Note: Descriptions are shown in the official language in which they were submitted.
CA 03036770 2019-03-13
WO 2018/051325
PCT/IL2017/000007
1
STABLE PHARMACEUTICAL FOAM
FIELD OF THE INVENTION
The invention relates to the field of pharmaceutical foams, such as
pharmaceutical foams comprising peptones prepared by enzymatic hydrolysis of
proteins.
BACKGROUND OF THE INVENTION
A foam is a substance that is formed by dispersing a gas in a liquid, such
that
bubbles of the gas are trapped in the liquid, with thin films of liquid
separating the
regions of gas.
Pure liquids comprising no dissolved particles (e.g. 100% H20) do not foam,
such that the addition of a surfactant is generally required in order to
reduce the
surface tension of the liquid, enabling mixing of the gas with the liquid to
form a
stable foam. Surfactants are usually amphiphilic in nature (i.e. having both a
hydrophilic group and a lipophilic group), with long hydrophobic chains.
Foams prepared from full-length proteins, which act as surfactants, are known.
Full-length proteins require denaturation in order to provide the required
surfactant
characteristics. In order to prepare the foam, an amphiphilic agent is
required i.e. a
molecule which has both a hydrophilic group and a hydrophilic group, allowing
the
strands of denatured proteins to form micelles, within which gas, such as air,
is
trapped. This characteristic allows forming bubbles of air which are stable
within the
liquid.
Foams are widely used in industry, such as in the food industry or as fire
extinguishing foams. Foams are potentially useful in a wide variety of medical
and
surgical procedures, e.g. for providing protection of a surface, delivery of a
drug, or to
serve as a barrier for numerous surgical procedures. Use of a liquid foam
enables fast
and efficient coverage of a large area with a minimal amount of liquid.
For most surgical procedures, it is essential that a foam used must be strong
and durable. The strength of a foam may be expressed as the force required for
the
compression of the foam (i.e. compression strength), which may be measured
using a
device such as manufactured by Instron or Lloyd, using a method similar to
that
CA 03036770 2019-03-13
WO 2018/051325 2
PCT/IL2017/000007
performed for the determination of the gelatin gel strength, the Bloom number.
The
Bloom number is a measure of the force (weight in grams) required to compress
a
given sample area a distance of 4 mm. A higher Bloom number indicates a
stronger
gel. Bloom number is proportional to the average molecular mass. A low Bloom
number (50-125) correlates to an average molecular mass of 20,000-25,000; a
medium Bloom number (175-225) correlates to an average molecular mass of
40,000-
50,000; while a high Bloom number (225-325) correlates to an average molecular
mass of 50,000-100,000.
Background art include US Patent Nos. 8,778,883; 8,512,740; 8,753,670;
8,741,335; 2,492,458; 6,454,787; 8,603,543; and 6,730,299; PCT Publication
Nos.
WO 2014/086996; 2014/071053; and 2010/088469; and European Patent No.
1257304.
SUMMARY OF THE INVENTION
The invention, in some aspects thereof, relates to a pharmaceutical (i.e. for
medical and/or surgical use) foam composition comprising a peptone prepared by
enzymatic hydrolysis of a full-length protein, wherein the foam is free of the
full-
length protein.
Aspects and embodiments of the invention are described in the specification
hereinbelow and in the appended claims.
It is generally known that for gels produced from full-length proteins, the
compression force of the gel is directly proportional to the average molecular
mass of
the protein. This feature of the effect of molecular weight on compression
force could
be correlated to foams prepared by denatured proteins.
The present Inventors have surprisingly found that peptones, comprising short
peptide lengths (e.g. of 90 or fewer amino acids), can be used to produce a
stable
foam, which has superior qualities, such as higher foam compression strength,
as
compared to known foams produced from full-length, homogeneous proteins,
having
higher average molecular mass.
Generally, peptones may be prepared from full-length proteins derived from
different sources (e.g. gelatin, casein or protein mixtures) to obtain peptide
fragments.
Peptide fragments are short chains of amino acid monomers linked by amide
bonds.
CA 03036770 2019-03-13
WO 2018/051325
PCT/IL2017/000007
3
Peptones may be obtained by different methods, such as by enzymatic, acidic,
and/or
alkali hydrolysis of full-length proteins.
The shortest peptides can be dipeptides, consisting of two amino acids joined
by a
single peptide bond.
Peptones used in the present invention are water-soluble mixtures comprising
peptides and optionally free amino acids, formed by enzymatic
hydrolysis/digestion
of a full-length protein. In some embodiments, the peptones are devoid of free
amino
acids.
According to an aspect of the present invention, there is provided a
pharmaceutical foam composition comprising a peptone prepared by hydrolysis of
a
full-length protein, wherein the foam is free of the full-length protein.
According to a further aspect of the present invention, there is provided a
pharmaceutical foam composition comprising a peptone prepared by enzymatic
hydrolysis of a full-length protein, wherein the foam is free of said full-
length protein.
According to a further aspect of the present invention, there is provided a
pharmaceutical foam composition comprising a peptone prepared by enzymatic-
digestion of a full length protein, wherein the foam is free of the full-
length protein.
According to a further aspect of the present invention, there is provided a
pharmaceutical foam composition comprising a protein hydrolysate prepared by
enzymatic hydrolysis of a full-length protein, wherein said foam is free of
the full-
length protein.
As used herein, the term "hydrolysate" refers to a material produced by
hydrolysis. The term "hydrolysis" usually means the cleavage of chemical bonds
by
the addition of water. In some embodiments the term "protein hydrolysis"
relates to
the breakdown of protein into smaller peptides and free amino acids. In some
embodiments the term "protein hydrolysis" relates to the breakdown of protein
by
hydrolysis of the peptide bonds. The term "protein hydrolysate" refers to a
product of
hydrolysis of a protein that typically comprises peptides and free amino
acids.
In the following aspects of the invention, the peptone or the protein
hydrolysate comprises enzymatically-digested protein or enzymatically-
hydrolyzed
protein.
CA 03036770 2019-03-13
WO 2018/051325
PCT/IL2017/000007
4
In some embodiments of any of the pharmaceutical foam compositions
disclosed herein, the peptone, the protein hydrolysate, or the enzymatically-
hydrolyzed protein is devoid of peptides of size greater than 11.7 kDa.
