Note: Descriptions are shown in the official language in which they were submitted.
A GUIDEWIRE WITH AN INTEGRATED OPTICAL FIBER
FIELD OF THE INVENTION
The present invention relates generally to medical
devices, and particularly to methods and systems for producing
integrated guidewires and for applying these guidewires in
medical procedures.
BACKGROUND OF THE INVENTION
Guidewires are used in various medical applications,
such as in neurology, cardiology and sinuplasty.
For example, U.S. Patent Application Publication
2003/0181894 describes a device and method for preventing
restenosis and streamlining an angioplasty procedure. The
device and method provide a fiber-optic guidewire, or,
alternatively, a light-conducting catheter, to decrease the
size of the angioplasty device, decrease the overall time of
the procedure, and increase the safety of the procedure.
U.S. Patent 5,441,497 describes a light diffusing
guidewire which has the ability to deliver light to luminal
surfaces such as blood vessels for the diagnosis and treatment
of medical conditions. The guidewire has an elongate body
portion having a proximal end and a distal (invasive) end. A
portion of the body portion transmits light from the proximal
end to a light diffusing element within the body portion near
the distal end.
U.S. Patent Application Publication 2006/0074442
describes a deflectable and torqueable hollow guidewire
device for removing occlusive material and passing through
occlusions and other materials in a body lumen. The hollow
guidewire generally comprises an elongate, tubular guidewire
body that has an axial lumen. A mechanically moving core
1
CA 3051888 2019-08-13
element is positioned at or near a distal end of the tubular
guidewire body and extends through the axial lumen.
U.S. Patent 5,372,587 describes a steerable tubular
sheath comprising an elongate flexible tubular body, having
a laterally deflectable distal tip. Lateral deflection of the
tip is accomplished by axial displacement of at least one
pull wire extending through the housing. The housing comprises
at least one central lumen extending axially therethrough,
for receiving medical implements, optical fibers, suction or
transmission of fluids such as for irrigation or drug
delivery.
SUMMARY OF THE INVENTION
An embodiment of the present invention that is described
herein provides an integrated guidewire including a wire and
an optical fiber. The wire is sized and shaped to move in an
anatomical material transportation system of a patient. The
optical fiber has proximal and distal ends, the proximal-end
is coupled to a device external to the patient, the optical
fiber is configured to transfer optical signals between the
distal-end and the device, and the wire and the optical fiber
are intertwined with respect to one another.
In some embodiments, the wire and the optical fiber are
fixed directly to one another at one or more coupling points
located between or at the distal-end and the proximal-end. In
other embodiments, the integrated guidewire includes an image
sensor configured to receive optical signals reflected from
an organ of the patient, and to produce, using the reflected
optical signals, an image of the organ. In yet other
embodiments, the intertwined wire and the optical fiber have,
2
CA 3051888 2019-08-13
between the distal-end and the proximal-end, multiple
windings around an axis of the integrated guidewire.
In an embodiment, a number of the windings sets stiffness
and flexibility levels of the integrated guidewire. In another
embodiment, the windings are distributed evenly between the
distal-end and the proximal-end. In yet another embodiment,
the windings are distributed unevenly between the distal-end
and the proximal-end.
In some embodiments, the integrated guidewire includes
at least one of an additional optical fiber and a flexible
tube configured to transfer fluids between the distal-end and
the proximal-end, and the at least one of the additional
optical fiber and flexible tube is intertwined with the wire
and the optical fiber. In other embodiments, the anatomical
material transportation system includes an anatomical system
of the patient selected from a list consisting of a
vasculature system, an ear-nose-throat (ENT) system, and a
neurological system.
There is additionally provided, in accordance with an
embodiment of the present invention, a method including
inserting into an anatomical material transportation system
of a patient an integrated guidewire that includes (i) a wire,
which is sized and shaped to move in the anatomical material
transportation system, and (ii) an optical fiber having
proximal and distal ends, the proximal-end is coupled to a
device external to the patient, the optical fiber is
configured to transfer optical signals between the distal-end
and the device, and the wire and the optical fiber are
intertwined with respect to one another. Anatomical
information is acquired from the patient by transferring
optical signals between the distal-end and the device.
3
CA 3051888 2019-08-13
There is further provided, in accordance with an
embodiment of the present invention, a method for producing
an integrated guidewire, the method includes providing a wire
which is sized and shaped to move in an anatomical material
transportation system of a patient. The wire and an optical
fiber are intertwined with respect to one another.
