Note: Descriptions are shown in the official language in which they were submitted.
,)*
85347506
1
Inhalation device for powdered drugs
This application is a divisional of Canadian Patent Application No. 2,860,884
and claims priority from therein.
The Invention refers to an inhalation device for powdered drugs to be received
by
a patient by an inhalation-caused air stream, comprising at least one powder
reservoir, metering means for repeatedly metering a powder dose from the
reservoir, a transportation mechanism for moving said metering means from a
filling position for receiving a powder dose into an emptying position for
releasing
said powder dose into a powder channel, and at least one activating device for
manual operation by the patient, said activating device being operatively
to connected to said transportation mechanism such that upon operation a
single
powder dose is being metered, said activating device comprising a dosage key
acting on said transportation mechanism when pressed by the patient.
A powder Inhaler of above-referred kind is for instance disclosed in
IS EP 1 616 592 B9.
In the field of treating bronchial diseases but also other diseases in which
medication can be affected by way of the respiratory tract, It is generally
known to
apply medicaments in powder form. Of course, in the art are also known devices
zo for atomization of solutions of suspensions to provide inhalable
aerosols.
The present invention relates to an inhaler for the administration of powdered
pharmaceuticals in form of a multi.dose dry powder inhaler, preferably with a
dosing counting or indexing means provided in the inhaler or on a cartridge
for
zs powdered pharmaceuticals.
As aforementioned, an inhaler of this kind is disclosed in EP 1 616 592 89.
This
reference refers to an inhaler for providing a large number of doses of a
pharmaceutical powder medicament from a single reservoir space which
30 medicament can be received by the patient by means of an air stream
which has
to be induced by suction to a mouthpiece by the patient
For multi-dose inhalers an important design aspect is the metering accuracy of
the
device.
CA 3051973 2019-08-14
85347506
2
Another important design aspect of inhalation devices of the above-referred
kind
are the use properties of the device.
The inhalation device has to be designed such that the user clearly may make
out
whether the device is ready for inhalation, and whether the device has a
certain
and sufficient residual amount of powder doses. Moreover, the device has to be
sufficient fail-safe and safe against operating errors of the user. For
instance
double dosing has to be prevented in any event by an appropriate design of the
metering technique.
In particular, EP 1 616 592 B9 refers to a locking mechanism, locking an
activation device and/or transportation mechanism of the inhaler after a pre-
determined number of metering cycles.
This known inhalation device comprises an activating device for manual
engagement by the patient for repeatedly metering a dose of medicament to be
administered to the patient, an advancing mechanism for advancing a counting
or
indexing means each time the activating device has been engaged by the patient
so that a dose of medicament has been released for administration to the
patient,
the counting or indexing means comprises an index, the index being detectable
by
a detection means of the inhaler, and the detection means being coupled to a
locking mechanism, the locking mechanism blocking the activation device and/or
any transportation mechanism of the inhaler delayed by a pre-determined number
of metering cycles since detection of the index. The activating device is
arrested
in a position different from the operating position indicating the blocking
state of
the inhaler. This arrangement allows to block further use of the inhaler after
removal of a number of doses from the reservoir space or an approximate number
of doses left in the reservoir space with a simple, inexpensive and reliable
mechanism so that an improved security of the patient using the inhaler can be
obtained, In this manner, a patient is prevented from trying to dose from an
empty
reservoir space causing an inappropriate lack of required medicine. Insofar,
the
known inhalation device provides an enhanced usability.
CA 3051973 2019-08-14
85347506
3
WO 2008/077623 Al discloses an inhalation device for powder drugs comprising
at least one storage chamber for accommodating a plurality of drug powder
doses
and a dosing device which includes at least one dosing slider which is
moveable
with a translatory movement in a dosing slider passage from a filling position
into
an emptying position, wherein the inhalation device further includes a device
for
inhalation-triggered automatic movement of the dosing slider from its filing
position into the emptying position and a return device for automatic movement
of
the dosing slider back into the filling position. The device for inhalation-
triggered
automatic movement of the dosing slider comprises a pivotally mounted spring-
io flap arranged into an air passage. The spring-loaded flap cooperates
with
a thrust rod which releases the device for inhalation-triggered automatic
movement of the dosing slider when the flap is deflected out of its rest
position by
an airflow through the air passage upon inhalation by the patient. This
requires a
predetermined minimum airflow in the air passage to be exceeded. For
comprehensive moisture protection for the medicament in the storage chamber of
the cartridge, the inhalation device according to WO 2008/077623 Al includes a
dosing slider passage which has a corresponding opening at its open ends to
which the dosing slider can issue with the dosing cavity, wherein a contact
surface
or a seal is provided around the opening and wherein the dosing slider further
has
a sealing surface, which is arranged in a plane approximately transverse
relationship with the direction of movement out of the filling position into
the
emptying position. The sealing function may be provided by elastic seal
disposed
on the contact surface of a sealing surface of the dosing slider. These
sealing
measures, however, do not contribute to the issue of reproducible triggering
forces.
