Note: Descriptions are shown in the official language in which they were submitted.
WO 2017/115262 PCT/IB2016/057999
1
METHODOLOGY FOR TREATING BIOMASS, COAL, MSW/ANY KIND OF WASTES AND
SLUDGES FROM SEWAGE TREATMENT PLANTS TO PRODUCE CLEAN/UPGRADED
MATERIALS FOR THE PRODUCTION OF HYDROGEN, ENERGY AND LIQUID FUELS-
CHEMICALS
The present invention refers to a method for treating agricultural or forestry
or urban origin
biomass or mixture of different origin's biomass feedstocks, low quality coal
such as peat,
lignite or subbituminous or/and bituminous coal, or/and mixtures of them,
garbage and
urban/industrial wastes, solid and/or liquid state, as well as sewage
treatment plant sludges
by means of removal of inorganic elements, such as silica, potassium, sodium,
chlorine,
sulfur, phosphorus, nitrogen and heavy metals such as zinc, mercury, copper,
lead,
chromium, etc., and the addition of new inorganic elements such as calcium,
magnesium,
titanium, zirconium, yttrium, aluminum and ammonium, in order to produce a
purified and
upgraded solid and/or liquid material which can be used as raw material in
thermochemical
conversion processes such as combustion, flash (t<1 sec)/fast pyrolysis
(1<t<l0sec), as well
as in the gasification for the production of energy, and/or hydrogen-rich gas
and liquid
hydrocarbons which can be further upgraded by applying commercially available
thermochemical conversion technologies for the production of pure hydrogen,
liquid fuels,
chemicals and energy with great economic and environmental benefits.
The excessive use of fossil fuels such as coal, oil and natural gas nowadays
for energy/heat
production as well as liquid and solid/gaseous transportation fuels causes
major
environmental problems such as emissions of sulfur and nitrogen oxides,
particulates, heavy
metals, methane and carbon dioxide. Additionally, the mining processes cause
pollution of the
local environment and especially of water, air and soil.
Aiming to reduce the gaseous/liquid and solid emissions caused by the use of
conventional
fuels and especially to reduce emissions of gases that contribute to the
greenhouse effect,
the use of renewable energy sources such as wind, solar, hydro and biomass is
encouraged.
Especially the use of biomass in solid and liquid form to produce liquid and
gaseous biofuels
which will not contribute to the greenhouse effect is highly important for
solving that problem.
In addition, the recycling and recovery of urban and/or industrial waste and
municipal waste
such as garbage, wastewater treatment sludges, etc., is nowadays one of the
biggest
environmental problems worldwide. Every year, millions of tons of waste
require safe as well
as economically viable disposal and recovery. The most common disposal method
is storing
wastes in dumps followed by incineration and recycling to produce new
materials. However,
the high content of garbage and wastes in alkali metals, chlorine, sulfur and
heavy metals
make their thermochemical application problematic, costly and very low
efficient.
The problems caused nowadays during the thermochemical incineration,
combustion,
gasification and pyrolysis of biomass is due to the ash composition. These
problems occur
especially when biomass derived from agricultural, forest and urban
environment such as
various kinds of straw, different kinds of waste from agricultural industries
such as cotton,
peanut olive, etc., as well as from trimmings and wood residues from
construction and
furniture production. Similar problems occur when you use low-quality coal
such as peat,
lignite and subbituminous/bituminous coal, used mainly for power and/or heat
generation on a
large scale, as well as urban and industrial origin wastes and wastewater
treatment sludges
which are intended to be used for safe, economically viable and
environmentally beneficial
treatment/deactivation/deposition. The ash of these specific biomass types is
very rich in
Date recue/Date received 2023-03-24
WO 2017/115262 PCT/IB2016/057999
2
alkali metals, chlorine, sulfur and phosphorus, therefore the gases, liquids
and solids
produced during the thermochemical conversion of that biomass types tend to
react with each
other and with any other inorganic compounds present during the conversion, as
well as with
the metal surfaces creating corrosion problems, deposits and agglomerates.
They also
generate emissions which result in great financial losses, environmental
problems and in the
inability to use certain types of biomass on a large scale, separate and/or
combined with solid
or gaseous fuels for power generation, liquid fuels and chemicals production.
Similarly, the
ash of many low-quality coal fuels such as peat, lignite and
subbituminous/bituminous coal
appears to be also rich in alkali metals, chlorine and sulfur, where the ash
composition differs
depending on the coal quality and the specific characteristics of each coal
deposit.
Consequently, similar problems, although of lower intensity compared to with
biomass use,
are observed, which lead to financial losses, environmental problems, and
limited efficiency in
the use of such coals, as well as to problems in their application as in the
case of gasification
of lignite with high sodium and chloride content for energy and /or liquid
fuels production.
Additionally, the remaining ash from urban and industrial origin wastes and
wastewater
treatment sludges is rich in alkali metals, chlorine, sulfur, and phosphorus
as well as in heavy
metals such as zinc, lead, copper, chromium etc., which makes their
thermochemical
application problematic, costly and very low efficient.
