Note: Descriptions are shown in the official language in which they were submitted.
CA 03064550 2019-11-21
WO 2018/231254
PCT/US2017/037991
COMPOSITIONS AND METHODS FOR TREATING TAUOPATHIES
Field
The present application relates generally to dosage regimens and formulations
for the
clinical use of anti-tau antibodies.
Background
Protein accumulation, modifications, and aggregation are pathological aspects
of
numerous neurodegenerative diseases. Pathologically modified and aggregated
tau including
hyperphosphorylated tau conformers are an invariant hallmark of tauopathies
and correlate
with disease severity.
The microtubule associated protein tau is abundant in the central nervous
system and
is produced primarily by neurons. Tau promotes the assembly of, maintains the
structure of,
and stabilizes microtubules. The tau proteins are derived from alternative
mRNA splice
variants that originate from a single gene and result in mature proteins that
vary in size from
352 to 441 amino acids. While the fetal brain contains a single tau isoform
(Tau-352), six tau
isoforms exist in the adult human brain. They differ from one another in
having three or four
microtubule binding repeats of 31-32 amino acids each, and two, one, or no
amino terminal
inserts of 29 amino acids each.
Tauopathies are a class of neurodegenerative diseases resulting from the
pathological
aggregation of Tau protein in so-called neurofibrillary tangles (NFT) in the
brain. Some
examples of tauopathies include progressive supranuclear palsy, Alzheimer's
disease,
frontotemporal dementia (FTD), corticobasal degeneration, and frontotemporal
lobar
degeneration.
There is a need in the art for methods of treating tauopathies. In order to
treat the
growing numbers of patients with tauopathies, there is a need for a
therapeutic antibody
against tau and appropriate dosage regimens and formulations for the clinical
use of such an
anti-human tau antibody.
Summary
This disclosure relates, in part, to dosage regimens and formulations of anti-
human
tau antibodies or tau-binding fragments thereof and their use in the treatment
of a tauopathy.
In one aspect, this disclosure provides a method of treating a tauopathy in a
human
subject in need thereof The method involves administering to the human subject
an anti-
human tau antibody at a fixed dose of 2000 mg. The anti-human tau antibody
comprises an
1
CA 03064550 2019-11-21
WO 2018/231254
PCT/US2017/037991
immunoglobulin heavy chain variable region (VH) and an immunoglobulin light
chain
variable region (VL). The VH comprises VH complementarity determining regions
(VH-
CDRs), wherein VH-CDR1 comprises or consists of the amino acid sequence of SEQ
ID
NO:16; VH-CDR2 comprises or consists of the amino acid sequence of SEQ ID
NO:17; and
VH-CDR3 comprises or consists of the amino acid sequence of SEQ ID NO:18. The
VL
comprises VL-CDRs, wherein VL-CDR1 comprises or consists of the amino acid
sequence
of SEQ ID NO:19; VL-CDR2 comprises or consists of the amino acid sequence of
SEQ ID
NO:20; and VL-CDR3 comprises or consists of the amino acid sequence of SEQ ID
NO:21.
In certain instances the anti-human tau antibody is administered to the human
subject
intravenously. In certain cases, the fixed dose of 2000 mg of the anti-human
tau antibody is
administered once every four weeks.
In another aspect, provided herein is a method of treating a tauopathy in a
human
subject in need thereof The method involves administering to the human subject
an anti-
human tau antibody at a fixed dose of 150 mg. The anti-human tau antibody
comprises an
immunoglobulin heavy chain variable region (VH) and an immunoglobulin light
chain
variable region (VL). The VH comprises VH complementarity determining regions
(VH-
CDRs), wherein VH-CDR1 comprises or consists of the amino acid sequence of SEQ
ID
NO:16; VH-CDR2 comprises or consists of the amino acid sequence of SEQ ID
NO:17; and
VH-CDR3 comprises or consists of the amino acid sequence of SEQ ID NO:18. The
VL
comprises VL-CDRs, wherein VL-CDR1 comprises or consists of the amino acid
sequence
of SEQ ID NO:19; VL-CDR2 comprises or consists of the amino acid sequence of
SEQ ID
NO:20; and VL-CDR3 comprises or consists of the amino acid sequence of SEQ ID
NO:21.
In certain instances the anti-human tau antibody is administered to the human
subject
intravenously. In certain cases, the fixed dose of 150 mg of the anti-human
tau antibody is
administered once every four weeks.
In another aspect, provided herein is a method of treating a tauopathy in a
human
subject in need thereof The method involves administering to the human subject
an anti-
human tau antibody at a fixed dose of 210 mg. The anti-human tau antibody
comprises an
immunoglobulin heavy chain variable region (VH) and an immunoglobulin light
chain
variable region (VL). The VH comprises VH complementarity determining regions
(VH-
CDRs), wherein VH-CDR1 comprises or consists of the amino acid sequence of SEQ
ID
NO:16; VH-CDR2 comprises or consists of the amino acid sequence of SEQ ID
NO:17; and
VH-CDR3 comprises or consists of the amino acid sequence of SEQ ID NO:18. The
VL
comprises VL-CDRs, wherein VL-CDR1 comprises or consists of the amino acid
sequence
2
CA 03064550 2019-11-21
WO 2018/231254
PCT/US2017/037991
of SEQ ID NO:19; VL-CDR2 comprises or consists of the amino acid sequence of
SEQ ID
NO:20; and VL-CDR3 comprises or consists of the amino acid sequence of SEQ ID
NO:21.
In certain instances the anti-human tau antibody is administered to the human
subject
intravenously. In certain cases, the fixed dose of 210 mg of the anti-human
tau antibody is
administered once every four weeks.
In another aspect, provided herein is a method of treating a tauopathy in a
human
subject in need thereof The method involves administering to the human subject
an anti-
human tau antibody at a fixed dose of 2100 mg. The anti-human tau antibody
comprises an
immunoglobulin heavy chain variable region (VH) and an immunoglobulin light
chain
variable region (VL). The VH comprises VH complementarity determining regions
(VH-
CDRs), wherein VH-CDR1 comprises or consists of the amino acid sequence of SEQ
ID
NO:16; VH-CDR2 comprises or consists of the amino acid sequence of SEQ ID
NO:17; and
VH-CDR3 comprises or consists of the amino acid sequence of SEQ ID NO:18. The
VL
comprises VL-CDRs, wherein VL-CDR1 comprises or consists of the amino acid
sequence
of SEQ ID NO:19; VL-CDR2 comprises or consists of the amino acid sequence of
SEQ ID
NO:20; and VL-CDR3 comprises or consists of the amino acid sequence of SEQ ID
NO:21.
In certain instances the anti-human tau antibody is administered to the human
subject
intravenously. In certain cases, the fixed dose of 2100 mg of the anti-human
tau antibody is
administered once every four weeks.
In one aspect, provided herein is a method of treating a tauopathy in a human
subject
in need thereof The method involves administering to the human subject an anti-
human tau
antibody at a fixed dose of 4200 mg. The anti-human tau antibody comprises an
immunoglobulin heavy chain variable region (VH) and an immunoglobulin light
chain
variable region (VL). The VH comprises VH complementarity determining regions
(VH-
CDRs), wherein VH-CDR1 comprises or consists of the amino acid sequence of SEQ
ID
NO:16; VH-CDR2 comprises or consists of the amino acid sequence of SEQ ID
NO:17; and
VH-CDR3 comprises or consists of the amino acid sequence of SEQ ID NO:18. The
VL
comprises VL-CDRs, wherein VL-CDR1 comprises or consists of the amino acid
sequence
of SEQ ID NO:19; VL-CDR2 comprises or consists of the amino acid sequence of
SEQ ID
NO:20; and VL-CDR3 comprises or consists of the amino acid sequence of SEQ ID
NO:21.
In certain instances the anti-human tau antibody is administered to the human
subject
intravenously. In certain cases, the fixed dose of 4200 mg of the anti-human
tau antibody is
administered once every four weeks.
3
CA 03064550 2019-11-21
WO 2018/231254
PCT/US2017/037991
The following embodiments apply to all of the above aspects. In some
instances, the
tauopathy is progressive supranuclear palsy, Alzheimer's disease, amyotrophic
lateral
sclerosis/parkinsonism- dementia complex, argyrophilic grain dementia, British
type amyloid
angiopathy, cerebral amyloid angiopathy, corticobasal degeneration,
Creutzfeldt- Jakob
disease, dementia pugilistica, diffuse neurofibrillary tangles with
calcification, Down's
syndrome, frontotemporal dementia (FTD), frontotemporal dementia with
parkinsonism
linked to chromosome 17, frontotemporal lobar degeneration, Gerstmann-
Straussler-
Scheinker disease, Hallervorden-Spatz disease, inclusion body myositis,
multiple system
atrophy, myotonic dystrophy, Niemann-Pick disease type C, non-Guamanian motor
neuron
disease with neurofibrillary tangles, Pick's disease, postencephalitic
parkinsonism, prion
protein cerebral amyloid angiopathy, progressive subcortical gliosis, globular
glial tauopathy,
subacute sclerosing panencephalitis, Tangle only dementia, multi-infarct
dementia, stroke,
chronic traumatic encephalopathy, traumatic brain injury, concussion,
seizures, epilepsy, or
acute lead encephalopathy. In one instance, the tauopathy is progressive
supranuclear palsy.
In another instance, the tauopathy is Alzheimer's disease. In certain cases,
the VH of the
anti-human tau antibody comprises or consists of SEQ ID NO:12, and the VL of
the anti-
human tau antibody comprises or consists of SEQ ID NO:13. In certain
instances, the anti-
human tau antibody comprises a heavy chain and a light chain, wherein the
heavy chain
comprises or consists of SEQ ID NO:14, and the light chain comprises or
consists of SEQ ID
NO:15.
