Note: Descriptions are shown in the official language in which they were submitted.
CA 03053663 2019-08-15
WO 2018/187852 PCT/CA2017/050646
BUTYRATE AND BETA-HYDROXYBUTYRATE COMPOSITIONS
AND METHODS OF USE THEREOF
FIELD OF THE INVENTION
[001] The field of the present invention relates to certain compositions
(and methods
of use thereof) that comprise a combination of short chain fatty acids (such
as butyrate)
and ketones (such as beta-hydroxybutyrate), which provide the various health
benefits
described herein.
BACKGROUND OF THE INVENTION
[002] Ketones (Background)
[003] It is well understood that dietary restriction in the form of calorie
deprivation
and/or a low carbohydrate / high fat diet (LCHFD) is conducive to ketogenesis.
Although hyperketonemia (> 0.5 mmol/L of serum ketones), when induced by such
dietary programs, has been shown to produce positive effects on biological
markers of
insulin resistance, serum glucose stabilization, diabetes, obesity, epilepsy,
cognitive
deficits, inflammation and even cancer, achievement and sustenance of
functional
serum ketone levels is a very difficult task. Achieving a state of ketosis
requires
dedication and sacrifice, while enduring states of malaise during energy
substrate
transition. For some, the achievement of ketosis is more difficult than for
others based
on metabolic, genetic, environmental, and lifestyle factors combined.
[004] Sustained ketosis is also a state desired by athletes in pursuit of
improved
performance, as a function of ketones serving as substrates for mitochondrial
ATP
generation. Research shows that ketones produce as much as 38% more ATP per
unit
carbon than glucose as substrates of the TCA cycle. In addition, research
shows ATP
1
CA 03053663 2019-08-15
WO 2018/187852 PCT/CA2017/050646
generation from ketones as substrates in the mitochondria instead of glucose
results in
fewer free radical by-products. Furthermore, ketones are shown to induce
transcription
and subsequent synthesis of endogenous antioxidants, thereby priming the
generation
of intracellular glutathione and other endogenous antioxidants to produce a
proactive
protection against oxidative stress.
[005] Athletes in ketosis and under physical load are shown to operate at full
power
output with a lower V02 max than those utilizing glucose (carbohydrate) as a
primary
source of ATP. When at elevated serum levels indicative of ketosis, ketones
are also
known to spare muscle (anticatabolic) when under stress, including stress
caused by
nutrient deprivation. Although ketosis is a metabolic state that does not fit
optimally for
everyone who attempts to achieve it, there are pharmacological benefits of
ketones,
such as beta-hydroxyl-butyrate / beta-hydroxybutyrate (BHB) and acetoacetate
when
they exist in hyperketonemic states. Healthy ketosis is represented by a state
where
ketones measure in the range 0.4 ¨ 5.0 mmol/L, while blood sugar remains
stable and
at around baseline of 4.2-5.0 mmol/L.
[006] Although it is known that ketones serve as substrates for efficient ATP
generation, the means by which such ketones also serve as ligands for various
receptors, such as hydroxyl carboxylic acid receptors (HCA), are not well
understood.
BHB is a known HCA2 receptor agonist and, therefore, it is expected to have
some
neuroprotective activity, vis-à-vis its activity on monocytes and macrophages.
[007] During carbohydrate deprivation, serum glucose declines and the
metabolism
can shift to fatty acid beta-oxidation and the production of ketones. This is
the essence
of endogenous ketone induction. Although fatty acids cannot readily cross the
blood
2
CA 03053663 2019-08-15
WO 2018/187852 PCT/CA2017/050646
brain barrier to serve neurons as an energy substrate amid carbohydrate
deprivation,
ketones are hydrophilic and can cross to serve efficiently as substrates for
neuron ATP
generation. Ketones can supply in excess of 50% of the brain's energy
requirements
during periods of glucose scarcity. The scarcity of glucose amid the
abundantly
available ketone causes cells to increase mitochondrial numbers, induce
endogenous
antioxidant generation, and activate various other protective mechanisms.
[008] It has further been established that many neurological disorders are
associated
with impaired mitochondrial activity, compromised mitochondrial numbers,
limited
endogenous antioxidant status, elevated free radical generation, and oxidation
amongst
other pathological features and hallmarks. Ketosis has been shown to improve
many of
these pathological features.
[009] In view of the foregoing, the exogenous supply of ketones may offer a
number
of pharmacological benefits, including both mental and physical benefits. A
daily supply
of exogenous ketones would alleviate the stress associated with diet
adherence, and
would allow for the pharmacological benefits of ketones to continue due to the
maintenance of elevated serum ketone levels (despite the temporary or
prolonged
increment of serum glucose and stored glycogen that may ensue as a function of
a
meal or few days off a ketogenic cycle). An exogenous supplement of ketones
would
also provide an immediate and efficient transition back to a ketogenic
lifestyle, without
the associated energy deficit that is typically associated with the cell-
switch-back to
serum ketones and fat as an energy (ATP) substrate. Metabolic support during
energy
substrate scarcity would be another substantial benefit of an exogenous ketone
supply,
particularly in the context of calorie or carbohydrate deprivation for weight
management
3
CA 03053663 2019-08-15
WO 2018/187852 PCT/CA2017/050646
or therapy of other types. An exogenously supply of ketones would further
serve as a
bridging energy source during a low-carbohydrate diet and fluctuations in
dietary habits,
whether those shifts are long-term initiatives or short-term breaks. An
exogenous
supply of ketones would serve to avert the state of malaise that is often
attendant to a
calorie / carbohydrate deprived state, and would improve appetite control and
support
cognitive alertness.
