Note: Descriptions are shown in the official language in which they were submitted.
CA 03046545 2019-06-10
WO 2018/112510 PCT/AU2017/051344
METAL-AIR FUEL CELL
FIELD
[0001] The present invention relates to a metal-air fuel cell and uses thereof
including use
as a long-life, mechanically rechargeable, direct-current power source for
devices and
products.
BACKGROUND
[0002] The reference in this specification to any prior publication, or
information derived from
it, or to any matter which is known, is not, and should not be taken as an
acknowledgement
or admission or any form of suggestion that the prior publication, or
information derived from
it, or known matter forms part of the common general knowledge in the field of
endeavour to
which this specification relates.
[0003] Many products, in particular household and portable devices are
designed to be
powered by batteries, such as AA-cell, C-cell and D-cell batteries.
Disadvantages when
using such traditional batteries include: relatively short operational
lifetime and limited shelf-
life i.e. expiry due to degradation of the internal (closed system) components
over time even
when not in use. These devices are therefore also alternatively powered by
other sources,
including solar power or kerosene.
[0004] Disadvantages of using kerosene as an alternative power source include:
high
monthly costs; environmental pollutant (millions of tonnes of CO2 and black
carbon released
into the atmosphere contributing to global warming); adverse impact on health
(e.g. lungs,
eyes, skin and general wellbeing); potential fire hazard (due to
flammability); safe storage
and regular purchase issues; not suitable as a power source for some products
and devices
(such as emergency beacons, radios, communications equipment and recharging
docks for
USB devices); and even the potential for poisoning caused by accidental
drinking due to
confusion as a beverage.
[0005] Disadvantages of using solar energy as an alternative power source
include:
variability in amount and duration of sunshine (particularly during winter);
impact of rain
(which can reduce solar potential to near zero during tropical rainy /
monsoonal season);
cloudy conditions and fog can reduce power generation (by approximately 10-
80%);
shadows and haze can also reduce the effectiveness of solar power; impact of
the sun's
latitude (angle of the sun) and need to adjust position of solar capture
device for effective
capture; must be located outdoors to capture sunshine leaving them at risk of
damage from
1
CA 03046545 2019-06-10
WO 2018/112510 PCT/AU2017/051344
external elements as well as theft; and limitations of solar devices
themselves in that they
are not rechargeable so must be disposed of at the end of their life.
[0006] Metal-air fuel cells, such as magnesium-air fuel cells can also be used
as an
alternative to traditional batteries. Metal-air fuel cells are considered to
offer certain
advantages including: high energy density; low price; and long storage
potential.
[0007] Generally speaking, metal-air fuel cells operate by suspension in an
ionic aqueous
solution, such as sea water or other saline solutions, which acts as the
electrolyte between
the air cathode and anode. The air cathode is exposed to oxygen to allow the
electrochemical reaction to occur. By-products of this electrochemical
reaction include:
a) release of hydrogen gas (and minute amounts of chlorine gas); and
b) waste materials from anode degradation (e.g. metal hydroxides).
[0008] Metal-air fuel cell technology is not without its disadvantages,
including: leakage of
the electrolyte from the cell; exposure of the electrodes to excess
electrolyte causing
performance interference; sealing problems; gas (e.g. hydrogen and chlorine)
build-up and
venting issues; dangerous temperature and pressure build up caused by runaway
exothermic redox reactions, waste management issues associated with anode
degradation
(e.g. impaired cathode life caused waste material accumulation within the fuel
cell in the
absence of regular cleaning and electrolyte replacement).
[0009] Magnesium-air fuel cells have a typical lifespan of 50 to 100 hours
before requiring
replacement of the anode. Performance of the air cathode also often diminishes
very rapidly
after only 100 to 200 hours of use, or even during storage after initial use.
Some metal-air
fuel cells require stringent regular maintenance and cleaning activities by
the user in order to
maximize air cathode life.
[0010] Typical metal-air fuel cell configurations are exemplified by: US
Patent Number
3,519,486 (7 July 1970), Huebscher, R.G. et. al.; and US Patent Number
3,963,519 (15 June
1976), Louie, H.P.
[0011] US3,519,486 describes a trapped electrolyte fuel cell that includes
internal
reservoir(s)/chamber(s) in the bottom of the cell to capture excess
electrolyte. The captured
excess electrolyte forms electrolyte pool(s) in which the electrodes and a
matrix are
positioned. The matrix is made of a material resistant to potassium hydroxide,
such as a
fibrous asbestos matting (column 2, lines 4-5). The cell must be sealed to
prevent leakage
of the electrolyte. Further, as the reservoir(s) are positioned in the bottom
of the cell, the cell
2
CA 03046545 2019-06-10
WO 2018/112510 PCT/AU2017/051344
must be positioned in an upright orientation to ensure electrolyte pooling and
operation of
the cell.
[0012] U53,963,519 describes another trapped electrolyte fuel cell with a
protective shield
spacer. The spacer provides structural strength to the cell and protects the
cathode while
permitting air to pass over the entire surface of the cathode. This design was
considered an
advance over earlier heavy-framed metal/air battery constructions that were
considered
unsuitable for use as primary and secondary lightweight metal-air cells of AA,
C and D cell
configurations. A liquid-tight configuration to internally seal the
electrolyte is described.
[0013] Neither U53,519,486 nor U53,963,519 describes a process for removing or
isolating
accumulated anode-degradation waste and/or venting by-products to alleviate
pressure
build-up.
[0014] Development of metal-air fuel cell technologies is ongoing. For example
Aqua Power
System, Japan is presently seeking to advance metal-air fuel cell technology
as described in
at least the following three PCT patent applications and marketed as their
"Realistic
Magnesium Air Fuel" (RMAF) system technology
(http://aquapowersystems.com/technology/how-aqua-powers-technology-works/,
website
accessed 19 December 2016).
[0015] W02014/097909 (Aqua Power System, Japan; also published as
U52015/0340704
Al), discloses a metal-air fuel cell with a layered cathode body including
water repellent and
electrically conductive carbon material(s). The resulting fuel cell is
described as highly water
repellent, air permeable, and leakage resistant.
