Note: Descriptions are shown in the official language in which they were submitted.
CA 03057548 2019-09-23
WO 2018/178243
PCT/EP2018/058088
1
PHARMACEUTICAL COMPOSITIONS
This invention relates to the use of (+)13-dihydrotetrabenazine and
combinations of
(+)-a-dihydrotetrabenazine, (-)-a-dihydrotetrabenazine and/or (+)13-
dihydrotetrabenazine, for the treatment of movement disorders, such as
Tourette's
syndrome.
Background of the Invention
Movement disorders can generally be classified into two categories:
hyperkinetic
movement disorders and hypokinetic movement disorders. Hyperkinetic movement
disorders are caused by an increase in muscular activity and can cause
abnormal
and/or excessive movements, including tremors, dystonia, chorea, tics,
myoclonus
and stereotypies.
Hyperkinetic movement disorders often are often psychological in nature and
arise
through improper regulation of amine neurotransmitters in the basal ganglia.
A particular hyperkinetic movement disorder is Tourette's syndrome, which is
an
inherited neurological condition characterised by multiple physical and vocal
tics.
The tics are usually repetitive, but random, physical movements or vocal
noises.
The vocal tics can be of various forms and include repeating one's own words,
the
words of others or other sounds. Onset usually occurs in children and
continues
through to adolescence and adulthood.
While the tics associated with Tourette's syndrome are temporarily
suppressible,
those affected can usually only suppress their tics for limited time periods.
There
is yet to be an effective treatment to cover all types of tics in all
patients, but certain
medicaments for tic suppression have been developed.
It is known that dopamine receptor antagonists display an ability to supress
tics in
Tourette's syndrome patients and a number dopamine receptor antagonists are
currently used in the suppression of Tourette's tics, such as fluphenazine,
haloperidol and pimozide.
Type 2 vesicular monoamine transporter (VMAT2) is a membrane protein
responsible for the transportation of monoamine neurotransmitters, such as
dopamine, serotonin and histamine, from cellular cytosol into synaptic
vesicles.
CA 03057548 2019-09-23
WO 2018/178243
PCT/EP2018/058088
2
Inhibition of this protein hinders presynaptic neurons from releasing
dopamine,
resulting in a depletion of dopamine levels in the brain.
VMAT2 inhibitors can be used to treat the symptoms of Tourette's syndrome.
Tetrabenazine (Chemical name: 1,3,4,6,7,11b-hexahydro-9,10-dimethoxy-3-(2-
.. methylpropyI)-2H-benzo(a)quinolizin-2-one) has been in use as a
pharmaceutical
drug since the late 1950s. Initially used as an anti-psychotic, tetrabenazine
is
currently used for treating hyperkinetic movement disorders such as
Huntington's
disease, hemiballismus, senile chorea, tic, tardive dyskinesia and Tourette's
syndrome, see for example Jankovic etal., Am. J. Psychiatry. (1999) Aug;
156(8):1279-81 and Jankovic etal., Neurology (1997) Feb; 48(2):358-62.
The primary pharmacological action of tetrabenazine is to reduce the supply of
monoamines (e.g. dopamine, serotonin, and norepinephrine) in the central
nervous
system by inhibiting the human vesicular monoamine transporter isoform 2
(hVMAT2). The drug also blocks post-synaptic dopamine receptors.
The central effects of tetrabenazine closely resemble those of reserpine, but
it
differs from reserpine in that it lacks activity at the VMAT1 transporter. The
lack of
activity at the VMAT1 transporter means that tetrabenazine has less peripheral
activity than reserpine and consequently does not produce VMAT1-related side
effects such as hypotension.
Tetrabenazine is an effective and safe drug for the treatment of a variety of
hyperkinetic movement disorders and, in contrast to typical neuroleptics, has
not
been demonstrated to cause tardive dyskinesia. Nevertheless, tetrabenazine
does
exhibit a number of dose-related side effects including causing depression,
parkinsonism, drowsiness, nervousness or anxiety, insomnia and, in rare cases,
neuroleptic malignant syndrome, see for example the introductory section of
W02016/127133 (Neurocrine Biosciences).
The chemical structure of tetrabenazine is as shown below.
CA 03057548 2019-09-23
WO 2018/178243
PCT/EP2018/058088
3
8 7
CH30 9 6
11b N5
CH30 4
11
3
1 2
0
Structure of tetrabenazine
The compound has chiral centres at the 3 and llb carbon atoms and hence can,
theoretically, exist in a total of four isomeric forms, as shown below.
7 8
8 7
CH30 9 6 CH30 9 6
11b N5 11b N5
CH30 1 4
CH30 1 4 11 Ho
H
3 3
1
1 2
RR 0 SS 0
7 8
7 8 CH30 9 6
CH30 9 6
11b N5
11b N5
CH30 1
CH30 1 11 Fc 11 H 4
4
3 1 2 3
1 2
SR 0
5 RS 0
Possible tetrabenazine isomers
The stereochemistry of each isomer is defined using the "R and S" nomenclature
developed by Cahn, Ingold and Prelog, see Advanced Organic Chemistry by Jerry
March, 41h Edition, John Wiley & Sons, New York, 1992, pages 109-114. In this
10 patent application, the designations "R" or "S" are given in the order
of the position
numbers of the carbon atoms. Thus, for example, RS is a shorthand notation for
3R,11bS. Similarly, when three chiral centres are present, as in the
dihydrotetrabenazines described below, the designations "R" or "S" are listed
in the
order of the carbon atoms 2, 3 and 11b. Thus, the 2R,3S,11bS isomer is
referred
to in short hand form as RSS and so on.
Commercially available tetrabenazine is a racemic mixture of the RR and SS
isomers and it would appear that the RR and SS isomers are the most
thermodynamically stable isomers.
CA 03057548 2019-09-23
WO 2018/178243 PCT/EP2018/058088
4
Tetrabenazine has somewhat poor and variable bioavailability. It is
extensively
metabolised by first-pass metabolism, and little or no unchanged tetrabenazine
is
typically detected in the urine. It is known that at least some of the
metabolites of
tetrabenazine are dihydrotetrabenazines formed by reduction of the 2-keto
group in
tetrabenazine.
Dihydrotetrabenazine (Chemical name: 2-hydroxy-3-(2-methylpropyI)-
1,3,4,6,7,11b-hexahydro-9,10-dimethoxy-benzo(a)quinolizine) has three chiral
centres and can therefore exist in any of the following eight optical isomeric
forms:
8 7
8 7
CH30 9 4 CH30 9 6
6
11b N5
11b N5
H
CH30 1 CH30 1
11 Ho. 4 H
3 so H 3
1 2 1 2
RRR OH SSS OH
8 7 8 7
CH30 9 6 CH30 9 6
11b N5 11b N5
CH30 1 11 H 4 CH30 1
3 so H 11 Hµ H
1 2 3
1 2 =õ,õ.õ...---õõ
SRR OH RSS OH
CH30 CH30
CH30 11b CH30
Ho% 11b H
3
2
OH OH
SSR RRS
CH30 CH30
N
CH30 11b CH30
Ho% 11b H
2 2
OH OH
RSR SRS
Dihydrotetrabenazine isomers
CA 03057548 2019-09-23
WO 2018/178243
PCT/EP2018/058088
The synthesis and characterisation of all eight dihydrotetrabenazine isomers
is
described by Sun et al. (Eur. J. Med. Chem. (2011), 1841-1848).
Of the eight dihydrotetrabenazine isomers, four isomers are derived from the
RR
and SS isomers of the parent tetrabenazine, namely the RRR, SSS, SRR and RSS
5 isomers.
The RRR and SSS isomers are commonly referred to as "alpha (a)"
dihydrotetrabenazines and can be referred to individually as (+)-a-
dihydrotetrabenazine and (-)-a-dihydrotetrabenazine respectively. The alpha
isomers are characterised by a trans relative orientation of the hydroxyl and
2-
methylpropyl substituents at the 2- and 3-positions - see for example,
Kilbourn et
al., Chirality, 9:59-62 (1997) and Brossi et al., Hely. Chim. Acta., vol. XLI,
No. 193,
pp1793-1806 (1958.
The SRR and RSS isomers are commonly referred to as "beta (8)" isomers and
can be referred to individually as (+)-8-dihydrotetrabenazine and (+8-
dihydrotetrabenazine respectively. The beta isomers are characterised by a cis
relative orientation of the hydroxyl and 2-methylpropyl substituents at the 2-
and 3-
positions.
Although dihydrotetrabenazine is believed to be primarily responsible for the
activity of the drug, there have been no studies published to date that
contain
evidence demonstrating which of the various stereoisomers of
dihydrotetrabenazine is responsible for its biological activity. More
specifically,
there have been no published studies demonstrating which of the stereoisomers
is
responsible for the ability of tetrabenazine to treat movement disorders such
as
Tourette's syndrome.
Schwartz et al. (Biochem. Pharmacol. (1966), 15: 645-655) describes metabolic
studies of tetrabenazine carried out in rabbits, dogs and humans. Schwartz et
al.
identified nine metabolites, five of which were unconjugated and the other
four of
which were conjugated with glucuronic acid. The five unconjugated metabolites
were the alpha- and beta-dihydrotetrabenazines, their two oxidised analogues
in
which a hydroxyl group has been introduced into the 2-methylpropyl side chain,
and oxidised tetrabenazine in which a hydroxyl group has been introduced into
the
2-methylpropyl side chain. The four conjugated metabolites were all compounds
in
which the 9-methoxy group had been demethylated to give a 9-hydroxy compound.
CA 03057548 2019-09-23
WO 2018/178243
PCT/EP2018/058088
6
The chirality of the various metabolites was not studied and, in particular,
there
was no disclosure of the chirality of the individual a- and 13-isomers.
Scherman etal., (Mol. Pharmacol. (1987), 33, 72-77 describes the
stereospecificity
of VMAT2 binding between racemic a- and 13- dihydrotetrabenazine. They
reported
that a-dihydrotetrabenazine had a 3- to 4-fold higher affinity for the
Chromaffin
Granule Monoamine Transporter than the 13-isomer, when studied in vitro.
However, Scherman et al. does not disclose the resolution or testing of the
individual enantiomers of the a- and 13-dihydrotetrabenazines.
Mehvar etal. (J. Pharm. Sci. (1987), 76(6), 461-465) reported a study of the
concentrations of tetrabenazine and dihydrotetrabenazine in the brains of rats
following administration of either tetrabenazine or dihydrotetrabenazine. The
study
showed that despite its greater polarity, dihydrotetrabenazine was able to
cross the
blood-brain barrier. However, the stereochemistry of the dihydrotetrabenazine
was
not disclosed.
Mehvar etal. (Drug Metabolism and Disposition (1987), 15:2, 250-255) describes
studies of the pharmacokinetics of tetrabenazine and dihydrotetrabenazine
following administration of tetrabenazine to four patients affected by tardive
dyskinesia. Oral administration of tetrabenazine resulted in low plasma
concentrations of tetrabenazine but relatively high concentrations of
dihydrotetrabenazine. However, the stereochemistry of the dihydrotetrabenazine
formed in vivo was not reported.
Roberts et al. (Eur. J. Clin. Pharmacol. (1986), 29: 703-708) describes the
pharmacokinetics of tetrabenazine and its hydroxy-metabolite in patients
treated
for involuntary movement disorders. Roberts et al. reported that tetrabenazine
was
extensively metabolised after oral administration resulting in very low plasma
concentrations of tetrabenazine but much higher concentrations of a
hydroxymetabolite. Although they did not describe the identity of the
hydroxymetabolites, they suggested that the high plasma concentrations of the
hydroxymetabolites may be therapeutically important (since the metabolites
were
known to be pharmacologically active) and that, in view of the disclosure in
Schwartz et al. (idem), the combination of cis and trans isomers (i.e. alpha
and
beta isomers) could be more therapeutically important than the parent drug.
CA 03057548 2019-09-23
WO 2018/178243
PCT/EP2018/058088
7
Michael Kilbourn and collaborators at the University of Michigan Medical
School
have published a number of studies relating to the various isomers of
dihydrotetrabenazines. In Med. Chem. Res. (1994), 5:113-126, Kilbourn etal.
describe the use (+/-)-a-[11C]-dihydrotetrabenazine as in vivo imaging agents
for
VMAT2 binding studies.
In Eur. J. Pharmacol (1995) 278, 249-252, Kilbourn etal. reported competition
binding studies using [3H]-tetrabenazine to study the in vitro binding
affinity of (+)-,
(-)-, and (+/-)-a-DHTBZ. The binding assays gave a Ki value of 0.97 nM for (+)-
a-
dihydrotetrabenazine and 2.2 pM for (-)-a-dihydrotetrabenazine, thereby
showing
that the (+) alpha isomer has much greater binding affinity for the VMAT2
receptor
than the (-) alpha isomer. However, no studies were reported, or conclusions
drawn, as to the usefulness of either isomer in the treatment of movement
disorders such as Tourette's syndrome.
In Chirality (1997) 9:59-62, Kilbourn etal. described studies aimed at
identifying
the absolute configuration of (+)-a-dihydrotetrabenazine from which they
concluded that it has the 2R, 3R, 11bR configuration shown above. They also
referred to the Schwartz et al. and Mehvar et al. articles discussed above as
indicating that the a- and 6-dihydrotetrabenazines are likely to be the
pharmacologically active agents in the human brain but they drew no explicit
conclusions as to the precise stereochemical identities of the active
metabolites of
tetrabenazine.
In Synapse (2002), 43:188-194, Kilbourn etal. described the use of (+)-a-[11C]-
dihydrotetrabenazine as an agent used to measure specific in vivo binding of
the
VMAT receptor, in "infusion to equilibrium methods". They found that (-)-a-
[11C]-
dihydrotetrabenazine produced a uniform brain distribution, consistent with
the
earlier observations that this enantiomer has a low VMAT affinity.
Sun et al. (idem) investigated the VMAT2 binding affinities of all eight
dihydrotetrabenazine isomers. They found that all of the dextrorotatory
enantiomers exhibited dramatically more potent VMAT2 binding activity than
their
.. corresponding laevorotatory enantiomers with the most active (+)-a-isomer
being
found to be the most active. However, Sun et al. did not carry out any
investigations into the relative efficacies of the individual isomers in
treating
movement disorders such as Tourette's syndrome.
CA 03057548 2019-09-23
WO 2018/178243
PCT/EP2018/058088
8
WO 2011/153157 (Auspex Pharmaceutical, Inc.) describes deuterated forms of
dihydrotetrabenazine. Many deuterated forms of dihydrotetrabenazine are
depicted but the application only provides sufficient information to allow a
small
number of the depicted compounds to be synthesised. Although racemic mixtures
of d6-a-dihydrotetrabenazine and d6-6-dihydrotetrabenazine as disclosed, these
mixtures were not resolved and the properties of the individual (+) and (-)
isomers
are not studied. Similarly, WO 2014/047167 (Auspex Pharmaceutical, Inc.)
describes number of deuterated forms of tetrabenazine and its derivatives.
Again,
the individual (+) and (-) isomers of deuterated forms of a- and 6-
.. dihydrotetrabenazine were not separated or studied.
It is evident that, up to the present, it has been unclear as to precisely
which
dihydrotetrabenazine isomers are responsible for the therapeutic properties
resulting from the administration of tetrabenazine. It has previously been
assumed
that (+)-a-dihydrotetrabenazine is the metabolite of tetrabenazine that is
primarily
responsible for its therapeutic effects (see WO 2015/171802 Neurocrine
Biosciences, Inc.), but this has not been demonstrated experimentally.
Summary of the Invention
As discussed above, the studies carried out by Schwartz et al. (referred to
above)
demonstrated that both alpha and beta isomers of tetrabenazine are formed as
metabolites of tetrabenazine. However, the precise stereochemical
configurations
of the alpha and beta isomers were not investigated.
Studies in human subjects carried out by the present applicants and described
in
Example 1 below have confirmed the findings of Schwartz et al. that major
metabolites of tetrabenazine are indeed alpha and beta dihydrotetrabenazines.
However, contrary to what has previously been suggested, the main metabolites
produced upon administration of tetrabenazine are the (-)-a-
dihydrotetrabenazine
isomer, which is essentially active as a VMAT2 binding agent, and the (+)-6-
dihydrotetrabenazine isomer, which is significantly less active than the (+)-a-
dihydrotetrabenazine isomer.
Thus, in a single dose study involving the administration of tetrabenazine to
adult
male humans, the Cmax figures for (+)-6-dihydrotetrabenazine and (-)-a-
dihydrotetrabenazine respectively were 103 and 72.94 ng/ml whereas the Cmax
figures for (+6-dihydrotetrabenazine and (+)-a-dihydrotetrabenazine
respectively
CA 03057548 2019-09-23
WO 2018/178243
PCT/EP2018/058088
9
were 5.28 and 2.61 ng/ml. The area under the curve (AUC) figures for each of
the
(+)13-dihydrotetrabenazine, (-)-a-dihydrotetrabenazine, (-)13-
dihydrotetrabenazine
and (+)-a-dihydrotetrabenazine metabolites respectively were 375.78, 305.84,
16.28 and 7.98. A similar distribution of metabolites was found when multiple
doses of tetrabenazine were administered.
The data suggest that (+)-a-dihydrotetrabenazine is not primarily responsible
for
the therapeutic properties of tetrabenazine. On the contrary, it appears that
(+)-a-
dihydrotetrabenazine may be responsible for a relatively small contribution to
the
therapeutic properties of tetrabenazine.
The importance of preparing enantiopure compositions for treatment of the
human
and animal body is known. It is well known that enantiomers may have different
biological properties, for example wherein one enantiomer is useful for the
treatment of a specific disease or condition and wherein the other enantiomer
is
toxic or produces unwanted side effects. An example of this is the drug
thalidomide, which was marketed as a sedative and also prescribed to pregnant
women to treat morning sickness, but it was later found that one enantiomer
caused birth defects in children of the women who had been administered
thalidomide during their pregnancy.
Guidance from the FDA regarding enantiomers states that applications for drug
substances and drug products should include a stereochemically specific
identity
test and/or a stereochemically selective assay method.
Even in cases where one of the enantiomers is medically useful and the other
is
inactive and shows no side effects, it may still be advantageous to remove the
inactive enantiomer in order to reduce the size of the dosage form
administered to
patients.
Combinations of (+)-a-dihydrotetrabenazine and (-)-a-dihydrotetrabenazine
It has now surprisingly been found that combinations of (+)- and (-)-a-
dihydrotetrabenazine are more effective in the reduction of locomotor activity
in
amphetamine induced rats than (+)-a-dihydrotetrabenazine alone, despite the
fact
that it has been previously reported that the (-)-a-dihydrotetrabenazine is a
poor
VMAT2 inhibitor.
CA 03057548 2019-09-23
WO 2018/178243
PCT/EP2018/058088
It has also been shown that both racemic and scalemic (i.e. non-racemic)
combinations exhibit enhanced efficacy
Studies confirmed the inactivity of (-)-a-dihydrotetrabenazine in treating
movement
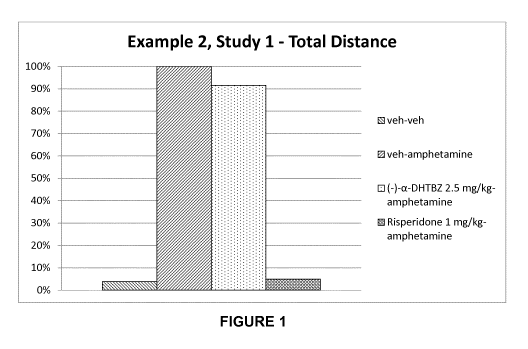
disorders when administered alone (see Example 2, Study 1). However, it was
5 unexpectedly found that when (-)-a-dihydrotetrabenazine was administered
in
combination with (+)-a-dihydrotetrabenazine, an improved effect was seen when
compared to the administration of the (+)-isomer alone (see Example 2, Studies
3
and 4). It is thought that this improved effect may be due to the binding of (-
)-a-
dihydrotetrabenazine to proteins other than VMAT2, which may play a role in
10 mediating hyperkinetic movement disorders.