In some embodiments, the peptone, the protein hydrolysate or the
enzymatically-hydrolyzed protein comprises peptides of size less than
10.01cDa.
In some embodiments peptones, protein hydrolysates or enzymatically-
hydrolyzed proteins, e.g. peptones or protein hydrolysates or enzymatically-
hydrolyzed proteins prepared from gelatin, consist mainly of chain lengths
below 10.0
IcDa (of approximately 90 or fewer amino acids), such as, for example, from
about
1000 Da up to about 10 lcDa, from about 300 Da to about 500 Da, or even below
300
Da.
In one embodiment, the peptone, protein hydrolysate or enzymatically-
hydrolyzed protein comprises peptides that are long, continuous, and
unbranched
peptide chains.
In one embodiment, the peptone, protein hydrolysate or enzymatically-
hydrolyzed protein comprises peptides of approximately 90 or fewer amino
acids.
In some embodiments, the full-length protein being hydrolyzed is a
combination of two or more types of full-length proteins.
In some embodiments, the full-length protein being hydrolyzed is a single type
of full-length protein.
In some embodiments, the full-length protein being hydrolyzed is selected
from the group consisting of a milk protein (such as casein), a collagen-
derived
protein (such as gelatin), an egg protein, a blood protein (such as albumin),
a yeast
protein, a plant protein, or combinations thereof.
In some embodiments, the full-length protein being hydrolyzed is selected
from the group consisting of casein and gelatin.
In some embodiments, the foam is stable.
In some embodiments, the enzymatic hydrolysis comprises use of a protease
selected from the group consisting of a serine protease, a cysteine protease,
a
CA 03036770 2019-03-13
WO 2018/051325
PCT/IL2017/000007
threonine protease, an aspartic protease, a glutamic protease, a
metalloprotease and a
combination thereof.
In some embodiments, the peptone, protein hydrolysate or enzymatically-
hydrolyzed protein is present in the foam at a concentration of higher than
about 0.05
5 to lower than about 20% w/v of the foam, such as, for example, at a
concentration of
higher than about 1.5 to lower than about 18.0 % w/v of the foam, or at a
concentration of higher than about 1.66 to lower than about 17.86% w/v of the
foam.
In some embodiments, the pharmaceutical foam composition further
comprises fibrin and/or fibrinogen, optionally at a concentration in the range
of from
about 0.1 mg/mL to about 10 mg/mL of the foam, such as, for example, at a
concentration in the range of from about 2.3 mg/mL to about 7 mg/mL of the
foam.
In some embodiments, the pharmaceutical foam composition further
comprises thrombin, optionally at a concentration in the range of from about
0.1
IU/mL to about 100 IU/mL of the foam.
According to a further aspect of the invention, there is provided a method for
preparing a pharmaceutical foam composition, comprising a step of: foaming a
solution of a peptone, peptide hydrolysate or enzymatically-hydrolyzed protein
with a
gas, the solution of a peptone, peptide hydrolysate or enzymatically-
hydrolyzed
protein is prepared by enzymatic hydrolysis of a full-length protein in an
aqueous
solution, wherein the solution of a peptone, peptide hydrolysate or
enzymatically-
hydrolyzed protein is free of the full-length protein.
According to a further aspect of the invention, there is provided a method for
preparing a pharmaceutical foam composition, comprising a step of: foaming a
liquid
solution of a peptone, peptide hydrolysate or enzymatically-hydrolyzed protein
with a
gas, the liquid solution of a peptone or a peptide hydrolysate is prepared by
enzymatic
hydrolysis of a full-length protein in a liquid, aqueous solution, wherein the
solution
of a peptone, peptide hydrolysate or enzymatically-hyrolyzed protein is free
of the
full-length protein.
As used herein, the term "foaming" refers to the process of preparing a foam
by mixing a liquid solution with a gas.
CA 03036770 2019-03-13
WO 2018/051325
PCT/IL2017/000007
6
Foaming may be achieved manually or automatically. For example, foaming
may be achieved by providing two containers (such as two syringes) in fluid
communication one with the other, wherein a liquid solution is present in a
first of the
two containers and a gas, such as air, is present in the second of the two
containers;
passing the liquid from the first syringe into the gas in the second syringe
or the gas
from the second syringe into the liquid from the first syringe; then passing
the liquid
and gas between the two syringes until a foam is achieved.
For example, foaming may be achieved by providing two containers (such as
two syringes) in fluid communication one with the other, wherein a liquid for
reconstitution is present in a first of the two containers and a gas, such as
air, together
with a peptone powder or protein hydrolysate is present in the second of the
two
containers; passing the liquid from the first syringe into the gas in the
second syringe
or the gas from the second syringe into the liquid from the first syringe;
then passing
the liquid and gas between the two syringes until a foam is achieved.
Alternatively, a liquid solution may be provided in a sealed container which
does not have fluid communication with a gas until an operating mechanism is
activated to bring the gas into contact with the liquid. Such a mechanism may
include,
for example, a pump device or a mechanism for breaking a seal of the sealed
container.
In one embodiment, the passing of the liquid between the two syringes is
performed at least 6 times.
As used herein, the term "aqueous solution" refers to a solution comprising
water and at least one solute dissolved therein. In one embodiment, the term
is
intended to exclude emulsions or solutions comprising an oil.
An emulsion is a mixture of two or more liquids that are normally immiscible
(urn-nixable or unblendable).
A "liquid" is, for example, a fluid that conforms to the shape of its
container
but retains a (nearly) constant volume independent of pressure, and/or a
flowable
material.
In some embodiments, the peptone or peptide hydrolysate comprises
enzymatically-digested protein.
CA 03036770 2019-03-13
WO 2018/051325
PCT/IL2017/000007
7
Hence, according to an aspect of the present invention, there is provided a
method for preparing a pharmaceutical foam composition comprising a step of:
foaming a solution of an enzymatically-digested protein with a gas, the
solution of a
an enzymatically-digested protein prepared by enzymatic hydrolysis of a full-
length
protein in an aqueous solution, wherein the solution of an enzymatically-
digested
protein is free of the full-length protein.
