Note: Descriptions are shown in the official language in which they were submitted.
SPECIFICATION
EXHAUST GAS TREATMENT SYSTEM AND EXHAUST GAS PURIFICATION METHOD
Technical field
[0001]
The present invention relates to exhaust gas post-treatment systems and
exhaust gas purification methods.
Specifically, the present invention relates to exhaust gas post-treatment
systems of which NOx removal performance is
enhanced using hydrogen, and exhaust gas purification methods.
Background art
[0002]
Currently, I) urea selective catalytic reduction (SCR) systems and 2)
hydrocarbon selective catalytic reduction
systems (hereinafter, "HC-SCR systems") have been mass-produced as post-
treatment systems for exhaust gases from
lean-burn engines (HC: hydrocarbon).
[0003]
1) Urea SCR systems use urea for the reduction of nitrogen oxides (NOx) and
have gained worldwide
popularity because of their high NOx removal rate; however, these systems face
challenges including the limited
improvement of catalytically active species and the requirement of high
temperatures due to urea's unreactiveness at
temperatures around and below 200 C. In addition, it is necessary to inject an
aqueous solution of urea into the vehicle,
forcing users to bear burdens. Furthermore, it is also necessary to treat the
ammonia derived from the urea that is left
over after the reduction.
[0004]
Compared with this, 2) HC-SCR systems use light oils as FIC for the reduction
of NOx as described in, for
example, Patent document 1. These systems are simple and cost effective;
however, they experience the issue of low
NOx removal rate. Hence, measures are required to reduce NOx in the engine in
advance, improve catalysts, and
carefully control the addition of light oils.
Related art documents
Patent documents
[0005]
[Patent document 1] JP-A-2012-97724
Summary of the invention
Problems to be solved by the invention
[0006]
1
CA 3060301 2019-10-28
The present invention was made in view of these circumstances, and an object
thereof is to provide
cost-effective exhaust gas treatment systems and exhaust gas purification
methods with high NOx removal rates.
Means to solve the problem
[0007]
As a result of intensive studies to achieve the above-mentioned object, the
present inventor has conceived the
invention that promotes HC-SCR reactions between light oil and NOx to enhance
NOx removal performance by adding,
when hydrocarbon is added to the diesel oxidation catalyst in a manner similar
to those conventionally used, hydrogen
(H2) along with the hydrocarbon.
[0008]
That is, the present invention is an exhaust gas treatment system in which H2
is added to a diesel oxidation
catalyst (DOC) along with hydrocarbon in a hydrocarbon selective catalytic
reduction (HC-SCR) system. In addition,
the present invention is an exhaust gas purification method including removing
NOx from an exhaust gas by adding H2 to
a diesel oxidation catalyst along with hydrocarbon in a hydrocarbon selective
catalytic reduction (HC-SCR) system.
[0009]
Furthermore, the present invention is an exhaust gas treatment system
including, in order of exhaust gas inflow,
an upstream diesel oxidation catalyst (DOC), a diesel particulate filter
(DPF), and a downstream diesel oxidation catalyst
(DOC).
Effect of the invention
[0010]
The present invention exhibits enhanced NOx removal performance compared with
conventional HC-SCR
systems. The enhancement of NOx removal performance at low temperatures is
particularly significant.
Brief description of the drawings
[0011]
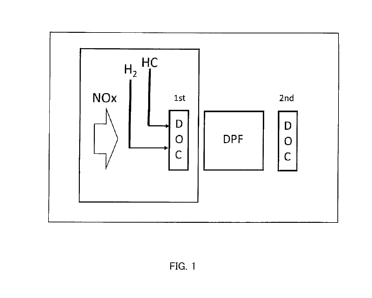
Fig. 1 is a diagrammatic representation of a system according to a first
embodiment;
Fig. 2 is a graph showing the relation between NOx removal rates and
temperatures;
Fig. 3 is a graph showing the relation between NOx removal rates and
concentrations of added H2 at a
temperature between 100 C and 200 C;
Fig. 4A is a graph showing the relation between muffler inlet temperatures and
engine operating times in the
1199 mode of FTP (US regulation);
Fig. 413 is a graph showing the average values at each time interval in Fig.
