Note: Descriptions are shown in the official language in which they were submitted.
CA 03047369 2019-06-17
1
DESCRIPTION
Title of Invention
METHOD FOR PRODUCING MAGNESIUM
ALLOY, AND MAGNESIUM ALLOY
Technical Field
The invention relate generally to a method for
producing a magnesium alloy and the magnesium alloy.
Background Art
Magnesium alloys are used for various uses because
of their advantageous characteristics, for example, as
a member which contributes to realization of reducing
the weight and a member which improves vibration-
damping properties. Moreover, magnesium alloys are
used in various fields due to their advantageous
characteristics as metal materials of being basic, for
example, as a sacrificial electrode member used to
prevent corrosion of a structure placed in a soil or
sea water, or a civil engineering work member. In
recent years, taking advantage of the properties of
decomposing in the living body, the applied technology
for members for medical treatments, such as stents and
plates are being developed.
Summary of Invention
Technical Problem
However, under various environments or conditions,
there is a demand of a magnesium alloy which has
performance required as a member to be applied (for
example, mechanical properties, such as strength), and
still has such properties that it stably dissolves or
decomposes in desired time. An object of the present
inventions is to provide a method of producing a
magnesium alloy controllable to decompose at a desired
rate while maintaining predetermined mechanical
properties, and such a magnesium alloy.
CA 03047369 2019-06-17
2
Means for Solving the Problem
The embodiment recited in Claim 1 is a method of
producing a magnesium alloy is provided, which is
characterized by adding: 7.8 to 9.2% by weight of Al,
0.20 to 0.80% by weight of Zn, 0.12 to 0.40% by weight
of Mn, and an amount Y [ppm] calculated by formulas
provided below, of Ni,
(1) when the decomposition rate X is lower than
500 mg/cm2/day,
Y = 48.385Ln(X) - 119.64 (Formula 1)
(2) when the decomposition rate X is 500 or higher
but lower than 1400 mg/cm2/day,
Y = 63.818exp(0.0032X) (Formula 2)
and controlling to have a desired decomposition rate.
The embodiment recited in Claim 2 is a magnesium
alloy controlled to have a desired decomposition rate,
characterized by comprising: 7.8 to 9.2% by weight of
Al, 0.20 to 0.80% by weight of Zn, 0.12 to 0.40% by
weight of Mn, and an amount Y [ppm] calculated by
formulas provided below, of Ni,
(1) when the decomposition rate X is lower than
500 mg/cm2/day,
Y = 48.385Ln(X) - 119.64 (Formula 1)
(2) when the decomposition rate X is 500 or higher
but lower than 1400 mg/cm2/day,
Y = 63.818exp(0.0032X) (Formula 2).
Advantageous Effects of Invention
According to the embodiment of Claim 1, a
magnesium alloy controlled to have a desired
decomposition rate can be produced. The thus produced
magnesium alloy, while maintaining a generally required
strength, can be dissolved after use to easily vanish.
According to the embodiment of Claim 2, a
magnesium alloy controlled to have a desired
CA 03047369 2019-06-17
3
decomposition rate can be obtained. Such a magnesium
alloy, while maintaining a generally required strength,
can be dissolved after use to easily vanish.
Brief Description of Drawings
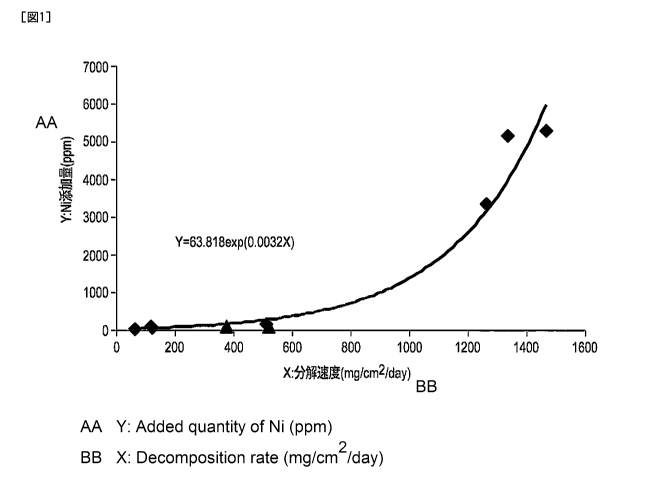
FIG. 1 is a graph showing a relationship between
the decomposition rate of a magnesium alloy and the
amount of nickel added.
