Note: Descriptions are shown in the official language in which they were submitted.
CA 03042686 2019-05-02
WO 2018/085563 PCT/US2017/059754
RESPIRATORY EARLY WARNING SCORING SYSTEMS AND METHODS
Reference to Related Applications
The present application claims priority to Provisional U.S. Application No.
62/416,416, filed November 2, 2016 and entitled "Respiratory Early Warning
Scoring
Systems and Methods," which is incorporated in its entirety.
Background of the Invention
1. Field of the Invention
This invention is directed to methods and devices for improving non-invasive
ventilation therapy. Specifically, the invention is directed to methods and
devices for
adjusting non-invasive ventilation therapy based on impedance measurements of
the patient.
2. Description of the Background
Physiological Monitoring - History and Evolution
Patient monitoring is essential because it provides warning to patient
deterioration
and allows for the opportunity of early intervention, greatly improving
patient outcomes.
For example, modern monitoring devices can detect abnormal heart rhythms,
blood oxygen
saturation, and body temperature, which can alert clinicians of a
deterioration that would
otherwise go unnoticed.
The earliest records of patient monitoring reveal that ancient Egyptians were
aware
of the correlation between peripheral pulse and the heart beat as early as
1550 BC. Three
millennia passed before the next significant advancement in monitoring was
made, with
Galileo using a pendulum to measure pulse rate. In 1887, Waller determined
that he could
passively record electrical activity across the chest by using electrodes and
correlated the
signal to activity from the heart. Waller's discovery paved the way for the
use of electrical
signals as a method to measure physiological signals. However, it would still
take time
before scientists recognized the advantages of monitoring a physiological
signal in a clinical
environment.
In 1925, MacKenzie emphasized the importance of continuous recording and
monitoring of physiological signals such as the pulse rate and blood pressure.
He
specifically stressed that the graphical representation of these signals is
important in the
assessment of a patient's condition. In the 1960s, with the advent of
computers, patient
1
CA 03042686 2019-05-02
WO 2018/085563 PCT/US2017/059754
monitors improved with the addition of a real-time graphical display of
multiple vital signs
being recorded simultaneously. Alarms were also incorporated into monitors and
were
triggered when signals, such as a pulse rate or blood pressure, reached a
certain threshold.
The first patient monitors were used on patients during surgery. As patient
outcomes
were shown to improve, monitoring of vital signs spread to other areas of the
hospital such
as the intensive care unit and the emergency department. For instance, pulse
oximetry was
first widely used in operating rooms as a method to continuously measure a
patient's
oxygenation non-invasively. Pulse oximetry quickly became the standard of care
for the
administration of general anesthetic and subsequently spread to other parts of
the hospital,
including the recovery room and intensive care units.
The Growing Need for Improved Patient Monitoring
The number of critically ill patients presenting to the emergency department
is
increasing at a great rate, and these patients require close monitoring. It
has been estimated
that between 1-8% of patients in the emergency department require a critical
care procedure
.. to be performed, such as a cardiovascular procedure or a thoracic and
respiratory procedure
(mechanical ventilation, catheter insertion, arterial cannulation).
Physiological scores, such as the Mortality Probability Model (MPM), the Acute
Physiology and Chronic Health Education (APACHE), the Simplified Acute
Physiological
Score (SAPS) and the Therapeutic Intervention Scoring System (TISS) have shown
significant improvements in patient outcomes. Monitoring sick patients by
using
physiological scores and vital signs in their early stages of illness, even
prior to organ failure
or shock, improves outcomes. Close monitoring of patients allows for
recognition of patient
degeneration and the administration of the appropriate therapy.
However, current scoring methods do not accurately predict patient outcomes in
approximately 15% of ICU patients, and it may be worse for patients in a
respiratory
intensive care unit, which provide care in hospitals with large number of
patients with acute
respiratory failure. Furthermore, differences in currently monitored vital
signs such as blood
oxygenation occur late in the progression of respiratory or circulatory
compromise. Often
the earliest sign of patient degradation is a change in a patient's breathing
effort or
respiratory pattern.
2
CA 03042686 2019-05-02
WO 2018/085563 PCT/US2017/059754
Respiratory rate is recognized as a vital indicator of patient health and is
used to
assess patient status. However, respiratory rate alone fails to indicate
important
physiological changes, such as changes in respiratory volumes. Metrics derived
from
continuous volume measurements have been shown to have great potential for
determining
patient status in a wide range of clinical applications. However, there are
currently no
adequate systems that can accurately and conveniently determine respiratory
volumes,
which motivates the need for a non-invasive respiratory monitor that can trace
changes in
breath volume.
Shortcomings of Current Methods
Currently, a patient's respiratory status is monitored with methods such as
spirometry and end tidal CO2 measurements. These methods are often
inconvenient to use
and inaccurate. While end tidal CO2 monitoring is useful during anesthesia and
in the
evaluation of intubated patients in a variety of environments, it is
inaccurate for non-
ventilated patients. The spirometer and pneumotachometer are limited in their
measurements are highly dependent on patient effort and proper coaching by the
clinician.
Effective training and quality assurance are a necessity for successful
spirometry. However,
these two prerequisites are not necessarily enforced in clinical practice like
they are in
research studies and pulmonary function labs. Therefore quality assurance is
essential to
prevent misleading results.
Spirometry is the most commonly performed pulmonary function test. The
spirometer and pneumotachometer can give a direct measurement of respiratory
volume. It
involves assessing a patient's breathing patterns by measuring the volume or
the flow of air
as it enters and leaves the patient's body. Spirometry procedures and
maneuvers are
standardized by the American Thoracic Society (ATS) and the European
Respiratory
Society (ERS). Spirometry can provide important metrics for evaluating
respiratory health
and diagnosing respiratory pathologies. The major drawback of mainstream
spirometers is
that they require the patient to breathe through a tube so that the volume
and/or flow rate of
his breath can be measured. Breathing through the apparatus introduces
resistance to the
flow of breath and changes the patient's breathing pattern. Thus it is
impossible to use these
devices to accurately measure a patient's normal breathing. Breathing through
the apparatus
3
CA 03042686 2019-05-02
WO 2018/085563 PCT/US2017/059754
requires a conscious, compliant patient. Also, in order to record the metrics
suggested by the
ATS and ERS, patients must undergo taxing breathing maneuvers, which excludes
most
elderly, neonatal, and COPD patients from being able to undergo such an
examination. The
outcomes of the procedures are also highly variable dependent on patient
effort and
coaching, and operator skill and experience. The ATS also recommends extensive
training
for healthcare professionals who practice spirometry. Also, many physicians do
not have the
skills needed to accurately interpret the data gained from pulmonary function
tests.
According to the American Thoracic Society, the largest source of intrasubject
variability is
improper performance of test. Therefore much of the intrapatient and
interpatient variability
in pulmonary function testing is produced by human error. Impedance-based
respiratory
monitoring fills an important void because current spirometry measurements are
unable to
provide continuous measurements because of the requirement for patient
cooperation and
breathing through a tube. Therefore there is a need for a device that provides
near-real-time
information over extended periods of time (vs. spirometry tests which last a
minute or less)
in non-intubated patients that can show changes in respiration related to a
provocative test or
therapeutic intervention.
In order to acquire acceptable spirometry measurements, as dictated by ATS
standards, healthcare professionals must have extensive training and take
refresher courses.
A group showed that the amount of acceptable spirometry measurements was
significantly
greater for those who did a training workshop (41% vs. 17%). Even with
acceptable
spirometry measurements, the interpretations of the data by primary physicians
were
deemed as incorrect 50% of the time by pulmonologists. However, it was noted
that aid
from computer algorithms showed improvement in interpreting spirograms when
adequate
spirometry measurements were collected.
Rigorous training is needed for primary care clinics to acquire acceptable
spirometry
measurements and make accurate interpretations. However, resources to train a
large
number of people and enforce satisfactory quality assurance are unreasonable
and
inefficient. Even in a dedicated research setting, technician performance
falls over time.
In addition to human error due to the patient and healthcare provider,
spirometry
contains systematic errors that ruin breathing variability measurements.
Useful
measurements of breath by breath patterns and variability have been shown to
be
4
CA 03042686 2019-05-02
WO 2018/085563 PCT/US2017/059754
compounded by airway attachments such as a facemask or mouthpiece. Also, the
discomfort
and inconvenience involved during measurement with these devices prevents them
from
being used for routine measurements or as long-term monitors. Other less
intrusive
techniques such as thermistors or strain gauges have been used to predict
changes in
volume, but these methods provide poor information on respiratory volume.
Respiratory
belts have also shown promise in measuring respiration volume, but groups have
shown that
they are less accurate and have a greater variability than measurements from
impedance
pneumography. Therefore, a system that can measure volume for long periods of
time with
minimal patient and clinician interaction is needed.
Pulmonary Function Testing and Preoperative, Postoperative Care
Preoperative care is centered on identifying what patient characteristics may
put the
patient at risk during an operation and minimizing those risks. Medical
history, smoking
history, age, and other parameters dictate the steps taken in preoperative
care. Specifically,
elderly patients and patients with pulmonary diseases may be at risk for
respiratory
complications when placed under a ventilator for surgery. In order to clear
these patients for
surgery, pulmonary function tests such as spirometry are performed which give
the more
information to determine whether the patient can utilize the ventilator. Chest
x-rays may
also be taken. However, these tests cannot be replicated mid-surgery, or in
narcotized
patients or those who cannot or will not cooperate. Testing may be
uncomfortable in a
postoperative setting and disruptive to patient recovery.
End Tidal CO2 and Patient Monitoring
End tidal CO2 is another useful metric for determining pulmonary state of a
patient.
The value is presented as a percentage or partial pressure and is measured
continuously
using a capnograph monitor, which may be coupled with other patient monitoring
devices.
These instruments produce a capnogram, which represents a waveform of CO2
concentration. Capnography compares carbon dioxide concentrations within
expired air and
arterial blood. The capnogram is then analyzed to diagnose problems with
respiration such
as hyperventilation and hypoventilation. Trends in end tidal CO2 are
particularly useful for
evaluating ventilator performance and identifying drug activity, technical
problems with
intubation, and airway obstruction. The American Society of Anesthesiologists
(ASA)
mandates that end-tidal CO2 be monitored any time an endotracheal tube or
laryngeal mask
5
CA 03042686 2019-05-02
WO 2018/085563 PCT/US2017/059754
is used, and is also highly encouraged for any treatment that involves general
anesthesia.
Capnography has also been proven to be more useful than pulse oximetry for
monitoring of
patient ventilation. Unfortunately, it is generally inaccurate and difficult
to implement in the
non-ventilated patient, and other complementary respiratory monitoring methods
would
have great utility.
Echocardiograms
Fenichel et al. determined that respiratory motion can cause interference with
echocardiograms if it is not controlled for. Respiratory motion can block
anterior echoes
through pulmonary expansion and it chances the angle of incidence of the
transducer ray
relative to the heart. These effects on the echocardiography signal can
decrease the accuracy
of measurements recorded or inferred from echocardiograms. Combining
echocardiography
with accurate measurement of the respiratory cycle can allow an imaging device
to
compensate for respiratory motion.
Impedance Pneumography
Impedance pneumography is a simple method that can yield respiratory volume
tracings without impeding airflow, does not require contact with the
airstream, and does not
restrict body movements. Furthermore, it may be able to make measurements that
reflect
functional residual capacity of the lungs.
While attempting to measure cardiac activity, Atzler and Lehmann noted
transthoracic electrical impedance changed with respiration. They regarded the
respiratory
impedance changes as artifacts and asked the patients to stop breathing while
measurements
were made. In 1940, while also studying cardiac impedance, Nyboer noticed the
same
respiratory impedance artifact in his measurement. He confirmed the origin of
the artifact by
being the first person to relate changes in transthoracic impedance to changes
in volume
using a spirometer by simultaneously recording both. Goldensohn and Zablow
took
impedance pneumography a step further by being the first investigators to
quantitatively
relate respired volume and transthoracic impedance. They reported difficulty
in separating
the cardiac signal artifacts and also noted artifacts during body movements.
However, after
comparing the impedance changes and respired volume changes by a least squares
regression, they importantly determined that the two are linearly related.
Other groups have
confirmed the linear relationship between transthoracic impedance changes and
respiratory
6
CA 03042686 2019-05-02
WO 2018/085563 PCT/US2017/059754
breaths and have found that approximately 90% of the spirometric signal can be
explained
by the thoracic impedance signal. While the relationship has been shown to be
linear, many
groups found the calibration constants for intrapatient and interpatient to be
highly variable
between trials. These differences in calibration constants can be attributed
to a variety of
physiological and electrode characteristics, which must be taken into account.
Transthoracic Impedance Theory
Electrical impedance is a complex quantity defined as the sum of the
resistance (R),
the real component, and the reactance (X), the imaginary component
(Z=R+jX=IZI& ). It is
used as the measurement of opposition to an alternating current.
Mathematically, impedance
is measured by the following equation, which is analogous to Ohm's law:
Z=V/I (1)
Where voltage=V, current,I, and impedance,Z. An object that conducts
electricity
with unknown impedance can be determined from a simple circuit. Applying a
known
alternating current across the object while simultaneously measuring the
voltage across it
.. and using equation (1) yields the impedance. The thorax represents a volume
conductor,
and because of that, the laws governing ionic conductors can be applied. In
addition, the
movement of organs and the enlargement of the thoracic cage during breathing
create a
change in conductivity, which can be measured. Impedance across the thorax can
be
measured by introducing a known current and measuring the change in voltage
across the
thorax with electrodes.
Origins of the Transthoracic Impedance Signal
The tissue layers that makeup the thorax and the abdomen, all influence the
measurement of transthoracic impedance. Each tissue has a different
conductivity that
influences the direction of current flow between electrodes. Beginning with
the outermost
.. layer, the surface of the body is covered by skin, which presents a high
resistivity but is only
about lmm thick. Under the skin is a layer of fat, which also has a high
resistivity. However,
the thickness of this layer is highly variable and depends on body location
and the body type
of the subject. Moving posterior to anterior, below the layer of skin and fat
are the postural
muscles, which are anisotropic. They have a low resistivity in the
longitudinal direction but
a high resistivity in all other directions, which leads to a tendency to
conduct current in a
direction that is parallel to the skin. Below the muscle are the ribs, which,
as bone, are
7
CA 03042686 2019-05-02
WO 2018/085563
PCT/US2017/059754
highly insulating. Therefore, current through the thorax can only flow between
bones. Once
current reaches the lungs, it is hypothesized that current travels through the
blood, which has
one of the lowest resistances of any body tissue. Aeration of the lungs
changes the size of
the lung and the pathway of current flow, and manifests itself as a change in
resistance or
impedance that can be measured.
Due to the anisotropic properties of the tissues, radial current flow through
the chest
is much less than would be expected. Much of the current goes around the chest
rather than
through it. As a result, impedance changes come from changes in thoracic
circumference,
changes in lung size, and movement of the diaphragm-liver mass. Measurements
at lower
thoracic levels are attributed to movement of the diaphragm and liver, and at
higher thoracic
levels they are attributed to aeration and expansion of the lungs. Therefore,
the impedance
signal is the sum of the change from the expansion and aeration of the lungs
and the
movement of the diaphragm-liver mass. Both the abdominal and thoracic
components are
needed in order to observe a normal respiratory signal. In addition, the
different origins of
impedance changes in the upper and lower thorax could explain why greater
linearity is
observed at higher thoracic levels.
Influences of Electrode Placement
Transthoracic impedance is measured with electrodes attached to the patient's
skin.
Geddes et al. determined that the electrode stimulation frequency should not
be below 20
kHz because of physiological tissue considerations. It is a matter of safety
and eliminating
interference from bioelectric events. In addition, impedance measurements of a
subject were
found to differ depending on subject position, including sitting, supine, and
standing. It was
shown that for a given change in volume, laying supine yielded the greatest
signal amplitude
and lowest signal to noise during respiration.
Another potential signal artifact comes from subject movements, which may move
electrodes and disturb calibrations. Furthermore, electrode movements may be
more
prevalent in obese and elderly patients, which may require repetitive lead
recalibration
during periods of long-term monitoring. Because of the calibration variability
between trials,
some have suggested that calibration should be performed for each individual
for a given
subject posture and electrode placement. However, a group was able to show
that careful
8
CA 03042686 2019-05-02
WO 2018/085563 PCT/US2017/059754
intrapatient electrode placement can reduce impedance differences between
measurements
to around 1%.
Despite having the same electrode placements, calibration constants and signal
amplitudes for individuals of different sizes showed variability. It was
determined that the
change in impedance for a given change in volume is the largest for thin-
chested people and
smaller for people who are more amply sized. These observed differences may be
due to the
greater amount of resistive tissue, such as adipose tissue and muscle, between
the electrodes
and lungs in larger subjects, yielding an overall smaller percent change in
impedance for a
given change in volume for larger subjects. On the other hand, it is
noticeable that in
children the cardiac component of the impedance trace is greater than in
adults. This may be
due to greater fat deposition around the heart in adults than in children,
which serves to
shield the heart from being incorporated into the impedance measurement.
Electrodes attached to the mid-axillary line at the level of the sixth rib
yielded the
maximum impedance change during respiration. However, the greatest linearity
between the
two variables was attained by placing the electrodes higher on the thorax.
Despite the high
degree of linearity reported, large standard deviations of impedance changes
during
respiration have been reported. However, the variability observed in impedance
measurements is comparable to those seen in measurements of other vital signs,
such as
blood pressure. Groups have shown that impedance pneumography methods are
sufficiently
accurate for clinical purposes. Furthermore, in the 40 years since these
studies, electrode
materials and signal processing of the impedance measurements have greatly
improved,
yielding even more reliable measurements. Digital signal processing allows for
the near
instantaneous filtering and smoothing of real-time impedance measurements,
which allows
for the minimization of artifacts and noise. More recently, respiratory
impedance has been
used successfully in long-term patient monitoring. As long as the electrodes
remain
relatively unmoved, the relationship of change in impedance to change in
volume is stable
for long periods of time.
Active Acoustic System
The most common use of acoustics in relationship to the lungs is to evaluate
sounds
.. that originate in the lungs acquired by the use of a stethoscope. One
frequently overlooked
property of lung tissue is its ability to act as an acoustic filter. It
attenuates various
9
CA 03042686 2019-05-02
WO 2018/085563 PCT/US2017/059754
frequencies of sound that pass through them to different extents. There is a
relationship
between the level of attenuation and the amount of air in the lungs. Motion of
the chest wall
also results in frequency shift of acoustic signals passing through the
thorax.
Potential for Detecting Abnormalities
Many useful indicators, such as the forced vital capacity (FVC) and forced
expiratory volume in one second (FEV1), can be extracted from monitoring the
volume trace
of a patient's respiration with impedance pneumography. The FVC and FEV1 are
two
benchmark indicators typically measured by a spirometer and are used to
diagnose and
monitor diseases such as COPD, asthma, and emphysema. In addition to
monitoring the
.. respiration, impedance pneumography can also simultaneously record the
electrocardiogram
from the same electrodes.
Breath-to-Breath Variability
Calculations such as breath to breath variability, coefficient of variance,
standard
deviation, and symmetry of a tidal volume histogram have been shown to be
dependent on
age and respiratory health. Compared to normal subjects it has been shown that
some of
these parameters, particularly coefficient of variance, are significantly
different in patients
with tuberculosis, pneumonitis, emphysema, and asthma. Furthermore, it has
been noted in
the literature that impedance measurements were satisfactory as long as the
electrodes did
not move on the patient . In general, it has been determined by many groups
that healthy
.. subjects show greater variability in breathing patterns than subjects in a
pulmonary disease
state.
The nonlinear analysis of respiratory waveforms has been used in a wide array
of
applications. In examining the regularity of nonlinear, physiologic data,
studies have shown
that within pulmonary disease states, patients exhibit a decrease in breath-to-
breath
complexity. This decrease in complexity has been demonstrated within chronic
obstructive
pulmonary disease, restrictive lung disease, and within patients that fail
extubation from
mechanical ventilation. Reduced variability has also been determined to be a
result of
sedation and analgesia. In broad terms, normal patients have greater breath to
breath
variability than those afflicted by some form of pulmonary disease or
compromise.
The respiratory pattern is nonlinear, like any physiologic data, as it is
influenced
by a multitude of regulatory agents within the body. Within the analysis of
breath-to-
CA 03042686 2019-05-02
WO 2018/085563 PCT/US2017/059754
breath variability, various entropy metrics are used to measure the amount of
irregularity
and reproducibility within the signal. These metrics can be used within the
analysis of
RVM tidal volume tracings in assessing not only breath-to-breath changes, but
intrabreath
variability, as well as magnitude, periodicity, and spatial location of the
curve.
Universal calibration of the system based off standardized patient
characteristic
data (Crapo) allows for the creation of a complexity index, and comparison of
a single
patient to what is defined as a normal level of complexity. This index would
be used to aid
clinicians in determining the appropriate time to extubate, determining the
severity of
cardiopulmonary disease, and also within the assessment of therapeutics. This
index
would be independent of the method in which data is collected, whether through
an
impedance based device, accelerometers, a ventilator, or an imaging device.