[0010] Butyrate (Background)
[0011] Short chain fatty acids, also known as volatile fatty acids, are those
typically
produced by the microbial community of the intestine. These microbes are often
referred to as probiotics or microbiota. Such
microbes comprise a significant
component of the immune system. These symbiotic microbes produce short chain
fatty
acids from dietary fiber, i.e., fatty acids that serve as signaling ligands
for various
receptors involved in inflammatory control, including the HCA2 receptor (the
above
described beta-hydroxybutyrate (ketone) serves as an agonist for such receptor
as
well).
[0012] The short chain fatty acids of the intestinal lumen include most
abundantly,
butyrate, propionate, and acetate. Research shows that butyrate fed mice
remain lean
(despite dietary calorie load); avoid metabolic problems; have increased
energy
expenditure in the form of body heat generation; and tend to have higher
physical
activity. Butyrate (BA) has been shown to lower serum cholesterol in various
studies
and by as much as 25% in some studies, and reduce serum triglycerides by as
much as
50% compared to controls. Butyrate has further been shown to lower fasting
insulin by
4
CA 03053663 2019-08-15
WO 2018/187852 PCT/CA2017/050646
nearly 50%, and increase insulin sensitivity by as much as 300%. Still
further, butyrate
administration has been shown to improve appetite and food portion control.
[0013] Research has further shown that butyrate is a key fuel for epithelial
cells of the
intestinal tract and that it may improve gut lining integrity. Similar to BHB,
butyrate is an
inhibitor of HDAC to induce global changes in genetic transcription of genes
encoding
oxidative stress resistance. This down regulation of gene transcription
results in
improved protection from free radical damage associated with strained or
extreme
metabolic conditions (and environmental toxins). This genetic optimization
provided by
butyrate also includes neuroprotection, similar to that exhibited by BHB.
[0014] Still further, lumen butyrate has been shown to directly preserve gut
friendly
bacteria in the microbiota, while adversely affecting pathogenic bacteria like
Escherichia
coli, Salmonella spp. and Campylobacter spp. Passive absorption of water in
the colon
depends on short chain fatty acid availability. Butyrate has been shown to
play a role in
healthy peristalsis to help normalize movement in cases of constipation or
diarrhea.
Butyrate serves to support optimal hydration and optimal bowel elimination
function.
Butyrate has also been shown to exhibit trophic effects on intestinal cell
proliferation,
improving villi, and general lining health. In addition, butyrate has been
shown to be a
potent promoter of intestinal regulatory T cells establishing yet another
immune
regulating mechanism that promotes better inflammatory control at the mucosal
lining.
Promotion of gastrointestinal health provides a formidable platform for
improved general
and systemic health.
[0015] To compound the benefits offered by ketosis (as described above), it is
known
from the literature that butyrate induces FGF21 in serum, liver and
adipocytes, which in
CA 03053663 2019-08-15
WO 2018/187852 PCT/CA2017/050646
turn stimulates fatty acid oxidation and hepatic ketone production. This
serves as an
inducing signal for ketosis, along with butyrate itself, thereby serving as a
direct
substrate for ketone production and energy generation. In short, butyrate
serves as a
significant synergistic force for ketosis induction; BHB ligand interactions
and
pharmacology; and general health, fitness and performance support.
[0016] As discussed above, an exogenous supply of ketones, such as BHB, will
provide an immediate alternative energy (ATP) source during periods of calorie
or
carbohydrate deprivation. However, concurrent butyrate supplementation in the
form of
sodium, calcium or potassium butyrate (or its esters) will prompt the body to
induce
endogenous ketone synthesis; will serve as a ligand to stimulate receptors
that the
ketone will act on; will contribute to the improvement of insulin and general
metabolic
health; will support inflammatory and general immune system health; will
improve
gastrointestinal health and integrity -- all in parallel with the benefits
that concurrent
supplementation of the sister ketone molecule will provide.
[0017] As the following will demonstrate, the compositions and methods of the
present
invention will be very useful for providing an exogenous supply of ketones, to
provide a
person with the numerous pharmacologic benefits described herein.
