Note: Descriptions are shown in the official language in which they were submitted.
CA 03064953 2019-11-19
WO 2018/218107 PCT/US2018/034571
TITLE: STABILIZED COMPOSITIONS FOR THE CONTROLLED DELIVERY OF
PROBIOTICS AND METHODS OF PRODUCTION THEREOF
CROSS-REFERENCE TO RELATED APPLICATION
[001] This application claims the benefit of priority of U.S. Provisional
Patent
Application No. 62/511,374 titled "Stabilized Compositions for the Controlled
Delivery of
Probiotics and Methods of Production Thereof," filed on May 26, 2017, which is
incorporated
herein in its entirety by this reference.
BACKGROUND OF THE DISCLOSURE
[002] Probiotics¨beneficial microorganisms that perform immune modulating
functions in humans¨have been used both topically and consumed internally to
promote general
health and aid in healing. Numerous studies have demonstrated the
effectiveness of probiotics in
promoting human health by aiding in metabolic functions, destroying
microorganisms that are
harmful to human health, and preventing the colonies of bad microorganisms
from developing
into problematic diseases or infections. Probiotics must be alive to perform
these activities that
promote human health.
[003] Probiotics occur naturally, but they are frequently depleted through
repeated
washing, infections of bad microorganisms, and weakened immune systems. It can
take days or
even weeks for probiotics to redevelop naturally. During this time, bad
microorganisms may
proliferate unchecked and cause a variety of health problems. Thus, it is
beneficial and desirable
to replenish depleted probiotics with live probiotic cultures.
[004] However, delivering live probiotic cultures commercially presents
challenges.
1
CA 03064953 2019-11-19
WO 2018/218107 PCT/US2018/034571
Live probiotic cultures are comprised of numerous different species of
microorganisms that
compete with each other for resources. Probiotics generally multiply
uncontrollably and
unpredictably. Thus, products with live probiotic cultures have short shelf-
lives and must be kept
refrigerated to ensure that the desired microorganism species are delivered in
the desired
proportions and quantities.
[005] There are many products on the market that purport to deliver
probiotic, but few
deliver live probiotic cultures, and none deliver live probiotic cultures and
remain shelf-stable
over a period of months without refrigeration. There remains a need for a
composition capable of
delivering controlled quantities and proportions of probiotics that remains
shelf-stable over a
long period of time without the need for refrigeration.
SUMMARY OF THE INVENTION
[006] The present invention provides stabilized compositions for the
controlled delivery
of probiotics and methods of producing such compositions. Specifically, the
stabilized
compositions of the present invention incorporate microencapsulated probiotic
beads into a
stable hydrogel matrix that release probiotics upon contact with any tissue.
The stabilized
composition of the present invention allows for a desired combination of
various probiotics to
remain alive in the quantities and proportions desired. Thus, the present
invention allows the
controlled delivery of probiotics tailored for specific applications.
[007] In one embodiment of the present invention a stabilized composition
for the
controlled delivery of probiotics may include an aqueous solution of
crystalloids having an
osmolality between about 150 mOsm/L and about 500 mOsm/L, wherein the aqueous
solution
comprises between about 55% to about 95% of the composition by weight. The
composition may
2
CA 03064953 2019-11-19
WO 2018/218107 PCT/US2018/034571
further include a colloid comprised of water insoluble polymers with the
ability to de-swell upon
electrical charge, wherein the colloid comprises between about 0.01% to about
1% of the
composition by weight. The composition may further include a plurality of oil
droplets, wherein
the plurality of oil droplets comprise between about 1% to about 50% of the
composition by
weight. The composition may further include a base solution, wherein the base
solution
comprises between about 0.01% to about 1% of the composition by weight. The
composition
may further include a destabilizing solution with an osmolality between 190
mOsm/L and about
900 mOsm/L comprising a solvent, about 0.05% to about 2% nitrogen by weight, a
natural acid
sufficient to produce a pH of about 3.0 to about 4.5, and a sufficient amount
of a salt to
contribute to an osmolality between about 190 mOsm/L and about 900 mOsm/L. The
composition may further include a plurality of microencapsulated probiotic
beads, wherein the
plurality of microencapsulated probiotic beads comprises between about 0.01%
to about 10% of
the composition by weight.
