Note: Descriptions are shown in the official language in which they were submitted.
CA 03062508 2019-11-05
W02018/211344
PCT/IB2018/052807
Transcatheter valve prosthesis for blood vessel
Field of invention
The present invention relates to an expandable prosthetic valve that
is designed to be positioned within a blood vessel, during the
repair of replacement of a native valve, for instance an aortic
valve.
Background
The clinical complications related to the implant of a transcatheter
heart valve prosthesis (TAVI) are mainly related to the fact that
it overlapps the diseased native valve. The heavy presence of tissue
calcifications, involving the valve apparatus and the surronding
tissues, influences the correct deployment of the prosthesis
creating the conditions for embolic episodes.
The different types of clinical complications, associated with the
TAVIs implant, are therefore mainly related to the dystrophic
calcifications of the native valve and to the inhomogeneous
deployment of the valve prosthesis, and are:
= The occurrence of moderate-severe peri-valvular leaks (grade
II)
= The occurrence of embolic events (blood clots and fibrous or
calcific emboli
The occurrence of moderate-severe peri-valvular leaks (PVL) after
transcatheter aortic valve prostheses implantation is at least 10%
with a peak of mortality around one year for this particular
patients' subgroup.
The clinical data, suitable for the second generation of
transcatheter heart valves, are substantially better than those of
the first generation for what concerns the PVL. In fact the
occurrence of moderate PVLs dropped to 3.4%, but different authors
documented higher percentages of PVL complications in patients with
"high calcium scored valves".
1
CA 03062508 2019-11-05
WO 2018/211344
PCT/IB2018/052807
The coronary occlusion is a kind of clinical complication generated
by two different causes, namely the mechanical occlusion of the
coronary ostia is induced by the aortic valve native leaflets or the
embolization of calcium debris during a TAVI implant procedure.
Despite the occurrence of this clinical complication is only 1% of
the TAVI implants it is letal in 50$ of the cases even with a delay
of few days after the implant procedure. The extension of TAVI
implants to the intermediate risk patients is further increasing of
serious events to a younger patient population.
The mechanical occlusion of the coronary ostia can occur because the
TAVI, during its deployment, is pushing outward the calcified native
leaflet creating an obstruction of coronary ostia. The same condition
can occur when a TAVI is implanted over a degenerated bioprosthesis.
In particular with some bioprostheses, such as the "stentless" ones,
the risk coronary ostia obstruction is more frequent when a TAVI is
implanted.
The procedural embolic events, so called "macro-embolic cerebral
events", are occurring during a TAVI implant procedure (during
predilation, implant or postdilation) and are mainly related to the
embolization of macro debris of calcium of fibroelatic particles
usually targeting the brain (strokes), the coronary arteries or the
peripheral organs. However the strokes are the most frightful
clinical events occurring, nowadays, at a rate of 2.7% against a
rate of 3.3% of the previous generations of TAVIs. This reduction
of strokes is related to the minor need of pre- and postdilation
during TAVI implant nevertheless this data are unclear since are
referring to aortic valves with a mild level of calcification.
The post-procedural micro-embolic cerebral events are documented in
at least 8% of the patients submitted to investigation. The high
incidence of new cerebral lesions after TAVI warrants for a longer-
term evaluation of neurocognitive function.
In this study conducted over a short-term follow-up period of 3
months, no impairment of neurocognitive function was observed
clinically, and the majority of lesions (80%) had resolved on 3-
month MRI. However, the issue of periprocedural brain embolization
and its potential effects on neurocognitive function may portend
greater clinical implications once the indication for TAVI is
broadened to include younger patients with long life expectancy.
Future research in the field of TAVI should thus be directed at
developing strategies to reduce the risk of embolization (eg, less
2
CA 03062508 2019-11-05
WO 2018/211344
PCT/IB2018/052807
traumatic, smaller-bore catheter systems, improved identification
of patients at risk for embolization and a potential use of cerebral
protection devices).
In some clinical studies at least 10% of the patients, submitted to
TAVI implant, show a neurological damage detectable during
psycometric tests. While this occurrence rate can be acceptable in
high risk and an old patient population it appears unacceptable in
lower-risk younger patients. Several clinical studies are ongoing
to better investigate this clinical condition.
Another kind of embolic events are the sub-acute and chronic
microembolic events occurring after the immediate post-procedural
time. The native aortic calcific valve is rough, with a warty
surface, immobilized acting like an atherosclerotic ulcerated
plaque. This condition is favouring the formation of microtrombi
that later-on embolize towards the brain and other peripheral organs.
