Note: Descriptions are shown in the official language in which they were submitted.
CA 03037712 2019-03-20
WO 2018/057624
PCT/US2017/052516
TITLE OF THE INVENTION
METHODS AND COMPOSITIONS FOR STIMULATION AND ENHANCEMENT OF
REGENERATION OF TISSUES
STATEMENT REGARDING FEDERALLY SPONSORED RESEARCH OR
DEVELOPMENT
N/A
BACKGROUND OF THE INVENTION
Tissue regeneration in humans is extremely limited and constitutes a major
challenge to the repair of damaged limb function. Injured tissues are able to
heal by regeneration,
by repair, or by a combination of these processes. Regeneration results in the
re-establishment of
the original tissue structure and function. Bodily structures comprised of
composite tissues, such as
a limb or digit, or an appendage, are made of numerous cell types arranged in
an organized and
iterative structure that is preserved from individual to individual. When
composite tissues or bodily
structures comprised of composite tissues are injured, regeneration requires
the coordinated growth,
and interactions of numerous cell types within the composite tissues to
regenerate a bodily structure
that effectively is indistinguishable from the original. Tissue regeneration
is a rapidly developing
field of biomedical research that aims at regenerating damaged tissues. But in
mammals and other
higher organisms, there is a failure of regeneration of composite or complex
tissues. Therefore,
there is a need for methods and compositions to regenerate damaged or injured
tissue and limbs
after injury.
BRIEF SUMMARY OF THE INVENTION
Methods and compositions are provided for enhancing or stimulating
regeneration of
tissues, including as non-limiting examples, limbs or organs in a subject. As
a result of such
enhancement or stimulation, certain medical benefits are achieved including
the treatment of tissue
injured by disease, disorders, trauma or other conditions where regeneration
of the injured tissues,
-1-
CA 03037712 2019-03-20
WO 2018/057624
PCT/US2017/052516
such as those involving the liver, lung, skin, soft tissues and muscle, heart,
nervous system,
intestines, hematopoietic and vascular system, would be beneficial. The
objective of the invention
is to enhance the rate and healing capacity of injured tissues. In certain
aspects, the method of the
invention includes identifying a subject having a tissue injury including, for
non-limiting examples,
a limb or organ injury, and administering a therapeutically effective amount
of an aminosterol such
as, for example, a MSI-1436 or a pharmaceutically acceptable salt thereof that
enhances or
stimulates tissue regeneration in the subject.
It has been discovered that administering to a subject a therapeutically
effective amount of
an aminosterol such as, for example, MSI-1436 or pharmaceutically acceptable
salt thereof,
enhances or stimulates regeneration of an injured issue to treat or prevent a
disease, disorder,
trauma or other condition. Subjects preferably can include mammals, and more
preferably can
include humans. Targeted issue can be selected from liver tissue, lung tissue,
skin soft tissues,
skeletal muscle, cardiac muscle, vascular tree, central and peripheral nervous
system,
gastrointestinal tract, exocrine and endocrine pancreas, skeletal system, and
hematopoietic tissues.
The invention shows that therapeutically effective amounts of MSI-1436
preferably including from
about 0.1 to about 20-mg/kg-body weight (equivalent to about 0.07 mg/kg to
about 2.67 mg/kg
body weight in a human) promotes the regeneration of injured tissue including,
for a non-limiting
example, limbs or organs.
In one aspect, the invention provides a method including the step of
administering to a
subject in need thereof a therapeutically effective amount of an aminosterol
or a pharmaceutically
acceptable salt thereof to stimulate or enhance regeneration of a tissue. In
one embodiment, the
invention provides prior to the administering step, identifying a subject
having an injury of the
tissue. In another embodiment, the invention provides a method wherein the
tissue is selected from
the group consisting of a liver tissue, a lung tissue, a skin soft tissue, a
skeletal muscle tissue, a
cardiac muscle tissue, a vascular tree tissue, a central nervous system
tissue, a peripheral nervous
system tissue, a gastrointestinal tract tissue, an exocrine pancreas tissue,
an endocrine pancreas
tissue, a skeletal system tissue, and an hematopoietic tissue. In yet other
embodiments, the
invention provides a method, wherein the aminosterol is MSI-1436 or wherein
the aminosterol is an
isomer of MSI-1436.
In another embodiment, the invention provides a method wherein the aminosterol
includes
a sterol nucleus and a polyamine, attached at any position on the sterol, such
that the aminosterol
exhibits a net charge of at least + 1, the charge being contributed by the
polyamine. In yet another
-2-
CA 03037712 2019-03-20
WO 2018/057624
PCT/US2017/052516
embodiment, the invention provides a method wherein the aminosterol is
modified to include at
least one of the following: a substitution of the sulfate, wherein the
substitution is selected from the
group consisting of a sulfonate, a phosphate, a carboxylate, and an anionic
moiety, and wherein the
substitution is chosen to circumvent metabolic removal of the sulfate moiety
and oxidation of the
cholesterol side chain; a replacement of a hydroxyl group by a non-
metabolizable polar substituent
to prevent its metabolic oxidation or conjugation; and a substitution of at
least one ring hydrogen
atom to prevent oxidative or reductive metabolism of the steroid ring system.
In a further
embodiment, the invention provides a method wherein the non-metabolizable
polar substituent is a
fluorine atom. In yet a further embodiment, the invention provides a method
wherein the
aminosterol is a derivative of MSI-1436 modified through medical chemistry to
improve at least
one of bio-distribution, ease of administration, metabolic stability, and a
combination of at least two
thereof
In addition, the invention provides a method wherein the therapeutically
effective amount of
MSI-1436 is from about 0.07 mg/kg to about 2.67 mg/kg body weight in a human.
In another
embodiment, the invention provides a method wherein the therapeutically
effective amount of MSI-
1436 is administered in combination with at least one additional active agent
to achieve an additive
or synergistic effect. In yet another embodiment, the invention provides a
method wherein the
active agent is administered concomitantly, as an admixture, separately and
simultaneously,
separately and concurrently, or separately and sequentially. In a further
embodiment, the invention
provides a method wherein a therapeutically effective amount of aminosterol is
administered in the
form of a liquid, a capsule, a tablet, intravenously, intraperitoneally,
inhaled, or topically. In yet a
further embodiment, the invention provides a method wherein the subject is a
mammal. In another
embodiment, the invention provides a method wherein the subject is a human.
In another aspect, the invention provides a method including administering to
a subject a
.. therapeutically effective amount of an aminosterol or a pharmaceutically
acceptable salt thereof to
stimulate or enhance regeneration of a tissue to treat or prevent a disorder,
disease, or condition
resulting from an injury of the tissue. In one embodiment, the method includes
prior to the
administering step, identifying the subject having the disorder, disease,
trauma or condition
resulting from an injury of the tissue. In another embodiment, the invention
provides a method
wherein the tissue is selected from the group consisting of: a liver tissue, a
lung tissue, a skin soft
tissue, a skeletal muscle tissue, a cardiac muscle tissue, a vascular tree
tissue, a central nervous
system tissue, a peripheral nervous system tissue, a gastrointestinal tract
tissue, a exocrine
-3-
CA 03037712 2019-03-20
WO 2018/057624
PCT/US2017/052516
pancreatic tissue, an endocrine pancreatic tissue, a skeletal system tissue,
and a hematopoietic
tissue. In yet another embodiment, the invention provides a method wherein the
aminosterol is
MSI-1436. In a further embodiment, the invention provides a method wherein the
aminosterol is an
isomer of MSI-1436. In an additional embodiment, the invention provides a
method wherein the
aminosterol comprises a sterol nucleus and a polyamine, attached at any
position on the sterol, such
that the aminosterol exhibits a net charge of at least + 1, the charge being
contributed by the
polyamine. In yet a further embodiment, the invention provides a method
wherein the aminosterol
is modified to include at least one of the following: a substitution of the
sulfate, wherein the
substitution is selected from the group consisting of a sulfonate, a
phosphate, a carboxylate, and an
-- anionic moiety, and wherein the substitution is chosen to circumvent
metabolic removal of the
sulfate moiety and oxidation of the cholesterol side chain; a replacement of a
hydroxyl group by a
non-metabolizable polar substituent to prevent its metabolic oxidation or
conjugation; and a
substitution of at least one ring hydrogen atom to prevent oxidative or
reductive metabolism of the
steroid ring system. In another embodiment, the invention provides a method
wherein the non-
-- metabolizable polar substituent is a fluorine atom.
In an additional embodiment, the invention provides a method wherein the
aminosterol is a
derivative of MSI-1436 modified through medical chemistry to improve at least
one of bio-
distribution, ease of administration, metabolic stability, and a combination
of at least two thereof In
another embodiment, the invention provides a method wherein the
therapeutically effective amount
-- of MSI-1436 is from about 0.07 mg/kg to about 2.67 mg/kg body weight in a
human. In yet another
embodiment, the invention provides a method wherein a therapeutically
effective amount of MSI-
1436 is administered in combination with at least one additional active agent
to achieve an additive
or synergistic effect. In a further embodiment, the invention provides a
method wherein the active
agent is administered concomitantly, as an admixture, separately and
simultaneously, separately
-- and concurrently, or separately and sequentially. In yet a further
embodiment, the invention
provides a method wherein the therapeutically effective amount of aminosterol
is administered in
the form of a liquid, capsule, tablet, intravenously, intraperitoneally,
inhaled, or topically. In yet a
further embodiment, the invention provides a method wherein the subject is a
mammal. In an
additional embodiment, the invention provides a method wherein the subject is
a human.
In another aspect, the invention provides a pharmaceutical composition
including a
therapeutically effective amount of an aminosterol to stimulate or enhance
regeneration of a tissue.
