Note: Descriptions are shown in the official language in which they were submitted.
TRANSCATHETER MITRAL VALVE PROSTHESIS
This application is divided from Canadian Patent Application Serial No.
2,797,863
filed on May 4, 2011.
BACKGROUND OF THE INVENTION
[0001] 1. Field of the Invention. The present invention generally
relates to medical
devices and methods, and more particularly relates to the treatment of valve
insufficiency,
such as mitral insufficiency, also referred to as mitral regurgitation. The
use of prosthetic
valves delivered by traditional surgical implantation methods, or by less
invasive
percutaneous catheter or minimally invasive transapical methods are one
possible
treatment for valvar insufficiency.
[0002] The heart of vertebrate animals is divided into four chambers, and is
equipped with
four valves (the mitral, aortic, pulmonary and tricuspid valves) that ensure
that blood
pumped by the heart flows in a forward direction through the cardiovascular
system. The
mitral valve of a healthy heart prevents the backflow of blood from the left
ventricle into the
left atrium of the heart, and comprises two flexible leaflets (anterior and
posterior) that
close when the left ventricle contracts. The leaflets are attached to a
fibrous annulus, and
their free edges are tethered by subvalvular chordae tendineae to papillary
muscles in the
left ventricle to prevent them from prolapsing into the left atrium during the
contraction of
the left ventricle.
[0003] Various cardiac diseases or degenerative changes may cause dysfunction
in any of
these portions of the mitral valve apparatus, causing the mitral valve to
become abnormally
narrowed or dilated, or to allow blood to leak (i.e. regurgitate) from the
left ventricle back
into the left atrium. Any such impairments compromise cardiac sufficiency, and
can be
debilitating or life threatening.
[0004] Numerous surgical methods and devices have accordingly been developed
to treat
mitral valve dysfunction, including open-heart surgical techniques for
replacing, repairing
or re-shaping the native mitral valve apparatus, and the surgical implantation
of various
prosthetic devices such as annuloplasty rings to modify the anatomy of the
native mitral
valve. More recently, less invasive transcatheter techniques for the 30
delivery of
replacement mitral valve assemblies have been developed. In such techniques, a
prosthetic valve is generally mounted in a crimped state on the end of a
flexible catheter
1
CA 3043737 2019-05-16
and advanced through a blood vessel or the body of the patient until the valve
reaches the
implantation site. The prosthetic valve is then expanded to its functional
size at the site of
the defective native valve.
[0005] While these devices and methods are promising treatments for valvar
insufficiency, they can be difficult to deliver, expensive to manufacture, or
may not be
indicated for all patients. Therefore, it would be desirable to provide
improved devices and
methods for the treatment of valvar insufficiency such as mitral
insufficiency. At least
some of these objectives will be met by the devices and methods disclosed
below.
[0006] 2. Description of the Background Art. By way of example, PCT
international
patent number PCT/US2008/054410 (published as PCT international publication
no.
W02008/103722), describes a transcatheter mitrel valve prosthesis that
comprises a
resilient ring, a plurality of leaflet membranes mounted with respect to the
ring so as to
permit blood flow therethrough in one direction, and a plurality of tissue-
engaging
positioning elements movably mounted with respect to the ring and dimensioned
to grip the
anatomical structure of the heart valve annulus, heart valve leaflets, and/or
heart wall.
Each of the positioning elements defines respective proximal, intermediate,
and distal
tissue engaging regions cooperatively configured and dimensioned to
simultaneously
engage separate corresponding areas of the tissue of an anatomical structure,
and may
include respective first, second, and third elongate tissue-piercing elements,
The valve
prosthesis may also include a skirt mounted with respect to the resilient ring
for sealing a
periphery of the valve prosthesis against a reverse flow of blood around the
valve
prosthesis.
[0007] PCT international patent number PCT/US2009/041754 (published as PCT
international publication no. W02009/134701), describes a prosthetic mitre!
valve
assembly that comprises an anchor or outer support frame with a flared upper
end and a
tapered portion to fit the contours of the native mitrel valve, and a tissue-
based one-way
valve mounted therein. The assembly is adapted to expand radially outwardly
and into
contact with the native heart tissue to create a pressure fit, and further
includes tension
members anchoring the leaflets of the valve assembly to a suitable location on
the heart to
function as prosthetic chordae tendineae.
[0008] Also known in the prior art are prosthetic mitral valve assemblies that
utilize a claw
structure for attachment of the prosthesis to the heart (see, for example,
U.S. patent
2
CA 3043737 2019-05-16
application publication no. US2007/0016286 to Hermann et al.), as are
prosthetic mitral
valve assemblies that rely on the application of axial rather than radial
clamping forces to
facilitate the self-positioning and self-anchoring of the prosthesis with
respect to the native
anatomical structure.
[0009] Another method which has been proposed as a treatment of mitral valve
regurgitation is the surgical bow tie method, which recently has been adapted
into a
minimally invasive catheter based treatment where an implant is used to clip
the valve
leaflets together. This procedure is more fully disclosed in the scientific
and patent literature,
such as in U.S. Patent No. 6,629,534 to St. Goar et al.
[0010] Other relevant publications include U.S. Patent Publication No.
2011/0015731 to
Carpentier et al.
BRIEF SUMMARY OF THE INVENTION
[0011] The present invention generally relates to medical devices and more
particularly
prosthetic valves used to treat mitral regurgitation. While the present
disclosure focuses on
the use of a prosthetic valve for treating mitral regurgitation, this is not
intended to be
limiting. The prosthetic valves disclosed herein may also be used to treat
other body valves
including other heart valves or venous valves. Exemplary heart valves include
the aortic
valve, the triscupsid valve, or the pulmonary valve.
[0011a] Accordingly, there is provided a delivery system for delivering a
prosthetic cardiac
valve to a patient's heart, said system comprising: an inner guidewire shaft
having a
lumen extending therethrough, the lumen adapted to slidably receive a
guidewire, wherein
the guidewire shaft is stationary; a hub shaft concentrically disposed over
the inner
guidewire shaft, wherein the hub shaft is stationary; a bell shaft slidably
and concentrically
disposed over the hub shaft, wherein the bell shaft is configured to be
advanced or
retracted relative to the hub shaft; a sheath slidably and concentrically
disposed over the
bell shaft, wherein the sheath is configured to be advanced or retracted
relative to the bell
shaft; and a handle near a proximal end of the delivery system, the handle
comprising an
actuator mechanism adapted to advance and retract the bell shaft and the
sheath.
[0012] In embodiments of the present subject matter, transcatheter mitral
valve
prostheses are provided. In certain embodiments, the mitral valve prosthesis
comprises a
tissue-type prosthetic one-way valve structure comprising a plurality of
leaflets affixed
within a self-expanding or expandable anchor (i.e. frame) portion having a
geometry that
expands into a low profile atrial skirt region, an annular region dimensioned
to
3
Date Recue/Date Received 2020-09-08
generally conform to a native mitrel valve annulus, a ventricular skirt region
that
displaces the native mitral valve leaflets, and a plurality of leaflet
commissures
extending into the sub-annular ventricular space (i.e. in the direction of the
outflow of
blood through the prosthesis) and configured to optimize the efficiency of the
prosthetic valve structure and the load distribution on the leaflets thereof.
The anchor
portion may also in preferred embodiments be asymmetrical along its
longitudinal axis,
with the atrial skirt region, the annular region and/or the ventricular skirt
region having
differently configured anterior and posterior aspects in order to facilitate
close
accommodation of the asymmetrical contours and features of a typical native
mitral
valve apparatus. This
asymmetry may result inherently from the structural
configuration of the anchor portion as discussed further below, and/or as a
consequence of shaping or forming steps employed during the manufacturing
process.
[0013] The prosthetic valve structure in preferred embodiments may comprise a
bicuspid or tricuspid valve in order, in part, to simplify manufacture of the
mitral valve
prosthesis, but as would be readily apparent to those of skill in the art,
other
configurations are possible. The leaflets may be fabricated from a single
piece or from
multiple pieces of standard biologic prosthetic materials, such as cryo- or
chemically-
preserved pericardium (e.g. bovine, equine, porcine, caprine, kangaroo), or
from
standard suitable synthetic prosthetic materials (e.g. fiber-reinforced matrix
materials)
well known in the art, and may be sewn or otherwise adhered to the anchor to
form
the valve leaflets in any standard suitable manner.
[0014] To optimize prosthetic valve efficiency and the load distribution on
the
prosthetic leaflets, the commissures extend generally axially in a
cantilevered fashion
downstream into the sub-annular space, and are capable of flexing radially and
laterally along their axial lengths to distribute the forces associated with
blood flow
through the prosthetic valve structure. In some embodiments, the commissures
define
(when the mitral valve prosthesis is in an expended state) a somewhat
frustoconical
aperture that narrows along the forward direction of blood flow in order to
aid in the
closure of the prosthetic valve structure during contraction of the ventricle.
To further
optimize efficiency and load distribution on the leaflets, the commissures may
be
shaped and dimensioned so as to provide for the attachment of the leaflets
along
4
CA 3043737 2019-05-16
arcuate seams, and may also be made selectively flexible at different points
or zones
along their axial length through, for example, the addition or deletion of
reinforcing
struts, or through variation of the thickness of the commissures in selected
regions.
[0016] The anchor portion of the mitral valve prosthesis is preferably
fabricated from
a single piece of metallic material that has been cut so as to permit the
mitral valve
prosthesis to be compressed into a compact, generally tubular delivery
configuration,
and expanded into the deployment configuration further described herein. In
self-
expanding embodiments, the anchor portion of the mitral valve prosthesis may
be
fabricated from a shape memory alloy (SMA) such as the nickel-titanium alloy
nitinol,
and in expandable embodiments, the anchor portion may be fabricated from any
metallic material, such as chromium alloy or stainless steel, that is suitable
for
implantation into the body. In some embodiments, the metallic material may be
of a
single thickness throughout entirety of the anchor portion, and in others may
vary in
thickness so as to facilitate variations in the radial force that is exerted
by the anchor
portion in specific regions thereof, to increase or decrease the flexibility
of the anchor
portion in certain regions, and/or to control the process of compression in
preparation
for deployment and the process of expansion during deployment.
[0016] When deployed, the atrial skirt region of the mitral valve prosthesis
extends
generally radially outwards so as to lie flat against and cover the atrial
surface of the
native mitral valve annulus, and to anchor the mitral valve prosthesis against
at least a
portion of the adjoining atrial surface of the heart. The atrial skirt region
has a low
axial profile (extending only slightly into the atrium of the heart) in order
to minimize
potentially thrombogenic turbulence in blood flow, and in preferred
embodiments, may
be covered with standard biologic or synthetic prosthetic materials of the
sort
described above in order to seal the atrial skirt region against the atrial
surface and to
facilitate the funnelling of atrial blood through the mitral valve prosthesis.
In some
embodiments, the atrial skirt region further comprises atrial barbs or prongs
to further
facilitate the anchoring of the deployed prosthesis to the atrial heart
surface. To
facilitate the orientation and alignment of the mitrel valve prosthesis within
the native
mitral valve upon deployment, particularly in embodiments where the anchor
portion is
longitudinally asymmetrical, the atrial skirt region of the anchor portion of
the mitral
5
CA 3043737 2019-05-16
valve prosthesis may preferably further comprise an alignment structure that
may be
differentiated (such as by angiography, computed tomography, etc.) from the
remainder of the atrial skirt region and thereby used as an orientation guide
during
deployment. Most preferably, the alignment structure may comprise an
elongation of
the anterior aspect of the atrial skirt region configured to expand radially
to
accommodate the aortic root portion of the atrial surface.
[0017] The annular region of the mitral valve prosthesis is dimensioned, as
noted
above, to generally conform to and anchor against a native mitral valve
annulus when
deployed. In preferred embodiments, the deployed annular region may define a
generally D-shaped annulus suitable for fitting the contours of a typical
native mitral
valve, and may be covered with standard biologic or synthetic prosthetic
materials of
the sort previously described to seal the annular region against the native
mitral valve
annulus.