Hence, according to an aspect of the present invention, there is provided a
method for preparing a pharmaceutical foam composition comprising a step of:
foaming a solution of an enzymatically-digested protein with a gas, the
solution of a
an enzymatically-digested protein prepared by enzymatic hydrolysis of a full-
length
protein in a liquid aqueous solution, wherein the liquid solution of an
enzymatically-
digested protein is free of the full-length protein.
In some embodiments of the method disclosed herein, the peptone, the peptide
hydrolysate or the enzymatically-digested protein comprises peptides of size
less than
10.0 kDa.
In some embodiments, the peptone, the peptide hydrolysate or the
enzymatically-digested protein comprises peptides of at least 1000 Da.
In some embodiments, the peptone, the protein hydrolysate or the
enzymatically-digested protein comprises peptides having a size in the range
of from
1000 Da to less than 10.0 kDa.
In some embodiments, prior to foaming, the solution of a peptone, protein
hydrolysate or enzymatically-digested protein is dried and prior to
preparation, is
reconstituted with a solution comprising water.
In some embodiments, the enzyme hydrolyses the full-length protein to
produce a peptone, protein hydrolysate or enzymatically-hydrolyzed protein
including
peptides of size less than 10.0 kDa.
.In some embodiments, the method further comprises, prior to foaming,
removing peptides of size greater than 11.7 kDa from the solution of a
peptone,
protein hydrolysate or enzymatically-hydrolyzed protein.
CA 03036770 2019-03-13
WO 2018/051325
PCT/IL2017/000007
8
In some embodiments, the method further comprises, prior to foaming,
removing peptides of size greater than 10 kDa from the solution of a peptone,
protein
hydrolysate or enzymatically-hydrolyzed protein.
In some embodiments, removing peptides of a selected size is performed by
filtration, e.g. passage through a size exclusion membrane e.g. in a
centrifugal
filtration device.
In some embodiments, the full-length protein being hydrolyzed is a
combination of proteins, such as 2, 3 or more different full-length proteins.
In some embodiments, the full-length protein being hydrolyzed is a single type
of protein.
In some embodiments, the full-length protein being hydrolyzed is casein.
In some embodiments, the full-length protein being hydrolyzed is gelatin.
In some embodiments, enzymatic hydrolysis is carried out with a protease
selected from the group consisting of a serine protease, a cysteine protease,
a
threonine protease, an aspartic protease, a glutamic protease, a
metalloprotease and a
combination thereof as long as the produced protein hydrolysate or peptone
comprises
peptides having a size in the range of from 1000 Da to less than 10.0 kDa
and/or as
long as the foaming ability of the peptone, protein hydrolysate or
enzymatically-
hydrolyzed protein is not compromised.
Solutions comprising peptone, protein hydrolysate or enzymatically-
hydrolyzed protein at a concentration of less than about 50% w/v of the
solution are
considered to be beneficial for use in preparing the foam as disclosed herein.
Hence,
in some embodiments, the peptone, protein hydrolysate or enzymatically-
hydrolyzed
protein is present at a concentration of lower than about 50 w/v of the
solution e.g. at
a concentration of higher than about 1 to lower than 50% w/v.
In some embodiments, the peptone, protein hydrolysate or enzymatically-
hydrolyzed protein is present in the solution at a concentration of higher
than about 1
to lower than about 40% w/v, such as, for example, at a concentration of
higher than
about 5 to lower than about 25% w/v.
In some embodiments, the method further comprises inactivating the enzyme
upon completion of the hydrolysis. Enzyme inactivation can be carried out by
altering
CA 03036770 2019-03-13
WO 2018/051325
PCT/IL2017/000007
9
the conditions required for enzymatic activity such as heating and/or pH
adjustment,
or by removing the enzyme (e.g. by affinity chromatography, size exclusion
etc.) as
long as the foaming ability of the peptone, protein hydrolysate or
enzymatically-
hydrolyzed protein is not compromised.
In some embodiments, the peptone, protein hydrolysate or enzymatically-
hydrolyzed protein and/or the foam are free of an active enzyme used to
prepare the
peptone, protein hydrolysate or enzymatically-hydrolyzed protein.
In some embodiments, the method further comprises adding fibrinogen to the
solution of a peptone, protein hydrolysate or enzymatically-hydrolyzed protein
prior
to foaming and after enzyme inactivation, optionally at a concentration in the
range of
from 1% w/v to up to about 30% w/v of the solution of a peptone, protein
hydrolysate
or enzymatically-hydrolyzed protein as long as the foaming ability of the
peptone,
protein hydrolysate or enzymatically-hydrolyzed protein is not compromised.
In some embodiments, the method further comprises adding thrombin to the
pharmaceutical foam composition, optionally at a concentration of from about
0.1
IU/mL to about 100 IU/mL of the pharmaceutical foam composition. In one
embodiment, the thrombin is added after foaming.
In some embodiments, there is provided a pharmaceutical foam obtained
according to any of the methods disclosed herein.
In some embodiments, there is provided the use of the pharmaceutical foam
composition disclosed herein, for providing hemostasis, sealing (such as of
pleural
tissue), anti-adhesion and/or wound healing.
According to an aspect disclosed herein, there is provided a kit comprising a
container comprising a peptone, protein hydrolysate or enzymatically-
hydrolyzed
protein, a device for obtaining a foam and optionally, a full-length protein
other than
the one subjected to hydrolysis.
In some embodiments, the full-length protein other than the one subjected to
hydrolysis is fibrinogen.
In some embodiments, the kit further comprises a container comprising
thrombin.
CA 03036770 2019-03-13
WO 2018/051325
PCT/IL2017/000007
In some embodiments of the kit as disclosed herein, the peptone, protein
hydrolysate or enzymatically-hydrolyzed protein comprises peptides of size
less than
10.0 kDa.
In one aspect, the invention provides a pharmaceutical foam composition
5 comprising a peptone, protein hydrolysate or enzymatically-hydrolyzed
protein
prepared by enzymatic hydrolysis of a full-length protein, wherein said foam
is free of
the full-length protein subjected to hydrolysis.
In some embodiments, the foam as disclosed herein is sturdier and more
durable than foams known in the art, having greater tensile strength,
determined by its
to increased resistance to compression.
