Note: Descriptions are shown in the official language in which they were submitted.
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
NOVEL INHIBITORS OF MAP4K1
TECHNICAL FIELD
The present patent application is directed to novel inhibitors of the mitogen-
activated
protein kinase kinase kinase kinase 1, also known as MAP4K1 or HPK1
(hematopoietic
progenitor kinase 1).
BACKGROUND OF THE INVENTION
Protein kinases represent a large family of proteins which play a variety of
crucial
roles in the regulation of a wide range of cellular processes. Such kinases
include Akt, Axl,
Aurora A, Aurora B, DYRK2, EPHAa2, FGFR3, FLT-3, VEGFr3, IGFLr, IKK2, JNK3,
VEGFr2, MEK1, MET, P70s6K, Plkl, RSK1, Src, TrkA, Zap70, cKit, bRaf, EGFR,
Jak2,
PI3K, NPM-Alk, c-Abl, BTK, FAK, PDGFR, TAK1, LimK, Fltl, PDK1, Erk and RON.
Inhibition of various protein kinases, especially selective inhibition, has
become an important
strategy in treating many diseases and disorders.
MAP4K1 is a serine/threonine kinase of the 5te20 family. MAP4K enzymes (MAP
kinase kinases) are generally involved at the highest level of a largely
linear kinase activation
pathway. A MAP4K will phosphorylate and activate a particular substrate which
is a MAP3K
(a MAP kinase kinase). A MAP3K in turn phosphorylates and activates a MAP2K (a
MAP
kinase kinase). A MAP2K in turn phosphorylates and activates a MAPK (MAP
kinase). The
MAP kinase is the final effector of the pathway and it in turn phosphorylates
a substrate to
control key cellular processes such as cell proliferation, cell
differentiation, gene expression,
transcription regulation, and apoptosis. The substrate of MAPK is generally a
nuclear protein,
such as nuclear factor kappa-B (NF-KB). Activation of the MAPK by its
phosphorylation by
an MAP2K results in translocation of this final enzyme in the cascade into the
nucleus.
MAP4K1, also known as HPK1, is primarily expressed in the immune system's
Tcells
and B cells, which are critical in regulation of the immune system.
Overstimulation of T cell
and B cell activation pathways can result in auto-immune diseases, while
understimulation of
these pathways can result in immune dysfunction, susceptibility to viral and
bacterial
infection and increased susceptibility to cancer. MAP4K1 is activated by its
interaction with
activated T cell receptors (TCRs) and B cell receptors (BCRs), so MAP4K1
activation serves
to convey the cellular activation signal from the surface of a T or B cell to
the effector
proteins in the nucleus. There is also evidence that MAP4K1 can be activated
via the TGF-I3
receptor, the erythropoietin receptor and the FAS protein (which is involved
in apoptosis
1
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
signaling). MAP4K1 activation ultimately results in activation of several
identified nuclear
effector proteins, including those involved in the NF-K1, AP-1, ERK2, and Fos
signaling
pathways.
MAP4K1 is considered a negative regulator of T cell receptor (TCR) activation
signals, and it is one of the effector molecules that mediates
immunosuppression of T cell
responses upon exposure to prostaglandin E2 (PGE2). Studies have shown that
MAPK1
activity dampens the strength of the T cell receptor signal transduction
cascade, and thus,
targeted genetic disruption of MAP4K1 results in strengthened TCR activation
signals.
One particularly important pathway that MAP4K1 appears to be involved with is
the
JNK pathway. MAP4K1 regulates the MAP3K's MEKK1, TAK1 and MLK3. These in turn
regulate the MAP2K's MKK4 and MKK7. These in turn regulate the MAPK JNK. JNK
then
regulates important transcription factors and other proteins, including p53,
SMAD4, NFAT-2,
NFAT-4, ELK1, ATF2, HSF1, c-Jun, and JunD. JNK has been implicated in
apoptosis,
neurodegeneration, cell differentiation and proliferation, inflammatory
conditions and
cytokine production.
The JNK signal transduction pathway is activated in response to environmental
stress
and by the engagement of several classes of cell surface receptors, including
cytokine
receptors, serpentine receptors and receptor tyrosine kinases. In mammalian
cells, the JNK
pathway has been implicated in biological processes such as oncogenic
transformation and
mediating adaptive responses to environmental stress. JNK has also been
associated with
modulating immune responses, including maturation and differentiation of
immune cells, as
well as effecting programmed cell death in cells identified for destruction by
the immune
system. Among several neurological disorders, JNK signaling is particularly
implicated in
ischemic stroke and Parkinson's disease, but also in other diseases as
mentioned further
below.
It is noteworthy that the MAPK p38a1pha was shown to inhibit cell
proliferation by
antagonizing the JNK-c-Jun-pathway. p38a1pha appears to be active in
suppression of
proliferation in both normal cells and cancer cells, and this strongly
suggests the involvement
of JNK in hyperproliferative diseases (see, e.g., Hui et al., Nature Genetics,
Vol. 39, No. 6,
June 2007). JNK signaling has also been implicated in diseases such as
excitotoxicity of
hippocampal neurons, liver ischemia, reperfusion, neurodegenerative diseases,
hearing loss,
deafness, neural tube birth defects, cancer, chronic inflammatory diseases,
obesity, diabetes,
in particular, insulin-resistant diabetes, and it has been proposed that
selective JNK inhibitors
2
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
are needed for treatment of various diseases with a high degree of specificity
and lack of
toxicity.
Because MAP4K1 is an upstream regulator of JNK, effective inhibitors of MAP4K1
would be useful in treating the same diseases which have been suggested or
implicated for
INK inhibitors, especially where such disease or dysfunction is manifested in
hematopoietic
cells such as T cells and B cells.
Targeted disruption of MAP4K1 (HPK1) alleles has been shown to confer T cells
with an elevated Thl cytokine production in response to TCR engagement.
Burakoff et al.,
Immunologic Research, 54(1): 262-265 (2012). HPK1¨/¨ T cells were found to
proliferate
more rapidly than the haplotype-matched wild-type counterpart and were
resistant to
prostaglandin E2 (PGE2)-mediated suppression. Most strikingly, mice that
received adoptive
transfer of HPK1¨/¨ T cells became resistant to lung tumor growth. Also, the
loss of HPK1
from dendritic cells (DCs) endowed them with superior antigen presentation
ability, enabling
HPK1¨/¨ DCs to elicit a more potent anti-tumor immune response when used as
cancer
vaccine. It was considered probable that blocking the MAP4K1 kinase activity
with a small
molecule inhibitor may activate the superior antitumor activity of both cell
types, resulting in
a synergistic amplification of anti-tumor potential. Given that MAP4K1 is not
expressed in
any major organs, it is less likely that a selective inhibitor of MAP4K1 would
cause any
serious side effects.
The relationship between MAP4K1 and PGE2 is particularly noteworthy because
PGE2 is the predominant eicosanoid product released by cancer cells, including
lung, colon
and breast cancer cells. Tumor-produced PGE2 is known to contribute
significantly to tumor-
mediated immune suppression.
Zhang et al., J. Autoimmunity, 37:180-189 (2011), described diminished HPK1
expression in CD4 T cells of lupus patients due to the selective loss of JMJD3
histone
demethylase binding to the HPK1 locus. This suggests that HPK1 is one of the
key molecules
involved in the maintenance of peripheral tolerance. Peripheral tolerance is
one of the major
obstacles to the development of effective anti-tumor immunity.
Several small molecule inhibitors of MAP4K1 have been reported, but they do
not
inhibit MAP4K1 selectively, or even preferentially. Such inhibitors include
staurosporine,
bosutinib, sunitinib, lestaurtinib, crizotinib, foretinib, dovitinib and KW-
2449. Staurosporine,
for example, broadly inhibits a wide range of protein kinases across both the
serine/threonine
and tyrosine kinase families. Bosutinib is primarily an inhibitor of the
tyrosine kinase BCR-
Abl, with additional activity against the Src family tyrosine kinases.
Sunitinib is a broad
3
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
inhibitor of tyrosine kinases. Lestaurtinib is primarily an inhibitor of the
FLT, JAK and TRK
family tyrosine kinases. Crizotinib is primarily an inhibitor of the c-met and
ALK tyrosine
kinases. Foretinib was under study as an inhibitor of the c-Met and VEGFR
tyrosine kinases.
Dovitinib is primarily an inhibitor of the FGFR receptor tyrosine kinase. KW-
2449 is an
experimental inhibitor primarily of the FLT3 tyrosine kinase.
Sunitinib inhibits MAP4K1 at nanomolar concentrations, but it is a broad-
spectrum
receptor tyrosine kinase inhibitor. Treating T-cells with sunitinib results in
enhanced cytokine
product similar to that observed with HPK1 ¨/¨ T cells, which suggests that in
T cells a
selective MAP4K1 inhibitor could produce the same enhanced immune response
phenotype.
Currently, there is a largely unmet need for an effective way of treating
disease and
disorders associated disrupted protein kinase signaling. Autoimmune diseases,
inflammatory
diseases, neurological and neurodegenerative diseases, cancer, cardiovascular
diseases,
allergies and asthma, are all diseases and disorder which can be affected by
dysfunctional
protein kinase signaling. Improved therapeutic compounds, compositions and
methods for the
treatment for these disease and disorders are urgently required. MAP4K1
inhibition is an
especially attractive target for cancer immunotherapy.
The major challenge currently faced in the field is the lack of MAP4K1
specific
inhibitors. The present disclosure provides novel, highly effective small-
molecule inhibitors
of MAP4K1.
SUMMARY OF THE INVENTION
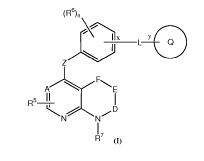
In one aspect, the present invention relates to a compound of formula (I)
(R6)n \
I X Y
L Q
Z
A..... R...'' E
5 II I
R -r,
N N / D
I
R7 (i)
stereoisomer, diastereoisomer, enantiomer or a pharmaceutically acceptable
salt
thereof,
wherein
A is selected from CH and N;
D is selected from CR1R2 and CO;
E is selected from (CR3R4)m, NR' and CO;
4
CA 03064975 2019-11-26
WO 2018/215668 PCT/EP2018/063957
F is selected from 0, CH2, CHOH and CO;
each occurrence of R5 is selected from hydrogen, halogen, cyano, hydroxyl and
Ci-
salkyl;
R7 is selected from hydrogen and Ci_salkyl;
each occurrence of R6 is selected from halogen, cyano, hydroxyl, Ci_salkyl,
haloCi-
salkyl, hydroxyCi_salkyl, C1-8alkoxy, Ci_salkoxyCi_salkyl, C3-6cycloalkyl and
C3-
6cycloalky1C1_8a1ky1;
Rl, R2, R3 and R4 which may be same or different, are each independently
selected
from hydrogen, amine, Ci_salkyl, C3_6cycloalkyl, haloCi_salkyl,
hydroxyCi_salkyl, C3-
6cycloalky1C1_8alkyl, C1_8alkoxy, 3-15 membered heterocyclyl, Ci_salky13-15
membered
heterocyclyl and CRaRbNRaRb;
Ra and Rb, which may be the same or different, are each independently selected
from
hydrogen and Ci_salkyl;
Z is selected from 0, NH and S;
x NH 0 0
./x
N AN N
L is selected from o H ' 5 and
=
NX,Y.
0 0
7
x and y represents point of attachment;
Ring Q is selected õ from
- (R8)
q
(R10),
)>. ,=
(R)p 0 _____________________________
and
5 5 5
Rio
N
=
each occurrence of R8 is selected from halogen, cyano, cyanoCi_salkyl,
cyanohaloCi-
salkyl, cyanoC3-6cycloalkyl, Ci_salkyl, haloC1_8alkyl, hydroxyC1_8alkyl,
hydroxyCi_shaloalkyl,
and -SO2R1;
5
CA 03064975 2019-11-26
WO 2018/215668 PCT/EP2018/063957
each occurrence of R9 is selected from halogen, cyano, hydroxyl, Ci_salkyl,
haloCi-
N
= N, '
f\L
salkyl, hydroxyCi_salkyl, Ci_salkoxy,
N 0 NH = r I N----\
c____ i
,,,\,...õNõ..........,.--...... .....õ--
N
N- 7................N
I N
5 5 5 5
0
\
/N---)
1 1 /5/õ..........õ.õ,N.,,,,........õ. N
N \
(
\--NH 0 /-/............,,õN.,........õ.õ,...
5
5 5 5 _____ /
I I
..,,---N'5.õ.. ..../NN....,
N
_N
5 0 ,õ,--
5 5
5
1 1
.........N.,..... ...........N,.....,
1 .
...õ."N======,..õ
1 =
N \ / .......,N.,........ /'^\ I
N N
I
N \N N
______________________ /N , 1 , r\l'/', 1
H
5 5 5 5 F5
/N
N
N
N c ) C )
S . / 1 c ) \
/ % ' N
1 1 1
t 5 o o ,
5 5 5 5 5
I
I......-'N'-.......
N I
N I
N I ...../ N',.......
N
N N
c ) \ /
*N- X
N N-
'''''N / 'OH A
H 5 H 5 5 / 5 F F 5 5 5
6
CA 03064975 2019-11-26
WO 2018/215668
:......,...,N,...õ....0 PCT/EP2018/063957
I
,../N,.õ,
(N
I 0 c NI N
1
NH, ,'
H <>
, , ,
I
I ..................................................................... 1 --
,õ..,''N'....... ...õ/NN,....
,.=
N
H H \ ___ N\
F ,
\ ,
I ----------------- I I I ----- I --
=N I
N -------------------------------------------------------------------- I
.õ..--N,..,
...õ...-'N',.,.., ..õ.....N,......40,0
/ \
N
I 1 1
,
/s:.
0 s
I I I
1 1
0
N N 1 /ON N--"N/ N
1 1 ___________ N
\ 1 N NH
' , , ,
,
HN 0 ---
I N%
N '
I 1 1
HNN<5.,õ.0
0
HN0 (N / ,,
N 1 CN
0 <>
CN CN, \ , N )V NN/
1
7
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
H 1 N
N
/
( ....,..--,,,,
1
i> N
I0 (:)NH2 CN ) /OH
HO
N u2N
N 5 5 " 5 5 5 5 5
5
1 1
1 1 1 1
CN HO and OH .
5
RM is selected from halogen, hydroxyl, cyano, Ci_salkyl, haloCi_salkyl,
C3_6cycloalkyl
and C6_14aryl; wherein C6_14aryl is optionally substituted with one or more
substituents
5 selected from halogen, hydroxyl, cyano, amide or Ci_salkyl;
'm' is 1 or 2;
'n' is 0, 1 or 2;
`p' is 0 or 1;
'q' is 0 or 1; and
T is 1 or 2.
The compounds of formula (I) may involve one or more embodiments. It is to be
understood that the embodiments below are illustrative of the present
invention and are not
intended to limit the claims to the specific embodiments exemplified. It is
also to be
understood that the embodiments defined herein may be used independently or in
conjunction
with any definition, any other embodiment defined herein. Thus the invention
contemplates
all possible combinations and permutations of the various independently
described
embodiments. For example, the invention provides compounds of formula (I) as
defined
above wherein A is N (according to an embodiment defined below); F is 0
(according to
another embodiment defined below); R7 is hydrogen (according to yet another
embodiment
defined below).
According to one embodiment specifically provided are compounds of formula
(I), in
which A is CH.
According to another embodiment specifically provided are compounds of formula
(I), in which A is N.
According to yet another embodiment specifically provided are compounds of
formula (I), in which D is CR1R2 or CO.
8
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
According to yet another embodiment specifically provided are compounds of
formula (I), in which Rl is hydrogen or Ci_salkyl (e.g. methyl) and R2 is
hydrogen, Ci_salkyl
(e.g. methyl, ethyl or isopropyl), hydroxyCi_salkyl (e.g. hydroxyl methyl),
C1_8alkoxyC1-
H2C
N
0
salkyl (e.g. methoxymethyl), 3-15 membered heterocycly1C1-8alkyl (e.g.
) or
CRaRbNRaRb. In this embodiment, Ra and Rb are independently hydrogen or
methyl. In
another embodiment, Ra and Rb are hydrogen. In yet another embodiment, Ra and
RID are
methyl.
According to yet another embodiment specifically provided are compounds of
formula (I), in which D is CH2, CH-CH3, CH-CH2-CH3, C(CH3)2, CH-CH(CH3)2, CH-
HC
CH2
I
......./N".......
CH2OH, CH-CH2-0-CH3, 0 5 CH-CH2-N(CH3)2 or CO.
According to yet another embodiment specifically provided are compounds of
formula (I), in which E is (CR3R4)m, NR' or CO.
According to yet another embodiment specifically provided are compounds of
formula (I), in which R3 is hydrogen or Ci_salkyl (e.g. methyl) and R4 is
hydrogen or Ci_salkyl
(e.g. methyl) and 'm' is 1 or 2.
According to yet another embodiment specifically provided are compounds of
formula (I), in which E is CH2, CH-CH3, C(CH3)2, (CH2)2, N-CH3 or CO.
According to yet another embodiment specifically provided are compounds of
formula (I), in which F is 0, CH2, CHOH or CO.
According to yet another embodiment specifically provided are compounds of
formula (I), in which R5 is hydrogen, halogen (e.g. chloro) or cyano.
According to yet another embodiment specifically provided are compounds of
formula (I), in which R5 is hydrogen, chloro or cyano.
According to yet another embodiment specifically provided are compounds of
formula (I), in which R7 is hydrogen or Ci_salkyl (e.g. methyl).
According to yet another embodiment specifically provided are compounds of
formula (I), in which R7 is hydrogen or methyl.
According to yet another embodiment specifically provided are compounds of
formula (I), in which Z is 0.
9
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
According to yet another embodiment specifically provided are compounds of
formula (I), in which Z is NH.
According to yet another embodiment specifically provided are compounds of
formula (I), in which Z is S.
According to yet another embodiment specifically provided are compounds of
formula (I), in which R6 is halogen (e.g. chloro or fluoro), Ci_salkyl (e.g.
methyl), haloCi-
salkyl (e.g. trifluoromethyl) or Ci_salkoxy (e.g. methoxy).
According to yet another embodiment specifically provided are compounds of
formula (I), in which R6 is chloro, fluoro, methyl, trifluoromethyl or
methoxy.
According to yet another embodiment specifically provided are compounds of
formula (I), in which 'n' is 0, 1 or 2.
According to yet another embodiment specifically provided are compounds of
..,,,<.....,...õ NH ...,...):,.,,
formula (I), in which L is 0 ; x and y represents point of attachment.
According to yet another embodiment specifically provided are compounds of
o
./x
,-"\N '
formula (I), in which L is H ' ; x and y represents point of attachment.
According to yet another embodiment specifically provided are compounds of
o
N N H
formula (I), in which L is H ; x and y represents point of
attachment.
According to yet another embodiment specifically provided are compounds of
formula (I), in which L is o o ; x and y represents point of
attachment.
According to yet another embodiment specifically provided are compounds of
formula (I), in which Ring Q is
Rio
1
,
)
'
,-- , ( R8)q 1 \N
..
-,____......
-- =-= >.
(R9)p .n ..
1 \ ( (Rio),
N .......0 N Or
.
5 5 5
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
According to yet another embodiment specifically provided are compounds of
- )>.
formula (I), in which Ring Q is
According to yet another embodiment specifically provided are compounds of
(R8)
q
formula (I), in which Ring Q is (R9 )p =
According to yet another embodiment specifically provided are compounds of
formula (I), in which R8 is halogen (e.g. chloro or bromo), cyano,
cyanoCi_salkyl (e.g.
cyanomethyl or cyanoisopropyl), cyanohaloC1_8alkyl (e.g. cyanodifluoromethyl),
cyanoC3_
6 cycloalkyl (e.g. cyanocyclopropane), Ci_salkyl (e.g. methyl), haloC1_8alkyl
(e.g.
trifluoromethyl or difluoromethyl), hydroxyCi_shaloalkyl (e.g. hydroxyl
difluoromethyl) or -
S02R1. In this embodiment, Rl is Ci_salkyl (e.g. methyl, ethyl or amine).
According to yet another embodiment specifically provided are compounds of
formula (I), in which R8 is chloro, bromo, cyano, cyanomethyl, cyanoisopropyl,
cyanodifluoromethyl, cyanocyclopropane, methyl, trifluoromethyl,
difluoromethyl, hydroxyl
difluoromethyl, -S02Me, -S02Et or -SO2NH2.
According to yet another embodiment specifically provided are compounds of
formula (I), in which R9 is halogen (e.g. fluoro or bromo), cyano, hydroxyl,
Ci_salkyl (e.g.
methyl), haloCi_salkyl (e.g. trifluoromethyl), Ci_salkoxy (e.g. methoxy), /N/
=
NH N
5 5 5 5 5
\ 5
0
FN]
= \
N NH 5 o \ __ 0 5
5 5 = 5
5
11
CA 03064975 2019-11-26
WO 2018/215668 PCT/EP2018/063957
I I
...,,,N,...... ...,,,N.,....... I
N
I
N N \
/ \ N
= 0 5 5 5 5 5
5 r 5
I I
NI
N . I ..,,===N"...õ
I ..../\_
cN
N I S =,' N
\ N
H I
t
F 0 0 ,'
5 5 5 5 5 5 5
71- N
zN N N N
\ ) ) ..
N N
¨NI\ /1\I
N N¨
I I I ''"+
'NI N /
/N-
5 5 5 H 5 H 5 5
5
NI
I
I I
N 0,.....N`....,
0 I
N ,õ===== \
C ) N
N CN <
n , N 0 __ cN z\
X N 1 'OH F F \----NH 2''N H
V
5 5 5 5 5 5 5 5
I I I I I
N
.õ...õNõ...... ,,,,N,,.... I
cN .,:=cly N (
.....õ,.Nõ.....
\ 5
5 OH, H 5 H 5 F 5
5 5
I I
N I
....õ,. Nõ,... I
N )1
Ni 0 it] <õ NI
..õ===== `....stso \sõ..Ø0õ ,....,...
N N 1 N N
N \ /
N I I I I 1
H H
5 5 5 5 5 5 5 5
%
0 =
..õ..-"N`.,..
I I
N
0 N
¨N N
/ N
\ 0
N N-NH 5 C N
5 5 5 5
5
12
CA 03064975 2019-11-26
WO 2018/215668 PCT/EP2018/063957
I
I HN, _.0
= I I I
HN, _..0 11 I
HN0 (N)
CN <>
0 N ' ...õ:õ1
CNõ.....,1 , NN
I
\ , I N N
5 5 5 5 5
CH3 I
1
1 1
N 11 11
OH
H2N/. 5 o 0NH5 5 5 HO 2 CN ) j
, 5 5 5 / \ o
CN HO
11
Or OH,
According to yet another embodiment specifically provided are compounds of
5 formula (I), in which R9 is fluoro, bromo, cyano, hydroxyl, methyl,
trifluoromethyl, methoxy,
N 0
N N
/,,......N.,,....,....õ ,N............ ,,,N..õ......õ/õ..- "'
5 5 5
5
---Si____\ ----
NH ..= 1
c_____ i -- \
/N
,, ----\
/ \. N N
1
7,.....,.....õ-N........,....
1 \ 5
---/
NH, =;' N
0
5
55
N 0
--- \ ________________________________________________________________ N
1
,,N.........N,.......õ..õ...s.N.,,,.-
../....õ,õõ,,N.,.........õ...õ. I :,''',
0 \ __ 0 0 , 0
5 / 5 5 5
5
I 1
.....õ.=N...õ..... ,.....,. N .õ.......
I
........, N --...õ,
I I
....,,,N,....... ..,,,N,........ N/ \ N/
N
N
N
N
5 5 5 5 5
5
13
CA 03064975 2019-11-26
WO 2018/215668 PCT/EP2018/063957
I ----------------------------- =
I
N
I
1
NN
S
N
1 1 F 0/ %0 N, 5 H 5 5
5 5 ' 5
I zN N N)
'. .' N c ) C N
c ) \
'I\1
(NN
õ
H H 5
5 5 5 5
I
N
I I I
o
KN /N 1 ..õ..-- N =====..õ..
N N/ I
../1õ...../N
\ \ c )
. N
. - - x ________________________________________ n si
\---NI-<
*N- N-
/ / 1-_1 H F F A 5 5 5 5
5 5 5
I
..,....,- N ====.õ,
N, N N õ,./N====õ,....
N 0 H 1
5 H 5 H
5
5 5 5
I
I
N I 1
I N
N N rti iti j, ,
"1""/
N
Y N\ 5 5 5 0 1
5 5 F 5 5 H 5
5
I I I ..
.......,,N,...... I
...õN.,..õ.40,
(:)
N 1 __ 1 n -------------------- NO.N/
N
N N-N
/ \ 5 5 5 5 5 5 5
14
CA 03064975 2019-11-26
WO 2018/215668 PCT/EP2018/063957
../. s.
0 = I
N
I I
I =
\ )
HNN.........0
N¨NH(N /
0
N
, 101 N
1\ , 5 5 CN 5 CN, 5
5
I
I H I
HN 0
)\1 ,
N'''...
NN I
) I N N hI2N0 ONH2 CN
5 5 5 5 5 5
I
11
11 H 11 II
OH
HO 5 5 CN HO or OH,
5 5
5
According to yet another embodiment specifically provided are compounds of
formula (I), in which Ring Q is
CF3 = CF, CF3 CF3
5 5 5
CF,
/ 0 CF CF3
0 CF3 L ,
.,
= N 0 N . * N (NNH
õ..-....,,N...õ,
NõNõ..) 1 ..............õ,N.,,, 5
........,....õ.NH 5 0 5
5 5
i 0 CF3
=
N
.--='- s',..
.,
./ ,
=
N'.---\
CF
5 5 5
5
CF,
.
,
NN NN
Br 5 0 5 5 5
CA 03064975 2019-11-26
WO 2018/215668 PCT/EP2018/063957
,
,. CF,
=
=
CF3 0
1
N1 ,
0 H
Nõ...............õ....õ, N....,
I :,.. I. C F3
/ 5 *
0
CF
5 5
,
CF ..,' CF õ,, CF3
0
CF
........N....,
.........N.....,
.........N.,...., c NN,.....
N
CF, N ,
1....**..'N
[---.... ...-1.... ,
N.,.....õ....õ... "........
LV N'Th
1...,õõ....N...,..õ,....5
5 5 5 5 5
CF
, . *
CFe
........N.,,,
' .
,
, = r N.,.....
.,,,k1....,
. .
N . :
......õ.N.õ..õ,.....,,,N........, , CN
CN
I
.......,N,..... 5
I N
H
5 5 5 5 5
,
,' CF3 l' CF3 ,=
CF i ilo CF
=,'' CF3
,
,
(....,N .......A....., .'.
CN
N
1
F 5 0 0 1 H 5 5 5 5
5
,
.
, CF,,
3
. CF, i , CF
,
. =
CF,
,
,
F F * N
erN N
N
'.
CN
CF
5 5 5 5
i CF,
,
F
c ) \ ........( 0F3 / 0 CF3
5 /
.= el ./
I 5 I 01 CN 0F35 CN
5 5 5
,
=
CF,
1 01
,
,
i 40 CF3 / CF ,,,/ CF3
, ,
= /40 C F3 i
, N
,
..,,..."N,....... )
0
OMe F 5 5 5 5 OH 1 \ /
0 \-N
---
/
5
16
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
CF33 ,. 10) CF3
, / C Fs
N ,
c
1
,
5 5 5 ' 5
i CF / CF,
CF3 0
. . ,
CF,
.'
õ' CF3 .
...õ,,,1\1,....
0 ....õõNõ...... ,
0
N erN
(:)....õ, NH
H A 6 \ ....-.,
..,..--- 0 OH
5
5 5 5 5
. CF,
/ CF, I
. ,
. CF3 / Ili = =
. CF3
. '
. .=
CF,
N
0
N.........N....,
NH (N)
N Y 5 5 5 5 5 5
: CF
= 0 3 i CF3 ri 0 CF3
,== CF3 / CF3
N
cN ,,,,,, 0 N
N N
N''''''''',
N N H 0 I 1 1 H
5 5 5 5 5 5
' CF3 /
. 1110 CF3
N
N N N3m111111/
Ni....N/
N \ 5 5 5 5 5
,I 0 CF3 CF3
, / 0 CF3
.
, .
,
= .
0 . ' CF3 =
.õ..,N,..õ.
...õ.....".õ
101
. 0
.= N
N L.,3 5
F, C.\ 1
5 N¨NH 5 CN 5 CN 5
17
CA 03064975 2019-11-26
WO 2018/215668 PCT/EP2018/063957
lio CF3 .,./ 0 CF
i 3
:" CF3
0
.=
CF3
=
,
,, HN
zN ./ CF3 =0
HN0
µI __ ( = CN
----,N.-----
--............ 5
F 1
5 5 5 5
0
,, CF3
l'i CF3 CF3
i Iliiii
CF3
N
<>./.....
`.....,
H2N 0 0 NH2 5 ON
, 0 5 l' CF 5 5
: CF3
= .. . 1 0
, ,' CF3
cHs
.........N . i
N
..........N..õ, .
......õN.õ.... .. ,
,
,= 0 SO2Me 11
i
.. ) I 41111 OH N
3 5 HO
5 5 CF 5 5 5
==1 Am SO2Me
/ CF
111W
,
F F
/ CH F2 .../ SO2 Et
0
N
I
5 5 5 5
=
',/ SO2Me
=,.." 0F3 .3...i CF3
CF3
........,N,......
N 11 H ...../ õI
so2NH2
5 L.N. CN 5 HO OH 5
5 5
CF3
Or OH ,
18
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
According to yet another embodiment specifically provided are compounds of
=
I
(R1 )t
formula (I), in which Ring Q is
. In this embodiment, Rl is Ci_salkyl (e.g.
methyl) or haloCi_salkyl (e.g. trifluoromethyl). In yet another embodiment,
't' is 2.
According to yet another embodiment specifically provided are compounds of
formula (I), in which Ring Q is
According to yet another embodiment specifically provided are compounds of
formula (I), in which Ring Q is
According to yet another embodiment specifically provided are compounds of
Rio
formula (I), in which Ring Q is
According to yet another embodiment specifically provided are compounds of
formula (I), in which Rl is Ci_salkyl (e.g. methyl) or C6_14aryl. In this
embodiment, C6_14aryl
is optionally substituted with one or more substituents selected from cyano,
or amide.
19
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
According to yet another embodiment specifically provided are compounds of
CN 0
401 CN NH2
NN
/\
formula (I), in which Ring Q is
Or
CH3
tRN
According to yet another embodiment specifically provided are compounds of
formula (I), in which `p' is 0 or 1.
According to yet another embodiment specifically provided are compounds of
formula (I), in which `q' is 0 or 1.
According to yet another embodiment, specifically provided are compounds of
formula (I), in which
A is CH or N;
D is CH2, CH-CH3, CH-CH2-CH3, C(CH3)2, CH-CH(CH3)2, CH-CH2OH, CH-CH2-0-
HC
CH3, 0, CH-CH2-N(CH3)2, CO or cycloalkyl;
E is CH2, CH-CH3, C(CH3)2, (CH2)2, N-CH3 or CO;
F is 0, CH2, CHOH or CO;
R5 is hydrogen, chloro or cyano;
R7 is hydrogen or methyl;
Z is 0, NH or S;
R6 is chloro, fluoro, methyl, trifluoromethyl or methoxy;
y
N 'N "NH'
Lis 0 H ' Or 0 0 =
5 5 5
CA 03064975 2019-11-26
WO 2018/215668 PCT/EP2018/063957
Ring Q is
---
(NN /
f----NN--- ,
r---NN------..
5 5 5
CF3 ,
.,' CF3
r.......µ CF30 ,
, '
NN......) N,N)
5 5 5
i CF3
.,
i 0 ,
.,
,
.,
,
0 0
-..,..............,õNH5 ..,.............,0 5
N.,....,,5)
5 5
CF= 3
01
/ CF,
,
,....N..,1 ,
(NN\
5 I
5 Br 5 0 5
0
, CF3
21 ,' Br
:; Iso CF 3
NN (NN
1
NN....) NN.....) ON
5 5 5
i/. CF3
/
CF 0 CF3
,
.,../N,.......
=
. , ..../N,....... ,
,
=== CF3 s ' . N
H ' , 0 CF305,..õ.....,
NN.,,,,......õõ...N.....õ
5 5 5 5 5
, 0 CF3
CFs
,
, ill CF,
,
,
. . CFs
L N I-.
= .....,N......., ,
.....,,N
Nrklõ....,
'''NI...,v
, CF, ........................,......
,
,
i
N...,..........,
H ...,,,,....,,,.N õ.........õ, 5 NI'''
I
õ......N,...., 5
5 5 5
,
CF ,
; CF,
,
,
/ so C F3 3 / cFe
CN
,
,
, /
'
, r N....,
,
,
,
/ NN CN
I N
H N
H
5 5 5 5 5 5
21
CA 03064975 2019-11-26
WO 2018/215668 PCT/EP2018/063957
,
,
õ'
CF
,
,
F F
erN ........N......,
N \ ...Z N .
. '
CF3
CN ./
CN
I......,.S.,
5 5 5 5 5
.' "i' cFs
. CF,
, .
. õ CF,
, I., CF3 õ
.
.
* / CF, '
7 N N N N
N
\ ...Z c ) ) N
c ) c"
........(
) N¨ ¨1\1'
''F 5 / 5 I I I5 I
5 5
5
F ,/ oli CF3
//'
CF3
1= CFCF
3 / / ' ao CF35 /.1 . 11) CN OF3 5 CN OMe F
5 5 5 5
:
CF3
CF, / CF s '
CF
=
C F3 / O F3
N erN
..,,,,N,....... C ) \ .........
.,../N,,,..
0
OH 1 /
0 IA--
/ N¨
I X
F F
5 5 5 5 5
5
r:
CF3
/ CF3
'
CF3
0.........N.,..,
N 0
c ) N
( ) ./.' CF3
NN......)
(NN/Nõ.....CN
N N
5 tH 5 NH 5 H A
5 5 5
i CF
.' . .. CF, ,.'
CF3
0 CF3 N
C ) .
./ 0 CF, ii CF3 == lei
N trõN ..õ.../N,......
....õ..,N,......
(:)......,,NH
6 \ ........( NH
N */ N
% ........."....õ 5
0 OH 5 H H
5 5 5 5
.' CF,
.
, . 3 õ' 0 CF3
, .
CF3 1.' CF . ,
O......õ..N,,, il CF CF, . 0
N ..õ..N.õ,.... cN 0
N
...õõ,N.3,.."
.........N.........
%,..õ.., ,...,...
Y N N N/ /
H H 0
5 5 5 5 5 5
5
22
CA 03064975 2019-11-26
WO 2018/215668 PCT/EP2018/063957
... 40 CF, / 0 CFs i Fs ,,, ,CF3 , ill CFs
... 0 CF,
. , õN, ,....,N,....,.. ...........N...., N
0
N N N""'''''= N N/
1
I 5 I 5 I 5 I .--rN,.......
5
5
/,''' 410 CF3
,
,'. CF3 /
, ,
' CF, ,
0 ,
.. CF,
.........."
,
=
=
/NN
/ NO.......,.< O./
i \
\ 5 F 5 1 N-NH 5
5 5 5
,
,
, CF3 /
/ CF3
,
/ ail
C F3
,,./ CF3
,
..õ,...,N,..,
N
III HN,.......0 N
µ _________________________________________________________ (
, / 0 CF3
'
CN CN 5 .............. 5
5 F
5
5
,
.. 0 CF3
,
. .
..= 0 c3 .
. ,..
CF,
,
,
. CF3 11
, HN...,...0
. HN,.......0 N
CN
<> (.> ..----..
----..N../...
1 5 5 5 5
.....)õ,,,,N
N
..."'N',.. 5 H2N 0
5
,
; CF3
1
O
' CF ; ,
CF3
' 3 .
, ' P
=
,
CH3 / ........NN
N
11 C ) .........N....,
N N 0 1
5 0 NH2 5 CN --'j I CF3 5 HO
5 5 5
5
0
; CF3 , /
CF3
,
% OH 0 el
SO2Me I I I
, .
% CHF2 I SO2Et
. .
= .
.1 -
,.....,
0
5 5 5 5
5
23
CA 03064975 2019-11-26
WO 2018/215668 PCT/EP2018/063957
010 -..,/ cF3 =, CF3
CF3
SO2NH2
. OH 11 11
ON 5 HO OH 5
5
'
..,, SO2Me
,
.; SO2Me 5/
I II 0 /
,õ..,N".....õ,
CF3 ...õ.'N"..,...
N C
F3
, 1
N
I
5 OH, 5 5 1 5 N........0
5
CN 0
0 0 CN NH2 CH3
I
/ ',............., N \
,
........RN ,/\.......N
1 \ N
A
5 Or / 5
5 `na' is 1 or 2; and
'n' is 0, 1 or 2.
According to an embodiment, specifically provided are compounds of formula (I)
with an ICso value of less than 500 nM, preferably less than 100 nM, more
preferably less
than 50 nM, with respect to MAP4K1 inhibition.
Compounds of the present invention include the compounds in Examples 1-261. It
should be understood that the formulas (I) structurally encompasses all
geometrical isomers,
stereoisomers, enantiomers and diastereomers, N-oxides, and pharmaceutically
acceptable
salts that may be contemplated from the chemical structure of the genera
described herein.
The present application also provides a pharmaceutical composition that
includes at
least one compound described herein and at least one pharmaceutically
acceptable excipient
(such as a pharmaceutically acceptable carrier or diluent). Preferably, the
pharmaceutical
composition comprises a therapeutically effective amount of at least one
compound described
24
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
herein. The compounds described herein may be associated with a
pharmaceutically
acceptable excipient (such as a carrier or a diluent) or be diluted by a
carrier, or enclosed
within a carrier which can be in the form of a tablet, capsule, sachet, paper
or other container.
Dosages employed in practicing the present invention will of course vary
depending,
e.g. on the particular disease or condition to be treated, the particular
compound used, the
mode of administration, and the therapy desired. The compound may be
administered by any
suitable route, including orally, parenterally, transdermally, or by
inhalation. In general,
satisfactory results, e.g. for the treatment of diseases as hereinbefore set
forth are indicated to
be obtained on oral administration at dosages of the order from about 0.01 to
2.0 mg/kg. In
larger mammals, for example humans, an indicated daily dosage for oral
administration will
accordingly be in the range of from about 0.75 to 300 mg, conveniently
administered once, or
in divided doses 2 to 4 times, daily or in sustained release form. Unit dosage
forms for oral
administration thus for example may comprise from about 0.2 to 75 or 150 mg or
300 mg,
e.g. from about 0.2 or 2.0 to 10, 25, 50, 75, 100, 150, 200 or 300 mg of the
compound
disclosed herein, together with a pharmaceutically acceptable diluent or
carrier therefor.
Pharmaceutical compositions comprising Compounds of the Invention may be
prepared using conventional diluents or excipients and techniques known in the
galenic art.
Thus oral dosage forms may include tablets, capsules, solutions, suspensions
and the like.
DETAILED DESCRIPTION OF THE INVENTION
Definitions
The terms "halogen" or "halo" means fluorine (fluoro), chlorine (chloro),
bromine
(bromo), or iodine (iodo).
The term "alkyl" refers to a hydrocarbon chain radical that includes solely
carbon and
hydrogen atoms in the backbone, containing no unsaturation, having from one to
eight carbon
atoms (i.e. Ci_salkyl), and which is attached to the rest of the molecule by a
single bond, such
as, but not limited to, methyl, ethyl, n-propyl, 1-methylethyl (isopropyl), n-
butyl, n-pentyl,
and 1,1-dimethylethyl (t-butyl). The term "C1_6alkyl" refers to an alkyl chain
having 1 to 6
carbon atoms. The term "C1_4alkyl" refers to an alkyl chain having 1 to 4
carbon atoms.
Unless set forth or recited to the contrary, all alkyl groups described or
claimed herein may
be straight chain or branched.
The term "alkoxy" denotes an alkyl group attached via an oxygen linkage to the
rest
of the molecule (i.e. C1-8 alkoxy). Representative examples of such groups are
-OCH3 and -
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
0C2H5. Unless set forth or recited to the contrary, all alkoxy groups
described or claimed
herein may be straight chain or branched.
The term "alkoxyalkyl" or "alkyloxyalkyl" refers to an alkoxy or alkyloxy
group as
defined above directly bonded to an alkyl group as defined above (i.e.
Ci_salkoxyCi_salkyl or
Ci_salkyloxyCi_salkyl). Example of such alkoxyalkyl moiety includes, but are
not limited to, -
CH2OCH3 (methoxymethyl) and -CH20C2H5 (ethoxymethyl). Unless set forth or
recited to
the contrary, all alkoxyalkyl groups described herein may be straight chain or
branched.
The term "haloalkyl" refers to at least one halo group (selected from F, Cl,
Br or I),
linked to an alkyl group as defined above (i.e. haloCi_salkyl). Examples of
such haloalkyl
moiety include, but are not limited to, trifluoromethyl, difluoromethyl and
fluoromethyl
groups. The term "haloC1_4alkyl" refers to at least one halo group linked an
alkyl chain
having 1 to 4 carbon atoms. Unless set forth or recited to the contrary, all
haloalkyl groups
described herein may be straight chain or branched.
The term "haloalkoxy" refers to an alkoxy group substituted with one or more
halogen atoms (i.e. haloCi_salkoxy). Examples of "haloalkoxy" include but are
not limited to
fluoromethoxy, difluoromethoxy, trifluoromethoxy, 2,2,2-trifluoroethoxy,
pentafluoroethoxy,
pentachloroethoxy, chloromethoxy, dichlorormethoxy, trichloromethoxy and 1-
bromoethoxy.
Unless set forth or recited to the contrary, all haloalkoxy groups described
herein may be
straight chain or branched.
The term "hydroxyCi_salkyl" refers to a Ci_salkyl group as defined above
wherein
one to three hydrogen atoms on different carbon atoms is/are replaced by
hydroxyl groups
(i.e. hydroxyCiAalkyl). Examples of hydroxyC1_4alkyl moieties include, but are
not limited to
-CH2OH and -C2H4OH.
The term "cyanoalkyl" refers to a alkyl group as defined above directly bonded
to
cyano group (i.e. cyanoCi_salkyl). Examples of such cyanoCi_salkyl moiety
include, but are
not limited to, cyanomethyl, cyanoethyl and cyanoisopropyl. Unless set forth
or recited to the
contrary, all cyanoalkyl groups described herein may be straight chain or
branched.
The term "cyanohaloalkyl" refers to cyanoalkyl group substituted with one or
more
halogen atoms (i.e.cyanohaloCi_salkyl). Example of cyanohaloalkyl include but
are not
limited to cyanodifluoromethyl. Unless set forth or recited to the contrary,
all cyanohaloalkyl
groups described herein may be straight chain or branched.
The term "cycloalkyl" denotes a non-aromatic mono or multicyclic ring system
of 3
to about 12 carbon atoms, (i.e.C3_12cycloalkyl). Examples of monocyclic
cycloalkyl include
but are not limited to cyclopropyl, cyclobutyl, cyclopentyl, and cyclohexyl.
Examples of
26
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
multicyclic cycloalkyl groups include, but are not limited to,
perhydronapthyl, adamantyl and
norbornyl groups, bridged cyclic groups or spirobicyclic groups, e.g.,
spiro(4,4)non-2-yl. The
term "C3_6cycloalkyl" refers to the cyclic ring having 3 to 6 carbon atoms.
Examples of "C3-
6cyc10a1ky1" include but are not limited to cyclopropyl, cyclobutyl,
cyclopentyl, or
cyclohexyl.