[0018] The ventricular skirt region expands when deployed in the ventricular
space
generally radially outwards against the native mitral valve, but not so far as
to obstruct
the left ventricular outflow tract, nor to contact the ventricular wall. To
anchor the
mitral valve prosthesis against the displaced native leaflets in the
ventricular space,
the maximal radial displacement of the fully deployed ventricular skirt region
is
selected to be slightly greater than the circumference of the native mitral
valve. In
preferred embodiments, the ventricular skirt region also comprises ventricular
and/or
native leaflet barbs or prongs to further anchor the deployed prosthesis
thereto. Most
preferably, the ventricular skirt region is asymmetrical and the prongs
thereof comprise
two trigonal anchoring tabs located in the anterior aspect of the ventricular
skirt region
for anchoring against the fibrous trigones on either side of the anterior
leaflet of the
native mitral valve, and one posterior ventricular anchoring tab located in
the posterior
aspect of the ventricular skirt region for anchoring over the posterior
leaflet of the
native mitral valve. Associated with these tabs are deployment control regions
as
described in further detail below.
[0019] The ventricular skirt region may also in some embodiments be covered
with
standard biologic or synthetic prosthetic materials of the sort previously
described in
order to seal the ventricular skirt region against the displaced native
leaflets, and
6
CA 3043737 2019-05-16
thereby to funnel ventricular blood (during contraction of the ventricle)
towards the
prosthetic valve structure to assist in the closure thereof during contraction
of the
ventricle.
[0020] The combined 3-zone anchoring of the mitral valve prosthesis against
the
atrial surface, the native valve annulus, and the displaced native leaflets
(supplemented, in preferred embodiments by a fourth zone of anchoring from the
trigonal and posterior ventricular anchoring) in the ventricular space
prevents the
prosthesis from migrating or dislodging from within the native valve annulus
during the
contraction of the atrium or the ventricle, and lessens the anchoring pressure
that is
required to be applied in any given anchoring zone as compared to a prosthesis
that is
anchored in only a single anchoring zone, or in any combination of these four
anchoring zones. The consequent reduction in radial force required to be
exerted
against the native structures in each zone minimizes the risk of obstruction
or
impingement of the nearby aortic valve or aortic root caused by the
displacement of
the native mitral valve apparatus. The combined 3 or 4-zone anchoring of the
mitral
valve prosthesis also facilitates the positioning and/or re-positioning of the
mitral valve
prosthesis as described below.
(00211 To deploy the mitral valve prosthesis within the native mitral valve
apparatus,
the prosthesis is first compacted and loaded into a suitably-adapted
conventional
catheter delivery system of the sort well known to those of skill in the art.
Preferably,
to facilitate later deployment, the commissures and associated prosthetic
valve
structure of the prosthesis are captured within an inner lumen of the catheter
delivery
system, and the remaining portions of the anchor region are captured within a
secondary outer lumen of the catheter delivery system. The loaded mitral valve
prosthesis may then be delivered (typically either transseptally or
transapically) in its
compacted form into the left atrium of the heart using a conventional catheter
delivery
system. The prosthesis is releasably attached to the catheter delivery system
via its
commissures, and shielded by the (preferably dual-lumen) delivery sheath
thereof
during transit into the atrial space. Once the prosthesis has been guided into
the left
atrium, the delivery sheath of the catheter delivery system is retracted as
described
below in order to permit expansion of the various regions of the prosthesis to
proceed.
7
CA 3043737 2019-05-16
Of course, in self-expanding embodiments, expansion of the prosthesis will
occur
spontaneously upon retraction of the delivery sheath, and in expandable
embodiments, a catheter inflation structure such as a balloon is required to
effect the
expansion.
[0022] Deployment of the mitral valve prosthesis may proceed differently
depending
upon the features of the particular embodiment of the prosthesis being
deployed. For
example, in asymmetrical embodiments that comprise trigonal anchoring tabs and
a
posterior ventricular anchoring tab in the ventricular skirt region (as well
as, preferably,
an alignment structure in the atrial region), these tabs may preferably be
deployed
before deployment of the remaining portions of the ventricular skirt regions
in order to
facilitate the anchoring of these tabs against the native fibrous trigones and
posterior
leaflet, respectively.
[0023] In the first general deployment step, the atrial skirt region of the
mitral valve
prosthesis is permitted to expand by retracting the corresponding portion of
the
catheter delivery sheath (or is balloon-expanded following the retraction of
the
corresponding portion of the delivery sheath) within the left atrium of the
heart, and the
expanded atrial skirt region is then positioned over the atrial surface of the
native
mitral valve and anchored against at least a portion of the adjoining atrial
surface of
the heart. In preferred embodiments where the atrial skirt region comprises an
alignment structure, this first general deployment step may be further broken
down into
two sub-steps, wherein the catheter delivery sheath is first retracted only so
far as to
permit expansion of the alignment structure (so that it may be visualized to
facilitate
manipulation of the delivery system in such a way as to orient the mitral
valve
prosthesis into a desired position), and then, once initial alignment of the
prosthesis
appears to be satisfactory, further retracted to permit the expansion,
positioning and
anchoring of the remaining portions of the atrial skirt region. In embodiments
where
the alignment structure comprises an elongation of the anterior aspect of the
atrial skirt
region, such initial alignment comprises the rotation and/or alignment of the
alignment
structure so that it is situated adjacent the aortic root and between the
fibrous trigones
of the native anterior leaflet.
8
CA 3043737 2019-05-16
[0024] Next, the annular region of the prosthesis is permitted to expand by
further
retraction of the catheter delivery sheath so as to engage the native mitral
valve
annulus (i.e. to contact the native valve annulus throughout at least a
majority thereof)
in order to create a second anchoring zone and to create a suitable opening
for blood
flow through the prosthetic valve structure.
[0025] Then, in embodiments that comprise trigonal anchoring tabs and a
posterior
ventricular anchoring tab in the ventricular skirt region, the catheter
delivery sheath is
further retracted so far as to permit the tabs to expand while the remainder
of the
ventricular skirt region of the prosthesis, including the deployment control
regions of
the tabs, remain sheathed. With the deployment control regions still retained
within
the delivery system and the atrial skirt region anchored against the atrial
surface, the
tabs project radially outward to facilitate engagement with the corresponding
features
of the native mitral valve. The posterior ventricular anchoring tab is aligned
in the
middle of the posterior leaflet of the mitral valve where there is an absence
of chordae
attachments to the posterior leaflet, and passed over the posterior leaflet to
seat
between the posterior leaflet and the ventricular wall. The two trigonal
anchoring tabs
are positioned on either side of the anterior leaflet with their heads
positioned at the
fibrous trigones. Slight rotation and realignment of the prosthesis can occur
at this
time.
[0026] Once the assembly has been satisfactorily positioned and the tabs
aligned,
the catheter delivery sheath may be further retracted to permit expansion of
the
remaining portions of the ventricular skirt region to secure the prosthesis
within the
mitral apparatus and seal the mitral annulus. Complete retraction of the outer
catheter
delivery sheath releases the ventricular skirt region and allows the anchoring
tabs to
proximate their anchoring location. As the prosthesis expands, the trigonal
tabs
anchor against the fibrous trigones, capturing the native anterior leaflet and
chordae
between the tabs and the anterior surface of the prosthetic valve assembly,
and the
posterior ventricular tab anchors between the ventricular wall and the
posterior leaflet,
capturing the posterior leaflet between the posterior anchoring tab and the
posterior
surface of the prosthetic valve assembly. The remaining portions of the
ventricular
skirt region expand out against the native mitral valve leaflets and adjacent
anatomy,
9
CA 3043737 2019-05-16
thereby creating a sealing funnel within the native leaflets and displacing
the native
leaflets from the prosthetic commissures to avoid obstruction of the
prosthetic valve
function. With the commissures of the prosthesis still captured within the
delivery
system, very minor adjustments may still made to ensure accurate positioning,
anchoring and sealing.
[0027] In embodiments that do not comprise trigonal anchoring tabs and a
posterior
ventricular anchoring tab in the ventricular skirt region, the retraction of
the catheter
delivery sheath from the ventricular skirt region may, of course, be performed
in one
step after the atrial skirt and annular regions of the prosthesis have been
initially
anchored, to permit the ventricular skirt region of the prosthesis to expand
against the
native mitral valve, and to additionally anchor the prosthesis against the
displaced
native leaflets in the ventricular space. Optionally, the mitral valve
prosthesis, which is
still at this point releasably attached to the catheter delivery system via
its
commissures, may be driven slightly further downstream into ventricular space
to
create a greater seating force as between the atrial skirt region and atrial
surface of
the heart, and to provide additional purchase for any ventricular and/or
native leaflet
barbs or prongs that may be present in the ventricular skirt region. In
embodiments
where one or more of the atrial skirt region, the annular region and the
ventricular skirt
region are covered with a suitable biologic or synthetic prosthetic material,
a seal may
also be formed between the respective regions of the prosthesis and the
associated
zone of the native mitral valve apparatus.
[0028] Finally, once satisfactory positioning of the prosthesis has been
achieved, the
commissures are released from the catheter delivery system, allowing the
catheter
delivery system to be withdrawn, and leaving the mitral valve prosthesis in
place as a
functional replacement for the native mitral valve apparatus. Upon release of
the
commissures, the prosthesis may further undergo a final stage of
foreshortening and
seating as any remaining pressure exerted by the delivery system is relased.
The
atrial skirt region may recoil slightly from this release in pressure, pulling
the prosthesis
slightly further up in to the left atrium, and thereby further seating the
ventricular skirt
region, including any associated barbs, prongs or tabs. In embodiments that
comprise
trigonal anchoring tabs, the seating thereof pulls the captured anterior
leaflet tightly
CA 3043737 2019-05-16
against the prosthesis, thereby avoiding or minimizing obstruction of the Left
Ventricular Outflow Tract (LVOT), and firmly seats the ventricular skirt
region in the
annulus to prevent paravalvular leakage. Once final deployment is complete,
the
delivery system is retracted and removed.
[0029] In a first aspect of the present invention, a method of anchoring a
prosthetic
valve in a patient's heart comprises providing the prosthetic valve, wherein
the
prosthetic valve comprises an anchor having an atrial skirt, an annular
region, a
ventricular skirt, and a plurality of valve leaflets, wherein the anchor has a
collapsed
configuration for delivery to the heart and an expanded configuration for
anchoring
.. with the heart, and positioning the prosthetic valve in the patient's
heart. The method
also comprises expanding the atrial skirt radially outward so as to lie over a
superior
surface of the patient's native mitral valve, anchoring the atrial skirt
against a portion of
the atrium, and radially expanding the annular region of the anchor to conform
with
and to engage the native mitral valve annulus. The method also comprises
radially
expanding the ventricular skirt thereby displacing the native mitre' valve
leaflets
radially outward.
[0030] At least a portion of the prosthetic valve may be covered with tissue
or a
synthetic material. Positioning the prosthetic valve may comprise
transseptally
delivering the prosthetic valve from the right atrium to the left atrium of
the heart, or
transapically delivering the prosthetic valve from a region outside the heart
to the left
ventricle of the heart.
[0031] Expanding the atrial skirt may comprise slidably moving a restraining
sheath
away from the atrial skirt thereby allowing radial expansion thereof. The
atrial skirt
may self-expand when the restraining sheath is removed therefrom. The method
may
further comprise applying a force on the prosthetic valve to ensure that the
atrial skirt
engages the superior surface of the mitral valve. The atrial skirt may
comprise a
plurality of barbs, and expanding the atrial skirt may comprise anchoring the
barbs into
the superior surface of the mitral valve. Expanding the atrial skirt may
comprise
sealing the atrial skirt against the superior surface of the native mitral
valve.