In another aspect, this disclosure features a pharmaceutical composition
comprising
an anti-human tau antibody. The pharmaceutical composition comprises an anti-
human tau
antibody at a concentration of 50 mg/ml or 60 mg/ml; histidine at a
concentration of 20 mM,
sucrose at a concentration of 250 mM, polysorbate-80 at a concentration of
0.05% (w/v). In
some instances, the pharmaceutical composition further comprises 50 [IM
diethylenetriamine
pentaacetic acid (DTPA). The anti-human tau antibody comprises a VH and a VL.
The VH
comprises a VH-CDR1 comprising or consisting of the amino acid sequence of SEQ
ID
NO:16; a VH-CDR2 comprising or consisting of the amino acid sequence of SEQ ID
NO:17;
and a VH-CDR3 comprising or consisting of the amino acid sequence of SEQ ID
NO:18. The
VL comprises a VL-CDR1 comprising or consisting of the amino acid sequence of
SEQ ID
NO:19; a VL-CDR2 comprising or consisting of the amino acid sequence of SEQ ID
NO:20;
and a VL-CDR3 comprising or consisting of the amino acid sequence of SEQ ID
NO:21. The
pharmaceutical composition has a pH of 6Ø
4
CA 03064550 2019-11-21
WO 2018/231254
PCT/US2017/037991
In some embodiments, the VH of the anti-human tau antibody comprises or
consists
of SEQ ID NO:12 and the VL of the anti-human tau antibody comprises or
consists of SEQ
ID NO:13. In some embodiments, the anti-human tau antibody comprises a heavy
chain and
a light chain, wherein the heavy chain comprises or consists of SEQ ID NO:14
and the light
chain comprises or consists of SEQ ID NO:15. In some embodiments, the
pharmaceutical
composition is used in for treating a tauopathy in a human subject in need
thereof by
intravenously administering to the human subject any of the above-described
pharmaceutical
compositions. In some embodiments, the anti-human tau antibody of the
pharmaceutical
composition is administered to a human subject at a fixed dose of 150 mg once
every four
weeks. In other embodiments, the anti-human tau antibody of the pharmaceutical
composition is administered to a human subject at a fixed dose of 210 mg once
every four
weeks. In yet other embodiments, the anti-human tau antibody of the
pharmaceutical
composition is administered to a human subject at a fixed dose of 700 mg once
every four
weeks. In further embodiments, the anti-human tau antibody of the
pharmaceutical
composition is administered to a human subject at a fixed dose of 2000 mg once
every four
weeks. In other embodiments, the anti-human tau antibody of the pharmaceutical
composition is administered to a human subject at a fixed dose of 2100 mg once
every four
weeks. In another embodiment, the anti-human tau antibody of the
pharmaceutical
composition is administered to a human subject at a fixed dose of 4200 mg once
every four
weeks. In certain instances, the pharmaceutical composition is administered
for at least 12
weeks (e.g., 12 weeks, 16 weeks, 20 weeks, 24 weeks, 30 weeks, 32 weeks, 36
weeks, 40
weeks, 48 weeks, 52 weeks). In certain instances, the tauopathy is progressive
supranuclear
palsy, Alzheimer's disease, amyotrophic lateral sclerosis/parkinsonism-
dementia complex,
argyrophilic grain dementia, British type amyloid angiopathy, cerebral amyloid
angiopathy,
corticobasal degeneration, Creutzfeldt- Jakob disease, dementia pugilistica,
diffuse
neurofibrillary tangles with calcification, Down's syndrome, frontotemporal
dementia (FTD),
frontotemporal dementia with parkinsonism linked to chromosome 17,
frontotemporal lobar
degeneration, Gerstmann-Straussler-Scheinker disease, Hallervorden-Spatz
disease, inclusion
body myositis, multiple system atrophy, myotonic dystrophy, Niemann-Pick
disease type C,
non-Guamanian motor neuron disease with neurofibrillary tangles, Pick's
disease,
postencephalitic parkinsonism, prion protein cerebral amyloid angiopathy,
progressive
subcortical gliosis, globular glial tauopathy, subacute sclerosing
panencephalitis, Tangle only
dementia, multi-infarct dementia, stroke, chronic traumatic encephalopathy,
traumatic brain
injury, concussion, seizures, epilepsy, or acute lead encephalopathy. In one
embodiment, the
CA 03064550 2019-11-21
WO 2018/231254
PCT/US2017/037991
tauopathy is progressive supranuclear palsy. In another embodiment, the
tauopathy is
Alzheimer's disease.
Unless otherwise defined, all technical and scientific terms used herein have
the same
meaning as commonly understood by one of ordinary skill in the art to which
this invention
belongs. Although methods and materials similar or equivalent to those
described herein can
be used in the practice or testing of the present invention, the exemplary
methods and
materials are described below. All publications, patent applications, patents,
and other
references mentioned herein are incorporated by reference in their entirety.
In case of
conflict, the present application, including definitions, will control. The
materials, methods,
and examples are illustrative only and not intended to be limiting.
Other features and advantages of the invention will be apparent from the
following
detailed description and from the claims.
Brief Description of Drawings
Fig. 1 provides the sequences of different forms of extracellular Tau (eTau),
eTaul
(SEQ ID NO:7); eTau2 (SEQ ID NO:8), eTau3 (SEQ ID NO:9), and eTau4 (SEQ ID
NO:10), compared to the human Tau-441 isoform (2N4R) sequence (SEQ ID NO:6).
Fig. 2 is a schematic representation of the study design for the single
ascending dose
study described in Example 1.
Fig. 3 is a graph depicting the exposure-response model (Bayesian Emax) of CSF
concentration versus eTau suppression.
Fig. 4 is a schematic representation of the study design for the multiple
ascending
dose study described in Example 3.
Fig. 5 is a table providing the baseline demographic characteristics of the
patients in
the multiple ascending dose study described in Example 3.
Fig. 6 is a table providing a summary of the adverse events for the multiple
ascending
dose study described in Example 3.
Fig. 7 is a table providing a summary of the serum PK parameters for BIIB092
(at day
57 of the study).
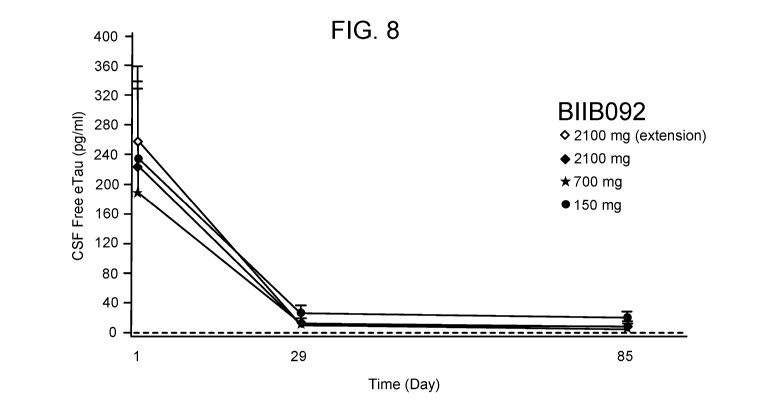
Fig. 8 is a graphical depiction of the mean change in eTau concentrations over
time.
There was a dose-dependent relationship between BIIB092 dose and the extent of
eTau
suppression in the CSF.
FIG. 9 is a table providing CSF free eTau as a percentage of baseline with
BIIB092
dose.
6
CA 03064550 2019-11-21
WO 2018/231254
PCT/US2017/037991
Detailed Description
This disclosure features dosage regimens and formulations of anti-human tau
antibodies and tau-binding fragments thereof and their use in the treatment of
tauopathies
(e.g., disorders related to aggregates of tau such as progressive supranuclear
palsy,
Alzheimer's disease, amyotrophic lateral sclerosis/parkinsonism- dementia
complex,
argyrophilic grain dementia, British type amyloid angiopathy, cerebral amyloid
angiopathy,
corticobasal degeneration, Creutzfeldt- Jakob disease, dementia pugilistica,
diffuse
neurofibrillary tangles with calcification, Down's syndrome, frontotemporal
dementia (FTD),
frontotemporal dementia with parkinsonism linked to chromosome 17,
frontotemporal lobar
degeneration, Gerstmann-Straussler-Scheinker disease, Hallervorden-Spatz
disease, inclusion
body myositis, multiple system atrophy, myotonic dystrophy, Niemann-Pick
disease type C,
non-Guamanian motor neuron disease with neurofibrillary tangles, Pick's
disease,
postencephalitic parkinsonism, prion protein cerebral amyloid angiopathy,
progressive
subcortical gliosis, subacute sclerosing panencephalitis, Tangle only
dementia, multi-infarct
dementia, stroke, chronic traumatic encephalopathy, traumatic brain injury,
concussion,
seizures, epilepsy, and acute lead encephalopathy).
Tau
Tau is a protein that plays a critical role in the pathogenesis of several
disorders
collectively referred to as tauopathies. There are several different isoforms
of the
microtubule-associated protein, which are provided below:
Isoform Fetal-tau of 352aa
MAEPRQEFEVMEDHAGTYGLGDRKDQGGYTMHQDQEGDTDAGLKAEEAGIGDT PSLE
DEAAGHVT QARMVS KS KDGT GS DDKKAKGADGKT KIAT PRGAAP PGQKGQANAT RI P
AKTPPAPKTPPSSGEPPKSGDRSGYSSPGSPGTPGSRSRTPSLPTPPTREPKKVAVV
RT PPKS PS SAKSRLQTAPVPMPDLKNVKSKIGS TENLKHQPGGGKVQIVYKPVDLS K
VTSKCGSLGNIHHKPGGGQVEVKS EKLDFKDRVQSKI GSLDNITHVPGGGNKKIET H
KLT FRENAKAKTDHGAEIVYKS PVVSGDTS PRHLSNVS SIGS I DMVDS PQLATLADE
VSASLAKQGL (SEQ ID NO:!)