The term "cycloalkylalkyl" refers to a cyclic ring-containing radical having 3
to about
6 carbon atoms directly attached to an alkyl group (i.e.
C3_6cycloalkylC1_8alkyl). The
cycloalkylalkyl group may be attached to the main structure at any carbon atom
in the alkyl
group that results in the creation of a stable structure. Non-limiting
examples of such groups
include cyclopropylmethyl, cyclobutylethyl, and cyclopentylethyl.
The term "cyanocycloalkyl" refers to a cyclic ring-containing radical having 3
to
about 6 carbon atoms directly attached to cyano group (i.e.
"cyanoC3_6cycloalkyl). Non-
limiting example of such groups include cyanocyclopropane.
The term "aryl" refers to an aromatic radical having 6 to 14 carbon atoms
(i.e. C6-
',aryl), including monocyclic, bicyclic and tricyclic aromatic systems, such
as phenyl,
naphthyl, tetrahydronapthyl, indanyl, and biphenyl.
The term "heterocyclic ring" or "heterocycly1" unless otherwise specified
refers to
substituted or unsubstituted non-aromatic 3 to 15 membered ring radical (i.e.
3 to 15
membered heterocycly1) which consists of carbon atoms and from one to five
hetero atoms
selected from nitrogen, phosphorus, oxygen and sulfur. The heterocyclic ring
radical may be
a mono-, bi- or tricyclic ring system, which may include fused, bridged or
spiro ring systems,
and the nitrogen, phosphorus, carbon, oxygen or sulfur atoms in the
heterocyclic ring radical
may be optionally oxidized to various oxidation states. In addition, the
nitrogen atom may be
optionally quaternized; also, unless otherwise constrained by the definition
the heterocyclic
ring or heterocyclyl may optionally contain one or more olefinic bond(s).
Examples of such
heterocyclic ring radicals include, but are not limited to azepinyl,
azetidinyl, benzodioxolyl,
benzodioxanyl, chromanyl, dioxolanyl, dioxaphospholanyl, decahydroisoquinolyl,
indanyl,
indolinyl, isoindolinyl, isochromanyl, isothiazolidinyl, isoxazolidinyl,
morpholinyl,
oxazolinyl, oxazolidinyl, 2-oxopiperazinyl, 2-oxopiperidinyl, 2-
oxopyrrolidinyl, 2-
oxoazepinyl, octahydroindolyl, octahydroisoindolyl, perhydroazepinyl,
piperazinyl, 4-
piperidonyl, pyrrolidinyl, piperidinyl, phenothiazinyl, phenoxazinyl,
quinuclidinyl,
tetrahydroisquinolyl, tetrahydrofuryl or tetrahydrofuranyl, tetrahydropyranyl,
thiazolinyl,
thiazolidinyl, thiamorpholinyl, thiamorpholinyl sulfoxide and thiamorpholinyl
sulfone. The
27
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
heterocyclic ring radical may be attached to the main structure at any
heteroatom or carbon
atom that results in the creation of a stable structure.
The term "heterocyclylalkyl" refers to a heterocyclic ring radical directly
bonded to
an alkyl group (i.e. 3 to 15 membered heterocyclylCi_salkyl). The 20
heterocyclylalkyl
radical may be attached to the main structure at any carbon atom in the alkyl
group that
results in the creation of a stable structure.
The term "heteroaryl" unless otherwise specified refers to 5 to 14 membered
aromatic
heterocyclic ring radical with one or more heteroatom(s) independently
selected from N, 0 or
S (i.e. 5 to 14 membered heteroaryl). The heteroaryl may be a mono-, bi- or
tricyclic ring
system. The heteroaryl ring radical may be attached to the main structure at
any heteroatom
or carbon atom that results in the creation of a stable structure. Examples of
such heteroaryl
ring radicals include, but are not limited to oxazolyl, isoxazolyl,
imidazolyl, furyl, indolyl,
isoindolyl, pyrrolyl, triazolyl, triazinyl, tetrazoyl, thienyl, oxadiazolyl,
thiazolyl, isothiazolyl,
pyridyl, pyrimidinyl, pyrazinyl, pyridazinyl, pyrazolyl, benzofuranyl,
benzothiazolyl,
benzoxazolyl, benzimidazolyl, benzothienyl, benzopyranyl, carbazolyl,
quinolinyl,
isoquinolinyl, quinazolinyl, cinnolinyl, naphthyridinyl, pteridinyl, purinyl,
quinoxalinyl,
quinolyl, isoquinolyl, thiadiazolyl, indolizinyl, acridinyl, phenazinyl and
phthalazinyl.
The term "pharmaceutically acceptable salt" includes salts prepared from
pharmaceutically acceptable bases or acids including inorganic or organic
bases and
inorganic or organic acids. Examples of such salts include, but are not
limited to, acetate,
benzenesulfonate, benzoate, bicarbonate, bisulfate, bitartrate, borate,
bromide, camsylate,
carbonate, chloride, clavulanate, citrate, dihydrochloride, edetate,
edisylate, estolate, esylate,
fumarate, gluceptate, gluconate, glutamate, glycollylarsanilate,
hexylresorcinate,
hydrabamine, hydrobromide, hydrochloride, hydroxynaphthoate, iodide,
isothionate, lactate,
lactobionate, laurate, malate, maleate, mandelate, mesylate, methylbromide,
methylnitrate,
methylsulfate, mucate, napsylate, nitrate, N-methylglucamine ammonium salt,
oleate, oxalate,
pamoate (embonate), palmitate, pantothenate, phosphate, diphosphate,
polygalacturonate,
salicylate, stearate, sulfate, subacetate, succinate, tannate, tartrate,
teoclate, tosylate,
triethiodide and valerate. Examples of salts derived from inorganic bases
include, but are not
limited to, aluminum, ammonium, calcium, copper, ferric, ferrous, lithium,
magnesium,
manganic, mangamous, potassium, sodium, and zinc.
The term "treating" or "treatment" of a state, disorder or condition includes:
(a)
preventing or delaying the appearance of clinical symptoms of the state,
disorder or condition
developing in a subject that may be afflicted with or predisposed to the
state, disorder or
28
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
condition but does not yet experience or display clinical or subclinical
symptoms of the state,
disorder or condition; (b) inhibiting the state, disorder or condition, i.e.,
arresting or reducing
the development of the disease or at least one clinical or subclinical symptom
thereof; or (c)
relieving the disease, i.e., causing regression of the state, disorder or
condition or at least one
of its clinical or subclinical symptoms.
The term "subject" includes mammals (especially humans) and other animals,
such as
domestic animals (e.g., household pets including cats and dogs) and non-
domestic animals
(such as wildlife).
A "therapeutically effective amount" means the amount of a compound that, when
administered to a subject for treating a state, disorder or condition, is
sufficient to effect such
treatment. The "therapeutically effective amount" will vary depending on the
compound, the
disease and its severity and the age, weight, physical condition and
responsiveness of the
subject to be treated.
The compounds of formula (I) may contain asymmetric or chiral centers, and,
therefore, exist in different stereoisomeric forms. It is intended that all
stereoisomeric forms
of the compounds of formula (I) as well as mixtures thereof, including racemic
mixtures,
form part of the present invention. In addition, the present invention
embraces all geometric
and positional isomers. Diastereomeric mixtures can be separated into their
individual
diastereomers on the basis of their physical chemical differences by methods
well known to
those skilled in the art, such as, for example, by chromatography and/or
fractional
crystallization. Enantiomers can be separated by converting the enantiomeric
mixture into a
diastereomeric mixture by reaction with an appropriate optically active
compound (e.g.,
chiral auxiliary such as a chiral alcohol or Mosher's acid chloride),
separating the
diastereomers and converting (e.g., hydrolysing) the individual diastereomers
to the
corresponding pure enantiomers. Enantiomers can also be separated by use of
chiral HPLC
column. The chiral centres of the present invention can have the S or R
configuration as
defined by the IUPAC 1974.
The terms "salt" or "solvate", and the like, is intended to equally apply to
the salt,
solvate and prodrug of enantiomers, stereoisomers, rotamers, tautomers,
positional isomers or
racemates of the inventive compounds.
PHARMACEUTICAL COMPOSITIONS
The compounds of the invention are typically administered in the form of a
pharmaceutical composition. Such compositions can be prepared using procedures
well
29
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
known in the pharmaceutical art and comprise at least one compound of the
invention. The
pharmaceutical compositions described herein comprise one or more compounds
described
herein and one or more pharmaceutically acceptable excipients. Typically, the
pharmaceutically acceptable excipients are approved by regulatory authorities
or are
generally regarded as safe for human or animal use. The pharmaceutically
acceptable
excipients include, but are not limited to, carriers, diluents, glidants and
lubricants,
preservatives, buffering agents, chelating agents, polymers, gelling agents,
viscosifying
agents, solvents and the like.
Examples of suitable carriers include, but are not limited to, water, salt
solutions,
alcohols, polyethylene glycols, peanut oil, olive oil, gelatin, lactose, terra
alba, sucrose,
dextrin, magnesium carbonate, sugar, amylose, magnesium stearate, talc,
gelatin, agar, pectin,
acacia, stearic acid, lower alkyl ethers of cellulose, silicic acid, fatty
acids, fatty acid amines,
fatty acid monoglycerides and diglycerides, fatty acid esters, and
polyoxyethylene.
The pharmaceutical compositions described herein may also include one or more
pharmaceutically acceptable auxiliary agents, wetting agents, suspending
agents, preserving
agents, buffers, sweetening agents, flavouring agents, colorants or any
combination of the
foregoing.
The pharmaceutical compositions may be in conventional forms, for example,
capsules, tablets, solutions, suspensions, injectables or products for topical
application.
Further, the pharmaceutical composition of the present invention may be
formulated so as to
provide desired release profile.
Administration of the compounds of the invention, in pure form or in an
appropriate
pharmaceutical composition, can be carried out using any of the accepted
routes of
administration of such compounds or pharmaceutical compositions. The route of
administration may be any route which effectively transports the active
compound of the
patent application to the appropriate or desired site of action. Suitable
routes of
administration include, but are not limited to, oral, nasal, buccal, dermal,
intradermal,
transdermal, parenteral, rectal, subcutaneous, intravenous, intraurethral,
intramuscular, and
topical.
Solid oral formulations include, but are not limited to, tablets, capsules
(soft or hard
gelatin), dragees (containing the active ingredient in powder or pellet form),
troches and
lozenges.
Liquid formulations include, but are not limited to, syrups, emulsions, and
sterile
injectable liquids, such as suspensions or solutions.
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
Topical dosage forms of the compounds include, but are not limited to,
ointments,
pastes, creams, lotions, powders, solutions, eye or ear drops, impregnated
dressings, and may
contain appropriate conventional additives such as preservatives, solvents to
assist drug
penetration.
Suitable doses of the compounds for use in treating the diseases and disorders
described herein can be determined by those skilled in the relevant art.
Therapeutic doses are
generally identified through a dose ranging study in humans based on
preliminary evidence
derived from the animal studies. Doses must be sufficient to result in a
desired therapeutic
benefit without causing unwanted side effects. Mode of administration, dosage
forms, and
suitable pharmaceutical excipients can also be well used and adjusted by those
skilled in the
art.
METHODS OF TREATMENT
The compounds of Formula I, as described herein are highly effective
inhibitors of the
MAP4K1 kinase, producing inhibition at nanomolar concentrations. MAP4K1
inhibitors
according to the invention are therefore useful for treatment and prophylaxis
of diseases
associated with protein kinase signaling dysfunction. Accordingly, without
being bound by
any theory, it is believed that inhibition of MAP4K1 could, for example,
reverse or prevent
the cellular dysfunction associated with perturbations of the INK signaling
pathway,
especially in T and B cells. Therefore, administration of a MAP4K1 inhibitor
as described
herein could provide a potential means to regulate MAPK signal transduction
pathways,
especially the INK pathway, and by extension provide a treatment for a variety
of diseases
and disorders including autoimmune, neurodegenerative, neurological,
inflammatory,
hyperproliferative, and cardiovascular diseases and disorders.
In addition, without being bound by theory, selective MAP4K1 inhibition, as
provided
by the Compounds of the Invention, may provide a novel means of cancer
treatment.
Traditional signal transduction strategies relate to interference with the
pathways that
promote tumor cell proliferation or metastasis. The present invention provides
instead a
means of enhancing the activity and effectiveness of the body's T cells, for
example, to
overcome the immunosuppressive strategies used by many cancers. The U.S. Food
and Drug
Administration (FDA) has recently approved some monoclonal antibody-based
treatments
that achieve the same result by interfering with T-cell surface receptors
which promote
inhibition of TCR activity (e.g., anti-CTLA-4 and anti-PD-1 antibodies,
marketed as
Ipilimumab and Pembrolizumab, respectively). The success of the treatments
demonstrate
proof of the concept that cancer can be effectively treated by interfering
with pathways which
31
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
inhibit TCR signaling, Targeting these pathways using a small molecule
inhibitor of
MAP4K1 should produce improved results using more patient-friendly
administration
techniques.
Therefore, in the third aspect, the invention provides a method for the
treatment or
prophylaxis of a disease or disorder which may be ameliorated by modulating
(e.g.,
inhibiting) MAP4K1-dependent signaling pathways, including the JNK pathway,
e.g.,
autoimmune, neurodegenerative, neurological, inflammatory, hyperproliferative,
and
cardiovascular diseases and disorders, comprising administering to a patient
in need thereof
an effective amount of the compound of Formula I as described herein, in free
or
pharmaceutically acceptable salt form.
In particular embodiments, administration of the compound of Formula I results
in
enhanced T cell receptor (TCR) signaling, such as resulting in an enhanced T
cell-mediated
immune response (e.g., increased T cell cytokine production).
In other particular embodiments, administration of the compound of Formula I
results
in increased T cell resistance to PGE2-mediated T cell suppression.
The disease or disorder may be selected from the group consisting of:
neurodegenerative diseases, such as Parkinson's disease or Alzheimer's
disease; stroke and
associated memory loss; autoimmune diseases such as arthritis; allergies and
asthma;
diabetes, especially insulin-resistant diabetes; other conditions
characterized by
inflammation, including chronic inflammatory diseases; liver ischemia;
reperfusion injury;
hearing loss or deafness; neural tube birth defects; obesity;
hyperproliferative disorders
including malignancies, such as leukemias, e.g. chronic myelogenous leukemia
(CML);
oxidative damage to organs such as the liver and kidney; heart diseases; and
transplant
rejections. In certain embodiments, the disease or disorder to be treated may
also relate to
impaired MAP4K1-dependent signaling. Impaired MAP4K1 signaling can lead to
reduced
immune cell, e.g. T and B cell, function which can permit or enhance the
escape of nascent
cancer cells from immune surveillance. Restoration of T and B cell function
via treatment
with a MAP4K1-inhibitor can therefore promote the clearance of carcinogenic
and pre-
carcinogenic cells from the body. Thus, in a particular embodiment, the
invention provides a
method for the treatment or prevention of hyperproliferative diseases, such as
cancer,
including melanomas, thyroid cancers, adenocarcinoma, breast cancer, central
nervous
system cancers such as glioblastomas, astrocytomas and ependymomas, colorectal
cancer,
squamous cell carcinomas, small and non-small cell lung cancers, ovarian
cancer,
endometrial cancer, pancreatic cancer, prostate cancer, sarcoma and skin
cancers. In
32
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
particular embodiments, owing to the unique role of immune cell dysfunction in
hematologic
cancers, the invention provides a method of treatment or prevention of
hematologic cancers
such as leukemias, acute myelogenous leukemia (AML), myelodysplastic
syndromes, chronic
myelogenous leukemia (CML), Hodgkin's lymphoma, non-Hodgkin's lymphoma,
megakaryoblastic leukemia, and multiple myeloma.
The MAP4K1 inhibitor compounds described herein for the treatment or
prophylaxis
of disease or disorder according to the foregoing methods may be used as a
sole therapeutic
agent or may be used in combination with one or more other therapeutic agents
useful for the
treatment of said diseases or disorders. Such other agents include inhibitors
of other protein
kinases in the JNK pathway, including, for example, inhibitors of INK (e.g.,
JNK1 or JNK2),
MKK4, MKK7, p38, MEKK (e.g., MEKK1, MEKK2, MEKK5), and GCK,
Therefore, in a particular embodiment, the MAP4K1 inhibitor of the invention
may be
administered in combination with inhibitors of INK (e.g., JNK1 or JNK2), MKK4,
MKK7,
p38, MEKK (e.g., MEKK1, MEKK2, MEKK5), and GCK.
In another aspect, the invention provides the following:
(0 the compound of Formula I as described herein, in free or
pharmaceutically acceptable salt form, for use in any of the methods or in
the treatment or prophylaxis of any disease or disorder as set forth herein,
(ii) a combination as described hereinbefore, comprising a MAP4K1 inhibitor
of the invention, e.g., the compound of Formula I as described herein, in
free or pharmaceutically acceptable salt form and a second therapeutic
agent useful for the treatment or prophylaxis of any disease or disorder set
forth herein;
(iii) use of the compound of Formula I in free or pharmaceutically acceptable
salt form, or the combination described herein, (in the manufacture of a
medicament) for the treatment or prophylaxis of any disease or condition
as set forth herein,
(iv) the compound of Formula I in free or pharmaceutically acceptable salt
form, the combination described herein or the pharmaceutical composition
of the invention as hereinbefore described for use in the treatment or
prophylaxis of any disease or condition as set forth herein.
GENERAL METHODS OF PREPARATION
33
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
The compounds, described herein, including those of general formula (I),
intermediates and specific examples are prepared through the synthetic methods
as depicted
in Schemes 1 to 14. Furthermore, in the following schemes, where specific
acids, bases,
reagents, coupling reagents, solvents, etc. are mentioned, it is understood
that other suitable
acids, bases, reagents, coupling reagents, solvents etc. may be used and are
included within
the scope of the present invention. The modifications to reaction conditions,
for example,
temperature, duration of the reaction or combinations thereof, are envisioned
as part of the
present invention. The compounds obtained using the general reaction sequences
may be of
insufficient purity. These compounds can be purified using any of the methods
for
purification of organic compounds known to persons skilled in the art, for
example,
crystallization or silica gel or alumina column chromatography using different
solvents in
suitable ratios. All possible geometrical isomers and stereoisomers are
envisioned within the
scope of this invention.
General schemes
A general approach for the preparation of compounds of the formulae (Ia)
(wherein
R5, R6, R7, A, E, F, D, Z, Q and n are as defined in the general description)
is depicted in
synthetic scheme 1.
Synthetic Scheme 1
Hal
(R6)n
WAN
' =R'
(R6)n 0
r R7 (2)
)1- elLX
'R'
HZ substitution reaction
0 R N.6
(1) (3) 17
H2N,Q coupling coupling H2N.Q
(4 reaction reaction
)
(4)
Hal (R6)n
n
A)F'E
"Thr Q
(R6). R5): AN N.6 ZL
eLXFE
HZ .Q R7 (2)
R5AN N.15
substitution reaction I7
(5) (la)
The substitution reaction of compound of formula (1) (wherein R' = Me or Et)
with
halogen bearing compound of formula (2) in the presence of a suitable reagent
and solvent
yields the compound of formula (3). The reaction may be carried out in the
presence of
34
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
suitable base such as cesium carbonate, potassium carbonate, sodium carbonate,
cesium
fluoride etc., and the solvent can be selected from DMF, DMSO, acetonitrile,
1,4-dioxane or
a mixture thereof. The reaction can also be performed by Buchwald reaction
using a suitable
base such as tripotassium phosphate, sodium or potassium tert-butoxide, cesium
carbonate,
etc., in the presence of palladium acetate as catalyst and a suitable hindered
ligand (eg.
XPhos, di-tBuXPhos, JohnPhos, DavePhos, Sphos, etc.) in an appropriate solvent
such as
toluene, 1,4-doxane, water or a mixture thereof (Ref: Angew. Chem. Int. Ed.
2006, 45, 4321)
The coupling reaction of the ester of formula (3) with amine of formula (4) in
the presence of
a suitable reagent and solvent affords the compound of general formula (Ia).
The suitable
base used in the reaction may be potassium tert-butoxide or trimethyl
aluminium solution.
The coupling reaction may be carried out in a suitable solvent or mixture
thereof. The
suitable solvent may be selected from dichloromethane, THF, toluene, or a
combination
thereof Alternatively, the coupling reaction of compound of formula (1) with
the amine of
formula (4) gives amide of formula (5) which on substitution reaction with
compound of
formula (2) furnishes the compound of general formula (I).
A general approach for the preparation of compounds of the formulae (Ha)
(wherein
Rl, R2, R3, R4, R5, R6, A, F, Z, Q and n are as defined in the general
description) (and P =
protecting group, like Boc or PMB) is depicted in synthetic scheme 2.
Synthetic Scheme 2
Hal R4 (R6)n
AL
J:
(R6)n A-Fi2 4 Z 0
R N N Ri R p
L .R, 15 (6) R31'' 1Link
0 R2--L
substitution reaction R1 N N R5
(1) 15 (7)
H2N.r, coupling coupling H2N.Q
(4)-` reaction reaction
(4)
Hal R4
F'(--"R3 (R6 )n
(R6)n
R N R1
I-' 6
) R4 Z 0
HZ 0 i. substitution reaction
R32ZF A
deprotection R R1 NN:LR5
(5) H (Ha)
The substitution reaction of compound of formula (1) (wherein R' = Me or Et)
with
halogen bearing compound of formula (6) in the presence of a suitable reagent
and solvent
yields the compound of formula (7). The reaction may be carried out in the
presence of
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
suitable base such as cesium carbonate, potassium carbonate, sodium carbonate,
cesium
fluoride etc., and the solvent can be selected from DMF, DMSO, acetonitrile,
1,4-dioxane or
a mixture thereof. The reaction can also be performed by Buchwald reaction
using a suitable
base such as tripotassium phosphate, sodium or potassium tert-butoxide, cesium
carbonate,
etc., in the presence of palladium acetate as catalyst and a suitable hindered
ligand (eg.
XPhos, di-tBuXPhos, JohnPhos, DavePhos, Sphos, etc.) in an appropriate solvent
such as
toluene, 1,4-doxane, water or a mixture thereof The coupling reaction of the
ester of formula
(7) with amine of formula (4) in the presence of a suitable reagent and
solvent directly affords
the deprotected final compound of general formula (Ha). The suitable base used
in the
reaction may be potassium tert-butoxide or trimethyl aluminium solution. The
coupling
reaction may be carried out in a suitable solvent or mixture thereof The
suitable solvent may
be selected from dichloromethane, THF, toluene, or a combination thereof.
Alternatively, the
coupling reaction of compound of formula (1) with the amine of formula (4)
gives amide of
formula (5) which on substitution reaction with compound of formula (6)
followed by
deprotection furnishes the compound of general formula (Ha). The reaction
conditions for the
alternative sequence may remain the same as described in scheme 1.
Deprotection reaction
may be carried out using hydrochloric acid or trifluoroacetic acid in suitable
solvent such as
methanol, ethanol, ethyl acetate, 1,4-dioxane, dichloroethane, etc.
A general approach for the preparation of compounds of the formulae (JIb)
(wherein
R5, R6, R7, A, E, F, D and n are as defined in the general description) is
depicted in synthetic
scheme 3.
Synthetic Scheme 3
F F
(R 6)n H2N OEt
6,
(R 6,
(9)0
/Or H F F (R \' I H F F
I OH N :Me0H NH2
0 coupling reaction 0 OEt NH3
HO N
TBDMS TBDMS o 0
(8) (10) (11)
Hal (R6)n (R 6)n
H F F
R5AN N b
0 N NI12 F F
0
117 (2)
b
ink)F'E 0 Burgess reagent 1.1 CN
in(ly'E
substitution
R5 A N' X N. b
reaction R5 N N'
(12) R7 (11b)
The benzoic acid derivative of formula (8) on coupling reaction with ethyl 2-
(3-
aminopheny1)-2,2-difluoroacetate (9) yields the amide derivative of formula
(10). The
reaction may be carried out via acid chloride formation using oxalyl chloride
or thionyl
36
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
chloride followed by coupling with the amine in the presence of suitable base
and solvent.
The suitable base for the reaction may be triethylamine, N,N-
diisopropylethylamine, pyridine
or DMAP and solvent may be selected from THF, chloroform, dichloromethane or
1,4-
dioxane. The reductive amination and deprotection of compound of formula (10)
using
ammonia solution in methanol at elevated temperature (above 50 C) affords the
compound
of formula (11). Substitution of compound of formula (11) with halogen
derivative (2) yields
the compound of formula (12). The reaction may be carried out in the presence
of suitable
base such as cesium carbonate, potassium carbonate, sodium carbonate, cesium
fluoride etc.;
and the solvent can be selected from DMF, DMSO, acetonitrile, 1,4-dioxane or a
mixture
thereof The amide group of compound of formula (12) converts to the nitrile
group by
reaction with Burgess Reagent to give compound of general formula (III)). The
reaction may
be carried out in a suitable solvent such as dichloromethane.
A general approach for the preparation of compounds of the formula (Ma) and
(Mb)
(wherein Rl, R2, R3, R4, R5, R6, A, F, Z, Q and n are as defined in the
general description)
(and wherein R' = Me or Et and P = protecting group, like Cbz, Boc or PMB) is
depicted in
synthetic scheme 4.
Synthetic Scheme 4
Hal
R4
eL,(1)1--R3
5)t 'L .-k-R2
1:1 N N Fit
(6)
HNQ (4) 1 )
(R6) 2 -
, (R6), 0
i. triphosgene, solvent i. substitution reaction
Z
ii. deprotection
HZ H H ii. deprotection
(13') (14') /
Hal
I 6 6
), )n protection ink)LxFLRR43 (R
A , 1µ-R2
0-NI-12
H (R 0
.
(R6)n R- N N. R1 04 7 -12N0NyTh 'R0 N. ,
lr Q R4
-NH L
H H
P (6) R 3"¨r FlA i. (4) ' or (4) 0 ... R3_),FINA
2
, 0.
HZ substitution reaction R2---L J1, 5 ii.
deprotection R24 AR 5
R1 N N R R1 N
(13)
15 (14) H
(111a)
0
coupling HOAQ
reaction
(4')
(R6), (R6),
0 0
, K
z'CNAQ z0N Q H
R4 H deprotection R4 E
R301' Link LA
R----c,I õ.:1...,5 R)r
õ.(r.,5
RI N iN ri R 1 N iN ri
1) (15) H
(111b)
37
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
The amino phenol compound of formula (13) on reaction with compound of formula
(6) yields the compound of formula (14). The reaction may be carried out in
the presence of
suitable base such as cesium carbonate, potassium carbonate, sodium carbonate,
cesium
fluoride etc.; and the solvent can be selected from DMF, DMSO, toluene,
acetonitrile, 1,4-
dioxane or a mixture thereof. The amine compound of formula (14) on step wise
reaction
with triphosgene and amine of formula (4) in THF; followed by deprotection
yields the urea
derivative of general formula (Ma). Deprotection reaction may be carried out
using
hydrochloric acid or trifluoroacetic acid in suitable solvent such as
methanol, ethanol, ethyl
acetate, 1,4-dioxane, dichloroethane, etc. Alternatively, Compound of general
formula (Ma)
may be synthesized by the reaction of a carbamate derivative of amine (4')
with compound
(14) in the presence of a suitable base and solvent. The suitable base for the
reaction may be
triethylamine, DIPEA, etc. and the suitable solvent may be DMSO. Compound of
general
formula (Ma) can also be synthesized by an alternative sequence of reaction
starting from Z-
protected analogue (13') of compound (13). The compound of formula (13') on
step wise
reaction with triphosgene and amine of formula (4) in THF; followed by Z-
deprotection
yields the urea derivative of formula (14'). Compound (14') on reaction with
compound of
formula (6) yields the compound general formula (Ma). The reaction conditions
may remain
same as discussed above.
Alternatively, the amine of formula (14) on reaction with acid compound of
formula
(4') yields the amide compound of formula (15). The coupling reaction may be
carried out in
the presence of suitable coupling agent such as HATU, EDCI.HC1 with or without
HOBt,
T3P or DCC. The reaction may be carried out in suitable solvent selected from
THF,
dichloromethane, dichloroethane, chloroform, 1,4-dioxane or a mixture thereof
The
compound of formula (16) on deprotection yields the compound of general
formula (Mb).
Deprotection reaction may be carried out using hydrochloric acid or
trifluoroacetic acid in
suitable solvent such as methanol, ethanol, ethyl acetate, 1,4-dioxane,
dichloroethane, etc.
A general approach for the preparation of compounds of the formula (IIc)
(wherein
Rl, R2, R3, R4, R5, R6, A, F, Q and n are as defined in the general
description) (and wherein
R' = Me or Et; P = protecting group, like Boc or PMB) is depicted in synthetic
scheme 5.
Synthetic Scheme 5
38
CA 03064975 2019-11-26
WO 2018/215668 PCT/EP2018/063957
(R6)n H2N.Q (R6)n,
(R6),,
(4) nitro reduction _H2N..._TrH
" -rr N,Q
021N 0 coupling 02N Q
0 0
reaction
(16) (17) (18)
Hal (R6)n, (R6)n,
R4
^¨R2 4 Q Q
,R F n FO
deprotection
`---1\
catalyst, base, solvent Fit N 171
N R- R1 N R-
P H (19) (11c)
The coupling reaction of the nitro ester of formula (16) with amine of formula
(4) in
the presence of a suitable reagent and solvent affords the compound of general
formula (17).
The suitable base used in the reaction may be potassium tert-butoxide or
trimethyl aluminium
solution. The coupling reaction may be carried out in a suitable solvent or
mixture thereof.
The suitable solvent may be selected from dichloromethane, THF, toluene, or a
combination
thereof Nitro reduction compound of formula (17) yields the amine of formula
(18). The
reaction may be carried out using iron powder in the presence of acetic acid
or ammonium
chloride in appropriate solvent such as methanol, ethanol, THF, water or a
mixture thereof.
The substitution reaction of compound of formula (18) with halogen bearing
compound of
formula (6) in the presence of a suitable reagent and solvent yields the
compound of formula
(19). The reaction may be performed using Buchwald coupling method in the
presence of
suitable base, catalyst, ligand and solvent. The reaction may be performed
using base such as
sodium or potassium tert-butoxide, cesium or potassium carbonate, etc.
palladium acetate can
be used as a catalyst along with a suitable ligand (eg. XPhos, t-BuXPhos,
JohnPhos) and
appropriate solvent can be selected from 1,4-dioxane, toluene, water or a
mixture thereof.
The compound of formula (19) on deprotection yields the compound of general
formula (IIc).
Deprotection reaction may be carried out using hydrochloric acid or
trifluoroacetic acid in
suitable solvent such as methanol, ethanol, ethyl acetate, 1,4-dioxane,
dichloroethane, etc.
A general approach for the preparation of compounds of the formula (lid) and
(He),
(III) and (IIg) (wherein 1V, R25 R35 R45 R55 65
K A, Q and n are as defined in the general
description) (and P = protecting group, like Boc or PMB) is depicted in
synthetic scheme 6.
Synthetic Scheme 6
39
n,
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
Hal ?
51F..y (1,16) Hal 0 RR42
(1,16)n.
H H
(R6)11, B 3 .
H R5 N N (3c N,c)
.0_11,11,Q
H R N N Ri 3y(x.,1,, 0 4 OH 0
0
0 0 0
base HO 0 substitution reaction Ri N N R5
Me0H
R iN R5
Boc õ1,...., solvent
11 N R- (24) (5a) P (21) p (22)
H
i. methylamine soln 1
ii. deprotection deprotection i.
trimethylsilane, TEA
ii. deprotection
(1,16)n, (1,16) 6
n.
(R6)n> H h
(R)õ,,, H
H
--''---rr"µQ
....iiN,_
4 OH 0--3-110 NµQ R4 0 0 0
R4 o- 0 Q
0 R31
0 5 R H 0 Q NaBEI4 3 ^ R3..rk
R1 NRNR-1 N:..R5 R 1.2 --
.. -
N N H
H
(11g) (Ile) (11d) R1 HN Not)
1,1'
The compound of formula (5a) with phenolic hydroxyl group on substitution with
halogen bearing compound of formula (20) yields the ether of formula (21). The
reaction may
be carried out in the presence of suitable base and solvent. Suitable base may
be potassium
carbonate, cesium carbonate, cesium fluoride etc. and the suitable solvent may
be DMF,
DMSO, 1,4-dioxane, etc. The compound of formula (21) on N-deprotection affords
the
compound of general formula (lid). Deprotection reaction may be carried out
using
hydrochloric acid or trifluoroacetic acid in suitable solvent such as
methanol, ethanol, ethyl
acetate, 1,4-dioxane, dichloroethane, etc.
Alternatively compound of formula (21) on reaction with sodium borohydride in
a
suitable solvent yields the gives the hydroxyl derivative of formula (22). The
suitable solvent
for the reaction may be THF, methanol or a mixture thereof. The compound (22)
undergoes
further reduction in the presence of trimethylsilane and trifluoroacetic acid
followed by N-
deprotection to furnish the compound of general formula (III). Deprotection
reaction may be
carried out using hydrochloric acid or trifluoroacetic acid in suitable
solvent such as
methanol, ethanol, ethyl acetate, 1,4-dioxane, dichloroethane, etc.
The compound of general formula (lid) on reduction using sodium borohydride
yields the compound of general formula (lie). The suitable solvent for the
reaction may be
THF, methanol or a mixture thereof In another embodiment the compound of
formula (5a)
on reaction with formyl derivative of formula (23) in the presence of base and
solvent affords
the compound of formula (24). Suitable base may be potassium carbonate, cesium
carbonate,
cesium fluoride etc. and the suitable solvent may be DMF, DMSO, 1,4-dioxane,
etc.
The reaction of compound of formula (24) with methylamine solution in the
presence
of catalytic amount of acetic acid followed by N-deprotection yields the
compound of general
formula (hg). Deprotection reaction may be carried out using hydrochloric acid
or
CA 03064975 2019-11-26
WO 2018/215668 PCT/EP2018/063957
trifluoroacetic acid in suitable solvent such as methanol, ethanol, ethyl
acetate, 1,4-dioxane,
dichloroethane, etc.
A general approach for the preparation of compounds of the formula (2a)
(wherein
Rl, R2, R3 and R4 are as defined in the general description) (and P =
protecting group, like
Boc or PMB) is depicted in synthetic scheme 7.
Synthetic Scheme 7
CI
R2 0R3
Rli<OH N
CI HN R3 CI Ri CI
N JJ:0 (26) R40 BBr3 N N 2
H R N-protecting 0 R4
p 1 R2 agent N
:(_Rs'
I base, solventl I I>c ,OH solvent
CI (28) R1 Lz..,
N CI N N base, solvent N N
H R3 Ra
NjC9-1 1; R-
(25) (27) I R> I<Br
N N (2a)
H R3 Fe
(29)
4,6-Dichloro-5-methoxypyrimidine (25) on reaction with appropriately
substituted
ethanolamine derivative of formula (26) in the presence of suitable base and
solvent yields
the compound of formula (27). The suitable base for the reaction may be
potassium carbonate
and solvent may be DMF or 1,4-dioxane. In attempt to cyclize the compound of
formula (27)
using boron tribromide in a suitable solvent such as THF, affords either of
the compound of
formula (28) or (29) or a mixture thereof in varied ratio, which on reaction
with a suitable
protecting agent in the presence of a suitable base and solvent furnishes the
compound of
formula (2a). The N-protecting agent can be tert-butyl dicarbonate (Boc
anhydride) or 4-
methoxybenzylchloride (PMB-C1). The suitable base for the reaction may be
triethylamine,
DIPEA, DMAP, or a mixture thereof and solvent can be selected from THF,
dichloromethane, 1,4-dioxane, DMF or a mixture thereof
A general approach for the preparation of compounds of the formula (2b)
(wherein R3
and R4 are as defined in the general description) is depicted in synthetic
scheme 8.
Synthetic Scheme 8
0
CI CI
H01)(0 CI 0 CI
N
R3 A
AlC13 OH R3R4 (31) N
A NOR
PMBA R-
NCI DCE I TPP, DEAD, solvent R3 R4 base,
solvent I
N CI N -CI N N
PMB
(25) (30) (32) (2b)
The demethylation reaction of 4,6-dichloro-5-methoxypyrimidine (25) with
aluminium chloride in a suitable solvent such as dichloroethane yields 4,6-
dichloropyrimidin-
41
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
5-ol (30) which on reaction with appropriately substituted glycolate ester of
formula (31)
under Mitsunobu condition affords the ethyloxy-ester of formula (32). The
Mitsunobu
reaction may be carried out in the presence of triphenylphosphine, diethyl
azodicarboxylate
(DEAD) or diisopropyl azodicarboxylate (DIAD) in THF as a solvent. The
compound of
formula (32) on reaction with 4-methoxybenzylamine (PMBA) in the presence of a
suitable
base and solvent at elevated temperature (more than 100 C) affords the
cyclized compound
of formula (2b). The suitable base for the reaction may be triethylamine,
DIPEA, DMAP, or
a mixture thereof and solvent can be selected from DMF, 1,4-dioxane, DMSO or a
mixture
thereof
A general approach for the preparation of compounds of the formula (2d)
(wherein Rl
and R2, are as defined in the general description) (and P = protecting group,
like Boc or PMB)
is depicted in synthetic scheme 9.
Synthetic Scheme 9
Rõ1 R2
CI 1
H2N)OH CI 1 CI
N'(:) (26a), Ne-CfRi R2 OH ,
B131-3 NI pi R`
k substitution I )10H solvent
N CI reaction N N )\IN)C)11
H H
(25) (27a) (33)
Cl CI
N-protecting
H J
, c,0
agent N 1IRNI ' TPP, DEAD N
I el R ' I
base, solvent N " '-'OH THF N^N<R2
P
(34)1" (2d)
4,6-Dichloro-5-methoxypyrimidine (25) on reaction with appropriately
substituted
ethanolamine derivative of formula (26a) in the presence of suitable base and
solvent yields
the compound of formula (27a). The suitable base for the reaction may be
potassium
carbonate and solvent may be DMF or 1,4-dioxane. In attempt to cyclize the
compound of
formula (27a) using boron tribromide in a suitable solvent such as THF,
affords the
compound of formula (33) which on N-protection with a suitable protecting
agent in the
presence of suitable base and solvent furnishes the compound of formula (34).
The N-
protecting agent can be tert-butyl dicarbonate (Boc anhydride) or 4-
methoxybenzylchloride
(PMB-C1). The suitable base for the reaction may be triethylamine, DIPEA,
DMAP, or a
mixture thereof and solvent can be selected from THF, dichloromethane, 1,4-
dioxane, DMF
or a mixture thereof The compound of formula (34) on self-Mitsunobu reaction
yields the
cyclized compound of formula (2d).
42
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
A general approach for the preparation of compounds of the formula (2e) and
(21)
(wherein P = protecting group, like Boc or PMB) is depicted in synthetic
scheme 10.
Synthetic Scheme 10
Br N-
protecting Br
Br
OH bromination OH Br
'Br (37) agent n (1(:) C 1 , 1
j , 1 j
NH2
N NH2 base, solvent N N
base, solvent N N
HBr N H
(35) (36) (38) (2e)
0
ClAci base, solvent
(39)
Br
Br N-protecting
0 agent 0 1 1
1 1 N 0 base, solvent N N 0
N
H 15
(40) (2f)
5
Bromination of 2-aminopyridin-3-ol (35) using bromine in absolute ethanol
yields the
hydrobromide salt of 2-amino-4-bromopyridin-3-ol (36). The compound (36) on
reaction
with dibromoethane (37) in the presence of suitable base and solvent at
elevated temperature
(>50 C) yields 8-bromo-3,4-dihydro-2H-pyrido[3,2-b][1,4]oxazine (38). The
suitable base
for the reaction may be potassium or cesium carbonate and solvent can be
selected from
10
acetonitrile, dichloromethane, 1,4-dioxane, THF or a mixture thereof Compound
(38) on N-
protection with a suitable protecting agent in the presence of suitable base
and solvent
furnishes the compound of formula (2e). The N-protecting agent can be tert-
butyl dicarbonate
(Boc anhydride) or 4-methoxybenzylchloride (PMB-C1). The suitable base for the
reaction
may be LiHMDS, triethylamine, DIPEA, DMAP, or a mixture thereof The suitable
solvent
15 can be selected from THF, dichloromethane, 1,4-dioxane, DMF or a mixture
thereof.
Alternatively, 2-amino-4-bromopyridin-3-ol hydrobromide (36) undergoes
cyclization with
chloroacetyl chloride (39) at elevated temperature (> 50 C) in the presence
of suitable base
solvent to yield 8-bromo-2H-pyrido[3,2-b][1,4]oxazin-3(4H)-one (40). The
suitable base for
the reaction may be sodium bicarbonate and solvent may be 2-butanone, water or
a mixture
thereof Compound (40) on N-protection with a suitable protecting agent in the
presence of
suitable base and solvent furnishes the compound of formula (2f). The N-
protecting agent can
be tert-butyl dicarbonate (Boc anhydride) or 4-methoxybenzylchloride (PMB-C1).
The
suitable base for the reaction may be cesium carbonate, and solvent can be
selected from
THF, 1,4-dioxane, DMF or a mixture thereof.
A general approach for the preparation of compounds of the formula (2g) is
depicted
in synthetic scheme 11.
43
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
Synthetic Scheme 11
ci ci OH CI 0 CI 0
CI,
Mg õ, /
N 0 (42) " 1 oxidation N 1 PMBA N
N CI solvent ks.tzõ. 1
solvent, ht
N CI N CI ven ea N N
(41) (43) (44) (2g) PMB
4,6-Dichloropyrimidine-5-carbaldehyde (41) on reaction with vinylmagnesium
chloride (42) in THF affords 1-(4,6-dichloropyrimidin-5-yl)prop-2-en- 1 -ol
(43) which on
oxidation yields 1-(4,6-dichloropyrimidin-5-yl)prop-2-en-1-one (44). The
oxidation reaction
may be carried out using Des -Martin periodinane in dichloromethane. The
compound of
formula (44) on reaction with 4-methoxybenzylamine in the presence of a
suitable base
(optional) and solvent at elevated temperature (> 45 C) affords the cyclized
compound of
formula (2g). The suitable base for the reaction may be triethylamine, DIPEA,
DMAP, or a
mixture thereof and solvent can be selected from DMF, 1,4-dioxane, DMSO or a
mixture
thereof
A general approach for the preparation of compounds of the formula (la)
(wherein R6
and n are as defined in the general description) and X is halogen is depicted
in synthetic
scheme 12.
Synthetic Scheme 12
(R6)n \ 16 OH (R6)nv (R 6 i
\
(46) n
Me0H/H+ , 4r0,
'W 0H
Ph 0
xl>1(3F1 base, solve: Ph(:) 0 0
(48)
0 (
(45) 47)
1 H2/Pd
n
(R6) (R6)
HO ...
nrcy
HO
f>
OH Me0H/H+
N inr0,
0 - 0
(49) (1a)
The compound of formula (45) on reaction with benzyl alcohol (46) in the
presence of
suitable base and solvent yields the compound of formula (47). The suitable
base for the
reaction may be potassium tert-butoxide and solvent may be DMSO. The compound
of
formula (47) undergoes esterification using sulfuric acid in methanol under
reflux conditions
to give the compound of formula (48) which on palladium (palladium on carbon 5-
10%, 50%
wet) catalyzed hydrogenation affords the compound of formula (la). The
hydrogenation
reaction may be performed in a suitable solvent such as ethanol, methanol,
ethyl acetate, or a
combination thereof Alternatively, the benzoic acid of formula (49) on
esterification reaction
using sulfuric acid in methanol affords the methyl ester of formula (la).