[008] In another embodiment of the present invention, a method of
producing a
stabilized composition for the controlled delivery of probiotics may include
mixing an aqueous
solution of crystalloids having an osmolality between 150 mOsm/L and about 900
mOsm/L with
a colloid comprised of water insoluble polymers with the ability to de-swell
upon electrical
charge to form a hydrogel mixture, wherein the aqueous solution comprises
between about 55%
to about 95% of the composition by weight and the colloid comprises between
about 0.01% to
about 1% of the composition by weight. The method may further include adding a
plurality of oil
droplets to the hydrogel mixture, wherein the plurality of oil droplets
comprise between about
1% to about 50% of the composition by weight. The method may further include
adjusting the
pH of the hydrogel mixture to about 6.2 to about 7.2 by adding a base
solution, wherein adjusting
3
CA 03064953 2019-11-19
WO 2018/218107 PCT/US2018/034571
the pH of the hydrogel mixture causes the lipophilic portions of the water
insoluble polymers to
surround the plurality of oil droplets, creating a stable gel matrix
interspersed with a plurality of
oil droplets, and wherein the base solution comprises between about 0.01% to
about 1% of the
composition by weight. The method may further include partially destabilizing
the gel matrix by
adding a destabilizing solution with an osmolality between 190 mOsm/L and
about 900 mOsm/L
comprising a solvent, about 0.05% to about 2% nitrogen by weight, a natural
acid sufficient to
produce a pH of about 3.0 to about 4.5, and a sufficient amount of a salt to
contribute to an
osmolality between about 190 mOsm/L and about 900 mOsm/L, wherein adding a
concentrated
salt solution causes some of the lipophilic portions of the water insoluble
polymers to detach
from the oil droplets. The method may further include adding a plurality of
microencapsulated
probiotic beads to the hydrogel mixture, wherein the microencapsulated
probiotic beads bind to
the detached lipophilic portions of the water insoluble polymers, and wherein
the
microencapsulated probiotic beads comprises between about 0.01% to about 20%
of the
composition by weight.
[009] In another embodiment of the present invention, a stabilized
composition for the
controlled delivery of probiotics is provided, wherein the composition is
produced by a method
that may include mixing an aqueous solution of crystalloids having an
osmolality between 150
mOsm/L and about 500 mOsm/L with a colloid comprised of water insoluble
polymers with the
ability to de-swell upon electrical charge to form a hydrogel mixture, wherein
the aqueous
solution comprises between about 55% to about 95% of the composition by weight
and the
colloid comprises between about 0.01% to about 1% of the composition by
weight. The method
may further include adding a plurality of oil droplets to the hydrogel
mixture, wherein the
plurality of oil droplets comprise between about 1% to about 50% of the
composition by weight.
4
CA 03064953 2019-11-19
WO 2018/218107 PCT/US2018/034571
The method may further include adjusting the pH of the hydrogel mixture to
about 6.2 to about
7.2 by adding a base solution, wherein adjusting the pH of the hydrogel
mixture causes the
lipophilic portions of the water insoluble polymers to surround the plurality
of oil droplets,
creating a stable gel matrix interspersed with a plurality of oil droplets,
and wherein the base
solution comprises between about 0.01% to about 1% of the composition by
weight. The method
may further include partially destabilizing the gel matrix by adding a
destabilizing solution with
an osmolality between 190 mOsm/L and about 900 mOsm/L comprising a solvent,
about 0.05%
to about 2% nitrogen by weight, a natural acid sufficient to produce a pH of
about 3.0 to about
4.5, and a sufficient amount of a salt to contribute to an osmolality between
about 190 mOsm/L
and about 900 mOsm/L, wherein adding a concentrated salt solution causes some
of the
lipophilic portions of the water insoluble polymers to detach from the oil
droplets. The method
may further include adding a plurality of microencapsulated probiotic beads to
the hydrogel
mixture, wherein the microencapsulated probiotic beads bind to the detached
lipophilic portions
of the water insoluble polymers, and wherein the microencapsulated probiotic
beads comprises
between about 0.01% to about 10% of the composition by weight.
[0010] In yet another embodiment of the present invention, the
crystalloids are mineral
salts, bicarbonates, or other water soluble elements and molecules.
[0011] In yet another embodiment of the present invention, the water
insoluble polymers
are organic polymers.
[0012] In yet another embodiment of the present invention, the water
insoluble polymers
are synthetic polymers.
[0013] In yet another embodiment of the present invention, the plurality
of oil droplets
CA 03064953 2019-11-19
WO 2018/218107 PCT/US2018/034571
are comprised of one or more oils selected from the group consisting of plant-
based oils, natural
fruit-based oils, silicones, dimethicones, esters, essential oils, and
eicosene.
[0014] In yet another embodiment of the present invention, the base
solution is selected
from the group consisting of aqueous sodium hydroxide, aqueous sodium citrate,
or aqueous
triethanolamine (TEA).
[0015] In yet another embodiment of the present invention, the
composition further
comprises a cosmetic preservative.
BRIEF DESCRIPTION OF THE DRAWINGS
[0016] FIG.1 shows a stable emulsion of oil droplets and a gel matrix
according to an
embodiment of the present invention.
