Note: Descriptions are shown in the official language in which they were submitted.
CA 03057853 2019-09-24
WO 2018/183421 PCT/US2018/024729
DEVICES AND METHODS FOR DIAGNOSIS OF SINUSITIS
CROSS REFERENCE TO RELATED APPLICATIONS
[0001] This patent application claims priority to U.S. provisional patent
application number
62/477,889, titled "DEVICES AND METHODS FOR DIAGNOSIS OF SINUSITIS" and filed
on March 28, 2017.
INCORPORATION BY REFERENCE
[0002] All publications and patent applications mentioned in this
specification are herein
incorporated by reference in their entirety to the same extent as if each
individual publication or
patent application was specifically and individually indicated to be
incorporated by reference.
FIELD
[0003] The present application relates to methods and devices for the
determination of the
presence of one or more pathogens associated with bacterial sinusitis from a
collected mucus
sample, and preferably the detection of three or more of the pathogens
associated with over 90%
of bacterial sinusitis.
BACKGROUND
[0004] Sinusitis, defined as inflammation of the sinus tissues, usually
as a complication to
viral infections from the common cold. Although there are over 1 billion
common colds in the
U.S., a small percentage of them lead to sinusitis. In fact, 29 million people
were diagnosed with
sinusitis in 2011 in the US. Often antibiotics are ordered as a treatment for
sinusitis and it is the
5th leading indication for the antibiotic prescriptions annually. Western EU
markets are
estimated to be over 43 million patients annually. The majority of these
patients are initially
seen by primary care physicians and then referred out to otolaryngologists,
also known as ENT's
if their symptoms do not resolve. Complicated cases of sinusitis eventually
lead to surgery and
there are 1.5 million patients in the U.S. each year that are candidates for
surgical procedures, in
which currently 500k patients elect to undergo some type of surgical
procedure. The direct costs
association with managing sinusitis amount to over $6 billion annually, with
another $3 billion
associated with indirect costs associated with sinusitis management.
[0005] The initial diagnosis of sinusitis remains a challenge for
physicians. A patient
presenting at a physician's office with a symptom complex of fever, headache
and fatigue, also
- 1 -
CA 03057853 2019-09-24
WO 2018/183421 PCT/US2018/024729
present in many different types of systemic diseases, could warrant a
diagnosis of sinusitis. As a
result, many patients with non-sinus related diseases such as migraine
disorders, chronic fatigue,
and chronic systemic disorders are misdiagnosed as sinusitis. An additional
objective laboratory
diagnostic testing would guide physicians as to the etiology of these common
symptoms of viral
upper respiratory tract infections, acute bacterial sinusitis and chronic
sinusitis and lead to
reduction of unnecessary antibiotic and steroid prescriptions provided to
patients.
[0006] Currently doctors typically decide on a treatment regime without
a definitive test to
determine if the patient has viral sinusitis, bacterial sinusitis, upper
respiratory infection, chronic
fatigue, or migraines, because it is difficult to diagnose the cause of
sinusitis as either viral or
bacterial etiology. Treatment often involves antibiotics, which are only
effective for a small
amount of these conditions. The majority of sinusitis cases are viral, with
some estimates that
about 90% of sinusitis cases are viral. Majority of all patients receive an
antibiotic that they do
not need, can make their condition worse, and can lead to antibiotic
resistance. Improved
methods of diagnosing sinusitis are needed. In particular, what is needed is a
definitive, rapid
test for the cause of sinusitis, which could save the physician time and
provide timely
information that will lead to fewer antibiotics being prescribed.
[0007] There are many advantages to determining the etiology of
sinusitis (e.g., as viral,
bacterial, etc.), including the reduction in health care costs, decreases in
antibiotic use and
concomitant bacterial drug resistance, and improvements in the level of care
for patients.
Described herein are bacterial sinusitis diagnostic apparatuses (e.g.,
devices, systems, kits, etc.)
and methods that may address many of the needs described herein. For example,
the sampling,
testing, and treatment apparatuses and methods described herein may allow for
rapid and
definitive diagnosis of bacterial sinusitis, permitting targeted treatment
with optimal antibiotics
based on the specific diagnosis. Such targeted treatment may avoid unnecessary
antibiotic
treatments for patients not suffering from bacterial sinusitis. A rapid
diagnosis may also result in
improved treatment for patients that test negative for bacterial sinusitis by
instead treating the
patient based on a negative test for bacterial sinusitis.
[0008] It has been particularly difficult to easily and quickly get a
sample of the bacteria
within a patient's sinus with little pain and discomfort for the patient.
Existing sampling devices,
including swabs and other such devices, may not adequately guide the patient
to the appropriate
location within the sinus to extract a sample. Described herein are
apparatuses (including
devices and systems) for taking a sample as well as methods for using them.
- 2 -
CA 03057853 2019-09-24
WO 2018/183421 PCT/US2018/024729
SUMMARY OF THE DISCLOSURE
[0009] Described herein are apparatuses (e.g., systems, kits, assays,
including lateral flow
assay kits) taking a sample as well as methods for using them; any of these
apparatuses may
include or be used to determine the presence of one or more of the three
pathogens associated
with over 90% of bacterial sinusitis from a collected mucus sample.
Specifically, these methods
and apparatuses may determine, as part of a single rapid assay, the presence
of one or more of:
Haemophilus influenzae, Moraxella catarrhalis and Streptococcus pneumoniae. In
particular,
described herein are sinus collection devices for collection of mucus samples
from patient
sinuses; these collection devices may be included as part of the assays
described herein.
[0010] The sinus collection devices (sampling devices) described herein are
intended for use
during a routine office visit to a physician. These devices may accurately and
quickly (with a
minimum of discomfort) allow the acquisition of a mucus sample from the middle
meatus region
of the sinus (while avoiding miss-targeting of the region and cross-
contamination). A collected
mucus sample may then be analyzed using any of the lateral flow assays
described herein. If the
test is positive for any of the three bacterial pathogens, the patient has
bacterial sinusitis and may
be prescribed an appropriate antibiotic and/or steroid regimen to address the
pathogenic bacteria.
If the test is negative, the patient may be treated for viral sinusitis and
antibiotics may not be
administered. Examples of the sampling devices and assays (e.g., lateral flow
assays) are
described herein. Although the majority of these examples describe
apparatuses, including
collection devices, that are adapted for use in the nasal cavity, any of these
apparatuses and
methods may be adapted for use in other regions. For example, a variation of
the sampling
device and/or the assay may be adapted for use in collecting mucus samples
from within the
sinus during sinus surgery procedures, from an ear (e.g., in the case of
otitis media, which is
usually caused by the same three pathogens as is bacterial sinusitis) or
elsewhere.
[0011] As will be described in greater detail below, these assays may be
configured as lateral
flow assays that include a single lysis solution (e.g., lysis buffer solution)
that is appropriate for
use with all three types of bacteria (e.g., H. influenzae, M. catarrhalis and
S. pneumoniae) in
order to expose the antigens specific to each one for detection. Any of the
assays described
herein may be adapted for use with the lysis buffer, and may include multiple
(e.g., three) pairs,
or defined pools, of antigen binding agents that bind antigens (e.g., surface
proteins) specific to
each type of bacteria (e.g., H.flu, M.cat, S.pneumo). The antigen binding
agents ("agents") may
be monoclonal or polyclonal antibodies, or antibody fragments (e.g., FAB
fragments, etc.) or
molecules including all or a portion of these. Pairs of such agents may bind
to different portions
of the same antigen. An agent specific to each type of bacteria (e.g., H.flu,
M.cat, S.pneumo)
may be bound to a solid phase substrate (e.g., membrane, particle, etc.) and
spatially arranged in
- 3 -
CA 03057853 2019-09-24
WO 2018/183421 PCT/US2018/024729
the assay and provide specific identification of H. influenzae, M. catarrhalis
and S. pneumoniae
by visual detection of binding, including by binding the antigen to the
tethered substrate and to a
labeled agent. The pairs or pools of antibodies may be chosen to have low
cross-reactivity,
while allowing comparable detection of H. influenzae, M. catarrhalis and S.
pneumoniae.
[0012] The antigen binding agent (or "agent" and may also be referred to
herein as an
indicator) may be chosen so that they are selective for the organism of
interest, binds cognate
antigen specifically, have minimal cross-reactivity to common contaminating
organisms and
minimal cross-reactivity with commensal organisms. These antigen binding
agents may also
have a high affinity to the target pathogen antigen, rapid association
kinetics, slow dissociation
kinetics, and be sensitive to low numbers of the pathogen. Finally these
antigen binding agents
may be compatible with lateral flow, and compatible with a conjugate. As
mentioned above, in
particular the antigen binding agents may also be compatible for use with a
common lysing
solution for all three pathogens.
[0013] As will be described in greater detail herein, finding a common
lysing solution that
may work with multiple types of pathogens, and particularly H.flu, M.cat and
S.pneumo, was
surprisingly difficult, as many commonly used lytic agents (detergents,
enzymes, etc.) did not
work with all three, resulting in incomplete lysis (clogging of the lateral
flow system), lysis that
was too slow (e.g., took longer than 15 minutes), or disrupted the surface
proteins, including the
antigens specific to each cell type.
[0014] Haemophilus influenzae (H. influenzae) may be detected using a pair
or pool of
antibodies that are specific to one or more antigen binding agents that are
relatively specific or
characteristic of H. influenzae. For example, the indicator for H. influenzae
may bind with
specificity to the OMP-P2 and/or OMP-P5 antigen binding site for the pathogen.
As described
herein, numerous primary candidate antibodies have been evaluated, and
screened for cross
reactivity between numerous (e.g., 30) commensal bacterial strains to assure
minimal cross
reactivity with the normal flora occurring in the healthy sinus. Other
examples of antigen
binding agents include antibodies that may be used are discussed in
US20140314876, herein
incorporated by reference in its entirety.
[0015] Similarly, Moraxella catarrhalis (M. catarrhalis) may be detected
using a pair or
pool of antigen binding agents that are specific to a marker for M.
catarrhalis (see, e.g.,
US7811589) such as Protein C and Protein D outer member proteins.
[0016] One or more antigen binding agents specific for Streptococcus
pneumoniae (S.
pneumonia) may also be directed to S. pneumoniae markers such as the PsaA
antigen.
[0017] Specifically described herein are assay kits for concurrently
detecting H. influenzae,
.. M. catarrhalis and S. pneumoniae from a mucosal samples. An assay kit may
include: a lysis
- 4 -
CA 03057853 2019-09-24
WO 2018/183421 PCT/US2018/024729
buffer to lyse cells within the sample and form a single sample solution,
wherein the lysis buffer
comprises between 0.01% and 5% (w/w) of the anionic surfactant and between
0.1% and 15%
(w/w) of the osmotic agent; a cartridge containing one or more solid phase
substrates holding a
first agent that that binds specifically to a first antigen specific to H.
influenzae but not M.
catarrhalis or S. pneumoniae, a second agent that binds specifically to a
second antigen specific
to M. catarrhalis but not H. influenzae or S. pneumoniae, and a third agent
that binds specifically
to a third antigen specific to S. pneumoniae but not M. catarrhalis or H.
influenzae, wherein the
first, second and third agents are bound to specific regions of the one or
more solid phase
substrates in the cartridge; one or more conjugation regions within the
cartridge, the one or more
conjugation regions in fluid communication with the one or more solid phase
substrates and
comprising a fourth agent that is labeled and that binds specifically to the
first antigen, a fifth
agent that is labeled and that binds specifically to the second antigen, and a
sixth agent that is
labeled and that bind specifically to the third antigen; one or more sample
inlets on the cartridge
in fluid communication with the one or more conjugation regions; and one or
more windows
through which the specific regions of the solid phase substrate to which the
first, second and
third agents are bound may be visualized.
[0018] The anionic surfactant of the lysis buffer may comprise sarkosyl
and wherein the
osmotic agent of the lysis buffer comprises sucrose. Any of these assay kits
may include a
diluting buffer, as described herein.
[0019] The cartridge may include a housing that encloses one or more (e.g.,
three, arranged
in parallel) solid phase substrates. For example, a cartridge may comprise a
plurality (e.g., 3) of
solid phase substrates, wherein each solid phase substrate holds one of the
first agent, the second
agent or the third agent. Alternatively, cartridge may comprise a single solid
phase substrate
holding each of the first agent, second agent and third agent. The first
antigen may be a cell
surface antigen specific to H. influenzae, the second antigen may be a cell
surface antigen
specific to M. catarrhalis and the third antigen may be a cell-surface antigen
specific to S.
pneumoniae.
[0020] Any of these cartridge regions may include a conjugation region.
The conjugation
region may hold the unbound antigen binding agent, which may be marked with a
marker (e.g., a
visualizable marker such as a colloidal metal, colored bead, etc.). The
antigen binding agent(s)
in the conjugation region may be in solution (e.g., in a pre-wetted
conjugation sponge or
conjugation pad, a fluid conjugation chamber, etc.). Alternatively, the
antigen binding agent
(e.g., antibody, FAB, etc.) may be lyophilized and stored in this region, and
the sample solution
may re-suspend the antigen binding agent, allowing it to bind before entering
the portion(s) of
the solid phase substrate to which antigen binding agent(s) are bound. In
variations having a
- 5 -
CA 03057853 2019-09-24
WO 2018/183421 PCT/US2018/024729
single solid phase substrate with discrete regions for each of the different
types of antigen
binding agents binding to specific bacterial types, a single conjugation
region (e.g., holding the
fourth agent, fifth agent and sixth agent) may be used. Any of these
cartridges may include
multiple conjugation regions. In particular, cartridges having parallel fluid
paths may include
multiple conjugation regions, where each conjugation region holds the labeled
antigen binding
agent specific to one of the types of bacteria corresponding to the bound
antigen binding agent
on the downstream solid phase substrate.
[0021] Any of these kits may include a cartridge a single sample inlet.
The single inlet may
feed into a single fluidics line or into a plurality (e.g., 3) of parallel
fluidic lines that may connect
to, e.g., a sample region or chamber (e.g., sample pad), a conjugation region
or chamber (e.g.,
conjugation pad), an incubation region or chamber (e.g., incubation pad), a
solid phase substrate
region (e.g., detection region, which may be combined with the incubation
region or chamber or
separate from it), and/or a waste chamber or region (e.g., absorbent pad). The
fluid path(s)
through the cartridge may include an air inlet. For example, an air inlet may
be present at an
opposite end of the fluid path from the sample input.
[0022] The one or more windows in the cartridge may allow viewing of the
solid phase
substrate, allowing detection (e.g., visual, optical, etc.) of binding of
antigen to the solid phase
substrate(s) in this region (e.g., the detection region) where the
tethered/bound antigen binding
agent specifically bound to the solid phase substrate. In some variations the
method includes
reading/detection of the binding using a reader including an optical reader
(e.g., florescent
reader, etc.), visual (e.g., manual or automatic) reading, etc. The cartridges
described herein may
be configured to be compatible with one or more readers, including optical
readers such as the
Quidel "Sophia" device that is an optical reader that uses fluorescent markers
(see, e.g.,
www.quidel.com/immunoassays/sofia-tests-kits) or the Becton Dickinson
"Veritor" System (see,
e.g., www.bd.com/ds/veritorsystem/poctesting.asp).
[0023] As mentioned, any of the antigen binding agents (e.g., any or all
of the first agent,
second agent, third agent, fourth agent, fifth agent, and sixth agent) may
comprise an antibody or
an antibody fragment.
[0024] The one or more solid phase substrates may be, for example, a
membrane or other
surface onto which an antigen binding agent is immobilized. The substrate may
be smooth,
porous, rough, etc. In some variations a single solid phase substrate is used
to which each of the
multiple antigen binding agents (e.g., the first, second and third agents,
each specific to an
antigen of one of M.cat, S.pneumo, or H.flu). Thus, in any of these
variations, the one or more
conjugation regions may be a single conjugation region, and the one or more
sample inlets may
- 6 -
CA 03057853 2019-09-24
WO 2018/183421 PCT/US2018/024729
be a single sample inlet, and the single solid phase substrate may be upstream
of the single
conjugation region that is upstream of the single sample inlet.
[0025] Any of these assay kits may also include a control region on the
solid phase substrate.
The control region may include an immobilized binding agent that binds to one
or more of the
soluble antigen binding agents in the assay (e.g., the first, second or third
agent) configured to
bind to one or more of the fourth agent, fifth agent, or sixth agent and an
absorbent pad,
downstream of the specific regions of the solid phase substrate to which the
first, second and
third agents are bound.
