Note: Descriptions are shown in the official language in which they were submitted.
,
LIQUID PHOTO INITIATING COMPOUND AND USES OF THE SAME
CLAIM FOR PRIORITY
[0001] This application claims the benefit of priority of Taiwan Patent
Application No.
107124654 filed on July 17, 2018.
BACKGROUND OF THE INVENTION
Field of the Invention
[0002] The present invention relates to a liquid photo initiating compound and
photopolymerizable compositions using the same.
Descriptions of the Related Art
[0003] A photoinitiator is a substance that forms free radicals via Norrish
Type I photo-
fragmentation after absorbing the energy of visible or UV light, which can
initiate
polymerization of monomers or oligomers to provide a curing effect.
[0004] In 1990, Ciba Specialty Corporation introduced Irgacure 369, which is
the first
photoinitiator specifically designed for dark color UV ink (related patent: US
5,077,402).
Irgacure 369 is in solid form and its structure is shown below.
[Irgacure 369]
1
CA 3039209 2021-04-15
410
0
N 0
[0005] Irgacure 369 has an outstanding photo speed and other advantages like
odorless prior
to and after curing. However, it suffers from poor solubility in most UV inks
and thus requires
additional grinding and heating processes to facilitate dissolution. Even
though, Irgacure 369
is prone to precipitate when dosage is over 4 wt% and when handled in cold
areas. Irgacure
369 has recently been shown to have reproductive toxicity and thus is under
radar in EU to be
prohibited from use in food contact applications.
[0006] In order to improve the solubility issue, Ciba Specialty Corporation
introduced
another solid photoinitiator product, i.e., Irgacure 379 (related patent: US
7,247,659 B2).
Irgacure 379 does have improved solubility in some monomers and resins than
Irgacure 369.
Still, it is not easy to use in its solid form that requires grinding and
heating processes in order
to dissolve into an ink composition. Furthermore, Irgacure 379 also has
reproductive toxicity.
[Irgacure 379]
0
o N
2
CA 3039209 2019-04-04
[0007] In recent years, the demand for low viscosity UV inks such as digital
UV and flexo
UV inks has increased significantly. A photoinitiator in liquid form with high
solubility is
therefore highly desired to the low viscosity ink as these inks do not provide
sufficient shearing
force to perform the grinding and dispersion processes for solid
photoinitiators.
[0008] Examples of liquid photoinitiators include the following compounds 93,
95, 96 and
100 as disclosed in US 5,077,402. But these compounds are not commercialized
because their
poor photospeeds as well as odor issues.
[US 5,077,402]
\o N/
Compound 93 Compound 95
Compound 96 Compound 100
3
CA 3039209 2019-04-04
[0009] TW 1277834 discloses a liquid photoinitiator in Example 3. Even though
the liquid
photoinitiator is odorless, it is not commercialized due to its poor photo
speed which is about
one tenth that of Irgacure 369.
[Example 3 of TW 1277834]
0
1110
"=24 El
N(Me)2
[0010] The following compound I is also a liquid photoinitiator and has a
photo speed
comparable to that of Irgacure 369. Unfortunately, the compound I has a
serious odor issue
after curing.
[Compound 1]
0
MN 411 N
[0011] TW 1564276 also discloses a liquid photoinitiator, i.e., the following
compound IIa,
which is comparable to Irgacure 369 in terms of photo speed and is odorless
prior to or after
curing. However, the compound 1Ia has high acute oral toxicity. Besides, it is
in dark-brown
color which is not satisfactory for some colors.
4
CA 3039209 2019-04-04
[Compound ha of TW 1564276]
0
0
[0012] Therefore, conventional liquid photoinitiators with individual defects
for dark and
black inks are far from satisfactory.
SUMMARY OF THE INVENTION
[0013] In view of the unsatisfaction of conventional photoinitiators, the
present invention
provides a photoinitiator derived from oxazolidine, which improves all the
aforementioned
defects associated conventional liquid photoinitiators for the black ink
system as: (1) it becomes
a liquid state at 50 C; (2) it has a photo speed comparable to that of
Irgacure" 369; (3) it is
odorless prior to and after curing; and (4) it does not have acute oral
toxicity. Furthermore,
its light color and high purity perfectly meet the requirements of the
industry regarding liquid
photoinitiators. Accordingly, the present invention involves at least the
objectives described
below.