Moreover, the existence of large amounts of chlorine in the structure of
polymers/plastics
such as polyvinyl chloride (PVC), which is present in a large amount of
plastics included in
solid wastes, results in the production of large quantities of dioxins (PCOD)
and furans
(PCDF), which are not only harmful for human health but also for other forms
of life. The
removal/destruction of these pollutants before being emitted to the
environment requires the
use of very expensive technologies of high accident risk.
Solving these problems will result in further use of biomass, urban and
industrial origin
wastes both in solid and liquid form and wastewater treatment sludges for the
production of
energy, liquid fuels and chemicals as well as for the economic and efficient
use of coal with
major economic and environmental benefits especially nowadays when the
imported energy
cost appears to be rising and greenhouse gases from solid fuels should be
reduced. The
increased use of biomass or/and urban and industrial origin wastes as well as
the more
efficient use of low-quality coals used on a general basis for energy
production are expected
to contribute decisively not only to the reduction of greenhouse gases and to
the emission of
sulfur oxides, nitrogen, heavy metals and particles that pollute the
environment and human
health, but also in the cost reduction of energy and fuel production.
The currently applied techniques and methods dealing with these problems
appear to have
only limited success and, as a consequence, the use of biomass in
thermochemical
conversion appears to be, worldwide, very limited, and restricted mainly in
feedstocks like
wood which presents fewer problems. As far as the use of low-quality coals is
concerned, the
specific problems limit their thermochemical conversion efficiency and lead to
the use of
larger amounts of feedstocks for the production of energy and fuels/chemicals,
causing the
increase on greenhouse gas emissions and the financially non-efficient
exploitation of the
coal deposits with larger content of alkali metals, chlorine and sulphur.
Various pretreatment
technologies have been proposed to reduce the problems caused by the
thermochemical
conversion of coal, biomass and waste but they have limited success with
disproportionately
high costs and they all fail to control and eliminate effectively all those
different factors such
as silicon, alkali metals, chlorine, sulfur, phosphorus, heavy metals,
nitrogen, etc. that lead to
the aforementioned problems. Examples of such methodologies which put a limit
on the
Date recue/Date received 2023-03-24
WO 2017/115262 PCT/IB2016/057999
3
above problems are described in: Bender (US 4,560,390), McMahon (US
4,304,571), Grant
(US 4,137,050).
The purpose of this invention is to achieve the upgrading and purification of
agro/forest/urban
origin biomass or mixture of biomasses of different origins, low quality coal
such as peat,
lignite or subbituminous and/or bituminous coal, or mixtures of them, urban
and industrial
origin wastes and wastewater treatment sludges by removing the harmful
inorganic elements
such as silicon, sodium, potassium, chlorine, sulfur, phosphorus, nitrogen and
heavy metals
such as cadmium, chromium, nickel, lead, mercury, arsenic etc., and/or by
deactivating them
so that they do not adversely affect the thermochemical conversion processes
such as
combustion, flash/fast pyrolysis and gasification which are used to produce
energy and/or
gaseous/liquid hydrocarbons in case of pyrolysis and gas in case of
gasification, which can
be used for the production of pure hydrogen and/or liquid fuels/chemicals
having zero
footprint regarding greenhouse gas emissions and high financial value.
The invention is able to minimize/eliminate corrosion problems, deposition,
ash
agglomeration, and gaseous emissions (potassium, sodium, chlorine, sulfur,
nitrogen and
phosphorus), heavy metals (Cu, Pb, Zn, Cr, Hg, As, Mo, etc.), dioxins and
furans (PCDD,
PCDF) during thermochemical incineration, combustion, gasification, pyrolysis
of the raw
material used, e.g. agro/forest/urban origin biomass or biomass mixtures of
different origins,
coal or coal mixtures of different origins, low quality coal such as peat,
lignite or
subbituminous/bituminous coal, garbage and urban/industrial wastes, solid
and/or liquid state,
as well as sewage treatment plant sludges.
The intended purpose as surprisingly found in the laboratory is achieved by
leaching of
agro/forest/urban origin biomass or biomass mixtures of different origins,
coal or coal
mixtures of different origins, low quality coal such as peat, lignite or
subbituminous/bituminous
coal, or mixtures of them, garbage and urban/industrial wastes, as well as
sewage treatment
plant sludges with aqueous solutions of inorganic and/or organic salts and
bases under
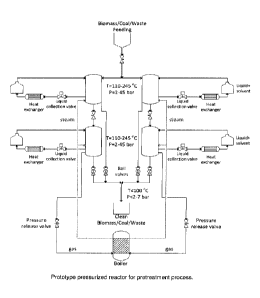
pressure using the reactor depicted in Figure 1. Mixtures of both organic and
inorganic
acids/salts can be used in the process to achieve the desired result
considering that the
proportion of acid is limited to less than 30% of the total mixture on a
weight basis and
preferably the extent of which does not lead to the creation of acidic
conditions having a pH
less than 5 in the solution under pressure.
The novelty of this invention is based on the fact that it's the first time
when the simultaneous
removal of all harmful inorganic elements (Si, K, Na, P, Cl, S, heavy metals,
nitrogen) and/or
their deactivation is possible to such a large extent that the resulting
upgraded/clean solid
and liquid materials are able to be used in thermochemical conversion
processes
(combustion, gasification, pyrolysis) without emissions, corrosion,
deposition, etc. problems at
the lowest possible cost and greater energy efficiency and financial benefit.