It is generally desirable to have a defined trigger threshold for
accomplishing such
inhalation-triggered automatic movement of the dosing slider. It will be
readily
apparent for a person skilled in the art that a certain bandwidth for the
required
triggering forces, i.e. a certain air flow variation for the suction to be
applied by the
patient is unavoidable due to the tolerances of the moveable parts of such art
inhaler.
CA 3051973 2019-08-14
A
85347506
4
US patent 6,071,498 discloses an inhalation device for powdered drugs with
prevention means for double or repeated dosages. The device contains a powder
reservoir, metering means for repeatedly metering a powder dose from the
reservoir, a transportation mechanism from moving said metering means from a
5 filling position for receiving a powder dose into an emptying position
for releasing
said powder dose into a powder channel, a dosage key, acting on said
transportation mechanism when pressed by the patient, said transportation
mechanism comprising a dosage lever acting on said metering means, wherein
said dosage lever is locked in the inhalation position of said metering means
after
10 said dosage key has been actuated by the user. In the locked position,
the
dosage lever is' engaged with a catch hook in a recess with a blocking edge in
the
shaft of a flap valve functioning as a trigger member. The engagement of the
metering lever/dosage lever with its catch hook in the recess is released by
moving the flap valve by means of the air stream caused by inhalation with
little
15 delay, so that the metering lever is pulled back into its initial
position under the
load of springs. The position of the catch hook within the recess generally
determines the position of the contact point of the catch hook relative to the
blocking edge of the recess. Depending on the mounting tolerances of the
metering lever and the flap valve as the moving parts of the inhaler, the
contact
20 point between the catch hook and the blocking edge within the recess may
have a
different position which actually results in more or less delay between
inhalation
by the user and release of the flap valve. A contact point between the catch
hook
of the metering lever within the recess may be very close to the blocking edge
with the result that release of the flap valve occurs almost instantaneously
after
25 the flap valve starts a pivoting movement. On the other hand, the
contact point
between the catch hook and the metering lever within the recess may be
relatively
far away from the blocking edge in the shaft so that upon pivoting movement of
the flap valve, there is a certain delay until the metering lever is
ultimately
released. In effect, this requires higher triggering forces as in the first
case.
However, it is desirable to keep the triggering forces for inhalation-
triggered
automatic metering as reproducible as possible. Such defined trigger threshold
ultimately results in a more constant volumetric flow during inhalation so
that for
instance if the powder medicament to be received is in the form of an adhesive
CA 3051973 2019-08-14
* P 85347506
mixture, the dry powder will be more effectively deagglomerated for releasing
the
drug particles from the powder formulation. In effect, the dosing accuracy
increases.
5 It is therefore an object of the present invention to provide
an inhalation device in
which at least a part of the metering cycle is inhalation-triggered and in
which the
the bandwidth for the required triggering forces is as small as possible.
Moreover, it is an object of the present invention to provide an inhaler of
the
above-referred kind which is further enhanced with regard to usability and in
view
of eventual mal operation.
According to one aspect of the present invention, there is provided an
inhalation
device for powdered drugs to be received by a patient by an inhalation-induced
airflow, comprising at least one powder reservoir, metering means for
repeatedly
metering a powder dose from the reservoir, a transportation mechanism for
moving said metering means from a filling position for receiving a powder dose
into an emptying position for releasing said powder into a powder channel, at
least
one activating device for manual operation by the patient, being operatively
connected to said transportation mechanism such that upon operation a single
powder dose is being metered, said activating device comprising a dosage key
acting on said transportation mechanism, wherein said transportation mechanism
comprises a dosage lever acting on said metering means, said dosage lever
locked in the inhalation position of said metering means after said dosage key
has
been properly actuated and wherein said dosage lever in the inhalation
position
engages a trigger member and being releasable by actuation of the trigger
member, wherein the trigger member comprises at least one cam surface and
wherein the dosage lever engages said cam surface tangentially in said locked
inhalation position.
With the inhalation device according to the invention, the metering cycle will
only
be completed and the transportation and/or metering means will only be reset
CA 3051973 2019-08-14
85347506
6
after inhalation of a metered quantity of powder by the patient. Inhalation
will
cause the trigger member to release said dosage lever which will reset the
metering means into its filling/receiving position. Since the dosage lever
only
engages said cam surface of the trigger member tangentially, the contact force
between the dosage lever and the trigger member is almost independent from the
tolerance of the components.
In other words, the trigger member comprises a cam surface which forms a curve
which is engaged or contacted by the dosage lever peripherally from outside
such
to that the fastening hook makes line contact with the cam surface. The
position of
the contact point/contact line between cam surface and the dosage lever is
almost
the same, i.e. remains stable, irrespective of the angular end position
(locked
position) of the trigger member relative to the dosage lever.
According to one advantageous aspect of the invention, the trigger member
comprises an inhalation-operated valve in an air duct communicating with said
powder channel, The inhalation-operated valve is operatively connected to said
dosage lever so that the dosage lever is releasable by action of the valve
initiated
by the suction generated by the patient during inhalation. The inhalation-
operated
valve may be for instance in the form of a spring-loaded flap which due to the
resilience of the spring is normally held in closed position.
The dosage lever may comprise a fastening hook engaging behind and/or under
said cam surface in the inhalation position.