[0032] Radially expanding the annular region may comprise slidably moving a
restraining sheath away from the annular region thereby allowing radial
expansion
11
CA 3043737 2019-05-16
thereof. The annular region may self-expand when the restraining sheath is
removed
therefrom. Radially expanding the annular region may comprise asymmetrically
expanding the annular region such that an anterior portion of the annular
region is
substantially flat, and a posterior portion of the annular region is
cylindrically shaped.
[0033] The ventricular skirt may further comprise a trigonal anchoring tab on
an
anterior portion of the ventricular skirt, and radially expanding the
ventricular skirt may
comprise anchoring the trigonal anchoring tab against a first fibrous trigon
on a first
side of the anterior leaflet of the native mitral valve. The native anterior
leaflet and
adjacent chordae tendineae may be captured between the trigonal anchoring tab
and
an anterior surface of the anchor. The ventricular skirt may further comprise
a second
trigonal anchoring tab on the anterior portion of the ventricular skirt, and
wherein
radially expanding the ventricular skirt may comprise anchoring the second
trigonal
anchoring tab against a second fibrous trigon opposite the first fibrous
trigon. The
native anterior leaflet and adjacent chordae tendineae may be captured between
the
second trigonal anchoring tab and an anterior surface of the anchor. The
ventricular
skirt may further comprise a posterior ventricular anchoring tab on a
posterior portion
of the ventricular skirt. Radially expanding the ventricular skirt may
comprise
anchoring the posterior ventricular anchoring tab over a posterior leaflet of
the native
mitral valve to seat between the posterior leaflet and a ventricular wall of
the heart.
Radially expanding the ventricular skirt may comprise slidably moving a
restraining
sheath away from the ventricular skirt thereby allowing radial expansion
thereof. The
ventricular skirt may self-expand when the restraining sheath is removed
therefrom.
[0034] The ventricular skirt may comprise a plurality of barbs, and expanding
the
ventricular skirt may comprise anchoring the barbs into heart tissue. The
prosthetic
valve may comprise a plurality of prosthetic valve leaflets, and radially
expanding the
ventricular skirt may comprise displacing the native mitral valve leaflets
radially
outward thereby preventing interference of the native mitral valve leaflets
with the
prosthetic valve leaflets. Radially expanding the ventricular skirt may
comprise
displacing the native mitral valve leaflets radially outward without
contacting a
ventricular wall, and without obstructing a left ventricular outflow tract.
Radially
expanding the ventricular skirt may comprise asymmetrically expanding the
ventricular
12
CA 3043737 2019-05-16
skirt such that an anterior portion of the ventricular skirt is substantially
flat, and a
posterior portion of the ventricular skirt is cylindrically shaped.
[0035] The atrial skirt may comprise an alignment element, and the method may
comprise aligning the alignment element relative to the patient's valve. The
valve may
comprise a mitral valve, and aligning may comprise aligning the alignment
element
with an aortic root and disposing the alignment between two fibrous trigones
of an
anterior leaflet of the mitral valve. Aligning may comprise rotating the
prosthetic valve.
The prosthetic valve may comprise a plurality of prosthetic leaflets coupled
to one or
more commissures, and the method may comprise releasing the commissures from a
delivery catheter. The prosthetic valve may comprise a tricuspid leaflet
configuration.
[0036] The prosthetic valve may have an open configuration in which the
prosthetic
valve leaflets are disposed away from one another, and a closed configuration
in
which the prosthetic valve leaflets engage one another. Blood flows freely
through the
prosthetic valve in the open configuration, and retrograde blood flow across
the
prosthetic valve is substantially prevented in the closed configuration. The
method
may comprise reducing or eliminating mitral regurgitation. The prosthetic
valve may
comprise a therapeutic agent, and the method may comprise eluting the
therapeutic
agent from the prosthetic valve into adjacent tissue.
[0037] In another aspect of the present invention, a prosthetic cardiac valve
comprises an anchor having an atrial skirt, an annular region, and a
ventricular skirt.
The anchor has a collapsed configuration for delivery to the heart and an
expanded
configuration for anchoring the prosthetic cardiac valve to a patient's heart.
The
prosthetic valve also comprises a plurality of prosthetic valve leaflets, each
of the
leaflets having a first end and a free end, wherein the first end is coupled
with the
anchor and the free end is opposite of the first end. The prosthetic cardiac
valve has
an open configuration in which the free ends of the prosthetic valve leaflets
are
disposed away from one another to allow antegrade bloodflow therepast, and a
closed
configuration in which the free ends of the prosthetic valve leaflets engage
one
another and substantially prevent retrograde bloodflow therepast.
[0038] At least a portion of the atrial skirt may be covered with tissue or a
synthetic
material. The atrial skirt may further comprise a plurality of barbs coupled
thereto, the
13
CA 3043737 2019-05-16
plurality of barbs adapted to anchor the atrial skirt into a superior surface
of the
patient's mita! valve. The atrial skirt may comprise a collapsed configuration
and an
expanded configuration. The collapsed configuration may be adapted for
delivery to
the patient's heart, and the expanded configuration may be radially expanded
relative
to the collapsed configuration and adapted to lie over a superior surface of
the
patient's native mitrel valve, thereby anchoring the atrial skirt against a
portion of the
atrium. The atrial skirt may self-expand from the collapsed configuration to
the radially
expanded configuration when unconstrained. The atrial skirt may comprise one
more
radiopaque markers. The atrial skirt may comprise a plurality of axially
oriented struts
connected together with a connector element thereby forming a series of peaks
and
valleys. Some of the peaks and valleys may extend axially outward further than
the
rest of the atrial skirt, thereby forming an alignment element.
[0039] At least a portion of the annular region may be covered with tissue or
a
synthetic material. The annular region may have a collapsed configuration and
an
expanded configuration. The collapsed configuration may be adapted for
delivery to
the patient's heart, and the expanded configuration may be radially expanded
relative
to the collapsed configuration and adapted to conform with and to engage the
native
mitrel valve annulus. The annular region may self-expand from the collapsed
configuration to the expanded configuration when unconstrained. The annular
region
may comprise an asymmetrically D-shaped cross-section having a substantially
flat
anterior portion, and a cylindrically shaped posterior portion. The annular
region may
comprise a plurality of axially oriented struts connected together with a
connector
element thereby forming a series of peaks and valleys. One or more of the
axially
oriented struts may comprise one or more suture holes extending therethrough,
the
suture holes sized to receive a suture.
[0040] At least a portion of the ventricular skirt may be covered with tissue
or a
synthetic material. The ventricular skirt may comprise an asymmetrically D-
shaped
cross-section having a substantially flat anterior portion, and a
cylindrically shaped
posterior portion. The ventricular skirt may have a collapsed configuration
and an
expanded configuration. The collapsed configuration may be adapted for
delivery to
the patient's heart, and the expanded configuration may be radially expanded
relative
14
CA 3043737 2019-05-16
to the collapsed configuration and adapted to displace the native mitral valve
leaflets
radially outward. The ventricular skirt may self-expand from the collapsed
configuration to the expanded configuration when unconstrained.
[0041] The ventricular skirt may further comprise a trigonal anchoring tab
disposed
on an anterior portion of the ventricular skirt. The trigonal anchoring tab
may be
adapted to being anchored against a first fibrous trigon on a first side of an
anterior
leaflet of the patient's mitral valve. Thus, the anterior leaflet and adjacent
chordae
tendineae may be captured between the trigonal anchoring tab and an anterior
surface
of the anchor. The ventricular skirt may further comprise a second trigonal
anchoring
tab that may be disposed on the anterior portion of the ventricular skirt. The
second
trigonal anchoring tab may be adapted to being anchored against a second
fibrous
trigon opposite the first fibrous trigon, such that the anterior leaflet and
adjacent
chordae tendineae are captured between the second trigonal anchoring tab and
the
anterior surface of the anchor. The ventricular skirt may further comprise a
posterior
ventricular anchoring tab disposed on a posterior portion of the ventricular
skirt. The
posterior ventricular anchoring tab may be adapted to being anchored over a
posterior
leaflet of the patient's mitral valve, such that the posterior ventricular
anchoring tab is
seated between the posterior leaflet and a ventricular wall of the patient's
heart. The
ventricular skirt may further comprise a plurality of barbs coupled thereto,
and that may
be adapted to anchor the ventricular skirt into heart tissue. The ventricular
skirt may
comprise a plurality of struts connected together with a connector element
thereby
forming a series of peaks and valleys. The one or more struts may comprise one
or
more suture holes extending therethrough, and that may be sized to receive a
suture.
[0042] The plurality of prosthetic valve leaflets may comprise a tricuspid
leaflet
configuration. At least a portion of the one or more prosthetic valve leaflets
may
comprise tissue or a synthetic material. One or more of the plurality of
prosthetic valve
leaflets may be disposed over one or more connmissure posts or struts that are
radially
biased inward relative to the ventricular skirt. The one or more commissure
posts or
struts may comprise one or more suture holes extending therethrough and that
may be
sized to receive a suture. The one or more prosthetic valve leaflets may be
coupled to
CA 3043737 2019-05-16
a commissure post or strut having a commissure tab adapted to releasably
engage the
commissure post or strut with a delivery device.
[0043] The prosthetic cardiac valve may further comprise an alignment element
coupled to an anterior portion of the anchor. The alignment element may be
adapted
to be aligned with an aortic root of the patient's heart and disposed between
two
fibrous trigones of an anerior leaflet of the patient's mitre! valve. The
alignment
element may be coupled with the atrial skirt. The prosthetic cardiac valve may
further
comprise a therapeutic agent coupled thereto, and adapted to being
controllably eluted
therefrom.
[0044] In still another aspect of the present invention, a delivery system for
delivering
a prosthetic cardiac valve to a patient's heart comprises an inner guidewire
shaft
having a lumen extending therethrough and adapted to slidably receive a
guidewire,
and a hub shaft concentrically disposed over the inner guidewire shaft The
delivery
system also comprises a bell shaft slidably and concentrically disposed over
the hub
shaft, a sheath slidably and concentrically disposed over the bell shaft, and
a handle
near a proximal end of the delivery system. The handle comprises an actuator
mechanism adapted to advance and retract the bell shaft and the sheath.
[0045] The system may further comprise the prosthetic cardiac valve which may
be
housed in the sheath in a radially collapsed configuration. The prosthetic
cardiac
valve may comprise an anchor having an atrial skirt, an annular region, and a
ventricular skirt. The prosthetic valve may also comprise a plurality of
prosthetic valve
leaflets. Each of the leaflets may have a first end and a free end. The first
end may
be coupled with the anchor and the free end may be opposite of the first end.
The
prosthetic cardiac valve may have an open configuration in which the free ends
of the
prosthetic valve leaflets are disposed away from one another to allow
antegrade
bloodflow therepast. The valve may have a closed configuration in which the
free
ends of the prosthetic valve leaflets engage one another and substantially
prevent
retrograde blood flow therepast.
[0046] Proximal retraction of the sheath relative to the bell shaft may remove
a
constraint from the prosthetic cardiac valve thereby allowing the prosthetic
cardiac
valve to self-expand into engagement with the patient's native heart tissue.
The
16
CA 3043737 2019-05-16
prosthetic cardiac valve may be releasably coupled with the hub shaft, and
proximal
retraction of the bell shaft relative to the hub shaft may release the
prosthetic cardiac
valve therefrom. The actuator mechanism may comprise a rotatable wheel. The
system may further comprise a tissue penetrating distal tip coupled to the hub
shaft.
The tissue penetrating distal tip may be adapted to pass through and expand an
incision in the patient's heart. The system may further comprise a pin lock
mechanism
releasably coupled with the handle. The pin lock mechanism may limit proximal
retraction of the sheath.
[0047] These and other embodiments are described in further detail in the
following
description related to the appended drawing figures.
BRIEF DESCRIPTION OF THE DRAWINGS
[0048] In the drawings, like reference numerals designate like or similar
steps or
components.