SUMMARY OF THE INVENTION
[0018] According to certain aspects of the invention, compositions are
provided that
include combinations of short chain fatty acids (e.g., butyrate) and ketones
(e.g., beta-
hydroxybutyrate), and/or derivatives of the foregoing. The compositions of the
present
invention offer a multitude of benefits and can be used for numerous
applications. For
example, oral formulations of such compositions may be used for sustaining
elevated
6
lumen and serum short chain fatty acid (SOFA) and/or ketone concentrations
intended
for therapeutic applications, such as body mass alteration, support of insulin
activity,
and support of cognitive activity (despite probiotic (microbiome) status and
diet). More
particularly, the compositions of the invention may be useful for treating or
preventing
obesity, insulin resistance, metabolic syndromes, cognitive deficits, IBS,
IBD, epilepsy,
atrophy, and catabolism.
[0019] According to additional aspects of the invention, methods for
increasing nuclear
factor erythroid 2 related factor 2 (Nrf2) levels in a group of cells are
provided. Such
methods generally comprise administering to the cells a butyrate / beta-
hydroxybutyrate
composition described herein ¨ e.g., a composition that comprises (a) purified
butyrate
or esters or propionate salts thereof and (b) purified beta-hydroxybutyrate or
esters or
propionate salts thereof. The invention provides that such compositions may
further be
used to increase Nrf2 levels, e.g., by increasing Nrf2 expression, in cells
that are
subjected to chemical-induced stress.
[0019a] In another aspect, there is provided a composition comprising: (a) a
butyrate
composition, selected from the group consisting of (i) a butyrate sodium salt,
(ii) a butyrate
calcium salt, (iii) a butyrate magnesium salt, and (iv) a combination of
(a)(i) ¨ (a)(iii); and (b)
a beta-hydroxybutyrate composition, selected from the group consisting of (i)
a beta-
hydroxybutyrate sodium salt, (ii) a beta-hydroxybutyrate calcium salt, (iii) a
beta-
hydroxybutyrate magnesium salt, and (iv) a combination of (b)(i) ¨ (b)(iii),
wherein: (x) the
composition is packaged in a form selected from the group consisting of an
oral capsule, in
powdered form, in an aqueous or non-aqueous liquid, in an oil-in-water or
water-in-oil
emulsion, in an elixir or syrup, and a paste; (y) the composition includes a
total butyrate to
total beta-hydroxybutyrate ratio of about 1:1; and (z) the composition is
effective to deliver
1000 - 5000 mg of a short chain fatty acid and 1000 ¨ 10,000 mg of ketone to a
person on a
daily basis.
7
Date Recue/Date Received 2021-02-09
[0020] The above-mentioned and additional features of the present invention
are
further illustrated in the Detailed Description contained herein.
BRIEF DESCRIPTION OF THE FIGURES
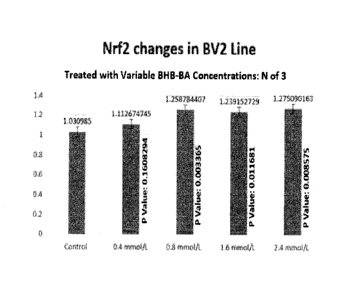
[0021] Figure 1: a bar graph that shows the impact of treating BV2 cells with
BHB-BA
complex on Nrf2 levels (when the cells were treated for 30 minutes at
concentrations of
BHB-BA ranging from 0.4 mM to 2.4mM).
[0022] Figure 2: a bar graph that shows the impact of treating LPS-stressed
BV2 cells
with BHB-BA complex on Nrf2 levels (when the cells were treated with BHB-BA
complex at concentrations ranging from 0.4 mM to 2.4 mM).
7A
Date Recue/Date Received 2021-02-09
CA 03053663 2019-08-15
WO 2018/187852 PCT/CA2017/050646
[0023] Figure 3: a bar graph that further shows the impact of treating LPS-
stressed
BV2 cells with BHB-BA complex on Nrf2 levels (when the cells were treated with
BHB-
BA complex at concentrations ranging from 0.4 mM to 2.4 mM).
[0024] Figure 4: a line graph that shows the results of certain MTT assays
using BV2
cells to measure cell stress and survival in the context of various BHB-BA and
BHB-only
treatment concentrations.
[0025] Figure 5: a line graph that summarizes a subject's (RG) serum glucose
levels
following administration of a BHB-BA combination of the present invention.
[0026] Figure 6: a line graph that summarizes a subject's (RG) serum ketone
levels
following administration of a BHB-BA combination of the present invention.
[0027] Figure 7: a line graph that summarizes a subject's (CD) serum glucose
levels
following administration of a BHB-BA combination of the present invention.
[0028] Figure 8: a line graph that summarizes a subject's (CD) serum ketone
levels
following administration of a BHB-BA combination of the present invention.
[0029] Figure 9: a line graph that summarizes a subject's (SD) serum glucose
levels
following administration of a BHB-BA combination of the present invention.
[0030] Figure 10: a line graph that summarizes a subject's (SD) serum ketone
levels
following administration of a BHB-BA combination of the present invention.
[0031] Figure 11: a line graph that summarizes a subject's (FC) serum glucose
levels
following administration of a BHB-BA combination of the present invention.
[0032] Figure 12: a line graph that summarizes a subject's (FC) serum ketone
levels
following administration of a BHB-BA combination of the present invention.
