Note: Descriptions are shown in the official language in which they were submitted.
CA 03037631 2019-03-14
WO 2018/064385
PCT/US2017/054108
HIGH RESOLUTION SYSTEMS, KITS, APPARATUS, AND METHODS FOR
BACTERIAL COMMUNITY RELATIONSHIP DETERMINATION AND OTHER
HIGH THROUGHPUT MICROBIOLOGY APPLICATIONS
CROSS-REFERENCE TO RELATED APPLICATIONS
This application is a continuation-in-part of U.S. Nonprovisional Patent
Application No.
15/135,377, filed on April 21, 2016, and also claims priority to U.S.
Provisional Patent
Application No. 62/400,841 filed on September 28, 2016, the disclosure of each
of which is
incorporated by reference in its entirety.
INCORPORATION OF SEQUENCE LISTING
This application includes a Sequence Listing which is being submitted in ASCII
format
via EFS-Web, named "GALT 007 PCT 5T25.txt," which is 3 KB in size and created
on
September 27, 2017. The contents of the Sequence Listing are incorporated
herein by reference
in their entirety.
TECHNICAL FIELD
The present disclosure relates generally to innovations in microbiology,
microfabrication,
chemistry, optics, robotics, and information technology. More specifically,
the present
disclosure relates to systems, apparatus, kits, and methods for high
throughput cultivation,
screening, isolation, sampling, and/or identification of biological entities
and/or nutrients.
BACKGROUND
Traditional techniques and tools for cultivating biological entities from
environmental
and other samples are often slow, laborious, and expensive. Even with these
techniques and
tools, often cells and other biological entities still defy all attempts at
culture, resulting in missed
information and/or product opportunities. Likewise, the screening of a
population of biological
entities for a particular metabolite, enzyme, protein, nucleic acid,
phenotype, mutation, metabolic
pathway, gene, adaptation, capability, and/or therapeutic benefit is
challenging, requiring
complex and expensive methods. For example, microbes live in extremely high-
risk
1
CA 03037631 2019-03-14
WO 2018/064385
PCT/US2017/054108
environments. To survive, microbes have developed amazing sets of biochemical
tools,
including novel enzymes, unique metabolites, innovative genetic pathways, and
strategies for
manipulating their environment and their microbial neighbors ¨ powerful
solutions that could
lead to new insights and products ranging from life-saving antibiotics to
fertilizers that improve
food production and security.
SUMMARY
The present disclosure provides microbiology systems, apparatus, kits, and
methods for
streamlining the cultivation workflow, supporting high throughput screening,
and/or developing
new insights and products in accordance with some embodiments.
In some embodiments, a method of analyzing a sample including a population of
biological entities is provided. The methods employs at least one
microfabricated device having
a top surface defining (or comprising) an array of microwells, wherein a
plurality of the
microwells of the array of microwells each comprise a unique tag nucleic acid
molecule
including: (1) a target-specific nucleotide sequence for annealing to a target
nucleic acid
fragment of one or more biological entities of interest that may be present in
the microwell, and
(2) a location-specific nucleotide sequence for identifying the location of
the microwell on the
microfabricated device. The method comprises: loading a sample onto the
microfabricated
device such that at least some microwells of the plurality of microwells each
include more than
one cell of a biological entity as well as an amount of a nutrient; incubating
the microfabricated
device at predetermined conditions; amplifying, in individual microwells, a
selected genetic
material of the cells (e.g., genomic DNA of the cells) of the biological
entities obtained from the
incubation in the plurality of microwells, thereby obtaining first amplicons
in at least a subset of
the plurality of microwells; sequencing an aggregate of the first amplicons
collected from the
subset the plurality of microwells to obtain sequencing data; and based on the
sequencing data
and the unique tag nucleic molecule included in each of the plurality of
microwells, obtaining an
identification of the biological entities present in each of the subset of the
plurality of microwells
of the at least one microfabricated device.
In some embodiments of the method, based on the identification of the
biological entities
present in each of the subset of the plurality of microwells, a presence or
absence of a
2
CA 03037631 2019-03-14
WO 2018/064385
PCT/US2017/054108
relationship between at least two different types of biological entities in
the population of
biological entities contained in the sample is determined. The relationship
can be one of a
dependent relationship, a symbiotic relationship, or a destructive
relationship. In some
embodiments, the presence or absence of a relationship can be determined by
comparing across
the plurality of microwells which types of biological entities are present or
not present in the
same microwells after incubation.
In some embodiments of the method, loading the sample onto the microfabricated
device
can be performed such that each microwell of the plurality of microwells
includes, on average, N
cells of any biological entity (the N cells can be same type or different
types), where N is a
number and equal to 2 or greater. For example, N can be a number between 2 and
100, between
2 and 50, between 2 and 20, between 2 and 10, etc.
In some embodiments of the method, the biological entities in the sample
comprise
bacteria. The bacteria can include bacteria of different strains, different
species, or different
genera. The population of the of the population of the biological entities in
the sample can
include a collection of microorganisms naturally occurring in a specific
environment. For
example, the biological entities can be a collection of microorganisms
obtained from human
stool, human gut, human skin, human nasal cavity, vagina, soil, rhizosphere,
water, etc. In other
embodiments, the biological entities in the sample can include viruses, fungi,
or eukaryotic cells.
In some embodiments of the method, prior to incubation, a membrane can be
applied on
the top surface of the microfabricated device to retain the biological
entities loaded in the
plurality of microwells. The nutrient can be pre-loaded in the microwells and
retained by the
membrane. The nutrient can include a plurality of different nutrients loaded
across the
microwells of the at least one microfabricated device. In such a case,
determining the
determining the presence or absence of a relationship between at least two
types of biological
entities can include determining such a relationship dependent on the
different nutrients. In
some embodiments, the nutrient can be included in a reservoir provided on the
membrane and
external to the microwells. In such a case, the membrane can be permeable to
the nutrient and
allows the nutrient to migrate from the reservoir into the microwells through
the membrane.
In some embodiments, the method further comprises transferring at least some
of the
cells of the biological entities obtained from the incubation to a target
location. In such a case,
the amplifying and sequencing can be performed on either the cells not
transferred to the target
3
CA 03037631 2019-03-14
WO 2018/064385
PCT/US2017/054108
location or the cells transferred to the target location.
In some embodiments, the unique tag nucleic acid molecule in each of the
plurality of
microwells constitutes a portion of a PCR primer used in the amplification.
In some embodiments, the surface density of the arrays of microwells of the at
least one
microfabricated device can be at least 150 microwells per cm2, at least 250
microwells per cm2,
at least 400 microwells per cm2, at least 500 microwells per cm2, at least 750
microwells per cm2,
at least 1,000 microwells per cm2, at least 2,500 microwells per cm2, at least
5,000 microwells
per cm2, at least 7,500 microwells per cm2, at least 10,000 microwells per
cm2, at least 50,000
microwells per cm2, at least 100,000 microwells per cm2, or at least 160,000
per cm2. In some
embodiments, each microwell of the arrays of microwells of the at least one
microfabricated
device can have a diameter of from about 51.tm to about 500 jim, from about
101.tm to about 300
or from about 201.tm to about 200
In some embodiments, a method of analyzing a sample including a population of
biological entities using at least one microfabricated device having a top
surface defining an
array of microwells is provided. The method comprises: loading into each of a
plurality of the
microwells of the array of microwells with a unique tag nucleic acid molecule
including: (1) a
target-specific nucleotide sequence for annealing to a target nucleic acid
fragment of one or more
biological entities of interest that may be present in the microwell, and (2)
a location-specific
nucleotide sequence for identifying the location of the microwell on the at
least microfabricated
device; loading a sample onto the microfabricated device such that at least
some microwells of
the plurality of microwells each includes more than one cell of a biological
entity as well as an
amount of a nutrient; incubating the microfabricated device at predetermined
conditions;
amplifying a selected genetic material of the cells of the biological entities
obtained from the
incubation in at least a subset of the plurality of microwells of the at least
one microfabricated
device, thereby obtaining first amplicons in the plurality of microwells;
sequencing an aggregate
of the first amplicons collected from the subset the plurality of microwells
to obtain sequencing
data; and based on the sequencing data and the unique tag nucleic molecule
included in each of
the plurality of microwells, obtaining an identification of the biological
entities present in each of
the subset of the plurality of microwells of the at least one microfabricated
device.
In some embodiments, a method of analyzing a microbiome of microorganisms
collected
from a specific environment is provided. The method employs at least one
microfabricated
4
CA 03037631 2019-03-14
WO 2018/064385
PCT/US2017/054108
device having a top surface defining an array of microwells, wherein a
plurality of the
microwells of the array of microwells each comprise a unique tag nucleic acid
molecule
including: (1) a target-specific nucleotide sequence for annealing to a target
nucleic acid
fragment of one or more biological entities of interest that may be present in
the microwell, and
(2) a location-specific nucleotide sequence for identifying the location of
the microwell on the
microfabricated device. The method includes: loading a sample prepared from
the microbiome
onto the at least one microfabricated device such that the plurality of
microwells each include, on
average, 2 to 20 cells of any microorganism of the microbiome as well as an
amount of a
nutrient; incubating the microfabricated device at predetermined conditions;
amplifying a
selected genetic material of the microorganisms obtained from the incubation
in at least a subset
of the plurality of microwells in the at least one microfabricated device,
thereby obtaining first
amplicons in the plurality of microwells; sequencing an aggregate of first
amplicons collected
from the subset of the plurality of microwells to obtain sequencing data; and
based on the
sequencing data and the unique tag nucleic molecule included in each of the
plurality of
microwells, obtaining an identification of microorganisms present in each of
the subset of the
plurality of microwells of the at least one microfabricated device; and based
on the identification
of the microorganisms present in the plurality of microwells, determining a
presence or absence
of a relationship between at least two different types of microorganisms in
the microbiome.
In some embodiments, a method of analyzing a relationship among microorganisms
of a
microbiome collected from a specific environment is provided. The method
comprises:
partitioning microorganisms of the microbiome into a plurality of portions
each including, on
average, 2 to 20 cells of any microorganism of the microbiome; incubating each
portion of the
plurality of portions of microorganisms in a separate compartment at
predetermined conditions in
the presence of a nutrient; identifying the microorganisms present in each of
at least a subset of
the isolated compartments after incubation; and determining a presence or
absence of a
relationship between at least two different types of the microorganisms in the
microbiome based
on a comparison across the subset of compartments which types of
microorganisms as identified
are present or not present in the same microwells.
It should be appreciated that all combinations of the foregoing concepts and
additional
concepts discussed in greater detail below (provided such concepts are not
mutually inconsistent)
are contemplated as being part of the inventive subject matter disclosed
herein. In particular, all
5
CA 03037631 2019-03-14
WO 2018/064385
PCT/US2017/054108
combinations of claimed subject matter appearing at the end of this disclosure
are contemplated
as being part of the inventive subject matter disclosed herein. It should also
be appreciated that
terminology explicitly employed herein that also may appear in any disclosure
incorporated by
reference should be accorded a meaning most consistent with the particular
concepts disclosed
herein.
Other systems, processes, and features will become apparent to those skilled
in the art
upon examination of the following drawings and detailed description. It is
intended that all such
additional systems, processes, and features be included within this
description, be within the
scope of the present invention, and be protected by the accompanying claims.
BRIEF DESCRIPTION OF THE DRAWINGS
The skilled artisan will understand that the drawings primarily are for
illustrative
purposes and are not intended to limit the scope of the inventive subject
matter described herein.
The drawings are not necessarily to scale; in some instances, various aspects
of the inventive
subject matter disclosed herein may be shown exaggerated or enlarged in the
drawings to
facilitate an understanding of different features. In the drawings, like
reference characters
generally refer to like features (e.g., functionally similar and/or
structurally similar elements).
FIG. 1 is a perspective view illustrating a microfabricated device or chip in
accordance
with some embodiments.
FIGS. 2A-2C are top, side, and end views, respectively, illustrating
dimensions of
microfabricated device or chip in accordance with some embodiments.
FIGS. 3A and 3B are exploded and top views, respectively, illustrating a
microfabricated
device or chip in accordance with some embodiments.
FIGS. 4A and 4B are diagrams illustrating a membrane in accordance with some
embodiments. FIG. 4C is an image of a membrane surface with impressions formed
from
contact with an array of wells in accordance with some embodiments.
FIG. 5A is a flowchart illustrating a method for isolating cells from a sample
in
accordance with some embodiments. FIG. 5B is a diagram illustrating a method
for isolating
6
CA 03037631 2019-03-14
WO 2018/064385
PCT/US2017/054108
cells from a soil sample in accordance with some embodiments.
FIG. 6 is a flowchart illustrating a method for isolating and cultivating
cells from a
sample in accordance with some embodiments.
FIG. 7 is a diagram illustrating a method for isolating and cultivating cells
from a
complex sample in accordance with some embodiments. Panel 716 shows the
output: isolated
strains of cultivated cells (SEQ ID NOs: 2-6).
FIGS. 8A-8C are diagrams illustrating picking by one pin or multiple pins in
accordance
with some embodiments.
FIGS. 9A-9D are images demonstrating picking of a well in accordance with some
embodiments.
FIGS. 10A-10D are diagrams illustrating a tool for picking a chip in
accordance with
some embodiments.
FIG. 11 is an image of a well that has been picked through a thin layer of
agar,
illustrating picking through a membrane or sealing layer in accordance with
some embodiments.
FIG. 12 is a diagram illustrating a cross-section of a chip 1200 in accordance
with some
embodiments.
FIG. 13 is a flowchart illustrating methods for screening in accordance with
some
embodiments.
FIG. 14 is a diagram illustrating a screening method in accordance with some
embodiments.
FIG. 15 is a series of images illustrating a screening example in accordance
with some
embodiments.
FIGS. 16A-16C are images illustrating recovery from a screen in accordance
with some
embodiments.
7
CA 03037631 2019-03-14
WO 2018/064385
PCT/US2017/054108
FIG. 17A is an exploded diagram illustrating a chip for screening in
accordance with
some embodiments. FIG. 17B is a fluorescence image of a chip following
screening in
accordance with some embodiments. FIG. 17C is an image showing a process of
picking a
sample from the chip following screening in accordance with some embodiments.
FIG. 18 is a flowchart illustrating a counting method in accordance with some
embodiments.
FIG. 19 is a diagram illustrating a counting method in accordance with some
embodiments. Panel 1916 shows the output: sequences and relative abundance of
cultivated cells
(SEQ ID NOs: 2-6).
FIG. 20 is a diagram illustrating an indexing system in accordance with some
embodiments.
FIGS. 21A-21E are diagrams illustrating a chip with well-specific chemistries
in
accordance with some embodiments.
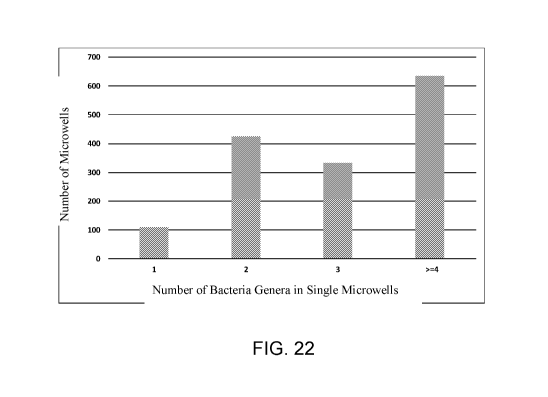
FIG. 22 is a diagram showing a summary of the number of genera of bacteria in
the
microwells of a microfabricated device in accordance with some embodiments.
DETAILED DESCRIPTION
The present disclosure relates generally to systems, kits, apparatus, and
methods for
isolation, culturing, adaptation, sampling, and/or screening of biological
entities and/or nutrients.
A microfabricated device (or a "chip") is disclosed for receiving a sample
comprising at least
one biological entity (e.g., at least one cell). The term "biological entity"
may include, but is not
limited to, an organism, a cell, a cell component, a cell product, and a
virus, and the term
"species" may be used to describe a unit of classification, including, but not
limited to, an
operational taxonomic unit (OTU), a genotype, a phylotype, a phenotype, an
ecotype, a history, a
behavior or interaction, a product, a variant, and an evolutionarily
significant unit.
A cell may be Archaea, Bacteria, or Eukaryota (e.g., fungi), mammalian cells,
etc. For
example, a cell may be a microorganism, such as an aerobic, anaerobic, or
facultative aerobic
microorganisms. A virus may be a bacteriophage. Other cell components/products
may include,
8
CA 03037631 2019-03-14
WO 2018/064385
PCT/US2017/054108
but are not limited to, proteins, amino acids, enzymes, saccharides, adenosine
triphosphate
(ATP), lipids, nucleic acids (e.g., DNA and RNA), nucleosides, nucleotides,
cell
membranes/walls, flagella, fimbriae, organelles, metabolites, vitamins,
hormones,
neurotransmitters, and antibodies.
A nutrient may be defined (e.g., a chemically defined or synthetic medium) or
undefined
(e.g., a basal or complex medium). A nutrient may include or be a component of
a laboratory-
formulated and/or a commercially manufactured medium (e.g., a mix of two or
more chemicals).
A nutrient may include or be a component of a liquid nutrient medium (i.e., a
nutrient broth),
such as a marine broth, a lysogeny broth (e.g., Luria broth), etc. A nutrient
may include or be a
component of a liquid medium mixed with agar to form a solid medium and/or a
commercially
available manufactured agar plate, such as blood agar.
A nutrient may include or be a component of selective media. For example,
selective
media may be used for the growth of only certain biological entities or only
biological entities
with certain properties (e.g., antibiotic resistance or synthesis of a certain
metabolite). A nutrient
may include or be a component of differential media to distinguish one type of
biological entity
from another type of biological entity or other types of biological entities
by using biochemical
characteristics in the presence of specific indicator (e.g., neutral red,
phenol red, eosin y, or
methylene blue).