In one advantageous embodiment of the inhalation device according to the
invention, the fastening hook comprises a planar contact surface making line
contact with the cam surface in the inhalation position. Due to the fact that
the
fastening hook only tangentially engages the cam surface, excursion of the
contact surface due to the tolerances of the parts of the inhaler is only
possible in
axial direction, which, however, has no impact on the required triggering
forces.
Triggering of the dosage lever is achieved by a pivot motion of the cam
surface so
that only a slight pivoting movement of the triggering member almost
CA 3051973 2019-08-14
85347506
7
instantaneously releases the dosage lever. Due to this design, the bandwidth
of
required air flow variation for the suction to be applied by the patient can
be kept
remarkably below 30 I/min.
In one advantageous embodiment, the inhalation-operated valve may include a
flap which fulfils a pivot movement upon inhalation, said cam surface being
provided on the flap or on a pivot shaft of the flap. The flap is for example
a major
component of the aforementioned valve. The pivot axis may extend in the centre
of gravity of the flap or nearby the centre of gravity of the flap. In the
event the
to cam surface is also arranged nearby the pivot axis or nearby the
centre of gravity
of the flap, easy triggering may be achieved.
According to yet another aspect of the present invention, there is provided an
inhalation device for powder drugs to be received by a patient by an
inhalation-
caused air stream/inhalation-caused air flow, comprising at least one powder
reservoir, metering means for repeatedly metering a powder dose from the
reservoir, a transportation mechanism for moving said metering means from a
filling position for receiving a powder dose into an emptying position for
releasing
said powder dose into a powder channel, at least one activating device for
manual
operation by the patient, said activating device being operatively connected
to
said transportation mechanism such that upon operation a single powder dose is
being metered, said activating device comprising a dosage key acting on said
transportation mechanism when pressed by the patient, and at least one powder
disintegration means, wherein said metering means and said transportation
means are arranged within a dosing compartment and said disintegration means
is arranged in a powder discharge compartment, and wherein said dosing
compartment is sealed against said powder discharge compartment.
A powder disintegration means in the sense of the instant application is a
means
for breaking up a powder agglomerate or releasing drug particles from a
carrier
material. Said powder disintegration means may include means for guiding
powder and air as well as a cyclone/air classifier.
CA 3051973 2019-08-14
CA 3051973 2020-04-03
85347506
8
Sealing the dosing compartment of the inhaler relative to said powder
discharge
compartment also remarkably reduces the variation in required air flow for
triggering.
Accordingly, this design avoids the possibility of sucking an airflow through
the
inhaler which by-passes the powder channel and/or an air duct of the device.
In one advantageous embodiment, the inhalation device according to the
invention
further comprises a main structural support member defining a valve chamber as
well
as support for a housing including at least two shells.
In a preferred embodiment, the valve chamber is sealed against the housing so
as to
define said dosing compartment and said powder discharge compartment.
A seal may be provided in the form of a resilient lip extending completely
around the
valve chamber in an uninterrupted fashion.
Alternatively, the sealing between the dosing compartment and the powder
discharge
compartment may be provided in form of a labyrinth seal. The valve chamber may
comprise a rigid sealing rib extending completely around the valve chamber and
engaging corresponding sealing grooves within the shells of the housing so as
to
form a labyrinth.
According to another aspect of the present invention, there is provided an
inhalation
device for powdered drugs to be received by a patient by an inhalation-caused
air
stream, comprising at least one powder reservoir, metering means for
repeatedly
metering a powder dose from the reservoir, a transportation mechanism for
moving
said metering means from a filling position for receiving a powder dose into
an
emptying position for releasing said powder dose into a powder channel, at
least one
activating device for manual operation by the patient, being operatively
connected to
said transportation mechanism such that upon operation a single powder dose is
being metered, said activating device comprising a dosage key acting on said
transportation mechanism when pressed by the patient, the inhalation device
further
comprising at least one powder disintegration means, wherein said metering
means
and said transportation mechanism are arranged within a dosing compartment and
said disintegration means is arranged in a powder discharge compartment, and
CA 3051973 2020-04-03
85347506
8a
wherein said dosing compartment is sealed against said powder discharge
compartment, further comprising a main structural support member defining a
valve
chamber as well as a support for a housing including at least two shells,
wherein the
valve chamber is sealed against the housing so as to define said dosing
compartment and said powder discharge compartment.