The present invention will be more fully understood from
the following detailed description of the embodiments
thereof, taken together with the drawings in which:
BRIEF DESCRIPTION OF THE DRAWINGS
Fig. 1 is a schematic, pictorial illustration of a
sinuplasty surgical system, in accordance with an embodiment
of the present invention;
Figs. 2A-20 are schematic, pictorial illustrations of an
integrated guidewire, in accordance with an embodiment of the
present invention;
Fig. 3 is a flow chart that schematically illustrates a
method for applying an integrated guidewire in medical
procedures, in accordance with an embodiment of the present
invention; and
Fig. 4 is a flow chart that schematically illustrates a
method for producing an integrated guidewire, in accordance
with an embodiment of the present invention.
DETAILED DESCRIPTION OF EMBODIMENTS
OVERVIEW
Embodiments of the present invention that are described
hereinbelow provide improved methods and systems for
producing and applying integrated guidewires comprising one
4
CA 3051888 2019-08-13
or more transferring devices, such as optical fibers and/or
fluid transferring tubes.
In principle, an optical fiber may be integrated in a
guidewire using various methods, such as by laser cutting a
spiral in a tube (to make the tube flexible) and feeding a
fiber optic through the tube. In another possible method, a
laser can be applied for cutting a spiral groove in a wire,
wherein the optical fiber in the groove. These methods,
however, are costly and difficult to apply in high volume
manufacturing (HVM). Moreover, some medical procedures
require drawing fluids out of a narrow lumen of a patient
body (e.g., a blood vessel in the brain), or administering
fluids thereto. Such procedures may be carried out using a
guidewire, but guidewires with integrated tubes are difficult
to manufacture.
Embodiments of the present invention provide improved
techniques for producing such integrated guidewires by
intertwining a wire and at least one of, (i) an optical fiber
and (ii) a flexible tube, with respect to one another.
In some embodiments, an integrated guidewire comprises
a wire, which is sized and shaped to move in an anatomical
material transportation system of a patient, such as in the
vasculature system of the brain, or in an ear-nose-throat
(ENT) system of the patient. In some embodiments, the
integrated guidewire further comprises one or more
transferring devices such as an optical fiber and a flexible
tube.
The proximal end of a transferring device is typically
coupled to a device that remains external to the patient body,
whereas the distal end of the transferring device is inserted
into the patient body. In some embodiments, the optical fiber
5
CA 3051888 2019-08-13
is configured to transfer optical signals between the distal-
end and the external device, and the flexible tube is
configured to transfer fluids and foreign material between
the distal-end and the external device. In an embodiment, the
wire and one or more transferring devices are intertwined
with respect to one another.
In some embodiments, the flexible tube may be coupled to
a reservoir of fluid external to the patient body, and
configured to transfer the fluid from the reservoir to an
organ in question. In other embodiments, the flexible tube
may be coupled to a pump, which is configured to draw fluids
and foreign material out of the patient body.
In an example embodiment, a physician may insert into
the patient brain, an integrated guidewire comprising a wire,
an optical fiber and a flexible tube, all are intertwined
with respect to one another. The physician may navigate the
integrated guidewire through the brain vasculature to a
location that is suspected to be clotted. In this embodiment,
the physician may investigate the clot by bringing the
integrated guidewire in close proximity thereto, and
illuminating the clot using the optical fiber. Based on the
information collected in the investigation, the physician may
apply the flexible tube for: (i) drawing the clot out of the
patient brain, or (ii) dissolving the clot by administering
a substance from the reservoir, or using any other suitable
technique, such as irrigation and/or a combination of
irrigation and suction of the clot out of the patient brain.
In another embodiment, the physician may sequentially
apply two integrated guidewires of different types, e.g., a
diagnostics guidewire comprising the intertwined wire and
optical fiber, and a treatment guidewire comprising the
6
CA 3051888 2019-08-13
intertwined wire and flexible tube. In this embodiment, the
physician may first apply the diagnostics guidewire so as to
investigate the clot, and subsequently retract the
diagnostics guidewire and insert the treatment guidewire for
treating the clot as described above.
The disclosed techniques improve the functionality of
integrated guidewires to carry out diagnostic and treatment
procedures, by enabling the integration of the wire with at
least an optical fiber and/or a flexible tube. Furthermore,
these techniques reduce the complexity and therefore cost of
producing such integrated guidewires.