A nutrient may include or be a component of an extract of or media derived
from a
.. natural environment. For example, a nutrient may be derived from an
environment natural to a
particular type of biological entity, a different environment, or a plurality
of environments. The
environment may include, but is not limited to, one or more of a biological
tissue (e.g.,
connective, muscle, nervous, epithelial, plant epidermis, vascular, ground,
etc.), a biological fluid
or other biological product (e.g., amniotic fluid, bile, blood, cerebrospinal
fluid, cerumen,
exudate, fecal matter, gastric fluid, interstitial fluid, intracellular fluid,
lymphatic fluid, milk,
mucus, rumen content, saliva, sebum, semen, sweat, urine, vaginal secretion,
vomit, etc.), a
microbial suspension, air (including, e.g., different gas contents),
supercritical carbon dioxide,
soil (including, e.g., minerals, organic matter, gases, liquids, organisms,
etc.), sediment (e.g.,
agricultural, marine, etc.), living organic matter (e.g., plants, insects,
other small organisms and
9
CA 03037631 2019-03-14
WO 2018/064385
PCT/US2017/054108
microorganisms), dead organic matter, forage (e.g., grasses, legumes, silage,
crop residue, etc.), a
mineral, oil or oil products (e.g., animal, vegetable, petrochemical), water
(e.g., naturally-
sourced freshwater, drinking water, seawater, etc.), and/or sewage (e.g.,
sanitary, commercial,
industrial, and/or agricultural wastewater and surface runoff).
A microfabricated device may define a high density array of microwells for
cultivating
the at least one biological entity. The term "high density" may refer to a
capability of a system
or method to distribute a number of experiments within a constant area. For
example, a
microfabricated device comprising a "high density" of experimental units may
include about 150
microwells per cm2 to about 160,000 microwells or more per cm2, as discussed
further herein.
Additional examples are shown in TABLE 1.
TABLE 1
Enm6iigtworside Spacing Density f
(pm) microwells (wells!cm2)
onormicrowellsommombetwetnzmomm=microwell$mom
LeiagMgMagagMaqiniMinitiOMMagjeiniMgagagagaga
500 500 100
100 100 2500
100 50 4489
100 10 8281
50 50 10000
50 10 27556
10 110889
10 5 444889
5 5 1000000
A microfabricated device may include a substrate with a series of functional
layers. The
series of functional layers may include a first functional layer defining a
first array of
15 experimental units (e.g., wells) and at least one subsequent functional
layer defining a
CA 03037631 2019-03-14
WO 2018/064385
PCT/US2017/054108
subsequent array of experimental units (e.g., microwells) in each experimental
unit of the
preceding functional layer. Each of the experimental units may be configured
to receive and
cultivate and/or screen biological entities and/or nutrients. In particular,
systems, kits, apparatus,
and methods described herein may be used for automated and/or high throughput
screening of
different conditions against a high density matrix of cells. For example,
systems, kits, apparatus,
and methods described herein may be used to test and compare the effect(s) of
one or more
different nutrients on the growth of microorganisms and/or screen for
metabolites, enzyme
activity, mutations, or other cell features.
FIG. 1 is a perspective view illustrating a microfabricated device or chip in
accordance
with some embodiments. Chip 100 includes a substrate shaped in a microscope
slide format with
injection-molded features on top surface 102. The features include four
separate microwell
arrays (or microarrays) 104 as well as ejector marks 106. The microwells in
each microarray are
arranged in a grid pattern with well-free margins around the edges of chip 100
and between
microarrays 104.
FIGS. 2A-2C are top, side, and end views, respectively, illustrating
dimensions of chip
100 in accordance with some embodiments. In FIG. 2A, the top of chip 100 is
approximately
25.5 mm by 75.5 mm. In FIG. 2B, the end of chip 100 is approximately 25.5 mm
by 0.8 mm. In
FIG. 2C, the side of chip 100 is approximately 75.5 mm by 0.8 mm.
After a sample is loaded on a microfabricated device, a membrane may be
applied to at
.. least a portion of a microfabricated device. FIG. 3A is an exploded diagram
of the
microfabricated device 300 shown from a top view in FIG. 3B in accordance with
some
embodiments. Device 300 includes a chip with an array of wells 302 holding,
for example, soil
microbes. A membrane 304 is placed on top of the array of wells 302. A gasket
306 is placed
on top of the membrane 304. A polycarbonate cover 308 with fill holes 310 is
placed on top of
the gasket 306. Finally, sealing tape 312 is applied to the cover 308.
A membrane may cover at least a portion of a microfabricated device including
one or
more experimental units, wells, or microwells. For example, after a sample is
loaded on a
microfabricated device, at least one membrane may be applied to at least one
microwell of a high
density array of microwells. A plurality of membranes may be applied to a
plurality of portions
11
CA 03037631 2019-03-14
WO 2018/064385
PCT/US2017/054108
of a microfabricated device. For example, separate membranes may be applied to
separate
subsections of a high density array of microwells.
A membrane may be connected, attached, partially attached, affixed, sealed,
and/or
partially sealed to a microfabricated device to retain at least one biological
entity in the at least
one microwell of the high density array of microwells. For example, a membrane
may be
reversibly affixed to a microfabricated device using lamination. A membrane
may be punctured,
peeled back, detached, partially detached, removed, and/or partially removed
to access at least
one biological entity in the at least one microwell of the high density array
of microwells.
A portion of the population of cells in at least one experimental unit, well,
or microwell
may attach to a membrane (via, e.g., adsorption). If so, the population of
cells in at least one
experimental unit, well, or microwell may be sampled by peeling back the
membrane such that
the portion of the population of cells in the at least one experimental unit,
well, or microwell
remains attached to the membrane.
FIGS. 4A and 4B are diagrams illustrating a membrane in accordance with some
embodiments. FIG. 4A shows a side view of a chip 400 defining an array of
wells filled with
content and a membrane 402 sealed on chip 400 over the array of wells, such
that the surface of
membrane 402 that was in contact with chip 400, when peeled off chip 400, has
impressions of
each of the wells with samples of the well contents attached (e.g., stuck)
thereto, as shown in
FIG. 4B. FIG. 4C is an image of a membrane surface with impressions formed
from contact
with an array of wells in accordance with some embodiments.
A membrane may be impermeable, semi-permeable, selectively permeable,
differentially
permeable, and/or partially permeable to allow diffusion of at least one
nutrient into the at least
one microwell of a high density array of microwells. For example, a membrane
may include a
natural material and/or a synthetic material. A membrane may include a
hydrogel layer and/or
filter paper. In some embodiments, a membrane is selected with a pore size
small enough to
retain at least some or all of the cells in a microwell. For mammalian cells,
the pore size may be
a few microns and still retain the cells. However, in some embodiments, the
pore size may be
less than or equal to about 0.2 um, such as 0.1 um. Membrane diameters and
pore sizes depend
on the material. For example, a hydrophilic polycarbonate membrane may be
utilized, for which
12
CA 03037631 2019-03-14
WO 2018/064385
PCT/US2017/054108
the diameter may range from about 10 mm to about 3000 mm, and the pore size
may range from
about 0.01 p.m to about 30.0 p.m. An impermeable membrane has a pore size
approaching zero.
In embodiments with an impermeable membrane, any nutrients must be provided in
a microwell
prior to being sealed with the membrane. A membrane that is gas permeable but
not liquid
permeable may allow oxygen into a microwell and carbon dioxide out of the
microwell. The
membrane may have a complex structure that may or may not have defined pore
sizes.
However, the pores may be on a nanometer scale. Other factors in selecting a
membrane may
include cost, ability to seal, and/or ability to sterilize.
A substrate may define an array of microchannels extended from a first surface
to a
second surface opposite the first surface. A microchannel may have a first
opening in the first
surface and a second opening in the second surface. A first membrane may be
applied to at least
a portion of the first surface such that at least some of the population of
cells in at least one
microchannel attach to the first membrane. A second detachable membrane may be
applied to at
least a portion of the second surface such that at least some of the
population of cells in at least
one microchannel attach to the second membrane. The population of cells in the
at least one
microchannel is sampled by peeling back the first membrane such that the at
least some of the
population of cells in the at least one microchannel remain attached to the
first membrane and/or
the second membrane such that the at least some of the population of cells in
the at least one
microchannel remain attached to the second membrane.
The term "high throughput" may refer to a capability of a system or method to
enable
quick performance of a very large number of experiments in parallel or in
series. An example of
a "high throughput" system may include automation equipment with cell biology
techniques to
prepare, incubate, and/or conduct a large number of chemical, genetic,
pharmacological, optical,
and/or imaging analyses to screen one or more biological entities for at least
one of a metabolite,
an enzyme, a protein, a nucleic acid, a phenotype, a mutation, a metabolic
pathway, a gene, an
adaptation, and a capability, as discussed herein. According to some
embodiments, "high
throughput" may refer to simultaneous or near simultaneous experiments on a
scale ranging from
at least about 96 experiments to at least about 10,000,000 experiments.
Systems, kits, apparatus, and methods disclosed herein may be used for high
throughput
13
CA 03037631 2019-03-14
WO 2018/064385
PCT/US2017/054108
screening of different conditions against a matrix of biological entities
(e.g., cells). A "wells-
within-wells" concept may be implemented by manufacturing (e.g.,
microfabricating) a substrate
or chip to have multiple levels of functional layers to whatever level is
required or desired (i.e.,
wells within wells within wells within wells, etc.). A first functional layer
may define an array
of experimental units (e.g., wells). Each of the experimental units presents a
second functional
layer by defining a subsequent array of experimental units (e.g., microwells).
This enables
multiple experiments or tests to be performed at the same time on a single
chip, thus enabling
high throughput operation.
For example, in FIGS. 3A and 3B, gasket 306 is placed on top of membrane 304,
which
is applied to an array of wells 302 on a microfabricated device 300 in
accordance with some
embodiments. Gasket 306 has only one opening. However, in further embodiments,
multiple
smaller gaskets with a smaller opening or a single gasket with more than one
smaller opening
may be placed on top of a device (either with or without a membrane), thereby
forming a
functional layer or an array of larger experimental units with a subsequent
functional layer or
subsequent array of experimental units (e.g., wells 302) located therein.
With multiple levels of functional layers, more than one nutrient or nutrient
formulation,
for example, can be tested simultaneously or near simultaneously. The same
format may be
used, for example, to screen for metabolites or specific capabilities of cells
or to wean
microorganisms from environmentally derived nutrients to other nutrients.
Experimental units are predetermined sites on a surface of a microfabricated
device. For
example, a surface of a chip may be designed to immobilize cells in a first
array of
predetermined sites. These predetermined sites may be wells, microwells,
microchannels, and/or
designated immobilization sites. For example, a surface may be manufactured to
define an array
of microwells. The array may be divided into sections by defining walls in the
substrate or
.. adding walls. For example, the surface may be manufactured to first define
a first array of wells,
in which an inner surface of each well, in turn, is manufactured to define a
second array of
microwells, microchannels, or immobilization sites. In another example, the
surface may be
manufactured to define an array of microwells, and another substrate (e.g.,
agar, plastic, or
another material) is applied to the surface to partition the surface and the
microwells defined
14
CA 03037631 2019-03-14
WO 2018/064385
PCT/US2017/054108
thereby. Each well, microwell, microchannel, and/or immobilization site may be
configured to
receive and grow at least one cell; however, in use, any given well,
microwell, microchannel, or
immobilization site may or may not actually receive and/or grow one or more
cells. Types of
experimental units may be interchangeable. For example, embodiments herein
that expressly
describe microwells are also intended to disclose embodiments in which the
microwells are at
least in part replaced with microchannels, immobilization sites, and/or other
types of
experimental units.
One or more portions of a microfabricated device may be selected, treated,
and/or coated
with a surface chemistry modifier to have a particular surface chemistry. For
example, at least a
portion of a substrate surface may be configured with first surface
characteristics that repel cells
and/or reduce cellular tendency to stick to the surface or second surface
characteristics that
attract cells and/or increase cellular tendency to attach to the surface.
Depending on the type of
target cell, the material and/or coating may be hydrophobic and/or
hydrophilic. At least a
portion of the top surface of the substrate may be treated to have first
surface characteristics that
repel target cells and/or reduce the tendency of target cells to stick to the
surface. Meanwhile, at
least a portion of the inner surface of each experimental unit, well, or
microwell may be treated
to have second surface characteristics that attract target cells and increase
the tendency of target
cells to occupy the experimental unit, well, or microwell. A surface of a
substrate may have a
plurality of portions with different surface characteristics.
A surface chemistry modifier may be applied using chemical vapor deposition,
electroporation, plasma treatment, and/or electrochemical deposition. The
surface chemistry
modifier may control surface potential, Lund potential, zeta potential,
surface morphology,
hydrophobicity, and/or hydrophilicity. The surface chemistry modifier may
include a silane, a
polyelectrolyte, a metal, a polymer, an antibody, and/or a plasma. For
example, the surface
chemistry modifier may include octadecyltrichlorosilane. The surface chemistry
modifier may
include a dynamic copolymer, such as polyoxyethylene (20) sorbitan monolaurate
and/or
polyethylene glycol p-(1,1,3,3-tetramethylbuty1)-phenyl ether. The surface
chemistry modifier
may include a static copolymer, such as poloxamer 407, poly(L-lysine), and/or
a poly(ethylene
glycol)-poly(1-lysine) block copolymer.
CA 03037631 2019-03-14
WO 2018/064385
PCT/US2017/054108
An apparatus for screening different conditions against a matrix of cells may
include a
substrate with a surface defining an array of microwells. Sections of the
microwell array may be
partitioned into subarrays (e.g., by larger wells or walls). The substrate may
be microfabricated.
Each microwell may receive and grow at least one biological entity (e.g.,
cell). The resulting
matrix of biological entities (e.g., cells) may be a high density matrix of
biological entities. The
first array and/or the second array may be planar, substantially planar,
and/or multi-planar (e.g.,
on a roll).
The term "high resolution" may refer to a capability of a system or method to
distinguish
between a number of available experiments. For example, a "high resolution"
system or method
.. may select an experimental unit from a microfabricated device comprising a
high density of
experimental units, in which the experimental unit has a diameter from about 1
nm to about 800
p.m. A substrate of a microfabricated device or chip may include about or more
than 10,000,000
microwells. For example, an array of microwells may include at least 96
locations, at least 1,000
locations, at least 5,000 locations, at least 10,000 locations, at least
50,000 locations, at least
100,000 locations, at least 500,000 locations, at least 1,000,000 locations,
at least 5,000,000
locations, or at least 10,000,000 locations.
The surface density of microwells may be from about 150 microwells per cm2 to
about
160,000 microwells per cm2 or more. A substrate of a microfabricated device or
chip may have
a surface density of microwells of at least 150 microwells per cm2, at least
250 microwells per
cm2, at least 400 microwells per cm2, at least 500 microwells per cm2, at
least 750 microwells per
cm2, at least 1,000 microwells per cm2, at least 2,500 microwells per cm2, at
least 5,000
microwells per cm2, at least 7,500 microwells per cm2, at least 10,000
microwells per cm2, at
least 50,000 microwells per cm2, at least 100,000 microwells per cm2, or at
least 160,000
microwells per cm2.
The dimensions of a microwell may range from nanoscopic (e.g., a diameter from
about 1
to about 100 nanometers) to microscopic or larger. For example, each microwell
may have a
diameter of about 1 p.m to about 800 p.m, a diameter of about 25 p.m to about
500 p.m, or a
diameter of about 30 p.m to about 100 p.m. A microwell may have a diameter of
about or less
than 1 p.m, about or less than 5 p.m, about or less than 10 p.m, about or less
than 25 p.m, about or
16
CA 03037631 2019-03-14
WO 2018/064385
PCT/US2017/054108
less than 50 p.m, about or less than 100 p.m, about or less than 200 p.m,
about or less than 300
p.m, about or less than 400 p.m, about or less than 500 p.m, about or less
than 600 p.m, about or
less than 700 p.m, or about or less than 800 p.m.
A microwell may have a depth of about 500 p.m to about 5000 p.m, a depth of
about 1 p.m
to about 500 p.m, or a depth of about 25 p.m to about 100 pm. A microwell may
have a depth of
about 1 p.m, about 5 p.m, about 10 p.m, about 25 pm, about 50 pm, about 100
p.m, about 200 p.m,
about 300 p.m, about 400 p.m, about 500 p.m, about 600 pm, about 700 p.m,
about 800 p.m, about
1000 p.m, about 1,500 p.m, about 2,000 p.m, about 3,000 p.m, or about 5,000
p.m.
Each microwell may have an opening that is round, hexagonal, or square. Each
.. microwell may include sidewalls. The sidewalls may have a cross-sectional
profile that is
straight, oblique, and/or curved. At least one unique location-specific tag,
as described further
below, may be disposed in at least one microwell of the high density array of
microwells to
facilitate identification of a species and a correlation of a species to a
specific microwell of the
high density array of microwells. The at least one unique tag may be disposed
and/or positioned
at the bottom of the microwell and/or on at least one side of the microwell.
The at least one
unique tag may include a nucleic acid molecule with a target-specific
nucleotide sequence for
annealing to a target nucleic acid fragment of the at least one biological
entity and a location-
specific nucleotide sequence for identifying the at least one microwell of the
high density array
of microwells.
For example, a substrate of a microfabricated device or chip may have a
surface with
dimensions of about 4 inches by 4 inches. The surface may define an array of
approximately 100
million microwells. The microwell array may be partitioned into about 100
subsections by walls
and/or the substrate may define an array of about 100 wells, with about one
million microwells
defined within each subsection or well totaling to approximately 100 million
microwells. For a
.. use case of testing different nutrients, microorganisms from an
environmental sample may be
loaded on the chip such that individual microorganisms or clusters of
microorganisms partition
into the microwells on the chip, each microwell being located at the bottom of
a larger well.
Each larger well may include an experimental unit such that about 100
different nutrients may be
tested in parallel or in series on the same chip, with each well providing up
to 1 million test
17
CA 03037631 2019-03-14
WO 2018/064385
PCT/US2017/054108
cases.
Target cells may be Archaea, Bacteria, or Eukaryota (e.g., fungi, plants, or
animals). For
example, target cells may be microorganisms, such as aerobic, anaerobic,
and/or facultative
aerobic microorganisms. Different nutrients may be tested in parallel or in
series on a
composition of target cells to analyze and compare, for instance, growth or
other effects on cell
population, cell components, and/or cell products. A composition of target
cells may be
screened for a cell component, product, and/or capability, such as one or more
of a virus (e.g., a
bacteriophage), a cell surface (e.g., a cell membrane or wall), a metabolite,
a vitamin, a hormone,
a neurotransmitter, an antibody, an amino acid, an enzyme, a protein, a
saccharide, ATP, a lipid,
a nucleoside, a nucleotide, a nucleic acid (e.g., DNA or RNA), a phenotype, a
mutation, a
metabolic pathway, a gene, and an adaptation.