4A;
Fig. 5 is a graph showing the relation between HC removal rates and
temperatures;
Fig. 6 is a graph showing the relation between NOx removal rates and
temperatures;
Fig. 7 is a graph showing the relation between NOx removal rates and HC
concentrations at I70 C;
Fig. 8 is a diagrammatic representation of a system according to a second
embodiment;
2
CA 3060301 2019-10-28
Fig. 9 is a graph showing the relation between NOx removal rates and
temperatures;
Fig. 10 is a graph showing the relation between NOx removal rates and
concentrations of added H2 at a
temperature between 100 C and 200 C;
Fig. 11A is a graph showing the relation between muffler inlet temperatures
and engine operating times in the
1199 mode of FTP (US regulation);
Fig. 11B is a graph showing the average values at each time interval in Fig.
11A;
Fig. 12 is a graph showing the relation between HC removal rates and
temperatures;
Fig. 13 is a graph showing the relation between NOx removal rates and
temperatures;
Fig. 14 is a graph showing the relation between NOx removal rates and HC
concentrations at 170 C
Detailed description of the invention
[0012]
Embodiments of the present invention are described below, but the scope of the
present invention is not
limited to the description including Examples.
[0013]
[First embodiment]
HC-SCR systems (exhaust gas treatment systems) convert harmful components
(e.g., NOx) in exhaust gas
from automobile engines into harmless components before the exhaust gas is
emitted into the atmosphere, and these
systems are usually disposed at the bottom of the automobiles.
[0014]
Fig. 1 illustrates a diagrammatic representation of an HC-SCR system according
to this embodiment. The
HC-SCR system comprises, in order of exhaust gas inflow, an upstream diesel
oxidation catalyst (upstream DOC;
denoted as "1st DOC" in Fig. 1), a diesel particulate filter (DPF), and a
downstream diesel oxidation catalyst
(downstream DOC; denoted as "2nd DOC" in Fig. 1).
[0015]
<Diesel oxidation catalyst (DOC)>
DOC converts, on itself, HC, CO, and NOx in exhaust gas into harmless
components. The DOC in the
present embodiment has two stages, an upstream DOC and a downstream DOC, in
order of exhaust gas inflow. The
downstream DOC is not an essential component.
[0016]
Examples of upstream DOC compositions include noble metals such as Pt and Pd,
and alumina, but any
composition can be used if it shows oxidation activity. In addition, two or
more noble metals can be used in a form
similar to that of an alloy. Cocatalysts such as Ce02 and ZrO2 can also be
used.
[0017]
Examples of substrates for supporting the upstream DOC include alumina
(Al2O3), lanthanum (La), and silica
(SiO2), but are not limited thereto.
[0018]
3
CA 3060301 2019-10-28
The upstream DOC has the role of converting HC and NOx, which are harmful
components in the exhaust gas
emitted from engines, into harmless components.
[0019]
In the HC-SCR system according to this embodiment, a light oil component is
added upstream of the upstream
DOC. Because the amount of HC in the exhaust gas is trace, the amount of HC in
the reaction system is intentionally
increased by the HC contained in the light oil component. Thus, purification
is performed by promoting the reduction
reaction between fIC and NOx in the exhaust gas. However, sufficient NOx
removal efficiency cannot be obtained only
by adding HC.
[0020]
The HC-SCR system according to this embodiment enhances NOx removal
performance by adding H2 along
with HC to the upstream DOC. It can be anticipated that this occurs because
the reaction intermediate of NOx can be
efficiently decomposed by reducing the surface of a catalyst such as Pt with
the addition of H2.
[0021]
Furthermore, by adding H2, the present invention also has the advantage that
NOx can be removed even at
such a low temperature that urea does not react (in an environment where the
urea SCR system does not function).
[0022]
The downstream DOC is typically provided downstream of the DPF and has the
role of removing excess HC
by oxidation. In the HC-SCR system, light oil is intentionally added in the
aforementioned manner, and the light oil
may be added more than the usual amount to remove NOx in some cases. Many HCs
that cannot be consumed or
removed by the upstream DOC or the DPF remain. The downstream DOC is provided
to remove such HCs.