High strength and durability is important for applications in which the
presence
of the foam is required over an extended period, such as for wound healing,
for sealing
procedures or for adhesion prevention. In some situations, hemostasis must be
ensured
over an extended period of time, for example in patients medicated with
anticoagulant
drugs. For sealing, the foam is required to have a high strength in order to
withstand the
stress resulting from specific applications, such as air sealing following
lung surgery.
For anti-adhesion applications, the durability of the foam is important in
order to
provide a sturdy physical barrier between different organs at the surgical
site. In some
embodiments, for wound healing, it is important that a matrix (e.g. foam) in
which the
cells can grow will remain durable throughout the initial healing phase.
In some embodiments, the foam as disclosed herein has reduced
irrn-nunogenicity and/or reduced allergenic properties as compared to foams
known in
the art, allowing for repeated application.
In some embodiments, the foam as disclosed herein has greater adhesiveness
than foams known in the art, which is highly advantageous in certain medical
applications to allow the material to remain in position at the site of
application. For
example, in some embodiments, the foam as disclosed herein has a mean adhesion
force to tissue of greater than 1 N/inch2, such as, for example at least 1
N/inch2, at
least 2 N/inch2, at least 3 N/inch2, at least 4 N/inch2, at least 5 N/inch2,
or even at least
6 N/inch2. In some embodiments, the mean adhesion force to tissue is in the
range of
from about 1 N/inch2 to about 6 N/inch2.
CA 03036770 2019-03-13
WO 2018/051325
PCT/IL2017/000007
11
In some embodiments, the foam as disclosed herein has greater stiffness than
foams known in the art, which is highly advantageous in certain medical
applications
i.e. for application to tissues where the foam must have strong cohesion to
seal fluid
or air leaks, especially where pressures may be elevated. For example, in some
embodiments, the foam as disclosed herein has a mean stiffness of at least 3
N/mm,
such as, for example, 3 N/mm, 4 N/mm, at least 5 N/mm at least 6 N/mm, at
least 7
N/mm, at least 8 N/mm, at least 9 N/mm, at least 10 N.mm, at least 11 N/mm, at
least
12 N/mm, at least 13 N/mm, at least 14 N/mm, at least 15 N/mm, at least 16
N/mm, at
least 17 N/mm, or even at least 18 N/mm. In some embodiments the mean
stiffness is
in the range of from about 3 N/nu-n to about 19 N/mm. Additionally, in some
embodiments, the foam must be able to remain intact, if the underlying tissue
is
expanding or contracting.
In some embodiments, the foam is stable, is not transient, and e.g. maintains
its foam structure including height, volume, and/or porosity/mean pore size,
for at
least one hour after formation.
As used herein, the term "stable" with regard to a foam (e.g. a non-dried
foam)
relates to a foam that can substantially support its own structure without
collapse at a
specified temperature. For example, foam which is stable in vitro at
physiological
temperature retains at least 80% (such as 90%, 95% or higher) of its original
structure
including height, volume, and/or porosity/mean pore size for at least 1 hour
at
ambient temperature. Typically, collapse is most evidently characterized by
the loss
of foam structure after foam formation. Collapse usually results in a
structure whose
volume is significantly smaller than the volume of the original prepared foam.
In some embodiments, the foam as disclosed herein has a faster in-vivo
degradation time than foams known in the art. Since peptones are already
partially
degraded proteins, these can be completely degraded more rapidly than native,
intact/folded proteins. This property may reduce one or more of inflammatory
reaction, foreign body reaction and post-surgical adhesions.
As used herein, the term "degradation time" means the time required for at
least 90% of the peptone components of the foam to be degraded in-vivo.
The desired degradation time of the foam is dependent on the intended use
(e.g. as sealant or hemostat), tissue type, amount used, chance of re-bleeding
or re-
CA 03036770 2019-03-13
WO 2018/051325
PCT/IL2017/000007
12
leaking, pressures involved, patient condition, etc. In general, it is desired
that a
sealant or hemostat be present long enough to allow for tissue repair, but to
not
impede tissue repair. For example, in some embodiments, it is preferred that a
foam
for use as a sealant or hemostat has a longevity of 4-5 days.
In some embodiments, the foam as disclosed herein is free of a non-protein
surfactant.
In some embodiments, the foam as disclosed herein is prepared in the absence
of a non-protein surfactant.
In some embodiments, the peptone, protein hydrolysate or enzymatically-
hydrolyzed protein used to prepare the foam (i.e. prior to foaming) has not
being
subjected to denaturation prior to foaming.
In some embodiments of the invention, the peptone is not denatured.
In some embodiments the full-length protein subjected to hydrolysis, to
prepare the peptone, the protein hydrolysate or enzymatically-hydrolyzed
protein has
not been subjected to denaturation prior to foaming.
In some embodiment the solution of a peptone, protein hydrolysate or
enzymatically-hydrolyzed protein is free from denatured proteins.
In some embodiments the solution of a peptone, peptide hydrolysate or
enzymatically-hydrolyzed protein is free from denatured proteins other than
the
hydrolyzing enzyme(s).
In some embodiment the solution of a peptone, peptide hydrolysate or
enzymatically-hydrolyzed protein includes other full-length protein, wherein
the other
full length protein is one that was not subjected to the enzymatic hydrolysis
In some
embodiments, the other full length protein is present in the peptone, protein
hydrolysate or enzymatically-hydrolyzed protein in addition to the hydrolyzing
enzyme, and the other full-length protein has not been subjected to
denaturation prior
to foaming.
= Typically, denaturation is a process of modifying the secondary and/or
tertiary
molecular structure of a protein/peptide e.g. by heating, by treatment with
alkali, acid,
urea, or detergent. When a protein is denatured, secondary and/or tertiary
structures
CA 03036770 2019-03-13
WO 2018/051325
PCT/IL2017/000007
13
are altered but the peptide bonds of the primary structure between the amino
acids are
left intact.
As used herein, the terms "comprising", "including", "having" and
grammatical variants thereof are to be taken as specifying the stated
features, integers,
steps or components but do not preclude the addition of one or more additional
features, integers, steps, components or groups thereof. These terms encompass
the
terms "consisting of' and "consisting essentially of'.
As used herein, the indefinite articles "a" and "an" mean "at least one" or
"one
or more" unless the context clearly dictates otherwise.