As surprisingly found in the laboratory, the combined use of the innovative
reactor illustrated
in Figure 1 with the appropriate applied conditions and inorganic/organic
compounds can lead
to the desired results. Although different commercially available pressurized
reactors could
also be used for the pretreatment, only the reactor in figure 1 ensures the
highest possible
process efficiency by carrying out the pretreatment in an integrated two-step
process
Date recue/Date received 2023-03-24
WO 2017/115262 PCT/IB2016/057999
4
demonstrating the maximum efficiency at the lowest cost, whereas commercial
reactors
require two separate processes using separate reactors,.
As depicted in Figure 1, the high pressure reactor consists of two separate
reactors in a
parallel mode. Each reactor contains an initial pressurized vessel where the
raw material and
the aqueous solution are mixed under ambient temperature and pressure having
materiaVaqueous phase ratio from 15 grams per liter up to 800 grams per liter
and solvent
concentration of 0.5-1.5% weight basis depending on the material used. This
does not
prevent the application of higher concentrations or reaction times considering
that this
achieves a better or different result. Consequently, if the material treatment
targets the silicon
structural removal during the first reaction step, the pressure vessel with
aqueous alkali
solution (base and/or salt) such as sodium, potassium, is heated between 110-
150 C and
pressure 2-10atm if the material is biomass while at temperatures from 130 C
up to 195 C
and pressure 4-20atm if the treated material is coal, garbage/waste for less
than five minutes
in case of biomass and less than 20 minutes in case of coal, garbage/waste. As
shown in
Figure 1, each pressurized vessel is equipped with a direct discharge valve
which
communicates with the interior of the reactor via a pipeline at the end of
which there is a 40
micron diameter solids filter. The immediate depressurization caused by the
discharge valve
opening after the end of the treatment process results in solid/liquid
separation letting the
liquid to be concentrated and cooled in the recover tank before being recycled
into the
process as shown in Figure 1 while the solid product is removed in the second
phase and is
transferred to the second pressurized vessel by opening the valve of the
pressurized
reactor's bottom.
Simultaneously, the parallel reactor operates one step back from the initial
reactor in order to
realize a process which is semi-batch but in progress at any time.
When the material reaches the second compartment of the pressurized reactor,
the second
pretreatment stage takes place. This step includes the leaching of the
material with an
aqueous solution of inorganic and/or organic salts. Mixtures of both organic
and inorganic
acids/salts can be used in the process to achieve the desired effect while the
proportion of
acid is limited to less than 30% of the total mixture on a weight basis and
preferably to such
an extent that will not create acidic conditions of pH less than 5 in the
pressurized solution.
The process conditions are temperature between 110-160 C and pressure 2-10atm
if the
material is biomass, temperature between 140-195 C and pressure 4-20atm if the
treated
material is coal, garbage/waste while pressure 4-45atm and temperature (140-
245 C) in case
of plastics/polymer materials especially if they have a chlorine containing
structure, for less
than 5 minutes in case of biomass and less than 20 minutes in case of coal,
garbage/waste
and plastics/polymer materials. Regarding organic and/or inorganic compounds,
they are/can
be used any water-soluble organic/inorganic salts of calcium, magnesium,
titanium,
zirconium, yttrium, aluminum and ammonium in proportions of 0.07% up to 4%
weight basis
in aqueous solution depending on the type of the treated material. In case of
biomass, solvent
concentration can be reduced to 0.5-1.5% and in case of coal and garbage/waste
typically
ranges between 0.5-4%. Additionally, all organic and/or inorganic acids that
create water-
soluble salts with the aforementioned cations can also be used. The acids
concentration
when acids/salt mixtures are applied is sufficiently low so that the pH of the
solution is always
higher than 5, preferably 6.5. Although the use of higher concentrations of
salts in the
solutions is feasible, it is not considered as necessary to achieve the
desired result. After the
end of the process, the solid-liquid separation as well as the solid removal
from the
Date recue/Date received 2023-03-24
WO 2017/115262 PCT/IB2016/057999
pressurized reactor takes place in the same way as previously described and as
shown in
Figure 1 which illustrates the process.
The conditions inside the pressurized reactor are always slightly
acidic/neutral/alkaline
5 depending on the use of suitable solvents as previously described. This fact
combined with
the low pressure (2-10atm) and temperatures (110-160 C) in case of biomass,
pressures (2-
20atm) and temperatures (110-195 C) in case of coal/garbage/waste and
pressures (4-
45atm) and temperatures (140-245 C) in case of plastics/polymer materials
especially when
they contain structural chlorine, results in the use of much cheaper materials
such as carbon
steel for manufacturing the pressurized reactors so that the process cost,
both capital and
operating, appears to be reduced by 50-80% compared to reactors that use much
higher
temperatures and pressures while their operating conditions are mildly to
strongly acidic.