44
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
A general approach for the preparation of compounds of the formula (la)
(wherein R6
and n are as defined in the general description) and X is halogen is depicted
in synthetic
scheme 13.
Synthetic Scheme 13
(R6) (R6)n (R6)n,
n
0
0 H H2N (i) NaNO2, HCI
+
HS2 I Me0H/H
I
HS
A 0
(51)
(50) (52) (1 b)
Et0S- K+
The amino benzoic acid derivative of formula (50) on diazotization reaction
using
sodium nitrite and hydrochloric acid followed by reaction with potassium ethyl
xanthate (51)
in the presence of a suitable base such as sodium bicarbonate yields the
thiophenol-benzoic
acid of formula (52). The suitable solvent for the reaction is water. The
benzoic acid of
.. formula (52) on esterification reaction using sulfuric acid in methanol
affords the methyl
ester of formula (lb).
A general approach for the preparation of compounds of the formula (4a)
(wherein R8
and R9 are as defined in the general description) is depicted in synthetic
scheme 14.
Synthetic Scheme 14
02N
p18
H
nitro H2N R8
9
9 reduction
Hal ___________________________ (54)
JI
substitution
reaction
(53) (55) (4a)
The compound of formula (53) (wherein 'hal' = halogen) on reaction with
compound
of formula (54) affords the compound of formula (55). The reaction may be done
in the
presence of suitable base and solvent. The suitable base may be sodium,
potassium or cesium
carbonate, sodium or potassium tert-butoxide, sodium hydride, cesium fluoride,
etc. The
solvent may be selected from THF, DMF, toluene, DMSO, chloroform,
dichloromethane,
acetonitrile, dichloroethane, 1,4-dioxane or a mixture thereof Nitro reduction
of compound
(55) yields the compound of formula (4a). The reaction may be carried out
using iron powder
in the presence of acetic acid or ammonium chloride in appropriate solvent
such as methanol,
ethanol, THF, water or a mixture thereof. Nitro reduction can also be done by
palladium
(palladium on carbon 5-10%, 50% wet) catalyzed hydrogenation. The
hydrogenation reaction
may be performed in a suitable solvent such as ethanol, methanol, ethyl
acetate, or a
combination thereof
Intermediates
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
Methods for the Synthesis of Intermediate A
Intermediate Al
tert-Butyl 4-chloro-6H-pyrimido[5,4-b][1,4]oxazine-8(7H)-carboxylate
CI
NICI
k j
N N
Boc
Step 1: 2((6-Chloro-5-methoxypyrimidin-4-yl)amino)ethanol
CI
NCNNOH
H
To a stirred solution of 4,6-dichloro-5-methoxypyrimidine (10 g, 55.8 mmol) in
1,4-dioxane
(100 mL) were added ethanolamine (3.42 mL, 56.9 mmol) and potassium carbonate
(9.26 g,
67.0 mmol) and the mixture was refluxed at 125 C for 8 h. The mixture was
cooled to RT
and partitioned between ethyl acetate and water. The organic layer was
separated and dried
over anhydrous sodium sulfate. The solution was filtered and concentrated
under reduced
pressure to yield 10.5 g of the desired product. 1H NMR (400 MHz, DMSO-d6) 6
3.44 (t, J =
5.6 Hz, 2H), 3.52 (t, J = 6.8 Hz, 2H), 3.72 (s, 3H), 4.80-4.85 (br s, 1H),
7.55 (s, 1H), 8.04 (s,
1H); ESI-MS (m/z) 204 (M+H)+.
Step 2: 4-Chloro-7,8-dihydro-6H-pyrimido[5,4-b][1,4]oxazine
CI
N0j
I
N N
H
A mixture of 2((6-chloro-5-methoxypyrimidin-4-yl)amino)ethanol (step 1
intermediate) (6.0
g, 29.4 mmol) and boron tribromide in dichloromethane (1.0M, 100 mL) was
refluxed for 3-4
h. The mixture was concentrated and the residue was diluted with water. The
solution was
.. neutralized with saturated sodium bicarbonate solution and the product was
extracted in ethyl
acetate. The organic layer was dried over anhydrous sodium sulfate, filtered
and concentrated
to yield 4.0 g of the desired compound. 1H NMR (400 MHz, DMSO-d6) 6 4.02 (t, J
= 10.4
Hz, 2H), 4.66 (t, J= 10.0 Hz, 2H), 8.46 (s, 1H), 10.31 (br s, 1H).
Step 3: tert-Butyl 4-chloro-6H-pyrimido[5,4-b][1,4]oxazine-8(7H)-carboxylate
To a stirred solution of 4-chloro-7,8-dihydro-6H-pyrimido[5,4-b][1,4]oxazine
(step 2
intermediate) (4.0 g, 23.3 mmol) in dichloromethane (40 mL) were added di-tert-
butyl
dicarbonate (Boc anhydride) (7.6 g, 34.9 mmol) at 0 C followed by
triethylamine (9.7 mL)
and the mixture was stirred at 0 C for 3 h and then 1 h at RT. DMAP (1.4 g,
11.6 mmol) was
46
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
added in small portions to the reaction mixture at 0 C and stirred for 1 h at
RT. The solvent
was evaporated under reduced pressure and the residue was diluted with ice-
water mixture.
The aqueous mixture was neutralized using sodium bicarbonate solution and the
product was
extracted in dichloromethane. The organic layer was dried over anhydrous
sodium sulfate,
filtered and concentrated. The residue thus obtained was purified by silica
gel column
chromatography to yield 1.5 g of the desired compound. 1H NMR (400 MHz, DMSO-
d6) 6
1.49 (s, 9H), 3.92 (t, J= 4.8 Hz, 2H), 4.12 (t, J= 4.4 Hz, 2H), 8.31 (s, 1H).
Intermediate A2
4-Chloro-8-(4-methoxybenzy1)-6H-pyrimido [5,4-b] [1,4] oxazin-7(8H)-one
CI
NO
k 1
N LBO
Step 1: 4,6-Dichloropyrimidin-5-ol
CI
NOH
I
N CI
A suspension of 4,6-dichloro-5-methoxypyrimidine (2.0 g, 11.2 mmol) and
aluminum
chloride (2.0 g, 15.0 mmol) in DCE (10 mL) was heated to reflux for 3-4 h. The
mixture was
concentrated under reduced pressure, cooled to 0 C and quenched with ice-
water. The
mixture was diluted with 1M HC1 (10 mL). The precipitated solid was filtered
and dried well
to yield 1.0 g of the desired compound. 1H NMR (400 MHz, DMSO-d6) 6 8.39 (s,
1H), 11.67
(br s, 1H).
Step 2: Ethyl 2-((4,6-dichloropyrimidin-5-yl)oxy)acetate
CI 0
0,
N
I
N CI
To a mixture of 4,6-dichloropyrimidin-5-ol (step 1 intermediate) (4.0 g, 24.2
mmol), ethyl
glycolate (3.02 g, 29.1 mmol) and triphenylphosphine (12.7 g, 48.5 mmol) in
THF (40 mL)
was slowly added diethyl azodicarboxylate (DEAD) (9.8 g, 48.5 mmol) at RT. The
mixture
was stirred overnight at RT. The mixture was diluted with diethyl ether and
filtered off the
precipitated solid. The filtrate was concentrated under reduced pressure and
the residue was
purified by silica gel column chromatography to yield 450 mg of the desired
compound. 1H
NMR (400 MHz, DMSO-d6) 6 1.34 (t, J= 7.2 Hz, 3H), 4.31 (q, J= 7.2 Hz, 2H),
4.80 (s, 2H),
8.51 (s, 1H).
47
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
Step 3: 4-Chloro-8-(4-methoxybenzy1)-6H-pyrimido[5,4-b][1,4]oxazin-7(8H)-one
To a solution of ethyl 2((4,6-dichloropyrimidin-5-yl)oxy)acetate (step 2
intermediate) (1.0 g,
3.98 mmol) in DMF (10 mL) were added 4-methoxybenzylamine (818 mg, 5.97 mmol)
followed by DIPEA (513 g, 3.98 mmol) at 0 C. The mixture was stirred
overnight at RT and
the 1 h at 130 C. The mixture was cooled and quenched with water. The aqueous
mixture
was extracted twice with dichloromethane. The combined organic layers were
washed with
water followed by brine and dried over anhydrous sodium sulfate. The solution
was filtered,
concentrated and the residue was purified by silica gel column chromatography
to yield 660
mg of the desired compound. 1H NMR (400 MHz, DMSO-d6) 6 3.71 (s, 3H), 5.05 (s,
2H),
5.12 (s, 2H), 6.86 (d, J= 8.8 Hz, 2H), 7.29 (d, J= 8.8 Hz, 2H), 8.44 (s, 1H).
The chemical structure, name and analytical data of the intermediate prepared
by
following the procedure described above are given below in Table 1.
Table 1: Chemical name, structure and analytical data of Intermediates A15-A17
Intermediate
No. Chemical Name and Structure Analytical Data
CI
1H NMR (400 MHz, DMSO-d6) 6 1.56
Al5 N:X 1
N NO (d, J = 6.8 Hz, 3H), 3.71 (s,
3H), 5.12
PMB (d, J = 6.8 Hz, 3H), 6.86 (d,
J = 8.4 Hz,
(R)-4-Chloro-8-(4-methoxybenzy1)- 2H), 7.27 (d, J = 8.8 Hz, 2H), 8.47 (s,
6-methyl-6H-pyrimido [5,4 - 1H); APCI-MS (m/z) 320 (M+H)+.
b][1,4]oxazin-7(8H)-one
CI
Nicc' 1H NMR (400 MHz, DMSO-d6) 6
1.66
r
A 1 6 NO
(d, J = 6.8 Hz, 3H), 3.79 (s, 3H), 4.90
N
PMB (q, J= 6.8 Hz, 1H), 5.24 (s, 2H), 6.83-
(S)-4-Chloro-8-(4-methoxybenzy1)- 6.86 (m, 2H), 7.42 (d, J = 8.8 Hz, 2H),
6-methyl-6H-pyrimido [5,4 - 8.45 (s, 1H); APCI-MS (m/z)
320
b][1,4]oxazin-7(8H)-one (M+H)+.
CI
NIC):(
N N 0 1H NMR (400 MHz, DMSO-d6) 6
1.61
Al7
(s, 6H), 3.79 (s, 3H), 5.24 (s, 2H), 6.84
PMB (d, J = 8.8 Hz, 2H), 7.40 (d,
J = 8.8 Hz,
4-Chloro-8-(4-methoxybenzy1)-6,6- 2H), 8.44 (s, 1H); APCI-MS (m/z) 334
dimethy1-6H-pyrimido [5,4- (M+H)+.
b][1,4]oxazin-7(8H)-one
Intermediate A3
(S)-tert-Butyl 4-chloro-6-methyl-6H-pyrimido[5,4-b][1,4]oxazine-8(7H)-
carboxylate
48
CA 03064975 2019-11-26
WO 2018/215668 PCT/EP2018/063957
CI
N
I
N N
60c
Step 1: (S)-1-((6-Chloro-5-methoxypyrimidin-4-yl)amino)propan-2-ol
CI
NrO
N N'()E1
H I
To a stirred solution of 4,6-dichloro-5-methoxypyrimidine (5.0 g, 27.9 mmol)
in 1,4-dioxane
(50 mL) were added potassium carbonate (4.6 g, 33.5 mmol) followed by (S)-(+)-
1-amino-2-
propanol (2.3 g, 30.7 mmol) at RT. The resultant mixture was stirred at 125 C
for 8 h. The
mixture was cooled to RT and diluted with ethyl acetate and water. The layers
were separated
and the organic layer was washed with water followed by brine. The solution
was dried over
anhydrous sodium sulfate, filtered and concentrated to yield 4.85 g of the
desired compound.
1H NMR (400 MHz, DMSO-d6) 6 1.05 (d, J = 6.4 Hz, 3H), 3.28-3.34 (m, 2H), 3.73
(s, 3H),
3.78-3.87 (m, 1H), 4.77 (d, J = 4.8 Hz, 1H), 7.46 (t, J = 5.6 Hz, 1H), 8.04
(s, 1H); ESI-MS
(m/z) 218 (M+H)+.
Step 2: (S)-4-((2-Bromopropyl)amino)-6-chloropyrimidin-5-ol
CI
NOH
N Br
H I
A mixture of (S)-1-((6-chloro-5-methoxypyrimidin-4-yl)amino)propan-2-ol
(step 1
intermediate) (4.8 g, 22.1 mmol) and boron tribromide (1M in dichloromethane,
25 mL) was
heated at 80 C for 18 h. The solvent was removed under reduced pressure and
the residue
was quenched with ice-cooled water. The aqueous solution was neutralized using
sodium
bicarbonate and extracted twice with ethyl acetate. The combined organic
layers were washed
with brine and dried over anhydrous sodium sulfate. The solution was filtered
and
concentrated to yield 3.9 g of the desired compound. 1H NMR (400 MHz, DMSO-d6)
6 1.63
(d, J= 6.4 Hz, 3H), 3.58-3.64 (m, 1H), 3.72-3.79 (m, 1H), 4.39-4.47 (m, 1H),
7.48 (t, J= 5.6
Hz, 1H), 7.86 (s, 1H), 9.99 (br s, 1H).
Step 3: (S)-tert-Butyl 4-chloro-6-methyl-6H-pyrimido[5,4-b][1,4]oxazine-8(7H)-
carboxylate
The titled compound was prepared by the reaction of (S)-4-((2-
bromopropyl)amino)-6-
chloropyrimidin-5-ol (step 2 intermediate) (500 mg, 2.69 mmol) with di-tert-
butyl
dicarbonate (881 mg, 4.84 mmol) in the presence of DMAP (296 mg, 2.48 mmol) in
dichloromethane (10 mL) as per the procedure described in step 3 of
Intermediate Al to yield
49
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
154 mg of the compound. 41 NMR (400 MHz, DMSO-d6) 6 1.50 (s, 9H), 1.63 (d, J =
6.8 Hz,
3H), 3.57-3.63 (m, 1H), 3.75-3.81 (m, 1H), 4.39 (q, J= 6.8 Hz, 1H), 8.27-8.33
(m, 1H); ESI-
MS (m/z) 285 (M)+.
The chemical structure, name and analytical data of the intermediate prepared
by
.. following the procedure described above are given below in Table 2.
Table 2: Chemical name, structure and analytical data of Intermediate A10
Intermediate
No. Chemical Name and Structure Analytical Data
CI
N y:' ) = 'N
I 1H NMR (400 MHz, DMSO-d6) 6 1.51
Al 0 N N
(s, 9H), 1.63 (d, J= 6.8 Hz, 3H), 3.57-
6oc
(R)-tert-Butyl 4-chloro-6-methyl- '
(q J =6.8 Hz, 1H), 8.30-8.34 (m, 1H).
6H-pyrimido[5,4-b][1,4]oxazine- '
8(7H)-carboxylate
Intermediate A4
tert-Butyl 4-chloro-7,7-dimethy1-6H-pyrimido[5,4-b][1,4]oxazine-8(7H)-
carboxylate
CI
N ,C).
I
N N
I3oc
Step 1: 246-Chloro-5-methoxypyrimidin-4-yl)amino)-2-methylpropan-1-ol
CI 1
N CXO
I V
N N.,OH
H
To a stirred solution of 4,6-dichloro-5-methoxypyrimidine (5.0 g, 27.9 mmol)
in 1,4-dioxane
(50 mL) were added potassium carbonate (4.6 g, 33.5 mmol) followed by 2-amino-
2-
methylpropanol (2.71 mL, 28.4 mmol) at RT. The resultant mixture was stirred
at 125 C for
8 h. The mixture was cooled to RT and diluted with ethyl acetate and water.
The layers were
separated and the organic layer was washed with water followed by brine. The
solution was
dried over anhydrous sodium sulfate, filtered and concentrated to yield 3.5 g
of the desired
compound. 1H NMR (400 MHz, DMSO-d6) 6 1.37 (s, 6H), 3.50 (d, J = 5.6 Hz, 2H),
3.74 (s,
3H), 5.10 (t, J= 6.0 Hz, 1H), 6.30 (s, 1H), 8.06 (s, 1H).
Step 2: 4-Chloro-6-((1-hydroxy-2-methylpropan-2-yl)amino)pyrimidin-5-ol
CA 03064975 2019-11-26
WO 2018/215668 PCT/EP2018/063957
CI
NOH
I V
N N,OH
H
To a solution of 2-((6-chloro-5-methoxypyrimidin-4-yl)amino)-2-methylpropan-1-
01 (step 1
intermediate) (3.5 g, 15.1 mmol) in dichloromethane (35 mL) was added boron
tribromide
(1M in THF, 75.8 mL) at 0 C and the mixture was stirred at RT for 16 h. The
reaction
mixture was quenched with methanol at 0 C and concentrated under reduced
pressure. The
residue was diluted with ethyl acetate and washed with aqueous sodium
bicarbonate solution.
The organic layer was washed with brine and dried over anhydrous sodium
sulfate. The
solution was filtered and concentrated to yield 2.5 g of the desired compound.
41 NMR (400
MHz, DMSO-d6) 6 1.36 (s, 6H), 3.48 (s, 2H), 5.13-5.15 (br s, 1H), 6.11 (s,
1H), 7.88 (s, 1H),
9.97 (s, 1H).
Step 3: tert-Butyl (6-chloro-5 -hydroxypyrimidin-4-y1)(1 -hydroxy-2-
methylprop an-2-
yl)carbamate
CI
NOH
I K,OH
N N
60c
To a stirred solution of 4-chloro-6-((1-hydroxy-2-methylpropan-2-
yl)amino)pyrimidin-5-ol
(step 2 intermediate) (2.5 g, 11.4 mmol) in dichloromethane (25 mL) were added
di-tert-butyl
dicarbonate (2.5 g, 11.4 mmol) at 0 C followed by triethylamine (2.4 mL, 17.1
mmol) and
the mixture was stirred at RT for 15 h. The solvent was evaporated under
reduced pressure
and the residue thus obtained was purified by silica gel column chromatography
to yield 1.9 g
of the desired compound. 1H NMR (400 MHz, DMSO-d6) 6 1.36 (br s, 6H), 1.50 (br
s, 9H),
3.50 (d, J= 6.0 Hz, 2H), 5.06 (t, J= 10.0 Hz, 1H), 6.55 (s, 1H), 8.20 (s, 1H).
Step 4: tert-Butyl 4-chloro-7,7-dimethy1-6H-pyrimido[5,4-b][1,4]oxazine-8(7H)-
carboxylate
To a stirred solution of tert-butyl (6-chloro-5-hydroxypyrimidin-4-y1)(1-
hydroxy-2-
methylpropan-2-yl)carbamate (step 3 intermediate) (1.9 g, 5.97 mmol) in
anhydrous THF (20
mL) were added triphenylphosphine (1.88 g, 7.18 mmol) and diisopropyl
azodicarboxylate
(DIAD) (1.39 mL, 7.17 mmol) at 0 C under nitrogen atmosphere. The mixture was
stirred
for 1 h at 0 C. The solvent was evaporated under reduced pressure and the
residue thus
obtained was purified by silica gel column chromatography to yield 350 mg of
the desired
compound. 1H NMR (400 MHz, DMSO-d6) 6 1.36 (s, 6H), 1.51 (s, 9H), 4.13 (s,
2H), 8.19 (s,
1H); ESI-MS (m/z) 300 (M+H)+.
51
CA 03064975 2019-11-26
WO 2018/215668 PCT/EP2018/063957
The chemical structure, name and analytical data of the intermediate prepared
by
following the procedure described above are given below in Table 3.
Table 3: Chemical name, structure and analytical data of Intermediates A5-A6
and All-A14
Intermediate
Chemical Name and Structure Analytical Data
No.
CI
k
N (),, lti NMR (400 MHz, DMSO-d6) 6 1.33
AS
(d, J = 5.6 Hz, 3H), 1.52 (s, 9H), 4.24
N N
60c (dd, J1 = 2.0 Hz, J2 = 11.2 Hz,
1H),
(R)-tert-Butyl 4-chloro-7-methyl- 4.36-4.39 (m, 1H), 4.58-4.61 (m,
1H),
6H-pyrimido[5,4-b][1,4]oxazine- 8.55 (s, 1H).
8(7H)-carboxylate
CI
NC)l 1F1 NMR (400 MHz, DMSO-d6) 6 1.34
J, ). (d, J = 5.2 Hz, 3H), 1.50 (s, 9H),
4.24
A6 N N "i/ (dd, J1 = 2.0 Hz, J2 = 11.2 Hz,
1H),
Boc
4.36-4.39 (m, 1H), 4.58-4.61 (m, 1H),
(S)-tert-Butyl 4-chloro-7-methyl-
8' 55 (s' '
1H). ESI-MS (m/z) 285
6H-pyrimido[5,4-b][1,4]oxazine- +
(M+H) .
8(7H)-carboxylate
CI
NJ:CI
1 Al 1 1F1 NMR (400 MHz, DMSO-d6) 6 0.97
(t, J = 7.6 Hz, 3H), 1.51 (s, 9H), 1.75-
N N
Boc 1.79 (m, 1H), 2.50-2.58 (br s, 2H),
(R)-tert-Butyl 4-chloro-7-ethyl-6H- 4.40-4.52 (br s, 2H), 8.55 (s, 1H); ESI-
PYrimido[5,4-b][1,4]oxazine-8(7H)- MS (m/z) 300 (M+H)t
carboxylate
Cl 1F1 NMR (400 MHz, DMSO-d6) 6 0.91
0
N:i ) (t, J = 7.6 Hz, 3H), 1.50 (s, 9H),
2.49-
Al2, N N ." 2.59 (m, 2H), 4.26 (dd, J1 = 2.4
Hz, .1-2
Boc = 11.2 Hz, 1H), 4.42 (t, J = 6.8 Hz,
(S)-tert-Butyl4-chloro-7-ethy1-6H- 1H), 4.50 (dd, J1 = 1.2 Hz, J2 = 11.2
pyrimido[5,4-b][1,4]oxazine-8(711)- Hz, 1H), 8.55 (s, 1H); ESI-MS (m/z)
carboxylate 300 (M+H)+.
CI
1F1 NMR (400 MHz, DMSO-d6) 6 0.93
NtC
N N
1 (d, J = 6.8 Hz, 3H), 1.04 (d, J =
6.8 Hz,
A13, 3H), 1.49 (s, 9H), 1.72-1.74 (m,
1H),
Boc
(R)-tert-Butyl 4-chloro-7-isopropyl-
2.38 (d, J = 3.6 Hz, 2H), 2.43 (d, J =
6H-pyrimido[5,4-b][1,4]oxazine-
6.4 Hz, 1H),+ 8.55 (s, 1H); ESI-MS
8(711)-carboxylate (m/z) 313 (MY.
CI
NA0 ), 1F1 NMR (400 MHz, DMSO-d6) 6 0.93
Al4 N N õr (d, J = 6.8 Hz, 3H), 1.05 (d, J =
6.8 Hz,
Boc 3H), 1.51 (s, 9H), 1.70-1.80 (m, 2H),
(S)-tert-Butyl 4-chloro-7-isopropyl- 2.50-2.51 (m, 2H), 8.55 (s, 1H); ESI-
6H-pyrimido[5,4-b][1,4]oxazine- MS (m/z) 314 (M+H)+.
8(7H)-carboxylate
52
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
Intermediate A7
tert-Butyl 8-bromo-2H-pyrido[3,2-b][1,4]oxazine-4(3H)-carboxylate
Br
.,0
1 j
1\111
Boc
Step 1: 2-Amino-4-bromopyridin-3-ol hydrobromide
Br
OH
, 1
-1\1NH2
HBr
To a solution of 2-aminopyridin-3-ol (45 g, 409 mmol) in absolute ethanol (225
mL) at 0-10
C was drop wise added bromine (64 mL, 818 mmol) and the mixture was stirred
for 3 days
at RT. The solvent was removed under reduced pressure at low temperature and
the residue
was cooled to 0 C. Ethyl acetate was added to the residue and stirred for 1
h. The solid was
filtered and dried well to yield 48 g of the desired product. 1H NMR (400 MHz,
DMSO-d6) 6
5.94 (br s, 2H), 6.65 (d, J = 5.6 Hz, 1H), 7.23 (d, J= 5.6 Hz, 1H).
Step 2: 8-Bromo-3,4-dihydro-2H-pyrido [3,2-b] [1,4] oxazine
Br
0
k j
N N
H
To a solution of 2-amino-4-bromopyridin-3-ol hydrobromide (step 1
intermediate) (15 g, 58.6
mmol) in acetonitrile (150 mL) were added cesium carbonate (57.1 g, 175 mmol)
followed by
1,2-dibromoethane (16.4 g, 87.7 mmol) and the mixture was refluxed for 48 h.
The mixture
was filtered and the filtrates was concentrated under reduced pressure. The
residue thus
obtained was purified by silica gel column chromatography to yield 3.0 g of
the titled
compound. 1H NMR (400 MHz, DMSO-d6) 6 3.33-3.43 (m, 2H), 4.13-4.20 (m, 2H),
6.71 (d,
J = 5.2 Hz, 1H), 7.02 (s, 1H), 7.40 (d, J = 5.2 Hz, 1H); ESI-MS (m/z) 215
(M+H)+.
Step 3: tert-Butyl 8-bromo-2H-pyrido[3,2-b][1,4]oxazine-4(3H)-carboxylate
To a solution of 8-bromo-3,4-dihydro-2H-pyrido[3,2-b][1,4]oxazine (step 2
intermediate)
(2.1 g, 9.76 mmol) in anhydrous THF (20 mL) were dropwise added lithium
bis(trimethylsilyl)amide (LiHMDS) (1M, 11.6 mL, 11.7 mmol) followed by di-tert-
butyl
dicarbonate (3.3 mL, 14.6 mmol) at 0 C and the mixture was stirred at the
same temperature
for 1 h. The reaction was quenched with saturated ammonium chloride solution
and extracted
53
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
with ethyl acetate. The organic layer was dried over anhydrous sodium sulfate,
filtered and
concentrated. The residue obtained was purified by silica gel column
chromatography to
yield 2.4 g of the desired compound. 1H NMR (400 MHz, DMSO-d6) 6 1.46 (s, 9H),
3.86 (d,
J = 4.4 Hz, 2H), 4.35 (d, J = 4.4 Hz, 2H), 7.38 (d, J = 5.2 Hz, 1H), 7.81 (d,
J= 5.2 Hz, 1H);
ESI-MS (m/z) 316 (M+H)+.
The chemical structure, name and analytical data of the intermediate prepared
by
following the procedure described above are given below in Table 4.
Table 4: Chemical name, structure and analytical data of Intermediates A26
Intermediate
No. Chemical Name and Structure
Analytical Data
BrO
1H NMR (400 MHz, DMSO-d6) 6 1.46
N N
(s, 9H), 3.81-3.86 (m, 2H), 4.23-4.28
A26 Boc
(m, 2H), 7.59 (d, J = 2.4 Hz, 1H), 8.04
tert-Butyl 7-bromo-2H-pyrido[3' 2-
(d, J = 2.0 Hz, 1H); ESI-MS (m/z) 215
b][1 ,4]oxazine- 4 (3 H)-c arb oxylate
(M+H-Boc)+.
Intermediate A8
8-Bromo-4-(4-methoxybenzy1)-2H-pyrido [3,2-b] [1,4]oxazin-3 (4H)-one
Br
0
I
1\1 N
PM B
Step 1: 8-Bromo-2H-pyrido [3,2-b] [1,4]oxazin-3(4H)-one
Br
I
)
H
To a solution of 2-amino-4-bromopyridin-3-ol hydrobromide (step 1-Intermediate
A7)
(12 g, 63.4 mmol) in a mixture of 2-butanone and water (1:1, 120 mL) was added
aqueous
solution of sodium bicarbonate (16 g, 190 mmol) at 0 C and the mixture was
stirred for 10
min. Chloroacetyl chloride (7.16 g, 63.4 mmol) was added to the mixture and
stirred for 3-4 h
at 0 C. Then the mixture was heated to 80 C and stirred for 10 h. The
mixture was cooled to
RT and extracted twice with ethyl acetate. The combined organic layers were
washed with
water, brine and dried over anhydrous sodium sulfate. The solvent was removed
under
reduced pressure and the residue obtained was purified by silica gel column
chromatography
to yield 2.5 g of the desired compound. 1H NMR (400 MHz, DMSO-d6) 6 4.77 (s,
2H), 7.26
(d, J = 5.2 Hz, 1H), 7.75 (d, J = 5.2 Hz, 1H), 11.44 (s, 1H); ESI-MS (m/z) 229
(M+H)+.
54
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
Step 2: 8-Bromo-4-(4-methoxybenzy1)-2H-pyrido [3,2-b] [1,4]oxazin-3 (4H)-one
To a solution of 8-bromo-2H-pyrido[3,2-b][1,4]oxazin-3(4H)-one (step 1
intermediate) (250
mg, 1.09 mmol) in DMF (5.0 mL) were added cesium carbonate (709 mg, 2.18 mmol)
followed by 4-methoxybenzyl chloride (256 mg, 1.63 mmol) and the mixture was
stirred for
3 h at RT. The mixture was quenched with water and the product was extracted
twice in ethyl
acetate. The combined organic layers were washed with water, brine and dried
over
anhydrous sodium sulfate. The solvent was removed under reduced pressure and
the residue
obtained was purified by silica gel column chromatography to yield 270 mg of
the desired
compound. 1H NMR (400 MHz, DMSO-d6) 6 3.78 (s, 3H), 4.80 (s, 2H), 5.30 (s,
2H), 6.83 (d,
J = 5.2 Hz, 2H), 7.16 (d, J = 5.2 Hz, 1H), 7.43 (d, J = 8.8 Hz, 2H), 7.86 (d,
J= 5.2 Hz, 1H).
Intermediate A9
4-Chloro-8-(4-methoxybenzy1)-7,8-dihydropyrido [2,3 -d]pyrimidin-5 (6H)-one
CI 0
N
I
NI\I
PMB
Step 1: 1-(4,6-Dichloropyrimidin-5-yl)prop-2-en-1-ol
CI OH
N '
I
N CI
To a stirred solution of 4,6-dichloropyrimidine-5-carbaldehyde (10 g, 56.5
mmol) in
anhydrous THF (100 mL) was slowly added vinylmagnesium chloride (1M, 67.6 mL,
67.8
mmol) at -20 C and the mixture was stirred for 3 h at RT. The reaction
mixture was
quenched with aqueous ammonium chloride solution and extracted with ethyl
acetate. The
organic layer was washed with brine and dried over anhydrous sodium sulfate.
The solution
was filtered and concentrated to yield 5.0 g of the desired compound. 1H NMR
(400 MHz,
DMSO-d6) 6 5.36-5.39 (m, 1H), 5.43 (s, 1H), 5.90-5.92 (br s, 1H), 6.15-6.23
(m, 1H), 8.73 (s,
1H).
Step 2: 1-(4,6-Dichloropyrimidin-5-yl)prop-2-en-1-one
ci 0
))N '
I
N CI
To a stirred solution of 1-(4,6-dichloropyrimidin-5-yl)prop-2-en-1-ol (step 1
intermediate)
(100 mg, 0.49 mmol) in dichloromethane (5.0 mL) was added Dess¨Martin
periodinane (415
mg, 0.97 mmol) at 0 C and the mixture was stirred overnight at RT. The
mixture was filtered
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
through celite and the filtrate was washed with water. The solution was dried
over anhydrous
sodium sulfate, filtered and concentrated. The residue was purified by silica
gel column
chromatography to yield 20 mg of the desired compound. 1H NMR (400 MHz, DMSO-
d6) 6
6.10 (d, J = 17.6 Hz, 1H), 6.33 (d, J = 10.8 Hz, 1H), 6.62-6.69 (m, 1H), 8.89
(s, 1H).
Step 3: 4-Chloro-8-(4-methoxybenzy1)-7,8-dihydropyrido [2,3 -d]pyrimidin-5
(6H)-one
To a solution of 1-(4,6-dichloropyrimidin-5-yl)prop-2-en-1-one (step 2
intermediate) (1.1 g,
5.40 mmol) in DMF (28 mL) was added 4-methoxybenzylamine (1.1 g, 8.12 mmol) at
RT.
The mixture was stirred overnight at 50 C. The mixture was cooled and
quenched with
water. The aqueous mixture was extracted twice with ethyl acetate. The
combined organic
layers were washed with water followed by brine and dried over anhydrous
sodium sulfate.
The solution was filtered, concentrated and the residue was purified by silica
gel column
chromatography to yield 900 mg of the desired compound. 1H NMR (400 MHz, DMSO-
d6) 6
3.29 (t, J = 6.0 Hz, 2H), 3.78-3.82 (m, 5H), 4.66 (d, J = 5.6 Hz, 2H), 6.89
(dd, ./i = 2.0 Hz, .1-2
= 6.8 Hz, 2H), 7.25 (d, J = 8.8 Hz, 2H), 8.38 (s, 1H).
Intermediate A 1 8
(R)-te rt-Butyl 4-chloro-7-(morpholinomethyl)-6H-pyrimido [5,4-b] [1,4]
oxazine-8 (7 H)-
carboxylate
ci
N:r\j(NDLI
Boo N
Co)
Step 1: (R)-Methyl 2-((tert-butoxycarbonyl)amino)-3-
((methylsulfonyl)oxy)propanoate
0
o-
(:)Nls
To a stirred solution (R)-methyl 2-((tert-butoxycarbonyl)amino)-3-
hydroxypropanoate (2.0 g,
9.13 mmol) in dichloromethane (20 mL) were added DIPEA (3.9 mL, 22.8 mmol)
followed
by methanesulfonyl chloride (Mesyl chloride) (850 L, 10.9 mmol) at 0 C and
the mixture
was stirred at 0 C for 1 h. The mixture was diluted with ethyl acetate and
water. The organic
layer was separated and washed with water followed by brine and dried over
anhydrous
sodium sulfate. The solution was filtered and concentrated under reduced
pressure to yield
2.95 g of the desired compound. 1H NMR (400 MHz, DMSO-d6) 6 1.41 (s, 9H), 3.20
(s, 3H),
3.67 (s, 3H), 4.35-4.45 (m, 3H), 7.53 (d, J = 8.0 Hz, 1H).
Step 2: (R)-Methyl 2-((tert-butoxycarbonyl)amino)-3-morpholinopropanoate
56
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
H 0
>01.r N...}Ø..._
0
,C,
To a stirred solution of (R)-methyl 2-((tert-butoxycarbonyl)amino)-3-
((methylsulfonyl)oxy)propanoate (step 1 intermediate) (2.9 g, 9.76 mmol) in
dichloromethane
(20 mL) were added morpholine (1.7 mL, 19.5 mmol) followed by N,N-
diisopropylethylamine (DIPEA) (3.3 mL, 19.5 mmol) at 0 C. The mixture stirred
at RT for
18 h. The reaction mixture was concentrated under reduced pressure and the
residue obtained
was purified by column chromatography to yield 1.46 g of the desired product.
1H NMR (400
MHz, DMSO-d6) 6 0.96 (t, J= 7.2 Hz, 3H), 2.28-2.51 (m, 10H), 3.72 (s, 2H),
8.09 (d, J = 8.4
Hz, 1H), 8.40 (s, 1H), 8.51 (dd, ./i = 2.4 Hz, J2 = 8.8 Hz, 1H).
Step 3: (R)-te rt-Butyl (1-hydroxy-3-morpholinopropan-2-yl)carbamate
H
If o H
0 r\i
0
To a solution of (R)-methyl 2-((tert-butoxycarbonyl)amino)-3-
morpholinopropanoate (step 2
intermediate) (1.4 g, 4.86 mmol) in THF (15 mL) was added DIBAL solution (1M
in toluene,
19.4 mL, 19.4 mmol) at -78 C and the mixture was stirred at the same
temperature for 2 h.
The reaction was quenched with brine and stirred for 1 h. The solution was
filtered and
washed with ethyl acetate. The combined organic layers were dried over
anhydrous sodium
sulfate, filtered and concentrated. The residue obtained was purified by
column
chromatography to yield 1.2 g of the desired product. 1H NMR (400 MHz, DMSO-
d6) 6 1.38
(s, 9H), 2.20-2.38 (m, 6H), 3.52-3.60 (m, 6H), 4.60 (t, J = 5.6 Hz, 1H), 8.41
(d, J = 8.8 Hz,
1H), 8.32 (s, 1H); ESI-MS (m/z) 260 (M+H)+.
Step 4: (R)-te rt-Butyl
(1-((4,6-dichloropyrimidin-5 -yl)oxy)-3 -morpho linoprop an-2-
yl)carbamate
N N
CI CI
(31 0
Boo. N.1.,4,,N ,>
H
The titled compound was prepared by the reaction of (R)-tert-butyl (1-hydroxy-
3-
morpholinopropan-2-yl)carbamate (step 3 intermediate) (1.2 g, 4.61 mmol) with
4,6-
dichloropyrimidin-5-ol (step 1 of Intermediate A2) (912 mg, 5.51 mmol) in the
presence of
DIAD (1.4 g, 6.92 mmol) and triphenylphosphine (1.8 g, 6.92 mmol) in THF (10
mL) as per
57
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
the procedure described in step 2 of Intermediate A2 to yield 2.96 g of the
compound. 1H
NMR (400 MHz, DMSO-d6) 6 1.18 (d, J = 6.0 Hz, 9H), 2.50-2.51 (m, 4H), 3.50-
3.55 (m,
4H), 3.90-4.0 (m, 1H), 4.09-4.13 (m, 1H), 4.21-4.23 (m, 1H), 4.74-4.80 (m,
2H), 8.32 (s, 1H);
ESI-MS (m/z) 406 (M+H)+.
Step 5: (R)-1-((4,6-Dichloropyrimidin-5-yl)oxy)-3-morpholinopropan-2-amine
hydrochloride
NN
CI )1LCI
H2N
HCI
To a solution of (R)-te rt-butyl (1-((4,6-dichloropyrimidin-5-yl)oxy)-3-
morpholinopropan-2-
yl)carbamate (step 4 intermediate) (2.9 g, 7.13 mmol) in ethyl acetate (10 mL)
was added
hydrochloric acid in 1,4-dioxane (4M, 40 mL) and the mixture was stirred at RT
for 3 h. The
mixture was concentrated under reduced pressure to give 536 mg of the desired
product. 1H
NMR (400 MHz, DMSO-d6) 6 3.28-3.66 (m, 6H), 3.90-4.05 (m, 4H), 4.44-4.48 (m,
3H), 8.76
(s, 1H), 9.01 (br s, 3H).
Step 6: (R)-4-Chloro-7-(morpholinomethyl)-7,8-dihydro-6H-pyrimido[5,4-
b][1,4]oxazine
ci
H-
C
0
To a solution of (R)-1-((4,6-dichloropyrimidin-5-yl)oxy)-3-morpholinopropan-2-
amine
hydrochloride (step 5 intermediate) (520 mg, 1.51 mmol) in DMF (5.0 mL) was
added
DIPEA (2.6 mL, 15.1 mmol) and the mixture was stirred overnight at RT. The
mixture was
quenched with water and extracted twice with ethyl acetate. The combined
organic extracts
were washed with water, brine and dried over anhydrous sodium sulfate. The
solution was
filtered, concentrated and the residue obtained was purified by column
chromatography to
yield 271 mg of the desired product. 1H NMR (400 MHz, DMSO-d6) 6 2.38-2.46 (m,
4H),
2.49-2.51 (m, 2H), 3.57 (t, J = 4.8 Hz, 4H), 3.77-3.82 (m, 1H), 4.15-4.16 (s,
2H), 7.89 (s,
1H), 8.09 (s, 1H); ESI-MS (m/z) 271 (M+H)+.
Step 7: (R)-te rt-Butyl 4-chloro-7-(morpholinomethyl)-6H-pyrimido[5,4-
b][1,4]oxazine-
8(7H)-carboxylate
The titled compound was prepared by the reaction of (R)-4-chloro-7-
(morpholinomethyl)-7,8-
dihydro-6H-pyrimido[5,4-b][1,4]oxazine (step 6 intermediate) (260 mg, 0.96
mmol) with di-
58
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
tert-butyl dicarbonate (230 mg, 1.05 mmol) in the presence of DMAP (106 mg,
0.86 mmol)
in dichloromethane (20 mL) as per the procedure described in step 3 of
Intermediate Al to
yield 231 mg of the compound. lti NMR (400 MHz, DMSO-d6) 6 1.50 (s, 9H), 3.51-
2.29 (m,
6H), 3.52 (d, J = 4.4 Hz, 4H), 4.03 (q, J = 7.2 Hz, 1H), 4.21 (dd, ./i = 2.4
Hz, J2 = 11.2 Hz,
1H), 8.27-8.33 (m, 1H); ESI-MS (m/z) 285 (M)+.
The chemical structure, name and analytical data of the intermediate prepared
by
following the procedure described above are given below in Table 5.
Table 5: Chemical name, structure and analytical data of Intermediate A23-A25
Intermediate
Chemical Name and Structure Analytical Data
No.
CI
A23 N0
1F1 NMR (400 MHz, DMSO-d6) 6 1.51
N I N ) ,O.
(s, 9H), 3.27 (s, 3H), 3.34-3.40 (m,
60c 2H), 4.24-4.28 (m, 1H), 4.51-
4.56 (m,
(R)-tert-Butyl 4-chloro-7-
1H), 4.63-4.69 (m, 1H), 8.31 (s, 1H);
(methoxymethyl)-6H-pyrimido [5,4- ESI-MS (m/z) 316 (M+H)+.
b][1,4]oxazine-8(7H)-carboxylate
CI
NCX0
N I N1','NI
1F1 NMR (400 MHz, DMSO-d6) 6 1.50
A24 0c
(s, 9H), 2.20 (s, 6H), 2.35-2.41 (m,
6
(R)-tert-Butyl 4-chloro-7-
2H), 4.16-4.22 (m, 1H), 4.54-4.61 (m,
((dimethylamino)methyl)-6H-
2H), 8.31 (s, 1H); ESI-MS (m/z) 329
(M+H)+.
pyrimido[5,4-b] [1,4]oxazine-8(7H)-
carboxylate
CI
N CI
:XI )
A25 CI N N
1F1 NMR (400 MHz, CDC13) 6 1.60 (s,
60c
9H), 3.96-4.00 (m, 2H), 4.38-4.42 (m,
tert-Butyl 2,4-dichloro-6H- 2H); ESI-MS (m/z) 307 (M+H)+.
pyrimido[5,4-b] [1,4]oxazine-8(7H)-
carboxylate
Intermediate A19
(S)-tert-Butyl
7-(((tert-butoxyc arbonyl)oxy)methyl)-4-chloro-6H-pyrimido [5,4-
b][1,4]oxazine-8(7H)-carboxylate
CI
0,N
1 Boc0,,=CNj NI"
thoc
Step 1: N-(6-Chloro-5-hydroxypyrimidin-4-yl)acetamide
59
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
CI
N c.CDH
NxI
N 0
To a solution of 4-amino-6-chloropyrimidin-5-ol (1.5 g, 10.3 mmol) in
dichloromethane (15
mL) was added acetyl chloride (0.8 mL, 10.3 mmol) followed by dropwise
addition of
triethylamine (4.2 mL, 30.9 mmol) at 0 C and the mixture was stirred at RT
for 3 h. The
mixture was diluted with ethyl acetate and water. The layers were separated
and the aqueous
layer was extracted twice with ethyl acetate. The combined organic extracts
were washed
with water followed by brine and dried over anhydrous sodium sulfate. The
solution was
filtered, concentrated and the residue thus obtained was purified by silica
gel column
chromatography to yield 790 mg of the desired compound. ESI-MS (m/z) 188
(M+H)+.