In the following the invention is disclosed by way of example with reference
to
accompanying drawings in which:
Figure 1 shows a perspective view of an embodiment of an inhalation
device
according to the invention with the mouthpiece cap opened,
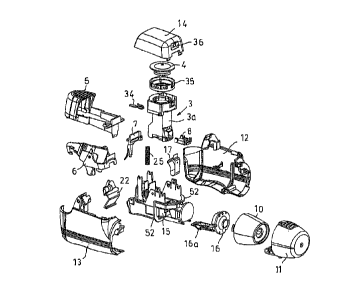
Figure 2 shows an exploded view of the inhaler according to the invention,
Figure 3 shows a partial longitudinal cross-sectional view through an
inhaler
according to a first embodiment of the invention,
. =
85347506 = ,
9
Figure 4 shows another longitudinal cross-sectional
cut through the
inhaler according to the first embodiment of the invention,
Figure 5 shows a perspective view of a dosage key according to the
invention,
Figure 6 shows a cross-sectional view of the inhaler
in a non-actuated
state of the dosage key,
Figures 7a, 7b show a cross-sectional view corresponding to
the view shown
in Figure 6 where the dosage key is partially pressed,
Figure 8a shows a cross-sectional view according to
Figures 6 and 7
where the dosage key is fully depressed,
Figure 8b shows a perspective view demonstrating the
engagement of
the dosage lever into the flap valve,
Figure 9 shows a perspective view of a dosage lever of the inhaler
according to the present invention,
Figure 10 shows a perspective view of a first preferred
embodiment of
an inhalation-operated valve closing the air duct of the inhaler
according to the invention,
Figure 1 la shows another perspective view of the
inhalation-operated
valve according to Figure 10,
Figure 11b shows a perspective rear view of an inhalation-operated valve
according to a second preferred embodiment,
Figure 12 shows a perspective side view of the inhaler
according to the
invention without its housing,
CA 3051973 2019-08-14
85347506 =
Figure 13 shows an enlarged detail of Figure 12 as a rear view,
Figure 14a shows an enlarged detail of the engagement between
the
dosage lever and an inhalation-operable flap valve of the
inhaler according to a first preferred embodiment of the
invention,
Figure 14b shows an enlarged detail of the engagement between
the
10 dosage lever and the flap valve according to the
first preferred
embodiment,
Figure 14c shows an enlarged detail of the engagement between
the
dosage lever and the flap valve according to a second
preferred embodiment,
Figure 15 shows another longitudinal sectional view through the
inhaler,
Figure 16 shows a perspective view of a powder cartridge of the
inhaler
according to the invention,
Figure 17 shows a perspective view of the inhaler housing,
Figure 18 shows a detail of the counter slide gliding over the
counter
ring's tooth,
Figure 19 shows a detail of the cartridge's locking ratchet
(locking lever
not displayed),
Figure 20 shows a detail of the locking lever and its engagement with
the dosage key and the counter ring.
The inhaler 1 shown in Figures 1 to 20 is an inhaler for powdered medicaments,
for providing a large number of doses of a pharmaceutical powder medicament
CA 3051973 2019-08-14
85347506 =
11
from a receptacle in the form of a powder cartridge. The powder cartridge 3
defines a reservoir 2 for receiving a large number of doses of a
pharmaceutical
powder/powdered drug. In the described embodiment, the typical number of
doses which may be obtained from one powder cartridge 3 may be in the range of
30 to 60 doses.
The reservoir 2 is sealingly covered by a lid 4 as can be seen from Figure 2.
The
lid is secured to the cartridge body 3a in the assembled state of the inhaler
in a
non-removeable fashion.
The powder medicament can be received by a patient by means of an air stream
caused by the user, i.e, induced by suction. Therefore, the inhaler further
comprises an activating device for manual engagement by the patient in the
form
of a dosage key 5 being connected to a transportation mechanism including a
dosage lever 6 and a locking lever 7. The dosage lever 6 acts on a dosage
slide 8
as a metering means being moveable from a filling position for receiving a
powder
dose into an emptying position for releasing said powder dose Into a powder
groove 16a of a cyclone 16 for deagglomeration of the powder in the cyclone
16.
From the powder groove 16a the patient can inhale the powdered drug through a
mouthpiece 10 via an air stream generated by the patient. If not in use, the
mouthpiece 10 is protected from dirt by a mouthpiece cover 11. The mouthpiece
cover 11 is secured to the inhaler housing fixedly, i.e. non-detachable.
The powder groove 16a of the cyclone 16 forms a part of a powder channel
through the cyclone 16 which functions as a disintegration means as this is
known
from the art. The powder medicament to be received by the patient may be in
form of an adhesive mixture. Adhesive mixtures consist of relatively large
crystals,
generally a-lactose-monohydrid, carrying the micronised drug particles on
their
surface. In the disintegration system, the dry powder will be deagglomerated
for
releasing the drug particles from the powder formulation. The cyclone 16, i.e.
the
disintegration means, generally includes an air circulation chamber as well as
several air supply channels which enter the circulation chamber tangentially
so
that a circular air flow pattern is created inside the circulation chamber. So
the
total respiratory flow through the inhaler does include a transportation
airflow for
CA 3051973 2019-08-14
85347506
12
traversing the powder dose in the powder groove and dragging the powder into
the circulation chamber, a cyclone air flow which tangentially enters the
circulation
chamber as well as eventually a bypass airflow for creating a so-called sheath
flow of clean air. A possible design for the disintegration means is for
instance
disclosed in the international patent publication WO 03/000325.
The disintegration means in the
following in a rather simplified form is referred to as a cyclone. In a also
rather
simplified form in the following the air path from the powder groove 16a to
the
mouthpiece opening is referred to as powder channel. It is, however, to be
to understood that the term "powder channel" does not necessarily refer to
one
distinct single powder channel but rather to a channel system as explained
above.