In one embodiment, the invention provides the aminosterol in a range from
about 0.07 mg/kg to
-4-
CA 03037712 2019-03-20
WO 2018/057624
PCT/US2017/052516
about 2.67 mg/kg body weight in a human. In an embodiment, the invention
provides a kit
including the pharmaceutical composition. In another embodiment, the invention
provides a
composition wherein the aminosterol is MSI-1436. In yet another embodiment,
the invention
provides a composition wherein the aminosterol is an isomer of MSI-1436. In a
further
embodiment, the invention provides a composition wherein the aminosterol
comprises a sterol
nucleus and a polyamine, attached at any position on the sterol, such that the
aminosterol exhibits a
net charge of at least + 1, the charge being contributed by the polyamine. In
yet a further
embodiment, the invention provides a composition wherein the aminosterol is
modified to include
at least one of the following: a substitution of the sulfate, wherein the
substitution is selected from
the group consisting of a sulfonate, a phosphate, a carboxylate, and an
anionic moiety, and wherein
the substitution is chosen to circumvent metabolic removal of the sulfate
moiety and oxidation of
the cholesterol side chain; a replacement of a hydroxyl group by a non-
metabolizable polar
substituent to prevent its metabolic oxidation or conjugation; and a
substitution of at least one ring
hydrogen atom to prevent oxidative or reductive metabolism of the steroid ring
system. In an
additional embodiment, the invention provides a composition wherein the non-
metabolizable polar
substituent is a fluorine atom.
In another embodiment, the invention provides a composition wherein the
aminosterol is a
derivative of MSI-1436 modified through medical chemistry to improve at least
one of bio-
distribution, ease of administration, metabolic stability, and a combination
of at least two thereof In
yet another embodiment, the invention provides a composition wherein the
composition includes at
least one additional active agent to achieve an additive or synergistic
effect.
In another aspect, the invention features a method of treatment for a subject
in need
thereof The method includes administering to the subject a therapeutically
effective amount of an
aminosterol or a pharmaceutically acceptable salt thereof to stimulate or
enhance regeneration or
growth of a plurality of stem cells of a tissue to treat or prevent a
condition selected from a disease,
a disorder, a trauma, and a health problem.
In one embodiment, this method of the invention includes prior to the
administering step,
identifying the subject having the condition.
In another embodiment, this method of the invention includes the tissue being
selected
from the group consisting of: a liver tissue, a lung tissue, a skin soft
tissue, a skeletal muscle tissue,
a cardiac muscle tissue, a vascular tree tissue, a central nervous system
tissue, a peripheral nervous
-5-
CA 03037712 2019-03-20
WO 2018/057624
PCT/US2017/052516
system tissue, a gastrointestinal tract tissue, a exocrine and endocrine
pancreas tissue, a skeletal
system tissue, and a hematopoietic tissue.
In still other embodiments of this method of the invention, the aminosterol is
MSI-1436 or
an isomer of MSI-1436.
In yet another embodiment of this method of the invention, the aminosterol
includes a
sterol nucleus and a polyamine, attached at any position on the sterol, such
that the aminosterol
exhibits a net charge of at least + 1, the charge being contributed by the
polyamine.
In additional embodiments of this method of the invention, the aminosterol is
modified to
include at least one of the following: a substitution of the sulfate, wherein
the substitution is
.. selected from the group consisting of a sulfonate, a phosphate, a
carboxylate, and an anionic
moiety, and wherein the substitution is chosen to circumvent metabolic removal
of the sulfate
moiety and oxidation of the cholesterol side chain; a replacement of a
hydroxyl group by a non-
metabolizable polar substituent to prevent its metabolic oxidation or
conjugation; and a substitution
of at least one ring hydrogen atom to prevent oxidative or reductive
metabolism of the steroid ring
system.
In another embodiment of this method of the invention, the non-metabolizable
polar
substituent is a fluorine atom.
In still another embodiment of this method of the invention, the aminosterol
is a derivative
of MSI-1436 modified through medical chemistry to improve at least one of bio-
distribution, ease
of administration, metabolic stability, and a combination of at least two
thereof
In yet another embodiment of this method of the invention, the therapeutically
effective
amount of the aminosterol is from about 0.07 mg/kg to about 2.67 mg/kg body
weight in a human.
In an additional embodiment of this method of the invention, the
therapeutically effective
amount of the aminosterol is administered in combination with at least one
additional active agent
to achieve an additive or synergistic effect. In other embodiments, the active
agent is administered
according to one of the group of administration methods consisting of: i)
administering
simultaneously but separately at least one dose of the active agent and at
least one dose of the
aminosterol; ii) administering together in an admixture at least one dose of
the active agent and at
least one dose of the aminosterol; iii) administering sequentially at least
one dose of the active agent
and at least one dose of the aminosterol, the at least one dose of the active
agent being administered
prior to administration of the at least one dose of the aminosterol; iv)
administering sequentially at
least one dose of the active agent and at least one dose of the aminosterol,
the at least one dose of
-6-
CA 03037712 2019-03-20
WO 2018/057624
PCT/US2017/052516
the active agent being administered following administration of the at least
one dose of the
aminosterol; and v) administering sequentially and together in an admixture at
least one dose of the
active agent and at least one dose of the aminosterol.
In still another embodiment of this method of the invention, the
therapeutically effective
amount of aminosterol is administered in the form of a liquid, a capsule, a
tablet, intravenously,
intraperitoneally, inhaled, or topically.
In a preferred embodiment of this method of the invention, the subject is a
mammal, and in
a more preferred embodiment, the subject is a human.
In another aspect, the invention features a pharmaceutical composition
including a
therapeutically effective amount of an aminosterol or a pharmaceutically
acceptable salt thereof for
stimulation or enhancement of growth or regeneration of a plurality of stem
cells of a tissue for the
treatment or prevention of a condition selected from the group consisting of a
disease, disorder,
trauma and health problem which affects the tissue.
In one embodiment, this pharmaceutical composition of the invention includes a
kit
containing the pharmaceutical composition.
In other embodiments of this pharmaceutical composition of the invention, the
aminosterol
is MSI-1436 or an isomer of MSI-1436.
In another embodiment of this pharmaceutical composition of the invention, the
aminosterol includes a sterol nucleus and a polyamine, attached at any
position on the sterol, such
that the aminosterol exhibits a net charge of at least + 1, the charge being
contributed by the
polyamine.
In still other embodiments of this pharmaceutical composition of the
invention, the
aminosterol is modified to include at least one of the following: a
substitution of the sulfate,
wherein the substitution is selected from the group consisting of a sulfonate,
a phosphate, a
carboxylate, and an anionic moiety, and wherein the substitution is chosen to
circumvent metabolic
removal of the sulfate moiety and oxidation of the cholesterol side chain; a
replacement of a
hydroxyl group by a non-metabolizable polar substituent to prevent its
metabolic oxidation or
conjugation; and a substitution of at least one ring hydrogen atom to prevent
oxidative or reductive
metabolism of the steroid ring system. In another embodiment, the non-
metabolizable polar
substituent is a fluorine atom.
In an additional embodiment of this pharmaceutical composition of the
invention, the
aminosterol is a derivative of MSI-1436 modified through medical chemistry to
improve at least
-7-
CA 03037712 2019-03-20
WO 2018/057624
PCT/US2017/052516
one of bio-distribution, ease of administration, metabolic stability, and a
combination of at least two
thereof
In another embodiment of this pharmaceutical composition of the invention, the
composition includes at least one additional active agent to achieve an
additive or synergistic effect.
In an additional embodiment of this pharmaceutical composition of the
invention, the
therapeutically effective amount comprises the aminosterol in a range from
about 0.07 mg/kg to
about 2.67 mg/kg body weight in a human.
In yet another aspect, this invention features a method of a method of growing
in vitro a
plurality of stem cells. The method includes adding an effective amount of an
aminosterol or a
pharmaceutically acceptable salt thereof to the plurality of stem cells
cultured from a tissue
extracted from a subject thereby stimulating or enhancing growth in vitro of
the plurality of stem
cells.
In one embodiment, this method of the invention includes the tissue being
selected from
the group consisting of: a liver tissue, a lung tissue, a skin soft tissue, a
skeletal muscle tissue, a
cardiac muscle tissue, a vascular tree tissue, a central nervous system
tissue, a peripheral nervous
system tissue, a gastrointestinal tract tissue, a exocrine and endocrine
pancreas tissue, a skeletal
system tissue, and a hematopoietic tissue.
In other embodiments of this method of the invention, the aminosterol is MSI-
1436 or an
isomer of MSI-1436.
In yet another embodiment of this method of the invention, the aminosterol
includes a
sterol nucleus and a polyamine, attached at any position on the sterol, such
that the aminosterol
exhibits a net charge of at least + 1, the charge being contributed by the
polyamine.
In additional embodiments of this method of the invention, the aminosterol is
modified to
include at least one of the following: a substitution of the sulfate, wherein
the substitution is
selected from the group consisting of a sulfonate, a phosphate, a carboxylate,
and an anionic
moiety, and wherein the substitution is chosen to circumvent metabolic removal
of the sulfate
moiety and oxidation of the cholesterol side chain; a replacement of a
hydroxyl group by a non-
metabolizable polar substituent to prevent its metabolic oxidation or
conjugation; and a substitution
of at least one ring hydrogen atom to prevent oxidative or reductive
metabolism of the steroid ring
system.
In another embodiment of this method of the invention, the non-metabolizable
polar
substituent is a fluorine atom.
-8-
CA 03037712 2019-03-20
WO 2018/057624
PCT/US2017/052516
In a preferred embodiment of this method of the invention, the subject is a
mammal, and in
a more preferred embodiment, the subject is a human.
These and other features, aspects, and advantages of the present invention
will become
better understood with regard to the following description and appended
claims.