The native aortic valve left in place as a source of microemboli has
been taken in account in several clinical studies that demonstrated
their role in the onset of vascular origin dementia. This evidence
creates a concern when the TAVI are implanted in younger patients
where an acceleration of the vascular dementia could impact in a
serious way on the social costs.
In summary the periprocedural clinical complications following a
TAVI implant are strongly related to the presence of the heavily
calcified aortic valve left in place. It brings, acutely, an
occurrence of macro-embolic cerebral events (strokes) and
haemodynamic consequences such as the PVLs resulting in a various
severity of aortic valve insufficiency. These unsatisfactory
clinical outcomes are closely related to an irregular deployment of
the transcatheter valve prostheses in concomitance of highly
calcified aortic native valves.
The longer term clinical complications are characterized by the
cerebral micro-embolizations generated by the native aortic valve
leaflets' left in place that become a source of emboli responsible
for vascular dementia.
The overall rate of clinical complications in TAVI is ranging between
5-% and 12-%. This occurrence is most probably underestimated because
it does not include patients with highly calcified and biscuspid
native valves.
3
CA 03062508 2019-11-05
WO 2018/211344
PCT/IB2018/052807
These evidences highlight the importance of protecting the
peripheral organs, in particular the brain and the heart, against
embolizations occurring during TAVIs procedures.
Nowadays, there are several devices on the market that protect the
organs from embolic products, acting as deflectors or anti-embolic
filters. In the case of the deflector, the protection system deflects
emboli from the brachiocephalic trunk and the left common carotid
artery towards the peripheral circulation. In the case of the anti-
embolic filters, they actually capture emboli with a mesh.
International patent application WO 2015/185870 discloses a
temporary valve prosthesis that is designed to be inserted into the
aortic root at the sinotubular junction.
The device comprises a filter that is contained within a valve having
a conical shape.
This above cited device provides some improvements with respect to
other prior art devices. It however shows some inconvenients, such
as a risk of leakage resulting from a blood back flow or the
difficulty to insert additional devices through the prosthesis due
to catheter dimensional constraints.
Summary of the invention
The inconvenients discussed in the previous chapter are solved with
the present invention that relates to a device as defined in the
claims.
More precisely, the present invention consists of an integrated
system providing, at the same time, an antiembolic protection, a
valve function as well as a self-centering conveyor for other
devices. The conveyor function is suitable for entering and centering
transcatheter devices operating on the diseased native valve
(devices for mitigation of native leaflet stiffness or partial/full
ablation of the native valve) or TAVI or other valves to be
implanted. This system can therefore optimize the overall TAVI
procedure and it could be very effective in reducing the acute pen-
procedural clinical complications that could arise especially in
complex procedures.
4
CA 03062508 2019-11-05
WO 2018/211344
PCT/IB2018/052807
The device according to the present invention is conceived to be
entirely collapsed inside a catheter and introduced in the patient's
artery with the aim to reach the aortic arch and to be deployed in
place. The device allows to be crossed by different transcatheter
devices performing procedures on the native valve while is providing
a temporary valve support and protecting the heart, the brain and
peripheral organs from any kind of embolizations.
The device can be completely or partially collapsed during the
procedure in order to be re-positioned. At the end of the procedure
the device is collapsed, retracted inside the shaft and fully
retrieved out from the patient.
This device preferably has a valve prosthesis contained inside a
shaped support structure that leak-free couples with the aortic
wall. A second structure, either internal or distal respect to the
support structure acts like an antiembolic filter. A third structure,
with a conical or funnel like shape called conveyor, can be either
internal or distal respect to the support structure and crosses the
inner lumen of the valve prosthesis. It has the function to create
a conduit across the device and to facilitate the introduction of
several transcatheter devices operating on a diseased aortic valve,
and the relevant alignment respect to the valve axis.
In one embodiment the valve prosthesis is anchored to the internal
surface of the support structure. In this case the expansion of the
external support structure, to get in contact with the aorta's wall,
is conditioned by the internal valve prosthesis. Therefore the
dimension of the device must be determined with accuracy at the time
of the intervention in order to avoid a prosthetic valve
insufficiency with a limited efficacy in term of haemodynamic
performance and antiembolic protection.
In another group of embodiments the valve prosthesis can be
considered independent from the antiembolic filter so that the
expansion of the last one, to fit to the aorta's wall, does not
interfere with the valve prosthesis function. This embodiment
requires that the external support structure and the inner valve
prosthesis are connected by a sort of diaphragm. In this way the
dimension of the inner valve prosthesis is independent from the
diameter change of the external support structure when fitting to
the aorta's wall. Several embodiments belong to this group, differing
in terms of positioning of the filter and conveyor elements and
5
CA 03062508 2019-11-05
WO 2018/211344
PCT/IB2018/052807
materials of the support structures, namely with embodiments having
the conveyor and/or the filter internal or outside the main support
structure and embodiments having all support structures made of
self-expanding metallic materials or inflatable structures or hybrid
ones.