A composition of cells may include an environmental sample extract and/or a
dilutant.
The environmental sample extract and/or the dilutant may include, but is not
limited to, one or
more of a biological tissue (e.g., connective, muscle, nervous, epithelial,
plant epidermis,
vascular, ground, etc.), a biological fluid or other biological product (e.g.,
amniotic fluid, bile,
blood, cerebrospinal fluid, cerumen, exudate, fecal matter, gastric fluid,
interstitial fluid,
intracellular fluid, lymphatic fluid, milk, mucus, rumen content, saliva,
sebum, semen, sweat,
urine, vaginal secretion, vomit, etc.), a microbial suspension, air
(including, e.g., different gas
contents), supercritical carbon dioxide, soil (including, e.g., minerals,
organic matter, gases,
liquids, organisms, etc.), sediment (e.g., agricultural, marine, etc.), living
organic matter (e.g.,
plants, insects, other small organisms and microorganisms), dead organic
matter, forage (e.g.,
grasses, legumes, silage, crop residue, etc.), a mineral, oil or oil products
(e.g., animal, vegetable,
petrochemical), alcohol, a buffer, an organic solvent, water (e.g., naturally-
sourced freshwater,
drinking water, seawater, etc.), and/or sewage (e.g., sanitary, commercial,
industrial, and/or
agricultural wastewater and surface runoff).
A method may include, prior to applying (e.g., loading) a composition
including cells to a
microfabricated device, preparing the composition by combining the cells with
an environmental
sample extract and/or a dilutant. The method further may include liquefying
the environmental
sample extract and/or the dilutant. A concentration of cells in a composition
may be adjusted to
18
CA 03037631 2019-03-14
WO 2018/064385
PCT/US2017/054108
target distribution of one cell per experimental unit, well, or microwell.
If a sample contains cells and/or viruses, the cells in the sample may be
lysed after they
are applied to a microfabricated device to release nucleic acid molecules.
Cells may be lysed
with chemical treatment such as alkaline exposure, detergents, sonication,
enzymatic proteinase
K, or lysozyme exposure. Cells may also be lysed by heating.
FIG. 5A is a flowchart illustrating a method for isolating cells from a sample
in
accordance with some embodiments. In step 500, a sample is obtained. In step
502, the sample
is homogenized and/or dispersed using at least one of a physical technique
(e.g., blending and/or
sonication) and a chemical technique (e.g., chelating agents, detergents,
and/or enzymes). In
step 504, cells in the homogenized and/or dispersed sample are separated by
density
centrifugation using, for example, Nycodenz non-particulate medium (available
from Progen
Biotechnik GmbH, Heidelberg, Germany).
FIG. 5B is a diagram illustrating a method for isolating cells from a soil
sample in
accordance with some embodiments. Panel 506 shows the soil sample. Panel 508
shows the
homogenized and/or dispersed sample in a test tube. Panel 510 shows the sample
after
centrifugation, separated into soluble debris 512, cells 514, insoluble debris
516, and
Nycodenz 518.
FIG. 6 is a flowchart illustrating a method for isolating and cultivating
cells from a
sample in accordance with some embodiments. In step 600, a sample is obtained.
In step 602, at
least one cell is extracted from the obtained sample. In step 604, at least
one high density
microwell array of a microfabricated device or chip is loaded with the at
least one extracted cell.
Step 604 may include preparing a cell concentration with the at least one
extracted cell, selecting
at least one nutrient/media, and/or selecting at least one membrane. In step
606, at least a portion
of the microwell array is sealed with the at least one selected membrane to
retain the cell
concentration with the microwells. In step 608, the chip is incubated. Step
608 may include
selecting a temperature, determining atmosphere (e.g., aerobic or anaerobic),
and/or timing
incubation). In step 610, the chip is split and/or substantially replicated
(using, e.g., a picker),
resulting in two portions of cultivated cells according to methods described
herein. For example,
the at least one membrane may be peeled off such that a portion of the
cultivated cells remain
19
CA 03037631 2019-03-14
WO 2018/064385
PCT/US2017/054108
attached or peeled off or punctured to sample the cultivated cells. In
optional step 612, one
portion of the cultivated cells is sacrificed for identification. Step 612 may
include PCR,
sequencing, and/or various data analytics. In step 614, strains of interest
are identified. Further
cultivation, testing, and/or identification may be performed with, for
example, the strains of
interest and/or the remaining portion of the cultivated cells.
FIG. 7 is a diagram illustrating a method for isolating and cultivating cells
from a
complex sample in accordance with some embodiments. Panel 700 shows examples
of complex
samples, specifically a microbiome sample 702 and a soil sample 704. In Panel
706, at least one
cell is extracted from the sample using, for example, the protocol illustrated
in FIGS. 5A and 5B.
In Panel 708, the at least one extracted cell (and any environmental extract
and/or dilutant) is
loaded on a microfabricated device or chip with at least one high density
microwell array 710.
Chip 710 and a reagent cartridge 712 may be loaded into an incubator 714. The
reagent may be
useful for adding liquid to maintain nutritional requirements for growth
and/or various screening
purposes. Panel 716 shows the output: isolated strains of cultivated cells.
To identify the species or taxonomic lineage of cells or microorganisms
growing in a
microwell requires techniques including, but not limited to, DNA sequencing,
nucleic acid
hybridization, mass spectrometry, infrared spectrometry, DNA amplification,
and antibody
binding to identify genetic elements or other species identifiers. Many
identification methods
and process steps kill the microorganisms and therefore prevent further
cultivation and study of
microorganisms of interest. To enable both the identification of cells or
microorganisms while
enabling subsequent cultivation, study, and further elaboration of particular
clones of interest,
further embodiments are designed for sampling each experimental unit, well, or
microwell across
a substrate or chip while maintaining the locational integrity and separation
of microorganism
populations across experimental units, wells, or microwells.
A substrate as described above may enable sampling a cell population using
further
systems, kits, apparatus, and methods. For example, a picking device may be
applied to a first
surface of the substrate. The device may include at least one protrusion
facing the first surface.
The at least one protrusion has a diameter less than the opening diameter of
each microwell,
well, or experimental unit. The at least one protrusion may be inserted into
at least one
CA 03037631 2019-03-14
WO 2018/064385
PCT/US2017/054108
microwell, well, or experimental unit holding a population of cells such that
a portion of the
population of cells in the at least one microwell, well, or experimental unit
adheres and/or
attaches to the at least one protrusion. The sample of the population of cells
in the at least one
microwell, well, or experimental unit may be withdrawn by removing the device
from the first
surface of the substrate such that the portion of the population of cells in
the at least one
microwell, well, or experimental unit remains adhered and/or attached to the
at least one
protrusion. Each protrusion may be a pin or a plurality or assembly of pins.
FIGS. 8A-8C are diagrams illustrating picking by one pin or multiple pins in
accordance
with some embodiments. Chip 800 is provided for inspection via a microscope
802 and picking
via picking control device 804. In FIG. 8A, picking control device 804
comprises an arm with a
single pin 806. In FIG. 8B, an arm with multiple pins 808 is shown. FIG. 8C is
a perspective
view of the chip during the picking process.
FIGS. 9A-9D are images demonstrating picking of a well in accordance with some
embodiments. In FIG. 9A, the well is full. In FIG. 9B, the pin is moved into
position. In FIG.
9C, the well is picked. In FIG. 9D, a sample is removed from the well.
FIGS. 10A-10D are diagrams illustrating a tool for picking a chip in
accordance with
some embodiments. In FIG. 10A, a tool comprising a plurality of pins is
aligned with a chip
having a plurality of wells. In FIG. 10B, the tool is lowered such that the
pins are dipped into the
wells. In FIG. 10C, the pins are shown with samples attached, and the samples
are transferred to
a new chip. Alternatively, in FIG. 10D, the tool is flipped such that the
samples may be
maintained in the tool itself.
FIG. 11 is an image of a well that has been picked through a thin layer of
agar,
illustrating picking through a membrane or sealing layer in accordance with
some embodiments.
Alternatively, when the at least one protrusion is inserted into the at least
one microwell,
well, or experimental unit, a portion of the population of cells in the at
least one the at least one
microwell, well, or experimental unit is volume displaced up and around the at
least one
protrusion such that at least some of the volume displaced portion is above
the first surface of the
substrate and/or the inner surface of the at least one microwell, well, or
experimental unit. The
21
CA 03037631 2019-03-14
WO 2018/064385
PCT/US2017/054108
method also includes sampling the population of cells in the at least one
microwell by collecting
at least some of the volume displaced portion of the population of cells.
A similar picking device may be applied to a second surface opposite the first
surface of
the substrate. The device may include at least one protrusion facing the
second surface. The at
least one protrusion has a diameter about equal to or less than a diameter of
at least one
microwell, well, or experimental unit. The at least one protrusion is pushed
against the second
surface at a location corresponding to the at least one microwell, well, or
experimental unit
holding a population of cells and/or inserted into the at least one microwell,
well, or
experimental unit holding the population of cells such that a portion of the
population of cells in
the at least one microwell, well, or experimental unit is displaced above the
first surface of the
substrate and/or the inner surface of the at least one microwell, well, or
experimental unit. The
displaced portion of the population of cells may then be collected. The
population of cells may
be located on a plug (e.g., a hydrogel or other soft material like agar) in
the at least one
experimental unit, well, or microwell such that when the at least one
protrusion is at least one of
pushed against the second surface and inserted into the at least one
microwell, the plug is
displaced, thereby displacing the portion of the population of cells.
The sample of the population of cells from the at least one experimental unit,
well, or
microwell may be deposited in a second location. The at least one protrusion
may be cleaned
and/or sterilized prior to further sampling. At least a portion of the at
least one protrusion may
be composed of a material, treated, and/or coated with a surface chemistry
modifier for surface
characteristics that favor attachment of cells. The at least one protrusion
may be an array of
protrusions. Upon applying the device to the first surface of the substrate,
the array of
protrusions may be inserted into a corresponding array of experimental units,
wells, or
microwells. The number of protrusions in the array of protrusions may
correspond to the number
of experimental units in the first array, the number of microwells in one
second array of
microwells, or the total number of microwells in the substrate.
Another device for sampling a cell population in a substrate includes at least
one needle
and/or nanopipette facing the first surface. The at least one needle and/or
nanopipette has an
external diameter less than the opening diameter of each microwell and an
internal diameter
22
CA 03037631 2019-03-14
WO 2018/064385
PCT/US2017/054108
capable of accommodating a target cell diameter. The at least one needle
and/or nanopipette is
inserted into at least one experimental unit, well, or microwell holding a
population of cells. The
sample of the population of cells in the at least one experimental unit, well,
or microwell is
withdrawn using pressure to pull a portion of the population of cells from the
at least one
experimental unit, well, or microwell into the device.
The sample of the population of cells from the at least one experimental unit,
well, or
microwell may be deposited in a second location. The at least one needle
and/or nanopipette
may be cleaned and/or sterilized prior to further sampling. The at least one
needle and/or
nanopipette may be an array of needles and/or nanopipettes. Upon applying the
device to the
.. first surface of the microfabricated substrate, the array of needles and/or
nanopipettes may be
inserted into a corresponding array of experimental units, wells, or
microwells. The number of
needles and/or nanopipettes in the array of needles and/or nanopipettes may
correspond to the
number of the experimental units in the first array, the number of microwells
in one second array
of microwells, or the total number of microwells in the substrate.
Another method for sampling a cell population in a substrate includes applying
focused
acoustic energy to at least one experimental unit, well, or microwell holding
a population of cells
in fluid. The focused acoustic energy may be applied in a manner effective to
eject a droplet
from the at least one microwell, such as, for example, acoustic droplet
ejection (ADE) (see, e.g.,
Sackmann et al., "Acoustical Micro- and Nanofluidics: Synthesis, Assembly and
Other
Applications," Proceedings of the 4th European Conference on Microfluidics
(December 2014)).
The droplet may include a sample of the population of cells in the at least
one experimental unit,
well, or microwell. The droplet may be directed into a second container or
surface or substrate.
A substrate may include at least a first piece including at least a portion of
the first
surface and a second piece including at least a portion of the second surface.
The first piece and
the second piece are detachably connected along at least a portion of a plane
parallel to the first
surface and the second surface. The plane divides the experimental units,
wells, or microwells.
A cell population in at least one experimental unit, well, or microwell is
sampled by detaching
the first piece and the second piece such that a first portion of the
population of cells in the at
least one experimental unit, well, or microwell remains attached to the first
piece and a second
23
CA 03037631 2019-03-14
WO 2018/064385
PCT/US2017/054108
portion of the population of cells in the at least one experimental unit,
well, or microwell remains
attached to the second piece.
FIG. 12 is a diagram illustrating a cross-section of a chip 1200 in accordance
with some
embodiments. Chip 1200 includes a substrate defining an array of wells 1202
filled with
contents 1204. The substrate comprises a first piece 1206 and a second piece
1208. The first
piece 1206 and the second piece 1208 are detachably connected along a plane
1210 parallel to
and bisecting the array of wells 1202. When the first piece 1206 and the
second piece 1208 are
detached, the wells 1202 and their contents 1204 are divided, resulting in two
copies of the
contents 1204 that preserve both the isolation and the location of the
contents 1204 on chip 1200.
Each microwell, experimental unit, or microchannel may include a partial
barrier that
partially separates the microwell, experimental unit, or microchannel into a
first portion and a
bottom portion such that a cell population is able to grow in both the first
portion and the bottom
portion. Prior to sampling the population of cells, the above methods may
include dispersing
and/or reducing clumps of cells in the population of cells. Dispersing and/or
reducing clumps of
cells in the population of cells may include, but is not limited to, applying
sonication, shaking,
and dispension with small particles.
The above methods further may include depositing the sample of the population
of cells
from the at least one experimental unit, well, or microwell in a second
location. The second
location may be a corresponding array of experimental units, wells, or
microwells. The second
location may be a single receptacle. The sample of the population of cells
from the at least one
experimental unit, well, or microwell may be maintained for subsequent
cultivation.
Alternatively, the remaining cells of the population of cells in the at least
one experimental unit,
well, or microwell may be maintained for subsequent cultivation.
The above methods further may include identifying at least one cell from the
sample of
the population of cells and/or the remaining cells of the population of cells.
This may include
performing DNA, cDNA, and/or RNA amplification, DNA and/or RNA sequencing,
nucleic acid
hybridization, mass spectrometry, and/or antibody binding. Alternatively, or
in addition, this
may include identifying an experimental unit, well, or microwell from which at
least one cell
originated. Each experimental unit, well, or microwell may be marked with a
unique tag
24
CA 03037631 2019-03-14
WO 2018/064385
PCT/US2017/054108
including a location-specific nucleotide sequence. To identify the
experimental unit, well, or
microwell, a location-specific nucleotide sequence may be identified in the
sequencing and/or
amplification reaction, and the location specific nucleotide sequence may be
correlated with the
at least one experimental unit, well, or microwell from which the at least one
cell originated.
A microfabricated device as described above may enable culturing cells in a
sample
derived from an environment using further systems, kits, apparatus, and
methods. For example,
a sample may be applied to the first surface of a substrate such that at least
one of the cells
occupies at least one microwell, well, or experimental unit. A semi-permeable
membrane is
applied to at least a portion of the first surface (e.g., at least a portion
of an inner surface of an
experimental unit or well) such that a nutrient can diffuse into the at least
one microwell, well, or
experimental unit. Meanwhile, escape of the occupying cells from the at least
one microwell,
well, or experimental unit is prevented and/or mitigated. A semi-permeable
membrane may be,
for example, a hydrogel layer. A semi-permeable membrane may be reversibly or
irreversibly
connected or affixed to the substrate using, for example, lamination. Thus,
the occupying cells
may be incubated in the at least one microwell, well, or experimental unit
with at least one
nutrient. The cells may be gradually transitioned over a period of time from
at least one nutrient
to at least one alternative nutrient or nutrient formulation using progressive
partial exchange,
thereby undergoing domestication or adaptation.
A first nutrient derived from the environment may be used to incubate the
cells
occupying at least one first experimental unit, well, or microwell, and a
second nutrient derived
from the environment may be used to incubate the cells occupying at least one
second
experimental unit, well, or microwell. The above methods may include comparing
the cells
occupying the at least one first experimental unit, well, or microwell with
the cells occupying at
least one second experimental unit, well, or microwell to analyze the first
nutrient and the second
nutrient.
For example, a method may include one or more of the following steps:
= Acquire a chip defining 1000 to 10 million or more microwells within a
number of
larger wells or flow cells, each microwell having a diameter of about 1 p.m to
about
800 p.m and a depth of about 1 p.m to about 800 p.m, the chip further having
one or
CA 03037631 2019-03-14
WO 2018/064385
PCT/US2017/054108
more surface chemistries configured to facilitate the movement of target
microorganisms into the microwells;
= Apply an environmental sample or a derivative of the environmental sample
to the chip
such that any target microorganisms become located in the microwells;
= Place one or more semi-permeable filters, hydrogel layers, or other
barriers on the chip
such that a barrier is created that allows nutrients to diffuse into the
microwells but
prevents and/or mitigates escape of microorganisms from the microwells;
= Incubate the chip with at least one nutrient (e.g., derived from the
environment);
= Gradually change the nutrient source by progressive partial exchange with
at least one
alternative nutrient (e.g., formulation); and
= Detect any growth of microorganisms in the microwells.
The target cells may be Archaea, Bacteria, or Eukaryota. Target viruses may be
bacteriophages. When viruses are targeted, the microwells of the chip may also
include host
cells in which the viruses may grow. Detecting the growth of the occupying
cells or viruses may
include detecting a change in biomass (e.g., DNA/RNA/protein/lipid),
metabolite presence or
absence, pH, consumption of nutrients, and/or consumption of gases. Detecting
the growth of
the occupying cells or viruses may include performing real-time sequential
imaging, microscopy,
optical density, fluorescence microscopy, mass spectrometry, electrochemistry,
amplification
(DNA, cDNA, and/or RNA), sequencing (DNA and/or RNA), nucleic acid
hybridization, and/or
antibody binding.