Isoform Tau-B of 381aa
MAEPRQEFEVMEDHAGTYGLGDRKDQGGYTMHQDQEGDTDAGLKES PLQT PT EDGS E
EPGSETSDAKST PTAEAEEAGIGDT PSLEDEAAGHVTQARMVSKSKDGTGSDDKKAK
7
CA 03064550 2019-11-21
WO 2018/231254
PCT/US2017/037991
GADGKTKIAT PRGAAPPGQKGQANATRI PAKT P PAPKT P PS S GEP PKS GDRS GYS S P
GS PGT PGSRSRT PS L PT P PTREPKKVAVVRT PPKS PS SAKSRLQTAPVPMPDLKNVK
SKIGSTENLKHQPGGGKVQIVYKPVDLSKVTSKCGSLGNIHHKPGGGQVEVKSEKLD
FKDRVQSKIGSLDNITHVPGGGNKKIETHKLT FRENAKAKTDHGAEIVYKS PVVSGD
TS PRHLSNVS SIGS I DMVDS PQLATLADEVSASLAKQGL (SEQ ID NO:2)
Isoform Tau-C of 410aa
MAEPRQEFEVMEDHAGTYGLGDRKDQGGYTMHQDQEGDTDAGLKES PLQT PT EDGS E
EPGSETSDAKST PTAEDVTAPLVDEGAPGKQAAAQPHTEI PEGTTAEEAGIGDT PS L
E DEAAGHVT QARMVS KS KDGT GS D DKKAKGADGKT KIAT PRGAAP PGQKGQANAT RI
PAKTPPAPKTPPSSGEPPKSGDRSGYSSPGSPGTPGSRSRTPSLPTPPTREPKKVAV
VRT PPKS PS SAKSRLQTAPVPMPDLKNVKSKIGSTENLKHQPGGGKVQIVYKPVDLS
KVTSKCGSLGNIHHKPGGGQVEVKSEKL DFKDRVQSKIGSLDNITHVPGGGNKKIET
HKLT FRENAKAKTDHGAEIVYKS PVVSGDTS PRHLSNVS SIGS I DMVDS PQLATLAD
EVSASLAKQGL (SEQ ID NO:3)
Isoform Tau-D of 383aa
MAEPRQEFEVMEDHAGTYGLGDRKDQGGYTMHQDQEGDTDAGLKAEEAGIGDT PSLE
DEAAGHVT QARMVS KS KDGT GS DDKKAKGADGKT KIAT PRGAAP PGQKGQANAT RI P
AKTPPAPKTPPSSGEPPKSGDRSGYSSPGSPGTPGSRSRTPSLPTPPTREPKKVAVV
RT PPKS PS SAKSRLQTAPVPMPDLKNVKSKIGS TENLKHQPGGGKVQI INKKLDLSN
VQSKCGSKDNIKHVPGGGSVQIVYKPVDLSKVT SKCGSLGNIHHKPGGGQVEVKSEK
LDFKDRVQSKIGSLDNITHVPGGGNKKIETHKLT FRENAKAKTDHGAEIVYKS PVVS
GDTS PRHLS NVS ST GS I DMVDS PQLATLADEVSASLAKQGL (SEQ ID NO:4)
Isoform Tau-E of 412aa
MAEPRQEFEVMEDHAGTYGLGDRKDQGGYTMHQDQEGDTDAGLKES PLQT PT EDGS E
EPGSETSDAKST PTAEAEEAGIGDT PSLEDEAAGHVTQARMVSKSKDGTGSDDKKAK
GADGKTKIAT PRGAAPPGQKGQANATRI PAKT P PAPKT P PS S GEP PKS GDRS GYS S P
GS PGT PGSRSRT PS L PT P PTREPKKVAVVRT PPKS PS SAKSRLQTAPVPMPDLKNVK
SKIGSTENLKHQPGGGKVQIINKKLDLSNVQSKCGSKDNIKHVPGGGSVQIVYKPVD
LSKVT SKCGSLGNI HHKPGGGQVEVKSEKLDFKDRVQSKIGS LDNIT HVPGGGNKKI
ETHKLT FRENAKAKTDHGAEIVYKS PVVSGDTS PRHL SNVS S TGS I DMVDS PQLATL
ADEVSASLAKQGL (SEQ ID NO:5)
8
CA 03064550 2019-11-21
WO 2018/231254
PCT/US2017/037991
Isoform Tau-F (2N4R) of 441aa
MAEPRQEFEVMEDHAGTYGLGDRKDQGGYTMHQDQEGDTDAGLKES PLQT PT EDGS E
EPGSETSDAKST PTAEDVTAPLVDEGAPGKQAAAQPHTEIPEGTTAEEAGIGDT PS L
E DEAAGHVT QARMVS KS KDGT GS D DKKAKGADGKT KIAT PRGAAP PGQKGQANAT RI
PAKTPPAPKTPPSSGEPPKSGDRSGYSSPGSPGTPGSRSRTPSLPTPPTREPKKVAV
VRT PPKS PS SAKSRLQTAPVPMPDLKNVKSKIGSTENLKHQPGGGKVQI INKKLDL S
NVQSKCGSKDNIKHVPGGGSVQIVYKPVDLSKVT SKCGSLGNIHHKPGGGQVEVKS E
KLDFKDRVQSKIGSLDNITHVPGGGNKKIETHKLT FRENAKAKTDHGAEIVYKS PVV
SGDTS PRHL SNVS SIGS I DMVDS PQLATLADEVSASLAKQGL (SE() ID NO:6)
Intracellular tau levels are increased in the brains of Alzheimer's disease
patients
when compared to non-demented controls (Barton, Am I Pathol., 137:497-502
(1990);
Khatoon, I Neurochem., 59:750-753 (1992)). This increase in the levels of
intracellular tau is
believed to be toxic to neurons since a reduction in the amount of
intracellular tau has been
shown to be protective in mouse models of neurodegeneration (Rapoport et al.,
Proc. Natl.
Acad. Sci. USA, 99:6364-6369 (2002); Robertson et al., Science, 316:750-754
(2007)), and
thus reducing the amount of intracellular tau can be therapeutically
beneficial.
Secreted N-terminally truncated tau species, designated extracellular Tau
(eTau), have
been identified. "eTau" as used herein, encompasses any Tau polypeptide that
can be
detected in cerebrospinal fluid (CSF) or interstitial fluid (ISF). In some
embodiments, eTau is
a polypeptide having a sequence set forth in one of SEQ ID NOS.: 7-10 of
Figure 1. The
eTau species varies from 171 amino acids for eTaul to 67 amino acids for
eTau4. Although
tau lacks a signal sequence, tau has been found released into culture medium
from
neuroblastoma cells, tau-expressing non-neuronal cells, induced pluripotent
stem cell-derived
human neurons, and mouse primary neurons. Thus, tau may be secreted
unconventionally or
associated with exosomes or other membrane vesicles. eTau has also been
detected in the
brain ISF of both wild type and P301S tau-expressing mice in microdialysis
studies. It has
also been seen in the brain ISF of patients following traumatic brain injury.
eTau has been
shown to regulate AP production and to increase neuronal hyperactivity (Bright
et al.,
Neurobiology and Aging, 36:693-709 (2015)). Treatment with an eTau-
neutralizing antibody
reduces eTau-mediated neuronal hyperactivity. See, e.g., WO 2014/02877. It has
been
proposed that the eTau-driven neuronal hyperactivity increase leads not only
to increased AP
secretion but also to a further increase in eTau secretion and thus, eTau and
AP create a feed
forward disease mechanism that perpetuates the disease. Thus, neutralizing
eTau can inhibit
the spread of tau and tau pathology in the brain, reduce central nervous
system AP levels and
9
CA 03064550 2019-11-21
WO 2018/231254
PCT/US2017/037991
the resulting neuronal hyperactivity, and potentially slow the clinical
progression into
dementia.
Anti-Human Tau Antibodies
One way of neutralizing tau is by using antibodies that bind tau. In certain
embodiments, these antibodies bind to an epitope within amino acids 1-25, 1-
18, 9-18, 13-24,
15-44, or 15-24 of SEQ ID NO:6. In a specific embodiment, an anti-tau antibody
binds to an
epitope within AGTYGLGDRK (SEQ ID NO:!!).