8
CA 03053663 2019-08-15
WO 2018/187852 PCT/CA2017/050646
DETAILED DESCRIPTION OF THE INVENTION
[0033] The following will describe, in detail, several preferred embodiments
of the
present invention. These embodiments are provided by way of explanation only,
and
thus, should not unduly restrict the scope of the invention. In fact, those of
ordinary skill
in the art will appreciate upon reading the present specification and viewing
the present
drawings that the invention teaches many variations and modifications, and
that
numerous variations of the invention may be employed, used and made without
departing from the scope and spirit of the invention.
[0034] According to certain preferred embodiments of the present invention,
compositions providing a person with an exogenous and therapeutically
effective supply
of ketones are disclosed. In certain preferred embodiments, the compositions
of the
present invention may consist essentially of (a) purified butyrate (or esters
or propionate
salts thereof) and (b) purified beta-hydroxybutyrate (or esters or propionate
salts
thereof). The invention provides that the compositions may further include
other
pharmacologically active agents, such as acetyl-L-carnitine, R-alpha lipoic
acid, green
tea extract, vitamins, and various combinations of such agents.
[0035] The invention provides that acetyl-L-carnitine (a source of L-carnitine
following
administration) has been shown to support cognition and mood; improve
Alzheimer's
symptoms; and support cardiovascular health. The
invention provides that a
composition of the invention that includes acetyl-L-carnitine will be designed
to support
mitochondrial fatty acid oxidation in the context of a low carbohydrate diet
and ketosis.
The invention provides that R-alpha lipoic acid will serve as an antioxidant
and will
support insulin sensitivity (i.e., to provide antioxidant protection in fat
and water
9
CA 03053663 2019-08-15
WO 2018/187852 PCT/CA2017/050646
mediums and improve serum glucose clearance to facilitate ketosis and ketone
prevalence as an energy substrate). The inclusion of high-epigallocatechin
gallate
(EGCG) and high-caffeine green tea extract will provide a natural source
caffeine to
support beta oxidation of fatty acids and ketosis induction; it will supply a
significant
amount of EGCG for optimal antioxidant support; and it will provide anti-
amylase activity
to inhibit or slow carbohydrate digestion to result in an impaired glycemic
index of and
serum contribution by dietary carbohydrate sources, which promotes a ketogenic
environment.
[0036] According to additional preferred embodiments of the present invention,
methods for providing a person with an exogenous and therapeutically effective
supply
of ketones are disclosed. In certain embodiments, the methods generally
include
providing a person with an exogenous supply of ketones (or precursors
thereof), by
orally administering one of the pharmacologic compositions described herein,
which is
effective to deliver 1000 -5000 mg of a short chain fatty acid (e.g.,
butyrate) and 1000 ¨
10,000 mg of ketone (e.g., beta-hydroxybutyrate) or, more preferably, which is
effective
to deliver 2000 - 5000 mg of butyrate and 5000¨ 10,000 mg of beta-
hydroxybutyrate to
a person on a daily basis. As described further below (and in the Examples),
such
compositions may be preferably delivered to a person in the form of oral
capsules or dry
powders.
[0037] Notwithstanding the preferred embodiments and Examples described
herein,
the invention provides that the compositions of the present invention may be
administered in any desired and effective manner, e.g., as pharmaceutical
compositions
or nutritional supplements for oral ingestion. More
particularly, for example,
CA 03053663 2019-08-15
WO 2018/187852 PCT/CA2017/050646
pharmaceutically acceptable compositions or nutritional supplements of the
invention
may comprise one or more of the compositions described herein with one or more
acceptable carriers. Regardless of the route of administration selected,
the
compositions may be formulated into acceptable dosage forms by conventional
methods known to those of skill in the art. For example, acceptable carriers
include, but
are not limited to, sugars (e.g., lactose, sucrose, mannitol, and sorbitol),
silicon dioxide,
starches, cellulose preparations (such as microcrystalline cellulose), calcium
phosphates (e.g., dicalcium phosphate, tricalcium phosphate and calcium
hydrogen
phosphate), sodium citrate, water, aqueous solutions, alcohols (e.g., ethyl
alcohol,
propyl alcohol, and benzyl alcohol), polyols (e.g., glycerol, propylene
glycol, and
polyethylene glycol), organic esters (e.g., ethyl oleate and tryglycerides),
biodegradable
polymers (e.g., polylactide-polyglycolide, poly(orthoesters), and
poly(anhydrides)),
elastomeric matrices, liposomes, microspheres, oils (e.g., corn, germ, olive,
castor,
sesame, cottonseed, and groundnut), cocoa butter, waxes, paraffins, silicones,
talc,
si I icylate, etc.
[0038] Each acceptable carrier used in a pharmaceutical composition or
nutritional
supplement of the invention must be "acceptable" in the sense of being
compatible with
the other ingredients of the formulation and not injurious to the subject.
Carriers
suitable for a selected dosage form and intended route of administration are
well known
in the art, and acceptable carriers for a chosen dosage form and method of
administration can be determined using ordinary skill in the art.