[0049] Fig. 1 is a schematic illustration of the left ventricle of a heart
showing blood
flow during systole with arrows.
[0050] Fig. 2 is a schematic illustration of the left ventricle of a heart
having
prolapsed leaflets in the mitral valve.
[0051] Fig. 3 is a schematic illustration of a heart in a patient suffering
from
cardiomyopathy where the heart is dilated and the leaflets do not meet.
[0052] Fig. 3A shows, normal closure of the leaflets.
[0053] Fig. 3B shows abnormal closure in the dilated heart.
[0054] Fig. 4 illustrates mitral valve regurgitation in the left ventricle of
a heart having
impaired papillary muscles.
[0055] Figs. 5A-5B illustrate the mitral valve.
[0056] Fig. 6 illustrates a bottom, partial cross-sectional view of an
exemplary
prosthetic mitral valve.
[0057] Fig. 7 is a perspective view of the anchor portion of the prosthetic
mitral valve
seen in Fig. 6.
[0058] Fig. 8A is a perspective view of a prosthetic mitral valve.
[0059] Fig. 8B is a top view from the atrium of the prosthetic valve in Fig.
8A.
17
CA 3043737 2019-05-16
[0060] Fig. 9A illustrates a perspective view of the prosthetic valve in Fig.
8A from the
atrium.
[0061] Fig. 9B illustrates a perspective view of the prosthetic valve in Fig.
8A from the
ventricle.
[0062] Fig. 10 illustrates the prosthetic valve of Fig. 8A uncovered and
unrolled in a
flat pattern.
[0063] Fig. 11 is a side view of a delivery device for implantation of a
prosthetic
valve.
[0064] Fig. 12 is a perspective exploded view of a proximal portion of the
delivery
device in Fig. 11.
[0065] Fig. 13 is a perspective exploded view of a distal portion of the
delivery device
in Fig. 11.
[0066] Fig. 14 is a cross-section of the a proximal portion of the delivery
device in
Fig. '11.
[0067] Figs. 15A-15C are cross-sectional views of a distal portion of the
delivery
device in Fig. 11.
[0068] Fig. 16 is a side view of another exemplary embodiment of a delivery
device
for implantation of a prosthetic valve.
[0069] Fig. 17 is a perspective view of the delivery device in Fig. 16.
[0070] Fig. 18 is a perspective exploded view of the delivery device in Fig.
16.
[0071] Figs. 19A-19B are side views of the delivery device in Fig. 16 during
various
stages of operation.
[0072] Fig. 20 illustrates a distal portion of the delivery device in Fig. 16
that is
adapted to engage a portion of a prosthetic valve.
[0073] Fig. 21 illustrates engagement of the delivery device in Fig. 16 with
the
prosthetic valve of Fig. 8A.
[0074] Figs. 22A-22G illustrate an exemplary method of transapically
delivering a
prosthetic mitral valve.
[0075] Figs. 23A-23G illustrate an exemplary method of transseptally
delivering a
prosthetic mitral valve.
[0076] Fig. 24 illustrates a prosthetic mitral valve implanted in the mitral
space.
18
CA 3043737 2019-05-16
[0077] Fig. 25 illustrates a bottom view of a mitral valve implanted in the
mitral space
looking upward from the left ventricle.
DETAILED DESCRIPTION OF THE INVENTION
[0078] Specific embodiments of the disclosed device, delivery system, and
method
will now be described with reference to the drawings. Nothing in this detailed
description is intended to imply that any particular component, feature, or
step is
essential to the invention.
[0079] Cardiac Anatomy. The left ventricle LV of a normal heart H in systole
is
.. illustrated in Fig. 1. The left ventricle LV is contracting and blood flows
outwardly
through the aortic valve AV, a tricuspid valve in the direction of the arrows.
Back flow
of blood or "regurgitation" through the mitral valve MV is prevented since the
mitral
valve is configured as a "check valve" which prevents back flow when pressure
in the
left ventricle is higher than that in the left atrium LA. The mitral valve MV
comprises a
pair of leaflets having free edges FE which meet evenly to close, as
illustrated in Fig.
1. The opposite ends of the leaflets LF are attached to the surrounding heart
structure
along an annular region referred to as the annulus AN. The free edges FE of
the
leaflets LF are secured to the lower portions of the left ventricle LV through
chordae
tendineae CT (also referred to herein as the chordae) which include a
plurality of
branching tendons secured over the lower surfaces of each of the valve
leaflets LF.
The chordae CT in turn, are attached to the papillary muscles PM which extend
upwardly from the lower portions of the left ventricle and interventricular
septum IVS.
[0080] Referring now to Figs. 2-4, a number of structural defects in the heart
can
cause mitral valve regurgitation. Ruptured chordae RCT, as shown in Fig. 2,
can
cause a valve leaflet LF2 to prolapse since inadequate tension is transmitted
to the
leaflet via the chordae. While the other leaflet LF1 maintains a normal
profile, the two
valve leaflets do not properly meet and leakage from the left ventricle LV
into the left
atrium LA will occur, as shown by the arrow.
[0081] Regurgitation also occurs in the patients suffering from cardiomyopathy
where
the heart is dilated and the increased size prevents the valve leaflets LF
from meeting
properly, as shown in Fig. 3. The enlargement of the heart causes the mitral
annulus
19
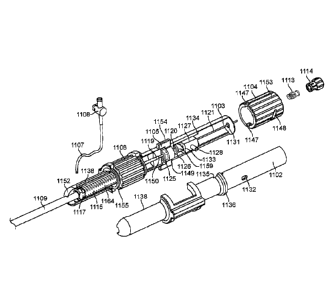
CA 3043737 2019-05-16
to become enlarged, making it impossible for the free edges FE to meet during
systole. The free edges of the anterior and posterior leaflets normally meet
along a
line of coaptation C as shown in Fig. 3A, but a significant gap G can be left
in patients
suffering from cardiomyopathy, as shown in Fig. 3B.
0082] Mitral valve regurgitation can also occur in patients who have suffered
ischemic heart disease where the functioning of the papillary muscles PM is
impaired,
as illustrated in Fig. 4. As the left ventricle LV contracts during systole,
the papillary
muscles PM do not contract sufficiently to effect proper closure. The leaflets
LF1 and
LF2 then prolapse, as illustrated. Leakage again occurs from the left
ventricle LV to
the left atrium LA, as shown by the arrow.
[0083] Fig. 5A more clearly illustrates the anatomy of a mitral valve MV which
is a
bicuspid valve having an anterior side ANT and a posterior side POST. The
valve
includes an anterior (aortic) leaflet AL and a posterior (mural) leaflet PL.
Chordae
tendineae CT couple the valve leaflets AL, PL with the antero-lateral
papillary muscle
.. ALPM and the postero-medial papillary muscle PM PM. The valve leaflets AL,
PL join
one another along a line referred to as the antero-lateral commissure ALC and
the
posterior-medial commissure PMC. The annulus AN circumscribes the valve
leaflets,
and two regions adjacent an anterior portion of the annulus, on opposite sides
of the
anterior leaflet are referred to as the left fibrous trigone LFT and also the
right fibrous
trigone RFT. These areas are indicted by generally by the solid triangles.
Fig. 5B
more clearly illustrates the left and right fibrous trigones, LFT, RFT.
[0084] While various surgical techniques as well as implantable devices have
been
proposed and appear to be promising treatments for mitral regurgitation,
surgical
approaches can require a lengthy recovery period, and implantable devices have
.. varying clinical results. Therefore, there still is a need for improved
devices and
methods for treating mitral regurgitation. While the embodiments disclosed
herein are
directed to an implantable prosthetic mitral valve for treating mitrel
regurgitation, one of
skill in the art will appreciate that this is not intended to be limiting, and
the device and
methods disclosed herein may also be used to treat other cardiac valves such
as the
.. tricuspid valve, aortic valve, pulmonary valve, etc, as well as other
valves in the body
such as venous valves.
CA 3043737 2019-05-16
[0085] Prosthetic Valve. Prosthetic valves have been surgically implanted in
the
heart as a treatment for mitral regurgitation. Some of these valves have been
valves
harvested from animals such as porcine valves, and others have been prosthetic
mechanical valves with or without a tissue covering. More recently, minimally
invasive
catheter technology has been used to deliver prosthetic valves to the heart.
These
valves typically include an anchor for securing the valve to the patient's
heart, and a
valve mechanism, either a mechanical valve, a valve with animal tissue, or
combinations thereof. The prosthetic valve once implanted, takes over for
malfunctioning native valve, thereby reducing or eliminating valvar
insufficiency. While
some of these valves appear promising, there still is a need for improved
valves. The
following discloses exemplary embodiments of a prosthetic valve, a delivery
system for
the prosthetic valve, and methods of delivering the valve that overcome some
of the
challenges associated with existing prosthetic valves.
[0086] Referring now to Figs. 6-7, exemplary embodiments of a mitre! valve
prosthesis generally designated with reference numeral 10 comprise tricuspid
tissue-
type prosthetic one-way valve structure 12 comprising leaflets 14 affixed
within self-
expanding or exapandable anchor portion 16 having a geometry that expands into
low
profile atrial skirt region 18, annular region 20, ventricular skirt region
22, and a
plurality of leaflet commissures 24 (also referred to herein as commissure
posts)
extending axially in a cantilevered fashion downstream into the sub-annular
space
defined by ventricular skirt region 22. Fig. 6 shows a partial cross-section
of the valve
10 from the patient's left ventricle looking upward toward the right atrium.
The atrial
skirt region 18 is anchored to a lower portion of the right atrium 19. The
valve leaflets
14 have an open position (not illustrated) and a closed position illustrated
in Fig. 6. In
the open position, the leaflets 14 are displaced away from one another to
allow blood
flow therepast, and in the closed position, the leaflets 14 engage one another
to close
the valve and prevent retrograde blood flow therepast. The valve commissures
24
may be configured to optimize the efficiency of the prosthetic valve structure
12 and
the load distribution on the leaflets 14 by providing for the attachment of
the leaflets 14
along arcuate seams 28 (best seen in Fig. 7), and by being made selectively
flexible at
21
CA 3043737 2019-05-16
different points or zones along their axial length through the
addition/deletion of reinforcing
struts.
[0087] Fig. 7 shows a perspective view of the anchor portion 16 of the valve
10 which has
been formed from a series of interconnected struts. The atrial skirt region 18
forms an
annular flanged region on the anchor to help secure an upper portion of the
prosthetic
valve in the atrium, and the annular region 20 is a cylindrical region for
anchoring the valve
along the native valve annulus. The ventricular skirt region 22 similarly is
cylindrically
shaped and helps anchor a lower portion of the valve in the patient's left
ventricle. Any
portion, or all of the anchor may be covered with tissue such as pericardium
or other
tissues disclosed herein, or a synthetic material such as DacronTM or ePTFE
may be used
to cover the anchor. The covering helps to seal the anchor to the native
valve, and this
helps funnel blood into and through the prosthetic valve, rather than around
the valve. In
some embodiments, the anchor may remain uncovered. The prosthetic valve has an
expanded configuration and a collapsed configuration. The collapsed
configuration has a
low profile cylindrical shape that is suitable for mounting on a delivery
system and delivery
is preferably made either transluminal^ on a catheter, or transapically
through the heart
wall. The expanded configuration (as illustrated) allow the prosthetic valve
to be anchored
into a desired position.
[0088] Fig. 8A illustrates a perspective view of a preferred embodiment of a
prosthetic
mitral valve with optional coverings removed to allow visibility of the anchor
struts. Fig. 8B
illustrates a top view of the prosthetic valve in Fig. 8A from the atrium
looking down into the
ventricle. The valve 800 includes an asymmetrical expanded anchor portion
having a D-
shaped cross-section. As shown, the anchor portion generally comprises
anterior 802 and
posterior 804 aspects along the longitudinal axis thereof, as well as atrial
806, annular 808
and ventricular 810 regions that correspond generally to the atrial skirt 18,
annular 20 and
ventricular skirt 22 regions of the embodiment described above in Figs. 6-7.