[0016] W02014/115880 (Aqua Power System, Japan; also published as
US2015/0364800
Al), provides a magnesium-air fuel cell with a comparatively shorter distance
between the
anode and the cathode to improve the electrochemical reaction. The height and
width of the
fuel cell, relative positioning of the anode and cathode, and use of a water
supply pipe
further including a reaction gas discharge pipe is said to generate a stable
supply of power
for a relatively long period of time. However, as the inlet to the reaction
gas discharge pipe
may be located within the cell, the reaction gas discharge pipe may
undesirably leak
electrolyte and/or gas.
[0017] W02014/115879 (Aqua Power System, Japan; also published as
US2015/0380693
Al), discloses a magnesium-air fuel cell that can be turned on and off by
virtue of a lid, that
when fastened brings the terminals into contact to switch the power 'on' and
when loosened,
turns the power 'off.'
3
CA 03046545 2019-06-10
WO 2018/112510 PCT/AU2017/051344
[0018] RMAF technology is understood to be incorporated into a number of
commercial
products including a water-activated 1.5V AA battery
(http://aquapowersystems.com/products/batteries/, website accessed 19 December
2016)
However, as disclosed in the website, the Aqua Power battery is configured as
a fixed-sized,
closed system which requires manual introduction of the electrolyte via a
small, hand-
operated pipette.
[0019] Fluidic, Inc., (US) is another company presently seeking to advance
metal-air fuel cell
technology. The Fluidic, Inc., platform technology is understood to be
incorporated into the
first commercialised rechargeable zinc-air battery
(http://fluidicenergy.com/technology/,
website accessed 19 December 2016).
[0020] Fluidic, Inc., describe various advances in metal-air fuel cell
technology including, for
example: use of a dopant to increase the conductivity of the metal fuel
oxidation product, i.e.
the anode is doped degenerately (W02014/062385, Fluidic, Inc.); use of
additives in the
ionically conductive medium to enhance electrodeposition and/or extending the
cell's
capacity (W02014/160144, Fluidic, Inc.); hetero-ionic aromatic additives
(W02014/160087,
Fluidic, Inc.); additives comprising poly(ethylene glycol) tetrahydrofurfuryl;
and control of the
concentration of additives in the ionic conductive medium (W02016/123113 and
W02012/030723, Fluidic, Inc.). Other claimed advances resulting from design
modifications
include: to accommodate a gaseous oxidant receiving space (W02013/066828,
Fluidic.
Inc.); a catch tray containing a catalyst material to catalyse the oxidation
of waste
particulates (W02012/012364, Fluidic, Inc.); an anode having a scaffolding
structure
(W02011/163553, Fluidic, Inc.); a fuel cell having a plurality of electrodes
(W02011/130178
and W02012/037026, Fluidic, Inc. respectively) and multiple fuel cell systems
(W02011/035176, W02012/106369 and W02010/065890, Fluidic, Inc. respectively).
[0021] In general, the Fluidic, Inc. metal-air fuel cell technology is akin to
conventional
rechargeable batteries in that the process is reversible because the anode is
not consumed
and further that the anode is "doped" or coated to stop it from degrading.
[0022] Despite numerous advances in metal-air fuel cell technology, there
remains an
ongoing need to overcome certain disadvantages associated with the technology
and to
provide new sources of direct current power particularly in the form of
batteries, for use in
devices and products which are affordable, accessible, environmentally
friendly (re-usable,
recyclable), have a long-life (shelf and/or operation), are reliable and safe.
4
CA 03046545 2019-06-10
PCT/AU2017/051344
Received 20/12/2018
SUMMARY
[0023] Throughout this specification and the claims which follow, unless the
context requires
otherwise, the word "comprise" and variations thereof such as "comprises" and
"comprising",
will be understood to include the inclusion of a stated integer or step or
group of integers or
steps but not the exclusion of any other integer or step or groups of integers
or steps.
[0024] The present invention provides a metal-air fuel cell comprising:
(a) an anode;
(b) a positionable air cathode;
(c) an absorbent material layer adapted to retain electrolyte, the
absorbent material layer
positioned intermediate the anode and the air cathode such that it contacts
the anode;
and
(d) an elastic air cathode positioning means adapted to position the air
cathode to ensure
that the air cathode remains in contact with the absorbent material layer
while
accommodating any change in volume of the absorbent material layer;
wherein the absorbent material layer functions as an ionic transfer bridge
between the anode
and the cathode by retaining electrolyte.
[0025] Preferably the anode, the absorbent material layer and the air cathode
are coaxially
arranged such that the air cathode substantially surrounds the absorbent
material layer and
the absorbent material layer substantially surrounds the anode.
[0026] Preferably the anode, the absorbent material layer and the air cathode
are provided
in a laminate or 'sandwich-layered' arrangement such that, for example, the
air cathode
overlies the absorbent material layer which in turn overlies the anode.
[0027] Preferably the elastic air cathode positioning means is positioned
around a cross-
sectional perimeter of the metal-air fuel cell.
[0028] Preferably the elastic air cathode positioning means is either
incorporated within or
provided separate to the air cathode, and is selected from: an 0-ring, a
deformable
polymeric material, an elastic (or rubber) band or an expandable mesh.
[0029] Preferably the metal-air fuel cell is contained within an open housing
unit.
[0030] Preferably the metal-air fuel cell is activated or re-activated for use
by allowing the
absorbent material layer to retain electrolyte (e.g. by dipping the metal-air
fuel cell in a
liquid).
AMENDED SHEET
IPEA/AU
CA 03046545 2019-06-10
WO 2018/112510 PCT/AU2017/051344
[0031] Preferably the absorbent material layer is pre-impregnated with ions to
form
electrolyte when the absorbent material layer retains water.
[0032] Preferably the absorbent material layer comprises a sub-layer of
absorbent material
that is pre-impregnated with ions, and a sub-layer of absorbent material that
is not pre-
impregnated with ions.
[0033] Preferably the absorbent material layer changes volume upon absorption
or depletion
of retained electrolyte, and/or capture of anode waste material.
[0034] Preferably the absorbent material layer comprises a woven or non-woven
fibrous
material or a combination thereof. It is further preferred that the absorbent
material layer
comprises fibrous cellulose, bamboo fibre or a combination thereof.
[0035] Preferably the anode comprises a magnesium alloy.
[0036] Preferably the air cathode comprises a sheet layer. It is further
preferred that the air
cathode is hydrophobic, air-permeable and comprises a layered Teflon material.
[0037] Preferably the metal-air fuel cell further comprises a paper separator
layer located
between the absorbent material layer and the air cathode to support and
contain the
absorbent material layer and/or further isolate and protect the cathode from
anode waste
precipitates.