[0039] The pharmaceutical compositions and nutritional supplements of the
invention
may, optionally, contain additional ingredients and/or materials commonly used
in
11
CA 03053663 2019-08-15
WO 2018/187852 PCT/CA2017/050646
pharmaceutical compositions and/or nutritional supplements. These ingredients
and
materials include (1) fillers or extenders, such as starches, lactose,
sucrose, glucose,
mannitol, and silicic acid; (2) binders, such as carboxymethylcellulose,
alginates,
gelatin, polyvinyl pyrrolidone, hydroxypropylmethyl cellulose, sucrose and
acacia; (3)
humectants, such as glycerol; (4) disintegrating agents, such as agar-agar,
calcium
carbonate, potato or tapioca starch, alginic acid, certain silicates, sodium
starch
glycolate, cross-linked sodium carboxy methyl cellulose and sodium carbonate;
(5)
solution retarding agents, such as paraffin; (6) absorption accelerators, such
as
quaternary ammonium compounds; (7) wetting agents, such as cetyl alcohol and
glycerol monosterate; (8) absorbents, such as kaolin and bentonite clay; (9)
lubricants,
such as talc, calcium stearate, magnesium stearate, solid polyethylene
glycols, and
sodium lauryl sulfate; (10) suspending agents, such as ethoxylated isostearyl
alcohols,
polyoxyethylene sorbitol and sorbitan esters, microcrystalline cellulose,
aluminum
metahydroxide, bentonite, agar-agar and tragacanth; (11) buffering agents;
(12)
excipients, such as lactose, milk sugars, polyethylene glycols, animal and
vegetable
fats, oils, waxes, paraffins, cocoa butter, starches, tragacanth, cellulose
derivatives,
polyethylene glycol, silicones, bentonites, silicic acid, talc, salicylate,
zinc oxide,
aluminum hydroxide, calcium silicates, and polyamide powder; (13) inert
diluents, such
as water or other solvents; (14) preservatives; (15) surface-active agents;
(16)
dispersing agents; (17) control-release or absorption-delaying agents, such as
hydroxypropylmethyl cellulose, other polymer matrices, biodegradable polymers,
liposomes, microspheres, aluminum monosterate, gelatin, and waxes; (18)
opacifying
agents; (19) adjuvants; (20) wetting agents; (21) emulsifying and suspending
agents;
12
CA 03053663 2019-08-15
WO 2018/187852 PCT/CA2017/050646
(22), solubilizing agents and emulsifiers, such as ethyl alcohol, isopropyl
alcohol, ethyl
carbonate, ethyl acetate, benzyl alcohol, benzyl benzoate, propylene glycol,
1,3-
butylene glycol, oils (in particular, cottonseed, groundnut, corn, germ,
olive, castor and
sesame oils), glycerol, tetrahydrofuryl alcohol, polyethylene glycols and
fatty acid esters
of sorbitan; (23) propellants, such as chlorofluorohydrocarbons and volatile
unsubstituted hydrocarbons, such as butane and propane; (24) antioxidants;
(25)
agents which render the formulation isotonic with the blood of the intended
recipient,
such as sugars and sodium chloride; (26) thickening agents; (27) coating
materials,
such as lecithin; (28) vitamins and minerals; (29) proteins that carry
therapeutic or
nutritional benefits, such as whey protein and other milk-derived proteins;
and (30)
sweetening, flavoring, coloring, perfuming and preservative agents. Each such
ingredient or material must be "acceptable" in the sense of being compatible
with the
other ingredients of the formulation and not injurious to the subject.
Ingredients and
materials suitable for a selected dosage form and intended route of
administration are
well known in the art, and acceptable ingredients and materials for a chosen
dosage
form and method of administration may be determined using ordinary skill in
the art.
[0040] Pharmaceutical compositions and nutritional supplements suitable for
oral
administration may be in the form of capsules, cachets, pills, tablets,
powders, granules,
a solution or a suspension in an aqueous or non-aqueous liquid, an oil-in-
water or
water-in-oil liquid emulsion, an elixir or syrup, or a paste. These
formulations may be
prepared by methods known in the art, e.g., by means of conventional pan-
coating,
mixing, granulation or lyophilization processes.
13
CA 03053663 2019-08-15
WO 2018/187852 PCT/CA2017/050646
[0041] Solid dosage forms for oral administration (capsules, tablets, pills,
powders,
granules and the like) may be prepared by mixing the active ingredient(s) with
one or
more acceptable carriers and, optionally, one or more fillers, extenders,
binders,
humectants, disintegrating agents, solution retarding agents, absorption
accelerators,
wetting agents, absorbents, lubricants, and/or coloring agents. Solid
compositions of a
similar type may be employed as fillers in soft and hard-filled gelatin
capsules using a
suitable excipient. A tablet may be made by compression or molding, optionally
with
one or more accessory ingredients. Compressed tablets may be prepared using a
suitable binder, lubricant, inert diluent, preservative, disintegrant, surface-
active or
dispersing agent. Molded tablets may be made by molding in a suitable machine.