[0014] It should be noted that neither US 5,077,402 nor US 7,732,504 B2
discloses the
photoinitiator of the present invention, even though the photoinitiators
disclosed in these patents
may have an oxazolidine structure. Furthermore, persons having ordinary skill
in the art
5
CA 3039209 2019-04-04
cannot expect the advantages of the photoinitiator of the present invention
based on the general
teaching of US 5,077,402 or US 7,732,504 B2. The advantages include being
liquid at a low
temperature of 50 C, high solubility in various monomers and oligomers,
odorless, comparable
photo speed to Irgacure 369, and no acute oral toxicity.
[0015] An objective of the present invention is to provide a liquid photo
initiating compound,
which is represented by the following Formula I:
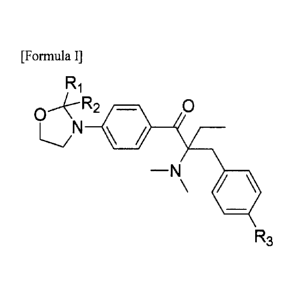
[Formula I]
Ri
O'eR2 0
-N
R3
wherein, in Formula I, RI and R2 are independently H or Ci-C3 alkyl, and R3 is
H or methyl.
Examples of Ci-C3 alkyl include methyl, ethyl, n-propyl and isopropyl.
[0016] In some embodiments of the present invention, the aforementioned
compound is
represented by the following Formula Ia:
[Formula Ia]
CA 3039209 2019-04-04 6
0 0
¨N
[0017] Another objective of the present invention is to provide a method of
initiating a
chemical reaction by using the aforementioned liquid photo initiating
compound.
[0018] Yet another objective of the present invention is to provide a
photopolymerizable
composition, comprising:
a first photoinitiator, which is the aforementioned liquid photo initiating
compound;
a photopolymerizable component; and
an optional solvent.
[0019] In some embodiments of the present invention, the photopolymerizable
component
is an olefinic unsaturated monomer, an olefinic unsaturated oligomer, or a
combination thereof
Examples of the olefinic unsaturated monomer include acrylate-based monomers.
Examples
of the olefinic unsaturated oligomer include acrylate-based oligomers.
[0020] In some embodiments of the present invention, the photopolymerizable
composition
further comprises a second photoinitiator selected from the group consisting
of acylphosphine
7
CA 3039209 2019-04-04
0
oxides, 9,10-dialkyloxyanthracene ( 'R ,
wherein R is alkyl), and combinations
thereof.
[0021] In
some embodiments of the present invention, the photopolymerizable composition
further comprises a photosensitizer selected from the group consisting of
benzophenones,
thioxanthones, Michler's ketones, anthraquinone, and combinations thereof.
[0022] In
some embodiments of the present invention, the photopolymerizable composition
further comprises an additive selected from the group consisting of pigments,
amine synergists,
and combinations thereof.
[0023] In
some embodiments of the present invention, the content of the first
photoinitiator
ranges from 0.1 wt% to 15 wt% based on the total weight of the
photopolymerizable
composition.
[0023a] In another aspect, the present invention resides in a compound of
Formula I:
Ri
0-k-R2 0
¨N
[Formula I] R3,
wherein, in Formula I, Ri and R2 are
independently H or Ci-C3 alkyl, and R3 is H or methyl.
[0023b] In another aspect, the present invention resides in use of the
aforementioned
compound as a liquid photo initiating compound.
8
Date Re9ue/Date Received 2020-10-13
[0024] To
render the above objectives, technical features and advantages of the present
invention more apparent, the present invention will be described in detail
with reference to
some embodiments hereinafter.
BRIEF DESCRIPTION OF THE DRAWINGS
Not applicable.
DESCRIPTION OF THE PREFERRED EMBODIMENT
8a
Date Re9ue/Date Received 2020-10-13
=
[0025] Hereinafter, some specific embodiments of the present invention will be
described in
detail. However, without departing from the spirit of the present invention,
the present
invention may be embodied in various embodiments and should not be limited to
the specific
embodiments described in the specification.