[0038] In an embodiment the metal-air fuel cell is adapted and/or used to
provide a direct
current power source for use to power the operation of a product or device.
Preferably the
product or device is selected from the group consisting of: torches (including
flashlights,
mag-lights, pen lights); lights and lighting products or devices (including
globe lights, LED
lights, strobe lights and Christmas lights); safety or temporary lighting
applications (including
for road works); lanterns (including camping lanterns and Chinese lanterns);
combination
products (including flashlight-lantern combinations convertible between
operation as a
flashlight and as a lantern); household products (including electrical
toothbrushes and
shavers) emergency beacons (including EPIRB and directional finders); radios
(analogue
and digital); communications equipment (including radios, CB radios and small
audio
devices); toys (i.e. battery powered), power banks for rechargeable products
and recharging
docks for USB devices (small electronic products including mobile phones, i-
pods, i-pads).
6
CA 03046545 2019-06-10
WO 2018/112510
PCT/AU2017/051344
Definitions
[0039] Unless otherwise herein defined, the following terms will be understood
to have the
general meanings which follow.
[0040] "Air permeability" means, with respect to a material, one that is
capable or has the
ability to allow air to flow, diffuse or otherwise pass through it.
[0041] "Absorbent" means, with respect to a material, one that is capable or
has the ability
or tendency to soak up or absorb a fluid (liquid or gas), in particular, a
liquid.
[0042] "Activate" means, with respect to the metal-air fuel cell of the
invention, to make
ready (active or operative) for use i.e. to generate electricity through a
redox reaction of the
metal-air fuel cell.
[0043] "Contractible" means, with respect to a material or object, capable of
or adapted to
decrease in size and/or volume by shrinking or contracting.
[0044] "Dipping" means the process of placing or immersing something briefly
into a liquid.
[0045] "Dry storage" means to process of storing in dry conditions, that is,
in a low humidity
environment and devoid of atmospheric moisture.
[0046] "Elastic" means, with respect to a material or object, one that is
capable of or has the
ability to resume its original size and shape spontaneously after being
stretched or
compressed or otherwise deformed.
[0047] "Electrolyte" means a solution (liquid or gel, preferably liquid) that
comprises ions and
is capable of or has the ability to conduct electricity.
[0048] "Expandable" means, with respect to a material or object, one that is
capable of or
adapted to increase in size and/or volume by expansion.
[0049] "Hydrophobicity" means, with respect to a material, one that is capable
of or has the
ability to repel (as opposed to attract or absorb) water.
[0050] "Mechanically rechargeable" means, with respect to a fuel-cell or
battery, the
replacement of the consumed anode, for example, in the case of a magnesium
anode, the
magnesium material is a storage medium for the electrons that are released
during the
chemical reaction and the magnesium material is consumed in the process.
7
CA 03046545 2019-06-10
WO 2018/112510
PCT/AU2017/051344
[0051] "Pressure build-up" means, with respect to gas(es), the build-up of
pressure due to
gas(es) in a sealed or closed system.
[0052] "Shelf-life" means, with respect to a product, the period, length or
duration of time for
which the product remains usable including fitness for its original purpose.
[0053] "Venting" means, with respect to gas(es), the process of releasing
gas(es) from a
sealed or closed system including for example, via an outlet.
[0054] "Waste" means unwanted material or by-product(s) resulting from a
process, such as
for example in the case of a magnesium metal-air fuel cell, the magnesium
hydroxide and/or
gases such as hydrogen and chlorine that are produced from the electrochemical
reaction in
the cell when in use.
[0055] "Wicking" means the process of absorbing or drawing a liquid into or
through a
material by capillary action.
BRIEF DESCRIPTION OF THE FIGURES
[0056] The invention is further described with respect to the accompanying
figures which
illustrate preferred embodiments of a metal-air fuel cell according to the
present invention.
Other embodiments of the invention are possible, and consequently, the
particularity of the
accompanying drawings is not to be understood as superseding the generality of
the
preceding description of the invention.
[0057] FIGURE 1A: Shows a cross-sectional cutaway side view of a traditional
magnesium-
air (Mg02) fuel cell as known in the art.
[0058] FIGURE 1B: Shows a cross-sectional partial cutaway side view of a
magnesium-air
fuel cell according to an embodiment of the invention.
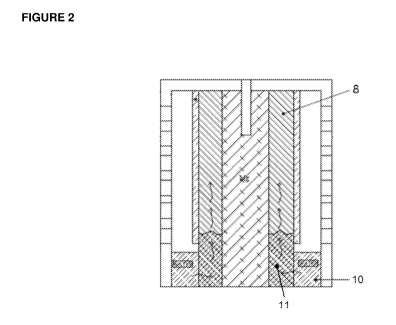
[0059] FIGURE 2: Shows a cross-sectional cutaway side view of the magnesium-
air fuel cell
of FIGURE 1B to illustrate the uptake (absorption) of the liquid electrolyte
(saline) by the
absorbent material layer when dipped in liquid electrolyte.
[0060] FIGURE 3A: Shows a cross-sectional cutaway side view of the traditional
Mg02 cell
of FIGURE lA to illustrate the cell's gas build-up (fuel-cell reaction by-
product) and venting
process when in use.
8
CA 03046545 2019-06-10
WO 2018/112510
PCT/AU2017/051344
[0061] FIGURE 3B: Shows a cross-sectional cutaway side view of the metal-air
fuel cell of
FIGURE 1B to illustrate the cell's gas build-up (fuel-cell reaction by-
product) and venting
process when in use.
[0062] FIGURE 4A: Shows a cross-sectional cutaway side view of the Mg02 cell
of FIGURE
1A, to illustrate build-up of corrosive magnesium anode waste precipitates
(fuel-cell reaction
by-product) on the cathode from use.
[0063] FIGURE 4B: Shows a cross-sectional cutaway side view of the metal-air
fuel cell of
FIGURE 1B, to illustrate prevention or reduction of corrosive magnesium anode
waste
precipitates (fuel-cell reaction by-product) build-up on the cathode from use.
[0064] FIGURE 5A: Shows a cross-sectional cutaway side view of the Mg02 cell
of FIGURE
1A, to illustrate the process of deposition and build-up of magnesium anode
waste
precipitates (fuel-cell reaction by-product) in the cell during use.