On the basis of the studies carried out to date, it is envisaged that
combinations of
(+)- and (-)-a-dihydrotetrabenazine will be useful in the prophylaxis or
treatment of
the disease states and conditions for which tetrabenazine is currently used or
proposed. Thus, by way of example, and without limitation, the
dihydrotetrabenazine compounds of the invention may be used for the treatment
of
movement disorders and in particular hyperkinetic movement disorders such as
Huntington's disease, hemiballismus, senile chorea, tic disorders, tardive
dyskinesia, dystonia and Tourette's syndrome.
Accordingly, in a first aspect, the invention provides a pharmaceutical unit
dosage
form comprising (+)-a-dihydrotetrabenazine and (-)-a-dihydrotetrabenazine, or
pharmaceutically acceptable salts thereof, and a pharmaceutically acceptable
excipient.
Typically, the combination of (+)-a-dihydrotetrabenazine and (-)-a-
dihydrotetrabenazine, or pharmaceutically acceptable salts thereof, is not
administered with a therapeutic effective amount of amantadine. In one
embodiment, the combination of (+)-a-dihydrotetrabenazine and (-)-a-
dihydrotetrabenazine, or pharmaceutically acceptable salts thereof, is not
administered with any amount of amantadine.
The (+)-a-dihydrotetrabenazine and/or (-)-a-dihydrotetrabenazine, or
pharmaceutically acceptable salts thereof, may be administered as an immediate
release unit dosage form.
CA 03057548 2019-09-23
WO 2018/178243
PCT/EP2018/058088
11
In another aspect, the invention provides a combination of (+)-a-
dihydrotetrabenazine and (-)-a-dihydrotetrabenazine, or pharmaceutically
acceptable salts thereof, for use in medicine.
(+)-a-Dihydrotetrabenazine is believed to have the chemical structure (I)
shown
below:
0
/
R) N
0
H
i IR
OH (I)
(-)-a-Dihydrotetrabenazine is believed to have the chemical structure (II)
shown
below:
0
/
s) N
0
µ'=
(s)
s)
H
OH (I1)
In another aspect, the invention provides a combination of (+)-a-
dihydrotetrabenazine and (-)-a-dihydrotetrabenazine, or pharmaceutically
acceptable salts thereof, for use in medicine.
In a further aspect, the invention provides a combination of (+)-a-
dihydrotetrabenazine and (-)-a-dihydrotetrabenazine, or pharmaceutically
acceptable salts thereof (pharmaceutical unit dosage form as hereinbefore
defined), for use in the treatment of a movement disorder.
In further embodiments, the invention provides:
= A method of treatment of a movement disorder in a subject in need thereof
(e.g. a mammalian subject such as a human), which treatment comprises
administering to the subject a combination of (+)-a-dihydrotetrabenazine
CA 03057548 2019-09-23
WO 2018/178243
PCT/EP2018/058088
12
and (-)-a-dihydrotetrabenazine, or pharmaceutically acceptable salts
thereof.
= The use of a combination of (+)-a-dihydrotetrabenazine and (-)-a-
dihydrotetrabenazine, or pharmaceutically acceptable salts thereof, for the
manufacture of a medicament for the treatment of a movement disorder.
The inventors have also found that low doses (i.e. of 20 mg or less per day)
of (+)-
a-dihydrotetrabenazine may be useful in the treatment of movement disorders
and
it is therefore envisaged that such low doses of (+)-a-dihydrotetrabenazine,
in
combination with (-)-a-dihydrotetrabenazine, will also be useful in treating
movement disorders.
Accordingly, in another embodiment, the invention provides a combination of
(+)-a-
dihydrotetrabenazine and (-)-a-dihydrotetrabenazine, or pharmaceutically
acceptable salts thereof, for use in a method of treatment of a movement
disorder,
wherein the method comprises administering to a subject (.e.g. a human
subject)
in need thereof an effective therapeutic amount of the combination sufficient
to
provide a dosage of from 1 mg to 20 mg of (+)-a-dihydrotetrabenazine per day.
In further aspects, the invention provides:
= A method of treatment of movement disorders, wherein the method
comprises administering to a subject (.e.g. a human subject) in need
thereof an effective therapeutic amount of a combination of (+)-a-
dihydrotetrabenazine and (-)-a-dihydrotetrabenazine, or pharmaceutically
acceptable salts thereof, sufficient to provide a dosage of from 1 mg to 20
mg of (+)-a-dihydrotetrabenazine per day.
= The use of a combination of (+)-a-dihydrotetrabenazine and (-)-a-
dihydrotetrabenazine, or pharmaceutically acceptable salts thereof, for the
manufacture of a medicament for the treatment of a movement disorder,
wherein the method comprises administering to a subject (.e.g. a human
subject) in need thereof an effective therapeutic amount of the combination
sufficient to provide a dosage of from 1 mg to 20 mg of (+)-a-
dihydrotetrabenazine per day.
CA 03057548 2019-09-23
WO 2018/178243
PCT/EP2018/058088
13
= The use of a (-)-a-dihydrotetrabenazine, or a pharmaceutically acceptable
salt thereof, for the manufacture of a medicament for administration in
combination with (+)-a-dihydrotetrabenazine or a pharmaceutically
acceptable salt thereof for a method of treatment of a movement disorder,
wherein the method comprises administering to a subject (.e.g. a human
subject) in need thereof an effective therapeutic amount of the combination
sufficient to provide a dosage of from 1 mg to 20 mg of (+)-a-
dihydrotetrabenazine per day.
In each of the foregoing embodiments (i.e. a combination for use, a method, or
a
use) employing a low dose of (+)-a-dihydrotetrabenazine or a pharmaceutically
acceptable salt thereof, the daily dose of (+)-a-dihydrotetrabenazine is from
1 mg
to 20 mg.
In particular embodiments, there is provided
= A combination for use, a method or a use as described herein, wherein the
treatment comprises administering to the subject an amount of (+)-a-
dihydrotetrabenazine from 1.5 mg to 20 mg (e.g. between 1.5 mg and 20
mg) per day.
= A combination for use, a method or a use as described herein, wherein the
treatment comprises administering to the subject an amount of (+)-a-
dihydrotetrabenazine from 2 mg to 20 mg (e.g. between 2 mg and 20 mg)
per day.
= A combination for use, a method or a use as described herein, wherein the
treatment comprises administering to the subject an amount of (+)-a-
dihydrotetrabenazine from 3 mg to 20 mg (e.g. between 3 mg and 20 mg)
per day.
= A combination for use, a method or a use as described herein, wherein the
treatment comprises administering to the subject an amount of (+)-a-
dihydrotetrabenazine from 2 mg to 15 mg (e.g. between 2 mg and 15 mg)
per day.
= A combination for use, a method or a use as described herein, wherein the
treatment comprises administering to the subject an amount of (+)-a-
CA 03057548 2019-09-23
WO 2018/178243
PCT/EP2018/058088
14
dihydrotetrabenazine from 3 mg to 15 mg (e.g. between 3 mg and 15 mg)
per day.
= A combination for use, a method or a use as described herein, wherein the
treatment comprises administering to the subject an amount of (+)-a-
dihydrotetrabenazine from 5 mg to 15 mg (e.g. between 5 mg and 15 mg)
per day.
The administration of (+)-a-dihydrotetrabenazine and (-)-a-
dihydrotetrabenazine
typically forms part of a chronic treatment regime. The (+)-a-
dihydrotetrabenazine
and (-)-a-dihydrotetrabenazine may therefore be administered to a patient for
a
.. treatment period of at least a week, more usually at least two weeks, or at
least a
month, and typically longer than a month. Where a patient is shown to respond
well to treatment, the period of treatment can be longer than six months and
may
extend over a period of years.
The chronic treatment regime may involve the administration of the (+)-a-
dihydrotetrabenazine and (-)-a-dihydrotetrabenazine every day, or the
treatment
regime may include days when no (+)-a-dihydrotetrabenazine or (-)-a-
dihydrotetrabenazine is administered.
The dosage administered to the subject may vary during the treatment period.
For
example, the initial dosage may be increased or decreased depending on the
subject's response to the treatment. A subject may, for example, be given an
initial
low dose to test the subject's tolerance towards the (+)-a-
dihydrotetrabenazine and
(-)-a-dihydrotetrabenazine, and the dosage thereafter increased as necessary
up
to the maximum daily intake of 20 mg. Alternatively, an initial daily dosage
administered to the patient may be selected so as to give an estimated desired
degree of VMAT2 blockage, following which a lower maintenance dose may be
given for the remainder of the treatment period, with the option of increasing
the
dosage should the subject's response to the treatment indicate that an
increase is
necessary.
It is envisaged that the quantity of (+)-a-dihydrotetrabenazine and (-)-a-
dihydrotetrabenazine required to achieve the desired therapeutic effect will
be
dependent on the weight of the subject to be treated. The quantities of (+)-a-
dihydrotetrabenazine and (-)-a-dihydrotetrabenazine administered to the
subject
can be expressed in a number of mg/kg, where in the kg relates the weight of
the
CA 03057548 2019-09-23
WO 2018/178243
PCT/EP2018/058088
subject to be treated. The appropriate dosage amount can therefore be
calculated
by multiplying the mg/kg amount by the weight of the subject to be treated.
Accordingly, in another aspect, the invention provides a combination of (+)-a-
dihydrotetrabenazine and (-)-a-dihydrotetrabenazine or pharmaceutically
5 acceptable salts thereof, for use in a method for the treatment of a
movement
disorder, wherein the treatment comprises administering to a subject an amount
of
the combination of from 0.01 mg/kg to 0.3 mg/kg (e.g. between 0.01 mg/kg and
0.3
mg/kg) per day provided that the total amount of (+)-a-dihydrotetrabenazine
administered per day is in the range from 1 mg to 20 mg.
10 In further aspects, the invention provides:
= A method of treatment of a movement disorder in a subject in need thereof
(e.g. a mammalian subject such as a human), which treatment comprises
administering to the subject a combination of (+)-a-dihydrotetrabenazine
and (-)-a-dihydrotetrabenazine, or pharmaceutically acceptable salts
15 thereof, in an amount from 0.01 mg/kg to 0.3 mg/kg (e.g. between 0.01
mg/kg and 0.3 mg/kg) per day, provided that the total amount of (+)-a-
dihydrotetrabenazine administered per day is in the range from 1 mg to 20
mg.
= The use of a combination of (+)-a-dihydrotetrabenazine and (-)-a-
dihydrotetrabenazine, or pharmaceutically acceptable salts thereof for the
manufacture of a medicament for the treatment of a movement disorder,
which treatment comprises administering to the subject the combination in
an amount from 0.01 mg/kg to 0.3 mg/kg (e.g. between 0.01 mg/kg and 0.3
mg/kg), provided that the total amount of (+)-a-dihydrotetrabenazine
administered per day is in the range from 1 mg to 20 mg.
In each of the foregoing embodiments (i.e. a combination for use, a method, or
a
use) wherein a combination of (+)-a-dihydrotetrabenazine and (-)-a-
dihydrotetrabenazine, or pharmaceutically acceptable salts thereof, is
administered
in an amount from 0.01 mg/kg to 0.3 mg/kg (e.g. between 0.01 mg/kg and 0.3
mg/kg) per day, the daily dose of (+)-a-dihydrotetrabenazine is from 1 mg to
20
mg.
In particular embodiments, there is provided:
CA 03057548 2019-09-23
WO 2018/178243
PCT/EP2018/058088
16
= A combination for use, a method or a use as described herein, wherein the
treatment comprises administering to the subject an amount of the
combination of from 0.02 mg/kg to 0.3 mg/kg (e.g. between 0.02 mg / kg
and 0.3 mg/kg) per day, provided that the total amount of (+)-a-
dihydrotetrabenazine administered per day is in the range from 1 mg to 20
mg.
= A combination for use, a method or a use as described herein, wherein the
treatment comprises administering to the subject an amount of the
combination of from 0.03 mg/kg to 0.3 mg/kg (e.g. between 0.03 mg/kg and
0.3 mg/kg) per day, provided that the total amount of (+)-a-
dihydrotetrabenazine administered per day is in the range from 1 mg to 20
mg.
= A combination for use, a method or a use as described herein, wherein the
treatment comprises administering to the subject an amount of the
combination from 0.04 mg/kg to 0.3 mg/kg (e.g. between 0.04 mg/kg and
0.3 mg/kg) per day, provided that the total amount of (+)-a-
dihydrotetrabenazine administered per day is in the range from 1 mg to 20
mg.
= A combination for use, a method or a use as described herein, wherein the
treatment comprises administering to the subject an amount of the
combination from 0.05 mg/kg to 0.3 mg/kg (e.g. between 0.05 mg/kg and
0.3 mg/kg) of (+)-a-dihydrotetrabenazine, provided that the total amount of
(+)-a-dihydrotetrabenazine administered per day is in the range from 1 mg
to 20 mg.
The combinations of the invention (and dosage forms containing the
combinations)
are useful in the treatment of movement disorders, and in particular
hyperkinetic
movement disorders such as Huntington's disease, hemiballismus, senile chorea,
tic disorders, tardive dyskinesia, dystonia and Tourette's syndrome.
More particularly, the combinations of the invention (and dosage forms
containing
.. the combinations) are for use in the treatment of a hyperkinetic movement
disorder
selected from tic disorders, Huntington's disease, tardive dyskinesia and
Tourette's
syndrome.
CA 03057548 2019-09-23
WO 2018/178243
PCT/EP2018/058088
17
In one particular embodiment, the combinations of the invention (and dosage
forms
containing the combinations) are for use in the treatment of tardive
dyskinesia.
In another particular embodiment, the combinations of the invention (and
dosage
forms containing the combinations) are for use in the treatment of Tourette's
syndrome.
The usefulness of the combinations of the invention in the treatment of
movement
disorders arises in part from the ability of (+)-a-dihydrotetrabenazine to
bind to the
vesicular monoamine transporter 2 (VMAT2).
Complete blocking of the VMAT2 proteins is considered undesirable as this can
lead to unwanted side effects, such as Parkinsonism. The present invention
provides plasma levels of (+)-a-dihydrotetrabenazine and (-)-a-
dihydrotetrabenazine that are sufficient to give effective treatment of
movement
disorders but do not block the VMAT2 proteins to an extent that causes
Parkinsonism and similar side effects. The levels of VMAT2 blocking can be
determined by competitive binding studies using Positron Emission Tomography
(PET). By co-administering a radioactive ligand with the compound of interest
at
various concentrations, the proportion of binding sites occupied can be
determined
(see for example, Matthews et al., "Positron emission tomography molecular
imaging for drug development", Br. J. Clin. Pharmacol., 73:2, 175-186).
Accordingly, in a further aspect, the invention provides a combination of (+)-
a-
dihydrotetrabenazine and (-)-a-dihydrotetrabenazine, or pharmaceutically
acceptable salts thereof, for use in a method for the treatment of a movement
disorder, wherein the treatment comprises administering to a subject an amount
of
the combination sufficient to cause a level of blocking of from 20% to 90% of
.. VMAT2 proteins in the subject.
In further aspects, the invention provides:
= A method of treatment of a movement disorder in a subject in need thereof
(e.g. a mammalian subject such as a human), which treatment comprises
administering to the subject a combination of (+)-a-dihydrotetrabenazine
and (-)-a-dihydrotetrabenazine, or pharmaceutically acceptable salts
thereof, sufficient to cause a level of blocking of from 20% to 90% of the
VMAT2 proteins in the subject.
CA 03057548 2019-09-23
WO 2018/178243
PCT/EP2018/058088
18
= The use of a combination of (+)-a-dihydrotetrabenazine and (-)-a-
dihydrotetrabenazine, or pharmaceutically acceptable salts thereof, for the
manufacture of a medicament for the treatment of a movement disorder in
a subject (e.g. a mammalian subject such as a human), which treatment
comprises administering to the subject an amount of the combination
sufficient to cause a level of blocking of from 20% to 90% of VMAT2
proteins in the subject.
= The use of (+)-a-dihydrotetrabenazine, or a pharmaceutically acceptable
salt thereof, for the manufacture of a medicament for use in combination
with (-)-a-dihydrotetrabenazine or a pharmaceutically acceptable salt
thereof for the treatment of a movement disorder in a subject (e.g. a
mammalian subject such as a human), which treatment comprises
administering to the subject an amount of the combination sufficient to
cause a level of blocking of from 20% to 90% of VMAT2 proteins in the
subject.
In further embodiments, there are provided:
= A combination for use, a method or a use as described herein, wherein the
treatment comprises administering to the subject an amount of the
combination sufficient to cause a level of blocking of from 25% to 85% of
the VMAT2 proteins in the subject.
= A combination for use, a method or a use as described herein, wherein the
treatment comprises administering to the subject an amount of the
combination sufficient to cause a level of blocking of from 30% to 85% of
the VMAT2 proteins in the subject.
= A combination for use, a method or a use as described herein, wherein the
treatment comprises administering to the subject an amount of the
combination sufficient to cause a level of blocking of from 35% to 85% of
the VMAT2 proteins in the subject.
= A combination for use, a method or a use as described herein, wherein the
treatment comprises administering to the subject an amount of the
combination sufficient to cause a level of blocking of from 40% to 85% of
the VMAT2 proteins in the subject.
CA 03057548 2019-09-23
WO 2018/178243
PCT/EP2018/058088
19
= A combination for use, a method or a use as described herein, wherein the
treatment comprises administering to the subject an amount of the
combination sufficient to cause a level of blocking of from 45% to 85% of
the VMAT2 proteins in the subject.
= A combination for use, a method or a use as described herein, wherein the
treatment comprises administering to the subject an amount of the
combination sufficient to cause a level of blocking of from 50% to 85% of
the VMAT2 proteins in the subject.
= A combination for use, a method or a use as described herein, wherein the
treatment comprises administering to the subject an amount of the
combination sufficient to cause a level of blocking of from 30% to 80% of
the VMAT2 proteins in the subject.
= A combination for use, a method or a use as described herein, wherein the
treatment comprises administering to the subject an amount of the
combination sufficient to cause a level of blocking of from 35% to 75% of
the VMAT2 proteins in the subject.
= A combination for use, a method or a use as described herein, wherein the
treatment comprises administering to the subject an amount of the
combination sufficient to cause a level of blocking of from 35% to 70% of
the VMAT2 proteins in the subject.
= A combination for use, a method or a use as described herein, wherein the
treatment comprises administering to the subject an amount of the
combination sufficient to cause a level of blocking of from 40% to 75% of
the VMAT2 proteins in the subject.
= A combination for use, a method or a use as described herein, wherein the
treatment comprises administering to the subject in need thereof, wherein
the method comprising administering to a subject an amount of the
combination sufficient to cause a level of blocking of from 45% to 75% of
the VMAT2 proteins in the subject.
= A combination for use, a method or a use as described herein, wherein the
treatment comprises administering to the subject in need thereof, wherein
CA 03057548 2019-09-23
WO 2018/178243
PCT/EP2018/058088
the method comprising administering to a subject an amount of the
combination sufficient to cause a level of blocking of from 35% to 80% of
the VMAT2 proteins in the subject.
= A combination for use, a method or a use as described herein, wherein the
5 treatment comprises administering to the subject in need thereof,
wherein
the method comprising administering to a subject an amount of the
combination sufficient to cause a level of blocking of from 40% to 80% of
the VMAT2 proteins in the subject.
= A combination for use, a method or a use as described herein, wherein the
10 treatment comprises administering to the subject an amount of the
combination sufficient to cause a level of blocking of from 45% to 80% of
the VMAT2 proteins in the subject.
