
aryl, or heteroaryl. For example, acyl includes groups such as (C1-C6)
alkanoyl (e.g.,
formyl, acetyl, propionyl, butyryl, valeryl, caproyl, t-butylacetyl, etc.),
(C3-
C6)cycloalkylcarbonyl (e.g., cyclopropylcarbonyl, cyclobutylcarbonyl,
cyclopentylcarbonyl, cyclohexylcarbonyl, etc.), heterocyclic carbonyl (e.g.,
pyrrolidinylcarbonyl, pyrrolid-2-one-5-carbonyl, piperidinylcarbonyl,
152
CA 3035442 2019-03-01
piperazinylcarbonyl, tetrahydrofuranylcarbonyl, etc.), aroyl (e.g., benzoyl)
and
heteroaroyl (e.g., thiopheny1-2-carbonyl, thiopheny1-3-carbonyl, furany1-2-
carbonyl,
furany1-3-carbonyl, 111-pyrroy1-2-carbonyl, 1H-pyrroy1-3-carbonyl,
benzorb]thiophenyl-
2-carbonyl, etc.). In addition, the alkyl, cycloalkyl, heterocycle, aryl and
heteroaryl
portion of the acyl group may be any one of the groups described in the
respective
defmitions. When indicated as being "optionally substituted", the acyl group
may be
unsubstituted or optionally substituted with one or more substituents
(typically, one to
three substituents) independently selected from the group of substituents
listed below in
the definition for "substituted" or the alkyl, cycloalkyl, heterocycle, aryl
and heteroaryl
portion of the acyl group may be substituted as described above in the
preferred and
more preferred list of substituents, respectively.
The term "alkyl" is intended to include both branched and straight chain,
substituted or unsubstituted saturated aliphatic hydrocarbon radicals/groups
having the
specified number of carbons. Preferred alkyl groups comprise about 1 to about
24
carbon atoms ("CI-C24") preferably about 7 to about 24 carbon atoms ("C7-
C24"),
preferably about 8 to about 24 carbon atoms ("C8-C24"), preferably about 9 to
about 24
carbon atoms ("C9-C24"). Other preferred alkyl groups comprise at about 1 to
about 8
carbon atoms ("C1-C8") such as about 1 to about 6 carbon atoms ("C1-C6"), or
such as
about Ito about 3 carbon atoms ("CI-C.2"). Examples of C1-C6 alkyl radicals
include,
but are not limited to, methyl, ethyl, propyl, isopropyl, n-butyl, tert-butyl,
n-pentyl,
neopentyl and n-hexyl radicals.
The term "alkenyl" refers to linear or branched radicals having at least one
carbon-carbon double bond. Such radicals preferably contain from about two to
about
twenty-four carbon atoms ("C2-C24") preferably about 7 to about 24 carbon
atoms ("C7-
C24"), preferably about 8 to about 24 carbon atoms ("Cg-C24"), and preferably
about 9 to
about 24 carbon atoms ("C9-C24"). Other preferred alkenyl radicals are "lower
alkenyl"
radicals having two to about ten carbon atoms ("C2-C10") such as ethenyl,
allyl,
propenyl, butenyl and 4-methylbutenyl. Preferred lower alkenyl radicals
include 2 to
about 6 carbon atoms ("C2-05"). The terms "alkenyl", and "lower alkenyl'',
embrace
radicals having "cis" and "tans" orientations, or alternatively, "E" and "Z"
orientations.
The term "alkynyl" refers to linear or branched radicals having at least one
carbon-carbon triple bond. Such radicals preferably contain from about two to
about
twenty-Baur carbon atoms ("C2-C24") preferably about 7 to about 24 carbon
atoms ("C7-
C24"), preferably about 8 to about 24 carbon atoms ("C8-C24"), and preferably
about 9 to
153
CA 3035442 2019-03-01
about 24 carbon atoms ("C9-C2.4"). Other preferred alkynyl radicals are "lower
alkynyl"
radicals having two to about ten carbon atoms such as propargyl, 1-propynyl, 2-
propynyl, 1-butyne, 2-butynyl and 1-pentynyl. Preferred lower allcynyl
radicals include 2
to about 6 carbon atoms ("C2-C6").
The term "cycloalkyl" refers to saturated carbocyclic radicals having three to
about twelve carbon atoms ("C3-C12"). The term "cycloalkyl" embraces saturated
carbocyclic radicals having three to about twelve carbon atoms. Examples of
such
radicals include cyclopropyl, cyclobutyl, cyclopentyl and cyclohexyl.
The term "cycloalkenyl" refers to partially unsaturated carbocyclic radicals
having three to twelve carbon atoms. Cycloalkenyl radicals that are partially
unsaturated
carbocyclic radicals that contain two double bonds (that may or may not be
conjugated)
can be called "cycloalk-yldienyl". More preferred cycloalkenyl radicals are
"lower
cycloalkenyl" radicals having four to about eight carbon atoms. Examples of
such
radicals include cyclobutenyl, cyclopentenyl and cyclohexenyl.
The term "alkylene," as used herein, refers to a divalent group derived from a
straight chain or branched saturated hydrocarbon chain having the specified
number of
carbons atoms. Examples of alkylene groups include, but are not limited to,
ethylene,
propylene, butylene, 3-methyl-pentylene, and 5-ethyl-hexylene.
The term "alkenylene," as used herein, denotes a divalent group derived from a
straight chain or branched hydrocarbon moiety containing the specified number
of
carbon atoms having at least one carbon-carbon double bond. Alkenylene groups
include, but are not limited to, for example, ethenylene, 2-propenylene, 2-
butenylene, 1-
methy1-2-buten-1 -ylene, and the like.
The term "alkynylene," as used herein, denotes a divalent group derived from a
straight chain or branched hydrocarbon moiety containing the specified number
of
carbon atoms having at least one carbon-carbon triple bond. Representative
alkynylene
groups include, but are not limited to, for example, propynylene, 1-
butynylene, 2-
methy1-3-hexynylene, and the like.
The term "alkoxy' refers to linear or branched oxy-containing radicals each
having alkyl portions of one to about twenty-four carbon atoms or, preferably,
one to
about twelve carbon atoms. More preferred alkoxy radicals are "lower alkoxy"
radicals
having one to about ten carbon atoms and more preferably having one to about
eight
carbon atoms, Examples of such radicals include methoxy, ethoxy, propoxy,
butoxy and
tert-butoxy.
154
CA 3035442 2019-03-01
The term "alkoxyalkyrrefers to alkyl radicals having one or more alkoxy
radicals attached to the alkyl radical, that is, to form monoallooxyalkyl and
dialkoxyalkyl
radicals.
The term "aryl", alone or in combination, means a carbocyclic aromatic system
containing one, two or three rings wherein such rings may be attached together
in a
pendent manner or may be fused. The term "aryl" embraces aromatic radicals
such as
phenyl, naphthyl, tetrahydronaphthyl, indane and biphenyl.
The terms "heterocyclyl", "heterocycle" "heterocyclic" or "heterocyclo" refer
to
saturated, partially unsaturated and unsaturated heteroatom-containing ring-
shaped
radicals, which can also be called "heterocyclyl", "heterocycloalkenyl" and
"heteroaryl"
correspondingly, where the heteroatoms may be selected from nitrogen, sulfur
and
oxygen. Examples of saturated heterocyclyl radicals include saturated 3 to 6-
membered
heteromonocyclic group containing 1 to 4 nitrogen atoms (e.g. pyrrolidinyl,
imidazolidinyl, piperidino, piperazinyl, etc.); saturated 3 to 6-membered
heteromonocyclic group containing 1 to 2 oxygen atoms and 1 to 3 nitrogen
atoms (e.g.
morpholinyl, etc.); saturated 3 to 6-membered heteromonocyclic group
containing 1 to 2
sulfur atoms and 1 to 3 nitrogen atoms (e.g., thiazolidinyl, etc.). Examples
of partially
unsaturated heterocyclyl radicals include dihydrothiophene, dihydropyran,
dihydrofuran
and dihydrothiazole. 1-leterocycly1 radicals may include a pentavalent
nitrogen, such as in
tetrazolium and pyridinium radicals. The term "heterocycle" also embraces
radicals
where heterocyclyl radicals are fused with aryl or cycloalkyl radicals.
Examples of such
fused bicyclic radicals include benzofuran, benzothiophene, and the like.
The term "heteroaryl" refers to unsaturated aromatic heterocyclyl radicals.
Examples of heteroaryl radicals include unsaturated 3 to 6 membered
heteromonocyclic
group containing 1 to 4 nitrogen atoms, for example, pyrrolyl, pyrrolinyl,
imidazolyl,
pyrazolyl, pyridyl, pyrimidyl, pyrazinyl, pyridazinyl, triazolyl (e.g., 4H-
1,2,4-triazoly1,
111-1,2,3-triazolyl, 2H-1,2,3-triazolyl, etc.) tetrazolyl (e.g. 1H-tetrazolyl,
2H-tetrazolyl,
etc.), etc.; unsaturated condensed heterocyclyl group containing 1 to 5
nitrogen atoms,
for example, indolyl, isoindolyl, indolizinyl, benzimidazolyl, quinolyl,
isoquinolyl,
indazolyl, benzotriazolyl, tetrazolopyridazinyl (e.g., tetrazolo[1,5-
b]pyridaziny1, etc.),
etc.; unsaturated 3 to 6-membered heteromonocyclic group containing an oxygen
atom,
for example, pyranyl, furyl, etc.; unsaturated 3 to 6-membered
heteromonocyclic group
containing a sulfur atom, for example, thienyl, etc.; unsaturated 3- to 6-
membered
heteromonocyclic group containing I to 2 oxygen atoms and 1 to 3 nitrogen
atoms, for
155
CA 3035442 2019-03-01
=
example, oxazolyl, isoxazolyl, oxadiazolyl (e.g., 1,2,4-oxadiazolyl, 1,3,4-
oxadiazolyl,
1,2,5-oxadiazolyl, etc.) etc.; unsaturated condensed heterocyclyl group
containing Ito 2
oxygen atoms and I to 3 nitrogen atoms (e.g. benzoxazolyl, benzoxadiazolyl,
etc.);
unsaturated 3 to 6-membered heteromonocyclic group containing 1 to 2 sulfur
atoms and
1 to 3 nitrogen atoms, for example, thiazolyl, thiachazoly1 (e.g., 1,2,4-
thiadiazolyl, 1,3,4-
thiadiazolyl, 1,2,5-thiadiazolyl, etc.) etc.; unsaturated condensed
heterocyclyl group
containing 1 to 2 sulfur atoms and Ito 3 nitrogen atoms (e.g., benzothiazolyl,
benzothiadiazolyl, etc.) and the like.
The term "heterocycloalkyl" refers to hcterocyclo-substituted alkyl radicals.
More
preferred heterocycloallcyl radicals are "lower heterocycloalkyl" radicals
having one to
six carbon atoms in the heterocyclo radical.
The term "alkylthio" refers to radicals containing a linear or branched alkyl
radical, of one to about ten carbon atoms attached to a divalent sulfur atom.
Preferred
alkylthio radicals have alkyl radicals of one to about twenty-four carbon
atoms or,
preferably, one to about twelve carbon atoms. More preferred alkylthio
radicals have
alkyl radicals which are "lower alkylthio" radicals having one to about ten
carbon atoms.
Most preferred are alkylthio radicals having lower alkyl radicals of one to
about eight
carbon atoms. Examples of such lower alkylthio radicals include methylthio,
ethylthio,
propylthio, butylthio and hexylthio.
The terms "aralkyl" or "arylallcyl" refer to aryl-substituted alkyl radicals
such as
benzyl, diphenylmethyl, triphenylmethyl, phenylethyl, and diphenylethyl.
The term "aryloxy" refers to aryl radicals attached through an oxygen atom to
other radicals.
The terms "aralkoxy" or "arylalkoxy" refer to aralk-yl radicals attached
through an
oxygen atom to other radicals.
The term "arninoalkyr refers to alkyl radicals substituted with amino
radicals.
Preferred aminoalkyl radicals have alkyl radicals having about one to about
twenty-four
carbon atoms or, preferably, one to about twelve carbon atoms. More preferred
aminoalkyl radicals are "lower aminoallcyl" that have alkyl radicals having
one to about
ten carbon atoms. Most preferred are aminoallcyl radicals having lower alkyl
radicals
having one to eight carbon atoms. Examples of such radicals include
aminomethyl,
aminoethyl, and the like.
The term "allcylatnino" denotes amino groups which are substituted with one or
two alkyl radicals. Preferred alkylamino radicals have alkyl radicals having
about one to
156
CA 3035442 2019-03-01
about twenty carbon atoms or, preferably, one to about twelve carbon atoms.
More
preferred alkylamino radicals are "lower alkylamino" that have alkyl radicals
having one
to about ten carbon atoms. Most preferred are alkylamino radicals having lower
alkyl
radicals having one to about eight carbon atoms. Suitable lower alkylamino may
be
monosubstituted N-alkylamino or disubstituted N,N-alkylamino, such as N-
methylamino, N-ethylamino, N,N-dimethylamino,N,N-diethylamino or the like.
The term "substituted" refers to the replacement of one or more hydrogen
radicals
in a given structure with the radical of a specified substituent including,
but not limited
to: halo, alkyl, alkenyl, alkynyl, aryl, heterocyclyt, thiol, alkylthio,
arylthio,
alkylthioalkyl, arylthioallcyl, alkylsulfonyl, alkylsulfonylalkyl,
arylsulfonylalkyl, alkoxy,
aryloxy, aralkoxy, arninocarbonyl, alkylaminocarbonyl, arylaminocarbonyl,
alkoxycarbonyl, aryloxycarbonyl, haloalkyt, amino, trifluoromethyl, cyano,
nitro,
alkylamino, arylamino, alkylaminoalkyl, arylaminoalkyl, aminoalkylamino,
hydroxy,
alkoxyalkyl, carboxyalkyl, alkoxycarbonylalkyl, aminocarbonylallcyl, acyl,
aralkoxycarbonyl, carboxylic acid, sulfonic acid, sulfonyt, phosphonic acid,
aryl,
heteroaryl, heterocyclic, and aliphatic. It is understood that the substituent
may be
further substituted.
For simplicity, chemical moieties that are defined and referred to throughout
can
be univalent chemical moieties (e.g., alkyl, aryl, etc.) or multivalent
moieties under the
appropriate structural circumstances clear to those skilled in the art. For
example, an
"alkyl" moiety can be referred to a monovalent radical (e.g. CH3-CH2-), or in
other
instances, a bivalent linking moiety can be "alkyl," in which case those
skilled in the art
will understand the alkyl to be a divalent radical (e.g., -CH2-CI-12-), which
is equivalent
to the term "alkylene." Similarly, in circumstances in which divalent moieties
are
required and are stated as being "alkoxy", "alkylamino", "aryloxy",
"alkylthio", "aryl",
"heteroaryl", "heterocyclic", "alkyl" "alkenyl", "alkynyl", "aliphatic", or
"cycloalkyl",
those skilled in the art will understand that the terms alkoxy", "alkylamino",
"aryloxy",
"alkylthio", "aryl", "hetcroaryl", "heterocyclic", "alkyl", "alkenyl",
"alkynyl",
"aliphatic", or "cycloalkyl" refer to the corresponding divalent moiety.