Detailed description of the invention
The invention will be better understood below, in association with
some illustrated examples.
Numerical references used in the figures
1 Guidewire
2 Balloon catheter tip
3 External shaft catheter of the device
3' Internal shaft catheter of the device
4 Device
4' Tethering struts connecting the device to the internal shaft
catheter 3'
4" Tethering struts connecting the conveyor 6 and the valve's
support stent 14
5 External support structure of the device
5' Anchoring holes to filter mesh
5" Combined internal structure (including elements 6 and 14)
5"' Tethering struts with keyholes connecting the structure 5"
(valve' support stent 14 and conveyor 6 combined in a single
element) with the external support structure 5
6 Conveyor (integrated in 4 or outside)
6' Internal lumen of conveyor
6" Distal conveyor's tube with a bi-directional normally closed
valve
6"' Anchoring holes for a conveyor placed outside the device 4
7 Leaflets of internal valve prosthesis
8 Coronary artery deflectors
9 Epiaortic vessel deflector
10 Mesh mounted on the internal or external surface of the support
structure 5 coupling with the aorta's internal surface with
antiembolic filter functions
11 Junction ring to the internal shaft catheter 3'
6
CA 03062508 2019-11-05
WO 2018/211344
PCT/IB2018/052807
11' Junction ring joining the device with the external convejor 6
12 Antiembolic filter mesh normally mounted on the conveyor 6
13 Junction diaphragm between the external support structure and
the internal valve's prosthetic structure 12
14 Valve's support stent
14' Leaflets' anchoring structure
14" Junction pillars between tethering struts 4" and valve's
support
Prosthetic valve
10 16 Inflatable structures
17 Mechanism to force open the valve leaflets 7
18 Radiopaque markers
7
CA 03062508 2019-11-05
WO 2018/211344
PCT/IB2018/052807
Brief description of the figures
Fig. 1: Device 4 closed inside the shaft 3 and positioned in the
aorta at level of the sino-tubular junction.
Fig. 2: Device 4 deployed in the ascending aorta, with open
prosthetic valve.
Fig. 3: Device 4 deployed in the ascending aorta, with closed
prosthetic valve.
Fig. 3a: Device 4, as in figure 3, deployed in the ascending aorta
showing the blood flow direction.
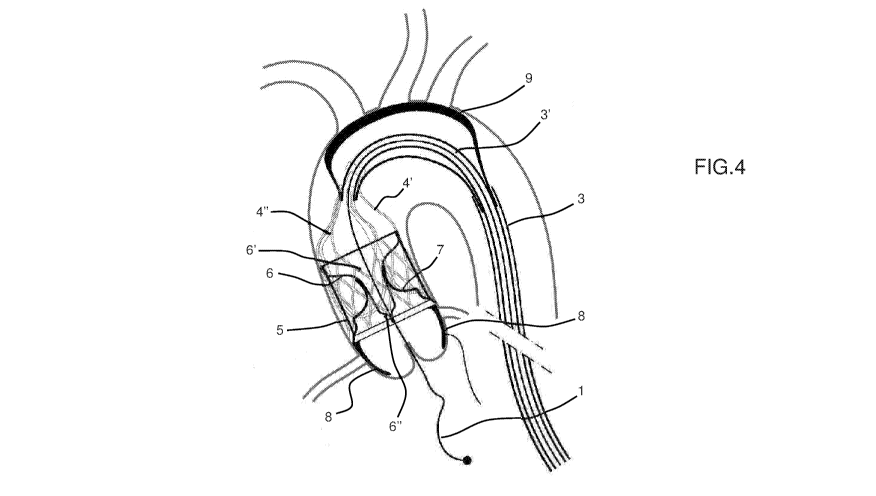
Fig. 4: Device 4 deployed in the ascending aorta with coronary
artery deflectors 8 and epiaortic vessels' deflector 9.
Fig. 4a: Device 4, as in figure 4, deployed in the ascending aorta
showing the blood flow direction. The deflectors block the
emboli but do not impede the blood perfusion.
Fig. 5: Hybrid device 4 in deployed configuration (long axis view).
Fig. 6: Hybrid device 4 in deployed configuration (short axis view
or ventricular view).
Fig. 7: Internal valve's support stent 14.
Fig. 7a: Internal valve's support stent 14: one configuration of
inflow profile.
Fig. 7b: Internal valve's support stent 14: alternative
configuration of inflow profile.
Fig. 7c: Internal valve's support stent 14: alternative
configuration of inflow profile.