SYSTEM DESCRIPTION
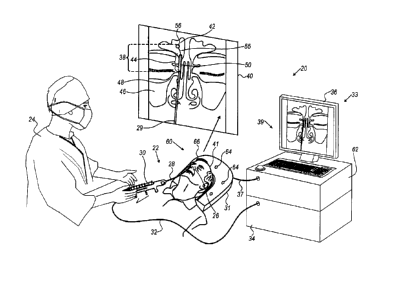
Fig. 1 is a schematic pictorial illustration of a
sinuplasty procedure using a surgical system 20, in accordance
with an embodiment of the present invention. In the example
of Fig. 1, system 20 comprises a catheter 28, which a
physician 24 inserts into a nose 26 of a patient 22 so as to
treat an ear-nose-throat (ENT) disease, such as infection in
one or more sinuses of patient 22. In other embodiments,
system 20 may be used in other medical procedures, such as in
diagnosing and treating a clot in the patient brain or other
organ. Additionally or alternatively, system 20 may be used
for administrating substances into, or suctioning material
out of an organ or an anatomical material transportation
system of patient 22, or for irrigating the organ or the
anatomical material transportation system of patient 22.
Reference is now made to an inset 40 that shows a frontal
anatomical view of the ENT system of patient 22. The ENT
system of patient 22 comprises a frontal sinus 42 and a
maxillary sinus 46. Ostia 44 and 48 connect between cavities
7
CA 3051888 2019-08-13
of the nose (not shown) and sinuses 42 and 46, respectively.
Catheter 28 comprises an integrated guidewire 29 having a
distal end 38. In the context of the present invention the
term "integrated guidewire" is also referred to below simply
as "guidewire" for brevity. In an embodiment, the tip of
distal end 38 may comprise a position sensor 56 attached at
the end of a residual end section 55 of guidewire 29.
Catheter 28 further comprises an inflatable balloon 50,
which may be configured in two positions, e.g., an expanded
(inflated) position and a collapsed position. When Balloon 50
is in the collapsed position, the catheter can be navigated
to the target location. The balloon is then inflated to the
expanded position using a suitable fluid (e.g., a saline
solution so as to anchor catheter 28 at the target location
(e.g., ostium 44) in the ENT system of patient 22.
Catheter 28 further comprises a handle 30, which is
located at the proximal end of catheter 28. Handle 30 is
configured to control the navigation of guidewire 29 and the
motion of balloon 50 along guidewire 29.
In some embodiments, system 20 further comprises a
location pad 60 placed at a known position external to patient
22 lying on table 31, pad 60 comprises field-generators 64
fixed on a frame 66. In the exemplary configuration shown in
Fig. 1, pad 60 comprises five field-generators 64, but may
alternatively comprise any other suitable number of field-
generators 64. Pad 60 further comprises a pillow (not shown)
placed under a head 41 of patient 22, such that field-
generators 64 are located at fixed, known positions external
to head 41.
In some embodiments, system 20 comprises a console 33,
which comprises a driver circuit 62 configured to drive, via
8
CA 3051888 2019-08-13
a cable 37, field-generators 64 with suitable signals so as
to generate magnetic fields in a predefined working volume in
space around head 41. In some embodiments, console 33
comprises a processor 34, typically a general-purpose
computer, with suitable front end and interface circuits for
receiving, via a cable 32, signals from catheter 28. Console
33 further comprises input devices 39 and a display 36, which
is configured to display data (e.g., images) received from
processor 34 or inputs inserted by a user (e.g., physician
24). In an embodiment, the position of position sensor 56 is
typically measured by magnetic position sensing of a catheter
position tracking system comprised in system 20.
This method of position sensing is implemented in various
medical applications, for example, in the CARTOTm system,
produced by Biosense Webster Inc. (Irvine, Calif.) and is
described in detail in U.S. Patents 5,391,199, 6,690,963,
6,484,118, 6,239,724, 6,618,612 and 6,332,089, in PCT Patent
Publication WO 96/05768, and in U.S. Patent Application
Publications 2002/0065455 Al, 2003/0120150 Al and
2004/0068178 Al, whose disclosures are all incorporated
herein by reference.
In some embodiments, system 20 may comprise an optical
module, comprising a light source (not shown) that may be
disposed in a suitable device of systems 20, such as in
console 33 or in handle 30, and an image sensor (not shown)
that may be mounted on distal end 38. In some embodiments,
the light source and image sensor are optically coupled to
one another via a light transferring device, such as an
optical fiber, shown in Figs. 2A, 2B and 2C below.