As used herein the term "about" refers to 10%.
Unless otherwise defined, all technical and scientific terms used herein have
the same meaning as commonly understood by one of ordinary skill in the art to
which the invention pertains. In addition, the descriptions, materials,
methods, and
examples are illustrative only and not intended to be limiting. Methods and
materials
similar or equivalent to those described herein can be used in the practices
of the
present invention.
As used herein, the term "enzymatic hydrolysis" means a full-length protein is
enzymatically hydrolyzed to a point at which the peptone solution is free of
the
original full-length protein.
In one embodiment, enzymatic hydrolysis according to the invention also
includes hydrolyzation to a point in which a given enzyme did not
hydrolyze/digest
all possible digestion sites of the full length protein that are recognized by
the
enzyme.
In some embodiments, the pharmaceutical foam composition disclosed herein
is substantially devoid and/or substantially free of the full-length protein
that was
subjected to enzymatic hydrolysis. As used herein, the term "substantially
free" or
"substantially devoid of' with regard to the full-length protein means that
the
composition contains less than 5 w/v %, less than 4 w/v %, less than 3 w/v %,
less
than 2 w/v %, less than 1 w/v %, less than 0.5 w/v %, less than 0.1 w/v % or
less than
0.05 w/v% of the full-length protein.
CA 03036770 2019-03-13
WO 2018/051325
PCT/IL2017/000007
14
As used herein, the term "solution of a peptone" refers to a solution, such as
a
liquid solution, comprising a peptone and optionally other components, such as
small
molecules, salts, active pharmaceutical ingredients, and coagulation factors.
As used herein, the term "solution of a peptide hydrolysate" refers to a
solution, such as a liquid solution, comprising the peptide hydrolysate and
optionally
other components, such as small molecules, salts, active pharmaceutical
ingredients,
and coagulation factors.
As used herein, the term "solution of an enzymatically-hydrolyzed protein"
refers to a solution, such as a liquid solution, comprising the enzymatically-
hydrolyzed protein and optionally other components, such as small molecules,
salts,
active pharmaceutical ingredients, and coagulation factors.
In some embodiments, the peptone is derived from a milk protein (such as
casein), a collagen-derived protein (such as gelatin, e.g., prepared from
skin, cartilage
or bones), an egg protein, a blood protein (such as albumin), a yeast protein,
a plant
protein, or combinations thereof.
In addition to containing small peptides, the resulting peptone solution may
also include fats, metals, salts, vitamins and many other biological
compounds.
According to an aspect, the invention provides a kit comprising a container
comprising a protein hydrolysate prepared by enzymatic hydrolysis of a full-
length
protein, a device for foaming the hydrolysate and optionally, a full-length
protein
other than that subjected to the enzymatic hydrolysis.
According to a further aspect, the invention provides a method for preparing a
pharmaceutical foam composition, comprising a step of: foaming a solution of a
protein hydrolysate with a gas, the solution of the protein hydrolysate
prepared by
enzymatic hydrolysis of a full-length protein in an aqueous solution, wherein
said
solution is free of said full-length protein.
Yet, according to a further aspect, the invention provides a method for
preparing a pharmaceutical foam composition comprising: enzymatically
hydrolyzing
a full-length protein in an aqueous solution until said solution is free of
said full-
length protein thereby obtaining a solution of a peptone or protein
hydrolysate; and
foaming said solution of said peptone or protein hydrolysate with a gas.
CA 03036770 2019-03-13
WO 2018/051325
PCT/IL2017/000007
In another aspect, the invention provides a pharmaceutical foam composition
obtained
according to the method of the invention.
According to an aspect of the present invention, there is provided a peptone-
based foam prepared by hydrolysis of a full-length protein, wherein the foam
is free
5 of the full-length protein that was subjected to hydrolysis.
The term "peptone based foam" means that the majority of the foam (more
than half of the total weight of the foam) is composed of peptone.
Other components such as fibrinogen, fibrin, thrombin, etc. may also be
present in the foam e.g. proteins other than the full-length protein that was
subjected
10 to hydrolysis can be present. For example the foam can comprise 1% to 100%
peptone out of the total dissolved components.
Proteins other than the full-length protein that was subjected to hydrolysis
can
be present in the foam in at concentrations of up to or equal to 49% while the
remaining components consist of peptone.
15 As used herein, the term "full-length" protein refers to a protein prior
to
hydrolysis/digestion.
In some embodiments, the ratio of air to liquid used in preparing the foam was
in the range of from 1:3 to 3:1 air:liquid. In some preferred embodiments, the
ratio of
air to liquid is in the range of from about 2:1 to about 3:1 air:liquid.
Protein molecules are often very large and are made up of hundreds to
thousands of amino acid units. Proteins include naturally occurring proteins
or
fragments thereof and/or synthetic proteins.
The foam can be dried or non-dried. A dry foam can be obtained by reducing
the concentration of water e.g. by air drying, vacuum drying, or freeze
drying.
The term "dry foam" refers to foam comprising water content of equal to or
less than 3% by weight based on the total weight of the foam composition
(w/w).
According to an aspect of the present invention, there is provided a method
for
promoting blood coagulation; sealing; prevention and/or reduction of adhesion;
and/or
wound healing comprising application of a pharmaceutical foam composition
according to the invention.
CA 03036770 2019-03-13
WO 2018/051325 16
PCT/IL2017/000007
All aspects and embodiments relating to peptone described herein above and
below also intend to relate to "peptide hydrolysate" or "enzymatically-
hydrolyzed
protein", where applicable.
BRIEF DESCRIPTION OF THE DRAWINGS
Some embodiments of the invention are described herein with reference to the
accompanying figures. The description, together with the figures, makes
apparent to a
person having ordinary skill in the art how some embodiments of the invention
may
be practiced. The figures are for the purpose of illustrative discussion and
no attempt
is made to show structural details of an embodiment in more detail than is
necessary
for a fundamental understanding of the invention. For the sake of clarity,
some objects
depicted in the figures are not to scale.
