Note: Descriptions are shown in the official language in which they were submitted.
CA 03055297 2019-09-03
WO 2018/165369 PCMJS2018/021461
TITLE OF THE INVENTION
High Nitrogen, Multi-Principal Element, High Entropy Corrosion Resistant Alloy
BACKGROUND OF THE INVENTION
FIELD OF THE INVENTION
This invention relates to corrosion resistant austenitic steel alloys and in
particular, to a
multi-principal element, high entropy, corrosion resistant alloy that includes
nitrogen.
DESCRIPTION OF THE RELATED ART
It is known that alloying elements such as chromium (Cr), molybdenum (Mo), and
nitrogen (N) improve corrosion resistance of steel alloys, particularly
resistance to localized
attack in chloride containing environments. The degree of corrosion resistance
can be predicted
by a pitting resistance equivalent number (PREN). A known equation for
determining the PREN
of an alloy is PREN = Cr (wt.%)+ 3.3 x Mo (wt.%)+ 16 x N (wt.%). Other
elements, such as
tungsten, copper, and vanadium have been proposed as beneficial alloying
additions for
corrosion resistance. Cr and Mo are strong ferrite formers and can lead to the
formation of sigma
phase and chi phase which adversely affect both pitting resistance and
mechanical properties. To
offset the adverse effects of using higher amounts of Cr and Mc), austenite
formers such as
nickel, cobalt, and copper may be added to the alloys. This practice has led
to the use of nickel-
base and cobalt-base alloys for the most severely corrosive environments. The
addition of N is
known to be generally beneficial to both corrosion resistance and strength,
but nitrogen solubility
and the unwanted precipitation of nitrides, especially at grain boundaries,
limits the total amount
of nitrogen that can be added. Nitrogen solubility becomes increasingly
limited as nickel and
cobalt contents increase.
Among the known austenitic, corrosion resistance alloys, there are nickel-base
and
cobalt-base alloys that include significant amounts of Mo. In those alloys, a
high Mo content is
stabilized by either a high nickel content or a high cobalt content. Most of
those alloys do not
contain a positive addition of N. Alloy N-155 which is sold under the
registered trademark
MULTIMETO has the following nominal composition in weight percent: 20% Ni, 20%
Co, 20%
Cr, 3% Mo. 2.5%W, 1.5% Mn, 1% Nb+Ta. 0.15% N, and 0.1% C. The balance of the
alloy is
1
iron and usual impurities. Those alloys have essentially a single base element
such as iron,
nickel, or cobalt.
Alloy design has traditionally not considered the contributions of the mixing
entropy to
alloy phase stability because the mixing entropy is relatively low in systems
with a single base
element. Because they do not have a single base element, high entropy alloys
(HEA) employ
configurational entropy to affect the stability of solid structural phases
within the alloy. By
definition, HEA are composed of a single solid solution phase or a mixture of
solid solution
phases. With the exception of a few studies, the solid solution phases have
either a body centered
cubic (BCC) or a face centered cubic (FCC) structure. HEA typically consist of
at least three
elements in equiatomic or close to equiatomic proportions to maximize the
configurational
entropy. According to Guo et al., "Phase stability in high entropy alloys:
Formation of solid-
solution phase or amorphous phase", Progress in Natural Science: Materials
International, vol.
21, pp. 433-446 (2011), an alloy that meets the following rules regarding
mixing enthalpy
(Atimix), mixing entropy (ASmix), and atomic size difference (6) is more
likely to provide a solid
solution structure.
-22 < AHmix < 7 kJ/mol
ASmix > 11 J/(K mol)
The parameters AHmix, 6, and ASmix are known and are defined in the technical
literature. See,
for example, Guo et al. at p. 434. The above-stated rules are based on
experimental results from
various published studies, but should be considered as broad guidelines.
The basic principles derived from the above-listed rules overlap with the Hume-
Rothery
rules relating to solid solution formation in alloys and are suitable starting
point for designing an
alloy with a solid solution structure. The mixing enthalpy should not be too
negative or too
positive in order to avoid the formation of intermetallic phases and to avoid
phase separation.
The atomic size difference between the constituent elements should be
minimized to prevent
lattice strain. Further, the mixing entropy should be maximized.
2
Date Recue/Date Received 2020-05-19
CA 03055297 2019-09-03
WO 2018/165369 PCT/US2018/021461
The electronegativity of the constituent elements should be similar among the
principal
elements. The solid solution phase that forms is also related to the valence
electron concentration
(VEC). Guo et al. also discloses that a single-phase FCC structure is
predicted when VEC is
.. greater than about 8, a single-phase BCC structure is predicted when the
VEC is less than about
6.87, and a mixed FCC/BCC structure is predicted when 6.87 < VEC < 8.