In other embodiments, system 20 may comprise a fluid
distribution module, which comprises a fluid reservoir (not
9
CA 3051888 2019-08-13
shown) filled with a fluid for medical use, such as irrigation
fluid for irrigating an organ, or some substance for treating
an infection or a tumor in an organ of patient 22. The
reservoir may be disposed in any suitable device of systems
20, such as in console 33 or in handle 30, or in a separate
tank of fluids.
In an embodiment, the fluids may be transferred from the
reservoir to distal end 38 via a fluid transferring device,
such as a flexible tube, shown in Figs. 2A, 2B and 20 below,
and disposed to a target organ through fluid distribution
holes (not shown) formed in distal end 38. In some
embodiments, the fluid transferring device may comprise a
flexible irrigation tube having one or more openings, e.g.,
irrigation holes (not shown). The flexible irrigation tube is
configured to transfer, via the irrigation holes, irrigation
fluid from the reservoir to an organ in question. In other
embodiments, the fluid transferring device is configured to
transfer a liquid substance, such as a drug, for treating
infection or a tumor in the organ in question.
In alternative embodiments, system 20 may comprise a
material evacuation module (not shown) comprising a suction
pump (not shown), which is configured to pump materials out
of an organ of patient 22, via the fluid transferring device,
e.g., into a sink (not shown) located external to patient 22.
During the sinuplasty procedure, physician 24 navigates
the tip of guidewire 29 into sinus 42. In some cases, e.g.,
when treating infection in the sinus, it is important for the
physician to anchor the distal tip of the catheter, for
example by inflating balloon 50 in ostium 44. In an
embodiment, balloon 50 may be 16 mm long and may have a
diameter of 5 mm, such as sinuplasty balloon produced by
CA 3051888 2019-08-13
Acclarent Inc. (catalog number RSP0516MFS), yet any other
suitable balloon with other dimensions may be used in the
disclosed techniques.
After inserting distal end 38 into the ENT system,
physician 36 navigates balloon 50 to ostium 44. Note that,
typically, balloon 50 does not comprise a position sensor and
is not otherwise imaged on display 36. To perform the
treatment safely and efficiently, it is important to position
balloon 50 accurately within ostium 44. For example,
positioning balloon 50 in the nose cavity, short of ostium
44, may not allow the physician to anchor end section 55
within sinus 42, whereas positioning the balloon within sinus
42, deeper than ostium 44, may disturb the physician in
treating the infection therein.
In the example of Fig. 1, balloon 50 is used for
anchoring end section 55 within sinus 42. In alternative
embodiments, any other suitable device may be positioned using
the disclosed techniques, instead of balloon 50. Such a device
may comprise, for example, an alternative anchoring device
for anchoring the end section or for any other diagnostic or
treatment purpose. For example, a balloon may be used for
treating cardiac arrhythmia at a pulmonary vein (PV) in a PV
isolation procedure. In other applications, a drug dispensing
device or a stent may be navigated to a specific location in
a human organ, using the techniques described above.
In some embodiments, processor 34 is configured to assist
physician 24 to position balloon 50 accurately within ostium
44. Fig. 1 shows only elements related to the disclosed
techniques, for the sake of simplicity and clarity. System 20
typically comprises additional modules and elements that are
not directly related to the disclosed techniques, and thus,
11
CA 3051888 2019-08-13
intentionally omitted from Fig. 1 and from the corresponding
description.
Processor 34 may be programmed in software to carry out
the functions that are used by the system, and to store data
in a memory (not shown) to be processed or otherwise used by
the software. The software may be downloaded to the processor
in electronic form, over a network, for example, or it may,
alternatively or additionally, be provided on non-transitory
tangible media, such as optical, magnetic or electronic memory
media. Alternatively, some or all of the functions of
processor 34 may be carried out by dedicated or programmable
digital hardware components.
In some embodiments, catheter 28 and guidewire 29 may be
used in applying medical procedures to various human
anatomical systems, such as but not limited to the vasculature
system, ENT system, neurological system and patient heart. In
an example embodiment, guidewire 29 may comprise an optical
fiber (shown in Fig. 2 below) that may be used for
illuminating the inner lumen of the blood vessels in the brain
of patient 22 so as to investigate a clot in the brain or a
tear in a blood vessel, or for any other diagnostics or
treatment purpose in any organ or material transportation
system of patient 22, as will be described in detail below.