In the Figures:
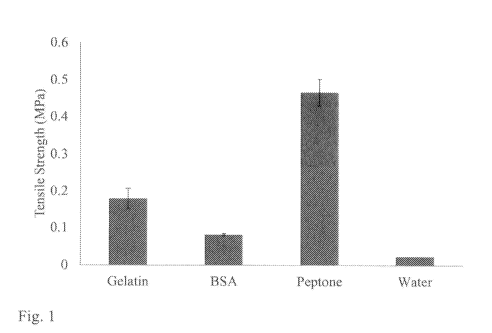
FIG. 1 is a bar graph showing the tensile strength of foams prepared from full-
length gelatin and full-length Bovine Serum Albumin (BSA), or from peptone
obtained by enzymatic hydrolysis of full-length gelatin;
FIG. 2 is a bar graph showing the tensile strengths of foams prepared from
full-length BSA, casein or gelatin and from peptone obtained by enzymatic or
acid
hydrolysis of the full-length casein or gelatin;
FIG. 3 is a bar graph showing the effect of peptone concentration on tensile
strength for peptone obtained by enzymatic hydrolysis of full-length gelatin;
FIG. 4 is a bar graph showing the effect of BAC2 concentration on tensile
strength for peptone obtained by enzymatic hydrolysis of full-length gelatin;
FIG. 5 is a bar graph showing the tensile strength of foams prepared from full-
length gelatin and from peptone derived from enzymatic hydrolysis of full-
length
gelatin in the presence and absence of fibrinogen;
FIG. 6 is a dot graph showing tissue adhesion strength of foams prepared from
peptones obtained by enzymatic hydrolysis of full-length gelatin or casein, as
compared to foams prepared from full-length albumin;
CA 03036770 2019-03-13
WO 2018/051325
PCT/IL2017/000007
17
FIG. 7 is a dot graph showing stiffness of foams prepared from peptones
obtained by enzymatic hydrolysis of full-length gelatin or casein, as compared
to
foams prepared from full-length albumin;
FIG. 8 shows scanning electron micrographs for foams prepared from full-
length gelatin (8A) and from foam prepared from peptone obtained by enzymatic
hydrolysis of full-length gelatin (8B);
FIG. 9 shows the effect of peptone peptide size on tensile strength of foams
prepared from full-length gelatin, from peptone derived from enzymatic
hydrolysis of
full-length gelatin and from peptone derived from enzymatic hydrolysis of full-
length
gelatin having peptides of less than about 10 kDa; and
FIG. 10 shows the effect of mixing peptones obtained by enzymatic hydrolysis
of full-length gelatin with full-length gelatin on the tensile strength of the
foam.
DESCRIPTION OF SOME EMBODIMENTS OF THE INVENTION
The invention, in some embodiments thereof, relates to a pharmaceutical foam
composition comprising peptone prepared by enzymatic hydrolysis of protein(s).
The principles, uses and implementations of the teachings herein may be
better understood with reference to the accompanying description. Upon perusal
of
the description, one skilled in the art is able to implement the invention
without undue
effort or experimentation.
Before explaining at least one embodiment in detail, it is to be understood
that
the invention is not necessarily limited in its application to the details of
construction
and the arrangement of the components and/or methods set forth in the
following
description. The invention is capable of other embodiments or of being
practiced or
carried out in various ways.
The phraseology and terminology employed herein are for descriptive purpose
and should not be regarded as limiting.
As shown in the Examples presented below, it was surprisingly found that
greater force was required to compress foams obtained from peptones as
compared to
foams prepared from full-length proteins.
CA 03036770 2019-03-13
W02018/051325 18
PCT/IL2017/000007
Further, unexpectedly, only peptones resulting from enzymatic hydrolysis, and
not from acid hydrolysis, were shown to yield durable foams.
In ex-vivo experiments it was further shown that foams obtained from
peptones have increased adhesive characteristics as compared to foams obtained
from
full-length proteins.
It was further surprisingly found that the presence of cross-linker was not
required to obtain sturdy foam from peptones, but can be optionally be added.
Further surprisingly, it was found that peptones comprising peptides of equal
to or less than 10 kDa provided sturdier foams than full-length proteins.
EXAMPLES
MATERIALS AND METHODS
Materials
BSA (Sigma, cat#A7030)
Gelatin from porcine skin (Sigma, cat#G1890)
Peptone obtained by enzymatic hydrolysis of gelatin (Sigma, cat#70951)
Casein (Sigma, cat#C3400)
Peptone obtained by enzymatic hydrolysis of casein (Sigma, cat#70172)
Peptone obtained by acidic hydrolysis of casein (Sigma, cat#70171)
BAC2 component of EVICEL , cats#3901,3902, 3905, Ethicon)
Thrombin (Thrombin component of EVICEL , cats#3901,3902, 3905,
Ethicon)
Water for preparation and dilution of solutions was deionized water.
Compression tests were performed using a Lloyd LF Plus device, with a 10
mm, flat bottom stencil, or an Instron.
CA 03036770 2019-03-13
WO 2018/051325
PCT/IL2017/000007
19
Example 1: Tensile strength of foams prepared from peptone and from full-
length
BSA and gelatin.
The force required for the compression of each of the following foams was
measured:
1. Foam comprising full-length gelatin, fibrinogen source (BAC2) and thrombin;
2. Foam comprising BSA, fibrinogen source (BAC2) and thrombin;
3. Foam comprising peptone obtained by enzymatic hydrolysis of full-length
gelatin, fibrinogen source (BAC2) and thrombin; and
4. Control foam comprising fibrinogen source (BAC2) and thrombin.
A 5% w/v aqueous solution of each of full-length gelatin, full-length BSA and
peptone obtained by enzymatic hydrolysis of full-length gelatin was prepared
(foam
nos. 1-3 from left to right). To 5 mL of each solution, 500 pL of a
concentrated BAC2
solution were added to provide a final concentration of 10 % BAC2, comprising
in
total about 35 mg fibrinogen. For control foam (no. 4), 5 mL water were added
to 500
pi concentrated BAC2 solution.
The solutions were foamed by using two syringes, interconnected with a 2 cm
Tyvec tubing (¨ 2 mm diameter). The solutions as prepared above were drawn
into
the first syringe, and 10 mL of air were drawn into the second syringe. The
solutions
were expelled back and forth between the first and second syringes, thereby
admixing
the solution with the air.