[0023]
Examples of downstream DOC compositions include noble metals such as Pt and
Pd, and alumina, similar to
the upstream DOC, but are not limited thereto. Moreover, an alloy and a
cocatalyst can be used similar to the upstream
DOC. Furthermore, the same examples of substrates for the upstream DOC can
also be used in this case.
[0024]
<Diesel particulate filter (DPF)>
DPF is a device that captures particulate matter (PM) contained in the exhaust
gas. There is no limit to the
types of DPF, and any known types can be used.
[0025]
The heat of the exhaust gas alone is insufficiently to raise the temperature
and the PM cannot be completely
burned off and tends to clog the DPF.
[0026]
Therefore, the DPF makes good use of the reaction heat generated by
intentionally adding light oil components
to the upstream DOC, thereby to remove the PM by burning it off.
[0027]
<Other structures>
4
CA 3060301 2019-10-28
A urea SCR catalyst that removes NOx with urea can be provided downstream of
the DPF. At low
temperatures, the upstream DOC can play the role of removing NOx by adding
light oil and H2 to the upstream DOC; at
high temperatures, the urea SCR catalyst can play the role of removing NOx by
adding urea to the urea SCR catalyst.
Accordingly, it is possible to enhance the NOx removal performance using the
hybrid effect.
[0028]
Examples of urea SCR catalyst compositions include those containing metals
such as Fe, Cu, and V, and
include Fe-zeolite, Cu-zeolite, and V205 but are not limited thereto.
[0029]
An ammonia slip catalyst (ASC) for removing excess ammonia can be provided
downstream of the urea SCR
catalyst. Examples of ASC compositions include combinations of a noble metal
such as Pt or Pd and an SCR catalyst
such as the Fe-zeolite or Cu-zeolite. The ASC works via the mechanism of
converting NOx from ammonia oxidation
with a noble metal catalyst into harmless components by catalytic reduction on
the ASC catalyst with in-flow ammonia.
[0030]
Next, the present invention is described by way of Examples, but the scope of
the present invention is not
limited to these Examples. It should be noted that "%" means "% by volume."
[0030]
<Example 1>
Changes in NOx gas removal characteristics were examined with H2
concentrations increased stepwise.
[0032]
- Composition of the catalyst
The catalyst that is used in Example 1 corresponds to the upstream DOC. A
specific composition of the
catalyst is Pt 6.0 g/L and dimensions are (p1.0 inch x 50 mm. The same applies
to the second to fourth Examples.
[0033]
- Composition of the simulated gas
C3I-16: 1300 ppmC, CO: 200 ppm, NO: 200 ppm, CO2: 5%, 02: 10%, H20: 5%, SO2: 2
ppm, H2: (see the graph
in Fig. 2), and N2: the balance. Note that "ppmC" is a unit of emission
concentration; it is a product of concentration in
ppm and the number of carbons.
[0034]
(Evaluation conditions)
- Catalytic heat treatment: 600 C, 50 hours
- Gas flow rate: 24 L/min (SV: 60000/h)
- Temperature: Measured while raising from room temperature to 500 C and then
decreasing at a rate of 10 C/min.
[0035]
The results of the Example mentioned above are shown in Figs. 2 and 3. Fig. 2
is a graph showing the
relation between the NOx removal rates and temperatures. Fig. 3 is a graph
showing the relation between the NOx
removal rates and H2 concentrations at a temperature between 100 C and 200 C
in which urea is not activated.
[0036]
CA 3060301 2019-10-28
From the graph in Fig. 2, it can be understood that the larger the amount of
the added H2 (concentration of the
added H2) is, the more the peaks of the removal rate are shifted to lower
temperatures. Among them, when the
concentration of the added H2 is 16000 ppm, it is estimated that the peak of
the removal rate is at 100 C or less, and it is
estimated that the removal reaction is actively occurring even at 100 C or
less.
[0037]
Also, from the graphs in Figs. 2 and 3, the maximum NOx removal rates get
higher with the increase in H2
concentrations up to a certain point, but when the H2 concentration is 16000
ppm, both of the maximum NOx removal
rate and the removal rate at a temperature between 100 C and 200 C decrease.