The pre-treatment consists of two stages in case of coal as well as
biomass/garbage/waste
whose ash contains silicon in large percent usually above 10% SiO2 in ash
basis, which
should be removed in order to reduce deposition problems, ash production and
to create new
high-value materials such as pure silicon. In any case and if necessary,
materials having less
silicon content in the ash can be used for upgrading. In that case, the first
pretreatment stage
is to remove the silicon from the treated material. This reaction is carried
out at temperatures
between 110-150 C and pressure 2-10atm when the material is biomass while at
130-195 C,
pressure 4-20atm when the material is coal and/or garbage/waste so that the
aqueous phase
remains in liquid form and is not converted to gas. Although higher
temperatures (200-350 C),
and pressures could be used, the financial cost of such an option combined
with the small
additional benefits for the process itself, make such a choice unprofitable.
This leaching
process is performed by using aqueous solutions of strong bases and/or salts
of strong bases
such as potassium, sodium. This does not prevent the use of other active
ingredients bringing
the same result. The reaction time is now limited below 5 minutes in case of
biomass while in
case of coal, waste, etc. materials the treatment time ranges from 5 to 20
minutes, the
solid/aqueous phase ratio can range from 15grams per liter to 800grams per
liter depending
on the treated material where the higher solid/liquid ratio is observed in
case of coal and
waste/litter, and the solution concentration of strong bases and/or salts of
strong bases below
1.5% weight basis where better results are obtained for concentrations of 0.5-
1% weight
basis. This does not prevent the application of higher concentrations or
reaction times if it is
considered to achieve a better or different result. This process leads to the
reaction of silicon
with the strong alkali forming a water soluble alkali compound such as the
KSiO3 while
removing silica from the treated material by over 80% and up to 100% applying
the
appropriate conditions. Simultaneously, short residence and reaction time
limit the reaction of
alkali metal with the organic phase of the material and consequently the
material loss. The
treated material is initially size reduced using appropriate equipment so that
the size of the
treated particles are limited below 5 mm and preferably less than 2 mm,
although larger
particle sizes can be used if the pretreatment conditions are modified.
After the end of the first pretreatment stage, the resulting material has
increased
concentration of alkali metals and minimum silicon content. The removal of the
liquid phase
from the solid one and the solid transfer from the first to the second
pressurized compartment
is described in the operating principle of the pressurized reactor. The
removed liquid is
recycled to the process several times until it is saturated in silicates. Then
it is further
processed for collecting the silicon dioxide which is a high value material
and can be used to
produce pure silicon by applying commercially available methods.
Date recue/Date received 2023-03-24
WO 2017/115262 PCT/IB2016/057999
6
In the second pressurized compartment, the material is now washed with an
aqueous solution
of inorganic and/or organic salts. Mixtures of both organic and inorganic
acids/salts can also
be used in the process to achieve the desired result considering that the
proportion of acid is
limited to less than 30% of the total mixture on a weight basis and preferably
the extent of
which does not lead to the creation of acidic conditions having a pH less than
5 in the
pressurized solution.
Regarding organic and/or inorganic compounds, they are/can be used any water-
soluble
organic/inorganic salts of calcium, magnesium, titanium, zirconium, yttrium,
aluminum and
fluoride in proportions of 0.07% up to 4% weight basis in aqueous solution
depending on the
type of the treated material. In case of biomass, solvent concentration can be
reduced to 0.5-
1.5% and in case of coal and garbage/waste typically ranges between 0.5-4%.
Additionally, all
organic and/or inorganic acids that create water-soluble salts with the
aforementioned cations
can also be used. The acids concentration when acids/salt mixtures are applied
is sufficiently
low so that the pH of the solution is always higher than 5, preferably 6.5.
Although the use of
higher concentrations of salts in the solutions is feasible, it is not
considered as necessary to
achieve the desired result.
Examples are salts of calcium acetate/citrate/nitrate and/or magnesium
acetate/citrate/nitrate
and/or ammonium acetate/citrate/nitrate. Also acetic acid, citric acid, nitric
acid could be used.
When magnesium, titanium, aluminum, yttrium, zirconium and/or ammonium salts
are used,
the addition of calcium salt to the mixture ranging from 1/10 up to 1/3 of the
total salts
concentration is always recommended for better results. However, the calcium
salts can be
used separately without the presence of other salts.
This reaction is carried out at temperatures between 110-160 C and pressure 2-
10atm when
the material is biomass while at 140-195 C, pressure 4-20atm when the material
is coal
and/or garbage/waste so that the aqueous phase remains in liquid form and is
not converted
to gas. Although higher temperatures (200-350 C) and pressures could be used,
the financial
cost of such an option combined with the small additional benefits for the
process itself, make
such a choice unprofitable. The reaction time is now limited below 5 minutes
in case of
biomass while in case of coal, waste, plastic etc. materials the treatment
time ranges from 5
to 20 minutes, the solid/aqueous phase ratio can range from 15grams per liter
to 800grams
per liter depending on the treated material where the higher solid/liquid
ratio is observed in
case of coal and waste/litter.
The applied ratios depend on the type and composition of the pretreated
material (e.g.
biomass, coal, garbage/waste, etc.) as well as on the desired properties which
are going to
be applied to the pretreated material. Regarding the creation of the aqueous
solution, any
kind of water from the public water system, source, etc., can be employed.
