Note: Descriptions are shown in the official language in which they were submitted.
CA 03064942 2019-11-25
WO 2018/220630
PCT/IL2018/050588
1
METHOD OF GENERATION BACTERIAL COMPOSITIONS COMPRISING A BIOFILM
WITH BENEFECIAL BACTERIA
FIELD AND BACKGROUND OF THE INVENTION
The present invention, in some embodiments thereof, relates to methods of
generating
bacterial compositions, more particularly, but not exclusively, to probiotic
compositions, those
beneficial to the environment and those used in industry.
Living microbial cells which are administered in adequate amounts, confer a
beneficial
physiological effect on the host, are known as "probiotics". Studies have
shown therapeutic
effects that probiotic bacteria can provide to the host in maintaining a
healthy gut and controlling
several types of gastrointestinal infections. Due to their perceived health
benefits, probiotic
bacteria have been increasingly incorporated into a variety of food and drink
products during the
last few decades. Some of the most common types of microorganisms used as
probiotics are the
lactic acid bacteria (LAB), which mainly belong to the genera Lactobacillus
and
Bifidobacterium. Both these genera are dominant inhabitants in the human
intestine and have a
long history of safe use and are considered as GRAS (generally recognized as
safe). To assure
their beneficial effects in the body, these organisms must survive during food
processing, storage
and the passage through the upper gastrointestinal tract (GIT) and arrive
alive to their site of
action. However, previous studies have shown low survival level of probiotic
bacteria in the final
food product and a considerable loss in their viability to high acidic
conditions of the stomach
and high bile concentration in the small intestine. In addition, probiotics
are usually available as
dry bacterial powders prepared mainly by freeze drying which has been
established as a
procedure that may cause fatal injury to cells. Therefore, there is a need to
develop novel
technologies aimed to improve the survival of health-promoting bacteria during
food production,
as well as through the storage and ingestion processes in order to maintain
delivery of probiotics
to humans.
In most natural ecosystems, bacteria prefer to grow in complex community of
multicellular cells called biofilm and not as free-living (planktonic) cells.
Biofilm mode of
growth is preferable also for bacteria that inhabit the intestinal tract.
Cells in a biofilm are bound
together by an extracellular matrix that mainly consists of polysaccharides
and other
macromolecules such as proteins, DNA, lipids and nucleic acids, which are
produced by the cells
themselves. Interactions between the species embedded in the biofilm and their
environment
result in the formation of a complex structure, capable of resisting to
environmental stress and
exposure to antimicrobial agents. Thus, biofilm formation represents a
strategy for persistence
under unfavorable conditions in diverse environments.
CA 03064942 2019-11-25
WO 2018/220630
PCT/IL2018/050588
2
One of the mostly studied biofilm formers is Bacillus subtilis, a spore-
forming non-
pathogenic bacterium, which is characterized by its ability to produce a
robust biofilm. Bacillus
species, principally B. subtilis, have gained recent interest as probiotic
microorganism since they
were shown to positively effect on host health status mainly by keeping a
favorable balance of
microflora in the gastrointestinal tract. Since B. subtilis spores are capable
of surviving extreme
pH conditions and low oxygen, high numbers of dormant but viable microbes may
reach the
lower intestine which may induce some beneficial effects through secretion of
active substances.
Furthermore, it was found that B. subtilis cells enhance growth and viability
of lactobacilli spp.,
possibly through the production of catalase and subtilisin (Hosoi, Ametani,
Kiuchi, &
Kaminogawa, 2000). It has also been reported that Y- polyglutamic acid
produced by B. subtilis
as part of an extracellular matrix could be used to improve the survival of
probiotic bacteria
during freeze drying (A. R. Bhat et al., 2013) and during storage (A. R. Bhat
et al., 2015).
Likewise, during simulated gastric juice which simulated the acidic conditions
of the stomach
(A. R. Bhat et al., 2015).
Additional background art includes US Application No. 20100203581 and Salas
Jara et
al., Microorganisms 2016, 4, 35; doi:10.3390.
SUMMARY OF THE INVENTION
According to an aspect of the present invention there is provided a method of
preparing a
bacterial composition comprising:
(a) in vitro co-culturing beneficial bacteria with biofilm-producing
bacteria in a
growth substrate under conditions that generate a biofilm which comprises the
beneficial bacteria
and the non-pathogenic bacteria; and
(b) isolating the biofilm from the growth substrate, thereby preparing the
bacterial
composition.
According to an aspect of the present invention there is provided a bacterial
composition
obtainable according to the methods described herein.
According to an aspect of the present invention there is provided a food/feed
product
comprising the bacterial composition described herein.
According to an aspect of the present invention there is provided a method of
improving
or maintaining the health of a subject comprising administering to the subject
a therapeutically
effective amount of the probiotic composition described herein, thereby
improving or
maintaining the health of the subject.
CA 03064942 2019-11-25
WO 2018/220630
PCT/IL2018/050588
3
According to an aspect of the present invention there is provided a method of
selecting an
agent or culturing condition which is advantageous for preparing a bacterial
composition, the
method comprising co-culturing beneficial bacteria with biofilm-producing
bacteria in a growth
substrate in the presence of the agent or under the culturing condition, so as
to generate a biofilm
comprising the beneficial bacteria and the biofilm-producing bacteria, wherein
a change in a
property of the biofilm is indicative of the agent or culturing condition
being advantageous for
preparing the bacterial composition.
According to embodiments of the present invention the biofilm-producing
bacteria are
non-pathogenic bacteria.
According to embodiments of the present invention the biofilm-producing
bacteria are of
the bacillus genus.
According to embodiments of the present invention the biofilm-producing
bacteria are of
the B. subtilis species.
According to embodiments of the present invention the biofilm-producing
bacteria are of
the strain 127185/2.
According to embodiments of the present invention the growth substrate
comprises
manganese.
According to embodiments of the present invention the growth substrate
comprises
dextrose.
According to embodiments of the present invention, when the biofilm-producing
bacteria
are of the bacillus genus, the growth substrate comprises manganese.
According to embodiments of the present invention the beneficial bacteria are
probiotic
bacteria.
According to embodiments of the present invention the beneficial bacteria are
genetically
modified to express a therapeutic polypeptide.
According to embodiments of the present invention the probiotic bacteria is of
the
lactobacillales order.
According to embodiments of the present invention the biofilm-producing
bacteria are of
the B. subtilis species.
According to embodiments of the present invention the probiotic bacteria are
of the L.
plantarum species.
According to embodiments of the present invention the beneficial bacteria are
used in
bioremediation.
CA 03064942 2019-11-25
WO 2018/220630
PCT/IL2018/050588
4
According to embodiments of the present invention the biofilm-producing
bacteria
express genes of the KinD-Spo0A pathway.
According to embodiments of the present invention the growth substrate
comprises a
growth medium.
According to embodiments of the present invention the growth medium is
selected from
the group consisting of LB, LB GM, milk and MRS.
According to embodiments of the present invention the biofilm-producing
bacteria are of
the bacillus genus and the beneficial bacteria are of the lactobacillales
order, the growth substrate
is LB GM, milk or MRS.
According to embodiments of the present invention the growth substrate is MRS.
According to embodiments of the present invention the conditions comprise a pH
of about
6.5-8.
According to embodiments of the present invention the conditions comprise a pH
of 6.8-
7.5.
According to embodiments of the present invention the growth substrate
comprises
acetoin.
According to embodiments of the present invention the method further comprises
dehydrating the biofilm following the isolating.
According to embodiments of the present invention the beneficial bacteria
comprises no
more than 50 bacterial species.
According to embodiments of the present invention the biofilm-producing
bacteria are a
single species of biofilm-producing bacteria.
According to embodiments of the present invention, at least 50 % of the
bacteria in the
composition are viable.
According to embodiments of the present invention the bacterial composition
comprises
no more than 50 bacterial species of beneficial bacteria.
According to embodiments of the present invention the bacterial composition
comprises a
single species of non-pathogenic bacteria.
According to embodiments of the present invention the bacterial composition is
edible.
According to embodiments of the present invention the bacterial composition is
a
probiotic bacterial composition.
According to embodiments of the present invention the bacterial composition is
formulated as a powder, a liquid or a tablet.
CA 03064942 2019-11-25
WO 2018/220630
PCT/IL2018/050588
According to embodiments of the present invention the biofilm-producing
bacteria are of
the bacillus genus.
According to embodiments of the present invention the biofilm-producing
bacteria are of
the B. subtilis species.
5 According to embodiments of the present invention the beneficial
bacteria are probiotic
bacteria.
According to embodiments of the present invention the probiotic bacteria are
of the
lactobacillales order.
According to embodiments of the present invention the agent alters the pH of a
medium
of the system.
Unless otherwise defined, all technical and/or scientific terms used herein
have the same
meaning as commonly understood by one of ordinary skill in the art to which
the invention
pertains. Although methods and materials similar or equivalent to those
described herein can be
used in the practice or testing of embodiments of the invention, exemplary
methods and/or
materials are described below. In case of conflict, the patent specification,
including definitions,
will control. In addition, the materials, methods, and examples are
illustrative only and are not
intended to be necessarily limiting.
BRIEF DESCRIPTION OF THE SEVERAL VIEWS OF THE DRAWINGS
Some embodiments of the invention are herein described, by way of example
only, with
reference to the accompanying drawings. With specific reference now to the
drawings in detail,
it is stressed that the particulars shown are by way of example and for
purposes of illustrative
discussion of embodiments of the invention. In this regard, the description
taken with the
drawings makes apparent to those skilled in the art how embodiments of the
invention may be
practiced.
In the drawings:
FIGs. 1A-B are graphs comparing B. subtilis and L. plantarum growth in co-
culture. The
co-culture generation had no effect on L. plantarum and B. subtilis growth
(compared to their
growth in pure culture), indicating that there are no antagonistic
interactions between these
bacteria.
FIG. 2 are photographs illustrating that modified MRS medium triggers biofilm
formation by B. subtilis. The effect of the pH modification of MRS on B.
subtilis NCIB3610
biofilm formation was analyzed using stereoscopic microscope.
CA 03064942 2019-11-25
WO 2018/220630
PCT/IL2018/050588
6
FIG. 3 are photographs illustrating that the combination of LB with MRS medium
triggers biofilm development by B. subtilis. The effect of LB medium enriched
with different
concentrations of MRS (pH 7) on colony (top row) and pellicle (bottom row)
biofilm formation.
FIGs. 4A-B are graphs illustrating that the combination of LB with MRS medium
triggers extracellular matrix production by B. subtilis. Increasing MRS
concentration induces
transcription of tapA-sipW-tasA (A) and epsA-0 (B) operons.
FIG. 5A are photographs illustrating that the biofilm stimulating effect of
MRS is
regulated by the matrix synthesis and biofilm forming signaling pathway
previously described in
B. subtilis. Colony development and pellicle formation on MRS (pH 7) by the
wild type (WT)
and various mutant strains were compared. The strains used here were as
follows: wild type
(NCIB3610), AkinCD (RL4577), AkinAB (RL4573), Aspo0A (RL4620), Zleps4tasA
(RL4566),
zlabrB (YC668).
FIG. 5B are photographs illustrating that the effect of MRS in WT cells is
comparable to
the matrix overproducing mutant cells (ZIabrB) in B. subtilis.
FIG. 6 are photographs illustrating that MRS induces colony biofilm formation
in
different Bacillus species. MRS (pH 7) medium strongly induced colony type
biofilm formation
of B. paralicheniformis M5303, B. licheniformis M5310, B. licheniformis S127,
B. subtilis
MS1577 and B. cereus 10987.
FIG. 7 are photographs illustrating that MRS induces pellicle formation in
different
Bacillus species. MRS (pH 7) medium strongly induced pellicle formation of B.
paralicheniformis M5303, B. licheniformis M5310, B. licheniformis S127, B.
subtilis MS1577
and B. cereus 10987.
FIGs. 8A-B are images illustrating that B. subtilis produces extracellular
matrix whilst
forming a dual-species biofilm with L. plantarum. 8A. CLSM images of co-
culture biofilm of B.
subtilis and L. plantarum in MRS pH 7 at 37 C and 50 rpm. From left to right:
images made
using fluorescent light, Nomarski differential interference contrast (DIC) and
merged image. Top
panel shows the expression of fluorescently tagged B. subtilis cells
constitutively express GFP.
Bottom panel shows expression of matrix producing B. subtilis cells express
CFP under the
control of tapA promoter. In all images L. plantarum cells are not stained.
8B. CLSM images of
co-culture biofilm of B. subtilis and L. plantarum in LBGM medium. From left
to right: images
made using fluorescent light, Nomarski differential interference contrast
(DIC) and merged
image. Top panel shows the expression of fluorescently tagged B. subtilis
cells constitutively
express GFP. Bottom panel shows expression of matrix producing B. subtilis
cells express CFP
under the control of tapA promoter. In all images L. plantarum cells are not
stained.
CA 03064942 2019-11-25
WO 2018/220630
PCT/IL2018/050588
7
FIGs. 9A-C are SEM images of (A) B. subtilis cells, (B) L. plantarum cells and
(C) dual
species biofilm composed of B. subtilis and L. plantarum.