It is assumed that this is because the
added H2 activates the reaction between H2 and NOx, and NOx removal is
occurring from a lower temperature. The
NOx removal rate at each temperature varies with the change in amount of the
added H2 as in the indicated experimental
results; thus, this means that, by adapting the H2 concentrations to different
engines, the required performance such as the
required removal rate and engine temperature can be achieved.
[0038]
Fig. 4 shows the relation between muffler inlet temperatures and engine
operating times in the 1199 mode of
the US Environmental Protection Agency (EPA) Federal Test Procedure, which is
a method that ought to be evaluated
for meeting the US regulatory compliance. Specifically, Fig. 4A shows
automobile muffler inlet temperatures at each
temperature, and Fig. 4B shows the average values at each time interval in
Fig. 4A.
[0039]
It can be understood from Figs. 4A and 4B that the operating temperatures of
the engine scarcely fall below
100 C except when the engine is starting up. That is, it can be understood
that, since most engine operating
temperatures in a temperature range around and below 200 C in which urea is
not activated come between 100 C and
200 C, it is preferable in the HC-SCR system that the amount of hydrogen
addition is regulated in such a manner that
high removal performance can be obtained in the range between 100 C and 200 C.
[0040]
<Example 2>
Changes in HC gas removal characteristics were examined with H2 concentrations
increased stepwise. The
evaluation conditions are the same as those in Example I.
[0041]
- Composition of the simulated gas
C3H6: 1300 ppmC, CO: 200 ppm, NO: 200 ppm, CO2: 5%, 02: 10%, H20: 5%, SO2: 2
ppm, Hz: (see the graph
in Fig. 5), and N2: the balance.
[0042]
The results of the Example mentioned above are shown in Fig. 5. Fig. 5 is a
graph showing the relation
between temperatures and HC removal rates.
[0043]
From the graph in Fig. 5, it can be understood that, in a temperature range
around and below 200 C in which
urea it not activated, the HC removal (reaction) rate increases and the peaks
of the removal rate are shifted to low
6
CA 3060301 2019-10-28
temperatures as in Example 1 with the increase in amount of the added H2
(concentration of the added H2). Above all,
when the concentration of the added H2 is 16000 ppm, the removal rate is
almost 100% at 100 C, and the removal
reaction occurs even at 100 C and less in which urea cannot be activated. It
can be understood that the estimation made
in Example 1 is correct.
[0044]
In summary, it can be anticipated that, from the results of Examples 1 and 2,
in the HC-SCR system of the
present invention, HC is used for NOx removal at least in the temperature
range in which urea is not activated, and the
enhancement of the HC activity is related to the addition of H2 (concentration
of the added Hz).
[0045]
<Example 3>
The relation among the presence/absence of H2, the presence/absence of HCs,
and the NOx removal rates was
examined. The evaluation conditions are the same as those in Example 1.
[0046]
- Composition of the simulated gas
C3H6: 0 or 1300 ppmC, CO: 200 ppm, NO: 200 ppm, CO2: 5%, 02: 10%, H2O: 5%,
SO2: 2 ppm, Hz: 0 or 2000
ppm, and N2: the balance.
[0047]
The results of the Example mentioned above are shown in Fig. 6. Fig. 6 is a
graph showing the relation
between the NOx removal rates and temperatures.
[0048]
From the graph in Fig. 6, in the temperature range around and below 200 C, the
NOx removal rate (with H2
and without HC) is exceptionally high, followed by (without H2, with HC) and
(with H2 and HC) in this order. On the
other hand, it can be understood that (without H2 and HC) shows almost no sign
of removal.
[0049]
That is, H2 alone does not exhibit superior NOx removal performance, and the
removal rate gets high when H2
is combined with HC. Therefore, it is understood that H2 can exhibits its
removal performance subject to be used in the
HC-SCR system. In addition, the removal rate is higher with the addition of H2
than with HC alone that represents the
conventional art. Thus, it is estimated that H2 promotes the HC-SCR reaction.
[0050]
<Example 4>
The relation among H2 concentrations and HC concentrations at 170 C and the
NOx removal rates was
examined. Note that 170 C is the temperature corresponded to the highest
removal rate (with H2 and HC) in Example 3.