In another exemplary embodiment, guidewire 29 may comprise a
flexible tube (shown in Fig. 2 below) that may be used for
irrigating an organ of patient 22 (e.g., for cooling the organ
during ablation), or for administering a substance into an
organ of patient 22 (e.g., for treating infection or a tumor),
or for suctioning some material to be removed out of an organ
of the patient.
12
CA 3051888 2019-08-13
INTEGRATED GUIDEWIRE COMPRISING AN OPTICAL FIBER AND/OR A
FLEXIBLE TUBE
Fig. 2A is a schematic, pictorial illustration of an
integrated guidewire 65, in accordance with an embodiment of
the present invention. Guidewire 65 may replace, for example,
guidewire 29 of Fig. 1 above. In some embodiments, guidewire
65 comprises a wire 70, which is sized and shaped to move in
an anatomical material transportation system of patient 22.
The transportation system may comprise ostia 44 and 48 and
sinuses 42 and 46 of the ENT system shown in the example of
Fig. 1, or blood vessels connecting between the heart (not
shown) and any organ of patient 22, such as the brain.
In some embodiments, wire 70 is made from any suitable
biocompatible material, such as an alloy of nickel-titanium,
stainless steel, titanium, nickel, or from gold, or platinum.
In other embodiments, wire 70 is made from two or more parts,
one of which comprises a core, made from any suitable
material, coated with a biocompatible material.
In some embodiments, guidewire 65 comprises a
transferring device 72 having a proximal end 73 and a distal
end 71, such that wire 70 and transferring device 72 are
intertwined with respect to one another. In the configuration
of Fig. 2A, wire 70 is laid out straight and aligned with a
longitudinal axis 78 of guidewire 65, whereas transferring
device 72 is wound around axis 78 and is coupled to an outer
surface of wire 70. In some embodiments, transferring device
72 may comprise an optical fiber which is coupled to a light
source (not shown), and is configured to transfer optical
signals between the light source and distal end 71.
In an example embodiment, physician 24 may apply handle
30 for moving guidewire 65 in a blood vessel of the patient
13
CA 3051888 2019-08-13
brain so as to bring distal end 71 adjacent to a clot in the
brain. In this embodiment, the optical fiber transfers light
from the light source toward the distal end so as to
illuminate a section of the brain and the clot, and further
transfers light that is reflected by the brain and clot back
to the image sensor at the proximal end as described in Fig.
1 above, so as to acquire anatomical information on the clot
and the brain section.
In another embodiment, transferring device 72 may
comprise a flexible tube, which is coupled to the fluid
reservoir described in Fig. 1 above, and is configured to
transfer fluids between the reservoir and distal end 71. In
an embodiment, transferring the fluid may be used for
irrigating an organ, for example, during an ablation
procedure. Alternatively, the flexible tube may be used for
administering medication from the reservoir to an organ of
patient 22, for treating the infection or tumor as described
above.
In alternative embodiments, transferring device 72 may
comprise the flexible tube described above, which is coupled
to a pump and configured to draw material, such as infection
or any undesired material, from the body of patient 22. In
these embodiments, distal end 71 of the flexible tube is
disposed in the organ in question, and proximal end 73 is
coupled to the pump and sink described in Fig. 1 above.
In some embodiments, during the production of integrated
guidewire 65, transferring device 72 is wound around wire 70,
thereby forming multiple windings, such as windings 74 and 76
having a predefined pitch size. It will be understood that
the pitch size and the number of windings may be varied so as
to obtain a desired tradeoff between flexibility and stiffness
14
CA 3051888 2019-08-13
of integrated guidewire 65. In the example embodiment of Fig.
2A, integrated guidewire 65 comprises eight windings of
transferring device 72 around wire 70. In another embodiment,
the guidewire may comprise only five windings, resulting in
lower stiffness and higher flexibility compared to the example
embodiment of Fig. 2A. In other words, a smaller pitch size
results in higher stiffness and lower flexibility of the
integrated guidewire.
Note that the pitch size may be uniform, or may vary,
along the integrated guidewire, so as to obtain different
levels of stiffness and flexibility along the integrated
guidewire.