SUMMARY OF THE INVENTION
In accordance with a first aspect of the present invention there is provided a
multi-
principal element, corrosion resistant alloy having the following composition
in weight percent:
Co about 13 to about 28
Ni about 13 to about 28
Fe+Mn about 13 to about 28
Cr about 13 to about 37
Mo about 8 to about 28
about 0.10 to about 1.00.
The alloy also includes the usual impurities found in corrosion resistant
alloys intended for the
same or similar use. In addition, one or both of W and V may be substituted
for some or all of
the Mo. The alloy provides a solid solution that is substantially all FCC
phase, but may include
minor amounts of secondary phases that do not adversely affect the corrosion
resistance and
mechanical properties provided by the alloy.
In accordance with another aspect of the present invention there is provided a
multi-
element, corrosion resistant, high entropy alloy having the atomic formula
(Fe,
Mn)aCobNieCrx(Mo, W, V)), wherein a and hare each 12-35 atomic percent (at.%),
c and x are
each 12-40 at.%, and y is 4-20 at.%. W and/or V may be substituted for some or
all of the Mo on
an equiatomic basis. The alloy also comprises from at least about 0.10% N up
to the solubility
limit.
Within the foregoing alloy compositions, the elements are selected to provide
the
following combination of parameters;
3
CA 03055297 2019-09-03
WO 2018/165369 PCT/US2018/021461
-6 kJ/mol < AFL, < 0 kJ/mol;
2.00% <3 <4.5%;
ASmix > 12 J/K mol; and
the valence electron concentration is greater than about 7.80.
It is contemplated that the alloy according to the present invention may
comprise or may
consist essentially of the elements described above, throughout the following
specification, and
in the appended claims. Here and throughout this application the term -
percent" and the symbol
mean percent by weight or percent by mass, unless otherwise indicated.
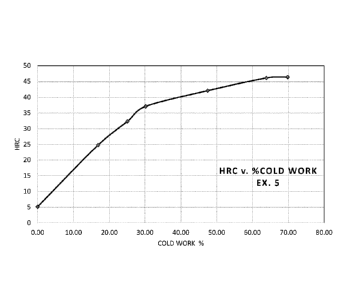
BRIEF DESCRIPTION OF THE DRAWING
The drawing is a graph of Rockwell C hardness (HRC) as a function of cold
working
percent for Example 5 of the alloy according to this invention.
DETAILED DESCRIPTION
By using the foregoing parameters in the design of multi-element alloy,
corrosion
resistant alloy, it is believed that higher amounts of elements such as
molybdenum, tungsten, and
vanadium, can be included in a CoCrNiMnFe base alloy to provide an FCC solid
solution
structure that is substantially free of undesired secondary phases. The alloy
also includes a small
amount of N as an interstitial element. An equiatomic or near-equiatomic
composition
comprising a combination of Cr, Mn, Fe, Co. and Ni provides the multi-element
base of the high
entropy alloy according to this invention. The combination of base elements is
chosen because it
meets the constraints for HEA outlined about. Interstitial elements such as N
have not been
studied extensively within the HEA design constructs and may require novel
design
considerations that go beyond the rules discussed above. Specifically, the use
of AHõ,,,, as an
average term should be avoided in order to properly design an alloy in which
nitride formation
does not occur. Relatively large additions of Mo, W, or V in conjunction with
N at or close to its
solubility limit provides a novel alloy system with potentially superior
corrosion resistance
compared to the known Fe-base, Ni-base, and Co-base stainless steel alloys.
4
CA 03055297 2019-09-03
WO 2018/165369 PCT/US2018/021461
Nickel and cobalt are present in the high entropy alloy of this invention to
help stabilize
the preferred FCC phase. Nickel and cobalt also benefit the desired single
phase nature of the
alloy by reducing the precipitation of undesirable ordered phases such as
sigma (G) and mu ( )
phases in the solid solution. In this way nickel and cobalt benefit the
ductility provided by the
alloy. Nickel and cobalt are relatively expensive elements and so their
contents are limited to
control the cost of making the alloy of this invention.
Chromium contributes to the general and localized corrosion resistance
provided by this
alloy. It is also believed that chromium helps to increase the solubility of
nitrogen in the alloy.
Too much chromium adversely affects the mechanical properties (e.g.,
ductility) and corrosion
resistance by promoting the precipitation of ordered phases, like sigma and/or
Chromium
nitrides.