FIG. 2 is an expanded graph showing an extracted
range of the amount of nickel added from 0 to 600 ppm
in FIG. 1.
FIG. 3 is a metallographic microscope photograph
of a portion of a magnesium alloy of Experiment 3.
FIG. 4 is a metallographic microscope photograph
of another portion of the magnesium alloy of Experiment
3.
FIG. 5 is a metallographic microscope photograph
of still another portion of the magnesium alloy of
Experiment 3.
FIG. 6 is a metallographic microscope photograph
of a magnesium alloy of Experiment 4.
FIG. 7 is a metallographic microscope photograph
of a magnesium alloy of Experiment 8.
FIG. 8 is a metallographic microscope photograph
of a magnesium alloy of Experiment 9.
FIG. 9 is a cross section of a vertical extruder
used for hot extrusion processing of the magnesium
alloy.
Mode for Carrying Out the Invention
Hereafter, a method of producing a magnesium alloy
according to an embodiment will be described in detail.
In the magnesium alloy production method of the
embodiment, 7.8 to 9.2% by weight of Al, 0.20 to 0.80%
by weight of Zn, 0.12 to 0.40% by weight of Mn, and an
amount Y [ppm] calculated by formulas provided below,
of Ni are added, and they are controlled to have a
CA 03047369 2019-06-17
4
desired decomposition rate [mg/cm2/day].
(1) When the decomposition rate X is lower than
500 mg/cm2/day,
Y = 48.385Ln(X) - 119.64 (Formula 1)
(2) when the decomposition rate X is 500 or higher
but lower than 1400 mg/cm2/day,
Y = 63.818exp(0.0032X) (Formula 2).
(a) Reason for setting the amount of Al to 7.8 to
9.2% by weight
When the Al content of the magnesium alloy is up
to about 10% by weight, the strength and proof strength
of the magnesium alloy improve with the increase in Al
content. When the Al content is 10% by weight or more,
the extrusion speed of the magnesium alloy
significantly decreases. On the other hand, when the
Al content is 6.0% by weight or less, precipitation of
Mg17A112, which is an intermetallic compound of Mg and
Al, is so small in amount that a pinning effect (an
effect of suppressing the growth of crystal grains to
be able to maintain fine recrystallized grains), cannot
be obtained, thereby creating coarse crystal grains.
For the reasons provided above, the amount of Al
is set to 7.8 to 9.2% by weight in order to ensure
precipitation of Mg17A112, fineness of the crystal
grains achieved thereby, improvement in strength and
proof strength, and ensuring extrudability.
(b) Reason for setting the amount of Zn to 0.20 to
0.80% by weight
When Zn is added to the magnesium alloy, an effect
of improving the proof strength and elongation by solid
solution strengthening and promoting the aging
precipitation (an effect of precipitating a solid
solution or the like with time progress) can be
obtained. As the Zn content increases, the tensile
CA 03047369 2019-06-17
strength and proof strength at room temperature
improve; however, when the Zn content is excessive,
there is a tendency that the toughness and strength
decrease.
5 Further, based on the experimental results set out
below, the amount of Zn is set to 0.20 to 0.80% by
weight.
(c) Reason for setting the amount of Mn to 0.12 to
0.40% by weight
When Mn is added to the magnesium alloy, an effect
of suppressing coarseness of recrystallization and an
effect of settling Fe, which is an impurity element,
can be obtained, and therefore 0.10% by weight or more
of Mn is essential. On the other hand, when the Mn
content is excessive, chances are high that the
intermetallic compound of Al and Mn becomes coarse,
which may give rise to a starting point of fatigue
fracture.
For the reasons provided above, the content of Mn
is set to 0.12 to 0.40% by weight.
