Note: Descriptions are shown in the official language in which they were submitted.
PROCESS FOR SEPARATING PARTICLES CONTAINING ALKALI METAL SALTS
FROM LIQUID HYDROCARBONS
CROSS REFERENCE TO RELATED APPLICATIONS
[0001] This claims the benefit of and priority to U.S. Provisional
Application No. 62/404119
filed October 4, 2016, U.S. Provisional Application No. 62/513871 filed June
1, 2017, and U.S.
Application No. 15/446299 filed March 1, 2017.
FIELD OF THE TECHNOLOGY
[0002] The present technology relates to a process for removing particles
containing alkali
metal salts, such as alkali metal sulfides, from liquid hydrocarbons. It
further relates to
processes for converting carbon-rich solids into fuels
BACKGROUND OF THE TECHNOLOGY
[0003] Liquid hydrocarbons, including many oil feedstocks, often contain
difficult to
remove sulfur in the form of organosulfur compounds as well as metals and
other heteroatom-
containing compounds that hinder usage of the hydrocarbons. Sulfur can cause
air pollution,
and can poison catalysts used in petroleum processing or catalysts designed to
remove
hydrocarbons and nitrogen oxide from motor vehicle exhaust. There has been a
worldwide trend
to limit the amount of sulfur in hydrocarbon fuels, such as gasoline, diesel,
and fuel oils,
including marine bunker fuels. Metals contained in the hydrocarbon stream can
also poison
catalysts typically utilized for removal of sulfur through standard and
improved hydro-
desulfurization processes whereby hydrogen reacts under extreme conditions to
break down the
sulfur bearing organosulfur molecules.
[0004] Sodium has been recognized as potentially effective for the
treatment of high-sulfur
hydrocarbons, including petroleum oil distillate, crude, heavy oil, bitumen,
and shale oil.
Sodium is capable of reacting with the oil and its contaminants to
dramatically reduce the sulfur,
nitrogen, oxygen, and metal content through the foiniation of sodium sulfide
compounds
(sulfide, polysulfide and hydrosulfide) as well as other byproducts. However,
removal of these
byproducts from the treated feedstock can be challenging. The suspensions
and/or emulsions
the byproducts may form often cannot be completely removed using standard
separation
techniques, and can be difficult to carry out efficiently on an industrial
scale. Indeed, no large
1
Date Regue/Date Received 2022-07-29
scale desulfurization using sodium metal or other alkali metals is in regular
commercial use due
in part to this problem.
BRIEF SUMMARY OF THF, TECHNOLOGY
[0005] The present technology provides a process for separating particles
containing alkali
metal salts from liquid hydrocarbons. Such mixtures result from the use of
alkali metals (in their
metallic state) to remove nitrogen, sulfur, oxygen and heavy metals from
liquid hydrocarbons
(e.g., oil feedstocks) contaminated with compounds containing such
heteroatoms. The process
includes heating a first mixture of elemental sulfur and particles comprising
an alkali metal
sulfide in a liquid hydrocarbon to a temperature of at least 150 C, to
provide a sulfur-treated
mixture comprising agglomerated particles. The process further includes
separating the
agglomerated particles from the sulfur-treated mixture to provide a
desulfurized liquid
hydrocarbon and separated solids. The present technology further provides
methods for removal
of residual alkali metal from the desulfurized liquid hydrocarbons and for
preparing the
separated solids for electrochemical regeneration of the alkali metal. The
desulfurized and
demetallized liquid hydrocarbons produced typically have less than 0.5 wt%
sulfur and less than
100 ppm alkali metal, and meet, for example, contaminant limits for bunker
fuel without further
processing. This process is also applicable to processes for converting carbon-
rich solids into
fuels.
[0006] The foregoing is a summary of the disclosure and thus by necessity
contains
simplifications, generalizations, and omissions of detail. Consequently, those
skilled in the art
will appreciate that the summary is illustrative only and is not intended to
be in any way
limiting. Other aspects, features, and advantages of the processes described
herein, as defined
by the claims, will become apparent in the detailed description set forth
herein and taken in
conjunction with the accompanying drawings.
BRIEF DESCRIPTION OF THE DRAWINGS
[0007] In order that the manner in which the above-recited and other features
and advantages of
the technology are obtained will be readily understood, a more particular
description of the
technology briefly described above will be rendered by reference to specific
embodiments
thereof that are illustrated in the appended drawings. Understanding that
these drawings depict
only typical embodiments of the technology and are not therefore to be
considered to be limiting
2
Date Regue/Date Received 2022-07-29
of its scope, the technology will be described and explained with additional
specificity and detail
through the use of the accompanying drawings in which:
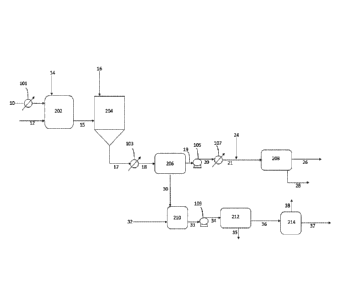
[0008] FIG. 1 shows a process flow diagram for an illustrative embodiment
of a process for
separating particles containing alkali metal sulfide from a liquid hydrocarbon
and optional
additional processes for removing residual alkali metal from the product
hydrocarbon and
preparing the separated solids for regeneration of alkali metal, as well as
the process for removal
of impurities from oil using an alkali metal which generates the mixtures of
particles and liquid
hydrocarbon.
[0009] FIG. 2 shows a process flow diagram for an illustrative embodiment
of the present
technology for converting coke to a liquid fuel while simultaneously reducing
or removing
sulfur, metals and other heteroatoms.
DETAILED DESCRIPTION OF THE TECHNOLOGY
[0010] The following temis are used throughout as defined below.