[0017] FIG. 2 shows a partially destabilized emulsion of oil droplets and
a gel matrix
according to an embodiment of the present invention.
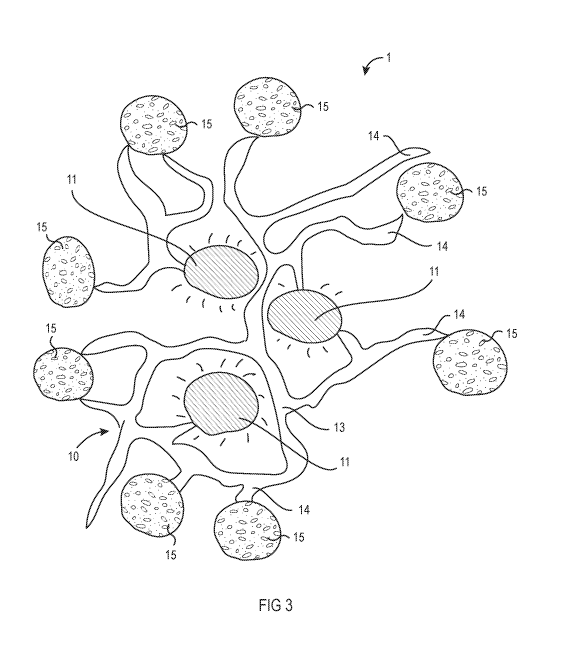
[0018] FIG. 3 shows a stable emulsion of microencapsulated probiotics
according to an
embodiment of the present invention.
DETAILED DESCRIPTION
[0019] The presently disclosed subject matter is presented with
sufficient details to
provide an understanding of one or more particular embodiments of broader
inventive subject
matters. The descriptions expound upon and exemplify particular features of
those particular
embodiments without limiting the inventive subject matters to the explicitly
described
embodiments and features. Considerations in view of these descriptions will
likely give rise to
6
CA 03064953 2019-11-19
WO 2018/218107 PCT/US2018/034571
additional and similar embodiments and features without departing from the
scope of the
presently disclosed subject matter.
[0020] Unless defined otherwise, all technical and scientific terms used
herein have the
same meaning as commonly understood to one of ordinary skill in the art to
which the presently
disclosed subject matter pertains. Although any methods, devices, and
materials similar or
equivalent to those described herein can be used in the practice or testing of
the presently
disclosed subject matter, representative methods, devices, and materials are
now described.
[0021] Following long-standing patent law convention, the terms "a",
"an", and "the"
refer to "one or more" when used in the subject specification, including the
claims. Thus, for
example reference to "an additive" can include a plurality of such additives,
and so forth.
[0022] Unless otherwise indicated, all numbers expressing quantities of
components,
conditions, and so forth used in the specification and claims are to be
understood as being
modified in all instances by the term "about". Accordingly, unless indicated
to the contrary, the
numerical parameters set forth in the instant specification and attached
claims are
approximations that can vary depending upon the desired properties sought to
be obtained by the
presently disclosed subject matter.
[0023] As used herein, the term "about", when referring to a value or to
an amount of
mass, weight, time, volume, concentration, and/or percentage can encompass
variations of, in
some embodiments +/-20%, in some embodiments, +/-10%, in some embodiments +/-
5%, in
some embodiments +/-1%, in some embodiments +/-0.5%, and in some embodiments,
+/-0.1%,
from the specified amount, as such variations are appropriate in the disclosed
products and
methods.
[0024] The presently disclosed subject matter provides a stable
composition for the
7
CA 03064953 2019-11-19
WO 2018/218107 PCT/US2018/034571
controlled delivery of probiotics and methods of production thereof.
Advantageously, the present
invention provides a composition that can maintain live probiotic cultures in
desired quantities
and proportions over long periods of time without the need for refrigeration,
allowing for the
delivery of live probiotic cultures in a variety of shelf-stable products.
[0025] According to some embodiments of the present invention, a
stabilized
composition for the controlled delivery of probiotics may comprise an aqueous
solution of
crystalloids having an osmolality between about 150 mOsm/L and about 500
mOsm/L, wherein
the aqueous solution comprises between about 55% to about 95% of the
composition by weight.
The composition may further comprise a colloid comprised of water insoluble
polymers with the
ability to de-swell upon electrical charge, wherein the colloid comprises
between about 0.01% to
about 1% of the composition by weight. The composition may further comprise a
plurality of oil
droplets, wherein the plurality of oil droplets comprise between about 1% to
about 50% of the
composition by weight. The composition may further comprise a base solution,
wherein the base
solution comprises between about 0.01% to about 1% of the composition by
weight. a
destabilizing solution with an osmolality between 190 mOsm/L and about 900
mOsm/L
comprising a solvent, about 0.05% to about 2% nitrogen by weight, a natural
acid sufficient to
produce a pH of about 3.0 to about 4.5, and a sufficient amount of a salt to
contribute to an
osmolality between about 190 mOsm/L and about 900 mOsm/L. The composition may
further
comprise a plurality of microencapsulated probiotic beads, wherein the
plurality of
microencapsulated probiotic beads comprises between about 0.01% to about 20%
of the
composition by weight.