FIG. 13 is a flowchart illustrating methods for screening in accordance with
some
embodiments. In step 1300, a sample is obtained. In step 1302, at least one
cell is extracted
from the obtained sample. In step 1304, at least one high density microwell
array of a
microfabricated device or chip is loaded with the at least one extracted cell.
Step 1304 may
include preparing a cell concentration with the at least one extracted cell,
selecting at least one
nutrient/media, and/or selecting at least one membrane. In step 1306, at least
a portion of the
26
CA 03037631 2019-03-14
WO 2018/064385
PCT/US2017/054108
microwell array is sealed with the at least one selected membrane to retain
the cell concentration
with the microwells. In step 1308, the chip is incubated. Step 1308 may
include selecting a
temperature, determining atmosphere (e.g., aerobic or anaerobic), and/or
timing incubation). A
genetic screen and/or a functional screen may be performed. In step 1310, a
genetic screen is
.. applied to the chip. In step 1312, the chip is split and/or substantially
replicated (using, e.g., a
picker), resulting in two portions of cultivated cells according to methods
described herein. For
example, the at least one membrane may be peeled off such that a portion of
the cultivated cells
remain attached or peeled off or punctured to sample the cultivated cells. In
optional step 1314,
one portion of the cultivated cells is sacrificed for identification. Step
1314 may include PCR,
.. sequencing, and/or various data analytics. In step 1316, strains of
interest are identified. Further
cultivation, testing, and/or identification may be performed with, for
example, the strains of
interest and/or the remaining portion of the cultivated cells. Alternatively,
in step 1318, a
functional screen is applied to the chip. In step 1320, one or more variables
are observed and, as
in step 1316, strains of interest are identified.
FIG. 14 is a diagram illustrating a screening method in accordance with some
embodiments. Panel 1400 shows examples of complex samples, specifically a
microbiome
sample 1402 and a soil sample 1404. In Panel 1406, at least one cell is
extracted from the
sample using, for example, the protocol illustrated in FIGS. 5A and 5B. In
Panel 1408, the at
least one extracted cell (and any environmental extract and/or dilutant) is
loaded on a
.. microfabricated device or chip with at least one high density microwell
array 1410. Chip 1410
and a reagent cartridge 1412 may be loaded into an incubator 1414. The reagent
may be useful
for adding liquid to maintain nutritional requirements for growth and/or
various screening
purposes. Panel 1416 shows the output: screen results and isolated strains of
cultivated cells.
FIG. 15 is a series of images illustrating a screening example in accordance
with some
embodiments. The images show portions of a chip with a membrane and an acid-
sensitive layer
applied thereon to screen for low pH. In image 1500, more than 1800, 50-[tm
microwells are
visible with nine clear hits 1502. Image 1504 is a magnified view of box 1504,
and image 1506
is a magnified view of one of the microwells with a hit 1502.
FIGS. 16A-16C are images illustrating recovery from a screen in accordance
with some
27
CA 03037631 2019-03-14
WO 2018/064385
PCT/US2017/054108
embodiments. In FIG. 16A, at least one well is picked using a microscope and a
picking device
with at least one pin. In FIG. 16B, a pin is removed and incubated in media.
In FIG. 16C,
growth is visible.
FIG. 17A is an exploded diagram illustrating a chip for screening in
accordance with
some embodiments. In FIG. 17A, chip 1700 includes a high density array of
microwells with,
for example, soil microbes in the microwells. Membrane 1702 is applied to chip
1700. Gasket
1704 is applied to chip 1700 over membrane 1702. Agar with fluorescent E. coil
bacteria 1706
is applied to chip 1700 over gasket 1704 and membrane 1702. FIGS. 17B and 17C
are images
illustrating a screening example in accordance with some embodiments. In this
example, the
screen is for clearance zones. FIG. 17B is a fluorescence image of a chip,
prepared like chip
1700 in FIG. 17A, following the screen. FIG. 17C is an image showing a process
of picking a
sample from this chip through the agar.
In some embodiments, a location on an apparatus may be correlated with a
portion of a
sample present at that location, after that portion of the sample (or a part
of the portion) is
removed from the apparatus. The apparatus may be or include a microarray. The
microarray
may comprise a plurality of locations for applying a sample, wherein each
location is marked
with a unique tag which may be used to identify the location from which a
portion of the sample
came, after that portion of the sample is removed from the microarray.
The disclosure relates to a method of identifying from which location on a
microarray a
portion of a sample comprising at least one nucleic acid molecule came, after
that portion of the
sample is removed from the microarray, the method comprising the steps of: (a)
applying one or
more portions of the sample onto one or more of a plurality of locations on
the microarray,
wherein each location is marked with a unique tag comprising a nucleic acid
molecule
comprising: (i) a location-specific nucleotide sequence; and (ii) a first
target-specific nucleotide
sequence; (b) allowing the target nucleic acid molecule found in at least one
portion of the
sample to anneal to a tag marking a location; (c) performing primer extension,
reverse
transcription, single-stranded ligation, or double-stranded ligation on the
population of annealed
nucleic acid molecules, thereby incorporating a location-specific nucleotide
sequence into each
nucleic acid molecule produced by primer extension, reverse transcription,
single-stranded
28
CA 03037631 2019-03-14
WO 2018/064385
PCT/US2017/054108
ligation, or double-stranded ligation; (d) combining the population of nucleic
acid molecules
produced in step (c); (e) sequencing the population of combined nucleic acid
molecules, thereby
obtaining the sequence of one or more location-specific nucleotide sequences;
and (f) correlating
the sequence of at least one location-specific nucleotide sequence obtained
from the population
of combined nucleic acid molecules to the location on the microarray marked
with a tag
comprising said location-specific nucleotide sequence; thereby identifying
from which location
on a microarray a portion of a sample comprising at least one nucleic acid
molecule came. In
some embodiments, a sample may include at least one cell and one or more
nucleic acid
molecules are released from the cell after step (a) and before step (b). A
sample may include at
least one cell, and the at least one cell replicates or divides after step (a)
and before step (b). A
portion of the portion of the sample may be removed from at least one location
before step (b)
and said portion of the portion of the sample may be stored in a separate
receptacle correlated to
the original location of the portion of the sample on the microarray. The
method of correlating
or identifying a location may further comprise the step of amplifying the
nucleic acid molecules
produced in step (c) or the population of combined nucleic acid molecules
produced in step (d).
The amplifying step may comprise polymerase chain reaction amplification,
multiplexed
polymerase chain reaction amplification, nested polymerase chain reaction
amplification, ligase
chain reaction amplification, ligase detection reaction amplification, strand
displacement
amplification, transcription based amplification, nucleic acid sequence-based
amplification,
.. rolling circle amplification, or hyper-branched rolling circle
amplification. Additional primers
may be added during an amplification reaction. For example, both 5' and 3'
primers may be
needed for a PCR reaction. One of the primers used during an amplification
reaction may be
complementary to a nucleotide sequence in the sample.
In some embodiments, a composition including cells and/or viruses may be
treated with a
nuclease before the composition is applied to a microfabricated device so that
contaminating
nucleic acid molecules are not amplified in subsequent steps.
The sequencing used in the disclosed methods and apparatuses may be any
process of
obtaining sequence information, including hybridization and use of sequence
specific proteins
(for example, enzymes). Sequencing may comprise Sanger sequencing, sequencing
by
hybridization, sequencing by ligation, quantitative incremental fluorescent
nucleotide addition
29
CA 03037631 2019-03-14
WO 2018/064385
PCT/US2017/054108
sequencing (QIFNAS), stepwise ligation and cleavage, fluorescence resonance
energy transfer,
molecular beacons, TaqMan reporter probe digestion, pyrosequencing,
fluorescent in situ
sequencing (FISSEQ), wobble sequencing, multiplex sequencing, polymerized
colony
(POLONY) sequencing (see, e.g., U.S. Patent Application Publication No.
2012/0270740, which
is incorporated by reference herein in its entirety); nanogrid rolling circle
(ROLONY)
sequencing (see, e.g., U.S. Patent Application Publication No. 2009/0018024,
which is
incorporated by reference herein in its entirety), allele-specific oligo
ligation assay sequencing,
or sequencing on a next-generation sequencing (NGS) platform. Non-limiting
examples of NGS
platforms include systems from Illuminag (San Diego, California) (e.g.,
MiSeqTM, NextSeqTM,
HiSeqTM, and HiSeq XTm), Life Technologies (Carlsbad, California) (e.g., Ion
TorrentTm), and
Pacific Biosciences (Menlo Park, California) (e.g., PacBiog RS II).
An organism or species may be identified by comparing the nucleic acid
sequence
obtained from that organism to various databases containing sequences of
organisms. For
example, ribosomal RNA sequence data is available in the SILVA rRNA database
project (Max
Planck Institute for Marine Microbiology, Bremen, Germany (www.arb-silva.de);
see, e.g.,
Quast et al., "The SILVA Ribosomal RNA Gene Database Project: Improved Data
Processing
and Web-Based Tools," 41 Nucl. Acids Res. D590-D596 (2013), and Pruesse et
al., "SINA:
Accurate High-Throughput Multiple Sequence Alignment of Ribosomal RNA Genes,"
28
Bioinformatics 1823-1829 (2012), both of which are incorporated by reference
herein in their
entirety). Other ribosomal RNA sequence databases include the Ribosomal
Database Project
(Michigan State University, East Lansing, Michigan (www.rdp.cme.msu.edu); see,
e.g., Cole et
al., "Ribosomal Database Project: Data and Tools for High Throughput rRNA
Analysis" 42
Nucl. Acids Res. D633-D642 (2014), which is incorporated by reference herein
in its entirety)
and Greengenes (Lawrence Berkeley National Laboratory, Berkeley, California
(www.greengenes.lbl.gov); see, e.g., DeSantis et al., "Greengenes, a Chimera-
Checked 16S
rRNA Gene Database and Workbench Compatible with ARE," 72 Appl. Environ.
Microbiol.
5069-72 (2006), which is incorporated by reference herein in its entirety).
The GenBank
genetic sequence database contains publicly available nucleotide sequences for
almost 260,000
formally described species (National Institutes of Health, Bethesda, Maryland
(www.ncbi.nlm.nih.gov); see, e.g., Benson et al., "GenBank," 41 Nucl. Acids
Res. D36-42
(2013).
CA 03037631 2019-03-14
WO 2018/064385
PCT/US2017/054108
The sequence used for matching and identification may include the 16S
ribosomal region,
18S ribosomal region or any other region that provides identification
information. The desired
variant may be a genotype (e.g., single nucleotide polymorphism (SNP) or other
type of variant)
or a species containing a specific gene sequence (e.g., a sequence coding for
an enzyme or
protein). An organism or species may also be identified by matching its
sequence to a custom
internal sequence database. In some cases, one may conclude that a certain
species or organism
is found at a location on the microarray if the sequence obtained from the
portion of the sample
at the location has at least a specified percentage identity (e.g., at least
90%, at least 91%, at
least 92%, at least 93%, at least 94%, at least 95%, at least 96%, at least
97%, at least 98%, at
least 99%, or 100% identity) to the known DNA, cDNA, or RNA sequence obtained
from that
species or microorganism.
The disclosure further relates to a method of manufacturing a microarray
comprising a
plurality of locations for applying a sample, wherein at least one location is
marked with a
unique tag, the method comprising the steps of: (a) synthesizing a plurality
of tags, wherein each
tag comprises a nucleic acid molecule comprising: (i) a location-specific
nucleotide sequence;
and (ii) a target-specific nucleotide sequence; and (b) placing a tag on at
least one location of the
plurality of locations on the microarray. In an alternative embodiment, the
disclosure relates to a
method of manufacturing a microarray comprising a plurality of locations for
applying a sample,
wherein at least one location is marked with a unique tag, the method
comprising the steps of: (a)
synthesizing a plurality of tags, wherein each tag comprises a nucleic acid
molecule comprising:
a target-specific nucleotide sequence and not comprising a location-specific
nucleotide sequence;
and (b) placing a tag on at least one location of the plurality of locations
on the microarray. The
target-specific sequence may be the same at every location in the microarray.
In either of the
above embodiments, step (a) may be performed before step (b). The placing step
(b) may
comprise placing the tag at each location by a liquid handling procedure (for
example, pipetting,
spotting with a solid pin, spotting with a hollow pin, or depositing with an
inkjet device). At
least one tag may include a nucleic acid molecule or a portion of a nucleic
acid molecule that is
pre-synthesized. Step (a) may be performed simultaneously with step (b). In
certain
embodiments, at least one tag comprises a nucleic acid molecule that is
synthesized at each
location by in situ synthesis. The synthesizing step (a) may comprise inkjet
printing synthesis or
photolithography synthesis.
31
CA 03037631 2019-03-14
WO 2018/064385
PCT/US2017/054108
Each location on a microarray may be configured to receive a portion of the
sample. A
location may be tagged or labeled with a nucleic acid molecule (e.g., an
oligonucleotide) that
comprises at least one of: (i) a location-specific nucleotide sequence (e.g.,
a barcode); and (ii) a
target-specific nucleotide sequence. A target-specific nucleotide sequence may
complement or
substantially complement a nucleotide sequence found in the sample. The order
of the
nucleotide sequences from the 5' end to the 3' end in the nucleic acid
molecule may be: (1) a
location-specific nucleotide sequence; and (2) a target-specific nucleotide
sequence.
Alternatively, the order of the nucleotide sequences from the 5' end to the 3'
end in the nucleic
acid molecule may be (2) then (1). The nucleic acid molecule may be attached
at its 5' end to the
microarray. One or more locations on the apparatus (e.g., microarray) may be
untagged or
unlabeled.
The terms "complementary" or "substantially complementary" may refer to the
hybridization, the base pairing, or the formation of a duplex between
nucleotides or nucleic
acids, such as, for instance, between the two strands of a double stranded DNA
molecule or
between an oligonucleotide primer and a primer binding site on a single
stranded nucleic acid.
Complementary nucleotides are, generally, A and T/U, or C and G. Two single-
stranded RNA
or DNA molecules are said to be substantially complementary when the
nucleotides of one
strand, optimally aligned and compared and with appropriate nucleotide
insertions or deletions,
pair with at least about 80% of the nucleotides of the other strand, usually
at least about 90% to
95%, and more preferably from about 98 to 100%. Alternatively, substantial
complementarity
exists when an RNA or DNA strand will hybridize under selective hybridization
conditions to its
complement. Typically, selective hybridization will occur when there is at
least about 65%
complementary over a stretch of at least 14 to 25 nucleotides, at least about
75%, or at least
about 90% complementary.
The term "selectively hybridize" or "selective hybridization" may refer to
binding
detectably and specifically. Polynucleotides, oligonucleotides and fragments
thereof selectively
hybridize to nucleic acid strands under hybridization and wash conditions that
minimize
appreciable amounts of detectable binding to nonspecific nucleic acids. "High
stringency" or
"highly stringent" conditions can be used to achieve selective hybridization
conditions as known
in the art and discussed herein. An example of "high stringency" or "highly
stringent"
32
CA 03037631 2019-03-14
WO 2018/064385
PCT/US2017/054108
conditions is a method of incubating a polynucleotide with another
polynucleotide, wherein one
polynucleotide may be affixed to a solid surface such as a membrane, in a
hybridization buffer of
6x SSPE or SSC, 50% formamide, 5xDenhardt's reagent, 0.5% SDS, 10011g/m1
denatured,
fragmented salmon sperm DNA at a hybridization temperature of 42 C for 12-16
hours,
followed by twice washing at 55 C using a wash buffer of lx SSC, 0.5% SDS.
The nucleic acid molecule that is part of a location tag may comprise at least
one
deoxyribonucleotide or at least one ribonucleotide. The nucleic acid molecule
may be single-
stranded or double-stranded. A nucleic acid molecule may be a double-stranded
molecule
having a single-stranded overhang.
In some embodiments, the location tag may be used to amplify a nucleic acid
molecule
that anneals to it. Thus, the location tag may comprise a nucleic acid
sequence that further
comprises an amplification primer binding site. An amplification primer
binding site may be at
least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at
least 22, at least 23, at least
24, at least 25, at least 26, at least 27, at least 28, at least 29, or at
least 30 nucleotides in length.
The order of the nucleotide sequences from the 5' end to the 3' end in the
nucleic acid molecule
may be, for example: (1) the amplification primer binding site; (2) the
location-specific
nucleotide sequence; and (3) the target-specific nucleotide sequence.
In some embodiments, a nucleic acid molecule may comprise a target-specific
nucleotide
sequence without comprising a location-specific nucleotide sequence. In
certain embodiments, a
nucleic acid molecule may comprise a target-specific nucleotide sequence
without comprising
either a location-specific nucleotide sequence or an amplification binding
site sequence. In
further embodiments, a nucleic acid molecule may comprise only a target -
specific nucleotide
sequence. In even further embodiments, a nucleic acid molecule may contain
only a target -
specific nucleotide sequence. The amplification primer binding site may be
capable of binding
to a polymerase chain reaction primer, a multiplexed polymerase chain reaction
primer, a nested
polymerase chain reaction primer, a ligase chain reaction primer, a ligase
detection reaction
primer, a strand displacement primer, a transcription based primer, a nucleic
acid sequence-based
primer, a rolling circle primer, or a hyper-branched rolling circle primer.
Additional primers
may be added to the microarray during an amplification reaction. For example,
both 5' and 3'
33
CA 03037631 2019-03-14
WO 2018/064385
PCT/US2017/054108
primers may be needed for a PCR reaction. Target-specific nucleotide sequences
may be
amplified in the locations containing target nucleic acid molecules and may be
detected by, for
example, qPCR, end point PCR, and/or dyes to detect amplified nucleic acid
molecules.
It may be desirable to sequence a nucleic acid molecule that anneals to a
location tag or
the amplified product based on such a nucleic acid molecule. The location tag
may comprise a
nucleic acid sequence that further comprises an adapter nucleotide sequence.