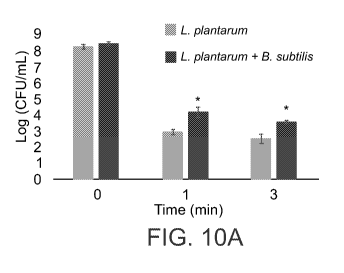
FIGs. 10A-B are graphs illustrating that dual species biofilm facilitates
survival of L.
plantarum exposed to unfavorable conditions. Survival of L. plantarum cells in
presence or
absence (control) of B. subtilis biofilm were determined during (A) heat
treatment at 63 C 1 to 3
min (B) storage at 4 C for 21 days. The values presented are the average of
at least three
independent experiments performed in duplicates. *p<0.05
FIGs. 11A-B are graphs illustrating that the extracellular matrix of B.
subtilis facilitates
increased survival of L. plantarum during heat treatment. A. The effect of
heat treatment at 63 C
for 3 min on WT B. subtilis and its derivatives, a mutant deficient in
exopolysaccharide
component and protein component of extracellular matrix (.4epsAtasA) and a
mutant deficient in
a repressor of the matrix genes (xlabrB; overproduces biofilm matrix) was
tested. The results
presented are the average of at least three independent experiments performed
in duplicates.
*p<0.05. B. The samples were grown in milk for 18 h at 30 C, 20 rpm.
Afterwards they were
heat treated at 63 C for 1 to 3 minutes. Control samples were not heat-
treated. The number of
viable L. plantarum cells was determined using CFU-method. *p<0.05
FIG. 12 is a graph illustrating that the presence of B. subtilis biofilm
increases survival of
L. plantarum during gastric and intestinal digestion in vitro (model system).
Survival of L.
plantarum cells in presence or absence (control) of B. subtilis biofilm were
determined during
gastro-intestinal digestion in vitro. The results presented are the average of
three independent
experiments performed in duplicates. *p<0.05
FIG. 13 is a graph of the growth curves of B. subtilis 3610NCIB in MRS (pH 7)
and LB.
FIG. 14 are photographs illustrating the effect of mutations in Histidine
kinases on
colony surface architecture and pellicle formation in MRS pH 7.
FIGs. 15A-C and CLSM images of fluorescently tagged B. subtilis cells (Pspank-
gfp)
following 24h incubation at LB medium in the presence and absence of acetoin.
FIGs. 16A-B are photographs illustrating that acetoin triggers the colony type
biofilm
formation by Bacillus subtilis
FIGs. 17A-D are photographs illustrating that the transcription of the tapA
operon
responsible for the matrix production in B. subtilis is highly upregulated by
acetoin. CLSM
images of B. subtilis cells that bear the PtapA¨cfp transcriptional fusion,
following 24h
incubation at LB medium that does not promote biofilm formation.
FIGs. 18A-B are photographs depicting the biofilm generated from the B.
subtilis strains
NCIB3610 and 127185/2 respectively.
CA 03064942 2019-11-25
WO 2018/220630
PCT/IL2018/050588
8
FIG. 19 is a graph illustrating the survival rate of L. plantarum grown in co-
culture
biofilm with B. subtilis during transition at in vitro model of the digestive
system. Cultures of
t=0 were the control of the experiment. Following the incubation in the
stomach-like fluid, the
co-culture of L. plantarum + B. subtilis 127185/2 showed the highest survival
rates. The L.
plantarum + B. subtilis NCIB3610 co-culture demonstrated a slightly higher
survival rate than
those of L. plantarum alone. After further incubation, in the intestinal-like
fluid, a significant
decrease occurred when the trend of survival rates in the different cultures
was maintained.
FIG. 20 is a graph illustrating the survival of L. plantarum grown in co-
culture biofilm
with B. subtilis in exposure to high acidity level. The sign '+' in the tested
cultures indicates a
growth with 50 rpm shaking, while the sign '-' indicates a growth without
shaking at all. In
general, there was a drastic decrease in the amount of L. plantarum that
survived in the transition
from a pH 7 to a pH 3 growth medium. The co-cultures of L. plantarum and B.
subtilis showed a
lower decrease in the survival rates of L. plantarum (compared to the mono-
culture of L.
plantarum) in transition to an acidic environment as with as well as without
shaking.
FIG. 21 are photographs illustrating that Mn2+ ions are involved in biofilm
formation by
B. subtilis in modified MRS. Effects of exclusion of certain MRS medium
components (Mg2+,
Mn2+, sodium acetate, dipotassium phosphate, dextrose, ammonium citrate) on
colony
development and pellicle formation by the WT B. subtilis cells were observed.
DESCRIPTION OF SPECIFIC EMBODIMENTS OF THE INVENTION
The present invention, in some embodiments thereof, relates to methods of
generating
bacterial compositions, more particularly, but not exclusively, to probiotic
compositions, those
beneficial to the environment and those used in industry.
Before explaining at least one embodiment of the invention in detail, it is to
be understood
that the invention is not necessarily limited in its application to the
details set forth in the
following description or exemplified by the Examples. The invention is capable
of other
embodiments or of being practiced or carried out in various ways.
Bacteria are economically important as these microorganisms are used by humans
for
many purposes. The beneficial uses of bacteria include the production of
traditional foods such
as yoghurt, cheese, and vinegar; biotechnology and genetic engineering,
producing substances
such as drugs and vitamins; agriculture; fibre retting; production of methane;
bioremediation and
biological control of pests.
To carry out their purpose, often times, bacteria are exposed to harsh
conditions which
reduce their viability and therefore their effectiveness.
CA 03064942 2019-11-25
WO 2018/220630
PCT/IL2018/050588
9
For example, to assure a probiotic' s beneficial effect in the body, these
organisms must
survive during food processing, storage and the passage through the upper
gastrointestinal tract
(GIT) and arrive alive to their site of action. However, previous studies have
shown low survival
level of probiotic bacteria in the final food product and a considerable loss
in their viability to
high acidic conditions of the stomach and high bile concentration in the small
intestine. In
addition, probiotics are usually available as dry bacterial powders prepared
mainly by freeze
drying which has been established as a procedure that may cause fatal injury
to cells.
Whilst carrying out research on bacterial biofilms, the present inventors
noticed that
under appropriate conditions a biofilm-producing bacteria may incorporate a
non-biofilm-
producing bacteria into its biofilm rendering it more resistant to extreme
temperatures (cold and
heat; Figures 10A-B and 11A-B respectively).
Specifically, the present inventors co-cultured bacteria of the B. subtilis
species together
with the probiotic bacteria L. plantarum. They showed that under particular
conditions the B.
subtilis bacteria generated a biofilm in which the L. plantarum cells were
incorporated within the
extracellular matrix thereof (Figure 9A). The biofilm-incorporated L.
plantarum were shown to
be both more heat-resistant and more cold-resistant, and further more acid-
resistant than control,
non-biofilm incorporated L. plantarum.
Taken together, the present inventors propose that biofilm-producing bacteria
can be used
to encapsulate a non-biofilm producing bacteria. Thus, the biofilm-producing
bacteria serve as a
protective carrier for the beneficial, non-biofilm producing bacteria.
Thus, according to a first aspect of the present invention, there is provided
a method of
preparing a bacterial composition comprising:
(a) in vitro co-culturing a beneficial bacteria with a biofilm-producing
bacteria in a
growth substrate under conditions that generate a biofilm which comprises the
beneficial bacteria
and the non-pathogenic bacteria;
(b) isolating the biofilm from the growth substrate, thereby preparing the
bacterial
composition.
The term "bacteria" as used herein refers to a prokaryotic microorganism,
including
archaea. The bacteria may be gram positive or gram negative. The bacteria may
also be
photosynthetic bacteria (e.g. cyanobacteria).
As used herein the term "beneficial bacteria" refers to any bacteria that
bring about a
positive effect on human beings.
In one embodiment, the beneficial bacteria do not produce a biofilm when
propagated as a
monoculture in a growth medium under standard culturing conditions.
CA 03064942 2019-11-25
WO 2018/220630
PCT/IL2018/050588
In another embodiment, the beneficial bacteria do not produce a biofilm when
propagated
as a monoculture in a growth medium under culturing conditions that are
optimal for their
propagation.
In still another embodiment, the beneficial-bacteria utilize the KinD-Spo0A
pathway (for
5
example express the genes histidine kinase kinD, spoOF, spo0B and/or spo0A) ¨
see for example
Shemesh and Chai, 2013 Journal of Bacteriology, 2013, Vol 195, No.12 pages
2747-2754, the
contents of which are incorporated herein by reference.
The beneficial bacteria may be one that is typically cultured in Man, Rogosa
and Sharpe
medium, MRS (solidified using agar or MRS broth).
10
The beneficial bacteria should typically not prevent (i.e. antagonize) the
biofilm-forming
capability of the biofilm-generating bacteria (e.g. B. subtilis). Methods of
determining whether
bacteria have antagonistic activity towards other bacteria when cultured
together are known in the
art (see for example Figures 1A-B). In one embodiment, the beneficial bacteria
are not soil
bacteria.
Any number of strains of beneficial bacteria may be cultured in the co-culture
of this
aspect of the present invention. In one embodiment, no more than 500 different
strains of
beneficial bacteria are cultured in a single culture, no more than 250
different strains of beneficial
bacteria are cultured in a single culture, no more than 100 different strains
of beneficial bacteria
are cultured in a single culture, no more than 90 different strains of
beneficial bacteria are
cultured in a single culture, no more than 80 different strains of beneficial
bacteria are cultured in
a single culture, no more than 70 different strains of beneficial bacteria are
cultured in a single
culture, no more than 60 different strains of beneficial bacteria are cultured
in a single culture, no
more than 50 different strains of beneficial bacteria are cultured in a single
culture, no more than
40 different strains of beneficial bacteria are cultured in a single culture,
no more than 30
different strains of beneficial bacteria are cultured in a single culture, no
more than 20 different
strains of beneficial bacteria are cultured in a single culture, no more than
10 different strains of
beneficial bacteria are cultured in a single culture, no more than 9 different
strains of beneficial
bacteria are cultured in a single culture, no more than 8 different strains of
beneficial bacteria are
cultured in a single culture, no more than 7 different strains of beneficial
bacteria are cultured in a
single culture, no more than 6 different strains of beneficial bacteria are
cultured in a single
culture, no more than 5 different strains of beneficial bacteria are cultured
in a single culture, no
more than 4 different strains of beneficial bacteria are cultured in a single
culture, no more than 3
different strains of beneficial bacteria are cultured in a single culture, no
more than 2 different
CA 03064942 2019-11-25
WO 2018/220630
PCT/IL2018/050588
11
strains of beneficial bacteria are cultured in a single culture only one
strain of beneficial bacteria
is cultured per single culture.
The beneficial bacterial strains of a single culture of this aspect of the
present invention
may belong to a single species or may belong to multiple species. Preferably,
the beneficial
bacterial strains of a culture belong to a single species of bacteria. In
other embodiments multiple
species of beneficial bacteria are cultured on a single culture. Preferably no
more than 10
different species of beneficial bacteria are cultured in a single culture, no
more than 9 different
species of beneficial bacteria are cultured in a single culture, no more than
8 different species of
beneficial bacteria are cultured in a single culture, no more than 7 different
species of beneficial
bacteria are cultured in a single culture, no more than 6 different species of
beneficial bacteria are
cultured in a single culture, no more than 5 different species of beneficial
bacteria are cultured in
a single culture, no more than 4 different species of beneficial bacteria are
cultured in a single
culture, no more than 3 different species of beneficial bacteria are cultured
in a single culture, no
more than 2 different species of beneficial bacteria are cultured in a single
culture only one
species of beneficial bacteria is cultured per single culture.
In one embodiment, the beneficial bacteria, when ingested promote the health
of a human
being. In another embodiment, the beneficial bacteria are used in industry to
generate a product
that is useful for human beings (e.g. methane, petroleum, insecticide etc.).
In another
embodiment, the beneficial bacteria are used in the food industry. In another
embodiment, the
beneficial bacteria are used in a silage inoculant. In still another
embodiment, the beneficial
bacteria are used in agriculture to support the growth of plants. In still
another embodiment, the
beneficial bacteria are used in bioremediation.
In one embodiment, the beneficial bacteria are probiotic bacteria.
The term "probiotic bacteria" as used herein refers to live bacteria which
when
administered in adequate amounts confer a health benefit on the host (e.g.
human).
Among the principal mechanisms of probiotic action, it is possible to find the
inhibition of
enteric pathogens by the production of lactic acid, hydrogen peroxide and
bacteriocins;
competitive exclusion of enteric pathogens by blocking adhesion sites,
competition for nutrients
and modulation of the immune system, including inflammation reduction. They
also provide
benefits to the host, such as lactose intolerance alleviation; cholesterol
decrease by assimilation,
sustenance of the intestinal normal microbiota and dysbiosis ameliorating
suppression of toxin
production, degradation of toxin receptors in the intestine, preservation of
normal intestinal pH,
increase intestinal motility and help to maintain the integrity of the
intestine permeability.
CA 03064942 2019-11-25
WO 2018/220630
PCT/IL2018/050588
12
In one embodiment the beneficial bacteria belong to the order Lactobacillales
(commonly
known as lactic acid bacteria (LAB)). These bacteria are Gram-positive, low-
GC, acid-tolerant,
generally nonsporulating, non-respiring, either rod- or coccus-shaped bacteria
that share common
metabolic and physiological characteristics. These bacteria produce lactic
acid as the major
metabolic end product of carbohydrate fermentation.
Preferably the beneficial bacteria of the Lactobacillales order are ones which
grow (and
are typically cultured) in MRS agar (MRS).
Exemplary contemplated genera of the order Lactobacillales include, but are
not limited
to Lactobacillus, Leuconostoc, Pediococcus, Lactococcus, Streptococcus,
Aerococcus,
Camobacterium, Enterococcus, Oenococcus, Sporolactobacillus, Tetragenococcus,
Vagococcus,
and Weissella.