= A combination for use, a method or a use as described herein, wherein the
treatment comprises administering to the subject an amount of the
15 combination sufficient to cause a level of blocking of from 50% to 80%
of
the VMAT2 proteins in the subject.
= A combination for use, a method or a use as described herein, wherein the
treatment comprises administering to the subject an amount of the
combination sufficient to cause a level of blocking of from 55% to 80% of
20 the VMAT2 proteins in the subject.
= A combination for use, a method or a use as described herein, wherein the
treatment comprises administering to the subject an amount of the
combination sufficient to cause a level of blocking of VMAT2 proteins in the
subject of from 30% to 70% (e.g. between 30% and 70%).
= A combination for use, a method or a use as described herein, wherein the
treatment comprises administering to the subject in need thereof, wherein
the method comprising administering to a subject an amount of the
combination sufficient to cause a blocking level of VMAT2 proteins in the
subject of from 30% to 65% (e.g. between 30% and 65%).
= A combination for use, a method or a use as described herein, wherein the
treatment comprises administering to the subject in need thereof, wherein
CA 03057548 2019-09-23
WO 2018/178243
PCT/EP2018/058088
21
the method comprising administering to a subject an amount of the
combination sufficient to cause a blocking level of VMAT2 proteins in the
subject of from 30% to 60% (e.g. between 30% and 60%).
= A combination for use, a method or a use as described herein, wherein the
treatment comprises administering to the subject in need thereof, wherein
the method comprising administering to a subject an amount of the
combination sufficient to cause a level blocking of VMAT2 proteins in the
subject of from 40% to 80% (e.g. between 40% and 80%).
= A combination for use, a method or a use as described herein, wherein the
treatment comprises administering to the subject in need thereof, wherein
the method comprising administering to a subject an amount of the
combination sufficient to cause a level of blocking of VMAT2 proteins in the
subject of from 40% to 75% (e.g. between 40% and 75%).
= A combination for use, a method or a use as described herein, wherein the
treatment comprises administering to the subject in need thereof, wherein
the method comprising administering to a subject an amount of the
combination sufficient to cause a level of blocking of VMAT2 proteins in the
subject of from 40% to 70% (e.g. between 40% and 70%).
= A combination for use, a method or a use as described herein, wherein the
treatment comprises administering to the subject in need thereof, wherein
the method comprising administering to a subject an amount of the
combination sufficient to cause a level of blocking of VMAT2 proteins in the
subject of from 40% to 65% (e.g. between 40% and 65%).
= A combination for use, a method or a use as described herein, wherein the
treatment comprises administering to the subject in need thereof, wherein
the method comprising administering to a subject an amount of the
combination sufficient to cause a level blocking of VMAT2 proteins in the
subject of from 40% to 60% (e.g. between 40% and 60%).
In each of the foregoing aspects and embodiments of the invention, the
combinations of (+)-a-dihydrotetrabenazine and (-)-a-dihydrotetrabenazine may
be
racemic.
CA 03057548 2019-09-23
WO 2018/178243
PCT/EP2018/058088
22
Alternatively, in each of the foregoing aspects and embodiments of the
invention,
the combinations of (+)-a-dihydrotetrabenazine and (-)-a-dihydrotetrabenazine
may
be scalemic (i.e. non-racemic).
In each of the combinations of the invention, the uses thereof and
pharmaceutical
dosage forms containing the combinations, as defined above, the ratio of (+)-a-
dihydrotetrabenazine to (-)-a-dihydrotetrabenazine in the combination can be,
for
example, from 0.5:1 to 20:1. In particular embodiments, the ratio of (+)-a-
dihydrotetrabenazine to (-)-a-dihydrotetrabenazine in the combination can be a
ratio in a range selected from:
(i) 1:1 to 20:1
(ii) 1:1 to 15:1
(iii) 1:1 to 12:1
(iv) 1:1 to 10:1
(v) 1:1 to 5:1
(vi) 1:1 to 4:1
(vii) 1:1 to 3:1
(viii) 1:1 to 2:1
(ix) 1.1:1 to 20:1
(x) 1.1:1 to 15:1
(xi) 1.1:1 to 12:1
(xii) 1.1:1 to 10:1
(xiii) 1.1:1 to 5:1
(xiv) 1.1:1 to 4:1
(xv) 1.1:1 to 3:1
(xvi) 1.1:1 to 2:1
(xvii) 1.2:1 to 20:1
(xviii) 1.2:1 to 15:1
(xix) 1.2:1 to 12:1
(xx) 1.2:1 to 10:1
(xxi) 1.2:1 to 5:1
(xxii) 1.2:1 to 4:1
(xxiii) 1.2:1 to 3:1
(xxiv) 1.2:1 to 2:1
CA 03057548 2019-09-23
WO 2018/178243
PCT/EP2018/058088
23
In each of the foregoing aspects and embodiments of the invention, the
combinations of (+)-a-dihydrotetrabenazine and (-)-a-dihydrotetrabenazine are
typically unaccompanied by other dihydrotetrabenazine isomers.
In some embodiments, minor amounts of other tetrabenazine isomers may be
present but these generally are present in amounts corresponding to no more
than
20% by weight (i.e. 0.2:1), compared to the total weight of the combination.
More
usually, other dihydrotetrabenazine isomers are present in amounts
corresponding
to no more than 10% or 5%, or 2%, or 1% by weight (i.e. 0.2:1), compared to
the
total weight of the combination. More preferably, other dihydrotetrabenazine
isomers are either completely absent, or are present in amounts of less than
1% by
weight, for example less than 0.5% by weight.
In each of the foregoing aspects and embodiments, the (+)-a-
dihydrotetrabenazine
and/or (-)-a-dihydrotetrabenazine can be administered as the free base or as a
pharmaceutically acceptable salt. In one embodiment, one or both of the (+)-a-
dihydrotetrabenazine and (-)-a-dihydrotetrabenazine are administered as
pharmaceutically acceptable salts. In another embodiment, both the (+)-a-
dihydrotetrabenazine and (-)-a-dihydrotetrabenazine are administered as free
bases. Unless stated otherwise, or unless the context indicates otherwise, the
quantities of (+)-a-dihydrotetrabenazine and (-)-a-dihydrotetrabenazine are
calculated as the amounts of the free base, or when the (+)-a-
dihydrotetrabenazine
or (-)-a-dihydrotetrabenazine is in the form of a pharmaceutically acceptable
salt,
the amount of (+)-a-dihydrotetrabenazine or (-)-a-dihydrotetrabenazine per se
present in the pharmaceutically acceptable salt.
In each of the foregoing aspects and embodiments of the invention relating to
combinations of (+)-a-dihydrotetrabenazine or (-)-a-dihydrotetrabenazine or
pharmaceutically acceptable salts thereof, typically, the (+)-a-
dihydrotetrabenazine
or (-)-a-dihydrotetrabenazine or pharmaceutically acceptable salt thereof, are
not
administered with a therapeutically effective amount of amantadine. More
particularly, the (+)-a-dihydrotetrabenazine or (-)-a-dihydrotetrabenazine, or
pharmaceutically acceptable salt thereof, are not administered with any amount
of
amantadine.
For example, with reference to pharmaceutical unit dosage forms, typically the
unit
dosage form does not comprise a therapeutically effective amount of amantadine
CA 03057548 2019-09-23
WO 2018/178243
PCT/EP2018/058088
24
and, more particularly, the pharmaceutical unit dosage form does not comprise
any
amount of amantadine.
Furthermore, in each of the foregoing aspects and embodiments of the invention
relating to (+)-a-dihydrotetrabenazine or (-)-a-dihydrotetrabenazine or
.. pharmaceutically acceptable salts thereof, the pharmaceutical unit dosage
form
may be other than an extended release or delayed release dosage form.
Thus, for example, the (+)-a-dihydrotetrabenazine or (-)-a-
dihydrotetrabenazine, or
pharmaceutically acceptable salt thereof, may be administered as an immediate
release unit dosage form.
.. (+)-13-dihydrotetrabenazine
As discussed above, the present inventors have found that (+)-a-
dihydrotetrabenazine does not appear to be primarily responsible for the
therapeutic properties of tetrabenazine. On the contrary, it appears that (+)-
a-
dihydrotetrabenazine may be responsible for a relatively small contribution to
the
.. therapeutic properties of tetrabenazine.
Therefore, despite the earlier findings that the (+)-a-isomer was 3- to 4-
times more
active than the (+)13-isomer, the fact that the (+)13-isomer is present in the
body
following administration of tetrabenazine in an amount over 50 times greater
suggests that (+)I3-dihydrotetrabenazine may make a major contribution to the
activity of tetrabenazine.
Investigations made by the present inventors indicate that (+)13-
dihydrotetrabenazine per se having the chemical name, (S,R,R)-3-isobuty1-9,10-
dimethyloxy-1,3,4,5,7,11b-hexahydro-2H-pyrido[2,1-a]isoquinolin-2-ol, and
having
the formula (111) shown below
0
/
R) N
0
H (R)
S)
OH
(111)
CA 03057548 2019-09-23
WO 2018/178243
PCT/EP2018/058088
is effective in the treatment of movement disorders, despite previous findings
that it
has a lower VMAT2 activity than (+)-a-dihydrotetrabenazine.
Accordingly, in a first aspect, the invention provides a unit dosage form
comprising
(+)-8-dihydrotetrabenazine, or a pharmaceutically acceptable salt thereof, and
a
5 pharmaceutically acceptable excipient.
In another aspect, the invention provides (+)-8-dihydrotetrabenazine, or a
pharmaceutically acceptable salt thereof, for use in medicine.
There is also provided a unit dosage form comprising (+)-8-
dihydrotetrabenazine,
or a pharmaceutically acceptable salt thereof, and a pharmaceutically
acceptable
10 excipient, which is substantially free of other dihydrotetrabenazine
isomers.
The unit dosage form can be one which is administered orally, for example a
capsule or tablet. Alternatively, the (+)-8-dihydrotetrabenazine or a
pharmaceutically acceptable salt thereof can be administered in a non-solid
dosage form such as a solution, syrup, suspension or gel.
15 In particular embodiments of the invention, there is provided:
= A unit dosage form comprising from 1 mg to 200 mg (e.g. between 1 mg
and 200 mg) of (+)-8-dihydrotetrabenazine, or a pharmaceutically
acceptable salt thereof, and a pharmaceutically acceptable excipient.
= A unit dosage form comprising from 1 mg to 150 mg (e.g. between 1 mg
20 and 150 mg) of (+)-8-dihydrotetrabenazine, or a pharmaceutically
acceptable salt thereof, and a pharmaceutically acceptable excipient.
= A unit dosage form comprising from 1 mg to 100 mg (e.g. between 1 mg
and 100 mg) of (+)-8-dihydrotetrabenazine, or a pharmaceutically
acceptable salt thereof, and a pharmaceutically acceptable excipient.
25 = A unit dosage form comprising from 1 mg to 80 mg (e.g. between 1 mg
and
80 mg) of (+)-8-dihydrotetrabenazine, or a pharmaceutically acceptable salt
thereof, and a pharmaceutically acceptable excipient.
CA 03057548 2019-09-23
WO 2018/178243
PCT/EP2018/058088
26
= A unit dosage form comprising from 3 mg to 200 mg (e.g. between 3 mg
and 200 mg) of (+)I3-dihydrotetrabenazine, or a pharmaceutically
acceptable salt thereof, and a pharmaceutically acceptable excipient.
= A unit dosage form comprising from 3 mg to 150 mg (e.g. between 3 mg
and 150 mg) of (+)I3-dihydrotetrabenazine, or a pharmaceutically
acceptable salt thereof, and a pharmaceutically acceptable excipient.
= A unit dosage form comprising from 3 mg to 100 mg (e.g. between 3 mg
and 100 mg) of (+)I3-dihydrotetrabenazine, or a pharmaceutically
acceptable salt thereof, and a pharmaceutically acceptable excipient.
= A unit dosage form comprising from 3 mg to 80 mg (e.g. between 3 mg and
80 mg) of (+)I3-dihydrotetrabenazine, or a pharmaceutically acceptable salt
thereof, and a pharmaceutically acceptable excipient.
= A unit dosage form comprising from 5 mg to 200 mg (e.g. between 5 mg
and 200 mg) of (+)I3-dihydrotetrabenazine, or a pharmaceutically
acceptable salt thereof, and a pharmaceutically acceptable excipient.
= A unit dosage form comprising from 5 mg to 150 mg (e.g. between 5 mg
and 150 mg) of (+)I3-dihydrotetrabenazine, or a pharmaceutically
acceptable salt thereof, and a pharmaceutically acceptable excipient.
= A unit dosage form comprising from 5 mg to 100 mg (e.g. between 5 mg
and 100 mg) of (+)I3-dihydrotetrabenazine, or a pharmaceutically
acceptable salt thereof, and a pharmaceutically acceptable excipient.
= A unit dosage form comprising from 5 mg to 80 mg (e.g. between 5 mg and
80 mg) of (+)I3-dihydrotetrabenazine, or a pharmaceutically acceptable salt
thereof, and a pharmaceutically acceptable excipient.
= A unit dosage form comprising from 3 mg to 60 mg (e.g. between 3 mg and
60 mg) of (+)I3-dihydrotetrabenazine, or a pharmaceutically acceptable salt
thereof, and a pharmaceutically acceptable excipient.
= A unit dosage form comprising from 5 mg to 60 mg (e.g. between 5 mg and
60 mg) of (+)I3-dihydrotetrabenazine, or a pharmaceutically acceptable salt
thereof, and a pharmaceutically acceptable excipient.
CA 03057548 2019-09-23
WO 2018/178243
PCT/EP2018/058088
27
= A unit dosage form comprising from 10 mg to 60 mg (e.g. between 10 mg
and 60 mg) of (+)-6-dihydrotetrabenazine, or a pharmaceutically acceptable
salt thereof, and a pharmaceutically acceptable excipient.
= A unit dosage form comprising from 15 mg to 60 mg (e.g. between 15 mg
and 60 mg) of (+)-6-dihydrotetrabenazine, or a pharmaceutically acceptable
salt thereof, and a pharmaceutically acceptable excipient.
= A unit dosage form comprising approximately 20 mg of (+)-6-
dihydrotetrabenazine, or a pharmaceutically acceptable salt thereof, and a
pharmaceutically acceptable excipient.
= A unit dosage form comprising approximately 30 mg of (+)-6-
dihydrotetrabenazine, or a pharmaceutically acceptable salt thereof, and a
pharmaceutically acceptable excipient.
= A unit dosage form comprising approximately 40 mg of (+)-6-
dihydrotetrabenazine, or a pharmaceutically acceptable salt thereof, and a
pharmaceutically acceptable excipient.
= A unit dosage form comprising approximately 50mg of (+)-6-
dihydrotetrabenazine, or a pharmaceutically acceptable salt thereof, and a
pharmaceutically acceptable excipient.
= A unit dosage form comprising approximately 60 mg of (+)-6-
dihydrotetrabenazine, or a pharmaceutically acceptable salt thereof, and a
pharmaceutically acceptable excipient.
The unit dosage forms defined and described above are typically for use in the
treatment of a hyperkinetic movement disorder such as Huntington's disease,
hemiballismus, senile chorea, tic disorders, tardive dyskinesia, dystonia and
Tourette's syndrome.
More particularly, the unit dosage forms described above are for use in the
treatment of a hyperkinetic movement disorder selected from tic disorders,
Huntington's disease, tardive dyskinesia and Tourette's syndrome.
In one particular embodiment, the unit dosage forms described above are for
use
in the treatment of tardive dyskinesia.
CA 03057548 2019-09-23
WO 2018/178243
PCT/EP2018/058088
28
In another particular embodiment, the unit dosage forms described above are
for
use in the treatment of Tourette's syndrome.
The present inventors have found that (+)13-dihydrotetrabenazine is useful in
the
blocking of the VMAT2 receptor in the treatment of movement disorders.
.. Accordingly, the invention provides a pharmaceutical composition comprising
(+)-
13-dihydrotetrabenazine or a pharmaceutically acceptable salt thereof and a
pharmaceutically acceptable excipient.
The invention also provides (+)13-dihydrotetrabenazine or a pharmaceutically
acceptable salt thereof for use as a VMAT2 inhibitor.
In further embodiments of the Invention, there are provided:
= (+)I3-Dihydrotetrabenazine or a pharmaceutically acceptable salt thereof
for use in the treatment of a hyperkinetic movement disorder.
= (+)I3-Dihydrotetrabenazine or a pharmaceutically acceptable salt thereof
for use in the treatment of Huntington's disease, hemiballismus, senile
chorea, tic disorders, tardive dyskinesia, dystonia or Tourette's syndrome.
= A method of treatment of a hyperkinetic movement disorder in a subject in
need thereof (e.g. a mammalian subject such as a human), which method
comprises administering to the subject a therapeutically effective amount of
(+)I3-dihydrotetrabenazine or a pharmaceutically acceptable salt thereof.
= A method of treatment of Huntington's disease, hemiballismus, senile
chorea, tic disorders, tardive dyskinesia, dystonia or Tourette's syndrome in
a subject in need thereof (e.g. a mammalian subject such as a human),
which method comprises administering to the subject a therapeutically
effective amount of (+)13-dihydrotetrabenazine or a pharmaceutically
acceptable salt thereof.
= The use of (+)-P-dihydrotetrabenazine or a pharmaceutically acceptable
salt thereof for the manufacture of a medicament for the treatment of a
hyperkinetic movement disorder.
= The use of (+)-P-dihydrotetrabenazine or a pharmaceutically acceptable
salt thereof for the manufacture of a medicament for the treatment of a of
CA 03057548 2019-09-23
WO 2018/178243
PCT/EP2018/058088
29
Huntington's disease, hemiballismus, senile chorea, tic disorders, tardive
dyskinesia, dystonia or Tourette's syndrome.
= (+)-P-dihydrotetrabenazine for use in a method for the treatment of a
movement disorder, wherein the treatment comprises administering to a
subject an amount of (+)I3-dihydrotetrabenzine from 1 mg to 200 mg (e.g.
between 1 mg and 200 mg) per day.
= A method of treatment of a movement disorder in a subject in need thereof
(e.g. a mammalian subject such as a human), which treatment comprises
administering to the subject an amount of (+)I3-dihydrotetrabenazine from 1
mg to 200 mg (e.g. between 1 mg and 200 mg) per day.
= The use of (+)-P-dihydrotetrabenazine for the manufacture of a medicament
for the treatment of a movement disorder, which treatment comprises
administering to the subject an amount of (+)I3-dihydrotetrabenazine from 1
mg to 200 mg (e.g. between 1 mg and 200 mg) per day.
= Typically, the (+)I3-dihydrotetrabenazine, or pharmaceutically acceptable
salt thereof, is not administered with an effective amount of amantadine. In
one embodiment, the (+)13-dihydrotetrabenazine, or pharmaceutically
acceptable salt thereof, is not administered with amantadine.
The (+)I3-dihydrotetrabenazine, or pharmaceutically acceptable salt
thereof, may be administered as an immediate release unit dosage form.
In further embodiments, there are provided:
= (+)13-dihydrotetrabenazine for use, a method or a use as described
herein,
wherein the treatment comprises administering to the subject an amount of
(+)I3-dihydrotetrabenazine from 1 mg to 150 mg (e.g. between 1 mg and
150) mg per day.
= (+)13-dihydrotetrabenazine for use, a method or a use as described
herein,
wherein the treatment comprises administering to the subject an amount of
(+)I3-dihydrotetrabenazine from 1 mg to 100 mg (e.g. between 1 mg and
100) mg per day.
CA 03057548 2019-09-23
WO 2018/178243
PCT/EP2018/058088
= (+)13-dihydrotetrabenazine for use, a method or a use as described
herein,
wherein the treatment comprises administering to the subject an amount of
(+)13-dihydrotetrabenazine from 1 mg to 80 mg (e.g. between 1 mg and 80)
mg per day.