[0011] As used herein and in the appended claims, singular articles such
as "a" and "an" and
"the" and similar referents in the context of describing the elements
(especially in the context of
the following claims) are to be construed to cover both the singular and the
plural, unless
otherwise indicated herein or clearly contradicted by context. Recitation of
ranges of values
herein are merely intended to serve as a shorthand method of referring
individually to each
separate value falling within the range, unless otherwise indicated herein,
and each separate
value is incorporated into the specification as if it were individually
recited herein. All methods
described herein can be performed in any suitable order unless otherwise
indicated herein or
otherwise clearly contradicted by context. The use of any and all examples, or
exemplary
language (e.g., "such as") provided herein, is intended merely to better
illuminate the
embodiments and does not pose a limitation on the scope of the claims unless
otherwise stated.
No language in the specification should be construed as indicating any non-
claimed element as
essential.
[0012] As used herein, "about" will be understood by persons of ordinary
skill in the art and
will vary to some extent depending upon the context in which it is used. If
there are uses of the
term which are not clear to persons of ordinary skill in the art, given the
context in which it is
used, "about" will mean up to plus or minus 10% of the particular term.
3
Date Recue/Date Received 2022-07-29
100131 The present technology provides a process for separating particles
containing alkali
metal salts, including alkali metal sulfides, from liquid hydrocarbons. The
separation process
may be employed as part of a series of processes for desulfurizing and
otherwise removing
metals and other heteroatom contaminants from liquid hydrocarbons as well as
carbon-rich
solids in a liquid hydrocarbon including but not limited to bunker oil, as
well as petroleum oil
distillate, crude, heavy oil, bitumen, shale oil, and refinery intermediate
streams (for example,
solvent deasphalting tar, steam cracked tar, atmospheric or vacuum residuals,
FCC slurry,
visbreaker tar, hydrotreater, hydrocracker or hydroconversion bottoms, coke
and asphalt).
100141 Liquid hydrocarbons contaminated with sulfur compound(s), and
optionally one or
more nitrogen compounds, oxygen compounds and heavy metals, may be
desulfurized and
decontaminated by contacting the hydrocarbons with a molten alkali metal (in
its metallic state)
such as sodium, potassium or lithium (or mixtures or alloys thereof) to remove
the heteroatoms,
and to provide a mixture of the liquid hydrocarbon and particles comprising
alkali metal
sulfides. A capping agent is typically used to cap the radicals formed when
sulfur and other
heteroatoms have been abstracted by the alkali metal. The capping agent may be
hydrogen, a
C 1-6 acyclic alkane, C2-6 acyclic alkene, hydrogen sulfide, ammonia, or a
mixture of any two or
more thereof. The contacting step may be carried out at a temperature of about
250 C to about
400 C, for example at about 250 C, about 300 C, about 350 C, about 400 C or a
range
between and including any two of the foregoing temperatures. In some
embodiments the
contacting takes place at about 300 C to about 400 C (e.g, 350 C). The
contacting may take
place at a pressure of about 400 to about 1500 psi, e.g., at about 400 psi,
about 500 psi, about
600 psi, about 750 psi, about 1000 psi, about 1250 psi, about 1500 psi or a
range between and
including any two of the foregoing values.
[0015] The amount of alkali metal in its metallic state used in the
contacting step will vary
with the level of heteroatom contaminants of the liquid hydrocarbon, the
temperature used and
other conditions. For example, 1-3 mole equivalents of metallic alkali metal
and 1-1.5 moles of
capping agent (e.g, hydrogen) may be needed per mole sulfur, nitrogen or
oxygen. In addition
an excess of metallic alkali metal may be used to drive the reaction towards
completion, e.g., a
10%, 15%, 20%, 25%, 30%, 40%, 50% or more excess on a mole equivalent basis
may be used.
In some embodiments the molten alkali metal used is sodium metal. Any suitable
source of
molten alkali metal may be used, including, but not limited to
electrochemically generated
sodium, e.g., per US 8,088,270.
4
Date Regue/Date Received 2022-07-29
[0016] The reaction of metallic alkali metal with heteroatom contaminants
in the liquid
hydrocarbons is relatively fast, being complete within a few minutes, if not
seconds. Mixing the
combination of liquid hydrocarbon and metallic alkali metal further speeds the
reaction and is
commonly used for this reaction on the industrial scale. Hence, in some
embodiments the
contacting step is carried out for 1 minute to about 5, about 6, about 7,
about 9, about 10, about
15 minutes, or about 20 minutes, or is conducted for a time ranging between
and including any
two of the foregoing values.
[0017] The foregoing desulfurization reaction produces a mixture that
includes the liquid
hydrocarbon and particles comprising alkali metal sulfides. The particles are
quite fine (e.g., <
gm) and cannot be completely removed by standard separation techniques (e.g.,
filtration or
centrifugation) at this stage, especially when the liquid hydrocarbons have a
high viscosity, e.g.,
bottoms and fuel oils. In addition, unreacted metallic alkali metal may be
present in such
mixtures. Further processing as described below is needed to separate the
particles, and provide
desulfurized liquid hydrocarbons. In some cases, where the liquid hydrocarbons
are especially
high viscosity, the hydrocarbon feeds are optionally thermally pretreated
before the reaction
with alkali metal, e.g., 300-450 C for 30-60 minutes. Such thermal treatment
can reduce the
heteroatom content of the liquid hydrocarbon, reducing the amount of alkali
metal needed, and
simplifying the subsequent separation step.
[0018] Surprisingly, it has been found that a separation process
comprising heating a first
mixture of elemental sulfur and particles comprising an alkali metal sulfide
(e.g., sodium
sulfide) in a liquid hydrocarbon to a temperature of at least 150 C provides
a sulfur-treated
mixture comprising agglomerated particles that are now separable. The
separation process thus
further includes separating the agglomerated particles from the sulfur-treated
mixture to provide
a desulfurized liquid hydrocarbon and separated solids.
[0019] The separation process may include mixing the first mixture during
heating.