[0026] The composition of the present invention comprises four primary
phases. The
aqueous phase is an aqueous solution of crystalloids. The crystalloids may be
any water soluble
8
CA 03064953 2019-11-19
WO 2018/218107 PCT/US2018/034571
molecules. Preferably, the crystalloids are mineral salts, bicarbonates,
glucose or other simple
sugars, antibodies, albumin, other proteins that mimic human plasma, or other
water soluble
elements. Examples of such crystalloids include, but are not limited to
potassium, sodium,
chlorine, magnesium, calcium, zinc, copper, selenium, oxygen, etc. The
specific combination of
crystalloids used in the present invention may be water soluble elements and
molecules sufficient
to promote the growth of the desired probiotic blend. The aqueous phase may
preferably contain
a sufficient concentration of crystalloids to achieve an osmolality similar to
that of human
plasma, or of about 250 mOsm/L to about 350 mOsm/L, but may have an osmolality
significantly less than or greater than 250 mOsm/L to about 350 mOsm/L
depending on the
specific application.
[0027] The aqueous phase may further include a base solution and a
destabilizing
solution, which are described in further details below.
[0028] The colloid phase of the composition comprises large water
insoluble molecules
polymers. Polymers suitable for use in the present invention should have an
effective use level of
between about .01% to about 1% to create a gel matrix, and de-swell upon
contact with an
electrical-charge. Said polymers may be of natural origin; for example,
cellulose, plant or animal
based gelatin, or collagen may be used as colloids. Synthetic polymers
including, but not limited
to acrylates, carbomers, PVP/eicosene copolymers, etc. and other commercially
available
synthetic polymers may also be used in the present invention.
[0029] The oil phase of the present invention may comprise any polar and
non-polar oils,
plant-based oils, natural fruit-based oils, esters, isododecane, essential
oils, synethetic oils,
dimethicone, silicone, PVP/eicosene copolymers and other similar oils. The oil
phase may
9
CA 03064953 2019-11-19
WO 2018/218107 PCT/US2018/034571
comprise between about 1% to about 50% of the composition by weight, depending
on the
specific application of the product.
[0030] The microencapsulated probiotic beads comprise microcapsules
possessing
Lewis-acid Lewis-base salt walls incorporating water-immiscible materials
enclosing dormant
probiotics. Preferably, the water-immiscible materials are oils or other
lipophilic materials,
making the microencapsulated probiotic beads attractive to lipophilic
molecules. The production
of such microcapsules are well-known in the art. For example, U.S. Patent No.
8,685,425 to
Speaker discloses exemplary methods of producing said microcapsules. The
microencapsulated
probiotic beads of the present invention may deliver any desired combination
of probiotics from
about 50 colony forming units per gram to about 1,000,000 and/or to TNTC (too
numerous to
count) colony forming units per gram as required by the specifically
contemplated application.
Table 1 shows the number of probiotic colony forming units per gram delivered
based on the
percentage of microencapsulated probiotic of the total composition by weight
in an exemplary
formulation of the present invention.
FN1-
FN1-200
Formula # RTO-190 FN1-200(a) FN1-200(b) FN1-200 (c) FN1-200(d)
(e)
(Control)
% of
microencapsulated
probiotic of total 0.0% 20.0% 0.06% 3.0% 0.3%
2.0%
composition by
weight
Total Bacteria Count
None
1.44 x 104
(colony forming TNTC 210 cfu/g 2.25 x 104 cfu/g 265 x 104 cfu/g
Detected
cfu/g
units/gram)
TABLE 1: CONTROLLED PROBIOTIC DELIVERY TABLE
[0031] In the compositions of the present invention, the colloids form a
gel matrix within
CA 03064953 2019-11-19
WO 2018/218107 PCT/US2018/034571
the aqueous phase. Oil droplets and microencapsulated probiotic beads are
interspersed within
the matrix, "anchored" at lipophilic portions of the colloids throughout the
matrix. The process
of creating such a configuration of the four primary phases is discussed in
further detail below.
In such a configuration, the microencapsulated probiotic beads remain
protected and stable over
a period of 18 months at temperatures as high as 40 degrees Celsius, as shown
in Table 2.