[0065] FIGURE 5B: Shows a cross-sectional cutaway side view of the metal-air
fuel cell of
FIGURE 1B to illustrate the capture or containment of magnesium anode waste by
the
absorption material in the cell during use.
[0066] FIGURE 6A: Shows a perspective view of a metal-air fuel cell according
to the
invention to illustrate the concentric layered (co-axial arrangement)
construction of the
internal anode rod, intermediate absorbent material layer, paper separator
layer and external
air cathode layer held or positioned in place with an elastic air cathode
positioning means
(such as an elastic 0-ring or mesh) to accommodate expansion (and contraction)
of the
absorbent material layer upon uptake/absorption (or depletion) of the adsorbed
liquid
electrolyte and/or collection of anode waste precipitates over time through
use.
[0067] FIGURE 6B: Shows a cross-sectional cutaway front view of the fuel cell
of Figure 6A
to illustrate the concentric layered (co-axial) construction before expansion
of the absorbent
material layer.
[0068] FIGURE 60: Shows a cross-sectional cutaway front view of the fuel cell
of Figure 6A
to illustrate the concentric layered (co-axial) construction after expansion
of the absorbent
material layer.
[0069] FIGURE 7: Shows an exploded view of a magnesium-air fuel cell according
to an
embodiment of the invention to illustrate its various components.
9
CA 03046545 2019-06-10
WO 2018/112510 PCT/AU2017/051344
[0070] FIGURE 8 shows a cross-sectional cutaway front view of a magnesium-air
fuel cell to
illustrate the absorbent material layer according to an embodiment of the
invention.
[0071] FIGURE 9: Presents the endurance (milliAmps) test results for prototype
Cells 1, 2
and 3 according to an embodiment of the invention over approximately 750 hours
(Example
1, Experiment 1).
[0072] FIGURE 10: Presents the comparative power output (milliAmps) test
results for a
traditional Mg02 cell and a prototype cell according to an embodiment of the
invention over
approximately 500 operating hours (Example 1, Experiment 2).
[0073] FIGURE 11: Presents the performance testing results (milliAmps) of
prototype Cells
1, 2 and 3 according to an embodiment of the invention over approximately 500
hours
(Example 1, Experiment 3).
DETAILED DESCRIPTION
[0074] FIGURE lA shows a traditional Mg02 cell as known in the art. The Mg02
cell
comprises a centrally positioned Mg anode (1) within a closed container (2)
containing
aqueous electrolyte (3) into which the anode is suspended. Air cathode (4) is
incorporated
into the outer wall of the container such that an redox reaction with the
outside atmosphere
can take place resulting in ionic exchanges occurring between the anode and
cathode via
the electrolyte.
[0075] As shown in FIGURE 3A, gas by-products generated by the Mg02 cell of
FIGURE lA
will build up in a void (12) within the closed system. These gas by-products
must be vented
(13) to the atmosphere through a vent hole (14) which allows the gas to vent
but prevents
electrolyte (3) leakage.
[0076] FIGURE 4A illustrates degradation of anode (16) of the Mg02 cell of
FIGURE 1A and
corresponding anode waste precipitates (e.g. magnesium hydroxide) build-up
(17) on the
cathode (4).
[0077] In contrast to FIGURE 1A, FIGURE 1B shows a metal-air fuel cell
according to an
embodiment of the invention in which a magnesium anode (5) is positioned
within an open
container (6) comprising one or more vents (7) and surrounded by an absorbent
material
layer (8) which in turn is surrounded by the air cathode (9).
CA 03046545 2019-06-10
WO 2018/112510
PCT/AU2017/051344
[0078] FIGURE 2 illustrates uptake of liquid electrolyte (10) by the metal-air
fuel cell of
FIGURE 1B. Electrolyte (10) is absorbed by the absorbent material layer (8)
via a wicking
action in the direction of arrows (11) when dipped into the electrolyte.
[0079] As shown in FIGURE 3B, the metal-air fuel cell of FIGURE 1B provides an
open
housing unit (6) with vents (7) that allow for gas exchange (including oxygen
intake) and
venting of the gas by-products in the direction of arrows (15).
[0080] While anode (5) is described as a magnesium anode, alternative metals,
alloys or
combinations of alloys to provide suitable anodes will generally be known to
those skilled in
the art. Suitable alternative metals include Li, Ca, Al, Zn and Fe.
Preferably, the anode
comprises a magnesium alloy such as "AZ31B" having the following composition:
Aluminium: 2.5 - 3.5
Copper: 0.05 max
Iron: 0.005 max
Magnesium: Balance
Manganese: 0.2 min
Nickel: 0.005 max
Silicon: 0.1 max
Zinc: 0.6 - 1.4
[0081] As the anode (5) may be adapted to be internally, preferably centrally,
located within
the metal-air fuel cell, the anode may generally be formed in the shape of a
rod or a cylinder
and can be formed through extrusion. Where the metal-air fuel cell is
alternatively
configured in a sandwich-layered (i.e. laminate) arrangement, the anode, the
absorbent
material layer and the air cathode may each be provided as a substantially
flat layer. This
configuration may be particularly desirable in replacing certain rectangular
battery shapes
such as existing 9V batteries.
[0082] Air cathodes (9) that may be suitable for use in the metal-air fuel
cell of the invention
will generally be known to those skilled in the art. Suitable properties of
the air cathode (9)
include hydrophobicity and air-permeability. Preferably the air cathode (9) is
in the form of a
sheet layer adapted to accommodate the change in volume of the absorbent
material layer
(8) upon expansion and contraction. Preferably the air cathode (9) is
hydrophobic and air-
permeable and comprises a layered Teflon material. Still more preferably the
air cathode (9)
may comprise a layered Teflon material, carbon and nickel plated wire.
11
CA 03046545 2019-06-10
WO 2018/112510
PCT/AU2017/051344
[0083] The absorbent material layer (8) may be a material with properties
making it suitable
for absorbing and retaining an absorbed amount of electrolyte. The absorbent
material layer
(8) essentially functions to transport the ions in the absorbed amount of
electrolyte between
the air cathode and the anode. It is therefore considered to act as an ionic
bridging system
(or ionic transfer bridge) which is required for operation of the cell.