Depending on conditions (e.g., whether mixing is used, and how high the
temperature is, etc.)
the first mixture may be heated for a longer or shorter period of time. In
some embodiments, the
period of time is at least 15 minutes. In others, the first mixture is heated
for a period of about
minutes to about 2 hours, e.g., about 15 minutes, about 30 minutes, about 45
minutes, about 1
hour, about 1.25 hours, about 1.5 hours, about 1.75 hours, about 2 hours, or a
range between and
including any two of the foregoing values.
5
Date Regue/Date Received 2022-07-29
100201 In some embodiments of the separation process, the first mixture is
heated to a
temperature of about 150 C to about 450 C, e.g., about 150 C, about 200 C,
about 250 C, about
300 C, about 350 C, about 400 C, about 450 C, or to a temperature in a range
between and
including any two of the foregoing values. In some embodiments, the first
mixture is heated to a
temperature of about 300 C to about 400 C.
100211 The pressure at which the separation process takes place is not
critical and may be
carried out at a wide range of pressures, including atmospheric pressure. In
some embodiments,
the pressure may be about 15 psi to about 1500 psi, e.g., about 15 psi, about
25 psi, about 50 psi,
about 100 psi, about 200 psi, about 300 psi, about 400 psi, about 500 psi,
about 750 psi, about
1000 psi, about 1250 psi, about 1500 psi, or a range between and including any
two of the
foregoing values. More commonly, the pressure may be about 100 psi to about
400 psi. The
pressurized gas in the vessels in which the separation process takes place
includes hydrogen
(predominantly), but can also include one or more of CO, CO2, H2S and C1-6
alkanes and
alkenes (e.g., methane, ethane, ethylene, propane, propene, butane, etc.).
100221 In addition to alkali metal sulfides, the first mixture may include
alkali metal in its
metallic state, also referred to herein as "residual alkali metal". This is
especially true where a
molar excess of metallic alkali metal was used to generate the mixture of
liquid hydrocarbons
and particles of alkali metal sulfide. In some embodiments of the separation
process, the first
mixture comprises 1-100 wt% alkali metal in its metallic state with respect to
the weight of
alkali metal in the alkali metal sulfide. The first mixture may also include
alkali metal oxides
and/or metals other than alkali metals.
100231 The amount of elemental sulfur to be added may range from about 0.5
equivalents to
about 2 or even about 3 equivalents of sulfur atoms per two equivalents of
free sodium atoms
present (i.e., those in the metallic state, not ionic). In some embodiments,
the amount of
elemental sulfur added is 0.5, 0.6, 0.7, 0.8, 0.9, 1.0, 1.1, 1.2, 1.5, 1.75,
2, 2.5, 3.0 equivalents or
a range between and including any two of the foregoing values. In some
embodiments 0.8 to 1.2
equivalents of elemental sulfur is added. Depending on how much elemental
sulfur has been
added, the agglomerated particles may comprise an alkali metal sulfide and/or
alkali metal
hydrosulfide (e.g., sodium sulfide (Na2S) or sodium hydrosulfide (NaHS)). It
was surprisingly
discovered that when more than a stoichiometric amount of elemental sulfur was
added (i.e.,
more than 1 equivalent of sulfur atoms per 2 equivalents of sodium atoms, that
sodium
6
Date Regue/Date Received 2022-07-29
hydrosulfide begins to form rather than the expected sodium polysulfide). Thus
in some
embodiments, the alkali metal hydrosulfide may predominate.
[0024] In some embodiments of the separation process, separating the
agglomerated
particles from the sulfur-treated mixture includes filtering, settling, or
centrifuging the sulfur
treated mixture to provide the separated solids. Separating the agglomerated
particles from the
sulfur-treated mixture may conveniently be performed by centrifuging the
sulfur-treated mixture
at, e.g., about 15 C to about 150 C. In some embodiments, the centrifuging
takes place at
about 120 C to about 140 C.
[0025] In some embodiments, the separating process further includes mixing
the separated
solids with an organic liquid (suitable for dissolving any liquid hydrocarbon
on the separated
solids) and separating the separated solids from the organic liquid to provide
washed solids.
Any suitable organic liquid may be used, including but not limited to toluene,
xylene, hexanes,
diesel (e.g., coker diesel) and/or condensate (e.g., BTX condensate). The wash
liquid containing
residual desulfurized liquid hydrocarbons may be sent to a recovery process
(e.g., distillation) to
recover the organic liquid for reuse or may be mixed with the desulfurized
liquid hydrocarbon as
product oil. The washed solids may be dried if desired using standard means
and electrolyzed
as described in US Patent No. 8,088,270, to recover metallic alkali metal
(e.g., sodium) for
reuse.
[0026] The desulfurized liquid hydrocarbon resulting from the separation
process typically
contains not more than 0.5 wt% sulfur. In some embodiments, the desulfurized
liquid
hydrocarbon contains not more than 0.4 wt%, 0.3 wt%, 0.2 wt%, 0.1 wt% or even
0.05 wt%
sulfur. In certain embodiments, especially those intended for blending with
lower sulfur content
hydrocarbons, the desulfurized liquid hydrocarbon contains slightly more than
0.5 wt% sulfur,
e.g., 0.6 wt%. Thus, in some embodiments the desulfurized liquid hydrocarbon
contains from
about 0.05 wt% to about 0.6 wt%. Once the alkali metal sulfide and metals have
been separated
from the liquid hydrocarbons, sulfur and metals are substantially removed, and
nitrogen is
moderately removed; also, both viscosity and density are reduced (API gravity
is increased).
[0027] Depending on the nature of the desulfurized liquid hydrocarbons,
there may be
considerable alkali metal content remaining, e.g., up to and sometimes
exceeding 1% by weight.
In some embodiments, such residual alkali metal is present at a level of about
400 ppm to about
10,000 ppm, e.g., about 400, about 600, about 800, about 1,000, about 1,200,
about 1,400, about
1,600, about 2,000, about 2,500, about 3,000, about 4,000, about 5,000, about
7,500 or even
7
Date Regue/Date Received 2022-07-29
about 10,000 ppm or in a range between and including any two of the foregoing
values. Some
of the alkali metal content may be associated ionically at the sites where
heavy metals originally
held position or ionically associated with napthenates, or finely dispersed in
the metallic state, or
ionically associated with sulfur, oxygen, or nitrogen which is still bonded to
the organic
molecules of the oil.