The terms "halogen" or "halo" as used herein, refers to an atom selected from
fluorine, chlorine, bromine and iodine.
The terms "compound" "drug", and "prodrug" as used herein all include
pharmaceutically acceptable salts, co-crystals, solvates, hydrates,
polymorphs,
157
CA 3035442 2019-03-01
enantiomers, diastereoisomers, racemates and the like of the compounds, drugs
and
prodrugs having the formulas as set forth herein.
Substituents indicated as attached through variable points of attachments can
be
attached to any available position on the ring structure.
As used herein, the term "effective amount of the subject compounds," with
respect to the subject method of treatment, refers to an amount of the subject
compound
which, when delivered as part of desired dose regimen, brings about management
of the
disease or disorder to clinically acceptable standards.
"Treatment" or "treating" refers to an approach for obtaining beneficial or
desired
clinical results in a patient. For purposes of this invention, beneficial or
desired clinical
results include, but are not limited to, one or more of the following:
alleviation of
symptoms, diminishment of extent of a disease, stabilization (i.e., not
worsening) of a
state of disease, preventing spread (i.e., metastasis) of disease, preventing
occurrence or
recurrence of disease, delay or slowing of disease progression, amelioration
of the
disease state, and remission (whether partial or total).
The term "labile" as used herein refers to the capacity of the prodrug of the
invention to undergo enzymatic and/or chemical cleavage in vivo thereby
forming the
parent parent drug. As used herein the term "prodnig" means a compounds as
disclosed
herein which is a labile derivative compound of a heteroaromatic NH-containing
parent
drag which when administered to a patient in vivo becomes cleaved by chemical
and/or
enzymatic hydrolysis thereby forming the parent drug such that a sufficient
amount of
the compound intended to be delivered to the patient is available for its
intended
therapeutic use in a sustained release manner.
Pharmaceutical Compositions
The pharmaceutical compositions of the present invention comprise a
therapeutically effective amount of a compound of the present invention
formulated
together with one or more pharmaceutically acceptable carriers or excipients.
As used herein, the term "pharmaceutically acceptable carrier or excipient"
means a non-toxic, inert solid, semi-solid, gel or liquid filler, diluent,
encapsulating
material or formulation auxiliary of any type. Some examples of materials
which can
serve as pharmaceutically acceptable carriers are sugars such as lactose,
glucose and
sucrose; cyclodextrins such as alpha- (a), beta- (p) and gamma- (7)
cyclodextrins;
starches such as corn starch and potato starch; cellulose and its derivatives
such as
sodium carboxymethyl cellulose, ethyl cellulose and cellulose acetate;
powdered
158
CA 3035442 2019-03-01
tragacanth; malt; gelatin; talc; excipients such as cocoa butter and
suppository waxes;
oils such as peanut oil, cottonseed oil, safflower oil, sesame oil, olive oil,
corn oil and
soybean oil; glycols such as propylene glycol; esters such as ethyl oleate and
ethyl
laurate; agar, buffering agents such as magnesium hydroxide and aluminum
hydroxide;
alginic acid; pyrogen-free water; isotonic saline; Ringer's solution; ethyl
alcohol, and
phosphate buffer solutions, as well as other non-toxic compatible lubricants
such as
sodium lauryl sulfate and magnesium stearate, as well as coloring agents,
releasing
agents, coating agents, sweetening, flavoring and perfuming agents,
preservatives and
antioxidants can also be present in the composition, according to the judgment
of the
formulator.
The pharmaceutical compositions of this invention may be administered orally,
parenterally, by inhalation spray, topically, rectally, nasally, buccally,
vaginally or via an
implanted reservoir. In a preferred embodiment, administration is parenteral
administration by injection.
The pharmaceutical compositions of this invention may contain any conventional
non-toxic pharmaceutically-acceptable carriers, adjuvants or vehicles. In some
cases, the
pH of the formulation may be adjusted with pharmaceutically acceptable acids,
bases or
buffers to enhance the stability of the formulated compound or its delivery
form. The
term parenteral as used herein includes subcutaneous, intracutaneous,
intravenous,
intramuscular, intraarticular, intraarterial, intrasynovial, intrasternal,
intrathecal,
intralesional and intracranial injection or infusion techniques.
Liquid dosage forms for oral administration include pharmaceutically
acceptable
emulsions, microemulsions, solutions, suspensions, syrups and elixirs. In
addition to the
active compounds; the liquid dosage forms may contain inert diluents commonly
used in
the art such as, for example, water or other solvents, solubilizing agents and
emulsifiers
such as ethyl alcohol, isopropyl alcohol, ethyl carbonate, ethyl acetate,
benzyl alcohol,
benzyl benzoate, propylene glycol, 1,3-butylene glycol, dimethylformamide,
oils (in
particular, cottonseed, groundnut, corn, germ, olive, castor, and sesame
oils), glycerol,
tetrahydrofurfuryl alcohol, polyethylene glycols and fatty acid esters of
sorbitan, and
mixtures thereof. Besides inert diluents, the oral compositions can also
include
adjuvants such as wetting agents, emulsifying and suspending agents,
sweetening,
flavoring, and perfuming agents.
Injectable preparations, for example, sterile injectable aqueous or oleaginous
suspensions, may be formulated according to the known art using suitable
dispersing or
159
CA 3035442 2019-03-01
wetting agents and suspending agents. The sterile injectable preparation may
also be a
sterile injectable suspension or emulsion, such as INTRALIPID , LIPOSYN or
OMEGAVEN , or solution, in a nontoxic parenterally acceptable diluent or
solvent, for
example, as a solution in 1,3-butanediol. TNTRALIPID is an intravenous fat
emulsion
containing 10-30% soybean oil, 1-10% egg yolk phospholipids, 1-10% glycerin
and
water. LIPOSYN is also an intravenous fat emlusion containing 2-15% safflower
oil,
2-15% soybean oil, 0.5-5% egg phosphatides 1-10% glycerin and water.
OMEGAVEN is an emulsion for infusion containing about 5-25% fish oil, 0.5-10%
egg phosphatides, 1-10% glycerin and water. Among the acceptable vehicles and
solvents that may be employed are water, Ringer's solution, USP and isotonic
sodium
chloride solution. In addition, sterile, fixed oils are conventionally
employed as a
solvent or suspending medium. For this purpose any bland fixed oil can be
employed
including synthetic mono- or diglycerides. In addition, fatty acids such as
oleic acid are
used in the preparation of injeetables.
The injectable formulations can be sterilized, for example, by filtration
through a
bacterial-retaining filter, or by incorporating sterilizing agents in the form
of sterile solid
compositions which can be dissolved or dispersed in sterile water or other
sterile
injectable medium prior to use.
Additional sustained release in accordance with the invention may be
accomplished by the use of a liquid suspension of crystalline or amorphous
material with
poor water solubility. The rate of absorption of the drug then depends upon
its rate of
dissolution, which, in turn, may depend upon crystal size and crystalline
form.
Alternatively, delayed absorption of a parenterally administered drug form is
accomplished by dissolving or suspending the drug in an oil vehicle.
Injectable depot
forms are made by forming microencapsule matrices of the drug in biodegradable
polymers such as polylactide-polyglycolide. Depending upon the ratio of drug
to
polymer and the nature of the particular polymer employed, the rate of drug
release can
be controlled. Examples of other biodegradable polymers include
poly(orthoesters) and
poly(anhydrides). Depot injectable formulations are also prepared by
entrapping the
drug in liposomes or microemulsions that are compatible with body tissues.
In one preferred embodiment, the formulation provides a sustained release
delivery system that is capable of minimizing the exposure of the prodrug to
water. This
can be accomplished by formulating the prodrug with a sustained release
delivery system
that is a polymeric matrix capable of minimizing the diffusion of water into
the matrix.
160
CA 3035442 2019-03-01
Suitable polymers comprising the matrix include polylactide (PLA) polymers and
the
lactide/glycolide (PLGA) co-polymers.
Alternatively, the sustained release delivery system may comprise poly-anionic
molecules or resins that are suitable for injection or oral delivery. Suitable
polyanionic
molecules include cyclodextrins and polysulfonates formulated to form a poorly
soluble
mass that minimizes exposure of the prodrug to water and from which the
prodrug
slowly leaves.
Compositions for rectal or vaginal administration are preferably suppositories
which can be prepared by mixing the compounds of this invention with suitable
non-
irritating excipients or carriers such as cocoa butter, polyethylene glycol or
a suppository
wax which arc solid at ambient temperature but liquid at body temperature and
therefore
melt in the rectum or vaginal cavity and release the active compound.
Solid dosage forms for oral administration include capsules, tablets, pills,
powders, and granules. hi such solid dosage forms, the active compound is
mixed with
at least one inert, pharmaceutically acceptable excipient or carrier such as
sodium citrate
or dicalcium phosphate and/or: a) fillers or extenders such as starches,
lactose, sucrose,
glucose, mannitol, and silicic acid, b) binders such as, for example,
carboxymethylcellulose, alginates, gelatin, polyvinylpyrrolidinone, sucrose,
and acacia,
c) humectants such as glycerol, d) disintegrating agents such as agar-agar,
calcium
carbonate, potato or tapioca starch, alginic acid, certain silicates, and
sodium carbonate,
e) solution retarding agents such as paraffin, f) absorption accelerators such
as
quaternary ammonium compounds, g) wetting agents such as, for example, cetyl
alcohol
and glycerol monostearate, h) absorbents such as kaolin and bentonite clay,
and i)
lubricants such as talc, calcium stearate, magnesium stearate, solid
polyethylene glycols,
sodium lauryl sulfate, and mixtures thereof. In the case of capsules, tablets
and pills, the
dosage form may also comprise buffering agents.
Solid compositions of a similar type may also be employed as fillers in soft
and
hard-filled gelatin capsules using such excipients as lactose or milk sugar as
well as high
molecular weight polyethylene glycols and the like.
The solid dosage forms of tablets, dragees, capsules, pills, and granules can
be
prepared with coatings and shells such as enteric coatings and other coatings
well known
in the pharmaceutical formulating art. They may optionally contain pacifying
agents
and can also be of a composition that they release the active ingredient(s)
only, or
preferentially, in a certain part of the intestinal tract, optionally, in a
delayed manner.
161
CA 3035442 2019-03-01
Examples of embedding compositions that can be used include polymeric
substances and
waxes.
Dosage forms for topical or transdermal administration of a compound of this
invention include ointments, pastes, creams, lotions, gels, powders,
solutions, sprays,
inhalants or patches. The active component is admixed under sterile conditions
with a
pharmaceutically acceptable carrier and any needed preservatives or buffers as
may be
required. Ophthalmic formulation, ear drops, eye ointments, powders and
solutions are
also contemplated as being within the scope of this invention.
The ointments, pastes, creams and gels may contain, in addition to an active
compound of this invention, excipients such as animal and vegetable fats,
oils, waxes,
paraffins, starch, tragacanth, cellulose derivatives, polyethylene glycols,
silicones,
bentonites, silicic acid, talc and zinc oxide, or mixtures thereof
Powders and sprays can contain, in addition to the compounds of this
invention,
excipients such as lactose, talc, silicic acid, aluminum hydroxide, calcium
silicates and
polyamide powder, or mixtures of these substances. Sprays can additionally
contain
customary propellants such as chlorofluorohydrocarbons.
Transdermal patches have the added advantage of providing controlled delivery
of a compound to the body. Such dosage forms can be made by dissolving or
dispensing
the compound in the proper medium. Absorption enhancers can also be used to
increase
the flux of the compound across the skin. The rate can be controlled by either
providing
a rate controlling membrane or by dispersing the compound in a polymer matrix
or gel.
For pulmonary delivery, a therapeutic composition of the invention is
formulated
and administered to the patient in solid or liquid particulate form by direct
administration
e.g., inhalation into the respiratory system. Solid or liquid particulate
forms of the active
compound prepared for practicing the present invention include particles of
respirable
size: that is, particles of a size sufficiently small to pass through the
mouth and larynx
upon inhalation and into the bronchi and alveoli of the lungs. Delivery of
aerosolized
therapeutics, particularly aerosolized antibiotics, is known in the art (see,
for example
U.S. Pat. No. 5,767,068 to VanDevanter et al., U.S. Pat. No. 5,508,269 to
Smith et al.,
and WO 98/43650 by Montgomery _
A discussion of pulmonary delivery of antibiotics is also found in U.S. Pat.
No.
6,014,969 .
By a "therapeutically effective amount" of a prodrug compound of the invention
is meant an amount of the compound which confers a therapeutic effect on the
treated
162
CA 3035442 2019-03-01
subject, at a reasonable benefit/risk ratio applicable to any medical
treatment. The
therapeutic effect may be objective (i.e., measurable by some test or marker)
or
subjective (i.e., subject gives an indication of or feels an effect).
In accordance with the invention, the therapeutically effective amount of a
prodrug of the invention is typically based on the target therapeutic amount
of the parent
drug. Information regarding dosing and frequency of dosing is readily
available for
many parent drugs from which the prodrugs of the invention are derived and the
target
therapeutic amount can be calculated for each prodrug of the invention. In
accordance
with the invention, the same dose of a prodrug of the invention provides a
longer
duration of therapeutic effect as compared to the parent drug. Thus if a
single dose of the
parent drug provides 12 hours of therapeutic effectiveness, a prodrug of that
same parent
drug in accordance with the invention that provides therapeutic effectiveness
for greater
than 12 hours will be considered to achieve a "sustained release".
The precise dose of a prodrug of the invention depends upon several factors
including the nature and dose of the parent drug and the chemical
characteristics of the
prodrug moiety linked to the parent drug. Ultimately, the effective dose and
dose
frequency of a prodrug of the invention will be decided by the attending
physician within
the scope of sound medical judgment. The specific therapeutically effective
dose level
and dose frequency for any particular patient will depend upon a variety of
factors
including the disorder being treated and the severity of the disorder; the
activity of the
specific compound employed; the specific composition employed; the age, body
weight,
general health, sex and diet of the patient; the time of administration, route
of
administration, and rate of excretion of the specific compound employed; the
duration of
the treatment; drugs used in combination or contemporaneously with the
specific
compound employed; and like factors well known in the medical arts.
EXAMPLES
The compounds and processes of the present invention will be better understood
in connection with the following examples, which are intended as an
illustration only and
not limiting of the scope of the invention. Various changes and modifications
to the
disclosed embodiments will be apparent to those skilled in the art and such
changes and
modifications including, without limitation, those relating to the chemical
structures,
substituents, derivatives, formulations and/or methods of the invention may be
made
without departing from the spirit of the invention and the scope of the
appended claims.
163
CA 3035442 2019-03-01
General methodology for the preparation of lactam compounds can be found in
the
following publications: U.S. Patent No. 7,160,888; U.S. Patent No. 5,462,934;
U.S.