BRIEF DESCRIPTION OF THE SEVERAL VIEWS OF THE DRAWINGS
These and other advantageous aspects of the invention will be evident from the
following
detailed description, which should be considered in conjunction with the
attached drawings,
wherein:
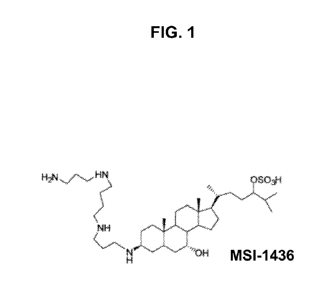
FIG. 1 illustrates the molecular structure of aminosterol MSI-1436;
FIG. 2 shows the effect of MSI-1436 on the regeneration of the caudal fin of
adult
zebrafish;
FIG. 3 shows the effect of MSI-1436 on the proliferation of reprogrammed
caudal fin
blastema cells in adult zebrafish;
FIG. 4 shows the effect of long-term MSI-1436 exposure on tissue overgrowth in
the caudal
fin of adult zebrafish;
FIG. 5 shows the effect of MSI-1436 on cardiomyocyte regenerative
proliferation in adult
zebrafish;
FIG. 6 shows the downregulation of the MSI-1436 target gene PTP1B in response
to heart
injury in adult zebrafish;
FIG. 7 shows the effect of MSI-1436 on genetically induced attenuation of
cardiac
regenerative proliferation in adult zebrafish hearts;
FIG. 8 shows the effect of MSI-1436 on mammalian cell proliferation;
FIG. 9a shows the effect of MSI-1436 in vivo administration in injured mice
muscle tissue
at three days post injury;
FIG. 9b shows the effect of MSI-1436 in vivo administration in injured mice
muscle tissue
at three to twenty one days post injury;
FIG. 9c shows the effect of MSI-1436 in vivo administration in injured mice
muscle tissue
at twenty one days post injury;
FIG. 10a shows the effect of previous MSI-1436 in vivo administration in re-
injured mice
muscle tissue at zero and three days following re-injury;
-9-
CA 03037712 2019-03-20
WO 2018/057624
PCT/US2017/052516
FIG. 10b shows the effect of previous MSI-1436 in vivo administration in re-
injured mice
muscle tissue at three days following re-injury;
FIG. ha shows the effect of MSI-1436 in vitro treatment of cultured mouse
skeletal muscle
stem cells at ninety-six hours following treatment; and
FIG. lib shows the effect of MSI-1436 in vitro treatment of cultured mouse
skeletal muscle
stem cells at ninety-six hours following treatment.
DETAILED DESCRIPTION OF THE INVENTION
The disclosures of U.S. Provisional Patent Application No. 61/740,291 filed
December 20,
2012, and U.S. Patent Application No. 14/137,259 filed December 20, 2013, are
incorporated
herein by reference in their entirety.
The present invention is directed to methods of enhancing or stimulating
regeneration of
tissues in a subject. Such tissues can include, as non-limiting examples,
limbs or organs. The
invention is unanticipated and based on the discovery of a previously unknown
property of the
aminosterol MSI-1436. The utility afforded by this invention includes all
applications in which
stimulation or enhancement of regeneration of a tissue including, for a non-
limiting example,
composite or complex tissues, would have benefit. These applications include,
for non-limiting
examples, conditions such soft tissue injury involving skin, dermis, or
muscle; cystic fibrosis
involving the lung; hepatic regrowth, following partial hepatectomy or in the
setting of cirrhosis;
cardiac muscle, in the setting of ischemic injury; the nervous system,
following traumatic injury;
the regrowth of amputated limbs; the possible regeneration of islet cells in
diabetes mellitus; the
recovery of intestinal epithelium in the setting of inflammatory bowel
disease.
1. Definitions
Unless defined otherwise, all technical and scientific terms used herein have
the same
meaning as commonly understood by one of ordinary skill in the art to which
this invention
belongs. Although any methods and materials similar or equivalent to those
described herein can be
used in the practice or testing of the invention, the preferred methods and
materials are now
described. All publications mentioned herein are incorporated herein by
reference.
Generally, nomenclatures used in connection with, and techniques of, cell and
tissue
culture, molecular biology, immunology, microbiology, genetics, protein, and
nucleic acid
chemistry and hybridization described herein are those well-known and commonly
used in the art.
-10-
CA 03037712 2019-03-20
WO 2018/057624
PCT/US2017/052516
The methods and techniques of the present invention are generally performed
according to
conventional methods well known in the art and as described in various general
and more specific
references that are cited and discussed throughout the present specification
unless otherwise
indicated. See, e.g., Sambrook et al. Molecular Cloning: A Laboratory Manual,
2d ed., Cold Spring
Harbor Laboratory Press, Cold Spring Harbor, N.Y. (1989); Ausubel et al.,
Current Protocols in
Molecular Biology, Greene Publishing Associates (1992, and Supplements to
2002); Harlow and
Lan, Antibodies: A Laboratory Manual, Cold Spring Harbor Laboratory Press,
Cold Spring Harbor,
N.Y. (1990); Principles of Neural Science, 4th ed., Eric R. Kandel, James H.
Schwart, Thomas M.
Jessell editors. McGraw-Hill/Appleton & Lange: New York, N. Y. (2000). Unless
defined
otherwise, all technical and scientific terms used herein have the same
meaning as commonly
understood by one of ordinary skill in the art.
The terms "animal," "patient," or "subject" as used herein, mean any animal
(e.g.,
mammals, including, but not limited to humans, primates, dogs, cattle, cows,
horses, kangaroos,
pigs, sheep, goats, cats, rabbits, rodents), transgenic non-human animals,
fish, amphibians, not
limited to frogs, and salamanders, reptiles, other vertebrates and
invertebrates and the like, which
are to be the recipient of a particular treatment. Typically, the terms
"animal" "subject" and
"patient" are used interchangeably herein in reference to a human subject. The
preferred animal,
patient, or subject is a mammal and more preferably a human.
As used herein, the terms "administer", "administering", and "administered"
refer to
providing the drug to the subject being tested or treated. In certain
embodiments, the aminosterol
e.g., MSI-1436 is administered in the form of a liquid, capsule, tablet,
intraperitoneally,
subcutaneously, intravenously, inhaled, that is intranasally, or topically,
but can include
microinjection, and/or direct application to the injured tissue including, for
non-limiting examples,
an injured limb or organ.
The terms, "enhance", "enhancing", and "enhanced" as used herein refer to
activities whose
effects are greater than that which is observed in a control or an untreated
group or subject.
Enhanced activity may be measured in vivo or in cell culture studies.
The terms "growth", grow", "grown", or "growing" as used herein, mean the
growth of
tissue, including but not limited to one or more tissues, limbs or organs,
following an injury of the
tissue resulting from a diseases, disorder, trauma or other condition and
includes but is not limited
to regeneration as described herein below.
-11-
CA 03037712 2019-03-20
WO 2018/057624
PCT/US2017/052516
The terms "injury of a tissue" and "tissue injury" as used herein, mean damage
of a tissue
that disrupts its physical structure resulting in the impairment of its
function.
The terms "injury of a limb" and "limb injury" as used herein, mean damage of
a limb such
as, for non-limiting examples, a finger, arm or foot, that involves a trauma
to any or all of the
tissues included in the limb.
The terms "injury of an organ" and "organ injury" as used herein, mean damage
of an organ
that involves a trauma to any or all of the tissues includes in the organ.
The term, "kit" as used herein, means any manufacture (e.g., a package or
container)
including at least one reagent, e.g., an aminosterol such as MSI-1436. In
certain embodiments, the
manufacture may be promoted, distributed, or sold as a unit for performing the
methods of the
invention.
The terms, "regenerate," "regenerating," or "regeneration" as used herein mean
the
restoration of a tissue, including but not limited to one or more tissues,
limbs or organs, to its
original state following an injury of the tissue resulting from a disease,
disorder, trauma or other
condition.
As used herein, the terms "stimulate", "stimulating", "stimulated" refer to an
activities
whose effects are greater than that which is observed in a control or an
untreated group. Stimulatory
effects may be measured in vivo or in cell culture studies.
As used herein, the terms "therapeutic activity" or "activity" may refer to an
activity whose
-- effect is consistent with a desirable therapeutic outcome in humans, or to
desired effects in non-
human mammals or in other species or organisms.
The term "therapeutically effective amount" as used herein means an amount
that achieves
the intended therapeutic effect of enhancing or stimulating regeneration of a
tissue, including but
not limited to one or more tissues, limbs or organs, in a subject. The full
therapeutic effect does not
necessarily occur by administration of one dose and may occur only after
administration of a series
of doses. Thus, a therapeutically effective amount may be administered in one
or more
administrations per day for successive days.
The terms "treat" "treating", "treated" or "treatment" in a subject having an
injury to a
tissue resulting from a disease, disorder, trauma or other condition as used
herein, mean taking
-- steps to obtain beneficial or desired results, including clinical results.
For purposes of this invention,
beneficial or desired clinical results include, but are not limited to
stimulating or enhancing
-12-
CA 03037712 2019-03-20
WO 2018/057624
PCT/US2017/052516
regeneration of a tissue, including but not limited to one or more tissues,
limbs or organs, in a
subject.
The term "tissue" and "tissues" as used herein, refer to single, composite
and/or complex
tissues which can form, for non-limiting examples, a limb or an organ.
2. Overview
It has been discovered that an aminosterol such as MSI-1436, is a new therapy
for tissue
regeneration. The molecular structure of MSI-1436 is illustrated in FIG. 1.
Specifically, MSI-1436
stimulates faster regeneration of missing limb and cardiac tissues by
amplifying growth factor
pathways important for tissue regeneration. Importantly, MSI-1436 treatment
rescues genetically
mediated defects in cardiomyocyte proliferation to normal levels. Certain
embodiments are directed
to identifying subjects having tissue injury and administering to the subject
a therapeutically
effective amount of an aminosterol such as MSI-1436 that enhances or
stimulates tissue
regeneration. In other embodiments, the invention is directed to
pharmaceutical formulations
comprising MSI-1436.
Vertebrate regeneration is robustly observed in a limited number of species,
including fish,
amphibian, and reptiles (K D Poss, Keating, & Nechiporuk, 2003; Sanchez
Alvarado & Tsonis,
2006). In mammals, regenerative activity is observed in early fetal
development, but becomes
highly restricted to specific organs in post-natal life (Kenneth D Poss,
2010).
In particular the human liver is known to functionally regenerate after
partial hepatectomy
(Taub, 2004). The epithelial lining of the small and large bowel can be
reconstituted after a severe
inflammatory insult (Simons & Clevers, 2011); restoration of the blood forming
tissues in the bone
marrow occurs following ablative therapies. Other tissues in the post-natal
human exhibit much less
regenerative potential. Indeed, extensive soft tissue injury to a limb is
repaired through a wound
healing process that lays down scar tissue, rather than one that restores the
prior tissue architecture.
In no case is it possible regenerate an appendage in a post-natal human.