[0028] Removal of the residual alkali metal from the oil is required
because the alkali metal
content is not permitted in most product applications, and most downstream
refining processes
are also sensitive to the presence of alkali metals. Also if a substantial
amount of alkali metal
were to leave the system, a large amount of make-up would be required to
sustain the process.
[0029] Hence, in another aspect the present technology provides a
demetallizing process
which includes adding a salt-forming substance to the desulfurized liquid
hydrocarbon to form a
second mixture, wherein the salt-forming substance converts the residual
alkali metal to an
alkali metal salt. Any suitable salt-forming substance may be used so long as
the resulting salt is
readily removed from the liquid hydrocarbons. In some embodiments, the salt-
forming
substance can be selected from the group consisting of elemental sulfur,
hydrogen sulfide,
formic acid, acetic acid, propanoic acid and water. In some embodiments,
acetic acid is used to
form sodium acetate salts, which are relatively easy to remove in their solid
form. Typically, the
amount of salt-forming substance added is equal to about 1 to about 4 times
the molar amount of
residual alkali metal, e.g., 1, 1.25, 1.5, 2, 2.5, 3, 3.5 mole equivalents or
a range between and
including any two of the foregoing values. For example, in some embodiments,
the amount is
equal to about 1 to about 2 mole equivalents.
[0030] In some embodiments, the addition of salt-forming substance may be
carried out at a
temperature of at least 150 C, e.g., a temperature of about 150 C, about 200
C, about 250 C,
about 300 C, about 350 C, about 400 C, about 450 C, or within a range between
and including
any two of the foregoing values. In some embodiments, the addition of salt-
forming substance
may be carried out at a temperature of about 150 C to about 450 C.
[0031] In certain embodiments, the addition of salt-fonning substance is
carried out at a
pressure of at least about 15 psi. In some embodiments the addition of salt-
forming substance is
carried out at a pressure of about 15 psi, about 25 psi, about 50 psi, about
100 psi, about 150 psi,
about 200 psi, about 250 psi, about 300 psi, about 400 psi, about 500 psi,
about 1,000 psi, about
1,500 psi, about 2,000 psi, about 2,500 psi or at a pressure in a range
between and including any
8
Date Regue/Date Received 2022-07-29
two of the foregoing values. For example, in some embodiments, the addition is
carried out at
about 50 psi to about 2,500 psi.
[0032] The demetallization process may include separating the alkali metal
salts from the
second mixture to provide a desulfurized and demetallized liquid hydrocarbon.
For example,
separating the alkali metal salts from the second mixture may include
filtering, settling, or
centrifuging the second mixture to remove the alkali metal salts and provide
the desulfurized
and demetallized liquid hydrocarbon.
[0033] The present embodiments of the present technology can be understood
by reference
to the drawings, wherein like parts are designated by like numerals
throughout. It will be readily
understood that the components of the present technology, as generally
described and illustrated
in the figure herein, could be arranged and designed in a wide variety of
different configurations.
Thus, the following more detailed description of the embodiments of the
methods and systems
of the present technology, as represented in FIG. 1 is not intended to limit
the scope of the
technology, as claimed, but is merely representative of present embodiments of
the technology.
In particular, although the present technology may be employed to separate any
mixture of alkali
metal sulfides from liquid hydrocarbons, FIG. 1 shows an embodiment in which
the mixture is
generated by reaction of alkali metal with a liquid hydrocarbon contaminated
with organosulfur
compounds. Optional processes for removing residual alkali metal from the
desulfurized
hydrocarbon and for preparing the separated alkali metal sulfides for further
processing are also
described.
[0034] As shown in FIG. 1, an oil feedstock 10 may be fed through an
optional heat
exchanger 101 to be preheated to about 150 C to about 350 C before entering
reactor 202. An
alkali metal 12 in its molten state is also fed to the reactor, along with a
radical capping agent 14
which may be hydrogen and/or a hydrocarbon such as methane, ethane, natural
gas, and the like.
The alkali metal is typically sodium metal but may also be lithium metal,
potassium metal, or
alloys or mixtures containing any two or more of these metals. The reactor may
be operated
batch-wise or continuously in the temperature range above the melting
temperature of the alkali
metal, typically between 150 ¨ 400 C but more preferably between 300 ¨ 360 C
to provide
faster reaction kinetics and reduce or avoid thermal cracking. The reaction
typically is carried
out at a pressure of 500 ¨ 2000 psi. Under these conditions, the alkali metal
often reacts with the
sulfur and other heteroatom contaminants in a matter of minutes (e.g., 1-20
minutes) to fouti
9
Date Regue/Date Received 2022-07-29
fine particles of alkali metal salts, including alkali metal sulfides. See,
e.g., U.S. Patent
8,088,270.
[0035] The resulting mixture of liquid hydrocarbon and alkali metal
salt/sulfide particles
exits the reactor through line 15 and is fed to a maturation vessel 204. There
it is combined with
elemental sulfur, which may conveniently (but is not require to be) in a
liquid form (e.g., at
130 C -160 C) via line 16 and heated to a temperature of at least about 150
C, typically with
mixing, for a period of 15 ¨ 120 minutes. More typically, the mixture is
heated to about 300 C
to about 450 C, or in some embodiments, about 300 C to about 400 C.
Although the
processes in reactor 202 and vessel 204 are preferably run continuously, they
may be performed
in the same vessel if the processes are run batch-wise. The sulfur-treated
mixture includes
agglomerated particles and exits vessel 204 via line 17, flowing into optional
heat exchanger 103
where heat is removed and optionally can be transferred back to the liquid
hydrocarbon feed via
the heat exchanger 101. The cooled sulfur-treated mixture (at, e.g., about 60¨
180 C, or even,
about 100-140 C) is fed via line 18 to a solid/liquid separation apparatus
206, which typically
includes a centrifuge but may also include a filter. The separated solids 30
exit the separation
apparatus for further processing. The resulting desulfurized liquid
hydrocarbon is free or
substantially free of solids but may contain about 400 to about 4,000 ppm or
more residual alkali
metal in its metallic form.