Commissures
(also referred to herein as commissure posts) 813 also correspond generally to
the leaflets
14 of the embodiment in Figs. 6-7. The prosthetic valve 800 has a collapsed
configuration
and an expanded configuration. The collapsed configuration is adapted to
loading on a
shaft such as a
22
Date Recue/Date Received 2020-09-08
delivery catheter for transluminal delivery to the heart, or on a shaft for
transapical
delivery through the heart wall. The radially expanded configuration is
adapted to
anchor the valve to the patient's native heart adjacent the damaged valve. In
order to
allow the valve to expand from the collapsed configuration to the expanded
configuration, the anchor portion of the valve may be fabricated from a self-
expanding
material such as a nickel titanium alloy like nitinol, or it may also be made
from spring
temper stainless steel, or a resilient polymer. In still other embodiments,
the anchor
may be expandable with an expandable member such as a balloon. In preferred
embodiments, the anchor is fabricated by laser cutting, electrical discharge
machining
(EDM), or photochemically etching a tube. The anchor may also be fabricated by
photochemically etching a flat sheet of material which is then rolled up with
the
opposing ends welded together.
10089] The atrial skirt portion 816 forms a flanged region that helps to
anchor the
prosthetic valve to the atrium, above the mitral valve. The atrial skirt
includes a
plurality of triangular fingers which extend radially outward from the anchor
to form the
flange. The posterior 804 portion of the atrial skirt 816 is generally round
or circular,
while a portion of the anterior 802 part of the atrial skirt 816 is flat.
Thus, the atrial skirt
region preferably has a 0-shaped cross-section. This allows the prosthetic
valve to
conform to the patient's cardiac anatomy without obstructing other portions of
the
heart, as will be discussed below. Each triangular finger is formed from a
pair of
interconnected struts. The triangular fingers of the atrial skirt generally
are bent
radially outward from the central axis of the prosthetic valve and lie in a
plane that is
transverse to the valve central axis. In some embodiments, the atrial skirt
lies in a
plane that is substantially perpendicular to the central axis of the valve.
The anterior
portion 802 of the atrial skirt 806 optionally includes an alignment element
814 which
may be one or more struts which extend vertically upward and substantially
parallel to
the prosthetic valve. The alignment element 814 may include radiopaque markers
(not
illustrated) to facilitate visualization under fluoroscopy. The alignment
element helps
the physician to align the prosthetic valve with the native mitral valve
anatomy, as will
be discussed later.
23
CA 3043737 2019-05-16
[0090] Disposed under the atrial skirt region is the annular region 820 which
also has
a collapsed configuration for delivery, and an expanded configuration for
anchoring the
prosthetic valve along the native valve annulus. The annular region is also
comprised
of a plurality of interconnected struts that form a series of cells,
preferably closed.
Suture holes 821 in some of the struts allow tissue or other coverings (not
illustrated)
to be attached to the annular region. Covering all or a portion of the anchor
with tissue
or another covering helps seal the anchor against the heart valve and adjacent
tissue,
thereby ensuring that blood is funneled through the valve, and not around it.
The
annular region may be cylindrical, but in preferred embodiments has a
posterior
portion 804 which is circular, and an anterior portion 802 which is flat,
thereby forming
a D-shaped cross-section. This D-shaped cross-section conforms better to the
native
mitral valve anatomy without obstructing blood flow in other areas of the
heart.
[0091] The lower portion of the prosthetic valve includes the ventricular
skirt region
828. The ventricular skirt region also has a collapsed configuration for
delivery, and an
expanded configuration for anchoring. It is formed from a plurality of
interconnected
struts that form a series of cells, preferably closed, that can radially
expand. The
ventricular skirt in the expanded configuration anchors the prosthetic valve
to the
ventricle by expanding against the native mitral valve leaflets. Optional
barbs 823 in
the ventricular skirt may be used to further help anchor the prosthetic valve
into the
ventricular tissue. Barbs may optionally also be included in the atrial skirt
portion as
well as the annular region of the anchor. Additionally, optional suture holes
821 in the
ventricular skirt may be used to help suture tissue or another material to the
ventricular
skirt region, similarly as discussed above. The anterior 802 portion of the
ventricular
skirt may be flat, and the posterior 804 portion of the ventricular skirt may
be circular,
similarly forming a D-shaped cross-section to anchor and conform to the native
anatomy without obstructing other portions of the heart. Also, the lower
portions of the
ventricular skirt serve as deployment control regions since the lower portions
can
remain sheathed thereby constraining the ventricular skirt from radial
expansion until
after the optional ventricular trigonal tabs and posterior tab have expanded,
as will be
explained in greater detail below.
24
CA 3043737 2019-05-16
[0092] The ventricular skirt portion may optionally also include a pair of
ventricular
trigonal tabs 824 on the anterior portion of the anchor (only 1 visible in
this view) for
helping to anchor the prosthetic valve as will be discussed in greater detail
below. The
ventricular skirt may also optionally include a posterior tab 826 on a
posterior portion
804 of the ventricular skirt for anchoring the prosthetic valve to a posterior
portion of
the annulus. The trigonal tabs 824 or the posterior tab 826 are tabs that
extend
radially outward from the anchor, and they are inclined upward in the upstream
direction.
[0093] The actual valve mechanism is formed from three commissures posts (also
referred to as commissures) 813 which extend radially inward toward the
central axis
of the anchor in a funnel or cone-like shape. The commissures 813 are formed
from a
plurality of interconnected struts that create the triangular shaped
commissures. The
struts of the commissures may include one or more suture holes 821 that allow
tissue
or a synthetic material to be attached to the commissures. In this exemplary
embodiment, the valve is a tricuspid valve, therefore it includes three
commissures
813. The tips of the commissures may include a commissure tab 812 (also
referred to
as a tab) for engaging a delivery catheter. In this embodiment, the tabs have
enlarged
head regions connected to a narrower neck, forming a mushroom-like shape. The
commissures may be biased in any position, but preferably angle inward
slightly
toward the central axis of the prosthetic valve so that retrograde blood flow
forces the
commissures into apposition with one another to close the valve, and antegrade
blood
flow pushes the commissures radially outward, to fully open the valve. Fig. 8B
is a top
view illustrating the prosthetic valve of Fig. 8A from the atrial side, and
shows the
preferred D-shaped cross-section.
[0094] Fig. 9A illustrates the prosthetic mitrel valve of Figs. 8A-8B with a
covering
870 coupled to portions of the anchor with suture 872. This view is taken from
an
atrial perspective. In this embodiment, the covering is preferably pericardium
which
may come from a number of sources as disclosed elsewhere in this
specification. In
alternative embodiments, the covering may be a polymer such as Dacron
polyester,
ePTFE, or another synthetic material. The covering is preferably disposed over
the
annular region 820 and the ventricular skirt region 828, and in some
embodiments the
CA 3043737 2019-05-16
anterior ventricular trigonal 824 tabs and the ventricular posterior tab 830
may also be
covered with the same or a different material. The covering helps seal the
anchor
against the adjacent tissue so that blood funnels through the valve mechanism.
In this
embodiment, the atrial skirt is left uncovered, as well as tabs 824, 830.
Additionally,
radiopaque markers 814a form a portion of the alignment element and facilitate
visualization of the prosthetic valve under fluoroscopy which is important
during
alignment of the valve.
[0095] Fig. 9B is a perspective view of the prosthetic mitral valve seen in
Fig. 9A, as
seen from the ventricle. The struts of the valve commissures are covered with
the
same material or a different material as the annular and ventricular regions
as
discussed above, thereby forming the tricuspid valve leaflets 813. Fig. 9B
shows the
valve in the closed configuration where the three leaflets are engaged with
one
another preventing retrograde blood flow. Commissure tabs 812 remain uncovered
and allow the commissures to be coupled with a delivery device as will be
explained
below. The prosthetic valve in Figs. 9A-9B may be sterilized so they are
suitable for
implantation in a patient using methods known in the art.
[0096] Fig. 10 illustrates the prosthetic valve of Fig. 9A with the covering
removed,
and the remaining anchor unrolled and flattened out. The prosthetic valve 800
is
formed from a plurality of interconnected struts. For example, the atrial
skirt region
806 includes a plurality of interconnected struts that form a series of peaks
and
valleys. The flat anterior region 802 of the prosthetic valve has its peaks
and valleys
axially offset from those of the remaining portion of the atrial skirt, and
this region
becomes a part of the alignment element 814. Radiopaque markers 814a are
disposed on either side of the offset peaks and valleys and help with
visualization
during implantation of the valve. An axially oriented connector joins the
struts of the
skirt region 806 with the struts of the annular region 808. The annular region
is also
comprised of a plurality of axially oriented and interconnected struts that
form peaks
and valleys. Connector struts couple struts of the annular region with the
struts of the
ventricular region 810. The ventricular region also includes a plurality of
interconnected struts that form peaks and valleys. Additionally, the struts
form the
leaflet commissures 813, the ventricular skirt 828, as well as the trigonal
and posterior
26
CA 3043737 2019-05-16
tabs 824, 830. Suture holes 821 are disposed along the struts of the annular
region as
well as the ventricular region to allow attachment of a cover such as
pericardium or a
polymer such as Dacron or ePTFE. Barbs 823 are disposed along the ventricular
skirt
828 to help anchor the prosthetic valve to adjacent tissue. Commissure tabs or
tabs
812 are disposed on the tips of the commissures 813 and may be used to
releasably
couple the prosthetic valve with a delivery system as will be described below.
One of
skill in the art will appreciate that a number of strut geometries may be
used, and
additionally that strut dimensions such as length, width, thickness, etc. may
be
adjusted in order to provide the anchor with the desired mechanical properties
such as
stiffness, radial crush strength, commissure deflection, etc. Therefore, the
illustrated
geometry is not intended to be limiting.
[0097] Once the flat anchor pattern has been formed by EDM, laser cutting,
photochemical etching, or other techniques known in the art, the anchor is
radially
expanded into a desired geometry. The anchor is then heat treated using known
processes to set the shape. Thus, the anchor may be loaded onto a delivery
catheter
in a collapsed configuration and constrained in the collapsed configuration
with a
constraining sheath. Removal of the constraining sheath will allow the anchor
to self-
expand into its unbiased pre-set shape. In other embodiments, an expandable
member such as a balloon may be used to radially expand the anchor into its
preferred
expanded configuration.
[0098] Delivery Systems. Figs. 11-15C show a delivery apparatus 1124 fashioned
to
deliver a prosthetic mitral valve to the heart transapically. However, one of
skill in the
art will appreciate that the delivery system may be modified and relative
motion of the
various components adjusted to allow the device to be used to deliver a
prosthetic
mitral valve transseptally. The delivery apparatus is generally comprised of a
handle
1101 that is the combination of a handle section 1102 and a handle section
1103 (best
seen in Fig. 12), as well as a flexible tip 1110 that can smoothly penetrate
the apex of
the heart, and a sheath catheter 1109 which houses several additional
catheters that
are designed to translate axially and will be described in detail below.
[0099] The handle 1101 includes a female threaded luer adaptor 1113 which
connects to a Tuohy Borst adaptor 1114 in order to provide a hemostatic seal
with a
27
CA 3043737 2019-05-16
0.035" diameter guide wire (not shown). The female threaded luer adaptor 1113
is in
threaded contact with the proximal section of the handle 1101 through a
threaded port
1131 (best seen in Fig. 12).
[0100] As can be seen in Fig. 11, the handle 1101 provides location for the
control
mechanisms used to position and deploy a prosthetic mitre! valve. The handle
1101
provides housing for a thumbwheel 1106 that can be accessed through a window
1137
that appears on both the top and bottom of the handle 1101. The thumbwheel 11
06
internally mates with a threaded insert 1115 (best seen in Fig. 12) that
actuates the
sheath catheter 1109, and the mechanics of this interaction will be explained
in detail
below.