[0026] For example, described herein are assay kits for concurrently
detecting H. influenzae,
M. catarrhalis and S. pneumoniae from a mucosal sample, the assay kit
comprising: a lysis
buffer to lyse cells within the sample and form a single sample solution,
wherein the lysis buffer
comprises between 0.01% and 5% (w/w) of the anionic surfactant and between
0.1% and 15%
(w/w) of the osmotic agent; a cartridge containing a solid phase substrates
holding a first agent
that that binds specifically to a first antigen specific to H. influenzae but
not M. catarrhalis or S.
pneumoniae, a second agent that binds specifically to a second antigen
specific to M. catarrhalis
but not H. influenzae or S. pneumoniae, and a third agent that binds
specifically to a third antigen
specific to S. pneumoniae but not M. catarrhalis or H. influenzae, wherein the
first, second and
third agents are bound to specific regions of the solid phase substrate; and a
conjugation region
within the cartridge, conjugation region in fluid communication with the solid
phase substrate
.. and comprising a fourth agent that is labeled and that binds specifically
to the first antigen, a
fifth agent that is labeled and that binds specifically to the second antigen,
and a sixth agent that
is labeled and that bind specifically to the third antigen; a sample inlet on
the cartridge in fluid
communication with the conjugation region; and one or more windows exposing
the specific
regions of the solid phase substrate to which the first second and third
agents are bound.
[0027] Also described herein are methods of concurrently detecting H.
influenzae, M.
catarrhalis and S. pneumoniae from a mucosal sample. For example a method of
concurrently
detecting H. influenzae, M. catarrhalis and S. pneumoniae from a mucosal
sample may include:
adding the sample to a lysis buffer to lyse cells within the sample and form a
single sample
solution, wherein the lysis buffer comprises both an anionic surfactant and an
osmotic agent;
adding the sample solution to a cartridge containing one or more solid phase
substrates holding a
first agent that that binds specifically to a first antigen specific to H.
influenzae but not M.
catarrhalis or S. pneumoniae, a second agent that binds specifically to a
second antigen specific
to M. catarrhalis but not H. influenzae or S. pneumoniae, and a third agent
that binds specifically
to a third antigen specific to S. pneumoniae but not M. catarrhalis or H.
influenzae, wherein the
.. first, second and third agents are bound to specific regions of the one or
more solid phase
- 7 -
CA 03057853 2019-09-24
WO 2018/183421 PCT/US2018/024729
substrates in the cartridge; and contacting the sample solution, either before
or after it is added to
the cartridge, with a fourth agent that is labeled and that binds specifically
to the first antigen, a
fifth agent that is labeled and that binds specifically to the second antigen,
and a sixth agent that
is labeled and that bind specifically to the third antigen.
[0028] In general, the agents that bind specifically to the antigens (e.g.,
first antigen, second
antigen, third antigen) described herein do not bind to antigens (proteins)
from the majority of
other commensal bacteria in the sinus specimen, in addition to having little
or any binding to
other antigens other than the intended/target antigen. For example, the
antigen binding agent
(e.g., antibody or antibody fragment) may bind specifically to the target
first antigen (e.g., from
H. flu), but not to non-target antigens (e.g., from M.cat or S.pneumo) .
[0029] In any of these methods, kits and compositions described herein,
the lysis buffer may
comprise between 0.01% and 5% (w/w) of the anionic surfactant and between 0.1%
and 15%
(w/w) of the osmotic agent. The anionic surfactant of the lysis buffer may
comprise between
0.01% and 5% (w/v) of sarkosyl and the osmotic agent of the lysis buffer may
comprises
between 0.1% and 15% (w/w) of sucrose.
[0030] Any of these methods may include adding a diluting buffer to the
sample solution
prior to adding it to the cartridge.
[0031] Adding the sample solution to the cartridge may include applying
a single bolus of
sample or applying multiple boluses of sample. For example, adding sample
solution to the
cartridge may comprise dividing the sample between a plurality of regions in
the cartridge,
wherein each region is in fluid communication with separate solid phase
substrates and wherein
each solid phase substrate holds one of the first agent, the second agent or
the third agent.
[0032] Adding the sample solution to the cartridge may comprise adding
the sample solution
to a single region in the cartridge that is in fluid communication with a
solid phase substrate
holding each of the first agent, second agent and third agent. In any of these
methods, kits, and
compositions described herein, the antigens to each bacterial type may be cell
surface antigens.
For example the first antigen may be a cell surface antigen specific to H.
influenzae, the second
antigen may be a cell surface antigen specific to M. catarrhalis and the third
antigen may be a
cell-surface antigen specific to S. pneumoniae.
[0033] Any of these methods may include passing the sample solution over
the one or more
solid phase substrates in the cartridge after contacting the sample solution
with the fourth, fifth
and sixth agents.
[0034] The step of contacting the sample solution with the fourth, fifth
and sixth agent may
comprise passing the sample through one or more portions of the cartridge
upstream from the
- 8 -
CA 03057853 2019-09-24
WO 2018/183421 PCT/US2018/024729
specific regions of the solid phase substrate in the cartridge to which the
first, second and third
agents are bound.
[0035] Any of the methods described herein may include visually
identifying which strain
(e.g., M.cat, S.pneumo, or H.flu) is present in the sample solution by
identifying that the fourth
agent has bound to the first antigen in the solid phase substrate region where
the first agent was
bound, and/or the fifth agent has bound to the second antigen in the solid
phase substrate region
where the second agent was bound, and/or that the sixth agent has bound to the
third antigen in
the solid phase substrate region where the third agent was bound.
[0036] The sample solution may be exposed (e.g., contacted with) the
labeled antigen
binding agents either before or after it is added to the cartridge. For
example, the sample solution
may be contacted with the fourth agent, fifth agent, and sixth agent before it
is added to the
cartridge, or the sample solution may be contacted with the fourth agent,
fifth agent, and sixth
agent after it is added to the cartridge.
[0037] For example, a method for concurrently detecting H. influenzae,
M. catarrhalis and S.
pneumoniae from a mucosal sample may include: adding the sample to a lysis
buffer to lyse cells
within the sample and form a single sample solution, wherein the lysis buffer
comprises between
0.01% and 5% (w/w) of the anionic surfactant and between 0.1% and 15% (w/w) of
the osmotic
agent; adding the sample solution to a cartridge containing a solid phase
substrate holding a first
agent that that binds specifically to a first antigen specific to H.
influenzae but not M. catarrhalis
or S. pneumoniae, a second agent that binds specifically to a second antigen
specific to M.
catarrhalis but not H. influenzae or S. pneumoniae, and a third agent that
binds specifically to a
third antigen specific to S. pneumoniae but not M. catarrhalis or H.
influenzae, wherein the first,
second and third agents are bound to specific separate regions of the solid
phase substrate;
contacting the sample solution with a fourth agent that is labeled and that
binds specifically to
the first antigen, a fifth agent that is labeled and that binds specifically
to the second antigen, and
a sixth agent that is labeled and that bind specifically to the third antigen;
and visually
identifying through a window in the cartridge that the fourth agent has bound
to the first antigen,
the fifth agent has bound to the second antigen, or the sixth agent has bound
to the third antigen.
[0038] Although the kits (e.g., assay kits, systems) described herein in
these examples are
configured to test for the presences of three bacteria (e.g., S. pneumoniae
but not M. catarrhalis
or H. influenzae), any of these kits and methods may be instead configured to
identify the
presence of two or more than three bacteria. In particular, any of the methods
and kits described
herein may be configured to determine the presence of S. pneumoniae and/or H.
influenzae,
which together account for approximately 70-75% of bacterial sinusitis.
- 9 -
CA 03057853 2019-09-24
WO 2018/183421 PCT/US2018/024729
[0039] Also described herein are nasal sampling devices that may be used
by themselves or
as part of a kit or system for testing a nasal (e.g., mucous) material,
particularly from the middle
meatus region of the sinus.
[0040] For example, a nasal sampling device for obtaining a sinus
secretion sample from a
subject's sinus may include: an elongate body having a distal end region that
is bent relative to a
proximal region by between 10 (e.g., 12, 15, 17, 18, 19, 20, etc.) degrees and
30 (e.g., 30, 35, 40,
45, etc.) degrees; a sample collector on a distal end of an extendable shaft,
wherein the sample
collector is configured to collect a sample of sinus fluid, further wherein
the sample collector is
housed entirely within the distal end of the elongate body in a retracted
position; and a control
coupled to the extendable shaft and configured to extend and retract the
sample collector in and
out of the distal end of the elongate body; wherein the nasal sampling device
has a retracted
configuration with the sample collector retracted and housed entirely within
the distal end of the
elongate body, a sampling configuration with the sample collector extended
distally out of a
distal opening of the distal end region of the elongate body a first distance
between 0.5 cm to 3
cm, and an elution configuration with the sample collector extended distally
out of the distal
opening of the distal end region of the elongate body a second distance that
is greater than the
first distance.
[0041] A nasal sampling device for obtaining a sinus secretion sample
from a subject's sinus,
wherein the nasal sampling device includes: an elongate body having a distal
end region that is
bent relative to a proximal region by between 10 (e.g., 12, 15, 17, 18, 19,
20, etc.) degrees and 30
(e.g., 30, 35, 40, 45, etc.) degrees; a sample collector on a distal end of an
extendable shaft,
wherein the sample collector is configured to collect a sample of sinus fluid,
further wherein the
sample collector is housed entirely within the distal end of the elongate body
in a retracted
position; and a control coupled to the extendable shaft, the control having a
first set point
wherein the sample collector is extended distally out of a distal opening of
the distal end region
of the elongate body a first distance between 0.5 cm to 3 cm, the control
having a second set
point, wherein the sample collector is retracted and housed entirely within
the distal end of the
elongate body, the control having a third set point, wherein the sample
collector is extended
distally out of the distal opening of the distal end region of the elongate
body a second distance
that is greater than the first distance.
[0042] Any of these nasal sampling devices may include a spacer (which
may also be a
protrusion, bump, deflector, etc.) on the extendable shaft proximal to the
sample collector,
wherein the spacer is configured to prevent the sample collector from
contacting an inner surface
of the elongate body when the sample collector is retracted into the distal
end of the elongate
body. Centering the sample collector in this manner may prevent the sample
collector from
- 10 -
CA 03057853 2019-09-24
WO 2018/183421 PCT/US2018/024729
getting contaminated by other bacteria (e.g., from regions other than the
sampling region) by
contacting the outer housing of the elongate body, which may contact other
regions; this may
also prevent prematurely releasing material or limiting the amount of material
held by the sample
collector (e.g., swab).
[0043] Any of the nasal sampling devices described herein may include a
releasable stop
configured to prevent the control from selecting the third set point until the
stop is released. Any
appropriate stop may be used, including an interference region between the
extendable shaft and
the elongate body and/or handle, a latch, etc. For example, the stop may
comprise a detachable
handle configured to releasably couple to a distal end of the extendable
shaft. The stop may
include a releasable connector connecting the extendable shaft to the stop.
[0044] In general, the dimensions of the nasal sampling may be
configured for use within the
nasal passages (e.g., sinus) so that the sample collector may be extended at
the correct region of
the apparatus to reach the desired portion of the sinus (e.g. the middle
meatus region, the upper
meatus region, the lower meatus region, etc.). Both the angle of the distal
end of the device
relative to more proximal regions as well as the size and shape of the device
may be configured
to allow external (through the nares/nostril) application of the device to
sample the mucosa. For
example the distal end region of the elongate body may be between 1.5 and 3.5
cm long (e.g.,
between 1 and 5 cm long, between 1 and 4 cm long, between 1.5 and 4 cm long,
between 2 and
3 cm long, etc.). Similarly, the proximal region of the elongate body may be
greater than 1 cm
long (e.g., greater than 1.5 cm, greater than 2 cm, greater than 3 cm, greater
than 4 cm, greater
than 5 cm, between 1 cm and 30 cm, between 1 cm and 20 cm, between 1 cm and 15
cm, etc.).
[0045] Similarly, the sample collector may be any appropriate size
(e.g., between 0.2 and 2
cm long, between 0.4 and 1.5 cm long, between 0.5 and 1.2 cm long, etc.). The
extendable shaft
may be any appropriate length (e.g., greater than 2 cm, greater than 5 cm,
greater than 10 cm,
between 1 cm and 30 cm, between 1 cm and 20 cm, between 1 cm and 15 cm,
between 1 cm and
12 cm, etc.). The extendable shaft may be configured (by operation of the
control) to extend
from the distal end region of the elongate body by a predetermined amount. For
example, as
mentioned above, in a sampling position the sample collector may be extended
from the distal
end by between 0.5 cm to 3 cm. In the elution configuration the extendable
shaft is extended
away from the elongate body further than in the sampling configuration. This
may be achieved
by advancing the extendable shaft relative to the elongate body, or by
retracting the distal end
region of the elongate body proximally, relative to the extendable shaft, or
in some variation by
removing all or a portion of the distal end region of the elongate shaft. For
example, in some
variations, the distance that the sample collector extends from the elongate
body in the elution
configuration (e.g., the second distance) may be 1.0 cm or greater than the
first distance. In
- 11 -
CA 03057853 2019-09-24
WO 2018/183421 PCT/US2018/024729
some variations, as will be described below, the extendable shaft includes
multiple (e.g., two or
more) stops.
[0046] As described herein, in general the sample collector may be a
swab, including in
particular a flocked swab. It may also be beneficial to use a swab having ends
which are
branched (e.g., bifurcated, or multiply-divided).
[0047] In any of these variations, the control on the nasal sampling
device may be coupled to
a handle at the proximal end of the device. For example, any of these
apparatuses may include a
handle body extending proximally from the elongate body, wherein the
extendable shaft extends
through the elongate body and into an internal channel within the handle body.
The extendable
shaft may generally be a flexible elongate shaft. The extendable shaft may be
configured to slide
within the elongate body.
[0048] Thus, any of the devices described herein may include a control
configured as a
slider. Other examples of controls may include dials, knobs, switches, or the
like. In some
variations a control that may be included (e.g., in addition to a slider or
other control) may be a
finger ring. In some variations a control comprises may be a compression
actuator configured to
be compressed to select the third set point in which the sample collector is
extended distally out
of the distal opening of the distal end region of the elongate body the second
distance. In
general, a control may be configured to be distally advanced to select the
first set point in which
the sample collector is extended distally out of a distal opening of the
distal end region of the
elongate body the first distance. In some variations a control comprises a
push button configured
to be depressed to select the third set point in which the sample collector is
extended distally out
of the distal opening of the distal end region of the elongate body the second
distance.
[0049] Any of these devices described herein may include a lock
configured to lock the
control at one or more of: the first set point, the second set point or the
(optional) third set point.
[0050] Any of the devices described herein may include a depth gauge
configured to display
a position of the sample collector to a user of the device. The distal end
region may be
configured to have an open configuration when the sample collector is advanced
out of the distal
end of the elongate body, and a closed configuration when the sample collector
is in the retracted
position.
[0051] Any of these devices may also include a depth stop to prevent the
sampling device
from being inserted too deep into a nasal and/or sinus cavity of a subject.
[0052] For example, a nasal sampling device for obtaining a sinus
secretion sample from a
subject's sinus may include: a hollow elongate body having a distal end region
that is bent
relative to a proximal region by between 10 (e.g., 12, 15, 17, 18, 19, 20,
etc.) degrees and 30
.. (e.g., 30, 35, 40, 45, etc.) degrees; a sample collector on a distal end of
an extendable shaft,
- 12 -
CA 03057853 2019-09-24
WO 2018/183421 PCT/US2018/024729
wherein the sample collector is configured to collect a sample of sinus fluid,
further wherein the
sample collector is housed entirely within the distal end of the elongate body
in a retracted
position; a control coupled to the extendable shaft, the control having a
first set point wherein the
sample collector is extended distally out of a distal opening of the distal
end region of the
elongate body a first distance between 0.5 cm to 3 cm, the control having a
second set point,
wherein the sample collector is retracted and housed entirely within the
distal end of the elongate
body, the control having a third set point, wherein the sample collector is
extended distally out
of the distal opening of the distal end region of the elongate body a second
distance that is 1.0
cm or greater than the first distance; and a projection on the extendable
shaft proximal to the
sample collector, wherein the projection is configured to prevent the sample
collector from
contacting an inner surface of the hollow elongate body when the sample
collector is retracted
into the distal end of the elongate body.
[0053] Also described herein are methods including methods of using a
nasal sampling
device. For example, a method for detecting one or more nasal bacteria in a
patient, using a
nasal sampling device including an elongate body having a distal end region
that is bent relative
to a proximal region by between 10 (e.g., 12, 15, 17, 18, 19, 20, etc.)
degrees and 30 (e.g., 30,
35, 40, 45, etc.) degrees, a sample collector on a distal end of an extendable
shaft, and a control
coupled to the extendable shaft, the control having a first set point wherein
the sample collector
is extended distally out of a distal opening of the distal end region of the
elongate body a first
distance, the control having a second set point, wherein the sample collector
is retracted and
housed entirely within the distal end of the elongate body, the control having
a third set point,
wherein the sample collector is extended distally out of the distal opening of
the distal end
region of the elongate body a second distance that is greater than the first
distance, may include:
advancing the distal end region of the nasal sampling device through a nares
of the patient until
the distal end region is adjacent to a middle meatus of a sinus; setting the
control to the first set
point to extend the sample collector into the middle meatus so that it
contacts a secretion fluid in
the middle meatus; setting the control to the second set point to retract the
sample collector
entirely within the distal end; withdrawing the nasal sampling device out of
the patient's nares;
and testing the secretion fluid with an immunoassay test after withdrawing the
nasal sampling
device.