[0036] The desulfurized liquid hydrocarbon is pumped from the separation
apparatus 206
through line 19 using pump 105, and is sent through line 20 into an optional
heat exchanger 107
which if present, heats the desulfurized liquid hydrocarbon to about 250 C to
about 350 C.
From there the desulfurized liquid hydrocarbon flows through line 21 and is
combined with a
salt forming substance such as acetic acid from line 24 as described above.
The combination of
acetic acid or other salt forming substance and the residual alkali metal
forms additional solids
comprising alkali metal salts. These solids are removed by a second separation
apparatus 208
(e.g., a centrifuge) to yield the desulfurized and demetallized liquid
hydrocarbon product 26 and
alkali metal salt solids 28.
[0037] The separated solids 30 from apparatus 206 are transported via
chute or other suitable
means to the washing tank 210, where they are mixed with an organic liquid 32
(as defined
above) such as toluene, xylene, hexanes, diesel, condensate or combinations of
any two or more
thereof or some other organic liquid suitable for washing. The now washed
solids are pumped
out of the washing tank through line 33 using pump 109 and through line 34 to
another solids-
Date Regue/Date Received 2022-07-29
liquids separation apparatus 212 where most of the organic liquid 35 is
recovered. If the
recovered organic liquid is, e.g., diesel, it is stored with other
desulfiffized liquid hydrocarbons
for later sale as product. If the recovered organic liquid is not a fuel
product, it is reused as a
wash liquid. The washed solids are transported to a dryer where any residual
organic wash
liquid is removed in a dryer 214. In the drying step, the washed solids are
heated in a non-
oxidizing atmosphere to a temperature of, e.g., 150 ¨ 350 C to recover the
wash liquid 38 which
may be returned back to the process as a wash liquid in the wash tank 210. The
dryer could be
any commercially available process including paddle dryers, spray dryers or
indirectly fired
kilns. The dried washed solids are ready for recycling by, e.g.,
electrochemical treatment to
recover the alkali metal in its metallic state as described, e.g., in US
Patent No. 8,088,270 or
8,747,660.
[0038] In another aspect, the present technology also provides processes
for converting
carbon-rich solids into fuels. Carbon-rich solids (at room temperature) are
solids that contain at
least 75 wt% carbon. Examples include petroleum coke, asphaltenes, and coal.
Such carbon-
rich solids generally have at least 0.5 wt% sulfur prior to treatment by the
present process.
Hence, in one aspect the present technology provides a process that includes
treating a slurry or
suspension of a carbon-rich solid having at least 0.5 wt% sulfur in a liquid
hydrocarbon with a
molten alkali metal and a capping agent as above (hydrogen, a C1-6 acyclic
alkane, C2_6 acyclic
alkene, hydrogen sulfide, ammonia, or a mixture of any two or more thereof).
This process is
carried out at an elevated temperature and pressure. The process converts at
least a portion of
the carbon-rich solids into a liquid fuel (e.g., residual fuel or refinery
feedstocks) and particles
comprising alkali metal sulfides. The liquid fuels have a reduced sulfur,
metal and heteroatom
content compared to the starting solids, e.g., not more than 0.2 or even 0.1
wt% sulfur. Liquid
fuels of the present technology include any hydrocarbon or hydrocarbon mixture
that is/are used
in a liquid state and may be burned as fuel. Hence liquid fuels include not
only gasoline,
kerosene and diesel, but fuel oil, residual fuel, and the like.
[0039] In some embodiments, the present methods include treating a slurry
or suspension of
petroleum coke in a liquid hydrocarbon with a molten alkali metal and one or
more capping
agents at an elevated temperature and pressure to convert at least a portion
of the petroleum coke
into a liquid fuel and inorganic solids in admixture with the liquid
hydrocarbon.
[0040] The carbon-rich solids, e.g., petroleum coke, are not soluble in
the liquid
hydrocarbon and are therefore present as a slurry or suspension. The slurry or
suspension may
11
Date Regue/Date Received 2022-07-29
contain, e.g., from 1 wt% to 20 wt% based on the total mass of the solids and
the liquid
hydrocarbon. It will be understood that the slurries/suspensions may include
any suitable
percentage of carbon-rich solids within this range such as 1,2, 3, 4,5, 6, 7,
8, 9, 10, 11, 12, 13,
14, 15, 16, 17, 18, 19, or 20 wt%, or a range between and including any two of
the foregoing
values, such as 1-15 wt%, 3-15 wt%, or 4 or 5 wt% - 10 or 11 wt%.
100411 The liquid hydrocarbon used in the slurry or suspension is selected
to provide both a
good slurry or suspension and dissolve the liquid fuels produced. Thus, heavy
hydrocarbons
with sufficient densities to fluidize the petroleum coke or other carbon-rich
solids may be used
including, e.g., hydrocarbons having densities of 800 to 1100 kg/m3 at room
temperature.
Suitable densities include 800, 900, 1000, and 1100 kg/m3 or a range between
and including any
two of the foregoing values. Suitable liquid hydrocarbons include virgin crude
oil, bitumen,
refinery intemiediates, such as fluid catalytic cracker slurry, hydrocracker
bottoms or vacuum
residual, shale oil, or a residual fuel oil, and may be the same or different
from the liquid fuel
being produced by the present process. In some embodiments, the liquid
hydrocarbon itself may
be part of a composition that is in need of desulfurization because it
includes sulfur and
optionally other heteroatoms such as nitrogen, oxygen and metals (e.g.,
vanadium).
100421 Before being suspending in the liquid hydrocarbon, the petroleum
coke or other
carbon-rich solids are crushed and milled to a powder or dust to reduce
particle size and promote
the reaction with the molten alkali metal. For example the solids may be
milled so that the
majority of particles have a diameter of not more than 1 mm. In some
embodiments the
majority of particles range in size from 1 urn to 1 mm.