As this can be taken from Figure 2, the inhaler 1 includes a three-part
housing
comprising shells 12 and 13 as well as cover 14 received on the shells 12 and
13
via snap-fit connection in a non-releasable fashion.
The heart of the inhaler 1 is formed by a valve chamber 15 including the
cyclone
16 and a cartridge body 3a.
Manual operation of the inhaler 1 by a patient functions via dosage key 5
which on
depression by the patient against the biasing force of a dosage key spring 17
acts
on a dosage lever 6.which is connected to the dosage slide 8 (see Figure 15).
Dosage slide 8 is slidably moveable within dosage slide passage 18 extending
below the reservoir 2 within the cartridge body 3a, as this for instance can
be
seen from Figure 15.
The dosage slide 8 (metering means) includes a dosage cavity 19 for receiving
a
metered dose of a powdered drug.
It should be mentioned that the cartridge body 3a not only defines the
reservoir 2
for receiving the powdered drug but also defines a dosage slide passage 16
extending below the reservoir 2 as well as a housing for receiving counting
and
indexing means as this is described hereinafter more detailed.
CA 3051973 2019-08-14
85347506
13
The dosage slide 8 is shown in Figure 15 in its emptying position where the
dosage cavity 19 is aligned with an opening 20 in the valve chamber 15
communicating with the powder groove 16a of the cyclone 16. The dosage slide 8
is moveable via dosage lever 6 between a filling position where the dosage
cavity
19 is aligned with an opening 21 of the reservoir 2 within the cartridge body
3a
and an inhalation/emptying position. In the filling position, the dosage
cavity 19
receives a metered quantity of powder. Upon actuation of the dosage key 5, the
dosage slide 8 will be advanced into the position shown in Figure 15, thereby
io releasing the powder dose into the powder groove 16a through the
opening 20.
In this position shown in Figure 15, the inhaler is ready for inhalation. In
the event
the patient applies suction via mouthpiece 10, this forces a flap valve 22 at
the
very end of an air duct 9 to swing open so that an air flow can freely
circulate from
the open end of the air duct 9 into a powder channel defined by the valve
chamber 15 and the powder groove 16a of the cyclone, into the mouthpiece. The
flap valve 22 includes the flap 22a and a shaft 22b which are in the disclosed
embodiment integrally formed.
The flap valve 22 according to a first embodiment in more detail is shown in
Figures 10 and 11a. The shaft 22b of the flap valve 22 at its ends is pivot
mounted
within valve chamber 15.
As this can be seen also from Figure 15, in the inhalation position the flap
valve
22 is engaged by a fastening hook 23 of the dosage lever 6.
A rear side view of the flap valve 22 is for instance shown in Figure 10. The
flap
22 has an angled/bent profile including three legs 22c, 22d and 22e, the first
leg
22c in the mounting position being inclined towards the closing direction of
the
flap valve 22, the second leg 22d being inclined rearwardly and the third leg
22e
extending in forward direction and approximately tangentially to the rotary
movement of the flap valve 22.
CA 3051973 2019-08-14
85347506
14
On the rear side of the first leg 22c of the flap 22a, a latching rlb 47 is
provided
which may be engaged by the fastening hook 23 of the dosage lever 6 in the
inhalation position. The fastening hook 23 of the dosage lever 6 at its
leading end
is provided with a barbed projection 50 which has a sloping face 51. The flap
valve 22 includes a flap valve lever 31 integrally formed with said shaft 22b.
The
distal end of the flap valve lever 31 is provided with a deflector surface 53.
Upon
actuation of the dosage key 5 and subsequent actuation and downward
movement of the dosage lever 6, a corresponding deflector surface 53' of a
latch
29 integrally formed with the hook 23 of the dosage lever 6 gets into abutment
with the deflector surface 53 of the flap valve lever 31. The flap valve lever
31 as
well as the hook of the dosage lever are thereby both being slightly
deflected, i.e.
bent aside and snap back in their initial position upon further downward
movement of the hook 23 of the dosage lever 6. Upon further downward
movement of the hook 23, the sloping face 51 of the barbed projection 50 abuts
is one edge of the latching rib 47. Thereby the hook 23 is bent aside
due to the
resilience of its material and snaps back behind the latching rib 47 in its
end
position thereby engaging the flap valve 22 and being releasable by an
inhalation-
triggered pivoting movement of the flap valve 22.
As this can particularly seen from figure 14b, which shows an enlarged
longitudinal cut through the flap 22a, the barbed projection 50 of the hook 23
engages the latching rib 47. When the inhaler 1 is ready for inhalation, the
latching rib 47 includes a curved bearing surface 54 facing the barbed
projection
50. The bearing surface 54 which defines a cam surface almost providing line
contact between the barbed projection 50 of the hook 23 and the latching rib
47
so that the contact force between the latching rib 47 and the hook 23 is
almost
independent from the tolerances of the components. To be more specific, the
planar surface of the barbed projection 50 of the hook engages the curved
bearing surface 54 only tangentially so that in effect excursion of the
contact
surface due to tolerances of the parts of the inhaler is only possible in
axial
direction, which, however, has no impact on the required triggering forces.