[0054] The secretion fluid may be tested using any of the method
described above (e.g.,
concurrently detecting H. influenzae, M. catarrhalis and S. pneumoniae from a
mucosal sample).
For example, testing the secretion fluid may include setting the control to
the third set point, so
that the sample collector is extended distally out of the distal opening of
the distal end region of
the elongate body a second distance that is greater than the first distance
and contacting the
- 13 -
CA 03057853 2019-09-24
WO 2018/183421 PCT/US2018/024729
sample collector with a buffer solution. Testing the secretion fluid may
comprise contacting the
secretion fluid with a lysing solution. For example, testing the secretion
fluid may comprise
contacting the secretion fluid with a lysing solution comprising both an
osmotic agent and an
anionic surfactant. In some variations, testing the secretion fluid comprises
contacting the
secretion fluid with a lysing solution comprising Sodium Lauroyl Sarcosinate
and sucrose to
form a sample fluid and contacting the immunoassay test with the sample fluid.
Testing the
secretion fluid may comprise testing the secretion fluid with one or more
agents that bind to: an
antigen specific to H. influenzae, an antigen specific to M. catarrhalis, or
an antigen specific to
S. pneumoniae. Testing the secretion fluid may comprises testing the secretion
fluid with one or
more agents that bind to each of: an antigen specific to H. influenzae, an
antigen specific to M.
catarrhalis, or an antigen specific to S. pneumoniae.
[0055] Also described herein are systems for detecting bacterial
sinusitis that generally
include a mucosal sampling device as described herein any of the assays/kits
described herein.
For example, a system for detecting bacterial sinusitis may include a nasal
sampling device for
obtaining a sinus secretion sample from a subject's sinus, wherein the nasal
sampling device
includes: an elongate body having a distal end region that is bent relative to
a proximal region by
between 10 (e.g., 12, 15, 17, 18, 19, 20, etc.) degrees and 30 (e.g., 30, 35,
40, 45, etc.) degrees; a
sample collector on a distal end of an extendable shaft, wherein the sample
collector is
configured to collect a sample of sinus fluid, further wherein the sample
collector is housed
.. entirely within the distal end of the elongate body in a retracted
position; a control coupled to the
extendable shaft, the control having a first set point wherein the sample
collector is extended
distally out of a distal opening of the distal end region of the elongate body
a first distance, the
control having a second set point, wherein the sample collector is retracted
and housed entirely
within the distal end of the elongate body, the control having a third set
point, wherein the
sample collector is extended distally out of the distal opening of the distal
end region of the
elongate body a second distance that is greater than the first distance; and
an immunoassay kit
for detecting at least one bacterial strain associated with bacterial
sinusitis infections.
[0056] The immunoassay kit may include a lysis buffer comprising both an
anionic
surfactant and an osmotic agent, such as an anionic surfactant between 0.01%
and 5% (w/w) and
an osmotic agent between 0.1% and 15% (w/w). In some variations the
immunoassay kit may
comprises a lysis buffer comprising sarkosyl and sucrose.
[0057] In any of these variations, the immunoassay kit may include a
cartridge, and the
cartridge may include a sample inlet for depositing a sample, a sample pad
onto which the
sample is absorbed prior to elution, a conjugate pad containing at least one
antibody complexed
with a detectable marker, a detector pad comprising at least one zone, wherein
the zone
- 14-
CA 03057853 2019-09-24
WO 2018/183421 PCT/US2018/024729
comprises antibodies directed to at least one bacterial antigen bound to the
detector pad, and a
visualization window for viewing the results of the assay.
[0058] The immunoassay kit may comprise a cartridge comprising a sample
inlet for
depositing a sample, a sample pad onto which the sample is absorbed prior to
elution, a
conjugate pad containing a plurality of antibodies complexed with a detectable
marker, a detector
pad comprising a plurality of different zones, wherein each zone comprises
antibodies directed to
at least one bacterial antigen bound to the detector pad, and a visualization
window for viewing
one or more of the zones of the detector pad. The kit may include a sampling
device with a
spacer on the extendable shaft proximal to the sample collector, wherein the
spacer is configured
to prevent the sample collector from contacting an inner surface of the
elongate body when the
sample collector is retracted into the distal end of the elongate body.
[0059] A system for detecting bacterial sinusitis may include: a nasal
sampling device for
obtaining a sinus secretion sample from a subject's sinus, wherein the nasal
sampling device
includes: an elongate body having a distal end region that is bent relative to
a proximal region by
.. between 10 (e.g., 12, 15, 17, 18, 19, 20, etc.) degrees and 30 (e.g., 30,
35, 40, 45, etc.) degrees; a
sample collector on a distal end of an extendable shaft, wherein the sample
collector is
configured to collect a sample of sinus fluid, further wherein the sample
collector is housed
entirely within the distal end of the elongate body in a retracted position; a
control coupled to the
extendable shaft, the control having a first set point wherein the sample
collector is extended
distally out of a distal opening of the distal end region of the elongate body
a first distance, the
control having a second set point, wherein the sample collector is retracted
and housed entirely
within the distal end of the elongate body, the control having a third set
point, wherein the
sample collector is extended distally out of the distal opening of the distal
end region of the
elongate body a second distance that is greater than the first distance; and
an immunoassay kit
.. for detecting multiple bacterial strains associated with bacterial
sinusitis infections, the kit
comprising a lysis buffer comprising both an anionic surfactant between 0.01%
and 5% (w/w)
and an osmotic agent between 0.1% and 15% (w/w).
[0060] Any of the apparatuses described herein, and particularly the
nasal sampling devices
described herein, may include guide attached or attachable to the apparatus
for assisting the user
.. (physician, technician, etc.) in controlling the insertion angle for the
apparatus so that an
appropriate sample may be taken. In particular, these apparatuses may be
configured a hand-
held sampling devices that include a projection region extending from the
proximal portion of
the apparatus that is at a fixed angle of between 20 and 45 relative (and
preferably between 30-
degrees, e.g., between 33-37 , e.g., approximately 35 relative to a long axis
of the device.
35 .. This reference marker may be a member (e.g., an arm, protrusion, etc.)
extending from the body
- 15 -
CA 03057853 2019-09-24
WO 2018/183421 PCT/US2018/024729
of the device, and/or it may be a leveling member (e.g., bubble level, etc.)
or an indicator light
that illuminates when the device is held at the proper orientation, or a sound
indicator, e.g.,
providing a specific tone when the device is held in the proper orientation,
or a marking on the
body of the device at an angle of between 30-40 (e.g., 33-37 ) relative to
the long axis that is
visible when held in the users hand, or some combination of these. In any of
these reference
markers, the proper orientation is when the long axis of the device is between
30-40 degrees
(e.g., 33-37 degrees) relative to a line perpendicular to the patient ground
surface when the
patient is laying down, or relative to a line parallel to the patient ground
surface, such as the
floor, if the patient is sitting/standing.
BRIEF DESCRIPTION OF THE DRAWINGS
[0001] FIG. 1 is an illustration of a healthy sinus and a sinus showing
symptoms of sinusitis.
[0002] FIG. 2 is a CT scan image of a patient exhibiting symptoms of
sinusitis.
[0003] FIGS. 3A-3F show an example of a method for sampling a sinus in
accordance with
some embodiments.
[0004] FIGS. 4A-4C illustrate aspects of a device configured to sample a
sinus in accordance
with some embodiments.
[0005] FIGS. 5A-5C illustrate aspects of a device configured to sample a
sinus in accordance
with some embodiments.
[0006] FIGS. 6A-6C illustrate aspects of a device configured to sample a
sinus in accordance
with some embodiments.
[0007] FIGS. 7A-7C illustrate aspects of a device configured to sample a
sinus in accordance
with some embodiments.
[0008] FIGS. 8A-8C illustrate aspects of a device configured to sample a
sinus in accordance
with some embodiments.
[0009] FIGS. 9A-9C illustrate aspects of a device configured to sample a
sinus in accordance
with some embodiments.
[0010] FIGS. 10A-10D illustrate aspects of a device configured to sample
a sinus in
accordance with some embodiments.
[0011] FIGS. 11A-11C illustrate aspects of a device configured to sample a
sinus in
accordance with some embodiments.
[0012] FIGS. 12A-12E illustrate aspects of a device configured to sample
a sinus in
accordance with some embodiments.
[0013] FIGS. 13A-13E illustrate aspects of a device configured to sample
a sinus in
accordance with some embodiments.
- 16-
CA 03057853 2019-09-24
WO 2018/183421 PCT/US2018/024729
[0014] FIGS. 14A-14E illustrate aspects of a device configured to sample
a sinus in
accordance with some embodiments.
[0015] FIG. 15A is a rendering of the lateral side of another variation
of a device configured
to sample from a sinus.
[0016] FIG. 15B is a rendering of the posterior side of the assembled
device of FIG. 15A.
[0017] FIGS. 15C and 15D show partially exploded views of the lateral
side and front of the
device of FIG. 15A.
[0018] FIG. 16 is an example of a proximal end of a sample collector
being inserted into a
distal end of the main body portion.
[0019] FIG. 17A illustrates one variation of a distal end of a sample
collector and a
corresponding sleeve into which the sample collector may be housed. Also shown
is at least one
coupler that joins the sleeve to the main body of the device.
[0020] FIG. 17B illustrates an alternative configuration for the at
least one coupler that joins
the sleeve to the main body of the device.
[0021] FIG. 18A shows an exploded view of one variation of a distal end of
a handle and a
proximal end of a main body region.
[0022] FIG. 18B illustrates a distal end of a thumb ring that is
separated from a proximal end
of a main body region of the device, similar to the view of FIG. 18A. The
thumb ring controller
region at the distal end (left side of FIGS. 18A and 18B) may be coupled into
the distal end of
the main body region.
[0023] FIG. 19 illustrates a sleeve for housing the distal end of the
swab. Both the sleeve
(protective cover) and the sample collector (including swab) are bent in a
predefined manner as
described herein.
[0024] FIG. 20 shows a sample collector proximal end coupled with the
distal end of the
main body.
[0025] FIG. 21 illustrates a proximal end region of a sample collector
that couples with the
handle.
[0026] FIG. 22 illustrates another variation of a sampling device having
a coupler and
releasable hold (e.g. releasable lock, or release lock) on the main body for
engaging the distal
handle.
[0027] FIG. 23A shows a coupler (shown as a snap-fit coupler) on the
handle and a
corresponding coupling channel on the main body.
[0028] FIG. 23B is an alternative view of the coupler of the handle and
a corresponding
coupling channel on the main body.
- 17 -
CA 03057853 2019-09-24
WO 2018/183421 PCT/US2018/024729
[0029] FIG. 23C is another view of a coupler of the handle and a
corresponding coupling
channel on the main body.
[0030] FIGS. 24A-24D show schematics of one example of a lateral flow
assay (having two
detection readouts, e.g., for two sets of antibodies) and components. FIG. 24A
shows an assay
housing, a sample pad for accepting the sample, a conjugate pad containing the
first antibody
with complexed detector molecule, a detection pad along which the sample will
run and come
into contact with zones of corresponding second antibodies bound to the
detection pad for each
antigen of interest. FIG. 24B shows a sample on the sample pad, the set of
first antibodies on the
conjugate pad, and zones on the detection pad holding different antibodies.
FIG. 24C shows an
eluting solution (dark) that runs across the detection pad and brings first
antibodies-detector
molecule coupled to corresponding antigens in contact with the second set of
antibodies. FIG.
24D shows the completed assay where the first antibodies-detector molecule
coupled to
corresponding antigens is now also bound to the corresponding second
antibodies for each
different antigen of interest.
[0031] FIGS. 25A-25K illustrate the operation of a sample collector as
described herein.
[0032] FIG. 26 schematically illustrates one variation of an assay
similar to the assay shown
in FIGS. 24A-24D for diagnosing sinusitis.
[0033] FIG. 27 is a table illustrating the effectiveness of various
lysis buffers on three of the
types of bacteria to be concurrently examined by the apparatuses and methods
described herein.
[0034] FIG. 28 is a table illustrating two examples of lysis buffers
compatible for the
concurrent detection of multiple different cell types (e.g., M.cat, S.pneumo
and H.flu) as
described herein.
[0035] FIG. 29 is a table illustrating two exemplary dilution buffers
compatible for the
concurrent detection of multiple different cell types as described herein. In
these examples, the
lysis buffer #1 (on left of FIG. 28) was used with dilution buffer #1 (on left
of FIG. 29), and lysis
buffer #2 (on right in FIG. 28) was used with dilution buffer #2 (on right in
FIG. 29).
[0036] FIGS. 30A-30C illustrate detection of each of S.pneumo, M.cat,
and H.flu,
respectively, using the kits and methods described herein. The concentration
of cells detected
(expressed as colony forming units (CFU)/sample) in this prototype show
thresholds for visual
detection from an exemplary lateral flow assay such as the one illustrated in
FIGS. 24A-24D and
26. FIG. 30A illustrates that the prototype assay detected the PsaA antigen
(the cell-surface
marker for S.pneumo) at bacterial concentrations ranging from 103-107 per 100
pi sample with
resolution at lx104. FIG. 30B illustrates that the prototype assay detected
the CD antigen (a cell-
surface marker for M.cat) at bacterial concentrations ranging from 104-107 per
100 vtl sample
with good resolution at 1x105. FIG. 30C illustrates that the prototype assay
detected the OMP-
- 18 -
CA 03057853 2019-09-24
WO 2018/183421 PCT/US2018/024729
P5 antigen (a cell-surface marker for H.flu) at bacterial concentrations
ranging from 105-107 per
100 tl sample with good resolution at 2x105.
[0037] FIG. 31 is one example of a cartridge having a single solid phase
substrate
(combining three separate assays, one each for a different bacterial type)
that can simultaneously
test for the presence of each of three different types of bacteria.
[0038] FIG. 32 is an example of a cartridge configured to simultaneously
test for the
presence of each of three different types of bacteria in parallel; the
cartridge include three
separate solid phase substrates and three fluidic pathways. Although the
example shown in FIG.
32 includes three separate inlet ports, a single port having three fluidic
paths may be used.
[0039] FIGS. 33A-33B illustrate a sample collector held in the proper
orientation for
insertion into the patient to collect the sample as described herein. In FIG.
33A, the sample
collector device includes a reference marker (shown schematically on the
proximal body region)
that is parallel to the ground surface for an upright, e.g., sitting or
standing, patient. In FIG. 33B,
the reference marker is perpendicular to the ground surface when the patient
is laying down.
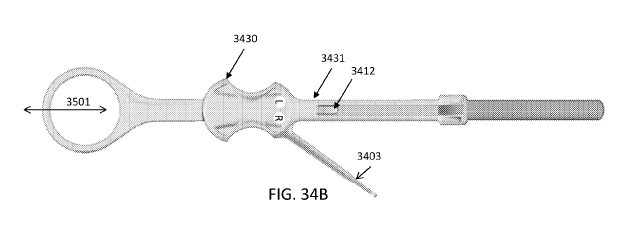
[0040] FIGS. 34A-34C illustrate examples of sample collector devices that
include a
reference marker that extends from the body of the sample collector at an
angle of between 30-
40 (e.g., between 33-37 , e.g., approximately 35 ). In this example, the
indicator is shown on
one side, indicating the orientation when the device is inserted into the
right nostril, so that it is
either perpendicular (when the patient is lying down) or parallel (when the
patient is
sitting/standing) to the ground surface of the patient. The indicator may be
switched to the other
side of the sample collector when the device is use on the patient's left
nostril. FIG. 34A shows
a left side view, FIG. 34B shows a top view, and FIG. 34C shows a right side
view.
[0041] FIGS. 35A-35C show another example of a sample collector device
from left, top and
right views, respectively. In this example, the indicator extends from the
body of the proximal
region of the device at a fixed 35 angle relative to the long axis of the
device.
[0042] FIG. 35D illustrates connection of the right-sided indicator to
the sample collector
device.
[0043] FIGS. 36A-36C show another example of a sample collector device
from left, top and
right views, respectively. In this example, the indicator extends from the
body of the proximal
region of the device at a fixed 35 angle relative to the long axis of the
device. The indicator is
an arm that may be attached to either the right side of the device or the left
side of the device to
provide guidance for either nostril.
[0044] FIGS. 37A-37C show another example of a sample collector device
from left, top and
right views, respectively. In this example, the indicator extends clips onto
the body of the device
and extends from the body at a fixed 35 angle relative to the long axis of
the device.
- 19-
CA 03057853 2019-09-24
WO 2018/183421 PCT/US2018/024729
[0045] FIG. 37D shows an example of the indicator extending from the
apparatus by
clipping onto the neck region of the main body of the collector device.
[0046] FIGS. 38A and 38B show to and side views, respectively, of an
extendable inner
(swab) portion of a device as described herein, including two stops at the
distal end region.
[0047] FIGS. 38C-38F illustrate details from indicated regions (C, D, E and
B, respectively)
in FIGS. 38A and 38B.
[0048] FIG. 38G is a perspective view of the extendable inner (swab)
portion of FIGS. 38A-
38F.