3. Background
MSI-1436 is a molecule similar in structure to squalamine but differing in the
nature of the
polyamine, that being a spermine in MSI-1436, and a spermidine in squalamine.
-13-
CA 03037712 2019-03-20
WO 2018/057624
PCT/US2017/052516
1414\/=,,MIN 96 04
:
ert\T"
MS-14a6
r.
et
====="'
SQUALA1VIINE
Aminosterol 1436 is an aminosterol isolated from the dogfish shark, which is
structurally
related to squalamine (U.S. Patent No. 5,840,936; Rao et al., 2000).
Aminosterol 1436 exhibits
antiviral activity against HIV in tissue culture (U.S. Patent No. 5,763,430)
via a mechanism
proposed to involve inhibition of a lymphocyte-specific NHE by 1436, resulting
in suppression of
cytokine responsiveness, and subsequent depression of the capacity of the
lymphocyte to support
HIV replication (U.S. Patent No. 5,763,430). Aminosterol 1436, however, has an
additional
pharmacological property, not shared with squalamine, namely potent appetite
suppression and
promotion of dose-dependent weigh loss (U.S. Patent No. 6,143,738; M Zasloff
et al., 2001; Ahima
et al., 2002). In addition, MSI-1436 corrected the diabetic phenotype in mice
with leptin receptor
lesions and leptin deficiency (Takahashi, Qi, Patel, & Ahima, 2004). Many of
the metabolic effects
of appear to a consequence of its activity on specific centers in the central
nervous system (Bence et
al., 2006).
The utility of MSI-1436 as an anti-infective has been demonstrated in vitro
against bacteria
and fungi (Rao et al., 2000). Like squalamine, MSI-1436 is a cationic
amphipathic substance
exhibiting an affinity for membranes composed of anionic phospholipids
(Selinsky et al., 1998;
Selinsky, Smith, Frangiosi, Vonbaur, & Pedersen, 2000). Like other such
agents, including
magainin and cationic antimicrobial peptides, MSI-1436 is believed to exert
antimicrobial action by
interacting electrostatically with the membranes of target microorganisms,
which generally display
-14-
CA 03037712 2019-03-20
WO 2018/057624
PCT/US2017/052516
anionic phospholipids on the membrane surface exposed to the environment,
subsequently
disturbing their functional integrity, and causing death of the targeted
microbe (Michael Zasloff,
2002; Salmi et al., 2008; Sills et al., 1998).
The utility of MSI-1436 in vivo has been demonstrated in models of obesity
(Lantz et al.,
2010), diabetes (Takahashi et al., 2004), and hepatic steatosis (Lantz et al.,
2010). No report of its
capacity to stimulate regeneration has been previously described or
demonstrated.
It has been reported that squalamine exerts its effects on human cells and
tissues at the
cellular level by displacing proteins bound electrostatically to negatively
charged membranes,
causing pleiotropic changes in the functional state of the cell (Alexander et
al., 2011; Yeung et al.,
2008; Sumioka, Yan, & Tomita, 2010; Michael Zasloff et al., 2011). It is
believed that the
fundamental mechanism of action of MSI-1436 also involves electrostatic
interactions with
negatively charged intracellular membranes, but the precise phospholipids
targeted and the specific
membranes onto which MSI-1436 localizes differ from squalamine.
At the cellular level MSI-1436 has been shown to inhibit a broadly acting
tyrosine
phosphatase PTP1B (Lantz et al., 2010). Specifically, MSI-1436 was shown to
inhibit the activity
of PTP1B in an in vitro enzyme assay (Lantz et al., 2010). In addition, the
addition of MSI-1436 to
hepatoma cells was shown to increase the level of phosphorylation of the
insulin receptor, and
known target of PTP1B (Lantz et al., 2010). Administration of MSI-1436 to a
mouse, resulted in an
increase in the recovery of phosphorylated insulin receptor from the
hypothalamus, associated with
the administration of insulin (Lantz et al., 2010). Furthermore the levels of
phosphorylated STAT3,
a downstream component of the insulin receptor circuit, were increased after
both insulin
administrations, compared to the extent observed following administration of
insulin alone (Lantz
et al., 2010).
It has been reported that squalamine exerts its effects at the cellular level
by displacing
proteins bound electrostatically to negatively charged membranes, causing
pleiotropic changes in
the functional state of the cell (Alexander et al., 2011; Sumioka et al.,
2010; Yeung et al., 2008;
Michael Zasloff et al., 2011). With respect to the disclosed invention, and
without being bound by
theory, it is believed that aminosterols, such as Aminosterol 1436, enter
certain tissues (via specific
transporters) and influence intracellular signaling by the electrostatic
mechanism proposed.
MSI-1436 is known to inhibit PTP1B, a phosphatase that normally "turns off'
the insulin
receptor after it has been occupied by insulin, and thereby "quiets" the
insulin response. PTP1B is
known to act on other tyrosine kinase receptors in addition to the insulin
receptor. The known
-15-
CA 03037712 2019-03-20
WO 2018/057624
PCT/US2017/052516
targets of PTP1B include, in addition to the insulin receptor, the insulin
related growth factor
receptor (IGFR), the epidermal growth factor receptor (EGFR), the fibroblast
growth factor
receptor (FGFR), colony stimulating growth factor receptor (CSFR), hepatocyte
stimulating factor
receptor (HSFR), and platelet derived growth factor receptor (PDGFR).
PTP1B is known to be localized within the cellular endoplasmic reticulum
(Frangioni,
Beahm, Shifrin, Jost, & Neel, 1992). It is believed to access the receptor
target by a mechanism that
involves the "kissing" of the cytoplasmic face of the plasma membrane, in
which the
phosphorylated site is localized, by a "ruffle" of the endoplasmic reticulum
containing PTP1B
(Frangioni et al., 1992). Thus, the physical contact of PTP1B, present on the
endoplasmic
reticulum, with the phosphorylated receptor present on the cytoplasmic face of
the plasma
membrane, is required for de-phosphorylation to occur.
Taken together, these properties of PTP1B suggest that MSI-1436 likely
displaces PTP1B
from the endoplasmic reticulum. We presume that MSI-1436 binds to the
endoplasmic reticulum,
neutralizes the electrostatic interactions that are involved in the attachment
of PTP1B, leading to its
release, and its ultimate "inactivation". In this scenario, inactivation is a
direct consequence of its
displacement from an intracellular site in which it normally operates, as
opposed to a direct effect
on the enzyme itself
Vertebrate regeneration is robustly observed in a limited number of species,
including fish,
amphibian, and reptiles (K D Poss, Keating, & Nechiporuk, 2003; Sanchez
Alvarado & Tsonis,
2006). In mammals, regenerative activity is observed in early fetal
development, but becomes
highly restricted to specific organs in post-natal life (Kenneth D Poss,
2010).
In particular the human liver is known to functionally regenerate after
partial hepatectomy
(Taub, 2004). The epithelial lining of the small and large bowel can be
reconstituted after a severe
inflammatory insult (Simons & Clevers, 2011); restoration of the blood forming
tissues in the bone
marrow occurs following ablative therapies. Other tissues in the post-natal
human exhibit much less
regenerative potential. Indeed, extensive soft tissue injury to a limb is
repaired through a wound
healing process that lays down scar tissue, rather than one that restores the
prior tissue architecture.
There are currently no known cases that we can identify which show
regeneration of an appendage
in a post- natal human.
The zebrafish is known to have the capacity to robustly regenerate various
tissues and
organs and has become a well characterized model for the systematic study of
the regenerative
process in vertebrates (K D Poss et al., 2003; Kenneth D Poss, 2010). In
particular, regeneration of
-16-
CA 03037712 2019-03-20
WO 2018/057624
PCT/US2017/052516
the heart and liver has been extensively explored in this animal (Curado &
Stainier, 2010; Kenneth
D Poss, Wilson, & Keating, 2002). Regrowth of the amputated tail fin has been
used a model for
the study of the regrowth of a vertebrate appendage. Recent reports have
highlighted the roles of
several well studied growth factor pathways in the regenerative processes of
the zebrafish,
including those of Wnts, insulin related growth factor, retinoic acid, TGFP
and fibroblast related
growth factor (Chablais & Jazwinska, 2010; Jazwinska, Badakov, & Keating,
2007; Yin et al.,
2008; Blum & Begemann, 2012; Lee, Grill, Sanchez, Murphy-Ryan, & Poss, 2005;
Stoick-Cooper
et al., 2007).
Although the precise mechanism by which MSI-1436 exerts its unprecedented and
unanticipated effects on tissue including as non-limiting examples limb or
organ regeneration in the
vertebrate are not as understood, without being bound by theory, it is
speculated that (1) since the
regenerative process involves the action of numerous growth factors that
utilize receptors that are
phosphorylated by tyrosine receptor kinases, and (2) that PTP1B normally turns
off these receptors
after activation, and (3) that MSI-1436 inhibits PTP1B, MSI-1436 potentiates
the activity of the
growth factors that are engaged in the regenerative process. By permitting the
activated receptor to
remain active for a longer period of time than normal, the magnitude of the
biological response to
the cognate growth factor would be enhanced. In a sense the "gain" on the
system would be
increased. Since the "master" regulators of the repair process are these
growth factors, and since the
spatiotemporal unfolding must require precise elaboration of these growth
factors, the enhancement
of regenerative rates by increasing the transduced "signal strength" through
reducing the
inactivation of the receptor must preserve the information critical for
effective regeneration. The
inactivation of PTP1B by MSI-1436 ensures that many different receptors will
be targeted, which
we believe to be favorable since many growth factors are utilized in the
complex restoration of a
limb.
4. Summary of Experimental Results and Embodiments of the Invention
In summary, the present invention herein disclosed describes an unprecedented
and
unanticipated discovery of the stimulatory and enhancement effects of an
aminosterol such as MSI-
1436 on the regeneration of certain tissues in the zebrafish.
= MSI-1436 treated animals exhibited ¨200% greater regenerated length when
compared to
the control or squalamine microinjected group, when evaluated at time point
prior to full repair of
the tail.