[0026] Unless it is additionally explained, the expressions "a,", "an", "the,"
or the like recited
in the specification (especially in the claims) should include both the
singular and the plural
forms.
[0027] Unless it is additionally explained, the term such as "first", "second"
or the like is
used to distinguish different elements or components, not terms supplying a
numerical limit.
[0028] Unless it is additionally explained, the term "alkyl" recited in the
specification
(especially in the claims) includes linear, branched and/or cyclic alkyl
groups.
[0029] Liquid photo initiating compound
[0030] The liquid photo initiating compound of the present invention is
represented by the
following Formula I:
[Formula I]
CA 3039209 2019-04-04 9
R
0 -* R2
N
R3
[0031] In formula I, RI and R2 are independently H or CI-C3 alkyl, and R3 is H
or methyl. In
some embodiments of the present invention, RI and R2 are independently methyl,
ethyl, n-
propyl or isopropyl, and R3 is H. In the appended examples, the first
photoinitiator is
represented by the following Formula Ia:
[Formula Ia]
0
¨N
[0032] The synthesis of the liquid photo initiating compound of the present
invention will be
described in the appended examples.
[0033] Uses of liquid photo initiating compound
[0034] The liquid photo initiating compound of the present invention is liquid
at a low
temperature of 50 C. Therefore, it can be used as a liquid photoinitiator
to trigger
CA 3039209 2019-04-04 10
=
polymerization and crosslinking of photopolymerizable monomers or oligomers
and thus,
provide a curing effect. The liquid photo initiating compound of the present
invention
surprisingly shows properties quite different from conventional
photoinitiators and has many
advantages. Specifically, the liquid photo initiating compound of the present
invention has
excellent photo speed performance, excellent solubility and light color and is
in liquid state at
a low temperature of 50 C. When used in a photopolymerizable system, the
influence of the
liquid photo initiating compound of the present invention on viscosity is very
minor. The
liquid photo initiating compound of the present invention also has long
storage stability.
Therefore, the liquid photo initiating compound of the present invention is
particularly useful
for low viscosity UV inks (e.g., an ink with a viscosity of less than 1000
cP), light color UV
inks, color resists, black matrixes and solder masks. Furthermore, the liquid
photo initiating
compound of the invention has high purity that can meet the chemical substance
registration
regulations of nations of the world.
[0035] Accordingly, the present invention also provides a photopolymerizable
composition,
comprising a first photoinitiator, a photopolymerizable component and an
optional solvent,
wherein the first photoinitiator is the compound represented by Formula I.
[0036] The species of the photopolymerizable component is not particularly
limited and can
be any substance that is photopolymerizable in the presence of a
photoinitiator, including the
substance that is generally used in photopolymerizable systems, like UV inks.
In some
embodiments of the present invention, the photopolymerizable component is
selected from the
CA 3039209 2019-04-04 11
group consisting of olefinic unsaturated monomers, olefinic unsaturated
oligomers and
combinations thereof. Examples of the olefinic unsaturated monomer include but
are not
limited to acrylate-based monomers, and examples of the olefinic unsaturated
oligomer include
but are not limited to acrylate-based oligomers.
[0037] The solvent is optional and can be any solvent that dissolves or
disperses the
components of the composition but does not react with the components,
including those
generally used in photopolymerizable systems such as UV inks. Examples of the
solvent
include but are not limited to water; aliphatic hydrocarbons, such as
dichloromethane,
trichloromethane, tetrachloromethane, n-hexane, and cyclohexane; aromatic
hydrocarbons,
such as toluene, benzene, and xylene; ketones, such as acetone, methyl ethyl
ketone, isobutyl
ketone and cyclohexanone; esters, such as ethyl acetate and butyl acetate;
alcohols, such as
methanol, ethanol, n-propanol and isopropanol; and ethers, such as
dimethylether, diethylether,
and methylethylether.
[0038] In the photopolymerizable composition of the present invention, the
amount of the
first photoinitiator is not particularly limited and can be optionally
adjusted by persons having
ordinary skill in the art. To obtain a better photocuring efficacy, the amount
of the
photoinitiator is usually from 0.1 wt% to 15 wt%, such as 1 wt%, 2 wt%, 3 wt%,
4 wt%, 5 wt%,
6 wt%, 7 wt%, 8 wt%, or 9 wt%, based on the total weight of the
photopolymerizable
composition. However, the present invention is not limited thereto.