The evaluation conditions are the same as those in Example 1.
[0051]
- Composition of the simulated gas
C3H6: see the graph, CO: 200 ppm, NO: 200 ppm, CO2: 5%, 02: 10%, H2O: 5%, SO2:
2 ppm, H2: (see the
graph in Fig. 7), and N2: the balance.
7
CA 3060301 2019-10-28
[0052]
The results of the Example mentioned above are shown in Fig. 7. Fig. 7 is a
graph showing the relation
between the NOx removal rates and HC concentrations at 170 C.
[0053]
From the graph in Fig. 7, it can be understood that, under the condition with
no H2 added, the NOx removal
rates do not increase even with increased HC concentrations. On the other
hand, under the condition with no HC added,
almost no difference was observed in removal rates regardless of the presence
or absence of H2. Furthermore, under the
condition with HC, the higher the H2 concentration was, the higher the NOx
removal rate was.
[0054]
[Second embodiment]
The invention described in this embodiment is, in an exhaust gas treatment
system comprising, in order of
exhaust gas inflow, a diesel oxidation catalyst removing NOx from the exhaust
gas using hydrocarbon, a diesel
particulate filter, and a urea SCR catalyst removing NOx, H2 is added to the
diesel oxidation catalyst along with
hydrocarbon.
[0055]
Furthermore, the invention described in this embodiment is the exhaust gas
treatment system including,
downstream of the urea SCR catalyst, a catalyst removing excess ammonia that
is a degradation product of the urea.
[0056]
The invention described in the embodiment resulted in the enhancement in NOx
removal performance
compared with conventional HC-SCR systems. In particular, the NOx removal
performance was enhanced in a wide
temperature range including lower temperatures in which urea is not activated.
[0057]
The exhaust gas treatment system according to this embodiment promotes HC-SCR
reactions between light oil
and NOx and enhances NOx removal performance by adding hydrogen (H2) along
with hydrocarbon when it is added to
the diesel oxidation catalyst. In addition, the exhaust gas system according
to the embodiment is a hybrid system that
can ensure high NOx removal performance over a wide temperature range by using
the urea SCR system in a high
temperature range in which urea is activated and using the HC-SCR system
utilizing H2 in a low temperature range in
which urea is not activated. Specifically, it is possible to achieve hybrid
systems that can ensure high NOx removal
performance at almost all temperatures in the engine's operating temperature
range by using, in the lower temperature
range, the HC-SCR system in which HC and H2 are both present and using the
urea SCR system in the high temperature
range. Details are described below.
[0058]
Fig. 8 shows a diagrammatic representation of an exhaust gas treatment system
according to this embodiment.
Exhaust gas treatment systems convert harmful components (e.g., NOx) in
exhaust gas from automobile engines into
harmless components before the exhaust gas is emitted into the atmosphere.
These systems are disposed at the bottom
of the automobiles. Exhaust gas treatment systems comprise, in order of
exhaust gas inflow, a diesel oxidation catalyst
(DOC), a diesel particulate filter (DPF), a urea SCR catalyst (urea SCR), and
an ammonia slip catalyst (ASC).
8
CA 3060301 2019-10-28
[0059]
<Diesel oxidation catalyst (DOC)>
DOC converts, over itself, HC, CO, and NOx in exhaust gas to harmless
components.
[0060]
Examples of upstream DOC compositions include noble metals such as Pt and Pd,
and alumina, but any
composition can be used if it shows oxidation activity. In addition, two or
more noble metals can be used in a form
similar to that of an alloy. Cocatalysts such as CeOz and ZrO2 can also be
used.
[0061]
Examples of substrates for supporting the upstream DOC include alumina
(Al2O3), lanthanum (La), and silica
(SiO2), but are not limited thereto.
[0062]
In the exhaust gas treatment system, a light oil component is added upstream
of the upstream DOC. Because
the amount of HC in the exhaust gas is trace, the amount of HC in the reaction
system is intentionally increased by the
HC contained in the light oil component. Thus, purification is performed by
promoting the reduction reaction between
HC and NOx in the exhaust gas. However, sufficient NOx removal efficiency
cannot be obtained only by adding HC.