[0049] FIG. 39A is an example of a distal end of a device with the inner
(swab) portion fully
.. extended, in a side view, showing the second locking feature preventing re-
entry of the swab
back into the distal end of the device, preventing contamination.
[0050] FIG. 39B is another, top perspective, view of the device of FIG.
39A.
DETAILED DESCRIPTION
[0051] Apparatuses (including devices, systems, kits, and assays) and
methods are disclosed
herein for diagnosing sinusitis, including obtaining a sample of sinus fluid
from a patient and/or
determining if the patient is infected with one or more of H. influenzae
(H.flu), M. catarrhalis
(M.cat) and S. pneumoniae (S.pneumo). For example, described herein are sample
devices for
accurately and quickly sampling sinus fluid within the sinus, such as the
middle meatus or
maxillary sinus, and assays for rapidly testing this sample to determine the
presence of bacteria,
viruses, and other diseases of interest. The fast diagnosis of the presence or
absence of the
diseases of interest can improve the treatment of the patient.
[0052] FIG. 1 illustrates a comparison between a healthy sinus and a
sinus with sinusitis.
The sinusitis can cause excess mucous in the frontal sinus and maxillary
sinus. Other symptoms
can include inflamed sinus lining and a sinus infection. FIG. 2 illustrates a
CT image of a patient
with chronic sinusitis. The arrows indicate the congested sinuses typical of
chronic sinusitis.
[0053] Testing the mucous / sinus fluid within the sinus, such as the
middle meatus or
maxillary sinus, can help diagnose the condition causing the discomforting
symptoms in the
patient. The sinus fluid can indicate a bacterial infection, viral infection,
or provide other
information to help diagnose and formulate an efficient and effective
therapeutic treatment.
Other examples of areas of the sinuses that can be tested using the devices
and methods
disclosed herein are the frontal sinuses, maxillary sinuses, ethmoid sinuses,
and sphenoid
sinuses. The devices disclosed herein can also have a tip geometry configured
to be advanced in
other passages within the body. For example the devices can be configured to
collect a sample
- 20 -
CA 03057853 2019-09-24
WO 2018/183421 PCT/US2018/024729
from the nasopharynx region, esophageal passage, from the middle ear, and
other portions of the
anatomy that a skilled artisan would want to sample.
[0054] FIGS. 3A-3D show an example of a method for sampling a sinus in
accordance with
some embodiments. FIGS. 3A-3D include a schematic illustrate of a portion of a
sinus 100
including the nares 102, middle meatus 103, ostium of the maxillary sinus 104,
maxillary sinus
106, and sinus fluid 108 within the maxillary sinus 106. FIG. 3B is a
schematic illustration of a
portion of a sampling device 110. Any of the sampling devices disclosed herein
can be used as
the sampling device 110 as illustrated in FIGS. 3A-3D. The sampling device 110
includes a
distal portion configured to be advanced through the nares 102 to an area
adjacent to the middle
meatus 103 and maxillary sinus 104 as shown in FIG. 3B. After the sampling
device 110 has
been advanced to a desired area adjacent to the middle meatus 103, the sample
collector 112 can
be advanced distally to contact and sample sinus fluid in the middle meatus
103 as shown in
FIG. 3C. After the sample of the sinus fluid has been obtained by the sample
collector 112, the
sample collector 112 can be retracted back into the sampling device 110. After
the sample
collector 112 has been retracted back into the sampling device 110, the
sampling device 110 can
be withdrawn from the nares 102 as shown in FIG. 3D. The sampling device 110
can be used to
sample either of the nares.
[0055] After the sinus fluid has been sampled using the sampling device
110, the sinus fluid
sample can be tested. FIG. 3E illustrates an example of a kit that can include
a sampling device
as described herein. The kit can include a lysis (e.g., buffer or lysis
buffer) solution 150,
diagnostic test 152, and packaging 154 in addition to the sample collector. As
will be described
in greater detail below, a sample collector 112 containing a sinus fluid
sample can be advanced
distally as described herein, and placed in contact with the lysis buffer
solution 150 to form the
sample solution in which bacterial cells (and particular the H. influenzae, M.
catarrhalis and S.
pneumoniae) will be lysed to expose markers that can be detected by the assay.
Thus, an aliquot
of the sample solution can be applied to the diagnostic test 152. The
diagnostic test 152 can
produce a color change or other indication visible to the medical technician
to indicate a positive
or negative result for one or more of the bacteria tested (e.g., for
sinusitis, H. influenzae, M.
catarrhalis and S. pneumoniae). FIG. 3F illustrates an example of a diagnostic
test 156 with
three different tests (one each for H. influenzae, M. catarrhalis and S.
pneumoniae) and a
control. FIG. 3F illustrates an example of positive responses to all three
different tests and the
control. In general, a diagnostic test 152 can contain a plurality of
immunoassay tests. The tests
can provide rapid results on the order of 1-30 minutes (e.g., 5-20 min, 5-17
min, 5-15 min, etc.).
Other configurations can be used for the immunoassay tests, for example
multiple testing strips
can be included in the diagnostic test 152 with each test strip testing for a
different pathogen on
-21 -
CA 03057853 2019-09-24
WO 2018/183421 PCT/US2018/024729
each strip, or some variation of a single sequential and one or more parallel
assays may be used.
In some embodiments the diagnostic test includes tests for two or more
pathogens. In some
embodiments the diagnostic test includes tests for three or more pathogens. In
some
embodiments the diagnostic test includes tests for four or more pathogens.
[0056] In some embodiments the immunoassay tests can include common
conditions
implicated in sinusitis, such as strep A, influenza A, and influenza B. In
some embodiments the
immunoassay tests can include strep A. In some embodiments the immunoassay
tests can include
influenza A. In some embodiments the immunoassay tests can include influenza
B.
[0057] In some embodiments the diagnostic tests can include bacterial
sinusitis tests.
Examples of bacterial sinusitis pathogens include: Haernophilus influenzae,
Moraxella
catarrhalis, and Streptococcus pneurnoniae. Other examples of diagnostic tests
that can be used
with the devices, kits, and methods disclosed herein include U.S. Patent
Publication No.
2014/0314876 to Das et al, titled "Proteomics Based Diagnostic Detection
Method for Chronic
Sinusitis", the disclosure of which is incorporated by reference herein in its
entirety.
[0058] The sample collection devices disclosed herein can include a distal
tip that is
configured to be advanced within the nare of the patient. The distal tip can
include a bend that is
configured to line up with the anatomy of most patients, such as the middle
meatus. In some
cases the bend has an angle of about 10 degrees to about 30 degrees relative
to a major axis of
the device. In some embodiments the distal tip can be flexible. The distal tip
can be made out of
a soft, biocompatible, and pliable material, such as a polymer. In some
embodiments the distal
tip can be made out of silicone. Other examples of biocompatible polymers
include
thermoplastic elastomer (TPE), thermoplastic vulcanizates (TPV), thermoplastic
polyolefins
(TPO), thermoplastic urethane (TPU) polymers, etc. Specific examples of
polymers that can be
used for the distal tip also include Kraton, Versaflex, Santoprene, etc. Other
biocompatible
polymers know by the skilled artisan can also be used. In some embodiments the
distal tip can
be made out of metal. It may be desirable (though not necessary) to have a
material hardness of
between about Durometer Shore A90 to D 85.
[0059] The distal tip can have an open end. In some embodiments the
distal tip includes a
covered or closed distal end. The covered or closed distal end can be opened
with distal
advancement of the sample collector. In some embodiments the covering can be
designed to be
punctured by the sample collector. In some embodiments the covering can be
designed to open
and close to reduce the chance of contamination of the sample collector. In
some embodiments
the covering or distal end can be designed to be resalable opened. For
example, the cover or
distal end can have a patterned opening. The sample collector can be pushed
through the
patterned opening and the patterned opening can close after the sample
collector is retracted.
- 22 -
CA 03057853 2019-09-24
WO 2018/183421 PCT/US2018/024729
The closed distal end or covering can prevent contamination of the sample
collector when the
device is advanced through the nare or retracted outside of the patient after
the sample has been
taken. In some embodiments the distal tip can have an open distal end.
[0060] The sample collector can be advanced distally past a distal end
of the distal tip to take
a sample of sinus fluid or other target fluid. The sample collector can be a
swab or contain
another absorbent material that can collect and hold fluid. The advancement of
the sample
collector can be done using an actuator. In some embodiments the actuator can
be slider or a
plurality of sliders. In some embodiments a handle portion engaged with the
sample collector can
be used to advance and retract the sample collector. In some embodiments the
mucous sample
can be collected using negative pressure. For example, the actuator can create
a negative pressure
in the environment surrounding the distal tip such that the mucous sample
flows into the sample
collector.
[0061] The device can include a safety or lock to reduce the inadvertent
advancement of the
sample collector while the device is in the nare of the patient. For example,
a button or slider
can be required to be pressed to allow further advancement of the actuator. In
some
embodiments the slider itself can be required to be depressed before it can
slide. In some cases
the safety can be a lock that can be deactivated prior to further advancing
the sample collector. In
some embodiments the slider can include two sliders that are simultaneously
depressed to allow
movement of the actuator. In some cases the actuator can move along a track
with notches to
catch or stop the actuator at the sample position and sample solution
position. In some cases the
actuator can move along a track with a stair type configuration that requires
shifting the actuator
at a stop position prior to further advancing or retracting the actuator.
[0062] In some embodiments the devices can be operated using a single
hand. For example,
one portion of the device can be held with one or more fingers while the
actuator or proximal end
of the device can be held and operated using the thumb. The devices can be
configured for
ambidextrous use. For example, the device can be ergonomically designed to
accommodate use
by the left hand and the right hand. The medical professional can use
whichever hand they
prefer to operate the device. In some embodiments the device can be operated
with both hands.
For example, the lab technician may prefer to use both hands to extend the
sample collector for
processing.
[0063] The device can include a marker to indicate the orientation of
the device, such as the
direction of the bend in the distal end. The marker can indicate the lateral
direction and/or the
left or right nares. The marker can include a colored portion of the device, a
label on the device,
or a projection on the exterior of the device indicating the orientation of
the bend in the distal
end.
-23 -
CA 03057853 2019-09-24
WO 2018/183421 PCT/US2018/024729
[0064] The device can be used to take a sample from either nostril. The
orientation of the
entire device can be rotated approximately 180 degrees for use on the other
nostril. In some
embodiments the device can have a rotatable portion that can be rotated, e.g.
by 180 degrees,
such that the device can be used for the other nostril. For example, the
distal portion of the tip
can be rotated relative to the handle of the device.
[0065] The device can have a multi-piece construction. The sample
collector can be part of a
removable handle. In some cases a portion of the handle can be removed prior
to being able to
expose the sample collector to the sample solution. In some embodiments a
portion of the distal
cover can be removed to access the sample collector.
[0066] The devices described herein can be used with an endoscope to
provide additional
guidance and visualization to assist the healthcare professional with
obtaining a sample from the
desired location.
[0067] After obtaining the sample the device can be removed from the
patient followed by
contacting the sample collector with a sample solution. The sample collector
can be advanced
distally past the distal end to contact the sample collector with the sample
solution. In some
embodiments the sample collector can be withdrawn proximally through an
interior of the device
followed by contacting the sample collector with the sample solution. In some
embodiments the
distal cover can be pulled back to expose the sample collector. In some
embodiments the distal
cover can have a multi-piece construction such that the cover can be removed
to expose the
sample collector. In some embodiments a separate slider can be used to advance
the sample
collector to a sample solution position for contact with the sample solution.
[0068] The device can include a depth gauge to provide information to
the user regarding the
location of the sample collector, such as the distance the sample collector
has been advanced.
[0069] In some embodiments the device can include a stop or guard
configured to engage
with the outside of the nose/nostril to prevent further advancement of the
device. In some
embodiments the stop or guard can be removed by the healthcare worker to
provide additional
visual guidance and clearance for endoscope.
[0070] The devices can have a naturally retracted position. For example,
a compression
element could provide a resting force to keep the sample collector in the
retracted position. The
compression element could pull the sample collector proximally after obtaining
the sample in the
absence of an actuating force applied by the user.
[0071] In some embodiments the hand held device can be configured to be
disposable after
obtaining a sample fluid from the patient. In some embodiments the hand held
devices can be
configured to be reusable. For example, the device could be sterilized after
obtaining a sample
fluid and used for subsequent sample collection from a second patient. In some
embodiments
- 24 -
CA 03057853 2019-09-24
WO 2018/183421 PCT/US2018/024729
the handle can be designed to be reused and a new sample collector or other
part can be
combined with the handle to form a device for obtaining a sample from a second
patient. The
sample collector could be provided separately as a single use cartridge to be
used with the
sterilized handle.
[0072] The sample collector can include a structure to facilitate opening
and/or closing of a
distal cover. For example, fins or a shoulder can be located adjacent to the
sample collector to
push open the distal cover and to hold the distal cover open during retraction
to prevent sample
loss caused by the distal cover squeezing the sample collector.
[0073] FIGS. 4-14 illustrate aspects of various embodiments of the hand
held sample
collecting devices disclosed herein.
[0074] FIGS. 4A-4C illustrate aspects of a device configured to sample a
sinus in accordance
with some embodiments. FIGS. 4A-4C illustrate a hand held sample collector 200
with a distal
section 202 and a proximal section 204. The distal section 202 includes a
distal end 206
configured to be introduced through the nares of the patient. The proximal
section 204 is
configured to slide relative to the distal section 202 to move the sample
collector 210, illustrated
as a swab, relative to the distal end 206. The device 200 is configured to be
gripped with a
human hand with a finger grip 212 on the distal portion 202 and a thumb grip
214 on the
proximal portion 204. The device 200 can be operated with one hand such that
movement of a
thumb on the thumb grip 214 can advance the proximal portion 204 relative to
the distal portion
202. The device 200 includes an opening or window 216 such that a shaft 218 of
the proximal
portion 204 can be observed. The window 216 can also be used to provide
orientation
information to the user, such as the lateral direction of the device. The
illustrated device 200
includes a depth gauge 220 aid the operator in determining the position of the
sample collector
210. The distal portion 206 can be advanced through the nares to the target
location followed by
advancing the proximal portion 204 and sample collector 210 relative to the
distal portion 206 to
contact the sinus fluid. After the sample has been collected, the sample
collector 210 is retracted
back into the distal portion 206 of the device 200 to shield the sample
collector 210 from the
sinuses while withdrawing the device 200. After the sample collector 210 has
been retracted
within the device 200, the device 200 can be removed from the patient. The
proximal portion
204 can be retracted proximally relative to the distal portion 202 to
completely separate the
proximal portion 204 and the distal portion 202 for sample testing as shown in
FIG. 4C. The
proximal portion 204 and sample collector 210 can be handled for the sample
testing and
processing. The sample collector 210 with the sinus sample can be tested using
the rapid
diagnostic testing methods disclosed herein.
- 25 -
CA 03057853 2019-09-24
WO 2018/183421 PCT/US2018/024729
[0075] FIGS. 5A-5C illustrate aspects of a device configured to sample a
sinus in accordance
with some embodiments. FIGS. 5A-5C illustrate a hand held sample collector 300
with a distal
section 302 and a proximal section 304. The distal section 302 includes a
distal end 306
configured to be introduced through the nares of the patient. A
compression/spring element 308
engages with a shaft 310 connected to the sample collector 312. The distal
section 302 includes
positioning markers 314. The positioning marker 314 can include a marker to
provide an
orientation of the device to the user, such as the "L" marking on the device
300 indicating the
lateral direction. A depth gauge 315 can be included on the distal section 302
to provide depth
positioning information to the user. The device 300 can be gripped using the
finger grip 316 and
compression element 308. The compression element 308 can be pushed forward to
advance the
shaft 310 and sample collector 312 distally relative to the distal portion 302
as shown in FIG. 5B
to retrieve a sample of sinus fluid. The compression element 308 provides a
force to retract the
sample collector 312 proximally in the absence of a force applied by the user.
The compression
element 308 can function as an automatic retraction of the sample collector
312 after a sample of
sinus fluid has been retrieved. The compression element 308 can be fully
pushed forward to
contact the sample collector 312 with the sample solution as shown in FIG. 5C.
Pushing the
sample collector 312 distally out of the device can minimize losses of the
collected sinus fluid.
[0076] FIGS. 6A-6C illustrate aspects of a device configured to sample a
sinus in accordance
with some embodiments. FIGS. 6A-6C illustrate a hand held sample collector 400
with a distal
section 402 and a proximal section 404. The distal section 402 includes a
distal end 406
configured to be introduced through the nares of the patient. The distal end
406 can be advanced
and retracted by an actuator, such as the slider 408. The slider 408 can slide
along the body of
the proximal section 404. The proximal section 404 includes a finger grip 410
and a thumb grip
411. The thumb grip 411 can pushed to advance the sample collector 412 as
illustrated in FIG.
6B. The thumb grip 411 can retract in the absence of an applied force to
retract the sample
collector back within the distal portion 406. The slider 408 can further
retract the distal end 406
to expose the sample collector 412 as shown in FIG. 6C for contact with a
sample solution while
minimizing sample loss. The slider 408 can provide orientation of the distal
end 406 to the user.