Fig. 7d: Internal valve's support stent 14: alternative
configuration of inflow profile.
Fig. 7e: Internal valve's support stent 14: alternative
configuration of inflow profile.
Fig. 8: External support structure 5.
Fig. 9: External support structure 5 and valve's support stent 14.
Fig. 9a: External support structure 5 and a combined internal
structure 5".
Fig. 9b: Combined internal structure 5" integrating the conveyor 6
and the valve's support stent 14. The structure 5" is
anchored to the external support structure 5 by means of
keyhole tethering struts 5"'.
Fig. 10a: Internal structure of the device 4 showing the interaction
of the conveyor with the prosthetic valve 15.
Fig. 10b: Different view of figure 10.
8
CA 03062508 2019-11-05
WO 2018/211344
PCT/IB2018/052807
Fig. 11: Device 4 assembled without the antiembolic filter mesh 10.
Fig. 12: Device 4 assembled with a self-expandable mesh
Fig. 13: Device as in figure 12 only with conveyor.
Fig. 14: Device as in figure 12 with external support structure and
internal valve anchored to its internal wall.
Fig. 15: Device as in figure 12 only represented with external
support structure.
Fig. 16: Device 4 with the convejor system placed outside the
device. In this embodiment it has been placed in series
sequentially and proximally to the device.
Fig. 16a: Another embodiment of the device 4 with the convejor system
placed outside the device.
Fig. 16b: Another embodiment of the device 4 with the convejor system
placed outside the device.
Fig. 16c: Another embodiment of the device 4 with the convejor system
placed outside the device.
Fig. 16d: Another embodiment of the device 4 with the convejor system
placed outside the device.
Fig. 16e: Another embodiment of the device 4 with the convejor system
placed outside the device.
Fig. 16f: Another embodiment of the device 4 with the convejor system
placed outside the device.
Fig. 17: Device 4 as in figure 16 but without the distal conveyor's
tube with bi-directional normally closed valve 6" and the
valve's support stent 14.
Fig. 18: Internal valve's support stent 14 and distal conveyor's
tube with bi-directional normally closed valve 6".
Fig. 19: Device 4 with inflatable structures supporting the device.
The conveyor is placed proximally to the device 4 as
described in figure 16.
Fig. 20: the device 4 as described in figure 19. The internal
structure is visible. The valve's support stent 14 is
visible.
9
CA 03062508 2019-11-05
WO 2018/211344
PCT/IB2018/052807
Procedure
In this chapter the procedure is described with the item description
referred to one embodiment that has the valve, filter, and conveyor
elements inside an external support structure (see figure 5). It is
intended that the procedure is also applied with the other
embidiments with different item mutual positioning.
The device is collapsed into the external shaft catheter 3 before
to introduce it into the arterial vessel (Figure 1). The distal
portion of the external shaft catheter is equipped with a balloon
catheter tip 2 deployed across the edge of the external shaft
catheter 3. The function of this balloon tip is to avoid any arterial
wall damage during the device traveling towards the ascending aorta
while ensuring precise positioning being inflated with radiopaque
solution. When the device is positioned at level of sino-tubular
junction the balloon tip is deflated and retracted outside the
patient's body. In figure 2 the device is deployed inside the
ascending aorta retracting the external shaft catheter 3. When the
device is deployed, the external support structures 5 are fitting
the aorta's wall in order to convey all blood into the device. The
device 4 is connected to the internal shaft catheter 3' by means of
struts or theters 4'. Internally, the device has two components
sustained by the external support structure 5: the conveyor 6 and
the valve prosthesis 15. The conveyor 6 is proximally fixed to the
proximal portion of the external support 5 and delimits "like a
funnel" a channel 6' inside the device. The role of the conveyor is
to allow devices (valvuloplasty balloons or TAVI, etc..) crossing
towards the aortic valve. For the specific embodiment described,
another function of the conveyor 6 is to support the antiembolic
filter. The role of the prosthetic valve, equipped with two, three
or more leaflets, is to avoid a massive blood flow regurgitation
during interventional procedures on the native aortic valve (e.g.
significant perivalvular leakage after valvuloplasty, TAVI implant
or in the future, an interventional ablation of the aortic valve).
The prosthetic valve 15 can be directly anchored to the distal edge
of the external support structure 5 but in the described embodiment
it is mounted on an independent valve support and joined to the
external support structure 5 by a diaphragm of fabric. The valve
function is granted by the coaptation of the leaflets that in closure
CA 03062508 2019-11-05
WO 2018/211344
PCT/IB2018/052807
phase adhere to the distal external surface of the conveyor 6.