In certain
embodiments, an adapter nucleotide sequence may not be found in the location
tag but is added
to the sample nucleic acid molecules in a secondary PCR reaction or by
ligation. An adapter
nucleotide sequence may be a generic adapter or an adapter for a specific
sequencing platform
.. (e.g., Illumina or Ion TorrentTm). An adapter nucleotide sequence may
include a sequencing
primer binding site. A sequencing primer binding site may be capable of
binding a primer for
Sanger sequencing, sequencing by hybridization, sequencing by ligation,
quantitative
incremental fluorescent nucleotide addition sequencing (QIFNAS), stepwise
ligation and
cleavage, fluorescence resonance energy transfer, molecular beacons, TaqMan
reporter probe
digestion, pyrosequencing, fluorescent in situ sequencing (FISSEQ), wobble
sequencing,
multiplex sequencing, polymerized colony (POLONY) sequencing (see, e.g., US
2012/0270740);
nanogrid rolling circle (ROLONY) sequencing (see, e.g., US 2009/0018024),
allele-specific
oligo ligation assay sequencing, sequencing on an NGS platform, or any
suitable sequencing
procedure. Non-limiting examples of NGS platforms include systems from
Illuminag (San
Diego, California) (e.g., MiSeqTM, NextSeqTM, HiSeqTM, and HiSeq XTm), Life
Technologies
(Carlsbad, California) (e.g., Ion TorrentTm), and Pacific Biosciences (Menlo
Park, California)
(e.g., PacBiog RS II).
A location-specific nucleotide sequence (e.g., a barcode) may be at least 2,
at least 3, at
least 4, at least 5, at least 6, at least 7, at least 8, at least 9, at least
10, at least 11, at least 12, at
least 13, at least 14, at least 15, at least 16, at least 17, at least 18, at
least 19, at least 20, at least
21, at least 22, at least 23, at least 24, at least 25, at least 26, at least
27, at least 28, at least 29, or
at least 30 nucleotides in length.
A target-specific nucleotide sequence may be at least 10, at least 11, at
least 12, at least
13, at least 14, at least 15, at least 16, at least 17, at least 18, at least
19, at least 20, at least 21, at
34
CA 03037631 2019-03-14
WO 2018/064385
PCT/US2017/054108
least 22, at least 23, at least 24, at least 25 nucleotides, at least 26, at
least 27, at least 28, at least
29, at least 30, at least 40, at least 50, at least 75, or at least 100
nucleotides in length.
At least one location on a microarray may be further marked with a unique
molecular
identifier tag. Unique molecular identifiers may be used to quantify growth
(e.g., growth of a
microorganism colony or replication of cells at the location). Unique
molecular identifiers may
be random nucleotide sequences. Methods using unique molecular identifiers and
examples of
unique molecular identifiers have been described in the art, see, e.g., WO
2013/173394, which is
incorporated by reference herein in its entirety. For example, a unique
molecular identifier tag
may have the nucleotide sequence NNNANNNCNNNTNNNGNNNANNNCNNN (SEQ ID
NO:1), wherein the Ns (equal random mix of ACGT) create a large encoding space
so that each
molecule amplified gets a unique (specific) DNA sequence barcode (4AN
barcodes, or 41'21 - 4
trillion in this example) This sequence can be counted without interference
from amplification
bias or other technical problems. The fixed bases in SEQ ID NO:1 (the A, C, G,
T) help with
reading the barcode accurately, e.g., handling indels.
The present disclosure encompasses using location-specific tags to monitor the
presence
or amount of more than one target-specific nucleotide sequence in a sample
(e.g., multiplexing).
At least one location on a microarray may be further marked with a second
unique tag
comprising a nucleic acid molecule comprising, for example: (i) an
amplification primer binding
site; (ii) a location-specific nucleotide sequence; and (iii) a target-
specific nucleotide sequence.
In some embodiments, a nucleic acid molecule may comprise a target-specific
nucleotide
sequence without comprising a location-specific nucleotide sequence. In
certain embodiments, a
nucleic acid molecule may comprise a target-specific nucleotide sequence
without comprising
either a location-specific nucleotide sequence or an amplification binding
site sequence. In
further embodiments, a nucleic acid molecule may comprise only a target -
specific nucleotide
sequence. In even further embodiments, a nucleic acid molecule may contain
only a target -
specific nucleotide sequence. The target-specific nucleotide sequence may be
at least 10, at least
11, at least 12, at least 13, at least 14, at least 15, at least 16, at least
17, at least 18, at least 19, at
least 20, at least 21, at least 22, at least 23, at least 24, at least 25
nucleotides, at least 26, at least
27, at least 28, at least 29, at least 30, at least 40, at least 50, at least
75, or at least 100
CA 03037631 2019-03-14
WO 2018/064385
PCT/US2017/054108
nucleotides in length. In certain embodiments, the target-specific sequence
may be the same at
every location in the microarray. Additional target-specific nucleotide
sequences may be
monitored. For example, one or more locations may be marked with at least 10,
at least 25, at
least 50, at least 75, or at least 100 unique tags, wherein each tag comprises
a target-specific
nucleotide sequence that is different from the other target-specific
nucleotide sequences in the
tags at that location.
Any genetic locus of interest may provide a target-specific nucleotide
sequence. For
example, sequences of bacterial 16S ribosomal RNA (rRNA), 18S ribosomal RNA,
poly(A)
RNA, an RNA polymerase gene, a DNA polymerase gene, the RecA gene, a
transposase gene,
.. ribosomal internal transcribed spacer (ITS) sequences, a gene encoding an
enzyme, control
region DNA sequences, binding site DNA sequences, or a portion of any of these
sequences may
serve as a target-specific nucleotide sequence. A disclosed system, kit,
apparatus, or method may
use one or more of the bacterial 16S rRNA primers described in Sundquist et
al., "Bacterial
Flora-Typing with Targeted, Chip-Based Pyrosequencing," 7:108 BMC Microbiology
(2007) and
Wang et al., "Conservative Fragments in Bacterial 16S rRNA Genes and Primer
Design for 16S
Ribosomal DNA Amplicons in Metagenomic Studies," 4:10 PLoS ONE e7401 (2009),
each of
which is incorporated herein by reference in its entirety.
A sample used in the disclosed apparatuses and methods may comprise a
plurality of
nucleic acid molecules. A sample may comprise at least one DNA molecule or at
least one RNA
molecule. A sample may comprise at least one nucleic acid molecule formed by
restriction
enzyme digestion. A sample may comprise at least one cell (e.g., an
archaebacterial cell, a
eubacterial cell, a fungal cell, a plant cell, and/or an animal cell). A
sample may comprise at
least one microorganism. A sample may comprise one or more viruses (e.g., a
bacteriophage),
for which host cells may need to be provided. A portion of a sample at a
location on a
microarray may be a single cell or a colony grown from a single cell. For
example, individual
microorganisms or cells may be placed in microwells and the individual
microorganisms or cells
may be allowed to divide or replicate so that a colony grows within each
microwell that had an
individual microorganism or cell placed in it. A location on a microarray may
thus contain a
single microorganism species or a mixed community of microorganism strains
that support one
another's growth. A sample may comprise any suitable dilutant. In non-limiting
examples, a
36
CA 03037631 2019-03-14
WO 2018/064385
PCT/US2017/054108
sample comprises soil, sewage, fecal matter, contents of a body cavity, a
biological fluid, living
organic matter, dead organic matter, a microbial suspension, naturally-sourced
freshwater,
drinking water, seawater, wastewater, supercritical carbon dioxide, a mineral,
a gas, a buffer,
alcohol, an organic solvent, and/or an oil. In some embodiments, a nucleic
acid molecule
comprising (a) (i) a location-specific nucleotide sequence and (ii) one or
more target-specific
nucleotide sequences; or (b) one or more target-specific nucleotide sequences
(i.e., not
comprising a location-specific nucleotide sequence) is placed on at least one
location on a
microarray before a portion of a sample is placed at the location. In other
embodiments, a
nucleic acid molecule comprising (a) (i) a location-specific nucleotide
sequence and (ii) one or
more target-specific nucleotide sequences; or (b) one or more target-specific
nucleotide
sequences (i.e., not comprising a location-specific nucleotide sequence) is
placed on at least one
location on a microarray after a portion of a sample is placed at the
location. In one example, a
sample or a portion of a sample may be placed on a microarray and incubated
before a nucleic
acid molecule comprising at least one of: (i) a location-specific nucleotide
sequence and (ii) a
target-specific nucleotide sequence is placed on the microarray. In some
embodiments, a portion
of the portion of a sample may be removed from at least one location on the
microarray and
stored in a separate receptacle or the microarray may be split either before
or after the nucleic
acid molecules are placed on at least one location on the microarray.
At least one location-specific tag may comprise a nucleic acid molecule or a
portion of a
nucleic acid molecule that is pre-synthesized and placed at the location by a
liquid handling
procedure. For instance, a liquid handling procedure may be pipetting,
spotting with a solid pin,
spotting with a hollow pin, or depositing with an inkjet device. A tag may be
generated at the
location using multiple nucleic acid molecules that are pre-synthesized
separately. At least one
tag may comprise a nucleic acid molecule that is synthesized at the location
by in situ synthesis
(e.g., by inkjet printing or by photolithography).
Digital Enumeration of Species
A high density chip device comprised of a surface having high density
microwells is
described herein. Microbes from a microbiome sample may be diluted and applied
to the device
such that wells contain approximately one microbe per occupied well. The chip
then may be
37
CA 03037631 2019-03-14
WO 2018/064385
PCT/US2017/054108
incubated such that the microbes replicate within the wells. Further, a DNA
based locational
indexing system is described herein to determine what species is present in
each well. This
indexing system may involve having PCR primers preloaded into each well that
contain
addressing barcodes that identify the well and a primer sequence targeted to a
specific genetic
element (e.g., 16S) in the microbial genome that provides species information
or targets a desired
genetic sequence. After incubation, the microbial DNA is released, the PCR
primers amplify the
target bacterial DNA region, and the amplicons from the various wells on a
chip are pooled and
then may be read next generation sequencing.
The systems, kits, apparatus, and methodologies described herein may be
utilized to
perform an absolute count of the number of each microbial species or variant
in a sample. Each
well may represent a digital event which represents the presence of a single
microbe in the
original diluted sample. The locational indexing system may allow a user to
determine what
bacterial species is in the well. A unit of measurement may be "there is a
bacterial species in a
well" and may be independent of the number of bacteria in the well.
In one example, a mixed sample of microbes includes 50% Species 1, 30% Species
2, and
20% Species 3. The sample is diluted then applied to the chip such that each
occupied well has,
for the most part, one microbe. The microbe replicates. Note the replication
rate may be
different for different species. Then, the chip is processed such that the DNA
from the microbes
in the wells is released and the 16S or some other target sequence is
amplified. The DNA
amplification products from each well may be pooled and sequenced using next
generation
sequencing. The next generation sequencing data may be analyzed to determine,
for each well,
what species is in each occupied well. Many wells may not be occupied at all.
The abundance
of each species may be determined by: the total number of wells occupied by
each species
divided by the total number of occupied wells. An absolute abundance
determination may be
made by multiplying the % abundance of each species from step by the total
number of microbes
in the original sample. The sequencing data may be compared to publicly
available sequence
datasets to determine what species is in each occupied well. For example,
ribosomal RNA
sequence data is available in the SILVA rRNA database project described above.
Other
ribosomal RNA sequence databases include the Ribosomal Database Project,
Greengenes, and
the GenBank genetic sequence database, also described above.
38
CA 03037631 2019-03-14
WO 2018/064385
PCT/US2017/054108
Current methods for estimating the abundance of microbial species in a sample
involve
the use of traditional techniques such as microscopy, staining, selective
media,
metabolic/physiological screens, and cultivation using petri dishes. These
methods are often
inaccurate due to lack of specificity (microscopy, staining,
metabolic/physiological screens) or
lack of ability to account for all species in a sample (selective media,
cultivation) whereby many
species do not grow well or do not grow at all with traditional approaches.
Current molecular methods for determining the relative abundance of microbial
species
in microbiome samples involve extracting microbial DNA from samples,
performing PCR
amplification of the 16S or some other DNA region that provide species or
other information,
then performing next generation sequencing (NGS) on the resulting PCR product.
The relative
abundance of each species in the original sample is inferred from the relative
frequency of the
species specific DNA sequence in the NGS data. There are many examples in the
literature of
this type of analysis and this method underpins much microbiome research.
The problem with the current methodology is that it does not control for
different
numbers of 16S gene that may exist in different microbes, PCR bias whereby
sequences from
different microbial species may be amplified at different rates, and
sequencing bias where
sequences from different microbial species may be sequenced at different
rates. The result is that
there is a lot of uncertainty with respect to the accuracy of relative
abundance data derived using
current methodologies.
The counting of different species may be based on the presence of a species in
a single
well. This is directly related to a single microbe from the original sample
partitioning into the
well during loading. Only PCR/NGS may be used to identify what microbial
species exists in
each well. The number of sequences identified does not form part of the
calculation. Hence, it
does not matter if there is PCR, NGS, or target sequence copy number variance
or bias in the
method.
Some embodiments may have applications in microbiome research, microbial
product
discovery and development, clinical diagnostics, and any other area where
accurate counts of
microbial species in a sample are required.
39
CA 03037631 2019-03-14
WO 2018/064385
PCT/US2017/054108
Accordingly, some embodiments may provide a much more accurate measurement of
the
relative abundance of each species in a microbiome sample, and the ability to
convert this
relative abundance measurement into an absolute abundance or a direct count of
each species in
the original sample (by accounting for the dilution and/or combining with a
measurement of the
total number of microbes in the original sample). Some embodiments may provide
new
applications for high density microfabricated chips (in addition to
cultivation and screening of
microbes).
FIG. 18 is a flowchart illustrating a counting method in accordance with some
embodiments. In step 1800, a sample is obtained. In step 1802, at least one
cell is extracted
from the obtained sample. In step 1804, at least one high density microwell
array of a
microfabricated device or chip is loaded with the at least one extracted cell.
Step 1804 may
include preparing a cell concentration with the at least one extracted cell,
selecting at least one
nutrient/media, and/or selecting at least one membrane. In step 1806, at least
a portion of the
microwell array is sealed with the at least one selected membrane to retain
the cell concentration
with the microwells. In step 1808, the chip is incubated. Step 1808 may
include selecting a
temperature, determining atmosphere (e.g., aerobic or anaerobic), and/or
timing incubation). In
step 1810, the cultivated cells may be sacrificed for identification. Step
1810 may include PCR,
sequencing, and/or various data analytics. In step 1812, information about the
sample (e.g., a
microbial community structure) may be assessed and/or determined.
FIG. 19 is a diagram illustrating a counting method in accordance with some
embodiments. Panel 1900 shows examples of complex samples, specifically a
microbiome
sample 1902 and a soil sample 1904. In Panel 1906, at least one cell is
extracted from the
sample using, for example, the protocol illustrated in FIGS. 5A and 5B. In
Panel 1908, the at
least one extracted cell (and any environmental extract and/or dilutant) is
loaded on a
microfabricated device or chip with at least one high density microwell array
1910. Chip 1910
and a reagent cartridge 1912 may be loaded into an incubator 1914. The reagent
may be useful
for adding liquid to maintain nutritional requirements for growth and/or
various screening
purposes. Panel 1916 shows the output: sequences and relative abundance of
cultivated cells.
CA 03037631 2019-03-14
WO 2018/064385
PCT/US2017/054108
Droplet-Based Platforms
A discrete droplet-based platform may be used to separate, cultivate, and/or
screen in
much the same way that chips are used. A droplet is an analog of a microwell
serving as a nano-
or picoliter vessel. Droplet generation methods, especially when combined with
cell-sorter-on-a-
chip type instrumentation, may be used to separate out microbes from a complex
environmental
sample. Droplet addition may be used to feed microbes. Droplet splitting may
be used for
sequencing or some other destructive testing while leaving behind a living
sample. All the prep
work necessary for sequencing may be done in droplet format as well.
Some embodiments may be used to get microbes out of a complex environment and
into
droplets. For example, a modular system for generating droplets containing
cell suspensions
may contain one or small numbers of cells. The aqueous drops may be suspended
in a
nonmiscible liquid keeping them apart from each other and from touching or
contaminating any
surfaces. Droplets may be generated at, for example, 30 Hz in each
microchannel, which
translates into millions per day.
A drop-based microfluidic system may encapsulate, manipulate, and/or incubate
small
drops (e.g., about 30 pL). Cell survival and proliferation is noted to be
similar to control
experiments in bulk solution. Droplets may be produced at several hundred Hz,
meaning
millions of drops can be produced in a few hours. A simple chip-based device
may be used to
generate droplets and the droplets may be engineered to contain a single cell.
Some embodiments may be used to screen cells in droplets. Fluorescence
screening of
droplets post-incubation may be done on-chip and at a rate of, for example,
500 drops per
second. Droplets may be flowed through a channel at the focus of an
epifluorescence
microscope that may be configured for a number of different measurements. This
may be a
particularly effective way to do screening for metabolites as the local
concentration is quite high
on account of being confined to a very small droplet.
Some embodiments may be used to sort droplets. Once cells have been isolated,
grown,
and/or screened, they may be sorted so that useful samples may be retrieved.
Droplets may be
sorted in an analogous way to the commonly used FACS machine.
41
CA 03037631 2019-03-14
WO 2018/064385
PCT/US2017/054108
Some embodiments may be used to split droplets. Some embodiments may require
the
ability to take a sample and split it in order to send one portion to
sequencing (a destructive
process) and retain another portion that is a viable culture. There are a
number of different ways
to split droplets including, but not limited to, constructing T-junctions with
carefully calculated
dimensions that result in drops splitting as they flow by or electrowetting
(taking care not to
cause cell lysis with voltages that are too high).
Some embodiments may be used to merge droplets and/or add a reagent to a
droplet. For
example, long term incubation of cells (e.g., weeks) requires the ability to
add liquid to maintain
nutritional requirements for growth. It also may be useful to be able to add
reagents for various
screening purposes. Droplet screening relies on being able to merge a droplet
containing a
compound-code with a droplet containing a single cell. The droplets then may
be incubated
and/or returned to an assay chip to identify compounds via their codes. This
may require the
ability to precisely merge drops on an as-needed basis.
Some embodiments may be used to perform PCR in droplets. PCR may be used in
order
to ultimately sequence a specific genetic element (e.g., the 16S region) in
order to identify
microbes. This may be used to determine what type of microbe is growing in
each well. In a
droplet-based system this approach may be used to determine what microbe is
present in each
droplet as long as the correct primer sequence is designed to amplify the
right region of the
genome.