During the
treatment with the aqueous solution of the organic and/or inorganic solvent
which is created
by mixing the specific organic and/or inorganic water-soluble salts and/or
acids, the alkali
metals (K, Na), sulfur, phosphorus, the heavy metals (Cu, Pb, Zn, Cr, Hg,
etc.) as well as the
chlorine present in the structure of the treated materials are transferred
into the aqueous
phase and are removed from the pretreated material mainly as inorganic/organic
salts.
Simultaneously, cations such as Ca, Mg, Al, Ti, Zr, NH4, etc., replace
hydrogen atoms and/or
alkali metals and others, inside the structure of the treated materials
thereby increasing the
concentration of these cations in the treated materials. This is concluded to
have a
surprisingly positive effect on the thermochemical conversion reactions such
as combustion,
flash/fast pyrolysis and gasification which favors the production of energy
without
Date recue/Date received 2023-03-24
WO 2017/115262 PCT/IB2016/057999
7
deposition/emission problems, the production of purified hydrogen-rich gas,
and/or pure liquid
phase hydrocarbons with high conversion efficiency which can be further used
for the
production of pure hydrogen and liquid fuels/chemicals with low financial
cost.
After the second pretreatment stage, the clean/upgraded solid/liquid end
product which now
contains very small to zero silicon, alkali metals, phosphorous, sulfur,
chlorine and heavy
metals concentration as well as increased cations concentration used in the
last process step
(Ca, Mg, Al, Ti, Zr, NH4, etc.,) is separated from the liquid phase in the
same way as
described in the operation of the pressurized reactor, while the
liquid/aqueous phase is
recycled back to the process.
Purification of the liquid phase from inorganic elements such as potassium,
sodium,
phosphorus, sulfur, chlorine, heavy metals is carried out after several loops
using ion
exchange resins when sign of saturation of the aqueous solution with the
specific
components is occurred.
In case that the pretreated material contains less than 10% SiO2 ash basis, or
only silicon
traces as in all kinds of plastics, RDF (refuse derived fuel), etc., biomass
such as peach
kernels, DDGS, etc., for which silicon is considered that there is no reason
to be removed
from the treated material, then the material is washed with an aqueous
solution of inorganic
and/or organic salts, mixtures of both organic and inorganic acids/salts
considering that the
proportion of acid is limited to less than 30% of the total mixture on weight
basis and
preferably the extent of which does not lead to the creation of acidic
conditions having a pH
less than 5 in the pressurized solution.
In this case, both separate pressurized compartments from each parallel
reactor illustrated in
Figure 1 can be used simultaneously for treating the material as the treatment
is now carried
out in one step. This results in treating the double amount of material
compared to the
previous case where treatment consisted of two stages. The treatment is
performed in the
same way described in detail in the operation characteristics of the
pressurized reactor.
Regarding organic and/or inorganic compounds, they are/can be used any water-
soluble
organic/inorganic salts of calcium, magnesium, titanium, zirconium, yttrium,
aluminum and
ammonium in proportions of 0.07% up to 4% weight basis in aqueous solution
depending on
the type of the treated material. In case of biomass, solvent concentration
can be reduced to
less than 1.5% while in case of coal and garbage/waste typically ranges
between 0.5-4%.
Additionally, all organic and/or inorganic acids that create water-soluble
salts with the
aforementioned cations can also be used. The acids concentration when
acids/salt mixtures
are applied is sufficiently low so that the pH of the solution is always
higher than 5, preferably
6.5. Although the use of higher concentrations of salts in the solutions is
feasible, it is not
considered as necessary to achieve the desired result.
Examples are salts of calcium acetate/citrate/nitrate and/or magnesium
acetate/citrate/nitrate
and/or ammonium acetate/citrate/nitrate. Also acetic acid, citric acid, nitric
acid can be used.
When magnesium, titanium, aluminum, yttrium, zirconium and/or ammonium salts
are used,
the addition of calcium salt to the mixture ranging from 1/10 up to 1/3 of the
total salts
concentration is always recommended for better results. However, the calcium
salts can be
used separately without the presence of other salts.
This reaction is carried out at temperatures between 110-160 C and pressure 2-
10atm when
the material is biomass, between 140-195 C, pressure 4-20atm when the material
is coal,
garbage/waste, between 140-245 C, pressure 4-45atm when the material is
plastic especially
Date recue/Date received 2023-03-24
WO 2017/115262 PCT/IB2016/057999
8
with high chlorine concentration such as poly-vinyl chloride, or other
synthetic material so that
the aqueous phase remains in liquid form and is not converted to gas. Although
higher
temperatures (250-350 C) and pressures could be used, the financial cost of
such an option
combined with the small additional benefits for the process itself, make such
a choice
unprofitable. The reaction time is now limited below 5 minutes in case of
biomass while in
case of coal, waste, plastic etc. materials, the treatment time ranges from 5
to 20 minutes, the
solid/aqueous phase ratio can range from 15grams per liter to 800grams per
liter depending
on the treated material where the higher solid/liquid ratio is observed in
case of coal, RDF
and waste/litter, longer reaction times can also be used if necessary
depending on the treated
material as well as on the desired properties which are going to be applied to
the pretreated
material.