Figures 2 and 2a respectively show the device 4 and the diseased
valve respectively in the closed and open positions.
In figure 3 the device is represented deployed as in figure 2 but
the device is equipped with an additional feature represented by two
coronary artery filters 8 and one epiaortic vessels filter 9. The
first one impedes possible debris embolizations into the coronary
ostia during an interventional procedure on the aortic valve. This
event despite being not very frequent is very often catastrophic.
The second one is aimed at avoiding possible residual debris,
accidentally not completely captured by the device 4, to embolize
towards the brain causing a stroke. This deflector can be deployed,
in case of high risk procedures, by further retracting the external
shaft catheter 3.
The above mentioned coronary artery and epiaortic vessels protection
system can be virtually applied in any of the specific embodiment
here-below described.
During the function the blood flow in systole crosses the native
aortic valve, opens the valve prosthesis and crosses the antiembolic
filter 6. Figure 4 details the blood flow direction in systole, with
the main flow pattern trough the aorta, together with the flow
pattern through the epiaortic vessels and a flow trough the coronary
artery, granted by a non complete sealing of the coronary ostia by
the native valve.
The embolic debris are captured and remain inside the structure in
between the conveyor 6 and the external support structure 5.
If needed the device can be left in place for a period in order to
allow a stabilization of the patient's haemodynamics and then
removed. In this case, a specific mechanism can be used that forces
the prosthetic valve open to verify the native valve functionality
restoring upon treatment and repeat the treatment if needed. The
above mentioned valve opening mechanism can be virtually applied
in any of the specific embodiment here-below described.
At the end of the procedure the devices that operated on the aortic
native valve are removed out from the internal lumen of the conveyor
6'. The device 4 is completely retrieved by pushing distally the
external shaft catheter 3. In this way, the device structures
gradually collaps until reaching the distal end of the device safely
keeping inside it all captured clots or calcium debris.
11
CA 03062508 2019-11-05
WO 2018/211344
PCT/IB2018/052807
The device 4 is conceived to provide an effective antiembolic
protection during interventional procedures on the native aortic
valve as well as support the blood circulation in case an aortic
valve insufficiency is present.
In particular a mild to severe valve insufficiency of the native
valve can occur after a balloon valvuloplasty, a suboptimal TAVI
implant or a TAVI misimplantation with consequent migration. This
last condition can be clinically catastrophic with limited
possibility of patient's survival.
In another future condition the device is absolutely necessary. It
is the case in which the diseased native aortic valve is removed
with an interventional off-pump procedure. In this complex procedure
during the dissection of the native valve an antiembolic protection
is mandatory and even more important an ancillary aortic valve
function is demanded in the meantime a sutureless valve prosthesis
is implanted. The device can answer to all these needs.
In a particular embodiment the valve that is integrating in one
single device the antiembolic filter and a valve prosthesis could
provide the two components detachable.
In the case of interventional ablation of the diseased native aortic
valve after its removal the prosthetic valve could be detached from
the device 4 and left in place as a permanent sutureless valve
prosthesis similar to a TAVI procedure.
Description of the device main elements
Valve Prosthesis
The valve allows to temporarily replace the diseased valve during
the procedure, while allowing hydrodynamic performances compatible
with clinical conditions of patients with aortic stenosis.
Support structure
The support structure can be either a single element structure 5"
or a multielement one. In the first case, it has the functions of
coupling with the aorta, support the valve and filter and act as
conveyor. In the second case, the external support 5 has the function
of coupling with the aorta and support other structures. The valve's
support stent 14 has the aim to support the valve leaflets; the
conveyor support 6 is here below described. The internal surface of
the support 5 (5") is covered by an antiembolic tissue mesh 12 in
12
CA 03062508 2019-11-05
WO 2018/211344
PCT/IB2018/052807
order to allow a better sealing of the device against the aortic
wall but also to impede emboli migration in case of limited contact.
Falter
The filter 12 allows to retain the emboli debris without
significantly alter the hydrodynamic characteristics of the valve.
In some embodiments, the filter and the conveyor fabric are joined
in a unique element.
Conveyor
The conveyor 6 is the introducer element of the TVAF: it makes an
easier in situ positioning of specific devices (i.e. TAVI) loaded
with external catheter 3, thanks to the geometry of its elements.
Typically, a series of elements interconnected: a conical support
structure with an antiembolic mesh lining, such as fabrics or
membranes, a distal cylindrical expandable tubular part with an
impermeable lining and a bi-directional normally closed valve.
In some embodiments, the conveyor 6 and the valve's support stent
14 are joined in a unique element 5".