Some embodiments may be used to sequence DNA out of droplets (e.g., generated
in the
PCR step) and/or prepare DNA libraries.
Location Specific Tags for High Density Chips
A high density chip device having a surface with a high density of microwells
may be
used. Microbes from a microbiome sample may be diluted and applied to the
device such that
wells contain approximately one microbe per occupied well. The chip may be
incubated such
that the microbe replicates within the well and the resulting population
represents a single
species. A DNA-based locational indexing system may be used to determine what
species is
present in each well. This indexing system may involve having PCR primers
preloaded into each
42
CA 03037631 2019-03-14
WO 2018/064385
PCT/US2017/054108
well that contain addressing barcodes that identify the well, and a primer
sequence targeted to a
specific genetic element (e.g., 16S in the microbial genome) that provides
species information.
After incubation the microbial DNA may be released, the PCR primers may
amplify the target
bacterial DNA region, and the amplicons from the various wells on a chip may
be pooled and
then read by next generation sequencing.
The above locational indexing system may involve incorporating a different
locational
code for each well of the microchip, or multiple locational codes may be
incorporated into each
well such that the total number of codes required to code a specified number
of wells is reduced.
For example, if there are 100 wells in a chip it would require 100 codes if
there is one code per
well. The same chip could be coded with only 20 codes if two codes were read
from each well
(i.e., 10 coding for the x axis of the grid and 10 coding for the y axis).
An example of a PCR strategy to incorporate two codes per well is provided in
TABLE 2.
TABLE 2
5'-Primers Amplified PCR Products
CODE1- TARGETSEQUENCEPRIMER1-3' CODE1 ¨ TARGETSEQUENCE- CODE
3'- TARGETSEQUENCEPRIMER2 ¨ CODE2 ¨ 2
5'
An example of a PCR strategy to incorporate three codes per well provided in
TABLE 3.
TABLE 3
5'- CODE1- TARGETSEQUENCEPRIMER1-3' CODE1 ¨ TARGETSEQUENCE- CODE
3'- TARGETSEQUENCEPRIMER2 ¨ CODE2 ¨ 2- ADAPTER - CODE3
ADAPTER 5'
3'- ADAPTER' ¨ CODE3' 5'
Three oligo primers are used to make a single PCR product. Advantages to this
system
using two oligos to put a multi-partite barcode on one end of the molecule may
include, for
43
CA 03037631 2019-03-14
WO 2018/064385
PCT/US2017/054108
example, reducing maximum length of oligos needed or making extra-long
barcodes.
This approach can be generalized to incorporate n barcodes per reaction. The
approach
can also have different implementations such that the barcodes are on the same
side of the target
sequence region. NGS sequencing adaptors may be added, and the full sequence
for the
population of barcoded PCR products may be read using next generation
sequencing.
In another implementation, a fixed code may be added to indicate sample number
or plate
number and allow pooling of multiple samples/plates in a run for two barcodes
as shown in
TABLE 4.
TABLE 4
Primers Amplified PCR Products
5'- PLATEA - CODE1- PLATEA - CODE1 ¨
TARGET SEQUENCEPRIMER1 -3 ' TARGETSEQUENCE- CODE 2
3'- TARGETSEQUENCEPRIMER2 ¨ CODE2 ¨5'
In another implementation, a fixed code may be added to indicate sample number
or plate
number and allow pooling of multiple samples/plates in a run for three
barcodes as shown in
TABLE 5.
TABLES
5'- PLATEA - CODE1- PLATEA - CODE1 ¨
TARGET SEQUENCEPRIMER1 -3 ' TARGETSEQUENCE- CODE 2-
3'- TARGETSEQUENCEPRIMER2 ¨ CODE2 ¨ ADAPTER - CODE3
ADAPTER 5'
3'- ADAPTER' ¨ CODE3' 5'
Note that in all cases the position of the barcode in the sequence conveys
information
hence the CODE1, CODE2, and CODE3 barcodes do not necessarily have to be
different from
each other in a particular well.
Making oligos and printing chips using a single code coding system are high
cost. For
44
CA 03037631 2019-03-14
WO 2018/064385
PCT/US2017/054108
example, a 10,000 well chip requires 10,000 single barcodes and 10,000
separate printing cycles
to place those barcodes into the wells on the chip. If a two-code system is
used, then potentially
only 200 barcodes are required with only 200 printing cycles to manufacture
chips. This
represents a significant saving in oligo cost, printing time and printing
capital investment.
The use of dual barcoded PCR primers, followed by amplification and sequencing
analysis, to provide locational data on DNA or DNA containing moieties
randomly partitioned
onto a microfabricated chip may have high utility and relatively low cost.
FIG. 20 is a diagram illustrating an indexing system in accordance with some
embodiments. Microwell chip 2000 has N rows and M columns, thereby producing N
x M
unique indices. A location of a microwell in chip 2000 may be considered to
have the
coordinates (N , M) . Each column has a common reverse primer
sequence (e.g., R1, R2, R3,...RM), and each row has a common forward primer
sequence (e.g., Fl, F2, F3,...FN). For example, a unique tag targeted to a
specific genetic
element in, for example, 16S ribosomal ribonucleic acid (rRNA) may include
forward primer
sequence F515 and reverse primer sequence R806. Following PCR of chip 2000,
the presence of
the targeted genetic element may be mapped back to a unique microwell of
origin based on the
presence of a forward primer sequence and a reverse primer sequence. For
example, the
presence of F515 and R806 directs a user to the microwell with coordinates
(515, 806) in
chip 2000.
Variability Reduction for PCR Amplification Product Across Microwells
Containing Bacteria
A DNA-based locational indexing system may be used to determine what species
is
present in each well. This indexing system may involve having PCR primers
preloaded into each
well that contain addressing barcodes that identify the well, and a primer
sequence targeted to a
specific genetic element (e.g. 16S) in the microbial genome that provides
species information.
After incubation the microbial DNA may be released, the PCR primers amplify
the target
bacterial DNA region, and the amplicons from the various wells on a chip are
pooled and then
read by next generation sequencing.
Some embodiments for limiting the variability in the amount of PCR product
across wells
CA 03037631 2019-03-14
WO 2018/064385
PCT/US2017/054108
may include limiting amount of PCR primer in the well during manufacture of
the chip such that
for the majority of possible sample DNA concentrations the amount of PCR
primer will limiting
in the DNA amplification reaction, hence the amount of PCR product produced
will be less
variable across wells.
Some embodiments for limiting the variability in the amount of PCR product
across wells
may include limiting the number of PCR cycles on the chip to less than 3
cycles, or less than 5
cycles, or less than 10 cycles or less than 15 cycles, or less than 20 cycles
or less than 25 cycles
or less than 30 cycles such that the amount of PCR product produced will be
less variable across
wells vs. a full cycle PCR amplification protocol.
Some embodiments for limiting the variability in the amount of PCR product
across wells
may include limiting the amount of nucleotides in the reaction mix so that the
number of PCR
amplicons produced is a more related to the amount of nucleotides than the
amount of DNA in
the original sample. Microwells with a large amount of target DNA will exhaust
the nucleotides
early in the cycling process while microwells with a small amount of target
DNA will exhaust
the nucleotides later in the cycling process, but produce around the same
amount of amplification
product.
Some embodiments for limiting the variability in the amount of PCR product
across wells
may include limiting the amount of nutrient available to microbes growing in
the wells such that
cells will replicate until the media is exhausted then stop replicating.
Some embodiments for limiting the variability in the amount of PCR product
across wells
may include placing a dye in each well that identifies PCR product such that
the signal gets
brighter as more PCR product is produced. The intensity of the dye during each
PCR cycle may
be monitored, and a sample may be taken from the well once the desired signal
intensity is
observed.
Some embodiments for limiting the variability in the amount of PCR product
across wells
may include using mixtures of hybridization beads covered with oligos
complementary to each
well-specific bar code to selectively hybridize amplified DNA from each well.
Once the beads
are saturated unbound DNA may be washed away releasing bound DNA from the
beads. The
46
CA 03037631 2019-03-14
WO 2018/064385
PCT/US2017/054108
amount of DNA from each well will then be normalized at the saturation limit
of the beads.
Some embodiments for limiting the variability in the amount of PCR product
across wells
may include incubating chips for a long period of time such that the fast
growing microbes
rapidly fill wells and cease replicating, and the slower growing microbes
gradually fill wells and
cease growing once approximately the same number of cells are in the wells
In some embodiments, use of barcoded primers and next generation sequencing
(NGS)
within the context of the chip format and method may be used to identify which
species is
growing in which well on the high density microchip. When an approximately
equal number of
bacteria occupy each microwell in the chip, the signal from each well in the
NGS data may be
approximately the same.
For example, in a typical NGS run generating 12 million sequence reads, if 24
chips are
sequenced in the run, each having 10,000 microwells, and there is the same
number of bacteria
per well, there is on average 50 reads per well.
However, different bacteria grow at different rates so it is likely that some
wells have few
bacteria and some wells will have many bacteria. This potentially skews the
NGS run so much
that the wells with few bacteria are not detected in the NGS analysis.
Hence, in the example of a typical NGS run generating 12 million sequence
reads, 24
chips are sequenced per run, each having 10,000 microwells, half of which have
100 times more
bacteria in them than the other half. The probability that the bacteria in the
slow growing wells
are detected is markedly reduced. In this case:
(10,000 x 24)/2 x 100 = 12,000,000
(1)
(10,000 x 24)/2 = 120,000
(2)
The low frequency wells are represented at 1% of total. So of 12,000,000 reads
in an
NGS run 120,000 will be from the low frequency wells ¨ i.e. average of 1 read
per well.
To minimize the impact of this phenomenon novel methods need to be developed
to help
equalize the amount of PCR product across wells so that all wells are detected
in the NGS run.
47
CA 03037631 2019-03-14
WO 2018/064385
PCT/US2017/054108
Silicon-Based Microwell Chips for Microbial Isolation, Growth, Screening, and
Analysis
A microfabricated device or chip may be composed at least in part of silicon
instead of or
in addition to plastic, glass, and/or polymers to allow for electrical
measurements on a well-by-
well basis. For example, the walls of each well may be isolated to create
microcapacitors. In
another example, an FET in each well such that the gate surface is exposed to
the contents of the
well. Instead of a purely silicon-based chip, thin metal layers may be
generated on top of an
existing chip by plating, vapor deposition, and/or arc/flame spraying. This
may add more
functionality to a chip, utilize alternate methods of manufacture which may be
cheaper and/or
cleaner, and/or allow miniaturization for handheld and/or portable devices.
Some embodiments may allow for monitoring growth by electrical measurement.
Impedance monitoring may be applied to measure microorganism (e.g., bacterial)
growth. For
example, impedance across a tube containing Escherichia colt (E. colt) is
compared to cell
counts therefrom in Ur et al., "Impedance Monitoring of Bacterial Activity,"
8:1 J. Med.
Microbiology 19-28 (1975), which is incorporated herein by reference in its
entirety.
Measurements may be taken on other types of bacteria including Pseudomonas,
Klebsiella, and
Streptococcus to demonstrate the effect is general. Wells may be filled with
different media in
order to test growth conditions across different formulations.
Some embodiments may allow for screening by electrical measurement. Electrical
measurements may be made on a well-by-well basis allowing for screening. One
example would
be pH. There are a number of different ways to get a pH-dependent response
from the gate of a
device in a well including, but not limited to, ISFETs and pH-meters. An array
of wells with
embedded pH sensors may determine, electrically, which wells contain microbes
that are
producing acidic or basic metabolites. A simple example is screening for the
production of lactic
acid from lactose. Bacteria is diluted out into wells, grown, and then fed
lactose. Wells that
record a drop in pH contain microbes capable of metabolizing lactose into
lactic acid.
Some embodiments may allow for electrical measurements of redox probes.
Another
way to leverage electrical measurements is to look at how bacteria in wells
affect a known redox
probe. Essentially, a system with well-defined response may be measured in the
presence of
bacteria and deviations from expected behavior may be attributed to the
bacterial samples. A
48
CA 03037631 2019-03-14
WO 2018/064385
PCT/US2017/054108
typical redox probe is something like ferricyanide; [Fe(CN)6]3-/4-. The
reduction of
ferricyanide to ferrocyanide is very well characterized such that small
changes in behavior,
particularly around electron transfer from the electrodes, are discoverable.
This system is "label
free" as it detects without having to directly modify the bacteria themselves.
Antibodies that can recognize microorganisms (e.g., E. coil) may be
immobilized on ITO
electrodes. Electron transfer resistance may be measured from the electrodes
to a ferricyanide
containing solution. E. coil binding to the electrode surface increases the
resistance proportional
to the concentration of E. coil on the surface. This is one example of a
family of measurements
that may be made to detect specific types of organisms or metabolites using
redox probes.
Working in silicon (or at least metals or metallized plastics) provides
advantages
including, but not limited to, less expensive production of chips (e.g., by
piggybacking on
existing technologies); integrated detection capability allowing small and/or
portable versions;
additional measuring capabilities not present in other materials (e.g., LCR,
CV, etc.); integration
of newly discovered chip-based detection modalities into existing devices; and
the combination
of electrical measurements and sequencing. These advantages would benefit any
customer using
interferometric detection.
Releasable Barriers to Protect Well-Specific Chemistries on a Chip
FIGS. 21A-21E are diagrams illustrating a chip with well-specific chemistries
in
accordance with some embodiments. In FIG. 21A, a microfabricated device or
chip is shown
with a plurality of microwells. In FIG. 21B, microwell-specific chemistries
have been disposed
in each microwell of the chip. In FIG. 21C, a sealant has been applied over
the microwell-
specific chemistries in each microwell of the chip, thereby preventing
interaction of the
chemistries with further additions to the wells. In FIG. 21D, samples are
loaded, and
experiments are performed on the samples in the microwells. In FIG. 21E, a
trigger (e.g., heat)
releases the microwell-specific chemistries for interaction with samples in
the wells.
Microwell chips may be manufactured, be cleaned, and/or have surfaces treated.
The
specific chemistries may be prepared separately and then deposited into wells
by, for example,
using a method and/or device that allows a specific set of chemicals to be
directed to a specific
49
CA 03037631 2019-03-14
WO 2018/064385
PCT/US2017/054108
well (or wells). A sealant then may be applied to protect the various
chemistries from the
environment and/or be removed/released/disbursed with some defined, external
trigger.
A microfabricated device or chip may be manufactured to a specific design, for
example,
cleaned and/or surface treated to improve wetting. PCR primers may be printed
or pin-spotted
into specific wells. The chip may be allowed to dry, and then a wax layer may
be deposited by
evaporation from an ethanol solution. Optimal concentration may be about 1%
v/v. Molten wax
may be applied directly or an aqueous or alcohol wax solution may be sprayed.
Alternatively,
spin coating or vapor deposition may be used. Various waxes may be used
including, but not
limited to, glyceryl stearate with and without polyethylene glycol, cetearyl
alcohol, 1-
hexadecanol, glyceryl ester of stearic acid, ceteareth-20 (CAS Registry No.
68439-49-6), and
some commercial products including, but not limited to, LotionproTM 165
(available from
Lotioncrafterg, Eastsound, Washington) and PolawaxTM (available from Croda,
Inc., Edison,
New Jersey). The underlying chemistry later may be released by, for example,
heating until the
wax melts. For these compositions it will be between 50 C and 70 C. It is
important to be low
enough not to damage any chemical component or to boil our aqueous solutions.
The key concept is of well-specific chemistry that is walled off from the chip
until the
experimenter triggers release. This method may be used for barcoding in wells,
but it also may
be applied more broadly to a whole range of different problems. Different
chemistries that may
be useful to seal on a chip include, but are not limited to, antibiotics,
fluors, dyes, PCR primers,
lysis-promoters, antibodies, and/or tests for various metabolites. While wax
is a good way to
seal things that can later be released by heating, other materials may be used
to seal and release
upon exposure to light, sonication, and/or some other trigger. The advantage
of this method over
simply adding reagents to the chip is one of control on a well-by-well basis.
A similar effect
might be achieved by printing chemicals into wells after doing microbial
experiments, but this
introduces problems with time (the print run may be as long as a day) and the
fact that it is
impossible to expose every well for the same amount of time if each well is
filled individually
after the microbes are on the chip. With a release mechanism every well can be
exposed at the
same time. In one example, wax may be deposited onto chips by solvent casting.
CA 03037631 2019-03-14
WO 2018/064385
PCT/US2017/054108
Isolated Microwells for Simplicity and/or Controlling Relative Abundance
A high-density chip device may comprise a surface having high-density
microwells.
Microbes from a microbiome sample, or other cell types, may be diluted and
applied to the
device such that wells contain approximately one microbe or cell per occupied
well. These
microwells may be sealed with semi-permeable membranes that allow nutrients to
diffuse into
the microwells but prevent all or at least some of the microbes or cells from
moving out of the
microwells.
A sample of microbes or cells may be prepared and then sealed into a chip
using an
impermeable or only-gas-permeable membrane. No reservoir of liquid sits on top
of the chip or
membrane and hence only the nutrient in the well at the time of sealing is
available to support
growth of the microbes or cells in the microwell. Two reasons for this feature
include:
(1) simplicity in construction and workflow as the device need not have semi-
permeable
membranes or reservoirs, nutrients do not have to be added, and there is less
potential for
contamination; and (2) a check on the relative abundance of fast-growers by
limiting their access
to nutrients. For a sample containing some fast-growers and some slow-growers,
the fast-
growers will rapidly be resource-limited in their respective microwells and
stop or slow growth
while the slow-growers continue to grow. This provides for slow growers to be
represented at a
higher relative abundance in the population of microbes across the chip,
compared to the case
where the fast growers do not have a limiting amount of nutrient. This becomes
important for
downstream processing when sequencing everything on a chip. It also provides a
better
detection limit for rare species as the rare species are not outgrown by fast
growing species to a
point that limits the ability of the system to detect them.
Current methods attempt to get all species to grow whether they are fast- or
slow-growing
by nature. This has the inevitable result that fast-growers dominate
communities and only
increase in relative abundance with time. Many types of downstream analysis
such as
sequencing or fluorescence screening cannot resolve every species in a given
population but only
those above a certain limiting relative abundance. If the goal is to preserve
diversity and detect
rare species, then the fast-growers need to be limited in some way.