The alloy also contains about 4 to about 20 atomic percent (at. %) or at least
about 8% up
to about 28% weight percent of molybdenum to benefit the alloy's resistance to
localized
corrosion such as pitting corrosion. Too much molybdenum promotes the
precipitation and
stabilization of topologically close packed phases which adversely affects the
corrosion
resistance and mechanical properties. Like chromium too much molybdenum
adversely affects
the ductility and processability of the alloy because it forms sigma phase at
relatively high
temperatures. Tungsten and/or vanadium can be substituted for some or all of
the molybdenum
on an equiatomic basis.
Manganese is present in the alloy of this invention because it benefits the
solubility of
nitrogen in the solid solution of the alloy. Too much manganese reduces the
solidus temperature
of the alloy which can adversely affect the intergranular strength during hot
working.
Iron contributes to the high entropy of mixing (AS.) that characterizes this
alloy and
helps to stabilize the desired single phase FCC structure of the alloy. Iron
is also present as a
substitute for some of the nickel and/or cobalt to help limit the cost of
producing the alloy.
Similar to chromium and molybdenum, too much iron can result in the
precipitation of sigma
phase which adversely affects the ductility of the alloy and its
processability.
5
CA 03055297 2019-09-03
WO 2018/165369 PCT/US2018/021461
At least about 0.10% nitrogen is also present in this alloy as an interstitial
element. The
addition of nitrogen helps to further stabilize the FCC phase and benefits the
localized corrosion
resistance provided by the alloy. As an interstitial element nitrogen also
contributes to the good
mechanical properties provided by the alloy such as its yield strength and
tensile strength.
Nitrogen may be present up to its solubility limit in the alloy, but
preferably is limited to not
more than about 1.00% in this alloy.
The alloy according to the present invention may also include copper to
benefit the
stability of the FCC phase structure. However, too much copper, reduces the
solidus temperature
of the alloy which can result in incipient intergranular liquation during hot
working of the alloy.
An alloy in accordance with this invention provides very good resistance to
corrosion,
especially pitting corrosion. In this regard the alloy is characterized by
having a pitting
resistance equivalent number (PREN) of at least 50 where the PREN is defined
as follows:
PREN = %Cr + 3.3x%Mo + 16x%N. Preferably, the alloy is characterized by a PREN
of at
least about 65 and better yet at least about 70.
The elements that constitute the alloy of this invention are selected to
provide the
following combination of parameters;
-6 kJ/mol < AH. < 0 kJ/mol;
2.00% < 6 < 4.5%;
AS. > 12 J/K mol; and
the valence electron concentration (VEC) is greater than about 7.80. ASmix is
mainly affected by
the number of main elements in the alloy and their concentrations. Preferably,
a minimum of
five equiatomic elements provide a ASmix that results in a stabilized alloy
microstructure. In the
five-element embodiment of the alloy it is expected that ASi, will be not more
than about 13-
13.5 J/K mol. However, in the copper-containing embodiment it is expected that
ASõõx will be
greater than 13-13.5 J/K mol. AfInilx is determined by the chemical affinity
of the constituent
elements and is preferably as close to zero as practicable to allow the
entropy to manage the
stability of the alloy. The parameter 6 is related to the difference in atomic
size of the constituent
6
CA 03055297 2019-09-03
WO 2018/165369 PCT/US2018/021461
elements. In this alloy, molybdenum is the largest atom and is the one that
most affects the value
of 6.
Valence electron concentration is the number of total electrons in the valence
band
including the "d" electrons. Cobalt and nickel have the higher VEC's, 9 and 10
respectively,
than the other elements. However, since this is an alloy, the VEC is
calculated as
VEC = Ci(VEC)i
Where Ci is the concentration of element i. Co and Ni affect the VEC in this
alloy. Preferably,
the alloy according to this invention provides a VEC greater than 8Ø
WORKING EXAMPLES
In order to demonstrate the properties provided by the alloys according to
this invention
six heats were vacuum induction melted and then cast as 40-1b. ingots. The
weight percent
compositions of the six heats are set forth in Table 1 below as Examples 1-6.
Table 1
Ex. 1 Ex. 2 Ex. 3 Ex. 4 Ex. 5 Ex. 6
0.137 0.229 0.130 0.167 0.227 0.128
Si 0.03 0.03 0.02 0.03 0.03 0.03
Cr 13.30 20.25 13.30 15.77 21.94 13.50
Ni 21.60 17.44 33.50 32.68 27.70 22.44
Mo 17.25 12.84 17.71 16.20 12.72 17.48
Co 26.45 25.40 17.27 16.93 19.84 25.81
Fe+Mn 21.23 23.81 18.07 18.22 17.54 20.61
After solidification it was determined that the ingots contained mainly a
solid solution consisting
essentially of an FCC structure with some interdendritic secondary phase(s).