Step 2: (S)-1-(4-Chloro-7-(hydroxymethyl)-6H-pyrimido [5,4-b] [1,4]
oxazin-8 (7 H)-
yl)ethanone
CI
N
N N
(D
To a suspension of potassium carbonate (1.47 g, 10.7 mmol) in acetonitrile (20
mL) were
added N-(6-chloro-5-hydroxypyrimidin-4-yl)acetamide (step 1 intermediate) (500
mg, 2.67
mmol) followed by (2R)-(-)-glycidyl tosylate (669 mg, 2.93 mmol) and the
mixture was
refluxed for 24 h. The mixture was cooled to RT and diluted with ethyl acetate
and water.
The layers were separated and the aqueous layer was extracted twice with ethyl
acetate. The
combined organic extracts were washed with water followed by brine and dried
over
anhydrous sodium sulfate. The solution was filtered, concentrated and the
residue thus
obtained was purified by silica gel column chromatography to yield 270 mg of
the desired
compound. 1H NMR (400 MHz, DMSO-d6) 6 2.12 (s, 3H), 3.96-4.16 (m, 1H), 4.16-
4.30 (m,
4H), 6.23 (s, 1H), 8.06 (s, 1H).
Step 3: (S)-(4-Chloro-7,8-dihydro-6H-pyrimido[5,4-b][1,4]oxazin-7-yl)methanol
CI
NYO
I N N (9. OH
A solution of (S)-1-(4-chloro-7-(hydroxymethyl)-6H-pyrimido[5,4-b][1,4]oxazin-
8 (7 H)-
yl)ethanone (step 2 intermediate) (150 mg, 0.62 mmol) in hydrochloric acid in
1,4-dioxane
(3.0 mL) was stirred at RT for 3 h. The mixture was concentrated under reduced
pressure and
the residue was diluted with water. The aqueous mixture was basified using
saturated sodium
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
bicarbonate solution till pH 8-9 at -20 C. The mixture was extracted twice
with ethyl acetate.
The combined organic extracts were dried over anhydrous sodium sulfate,
filtered and
concentrated under reduced pressure to give 170 mg of the desired product. 1H
NMR (400
MHz, DMSO-d6) 6 3.34-3.41 (m, 1H), 3.47-3.57 (m, 2H), 4.11-4.32 (m, 2H), 5.09
(t, J = 5.6
Hz, 1H), 7.88 (s, 1H), 8.21-8.22 (br s, 1H).
Step 4: (S)-tert-Butyl 7-(((tert-butoxycarbonyl)oxy)methyl)-4-chloro-6H-
pyrimido[5,4-
b][1,4]oxazine-8(7H)-carboxylate
The titled compound was prepared by the reaction of (S)-(4-chloro-7,8-dihydro-
6H-
pyrimido[5,4-b][1,4]oxazin-7-yl)methanol (step 3 intermediate) (170 mg, 0.84
mmol) with
di-tert-butyl dicarbonate (476 mg, 2.18 mmol) in the presence of DMAP (246 mg,
2.01
mmol) in dichloromethane (10 mL) as per the procedure described in step 3 of
Intermediate
Al to yield 100 mg of the compound. 1H NMR (400 MHz, DMSO-d6) 6 1.37 (s, 9H),
1.50 (s,
9H), 4.02-4.13 (m, 1H), 4.15-4.35 (m, 1H), 4.31 (dd, J, = 2.8 Hz, .1-2 = 11.2
Hz, 1H), 4.55-
4.79 (m, 1H), 4.80-4.81 (m, 1H), 8.33 (s, 1H).
Intermediate A20
tert-Butyl 9-bromo-3 ,4-dihydropyrido [3,2-b] [1,4] oxazepine-5 (2H)-
carboxylate
Br
_0
N^N
Boc
Step 1: 4-Bromo-3 -(3 -chloropropoxy)pyridin-2-amine
Br CI
I
NNH2
The titled compound was prepared by the reaction of 2-amino-4-bromopyridin-3-
ol
hydrobromide (step 1 of Intermediate A7) (1.0 g, 3.90 mmol) and 1-bromo-3-
chloropropane
(925 mg, 5.80 mmol) in the presence of cesium carbonate (3.8 g, 11.7 mmol) in
acetonitrile
(10 mL) as per the procedure described in step 2 of Intermediate A7 to yield
400 mg of the
compound. 1H NMR (400 MHz, DMSO-d6) 6 2.19-2.25 (m, 2H), 3.95 (t, J = 6.0 Hz,
2H),
4.39 (t, J= 5.6 Hz, 2H), 6.17 (s, 2H), 6.74 (d, J= 5.6 Hz, 1H), 7.55 (d, J =
5.6 Hz, 1H).
Step 2: 9-Bromo-2,3 ,4,5 -tetrahydropyrido [3,2-b] [1,4] oxazepine
Br 0
61
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
To a stirred solution of 4-bromo-3-(3-chloropropoxy)pyridin-2-amine (step 1
intermediate)
(400 mg, 1.20 mmol) in DMF (5.0 mL) was added sodium hydride (60% w/w, 96 mg,
2.40
mmol) at RT. The mixture stirred at 80 C for 1 h. The reaction mixture was
quenched with
water and diluted with ethyl acetate. The layers were separated and the
aqueous layer was
extracted twice with ethyl acetate. The combined organic layers were washed
with saturated
sodium bicarbonate solution followed by brine. The organic layer was dried
over anhydrous
sodium, filtered and concentrated under reduced pressure to yield 250 mg of
the desired
product. 1H NMR (400 MHz, DMSO-d6) 6 1.94-1.98 (m, 2H), 3.25-3.29 (m, 2H) 4.15
(t, J =
6.0 Hz, 2H), 6.39 (br s, 1H), 6.86 (d, J = 5.6 Hz, 1H), 7.52 (d, J= 5.2 Hz,
1H),
Step 3: tert-Butyl 9-bromo-3,4-dihydropyrido [3,2-b] [1,4] oxazepine-5 (2H)-
carboxylate
The titled compound was prepared by the reaction of 9-bromo-2,3,4,5-
tetrahydropyrido[3,2-
b][1,4]oxazepine (step 2 intermediate) (2.0 g, 8.77 mmol) and di-tert-butyl
dicarbonate (2.84
g, 13.0 mmol) in the presence of lithium bis(trimethylsilyl)amide (LiHMDS)
(1M, 11 mL,
10.6 mmol) in anhydrous THF (20 mL) as per the procedure described in step 3
of
.. Intermediate A7 to yield 2.1 g of the compound. 1H NMR (400 MHz, DMSO-d6) 6
1.35 (s,
9H), 1.94-1.97 (m, 2H), 3.60-3.63 ( br s, 2H), 4.12-4.14 (br s, 2H), 7.63 (d,
J = 5.2 Hz, 1H),
8.02 (d, J = 5.2 Hz, 1H).
Intermediate A21
tert-Butyl 8-bromo-7-nitro-2H-pyrido[3,2-b][1,4]oxazine-4(3H)-carboxylate
Br
02N 0
I j
N N
60c
Step 1: 8-Bromo-7-nitro-3,4-dihydro-2H-pyrido [3,2-b] [1,4]oxazine
Br
02N ko
k
1\1 N
To a solution of 8-bromo-3,4-dihydro-2H-pyrido[3,2-b][1,4]oxazine (step 2 of
Intermediate
A7) (802 mg, 3.73 mmol) in trifluoroacetic acid (5.0 mL) was added potassium
nitrate (415
mg, 4.10 mmol) at 0 C. The mixture was gradually warmed to RT and stirred
overnight at
RT. The mixture was quenched with ice-water and neutralized with aqueous
sodium
hydroxide solution at 0 C. The precipitated solid was filtered, washed with
water and dried.
The crude solid was triturated with diethyl ether and dried well to yield 732
mg of the desired
62
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
compound. 1H NMR (400 MHz, DMSO-d6) 6 3.52 (t, J = 4.0 Hz, 2H), 4.26 (t, J =
4.0 Hz,
2H), 8.46 (s, 1H), 8.60 (s, 1H).
Step 2: tert-Butyl 8-bromo-7-nitro-2H-pyrido [3,2-b] [1,4]oxazine-4(3H)-
carboxylate
The titled compound was prepared by the reaction of 8-bromo-7-nitro-3,4-
dihydro-2H-
pyrido[3,2-b][1,4]oxazine (step 1 intermediate) (875 mg, 3.36 mmol) with di-
tert-butyl
dicarbonate (1.15 mL, 5.04 mmol) in the presence of triethylamine (0.94 mL,
6.72 mmol) and
DMAP (41 mg, 0.34 mmol) in dichloromethane (20 mL) as per the procedure
described in
step 3 of Intermediate A4 to yield 1.02 g of the compound. 1H NMR (400 MHz,
DMSO-d6) 6
1.49 (s, 9H), 3.94 (t, J= 4.4 Hz, 2H), 4.46 (t, J= 4.4 Hz, 2H), 8.58 (s, 1H).
Methods for the Synthesis of Intermediate B
Intermediate B1
Preparation of methyl 4-chloro-3-hydroxybenzoate
CI al
0,
HO
0
To a stirred solution of 4-chloro-3-hydroxybenzoic acid (690 mg, 4.00 mmol) in
methanol
(8.0 mL) was added sulfuric acid (10 L, 0.20 mmol) and the mixture was heated
at 75 C for
14 h. The mixture was concentrated, residue was cooled to 0 C and neutralized
using
aqueous sodium bicarbonate solution. The mixture was extracted twice with
ethyl acetate.
The combined organic layers were washed with water followed by brine and dried
over
anhydrous sodium sulfate. The solution was filtered, concentrated to yield 902
mg of the
desired product. 1H NMR (400 MHz, DMSO-d6) 6 3.91 (s, 3H), 5.66 (d, J = 4.4
Hz, 1H),
7.39 (d, J = 8.3 Hz, 1H), 7.56 (dd, J = 8.3, 2.0 Hz, 1H), 7.69 (d, J = 2.0 Hz,
1H); ESI-MS
(m/z) 187 (M+H)+.
The chemical structure, name and analytical data of the intermediate prepared
by
following the procedure described above are given below in Table 6.
Table 6: Chemical name, structure and analytical data of Intermediates B2-B12
Intermediate
No. Chemical Name and Structure Analytical Data
B2 HO 00 0,
ESI-MS (m/z) 153 (M+H)+.
0
Methyl 3-hydroxybenzoate
1H NMR (400 MHz, CDC13) 6 2.30 (s,
B3 HO 40 0, 3H), 3.90 (s, 3H), 5.33 (s,
1H), 7.18 (d,
0 J = 8.1 Hz, 1H), 7.52-7.53 (m,
2H);
Methyl 3-hydroxy-4- ESI-MS (m/z) 167 (M+H)+.
63
CA 03064975 2019-11-26
WO 2018/215668 PCT/EP2018/063957
Intermediate
Chemical Name and Structure Analytical Data
No.
methylbenzoate
F
B4 a
HO 0, CAS # 214822-96-5; ESI-MS (m/z)
O 171(M+H)+.
Methyl 4-fluoro-3-hydroxybenzoate
CI
B5 HO
1I-1 NMR (400 MHz, DMSO-d6) 6 3.95
101 (s, 3H), 6.12 (br s, 1H), 7.10 (s,
1H),
O 7.49 (s, 1H), 7.60 (s, 1H).
Methyl 3-chloro-5-hydroxybenzoate
40 0, 1I-1 NMR (400 MHz, DMSO-d6) 6 2.48
B6 HO (s, 3H), 3.91 (s, 3H), 7.15-7.25
(m,
0
1H), 7.43 (dd, Ji = 0.8 Hz, .12 = 8.0 Hz,
Methyl 3-hydroxy-2-
1H).
methylbenzoate
CI al
1I-1 NMR (400 MHz, DMSO-d6) 6 2.42
0,
B7 HO (s, 3H), 3.81 (s, 3H), 7.25 (d, J =
8.0
O Hz, 1H), 7.31 (dd, ./1 = 0.8 Hz, .12 = 8.8
Methyl 4-chloro-3-hydroxy-2-
Hz, 1H), 9.50 (s, 1H).
methylbenzoate
al a 1I-1 NMR (400 MHz, DMSO-d6) 6 3.83
B8 HO 0, (s, 3H), 6.95 (dd, Ji = 2.8 Hz, .12
= 8.8
O Hz, 1H), 7.16 (s, 1H), 7.35 (d, J = 8.8
Methyl 2-chloro-5-hydroxybenzoate Hz, 1H), 10.08 (s, 1H).
,0 a
0, 1I-1 NMR (400 MHz, DMSO-d6) 6 3.83
B9 HO
(s, 6H), 7.01 (d, J = 8.4 Hz, 1H), 7.36
O (s, 1H), 7.43 (dd, Ji = 2.0 Hz, .12 = 8.0
Methyl 3-hydroxy-4- Hz, 1H), 9.44 (s, 1H).
methoxybenzoate
CF3 1I-1 NMR (400 MHz, DMSO-d6) 6 3.87
(s, 3H), 7.31-7.32 (m, 1H), 7.60-7.62
B10 HO 1.1 0 , (m, 2H), 10.67 (s, 1H).
0
Methyl 3-hydroxy-5-
(trifluoromethyl)benzoate
B11 al F
iCo 1I-1 NMR (400 MHz, DMSO-d6) 6 3.83
HO (s, 3H), 6.99-7.03 (m, 1H), 7.12-
7.22
O (m, 2H), 9.79 (s, 1H).
Methyl 2-fluoro-5-hydroxybenzoate
0
B12 a al o' CAS # 3964-57-6; ESI-MS (m/z) 187
HO (M+H)+.
Methyl 3-chloro-4-hydroxybenzoate
Intermediate B13
64
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
Methyl 3-hydroxy-4-(trifluoromethyl)benzoate
F3c
HO 0,
0
Step 1: Methyl 3-(benzyloxy)-4-(trifluoromethyl)benzoate
F3
io 0
0
A mixture of 3-fluoro-4-(trifluoromethyl)benzoic acid (1.0 g, 4.80 mmol) and
benzyl alcohol
(3.67 mL, 36.4 mmol) and potassium tert-butoxide (1.58 g, 14.2 mmol) in DMSO
was stirred
at RT for 2 days. The mixture was diluted with ethyl acetate and water. The
aqueous layer
was separated and acidified with conc. HC1 and extracted twice with ethyl
acetate. The
combined organic layers were washed with water followed by brine and dried
over anhydrous
sodium sulfate. The solution was filtered and concentrated. The residue was
dissolved in
methanol (5.0 mL). Sulfuric acid (0.5 mL) was added to the mixture and
refluxed for 3 h. The
mixture was concentrated, residue was cooled to 0 C and neutralized using
aqueous sodium
bicarbonate solution. The mixture was extracted twice with ethyl acetate. The
combined
organic layers were washed with water followed by brine and dried over
anhydrous sodium
sulfate. The solution was filtered, concentrated to yield 510 mg of the
desired product. 1H
NMR (400 MHz, DMSO-d6) 6 3.97 (s, 3H), 5.27 (s, 2H), 7.35-7.50 (m, 5H), 7.69-
7.70 (m,
2H), 7.74 (s, 1H).
Step 2: Methyl 3-hydroxy-4-(trifluoromethyl)benzoate
A solution of methyl 3-(benzyloxy)-4-(trifluoromethyl)benzoate (step 1
intermediate) (500
mg, 2.27 mmol) in methanol (5.0 mL) with catalytic amount of 10% palladium on
carbon
(50% wet) was hydrogenated at RT for 16 h. The mixture was filtered through
celite and the
celite bed was rinsed with methanol. The combined filtrate and washings were
concentrated
and the residue thus obtained was purified by silica gel column chromatography
to yield 400
mg of the desired product. 1H NMR (400 MHz, DMSO-d6) 6 3.87 (s, 3H), 7.47 (d,
J = 8.0
Hz, 1H), 7.60 (s, 1H), 7.66 (d, J= 8.0 Hz, 1H), 11.12 (br s, 1H).
Intermediate B14
Methyl 3-mercapto-4-methylbenzoate
el 0,
HS
0
Step 1: 3-Mercapto-4-methylbenzoic acid
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
SI O
HS H
0
To a stirred suspension of 3-amino-4-methylbenzoic acid (2.0 g, 13.22 mmol) in
conc.
hydrochloric acid (8.0 mL) and water (20 mL) at 0 C was added a solution of
sodium nitrite
(958 mg, 13.88 mmol) in water (20 mL) over a period of 20 min. The
diazotization mixture
was added lot wise to a stirred mixture of potassium ethyl xanthate (2.5 g,
15.8 mmol) and
2M aqueous sodium carbonate solution (22 mL, 43.6 mmol) over a period of 20
min. The
mixture was heated for 1 h at 45 C. The mixture was acidified with conc.
hydrochloric acid
and the product was extracted twice in ethyl acetate. The combined organic
extracts were
washed with water and dried over anhydrous sodium sulfate. The residue
obtained after
removal of solvents was refluxed overnight with potassium hydroxide (2.9 g,
52.9 mmol) in a
mixture of ethanol and water (1:1, 20 mL). The reaction mixture was cooled to
RT and
acidified with conc. hydrochloric acid and extracted thrice with ethyl
acetate. The combined
organic extracts were washed with water, dried over anhydrous sodium sulfate,
filtered and
concentrated. The crude product was purified by silica gel column
chromatography to yield
760 mg of the desired product. 1H NMR (400 MHz, DMSO-d6) 6 2.23 (s, 3H), 5.64
(s, 1H),
7.29 (d, J= 8.0 Hz, 1H), 7.60 (dd, J, = 1.6 Hz, J2 = 8.0 Hz, 1H), 7.98 (s,
1H), 12.90 (s, 1H);
ESI-MS (m/z) 167 (M-H)-.
Step 2: Methyl 3-mercapto-4-methylbenzoate
To a stirred solution of 3-mercapto-4-methylbenzoic acid (760 g, 4.52 mmol) in
methanol (10
mL) was added sulfuric acid (250 L) and the mixture was refluxed for 2 h. The
mixture was
concentrated, residue was cooled to 0 C and neutralized using aqueous sodium
bicarbonate
solution. The mixture was extracted twice with ethyl acetate. The combined
organic layers
were washed with water followed by brine and dried over anhydrous sodium
sulfate. The
solution was filtered, concentrated to yield 502 mg of the desired product. 1H
NMR (400
MHz, DMSO-d6) 6 2.28 (s, 3H), 3.83 (s, 3H), 5.75 (s, 1H), 7.32 (d, J= 8.0 Hz,
1H), 7.62 (dd,
= 1.6 Hz, .1-2 = 7.6 Hz, 1H), 8.01 (s, 1H).
Methods for the Synthesis of Intermediate C
Intermediate Cl
4-((4-Ethylpiperazin-1-yl)methyl)-3-(trifluoromethyl)aniline
H2N CFr,,
N
1\1,)
Step 1: 1-(Bromomethyl)-4-nitro-2-(trifluoromethyl)benzene
66
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
02N 0 CF3
Br
To a solution of 2-methyl-5-nitrobenzotrifluoride (10 g, 48.5 mmol) and AIBN
(800 mg, 4.85
mmol) in ethylene dichloride (150 mL) was added N-bromosuccinimide (8.6 g,
48.5 mmol) at
RT and the reaction mixture was refluxed for 18 h. The mixture was
concentrated and the
residue was diluted with water and ethyl acetate. The layers were separated
and the aqueous
layer was extracted with ethyl acetate. The combined organic layers were
washed with water
and brine. The solution was dried over anhydrous sodium sulfate, filtered and
concentrated.
The residue obtained was purified by silica gel column chromatography to yield
6.21 g of the
desired compound. 1H NMR (400 MHz, DMSO-d6) 6 4.88 (s, 2H), 8.04 (d, J = 8.4
Hz, 1H),
8.43 (s, 1H), 8.54 (dd, ./i = 2.4 Hz, .1-2 = 8.4 Hz, 1H).
Step 2: 1-Ethyl-4-(4-nitro-2-(trifluoromethyl)benzyl)piperazine
02N
r N
N)
To a stirred solution of 1-(bromomethyl)-4-nitro-2-(trifluoromethyl)benzene
(step 1
intermediate) (4.0 g, 14.1 mmol) in dichloromethane (40 mL) were added N-
ethylpiperazine
(1.88 mL, 14.7 mmol) followed by N,N-diisopropylethylamine (DIPEA) (3.27 mL,
19.1
mmol) at RT. The mixture stirred at RT for 16 h. The reaction mixture was
diluted with
dichloromethane and washed with saturated sodium bicarbonate solution followed
by brine.
The organic layer was dried over anhydrous sodium, filtered and concentrated
under reduced
pressure. The residue obtained was purified by column chromatography to yield
3.5 g of the
desired product. 1H NMR (400 MHz, DMSO-d6) 6 0.96 (t, J = 7.2 Hz, 3H), 2.28-
2.51 (m,
10H), 3.72 (s, 2H), 8.09 (d, J= 8.4 Hz, 1H), 8.40 (s, 1H), 8.51 (dd, ./1 = 2.4
Hz, J2 = 8.8 Hz,
1H).
Step 3: 4-((4-Ethylpiperazin-1-yl)methyl)-3-(trifluoromethyl)aniline
A solution of 1-ethyl-4-(4-nitro-2-(trifluoromethyl)benzyl)piperazine (step 2
intermediate)
(800 mg, 2.52 mmol) in methanol (20 mL) with catalytic amount of 10% palladium
on
carbon (50% wet) was hydrogenated at RT for 16 h. The mixture was filtered
through celite
and the celite bed was rinsed with methanol. The combined filtrate and
washings were
concentrated and the residue thus obtained was purified by silica gel column
chromatography
to yield 600 mg of the desired product. 1H NMR (400 MHz, DMSO-d6) 6 0.97 (t, J
= 7.2 Hz,
3H), 2.26-2.51 (m, 10H), 3.38 (s, 2H), 5.44 (s, 2H), 6.74 (dd, ./i = 2.4 Hz,
J2 = 8.4 Hz, 1H),
6.85 (s, 1H), 7.29 (t, J = 8.4 Hz, 1H).
67
CA 03064975 2019-11-26
WO 2018/215668 PCT/EP2018/063957
The chemical structure, name and analytical data of the intermediate prepared
by
following the procedure described above are given below in Table 7.
Table 7 Chemical name, structure and analytical data of Intermediates C2-C15
Intermediate
Chemical Name and Structure Analytical Data
No.
H2N 0cFrN.... lti
NMR (400 MHz, DMSO-d6) 6 2.19
N,) (s, 3H), 2.51 (br s, 8H), 3.33 (s,
2H),
C2
4-((4-Methylpiperazin-1-yl)methyl)- 5.44 (s, 2H), 6.75 (dd, ./1 = 2.0 Hz, J2 =
3-(trifluoromethyl)aniline 8.0
Hz, 1H), 6.84 (s, 1H), 7.29 (d, J =
8.0 Hz, 1H).
1F1 NMR (400 MHz, DMSO-d6) 6 0.95
H2N cFN,1.1
I. N) (d,
J = 6.4 Hz, 6H), 2.33-2.60 (m, 9H),
C3 4-((4-Isopropylpiperazin-1-
3.37 (d, J = 9.9 Hz, 2H), 5.43 (s, 2H),
6.75 (dd, ./1 = 1.6 Hz, J2 = 8.0 Hz, 1H),
yl)methyl)-3-
6.84 (s, 1H), 7.29 (d, J = 11.6 Hz, 1H).
(trifluoromethyl)aniline
1F1 NMR (400 MHz, DMSO-d6) 6 0.88
(d, J = 6.4 Hz, 3H), 1.05-1.15 (m, 2H),
H2N 0cFr----.....---
1.29-1.34 (m, 1H), 1.54 (d, J = 12.0
N.,,,..- Hz, 2H), 1.86 (d, J = 11.2 Hz, 2H),
C4
4-((4-Methylpiperidin-1-yl)methyl)- 2.72 (d, J= 11.6 Hz, 2H), 3.34 (s, 2H),
3-(trifluoromethyl)aniline 5.42 (s, 2H), 6.75 75 (dd, ./1 = 2.0 Hz,
J2 = 8.4 Hz, 1H), 6.84 (s, 1H), 7.30 (d,
J = 8.4 Hz, 1H); ESI-MS (m/z) 273
(M+H)+.
1F1 NMR (400 MHz, DMSO-d6) 6 2.31
H2N 40cFro (br
s, 4H), 3.17 (d, J = 5.2 Hz, 2H),
N,.) 3.39 (s, 2H), 3.55 (t, J = 4.4 Hz,
2H),
C5
4-(Morpholinomethyl)-3-
5.46 (s, 2H), 6.75 (dd, ./1 = 2.0 Hz, J2 =
(trifluoromethyl)aniline 8.4
Hz, 1H), 6.86 (s, 1H), 7.30 (d, J =
8.4 Hz, 1H); ESI-MS (m/z) 261
(M+H)+.
1FI NMR (400 MHz, DMSO-d6) 6 1.39
H2N iocFr.N.Boc (s,
9H), 2.27 (d, J = 4.8 Hz, 4H), 2.60
N,.) (d,
J = 5.2 Hz, 4H), 3.20-3.40 (m, 2H),
C6
tert-Butyl 4-(4-amino-2-
5.76 (s, 2H), 6.75 (dd, ./1 = 2.0 Hz, J2 =
(trifluoromethyl)benzyl)piperazine- 8.0 Hz, 1H), 6.85 (s, 1H), 7.30 (d, J =
1-carboxylate 8.0 Hz, 1H); ESI-MS (m/z) 360
(M+H)+.
1F1 NMR (400 MHz, DMSO-d6) 6 0.96
H2N C7 0 1\1) r.N
(t, J= 4.8 Hz, 3H), 2.11-2.51 (m, 13H),
,
4-((4-Ethylpiperazin-1-yl)methyl)-
3.21 (s, 2H), 4.85 (s, 2H), 6.29 (dd, ./1
= 2.4 Hz, J2 = 8.0 Hz, 1H), 6.35 (s,
3-methylaniline
1H), 6.78 (d, J = 8.0 Hz, 1H).
0 1FI
NMR (400 MHz, DMSO-d6) 6 1.97
C8 H2N so cFrN,Ic (s,
3H), 2.27 (t, J = 4.8 Hz, 2H), 2.33
N1) (t,
J = 4.8 Hz, 2H), 3.33-3.42 (m, 6H),
68
CA 03064975 2019-11-26
WO 2018/215668 PCT/EP2018/063957
Intermediate
Chemical Name and Structure Analytical Data
No.
1-(4-(4-Amino-2- 5.76 (s, 2H), 6.77 (dd, J, = 2.0
Hz, .1-2 =
(trifluoromethyl)benzyl)piperazin-1- 10.0 Hz, 1H), 6.86 (s, 1H), 7.32 (t, J =
yl)ethanone 8.4 Hz, 1H).
1H NMR (400 MHz, DMSO-d6) 6 0.83
H2N CFN
(t, J= 7.2 Hz, 3H), 1.38-1.43 (m, 2H),
C9 2.18-2.46 (m, 12H), 5.76 (s, 2H),
6.75
4-((4-Propylpiperazin-1-yl)methyl)-
(d, J = 8.4 Hz, 1H), 6.84 (s, 1H), 7.28
3-(trifluoromethyl)aniline
(d, J = 8.4 Hz, 1H); ESI-MS (m/z) 302
(M+H)+.
1H NMR (400 MHz, DMSO-d6) 6 0.95
cF3
(t, J= 3.6 Hz, 3H), 2.30-2.51 (m, 8H),
C10
1\1,) 3.17-3.58 (m, 4H), 5.76 (s, 2H), 6.71
H2N (s, 1H), 6.78 (s, 1H), 6.85 (s,
1H); ESI-
3-((4-Ethylpiperazin-1-yl)methyl)- MS (m/z) 288 (M+H)+.
5-(trifluoromethyl)aniline
cF3 1H NMR (400 MHz, DMSO-d6) 6 2.13
C11 (s, 6H), 3.29 (s, 2H), 5.0-5.3 (s,
2H),
H2N 6.69 (s, 1H), 6.72 (s, 1H), 6.76
(s, 1H);
34(Dimethylamino)methyl)-5- ESI-MS (m/z) 219 (M+H)+.
(trifluoromethyl)aniline
1H NMR (400 MHz, DMSO-d6) 6
H2N = 1.07-1.61 (m, 2H), 1.60-1.91 (m,
3H),
2.13 (s, 6H), 2.28-2.49 (m, 2H), 2.55-
C12
2.61 (m, 1H), 2.69-2.71 (m, 1H), 5.42
1-(4-Amino-2- (s, 2H), 6.75 (d, J, = 2.0 Hz, Ji = 8.8
(trifluoromethyl)benzy1)-N,N- Hz, 1H), 6.84 (s, 1H), 7.28 (d, J =
8.4
dimethylpyrrolidin-3-amine Hz, 1H); ESI-MS (m/z) 288 (M+H)+.
H2N = cF(s)
I
,)"=N\
C13
(S)-1-(4-Amino-2- ESI-MS (m/z) 288 (M+H)+.
(trifluoromethyl)benzy1)-N,N-
dimethylpyrrolidin-3-amine
1
\1¨
H NMR (400 MHz, DMSO-d6) 6
1
H2N = ,) 1.81-2.03 (m, 4H), 2.25 (s, 6H),
2.54-
C14 11 2.89 (m, 5H), 3.88 (q, J = 16.0 Hz,
(R)-1-(4-Amino-2- 2H), 8.09 (d, J= 8.4 Hz, 1H), 8.39
(dd,
(trifluoromethyl)benzy1)-N,N- J, = 2.4 Hz, Ji = 8.4 Hz, 1H), 8.51
(s,
dimethylpyrrolidin-3-amine 1H).
0 1H NMR (400 MHz, DMSO-d6) 6 0.98
(:) CF3.N (t, J= 7.2 Hz, 3H), 2.31-2.51 (m,
10H),
C15 N1,) 3.67 (s, 2H), 3.89 (s, 3H), 7.96
(d, J =
Methyl 4-((4-ethylpiperazin-1- 8.0 Hz, 1H), 8.17 (s, 1H), 8.22 (d,
J =
yl)methyl)-3- 8.4 Hz, 1H); ESI-MS (m/z) 331
(trifluoromethyl)benzoate (M+H)+.
69
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
Intermediate
No. Chemical Name and Structure Analytical Data
H2N SO2Me
1H NMR (400 MHz, DMSO-d6) 6 0.96
(t, J = 7.2 Hz, 3H), 2.27 (q, J = 7.2 Hz,
C104 N
2H), 2.32-2.38 (m, 6H), 3.32-3.43 (m,
C N
2H), 3.41 (s, 3H), 3.64 (s, 2H), 5.63 (s,
2H), 6.74 (dd, Ji = 2.4, .1-2 = 8.0 Hz,
1H), 7.08 (d, J= 8.4 Hz, 1H),7.21 (d, J
4-((4-ethylpiperazin-1-yl)methyl)-3- - 2.4 Hz, 1H).
(methylsulfonyl)aniline
Intermediate C16
4-(2-(Dimethylamino)ethoxy)-3-(trifluoromethyl)aniline
H2N oF3
N
Step 1: N,N-Dimethy1-2-(4-nitro-2-(trifluoromethyl)phenoxy)ethanamine
02N is cF3
To a stirred solution of N,N-dimethylethanolamine (2.9 mL, 48.6 mmol) in DMF
(10 mL)
was added sodium hydride (60% w/w, 421 mg, 10.5 mmol) at 0 C and stirred for
30 min. 1-
Fluoro-4-nitro-2-(trifluoromethyl)benzene (2.0 g, 9.56 mmol) was added to the
mixture and
stirred at RT for 2 h. The reaction mixture was quenched with water extracted
twice with
ethyl acetate. The combined organic layers were washed with water followed by
brine, dried
over anhydrous sodium, filtered and concentrated under reduced pressure. The
residue
obtained was purified by column chromatography to yield 2.3 g of the desired
product. The
compound was as such taken forward to the next step without characterization.
Step 2: 4-(2-(Dimethylamino)ethoxy)-3-(trifluoromethyl)aniline
The titled compound was prepared by the catalytic hydrogenation reaction of
N,N-dimethy1-
2-(4-nitro-2-(trifluoromethyl)phenoxy)ethanamine (2.2 g, 7.91 mmol) in
methanol (15 mL) as
per the procedure described in step 3 of Intermediate Cl to yield 1.8 g of the
compound. 1H
NMR (400 MHz, DMSO-d6) 6 2.20 (s, 6H), 2.58 (t, J = 6.0 Hz, 2H), 4.0 (t, J =
6.0 Hz, 2H),
5.03 (s, 2H), 6.77 (dd, J, = 2.8 Hz, J1 = 8.4 Hz, 1H), 6.81-6.82 (br s, 1H),
6.97 (d, J = 8.8 Hz,
1H).
The chemical structure, name and analytical data of the intermediate prepared
by following
the procedure described above are given below in Table 8.
Table 8: Chemical name, structure and analytical data of Intermediates C17 and
C67
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
Intermediate
No. Chemical Name and Structure
Analytical Data
1F1 NMR (400 MHz, DMSO-d6) 6 0.97
H2N
(t, J= 7.2 Hz, 3H), 1.59-1.63 (m, 2H),
1.84-1.90 (m, 2H), 2.19 (br
s, 2H),
C17 IW 0
2.32 (q, J = 7.2 Hz, 2H), 2.50-2.63 (m,
4-((1-Ethylpiperidin-4-yl)oxy)-3-
2H), 4.28-4.30 (m, 1H), 5.02 (br s,
(trifluoromethyl)aniline
2H), 6.74-6.81 (m, 2H), 6.98 (d, J = 8.8
Hz, 1H); ESI-MS (m/z) 289 (M+H)+.
H2N = cF3
NMR (400 MHz, DMSO-d6) 6
1.73-1.78 (m, 2H), 1.93-1.98 (m, 2H),
0
C67
2.28-2.51 (m, 5H), 2.74 (br s, 2H), 4.46
LNJ
(br s, 1H), 5.15 (s, 2H), 6.79 (dd, J, =
1.2 Hz, J2 = 8.0 Hz, 1H),6.97 (d, J =
2-((1-Methylpiperidin-4-yl)oxy)-5- 8.4 Hz, 1H), 8.09 (s, 1H); APCI-MS
(trifluoromethyl)aniline (m/z) 275 (M+H)+.
Intermediate C18
3 -(4-Isopropylpip erazin-l-y1)-5 -(trifluoromethyl)aniline
cF3
H2N NTh
LN
To a stirred solution of 3-bromo-5-(trifluoromethyl)aniline (1.5 g, 6.25 mmol)
in DMF (10
mL) were added 1-isoprpylpiperazine (3.6 mL, 24.4 mmol), cesium carbonate
(4.06 g, 12.5
mmol), copper (I) iodide (595 mg, 3.12 mmol) and 8-hydroxyquinoline (272 mg,
1.87 mmol)
at RT. The mixture was heated in a sealed tube at 120 C for 16 h. The
reaction mixture was
cooled to RT and diluted with ethyl acetate. The solution was washed with
water followed by
brine. The organic layer was dried over anhydrous sodium sulfate, filtered and
concentrated
under reduced pressure. The residue obtained was purified by column
chromatography to
yield 660 mg of the desired product. 1I-1 NMR (400 MHz, DMSO-d6) 6 1.00 (d, J
= 6.4 Hz,
6H), 2.50-2.55 (m, 4H), 2.62-2.69 (m, 1H), 3.07 (t, J= 4.8 Hz, 4H), 5.33-5.35
(br s, 2H), 6.28
(s, 1H), 6.32 (s, 2H); ESI-MS (m/z) 288 (M+H)+.
The chemical structure, name and analytical data of the intermediate prepared
by
following the procedure described above are given below in Table 9.
Table 9: Chemical name, structure and analytical data of Intermediates C29-C23
Intermediate
ChemicalNo. Name and Structure
Analytical Data
71
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
Intermediate
Chemical Name and Structure Analytical Data
No.
CF3
C19 = N 1H NMR (400 MHz, DMSO-d6) 6
H2N 2.22 (s, 3H), 2.43-2.50 (m, 4H),
3.09
(t, J = 4.8 Hz, 4H), 5.33 (s, 2H), 6.29
3-(4-Methylpiperazin-1-y1)-5- (s, 1H), 6.33 (s, 2H).
(trifluoromethyl)aniline
CF3 1H NMR (400 MHz, DMSO-d6) 1.02
(t, J= 3.2 Hz, 3H), 2.31-2.38 (m, 2H),
C20 H2N 2.46-2.51 (m, 4H), 3.09 (t, J=
4.8 Hz,
4H), 5.34 (s, 2H), 6.29 (s, 1H), 6.33
3-(4-Ethylpiperazin-1-y1)-5- (s, 2H); ESI-MS (m/z) 274
(M+H)+.
(trifluoromethyl)aniline
CF3 1H NMR (400 MHz, DMSO-d6) 0.85
(t, J= 7.6 Hz, 3H), 1.34-1.49 (m, 2H),
C21 2.17-2.34 (m, 2H), 2.44-2.54 (m, 4H),
H2N
3.09 (br s, 4H), 5.36 (s, 2H), 1
6.28-
\1.7\
6.32 (br s
3-(4-Propylpiperazin-1-y1)-5-
" 3H). ESI-MS (m/z) 288
+
(trifluoromethyl)aniline (M+H).
CF3 1H NMR (400 MHz, DMSO-d6)
C22 H2N N
0.10 (m, 2H), 0.45-0.50 (m, 2H),
0.83-0.87 (m, 1H), 2.19-2.22 (m, 2H),
A
2.49-2.55 (m, 4H), 3.10 (t, J= 4.8 Hz,
3-(4- 4H), 5.36 (s, 2H), 6.29 (s,1H),
6.33 (s,
(Cyclopropylmethyl)piperazin-1- 2H); ESI-MS (m/z) 300 (M+H)+.
y1)-5-(trifluoromethyl)aniline
1H NMR (400 MHz, DMSO-d6) 0.92
CF3
(d, J = 6.4 Hz, 3H), 1.12-1.23 (m,
C23 40 H2N Na 2H), 1.47-1.53 (m, 1H),
1.64-1.67 (m,
2H), 2.65 (dt, J, = 2.4 Hz, J2 = 12.4
Hz, 2H), 3.58-3.61 (m, 2H), 5.31 (s,
3-(4-Methylpiperidin-1-y1)-5- 2H), 6.25 (s, 1H), 6.32 (d, J =
10.8
(trifluoromethyl)aniline Hz, 2H).
Intermediate C24
4-(4-Methylpiperazin-1-y1)-3-(trifluoromethyl)aniline
H2N CF3
LN
Step 1: 1-Methyl-4-(4-nitro-2-(trifluoromethyl)phenyl)piperazine
02N rdi., CF3
LN
72
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
A mixture of 2-fluoro-5-nitrobenzotrifluride (5.0 g, 23.9 mmol) and 1-
methylpiperazine (7.18
g, 71.7 mmol) in DMSO (20 mL) was heated at 100 C for 5 h. The mixture was
cooled to
RT and diluted with ethyl acetate and water. The organic layer was separated;
and the
aqueous layer was extracted with ethyl acetate. The combined organic layers
were washed
with water followed by brine and concentrated under reduced pressure to give
5.2 g of the
desired compound. 1H NMR (400 MHz, DMSO-d6) 6 2.23 (s, 3H), 2.45-2.51 (m, 4H),
3.13 (t,
J= 4.8 Hz, 4H), 7.54 (d, J= 8.8 Hz, 1H), 8.38 (d, J= 2.8 Hz, 1H), 8.40 (d, J=
1.6 Hz, 1H).
Step 2: 4-(4-Methylpip erazin-l-y1)-3 -(trifluoromethyl)aniline
The titled compound was prepared by the catalytic hydrogenation reaction of 1-
methyl-4-(4-
nitro-2-(trifluoromethyl)phenyl)piperazine (step 1 intermediate) (5.0 g, 17.3
mmol) in
methanol (200 mL) as per the procedure described in step 3 of Intermediate Cl
to yield 4.12
g of the compound. 1H NMR (400 MHz, DMSO-d6) 6 2.19 (s, 3H), 2.49-2.51 (m,
4H), 2.71
(t, J = 4.4 Hz, 4H), 5.34 (s, 2H), 6.76 (d, J = 8.8 Hz, 2H), 7.22 (d, J = 8.4
Hz, 1H); ESI-MS
(m/z) 260 (M+H)+.
The chemical structure, name and analytical data of the intermediate prepared
by
following the procedure described above are given below in Table 10.
Table 10: Chemical name, structure and analytical data of Intermediates C25-
C26
Intermediate
No. Chemical Name and Structure Analytical Data
H2N cF3
1H NMR (400 MHz, DMSO-d6) 6
2.70 (d, J = 4.4 Hz, 4H), 3.64 (t, J
C25
= 3.6 Hz, 4H), 5.37 (s, 2H), 6.78
(dd, J, = 2.4 Hz, J2 = 8.4 Hz,
4-Morpholino-3-
1H), 6.82 (s, 1H), 7.25 (d, J = 8.8
(trifluoromethyl)aniline
Hz, 1H) ; ESI-MS (m/z) 247
(M+H)+.
cF3
H2N 40 N 1H NMR (400 MHz, DMSO-d6)
C26
6
3.06 (d, J = 4.8 Hz, 4H), 3.71 (d,
Lo
J = 4.8 Hz, 4H), 5.39 (s, 2H),
3 -Morpho lino-5 - 6.32-6.34 (m, 3H).
(trifluoromethyl)aniline
Intermediate C27
tert-Butyl 4-(4-amino-2-(trifluoromethyl)phenyl)piperazine-1-carboxylate
H2N cF3
C,N'Boc
Step 1: tert-Butyl 4-(4-nitro-2-(trifluoromethyl)phenyl)piperazine-1-
carboxylate
73
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
02N 40 cF3
N'
L,....õ N.
Boc
To a stirred solution of 2-fluoro-5-nitrobenzotrifluride (5.0 g, 23.9 mmol) in
acetonitrile (50
mL) were added potassium carbonate (6.6 g, 47.8 mmol) and tert-butyl
piperazine-l-
carboxylate (4.9 g, 26.3 mmol) at RT and the mixture refluxed 24 h. The
mixture was cooled
to RT and diluted with ethyl acetate and water. The layers were separated and
the aqueous
layer was extracted with ethyl acetate. The combined organic layers were
washed with water
followed by brine and concentrated under reduced pressure to give 5.23 g of
the desired
compound. 1H NMR (400 MHz, DMSO-d6) 6 1.43 (s, 9H), 3.06 (t, J = 5.2 Hz, 4H),
3.47 (t, J
= 4.4 Hz, 4H), 7.61 (d, J = 8.8 Hz, 1H), 8.39-8.45 (m, 2H).
Step 2: tert-Butyl 4-(4-amino-2-(trifluoromethyl)phenyl)piperazine-1-
carboxylate
The titled compound was prepared by the catalytic hydrogenation reaction of
tert-butyl 4-(4-
nitro-2-(trifluoromethyl)phenyl)piperazine-1-carboxylate (step 1 intermediate)
(3.0 g, 8.00
mmol) in methanol (200 mL) as per the procedure described in step 3 of
Intermediate Cl to
yield 2.3 g of the compound. 1H NMR (400 MHz, DMSO-d6) 6 1.41 (s, 9H), 2.66
(t, J = 4.8
Hz, 4H), 3.34 (s, 4H), 5.38 (m, 2H), 6.75 (dd ./1 = 2.4 Hz, J2 = 8.4 Hz, 1H),
6.81 (s, 1H), 7.23
(d, J = 8.4 Hz, 1H); ESI-MS (m/z) 346 (M+H)+.