For example, the distal end can be curved in the same direction/side as the
location of the slider
as shown in device 400. The bumps 414 on the distal portion 406 can function
as a depth gauge
to provide additional positioning and orientation information to the user.
[0077] FIGS. 7A-7C illustrate aspects of a device configured to sample
sinus fluid in
accordance with some embodiments. The hand held sample collector 500 includes
a distal
section 502 and a proximal section 504. The distal section 502 includes a
distal end 506 with an
outer covering 508 having a patterned cut distal end section 510 that allows
the sample collector
- 26 -
CA 03057853 2019-09-24
WO 2018/183421 PCT/US2018/024729
512 to advance distally past the covering 508. The covering 508 can be made
out of a flexible
and biocompatible material such as silicone. The device 500 includes a finger
grip 514 and
thumb grip 516. The thumb grip 516 can be advanced to push the sample
collector 512 distally
past the covering 508 as shown in FIG. 7B. The sample collector 512 advances
past the
patterned cut distal end section 510 to contact the sinus fluid. The sample
collector 512 can be
retracted and covered while removing the device from the user to prevent
contaminating the
sample collector with mucous from areas besides the targeted sinus fluid. The
thumb grip 516
can include markings or a colored section 518 to provide orientation
information to the user,
such as the direction of the bend in the distal section 502. The ridge 520 on
the distal section 502
.. can function as a depth gauge to provide additional positioning and
orientation information to the
user. The covering 508 can be pulled back to expose the sample collector 512
to the sample
solution as shown in FIG. 7C. The covering 508 can be patterned or scored to
fold back as it is
pulled back away from the sample collector 512. Retracting the covering 508
relative to the
sample collector 512 can help minimize the sample loss from the sample
collector 512.
[0078] FIGS. 8A-8C illustrate aspects of a device configured to sample a
sinus in accordance
with some embodiments. The hand held sample collector 600 includes a distal
section 602 and a
proximal section 604. The distal section has a two piece construction with a
first distal sleeve
portion 606 and second distal sleeve portion 608. The proximal section 604
includes a finger
grip section 610 and a thumb grip 612. The finger grip section 610 includes a
marking 611 to
label the lateral side of the device to let the user know the orientation of
the bend in the distal
section 602 of the device 600. The thumb grip 612 can be pushed distally to
expose the sample
collector 614 to collect a sample of sinus fluid as shown in FIG. 8B. The
thumb grip 612 can be
retracted to retract the sample collector 614 having the sinus fluid sample
back within the distal
section 602 prior to removing the device 600 from the patient to avoid
contaminating the
.. collected sample. The sample collector 614 can be processed after the
device 600 is removed
from the patient. The sample collector 614 can be exposed to the sample
solution by removing
the first distal sleeve portion 606 and second distal sleeve portion 608 as
shown in FIG. 8C.
[0079] FIGS. 9A-9C illustrate aspects of a device configured to sample a
sinus in accordance
with some embodiments. The hand held device 700 has a pen type shape with a
distal section
702 and a proximal section 704. The distal section 702 includes a distal tip
706 with a patterned
opening 708. The sample collector 710 can be advanced past the patterned
opening by
advancing the slider 712. The device 700 includes two sliders 712 on a central
portion 713 of
the device. The body of the device 700 can include a marker 714 to provide
information on the
orientation of the device to the user, such as the direction of the bend of
the distal section 702.
The device 700 can include a handle 716 on the proximal section 704. The
device 700 can be
-27 -
CA 03057853 2019-09-24
WO 2018/183421 PCT/US2018/024729
gripped by the user using the thumb and middle finger. The slider 712 can have
multiple
positions. A retracted position is shown in FIG. 9A. A partially advanced
slider 712 position is
shown in FIG. 9B with the sample collector 710 advanced distally past the
patterned opening
708. The slider 712 can be advanced further to expose the sample collector 710
to the sample
solution as shown in FIG. 9C. The slider 712 has a retracted position, sample
position, and
testing position. The device 700 can include a lock at each of the retracted
position, sample
position, and testing position. The slider 712 can be configured such that
both sliders 712 are
pushed to allow movement of the slider 712. The device 700 can be used for
either nostril by
rotating by 180 degrees. The distal tip 706 can be made out of a soft polymer
material like
silicone to be comfortable for the patient. The shape of the engagement 720
between the distal
tip 702 with a handle portion can be contoured as shown in FIGS. 9A-9C such
that the contour of
the engagement 720 can provide depth and orientation information to the user
of the device. The
patterned opening 708 can open like an alligator jaw to protect the sample
collector 710 from
being contaminated by nasal mucous or bacterial or cross contamination while
the device is
advanced in the nostril or retracted from the nostril after sampling. The
device can include a
shoulder or fin 718 adjacent to the sample collector 710 to facilitate opening
of patterned
opening 708. The shoulder or fin 718 can push open the patterned opening 708
and also assist
with pushing open and holding open the patterned opening 708 when the sample
collector 710 is
retracted to minimize sample loss.
[0080] FIGS. 10A-10D illustrate aspects of a device configured to sample a
sinus in
accordance with some embodiments. The hand held device 800 has a distal
section 802 and
proximal section 804. The proximal section 804 includes a handle 806 with a
slider 812. The
distal section 802 includes a distal portion 808. The distal portion 808
includes an orientation
marker 810 to display the orientation of the bend in the distal portion 808.
The distal portion 808
can be rotated 180 relative to the handle 806 so that the device 800 can be
used for either nostril.
The distal portion 808 can be made out of a soft material such as silicone to
improve patient
comfort. The slider 812 can be advanced to the sample mark 814 to distally
advance the sample
collector 816 past the distal portion 808 as shown in FIG. 10C. The sample
collector 816 can be
further advanced using the slider 812 as shown in FIG. 10D to contact the
sample solution.
[0081] FIGS. 11A-11C illustrate aspects of a device configured to sample a
sinus in
accordance with some embodiments. FIGS. 11A-11C illustrate a hand held sample
collector 900
with a distal section 902 and a proximal section 904. The distal section 902
includes a distal end
906 configured to be introduced through the nares of the patient. The proximal
section 904 is
configured to slide relative to the distal section 902 to move the sample
collector 910, illustrated
as a swab, relative to the distal end 906. The device 900 is configured to be
gripped with a
- 28 -
CA 03057853 2019-09-24
WO 2018/183421 PCT/US2018/024729
human hand with a finger grip 912 on the distal portion 902 and a thumb grip
914 on the
proximal portion 904. The device 900 includes a marking 916 to indicate the
orientation of the
bend in the distal end 906. The device can include a collar 908 to prevent
further advancement
of the distal end 906 after it has been inserted in the flares. The device 900
can be operated with
one hand such that movement of a thumb on the thumb grip 914 can advance the
proximal
portion 904 relative to the distal portion 902. The distal end 906 can be
advanced through the
nares to the target location followed by advancing the proximal portion 904
and sample collector
910 relative to the distal portion 906 to contact the sinus fluid as shown in
FIG. 11B. After the
sample has been collected the device 900 can be removed from the patient and
the proximal
portion 904 can be retracted proximally relative to the distal portion 902 to
completely separate
the proximal portion 904 and the distal portion 902 as shown in FIG. 11C. The
sample collector
910 with the sinus sample can be tested using the rapid diagnostic testing
methods disclosed
herein.
[0082] FIGS. 12A-12E illustrate aspects of a device configured to sample
a sinus in
accordance with some embodiments. The hand held device 1000 has a pen type
shape with a
distal section 1002 and a proximal section 1004. The distal section 1002
includes a distal tip
1006 with a patterned opening 1008. The sample collector 1010 can be advanced
past the
patterned opening by advancing the slider 1012. The device 1000 includes two
sliders 1012 on a
central portion 1013 of the device. The body of the device 1000 can include a
removable depth
gauge 1018. The depth gauge 1018 can be rotated 90 degrees to a major axis of
the device 1000
to maximize visualization of the nasal cavity. FIG. 12B illustrates the device
1000 with the
depth gauge 1018 removed. The body of the device 1000 can include a marker
1014 to provide
information on the orientation of the device to the user, such as the
direction of the bend of the
distal section 1002. The device 1000 can include a handle 1016 on the proximal
section 1004.
The device 1000 can be gripped by the user using the thumb and middle finger.
[0083] The slider 1012 can have multiple positions. A retracted position
is shown in FIGS.
12A and 12C. A partially advanced slider 1012 position is shown in FIG. 12D
with the sample
collector 1010 advanced distally past the patterned opening 1008. The slider
1012 can be
advanced further to expose the sample collector 1010 to the sample solution as
shown in FIG.
12E. Thus the slider 1012 has a retracted position, sample position, and
testing position. The
device 1000 can include a lock at each of the retracted position, sample
position, and testing
position. The slider 1002 can be configured such that both sliders 1012 are
pushed to allow
movement of the slider 1012. The device 1000 can be used for either nostril by
rotating by 180
degrees. The distal tip 1006 can be made out of a soft polymer material like
silicone to be
comfortable for the patient. The patterned opening 1008 can open like an
alligator jaw to protect
- 29 -
CA 03057853 2019-09-24
WO 2018/183421 PCT/US2018/024729
the sample collector 1010 from being contaminated by nasal mucus or bacterial
or cross
contamination while the device is advanced in the nostril or retracted from
the nostril after
sampling. The device can include a depth gauge 1018 adjacent to the sample
collector 1010.
The device 1000 can optionally include a safety that makes it difficult to
accidentally fully push
the sample collector past the sample configuration (FIG. 12D) while the device
is deployed in
the patient. The device 1000 can allow for improved endoscopic camera access
for the user. For
example, the depth gauge 1018 can be removed to improve clearance for a camera
or for
visualization as shown in FIG. 10B.
[0084] FIGS. 13A-13E illustrate aspects of a device configured to sample
a sinus in
accordance with some embodiments. The hand held device 1100 has a distal
section 1102 and a
proximal section 1104. The distal section 1102 includes a distal tip 1106 with
a patterned
opening 1108. The sample collector 1110 can be advanced past the patterned
opening by
advancing the handle 1113. The body of the device 1100 can include a removable
depth gauge
1118. FIG. 13B illustrates the device 1100 with the depth gauge 1118 removed.
The different
appearance of the body of the device 1100 and the distal section 1102 can also
provide depth and
orientation information to the user. The body of the device 1100 can include a
marker 1114 to
provide information on the orientation of the device to the user, such as the
direction of the bend
of the distal section 1102. The device can be held by the handle 1113 and
finger grips 1112. A
retracted position is shown in FIGS. 13A and 13C. An advanced handle 1113
position is shown
in FIG. 13D with the sample collector 1110 advanced distally past the
patterned opening 1108.
The handle 1113 can be removed as shown in FIG. 13E. The bottom side of the
device 1100
includes a slider 1120 configured to advance the sample collector 1110 to
contact the sample
solution as shown in FIG. 13E. The device 1100 can be used for either nostril
by rotating by 180
degrees. The device 1100 can optionally include a safety that makes it
difficult to accidentally
fully push the sample collector past the sample configuration while the device
is deployed in the
patient. The device 1100 can allow for improved endoscopic camera access for
the user.
[0085] FIGS. 14A-14E illustrate aspects of a device configured to sample
a sinus in
accordance with some embodiments. The hand held device 1200 has a distal
section 1202 and a
proximal section 1204. The distal section 1202 includes a distal tip 1206 with
a patterned
opening 1208. The sample collector 1210 can be advanced past the patterned
opening 1208 by
advancing the handle 1213. The handle 1213 can be advanced and retracted by
the user's thumb.
The body of the device 1200 can include a removable depth gauge 1218. FIG. 14B
illustrates
the device 1200 with the depth gauge 1218 removed. The different appearance of
the body of
the device 1200 and the distal section 1202 can also provide depth and
orientation information to
the user. The body of the device 1200 can include a marker 1214 to provide
information on the
-30-
CA 03057853 2019-09-24
WO 2018/183421 PCT/US2018/024729
orientation of the device to the user, such as the direction of the bend of
the distal section 1202.
The device can be held by the handle 1213 and finger grips 1212. A retracted
position is shown
in FIGS. 14A and 14C. An advanced handle 1213 position is shown in FIG. 14D
with the sample
collector 1210 advanced distally past the patterned opening 1208. The handle
1213 can be
removed as shown in FIG. 14E. The bottom side of the device 1200 includes a
slider 1220
configured to advance the sample collector 1210 to contact the sample solution
as shown in FIG.
14E. In some embodiments the handle 1213 can be configured such that it has to
be removed
prior to being able to advance the slider. The device 1200 can be used for
either nostril by
rotating by 180 degrees. The device 1200 can optionally include a safety that
makes it difficult
to accidentally fully push the sample collector past the sample configuration
while the device is
deployed in the patient. The device 1200 can allow for improved endoscopic
camera access for
the user.
[0086] FIGS. 15A-21 illustrates devices configured to sample a sinus in
accordance with
some embodiments. For example, in FIGS. 15A-15D, hand held device 1300 has a
distal section
1302 and a proximal section 1304. Hand held device 1300 includes a sample
collector 1310
housed within a sleeve 1305 with a sleeve opening 1307, a main body 1330, a
(thumb ring)
handle 1313 and a depth stop 1318. Sleeve 1305 couples to main body 1330 via a
couplers,
1332. Couplers 1332 can have a double tip lock feature as shown in FIG. 17A or
a single locking
tip feature as shown in FIG. 17B. The main body may also function as a handle,
and (as shown)
includes concave gripping regions that may be grasped.
[0087] Sample collector 1310 (e.g., swab) can be advanced past sleeve
opening 1307 by the
ring handle 1313, which may be configured to extend the sample collector a
predetermined
distance from the distal end. This first predetermined distance is configured
to extend into the
correct sample region (e.g., the sinus, such as the middle meatus or maxillary
sinus), while
avoiding regions distal to these regions which may otherwise contaminate the
sample. Handle
ring 1313 includes a connector (shown as a handle ring cavity) 1315 that is
able to engage a
proximal end of the sample collector 1310 while both elements are retained
within main body
1330. Sample collector includes at least one notch 1311 for coupling to handle
ring 1313 as
shown in FIG. 16. FIG. 21 shows an alternative design for coupling sample
collector 1310 with
handle 1313, which includes one or more notch 1311 (two are shown in this
example) that is
vertically arranged and parallel to the longitudinal axis of sample collector
1310. Sample
collector 1310 can be advanced past sleeve opening 1307 the first
predetermined distance (e.g.,
between 5 and 20 mm (e.g., between 7 mm and 15 mm, between 8 mm and 12 mm,
etc.) by
advancing handle 1313. Handle 1313 can be advanced and retracted by the user's
thumb.
Having the handle 1313 limited to only advancing the sample collector 1310 the
first pre-
- 31 -
CA 03057853 2019-09-24
WO 2018/183421 PCT/US2018/024729
determined distance from sleeve opening 1307 may prevent a longer portion of
sample collector
1310 from being extended further into the sinus of the subject and causing
discomfort and pain,
and/or contamination. Once a sample has been collected on sample collector
1310, sample
collector 1310 may be retracted within sleeve 1305, and device 1300 removed
from subject's
sinus and nasal cavity, the sample can be processed for assaying.
[00881 In both the designs shown in FIGS. 18A and 18B, handle 1313 can
be removed.
Removal may also release a locking mechanism that prevents the second
actuator/control (shown
as slider 1312 in FIG. 15A) from extending the sample collector. Removal may
be achieved
with minimal force by pulling it in an opposite lateral direction from the
body of device 1300.
Once handle 1313 has been removed from device 1300, slider 1312, shown
disposed
longitudinally along device 1300 in FIG. 15A can advance the sample collector
1310 beyond
sleeve opening 1307 for processing a second predefined distance that is
typically further than the
first predefined distance. Thus, the distance that slider 1312 can advance
sample collector 1310
may be greater than the distance sample collector 1310 can be extended with
handle 1313. This
second predetermined distance may allow the sample collector 1310 to be
inserted into a sample
(e.g., lysis buffer) tube for processing the sample, as described in greater
detail below.
[0089] Any of these devices may also include a centering element 1340
(which may also be
referred to a spacer or centering element) between the sample collector and
the main body.
Spacer 1340 in FIG. 17A is a protrusion on either the sample collector (e.g.,
near the distal end)
or the main body that prevents sample collector 1310 from contacting the inner
sides of sleeve
1305 when sample collector 1310 is extended and retracted from sleeve 1305.
Preventing
sample collector 1310 from contacting the internal sides of sleeve 1315 is
especially important
when retracting sample collector 1310 because if the sample-containing sample
collector 1310
scrapes past the internal sides of sleeve 1305 as it is being retracted, a
portion of the sample
collected on that surface of sample collector 1310 will be lost, thus
diminishing the amount of
sample (e.g., cells or bacteria) available for processing and detection.
[0090] In the variations shown in FIG. 22, hand held sample device 1400
has a distal section
1402 and a proximal section 1404. Hand held device 1400 includes a sample
collector 1410
housed within a sleeve 1405 with a sleeve opening 1407, a main body 1430, a
(thumb ring)
handle 1413 and a depth stop 1418.