The 40-lb ingots
were homogenized, forged to 0.75" square bars, and then solution annealed at
2250 F for 2.5 hrs.
7
CA 03055297 2019-09-03
WO 2018/165369 PCT/US2018/021461
followed by water quenching. It was determined that the alloy had a solid
solution structure
consisting substantially of the FCC phase in the solution-annealed-and-
quenched condition.
Test specimens for critical pitting temperature testing, potentiodynamic
testing, and slow
strain rate testing were obtained from the solution annealed 0.75" square bars
prepared from each
ingot. Critical pitting temperature (CPT) testing was performed in a 1 M
solution of NaCl at 0.7
volts with a nitrogen gas purge in accordance with ASTM Standard Test
Procedure G150. The
results of the CPT testing are shown in Table 2 below.
Table 2
CPT
Ex. 1 >99.7 C
Ex. 2 90.65 C
Ex. 3 >95 C
Ex. 4 >95 C
Ex. 5 >95 C
Ex. 6 >95 C
Cyclic polarization potentiodynamic testing was performed based on ASTM
Standard
Test Procedure G61. Voltage values at the knee of the curve, at 50 pA/cm2, and
at 100 pA/cm2
were measured for two sets of samples prepared from solution annealed 0.75"
square bars. The
results of the potentiodynamic pitting tests are shown in Table 3 below
including the pitting
potentials and the repassivation potentials in millivolts (mV).
8
CA 03055297 2019-09-03
WO 2018/165369 PCT/US2018/021461
Table 3
Pitting Potential Pitting Potential Pitting Potential @ Repassivation
@ Knee @ 500A/cm2 100pA/cm2 Potential
Ex. 1 891.5 949.3 961.9 846.3
Ex. 2 887.2 946.4 956.3 784.2
Ex. 3 937.9 966.1 974.6 853.3
Ex. 4 914.8 950.7 956.7 858
Ex. 5 921.3 961.2 965.8 867
Ex. 6 943.2 967.2 973.1 849
Another set of samples were obtained from the 0.75 in. bars of each example
for testing
resistance to corrosion in acidic solutions. The samples were tested after
immersion in a boiling
aqueous solution containing 85% by volume of phosphoric acid (H3PO4).
Additional samples
were tested after immersion in a boiling aqueous solution containing 60% by
volume of nitric
acid (HNO3). Further samples were tested after immersion in an of acid mixture
in accordance
ASTM Standard Test Procedure G28-02, Practice A. A fourth set of samples were
tested after
immersion in an of acid mixture in accordance ASTM Standard Test Procedure G28-
02, Practice
B. The results of the acidic corrosion tests for each example are presented in
Table 4 including
the weight loss in mills per year (mpy). Table 4 includes a qualitative
assessment of the severity
of intergranular attack for the specimens tested in accordance with ASTM G28-
02, Methods A
and B.
Table 4
Heat
85% H3PO4 65% HNO3 AS TM G28-B ASTM G28-A
Sample
Ex. 1 60.5 568.5 112.1 Light IGA 799.7
Severe IGA
Ex. 2 609.9 96.7 5.8 NVA
712.6 Severe IGA
Ex. 3 N/A* 904.5 N/A* N/A
480.4 Severe IGA
Ex. 4 55.55 335.8 21.35 NVA 139.2 Light
IGA
Ex. 5 124 22.4 2 NVA 16.5 NVA
9
CA 03055297 2019-09-03
WO 2018/165369 PCT/US2018/021461
Ex. 6 74.85 1125.7 81.95
Light IGA 471.4 Severe IGA
IGA= Intergranular attack.
NVA= Nonvisible attack
*The test could not be completed because of technical difficulties and
insufficient material for
retesting.
The data presented in Tables 2, 3, and 4 show that all the examples provided
very good
resistance to pitting in a chloride-containing environment, as well as good
resistance to
intergranular corrosion in acidic environments.
Slow strain rate testing of specimens from Examples 1, 2, 4, and 5 was
performed in each
of three different environments: ambient air, a 3.5% NaC1 solution at boiling
temperature, and a
3.5% NaCl solution at boiling temperature with a pH of 1Ø The results of the
slow strain rate
testing are shown in Table 5 below including the percent elongation (% El.),
the percent
reduction in area (%RA), and the number of hours to fracture (Hours). Also
shown in Table 5
are the results of each tested property presented as a percentage of the same
property measured in
air. In the last column of Table 5 is shown the "% of Air - Composite" which
is the average of
the % El. Air Avg, %RA Air Avg, and Hr Air Avg. It is calculated as (%El. Air
Avg. + %RA
Air Avg. + Hrs. Air Avg.)/3.