[0101] Fig. 11 also shows a deployment thumbwheel 1104 that provides linear
translation to a deployment catheter 1120 (best seen in Fig. 12) when turned,
since
the turning motion of the deployment thumbwheel 1104 acts as a power screw,
pushing the peg 1128 forward and distally from the user. The mechanics behind
the
peg 1128 will be further detailed below. The thumbwheel lock 1105 provides a
security measure against unwanted rotation of the deployment thumbwheel 1104
by
acting as a physical barrier to rotation. In order to turn the deployment
thumbwheel
1104 the user must push forward the thumbwheel lock 1105, disengaging it from
two
slots 1147 (seen in Fig. 12) in the deployment thumbwheel 1105.
[0102] As can also be seen in Fig. 11, a bleed valve 1108 and fluid line 1107
are
connected to an internal mechanism in the distal portion of the handle 1101,
which
provides a hemostatic seal for the sheath catheter 1109. The details of this
connection
will be described below.
[0103] Internal mechanics of the delivery apparatus 1124 are illustrated in
detail in
Fig. 12, and the following descriptions will reveal the interactions between
individual
components, and the manner in which those components combine in order to
achieve
a prosthetic heart valve delivery apparatus.
[0104] As seen in Fig. 12, a handle section 1103 and handle section 1102
combine
to create a handle 1101 that forms the basis of the delivery apparatus 1124.
In order
to advance the sheath catheter 1109 during valve loading, or retract the
sheath
catheter 1109 during deployment, a rotatable thumbwheel 1106 is in threaded
contact
28
CA 3043737 2019-05-16
(internal threads 1129 seen in Fig. 14) with a threaded insert 1115 (external
threads
1130 of Fig. 13) that translates linearly along the axis of the delivery
apparatus, from a
proximal position to a distal position. The sheath catheter 1109 is in mating
contact
with the threaded insert 1115 and is fastened through the use of a collar 1117
that
aligns and mates the collar with the insert. The collar 1117 is fastened with
screws
1116 (best seen in DETAIL A in Fig. 14) to the threaded insert 1115 and
contains a
fluid port 1142 (best seen in DETAIL A in Fig. 14) that provides location for
the fluid
line 1117 so that hemostasis can be maintained between the patient and
delivery
apparatus. An 0-ring 1118 (best seen in DETAIL A in Fig. 14) seals the
stationary
catheter 1119 (best seen in Fig. 14) against the sheath catheter 1109. The
fluid line
1107 also provides a means of visually locating the sheath catheter 1109 with
respect
to position, as a slot 1138 in the handle 1101 allows the fluid line 1107 to
translate with
the sheath catheter 1109 (through a hole 1151 (best seen in DETAIL A in Fig.
14)
during operation, and this translation is highly visible. In order to prevent
rotation of
the threaded insert during translation, a flat face 1164 has been machined
onto both
sides of the threaded insert 1115. The flat faces 1164 remain in contact with
bosses
1139 and 1140 that are located on both handle section 1102 and handle section
1103
so that the bosses 1139 and 1140 act to grip the threaded insert 1115 and
prevent
rotation. A textured pattern 1155 allows the user to easily turn the
thumbwheel 1106
.. in the surgical field. Detents 1141 (best seen in Fig. 14) locate flanges
63 (seen in Fig.
14) on the thumbwheel 1116 in order to allow for rotation.
[01051 The manner in which individual catheters (there are four catheters)
move with
respect to each other is illustrated in Fig. 12. Sheath catheter 1109 provides
housing
for the stationary catheter 1119, which in turn provides housing for the
movable hub
catheter 1120. The hub catheter 1120 translates linearly with respect to the
nose
catheter 1121 which can also be translated with respect to each previous
catheter, and
the handle 1101. The stationary catheter 1119 is mated to a handle section
1103 in
an internal bore 1150 which also forms a seal between the stationary catheter
1119
and the hub catheter 1120. The distal portion of the stationary catheter 1119
is formed
in the shape of a bell 1122 (see DETAIL A in Fig. 15A) which acts as a housing
to
retain the hub capture 1123 (seen in DETAIL A in Fig. 15A).
29
CA 3043737 2019-05-16
[0106] As previously stated a thumbwheel lock 1105 prevents rotation of the
deployment thumbwheel 1104. In order to provide a seating force that keeps the
thumbwheel lock 1105 in a locked position until manipulated, a spring 1125 is
housed
in an internal bore 62 (best seen in Fig. 14) and abuts against a shoulder
1161 (best
seen in Fig. 14) that is located inside the thumbwheel lock 1105. This spring
1125
maintains the leading edge 1149 of the thumbwheel lock 1105 in a locked
position
within the two slots 1147 of the deployment thumbwheel 1104. Gripping texture
1154
is provided on the thumbwheel lock 1105 for ease of use. In order to locate
and retain
the thumbwheel lock 1105 inside of the handle 1101, a slot 1135 has been
provided in
both a handle section 1102 and a handle section 1103.
[0107] As shown in Fig. 12, a sliding block 1127 is housed inside of flat
parallel faces
1134 which appear on the inside of the handle 1101. This sliding block 1127 is
in
mating contact with hub catheter 1120 and is the physical mechanism that
linearly
actuates the catheter. A spring 1126 is mounted on an external post 1159 and
abuts
against a shoulder 1133 that is located on the distal end of the sliding block
1127.
This spring 1126 forces a peg 1128 (located inside a thru-hole 1156 of Fig.
14) into
contact with the proximal edge of an angled slot 1148 that is cut into the
deployment
thumbwheel 1104. The deployment thumbwheel 1104 is contained between a
shoulder 1136 and a snap ring (not shown), both of which are features of the
handle
1101. Gripping texture 1153 on the deployment thumbwheel 1104 allows the user
to
easily rotate the thumbwheel in a clockwise direction, actuating the peg 1128
to ride
distally along the slot 1148 and move the sliding block 1127, which pushes the
hub
catheter 1120 and hub 1123 (best seen in DETAIL A of Fig. 15A) forward and out
of
the bell 1122 (seen in DETAIL A of Fig. 15A). A slot 1132 appears in a handle
section
1102 and a handle section 1103 and prevents the peg 1128 from translating
beyond a
desired range.
[0108] A nose catheter 1121 extends from a Tuohy Borst adaptor 1114 on the
proximal end of the handle 1101, and internally throughout the handle and the
respective catheters (sheath catheter 1109, stationary catheter 1119, and hub
catheter
1120), terminating inside the rigid insert 1112 (seen in Fig. 15A) of the
flexible tip 1110
(seen in Fig. 15A) that abuts with the distal end of the sheath catheter 1109.
CA 3043737 2019-05-16
[0109] Fig. 13 displays an exploded view of the tip section of the delivery
apparatus
1124, and shows the relation between prosthetic mitre! valve 1165 and the
internal
and external catheters. When crimped and loaded, the prosthetic mitral valve
1165 is
encased between the internal surface of the sheath catheter 1109 and the
external
surface of the nose catheter 1121. In order to capture and anchor the
prosthetic mitre!
valve 1165 within the delivery apparatus 1124, three commissure tabs 1160
(circumferentially spaced at 1200 apart) appearing on the proximal end of the
prosthetic mitral valve 1165 provide points of contact between the valve and
three
slots 1143 (seen in Fig. 15A) that are machined into the outer surface of the
hub 1123
(circumferentially spaced at 1200 apart). After first advancing the hub
catheter 1120
(Fig. 15A) by rotating the deployment thumbwheel 1104 (seen in Fig. 12)
clockwise,
the three commissure tabs 1160 can be captured within the three slots 1143
(seen in
Fig. 15A). The hub 1123 can then be retracted into the bell 1122 by releasing
the
deployment thumbwheel 1104 (seen in Fig. 12). In this position the prosthetic
mitre!
valve 1165 is anchored to the delivery apparatus 1124, and further crimping of
the
valve will allow the sheath catheter 1109 to be advanced over the valve.
[0110] Figs. 15A-15C further detail the manner in which loading of the
prosthetic
mitral valve 1165 (seen in Fig. 13) into the delivery apparatus 1124 can be
achieved.
Initially, the flexible tip 1110 is abutted against the distal edge 1157 of
the sheath
catheter 1109. The flexible tip 1110 is comprised of a rigid insert 1112, and
a soft and
flexible tip portion 1111 which is over-molded onto the rigid insert 1112. The
shoulder
1145 and tapered face 1146 of the rigid insert 1112 act to guide and locate
the distal
edge 1157 of the sheath catheter 1109, so that the catheter may rest against
and be
stiffened by the flexible tip 1110, and be more easily introduced into the
apex of the
heart.
[0111] An initial position from which loading can be achieved is illustrated
in Fig. 15A.
As a first step in the loading of a prosthetic mitral valve 1165 (seen in Fig.
13) into the
delivery apparatus 1124, the sheath catheter 1109 is withdrawn by rotation of
the
thumbwheel 1106 in a clockwise direction. The distal edge 1157 of the sheath
catheter 1109 is retracted until it passes the distal edge of the bell 1122,
as illustrated
in DETAIL A of Fig. 15B. As a second step in the loading of a prosthetic
mitral valve
31
CA 3043737 2019-05-16
1165 (seen in Fig. 13) into the delivery apparatus 1124, the hub 1123 is
advanced
from beneath the bell 1122 by clockwise turning of the deployment thumbwheel
1104
(seen in Fig. 12), as illustrated in DETAIL A of Fig. 15C. The deployment
thumbwheel
may only be turned once the thumbwheel lock 1105 (see Fig. 12) has been set in
the
forward position, disengaging it from contact with the thumbwheel. Advancement
of
the hub 1123 uncovers three slots 1143 into which three commissure tabs 1160
of the
prosthetic mitre! valve 1165 (seen in Fig. 13) will fit and be anchored. After
anchoring
of the commissure tabs 1160 into the slots 1143 by retraction of the hub 1123
has
been achieved, a third step in the loading of a prosthetic mitral valve 1165
(seen in
Fig. 13) into the delivery apparatus 1124 may be performed. The prosthetic
mitral
valve 1165 (seen in Fig. 13) can be crimped down to a minimum diameter by a
loading
mechanism (not shown), and then the sheath cannula 1109 can be advanced
forward
so as to cover the valve, by rotation of the thumbwheel 1106 in a counter-
clockwise
direction. The delivery apparatus 1124 and prosthetic mitral valve 1165 are
then
ready for deployment.
[0112] Figs. 16-19B illustrate another exemplary embodiment of a delivery
device for
implanting a prosthetic valve in the heart transapically. However, one of
skill in the art
will appreciate that the delivery system may be modified and relative motion
of the
various components adjusted to allow the device to be used to deliver a
prosthetic
transseptally. The delivery apparatus is generally comprised of a handle 1601
that is
the combination of two halves (1610 and 1635), as well as a tip 1603 that can
smoothly penetrate the apex of the heart, and a flexible sheath 1602 which is
comprised of concentric catheters that are designed to translate axially and
will be
described in detail below.
[0113] The handle 1601 includes a handle cap 1611 which connects to a female
threaded luer adaptor 1612 in order to provide a sealable exit for a 0.035"
diameter
guide-wire (not shown). The handle cap 1611 is attached to the handle 1601
with
threaded fasteners 1613. The female threaded luer adaptor 1612 is in threaded
contact with the handle cap 1611 through a tapped port, and when fully
inserted
squeezes against an o-ring (1636 best seen in Fig. 18) which seals against the
outer
diameter of a guide-wire catheter (1621 best seen in Fig. 18).
32
CA 3043737 2019-05-16
[0114] As can be seen in Fig. 17, the handle 1601 provides location for the
control
mechanisms used to position and deploy a prosthetic mitral valve. The handle
1601
provides housing for a thumbwheel 1616 that can be accessed through a window
1606
that appears on both the top and bottom of the handle 1601. The thumbwheel
1616
internally mates with a threaded insert (1627 in Fig. 18) that actuates the
sheath
catheter 1604, and the mechanics of this interaction will be explained in
detail below.