Internal and external shaft catheters
The internal shaft catheter 3' support the device 4, permanently
in the default set-up. The internal shaft catheter is protected by
the external shaft catheter 3 that has the function to guide the
device in position and to allow the deployment/recapture of the
device 4.
13
CA 03062508 2019-11-05
WO 2018/211344
PCT/IB2018/052807
The present invention is of course not limited to the embodiments
and examples discussed in the present document. Therefore the
disclosures should not be limited by any particular element
hereinafter described.
More into details, as far as concerns the materials: the support
structures are here described as made for most of the embodiments
by self-expanding metallic materials like nitinol, but also other
metallic and non metallic materials with similar characteristics can
apply and also non self-expandable sructures like polymeric
inflatable ones can apply; the filter is described as a polymeric
woven fabric, but also non-woven (i.e. membrane with calibrated
holes) and or metallic materials with similar characteristics can
apply; the valve is described as a polymeric woven fabric coated for
ensuring leak-free characteristics, but also non-woven with similar
characteristics can apply; the catheters comprises a polymeric tube,
but also a metal-reinforced polymeric tube.
As far as concerns the techonologies: the metallic support structures
are described as obtained by laser cutting tubes or welded sheets,
by woven (i.e. by a plurality strands) and single wire structures;
the coupling between the different elements of the device can be
either glueing, soldering, welding (i.e. ultrasound), adhering,
sewing, and other applicable methods; the valve can be obtained by
coating of a fabric, but also other synthetic or natural materials
can also apply, such as a polymeric membrane.
As far as concerns the embodiments: in the description, embodiments
deemed to be used with femoral access to restore the diseased aortic
valve are shown. At the same time, also embodiments with access
different from femoral can also apply. A specific embodiment where
the valve part can be disloged by the rest of the assembly can
apply, in order to be used as a TAVI or a sutureless valve prosthesis.
In this case, the valves' leaflets can be manufactured with material
different respect to polymeric ones, such as pericardial tissue or
other and the valve structure can also have specific retrieval
elements.
Moreover, also embodiments to restore other diseased heart valves
can apply.
The device can also apply in other technical fields, such as the
interventional radiology, as a valved, or not, filter for carotid
14
CA 03062508 2019-11-05
WO 2018/211344
PCT/IB2018/052807
artery protection as well as a repositionable/recapturable venous
valve with antiembolic filter. In this cases specific embodiments
and dimension for the different elements to be used amongst the
default set-up (valve, filter, conveyor and relevant support
structures, catheters) and the expected use (acute, subacute,
chronic) will apply.
In terms of dimensions, those related to the specific use will apply,
such as the anatomy dimensions of the health and disesed organs to
be treated, the access size for the different transcatheter approach,
the filter size to protect from the embolization in the coronary and
epiaortic arteries.
Figures from 5 to 11 show one embodiment, herebelow referred as
hybrid device being the techonology used for manufacturing the self-
expandable nitinol structures laser cutting for the external support
structure 5 and valve's support stent 14 and braiding or wiring for
the conveyor 6.
In this embodiment, the external support structure 5 and the inner
valve's support stent 14 are connected by a sort of diaphragm 13,
thus ensuring deployment of the inner prosthetic valve 15 independent
respect to the external support 5 and antiembolic filter elements.
The conveyor 6, which also acts as the filter support, is positioned
inside the external structure 5 in order to reduce the overall device
length.
In figure 5 a long axis view of the device in deployed configuration
shows the rings that permanently joints the internal shaft catheter
3' to the external self-expandable support structure 5 and the
conveyor 6 and valve's support stent 14 by means of the tethering
struts 4' and 4". In figure 5 it is also shown the coupling between
the external support structure 5 and the internal mesh lining 10,
that ensures a leak free contact to the aortic walls.
In figure 6 a short axis (ventricular) view of the device 4, in a
deployed configuration, shows the anchoring holes between the
external structure 5 and the mesh 10, that is reverted at the distal
side and is joined to the valve prosthesis 15 leaflets 7, these
latter covering the external side of the self-expandable material
internal valve's support stent 14. The absence of leakage in the
diastolic phase is guaranteed by the impermeable mesh of the leaflet
elements 7 and of the mesh 10 together with the configuration of the
conveyor conduit, which is distally equipped with a bi-directional
normally closed valve 6". Both in systole and diastole the valve
CA 03062508 2019-11-05
WO 2018/211344
PCT/IB2018/052807
6" remains closed, in order to prevent any blood and possible embolic
particles leakage; when the transcatheter devices are introduced,
the distal conveyor's tube 6" extends in diameter facilitating
their introduction maintaining a proper alignment, whilst the valve
6" allows a virtually leak free crossing of the device. The valve
6" can be either directly operated by the delivery system or
automatically, remaining strictly closed at the systolic and
diastolic differential pressure, but capable to be crossed by the
insterted device delivery system, whilst maintaining a leak free
coupling.