[0063]
The exhaust gas treatment system enhances NOx removal performance by adding H2
along with HC to the
DOC. It can be anticipated that this occurs because the reaction intermediate
of NOx can be efficiently decomposed by
reducing the surface of a catalyst with the addition of H2.
[0064]
Furthermore, by adding Hz, the present invention also has the advantage that
NOx removal performance is
enhanced in such a low temperature range that urea is not activated (in an
environment where it cannot function as the
urea SCR system).
[0065]
<Diesel particulate filter (DPF)>
DPF is a device that captures particulate matter (PM) contained in the exhaust
gas. There is no limit to the
types of DPF, and any known types can be used.
[0066]
The heat of the exhaust gas alone is insufficiently to raise the temperature
and the PM cannot be completely
burned off and tends to clog the DPF.
[0067]
Therefore, the DPF makes good use of the reaction heat generated by
intentionally adding light oil components
to the DOC, thereby to remove the PM by burning it off.
[0068]
<Urea SCR catalyst (urea SCR)>
A urea SCR is a catalyst for removing NOx with urea and is provided downstream
of the DPF. In a low
temperature range in which urea is not activated, the DOC can play the role of
removing NOx by adding light oil and H2
9
CA 3060301 2019-10-28
to the DOC; in a high temperature range, the urea SCR can play the role of
removing NOx by adding urea to the urea
SCR. Accordingly, it is possible to enhance the NOx removal performance using
the hybrid effect over a wide
temperature range.
[0069]
Examples of urea SCR compositions include those containing metals such as Fe,
Cu, and V, and include
Fe-zeolite, Cu-zeolite, and V205 but are not limited thereto.
[0070]
<Ammonia slip catalyst (ASC)>
ASC is a catalyst for removing excess ammonia that did not participate in the
reaction in the urea SCR and is
provided downstream of the urea SCR.
[0071]
Examples of ASC compositions include combinations of a noble metal catalyst
such as Pt or Pd and a urea
SCR catalyst such as the Fe-zeolite or Cu-zeolite.
[0072]
In the ASC, ammonia is oxidized into NOx on a noble metal catalyst and that
NOx is reacted with ammonia
newly flowing from the urea SCR on the urea SCR catalyst to convert the
ammonia into nitrogen and water, thereby
converting both ammonia and NOx into harmless components. Note that ASC is not
an essential component.
[0073]
<Other structures>
Besides, a DOC (hereinafter, "upstream DOC") can be provided and one DOC (not
shown; hereinafter,
"downstream DOC") that removes excess HC by oxidation can be provided
downstream of the DPF. In the exhaust gas
treatment system according to this embodiment, light oil may be added more
than the usual amount to remove NOx in
some cases. In such cases, many HCs that cannot be consumed or removed by the
upstream DOC remain.
Specifically, unlike DOCs, urea SCRs usually contain no platinum group metal.
Thus, excess HCs that could not be
removed are accumulated on a urea SCR or reach the ASC through the urea SCR in
some cases. The downstream DOC
is provided to remove such excess HCs.
[0074]
Examples of downstream DOC compositions include noble metals such as Pt and
Pd, and alumina, similar to
the upstream DOC, but are not limited thereto. Moreover, an alloy and a
cocatalyst can be used similar to the upstream
DOC. Furthermore, the same examples of substrates for the upstream DOC can
also be used in this case.
[0075]
The downstream DOC can be provided between the DPF and the urea SCR, between
the urea SCR and the
ASC, or downstream of the ASC.
[0076]
Next, the present invention is described by way of Examples, but the scope of
the present invention is not
limited to these Examples. It should be noted that "%" means "% by volume."
[0077]
CA 3060301 2019-10-28
<Example 5>
Changes in NOx removal characteristics were examined with H2 concentrations
increased stepwise.
[0078]
- Composition of the catalyst
The catalyst that is used in Example 5 corresponds to the DOC. A specific
composition of the catalyst is Pt
6.0 g/L and dimensions are (p1.0 inch x 50 mm. The same applies to the sixth
to eighth Examples.