In some embodiments, transferring device 72 and wire 70
are cemented to one another at selected locations along
integrated guidewire 65, typically including distal end 71
and proximal end 73. For example, transferring device 72 and
wire 70 may be coupled to one another at least at one coupling
point located between distal-end 71 and proximal-end 73. In
the context of the present invention, the term "cemented"
refers to coupling between transferring device 72 and wire 70
using any suitable coupling technique, such as gluing or
soldering. In some embodiments, the materials used for
cementing between device 72 and wire 70 are typically
biocompatible, or coated with a biomaterial after the
cementing process.
The various configurations of integrated guidewire 65
are depicted purely by way of example. In alternative
embodiments, guidewire 65 may comprise any suitable number
and types of transferring devices wound around wire 70 in any
suitable winding configuration. For example, integrated
CA 3051888 2019-08-13
guidewire 65 may comprise two or more transferring devices
intertwined around axis 78, on the outer surface of wire 70.
Fig. 2B is a schematic, pictorial illustration of an
integrated guidewire 75, in accordance with another
embodiment of the present invention. Guidewire 75 may replace,
for example, guidewire 29 of Fig. 1 above. In some
embodiments, integrated guidewire 75 comprises a wire 80
having similar properties to wire 70 of Fig. 2A, and a
transferring device 82 having a proximal end 83 and a distal
end 81, such that wire 80 and transferring device 82 are
intertwined with respect to one another.
In the configuration of Fig. 2B, transferring device 82
is laid out straight and aligned with a longitudinal axis 88
of guidewire 75, whereas wire 80 is wound around axis 88 and
is coupled to an outer surface of transferring device 82.
In some embodiments, transferring device 82 may comprise
an optical fiber which is coupled to the light source (not
shown), described in Fig. 1 above, and is configured to
transfer optical signals between the light source and distal
end 81, as also described in Fig. 2A above.
In another embodiment, transferring device 82 may
comprise a flexible tube, which is coupled to the fluid
reservoir described in Fig. 1 above. In the form of the
flexible tube, transferring device 82 is configured to
transfer fluids, such as irrigation fluids or medication
substance, between the reservoir and distal end 81, as
described in Fig. 2A above.
In alternative embodiments, transferring device 82 may
comprise a flexible tube, which is coupled to a pump and
configured to draw material, such as infection or any
undesired material, from the body of patient 22. In these
16
CA 3051888 2019-08-13
embodiments, distal end 81 of the flexible tube is disposed
in the organ in question, and proximal end 83 is coupled to
the pump and sink described in Fig. 1 above.
In some embodiments, during the production of integrated
guidewire 75, wire 80 is wound around transferring device 82,
thereby forming multiple windings, such as windings 84 and 86
having an even or a variable pitch size. In these embodiments,
the windings are distributed evenly along integrated
guidewire 75, between distal end 81 and proximal end 83. As
described in Fig. 2A above, the pitch size and the number of
windings may vary along axis 88 so as to determine the
flexibility and stiffness of each section of integrated
guidewire 75.
In some embodiments, transferring device 82 and wire 80
are cemented to one another, using the techniques described
in Fig. 2A above.
Fig. 20 is a schematic, pictorial illustration of an
integrated guidewire 85, in accordance with another
embodiment of the present invention. Guidewire 85 may replace,
for example, guidewire 29 of Fig. 1 above. In some
embodiments, integrated guidewire 85 comprises a wire 90
having similar properties to wire 70 of Fig. 2A, and a
transferring device 92 having a proximal end 93 and a distal
end 91.
During the production of integrated guidewire 85,
transferring device 92 and wire 90 are intertwined with
respect to one another as in a twisted pair configuration,
and subsequently, are cemented to one another, using the
cementing techniques described in Fig. 2A above.
In some embodiments, during the production of guidewire
85, transferring device 92 and wire 90 are coupled to one
17
CA 3051888 2019-08-13
another, permanently or temporarily, at one end (e.g., at
proximal end 93). Subsequently, device 92 and wire 90 are
braided relative to one another around a longitudinal axis 98
of guidewire 85, such that neither wire 90 nor transferring
device 92 are laid out straight.
After shaping guidewire 85 as a braid, device 92 and
wire 90 may be permanently coupled to one another, typically
at the distal and proximal ends, using the techniques
described in Fig. 2A above.