An exemplary anti-human tau antibody is designated "BIIB092." BIIB092 is a
humanized IgG4/kappa antibody that recognizes human tau. The heavy chain
variable
domain of the BIIB092 antibody has the following amino acid sequence:
EVHLVESGGA LVKPGGSLRL SCAASGFSFS KYGMSWVRQA PGKGLEWVAT
ISSSGSRTYY
PDSVKGRFTI SRDNAKNTLY LQMNSLRAED TAMYYCSISW DGAMDYWGQG TTVTVSS
(SEQ ID NO:12)
The light chain variable domain of the BIIB092 antibody has the following
amino acid
sequence:
DVVMTQSPLS LPVTLGQPAS ISCKSSQSIV HSNGNTYLEW YLQKPGQSPQ
LLVYKVSNRF
SGVPDRFSGS GSGTDFTLKI SRVEAEDVGT YYCFQGSLVP WAFGGGTKVE IK
(SEQ ID NO:13)
The heavy chain of the BIIB092 antibody has the following amino acid sequence:
EVHLVESGGA LVKPGGSLRL SCAASGFSFS KYGMSWVRQA PGKGLEWVAT
ISSSGSRTYY
PDSVKGRFTI SRDNAKNTLY LQMNSLRAED TAMYYCSISW DGAMDYWGQG
TTVTVSSAST
KGPSVFPLAP CSRSTSESTA ALGCLVKDYF PEPVTVSWNS GALTSGVHTF
PAVLQSSGLY
SLSSVVTVPS SSLGTKTYTC NVDHKPSNTK VDKRVESKYG PPCPPCPAPE
FLGGPSVFLF
PPKPKDTLMI SRTPEVTCVV VDVSQEDPEV QFNWYVDGVE VHNAKTKPRE
EQFNSTYRVV
SVLTVLHQDW LNGKEYKCKV SNKGLPSSIE KTISKAKGQP REPQVYTLPP
SQEEMTKNQV
SLTCLVKGFY PSDIAVEWES NGQPENNYKT TPPVLDSDGS FFLYSRLTVD
KSRWQEGNVF
SCSVMHEALH NHYTQKSLSL SLGK (SEQ ID NO:14)
The light chain of the BIIB092 antibody has the following amino acid sequence:
DVVMTQSPLS LPVTLGQPAS ISCKSSQSIV HSNGNTYLEW YLQKPGQSPQ
LLVYKVSNRF
SGVPDRFSGS GSGTDFTLKI SRVEAEDVGT YYCFQGSLVP WAFGGGTKVE
IKRTVAAPSV
CA 03064550 2019-11-21
WO 2018/231254
PCT/US2017/037991
FIFPPSDEQL KSGTASVVCL LNNFYPREAK VQWKVDNALQ SGNSQESVTE
QDSKDSTYSL
SSTLTLSKAD YEKHKVYACE VTHQGLSSPV TKSFNRGEC (SEQ ID NO:15)
In some embodiments, the anti-human tau antibody or tau-binding fragment
thereof
comprises the three light chain variable domain CDRs of BIIB092. In some
embodiments,
the anti-human tau antibody or tau-binding fragment thereof comprises the
three heavy chain
variable domain CDRs of BIIB092. In still other embodiments, the anti-human
tau antibody
or tau-binding fragment thereof comprises the three heavy chain variable
domain CDRs and
the three light chain variable domain CDRs of BIIB092. The CDRs can be based
on any CDR
definition in the art, e.g., the definitions of Kabat, Chothia, Chothia from
Abysis, enhanced
Chothia/AbM, or based on the contact definition. CDR sequences of BIIB092 are
provided in
Table 1 below.
Table 1: Sequences of the CDRs of BIIB092
Domain Amino Acid Sequence
VH CDR1 KYGMS (SEQ ID NO:16)
VH CDR2 TISSSGSRTYYPDSVKG (SEQ ID NO:17)
VH CDR3 SWDGAMDY (SEQ ID NO:18)
VL CDR1 KSSQSIVHSNGNTYLE (SEQ ID NO:19)
VL CDR2 KVSNRFS (SEQ ID NO:20)
VL CDR3 FQGSLVPWA (SEQ ID NO:21)
In some embodiments, the anti-human tau antibody or tau-binding fragment
thereof
comprises a VH CDR1 comprising or consisting of the amino acid sequence set
forth in SEQ
ID NO:16, a VH CDR2 comprising or consisting of the amino acid sequence set
forth in SEQ
ID NO:17; and a VH CDR3 comprising or consisting of the amino acid sequence
set forth in
SEQ ID NO:18; a VL CDR1 comprising or consisting of the amino acid sequence
set forth in
SEQ ID NO:19, a VL CDR2 comprising or consisting of the amino acid sequence
set forth in
SEQ ID NO:20; and a VL CDR3 comprising or consisting of the amino acid
sequence set
forth in SEQ ID NO:21.
In some embodiments, the anti-human tau antibody or tau-binding fragment
thereof
comprises a VH comprising or consisting of the amino acid sequence set forth
in SEQ ID
NO:12. In some embodiments, the anti-human tau antibody or tau-binding
fragment thereof
comprises a VL comprising or consisting of the amino acid sequence set forth
in SEQ ID
NO:13. In some embodiments, the anti-human tau antibody or tau-binding
fragment thereof
comprises a VH comprising or consisting of the amino acid sequence set forth
in SEQ ID
NO:12 and a VL comprising or consisting of the amino acid sequence set forth
in SEQ ID
NO:13.
11
CA 03064550 2019-11-21
WO 2018/231254
PCT/US2017/037991
In some embodiments, the anti-human tau antibody or tau-binding fragment
thereof
comprises a heavy chain comprising or consisting of the amino acid sequence
set forth in
SEQ ID NO:14. In some embodiments, the anti-human tau antibody or tau-binding
fragment
thereof comprises a light chain comprising or consisting of the amino acid
sequence set forth
in SEQ ID NO:15. In some embodiments, the anti-human tau antibody or tau-
binding
fragment thereof comprises a heavy chain comprising or consisting of the amino
acid
sequence set forth in SEQ ID NO:14 and a light chain comprising or consisting
of the amino
acid sequence set forth in SEQ ID NO:15.
In certain embodiments, the anti-human tau antibody is an IgG antibody. In
specific
embodiments, the anti-human tau antibody has heavy chain constant region
chosen from, e.g.,
IgGl, IgG2, IgG3, IgG4, IgM, IgAl, IgA2, IgD, and IgE. In one embodiment, the
anti-
human tau antibody is of the IgG4 isotype. In another embodiment, the anti-
human tau
antibody is of the IgG1 isotype. In yet another embodiment, the anti-human tau
antibody is
of the IgG2 isotype. In another embodiment, the anti-human tau antibody is of
the IgG3
isotype. In further embodiments, the anti-human tau antibody has a light chain
constant
region chosen from, e.g., a human kappa light chain constant region or a human
lambda light
chain constant region. In a certain embodiment, the anti-human tau antibody is
an
IgG4/human kappa light chain antibody.
In some embodiments, the anti-human tau antibody is a full-length (whole)
antibody
or substantially full-length. The protein can include at least one, and
preferably two, complete
heavy chains, and at least one, and preferably two, complete light chains. In
some
embodiments, the anti-human tau antibody is a tau-binding fragment. In some
instances, the
tau-binding antibody fragment is a Fab, a Fab', an F(ab')2, a Facb, an Fv, a
single chain Fv
(scFv), a sc(Fv)2, or a diabody.
The heavy chain and light chain of the anti-human tau antibodies disclosed
herein
may also include signal sequences. The signal sequences can be selected from
those known
in the art, for example, MDMRVPAQLLGLLLLWFPGSRC (SEQ ID NO:22) or
MDMRVPAQLLGLLLLWLPGARC (SEQ ID NO:23).
Antibodies, such as BIIB092, or tau-binding fragments thereof can be made, for
example, by preparing and expressing synthetic genes that encode the recited
amino acid
sequences or by mutating human germline genes to provide a gene that encodes
the recited
amino acid sequences. Moreover, this antibody and other anti-human tau
antibodies can be
produced, e.g., using one or more of the following methods.
12
CA 03064550 2019-11-21
WO 2018/231254
PCT/US2017/037991
Methods of Producing Anti-Human tau Antibodies
Anti-human tau antibodies or tau-binding fragments may be produced in
bacterial or
eukaryotic cells. Some antibodies, e.g., Fab's, can be produced in bacterial
cells, e.g., E. coil
cells. Antibodies can also be produced in eukaryotic cells such as transformed
cell lines (e.g.,
CHO, 293E, COS). In addition, antibodies (e.g., scFv's) can be expressed in a
yeast cell such
as Pichia (see, e.g., Powers et al., J Immunol Methods. 251:123-35 (2001)),
Hanseula, or
Saccharomyces. To produce the antibody of interest, a polynucleotide or
polynucleotides
encoding the antibody is/are constructed, introduced into an expression vector
or expression
vectors, and then expressed in suitable host cells. To improve expression, the
nucleotide
sequences of the light and heavy chain genes can be recoded without changing
(or minimally
changing ¨ e.g., removal of a C-terminal residue of the heavy or light chain)
the amino acid
sequence. The areas for potential recoding include those associated with
translation initiation,
codon usage, and possible unintended mRNA splicing. Polynucleotides encoding
an anti-
human tau antibody comprising the VH and/or VL, HC and/or LC of the tau
antibodies
described herein would be readily envisioned by the ordinarily skilled
artisan.
Standard molecular biology techniques are used to prepare the recombinant
expression vector(s), transfect the host cells, select for transformants,
culture the host cells,
and recover the anti-human tau antibody.
If the anti-human tau antibodies or tau-binding fragments are to be expressed
in
bacterial cells (e.g., E. coil), the expression vector should have
characteristics that permit
amplification of the vector in the bacterial cells. Additionally, when E. coil
such as JM109,
DH5a, HB101, or XL1-Blue is used as a host, the vector must have a promoter,
for example,
a lacZ promoter (Ward et al., 341:544-546 (1989), araB promoter (Better et
al., Science,
240:1041-1043 (1988)), or T7 promoter that can allow efficient expression in
E. coil.
Examples of such vectors include, for example, M13-series vectors, pUC-series
vectors,
pBR322, pBluescript, pCR-Script, pGEX-5X-1 (Pharmacia), "QIAexpress system"
(QIAGEN), pEGFP, and pET (when this expression vector is used, the host is
preferably
BL21 expressing T7 RNA polymerase). The expression vector may contain a signal
sequence for antibody secretion. For production into the periplasm of E. coil,
the pelB signal
sequence (Lei et al., I Bacteriol., 169:4379 (1987)) may be used as the signal
sequence for
antibody secretion. For bacterial expression, calcium chloride methods or
electroporation
methods may be used to introduce the expression vector into the bacterial
cell.