Test Description 0 Months 3 Months 6 Months 12 Months 18 Months
pH 5.8 6.1 6.0 5.9 5.9
Probiotic Aerobic 6.9 x 105 5.9 x 105 N/A 5.3 x 105 2.61 x 104
Plate Count (cfu/ml) cfu/g cfu/g cfu/g cfu/g
Mold/Yeast Count Non- Non- Non- Non- Non-
(cfu/ml) detectable detectable detectable detectable detectable
Undesirable Biotics Non- Non- Non- Non- Non-
detectable detectable detectable detectable detectable
TABLE 2: 18-MONTH STABILITY TABLE
[0032] A cosmetic preservative may be added to the final composition to
aid in shelf-
stability.
[0033] According to some embodiments of the present invention, a method
of producing
a stabilized composition for the controlled delivery of probiotics may
comprise mixing an
aqueous solution of crystalloids having an osmolality between 150 mOsm/L and
about 500
mOsm/L with a colloid comprised of water insoluble polymers with the ability
to de-swell upon
electrical charge to form a hydrogel mixture, wherein the aqueous solution
comprises between
about 55% to about 95% of the composition by weight and the colloid comprises
between about
0.01% to about 1% of the composition by weight. The method may further
comprise adding a
plurality of oil droplets to the hydrogel mixture, wherein the plurality of
oil droplets comprise
11
CA 03064953 2019-11-19
WO 2018/218107 PCT/US2018/034571
between about 1% to about 50% of the composition by weight. The method may
further
comprise adjusting the pH of the hydrogel mixture to about 6.2 to about 7.2 by
adding a base
solution, wherein adjusting the pH of the hydrogel mixture causes the
lipophilic portions of the
water insoluble polymers to surround the plurality of oil droplets, creating a
stable gel matrix
interspersed with a plurality of oil droplets, and wherein the base solution
comprises between
about 0.01% to about 1% of the composition by weight. The method may further
comprise
partially destabilizing the gel matrix by adding a destabilizing solution with
an osmolality
between 190 mOsm/L and about 900 mOsm/L comprising a solvent, about 0.05% to
about 2%
nitrogen by weight, a natural acid sufficient to produce a pH of about 3.0 to
about 4.5, and a
sufficient amount of a salt to contribute to an osmolality between about 190
mOsm/L and about
900 mOsm/L, wherein adding a concentrated salt solution causes some of the
lipophilic portions
of the water insoluble polymers to detach from the oil droplets. The method
may further
comprise adding a plurality of microencapsulated probiotic beads to the
hydrogel mixture,
wherein the microencapsulated probiotic beads bind to the detached lipophilic
portions of the
water insoluble polymers, and wherein the microencapsulated probiotic beads
comprises
between about 0.01% to about 20% of the composition by weight.
[0034] Referring now to FIG. 1, a stable emulsion 1 of a gel matrix 10
and oil droplets 11
is shown. The aqueous solution 12 and colloids 13 are mixed to from a hydrogel
mixture. Once
oil droplets 11 are added to the hydrogel mixture, the oil droplets 11 are
attracted to the
lipophilic portions of the colloids 13, preventing the oil droplets 11 from
aggregating into larger
pools of oil. Once the pH of the hydrogel mixture is increased to between
about 6.2 to about 7.2
by adding a base solution, the colloids 13 form a matrix 10 dispersed within
the water phase,
with oil droplets 11 interspersed within the matrix 10 "anchored" to
lipophilic portions of the
12
CA 03064953 2019-11-19
WO 2018/218107 PCT/US2018/034571
colloids 13. Thus, the stable emulsion 1 of a gel matrix 10 and oil droplets
11 as shown in FIG.1
is formed. The base solution used to neutralize the hydrogel mixture may be
comprised of any
base or alkaline pH adjusters, but is preferably sodium hydroxide, sodium
citrate, or
triethanolamine (TEA) in exemplary embodiments.
[0035] Referring now to FIG. 2, the stable emulsion 1 of a gel matrix 10
and oil droplets
11 is partially destabilized to create free lipophilic "anchor" sites 14 for
the microencapsulated
probiotic beads 15 (not shown in FIG. 2). Destabilization is achieved by
adding a destabilizing
solution to the emulsion. Suitable destabilizing solutions may comprise a
solvent, preferably
water. Said solutions may further comprise about 0.05% to about 2% nitrogen of
the solution by
weight, a sufficient amount of a natural source of acid to produce a pH of
about 3.0 to about 4.5
in the destabilizing solution, and a sufficient amount of a salt to contribute
to an osmolality
between about 190 mOsm/L and about 900 mOsm/L in the destabilizing solution.
The salt may
be any salt, but is preferably sodium chloride in exemplary embodiments.