Figure 7 shows the self-expandable internal valve's support stent
14, which supports both the commisures of the leaflet 7 and the
overall inflow profile of the said leaflets 7 with specific joints
14', which contoures the structure from the external side. This
configuration allows minimization of the pressure drop in the
systolic phase thanks to a wide and cylindrical leaflet opening and
minimization of the closure and leakage backflow regurgitation
during the diastolic phase. The tethering struts 4" allow a direct
joining with the internal shaft catheter 3' with adequate
independence respect to the external support 5.
Figures 7a, 7b, 7c, 7d and 7e show alternative configurations of
inflow profiles for ensuring at the same time adequate retrievability
and radial stiffness.
Figure 8 shows the self-expandable external support structure 5,
which support the conveyor 6 and relevant filter mesh 12 at the
anchoring holes 5' side and the coupling of the mesh 10 with the
inflow side of the leaflets 7.
In figure 9 both external 5 and internal 14 self-expandable
structures are shown without the relevant mesh, in order to outline
the mutual positioning of the tethering structures that joints them
to the the internal catheter 3', together with the holes for
connecting to the conveyor 6 and leaflets 7 elements.
The internal elements, called conveyor 6 and valve's stent support
14, can be combined in a single element 5" to be joined to the
external structure 5 by a tethered struts with keyholes 5"' as
described in figures 9a and 9b.
In figure 10, the outflow side of conveyor 6, supporting the filter
12, and the prosthetic valve 15 elements are shown, together with
the self-expandable internal valve's support stent 14 and the
16
CA 03062508 2019-11-05
WO 2018/211344
PCT/IB2018/052807
tethering struts connecting said structure to the internal shaft
catheter 3'.
As far as concerns the conveyor and filter elements, the conical
shape of the conveyor guarantees first a smooth and easy crossing
by the devices loaded with external catheter different than the
external 3; second, it is covered with a filter 12 of adequate mesh
and surface, in order to minimize relevant pressure drop in the
systolic phase and filter any possible embolization debris deriving
from the procedure and maintain it in the collection chamber obtained
between the mesh 12 and 10; third, it guarantees a smooth retrieve.
The distal end of the conveyor is cilindrical with axis aligned with
the diseased valve to be treated, to guarantee a proper alignment
of the loaded device. Furthermore, this cilindrical part has radial
compliance adequate to minimize the force to be applied for loading
and retrieving the device through the delivery system.
In figure 10a the same elements are viewn from the inflow side
(ventricle view), with the bi-directional normally closed valve at
the distal part of the conveyor shown, that guarantees no flow both
in systole, to impede any embolization to cross the device 4, nor
in diastole, to minimize overall leakage, whilst allowing the loaded
device crossing through the device 4.
As far as it concerns the valve, figure 10 and 10a show the prosthetic
valve body 15 from the outflow and inflow side. The trileaflet
configuration was selected, with leaflets made of a low thickness
polymeric fabric elastomerically coated and installed outside the
supporting structure 14 in order to guarantee a wide leaflet
cylindrical open configuration. This design configuration guarantees
optimal pliability/foldability and at the same type relatively low
extensibility, thus optimal hemodynamics and mechanical
characteristics. Design and materials allow adequate hemodynamic
performance in terms of low pressure drop in systole, thanks to the
large orifice area and leaflets foldability, and low regurgitation
in diastole, thanks to the leak free characteristic of the leaflets
and relevant foldability that allows a proper coupling at closure
of the said leaflets respect to the distal conveyor leak free tube
body 6".
Figure 11 shows the configuration of the device 4 assembled without
the mesh 10, in order to visualize the mutual positioning of the
conveyor/filter and of the valve respect to the relevant external 5
and valve's support stent 14 support structures.
17
CA 03062508 2019-11-05
WO 2018/211344
PCT/IB2018/052807
Radiopaque markers are placed in order to better detect specific
locations, such as the posts and side access, and internal catheter
locations, such as the aortic arch level. Materials, joining
mechanism and number of elements are selected based at the state of
the art and based on the current procedures.
Figures from 12 to 15 show an alternative embodiment, configured as
well as the hybrid one with a conveyor internal to the body, in
order to minimize overall length, but with both the external support
structure 5 and the conveyor 6 made of a superelastic metallic mesh,
therefore referred as mesh embodiment. Another difference respect
to the hybrid embodiment is that in the mesh embodiment the external
support structure 5 directly provides an anchoring surface for the
leaflet of prosthetic valve 15.