[0084] The absorbent material layer (8) may be made from an absorbent material
that is
able to absorb and hold or retain electrolyte by a process of wicking,
drawing, capillary
action, or similar. The absorbent material may be selected based on its
possessing one or
more of, preferably all of the following properties:
= wicking and electrolyte retaining ability;
= ability to expand to accommodate an increase in volume due to the
absorption of the
liquid electrolyte and/or retention of the anode waste materials;
= ability to encapsulate solid particles so as to capture and/or retain the
solid waste;
= ability to function as an "ionic bridge"; and/or
= ability to allow for exchange or diffusion of gases (i.e. for oxygen gas
diffusion process
and release of gas by-products during operation of the cell).
[0085] The absorbent material layer (8) may be made from a combination of air-
permeable
water absorbing, hydrophilic and/or hydrophobic materials and be conductive or
non-conductive. Suitable materials may include woven or non-woven materials or
combinations thereof produced from microfiber, rayon, cotton, cotton wool,
hemp, wool,
hessian, natural fiberwood pulp, aerogel composites, bamboo fibre pulp and/or
any suitable
combination thereof. Preferably the absorbent material layer comprises fibrous
cellulose,
bamboo fibre pulp or a combination thereof.
[0086] The performance of the absorbent material layer (8) may be enhanced
with additives
such as, for example, the addition of sphagnum and polyacrylate as well as
other super
absorbent gels derived from petroleum which will be familiar to those in the
art.
[0087] The cell may be activated or re-activated for use when the absorbent
material layer
comprises an absorbed amount of electrolyte. The absorbent material layer (8)
may
comprise an absorbed amount of electrolyte following absorption of an
electrolyte or water
(when the absorbent material layer is pre-impregnated with ions). A preferred
alternative
arrangement of the absorbent material layer is set out and described in
relation to FIGURE
8, which will be subsequently described in further detail.
12
CA 03046545 2019-06-10
WO 2018/112510 PCT/AU2017/051344
[0088] The types of electrolyte that may be suitable for use in the metal-air
fuel cell of the
invention will be generally known to those in the art. Suitable examples may
include but are
not limited to an aqueous solution comprising ions such as NaCI (e.g. salt
water, sea water
and saline solutions), electrolytes (e.g. sports drinks), urine and alkaline
solutions (e.g. KOH)
and water (e.g. when the absorbent material is pre-impregnated with ions).
[0089] As shown in FIGURE 4B, anode waste precipitates (18) are captured
within the
absorbent material layer (8) of the metal-air fuel cell of FIGURE 1B and are
prevented from
making direct contact with the air cathode (9). This process is similarly
illustrated in
FIGURES 5A and 5B respectively, in which 5A illustrates anode waste
precipitates build-up
(17) on the cathode (4) of the Mg02 cell of FIGURE 1A, while FIGURE 5B
illustrates capture
of anode waste precipitates (18) within the absorbent material layer (8) of
the metal-air fuel
cell of FIGURE 1B.
[0090] FIGURE 6A presents an metal-air fuel cell according to the invention
which provides
a co-axial arrangement of internal anode rod (19), substantially surrounded by
absorbent
material layer (20), which is in turn substantially surrounded by a paper
separator layer
(20A). The paper separator layer (20A) is in turn substantially surrounded by
air cathode
layer (21). Air cathode layer (21) is positioned by an elastic air cathode
positioning means
(22), such as an elastic 0-ring or mesh, to retain contact with the absorbent
material layer
(20). The metal-air fuel cell is therefore able to accommodate expansion (and
contraction) of
the absorbent material layer upon uptake (or depletion) of the liquid
electrolyte and/or
collection of anode waste precipitates over time. In an alternative
arrangement, the elastic
air cathode positioning means could be incorporated into the air cathode layer
such as by
weaving elastic material into the air cathode.
[0091] A cross-sectional view of the fuel cell of FIGURE 6A is also presented
to illustrate the
absorbent material layer (20) before expansion (FIGURE 6B) and after expansion
(FIGURE
60). As shown in FIGURES 6B and 60, the air cathode layer (21) remains
positioned in
contact with the absorbent material layer (20), and paper separator layer
(20A), by virtue of
the elastic air cathode positioning means.
[0092] FIGURE 7 shows an exploded view of a metal air fuel cell according to
the invention
to illustrate some of its various components. Magnesium anode (28), absorbent
material
layer (29), paper separator layer (29A) and air cathode (30) are co-axially
arranged such that
the air cathode (30) surrounds the paper separator layer (29A), which
surrounds the
absorbent material layer (29), which in turn surrounds the magnesium anode
(28). Elastic
air cathode positioning means (27) ¨ formed of 0-rings in the present
embodiment ¨ ensure
13
CA 03046545 2019-06-10
WO 2018/112510 PCT/AU2017/051344
contact between the air cathode (30) and the absorbent material layer (29).
The co-axial
arrangement of electrodes is positioned within a vented housing (32). The
vented housing
(32) is closed at respective ends by a top lid (25) and a bottom lid (33),
each held in place by
a screw (24) secured to the anode (28). A contact ring (23) situated outside
the top lid (25)
provides a terminal from air cathode (30) and is connected to air cathode (30)
by contact tab
(31). Rubber or plastic 0-rings (27A) and plastic washers (26) seal each end
of the
electrode arrangement and any electrolyte retained therein from other
components to protect
against corrosion.
[0093] As demonstrated by FIGURE 7, components of the metal-air fuel cell may
be easily
replaced by unscrewing one of the screws (24) holding either the top lid (25)
or the bottom
lid (33) in place to access the arrangement of electrodes. The magnesium anode
(28) (and
air cathode (30)) may therefore easily be replaced, providing an energy source
that can be
mechanically-recharged in a simple manner.
[0094] FIGURE 8 shows a cutaway view of a metal-air fuel cell of the invention
to illustrate a
preferred embodiment of the absorbent material layer. The metal-air fuel cell
comprises in
sequence moving inward from the outer circumference: air cathode positioning
means 35, air
cathode 34, paper separator layer 36 to support and contain the absorbent
material layer,
and further isolate and protect the cathode from anode waste precipitates, pre-
impregnated
sub-layer 37 of the absorbent material layer (being pre-impregnated with
ions), non-
impregnated sub-layer 38 of the absorbent material layer (being not pre-
impregnated with
ions), and anode 39.