The
tablets, and other solid dosage forms, such as capsules, pills and granules,
may
optionally be scored or prepared with coatings and shells, such as enteric
coatings and
other coatings well known in the art. The tablets, and other solid dosage
forms, may
also be formulated so as to provide slow or controlled release of the active
ingredient
therein. They may be sterilized by, for example, filtration through a bacteria-
retaining
filter. These compositions may also optionally contain opacifying agents that
release the
active ingredient only, or preferentially, in a certain portion of the
gastrointestinal tract,
optionally, in a delayed manner. The active ingredient can also be in a
microencapsulated form.
[0042] Liquid dosage forms for oral administration include acceptable
emulsions,
microemulsions, solutions, suspensions, syrups, and elixirs. The liquid dosage
forms
may contain suitable inert diluents commonly used in the art. Besides inert
diluents, the
oral compositions may also include adjuvants, such as wetting agents,
emulsifying and
14
CA 03053663 2019-08-15
WO 2018/187852 PCT/CA2017/050646
suspending agents, sweetening, flavoring, coloring, perfuming and preservative
agents.
Suspensions may contain suspending agents.
[0043] According to still further preferred embodiments, the present invention
encompasses methods of using the beta-hydroxybutyrate / butyrate (BHB-BA)
compositions described herein to modulate "nuclear factor erythroid 2 related
factor 2"
(Nrf2) levels. More particularly, the present invention encompasses methods of
using
the BHB-BA compositions described herein to increase Nrf2 levels in targeted
cells.
Nrf2 is a transcription factor that transcribes a series of endogenous
antioxidant defense
systems. The transcription factor nucleotranslocates and binds the
antioxidant
response element (ARE) to transcribe cytoprotective genes. Nrf2 has been shown
to
transcribe the endogenous antioxidant peptides hem eoxygenase-1, catalase
(CAT),
superoxide dismutase (SOD), and glutathione peroxidase (GSH/GPx) in response
to
stress as a preservation mechanism.
[0044] In addition, Nrf2 induction or overexpression has been shown to
heighten
cellular defense mechanisms during metabolic stress and convey neuroprotection
during toxin-induced mitochondrial stress to the point of reduced lesion
development.
This cellular protection is also observed in the context of chemotherapy where
concurrent Nrf2 induction protects healthy cells. Still further, Nrf2
induction has been
shown to protect cells from [PS-induced inflammatory activity and mortality;
Nrf2
signaling pathways are showing promise as a counteraction to mitochondrial
dysfunction in Parkinson's disease; Nrf2 induction has been shown to convey
critical
defense against elevated serum-glucose-induced oxidative injury to cardiac
muscle
cells; and Nrf2 induction has been shown to provide a protective endogenous
system
CA 03053663 2019-08-15
WO 2018/187852 PCT/CA2017/050646
against ischemic injury. Furthermore, a diabetic condition has been shown to
be
associated with downregulation of Nrf2 activity via ERK (a factor speculated
to be a
contributor to stress-induced insulin resistance in cardiac cells). Indeed,
studies show
that Nrf2 activation can be used as a therapeutic application to improve
metabolic
disorder and relieve renal damage associated with diabetes.
[0045] Accordingly, the widespread existence of Nrf2 and its role in cellular
protection
as a master regulator of antioxidant defense makes it a viable target for
upregulation
against toxicity in most organs and tissues of the body. In other words, there
is a
continued and growing demand for compositions that are associated with Nrf2
induction
and overexpression ¨ which, as demonstrated in Example 6 below, the BHB-BA
compositions described herein have been shown to satisfy.
EXAMPLES
[0046] Example 1 - The following Example describes a composition of the
present
invention, which includes butyrate salts (and/or esters or propionate salts
thereof), in
combination with beta-hydroxybutyrate salts (and/or esters or propionate salts
thereof).
In this Example, the target subject will preferably receive, on a daily basis,
1000 - 5000
mg of a short chain fatty acid (e.g., butyrate) and 1000 ¨ 10,000 mg of ketone
(e.g.,
beta-hydroxybutyrate) - or, more preferably, 2000 - 5000 mg of butyrate and
5000 ¨
10,000 mg of beta-hydroxybutyrate. The invention provides that additional
optimizing
ingredients may be included in the formulation. For example, acetyl carnitine
may be
included, to provide a fatty acid transport mechanism facilitator. In
addition, R-alpha
lipoic acid may be included to improve insulin efficacy and to drive serum
glucose levels
down to be conducive to ketogenesis and ketone body prevalence as an ATP
substrate.
16
CA 03053663 2019-08-15
WO 2018/187852 PCT/CA2017/050646
[0047] In the following Example, the invention provides that an approximate
2:1
(butyrate : beta-hydroxybutyrate) ratio may be employed. Alternatively, the
invention
provides that a 1:1 ratio can also be used, or other ratios that are deemed
suitable for
the mode of delivery and the body mass of the target subject (person). The
following
provides an example formulation of the compositions of the present invention,
showing
a capsule formulation (at 1:1 and 2:1 ratios of butyrate and beta-
hydroxybutyrate) and a
powder formulation.