-17-
CA 03037712 2019-03-20
WO 2018/057624
PCT/US2017/052516
= In other words, the rate of regeneration was 200% greater for those
animals receiving
MSI-1436 than those that had received either phosphate buffered solution (PBS)
or squalamine.
In addition, the present invention herein disclosed describes the
unprecedented and
unanticipated discovery of the stimulatory and enhancement effects of an
aminosterol such as MSI-
1436 in the proliferation of stem cells. Stem cells treated in vitro with MSI-
1436 exhibited 75%
greater stem cell proliferation when compared to the control stem cells
treated in vitro with saline.
Methods of Treatment
Embodiments of the invention are provided for enhancing or stimulating the
regeneration or
growth of tissues including as non-limiting examples, limbs and organs in a
subject. It is possible to
treat diseases, disorders, trauma or other conditions where regenerative
restoration of tissues would
be beneficial, such as those involving the liver, lung, skin, soft tissues and
muscle, heart, nervous
system, intestines, hematopoietic and vascular system. The objective is to
enhance the rate and
healing capacity of these tissues.
Regenerating tissues in a subject involves identifying a subject having tissue
injury and
administering a therapeutically effective amount of an aminosterol such as MSI-
1436 or a
pharmaceutically acceptable salt thereof and/or an additional active agent to
subjects having the
tissue injury. It is now possible in view of the new discoveries to administer
to subjects having a
disorder, disease, trauma or condition (e.g., cirrhosis, hepatitis, muscular
dystrophy, neurogenic
myopathies, type I diabetes mellitus, cystic fibrosis) as the result of an
injury to a tissue a
therapeutically effective amount of an aminosterol e.g., MSI-1436 or
pharmaceutically acceptable
salt thereof Such administration can ultimately stimulate or enhance tissue
including as non-
limiting examples, limb and/or organ regeneration from a tissue injury that
occurs and therefore
treat the disorder, disease, trauma or condition. Treatments of these
disorders, diseases, traumas or
conditions are possible where regenerative restoration of tissues would be
beneficial, such as those
involving the liver, lung, skin, soft tissues, and muscle, heart, nervous
system, intestines,
hematopoietic and vascular system. Subjects in these embodiments are
preferably mammal, and
even more preferably human, and targeted tissue may be selected from liver
tissue, lung tissue, skin
soft tissues, skeletal muscle, cardiac muscle, vascular tree, central and
peripheral nervous system,
gastrointestinal tract, exocrine and endocrine pancreas, skeletal system, and
hematopoietic tissues.
In certain embodiments of the invention, the aminosterol is an isomer of MSI-
1436. The
aminosterol may comprise a sterol nucleus and a polyamine, attached at any
position on the sterol,
-18-
CA 03037712 2019-03-20
WO 2018/057624
PCT/US2017/052516
such that the molecule exhibits a net charge of at least + 1, the charge being
contributed by the
polyamine.
In other embodiments of the invention, the aminosterol is modified to include
one or more
of the following: (1) substitutions of the sulfate by a sulfonate, phosphate,
carboxylate, or other
anionic moiety chosen to circumvent metabolic removal of the sulfate moiety
and oxidation of the
cholesterol side chain; (2) replacement of a hydroxyl group by a non-
metabolizable polar
substituent, such as, for example, a fluorine atom, to prevent its metabolic
oxidation or conjugation;
and (3) substitution of various ring hydrogen atoms to prevent oxidative or
reductive metabolism of
the steroid ring system. Yet, in other embodiments of the invention, the
aminosterol is a derivative
of MSI-1436 modified through medical chemistry techniques known to one or
ordinary skill in the
art to improve bio-distribution, ease of administration, metabolic stability,
or any combination
thereof
In the embodiments described herein, therapeutically effective amounts of MSI-
1436 from
about 0.1 to about 20-mg/kg-body weight (equivalent to about 0.07 mg/kg to
about 2.67 mg/kg
body weight in a human) were shown to regenerate tissue and limbs. More
preferably,
therapeutically effective amounts of MSI-1436 include doses from about 0.1 to
10 mg/kg-body
weight (equivalent to about 0.07 mg/kg to about 1.33 mg/kg body weight in a
human) and most
preferably, therapeutically effective amounts of MSI-1436 include doses from
about 0.1 to 5
mg/kg-body weight (equivalent to about 0.07 mg/kg to about 2.67 mg/kg body
weight in a human).
An additional active agent can be administered in combination with MSI-1416.
Active agents
include, but are not limited to, anti-infective agents, anti-inflammatory
compounds, hematopoietic
growth factors, anti-metabolites such as those used in cancer, pain
medications, anti-emetics, anti-
hypertensive agents, and cholesterol lowering agents. The amount of active
agent will vary
depending on many factors, including, but not limited to the severity of the
tissue including as non-
limiting examples, the limb or organ degeneration, the size of injury, the
location of injury, the age,
sex, and immune status of the subject. Various factors known to those skilled
in the art affect the
actual therapeutic amounts used in vivo, especially in humans. The aminosterol
such as, for
example, the MSI-1436 can also be administered in combination with at least
one additional active
agent such as one of the known growth factors (e.g., hematopoietic,
epithelial, platelet derived, or
vascular growth factors) to achieve either an additive or synergistic effect.
The active agent can be
administered (a) concomitantly; (b) as an admixture; (c) separately and
simultaneously or
concurrently; or (d) separately and sequentially. In certain embodiments, the
aminosterol e.g., MSI-
-19-
CA 03037712 2019-03-20
WO 2018/057624
PCT/US2017/052516
1436 is administered in the form of a liquid, capsule, tablet, intravenously,
intraperitoneally,
inhaled, or topically.
Embodiments of the invention are also provided for enhancing or stimulating
the growth or
proliferation of stem cells, for the treatment of conditions including
disease, disorders, trauma or
other health problems where such enhancement or stimulation would be
beneficial. Such methods
involve administration of an effective amount of an aminosterol or a
pharmaceutically acceptable
salt thereof to promote the stem cell growth or proliferation, and include
identifying a subject in
need thereof In various embodiments of this aspect of the invention, the
methods employ the
various aminosterols and the therapeutic effects amounts of MSI-1436 described
above.
Pharmaceutical Compositions or Formulations and Administration
The "therapeutic agents" (e.g., aminosterols such as MSI-1436) may be present
in the
pharmaceutical compositions in the form of pharmaceutically acceptable salts
of pharmaceutically
acceptable acids and bases. They may be present in amorphous form or in
crystalline forms,
including hydrates and solvates. Preferably, the pharmaceutical compositions
comprise a
therapeutically effective amount.
Pharmaceutically acceptable salts of the therapeutic agents described herein
include those
salts derived from pharmaceutically acceptable inorganic and organic acids and
bases. Examples of
suitable acid salts include acetate, adipate, alginate, aspartate, benzoate,
benzenesulfonate,
bisulfate, butyrate, citrate, camphorate, camphorsulfonate,
cyclopentanepropionate, digluconate,
dodecylsulfate, ethanesulfonate, formate, fumarate, glucoheptanoate,
glycerophosphate, glycolate,
hemi sulfate, heptanoate, hexanoate, hydrochloride, hydrobromide, hydroiodide,
2-
hydroxyethanesulfonate, lactate, maleate, malonate, methanesulfonate, 2-
naphthalenesulfonate,
nicotinate, nitrate, oxalate, palmoate, pectinate, persulfate, 3-
phenylpropionate, phosphate, picrate,
pivalate, propionate, salicylate, succinate, sulfate, tartrate, thiocyanate,
tosylate and undecanoate
salts. Other acids, such as oxalic, while not in themselves pharmaceutically
acceptable, may be
employed in the preparation of salts useful as intermediates in obtaining
pharmaceutically
acceptable acid addition salts. Salts derived from appropriate bases include
alkali metal (e.g.,
sodium and potassium), alkaline earth metal (e.g., magnesium), ammonium and
salts.
The therapeutic agents of the present invention are also meant to include all
stereochemical
forms of the therapeutic agents (i.e., the R and S configurations for each
asymmetric center).
Therefore, single enantiomers, racemic mixtures, and diastereomers of the
therapeutic agents are
-20-
CA 03037712 2019-03-20
WO 2018/057624
PCT/US2017/052516
within the scope of the invention. Also within the scope of the invention are
steric isomers and
positional isomers of the therapeutic agents.
In a preferred embodiment, the therapeutic agents of the present invention are
administered
in a pharmaceutical composition that includes a pharmaceutically acceptable
carrier, adjuvant, or
vehicle. The term "pharmaceutically acceptable carrier, adjuvant, or vehicle"
refers to a non-toxic
carrier, adjuvant, or vehicle that does not destroy or significantly diminish
the pharmacological
activity of the therapeutic agent with which it is formulated.
Pharmaceutically acceptable carriers, adjuvants or vehicles that may be used
in the
compositions of this invention encompass any of the standard pharmaceutically
accepted liquid
carriers, such as a phosphate- buffered saline solution, water, as well as
emulsions such as an
oil/water emulsion or a triglyceride emulsion. Solid carriers may include
excipients such as starch,
milk, sugar, certain types of clay, stearic acid, talc, gums, glycols, or
other known excipients.
Carriers may also include flavor and color additives or other ingredients. The
formulations of the
combination of the present invention may be prepared by methods well-known in
the
pharmaceutical arts and described herein. Exemplary acceptable pharmaceutical
earners have been
discussed above.
The pharmaceutical compositions of the present invention are preferably
administered
intraperitoneally, or orally, preferably as solid compositions. However, the
pharmaceutical
compositions may be administered parenterally, by inhalation spray, topically,
rectally, nasally,
buccally, vaginally or via an implanted reservoir. Sterile injectable forms of
the pharmaceutical
compositions may be aqueous or oleaginous suspensions. These suspensions may
be formulated
according to techniques known in the art using suitable dispersing or wetting
agents and suspending
agents. The sterile injectable preparation may also be a sterile injectable
solution or suspension in a
non-toxic parenterally acceptable diluent or solvent, for example as a
solution in 1, 3-butanediol.
Among the acceptable vehicles and solvents that may be employed are water,
Ringer's solution and
isotonic sodium chloride solution. In addition, sterile, fixed oils are
conventionally employed as a
solvent or suspending medium.