The system
could also be calibrated to a specific patient and focus on intra-subject
variability while
detecting rapid changes within any of the respiratory parameters.
Nonlinear Analysis of Interbreath Intervals
In addition to variability metrics, some groups have found that nonlinear
analysis of
instantaneous interbreath intervals are highly correlated to the success of
weaning from a
mechanical ventilator. These metrics are useful indicators of pulmonary health
and can
assist in clinical decisions. The inability for a patient to separate from a
mechanical
ventilator occurs in approximately 20% of patientsand current methods to
predict successful
separation are poor and add little to a physician's decision. In a study with
33 subjects under
mechanical ventilation for greater than 24 hours, it was found that 24
subjects were
successfully weaned from ventilation while 8 subjects failed (data from one
subject was
removed). The reasons of failure were cited as hypoxia in five subjects, and
tachypnea,
hypercapnia, and upper airway edema for the remaining three, all of which are
diseases that
can be potentially identified by an impedance pneumography system. The primary
finding in
this study was that the nonlinear analysis of instantaneous breath intervals
for those who
failed to separate from the mechanical ventilator was significantly more
regular than those
who separated successfully. Furthermore, it was shown that the respiratory
rate did not
differ between the two groups. The metrics derived from nonlinear analysis of
impedance
pneumography measurements can successfully predict patient outcomes. In
addition, these
11
CA 03042686 2019-05-02
WO 2018/085563 PCT/US2017/059754
metrics have been shown to be robust and did not significantly change when
artifacts such
as coughing were introduced.
Detection of Decreased Ventilation States
The respiratory trace produced by impedance pneumography as well as the
average
impedance of a subject can indicate states of decreased ventilation or changes
in fluid
volume in the thorax. This type of monitoring would be useful for the care of
anesthetized
patients. Respiratory monitoring with impedance pneumography in anaesthetized
or
immobile patients is shown to be accurate and reliable for long periods,
especially during
the critical period in the recovery room after surgery . Investigators have
determined that
fluid in the thorax or lungs can lead to measurable changes in impedance,
which can be used
to determine common problems for patients in the recovery room such as
pulmonary edema
or pneumonia.
In addition to measuring changes in fluid volume in the thorax, changes in
tidal
volume and upper airway resistance are immediately apparent in impedance
measurements.
Investigators found that endotracheal clamping of anaesthetized patients still
produced a
diminished impedance signal despite the patient's effort to breathe, thereby
giving a correct
indication of ventilation. It has also been shown that impedance measurements
provide
quantitative assessment of the ventilation of each lung. Differences in
impedance
measurements were observed in patients with unilateral pulmonary lesions, with
a pair of
electrodes on the injured side of the thorax producing a less pronounced
signal than the
normal side.
Respiratory Monitors
While certain contact probes record respiratory rate, to date, no device or
method has
been specifically devised to record or to analyze respiratory patterns or
variability, to
correlate respiratory patterns or variability with physiologic condition or
viability, or to use
respiratory patterns or variability to predict impending collapse. Heart rate
variability
algorithms only report on variations in heart rate, beat-to-beat. It is
desirable to use
respiratory rate variability algorithms to incorporate variability in
respiratory intensity, rate,
and location of respiratory motion. Marked abnormalities in respiration as
noted by changes
in intensity, in rate, in localization of respiratory effort, or in
variability of any of these
parameters provide an early warning of respiratory or cardiovascular failure
and may
12
CA 03042686 2019-05-02
WO 2018/085563 PCT/US2017/059754
present an opportunity for early intervention. Development of a device to
record these
changes and creation of algorithms that correlate these respiratory changes
with severity of
illness or injury would provide not only a useful battlefield tool, but also
one of importance
in the hospital critical care setting to help evaluate and treat critically
ill patients. Use in the
clinic or home setting could be of use to less critically ill patients that
nonetheless would
benefit from such monitoring. For example, respiratory rate drops and
respirations become
"shallow" if a patient is overly narcotized. Respiratory rate and respiratory
effort rise with
stiff lungs and poor air exchange due to pulmonary edema or other reasons for
loss of
pulmonary compliance. However, the implications of the rate, which is the only
parameter
objectively monitored is frequently not identified soon enough to best treat
the patient. A
system that could provide a real time, quantitative assessment of work of
breathing and
analyze the trend of respiratory rate, intensity, localization, or variability
in any or all of
these parameters is needed for early diagnosis and intervention as well as
therapeutic
monitoring. Such a system is needed to judge the depth of anesthesia, or the
adequacy or
overdose of narcotic or other pain relieving medications.
PCA and Feedback Controls
Patient Controlled Analgesia (PCA) is a method of postoperative pain control
that
includes patient feedback. The administration of opiates can suppress
respiration, heart rate,
and blood pressure, hence the need for careful and close monitoring. The
system comprises
a computerized pump that contains pain medication that can be pumped into the
patient's IV
line. Generally, in addition to a constant dose of pain medication, the
patient may press a
button to receive care in the form of additional medication. However, patients
are
discouraged from pressing the button if they are getting too drowsy as this
may prevent
therapy for quicker recovery. There are also safeguards in place that limit
the amount of
medication given to a patient in a given amount of time to prevent overdose.
Pulse
oximeters, respiratory rate and capnograph monitors may be used to warn of
respiratory
depression caused by pain medication and cut off the PCA dose, but each has
serious
limitations regarding at least accuracy, validity, and implementation.
Breathing Assistance Devices
Chronic obstructive pulmonary disease ("COPD"), emphysema, and other ailments
have an effect of lowering the ability for the patient to provide efficient
exchange of air and
13
CA 03042686 2019-05-02
WO 2018/085563 PCT/US2017/059754
provide adequate respiration. COPD is a lung disease that makes it hard to
breathe. It is
caused by damage to the lungs over many years, usually from smoking. COPD is
often a
mix of two diseases: chronic bronchitis and emphysema. In chronic bronchitis,
the airways
that carry air to the lungs get inflamed and make a lot of mucus. This can
narrow or block
the airways, making it hard for you to breathe. In a healthy person, the tiny
air sacs in the
lungs are like balloons. As a person breathes in and out, the air sacs get
bigger and smaller
to move air through the lungs. However, with emphysema, these air sacs are
damaged and
lose their stretch. Less air gets in and out of the lungs, which causes
shortness of breath.
COPD patients often have difficulty getting enough oxygenation and/or CO2
removal and
their breathing can be difficult and labored.
Cystic fibrosis ("CF"), also known as mucoviscidosis, is a genetic disorder
that
affects mostly the lungs but also the pancreas, liver, kidneys and intestine.
Long-term issues
include difficulty breathing and coughing up sputum as a result of frequent
lung infections.
Other symptoms include sinus infections, poor growth, fatty stool, clubbing of
the finger
and toes, and infertility in males among others.
There are numerous therapies used to help alleviate the symptoms of COPD, CF,
emphysema, and other breathing problems. For example, the patient may wear a
High-
Frequency Chest Wall Oscillation ("HFCWO") vest or oscillator. The HFCWO vest
is an
inflatable vest attached to a machine that vibrates it at high frequency. The
vest vibrates the
chest to loosen and thin mucus. Alternatively, a patient may us a continuous
positive airway
pressure ("CPAP") or bilevel positive airway pressure ("BiPAP") device to
provide mild air
pressure on a continuous basis to keep the airways continuously open in a
patient who is
able to breathe spontaneously on his or her own. Other mechanical ventilation
therapies
include, but are not limited to cough assist systems, oxygen therapy, suction
therapy, CHFO
("Continuous High Frequency Oscillation"), ventilators, medicated aerosol
delivery
systems, and other non-invasive ventilation methods.
Each of these therapeutic methods has a common drawback, there is no way of
knowing how much air is actually getting into the lungs. Some therapies use
air pressure
feedback to time effective oxygen therapy. This can be inaccurate and is not a
direct
measurement of oxygen ventilation. Furthermore, therapies using masks can be
inaccurate
due to leakage and problems associated with mask placement. Additionally,
kinking and
14
CA 03042686 2019-05-02
WO 2018/085563
PCT/US2017/059754
malfunctions in the pneumatic airway circuits can provide and inaccurate
measure of the
amount of air which is getting into the lungs.
Summary of the Invention
The present invention overcomes the problems and disadvantages associated with
current strategies and designs and provides new systems and methods of
monitoring
patients.
One embodiment of the invention is directed to an early warning scoring
system.
The system comprises a computing device, a plurality of sensors for acquiring
physiological
signals from a patient, wherein the sensors are functionally connected to the
computing
device, and at least one alarm adapted to output an alert upon an early
warning score (EWS)
exceeding a predetermined level. Wherein the computing device receives the
physiological
signals from the sensors, analyzes the physiological signals, and based on the
analyzed
signals, calculates the early warning score, and compares to the early waring
score to
predetermined limits and, if the score is outside the limits, triggers an
alarm or actuates or
modifies a treatment or medical intervention.
Preferably, at least one sensor is a bioelectrical impedance sensor and the
computing
device provides an assessment of minute ventilation, tidal volume, and/or
respiratory rate of
the patient based on the bioelectrical impedance signal. Preferably, the EWS
calculation
includes at least one of the minute ventilation, tidal volume, and/or
respiratory rate of the
patient. In a preferred embodiment, the EWS calculation includes minute
ventilation and
does not include respiratory rate. Preferably, the EWS is indicative of at
least one of
respiratory failure, sepsis, cardiac failure, congestive heart failure, renal
failure, over-
hydration, pulmonary edema, hyper metabolic state, overexertion, traumatic
brain injury,
pulmonary embolus, opioid induced respiratory depression, over sedation.
The sensors preferably obtain patient data relating to at least one of minute
ventilation, tidal volume, respiratory rate, oxygen saturation, temperature,
blood pressure,
pulse or heart rate, blood oxygen levels, and brain activity. Preferably, the
at least one
alarm is at least one of audible or visual. In a preferred embodiment, at
least two sensors are
placed on the torso of the patient and a physiological bioelectrical impedance
signal is
measured transthoracically. Preferably, the computing device further obtains
patient data
CA 03042686 2019-05-02
WO 2018/085563 PCT/US2017/059754
comprising alertness, voice, pain, and unresponsiveness (AVPU) of the patient,
and the
EWS calculation includes the patient's AVPU data. Preferably, the system is
non-invasive.
Preferably, the EWS calculation includes the patient's disease state and/or
circumstance. In a preferred embodiment, the EWS calculation includes the
patient's age,
demographics, condition, and/or data from the patient's electronic health
records.
Preferably, the system is a triage system, a mobilization protocol system, a
training protocol
system, or an activity and/or nutrition regimen system.
Another embodiment of the invention is directed to a method of calculating an
early
warning score (EWS). The method comprises the steps of coupling a plurality of
sensors for
acquiring physiological signals to a patient, receiving the physiological
signals from the
sensors, analyzing the physiological signals, based on the analyzed signals,
calculating the
EWS, and comparing the early waring score to predetermined limits and, if the
score is
outside the limits, triggering an alarm or actuating or modifying a treatment
or medical
intervention.
In a preferred embodiment, at least one sensor is a bioelectrical impedance
sensor
and the method further provides an assessment of minute ventilation, tidal
volume, and/or
respiratory rate of the patient based on the bioelectrical impedance signal.
Preferably, the
EWS calculation includes at least one of the minute ventilation, tidal volume,
and/or
respiratory rate of the patient. Preferably, the EWS calculation includes
minute ventilation
and does not include respiratory rate. In a preferred embodiment, the EWS is
indicative of
at least one of respiratory failure, sepsis, cardiac failure, congestive heart
failure, renal
failure, over-hydration, pulmonary edema, hyper metabolic state, overexertion,
traumatic
brain injury, pulmonary embolus, opioid induced respiratory depression, over
sedation.
Preferably, the sensors obtain patient data relating to at least one of minute
ventilation, tidal volume, respiratory rate, oxygen saturation, temperature,
blood pressure,
pulse or heart rate, blood oxygen levels, and brain activity. Preferably, the
alert is at least
one of audible or visual. Preferably, at least two sensors are placed on the
torso of the
patient and a physiological bioelectrical impedance signal is measured
transthorasically.
The method preferably further comprises obtaining patient data comprising
alertness,
voice, pain, and unresponsiveness (AVPU) of the patient, wherein the EWS
calculation
16
CA 03042686 2019-05-02
WO 2018/085563
PCT/US2017/059754
includes the patient's AVPU data. Preferably, the method is non-invasive.
Preferably, the
EWS calculation includes the patient's disease state and/or circumstance. In a
preferred
embodiment, the EWS calculation includes the patient's age, demographics,
condition,
and/or data from the patient's electronic health records. Preferably, the
method is a triage
method, a mobilization protocol method, a training protocol method, or an
activity and/or
nutrition regimen method.
Other embodiments and advantages of the invention are set forth in part in the
description, which follows, and in part, may be obvious from this description,
or may be
learned from the practice of the invention.
Description of the Figures
The invention is described in greater detail by way of example only and with
reference to the attached drawings, in which:
Figure 1 is a perspective view of a four-lead embodiment of the invention.
Figure 2 is a diagram of the Posterior Left to Right electrode configuration.
Figure 3 is a diagram of the Posterior Right Vertical electrode configuration.
Figure 4 is a diagram of the Anterior-Posterior electrode configuration.
Figure 5 is a diagram of the Anterior Right Vertical electrode configuration.
Figure 6 is a perspective view of two four-lead configurations connected to
each other by a
multiplexer.
Figure 7 is a diagram of the ICG electrode configuration.
Figure 8 is a perspective view of a four-lead embodiment of the invention
connected to a
spirometer.
Figure 9 is a perspective view of a four-lead embodiment of the invention
connected to a
ventilator.
Figure 10 is an RVM measurement (impedance) versus volume plot for slow,
normal, and
erratic breathing maneuvers.
Figure 11 is a set of RVM and volume plots against time for normal breathing.
Figure 12 is a set of RVM and volume plots against time for slow breathing.
Figure 13 is a set of RVM and volume plots against time for erratic breathing.
17
CA 03042686 2019-05-02
WO 2018/085563 PCT/US2017/059754
Figure 14 is a plot of calibration coefficients against BMI for four different
electrode
configurations.
Figure 15 is a spirometry plot that exhibits volume drift.
Figure 16 is a volume vs. impedance plot that is affected by volume drift.
Figure 17 is a spirometry plot that is corrected for volume drift.
Figure 18 is a plot of volume vs. impedance, comparing data that is
uncorrected and
corrected for volume drift.
Figure 19 is a flow chart that describes data analysis for the invention.
Figure 20 is a preferred embodiment of the invention that utilizes a speaker
and a
microphone.
Figure 21 is a preferred embodiment of the invention that utilizes a speaker
and an array of
microphones.
Figure 22 is a preferred embodiment of the invention that utilizes an array of
speakers and a
microphone.
Figure 23 is a preferred embodiment of the invention that utilizes a vest for
the sensors.
Figure 24 is a preferred embodiment of the invention that utilizes an array
built into a piece
of cloth for the sensors.
Figure 25 is a preferred embodiment of the invention that utilizes a net of
sensors.
Figure 26 is a preferred embodiment of the invention that utilizes a wireless
transmitter and
receiver.
Figure 27 shows graphs of impedance versus time and volume versus time for
simultaneously recorded data.
Figure 28 illustrates an embodiment of a system of the invention.
Figure 29 illustrates an embodiment of the device of the invention.
Figures 30-32 illustrate preferred embodiments of devices of the invention.
Figures 33-38 depict different embodiments of lead placement.
Figure 39 depicts an embodiment of a modified Howland circuit for compensating
for
parasitic capacitances.
Figure 40 depicts an embodiment of the invention wherein the impedance
measuring device
is in data communication with a HFCWO vest.
18
CA 03042686 2019-05-02
WO 2018/085563 PCT/US2017/059754
Figure 41 depicts an embodiment of the invention wherein the impedance
measuring device
is in data communication with a mechanical ventilation therapy device.
Figure 42 depicts an embodiment of the invention wherein the impedance
measuring device
is in data communication with a oxygenation therapy device.
Figure 43 depicts an embodiment of the invention wherein the impedance
measuring device
is in data communication with a suction therapy device.
Figure 44 depicts an embodiment of the invention wherein the impedance
measuring device
is in data communication with a cough assist device.
Description of the Invention
As embodied and broadly described herein, the disclosures herein provide
detailed
embodiments of the invention. However, the disclosed embodiments are merely
exemplary
of the invention that may be embodied in various and alternative forms.
Therefore, there is
no intent that specific structural and functional details should be limiting,
but rather the
intention is that they provide a basis for the claims and as a representative
basis for teaching
one skilled in the art to variously employ the present invention.
One embodiment of the present invention is directed to a device for assessing
a
patient, individual or animal that collects impedance measurements by placing
multiple
electrode leads and/or speakers and microphones on the body. Preferably at
least one
impedance measuring element and a microphone/speaker functionally connected to
a
programmable element, programmed to provide an assessment of at least one
respiratory
parameter of the subject.
Preferably, the impedance measurement is based on a plurality of remote probe
data
sets, and wherein the programmable element is further programmed to enhance at
least one
of the plurality of remote probe data sets; or to stabilize at least one of
the plurality of
remote probe data sets; or to analyze each of the plurality of remote probe
data sets for
dynamic range and signal to noise ratio (SNR) values. Preferably, the device
probes are
maintained in several lead configurations. In one embodiment, variations in
lead
configuration allow for flexibility depending on the subject and test being
performed. In
other embodiments, variations in lead configuration allow for variability in
patient anatomy.
19
CA 03042686 2019-05-02
WO 2018/085563 PCT/US2017/059754
Preferably, the device maintains settings to identify valid lead
configurations. Preferably,
the device maintains settings to identify valid lead attachment.
Preferably, the device or method as described in a protocol embedded in the
machine
instructs as to lead placement. Preferably, appropriate lead contact is
verified by the device.
Preferably, the device alerts the operator as to inadequate or inappropriate
lead placement.
Preferably, the device monitors continuously or intermittently and maintains
alarms
to indicate when a respiratory parameter reflects a loss in ventilation or
other vital function.
The alarm is set based on a respiratory sufficiency index, on minute
ventilation, on
respiratory rate, on tidal volume, on an inspiratory volume or flow parameter,
on an
expiratory volume or flow parameter, on variability of respiratory rate,
volume, flow or
other parameter generated. For example, the alarm goes off if the monitor
detects a decrease
in either respiratory frequency or depth or minute ventilation associated with
hypoventilation or detects an increase in any or all of these parameters that
would suggest
hyperventilation. An alarm is used on a hospital floor in comparing the
patient's current
respiratory status with a baseline level based on specific individual
calibration to ventilator
or spirometer. Preferably, the alarm is set based on parameters taken for the
given
individual from a ventilator or spirometer. More preferably the baseline level
is based on
one or more of the following: demographic, physiologic and body type
parameters. An
alarm is also used to alert for narcotic induced respiratory depression at a
point that is
determined to be detrimental to the patient. Preferably, the ranges of values
beyond which
alarms will be triggered are chosen by the physician or care giver for one or
more of the
following: respiratory rate, tidal volume, minute ventilation, respiratory
sufficiency index,
shape of the respiratory curve, entropy, fractal or other analysis parameters
associated with
respiratory variability or complexity.
In another embodiment, the RVM measurements taken at any given point in time
is
recorded as baseline. These recorded values are correlated to subjective
impression by a
physician or other health care worker of patient status. Subsequently, RVM is
monitored and
an alarm set to alert health care staff if a 10%, 20% or other selected
percentage change in
respiratory volumes, minute ventilation curve characteristics, or variability
is noted.
The following illustrate embodiments of the invention, but should not be
viewed as limiting
the scope of the invention.
CA 03042686 2019-05-02
WO 2018/085563 PCT/US2017/059754
Impedance Plethysmograph
As embodied and broadly described herein are provided detailed embodiments of
the
invention. The embodiments are merely exemplary of the invention that may be
embodied
in various and alternative forms. Therefore, there is no intent that specific
structural and
functional details should be limiting, but rather the intention is that they
provide a basis for
the claims and as a representative basis for teaching one skilled in the art
to variously
employ the present invention.
The invention preferably comprises an impedance pneumograph with integrated
electronics to convert measured impedance values to volume and display the
volume to an
end-user through an electronic interface or printed reports employing
numerical or graphical
representations of the data. The impedance measuring device comprises
circuitry, at least
one microprocessor and preferably at least four leads. Preferably, where at
least two leads
are used for injecting current into the subject's body and at least two are
used for reading the
.. voltage response of said patient's body.
In one embodiment, the device preferably comprises an integrated module to
simulate a patient and allow for automated system testing and demonstrations.
Automated
system tests improve the performance of the device and ensure that it is
functioning
correctly before use.
In the preferred embodiment, the device utilizes an analog divider to
compensate for
slight deviations in the injected current and increase the accuracy of
acquired data. The
analog divider in the preferred embodiment would be placed after the
demodulator and
before the rectifier. In other embodiments the analog divider may be placed in
other
locations in the circuit including, but not limited to, after the precision
rectifier or before the
demodulator.