After the end of the pretreatment process, the clean/upgraded solid/liquid end
product which
now contains very small to zero silicon, alkali metals, phosphorous, sulfur,
chlorine and heavy
metals concentration as well as increased cations concentration used in the
last process step
(Ca, Mg, Al, Ti, Zr, NH4, etc.,) is separated from the liquid phase in the
same way as
described in the operation of the pressurized reactor, while the
liquid/aqueous phase is
recycled back to the process.
Purification of the liquid phase from inorganic elements such as potassium,
sodium,
phosphorus, sulfur, chlorine, heavy metals is carried out after several loops
using ion
exchange resins when sign of saturation of the aqueous solution with the
specific
components is occurred.
The following examples are presented in order to indicate the effect of the
invention on
various materials such as biomass, coal, tires. However, the implementation
and results of
the method are not limited by the examples given here.
Exam ple 1
Wheat straw is treated at elevated pressure using the reactor shown in Figure
1. Since this
material contains a large proportion of silicon in the ash, its pretreatment
is focused in the first
stage on trying to remove the silicon from the ash. In order to achieve that,
the sample is
treated in the first stage using sodium hydroxide in the first compartment of
the pressurized
reactor. The applied conditions are the following: Temperature 147 C, pressure
5-9atm,
solid/liquid ratio 10% w/w dry basis, leaching time 4.8 minutes, solvent
concentration 1% w/w,
material particle size <1mm. After the first step of pretreatment the sample
is moved to the
second pressurized compartment where it is treated in the second step aiming
at the removal
of alkali metals, phosphorus, chlorine, sulfur, as well as the deactivation of
components
remaining in the material structure after the end of the process by means of
appropriate salts
so that they will be no longer a problem for the further thermochemical
treatment of the
treated material. The applied conditions are the following: Temperature 148 C,
pressure 5-
9atm, solid/liquid ratio 10% w/w dry basis, leaching time 4.9 minutes, solvent
concentration
1.2% w/w and calcium chloride as solvent. After the pretreatment, the sample
is dried at
C. The final solid sample appears to have increased ease of milling requiring
30-40% less
energy than the original raw wheat straw while it favors the production of
greater strength
45 pellets requiring reduced energy consumption by 30-50% compared again to
the original raw
straw. The ash content of the final treated material appears to be reduced by
more than 30%,
the silicon concentration appears to be reduced by 80%, while the
concentrations of chlorine
and active alkali metals are practically zero. The sulfur and phosphorus
concentrations
appear significantly reduced by 60-70% for sulfur and from 60% up to 70% for
phosphorous.
50 At the same time, the calcium concentration is significantly increased and
is now more than
Date recue/Date received 2023-03-24
WO 2017/115262 PCT/IB2016/057999
9
60% of the treated material ash. Both raw and treated material ash is
thermally treated in a
high temperature oven starting from 600 C followed by 50 C steps. Table 2
shows the results
of thermal treatment. It is clear that the ash of the treated material appears
to have
significantly increased thermal resistance while the ash melting point is
increased to 1550 C,
from 800 C in case of raw material.
Example 2
Olive kernel is treated at elevated pressure using the reactor shown in Figure
1. Since this
material contains a large proportion of silicon in the ash, its pretreatment
is focused in the first
stage on trying to remove the silicon from the ash. In order to achieve that,
the sample is
treated in the first stage using potassium hydroxide in the first compartment
of the
pressurized reactor. The applied conditions are the following: Temperature 147
C, pressure 5-
9atm, solid/liquid ratio 10% w/w dry basis, leaching time 4.2 minutes, solvent
concentration
0.8% w/w, material particle size <1mm. After the first step of pretreatment
the sample is
moved to the second pressurized compartment where it is treated in the second
step aiming
at the removal of alkali metals, phosphorus, chlorine, sulfur, as well as the
deactivation of
components remaining in the material structure after the end of the process by
means of
appropriate salts so that they will be no longer a problem for the further
thermochemical
treatment of the treated material. The applied conditions are the following:
Temperature
138 C, pressure 5-7atm, solid/liquid ratio 15% w/w dry basis, leaching time
4.9 minutes,
solvent concentration 1.2% w/w and calcium nitrate as solvent. After the
pretreatment, the
sample is dried at 50 C. The final solid sample appears to have increased ease
of milling
requiring 30-40% less energy than the original raw olive kernel while it
favors the production
of greater strength pellets requiring reduced energy consumption by 30-50%
compared again
to the original raw olive kernel. The ash content of the final treated
material appears to be
reduced by more than 40%, the silicon concentration appears to be reduced by
90%, while
the concentrations of chlorine and active alkali metals are practically zero.
The sulfur and
phosphorus concentrations appear significantly reduced by 40% for sulfur and
from 60% up
to 80% for phosphorous. The concentration of nitrogen is reduced by 45%. At
the same time,
the calcium concentration is significantly increased and is now more than 60%
of the treated
material ash. Both raw and treated material ash is thermally treated in a high
temperature
oven starting from 600 C followed by 50 C steps. Table 2 shows the results of
thermal
treatment. It is clear that the ash of the treated material appears to have
significantly
increased thermal resistance while the ash melting point is increased to 1450
C, from 850 C
in case of raw material.