100431 In the present processes, molten alkali metal (i.e., in its
metallic state) is believed to
react with the coke (or other carbon-rich solids) and form liquid hydrocarbons
that can be used
as fuels. In some embodiments, the alkali metal may be lithium, sodium,
potassium or an alloy
thereof. While not wishing to be bound by theory, it is believed that the
during the present
processes, the alkali metal, e.g., sodium, reacts with the coke and hydrogen
according to the
following reaction:
R-S-R' + 2Na ¨> R + R'= + Na2S (equation 1)
R + R'= + H2 -) R-H + R'-H (equation 2)
12
Date Regue/Date Received 2022-07-29
[0044] In these chemical equations, R and R' represent carbon rich organic
constituents
found in coke, each covalently bonded to a sulfur atom. It is believed sodium
oxidizes, giving up
an electron to the carbon rich organic constituents R and R' to form reactive
radicals. These
radicals may react with capping agents such as hydrogen (i.e., H2) to form the
carbon rich
hydrocarbon molecules R-H and R'-H. The new molecules R-H and R'-H are
shorter, and have
lower specific gravity than the original molecule R-S-R' and are now suitable
for dissolution
into a hydrocarbon liquid, thus increasing the mass and volume of the
resulting liquid phase.
Solid phase comprises inorganic constituents including sodium sulfide and any
coke which does
not enter solution but also includes other inorganic constituents such as
metals, alkali oxides and
hydroxides, and nitrides. The solids can be separated from the liquid by one
of many methods
including centrifugation, filtration or gravimetric settling.
[0045] The reaction conditions for conversion of carbon-rich solids such
as petroleum coke
into fuel are within the same range for desulfurization of liquid hydrocarbon
alone and are
described above. For example, the radical capping agent may be hydrogen,
methane or other
agents as described above for desulfurization of liquid hydrocarbons. Likewise
the conversion
reaction is run at the same range of elevated pressures and temperatures as
the desulfurization
reaction described above.
[0046] Utilizing the present processes, a significant portion of the
carbon-rich solids is
converted into liquid fuel and particles comprising alkali metal sulfides. For
example, the
portion of petroleum coke converted to liquid fuel and particles comprising
alkali metal sulfides
may be at least 20 wt%. In some embodiments the portion converted is at least
20, 30, 40, or 50
wt% or a range between and including any two of the foregoing values, e.g., 20-
50 wt%.
[0047] After the reaction with alkali metal and capping agent, the alkali-
metal treated slurry
may contain not only unreacted particles of the carbon-rich solids, but
particles including alkali
metal salts. These particles may be separated from the liquid hydrocarbon/fuel
mixture using
the same elemental sulfur process as herein described. In addition, any of the
subsequent
processes described herein for processing the sulfur-treated liquid
hydrocarbon and separated
solids may also be employed. Alternatively, to aid in separation of alkali
metal sulfide-
containing solids form the fuel slurry, water or hydrogen sulfide may be used
in place of sulfur,
while mixing at the same temperatures and pressures described herein. In
favorable cases, the
separation process of U.S. Patent No. 9,688,920.
13
Date Regue/Date Received 2022-07-29
[0048] The processes of the present technology may further include
separating the particles
comprising alkali metal sulfides and any remaining carbon-rich solids (e.g.,
petroleum coke)
from the mixture of liquid hydrocarbon and liquid fuel. The same techniques
described above
for first mixture of liquid hydrocarbon and particles may also be used for
this aspect as well.
[0049] Those skilled in the art will appreciate that additional heaters
and coolers may be
located between the various vessels and reactors to heat or cool the oil or
slurry to appropriate
process operating temperatures or to more easily recover heat generated in the
process.
[0050] FIG. 2 shows an illustrative embodiment of the present processes.
Finely milled
(e.g., majority of particles between 1 m and 1 mm) petroleum coke 40 is added
to a stream of
liquid hydrocarbon, e.g., oil feedstock 10. The resulting slurry or suspension
is fed to a reactor
202 where it is combined with an alkali metal 12 (e.g., metallic sodium) and a
radical capping
agent 14, such as hydrogen gas, methane and/or other hydrocarbons, hydrogen
sulfide, or
ammonia, (or mixtures of any two or more) at elevated temperature and
pressure. Under the
reaction conditions the alkali metal is in a molten state. The reactants are
mixed in the reactor by
mixer 306 for a period of time (e.g., 5-60 minutes) to allow the conversion of
the coke into a
liquid fuel to take place and heteroatoms (such as sulfur and nitrogen) and
other heavy metals to
be removed from the liquid hydrocarbon feedstock 10 and coke 40. The product,
a mixture of
desulfurized feedstock, liquid fuel and inorganic solids such as alkali metal
sulfides, nitrides,
heavy metals and unreacted coke are sent to the maturation vessel 204, where
the mixture is
treated with elemental sulfur 16 as described above for FIG. 1. The
(optionally cooled) sulfur-
treated mixture is fed to a solid/liquid separation apparatus 206, which
typically includes a
centrifuge but may also include a filter. The separated solids 30 exit the
separation apparatus for
further processing, and the upgraded oil feedstock 39 containing the liquid
fuel produced from
the petroleum coke may be further treated as described above for FIG. 1. The
solids 30 may
include, e.g., alkali metal sulfides, alkali metal nitrides, heavy metals, and
unreacted residual
coke. The solids 110 may also be further processed as described for FIG. 1 to
regenerate the
alkali metal.
EXAMPLES
Example 1 ¨ Desulfiirization with Sodium Followed by Sulfur Treatment and
Solids Separation
14
Date Regue/Date Received 2022-07-29
[0051] A
vacuum residuum oil feedstock was treated with sodium metal in a pilot plant
using a continuous system essentially as shown in FIG. 1 under the following
conditions to yield
a mixture of treated oil and fine particles comprising sodium sulfide.