In the preferred embodiment, the device utilizes adaptive electronics driven
by a
microprocessor to maintain the appropriate gains on the different amplifiers
in the circuit to
prevent the signal from going out of range. The microprocessor tracks the set
gains at each
of the hardware amplifiers and compensates appropriately during its
calculations so that it
always outputs an appropriate value.
21
CA 03042686 2019-05-02
WO 2018/085563 PCT/US2017/059754
The impedance measuring device is preferably connected to computer via a
digital
interface (e.g. USB, Fire wire, serial, parallel, or other kind of digital
interface). The digital
interface is used to prevent data from corruption during transfer.
Communication over this
interface is preferably encrypted to further ensure data integrity as well as
protect the
invention from usage of counterfeit modules (either measuring device or
computer).
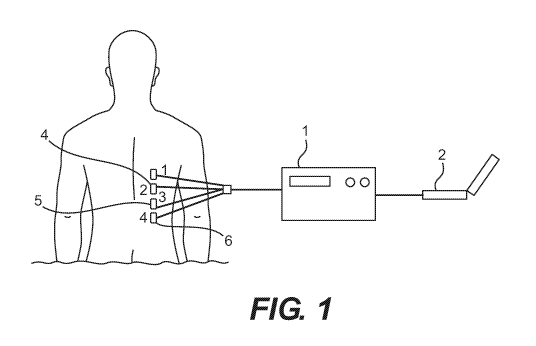
Referring now to a preferred embodiment of the invention in more detail, in
Figure 1
there is shown an impedance plethysmograph, comprising a radio frequency
impedance
meter 1, a programmable element 2 contained on a PC linked to the meter, which
is
connected to the patient by four leads, namely a first lead 3, a second lead
4, a third lead 5,
and a fourth lead 6. Each lead is preferably connected to a surface electrode,
namely a first
surface electrode, a second surface electrode, a third surface electrode, and
a fourth surface
electrode.
In further detail, still referring to the embodiment of Figure 1, the
electrodes can be
made of a conductive material such as AgC1, coated with an adhesive,
conductive material
such as a hydrogel or hydrocolloid. The leads can be made of any conductive
material such
as copper wire and are preferably coated with insulating material such as
rubber. In a
preferred embodiment, wireless electrodes are utilized to provide current and
collect and
transmit data. Preferably, this lead composition is coupled with Bluetooth
technology and a
receiver.
Leads 1 and 4 are connected to a current source with a constant frequency
preferably
greater than 20 KHz, which is great enough to avoid interfering with
biological signaling.
The amplitude of the current source is preferably less than 50 mA, and below
the level that
would cause fibrillation at the chosen frequency. The differential voltage
between leads 2
and 3 is used to calculate the impedance according to ohm's law. By sampling
the voltage
measurements taken by the impedance meter, the programmable element (such as a
PC)
tracks and plots changes in thoracic impedance that correspond to biological
functions such
as heartbeat and breathing. The changes in impedance are then used to monitor
pulmonary
function. Preferably, the device is calibrated by a method laid out herein to
calculate the
lung volumes and display them to an operator.
With reference to Figure 28, an exemplary and preferred system includes at
least one
general-purpose computing device 100, including a processing unit (CPU) 120,
and a
22
CA 03042686 2019-05-02
WO 2018/085563 PCT/US2017/059754
system bus 110 that couples various system components including the system
memory such
as read only memory (ROM) 140 and random access memory (RAM) 150 to the
processing
25 unit 120. Other system memory 130 may be available for use as well. The
invention
preferably operates on a computing device with more than one CPU 120 or on a
group or
cluster of computing devices networked together to provide greater processing
capability.
The system bus 110 may be any of several types of bus structures including a
memory bus
or memory controller, a peripheral bus, and a local bus using any of a variety
of bus
architectures. A basic input/output (BIOS) stored in ROM 140 or the like,
preferably
provides the basic routine that helps to transfer information between elements
within the
computing device 100, such as during start-up. The computing device 100
further preferably
includes storage devices such as a hard disk drive 160, a magnetic disk drive,
an optical disk
drive, tape drive or the like. The storage device 160 is connected to the
system bus 110 by a
drive interface. The drives and the associated computer readable media provide
nonvolatile
storage of computer readable instructions, data structures, program modules
and other data
.. for the computing device 100. The basic components are known to those of
skill in the art
and appropriate variations are contemplated depending on the type of device,
such as
whether the device is a small, handheld computing device, a desktop computer,
a laptop
computer, a computer server, a wireless devices, web-enabled devices, or
wireless phones,
etc.
In some embodiments, the system is preferably controlled by a single CPU,
however,
in other embodiments, one or more components of the system is controlled by
one or more
microprocessors (MP). Additionally, combinations of CPUs and MPs can be used.
Preferably, the MP is an embedded microcontroller, however other devices
capable of
processing commands can also be used.
Although the exemplary environment described herein employs the hard disk, it
should be appreciated by those skilled in the art that other types of computer
readable media
which can store data that are accessible by a computer, such as magnetic
cassettes, flash
memory cards, digital versatile disks, cartridges, random access memories
(RAMs), read
only memory (ROM), a cable or wireless signal containing a bit stream and the
like, may
also be used in the exemplary operating environment. To enable user
interaction with the
computing device 100, an input device 190 represents any number of input
mechanisms,
23
CA 03042686 2019-05-02
WO 2018/085563 PCT/US2017/059754
such as a microphone for speech, a touch sensitive screen for gesture or
graphical input,
electrical signal sensors, keyboard, mouse, motion input, speech and so forth.
The device
output 170 can be one or more of a number of output mechanisms known to those
of skill in
the art, for example, printers, monitors, projectors, speakers, and plotters.
In some
.. embodiments, the output can be via a network interface, for example
uploading to a website,
emailing, attached to or placed within other electronic files, and sending an
SMS or MMS
message. In some instances, multimodal systems enable a user to provide
multiple types of
input to communicate with the computing device 100. The communications
interface 180
generally governs and manages the user input and system output. There is no
restriction on
the invention operating on any particular hardware arrangement and therefore
the basic
features here may easily be substituted for improved hardware or firmware
arrangements as
they are developed.
Embodiments within the scope of the present invention may also include
computer
readable media for carrying or having computer-executable instructions or data
structures
stored thereon. Such computer-readable media can be any available media that
can be
accessed by a general purpose or special purpose computer. By way of example,
and not
limitation, such computer-readable media can comprise RAM, ROM, EEPROM, CD-ROM
or other optical disk storage, magnetic disk storage or other magnetic storage
devices, or any
other medium which can be used to carry or store desired program code means in
the form
.. of computer-executable instructions or data structures. When information is
transferred or
provided over a network or another communications connection (either
hardwired, wireless,
or combination thereof) to a computer, the computer properly views the
connection as a
computer-readable medium. Thus, any such connection is properly termed a
computer
readable medium. Combinations of the above should also be included within the
scope of
the computer-readable media.
Computer-executable instructions include, for example, instructions and data
which
cause a general purpose computer, special purpose computer, or special purpose
processing
device to perform a certain function or group of functions. Computer-
executable instructions
also include program modules that are executed by computers in stand-alone or
network
environments. Generally, program modules include routines, programs, objects,
components, and data structures, etc. that perform particular tasks or
implement particular
24
CA 03042686 2019-05-02
WO 2018/085563 PCT/US2017/059754
abstract data types. Computer-executable instructions, associated data
structures, and
program modules represent examples of the program code means for executing
steps of the
methods disclosed herein. The particular sequence of such executable
instructions or
associated data structures represents examples of corresponding acts for
implementing the
functions described in such steps.
Those of skill in the art will appreciate that other embodiments of the
invention may
be practiced in network computing environments with many types of computer
system
configurations, including personal computers, hand-held devices, multi-
processor systems,
microprocessor-based or programmable consumer electronics, network PCs,
minicomputers,
mainframe computers, and the like. Networks may include the Internet, one or
more Local
Area Networks ("LANs"), one or more Metropolitan Area Networks ("MANs"), one
or
more Wide Area Networks ("WANs"), one or more Intranets, etc. Embodiments may
also
be practiced in distributed computing environments where tasks are performed
by local and
remote processing devices that are linked (either by hardwired links, wireless
links, or by a
combination thereof) through a communications network. In a distributed
computing
environment, program modules may be located in both local and remote memory
storage
devices.
Figure 2 is a schematic of an embodiment of a system 200 of the invention. The
electrical source originates from signal source 205. Preferably, an adjustable
function
generator 210 (e.g. a XR2206 chip) is used to generate the electrical source.
The function
generator 210 is preferably adjustable via a microprocessor (MP) 275 or
manually. In some
embodiments, the function generator can be tuned in order to improve the
signal. Tuning
can occur once or multiple times. Bio-impedance spectroscopy can be used to
detect levels
of hydration at different frequencies, which can be used to calibrate function
generator 210.
Similarly, body fat percentages can be calculated. Signal source 205 also
comprises a
current generator 215 (e.g. a Howland circuit). Current generator 215
preferably keeps the
source current constant despite changes in pad contact (unless the contact is
totally broken).
In the preferred embodiment, current generator 215 can be tuned to improve
performance,
which can be done manually or automatically by the MP 275. The impedance
measuring
subsystem may utilize current generating components at one or more
frequencies, which
may be active simultaneously, or sequentially. Voltage measuring components
may be
CA 03042686 2019-05-02
WO 2018/085563 PCT/US2017/059754
functionally connected to one or more electrodes. The impedance measuring
subsystem may
utilize non-sinusoidal current, such as narrow current pulses. The system may
integrate
additional sensors, such as accelerometers, moisture and acoustics sensors,
capnography or
oximetry sensors.
In preferred embodiments, the pad contact quality is monitored and a warning
is
produced when the pad contact is broken or too poor quality for the
electronics to
compensate. Signal source 205 may also comprise a current monitor 220 to
calculate
impedance. In a preferred embodiment, signal source 205 also comprises a
patient simulator
225. Patient simulator 225 can simulate changes in the impedance with
parameters similar
to a real patient. Patient simulator 225 can be used for testing system 200 as
well as
calibration of the circuitry.
The signal from signal source 205 passes through patient 230 and is received
by
sensor 235. Preferably, sensor 230 comprises an input amplifier 240. Input
amplifier 240
suppresses the effect of poor or variable pad contact on measurement. The gain
of input
amplifier 240 is preferably controlled by the MP 275 to provide an enhanced
signal to the
other modules. Sensor 230 preferably also comprises a signal filter 245 to
remove
interference from the power grid, etc. Signal filter 245 may be a standard
high-pass filter (as
on Figure 30), a demodulator (as on Figure 31), or another signal filter.
Synchronous
demodulators are often used for detecting bio-impedance changes and stripping
out motion
artifacts in the signal.
In a preferred embodiment, the signal is split into two paths (as on Figure
32). The
first path demodulates the measured signal using the generator signal as a
carrier. The
second path uses a 90-degree phase rotating circuitry before demodulation.
Both
demodulated signals can be converted into RMS values using voltage-to-RMS
converters.
Measured separately, the signals are summed and then the square root is
calculated. This
allows for compensation for any phase shift in the subject and for separate
measurements of
resistance and reactance, which provides valuable information for motion
artifact
compensation as well as hydration levels, fat percentages, and calibration
coefficient
calculations.
Additionally, sensor 230 may comprise an analog divider 250, which divides the
measured voltage signal by the signal from the current monitoring circuit to
calculate
26
CA 03042686 2019-05-02
WO 2018/085563 PCT/US2017/059754
impedance. Sensor 230 preferably also comprises a precision rectifier or root
mean square to
direct current (RMS-to-DC) chip 255 with a low pass filter to remove the
carrier frequency.
The output of sensor 230 is preferably a DC signal proportional to the
patient's impedance.
Sensor 230 may also comprise a band-pass filter 260 to select only the
respiratory rates by
filtering out the portion of the signal not corresponding to the respiration.
Band-pass filter
260 may be calibrated manually or automatically by the MP 275. Preferably,
sensor 230
comprises a multiplexor 265 controlled by the MP 275 to accommodate multiple
probe
pairs. Preferably there are 2 probe pairs, however more or fewer probe pairs
are
contemplated. Sensor 230 may also comprise an output amplifier 270. Output
amplifier 270
is preferably controlled by the MP 275 and provides a signal to an analog-to-
digital
converter (ADC) 280 for high precision digitization. Oversampling is used to
reduce
measurement noise which may originate from different sources (e.g., thermal,
electronic,
biological, or EM interference). MP 275 commands ADC to take measurements with
as high
a cadence as possible and then averages the obtained data over the time
intervals
corresponding to the sampling frequency. The sampling frequency is the
frequency of the
impedance sampling as it is presented to the computer by the impedance
measuring device.
The frequency is preferably set sufficiently high to monitor all the minute
features of
respiration.
Using controllable gains and oversampling preferably allows the system to
measure
the impedance with extremely high effective precision (estimated 28-bit for
current
implementation, or 4 parts per billion).
Both signal source 205 and sensor 230 are controlled by MP 275. MP 275
preferably
comprises at least one ADC 280 monitoring the signal processing, and at least
one digital
output 285 to control the digital potentiometers, multiplexors, op-amps,
signal generator,
and other devices. Preferably, MP 275 and a computer interface (e.g., via a
USB interface, a
serial interface, or a wireless interface).
Preferably, the MP computes values for respiratory rate (RR), tidal volume
(TV) and
minute ventilation (MV) as well as, tracks the trends in computed RR, TV, or
MV values
and performs statistical, factor, or fractal analysis on trends in real-time.
The MP may
tracks instantaneous and cumulative deviations from predicted adequate values
for RR, TV,
or MV and computes a respiratory sufficiency index (RSI).
27
CA 03042686 2019-05-02
WO 2018/085563 PCT/US2017/059754
In a preferred embodiment, the device has the capability to measure and record
other
parameters including but not limited to: cardiac output, end tidal CO2, oxygen
perfusion,
ECG and other electrophysologic measurements of the heart. In a preferred
embodiment, the
impedance measuring device measures impedance cardiography and impedance
pneumography simultaneously. Preferably, the additional parameters are
displayed on-
screen. Preferably, the respiratory impedance data are combined with the
additional
parameters in a meaningful way to act as an adjunct to diagnosis. Preferably,
the impedance
data alone, or combined with one or more additional parameters are used to
provide a
diagnosis of a disease state.
In one embodiment, measurements are taken from each side of the chest
independently and used to evaluate both general pulmonary status and
differences between
right and left lung aeration or chest expansion. An example of this is, in the
case of rib
fractures, where there can be changes attributed to damage including pulmonary
contusion,
decrease in motion due to splinting or pneumothorax where both sides of the
chest are
monitored independently to provide side specific data. Other sources of
localized
pulmonary pathology can be evaluated including pneumonia, hydrothorax,
chylothorax,
hemothorax, hemo/pneumothorax, atelectasis, tumor, and radiation injury.
In another embodiment, information from the device is used with information
from an
echocardiogram, radionuclide study or other method of imaging the heart. In a
preferred
embodiment the device assists in the diagnosis of myocardial ischemia with one
of the
following: ekg, advanced electrophysiologic studies, cardiac catheterization,
echocardiogram, stress testing, radionuclide testing, CT, MRI, cardiac output
monitoring by
impedance measurement. In one embodiment the device provides information that
is used to
help with collection of other signals that vary with respiration such as
respiratory sounds,
cardiac information, radiation detection devices, radiation therapy devices,
ablation devices.
In a preferred embodiment the device can assist with the timing or data
collection by another
modality and/or using characteristics of the respiratory curve to correct data
that is collected.
In one embodiment, the device provides information about breath-to-breath
variability or respiratory complexity to be used in conjunction with cardiac
beat to beat
variability or complexity to provide otherwise unavailable information about
cardiac,
pulmonary systems, or overall metabolic or neurologic status.
28
CA 03042686 2019-05-02
WO 2018/085563 PCT/US2017/059754
Lead Configuration
The proposed respiratory parameters evaluation technique relies on a highly
linear
relation between the parameters and measured impedance. It is not true for
every electrode
placement. Extensive research was conducted to select best electrode placement
which
preferably satisfies following conditions:
1) Highly linear relation between respiratory volume and measured impedance
variations (i.e. correlation values above 96%).
2) Low level of artifacts due to patient motion.
3) Low variation between repetitive electrode applications.
4) Easy application in common clinical situation.
Capability for use with "universal calibration," which reliably determines
scaling factors
that depend on measurable patient body parameters without preliminary
calibration with
ventilator/spirometer.
Preferably, electrodes are attached horizontally to the mid-axillary line at
the level of
the sixth rib. Preferably, one electrode is placed at a stable location, such
as immediately
below the clavicle or at the sternal notch, and another electrode is place at
the bottom of the
ribcage or at the level of the xiphoid at the midaxillary line. However, the
electrodes can be
placed higher or lower on the thorax. Furthermore, electrodes may be placed in
other
locations and configurations (e.g. vertically along the thorax, at an angle
across the thorax,
or from a position on the front of the patient to a position on the back of
the patent),
depending on the subject to be tested, the test to be preformed, and other
physiological
concerns (e.g. if the patient has a pacemaker or other artificial device).
Preferably at least one impedance measuring element is present on one or more
electrode leads. Preferably, two or more electrodes are arranged in a linear
array, grid-like
pattern, or in an anatomically influenced configuration. Preferably, four
remote probes are
arranged in a linear array. In another embodiment, multiple electrode leads
are arranged as a
net, vest, or array. Preferably, the one or more probes, electrode leads or
sensors are placed
on the thorax or abdomen of the subject. Preferably, the device uses single
use electrodes. In
other embodiments, the electrodes are hydrogel, hydrocolloids, or solid gels.
Preferably, the
electrode utilizes AgC1, nickel, or carbon sensors. Preferably, the electrodes
come with soft
cloth, foam, microporous tape, clear tape backing or another adhesive.
Preferably, different,
29
CA 03042686 2019-05-02
WO 2018/085563 PCT/US2017/059754
size appropriate electrodes exist for adults and neonates, with the adult
electrodes larger
than the neonatal ones, which are preferably 1" by 3/8" or less (2.54 cm by
0.95 cm or less).
In other embodiments, sensor electrodes are the same as the probes that
deliver electrical
impulses to the body, or are different from the delivery electrodes, or are
wireless and
.. transmit data to a remote sensor. In another embodiment, the delivery
probes are themselves
sensors. In one embodiment, the stimulating electrode is battery powered.
Preferably, the at
least one respiratory parameter is recorded for a duration of 30 seconds,
continuously,
intermittently, for up to at least 3, 5, 10, 20, or 50 of the subject's
breaths, for up to at least
100 of the subject's breaths, for up to at least 1000 of the subject's
breaths, or for another
duration. Preferably, the subject's impedance cardiogram is simultaneously
recorded.
Preferably, the at least one impedance measuring element comprises one or more
remote probes or electrode leads, or leads similar to standard EKG leads or
similar to the
leads used for measuring cardiac impedance, and wherein the programmable
element is
further programmed to analyze one or more remote probe or electrode lead data
sets
collected from the one or more remote probes or electrode leads.
In one embodiment of the invention, the impedance measurement subsystem reads
impedance from multiple channels. In a preferred embodiment, a secondary
voltage sensing
channel is arranged at an angle to a primary voltage sensing channel. In one
embodiment,
the two channels share current generating electrodes. In one embodiment, the
two channels
also share one of the voltage sensing electrodes. Data from the two or more
channels may be
used in an adaptive algorithm to determine and suppress noise from motion.
Lead configuration is critical for the performance of the device in any
embodiment.
Preferably, one or more leads are placed on the thorax. In one embodiment,
leads are placed
on the thorax and abdomen to measure breathing from different regions of the
body such as
the thorax or the abdomen. Differences in the location of body motion
associated with
breathing produces information that is useful clinically for diagnosis of
physiologic state
and monitoring of disease and can be compensated for in calculations. Leads
are placed on
the thorax, neck and head in alternate configurations. In one embodiment,
leads are placed
in different configurations based on anatomic locations and spaced either
according to
specific measured distances or anatomic landmarks or a combination of both. In
one
embodiment, modifications of the spacing relative to body size are
implemented. Preferably
CA 03042686 2019-05-02
WO 2018/085563 PCT/US2017/059754
these modifications are related to anatomic landmarks. In a preferred
embodiment, the
spacings remain relatively the same for patients of all sizes from neonates to
obese patients,
ranging from 250g to 400kg. In another embodiment, the spacings vary based on
an
algorithm reflecting body size and habitus. Other configurations have the
advantage of
.. determining differential motion of one hemithorax vs. the other which is
useful in
diagnosing or monitoring unilateral or asymmetric pathology such as
pneumothorax,
hemothorax, empyema, cancer.
Referring now to Figure 2, there is shown one embodiment with a specific
electrode
configuration called Posterior Left to Right (PLR), in which the first
electrode 7 is placed 6
inches to the left of the spine at the level of the xiphoid process, the
second electrode 8 is
placed 2 inches to the left of the spine at the level of the xiphoid process,
the third electrode
9 is placed 2 inches to the right of the spine at the level of the xiphoid
process, and the
fourth electrode 10 is placed six inches to the right of the spine level with
the xiphoid
process. The advantage of placing the electrodes in this configuration is that
both lungs are
factored into the reading and high level of signal.