According to a preferred embodiment, the beneficial bacteria of this aspect of
the present
invention belong to the genus lactobacillus. Exemplary species of
lactobacillus contemplated by
the present invention include but are not limited to L. acetotolerans, L.
acidifarinae, L.
acidipiscis, L. acidophilus, L. agilis, L. algidus, L. alimentarius, L.
amylolyticus, L. amylophilus,
L. amylotrophicus, L. amylovorus,L. animalis, L. antri, L. apodemi, L.
aviarius, L. bifermentans,
L. brevis, L. buchneri, L. camelliae, L. casei, L. catenaformis, L. ceti, L.
coleohominis, L.
collinoides, L. composti, L. concavus, L. coryniformis, L. crispatus, L.
crustorum, L. curvatus, L.
delbrueckii subsp. bulgaricus, L. delbrueckii subsp. delbrueckii, L.
delbrueckii subsp. lactis, L.
dextrinicus, L. diolivorans, L. equi, L. equigenerosi, L. farraginis, L.
farciminis, L. fermentum, L.
fornicalis, L. fructivorans, L. frumenti, L. fuchuensis, L. gallinarum, L.
gasseri, L. gastricus, L.
ghanensis, L. hilgardii, L. homohiochii, L. iners, L. ingluviei, L.
intestinalis, L. jensenii, L.
johnsonii,L. kalixensis, L. kefiranofaciens, L. kefiri, L. kimchii, L.
kitasatonis, L. kunkeei, L.
leichmannii, L. lindneri, L. malefermentans, L. mali, L. manihotivorans, L.
mindensis, L.
mucosae, L. murinus, L. nagelii, L. namurensis, L. nantensis, L.
oligofermentans, L. oris, L.
panis, L. pantheris, L. parabrevis, L. parabuchneri, L. paracasei, L.
paracollinoides, L.
parafarraginis, L. parakefiri, L. paralimentarius, L. paraplantarum, L.
pentosus, L. perolens, L.
plantarum, L. pontis, L. protectus, L. psittaci, L. rennini L. reuteri, L.
rhamnosus, L. rimae, L.
rogosae, L. rossiae, L. ruminis, L. saerimneri, L. sakei, L. salivarius, L.
sanfranciscensis, L.
satsumensis, L. secaliphilus, L. sharpeae, L. siliginis, L. spicheri, L.
suebicus, L. thailandensis, L.
ultunensis, L. vaccinostercus, L. vaginalis, L. versmoldensis, L. vini, L.
vitulinus, L. zeae and L.
zymae.
In one particular embodiment, the species of lactobacillus is L. plantarum.
CA 03064942 2019-11-25
WO 2018/220630
PCT/IL2018/050588
13
The beneficial bacteria of this aspect of the present invention may generate a
fermentation
product. Examples of fermentation products include but are not limited to pre-
biotics, biofuels,
methanol, ethanol, propanol, butanol, alcohol fuels, proteins, recombinant
proteins, vitamins,
amino acids, organic acids (for e.g. lactic acid, propionic acid, acetic acid,
succinic acid, malic
acid, glutamic acid, aspartic acid and 3-hydroxypropionic acid), enzymes,
antigens, antibiotics,
organic chemicals, bioremediation treatments, preservatives and metabolites.
Thus, the beneficial bacteria may be genetically modified to express a
beneficial
polypeptide.
The beneficial polypeptides may be intracellular polypeptides (e.g., a
cytosolic protein),
transmembrane polypeptides, or secreted polypeptides. Heterologous production
of proteins is
widely employed in research and industrial settings, for example, for
production of therapeutics,
vaccines, diagnostics, biofuels, and many other applications of interest.
Exemplary therapeutic
proteins that can be produced by employing the subject compositions and
methods, include but
are not limited to certain native and recombinant human hormones (e.g.,
insulin, growth
hormone, insulin-like growth factor 1, follicle-stimulating hormone, and
chorionic gonadotropin),
hematopoietic proteins (e.g., erythropoietin, C-CSF, GM-CSF, and IL-11),
thrombotic and
hematostatic proteins (e.g., tissue plasminogen activator and activated
protein C), immunological
proteins (e.g., interleukin), antibodies and other enzymes (e.g.,
deoxyribonuclease I). Exemplary
vaccines that can be produced by the subject compositions and methods include
but are not
limited to vaccines against various influenza viruses (e.g., types A, B and C
and the various
serotypes for each type such as H5N2, H1N1, H3N2 for type A influenza
viruses), HIV, hepatitis
viruses (e.g., hepatitis A, B, C or D), Lyme disease, and human papillomavirus
(HPV). Examples
of heterologously produced protein diagnostics include but are not limited to
secretin, thyroid
stimulating hormone (TSH), HIV antigens, and hepatitis C antigens.
Proteins or peptides produced by the heterologous polypeptides can include,
but are not
limited to cytokines, chemokines, lymphokines, ligands, receptors, hormones,
enzymes,
antibodies and antibody fragments, and growth factors. Non-limiting examples
of receptors
include TNF type I receptor, IL-1 receptor type II, IL-1 receptor antagonist,
IL-4 receptor and
any chemically or genetically modified soluble receptors. Examples of enzymes
include
acetylcholinesterase, lactase, activated protein C, factor VII, collagenase
(e.g., marketed by
Advance Biofactures Corporation under the name Santyl); agalsidase-beta (e.g.,
marketed by
Genzyme under the name Fabrazyme); dornase-alpha (e.g., marketed by Genentech
under the
name Pulmozyme); alteplase (e.g., marketed by Genentech under the name
Activase); pegylated-
asparaginase (e.g., marketed by Enzon under the name Oncaspar); asparaginase
(e.g., marketed
CA 03064942 2019-11-25
WO 2018/220630
PCT/IL2018/050588
14
by Merck under the name Elspar); and imiglucerase (e.g., marketed by Genzyme
under the name
Ceredase). Examples of specific polypeptides or proteins include, but are not
limited to
granulocyte macrophage colony stimulating factor (GM-CSF), granulocyte colony
stimulating
factor (G-CSF), macrophage colony stimulating factor (M-CSF), colony
stimulating factor
(CSF), interferon beta (IFN-beta), interferon gamma (IFNgamma), interferon
gamma inducing
factor I (IGIF), transforming growth factor beta (IGF-beta), RANTES (regulated
upon activation,
normal T-cell expressed and presumably secreted), macrophage inflammatory
proteins (e.g.,
MP-1-alpha and MIP-1-beta), Leishmnania elongation initiating factor (LEIF),
platelet derived
growth factor (PDGF), tumor necrosis factor (TNF), growth factors, e.g.,
epidermal growth
factor (EGF), vascular endothelial growth factor (VEGF), fibroblast growth
factor, (FGF), nerve
growth factor (NGF), brain derived neurotrophic factor (BDNF), neurotrophin-2
(NT-2),
neurotrophin-3 (NT-3), neurotrophin-4 (NT-4), neurotrophin-5 (NT-5), glial
cell line-derived
neurotrophic factor (GDNF), ciliary neurotrophic factor (CNTF), TNF alpha type
II receptor,
erythropoietin (EPO), insulin and soluble glycoproteins e.g., gp120 and gp160
glycoproteins.
The gp120 glycoprotein is a human immunodeficiency virus (WIV) envelope
protein, and the
gp160 glycoprotein is a known precursor to the gp120 glycoprotein. Other
examples include
secretin, nesiritide (human B-type natriuretic peptide (hBNP)) and GYP-I.
Contemplated bacteria for the expression of human interferon beta lb include
for
example E.coli.
Contemplated bacteria for the expression of human interferon gamma include for
example E.coli.
Contemplated bacteria for the expression of human growth hormone include for
example
E.coli.
Contemplated bacteria for the expression of human insulin include for example
E.coli.
Contemplated bacteria for the expression of interleukin II include for example
E.coli.
According to a particular embodiment, the beneficial polypeptide is an
antibody (e.g.
Humira, Remicade, Rituxan, Enbrel, Avastin, Herceptin).
Contemplated bacteria for the expression of antibodies include for example
E.coli,
Bacillus brevis, Bacillus subtilis and Bacillus megaterium.
Other beneficial bacteria contemplated by the present invention include those
used as
bacterial vaccines. Exemplary vaccines contemplated by the present invention
include, but are
not limited to Vivotif Berna Vaccine (typhoid vaccine, live), Prevnar 13
(pneumococcal 13-
valent vaccine), Menactra (meningococcal conjugate vaccine), ActHIB
(haemophilus b
conjugate (prp-t) vaccine), Bexsero (meningococcal group B vaccine), Biothrax
(anthrax vaccine
CA 03064942 2019-11-25
WO 2018/220630
PCT/IL2018/050588
adsorbed), Hiberix (haemophilus b conjugate (prp-t) vaccine), HibTITER
(haemophilus b
conjugate (hboc) vaccine), Liquid PedvaxHIB (haemophilus b conjugate (prp-omp)
vaccine),
MenHibrix (haemophilus b conjugate (prp-t) vaccine/meningococcal conjugate
vaccine),
Menomune A / C / Y / W-135 (meningococcal polysaccharide vaccine), Menveo
5 (meningococcal conjugate vaccine), Pneumovax 23 (neumococcal 23-
polyvalent vaccine),
Prevnar (pneumococcal 7-valent vaccine), Te Anatoxal Berna (tetanus toxoid),
Tetanus Toxoid
Adsorbed (tetanus toxoid), TheraCys (bcg), Tice BCG (bcg), Trumenba
(meningococcal group B
vaccine), Typhim Vi (typhoid vaccine, inactivated), Vaxchora, cholera vaccine,
live and Vivotif
Berna (typhoid vaccine, live).
10 Other contemplated beneficial bacteria are those that are useful in
bioremediation. Such
remediation includes heavy metals, chemical, radiation and hydrocarbon
contamination.
Examples of bacteria that may be used for bioremediation are listed herein
below:
Pseudomonas putida: Pseudomonas putida is a gram-negative soil bacterium that
is
involved in the bioremediation of toluene, a component of paint thinner. It is
also capable of
15 degrading naphthalene, a product of petroleum refining, in contaminated
soils.
Dechloromonas aromatica: Dechloromonas aromatica is a rod-shaped bacterium
which
can oxidize aromatics including benzoate, chlorobenzoate, and toluene,
coupling the reaction
with the reduction of oxygen, chlorate, or nitrate. It is the only organism
able to oxidize benzene
anaerobically. Due to the high propensity of benzene contamination, especially
in ground and
surface water, D. aromatic is especially useful for in situ bioremediation of
this substance.
Nitrifiers and Denitrifiers: Industrial bioremediation is used to clean
wastewater. Most
treatment systems rely on microbial activity to remove unwanted mineral
nitrogen compounds
(i.e. ammonia, nitrite, nitrate). The removal of nitrogen is a two stage
process that involves
nitrification and denitrification. During nitrification, ammonium is oxidized
to nitrite by
organisms like Nitrosomonas europaea. Then, nitrite is further oxidized to
nitrate by microbes
like Nitrobacter hamburgensis. In anaerobic conditions, nitrate produced
during ammonium
oxidation is used as a terminal electron acceptor by microbes like Paracoccus
denitrificans. The
result is N2 gas. Through this process, ammonium and nitrate, two pollutants
responsible for
eutrophication in natural waters, are remediated.
Deinococcus radiodurans: Deinococcus radiodurans is a radiation-resistant
extremophile bacterium that is genetically engineered for the bioremediation
of solvents and
heavy metals. An engineered strain of Deinococcus radiodurans has been shown
to degrade ionic
mercury and toluene in radioactive mixed waste environments.
CA 03064942 2019-11-25
WO 2018/220630
PCT/IL2018/050588
16
In anaerobic conditions, nitrate produced during ammonium oxidation is used as
a
terminal electron acceptor by microbes like Paracoccus denitrificans. The
result is dinitrogen
gas. Through this process, ammonium and nitrate, two pollutants responsible
for eutrophication
in natural waters, are remediated.
Methylibium petroleiphilum: Methylibium petroleiphilum (formally known as PM1
strain) is a bacterium capable of methyl tert-butyl ether (MTBE)
bioremediation. PM1 degrades
MTBE by using the contaminant as the sole carbon and energy source.
Akanivorax borkumensis: Alcanivorax borkumensis is a marine rod-shaped
bacterium
which consumes hydrocarbons, such as the ones found in fuel, and produces
carbon dioxide. It
grows rapidly in environments damaged by oil, and has been used to aid in
cleaning the more
than 830,000 gallons of oil from the Deepwater Horizon oil spill in the Gulf
of Mexico. Other
contemplated bacteria that can be used to clean up oil include Colwellia and
Neptuniibacter.
As mentioned, the method of this aspect of the present invention contemplates
culturing
the beneficial bacteria with a biofilm-producing bacteria.
The term "biofilm" as used herein refers to a community of bacteria that are
comprised
(e.g. embedded or encapsulated) in a matrix of extracellular polymeric
substances that they have
produced. Typically, the bacteria when present in the biofilm exhibit an
altered phenotype with
respect to growth rate and gene transcription in comparison to freely floating
planktonic bacteria.
Examples of extracellular polymeric substances which may be present in the
biofilm include
exopolysaccharides (such as those synthesized by the products of the epsA-0
operon) and
amyloid fibers (such as those encoded by tapA-sipW-tasA operon). Thus, the
matrix typically
comprises extracellular DNA and protein, as well as carbohydrates.
It will be appreciated that the biofilm-producing bacteria may also be
beneficial bacteria.
The biofilm-producing bacteria are typically of a different order and/or genus
than the
beneficial bacteria which are incorporated into the biofilm. Thus, the biofilm-
producing bacteria
and the beneficial bacteria may be of distinct strains, species, genus and/or
order.
Preferably, the biofilm-producing bacteria is non-pathogenic (i.e. do not
cause physical
harm to, or disease in) a human being.