[0091] Unlike the previously discussed embodiment where the handle is
coupled to the
sample collector with the main body, and where upon obtaining a sample, the
user can disengage
the handle by pulling the handle laterally away from the rest of the device,
in the variations
shown in FIG. 17, the device 1400 may include an additional safety feature to
prevent
inadvertent uncoupling of handle 1413 from sample collector 1410 and main body
1430. In this
-32-
CA 03057853 2019-09-24
WO 2018/183421 PCT/US2018/024729
variation, main body 1430 includes a channel 1439. Channel 1439 is of a
predetermined length
with respect to main body 1430. In operation, handle 1413 couples with sample
collector 1410
and channel 1439 provides is of a predetermined length that handle 1413 can
only slide a given
distance within main body 1430 when coupled. The predetermined distance
corresponds to the
distance sample collector 1410 can extend from sleeve 1405. In any of the
device variations
described herein, the handle may include a release 1442. Release 1442, when
engaged with
channel 1439, may allow handle 1413 to extend and retract sample collector
1410 (e.g., the
predetermined first length). To release handle 1413, a user presses and slide
release element
1442 to allow release element 1442 to disengage from main body 1430 of device
1400. Then,
similar to other embodiments, device 1400 includes a slider 1420 that can
extend sample
collector1410 a second predetermined distance (that may be greater than the
first predetermined
distance possible when sample collector 1410 is extended by handle 1413).
Methods of using extraction device
[0092] Also disclosed herein are methods of using the devices described
above, as well as
sinusitis assays using them.
[0093] For example, the following steps can be taken to obtain a sample
of a patient's
infected mucous. As will be described in greater detail below, an alignment
guide may be
included as part of the apparatus to guide the user (e.g., physician, nurse,
technician, etc.) in
using the device to take a mucosal sample from the proper region of the
patient's sinus. For
example, when the patient is seated or lying down, a user may place the
sampling device (or
other embodiment of the device) so that the alignment guide is oriented
properly relative to the
long axis of the patient's body (e.g., parallel to the long axis of the
patient's body or
perpendicular to the long axis). The nostril intended to be sampled is first
determined, and either
a corresponding device is used or a positionable alignment guide is configured
for use in the
selected nostril. For example, the alignment guide may be attached in the
appropriate R or L
position on the device. If the patient is lying down, the alignment guide may
be aligned
approximately perpendicular to the floor and inserted into the subject's nasal
passage. If the
patient is in a sitting or standing position, the alignment guide may be
aligned approximately
parallel to the floor and likewise inserted into the subject's nasal passage.
The alignment guide
may therefore position the device, when oriented in the proper left or right
nostril so that the
device is at an approximately 33 to 37 degrees angle with respect to the
frontal plane, including
the longitudinal axis, of the patient's body (e.g., the long axis, between the
feet and head). The
frontal plane of the patient's body is typically parallel to the surface on
which the patient is
lying, when lying flat, on their back. Insert sleeve of the device (e.g., the
distal end) into the
- 33 -
CA 03057853 2019-09-24
WO 2018/183421 PCT/US2018/024729
subject's nasal passage, ensuring that the angle of sleeve is pointed downward
to follow the
natural curvature of the nasal passage. Discretion should be used where user
experiences
resistance when inserting the device into the nasal cavity of the subject. The
depth stop on the
device may provide a safety measure and prevent the user from inadvertently
inserting more of
the device into the subject's nasal cavity than is needed or safe.
[0094] Next, user pushes the slider forward to expose the swab's distal
end to the sinus
middle meatus. In other embodiments, the thumb ring is coupled to the swab
element. There,
the user may use the thumb ring by inserting his thumb through the thumb ring
aperture and
sliding the thumb ring forward to expose the distal end of the swab for
sampling the sinus region.
Once a sample has been collected, the user can retract the sample collector
distal end using the
slider. In other embodiments, the user can retract the distal end of the swab
back into the sleeve
by pulling the thumb ring proximal end away from the main body. The device
then can be
removed from the nasal cavity of the subject and the sample collected can be
now tested for
bacterial presence.
[0095] FIGS. 25A-25K illustrate another method of operating the sinus
mucosal sample
devices described herein. In FIG. 25A, the sinus collection device is inserted
into the patient's
nose by placing the device (probe) at an approximately 45 degree angle
relative to the floor
(shown by arrows), with the patient in an upright, sitting position. As shown
in FIG. 25B, the
collection device is then placed into the sinus by inserting the sinus
collection device tip through
the nasal cavity and into sinus middle meatus until the depth stop of the
device is engaged (e.g.,
the stop touches nasal entry), or until a slight tissue resistance is felt.
FIG. 25C illustrates the
collection device properly positioned into the middle meatus region.
[0096] After positioning the collection device, as shown in FIG. 25D,
the thumb ring may be
pushed to expose the swab tip and collect specimen, by pushing in the
direction of the arrow to
expose swab tip to sinus fluids. The tip of the swab is extended a first
predetermined distance
into the proper region of the sinus, as shown in FIG. 25E, showing the swab
tip exposed for fluid
collection. Thereafter, the swab tip may be retracted into collection device
by reversing the
thumb motion and retracting the swab tip into collection device, as shown in
FIG. 25F. FIG.
25G shows the swab tip fully retracted into Collection device. Thereafter, the
collection device
may be removed from the nose, as shown in FIG. 25H. The collection device,
once removed
from nose, is ready for assay testing, which may be performed in a physician's
office or other lab
area.
[0097] For processing, the distal controller, e.g., the distal handle or
thumb loop, may be
removed, as shown in FIG. 25i. Removing the handle in any of these devices may
release a
limiter or lock that prevents the swab from extending distally. The collection
device may be fully
- 34-
CA 03057853 2019-09-24
WO 2018/183421 PCT/US2018/024729
or partially extended before testing the sample. For example, FIG. 25K shows a
device with the
swab fully extended for processing; the swab portion (circled) was fully
extended by sliding the
second (distal) controller distally. Once fully extended, the swab may be
processed by inserting
into a buffer (e.g., lysing buffer) as described in greater detail below.
[0098] In any of the variations described herein, the apparatus may be
configured so that the
extendable portion (e.g., including the swab) has one or more (e.g., two)
stops on it to prevent
overextension and contamination. For example, a collection device may be
prevented from
being extended in its most extended form (e.g., when the thumb ring removed,
as illustrated
above) to prevent inadvertently applied pressure on the swab tip from forcing
the swab shaft
back into the protective device tip. This may otherwise lead to contaminating
the mucous
collection with fluid from the outside of the protective sheath. Thus, as show
in FIGS. 38A-38G
and 39A-39B, the extendable portion 3900 may include a second stop 3905 that
prevents the
swab for easily being pushed back into the sheath during processing.
[0099] As shown in FIGS. 38A-38B, the extendable (swab) inner portion
includes a pair of
stops 3901, 3905 that limit the movement of the swab during extension,
including during final
extension so that the swab may be added to a solution, as described herein.
FIGS. 39C-39F
illustrate enlarged views of various portions of this example of an extendable
element. The stops
are shown as projections, tabs, or protrusions extending from the structure
that may engage a
separate stop portion. FIG. 39A shows an image of one example of the
extendable (swab-
containing) inner portion shown in FIGS. 38A-39B, including the two stops.
FIG. 39B is
another view of the end region including the stops 3901, 3903. The proximal
stop prevents the
device from being withdrawn back into the distal end after being fully
extended for transfer to a
sampling fluid, preventing contamination. When the device is fully extended
the lock pushes
past the tip of the sheath. Due to the spring action of the swab shaft, the
extension stop hits the
distal tip of the sheath and prevents it from easily backing back into the
sheath.
Sample assay
[0100] Also described herein are assays that can be used to detect and
diagnose bacterial
sinusitis. More specifically, the assays may utilize antigen binding agents
(e.g., antibodies,
antibody fragments, etc.) for detecting markers specific to the types of
bacteria contained with
the sinus secretions of the subject. The methods disclosed herein may allow
detection of
signature antigens that are associated with specific bacterial pathogens
within the paranasal sinus
cavity, and may thus allow a caregiver better insight as to whether
prescribing an antibiotic is
beneficial. The assays may also provide information that aids a caregiver in
deciding which
- 35 -
CA 03057853 2019-09-24
WO 2018/183421 PCT/US2018/024729
antibiotic regimen would provide the most favorable outcome and most
importantly, reduce the
use of broad-spectrum antibodies in cases where such treatment would not
effective.
[0101] These assays may utilize biomarkers that are specific antigens
indicating the presence
of the organism or pathological process of interest. "Biomarkers" are
naturally occurring
molecule, gene, or characteristic by which a particular pathological or
physiological process,
disease, or the like can be identified or characterized. The term "biomarker"
may refer to a
protein measured in sample whose concentration reflects the severity or
presence of some
disease state. Biomarkers may be measured to identify risk for, diagnosis of
or progression of a
pathological or physiological process, disease or the like. Exemplary
biomarkers include
proteins, hormones, prohormones, lipids, carbohydrates, DNA, RNA and
combinations thereof.
Although the examples of assays described herein are specific to antigen
binding agents such as
antibodies in a sandwich-type lateral flow assays, other assays, including
nucleotide
hybridization, enzymatic and ligand-receptor type assays may also or
alternatively be used.
[0102] In some variations, the assay is capable of detecting at least
one biomarker, and more
preferably two biomarkers, including biomarkers from each of a plurality of
bacterial types
linked to sinusitis. The assays can be further modified to detect greater than
two biomarkers
(e.g., preferably three). Furthermore, detecting the biomarkers can mean
detecting a portion of
the proteins, hormones, prohormones, lipids, carbohydrates, DNA, RNA and
combinations
thereof. The biomarkers may also be a biologically active variant of the
naturally occurring
molecule of interest. For example, a protein or DNA biomarker can have at
least 65%, at least
70%, at least 80%, at least 85%, 86%, 87%, 88%, or 89%, and more typically
90%, 91%, 92%,
93%, 94%, and most common, 95%, 96%, 97%, 98% or 99% conformity or sequence
identity to
the native molecule.
Assays and kits
[0103] Any of the assays described herein may be part of a kit that allow a
user to easily
perform the assays for detecting antigens that are the primary cause bacterial
sinusitis. The kits
may include the sampling device that is described above. The kits can also
include a means for
lysing the cells in order to expose the target antigens of interest, such as a
lysis buffer, and a
means for delivering the lysed supernatant to the assay portion of the kit.
[0104] A first critical step in obtaining accurate results is in properly
processing the sample
extracted from the sampling device. Proper processing includes formulating an
appropriate lysis
buffer. While finding a lysis buffer that can lyse one particular type of cell
is fairly
straightforward, it is much more challenging to prepare a buffer composition
that is able to lyse
multiple bacterial cells of interest while protecting the bioactivity of the
antigens of interest and
- 36 -
CA 03057853 2019-09-24
WO 2018/183421 PCT/US2018/024729
while lysing in a reasonable amount of time (e.g., less than 15 minutes). Most
cells can be lysed
by mechanical means, such as sonification or freeze/thaw cycles, but such
methods may require
additional equipment. Thus, in some instances, it is preferable to use milder
methods, such as
detergents, for disrupting the cell membrane. Detergents may disrupt the lipid
layer surrounding
.. cells by solubilizing proteins and interrupting the lipid-lipid, protein-
lipid, and/or protein-protein
interactions. The appropriate detergent composition also depends upon the type
of cells to be
lysed, be it animal, bacteria, or yeast.
[0105] In the developing the "triple" assay for detecting one or more of
M. catarrhalis, S.
pneumoniae, and H. influenzae described herein, various lysis buffers were
tested for their ability
to lyse all of the bacterial cells of interest, namely M. catarrhalis, S.
pneumoniae, and H.
influenzae. For example, while N-Lauroylsarcosine effectively lysed NTHI and
exposed antigens
for recognition by their antigens, it did not effectively lyse M. catarrhalis.
Next, TritonX-100, a
commonly used lysis buffer was also tested and appeared to lyse M.
catarrhalis, but did not
work well with NTHI (nontypeable Haemophilus influenzae) The inventors
determined that the
addition of sucrose to a lysis buffer containing N-Lauroylsarcosine (e.g.,
Sarkosyl) was effective
in lysing all three bacterial cell lines of interest. Without being limited to
a particular theory of
operation, the addition of an appropriate percentage of sucrose to the N-
Lauroylsarcosine lysis
buffer may provide an osmotic shock to the cell membranes of the more lysis-
resistant cell
membranes to achieve appropriate lysing.
[0106] FIG. 27 illustrates various examples of lysis buffers that have been
examined,
indicating their effectiveness with different types of the bacterial strains
of interest. Although
many of the buffers identified are effective in lysing one of the types of
bacteria, in FIG. 27, only
the 7% sucrose with 1.3% Sarkosyl was effective (and efficient) in lysing all
three of M.
catarrhalis, S. pneumoniae, and H. influenzae. For example, 0.1% Triton X-100
with lysozyme
effectively/efficiently lysed M. catarrhalis but not S. pneumoniae or H.
influenzae, while 1%
Sarkosyl effectively/efficiently lysed H. influenzae but not S. pneumoniae,
and M. catarrhalis.
None of B-PER reagent, 0.1% Triton X-100 without lysozyme, RIPA buffer, 0.1%
Tween 20,
0.1% IGEPAL, 0.1% Tergitol or 0.1% Brij 35 was sufficiently effective in
lysing any of M.
catarrhalis, S. pneumoniae, and H. influenzae. Thus, it was surprising that
only the combination
of sucrose with Sodium lauroyl sarcosinate, (e.g., 7% sucrose with 1.3%
Sarkosyl) was able to
lyse all three types of bacterial cells at near-comparable levels. Although
only 7% sucrose with
1.3% Sarkosyl, other combinations of sucrose (e.g., between 3% and 15%
sucrose, and between
0.5% and 3% Sarkosyl, etc.) may be effective. Thus, the combination of osmotic
and anionic
detergent disrupting cell wall appears to be most effective.
- 37 -
CA 03057853 2019-09-24
WO 2018/183421 PCT/US2018/024729
[0107] Thus, in one variation, a lysis buffer appropriate for use with
all of Haemophilus
influenzae, Moraxella catarrhalis and Streptococcus pneumoniae includes
between 5-15%
sucrose (e.g., 7% sucrose), EDTA, PMSF, 1.3% sarkosyl (Sodium lauroyl
sarcosinate), 50 mM
Tris at a pH of 8Ø
[0108] The assay may include antigen binding agents (e.g., antibodies,
antibody fragments,
etc.) that specifically bind the protein biomarker of interest and components
for immunoassay to
detect the protein biomarkers using associated antibodies. The kits can also
contain instructions
on carrying out the sampling, performing the assay, and any of the methods
associated with this
invention.
[0109] The present invention provides for methods for detecting at least
one biomarker that
is specific to a biofilm protein profile for a pathogenic bacteria. In
general, immunological
methods are well-known in the art, and performed routinely for diagnostic and
research
purposes. ELISA (enzyme-linked immunosorbent assay) is a powerful tool in
studying
antibodies and antigens and their concentrations in a sample. ELISA can be
used to detect the
presence of antigens that are recognized by an antibody or conversely, ELISA
can be used to test
for antibodies that recognize an antigen. An immunoassay that utilizing the
ELISA platform is
the two antibody sandwich ELISA.
[0110] Sandwich ELISA is used to determine antigen concentration in
unknown samples. If
a pure antigen standard is available, the assay can determine the absolute
amount of antigen in an
unknown sample. Sandwich ELISA requires two antibodies that bind to epitopes
that do not
overlap on the antigen. This can be accomplished with either two monoclonal
antibodies that
recognize discrete sites or a batch of affinity-purified polyclonal
antibodies. A purified first
antibody (the capture antibody) is bound to a solid phase. A sample containing
the
corresponding first antigen is added and allowed to complex with the bound
first antibody.
Unbound first antigen is washed away and a second antibody with a label (the
detecting
antibody) is allowed to bind to the first antigen, thus forming a "sandwich".
The assay is then
either quantitative or qualitative amount of the second/detecting antibody
bound. It is also
possible to first bind the antigen to the labeled/detecting antibody and then
expose the antigen-
labeled antibody complex to the bound antibody.
[0111] The present invention allows for greater than one sandwich ELISA
assay, in order to
concurrently detect one or more of Haemophilus influenzae, Moraxella
catarrhalis and
Streptococcus pneumoniae, which together may account for >90% of bacterial
sinusitis. In some
variations, additional pathogens may also be detected (e.g., Pseudomonas
aeruginosa). In some
embodiments, the assay contains two, three, or more distinct antigen and
antibody pairings such
that more than one antigen can be detected with one single assay. The results
of the presence of
- 38 -
CA 03057853 2019-09-24
WO 2018/183421 PCT/US2018/024729
at least one or more antigen can be qualitatively obtained, meaning that there
is a threshold
concentration of the targeted antigen or antigens within the sample. Also, the
assay can provide
more quantitative measure of the presence of one or more antigens by
comparison with a
standard or reference. A standard or reference refers to a sample that has a
known antigen and
biomarker, and in some cases, a known concentration of the known antigen and
biomarker or
antigens and corresponding biomarkers of interest.