Referring to Figure 3, there is shown the second specific electrode
configuration
called Posterior Vertical Right (PVR), in which the first electrode 11 is
placed midway
between the midaxillary line and the spine just beneath the scapula, the
second electrode 12
is placed two inches beneath electrode 1, the third 13 electrode is placed two
inches beneath
.. electrode 2, and the fourth electrode 14 is placed beneath electrode 3. The
advantages of this
configuration are the reduction of electrode movement due to thoracic
expansion and less
cardiac interference. This position has the benefit of little to no volume
change between
electrodes and less heart noise.
Referring to Figure 4, there is shown the third specific electrode
configuration called
Anterior to Posterior (AP), in which the first electrode 15 is placed 6 inches
to the right of
the right midaxillary line at the level of the xiphoid process, the second
electrode 16 is
placed 2 inches to the right of the right midaxillary line at the level of the
xiphoid process,
the third electrode 17 is placed 2 inches to the left of the right midaxillary
line at the level of
the xiphoid process, and the fourth electrode 18 is placed 2 inches to the
left of the right
midaxillary line at the level of the xiphoid process. This position captures
the most volume
change, which is useful for determination of localization of breathing.
31
CA 03042686 2019-05-02
WO 2018/085563 PCT/US2017/059754
Referring to Figure 5, there is shown the fourth specific electrode placement
called
Anterior Vertical Right (AVR), in which the first electrode 19 is placed
immediately
beneath the clavicle midway between the xiphoid and midaxillary line, the
third electrode 20
is placed at the level of the xiphoid in line with the first electrode, the
second electrode 21 is
.. placed 4 inches above the third electrode, and the fourth electrode 22 is
placed 4 inches
below the third electrode. This position is useful for neonates and other
patients whose
characteristics prevent the operator from placing leads on the posterior.
Other four-probe
positions are placed vertically and horizontally on the abdomen and thorax,
equidistant from
each other or at specifically measured distances. Probe positions are also
placed at
physiological landmarks such as the iliac crest or third intercostal space.
Probe placement on
both the abdomen and thorax allows the relationship between chest and
abdominal breathing
to be determined. This relationship assists in diagnosis and monitoring of
therapeutics.
In addition to the aforementioned four-probe configurations, these
configurations
can be modified to include more probes by adding probes equidistant between
the positions,
for example, by adding electrodes in between electrodes 1 and 2, 2 and 3, 3
and 4 in the AP
configuration two inches from each electrode in line with the placement. With
a large
number of electrodes, they can be placed in a grid pattern equidistant from
each other; this
configuration will be further discussed below. Other placements for 2 or more
leads include
around the thorax at equidistant points at a constant height such as the
xiphoid process. The
specific placement for the 24 lead system is within a linear array with 12
leads equally
spaced in a linear on the chest and back respectively. Such a grid or array
can be
implemented within a net or vest to be worn by the patient. In one embodiment,
the device
provides a table describing lead placement alternatives and provides a
measurement device
to assist in probe placement. In one embodiment, measured distances between
leads are
confirmed automatically by the leads which have positioning sensors and/or
sensors which
can determine distance from one sensor to another sensor or sensors.
Referring now to Figure 6, there is shown several electrode configurations 23,
connected together by means of an analog multiplexer 24 and connected to a
radio
frequency impedance meter 25 and a programmable element 26 such as a PC. There
is
shown an embodiment of the device implementing the lead and multiplexor
configurations
shown in the previous figures, Figures 2 and 3. In Figure 6, each lead is
connected to several
32
CA 03042686 2019-05-02
WO 2018/085563 PCT/US2017/059754
different electrodes by means of a multiplexer. The advantage of this
configuration is that it
allows the device to digitally switch the electronic inputs and outputs of the
DAS and
effectively switch the electrode configuration in order to gather data on
impedance in
several directions nearly simultaneously. For example, a 12-electrode system
is comprised
of four different sets of leads, with the first set going to the corresponding
first electrode in
each configuration, the second set of leads going to the corresponding second
electrode in
each configuration, and so forth.
Electrode configurations are also made to correspond with anatomic positions
on the
thorax, abdomen, and limbs, such as a resting ICG position shown in Figure 7
where the
first electrode 27 is place on the forehead, the second 28 above the left
clavicle, the third 29
on the midaxillary line level with the xiphoid, and the fourth 30 on the
midaxillary line
immediately above the iliac crest.
Each electrode configuration will be affected by motion in different ways. For
instance, movement of the right arm will cause a motion artifact on any lead
placement
which traces impedance across the right pectoral, latissimus, trapezius
muscles, and other
muscles of the chest and upper back. By noting differences between the shapes,
derivatives
or magnitudes of simultaneously recorded signals from different lead
placements, local
motion artifacts can be identified and subtracted from the impedance signal.
In one embodiment, the probes are manufactured in a linear strip with a
delivery and
sensor pair at each end and having a fixed distance between the delivery and
sensor
electrode to form a discrete pad. In a preferred embodiment, there is a
compliant strip in-
between the two pads that can be stretched to permit appropriate patient
specific positioning
based on anatomic landmarks. Preferably the material, once stretched, will
maintain its
extended configuration.
Probes
Referring now to Figure 23, there is shown an embodiment of the device in
which
the one or more remote probes, which are embodied as surface electrodes,
speakers and/or
microphones, are integrated into a vest 46 connected to an impedance
plethysmograph 47
using a cable. The advantage of this embodiment is that the position of leads
is determined
by the manufacturer of the vest, and thus they are standardized. That is, the
use of the vest
eliminates operator error with respect to lead configuration. In an alternate
embodiment, the
33
CA 03042686 2019-05-02
WO 2018/085563 PCT/US2017/059754
probes and actuators are wireless. In an alternate embodiment, the vest also
includes leads
that cover the abdomen.
Referring now to Figure 24, there is shown an embodiment of the device in
which
the one or more remote probes are integrated into an array 48 where the
electrodes are
connected by a compliant piece of cloth or netting which is be pressed gently
onto the
patient's skin. The benefit of this configuration is that the inter-electrode
distance is
standardized by the array manufacturer, thus lessening operator dependent
error with respect
to electrode configuration.
Referring now to Figure 25, there is shown an embodiment of the device in
which
the one or more remote probes are connected to each other by strings, forming
a net 49
which can be applied to the patient's skin quickly and effectively. The
benefit of said
embodiment is that the inter-electrode distance as well as the relative
positions of electrodes
to one another are standardized, thus lessening the effects of operator
dependent error. In
another embodiment, elastic stretch of the strings provides probe adjustment
for different
body habitus. Preferably, the stretch material would provide a measurement of
the distance
either to be read on the material or by relaying information relative to
stretch to the device.
Preferably, the strings would have attached displacement sensors such as
linear
displacement transducers or strain gauges functionally connected to the
programmable
element to relay information about the length each string of the net is
stretched. Preferably,
the programmable element is further programmed to account for changes in lead
placement
relayed to it from the displacement sensors.
Referring now to Figure 26, there is shown an embodiment of the device in
which
the one or more remote probes are functionally connected to a remote
transmitter 50, and in
which the programmable element 51 is connected to a remote receiver. The
communication
protocols proposed for the system range from a limited scope to a vastly
networked system
of several nodes. This provides a foundation for an unlimited number of use
cases. In one
embodiment of the remote communication protocol a close range high frequency
system
such as Bluetooth v4.0 is used. This emulates a wireless solution of what a RS-
232 wired
connection would provide. This enables the communication of two devices in
close range
quickly and securely. In another embodiment a roughly 802.11 compliant
protocol is used to
generate a mesh network comprised of the nearest devices. This mesh network
incorporates
34
CA 03042686 2019-05-02
WO 2018/085563
PCT/US2017/059754
all of the devices in a given unit. The unit size is without bound since the
addition of
individual nodes increases the range (range and unit size are directly
proportional since the
network is comprised and governed by the nodes themselves ¨ no underlying
infrastructure
is required). Only a vast outlier is left out of this network. This means that
in order for the
outlier to be omitted the nearest currently connected node must be
unequivocally out of
range for the outlier to communicate with. These services, specifically the
hardware, are
capable of running / polling without the usage of a main CPU (minimizes
battery usage).
This is useful because when a device is not being read it can just act as a
relay node. The
nature of the system minimizes power requirements (increasing longevity of
service),
supports asymmetric links / paths, and enables each node to play multiple
roles in order to
benefit the network.
Another embodiment requires connection to a LAN or WAN network, the remote
procedure is catalyzed by a user-driven event (button press, etc). This
generates a unique
identifier, for a digital receipt of the data transaction, on each phone
coupled with device
specific information. This information is supplemented with a GPS location to
distinguish
the devices locations. Since the data transmission was initiated by both
parties at a precise
time, coupled with GPS information, the system is capable of securely
identifying both
parties by location, UID, and device identifier. All methods are secured with
anonymity
heuristics and encryption. This will prevent snooping of data, a problem
presented by a
"man-in-the-middle" attack.
Another embodiment of the device utilizes one or more electrical probes
implanted
in the body. In one embodiment of the invention, the implanted probes are
connected to a
cardiac pacemaker. In another embodiment, the implanted probes are connected
to an
internal automated defibrillator. In another embodiment, the implanted probes
are
connected to a phrenic nerve stimulator. In another embodiment the implanted
probes are
connected to a delivery pump for pain medication, local anesthesia, baclofen,
or other
medication. In another embodiment, the implanted probes are connected to
another
implanted electronic device. Preferably the connections are wireless.
Referring now to Figure 33, electrode configuration XidMar is show.
Configuration XidMar is a two channel configuration with electrode 1 on the
xiphoid
process and electrode 4 on the right midaxillary line, horizontally aligned
with electrode 1.
CA 03042686 2019-05-02
WO 2018/085563
PCT/US2017/059754
Electrode 2a is 1 inch to the left of electrode 1, while electrode 3a is 1
inch to the right of
electrode 4. Electrodes 2a and 3a are used to record the voltage signal on
channel a.
Channel b is recorded using electrodes 2b and 3b which are found 1 inch below
the
corresponding channel a electrodes.
Figure 34 shows the StnMar electrode configuration in which electrode 1 is
located
just below the sternal notch and electrode 4 is located on the right
midaxillary line,
horizontally aligned with the xiphoid process. Electrode 2a is located 1 inch
below
electrode 1, and electrode 3a is located 1 inch to the right of electrode 4.
Channel b is at an
angle approximately 45 degrees to channel a. Electrode 2b is located on the
xiphoid
process and electrode 3b is located 1 inch below electrode 3a.
Figure 35 shows the StnIMar electrode location in which electrode 1 is located
just
below the sternal notch and electrode 4 is located on the inferior right
midaxillary line at
the bottom of the rib cage. Electrode 2a is located 1 inch below electrode 1,
and 3a is
located 1 inch to the right of 4. Electrode 2b is located on the xiphoid
process and
electrode 3b is located 1 inch below electrode 3a.
Figure 36 shows the McrMar electrode configuration in which electrode 1 is
located on the right midclavicular line just below the clavicle and electrode
4 is located on
the right midaxillary line horizontally aligned with the xiphoid process.
Electrode 2a is
located 1 inch below electrode 1 and electrode 3a is located 1 inch to the
right of electrode
4. Electrode 2b is located on the xiphoid process, and electrode 3b is located
1 inch below
electrode 3a.
Figure 37 shows the McrIMar electrode configuration in which electrode 1 is
located on the right midclavicular line just below the clavicle and electrode
4 is located on
the inferior midaxillary line approximately at the bottom of the ribcage.
Electrode 2a is
located 1 inch below electrode 1 and electrode 3a is located 1 inch to the
right of electrode
4. Electrode 2b is located on the xiphoid process and electrode 3b is located
1 inch below
electrode 3a.
Figure 38 shows the MclMar electrode configuration in which electrode 1 is
located on the left mixclavicular line just below the clavicle and electrode 4
is located on
the right midaxillary line, horizontally aligned with the xiphoid process.
Electrode 2a is
located 1 inch below electrode 1 and electrode 3a is located 1 inch to the
right of electrode
36
CA 03042686 2019-05-02
WO 2018/085563
PCT/US2017/059754
4. Electrode 2b is located on the xiphoid process and electrode 3b is located
1 inch below
electrode 3a.
The electrode configurations shown in Figures 34-38 can utilize either channel
a,
channel b, or both simultaneously to measure data.
In one embodiment of the invention, the system is adapted to perform an
impedance tomography scan utilizing a one or more pairs of source electrodes
and one or
more voltage sensing electrodes. The scan is completed by taking a series of
measurements with a movable electrode which is applied to the skin. The
movable
electrode forms a voltage measuring pair for impedance reading with at least
one other
electrode. The movable electrode may be coated in hydrogel which may be
applied
multiple times. In another embodiment of the invention, the electrode contains
a hydrogel
dispenser for each application. In this embodiment, hydrogel is stored in an
internal pouch
or syringe and there are devices, such as a mechanical button or squeeze tube,
which
allows the user to dispense hydrogel onto the electrode. In one embodiment of
the device
of the invention, the system directs the user to sweep the movable electrode
between
predetermined points on the body as indicated on the user interface or on a
reference card.
In another embodiment, the user may place the movable electrode from point to
point and
the system senses the location of the electrode using a camera, sonar, radar
or other
device.
The secure adhesion of electrodes is determines the quality of impedance
readings.
In one embodiment of the invention, the system detects the quality of adhesion
and reports
an index of adhesion to the user. In another embodiment, the system reports
problems with
adhesion if the index crosses a specific threshold. In a preferred embodiment
of the
invention, there are multiple voltage sensing channels arranged in a straight
line. This can
be accomplished using five electrodes arranged in a line. Referring to the
five electrodes
by letter, electrodes A and B are placed close together on one end of the
line, electrodes D
and E are placed close together on the other end of the line. Pair A-B and
pair D-E may be
placed 3-24" apart from each other. Electrode C is placed somewhere between
the two
pairs. Impedance is measured on three channels, B-C, C-D and B-D. If all the
electrodes
are adhered well, the sum of ZBC and ZCD should be close to ZBD. The
difference between
37
CA 03042686 2019-05-02
WO 2018/085563
PCT/US2017/059754
the measures, or the ratio of the difference to the full measurement can be
used to
determine the index of adhesion quality.
In one embodiment of the invention, Electrode C is not placed in a straight
line
with the other pairs of electrodes. In this case, impedance is measured on
channels B-C
and B-D. The ratio between the impedance on the two channels ZBC and ZBD is
used to
determine the index of adhesion quality. In another embodiment of the
invention, the
current driven through electrodes A and E is measured. The current measurement
or
variability in the current measurement can be used to determine the index of
adhesion for
electrodes A and E.
Electrical connectors have inherent capacitance which can affect impedance
measurements. In one embodiment of the invention, the system compensates for
the
capacitance of cables, leads or other electrical connection between the
impedance
measuring subsystem and the patient-connected electrodes. In one embodiment,
this is
accomplished by an inductor within the impedance measuring subsystem. In
another
embodiment, a compensating inductor is integrated into a patient cable or
leads which
connect the impedance measuring subsystem to the patient-connected electrode
pads. In
another embodiment a compensating inductor is embedded into an integrated
electrode
PadSet. In another embodiment, a modification of a Howland circuit which
consists of
capacitors Ci and C2 with values chosen to compensate for parasitic
capacitances Cc is
used (see Figure 39).
To achieve high clinical relevancy and good definition of respiratory curves,
the
impedance measurement subsystem should to be able to determine small
variations in
patient impedances on top of a relatively high baseline background with a high
resolution.
Therefore, there are stringent requirements on the absolute and relative
impedance
measurement errors. To obtain sufficient precision one or more of the
following design
solutions can be used: (1) the electronic design can be based on high
precision/low
temperature drift electronic components; (2) a high precision analog divider
can be used to
obtain the ratio between measured voltage and monitored source current,
compensating
for variations in the source current; (3) the same voltage can be used for
source current
generation and as an ADC reference, compensating for variations in the
reference voltage;
(4) external calibrated impedance standards can be used to calibrate and
verify the
38
CA 03042686 2019-05-02
WO 2018/085563 PCT/US2017/059754
impedance measurement subsystem performance. The calibrated system is
preferably
connected to the impedance standard with the same trunk cables used for
patient
measurements, providing verification of overall system performance. (5) The
impedance
measuring subsystem can have a built-in calibrated impedance standard,
allowing on-site
verification and recalibration. In one embodiment built-in standard is
attached to the
system via an external service port. The calibration is conducted by
connecting the
"patient" end of trunk cable back to the service port on the device and
running calibration
procedure available through the device's GUI. (6) The calibration can be
compleated by
varying impedance of the built-in standard over the whole range of the
measured patient
impedances to derive a device model, which can be used during patient
measurements to
achieve high-precision results. (6) The temperature model of the device can be
derived by
placing the device into a thermostat and measuring drift in the measured value
as a
function of internal device temperature. The internal device temperature can
be monitored
via a built-in thermal sensor. During patient measurement, a measurement
correction is
calculated using the thermal sensor's reading and applied to the measured
values.
Active Acoustic System
For acoustic measurement of lung volumes, preferably the device comprises at
least
one speaker and at least one microphone. Preferably the at least one speaker
and microphone
are arranged as a net, vest, or array. Preferably the at least one speaker
switches between
discrete frequencies or broadcasts broad spectrum noise. Preferably, numerous
speakers are
active simultaneously, broadcasting different acoustic signals. Preferably,
numerous
microphones are active simultaneously and record the measured acoustic
properties of the
thorax which can be correlated to lung volume as well pathologies of the
lungs. Preferably,
the microphones also record sounds that originate in the lungs such as
wheezing, squawks,
and crackles, which can be indicators of numerous chronic and acute pulmonary
diseases.
Preferably the lung sounds are recorded and identified as they are modified by
the active
signal. Preferably an algorithm analyzes the number and position of wheezes,
squawks, and
crackles to predict asthma and other pulmonary diseases. In one embodiment,
acoustic data
are combined with impedance data to help time the acoustic measurements
relative to the
respiratory cycle. In one embodiment acoustic data are combined with impedance
data for
39
CA 03042686 2019-05-02
WO 2018/085563 PCT/US2017/059754
the purposes of diagnosis or monitoring of disease. An example of this is
congestive heart
failure where stiffness creates characteristic changes in impedance curves and
there are also
changes in lung sounds associated with congestive heart failure. Combination
of the data
provides additional information.
Referring now to Figure 20, there is shown a device in which a speaker 38 is
attached to the chest of a patient, and insulated with sound dampening foam
39. A
microphone 40 is attached to the patient's back and is insulated with sound
dampening
foam. Both the speaker and the microphone are functionally connected to a
programmable
element 41, for example a computer with installed analysis software such as
MATLAB. The
output element provides data relating to the patient's respiration to the
operator in real time.
The speaker generates an acoustic signal which is recorded by the microphone.
Signal
generation and recording are timed and synchronized by the programmable
element.
Analysis software uses features of the recorded sound wave to evaluate the
acoustic
properties of the thorax, which can be used to estimate lung volume. Said
signal features
include but are not limited to: frequency-dependent phase shift, and amplitude
attenuation.
Preferably, the speaker switches between discrete frequencies of sound or
generates broad
spectrum white noise.
In another embodiment of the device, the microphone is also used to detect
sounds
which originate within the lungs such as crackles, squawks and wheezes. In one
embodiment, the programmable element of the device will employ software
algorithms to
detect associate acoustic patterns and inform physicians. In one embodiment,
the acoustic
system will interface with an impedance based system as well.
Referring now to Figure 21, there is shown an embodiment of the device in
which an
array of microphones 42 is used to record transmitted sound from different
regions of the
thorax. Preferably microphones record simultaneously. Preferably, the
programmable
element 43 selects the microphone with the best signal to noise ratio for
analysis. Preferably,
the programmable element combines the data from different channels in order to
maximize
the accuracy of lung volume estimates and localize pathologies of the lungs
including tumor
formation, bleeding, and tissue degradation.
Referring now to Figure 22, there is shown an embodiment of the device in
which an
array of speakers 44 is used to generate acoustic waves. Preferably the
programmable
CA 03042686 2019-05-02
WO 2018/085563 PCT/US2017/059754
element 45 controls each of the speakers individually, and switches between
speakers to
allow the device to measure acoustic properties of the thorax in many
different directions.
Preferably, the programmable element will activate each speaker simultaneously
with
signals of unique frequencies so that the signal from each speaker can be
separated in the
recorded signals. Preferably, the programmable element combines the data from
different
channels in order to maximize the accuracy of lung volume estimates and
localize
pathologies of the lungs including tumor formation, bleeding, and tissue
degradation.
Patient Data Entry
Preferably, the device software maintains a user-friendly GUI (Graphical User
Interface). Preferably, the GUI contains a color coding system to aid
operators in quickly
making diagnoses and decisions for patient care. In one embodiment, the GUI
presents a
numerical RVM measurement. In one embodiment the GUI presents a respiratory
sufficiency index (RSI). In one embodiment, the GUI presents a respiratory
waveform.