(d) Reason for adding Ni
Generally, when nickel is added to a magnesium
alloy, it becomes easily corrodible; therefore Ni is
not added. However, the inventors of the present
embodiments carried out numerous experiments, and have
found a method of producing a magnesium alloy
controlled to have a desired decomposition rate by
adjusting the amount of nickel added as indicated by
the above-provided formula (1) or (2).
Hereafter, each experiment will be described with
reference to accompanying drawings provided in FIGS. 1
to 9.
Magnesium alloys were produced and evaluated in
Experiments 1 to 9. Note that in the production of the
magnesium alloys of Experiments 1 to 9, an AZ-based
CA 03047369 2019-06-17
6
alloy (Mg-Al-Zn based alloy), AZ80A alloy in the ASTM
standard was used as a base metal. This is a magnesium
alloy which can contain Al, Zn and Mn in the ranges
provided above. The composition ratio of the AZ80A
alloy is provided in Table 1 below.
P
Table 1 Composition ratio of AZ80A alloy
Composition ratio (% by weight)
NNNNNN\ Al Zn Mn Si Fe Cu Ni Mg
AZ80A 7.8 to 0.20 to 0.12 to
0.005 '-0.005
Bal.
alloy 9.2 0.8 0.5
CA 03047369 2019-06-17
8
For example, the AZ80A alloy of a temper
designation of F exhibit mechanical properties of a
tensile strength of 295 MPa or higher, a 0.2%-proof
strength of 195 MPa or higher, and an elongation of 9%
or higher. Note that the temper designation of F
indicates a material obtained directly from the
manufacturing process which does not include any
special adjustment in hardening or heat treatment.
<Experiment 1>
An AZ80A alloy was used as the base metal, and
70 ppm of Ni was added thereto. Then, they were melted
and subjected to die casting, thus preparing a billet.
The billet was subjected to a hot extrusion
processing described below using a vertical extruder
shown in FIG. 9, and thus a magnesium alloy of
Experiment 1 was obtained.
Here, the extrusion processing will be described
with reference to FIG. 9. A container 1 and a dice 5
in which a through hole 3 is formed were fixed, and a
billet 7 having a diameter of 60 mm and a length of
70 mm was accommodated in the container 1. Next, a fix
lock 9 was set on the billet 7, and a stem 11 was set
on the fix lock 9. The fix lock 9 was placed to
mediate between the billet 7 and the stem 11.
Subsequently, the billet 7 was pressurized by the stem
11 at a temperature of 350 C towards the through hole 3
formed in the dice 5. Then, via the through hole 3,
extrusion was carried out at a speed of 0.5 m/min,
thereby producing an extruded material having a
diameter of 10 mm. Note that in the extrusion, a load
of about 400 tons was applied.
<Experiment 2>
An AZ80A alloy was used as the base metal, and
100 ppm of Ni was added thereto. Then, they were
melted and subjected to die casting, thus preparing a
CA 03047369 2019-06-17
9
billet.
The billet was subjected to a hot extrusion
processing similar to that of Experiment 1, and thus a
magnesium alloy of Experiment 2 was obtained.
<Experiment 3>
An AZ80A alloy was used as the base metal, and
120 ppm of Ni was added thereto. Then, they were
melted and subjected to die casting, thus preparing a
billet.
The billet was subjected to a hot extrusion
processing similar to that of Experiment 1, and thus a
magnesium alloy of Experiment 3 was obtained.
<Experiment 4>
An AZ80A alloy was used as the base metal, and
180 ppm of Ni was added thereto. Then, they were
melted and subjected to die casting, thus preparing a
billet.
The billet was subjected to a hot extrusion
processing similar to that of Experiment 1, and thus a
magnesium alloy of Experiment 4 was obtained.
<Experiment 5>
An AZ80A alloy was used as the base metal, and
3380 ppm of Ni was added thereto. Then, they were
melted and subjected to die casting, thus preparing a
billet.
The billet was subjected to a hot extrusion
processing similar to that of Experiment 1, and thus a
magnesium alloy of Experiment 5 was obtained.
<Experiment 6>
An AZ80A alloy was used as the base metal, and
5100 ppm of Ni was added thereto. Then, they were
melted and subjected to die casting, thus preparing a
billet.