For an example that demonstrates this idea, consider the simple case of a
sample
51
CA 03037631 2019-03-14
WO 2018/064385
PCT/US2017/054108
containing two species: one doubles every day and the other doubles every week
as shown in
TABLE 6. If the slow-grower is rare to begin with, at 5% relative abundance,
it soon becomes
very rare as both species grow.
TABLE 6
Fast grower 19 38 76 152 2432 311296
Slow grower 1 1 1 1 2 3
Total 20 39 77 153 2434 311299
Rel. ab. fast 0.950 0.974 0.987 0.993 0.999 1.000
Rel. ab. 0.050 0.026 0.013 0.007 0.001 0.000
slow
If the fast-growers are limited by competition for nutrition and/or physical
space to grow,
then the relative abundance of the slow-growers will start to increase after
some time has elapsed
as shown in TABLE 7.
TABLE 7
Fast grower 19 38 50 50 50 50
Slow grower 1 1 1 1 2 3
Total 20 39 51 51 52 53
Rel. ab. fast 0.950 0.974 0.980 0.980 -- 0.962 --
0.943
Rel. ab. slow 0.050 0.026 0.020 0.020 0.038 0.057
High Density Microfabricated Arrays for Biobanking Cells
Biobanks are designed to give researchers access to a large number of samples
from a
large population in order to drive certain types of research, such as disease-
related biomarker
discovery. The current state of the art in biobanking provides for samples to
be stored in tubes or
low-density plate format such as a 96-well or 384-well plate. This works when
the number of
samples to be stored is relatively low in number and the samples themselves
are discrete, isolated
populations. Current approaches to biobanking become very cumbersome when
storing samples,
such as microbiome samples, where the number of samples may be high and the
number of
different species or variants in each sample may extend from hundreds to
thousands or many
52
CA 03037631 2019-03-14
WO 2018/064385
PCT/US2017/054108
millions per sample. Using current methods, a laborious isolation protocol
must be implemented
to separate out individual species or variants either prior to or subsequent
to the storage step in
order to access a desired species or variant.
The systems, kits, apparatus, and methodologies described herein may be
applied to
biobank cells, microbes, viruses, and other biological entities. A high-
density chip device
comprised of a surface having high density microwells may have thousands,
hundreds of
thousands, or millions of microwells per chip. For example, microbes from a
microbiome
sample (or another biological entity such as a different type of cell or a
virus) may be diluted and
applied to the device such that wells contain approximately one microbe per
occupied well. The
chip then may be incubated such that the microbe replicates within the well
and the resulting
population represents a single species. A nucleic acid-based locational
indexing system may be
utilized to determine what species is present in each well. This indexing
system may involve
having PCR primers preloaded into each well. The PCR primers may contain
addressing
barcodes that identify the well, and a primer sequence targeted to a specific
genetic element (e.g.,
16S) in the microbial genome that provides species information or targets a
desired genetic
sequence. After incubation the microbial DNA is released, and the PCR primers
amplify the
target bacterial DNA region. The amplicons from the various wells on a chip
may be pooled and
then read by next generation sequencing.
Using high-density microfabricated chips for biobanking provides for a
multitude of
.. species or variants within each sample to be stored as separate populations
without the need to
implement a laborious isolation protocol either before or after storage. Using
the DNA-based
locational indexing system or a custom assay enables a simpler, generic
approach to identifying
genetic signatures or characteristics of the contents of each microwell to
give information such
as, for example, species information. Additionally, the chip devices provide
for an extremely
space effective method of storing cell isolates. For example, a single
microscope slide
dimensioned chip with 100,000 wells occupies substantially less space that the
corresponding
traditional storage formats. The chip format also may be useful for properly
archiving and
curating samples and/or for managing subject (e.g., patient) information
databases by having one
chip contain many different samples from a single subject.
53
CA 03037631 2019-03-14
WO 2018/064385
PCT/US2017/054108
In order to bank cells using this apparatus, cells may be disposed and/or
positioned in
microwells of the apparatus. The cells in the microwells may be treated to
ameliorate the impact
of storage then placed in appropriate storage conditions. For example, cells
may be treated with
agents such as glycerol to ameliorate the impact of freezing. Then the chip
may be placed in
appropriate storage conditions such as a freezer. The cells may be dehydrated,
lyophilized,
and/or freeze dried, and the chip may be placed in appropriate conditions to
safeguard the dried
cells. Additional structures may be added to the chip to further enhance its
utility as a storage
device. For example, a membrane or another structural element may be placed on
top of the chip
to seal at least some of the wells prior to storage.
A chip may be loaded with cells such that a portion of microwells on the chip
are
occupied by approximately one cell each. The chip is incubated to allow for
replication of cells.
The chip and its contents are substantially replicated, and either the
original chip or the copy chip
is used to identify the cells or species or gene signatures present in each
well of the chip (by, e.g.,
using the locational indexing system described above). The chip is treated
and/or stored in
appropriate conditions. The replication step and/or the identification step
may take place after
storage rather than before storage.
One or more cells from each strain of a pre-existing set of isolated strains
may be
disposed into separate microwells on a chip. The position of the microwell
into which each
strain or cell type was placed may be recorded, and the chip may be treated
and/or stored in
appropriate conditions.
A version of the chip may be created in which preservative chemistry is
sequestered
underneath a wax barrier in each well. Isolates may be allowed to grow sealed
up inside a chip
and then preserved at a later date by heat-induced release of the
preservatives before banking.
Such apparatus may be used to store/biobank mixed microbiome samples such as
microbiome samples from soil, human gut, seawater, oral cavity, skin, etc. A
chip may be used
to store other types of biological entities such as fungi, archaebacteria,
human cells (including
reproductive cells), animal cells, and viruses.
The DNA locational indexing system may be used across all biological entity
types to
54
CA 03037631 2019-03-14
WO 2018/064385
PCT/US2017/054108
generate information regarding the content of each well. An entire chip may be
screened for
desired activity using custom assays such as antibody- or substrate-based
assays. For example, a
chip with banked populations of T cells may be screened for a particular
immunological activity.
In one illustrative example, human stool microbiome samples may be collected
from
study participants. The stool microbes from each individual may be biobanked
on chips to
maintain both a record of that individual's microbiome as well as a sample of
the microbiome to
use as a source of target microbes at a later date. In another example, mixed
populations of T
cells or other immunological cells may be sampled from individuals during
clinical or research
studies or as part of a therapeutic workflow (e.g., cell therapy using ex vivo
treated cells). In yet
another example, soil biomes may be stored in conjunction with seed banking.
High Resolution Picking
A high accuracy/precision picking apparatus or system may be designed to
execute
various picking functions from and/or to microwells on a microfabricated chip
described above.
A target substrate or chip may be a microscope slide format (approximately 25
mm x 75 mm x 1
mm) with injection-molded features on one surface. Microwells may be arranged
in a grid
pattern with about 4-8mm well-free edge around the edges of the chip. Well
size and spacing
may be determined based on picker capability. Microwells may be square with a
size from about
[tm to about 200 [tm along each edge and spacing in from about 25 [tm to about
100 [tm
between well edges. Microwells may be circular or hexagonal instead. Well
depths may be from
20 about 25 [tm to about 100 [tm. For example, a 75mm x 25mm slide with a 7
mm edge, 100 [tm
square wells with 100 micron edge-to-edge spacing will have about 16,775
microwells.
A high accuracy/precision picking system may be designed to execute various
picking
functions from and/or to portions of a membrane corresponding to microwells on
a
microfabricated chip, as described above. A membrane may be a thin sheet that
has previously
25 been used to seal growing bacteria into microwells. When peeled off the
chip the membrane may
retain an imprint of the microwell array as well as a sample of bacteria on
its surface after
separation from the chip. Thus, the peeled membrane may act as a replicate of
the bacteria
growing in the chip.
CA 03037631 2019-03-14
WO 2018/064385
PCT/US2017/054108
A high resolution picker may receive data input from a user. The input may
include at
least one pair of chip microwell coordinates such that the picker picks from
and/or to the at least
one input pair of coordinates. A sterilization routine may be performed
between cycles. A high
resolution picker may be capable of operating in an anaerobic chamber.
A high resolution picker system may receive a chip and align the picker with
the chip, for
example, using fiducial markers and/or reference wells. The picker may pick,
for example, cells
growing in microwells on the chip into, for example, 96- or 384-well plates
containing growth
media. The picker may include a single picking pin or a plurality of picking
pins.
A high resolution picker system may receive a membrane and align the picker
with the
membrane, for example, using reference well marks on the membrane. The picker
may pick, for
example, cells from the membrane into, for example, 96- or 384-well plates
containing growth
media. The picking pin(s) may have a different shape (e.g., mushroom-shaped)
and/or surface
(e.g., texture) for picking from a membrane. The system also may include one
or more
mechanisms (e.g., a floating pin and/or vacuum) to hold and/or flatten the
membrane.
A high resolution picker system may enable chip replication via a chip-to-chip
transfer.
The picker may receive and align a first chip based on fiducial markers and/or
reference wells.
The picker also may receive and align a second chip based on fiducial markers
and/or reference
wells. The picker may transfer, for example, cells growing in microwells on
the first chip to
microwells on the second chip.
A high throughput system for automatically picking a target species of a
plurality of
species of at least one biological entity cultivated in a microfabricated
device may include a port
for receiving the microfabricated device. The microfabricated device defines a
high density
array of microwells. Each microwell of the high density array of microwells is
configured to
isolate and cultivate at least one species of the at least one biological
entity and includes at least
one tag of a plurality of unique tags. Each tag of the plurality of unique
tags includes a nucleic
acid molecule, which includes a target-specific nucleotide sequence for
annealing to the at least
one biological entity and a location-specific nucleotide sequence correlating
to at least one
microwell of the high density array of microwells. The system also includes a
high-resolution
picking apparatus with at least one protrusion for picking the at least one
biological entity from
56
CA 03037631 2019-03-14
WO 2018/064385
PCT/US2017/054108
at least one microwell of the high density array of microwells. The system
further includes an
input device for receiving an indication of at least one target-specific
nucleotide sequence and at
least one processor communicatively coupled to the input device and the high-
resolution picking
apparatus. The at least one processor acquires the indication of the at least
one target-specific
nucleotide sequence from the input device, compares the at least one target-
specific nucleotide
sequence to the plurality of unique tags, determines at least one microwell of
the high density
array of microwells including the target species based on the comparison, and
controls the high-
resolution picking apparatus to pick the at least one biological entity from
the at least one
determined microwell of the high density array of microwells.
A high throughput system is disclosed for automatically picking a target
species of a
plurality of species of at least one biological entity cultivated in a
microfabricated device. The
microfabricated device defines a high density array of microwells, each
microwell of the high
density array of microwells being associated with at least one unique primer
of the plurality of
unique primers. The system includes a port for receiving a membrane removed
from the
microfabricated device, the membrane having sealed each microwell of the high
density array of
microwells to retain the at least one biological entity in the high density
array of microwells,
such that portions of the at least one biological entity corresponding to the
high density array of
microwells remain attached to the membrane following removal of the membrane.
The system
also includes a high-resolution picking apparatus including at least one
protrusion for picking the
at least one biological entity from at least one membrane location
corresponding to at least one
microwell of the high density array of microwells, an input device for
receiving an indication of
at least one target-specific nucleotide sequence associated with the target
species, and at least
one processor communicatively coupled to the input device and the high-
resolution picking
apparatus. The at least one processor acquires the indication of the at least
one target-specific
nucleotide sequence from the input device, compares the at least one target-
specific nucleotide
sequence to the plurality of unique tags, determines at least one membrane
location
corresponding to at least one microwell of the high density array of
microwells comprising the
target species based on the comparison, and controls the high-resolution
picking apparatus to
pick the portions of the at least one biological entity from the at least one
determined membrane
location.
57
CA 03037631 2019-03-14
WO 2018/064385
PCT/US2017/054108
The above-described embodiments can be implemented in any of numerous ways.
For
example, embodiments may be implemented using hardware, software or a
combination thereof
When implemented in software, the software code can be executed on any
suitable processor or
collection of processors, whether provided in a single computer or distributed
among multiple
computers.
Further, it should be appreciated that a computer may be embodied in any of a
number of
forms, such as a rack-mounted computer, a desktop computer, a laptop computer,
or a tablet
computer. Additionally, a computer may be embedded in a device not generally
regarded as a
computer but with suitable processing capabilities, including a Personal
Digital Assistant (PDA),
a smart phone or any other suitable portable or fixed electronic device.
Also, a computer may have one or more input and output devices. These devices
can be
used, among other things, to present a user interface. Examples of output
devices that can be
used to provide a user interface include printers or display screens for
visual presentation of
output and speakers or other sound generating devices for audible presentation
of output.
Examples of input devices that can be used for a user interface include
keyboards, and pointing
devices, such as mice, touch pads, and digitizing tablets. As another example,
a computer may
receive input information through speech recognition or in other audible
format.
Such computers may be interconnected by one or more networks in any suitable
form,
including a local area network or a wide area network, such as an enterprise
network, and
intelligent network (IN) or the Internet. Such networks may be based on any
suitable technology
and may operate according to any suitable protocol and may include wireless
networks, wired
networks or fiber optic networks.
The various methods or processes outlined herein may be coded as software that
is
executable on one or more processors that employ any one of a variety of
operating systems or
platforms. Additionally, such software may be written using any of a number of
suitable
programming languages and/or programming or scripting tools, and also may be
compiled as
executable machine language code or intermediate code that is executed on a
framework or
virtual machine.
58
CA 03037631 2019-03-14
WO 2018/064385
PCT/US2017/054108
Bacterial Community Relationship Determination
In some embodiments, the systems, kits, apparatus, and methods described above
may be
used to determine bacterial community relationships. The microwell array on
the
microfabricated device (or chip) with locational indexing system described
herein (e.g., by
placing in each microwell oligonucleotides containing coding information that
identifies their
specific microwell, as well as a primer sequence that targets a nucleic acid
sequence) may be
used to analyze syntrophic or other types of inter variant or inter species
relationships in a
microbiome sample in a massively parallel way.
In accordance with some embodiments of the present invention, a method of
analyzing a
sample including a population of biological entities is provided. The method
utilizes at least one
microfabricated device having a top surface defining an array of microwells,
wherein a plurality
of the microwells of the array of microwells each comprise a unique tag
nucleic acid molecule
including: (1) a target-specific nucleotide sequence for annealing to a target
nucleic acid
fragment of one or more biological entities of interest that may be present in
the microwell, and
(2) a location-specific nucleotide sequence for identifying the location of
the microwell on the
microfabricated device. In the method, a sample is loaded onto the
microfabricated device such
that at least some microwells of the plurality of microwells each include more
than one cell of a
biological entity. An amount of a nutrient is also loaded into each of the
plurality of microwells.
This can be done as a separate step, or the nutrient and the biological
entities can be loaded
together simultaneously. The microfabricated device with the loaded microwells
is incubated at
predetermined conditions. Then amplification is performed on-chip in each
individual microwell
of the plurality of microwells, e.g., by performing an PCR amplification on a
selected genetic
material of the cells of the biological entities (such as genomic DNA of
bacteria, fungi, or
eukaryotic cells) obtained from the incubation, thereby obtaining first
amplicons in the plurality
of microwells. It is possible that the amplification may not be successful in
each and every
microwell where the amplification is performed. The first amplicons are
collected/pooled from
the plurality of microwells, and sequenced to obtain sequencing data. Based on
the sequencing
data and the unique tag nucleic molecule included in each of the plurality of
microwells, the
59
CA 03037631 2019-03-14
WO 2018/064385
PCT/US2017/054108
biological entities present in at least a subset of the plurality of
microwells is identified. In some
embodiments, based on the identification of the biological entities present in
each of the plurality
of microwells, a presence or absence of a relationship is determined between
at least two
different types of biological entities in the population of biological
entities contained in the
sample. The relationship can be a dependent relationship, a symbiotic
relationship, or a
destructive relationship. The presence or absence of a relationship can be
determined by
comparing across the plurality of microwells which types of biological
entities are present or not
present in the same microwells after incubation.
For example, cells isolated from a complex microbiome sample (i.e. containing
one or
more species or variants) can be distributed into microwells of a
microfabricated device with
proper dilution and a distribution method that results in, on average, more
than one cell per
microwell. The distribution method could, for example, be by applying a
solution containing
microbes onto the array such that microbes are randomly distributed into
microwells. The
concentration of microbes in the solution can be adjusted to target a desired
average number or
distribution of bacteria per microwell. Alternatively, microbes could be
placed directly into
microwells with a device such as a cell sorter.
The target average number of cells per microwell can be varied depending on
the number
of the microbes in the sample and the complexity of the dependence being
assessed. For
example, it may be two cells per microwell, 5 cells per microwell, 10 cells
per microwell, 50
cells per microwell, 100 cells per microwell, or other number of cells per
microwell. In some
embodiments, the average number of cells per microwell is between 2 and 20. In
other
embodiments, the average number of cells per microwell can be between 5 and
50, between 10
and 100, etc. As the distribution method may have variations, there may be
microwells with no
cells in them, some microwells with one cell in them and some microwells with
more than the
desired number of cells in them.
In embodiments of the method, the population of biological entities in the
sample
comprise bacteria. The bacteria can include bacteria of different strains,
different species, or
different genera. The population of the biological entities can include a
collection of
microorganisms naturally occurring in a specific environment. For example, the
biological
CA 03037631 2019-03-14
WO 2018/064385
PCT/US2017/054108
entities can be a collection of microorganisms obtained from a human stool,
human gut, human
skin, human nasal cavity, vagina, soil, rhizosphere, water, etc. In some other
embodiments, the
biological entities can include eukaryotic cells.
After loading on the microfabricated device, the cells can be cultivated by
incubating the
microfabricated device at suitable conditions. The incubation can be performed
in various ways.
For example, prior to incubation, a membrane can be applied on the top surface
of the
microfabricated device to retain the cells loaded in the microwells. The
nutrient can be pre-
loaded in the microwells and retained by the membrane. In some embodiments,
the nutrient can
be loaded in a reservoir provided on the membrane and external to the
microwells. The
membrane is permeable to the nutrient and allows the nutrient to migrate from
the reservoir into
the microwells.