In the software present in all embodiments of the device, patient data is
preferably
recorded by the user prior to testing. The user is prompted to enter patient
data. The data
recorded includes any or all of the following: patient height, weight, chest
circumference
during maximum inspiration, chest circumference during normal end-expiration,
age,
gender, ethnicity, and smoking history. In one embodiment, posture when
testing is also
input into the device within the programmable GUI. Variations in posture may
lead to
different breathing patterns and tidal volumes. The device accepts posture
inputs such as
supine and seated and standing. The ability to test patients in multiple
postures is helpful
with noncompliant patients such as neonates or obtunded patients.
In one embodiment, the device calculates BMI. In a preferred embodiment, an
algorithm in the device or on a look up table calculates a "calibration
coefficient" that
corrects for patient size and body habitus to provide a universal calibration
to deliver an
absolute measurement. The calibration coefficient may be obtained by combining
patient
information with the data recorded off the probes applied. Preferably, the
physical location
of the probes is also entered. During the data acquisition, the calibration
algorithm may
validate the data and their consistency with the patient information entered,
and may suggest
combination of the input parameters that is most consistent with the data
recorded, as well
as a suggestion for the operator to re-check the patient's information. As
data is being
41
CA 03042686 2019-05-02
WO 2018/085563 PCT/US2017/059754
acquired, the calibration algorithm may suggest and/or perform re-adjustment
based on
signal pattern recorded off probes, and/or provided by an operator as normal
or abnormal. In
another embodiment, the device calculates BSA or another index of body shape
or size. In
one embodiment, the system displays predictive values for patient results
based on the
aforementioned patient data. In one embodiment, the device also provides a
percentage
comparison against these values within displayed results to further inform the
clinician of
patient parameters or condition based on standard tables of spirometric data
created by
Knudsen, Crapo, or others. In one embodiment, the patient's demographics
and/or body
measurements are entered and the device suggests the lead configuration and/or
the spacing
of the leads and/or the size or characteristics of the lead for that patient.
In one embodiment, the device assesses signal variation and adjusts display
parameters, calibration parameters and or intermediate calculations in
response to the
variation. In one embodiment the device assesses variation in one or more
features of the
signal including baseline, mean, minimum, maximum, dynamic range, amplitude,
rate,
depth, or second or third order derivatives of any items in the list.
In one embodiment, the device calculates a calibration coefficient to convert
a raw or
processed impedance trace to a respiratory volume trace. In one embodiment,
the calibration
coefficient is calculated from a range of physiological and demographic
parameters. In one
embodiment, the device of the invention automatically adjusts the calibration
coefficient in
response to variation in the parameters. In one embodiment, the device
automatically adjusts
the calibration coefficient in response to one or more of: respiratory rate,
baseline
impedance, or mean impedance.
In one embodiment, the device includes one or more of, respiratory rate,
baseline
impedance, or mean impedance in the calculation of the coefficient, or a
correction factor
for the calibration coefficient. In embodiments in which the calibration
coefficient is based
on a time-variable parameter, such as respiratory rate, baseline impedance or
mean
impedance, the device automatically adjusts the calibration coefficient to
account for the
variation in the parameter.
In one embodiment, the device adjusts the calibration coefficient based on the
assessment of variation in the signal. In one embodiment where the calibration
coefficient is
42
CA 03042686 2019-05-02
WO 2018/085563 PCT/US2017/059754
used to convert a raw impedance signal to a respiratory volume trace, the
calibration
coefficient is based partially on respiratory rate.
In one embodiment, the device adjusts the display of a dataset in response to
variation in the dataset. The dataset is made up of a raw signal from a
sensor, a processed
signal from a sensor, or the calculated metrics or parameters.
In one embodiment, the device adjusts the minimum of the y-axis on a displayed
chart in response to variation in the dataset. In one embodiment, the minimum
of the y-axis
on a displayed chart is equal to the minimum of the dataset. In one embodiment
the
minimum of the y-axis on the displayed chart is equal to the minimum of the
dataset within
a specific window. In one embodiment, the window over which the relevant
minimum of the
dataset is calculated is the same as the window over which the data is
displayed. In one
embodiment, the minimum of the y-axis on the displayed chart is equal to the
minimum of
the dataset within the display window minus a coefficient or percentage of the
minimum
value.
In one embodiment, the device adjusts the range of the y-axis of the displayed
dataset is to account for variation in the dataset. In one embodiment the
range of the y-axis
of a displayed dataset is equal to the dynamic range of the dataset. In one
embodiment the
range of the y-axis of the displayed dataset is equal to the dynamic range of
the dataset
within a specific window. In one embodiment, the y-axis of the displayed
dataset is equal to
the dynamic range of the dataset within a specific window, plus a constant, or
a percentage
of the dynamic range.
In one embodiment, the device adjusts the range of the y-axis of a displayed
dataset
based on statistics of a feature of the dataset. In one embodiment, the device
sets the range
of the y-axis to be equal to the mean amplitude of the signal plus the
standard deviation of
the amplitude of the signal within a specified window multiplied by a
coefficient. In one
embodiment, the device adjusts the range of the y-axis of a displayed dataset
to be equal to
the mean amplitude of the signal plus the variance of the amplitude of the
signal within a
specified window multiplied by a coefficient. In one embodiment, the device
calculates the
amplitude of respirations in the dataset. The device then removes outliers at
the high end,
low end or which have features which appear unrelated to the intended measured
parameter.
43
CA 03042686 2019-05-02
WO 2018/085563 PCT/US2017/059754
The device then adjusts the range of the y-axis to be equal to the mean of the
amplitude of
the dataset plus the standard deviation of the dataset multiplied by a
coefficient.
In one embodiment, the device automatically adjusts the midpoint of the y-axis
of a
chart of a dataset in response to variation in the dataset. In one embodiment,
the device sets
the y-axis to be equal to the mean of the dataset within a specific window. In
another
embodiment, the device sets the y-axis to the equal to the median of the
dataset within a
specific window. In one embodiment, the device sets the midpoint of the y-axis
to the result
of a function of the statistics of the dataset.
Calibration Method
The calibration coefficient is calculated in a novel way. In the preferred
embodiment, the device contains circuitry and software that automatically
calibrates the
device. In one embodiment, calibration is aided by data acquired through
bioelectrical
impedance analysis, a process which measures tissue impedance on one or more
channels at
various frequencies. In this embodiment, data from bioelectrical impedance
analysis may be
used to calculate certain characteristics of the subject including, but not
limited to, hydration
level, baseline impedance and body composition. A low level of hydration
causes the
electrical impedance of the body to be greater. A high level of fat in the
body would also
cause an increase in the average electrical impedance of the body, but likely
a decrease in
overall impedance as electricity passes through the path of least resistance.
Muscle is much
more vascular than fat and contains more conductive electrolytes, so a
muscular patient's
body would have much lower electrical impedance than a similarly size person
who was not
very muscular. Scaling the calibration factor based on these inputs makes it
more accurate.
Calibration of the device of the invention preferably comprises predictions
for
respiratory rate, tidal volume and minute ventilation based on the metabolic
requirements of
body tissue. Predictions preferably involve multiplying the patient's measured
body weight,
or ideal body weight by a volume of air, or volume of air per minute required
by a unit of
body weight. The ideal body weight is determined from a patient's height,
race, and/or age
and may further be determined with one or more of the Devine, Robinson, Hamwi,
and
Miller formulas.
In one embodiment, the calibration coefficient is calculated from a patient's
demographic information, including but not limited to: sex, age, and race. In
another
44
CA 03042686 2019-05-02
WO 2018/085563
PCT/US2017/059754
embodiment, the calibration coefficient is calculated from a patient's
physiological
measurements including but not limited to body type, height, weight, chest
circumference
measured at different points of the respiratory cycle, body fat percent, body
surface area,
and body mass index. In another embodiment the calibration coefficient is
calculated
based on the measured value of the ECG signal recorded at different points. In
more
detail, the ECG is recorded by electrodes at various locations on the thorax
and abdomen.
In one embodiment, the differential voltage recordings at different electrodes
are used to
calculate the average baseline impedance and estimate the resistivity of the
patient's
thorax in various directions. In another embodiment the calibration
coefficient is
calculated based on the patient's baseline impedance to an external current
source as
measured between electrodes in a bipolar configuration, tetrapolar
configuration or other
configuration comprising 2 or more leads. The locations of these electrodes
are placed in a
range of configurations over the whole body. In another embodiment,
demographic
characteristics are combined with baseline impedance measurements for
calibration. In
another embodiment anatomic information is combined with baseline impedance
measurements for calibration. In a preferred embodiment, known volumes
recorded on a
spirometer or ventilator are combined with demographic information and
baseline
impedance. In such embodiments, the system may simultaneously measure
impedance
and volume (using a spirometer, ventilator, or other similar device). The
system then
computes a specific transformation between impedance and volume as an input to
the
conversion algorithm
In another embodiment, a dynamic calibration based on additional parameters
obtained using the impedance measuring subsystem and consisting of overall
patient
impedance (including skin and fat layer impedances), internal organs impedance
(baseline
impedance) and its variations, and the shape of the respiratory curve is
implemented.
Ongoing or intermittent checks of calibration are preferably undertaken. In a
preferred embodiment of the device, calibration is recalculated with the
recording of each
sample. In another embodiment, the device is regularly recalibrated based on a
timer
function. In another embodiment, the device is recalibrated whenever the
baseline
impedance varies from the baseline by a certain threshold such as 10%. In
another
embodiment, the device is recalibrated whenever tidal volume or minute volume
varies
CA 03042686 2019-05-02
WO 2018/085563 PCT/US2017/059754
from baseline levels or predicted levels by a certain threshold, such as 20%,
where
predicted values are calculated using the formulas published by Krappo,
Knudson, and
others.
Ongoing or intermittent checks of calibration may be undertaken. Preferably
this
involves an internal check to internal phantom.
Preferably ongoing or intermittent checks of baseline impedance are be used to
recalibrate or reaffirm calibration. Preferably ongoing or intermittent
readings from each
hemithorax individually or in combination are used to recalibrate or provide
data for
recalibration.
Preferably, recalibration is performed automatically or by alerting a
caregiver of
required modification or requiring additional steps to be taken by the
caregiver, such as
recalibrating with a ventilator or spirometer.
In one embodiment calibration is done through measurement electrode pairs. In
another embodiment, calibration is done through additional electrodes. In
another
embodiment, calibration is done all or in part by repurposing measurement
electrodes and
using the sensor as the delivery electrodes and the delivery electrodes as the
sensor
electrodes.
Preferably the calibration electrodes are placed in specific locations and/or
at
specific distances apart on the abdomen and thorax. In another embodiment, one
or more of
the leads are placed a specified distance apart on the forehead. In another
embodiment of the
device, the magnitude of the ICG signal across an acceptable electrode
configuration with or
without an estimation of the heart volume is used to determine the baseline
impedance and
calibrate the RVM data to respiratory volume. Preferably the calibration
coefficient is
calculated using a combination of the 5 previously mentioned methods.
Universal Calibration
While relations between respiratory and impedance variations are highly
linear, the
"scaling factor" between those values vary significantly from one patient to
another. There
is also day-to-day variation for the same patient. The day-to-day variations
are correlated
to some extent with physiological parameters measured by the RMV device and
can be
significantly compensated for. The residual day-to-day variations for the same
patient are
smaller than typical measurement error. In a preferred embodiment, this
residual variation
46
CA 03042686 2019-05-02
WO 2018/085563 PCT/US2017/059754
can be managed with existing ancillary measurements. In a preferred
embodiment, this
residual variation can be managed using ongoing or intermittent recalibration
by any of
the methods previously described.
In one embodiment, the "scaling factor" varies between patients by about an
order
of magnitude. In a preferred embodiment, this factor can be determined
precisely by
preliminary calibration with a spirometer or ventilator data or other data
set. In a preferred
embodiment, the RMV device is used for measurement of respiratory parameters
without
preliminary calibration. Preferably, a reliable procedure of deducing this
factor from
measurable patient physiological parameters is used for calibration. Such
procedure
allows the determination of the "scaling parameter" with sufficient precision
to satisfy
measurement requirements for all proposed device applications.
In one embodiment, measurements of respiratory motion derived from a
technology
including impedance plethysmography, accelerometers placed on the body, video
images,
acoustic signals or other means of tracking motion of the thorax, abdomen or
other body
parts is calibrated or correlated with another technology that assesses
respiratory status. In a
preferred embodiment, respiratory motion detection derived from impedance
measurements
is calibrated with spirometry. In one embodiment respiratory motion detection
is calibrated
or correlated with end tidal CO2 measurements. In one embodiment, respiratory
motion
detection is calibrated or correlated with ventilator measurements of flow
and/or volume. In
one embodiment, respiratory motion is calibrated with a full-body
plethysmograph. In one
embodiment, baseline RVM measurements of a given patient are taken in
conjunction with
standard spirometry measurements and a calibration coefficient for that
particular patient is
derived. Later in the postoperative period or otherwise, the calibration
coefficients are used
to obtain quantitative lung volume measurements for that patient. In a
preferred
embodiment, such calibration coefficients are combined with current baseline
impedance or
other physiologic measurements for ongoing or intermittent calibration. In one
embodiment, preoperative measurements are used to derive a calibration
coefficient which
is then used, alone or in combination with other data, to obtain quantitative
lung volume
measurements to use in management of the patient after surgery or in other
situations. In
another embodiment, the calibration coefficient is derived from lung volume or
flow
47
CA 03042686 2019-05-02
WO 2018/085563 PCT/US2017/059754
measurements obtained on an intubated patient from measurements recorded from
a
mechanical ventilator.
Preferably the device is linked to a spirometer, ventilator or
pneumotachometer to
provide volume or flow calibration. Preferably, the device is linked to a
spirometer or
ventilator or pneumotachometer to provide volume calibration. In one
embodiment, the
operator will run the patient through a brief breathing test regimen of one or
more of the
following: at least one tidal breathing sample, at least one forced vital
capacity (FVC)
sample, at least one measurement of minute ventilation sample, and at least
one maximum
voluntary ventilation (MVV) sample. The device will be calibrated based on the
results of
the spirometer tests relative to the impedance measurements. In a preferred
embodiment,
calibration will be implemented from measurements taken during tidal
breathing. In
particular, for patients who are unable to comply with the procedure, a simple
tidal
breathing sample will be taken, which requires no coaching or compliance. The
tidal
breathing sample is collected over 15 seconds, 30 seconds, 60 seconds, or
another time
frame.
In one embodiment, a calibration coefficient for a given individual is
calculated
based on combined spirometry and RVM data and applied to deliver an absolute
volume
measurement for RVM measurements taken at a future time. Preferably, this
absolute
volume measurement will be validated or modified at the future time using
calibration
capabilities intrinsic to the hardware and current measurements derived from
the device. In
a preferred embodiment, an algorithm is applied to RVM data based on patient
demographics, existing normal spirometry data for varying patient demographics
found in
the work of Knudsen, Crapo, and others and/or other anatomic or physiologic
measurements
to provide a universal calibration to deliver absolute volume measurements
without the need
for individual calibration with a spirometer or ventilator.
Preferably, the device may be used in conjunction with ECG or ICG data to
produce
further calibration of impedance data by utilizing parameters derived ECG and
ICG such as
heart rate and SNR. Preferably, ECG or ICG data will help validate proper
electrode
placement. In another embodiment, the electrical activity of the heart is used
to enhance the
device calibration. Preferably the device can measure the following cardiac,
pulmonary and
other physiology parameters and features: Heart Rate (HR), baseline impedance,
impedance
48
CA 03042686 2019-05-02
WO 2018/085563
PCT/US2017/059754
magnitude, Pre-ejection Period (PEP), Left Ventricular Ejection Time (LVET),
Systolic
Time Ration (STR), Stroke Volume (SV), Cardiac Output (CO), Cardiac Index
(CI),
Thoracic Fluid Content (TFC), Systolic Blood Pressure (SBP), Diastolic Blood
Pressure
(DBP), Mean Arterial Pressure (MAP), Mean Central Venous Pressure (CVP),
Systemic
Vascular Resistance (SVR), Rate Pressure Product (RPP), Heather Index (HI),
Stroke
Volume Index (SVI), and Waveform Accuracy Value (WAV). Baseline values
calculated
from patient characteristics for these features are utilized to derive the
calibration coefficient
as well as calculate an index of overall respiratory sufficiency. Conversely,
RVM data can
be used to enhance accuracy or utility of ICG data such as Heart Rate (HR),
baseline
impedance, impedance magnitude, Pre-ejection Period (PEP), Left Ventricular
Ejection
Time (LVET), Systolic Time Ration (STR), Stroke Volume (SV), Cardiac Output
(CO),
Cardiac Index (CI), Thoracic Fluid Content (TFC), Systolic Blood Pressure
(SBP), Diastolic
Blood Pressure (DBP), Mean Arterial Pressure (MAP), Mean Central Venous
Pressure
(CVP), Systemic Vascular Resistance (SVR), Rate Pressure Product (RPP),
Heather Index
(HI), Stroke Volume Index (SVI), and Waveform Accuracy Value (WAV).
In particular, for patients who are unable to comply with a more complicated
procedure, a simple tidal breathing sample of respirations at rest is taken,
which requires no
coaching or compliance. Analysis of these data provides information relative
to pulmonary
physiology and respiratory status that could not otherwise be obtained.
Referring now to Figure 8, there is shown an impedance plethysmograph 31 and a
spirometer 32 both functionally connected to the same programmable element 33.
Volume
data from the spirometer is preferably sampled simultaneously or nearly
simultaneously
with the impedance reading of the impedance plethysmograph. Referring now to
Figure 9,
there is shown a patient who is connected to a ventilator 34 as well as the
impedance
plethysmograph 35, both functionally connected to a programmable element 36.
The volume
of the ventilator is sampled simultaneously with the impedance reading of the
impedance
plethysmograph. Referring now to the graph in Figure 10, there is shown a
graph of volume
versus impedance for a given patient undergoing various breathing maneuvers
while data
was simultaneously collected using the impedance plethysmograph and a
spirometer. The
trace represented by Figure 11 with volume over time is normal breathing. The
trace
represented by Figure 12 is slow breathing and the trace represented Figure 13
is erratic
49
CA 03042686 2019-05-02
WO 2018/085563 PCT/US2017/059754
breathing. In one embodiment, the slope of the line of best fit 37 is used as
the RVM
calibration coefficient to compute volume from impedance. In another
embodiment, an
algorithm utilizing the slope, shape and/or other curve characteristics and/or
other
demographic or body habitus characteristics of the patient is used to
calculate the calibration
coefficient.
In one embodiment a simple numerical value is obtained from a ventilator or
spirometer for tidal volume or minute ventilation for use in calibration of
the device. One
embodiment is comprised of a combined system in which RVM and volume
measurements
are taken simultaneously, nearly simultaneously, or sequentially by means of a
spirometer,
pneumotachometer, ventilator or similar device and the combined data utilized
to create an
individual calibration coefficient for the calculation of absolute volume from
RVM
measurements for a given individual.
Example:
One method of calibration has already been utilized in a small-scale study.
Measurements of height, weight, chest circumference at maximum inspiration and
normal
expiration, distance from suprasternal notch to xiphoid, distance from under
mid-clavicle to
end of rib cage in midaxillary line, distance from end of rib cage to iliac
crest in midaxillary
line, and abdominal girth at umbilicus were taken and recorded. Electrodes
were positioned
at the Posterior Left to Right, Posterior Right Vertical, and Anterior-
Posterior, and ICG
configuration discussed above. The four probes of the impedance measurement
device were
connected to the electrodes that corresponded to one of the configurations
above. The ICG
position was connected first and only used to measure resting ICG of the
subject in a supine
position. The leads were then reconfigured to connect to the Posterior Left to
Right position.
Once the leads were positioned correctly and the subject was supine, the
subject performed
breathing tests which were measured simultaneously by the impedance
measurement device
and a spirometer for a sampling time of about 30 seconds. The breathing tests
performed
were normal tidal breathing (3 runs), erratic breathing (2 runs), slow
breathing (2 runs),
Forced Vital Capacity (FVC) (3 runs), and Maximum Ventilatory Volume (MVV) (2
runs).
FVC and MVV were performed according to ATS procedures. Normal, erratic, and
slow
tests were measured by a bell spirometer, and FVC and MVV were measured by a
turbine
spirometer. Preferably, the calibration can be run all together on any type of
spirometer that
CA 03042686 2019-05-02
WO 2018/085563 PCT/US2017/059754
meets ATS standards. Once all breathing tests were complete, the leads were
repositioned to
a new configuration, and the tests were run again until all configurations had
been tested.
The data was collected on PC for the impedance data and turbine spirometer
data, and on
another PC for the bell spirometer data. The data was then merged onto one PC
and loaded
into MATLAB. Preferably, MATLAB or other software packages that utilize signal
processing are used. Preferably, the data is loaded onto a PC or other
computing station.
Once the data was merged, the impedance and volume data from each breathing
test were
matched together using a GUI-based program. Correlation coefficients and
calibration
coefficients were produced for each of the test runs by comparing the
impedance and
volume traces using MATLAB. This data then was utilized in Excel to predict
calibration
coefficients based on patient characteristics. Preferably, the data can be
imported into and
analyzed in any software with a statistical package.