At the final step of the preparation, 20 IU Thrombin in 40 mM CaCl2 in a
volume of 200 1 were added to the foam by adding the thrombin solution to one
syringe and expelling the foam back and forth one more time. The prepared foam
was
expelled to rim height into a well of a 24-tissue culture plate. The foam was
allowed
to stand for one hour at room temperature. The force required for compression
was
then evaluated using a 10 mm2 stencil, pressing at a rate of 5 mm/min for a
total
length of 12 mm in triplicate. The results were recorded and analyzed.
As seen in Figure 1, it was surprisingly found that foam prepared from the
peptone obtained by enzymatic hydrolysis of full-length gelatin required the
highest
force for compression of the foam. As shown by the control sample (Water), the
force
required for compression of BAC2 alone was negligible. Furthermore, full-
length
CA 03036770 2019-03-13
WO 2018/051325 0
PCT/IL2017/000007
2
BSA (66.5 kDa), a globular protein, was shown to require smaller force for
compression than that required for gelatin.
Example 2: Tensile strength of foams prepared from peptone obtained by
enzymatic
or acid hydrolysis of selected full-length proteins.
In order to study the effect of different hydrolysis mechanisms by which
peptones were obtained from full-length proteins on the compression force, the
force
required for compression of foams prepared from an aqueous solution of
peptones
obtained by enzymatic or acid hydrolysis of casein was measured. For further
comparison, the force required for compression of foams obtained from full-
length
gelatin, BSA and casein were also measured.
A 5% w/v aqueous solution of each of full-length gelatin, BSA and casein;
peptone obtained by enzymatic or acidic hydrolysis of casein; and peptone
obtained
by enzymatic hydrolysis of gelatin was prepared. Foam was prepared in two 50
mL
syringes. The first syringe was loaded with 20 mL of a 5% protein solution and
2 mL
BAC2. In the second syringe 40 mL air were loaded. Following foaming by
vigorous
admixing of the air into the liquid the prepared material was expelled into a
cup with a
diameter of 60 mm, at a height of 20 mm.
The force required for compression was evaluated at 0.5 mm/sec for a depth of
4 mm. Foams prepared from full-length gelatin and peptones were tested in
triplicate,
foams prepared from full-length BSA and casein were tested in duplicate.
Results are presented in Figure 2.
As shown in Figure 2, the force required for compression of the foam prepared
from peptone obtained by enzymatic hydrolysis of casein or gelatin was
significantly
higher than that of foam prepared from the respective full-length proteins,
indicating a
reverse correlation between the chain length and the compression force
required. In
contrast, foam prepared from peptone obtained by acid hydrolysis of casein was
found
to be less stable upon compression than foam prepared from full-length casein.
It was
further noted that very similar results were seen with foams prepared from
peptones
obtained from gelatin and from casein.
CA 03036770 2019-03-13
WO 2018/051325 1
PCT/IL2017/000007
2
Example 3: Effect of peptone concentration on tensile strength of foam.
A 50% w/v aqueous solution of peptone obtained by enzymatic hydrolysis of
full-length gelatin was prepared by dissolving 50 g peptone powder in 100 mL
water.
The solution was diluted with water to obtain 1%, 5%, 10%, and 25% aqueous
solutions of peptone.
5 mL of each solution was foamed as described in Example 1.
At the final step of the preparation, 200 i.tL of a 100 IU/mL Thrombin
solution
in 40 mM CaCl2 were added to the foam, and the final foam prepared and the
force
required for compression tested in quadruplicate, substantially as described
in
Example 1 above, except that pressing to a depth of 4 mm (instead of 12 mm) at
5
mm/sec was performed. Results are presented in Figure 3.
The results show that for foam comprising a concentration of between 1 to
25% peptone w/v in water, the force required for compression was directly
proportional to the peptone concentration. Peptone concentrations of equal to
or
greater than 50% w/v resulted in reduced foam quality as reflected in the
lower force
required for compression. .
Example 4: Effect of BAC2 concentration on tensile strength of foam.
A 5% aqueous solution of peptone obtained by enzymatic hydrolysis of gelatin
was prepared.
Four samples, each comprising 5 mL peptone solution were prepared. Each
sample was foamed as described in Example 1.
At the final step of the preparation, BAC2, at concentration of 1%, 5%, 10%
or 30% w/v was added, wherein each percent of BAC2 comprised about 7 mg
fibrinogen. The, final foam was prepared and tested in quadruplicates as
described in
Example 3. Results are presented in Figure 4.
The results show that for foam comprising a concentration of between 1 to
30% BAC2, the force required for compression was directly proportional to the
peptone concentration.
CA 03036770 2019-03-13
WO 2018/051325 22
PCT/IL2017/000007
Example 5: Tensile strength of foams prepared from peptone and from gelatin in
the presence and absence of fibrinogen.
In order to test for the requirement of a protein cross-linker, the tensile
strength of foams prepared from 5% w/v aqueous solution of each of full-length
gelatin, and peptone obtained by enzymatic hydrolysis of full-length gelatin,
in the
presence and absence of fibrinogen (provided by BAC2), were measured. Results
are
presented in Figure 5.
Foams were prepared substantially as described in Example 1, except that
foams comprising about 35 mg fibrinogen and 20 IU thrombin, as well as foams
devoid of BAC2 were prepared. Triplicates of the samples were tested.
As shown in Figure 5, the increased force required for compression of foams
prepared from peptone obtained by enzymatic hydrolysis of full-length gelatin
as
compared to those prepared from full-length gelatin was observed in both the
presence and absence of fibrinogen provided by BAC2.
Use of an alternative cross-linker, 4-armed PEG was also tested. However,
foams cross-linked with the 4-aimed PEG showed breakdown of the foam, which -
could therefore not be evaluated.
Example 6: Tissue adhesion.
Aqueous solutions were prepared as follows:
5% w/v full-length albumin + 30 mg/mL concentrated BAC2 + 2 IU/mL
EVICEL Thrombin (1:3 ratio of liquid:air);
5% w/v peptone obtained by enzymatic hydrolysis of gelatin + 30 mg/mL
concentrated BAC2 + 3 IU/mL EVICEL Thrombin (1:3 ratio of liquid:air); and
5% w/v peptone obtained by enzymatic hydrolysis of casein + 30 mg/mL
concentrated BAC2 + 10 IU/mL EVICEL Thrombin (1:3 ratio of liquid:air).