In the example of Fig. 2C, integrated guidewire 85 has
a uniform pitch 94 substantially smaller than pitch 84 of
guidewire 75, resulting in thirteen windings per 5 mm of
linear length in guidewire 85 compared to eight windings per
5 mm in guidewire 75. As a result, guidewire 85 has higher
stiffness and lower flexibility compared to guidewire 75,
assuming wires 80 and 90 are substantially identical, and
using substantially identical transferring devices 82 and 92.
Note that in other configurations the size of pitch 94 may
vary along axis 98 of guidewire 85, so as to obtain the
desired stiffness and flexibility as each section of
integrated guidewire 85. In case increased stiffness is
desired, the twisted pair of wire 90 and transferring device
92 may be fixed directly together using any suitable fixation
technique, e.g., epoxy, polyurethane, staples or crimping
bands.
In some embodiments, transferring device 92 may comprise
an optical fiber which is coupled to the light source (not
shown), described in Fig. 1 above, and is configured to
transfer optical signals between the light source and distal
end 91, as also described in Fig. 2A above.
18
CA 3051888 2019-08-13
In another embodiment, transferring device 92 may
comprise a flexible tube, which is coupled to the fluid
reservoir described in Fig. 1 above. In the form of the
flexible tube, transferring device 92 is configured to
transfer fluids, such as irrigation fluids or medication
substance, between the reservoir and distal end 91, as
described in Fig. 2A above.
In alternative embodiments, transferring device 92 may
comprise a flexible tube, which is coupled to a pump and
configured to draw material, such as infection or any
undesired material, from the body of patient 22. In these
embodiments, distal end 91 of the flexible tube is disposed
in the organ in question, and proximal end 93 is coupled to
the pump and sink described in Fig. 1 above.
The particular configurations of integrated guidewires
65, 75 and 85 are shown by way of example, in order to
illustrate certain problems that are addressed by embodiments
of the present invention and to demonstrate the application
of these embodiments in enhancing the performance of a medical
system such as system 20.
Embodiments of the present invention, however, are by no
means limited to this specific sort of example integrated
guidewires, and the principles described herein may similarly
be applied to other sorts of integrated guidewires. In an
alternative embodiment, another type of integrated guidewire
may comprise two or more transferring devices. In an example
embodiment, the guidewire may comprise a single wire and two
flexible tube, a first tube for pumping fluids to the organ
in question and a second tube for drawing material out of the
patient body. The wire and the flexible tubes may be wound
around the longitudinal axis of the integrated guidewire,
19
CA 3051888 2019-08-13
using any suitable winding technique, such as the techniques
described in Figs. 2A-2C above. This configuration may be
used to remove undesired material from patient body using a
flux of incoming fluid from the first tube and using the
second tube for drawing a mixture of the undesired material
and the incoming fluid. In alternative embodiments, the
integrated guidewire may comprise an optical fiber and a
suction tube, for example, so as to investigate the clot in
the brain, and if medically applicable, to draw the clot out
of the brain thereafter, using the suction tube.
In other embodiments, the integrated guidewire may
comprise any combination of one or more wires, wound around
an axis with one or more optical fibers, and/6r one or more
flexible tubes, and/or any other one or more suitable types
of transferring devices.
Fig. 3 is a flow chart that schematically illustrates a
method for applying integrated guidewire 75 in various medical
procedures, in accordance with an embodiment of the present
invention. Note that integrated guidewire 75 was selected
purely by way of example. In alternative embodiments, any
other suitable type of integrated guidewire, such as
guidewires 65 and 85 depicted above, may be applied in
addition to, or instead of guidewire 75.
The method begins at a guidewire insertion step 100,
with physician 24 inserting integrated guidewire 75 into an
anatomical material transportation system of patient 22. As
described in Fig. 2B above, guidewire 75 comprises wire 80
intertwined with transferring device 82, such as the optical
fiber or the flexible tube.
At a navigation step 102, physician 24 navigates
integrated guidewire 75 to an organ in question, such as the
CA 3051888 2019-08-13
brain or frontal sinus 42 of patient 22. At a first decision
step 104, physician 24 checks whether investigation of a
potential clot in the brain of patient 22 is required.
When at step 104 physician 24 decides to investigate the
potential clot and assuming guidewire 75 comprises the optical
fiber, physician 24 may apply guidewire 75 to acquire
anatomical information, such as images of the clot, at an
anatomical image acquisition step 106. After concluding the
acquisition of clot images, physician 24 may retract guidewire
75 out of the body of patient 22, at a guidewire retraction
step 118.