[0036] Referring now to FIG. 3, microencapsulated probiotic beads 15
comprising
between about 0.01% to about 20% of the composition by weight are added to the
destabilized
emulsion. The microencapsulated probiotic beads 15 will bind to the open
lipophilic anchor sites
14 of the colloids 13, once again forming a stabilized emulsion of gel matrix
10, oil droplets 11,
and microencapsulated probiotic beads 15. As shown in Table 1, the amount of
probiotics
delivered can be controlled by varying the amount of microencapsulated
probiotic beads 15
incorporated into the composition.
[0037] The following examples relate to the production and the
composition of the
stabilized compositions for the controlled delivery of probiotics according to
embodiments of the
13
CA 03064953 2019-11-19
WO 2018/218107 PCT/US2018/034571
present invention. All percentages provided in the examples are percentage by
weight of the total
weight of the final composition.
[0038] EXAMPLE 1
[0039] Exemplary formulation FN1-200(a) described in Table 1 was produced
by
[0040] (a) combining in a first vessel 42.42% water, polysaccharides,
NaCl, minerals,
enzymes, and vitamins sufficient to yield and osmolality of 290 mOsm/L; 0.1%
phytic acid;
0.1% ascorbic acid; 3.0% glycerin; 0.3% carbomer/polymer;
[0041] (b) combining in a second vessel 3.0% octyldodecyl myristate; 3.0%
avocado oil;
6.0% jojoba seed oil; 5.0% squalene; 0.98% tocopherol; 5.0% cannabis sativa
seed oil; 2.0%
bisabolol; 3.0% hypericum perforatum oil; 1.0% hippophae rhamnoides fruit oil
and heating the
ingredients to about 60 about 70 degrees Celsius;
[0042] (c) adding the contents of the second vessel to the contents of
the first vessel
while mixing with sufficient sheer force to create a stable emulsion;
[0043] (d) allowing the stable emulsion to cool to about 30 degrees
Celsius;
[0044] (e) adjusting the pH of the stable emulsion by adding 0.6% sodium
hydroxide;
[0045] (f) destabilizing the mixture with 3.5% saline with an osmolality
of 290 mOsm/L;
[0046] (g) adding 3.0% probiotic microbeads;
[0047] (h) adding 1.0% cosmetic preservative.
14
CA 03064953 2019-11-19
WO 2018/218107 PCT/US2018/034571
[0048] EXAMPLE 2
[0049] Exemplary formulation FN1-200(b) described in Table 1 was produced
by
[0050] (a) combining in a first vessel 62.36% water, polysaccharides,
NaCl, minerals,
enzymes, and vitamins sufficient to yield and osmolality of 290 mOsm/L; 0.1%
phytic acid;
0.1% ascorbic acid; 3.0% glycerin; 0.3% carbomer/polymer;
[0051] (b) combining in a second vessel 3.0% octyldodecyl myristate; 3.0%
avocado oil;
6.0% jojoba seed oil; 5.0% squalene; 0.98% tocopherol; 5.0% cannabis sativa
seed oil; 2.0%
bisabolol; 3.0% hypericum perforatum oil; 1.0% hippophae rhamnoides fruit oil
and heating the
ingredients to about 60 about 70 degrees Celsius;
[0052] (c) adding the contents of the second vessel to the contents of
the first vessel
while mixing with sufficient sheer force to create a stable emulsion;
[0053] (d) allowing the stable emulsion to cool to about 30 degrees
Celsius;
[0054] (e) adjusting the pH of the stable emulsion by adding 0.6% sodium
hydroxide;
[0055] (f) destabilizing the mixture with 3.5% saline with an osmolality
of 290 mOsm/L;
[0056] (g) adding 0.6% probiotic microbeads;
[0057] (h) adding 1.0% cosmetic preservative.
[0058] EXAMPLE 3
[0059] Exemplary formulation FN1-200(c) described in Table 1 was produced
by
CA 03064953 2019-11-19
WO 2018/218107 PCT/US2018/034571
[0060] (a) combining in a first vessel 59.42% water, polysaccharides,
NaCl, minerals,
enzymes, and vitamins sufficient to yield and osmolality of 290 mOsm/L; 0.1%
phytic acid;
0.1% ascorbic acid; 3.0% glycerin; 0.3% carbomer/polymer;
[0061] (b) combining in a second vessel 3.0% octyldodecyl myristate; 3.0%
avocado oil;
6.0% jojoba seed oil; 5.0% squalene; 0.98% tocopherol; 5.0% cannabis sativa
seed oil; 2.0%
bisabolol; 3.0% hypericum perforatum oil; 1.0% hippophae rhamnoides fruit oil
and heating the
ingredients to about 60 about 70 degrees Celsius;
[0062] (c) adding the contents of the second vessel to the contents of
the first vessel
while mixing with sufficient sheer force to create a stable emulsion;
[0063] (d) allowing the stable emulsion to cool to about 30 degrees
Celsius;
[0064] (e) adjusting the pH of the stable emulsion by adding 0.6% sodium
hydroxide;
[0065] (f) destabilizing the mixture with 3.5% saline with an osmolality
of 290 mOsm/L;
[0066] (g) adding 3.0% probiotic microbeads;
[0067] (h) adding 1.0% cosmetic preservative.