The chemical structure, name and analytical data of the intermediate prepared
by
following the procedure described above are given below in Table 11.
Table 11: Chemical name, structure and analytical data of Intermediates C99
Intermediate
No. Chemical Name and Structure
Analytical Data
H2N 40 c _F3
1H NMR (400 MHz, DMSO-d6) 6
2.24 (s, 3H), 2.84-2.92 (m, 8H),
C99 (1\1
5.10 (s, 2H), 6.85 (d, J = 1.2 Hz,
1H), 6.97 (d, J = 2.0 Hz, 1H),
2-(4-Methylpiperazin-1-y1)-5- 7.01 (d, J = 8.0 Hz, 1H); ESI-MS
(trifluoromethyl)aniline (m/z) 260 (M+H)+.
Intermediate C28
4-((4-Ethylpiperazin-1-yl)methyl)aniline
H2N 0
N,)
Step 1: 1-Ethyl-4-(4-nitrobenzyl)piperazine
02N
N...)
74
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
The titled compound was prepared by the reaction of 1-(bromomethyl)-4-
nitrobenzene (1.8
g, 8.32 mmol) with N-ethylpiperazine (1.16 mL, 9.16 mmol) in the presence of
N,N-
diisopropylethylamine (DIPEA) (2.15 mL, 12.5 mmol) in dichloromethane (10 mL)
as per
the procedure described in step 2 of Intermediate Cl to yield 1.23 g of the
compound. 1H
NMR (400 MHz, DMSO-d6) 6 0.98 (t, J = 7.2 Hz, 3H), 2.34-2.40 (m, 10H), 3.59
(s, 2H),
7.58 (d, J = 8.4 Hz, 2H), 8.19 (d, J = 8.4 Hz, 2H).
Step 2: 4-((4-Ethylpiperazin-1-yl)methyl)aniline
To a stirred solution of 1-ethyl-4-(4-nitrobenzyl)piperazine (step 1
intermediate) (200 mg,
0.88 mmol) in a mixture of methanol and water (1:1, 20 mL) were added ammonium
chloride
(215 mg, 4.08 mmol) followed by iron powder (224 mg. 4.028 mmol) in small
portions at
100 C. The mixture was stirred at 100 C for 2 h. The mixture was
concentrated and the
residue was diluted with a mixture of ethyl acetate and water. The organic
layer was
separated and washed with water followed by brine and dried over anhydrous
sodium sulfate.
The solution was filtered, concentrated and the residue obtained was purified
by column
chromatography to yield 3.5 g of the desired compound. 1H NMR (400 MHz, DMSO-
d6) 6
0.95 (t, J = 7.2 Hz, 3H), 2.24-2.29 (m, 10H), 3.23 (s, 2H), 4.93 (br s, 2H),
6.48 (d, J= 8.4 Hz,
2H), 6.89 (d, J = 8.4 Hz, 2H).
The chemical structure, name and analytical data of the intermediate prepared
by
following the procedure described above are given below in Table 12.
Table 12: Chemical name, structure and analytical data of Intermediates C29-
C32 and C77-
C78
Intermediate
No. Chemical Name and Structure Analytical Data
1H NMR (400 MHz, DMSO-d6) 6
H2N 0 CI r=Nr,-..
0.97 (t, J = 7.2 Hz, 3H), 2.27-
N.,.)
C29
2.35 (m, 10H), 3.34 (s, 2H), 5.29
3-C hloro-4-((4-ethylpiperazin-1-
(s, 2H), 6.47 (dd, ./1 = 2.4 Hz, J2
yl)methyl)aniline
= 8.4 Hz, 1H), 6.57 (s, 1H), 7.02
(d, J= 8.0 Hz, 1H).
1H NMR (400 MHz, DMSO-d6) 6
0.97 (t, J = 6.8 Hz, 3H), 2.28-
H2N 0 Br -N,-
2.36 (m, 7H), 3.33 (d, J = 5.6 Hz,
C30 N,)
5H), 5.26 (s, 2H), 6.51 (dd, ./i =
3-Bromo-4-((4-ethylp ip erazin-1-
2.0 Hz, J2 = 10.0 Hz, 1H), 6.77
yl)methyl)aniline
(s, 1H), 7.02 (d, J = 8.4 Hz, 1H);
ESI-MS (m/z) 299 (M+H)+.
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
Intermediate
Chemical Name and Structure Analytical Data
No.
cF=3
(R) Boc
1H NMR (400 MHz, DMSO-d6)
H2N
6
N-NõNj
1.42-1.57 (m, 11H), 2.90 (s, 3H),
C31
3.02 (s, 2H), 3.08-3.14 (m, 2H),
3.64-3.71 (m, 3H), 7.28-7.34 (m,
(R)-te rt-butyl (1-(4-amino-2-
1H), 7.55 (dd, Ji = 2.0 Hz, .1-2 =
(trifluoromethyl)benzyl)pyrrolidin-3-
8.0 Hz, 1H), 7.78 (s, 1H).
yl)(methyl)carbamate
cF3
(S) Boc
C32 H2N =
ESI-MS (m/z) 274 (M+H-Boc).
(S)-te rt-butyl (1-(4-amino-2-
(trifluoromethyl)benzyl)pyrrolidin-3-
yl)(methyl)carbamate
H2N cF3
1H NMR (400 MHz, DMSO-d6) 6
1.62-1.66 (m, 1H), 1.85-1.91 (m,
C77
1H), 2.11 (s, 6H), 2.28-2.76 (m,
l_j \
4H), 3.35-3.54 (br s, 3H), 5.76 (s,
(R)-1-(3-Amino-5-
2H), 6.69 (s, 1H), 6.71 (s, 1H),
(trifluoromethyl)benzy1)-N,N-
6.76 (s, 1H).
dimethylpyrrolidin-3-amine
H2N cF3
1H NMR (400 MHz, DMSO-d6) 6
2.00-2.23 (m, 2H), 2.55 (s, 6H),
C78 l_
2.61-2.67 (m, 1H), 2.79-2.85 (m,
j \
3H), 3.34-3.37 (m, 1H), 3.60 (d, J
(S)-1-(3-Amino-5-
= 3.2Hz, 2H), 6.81 (s, 1H), 6.87
(trifluoromethyl)benzy1)-N,N-
(s, 1H), 6.91 (s, 1H).
dimethylpyrrolidin-3-amine
Intermediate C33
N1-(4-Amino-2-(trifluoromethyl)benzy1)-N1,N2,N2-trimethylethane-1,2-diamine
H2N CF3
Step 1: tert-Butyl (4-methyl-3-(trifluoromethyl)phenyl)carbamate
Boc'N CF3
To a solution of 4-methyl-3-(trifluoromethyl)aniline (2.5 g, 14.2 mmol) in a
mixture of 1,4-
dioxane (20 mL) and water (20 mL) were added sodium carbonate (2.3 g, 21.3
mmol) and
di-tert-butyl dicarbonate (3.72 g, 17.0 mmol) and the mixture was stirred at
RT for 16 h. The
reaction mixture was diluted with water and ethyl acetate. The layers were
separated and the
aqueous layer was extracted with ethyl acetate. The combined organic layers
were washed
76
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
with water and brine. The solution was dried over anhydrous sodium sulfate,
filtered and
concentrated to yield 3.85 g of the desired compound. 1H NMR (400 MHz, DMSO-
d6) 6 1.48
(s, 9H), 2.35 (s, 3H), 7.31 (d, J= 8.4 Hz, 1H), 7.54 (d, J = 8.8 Hz, 1H), 7.87
(s, 1H), 9.59 (s,
1H).
.. Step 2: tert-Butyl (4-(bromomethyl)-3-(trifluoromethyl)phenyl)carbamate
Boc'N CF3
Br
To a solution of tert-butyl (4-methyl-3-(trifluoromethyl)phenyl)carbamate
(step 1
intermediate) (3.8 g, 13.8 mmol) in carbon tetrachloride (30 mL) were added N-
bromosuccinimide (4.13 g, 27.6 mmol) and AIBN (226 mg, 1.38 mmol) at RT and
the
.. reaction mixture was refluxed for 16 h. The mixture was concentrated and
the residue was
diluted with water and ethyl acetate. The layers were separated and the
aqueous layer was
extracted with ethyl acetate. The combined organic layers were washed with
water and brine.
The solution was dried over anhydrous sodium sulfate, filtered and
concentrated. The residue
obtained was purified by silica gel column chromatography to yield 3.05 g of
the desired
compound. 1H NMR (400 MHz, DMSO-d6) 6 1.49 (s, 9H), 4.62 (s, 2H), 6.68 (s,
1H), 7.49-
7.57 (m, 2H), 7.71 (s, 1H).
Step 3: tert-Butyl (4-4(2-
(dimethylamino)ethyl)(methyl)amino)methyl)-3-
(trifluoromethyl)phenyl)carbamate
Boc CF3'N 40
.. To a solution of tert-butyl (4-(bromomethyl)-3-
(trifluoromethyl)phenyl)carbamate (step 2
intermediate) (200 mg, 0.56 mmol) in THF (10 mL) were added N,N,N'-
trimethylethylenediamine (176 L, 1.35 mmol) and triethylamine (236 L, 1.69
mmol) at RT
and the reaction mixture was stirred at RT for 2 h. The mixture was diluted
with water and
ethyl acetate. The layers were separated and the aqueous layer was extracted
with ethyl
acetate. The combined organic layers were washed with water and brine. The
solution was
dried over anhydrous sodium sulfate, filtered and concentrated. The residue
obtained was
purified by silica gel column chromatography to yield 315 mg of the desired
compound. 1H
NMR (400 MHz, DMSO-d6) 6 1.48 (s, 9H), 2.12 (s, 9H), 2.35 (q, J = 4.4 Hz, 2H),
2.41-2.44
(m, 2H), 3.53 (s, 2H), 7.62 (s, 2H), 7.87 (s, 1H), 9.66 (s, 1H); ESI-MS (m/z)
376 (M+H)+.
Step 4: N1-(4-Amino-2-(trifluoromethyl)benzy1)-N1,N2,N2-trimethylethane-1,2-
diamine
77
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
To a solution of tert-butyl (4-4(2-(dimethylamino)ethyl)(methyl)amino)methyl)-
3-
(trifluoromethyl)phenyl)carbamate (step 3 intermediate) (300 mg, 0.80 mmol) in
dichloromethane (5.0 mL) was added trifluoroacetic acid (2.0 mL) and the
mixture was
stirred at RT for 3 h. The reaction mixture was diluted with ethyl acetate and
the organic
solution was washed with saturated sodium bicarbonate solution followed by
water and brine.
The solution was dried over anhydrous sodium sulfate, filtered and
concentrated to yield 102
mg of the desired compound. 1H NMR (400 MHz, DMSO-d6) 6 2.11 (s, 9H), 2.34-
2.39 (m,
4H), 3.33-3.40 (br s, 2H), 5.42 (s, 2H), 6.75 (dd, ./i = 2.0 Hz, .1-2 = 8.0
Hz, 1H), 6.85 (s, 1H),
7.32 (d, J = 8.4 Hz, 1H).
Intermediate C34
N1-(2-(Dimethylamino)ethyl)-N1-methy1-5-(trifluoromethyl)benzene-1,3-diamine
cF3
FI2N N1')
Step 1: Di-tert-butyl (3-bromo-5-(trifluoromethyl)phenyl)imidodicarbonate
c3
(Boc)2N Br
15 The titled compound was prepared by the reaction of 3-bromo-5-
trifluoromethylaniline (5.0
g, 20.8 mmol) with di-tert-butyl dicarbonate (11.3 g, 52.1 mmol) in the
presence of DMAP
(254 mg, 2.08 mmol) in dichloromethane (20 mL) as per the procedure described
in step 3 of
Intermediate Al to yield 3.8 g of the product. 1H NMR (400 MHz, DMSO-d6) 6
1.41 (s,
18H), 7.77 (d, J= 6.0 Hz, 1H), 7.93-7.96 (m, 1H), 8.05 (s, 1H).
20 Step 2: Di-
tert-butyl (3 -42-(dimethylamino)ethyl] (methyl)amino)-5 -
(trifluoromethyl)phenyl)imidodicarbonate
cF3
='
(Boc)2N N1
To a solution of di-tert-butyl (3-bromo-5-
(trifluoromethyl)phenyl)imidodicarbonate (step 1
intermediate) (2.2 g, 4.89 mmol) in 1,4-dioxane (20 mL) were added N,N,N' -
25 trimethylethylenediamine (636 L, 4.89 mmol), sodium tert-butoxide (1.40
g, 14.7 mmol),
tris(dibenzylideneacetone)dipalladium(0) (Pd2(dba)3) (447 mg, 0.49 mmol) and
(2-
biphenyl)di-tert-butylphosphinetriethylamine (JohnPhos) (37 mg, 1.47mmol) at
RT and the
reaction mixture was stirred at 45 C for 4 h. The mixture was cooled to RT
and filtered
78
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
through celite. The filtrate was concentrated and the residue was dissolved in
ethyl acetate.
The organic solution was washed with 0.1 N HC1 followed by water, dried over
anhydrous
sodium sulfate, filtered and concentrated. The residue obtained was purified
by silica gel
column chromatography to yield 2.01 g of the desired compound. The compound
was as such
carried forward to the next step without characterization.
Step 3: N1-(2-(Dimethylamino)ethyl)-N1-methy1-5-(trifluoromethyl)benzene-1,3-
diamine
The titled compound was prepared by the reaction of di-tert-butyl (34(2-
(dimethylamino)ethyll(methyl)amino)-5-(trifluoromethyl)phenyl)imidodicarbonate
(step 2
intermediate) (100 mg, 0.22 mmol) with trifluoroacetic acid (0.5 mL) in
dichloromethane (3.0
mL) as per the procedure described in step 4 of Intermediate C33 to yield 50
mg of the
compound. 1H NMR (400 MHz, DMSO-d6) 6 2.17 (s, 6H), 2.34 (t, J = 7.2 Hz, 2H),
3.3-3.37
(m, 5H), 5.29 (s, 2H), 6.07 (s, 1H), 6.10 (s, 1H), 6.14 (s, 1H); ESI-MS (m/z)
262 (M+H)+.
The chemical structure, name and analytical data of the intermediate prepared
by
following the procedure described above are given below in Table 13.
Table 13: Chemical name, structure and analytical data of Intermediate C35
Intermediate
No. Chemical Name and Structure Analytical Data
cF3
1H NMR (400 MHz, DMSO-d6) 6
H2N N 1.41-1.47 (m, 2H), 1.81 (d, J
= 12.8
C35 Hz, 2H), 2.21 (s, 7H), 2.62-
2.69 (m,
2H), 3.64 (d, J= 12.8 Hz, 2H), 5.34 (s,
1 -(3 -Amino-5 - 2H), 6.26 (s, 1H), 6.33 (d, J
= 7.2 Hz,
(trifluoromethyl)pheny1)-N,N- 2H); ESI-MS (m/z) 288 (M+H)+.
dimethylpiperidin-4-amine
Intermediate C36
1-(3 -Aminophenyl)cycloprop anecarbonitrile
H2N
ON
Step 1: 1-(3-Nitrophenyl)cyclopropanecarbonitrile
o2N =ON
To a stirred solution of 2-(3-nitrophenyl)acetonitrile (500 mg, 3.08 mmol) in
DMSO (10 mL)
were added 1,2-dibromoethane (266 L, 3.08 mmol) and sodium hydride (60% w/w,
123 mg,
3.08 mmol) at RT. The mixture stirred at RT for 16 h. The reaction mixture was
quenched
79
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
with aqueous sodium sulfate solution, diluted with ethyl acetate and the
organic layer was
washed with saturated sodium bicarbonate solution followed by brine. The
organic layer was
dried over anhydrous sodium, filtered and concentrated under reduced pressure.
The residue
obtained was purified by column chromatography to yield 635 mg of the desired
product. 41
NMR (400 MHz, DMSO-d6) 6 1.69 (t, J = 2.8 Hz, 2H), 1.87 (t, J= 2.8 Hz, 2H),
7.70 (t, J=
8.0 Hz, 1H), 7.78-7.80 (m, 1H), 8.17-8.19 (m, 2H).
Step 2: 1-(3 -Aminophenyl)cycloprop ane carbonitrile
To a stirred solution of 1-(3-nitrophenyl)cyclopropanecarbonitrile (step 1
intermediate) (630
mg, 3.35 mmol) in a mixture of ethyl acetate and water (1:1, 20 mL) were added
ammonium
chloride (1.79 g, 33.4 mmol) followed by iron powder (747 mg. 13.4 mmol) in
small portions
at 100 C. The mixture was stirred at 100 C for 2 h. The mixture was
concentrated and the
residue was diluted with a mixture of ethyl acetate and water. The organic
layer was
separated and washed with water followed by brine and dried over anhydrous
sodium sulfate.
The solution was filtered, concentrated and the residue obtained was purified
by column
chromatography to yield 350 mg of the desired compound. 1H NMR (400 MHz, DMSO-
d6) 6
1.36 (t, J = 4.8 Hz, 2H), 1.66 (t, J = 4.8 Hz, 2H), 5.22 (br s, 2H), 6.36-6.38
(m, 1H), 6.46-
6.48 (m, 1H), 6.57 (s, 1H), 6.99 (t, J = 4.0 Hz, 1H).
Intermediate C37
(S)-3 -(3 -F luoropyrrolidin-1 -y1)-5 -(trifluoromethyl)aniline
cF3
0 No....
H2N F
Step 1: (S)-3 -F luoro-1-(3 -nitro-5 -(trifluoromethyl)phenyl)pyrro lidine
cF3
40 02N 0....
F
The
titled compound was prepared by the reaction of 1 -bromo-3 -nitro-5 -
(trifluoromethyl)benzene (1.2 g, 4.44 mmol) with (5)-3-fluoropyrrolidine (593
mg, 6.66
mmol) in the presence of cesium carbonate (4.34 g, 13.3 mmol),
tris(dibenzylideneacetone)dipalladium(0) (Pd2(dba)3) (81 mg, 0.09 mmol) and (
)-2,2'-
bis(diphenylphosphino)-1,1'-binaphthalene (rac-BINAP) (83 mg, 0.13 mmol) in
1,4-dioxane
(20 mL) as per the procedure described in step 2 of Intermediate C34 to yield
109 mg of the
desired compound. 1H NMR (400 MHz, DMSO-d6) 6 2.16-2.22 (m, 1H), 2.27-2.33 (m,
1H),
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
3.47-3.58 (m, 1H), 3.60-3.67 (m, 2H), 3.72 (s, 1H), 5.43-5.57 (m, 1H), 7.20
(s, 1H), 7.52 (s,
1H), 7.62 (s, 1H); ESI-MS (m/z) 279 (M+H)+.
Step 2: (S)-3 -(3 -F luoropyrro lidin-l-y1)-5 -(trifluoromethyl)aniline
The titled compound was prepared by the catalytic hydrogenation of (S)-3-
fluoro-1-(3-nitro-
5-(trifluoromethyl)phenyl)pyrrolidine (step 1 intermediate) (100 mg, 0.36
mmol) in the
presence of palladium on carbon (10% w/w, 50% wet) as per the procedure
described in step
3 of Intermediate Cl to yield 55 mg of the compound. 1H NMR (400 MHz, DMSO-d6)
6
2.10-2.26 (m, 2H), 3.27-3.57 (m, 4H), 5.33-5.36 (br s, 2H), 5.49 (br s, 1H),
5.98 (s, 2H), 6.18
(s, 1H).
The chemical structure, name and analytical data of the intermediate prepared
by
following the procedure described above are given below in Table 14.
Table 14: Chemical name, structure and analytical data of Intermediates C38-
C39
Intermediate
No. Chemical Name and Structure Analytical Data
cF3
1411
C38 H2N , F
The crude amine was as such carried
forward to the next step.
(R)-3 -(3 -F luoropyrrolidin-l-y1)-5 -
(trifluoromethyl)aniline
cF3
40
1H NMR (400 MHz, DMSO-d6) 6 1.44
C39 H2N N
(s, 9H), 3.07 (t, J = 5.2 Hz, 4H), 3.43
LN.Boc
(t, J = 4.4 Hz, 4H), 5.39 (s, 2H), 6.32-
tert-Butyl 4-(3-amino-5-
6.35 (m, 3H).
(trifluoromethyl)phenyl)piperazine-
1-c arbo xylate
Intermediate C40
tert-Butyl 4-(3 -amino-5 -(trifluoromethyl)phenyl)pip eridine-l-carboxylate
c3
H2N
N.Boc
Step 1: tert-Butyl
4-(3 -nitro-5 -(trifluoromethyl)pheny1)-5 ,6-dihydropyridine-1(2H)-
carboxylate
81
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
CF3
02N I*
I N.Boc
To a solution of 1-bromo-3-nitro-5-(trifluoromethyl)benzene (1.0 g, 3.70 mmol)
in 1,4-
dioxane (35 mL) were added tert-butyl 4-(4,4,5 ,5-tetramethy1-1,3,2-
dioxaborolan-2-y1)-5,6-
dihydropyridine-1(2H)-carboxylate (1.1 g, 3.70
mmol),
tetrakis(triphenylphosphine)palladium(0) (213 mg, 0.18 mmol) and saturated
aqueous sodium
bicarbonate solution (15 mL) and the mixture was heated at 120 C for 6 h. The
mixture was
cooled to RT and diluted with ethyl acetate. The organic layer was washed with
water
followed by brine, dried over anhydrous sodium sulfate, filtered and
concentrated. The
residue obtained was purified by silica gel column chromatography to yield 891
mg of the
desired compound. 1H NMR (400 MHz, DMSO-d6) 6 1.52 (s, 9H), 2.60 (br s, 2H),
3.71 (t, J
= 5.6 Hz, 2H), 4.18 (br s, 2H), 6.32 (br s, 1H), 7.94 (s, 1H), 8.39 (s, 1H),
8.42 (s, 1H).
Step 2: tert-Butyl 4-(3 -amino-5 -(trifluoromethyl)phenyl)pip eridine-1 -
carboxylate
The titled compound was prepared by the catalytic hydrogenation of tert-butyl
4-(3-nitro-5-
(trifluoromethyl)phenyl)piperidine-1-carboxylate (step 1 intermediate) (880
mg, 2.35 mmol)
in the presence of palladium on carbon (10% w/w, 50% wet) (catalytic) as per
the procedure
described in step 3 of Intermediate Cl to yield 728 mg of the compound. 1H NMR
(400 MHz,
DMSO-d6) 6 1.50 (s, 9H), 1.55-1.66 (m, 2H), 1.82 (d, J= 12.4 Hz, 2H), 2.58-
2.66 (m, 1H),
2.80 (t, J= 12.0 Hz, 2H), 4.26 (br s, 2H), 6.69 (s, 1H), 6.78 (s, 1H), 6.86
(s, 1H).
Intermediate C41
3 -(1 -Methylpip eridin-4-y1)-5 -(trifluoromethyl)aniline
cF3
H2N
To a stirred suspension of lithium aluminium hydride (275 mg, 7.25 mmol) in
THF (20 mL)
was slowly added a solution of tert-butyl 4-(3-amino-5-
(trifluoromethyl)phenyl)piperidine-1-
carboxylate (Intermediate C40) (500 mg, 1.45 mmol) in THF (20 mL) at RT and
the mixture
was heated at 90 C for 18 h. The mixture was cooled to RT and quenched with
saturated
aqueous sodium sulfate solution. The mixture was filtered and the filtrate was
diluted with
ethyl acetate and water. The layers were separated and the aqueous layer was
extracted with
ethyl acetate. The combined organic layers were dried over anhydrous sodium
sulfate, filtered
82
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
and concentrated. The residue obtained was purified by silica gel column
chromatography to
yield 181 mg of the desired compound. 1H NMR (400 MHz, DMSO-d6) 6 1.56-1.59
(m, 2H),
1.62-1.71 (m, 2H), 1.89-1.90 (m, 2H), 2.17 (s, 3H), 2.3-2.51 (m, 1H), 2.83 (d,
J = 7.6 Hz,
2H), 5.48 (s, 2H), 6.29 (s, 1H), 6.73 (s, 2H).
Intermediate C42
4-(3 -Amino-5 -(trifluoromethyl)phenyl)thiomorpholine 1,1-dioxide
C F3
H2N
S=C:1
'b
Step 1: 4-(3 -Nitro-5 -(trifluoromethyl)phenyl)thiomorpholine
CF3
v211 1011
m
The titled compound was prepared by the reaction of 1 -bromo-3 -nitro-5 -
(trifluoromethyl)benzene (2.0 g, 7.40 mmol) with thiomorpholine (1.1 g, 11.1
mmol) in the
presence of cesium carbonate (7.2 g, 22.2 mmol),
tris(dibenzylideneacetone)dipalladium(0)
(Pd2(dba)3) (135 mg, 0.15 mmol) and ( )-2,2'-bis(diphenylphosphino)-1,1'-
binaphthalene
(rac-BINAP) (138 mg, 0.22 mmol) in 1,4-dioxane (30 mL) as per the procedure
described in
step 2 of Intermediate C34 to yield 1.05 g of the desired compound. 1H NMR
(400 MHz,
DMSO-d6) 6 2.66-2.69 (m, 4H), 3.79-3.81 (m, 4H), 7.60 (s, 1H), 7.69 (s, 1H),
7.86 (s, 1H).
Step 2: 4-(3 -Nitro-5 -(trifluoromethyl)phenyl)thiomorpholine 1,1-dioxide
cF3
02N
sr(D
'b
To a solution of 4-(3-nitro-5-(trifluoromethyl)phenyl)thiomorpholine (step 1
intermediate)
(1.0 g, 3.42 mmol) in dichloromethane (20 mL) was added mCPBA (1.7 g, 10.3
mmol) at RT
and the mixture was stirred at RT for 2 h. The mixture was diluted with
saturated sodium
bicarbonate solution and the layers were separated. The aqueous layer was
extracted with
ethyl acetate. The combined organic layers were washed with brine and dried
over anhydrous
sodium sulfate. The solution was filtered, concentrated and the residue was
purified by silica
gel column chromatography to yield 523 mg of the desired product. 1H NMR (400
MHz,
83
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
DMSO-d6) 6 3.19 (t, J= 5.2 Hz, 4H), 4.01 19 (t, J= 5.2 Hz, 4H), 7.75 (s, 1H),
7.97 (s, 1H),
8.0 (s, 1H).
Step 3: 4-(3 -Amino-5 -(trifluoromethyl)phenyl)thiomorpholine 1,1-dioxide
The titled compound was prepared by the catalytic hydrogenation of 4-(3-nitro-
5-
(trifluoromethyl)phenyl)thiomorpholine 1,1-dioxide (step 2 intermediate) (510
mg, 1.57
mmol) in the presence of palladium on carbon (10% w/w, 50% wet) (catalytic) as
per the
procedure described in step 3 of Intermediate Cl to yield 346 mg of the
compound. 1H NMR
(400 MHz, DMSO-d6) 6 3.12 (s, 4H), 3.72 (s, 4H), 5.43 (s, 2H), 6.35 (s, 1H),
6.42 (d, J= 6.8
Hz, 2H).
Intermediate C43
4-Amino-N-(2-(dimethylamino)ethyl)-2-(trifluoromethyl)benzamide
H2N cF3
0
Step 1: N-(2-(Dimethylamino)ethyl)-4-nitro-2-(trifluoromethyl)benzamide
o2N cF3
PH
To a stirred solution of 4-nitro-2-(trifluoromethyl)benzoic acid (500 mg, 2.12
mmol) in
dichloromethane (20 mL) were added triethylamine (612 L, 4.24 mmol), N1,N1-
dimethylethane-1,2-diamine hydrochloride (633 L, 5.0 mmol) followed by HATU
(967 mg,
2.54 mmol) at RT. The mixture was stirred at RT for 18 h before quenching it
with water.
The layers were separate and the aqueous layer was extracted twice with ethyl
acetate. The
combined organic layers were washed with brine and dried over anhydrous sodium
sulfate.
The solution was filtered and concentrated under reduced pressure to yield 311
mg of the
titled compound. 1H NMR (400 MHz, DMSO-d6) 6 2.23 (s, 6H), 2.45-2.50 (m, 2H),
3.33-
3.38 (m, 2H), 7.80 (d, J = 8.4 Hz, 1H), 8.49 (s, 1H), 8.56 (dd, J, = 2.0 Hz,
.1-2 = 8.4 Hz, 1H),
8.76 (t, J = 5.2 Hz, 1H); ESI-MS (m/z) 306 (M+H)+.
Step 2: 4-Amino-N-(2-(dimethylamino)ethyl)-2-(trifluoromethyl)benzamide
The titled compound was prepared by the catalytic hydrogenation of N-(2-
(dimethylamino)ethyl)-4-nitro-2-(trifluoromethyl)benzamide (step 1
intermediate) (300 mg,
0.98 mmol) in the presence of palladium on carbon (10% w/w, 50% wet) in
methanol (10
mL) as per the procedure described in step 3 of Intermediate Cl to yield 151
mg of the
compound. 1H NMR (400 MHz, DMSO-d6) 6 2.16 (s, 6H), 2.33 (t, J= 7.2 Hz, 2H),
3.18-3.26
84
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
(m, 2H), 5.79 (s, 2H), 6.73 (dd, Ji = 2.0 Hz, .12 = 10 Hz, 1H), 6.87 (s, 1H),
7.16 (d, J = 8.4
Hz, 1H), 8.0 (t, J= 5.6 Hz, 1H); ESI-MS (m/z) 276 (M+H)+.
Intermediate C44
(4-Amino-2-(trifluoromethyl)phenyl)(4-ethylpip erazin-l-yl)methanone
H2N is CF3r,-...N..-õ,
0
Step 1: (4-Ethylpiperazin-1-y1)(4-nitro-2-(trifluoromethyl)phenyl)methanone
02N sCFrN
N,)
0
To a stirred solution of 4-nitro-2-(trifluoromethyl)benzoic acid (2.0 g, 8.51
mmol) in
dichloromethane (20 mL) were added triethylamine (8.5 mL, 59.5 mmol), N-
ethylpiperazine
.. (1.24 mL, 9.35 mmol) followed by T3P (11.1 mL, 18.7 mmol) at RT. The
mixture was stirred
at RT for 16 h before quenching it with water. The layers were separate and
the aqueous layer
was extracted twice with ethyl acetate. The combined organic layers were
washed with brine
and dried over anhydrous sodium sulfate. The solution was filtered and
concentrated under
reduced pressure and the residue obtained was purified by silica gel column
chromatography
to yield 2.2 g of the titled compound. 1H NMR (400 MHz, DMSO-d6) 6 0.99 (d, J
= 6.8 Hz,
3H), 2.19-2.50 (m, 6H), 3.03-3.16 (m, 2H), 3.58-3.72 (m, 2H), 7.82 (d, J = 8.4
Hz, 1H), 8.53-
8.58 (m, 2H).
Step 2: (4-Amino-2-(trifluoromethyl)phenyl)(4-ethylpiperazin-1-y1)methanone
The titled compound was prepared by the catalytic hydrogenation of (4-
ethylpiperazin-1-
yl)(4-nitro-2-(trifluoromethyl)phenyl)methanone (step 1 intermediate) (2.0 g,
6.04 mmol) in
the presence of 10% palladium on carbon (50% wet) in methanol (50 mL) as per
the
procedure described in step 3 of Intermediate Cl to yield 1.9 g of the
compound. 1H NMR
(400 MHz, DMSO-d6) 6 0.98 (t, J= 7.2 Hz, 3H), 2.19-2.50 (m, 6H), 3.09-3.18 (m,
2H), 3.57-
3.58 (m, 2H), 5.78 (s, 2H), 6.78 (dd, ./1 = 2.0 Hz, .12 = 8.4 Hz, 1H), 6.88
(s, 1H), 7.01 (d, J =
8.0 Hz, 1H).
Intermediate C45
Ethyl 2-(3-aminopheny1)-2,2-difluoroacetate
F F
H2N
0
Step 1: Ethyl 2,2-difluoro-2-(3-nitrophenyl)acetate
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
F F
02N is0
To a stirred suspension of 1-iodo-3-nitrobenzene (5.0 g, 20.1 mmol) and copper
powder (5.0
g, 80.3 mmol) in DMSO (20 mL) was added ethyl 2-bromo-2,2-difluoroacetate (5.1
mL, 40.2
mmol) at RT. The mixture was stirred overnight at 60 C in a sealed tube. The
reaction
mixture was cooled to RT and quenched with aqueous ammonium chloride solution.
The
aqueous mixture was poured into water and extracted twice with ethyl acetate.
The combined
organic layers were dried over anhydrous sodium sulfate and concentrated under
reduced
pressure. The crude material obtained was purified by silica gel column
chromatography to
yield 3.2 g of the desired product. 41 NMR (400 MHz, DMSO-d6) 6 1.23 (t, J =
7.2 Hz, 3H),
4.26 (q, J = 7.2 Hz, 2H), 7.89 (t, J = 8.4 Hz, 1H), 8.09 (dd, ./1 = 0.8 Hz,
.12 = 8.0 Hz, 1H), 8.33
(t, J = 2.0 Hz, 1H), 8.47 (dd, ./i = 0.8 Hz, .12 = 6.8 Hz, 1H); ESI-MS (m/z)
245 (M+H)+.
Step 2: Ethyl 2-(3-aminopheny1)-2,2-difluoroacetate
The titled compound was prepared by the reaction of ethyl 2,2-difluoro-2-(3-
nitrophenyl)acetate (step 1 intermediate) (600 mg, 2.44 mmol) with iron powder
(567 mg,
10.2 mmol) and ammonium chloride (1.30 g, 24.4 mmol) in a mixture of ethyl
acetate and
water (7:2, 9.0 mL) as per the procedure described in step 2 of Intermediate
C28 to yield 350
mg of the compound. 1H NMR (400 MHz, DMSO-d6) 6 1.23 (t, J = 7.2 Hz, 3H), 4.20
(q, J =
7.2 Hz, 2H), 5.53 (br s, 2H), 6.65 (d, J = 7.6 Hz, 1H), 6.71 (d, J = 8.0 Hz,
1H), 6.75 (s, 1H),
7.15 (t, J = 7.6 Hz, 1H); ESI-MS (m/z) 216 (M+H)+.
Intermediate C46
3 -(4,4-Difluoropip eridin-l-y1)-5 -(trifluoromethyl)aniline
H2N CF3
F F
Step 1: 4,4-Difluoro-1-(3 -nitro-5 -(trifluoromethyl)phenyl)pip eridine
02N CF3
F F
The titled compound was prepared by the reaction of 1-bromo-3 -nitro-5 -
(trifluoromethyl)benzene (250 mg, 0.93 mmol) with 4,4-difluoropiperidine (354
mg, 2.77
86
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
mmol) in the presence of sodium tert-butoxide (140 mg, 1.45 mmol),
tris(dibenzylideneacetone)dipalladium(0) (Pd2(dba)3) (44 mg, 0.05 mmol) and
1,1-
bis(diphenylphosphinoferrocene (54 mg, 0.10 mmol) in 1,4-dioxane (3.0 mL) as
per the
procedure described in step 2 of Intermediate C34 to yield 150 mg of the
desired compound.
1H NMR (400 MHz, DMSO-d6) 6 2.06 2.09 (m, 4H), 3.60 (t, J = 5.60 Hz, 4H), 7.72
(s, 1H),
7.67 (s, 1H), 7.97 (s, 1H).
Step 2: 3 -(4,4-Difluoropip eridin-l-y1)-5 -(trifluoromethyl)aniline
The titled compound was prepared by the catalytic hydrogenation of 4,4-
difluoro-1-(3-nitro-
5-(trifluoromethyl)phenyl)piperidine (step 1 intermediate) (220 mg, 0.71 mmol)
in the
presence of palladium on carbon (10% w/w, 50% wet) in methanol (5.0 mL) as per
the
procedure described in step 3 of Intermediate Cl to yield 70 mg of the
compound. 1H NMR
(400 MHz, DMSO-d6) 6 1.97-2.07 (m, 4H), 3.28-3.33 (m, 4H), 5.39 (s, 2H), 6.32
(s, 1H),
6.40 (s, 2H); ESI-MS (m/z) 281 (M+H)+.
The chemical structure, name and analytical data of the intermediate prepared
by
following the procedure described above are given below in Table 15.
Table 15: Chemical name, structure and analytical data of Intermediates C47-
C49 and C63
Intermediate
No. Chemical Name and Structure Analytical Data
H2N 0 CF 3 1H NMR (400 MHz, DMSO-d6) 6
0.33-0.33 (m, 2H), 0.43-0.45 (m, 2H),
N 1.62-1.67 (m, 1H), 2.64-2.65
(m, 4H),
C47 CN) 3.05 (t, J= 4.8 Hz, 4H), 5.35
(s, 2H),
A 6.28 (s, 1H), 6.32 (s, 2H);
ESI-MS
(m/z) 286 (M+H)+.
3 -(4-Cyc lopropylpip erazin-l-y1)-5 -
(trifluoromethyl)aniline
H2N s 0F3 1H NMR (400 MHz, DMSO-d6) 6
1.72-1.79 (m, 2H), 1.88-1.98 (m, 2H),
,N, 3.06-3.10 (m, 2H), 3.11-3.34
(m, 2H),
C48 4.76-4.98 (m, 1H), 5.36 (s,
2H), 6.23
Y (s, 1H), 6.36 (s, 2H).
F
3 -(4-F luoropip eridin-l-y1)-5 -
(trifluoromethyl)aniline
H2N 0 c3 1H NMR (400 MHz, DMSO-d6) 6
1.88-1.99 (m, 1H), 2.01-2.02 (m, 1H),
2.54 (s, 2H), 3.02 (d, J= 10.0 Hz, 1H),
C49 c1\12,,R)
3.19-3.34 (m, 2H), 4.37 (m, 1H), 5.27
OH (br s, 2H), 5.91-5.92 (br s,
2H), 6.12 (s,
(R)-1-(3 -Amino-5 - 1H); ESI-MS (m/z) 247 (M+H)+.
(trifluoromethyl)phenyl)pyrrolidin-
3-ol
87
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
Intermediate
No. Chemical Name and Structure Analytical Data
H2N c3 1H NMR (400 MHz, DMSO-d6) 6
1.16
(d, J = 10.0 Hz, 6H), 2.23 (t, J = 10.8
Hz, 2H), 3.50 (d, J = 12.0 Hz, 2H),
C63 C 3.64-3.68 (m, 2H), 5.36 (s,
2H), 6.30
s' 0 (s, 1H), 6.35 (d, J = 11.2 Hz,
2H); ESI-
3-((2R,6S)-2,6- MS (m/z) 275 (M+H)+.
Dimethylmorpholino)-5-
(trifluoromethyl)aniline
Intermediate C50
N-(3 -Amino-5 -(trifluoromethyl)phenyl)acrylamide
cF3
H2N NH
5 To a stirred solution of 5-(trifluoromethyl)benzene-1,3-diamine (200 mg,
1.13 mmol) in
dichloromethane (9.0 mL) were added triethylamine (164 L, 1.13 mmol) followed
by
acryloyl chloride (31 L, 0.34 mmol) at 0 C. The mixture was stirred
overnight at RT. The
mixture was quenched with water and extracted twice with ethyl acetate. The
combined
organic layers were washed with water followed by brine and dried over
anhydrous sodium
10 sulfate. The solution was filtered, concentrated and the residue was
purified by silica gel
column chromatography to yield 60 mg of the desired product. ESI-MS (m/z) 231
(M+H)+.
Intermediate C51
6-Methyl-5 -(trifluoromethyl)pyridin-3 -amine
H2N F3
15 Step 1: Diethyl 2-(5-nitro-3-(trifluoromethyl)pyridin-2-yl)malonate
02N -CF3
CO2Et
CO2Et
To a stirred solution of diethylmalonate (5.0 g, 23.5 mmol) in THF (25 mL) was
added
potassium tert-butoxide (1M in THF, 70.5 mmol) at -10 C and the mixture was
stirred 10
min at the same temperature followed by 30 min at RT. A solution of 2-chloro-5-
nitro-3-
20 (trifluoromethyl)pyridine (5.0 g, 23.52 mmol) in THF (25 mL) was added
slowly to the
mixture at 0 C and the resultant mixture was stirred for 4-5 h at RT. The
mixture was
quenched with aqueous ammonium chloride solution and extracted twice with
ethyl acetate.
88
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
The combined organic layers were washed with water followed by brine and dried
over
anhydrous sodium sulfate. The solution was filtered, concentrated to yield 5.1
g of the desired
product. The crude amine was as such carried forward to the next step.
Step 2: 2-Methyl-5 -nitro-3 -(trifluoromethyl)pyridine
02NcF3
1
A solution of diethyl 2-(5-nitro-3-(trifluoromethyl)pyridin-2-yl)malonate
(step 1
intermediate) (5.0 g, 14.3 mmol) in 50% sulfuric acid (50 mL) was heated at 85-
90 C for 2-3
h. The mixture was cooled to 0 C and basified with 3N NaOH solution. The
aqueous
solution was extracted twice with diethyl ether. The combined organic layers
were washed
with brine and dried over anhydrous sodium sulfate. The solution was filtered,
concentrated
to yield 3.2 g of the desired product. 1H NMR (400 MHz, DMSO-d6) 6 2.79 (s,
3H), 8.73 (d,
J = 2.4 Hz, 1H), 9.50 (d, J = 2.4 Hz, 1H).
Step 3: 6-Methyl-5 -(trifluoromethyl)pyridin-3 -amine
The titled compound was prepared by the reaction of 2-methyl-5-nitro-3-
(trifluoromethyl)pyridine (250 mg, 1.21 mmol) with iron powder (679 mg, 12.1
mmol) and
ammonium chloride (519 mg, 9.70 mmol) in a mixture of ethanol and water (6:1,
3.5 mL) as
per the procedure described in step 2 of Intermediate C28 to yield 170 mg of
the compound.
1H NMR (400 MHz, DMSO-d6) 6 2.41 (s, 3H), 5.57 (br s, 2H), 7.19 (d, J= 2.8 Hz,
1H), 8.03
(d, J= 2.0 Hz, 1H).
Intermediate C52
tert-Butyl 4-(3 -amino-5 -(trifluoromethyl)pheny1)-1,4- diazep ane-1 -
carboxylate
H2N CF3
'Boc
Step 1: tert-Butyl 4-(3 -nitro-5 -(trifluoromethyl)pheny1)-1,4-diazep ane-1 -c
arbo xylate
02N io cF3
'Boc
The titled compound was prepared by the reaction of 1 -bromo-3 -nitro-5 -
(trifluoromethyl)benzene (100 mg, 0.37 mmol) with tert-butyl 1,4-diazepane- 1 -
carboxylate
89
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
(222 mg, 1.11 mmol) in the presence of sodium tert-butoxide (53 mg, 0.55
mmol),
tris(dibenzylideneacetone)dipalladium(0) (Pd2(dba)3) (20 mg, 0.02 mmol) and
1,1-
bis(diphenylphosphinoferrocene (24 mg, 0.04 mmol) in 1,4-dioxane (1.0 mL) as
per the
procedure described in step 2 of Intermediate C34 to yield 85 mg of the
desired compound.
1H NMR (400 MHz, DMSO-d6) 6 1.42 (s, 9H), 1.630 (br s, 2H), 1.99-2.02 (m, 2H),
3.30-3.40
(m, 2H), 3.67-3.69 (m, 4H), 7.14 (s, 1H), 7.66 (s, 1H), 7.74 (s, 1H).