[0048] Capsules (1:1 butyrate / beta-hydroxybutyrate ratio)
Component Amount (mg)
Butyrate Sodium Salt 100
Butyrate Calcium Salt 100
Butyrate Magnesium Salt 100
Beta Hydroxybutyrate Sodium Salt 100
Beta Hydroxybutyrate Calcium Salt 100
Beta Hydroxybutyrate Magnesium Salt 200
Acetyl-L Carnitine 70
R alpha lipoic acid 12
Green Tea Extra (14% Caffeine) 70
Total per capsule 852
[0049] Capsules (2:1 butyrate / beta-hydroxybutyrate ratio)
Component Amount (mg)
Butyrate Sodium Salt 50
Butyrate Calcium Salt 50
Butyrate Magnesium Salt 100
Beta Hydroxybutyrate Sodium Salt 100
Beta Hydroxybutyrate Calcium Salt 100
17
CA 03053663 2019-08-15
WO 2018/187852
PCT/CA2017/050646
Beta Hydroxybutyrate Magnesium Salt 200
Acetyl-L Carnitine 70
R alpha lipoic acid 12
Green Tea Extra (14 % Caffeine) 70
Total per capsule 852
[0050] Powder Pouch / Canister
Component Amount (mg)
Powdered Cream / Butter Powder 3000
Coconut Fat/Cream Powder 2000
Butyrate Sodium Salt 1000
Butyrate Calcium Salt 1000
Butyrate Magnesium Salt 1000
Beta Hydroxybutyrate Sodium Salt 1000
Beta Hydroxybutyrate Calcium Salt 1000
Beta Hydroxybutyrate Magnesium Salt 1000
Resistant Starch 10000
Agave Extract 2000
Stevia 90
Berry Flavor 1000
Vitamin B1 diphosphate 5
Vitamin B2 Riboflavin 5
Vitamin B2 Riboflavin 51-phosphate 5
Niacin B3 10
Niacinaminde B3 5
NADH B3 5
Vitamin B5 panthenol 5
Vitamin B6 pyridoxine HCI 5
Vitamin B6 pyridoxine 5'-phosphate 5
18
CA 03053663 2019-08-15
WO 2018/187852 PCT/CA2017/050646
Vitamin B7 Biotin 0.5
Vitamin B9 Folic Acid 1
Vitamin B12 1
Inositol 0.3
Choline Bitartrate 0.3
Acetyl-L Carnitine 700
R alpha lipoic acid 12
Green Tea Extra (14% Caff total) 70
Total 24,843
[0051] Example 2 ¨ The following Example describes another composition of the
present invention, which includes butyrate salts (and/or esters or propionate
salts
thereof), in combination with beta-hydroxybutyrate salts (and/or esters or
propionate
salts thereof). In this Example, the composition was formulated as individual
capsules,
which comprised the components set forth in the table below.
Component Amount (mg) / % Total
Calcium Butyrate Powder 50 (8.0%)
Magnesium Butyrate Powder 150 (23.8%)
Sodium Beta Hydroxybutyrate Powder 102 (16.2%)
Magnesium Beta Hydroxybutyrate Powder 306 (48.6%)
Other (Magnesium Stearate; Silicon
22 (3.4%)
Dioxide; Capsule Vegetable Oil)
Total 630 (100%)
[0052] Example 3 ¨ The capsule described in Example 2 was administered to a
male
subject, 49 years of age with a body weight of 190 pounds, who exhibited above-
average fitness. The subject was administered a total of five (5) capsules at
time = 0
(following approximately four (4) hours of fasting), and the subject's serum
glucose and
19
CA 03053663 2019-08-15
WO 2018/187852 PCT/CA2017/050646
serum ketone levels were subsequently measured at the time points listed in
the table
below.
Time (Minutes / Post-Admin) 0 15 30 45 60
Serum Glucose (mM/L) 4.3 4.1 4.2 4.6 4.7
Serum Ketone (mM/L) 0.25 0.4 0.4 0.5 0.4
[0053] Example 4 - In this Example, the capsule described in Example 2 was
administered to the male subject described in Example 3. The subject was
administered a total of eight (8) capsules at time = 0 (following
approximately twelve
(12) hours of fasting), and the subject's serum glucose and serum ketone
levels were
subsequently measured at the time points listed in the table below.
Time (Minutes! Post-Admin) 0 15 45 60 75 90 105
Serum Glucose (mM/L) 4.7 5.3 5.6 5.4 5.6 6.0
4.9
Serum Ketone (mM/L) 0.2 0.2 0.4 0.4 0.4 0.2
0.2
[0054] Example 5 - In this Example, the capsule described in Example 2 was
administered to the male subject described in Example 3. The subject was
administered a total of six (6) capsules at time = 0 (following approximately
three (3)
hours of fasting), and the subject's serum glucose and serum ketone levels
were
subsequently measured at the time points listed in the table below.