[0095] According to the preferred embodiment illustrated the configuration of
the absorbent
material allows for control of the dissolution of ions into the retained
electrolyte. This may in
turn allow for control of matters such as dissipation or concentration of heat
of solution
arising when ions are dissolved into solution. This configuration may also
allow control over
the composition of electrolyte within sub-layers of the absorbent material
layer particularly
where, for example, pre-impregnated ions have a slow dissolution rate. This
may in turn
allow control of reaction-rates and temperature within the absorbent material
layer and the
metal-air fuel cell, noting that the redox reaction between the cathode and
anode will
typically be exothermic. In this way, higher temperatures caused by runaway
redox
reactions may be contained within particular zones or sub-layers of the
absorbent material
layer (e.g. sub-layer 37 as provided in the embodiment shown in FIGURE 8) that
allow for
greater evaporative cooling and resultant reduction in electrolyte content to
restrict the
runaway redox reaction.
14
CA 03046545 2019-06-10
WO 2018/112510
PCT/AU2017/051344
[0096] As suggested by the drawings and preceding description, the metal-air
fuel cell of the
invention advantageously allows for expansion and contraction of the absorbent
material
layer upon absorption/release of electrolyte and/or capture of anode waste
material. Further,
potential advantages of the invention may include:
= the metal-air fuel cell may be simply and conveniently activated and re-
activated on
demand by dipping the absorbent material into electrolyte, and deactivated by
being
allowed to dry out between uses. This provides a metal-air fuel cell with a
"dormant"
mode in which components are not consumed, and the associated potential for a
long shelf-life without any appreciable or significant loss in performance
power of the
cell;
= by providing a novel wicking and retention system for the electrolyte,
the invention
that may allow for configurations avoiding bulky water vessels and requiring
less
electrolyte, thereby reducing the weight of the cell while eliminating the
potential for
electrolyte leakage caused by, for example, tipping the fuel-cell from an
upright
position;
= by providing the metal-air fuel cell in an open housing unit, the
invention may
overcome disadvantages present in closed metal-air fuel cell systems, such as
gas
pressure build up and effective sealing of electrolyte, while also improving
oxygen
intake and venting of by-products;
= convenient replacement and recycling of components. The anode, the
absorbent
material layer, and the air cathode may be simply and conveniently replaced
and
recycled to provide an environmentally-friendly mechanically rechargeable
device;
and/or
= the novel wicking and retention system for the electrolyte may allow for
increased
thermal control to prevent a runaway exothermic reaction. Metal-air fuel cells
work
by creating an exothermic redox reaction between the anode and the cathode. In
traditional metal-air fuel cells this creates the potential for runaway
exothermic
reactions, in which pressure and heat within the cell may rise to dangerous
levels.
The absorbent material layer of the invention allows for improved evaporation
and
venting of electrolyte as temperatures increase within the cell. This may in
turn
control runaway reactions by reducing available electrolyte through
evaporation,
thereby slowing the reaction. Any redox reaction within the cell would
completely
stop upon full evaporation of the electrolyte.
[0097] The metal-air fuel cells of the present application are, depending on
their size,
considered to potentially provide a power source equivalent to the use of 90-
100 traditional
CA 03046545 2019-06-10
WO 2018/112510
PCT/AU2017/051344
AA batteries. This is based on the known electrical storage of the materials
in comparison to
standard carbon AA dry cell batteries. An AA standard carbon battery has a
storage
capacity of less than one watt-hour (Wh) of energy (see for example,
http://www.allaboutbatteries.com/Energy-tables.html, website accessed 19
December 2016).
Table: Energy storage in AA batteries (table reproduced from
http://www.allaboutbatteries.com/Energy-tables.html, website accessed 19
December 2016)
Battery Type Avg. voltage Milli-Amp hours Watt-hours
Joules
during discharge (mAh) Wh J
Alkaline Long-life 1.225 2122 2.60 9360
Carbon-zinc 1.1 591 0.65 2340
Nickel-Cadmium 1.2 1000 1.20 4320
NiMH 1.2 2100 2.52 9072
Lithium Ion 3.6 853 3.1 11050
[0098] The magnesium alloy used has a storage capacity of one watt-hour (Wh)
for every
gram of material by weight. Accordingly, a 50 g magnesium alloy rod provides a
potential 50
watt hours or storage whereas a heavier or larger magnesium rod, for example
150 grams
provides 150 watt-hours of storage. The reference for Magnesium Galvanic
Energy Density
is as follows.
Metal-Air Types Magnesium Aluminium Zinc
Specific Gravity 1.74 2.70 7.13
SHE -2.363 -1.662 -0.763
Energy # electrons 2 3 2
Open circuit voltage 1.7 1.2 1.3
Anode composition >90% 99.999% 99.99%
Current capacity 2200 2500 (alloy) 740
Ah/kg
Electrolyte Salt water KOH KOH
pH electrolyte 6 ¨ 8 13 ¨ 14 13 - 14
EXAMPLES
[0099] To test performance of the metal-air fuel cells according to the
invention, a series of
experiments were conducted using prototype cells constructed in accordance
with the
invention. In Experiment 1 prototype cells were tested alone. In Experiments 2
and 3,
prototype cells were tested against a traditional Mg02 fuel cell constructed
substantially in
accordance with FIGURES 1A, 3A and 4A.
Experiment Parameters
[0100] Identical anode and cathode materials were used in all tests.
16
CA 03046545 2019-06-10
WO 2018/112510
PCT/AU2017/051344
Magnesium Anode
[0101] An extruded magnesium AZ31B rod (anode) having the following
composition
resulting in a magnesium anode composition of approximately (typically) 96%
pure
magnesium:
Aluminium: 2.5 - 3.5
Copper: 0.05 max
Iron: 0.005 max
Magnesium: Balance
Manganese: 0.2 min
Nickel: 0.005 max
Silicon: 0.1 max
Zinc: 0.6 - 1.4
Air Cathode
[0102] An air cathode with sufficient hydrophobicity, consisting of layered
Teflon material,
carbon and nickel plated wire.
Configuration of traditional Mg02 fuel cell
[0103] The traditional Mg02 cell was of commonly understood configuration such
that the
magnesium anode was contained within a vessel of 5% saline water solution
electrolyte, and
the air cathode formed part of the vessel wall structure.