Patent No. 4,914,094; U.S. Patent No. 4,234,584; U.S. Patent No. 4,514,401;
U.S. Patent
No. 5,462,934; U.S. Patent No. 4,468,402; WO 2006/090273 A2; WO 2008/150848
Al;
WO 2006/112464 Al; WO 2008/132600 Al.
cH20 110 Cl
CI
DMF, NEt3
40
HN
Example
Aripiprazole
0 or:
BnNCO, DMAP, a so
NEt,
Example 2
Preparation of 7-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butoxy)-1-
(bydroxymethyl)-3,4-dthydroquinolin-2(1H)-one (Example 1: Compound Al)
A mixture of Aripiprazole (20g, 45 mmol), triethylamine (1mL, 7.1 mmol),
formaldehyde (37% aqueous solution, 70 mL) and dimethylformamide (200 mL) was
heated to 80 C for 20 h. The reaction mixture was cooled, diluted with ethyl
acetate (400
mL) and washed with water/brine (1:1, 3 x 500 mL). The organic phase was dried
over
MgSO4, filtered and evaporated to dryness under vacuum to give hemi-aminal Al
as a
white solid (18.6 g, containing 25% Aripiprazole, 65% yield based on Al).
NMR (CDC13, 3001VIliz) complex mixture of signals due to contamination with
Aripiprazolc, main signal 8 5.34 (s, 2H, OHCH2N); nilz (M+H) 478 and 480.
(7-(4-(4-(2,3-dichlorophenyl)piperazin-l-yl)butoxy)-2-oxo-3,4-dihydroquinolin-
1(211)-yl)metbyl benzylcarbamate (Example 2: Compound 28)
To a solution of hemi-aminal, Al, from Example 1(4 g, 8.4 mmol), 4-
dimethylaminopyridine (0.15g, 1.3 mmol) and triethylamine (1.1 mL, 7.5 mmol)
in
dichlorornethane (30 mL) was added benzylisocyanate (1.03 mL, 8.3 mmol) and
the
reaction mixture stirred for 24 hours. The reaction mixture was then heated at
35 C for
20 hours, cooled and washed with water/brine (1:1, 50 mL). The organic phase
was dried
over MgSO4, filtered and evaporated under vacuum. The residue was further
purified by
chromatography on silica eluting with ethyl acetate/dichloromethane/methanol
(1:1:0.1)
164
CA 3035442 2019-03-01
to give the desired product as an off white foam (530 mg, 14% yield). 111NMR
(CDC13,
300MHz) 8 1.58-1.88 (m, 414), 2.48 (t, 211), 2.60-2.72 (m, 6H), 2.85 (n, 2H),
300-3.12
(in, 411), 3.96 (t, 2H), 4.40 (d, 211), 5.13 (NH), 5.96 (s, 211), 6.58 (dd,
1H), 6.79 (d, 111),
6.92-6.98 (m, 1H), 7.04 (d, 1H), 7.12-7.16 (in. 1H), 7.23-7.35 (m, 6H); m/z
(M+11)
611.12 and 613.10.
The following compounds were prepared in an analogous fashion to Example 2.
(7-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butoxy)-2-oxo-3,4-dihydroquinolin-
1(211)-yl)methyl ethyl carbonate (Example 3: Compound 79) The desired product
was isolated as a yellow oil (830 mg, 24% yield). Ili NMR (c16-DMSO, 300MHz)
61.78
(t, 3H), 1.52-1.61 (m, 211), 1.63-1.76 (in, 2H), 2.31-2.40 (n, 2H), 2.40-2.60
(in, 6H),
2.73-2.80 (m, 2H), 2.91-2.99 (n, 4H), 3.96 (t, 311), 4.11 (q, 211), 5.87(s,
2H), 6.60-6.70
(n, 2H), 7.07-7.12 (in, 2H), 7.24-7.30 (m, 211); rn/z (M4-14) 550.48 and
552.40.
butyl (7-(4-(4-(2,3-dichlorophenyl)piperazin-l-yl)butoxy)-2-oxo-3,4-
dihydroquinolin-1(211)-yl)methyl carbonate (Example 4: Compound 80) The
desired product was isolated as a yellow oil (750mg, 21% yield). 'H NMR
(CDC13,
300MHz) 8 0.92 (t, 311), 1.33-1.45 (m, 211), 1.59-1.80 (in, 4H), 1.80-1.92
(in, 211), 2.49
(t, 2H), 2.58-2.75 (in, 6H), 2.85 (t, 2H), 3.00-3.13 (m, 4H), 3.98 (t, 2H),
4.18 (t, 211),
5.92 (s, 211), 6.58 (dd, 1H), 6.67 (d, 1H), 6.92-6.99 (m, 1H), 7.03 (dd, 114),
7.10-7.20 (m,
2H); m/z (1v114) 578.10 and 580.08.
(7-(4-(4-(2,3-dichlorophenyl)piperaAn-1-yl)butoxy)-2-oxo-3,4-dihydroquinolin-
1(211)-yl)methyl hexyl carbonate (Example 5: Compound 81) The desired product
was isolated as a yellow oil (1.77g, 62% yield). 114 NMR (d6-DMSO, 300MHz) 8
0.80
(t, 314), 1.15-1.30 (m, 611), 1.50-1.60 (in, 4H), 1.65-1.73 (m, 2H), 2.35 (t,
2H), 2.41-2.60
(n, 6H), 2.78 (t, 211), 2.88-3.00 (n, 411), 3.95 (t, 2H), 4.06 (t, 211), 5.86
(s, 214), 6.60-
6.70 (n, 211), 7.05-7.15 (n, 2H), 7.22-7.28 (m 2H); m/z (WH) 606.15 and
608.15.
decyl (7-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butoxy)-2-oxo-3,4-
dihydroquinolin-1(2H)-yl)methyl carbonate (Example 6: Compound 82) The
desired product was isolated as a yellow oil (1.42g, 46% yield). 111 NMR (d6-
DMSO,
300MHz) 60.79 (m, 3H), 1.13-1.30 (m, 14H), 1.48-1.60 (m, 411), 1.65-1.75 (n,
211),
2.33 (t, 211), 2.41-2.60 (in, 611), 2.72-2.80 (in, 2H), 2.89-2.98 (m, 4H),
3.95 (t, 211), 4.05
(t, 2H), 5.86 (s, 2H), 6.60-6.70 (m, 211), 7.05-7.13 (m, 2H), 7.22-7.28 (m,
211); m/z
(WH) 662.56 and 664.54.
165
CA 3035442 2019-03-01
(7-(4-(4-(2,3-dichlorophenyl)piperazin-l-yl)butoxy)-2-oxo-3,4-dihydroquinolin-
1(2H)-yl)methyl hexadecyl carbonate (Example 7: Compound 83) The desired
product was isolated as a yellow oil (1.55g, 44% yield). 'H NMR (d6-DMSO,
300MHz)
0.80 (t, 3H), 1.10-1.29 (m, 26H), 1.49-1.60 (m, 4H), 1.65-1.75 (in, 2H), 2.33
(t, 2H),
2.43-2.55 (m, 6H), 2.78 (t, 2H), 2.90-2.95 (m, 4H), 3.95 (1, 2H), 4.05 (t,
2H), 5.84 (s,
2H), 6.60-6.68 (in, 2H), 7.05-7.12 (n, 2H), 7.24-7.29 (m, 2H); ni/z (M-CoH2o)
606.52
and 608.54.
(7-(4-(4-(2,3-dichlorophenyl)piperazin-l-yl)butoxy)-2-oxo-3,4-dihydroquinolin-
1(211)-yl)methyl morpholine-4-carboxylate (Example 8: Compound 49) The desired
product was isolated as a yellow oil (1.52g, 55% yield). 1H NMR (d6-DMSO,
300MHz)
8 1.50-1.75 (m, 4H), 2.35 (t, 2H), 2.42-2.61 (in, 6H), 2.70-2.82 (m, 2H), 2.88-
3.00 (m,
411), 3.26-3.40 (in, 4H), 3.40-3.60 (m, 4H), 3.94 (t, 2H), 5.81 (s, 2H), 6.61
(dd, 1H), 6.68
(d, 1H), 7.05-7.13 (in, 2H), 7.20-7.30 (ni, 2H); raiz (1v111) 591.11 and
593.15.
(7-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butoxy)-2-oxo-3,4-dihydroquinolin-
1(2H)-yl)methyl diethylcarbamate (Example 9: Compound 84) The desired product
was isolated as a yellow oil (0.83g, 31% yield). 11INMR (CDC13, 300MHz) 8 1.00-
1.20
611), 1.65-1.88 (in, 4H), 2.45-2.52 (m, 2H), 2.58-2.83 (in, 6H), 2.82-2.90 (m,
2H),
3.00-3.12 (m, 4H), 3.18-3.38 (m, 4H), 3.97 (t, 2H), 5.91 (s, 2H), 6.58 (dd,
111), 6.77 (d,
1H), 6.94-6.98 (m, 1H), 7.06 (d, 1H), 7.15-7.20 (m, 211); ink (MIT) 577.48 and
579.46.
(7-(4-(4-(2,3-dichlorophenyppiperazin-l-yl)butoxy)-2-oxo-3,4-dihydroquinolin-
1(21)-y1)methyl lsopentyl carbonate (Example 10: Compound 85) To a solution of
phosgene (20% in toluene, 54 mL, 110 mmol) in tetrahydrofuran (100 mL) was
added a
solution of 3-methyl-1-butanol (1.7 mL, 15.7 mmol) in tetrahydrofuran (50 mL)
over 1
hour. After 4 hours the volatiles were removed under vacuum and the residue
added to a
solution of the hemi-aminal Al (3 g, 4.7 mmol), 4-dimethylaminopyridine (0.3
g, 1.9
mmol), pyridine (10 mL) and triethylamine (1.3 mL, 9.4 mmol) in
diehloromethane (30
mL). After being stirred for 72 hours, the reaction mixture was diluted with
ethyl acetate
(100 mL) and washed with 5% aqueous NaHCO3/brine (1:1, 100 mL). The organic
phase
was dried over MgSO4, filtered and evaporated under vacuum. The residue was
further
purified by chromatography on silica eluting with ethyl
acetate/dichloromethane/methanol (1:1:0.1) to give the desired product as a
yellow oil
(1.54 g, 55% yield). 111 NMR (CDC13, 300MHz) 8 1.90-1.95 (m, 6H), 1.50-1.60
(in,
4H), 1.65-1.79 (in, 2H), 1.79-1.89 (n, 2H), 2.50 (t, 2H), 2.60-2.72 (m, 6H),
2.82-2.90
(m, 211), 3.02-3.11 (m, 4H), 3.98 (t, 2H), 4.21 (t, 211), 5.92 (s, 2H), 6.56
(dd, 1H), 6.67
166
CA 3035442 2019-03-01
(d, 1H), 6.95-7.00 (m, 1H), 7.05 (d, 1H), 7.13-7.19 (in, 2H); m/z (WE) 592.48
and
594.46.
(7-(4-(4-(2,3-dichlorophenyl)piperazin-l-yl)butoxy)-2-oxo-3,4-dihydroquinolin-
1(2H)-y1)methyl acetate (Example 11: Compound 1)
0 is
CI r---N-------- acetic anhydride CI io
THF, 60 C
HOH2C.N H3C--esi
0 0
A solution of Compound-Al from Example-1, (50.63 g, 0.105 mol) in anhydrous
tetrahydrofuran (THF, 80 mL) was treated with acetic anhydride (15.3 mL, 0.16
mol)
and heated for 2.0 hours at 60 C (oil-bath). To the above solution,
triethylamine (2.0
mL, 0.014 mol) was added and stirred for 16 hours at 60 C. The solvent was
removed
using a rotator evaporator. To the resulting crude mixture, ethyl acetate (150
mL) and
heptane (50 mL) was added. The solution was washed with NaHCO3 (5% aqueous
solution, 250 mL). After separation of the two layers, pH of the aqueous layer
was
adjusted to above 7. The aqueous layer was further extracted using the organic
mixture.
The organic layer was separated and washed with 5% NaHCO3 solution, followed
by
deionized water, and brine. The solution was dried using anhydrous MgSO4,
filtered and
evaporated under vacuum. The resulting product was purified using silica gel
column
chromatography using ethanol: ethyl acetate (5:95) as the eluent. Fractions
containing
the desired product were combined and d-tartaric acid (12.5 g dissolved in
60:5 ethanol:
water) was added, resulting in the precipitation of the desired product (48.78
g, 89%
yield). 1H NMR (CDCI3, 300MHz) 51.73 (m, 2H), 1.84 (m, 2H), 2.12 (s, 311),
2.50 (t,
2H), 2.68 (m, 6H), 2.87 (dd, 211), 3.08 (m, 411), 3.98 (t, 211), 5.91 (s,
211), 6.59 (m, 2H),
6.96 (dd, 1H), 7.08 (dd, 1H), 7.15 (m, 2H).
The following compounds were prepared in an analogous fashion to Example 11.
(7-(4-(4-(2,3-dichlorophenyl)piperazin-l-yl)butoxy)-2-oxo-3,4-dihydroquinolin-
1(21-1)-yllmethyl dodecanoate (Example 12: Compound 7) The desired product was
isolated as a crystalline solid (0.3 g, 21 % yield). The molecular weight was
confirmed
by mass spectrometer analysis. Figure 2-6 shows the PXRD, IR, Raman, TGA
spectrum
of the desired product. 1H NMR (CDCI3, 300MHz) 60.87 (t, 3H), 1.24 (m, 16H),
1.62
(to, 2H), 1.83 (m, 2H), 1.86 (m, 2H), 2.36 (t, 2H), 2.49 (t, 211), 2.68 (m,
6H), 2.86 (dd,
167
CA 3035442 2019-03-01
2H), 3.08 (m, 4H), 3.97 (t, 2H), 5.91 (s, 2H), 6.59 (m, 2H), 6.96 (dd, 1H),
7.07 (dd, 1H),
7.14 (m, 2H). See Figures x-y for further characterization (PXRD, IR, Raman,
TGA and
DSC spectra) of Compound 7.
(7-(4-(4-(2,3-dichlorophenyl)piperazin-l-yl)butoxy)-2-oxo-3,4-dihydroquinolin-
1(211)-yl)methyl palmitate (Example 13: Compound 10)
CI 0 ) =
paimitic anhydride
ci N,
THE, 60 C
HOH2C'N
0 0
H3C 0
The desired product was isolated as a crystalline solid (4.2 g, 70 % yield).
The
molecular weight (716.6) was confirmed by mass spectrometer analysis. 11-1 NMR
(CDC13, 300MHz) 5 0.88 (t, 3H),1.25 (m, 24 H), 1.64 (m, 2H), 1.72 (m, 2H),
1.84 (m,
2H), 2.36 (t, 2H), 2.49 (t, 2H), 2.68 (m, 6H), 2.86 (dd, 2H), 3.08 (m, 4H),
3.97 (t, 2H),
5.92 (br s, 2H), 6.59 (dd, 1H), 6.60 (s, IH), 6.96 (dd, 1H), 7.07 (d, 1H),
7.14 (m, 2H).
(7-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butoxy)-2-oxo-3,4-dihydroquinolin-
1(211)-yl)methyl decanoate (Example 14: Compound 6)
o tornt"-"--' 1. NaH/1,4-DIOXANE a
ci so N,)
HN 2.