Any number of strains of biofilm-producing bacteria may be cultured in the co-
culture of
this aspect of the present invention. In one embodiment, no more than 500
different strains of
biofilm-producing bacteria are cultured in a single culture, no more than 250
different strains of
biofilm-producing bacteria are cultured in a single culture, no more than 100
different strains of
biofilm-producing bacteria are cultured in a single culture, no more than 90
different strains of
biofilm-producing bacteria are cultured in a single culture, no more than 80
different strains of
CA 03064942 2019-11-25
WO 2018/220630
PCT/IL2018/050588
17
biofilm- bacteria are cultured in a single culture, no more than 70 different
strains of biofilm-
producing bacteria are cultured in a single culture, no more than 60 different
strains of biofilm-
producing bacteria are cultured in a single culture, no more than 50 different
strains of biofilm-
producing bacteria are cultured in a single culture, no more than 40 different
strains of biofilm-
.. producing bacteria are cultured in a single culture, no more than 30
different strains of biofilm-
producing bacteria are cultured in a single culture, no more than 20 different
strains of biofilm-
producing bacteria are cultured in a single culture, no more than 10 different
strains of biofilm-
producing bacteria are cultured in a single culture, no more than 9 different
strains of biofilm-
producing bacteria are cultured in a single culture, no more than 8 different
strains of biofilm-
producing bacteria are cultured in a single culture, no more than 7 different
strains of the biofilm-
producing bacteria are cultured in a single culture, no more than 6 different
strains of the biofilm-
producing bacteria are cultured in a single culture, no more than 5 different
strains of the biofilm-
producing bacteria are cultured in a single culture, no more than 4 different
strains of the biofilm-
producing bacteria are cultured in a single culture, no more than 3 different
strains of biofilm-
producing bacteria are cultured in a single culture, no more than 2 different
strains of biofilm-
producing bacteria are cultured in a single culture or only one strain of
biofilm-producing
bacteria are cultured per single culture.
The biofilm-producing bacterial strains of a single culture of this aspect of
the present
invention may belong to a single species or may belong to multiple species.
Preferably, the
biofilm-producing bacterial strains of a culture belong to a single species of
bacteria. In other
embodiments multiple species of biofilm-producing bacteria are cultured on a
single culture.
Preferably no more than 10 different species of biofilm-producing bacteria are
cultured in a
single culture, no more than 9 different species of biofilm-producing bacteria
are cultured in a
single culture, no more than 8 different species of biofilm-producing bacteria
are cultured in a
single culture, no more than 7 different species of biofilm-producing bacteria
are cultured in a
single culture, no more than 6 different species of biofilm-producing bacteria
are cultured in a
single culture, no more than 5 different species of biofilm-producing bacteria
are cultured in a
single culture, no more than 4 different species of biofilm-producing bacteria
are cultured in a
single culture, no more than 3 different species of biofilm-producing bacteria
are cultured in a
single culture, no more than 2 different species of biofilm-producing bacteria
are cultured in a
single culture or only one species of biofilm-producing bacteria is cultured
per single culture.
In one embodiment, the biofilm-producing bacteria belong to the genus
Bacillus.
As used herein, "the genus Bacillus" includes all members known to those of
skill in the
art, including but not limited to B. subtilis, B. licheniformis, B. lentus, B.
brevis, B.
CA 03064942 2019-11-25
WO 2018/220630
PCT/IL2018/050588
18
stearothermophilus, B. alkalophilus, B. amyloliquefaciens, B. clausii, B.
halodurans, B.
megaterium, B. coagulans, B. circulans, B. lautus, and B. thuringiensis. It is
recognized that the
genus Bacillus continues to undergo taxonomical reorganization. Thus, it is
intended that the
genus include species that have been reclassified, including but not limited
to such organisms as
B. stearothermophilus, which is now named "Geobacillus stearothermophilus."
The production
of resistant endospores in the presence of oxygen is considered the defining
feature of the genus
Bacillus, although this characteristic also applies to the recently named
Allcydobacillus,
Amphibacillus, Aneurinibacillus, Anoxybacillus, Brevibacillus, Filobacillus,
Gracilibacillus,
Halobacillus, Paenibacillus, Salibacillus, Thermobacillus, Ureibacillus, and
Virgibacillus.
In one embodiment, the biofilm-producing bacteria are of the species B.
subtilis.
Exemplary strains of B. subtilis contemplated by the present invention
include, but are
not limited to B. subtilis MS1577 and 127185/2 (MS302; dairy isolate) and
NCIB3610.
Exemplary strains of B paralicheniformis contemplated by the present invention
include,
but are not limited to B. paralicheniformis MS 303, and B. paralicheniformis
S127.
Exemplary strains of B.licheniformis contemplated by the present invention
include, but
are not limited to B.licheniformis MS310, and B. licheniformis M5307.
According to a particular embodiment, the biofilm-producing bacteria does not
comprise
the species B. cereus.
In order to generate a co-culture, typically both the beneficial culture and
the biofilm-
generating culture are cultured separately to generate a starter culture. The
medium and
conditions of the starter culture are typically selected so as to optimize
growth of each of the
bacteria.
Contemplated started cultures include a dried starter culture, a dehydrated
starter culture,
a frozen starter culture, or a concentrated starter culture.
The starter culture is grown for at least two hours, 4 hours, 8 hours, 12
hours until a
sufficient amount of bacteria are propagated.
According to a particular embodiment, the method includes a method of co-
culturing,
whereby the beneficial bacteria is of the genus lactobacillus (e.g. the
species L. plantarum) and
the biofilm-producing bacteria is of the genus Bacillus (e.g. of the species
B. subtilis).
The method of co-culturing the beneficial bacteria with the biofilm producing
bacteria is
selected such that it enables the proliferation of both types of
microorganisms and incorporation
of both microorganisms into the biofilm.
CA 03064942 2019-11-25
WO 2018/220630
PCT/IL2018/050588
19
In one embodiment, the co-culturing is carried out in (or on) a growth
substrate that is
typically used to culture the beneficial bacteria. The growth substrate may be
a solid or a liquid
medium. Preferably, the co-culture is shaken during the culturing.
Examples of growth substrates that can be used to culture bacteria include but
are not
limited to MRS medium, LB medium, TBS medium, yeast extract, soy peptone,
casein peptone
and meat peptone.
Further examples of media are listed in Table 1 herein below.
Table I
Abiotrophia media - Recipe for medium appropriate for growth of Abiotrophia
genus
Acetamide Medium - Recipe for Acetamide medium.
Acetobacter Medium - Recipe for medium appropriate for the growth of
Acetobacter genus.
Actinoplanes Medium - Media used to grow certain Actinoplanes species
Agrobacterium Agar Recipe - Agar appropriate for growth of Agrobacterium genus
Alicyclobacillus Agar - Recipe for Alicyclobacillus Agar.
Alicyclobacillus Medium - Recipe for Alicyclobacillus Medium.
Allantoin mineral agar - Recipe for the preparation of Allantoin minimal agar.
Allantoin mineral medium - Recipe for the preparation of allantoin minimal
medium.
Ashbya Full Medium - Recipe for the production of Ashbya full medium.
Azotobacter Agar - Agar appropriate for growth of Azobacter genus.
Bennett's Medium - media used for growth of some Actinoplanes species.
Bacillus agar - Agar used to grow some Bacillus species.
Bacillus broth - Agar used to grow some Bacillus species.
CA 03064942 2019-11-25
WO 2018/220630 PCT/IL2018/050588
20
Bacillus schlegelii Medium - Medium appropriate for the growth of Bacillus
schlegelii.
Bifidobacterium Medium - Recipe for Bifidobacterium medium.
Blue green algae agar - Recipe for blue green algae agar.
Blue green algae broth - Recipe for blue green algae broth.
Brain Heart Infusion Glucose Agar - Recipe for Brain Heart Infusion Glucose
Agar.
Caulobacter Agar - Recipe for Caulobacter Agar.
Caulobacter Medium - Recipe for Caulobacter Medium.
Cantharellus Agar Recipe - Recipe for Cantharellus agar.
CASO agar - Recipe for CASO agar.
Clostridium thermocellum Medium - Recipe for medium appropriate for growth of
Clostridium
thermocellum
Corynebacterium agar - Recipe for Corynebacterium agar.
Creatinine Medium - Recipe for the production of creatinine medium.
Czapek Agar (CZA) - Recipe for Czapek Agar (CZA).
Desulfovibrio Medium - Recipe for Desulfovibrio Medium.
Gluconobacter agar - Recipe for Gluconobacter agar.
Glucose Peptone Yeast Extract Agar (GPYA) - Recipe for Glucose Peptone Yeast
Extract Agar
(GPYA).
Glucose Yeast Extract Agar - Recipe for Glucose Yeast Extract Agar.
Halobacterium agar - Recipe for the preparation of Halobacterium agar.
CA 03064942 2019-11-25
WO 2018/220630 PCT/IL2018/050588
21
Halobacteria Medium - Recipe for Halobacteria Medium.
LB Agar - Recipe for the preparation of LB agar bacterial media.
LB broth - Recipe for the preparation of LB broth bacterial media.
LB broth (low salt) - Recipe for the preparation of low salt LB broth
bacterial media.
Luminous Medium - Recipe for Luminous Medium.
M17 media - Recipe for the preparation of M17 media.
M9 minimal media - Minimal salts bacterial media.
Mannitol agar - Recipe for mannitol agar.
Mannitol broth - Recipe for mannitol broth.
Marine agar - Recipe for marine agar. Used for the growth of several marine
bacteria.
Marine broth - Recipe for marine broth. Used for the growth of several marine
bacteria.
Methylamine Salts Agar - Recipe for methylamine salts agar
Methylamine Salts Medium - Recipe for methylamine salts medium
Modified Chopped Meat Medium - Used for the growth of several anaerobic
bacteria.
MY medium - Maltose yeast extract bacterial growth medium.
N4 Mineral Medium - Recipe for the production of N4 mineral medium.
Nitrosomonas europaea medium - Recipe for the production of Nitrosomonas
europaea medium.
Nutrient agar - Recipe for nutrient agar suitable for growth of many bacterial
species.
CA 03064942 2019-11-25
WO 2018/220630 PCT/IL2018/050588
22
Nutrient broth - Recipe for nutrient broth suitable for growth of many
bacterial species.
MRS media - Recipe for MRS media. MRS media has been used for the recovery of
lactic acid
bacteria (LAB) from various food products.
MS-Medium - Recipe for MS-medium.
N-Z amine agar with soluble starch and glucose - Agar used to grow some
Actinomadura species
NZCYM - NZ amine, NaCl, bacto-yeast extract, casamino acids, and magnesium
sulfate.
NZM - NZ amine, NaCl, and magnesium sulfate.
NZYM - NZ amine, NaCl, bacto-yeast extract, and magnesium sulfate.
Oatmeal agar - agar used to grow some Actinomadura species.
Oenococcus Medium - Recipe for the preparation of Oenococcus medium.
Osmophilic Agar - Recipe for Osmophilic Agar.
Osmophilic Medium - Recipe for Osmophilic Medium.
Phenol red lactose broth - turns yellow when lactose is fermented.
Potato-Carrot Medium - agar used to grow some Actinoplanes species.
Propionibacterium Agar Recipe - Agar appropriate for the growth of
Propionibacterium.
Propionibacterium Medium Recipe - Medium appropriate for the growth of
Propionibacterium.
PYS agar - agar used to grow some Actinomadura species.
R Medium - R Medium Recipe.
Rolled Oats Mineral Agar - Recipe for Rolled Oats Mineral Agar.
CA 03064942 2019-11-25
WO 2018/220630 PCT/IL2018/050588
23
Saccharose agar - Recipe for the production of saccharose agar
Saccharose medium - Recipe for the production of saccharose medium
SOB media - Tryptone/yeast extract bacterial media.
SOC media - Tryptone/yeast extract bacterial media.
5% Sorbitol agar - Recipe for the production of 5% sorbitol agar.
5% Sorbitol medium - Recipe for the production of 5% sorbitol medium.
Sour Dough Medium - Recipe for the preparation of sour dough medium.
Starch - Mineral Salt (STMS) Agar - Recipe for starch - mineral salt (STMS)
agar.
Styrene Mineral Salts Medium - Recipe for Styrene Mineral Salts medium.
Terrific broth - Recipe for the preparation of terrific broth bacterial media.
Thermus Agar - Recipe for agar appropriate for the growth of Therums genus
Thermus Medium - Recipe for media appropriate for the growth of Therums genus
Thiobacillus Medium F2 - Recipe for the production of Thiobacillus medium F2
Tomato Juice Agar - Recipe for the preparation of tomato juice agar.
Tomato Juice Medium - Recipe for the preparation of tomato juice medium.
Tomato Juice Yeast Extract Agar - Recipe for the preparation of tomato juice
yeast extract agar.
Tomato Juice Yeast Extract Medium - Recipe for the preparation of tomato juice
yeast extract
medium.
TSY agar - Trypticase soy yeast agar Recipe.
CA 03064942 2019-11-25
WO 2018/220630 PCT/IL2018/050588
24
TSY broth - Trypticase soy yeast broth Recipe.
TYG Medium - Tryptone, yeast, glucose bacterial growth medium.
TYX Medium - Tryptone, yeast, xylose bacterial growth medium.
Urea Medium - Recipe for the preparation of urea medium
Uric Acid Medium - Recipe for the preparation of uric acid medium
Whey Agar - Recipe for the preparation of whey agar.
Whey Medium - Recipe for the preparation of whey medium.
Wickerham Salt Agar - Recipe for Wickerham Salt Agar.
Wickerham Salt Medium - Recipe for Wickerham Salt Medium.
Yeast Extract Glucose Medium - Yeast Extract Glucose medium recipe
YEL Agar - Recipe for YEL Agar.
YMF agar recipe - Recipe for preparation of YMF agar.
YMF medium recipe - Recipe for preparation of YMF medium.