[0115] Fig. 17 also shows a first hemostasis tube 1617 that is inserted
internally
through a slot 1605, and that mates with a first hemo-port through a hole
(1625 and
1626 in Fig. 18 respectively). The first hemostasis tube 1617 allows for fluid
purging
between internal catheters. The position of the first hemostasis tube 1617
along the
slot 1605 provides a visual cue as to the position of the sheath catheter
1604, and
relative deployment phase of a prosthetic mitral valve (not shown). The
relationship
between the connection of the first hemostasis tube 1617 and the sheath
catheter
1604 will be described below.
[0116] As can also be seen in Fig. 17, a second hemostasis tube 1614 is
inserted
into the handle 1601 and mated to a second hemo-port (1629 in Fig. 18) in
order to
allow fluid purging between internal catheters, and details of this insertion
will be
described below. Finally, a pin lock 1608 provides a security measure against
premature release of a prosthetic mitral valve, by acting as a physical
barrier to
translation between internal mechanisms. Pin lock prongs 1615 rely on spring
force to
retain the pin lock 1608 in the handle 1601, and a user must first pull out
the pin lock
1608 before final deployment of a prosthetic valve.
[0117] Fig. 17 also shows how the handle 1601 is fastened together by use of
threaded fasteners and nuts (1607 and 1639 of Fig. 18 respectively), and
countersunk
locator holes 1609 placed throughout the handle length.
[0118] Internal mechanisms of the delivery system are illustrated in detail in
Fig. 18,
and the following descriptions will reveal the interactions between individual
components, and the manner in which those components combine in order to
create a
system that is able to deliver a prosthetic mitral valve preferably
transapically.
[0119] As seen in Fig. 18, the flexible sheath 1602 is comprised of four
concentrically
nested catheters. In order from smallest to largest in diameter, the
concentrically
33
CA 3043737 2019-05-16
nested catheters will be described in detail. The innermost catheter is a
guide-wire
catheter 1621 that runs internally throughout the entire delivery system,
beginning at
the tip 1603 and terminating in the female threaded luer adaptor 1612. The
guide-wire
catheter 1621 is composed of a lower durometer, single lumen Pebax extrusion
and is
stationary. It provides a channel through which a guide-wire (not shown) can
communicate with the delivery system. The next catheter is the hub catheter
1622
which provides support for the hub 1620 and is generally comprised of a higher
durometer, single lumen PEEK extrusion. The hub catheter 1622 is in mating
connection with both the hub 1622 at the distal end, and a stainless steel
support rod
1634 at the proximal end. The stainless steel support rod 1634 is held fixed
by virtue
of a stopper 1637 that is encased in the handle 1601. The hub catheter 1622 is
stationary, and provides support and axial rigidity to the concentrically
nested
catheters. The next catheter is the bell catheter 1624, which provides housing
to the
hub 1620 and is generally comprised of a medium durometer, single lumen Pebax
extrusion, including internal steel braiding and lubricious liner, as well as
a radiopaque
marker band (not shown). The bell catheter 1624 translates axially, and can be
advanced and retracted with respect to the hub 1620. The bell catheter 1624 is
in
mating connection with the second hemo-port 1629 at the proximal end, and
hemostasis between the bell catheter 1624 and the stainless steel support rod
1634
can be achieved by purging the second hemostasis tube 1614. The bell catheter
1624
is bumped up to a larger diameter 1623 on the distal end in order to
encapsulate the
hub 1620. The outermost and final catheter is the sheath catheter 1604 which
provides housing for a prosthetic mitral valve (not shown), and which is able
to
penetrate the apex of the heart (not shown), by supporting and directing a tip
1603 and
assisting in the dilation of an incision in the heart wall muscle. The sheath
catheter
1604 is generally comprised of a medium durometer, single lumen Pebax
extrusion,
including internal steel braiding and lubricious liner, as well as radiopaque
marker
band (not shown). The sheath catheter 1604 translates axially, and can be
advanced
and retracted with respect to the hub 1620. The sheath catheter 1604 is in
mating
connection with the first hemo-port 1625 at the proximal end, and hemostasis
between
34
CA 3043737 2019-05-16
the sheath catheter 1604 and the bell catheter 1624 can be achieved by purging
the
first hemostasis tube 1617.
[0120] As seen in Fig. 18, the proximal end of the sheath catheter 1604 is in
mating
contact with a first hemo-port 1625. The first hemo-port is in mating contact
with a
threaded insert 1627, and an o-ring 1638, which is entrapped between the first
hemo-
port 1625 and the threaded insert 1627 in order to compress against the bell
catheter
1624, creating a hemostatic seal. As the thumbwheel 1616 is rotated, the screw
insert
1627 will translate, and the sheath catheter 1624 can be retracted or advanced
by
virtue of attachment. In order to provide adequate stiffness to dilate heart
wall tissue,
.. the distal edge of the sheath catheter 1604 will abut against a shoulder
1618 located
on the tip 1603. This communication allows the tip 1603 to remain secure and
aligned
with the sheath catheter 1604 during delivery, and creates piercing stiffness.
[01211 Fig. 18 also details the mechanism through which the bell catheter 1624
can
be retracted or advanced with respect to the hub 1620. The thumbwheel 1616 can
be
rotated to such an extent that the screw insert 1627 will be brought into
contact with
two pins 1628 that are press fit into the second hemo-port 1629. As the bell
catheter
1624 is in mating contact with the second hemo-port 1629, further rotation of
the
thumbwheel 1616 will cause the second hemo-port 1629 to translate and press
against a spring 1633 by virtue of connection to a second hemo-port cap 1632.
This
advancement will cause the bumped larger diameter section 1623 of the bell
catheter
1624 to be retracted from the hub 1620. As the thumbwheel 1616 is rotated in
the
opposite direction, restoring force produced by the spring 1633 will cause the
second
hemo-port 1629 to be pushed in the opposite direction, drawing the bumped
larger
diameter section 1623 of the bell catheter 1624 back over the hub 1620, an
action that
is necessary during the initial loading of a valve prosthesis.
[0122] Fig. 18 further details the manner in which hemostasis is achieved
between
the stainless steel support rod 1634 and the bell catheter 1624. An o-ring
1631 is
compressed between the second hemo-port 1629 and the second hemo-port cap
1632, creating a seal against the stainless steel support rod 1634. Hemostasis
between the bell catheter 1624 and the stainless steel support rod 1634 can be
CA 3043737 2019-05-16
achieved by purging the second hemostasis tube 1614, which is in communication
with the void to be purged through a slot and hole 1630.
[0123] The deployment process and actions necessary to activate the mechanisms
responsible for deployment are detailed in Figs. 19A-19B. When performed in
the
reverse order, these actions also necessitate the first loading of a valve
(not shown)
prior to surgery.
[0124] As seen in Fig. 19A, manipulation of the thumbwheel 1616 will provide
translational control of the sheath catheter 1604. In order to effect the
deployment of a
heart valve (not shown), the user must withdraw the sheath catheter 1604 from
contact
with the shoulder 1618 of the tip 1603 until it passes the larger diameter
section 1623
of the bell catheter 1624. A heart valve (not shown) will reside
concentrically above
the guide-wire catheter 1621 in the position indicated by the leader for 1621
in Fig.
19A, similarly as to the embodiment illustrated in Fig. 13. The sheath
catheter 1604
can be withdrawn until the screw insert 1627 comes into contact with the pin
lock
1608. The pin lock 1608 must then be removed before further travel of the
screw
insert 1627 can be achieved.
[0125] As seen in Fig. 19B, the pin lock 1608 is removed from the handle 1601
in
order to allow further translation of the sheath catheter 1604. When the
sheath
catheter 1604 is fully retracted, the larger diameter section 1623 of the bell
catheter
1624 is also fully retracted, which completely frees the heart valve (not
shown) from
the delivery system. Three hub slots 1619, spaced circumferentially at 120
from each
other provide the anchoring mechanism and physical link between delivery
system and
heart valve. Once the larger diameter section 1623 of the bell catheter 1624
has been
withdrawn, the hub slots 1619 become uncovered which allows the heart valve
anchor
(not shown) to fully expand.
[0126] Fig. 20 illustrates a distal portion of the delivery device in Fig. 16.
Three hub
slots 1619 are slidably disposed distally relative to the large diameter tip
1623 of bell
catheter 1624. These slots allow engagement with a prosthetic valve. The valve
may
be releasably held by the slots by disposing the commissure tabs or tabs 812
of the
prosthetic valve into slots 1619 and then retracting the slots 1619 under tip
1623 of
bell catheter 1624. The prosthetic valve may be released from the delivery
catheter by
36
CA 3043737 2019-05-16
advancing the slots distally relative to the bell catheter so that the loading
anchors
or tabs 812 may self-expand out of and away from slots 1619 when the
constraint
of tip 1623 on bell catheter 1624 has been removed.
[0127] Fig. 21 illustrates a prosthetic mitral valve 800 (as discussed above
with reference to Fig. 8A) with the anchor tabs 812 disposed in the hub slots
(not
visible), and bell catheter 1623 advanced thereover. Thus, even though most of
the
prosthetic valve 800 has self-expanded into its expanded configuration, the
valve
commissures remain in a collapsed configuration with the tabs 812 captured in
slots 1619. Once the constraint provided by bell catheter 1623 has been
removed
from the slots 1619, the tabs 812 may self-expand out of slots 1619, the
commissures will open up to their unbiased position. The prosthetic valve is
then
disconnected and free from the delivery device.
[0128] Transapical Delivery Methods. Figs. 22A-22G illustrate an exemplary
method of transapically delivering a prosthetic mitral valve. This embodiment
may
use any of the prosthetic valves described herein, and may use any of the
delivery
devices described herein. Fig. 22A illustrates the general transapical pathway
that
is taken with entry into the heart at the apex 2202, through the left
ventricle 2204,
across the mitral valve 2206 and into the left atrium 2208. The aortic valve
2210
remains unaffected. Transapical delivery methods have been described in the
patent and scientific literature, such as in International PCT Publication No.
W02009/134701.
[0129] In Fig. 22B a delivery device 2214 is introduced through an incision in
the
apex 2202 and over a guidewire GW through the ventricle 2204, past the mitral
valve 2206 with a distal portion of the delivery device 2214 disposed in the
atrium
2208. The delivery device has a rounded tip 2212 that is configured to pass
through and dilate the incision, and can be advanced through the heart without
causing unwanted trauma to the mitral valve 2206 or adjacent tissue. Suture
2216
may be stitched around the delivery device 2214 at the apex 2202 using a purse
string stitch or other patterns known in the art in order to prevent excessive
bleeding and to help hold the delivery device in position.
37
CA 3043737 2019-05-16
[0130] In Fig. 22C, the outer sheath 2214a of the delivery device 2214 is
retracted
proximally relative to the prosthetic mitral valve 2220 (or the prosthetic
mitral valve is
advanced distally relative to the outer sheath 2214a) to expose the alignment
element
2218 and a portion of the atrial skirt region 2222 on the prosthetic mitral
valve 2220
which allows the atrial skirt region 2222 to begin to partially radially
expand outward
and flare open. Alignment element 2218 may include a pair of radiopaque
markers
2218a which facilitate visualization under fluoroscopy. The physician can then
align
the alignment element so that the radiopaque markers 2218a are disposed on
either
side of the anterior mitral valve leaflet. Delivery device 2214 may be rotated
in order
to help align the alignment element. The alignment element is preferably
situated
adjacent the aortic root and between the fibrous trigones of the native
anterior leaflet.
[0131] In Fig. 22D once alignment has been obtained, the sheath 2214a is
further
retracted proximally, allowing radial expansion of the atrial skirt 2222 which
flares
outward to form a flange. Proximal retraction of the delivery device 2214 and
prosthetic valve 2220 seat the atrial skirt 2222 against an atrial surface
adjacent the
mitral valve 2206 thereby anchoring the prosthetic valve in a first position.