0 o 0
The chloromethyl ester above is dried over 4 A molecular sieves. A solution of
aripiprazole (45 grams, 0.1 mol) in 1,4-dioxane (800 mL) was sonicated to
dissolve the
aripiprazole completely, and then treated with NaH (38 g, 0.95 mol, 60%
dispersion) in
one portion. After stirring this reaction mixture for 15 minutes at room
temperature, the
reaction mixture was treated dropwise with chloromethyl ester (0.3 mol.) and a
catalytic
amount of sodium iodide (0.05 mol). The resultant cloudy mixture was heated to
90 C
for 2 hours, cooled to ambient temperature and poured into water. The product
was
extracted with ethyl acetate, and the combined ethyl acetate layers washed
with brine,
dried over sodium sulfate, filtered and concentrated under reduced pressure.
Column
chromatography over silica gel provided the desired product (12.5 gram, 70%
yield). 11-1
NMR (CDC13, 300MHz) 8 0.87 (t, 3H), 1.20 (m, 12H), 1.63 (m, 2H), 1.70 (m, 2H),
1.83
(m, 2H), 2.35 (t, 2H), 2.50 (t, 2H), 2.68 (m, 6H), 2.86 (t, 2H), 3.08 (m, 4H),
3.97 (t, 2H),
168
CA 3035442 2019-03-01
5.92 (s, 2H), 6.58 (dd, 114), 6.61 (d, 1H), 6.94 (dd, 1H), 7.06 (d, 111), 7.14-
7.17 (m, 2H);
iniz (M4-14) 632.88.
The following compounds (Examples 15-29) were prepared in an analogous fashion
to
Example 2:
(7-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butoxy)-2-oxo-3,4-dihydroquinolin-
1(211)-yl)methyl benzoate (Example 15, Compound 31) The desired product was
isolated as a yellow oil.
TH NMR (CDC13, 300MHz) 6 1.60-1.85 (m, 4H), 2.45 (t, 2H), 2.55-2.70 (m, 411),
2.70-
2.78 (m, 2H), 2.85-2.92 (m, 2H), 3.00-3.10 (rn, 4H), 3.94 (t, 2H), 6.16 (s,
211), 6.60 (d,
111), 6.72 (dd, 111), 6.90-6.95 (n, 1H), 7.05-7.18 (m, 211), 7.35-7.42 (m,
2H), 7.52-7.60
(m, 111), 8.00-8.08 (m, 2H). m/z (MB) 582.3.
(7-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butoxy)-2-oxo-3,4-dihydroquinolin-
1(2H)-yl)methyl butyrate (Example 16, Compound 2) The desired product was
isolated by chromatography on silica eluting with ethyl
acetate/dichloromethane/methanol (1:1:0.1) to give a yellow oil (2.0g, 87%
yield). Ili
NMR (CDC13, 300MHz) 30.94 (t, 3H), 1.60-1.90 (m, 6H), 2.34 (t, 2H), 2.51 (t,
211),
2.61-2.73 (m, 6H), 2.82-2.90 (m, 2H), 3.02-3.12 (m, 411), 3.96 (t, 2H), 5.91
(s, 1H), 6.55-
6.61 (m, 211), 6.93-6.98 (in, 1H), 7.05 (d, 1H), 7.11-7.18 (m, 2H). miz (M+H)
548.2 and
550.2.
(7-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butoxy)-2-oxo-3,4-dihydroquinolin-
1(211)-yl)methyl hexanoate (Example 17, Compound 4) The desired product was
isolated as a yellow solid (3.69g, 87% yield). 'H NMR (CDC13, 300MHz) 8. 0.78
(t, 3H),
1.11-1.28 (m, 4H), 1.40-1.78 (m, 6H), 2.20-2.40 (in, 4H), 2.40-2.60 (in, 611),
2.73-2.81
(m, 2H), 2.85-3.00 (m, 411), 3.88-4.00 (m, 211), 5.75-5.83 (m, 211), 6.55-6.62
(m, 2H),
7.03-7.12 (m, 2H), 7.20-7.26 (m, 2H). raiz (MI) 576.4 and 578.4.
(7-(4-(4-(2,3-dichlorophenyl)plperazin-1-y1)butoxy)-2-oxo-3,4-dihydroquinolin-
1(2H)-y1)methyl tetradecanoate (Example 18, Compound 8) The desired product
was
isolated as a pale yellow solid (5.3g, 74% yield). 1H NMR (CDC13, 300MHz) 5
0.87 (t,
3H), 1.07-1.37 (m, 22H), 1.55-1.70 (m, 211), 1.70-1.90 (m, 411), 2.34 (t,
211), 2.53 (t,
_
2H), 2.65-2 78 (m, 611), 2.82-2.90 (in, 211), 3.02-3.12 (m, 411), 3.96 (t,
2H), 5.91 (s, 2H),
6.55-6.62 (m, 211), 6.92-6.98 (m, 1H), 7.05 (d, 111), 7.11-7.18 (m, 211). miz
(M+H) 688.4
and 690.4.
169
CA 3035442 2019-03-01
(7-(4-(4-(2,3-dichlorophenyppiperazin-l-y1)butoxy)-2-oxo-3,4-dihydroquinolin-
1(211)-yl)methyl octanoate (Example 19, Compound 5) The desired product was
isolated as a yellow oil (2.2g, 87% yield). 'H NMR (CDC13, 300MHz) 6 0.82 (t,
3H),
1.15-1.35 (m, 10H, 1.55-1.87 (m, 6H), 2.34 (t, 2H), 2.53 (t, 2H), 2.65-2.73
(m, 4H), 2.85
(dd, 2H), 3.01-3.11 (m, 4H), 3.95 (t, 2H), 5.85-5.92 (m, 2H), 2.53-2.60 (m,
2H), 6.91-
6.97 (m, 1H), 7.05 (d, 1H), 7.10-7.16 (m, 2H). m/z (M+H) 604.3 and 606.3.
(7-(4-(4-(2,3-dichlorophenyl)piperazin-l-yl)butoxy)-2-oxo-3,4-dihydroquinolin-
1(211)-yl)methyl isopropyl carbonate (Example 20, Compound 48) The desired
product was isolated as an orange oil (2.4g, 68% yield). NMR (CDC13,
300MHz) 8
1.31 (d, 6H), 1.62-1.77 (m, 2H), 1.77-1.89 (m, 2H), 2.48 (t, 2H), 2.60-2.71
(m, 6H),
2.81-2.90 (m, 2H), 3.01-3.11 (m, 4H), 3.98 (t, 2H), 4.89-4.97 (m, 1H), 5.92
(s, 2H), 6.57
(d, 1H), 6.68 (d, 1H), 6.91-7.00 (m, 1H), 7.05 (dd, 1H), 7.11-7.18 (m, 2H).
m/z (M4H)
564.3 and 566.3.
(7-(4-(4-(2,3-dichlorophenyl)piperazin-l-yl)butoxy)-2-oxo-3,4-dihydroquinolin-
1(211)-yOmethyl methylcarbamate (Example 21, Compound 47) The desired product
was isolated as a yellow solid (1.3g, 52% yield). 'H NMR (CDC13, 300MHz) 6
1.68-1.88
(m, 4H), 2.49 (dd, 2H), 2.60-2.73 (m, 611), 2.80-2.90 (m, 5H), 3.02-3.12 (m,
4H), 3.95-
4.02 (m, 2H), 5.90 (s, 2H), 6.57 (d, 1H), 6.77 (d, 1H), 6.93-6.70 (m, 1H),
7.05 (d, 1H),
7.10-7.19 (m, 2H). m/z (M+H) 535.5 and 537.5.
(7-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butoxy)-2-oxo-3,4-dihydroquinolin-
1(211)-y1)methyl decylcarbamate (Example 22, Compound 46) The desired product
was isolated as a yellow solid (0.50g, 14% yield). NMR (CDC13,
300MHz) 60.86 (t,
3H), 1.18-1.35 (m, 16H), 1.42-1.53 (m, 2H), 1.67-1.79 (m, 2H), 1.79-1.87 (m,
211), 2.48
(t, 2H), 2.58-2.72 (m, 4H), 2.80-2.90 (m, 2H), 3.01-3.12 (m, 4H), 3.15-3.22
(m, 2H),
3.98 (t, 2H), 4.78 (NH), 5.90 (s, 2H), 6.58 (d, 1H), 6.78 (d, 111), 6.93-7.00
(m, 1H), 7.04
(d, 111), 7.10-7.16 (m, 2H). rrilz (WH) 661.6 and 663.6.
(7-(4-(4-(2,3-dichlorophenyl)piperazin-1-Abutoxy)-2-oxo-3,4-dihydroquinolin-
1(211)-yOmethyl isobutyrate (Example 23, Compound 32)
114 NMR (CDC13, 300MHz) 6 1.18 (d, 6H), 1.68-1.88 (m, 4H), 2.45-2.73 (m, 911),
2.87
(dd, 2H), 3.03-3.12 (m, 211), 3.95 (t, 211), 5.91 (s, 211), 6.55-6.60 (m,
211), 6.93-6.97 (m,
111), 7.04-7.09 (in, 1H), 7.12-7.19 (m, 2H). rri/z (M+H) 548.15.
170
CA 3035442 2019-03-01
(7-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butoxy)-2-oxo-3,4-dihydroquinolin-
1(2H)-yl)methyl cyclopentanecarboxylate (Example 24, Compound 33)
1H NMR (CDC13, 300MHz) 8 1.47-1.93 (in, 13H), 2.50-2.60 (m, 211), 2.60-2.90
(in, 8H),
3.02-3.15 (m, 4H), 3.95 (t, 211), 5.89 (s, 2H), 6.50-6.60 (m, 211), 690-6.95
(m, I H), 7.02-
7.07 (n, 1H), 7.10-7.19 (n, 2H). rn/z (M+H) 574.15.
(7-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butoxy)-2-oxo-3,4-dihydroquinolin-
1(211)-ypmethyl cyclobutanecarboxylate (Example 25, Compound 34)
1H NMR (CDC13, 300MHz) 8 1.82-1.91 (m, 311), 1.22-1.30 (n, 2H), 1.75-2.05 (n,
611),
2.05-2.40 (m, 611), 2.68-2.73 (n, 211), 2.84-2.90 (m, 211), 3.06-3.22 (m, 4H),
3.96 (t,
2H), 5.91 (s, 2H), 6.55-6.59 (m, 211), 6.97 (dd, 111), 7.07 (d, 111), 7.12-
7.18 (m, 211). m/z
(WE) 560.19.
(7-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butoxy)-2-oxo-3,4-dihydroquinolin-
1(211)-yOmethyl cyclohexaneearboxylate (Example 26, Compound 35)
1H NMR (CDC13, 300MHz) 8 1.15-1.35 (m, 311), 1.35-1.55 (n, 2H), 1.55-1.95 (m,
1011),
2.21-2.40 (m, 111), 2.52-2.60 (m, 1H), 2.62-3.00 (m, 8H), 3.02-3.12 (m, 411),
3.95 (t,
2H), 5.89 (s, 211), 6.50-6.60 (in, 2H), 6.93-6.97 (m, 111), 7.02-7.06 (n, 1H),
7.10-7.15
(m, 2H). m/z (NITI) 588.24.
(7-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butoxy)-2-oxo-3,4-dihydroquinolin-
1(211)-yl)methyl 2-(2-methoxyethoxy)acetate (Example 27, Compound 40)
1H NMR (CDC13, 300MHz) 8 1.56-1.90 (m, 6H), 2.43-2.55 (m, 211), 2.55-2.80 (in,
4H),
2.81-2.90 (n, 211), 3.37 (s, 3H), 3.55-3.61 (m, 211), 3.72-3.79 (in, 211),
4.20 (s, 211), 5.97
(s, 211), 6.55-6.59 (m, 2H), 6.91-6.98 (m, 1H), 7.09 (d, 111), 7.11-7.15 (m,
2H). m/z
(MPH) 594.17.
(7-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butoxy)-2-oxo-3,4-dihydroquinolin-
1(211)-yl)methyl 2-(2-(2-methoxyethoxy)ethoxy)acetate (Example 28, Compound
41)
1H NMR (CDC13, 300MHz) 8 1.65-1.93 (m, 6H), 2.49-2.60 (m, 2H), 2.61-2.77 (m,
411),
2.81-2.90 (m, 2H), 3.02-3.20 (n, 4H), 3.36 (s, 3H), 3.51-3.57 (in, 2H), 3.60-
3.70 (m,
4H), 3.72-3.78 (m, 2H), 3.92-3.99 (m, 2H), 4.20 (s, 211), 5.97 (s, 2E1), 6.55-
6.59 (in, 2H),
6.95-6.99 (m, 1H), 7.05-7.09 (n, 111), 7.11-7.18 (in, 2H). m/z (MPH) 638.30.
(7-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butoxy)-2-oxo-3,4-dihydroquinolin-
1(21)-yl)methyl pivalate (Example 29, Compound 42)
'H NMR (CDC13, 300MHz) 8 1.21 (s, 911), 1.65-1.88 (m, 411), 2.45-2.55 (m, 2H),
2.60-
2.73 (m, 611), 2.82-2.91 (m, 2H), 3.02-3.13 (m, 411), 3.95 (t, 2H), 5.89 (s,
211), 6.54-6.60
(m, 211), 6.92-6.99 (m, 1H), 7.06 (d, 1H), 7.13-7.17 (m, 211); nliz (M-11)
562.39.
171
CA 3035442 2019-03-01
(7-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butoxy)-2-oxo-3,4-diltydroquinolin-
1(211)-yl)methyl 2-hydroxyethylcarbamate (Example 30, Compound 36)
2-(((7-(4-(4-(2,3-dichlorophenyppiperazin-l-ypbutoxy)-2-oxo-3,4-
dihydroquinolin-
1(211)-yOmethoxy)carbonylamino)ethyl methacrylate (2.0g) was synthesized in a
similar
manner to Example 2. This was reacted with 16% NH3/Me0H at room temperature
for
18 hours and then concentrated at 40 C. The residue was purified by silica
chromatography eluting with 1:1:0.1 to 1:1:0.2 DCM/Et0Ac/Me0H. The resulting
yellow oil was re-crystallised from Et0Aciheptane to give the title compound
as a white
solid (1.2g, 67%).
NMR (CDC13, 300MHz) 8 1.60-1.88 (m, 411), 2.40-2.50 (m, 2H), 2.50-2.75 (m,
6H),
2.75-2.89 (m, 2H), 2.95-3.15 (m, 4H), 3.20-3.40 (m, 2H), 2.58-3.78 (m, 2H),
3.89-4.05
(tn, 2H), 5.30-5.45 (in, NH), 5.91 (s, 211), 6.55 (dd, I H), 6.73 (d, IH),
6.91-6.96 (m, 111),
6.98-7.03 (m, 1H), 7.04-7.18 (m, 2H). m/z (WH) 565.16.