[0112] An optical (including, but not limited to visual) indicator may
be used to indicate the
presence of an antigen. The visual indicator is typically displayed on a
region of the assay. The
visual indicator can be colorimetric. The visual indicator can also be a
symbol, such as a line,
that indicates the presence of a particular antigen. The immunoassay may
contain labeling next
to the regions where different indicators for the presence of various antigens
will be shown.
Having the labeling will alert the user as to which particular antigen is or
antigens are present
with the sample. A caregiver user will then have a better knowledge as to
which antibiotic, if
any, should be provided to the subject.
[0113] The immunoassay can be of any suitable format. In some examples, the
immunoassay can be performed using a dipstick format where the sample solution
is drawn up
the "dipstick" type assay with capillary action. The immunoassay can also have
a largely
horizontal format such as a lateral flow assay. In this latter formats, the
sample extracted from
the subject's nose is treated such that the cells are lysed in an appropriate
buffer, freeing the
proteins and providing the sample solution. An aliquot of the sample solution
then can be placed
in a sample reservoir on the assay or other region noted on the assay and
migrated across regions
of the assay that contain bound antibodies. Furthermore, the dipstick or flat
format
immunoassays can have a solid support made of any suitable material, such as
nitrocellulose or
polyvinylidene difluoride (PVDF) or other membranes, dipstick, wells, or
tubes.
[0114] In this present example, a combined immunoassay for three different
antigens are
described. The three antigens of interest and described below are all
associated with bacterial
sinusitis, but the overall concept of having a multiple antigen test on one
assay can be applied to
other antigen/antibody systems as well. An example of a lateral flow assay
1500 is shown in
FIGS. 24A-24D. Lateral flow assay 1500 includes a housing 1509 having a sample
inlet 1501
(e.g., sample port) for depositing an aliquot of sample and detection window
1511 for visualizing
the results of the assay. A sample pad 1503 a conjugate pad 1505, and a
detection pad 1507 are
included within a housing 1509. Sample pad 1503 will hold the sample to be run
through the
assay. Sample pad 1503 is in contact with conjugate pad 1505. Conjugate pad
1505 contains the
first antigen binding agent (e.g., shown here as an antibody) specific for at
least one antigen. In
some examples, the conjugate pad may contain two distinct antigen binding
agent (e.g.,
- 39 -
CA 03057853 2019-09-24
WO 2018/183421 PCT/US2018/024729
antibodies) that recognize and bind to two distinct antigens. In other
examples, the conjugate
pad may contain three distinct antigen binding agents that recognize and bind
three distinct
antigens. When a sample is run through sample pad 1503 past conjugate pad
1505, any antigen
or antigens of interest will bind to corresponding antibodies. The antibodies
contained in
conjugate pad 1505 may be linked to a detector molecule. The complex or
complexes of antigen
with detector-labeled antigen binding agent is then delivered across detection
pad 1507 where a
second antigen binding agent (e.g., antibody or antibodies) corresponding to
the one more
antigens are immobilized on a solid support. In this example, distinct
antibodies are affixed in
strips across detection pad 1507 as shown in FIGS. 24B and 24C. Thus, when the
corresponding
antigen complexed with detector-labeled antibody is eluted past the different
regions of detection
pad 1507, the antigen complexed with detector-labeled antibody will bind to
the corresponding
second immobilized antibody and produce a signal as shown in FIGS. 24C and
24D. In any
variation described herein, the pattern of the immobilized antigen binding
agent may be any
appropriate pattern and/or density. For example, one (or preferably all) of
the bound antigen
binding agent may be arranged on the solid phase substrate into a character,
symbol, letter, word,
pictogram, etc. In some variations the antigen binding agent is arranged into
a letter (e.g.,
spelling the initial or type of bacteria (e.g., H.flu, M.cat, S.pneumo, etc.).
[0115] For example, an antigen profile for NTHI may include outer
membrane proteins
(OMP), specifically, OMP P5 and OMP P2. It has been verified that presence of
OMP P5 and
OMP P2 within NTHI biofilm supernatant and thus detection of OMP P5 and OMP P2
with a
sample is indicative of NTHI infection. Corresponding antibodies were
developed to both OMP
P5 and OMP P2. For M. catarrhalis, antibodies to Protein C and Protein D outer
member
proteins (OMP-CD) may be used. For S. pneumoniae, the PsaA (pneumoccal surface
adhesion
A) protein may be a viable antigen to indicate the presence of S. pneumoniae.
PsaA is a surface-
exposed common 37-kilodalton multi-functional lipoprotein detected on all
known serotypes of
Streptococcus pneumoniae.
[0116] FIGS. 31 and 32 illustrate example of other types of cartridge
configurations that may
be useful. For example FIG. 31 illustrates schematically how three different
assays for different
bacteria may be combined into a single assay, using a single lysis buffer for
all three types of
bacteria. In this example, the cartridge 3100 includes a single port 3103. The
cartridge also
includes a window 3109 into an inner region of the cartridge, showing the
solid phase substrate
on which the assay is being run.
[0117] FIG. 32 is another variation of cartridge in which each assay is
run in parallel and the
report out includes three separate windows and/or three separate solid phase
substrates. Both of
the examples shown in FIGS. 31 and 32 include a control band that is formed as
a positive
- 40 -
CA 03057853 2019-09-24
WO 2018/183421 PCT/US2018/024729
control that the labeled antigen binding agent has diffused through the
device. As mentioned
above, any of these device may include a vent or opening at the end opposite
to the cartridge.
Example 1 (Lysis buffer)
[0118] As shown in FIG. 27, different lysis buffers were tested to
determine the best buffer
composition for successfully lysing the cells corresponding to Haemophilus
influenzae (H.flu),
Moraxella catarrhalis (M.cat) and Streptococcus pneumoniae (S.pneumo). Some of
the potential
candidates tested included N-Lauroylsarcosine, Triton X100, Sarkosyl, Sarkosyl
and sucrose,
and Bugbuster (Novogen). Standardized samples with known concentrations of
Haemophilus
influenzae, Moraxella catarrhalis and Streptococcus pneumoniae were used to
test the
effectiveness of each of these buffers. Based on the lysing data, the sucrose
and Sarkosyl lysis
buffer composition appeared to be the most effective in being able to lyse all
three cell types.
Importantly, many combinations (including combinations not including sarkosyl
and sucrose)
did not work for all three cell types and therefore may not be compatible with
a combined assay
for detection of all three cell types.
[0119] Thus, in general, only lysis buffers having an osmotic agent (e.g.,
sucrose) and an
anionic surfactant (sarkosyl, sodium lauroyl sarcosinate, which may be
referred to as an ionic
surfactant, anionic detergent or ionic detergent) was compatible with the
assays for all three of
M. cat, S. pneumo, and H. flu. In particular, lysis buffers having an osmotic
agent between 0.1%
and 15% (w/w or w/v, e.g., between 0.5% and 12%, 0.5% and 10%, etc.) and an
anionic
surfactant between 0.01% and 5% (w/w or w/v, e.g., 0.05% and 5%, 0.1% and 5%,
0.05% and
3%, etc.) were effective, whereas other lysis buffers having non-ionic
detergents/surfactants,
enzymatic agents, or either osmotic agents alone or anionic/ionic surfactants
alone were not
effective. For example the lysis buffer may include an osmotic agent within a
range having a
lower value of 0.1%, 0.2%, 0.3%, 0.5%, 0.6%, 0.7%, 0.8%, 0.9%, 1%, 1.1%, 1.2%,
1.3%, 1.4%,
1.5%, 1.6%, 1.7%, and an upper value of 1.3%, 1.5%, 1.7%, 2%, 2.5%, 3%, 4%,
5%, 7.5%,
10%, 12%, 15%, 20%, etc., where the lower value is less than the upper value,
and an anionic
detergent within a range having a lower value of 0.05%, 0.1%, 0.15%, 0.2%,
0.25%, 0.3%,
0.35%, 0.4%, 0.45%, 0.5%, etc., and an upper value of 1.3%, 1.4%, 1.5%, 1.6%,
1.7%, 1.8%,
1.9%, 2%, 2.1%, 2.2%, 2.3%, 2.4%, 2.5%, 3%, 4%, 5%, 7.5%, 10%, 12.5%, 15%,
17.5%, 20%,
etc., where the lower value is less than the upper value. Examples of anionic
surfactants (e.g.,
detergents) include alkylbenzenesulfonates, sulfates, sulfonates, and
phosphate esters, including
in particular sarkosyl (sodium lauroyl sarcosinate). Surprisingly, only lysis
buffers containing
the combination of an osmotic agent and an anionic surfactant within the
specified ranges were
-41 -
CA 03057853 2019-09-24
WO 2018/183421 PCT/US2018/024729
compatible for use in the assay looking for epitopes specific to each of the
three cell types
(M.cat, S.pneumo, H.flu).
[0120] FIG. 28 illustrates two exemplary lysis buffer that may be used
(and were used to
generate exemplary readings in a lateral flow assays, such as the ones shown
in FIGS. 30A-30C,
and may be used as part of the assay (e.g., kits, systems, etc.) described
herein. FIG. 29
illustrates two exemplary dilution buffers used as part of the assay (e.g.,
kits, systems, etc.)
described herein.
Example 2 ( Swabbing material selection)
[0121] Experiments testing the optimal sampling swab material was also
performed.
Because the region where the sample is to be collected, a subject's nasal and
sinus cavity, is a
fairly sensitive area, it is important to be able to quickly and effectively
gather enough sample
material for assaying. Also, it would be desirable only sample the subject's
nasal and sinus
cavities once because repeated sampling can cause irritation to the subject's
nose and sinus
cavities. In addition, any attempts to gather sample after a first try may
elicit an autonomic
response of excess mucous in the nasal passage that may dilute the sample
collected or blood.
While materials as cotton swabs and gauze can be used, two commercially
available swab
materials were tested for their ability to quickly take up sample. Hydraflock,
Ultraflock, and
Purflock were tested for their ability to take up water, ATS-M (artificial
test soil) lab soil
containing mucin, and bacteria in ATS lab soil in a given time interval. In
addition to the uptake
analysis, materials were also analyzed for their ability to release sample.
Hydraflock showed
100% recovery and release at 10 and 30 seconds. In contrast materials such as
Purflock showed
only 85% recovery and release.
[0122] Thus, in general, the sample collector (swab or swab material)
may be a flocked
material that is coupled to a shaft (e.g., an extendable and/or slideable
shaft, rod, member, etc.).
Flocked materials may minimize entrapment of the cells, while efficiently
absorbing them for
later release. In particular, flocked swabs having split (e.g., bifurcated, or
multiply-split) distal
ends of the fibers, such as the hydroflock material described above work
surprisingly well, even
compared to other flocked materials.
Example 3: assay
[0123] Following sampling with a device as described above for the
collection device, the
swab with the sample is inserted into the lysis buffer by fully extending the
swab tip and
inserting into an appropriate volume of lysis buffer, as shown in FIG. 26. In
this example, the
swab positive end goes into a tube with the lysis buffer (the swab may be the
swab of a sample
- 42 -
CA 03057853 2019-09-24
WO 2018/183421 PCT/US2018/024729
device, such as shown in FIG. 25K). The swab may be mixed or agitated in the
buffer, and
removed (e.g., after between 1-30 sec). The bacteria may be lysed within the
sample buffer by
the action of the lysing buffer. See FIGS. 28 and 29 for examples of lysing
buffer (FIG. 28) and
dilution buffer (FIG. 29). The sample buffer may then be directly applied to
the assay or it may
be diluted (e.g., 1:10) in the lysis buffer or a second buffer (a dilution
buffer, e.g., a Tris buffer),
which may aid in wicking on the membrane. 100-200 microliters of sample may
then be loaded
into the port on a lateral flow cartridge, possibly followed by loading an
additional volume (e.g.,
100-200 microliters) of buffer to induce wicking (as a "chaser"). An assay
configured similar to
that shown in FIGS. 24A-24D and described above may be used (e.g., a lateral
flow assay) in
which there are three separate regions arranged in sequence (adjacent each
other) followed by a
control region. This configuration is one variation of a multiplexed
arrangement. Alternatively
or additional one or more of the bound antibody-containing regions
(sensing/detection regions)
may be present in a parallel region that is fed by the same or a different
port. In these examples
the antigen binding agents are antibodies, including a pair of antibodies is
directed to each
antigen specific to one of the bacterial types; a tethered (e.g., in the
detection region) antibody
(e.g., antigen binding agent) and a soluble (detection) antibody (antigen
binding agent) which
may be linked to a visible marker (readout) such as colloidal gold or a
visible dye (e.g., colored
latex bead). In some variations the assay may be read in about 5 minutes
(e.g., following 2-3
min of lysis or less and 3-5 min on the cartridge or cassette). The time to a
positive or negative
result (as shown by the positive control, which may be cross-reactive to the
detection antibody,
e.g., tethered anti-mouse antibody within a downstream control region), may
depend on the
wicking of the sample in the membrane. Typically after adding the sample to
the assay device
("cartridge" or "cassette"), the sample wicks through a sample pad into a
conjugate pad, then
into the membrane, where the markers may be captured by capture antibody,
concentrating
antibody also bound to the marker (antigen) and having a readout (e.g.,
colloidal gold) so that it
can be visualized.
[0124] As mentioned, the capture antibodies may be laid down on the
membrane in different
characteristic positions (e.g., marked/labeled), and may depend on the wicking
capacity of the
membrane.
[0125] FIGS. 30A-30C illustrate proof-of-concept data showing sensitivity
ranges for assays
as described herein for detection of each of S.pneumo, M.cat, and H.flu,
respectively.
[0126] In FIG. 30A, an assay using a first antigen binding agent that is
labeled and that binds
to OMP-P2 and/or OMP-P5 and a fourth tethered antigen binding agent that binds
specifically to
another region of the same antigen (e.g., OMP-P2 and/or OMP-P5) were used. The
first and
fourth antigen binding agents specific to H. influenzae but not M. catarrhalis
or S. pneumoniae.
-43 -
CA 03057853 2019-09-24
WO 2018/183421 PCT/US2018/024729
In this example, a lateral-flow assay was prepared with these antigen binding
agents, a mucosal
sample (which may be taken using a sampling device such as the ones shown in
FIGS. 15A-23C
and the steps of FIGS. 25A-25K). To generate the curve shown, the sample used
may be from a
cultured example of the bacteria, so that the concentration may be determined
accurately. The
sample was re-suspended a lysis buffer such as the buffer shown in FIG. 28
(e.g., lysis Buffer
#1), so that all three types of bacteria being sampled (e.g., H.flu, M.cat,
and S.pneumo) were
lysed appropriate following approximately 30 sec to 1 min of lysis. The
solution was then
diluted and applied to the sample port 3201 of a lateral flow cartridge such
as the one shown in
FIG. 32, so that it may be fluidically channeled a conjugation region (e.g.,
chamber or bad)
containing the labeled and untethered first antigen binding agent. In this
example, the antigen
binding agent is a monoclonal antibody conjugated to a particle (e.g.,
colloidal gold or dyed
bead) that can be visualized through the window of the cartridge after
travelling (e.g., via
capillary action) from the conjugation region onto and/or across the portion
of the solid phase
structure to which the fourth antigen binding agent is tethered. As
illustrated in FIG. 30A,
different concentrations of sample bacteria (H.flu) were used to generate the
sensitivity curve. In
FIGS. 30A-30C, the detection was performed manually and visually, however
higher sensitivity
may be achieved by using a reader (e.g., optical reader) as mentioned above.
[0127] In this example, as described above, a single lysis buffer was
used for lyisng the
sample so that multiple (e.g., M.cat, H.flu and S.pneumo) bacterial types
could be
simultaneously tested for from the same sample, even after a very brief lysis
(e.g., between 5
seconds and 15 minutes, between 5 seconds and 10 minutes, between 5 seconds
and 5 minutes,
between 5 seconds and 4 minutes, between 5 seconds and 3 minutes, between 5
seconds and 2
minutes, between 5 seconds and 1 minute, between 5 seconds and 45 seconds,
etc., or less than
15 minutes, less than 10 minutes, less than 5 minutes, less than 1 minute,
etc.). The particular
composition (and combination) of lysing agents described herein are
surprisingly effective at
quickly, completely and gently lying the multiple different types/classes of
bacteria without
disrupting the antigens or their ability to be recognized by the antigen
binding agents used.
[0128] In FIGS. 30B, M.cat was detected in parallel (e.g., by loading a
sample of the lysed
solution into the second port 3207 of the cartridge shown in FIG. 32, and
S.pneumo was detected
by loading the solution into the third port 3209 in FIG. 32. Alternatively
concurrent detection
may be performed using a cartridge 3100 such as the one shown in FIG. 31. With
respect to
detection of Moraxella catarrhalis (M.cat) a pair of antigen binding agents
(second and fifth
antigen binding agents or just agents) that are specific to the antigen
Protein C (an outer member
protein) at different portion of the antigen were used; the second antigen
biding agent is labeled
as described above (e.g., using colloidal gold), and the fifth antigen binding
agent is tethered to a
- 44 -
CA 03057853 2019-09-24
WO 2018/183421 PCT/US2018/024729
specific region of the solid phase substrate (e.g., a membrane within the
cartridge). Similarly
detection of Streptococcus pneumoniae (S.pneumo) in FIG. 30C may be performed
using the
lateral flow cartridge such as the one shown in FIG. 32 (applying the sample
into the port 3209).