Configuration of Prototype cells
[0104] The prototype cells consisted of a 45 gram magnesium anode and an air
cathode
consisting of layered Teflon material, carbon and nickel plated wire. The
anode was
encapsulated in an absorbent material (woven cotton material rolled and woven
into a mat-
like substance). The air cathode was wrapped around the absorbent material and
secured
with elastic 0-rings to allow for expansion.
Test Methodology
[0105] Electronic measurements were conducted using hand-held multimeters, of
well-
known voltage and amperage measurement accuracy (typically well within 1%) and
stored in
a climate-controlled environment. The instruments were allowed to warm up
before
measurements commenced.
17
CA 03046545 2019-06-10
WO 2018/112510
PCT/AU2017/051344
[0106] The tests were conducted on a 24-hour basis in a climate controlled
laboratory with
typically 65% humidity and a constant air temperature of 25 degrees Celsius.
The traditional
Mg02 cells were maintained by carefully emptying and replacing the saline
electrolyte every
24 hours, while the prototype cells were dipped, for approximately 10 seconds,
in the same
saline solution every 24 hours.
[0107] An electronic load in the form of a high-efficiency DC to DC converter
circuit
powering 3 LEDs was used. This circuit was specifically designed to maximize
load on the
cells at all times, whilst maximizing brightness of the LEDs.
Experiment 1: Improvement in lifetime and performance of metal-air fuel cell
(or endurance
testing)
[0108] The experiment was conducted using three identically constructed
prototype cells
labelled as Cell 1, Cell 2 and Cell 3. The target was to generate at least 250
milliamps at
over 1.2 volts (under constant electrical load) for 250 hours (the claimed run
time in
products).
[0109] Cell 1 and Cell 2 had a continuous electrical load (i.e. 24 hours per
day) while control
Cell 3 ran for four (4) hours per day under electrical load.
[0110] The voltage of each cell was not recorded daily but on a regular random
basis. All
cells maintained over 1.2V for the duration of the test and typically between
1.3V and 1.65V.
Results
[0111] The results are presented in Table 1 below and Figure 9.
Table 1: Endurance testing results (milliAmps) of Cells 1, 2 and 3 according
to an
embodiment of the invention over approximately 750 hours
Cell 1 Cell 3
Hours (24/day) Cell 2 (24/day) (4/day)
MilliAmps MilliAmps MilliAmps
24 520 470 420
48 270 380 380
72 250 290 360
96 210 510 510
120 190 250 310
144 180 540 530
18
CA 03046545 2019-06-10
WO 2018/112510 PCT/AU2017/051344
Cell 1 Cell 3
Hours (24/day) Cell 2 (24/day) (4/day)
MilliAmps MilliAmps MilliAmps
168 680 610 590
192 420 520 590
216 450 610 490
240 410 590 520
264 440 360 350
288 380 450 390
312 490 380 510
336 440 260 660
360 450 230 510
384 300 330 330
408 240 300 280
432 280 290 480
456 430 410 480
480 380 330 380
504 280 490 580
528 330 450 420
552 380 430 360
576 430 580 500
600 320 500 480
624 460 350 410
648 250 370 380
672 240 310 280
696 260 380 410
720 250 350 170
744 180 340 280
768 150 240 280
[0112] The results show that the prototype cells exceeded the 250 milliamp
target threshold
after 264 hours, as follows:
Cell 1: 440 mA
Cell 2: 360 mA
Cell 3: 350 mA
19
CA 03046545 2019-06-10
WO 2018/112510
PCT/AU2017/051344
[0113] The test duration was extended and after 504 hours, again the 250 mA
threshold was
still exceeded as follows:
Cell 1: 280 mA
Cell 2: 490 mA
Cell 3: 580 mA
[0114] Cells 2 and 3 continued to show improved performance.
[0115] At the 740-hour mark, two of the three cells still exceeded the 250 mA
target power
output threshold, as follows:
Celli: 180 mA
Cell 2: 340 mA
Cell 3: 280 mA
[0116] After several repeated experiments with similar observations and
results it was
concluded that the cells exceeded lifecycle endurance expectations (i.e. the
250 mA target)
by a factor of three.
[0117] Based on these results, it is anticipated that the cells would, in most
applications
perform for at least 250 hours and in many cases in excess of 500 hours with a
satisfactorily
or target power output of above 250 mA and 1.2 volts.
Experiment 2: Improvement in lifetime and performance of cathode
[0118] In order to test the lifetime and performance of the cathode, a
comparative
experiment was conducted using identically constructed prototype cells
(labelled as
Prototype Cell 1 and Prototype Cell 2) and identically constructed traditional
Mg02 cells
(labelled as Traditional Cell 1 and Traditional Cell 2).
Results
[0119] The results are presented in Table 2 below and Figure 10.
Table 2: Comparative power output (milliAmps) of a traditional Mg02 cell and a
prototype
cell according to an embodiment of the invention over approximately 500
operating hours
Traditional Cell 1 Traditional Cell 2 Prototype Cell 1
Prototype Cell 2
Hours Power Output Power Output Power Output Power
Output
(mA) (mA) (mA) (mA)
24 1330 1110 420 680
CA 03046545 2019-06-10
WO 2018/112510
PCT/AU2017/051344
Traditional Cell 1 Traditional Cell
2 Prototype Cell 1 Prototype Cell 2
Hours Power Output Power Output Power Output Power
Output
(mA) (mA) (mA) (mA)
48 1510 1210 360 420
72 1060 910 510 450
96 950 720 440 410
120 720 740 310 440
144 540 300 530 380
168 370 280 590 490
192 400 270 590 440
216 250 230 490 450
240 280 310 520 310
264 220 230 350 240
288 170 230 390 280
312 300 200 510 430
336 123 204 660 380
360 160 170 510 280
384 140 160 330 330
408 120 140 280 380
432 140 100 480 430
456 120 100 480 320
480 130 110 380 460
504 110 60 580 420
[0120] The results show that the Mg02 traditional cell, after a commonly
observed initial
first-activation power peak, rapidly declined in electrical performance, and
then subsequently
continued to degrade at a steady pace. After 500 hours of continuous operation
the traditional Mg02 cells failed to generate more than 10% of the original
initial electrical
power output.
[0121] In comparison, after 500 hours of continuous operation, the prototype
cell was able to
maintain over 70% of the original electrical power output in one instance and
over 100% of
the output in another ¨ clearly showing power output improvement well over the
initial first-
activation output.