Due to
this design the required triggering forces are only subject to minor
variations so
that triggering of the flap valve 22 is fairly reproducible. It is readily
apparent from
Figure 15 that if the flap 22a moves in clockwise direction, the hook 23 is
released
CA 3051973 2019-08-14
= 0
85347506 ,
almost instantaneously. As a result, the dosage lever may swing upwards driven
by the force of the dosage lever spring 25. This upward movement will cause
the
dosage slide 8 to return to its powder receiving position.
5 Another embodiment of the flap valve 22 is shown in Figures
11 b and 14c. Same
parts of the flap valve 22 are denoted by the same reference numerals.
The flap valve 22 according to this embodiment comprises a relatively simple
flat
flap 22a which is not bent or angled in itself.
As this can be taken from Figure 14c, the fastening hook 23 engages the shaft
22b of the flap valve 22 when the inhaler 1 is ready for inhalation.
The shaft 22b of the flap valve 22 (see for instance Figure 11b) has a cut out
area
24 which is arranged approximately in the middle of the shaft 22b such that,
if the
flap 22a swings open (in Figures 14c and lib in clockwise direction), the
fastening hook 23 of the dosage lever 6 tangentially engaging the shaft 22b
approximately in the middle of the shaft 22b, will be released so that the
dosage
lever 6 driven by the force of the dosage lever spring 25 may return to its
initial
position, thereby moving the dosage slide 8 back into the filling position for
receiving a powdered dose from the reservoir/powder cartridge. Upon actuation
of
the dosage key 5 the dosage lever 6 will be moved downward while partially
pivoting the flap 22a by contact of the latch 29 of the dosage lever 6 with
the flap
valve lever 31. After partially pivoting the flap valve 22 swivels back to its
starting
position by the force of its molded, integrally formed spring 32. The pivot
motion of
the flap 22a caused by the contact of the latch 29 with the flap valve lever
31
allows the latch 29 to engage behind the mechanical stop 30 of the flap valve
lever 31 upon early release and upward movement of the fastening hook 23.
In the area of the cut out portion 24 of the shaft 22b, the shaft 22b has only
a
semi-circle cross-section, the leading end of the fastening hook 23 engages
the
remainder of the cross-section of the shaft only tangentially and only in a
very
limited surface area (line contact) so that the contact force between the
shaft 22b
and the fastening hook 23 is almost independent from the tolerances of the
CA 3051973 2019-08-14
85347506 = =
16
components. Due to this design, in particular due to the fact that a planar
contact
surface of the fastening hook 23 contacts a curved surface area of the
remainder
of the cross section of the shaft 22b, the required triggering forces are only
subject to minor variations so that triggering of the flap valve 22 is fairly
reproducible. Only a slight rotation/pivoting movement of the shaft 22b and
the
flap 22a will set the fastening hook 23 free so that the dosage lever 6 may
swing
upwards driven by the force of the dosage lever spring 25, thereby finishing
the
inhalation cycle.
to A perspective view of the dosage key 5 is shown in Figure 5. The
dosage key 5 is
held in its initial position/starting position by dosage key spring 17 which
abuts a
tongue member 26 integrally formed with the dosage key 5.
Said dosage key 5 includes an actuator blade 27 being formed as a flexible
ts arm/leg also integrally formed with the dosage key 5 and extending
downwards in
the mounting position shown in Figure 5. As this can be seen from the
operating
sequence shown in Figures 6 to 8a, valve chamber 15 is provided with a beveled
edge 28 forming a kind of cam surface for the actuator blade 27 upon
depression
of the dosage key 5.
Figure 6 shows a cross-sectional cut through the inhaler 1 where the dosage
key
5 is in its not-operated starting position. The actuator blade 27 in this
state is not
engaged with the transportation mechanism, i.e. with the dosage lever 6.
Upon depression of the dosage key 5, the actuator blade 27 moves downwards
and engages the beveled edge 28 of the valve chamber such that the actuator
blade 27 due its inherent flexibility is deflected/bent from a first position
shown in
Figure 6 to a second position in Figure 7a where it engages at the same time
the
dosage lever 6. By a further movement of the dosage key 5 and the actuator
blade 27, the actuator blade 27 urges the dosage lever 6 downwards against the
biasing force of dosage lever spring 25. Upon full depression of the dosage
key 5,
which is shown in Figure 8a, actuator blade 27 snaps back in its non-deflected
and disengaged position. In this position the fastening hook 23 of the dosage
CA 3051973 2019-08-14
85347506 =
17
lever 6 engages the latching rib 47 of the flap valve 22 as this is also shown
in
Figure 8a. The device/inhaler is now ready for inhalation.
In the following the double dosing prevention mechanism of the inhaler
according
to the invention will be described, first referring to the first embodiment of
the flap
valve 22 according to the invention.
As this has been mentioned before, the dosage lever 6 in the area of its
trailing
end (left hand side in Figure 9) is provided with latch 29. Upon downward
movement of the dosage lever as a result of the actuation of the dosage key 5,
first of all the complementary deflection surface 53' of the latch 29 gets
into
abutment with the deflector surface 53 of the flap valve lever 31. As a
result, the
flap valve lever 31 is bent aside/deflected while being passed by the
fastening
hook 23 and snaps back into its initial position upon further downward
movement
is of the dosage lever 6, which ultimately will result in engagement of
the hook and
the latching rib 47.