CA 3039209 2019-04-04 12
[0039] In addition to the first photoinitiator, the photopolymerizable
composition of the
present invention can optionally further include other components that are
advantageous to the
efficacy of photoinitiation, like photosensitizing components or other photo
initiating
components. Therefore, in some embodiments of the present invention, the
photopolymerizable composition further comprises a second photoinitiator, a
photosensitizer,
or a combination thereof.
[0040] The second photoinitiator can be any photo initiating component other
than the first
photoinitiator. Examples of the second photoinitiator include but are not
limited to
acylphosphine oxides, 9,10-dialkyloxyanthracene, and combinations thereof. The
species of
.. the photosensitizer is not particularly limited. Examples of the
photosensitizer include but are
not limited to benzophenones, thioxanthones, Michler's ketones, anthraquinone,
and
combinations thereof. In the appended examples, 2,4-diethylthioxanthone
(product name:
Chivacure DETX) is used as a second photosensitizer in the photopolymerizable
composition.
The amount of the second photoinitiator or the photosensitizer is not
particularly limited and
can be optionally adjusted by persons having ordinary skill in the art
depending on the need.
[0041] The photopolymerizable composition of the present invention may
optionally further
comprise one or more additives to impart desired properties to the cured
product of the
composition. For example, a pigment can be added to provide color to the cured
product, and
an amine synergist can be added to improve curing performance, for example, to
improve the
surface curing speed of composition and to enhance the mirror surface effect
after curing.
CA 3039209 2019-04-04 13
Examples of the pigment include titanium oxide, carbon black, cadmium red,
molybdate red,
chrome yellow, cadmium yellow, titanium yellow, chromium oxide, cobalt
titanate green,
cerulean blue, copper blue, azo pigments, phthalocyanine pigments,
quinacridone based
pigments, isoindolinonc based pigments, perylene based pigments, thio indigo
based pigments,
and metal complex pigments. As used herein, an amine synergist is well-known
to persons
having ordinary skill in the art and refers to a reactive amine that can
promote the photommation,
such as a low-molecular weight tertiary amine. Examples of the amine synergist
include but
are not limited to methyldiethanolamine, dibutylethanolamine, triethylamine
and
triethanolamine. Commercially available products of the amine synergist
include Chivacure
115, Chivacure EPD, Chivacure OPD, etc. available from Chitec Technology
Co., Ltd.
[0042] The present invention is further illustrated by the following
embodiments.
[0043] Examples
[0044] Example 1: preparation of liquid photo initiating compound represented
by
Formula Ia
[0045] 34 g 2 -(dimethylamino)-1 - [(2 -hydroxyethyflami no 1pheny11-2 -(pheny
lmethyl)- 1 -
butanone (CAS 862589-48-8); the synthesis method of it may refer to TW
1277834), 13.0 g 2,2-
dimethoxybutane (CAS 3453-99-4), 0.17 g PTSA (p-toluenesulfonic acid), and 100
mL toluene
were added in sequence to a 250 mL three-necked flask at room
14
Date Re9ue/Date Received 2020-07-13
temperature and then the reaction as shown below was carried out at reflux.
The by-product
methanol produced by this reaction was removed by Dean-Stark evaporator.
OMe
)</
HO HN Me0 0
0 N
PTSA, toluene
¨N
¨N
Ia \
[0046] The reaction was monitored by High Performance Liquid Chromatography
(HPLC).
After the reaction was completed, the reaction product was cooled to room
temperature and
washed with 17 g pure water for three times. The organic layer was collected
and then
concentrated under vacuum to obtain the compound represented by Formula Ia
(hereinafter
"photo initiating compound 1a") as a yellowish and viscous liquid. The yield
is 95%.