[0068] EXAMPLE 4
[0069] Exemplary formulation FN1-200(d) described in Table 1 was produced
by
[0070] (a) combining in a first vessel 62.12% water, polysaccharides,
NaCl, minerals,
enzymes, and vitamins sufficient to yield and osmolality of 290 mOsm/L; 0.1%
phytic acid;
0.1% ascorbic acid; 3.0% glycerin; 0.3% carbomer/polymer;
16
CA 03064953 2019-11-19
WO 2018/218107 PCT/US2018/034571
[0071] (b) combining in a second vessel 3.0% octyldodecyl myristate; 3.0%
avocado oil;
6.0% jojoba seed oil; 5.0% squalene; 0.98% tocopherol; 5.0% cannabis sativa
seed oil; 2.0%
bisabolol; 3.0% hypericum perforatum oil; 1.0% hippophae rhamnoides fruit oil
and heating the
ingredients to about 60 about 70 degrees Celsius;
[0072] (c) adding the contents of the second vessel to the contents of
the first vessel
while mixing with sufficient sheer force to create a stable emulsion;
[0073] (d) allowing the stable emulsion to cool to about 30 degrees
Celsius;
[0074] (e) adjusting the pH of the stable emulsion by adding 0.6% sodium
hydroxide;
[0075] (f) destabilizing the mixture with 3.5% saline with an osmolality
of 290 mOsm/L;
[0076] (g) adding 0.3% probiotic microbeads;
[0077] (h) adding 1.0% cosmetic preservative.
[0078] EXAMPLE 5
[0079] Exemplary formulation FN1-200(e) described in Table 1 was produced
by
[0080] (a) combining in a first vessel 60.42% water, polysaccharides,
NaCl, minerals,
enzymes, and vitamins sufficient to yield and osmolality of 290 mOsm/L; 0.1%
phytic acid;
0.1% ascorbic acid; 3.0% glycerin; 0.3% carbomer/polymer;
[0081] (b) combining in a second vessel 3.0% octyldodecyl myristate; 3.0%
avocado oil;
6.0% jojoba seed oil; 5.0% squalene; 0.98% tocopherol; 5.0% cannabis sativa
seed oil; 2.0%
bisabolol; 3.0% hypericum perforatum oil; 1.0% hippophae rhamnoides fruit oil
and heating the
17
CA 03064953 2019-11-19
WO 2018/218107 PCT/US2018/034571
ingredients to about 60 about 70 degrees Celsius;
[0082] (c) adding the contents of the second vessel to the contents of
the first vessel
while mixing with sufficient sheer force to create a stable emulsion;
[0083] (d) allowing the stable emulsion to cool to about 30 degrees
Celsius;
[0084] (e) adjusting the pH of the stable emulsion by adding 0.6% sodium
hydroxide;
[0085] (f) destabilizing the mixture with 3.5% saline with an osmolality
of 290 mOsm/L;
[0086] (g) adding 2.0% probiotic microbeads;
[0087] (h) adding 1.0% cosmetic preservative.
[0088] EXAMPLE 6
[0089] Exemplary formulation FN1-126 described in Table 2 was produced by
[0090] (a) combining in a first vessel 60.42% water, polysaccharides,
NaCl, minerals,
enzymes, and vitamins sufficient to yield and osmolality of 320 mOsm/L; 0.5%
ascorbic acid;
3.0% glycerin; 0.3% carbomer/polymer; 0.02% sodium phytate;
[0091] (b) combining in a second vessel 3.0% octyldodecyl myristate; 1.0%
avocado oil;
2.0% jojoba seed oil; 3.0% squalene; 0.98% tocopherol; 2.0% cannabis sativa
seed oil; 2.0%
bisabolol; 3.0% hypericum perforatum oil; 3.0% hippophae rhamnoides fruit oil
and heating the
ingredients to about 60 about 70 degrees Celsius;
[0092] (c) adding the contents of the second vessel to the contents of
the first vessel
while mixing with sufficient sheer force to create a stable emulsion;
18
CA 03064953 2019-11-19
WO 2018/218107 PCT/US2018/034571
[0093] (d) allowing the stable emulsion to cool to about 30 degrees
Celsius;
[0094] (e) adjusting the pH of the stable emulsion by adding 0.6% sodium
hydroxide;
[0095] (f) destabilizing the mixture with 4.5% saline with an osmolality
of 320 mOsm/L;
[0096] (g) adding 3.0% probiotic microbeads;
[0097] (h) adding 1.0% cosmetic preservative.