The antigen in this example is a PsaA antigen that is recognized by both the
third and sixth
antigen binding agents, where the third agent is labeled and the sixth is
tethered. Thus, the
second agent binds specifically to a second antigen specific to M. catarrhalis
but not H.
influenzae or S. pneumoniae. Similarly, the third and sixth agents bind
specifically to the third
antigen specific to S. pneumoniae but not M. catarrhalis or H. influenzae.
[0129] In FIGS. 30A-30C, the sensitivities for detection of cells
(expressed as colony
forming units (CFU)/sample) show thresholds for visual detection between 103-
105 per 100 .1
sample. As mentioned above, this sensitivity may be increased, for example by
using a reader to
read the cartridge.
Insertion Guides
[0130] Any of the apparatuses, e.g., sampling devices such as those
shown in FIGS. 4A-23C
described above, may include an indicator for guiding insertion of the
apparatus into a patient's
nostrils.
[0131] For example, any of these apparatuses may include an alignment
guide, which may
also be referred to herein as an indicator, indicator guide, or guide. The
alignment guide may
help a user in correctly estimating the insertion angle of the device into the
nose and sinus, so
that a sample may be taken as necessary. In general, the angle of the long
axis of the sampling
tool (typically no including the distal bend) may be between 30-40 degrees
(e.g., 33-37 degrees,
etc.) relative to the long axis of the sampling device or tool. This is
illustrated in FIGS. 33A and
33B. In FIG. 33A, the patient is shown sitting or standing in a chair or on
the ground (upright
relative to the patient "ground" 3305) and the alignment guide indicates that
the long axis of the
device is at an angle, a, of between 33-37 degrees (e.g., approximately 35 )
relative to the
ground. The alignment guide 3309 may include a sensor for automatically
detecting this angle
(e.g., including an accelerometer) or a bubble-level, or the like. In some
variations the alignment
guide is an extension (e.g., arm, protrusion, etc.) extending from the body of
the device, as will
be described in greater detail below. For example, when the alignment guide is
a marking on
and/or extension from the body of the sampling device at a 33-37 angle
relative to the long axis
of the device, the user may align the device so that the markings and/or
extension are parallel to
the ground, as shown in FIG. 33A. When the patient is laying down, as shown in
FIG. 33B, the
device may be oriented at an approximately 55 angle relative to the patient
ground 3305', so
-45 -
CA 03057853 2019-09-24
WO 2018/183421 PCT/US2018/024729
that the insertion of the device tip reaches the middle meatus portion of the
sinus in the proper
orientation.
[0132] As mentioned, and as shown in FIGS. 34A-47D, the alignment guide
(indicator) may
be an extension, such a removable arm, that extends from the central body
portion of the device,
also referred to herein as a proximal handle. Removable indicators may be
useful where the
device inserted at complementary angles depending on if the device is to be
inserted into the
patient's left nostril or their right nostril. Because the distal end of the
device is bent, as
described herein, the indicators may therefore be different. Although (as
shown schematically in
FIG. 33A) a pair of alignment guides/indicators may be use, e.g., each at an
angle of between 33-
37 relative to the long axis of the device, in some variations it may be
preferable to include a
removable/repositionable alignment guide/indicator that can be switched
between left and right
sides of the device. For example, the indicator 3403 shown in FIGS. 34B and
34C may snap
onto either the right side ("R") of the device, as shown in FIG. 34B, or it
may be removed and
snapped onto the left side ("L").
[0133] Observations of primary care healthcare providers using the sampling
devices
described herein indicated that without the alignment guides described, these
devices were often
inserted at too great of an angle (e.g., >37 ), resulting in pain and/or poor
sampling.
[0134] In the example shown in FIGS. 35A-35D, the alignment
guide/indicator 3503 snaps
onto a face of the device, as shown in FIG. 35D. A different right side vs.
left side indicator may
be use, or in some variations the same indicator may be used for both left and
right sides, and the
indicator may indicate "left" or "right" patient nostril when attached to the
device.
[0135] In FIGS. 36A-36C the indicator 3603 is shown as a clip-on that
couples to the
sampling device (e.g., the proximal body/handle portion), a shown.
[0136] In the variations shown in FIGS. 34A-36C, the nasal sampling
device (which may be
used for obtaining a sinus secretion sample from a subject's sinus) includes a
proximal handle
3413 (shown as a ring handle) that is configured to be held in a human hand.
This proximal
handle may be configured to releasable couple with a flexible elongate and
extendable shaft that
is housed within an elongate body 3431. The elongate body also includes a
second handle region
3430; the elongate body may be referred to (as above) as a main body. The
elongate body
extends distally from the proximal handle and typically has a distal portion
3402 that is
configured to be inserted into a nare of a patient. The distal portion of the
elongate body is bent
relative to a proximal region by between 10 degrees and 30 degrees (e.g.,
between 12 and 30
degrees, between 15 and 30 degrees, etc.). The distal portion may be
configured as a housing
that may be removable from the rest of the elongate body.
- 46 -
CA 03057853 2019-09-24
WO 2018/183421 PCT/US2018/024729
[0137] As mentioned, each of these devices also includes a sample
collector (not visible in
FIGS. 34A-36C) that is on a distal end of the extendable shaft and is
configured to collect a
sample of sinus fluid for diagnostic testing. The sample collector is housed
entirely within the
distal end of the elongate body in a retracted position (as shown in FIGS. 34A-
36C). These
devices also include a control 3412 on the extendable shaft. The control is a
slider and may be
configured for being thumb or finger operated. The control may be visible
through the elongate
body (e.g., a window or opening through the elongate body) as shown in FIG.
34B. The control
typically has a first position wherein the sample collector is extended
distally out of a distal
opening of the distal end region of the elongate body (not shown), e.g., a
first distance of
between about 0.2 cm to about 3 cm. The control may also have a second
position (shown in
FIG. 34B), in which the sample collector is housed entirely within the distal
end of the elongate
body in the retracted position. The control also has a third position (not
shown), wherein the
sample collector is extended distally out of the distal opening of the distal
end region of the
elongate body a second distance that is greater than the first distance (e.g.,
greater than 2 cm,
greater than 2.5 cm, greater than 3 cm, greater than 3.5 cm, greater than 4
cm, etc.).
[0138] The control may be used to move the tip by itself or in
conjunction with the handle.
In some variations the control is used after the handle has been detached, so
that the tip may be
extended to the third position; with the handle attached to the elongate
member, the tip may be
prevented from extending to the third position.
[0139] FIGS. 37A-37D illustrate an example in which the indicator
(alignment guide 3703)
clips onto the proximal handle/body portion, as illustrated in FIG. 37D. In
all of the examples
shown in FIGS. 34A-37D, the indicator is held at a fixed angle of
approximately 350 relative to
the long axis of the device 3501.
[0140] For example, described herein are hand held device having an
indicator for guiding
insertion of the device into a patient's nostril, the device comprising: a
proximal handle
configured to be gripped with a human hand; a bent distal portion configured
to be inserted into a
nare of a patient; a cover configured to cover the bent portion; a sample
collector configured to
collect a sample of sinus fluid for later diagnostic testing, the sample
collector having a retracted
position within the distal portion and an advanced position extending distally
past a distal end of
the distal portion and the cover, wherein the cover is configured to prevent
contamination of the
sample collector in the retracted position; an actuator configured to advance
the sample collector
to the advanced position and to retract the sample collector to the retracted
position; and an
indicator on or adjacent to the proximal handle portion extending from a long
axis of the device
at an angle of between 33 and 37 degrees, wherein the indicator is configured
to be oriented
perpendicular to a longitudinal axis of the patient's body.
-47 -
CA 03057853 2019-09-24
WO 2018/183421 PCT/US2018/024729
[0141] The indicator may be removable or permanently attached to the
device. For example,
the indicator may be configured to removably attach to the device on either a
right side of the
device of a left side of the device. The indicator may be a snap-on
attachment, a slide-on
attachment, a screw-on attachment, a clamping attachment, etc.
[0142] In variations in which the indicator includes a projection (e.g.,
arm, member, etc.)
extending from the body (e.g., proximal handle region) of the device, the
indicator may extend
more than 1 cm from the device (e.g., from the proximal handle portion of the
device).
[0143] Any of these devices may include a left side indicator and a
right-side indicator. For
example, the indicator may comprise a first arm extending from the long axis
at an angle of
between 33 and 37 degrees on a first side of the device and a second arm
extending from the
long axis at an angle of between 33 and 37 degrees on a second side of the
device.
[0144] For example a hand held device having an indicator for guiding
insertion of the
device into a patient's nostril may include: a proximal handle configured to
be gripped with a
human hand; a bent distal portion configured to be inserted into a nare of a
patient; a cover
configured to cover the bent portion; a sample collector configured to collect
a sample of sinus
fluid for later diagnostic testing, the sample collector having a retracted
position within the distal
portion and an advanced position extending distally past a distal end of the
distal portion and the
cover, wherein the cover is configured to prevent contamination of the sample
collector in the
retracted position; an actuator configured to advance the sample collector to
the advanced
position and to retract the sample collector to the retracted position; and an
indicator on or
adjacent to the proximal handle portion configured to indicate when a long
axis of the device is
oriented perpendicular to a surface on which the patient is laying.
[0145] The indicator may include a bubble level oriented at an angle of
between 33-37
degrees relative to the long axis of the device. The indicator may comprises
an accelerometer
(e.g., showing orientation relative to "down"); this accelerometer may include
logic to indicate
when the device is oriented at either 35 relative to the ground (e.g., in a
standing patient) and/or
approximately 550 when a patient laying down flat, relative to ground (e.g., a
table). Any of
these indicators on these devices may include an audio output, visual output,
etc.
[0146] Any of the methods (including user interfaces) described herein
may be implemented
as software, hardware or firmware, and may be described as a non-transitory
computer-readable
storage medium storing a set of instructions capable of being executed by a
processor (e.g.,
computer, tablet, smartphone, etc.), that when executed by the processor
causes the processor to
control perform any of the steps, including but not limited to: displaying,
communicating with
the user, analyzing, modifying parameters (including timing, frequency,
intensity, etc.),
determining, alerting, or the like.
- 48 -
CA 03057853 2019-09-24
WO 2018/183421 PCT/US2018/024729
[0147] When a feature or element is herein referred to as being "on"
another feature or
element, it can be directly on the other feature or element or intervening
features and/or elements
may also be present. In contrast, when a feature or element is referred to as
being "directly on"
another feature or element, there are no intervening features or elements
present. It will also be
understood that, when a feature or element is referred to as being
"connected", "attached" or
"coupled" to another feature or element, it can be directly connected,
attached or coupled to the
other feature or element or intervening features or elements may be present.
In contrast, when a
feature or element is referred to as being "directly connected", "directly
attached" or "directly
coupled" to another feature or element, there are no intervening features or
elements present.
Although described or shown with respect to one embodiment, the features and
elements so
described or shown can apply to other embodiments. It will also be appreciated
by those of skill
in the art that references to a structure or feature that is disposed
"adjacent" another feature may
have portions that overlap or underlie the adjacent feature.
[0148] Terminology used herein is for the purpose of describing
particular embodiments
only and is not intended to be limiting of the invention. For example, as used
herein, the singular
forms "a", "an" and "the" are intended to include the plural forms as well,
unless the context
clearly indicates otherwise. It will be further understood that the terms
"comprises" and/or
"comprising," when used in this specification, specify the presence of stated
features, steps,
operations, elements, and/or components, but do not preclude the presence or
addition of one or
more other features, steps, operations, elements, components, and/or groups
thereof. As used
herein, the term "and/or" includes any and all combinations of one or more of
the associated
listed items and may be abbreviated as "/".
[0149] Spatially relative terms, such as "under", "below", "lower",
"over", "upper" and the
like, may be used herein for ease of description to describe one element or
feature's relationship
to another element(s) or feature(s) as illustrated in the figures. It will be
understood that the
spatially relative terms are intended to encompass different orientations of
the device in use or
operation in addition to the orientation depicted in the figures. For example,
if a device in the
figures is inverted, elements described as "under" or "beneath" other elements
or features would
then be oriented "over" the other elements or features. Thus, the exemplary
term "under" can
encompass both an orientation of over and under. The device may be otherwise
oriented (rotated
90 degrees or at other orientations) and the spatially relative descriptors
used herein interpreted
accordingly. Similarly, the terms "upwardly", "downwardly", "vertical",
"horizontal" and the
like are used herein for the purpose of explanation only unless specifically
indicated otherwise.
[0150] Although the terms "first" and "second" may be used herein to
describe various
features/elements (including steps), these features/elements should not be
limited by these terms,
- 49 -
CA 03057853 2019-09-24
WO 2018/183421 PCT/US2018/024729
unless the context indicates otherwise. These terms may be used to distinguish
one
feature/element from another feature/element. Thus, a first feature/element
discussed below
could be termed a second feature/element, and similarly, a second
feature/element discussed
below could be termed a first feature/element without departing from the
teachings of the present
invention.
[0151] Throughout this specification and the claims which follow, unless
the context
requires otherwise, the word "comprise", and variations such as "comprises"
and "comprising"
means various components can be co-jointly employed in the methods and
articles (e.g.,
compositions and apparatuses including device and methods). For example, the
term
"comprising" will be understood to imply the inclusion of any stated elements
or steps but not
the exclusion of any other elements or steps.
[0152] In general, any of the apparatuses and methods described herein
should be understood
to be inclusive, but all or a sub-set of the components and/or steps may
alternatively be
exclusive, and may be expressed as "consisting of" or alternatively
"consisting essentially of"
the various components, steps, sub-components or sub-steps.
[0153] As used herein in the specification and claims, including as used
in the examples and
unless otherwise expressly specified, all numbers may be read as if prefaced
by the word "about"
or "approximately," even if the term does not expressly appear. The phrase
"about" or
"approximately" may be used when describing magnitude and/or position to
indicate that the
value and/or position described is within a reasonable expected range of
values and/or positions.
For example, a numeric value may have a value that is +/- 0.1% of the stated
value (or range of
values), +/- 1% of the stated value (or range of values), +/- 2% of the stated
value (or range of
values), +/- 5% of the stated value (or range of values), +/- 10% of the
stated value (or range of
values), etc. Any numerical values given herein should also be understood to
include about or
approximately that value, unless the context indicates otherwise. For example,
if the value "10"
is disclosed, then "about 10" is also disclosed. Any numerical range recited
herein is intended to
include all sub-ranges subsumed therein. It is also understood that when a
value is disclosed that
"less than or equal to" the value, "greater than or equal to the value" and
possible ranges between
values are also disclosed, as appropriately understood by the skilled artisan.
For example, if the
value "X" is disclosed the "less than or equal to X" as well as "greater than
or equal to X" (e.g.,
where X is a numerical value) is also disclosed. It is also understood that
the throughout the
application, data is provided in a number of different formats, and that this
data, represents
endpoints and starting points, and ranges for any combination of the data
points. For example, if
a particular data point "10" and a particular data point "15" are disclosed,
it is understood that
greater than, greater than or equal to, less than, less than or equal to, and
equal to 10 and 15 are
- 50 -
CA 03057853 2019-09-24
WO 2018/183421 PCT/US2018/024729
considered disclosed as well as between 10 and 15. It is also understood that
each unit between
two particular units are also disclosed. For example, if 10 and 15 are
disclosed, then 11, 12, 13,
and 14 are also disclosed.
[0154] Although various illustrative embodiments are described above,
any of a number of
changes may be made to various embodiments without departing from the scope of
the invention
as described by the claims. For example, the order in which various described
method steps are
performed may often be changed in alternative embodiments, and in other
alternative
embodiments one or more method steps may be skipped altogether. Optional
features of various
device and system embodiments may be included in some embodiments and not in
others.
Therefore, the foregoing description is provided primarily for exemplary
purposes and should
not be interpreted to limit the scope of the invention as it is set forth in
the claims.
[0155] The examples and illustrations included herein show, by way of
illustration and not of
limitation, specific embodiments in which the subject matter may be practiced.
As mentioned,
other embodiments may be utilized and derived there from, such that structural
and logical
substitutions and changes may be made without departing from the scope of this
disclosure.
Such embodiments of the inventive subject matter may be referred to herein
individually or
collectively by the term "invention" merely for convenience and without
intending to voluntarily
limit the scope of this application to any single invention or inventive
concept, if more than one
is, in fact, disclosed. Thus, although specific embodiments have been
illustrated and described
.. herein, any arrangement calculated to achieve the same purpose may be
substituted for the
specific embodiments shown. This disclosure is intended to cover any and all
adaptations or
variations of various embodiments. Combinations of the above embodiments, and
other
embodiments not specifically described herein, will be apparent to those of
skill in the art upon
reviewing the above description.
-51 -