Foams were prepared from 5 mL of each solution, substantially as described
above for Example 1, except that the amount of BAC2 added to each foam was
identical, and the amount of thrombin was adjusted to achieve a comparable
fibrinogen polymerization rate.
CA 03036770 2019-03-13
WO 2018/051325 23
PCT/IL2017/000007
Five replicates were tested for each formulation. The liquid: air ratio for
each
foam preparation was 1: 3, providing a homogeneous foam, without large air
pockets
or bubbles.
Foams were tested for adhesion to tissue using ASTM F2258 (Standard Test
Method for Strength Properties of Tissue Adhesives in Tension). Freshly
harvested
porcine pleura, as a tissue substrate, was mounted on 1 inch x 1 inch plates
secured to
the load cell and bottom grip of an INSTRON (Tensile Tester model 5565 with
10N
Load Cell) device for tensile strength measurement. The crosshead and load
cell were
lowered to ensure alignment of the two tissue surfaces. A 3 mm gap between the
tissue surfaces was maintained for each sample.
Before expressing the foam, the crosshead was moved away from the bottom.
Each formulation was prepared immediately before testing and approximately 3
mL
of formulation was expelled on the tissue surface for each sample. Excess
material
was wiped away from the perimeter of the fixture immediately after the top
plate was
.. returned to the initial gap height. A 15-minute time period was allowed for
complete
polymerization of the foam before testing. The cross head moved in a vertical
direction at 5 mm/min until the test was stopped. The load-extension output
for each
sample was recorded by the INSTRON control software. The peak adhesive force,
stiffness and failure mode was recorded for each sample. Tissue adhesion
results are
presented in Figure 6. Stiffness (material strength) results are presented in
Figure 7.
As seen in Figures 6 and 7, tissue adhesion was greater with foams prepared
from gelatin peptone or casein peptone as compared to intact albumin. Foams
prepared from casein peptone had the highest maximum adhesion and stiffness.
Mean
maximum adhesion scores were as follows: intact albumin 0.97 N; gelatin
peptone
.. 1.19 N; and casein peptone 1.58 N.
For all formulations, the failure mode was adhesive, i.e. failure occurred at
the
tissue: foam interface, and not cohesive i.e. failure did not occur within the
test article.
Example 7: Scanning Electron Microscope (SEM) studies.
Foams were prepared from aqueous solutions of 5% w/v full-length gelatin
.. and 5% w/v peptone obtained by enzymatic hydrolysis of full-length gelatin,
with the
addition of BAC2 and thrombin, as described above for Example 1.
CA 03036770 2019-03-13
WO 2018/051325 24
PCT/IL2017/000007
Figures 8A and 8B show electron micrographs for foams prepared from full-
length gelatin (8A) and from foam prepared from peptone obtained by enzymatic
hydrolysis of gelatin (8B).
As seen in Figures 8A and 8B, foams prepared from peptone had higher
density and smaller air pockets than foam prepared from full-length protein.
It is
expected that the foam prepared from gelatin would be less stable due to the
large
bubble structure, while the foam obtained from the peptone would be more
stable and
more rigid. It is hypothesized that the differences may be due to the greater
hydrophobicity of the full-length protein.
Example 8: Effect of peptide size on tensile strength.
In order to investigate the effect of peptide size on tensile strength,
aqueous
solutions of 5% w/v full-length gelatin and 5% w/v of peptone obtained by
enzymatic
hydrolysis of full-length gelatin were prepared.
10 mL, of the solution comprising the peptide was subjected to centrifugation
through a Amicon Ultra centrifugal filters, Ultra-15 with a 10 kDA cut-off
centrifugation filter in a centrifugal filtration device (Sigma, Z706345). The
device
was subjected to 3500 G centrifugal force for 10 minutes at room temperature,
ensuring that the filtered solution included only peptides with a length of
less than 10
kDa.
Foams were prepared from 5 mL of each of the full-length gelatin solution,
and of the solutions comprising peptone, with and without filtration
centrifugation, as
described in Example 1. Force required for compression of the foams was tested
in
quadruplicates as described in Example 1. Results are presented in Figure 9.
As shown in Figure 9, foam prepared from a solution of peptone comprising
only peptides of length less than 10 kDa required greater force for
compression.
Admixing the full length protein with enzymatically hydrolyzed peptides
decreased
the required compression force. Interestingly the mixtures resulted in lower
compression force as compared to the homogenous solutions of either the full
length
or the enzymatically hydrolyzed solutions.
CA 03036770 2019-03-13
WO 2018/051325 25
PCT/IL2017/000007
Example 9: Effect on tensile strength of mixing peptones with full-length
protein.
5% aqueous solutions of full-length gelatin and of peptone obtained by
enzymatic hydrolysis of full-length gelatin were prepared.
Samples comprising a mixture of full-length gelatin and peptone, in ratios of
gelatin: peptone 40:60 and 95:5 were also prepared.
Foams were prepared from 5 mL of each of full-length gelatin, peptone alone,
and gelatin: peptone mixtures at each of the two ratios, as described in
Example 1.
Force required for compression of each foam was tested in quadruplicates as
described in Example 1. Results are presented in Figure 10.
As seen in Figure 10, foams prepared from solutions comprising an admixture
of full-length gelatin with peptone obtained by enzymatic hydrolysis of full-
length
gelatin required less force for compression than foams comprising either full-
length
gelatin or peptone alone.
It is appreciated that certain features of the invention, which are, for
clarity,
described in the context of separate embodiments, may also be provided in
combination in a single embodiment. Conversely; various features of the
invention,
which are, for brevity, described in the context of a single embodiment, may
also be
provided separately or in any suitable subcombination or as suitable in any
other
described embodiment of the invention. Certain features described in the
context of
various embodiments are not to be considered essential features of those
embodiments, unless the embodiment is inoperative without those elements.
Although the invention has been described in conjunction with specific
embodiments thereof, it is evident that many alternatives, modifications and
variations
will be apparent to those skilled in the art. Accordingly, it is intended to
embrace all
such alternatives, modifications and variations that fall within the scope of
the
appended claims.
Citation or identification of any reference in this application shall not be
construed as an admission that such reference is available as prior art to the
invention.