Step 2: tert-Butyl 4-(3 -amino-5 -(trifluoromethyl)pheny1)-1,4-diazep ane-l-
carboxylate
The titled compound was prepared by the reaction of tert-butyl 4-(3-nitro-5-
(trifluoromethyl)pheny1)-1,4-diazepane-l-carboxylate (step 1 intermediate )(80
mg, 0.21
mmol) with iron powder (114 mg, 2.05 mmol) and ammonium chloride (88 mg, 1.64
mmol)
in ethanol (2.0 mL) as per the procedure described in step 2 of Intermediate
C28 to 35 mg of
the compound. 1H NMR (400 MHz, DMSO-d6) 6 1.34 (s, 9H), 1.71-1.99 (m, 2H),
3.16-3.32
(m, 2H), 3.45-3.56 (m, 4H), 5.23-5.24 (m, 4H), 6.12-6.15 (m, 3H).
The chemical structure, name and analytical data of the intermediate prepared
by
following the procedure described above are given below in Table 16.
Table 16: Chemical name, structure and analytical data of Intermediates C53-
056 and C69-
C70
Intermediate
No. Chemical Name and Structure Analytical Data
H2N cF3
1H NMR (400 MHz, CDC13): 6 1.29 (d,
J = 6.8 Hz, 3H), 1.30 (s, 9H), 2.81(dt,
= 3.6 Hz, .12 = 7.6 Hz, 1H), 3.0 (dd ,
C53 C(a
J, = 4.0 Hz, J2 = 12.0 Hz, 1H), 3.27
oc (dt, ./1 = 3.6 Hz, J2 = 7.6 Hz,
1H),
B
(S)-tert-Butyl 4-(3-amino-5-
3.36-3.39 (m, 1H), 3.50-3.53 (m, 1H),
(trifluoromethyl)pheny1)-2-
3.93-3.96 (m, 1H), 4.35 (br s, 1H), 6.33
methylpiperazine-l-carboxylate
(s, 1H), 6.44 (s, 1H), 6.52 (s, 1H).
H2N CF3
1H NMR (400 MHz, CDC13): 6 1.28 (d,
J = 6.8 Hz, 3H), 1.30 (s, 9H), 2.81(dt,
= 3.6 Hz, .12 = 7.6 Hz, 1H), 3.0 (dd ,
C54 4R),
= 4.0 Hz, J2 = 12.0 Hz, 1H), 3.27
(dt, ./1 = 3.6 Hz, J2 = 7.6 Hz, 1H),
6oc
(R)-tert-Butyl 4-(3-amino-5-
3.36-3.39 (m, 1H), 3.50-3.53 (m, 1H),
(trifluoromethyl)pheny1)-2-
3.93-3.96 (m, 1H), 4.35 (br s, 1H), 6.33
methylpiperazine-l-carboxylate
(s, 1H), 6.44 (s, 1H), 6.52 (s, 1H).
CA 03064975 2019-11-26
WO 2018/215668 PCT/EP2018/063957
Intermediate
Chemical Name and Structure Analytical Data
No.
H2N CF3
1H NMR (400 MHz, DMSO-d6) 6 1.34
(s)r, (s, 9H), 1.90 (br s, 2H), 2.92 (t,
J= 9.6
C55 N2(s) Hz, 1H), 3.18 (t, J = 8.0 Hz, 1H),
3.51
Boc (t, J = 8.4 Hz, 2H), 4.37-4.43 (m,
2H),
(1S,4S)-tert-Butyl 5-(3-amino-5-
5.03 (s, 2H), 6.00 (d, J = 10.8 Hz, 2H),
(trifluoromethyl)pheny1)-2,5-
6.17 (s, 1H).
diazabicyclo[2.2.1]heptane-2-
carboxylate
H2N CF3
C56 c(s) ESI-MS (m/z) 247 (M+H)+.
OH
(S)-1-(3-Amino-5-
(trifluoromethyl)phenyl)pyrrolidin-
3-ol
H2N c3
1H NMR (400 MHz, DMSO-d6) 6 0.91
C69
(d, J = 6.4 Hz, 3H), 1.42 (s, 9H), 2.80-
3.0 (m, 2H), 3.30-3.40 (m, 2H), 3.60-
Boc 3.65 (m, 2H), 3.90 (br s, 1H), 5.37
(s,
(S)-tert-Butyl 4-(3-amino-5- 2H), 6.28-6.29 (br s, 3H).
(trifluoromethyl)pheny1)-3-
methylpiperazine-1-carboxylate
H2N CF
The crude amine was as such carried
C70 (N) forward to the next step due to
poor
solubility and instability in solution
Boo
(R)-tert-Butyl 4-(3-amino-5- form.
(trifluoromethyl)pheny1)-3-
methylpiperazine-1-carboxylate
Intermediate C57
3-(4-Methy1-1,4-diazepan-1-y1)-5-(trifluoromethyl)aniline
H2N ao CF3
N\
Step 1: 1-(3-Nitro-5-(trifluoromethyl)pheny1)-1,4-diazepane
91
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
02N CF3
cN.,)
NH
A solution of tert-butyl 4-(3 -nitro-5 -(trifluoromethyl)pheny1)-1,4-diaz
epane-1 -carboxylate
(step 1-Intermediate C52) (600 mg, 1.54 mmol) in hydrochloric acid in 1,4-
dioxane (5.0 mL)
was stirred at RT for 3 h. The mixture was concentrated under reduced pressure
and the
residue was diluted with water. The aqueous mixture was basified using
saturated sodium
bicarbonate solution till pH 8-9 at -20 C. The mixture was extracted twice
with
dichloromethane. The combined organic extracts were dried over anhydrous
sodium sulfate,
filtered and concentrated under reduced pressure to give 250 mg of the desired
product. The
crude amine was as such carried forward to the next step.
Step 2: 1-Methyl-4-(3 -nitro-5 -(trifluoromethyl)pheny1)-1,4-diazep ane
02N CF3
N\
To a stirred solution of 1-(3-nitro-5-(trifluoromethyl)pheny1)-1,4-diazepane
(step 1
intermediate) (250 mg, 0.87 mmol) in acetonitrile (35 mL) were added methyl
iodide (126
mg, 0.89 mmol) and potassium carbonate (125 mg, 0.91 mmol) at 0 C and the
mixture was
stirred overnight at RT. The solvent was removed under reduced pressure and
the residue was
dissolved in dichloromethane. The solution was washed with water, dried over
anhydrous
sodium sulfate, filtered and concentrated under reduced pressure. The residue
was purified by
silica gel column chromatography to yield 160 mg of the desired product. 1H
NMR (400
MHz, DMSO-d6) 6 1.90-1.92 (m, 2H), 2.26 (s, 3H), 2.26-2.51 (m, 2H), 2.64 (t, J
= 4.8 Hz,
2H), 3.55 (t, J= 6.0 Hz, 2H), 3.65 (t, J= 4.8 Hz, 2H),7.32 (s, 1H), 7.56 (s,
1H), 7.63 (t, J =
2.0 Hz, 1H); ESI-MS (m/z) 304 (M+H)-1.
Step 3: 3 -(4-Methyl-1,4-diazep an-1 -y1)-5 -(trifluoromethyl)aniline
The titled compound was prepared by the reaction of 1-methy1-4-(3-nitro-5-
(trifluoromethyl)pheny1)-1,4-diazepane (step 2 intermediate) (180 mg, 0.59
mmol) with iron
powder (331 mg, 5.92 mmol) and ammonium chloride (253 mg, 4.73 mmol) in
ethanol (3.0
mL) as per the procedure described in step 2 of Intermediate C28 to 140 mg of
the
compound. The crude amine was as such carried forward to the next step due to
poor
solubility and instability in solution form.
92
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
The chemical structure, name and analytical data of the intermediate prepared
by
following the procedure described above are given below in Table 17.
Table 17: Chemical name, structure and analytical data of Intermediate C58
Intermediate
No. Chemical Name and Structure Analytical Data
H2N u3
1H NMR (400 MHz, DMSO-d6) 6 2.56
(t, J = 5.2 Hz, 4H), 3.12 (t, J = 4.8 Hz,
4H), 3.32-3.34 (br s, 3H), 5.36 (s, 2H),
C58 CN)
6.29 (s, 1H), 6.34 (s, 2H); ESI-MS
(m/z) 284 (M+H)+.
3 -(4-(Prop-2-yn-1 -yl)pip erazin-1 -
y1)-5 -(trifluoromethyl)aniline
Intermediate C59
3 -(4-(Oxetan-3 -yl)pip erazin-l-y1)-5 -(trifluoromethyl)aniline
40 H2N NTh
\-6
Step 1: 1-(3 -Nitro-5 -(trifluoromethyl)pheny1)-4-(oxetan-3 -yl)pip erazine
cF3
N^i
\-6
A mixture of zinc chloride (277 mg, 2.03 mmol) and molecular sieves (200 mg)
was dried
under vacuum followed with addition of 1-(3-nitro-5-
(trifluoromethyl)phenyl)piperazine
hydrochloride (100 mg, 0.40 mmol) and oxetan-3-one (150 mg, 2.08 mmol) in
methanol (1.0
mL). The resultant mixture was stirred at RT for 2 h. The mixture was cooled
to 0 C and was
added sodium cyanoborohydride (126 mg, 2.08 mmol) in small portions. The
mixture was
stirred for 2 h at RT. The mixture was filtered through celite and the
filtrate was diluted with
sodium bicarbonate solution. The mixture was again passed through celite and
the filtrate was
diluted with ethyl acetate. The layers were separated and the aqueous layer
was extracted
with ethyl acetate. The combined organic layers were washed with water, dried
over
anhydrous sodium sulfate, filtered and concentrated under reduced pressure.
The residue was
purified by silica gel column chromatography to yield 90 mg of the desired
product. 1H
NMR (400 MHz, DMSO-d6) 6 2.42-2.51 (m, 4H), 3.43-3.47 (m, 4H), 4.34-4.37 (m,
1H), 4.48
(t, J= 6.0 Hz, 2H), 4.56-4.68 (m, 2H), 7.64 (s, 1H), 7.75 (s, 1H), 7.90 (t, J=
2.0 Hz, 1H).
93
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
Step 2: 3 -(4-(Oxetan-3 -yl)pip erazin-l-y1)-5 -(trifluoromethyl)aniline
The titled compound was prepared by the reaction of 1-(3-nitro-5-
(trifluoromethyl)pheny1)-4-
(oxetan-3-yl)piperazine (step 1 intermediate ) (100 mg, 0.30 mmol) with iron
powder (179
mg, 3.19 mmol) and ammonium chloride (138 mg, 2.55 mmol) in ethanol (4.0 mL)
as per the
procedure described in step 2 of Intermediate C28 to 70 mg of the compound. 1H
NMR (400
MHz, DMSO-d6) 6 2.388 (t, J = 4.8 Hz, 4H), 3.12 (t, J = 4.8 Hz, 4H), 3.42-3.45
(m, 1H),
4.47 (t, J = 5.6 Hz, 2H), 4.56 (t, J = 6.4 Hz, 2H), 5.37 (s, 2H), 6.30 (s,
1H), 6.34 (s, 2H).
Intermediate C60
(R)-1-(3 -Amino -5 -(trifluoromethyl)pheny1)-N,N-dimethylpyrro lidin-3 -amine
H2N I* CF3
^
N-
/
To a stirred solution of 3-bromo-5-(trifluoromethyl)aniline (200 mg, 0.83
mmol) and (R) -
N ,N - dime thy 1py r r oli din -3 - amin e (200 mg, 1.75 mmol) in DMF (2.0
mL) were added copper
iodide (83 mg, 0.44 mmol) followed by 8-hydroxyquinoline (40 mg, 0.26 mmol) at
RT in a
sealed tube. The mixture was stirred overnight at 120 C. The mixture was
cooled to RT and
diluted with water. The aqueous mixture was extracted twice with ethyl
acetate. The
combined organic layers were washed with water, dried over anhydrous sodium
sulfate,
filtered and concentrated under reduced pressure. The residue was purified by
silica gel
column chromatography to yield 80 mg of the desired product. 1H NMR (400 MHz,
DMSO-
d6) 6 1.73-1.83 (m, 1H), 2.12-2.17 (m, 6H), 2.74-2.78 (m, 1H), 2.98 (t, J =
8.4 Hz, 1H), 3.16-
3.22 (m, 1H), 3.33-3.41 (m, 4H), 5.28 (s, 2H), 5.95 (s, 1H), 6.14 (s, 1H); ESI-
MS (m/z) 274
(M+H)+.
The chemical structure, name and analytical data of the intermediate prepared
by
following the procedure described above are given below in Table 18.
Table 18: Chemical name, structure and analytical data of Intermediate C61
Intermediate
No. Chemical Name and Structure
Analytical Data
H2N . cF3
1H NMR (400 MHz, DMSO-d6) 6
1.77-1.83 (m, 1H), 2.12-2.20 (m, 5H),
C61 (..N
2.49-2.99 (m, 1H), 2.99 (t, J = 8.4 Hz,
\ i(S)
1H), 3.15-3.41 (m, 5H), 5.28 (s, 2H),
N-
5.94 (s, 2H), 76.14 (s, 1H); ESI-MS
/
(S)-1-(3 -Amino-5 - (m/z) 274 (M+H)+.
94
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
(trifluoromethyl)pheny1)-N,N-
dimethylpyrro lidin-3 -amine
Intermediate C62
tert-Butyl 3 -amino-5 -(trifluoromethyl)b enzyl(isopropyl)carb amate
H2N CF3
N.Boc
Step 1: N-(3 -Nitro-5 -(trifluoromethyl)b enzyl)propan-2-amine
02N so CFNH
A solution of 2-propanamine (408 mg, 7.04 mmol) in dichloromethane (5.0 mL)
was cooled
to 0 C and to that was slowly added a solution of 1-(bromomethyl)-3-nitro-5-
(trifluoromethyl)benzene (500 mg, 1.76 mmol) in dichloromethane (5.0 mL). The
mixture
was warmed to RT and stirred for 18 h. The mixture was poured into water and
the layers
were separated. The aqueous layer was extracted with dichloromethane. The
combined
organic layers were washed with water, dried over anhydrous sodium sulfate,
filtered and
concentrated under reduced pressure to yield 170 mg of the desired product. 1H
NMR (400
MHz, DMSO-d6) 6 1.13 (d, J = 6.0 Hz, 6H), 2.96 (br s, 1H), 4.11 (br s, 2H),
8.32 (s, 1H),
8.43 (s, 1H), 8.64 (s, 1H).
Step 2: tert-Butyl isopropy1(3-nitro-5-(trifluoromethyl)benzyl)carbamate
02N 40 cF3
N.Boc
The titled compound was prepared by the reaction of N-(3 -nitro-5 -
(trifluoromethyl)benzyl)propan-2-amine (step 1 intermediate) (170 mg, 0.65
mmol) with di-
tert-butyl dicarbonate (156 mg, 0.71 mmol) in the presence of DIPEA (168 mg,
1.30 mmol)
in dichloromethane (5.0 mL) as per the procedure described in step 3 of
Intermediate Al to
yield 240 mg of the product. 1H NMR (400 MHz, DMSO-d6) 6 1.15 (d, J = 6.8 Hz,
6H), 1.57
(s, 9H), 4.47 (br s, 3H), 7.85 (s, 1H), 8.32 (s, 1H), 8.38 (s, 1H).
Step 3: tert-Butyl 3-amino-5-(trifluoromethyl)benzyl(isopropyl)carbamate
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
The titled compound was prepared by the reaction of tert-butyl isopropy1(3-
nitro-5-
(trifluoromethyl)benzyl)carbamate (step 2 intermediate ) (235 mg, 0.65 mmol)
with iron
powder (363 mg, 6.50 mmol) and ammonium chloride (278 mg, 5.20 mmol) in
ethanol (5.0
mL) and water (1.5 mL) as per the procedure described in step 2 of
Intermediate C28 to 170
mg of the compound. 1H NMR (400 MHz, DMSO-d6) 6 1.05 (d, J = 6.8 Hz, 6H), 1.42
(s,
9H), 4.21 (br s, 3H), 5.56 (s, 2H), 6.62 (s, 1H), 6.67 (d, J= 8.4 Hz, 2H).
Intermediate C64
3 -(1-Methyl-1H-pyrazol-4-y1)-5 -(trifluoromethyl)aniline
H2N
N-N
Step 1: 4-(3 -Nitro-5 -(trifluoromethyl)pheny1)-1-(tetrahydro-2H-pyran-2-y1)-
1H-pyrazo le
02N is cF3
7,
N-N
THF;
The titled compound was prepared by the reaction of 1-bromo-3 -nitro-5 -
(trifluoromethyl)benzene (1.0 g, 3.70 mmol) with 1-(tetrahydro-2H-pyran-2-y1)-
4-(4,4,5,5-
tetramethy1-1,3,2-dioxaborolan-2-y1)-1H-pyrazole (1.12 g, 4.07 mmol) in the
presence of
cesium carbonate (1.80 g, 5.50 mmol), and 1,1'-bis(diphenylphosphino)ferrocene-
palladium(II)dichloride dichloromethane complex (150 mg, 0.18 mmol) in 1,4-
dioxane (10
mL) and water (2.0 mL) as per the procedure described in step 2 of
Intermediate C34 to yield
730 mg of the desired compound1H NMR (400 MHz, DMSO-d6) 6 1.53-1.68 (m, 2H),
1.69-
1.74 (m, 1H), 1.95-2.0 (m, 2H), 2.07-2.10 (m, 1H), 3.65-3.70 (m, 1H), 3.94-
3.97 (m, 1H),
5.45 (dd , ./i = 2.4 Hz, .1-2 = 10.0 Hz, 1H), 7.86 (s, 1H), 8.32 (s, 1H), 8.51
(s, 1H), 8.75 (s,
1H), 8.87 (s, 1H).
Step 2: 4-(3 -Nitro-5 -(trifluoromethyl)pheny1)-1H-pyrazo le
o2N is cF3
7,
HN-N
The titled compound was prepared by the reaction of 4-(3-nitro-5-
(trifluoromethyl)pheny1)-1-
(tetrahydro-2H-pyran-2-y1)-1H-pyrazole (step 1 intermediate) (350 mg, 1.03
mmol) with
hydrochloric acid in ethyl acetate (10 mL) in methanol (5.0 mL) as per the
procedure
96
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
described in step 1 of Intermediate C57 to yield 257 mg of the compound. 1H
NMR (400
MHz, DMSO-d6) 6 8.25 (s, 1H), 8.29 (s, 1H), 8.65 (s, 1H), 8.71 (s, 1H), 8.72
(d, J= 2.0 Hz,
1H), 13.24 (s, 1H).
Step 3: 1-Methyl-4-(3 -nitro-5 -(trifluoromethyl)pheny1)-1H-pyrazo le
02N cF3
N-N
The titled compound was prepared by the reaction of 4-(3-nitro-5-
(trifluoromethyl)pheny1)-
1H-pyrazole (step 2 intermediate) (100 mg, 0.39 mmol) with methyl iodide (82
mg, 0.58
mmol) in the presence of sodium hydride (60% w/w, 17 mg, 0.42 mmol) in DMF
(5.0 mL) as
per the procedure described in step 2 of Intermediate C57 to yield 60 mg of
the compound.
1H NMR (400 MHz, DMSO-d6) 6 3.89 (s, 3H), 8.24 (s, 2H), 8.41 (s, 1H), 8.59 (s,
1H), 8.66
(s, 1H).
Step 4: 3 -(1-Methyl-1H-pyrazol-4-y1)-5 -(trifluoromethyl)aniline
The titled compound was prepared by the catalytic hydrogenation of 1-methy1-4-
(3-nitro-5-
(trifluoromethyl)pheny1)-1H-pyrazole (step 3 intermediate) (55 mg, 0.20 mmol)
in the
presence of palladium on carbon (10% w/w, 50% wet, 20 mg) in methanol (5.0 mL)
and THF
(2.0 mL) as per the procedure described in step 3 of Intermediate Cl to yield
35 mg of the
compound. 1H NMR (400 MHz, DMSO-d6) 6 3.85 (s, 3H), 5.56 (s, 2H), 6.68 (s,
1H), 6.95 (s,
2H), 7.79 (s, 1H), 8.12 (s, 1H).
The chemical structure, name and analytical data of the intermediate prepared
by
following the procedure described above (step 1 and 4) are given below in
Table 19.
Table 19: Chemical name, structure and analytical data of Intermediate C65
Intermediate
No. Chemical Name and Structure Analytical Data
H2N cF3
1H NMR (400 MHz, DMSO-d6) 6
1.54-1.56 (m, 2H), 1.63-1.70 (m, 1H),
1.71-1.96 (m, 2H), 2.08-2.17 (m, 1H),
C65
3.61-3.67 (m, 1H), 3.92-3.95 (m, 1H),
N-N
THF;
5.40 (dd , ./i = 2.8 Hz, .1-2 = 10.0 Hz,
3-(1-(Tetrahydro-2H-pyran-2-y1)-
1H), 5.55 (s, 2H), 6.71 (s, 1H), 7.00 (s,
1H-pyrazol-4-y1)-5-
1H), 7.04 (s, 1H), 7.87 (s, 1H), 8.35 (s,
(trifluoromethyl)aniline 1H)
Intermediate C66
3 -((l-Methylazetidin-3 -yl)oxy)-5 -(trifluoromethyl)aniline
97
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
H2N C_F3
Step 1: tert-Butyl 3 -(3 -nitro-5 -(trifluoromethyl)phenoxy)azetidine-l-c
arboxylate
02N 401 cF3
O
\.-N.Boc
The titled compound was prepared by the reaction of 3-nitro-5-
(trifluoromethyl)phenol (1.0
g, 4.83 mmol) with N-Boc-3-hydroxyazetidine (1.0 g, 5.80 mmol) in the presence
of
triphenylphosphine (1.9 g, 7.20 mmol) and diisopropyl azodicarboxylate (DIAD)
(1.35 mL,
7.20 mmol) in THF (10 mL) as per the procedure described in step 2 of
Intermediate A2 to
yield 1.52 g of the compound. 1H NMR (400 MHz, DMSO-d6) 6 1.39 (s, 9H), 3.84-
3.88 (m,
2H), 4.36 (br s, 2H), 5.27-5.32 (m, 1H), 7.82 (s, 1H), 7.88-7.90 (m, 1H), 8.11
(s, 1H).
Step 2: 3 -(3 -Nitro-5 -(trifluoromethyl)phenoxy)az etidine hydrochloride
02N is
\--NH HCI
The titled compound was prepared by the reaction of tert-butyl 3-(3-nitro-5-
(trifluoromethyl)phenoxy)azetidine-1-carboxylate (step 1 intermediate) (1.50
g, 5.73 mmol)
with hydrochloric acid in ethyl acetate (20 mL) in ethyl acetate (4.0 mL) as
per the procedure
described in step 1 of Intermediate C57 to yield 918 mg of the compound
(isolated a
hydrochloride salt). 1H NMR (400 MHz, DMSO-d6) 6 4.02-4.06 (m, 2H), 4.46-4.51
(m, 2H),
5.39-5.42 (m, 1H), 7.77 (d, J = 1.5 Hz, 1H), 7.95 (t, J = 2.0 Hz, 1H), 8.14
(s, 1H), 9.68 (br s,
2H); ESI-MS (m/z) 263 (M+H-HCl).
Step 3: 1-Methyl-3 -(3 -nitro-5 -(trifluoromethyl)phenoxy)azetidine
02N CF3
Toa stirred solution of 3-(3-nitro-5-(trifluoromethyl)phenoxy)azetidine
hydrochloride (step 2
intermediate) (900 mg, 3.01 mmol) in dichloroethane (10 mL) were added
formaldehyde
(37%, 135 mg, 4.52 mmol) and sodium triacetoxyborohydride (STAB) (958 mg, 4.52
mmol)
and the mixture was stirred at RT for 18 h. The mixture was concentrated under
reduced
pressure and the residue obtained was purified by silica gel column
chromatography to yield
98
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
790 mg of the compound. 1H NMR (400 MHz, DMSO-d6) 6 2.29 (s, 3H), 3.02-3.06
(m, 2H),
3.72-3.76 (m, 2H), 5.08 (t, J = 5.6 Hz, 1H), 7.67 (s, 1H), 7.85 (t, J = 2.0
Hz, 1H), 8.07 (s,
1H); ESI-MS (m/z) 277 (M+H)+.
Step 4: 3 -((l-M ethylazetidin-3 -yl)oxy)-5 -(trifluoromethyl)aniline
The titled compound was prepared by the reaction of 1-methy1-3-(3-nitro-5-
(trifluoromethyl)phenoxy)azetidine (step 3 intermediate ) (780 mg, 2.82 mmol)
with iron
powder (780 mg, 14.1 mmol) and ammonium chloride (1.5 g, 28.2 mmol) in
methanol (10
mL) and water (10 mL) as per the procedure described in step 2 of Intermediate
C28 to 623
mg of the compound. 1H NMR (400 MHz, DMSO-d6) 6 2.27 (s, 3H), 2.92-2.95 (m,
2H), 3.69
(dt, ./i =2.0 Hz, ./i = 6.0 Hz, 2H), 4.67-4.70 (m, 1H), 5.61 (s, 2H), 6.17 (s,
1H), 6.23 (t, J= 1.6
Hz, 1H), 6.45 (s, 1H); ESI-MS (m/z) 247 (M+H)+.
Intermediate C68
1-((4-Fluorophenyl)carbamoyl)cyclopropanecarboxylic acid
HO
0 0 [W
To a stirred solution of cyclopropane-1,1-dicarboxylic acid (400 mg, 3.05
mmol) in
dichloromethane (10 mL) were added thionyl chloride (222 L, 3.05 mmol)
followed by
catalytic amount of DMF and the mixture was stirred at RT for 2 h. The mixture
was
concentrated under reduced pressure and the residue was dissolved in THF (10
mL). The
solution was cooled to 0 C; 4-fluoroaniline (0.29 mL, 3.05 mmol) was added to
the reaction
mixture followed by triethylamine (0.43 mL, 3.08 mmol). The resultant mixture
was stirred
overnight at RT. The mixture was diluted with water and extracted twice with
ethyl acetate.
The combined organic extracts were washed with saturated sodium bicarbonate
solution,
water and brine. The solution was dried over anhydrous sodium sulfate,
filtered and
concentrated. The residue thus obtained was purified by column chromatography
to yield 270
mg of the desired product. 1H NMR (400 MHz, DMSO-d6) 6 1.41 (s, 4H), 7.12-7.17
(m, 2H),
7.61-7.64 (m, 2H), 10.51 (s, 1H), 12.81 (s, 1H).
Intermediate C71
3 -((1S,4S)-5 -M ethy1-2,5-diazabicyclo [2 .2 .1]heptan-2-y1)-5-
(trifluoromethyl)aniline
H2N c_F3
(s)c%
N (s)
99
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
Step 1: (1S,4S)-tert-Butyl
5 -(3 -nitro-5 -(trifluoromethyl)pheny1)-2,5 -
diazabicyclo[2.2.1]heptane-2-carboxylate
02N 0_ F3
(s)1
(s)
Boc
The
titled compound was prepared by the reaction of 1-bromo-3 -nitro-5 -
(trifluoromethyl)benzene (2.7 g, 13.6 mmol) with (1S,4S)-tert-butyl 2,5-
diazabicyclo[2.2.1]heptane-2-carboxylate (500 mg, 2.52 mmol) in the presence
of sodium
tert-butoxide (350 mg, 3.64 mmol), tris(dibenzylideneacetone)dipalladium(0)
(Pd2(dba)3)
(138 mg, 0.15 mmol) and Xantphos (175 mg, 0.30 mmol) in toluene (10 mL) as per
the
procedure described in step 2 of Intermediate C34 to yield 840 mg of the
desired compound.
1H NMR (400 MHz, DMSO-d6) 6 1.60 (s, 9H), 2.06 (d, J = 5.2 Hz, 2H), 3.26-3.51
(m, 4H),
4.52 (s, 1H), 4.61-4.75 (m, 1H), 6.99 (s, 1H), 7.51 (s, 1H), 7.76 (s, 1H).
Step 2:
(1S,4S)-2-(3 -Nitro-5 -(trifluoromethyl)pheny1)-2,5 -diaz abicyclo [2 .2
.1]heptane
hydrochloride
02N so CF3
(s)
H HCI
The titled compound was prepared by the reaction of (1S,4S)-tert-butyl 5-(3-
nitro-5-
(trifluoromethyl)pheny1)-2,5-diazabicyclo[2.2.1]heptane-2-carboxylate (step 1
intermediate)
(400 mg, 1.13 mmol) with hydrochloric acid in 1,4-dioxane (4.0 mL) in ethanol
(8.0 mL) as
per the procedure described in step 1 of Intermediate C57 to yield 280 mg of
the compound.
The crude amine was as such carried forward to the next step.
Step 3:
(1S,4S)-2-Methy1-5 -(3 -nitro-5 -(trifluoromethyl)pheny1)-2,5-
diazabicyclo[2.2.1]heptane
02N u3
(s)
The titled compound was prepared by the reaction of (1S,4S)-2-(3-nitro-5-
(trifluoromethyl)pheny1)-2,5-diazabicyclo[2.2.1]heptane hydrochloride (step 2
intermediate)
(275 mg, 0.94 mmol) with methyl iodide (147 mg, 1.03 mmol) in the presence of
potassium
100
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
carbonate (142 mg, 1.03 mmol) in acetonitrile (12 mL) as per the procedure
described in step
2 of Intermediate C57 to yield 60 mg of the compound. The crude amine was as
such carried
forward to the next step.
Step 4: 3 -((1S,4S)-5 -Methyl-2,5 -diaz abicyclo [2 .2 .1]heptan-2-y1)-5 -
(trifluoromethyl)aniline
The titled compound was prepared by the catalytic hydrogenation of (1S,4S)-2-
methy1-5-(3-
nitro-5-(trifluoromethyl)pheny1)-2,5-diazabicyclo[2.2.1]heptane (step 3
intermediate) (60 mg,
0.19 mmol) in the presence of palladium on carbon (10% w/w, 50% wet, 20 mg) in
methanol
(10 mL) as per the procedure described in step 3 of Intermediate Cl to yield
60 mg (crude) of
the compound. The crude amine was as such carried forward to the next step due
to poor
solubility and instability in solution form.
The chemical structure, name and analytical data of the intermediate prepared
by
following the procedure described above are given below in Table 20.
Table 20: Chemical name, structure and analytical data of Intermediate C72-C73
and C75-
C76
Intermediate
No. Chemical Name and Structure Analytical Data
H2N cF3
The crude amine was as such carried
C72 C (s
forward to the next step due to poor
solubility and instability in solution
form.
(S)-3 -(2,4-Dimethylpip erazin-l-y1)-
5 -(trifluoromethyl)aniline
H2N c_F3
1H NMR (400 MHz, DMSO-d6) 6 1.01
(d, J = 6.4 Hz, 3H), 2.02 (td , J, = 3.6
C73 N
Hz, J2 = 11.2 Hz, 1H), 2.20 (d, J = 3.6
C Hz, 3H), 2.61-2.64 (m, 1H), 2.76-2.79
(m, 2H), 2.94 (dt, J, = 3.2 Hz, J2 = 11.6
Hz 1H) 3.18-3.21 (m, 1H), 3.89-3.92
(R)-3-(2,4-Dimethylpiperazin-1-y1)- "
(m, 1H), 5.32 (s, 2H), 6.25 (s, 1H),
5 -(trifluoromethyl)aniline
6.29 (d, J = 1.6 Hz, 2H).
H2N c _F3
1H NMR (400 MHz, DMSO-d6) 6 1.04
(d, J = 6.0 Hz, 3H), 2.06-2.17 (m, 1H),
C75
2.20 (s, 3H), 2.32-2.37 (m, 2H), 2.68-
C (
2.81 (m, 2H), 3.42-3.48 (m, 2H), 5.35
(s, 2H), 6.28 (s, 1H), 6.33 (s, 2H) ESI-
(R)-3-(3,4-Dimethylpiperazin-1-y1)-
MS (m/z) 274 (M+H)+.
5 -(trifluoromethyl)aniline
101
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
Intermediate
No. Chemical Name and Structure Analytical Data
H2N c_F3
1H NMR (400 MHz, DMSO-d6) 6 1.04
C76
C
(d, J = 5.6 Hz, 3H), 2.08-2.16 (m, 1H),
2.20 (s, 3H), 2.22-2.37 (m, 1H), 2.68-
2.80 (m, 3H), 3.43-3.48 (m, 2H), 5.35
(S)-3-(3,4-Dimethylpiperazin-1-y1)- (s, 2H), 6.28 (s, 1H), 6.33 (s, 2H).
-(trifluoromethyl)aniline
Intermediate C74
4-(3 -Amino-5 -(trifluoromethyl)phenyl)morpho lin-3 -one
H2N c _ F3
0,1\H
5 Step 1: 4-(3 -Nitro-5 -(trifluoromethyl)phenyl)morpholine
02N so c3
(o)
The titled compound was prepared by the reaction of
1-bromo-3-nitro-5-
(trifluoromethyl)benzene (2.0 g, 7.40 mmol) with morpholine (1.61 g, 18.5
mmol) in the
presence of N,N-diisopropylethylamine (DIPEA) (2.7 mL, 14.8 mmol) in DMSO (10
mL) as
per the procedure described in step 2 of Intermediate Cl to yield 1.1 g of the
compound. 1H
NMR (400 MHz, DMSO-d6) 6 3.36 (t, J = 5.2 Hz, 4H), 3.75 (t, J = 4.8 Hz, 4H),
7.64 (s, 1H),
7.78 (s, 1H), 7.91 (t, J = 2.4 Hz, 1H).
Step 2: 4-(3 -Nitro-5 -(trifluoromethyl)phenyl)morpho lin-3 -one
02N is c3
Oy\H
401)
A suspension of 4-(3-nitro-5-(trifluoromethyl)phenyl)morpholine (step 1
intermediate) (1.1 g,
3.98 mmol), benzyltributylammonium chloride (7.5 g, 23.9 mmol) and K1VIn04
(3.77 g, 23.9
mmol) in dichloromethane (70 mL) was heated overnight at 70 C. The mixture
was cooled
to RT and diluted with ethyl acetate. The suspension was filtered through
celite and the bed
was washed with ethyl acetate. The combined filtrate and washings were
concentrated under
reduced pressure and the residue obtained was purified by silica gel column
chromatography
102
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
to yield 340 mg of the desired compound. 41 NMR (400 MHz, DMSO-d6) 6 3.91-3.94
(m,
2H), 3.94-4.03 (m, 2H), 4.29 (s, 2H), 8.37 (d, J= 10.8 Hz, 2H), 8.66 (t, J =
1.6 Hz, 1H).
Step 3: 4-(3 -Amino-5 -(trifluoromethyl)phenyl)morpho lin-3 -one
The titled compound was prepared by the reaction of 4-(3-nitro-5-
(trifluoromethyl)phenyl)morpholin-3-one (step 2 intermediate ) (300 mg, 1.14
mmol) with
iron powder (636 mg, 11.3 mmol) and ammonium chloride (487 mg, 9.10 mmol) in
ethanol
(10 mL) and water (4.0 mL) as per the procedure described in step 2 of
Intermediate C28 to
150 mg of the compound. 1H NMR (400 MHz, DMSO-d6) 6 3.69 (t, J = 5.2 Hz, 2H),
3.95 (t,
J=4.8 Hz, 2H), 4.19 (s, 2H), 5.72 (s, 2H), 6.76 (s, 2H), 6.82 (d, J= 12.4 Hz,
2H).
Intermediate C79
3'-Amino-5'-(trifluoromethyl)-[1,1'-bipheny1]-4-carbonitrile
H2N CF3
ON
Step 1: 3'-Nitro-5'-(trifluoromethyl)-[1,1'-biphenyl]-4-carbonitrile
02N CF3
ON
To a solution of 1-bromo-3-nitro-5-(trifluoromethyl)benzene (250 mg, 0.92
mmol) in 1,4-
dioxane (10 mL) and water (1.0 mL) were added 4-(4,4,5,5-tetramethy1-1,3,2-
dioxaborolan-
2-yl)benzonitrile (215 mg, 0.94 mmol),
[1,1'-
bis(diphenylphosphino)ferrocene]dichloropalladium(II) complex with
dichloromethane (39
mg, 0.05 mmol) and potassium carbonate (269 mg, 1.95 mmol) and the mixture was
heated at
100 C for 18 h. The mixture was cooled to RT and diluted with ethyl acetate.
The solution
was filtered through celite. The filtrate was washed with water followed by
brine, dried over
anhydrous sodium sulfate, filtered and concentrated. The residue obtained was
purified by
silica gel column chromatography to yield 350 mg of the desired compound. 1H
NMR (400
MHz, DMSO-d6) 6 8.03 (dd, ./i = 1.6 Hz, .12 = 3.6 Hz, 2H), 8.14 (dd, ./i = 2.0
Hz, .12 = 6.4
.. Hz, 2H), 8.60 (s, 2H), 8.81 (t, J = 1.6 Hz, 1H).
Step 2: 3'-Amino-5'-(trifluoromethy1)41,1'-biphenyl]-4-carbonitrile
103
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
The titled compound was prepared by the reaction of 3'-nitro-5'-
(trifluoromethy1)41,1'-
biphenyl]-4-carbonitrile (step 2 intermediate) (150 mg, 0.51 mmol) with iron
powder (286
mg, 5.13 mmol) and ammonium chloride (219 mg, 4.10 mmol) in ethanol (5.0 mL)
and water
(2.0 mL) as per the procedure described in step 2 of Intermediate C28 to 50 mg
of the
compound. 1H NMR (400 MHz, DMSO-d6) 6 5.80 (s, 2H), 6.92 (s, 1H), 7.08 (s,
1H), 7.12 (s,
1H), 7.82 (d, J= 8.4 Hz, 2H), 7.93 (d, J = 8.4 Hz, 2H).
The chemical structure, name and analytical data of the intermediate prepared
by
following the procedure described above are given below in Table 21.
Table 21: Chemical name, structure and analytical data of Intermediate C80
Intermediate
No. Chemical Name and Structure Analytical Data
H2N cF3 1H NMR (400 MHz, DMSO-d6) 6
5.75 (s, 2H), 6.91 (s, 1H), 7.11 (s,
C80
2H), 7.67 (t, J = 7.6 Hz, 1H), 7.84-
7.87 (m, 1H), 7.96 (dt, ./i = 1.2 Hz, .1-2
CN
= 6.8 Hz, 1H), 8.11 (s, 1H); ESI-MS
3 '-Amino-5 '-(trifluoromethyl)- [1,1'- (m/z) 261 04-Hy.
biphenyl] -3 -carbonitrile
Intermediate C81
4-(1-(4-Ethylpiperazin-1-yl)ethyl)-3-(trifluoromethyl)aniline
H2N CF _ 3
(Nj
Nc
Stepl : 1 -(4-Nitro-2-(trifluoromethyl)phenyl)ethanone
02N 10 CF3
0
To a stirred solution of 1-bromo-4-nitro-2-(trifluoromethyl)benzene (500 mg,
1.85 mmol) and
tributy1(1-ethoxyvinyl)tin (806 mg, 2.22 mmol) in DMF (5.0 mL) was added
tetrakis(triphenylphosphine)palladium(0) (136 mg, 0.09 mmol) after purging
argon for 15
min. The mixture was heated at 90 C for 18 h. The mixture was cooled to RT
and poured
into dilute hydrochloric acid (50 mL). The solution was stirred at RT for 1 h.
The aqueous
mixture was extracted with ethyl acetate. The organic layer was dried over
anhydrous sodium
sulfate, filtered and concentrated under reduced pressure. The residue was
purified by silica
104
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
gel column chromatography to yield 125 mg of the desired compound. 1H NMR (400
MHz,
DMSO-d6) 6 2.65 (s, 3H), 7.66 (d, J= 8.4 Hz, 1H), 8.50 (m, 1H), 78.60 (s, 1H).
Step 2: 1-(4-Nitro-2-(trifluoromethyl)phenyl)ethanol
02N 40 CF
_ 3
OH
To a solution of 1-(4-nitro-2-(trifluoromethyl)phenyl)ethanone (step 1
intermediate) (120 mg,
0.52 mmol) in methanol (5.0 mL) was added sodium borohydride (20 mg, 0.52
mmol) at 0 C
and the mixture was stirred at RT for 3 h. The reaction was quenched with
acetone (2.0 mL)
and diluted with water. The aqueous mixture was extracted twice with
chloroform. The
combined organic extracts were washed with water, dried over anhydrous sodium
sulfate,
filtered and concentrated under reduced pressure to yield 120 mg of the
desired compound.
1H NMR (400 MHz, CDC13) 6 1.53 (d, J = 6.4 Hz, 3H), 2.15 (d, J = 2.8 Hz, 1H),
5.42-5.43
(br s, 1H), 8.11 (d, J = 8.8 Hz, 1H), 8.46 (dd, J, = 2.4 Hz, J2 = 8.8 Hz, 1H),
8.52 (s, 1H).
Step 3: 1-Ethy1-4-(1-(4-nitro-2-(trifluoromethyl)phenyl)ethyl)piperazine
02N so cF3
cNj
To a stirred solution of 1-(4-nitro-2-(trifluoromethyl)phenyl)ethanol (step 2
intermediate)
(600 mg, 2.56 mmol) in dichloromethane (5.0 mL) were added triethylamine (1.0
mL, 7.68
mmol) followed by methanesulfonyl chloride (Mesyl chloride) (587 mg, 5.12
mmol) at 0 C
and the mixture was stirred at RT for 1 h. The reaction was quenched with ice-
water mixture
and extracted with dichloromethane. The organic layer was dried over anhydrous
sodium
sulfate and concentrated under reduced pressure at 25 C. The residue was
dissolved in DMF
(10 mL) and cooled to 0 C. To that solution were added potassium carbonate
(706 mg, 5.12
mmol) followed by N-ethylpiperazine (292 mg, 2.56 mmol) and the mixture was
stirred
overnight at RT. The reaction was quenched with ice-water mixture and
extracted with
dichloromethane. The organic layer was dried over anhydrous sodium sulfate and
concentrated under reduced pressure. The residue obtained was purified by
silica gel column
chromatography to yield 320 mg of the desired compound. 1H NMR (400 MHz,
CDC13) 6
1.09 (t, J= 7.2 Hz, 3H), 1.32 (d, J= 10.4 Hz, 3H), 2.30-2.46 (m, 10H), 3.77
(q, J = 4.8 Hz,
1H), 8.15 (d, J= 8.8 Hz, 1H), 8.40 (dd, J, = 2.4 Hz, J2 = 8.8 Hz, 1H), 8.51
(s, 1H).
Step 4: 4-(1-(4-Ethylpiperazin-1-yl)ethyl)-3-(trifluoromethyl)aniline
105
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
The titled compound was prepared by the reaction of 1-ethy1-4-(1-(4-nitro-2-
(trifluoromethyl)phenyl)ethyl)piperazine (step 3 intermediate ) (220 mg, 0.66
mmol) with
iron powder (371 mg, 6.64 mmol) and ammonium chloride (283 mg, 5.31 mmol) in
ethanol
(5.0 mL) and water (1.0 mL) as per the procedure described in step 2 of
Intermediate C28 to
.. 80 mg of the compound. 1H NMR (400 MHz, DMSO-d6) 6 1.03 (t, J= 6.4 Hz, 3H),
1.17-1.23
(m, 5H), 2.51-2.92 (m, 7H), 3.36 (m, 2H), 5.76 (s, 2H), 6.78-6.81 (br s, 2H),
7.38 (d, J = 8.4
Hz, 1H).