It is again referred to Figure 9 which shows a perspective view of the dosage
lever
6. In the area of its trailing end (left hand side in Figure 9), the dosage
lever 6 is
provided with a latch 29 for engaging a mechanical stop 30 of a flap valve
lever 31
integrally formed with said shaft 22b on a return movement of the dosage lever
6.
In the event the dosage key 5 will be pressed and released too early, i.e.
prior to
the engagement of the fastening hook 23 into the latching rib 47 of the flap
valve
22, latch 29 of the dosage lever 6 upon upward movement of the dosage lever 6
will abut said mechanical stop 30 of the flap valve lever 31. Accordingly, the
dosage lever 6 locks into the flap valve 22 in a middle position. This middle
position lock provides a double dosing prevention mechanism. In this middle
position lock, i.e. first locked position, the relationship of lever is such
that the
forces required for releasing the dosage lever 6 can not be brought up simply
by
inhalation. If the dosage lever 6 does not lock into flap valve 22 in the end
position, e.g. when the dosage key 5 is not pressed all the way down, the
dosage
lever 6 will not return to its initial starting position, i.e. will be locked
in the middle
position. Accordingly, no additional powder dose will be released from the
CA 3051973 2019-08-14
85347506
18
reservoir 2. The dosage lever 6 and the dosage slide 8 will only return into
their
starting position after inhalation-triggered actuation of the flap valve 22,
thereby
releasing the fastening hook 23 of the dosage lever 6.
A double-dosing prevention mechanism is also provided with the design of the
flap valve 22 of the second embodiment according to Figure 14c. Upon actuation
of the dosage key 5, the dosage lever 6 will be moved downward while partly
pivoting the flap 22a by contact of the latch 29 of the dosage lever with the
flap
valve lever 31. After partly pivoting the flap upon downward movement of the
dosage lever, the flap valve 22 will return into its closed position due to
the
resilience of a spring 32 integrally molded with the flap valve 22. Latch 29
of the
dosage lever 6 upon an early upward movement of the dosage lever 6 will abut
the mechanical stop 30 of the flap valve lever 31. Accordingly, the dosage
lever 6
locks into the flap valve 22 in a middle position.
Dosage lever 6 includes a cam-like actuating element 33 which upon each
actuation moves a counter slide 34 of the cartridge so that a counter ring 35
of the
cartridge is moved by one count towards a lower dose. The degree of the
cartridge's content is accordingly visible in a display window 36 of the
cartridge
body 3a indexing the fill status of the cartridge. Details of the counter
slide 34
acting on the counter ring 35 may be taken from Figure 18. Counter ring 35
which
is designed as a ratchet ring with teeth 37 is ratably inserted into a collar
of the
cartridge body 3a. Upon actuation of the dosage lever 6, the actuating element
33
moves the counter slide 34, the counter slide engaging the counter ring's
teeth 37,
thereby moving the counter ring 35 so that the next index number is indicated
in
the display window 36. The counter ring 35 for instance provides a visual
indication of a dose count for each 5th dosing steplmetering cycle. The
counter
ring for instance shows thirteen numbers and indicates a countdown from 60 to
0
upon each metering cycle. Each tooth of the counter ring 35 represents one
metering cycle.
As this also can be taken from Figure 4, the counter slide 34 includes a pawl
43
integrally formed with the counter slide 34. The pawl 43 is biased towards the
counter ring 35 that it firmly engages the teeth 37 of the counter ring 35.
The teeth
CA 3051973 2019-08-14
85347506
19
37 are unsymmetrical insofar as they have one sloping flank and one vertically
extending flank, the sloping flank representing the leading flank with respect
to the
rotational direction of the counter ring 35.
s The counter slide 34 is moveable back and forth within a sliding
channel 44 of the
cartridge body 3a. The cam-like actuating element 33 of the dosage lever 6
extends into the sliding channel 44 and into a recess 45 of the horizontally
extending part of the counter slide 34. Engagement of the actuating element 33
with the counter slide 34 transforms a pivoting movement of the actuating
element
33 into a linear movement of the counter slide 34.
Upon depression of the dosage key 5, the dosage lever will be pivoted such
that
the actuating element 33 is pivoted towards the left hand side in Figure 4. At
the
same time, the lower leading end 46 of the dosage lever (see Figures 9 and 12)
is pushes the dosage slide 8 into its emptying/inhalation position. The
counter slide
34 is thereby moved in the opposite direction. While the counter slide 34
fulfills
this movement, the pawl 43 engages the vertical flank of the respective tooth
37
of the counter ring, moving the counter ring one count/step. After release of
the
dosage lever 6, the counter slide 34 will be moved backwards into its starting
position. Due to the resilience of the pawl 43, the pawl may glide over the
sloping
flank of the respective tooth, thereby snapping back behind the tooth. As this
can
be seen from Figure 19, the counter mechanism includes a locking ratchet 38
engaging the counter ring teeth 37. Due to the geometry of the teeth 37, the
locking ratchet 38, which is also a resilient member, blocks an anti-clockwise
rotation of the counter ring 35.