(7-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butoxy)-2-oxo-3,4-dihydroquinolin-
1(211)-yl)methyl bis(2-hydroxyethyl)carbamate (Example 31, Compound 37)
To a solution of hemiaminal Al (2g, 0.0042mo1) in dichloromethane (30mL) at
room
temperature was added pyridine (0.68mL), followed by p-
nitrophenylchloroformate
(1.27g, 0.0063mo1). After 90 minutes diethanolamine (3.5g, 0.0334mo1) and
triethylamine (1.2mL, 0.084mo1) were added. After 3h the reaction was diluted
with
dichloromethane and washed with sat. NaHCO3, dried over MgS0.4 and evaporated.
The
residue was purified on silica eluting with 1:1:0.I to 1:1:0.2 DCMJEt0Ac/Me0H
to give
the title compound as a colourless gum (0.83g, 33%).
NMR (CDC13, 300MHz) 8 1.70-1.82 (m, 4H), 2.42-2.52 (m, 2H), 2.59-2.79 (m, 6H),
2.80-2.90 (m, 2H), 3.00-3.12 (m, 4H), 3.40-3.48 (m, 2H), 3.50-3.58 (m, 2H),
3.61-3.70
(m, 211), 3.85-3.90 (m, 2H), 3.99406 (m, 2H), 5.90 (m, 211), 6.57 (d, 111),
6.70 (dd,
1H), 6.92-6.98 (m, 1H), 7.07 (d, 1H), 7.10-7.20 (m, 2H). rn/z (M+H) 609.21.
(7-(4-(4-(2,3-dlchlorophenyl)piperazin-l-yl)butoxy)-2-oxo-3,4-dihydroquinolin-
1(211)-yl)methyl 4-methylpiperazine-1-carboxylate (Example 32, Compound 38)
Compound 141 was synthesizedin a similar manner to Example 28.
NMR (CDC13, 300MHz) 6 1.68-1.88 (m, 4H), 2.25-2.42 (m, 711), 2.45-2.55 (m,
2H),
2.61-2.76 (m, 6H), 2.85 (dd, 2H), 3.02-3.16 (m, 411), 3.40-3.60 (m, 4H), 3.97
(t, 2H),
5,92 (s, 2H), 6.59 (d, 1H), 6.74 (d, 1H), 6.92-6.98 (m, 1H), 7.02-7.07 (m,
Hi), 7.10-7.16
(m, 2H). tn/z (MPH) 604.24.
172
CA 3035442 2019-03-01
(7-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butoxy)-2-oxo-3,4-dihydroquinolin-
1(2H)-yl)methyl 1,4'-bipiperidine-l'-carboxylate (Example 33, Compound 39)
Compound 142 was synthesized in a similar manner to Example 28.
IH NMR (CDC13, 300MHz) 8 1.26-2.06 (m, 14H), 2.31-2.91 (m, 17H), 2.95-3.18 (m,
4H), 3.97 (t, 2H), 4.0-4.37 (m, 2H), 5.91 (s, 2H), 6.58 (dd, 1H), 6.74 (d,
1H), 6.90-6.99
(m, 1H), 7.05 (d, 111), 7.11-7.18 (m, 2H); m/z (MPH) 672.25.
7-(4-(4-(2,3-dicblorophenyl)plperazin-1-yl)butory)-1-(methoxymethyl)-3,4-
dihydroquinolin-2(111)-one (Example 34, Compound 100)
To a mixture of hemiaminal Al (2.0g, 4.2mmo1) in dichloromethane (20mL) was
added
thionyl chloride (1.5mL, 12.6nuno1) and stirred for 2h at room temperature. To
the
reaction mixture was added methanol (10mL) and stirred a further 2h. The
reaction
poured into NaHCO3 (aq) and extracted with dichloromethane. The organic phase
dried
over MgSO4, evaporated and the residue purified on silica eluting with 1:1:0.1
dichloromethane/ethyl acetate/methanol to give the title compound as a cream
solid
(1.3g,63%).
1H NMiR (CDC13, 300MHz) 8 1.65-1.83 (m, 4H), 2.47 (t, 211), 2.58-2.70 (m, 6H),
2.82
(dd, 2H), 2.99-3.01 (m, 4H), 3.38 (s, 3H), 3.96 (t, 2H), 5.27 (s, 2H), 6.55
(dd, 1H),6.88
(dd, 1H), 6.91-6.96 (in, 1H), 7.03 (d, 1H), 7.08-7.15 (m, 213). m/z (MPH)
492.05.
1-(7-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butory)-2-oxo-3,4-
dihydroquinolin-
1(2H)-y1)-2-ethory-2-oroethyl decanoate (Example 35, Compound 111)
A mixture of Aripiprazole (2.0g, 4.5mmo1), ethyl glyoxylate (50% soln. in
toluene,
2.7mL), K2CO3 (0.49g, 3.6mmo1), tetrabutylammonium bromide (0.57g, 1.8mmol)
and
dichloromethane (20mL) was heated at reflux for 4h. The reaction mixture was
cooled
and quickly washed with water, dried over MgSO4 and filtered. The resulting
solution
was treated with pyridine (1.8mL, 22.2mmo1) and then decanoylchloride (4.6mL,
22.2mmol). After being stirred for 3h, methanol (1mL) was added and stirred a
further
10min. The reaction mixture was washed with sat.NaHCO3 (aq), dried over MgSO4
and
evaporated. The residue was purified on silica eluting with 1:1:0.1
dichloromethane/ethyl
acetate/methanol to give the title compound as a yellow oil (1.2g, 38%).
11-1 NMR (CDC13, 300MHz) 60.86 (t, 31-1), 1.11 (t..3H), 1.05 ,1.40 (m, 1211),
1.59 -1.75
(m, 211), 1.75-1.98 (m, 4H), 2.40-2.54 (m, 213), 2.60-3.07 (in, 1013), 3.15-
3.32 (m, 411),
3.89-3.99 (m, 213), 4.09-4.21 (in, 2H), 6.57 (dd, 1H), 6.67 (d, 1H), 6.95-7.00
(m, 1H),
7.08 (dd, 1H), 7.12-7.20 (m, 2H), 7.27-7.32 (m, 111). m/z (MPH) 704.38.
173
CA 3035442 2019-03-01
(7-(4-(4-(2,3-dichlorophenyl)piperazin-l-yl)butoxy)-2-oxo-3,4-dihydroquinolin-
1(211)-yl)methyl 4-acetamidobutanoate (Example 36, Compound 44)
To a suspension of hemiaminal Al (2.6g, 5.5mmo1) in dichloromethane (30mL) was
added triethylamine (2.3mL, 16.4mmo1), followed by addition of methanesulfonyl
chloride (0.47g, 6.0nuno1) over 3min. The reaction mixture was stirred for
25tnin and
then N-acetyl-4-aminobutyric acid (1.6g, 10.1nunol) added. The reaction
mixture was
then heated at reflux for 18h, cooled and washed with sat. NaHCO3 (aq). The
organic
phase was dried over MgSO4, filtered and evaporated. The residue was further
purified
on silica eluting with 1:1:0.1 to 1:1:0.2 dichloromethane/ethyl
acctate/methanol to give
the title compound as an off white solid (1.1g, 34%).
IFINMR (CDCI3, 300MHz) 5 1.70-1.80 (m, 2H), 1.80-1.90 (m, 4H), 1.97 (s, 3H),
2.41
(t, 2H), 2.50-2.57 (m, 2H), 2.60-2.75 (in, 6H), 2.83-2.88 (m, 2H), 3.03-3.12
(in, 4H),
3.24-3.32 (m, 2H), 3.95-4.00 (m, 2H), 5,85-5.92 (m, 3H), 6.58 (d, 2H), 6.92-
6.96 (in,
1H), 7.05 (d, 1H), 7.12-7.16 (m, 2H).). nth (WTI) 605.08.
(7-(4-(4-(2,3-dichlorophenyl)piperazin-l-yl)butoxy)-2-oxo-3,4-dihydroquInolin-
1(211)-yl)methyl 4-octanamidobutanoate (Example 37, Compound 45)
Compound 149 (1.4g) was synthesized in a similar manner to Compound 148.
11-1 NMR (d.6-DMSO, 300MHz) 8 0.79 (t, 3H), 1,10-1.28 (m, 8H), 1.38-1.48 (in,
2H),
1.50-1.77 (m, 6H), 1.93-2.00 (m, 2H), 2.25-2.40 (m, 4H), 2.40-2.60 (m, 611),
2.72-2.81
(m, 2H). 2.87-3.02 (m, 6H), 3.90-4.00 (m, 2H), 5.82 (s, 2H), 6.58-6.63 (m,
2H), 7.04-
7.02 (m, 2H), 7.20-7.30 (m, 2H). miz (ME) 689.47.
(5-(2-(4-(benzoldlisothiazol-3-yl)piperazin-1-yl)ethyl)-6-chloro-2-oxoindolin-
1-
yl)methyl hexanoate (Example 38, Compound 322)
DCM Pwaformalciehyde
SOCl2 0 Cat Zinc chloride 0
OH
o
STEP 1: Thionyl chloride (12.31g, 103 mmol) followed by catalytic amount of
N,N-
dimethyl formamide (DMF, 0.1 la) was added to a solution of Hexanoic acid (10
g, 86
mmol) in dichloromethane ( DCM, 100 mL) at 25-30 C. The reaction solution was
stirred at same temperature for 2 hours u-r..r e.caosphere,
upou completion of
the starting material by TLC analysis. The volatiles were evaporated under
reduced
pressure below 40 C. which provided a viscous liquid material, hexanoyl
chloride
(about 10.5 g).
174
CA 3035442 2019-03-01
STEP 2: To the above hexanoyl chloride, para maldehyde (3.8 g, 128 mmol) and.
anhydrous ZnCl2 (0.232 g, 17 mmol) were added at 25-30 C under inert
atmosphere and
then heated to 90 C. The thick mass was stirred at 90-95 C for 5 hours,
which after
cooling provided crude product, chloromethyl hexanoate which was purified by
silica gel
column chromatography.
111-NMR (CDC13, 500 MHz): 65.70 (s, 2H), 2.39-2.33 (m, 2H), 1.69-1.61(m, 2H),
1.33-1.28 (m., 4H), 0.90-0.88 (t, J=7, 3H).
S'N=` Nr-ThN
0 0
TEA, DMAP N\_,N
0 CI
STEP 3: Chloromethyl hexanoate (3.18 g, 19.0 mmol) in dichloromethanc (6 mL)
was
added to a suspension of Ziprasidone free base (4.0 g, 9.6 nunol), triethyl
amine (4.0 mL,
27 mmol) and 4-dimethylamino pyridine (DMAP, 0.708 g, 5 mmol) in dichloride
methane (240 mL) at 25-30 C. The reaction solution was stirred for 24 h at
same
temperature. The crude mixture was washed with water (100 mL) followed by
brine
solution (100 mL), upon solvent evaporation under vacuum below 40 C provided
crude
title product, Compound 322, which was further purified by silica gel column
chromatography. (1.4 g, 27% yield)
'H-NMR(CDC13, 500 MHz): 8 7.92-7.90 (d, J=7.5,1H) , 7.82-7.80 (d, J=7.5,1H)
,7.48-7.45 (t, J=7.5,1H), 7.37-7.34 (t, J=7.5,1H) , 7.17 (s,1H),7.05 (s, 1H),
5.72 (s, 2H),
3.60-3.55 (m,6H) , 2.98-2.95 (t, J=7.5,2H) , 2.79-2.78 (m, 4H),2.68-2.65 (t,
2H),2.35-2.32 (t, J=7.5,2H) , 1.64-1.61 (t, J=7.5, 2H), 1.29-1.25 (m, 4H),
0.88-0.85 (t,
J=7, 3H).
Mass (m/z = 541 + 1].
(5-(2-(4-(benzo [ isothiazol-3-yl)piperazin- 1 -yl)ethy1)-6-chloro-2-
oxoindolin-1-
yl)methyl dodecanoate (Exampt 3), (..6.1iApialid -324)
Compound 324 was synthesized in a similar manner to Compound 322, Example 38.
1H-NMR(CDC13, 500 MHz): 8 7.92-7.90 (d, J=7.5, 1H) , 7.82-7.80 (d, J=7.5,1H),
7.48-7.45 (t, J=7.5, 1H), 7.37-7.34 (t, J=7.5, 1H) , 7.17 (s, 1H),7.05 (s,
1H), 5.72 (s, 2H),
175
CA 3035442 2019-03-01
3:60-3.55 (m, 6H) , 2.98-2.95 (t, J-8, 2H) , 2.79-2.77 (m, 4H),2.68-2.65 (t,
J=8,
2H),2.34-2.31 (t, J=7,2H) , 1.63-1.60 (m, ,2H), 1.24(s, 16H), 0.89-0.86 (t,
J=7, 3H).
Mass (m/z) = 625.5 [1\4+ + 1].
(5-(2-(4-(benzoldlisothiazol-3-yl)piperazin-1-yl)ethyl)-6-chloro-2-oxoindolin-
1-
yl)methyl palmitate (Example 40, Compound 326)
111-NMR (CDC13, 500 MHz): 8 7.92-7.90 (d, J=7.5, 1H) ,7.82-7.80 (d, J=7 .5,
1H),
7.48-7.45 (t, J=7.5, 1H), 7.37-7.34 (t, .1=7.5, 1H), 7.17 (s, IH), 7.05 (s,
111), 5.72 (s, 2H),
3.60-3.55 (in, 6H), 2.98-2.95 (t, J=8, 2H) , 2.79-2.77 (in, 4H), 2.68-2.65 (t,
J=8, 2H),
2.34-2.31 (t, J=8, 2H) , 1.63-1.56 (m, 2H), 1.25-1.23 (m, 24H), 0.88-0.86 (t,
.1=7, 2H).
Mass (m/z) = 681.5 [M. + 1].
(7-1(4-bipheny1-3y1 methyl)piperazin-1-y11-2-oxobenzo[d] oxazol-3(211)-
yl)methyl
acetate (Example 41, Compound 416).
OA NH 0
0
0 0 NI"-
`scric
*
--"Th0 I \N 411
Step 1: Synthesis of chloromethyl acetate: Acetyl chloride (5 g, 0.06 mol) was
added
dropwise to a mixture of paraformakihyde (8.5 g, 0.06 mol) and anhydrous zinc
chloride
(0.175 g, 0.02 mol) at 0 C under Argon. The reaction mixture was warmed to
room
temperature and stirred for I hour, then heated to 90 C for 18 hours. The
solid was
filtered off washed with dichloromethane, and the filtrate was concentrated
under
vacuum at 37 C to provide the desired product (6.6 g, 94% yield). The product
was used
directly (without purification) in to next step and stored with activated
molecular sieves
(4 A).
Step 2: Synthesis of iodomethyl acetate: Sodium iodide (27.6 g, 0.18 mol) was
added to
a solution of chloromethyl acetate (6.6 g, 0.06 mol) in acetonitrile (66 mL).
The reaction
flask was covered in aluminum foil to exclude light and stirred at ambient
temperature
for 15 hours. The reaction mixture was partition between dichloromethane and
water,
and the aqueous layer was extracted with dichloromethane. The combine organics
were
washed with aqueous saturated NaHCO3, 10% aqueous sodium sulfite solution, and
brine then dried with sodium ,inViutc...'uii&coa,entraied to give the product
(1.13 g, 12%
yield) as a yellow oil.