If the antibody is to be expressed in animal cells such as CHO, COS, and
NIH3T3
cells, the expression vector includes a promoter necessary for expression in
these cells, for
13
CA 03064550 2019-11-21
WO 2018/231254
PCT/US2017/037991
example, an SV40 promoter (Mulligan etal., Nature, 277:108 (1979)) (e.g.,
early simian
virus 40 promoter), MMLV-LTR promoter, EFla promoter (Mizushima et al.,
Nucleic Acids
Res., 18:5322 (1990)), or CMV promoter (e.g., human cytomegalovirus immediate
early
promoter). In addition to the nucleic acid sequence encoding the
immunoglobulin or domain
thereof, the recombinant expression vectors may carry additional sequences,
such as
sequences that regulate replication of the vector in host cells (e.g., origins
of replication) and
selectable marker genes. The selectable marker gene facilitates selection of
host cells into
which the vector has been introduced (see e.g., U.S. Pat. Nos. 4,399,216,
4,634,665 and
5,179,017). For example, typically the selectable marker gene confers
resistance to drugs,
such as G418, hygromycin, or methotrexate, on a host cell into which the
vector has been
introduced. Examples of vectors with selectable markers include pMAM, pDR2,
pBK-RSV,
pBK-CMV, pOPRSV, and p0P13.
In one embodiment, the anti-human tau antibodies are produced in mammalian
cells.
Exemplary mammalian host cells for expressing an antibody include Chinese
Hamster Ovary
(CHO cells) (including dhfr- CHO cells, described in Urlaub and Chasin (1980)
Proc. Natl.
Acad. Sci. USA 77:4216-4220, used with a DHFR selectable marker, e.g., as
described in
Kaufman and Sharp (1982) Mol. Biol. 159:601-621), human embryonic kidney 293
cells
(e.g., 293, 293E, 293T), COS cells, NIH3T3 cells, lymphocytic cell lines,
e.g., NSO myeloma
cells and SP2 cells, and a cell from a transgenic animal, e.g., a transgenic
mammal. For
example, the cell is a mammary epithelial cell. In a specific embodiment, the
mammalian
cell is a CHO-DG44I cell.
In an exemplary system for antibody expression, a recombinant expression
vector
encoding both the anti-human tau antibody heavy chain and the anti-human tau
antibody light
chain of an anti-human tau antibody (e.g., BIIB092) is introduced into dhfr-
CHO cells by
calcium phosphate-mediated transfection. Within the recombinant expression
vector, the
antibody heavy and light chain genes are each operatively linked to
enhancer/promoter
regulatory elements (e.g., derived from 5V40, CMV, adenovirus and the like,
such as a CMV
enhancer/AdMLP promoter regulatory element or an 5V40 enhancer/AdMLP promoter
regulatory element) to drive high levels of transcription of the genes. The
recombinant
expression vector also carries a DHFR gene, which allows for selection of CHO
cells that
have been transfected with the vector using methotrexate
selection/amplification. The
selected transformant host cells are cultured to allow for expression of the
antibody heavy
and light chains and the antibody is recovered from the culture medium.
14
CA 03064550 2019-11-21
WO 2018/231254
PCT/US2017/037991
Antibodies can also be produced by a transgenic animal. For example, U.S. Pat.
No.
5,849,992 describes a method of expressing an antibody in the mammary gland of
a
transgenic mammal. A transgene is constructed that includes a milk-specific
promoter and
nucleic acids encoding the antibody of interest and a signal sequence for
secretion. The milk
produced by females of such transgenic mammals includes, secreted-therein, the
antibody of
interest. The antibody can be purified from the milk, or for some
applications, used directly.
Animals are also provided comprising one or more of the nucleic acids
described herein.
The antibodies of the present disclosure can be isolated from inside or
outside (such
as medium) of the host cell and purified as substantially pure and homogenous
antibodies.
Methods for isolation and purification commonly used for antibody purification
may be used
for the isolation and purification of antibodies, and are not limited to any
particular method.
Antibodies may be isolated and purified by appropriately selecting and
combining, for
example, column chromatography, filtration, ultrafiltration, salting out,
solvent precipitation,
solvent extraction, distillation, immunoprecipitation, SDS-polyacrylamide gel
electrophoresis, isoelectric focusing, dialysis, and recrystallization.
Chromatography
includes, for example, affinity chromatography, ion exchange chromatography,
hydrophobic
chromatography, gel filtration, reverse-phase chromatography, and adsorption
chromatography (Strategies for Protein Purification and Characterization: A
Laboratory
Course Manual. Ed Daniel R. Marshak et al., Cold Spring Harbor Laboratory
Press, 1996).
Chromatography can be carried out using liquid phase chromatography such as
HPLC and
FPLC. Columns used for affinity chromatography include protein A column and
protein G
column. Examples of columns using protein A column include Hyper D, POROS, and
Sepharose FF (GE Healthcare Biosciences). The present disclosure also includes
antibodies
that are highly purified using these purification methods.
Anti-Human Tau Antibody Formulations
Any of the anti-human tau antibodies described herein can be formulated as a
pharmaceutical composition. The pharmaceutical composition can comprise 10
mg/mL, 60
mg/mL or 50 mg/mL of an anti-human tau antibody described herein. In a
particular
embodiment, the pharmaceutical composition comprises 50 mg/mL of an anti-human
tau
antibody described herein. In addition, the pharmaceutical composition
includes histidine at
a concentration of 20 mM, sucrose at a concentration of 250 mM, and
polysorbate-80 at a
concentration of 0.05% (w/v). In certain cases, the pharmaceutical composition
further
CA 03064550 2019-11-21
WO 2018/231254
PCT/US2017/037991
includes 50 [tM diethylenetriamine pentaacetic acid (DTPA). The pharmaceutical
composition has a pH of 6Ø
In some instances, the anti-human tau antibody of the pharmaceutical
composition
comprises an immunoglobulin heavy chain variable region (VH) comprising VH
complementarity determining regions (VH-CDRs) and an immunoglobulin light
chain
variable region (VL) comprising VL-CDRs. The VH-CDR1 comprises or consists of
the
amino acid sequence of SEQ ID NO:16; VH-CDR2 comprises or consists of the
amino acid
sequence of SEQ ID NO:17; and VH-CDR3 comprises or consists of the amino acid
sequence of SEQ ID NO:18. The VL-CDR1 comprises or consists of the amino acid
sequence of SEQ ID NO:19; VL-CDR2 comprises or consists of the amino acid
sequence of
SEQ ID NO:20; and VL-CDR3 comprises or consists of the amino acid sequence of
SEQ ID
NO:21.
In certain cases, the anti-human tau antibody of the pharmaceutical
composition
comprises a VH comprising or consisting of SEQ ID NO:12. In certain cases, the
anti-human
tau antibody of the pharmaceutical composition comprises a VL comprising or
consisting of
SEQ ID NO:13. In certain cases, the anti-human tau antibody of the
pharmaceutical
composition comprises a VH comprising or consisting of SEQ ID NO:12 and a VL
comprising or consisting of SEQ ID NO:13.
In certain cases, the anti-human tau antibody of the pharmaceutical
composition
comprises a heavy chain comprising or consisting of SEQ ID NO:14. In certain
cases, the
anti-human tau antibody of the pharmaceutical composition comprises a light
chain
comprising or consisting of SEQ ID NO:15. In certain cases, the anti-human tau
antibody of
the pharmaceutical composition comprises a heavy chain comprising or
consisting of SEQ ID
NO:14 and alight chain comprising or consisting of SEQ ID NO:15.
Indications
The anti-human tau antibodies described herein are expected to be useful in
the
treatment of tauopathies. Tauopathies are a class of neurodegenerative
diseases associated
with the pathological aggregation of tau protein in neurofibrillary or
gliofibrillary tangles in
the human brain.
Exemplary tauopathies include progressive supranuclear palsy, Alzheimer's
disease,
amyotrophic lateral sclerosis/parkinsonism- dementia complex, argyrophilic
grain dementia,
British type amyloid angiopathy, cerebral amyloid angiopathy, corticobasal
degeneration,
Creutzfeldt- Jakob disease, dementia pugilistica, diffuse neurofibrillary
tangles with
16
CA 03064550 2019-11-21
WO 2018/231254
PCT/US2017/037991
calcification, Down's syndrome, frontotemporal dementia (FTD), frontotemporal
dementia
with parkinsonism linked to chromosome 17, frontotemporal lobar degeneration,
Gerstmann-
Straussler-Scheinker disease, Hallervorden-Spatz disease, inclusion body
myositis, multiple
system atrophy, myotonic dystrophy, Niemann-Pick disease type C, non-Guamanian
motor
neuron disease with neurofibrillary tangles, Pick's disease, postencephalitic
parkinsonism,
prion protein cerebral amyloid angiopathy, progressive subcortical gliosis,
globular glial
tauopathy, subacute sclerosing panencephalitis, Tangle only dementia, multi-
infarct
dementia, stroke, chronic traumatic encephalopathy, traumatic brain injury,
concussion,
seizures, epilepsy, and acute lead encephalopathy.
In one embodiment, the anti-human tau antibodies described herein are used to
treat
progressive supranuclear palsy.
In another embodiment, the anti-human tau antibodies described herein are used
to
treat Alzheimer's disease.
Methods of Treatment
The disclosure features methods of treating human subjects with a tauopathy
(see
above) with an anti-human tau antibody disclosed herein or a pharmaceutical
composition
disclosed herein. In one embodiment, the tauopathy is progressive supranuclear
palsy. In
another embodiment, the tauopathy is Alzheimer's disease.