Intermediate C82
N-(3 -Amino-5 -(trifluoromethyl)phenyl)propionamide
H2N CF 40 3
HN,f0
Step 1: N-(3 -Bromo-5 -(trifluoromethyl)phenyl)propionamide
Br CF _ 3
HN,f0
To a stirred mixture of propionic acid (1.54 g, 20.8 mmol) and 3-bromo-5-
(trifluoromethyl)aniline (5.0 g, 20.8 mmol) in dichloromethane (15 mL) were
added EDCI.
HC1 (7.9 g, 41.6 mmol), HOBt (2.8 g, 20.8 mmol) and DIPEA (7.0 mL, 41.6 mmol)
at 0 C.
The mixture was stirred at RT for 3 h. The mixture was diluted with water and
ethyl acetate.
The organic layer was separated and the aqueous layer was extracted with ethyl
acetate. The
combined organic extracts were washed with water and brine. The solution was
dried over
anhydrous sodium sulfate, fileted and concentrated under reduced pressure. The
residue
obtained was purified by silica gel column chromatography to yield 2.3 g of
the desired
compound. 1H NMR (400 MHz, DMSO-d6) 6 0.90 (t, J = 7.2 Hz, 3H), 2.36 (q, J =
7.6 Hz,
2H), 7.60 (s, 1H), 7.98 (s, 1H), 8.11 (s, 1H), 10.35 (s, 1H).
Step 2: N-(34(Diphenylmethylene)amino)-5-(trifluoromethyl)phenyl)propionamide
Ph N CF3
Ph 1.1
HN,f0
The titled compound was prepared by the reaction of N-(3 -bromo-5 -
(trifluoromethyl)phenyl)propionamide (step 1 intermediate) (1.0 g, 3.37 mmol)
with
benzophenone imine (918 mg, 5.06 mmol) in the presence of cesium carbonate
(2.2 g, 6.76
106
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
mmol), tris(dibenzylideneacetone)dipalladium(0) (Pd2(dba)3) (232 mg, 0.25
mmol) and ( )-
2,2'-bis(diphenylphosphino)-1,1'-binaphthalene (rac-BINAP) (105 mg, 0.16 mmol)
in 1,4-
dioxane (10 mL) as per the procedure described in step 2 of Intermediate C34
to yield 1.05 g
of the desired compound. 1H NMR (400 MHz, DMSO-d6) 6 1.05 (t, J = 7.6 Hz, 3H),
2.30 (q,
.. J= 7.6 Hz, 2H), 6.61 (s, 1H), 7.17-7.36 (m, 3H), 7.47-7.69 (m, 9H), 1.06
(s, 1H); ESI-MS
(m/z) 397 (M+H)+.
Step 3: N-(3 -Amino-5 -(trifluoromethyl)phenyl)propionamide
The titled compound was prepared by the reaction of N-(3-
((diphenylmethylene)amino)-5-
(trifluoromethyl)phenyl)propionamide (step 2 intermediate) (1.0 g, 2.53 mmol)
with
.. hydrochloric acid in 1,4-dioxane (5.0 mL) in THF (10 mL) as per the
procedure described in
step 1 of Intermediate C57 to yield 431 mg of the compound. 1H NMR (400 MHz,
DMSO-
d6) 6 1.06 (t, J = 7.6 Hz, 3H), 2.29 (q, J = 7.6 Hz, 2H), 5.58 (s, 2H), 6.51
(s, 1H), 7.07 (s,
1H), 7.10 (s, 1H), 9.84 (s, 1H).
Intermediate C83
244-Amino-2-(trifluoromethyl)pheny1)-2-methylpropanenitrile
H2N 0CF3
CN
Step 1: 2-(4-Nitro-2-(trifluoromethyl)phenyl)acetonitrile
o2N 0 c
_ F3
CN
A mixture of 2-chloro-5-nitrobenzotrifluoride (3.0 g, 13.3 mmol), potassium
carbonate (367
mg, 2.66 mmol), potassium iodide (3.3 g, 19.9 mmol) and ethyl cyanoacetate
(1.80 g, 15.9
mmol) in DMF (20 mL) was stirred at RT for 72 h. The mixture was quenched with
10%
aqueous citric acid solution and extracted twice with ethyl acetate. The
combined organic
extracts were dried over anhydrous sodium sulfate, filtered and concentrated
under reduced
pressure. The resulting residue was dissolved in a mixture of water (25 mL)
and acetic acid
(10 mL) and was added 37% hydrochloric acid at RT. The mixture was heated at
100 C for
h. The reaction mixture was cooled to RT and quenched with 10% aqueous
potassium
carbonate solution. The aqueous mixture was extracted twice with ethyl
acetate. The
combined organic extracts were dried over anhydrous sodium sulfate, filtered
and
concentrated under reduced pressure. The residue obtained was purified by
silica gel column
30 chromatography to yield 2.3 g of the desired compound. 1H NMR (400 MHz,
DMSO-d6) 6
107
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
4.40 (s, 2H), 8.02 (d, J = 8.8 Hz, 1H), 8.49 (s, 1H), 8.60 (dd, Ji = 2.4 Hz,
J2 = 8.4 Hz, 1H) ;
ESI-MS (m/z) 229 (M-H)-.
Step 2: 2-Methyl-2-(4-nitro-2-(trifluoromethyl)phenyl)propanenitrile
02N 40 CF3
CN
To a stirred solution of 2-(4-nitro-2-(trifluoromethyl)phenyl)acetonitrile
(step 1 intermediate)
(500 mg, 2.17 mmol) in THF (15 mL) were added methyl iodide (925 mg, 6.51
mmol)
followed by potassium tert-butoxide solution (1M, 6.5 mL, 6.51 mmol) at 0 C
and the
mixture was stirred at RT for 18 h. The mixture was diluted with aqueous
ammonium
chloride solution and extracted with ethyl acetate. The organic extract was
washed with brine
and dried over anhydrous sodium sulfate, filtered and concentrated under
reduced pressure.
The residue obtained was purified by silica gel column chromatography to yield
70 mg of the
desired compound (Fraction-1) along with 400 mg of 2-(4-nitro-2-
(trifluoromethyl)phenyl)propanenitrile (Fraction-2). The fraction-2 (400 mg,
1.64 mmol) was
further reacted with methyl iodide (465 mg, 3.27 mmol) in the presence of
potassium tert-
butoxide solution (1M, 3.27 mL, 3.27 mmol) in THF (20 mL) as per the procedure
described
above to yield 310 mg of the titled compound. 1H NMR (400 MHz, DMSO-d6) 6 1.88
(s,
6H), 8.11 (d, J= 9.2 Hz, 1H), 8.55 (s, 2H).
Step 3: 2-(4-Amino-2-(trifluoromethyl)pheny1)-2-methylpropanenitrile
The titled compound was prepared by the reaction of 2-methyl-2-(4-nitro-2-
(trifluoromethyl)phenyl)propanenitrile (step 2 intermediate) (300 mg, 1.15
mmol) with iron
powder (321 mg, 5.76 mmol) and ammonium chloride (308 mg, 5.76 mmol) in
methanol (10
mL) and water (10 mL) as per the procedure described in step 2 of Intermediate
C28 to 137
mg of the compound. 1H NMR (400 MHz, DMSO-d6) 6 1.72 (s, 6H), 5.71 (s, 2H),
6.79 (dd,
= 1.6 Hz, .1-2 = 8.4 Hz, 1H), 6.99 (s, 1H), 7.35 (d, J = 8.8 Hz, 1H); ESI-MS
(m/z) 229
(M+H)+.
Intermediate C84
N-(3 -Amino-5 -(trifluoromethyl)pheny1)-2-(dimethylamino)acetamide
H2N io
HN,e0
LI\J
108
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
The titled compound was prepared by the reaction of 5-(trifluoromethyl)benzene-
1,3-diamine
(1.1 g, 6.26 mmol) with N,N-dimethylglycine (644 mg, 6.25 mmol) in the
presence of EDCI.
HC1 (2.39 mg, 12.5 mmol), HOBt (843 mg, 6.25 mmol) and DIPEA (2.15 mL, 12.5
mmol) in
dichloromethane (10 mL) as per the procedure described in step 1 of
Intermediate C82 to
yield 230 mg of the compound. 1H NMR (400 MHz, DMSO-d6) 6 2.62 (s, 6H), 3.04
(s, 2H),
5.60 (s, 2H), 6.52 (s, 1H), 7.13 (s, 1H), 7.18 (s, 1H), 9.72 (s, 1H); ESI-MS
(m/z)
3261(M+H)+.
The chemical structure, name and analytical data of the intermediate prepared
by
following the procedure described above are given below in Table 22.
Table 22: Chemical name, structure and analytical data of Intermediate C85
Intermediate
No. Chemical Name and Structure Analytical Data
cF3
1H NMR (400 MHz, DMSO-d6) 6
0.84 (d, J = 6.0 Hz, 6H), 2.27-2.30
(m, 1H), 3.12-3.15 (m, 1H), 3.22-
C85 H2N 14 N)CC
H N T.,
3.26 (m, 1H), 3.37-3.41 (m, 3H),
5.61 (s, 2H), 6.52 (s, 1H), 7.05 (s,
N-(3 -Amino-5 -
1H), 7.12 (s, 1H), 9.89 (s, 1H); ESI-
(trifluoromethyl)pheny1)-1- MS (m/z) 302 (M+H)+.
isopropylazetidine-3 -carboxamide
Intermediate C86
1-(3 -Amino-5 -(trifluoromethyl)pheny1)-N,N-dimethylazetidin-3 -amine
H2N 0 cF3
N
?
N
Step 1: N,N-Dimethy1-1-(3 -nitro-5 -(trifluoromethyl)phenyl)azetidin-3 -amine
02N 40 cF3
N
?
The
titled compound was prepared by the reaction of 1-bromo-3 -nitro-5 -
(trifluoromethyl)benzene (200 mg, 0.74 mmol) with N,N-dimethylazetidin-3-amine
hydrochloride (256 mg, 1.48 mmol) in the presence of sodium tert-butoxide (355
mg, 3.70
mmol), tris(dibenzylideneacetone)dipalladium(0) (Pd2(dba)3) (34 mg, 0.04 mmol)
and
Xantphos (30 mg, 0. 05 mmol) in 1,4-dioxane (4.0 mL) as per the procedure
described in
109
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
step 2 of Intermediate C34 to yield 155 mg of the desired compound. 1H NMR
(400 MHz,
DMSO-d6) 6 2.13 (s, 6H), 3.24-3.27 (m, 1H), 3.79 (t, J = 5.2 Hz, 2H), 4.08 (t,
J = 7.6 Hz,
2H), 7.07 (s, 1H), 7.37 (s, 1H), 7.63 (s, 1H); ESI-MS (m/z) 290 (M+H)+.
Step 2: 1-(3 -Amino-5 -(trifluoromethyl)pheny1)-N,N-dimethylazetidin-3 -amine
The titled compound was prepared by the reaction of N,N-dimethy1-1-(3-nitro-5-
(trifluoromethyl)phenyl)azetidin-3-amine (step 1 intermediate) (150 mg, 0.52
mmol) with
iron powder (290 mg, 5.19 mmol) and ammonium chloride (222 mg, 4.15 mmol) in
ethanol
(4.0 mL) and water (2.0 mL) as per the procedure described in step 2 of
Intermediate C28 to
95 mg of the compound. 1H NMR (400 MHz, DMSO-d6) 6 2.14 (s, 6H), 3.13-3.18 (m,
1H),
3.50 (t, J = 5.6 Hz, 2H), 3.86 (t, J = 7.2 Hz, 2H), 5.36 (s, 2H), 5.82 (s,
2H), 6.19 (s, 1H); ESI-
MS (m/z) 260 (M+H)+.
Intermediate C87
(E)-3 -(3 -Amino-5 -(trifluoromethyl)phenyl)acrylamide
H2N cF3
H2N
Step 1: 3 -(3 -nitro-5 -(trifluoromethyl)phenyl)propiolamide
02N is 0F3
H2N 0
To a solution of 1-bromo-3-nitro-5-(trifluoromethyl)benzene (1.0 g, 3.70 mmol)
in degassed
DMF (10 mL) were added propiolamide (511 mg, 7.40 mmol),
bis(triphenylphosphine)palladium(II)dichloride (129 mg, 0.18 mmol),
copper(II)iodide (71
mg, 0.37 mmol) and triethylamine (1.54 mL, 11.1 mmol) at RT. The resultant
mixture was
heated at 120 C for 30 min in a microwave reactor. The mixture was cooled to
RT and
quenched with water. The product was extracted in ethyl acetate. The organic
layer was
washed with brine, dried over anhydrous sodium sulfate, filtered and
concentrated under
reduced pressure. The residue obtained was purified by silica gel column
chromatography to
yield 480 mg of the desired compound. 1H NMR (400 MHz, DMSO-d6) 6 7.92 (br s,
2H),
8.32 (s, 1H), 8.46 (s, 1H), 8.57-8.66 (m, 1H).
Step 2: (E)-3 -(3 -Amino-5 -(trifluoromethyl)phenyl)acrylamide
110
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
To a stirred solution of 3-(3-nitro-5-(trifluoromethyl)phenyl)propiolamide
(step 1
intermediate) (201 mg, 0.78 mmol) in a mixture of methanol and water (3:1, 10
mL) was
added ammonium chloride (416 mg, 7.78 mmol) and the mixture was heated to 80
C. Zinc
dust (254 mg, 3.84 mmol) was added to the mixture in small portions and
stirred at for 1 h at
80 C. The mixture was cooled to RT and diluted with ethyl acetate. The
solution was filtered
through celite. The filtrate was washed with ethyl acetate and the combined
organic layers
were washed with water, followed by brine. The organic layer was dried over
anhydrous
sodium sulfate, filtered and concentrated. The residue obtained was purified
by column
chromatography to yield 65 mg of the desired compound. 41 NMR (400 MHz, DMSO-
d6) 6
5.73 (s, 2H), 6.58 (d, J= 16.0 Hz, 1H), 6.84 (s, 1H), 6.95 (d, J= 7.6 Hz, 2H),
7.16 (s, 1H),
7.30 (d, J= 15.6 Hz, 1H), 7.55 (s, 1H).
The chemical structure, name and analytical data of the intermediate prepared
by
following the procedure described above are given below in Table 23.
Table 23: Chemical name, structure and analytical data of Intermediate C88,
C93, C96,
C102-103, and 105
Intermediate
No. Chemical Name and Structure Analytical Data
F3c 1H NMR (400 MHz, DMSO-d6) 6
41 = \ N 5.87 (s, 2H), 6.94 (s, 1H),
6.99 (s,
I C88 2H), 7.54 (d, J = 6.0 Hz, 2H), 8.64
H2N (d, J = 6.4 Hz, 2H), ESI-MS
(m/z)
3 -(Pyridin-4-ylethyny1)-5 - 263 (M+H)+.
(trifluoromethyl)aniline
F3c 1H NMR (400 MHz, DMSO-d6) 6
41. = OH 1.44 (s, 6H), 5.49 (s, 1H),
5.73 (s,
C93 H2N 2H), 6.71 (s, 1H), 6.81 (dd, J = 5.6,
1.6 Hz, 2H).
443 -Amino-5 -
(trifluoromethyl)pheny1)-2-methylbut-
3 -yn-2-ol
F3c 1H NMR (400 MHz, DMSO-d6) 6
. ¨ 4.28 (d, J = 6.0 Hz, 2H),
5.36 (t, J=
C96 H2N OH 6.0 Hz, 1H), 5.76 (s 2H), 6.76 (s,
3 -(3 -Amino-5 - 1H), 6.84 (dd, J = 5.6, 1.6
Hz, 2H).
(trifluoromethyl)phenyl)prop-2-yn-1-
ol
F3c 1H NMR (400 MHz, DMSO-d6) 6
C102 . ¨ OH 1.37 (d, J = 5.2 Hz, 3H),
4.57 (br s,
H2N
1H), 5.49 (s, 1H), 5.75 (s, 2H), 6.74
4-(3 -amino-5 - (s, 1H), 6.80-6.84 (m, 2H).
(trifluoromethyl)phenyl)but-3-yn-2-ol
111
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
Intermediate
No. Chemical Name and Structure Analytical Data
1H NMR (400 MHz, DMSO-d6) 6
CF 3 1.35 (d, J = 6.4 Hz, 3H), 4.54 (t, J =
6.0 Hz, 1H),5.36 (d, J = 5.2 Hz, 1H),
C103 H2N . OH 5.93 (s, 2H), 6.72 (dd, J1 = 2.0 Hz, .12
4-(4-Amino-2-
= 8.4 Hz, 1H), 6.89 (d, J = 2.4 Hz,
1H), 7.24 (d,
(trifluoromethyl)phenyl)but-3-yn-2-ol
J = 8.4 Hz, 1H); ESI-
MS 230
1H NMR (400 MHz, DMSO-d6) 2.49
.5__/ _cF3
(t, J = 6.8 Hz, 2H), 3.54 (q, J = 6.8
C105 H 2N \ /
OH
Hz, 2H), 4.84 (t, J = 5.6 Hz, 1H),
=
5.86 (s, 2H), 6.70 (dd, J1 = 2.0 Hz, .12
4-(4-Amino-2-
= 8.0 Hz, 1H), 6.86 (d, J = 2.4 Hz,
(trifluoromethyl)phenyl)but-3-yn-1-ol 1H), 7.23 (d, J = 8.4 Hz, 1H); ESI-
MS (m/z) 230 (M+H)+.
Intermediate C89
3 -(3 -Amino-5 -(trifluoromethyl)phenyl)propio lamide
H2N 0 CF3
11
0 NH2
The
titled compound was prepared by the reaction of 3 -(3 -nitro-5 -
(trifluoromethyl)phenyl)propiolamide (Intermediate C87-step 1 intermediate)
(200 mg, 0.77
mmol) with iron powder (216 mg, 3.87 mmol) and ammonium chloride (42 mg, 0.77
mmol)
in a mixture of ethanol, THF and water (2:1:1, 10 mL) as per the procedure
described in step
2 of Intermediate C28 to yield 110 mg of the compound. 1H NMR (400 MHz, DMSO-
d6) 6
5.90 (s, 2H), 714-7.21 (m, 3H), 7.71 (s, 1H), 8.17 (s, 1H).
Intermediate C90
(E)-3 -(3 -Amino-5 -(trifluoromethyl)phenyl)acrylonitrile
H2N 0 cF3
CN
To a solution of 3-bromo-5-(trifluoromethyl)aniline (502 mg, 2.09 mmol) in
degassed DMF
(5.0 mL) were added acrylonitrile (97 L, 2.51
mmol),
tetrakis(triphenylphosphine)palladium(0) (121 mg, 0.10 mmol), and
triethylamine (0.87 mL,
6.27 mmol) at RT. The resultant mixture was heated at 130 C for 30 min in a
microwave
112
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
reactor. The mixture was cooled to RT and quenched with water. The product was
extracted
in ethyl acetate. The organic layer was washed with brine, dried over
anhydrous sodium
sulfate, filtered and concentrated under reduced pressure. The residue
obtained was purified
by silica gel column chromatography to yield 206 mg of the desired compound.
41 NMR
(400 MHz, DMSO-d6) 6 5.79 (s, 2H), 7.44 (d, J= 16.8 Hz, 1H), 7.92-7.96 (m,
2H), 7.12 (s,
1H), 7.59 (d, J = 16.8 Hz, 1H).
Intermediate C91
445 -Amino-3 -(tert-butyl)-1H-pyrazol-1-y1)b enzonitrile
ON
140
H 2 N
To a solution of 4-hydrazinylbenzonitrile (3.0 g, 22.55 mmol) in ethanol (30
mL) were added
pivaloyl acetonitrile (3.38 g, 37.1 mmol) followed by PTSA (6.42 g, 33.8 mmol)
and the
mixture was heated to reflux using Dean-Stark apparatus for 16 h. The
precipitated solid was
filtered and dried to yield 3.5 g of the desired compound. 1H NMR (400 MHz,
DMSO-d6) 6
1.22 (s, 9H), 5.50 (s, 2H), 7.83-7.93 (m, 5H); ESI-MS (m/z) 241 (M+H)+.
Intermediate C92
3 -(5 -Amino-3 -(tert-butyl)-1H-pyrazol-1-y1)benzonitrile
CN
H2N \NIN
To a solution of 3-hydrazinylbenzonitrile (500 mg, 3.75 mmol) in hydrochloric
acid in 1,4-
dioxane (10 mL) was added pivaloyl acetonitrile (565 mg, 4.51 mmol) and the
mixture was
heated to reflux for 16 h. The mixture was cooled to RT and diluted with
water. The solution
was basified using saturated aqueous sodium bicarbonate solution and extracted
twice with
ethyl acetate. The combined organic extracts were dried over anhydrous sodium
sulfate,
filtered and concentrated under reduced pressure to yield 830 mg of the
desired compound.
1H NMR (400 MHz, DMSO-d6) 6 1.22(s, 9H), 5.45 (s, 2H), 7.62-7.73 (m, 2H), 7.94-
8.03 (m,
3H); ESI-MS (m/z) 241 (M+H)+.
Intermediate C94
3 -(methylsulfonyl)aniline
113
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
0,
H2N 401
Step 1: 1-(Methylsulfony1)-3-nitrobenzene
o2N sso,
To a solution of 1-iodo-3-nitrobenzene (500 mg, 2.01 mmol) in degassed DMSO
(5.0 mL)
were added sodium methanesulfinate (413 mg, 4.01 mmol), N,N-
dimethylethylenediamine
(106 mg, 1.20 mmol) copper(I)trifluoromethane sulfonate toluene complex (311
mg, 0.60
mmol) and the mixture was evacuated and flushed with nitrogen for thrice. The
mixture was
heated at 120 C for 3 h. The mixture was cooled to RT, quenched with
saturated ammonium
chloride solution and extracted twice with ethyl acetate. The combined organic
layers were
washed with brine, dried over anhydrous sodium sulfate, filtered and
concentrated under
reduced pressure. The residue obtained was purified by silica gel column
chromatography to
yield 360 mg of the desired compound. The compound was as such taken forward
to the next
step without characterization.
Step 2: 3-(methylsulfonyl)aniline
The titled compound was prepared by the catalytic hydrogenation of 1-
(methylsulfony1)-3-
nitrobenzene (step 1 intermediate) (351 mg, 1.74 mmol) in the presence of 10%
palladium on
carbon (50% wet, 150 mg) in methanol (5.0 mL) as per the procedure described
in step 3 of
Intermediate Cl to yield 230 mg of the compound. ESI-MS (m/z) 172 (M+H)+.
The chemical structure, name and analytical data of the intermediate prepared
by
following the procedure described above are given below in Table 24.
Table 24: Chemical name, structure and analytical data of Intermediate C98
Intermediate
No. Chemical Name and Structure Analytical Data
0, j
H2N 401
1H NMR (400 MHz, DMSO-d6) 6
1.09 (t, J = 7.2 Hz, 3H), 3.16 (q, J =
C98
7.2 Hz, 2H), 5.67 (s, 2H), 6.85 (d, J =
7.6 Hz, 1H), 6.93 (d, J = 7.6 Hz, 1H),
3 -(Ethylsulfonyl)aniline
7.03 (s, 1H), 7.26 (t, J = 8.0 Hz, 1H).
Intermediate C95
3 -(3 -Methoxyprop-1 -yn-1 -y1)-5 -(trifluoromethyl)aniline
114
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
H2N 40 CF3
'0
Step 1: 3 -(3 -Nitro-5 -(trifluoromethyl)phenyl)prop-2-yn-1-01
02N c3
HO
The titled compound was prepared by the reaction of 1-bromo-3 -nitro-5 -
(trifluoromethyl)benzene (502 mg, 1.86 mmol) with propargyl alcohol (219 L,
3.72 mmol)
in the presence of bis(triphenylphosphine)palladium(II)dichloride (65 mg, 0.09
mmol),
copper iodide (35 mg, 0.18 mmol) and triethylamine (0.8 mL, 5.58 mmol) in DMF
(5.0 mL)
as per the procedure described in step 1 of Intermediate C87 to yield 205 mg
of the
compound. 1H NMR (400 MHz, DMSO-d6) 6 4.37 (d, J = 6.0 Hz, 2H), 5.50 (t, J =
6.0 Hz,
1H), 8.26 (s, 1H), 8.46 (d, J = 2.0 Hz, 2H).
Step 2: 1-(3 -Methoxyprop-1-yn-l-y1)-3 -nitro-5 -(trifluoromethyl)b enzene
02N c3
'0
The titled compound was prepared by the reaction of 3 -(3 -Nitro-5 -
(trifluoromethyl)phenyl)prop-2-yn-l-ol (step 1 intermediate) (738 mg, 3.01
mmol) with
methyl iodide (0.37 mL, 6.02 mmol) in the presence of sodium hydride (60%w/w,
240 mg,
6.02 mmol) in THF (10 mL) as per the procedure described in step 2 of
Intermediate C57 to
yield 390 mg of the compound. 1H NMR (400 MHz, DMSO-d6) 6 3.38 (s, 3H), 4.41
(s, 2H),
8.36 (s, 1H), 8.49 (s, 1H), 8.53 (s, 1H).
Step 3: 3 -(3 -Methoxyprop-1-yn-l-y1)-5 -(trifluoromethyl)aniline
The titled compound was prepared by the reaction of 1-(3-methoxyprop-1-yn-1-
y1)-3-nitro-5-
(trifluoromethyl)benzene (step 2 intermediate) (465 mg, 1.79 mmol) with iron
powder (501
mg, 8.97 mmol) and ammonium chloride (95 mg, 1.79 mmol) in a mixture of
ethanol, THF
and water (3:2:1, 12 mL) as per the procedure described in step 2 of
Intermediate C28 to
yield 312 mg of the compound. 1H NMR (400 MHz, DMSO-d6) 6 3.34 (s, 3H), 4.31
(s, 2H),
5.78 (s, 2H), 7.54 6.81 (s, 1H), 6.86 (s, 1H), 6.87 (s, 1H); ESI-MS (m/z) 230
(M+H)-1.
115
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
Intermediate C97
3 -(Difluoromethyl)aniline
H2N 0 CHF2
Step 1: 1 -(Difluoromethyl)-3 -nitrobenzene
02N I* CHF2
To a stirred solution of 3-nitrobenzaldehyde (506 mg, 3.35 mmol) in anhydrous
dichloromethane (10 mL) was dropwise added DAST (1.32 mL, 10.04 mmol) at -78
C. The
mixture was gradually warmed up to RT and stirred for 3 h. The mixture was
quenched with
saturated sodium bicarbonate solution and extracted twice with ethyl acetate.
The combined
organic extracts were washed with brine and dried over anhydrous sodium
sulfate. The
solution was filtered and concentrated under reduced pressure and the residue
obtained was
purified by silica gel column chromatography to yield 332 mg of the desired
compound. The
compound was as such taken forward to the next step without characterization.
Step 2: 3-(Difluoromethyl)aniline
The titled compound was prepared by the catalytic hydrogenation of 1-
(difluoromethyl)-3-
nitrobenzene (step 1 intermediate) (320 mg, 1.85 mmol) in the presence of 10%
palladium on
carbon (50% wet, 100 mg) in methanol (5.0 mL) as per the procedure described
in step 3 of
Intermediate Cl to yield 160 mg of the compound. ESI-MS (m/z) 144 (M+H)+.
Intermediate C100
2-(3 -Aminopheny1)-2,2-difluoro ethanol
F F
H2N 0 OH
Step 1: 2,2-Difluoro-2-(3-nitrophenyl)ethanol
F F
02N OH
The titled compound was prepared by the reaction of ethyl 2,2-difluoro-2-(3-
nitrophenyl)acetate (Step 1 of C45) (408 mg, 1.66 mmol) with sodium
borohydride (126 mg,
3.32 mmol) in ethanol (10 mL) as per the procedure described in step 2 of
Intermediate C81
to yield 271 mg of the compound. 1H NMR (400 MHz, DMSO-d6) 6 3.89-4.01 (m,
2H), 5.76
(t, J= 6.4 Hz, 1H), 7.82 (t, J= 7.6 Hz, 1H), 8.01 (dd, ./1 = 0.4 Hz, .1-2 =
7.6 Hz, 1H), 8.31 (s,
1H), 8.36-8.41 (m, 1H).
116
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
Step 2: 2-(3-Aminopheny1)-2,2-difluoroethanol
The titled compound was prepared by the catalytic hydrogenation of 2,2-
difluoro-2-(3-
nitrophenyl)ethanol (step 1 intermediate) (260 mg, 1.28 mmol) in the presence
of 10%
palladium on carbon (50% wet, 100 mg) in methanol (8.0 mL) as per the
procedure described
in step 3 of Intermediate Cl to yield 75 mg of the compound. 1H NMR (500 MHz,
DMSO-
d6) 6 3.70-3.77 (m, 2H), 5.31 (s, 2H), 5.53 (s, 1H), 6.58-6.65 (m, 2H), 6.68
(s, 1H), 7.05-7.10
(m, 1H); ESI-MS (m/z) 174 (M+H)+.
Intermediate C101
3 -(3 -Amino-5 -(trifluoromethyl)phenyl)propio lonitrile
H2N cF3
CN
Step 1: 3 -(3 -Nitro-5 -(trifluoromethyl)phenyl)propiolonitrile
02N cF3
CN
To a stirred solution of 3-(3-nitro-5-(trifluoromethyl)phenyl)propiolamide
(step 1 of C87)
(100 mg, 0.38 mmol) in anhydrous dichloromethane (5.0 mL) was added Burgess
reagent
(110 mg, 0.46 mmol) at 0 C and the mixture was stirred at RT for 2 h. The
mixture was
concentrated under reduced pressure and the residue was purified by silica gel
column
chromatography to yield 60 mg of the titled compound. 1H NMR (400 MHz, DMSO-
d6) 6
8.72 (d, J = 1.2 Hz, 1H), 8.77 (s, 1H), (d, J = 1.2 Hz, 1H), 8.99 (dd, J, =
1.6 Hz, J2 = 2.4 Hz,
1H).
Step 2: 3 -(3 -Amino-5 -(trifluoromethyl)phenyl)propio lonitrile
The titled compound was prepared by the reaction of 3-(3-nitro-5-
(trifluoromethyl)phenyl)propiolonitrile (step 1 intermediate) (251 mg, 1.04
mmol) with iron
powder (292 mg, 5.22 mmol) and ammonium chloride (56 mg, 1.04 mmol) in a
mixture of
ethanol, THF and water (2:2:1, 25 mL) as per the procedure described in step 2
of
Intermediate C28 to yield 121 mg of the compound. 1H NMR (400 MHz, DMSO-d6) 6
6.04
(s, 2H), 7.06-7.11 (m, 2H), 7.20 (s, 1H).
Intermediate C106
3 -(4-Methylpip erazin-l-y1)-5 -(methylsulfonyl)aniline
117
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
H2N SO2Me
cNj
Step 1: 1-(3-Iodo-5-nitropheny1)-4-methylpiperazine
02N I
cNj
The titled compound was prepared by the reaction of 1-fluoro-3-iodo-5-
nitrobenzene (1.0 g,
3.75 mmol) 1-methylpiperazine (1.9 g, 18.7 mmol) in DMSO (10 mL) as per the
procedure
described in step 1 of Intermediate C24 to yield 901 mg of the compound. 1H
NMR (400
MHz, DMSO-d6) 6 2.20 (s, 3H), 2.40-2.45 (m, 4H), 3.25-3.30 (m, 4H), 7.61-7.67
(m, 2H),
7.77-7.80 (m, 1H); ESI-MS (m/z) 348 (M+H)+.
Step 2: 1-Methyl-4-(3-(methylsulfony1)-5-nitrophenyl)piperazine
02N SO2Me
(Nj
The titled compound was prepared by the reaction of 1-(3-iodo-5-nitropheny1)-4-
methylpiperazine (step 1 intermediate) (481 mg, 1.38 mmol) with sodium
methanesulfinate
(282 mg, 2.77 mmol), in the presence of N,N-dimethylethylenediamine (73 mg,
0.83 mmol)
and copper(I)trifluoromethane sulfonate toluene complex (215 mg, 0.42 mmol) as
per the
procedure described in step 1 of Intermediate C94 to yield 310 mg of the
compound. 1H
NMR (400 MHz, DMSO-d6) 6 2.20 (s, 3H), 2.44-2.49 (m, 4H), 2.54 (s, 3H), 3.38-
3.42 (m,
4H), 7.74-7.77 (m, 1H), 7.89-7.94 (m, 2H); ESI-MS (m/z) 300 (M+H)+.
Step 3: 3 -(4-Methylpip erazin-l-y1)-5 -(methylsulfonyl)aniline
The titled compound was prepared by the catalytic hydrogenation of 1-methyl-4-
(3-
(methylsulfony1)-5-nitrophenyl)piperazine (step 2 intermediate) (301 mg, 1.00
mmol) in the
presence of 10% palladium on carbon (50% wet, 150 mg) in methanol (10 mL) as
per the
procedure described in step 3 of Intermediate Cl to yield 110 mg of the
compound. 1H NMR
(400 MHz, DMSO-d6) 6 2.22 (s, 3H), 2.41-2.47 (m, 4H), 3.08 (s, 3H), 3.09-3.14
(m, 4H),
5.45 (s, 2H), 6.36-6.39 (m, 1H), 6.51-6.57 (m, 2H); ESI-MS (m/z) 270 (M+H)+.
118
CA 03064975 2019-11-26
WO 2018/215668 PCT/EP2018/063957
Examples
General procedures for the synthesis of the examples are described in Method A-
R. All
the examples were prepared by following either of the methods described below
from the
combination of appropriate intermediates. Name, structure, Intermediate/method
used and
characterization data for individual examples are given in Table 25.
Method A
Preparation of
3 -((7, 8-dihydro-6H-pyrimido [5,4-b] [1,4] oxazin-4-yl)oxy)-N-(4-((4-
ethylpip erazin-1 -yl)methyl)-3 -(trifluoromethyl)phenyl)b enzamide
0 H
0 N s CFrN
N,)
N C'C) 0
NJ.NJ
H
Step 1:
N-(4-((4-Ethylpip erazin-1 -yl)methyl)-3 -(trifluoromethyl)p heny1)-3 -
hydroxybenzamide
0 H
HO N 0 CFrNI,
0 1\1)
A mixture of 4-((4-ethylpiperazin-1-yl)methyl)-3-(trifluoromethyl)aniline
(Intermediate Cl)
(500 g, 1.74 mmol) and methyl 3-hydroxybenzoate (Intermediate B2) (317 mg,
1.91 mmol)
in anhydrous THF (5.0 mL) was cooled to -20 C and added potassium tert-
butoxide (1M, 10
mL, 10.44 mmol). The mixture was stirred at RT for 3 h. The reaction mixture
was cooled to
-20 C and quenched with saturated sodium bicarbonate solution. The aqueous
mixture was
extracted twice with ethyl acetate and the combined organic layers were washed
with water
followed by brine. The organic layer was dried over anhydrous sodium sulfate,
filtered and
concentrated to yield 220 mg of the desired product. 1H NMR (400 MHz, DMSO-d6)
6 0.98
(t, J = 6.8 Hz, 3H), 2.31-2.50 (m, 8H), 2.51 (br s, 2H), 3.56 (s, 2H), 6.98-
7.01 (br d, 1H),
7.31-7.35 (m, 2H), 7.39 (d, J = 6.4 Hz, 1H), 7.69 (d, J = 8.8 Hz, 1H), 8.03
(d, J = 8.4 Hz,
1H), 8.21 (s, 1H), 9.79 (s, 1H), 10.42 (s, 1H); ESI-MS (m/z) 408 (M+H)+.
Step 2: tert-Butyl 4-
(3-((4-((4-ethylpiperazin-1-yl)methyl)-3-
(trifluoromethyl)phenyl)carbamoyl)phenoxy)-6H-pyrimido [5,4-b] [1,4] oxazine-8
(7 H)-
carboxylate
119
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
so H
O N 0 CFrN
NO 0 N,)
I j
N N
6oc
To a stirred solution of N-(4-((4-ethylpiperazin-1-yl)methyl)-3-
(trifluoromethyl)pheny1)-3-
hydroxybenzamide (step 1 intermediate) (200 mg, 0.49 mmol) and tert-butyl 4-
chloro-6H-
pyrimido[5,4-b][1,4]oxazine-8(7H)-carboxylate (Intermediate Al) (134 mg, 0.49
mmol) in
DMF (5.0 mL) was added cesium carbonate (241 mg, 0.74 mmol) and the mixture
was stirred
at 130 C for 2 h. The mixture was cooled to RT and diluted with a mixture of
ethyl acetate
and water. The organic layer was separated and washed with water followed by
brine. The
solution was dried over anhydrous sodium sulfate, filtered and concentrated
under reduced
pressure. The residue thus obtained was purified by silica gel column
chromatography to
yield 245 mg of the desired product. 1H NMR (400 MHz, DMSO-d6) 6 0.98 (t, J =
7.2 Hz,
3H), 1.50 (s, 9H), 2.39-2.50 (m, 10H), 3.56 (s, 2H), 3.91 (t, J= 4.0 Hz, 2H),
4.39 (t, J = 4.0
Hz, 2H), 7.46 (dd , ./1 = 1.6 Hz, .1-2 = 8.0 Hz, 1H), 7.62 (t, J = 8.0 Hz,
1H), 7.71 (d, J = 8.4
Hz, 1H), 7.78 (s, 1H), 7.88 (d, J= 7.6 Hz, 1H), 8.04 (d, J = 8.0 Hz, 2H), 8.20
(s, 1H), 10.52
(s, 1H); ESI-MS (m/z) 643 (M+H)+.
Step 3: 3 -((7,8-dihydro-6H-pyrimido [5 ,4-b] [1,4]oxazin-4-yl)oxy)-N-(4-((4-
ethylpip erazin-1-
yl)methyl)-3 -(trifluoromethyl)phenyl)b enz amide
To a stirred solution of tert-butyl 4-(3-((4-((4-ethylpiperazin-l-yl)methyl)-3-
(trifluoromethyl)phenyl)carbamoyl)phenoxy)-6H-pyrimido [5,4-b] [1,4] oxazine-8
(7 H)-
carboxylate (step 2 intermediate) (220 mg, 0.34 mmol) in ethanol (3.0 mL) was
added
hydrochloric acid in 1,4-dioxane (7.0 mL) at 0 C and the mixture was stirred
at RT for 3 h.
The mixture was diluted with ethyl acetate and water. The organic layer was
washed with
water, saturated sodium bicarbonate solution followed by brine. The solution
was dried over
anhydrous sodium sulfate, filtered and concentrated under reduced pressure to
give 105 mg of
the desired product.
Method B
Preparation of 4-chloro-3-((7,8-dihydro-6H-pyrimido [5,4-b][1,4]oxazin-4-
yl)oxy)-N-(4-(42-
(dimethylamino)ethyl)(methyl)amino)methyl)-3-(trifluoromethyl)phenyl)benzamide
CI
H
O N 0 CF
N C) 0 N,---..N.--
I, ) I
N N
H
120
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
Step 1: tert-Butyl
4-(2-chloro-5-(methoxycarbonyl)phenoxy)-6H-pyrimido [5,4-
b.] [1,4]oxazine-8(7H)-carboxylate
ci a
0,
NO
0
0
1 )
NN
Boo
To a stirred solution of methyl 4-chloro-3-hydroxybenzoate (Intermediate B1)
(226 mg, 1.21
mmol) and tert-butyl 4-chloro-6H-pyrimido[5,4-b][1,4]oxazine-8(7H)-carboxylate
(Intermediate Al) (300 mg, 1.10 mmol) in DMF (10 mL) was added cesium
carbonate (540
mg, 1.65 mmol) and the mixture was stirred at 130 C for 3 h. The mixture was
cooled to RT
and quenched with saturated aqueous solution of sodium bicarbonate. The
aqueous mixture
was extracted twice with ethyl acetate. The combined organic layers were
washed with water
followed by brine. The solution was dried over anhydrous sodium sulfate,
filtered and
concentrated under reduced pressure. The residue thus obtained was purified by
silica gel
column chromatography to yield 334 mg of the desired product. 1H NMR (400 MHz,
DMSO-
d6) 6 1.50 (s, 9H), 3.86 (s, 3H), 3.94 (t, J= 4.4 Hz, 2H), 4.40 (t, J= 4.0 Hz,
2H), 7.79 (d, J =
8.4 Hz, 1H), 7.85-7.88 (m, 2H), 8.04 (s, 1H).
Step 2: 4-
Chloro-3-((7,8-dihydro-6H-pyrimido [5,4-b][1,4]oxazin-4-yl)oxy)-N-(4-(42-
(dimethylamino)ethyl)(methyl)amino)methyl)-3-(trifluoromethyl)phenyl)benzamide
The titled compound was prepared by the reaction of tert-butyl 4-(2-chloro-5-
(methoxycarbonyl)phenoxy)-6H-pyrimido[5,4-b][1,4]oxazine-8(7H)-carboxylate
(step 1
intermediate) (100 mg, 0.24 mmol) and N1-(4-amino-2-(trifluoromethyl)benzy1)-
N1,N2,N2-
trimethylethane-1,2-diamine (Intermediate C33) (58 mg, 0.22 mmol) in the
presence of
potassium tert-butoxide (1M, 1.42 mL, 1.42 mmol) in anhydrous THF (20 mL) as
per the
procedure described in step 1 of Method A to yield 20 mg of the product.
Method B'
Preparation of 4-chloro-3-((7,8-dihydro-6H-pyrimido [5,4-b] [1,4] oxazin-4-
yl)oxy)-N-(3 -
(pip erazin-l-y1)-5 -(trifluoromethyl)phenyl)b enzamide
ci a
H
N 0 cF3
0 w
,,, 0
NO
, 1 j
NN
(N
H )
N
H
121
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
Step 1: tert-Butyl
4-(3-(4-chloro-3-((7,8-dihydro-6H-pyrimido [5,4-b] [1,4] oxazin-4-
yl)oxy)b enzamido)-5 -(trifluoromethyl)phenyl)pip erazine-l-carboxylate
ci Ai
H
N
0 so cF3
N:CX10) 0
N N
H N
ci
ri
Boc
The titled compound was prepared by the reaction of tert-butyl 4-(2-chloro-5-
(methoxycarbonyl)phenoxy)-6H-pyrimido[5,4-b][1,4]oxazine-8(7H)-carboxylate
(step 1-
Method B) (150 mg, 0.34 mmol) and tert-butyl 4-(3-amino-5-
(trifluoromethyl)phenyl)piperazine-1-carboxylate (Intermediate C39) (107 mg,
0.31 mmol) in
the presence of potassium tert-butoxide (1M, 2.0 mL, 2.06 mmol) in anhydrous
THF (10 mL)
as per the procedure described in step 1 of Method A to yield 118 mg of the
product. 1H
NMR (400 MHz, DMSO-d6) 6 1.43 (s, 9H), 3.19-3.21 (m, 4H), 3.46-3.52 (m, 6H),
4.21 (t, J
= 4.0 Hz, 2H), 6.99 (s, 1H), 7.61-7.89 (m, 7H), 10.40 (s, 1H); ESI-MS (m/z)
635 (M+H)+.