Oil Feed Rate: 66 kg/h
Sodium Feed Rate: 2.1 kg/h
Hydrogen Feed Rate: 250 g/h
Reaction Temperature: 352 C
Reaction Pressure: 1425 psi
[0052]
Aliquots of the mixture of oil and fine particles were mixed with elemental
sulfur
under the following batch conditions (Table 1). The amount of sulfur remaining
in the oil was
measured.
Table 1
Trial Oil Added Sulfur Temperature Pressure Hold
Time Wt%
(g) Added (g) ( C) (psi) (min) Sulfur
1A 200.3 0.678 350 300-350 60 0.17
1B 199.9 2.861 350 300-350 60 0.18
200 5.06 350 300-350 60 0.21
1D 200.1 8.371 350 300-350 60 0.54
Example 2¨ Continuous Desulfurization with Sodium Followed by Continuous
Sulfur
Treatment and Solids Separation
[0053] A
vacuum residuum oil feedstock was treated with sodium metal in a pilot plant
using a continuous system essentially as shown in FIG. 1 under the following
four trial
conditions to yield a mixture of treated oil and fine particles comprising
sodium sulfide.
Date Regue/Date Received 2022-07-29
Table 2
Trial Oil feed rate Sodium feed Hydrogen feed Reaction Reaction P
(kg/h) rate (g/min) rate (g/h) Temp. (deg C) (psi)
2A 65.8 35 250 378 750
2B 65.8 36 250 378 750
2C 65.7 35 250 350 750
2D 65.9 35 279 358 750
[0054] Trials 2A-2D provided four mixtures of sodium-reacted oil feedstock
and particles
containing sodium sulfide that were processed in a continuous fashion at a
pilot plant using the
present separation process in accordance with FIG. 1 and the conditions of
Trials 3A to 3D
shown below in Table 3. The amount of sulfur was measured in the desulfurized
liquid
hydrocarbons.
Table 3
Conditions Sulfur Feed Temperature Pressure Hold Time Wt%
Rate ( C) (psi) (min) Sulfur
(g/min)
3A 12.2 343 280 70 0.37
3B 35 . 350 300 70 0.4
3C 45 346 300 70 0.45
3D 10 350 300 70 0.48
Example 3 ¨Removal of Residual Sodium from Desulfurized Liquid Hydrocarbons.
[0055] After desulfurization and separation of solids in accordance with
the procedure of
Example 2, the desulfurized liquid hydrocarbons were treated with a mixture of
acetic acid and
sodium acetate in accordance with the process of FIG. 1 and under the
conditions shown below
in Table 4.
16
Date Regue/Date Received 2022-07-29
Table 4
Trials* Initial [Nal Acetic Temperature Time (min) Final [Na]
(PPm) Acid/Na ratio ( C) (PP111)
4A (Batch) 1500 1.0 350 30 72
4B 530 2.3 323 55 82
4C 530 6.4 313 5 72
4D 530 6.4 245 5 64
4E 530 6.4 267 55 11
*Continuous conditions except as indicated
Example 4 - Desulfurization of Petroleum Coke
[0056] Ten grams of petroleum coke were mixed with 90 grams of a residual
fuel oil, heated
to 80 C, then centrifuged at the same temperature. The petroleum coke solid
separated from the
residual fuel oil. The petroleum coke and residual fuel had the compositions
shown in Table 5
below.
Table 5
Substance C% H% N% S%
V(ppm) Ni(ppm)
Petroleum Coke 82.05 3.56 1.84 5.97 502 176
Residual Fuel Oil 85.9 10.87 0.50 1.50 89 37
Example 5:
[0057] A 5 weight % mixture of the petroleum coke with balance residual
fuel oil from
Example 4 was prepared. 700 grams was placed into a 1.8L Parr reactor with a
gas induction
impellor and cooling loop. The reactor was purged with hydrogen. After
purging, the sample
was heated to operating temperature of 358 C and pressure of 1514 psig. 22.71
grams molten
sodium was pumped into the reactor using an electromagnetic pump. Hydrogen
pressure was
maintained by pumping hydrogen into the system while measuring the flow. At
the end of the
run the reactor contents were allowed to cool. Gases were slowly releasecL The
flow rate of
gases were measured using flow meters and analyzed using an Agilent
Technologies Gas
Chromatograph, Model 7890A. The reactor contents were centrifuged to separate
the solids
from the liquids and the solids were then rinsed with toluene to remove
adhered liquids. The
toluene rinse was evaporated using a rotary evaporator and the remaining
liquid was added to
the centrifuged liquid. The solids were rinsed again with pentane. The pentane
rinse was
evaporated off using a rotary evaporator and the remaining liquid was combined
with the liquid
from the centrifuge. The solids and liquids were characterized in the same
manner as the original
17
Date Regue/Date Received 2022-07-29
sample and a mass balance was calculated to determine the liquid yield. The
final liquid had the
composition shown in Table 6 below.
Table 6
Substance C% H% N% S%
V(ppm) Ni(ppm)
Feed Mixture 85.71 10.50 0.57 1.72 109.5
44.1
Liquid Product 86.14 11.10 0.50 0.26 1.0 7.0
[0058] Hydrogen consumption was 14.7 standard liters. The liquid yield was
94.8 wt%.
Sulfur was substantially removed as well as a small amount of nitrogen. Of the
35 grams of
coke charged, bearing nearly 6% sulfur, approximately 11.3 grams went into the
product liquid
where the product was only 0.26% sulfur. Thus excluding the sulfur portion of
the coke, there
was 33 grams charged. Thus, approximately 34 wt% of the solid coke entered the
liquid phase
fuel product. The initial residual fuel had a specific gravity of 994 kg/m3
while the desulfurized
product with the coke additional was 980 kg/m3. The portion of the materials
considered
residuum were as shown in Table 7.