[0079]
- Composition of the simulated gas
C3H6: 1300 ppmC, CO: 200 ppm, NO: 200 ppm, CO2: 5%, 02: 10%, H2O: 5%, SO2: 2
ppm, Hz: (see the graph
in Fig. 9), and Nz: the balance. Note that "ppmC" is a unit of emission
concentration; it is a product of concentration in
ppm and the number of carbons.
[0080]
(Evaluation conditions)
- Catalytic heat treatment: 600 C, 50 hours
- Gas flow rate: 24 L/min (SV: 60000/h)
- Temperature: Measured while raising from room temperature to 500 C and
then decreasing at a rate of 10 C/min.
[0081]
The results of the Example mentioned above are shown in Figs. 9 and 10. Fig. 9
is a graph showing the
relation between the NOx removal rates and temperatures. Fig. 10 is a graph
showing the relation between the NOx
removal rates and H2 concentrations at a temperature between 100 C and 200 C
in which urea is not activated.
[0082]
From the graph in Fig. 9, it can be understood that the larger the amount of
the added H2 (concentration of the
added H2) is, the more the maximum removal rates are shifted to lower
temperatures. Among them, when the
concentration of the added H2 is 16000 ppm, it is presumed that the maximum
removal rate is at 100 C or less, and it is
presumed that the removal reaction is actively occurring even at 100 C or
less.
[0083]
On the other hand, from the graph in Fig. 9, it is presumed that the maximum
NOx removal rate increases with
the increase in H2 concentration up to the H2 concentration of 8000 ppm but
then decreases when the H2 concentration
reaches 16000 ppm.
[0084]
Furthermore, from the graph in Fig. 10, it is presumed that the NOx removal
rate between 100 C and 200 C
increases with the increase in H2 concentration up to the H2 concentration of
8000 ppm but then decreases when the H2
concentration reaches 16000 ppm.
[0085]
It is assumed that this is because the added H2 activates the reaction between
H2 and NOx, and NOx removal is
occurring from a lower temperature. The NOx removal rate at each temperature
varies with the change in amount of the
added H2 as in the indicated experimental results; thus, this means that, by
adapting the H2 concentrations to different
11
CA 3060301 2019-10-28
engines, various kinds of required performance, such as temperature ranges
where a specific NOx removal rate or a high
NOx removal rate is required, can be met.
[0086]
On the other hand, focusing on a temperature range of 200 C and above in which
urea is activated, as shown in
the graph in Fig. 9, it can be understood that the NOx removal rate decreases
with the increase in temperature even with
the addition of H2. Accordingly, it can be understood that sufficient NOx
removal performance cannot be expected in
the temperature range of 200 C and above in which urea is activated merely by
adding H2.
[0087]
Fig. 11 shows the relation between muffler inlet temperatures and engine
operating times in the 1199 mode of
the US Environmental Protection Agency (EPA) Federal Test Procedure, which is
a method that ought to be evaluated
for meeting the US regulatory compliance. Specifically, Fig. 11A shows
automobile muffler inlet temperatures at each
temperature, and Fig. 11B shows the average values at each time interval in
Fig. 11A.
[0088]
It can be understood from Figs. 11A and 11B that the operating temperatures of
the engine scarcely fall below
100 C except when the engine is starting up. That is, it can be understood
that, since most engine operating
temperatures in a temperature range around and below 200 C in which urea is
not activated come between 100 C and
200 C, it is preferable in the exhaust gas treatment system that the amount of
hydrogen addition is regulated in such a
manner that high NOx removal performance can be obtained in the range between
100 C and 200 C.
[0089]
<Example 6>
Changes in NOx removal characteristics with HC were examined with H2
concentrations increased stepwise.
The evaluation conditions are the same as those in Example 1.
[0090]
- Composition of the simulated gas
C3H6: 1300 ppmC, CO: 200 ppm, NO: 200 ppm, CO,: 5%, 02: 10%, H2O: 5%, SO2: 2
ppm, H2: (see the graph
in Fig. 12), and N2: the balance.
[0091]
The results of the Example mentioned above are shown in Fig. 12. Fig. 12 is a
graph showing the relation
between temperatures and HC removal rates.