In certain embodiments, the method comprises administering to the human
subject in
need thereof an anti-human tau antibody in an amount effective to reduce
significantly the
level of tau (e.g., total Tau and/or free Tau) in an extracellular fluid
(e.g., cerebrospinal fluid
(C SF), interstitial fluid (ISF), blood, or a blood fraction (e.g., serum or
plasma)) in the
individual. "Free Tau" refers to a tau polypeptide that is not bound to an
anti-human tau
antibody. In one embodiment, the free Tau is extracellular Tau (eTau). "Total
Tau" includes
free Tau and Tau that is bound to an anti-human tau antibody. In one
particular embodiment,
the method comprises administering to the human subject in need thereof an
anti-human tau
antibody in an amount effective to reduce significantly the level of free
eTau. In some
embodiments, the level of tau (e.g., total Tau and/or free Tau) is
significantly reduced within
36 hours of administration of the anti-human tau antibody. In some
embodiments, the level of
tau (e.g., total Tau and/or free Tau) is significantly reduced within 24 hours
of administration
of the anti-human tau antibody. In some embodiments, the level of free eTau is
significantly
reduced within 24 hours of administration of the anti-human tau antibody. In
some cases, an
effective amount of an anti-human tau antibody is an amount that is effective
to reduce
17
CA 03064550 2019-11-21
WO 2018/231254
PCT/US2017/037991
significantly the level of tau (e.g., total Tau and/or free Tau) in an
extracellular fluid within
48 hours, 36 hours, within 24 hours, within 12 hours, within 8 hours, within 4
hours, within 2
hours, within 1 hour, within 30 minutes, within 15 minutes, or within 5
minutes, of
administration of the anti-human tau antibody. For example, in some cases, an
effective
amount of an anti-human tau antibody is an amount that is effective to reduce
significantly
the level of Tau (e.g., total Tau and/or free Tau) in an extracellular fluid
within from 5
minutes to about 10 minutes, from about 10 minutes to about 15 minutes, from
about 15
minutes to about 30 minutes, from about 30 minutes to about 1 hour, from about
1 hour to
about 2 hours, from about 2 hours to about 4 hours, from about 4 hours to
about 8 hours, from
about 8 hours to about 12 hours, from about 12 hours to about 24 hours, from
about 24 hours
to about 36 hours, from about 24 to about 48 hours, or from about 36 hours to
about 48 hours.
A significant reduction in the level of tau (e.g., total Tau and/or free Tau)
in an
extracellular fluid (e.g., CSF, ISF, blood, or a blood fraction (e.g., serum
or plasma)) of an
individual is an at least 30% reduction, at least 35% reduction, at least 40%
reduction, at least
45% reduction, at least 50% reduction, an at least 55% reduction, an at least
60% reduction,
an at least 65% reduction, an at least 70% reduction, an at least 75%
reduction, an at least
80% reduction, an at least 85% reduction, an at least 90% reduction, an at
least 95%
reduction, or a greater than 90% reduction. In some instances, the significant
reduction is a
statistically significant reduction. In some instances, the significant
reduction is a clinically
significant reduction. In some embodiments, the level of tau (e.g., total Tau
and/or free Tau)
in an extracellular fluid is reduced to a normal, control level (e.g., about
100 pg/ml). In some
embodiments, the level of Tau (e.g., total Tau and/or free Tau) in an
extracellular fluid is
reduced to an undetectable level. In some cases, the extracellular fluid is
CSF. In other cases,
the extracellular fluid is ISF. In other cases, the extracellular fluid is
plasma. In other cases,
the extracellular fluid is whole blood. In other cases, the extracellular
fluid is serum.
In certain instances, an anti-human tau antibody described herein is
administered to
the human subject at a fixed dose of 2000 mg. In certain instances, an anti-
human tau
antibody is administered to the human subject at a fixed dose of 2100 mg. In
other instances,
an anti-human tau antibody is administered to the human subject at a fixed
dose of 150 mg. In
further instances, an anti-human tau antibody is administered to the human
subject at a fixed
dose of 4200 mg. In certain embodiments, the above-noted fixed doses of an
anti-human tau
antibody described herein are administered to the human subject once every
four weeks.
In certain cases, an anti-human tau antibody described herein is administered
to the
human subject as part of a pharmaceutical composition. In one embodiment, the
18
CA 03064550 2019-11-21
WO 2018/231254
PCT/US2017/037991
pharmaceutical composition comprises 50 mg/mL of the anti-human tau antibody,
20 mM
histidine, 250 mM sucrose, and 50 p,M DTPA. The pharmaceutical composition has
a pH of
6Ø In certain embodiments, the pharmaceutical composition is administered to
the human
subject in an amount sufficient to deliver a fixed dose of 2000 mg of the anti-
human tau
antibody. In certain embodiments, the pharmaceutical composition is
administered to the
human subject in an amount sufficient to deliver a fixed dose of 2100 mg of
the anti-human
tau antibody. In certain embodiments, the pharmaceutical composition is
administered to the
human subject in an amount sufficient to deliver a fixed dose of 700 mg of the
anti-human
tau antibody. In certain embodiments, the pharmaceutical composition is
administered to the
human subject in an amount sufficient to deliver a fixed dose of 150 mg of the
anti-human
tau antibody. In certain embodiments, the pharmaceutical composition is
administered to the
human subject in an amount sufficient to deliver a fixed dose of 210 mg of the
anti-human
tau antibody. In certain embodiments, the pharmaceutical composition is
administered to the
human subject in an amount sufficient to deliver a fixed dose of 4200 mg of
the anti-human
tau antibody.
In certain embodiments of these methods, the anti-human tau antibody is
administered
to the human subject in need thereof by an intravenous route.
In certain embodiments, the anti-human tau antibody comprises an
immunoglobulin
heavy chain variable region (VH) and an immunoglobulin light chain variable
region (VL),
wherein the VH comprises VH complementarity determining regions (VH-CDRs),
wherein
VH-CDR1 comprises or consists of the amino acid sequence of SEQ ID NO:16; VH-
CDR2
comprises or consists of the amino acid sequence of SEQ ID NO:17; and VH-CDR3
comprises or consists of the amino acid sequence of SEQ ID NO:18; and the VL
comprises
VL-CDRs, wherein VL-CDR1 comprises or consists of the amino acid sequence of
SEQ ID
NO:19; VL-CDR2 comprises or consists of the amino acid sequence of SEQ ID
NO:20; and
VL-CDR3 comprises or consists of the amino acid sequence of SEQ ID NO:21.
In certain embodiments, the VH of the anti-human tau antibody comprises or
consists
of SEQ ID NO:12 and the VL comprises or consists of SEQ ID NO:13.
In certain embodiments, the anti-human tau antibody comprises a heavy chain
and a
light chain, wherein the heavy chain comprises or consists of SEQ ID NO:14 and
the light
chain comprises or consists of SEQ ID NO:15.
The following example is not to be construed as limiting the scope of the
invention in
any way.
19
CA 03064550 2019-11-21
WO 2018/231254
PCT/US2017/037991
Examples
Example 1: A Single Ascending Dose Study of an Anti-Human Tau Antibody,
BIIB092
BIIB092 is a humanized antibody that recognizes human extracellular Tau
(eTau).
The purposes of this study was to evaluate the safety, tolerability, and
pharmacokinetics (PK)
of BIIB092 as well as the pharmacodynamic (PD) effects of BIIB092 on
extracellular tau
(eTau) after a single intravenous (IV) infusion of BIIB092 in healthy human
subjects.
Specifically, BIIB092 was tested to determine its efficacy in preventing
transmission of tau
pathology by binding and reducing free eTau in human CSF.
This study was a randomized, double blind, placebo controlled single ascending
dose
trial. Healthy subjects (age: 21-65) in 6 ascending dose cohorts (21mg, 70mg,
210mg,
700mg, 2100mg and 4200mg of BIIB092) comprised of 8 subjects per cohort were
administered a single intravenous (IV) infusion of BIIB092 (6 subjects) or
placebo (2
subjects). See, FIG. 2. Safety assessments, and serum and CSF samples
(including 4 lumbar
punctures) were collected over 12 weeks. Pharmacokinetic parameters (in serum
and CSF)
and pharmacodynamic measures (CSF concentrations of free eTau) and
corresponding
change and percent change from baseline were evaluated.
Increases in peak (Cmax) and exposure (AUC[INF1) of BIIB092 in serum appeared
to
be dose proportional. The terminal elimination half-life of BIIB092 was
approximately 25
days. CSF concentrations of BIIB092 increased with dose and appeared dose-
proportional.
CSF-to-serum ratio of BIIB092 was approximately 0.2% and similar across the
dose range.
Most adverse events were mild. There were no serious adverse events or
discontinuations due
to adverse events. The extent and duration of suppression of eTau increased
with dose.
Following single doses of BIIB092, suppression of CSF eTau at 28 days ranged
from 65% to
96% at doses ranging from 70mg to 4200mg.
The ability of BIIB092 to robustly suppress CSF concentrations of free eTau in
this
phase 1 study suggests that BIIB092 has utility for the treatment of human
tauopathies (e.g.,
progressive supranuclear palsy). Single doses of BIIB092 administration were
safe and well
tolerated at doses up to 4200mg.
CA 03064550 2019-11-21
WO 2018/231254
PCT/US2017/037991
Example 2: Bayesian Emax Model
An exposure-response model (Bayesian Emax) of CSF concentration versus eTau
suppression was constructed (see, FIG. 3). The Bayesian Emax model captured
the observed
eTau suppression reasonably well.