[0047] The photo initiating compound Ia was subjected to nuclear magnetic
resonance
analysis, and the results are as follows:
Nuclear magnetic NMR(500 MHz, CDC13): 0.70 (t, J = 7.5 Hz, 311), 0.82
(t, J = 7.5
resonance analysis: Hz, 3H), 1.85-1.89 (m, 1H), 1.94-1.99 (m, 1H), 2.04-2.09
(m, 114),
2.13-2.18(m, 1H), 2.36 (s, 6H),3.16-3.23 (m, 2H), 3.18 (d, J = 14.0Hz,
1H), 3.22 (d, J =14.0Hz, 1H), 4.06-4.10 (m, 1H), 4.12-4.26 (m, 1H),
6.60 (d, J = 9.0 Hz, 2H), 7.16-7.28 (m, 6H), 8.34 (d, J = 9.0 Hz, 211)
13C NMR(500 MHz, CDC13): 7.7, 9.8, 23.8, 27.8, 31.0, 35.5, 39.1,
48.4, 63.2, 73.8, 96.2, 111.5, 125.8, 125.9, 127.9, 131.3, 132.2, 139.6,
147.1, 201.6
CA 3039209 2019-04-04 15
[0048] Example 2: Preparation of a liquid photo initiating compound
represented by
Formula lb
[0049] 34 g 2 -(dimethylamino)-1 - [(2 -hydroxyethyl)ami no ] pheny1]-2 -
(pheny lmethyl)- 1 -
butanone, 12.0 g 2,2-dimethoxypropane (CAS 77-76-9), 0.17 g PTSA (p-
toluenesulfonic acid),
and 100 mL toluene were added to a 250 mL three-necked flask at room
temperature in sequence
and the reaction as shown below was carried out at a condition the same as the
previous example.
0 Me
/ 0
HO HN MeO)< 0
¨N PTSA, toluene
lb ¨N
[0050] The reaction was monitored by High Performance Liquid Chromatography
(HPLC).
After the reaction was completed, the reaction product was extracted with 17 g
pure water for
.. three times. The organic layer was collected and then concentrated under
vacuum to obtain
the compound represented by Formula Ib (hereinafter referred to as "photo
initiating compound
lb") as a yellowish and viscous liquid. The yield is 93%.
[0051] The photo initiating compound Ib was subjected to nuclear magnetic
resonance
analysis, and the results are as follows:
Nuclear magnetic 1H NMR(500 MHz, CDC13): 0.69 (t, J = 7.5Hz, 3H), 1.64 (s,
6H), 1.83-
resonance analysis: 1.90 (m, 1H), 2.02-2.17 (m, 1H), 2.36 (s, 6H), 3.17 (d,
J = 14.0Hz,
16
Date Re9ue/Date Received 2020-07-13
=
1H), 3.22 (d, J = 14.0Hz, Hi), 3.53 (t, J = 6.5Hz, 2H), 4.11 (t, J =
6.5Hz, 2H), 6.60 (d, J = 9.0Hz, 2H), 7.15-7.28(m, 6H), 8.35(d, J =
9.0Hz, 2H)
'3C NMR(500 MHz, CDC13): 9.8, 25.4, 27.8, 35.5, 39.1, 47.8, 62.9,
73.9, 93.9, 111.8, 125.8, 125.9, 127.9, 131.3, 132.2, 139.6, 147.0,
201.6
[0052] Example 3: Solubility test
[0053] Mixtures I to III were prepared according to the composition provided
below, and
then 6 parts by weight of photoinitiator Irgacure 369 (available from IGM),
photoinitiator R-
gen 919 (available from Chitec Technology Co., Ltd.) or photo initiating
compound Ia was
added to each of the mixtures I to III. The obtained mixtures were
ultrasonicated for 1 hour
at room temperature, and the dissolution of each of Irgacure 369, R-gen 919
and photo
initiating compound Ia was observed and described in the following Table 1.