The billet was subjected to a hot extrusion
processing similar to that of Experiment 1, and thus a
CA 03047369 2019-06-17
magnesium alloy of Experiment 6 was obtained.
<Experiment 7>
An AZ80A alloy was used as the base metal, and
5300 ppm of Ni was added thereto. Then, they were
5 melted and subjected to die casting, thus preparing a
billet.
The billet was subjected to a hot extrusion
processing similar to that of Experiment 1, and thus a
magnesium alloy of Experiment 7 was obtained.
10 <Experiment 8 (Comparative Example)>
An AZ80A alloy was used as the base metal, and
120 ppm of Ni was added thereto. Then, they were
melted and subjected to die casting, thus preparing a
billet.
To this billet, a hot extrusion processing was not
carried out, and thus a magnesium alloy of Experiment 8
was obtained.
<Experiment 9 (Comparative Example)>
An AZ80A alloy was used as the base metal, and
140 ppm of Ni was added thereto. Then, they were
melted and subjected to die casting, thus preparing a
billet.
To this billet, a hot extrusion processing was not
carried out, and thus a magnesium alloy of Experiment 9
was obtained.
<< Evaluation 1>>
The composition ratio of each of the magnesium
alloy of Experiments 1 to 9 was measured based on a
high-frequency inductively coupled plasma optical
emission spectrometry (ICP optical emission
spectrometry). The results of the magnesium alloys of
Experiments 1 to 9 in composition ratio are provided in
Table 2 below.
Table 2 Composition ratios of magnesium alloys in Experiments 1 to 9
Composition ratio (% by weight)
Al Zn Mn Si Fe Cu
Ni Mg
Experiment 1 9.1 0.64 0.25 0.02
<0.002 <0.002 0.0074 Bal.
Experiment 2 8.9 0.60 0.23 0.02
<0.002 <0.002 0.0100 Bal. P
Experiment 3 8.2 0.56 0.23 0.02
<0.002 <0.002 0.0123 Bal. ..
,
Experiment 4 8.8 0.59 0.24 0.02
<0.002 <0.002 0.0180 Bal. .
Experiment 5 7.9 0.58 0.22 0.02
<0.002 <0.002 0.3380 Bal. ,
,
Experiment 6 8.1 0.59 0.22 0.02
<0.002 <0.002 0.5160 Bal. 1--
,
,
_
,
Experiment 7 7.8 0.52 0.19 0.02 0.001 0.004
0.5300 Bal.
Experiment 8
8.2 0.56 0.23 0.02 <0.002 <0.002 0.0123 Bal.
(Comparative Example)
Experiment 9
8.4 0.61 0.24 0.02 <0.002 <0.002 0.0142 Bal.
(Comparative Example)
CA 03047369 2019-06-17
12
<< Evaluation 2
The magnesium alloys of Experiments 1 to 9 were
immersed in a 2%-KC1 solution of 93 C, and the
decomposition rates thereof were measured. The results
are indicated in Table 3 below.
Table 3 Amount of Ni added and decomposition
rate of magnesium alloys Experiments 1 to 9
Amount of Ni added Decomposition rate
(Pim) (mg/cm2/day)
Experiment 1 74 58
P
Experiment 2 100 115
.
_. .
.
,
Experiment 3 123 113
.
.
Experiment 4 _ 180 501
,
Experiment 5 3380 1242
1---- ,
0
w
,
,
Experiment 6 5160 1313
,
Experiment 7 5300 1441
Experiment 8
123 367
(Comparative Example) ...
Experiment 9
142 507
(Comparative Example)
CA 03047369 2019-06-17
14
Based on the results of Table 3 provided above, a
graph was created, in which the results indicated in
FIG. 1 were plotted. The correlations between the
plots of FIG. 1 mentioned above were analyzed (except
for Experiments 8 and 9, which are comparative
examples), and as a result, Formula 2, which is an
expression indicating the relationship between the
amount of nickel added and the decomposition rate was
obtained.