YMG agar - Recipe for yeast and malt extract with glucose agar. This agar is
used for a number
of Streptomyces species.
YMG media - Recipe for yeast and malt extract with glucose media. This media
is used for a
number of Streptomyces species.
YPD Agar - Yeast extract/peptone/dextrose bacterial agar.
YPD media - Yeast extract/peptone/dextrose bacterial media.
YPG media - Yeast extract/peptone/galactose bacterial media.
CA 03064942 2019-11-25
WO 2018/220630
PCT/IL2018/050588
25
YPM Agar - Recipe for YPM agar.
YPM Medium - Recipe for YPM medium.
YT (2x) - Yeast extract/tryptone bacterial media.
Thus, for example in the case of the beneficial bacteria being of the genus
lactobacillus
(e.g. the species L. plantarum) and the biofilm-producing bacteria being of
the genus Bacillus
(e.g. of the species B. subtilis), the co-culture may be carried out in a
growth substrate which
comprises LBGM, milk or MRS. Other media that can be used to generate the co-
culture of the
present invention include MSgg minimal medium (Shemesh, M., et al (2010). J
Bacteriol 192,
6352-6356); LB enriched with lactose: Duanis-Assaf D., et al (2016) Front.
Microbiol. 6:1517;
LB with addition of butyric acid: Pasvolsky R., et al., Int. J. Food
Microbiol. 181C:19-27.
Typically, the culturing conditions are selected that encourage incorporation
of both the different
bacteria into the biofilm.
The present inventors have uncovered particular components of a growth medium
that
are important for biofilm generation of bacteria being of the genus Bacillus
(e.g. of the species B.
subtilis) ¨ see Figure 21. Thus, the present inventors propose that the medium
used for co-
culturing a beneficial bacteria with Bacillus bacteria comprises manganese. In
another
embodiment, the medium comprises dextrose. In still another embodiment, the
medium used for
co-culturing comprises both manganese and dextrose.
Thus according to another aspect of the present invention there is provided a
method of
selecting an agent or culturing condition which is advantageous for preparing
a bacterial
composition, the method comprising co-culturing beneficial bacteria with a
biofilm-producing
bacteria in a growth substrate in the presence of the agent or under the
culturing condition so as
to generate a biofilm comprising the beneficial bacteria and the biofilm-
producing bacteria,
wherein a change in a property of the biofilm is indicative of the agent or
culturing condition
being advantageous for preparing the bacterial composition.
Exemplary conditions of the co-culture that may be altered include the
properties of the
surface on which the culture is carried out (for example the surface chemistry
of the solid
surface, including but not limited to functional groups, electrostatic charge,
coating; surface
roughness, surface topography, including but not limited to grooves, cavities,
ridges, pores,
hexagonally packed (HP) pillars, equilateral triangles surrounded by HP
pillars, and the Sharklet
topography etc.). The solid surface may be of a defined geometry and/or
topography such that it
CA 03064942 2019-11-25
WO 2018/220630
PCT/IL2018/050588
26
promotes encapsulation/incorporation of the beneficial bacteria into the
biofilm. Furthermore,
the solid surface may be of a defined geometry and/or topography such that it
promotes
generation of a biofilm of a particular thickness. Other topographical
patterns contemplated by
the present invention are described in Graham and Cady, Coatings, 2014, 4,
pages 37-59, the
contents of which are incorporated herein by reference.
Exemplary solid surfaces on which the culturing can be carried out include a
wide range
of substrates, ranging from various polymeric materials (silicone,
polystyrene, polyurethane, and
epoxy resins) to metals and metal oxides (silicon, titanium, aluminum, silica,
and gold).
Fabrication techniques (soft lithography and double casting molding
techniques, microcontact
printing, electron beam lithography, nanoimprint lithography,
photolithography,
electrodeposition methods, etc.) can be carried out on such materials in order
to alter the
topography of the solid surface.
Other conditions of the co-culture that may be altered include, but are not
limited to
environmental parameters such as pH, nutrient concentration, the ratio between
the beneficial
bacteria: biofilm producing bacteria and temperature.
In one embodiment, the co-culturing is carried out in a bioreactor.
As used herein, the term "bioreactor" refers to an apparatus adapted to
support the
biofilm of the invention.
The bioreactor will generally comprise one or more supports for the biofilm
which may
form a film thereover, and wherein the support is adapted to provide a
significant surface area to
enhance the formation of the biofilm. The bioreactors of the invention may be
adapted for
continuous throughput.
It will be appreciated that when the biofilm is generated in a bioreactor
system, the
conditions of the co-culture can be altered by altering the microfluidics
(e.g. sheer stress) of the
system.
As mentioned, the agents or conditions are selected that bring about an
advantageous
change in a property of the biofilm. In one embodiment, the property is an
amount of biofilm. In
one embodiment, the property is a thickness of biofilm. In another embodiment,
the property is a
density of the biofilm. In yet another embodiment, the property is the rate in
which the biofilm
is formed. In still another embodiment, the property is the amount of
beneficial bacteria which is
incorporated into the biofilm. In still another embodiment, the property is
the resistance to
temperature and/or pH.
In still another embodiment, the property is the amount of beneficial bacteria
released
from the biofilm over a period of time. This may be of particular relevance
when a controlled
CA 03064942 2019-11-25
WO 2018/220630
PCT/IL2018/050588
27
release of the beneficial bacteria is required. For example, it may be
advantageous to
incorporate bacteria which are beneficial for the skin, scalp or dental
applications in biofilms of
which the rate of release of the beneficial bacteria therefrom is selected for
maximum therapeutic
effect.
The present inventors have now found that altering the pH of the growth
substrate to
higher than 6, encourages bacteria that utilize the KinD-Spo0A pathway (e.g.
being of the genus
Bacillus, such as of the species B. subtilis) to be incorporated into a
biofilm when cultured in
MRS.
In one embodiment, the co-culturing of the beneficial bacteria being of the
genus
lactobacillus (e.g. the species L. plantarum) and the biofilm-producing
bacteria being of the
genus Bacillus (e.g. of the species B. subtilis), carried out in, or on LBGM,
milk or MRS (and
more specifically MRS) is effected at a pH of between 6.5 and 9; 6.5-and 8;
6.5 and 7.5; 6.8 and
9; 6.8 and 8; 6.8 and 7.5.
In a particular embodiment, when the co-culturing is effected in milk the
biofilm
producing bacteria is not B. subtilis MS1577 or 3610.
The co-culturing of this aspect of the present invention may be carried out in
the presence
of additional agents that serve to increase propagation of the bacteria and/or
enhance biofilm
formation. Such agents include for example acetoin.
The amount of acetoin and the timing of addition may be altered so as to
promote optimal
biofilm production. In one embodiment, about 0.01 - 5 % acetoin is used. In
another
embodiment, about 0.01 - 4 % acetoin is used. In another embodiment, about
0.01 ¨ 3 % acetoin
is used. In another embodiment, about 0.01 - 2 % acetoin is used. In another
embodiment, about
0.01 - 1 % acetoin is used. In another embodiment, about 0.01 ¨ 0.5 % acetoin
is used.
Thus, the present inventors contemplate a culture comprising acetoin, a
biofilm
comprising a bacillus bacteria and a culture medium. In one embodiment, the
culture medium is
one which is mentioned in Table 1 (for example LB).
In one embodiment, about 0.05 - 5 % acetoin is used. In another embodiment,
about 0.05
- 4 % acetoin is used. In another embodiment, about 0.05 ¨ 3 % acetoin is
used. In another
embodiment, about 0.05 - 2 % acetoin is used. In another embodiment, about
0.05 - 1 % acetoin
is used. In another embodiment, about 0.05 ¨ 0.5 % acetoin is used.
In one embodiment, about 0.1 - 5 % acetoin is used. In another embodiment,
about 0.1 -
4 % acetoin is used. In another embodiment, about 0.1 ¨ 3 % acetoin is used.
In another
embodiment, about 0.1 - 2 % acetoin is used. In another embodiment, about 0.1 -
1 % acetoin is
used. In another embodiment, about 0.1 ¨ 0.5 % acetoin is used.
CA 03064942 2019-11-25
WO 2018/220630
PCT/IL2018/050588
28
The co-cultures of this aspect of the present invention are propagated for a
length of time
sufficient to generate a biofilm which incorporates both the beneficial
bacteria and the biofilm
generating bacteria.
According to one embodiment the co-cultures are grown to maximal plateau
growth
phase of the beneficial bacteria, at which time they may be harvested for
maximal biofilm
production.
According to another embodiment the co-cultures are grown to maximal plateau
growth
phase of the biofilm-producing bacteria, at which time they may be harvested
for maximal
biofilm production.
Thus, the bacteria may be cultured for at least 3 hours, at least 6 hours, at
least 12 hours,
at least 24 hours, 2 days, 3 days, 4 days, 5 days, 6 days or 7 days or longer.
In one embodiment,
the bacteria are not cultured for longer than 1 week, 2 weeks, 3 weeks, 4
weeks, 5 weeks or 6
weeks.
Once sufficient quantities of beneficial bacteria are propagated (and
encapsulated in the
biofilm), the biofilm is harvested (i.e. removed from the growth substrate).
Following isolation from the growth substrate, the biofilm (and/or bacteria
incorporated
therein) may be subject to drying (i.e. dehydrating), freezing, spray drying,
or freeze-drying.
Preferably, the biofilm is treated in a way that preserves the viability of
the bacteria.
Thus, according to another aspect of the present invention there is provided a
bacterial
composition obtainable according to the methods described herein.
The biofilm-producing bacteria is present in the bacterial composition in an
amount of
from 103 to 1015 colony forming units per gram of the bacterial composition
(e.g. probiotic
composition).
The amount (in weight) of non-cellular material (e.g. exopolysaccharides
and/or amyloid
fibers) in the composition may be higher than the amount (in weight) of
cellular material (e.g.
bacterial cells). For example, the weight of non-cellular material (e.g.
exopolysaccharides and/or
amyloid fibers) in the composition may be at least 5 %, 10 %, 20 %, 30 %, 40
%, 50 %, 60 %, 70
%, 80 %, 90 % or 100 % higher than the weight of cellular material (e.g.
bacterial cells) in the
composition.
The amount (in weight) of non-cellular material (e.g. exopolysaccharides
and/or amyloid
fibers) in the composition may be lower than the amount (in weight) of
cellular material (e.g.
bacterial cells). For example, the weight of cellular material (e.g. bacterial
cells) in the
composition may be at least 5 %, 10 %, 20 %, 30 %, 40 %, 50 %, 60 %, 70 %, 80
%, 90 % or 100
CA 03064942 2019-11-25
WO 2018/220630
PCT/IL2018/050588
29
% higher than the weight of non-cellular material (e.g. e.. exopolysaccharides
and/or amyloid
fiber) in the composition.
Thus, the weight ratio of non-cellular material (e.g. exopolysaccharides):
bacterial cells in
the compositions described herein may be between 99:1 ¨ 1:99. In some
embodiments the
weight ratio of non-cellular material (e.g. exopolysaccharides): bacterial
cells in the compositions
described herein may be between 99:1 ¨ 50:50. In some embodiments the weight
ratio of non-
cellular material (e.g. exopolysaccharides): bacterial cells in the
compositions described herein
may be between 99:1 ¨ 70:30.
In one embodiment, the bacterial composition is a probiotic composition.
In some embodiments, the probiotic composition comprises from about 103 to
1015
colony forming units ("CFUs") of the biofilm-producing microorganism per gram
of finished
product. In some embodiments, the probiotic composition comprises from about
104 to about
1014 CFUs of the biofilm-producing microorganism per gram of finished product.
In some
embodiments, the probiotic composition comprise from about 105 to about 1015
CFUs of biofilm-
producing microorganism per gram of finished product. In some embodiments, the
probiotic
composition comprises from about 106 to 1011 colony forming units of the
biofilm-producing
microorganism per gram of finished product. In some embodiments, the probiotic
composition
comprises from about 102 to about 105 colony forming units of the biofilm-
producing
microorganism per gram of finished product.
It will be appreciated that at least 20 %, 30 %, 40 %, 50 %, 60 %, 70 %, 80 %,
90% or
more of the beneficial bacteria of the composition are viable (i.e.
propagate). Furthermore, at
least 20 %, 30 %, 40 %, 50 %, 60 %, 70 %, 80 %, 90% or more of the biofilm-
producing bacteria
of the composition are viable (i.e. propagate).
According to a particular embodiment, the bacterial composition is a probiotic
composition.
Exemplary beneficial bacteria that may be present in the probiotic composition
are those
that belong to the genus lactobacillus (as described herein above).
The probiotic composition may comprise additional beneficial bacteria such as
those
belonging to the Bifidobacterium genus. Contemplated species of
Bifidobacterium that may be
present in the probiotic composition of this aspect of the present invention
include, but are not
limited to Bifidobacterium ion gum, Bifidobacterium bifidum, Bifidobacterium
breve,
Bifidobacterium infantis, Bifidobacterium adolecentis, Bifidobacterium lactis,
and
Bifidobacterium animalis. In some embodiments, the probiotic composition
comprises a species
that belongs to the genus lactobacillus e.g. Lactobacillus plantarum and at
least two
CA 03064942 2019-11-25
WO 2018/220630
PCT/IL2018/050588
microorganisms selected from the following Bifidobacterium ion gum,
Bifidobacterium bifidum,
Bifidobacterium breve, Bifidobacterium infantis, Bifidobacterium adolecentis,
Bifidobacterium
lactis, and Bifidobacterium animalis.
In one embodiment, the bacterial compositions disclosed herein are in any form
suitable
5 for administering the composition to a mammalian subject. In some
embodiments, the
composition is in the form of a tablet, a powder or a liquid. If provided as a
powder, combining
the powder with a suitable liquid (e.g., liquid dairy product, fruit or
vegetable juice, blended fruit
or vegetable juice product, etc.) is specifically contemplated.