Mixture I: a mixture of 50 parts by weight of 1,6-hexanediol diacrylate
(product name:
EM221, Eternal Materials Co., Ltd.) and 50 parts by weight of polyester
tetraacrylate (product name: Oligomer 6325-100, Eternal Materials Co.,
Ltd.) with a viscosity of 41.2 cP
Mixture II: a mixture of 50 parts by weight of trimethylolpropane
triacrylate (product
name: EM231, Eternal Materials Co., Ltd.) and 50 parts by weight of
polyester tetraacrylate (product name: Oligomer 6325-100, Eternal
Materials Co., Ltd.) with a viscosity of 349.5 cP
CA 3039209 2019-04-04 17
Mixture III: a
mixture of 50 parts by weight of ethoxylated pentaerythritol tetraacrylate
(product name: EM2411, Eternal Materials Co., Ltd.) and 50 parts by weight
of polyester tetraacrylate (product name: Oligomer 6325-100, Eternal
Materials Co., Ltd.) with a viscosity of 433.0 cP
[0054] Table 1: Dissolution of Irgacure 369, R-gen 919 and photo initiating
compound Ia
after one-hour ultrasonication at room temperature
Photoinitiator Mixture I Mixture II Mixture III
Irgacure 369 Completely Only a small part
Only a small part
dissolved was dissolved was dissolved
R-gen 919 Completely Completely Completely
dissolved dissolved dissolved
Photo initiating Completely Completely Completely
compound Ia dissolved dissolved dissolved
[0055] As shown in Table 1, the photo initiating compound Ia completely
dissolved in each
of the three mixtures after one-hour ultrasonication at room temperature. The
results indicate
that the photo initiating compound Ia is much better than the solid
photoinitiator Irgacure 369
and comparable to R-gen 919 in terms of solubility.
[0056] Example 4: Viscosity variation test
[0057] 4 parts by weight of photoinitiators Irgacure 369, R-gen 919, and
photo initiating
compound la were individually added to each of the mixtures Ito III, and the
obtained mixtures
were exposed to a 0 C atmosphere for 7 days. The viscosities before and after
the exposure
were measured and tabulated in the following Table 2.
[0058] Table 2: Viscosities (cP) before and after exposure to 0 C atmosphere
for 7 days
Photoinitiator Mixture I Mixture II Mixture III
CA 3039209 2019-04-04 18
=
=
Initial 7 days Initial _ 7 days
Initial 7 days
None 41.2 50.1 349.5 _ 401.0 433.0 548.0
Irgacure 369 52.7 60.4 385.0 Precipitate
487.0 Precipitate
R-gen 919 42.2 51.0 354.0 408.0 445.5 544.0
Photo initiating 41.6 51.2 351.3 406.7 433.1 547.8
compound Ia
[0059] As shown in Table 2, the photo initiating compound IA has the lowest
influence on
the viscosity of the mixture. In addition, after the exposure to a 0 C
atmosphere for 7 days,
the viscosity variation of the mixture with the photo initiating compound Ia
is similar to that of
the mixture without photoinitiator, and no precipitate was observed in the
mixture with the
photo initiating compound Ia. The results indicate that the photo initiating
compound Ia is
better than Irgacure 369 in terms of solubility and resin compatibility and
therefore is very
useful.
[0060] Example 5: Photo speed performance test
[0061] The black flexo formulation containing Oligomer 6325-100, EM2411 and
carbon
black (viscosity: 1750 cP) was mixed with a photoinitiator to carry out the
photo speed
performance test. Specifically, 100 parts by weight of the black flexo
formulation and 2 parts
by weight of Chivacure DETX (available from Chitec Technology Co., Ltd.) were
mixed, and
then 6 parts by weight of Irgacure 369, R-gen 998 (available from Chitec
Technology Co.,
Ltd.), R-gen 919, or photo initiating compound Ia was added to the obtained
mixture. The
resultant mixture was coated on a printable pearlescent film (wet film
thickness: 25.15 um);
and the coated printable pearlescent film was exposed to an UV light (Fusion
F300, D-bulb,
CA 3039209 2019-04-04 19
300 W/inch) for curing. The photo speed performance was observed and tabulated
in the
following Table 3.
[0062] Table 3: Photo speed performance of photoinitiator
Photoinitiator Irgacure 369 R-gen" 998 R-gen 919 Photo
initiating
compound Ia
Photo speed
120 70 120 120
(m/minute)
[0063] Theoretically, photoinitiators with electron withdrawing groups, such
as the photo
.. initiating compound Ia, usually have a lower photo speed due to the blue
shift effect. However,
as shown in Table 3, the photo initiating compound Ia surprisingly has an
outstanding photo
speed which is comparable to those of Irgacure 369 and R-gen 919 and
significantly better
than that of R-gen 998.