Step 3: n-Butyl lithium (1.6 M in hexane; 3.8 mL, 0.007 mol) was added drop
wise
from a syringe to a stirred solution of bifeprunox (1.46 g, 0.003 mol) in
tetrahydrofuran
176
CA 3035442 2019-03-01
at -78 C. After 1 hour a solution of iodomethyl acetate (1.13 g, 0.005 mol)
was added
drop-wise at -70 C. The reaction mixture was stirred for 15 hours. The
reaction mixture
was dumped in a saturated aqueous solution of ammonium chloride and extracted
with
ethyl acetate. The combined organic layers were washed with IN solution of
NaOH and
brine, then dried with sodium sulphate and concentrated under vacuum.
Purification by
flash chromatography provided compound 416. (0.25 g, 14% yield). 1H NMR (DMSO,
400MHz) 8 2.034 (s, 3H), 2.565 (s, 4H), 3.183 (s, 4H), 3.597 (s, 2H), 5.765
(s, 2H),
6.696-6.717 (d, 1H), 6.882-6.901 (d, 111), 7.091-7.182 (t, 1H), 7.315-7.370
(q, 2H),
7.404-7.473 (m, 311), 7.515-7.555 (d, 1H), 7.59 (d, 1H), 7.639-7.657 (d, 2H).
miz (M+H)
457.
(7-[(4-bipheny1-3y1methyl)piperazin-1-y11-2-oxobenzo[d]oxazol-3(2H)-yl)methyl
butyrate (Example 42, Compound 417).
Compound 417 was prepared in a similar manner to Example 41 using butanoyl
chloride.
Purification by flash chromatography provided the desired product (1.25 g, 45%
yield).
1H NMR (DMSO, 400MHz) 5 1.065 (t, 3H),1.448-1.54 (m, 2H), 2.284-2.320 (t, 2H),
2.564 (s, 411), 3.184 (s, 4H), 3.597 (s, 2H), 5.787(s, 2H), 6.694-6.713 (d,
1H), 6.878-
6.896 (d, 1H), 7.092-7.133 (t, 7.315-7.370 (q, 211), 7.422-7.533 (m, 3H),
7.535-
7.555 (d, 1H), 7.639 (d, 1H), 7.657-7.660 (d, 2H). In/z (W-H)485.
(7-[(4-bipheny1-3y1 methyl)piperazin-l-y11-2-oxobenzold1oxazol-3(211)-
y1)methyl
hexanoate (Example 43, Compound 413).
Compound 413 was prepared in a similar manner to Example 41 using hexanoyl
chloride. Purification by flash chromatography provided the desired product
(0.6 g,
60% yield). 1H NMR (DMSO, 400MHz) 5 0.774 (t, 3H),1.114-1.187 (in, 4H), 1.433-
1.506 (rn , 2H) , 2.291-2.328(11, 211), 2.564 (s, 411), 3.182 (s, 4H), 3.597
(s, 2H), 5.783(s,
2H), 6.693-6.713 (d, 1H), 6.870-6.890 (d, 1H), 7.090-7.130 (t, 111), 7.314-
7.351 (q, 2H),
7.422-7.472 (m, 311), 7.535-7.554 (d, 1H), 7.589 (d, IH), 7.638-7.656 (d, 2H).
rri/z
(M+H)513.
(7-[(4-biphenyl-3y1 methyl)piperazin-1-y1]-2-oxobenzo[d]oxazol-3(2H)-y1)methyl
palmitate (Example 44, Compound 422).
Compound 422 was prepared in a similar manner to Example 41 using palmitoyl
chloride. Purificationisiills-h chromatography provided the desired product
(0.5 g,
47% yield). 1H NMR (DMSO, 400MHz) 5 0.819 (t, 3H),1.127-1.302 2211), 1.437-
1.454 (t , 2H) , 2.287-2.305(t, 2H), 2.564 (s, 411), 3.182 (s, 411), 3.596 (s,
2H), 5.784(s,
2H), 6.688-6.708 (d, 111), 6.863-6.882 (d, 1H), 7.083-7.124 (t, 1H), 7.331-
7.368 (q, 2H),
177
CA 3035442 2019-03-01
7.400-7.470 (in, 311), 7.534-7,553 (d, Hi), 7.587 (d, 1H), 7.635-7.653 (d,
2H). m/z
(M+H)653.
(7-[(4-biphenyl-3y1 methyl)piperazin-1-y1]-2-oxobenzoldloxazol-3(211)-
yl)methyl
decanoate (Example 45, Compound 419).
Compound 419 was prepared in a similar manner to Example 41 using decanoyl
chloride. Purification by flash chromatography provided the desired product
(0.8 g,
77% yield). 1H NMR (DMSO, 400MHz) 5 0.795-0.829 (t, 3H),1.140-1.211 (in, 12H),
1,438-1.471 (t, 2H) , 2.288-2.324(t, 2H), 2.562 (s, 4H), 3.181 (s, 4H), 3.595
(s, 2H),
5.783(s, 2H), 6.689-6.709 (d, 1H), 6.856-6.884 (d, 1H), 7.083-7.124 (t, 1H),
7.311-7.367
(q, 211), 7.400-7.470 (m, 3H), 7.533-7.552 (d, 1H), 7.587 (d, 111), 7.635-
7.653 (d, 2H).
m/z (M+H)569.
(7-[(4-biphenyl-3y1 methyl)piperazin-1-y11-2-oxobenzo[d)oxazol-3(211)-
yl)methyl
isobutyrate (Example 46, Compound 414).
Compound 414 was prepared in a similar manner to Example 41 using isobutyryl
chloride. Purification by flash chromatography provided the desired product
(0.3 g, 15%
yield). 1H NMR (DMSO, 400MHz) 5 1.027-1.044 (d, 6H),2.478-2.553 (m, 111),
2.562
(s, 4H), 3.185 (s, 4H), 3.597 (s, 2H), 5.785(s, 211), 6.692-6.713 (d, 1H),
6.873-6.892 (d,
111), 7.093-7.134 (t, 1H), 7.315-7.369 (q, 2H), 7.403-7.472 (in, 3H), 7.533-
7.555 (d, 1H),
7.590 (d, 111), 7.657-7.660 (d, 2H). m/z (M+H)485.
(744-(4-(2,3-dichloronbenyl)vinerazin-1-vnbutory)-2-oxopuinolin-1(2H)-
14)methvl
butyrate (Example 47, Compound 151).
(7-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butoxy)-2-oxo-3,4-dihydroquinolin-
1(2H)-
yl)methyl butyrate (Compound 2) was prepared as described in Example 16,
supra.
DDQ,TFA
ci ON CI
CI 0 0 CI 0 0
Compound 2 Compound 151
To a stirred solution of (7-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butoxy)-2-
oxo-3,4-
dihydroqumoliii-42H)-yOincihyl butyrate (3.26 g, 5.94 mrnol) in THF (100 mL)
was
added TFA (2.74 mL, 35.63 mmol) followed by 2,3-dichloro-5,6-
dicyanobenz,oquinone
(DDQ; 7.01 g, 30.88 mmol) in THF (40 mL). The reaction was stirred at room
temperature over the weekend. The reaction was quenched with water (100 mL)
and then
178
CA 3035442 2019-03-01
poured into water (600 mL) and dichloromethane (100 mL). Solid NaHCO3 (100 g)
was
added and the mixture stirred for approximately 30 minutes. Dichloromethane
(200 mL)
was added and the mixture filtered. The collected filtrate was transferred to
a separating
funnel and the layers separated. The aqueous layer was extracted with
dichloromethane
(2 x 100 mL) and the combined organics washed with water (3 x 100 mL, brine
(100
mL) and dried over MgSO4. After filtration, the volatiles were removed. The
crude
material was purified by silica chromatography eluting 0-4% Methanol / (1:1
ethyl
acetate / dichloromethane). The oil was recrystallized from methanol to give
Compound
151. (2.03 g, 3.72 rnmol, 63% yield).
'H-NMR (300MHz, CDC13) 8 7.63 (1H, d), 7.45 (1H, d), 7.19-7.06 (2H, m), 6.99-
6.90
(1H, m), 6.88-6.78 (2H, m), 6.52 (1H, d), 6.33 (2H, s), 4.06 (2H, t), 3.17-
2.99 (4H, bs),
2.74-2.43 (6H, m), 2.35 (2H, t), 1.94-1.54 (6H, m), 0.93 (3H, t).
The following compounds were synthesized in a similar manner to Example 47
from their corresponding 3,4 dihvdro precursors:
(7-(4-(4-(2,3-dichlorophenyl)piperazin-l-yObutoxy)-2-oxoquinolin-1(211)-
y1)methyl
palmitate (Example 48, Compound 159)
Compound 159 was synthesized in a similar manner to Example 47 from Compound
10.
2.04 g. 1H-NMR (400MHz, CDC13) 8 7.62 (1H, d), 7.44 (1H, d), 7.18-7.10 (2H,
m),
6.98-6.91 (1H, m), 6.87-6.80 (2H, m), 6.52 (1H, d), 6.32 (2H, s), 4.05 (2H,
t), 3.15-2.99
(4H, bs), 2.74-2.44 (6H, m), 2.35 (2H, t), 1.92-1.83 (2H, m), 1.80-1.68 (2H,
m) 1.66-1.55
(2H, m), 1.32-1.14 (24H, m), 0.87 (3H, t).
(7444442,3 -dichlorophenyl)piperazin- 1 -yl)butoxy)-2-oxoquinolin-1(21-1)-y1)m
ethyl
laurate (Example 49, Compound 156)
Compound 156 was synthesized in a similar manner to Example 47 from Compound
7.
1.37 g. 111-NMR (400MHz, CDC13) 8 7.62 (1H, d), 7.43 (1H, d), 7.17-7.10 (2H,
m),
6.96-6.92 (1H, m), 6.87-6.80 (2H, m), 6.51 (1H, d), 6.33 (2H, s), 4.06 (2H,
t), 3.12-3.01
(4H, bs), 2.71-2.59 (4H, bs), 2.50 (2H, t), 2.35 (2H, t), 1.92-1.83 (2H, m),
1.78-1.69 (2H,
m) 1.66-1.55 (2H, m), 1.32-1.16 (16H, m), 0.86 (3H, t).
(7-(4-(4-(2,3-dichloroph enyl)piperazin- 1 -yl)butoxy)-2-oxoquin oli n-1 (2H)-
y1) methyl
stearafe (Elaimple 50, Compound 160)
Compound 160 was synthesized in a similar manner to Example 47 from Compound
11.
1.38 g1H-NMR (400MHz, CDC13) 8 7.62 (1H, d), 7.44 (1H, d), 7.17-7.11 (211, m),
6.97-
6.92 (1H, m), 6.87-6.79 (21-1, m), 6.51 (1H, d), 6.32 (2H, s), 4.05 (2H, t),
3.13-3.00 (4H,
179
CA 3035442 2019-03-01
bs), 2.73-2.58 (4H, bs), 2.50 (2H, t), 2.35 (2H, t), 1.92-1.83 (2H, m), 1.79-
1.69 (2H, m)
1.66-1.55 (2H, m), 1.32-1.14 (28H, m), 0.87 (3H, t).
(7-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butoxy)-2-oxoquinolin-1(2H)-
yl)methyl
acetate (Example 51, Compound 150)
Compound 150 was synthesized in a similar manner to Example 47 from Compound
1.
1.61 g 1H-NMR (300MHz, CDC13) 8 7.63 (1H, d), 7.45 (IH, d), 7.18-7.11 (2H, m),
6.98-6.92 (1H, m), 6.90-6.80 (2H, m), 6.52 (111, d), 6.32 (2H, s), 4.07 (2H,
t), 3.14-3.01
(4H, bs), 2.73-2.59 (4H, bs), 2.51 (2H, t), 2.12 (3H, s), 1.95-1.82 (2H, m),
1.82-1.68 (2H,
m).
(7444442,3-dichlorophenyl)piperazin-1-yl)butoxy)-2-oxoquinolin-1(2H)-yl)methyl
2,2-dimethylbutanoate (Example 52, Compound 165)
Compound 165 was synthesized in a similar manner to Example 47 from Compound
16.
1.02 g 11-1-NMR (400MHz, CDCI3) 6 7.61 (1H, d), 7.43 (IH, d), 7.17-7.10 (2H,
m),
6.97-6.92 (IH, m), 6.83-6.79 (2H, m), 6.51 (1H, d), 6.31 (211, s), 4.05 (2H,
t), 3.12-3.02
(4H, bs), 2.71-2.60 (4H, bs), 2.50 (2H, t), 1.92-1.83 (2H, m), 1.78-1.68 (2H,
m) 1.55
(2H, q), 1.15 (6H, s), 0,81 (3H, t).
(24N-(14144-fluorobenzv1)-1H-benzo di i midazol-2-vbnineridin-4-vH-N-
methvlamino)-6-oxonvrimidin-1(611)-vbniethyl octanoate (Example 53, Compound
Step 1:
0 0
ZnC1 _
CI + CH20
CI 0
Octanoyl chloride (10 g, 0.06 mol) was added drop wise to a mixture of
paraformaldehyde (8.07 g, 0.06 mol) and anhydrous zinc chloride (0.163 g,
0.0012 mol)
at 0 C under Argon. After the addition was completed, the reaction mixture
was stirred
at 25 C for 1 hour, and then heated to 90 C for 16 hour. The solid was
filtered off and
washed with dichloromethane. The filtrate was concentrated in vacuo at 37 C to
provide
the desired chloromethyl octanoate (9.5 g, 84% yield), which was used directly
(without
purification) in the next step. This product was stored over activated
moleculor gicves (4
"A) to keep it dry.
180
CA 3035442 2019-03-01
Step 2:
0 0
Nal
Ir",0 CI 0
Sodium iodide (21.7 g, 0.1449 mol) was added to a solution of chloromethyl
octanoate
(9.5 g, 0.0483 mol) in of acetonitrile (100 m1). The flask was covered in
aluminum foil to
protect from light and stirred at 25 C for 16 hours. The reaction mixture was
partitioned
between dichloromethane and water the aqueous layer was further extracted with
dichloromethane. The combined organic extracts were washed with aqueous
saturated
NaHCO3, 10% aqueous sodium sulfite solution and brine, and finally dried with
sodium
sulphate and concentrated in vacuo to provide the product (8.4 g, 71 % yield)
as a yellow
oil. This product was taken into the next step without further purification.
Step 3:
N 0
NH
401
N _____________________________ +=
________________________ )¨N
N 0
Nr- eW
n-Butyl lithium (15 M in hexane; 14.6 ml, 0.0042 mol) was added drop wise to a
stirred
solution of 2-(N-(1-(1-(4-fluoroberizy1)-1H-benzo[d]imidazol-2-y1)piperidin-4-
y1)-N-
methylamino)pyrimidin-4(3H)-one (Mizolastine, 14.3 g, 0.00696 mol) in
tetrahydrofuran
(50 ml) at -78 C. After 1 hour the reaction mixture was treated drop-wise
with a
181
CA 3035442 2019-03-01
iodomethyl octanoate (2.5 g, 0.0231 mol) at -70 C. The reaction mixture was
stirred at
25 C for 16 hours. The reaction mixture was poured into ammonium chloride
solution
and extracted with ethyl acetate. The combined organic was washed with aqueous
sodium hydroxide (IN) and brine, and then dried with sodium sulphate and
concentrated
in vacuo. Flash chromatography provided the desired product (0.45 g, 17%
yield).