The pharmaceutical compositions employed in the present invention may be
orally
administered in any orally acceptable dosage form, including, but not limited
to, solid forms such as
capsules and tablets. In the case of tablets for oral use, carriers commonly
used include
microcrystalline cellulose, lactose and cornstarch. Lubricating agents, such
as magnesium stearate,
are also typically added. When aqueous suspensions are required for oral use,
the active ingredient
-21-
CA 03037712 2019-03-20
WO 2018/057624
PCT/US2017/052516
may be combined with emulsifying and suspending agents. If desired, certain
sweetening, flavoring
or coloring agents may also be added.
The pharmaceutical compositions employed in the present invention may also be
administered by nasal aerosol or inhalation. Such pharmaceutical compositions
may be prepared
according to techniques well-known in the art of pharmaceutical formulation
and may be prepared
as solutions in saline, employing benzyl alcohol or other suitable
preservatives, absorption
promoters to enhance bioavailability, fluorocarbons, and/or other conventional
solubilizing or
dispersing agents.
Should topical administration be desired, it can be accomplished using any
method
commonly known to those skilled in the art and includes but is not limited to
incorporation of the
pharmaceutical composition into creams, ointments, or transdermal patches.
The passage of agents through the blood-brain barrier to the brain can be
enhanced by
improving either the permeability of the agent itself or by altering the
characteristics of the blood-
brain barrier. Thus, the passage of the agent can be facilitated by increasing
its lipid solubility
through chemical modification, and/or by its coupling to a cationic carrier.
The passage of the agent
can also be facilitated by its covalent coupling to a peptide vector capable
of transporting the agent
through the blood-brain barrier. Peptide transport vectors known as blood-
brain barrier
permeabilizer compounds are disclosed in U.S. Patent No. 5,268, 164. Site-
specific
macromolecules with lipophilic characteristics useful for delivery to the
brain are disclosed in U.S.
Patent No. 6,005,004.
Examples of routes of administration include parenteral, e.g., intravenous,
intradermal,
subcutaneous, inhalation, transdermal (topical), transmucosal, and rectal or
oral administration.
Solutions or suspensions used for parenteral, intradermal, or subcutaneous
application can include
the following components: a sterile diluent such as water for injection,
saline solution, fixed oils,
polyethylene glycols, glycerine, propylene glycol or other synthetic solvents;
antibacterial agents
such as benzyl alcohol or methyl parabens; antioxidants such as ascorbic acid
or sodium bisulfite;
chelating agents such as ethylenediaminetetraacetic acid; buffers such as
acetates, citrates or
phosphates and agents for the adjustment of tonicity such as sodium chloride
or dextrose. The pH
can be adjusted with acids or bases, such as hydrochloric acid or sodium
hydroxide. The parenteral
preparation can be enclosed in ampoules, disposable syringes or multiple dose
vials made of glass
or plastic. Pharmaceutical compositions suitable for injection include sterile
aqueous solutions
(where water soluble) or dispersions and sterile powders for the
extemporaneous preparation of
-22-
CA 03037712 2019-03-20
WO 2018/057624
PCT/US2017/052516
sterile injectable solutions or dispersions. For intravenous administration,
suitable carriers comprise
physiological saline, bacteriostatic water, Cremophor ELTM (BASF, Parsippany,
N.J.) or phosphate
buffered saline (PBS). In all cases, the composition must be sterile and
should be fluid to the extent
that easy syringability exists. It should be stable under the conditions of
manufacture and storage
and must be preserved against the contaminating action of microorganisms such
as bacteria and
fungi. The carrier can be a solvent or dispersion medium containing, for
example, water, ethanol,
polyol (for example, glycerol, propylene glycol, and liquid polyetheylene
glycol, and the like), and
suitable mixtures thereof The proper fluidity can be maintained, for example,
by the use of a
coating such as lecithin, by the maintenance of the selected particle size in
the case of dispersion
and by the use of surfactants. Prevention of the action of microorganisms can
be achieved by
various antibacterial and antifungal agents, for example, parabens,
chlorobutanol, phenol, ascorbic
acid, thimerosal, and the like. In some cases, isotonic agents are included in
the composition, for
example, sugars, polyalcohols such as manitol, sorbitol, or sodium chloride.
Prolonged absorption
of an injectable composition can be achieved by including in the composition
an agent that delays
.. absorption, for example, aluminum monostearate or gelatin.
Sterile injectable solutions can be prepared by incorporating the active
compound in the
specified amount in an appropriate solvent with one or a combination of
ingredients enumerated
above, as needed, followed by filtered sterilization. Generally, dispersions
are prepared by
incorporating the active compound into a sterile vehicle that contains a basic
dispersion medium
and other ingredients selected from those enumerated above or others known in
the art. In the case
of sterile powders for the preparation of sterile injectable solutions, the
methods of preparation
include vacuum drying and freeze-drying which yields a powder of the active
ingredient plus any
additional desired ingredient from a previously sterile-filtered solution
thereof
Oral compositions generally include an inert diluent or an edible carrier. For
the purpose of
oral therapeutic administration, the active compound can be incorporated with
excipients and used
in the form of tablets, troches, or capsules, e.g., gelatin capsules. Oral
compositions can also be
prepared using a fluid carrier for use as a mouthwash. Pharmaceutically
compatible binding agents,
and/or adjuvant materials can be included as part of the composition. The
tablets, pills, capsules,
troches and the like can include any of the following ingredients, or
compounds of a similar nature:
a binder such as microcrystalline cellulose, gum tragacanth or gelatin; an
excipient such as starch or
lactose, a disintegrating agent such as alginic acid, Ptimogel, or cornstarch;
a lubricant such as
-23-
CA 03037712 2019-03-20
WO 2018/057624
PCT/US2017/052516
magnesium stearate or sterotes; a glidant such as colloidal silicon dioxide; a
sweetening agent such
as sucrose or saccharin; or a flavoring agent such as peppermint.
For administration by inhalation, the compounds are delivered in the form of
an aerosol
spray from pressured container or dispenser that contains a suitable
propellant, e.g., a gas such as
carbon dioxide, or a nebulizer.
Systemic administration can also be by transmucosal or transdermal means. For
transmucosal or transdermal administration, penetrants appropriate to the
barrier to be permeated
are used in the formulation. Such penetrants are generally known in the art,
and include, for
example, for transmucosal administration, detergents, bile salts, and fusidic
acid derivatives.
Transmucosal administration can be accomplished through the use of nasal
sprays or suppositories.
For transdermal administration, the active compounds are formulated into
ointments, salves, gels,
or creams as generally known in the art.
The compounds can also be prepared in the form of suppositories (e.g., with
conventional
suppository bases such as cocoa butter and other glycerides) or retention
enemas for rectal delivery.
Examples
The invention is illustrated herein by the experiments described by the
following examples,
which should not be construed as limiting. The contents of all references,
pending patent
applications and published patents, cited throughout this application are
hereby expressly
.. incorporated by reference. Those skilled in the art will understand that
this invention may be
embodied in many different forms and should not be construed as limited to the
embodiments set
forth herein. Rather, these embodiments are provided so that this disclosure
will fully convey the
invention to those skilled in the art. Many modifications and other
embodiments of the invention
will come to mind in one skilled in the art to which this invention pertains
having the benefit of the
teachings presented in the foregoing description. Although specific terms are
employed, they are
used as in the art unless otherwise indicated.
Example 1: Pharmacological Effects of Aminosterol MSI-1436 Administration on
the Zebrafish
Appendage
The purpose of this example was to evaluate the pharmacological effect of
aminosterol
1436 administration on the regeneration of the caudal or tail fins of
zebrafish. Wildtype adult
zebrafish were treated with MSI-1436 or squalamine or a control of phosphate
buffered saline
-24-
CA 03037712 2019-03-20
WO 2018/057624
PCT/US2017/052516
solution (PBS) over a four-day period via daily intraperitoneal
microinjections. The adult zebrafish
were bred and housed in the Yin laboratory at Mount Desert Island Biological
Laboratory. The
microinjections of PBS, squalamine and MSI-1436 were performed for the
duration of the
experiments at concentrations of 0.125 mg/kg which corresponds to 50 ng/300 mg
body weight
which translates to 5-200 mg per dose in humans. On average, a Sul volume of
10 ug/ml solution
was delivered into each animal with a custom made 10 ul glass syringe
(Hamilton part # 80008).
The tail or caudal fins were amputated following the second day of
intraperitoneal
microinjections. These amputations were accomplished using a razor blade to
remove
approximately 50% of the caudal fins each in a manner perpendicular to the
direction of bony ray
growth. The zebrafish were maintained at standard conditions of 28 C at all
times during the
experiments. At 4 days post-amputation (4 dpa) through 14 dpa, caudal fins
were imaged with an
Olympus MVX10 stereomicroscope and the length of regenerated tissue from the
amputation plane
was quantified using Adobe Photoshop.
FIG. 2 shows the experimental results. (The dashed lines correspond to the
amputation
planes; the scale bar is 1 mm; * corresponds to the Student's ttest p-value <
0.01; error bars
correspond to SEM; n corresponds to 8-10 fish per group.) MSI-1436 stimulated
appendage
regeneration 2-fold four days after amputation (4 dpa), as shown in FIG. 2.
MSI-1436 treated
animals exhibited approximately 200% greater regenerated length when compared
to the control
and the squalamine microinjected groups, when evaluated at 4 days post-
amputation (4 dpa) prior
to full repair of the tail. In other words, the rate of regeneration was 200%
greater for those animals
receiving MSI-1436 than for those receiving either phosphate buffered saline
(PBS) or squalamine.
Each of the experiments was conducted with at least 6 animals per group. Each
experiment was
repeated four times and each time, the same enhancement of regeneration was
observed in the
caudal fins of the adult zebrafish. FIG. 1 reflects the results of all four
experiments. Although the
rate of regeneration was increased by the administration of MSI-1436, the
restored tail was
anatomically normal.
The zebrafish caudal fins from the control and MSI-1436 treated animals were
extracted at
four days post-amputation (4 dpa) and stained with an antibody directed
against phosphorylated
histone 3 (H3P) as a marker of cell proliferation. FIG. 3 shows the results of
this study.