[0122] It is well-known that the pores of air cathodes in Mg-Air cells become
increasingly
blocked by precipitates that form during discharge and consumption of
magnesium (e.g.
magnesium hydroxide precipitates). This in turn negatively affects oxygen gas
diffusion such
that the reaction diminishes, degrading the air-cathode performance over time.
The
21
CA 03046545 2019-06-10
WO 2018/112510
PCT/AU2017/051344
microstructure and air-permeability of carbon-based air-cathode materials is
thus a critical
factor that affects the electrochemical performance of Mg02 cells.
[0123] Upon review, the air-cathodes of the traditional Mg02 cells were
completely
degraded, covered in a solid white material (solidified magnesium hydroxide)
and were also
observed to be badly corroded and therefore no longer useable.
[0124] In comparison, the air cathodes in the prototype cells appeared normal
and were re-
used for future experiments with little or no loss of performance.
[0125] After several repeated experiments with identical observations and
identical results it
was concluded that, in contrast to traditional Mg02 cell configuration, the
prototype cells
demonstrated improved lifetime and performance of the cathode.
Experiment 3: Improvement in performance of metal-air fuel cell associated
with waste
accumulation in the absorbent material layer
[0126] A series of experiments and tests were performed to characterise the
benefits and
effectiveness of the waste management system incorporated into the cells of
the present
application.
[0127] The absorbent material layer for each cell tested according to an
embodiment of the
invention comprised of a fibrous cellulose / bamboo material having a similar
consistency to
female sanitary products. The expandable, anode-encapsulating absorbent
material layer
functioned to provide an "ionic bridge" between the anode and cathode after
dipping into an
electrolyte solution (salt water) in order to initiate and sustain the ionic
reaction which may
last up to several days. As demonstrated by the following results, the ionic
exchange
process was observed to improve in the cell over time with the accumulation of
anode waste
material (magnesium hydroxide) generated as a by-product of the reaction.
Results
[0128] The results are presented in Table 3 below and Figure 11.
Table 3: Performance testing results (milliAmps) of Cells 1, 2 and 3 according
to an
embodiment of the invention over approximately 500 hours
Cell 1 Cell 2 Cell 3
Hours (mA) (mA) (mA)
24 440 880 450
22
CA 03046545 2019-06-10
WO 2018/112510
PCT/AU2017/051344
Cell 1 Cell 2 Cell 3
Hours (mA) (mA) (mA)
48 580 780 380
72 530 650 310
96 460 470 240
120 460 290 170
144 500 160 140
168 340 170 100
192 330 210 120
216 360 170 140
240 290 220 150
264 280 230 150
288 350 320 350
312 410 250 220
336 400 300 280
360 400 300 300
384 410 280 300
408 390 290 320
432 340 240 350
456 360 300 360
480 320 220 420
504 300 250 430
[0129] The results show that as the magnesium hydroxide "waste" accumulates,
the electrical performance (electrical output) of the cells actually improved
until the
magnesium anode material was fully consumed. This is evident from the 100-hour
mark
onwards in all performed tests. That is, once the initial drop in power has
stabilized, the
results show that the cell output subsequently progressively improves and as a
result, the
electrical performance (power output) increases.
[0130] Without wishing to be bound by theory, the inventors believe that this
unexpected
phenomenon may take place as a result of any or all of the following:
= anode waste captured over time within the absorbent material layer
provides better
conductive or ionic paths to enhance the reaction;
= as the cell expands during accumulation of waste, more electrolyte is
adsorbed by
the absorbent material and made available for reaction;
23
CA 03046545 2019-06-10
WO 2018/112510 PCT/AU2017/051344
= the surface area of the cell where the reaction is taking place is
increased allowing
for increased reaction; and/or
= the pores of the air cathode are protected from magnesium hydroxide
precipitates so
that oxygen gas diffusion is not significantly impaired.
[0131] Based on the results of the experiments and observations, it was
considered that, the
unique design, engineering and operation of metal-air fuel cells according to
the invention
allows for filtering and/or capturing anode waste precipitates within the
absorbent material,
which in turn:
= protects pores found within the air-cathode from blockage by waste
particulates, in
turn allowing for critical oxygen gas diffusion across the air cathode;
= effectively prevent the waste precipitates otherwise degrading the air-
cathode;
through salt ingress or corrosion (e.g. salt migration due to "salt creep"
i.e. salt crystal
migration resulting in salt ingress and/or corrosion), potentially also
improving the life
of the cathode and other cell components such as contacts, wiring and/or
electronics;
= render regular internal cleaning of the cell to remove accumulated waste
unnecessary; and/or
= may actually enhance the performance of the cell as the waste
precipitates collect or
are captured within the absorbent material.
[0132] Metal-air fuel cells according to the invention may offer an
affordable, low-cost power
source for use in the developing world. It is anticipated that such metal-air
fuel could provide
approximately five (5) hours of light usage per day for less that the cost of
0.05 USD/day in
the first year (including the initial cost of device). This would reduce in
subsequent years to
0.01 USD/day. It is further noted that all constituent parts are exchangeable
and replaceable
(being ideal for third-world applications) and the entire cell is inherently
safe as even short
circuits have no detrimental effects other than the consumption of the anode
metal.
[0133] Metal-air fuel cells according to the invention may therefore provide
to provide a
portable, light-weight (i.e. light and sturdy unit construction that avoids
bulky water vessels
and/or containers), eco-friendly, exchangeable, powerful (i.e. may be powerful
enough to
drive a myriad of electrical and electrical applications not previously
possible with certain
known metal-air fuel cells), scalable and miniaturised direct current
generator (i.e. may be
miniaturised to be used in "classic" previously battery dominated areas such
as D-cells and
other battery only form factors); and/or to offer the potential of an
environmentally friendly, or
"green", power source for products and devices (when compared to, for example,
traditional
batteries, solar and kerosene as the constituent parts are eco-friendly and
commonly
24
CA 03046545 2019-06-10
PCT/AU2017/051344
Received 20/12/2018
available allowing for excellent recyclability of the entire device, the
cathode itself is also
removable and suitable for recycling.
[0134] Modifications and variations as would be deemed obvious to the person
skilled in the
art are included within the ambit of the present invention as claimed in the
appended claims.
[0135] Unless the context otherwise requires, "position" means, when used as a
verb, to put
or arrange something in a new location or shape, and "positioning" and
"positionable" are to
be construed accordingly.
AMENDED SHEET
IPEA/AU