NMR (DMSO, 400MHz) 8 0.815 (t, 3H),1.117-1.235 (m, 10H), 1.474-1.491 (t , 2H)
,
1.638-1.665 (d, 2H), 1.992-2.010 (in, 2H), 2.292-2.230 (t, 2H), 2.992 (s, 3H),
3.027-
3.088 (t, 2H), 3.55-3.62 (t, 2H), 4.625 (s, 1H) 5.311 (s, 2H), 6.040 (s, 2H),
6.110-6.124
(d, 1H), 7.014-7.076 (m, 2H), 7.148-7.253 (m, 5H), 7.442-7.460 (d, 1H), 8.187-
8.201 (d,
1H). ni/z (M+H) 589.
(24N-(1-(1-(4-fluorobenzv1)-1H-benzoldlimidazol-2-14)piperidin-4-v1)-N-
methvlamino)-6-oxopyrimidin-1(6111-vDmethvl laurate (Example 54, Compound
:TO
Compound 706 was synthesized using a similar procedure as Example 53 using
lauroyl
chloride.
11-1-NMR (DMSO, 400MHz) 60.791-0.826 (t, 3H),1.134-1.210 (m, 16H), 1.446 (t ,
2H) ,
1.642-1.925 (d, 2H), 1.956-2.008 (in, 2H), 2.266-2.301 (t, 2H), 2.968 (s, 3H),
3.003-
3.063 (t, 2H), 3.31-3.62 (t, 2H), 4.625 (s, 1H) 5.286 (s, 2H), 6.015 (s, 2H),
6.085-6.099
(d, 114), 7.015-7.072 (m, 2H), 7.122-7.215 (in, 5H), 7.418-7.436 (d, 1H),
8.159-8.172 (d,
114). m/z (ME) 645.5.
(5-(4-(2-(5-ethylpyridin-2-yl)ethoxy)benzy1)-2,4-dioxothiazolidin-3-yl)methyl
hexanoate (Example 55, Compound 1003)
0
NH 4.
0
Step 1; Chloromethyl hexanoate was synthesized from hexanoyl chloride in a
similar
process as described above in Example 53, step 1.
182
CA 3035442 2019-03-01
Step 2: Iodomethyl hexanoate was synthesized from chloromethyl hexanoate in a
similar process as described above in Example 53, step 2.
Step 3: A solution of Pioglitazone (3.0 g, 0.0084 mot) in dimethyl formamide
was
treated with dry K2CO3 (3.48 g, 0.0252) at 25 C. After 40 minutes a solution
of
Iodomethyl hexanoate (4.29 g, 0.0168 mol) was added drop-wise. The reaction
mixture
was stirred for 15 hours, then dumped into water and extracted with ethyl
acetate. The
combined organic layers were dried with sodium sulphate and concentrated under
vacuum. The product was purified by flash chromatography to obtain the desired
product
(1.9 g, 44% yield).
'FINMR (CDC13, 400MHz) 8 0.86-0.90 (t, 3H), 1.22-1.29 (m, 8H), 1.58-1.62 (t,
2H),
2.27-2.31 (t, 2H), 2.62-2.64 (d, 2H), 3.04-3.099 (q, 1H), 3.21-3.25 (t, 2H),
3.452-3.497
(q, 1H), 4.30-4.34 (t, 2H), 4.46-4.48 (d, 1H),5.513-5.51(d, 2H), 6.81-6.85 (t,
2H), 7.09-
7.11 (d, 2H), 7.18-7.20 (d,1H),7.46-7.48(q,1H),8.38-8.39(d,1H) mez (MPH) 485.
(544-(2-(5-ethylpyridin-2-yl)ethoxy)benzy1)-2,4-dioxothiazolidin-3-yl)methyl
laurate (Example 56, Compound 1006)
Compound 1006 was synthesized using a similar procedure as Example 55 using
lauroyl
chloride.
1H NMR (CDC13, 400MHz) 8 0.802-0.836 (t, 311), 1.133-1.171 (t, 4H), 1.197-
1.235 (d,
15H), 1.308 (s, H), 1.419-1.452 (t, 2H), 2.172.254(q, 21-1) ,2.533-2.590 (q,
2H), 3.044-
3.118 (m, 3H), 4.251-4.284 (t, 2H), 4.97-5.005 (q, 1H), 5.345-5.413 (q, 2H),
6.82-6.841
(d, 2H), 7.09-7.11 (d, 2H) ,7.23-7.25 (d,1H),7.53-7.55(q,1H), 8.33-8.34 (d,1H)
m/z
(MH) 569.
(5-(4-(2-(5-ethylpyridin-2-yl)ethoxy)benzy1)-2,4-dioxothiazolidin-3-y1)methyl
palmitoate (Example 57, Compound 1008)
Compound 1008 was synthesized using a similar procedure as Example 55 using
palmitoyl chloride.
111NMR (CDC13, 400MHz) 80.870 (s, 3H), 1.23-1.26 (t, 27H), 1.57-1.61 (t,
2H),2.27-
2.31 (t, 2H), 2.61.265(t, 2H) ,3.06-310 (t, 1H), 3.22-3.25 (t, 2H), 3.45-3.46
(d, 1H),
4.31-4.34 (t, 2H), 4.45-4.49 (q, 1H), 5.487-5.541 (q, 2H),6.83-6.85 (d, 2H),
7.09-7.11 (d,
2H) ,7.19-7.26 (t, 1H), 7.47-7.49(q,1H), 8_393-8.397 (d,1H) rniz. (M+H) 625.
(5-(4-(2-(5-ethylpyridin-2-yl)etboxy)benzy1)-2,4-dioxothiazolidin-3-y1)methyl
stearoate (Example 58, Compound 1009)
Compound 1009 was synthesized using a similar procedure as Example 55 using
stearoyl
chloride.
183
CA 3035442 2019-03-01
111 NMR (CDC13, 400MHz) 60.874-0.894 (t, 3H), 1.222-1.260 (t, 30H), 1.570-
1.603 (d,
1H),2.27-2.31 (t, 2H), 2.609-2.266(q, 2H) .3.04-3.10 (q, 111), 3.20-3.24 (t,
2H), 3.46-
3.50 (q, 1H), 4.302-4.335 (t, 2H), 4.453-4.487 (q, 1H), 5.488-5.552 (q,
2H),6.83-6.86 (d,
2H), 7.09-7.11 (d, 2H) ,7.17-7.19 (d, 111), 7.44-7.47(d,1H), 8.386-8.391
(d,1H) m/z
(MPH) 653.
(5-(4-(2-(5-ethylpyridin-2-yl)ethoxy)benzy1)-2,4-dioxothiazolidin-3-yl)methyl
myristoate (Example 59, Compound 1007)
Compound 1007 was synthesized using a similar procedure as Example 55 using
myristoyl chloride.
1H NMR (CDC13, 400MHz) 60.854-0.887 (t, 311), 1.226-1.262 (t, 24H), 1.57-1.604
(t,
2H),2.27-2.308 (t, 2H), 2.609-2.265(t, 2H) , 3.035-3.094 (q, 1H), 3.223-3.256
(t, 211),
3.456-3.500 (q, 111), 4.307-4.340 (t, 2H), 4.463-4.487 (t, 111), 5.487-5.540
(q, 2H),6.832-
6.852 (d, 2H), 7.092-7.114 (d, 211) ,7.198-7.217 (d, 1H), 7.475-7.491(d,1H),
8.393-8.397
(d,1H) m/z (M+H) 596.
(5-(4-(2-(5-ethylpyridin-2-yl)ethoxy)benzy1)-2,4-dioxothiazolidin-3-y1)methyl
butyrate (Example 60, Compound 1002)
Compound 1002 was synthesized using a similar procedure as Example 55 using
butyroyl chloride.
'H NMR (CDC13, 400MHz) 6 0.798-0.835 (t, 311), 1.133-1.212 (q, 4H), 1.417-
1.509 (m,
2H), 2.210-2.246 (t, 2H), 2.482-2.2591(q, 2H) ,3.047-3.118 (q, 3H), 4.253-
4.286 (t, 2H),
4.983-5.016 (q, 1H), 5.353-5.415 (q, 2H), 6.824-6.845 (d, 2H), 7.097-7.118 (d,
2H),
7.239-7.258 (d, 111), 7.538-7.563 (d,1H), 8.340-8.365 (d,1H) m/z (MPH) 458.
(544-(2-(5-ethylpyridin-2-ypethoxy)benzy1)-2,4-dioxothiazolidin-3-yl)methyl
cyclohexanecarboxylate (Example 60, Compound 1015)
Compound 1015 was synthesized using a similar procedure as Example 55 using
cyclohexanecarbonyl chloride.
11-1NMR (CDC13, 400MHz) 1.181-1.293 (m, 711), 1.359-1.449 (m, 2H), 2.624 (s,
1H),
1.714-1.738 (t, 211) , 1.843-1.874(q,2H),2.244-2.319(m,1H),2.607-
2.664(q,2H),3.049-
3.107(q,1H),3.22.-3.253(t,211),3.340-3.485(q,1H), 5.481-5.534 (q, 211), 6.831-
6.853 (d,
2H), 7.091-7.113 (d, 2H) ,7.193-7.213 (d, 1H),_7.465-7,590 (q,1H), 8.392-8.396
(d,1H)
m/z (M+11) 497.
184
CA 3035442 2019-03-01
General Scheme for Synthesis
AgCO3, 2-Me THF 410
RN
1110
CI
CI 0 CI 0 0 0
R
((7-(4-(4-(2,3-dicbloronhenvbninerazin-1-v1)butoxy)ouinolin-2-v1)oxv)methvl
bexvl
carbonate (Example 61, Compound 1240)
To a solution of dehydro-Aripiprazole (1.5 g, 3.36 mmol) in 2-
methyltetrahydrofuran (30
mL) was added silver carbonate (1.853 g, 6.72 mmol) and hexyl iodomethyl
carbonate
(2.021 g, 7.05 mmol) in 2-methyltetrahydrfuran (4 mL) at room temperature. The
reaction was stirred for 4.5 days. The reaction was quenched with E120 (30 mL)
and
filtered through celite7:4The reaction was extracted with ethyl acetate (3 x
20 mL),
washed with brine (20 mL), dried over MgSO4 and concentrated. The product was
purified by column chromatography on silica eluting with 1 11 ethyl acetate to
dichloromethane to 2% Me0H in 1 : 1 ethyl acetate to dichloromethane to
provide
Compound-1240 (1.08 g) as a yellow oil.
'H-NMR (300 MHz, CDCI3) 8 7.96 (1H, d), 7.60 (111, d), 7.21 (1H, m), 7.14 (2H,
m),
7.03 (1H, dd), 6.94 (111, in), 6.81 (1H, d), 6.26 (211, s), 4.18(211, m), 4.12
(2H, t), 3.09
(4H, m), 2.68(411, m), 2.53 (2H, m), 1.91 (211, m), 1.78 (2H, m), 1.63 (2H,
in), 1.28 (6H,
m), 0.86 (3H, t). [M+H] = 604.2
((7-(4-(442,3-dichloronbenvllninerazin-1-v1)butoxy)ouinolin-2-vfloxv)methrl
octanoate (Examnle 62, Compound 1206)
To a solution of dehydro-Aripiprazole (1.0 g, 2.24 mmol) in 2-
methyltetrahydrofuran (25
mL) was added silver carbonate (0.864 g, 3.13 mmol) and iodomethyl octanoate
(0.764
g, 2.68 mmol) at room temperature. The reaction was stirred for 5 days. The
reaction
TM
was quenched with H20 (30 mL) and filtered through celite. The reaction was
extracted
with ethyl acetate (3 x 20 mL), washed with 5% w/v sodium sulfite solution (15
mL),
brine (20 mL), dried over MgSO4 and concentrated. The product was purified by
column chromatography on silica eluting with 0- 70% ethyl acetate in heptane
to
provide Compound 1206 (0.602 g) as a pale orange oil
11-1-NMR (300 MHz, CDC13) 8 7.95 (1H, d), 7.60 (1H, d), 7.21 (1H, m), 7.14
(2H, in),
7.07 (111, dd), 6.95 (111, m), 6.79(111, d), 6.24 (2H, s), 4.12 (2H, m), 3.09
(4H, m), 2.68
185
CA 3035442 2019-03-01
(4H, m), 2.54 (2H, m), 2.36 (211, t), 1.90 (2H, m), 1.77 (2H, m), 1.61 (4H,
m), 1.23 (611,
m), 0.83 (3H, t). [M+H] = 602.2
0-(4-(4-(2,3-dichlorophenVI)DiDerazin-1-vnbutoxy)cluinolin-2-11)oxv)methyl
dodecanoate (Example 63. Compound 1208)
The experimental procedure was carried out in the same manner as for Compound-
1206
m Example 62, to give 1208 (0.738 g) as a yellow oil.
111-NMR (300 MHz, CDC13) E. 7.95 (1H, d), 7.60 (111, d), 7.20 (1H, d), 7.14
(211, m),
7.05 (111, dd), 6.95 (1H, in), 6.80 (111, d), 6.24 (2H, s), 4.13 (2H, m), 3.09
(4H, m), 2.68
(4H, m), 2.54 (2H, m), 2.36 (2H, t), 1.93 (2H, m), 1.80 (2H, m), 1.60 (4H, m),
1.23 (14H,
m), 0.86 (311, 0. [M+Hr = 658.4.
((7-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butoxy)quinolin-2-yl)oxy)metbyl
butyrate (Example 64, Compound 1202)
The experimental procedure was carried out in the same manner as for Compound-
1206
in Example 62, to give 1202 (0.695 g) as a yellow oil.
111-NMR (300 MHz, CDC13) 8 7.95 (111, d), 7.61 (1H, d), 7.20(111, d), 7.14
(214, In),
7.04 (1H, dd), 6.96 (1H, m), 6.79 (1H, d), 6.25 (2H, s), 4.13 (2H, m), 3.09
(4H, ca), 2.69
(4F1, in), 2.54 (2H, m), 2.35 (2H, t), 1.91 (2H, m), 1.78 (2H, m), 1.66 (211,
m), 0.94 (311,
t). [M+H]+ = 546.1.
Example 65: ((7-(4-(4-(2,3-dichlorophenyl)piperazin-1-vl)butoxy)quinolin-2-
2,2-dimethvItetradecanoate (Compound 1213) and (74444423-
dichlorophenvlbinerazin-1-v1)butoxv)-2-oxocluinolin-1(211methyl 2,2-
dimethvitetradecanoate (Compound 255)
The experimental procedure was carried out in the same manner as for Compound-
1206
in Example 62 to give both Compound-255 and Compound-1212. Compound-1213 was
isolated (0.586 g) as a yellow oil, and Compound-255 was isolated (0.156 g) as
a yellow
oil.