After incubation, the microfabricated device can be replicated or split as
described
previously to make a replicate of the contents of the microwells by removing a
small sample
from each microwell. On-chip PCR or other means of nucleic acid amplification
can then be run
to generate libraries of amplification product or amplicons with appropriate
indexing such that
one can generate a list of the cell contents (genus, species, strains,
variants or other) of each
microwell for analysis.
By observing which genus, species, or other types of microbes grow with each
other in
the same microwells across the array of microwells of the microfabricated
device, potential
dependencies or relationships that exist within that microbiome can be
determined. For example,
the relationship can be a dependent relationship, a symbiotic relationship, an
inhibiting
relationship, or independence. For illustration, a dependent relationship
between two species is
one where one or both species are dependent upon the other in order to
survive, thrive or
proliferate. When both species depend on each other to survive, thrive or
proliferate, they are
considered interdependent. In the case where the first species is dependent
upon the second, it
will be for the most part only detected growing in the presence of the second
species. In the case
where the first and second species are dependent upon each other, neither
species will be found
growing in the absence of the other. A symbiotic relationship between two
species is where a
first species can survive, thrive or proliferate in the absence of a second
species, but grows or
61
CA 03037631 2019-03-14
WO 2018/064385
PCT/US2017/054108
proliferates more favorably in the presence of the second species. An
inhibiting or destructive
relationship between two species is one where one species inhibits the growth
of or kills the
second species. If a first species is inhibited by the second species, then
the first species will not
be detected, or only be detected at low levels whenever the second species is
present.
Independence (or lack of a relationship, absence of a relationship) between
two species refers to
the situation where both the first and second species are able to grow
successfully both together
and separately. Both the first and second species will be detected growing
both together and
separately.
One could employ various abundance thresholds to analyze the result from the
microwells to determine the potential relationship between the two species.
For example, one
could consider an abundance of a first species of less than 1% to be
equivalent to zero; or an
abundance of a first species of less than 5% to signify inhibition. The exact
nature and level of
the cut-offs will vary between different biological systems and compositions.
The method can be
used to analyze inter species relationships across an entire microbiome
containing many
hundreds or thousands of different components/species/variants.
Consider a community with two elements or species A and B. They are loaded on
a chip,
grown, and then indexed and sequenced. The sequence data can be studied to
determine which
species grows with which other species and therefore provides insights into
their relationships
and dependencies. If A and B can coexist, the expected result is some
microwells with A without
B, some with B without A, and some with both A and B. If A destroys B, then
there will be
multiple instances of A, multiple instances of B and minimal or no instances
of A + B. If A and
B has a true mutual symbiotic relationship and there will be multiple examples
of A + B and
minimal or no examples of A or B alone.
More particularly, to determine a relationship between cells of two biological
entities of
interest, say Microbe A and another microbe (Microbe B, Microbe C, etc.), in a
microbiome
sample, the following example procedure can be used. The procedure is only
illustrative and
does not limit the invention.
1. Take the sample, such as soil.
62
CA 03037631 2019-03-14
WO 2018/064385
PCT/US2017/054108
2. Separate the microbial cells in the soil from the rest of the soil to
produce a suspension
of microbial cells. This can be done by filtering and centrifugation using a
medium such as
nycodenz.
3. Count microbial cells in the suspension using techniques such as counting
cells in a
cell counting slide (such as a haemocytometer) under a microscope or with a
flow cytometer.
4. Take an microfabricated device as described herein, where each microwell is
preloaded
with a PCR primer that incorporates i) a barcode nucleotide sequence that are
specific to the
individual microwell (e.g., the row and column number of the microwell, or
other relative
location of the microwell on the microfabricated device), and ii) a nucleotide
sequence that
targets the 16S rRNA gene region of the bacteria. (For analysis of microbiome
comprising fungi,
the nucleotide sequence can be targeting ITS gene region) The primers can be
protected by a
wax coating. Both the primers and the wax can be printed into the microwells
using high
resolution printing devices.
5. Dilute the cell suspension so that when loaded onto an microfabricated
device of the
present invention, the expected (or average) number of cells per occupied
microwell is 5. Some
microwells can have fewer than five cells and some can have more. The cells
can be suspended
in appropriate growth media.
6. Load the microwells by pipetting the dilute cell suspension on the surface
of the
microfabricated device such that all microwells are covered with liquid. A
portion of the
bacterial suspension enters the microwells.
7. Either: (a) place an impermeable or a gas permeable membrane over the
microwells, or
(b) place a permeable membrane over the microwells, then a nutrient reservoir
with nutrient.
8. Incubate the microfabricated device for a period and at suitable conditions
(temperature, humidity, atmosphere, etc.) appropriate to the species being
studied.
9. Replicate the microfabricated device using methods described herein. Add
membrane
to one or both replicates, and optionally incubate some more.
10. Take the replicate of the chip containing the PCR primers, immerse in
liquid nitrogen
63
CA 03037631 2019-03-14
WO 2018/064385
PCT/US2017/054108
then remove from liquid nitrogen, then remove membrane and add a PCR mix to
the microwells,
cover the microwells with oil and perform on-chip PCR by amplifying genomic
DNA of the
bacteria in a flatbed PCR thermocycler.
11. Pool the PCR products or amplicons from the microwells on the chip, and
add
sequencing adaptors and sample specific barcodes for a second PCR reaction.
12. Sequence the amplicons in a Next Generation Sequencing system such as
Illumina
Miseq. This can give a read out of the majority of the location-specific
barcodes on the
microwells and the 16S sequence will indicate which microbes are growing in
which microwells.
Given the nature of 16S analysis this information will generally be at the
genus or species level.
13. Analyze results to determine what species or genera grew in each
microwell.
14. Identify those wells containing Microbe A. Identify for each microwell
containing
Microbe A what other species are growing in the same microwell. As there are a
limited number
of species in each well it is possible to identify which additional species
may support the growth
of Microbe A. For example, the result may be that Microbe A only grows in the
presence of
Microbe B, Microbe C or Microbe D.
15. The experiment can be repeated using a higher dilution level to target
only 2 microbes
per well ¨ this can provide confirmatory information.
The method described above can be used to map relationships across an entire
microbiome, including i) what species always grow with other species
(indicating dependency
(including interdependency)), ii) what species never grow with certain other
species (indicating
destructive or inhibiting relationship), iii) what species are able to grow
independent of other
species. This data can be used to create a map of relationship across the
entire microbiome and
identify clusters of microbes sharing interdependent relationships. This
information can be used,
for example, to predict which microbes in a microbiome sample may be easy to
cultivate
(because they grow independently) and which microbes may be difficult to
cultivate
independently as they depend on other species for critical growth factors.
The above method can also be used as a discovery tool for novel antibiotics.
For
64
CA 03037631 2019-03-14
WO 2018/064385
PCT/US2017/054108
example, microfabricated chips are loaded such that any one of microwells
thereon containing
only one species is mathematically unlikely, e.g., by dilution to a
predetermined concentration or
using a cell sorter to apply the microbes in solution to the microwells on the
array. If any
microwell contains only one species, the species is likely to produce
antibiotics (i.e., it is likely
that other species which were previously in the microwell have been killed by
an antibiotic
secreted by the surviving species). A viable sample of the putative anti-
biotic producer can be
amplified and further tested via splitting of the chips.
Different microbial growth media can be used on the array to understand the
effects of
media formulations (or nutrients) on relationships between bacterial variants
or species, e.g.,
promoting or retarding any interdependent relationship, and the underlying
chemistry for such
effects. For example, multiple microfabricated devices containing different
media formulations
or nutrients can be loaded with microbes from a microbiome sample and
processed as outlined
above. Differences in the growth dependency map observed between arrays would
indicate how
each media influence syntrophic relationships or other interactions between
different bacterial
variants or species. Medium X may be formulated such that it has no free amino
acids. Medium
Y may be formulated such that is it the same as Medium X but contains free
amino acids.
Suppose one finds that Species A only grows in the same microwells as Species
B when using
Medium X, but Species A can grow independent of Species B when using Medium Y.
This
suggests that Species A may require amino acids secreted by Species B to grow.
Using multiple
.. formulations of media and analyzing across all microwells a map of
syntrophic relationships and
an indication of the underlying nature of these relationships can be developed
for the
microbiome.
Alternatively, different media formulations can be added into microwells
located on
different sub-area of a single microfabricated device and inoculated with the
particular microbial
species or microbiome under study. Differences in observed growth dependencies
due to
different media will provides insights into the underlying nature of the
relationship.
Further, the effects of different media formulation on the bacterial
relationships as well as
individual bacterial growth can be determined. For example, relationships
within a microbiome
of interest can be studied according to the method described herein and it is
determined that
CA 03037631 2019-03-14
WO 2018/064385
PCT/US2017/054108
microbe G only grows in the presence of microbe K with a given growth medium.
The
microbiome can be reanalyzed using a number of different media on different
microfabricated
chips. It is determined that for a certain media formulation, microbe G is
able to grow in the
absence of microbe K. Then, this particular media formulation is deconstructed
and the various
components used in further experiments to determine which component
facilitates the growth of
microbe Gin the absence of microbe K.
Alternatively, the above can be accomplished in the following manner:
Relationships
within a microbiome of interest are studied and it is determined that microbe
G only grows in the
presence of microbe K with a given medium. An array is then made such that
different media
formulations are placed in different microwells on the array. This may be
performed by, for
example, using a highly accurate printing device such as an inkjet printer to
deliver different
media formulations in different wells. Then the array is loaded with a mixture
of microbe G and
microbe K. The above-described locational indexing system can then be used to
determine in
which microwells i) only G grows, ii) only K grows, iii) neither G nor K
grows, or iv) both G
and K grow. Such growth information is correlated with the media formulation
and used to infer
what components may be important in the growth relationship.
While theoretically the above studies of microbial interrelations may be done
on
traditional platforms (e.g., petri dishes), using the microfabricated chips
with arrays of
microwells of the present invention allows such studies to be done in a
massively parallel
manner and in a highly multiplexed format, which does not require any a priori
knowledge of
how each species will behave. A single experiment or a small number of
experiments can enable
the identification of a large number of relational dependencies in a complex
microbiome.
Coupled with the ability to sample each well (by replicating the contents of
the microwells)
before getting sequence data to build the relationship map, if any
relationship of interest is
discovered, the sample in which such a relationship is discovered could have
already been
replicated, allowing a viable sample of the microbe to be reserved for further
experimentation.
In the following examples, each microwell on a panel consisting of 2500
(50x50)
microwells (each is square with edges of 100[tm by 100[tm and 100 [tm deep) of
a
microfabricated chip is loaded with a unique tag oligo which serve as PCR
amplification primers
66
CA 03037631 2019-03-14
WO 2018/064385
PCT/US2017/054108
directed to the 16S bacterial gene region. Each tag oligo includes a barcode
sequence to identify
the specific location of the microwell in which the tag oligo is loaded. The
microwells are
arranged in a grid pattern, therefore the barcode sequence herein includes a
subsequence to
identify the column of the microwell and another subsequence to identify the
row.
The methods used are as follows:
Chip PCR Protocol
1. Diluted the bacteria in TSB medium 1:10.
2. Loaded 3 [iL bacteria into each panel of the chip and seal with PCR
sealer.
3. Incubated the chip with bacteria at 30 C incubator overnight.
4. Immersed the chip in liquid nitrogen to facilitate lysis
5. Removed seal.
6. Loaded 3 [iL chip PCR buffer, and sealed the chip with PCR sealer.
7. Loaded the chip in Life Tech QuantStudio 3D Digital PCR System and run
the
chip PCR. PCR amplification protocol: 96 C 12 min; 39 Cycles for: 60 C 2 min,
98 C 40 sec;
60 C 2 min; 10 C hold.
8. NGS library building: in order to add the sample index and NGS adaptor
for each
chip sample, a second PCR amplification was run to prepare the NGS library.
After confirming
presence of the right products with gel electrophoresis, the amplicon was
purified with Kapa
purification beads, and a final aliquot of 25 [iL of amplicon product was
collected for NGS.
Illumina MiSeq and Bioinformatics
1. Loaded 10 pM DNA library on the MiSeq and ran the NGS
2. Demultiplexed the data using the unique tag information and located each
NGS
read to each microwell of the microfabricated device. NGS read data were
thresholded to 5000
67
CA 03037631 2019-03-14
WO 2018/064385
PCT/US2017/054108
reads per well.
The panel of microwells was loaded with an 8 species mock community of live
bacteria
and incubated overnight. The mock community consisted of Acinetobacter
calcoaceticus,
Bacillus cereus, Bacillus subtilis, E. coli, Pseudomonas aeruginosa,
Salmonella enterica,
Serratia marcences and Staphylococcus warneri. After chip PCR and NGS as
described above,
the NGS data show that there were total NGS reads of about 7,514,030. Bacteria
were detected
in 1503 microwells (about 60% of all microwells in the panel). In 92% of these
microwells
multiple bacterial genera were detected. In 108 microwells a single bacterial
genus was detected,
the majority of which were E. coli. There were also microwells that each
contain multiple
species where E. coli. was the most abundant species in these microwells. One
of the explanation
is E. coli grown quickly and occupy major of the wells. To understand the
distribution of number
of bacterial genera grown in each microwell, the data were summarized and
shown in Figure 22.
There were 108 wells that had single genus bacteria detected, 426 wells that
had two bacteria
genera detected, 334 wells that had three genera detected, and 635 wells that
had 4 or more
genera detected.
While various inventive embodiments have been described and illustrated
herein, those of
ordinary skill in the art will readily envision a variety of other means
and/or structures for
performing the function and/or obtaining the results and/or one or more of the
advantages
described herein, and each of such variations and/or modifications is deemed
to be within the
scope of the inventive embodiments described herein. More generally, those
skilled in the art
will readily appreciate that all parameters, dimensions, materials, and
configurations described
herein are meant to be exemplary and that the actual parameters, dimensions,
materials, and/or
configurations will depend upon the specific application or applications for
which the inventive
teachings is/are used. Those skilled in the art will recognize, or be able to
ascertain using no
more than routine experimentation, many equivalents to the specific inventive
embodiments
described herein. It is, therefore, to be understood that the foregoing
embodiments are presented
by way of example only and that, within the scope of the appended claims and
equivalents
thereto, inventive embodiments may be practiced otherwise than as specifically
described and
claimed. Inventive embodiments of the present disclosure are directed to each
individual feature,
system, article, material, kit, and/or method described herein. In addition,
any combination of
68
CA 03037631 2019-03-14
WO 2018/064385
PCT/US2017/054108
two or more such features, systems, articles, materials, kits, and/or methods,
if such features,
systems, articles, materials, kits, and/or methods are not mutually
inconsistent, is included within
the inventive scope of the present disclosure.
Also, various inventive concepts may be embodied as one or more methods, of
which an
example has been provided. The acts performed as part of the method may be
ordered in any
suitable way. Accordingly, embodiments may be constructed in which acts are
performed in an
order different than illustrated, which may include performing some acts
simultaneously, even
though shown as sequential acts in illustrative embodiments.
All publications, patent applications, patents, and other references mentioned
herein are
incorporated by reference in their entirety.
All definitions, as defined and used herein, should be understood to control
over
dictionary definitions, definitions in documents incorporated by reference,
and/or ordinary
meanings of the defined terms.
The indefinite articles "a" and "an," as used herein in the specification and
in the claims,
unless clearly indicated to the contrary, should be understood to mean "at
least one."
The phrase "and/or," as used herein in the specification and in the claims,
should be
understood to mean "either or both" of the elements so conjoined, i.e.,
elements that are
conjunctively present in some cases and disjunctively present in other cases.
Multiple elements
listed with "and/or" should be construed in the same fashion, i.e., "one or
more" of the elements
so conjoined. Other elements may optionally be present other than the elements
specifically
identified by the "and/or" clause, whether related or unrelated to those
elements specifically
identified. Thus, as a non-limiting example, a reference to "A and/or B", when
used in
conjunction with open-ended language such as "comprising" can refer, in one
embodiment, to A
only (optionally including elements other than B); in another embodiment, to B
only (optionally
including elements other than A); in yet another embodiment, to both A and B
(optionally
including other elements); etc.
As used herein in the specification and in the claims, "or" should be
understood to have
the same meaning as "and/or" as defined above. For example, when separating
items in a list,
69
CA 03037631 2019-03-14
WO 2018/064385
PCT/US2017/054108
"or" or "and/or" shall be interpreted as being inclusive, i.e., the inclusion
of at least one, but also
including more than one, of a number or list of elements, and, optionally,
additional unlisted
items. Only terms clearly indicated to the contrary, such as "only one of' or
"exactly one of," or,
when used in the claims, "consisting of," will refer to the inclusion of
exactly one element of a
number or list of elements. In general, the term "or" as used herein shall
only be interpreted as
indicating exclusive alternatives (i.e. "one or the other but not both") when
preceded by terms of
exclusivity, such as "either," "one of," "only one of," or "exactly one of"
"Consisting
essentially of," when used in the claims, shall have its ordinary meaning as
used in the field of
patent law.
As used herein in the specification and in the claims, the phrase "at least
one," in
reference to a list of one or more elements, should be understood to mean at
least one element
selected from any one or more of the elements in the list of elements, but not
necessarily
including at least one of each and every element specifically listed within
the list of elements and
not excluding any combinations of elements in the list of elements. This
definition also allows
that elements may optionally be present other than the elements specifically
identified within the
list of elements to which the phrase "at least one" refers, whether related or
unrelated to those
elements specifically identified. Thus, as a non-limiting example, "at least
one of A and B" (or,
equivalently, "at least one of A or B," or, equivalently "at least one of A
and/or B") can refer, in
one embodiment, to at least one, optionally including more than one, A, with
no B present (and
optionally including elements other than B); in another embodiment, to at
least one, optionally
including more than one, B, with no A present (and optionally including
elements other than A);
in yet another embodiment, to at least one, optionally including more than
one, A, and at least
one, optionally including more than one, B (and optionally including other
elements); etc.
In the claims, as well as in the specification above, all transitional phrases
such as
"comprising," "including," "carrying," "having," "containing," "involving,"
"holding,"
"defining," "composed of," and the like are to be understood to be open-ended,
i.e., to mean
including but not limited to. Only the transitional phrases "consisting of'
and "consisting
essentially of' shall be closed or semi-closed transitional phrases,
respectively, as set forth in the
United States Patent Office Manual of Patent Examining Procedures, Section
2111.03.