[0132] Fig. 22E shows that further proximal retraction of sheath 2214a exposes
and
axially removes additional constraint from the prosthetic valve 2220, thereby
allowing
more of the valve to self-expand. The annular region 2224 expands into
engagement
with the mitrel valve annulus and the ventricular trigonal tabs 2226 and the
posterior
tab 2228 radially expand. Portions of the ventricular skirt serve as
deployment control
regions and prevent the entire ventricular skirt from expanding because they
are still
constrained. The tabs are captured between the anterior and posterior mitrel
valve
leaflets and the ventricular wall. The posterior ventricular anchoring tab
2228 is
preferably aligned in the middle of the posterior mitral valve leaflet where
there is an
absence of chordae attachments, and is passed over the posterior leaflet to
seat
between the posterior leaflet and the ventricular wall. The two ventricular
trigonal
anchoring tabs 2226 are positioned on either side of the anterior leaflet with
their
heads positioned at the fibrous trigones. Slight rotation and realignment of
the
prosthesis can occur at this time. As the prosthesis expands, the anterior
trigonal tabs
anchor against the fibrous trigones, capturing the native anterior leaflet and
chordae
38
CA 3043737 2019-05-16
between the tabs and the anterior surface of the prosthetic valve, and the
posterior
ventricular tab anchors between the ventricular wall and the posterior
leaflet,
capturing the posterior leaflet between the posterior anchoring tab and the
posterior
surface of the prosthetic valve assembly.
[0133] Fig. 22F shows that further retraction of sheath 2214a releases the
ventricular
trigonal tabs and the posterior tab and the deployment control regions of the
ventricular skirt 2230 are also released and allowed to radially expand
outward
against the native mitral valve leaflets. This creates a sealing funnel within
the native
leaflets and helps direct blood flow through the prosthetic mitral valve. With
the
commissures of the prosthesis still captured within the delivery system, very
minor
adjustments may still be made to ensure accurate positioning, anchoring and
sealing.
The prosthetic valve is now anchored in four positions. The anchor tabs 2232
are then
released from the delivery device by retraction of an inner shaft, allowing
the tabs to
self-expand out of slots on the delivery catheter as previously discussed
above and
shown in Fig. 22G.
The prosthetic valve is now implanted in the patient's heart and takes over
the native
mitral valve. The delivery device 2214 may then be removed from the heart by
proximally retracting it and removing it from the apex incision. The suture
2216 may
then be tied off, sealing the puncture site.
[0134] Transseptal Delivery Methods. Figs. 23A-23G illustrate an exemplary
method
of transseptally delivering a prosthetic mitral valve. This embodiment may use
any of
the prosthetic valves described herein, and may use any of the delivery
devices
described herein if modified appropriately. One of skill in the art will
appreciate that
relative motion of the various shafts in the delivery system embodiments
disclosed
above may need to be reversed in order to accommodate a transseptal approach.
Fig.
23A illustrates the general transseptal pathway that is taken with the
delivery device
passing up the vena cava 2302 into the right atrium 2304. A transseptal
puncture
2306 is created through the atrial septum, often through the foramen ovate, so
that
the device may be passed into the left atrium 2308, above the mitral valve
2310 and
adjacent the left ventricle 2312. Transseptal techniques have been published
in the
patent and scientific literature, such as in U.S. Patent Publication No.
2004/0181238
to Zarbatany et al.
39
CA 3043737 2019-05-16
[0135] In Fig. 23B a delivery device 2314 is passed over a guidewire GW
through the
vena cava 2302 into the right atrium 2306. The delivery device 2314 is then
transseptally passed through the atrial wall into the left atrium 2308
adjacent the mitral
valve 2310. The guidewire GW may be disposed across the mitral valve 2310 in
the
left ventricle 2312. The distal tip of the delivery device typically includes
a nose cone
or other atraumatic tip to prevent damaging the mitral valve or adjacent
tissue.
[0136] In Fig. 23C, the outer sheath 2214a of the delivery device 2214 is
retracted
proximally relative to the prosthetic mitral valve 2319. Alternatively, a
distal portion
2314b of the delivery device 2214 may be advanced distally relative to the
prosthetic
valve 2319 to expose the alignment element 2316 and a portion of the atrial
skirt
region 2318 on the prosthetic mitral valve 2319 which allows the atrial skirt
region
2318 to begin to partially radially expand outward and flare open. Alignment
element
2316 may include a pair of radiopaque markers 2316a which facilitate
visualization
under fluoroscopy. The physician can then align the alignment element so that
the
radiopaque markers 2316a are disposed on either side of the anterior mitral
valve
leaflet. The alignment element is preferably situated adjacent the aortic root
and
between the fibrous trigones of the native anterior leaflet. Delivery device
2214 may
be rotated in order to help align the alignment element.
[0137] In Fig. 23D once alignment has been obtained, the distal portion 2314b
is
further advanced distally allowing radial expansion of the atrial skirt 2318
which flares
outward to form a flange. Distally advancing the delivery device 2214 and
prosthetic
valve 2319 seats the atrial skirt 2318 against an atrial surface adjacent the
mitral valve
2310 thereby anchoring the prosthetic valve in a first position.
[0138] Fig. 23E shows that further distal advancement of distal portion 2314b
exposes and axially removes additional constraint from the prosthetic valve
2319,
thereby allowing more of the valve to self-expand. The annular region 2320
expands
into engagement with the mitral valve annulus and the ventricular trigonal
tabs 2324
and the posterior tab 2322 radially expand. Portions of the ventricular skirt
serve as
deployment control regions since they remain constrained and thus the entire
ventricular skirt cannot expand. The tabs are captured between the anterior
and
posterior mitral valve leaflets and the ventricular wall. The posterior
ventricular
CA 3043737 2019-05-16
anchoring tab 2322 is preferably aligned in the middle of the posterior mitral
valve
leaflet where there is an absence of chordae attachments, and is passed over
the
posterior leaflet to seat between the posterior leaflet and the ventricular
wall. The two
ventricular trigonal anchoring tabs 2324 are positioned on either side of the
anterior
leaflet with their heads positioned at the fibrous trigones. Slight rotation
and
realignment of the prosthesis can occur at this time. As the prosthesis
expands, the
anterior trigonal tabs anchor against the fibrous trigones, capturing the
native anterior
leaflet and chordae between the tabs and the anterior surface of the
prosthetic valve,
and the posterior ventricular tab anchors between the ventricular wall and the
posterior
leaflet, capturing the posterior leaflet between the posterior anchoring tab
and the
posterior surface of the prosthetic valve assembly.
[0139] Fig. 23F shows that further distal advancement of distal portion 2314b
releases the ventricular trigonal tabs and the posterior tab and the
ventricular skirt
2326 is also released and allowed to radially expand outward against the
native mitral
valve leaflets without engaging the ventricular wall. This creates a sealing
funnel
within the native leaflets and helps funnel blood flow through the prosthetic
valve.
With the commissures of the prosthetic valve still captured by the delivery
system, very
minor adjustments may still be made to ensure accurate positioning, anchoring
and
sealing. The prosthetic valve is now anchored in four positions. The anchor
tabs 2328
are then released from the delivery device by further advancement of an inner
shaft,
allowing the tabs to self-expand out of slots on the delivery catheter as
previously
discussed above and shown in Fig. 23G. The prosthetic valve is now implanted
in the
patient's heart and takes over the native mitral valve. The delivery device
2314 may
then be removed from the heart by proximally retracting it back through the
atrial
septum, and out of the vena cava.
[0140] Fig. 24 shows the prosthetic valve 2418 anchored in the mitral space
after
transapical or transseptal delivery. Prosthetic valve 2418 is preferably the
prosthetic
mitral valve illustrated in Fig. 8A, and delivered by methods shown in Figs.
22A-220 or
Figs. 23A-23G. The prosthetic valve 2418 has radially self-expanded into
engagement
with the mitral valve to anchor it in position without obstructing other
portions of the
heart including the left ventricular outflow tract such as aortic valve 2402.
The anterior
41
CA 3043737 2019-05-16
trigonal tabs 2408 (only 1 seen in this view) and the posterior ventricular
tab 2405 are
radially expanded outward from the rest of the ventricular skirt 2410 and the
anterior
leaflet 2406 and posterior leaflet 2404 are captured between the respective
tab and
the ventricular skirt 2410 to form an anchor point. The ventricular skirt 2410
is also
radially expanded outward to engage and press outwardly at least some of the
chordae tendineae and papillary muscles but preferably without pressing
against the
ventricular wall. The annular region 2416 is expanded radially outward to
engage and
press against the mitral valve annulus, and the atrial skirt 2414 has also
expanded
outwardly to form a flange that rests on top of the mitral valve against the
atrium.
Thus, the prosthetic valve 2418 is anchored in four positions in the mitral
space which
prevents the prosthetic valve from migrating or dislodging during contraction
of the
heart. Moreover, using four anchor points lessens the anchoring pressure that
is
required to be applied in any given anchoring zone as compared to a prosthesis
that is
anchored in only a single anchoring zone, or in any combination of these four
anchoring zones. The consequent reduction in radial force required to be
exerted
against the native structures in each zone minimizes the risk of obstruction
or
impingement of the nearby aortic valve or aortic root caused by the
displacement of
the native mitral valve apparatus. Valve leaflets 2420 form a tricuspid valve
which
opens with antegrade blood flow and closes with retrograde blood flow. Tab
2412 on
a tip of the commissures 2421 (best seen in Fig. 25) remains free after
disengagement
from the delivery device.
[0141] Fig. 25 illustrates the prosthetic valve 2418 of Fig. 24 anchored in
the mitral
space and viewed from the left ventricle, looking upward toward the atrium. As
previously mentioned, the prosthetic valve 2418 may be transapically or
transseptally
delivered and is preferably the prosthetic mitral valve illustrated in Fig.
8A, delivered by
methods shown in Figs. 22A-22G or Figs. 23A-23G. This view more clearly
illustrates
anchoring and engagement of the prosthetic mitral valve 2418 with the adjacent
tissue.
For example, the three valve leaflets 2420 forming the tricuspid valve are
shown in the
open position, allowing blood flow therepast. Additionally, the anterior
trigonal tabs
2408 and the posterior ventricular tab 2405 are shown radially expanded
outward into
engagement with the ventricular heart tissue 2425. The anterior portion of the
42
CA 3043737 2019-05-16
prosthetic valve in between anterior trigonal tabs 2408 is approximately flat
to match
the corresponding flat anatomy as previously discussed above. The flat shape
of the
anterior portion of the prosthetic valve prevents the prosthetic valve from
impinging on
and obstructing adjacent anatomy such as the left ventricular outflow tract
including
the aortic valve. Fig. 25 also illustrates how the ventricular skirt 2410
expands radially
outward against the native mitral valve leaflets.
[0142] Drug Delivery. Any of the prosthetic valves may also be used as a drug
delivery device for localized drug elution. The therapeutic agent may be a
coated on
the prosthetic valve, on the tissue covering the anchor, on both, or otherwise
carried
by the prosthetic valve and controllably eluted therefrom after implantation.
Exemplary
drugs include anti-calcification drugs, antibiotics, anti-platelet aggregation
drugs, anti-
inflammatory drugs, drugs which inhibit tissue rejection, anti-restenosis
drugs, anti-
thrombogenic drugs, thrombolytic drugs, etc. Drugs which have these
therapeutic
effects are well known to those of skill in the art.
[0143] Although the exemplary embodiments have been described in some detail
for
clarity of understanding and by way of example, a variety of additional
modifications,
adaptations and changes may be clear to those of skill in the art. One of
skill in the art
will appreciate that the various features described herein may be combined
with one
another or substituted with one another. Hence, the scope of the present
invention is
limited solely by the appended claims.
43
CA 3043737 2019-05-16