[0064] Example 6: Photoinitiator dosage test
[0065] The commercially available black flexo ink without a photoinitiator was
mixed with
a photoinitiator to carry out the photo speed performance test, and the effect
of the amount of
photo initiating compound Ia on the photo speed performance was evaluated.
Specifically,
100 parts by weight of the black Ilex formulation and 2 parts by weight of
Chivacure DETX
(photoinitiator; Chitec Technology Co., Ltd.) were mixed, and then a specific
amount of
Irgacure 369 or photo initiating compound Ia as shown in Table 4 was added to
the obtained
mixture. The resultant mixture was coated on a PET film with primer (means for
improving
adhesion) (dry film thickness: 5 gm). The coated PET film was exposed to an UV
light
CA 3039209 2019-04-04 20
(wavelength: 365 nm; 4 W/inch) for curing. The photo speed performance in each
case was
observed and tabulated in the following Table 4.
[0066] Table 4: Photo speed performance of photoinitiator in different amounts
Amounts Curing
performance (times of UV light treatment)
(parts by weight) 1 2 3 4 5
4 X X X A
Irgacure 369 ___________________________________________________________
8 Incomplete dissolution
4 X X X A 0
Photo initiating _________________________________________________________
8 A 0
compound Ia _____________________________________________________________
12 0
Description for curing results:
X Not tack-free
A Tack-free but not through-cure
0 Tack-free and through-cure
[0067] As shown in Table 4, the tested black flexo ink is an ink system that
is difficult to be
photocured, and the photocuring efficiency can be improved by increasing the
amount of the
photoinitiator. However, when 8 parts by weight of Irgacure 369 was added,
like the result
of the solubility test, the photoinitiator could not be completely dissolved
in the ink system,
making it impossible to carry out a photo speed test. By contrast, when the
amount of the
photo initiating compound Ia was increased, no solubility issue was observed
even if the amount
reaches 12 parts by weight. Furthermore, increasing the amount of the photo
initiating
compound Ia from 4 parts by weight to 8 parts by weight can significantly
improve the
CA 3039209 2019-04-04 21
=
photocuring speed; and only one time of UV light treatment was required to
achieve sufficient
curing when the amount of the photo initiating compound Ia was 12 parts by
weight.
[0068] Example 7: Acute oral toxicity test
[0069] The median lethal dose (LD50) test was carried out for each of
Irgacureg 369, R-geng
998, R-gen 919 and photo initiating compound Ia in accordance with Test
Guidelines 423:
acute oral toxicity, Organization for Economic Co-operation and Development
(OECD). The
results are tabulated in the following Table 5.
[0070] Table 5: Median lethal dose (LD50) of photoinitiator
Photoinitiator Irgacure 369 R-gen 998 R-geng 919 Photo initiating compound
Ia
LD50 (mg/kg) >5000 2500 500 >5000
[0071] As shown in Table 5, the median lethal dose (LD50) of the photo
initiating compound
Ia is comparable with that of Irgacure" 369, both exceeding 5000 mg/kg, which
is considered
as no acute oral toxicity.
[0072] As can be seen from the results of Examples 1 to 7, the liquid photo
initiating
compound of the present invention has excellent photo speed performance,
excellent solubility,
light color and no acute oral toxicity. The liquid photo initiating compound
of the present
invention is in liquid state at a low temperature of 50 C. When being used
in a
photopolymerizable system, the influence of the liquid photo initiating
compound of the present
invention on viscosity is very minor. The liquid photo initiating compound of
the present
CA 3039209 2019-04-04 22
invention also has long storage stability. Therefore, the liquid photo
initiating compound of
the present invention is particularly useful for low viscosity UV inks, light
color UV inks, color
resists, black matrixes and solder masks.
[0073] The above examples are used to illustrate the principle and efficacy of
the present
invention and show the inventive features thereof. People skilled in this
field may proceed
with a variety of modifications and replacements based on the disclosures and
suggestions of
the invention as described without departing from the principle and spirit
thereof. Therefore,
the scope of protection of the present invention is that as defined in the
claims as appended.
CA 3039209 2019-04-04 23