5 = (+)13-dihydrotetrabenazine for use, a method or a use as described
herein,
wherein the treatment comprises administering to the subject an amount of
(+)13-dihydrotetrabenazine from 3 mg to 200 mg (e.g. between 3 mg and
200) mg per day.
= (+)13-dihydrotetrabenazine for use, a method or a use as described
herein,
10 wherein the treatment comprises administering to the subject an amount
of
(+)13-dihydrotetrabenazine from 3 mg to 150 mg (e.g. between 3 mg and
150) mg per day.
= (+)13-dihydrotetrabenazine for use, a method or a use as described
herein,
wherein the treatment comprises administering to the subject an amount of
15 (+)I3-dihydrotetrabenazine from 3 mg to 100 mg (e.g. between 3 mg and
100) mg per day.
= (+)13-dihydrotetrabenazine for use, a method or a use as described
herein,
wherein the treatment comprises administering to the subject an amount of
(+)13-dihydrotetrabenazine from 5 mg to 200 mg (e.g. between 5 mg and
20 200) mg per day.
= (+)13-dihydrotetrabenazine for use, a method or a use as described
herein,
wherein the treatment comprises administering to the subject an amount of
(+)13-dihydrotetrabenazine from 5 mg to 150 mg (e.g. between 5 mg and
150) mg per day.
25 = (+)13-dihydrotetrabenazine for use, a method or a use as described
herein,
wherein the treatment comprises administering to the subject an amount of
(+)13-dihydrotetrabenazine from 5 mg to 100 mg (e.g. between 5 mg and
100) mg per day.
= (+)13-dihydrotetrabenazine for use, a method or a use as described
herein,
30 wherein the treatment comprises administering to the subject an amount
of
CA 03057548 2019-09-23
WO 2018/178243
PCT/EP2018/058088
31
(+)13-dihydrotetrabenazine from 1 mg to 70 mg (e.g. between 1 mg and 70)
mg per day.
= (+)13-dihydrotetrabenazine for use, a method or a use as described
herein,
wherein the treatment comprises administering to the subject an amount of
(+)13-dihydrotetrabenazine from 1 mg to 60 mg (e.g. between 1 mg and 60
mg) per day.
= (+)13-dihydrotetrabenazine for use, a method or a use as described
herein,
wherein the treatment comprises administering to the subject an amount of
(+)13-dihydrotetrabenazine from 1 mg to 50 mg (e.g. between 1 mg and 50
mg) per day.
= (+)13-dihydrotetrabenazine for use, a method or a use as described
herein,
wherein the treatment comprises administering to the subject an amount of
(+)13-dihydrotetrabenazine from 5 mg to 70 mg (e.g. between 5 mg and 70
mg) per day.
= (+)13-dihydrotetrabenazine for use, a method or a use as described herein,
wherein the treatment comprises administering to the subject an amount of
(+)13-dihydrotetrabenazine from 5 mg to 60 mg (e.g. between 5 mg and 60
mg) per day.
= (+)13-dihydrotetrabenazine for use, a method or a use as described
herein,
wherein the treatment comprises administering to the subject an amount of
(+)13-dihydrotetrabenazine of approximately 10 mg per day.
= (+)13-dihydrotetrabenazine for use, a method or a use as described
herein,
wherein the treatment comprises administering to the subject an amount of
(+)13-dihydrotetrabenazine of approximately 15 mg per day.
= (+)13-dihydrotetrabenazine for use, a method or a use as described herein,
wherein the treatment comprises administering to the subject an amount of
(+)13-dihydrotetrabenazine of approximately 20 mg per day.
= (+)13-dihydrotetrabenazine for use, a method or a use as described
herein,
wherein the treatment comprises administering to the subject an amount of
(+)I3-dihydrotetrabenazine of approximately 30 mg per day.
CA 03057548 2019-09-23
WO 2018/178243
PCT/EP2018/058088
32
= (+)-8-dihydrotetrabenazine for use, a method or a use as described
herein,
wherein the treatment comprises administering to the subject an amount of
(+)-8-dihydrotetrabenazine of approximately 40 mg per day.
In each case, the quantity of (+)-8-dihydrotetrabenazine specified may be
administered once per day or in several (e.g. two) doses per day.
In some embodiments, the quantity of (+)-8-dihydrotetrabenazine specified is
administered once daily.
The administration of (+)-8-dihydrotetrabenazine typically forms part of a
chronic
treatment regime. The (+)-8-dihydrotetrabenazine may therefore be administered
to a patient for a treatment period of at least a week, more usually at least
two
weeks, or at least a month, and typically longer than a month. Where a patient
is
shown to respond well to treatment, the period of treatment can be longer than
six
months and may extend over a period of years.
The chronic treatment regime may involve the administration of the (+)-8-
dihydrotetrabenazine every day, or the treatment regime may include days when
no (+)-8-dihydrotetrabenazine is administered.
The dosage administered to the subject may vary during the treatment period.
For
example, the initial dosage may be increased or decreased depending on the
subject's response to the treatment. A subject may, for example, be given an
initial
low dose to test the subject's tolerance towards the (+)-8-
dihydrotetrabenazine,
and the dosage thereafter increased as necessary up to the maximum daily
intake
of 80 mg (or other daily intakes as described above). Alternatively, an
initial daily
dosage administered to the patient may be selected so as to give an estimated
desired degree of VMAT2 blockage, following which a lower maintenance dose
may be given for the remainder of the treatment period, with the option of
increasing the dosage should the subject's response to the treatment indicate
that
an increase is necessary.
The quantity of (+)-8-dihydrotetrabenazine required to achieve the desired
therapeutic effect may be dependent on the weight of the subject to be
treated.
The quantities of (+)-8-dihydrotetrabenazine administered to the subject can
be
defined in terms of the weight in milligrams of (+)-8-dihydrotetrabenazine
administered to the subject per kilogram of the subject's body weight, which
can be
CA 03057548 2019-09-23
WO 2018/178243
PCT/EP2018/058088
33
abbreviated to mg/kg. The appropriate dosage amount can therefore be
calculated
by multiplying the mg/kg amount by the weight of the subject to be treated.
Accordingly, the invention also provides:
= (+)-8-dihydrotetrabenazine for use in a method for the treatment of a
movement disorder, wherein the treatment comprises administering to a
subject an amount of (+)-8-dihydrotetrabenazine from 0.01 mg/kg to 2.0
mg/kg (e.g. between 0.01 mg/kg and 2.0 mg/kg) per day provided that the
total amount of (+)-8-dihydrotetrabenazine administered per day is in the
range from 1 mg to 80 mg (or such other range as defined above).
= A method of treatment of a movement disorder in a subject in need thereof
(e.g. a mammalian subject such as a human), which treatment comprises
administering to the subject an amount of (+)-8-dihydrotetrabenazine from
0.01 mg/kg to 2.0 mg/kg (e.g. between 0.01 mg/kg and 2.0 mg/kg) per day,
provided that the total amount of (+)-8-dihydrotetrabenazine administered
per day is in the range from 1 mg to 80 mg (or such other range as defined
above).
= The use of (+)-8-dihydrotetrabenazine for the manufacture of a medicament
for the treatment of a movement disorder, which treatment comprises
administering to the subject an amount of (+)-8-dihydrotetrabenazine from
0.01 mg/kg to 2.0 mg/kg (e.g. between 0.01 mg/kg and 2.0 mg/kg) per day,
provided that the total amount of (+)-8-dihydrotetrabenazine administered
per day is in the range from 1 mg to 80 mg (or such other range as defined
above).
In further embodiments, there is provided:
= (+)-8-dihydrotetrabenazine for use, a method or a use as described
herein,
wherein the treatment comprises administering to the subject from 0.01
mg/kg to 1.5 mg/kg (e.g. between 0.01 mg/kg and 1.5 mg/kg) of (+)-8-
dihydrotetrabenazine per day, provided that the total amount of (+)-8-
dihydrotetrabenazine administered per day is in the range from 1 mg to 80
mg (or such other range as defined above).
= (+)-8-dihydrotetrabenazine for use, a method or a use as described
herein,
wherein the treatment comprises administering to the subject from 0.1
CA 03057548 2019-09-23
WO 2018/178243
PCT/EP2018/058088
34
mg/kg to 1.5 mg/kg (e.g. between 0.1 mg / kg and 1.5 mg / kg) of ()-I3-
dihydrotetrabenazine, provided that the total amount of ()-I3-
dihydrotetrabenazine administered per day is in the range from 1 mg to 80
mg (or such other range as defined above).
= (+)-6-dihydrotetrabenazine for use, a method or a use as described
herein,
wherein the treatment comprises administering to the subject from 0.25
mg/kg to 1.5 mg/kg (e.g. between 0.25 mg / kg and 1.5 mg / kg) of ()-I3-
dihydrotetrabenazine, provided that the total amount of ()-I3-
dihydrotetrabenazine administered per day is in the range from 1 mg to 80
mg (or such other range as defined above).
= (+)-6-dihydrotetrabenazine for use, a method or a use as described
herein,
wherein the treatment comprises administering to the subject from 0.01
mg/kg to 1.25 mg/kg (e.g. between 0.01 mg / kg and 1.25 mg / kg) of ()-I3-
dihydrotetrabenazine per day, provided that the total amount of (+)-6-
dihydrotetrabenazine administered per day is in the range from 1 mg to 60
mg (or such other range as defined above).
= (+)-6-dihydrotetrabenazine for use, a method or a use as described
herein,
wherein the treatment comprises administering to the subject from 0.1
mg/kg to 1.25 mg/kg (e.g. between 0.1 mg / kg and 1.25 mg / kg) of (+)-6-
dihydrotetrabenazine, provided that the total amount of ()-I3-
dihydrotetrabenazine administered per day is in the range from 1 mg to 60
mg (or such other range as defined above).
= (+)-6-dihydrotetrabenazine for use, a method or a use as described
herein,
wherein the treatment comprises administering to the subject from 0.25
mg/kg to 1.25 mg/kg (e.g. between 0.25 mg / kg and 1.25 mg / kg) of ()-I3-
dihydrotetrabenazine, provided that the total amount of ()-I3-
dihydrotetrabenazine administered per day is in the range from 1 mg to 60
mg (or such other range as defined above).
In each of the foregoing aspects and embodiments, the (+)-6-
dihydrotetrabenazine
can be administered as the free base or as a pharmaceutically acceptable salt.
Unless the context indicates otherwise, references herein to (+)-6-
dihydrotetrabenazine also include pharmaceutically acceptable salts thereof.
CA 03057548 2019-09-23
WO 2018/178243
PCT/EP2018/058088
In one general embodiment, the (+)-6-dihydrotetrabenazine is administered as a
pharmaceutically acceptable salt.
In another general embodiment, the (+)-6-dihydrotetrabenazine is administered
as
a free base.
5 Where quantities or ranges of quantities of (+)-6-dihydrotetrabenazine
are stated
herein, these are calculated as the amounts of the free base, or when the (+)-
6-
dihydrotetrabenazine is in the form of a pharmaceutically acceptable salt, the
amount of (+)-6-dihydrotetrabenazine free base present in the pharmaceutically
acceptable salt.
10 Complete blocking of the VMAT2 proteins is considered undesirable as
this can
lead to unwanted side effects such as Parkinsonism. The present invention
provides plasma levels of (+)-6-dihydrotetrabenazine that are sufficient to
give
effective treatment of movement disorders but do not block the VMAT2 proteins
to
an extent that causes Parkinsonism and similar side effects. The levels of
VMAT2
15 blocking can be determined by competitive binding studies using Positron
Emission Tomography (PET). By co-administering a radioactive ligand with the
compound of interest at various concentrations, the proportion of binding
sites
occupied can be determined (see for example, Matthews et al., "Positron
emission
tomography molecular imaging for drug development", Br. J. Clin. Pharmacol.,
20 73:2, 175-186). Accordingly, the invention also provides:
= (+)-6-dihydrotetrabenazine for use in a method for the treatment of a
movement disorder, wherein the treatment comprises administering to a
subject an amount of (+)-6-dihydrotetrabenazine sufficient to cause a level
of blocking of up to 90% of the VMAT2 proteins in the subject.
25 = A method of treatment of a movement disorder in a subject in need
thereof
(e.g. a mammalian subject such as a human), which treatment comprises
administering to the subject an amount of (+)-6-dihydrotetrabenazine
sufficient to cause a level of blocking of up to 90% of the VMAT2 proteins in
the subject.
30 = The use of (+)-6-dihydrotetrabenazine for the manufacture of a
medicament
for the treatment of a movement disorder, which treatment comprises
administering to the subject an amount of (+)-6-dihydrotetrabenazine
CA 03057548 2019-09-23
WO 2018/178243
PCT/EP2018/058088
36
sufficient to cause a level of blocking of up to 90% of the VMAT2 proteins in
the subject.
In further embodiments, there is provided:
= (+)-8-dihydrotetrabenazine for use, a method or a use as described
herein,
wherein the treatment comprises administering to the subject an amount of
(+)-8-dihydrotetrabenazine sufficient to cause a level of blocking of up to
85% of the VMAT2 proteins in the subject.
= (+)-8-dihydrotetrabenazine for use, a method or a use as described
herein,
wherein the treatment comprises administering to the subject an amount of
(+)-8-dihydrotetrabenazine sufficient to cause a level of blocking of up to
80% of the VMAT2 proteins in the subject.
= (+)-8-dihydrotetrabenazine for use, a method or a use as described
herein,
wherein the treatment comprises administering to the subject an amount of
(+)-8-dihydrotetrabenazine sufficient to cause a level of blocking of up to
75% of the VMAT2 proteins in the subject.
= (+)-8-dihydrotetrabenazine for use, a method or a use as described
herein,
wherein the treatment comprises administering to the subject an amount of
(+)-8-dihydrotetrabenazine sufficient to cause a level of blocking of up to
70% of the VMAT2 proteins in the subject.
= (+)-8-dihydrotetrabenazine for use, a method or a use as described herein,
wherein the treatment comprises administering to the subject an amount of
(+)-8-dihydrotetrabenazine sufficient to cause a level of blocking of from
20% to 90% of the VMAT2 proteins in the subject.
= (+)-8-dihydrotetrabenazine for use, a method or a use as described
herein,
wherein the treatment comprises administering to the subject an amount of
(+)-8-dihydrotetrabenazine sufficient to cause a level of blocking of from
25% to 85% of the VMAT2 proteins in the subject.
= (+)-8-dihydrotetrabenazine for use, a method or a use as described
herein,
wherein the treatment comprises administering to the subject an amount of
CA 03057548 2019-09-23
WO 2018/178243
PCT/EP2018/058088
37
(+)-P-dihydrotetrabenazine sufficient to cause a level of blocking of from
30% to 80% of the VMAT2 proteins in the subject.
= (+)13-dihydrotetrabenazine for use, a method or a use as described
herein,
wherein the treatment comprises administering to the subject an amount of
(+)13-dihydrotetrabenazine sufficient to cause a level of blocking of from
35% to 75% of the VMAT2 proteins in the subject.
= (+)13-dihydrotetrabenazine for use, a method or a use as described
herein,
wherein the treatment comprises administering to the subject an amount of
(+)-P-dihydrotetrabenazine sufficient to cause a level of blocking of from
35% to 70% of the VMAT2 proteins in the subject.
= (+)13-dihydrotetrabenazine for use, a method or a use as described
herein,
wherein the treatment comprises administering to the subject an amount of
(+)-P-dihydrotetrabenazine sufficient to cause a level of blocking of from
40% to 75% of the VMAT2 proteins in the subject.
= (+)13-dihydrotetrabenazine for use, a method or a use as described herein,
wherein the treatment comprises administering to the subject in need
thereof, wherein the method comprising administering to a subject an
amount of (+)-P-dihydrotetrabenazine sufficient to cause a level of blocking
of from 45% to 75% of the VMAT2 proteins in the subject.
= (+)13-dihydrotetrabenazine for use, a method or a use as described herein,
wherein the treatment comprises administering to the subject in need
thereof, wherein the method comprising administering to a subject an
amount of (+)-P-dihydrotetrabenazine sufficient to cause a level of blocking
of from 35% to 80% of the VMAT2 proteins in the subject.
= (+)13-dihydrotetrabenazine for use, a method or a use as described herein,
wherein the treatment comprises administering to the subject in need
thereof, wherein the method comprising administering to a subject an
amount of (+)-P-dihydrotetrabenazine sufficient to cause a level of blocking
of from 40% to 80% of the VMAT2 proteins in the subject.
= (+)13-dihydrotetrabenazine for use, a method or a use as described herein,
wherein the treatment comprises administering to the subject an amount of
CA 03057548 2019-09-23
WO 2018/178243
PCT/EP2018/058088
38
(+)-8-dihydrotetrabenazine sufficient to cause a level of blocking of from
45% to 80% of the VMAT2 proteins in the subject.
= (+)-8-dihydrotetrabenazine for use, a method or a use as described
herein,
wherein the treatment comprises administering to the subject an amount of
(+)-8-dihydrotetrabenazine sufficient to cause a level of blocking of from
50% to 80% of the VMAT2 proteins in the subject.
= (+)-8-dihydrotetrabenazine for use, a method or a use as described
herein,
wherein the treatment comprises administering to the subject an amount of
(+)-8-dihydrotetrabenazine sufficient to cause a level of blocking of from
55% to 80% of the VMAT2 proteins in the subject.
= (+)-8-dihydrotetrabenazine for use, a method or a use as described
herein,
wherein the treatment comprises administering to the subject an amount of
(+)-8-dihydrotetrabenazine sufficient to cause a level of blocking of VMAT2
proteins in the subject of from 30% to 70% (e.g. between 30% and 70%).
= (+)-8-dihydrotetrabenazine for use, a method or a use as described herein,
wherein the treatment comprises administering to the subject in need
thereof, wherein the method comprising administering to a subject an
amount of (+)-8-dihydrotetrabenazine sufficient to cause a blocking level of
VMAT2 proteins in the subject of from 30% to 65% (e.g. between 30% and
65%).
= (+)-8-dihydrotetrabenazine for use, a method or a use as described
herein,
wherein the treatment comprises administering to the subject in need
thereof, wherein the method comprising administering to a subject an
amount of (+)-8-dihydrotetrabenazine sufficient to cause a blocking level of
VMAT2 proteins in the subject of from 30% to 60% (e.g. between 30% and
60%).
= (+)-8-dihydrotetrabenazine for use, a method or a use as described
herein,
wherein the treatment comprises administering to the subject in need
thereof, wherein the method comprising administering to a subject an
amount of (+)-8-dihydrotetrabenazine sufficient to cause a level blocking of
VMAT2 proteins in the subject of from 40% to 80% (e.g. between 40% and
80%).
CA 03057548 2019-09-23
WO 2018/178243
PCT/EP2018/058088
39
= (+)13-dihydrotetrabenazine for use, a method or a use as described
herein,
wherein the treatment comprises administering to the subject in need
thereof, wherein the method comprising administering to a subject an
amount of (+)-P-dihydrotetrabenazine sufficient to cause a level of blocking
of VMAT2 proteins in the subject of from 40% to 75% (e.g. between 40%
and 75%).
= (+)13-dihydrotetrabenazine for use, a method or a use as described
herein,
wherein the treatment comprises administering to the subject in need
thereof, wherein the method comprising administering to a subject an
amount of (+)I3-dihydrotetrabenazine sufficient to cause a level of blocking
of VMAT2 proteins in the subject of from 40% to 70% (e.g. between 40%
and 70%).
= (+)13-dihydrotetrabenazine for use, a method or a use as described
herein,
wherein the treatment comprises administering to the subject in need
thereof, wherein the method comprising administering to a subject an
amount of (+)-P-dihydrotetrabenazine sufficient to cause a level of blocking
of VMAT2 proteins in the subject of from 40% to 65% (e.g. between 40%
and 65%).