Then both the untreated and the treated material are used in fast pyrolysis
tests (t = 2sec) at
600 C. These tests showed that the material conversion into gaseous and liquid
products was
increased from 80% to 93% at 600 C after pretreatment. At the same time,
although SO2 was
produced in the final gaseous and liquid products during pyrolysis of the raw
material, there
was no presence of SO2 in case of the treated material. Additionally, the
production of liquid
hydrocarbons appears to be decreased by more than 85% in case of the treated
sample
while the primary end product is a gas mixture rich in H2, CO, CH4, and other
hydrocarbons.
Example 3
Coal (HSMc) from a US Mine is treated at elevated pressure using the reactor
shown in
Figure 1. Since this material contains a large proportion of silicon in the
ash, its pretreatment
is focused in the first stage on trying to remove the silicon from the ash. In
order to achieve
that, the sample is treated in the first stage using sodium hydroxide in the
first compartment of
the pressurized reactor. The applied conditions are the following: Temperature
165 C,
pressure 10-20atm, solid/liquid ratio 35% w/w dry basis, leaching time 19
minutes, solvent
Date recue/Date received 2023-03-24
WO 2017/115262 PCT/IB2016/057999
concentration 3.8% w/w, material particle size <1mm. After the first step of
pretreatment the
sample is moved to the second pressurized compartment where it is treated in
the second
step aiming at the removal of alkali metals, phosphorus, chlorine, sulfur, as
well as the
deactivation of components remaining in the material structure after the end
of the process by
5 means of appropriate salts so that they will be no longer a problem for the
further
thermochemical treatment of the treated material. The applied conditions are
the following:
Temperature 195 C, pressure 18-20atm, solid/liquid ratio 35% w/w dry basis,
leaching time 20
minutes, solvent concentration 4% w/w and calcium chloride as solvent. After
the
pretreatment, the sample is dried at 50 C. The ash content of the final
treated material
10 appears to be reduced by more than 35%, the silicon concentration
appears to be reduced by
70%, while the concentrations of chlorine and active alkali metals are
practically zero. The
sulfur concentration appears to be significantly reduced by 50-70%, nitrogen
concentration is
reduced by 55%, while the concentration of heavy metals such as Hg, Pb, Ni,
Cd, As, etc.
appears to be reduced by 60-95%. At the same time, the calcium concentration
is significantly
increased and is now more than 50% of the treated material ash. Both raw and
treated
material ash is thermally treated in a high temperature oven starting from 800
C followed by
50 C steps. Table 2 shows the results of thermal treatment. It is clear that
the ash of the
treated material appears to have significantly increased thermal resistance
while the ash
melting point is increased to 1450 C, from 1300 C in case of raw material.
Then both the untreated and the treated material are used in fast pyrolysis
tests (t = 2sec) at
600 C and 800 C. These tests showed that the material conversion into gaseous
and liquid
products was increased from 41 to 75% at 600 C and from 70 to 85% at 800 C
after
pretreatment. At the same time, although SO2 was produced in the final gaseous
and liquid
products during pyrolysis of the raw material, there was 90% reduction in the
presence of SO2
in case of the treated material. Additionally, the production of liquid
hydrocarbons appears to
be decreased by more than 80% in case of the treated sample while the primary
end product
is a gas mixture rich in H2, CO, CH4, and other hydrocarbons.
Example 4
Coal (EBWM) from a US Mine is treated at elevated pressure using the reactor
shown in
Figure 1. Since this material contains a large proportion of silicon in the
ash, its pretreatment
is focused in the first stage on trying to remove the silicon from the ash. In
order to achieve
that, the sample is treated in the first stage using sodium hydroxide in the
first compartment of
the pressurized reactor. The applied conditions are the following: Temperature
185 C,
pressure 15-20atm, solid/liquid ratio 28% w/w dry basis, leaching time 15
minutes, solvent
concentration 3% w/w, material particle size <1mm. After the first step of
pretreatment the
sample is moved to the second pressurized compartment where it is treated in
the second
step aiming at the removal of alkali metals, phosphorus, chlorine, sulfur, as
well as the
deactivation of components remaining in the material structure after the end
of the process by
means of appropriate salts so that they will be no longer a problem for the
further
thermochemical treatment of the treated material. The applied conditions are
the following:
Temperature 195 C, pressure 18-20atm, solid/liquid ratio 28% w/w dry basis,
leaching time 15
minutes, solvent concentration 2.5% w/w and calcium nitrate/calcium chloride
ratio: 50/50 as
solvent. After the pretreatment, the sample is dried at 50 C. The ash content
of the final
treated material appears to be reduced by more than 40%, the silicon
concentration appears
to be reduced by 80%, while the concentrations of chlorine and active alkali
metals are
practically zero. The sulfur concentration appears to be significantly reduced
by 60-70%,
while the concentration of heavy metals such as Hg, Pb, Ni, Cd, As, etc.
appears to be
reduced by 60-98%. At the same time, the calcium concentration is
significantly increased
and is now more than 50% of the treated material ash. Both raw and treated
material ash is
Date recue/Date received 2023-03-24
WO 2017/115262 PCT/IB2016/057999
11
thermally treated in a high temperature oven starting from 800 C followed by
50 C steps.
Table 2 shows the results of thermal treatment. It is clear that the ash of
the treated material
appears to have significantly increased thermal resistance while the ash
melting point is
increased to 1450 C, from 1300 C in case of raw material.