Example 3: Multiple Ascending Dose Study of an Anti-Human Tau Antibody,
BIIB092, in
Patients with Progressive Supranuclear Palsy
The purpose of this study was to assess the safety, tolerability,
pharmacokinetic (PK)
and pharmacodynamic (PD) effects of BIIB092 on free extracellular tau (eTau)
after
intravenous (IV) infusions of BIIB092 every 4 weeks (Q4W) in patients with
progressive
supranuclear palsy (PSP). Specifically, this study was designed to evaluate
the safety profile
of BIIB092 and its ability to reduce free eTau in the CSF of patients with
PSP.
The baseline demographic characteristics for this study are shown in FIG. 4.
This
study was a randomized, double-blind, placebo-controlled, multiple ascending
dose trial of 48
patients with PSP, of whom 12 (25%) received placebo. Three ascending dose
panels (150
mg, 700 mg, and 2100 mg) comprised of 8 patients per panel, were administered
IV infusions
of BIIB092 (6 patients) or placebo (2 patients) Q4W for 12 weeks; an
additional 24 patients
were treated with BIIB092 at a dose of 2100 mg (18 patients) or placebo (6
patients)
administered Q4W for 12 weeks. See, FIG. 5. All patients were also offered the
opportunity
to participate in an open-label extension study. Safety assessments and serum
and CSF
samples were collected over the 12 weeks. PK parameters (in serum and CSF), PD
measures
(concentrations of CSF free eTau), and corresponding change and percent change
from
baseline were evaluated. Clinical outcome measures including the PSP Rating
Scale and
Schwab and England Activities of Daily Living Scale were also employed.
Patients' mean age was 67.4 5.5 years; 54.2% were female. Concentrations of
BIIB092 in serum and CSF increased with dose. The percentages of patients
experiencing
adverse events (AEs) were similar in the BIIB092 and placebo groups (-75%).
Most AEs
were mild. See, FIG. 6. There were no deaths or discontinuations due to AEs. A
summary of
the serum PK parameters for BIIB092 is provided in FIG. 7. Mean suppression of
CSF free
eTau was approximately 90-96% (Day 29) and 91-97% (Day 85) at doses ranging
from 150
mg to 2100 mg. Although CSF and serum exposures and reductions of CSF free
eTau
increased with BIIB092 dosage, significant reductions of CSF free eTau were
observed with
all dosages employed in the study. See, FIGs. 8 and 9.
21
CA 03064550 2019-11-21
WO 2018/231254 PCT/US2017/037991
Administration of multiple doses of BIIB092 was safe and well tolerated at
doses up
to 2100 mg in patients with PSP.
Example 4: BIIB092 Dose Selection Based on Simulated PK and eTau Suppression
Using the estimated PK-PD model parameters and the variability around these
parameters in PSP patients, 1000 profiles were simulated and the time course
of PK in serum
and CSF and PD (eTau) in CSF were obtained for two doses, namely 2000 mg and
700 mg of
BIIB092 administered once every 4 weeks (Q4W). eTau concentrations were
converted to
percent suppression, relative to each subject's baseline value, prior to
summarization based
on the simulations. Table 1 below shows summary statistics of percent
suppression of eTau
relative to baseline levels at 4 weeks, 12 weeks, 24 weeks, and 48 weeks
following the 2000
mg Q4W dosing regimen.
Table 1: Summary Statistics of Percent Suppression of eTau Relative to
Baseline Following
2000 mg Q4W (Simulated)
Statistic 4 Weeks 12 Weeks 24 Weeks 48 Weeks
1000 1000 1000 1000
Median 96.46 97.46 97.57 97.58
Minimum 93.87 94.80 94.82 94.82
Maximum 98.25 98.92 99.03 99.04
10th percentile 95.35 96.34 96.43 96.43
90th percentile 97.37 98.25 98.39 98.40
Table 2 shows summary statistics of percent suppression of eTau relative to
baseline
levels at 4 weeks, 12 weeks, 24 weeks, and 48 weeks following the 700 mg Q4W
dosing
regimen.
Table 2: Summary Statistics of Percent Suppression of eTau Relative to
Baseline
Following 700 mg Q4W (Simulated)
Statistic 4 Weeks 12 Weeks 24 Weeks 48 Weeks
1000 1000 1000 1000
Median 93.28 94.75 94.94 94.95
Minimum 88.75 89.97 90.04 90.04
Maximum 96.31 97.68 97.96 98.00
10th percentile 91.85 93.15 93.27 93.28
22
CA 03064550 2019-11-21
WO 2018/231254 PCT/US2017/037991
90th percentile 94.58 96.02 96.28 96.30
Both 2000 mg and 700 mg doses of BIIB092, administered Q4W lead to robust
suppression of eTau at trough. The 2000 mg dose of BIIB092 is associated with
slightly
higher suppression (3 to 5%) at trough than the 700 mg dose. Ninety percent of
all subjects
that are dosed with the 2000 mg Q4W dose are expected to have a percentage
suppression of
eTau at or above 96% at trough. Ninety percent of all subjects that are dosed
the 700 mg
Q4W dose are expected have a percentage suppression of eTau at or above 93% at
trough.
Given the robust and persistent suppression of eTau up to 12 weeks postdose at
doses
at and above 210 mg that were studied, and the -2x higher CSF concentrations
of BIIB092
observed in PSP patients compared to healthy subjects, dosing BIIB092 can also
be done on a
less frequent basis, e.g., once every 12 weeks (Q12W). Simulations were
performed using the
same PK-PD model for a 2000 mg dose, administered Q12W. eTau suppression is
summarized in Table 3.
Table 3: Summary Statistics of Percent Suppression of eTau Relative to
Baseline Following
2000 mg Q12W (Simulated)
Statistic 4 Weeks 12 Weeks 24 Weeks 28 Weeks 48
Weeks
(trough)
(trough) (trough)
1000 1000 1000 1000 1000
Median 96.47 90.08 90.35 96.64 90.36
Minimum 93.46 74.05 74.13 93.54 74.13
Maximum 98.22 95.54 96.05 98.37 96.15
10th percentile 95.35 86.01 86.20 95.46 86.21
90th percentile 97.37 92.79 93.11 97.54 93.15
Ninety percent of all subjects that are administered the 2000 mg Q12W dose are
expected
have a
percentage suppression of eTau at or above 86% at trough (i.e., end of the 12-
week dosing
interval). However, subjects are expected to be at or above 95% suppression
during the one
month immediate to the infusion, with slightly attenuated suppression in the
ensuing 2
months.
Overall, Q12W dosing is also expected to be associated with robust and
persistent lowering
of
23
CA 03064550 2019-11-21
WO 2018/231254 PCT/US2017/037991
eTau and may be preferred by patients and caregivers.
Example 5: BIIB092 Formulation Optimization for Excipient Content
In this excipient optimization stability, BIIB092 was evaluated at 10 mg/mL in
20
mM
histidine buffer at pH 5.5, 6.0, and 6.5, containing 0.05% PS-80 and 50 pM
DTPA, plus
either 250 mM sucrose or 250 mM sorbitol. In addition, BIIB092 was evaluated
at 20 mg/mL
and 50 mg/mL in 20 mM histidine at pH 6.0 containing 0.05% PS-80, 50 pM DTPA,
and
250 mM sucrose. A list of the formulations is shown in Table 4.
Table 4. Summary of Formulations for BIIB092 Excipient Optimization Study
Formulation ii Buffer Sucrose
Sorbitol PS-80 DTPA API ConC:1
pH :.:
ii Abbreviation iiii iii (mM) (mM) õ: (%) (nM)
(mg/mL)
...
....
.. ...: :::: .....: .....:
..
: :: ..... ... ...
= ..
.== ::: :: : ::
......: .== .==
El 20 mM 5.5 250 0 0.05 50 10
E2 20 mM 6.0 250 0 0.05 50 10
E3 20 mM 6.5 250 0 0.05 50 10
E4 20 mM 5.5 0 250 0.05 50 10
ES 20 mM 6.0 0 250 0.05 50 10
E6 20 mM 6.5 0 250 0.05 50 10
E7 20 mM 6.0 250 0 0.05 50 20
E8 20 mM 6.0 250 0 0.05 50 50
All formulations were stored at 40 2 C/75 5%RH for up to three months and at 5
3 C and
25 2 C/60 5%RH up to 12 months. Initial and time point samples were evaluated
for API
stability by appearance, pH, Am, HIAC, SEC, CEX, and potency ELISA. Peptide
mapping
analysis was performed only at the initial, two month, and 12-month time
points. Microflow
imaging was evaluated only at the three-month time point. The test methods are
shown in
Table
and summary of testing at each time point is shown in Table 6.
24
CA 03064550 2019-11-21
WO 2018/231254 PCT/US2017/037991
Table 5. Analytical Testing for BIIB092 Excipient Optimization Study
Method Result Reporting
Appearance physical state, color, clarity
pH pH
Protein Content by UV mg/mL
CEX %acidic variants, %main peak, %basic variants
SEC %monomer, %HMW, %LMW
HIAC cumulative counts per mL at? 2 p.m, > 5 p.m,? 10 p.m,
> 25 p.m
Particulates (?2 p.m, > 5 p.m,? 5 p.m with AR? 0.85,
MFI > 10 p.m, > 25 p.m)
Potency ELISA %Relative Potency 3
Peptide Mapping IPN007 chemical degradation 3
'Testing was performed on 3 x 0.5 mL runs, included data from all three runs
2 MFI was only tested and reported at the three-month time point
3ELISA and peptide mapping testing were performed only at the initial, two
month, and final
time points
Table 6. Pull Schedule for BIIB092 Excipient Optimization Study
mmummmmmmmmmmliloitths
Stol.age Condition Initial
1 ............. 6
õAl. 9 12
3 C B A
25 2 C/60 5%RH A B A
40 2 C/75 5%RH B A
A = Testing according to Table 2, excluding MFI.
B = Testing according to Table 2, excluding ELISA, MFI and
peptide map.