= (+)13-dihydrotetrabenazine for use, a method or a use as described
herein,
wherein the treatment comprises administering to the subject in need
thereof, wherein the method comprising administering to a subject an
amount of (+)-P-dihydrotetrabenazine sufficient to cause a level blocking of
VMAT2 proteins in the subject of from 40% to 60% (e.g. between 40% and
60%).
The movement disorder can be a hyperkinetic movement disorder such as
Huntington's disease, hemiballismus, senile chorea, tic disorders, tardive
dyskinesia, dystonia, myoclonus and Tourette's syndrome. In one Embodiment,
the movement disorder is Tourette's syndrome
In each of the foregoing embodiments, the (+)I3-dihydrotetrabenazine is
accompanied by no more than 20% by weight, relative to the (+)13-
dihydrotetrabenazine, of any other isomer of dihydrotetrabenazine.
CA 03057548 2019-09-23
WO 2018/178243
PCT/EP2018/058088
More usually the (+)13-dihydrotetrabenazine is accompanied by no more than 10%
by weight, relative to the (+)13-dihydrotetrabenazine, of any other isomer of
dihydrotetrabenazine; preferably no more than 5% by weight, relative to the ()-
I3-
dihydrotetrabenazine, of any other isomer of dihydrotetrabenazine; and more
5 preferably no more than 2% by weight, relative to the (+)13-
dihydrotetrabenazine,
of any other isomer of dihydrotetrabenazine. Most preferably the ()-I3-
dihydrotetrabenazine is accompanied by less than 1% (e.g. less than 0.5% or
less
than 0.1%) relative to the (+)13-dihydrotetrabenazine, of any other isomer of
dihydrotetrabenazine.
10 Thus, the (+)13-dihydrotetrabenazine typically has an isomeric purity of
at least
80%.
The term "isomeric purity" in the present context refers to the amount (+)13-
dihydrotetrabenazine present relative to the total amount or concentration of
dihydrotetrabenazines of all isomeric forms. For example, if 90% of the total
15 dihydrotetrabenazine present in the composition is (+)13-
dihydrotetrabenazine,
then the isomeric purity is 90%.
The (+)13-dihydrotetrabenazine of the invention may have an isomeric purity of
greater than 82%, greater than 85%, greater than 87%, greater than 90%,
greater
than 91%, greater than 92%, greater than 93%, greater than 94%, greater than
20 95%, greater than 96%, greater than 97%, greater than 98%, greater than
99%,
greater than 99.5%, or greater than 99.9%.
In each of the foregoing aspects and embodiments of the invention relating to
(+)-
13-dihydrotetrabenazine or pharmaceutically acceptable salts thereof,
typically, the
(+)13-dihydrotetrabenazine, or pharmaceutically acceptable salt thereof, are
not
25 administered with a therapeutically effective amount of amantadine. More
particularly, the (+)13-dihydrotetrabenazine, or pharmaceutically acceptable
salt
thereof, are not administered with any amount of amantadine.
For example, with reference to pharmaceutical unit dosage forms, typically the
unit
dosage form does not comprise a therapeutically effective amount of amantadine
30 and, more particularly, the pharmaceutical unit dosage form does not
comprise any
amount of amantadine.
CA 03057548 2019-09-23
WO 2018/178243
PCT/EP2018/058088
41
Furthermore, in each of the foregoing aspects and embodiments of the invention
relating to (+)I3-dihydrotetrabenazine or pharmaceutically acceptable salts
thereof,
the pharmaceutical unit dosage form may be other than an extended release or
delayed release dosage form.
Thus, for example, the (+)13-dihydrotetrabenazine, or pharmaceutically
acceptable
salt thereof, may be administered as an immediate release unit dosage form.
Combinations of (+)-13-dihydrotetrabenazine with (-)-a-dihydrotetrabenazine
and/or (+)-a-dihydrotetrabenazine
In a further aspect, it is envisaged that combinations of (+)I3-
dihydrotetrabenazine,
having the formula (III),
0
N
0
(R)
S)
OH
(III)
and
(-)-a-dihydrotetrabenazine, having the formula (II),
N
0
H.%==
ss)1 ,,,,
OH
(II)
and/or (+)-a-dihydrotetrabenazine, having the formula (I),
CA 03057548 2019-09-23
WO 2018/178243
PCT/EP2018/058088
42
0
/
R) N
0
H lir)
oH
(I)
will be useful in the prophylaxis or treatment of inter alia the disease
states and
conditions for which tetrabenazine is currently used or proposed. Thus, by way
of
example, and without limitation, it is envisaged that these combinations of
dihydrotetrabenazine isomers may be used for the treatment of hyperkinetic
movement disorders such as Huntington's disease, hemiballismus, senile chorea,
tic disorders, tardive dyskinesia, dystonia and, in particular, Tourette's
syndrome.
Accordingly, in a first aspect, the invention provides a pharmaceutical
combination
comprising:
(a) (+)I3-dihydrotetrabenazine, or a pharmaceutically acceptable salt
thereof;
and one or both of:
(b) (-)-a-dihydrotetrabenazine, or a pharmaceutically acceptable salt
thereof;
and
(c) (+)-a-dihydrotetrabenazine, or a pharmaceutically acceptable salt
thereof.
In one embodiment, the invention provides a pharmaceutical combination
comprising:
(a) (+)I3-dihydrotetrabenazine, or a pharmaceutically acceptable salt
thereof;
and
(b) (-)-a-dihydrotetrabenazine, or a pharmaceutically acceptable salt
thereof.
In another embodiment, the invention provides a pharmaceutical combination
comprising:
(a) (+)I3-dihydrotetrabenazine, or a pharmaceutically acceptable salt
thereof;
and
(c) (+)-a-dihydrotetrabenazine, or a pharmaceutically acceptable salt
thereof.
CA 03057548 2019-09-23
WO 2018/178243
PCT/EP2018/058088
43
In another embodiment, the invention provides a pharmaceutical combination
comprising:
(a) (+)13-dihydrotetrabenazine, or a pharmaceutically acceptable salt
thereof;
(b) (-)-a-dihydrotetrabenazine, or a pharmaceutically acceptable salt
thereof;
and
(c) (+)-a-dihydrotetrabenazine, or a pharmaceutically acceptable salt
thereof.
The (+)13-dihydrotetrabenazine, (-)-a-dihydrotetrabenazine and (+)-a-
dihydrotetrabenazine may be referred to herein collectively as "the
dihydrotetrabenazine isomers of the invention" or "isomers of
dihydrotetrabenazine" or "the dihydrotetrabenazines", unless the context
indicates
otherwise. When describing types of pharmaceutical formulation, they may also
be
referred to collectively as the "active compounds".
The pharmaceutical combination may be substantially free of (-)13-
dihydrotetrabenazine. Accordingly, the invention also provides a
pharmaceutical
combination as described herein, wherein the unit dosage form is substantially
free
of (-)13-dihydrotetrabenazine.
By "substantially free of (-)13-dihydrotetrabenazine" is meant that the %
weight of
(-)13-dihydrotetrabenazine present compared to the total weight of all isomers
of
dihydrotetrabenazine is less than 5%, preferably less than 3%, more preferably
less than 2% and most preferably less than 1%.
The relative proportions of the (+)13-dihydrotetrabenazine, (-)-a-
dihydrotetrabenazine and (+)-a-dihydrotetrabenazine may be expressed in terms
of parts by weight of the individual isomers. Thus, for example, the unit
dosage
forms may comprise from 35 to 75 parts by weight of (+)13-dihydrotetrabenazine
and from 25 to 55 parts by weight of an a-dihydrotetrabenazine (which may be
either (+)-a-dihydrotetrabenazine or (-)-a-dihydrotetrabenazine or a mixture
thereof). It will be appreciated that the proportions expressed above as parts
by
weight could instead be expressed in terms of molar ratios (as all of the
isomers
have the same molecular weight), in which case the relative proportions of the
isomers could be expressed as a molar ratio of (+)-P-dihydrotetrabenazine : a-
dihydrotetrabenazine (which may be either (+)-a-dihydrotetrabenazine or (-)-a-
dihydrotetrabenazine or a mixture thereof) of 35-70 : 25-55.
CA 03057548 2019-09-23
WO 2018/178243
PCT/EP2018/058088
44
In one embodiment, a pharmaceutical combination of the invention comprises:
(a) 40-65 parts by weight of (+)I3-dihydrotetrabenazine, or a
pharmaceutically
acceptable salt thereof; and
(c) 40-65 parts by weight of (+)-a-dihydrotetrabenazine, or a
pharmaceutically
acceptable salt thereof.
For example, the pharmaceutical combination may comprise:
(a) 45-55 parts by weight of (+)I3-dihydrotetrabenazine, or a
pharmaceutically
acceptable salt thereof; and
(c) 45-55 parts by weight of (+)-a-dihydrotetrabenazine, or a
pharmaceutically
acceptable salt thereof.
In one particular embodiment, the pharmaceutical combination comprises (+)13-
dihydrotetrabenazine and (+)-a-dihydrotetrabenazine in approximately equimolar
amounts.
In another embodiment, a pharmaceutical combination of the invention
comprises:
(a) 45-65 parts by weight of (+)I3-dihydrotetrabenazine, or a
pharmaceutically
acceptable salt thereof;
(b) 30-50 parts by weight of (-)-a-dihydrotetrabenazine, or a
pharmaceutically
acceptable salt thereof; and optionally
(c) 0.1-5 parts by weight of (+)-a-dihydrotetrabenazine, or a
pharmaceutically
acceptable salt thereof.
In another embodiment, a pharmaceutical combination of the invention
comprises:
(a) 45-65 parts by weight of (+)I3-dihydrotetrabenazine, or a
pharmaceutically
acceptable salt thereof;
(b) 30-50 parts by weight of (-)-a-dihydrotetrabenazine, or a
pharmaceutically
acceptable salt thereof; and optionally
(c) 0.1-3 parts by weight of (+)-a-dihydrotetrabenazine, or a
pharmaceutically
acceptable salt thereof.
In another embodiment, a pharmaceutical combination of the invention
comprises:
CA 03057548 2019-09-23
WO 2018/178243
PCT/EP2018/058088
(a) 45-65 parts by weight of (+)I3-dihydrotetrabenazine, or a
pharmaceutically
acceptable salt thereof;
(b) 30-50 parts by weight of (-)-a-dihydrotetrabenazine, or a
pharmaceutically
acceptable salt thereof; and optionally
5 (c) 0.1-2 parts by weight of (+)-a-dihydrotetrabenazine, or a
pharmaceutically
acceptable salt thereof.
In a further embodiment, a pharmaceutical combination of the invention
comprises:
(a) 45-65 parts by weight of (+)I3-dihydrotetrabenazine, or a
pharmaceutically
acceptable salt thereof;
10 (b) 30-50 parts by weight of (-)-a-dihydrotetrabenazine, or a
pharmaceutically
acceptable salt thereof; and optionally
(c) 0.1-1.5 parts by weight of (+)-a-dihydrotetrabenazine, or a
pharmaceutically
acceptable salt thereof.
By pharmaceutical combination is meant a combination of the three
15 dihydrotetrabenazines (a) and (b) and/or (c) in a form that is suitable
for
administration to a subject, typically a human or other animal subject. The
term
therefore excludes crude reaction mixtures, partially purified reaction
products,
whole blood samples or blood fraction samples such as plasma or other
biological
samples such as urine samples containing the combinations. It also excludes
20 simple solutions of the combinations in non-pharmaceutically acceptable
solvents
(e.g. chloroform, dichloromethane) that are not normally used in pharmacy.
The pharmaceutical combinations may be in the form of mixtures of the pure
compounds or the combinations may comprise one or more pharmaceutically
acceptable excipients.
25 Typically, the pharmaceutical combinations comprise a pharmaceutically
acceptable excipient and are formulated as unit dosage forms containing
defined
amounts of the dihydrotetrabenazines (a), (b) and/or (c).
In the pharmaceutical combinations of the invention, one or more of the three
dihydrotetrabenazines (a), (b) and (c) may be formulated separately but used
in
30 combination. More typically, however, the three dihydrotetrabenazines
(a), (b) and
CA 03057548 2019-09-23
WO 2018/178243
PCT/EP2018/058088
46
(c) are formulated together in a pharmaceutical composition, and in particular
a
unit dosage form.
In a unit dosage form of the invention containing a combination as defined
herein,
the sum of the amounts of the three isomers (+)-P-dihydrotetrabenazine, (-)-a-
dihydrotetrabenazine and (+)-a-dihydrotetrabenazine (the "total amount") may
be
selected so that it does not exceed 100mg.
Typically, the pharmaceutical unit dosage form does not comprise an effective
amount of amantadine. In one embodiment the pharmaceutical unit dosage form is
one that does not comprise amantadine.
The pharmaceutical unit dosage form may be an immediate release unit dosage
form.
In particular embodiments:
= the total amount of the three isomers does not exceed 75mg; or
= the total amount of the three isomers does not exceed 50mg; or
= the total amount of the three isomers does not exceed 40mg; or
= the total amount of the three isomers does not exceed 30mg; or
= the total amount of the three isomers does not exceed 20mg.
The unit dosage form can be one which is administered orally, for example a
capsule or tablet.
The unit dosage form can be one which is administered orally, for example a
capsule or tablet.
The pharmaceutical combinations as defined herein are provided for use in
medicine.
More particularly, the pharmaceutical combinations (and unit dosage forms)
defined and described above are provided for use in the treatment of a
hyperkinetic movement disorder such as Huntington's disease, hemiballismus,
senile chorea, tic disorders, tardive dyskinesia, dystonia and Tourette's
syndrome.
CA 03057548 2019-09-23
WO 2018/178243
PCT/EP2018/058088
47
More particularly, the pharmaceutical combinations (and unit dosage forms)
described above are for use in the treatment of a hyperkinetic movement
disorder
selected from tic disorders, Huntington's disease, tardive dyskinesia and
Tourette's
syndrome.
In one particular embodiment, the pharmaceutical combinations (and unit dosage
forms) described above are for use in the treatment of tardive dyskinesia.
In another particular embodiment, the pharmaceutical combinations (and unit
dosage forms) described above are for use in the treatment of Tourette's
syndrome.
In further aspects, the invention provides:
= A pharmaceutical combination as defined herein for use in the treatment
of
a hyperkinetic movement disorder.
= A method of treatment of a hyperkinetic movement disorder in a subject in
need thereof (e.g. a mammalian subject such as a human), which method
comprises administering to the subject a therapeutically effective amount of a
pharmaceutical combination as defined herein.
= The use of a pharmaceutical combination as defined herein for the
manufacture of a medicament for the treatment of a hyperkinetic movement
disorder.
= A unit dosage form for use, a pharmaceutical combination for use, a
method or a use as described herein wherein the hyperkinetic movement disorder
is selected from Huntington's disease, hemiballismus, senile chorea, tic
disorders,
tardive dyskinesia, dystonia and Tourette's syndrome.
A unit dosage form for use, a pharmaceutical combination for use, a method or
a
use as described herein wherein the hyperkinetic movement disorder is
Tourette's
syndrome.
In each case, the combination of (+)-P-dihydrotetrabenazine, (-)-a-
dihydrotetrabenazine; and optionally (+)-a-dihydrotetrabenazine is typically
administered once per day.
CA 03057548 2019-09-23
WO 2018/178243
PCT/EP2018/058088
48
Complete blocking of VMAT2 is considered undesirable as this can lead to
unwanted side effects, such as Parkinsonism. The present invention provides
plasma levels of dihydrotetrabenazines that are sufficient to give effective
treatment of movement disorders but do not block VMAT2 to an extent that
causes
Parkinsonism and similar side effects. The levels of VMAT2 blocking can be
determined by competitive binding studies using Positron Emission Tomography
(PET). By co-administering a radioactive ligand with the compound of interest
at
various concentrations, the proportion of binding sites occupied can be
determined
(see for example, Matthews et al., "Positron emission tomography molecular
.. imaging for drug development", Br. J. Clin. Pharmacol., 73:2, 175-186).
Accordingly, the invention also provides:
= A unit dosage form for use, a pharmaceutical combination for use, a
method or a use as described herein, wherein the treatment comprises
administering to the subject an amount of the unit dosage form or
combination sufficient to cause a blocking level of greater than 20% of
VMAT2 proteins in the subject.
= A unit dosage form for use, a pharmaceutical combination for use, a
method or a use as described herein, wherein the treatment comprises
administering to the subject an amount of the unit dosage form or
combination sufficient to cause a blocking level of greater than 30% of
VMAT2 proteins in the subject.
= A unit dosage form for use, a pharmaceutical combination for use, a
method or a use as described herein, wherein the treatment comprises
administering to the subject an amount of the unit dosage form or
combination sufficient to cause a blocking level of greater than 40% of
VMAT2 proteins in the subject.
= A unit dosage form for use, a pharmaceutical combination for use, a
method or a use as described herein, wherein the treatment comprises
administering to the subject an amount of the unit dosage form or
combination sufficient to cause a blocking level of less than 90% of VMAT2
proteins in the subject.
CA 03057548 2019-09-23
WO 2018/178243
PCT/EP2018/058088
49
= A unit dosage form for use, a pharmaceutical combination for use, a
method or a use as described herein, wherein the treatment comprises
administering to the subject an amount of the unit dosage form or
combination sufficient to cause a blocking level of less than 85% of VMAT2
proteins in the subject.
= A unit dosage form for use, a pharmaceutical combination for use, a
method or a use as described herein, wherein the treatment comprises
administering to the subject an amount of the unit dosage form or
combination sufficient to cause a blocking level of less than 80% of VMAT2
proteins in the subject.
= A unit dosage form for use, a pharmaceutical combination for use, a
method or a use as described herein, wherein the treatment comprises
administering to the subject an amount of the unit dosage form or
combination sufficient to cause a blocking level of less than 75% of VMAT2
proteins in the subject.
= A unit dosage form for use, a pharmaceutical combination for use, a
method or a use as described herein, wherein the treatment comprises
administering to the subject an amount of the unit dosage form or
combination sufficient to cause a blocking level of less than 70% of VMAT2
proteins in the subject.
= A unit dosage form for use, a pharmaceutical combination for use, a
method or a use as described herein, wherein the treatment comprises
administering to the subject an amount of the unit dosage form or
combination sufficient to cause a level of blocking of VMAT2 proteins in the
subject of from 20% to 90% (e.g. between 20% and 90%).
= A unit dosage form for use, a pharmaceutical combination for use, a
method or a use as described herein, wherein the treatment comprises
administering to the subject an amount of the unit dosage form or
combination sufficient to cause a level of blocking of VMAT2 proteins in the
subject of from 30% to 80% (e.g. between 30% and 80%).
= A unit dosage form for use, a pharmaceutical combination for use, a
method or a use as described herein, wherein the treatment comprises
CA 03057548 2019-09-23
WO 2018/178243
PCT/EP2018/058088
administering to the subject an amount of the unit dosage form or
combination sufficient to cause a level of blocking of VMAT2 proteins in the
subject of from 30% to 75% (e.g. between 30% and 75%).
= A unit dosage form for use, a pharmaceutical combination for use, a
5 method or a use as described herein, wherein the treatment comprises
administering to the subject an amount of the unit dosage form or
combination sufficient to cause a level of blocking of VMAT2 proteins in the
subject of from 30% to 70% (e.g. between 30% and 70%).
= A unit dosage form for use, a pharmaceutical combination for use, a
10 method or a use as described herein, wherein the treatment comprises
administering to the subject an amount of the unit dosage form or
combination sufficient to cause a level of blocking of VMAT2 proteins in the
subject of from 30% to 65% (e.g. between 30% and 65%).