Then both the untreated and the treated material are used in fast pyrolysis
tests (t = 2sec) at
600 C and 800 C. These tests showed that the material conversion into gaseous
and liquid
products was increased from 48 to 73% at 600 C and from 65 to 80% at 800 C
after
pretreatment. At the same time, although SO2 was produced in the final gaseous
and liquid
products during pyrolysis of the raw material, there was 92% reduction in the
presence of SO2
.. in case of the treated material. Additionally, the production of liquid
hydrocarbons appears to
be decreased by more than 80% in case of the treated sample while the primary
end product
is a gas mixture rich in H2, CO, CFI4, and other hydrocarbons.
Example 5
Used car tires are treated at elevated pressure using the reactor shown in
Figure 1 utilizing
calcium nitrate as solvent. Since this material does not contain a large
proportion of silicon in
the ash, the pretreatment is focused on the removal of alkali metals,
phosphorus, chlorine,
sulfur, as well as the deactivation of components remaining in the material
structure after the
end of the process by means of appropriate salts so that they will be no
longer a problem for
the further thermochemical treatment of the treated material. The applied
conditions are the
following: temperature 147 C, pressure 5-7atm, solid/liquid ratio 20% w/w dry
basis, leaching
time 7.5 minutes, solvent concentration 3% w/w, material particle size <3mm.
After the
pretreatment, the sample is dried at 50 C. After the pretreatment, 2.1% weight
increase of the
treated dry material is noticed because of the calcium absorption by the
material. Sample
analysis by electron microscopy SEM-EDX confirms the significantly increased
calcium
concentration in the sample as well as the absence of chlorine and alkali
metals while the
sulfur concentration appears to be significantly reduced by 17-35 A. Then both
the untreated
and the treated material are used in fast pyrolysis tests (t = 2sec) at 600 C
and 800 C. These
tests showed that the material conversion into gaseous and liquid products was
increased
from 37 to 75% at 600 C and from 73 to 93.5% at 800 C after pretreatment. At
the same time,
although SO2 was produced in the final gaseous and liquid products during
pyrolysis of the
raw material, there was no presence of SO2 in case of the treated material.
Additionally, the
production of liquid hydrocarbons appears to be decreased by more than 80% in
case of the
treated sample while the primary end product is a gas mixture rich in H2, CO,
CH4, and other
hydrocarbons.
45
Date recue/Date received 2023-03-24
WO 2017/115262 PCT/IB2016/057999
12
TABLE 1 Ash analysis and characterization of biomass, coal
Analysis Wheat Pretreated Olive Pretreated Coal
Pretreated Coal Pretreated
( /0) Straw Wheat Kernel Olive (HSMc) Coal (EBWM)
Coal
Straw Kernel (HSMc) (EBWM)
Ash 8.34 5.6 4.3 2.5 8.58 6.4 15.12 ' 10.3
Content
*K20 1.31 ' 0.2 1.22 ' 0.16 ' 0.14 0.03 0.45
0.04
*Na20 0.56 0.09 0.03 0.02 0.07 0.02 0.12 0.019
SiO2 - 5 1 0.57 ' 0.07 ' 3= .38 0.83 6.8
0.78
CaO 0.39 2.9 0.97 1.7 - 0= .23 3.6 0.74
5.7
P205 0.35 0.05 0.2 0.05 rid nd nd rid
SO2 0.13 0.02 0.05 0.03 0.35 0.1 0.73 0.21
Cl - 0.13 ' 0.00 0.12 0.00 - 0.047 0.00 0.15 '
0.00
Analysis
(PPrn)
Cd nd nd rid Nd 0.38 0.039 1.2 0.12
As ' nd nd nd Nd ' 5.7 0.95 5.98
0.32
Ni nd nd nd Nd - 3= 1.9 2.15 33.86
1.55
Hg nd rid rid Nd 0.2 0.04 0.17 0.04
Zn nd rid rid Nd - 34.18 4.76 55.78
2.59
Pb nd rid rid Nd - 155.7 1.32 9.96 '
0.71 '
Cr nd rid rid Nd 11.77 4.01 27.89 2.18
Cu nd rid nd ' Nd 81.66 2.88 41.83 1.47
nd: not detected, *: non reactive forms in the case of the pretreated sample
TABLE 2 Thermal behavior of ash from raw and pretreated biomass types and coal
Ash samples Melting point ( C)
Raw olive kernel 850
Pretreated olive kernel 1450
Raw wheat straw 800
'
Pretreated wheat straw 1550
Raw coal (HSMc) 1300
,
Pretreated coal (HSMc) 1450
Raw coal (EBWM) 1300
Pretreated coal (EBWM) 1450
- _
Date recue/Date received 2023-03-24