In some embodiments, the bacterial compositions disclosed herein are
administered to a
10 subject prior to, concomitant with or following administration of an
antibiotic agent. The
conditions of the co-culture may be such that the biofilm which is generated
releases the
beneficial bacteria in the body such that they are not subject to the activity
of the antibiotic
agent.
In some embodiment, the bacterial compositions described herein are formulated
for
15
topical administration - e.g. in a cream, a gel, a lotion, a shampoo, a
rinse. The bacterial
compositions may be administered to the skin or the scalp. The bacterial
compositions may be
useful for dental applications. For such applications they may be administered
to the gums.
In some embodiments the compositions described herein are incorporated into a
food
product. The term "food product" as used herein refers to any substance
containing nutrients that
20 can be ingested by an organism to produce energy, promote health and
wellness, stimulate
growth, and maintain life. The term "enriched food product" as used herein
refers to a food
product that has been modified to include the composition comprising
composition described
herein, which provides a benefit such as a health/wellness-promoting and/or
disease-
preventing/mitigating/treating property beyond the basic function of supplying
nutrients.
25 The probiotic composition can be incorporated into any food product.
Exemplary food
products include, but are not limited to, protein powder (meal shakes), baked
goods (cakes,
cookies, crackers, breads, scones and muffins), dairy-type products (including
but not limited to
cheese, yogurt, custards, rice pudding, mousses, ice cream, frozen yogurt,
frozen custard),
desserts (including, but not limited to, sherbet, sorbet, water-ices, granitas
and frozen fruit
30 purees), spreads/margarines, pasta products and other cereal
products, meal replacement
products, nutrition bars, trail mix, granola, beverages (including, but not
limited to, smoothies,
water or dairy beverages and soy-based beverages), and breakfast type cereal
products such as
oatmeal. For beverages, the probiotic composition described herein may be in
solution,
suspended, emulsified or present as a solid.
CA 03064942 2019-11-25
WO 2018/220630
PCT/IL2018/050588
31
In one embodiment, the enriched food product is a meal replacement product.
The term
"meal replacement product" as used herein refers to an enriched food product
that is intended to
be eaten in place of a normal meal. Nutrition bars and beverages that are
intended to constitute a
meal replacement are types of meal replacement products. The term also
includes products
which are eaten as part of a meal replacement weight loss or weight control
plan, for example
snack products which are not intended to replace a whole meal by themselves,
but which may be
used with other such products to replace a meal or which are otherwise
intended to be used in the
plan. These latter products typically have a calorie content in the range of
from 50-500
kilocalories per serving.
In another embodiment, the food product is a dietary supplement. The term
"dietary
supplement" as used herein refers to a substance taken by mouth that contains
a "dietary
ingredient" intended to supplement the diet. The term "dietary ingredients"
includes, but is not
limited to, the composition comprising the probiotic composition as described
herein as well as
vitamins, minerals, herbs or other botanicals, amino acids, and substances
such as enzymes,
organ tissues, glandulars, and metabolites.
In yet another embodiment, the food product is a medical food. The term
"medical food"
as used herein means a food which is formulated to be consumed or administered
entirely under
the supervision of a physician and which is intended for the specific dietary
management of a
disease or condition for which distinctive nutritional requirements, based on
recognized
scientific principles, are established by medical evaluation.
It is also well established that the addition of probiotic microorganisms to
animal feed
can improve animal efficiency and health. Specific examples include increased
weight gain-to-
feed intake ratio (feed efficiency), improved average daily weight gain,
improved milk yield, and
improved milk composition by dairy cows as described by U.S. Pat. Nos.
5,529,793 and
5,534,271. The administration of probiotic organisms can also reduce the
incidence of
pathogenic organisms in cattle, as reported by U.S. Pat. No. 7,063,836.
Thus, according to another embodiment, the probiotic composition described
herein can
be incorporated into an animal feed.
In one embodiment, the probiotic composition is designed for continual or
periodic
administration to ruminal, cecal or intestinal fermentors throughout the
feeding period in order to
reduce the incidence and severity of diarrhea and/or overall health. In this
embodiment, the
probiotic composition can be introduced into the rumen, cecum and/or
intestines of the animal.
In yet another embodiment, the probiotic composition described herein are
incorporated
into a pharmaceutical product or composition. Pharmaceutical compositions
comprise a
CA 03064942 2019-11-25
WO 2018/220630
PCT/IL2018/050588
32
prophylactically or therapeutically effective amount of the composition
described herein and
typically one or more pharmaceutically acceptable carriers or excipients
(which are discussed
below).
The disclosure contemplates formulations of the bacterial compositions
described herein
that are, in some embodiments, powdered, tableted, encapsulated or otherwise
formulated for
oral administration. The compositions may be provided as pharmaceutical
compositions,
nutraceutical compositions (e.g., a dietary supplement), or as a food or
beverage additive, as
defined by the U.S. Food and Drug Administration. The dosage form for the
above compositions
are not particularly restricted. For example, liquid solutions, suspensions,
emulsions, tablets,
pills, capsules, sustained release formulations, powders, suppositories,
liposomes, microparticles,
microcapsules, sterile isotonic aqueous buffer solutions, and the like are all
contemplated as
suitable dosage forms.
The compositions typically include one or more suitable diluents, fillers,
salts,
disintegrants, binders, lubricants, glidants, wetting agents, controlled
release matrices, colorings,
flavoring, carriers, excipients, buffers, stabilizers, solubilizers,
commercial adjuvants, and/or
other additives known in the art.
Any pharmaceutically acceptable (i.e., sterile and acceptably non-toxic as
known in the
art) liquid, semisolid, or solid diluent that serves as a pharmaceutical
vehicle, excipient, or
medium can be used. Exemplary diluents include, but are not limited to,
polyoxyethylene
sorbitan monolaurate, magnesium stearate, calcium phosphate, mineral oil,
cocoa butter, and oil
of theobroma, methyl- and propylhydroxybenzoate, talc, alginates,
carbohydrates, especially
mannitol, .alpha.-lactose, anhydrous lactose, cellulose, sucrose, dextrose,
sorbitol, modified
dextrans, gum acacia, and starch.
Pharmaceutically acceptable fillers can include, for example, lactose,
microcrystalline
cellulose, dicalcium phosphate, tricalcium phosphate, calcium sulfate,
dextrose, mannitol, and/or
sucrose. Salts, including calcium triphosphate, magnesium carbonate, and
sodium chloride, may
also be used as fillers in the pharmaceutical compositions.
Binders may be used to hold the composition together to form a hard tablet.
Exemplary
binders include materials from organic products such as acacia, tragacanth,
starch and gelatin.
.. Other suitable binders include methyl cellulose (MC), ethyl cellulose (EC)
and carboxymethyl
cellulose (CMC).
In some embodiments, an enriched food product further comprises a
bioavailability
enhancer, which acts to increase the absorption of the sorbable natural
product(s) by the body.
Bioavailability enhancers can be natural or synthetic compounds. In one
embodiment, the
CA 03064942 2019-11-25
WO 2018/220630
PCT/IL2018/050588
33
enriched food product comprising the composition described herein further
comprises one or
more bioavailability enhancers in order to enhance the bioavailability of the
bioactive natural
product(s).
Natural bioavailability enhancers include ginger, caraway extracts, pepper
extracts and
chitosan. The active compounds in ginger include 6-gingerol and 6-shogoal.
Caraway oil can
also be used as a bioavailability enhancer (U.S. Patent Application
2003/022838). Piperine is a
compound derived from pepper (Piper nigrum or Piper longum) that acts as a
bioavailability
enhancer (see U.S. Pat. No. 5,744,161). Piperine is available commercially
under the brand name
BioperineRTM (Sabinsa Corp., Piscataway, N.J.). In some embodiments, the
natural
bioavailability enhancers is present in an amount of from about 0.02% to about
0.6% by weight
based on the total weight of enriched food product.
Examples of suitable synthetic bioavailability enhancers include, but are not
limited to
surfactants including those composed of PEG-esters such as are commercially
available under
the tradenames: GelucireRTM, LabrafilRTM, LabrasolRTM, LauroglycolRTM, Pleurol
OleiqueRTM
(Gattefosse Corp., Paramus, N.J.) and CapmulRTM (Abitec Corp., Columbus,
Ohio).
The amount and administration regimen of the composition is based on various
factors
relevant to the purpose of administration, for example human or animal age,
sex, body weight,
hormone levels, or other nutritional need of the human or animal. In some
embodiments, the
composition is administered to a mammalian subject in an amount from about
0.001 mg/kg body
weight to about 1 g/kg body weight.
A typical regimen may comprise multiple doses of the composition. In one
embodiment,
the composition is administered once per day. The composition may be
administered to an
individual at any time. In some embodiments, the composition is administered
concurrently, or
prior to or at the consumption of a meal.
In some embodiments the bacterial compositions of this aspect of the present
invention
are formulated for use as an agricultural product. The bacterial compositions
may be added to an
agricultural carrier such as soil or plant growth medium. Other agricultural
carriers that may be
used include fertilizers, plant-based oils, humectants, or combinations
thereof. Alternatively, the
agricultural carrier may be a solid, such as diatomaceous earth, loam, silica,
alginate, clay,
bentonite, vermiculite, seed cases, other plant and animal products, or
combinations, including
granules, pellets, or suspensions. Mixtures of any of the aforementioned
ingredients are also
contemplated as carriers, such as but not limited to, pesta (flour and kaolin
clay), agar or flour-
based pellets in loam, sand, or clay, etc. Formulations may include food
sources for the cultured
organisms, such as barley, rice, or other biological materials such as seed,
leaf, root, plant
CA 03064942 2019-11-25
WO 2018/220630
PCT/IL2018/050588
34
elements, sugar cane bagasse, hulls or stalks from grain processing, ground
plant material or
wood from building site refuse, sawdust or small fibers from recycling of
paper, fabric, or wood.
Other suitable formulations will be known to those skilled in the art.
In one embodiment, the agricultural formulation comprises a fertilizer.
Preferably, the
fertilizer is one that does not reduce the viability of the bacterial
composition by more than 20 %,
30 %, 40 %, 50 % or more.
In some cases, it is advantageous for the agricultural formulation to contain
agents such
as herbicide, a nematicide, an insecticide, a plant growth regulator, a
rodenticide, and a nutrient.
Such agents are ideally compatible with the plant onto which the formulation
is applied (e.g., it
should not be deleterious to the growth or health of the plant). Furthermore,
the agent is ideally
one which does not cause safety concerns for human, animal or industrial use
(e.g., no safety
issues, or the compound is sufficiently labile that the commodity plant
product derived from the
plant contains negligible amounts of the compound).
The agricultural formulations comprising the biofilm of the present invention
typically
contains between about 0.1 to 95% by weight, for example, between about 1% and
90%,
between about 3% and 75%, between about 5% and 60%, between about 10% and 50%
in wet
weight of the biofilm-incorporated beneficial bacterial population of the
present invention. It is
preferred that the formulation contains at least about 102 CFU or spores per
ml of formulation, at
least about 103 CFU or spores per ml of formulation, at least about 104 CFU or
spores per ml of
formulation, at least about 105 CFU or spores per ml of formulation, at least
about 106 CFU or
spores per ml of formulation, or at least about 107 CFU or spores per ml of
formulation.
The present inventors also contemplate that the presently disclosed
agricultural
composition may be comprised in an article of manufacture which further
comprises an agent
which promotes the growth of plants.
The agents may be formulated together with the biofilm in a single
composition, or
alternatively packaged separately, but in a single container.
Suitable agents are described herein above. Other suitable agents include
fertilizers,
pesticides (an herbicide, a nematocide, a fungicide and/or an insecticide), a
plant growth
regulator, a rodenticide, and a nutrient, as further described herein below.
In one embodiment, the agent which promotes the growth of the plant lacks anti-
bacterial activity.
As used herein the term "about" refers to 10 %
The terms "comprises", "comprising", "includes", "including", "having" and
their
conjugates mean "including but not limited to".
CA 03064942 2019-11-25
WO 2018/220630
PCT/IL2018/050588
The term "consisting of' means "including and limited to".
The term "consisting essentially of" means that the composition, method or
structure may
include additional ingredients, steps and/or parts, but only if the additional
ingredients, steps
and/or parts do not materially alter the basic and novel characteristics of
the claimed
5 composition, method or structure.
As used herein, the singular form "a", "an" and "the" include plural
references unless the
context clearly dictates otherwise. For example, the term "a compound" or "at
least one
compound" may include a plurality of compounds, including mixtures thereof.
Throughout this application, various embodiments of this invention may be
presented in
10 a range format. It should be understood that the description in range
format is merely for
convenience and brevity and should not be construed as an inflexible
limitation on the scope of
the invention. Accordingly, the description of a range should be considered to
have specifically
disclosed all the possible subranges as well as individual numerical values
within that range. For
example, description of a range such as from 1 to 6 should be considered to
have specifically
15 disclosed subranges such as from 1 to 3, from 1 to 4, from 1 to 5, from
2 to 4, from 2 to 6, from
3 to 6 etc., as well as individual numbers within that range, for example, 1,
2, 3, 4, 5, and 6. This
applies regardless of the breadth of the range.
Whenever a numerical range is indicated herein, it is meant to include any
cited numeral
(fractional or integral) within the indicated range. The phrases
"ranging/ranges between" a first
20 indicate number and a second indicate number and "ranging/ranges from" a
first indicate
number "to" a second indicate number are used herein interchangeably and are
meant to include
the first and second indicated numbers and all the fractional and integral
numerals therebetween.