(Arrowheads highlight subset of H3P positive cells; * corresponds to Student's
ttest p-value <
0.001; error bars correspond to SEM; n corresponds to 6 fish per group). The
blastema cells of
MSI-1436 treated caudal fins demonstrated a significant 2-fold increase in
cellular proliferation.
-25-
CA 03037712 2019-03-20
WO 2018/057624
PCT/US2017/052516
Wildtype adult zebrafish were subjected to caudal fin amputation and treated
daily with
either control PBS or MSI-1436 as described above in the context of FIG. 1 for
14 consecutive days
during regeneration. The results of this study are shown in FIG. 4. .
(Arrowhead = amputation
plane; n=4-6 per group). At the end of 14 days of microinjections, the MSI-
1436 treated animals
did not display any overgrowth of the regenerated tissue, as shown in FIG. 4.
By all measurements,
the MSI-1436-treated and control groups displayed the same amount of
regenerated tissue at 14
days post-amputation (14 dpa), a time when zebrafish tail regeneration is
normally completed.
Example 2: Pharmacological Effects of Aminosterol MSI-1436 Administration on
the Injured
Zebrafish Heart
The pharmacological effect of aminosterol MSI-1436 administration on the
regeneration of
zebrafish adult hearts was tested using the same experimental paradigm
described under the first
study of example 1.
Adult zebrafish of 6-8 months old, were subjected to partial ventricular
resection procedures
followed by daily treatments with intraperitoneal microinjections of PBS
(control) or squalamine or
MSI-1436 at concentrations of 0.125 mg/kg. The ventricular resection
procedures removed ¨20%
of the ventricular apex of each heart and challenged each animal to regenerate
new heart tissue. At
three days post-amputation (3 dpa), hearts were extracted, fixed,
cyrosectioned and stained with
antibodies directed against Mef2 and PCNA. Cardiomyocyte proliferation indices
were determined
.. for each group by representing Mef2+PCNA+cells as a percent of total
Mef2+cells within each
heart. The results of this study are shown in FIG. 5. (* = Student's ttest p-
value < 0.05; error bars=
SEM; n = 4 per group). Animals treated with the MSI-1436 microinjections
demonstrated ¨2-3-fold
greater rate of heart regeneration, as quantified by cardiomyocyte
proliferation indices when
compared to animals microinjected with the PBS alone or the squalamine alone,
as illustrated in
.. FIG. 5. These results were reproducible in three separate experiments of 4-
6 animals per group.
Wildtype adult zebrafish were subjected to a partial resection procedure as
described above
and allowed to regenerate for 24 hours post-amputation (24 hpa) or 5 days post-
amputation (5 dpa)
and hearts were extracted for total RNA isolation for quantitative PCR
studies. The results are
shown in FIG. 6. (* = Student's ttest p-value < 0.05; error bars= SEM; n = 4
per group) Injury to
.. untreated adult zebrafish hearts prompted a decrease in expression of
PTP1B, a known target gene
regulated by MSI-1436, as depicted by FIG. 6. Levels of PTP1B significantly
decreased during the
-26-
CA 03037712 2019-03-20
WO 2018/057624
PCT/US2017/052516
early phases of heart injury, suggesting PTP1B normally functions to repress
heart regeneration
genetic programs.
To determine if MSI-1436 is capable of rescuing defects in heart regeneration,
we
microinjected antisense oligonucleotides to bind and remove activity of the
let-7 family of small
RNAs (miRNAs). Wildtype adult zebrafish were injured via the partial
ventricular resection
procedure described above and treated with either 1) control of PBS, 2) MSI-
1436, 3) miRNA
depletion, and 4) miRNA depletion and MSI-1436. At three days post-amputation
(3 dpa), hearts
were extracted and stained to detect Mef2 +PCNA + cells. Cardiomyocyte
proliferation indices
were determined as a percent of Mef2+PCNA+cells within the total cardiomyocyte
population in
each heart. The results of this study are shown in FIG. 7. (* = Student's
ttest p-value < 0.05; error
bars = SEM; n = 8-12 hearts per group). The inclusion of MSI-1436 enhanced
cardiomyocyte
proliferation indices by 2-fold when compared to control hearts. Depletion of
the let-7 family of
miRNAs with antisense nucleotides inhibited cardiomyocyte proliferation index
by approximately
45%. In animals treated with miRNA depletion and MSI-1436, the cardiomyocyte
proliferation
index was restored to wildtype control levels.
Example 3: Pharmacological Effects of Aminosterol MSI-1436 Administration on
Normal Green
Monkey Kidney Cells
This study was conducted to determine the effects of MSI-1436 on the cellular
proliferation
of normal, uninjured mammalian cells. The results of the study are shown in
FIG. 8.
Normal, green monkey kidney cells were cultured with either control media of
PBS or
supplemented with 0.1 g/m1 or 1.0 i_rg/m1 of MSI-1436. Growth media with and
without MSI-1436
were replaced daily and total cell numbers were determined for each group over
a period of 2-
weeks. Cellular proliferation plots over the course of two-weeks show normal
growth curves for all
groups. MSI-1436 treatment did not promote overgrowth or hyper-proliferation
of these
mammalian cells. This suggests MSI-1436 does not have aberrant proliferative
activity on normal,
uninjured mammalian cells.
Example 4: Effects of Aminosterol MSI-1436 Administration on Injured Mouse
Skeletal Muscle
Tissue
To quantify the effect of MSI-1436 on skeletal muscle repair and regeneration,
we used
Pwc7CreER/LuSEAP mice expressing a luciferase reporter in skeletal muscle stem
cells, which are
termed satellite cells. Expression of luciferase is coupled to expression of
the Pax7 gene, which is a
-27-
CA 03037712 2019-03-20
WO 2018/057624
PCT/US2017/052516
well described marker of satellite cell activation (Brack AS, Rando TA. Tissue-
specific stem cells:
lessons from the skeletal muscle satellite cell. Cell Stem Cell 2012;10(5):504-
14).
The right anterior tibialis skeletal muscle in Pax7CreER/LuSEAP mice was
injected in vivo
with 30 1 of 1.2% BaC12 solution (w/v in double distilled H20) to induce
muscle tissue injury.
After twenty-four hours, the aminosterol MSI-1436 was administered to the mice
by intraperitoneal
injection (IP) at a dose of 0.125 mg/kg and then every 3 days thereafter for
21 days. Luciferase
luminescence was measured in vivo as photon emission from the tissue surface
using a Xenogen
IVIS-Spectrum System (Caliper Life Sciences). Luminescence is expressed as
radiance
(p/sec/cm2/sr). Increased radiance indicates increased Pax7 expression and
increased satellite cell
activation. All studies were conducted in a randomized and blinded fashion.
FIG. 9a provides a comparison of luciferase luminescence in a control non-MSI-
1436
treated mouse and a mouse treated with 0.125 mg/kg MSI-1436 at 3 days post-
injury (dpi). The
MSI-1436 treated mouse shows much higher luminescence, which is evidenced by
the red, yellow
and green colors not present in the control mouse. Radiance is shown on the
color scale to the right
and ranges from lower (purple) to higher (red) levels.
FIG. 9b shows relative Pax7 gene expression from 3 to 21 dpi expressed as
luciferase
luminescence detected in MSI-1436 treated mice over luminescence detected in
control non-MSI-
1436 treated mice. One-to-one luminescence is shown by the dotted red line. At
7, 14 and 21 dpi,
Pax7expression and satellite cell activation are significantly (*P<0.02-0.04)
higher in MSI-1436
.. treated mice.
FIG. 9c compares the muscle tissue of control non-MSI-1436 treated mice and
mice treated
with 0.125 mg/kg MSI-1436 at 21 dpi. Normal muscle morphology was observed in
both the
control and the MSI-1436 treated muscle tissue. Thus, enhanced muscle
satellite cell activation
resulted in normal muscle regeneration.
Example 5: Effects of Previous Aminosterol MSI-1436 Administration on Re-
injured Mouse
Muscle Tissue
The right anterior tibialis skeletal muscle of Pax7CreER/LuSEAP mice was
injected with 30
1 of 1.2% BaC12 solution (w/v in double distilled H20) to induce injury of the
muscle tissue. After
twenty-four hours, the aminosterol MSI-1436 was administered to the mice by
intraperitoneal
injection (IP) at a dose of 0.125 mg/kg and then every 3 days thereafter for
21 days. Luciferase
luminescence was measured in vivo as photon emission from the tissue surface
using a Xenogen
IVIS-Spectrum System (Caliper Life Sciences). Luminescence is expressed as
radiance
-28-
CA 03037712 2019-03-20
WO 2018/057624
PCT/US2017/052516
(p/sec/cm2/sr). Increased radiance indicates increased Pax7 expression and
increased satellite cell
activation. All studies were conducted in a randomized and blinded fashion.
Two weeks following the initial 21-day testing period, the right anterior
tibialis skeletal
muscle of control and MSI-1436 treated mice was reinjured by injection of 30
1 of 1.2% BaC12
solution (w/v in double distilled H20). Fig. 10a shows images of luciferase
luminescence detected
in the control non-previously MSI-1436 treated mouse and the previously MSI-
1436 treated mouse
at 0 dpi and at 3 dpi following re-injection with BaC12. At 0 dpi (i.e., prior
to injury), both the
control non-previously MSI-1436 treated mouse and the mouse previously treated
with MSI-1436
show similar degrees of luminescence. However, at 3 dpi, the mouse previously
treated with MSI-
1436 shows much higher luminescence, which is evidenced by the red, yellow and
green colors not
present in the control mouse. Radiance is shown on the color scale to the
right and ranges from
lower (purple) to higher (red) levels.
FIG. 10b is a graph of radiance measured from a mouse not previously treated
with MSI-
1436 compared to a mouse treated previously with MSI-1436. At 3 dpi, radiance
is approximately
75% higher in the mouse previously treated with MSI-1436. Thus, the
experimental results show
that activation of skeletal muscle satellite cells is greater in the mouse
previously treated with MSI-
1436. It is concluded that MSI-1436 keeps skeletal satellite cells in a primed
state for activation in
response to a subsequent injury. This conclusion suggests that treatment with
MSI-1436 could be
intermittent rather than continuous in patients suffering repeated muscle
injury from a disease such
as, for a non-limiting example, muscular dystrophy, and in particular,
Duchenne muscular
dystrophy. Intermittent treatment has the potential for reduced potential side
effects and treatment
costs.