[0092]
From the graph in Fig. 12, it can be understood that, in a temperature range
around and below 200 C in which
urea it not activated, the removal (reaction) rate with HC increases and the
peaks of the removal rate are shifted to low
temperatures with the increase in amount of the added H2 (concentration of the
added H2). Above all, when the
concentration of the added H2 is 16000 ppm, the removal rate is almost 100% at
100 C, and it is estimated that NOx
removal with HC occurs even at 100 C and less in which urea is not activated.
[0093]
12
CA 3060301 2019-10-28
In summary, it can be understood that, from the results of Examples 5 and 6,
NOx is efficiently removed with
HC at least in the temperature range between 100 C and 200 C of the
temperature range in which urea is not activated.
In addition, the HC activity in this temperature range is enhanced depending
on the concentration of the added H2.
[0094]
<Example 7>
The relation among the presence/absence of H2, the presence/absence of HCs,
and the NOx removal rates was
examined. The evaluation conditions are the same as those in Example 5.
[0095]
- Composition of the simulated gas
C3H6: 0 or 1300 ppmC, CO: 200 ppm, NO: 200 ppm, CO2: 5%, 02: 10%, H20: 5%,
SO2: 2 ppm, Hz: 0 or 2000
ppm, and N2: the balance.
[0096]
The results of the Example mentioned above are shown in Fig. 13. Fig. 13 is a
graph showing the relation
between the NOx removal rates and temperatures.
[0097]
From the graph in Fig. 13, it can be understood that for the H2 concentration
of 2000 ppm, the HC removal rate
exceeds 90% only after the temperature reaches 170 C. Then, in the case where
the H2 concentration is 2000 ppm, it
can be anticipated that the HC-SCR reaction proceeds well at around 170 C.
Thus, focusing on the NOx removal rate at
170 C in the graph in Fig. 13, it can be understood that (with H2 and HC)
exhibits the highest rate.
[0098]
That is, compared with the base condition (without H2 and with HC), H2 alone
does not enhance the NOx
removal performance. In contrast, it can be understood that, by using H2 in
combination with HC, higher removal rates
can be achieved than those obtained under the base condition. Furthermore, it
can be understood that higher removal
rates are obtained with the addition of H2 than HC alone. From this result, it
is presumed that H2 promotes the HC-SCR
reactions.
[0099]
On the other hand, focusing on a temperature range of 200 C and above in which
urea is activated, it can be
understood that the NOx removal rate in the case of (with H2 and HC) decreases
with the increase in temperature similar
to the case of Example 5.
[0100]
<Example 8>
The relation among H2 concentrations and HC concentrations at 170 C and the
NOx removal rates was
examined. Note that 170 C is the temperature corresponded to the highest
removal rate (with H2 and HC) in Example 7.
The evaluation conditions are the same as those in Example 5.
[0101]
- Composition of the simulated gas
13
CA 3060301 2019-10-28
C3H6: see the graph, CO: 200 ppm, NO: 200 ppm, CO2: 5%, 02: 10%, H20: 5%, SO2:
2 ppm, Hz: (see the
graph in Fig. 14), and N2: the balance.
[0102]
The results of the Example mentioned above are shown in Fig. 14. Fig. 14 is a
graph showing the relation
between the NOx removal rates and HC concentrations at 170 C.
[0103]
From the graph in Fig. 14, under the condition with no HC added, almost no
difference was observed in
removal rates regardless of the presence or absence of H2. Under the condition
with HC, the higher the H2
concentration was, the higher the NOx removal rate was. Note that under
conditions where on H2 was added, the NOx
removal rates were kept low and changed little regardless of the concentration
of HC.
[0104]
This result revealed that NOx removal rates are dependent on H2 concentrations
on the premise that HC is
present together with Hz.
[0105]
From the above, it can be understood that NOx removal rates significantly
increase by adding H2 to DOC
along with hydrocarbon in a low temperature range around and below 200 C in
which urea is not activated. In contrast,
in a high temperature range around and above 200 C in which urea is activated,
no increase in NOx removal rate by the
addition of H2 can be found.
14
CA 3060301 2019-10-28