Table 7
Substance Fraction boiling above
524C
Petroleum coke 100%
Residual fuel 63.1%
Treated mixture product 53.8%
Example 6:
[0059] Example 5 was repeated except rather than 5 wt% petroleum coke, 10
weight % coke
was prepared then operated in the same manner except only 21.07 grams sodium
was added and
the operating pressure was slightly lower at 1499 psig. The final liquid had
the composition
shown in Table 7.
Table 7
Substance C% H% N% S%
V(ppm) Ni(ppm)
Feed Mixture 85.52 10.14 0.63 1.95 109.5
44.1
Liquid Product 87.06 10.92 0.52 0.17 2.3 5.4
[0060] Hydrogen consumption was 17.1 standard liters. The liquid yield was
85.45 wt%.
Sulfur was substantially removed as well as a small amount of nitrogen. Of the
70 grams coke
18
Date Regue/Date Received 2022-07-29
charged, bearing nearly 6% sulfur, approximately 19.4 grams went into the
product liquid where
the product was only 0.17% sulfur. Thus excluding the sulfur portion of the
coke, there was 65.9
grams charged. Thus approximately 29% of the solid coke entered the liquid
phase fuel product.
The initial residual fuel had a specific gravity of 994 kg/m' while the
desulfurized product with
the coke additional was 984 kg/m3.
[0061] Based on the specific gravity in the desulfurized product, the
final fuel is still a
residual fuel but now it meets the sulfur specification where there will be
very large demand and
will command a price similar to distillate while the starting materials,
especially the coke would
be priced substantially below the price of the product.
[0062] The solids can be treated and according to the procedures of Gordon
et. al. in U.S
Pat. No 8,088,270 to regenerate the sodium so it may be recycled to the
process.
[0063] Of course, other hydrocarbon liquids capable of dissolving organic
molecules rich in
carbon could have been utilized rather than the one selected. For example, if
the dissolving
liquid already has a low sulfur content then less sodium could be added to the
reaction and less
hydrogen donation required. It may be noted than when 5% coke was added, 34%
of the
desulfurized coke entered the liquid phase while when 10% coke was added only
29% of the
desulfurized coke entered the liquid phase. But if the coke has extremely low
value and the
product is priced similar to distillate, then accepting lower portions
entering the liquid phase
may still be desirable. Conversely, adding a lower relative amount of coke the
portion entering
the liquid phase is expected to be greater. Also, other liquids may be
conducive to increasing the
solubility of the treated coke. For example, asphaltenes are soluble in
toluene. Adding particular
hydrocarbons may be conducive to increasing the solubility of the molten
sodium treated
petroleum coke and are part of the scope of this invention.
EQUIVALENTS
[0064] While certain embodiments have been illustrated and described, a
person with
ordinary skill in the art, after reading the foregoing specification, can
affect changes,
substitutions of equivalents and other types of alterations to the processes
of the present
technology and products thereof as set forth herein. Each aspect and
embodiment described
above can also have included or incorporated therewith such variations or
aspects as disclosed in
regard to any or all of the other aspects and embodiments.
19
Date Recue/Date Received 2022-07-29
[0065] The present technology is also not to be limited in terms of the
particular aspects
described herein, which are intended as single illustrations of individual
aspects of the present
technology. Many modifications and variations of this present technology can
be made without
departing from its spirit and scope, as will be apparent to those skilled in
the art. Functionally
equivalent methods within the scope of the present technology, in addition to
those enumerated
herein, will be apparent to those skilled in the art from the foregoing
descriptions. Such
modifications and variations are intended to fall within the scope of the
appended claims. It is to
be understood that this present technology is not limited to particular
methods, feedstocks,
compositions, or conditions, which can, of course, vary. It is also to be
understood that the
terminology used herein is for the purpose of describing particular aspects
only, and is not
intended to be limiting. Thus, it is intended that the specification be
considered as exemplary
only with the breadth, scope and spirit of the present technology indicated
only by the appended
claims, definitions therein and any equivalents thereof.
[0066] The embodiments, illustratively described herein may suitably be
practiced in the
absence of any element or elements, limitation or limitations, not
specifically disclosed herein.
Thus, for example, the terms "comprising," "including," "containing," etc.
shall be read
expansively and without limitation. Additionally, the temis and expressions
employed herein
have been used as terms of description and not of limitation, and there is no
intention in the use
of such terms and expressions of excluding any equivalents of the features
shown and described
or portions thereof, but it is recognized that various modifications are
possible within the scope
of the claimed technology. Additionally, the phrase "consisting essentially
of' will be
understood to include those elements specifically recited and those additional
elements that do
not materially affect the basic and novel characteristics of the claimed
technology. The phrase
"consisting of' excludes any element not specified.
[0067] In addition, where features or aspects of the disclosure are
described in terms of
Markush groups, those skilled in the art will recognize that the disclosure is
also thereby
described in terms of any individual member or subgroup of members of the
Markush group.
Each of the narrower species and subgeneric groupings falling within the
generic disclosure also
form part of the invention. This includes the generic description of the
invention with a proviso
or negative limitation removing any subject matter from the genus, regardless
of whether or not
the excised material is specifically recited herein.
Date Regue/Date Received 2022-07-29
[0068] As will be understood by one skilled in the art, for any and all
purposes, particularly
in terms of providing a written description, all ranges disclosed herein also
encompass any and
all possible subranges and combinations of subranges thereof. Any listed range
can be easily
recognized as sufficiently describing and enabling the same range being broken
down into at
least equal halves, thirds, quarters, fifths, tenths, etc. As a non-limiting
example, each range
discussed herein can be readily broken down into a lower third, middle third
and upper third, etc.
As will also be understood by one skilled in the art all language such as "up
to," "at least,"
"greater than," "less than," and the like, include the number recited and
refer to ranges which
can be subsequently broken down into subranges as discussed above. Finally, as
will be
understood by one skilled in the art, a range includes each individual member.
[0069] Other embodiments are set forth in the following claims, along with
the full scope of
equivalents to which such claims are entitled.
21
Date Regue/Date Received 2022-07-29