Figure 12 shows a lateral view of the mesh assembly, with the
external cylindrical structure 5 and the mesh 10, the conveyor 6 and
relevant mesh 12, the prosthetic valve 15, together with the relevant
coupling between the elements.
The coupling elements of the superelastic metallic external
structure 5 are as follows: a tethering structure 4', which is
permanently joined to the internal catheter 3' by means of a ring
11, sustains the external structure 5 and the inflow side of the
conveyor 6, whilst allowing the mutual sliding of the elements to
allow proper self expanding and retrieval; a cylindrical tube mesh
10, acts as a mutual joint elements between the external structure
5 and the prosthetic valve 15, namely with sewing/ultrasound welding
them at the inflow and outflow sides to the tube 10 and to the valve
along its inflow side profile.
In figure 13 the conveyor 6 is shown in its coupling to the internal
catheter 3' by means of the tethering structure 4", in its conical
part and in relevant conveyor distal tube 6" equipped with the bi-
directional normally closed valve. The same features already
depicted for the preferred assembly here apply.
In figure 14 the external support structure 5 and internal valve's
support stent 14 anchored to its internal wall are shown. This
embodiment is different respect to the hybrid one because it misses
an internal metallic support structure in order to optimize the low
profile characteristics of the device rather than having an
independent valve anchoring.
18
CA 03062508 2019-11-05
WO 2018/211344
PCT/IB2018/052807
In figure 15 the sliding coupling amongst the external support
structure 5 and the tethering struts 4" is shown from the outflow
side.
Figures from 16 to 18 show a device 4 derived from the hybrid, but
with the conveyor 6 system placed proximally outside the device.
As one can see in figure 16, this embodiment can guarantee, in
principle, an alignment of the loaded device better than the previous
ones due to a longer distal conveyor tube and an easier retrieval
inside small caliper external catheter 3 thanks to the reduced number
of elements put one inside the other. At the same time, due to the
higher legnth respect to the embodiments with internal conveyor, the
coupling at 11' must be flexible in order to follow the aortic arch
pattern at the proximal conveyor side, whilst guaranteeing a stable
anchoring to the aorta at the distal side.
Figures 16, 16a, 17, 18 show the elements similar to the hybrid ones
(namely the coupling amongst the prosthetic valve 15 and its valve's
support stent 14, the coupling amongst the mesh 10 and the external
structure 5) and the main differences: the conveyor cone 6 is
proximal, it is placed outside of the external structure 5, and it
is half distally covered with a filtering mesh that can have only
the mechanical function of driving the movement of the loaded devices
towards the internal lumen of the conveyor; the antembolic filter
mesh 12, viceversa, is in the conical part of the external structure,
distal respect to the ring 11'.
In the following figures, some alternative embodiments of the
external support 5 and valve's support stent 14 are shown, without
the conveyor system.
Figures 16b, 16c, 16d and 16e show, respectively, two laser cut and
two braided alternative embodiments of the external structure 5 of
the hybrid device 4, with different ratio between diamonds and
straight elements in order to be more oriented to radial stiffness
or retrievability characteristics.
Figure 16f shows a self expanding structure that combines the
characteristics of the external 5 and valve's stent 14 support
structures in one, devoting the last one on holding only the leaflets
posts. This embodiment is intended to minimize the radial thickness
of the supporting structure in order to maximize the retrievability.
Figure 16g shows a self expanding structure similar to the hybrid
mesh, in which two diamond structures at the inflow and outflow
sides of the valve are joined by linear elements in order to avoid
19
CA 03062508 2019-11-05
WO 2018/211344
PCT/IB2018/052807
overall length variation of this region at retrieval and a skirt
element.
In figures from 19 to 20 a specific embodiment of an inflatable
device is described.
The use of inflatable structures has the aim to minimize the number
of different materials involved in the manufacturing and it allows
a reduced encumbrance of the collapsed device. Moreover it allows
an easy positioning of the device thanks to the radiopaque
characterisitcs of the CO2 filler.
Several different embodiments can apply to the inflatable group,
starting from a device 4 with all inflated support structures, with
conveyor 6 inside the external support structure 5 and the prosthetic
valve 15 directly joined to it and ending to a device 4 with
longitudinal elements of the external support structure 5 and valve's
support stent 14 made of self-expanding materials, such as nitinol,
and conveyor external to the structure 5.
As far as concerns the opening mechanism for the valve, which is
intended to be used at the end of the restoring procedure to verify
the relevant outcomes on the diseased valve, different embodiments
can apply, acting directly on the leaflets 7 and / or on the valve's
support stent 14 by means of shaft mechanisms, either pushing
/pulling or rotating, proximally holded inside the internal catheter
3' and commanded by the delivery system.
20