Referring now to Figure 14, depicted is a graph of BMI versus the calibration
coefficient for 7 patients. BMI is shown on the x-axis, and calibration
coefficient is shown
on the y-axis. The linear relationship between height and the calibration
coefficient in
configuration D (PRR placement as described earlier) is indicative of its
utility in
determining the calibration coefficient. Other physiological parameters such
as height
weight, body surface area, race, sex, chest circumference, inter-mammary
distance, age also
have important relationships with the calibration coefficient, and in one
embodiment any or
all of these parameters aid in accurate determination of the calibration
coefficient. A
combination of statistical analysis and an expert system is used to determine
a given
patient's correlation coefficient based on the input of said physiological
parameters. Such
methods may include principal component analysis, artificial neural networks,
fuzzy logic,
and genetic programming and pattern analysis. In a preferred embodiment, test
data from a
pilot study is used to train the expert systems. In a preferred embodiment,
existing data
regarding patient demographics and pulmonary function are used to train the
expert system.
Preferably, a combination of test data from a pilot study and existing
pulmonary function
datasets are use to train the expert system.
One problem that is encountered with some spirometers is volume drift, where a
greater amount of air is inspired rather than expired. Additionally, prolonged
spirometry
testing provides increase in resistance to pulmonary flow that can alter the
physiology
51
CA 03042686 2019-05-02
WO 2018/085563 PCT/US2017/059754
and/or can change the respiratory flows and/or volumes. These patterns can
disrupt the
correlation coefficient for the test by altering the volume so that it trends
downwards while
the impedance trace stays constant. Figure 15 shows a volume curve that
exhibits volume
drift. Figure 16 shows a volume versus impedance curve for that set where the
volume drift
damages the fit of the plot. In one embodiment, the device corrects for the
problem by
subtracting out a line with a constant slope value. After using this mean flow
method, the
curves do not trend up or down as seen in Figure 17 and the volume versus
impedance data
stays much tighter as seen in Figure 18, and the volume versus impedance data
stays much
tighter, giving higher correlations and better correlation coefficients. In
one embodiment,
volume drift subtraction is used in calibration. In one embodiment volume
drift subtraction
is used in deriving the calibration coefficient. The same utility is also
achieved by
differentiating the volume curve to get flow, subtracting the DC offset
between intervals that
have the same lung volume at the start and end point, and then integrating to
get flow
without the drift artifact.
In another embodiment of the device, the calibration coefficient is determined
by
comparing the RVM data trace and calculated values compared to predicted
values for the
patient's tidal volume, FVC, FEV1 etc. based on standard tables of spirometric
data created
by Knudsen, Crapo, or others known to those skilled in the art.
Data Analysis
Referring now to Figure 19, there is shown a flow chart that displays the
progression
of data through the analysis software. Raw data is recorded by the impedance
meter,
digitized using an analog to digital converter, and inputted to the
programmable element
through a standard data port. Data processing strips the signal of noise and
motion artifacts.
Analysis algorithms calculate the volume trace as well as medically relevant
information
including but not limited to: frequency and time domain plots of the impedance
and/or
calculated volume traces, respiratory rate, tidal volume, and minute
ventilation. In one
embodiment, the analysis algorithm to convert impedance into volume traces
utilizes either
calibration in conjunction with spirometer or ventilator data, or in another
embodiment,
calibration based on physiological parameters. The algorithm produces a
correlation
coefficient which, when multiplied with the impedance data, converts the
impedance scale
into a volume scale. In addition, the algorithms take variability of the above
metrics into
52
CA 03042686 2019-05-02
WO 2018/085563 PCT/US2017/059754
account and automatically calculate a standardized index of respiratory
sufficiency (RSI).
This RSI contains information that integrates information from one or more
measurements
and/or utilizes the range of acceptable values of the following measurements
individually
and in combination to provide a single number related to respiratory
sufficiency or
insufficiency: respiratory rate, respiratory volume, respiratory curve
characteristics,
respiratory variability or complexity as previously prescribed.
In one embodiment, one of the following methods are used in calculation of the
RSI:
change in patient status from previous measurement, second derivative of
change in patient
status from previous measurements, multivariate analysis, pattern analysis,
spectral analysis,
neural networks, self-teaching system for individual, self-teaching system for
patient
population.
In one embodiment, the RSI also includes data from the following: oxygen
saturation, Tcp02, TcpCO2, end tidal CO2, sublingual CO2, heart rate, cardiac
output,
oncotic pressure, skin hydration, body hydration, and BMI. The advantage of
this index is
that it can be understood by untrained personnel and it can be linked to
alarms to notify
physicians or other caregivers in case of rapidly deteriorating health. After
computation,
processed metrics pass to the output module, which may be embodied as a
printer or
displayed on a screen or delivered by oral, visual, or textual messaging.
In one embodiment, the device notes a pattern in the curve recorded during the
inspiratory or expiratory phase of respiration. In one embodiment, the device
notes a pattern
in the respiratory variability in rate, volume and/or location of respiration.
In one
embodiment the pattern is noted in the shape of the respiratory curve. In one
embodiment,
the pattern analysis includes the values derived from the slope of
inspiration. In one
embodiment, the pattern analysis includes the values derived from the slope of
expiration. In
one embodiment, the pattern analysis includes a combination of parameters
which could
include any or all of the following: respiratory rate, minute ventilation,
tidal volume, slope
of inspiration, slope of expiration, respiratory variability. In one
embodiment, these
parameters are used within the calculation of a Respiratory Health Index (RHI)
that provides
a standardized quantitative measure of adequacy of ventilation. In one
embodiment, the RHI
is coupled with alarms that sound either when respiration falls below what is
deemed as
adequate, or within the range that is deemed adequate, if the patient
experiences a very
53
CA 03042686 2019-05-02
WO 2018/085563 PCT/US2017/059754
sudden change. In one embodiment, the device provides information to calculate
an RHI.
Preferably the device calculates and displays the RHI. In one embodiment, the
Respiratory
Health Index is compared against a universal calibration based on patient
characteristics. In
one embodiment, the RHI provides quantitative data with the system calibrated
to a specific
patient.
Referring now to Figure 27, the time delay or phase lag of an impedance signal
and a
volume signal is shown. In this particular figure, the delay was found to be
0.012 seconds.
Phase lag between volume and impedance signals is an important issue that is
addressed in
one embodiment. There is a time lag between impedance and volume signals due
to the
elastic and capacitive nature of the pleura and lung tissue, which creates a
slight delay
between the diaphragm moving and air flowing in the lung. In one embodiment,
this phase
difference is used as a measure of lung stiffness and airway resistance.
Frequency phase
analysis allows the user to find the phase angle. A larger phase offset is
indicative of a high
degree of airway resistance to motion. Calculation of the phase angle is
accomplished by
comparing simultaneously recorded and synchronized RVM curves with flow,
volume or
pressure curves recorded by a spirometer, pneumotachometer, ventilator or
similar device.
In one embodiment the phase lag between volume and impedance signals is a
component of
the algorithm that is used to calibrate the system to a given individual. In
one embodiment
the phase lag is used to calibrate the system for a universal calibration.
When calculating the
calibration coefficient using an external pressure, flow, or volume measuring
device, the
leading curve is shifted by the magnitude of the phase lag so as to correlate
temporally with
the trailing curve. This embodiment increases the accuracy of the calibration
algorithm.
When no external pressure, flow, or volume measuring device is used for
calibration, a
virtual phase lag is calculated based on patient characteristics, including
demographic
information, physiological measurements, and pulmonary function test metrics.
In one embodiment, phase lag is corrected for by RVM algorithms in aligning
both
impedance and volume. In one embodiment, phase lag data is presented
independently as a
standardized index to demonstrate a measure of lung compliance and stiffness.
In one
embodiment, phase lag data is integrated within the Respiratory Health Index
as a measure
of respiratory status.
54
CA 03042686 2019-05-02
WO 2018/085563 PCT/US2017/059754
In one embodiment, frequency domain analysis is applied to the RVM
measurements. Preferably, at least one frequency domain plot such as a Fourier
transform is
displayed to the operator. Preferably, at least one 2-dimensional frequency
domain image of
the RVM data such as a spectrograph is displayed to the operator, where one
dimension is
frequency and the other is time, and the magnitude of the signal at each
location is
represented by color. Preferably, the frequency domain information is used to
assess
respiratory health or pathologies. Preferably, an alarm will alert a medical
professional if the
frequency domain data indicates rapid deterioration of patient health.
In a preferred embodiment, RVM measurements are used as the basis for
complexity
analysis. In one embodiment, complexity analysis is performed on the RVM
signal alone.
Preferably, RVM measurements are used in combination with other physiologic
measurements such as heart rate, urine output, EKG signal, impedance
cardiogram, EEG or
other brain monitoring signal.
In a preferred embodiment, RVM measurements are utilized as a component of
complexity analysis in combination with data provided by a device used to
treat or monitor
the patient including: the ventilator measurement of the patient generated
respiratory
pressure, the ventilator measurement of the patient generated respiratory
flow, the ventilator
measurement of the patient generated respiratory volume, the ventilator
measurement of the
ventilator generated respiratory pressure, the ventilator measurement of the
ventilator
generated respiratory flow, the ventilator measurement of the ventilator
generated
respiratory volume an infusion pump, or other devices used to treat the
patient, RVM
measurements may be used to quantify breath-to-breath variability. One
embodiment of the
device is used to define a specified point along the respiratory curve with
which to calculate
breath-to-breath variability in respiratory rate such as the peak of
inspiration or nadir of
expiration. Preferably, peaks or nadirs of each respiration are automatically
identified. In
one embodiment, the device provides data with describing breath-to-breath
variability in
volume inspired. In one embodiment, the device provides data describing breath-
to-breath
variability or complexity in the slope or other characteristics of the
respiratory volume or
flow curve. In one embodiment, the device provides data with which to
calculate variability
.. or complexity associated with the location of respiratory effort, such as
chest vs. abdominal
or one hemithorax vs. the other, by collecting data from different locations
on the body with
CA 03042686 2019-05-02
WO 2018/085563 PCT/US2017/059754
the same or different electrode pairings. Preferably, the device calculates
breath-to-breath
variability or complexity of one or more of these parameters. Preferably, the
device presents
the variability or complexity analysis in a form that is easy to interpret by
the user. In one
embodiment, the device combines data from more than one source of variability
or
complexity among the following: respiratory rate, respiratory volume, location
of
respiratory effort, slope or other characteristic of the respiratory volume or
flow curves, to
provide an advanced assessment of respiratory function. In one embodiment, the
device
analyzes the variability or complexity data intermittently or continuously and
presents the
data at intervals such as every 10 minutes, every 30 minutes, or every hour.
Preferably, the
device presents the variability analysis in less than 10 minutes, less than 5
minutes, less than
1 minute, or in near real time. In one embodiment, the variability or
complexity of any of
the respiratory parameters may be quantified by linear or nonlinear analysis
methods.
Preferably, the variability or complexity of any of the respiratory parameters
may be
quantified by nonlinear dynamical analysis. In one embodiment, approximate
entropy is
.. used by the device for data analysis. In one embodiment, variability or
complexity analysis
of the data is combined with volume data to provide a combined index of
respiratory
function. In one embodiment, variability or complexity analysis data is
combined with other
parameters and presented as a Respiratory Sufficiency Index or a Respiratory
Health Index.
In a preferred embodiment, RVM measurements or the complexity analysis of the
RVM signal is utilized as at least a part of the information used in goal
directed therapy. In a
preferred embodiment, RVM measurements or the complexity analysis of the RVM
signal
provide information for decision support. In a preferred embodiment RVM
measurements or
the complexity analysis of RVM signal is utilized as at least a part of the
patient data
required for a controlled loop system.
Use in Imaging
In one embodiment of the device, the respiratory cycle is measured by one or
more
methods including but not limited to impedance pneumography, end tidal CO2, or
pulse
oximetry while the heart is imaged or otherwise measured using
echocardiography which
may be embodied as 2D echo, 3D echo or any other type of echocardiography.
Time series
data from the echocardiogram is marked as having a certain accuracy rating
based on the
respiratory motion recorded by the respiratory monitor. In one embodiment,
56
CA 03042686 2019-05-02
WO 2018/085563 PCT/US2017/059754
echocardiography data below an accuracy threshold is discarded. In another
embodiment,
echocardiography data is weighted based on its accuracy rating where the least
accurate data
is weighted lowest. The device generates a composite image or video of the
heart and
cardiac motion based on the most accurate echocardiogram data. In one
embodiment,
echocardiography data is recorded over more than one cardiac cycle, then after
analysis and
accuracy rating, the best data is used for generating a composite image of the
heart or video
of the cardiac cycle.
Other embodiments include combining respiratory cycle measurement and
quantification with other cardiac imaging techniques for the purpose of
improving accuracy.
The methods of cardiac imaging may include Doppler flow measurements,
radionuclide
study, gated CT, and gated MRI. Other embodiments include combining
respiratory cycle
measurement by RVM with other diagnostic or therapeutic modalities of the
chest,
abdomen, and other body parts, including diagnostic CT or MRI, catheter
directed therapy,
directed cardiac ablation, radioablation of tumor, radiation of tumor. In a
preferred
embodiment, RVM and cardiac impedance data are utilized together for timing of
data
collection or data analysis of diagnostic imaging or anatomically directed
therapy.
In another embodiment of the device, the respiratory impedance measurements or
data from complexity analysis of RVM measurements are used to generate an
image of the
lungs. In another embodiment of the device, data from complexity analysis of
RVM
measurements and cardiac impedance measurements are used to generate an image
of the
heart and lungs. In the preferred embodiment, the heart and lungs are imaged
simultaneously. In one embodiment, the device is used for generating 2D
images, videos, or
models of the heart and/or lungs. In the preferred embodiment, the device
generates 3D
images, videos or models of the heart and/or lungs.
Detecting Pathologies and Improving Monitoring
In one embodiment, the device provides RVM data which, with our without
variability or complexity analysis, is used to aid in decision making such as
extubation or
intubation for mechanical ventilation. In one embodiment the device provides
RVM data
which, with or without variability or complexity analysis, aids in decision
making regarding
drug administration or other therapeutic intervention. In one embodiment, the
device uses
variability or complexity information alone or with volume data as part of an
open or closed
57
CA 03042686 2019-05-02
WO 2018/085563
PCT/US2017/059754
loop control system to adjust ventilatory settings. In one embodiment, the
device uses
variability or complexity information, alone or with volume data or other
analysis of the
respiratory curve provided by RVM, as part of an open or closed loop control
system to
adjust doses of medications. This embodiment is useful for premature infants
to optimize the
management of a pressure ventilator, and for patients with uncuffed
endotrachial tubes. In
one embodiment, the device uses variability or complexity information, alone
or with
volume data or other analysis of the respiratory curve provided by RVM, as
part of a patient
management system that monitors patient status, recommends medication
delivery, and,
then, reassesses the patient to direct further action.
In one embodiment the device uses variability or complexity analysis of the
RVM
signal alone, volume data alone, curve analysis alone, or any of these in
combination to
trigger alarms indicating change in patient status. In another embodiment,
symbol-
distribution entropy and bit-per-word entropy are used to measure the
probability of patterns
within the time series. In another embodiment, similarity of distributions
methodology is
used. In one embodiment, the device sounds an alarm when it detects a change
in
respiratory complexity or a respiratory complexity below a specified threshold
or more
constrained breathing patterns associated with pulmonary pathology or disease
states. In one
embodiment, the device sounds an alarm when it detects a change in a combined
measurement of respiratory and heart rate complexity beyond a specified
threshold.
An early warning score (EWS) is a guide used by medical services to determine
the
degree of illness of a patient. There are several variants of the, but in
general, the and
scoring are similar to table 1 below.
58
CA 03042686 2019-05-02
WO 2018/085563 PCT/US2017/059754
Score 3 2 1 0 1 2 3
Respiratory rate
>35 31-35 21-30 9-20 <7
(breaths/min)
Sp02 (%) <85 85-89 90-92 >92
Temperature (C) >38.9 38-38.9 36-37.9
35-35.9 34-34.9 <34
Systolic BP (mmHg) >199 100-199 80-99 70-79 <70
Heart rate (bpm) >129 110-129 100-109 50-99 40-49 30-39
<30
AVPU Alert Verbal Pain Unresponsive
Table 1
Within hospitals, the EWS is often used as part of a "track-and-trigger"
system
whereby an increasing score produces an escalated response varying from
increasing the
frequency of patient's observations (for a low score) up to urgent review by a
rapid response
or Medical Emergency Team. EWS scores are tools used by hospital care teams to
recognize the early signs of clinical deterioration in order to initiate early
intervention and
management, such as increasing nursing attention, informing the provider, or
activating a
rapid response or medical emergency team. These tools typically involve
assigning a
numeric value to several physiologic parameters (e.g., systolic blood
pressure, heart rate,
oxygen saturation, respiratory rate, level of consciousness, and urine output)
to derive a
composite score that can be used to identify a patient at risk of
deterioration. Most are based
on an aggregate weighted system in which the elements are assigned different
points for the
degree of physiological abnormality. Observational studies suggest that
patients often show
signs of clinical deterioration up to 24 hours prior to a serious clinical
event requiring an
intensive intervention. Delays in treatment or inadequate care of patients on
general
hospital wards may result in increased admissions to the intensive care unit
(ICU), increased
length of hospital stay, cardiac arrest, or death.
59
CA 03042686 2019-05-02
WO 2018/085563 PCT/US2017/059754
In one embodiment of the device, MV, TV and/or RR become part of an existing
early warning scoring system, either as a replacement of or in addition to the
standard
respiratory rate that is currently a part of most EWS systems, for use to help
prevent or
predict evolving patient compromise, disease state, or distress. Such
compromise, disease
state, or distress may be one or more of respiratory failure, sepsis, cardiac
failure, congestive
heart failure, renal failure, over-hydration, pulmonary edema, hyper metabolic
state,
overexertion, traumatic brain injury, pulmonary embolus, opioid induced
respiratory
depression, over sedation. Preferably, the device is attached to the patient
using one or more
sensors to obtain one or more of the patient's impedance levels (used to
determine MV, TV
and/or RR as described herein), MV, TV and/or RR, oxygen saturation,
temperature, blood
pressure, pulse or heart rate, blood oxygen levels, brain activity, blood lab
tests (e.g.
complete blood count (CBC)) or another physiological status. The incoming data
from the
sensors is collected an analyzed to output an early warning score for the
patient. If the score
exceeds a predetermined level, one or more alarms (audible and/or visual) may
be activated.
Additionally, clinicians may input information about the patient's condition,
including, but
not limited to alertness, voice, pain, and unresponsiveness (commonly referred
to as
AVPU). Preferably, minute ventilation is used instead of or in conjunction
with respiration
rate and in combination with other sensor data to derive the patients state
and output the
early warning score. Preferably, the sensors do not impede breathing or
obstruct the
patient's airways. Preferably, the sensors are non-invasive.
In another embodiment of the device, MV, TV and/or RR become one of the
foundational pieces of a new, improved early warning scoring system upon which
a
predictive algorithm is based for use to help prevent or predict evolving
patient compromise,
disease state, or distress. Such compromise, disease state, or distress may be
one or more of
respiratory failure, sepsis, cardiac failure, congestive heart failure, renal
failure, over-
hydration, pulmonary edema, hyper metabolic state, overexertion, traumatic
brain injury,
pulmonary embolus, opioid induced respiratory depression, over sedation.
Preferably, the
device is attached to the patient using one or more sensors to obtain one or
more of the
patient's impedance levels (used to determine MV, TV and/or RR as described
herein), MV,
TV and/or RR, oxygen saturation, temperature, blood pressure, pulse or heart
rate, blood
oxygen levels, brain activity, blood lab tests (e.g. complete blood count
(CBC)) or another
CA 03042686 2019-05-02
WO 2018/085563 PCT/US2017/059754
physiological status. The incoming data from the sensors is collected and
analyzed to output
an early warning score for the patient. If the score exceeds a predetermined
level, one or
more alarms (audible and/or visual) may be activated. Additionally, clinicians
may input
information about the patient's condition, including, but not limited to
alertness, voice, pain,
and unresponsiveness (commonly referred to as AVPU). Preferably, minute
ventilation is
used instead of or in conjunction with respiration rate and in combination
with other sensor
data to derive the patients state and output the early warning score.
Preferably, the sensors
do not impede breathing or obstruct the patient's airways. Preferably, the
sensors are non-
invasive.
With the better respiratory data provided by the device, the weighting of the
algorithm is modified and then applied for use to help prevent or predict
evolving patient
compromise, disease state, or distress. Such compromise, disease state, or
distress may be
one or more of respiratory failure, sepsis, cardiac failure, congestive heart
failure, renal
failure, over-hydration, pulmonary edema, hyper metabolic state, overexertion,
traumatic
brain injury, pulmonary embolus, opioid induced respiratory depression, over
sedation.
Preferably, the device is attached to the patient using one or more sensors to
obtain one or
more of the patient's impedance levels (used to determine MV, TV and/or RR as
described
herein), MV, TV and/or RR, oxygen saturation, temperature, blood pressure,
pulse or heart
rate, blood oxygen levels, brain activity, blood lab tests (e.g. complete
blood count (CBC))
.. or another physiological status. The incoming data from the sensors is
collected and
analyzed to output an early warning score for the patient. If the score
exceeds a
predetermined level, one or more alarms (audible and/or visual) may be
activated.