As used herein the term "method" refers to manners, means, techniques and
procedures
for accomplishing a given task including, but not limited to, those manners,
means, techniques
25 and procedures either known to, or readily developed from known manners,
means, techniques
and procedures by practitioners of the chemical, pharmacological, biological,
biochemical and
medical arts.
As used herein, the term "treating" includes abrogating, substantially
inhibiting, slowing
or reversing the progression of a condition, substantially ameliorating
clinical or aesthetical
30 symptoms of a condition or substantially preventing the appearance of
clinical or aesthetical
symptoms of a condition.
It is appreciated that certain features of the invention, which are, for
clarity, described in
the context of separate embodiments, may also be provided in combination in a
single
embodiment. Conversely, various features of the invention, which are, for
brevity, described in
CA 03064942 2019-11-25
WO 2018/220630
PCT/IL2018/050588
36
the context of a single embodiment, may also be provided separately or in any
suitable
subcombination or as suitable in any other described embodiment of the
invention. Certain
features described in the context of various embodiments are not to be
considered essential
features of those embodiments, unless the embodiment is inoperative without
those elements.
Various embodiments and aspects of the present invention as delineated
hereinabove and
as claimed in the claims section below find experimental support in the
following examples.
EXAMPLES
Reference is now made to the following examples, which together with the above
descriptions
illustrate some embodiments of the invention in a non limiting fashion.
Generally, the nomenclature used herein and the laboratory procedures utilized
in the present
invention include molecular, biochemical, microbiological and recombinant DNA
techniques.
Such techniques are thoroughly explained in the literature. See, for example,
"Molecular
Cloning: A laboratory Manual" Sambrook et al., (1989); "Current Protocols in
Molecular
Biology" Volumes I-III Ausubel, R. M., ed. (1994); Ausubel et al., "Current
Protocols in
Molecular Biology", John Wiley and Sons, Baltimore, Maryland (1989); Perbal,
"A Practical
Guide to Molecular Cloning", John Wiley & Sons, New York (1988); Watson et
al.,
"Recombinant DNA", Scientific American Books, New York; Birren et al. (eds)
"Genome
Analysis: A Laboratory Manual Series", Vols. 1-4, Cold Spring Harbor
Laboratory Press, New
York (1998); methodologies as set forth in U.S. Pat. Nos. 4,666,828;
4,683,202; 4,801,531;
5,192,659 and 5,272,057; "Cell Biology: A Laboratory Handbook", Volumes I-III
Cellis, J. E.,
ed. (1994); "Culture of Animal Cells - A Manual of Basic Technique" by
Freshney, Wiley-Liss,
N. Y. (1994), Third Edition; "Current Protocols in Immunology" Volumes I-III
Coligan J. E., ed.
(1994); Stites et al. (eds), "Basic and Clinical Immunology" (8th Edition),
Appleton & Lange,
Norwalk, CT (1994); Mishell and Shiigi (eds), "Selected Methods in Cellular
Immunology", W.
H. Freeman and Co., New York (1980); available immunoassays are extensively
described in the
patent and scientific literature, see, for example, U.S. Pat. Nos. 3,791,932;
3,839,153; 3,850,752;
3,850,578; 3,853,987; 3,867,517; 3,879,262; 3,901,654; 3,935,074; 3,984,533;
3,996,345;
4,034,074; 4,098,876; 4,879,219; 5,011,771 and 5,281,521; "Oligonucleotide
Synthesis" Gait,
M. J., ed. (1984); "Nucleic Acid Hybridization" Hames, B. D., and Higgins S.
J., eds. (1985);
"Transcription and Translation" Hames, B. D., and Higgins S. J., eds. (1984);
"Animal Cell
Culture" Freshney, R. I., ed. (1986); "Immobilized Cells and Enzymes" IRL
Press, (1986); "A
Practical Guide to Molecular Cloning" Perbal, B., (1984) and "Methods in
Enzymology" Vol. 1-
317, Academic Press; "PCR Protocols: A Guide To Methods And Applications",
Academic
CA 03064942 2019-11-25
WO 2018/220630
PCT/IL2018/050588
37
Press, San Diego, CA (1990); Marshak et al., "Strategies for Protein
Purification and
Characterization - A Laboratory Course Manual" CSHL Press (1996); all of which
are
incorporated by reference as if fully set forth herein. Other general
references are provided
throughout this document. The procedures therein are believed to be well known
in the art and
are provided for the convenience of the reader. All the information contained
therein is
incorporated herein by reference.
EXAMPLE 1
Biofilm Formation
MATERIALS AND METHODS
Strains and growth conditions: The probiotic bacterial strain used in this
study was
Lactobacillus plantarum. This strain routinely is grown in either MRS (Man,
Rogosa & Sharpe)
broth or MRS broth solidified using 1.5% agar (DifcoTm). The Bacillus subtilis
wild strain
NCIB3610 and its derivatives are typically cultured in LB (10 g of tryptone, 5
g of yeast extract,
5 g of NaCl per liter) broth or LB solidified with 1.5% agar. Prior to their
use, L. plantarum and
B. subtilis were grown on a hard agar plate for 48 h or overnight,
respectively, both at 37 QC C. A
starter culture of each strain was prepared using a single bacterial colony,
L. plantarum
inoculated into 5 mL MRS broth for 8 h without agitation and B. subtilis into
LB medium for 5
hours at 37 QC 150 rpm, until it reached an 0D600 of approximately 1.5. For co-
culture
experiments, MRS medium at pH 7 was used since it was found to be effective in
promoting
biofilm formation by B. subtilis and suitable for co-culture cultivation of B.
subtilis and probiotic
lactic acid bacteria (LAB). B. subtilis cells were mixed with an equal amount
of L. plantarum
cells to a final concentration of 108 cells/mL of each strain, and then
diluted 1:100 into MRS pH
7. The cells in mixed cultures were incubated aerobically at 37 C at 50 rpm
for 7-8 h.
Mono-species biofilms of B. subtilis were generated in MRS (Hy-lab) medium or
in MRS
supplemented with LB at different ratio (1:5, 1:2, 5:1) at 30 C. In order to
determine optimal
conditions for Bacillus strains to form biofilm, the pH of MRS was gradually
elevated from 6 to
8 using 1M NaOH. All strains used in this study are listed in Table 2 and are
isogenic unless
otherwise indicated.
CA 03064942 2019-11-25
WO 2018/220630
PCT/IL2018/050588
38
Table 2
Strain Genotype
NCIB3610 Undomesticated wild strain of
B. subtilis capable of forming
robust biofilms
RL4566 zlkinA::tet
RL4563 zlkinB::kan
RL4565 AkinC: .cat
RL4569 zlkinD::mls
RL4570 zlkinE::mls
RL4573 AkinA::tet, zlkinB::kan
RL4577 AkinC: :cat, zlkinD::mls
RL4620 Aspo0A:kan
RL4582 PtapA-/acZ at the amyE locus
in 3610, SpecR
YC668 zlabrB: :kan
B.paralichernformis MS 303
B.licheniformis MS310
B. lichernformis S127
B. subtilis MS1577
B. cereus 10987
Assay for colony and pellicle biofilm formation: For colony architecture
analysis, 3 pL
of starter cultures were spotted onto MRS agar plates or control LB and
incubated at 30 C for
72 h. For pellicle formation analysis, the starter cultures were diluted 1:100
into 3.5 mL MRS
broth or control LB in a 12-well plates and incubated without agitation at 30
C for 48 h. Images
were taken using a Zeiss Stemi 2000-C microscope with an axiocam ERc 5s camera
(Zeiss,
Germany).
13-galactosidase assay: Cells were harvested from colonies grown in either LB,
LB
supplemented with MRS in different ratio (1:1, 1:5, and 5:1) or MRS with pH
adjustment to 7 on
solid medium at 30 C and resuspended in phosphate-buffered saline (PBS)
solution. Typical
long bundled chains of cells in the biofilm colony were disrupted using mild
sonication. The
optical density (OD) of the cell samples were normalized to an 0D600 of 1.0 in
PBS. One
CA 03064942 2019-11-25
WO 2018/220630
PCT/IL2018/050588
39
milliliter of bacterial cell suspensions were collected and assayed according
to standard
procedure.
Growth curve analysis of L. plantarum during growth in co-culture: Overnight
cultures
of B. subtilis and L. plantarum were grown in LB or MRS, respectively, to the
stationary phase
.. and diluted 1:100 into 25 mL of modified MRS broth with an elevated pH (up
to 7). Co-culture
samples generated as described above were grown for 8 h aerobically at 37 C
and 150 rpm. B.
subtilis and L. plantarum mono-species cultures were also prepared and used as
control samples.
Every hour, 1 mL was collected from each culture for microbial counting by
colony forming
units (CFU) count method. This was done by making appropriate dilutions using
PBS buffer and
.. plating them on MRS agar. The plates were incubated aerobically at 37 C for
48 h.
Visualizing biofilm forming cells using confocal laser scanning microscopy
(CLSM):
L. plantarum cells were grown in co-culture as described above with B.
subtilis (YC161)
aborting GFP or B. subtilis (YC189) aborting CFP in modified MRS broth. Cell
suspensions of
each bacterium grown as monospecies culture served as control samples. One
milliliter of each
culture was collected and centrifuged at 5000 rpm for 2 minutes. After
removing supernatant, the
cells were washed with 1 mL of PBS buffer and then following centrifugation
(at 5000 rpm for 2
minutes) resuspended in 100 1 of the same buffer. 5 1 from each sample were
placed on a
microscopy glass slide and visualized in a transmitted light microscope using
Nomarski
differential interference contrast (DIC).
Scanning electron microscopy (SEM) analysis: The cells of co-culture grown as
described above were placed on glass slides coated with poly-lysine for
overnight. Afterwards,
glass slides were washed twice using DDW to remove unattached cells and medium
remnants.
The slides were exposed to 40 pi of 4% formaldehyde and incubated for 15 min
at room
temperature. The glass slides were washed once again using DDW and analyzed by
SEM.
Analysis of survival rates following heat and cold treatment: Co-culture
samples
generated as described above were grown for 7-8 h aerobically at 37 C and 50
rpm. L.
plantarum cells grown as a monoculture were used as a control. The samples
were taken to
challenge tests such as heat or cold treatments. The samples were taken prior
and post treatment,
sonicated to break biofilm bundles (Time: 20 sec, Pulse: 10 sec, Pause: 5 sec,
Amp: 30 %) and
conducted to CFU counting on MRS agar plates.
Analysis of L. plantarum survival during transition within in vitro digestion
system: In
order to study the survival ability of L. plantarum during transition in the
gastro-intestinal tract,
samples of L. plantarum in mono-culture and co-culture with B. subtilis cells
were monitored for
4 h using in vitro digestion model (Minekus et al., 2014). To simulate the
gastric phase of
CA 03064942 2019-11-25
WO 2018/220630
PCT/IL2018/050588
digestion, 5 mL aliquot of suspension from each sample were mixed 1:1 with
simulated gastric
fluid (SGF) up to a final volume of 10 mL. Porcine pepsin (SIGMA P9700) was
added to
achieve 2000 U mL-1 in the final digestion mixture, followed by CaCl2 to
achieve 0.075 mM in
the final digestion mixture. The pH was reduced to 3.0 with 1M HC1 and the
samples were
5 placed in a water bath with a magnetic stirrer for 2 h at 37 C. Each
sample was divided into 2
tubes each containing 5 mL. 50 1 of PMSF (phenylmethylsulfonyl fluoride; SIGMA
P7626) was
added to 1 tube to stop the reaction and then the survivability of L.plantarum
was checked. The
other tube was used in the next digestion phase- the intestinal. To simulate
intestinal phase of
digestion, 2.5 mL of gastric chyme was mixed 1:1 with simulated intestinal
fluid (S IF) up to a
10 final volume of 5mL. 1M NaOH was added to neutralize the mixture to pH
7.0 and pancreatic
enzymes were added to the digestion mixture to achieve following activities in
the final mixture:
porcine trypsin (SIGMA T0303) (100 U mL-1), bovin chymotrypsin (SIGMA C4129)
(25 U mL-
-
1), porcine pancreatic a amylase (SIGMA A3176) (200 U mL 1), porcine
pancreatic lipase
(SIGAM L3126) (2000 U mL-1). In addition, bile salts (SIGMA T4009) were added
to give a
15 .. final concentration of 10 mM in the final mixture and then the samples
were incubated again for
2.5 h. One milliliter from each sample collected after gastric and intestinal
phases and the
numbers of viable L. plantarum cells were determined using CFU counting method
as described
above.
20 RESULTS
Development of a system for mutual growth of B. subtilis and L. plantarum in
co-culture
It was previously shown that biofilms have an increased tolerance toward
various
unfavorable environmental conditions, apparently due to production of
extracellular matrix
(Friedman, Kolter, & Branda, 2005). The present inventors thus hypothesized
that extracellular
25 matrix produced by robust biofilm former bacterium B. subtilis may
provide increased protection
to other species such as probiotic bacteria during their growth in co-culture
biofilm system. To
this end, a specialized medium was developed where L. plantarum and B.
subtilis are able to
grow in co-culture. It was found that by modifying the pH of the MRS to pH 7,
it was possible to
grow these bacteria in co-culture. As shown in Figure 13, the co-culture
cultivation had no effect
30 on L. plantarum and B. subtilis growth (compared to their growth in pure
culture), indicating that
there are no antagonistic interactions between these bacteria at given
conditions. Surprisingly, it
was found that modification of MRS medium promotes strong biofilm formation by
B. subtilis
(Figure 2). Since B. subtilis appears to be sensitive to acidic pH, the pH of
MRS medium used
for co-culture cultivation was gradually elevated in order to find a pH value
suitable for Bacillus
CA 03064942 2019-11-25
WO 2018/220630
PCT/IL2018/050588
41
growth. The increase of pH from 6 to 8 led to a proportional increase in
robustness of biofilm
phenotype of both colony and pellicle biofilm (Figure 2). When the pH was
adjusted to 6 weak
growth on solid MRS medium was seen and no growth in liquid medium. With pH
adjustment to
6.5, not only bacterial growth in both solid and liquid MRS was observed, but
surprisingly the
beginning of biofilm formation on solid medium was also observed. Following an
increase of pH
to 7 and 8, an extremely robust biofilm phenotype in both growth setups was
observed. Next, the
growth rates of B. subtilis in MRS pH 7 and LB were compared. As can be seen
in Figure 13, a
minor delay was observed at the beginning of microbial growth in MRS compare
to LB.