FIG. 2 is an expanded graph extracting the range
of the amount of nickel added from 0 to 600 ppm in
FIG. 1. In a similar manner to that of FIG. 1, the
correlations between the plots of FIG. 2 were analyzed
(except Experiments 8 and 9, which are comparative
examples), and as a result, Formula 1 was obtained.
From the above, it can be concluded that in the
production of magnesium alloys whose decomposition rate
is less than 500 mg/cm2/day, the amount of nickel
added, which corresponds to a desired decomposition
rate is calculated using Formula 1, and thus a
magnesium alloy controlled to have the desired
decomposition rate can be produced. Moreover, in the
production of a magnesium alloy having a decomposition
rate of 500 or higher but less than 1400 mg/cm2/day, a
magnesium alloy controlled to have a desired
decomposition rate can be produced using Formula 2.
<< Evaluation 3>>
Cross sections of cut Magnesium alloys of
Experiments 3, 4, 8 and 9 were observed using a
metallographic microscope, and for each case, the
crystal grain diameter was measured based on a
planimetry of JIS H 0542 (crystal granularity test for
magnesium alloy rolled plates). The results are
indicated in Table 4 below, and also metallographic
CA 03047369 2019-06-17
microscope photographs are provided.
Table 4 The amount of Ni added, decomposition rate and crystal grain
diameter of each of magnesium alloys in Experiments 3, 4, 8 and 9
Amount of Ni added Decomposition rate
Crystal grain P
(PPm)
(mg/cm2/day) diameter (pm)
,
Experiment 3 123
113 26,35,39
Experiment 4 180
501 15 0
,
,
Experiment 8
,
123
367 156
,
(Comparative Example)
Experiment 9
142
507 141
(Comparative Example)
,
CA 03047369 2019-06-17
17
Crystal grain diameters of 26 pm, 35 pm and 39 pm
in Experiment 3 shown in Table 4 provided above were
measured from metallographic microscope photographs of
FIGS. 3, 4 and 5, respectively.
FIG. 6 is a metallographic microscope photograph
showing a cross section of the cut magnesium alloy of
Experiment 4, and the crystal grain diameter was 15 pm.
FIG. 7 is a metallographic microscope photograph
showing a cross section of the cut magnesium alloy of
Experiment 8, and the crystal grain diameter was
156 pm. The black perlite-like sections are of the
Mg17A112 phase, and it can be seen that the phase is
unevenly located.
FIG. 8 is a metallographic microscope photograph
showing a cross section of the cut magnesium alloy of
Experiment 9, and the crystal grain diameter was
141 pm. As in the case of the magnesium alloy of
Experiment 8, the Mg17A112 phase is unevenly located.
As is clear from Table 4 provided above, a
correlation was observed also between the decomposition
rate and the crystal grain diameter of the magnesium
alloys. More specifically, as compared with the
magnesium alloys of Experiments 8 and 9, the magnesium
alloys of Experiments 3 and 4, which had smaller
crystal grain diameters, exhibited low values in the
decomposition rate as well.
(I) Relationship between decomposition rate and
crystal grain diameter
Magnesium alloys having an Al content of 6% by
weight or more, such as of Experiments 1 to 9 consist
of a chemically unstable matrix phase, an Mg17A112
phase, which is an intermetallic compound phase which
contains a great amount of Al, which is chemically
stable, and an Al-Mn phase.
CA 03047369 2019-06-17
18
In such magnesium alloys, progress of
decomposition is promoted preferentially from a
chemically unstable matrix phase. The progress of
decomposition depends on the Mg17A112 phase
precipitates to surround crystal grain boundaries
mainly. Therefore, the finer the grains are, the more
stably, the decomposition becomes controllable.
On the other hand, as the grains of a magnesium
alloy are larger, the more coarsely and unevenly
Mg17A112 precipitates, and thus the decomposition rate
increases and further it is very much likely that the
decomposition rate varies greatly. Therefore, it is
difficult to control the decomposition rate.