Additionally, clinicians may input information about the patient's condition,
including, but
not limited to alertness, voice, pain, and unresponsiveness (commonly referred
to as
.. AVPU). Preferably, minute ventilation is used instead of or in conjunction
with respiration
rate and in combination with other sensor data to derive the patients state
and output the
early warning score. Preferably, the sensors do not impede breathing or
obstruct the
patient's airways. Preferably, the sensors are non-invasive.
In another embodiment the device provides information for the early detection
of
sepsis or other infections, by using MV, TV, and/or RR measurements in
conjunction with
other factors, like temperature, blood pressure, pulse rate, blood work (e.g.
CBC), etc. but
61
CA 03042686 2019-05-02
WO 2018/085563 PCT/US2017/059754
reduces the emphasis commonly associated with temperature and pulse rate while
increasing
the emphasis on other (e.g. respiratory) parameters (e.g. MV, TV, and/or RR).
In another embodiment, different physiologic components are added to the EWS.
In
another embodiment one or more of the existing parameters in the EWS is
removed from the
formula used to help prevent or predict evolving patient compromise, disease
state, or
distress. Such compromise, disease state, or distress may be one or more of
respiratory
failure, sepsis, cardiac failure, congestive heart failure, renal failure,
over-hydration,
pulmonary edema, hyper metabolic state, overexertion, traumatic brain injury,
pulmonary
embolus, opioid induced respiratory depression, over sedation. Preferably, the
device is
attached to the patient using one or more sensors to obtain one or more of the
patient's
impedance levels (used to determine MV, TV and/or RR as described herein), MV,
TV
and/or RR, oxygen saturation, temperature, blood pressure, pulse or heart
rate, blood oxygen
levels, brain activity, blood lab tests (e.g. complete blood count (CBC)) or
another
physiological status. The incoming data from the sensors is collected and
analyzed to output
an early warning score for the patient. If the score exceeds a predetermined
level, one or
more alarms (audible and/or visual) may be activated. Additionally, clinicians
may input
information about the patient's condition, including, but not limited to
alertness, voice, pain,
and unresponsiveness (commonly referred to as AVPU). Preferably, minute
ventilation is
used instead of or in conjunction with respiration rate and in combination
with other sensor
data to derive the patient's state and output the early warning score.
Preferably, the sensors
do not impede breathing or obstruct the patient's airways. Preferably, the
sensors are non-
invasive.
In one embodiment, pre-defined criteria and ranges for the early warning
scoring
system are based on (and may be adjusted according to) patient's disease state
(obstructive
sleep apnea (OSA), congestive heart failure (CHF), systemic inflammatory
response
syndrome (SIRS), sepsis, renal disease, etc.) either externally entered
(manually or
automatically (e.g. from an electronic health record (EHR))) or as determined
by the device
itself. In one embodiment, pre-defined criteria and ranges for the early
warning scoring
system are based on (and may be adjusted according to) the patient's
circumstance
(endoscopic procedure, surgery, post-operative state, etc.) either externally
entered
(manually or automatically (e.g. from an EHR)) or as determined by the device
itself. The
62
CA 03042686 2019-05-02
WO 2018/085563
PCT/US2017/059754
early warning score may be adjusted based on the patient's age, demographics,
condition
(e.g. pregnancy), or other feature. In one embodiment, pre-defined criteria
and ranges for
the early warning scoring system are based on (and may be adjusted according
to) a
combination of patient's circumstance (endoscopic procedure, surgery, post-
operative state,
etc.) and disease state (OSA, CHF, COPD, pulmonary fibrosis, asthma, sepsis,
renal disease,
etc.) either externally entered (manually or automatically (e.g. from an EHR))
or as
determined by the device itself.
In one embodiment, MV, TV and/or RR become part of a triage system (such as
the
Aldrete scoring system used for discharge criteria from a PACU) to help make
decisions
regarding patient care, patient medication or patient nutrition. The triage
system preferably
is similar to the early warning scoring system. In one embodiment, pre-defined
criteria and
ranges for the triage system are based on (and may be adjusted according to) a
combination
of a triage criteria (Aldrete score, etc.) and disease state (OSA, CHF,
sepsis, renal disease,
etc.) either externally entered (manually or automatically (e.g. from an EHR))
or as
determined by the device itself.
In one embodiment, MV, TV, and/or RR become part of a modified early warning
scoring system (which computes a modified early warning score used to trigger
an alarm
and/or actuates an external system, which delivers or controls treatment or
medical
intervention) used for detecting changes in patient condition, disease state
(e.g. CHF,
COPD, OSA, asthma, sepsis, cranial hemorrhage, ARDS, etc.), identify patients
at-risk or in
need of additional or advanced care, help initiate or modify patient care,
determine the
effectiveness or ineffectiveness of interventions. The modified early scoring
system
preferably is similar to the early warning system. In one embodiment pre-
defined ranges and
criteria for the modified early scoring system are based on (and may be
adjusted according
to) a combination of a triage criteria (Aldrete score, etc.) and disease state
(OSA, asthma,
pulmonary fibrosis, COPD, CHF, sepsis, renal disease, etc.) either externally
entered
(manually or automatically (e.g. from an EHR)) or as determined by the device
itself. The
modified early warning scoring system may be adjusted based on the patient's
age,
demographics, condition (e.g. pregnancy), or other feature.
In one embodiment, MV, TV, and/or RR become part of a pediatric early warning
scoring system (which computes a pediatric early warning score used to trigger
an alarm
63
CA 03042686 2019-05-02
WO 2018/085563 PCT/US2017/059754
and/or actuate an external system, which delivers or controls treatment or
medical
intervention) used for detecting changes in patient condition, disease state
(e.g.
brochopulmonary dysplasia, asthma, cystic fibrosis, etc.), identify patients
at-risk or in need
of additional or advanced care, help initiate or modify patient care,
determine the
effectiveness or ineffectiveness of interventions. The pediatric early scoring
system
preferably is similar to the early warning system. In one embodiment pre-
defined ranges and
criteria for the pediatric early scoring system are based on (and may be
adjusted according
to) a combination of a triage criteria (Aldrete score, etc.) and disease state
(OSA, CHF,
sepsis, renal disease, etc.) either externally entered (manually or
automatically (e.g. from an
EHR)) or as determined by the device itself. The modified early warning
scoring system
may be adjusted based on the patient's age, demographics, condition (e.g.
pregnancy), or
other feature.
In one embodiment, MV, TV and/or RR become part of a PACU/ICU/hospital
floor/home/rehab/nursing home mobilization protocol to help make decisions
regarding
patient care or patient nutrition. The mobilization protocol system preferably
is similar to
the early waring scoring system. In one embodiment, pre-defined criteria and
ranges for the
mobilization protocol system are based on (and may be adjusted according to) a
combination of PACU/ICU/hospital floor/home/rehab/nursing home mobilization
protocol
and disease state (OSA, CHF, sepsis, renal disease, etc.) either externally
entered (manually
or automatically (e.g. from an EHR)) or as determined by the device itself.
In one embodiment, MV, TV and/or RR become part of a
fitness/wellness/rehab/athletic training/performance protocol to help make
decisions
regarding modification of training regimen or nutrition. The training protocol
system
preferably is similar to the early waring scoring system. In one embodiment,
pre-defined
criteria and ranges are based on (and may be adjusted according to) a
combination of
fitness/wellness/rehab/athletic training/performance protocol and disease
state (OSA, CHF,
sepsis, renal disease, etc.) either externally entered (manually or
automatically (e.g. from an
EHR)) or as determined by the device itself.
In one embodiment, MV, TV and/or RR become part of a system for monitoring or
adjusting patient care based on activity and/or nutrition for patients with
different metabolic
states such as diabetes, cachexia, obesity, sepsis, anabolism, catabolism etc.
to help make
64
CA 03042686 2019-05-02
WO 2018/085563 PCT/US2017/059754
decisions regarding modification of activity or nutrition regimens. Regimens
can be
modified continuously with an open or closed-loop feedback system, or
intermittently on a
pre-defined schedule, or as alerted by the system. The activity or nutrition
regimen system
preferably is similar to the early warning scoring system. In one embodiment
of the device,
pre-defined criteria and ranges are based on (and may be adjusted according
to) a
combination of a system for monitoring or adjusting patient care based on
activity and/or
nutrition for patients with different metabolic states such as diabetes,
cachexia, obesity,
sepsis, etc. and disease state (OSA, CHF, sepsis, renal disease, etc.) either
externally entered
(manually or automatically (e.g. from an EHR)) or as determined by the device
itself.
In one embodiment, an activity is considered to be "normal" over a given time
frame
if outputs from it (measured or computed) fall within predefined or adjustable
limits. In
another embodiment, activity is structured to elicit or enhance certain
measurement, for
example MV, and thus used to evaluate metabolic state with specific active
stimulus.
In one embodiment, RVM measurements are integrated into an open or closed
feedback loop to report adequacy of ventilation by ensuring safe dosage of
medication by
monitoring ventilation for warning signs of respiratory arrest. In a preferred
embodiment,
RVM is integrated into a system with a ventilator providing an open or closed
feedback loop
by which ventilator adjustments are made. Differences between RVM measurements
and
ventilator or spirometer generated volume or flow measurements can be used to
provide
information for diagnosis and guidance of therapy. By using RVM monitoring
with or
without additional information from end tidal CO2 or pulse oximetry
measurements, this
embodiment automatically weans the patient by gradually decreasing ventilatory
support
and observing RVM and other parameters and alerts the physician of readiness
for
extubation, or alerts for failure to progress. This combined system with
either pulse
oximetry or ETCO2 or both could be used as an open or closed loop system to
deliver
narcotics or other respiratory depressant drugs such as benzodiazepines or
propofol.
In one embodiment, the analysis algorithm detects the presence of specific
respiratory patterns maintained in the expert system database and informs the
physician or
other health care provider about the possibility of associated pathology. In
one embodiment,
the respiratory pattern for a given pathology is recognized and in a preferred
embodiment,
quantified. In another embodiment the pathology is localized.
CA 03042686 2019-05-02
WO 2018/085563 PCT/US2017/059754
In a preferred embodiment, the device recognizes a specific patterns related
to
respiratory volume, curve, variability or complexity or other analysis of RVM
data.
In one embodiment, the device recognizes the pattern associated with impending
respiratory failure or respiratory arrest and delivers an audible and/or
visible alert or
warning. In one embodiment, the device analyzes the respiratory data or the
trend in the data
and makes a recommendation for intubation and mechanical ventilation. In one
embodiment, the device analyses the respiratory pattern data and adjusts the
level of
infusion of a narcotic or other respiratory depressant drug such as propafol.
In one embodiment, the device recognizes the respiratory pattern associated
with a
specific disease entity or pathology such as congestive heart failure, or
asthma or COPD or
narcotic induced respiratory depression or impending respiratory failure. In
one
embodiment, the device alerts the physician to this pathology. In one
embodiment the device
quantifies the degree of the pathology. In one embodiment, the device
recognizes a pattern
of congestive heart failure and provides data regarding the trending toward
improvement or
deterioration with time or as associated therapeutic intervention.
Preferably, the impedance measuring element of the device can produce
Impedance
Cardiograph (ICG) measurements. Preferably, the device detects impedance
variability
associated with heart rate variability. Preferably the device detects
impedance variability
associated with variability of the respiratory waveform or other respiratory
parameter and
utilizes the heart rate and respiratory rate, volume or waveform variability
to predict cardiac,
respiratory and pulmonary complications. Preferably, the device maintains
alarms for
predetermined limits associated with unsafe pulmonary variability or
complexity or
combined heart rate and respiratory variability or complexity.
In another embodiment, End Tidal CO2 (ETCO2) is used in addition to or instead
of
subjective assessment to determine the RVM baseline. In one embodiment, RVM is
coupled
with ETCO2 measurements to provide additional information regarding
respiratory status.
In another embodiment RVM is coupled with pulse oximetry to provide
information
about both ventilation/respiration and oxygenation. A more complex RVM system
couples
standard RVM measurements with both or either ETCO2 or pulse oximetry. This
combined
device provides further information about breathing for sedated patients and
enhances
patient monitoring.
66
CA 03042686 2019-05-02
WO 2018/085563 PCT/US2017/059754
In a preferred embodiment, measurments of lung volumes and minute ventilation
are
used to assess the adequacy of the patient after extubation in a quantitative
way. Minute
ventilation is specifically used for patients undergoing surgery. Preferably,
a preoperative
measurement of tidal volume or minute ventilation is obtained as a baseline
for the specific
patient. Preferably the baseline is used post-operatively as a comparison
between
preoperative and postoperative respiratory status. The trend of tidal volume
or minute
ventilation is used to monitor a patient during surgery or a procedure or
during post-
operative recovery in the Post Anesthesia Care Unit, in the Intensive Care
Unit, or on the
hospital floor. This trend gives an accurate measure of differences and
changes in the
patient's breathing from preprocedure baseline and can denote when the patient
returns to a
baseline level of breathing. In a preferred embodiment, the device directly
aids the physician
to make an appropriate extubation decision by defining an adequate level of
breathing
specific to that patient. In one embodiment, absolute lung volumes are
compared with
precalibrated data derived from patient characteristics, and are used in
determining the
presence of restrictive and/or obstructive lung disease and other respiratory
conditions.
Absolute volume data can be especially useful within the PACU and ICU as a
complement
to existing quantitative data.
Use in PCA feedback and Drug Dosing Optimization
One use of the device is to use cardiac and/or respiratory data measured and
recorded by one, several, or a combination of the technologies listed herein,
to determine the
effect of one or more drugs or other medical interventions on the patient. In
an embodiment,
the respiratory monitor is used to judge the side effects of analgesic drugs
on the body and
prevent or assist in the prevention of respiratory failure or other
compromises due to adverse
reaction or overdose.
In a preferred embodiment, the device is paired with or integrated into a
patient
controlled analgesia (PCA) system. This is accomplished electronically through
communication between the device of the invention and an electronic PCA
system, or by an
integrated monitor/PCA system or by a setting in the monitor indicating that
the patient is
being administered PCA. In this embodiment, the administration of analgesia or
anesthesia
is limited based on the risk of respiratory or other complications predicted
by the device. If
the PCA system is not electronic, or analgesic drugs are being delivered by
personnel, the
67
CA 03042686 2019-05-02
WO 2018/085563 PCT/US2017/059754
device makes recommendations as to when the risk of respiratory complication
is high and
the dosage should be lowered.
Another embodiment of the device of the invention is a diagnostic/therapeutic
platform. The monitoring device is paired with one or more of the following:
pharmaceutical regimens, therapeutic regimens, use of inhaler, use of
nebulizer, use of
pharmaceutical targeting respiratory system, use of pharmaceutical targeting
cardiovascular
system, use of pharmaceutical targeting asthma, COPD, CHF, cystic fibrosis,
bronchopulmonary dysplasia, pulmonary hypertension, other diseases of the
lungs. This
embodiment of the device is used to judge the effectiveness of possible
medical and
nonmedical interventions on respiratory state or respiratory health and
suggest changes in
regimen for optimization and/or suggest appropriate interventions when the
patient is at risk
for complications.
In one embodiment RVM is paired with behavioral algorithms or algorithm that
includs information about any of the following patient medical status,
environmental factors,
and behavioral factors of a demographic group or of the patient in general. In
a preferred
embodiment, one of the algorithms described above could denote the necessity
for obtaining
an RVM measurement. More preferably, the RVM measurements are used in
conjunction
with behavioral/medical/environmental algorithmic data to provide information
to indicate
action or therapy. An example of the use of this embodiment of the device
would be an
algorithm which includes the patient's previous respiratory complications or
chronic
respiratory illness, and/or allergies as inputs along with behavioral events
known to
exacerbate said conditions. By including information from the patient's
schedule (e.g.
attending an outdoor event during allergy season, or participating in a
sporting competition),
the system recommends that he take an RVM measurement then makes
recommendations
about whether to maintain normal dosing of medication or increase it. The
software can also
recommend that the patient bring medication with him to the event, and
generally remind
the patient to take his medication. Another example could be that the patient
had an asthma
attack or other respiratory complication. RVM data could be utilized to assess
the severity of
this attack by any of the measured parameters including minute ventilation,
tidal volume,
time for inspiration vs. expiration (i.e. ratio), shape of the respiratory
curve during normal
breathing, shape of the respiratory curve during the deepest possible breath
or other
68
CA 03042686 2019-05-02
WO 2018/085563 PCT/US2017/059754
respiratory maneuver. The data could then prompt independently or be used in
conjunction
with other information to make a decision for the patient to perform an action
including one
of the following: do nothing, rest, use an inhaler, take a pharmaceutical, use
a nebulizer, go
to the hospital. Information as to the action required could be part of a
behavioral or other
algorithm designed for the specific patient or a group of patients with a
similar disorder,
patients with a similar demographic, patients with a specific medical,
anatomic or
behavioral profile or patients in general. Preferably, after the action, the
patient is instructed
to repeat the RVM measurement to assess the adequacy of therapy. Preferably
his repeat
measurement is compared to the measurement before the therapy or other
intervention and
changes are noted. Additional information from this comparison or just data
taken after
therapy is used alone or in combination with other patient data to make
further medical
decisions or recommendations for action.
For example, an asthmatic is having symptoms and decides to or is instructed
by a
disease management algorithm to obtain an RVM measurement. The RVM data is
analyzed
by the device, utilized independently or compared to his historic baseline or
the last
measurement taken. Based on these, with or without other patient specific
inputs such as
heart rate, the device recommends he use his inhaler. A second set of RVM data
is then
taken. The RVM data is compared to the previous RVM data taken prior to
treatment. The
device then follows a decision tree and tells the patient he has improved and
needs no
further therapy, that he needs to repeat the dosage, that he needs to call his
physician, or that
he immediately needs to go to the hospital. In a preferred embodiment, the RVM
data is
combined with behavioral algorithms developed for a demographic or for a
specific patient
to optimize recommendations for the patient.
PACU/ICU Usage
In one embodiment, the device is used within a Postoperative Anesthesia Care
Unit
(PACU) setting, as either a standalone monitor or as an accompaniment to or
incorporated in
an existing monitor. Within the PACU, RVM volume is calculated and compared
against
pre-calibrated data derived taking into account BMI, height, weight, chest
circumference,
and other parameters. The device is used to complement existing quantitative
data that
supports decision making within the PACU. In one embodiment, within the
operating room,
RVM data is correlated with end tidal carbon dioxide measurements to provide a
more
69
CA 03042686 2019-05-02
WO 2018/085563 PCT/US2017/059754
comprehensive assessment of respiratory status. RVM derived measurements
including
minute ventilation are used to compare a patient's status before, during, and
after surgery or
a procedure and to document the effect of anesthesia/narcotic induced
respiratory
depression. RVM is used to support more subjective assessments made by
clinicians in the
PACU by providing a quantitative justification for certain decisions,
including the decision
to re-intubate. The device also supports subjective assessment regarding
patients on the
hospital floor as a monitor for decline in respiratory status and an alarm for
the need to re-
intubate or perform another intervention to improve respiratory status.
Preferably, RVM
measurements will assist in regulation of narcotic pain medication, sedative
drugs such as
benzodiazepines, or other drugs with respiratory depressive effects. In one
embodiment, the
above mentioned uses regarding the RVM in a PACU setting are implemented
within the
ICU setting such as a Neonatal ICU, Surgical ICU, Medical ICU, Pulmonary ICU,
Cardiac
ICU, Coronary Care Unit, Pediatric ICU, and Neurosurgical ICU. In another
embodiment,
the RVM device is used in the setting of a step down unit or standard hospital
bed to follow
respiratory status.
Later in the postoperative period or otherwise, measurements of the
respiratory
pattern, including tidal volumes, respiratory rate, minute ventilation,
variability in
interbreath interval or volume, or RVM signal complexity can be compared to
baseline
values measured before surgery. This can directly aid the extubation decision
by defining
what is an adequate level of breathing specific to that patient. In another
embodiment of the
device, RVM monitoring identifies problems that are commonly associated with
ventilators,
such as poor endotracheal tube positioning, hyperventilation, hypoventilation,
rebreathing
and air leaks. The system also identifies air leaks through a chest tube or
cuffless tube. Air
leaks would cause a downward trend to appear on any direct volume measurement
which
would not be present on the impedance trace, thus the device can detect and
report air leaks
in devices which directly measure volume or flow. In a preferred embodiment,
the system
identifies abnormalities and trends specific to a hemithorax such as those
related to the
following pathologies: pneumothorax, pulmonary contusion, rib fractures,
hemothorax,
chylothorax, hydrothorax, and pneumonia.
In one embodiment, the device is used during Monitored Anesthesia Care (MAC)
to
monitor respiratory status, assist in drug and fluid administration, provide
indication of
CA 03042686 2019-05-02
WO 2018/085563 PCT/US2017/059754
impending or existing respiratory compromise or failure, and assist in the
decision to
intubate if necessary.