= A unit dosage form for use, a pharmaceutical combination for use, a
15 method or a use as described herein, wherein the treatment comprises
administering to the subject an amount of the unit dosage form or
combination sufficient to cause a level of blocking of VMAT2 proteins in the
subject of from 30% to 60% (e.g. between 30% and 60%).
= A unit dosage form for use, a pharmaceutical combination for use, a
20 method or a use as described herein, wherein the treatment comprises
administering to the subject an amount of the unit dosage form or
combination sufficient to cause a level of blocking of VMAT2 proteins in the
subject of from 40% to 80% (e.g. between 40% and 80%).
= A unit dosage form for use, a pharmaceutical combination for use, a
25 method or a use as described herein, wherein the treatment comprises
administering to the subject an amount of the unit dosage form or
combination sufficient to cause a level of blocking of VMAT2 proteins in the
subject of from 40% to 75% (e.g. between 40% and 75%).
= A unit dosage form for use, a pharmaceutical combination for use, a
30 method or a use as described herein, wherein the treatment comprises
administering to the subject an amount of the unit dosage form or
CA 03057548 2019-09-23
WO 2018/178243
PCT/EP2018/058088
51
combination sufficient to cause a level of blocking of VMAT2 proteins in the
subject of from 40% to 70% (e.g. between 40% and 70%).
= A unit dosage form for use, a pharmaceutical combination for use, a
method or a use as described herein, wherein the treatment comprises
administering to the subject an amount of the unit dosage form or
combination sufficient to cause a level of blocking of VMAT2 proteins in the
subject of from 40% to 65% (e.g. between 40% and 65%).
= A unit dosage form for use, a pharmaceutical combination for use, a
method or a use as described herein, wherein the treatment comprises
administering to the subject an amount of the unit dosage form or
combination sufficient to cause a level of blocking of VMAT2 proteins in the
subject of from 40% to 60% (e.g. between 40% and 60%).
In each of the foregoing aspects and embodiments of the invention relating to
combinations of dihydrotetrabenazines (a) and (b) and/or (c), typically the
combinations are not administered with a therapeutically effective amount of
amantadine. More particularly, the combinations, are not administered with any
amount of amantadine.
For example, with reference to pharmaceutical unit dosage forms, typically the
unit
dosage form does not comprise a therapeutically effective amount of amantadine
and, more particularly, the pharmaceutical unit dosage form does not comprise
any
amount of amantadine.
Furthermore, in each of the foregoing aspects and embodiments of the invention
relating to combinations of dihydrotetrabenazines (a) and (b) and/or (c), the
pharmaceutical unit dosage form may be other than an extended release or
delayed release dosage form.
Thus, for example, the combinations of dihydrotetrabenazines (a) and (b)
and/or
(c) may be administered as an immediate release unit dosage form.
Free bases and salts
In each of the foregoing aspects and embodiments of the invention, all
references
herein to the individual isomers of dihydrotetrabenazine refer to both the
free
bases and salts thereof, unless the context indicates otherwise.
CA 03057548 2019-09-23
WO 2018/178243
PCT/EP2018/058088
52
The salts are typically acid addition salts.
The salts can be synthesized from the parent compound by conventional chemical
methods such as methods described in Pharmaceutical Salts: Properties,
Selection, and Use, P. Heinrich Stahl (Editor), Camille G. Wermuth (Editor),
ISBN:
3-90639-026-8, Hardcover, 388 pages, August 2002. Generally, such salts can be
prepared by reacting the free base form of the compound with the acid in water
or
in an organic solvent, or in a mixture of the two; generally, nonaqueous media
such
as ether, acetone, ethyl acetate, ethanol, isopropanol, or acetonitrile are
used.
Acid addition salts may be formed with a wide variety of acids, both inorganic
and
organic. Examples of acid addition salts include salts formed with an acid
selected
from the group consisting of acetic, 2,2-dichloroacetic, adipic, alginic,
ascorbic (e.g.
L-ascorbic), L-aspartic, benzenesulphonic, benzoic, 4-acetamidobenzoic,
butanoic, (+) camphoric, camphor-sulphonic, (+)-(1S)-camphor-10-sulphonic,
capric, caproic, caprylic, cinnamic, citric, cyclamic, dodecylsulphuric,
ethane-1,2-
disulphonic, ethanesulphonic, 2-hydroxyethanesulphonic, formic, fumaric,
galactaric, gentisic, glucoheptonic, D-gluconic, glucuronic (e.g. D-
glucuronic),
glutamic (e.g. L-glutamic), a-oxoglutaric, glycolic, hippuric, hydrobromic,
hydrochloric, hydriodic, isethionic, (+)-L-lactic, ( )-DL-lactic, lactobionic,
maleic,
malic, (-)-L-malic, malonic, ( )-DL-mandelic, methanesulphonic, naphthalene-2-
sulphonic, naphthalene-1,5-disulphonic, 1-hydroxy-2-naphthoic, nicotinic,
nitric,
oleic, orotic, oxalic, palmitic, pamoic, phosphoric, propionic, L-
pyroglutamic,
salicylic, 4-amino-salicylic, sebacic, stearic, succinic, sulphuric, tannic,
(+)-L-
tartaric, thiocyanic, p-toluenesulphonic, undecylenic and valeric acids, as
well as
acylated amino acids and cation exchange resins.
The salt forms of the compounds of the invention are typically
pharmaceutically
acceptable salts, and examples of pharmaceutically acceptable salts are
discussed
in Berge et al., 1977, "Pharmaceutically Acceptable Salts," J. Pharm. Sci.,
Vol. 66,
pp. 1-19. However, salts that are not pharmaceutically acceptable may also be
prepared as intermediate forms which may then be converted into
.. pharmaceutically acceptable salts. Such non-pharmaceutically acceptable
salts
forms, which may be useful, for example, in the purification or separation of
the
compounds of the invention, also form part of the invention.
The isomers of dihydrotetrabenazine may contain one or more isotopic
substitutions, and a reference to a particular element includes within its
scope all
isotopes of the element. For example, a reference to hydrogen includes within
its
CA 03057548 2019-09-23
WO 2018/178243
PCT/EP2018/058088
53
scope 1H, 2H (D), and 3H (T). Similarly, references to carbon and oxygen
include
within their scope respectively 110, 120, 130 and 140 and 160 and 180.
Typically, the isomers of dihydrotetrabenazine of the invention does not
contain
isotopes (such as 110 or 3H) in amounts higher than their natural abundance.
In one embodiment, the percentage of the total hydrogen atoms in the (+)-a-
dihydrotetrabenazine and (-)-a-dihydrotetrabenazine that are deuterium atoms
is
less than 2%, more typically less than 1%, more usually less than 0.1%,
preferably
less than 0.05% and most preferably no more than 0.02%.
In an analogous manner, a reference to a particular functional group also
includes
within its scope isotopic variations, unless the context indicates otherwise.
The isotopes may be radioactive or non-radioactive. In one embodiment of the
invention, the isomers of dihydrotetrabenazine contain no radioactive
isotopes.
Such compounds are preferred for therapeutic use. In another embodiment,
however, the one or more of the isomers of dihydrotetrabenazine may contain
one
or more radioisotopes. Compounds containing such radioisotopes may be useful
in a diagnostic context.
References to the isomers of dihydrotetrabenazine include any solvates formed
by
the compounds.
Examples of solvates are solvates formed by the incorporation into the solid
state
structure (e.g. crystal structure) of the compounds of the invention of
molecules of
a non-toxic pharmaceutically acceptable solvent (referred to below as the
solvating
solvent). Examples of such solvents include water, alcohols (such as ethanol,
isopropanol and butanol) and dimethylsulphoxide. Solvates can be prepared by
recrystallising the compounds of the invention with a solvent or mixture of
solvents
containing the solvating solvent. Whether or not a solvate has been formed in
any
given instance can be determined by subjecting crystals of the compound to
analysis using well known and standard techniques such as thermogravimetric
analysis (TGA), differential scanning calorimetry (DSC) and X-ray
crystallography.
The solvates can be stoichiometric or non-stoichiometric solvates.
Particular examples of solvates are hydrates such as hemihydrates,
monohydrates
and dihydrates.
CA 03057548 2019-09-23
WO 2018/178243
PCT/EP2018/058088
54
For a more detailed discussion of solvates and the methods used to make and
characterise them, see Bryn et al., Solid-State Chemistry of Drugs, Second
Edition,
published by SSCI, Inc of West Lafayette, IN, USA, 1999, ISBN 0-967-06710-3.
Alternatively, rather than existing as a hydrate, the compound of the
invention may
be anhydrous. Therefore, in another embodiment, one or more of the isomers of
dihydrotetrabenazine are in an anhydrous form.
Methods for the Preparation of the Dihydrotetrabenazine Isomers
(+)-a-Dihydrotetrabenazine and (-)-a-dihydrotetrabenazine can be prepared from
tetrabenazine according to the synthetic route shown in Scheme 1.
CA 03057548 2019-09-23
WO 2018/178243 PCT/EP2018/058088
0 0
N + N
0 sH 0 H)
H s= Hssµs
0 0
(RR) (SS)
NaBH4
Y
0 0
N + N
0 H 0
H sss H"s% H)
15H OH
(RRR) (SSS)
0 0
N + N
Hss Hs
OH H
(SRR) (RSS)
Resolution of isomers
(I) (II)
Scheme 1
Racemic tetrabenazine (3-isobuty1-9,10-dimethyoxy-1,3,4,6,7,11b-hexahydro-2H-
pyrido[2,1,a]isoquinolin-2-one) containing the RR and SS isomers of
tetrabenazine
5 is reduced with sodium
borohydride to afford a mixture of four
dihydrotetrabenazine isomers of which a racemic mixture of the a-
dihydrotetrabenazine (RRR and SSS isomers) constitutes the major product and a
CA 03057548 2019-09-23
WO 2018/178243
PCT/EP2018/058088
56
racemic mixture of the 6-dihydrotetrabenazines (the SRR and RSS isomers)
constitutes a minor product. The 6-dihydrotetrabenazines can be removed during
an initial purification procedure, for example by chromatography or
recrystallization
and then the racemic a-dihydrotetrabenazines resolved by well known methods
such as chiral chromatography or the formation of diastereoisomeric salts by
reaction with chiral acids followed by separation by recrystallisation.
For example, by recrystallisation of the racemic mixture with di-p-toluoyl-L-
tartaric
acid or (R)-(-)-camphorsulfonic acid or by chiral chromatography, the (+)-a-
dihydrotetrabenazine isomer (1) ((2R, 3R, 11bR)-3-isobuty1-9,10-dimethoxy-
1,3,4,6,7,11b-hexahydro-2H-pyrido[2,1,a]isoquinolin-2-ol) can be obtained.
By recrystallisation of the racemic mixture with di-p-toluoyl-R-tartaric acid
or (L)-
(+)-camphorsulfonic acid or by chiral chromatography, the (-)-a-
dihydrotetrabenazine isomer (1) ((2S, 3S, 11bS)-3-isobuty1-9,10-dimethoxy-
1,3,4,6,7,11b-hexahydro-2H-pyrido[2,1,a]isoquinolin-2-ol) can be obtained.
(+)-a-Dihydrotetrabenazine and (-)-a-dihydrotetrabenazine can also be prepared
according to Yao etal., "Preparation and evaluation of tetrabenazine
enantiomers
and all eight stereoisomers of dihydrotetrabenazine as VMAT2 inhibitors", Eur.
J.
Med. Chem., (2011), 46, pp. 1841 ¨1848.
(+)-I3-Dihydrotetrabenazine (compound of formula (Ill)) can be prepared from
tetrabenazine according to the synthetic route shown in Scheme 2.
CA 03057548 2019-09-23
WO 2018/178243
PCT/EP2018/058088
57
O 0
N + N
0 sH 0
0 0
(RR) (SS)
L-Selectride
O 0
N + N
0 sH 0
Hoo H)
H ss
OH aH
(SRR) (RSS)
O 0
N + N
0 sH 0
H00 H)
H ss
(5H OH
(RRR) (SSS)
Resolution of isomers
(III)
Scheme 2
Racemic tetrabenazine (3-isobuty1-9,10-dimethyoxy-1,3,4,6,7,11b-hexahydro-2H-
pyrido[2,1,a]isoquinolin-2-one) containing the RR and SS isomers of
tetrabenazine
is reduced with sodium borohydride to afford a mixture of four
dihydrotetrabenazine isomers of which a racemic mixture of the 13-
CA 03057548 2019-09-23
WO 2018/178243
PCT/EP2018/058088
58
dihydrotetrabenazines (SRR and RSS isomers) constitutes the major product and
a racemic mixture of the a-dihydrotetrabenazines (the RRR and SSS isomers)
constitutes a minor product. The a-dihydrotetrabenazines can be removed during
an initial purification procedure, for example by chromatography or
recrystallization
and then the racemic 13-dihydrotetrabenazines resolved (e.g. by
recrystallisation
with di-p-toluoyl-L-tartaric acid or (R)-(-)-camphorsulfonic acid or by chiral
chromatography), to afford (+)13-dihydrotetrabenazine (Ill) ((2S, 3R, 11bR)-3-
isobuty1-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2H-pyrido[2,1,a]isoquinolin-2-
01).
The stereochemical configuration of (+)13-dihydrotetrabenazine can be
determined, so example by forming a salt such as the mesylate salt in
crystalline
form and the structure identified by X-ray crystallography.
(+)-a-Dihydrotetrabenazine, (-)-a-dihydrotetrabenazine and (+)13-
dihydrotetrabenazine can also be prepared according to Yao etal., "Preparation
and evaluation of tetrabenazine enantiomers and all eight stereoisomers of
dihydrotetrabenazine as VMAT2 inhibitors", Eur. J. Med. Chem., (2011), 46, pp.
1841 ¨ 1848.
Once prepared and purified, the (+)I3-dihydrotetrabenazine, (-)-a-
dihydrotetrabenazine and, where present the (+)-a-dihydrotetrabenazine, or
their
respective salts, can be mixed in the required proportions.
Pharmaceutical Formulations and Methods of Treatment
The pharmaceutical unit dosage forms of the invention can be in any form
suitable
for oral, parenteral, topical, intranasal, intrabronchial, ophthalmic, otic,
rectal, intra-
vaginal, or transdermal administration. Where the compositions are intended
for
parenteral administration, they can be formulated for intravenous,
intramuscular,
.. intraperitoneal, subcutaneous administration or for direct delivery into a
target
organ or tissue by injection, infusion or other means of delivery.
Pharmaceutical unit dosage forms suitable for oral administration include
tablets,
capsules, caplets, pills, lozenges, syrups, solutions, sprays, powders,
granules,
elixirs and suspensions, sublingual tablets, sprays, wafers or patches and
buccal
patches.
Particular examples of pharmaceutical unit dosage forms containing the
combinations of the invention are capsules and tablets.
CA 03057548 2019-09-23
WO 2018/178243
PCT/EP2018/058088
59
Pharmaceutical unit dosage forms containing the combinations of the invention
can
be formulated in accordance with known techniques, see for example,
Remington's
Pharmaceutical Sciences, Mack Publishing Company, Easton, PA, USA.
Thus, tablet compositions can contain a unit dosage of the combination of
active
compounds together with an inert diluent or carrier such as a sugar or sugar
alcohol, e.g.; lactose, sucrose, sorbitol or mannitol; and/or a non-sugar
derived
diluent such as sodium carbonate, calcium phosphate, talc, calcium carbonate,
or
a cellulose or derivative thereof such as methyl cellulose, ethyl cellulose,
hydroxypropyl methyl cellulose, and starches such as corn starch. Tablets may
also contain such standard ingredients as binding and granulating agents such
as
polyvinylpyrrolidone, disintegrants (e.g. swellable crosslinked polymers such
as
crosslinked carboxymethylcellulose), lubricating agents (e.g. stearates),
preservatives (e.g. parabens), antioxidants (e.g. BHT), buffering agents (for
example phosphate or citrate buffers), and effervescent agents such as
citrate/bicarbonate mixtures. Such excipients are well known and do not need
to
be discussed in detail here.
Capsule formulations may be of the hard gelatin or soft gelatin variety and
can
contain the active component in solid, semi-solid, or liquid form. Gelatin
capsules
can be formed from animal gelatin or synthetic or plant derived equivalents
thereof.
The solid dosage forms (e.g.: tablets, capsules etc.) can be coated or un-
coated,
but typically have a coating, for example a protective film coating (e.g. a
wax or
varnish) or a release controlling coating. The coating (e.g. a Eudragit TM
type
polymer) can be designed to release the active component at a desired location
within the gastro-intestinal tract. Thus, the coating can be selected so as to
degrade under certain pH conditions within the gastrointestinal tract, thereby
selectively release the compound in the stomach or in the ileum or duodenum.
Instead of, or in addition to, a coating, the isomers of dihydrotetrabenazine,
or
pharmaceutically acceptable salts thereof making up the combinations of the
invention can be presented in a solid matrix comprising a release controlling
agent,
for example a release delaying agent which may be adapted to selectively
release
the compound under conditions of varying acidity or alkalinity in the
gastrointestinal
tract. Alternatively, the matrix material or release retarding coating can
take the
form of an erodible polymer (e.g. a maleic anhydride polymer) which is
CA 03057548 2019-09-23
WO 2018/178243
PCT/EP2018/058088
substantially continuously eroded as the dosage form passes through the
gastrointestinal tract.
Compositions for topical use include ointments, creams, sprays, patches, gels,
liquid drops and inserts (for example intraocular inserts). Such compositions
can
5 be formulated in accordance with known methods.
Compositions for parenteral administration are typically presented as sterile
aqueous or oily solutions or fine suspensions, or may be provided in finely
divided
sterile powder form for making up extemporaneously with sterile water for
injection.
Examples of formulations for rectal or intra-vaginal administration include
10 pessaries and suppositories which may be, for example, formed from a
shaped
mouldable or waxy material containing the active compound.
Compositions for administration by inhalation may take the form of inhalable
powder compositions or liquid or powder sprays, and can be administrated in
standard form using powder inhaler devices or aerosol dispensing devices. Such
15 devices are well known. For administration by inhalation, the powdered
formulations typically comprise the combination of dihydrotetrabenazine
isomers,
or pharmaceutically acceptable salts thereof together with an inert solid
powdered
diluent such as lactose.
The isomers of dihydrotetrabenazine, and their respective salts can be
formulated
20 separately and used in combination, or they can be formulated together.
When
formulated together, they can be provided as a mixture to which one or more
pharmaceutical excipients is (are) added before processing (e.g. compressing
to
form a tablet or filling into a capsule) to form a pharmaceutical composition
such as
a unit dosage form. Alternatively, they can be added separately to an
excipient or
25 mixture of excipients and processed together. In a further alternative,
at least some
of the dihydrotetrabenazine isomers can be formulated separately in different
granules, pellets, microbeads or mini-tablets and then brought together and
processed to give a pharmaceutical composition (e.g. by filling into a capsule
or
compressing to form a tablet). As another alternative, the different isomers
of the
30 dihydrotetrabenazine isomers can be contained within different layers in
a multi-
layered tablet.
CA 03057548 2019-09-23
WO 2018/178243
PCT/EP2018/058088
61
Particular pharmaceutical compositions of the invention are compositions
selected
from:
= Sublingual compositions;
= Intranasal;
= Pellets or tablets formulated to provide release kinetics corresponding
to
zero order release of the active compound;
= Pellets or tablets formulated to provide first fast release followed by
constant rate release (zero order) of the active compound;
= Pellets or tablets formulated to provide a mixture of first order and
zero
order release of the active compound; and
= Pellets or tablets formulated to provide a combination of zero order and
first
order release of the active compound; and optionally a further order of
release of the active compound selected from second, third and fourth
orders of release and combinations thereof.