At a second decision step 108, physician 24 checks
whether irrigation of an organ of patient 22 is required, for
example, to open a block at ostium 44 of the ENT system. When
at step 108 the physician decides that irrigation is required,
physician 24 may apply guidewire 75 to irrigate ostium 44, at
an irrigation step 110.
After irrigating ostium 44, physician 24 may retract
guidewire 75 out of the body of patient 22, at guidewire
retraction step 118.
At a third decision step 112, in an embodiment, physician
may identify an infection in frontal sinus 42 and has to
decide whether to administer a suitable substance, such as an
antibiotic drug, for treating the infection. In another
embodiment, based on the images acquired at step 106,
physician 24 may consider to dissolve the clot in the brain
of patient 22. At a substance administration step 114,
physician may apply guidewire 75 to administrate the substance
into the organ in question of patient 22. For example, by
administering the antibiotic drug into frontal sinus 42, or
21
CA 3051888 2019-08-13
by administering a material adapted to dissolve the clot in
the brain of patient 22.
After concluding substance administration step 114,
physician 24 may retract guidewire 75 out of the body of
patient 22, at guidewire retraction step 118.
At an alternative procedure step 116, physician 24 may
decide to treat the infection in frontal sinus 42 and/or the
clot in the brain of patient 22 using alternative techniques
to substance administration. For example, physician 24 may
apply guidewire 75 having the suction tube described, for
example, in Fig. 2A above. In this embodiment, physician 24
may apply the suction tube of guidewire 75 to draw the
infection from sinus 42 and/or to draw the clot from the brain
of patient 22.
After concluding the material drawing at step 116,
physician 24 may retract guidewire 75 out of the body of
patient 22, at guidewire retraction step 118.
In some embodiments, the procedures described at steps
106, 110, 114 and 116 may be carried out separately, such
that retraction step 118 concludes the method for each
procedure. In other embodiments, two or more of steps 106,
110, 114 and 116 may be carried out sequentially or
simultaneously without retracting guidewire 75 between the
respective steps. For example, as described above, any
integrated guidewire, such as guidewire 75, may comprise two
transferring devices 82, such as an optical fiber and a
suction tube. In these embodiments, physician may apply the
optical fiber to investigate the clot, as described at step
106 above, subsequently apply the suction tube so as to draw
the clot out of the brain of patient 22, and only then retract
guidewire 75 out.
22
CA 3051888 2019-08-13
=
Fig. 4 is a flow chart that schematically illustrates a
method for producing integrated guidewire 65, in accordance
with an embodiment of the present invention. Note that
integrated guidewire 65 was selected purely by way of example.
In alternative embodiments, any other suitable type of
integrated guidewire, such as guidewires 75 and 85 depicted
above, may be produced in addition to, or instead of guidewire
65.
The method begins with providing wire 70, which is sized
and shaped to move in the blood vessels or in any other
anatomical material transportation system of patient 22, at
a wire provision step 200. Note that wire 70 may alternatively
be produced, for example, by cutting and shaping a section
from a continuous wire. At an intertwining step 202, a
production operator forms integrated guidewire 65 by
intertwining between wire 70 and one or more transferring
devices 72, which comprise one or more optical fibers and/or
one or more flexible tubes as described above.
At a coupling step 202, which concludes the production
method of integrated guidewire 65, a production operator
couples the proximal end of the integrated guidewire to a
medical device or system located externally to the body of
patient 22. As described above, the medical devices or systems
may comprise at least one of handle 30, console 33, a fluid
reservoir, a pump, a light source, an image sensor, and any
other suitable device, apparatus and/or system.
Although the embodiments described herein mainly address
sinuplasty procedures, the methods and systems described
herein can also be used in other applications, such as in
neurology, cardiology and the vasculature system.
23
CA 3051888 2019-08-13
It will thus be appreciated that the embodiments
described above are cited by way of example, and that the
present invention is not limited to what has been particularly
shown and described hereinabove. Rather, the scope of the
present invention includes both combinations and sub-
combinations of the various features described hereinabove,
as well as variations and modifications thereof which would
occur to persons skilled in the art upon reading the foregoing
description and which are not disclosed in the prior art.
Documents incorporated by reference in the present patent
application are to be considered an integral part of the
application except that to the extent any terms are defined
in these incorporated documents in a manner that conflicts
with the definitions made explicitly or implicitly in the
present specification, only the definitions in the present
specification should be considered.
24
CA 3051888 2019-08-13