As this can be seen from Figure 12, the inhaler 1 includes a locking lever 7
which
is pivotably mounted in the valve chamber 15 between dosage key 5 and dosage
lever 6. The locking lever 7 includes a blocking arm 39 and a spring leg 40.
During
assembly of the powder cartridge 3, the locking lever 7 is pushed downwards,
the
blocking arm 39 and the spring leg 40 thereby being moved backwards. In this
position, the dosage key 6 may be freely moved downwards against the biasing
force of the spring leg 40 as shown in Figure 12.
CA 3051973 2019-08-14
= 85347506 =
The dosage key 5 is also freely moveable against the biasing force of the
dosage
key spring 17 as shown in Figure 15.
The counter ring 35 includes a notch 41 being engageable by a tongue 42 of the
5 locking lever 7.
The notch is arranged on the counter ring 35 such that, after a pre-determined
number of doses has been delivered, the locking lever 7 engages the notch in
the
counter ring with a pivoting movement caused by the action of spring leg 40
10 actuated by the dosage key 5. Upon upward movement of the locking
lever 7, the
blocking arm 39 of the locking lever 7 is pushed forward (towards the
mouthpiece
10) and engages the dosage key 5 in its lowest position such that the dosage
key
5 stays blocked in its lowest position after the last inhalation. It is
impossible to
perform another activation of the empty device.
As this can be seen from Figures 5 and 20, the dosage key 5 includes an
actuation rib 55 acting on the spring leg 40 only while being In depressed
condition in order to avoid fatigue of the spring leg 40. The spring leg 40 is
accordingly only biased if the dosage key 5 is being pressed or held downward.
Apart from the indexing means in the form of the counter ring, the device
includes
another inhalation control window 48 indexing whether the device is ready for
inhalation or not. The inhalation control window shows for instance a green-
colored flag in the event the device is ready for inhalation. This is because
in the
activated status of the inhaler 1 a green colored tab 49 of the dosage lever 6
covers a red colored flag in the inhalation control window 48. The reset of
the
device from the inhalation position into the starting position takes place
during
inhalation by means of an airflow upon inhalation. Flap valve 22 is deflected
thus
releasing the dosage lever 6 as this has been described in detail before.
In order to ensure leak tightness of the air duct 9, the shells 12 and 13 may
be
sealed against valve chamber 15 by means of one or more sealing ribs which
extend around valve chamber 15. The sealing rib may be in form of a
thermoplastic elastomer which has been co-injection molded with valve chamber
CA 3051973 2019-08-14
85347506
21
15. Alternatively, the sealing rib 52 may be designed as a resilient ring
which has
been mounted into a sealing groove during assembly of the inhaler.
In a particularly preferred embodiment of the inhaler according to the
invention,
the shells 12 and 13 are sealed against the valve chamber by a labyrinth seal
which completely extends around the valve chamber 15, so that the valve
chamber 15 including the cyclone 16 and the powder groove 16a is effectively
sealed against the dosing compartment of the inhaler. The labyrinth seal is
provided by a sealing rib 52 completely extended around the valve chamber 15
in and in the assembled state of the inhaler 1 engaging a corresponding
sealing
groove in the shells 12 and 13. This sealing assists in keeping the triggering
forces for the flap valve 22 as reproducible as possible. The bandwidth for
the
required triggering forces normally corresponds to an air flow variation of 30
l/min
for the suction to be applied by the patient. Sealing the valve chamber of the
Is inhaler 1 against the shells 12, 13 remarkably reduces this
variation in required air
flow for triggering the flap valve 22. Accordingly, this design avoids the
possibility
of sucking an air flow through the inhaler which bypasses the powder channel
and/or the air duct 9.
CA 3051973 2019-08-14
85347506
22
Reference numerals
1 inhaler
2 reservoir
3 cartridge
3a cartridge body
4 lid
5 dosage key
6 dosage lever
io 7 locking lever
8 dosage slide
9 air duct
mouthpiece
11 mouthpiece cap
Is 12, 13 shells
14 cover
valve chamber
16 cyclone
16a powder groove
17 dosage key spring
18 dosage slide passage
19 dosage cavity
20 opening
21 opening
22 flap valve
22a flap
22b shaft of flap valve
22c first leg of flap valve
22d second leg of flap valve
22e third leg of flap valve
23 fastening hook
24 cut-out portion
25 dosage lever spring
26 tongue member
CA 3051973 2019-08-14
85347506
23
27 actuator blade
28 beveled edge
29 latch
30 mechanical stop
s 31 flap valve lever
32 spring of flap valve
33 actuating element
34 counter slide
35 counter ring
36 display window
37 teeth
38 locking ratchet
39 blocking arm
40 spring leg
is 41 notch
42 tongue
43 pawl
44 sliding channel
45 recess
46 leading end of dosage lever
47 latching rib
48 inhalation control window
49 tab
50 barbed projection
51 sloping face
52 sealing rib
53 deflector surface
53' deflector surface
54 bearing surface
55 actuation rib
CA 3051973 2019-08-14