Pellets and tablets formulated to provide release kinetics of the types
defined
above can be prepared according to methods well known the skilled person; for
example as described in Remington's Pharmaceutical Sciences (idem) and
"Remington - The Science and Practice of Pharmacy, 21st edition, 2006, ISBN 0-
7817-4673-6.
The combinations of the invention will generally be presented in
pharmaceutical
unit dosage form and, as such, will typically contain sufficient compound to
provide
a desired level of biological activity, as described above.
The combinations of the invention will be administered to a subject (patient)
in
need thereof (for example a human or animal patient) in an amount sufficient
to
.. achieve the desired therapeutic effect, as described above.
Brief Description of the Drawings
Figure 1 shows the average total distance travelled by rats when treated with
vehicle (with or without amphetamine induction) and (-)-a-dihydrotetrabenazine
at
a dose of 2.5 mg/kg and risperidone at a dose of 1 mg/kg in amphetamine-
induced
rats, as described in Example 2, Study 1 below.
CA 03057548 2019-09-23
WO 2018/178243
PCT/EP2018/058088
62
Figure 2 shows the average total stereotypic behaviour by rats when treated
with
vehicle (with or without amphetamine induction) and (-)-a-dihydrotetrabenazine
at
a dose of 2.5 mg/kg and risperidone at a dose of 1 mg/kg in amphetamine-
induced
rats, as described in Example 2, Study 1 below.
Figure 3 shows the average total distance travelled by rats when treated with
vehicle (with or without amphetamine induction) and (+)-a-dihydrotetrabenazine
at
doses of 0.1 mg/kg and 0.25 mg/kg and risperidone at a dose of 1 mg/kg in
amphetamine-induced rats, as described in Example 2, Study 2 below.
Figure 4 shows the average total stereotypic behaviour by rats when treated
with
vehicle (with or without amphetamine induction) and (+)-a-dihydrotetrabenazine
at
doses of 0.1 mg/kg and 0.25 mg/kg and risperidone at a dose of 1 mg/kg in
amphetamine-induced rats, as described in Example 2, Study 2 below.
Figure 5 shows the average total distance travelled by rats when treated with
vehicle (with or without amphetamine induction) and (-)-a-dihydrotetrabenazine
at
a dose of 2 mg/kg, a combination of (+)-a-dihydrotetrabenazine at a dose of 2
mg/kg and (-)-a-dihydrotetrabenazine at a dose of 2 mg/kg, and risperidone at
a
dose of 1 mg/kg in amphetamine-induced rats, as described in Example 2, Study
3
below.
Figure 6 shows the average total stereotypic behaviour by rats when treated
with
vehicle (with or without amphetamine induction) and (-)-a-dihydrotetrabenazine
at
a dose of 2 mg/kg, a combination of (+)-a-dihydrotetrabenazine at a dose of 2
mg/kg and (-)-a-dihydrotetrabenazine at a dose of 2 mg/kg, and risperidone at
a
dose of 1 mg/kg in amphetamine-induced rats, as described in Example 2, Study
3
below.
Figure 7 shows the average total distance travelled by rats when treated with
vehicle (with or without amphetamine induction) and combinations of (+)-a-
dihydrotetrabenazine and (-)-a-dihydrotetrabenazine in varying ratios, and
risperidone at a dose of 1 mg/kg in amphetamine-induced rats, as described in
Example 2, Study 4 below.
Figure 8 shows the average total stereotypic behaviour by rats when treated
with
vehicle (with or without amphetamine induction) and combinations of (+)-a-
dihydrotetrabenazine and (-)-a-dihydrotetrabenazine in varying ratios, and
CA 03057548 2019-09-23
WO 2018/178243
PCT/EP2018/058088
63
risperidone at a dose of 1 mg/kg in amphetamine-induced rats, as described in
Example 2, Study 4 below.
Figure 9 shows the average total distance travelled by rats when treated with
vehicle (with or without amphetamine induction), (+)13-dihydrotetrabenazine
and
combinations of (+)-a-dihydrotetrabenazine and (+)13-dihydrotetrabenazine in
amphetamine-induced rats, as described in Example 2, Study 5 below.
Figure 10 shows the average total stereotypic behaviour by rats when treated
with
vehicle (with or without amphetamine induction), (+)13-dihydrotetrabenazine
and
combinations of (+)-a-dihydrotetrabenazine and (+)13-dihydrotetrabenazine in
amphetamine-induced rats, as described in Example 2, Study 5 below.
Figure 11 shows the average total distance travelled by rats when treated with
vehicle (with or without amphetamine induction), (+)-a-dihydrotetrabenazine
alone,
(+)-a-dihydrotetrabenazine in combination with (-)13-dihydrotetrabenazine,
(+)13-
dihydrotetrabenazine in combination with (-)-a-dihydrotetrabenazine, (+)-[3-
dihydrotetrabenazine in combination with (-)13-dihydrotetrabenazine, and (+)-a-
dihydrotetrabenazine in combination with (+)13-dihydrotetrabenazine in
amphetamine-induced rats, as described in Example 2, Study 6 below.
Figure 12 shows the stereotypic behaviour (distance over time) by rats when
treated with vehicle (with or without amphetamine induction), (+)-a-
.. dihydrotetrabenazine alone, (+)-a-dihydrotetrabenazine in combination with
(-)13-
dihydrotetrabenazine, (+)13-dihydrotetrabenazine in combination with (-)-a-
dihydrotetrabenazine, (+)13-dihydrotetrabenazine in combination with (-)--
dihydrotetrabenazine, and (+)-a-dihydrotetrabenazine in combination with ()-I3-
dihydrotetrabenazine in amphetamine-induced rats, as described in Example 2,
Study 6 below.
EXAMPLES
The following non-limiting examples illustrate the synthesis and properties of
the
compositions of the invention.
EXAMPLE 1
An investigation into the nature of the dihydrotetrabenazine metabolites
formed
after administration of tetrabenazine to human subjects
CA 03057548 2019-09-23
WO 2018/178243 PCT/EP2018/058088
64
A pharmacokinetic study was carried out in healthy adult male volunteers under
fasting conditions at a dose of single and multiple oral administration of
25mg
tablets once a day to ascertain the plasma levels of +/-a and +113 dihydro-
tetrabenazine. The data are summarised below.
Table 1 summarises the pharmacokinetic data obtained following single-dose
oral
administration of tetrabenazine at a dose level of 25 mg (fasting, N = 08).
Table 1
Analyte T' C AUC(0-t) AUC(0-inf.) Kei
Half-life Extrapolated
Mean '
(h) (ng/mL) (ng.h/mL) (ng.h/mL) NA (h)
Tetrabenazine 0.87 0.58 1.87 2.42 0.19 4.35 27.54
(+) a-DHTBZ 1.16 2.61 7.98 10.83 0.17 4.79 32.10
(-) a-DHTBZ 0.938 72.94 305.84 351,80 0.10 7.89 10.59
(+) 6-DHTBZ 1.125 103.00 375.78 410.46 0.13
5.80 5.03
(-) 6-DHTBZ 1.03 5.28 16.28 18.77 0.45 12.98 17.66
Table 2 summarises the pharmacokinetic data obtained following multiple-dose
oral administration of tetrabenazine at a dose level of 25mg (fasting, N =
07).
Table 2
T
Analyte maxss c max" cminss AUC(0-t) Otaa
Cavg
Mean
(h) (ng/mL) (ng/mL) (ng.h/mL) (ng/mL) (ng.h/mL)
Tetrabenazine 96.89 0.73 0.01 2.79 0.10 0.12
(+) a-DHTBZ 97.18 3.31 0.00 13.74 0.44 0.57
(-) a-DHTBZ 96.96 98.34 5.61 474.17 6.10 19.76
(+) 6-DHTBZ 97.11 144.76 5.45 598.76 5.54 24.95
(-) 6-DHTBZ 97.11 7.78 0.16 25.17 0.57 1.05
CA 03057548 2019-09-23
WO 2018/178243
PCT/EP2018/058088
The data presented in Tables 1 and 2 demonstrate that, in humans, the major
metabolites are the (-)-a-dihydrotetrabenazine isomer, which is essentially
active
as a VMAT2 binding agent, and the (+)-6-dihydrotetrabenazine isomer, which is
significantly less active than the (+)-a-dihydrotetrabenazine isomer. (-)-6-
5 Dihydrotetrabenazine and (+)-a-dihydrotetrabenazine were shown to be
minor
metabolites
The data suggest that (+)-a-dihydrotetrabenazine is not primarily responsible
for
the therapeutic properties of tetrabenazine. On the contrary, it appears that
(+)-a-
dihydrotetrabenazine may be responsible for a relatively small contribution to
the
10 therapeutic properties of tetrabenazine.
EXAMPLE 2
Investigation of the effect of combinations of (+)-a-dihydrotetrabenazine and
(-)-a-
dihydrotetrabenazine on locomotor activity and stereotypies in rats
The effects of combinations of (+)-a-dihydrotetrabenazine and (-)-a-
15 dihydrotetrabenazine on locomotor activity and stereotypies in rats were
investigated and compared to the effects of the individual (+)-a-
dihydrotetrabenazine and (-)-a-dihydrotetrabenazine isomers.
MATERIALS AND METHODS
Equipment
20 Open field arena, Med Associates Inc.
Plastic syringes 1 ml, Terumo. Ref: 55-01T1
Animal feeding needle 15 G, lnstech Solomon, Cat: 72-4446
Sartorius Mechatronics Scale A22101, Sartorius Weighting Technology, Germany
Needle 27 G Terumo Myjector, 0,5 ml, Ref: 8300010463
25 Plastic syringes 3 ml, Soft-Ject, Ref: 8300005761
BD Microtainer K2EDTA tubes Ref: 365975
Matrix 0,75 ml, Alphanum Tubes, Thermo Scientific, Ref: 4274
Microplate Devices, Uniplate 24 wells, 10 ml, Ref: 734-1217
Thermo Electron Corp. Heraeus Fresco 17, refrigerated centrifuge
30 Test Animals
CA 03057548 2019-09-23
WO 2018/178243
PCT/EP2018/058088
66
All animal experiments were carried out according to the National Institute of
Health (NIH) guidelines for the care and use of laboratory animals, and
approved
by the National Animal Experiment Board, Finland. Male CD (Charles River
Laboratories, Germany) at weight range of 200-250 g (165-200 g upon arrival)
were used for the experiments. Animals were housed at a standard temperature
(22 1 C) and in a light-controlled environment (lights on from 7 am to 8 pm)
with
ad libitum access to food and water.
Methods
Locomotor activity of the rats was tested in open field arena. The open field
test
was performed during the rat light cycle and under a normal lighting evenly
distributed to the test chambers. The paths of the rats were recorded by
activity
monitor (Med. Associates Inc.).
Dosing the vehicle, amphetamine, (+)-a-DHTBZ, (-)-a-DHTBZ,(+)-6-DHTBZ, (+6-
DHTBZ or risperidone was done prior to LMA test. The rats were placed in the
center of the arena, and the path was recorded for 30 minutes. After 30
minutes of
testing vehicle or amphetamine was dosed and the rat was placed in the center
of
the arena, and the path was recorded for 60 minutes, the total testing time
being
90 minutes.
Endpoint, Blood Samples and Tissue Processing
Within 10 minutes from the end of the test animals were euthanized by an
overdose of CO2. The terminal blood sample was collected with cardiac puncture
from all compound treated rats from each group excluding vehicle rats. 0.5 ml
of
blood was collected with syringe attached to 18 G needle and moved into
precooled K2-EDTA microtubes. The EDTA microtube was inverted several times
to mix up the EDTA and blood. Tubes were then immediately put on wet ice and
centrifuged (Heraeus Fresco 17) within 10-15 minutes of collecting (9.6 x1000
G/
10 x 1000 RPM, +4 C for 2 min), and 200 pl of plasma was collected in 96-tube
plates (Matrix Technologies ScreenMates 0.75 ml Alphanumeric Round-Bottom
Storage tubes, PP) on dry ice according to sample map.
After collection of blood the neck was dislocated at the base of the skull.
Brain was
collected and weighed. Brain weights were recorded and the brain was frozen on
dry ice on the 24 well plate.
The plasma and brain samples were stored at ¨80 C on dry ice until sent for
analysis.
CA 03057548 2019-09-23
WO 2018/178243 PCT/EP2018/058088
67
STUDY 1
Animals were grouped as follows:
= Group 1: 10 rats treated with Vehicle (t= 0 min) and Vehicle (t= 30 min)
= Group 2: 10 rats treated with Vehicle (t= 0 min) and Amphetamine (t= 30
min)
= Group 3: 10 rats treated with (-)-a-DHTBZ 2.5 mg/kg (t=0 min) and
Amphetamine (t= 30 min)
= Group 4: 10 rats treated with risperidone 1 mg/kg (t=0 min) and
Amphetamine (t= 30 min)
Results
1. Distance Travelled
Rats dosed with either vehicle, (-)-a-DHTBZ 2.5 mg/kg or risperidone 1 mg/kg
were
subjected to LMA testing first for 30 min and then for 60 minutes after
vehicle or
amphetamine challenge. Resulting locomotor activity was evaluated in 3 min
bins
and as a total over the testing period. The normalised total distance
travelled over
the testing time is presented in Figure 1.
When compared to the vehicle-vehicle group the vehicle-amphetamine and (-)-a-
DHTBZ 2.5 mg/kg were significantly different. When compared to vehicle-
amphetamine group the vehicle-vehicle and risperidone 1 mg/kg were
significantly
different.
2. Stereotypic Behaviour
Rats dosed with either vehicle, (-)-a-DHTBZ 2.5 mg/kg or risperidone 1 mg/kg
were
subjected to LMA testing first for 30 min and then for 60 minutes after
vehicle or
amphetamine challenge. Resulting stereotypic activity was evaluated in 3 min
bins
and as a total over the testing period. The normalised total stereotypic
behaviour
over the testing time is presented in Figure 2.
When compared to the vehicle-vehicle group the vehicle-amphetamine and (-)-a-
DHTBZ 2.5 mg/kg were significantly different. When compared to vehicle-
amphetamine group the vehicle-vehicle and risperidone 1 mg/kg were
significantly
different.
Conclusions
CA 03057548 2019-09-23
WO 2018/178243
PCT/EP2018/058088
68
This study evaluated the effect of (-)-a-DHTBZ at dose 2.5 mg/kg and
risperidone
at dose 1 mg/kg on amphetamine induced locomotor activity in male CD rats.
(-)-a-DHTBZ at dose 2.5 mg/kg did not lead to lower locomotor activity or
reduced
stereotypic behaviour when compared to the vehicle-amphetamine group. The rats
.. dosed with (-)-a-DHTBZ at dose 2.5 mg/kg were less focused on what was
going
on around. The rats dosed with (-)-a ¨DHTBZ were equally active when compared
to the vehicle-amphetamine dosed animals suggesting that (-)-a ¨DHTBZ does not
have a sedative effect similar to risperidone.
STUDY 2
.. The effects on stereotypic behaviour and the distance travelled in rats
following
administration of (+)-a-dihydrotetrabenazine dosed at 0.1 mg/kg to 0.25 mg/kg,
as
well as risperidone at 1 mg/kg, were studied.
Animals were grouped as follows:
= Group 1: 10 rats treated with Vehicle (t= 0 min) and Vehicle (t= 30 min)
= Group 2: 10 rats treated with Vehicle (t= 0 min) and Amphetamine (t= 30
min)
= Group 3: 10 rats treated with (+)-a-DHTBZ 0.1 mg/kg (t=0 min) and
Amphetamine (t=30 min)
= Group 4: 10 rats treated with (+)-a-DHTBZ 0.25 mg/kg (t=0 min) and
Amphetamine (t=30 min)
= Group 5: 10 rats treated with risperidone 1 mg/kg (t=0 min) and
Amphetamine (t= 30 min)
Results
1 Distance Travelled
.. Rats dosed with either vehicle, (+)-a-DHTBZ 0.1 mg/kg, (+)-a-DHTBZ 0.25
mg/kg,
or Risperidone 1 mg/kg were subjected to LMA testing first for 30 min and then
for
60 minutes after vehicle or amphetamine challenge. Resulting locomotor
activity
was evaluated in 3 min bins and as a total over the testing period. The
normalised
total distance travelled over the testing time is presented in Figure 3.
.. When compared to vehicle-amphetamine group the vehicle-vehicle, (+)-a-DHTBZ
0.25 mg/kg and risperidone 1 mg/kg were significantly different.
CA 03057548 2019-09-23
WO 2018/178243
PCT/EP2018/058088
69
2 Stereotypic Behaviour
Rats dosed with either vehicle, (+)-a-DHTBZ 0.1 mg/kg, (+)-a-DHTBZ 0.25 mg/kg,
or Risperidone 1 mg/kg were subjected to LMA testing first for 30 min and then
for
60 minutes after vehicle or amphetamine challenge. Resulting stereotypic
activity
was evaluated in 3 min bins and as a total over the testing period. The
normalised
total stereotypic behaviour over the testing time is presented in Figure 4.
When compared to vehicle-amphetamine group the vehicle-vehicle, (+)-a-DHTBZ
0.1 mg/kg, (+)-a-DHTBZ 0.25 mg/kg and risperidone 1 mg/kg were significantly
different.
Conclusions
This study evaluated the effect of (+)-a-DHTBZ at doses 0.1 mg/kg and 0.25
mg/kg
and risperidone at dose 1 mg/kg on amphetamine induced locomotor activity in
male CD rats.
(+)-a-DHTBZ at 0.25 mg/kg and risperidone 1 mg/kg led to lower locomotor
activity
when compared to the vehicle-amphetamine group. (+)-a-DHTBZ at both the
tested doses and risperidone 1 mg/kg led to reduced stereotypic behaviour when
compared to the vehicle-amphetamine group.
STUDY 3
Animals were grouped as follows:
Group 1: 10 rats treated with Vehicle (t= 0 min) and Vehicle (t= 30 min)
Group 2: 10 rats treated with Vehicle (t= 0 min) and Amphetamine (t= 30 min)
Group 3: 10 rats treated with (+)-a-DHTBZ 2 mg/kg (t=0 min) and Amphetamine
(t=
min)
Group 4: 10 rats treated with (+)-a-DHTBZ 2mg/kg with (-)-a-DHTBZ 2mg/kg (t=0
25 min) and Amphetamine (t=30 min)
Group 5: 10 rats treated with risperidone 1 mg/kg (t=0 min) and Amphetamine
(t=
30 min)
Results
1 Distance Travelled
30 Rats dosed with either vehicle, (+)-a-DHTBZ 2 mg/kg, the combination of
(-)-a-
DHTBZ 2 mg/kg and (+)-a-DHTBZ 2 mg/kg or Risperidone 1 mg/kg were subjected
CA 03057548 2019-09-23
WO 2018/178243
PCT/EP2018/058088
to LMA testing first for 30 min and then for 60 minutes after vehicle or
amphetamine challenge. Resulting locomotor activity was evaluated in 3 min
bins
and as a total over the testing period. The normalised total distance
travelled over
the testing time is presented in Figure 5.
5 When compared to the vehicle-vehicle group the vehicle-amphetamine was
significantly different. When compared to vehicle-amphetamine group the
vehicle-
vehicle, (+)-a-DHTBZ 2 mg/kg, the combination of (-)-a-DHTBZ 2 mg/kg and (+)-a-
DHTBZ 2 mg/kg and risperidone 1 mg/kg were significantly different.
2 Stereotypic Behaviour
10 Rats dosed with either vehicle, (+)-a-DHTBZ 2 mg/kg, the combination of
(-)-a-
DHTBZ 2 mg/kg and (+)-a-DHTBZ 2 mg/kg or Risperidone 1 mg/kg were subjected
to LMA testing first for 30 min and then for 60 minutes after vehicle or
amphetamine challenge. Resulting stereotypic activity was evaluated in 3 min
bins
and as a total over the testing period. The normalised total stereotypic
behaviour
15 over the testing time is presented in Figure 6.