Example 6: Effect of Aminosterol MSI-1436 Treatment of Cultured Mouse Skeletal
Muscle Cells
Cultured mouse skeletal muscle satellite cells were treated in vitro with
saline (control) or
0.365 iJV1 MSI-1436. Ninety-six (96) hours after treatment, the cells were
fixed and stained with 5¨
ethyny1-2'¨deoxyuridine (EdU) and 4', 6-diamidino-2-phenylindole (DAPI). FIG.
1 la shows
images of cells stained with DAPI, which labels cell nuclei, and EdU, which
stains DNA of
proliferating cells. FIG. 1 lb shows the percentage of EdU positive cells. MSI-
1436 treatment
increased satellite cell proliferation in vitro by ¨75%.
In the present specification, the invention has been described with reference
to specific
embodiments thereof It will, be evident, however, that various modifications
and changes may be
made thereto without departing from the broader spirit and scope of the
invention. The specification
-29-
CA 03037712 2019-03-20
WO 2018/057624
PCT/US2017/052516
and drawings are, accordingly, to be regarded in an illustrative rather than a
restrictive sense. The
contents of all references, pending patent applications and published patents,
cited throughout this
application (including the Appendix and reference lists) are hereby expressly
incorporated by
reference as if set forth herein in their entirety, except where terminology
is not consistent with the
definitions herein. Although specific terms are employed, they are used as in
the art unless
otherwise indicated.
References Cited
All citations (e.g., scientific journal publications, patents, and other
reference material)
mentioned herein are hereby incorporated herein by reference to the same
extent as if each
individual citation was specifically and individually indicated to be
incorporated by reference.
1. Ahima, R. S., Patel, H. R., Takahashi, N., Qi, Y., Hileman, S. M., &
Zasloff, M. A. (2002).
Appetite suppression and weight reduction by a centrally active aminosterol.
Diabetes,
51(7), 2099-2104.
1. Alexander, R. T., Jaumouille, V., Yeung, T., Furuya, W., Peltekova, I.,
Boucher, A.,
Zasloff, M., et al. (2011). Membrane surface charge dictates the structure and
function of the
epithelial Na+/H+ exchanger. The EMBO journal, 30(4), 679-91.
doi:10.1038/emboj .2010.356.
2. Bence, K. K., Delibegovic, M., Xue, B., Gorgun, C. Z., Hotamisligil, G.
S., Neel, B. G., &
Kahn, B. B. (2006). Neuronal PTP1B regulates body weight, adiposity and leptin
action. Nat Med,
12(8), 917-924. doi:10.1038/nm1435.
3. Blum, N., & Begemann, G. (2012). Retinoic acid signaling controls the
formation,
proliferation and survival of the blastema during adult zebrafish fin
regeneration. Development
(Cambridge, England), 139(1), 107-16. doi:10.1242/dev.065391.
4. Chablais, F., & Jazwinska, A. (2010). IGF signaling between blastema and
wound
epidermis is required for fin regeneration. Development (Cambridge, England),
137(6), 871-9.
doi :10.1242/dev.043885.
5. Curado, S., & Stainier, D. Y. R. (2010). deLiver'in regeneration: injury
response and
development. Seminars in liver disease, 30(3), 288-95. doi:10.1055/s-0030-
1255357.
6. Frangioni, J. V., Beahm, P. H., Shifrin, V., Jost, C. A., & Neel, B. G.
(1992). The
nontransmembrane tyrosine phosphatase PTP-1B localizes to the endoplasmic
reticulum via its 35
amino acid C-terminal sequence. Cell, 68(3), 545-560. doi:10.1016/0092-
8674(92)90190-N.
-30-
CA 03037712 2019-03-20
WO 2018/057624
PCT/US2017/052516
7. Jazwinska, A., Badakov, R., & Keating, M. T. (2007). Activin-betaA
signaling is required
for zebrafish fin regeneration. Curr Biol, 17(16), 1390-1395. doi:S0960-
9822(07)01697-1 [pi]
10.1016/j.cub.2007.07.019.
8. Lantz, K. a, Hart, S. G. E., Planey, S. L., Roitman, M. F., Ruiz-White,
I. a, Wolfe, H. R., &
McLane, M. P. (2010). Inhibition of PTP1B by trodusquemine (MSI-1436) causes
fat-specific
weight loss in diet-induced obese mice. Obesity (Silver Spring, Md.), 18(8),
1516-23.
doi :10.1038/oby.2009.444.
9. Lee, Y., Grill, S., Sanchez, A., Murphy-Ryan, M., & Poss, K. D. (2005).
Fgf signaling
instructs position-dependent growth rate during zebrafish fin regeneration.
Development, 132(23),
.. 5173-5183.
10. Poss, K D, Keating, M. T., & Nechiporuk, A. (2003). Tales of
regeneration in zebrafish.
Dev Dyn, 226(2), 202-210.
11. Poss, Kenneth D. (2010). Advances in understanding tissue regenerative
capacity and
mechanisms in animals. Nature reviews. Genetics, 11(10), 710-22.
doi:10.1038/nrg2879.
12. Poss, Kenneth D, Wilson, L. G., & Keating, M. T. (2002). Heart
regeneration in zebrafish.
Science (New York, N.Y.), 298(5601), 2188-90. doi:10.1126/science.1077857
13. Rao, M. N., Shinnar, A. E., Noecker, L. A., Chao, T. L., Feibush,
B., Snyder, B.,
Sharkansky, I., et al. (2000). Aminosterols from the dogfish shark Squalus
acanthias. J Nat Prod,
63(5), 631-635..
14. Salmi, C., Loncle, C., Vidal, N., Laget, M., Letourneux, Y., & Brunel,
J. M. (2008).
Antimicrobial activities of 3-amino- and polyaminosterol analogues of
squalamine and
trodusquemine. J Enzyme Inhib Med Chem, 23(6), 860-865.
doi:10.1080/14756360701809910.
15. Selinsky, B. S., Smith, R., Frangiosi, A., Vonbaur, B., & Pedersen,
L. (2000). Squalamine is
not a proton ionophore. Biochim Biophys Acta, 1464(1), 135-141.
16. Selinsky, B. S., Zhou, Z., Fojtik, K. G., Jones, S. R., Dollahon, N.
R., & Shinnar, A. E.
(1998). The aminosterol antibiotic squalamine permeabilizes large unilamellar
phospholipid
vesicles. Biochim Biophys Acta, 1370(2), 218-234.
17. Sills, A. K., Williams, J. I., Tyler, B. M., Epstein, D. S., Sipos, E.
P., Davis, J. D., Mclane,
M. P., et al. (1998). Squalamine Inhibits Angiogenesis and Solid Tumor Growth
In Vivo and
.. Perturbs Embryonic Vasculature. Cancer Res. 1998 Jul 1; 58(13): 2784-2792.
18. Simons, B. D., & Clevers, H. (2011). Stem cell self-renewal in
intestinal crypt.
Experimental cell research, 317(19), 2719-24. doi:10.1016/j.yexcr.2011.07.010.
-31-
CA 03037712 2019-03-20
WO 2018/057624
PCT/US2017/052516
19. Stoick-Cooper, C. L., Weidinger, G., Riehle, K. J., Hubbert, C., Major,
M. B., Fausto, N., &
Moon, R. T. (2007). Distinct Wnt signaling pathways have opposing roles in
appendage
regeneration. Development, 134(3), 479-489. doi:dev.001123 [pi]
10.1242/dev.001123.
20. Sumioka, A., Yan, D., & Tomita, S. (2010). TARP phosphorylation
regulates synaptic
AMPA receptors through lipid bilayers. Neuron, 66(5), 755-67.
doi:10.1016/j.neuron.2010.04.035.
21. Sanchez Alvarado, A., & Tsonis, P. A. (2006). Bridging the regeneration
gap: genetic
insights from diverse animal models. Nature reviews. Genetics, 7(11), 873-84.
doi:10.1038/nrg1923.
22. Takahashi, N., Qi, Y., Patel, H. R., & Ahima, R. S. (2004). A novel
aminosterol reverses
diabetes and fatty liver disease in obese mice. J Hepatol, 41(3), 391-398.
doi :10.1016/j jhep.2004.05.006 S0168827804002119 [pi].
23. Taub, R. (2004). Liver regeneration: from myth to mechanism. Nat Rev
Mol Cell
Bio1,5(10), 836-847. doi:10.1038/nrm1489 nrm1489 [pi].
24. Yeung, T., Gilbert, G. E., Shi, J., Silvius, J., Kapus, A., &
Grinstein, S. (2008). Membrane
phosphatidylserine regulates surface charge and protein localization. Science
(New York, N.Y.),
319(5860), 210-3. doi:10.1126/science.1152066.
25. Yin, V. P., Thomson, J. M., Thummel, R., Hyde, D. R., Hammond, S. M., &
Poss, K. D.
(2008). Fgf-dependent depletion of microRNA-133 promotes appendage
regeneration in zebrafish.
Genes Dev, 22(6), 728-733. doi:22/6/728 [pi] 10.1101/gad.1641808.
26. Zasloff, M, Williams, J. I., Chen, Q., Anderson, M., Maeder, T.,
Holroyd, K., Jones, S., et
al. (2001). A spermine-coupled cholesterol metabolite from the shark with
potent appetite
suppressant and antidiabetic properties. Int J Obes Relat Metab Disord, 25(5),
689-697.
doi :10.1038/sj .ij o.0801599.
27. Zasloff, Michael. (2002). Organisms, 415(January), 389-395.
28. Zasloff, Michael, Adams, A. P., Beckerman, B., Campbell, A., Han, Z.,
Luijten, E., Meza,
I., et al. (2011). Squalamine as a broad-spectrum systemic antiviral agent
with therapeutic potential.
Proceedings of the National Academy of Sciences of the United States of
America, 108(38),
15978-83. doi:10.1073/pnas.1108558108.
-32-