[0098] EXAMPLE 7
[0099] Another exemplary formulation according to an embodiment of the
present
invention is produced by
[00100] (a) combining in a first vessel 59.42% water, polysaccharides,
NaCl, minerals,
enzymes, and vitamins sufficient to yield and osmolality of 700 mOsm/L; 0.1%
phytic acid;
0.1% ascorbic acid; 3.0% butylene glycol; 0.3% carbomer/polymer;
[00101] (b) combining in a second vessel 3.0% capryliccapric
triglycerides; 3.0% avocado
oil; 6.0% jojoba seed oil; 5.0% squalene; 0.98% tocopherol; 5.0% cannabis
sativa seed oil; 2.0%
bisabolol; 3.0% hypericum perforatum oil; 1.0% hippophae rhamnoides fruit oil
and heating the
ingredients to about 60 about 70 degrees Celsius;
[00102] (c) adding the contents of the second vessel to the contents of
the first vessel
while mixing with sufficient sheer force to create a stable emulsion;
[00103] (d) allowing the stable emulsion to cool to about 30 degrees
Celsius;
[00104] (e) adjusting the pH of the stable emulsion by adding 0.6% sodium
hydroxide;
19
CA 03064953 2019-11-19
WO 2018/218107 PCT/US2018/034571
[00105] (f) destabilizing the mixture with 3.5% saline with an osmolality
of 290 mOsm/L;
[00106] (g) adding 3.0% probiotic microbeads;
[00107] (h) adding 1.0% cosmetic preservative.
[00108] EXAMPLE 8
[00109] Another exemplary formulation according to an embodiment of the
present
invention is produced by
[00110] (a) combining in a first vessel 59.42% water, aloe juice,
polysaccharides, NaCl,
minerals, enzymes, and vitamins sufficient to yield and osmolality of 260
mOsm/L; 0.1% phytic
acid; 0.1% ascorbic acid; 3.0% ethylhexylglycerin; 0.3% carbomer/polymer;
[00111] (b) combining in a second vessel 3.0% ester mixture of dicaprylyl
maleate and
ethylhexyl palmitate; 3.0% avocado oil; 6.0% jojoba seed oil; 5.0% squalene;
0.98% tocopherol;
5.0% cannabis sativa seed oil; 2.0% bisabolol; 3.0% hypericum perforatum oil;
1.0% hippophae
rhamnoides fruit oil and heating the ingredients to about 60 about 70 degrees
Celsius;
[00112] (c) adding the contents of the second vessel to the contents of
the first vessel
while mixing with sufficient sheer force to create a stable emulsion;
[00113] (d) allowing the stable emulsion to cool to about 30 degrees
Celsius;
[00114] (e) adjusting the pH of the stable emulsion by adding 0.6% sodium
hydroxide;
[00115] (f) destabilizing the mixture with 3.5% saline with an osmolality
of 290 mOsm/L;
[00116] (g) adding 3.0% probiotic microbeads;
CA 03064953 2019-11-19
WO 2018/218107 PCT/US2018/034571
[00117] (h) adding 1.0% cosmetic preservative.
[00118] EXAMPLE 9
[00119] Another exemplary formulation according to an embodiment of the
present
invention is produced by
[00120] (a) combining in a first vessel 59.42% water, polysaccharides,
NaCl, minerals,
enzymes, and vitamins sufficient to yield and osmolality of 190 mOsm/L; 0.2%
citric acid; 3.0%
propendoil; 0.3% carbomer/polymer;
[00121] (b) combining in a second vessel 3.0% C10-18 triglycerides; 3.0%
avocado oil;
6.0% jojoba seed oil; 5.0% squalene; 0.98% tocopherol; 5.0% cannabis sativa
seed oil; 2.0%
bisabolol; 3.0% hypericum perforatum oil; 1.0% hippophae rhamnoides fruit oil
and heating the
ingredients to about 60 about 70 degrees Celsius;
[00122] (c) adding the contents of the second vessel to the contents of
the first vessel
while mixing with sufficient sheer force to create a stable emulsion;
[00123] (d) allowing the stable emulsion to cool to about 30 degrees
Celsius;
[00124] (e) adjusting the pH of the stable emulsion by adding 0.6% sodium
hydroxide;
[00125] (f) destabilizing the mixture with 3.5% saline with an osmolality
of 190 mOsm/L;
[00126] (g) adding 3.0% probiotic microbeads;
[00127] (h) adding 1.0% cosmetic preservative.
[00128] Those skilled in the art will recognize improvements and
modifications to the
21
CA 03064953 2019-11-19
WO 2018/218107 PCT/US2018/034571
embodiments of the present disclosure. All such improvements and modifications
are considered
within the scope of the concepts disclosed herein and the claims that follow.
22