However, higher rate growth was noted later for B. subtilis cells grown in MRS
compare to LB.
The modified MRS medium promotes biofilm formation and matrix gene expression
through
KinD-Spo0A pathway
To evaluate the potential of MRS medium in promoting biofilm development and
matrix
genes expression, LB medium (that is usually used to culture B. subtilis) was
enriched with
different amounts of MRS (1:1, 1:5, and 5:1). Directly proportional
correlation between biofilm
.. phenotype and increase in MRS concentration was shown (Figure 3). The
effect of increasing
MRS concentration on matrix gene expression in B. subtilis using tapA and eps
operons was also
investigated, since their products are major components of extracellular
matrix. It was found that
the expression of tapA increased proportionally with the concentration of MRS
in LB (Figures
4A-B). The expression of eps increased proportionally to the concentration of
MRS up to 80%
MRS, than a decrease of expression for 100% was detected (Figures 5A-B).
Next, the present inventors determined whether MRS triggers biofilm formation
through
the Kin-Spo0A pathway previously described for B. subtilis (Shemesh and Chai,
2013 Journal of
Bacteriology, 2013, Vol 195, No.12 pages 2747-2754). They tested different B.
subtilis mutants
for biofilm formation (zIkinA, AkinB, AkinC, AkinD, AkinE, AkinAB, AkinCD,
Aspo0A,
.. zlepszltasA) or overproducing biofilm (zIabrB). Firstly, they determined
biofilm phenotype of
mutants deficient in histidine kinases responsible for sensing environmental
signals that induce
biofilm formation. They found that single mutants in either kinases did not
show significant
defect in biofilm phenotype, although the zlkinC and AkinD mutants showed a
slight decrease in
biofilm formation compared to control (Figure 14). However, the AkinCD double
mutant showed
the total abolishment of biofilm phenotype (Figure 5A). On the other hand,
double mutation in
AkinAB did not prevent biofilm formation, although some changes were observed
in biofilm
phenotype (in case of colony type biofilm). Mutation in master transcriptional
regulator spo0A as
well as double mutation in eps and tasA fully abolished biofilm formation
(Figure 5A). Mutation
CA 03064942 2019-11-25
WO 2018/220630
PCT/IL2018/050588
42
in the transcriptional repressor ZlabrB did not lead to an additional increase
in biofilm formation
compared to control WT cells (Figure 5B). This result emphasize once again the
dramatic
increase in matrix production during growth of B. subtilis WT cells in
modified MRS medium.
In order to investigate whether the biofilm-promoting effect of MRS is
conserved among
Bacillus species, other B. subtilis strains were tested as well as other
Bacillus species. A biofilm
promoting effect was seen as judged by wrinkled colonies (Figure 6) and robust
floating pellicles
(Figure 7).
Growth of B. subtilis and L. plantarum in co-culture results in dual species
biofilm
development
The modified MRS medium was used to investigate dual species biofilm by co-
culturing
fluorescently tagged B. subtilis cells, which constitutively express GFP
(YC161), together with
L. plantarum cells. Generated biofilm was visualized using CLSM. As can be
seen in Figure 8A
(top panel), the generated biofilm consisted of both fluorescent and non-
fluorescent cells. L.
plantarum cells were surrounded by B. subtilis cells which attached to each
other to form a
biofilm-related structure (bundle). This is further illustrated in Figure 8B
which illustrates the
co-cultured biofilm of B. subtilis and L. plantarum in LBGM medium.
Since biofilm formation in B. subtilis depends on the synthesis of
extracellular matrix,
the present inventors sought to determine whether the production of
extracellular matrix takes
place during dual species biofilm development. The level of the matrix gene
expression in the
formed biofilm was analyzed using transcriptional fusion of the promoter for
tapA-sipW-tasA
(operon responsible for synthesis of protein components of biofilm matrix in
B. subtilis) to the
cfp gene encoding cyan fluorescent protein (YC189), as described previously
(Shemesh, Kolter,
& Losick, 2010, J Bacteriol 192, 6352-6356) (PtapA-cfp). Notable CFP
expression was observed,
indicating that the tapA-sipW-tasA operon is been activated and therefore
matrix production was
induced in the dual species biofilm (Figures 8A-B, bottom panel). To determine
whether L.
plantarum cells could be surrounded with extracellular polymeric substances
derived from B.
subtilis biofilm formation, the dual species biofilm was analyzed using SEM
(Figures 9A-C).
The obtained images (Figure 9C) demonstrate formation of 3-dimentional and
heterogeneous
structure of biofilm where L. plantarum cells appeared to be incorporated
within the extracellular
matrix produced by B. subtilis. Importantly, B. subtilis cells grown as
monoculture form also
biofilm characterized with homogenous structure in which long filaments of the
cells are bound
together by an extracellular matrix (Figure 9A). In contrast, the L. plantarum
cells could not
form notable biofilm in monospecies culture. The observations described above
indicate that the
CA 03064942 2019-11-25
WO 2018/220630
PCT/IL2018/050588
43
extracellular matrix produced by B. subtilis cells could be shared with L.
plantarum cells and
thus provide them with possible protection against environmental stresses.
The dual species biofilm facilitates survival of L. plantarum in hostile
environments
In order to determine whether the matrix produced by B. subtilis in the co-
culture biofilm
might provide defense to L. plantarum against unfavorable environment
conditions, the survival
of L. plantarum cells was tested during heat treatment (conditions that
simulate industrial
processing such as pasteurization) as well as during refrigerating (conditions
that simulates
storage conditions). For heat treatment pasteurization, L. plantarum cells
grown in co-culture
biofilm were exposed to heating at 63 C for 1 and 3 min. For cold treatment,
L. plantarum cells
grown in co-culture biofilm were stored for up to 21 days at 4 C. L.
plantarum cells that grew in
monospecies culture were used as control. Following 1 and 3 min of heat
treatment, L.
plantarum cells grown in co-culture biofilm resulted in an increase of around
1.25 Log CFU/mL
and 1.06 Log CFU/mL, respectively, in the number of viable L. plantarum cells,
compare to
control (Figures 10A-B). Furthermore, the results from the cold treatment
experiment showed
that L. plantarum cells grown in co-culture biofilm were much more protected
throughout the
storage conditions demonstrating an increase of around 0.44 to 0.89 Log CFU/mL
in their
viability (Figures 10A-B).
Extracellular matrix produced during formation of dual species biofilm
facilitates survival of
L. plantarum during heat treatment
To further prove that increased resistance of L. plantarum to unfavorable
environment
conditions is facilitated by extracellular matrix, co-cultures of L. plantarum
and B. subtilis
mutant strains (either deficient in biofilm formation (zlepszltasA) or an
overproducing biofilm
matrix (zIabrB)) were generated. The co-cultures were subjected to heat
treatment pasteurization.
L. plantarum cells grown in mono-species culture and in co-culture with wild
type B. subtilis
were used as control. As shown in Figure 11A, L. plantarum cells grown with
the cells of
zlepszltasA double mutant did not show a significant difference in their
survival level compare to
L. plantarum grown in mono-species culture. However, a significant increase in
survival of L.
plantarum cells grown in co-culture with wild type B. subtilis was observed.
Interestingly, an
increase of about 1.78 Log CFU/mL in survival rates of the L. plantarum cells
grown in the
presence of zlabrB mutant cells, compared to survival rates of L. plantarum
grown in mono-
culture was observed.
CA 03064942 2019-11-25
WO 2018/220630
PCT/IL2018/050588
44
In another experiment, the samples were grown in milk for 18 hours at 30 C,
20 rpm.
Afterwards they were heat treated at 63 C for 1 to 3 min. Control samples were
not heat treated.
The number of viable L. plantarum cells was determined using CFU-method.
*p<0.05. As
illustrated in Figure 11B, B. subtilis biofilm facilitates L. plantarum
survival during heat in milk.
Extracellular matrix produced during formation of dual species biofilm
facilitates survival of
L. plantarum under the conditions resembling the human digestion system
In order to study the survival ability of L. plantarum during transition in
the gastro-
intestinal tract, the survival rate of L. plantarum cells was examined using
an in vitro digestion
model (Figure 12). After 2 h of incubation in simulate gastric conditions, an
increase in viable
cell concentration of around 0.86 Log CFU/mL was observed for L. plantarum
cells grown in
co-culture biofilm with B. subtilis, compared to mono-culture L. plantarum
cells. Afterwards,
cells were incubated 2 h under simulated intestinal conditions and increase of
around 0.9 Log
CFU/mL in viable cell concentration was observed for L. plantarum cells
protected by biofilm,
compared to free living L. plantarum cells.
EXAMPLE 2
Acetoin enhances biofilm formation
Food products are often enriched by different food additives which may improve
organoleptic and sensory characteristics of the products. Among those
additives there are
important small molecules such as acetoin which can improve the flavor of
different food
products. Acetoin is a neutral molecule which widely exists in nature. Some
microorganisms,
higher plants, insects, and higher animals have the ability to synthesize
acetoin. Those additives
can affect the physiology of many bacteria associated with human health, and
affect
development of multicellular community of bacterial cells known as a biofilm.
Biofilm
formation depends on the synthesis of an extracellular matrix that holds the
constituent cells
together. In Bacillus subtilis, a prebiotic bacteria, the matrix has two main
components, an
exopolysaccharide synthesized by the products of the epsA-0 operon, and
amyloid fibers
encoded by tapA-sipW-tasA operon.
RESULTS
As illustrated in Figures 15A-C, acetoin triggers the biofilm bundles
formation in
Bacillus subtilis. In the absence of acetoin, no biofilm formation is observed
when grown in LB
medium (Figure 15A). Figures 16A-B illustrate that acetoin triggers a colony
type biofilm
CA 03064942 2019-11-25
WO 2018/220630
PCT/IL2018/050588
formation in Bacillus subtilis. Transcription of the tapA operon responsible
for the matrix
production in B. subtilis was shown to be highly upregulated by acetoin
(Figures 17A-D).
The results indicate that cells of B. subtilis develop into a complex bundle
during growth
in the presence of acetoin. The cells express high levels of the extracellular
matrix components,
5 in response to acetoin, which are crucial for biofilm formation.
EXAMPLE 3
The objective of this experiment was to test the ability of NCIB3610 (isolated
from soil)
and 127185/2 (isolated from dairy environment) to protect L. plantarum against
hostile
10 environmental conditions during growth in co-culture system.
MATERIALS AND METHODS
The growth medium selected for the co-culture system of B. subtilis and L.
plantarum was
modified (pH adjusted) MRS medium.
Characterization of biofilm formation was performed using a stereoscopic
microscope or
15 confocal laser scanning microscope (for colony or bundles type biofilm,
respectively).
The experiments that examined the survival of L. plantarum grown in co-culture
biofilm with
B. subtilis during transition in vitro model of GI tract and in exposure to
low pH were performed
using CFU method, as described herein above.
RESULTS
20 Figures 18A-B are photographs depicting the biofilm generated from the
B. subtilis
strains NCIB3610 and 127185/2 respectively.
The L. plantarum count that survived in the co-culture with NCIB3610 was
higher than
L. plantarum that grew in mono-culture. This effect was enhanced when the
culture was shaken
(Figure 19). Furthermore, the number of L. plantarum that survived co-cultures
with NCIB3610
25 or 127185/2 under acidic conditions was 30 times greater than the single
culture grew at the
same conditions (Figure 20).
EXAMPLE 4
In order to elucidate a key component of MRS medium for triggering biofilm
development,
30 we analyzed the contribution of each component ¨ Mg2+, Mn2+, sodium
acetate, dipotassium
phosphate, dextrose, ammonium citrate ¨ involved in colony-type biofilm
formation by B.
subtilis. Interestingly, the most defective biofilm phenotype was observed in
the absence of
Mn2+: B. subtilis could not form a developed pellicle as well as colony-type
biofilm on MRS
medium un-supplemented with Mn2+ (Figure 21). It is noticeable that the
biofilms generated in
CA 03064942 2019-11-25
WO 2018/220630
PCT/IL2018/050588
46
the absence of dextrose showed some inhibition, with a wrinkling phenotype,
whereas those
generated in the absence of Mn2+ were completely flat (Figure 21). These
results led as to
conclude that the presence of Mn2+ in MRS medium is most crucial for biofilm
development by
B. subtilis.
Although the invention has been described in conjunction with specific
embodiments
thereof, it is evident that many alternatives, modifications and variations
will be apparent to those
skilled in the art. Accordingly, it is intended to embrace all such
alternatives, modifications and
variations that fall within the spirit and broad scope of the appended claims.
All publications, patents and patent applications mentioned in this
specification are herein
.. incorporated in their entirety by reference into the specification, to the
same extent as if each
individual publication, patent or patent application was specifically and
individually indicated to
be incorporated herein by reference. In addition, citation or identification
of any reference in this
application shall not be construed as an admission that such reference is
available as prior art to
the present invention. To the extent that section headings are used, they
should not be construed
as necessarily limiting.