As described above, from the view point of stable
control of the decomposition rate, it is desirable that
the grains of the magnesium alloy be finer in order to
distribute the Mig17A112 phase more uniformly. It can
be seen from the metallographic microscope photograph
of FIG. 5 that the maximum crystal grain diameter of
the magnesium alloy of Experiment 3 is 100 pm. On the
other hand, the crystal grain diameter of the magnesium
alloy of Experiment 4 is 15 pm. Here, as is clear from
the results of Experiment 3 indicated in Table 4
provided above, the size of grains varies from one cut
section to another, and thus the crystal grain diameter
may be 10 pm in some other section. Therefore, the
crystal grain diameter of the magnesium alloy should
desirably be, 10 to 100 pm, to be specific. Note that
the maximum crystal grain diameter described above is
meant an arithmetical average of the maximum diameter
and the minimum diameter of the largest crystal grain
in the metallographic microscope photograph of FIG. 5.
The Mg17A112 phase preferentially precipitates
discontinuously in crystal grain boundaries after
CA 03047369 2019-06-17
19
dynamic recrystallization occurs in such a magnesium
alloy in the process of cooling. Therefore, the finer
the grains, the higher the possibility that they are
distributed even more uniformly in crystal grain
boundaries. Moreover, as the chemically unstable
matrix phases become more equal to each other in a
fixed size, the variation in decomposition rate can be
reduced for control. In consideration of the above,
the crystal grain diameter of the magnesium alloy
should desirably be 10 to 50 pm, in which they cannot
easily form duplex grains.
(II) Relationship between crystal grain diameter
and extrusion
In the magnesium alloys, the crystal grain
diameters are made finer by extrusion, and also
Mg17A112 precipitates, thus securing the strength of
the extruded material. With a great amount of
precipitation of Mg17A112, the growth of the crystal
grains of the magnesium alloy is suppressed, thus
making it possible to maintain fine recrystallized
grains.
<< Evaluation 4>>
The magnesium alloys of Experiments 1, 2, 4 and 7
were subjected to tension test at room temperature to
measure mechanical properties, tensile strength, 0.2%-
proof strength, and elongation. The tension test was
carried out after shaping the magnesium alloys each
into that of No. JIS14A test sample piece, at a speed
of an initial strain rate (1x10-3[s-1]) at room
temperature. The results of the mechanical properties
of the magnesium alloys of Experiments 1, 2, 4 and 7
are indicated in Table 5 below.
Table 5 Mechanical properties of magnesium alloys of Experiments 1, 2, 4 and 7
P
Tensile strength 0.2%-proof strength Elongation
,
(MPa) (MPa) (%)
Experiment 1 334 223 10
,
,
Experiment 2 340 235 9
Iv
D
,
,
Experiment 4 337 219 11
,
Experiment 7 322 213 18
CA 03047369 2019-06-17
21
As is clearly from Table 5 provided above, the
magnesium alloys of the experiments exhibit a tensile
strength of 320 MPa or higher, a 0.2% proof strength of
210 MPa or higher, and an elongation of 9% or higher,
and thus have a strength which satisfies the mechanical
properties of the AZ80A alloy in the ASTM standards.
As described above, the magnesium alloys according
to the embodiments, such as of those of Experiments 1
to 9, can be dissolved after use and easily vanished.
These magnesium alloys can be dissolved and vanished at
a desired decomposition rate in accordance with any
environment to be employed.
The present invention is not limited to the above-
described embodiments, but can be modified in various
ways in a range which it does not fall out of the
essence of the invention.
For example, in the above-described experiments,
the magnesium alloys were prepared from an AZ80A alloy
as the base metal, but MB3 or M53, as well, of JIS
standards can be used as a base metal. The MB3 and MS3
are magnesium alloys which may contain Al, Zn and Mn in
the ranges described above, and the composition ratios
of MB3 and MS3 are indicated in Table 6 below.
P
.
.
Table 6 Composition ratios of MB3 and MS3
,
\
Composition ratio (% by weight)
.
. Al Zn Mn Si Fe Cu Ni Others
Mg ,
,
.
Iv
.
,
M63, 7.5 to 0.2 to 0.10 to
N) ,
.._0.10 -Ø005 -Ø05 Ø005 -
0.30 Bal. ,
MS3 9.2 1.0 0.40