Compound-1213: '11.-NMR (300 MHz, CDCI3) 57.93 (111, d), 7.59 (1H, d), 7.16
(3H,
m), 7.03 (1H, dd), 6.97 (1H, m), 6.78 (1H, d), 6.22 (21-1, s), 4.12 (2H, m),
3.10 (4H, m),
2.73 (4H, m), 2.57 (2H, t), 1.91 (2H, m), 1.80(211, m), 1.46 (2H, d), 1.01 ¨
1.33 (2614,
m), 0.87 (311, t). [M+Hr = 714.3.
Compound-255: '11-NMR (300 MHz, CDC13) 8 7.60 (1H, d), 7.42 (1H, d), 7.15 (2H,
m),
6.96 (1H, m), 6.82 (2H, m), 6.51 (111, d), 6.32 (2H, s), 4.04 (2H, t), 3.07
(4H, m), 2.66
(4H, in), 2.49 (2H, m), 1.87(211, m), 1.76 (2H, m), 1.45(211, m), 1.01 ¨
1.36(2611, m),
0.87 (3H, t). [M+H] = 714.3.
186
CA 3035442 2019-03-01
((7-(4-(4-(2,3-dichlorophenvI)piperazin-l-vl)butoxvlquinolin-2-v1)oxy)methyl
diethvlcarbamate (Example 66, Compound 1247)
The experimental procedure was carried out in the same manner as for Compound-
1206
in Example 62. The reaction was incomplete after 5 days at room temperature.
The
reaction was heated to 60 C for two days before following the same work-up
and
purification procedures as in Example-62 to give Compound-1247 (0.053 g) as a
yellow
oil.
1H-NMR (300 MHz, CDC13) 8 7.94 (111, d), 7.60 (1H, d), 7.20 (1H, m), 7.15 (2H,
m),
7.04 (1H, dd), 6.95 (1H, m), 6.81 (1H, d), 6.24 (2H, s), 4.11 (2H, m), 3.28
(4H, m), 3.09
(4H, in), 2.70 (4H, m), 2.54 (2H, m), 1.90(211, m), 1.78 (211, in), 1.13 (311,
q), 1.03 (3H,
q). [M+H]* = 575.2.
((7-(4-(4-(2,3-dichlorophenvI)niperazin-1-vIlbutoxy)auinolin-2-v1)oxv)methyl
nivalate (Example 67, Compound 1215)
The experimental procedure was carried out in the same manner as for Compound-
1206
in Example-62 to give Compound 1215 (0.555 g) as a yellow oil.
1H-NMR (300 MHz, CDC13) 6 7.95 (1H, d), 7.60(111, d), 7.15 (3H, m), 7.05 (1H,
dd),
6.97 (1H, m), 6.79 (I H, d), 6.22 (2H, s), 4.12 (2H, m), 3.10(411, m), 2.68
(4H, m), 2.54
(211, m), 1.91 (2H, m), 1.78 (2H, m), 1.19 (9H, s). [M+Hr = 560.1.
Example 68: Pharmacokinetic Evaluation in Rats
Pharmacokinetic Evaluation of Prodrugs in Rats Following Intramuscular
Injection
Animals: Male Sprague¨Dawley rats (Charles River Laboratories, Wilmington,
MA) were obtained. Approximately 24 rats were used in each study. Rats were
approximately 350-375 g at time of arrival. Rats were housed 2 per cage with
ad libitum
chow and water. Environmental conditions in the housing room: 64-67 F, 30% to
70%
relative humidity, and 12:12-h light: dark cycle. All experiments were
approved by the
institutional animal care and use committee.
Pharmacokinetics study: Rats were dosed IM by means of a 25 gauge, 5/8 inch
needle with 1 cc syringe 0.3mL suspension was withdrawn from the vial
containing the
test compound (see Table E). The mouse was injected in the muscles of the hind
limb
after anesthesia with isoftourane. Blood samples were collected via a lateral
tail vein
after brief anesthesia with Isoflurane. A 271/2G needle and Ice syringe
without an
anticoagulant was used for the blood collection. Approximately 350p,L of whole
blood
was collected at each sampling time-point of 6 hours, 24 hours and 2, 5, 7, 9,
12, 14, 21,
28, 35 days after administration. Once collected, whole blood was immediately
187
CA 3035442 2019-03-01
transferred to tubes containing K2 EDTA, inverted 10-15 times and immediately
placed
on ice. The tubes were centrifuged for 2 minutes at >14,000 g's (11500 RPMs
using
Eppendorf Centrifuge 5417C, F45-30-11 rotor) at room temperature to separate
plasma.
Plasma samples were transferred to labeled plain tubes (MICROTAINERC) and
stored
frozen at <-70 C.
Data Analysis: Drug concentrations in plasma samples were analyzed by liquid
chromatography¨mass spectroscopy using appropriate parameters for each
compound.
Half-life, volume of distribution, clearance, maximal concentration, and AUC
were
calculated by using WinNonlin Version 5.2_software (Pharsight, St. Louis, MO).
Results and Discussion: The Results are shown in Table J. As shown in Table J,
each of the compounds tested provides a plasma concentration that is extended
as
compared to the parent drug when administered alone.
Table J
API Form used Excipients Dose AU C4 AUCo-T
(Compound No.) **(mpg) (ng'clay/mL) __ (ng*day/mL)
82 solution in ethyl oleate 57 204 NC
2 Recrystallized crystalline 67 1016.9
1139.8
suspension in 1% HPMC in
PBS + 0.2% Tween 20
81 solution in ethyl oleate 56 584 NC
48 Milled crystalline suspension 70.00 2238
2264.6
in 1% HPMC in PBS + 0.2%
Tween 20. Measured and
diluted to correct
concentration*
5 Ethyl oleate emulsion in water 67 1728.6 1742
with DPPC, Glycerol and
NCOH
6 solution in ethyl oleate 67 67 327
6 Oil emulsion in water with 67 1490.3
1678.1
DPPC and Glycerol
47 Milled crystalline suspension loco 113 176
in 1% HPMC
85 MiIki ci rtalline suspension 67 1233.9
1348
in 1% IPMC in PBS + 0.2%
Twee;m20. Measured and
diluted to correct
concentration
1 Crystalline material 56.7 1673 1938
suspended in 1% HPMC
Trademark"
188
Date Recue/Uate Received 2020-1U-06
7 Recrystallized crystalline 67 512.0 1169.5
suspention in 1% HPMC in
PBS + 0.2% Tween 20
32 Milled crystalline suspension 67 1334.4 1486
in 1% HPMC in PBS + 0.2%
Tween 20. Measured and
diluted to correct
concentration*
8 Milled crystalline suspention 24 580.3 666.1
in 1% HPMC in PBS + 0.2%
Tween 20
49 Milled crystalline suspension 71.1 152 1997.
in 1% HPMC
34 Milled crystalline suspension 43.33 2050
2095.8
in 1% HPMC in PBS + 0.2%
Tween 20. Measured and
diluted to correct
concentration*
79 Prodrug solution in ethyl 67 954 NC
oleate
79 Recrystallized crystalline 67 907.4 940
suspension in 1% HPMC in
PBS + 0.2% Tween 20
31 Recrystallized crystalline 67 819.0 997
suspension in 1% HPMC in
PBS + 0.2% Tween 20
Recrystallized crystalline 67 302 786.6
suspension in 1% HPMC in
PBS + 0.2% Tween 20
4 Recrystallized crystalline 67 1455.4 1678
suspension in 1% HPMC in
PBS + 0.2% Tween 20
1002 Crystaline material in 2%CMC, 67 5350 5972
0.2% Tween 20, PBS buffer 302
rnOsm/Kg, pH 6.7
1008 Crystalline matena in 2%CMC, 67 5000 6763
0.2% Tween 20, PBS buffer 302
mOsm/Kg, pH 6.7
Example 69: Pharmacokinetic study for Pioglitazone. Compounds 1002 and 1008
PK profile of compounds 1002 and 1008 was compared to pioglitazone using a
similar
5 model as described above. 20 mg of pioglitazone or 20 mg equilant of
pioglitazone
prodrug was administered intramuscularly. The results are tabulated in Table
J, supra.
Figure 10 shows the PK profile and comparison with pioglitazone.
Example 70- Pharmacodynamic Studies Using an Amphetamine-Induced
-
LOCObuaiion Model -
10 Introduction: Prodrugs of the invention useful in the treatment of
schizophrenia
and bipolar disorder show predictive validity in rodent models of
hyperlocomotion. D-
Amphetamine-induced locomotion is postulated to mimic the dopaminergic
189
CA 3035442 2019-03-01
hyperactivity which forms the basis for the "dopamine hypothesis" of
schizophrenia.
The AMPH-induced hyperactivity model provides a simple, initial screen of
antipsychotic compound efficacy. See, Fell et al., Journal ofPhannacology and
Experimental Therapeutics (2008) 326:209-217. Amphetamine induced
hyperactivity
was used to screen various doses of orally administered (PO) prodrug
formulations of
aripiprazole to measure pharmacodynamic efficacy in an acute hyperlocomotion
paradigm. The hypothesis of the study is that PO administration of
aripiprazole prodmg
formulations, which result in plasma concentrations of ¨100-200 ng/ml, will
produce a
significant attenuation of AMPH-induced locomotion.
General behavior and activity can be measured in experimental animals
(typically
rats and mice) in order to assess psychomotor stimulant properties, anxiogenic
/
anxiolytic or sedative properties of a drug. As such, open-field studies can
provide
insight into the behavioral effects of test compounds, Certain prodrugs of the
present
invention are useful in the treatment of schizophrenia and bipolar disorder.
Aripiprazole
is a parent lactam containing drug from which some of the prodrugs of the
invention are
derived that is useful in the treatment of schizophrenia and bipolar disorder.
Such
aripiprazole prodrugs of the invention show predictive validity in rodent
models of
hyperlocomotion. D-Amphetamine-induced locomotion is postulated to mimic the
dopaminergic hyperactivity which forms the basis for the "dopamine hypothesis"
of
schizophrenia. Likewise, glutamate NMDA receptor antagonist (MK-801, PCP,
etc.)
induced locomotion is postulated to mimic the NMDA hypoactivity hypothesis of
schizophrenia (Felt et al., supra). These tests of drug-induced hyperactivity
provide
simple, initial screens of antipsychotic compound efficacy. Amphetamine
induced
hyperactivity will be used to screen various prodrugs of aripiprazole,
administered PO in
oil solutions, to measure pharmacodynarnic efficacy. The results of the D-AMPH
induced locomotion done in this study will be compared to the historical
results of
subcutaneous (S.C.) aripiprazole administration on D-AMPH. The hypothesis of
the
study is that PO exposure to aripiprazole prodrugs, which results in
aripiprazole
concentrations of 100-200ng/m1 at locomotor testing, will display efficacy in
in-vivo
measures of antipsychotic efficacy.
. .
. .
Materials: Experimental animals: 12, Sprague Dawley rats were purchased from
Charles River Laboratory. The rats were approximately 90 days old, and weighed
in the
range of 350-275 grams upon receipt from the supplier. One rat was placed in a
cage and
190
CA 3035442 2019-03-01
allowed to acclimate for about 1 week. The rats were provided with food and
water ad
Dosing solution of D-Amphetamine (D-AMPH): D-AMPH was purchased from
Sigma Aldrich. D-amphetamine HCI was prepared in 0.9% saline to a
concentration of
1.5mg/inl. D-Amphetamine was given I.P. per body weight at a dose of ImUkg
(=1.5mg/kg). Salt form correction was not used in accordance with historical
literature.
D-Amphetamine was prepared fresh from solid form 30 min. prior to each test
period.
Dosina solutions of prodrua derivatives of aribiprazole:
Table K:
Study Dose
Dose
Group Formulation (Route) volume
mg/rat
mL
Compound-7 oral oil
A Solution (PO) 7.5 1.5 4
Compound-4 oral oil
= Solution (PO) 20 1.5 4
Compound-4 oral oil
= Solution (PO) 10 1.5 4
Compound-7 oral oil
= Solution (PO) 10 1.5 4
Compound-4 oral oil
= Solution (PO) 0.66 1.5 4
Compound-7 oral oil
= Solution (PO) 20 1.5 4
= Saline (PO) 0 1.5 4
191
CA 3035442 2019-03-01
Behavior Box: The behavior chambers were purchased from Med Associates,
Inc. of St. Albans, VT, Model ENV-515. Software for measuring animal movement
is
provided with the behavior chamber by the supplier.
Methods: Following 1 week habituation to the animal facility, the activity
assessments commenced. The animals were initially acclimated to the behavior
box for
about 15 minutes before they were removed from the box and injected PO with
1.5 ml of
an aripiprazole prodrug compound of the invention, at concentrations which
produce PK
levels of 100-200 ng/ml approximately 1 hour after administration. After an
additional
minutes the animals were placed back in the behavior box for an additional 30
minute
10 drug-baseline test session. The mice were then administered by IP
injection, D-AMPH
(1.5 mg/kg) followed by a 60 minute experimental behaviorial measurement
period. The
parameters that were measured were a) total distance measured (primary
measure), b)
total number of ambulatory moves (second measure), c) total number of vertical
moves
(secondary measure) and d) time spent immobile (secondary measure).
15 Blood Sampling: Tail vein blood was taken on experiment days
immediately
following locomotor activity measurements (2-hours post-prodrug
administration) and
again the following day a time-point corresponding to 22 hours post-prodrug
administration. Blood samples were collected via a lateral tail vein after
anesthesia with
Isoflurane. A 271/2 G syringe without an anticoagulant was used for the blood
collection,
and the whole blood transferred to pre-chilled (wet ice) tubes containing 1(2
EDTA.
0.5ml of blood per animal was collected per time point. The tubes were
inverted 15-20
times and immediately returned to the wet ice until being centrifuged for 2
minutes >
14,000g to separate plasma. The plasma samples prepared in this manner were
transferred to labeled plain tubes (MICROTAINER ) and stored frozen at < -70
C.
Behavioral Data Acquisition: Behavioral data was captured electronically by
the
software package associated with the behavior chambers. Data was transformed
and
analyzed via GRAPHPAD PRISM 5 software (GraphPad Software, Inc., La Jolla,
CA).
The data was analyzed using a 2-way repeated measures ANOVA.
Results and Discussion: The results are shown in Figures 6 and 7. The results
indicate
that orally administered D-AMPH caused a significant increase in the total
distance
traveled by the mice as compared to mice who were administered only saline.
The
192
CA 3035442 2019-03-01
results also indicate that aripiprazole prodrug compound 4 of the invention
significantly
inhibited the increases in distance traveled caused by D-AMPH. The inhibition
of
distance travelled by compound 4 did not appear to be dose dependent.
Likewise,
aripiprazole prodrug compounds 7 and 47 did appear to significantly inhibit
increases in
distance traveled caused by D-ANIPH at the higher dose of 20 mg. This data
indicates
that in accordance with the invention, the prodrug compounds are cleaved in
vivo to
release the parent drug (aripiprazole in this example) to provide the expected
pharmacological effects on the animal.
193
CA 3035442 2019-03-01