When compared to vehicle-amphetamine group the vehicle-vehicle, (+)-a-DHTBZ
2 mg/kg, the combination of (-)-a-DHTBZ 2 mg/kg and (+)-a-DHTBZ 2 mg/kg and
risperidone 1 mg/kg were significantly different.
Conclusions
20 This study evaluated the effect of compounds (+)-a-DHTBZ at a dose of 2
mg/kg,
the combination of (+)-a-DHTBZ and (-)-a-DHTBZ at dose 2 mg/kg and risperidone
at dose 1 mg/kg on amphetamine induced locomotor activity in male CD rats.
(+)-a-DHTBZ at the tested dose, the combination of (+)-a-DHTBZ and (-)-a-DHTBZ
at doses 2 mg/kg and risperidone 1 mg/kg led to lower locomotor activity when
25 compared to the vehicle-amphetamine group. (+)-a-DHTBZ at the tested
dose, the
combination of (+)-a-DHTBZ and (-)-a-DHTBZ at doses 2 mg/kg and risperidone 1
mg/kg led to reduced stereotypic behaviour when compared to the vehicle-
amphetamine group.
Amphetamine induced locomotor activity was less in rats treated with the
30 combination of (+)-a-DHTBZ and (-)-a-DHTBZ than in rats treated with (+)-
a-
DHTBZ only, despite it being shown that the (-)-a-isomer provides very little,
if any,
reduction induced locomotor activity.
STUDY 4
CA 03057548 2019-09-23
WO 2018/178243
PCT/EP2018/058088
71
Animals were grouped as follows:
= Group 1: 10 rats treated with Vehicle (t= 0 min) and Vehicle (t= 30 min)
= Group 2: 10 rats treated with Vehicle (t= 0 min) and Amphetamine (t= 30
min)
= Group 3: 10 rats treated with (+)-a-DHTBZ 0.5 mg/kg (t=0 min) and (-)-a-
DHTBZ 0.5 mg/kg and Amphetamine (t=30 min)
= Group 4: 10 rats treated with (+)-a-DHTBZ 1.0 mg/kg (t=0 min) and (-)-a-
DHTBZ 0.5 mg/kg and Amphetamine (t=30 min)
= Group 5: 10 rats treated with (+)-a-DHTBZ 1.0 mg/kg (t=0 min) and (-)-a-
DHTBZ 1.0 mg/kg and Amphetamine (t=30 min)
= Group 6: 10 rats treated with (+)-a-DHTBZ 1.5 mg/kg (t=0 min) and (-)-a-
DHTBZ 1.0 mg/kg and Amphetamine (t=30 min)
Results
1 Distance Travelled
.. Rats dosed with either vehicle, the combination of (+)-a-DHTBZ 0.5 mg/kg
and (-)-
a-DHTBZ 0.5 mg/kg, the combination of (+)-a-DHTBZ 1 mg/kg and (-)-a-DHTBZ
0.5 mg/kg, the combination of (+)-a-DHTBZ 1 mg/kg and (-)-a-DHTBZ 1 mg/kg or
the combination of (+)-a-DHTBZ 1.5 mg/kg and (-)-a-DHTBZ 1 mg/kg were
subjected to LMA testing first for 30 min and then for 60 minutes after
vehicle or
.. amphetamine challenge. Resulting locomotor activity was evaluated in 3 min
bins
and as a total over the testing period. The normalised total distance
travelled over
the testing time is presented in Figure 7.
When compared to vehicle-amphetamine group the vehicle-vehicle, (+)-a-DHTBZ,
the combination of (+)-a-DHTBZ 0.5 mg/kg and (-)-a-DHTBZ 0.5 mg/kg, the
combination of (+)-a-DHTBZ 1 mg/kg and (-)-a-DHTBZ 0.5 mg/kg, the combination
of (+)-a-DHTBZ 1 mg/kg and (-)-a-DHTBZ 1 mg/kg and the combination of (+)-a-
DHTBZ 1.5 mg/kg and (-)-a-DHTBZ 1 mg/kg were significantly different.
2 Stereotypic Behaviour
Rats dosed with either vehicle, the combination of (+)-a-DHTBZ 0.5 mg/kg and (-
)-
a-DHTBZ 0.5 mg/kg, the combination of (+)-a-DHTBZ 1 mg/kg and (-)-a-DHTBZ
0.5 mg/kg, the combination of (+)-a-DHTBZ 1 mg/kg and (-)-a-DHTBZ 1 mg/kg or
the combination of (+)-a-DHTBZ 1.5 mg/kg and (-)-a-DHTBZ 1 mg/kg were
subjected to LMA testing first for 30 min and then for 60 minutes after
vehicle or
CA 03057548 2019-09-23
WO 2018/178243
PCT/EP2018/058088
72
amphetamine challenge. Resulting stereotypic activity was evaluated in 3 min
bins
and as a total over the testing period. The normalised total stereotypic
behaviour
over the testing time is presented in Figure 8.
When compared to the vehicle-vehicle group the combination of (+)-a-DHTBZ 1
mg/kg and (-)-a-DHTBZ 0.5 mg/kg was significantly different. When compared to
vehicle-amphetamine group the vehicle-vehicle, the combination of (+)-a-DHTBZ
0.5 mg/kg and (-)-a-DHTBZ 0.5 mg/kg, the combination of (+)-a-DHTBZ 1 mg/kg
and (-)-a-DHTBZ 0.5 mg/kg, the combination of (+)-a-DHTBZ 1 mg/kg and (-)-a-
DHTBZ 1 mg/kg and the combination of (+)-a-DHTBZ 1.5 mg/kg and (-)-a-DHTBZ
1 mg/kg were significantly different.
Conclusions
This study evaluated the effect of the combination of (+)-a-DHTBZ and (-)-a-
DHTBZ at doses of 0.5 mg/kg + 0.5 mg/kg, 1 mg/kg + 0.5 mg/kg, 1 mg/kg + 1
mg/kg and 1.5 mg/kg + 1 mg/kg on amphetamine induced locomotor activity in
male CD rats.
The combination of (+)-a-DHTBZ and (-)-a-DHTBZ at all the tested combinations
and risperidone 1 mg/kg led to lower locomotor activity when compared to the
vehicle-amphetamine group. The combination of (+)-a-DHTBZ and (-)-a-DHTBZ at
all the tested doses and risperidone 1 mg/kg led to reduced stereotypic
behaviour
when compared to the vehicle-amphetamine group.
It would appear that there are interactions between the (+)-a-DHTBZ and (-)-a-
DHTBZ affecting the ability of (+)-a-DHTBZ to block the amphetamine induced
hyperactivity.
Comparing the data for rats dosed with a combination of (+)-a-DHTBZ at a dose
of
lmg/kg and (-)-a-DHTBZ at a dose of 0.5 mg/kg and rats dosed with a
combination
of (+)-a-DHTBZ at a dose of 1mg/kg and (-)-a-DHTBZ at a dose of 1 mg/kg, given
the demonstrated lack of efficiency of the (-)-a-isomer in isolation, it would
not be
expected that increases in the amount of (-)-a-isomer in the combination
treatment
would lead to a reduction in the locomotor activity in the tested rats.
STUDY 5
Animals were grouped as follows:
= Group 1: 10 rats treated with Vehicle (t= 0 min) and Vehicle (t= 30 min)
CA 03057548 2019-09-23
WO 2018/178243
PCT/EP2018/058088
73
= Group 2: 10 rats treated with Vehicle (t= 0 min) and Amphetamine (t= 30
min)
= Group 3: 10 rats treated with (+)-6-DHTBZ 2.5 mg/kg (t=0 min) and
Amphetamine (t=30 min)
= Group 4: 10 rats treated with (+)-6-DHTBZ 5 mg/kg (t=0 min) and
Amphetamine (t=30 min)
= Group 5: 10 rats treated with and (+)-6-DHTBZ 2.5 mg/kg and (+)-a-DHTBZ
2.5 mg/kg (t=0 min) and Amphetamine (t=30 min)
Results
1 Distance Travelled
Rats dosed with either vehicle, (+)-6-DHTBZ 2.5 mg/kg, (+)-6-DHTBZ 5 mg/kg or
(+)-6-DHTBZ 2.5 mg/kg and (+)-a-DHTBZ 2.5 mg/kg were subjected to LMA
testing first for 30 minutes and then for 60 minutes after vehicle or
amphetamine
challenge. Resulting locomotor activity was evaluated in 3 minute bins and as
a
total over the testing period. The normalised total distance travelled over
the
testing time is presented in Figure 9.
When compared to the vehicle-vehicle group the vehicle-amphetamine, (+)-6-
DHTBZ 2.5 mg/kg and (+)-6-DHTBZ 5 mg/kg. When compared to vehicle-
amphetamine group the vehicle-vehicle, the combination of (+)-6-DHTBZ 2.5
mg/kg and (+)-a-DHTBZ 2.5 mg/kg, (+)-6-DHTBZ 2.5 mg/kg and (+)-6-DHTBZ 5
mg/kg, were significantly different.
2 Stereotypic Behaviour
Rats dosed with either vehicle, (+)-6-DHTBZ 2.5 mg/kg, (+)-6-DHTBZ 5 mg/kg or
(+)-6-DHTBZ 2.5 mg/kg and (+)-a-DHTBZ 2.5 mg/kg were subjected to LMA
testing first for 30 minutes and then for 60 minutes after vehicle or
amphetamine
challenge. Resulting stereotypic activity was evaluated in 3 minute bins and
as a
total over the testing period. The normalised total stereotypic behaviour over
the
testing time is presented in Figure 10.
When compared to the vehicle-vehicle group the vehicle-amphetamine, (+)-6-
DHTBZ 2.5 mg/kg and (+)-6-DHTBZ 5 mg/kg were significantly different. When
compared to vehicle-amphetamine group the vehicle-vehicle, the combination of
CA 03057548 2019-09-23
WO 2018/178243
PCT/EP2018/058088
74
(+)-6-DHTBZ 2.5 mg/kg and (+)-a-DHTBZ 2.5 mg/kg, (+)-6-DHTBZ 2.5 mg/kg and
(+)-6-DHTBZ 5 mg/kg were significantly different.
STUDY 6
Animals were grouped as follows:
= Group 1: 10 rats treated with Vehicle (t= 0 min) and Vehicle (t= 30 min)
= Group 2: 10 rats treated with Vehicle (t= 0 min) and Amphetamine (t= 30
min)
= Group 3: 10 rats treated with (+)-a-DHTBZ 1 mg/kg (t=0 min) and
amphetamine (t =30 min)
= Group 4: 10 rats treated with (+)-a-DHTBZ 1 mg/kg plus (-)-a-DHTBZ 1
mg/kg (t=0 min); and amphetamine (t=30 min)
= Group 5: 10 rats treated with (+)-a-DHTBZ 1 mg/kg plus (-)-6-DHTBZ 1
mg/kg (t=0 min) and t=30 min) amphetamine
= Group 6: 10 rats treated with (+)-6-DHTBZ 1 mg/kg plus (-)-a-DHTBZ 1
mg/kg (t=0 min) and amphetamine (t=30 min)
= Group 7: 10 rats treated with (+)-6-DHTBZ 1 mg/kg plus (-)-6-DHTBZ 1
mg/kg (t=0 min); and amphetamine (t=30 min)
= Group 8: 10 rats treated with (+)-a-DHTBZ 1 mg/kg plus (+)-6-DHTBZ 1
mg/kg (t=0 min); and amphetamine (t=30 min)
= Group 9: 10 rats treated with risperidone 1 mg/kg (t=0 min); and
amphetamine (t=30 min)
Results
1 Distance Travelled
Rats dosed with either vehicle or dihydrotetrabenazine were subjected to LMA
testing first for 30 minutes and then for 60 minutes after vehicle or
amphetamine
challenge. Resulting locomotor activity was evaluated in 3 minute bins and as
a
total over the testing period. The unnormalised total distance travelled over
the
testing time is presented in Figure 11.
When compared to the vehicle-vehicle group, the vehicle-amphetamine, (+)-6-
DHTBZ 1 mg/kg plus (-)-a-DHTBZ 1 mg/kg and (+)-6-DHTBZ 1 mg/kg plus (-)-[3-
CA 03057548 2019-09-23
WO 2018/178243
PCT/EP2018/058088
DHTBZ 1 mg/kg groups were significantly different. When compared to the
vehicle-
amphetamine group, the vehicle-vehicle, all of groups 1 and 3 to 9 were
significantly different.
2 Stereotypic Behaviour
5 Rats dosed with either vehicle or dihydroterabenazine were subjected to
LMA
testing first for 30 minutes and then for 60 minutes after vehicle or
amphetamine
challenge. Resulting stereotypic activity was evaluated in 3 minute bins and
as a
total over the testing period. The unnormalised total stereotypic behaviour
over the
testing time is presented in Figure 12.
10 When compared to the vehicle-vehicle group, the vehicle-amphetamine, ()-
I3-
DHTBZ 1 mg/kg plus (-)-a-DHTBZ 1 mg/kg and (+)-6-DHTBZ 1 mg/kg plus (-)--
DHTBZ 1 mg/kg groups were significantly different. When compared to the
vehicle-
amphetamine group, the vehicle-vehicle, all of groups 1 and 3 to 9 were
significantly different.
15 Comments
Study 1 evaluated the effect of (-)-a-DHTBZ at a dose of 2.5 mg/kg and
risperidone
at a dose of 1 mg/kg on amphetamine induced locomotor activity in male CD
rats.
(-)-a-DHTBZ at a dose of 2.5 mg/kg did not lead to lower locomotor activity or
reduced stereotypic behaviour when compared to the vehicle-amphetamine group.
20 The rats dosed with (-)-a-DHTBZ at a dose of 2.5 mg/kg were less focused
on
what was going on around them. The rats dosed with (-)-a ¨DHTBZ were equally
active when compared to the vehicle-amphetamine dosed animals suggesting that
(-)-a ¨DHTBZ does not have an effect on movement similar to risperidone.
Study 2 evaluated the effect of (+)-a-DHTBZ at doses 0.1 mg/kg and 0.25 mg/kg
25 and risperidone at dose 1 mg/kg on amphetamine induced locomotor
activity in
male CD rats.
(+)-a-DHTBZ at 0.25 mg/kg and risperidone 1 mg/kg led to lower locomotor
activity
when compared to the vehicle-amphetamine group. (+)-a-DHTBZ at both the
tested doses and risperidone 1 mg/kg led to reduced stereotypic behaviour when
30 compared to the vehicle-amphetamine group.
CA 03057548 2019-09-23
WO 2018/178243
PCT/EP2018/058088
76
Study 3 evaluated the effect of (+)-a-DHTBZ at a dose of 2 mg/kg, the
combination
of (+)-a-DHTBZ and (-)-a-DHTBZ at dose 2 mg/kg and risperidone at dose 1 mg/kg
on amphetamine induced locomotor activity in male CD rats.
(+)-a-DHTBZ at all the tested dose, the combination of (+)-a-DHTBZ and (-)-a-
DHTBZ at doses of 2 mg/kg and risperidone at 1 mg/kg led to lower locomotor
activity when compared to the vehicle-amphetamine group. (+)-a-DHTBZ at all
the
tested dose, the combination of (+)-a-DHTBZ and (-)-a-DHTBZ at doses of 2
mg/kg and risperidone 1 mg/kg led to reduced stereotypic behaviour when
compared to the vehicle-amphetamine group.
Amphetamine induced locomotor activity was less in rats treated with the
combination of (+)-a-DHTBZ and (-)-a-DHTBZ than in rats treated with (+)-a-
DHTBZ only, despite it being shown that the (-)-a-isomer provides very little,
if any,
reduction in induced locomotor activity.
Study 4 evaluated the effect of the combination of (+)-a-DHTBZ and (-)-a-DHTBZ
at doses 0.5 mg/kg + 0.5 mg/kg, 1 mg/kg + 0.5 mg/kg, 1 mg/kg + 1 mg/kg and 1.5
mg/kg + 1 mg/kg on amphetamine induced locomotor activity in male CD rats.
The combination of (+)-a-DHTBZ and (-)-a-DHTBZ at all the tested combinations
and risperidone 1 mg/kg led to lower locomotor activity when compared to the
vehicle-amphetamine group. The combination of (+)-a-DHTBZ and (-)-a-DHTBZ at
all the tested doses and risperidone 1 mg/kg led to reduced stereotypic
behaviour
when compared to the vehicle-amphetamine group.
Comparing the data for rats dosed with a combination of (+)-a-DHTBZ at a dose
of
lmg/kg and (-)-a-DHTBZ at a dose of 0.5 mg/kg and rats dosed with a
combination
of (+)-a-DHTBZ at a dose of 1mg/kg and (-)-a-DHTBZ at a dose of 1 mg/kg, given
the demonstrated lack of efficiency of the (-)-a-isomer in isolation, it was
surprising
that increasing the amount of (-)-a-isomer in the combination treatment led to
a
reduction in the locomotor activity in the tested rats.
Study 5 evaluated the effect of (+)13 -DHTBZ at doses 2.5 mg/kg and 5 mg/kg
and
the combination of (+)-a-DHTBZ at dose 2.5 mg/kg and (+)13-DHTBZ at dose 2.5
mg/kg on amphetamine induced locomotor activity in male CD rats.
(+)13 -DHTBZ 2.5 mg/kg, (+)13 -DHTBZ 5 mg/kg, and the combination of (+)-a-
DHTBZ 2.5 mg/kg and (+)13-DHTBZ 2.5 mg/kg led to lower locomotor activity when
compared to the vehicle-amphetamine group. (+)13 -DHTBZ 2.5 mg/kg, (+)13 -
DHTBZ 5 mg/kg and the combination of (+)-a-DHTBZ 2.5 mg/kg and (+)13-DHTBZ
CA 03057548 2019-09-23
WO 2018/178243
PCT/EP2018/058088
77
2.5 mg/kg also led to reduced stereotypic behaviour when compared to the
vehicle-amphetamine group. The rats dosed with (+)I3-DHTBZ at dose 5 mg/kg
were less focused on what was going on around them and the rats that received
the (+)I3-DHTBZ 5 mg/kg were observed to have tensed limbs and were partially
missing their righting reflex at the end of the test.
Study 6 evaluated the effect of (+)-a-DHTBZ at a dose of 1 mg/kg, a
combination
of (+)-a-DHTBZ at a dose of 1 mg/kg plus (-)-a-DHTBZ at a dose of 1 mg/kg, a
combination of (+)-a-DHTBZ at a dose of 1 mg/kg plus (-)I3-DHTBZ at a dose of
1
mg/kg, a combination of (+)I3-DHTBZ at a dose of 1 mg/kg plus (-)-a-DHTBZ at a
dose of 1 mg/kg, a combination of (+)I3-DHTBZ at a dose of 1 mg/kg plus (-)13-
DHTBZ at a dose of 1 mg/kg, a combination of (+)-a-DHTBZ at a dose of 1 mg/kg
plus (+)I3-DHTBZ at a dose of 1 mg/kg, and risperidone at a dose of 1 mg/kg
(t=0
min) on amphetamine induced locomotor activity in male CD rats.
The vehicle, risperidone and all of the dihydrotetrabenazine-containing led to
lower
locomotor activity and led to reduced stereotypic behaviour when compared to
the
vehicle-amphetamine group.
The results obtained from the six studies indicate that combinations of (+)13-
dihydrotetrabenazine, (-)-a-dihydrotetrabenazine and (+)-a-
dihydrotetrabenazine
will be useful in the treatment of movement disorders.
Equivalents
It will readily be apparent that numerous modifications and alterations may be
made to the specific embodiments of the invention described above without
departing from the principles underlying the invention. All such modifications
and
alterations are intended to be embraced by this application.