C = Testing according to Table 2, excluding ELISA and peptide
map.
D = Testing of formulations E1-E3, E7, and E8 according to Table 2, except
MFI, ELISA
and peptide map.
E = Testing of formulations E1-E3, E7, and E8 according to Table 2, except
MFI.
Appearance
All samples at all time points and conditions except for one were assessed to
be clear,
colorless solutions, free of any visible product-related particulates. One
sample (E3, six
month, 25 2 C/60 5% RH) was noted to contain a single large white particle,
which was
considered to be a contaminant and prevented further testing of that
particular sample.
CA 03064550 2019-11-21
WO 2018/231254
PCT/US2017/037991
Measurement of pH
All samples at all time points and conditions exhibited pH values that were
within
0.1 pH units of the nominal values for each formulation. Any observed
differences in pH
were therefore within the variability of the method.
Protein Content by Ultraviolet/Visible Spectroscopy
All samples at all time points and conditions exhibited protein concentrations
that did
not
differ significantly from their respective initial values. Formulations
targeted to 10 mg/mL
(El ¨ E6) all exhibited protein concentrations between 9.8 ¨ 11.4 mg/mL. Note
that the
measured concentrations of the sorbitol formulations (E4 ¨ E6) were
approximately 0.5
mg/mL higher than those of the sucrose formulations (El ¨ E3), but this merely
reflects
slightly higher concentrations from sample preparation. Formulation E7
(targeted to 20
mg/mL) ranged from 19.0 ¨ 20.2 mg/mL, and E8 (targeted to 50 mg/mL) ranged
from 50.0 ¨
53.7 mg/mL. Protein content by A280 demonstrated no significant trends in
protein
concentration throughout the stability time points for any of the formulations
tested.
Particle Count (HIAC)
In all formulations, increases in the number of larger particles (10 pm and 25
pm)
were observed across the stability time points. However, there was no
dependence of particle
formation on storage temperature. Formulations El ¨ E3 exhibited overall
increases in large
particle counts out to the 12-month time point; any differences between the
three
formulations are likely due to the variability of the method. While the trends
were not
extremely strong, relative to the noise, it should be noted that the absolute
magnitude of
counts was considerable in the samples. Particle increases were especially
pronounced in the
E7 and E8 formulations (with 20 mg/mL and 50 mg/mL of API, respectively);
particles at 10
pm in E7 increased from 12 counts at the initial time point to 1161 counts for
the nine month
/ 25 C/60% RH sample, particles at 10 pm in E8 increased from 18 counts at the
initial time
point to 1003 counts for the nine month / 25 C/60% RH sample. Particle counts
in these two
samples at other longer range stability time points and conditions were also
much higher than
the initial particle counts. Differences between storage temperatures are
likely due to
variability of the method. For the formulations E4 - E6, which were only
tested out to three
26
CA 03064550 2019-11-21
WO 2018/231254
PCT/US2017/037991
months, particle counts out to that time point were comparable to those
observed in El ¨ E3,
and smaller than those observed in E7 ¨ E8.
Size Exclusion Chromatography
At each time point, small but significant decreases in percent HMW were
observed
for all formulations as stability condition temperature increased. Generally,
the temperature-
dependent differences in HMW impurities were smaller for formulations (El ¨
E3) prepared
with 250 mM sucrose than for formulations (E4 ¨ E6) prepared with 250 mM
sorbitol. At the
12-month time
point, formulations E2 and E3 exhibited the highest main peak purity,
indicating the least
aggregation and fragmentation of the API in these formulations.
Within the time point data for each formulation, there is no solid evidence
for
increasing HMW content at any storage condition. The difficulty in comparing
time points
makes a more distinct conclusion difficult. However, some comparison can be
made between
time points, when the reference standard performance in each sample queue is
comparable.
Two such time points were the initial and final (12 month) time points, for
which all
reference standard injections exhibited 1.5 0.1% HMW. At these two time
points, there was
little difference in HMW content for the study samples.
For each time point, the percent LMW did not appear to change significantly
(increases of between 0.1 ¨ 0.3% for some formulations and time points /
conditions, others
saw no
change in percent LMW).
Cation Exchange Chromatography
Percent acidic variants increased over time for all formulations, and
demonstrated
greater increases with increasing temperature, and appeared to be lower with
lower pH.
Conversely, percent basic variants, while increasing over time and with higher
stability
temperature, demonstrated lower variants with increasing pH. There did not
appear to be any
significant differences in either percent acidic or percent basic variants in
samples formulated
at higher concentrations (E7 ¨ 20 mg/mL, E8 ¨ 50 mg/mL) versus comparable
sample at 10
mg/mL at the same pH (6.0).
Overall, the percent main peak for the samples that were tested out to twelve
months
(El ¨ E3, E7 ¨ E8) appeared to be comparable for those samples at all
concentrations that
27
CA 03064550 2019-11-21
WO 2018/231254
PCT/US2017/037991
were formulated at pH 5.5 and 6Ø The sample formulated at pH 6.5 (E3)
exhibited
somewhat lower percent main peak versus those samples at lower pH (70.6% for
E3 versus
76.9% for E2 at 12 month /25 C/60% RH).
Potency by Enzyme-Linked Immunosorbent Assay
Samples demonstrated potency determinations ranging from 82 ¨ 126%. There did
not
appear to be any trends in percent potency with respect to either formulation
type or stability
time points /conditions.
Peptide Mapping
Peptide maps were interpreted in light of the identifications made by LC/MS.
For
deamidation of asparagine at light chain site N33 or N35, no significant
differences from
frozen reference standard were observed in formulations stored at 5 C, even
out to 12
months. At room temperature (25 C / 60% RH), deamidation above background
could be
detected in the pH 6.5 formulations (E3 and E6) at two months. Significant
deamidation
(>2% increase versus reference) was observed in a pH-dependent manner after 12
months at
25 C / 60% RH. Similar increases were observed after two months at 40 C / 75%
RH, with a
clear dependence on pH, but with equivalent results in sorbitol and sucrose.
Note that levels
of modification in reference standard measured by UV (6.1¨ 8.3%) were
reasonably similar
to those measured for the parent material by LC/MS (3.0%).
Deamidation of asparagine at heavy chain site N381 or N386 responded very
similarly to
the light chain deamidation site. No significant differences from frozen
reference standard
were observed in formulations stored at 5 C, even out to 12 months. At room
temperature
(25 C / 60% RH), deamidation above background could be detected in the pH 6.5
formulations (E3 and E6) at two months. Significant deamidation (>2% increase
versus
reference) was observed in a pH-dependent manner after 12 months at 25 C / 60%
RH.
Similar increases were observed after two months at 40 C / 75% RH, with a
clear
dependence on pH, but with equivalent results in sorbitol and sucrose. The
levels of
modification in reference standard measured by UV (5.9 ¨ 6.8%) were similar to
those
measured for the parent material by LC/MS (3.3%).
For deamidation of asparagine at heavy chain site N312, no significant
differences
from
frozen reference standard were observed in the study overall. Even at the 25 C
/
28
CA 03064550 2019-11-21
WO 2018/231254
PCT/US2017/037991
60% RH, 12 month and 40 C / 75% RH, two-month time points, the peak
percentages
were not significantly different from the frozen reference standard. This may
indicate that
this asparagine site is not susceptible to deamidation, or that additional
species are
present at the migration times of the peak identified as modified asparagine,
masking the
deamidation response. In support of this theory, total N312 modification was
somewhat
higher in reference standard by UV analysis (5.1 ¨ 9.8%) than in the parent
material by
LC/MS (2.8%). Because the succinimide form of the Asn/Asp intermediate was
most
abundant in the LC/MS data, actual deamidation may simply be low (<1%) at
all time points.
Proline and lysine hydroxylation, although present in significant abundance in
the
reformulated samples, did not appear to change with time, regardless of
storage temperature.
Hydroxylation of P189 was very consistent and unchanged at all time points.
Interestingly,
this proline hydroxylation was lower in the frozen reference standard. The
levels of proline
hydroxylation in reference standard measured by UV (1.1¨ 1.6%) were reasonably
similar to
those measured by LC/MS (1.0%). Measurement of K121 hydroxylation produced
much
larger differences in quantities. However, because no consistent trends could
be observed, it
is concluded these differences represent analytical noise. The levels of
lysine hydroxylation
in reference standard measured by UV (4.1 ¨ 7.9%) were reasonably similar to
those
measured in the parent material by LC/MS (2.8%).
Methionine oxidation at heavy chain site M425 was fairly conducive to
analysis.
Small, but consistent, levels of oxidation were seen in all formulations.
Oxidation increased
slowly as a function of time and temperature, but was not affected by the pH
or formulation
components. Even after two months at 40 C / 75% RH, the oxidation increased
only by about
0.5%. The levels of modification in reference standard measured by UV (0.2
¨0.6%) were
very similar to those measured in the parent material by LC/MS (0.3%).
Particle Count (Micro-Flow Imaging)
Particle counting by MFI was performed only at the three-month time point.
Overall, samples
formulated with 250 mM sorbitol appeared to demonstrate lower particle counts
at larger
particle sizes (10 pm and 25 pm). Among those samples formulated in 250 mM
sucrose,
formulation E2
appeared to demonstrate slightly lower particle counts overall. There did not
appear to be a
significant difference in particle counts between formulations at differing
API concentration.
29
CA 03064550 2019-11-21
WO 2018/231254
PCT/US2017/037991
Other Embodiments
While the invention has been described in conjunction with the detailed
description
thereof, the foregoing description is intended to illustrate and not limit the
scope of the
invention, which is defined by the scope of the appended claims. Other
aspects, advantages,
and modifications are within the scope of the following claims.