Table 5
Environment %El. %El. %RA %RA Hours Hrs. as % of Air
as % as % % of Composite
of of Air
Air Air Avg.
Avg. Avg.
Ex. 1 Air 92.8 75.0 59.1
3.5 NaCl @ 92.6 99.8 73.0 97.3 46.4 78.5
91.9
Boiling
3.5 NaCl @ 88.6 95.4 73.0 97.3 42.9 72.6
88.4
Boiling, pH
1.0
Ex. 2 Air 91.1 72.8 45.5
3.5 NaCl @ 92.0 100 65.9 90.5 41.5 91.2
93.9
Boiling
CA 03055297 2019-09-03
WO 2018/165369 PCT/US2018/021461
3.5 NaC1 @ 87.9 96.5 65.0 89.3 42.5 93.4
93.1
Boiling, pH
1.0
Ex. 4 Air 87.7 74.8 61.5
3.5 NaCl @ 87.2 99.43 75 100 60.3 98.05
99.1
Boiling
3.5 NaC1 @ 81.4 92.82 70 93.58 54.1 87.97
91.4
Boiling, pH
1.0
Ex. 5 Air 90.7 79.5 63.1
3.5 NaC1 @ 87.1 96.03 70.2 88.30 60.2 95.4 93.2
Boiling
3.5 NaC1 @ 73.7 81.26 54.3 68.30 50.7 80.35 76.6
Boiling, pH
1.0
The results presented in Table 5 show that Examples 1, 2, 4, and 5 are
practically
immune to boiling 3.5% NaCl, even at a pH of 1.0, thereby showing the good
corrosion
resistance in the boiling sodium chloride environment.
Two sets of longitudinal tensile samples were prepared from the bars of
Examples 4, 5,
and 6, one set for mechanical testing at room temperature (25 C) and the
other set for testing at
a cryogenic temperature (-100 C). The results of the room temperature tensile
testing are
presented in Table 6 and the results of the cryogenic tensile testing are
presented in Table 7. For
both sets of tests the results include the 0.2% offset yield strength (Y.S)
and the ultimate tensile
strength (U.T.S.) in ksi (MPa), the percent elongation in 4 diameters (%El.),
and the percent
reduction in area (%R.A.).
11
CA 03055297 2019-09-03
WO 2018/165369 PCT/US2018/021461
Table 6
Y.S. U.T.S.
%El. %R.A.
Ex. ksi MPa ksi MPa
4 51.3 354 111 765 73 29
55.9 385 118 813 72 29
6 54.4 375 112 772 70 26
Table 7
Y.S. U.T.S.
%El. %R.A.
Ex. ksi MPa ksi MPa
4 72.8 502 138 951 78 26
5 77.2 532 148 1020 76 27
6 74.6 514 139 958 87 23
5
One of the important properties in this alloy is the very high ductility
provided by the
alloy as demonstrated by the high elongation values set forth in Tables 6 and
7. By way of
example, the percent elongation provided by the alloy is up to 73% at room
temperature which
compares very favorably to 58% elongation provided by the known stainless
steels. However,
more important is the capability to provide that level of ductility even at
cryogenic temperatures
without adversely affecting the tensile strength provided by the alloy as
shown in Table 7.
In addition to the exceptional corrosion resistance and mechanical properties
provided by
the alloy according to the invention as presented in Tables 2 through 7, this
alloy provides
excellent cold processability as demonstrated by its cold work hardening
capability. In this
regard, the alloy is able to provide a Rockwell C-scale hardness (HRC) of
about 37 after about
30% cold work, where the percent cold work is defined by the equation below:
12
CA 03055297 2019-09-03
WO 2018/165369 PCT/US2018/021461
Initial Area ¨ final Area
%CW = ________________________________________________
Initial Area
In order to demonstrate the good cold processability provided by this alloy,
material from
Example 5 was cold worked to increasing percent reductions in cross-sectional
area and the HRC
was measured at several intervals. The results are shown is shown the drawing
figure as a graph
of the measured HRC values as a function of the percent cold reduction. The
graphed data shows
the unexpectedly high ductility provided by this alloy allows the alloy to be
cold worked up to
70% or more while reaching a hardness of about 45 HRC.
The terms and expressions which are employed in this specification are used as
terms of
description and not of limitation. There is no intention in the use of such
terms and expressions
of excluding any equivalents of the features shown and described or portions
thereof. It is
recognized that various modifications are possible within the invention
described and claimed
herein.
13