In another embodiment of the device, RVM monitoring identifies problems that
are
commonly associated with ventilators, such as poor endotracheal tube
positioning,
hyperventilation, hypoventilation, rebreathing and air leaks. In one
embodiment RVM
measurements are combined with data derived from the ventilator to provide
additional data
regarding physiology. An example of this is that differences can be recorded
in RVM
measurements vs. inspired or expired flows or volumes measured on the
ventilators to assess
"work of breathing" in a quantitative fashion.
In another embodiment, RVM measurements are taken after surgery in a patient
who
is still under the effects of anesthesia or pain medication to monitor patient
recovery.
Recording a baseline tidal volume curve for a patient during normal
preoperative conditions
provides a comparison baseline for monitoring during and after surgery.
Returning to a
similar tidal volume curve is one signal of respiratory recovery after being
taken off a
ventilator. In this embodiment of the invention, the device is used to
evaluate the success of
extubation and determine if reintubation is necessary. The invention described
herein allows
these measurements to be taken noninvasively and without being in the stream
of
inspired/expired air or impeding airway flow or contaminating the airway
circuit.
In one embodiment, the device is used within outpatient surgicenters,
specifically
geared towards patients receiving Monitored Anesthesia Care, including
patients undergoing
orthopedic procedures, cataract surgery and endoscopy of the upper and lower
GI tract.
Diagnostic Usage
In one embodiment, the device is used to quantify respiratory parameters
during
performance based tests. In a preferred embodiment, the device is used to
quantify
.. respiratory parameters in tests of cardiovascular function including stress
tests. In a
preferred embodiment, the device is used in combination with one of the
following tests to
assess impact of the test on respiration. In a preferred embodiment, the
device reports effects
of exercise or a particular drug like dopamine on the overall physiology or
metabolism of
the body as reflected by changes in respiratory volumes, patterns, rate or
combinations
thereof including advanced analysis of breath-to-breath
variability/complexity, fractal or
71
CA 03042686 2019-05-02
WO 2018/085563 PCT/US2017/059754
entropy based analyses as described elsewhere. In a preferred embodiment, the
device is
used to evaluate the safety of a given level of exercise or pharmacologic
stress.
In a preferred embodiment, variability or complexity analysis of RVM
measurements is undertaken in concert with standard pulmonary function
testing. In a
preferred embodiment, variability or complexity analysis of RVM measurements
is
undertaken with or without heart rate variability/complexity analysis in
concert with
standard cardiovascular physiology testing such as stress testing, walking
tests for
claudication, or other performance based testing.
In a preferred embodiment, the device is used to evaluate the effects of drugs
on the
.. respiratory system including bronchodilators for diagnostic purposes,
monitoring of
therapeutics, optimization including effects on both heart and lungs. More
preferably, the
device above combines respiratory information obtained by impedance or other
methods
described with EKG information about heart rate, heart rate variability, EKG
evidence of
ischemia or arrhythmia. In a preferred embodiment, the device is used to
evaluate the effects
of bronchoconstrictors as in a provocative test. In various embodiments, the
device obtains
continuous or intermittent RVM measurements. In a preferred embodiment, the
device
provides trending of RVM data.
In a preferred embodiment, the device is used to evaluate the effects of
metabolic
stimulants, cardiovascular drugs including beta blockers, alpha adrenergic
agonists or
blockers, beta adrenergic agonists or blockers. In a preferred embodiment, the
device is used
during a stress test to demonstrate level of effort placed or to demonstrate
an unsafe
condition relative to the pulmonary system to terminate or modify the test.
Stress Introduced
to the patient is created by various means including but not limited to,
exercise and/or the
delivery of a drug. In a preferred embodiment, the device indicates or works
with other
technologies described earlier to indicate the level of overall exercise. In a
preferred
embodiment, the device is used as a free-standing device for measuring the
effects of
exercise or other stimulant on the pulmonary system.
In another embodiment of the device, the respiratory information is combined
with
cardiac information to define the level of exertion related to EKG changes
associated with
cardiac disease. In another embodiment of the device, the system combines
respiratory
information with cardiac information to determine the level of exertion of an
athlete.
72
CA 03042686 2019-05-02
WO 2018/085563 PCT/US2017/059754
In another embodiment, the device provides warning of potential negative
impact of
the level of exercise on overall health or on cardiac status, with or without
pairing
respiratory signals with cardiac impedance or EKG measurements in the home,
athletic
field, military environment or out of hospital setting. One embodiment of the
device is a
holter monitor which outputs values for one or more of the following:
respiratory effort,
level of activity, state of physiology, or metabolism associated with
different rhythms,
depolarization or other cardiac pathophysiology.
One embodiment of the invention is similar to a holter monitor which monitors
one
or more physiological parameters over hours to days in a hospital, home, or
other setting.
One embodiment of the device is combined with a holter monitor or critical
care monitor
which specifically monitors decompensation effects related to heart failure. A
similar
embodiment of the device monitors and outputs measurements of "lung water". In
one
embodiment, the device is included in a disease management system for
congestive heart
failure.
In a most preferred embodiment, the device provides a continuous measurement
which can be run for long periods of time and can deliver a time curve
demonstrating the
effects of exercise or a drug for diagnosis, therapeutic monitoring or drug
development.
One embodiment of the device provides trending data over minutes to hours to
days
for patients with a variety of disease states including chronic obstructive
pulmonary disease,
congestive heart failure, pulmonary hypertension, pulmonary fibrosis, cystic
fibrosis,
interstitial lung disease, restrictive lung disease, mesothelioma, post
thoracic surgery, post
cardiac surgery, post thoracotomy, post thoracostomy, post rib fracture, post
lung contusion,
post pulmonary embolus, cardiac ischemia, cardiomyopathy, ischemic
cardiomyopathy,
restrictive cardiomyopathy, diastolic cardiomyopathy, infectious
cardiomyopathy,
hypertrophic cardiomyopathy. Preferably the device provides information about
changes in
respiration in these disease states related to interventions or provocative
testing procedures.
In one embodiment of the device of the invention, the system is used to
diagnose
various diseases. In a preferred embodiment, the device is used to assess the
risk of
developing pneumonia. In another embodiment, the device is used to assess the
risk that a
pneumonia therapy is not effective, and suggest corrective action. Another
embodiment of
the invention is used for the evaluation of functional deterioration or
recovery associated
73
CA 03042686 2019-05-02
WO 2018/085563 PCT/US2017/059754
with diseases including but not limited to: pneumonia, heart failure, cystic
fibrosis,
interstitial fibrosis, hydration levels, congestion due to heart failure,
pulmonary edema,
blood loss, hematoma, hemangioma, buildup of fluid in the body, hemorrhage, or
other
diseases. This information may be used for diagnosis as above or be integrated
with
respiratory volume measurements or other physiological measurements that may
be
measured by the device or input into the device to provide a comprehensive
respiratory
sufficiency index (cRSI).
In one embodiment, disease specific modules can be created to gather disease
specific information, employ disease specific algorithms and deliver either
optimized
respiratory volume data or respiratory diagnostic data related to the specific
disease.
In a preferred embodiment of the invention, respiratory curve analysis is used
to diagnose
medical conditions. In one embodiment, the system utilizes provocative tests
to determine
measurements or estimates of one or more of the following: tidal volume,
residual volume,
expiratory reserve volume, inspiratory reserve volume, inspiratory capacity,
inspiratory vital
capacity, vital capacity, functional residual capacity, residual volume,
forced vital capacity,
forced expiratory volume, forced expiratory flow, forced inspiratory flow peak
expiratory
flow, and maximum voluntary ventilation. In this embodiment, diagnostic tools
such as flow
volume loops are generated by software running on the system for diagnosis of
various
cardiopulmonary or other disorders.
Respiratory curve analysis can also be used to assess cardiopulmonary or other
disorders without provocative tests. In one embodiment, an algorithm monitors
trends in
TV, MV and RR to provide a metric of respiratory sufficiency or respiratory
sufficiency
index (RSI). In another embodiment, an algorithm analyzes individual breaths
as an input to
diagnose respiratory conditions. In this embodiment, one or more of the
following
parameters are calculated on a breath by breath basis: inspiratory time (It),
expiratory time
(Et), It:Et ratio, percent inspiratory time, tidal impedance, tidal volume and
area under the
curve. In this embodiment, the various parameters are outputted through the
system's user
interface or printable report for the user to assess respiratory disease
state. In a preferred
embodiment, an algorithm analyzes the parameters to act as a diagnostic aid.
In this
embodiment, the system outputs an index of disease severity or a
positive/negative reading
for the disease.
74
CA 03042686 2019-05-02
WO 2018/085563 PCT/US2017/059754
In one embodiment, the device is implanted. In a preferred embodiment, the
device
is powered from a pacemaker-like battery. In one embodiment the device is
combined with a
pacemaker or defibrillator. In one embodiment the device is adjusted or
calibrated or
interrogated using an external component.
Figure 40 depicts an embodiment of the invention wherein the impedance
measuring
device is in data communication with a High-Frequency Chest Wall Oscillation
("HFCWO") vest. It has recently been observed that during vest oscillation
therapy, the
Minute Ventilation of a patient is reduced by up to 50%. The improvement in
efficiency
may provide significant health benefits for a patient who is having difficulty
providing
oxygenation of their bloodstream during breathing. In a preferred embodiment,
the
HFCWO vest automatically provides therapy levels (frequency, intensity,
length) which
have been developed to optimize the 02 to CO2 transfer in the lungs. The goal
is to
optimize the oxygen and CO2 transfer by the use of the HFCWO vest. By
increasing the
turbulence in the lungs during inhalation and exhalation better oxygen and CO2
transfer can
be achieved. Preferably, a decrease in work of breathing decreases the chance
of respiratory
failure. In addition, patients who are receiving oxygen therapy could combine
the oxygen
therapy with the HFCWO vest therapy to maximize oxygenation, improve CO2
removal and
decrease work of breathing, thereby preferably extending life.
Typically, HFCWO vest therapy provides for a 10 min treatment to eliminate
exudate. The use of this product preferably allows for better oxygenation. The
use of the
product could be continuously up to 24hrs/day. The system could be customized
to activate
when the patient requires the additional oxygenation efficiency, e.g. during
active times
such as walking. As opposed to exudate removal the parameters of oscillation
could be
optimized to minimize patient discomfort while maximizing oxygen transfer in
the lungs.
As shown in figure 40, a sensor for acquiring a physiological bioelectrical
impedance signal from the patient is preferably functionally connected to a
computing
device. The computing device preferably analyzes the physiological
bioelectrical
impedance signal, and provides an assessment of minute ventilation and tidal
volume of the
patient based on the analyzed bioelectrical impedance signal. The computing
device also
preferably monitors the signal over time and provides a signal to the HFCWO
vest.
CA 03042686 2019-05-02
WO 2018/085563 PCT/US2017/059754
Preferably, the HFCWO vest automatically adjusts therapy levels (frequency,
intensity, length) based on the levels of physiologic parameters including
tidal volume,
minute ventilation, and respiratory rate during therapy as determined by the
computing
device. In addition, the general session-to-session lung performance can be
tracked (TV,
RR, MV) to demonstrate effectiveness of the therapy and the need to extend or
modify the
therapy levels. The goal is to optimize the oxygen and CO2 transfer by the use
of the
HFCWO vest to increase the turbulence in the lungs during inhalation and
exhalation.
In addition, the shape of the bioimpedance exhalation/inhalation curve can be
an
indicator of the success of the therapy. Appropriate curves for maximizing
oxygen transfer
can be identified and the levels of the HFCWO vest (frequency, intensity,
length of therapy,
Baseline compression) can be adjusted to get the desired respiratory curve and
necessary
oxygenation and/or CO2 extraction and to minimize the work of breathing.
Additionally, a pulse oximeter can be added to the system as an indicator of
the
success of the enhanced compression therapy and improved oxygenation. The
levels of
therapy can be optimized by watching the oxygenation response over time. CO2
monitoring
can be added to the system with either end tidal or transcutaneous CO2
monitoring.
In addition, patients who are receiving oxygen therapy could combine the
oxygen therapy
with the HFCWO vest therapy to preferably maximize oxygenation, improve CO2
removal,
decrease work of breathing, and extend life.
Figure 41 depicts an embodiment of the invention wherein the impedance
measuring
device is in data communication with a mechanical ventilation therapy device.
The
mechanical ventilation therapy device may be a CHFO system, a ventilator, a
CPAP, a
BiPAP, a CPEP (Continuous Positive Expiratory Pressure), or another non-
invasive
ventilation device. Preferably, the system includes a sensor for acquiring a
physiological
bioelectrical impedance signal from a patient and is functionally connected to
a computing
device. The computing device preferably analyzes the physiological
bioelectrical
impedance signal and outputs an assessment of minute ventilation and tidal
volume of the
patient based on the analyzed bioelectrical impedance signal. The system may
also monitor
the signal over time and provide a signal to the mechanical ventilation
device. The
mechanical ventilation device preferably causes better oxygenation efficiency
in the lungs.
76
CA 03042686 2019-05-02
WO 2018/085563
PCT/US2017/059754
The mechanical ventilation device preferably can adjust the frequency,
intensity, of the
oscillations and/or the base line inhalation and exhalation pressures.
A bioelectric feedback signal provides indication for the success of
oxygenation.
The characteristic values for tidal volume, minute volume, and respiratory
rate will change.
By monitoring the change, the system can automatically adjust the mechanical
ventilation
device's parameters to optimize physiological response and the efficiency of
the system.
Additionally a pulse oximeter can be added to the system as an indicator of
the success of
the mechanical ventilation therapy. Improved oxygenation and CO2 transfer can
preferably
be achieved or a decrease in work of breathing can preferably be achieved to
decrease the
chance of respiratory failure. The levels of therapy can be further optimized
by watching
the oxygenation response over time. In addition, the overall length of therapy
can be
adjusted. The general session-to-session lung performance can be tracked (TV,
RR, MV) to
demonstrate effectiveness of the ventilation and the need to extend or modify
the therapy
levels.
In addition, the characteristic shape of the bioimpedance inhalation and
exhalation
curve is an indicator of the success of the therapy. By tailoring the therapy
to get the desired
expulsion curve, the system can optimize oxygenation efficiency. Appropriate
curves for
maximizing ventilation can be determined and the adjustment levels of the
Ventilator
(frequency, intensity, length of therapy, Baseline Pressure) can be adjusted
to get the desired
respiratory curve. In addition, patients who are receiving oxygen therapy
could combine
oxygen therapy with mechanical ventilation therapy to maximize oxygenation and
extend
life. Additionally, the level of compliance to using the system and getting
the adequate
therapy can be monitored by analyzing the volume of air coming in and out of
the lungs.
By using the Tidal Volume, MV, and RR the relative success of opening up the
airways can be determined.
Mechanical ventilation therapy can be combined with aerosol delivery to
provide an
additional therapy regimen. As the aspiration of aerosol will inherently
modify the
impedance characteristic of the lung, the level of respiration and the effect
of these two
combined treatments can also be optimized. For example during the treatment
the Tidal
Volume and the characteristic inhalation and expulsion curves can be monitored
before,
77
CA 03042686 2019-05-02
WO 2018/085563 PCT/US2017/059754
during, and after treatment to ensure appropriate optimization of the positive
expiratory
presssure on expansion of the lung and airways or an adequately cleared lung.
Figure 42 depicts an embodiment of the invention wherein the impedance
measuring
device is in data communication with an oxygenation therapy device. The system
preferably includes a sensor for acquiring a physiological bioelectrical
impedance signal
from a patient and is functionally connected to a computing device. The
computing device
preferably analyzes the physiological bioelectrical impedance signal and
provides outputs an
assessment of minute ventilation and tidal volume of the patient based on the
analyzed
bioelectrical impedance signal. The computing device additionally preferably
monitors the
signal over time and provides a signal to an oxygen therapy system.
Preferably, the oxygen
therapy provides oxygen via a mask or nose cannula. The bioelectric feedback
signal
provides indication for the success of the level of the expansion of the
airways. The
characteristic shape of the bioimpedance expansion curve is an indicator that
the air is
getting into the lungs.
By combining the pressure monitoring of the inhalation and exhalation with the
impedance signal, the oxygenation therapy system can synchronize the delivery
of oxygen
to the cannula to ensure optimal oxygen uptake through the nose cannula.
For oxygen therapy using a mask, the feedback mechanism of the oxygen delivery
can be optimized as well. In addition, by using both the impedance signal as
well as the
mask pressure, the oxygen system can more reliably determine how well the mask
is applied
to the patient and how well the circuit is maintained (kink free and leak
free).
Figure 43 depicts an embodiment of the invention wherein the impedance
measuring
device is in data communication with a suction therapy device. The system
preferably
includes a sensor for acquiring a physiological bioelectrical impedance signal
from a patient
and is functionally connected to a computing device. The computing device
preferably
analyzes the physiological bioelectrical impedance signal and provides an
output of an
assessment of minute ventilation and tidal volume of the patient based on the
analyzed
bioelectrical impedance signal. The computing device preferably also monitors
the signal
over time and provides a signal to the suction therapy device.
Suction therapy preferably causes the mobilization of fluid in the lungs. The
suction
therapy can be adjusted for frequency and intensity of the oscillations. Also,
the base line
78
CA 03042686 2019-05-02
WO 2018/085563 PCT/US2017/059754
inhalation and exhalation pressures can be adjusted and the overall length of
therapy can be
adjusted.
The bioelectric feedback signal preferably provides an indication for the
success of
the mobilization of secretions. As the suction draws fluid, the characteristic
values for tidal
volume, minute volume, and respiratory rate will change. By monitoring the
change, the
system can preferably automatically adjust the suction parameters to optimize
physiological
response.
In addition the characteristic shape of the bioimpedance expulsion curve is an
indicator of the success of the therapy. By tailoring the therapy to get the
desired expulsion
curve the system can optimize the mobilization of fluid from the patient.
Fluid clearance can be combined with aerosol delivery to provide another
therapy
regimen. As the aspiration of aerosol will inherently modify the impedance
characteristic
of the lung, the level of respiration and the effect of these two combined
treatments can also
be optimized. For example during the treatment the tidal volume and the
characteristic
inhalation and expulsion curves can be monitored before, during, and after
treatment to
ensure appropriate outcome of an adequately cleared lung.
Figure 44 depicts an embodiment of the invention wherein the impedance
measuring
device is in data communication with a cough assist device. The system
preferably includes
a sensor for acquiring a physiological bioelectrical impedance signal from a
patient and is
functionally connected to a computing device. The computing device preferably
analyzes
the physiological bioelectrical impedance signal and provides an output of an
assessment of
minute ventilation and tidal volume of the patient based on the analyzed
bioelectrical
impedance signal. The computing device preferably also monitors the signal
over time and
provides a signal to the cough assist device.
The cough assist device is preferably a non-invasive therapy that stimulates a
cough
to remove secretions in patients with compromised peak cough flow. It is
designed to keep
lungs clear of mucus. Retained secretions collect in the lungs, creating an
environment for
infection. Mechanical Insufflation/Ex-sulflation (MI/E) therapy products are
important for
patients who have weakened cough and are unable to remove secretions from the
large
airways without assistance. The system supplies positive pressure (inhale) to
inflate the
lungs, then quickly shifts to supply negative pressure (exhale), during this
process secretions
79
CA 03042686 2019-05-02
WO 2018/085563 PCT/US2017/059754
are sheared and can be expectorated or removed with suction. After the exhale,
the system
pauses and maintains a resting positive pressure flow to the patient. A
facemask or
mouthpiece can be used on endotracheal and tracheostomy (i.e. for patients
with an
appropriate adapter).
Preferably, the cough assist device automatically adjusts characteristic
therapy levels
(frequency, intensity, length of therapy, inhalation pressure, exhalation
pressure) based on
the levels of tidal volume, minute ventilation, and respiratory rate during
therapy. In
addition, the general within session and the session-to-session lung
performance can be
tracked to demonstrate effectiveness of the therapy (before, during, and after
and across
many sessions). Graphs could be provided to document breathing characteristics
of the
patient and to demonstrate improvement to the patient over time.
In addition, the characteristic shape of the bioimpedance expansion curve is
an
indicator of the success of each individual cough. Appropriate curves for
maximizing
exudate removal can be identified and the adjustment levels of the Cough
assist System
(frequency, intensity, length of therapy, inhalation pressure, and exhalation
pressure) can be
adjusted to get the desired cough expulsion curve. Characteristics of the
cough assist can be
adjusted to ensure the optimal results are provided for each individual
patient.
Other embodiments and technical advantages of the invention are set forth
below
and may be apparent from the drawings and the description of the invention
which follow,
.. or may be learned from the practice of the invention.
Other embodiments and uses of the invention will be apparent to those skilled
in the
art from consideration of the specification and practice of the invention
disclosed herein. All
references cited herein, including all publications, U.S. and foreign patents
and patent
applications, are specifically and entirely incorporated by reference. The
term comprising,
where ever used, is intended to include the terms consisting and consisting
essentially of.
Furthermore, the terms comprising, including, and containing are not intended
to be
limiting. It is intended that the specification and examples be considered
exemplary only
with the true scope and spirit of the invention indicated by the following
claims.