Time (Minutes! Post-Admin) 0 15 30 45 60 75
Serum Glucose (mM/L) 4.5 4.1 4.8 4.9 4.2 4.2
Serum Ketone (mM/L) 0.2 0.2 0.3 0.4 0.3 0.2
[0055] Example 6 - In this Example, four different subjects (RG, CD, SD, and
FC)
were each administered a combination of butyrate salts and beta-
hydroxybutyrate salts
at a 1:1 ratio (9.3 grams of such combination was solubilized in 350 mL of
water). Each
CA 03053663 2019-08-15
WO 2018/187852 PCT/CA2017/050646
subject's serum glucose and serum ketone levels were subsequently measured at
the
time points listed in the applicable table below (the following data are
further
summarized in Figures 5 - 12).
RG
Time (Minutes / Post-Admin) 0 20 30 60 70
Serum Glucose (mM/L) 5.0 5.9 6.3 5.3 5.2
Serum Ketone (mM/L) 0.2 0.7 1.2 1.1 0.4
CD
Time (Minutes / Post-Admin) 0 20 30 40 50 60 80
Serum Glucose (mM/L) 5.4 5.1 5.3 6.2 5.0 5.4 4.8
Serum Ketone (mM/L) 0.2 0.3 0.4 0.5 0.5 0.4 0.2
SD
Time (Minutes / Post-Admin) 0 20 40 50 60
Serum Glucose (mM/L) 5.1 4.9 4.6 4.7 4.7
Serum Ketone (mM/L) 0.2 0.4 0.2 0.2 0.2
FC
Time (Minutes / Post-Admin) 0 20 40 60 80
Serum Glucose (mM/L) 5.1 4.9 4.9 5.0 5.0
Serum Ketone (mM/L) 0.2 0.4 0.6 0.4 0.4
[0056] As shown in Examples 3 - 6, a composition of the present invention was
effective to induce healthy ketosis in a subject (represented by a state where
ketones
are elevated), while maintaining a relatively stable blood sugar level.
21
CA 03053663 2019-08-15
WO 2018/187852 PCT/CA2017/050646
[0057] Example 7 ¨ The following Example demonstrates the effects of the beta-
hydroxybutyrate (BHB) / butyrate (BA) compositions described herein on
"nuclear factor
erythroid 2 related factor 2" (Nrf2). Specifically, Figure 1 shows the impact
of treating
BV2 cells (human microglia, brain-resident macrophage cells) with BHB-BA
complex on
Nrf2 levels. In
this Example, the BV2 cells were treated for 30 minutes at
concentrations of BHB-BA complex ranging from 0.4 mM to 2.4 mM. Figure 1 shows
that the BHB-BA treatments increased Nrf2 levels by more than 22% over control
(i.e.,
over the endogenous Nrf2 levels in the treated cells).
[0058] While Figure 1 shows the impact of BHB-BA complex on Nrf2 levels in
resting
cells, Figure 2 shows the protective effects of BHB-BA complex (at
concentrations
ranging from 0.4 mM to 2.4 mM) when the BV2 cells are pretreated with the BHB-
BA
complex, prior to application of lipopolysacharride (LPS) induced stress.
More
specifically, the BV2 cells were pretreated with BHB-BA complex, prior to
being
subjected to LPS-induced stress for 30 minutes. In this Example, Nrf2 was
shown to
increase by as much as 30% over the control at 1.6 mM of BHB-BA complex,
thereby
preparing the stressed cells to compensate with a greater counterforce than
that seen in
resting non-stressed (non-LPS stimulated) cells. Nrf2 status was shown to
level off at
higher BHB-BA levels, which indicates there is no need to escalate BHB-BA
levels
beyond such levels (when elevation of Nrf2 levels is desired). Similar to
Figure 2,
Figure 3 shows yet additional and confirmatory data collected after treatment
of BV2
cells with BHB-BA complex at concentrations ranging from 0.4 mM to 2.4 mM
(wherein
the cells were also stimulated with LPS for 30 minutes after pretreatment with
the BHB-
BA complex).
22
CA 03053663 2019-08-15
WO 2018/187852 PCT/CA2017/050646
[0059] Figure 4 shows the results of certain MTT assays (colorimetric assays
for
assessing cell metabolic activity) using BV2 cells to measure cell stress and
survival in
the context of various BHB-BA treatment concentrations (based on BHB
concentrations
established by the treatment). As shown in Figure 4, the percent survival
scores
demonstrate that BHB-BA treatment achieves a healthier response in relation to
the
less preferable / survival outcome in the BHB-only group.
[0060] The many aspects and benefits of the invention are apparent from the
detailed
description, and thus, it is intended for the following claims to cover all
such aspects and
benefits of the invention that fall within the scope and spirit of the
invention. In addition,
because numerous modifications and variations will be obvious and readily
occur to
those skilled in the art, the claims should not be construed to limit the
invention to the
exact construction and operation illustrated and described herein.
Accordingly, all
suitable modifications and equivalents should be understood to fall within the
scope of
the invention as claimed herein.
23