Step 2: 4-Chloro-3-((7,8-dihydro-6H-pyrimido [5 ,4-b] [1,4] oxazin-4-yl)oxy)-N-
(3-(pip erazin-
1-y1)-5 -(trifluoromethyl)phenyl)b enz amide
The titled compound was prepared by the reaction of tert-butyl 4-(3-(4-chloro-
3-((7,8-
dihydro-6H-pyrimido [5,4-b] [1,4] oxazin-4-yl)oxy)b enzamido)-5 -
(trifluoromethyl)phenyl)piperazine-l-carboxylate (step 1 intermediate) (110
mg, 0.17 mmol)
with hydrochloric acid in 1,4-dioxane (4M, 20 mL) in ethanol (4.0 mL) as per
the procedure
described in step 3 of Method A to yield 54 mg of the product.
Method C
Preparation of 4-chloro-3-((7,8-dihydro-6H-pyrimido [5,4-b] [1,4]oxazin-4-
yl)amino)-N-(4-
((4-ethylpiperazin-1-yl)methyl)-3 -(trifluoromethyl)phenyl)b enz amide
CI 0
H
HN N 0 CFn\j.=
NjC0) 0 N,
N N
H
Step 1:
4-C hloro-N-(4-((4-ethylpip erazin-l-yl)methyl)-3 -(trifluoromethyl)pheny1)-3 -
nitrobenzamide
CI 0
H
02N N
0 N,
122
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
To a stirred solution of 4-chloro-3-nitrobenzoic acid (1.0 g, 4.97 mmol) in
dichloromethane
(10 mL) were added oxalyl chloride (500 L, 5.30 mmol) followed by catalytic
amount of
DMF and the mixture was stirred at RT for 2 h. The mixture was concentrated
under reduced
pressure and the residue was dissolved in dichloromethane. The solution was
cooled to 0 C;
4-((4-ethylpiperazin-1-yl)methyl)-3-(trifluoromethyl)aniline (Intermediate Cl)
(1.0 g, 3.30
mmol) was added to the reaction mixture followed by DIPEA (1.5 mL, 8.30 mmol).
The
resultant mixture was stirred overnight at RT. The mixture was diluted with
water and
extracted twice with ethyl acetate. The combined organic extracts were washed
with saturated
sodium bicarbonate solution, water and brine. The solution was dried over
anhydrous sodium
sulfate, filtered and concentrated. The residue thus obtained was purified by
column
chromatography to yield 1.43 g of the desired product. 1H NMR (400 MHz, DMSO-
d6) 6
0.98 (t, J = 7.2 Hz, 3H), 2.28-2.39 (m, 10H), 3.57 (s, 2H), 7.74 (d, J = 8.4
Hz, 1H), 7.98-8.04
(m, 2H), 8.16 (s, 1H), 8.27 (dd, ./i = 2.4 Hz, .12 = 8.4 Hz, 1H), 8.65 (s,
1H), 10.77 (s, 1H).
Step 2:
3 -Amino-4-chloro-N-(4-((4-ethylpip erazin-l-yl)methyl)-3 -
(trifluoromethyl)phenyl)benzamide
CI a
H
H2N N 0 oFrN,
0 I\1.)
To a stirred solution
of 4-chloro-N-(4-((4-ethylpip erazin-l-yl)methyl)-3-
(trifluoromethyl)pheny1)-3-nitrobenzamide (step 1 intermediate) (1.4 g, 2.97
mmol) in a
mixture of ethyl methanol and water (1:1, 60 mL) were added ammonium chloride
(1.6 g,
29.7 mmol) followed by iron powder (830 mg. 14.8 mmol) in small portions at 80
C. The
mixture was stirred at 80 C for 2 h. The mixture was cooled to RT, filtered
and concentrated.
The residue was diluted with a mixture of ethyl acetate and water. The organic
layer was
separated and washed with water followed by brine and dried over anhydrous
sodium sulfate.
The solution was filtered, concentrated and the residue obtained was purified
by column
chromatography to yield 732 mg of the desired compound. 1H NMR (400 MHz, DMSO-
d6) 6
0.98 (t, J = 7.2 Hz, 3H), 2.28-2.39 (m, 10H), 3.55 (s, 2H), 5.60 (s, 2H), 7.13
(dd, ./i = 2.4 Hz,
.12 = 8.4 Hz, 1H), 7.35 (d, J= 8.4 Hz, 2H), 7.69 (d, J= 8.4 Hz, 1H), 8.01 (dd,
./i = 2.0 Hz, .12
= 8.8 Hz, 1H), 8.18 (s, 1H), 10.41 (s, 1H); ESI-MS (m/z) 441 (M+H)+.
Step 3: tert-Butyl
4-((2-chloro-5-((4-((4-ethylpiperazin-1-yl)methyl)-3 -
(trifluoromethyl)phenyl)carbamoyl)phenyl)amino)-6H-pyrimido [5,4-b] [1,4]
oxazine-8 (7 H)-
carboxylate
123
CA 03064975 2019-11-26
WO 2018/215668 PCT/EP2018/063957
CI a
H
HN N CFrN
..0 0 IW I\1)
k )
N N
I3oc
To
a stirred solution of 3 -amino-4-chloro-N-(4-((4-ethylpip erazin-l-yl)methyl)-
3 -
(trifluoromethyl)phenyl)benzamide (step 2 intermediate) (150 mg, 0.34 mmol)
and tert-butyl
4-chloro-6H-pyrimido[5,4-b][1,4]oxazine-8(7H)-carboxylate (Intermediate Al)
(102 mg,
0.37 mmol) in toluene (5.0 mL) were added sodium tert-butoxide (36 mg, 0.37
mmol),
tris(dibenzylideneacetone)dipalladium(0) (Pd2(dba)3) (12 mg, 0.01 mmol) and (
)-2,2'-
bis(diphenylphosphino)-1,1'-binaphthalene (rac-BINAP) (13 mg, 0.02 mmol) and
the
mixture was stirred at 140 C for 25 h in a sealed tube. The mixture was
diluted with ethyl
acetate and the organic mixture was washed with water followed by brine. The
organic layer
was dried over anhydrous sodium sulfate, filtered and concentrated. The
residue obtained was
purified by column chromatography to yield 67 mg of the desired compound. 1H
NMR (400
MHz, DMSO-d6) 6 0.98 (t, J = 6.8 Hz, 3H), 1.49 (s, 9H), 2.30-2.39 (m, 10H),
3.57 (s, 2H),
3.90 (t, J= 8.0 Hz, 2H), 4.15 (t, J= 8.0 Hz, 2H), 7.72 (t, J= 7.2 Hz, 2H),
7.78 (m, 1H), 8.02-
8.04 (m, 2H), 8.20 (s, 1H), 8.43 (s, 1H), 8.55 (s, 1H), 10.56 (s, 1H); ESI-MS
(m/z) 674 (M-
H)-.
Step 4:
4-Chloro-3 -47,8-dihydro-6H-pyrimido [5,4-b] [1,4] oxazin-4-yl)amino)-N-(4-((4-
ethylpip erazin-l-yl)methyl)-3 -(trifluoromethyl)phenyl)b enzamide
The titled compound was prepared by the reaction of tert-Butyl 4-((2-chloro-5-
((4-((4-
ethylpiperazin-l-yl)methyl)-3 -(trifluoromethyl)phenyl)carb
amoyl)phenyl)amino)-6H-
pyrimido[5,4-b][1,4]oxazine-8(7H)-carboxylate (step 3 intermediate) (60 mg,
0.09 mmol)
with hydrochloric acid in 1,4-dioxane (4.0 mL) in ethanol (4.0 mL) as per the
procedure
described in step 3 of Method A to yield 19 mg of the product.
Method D
Preparation of
4-chloro-N-(3-(cyanomethyl)pheny1)-3-47,8-dihydro-6H-pyrimido [5,4-
b][1,4]oxazin-4-yl)oxy)benzamide
ci AI
H
N
0 0 CN
iNic,0 0
NJ.NJ
H
124
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
To a stirred solution of tert-butyl 4-(2-chloro-5-(methoxycarbonyl)phenoxy)-6H-
pyrimido[5,4-b][1,4]oxazine-8(7H)-carboxylate (Step 1-Method B) (100 mg, 0.24
mmol) in
toluene (5.0 mL) were added 2-(3-aminophenyl)acetonitrile (31 mg, 0.24 mmol)
and
trimethyl aluminium solution (2M, 237 L, 0.47 mmol) at 0 C. The mixture was
stirred at
RT for 2 h. The mixture was poured into ice-water and extracted twice with
ethyl acetate. The
combined organic extracts were washed with brine and dried over anhydrous
sodium sulfate.
The solution was filtered and concentrated under reduced pressure. The residue
thus obtained
was purified by silica gel column chromatography to yield 68 mg of the desired
product.
Method E
Preparation of 4-chloro-N-
(3 -(cyano difluoromethyl)pheny1)-3 47, 8-dihydro-6H-
pyrimido [5 ,4-b] [1,4] oxazin-4-yl)oxy)b enzamide
ci
H F F
N
0 W 0 CN
, 0
NAC21 j
N N
H
Step 1: Ethyl
2-(3 -(3 -((te rt-butyldimethylsilyl)oxy)-4-chlorob enz amido)pheny1)-2,2-
difluoroacetate
ci
H F F
0 WI N la C)
TBDMS 0 'W 0
To a stirred solution of 3-((tert-butyldimethylsilyl)oxy)-4-chlorobenzoic acid
(600 mg, 2.09
mmol) in dichloromethane (10 mL) were added oxalyl chloride (364 L, 4.19
mmol)
followed by catalytic amount of DMF and the mixture was stirred at RT for 2 h.
The mixture
was concentrated under reduced pressure and the residue was dissolved in
dichloromethane
(10 mL). The solution was cooled to 0 C; ethyl 2-(3-aminopheny1)-2,2-
difluoroacetate
(Intermediate C45) (495 mg, 2.30 mmol) was added to the reaction mixture
followed by
DIPEA (1.12 mL, 6.27 mmol). The resultant mixture was stirred overnight at RT.
The
mixture was diluted with water and extracted twice with ethyl acetate. The
combined organic
extracts were washed with saturated sodium bicarbonate solution, water and
brine. The
solution was dried over anhydrous sodium sulfate, filtered and concentrated.
The residue thus
obtained was purified by column chromatography to yield 655 mg of the desired
product. 1H
NMR (400 MHz, DMSO-d6) 6 0.27-0.35 (m, 6H), 1.02 (s, 9H), 1.27 (t, J= 6.8 Hz,
3H), 4.33
(q, J = 7.2 Hz, 2H), 7.33 (d, J = 7.6 Hz, 1H), 7.51-7.64 (m, 4H), 7.97 (d, J =
8.4 Hz, 1H),
8.21 (s, 1H), 10.54 (s, 1H); ESI-MS (m/z) 484 (M+H)-1.
125
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
Step 2: N-(3 -(2-Amino-1,1-difluoro-2-oxo ethyl)pheny1)-4-chloro-3 -hydroxyb
enzamide
ci al
H F F
N 0 NH2
HO
0 0
A mixture of ethyl 2-(3-(3-((tert-butyldimethylsilyl)oxy)-4-
chlorobenzamido)pheny1)-2,2-
difluoroacetate (step 1 intermediate) (650 mg, 1.34 mmol) and ammonia solution
(7M in
methanol, 10 mL) was heated at 80 C for 4 h in a sealed tube. The reaction
mixture was
cooled and concentrated under reduced pressure to yield 560 mg of the desired
product. 1H
NMR (400 MHz, DMSO-d6) 6 7.32 (d, J = 8.0 Hz, 1H), 7.43-7.53 (m, 4H), 7.92 (d,
J = 8.4
Hz, 1H), 8.03 (s, 2H), 8.37 (s, 1H), 10.47 (s, 1H), 10.51 (s, 1H); ESI-MS
(m/z) 341 (M+H)+.
Step 3:
N-(3 -(2-Amino-1,1-difluoro-2-oxo ethyl)pheny1)-4-chloro-3 -47,8-dihydro-6H-
pyrimido [5,4-b] [1,4] oxazin-4-yl)oxy)benzamide
ci al
H F F
N ao NH2
0 w
N0) 0 0
I
N N
H
To a stirred solution of N-(3-(2-amino-1,1-difluoro-2-oxoethyl)pheny1)-4-
chloro-3-
hydroxybenzamide (step 2 intermediate) (250 mg, 0.73 mmol) and tert-butyl 4-
chloro-6H-
pyrimido[5,4-b][1,4]oxazine-8(7H)-carboxylate (Intermediate Al) (200 mg, 0.74
mmol) in
DMF (5.0 mL) was added cesium carbonate (360 mg, 1.10 mmol) and the mixture
was stirred
at 130 C for 3 h. The mixture was cooled to RT and quenched with saturated
aqueous
solution of sodium bicarbonate. The aqueous mixture was extracted twice with
ethyl acetate.
The combined organic layers were washed with water followed by brine. The
solution was
dried over anhydrous sodium sulfate, filtered and concentrated under reduced
pressure. The
residue thus obtained was purified by silica gel column chromatography to
yield 45 mg of the
desired product. 1H NMR (400 MHz, DMSO-d6) 6 3.51 (br s, 2H), 4.19-4.22 (m,
2H), 7.33
(d, J = 7.6 Hz, 1H), 7.51 (d, J = 8.4 Hz, 1H), 7.66-7.91 (m, 6H), 7.94 (d, J =
8.0 Hz, 1H),
8.07 (m, 1H), 8.38 (s, 1H), 10.52 (s, 1H); ESI-MS (m/z) 476 (M+H)+.
Step 4: 4-C hloro-N-(3 -(cyano difluoromethyl)pheny1)-3 -47,8-dihydro-6H-
pyrimido [5,4-
b] [1,4]oxazin-4-yl)oxy)benzamide
To
a solution of N-(3 -(2-amino-1,1-difluoro-2-oxo ethyl)pheny1)-4-chloro-3 -
((7,8-dihydro-
6H-pyrimido [5,4-b][1,4]oxazin-4-yl)oxy)benzamide (step 3 intermediate) (40
mg, 0.08
mmol) in dichloromethane (3.0 mL) was added Burgess reagent (76.8 mg, 0.32
mmol) and
the mixture was stirred at RT for 3 h. The reaction mixture was concentrated
under reduced
126
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
pressure and the residue obtained was purified by column chromatography to
yield 7.0 mg of
the desired compound.
Method F
Preparation of 4-chloro-3-((7,8-dihydro-6H-pyrimido [5,4-b] [1,4] oxazin-4-
yl)oxy)-N-(3 -
hydroxy-5-(trifluoromethyl)phenyl)benzamide
CI a
H
N
0 5 CF
C) 3
N 0
k N Nj OH
H
Step 1: 4-Chloro-3-((7,8-dihydro-6H-pyrimido [5 ,4 -b.] [1,4] oxazin-4-yl)oxy)-
N-(3 -methoxy-5 -
(trifluoromethyl)phenyl)benzamide
CI a
H
N CF $ 3
0
0
V 1 j
OCH3
N N
H
The titled compound was prepared by the reaction of tert-butyl 4-(2-chloro-5-
(methoxycarbonyl)phenoxy)-6H-pyrimido[5,4-b][1,4]oxazine-8(7H)-carboxylate
(step 1-
Method B) (100 mg, 0.24 mmol) and 3-methoxy-5-(trifluoromethyl)aniline (45 mg,
0.24
mmol) in the presence of potassium tert-butoxide (1M, 1.42 mL, 1.42 mmol) in
anhydrous
THF (3.0 mL) as per the procedure described in step 1 of Method A to yield 75
mg of the
product. 1H NMR (400 MHz, DMSO-d6) 6 3.51 (br s, 2H), 3.83 (s, 3H), 4.20 (t, J
= 4.0 Hz,
2H), 7.00 (s, 1H), 7.69-7.90 (m, 7H), 10.56 (s, 1H); ESI-MS (m/z) 481 (M+H)+.
Step 2: 4-Chloro-3-((7,8-dihydro-6H-pyrimido [5,4-b] [1,4] oxazin-4-yl)oxy)-N-
(3 -hydroxy-5 -
(trifluoromethyl)phenyl)benzamide
To a solution of 4-chloro-3-((7,8-dihydro-6H-pyrimido[5,4-b][1,4]oxazin-4-
yl)oxy)-N-(3-
methoxy-5-(trifluoromethyl)phenyl)benzamide (step 1 intermediate) (70 mg, 0.14
mmol) in
dichloromethane (2.0 mL) was added boron tribromide (1M in dichloromethane,
1.2 mL, 1.16
mmol) at 0 C and the mixture was stirred at RT for 4 h. The reaction mixture
was quenched
with methanol at 0 C and concentrated under reduced pressure. The residue was
diluted with
ethyl acetate and washed with aqueous sodium bicarbonate solution. The organic
layer was
washed with brine and dried over anhydrous sodium sulfate. The solution was
filtered,
concentrated and the residue was purified by flash column chromatography to
yield 25 mg of
the desired compound.
127
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
Method G
Preparation of 4-chloro-N-(4-((4-ethylpip erazin-l-yl)methyl)-3 -
(trifluoromethyl)pheny1)-3 -
((5 -oxo-5 ,6,7,8-tetrahydropyrido [2,3 -d]pyrimidin-4-yl)oxy)b enz amide
CI Al
0 N CFrN
N-3::) 0 N,)
/
HN
Step 1: 4-C
hloro-N-(4-((4-ethylpip erazin-l-yl)methyl)-3 -(trifluoromethyl)pheny1)-3 -
hydroxybenzamide
CI opHO N CFN
0 Ir N,)
The titled compound was prepared by the reaction of methyl 4-chloro-3-
hydroxybenzoate
(Intermediate B 1) (260 mg, 1.39 mmol) and 4-((4-ethylpiperazin-1-yl)methyl)-3-
(trifluoromethyl)aniline (Intermediate Cl) (400 mg, 1.39 mmol) in the presence
of potassium
tert-butoxide (1M, 8.4 mL, 8.39 mmol) in anhydrous THF (8.0 mL) as per the
procedure
described in step 1 of Method A to yield 200 mg of the product. 1H NMR (400
MHz, DMSO-
d6) 6 0.98 (t, J = 7.2 Hz, 3H), 2.33-2.55 (m, 6H), 3.33 (br s, 4H), 3.56 (s,
2H), 7.42-7.53 (m,
3H), 7.70 (d, J = 8.4 Hz, 1H), 8.01 (dd, ./i = 2.0 Hz, .1-2 = 8.4 Hz, 1H),8.32
(s, 1H), 10.50 (s,
1H), 10.60 (br s, 1H).
Step 2: 4-Chloro-N-(4-((4-ethylpiperazin-1-yl)methyl)-3 -
(trifluoromethyl)pheny1)-3 4(844-
methoxyb enzy1)-5 -oxo-5 ,6,7,8-tetrahydropyrido [2,3 -d] pyrimidin-4-yl)oxy)b
enzamide
CI Ai
0 N CFN
0 N)
/
Pmd
To a stirred solution of
4-chloro-N-(4-((4-ethylpip erazin-l-yl)methyl)-3-
(trifluoromethyl)pheny1)-3-hydroxybenzamide (step 1 intermediate) (872 mg,
1.97 mmol)
and
4-chloro-8-(4-methoxybenzy1)-7,8-dihydropyrido [2,3 -d]pyrimidin-5 (6H)-one
(Intermediate A9) (600 mg, 1.97 mmol) in DMF (20 mL) was added cesium
carbonate (3.2 g,
9.85 mmol) and the mixture was stirred at 50 C for 3 h. The mixture was
cooled to RT and
quenched with water. The aqueous mixture was extracted twice with ethyl
acetate. The
combined organic layers were washed with water followed by brine. The solution
was dried
over anhydrous sodium sulfate, filtered and concentrated under reduced
pressure. The residue
128
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
thus obtained was purified by silica gel column chromatography to yield 400 mg
of the
desired product. 1H NMR (400 MHz, DMSO-d6) 6 0.99 (t, J = 7.2 Hz, 3H), 2.40-
2.51 (m,
10H), 2.68 (t, J = 7.6 Hz, 2H), 3.57 (s, 2H), 3.65 (t, J = 6.8 Hz, 2H), 3.74
(s, 3H), 4.90 (s,
2H), 6.92 (d, J = 8.8 Hz, 2H), 7.29 (d, J = 8.4 Hz, 2H), 7.72 (d, J = 8.8 Hz,
1H), 7.78 (d, J =
8.0 Hz, 1H), 7.91-7.94 (br s, 2H), 8.04 (dd, Ji = 1.6 Hz, .1-2 = 8.4 Hz, 1H),
8.18 (s, 1H), 8.24
(s, 1H), 10.58 (s, 1H); ESI-MS (m/z) 709 (M+H)+.
Step 3: 4-chloro-N-(4-((4-ethylpip erazin-l-yl)methyl)-3 -
(trifluoromethyl)pheny1)-3 -45 -oxo-
5,6,7,8-tetrahydropyrido [2,3 -d]pyrimidin-4 -yl)oxy)b enzamide
A solution of 4-chloro-N-(4-((4-ethylpiperazin-1-yl)methyl)-3-
(trifluoromethyl)pheny1)-3-
((8-(4-methoxyb enzy1)-5 -oxo-5 ,6,7,8-tetrahydropyrido [2,3 -d]pyrimidin-4-
yl)oxy)b enz amide
(step 2 intermediate) (40 mg, 0.06 mmol) in a mixture of dichloroethane and
trifluoroacetic
acid (1:1, 600 L) was stirred overnight at 80 C. The mixture was cooled to
RT and then to
0 C before the addition of aqueous sodium bicarbonate solution till pH 7-8.
The aqueous
mixture was extracted twice with dichloromethane and the combined organic
layers were
washed with water followed by brine. The solution was dried over anhydrous
sodium sulfate,
filtered and concentrated under reduced pressure. The residue thus obtained
was purified by
silica gel column chromatography to yield 12 mg of the desired product.
Method G'
Preparation of 4-chloro-N-(3 -(4-methylpip erazin-l-y1)-5 -
(trifluoromethyl)pheny1)-3 -47-oxo-
7,8-dihydro-6H-pyrimido [5,4-b] [1,4]oxazin-4-yl)oxy)benzamide
CI
H
N 0 u3
0 Wi
0
.ciD
NC 1 1
1\l'N'.0 C\ij
H
N
I
Step 1:
4-C hloro-3 -hydroxy-N-(3 -(4-methylpip erazin-l-y1)-5 -
(trifluoromethyl)phenyl)benzamide
ci HO IN H
N
I 40 cF3
0
(Nj
N
I
.. The titled compound was prepared by the reaction of methyl 4-chloro-3-
hydroxybenzoate
(Intermediate B1) (3.6 g, 19.3 mmol)
and 3 -(4-methylpip erazin-l-y1)-5 -
129
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
(trifluoromethyl)aniline (Intermediate C19) (5.0 g, 19.3 mmol) in the presence
of potassium
tert-butoxide (1M, 115 mL, 115 mmol) in anhydrous THF (50 mL) as per the
procedure
described in step 1 of Method A to yield 2.6 g of the product. 1H NMR (400
MHz, DMSO-
d6) 6 2.23 (s, 3H), 2.47 (s, 4H), 3.21 (br s, 4H), 6.95 (s, 1H), 7.42-7.44 (m,
1H), 7.51-7.53 (m,
2H), 7.60 (s, 1H), 7.65 (s, 1H), 10.32 (s, 1H), 10.60 (s, 1H).
Step 2:
4-Chloro-3 -48-(4-methoxyb enzy1)-7-oxo-7,8-dihydro-6H-pyrimido [5,4-
b][1,4] oxazin-4-yl)oxy)-N-(3 -(4-methylpip erazin-l-y1)-5 -
(trifluoromethyl)phenyl)b enz amide
ci
0 CF3
NC''
N N-0 rN
PMBN)
To a stirred solution
of 4-chloro-3 -hydroxy-N-(3 -(4-methylpip erazin-l-y1)-5 -
(trifluoromethyl)phenyl)benzamide (step 1 intermediate) (80 mg, 0.19 mmol) and
4-chloro-
8-(4-methoxybenzy1)-6H-pyrimido[5,4-b][1,4]oxazin-7(8H)-one (Intermediate A2)
(118 mg,
0.38 mmol) in DMSO (0.5 mL) was added cesium fluoride (88 mg, 0.58 mmol) and
the
mixture was stirred at 118 C for 5-7 h. The mixture was cooled to RT and
quenched with
water. The precipitated solid was collected through filtration and dried under
vacuum. The
crude compound was purified by flash column chromatography to yield 110 mg of
the
desired product. 1H NMR (400 MHz, DMSO-d6) 6 2.24 (s, 3H), 2.52-2.56 (m, 4H),
3.18-3.22
(m, 4H),3.72 (s, 3H), 5.04 (s, 2H), 5.15 (s, 2H), 6.88 (d, J= 8.8 Hz, 2H),
6.97 (s, 1H), 7.32
(d, J = 8.8 Hz, 2H), 7.59 (s, 1H), 7.64 (s, 1H), 7.82 (d, J = 8.0 Hz, 1H),
7.93-7.98 (m, 2H),
8.22 (s, 1H), 10.41 (s, 1H).
Step 3: 4-Chloro-N-(3 -(4-methylpip erazin-l-y1)-5 -(trifluoromethyl)pheny1)-3
dihydro-6H-pyrimido [5,4-b] [1,4] oxazin-4-yl)oxy)b enzamide
The titled compound was prepared by the reaction of 4-chloro-3-48-(4-
methoxybenzy1)-7-
oxo-7,8-dihydro-6H-pyrimido [5 ,4-b] [1,4]oxazin-4-yl)oxy)-N-(3 -(4-methylpip
erazin-l-y1)-5 -
(trifluoromethyl)phenyl)benzamide (step 2 intermediate) (100 mg, 0.15 mmol)
with
trifluoroacetic acid (2.0 mL) as per the procedure described in step 3 of
Method G to yield 40
mg of the product.
Method H
Preparation of 4-chloro-N-(4-((4-ethylpip erazin-l-yl)methyl)-3 -
(trifluoromethyl)pheny1)-3 -
45,6,7,8-tetrahydropyrido [2,3 -d] pyrimidin-4-yl)oxy)b enzamide
130
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
CI AI
H
N 0 CFrN
N 0 1W N,)
k
N N
H
Step
1: 4-C hloro-N-(4-((4-ethylp ip erazin-l-yl)methyl)-3 -
(trifluoromethyl)pheny1)-3 -45-
hydroxy-8-(4-methoxyb enzy1)-5 ,6,7,8-tetrahydropyrido [2,3 -d]pyrimidin-4-
yl)oxy)b enzamide
ci a
H
OH 0 N CFrN
)11\1 0 Ir N,.)
I
1\1N
1;1\AB
To a solution of 4-chloro-N-(4-((4-ethylpiperazin-1-yl)methyl)-3-
(trifluoromethyl)pheny1)-3-
((8-(4-methoxybenzy1)-5-oxo-5,6,7,8-tetrahydropyrido [2,3 -d]pyrimidin-4-
yl)oxy)b enz amide
(Step 2-Method G) (500 mg, 0.76 mmol) in THF (5.0 mL) at 0 C were added
sodium
borohydride (54 mg, 0.71 mmol) followed by added methanol (1.0 mL) dropwise.
The
mixture was stirred for 3-4 h at RT. The reaction was quenched with water and
extracted
twice with chloroform. The combined organic layers were washed with water
followed by
brine. The solution was dried over anhydrous sodium sulfate, filtered and
concentrated under
reduced pressure. The residue thus obtained was purified by silica gel column
chromatography to yield 400 mg of the desired product. 1H NMR (400 MHz, DMSO-
d6)
6
(t, J = 7.2 Hz, 3H), 1.70-1.74 (m, 1H), 1.92 (d, J = 2.8 Hz, 1H), 2.45-2.56
(m,
10H), 3.50-3.60 (m, 4H), 3.78 (s, 3H), 4.77 (d, J = 14.8 Hz, 1H), 4.89 (d, J =
15.2 Hz, 1H),
5.03 (br s, 1H), 5.26 (d, J = 4.4 Hz, 1H), 6.90 (dd, ./i = 2.0 Hz, ./i = 6.8
Hz, 2H), 7.24 (d, J=
8.8 Hz, 2H), 7.71-8.18 (m, 7H), 10.59 (s, 1H); ESI-MS (m/z) 711 (M+H)+.
Step 2: 4-Chloro-N-(4-((4-ethylpiperazin-1-yl)methyl)-3-
(trifluoromethyl)pheny1)-3-((8-(4-
methoxybenzy1)-5,6,7,8-tetrahydropyrido [2,3 -d]pyrimidin-4-yl)oxy)b enzamide
CI a
H
O N CFrN
0 ir N,)
I
NI\I
14/1B
To a stirred mixture of
4-chloro-N-(4-((4-ethylpip erazin-l-yl)methyl)-3-
(trifluoromethyl)pheny1)-3 -45 -hydroxy-8-(4-methoxyb enzy1)-5 ,6,7,8-
tetrahydropyrido [2,3 -
d]pyrimidin-4-yl)oxy)benzamide (step 1 intermediate) (400 mg, 0.56 mmol) in
triethylsilane
(16 mL) at 0 C was added trifluoroacetic acid (8.0 mL) dropwise and the
mixture was stirred
131
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
for 3-4 h at RT. The mixture was cooled to 0 C and neutralized using aqueous
sodium
bicarbonate solution. The aqueous mixture was extracted twice with chloroform.
The
combined organic layers were washed with water followed by brine. The solution
was dried
over anhydrous sodium sulfate, filtered and concentrated under reduced
pressure. The residue
thus obtained was purified by silica gel column chromatography to yield 200 mg
of the
desired product. 1H NMR (400 MHz, DMSO-d6) 6 1.09 (m, 3H), 1.89 (m, 2H), 2.50
(br s,
10H), 2.76 (m, 2H), 3.51-3.62 (m, 4H), 3.73 (s, 3H), 4.78 (s, 2H), 6.90 (d, J
= 8.4 Hz, 2H),
7.22 (d, J= 8.8 Hz, 2H), 7.71-7.92 (m, 4H), 8.00 (s, 1H), 8.06 (d, J= 7.2 Hz,
1H), 8.18 (s,
1H), 10.57 (s, 1H); ESI-MS (m/z) 695 (M+H)+.
Step 3: 4-C hloro-N-(4-((4-ethylpip erazin-l-yl)methyl)-3 -
(trifluoromethyl)pheny1)-3 -
45,6,7,8-tetrahydropyrido [2,3 -d]pyrimidin-4-yl)oxy)b enzamide
The titled compound was prepared by the reaction of 4-chloro-N-(444-
ethylpiperazin-1-
yl)methyl)-3-(trifluoromethyl)pheny1)-348-(4-methoxybenzy1)-5,6,7,8-
tetrahydropyrido [2,3 -
d]pyrimidin-4-yl)oxy)benzamide (step 2 intermediate) (100 mg, 0.14 mmol) with
trifluoroacetic acid (2.0 mL) as per the procedure described in step 3 of
Method G to yield 25
mg of the product.
Method I
Preparation of 1-(4-chloro-3-((7,8-dihydro-6H-pyrimido [5,4-b] [1,4]oxazin-4-
yl)oxy)pheny1)-
3 -(4-((4-ethylpip erazin-l-yl)methyl)-3 -(trifluoromethyl)phenyl)urea
ci 0
NA SI N
O CF-'1\i'=
NO H H
I j
NN
H
Step 1: tert-Butyl 4-(5-amino-2-chlorophenoxy)-6H-pyrimido[5,4-b][1,4]oxazine-
8 (7 H)-
carboxylate
CI A
O NH2
c,
t 1 j
NII
Boc
The titled compound was prepared by the reaction of 5-amino-2-chlorophenol (80
mg, 0.55
mmol) and tert-butyl 4-chloro-6H-pyrimido[5,4-b][1,4]oxazine-8(7H)-carboxylate
(Intermediate Al) (150 mg, 0.55 mmol) in the presence of cesium carbonate (540
mg, 1.65
mmol) in DMF (3.0 mL) as per the procedure described in step 2 of Method G to
yield 130
132
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
mg of the compound. 41 NMR (400 MHz, DMSO-d6) 6 1.50 (s, 9H), 3.92 (t, J = 4.4
Hz, 2H),
4.37 (t, J = 4.0 Hz, 2H), 5.44 (s, 2H), 6.41 (d, J = 6.4 Hz, 1H), 6.47 (dd,
./i = 2.4 Hz, J2 = 8.4
Hz, 1H), 7.13 (d, J= 8.8 Hz, 1H), 8.04 (s, 1H); ESI-MS (m/z) 379 (M+H)-1.
Step 2: te rt-Butyl 4-(2-chloro-5 -(3 -(4-((4-ethylpip erazin-
l-yl)methyl)-3 -
(trifluoromethyl)phenyl)ureido)phenoxy)-6H-pyrimido [5,4-b] [1,4] oxazine-
8(7H)-carboxylate
ci
0 lei0 N
A r,I\L
N N CF3 ¨
,0 H H
NC 1 )
NN
6oc
To a stirred solution of 4-((4-ethylpiperazin-1-yl)methyl)-3-
(trifluoromethyl)aniline
(Intermediate Cl) (150 mg, 0.52 mmol) in THF (20 mL) was added triphosgene (54
mg, 0.18
mmol) and the resulting mixture was stirred at 70 C. After 1 h of heating the
mixture was
concentrated under reduced pressure and the residue was dissolved in THF (20
mL). That
solution was added dropwise to a stirred mixture of tert-butyl 4-(5-amino-2-
chlorophenoxy)-
6H-pyrimido[5,4-b][1,4]oxazine-8(7H)-carboxylate (step 1 intermediate) (150
mg, 0.40
mmol) and triethylamine (172 L, 1.19 mmol) in THF (20 mL) at RT. The
resultant mixture
was stirred for 2 h at 70 C. The mixture was cooled to RT and quenched with
water. The
aqueous mixture was extracted twice with ethyl acetate. The combined organic
layers were
washed with water followed by brine. The solution was dried over anhydrous
sodium sulfate,
filtered and concentrated under reduced pressure. The residue thus obtained
was purified by
silica gel column chromatography to yield 100 mg of the desired product. 1H
NMR (400
MHz, DMSO-d6) 6 1.09 (t, J= 7.2 Hz, 3H), 1.50 (s, 9H), 2.42-2.51 (m, 10H),
3.54 (br s, 2H),
3.94 (t, J= 8.4 Hz, 2H), 4.40 (t, J= 7.6 Hz, 2H), 7.29 (dd, ./i = 2.4 Hz, .1-2
= 8.0 Hz, 1H), 7.48
(d, J = 8.8 Hz, 1H), 7.55-7.94 (m, 3H), 7.95 (s, 1H), 8.05 (s, 1H), 9.15 (s,
1H), 9.20 (s, 1H).
Step 3: 1-(4-Chloro-3-((7,8-dihydro-6H-pyrimido [5,4-b] [1,4]oxazin-4-
yl)oxy)pheny1)-3 -(4-
((4-ethylpip erazin-l-yl)methyl)-3 -(trifluoromethyl)phenyOurea
The titled compound was prepared by the reaction of tert-butyl 4-(2-chloro-5-
(3-(4-((4-
ethylpiperazin-l-yl)methyl)-3-(trifluoromethyl)phenyl)ureido)phenoxy)-6H-
pyrimido [5,4-
b][1,4]oxazine-8(7H)-carboxylate (step 2 intermediate) (100 mg, 0.14 mmol)
with
hydrochloric acid in 1,4-dioxane (2.0 mL) as per the procedure described in
step 3 of Method
A to yield 52 mg of the product.
Method I'
133
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
Preparation of 1-(4-((7,8-dihydro-6H-pyrimido [5,4-b] [1,4] oxazin-4-
yl)oxy)pheny1)-3 -(3 -
(trifluoromethyl)phenyl)urea
H H
N N CF3
NO
140
0
I )
NN
Step 1: Benzyl (4-(3-(3-(trifluoromethyl)phenyl)ureido)phenyl) carbonate
H H
NN CF3
Cbz0 , W 0
The titled compound was prepared by the reaction of 3-(trifluoromethyl)aniline
(218 mg, 1.34
mmol), triphosgene (138 mg, 0.47 mmol) and 4-aminophenylbenzylcarbonate (250
mg, 0.89
mmol) in the presence of triethylamine (361 mg, 3.57 mmol) in THF (5.0 mL) as
per the
procedure described in step 2 of Method I to yield 200 mg of the titled
compound. 1H NMR
(400 MHz, DMSO-d6) 6 5.07 (s, 2H), 6.95 (d, J = 2.0 Hz, 2H), 7.29-7.57 (m,
10H), 8.01 (s,
1H), 8.61 (s, 1H), 8.98 (s, 1H).
Step 2: 1-(4-Hydroxypheny1)-3 -(3 -(trifluoromethyl)phenyl)urea
H H
NN c3
HO 0
A solution of benzyl (4-(3-(3-(trifluoromethyl)phenyl)ureido)phenyl)carbonate
(step 1
intermediate) (200 mg, 0.47 mmol) in methanol (5.0 mL) with catalytic amount
of 10%
palladium on carbon (50% wet, 200 mg) was hydrogenated at RT for 18 h. The
mixture was
diluted with chloroform filtered through celite and the celite bed. The
filtrate was
concentrated and the residue thus obtained was purified by silica gel column
chromatography
to yield 48 mg of the desired product. 1H NMR (400 MHz, DMSO-d6) 6 6.69 (d, J
= 6.8 Hz,
2H), 7.22 (d, J = 8.8 Hz, 2H), 7.27 (d, J = 7.6 Hz, 1H), 7.48-7.53 (m, 2H),
8.0 (s, 1H), 8.44
(s, 1H), 8.91 (s, 1H), 9.11 (s, 1H); ESI-MS (m/z) 397 (M+H)+.
Step 3: tert-Butyl 44443 -(3 -(trifluoromethyl)phenyl)ureido)phenoxy)-6H-
pyrimido [5,4-
[1,4]oxazine-8(7H)-carboxylate
H H
NN CF3
0 WI
N:CC3')
N N
13oc
134
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
The titled compound was prepared by the reaction of 1-(4-hydroxypheny1)-3-(3-
(trifluoromethyl)phenyl)urea (step 2 intermediate) (50 mg, 0.17 mmol) and tert-
butyl 4-
chloro-6H-pyrimido[5,4-b][1,4]oxazine-8(7H)-carboxylate (Intermediate Al) (59
mg, 0.22
mmol) in the presence of cesium carbonate (109 mg, 0.34 mmol) in DMF (0.5 mL)
as per the
procedure described in step 2 of Method G to yield 30 mg of the compound. 1H
NMR (400
MHz, DMSO-d6) 6 1.48 (s, 9H), 3.34-3.39 (m, 1H), 3.91 (t, J= 4.4 Hz, 2H), 4.36
(t, J= 4.0
Hz, 2H), 7.10-7.12 (m, 2H), 7.50-7.58 (m, 4H), 8.02-8.04 (br s, 2H), 8.67 (s,
1H), 9.07 (s,
1H); ESI-MS (m/z) 432 (M+H)-1.
Step 4:
1-(4-((7,8-Dihydro-6H-pyrimido[5,4-b] [1,4]o xazin-4-yl)oxy)pheny1)-3 -(3 -
(trifluoromethyl)phenyl)urea
The titled compound was prepared by the reaction of tert-butyl 4444343-
(trifluoromethyl)phenyl)ureido)phenoxy)-6H-pyrimido [5,4-b] [1,4] oxazine-
8(7H)-carboxylate
(step 3 intermediate) (30 mg, 0.06 mmol) with hydrochloric acid in 1,4-dioxane
(1.0 mL) as
per the procedure described in step 3 of Method A to yield 11 mg of the
product.
Method I"
Preparation of 1-(3-(tert-buty1)-1-(4-cyanopheny1)-1H-pyrazol-5-y1)-3-(4-43,4-
dihydro-2H-
pyrido [3 ,2-b] [1,4] oxazin-8-yl)oxy)phenyl)urea
CN
4Ik
H H
0
N N N Is 1 ,N
0
ri:))
N N
H
Step 1: tert-Butyl
8-(4-(((benzyloxy)carbonyl)amino)phenoxy)-2H-pyrido [3,2-
b] [1,4]oxazine-4(3H)-carboxylate
H
NO 40
0 'S
0
x ,0
N N
Boc
A suspension of tert-butyl 8-bromo-2H-pyrido[3,2-b][1,4]oxazine-4(3H)-
carboxylate
(Intermediate A7) (400 mg, 1.27 mmol), benzyl (4-hydroxyphenyl)carbamate (370
mg, 1.52
mmol) and tripotassium phosphate (966 mg, 4.56 mmol) in toluene (10 mL) was
degassed
and added palladium acetate (52 mg, 0.24 mmol) followed by 2-di-tert-
butylphosphino-
135
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
2',4',6'-triisopropylbiphenyl (t-BuXPhos) (129 mg, 0.30 mmol) at RT. The
mixture was
refluxed at 120 C for 16 h. The mixture was cooled to RT, diluted with
diethyl ether and
filtered through celite. The filtrate was concentrated and the residue thus
obtained was
purified by flash column chromatography to yield 198 mg of the desired
product. 'I-1 NMR
(400 MHz, DMSO-d6) 6 1.47 (s, 9H), 3.86 (t, J= 4.4 Hz, 2H), 4.27 (t, J = 4.4
Hz, 2H), 5.16
(s, 2H), 6.45 (d, J = 5.6 Hz, 1H), 7.04-7.09 (m, 2H), 7.34-7.46 (m, 5H), 7.52
(d, J = 8.8 Hz,
2H), 7.79 (d, J= 5.6 Hz, 1H), 9.86 (s, 1H); ESI-MS (m/z) 478 (M+H)+.
Step 2: tert-Butyl 8-(4-aminophenoxy)-2H-pyrido[3,2-b][1,4]oxazine-4(3H)-
carboxylate
Ai NH2
0
1 )
N N
Lc
The titled compound was prepared by the hydrogenation reaction of tert-butyl 8-
(4-
(((benzyloxy)carbonyl)amino)phenoxy)-2H-pyrido [3,2-b] [1,4] oxazine-4(3H)-
carboxylate
(step 1 intermediate) (230 mg, 0.48 mmol) I the presence of 10% palladium on
carbon (50%
wet, 40 mg) as per the procedure described in step 2 of Method I' to yield 127
mg of the
desired compound. The compound was as such taken forward to the next step
without
characterization.
Step 3: tert-Butyl 84443 -(3 -(tert-butyl)-1-(4-cyanopheny1)-
1H-pyrazol-5 -
yl)ureido)phenoxy)-2H-pyrido [3,2-b] [1,4] oxazine-4(3H)-c arbo xylate
ON
410
H H
Al NyN . NIN
0 1
0
I CILJ
N N
60c
To a stirred solution of 4-(5-amino-3-(tert-buty1)-1H-pyrazol-1-
y1)benzonitrile (Intermediate
C91) (50 mg, 0.21 mmol) in a mixture of dichloromethane (6.0 mL) and saturated
sodium
bicarbonate solution (4.0 mL) at 0 C was added diphosgene (100 L, 0.83 mmol)
and the
mixture was stirred at RT for 1.5 h. The mixture was diluted with
dichloromethane, washed
with brine, dried over anhydrous sodium sulfate and concentrated under reduced
pressure to
give the isocyanate. The isocyanate intermediate was suspended in a mixture of
THF (10 mL)
and acetonitrile (1.0 mL) and cooled 0 C. To that mixture were added tert-
butyl 8-(4-
136
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
aminophenoxy)-2H-pyrido[3,2-b][1,4]oxazine-4(3H)-carboxylate (step 2
intermediate) (71
mg, 0.21 mmol) followed by DIPEA (142 L, 0.83 mmol) and the resultant mixture
was
stirred at RT for 16 h. The mixture was diluted with ethyl acetate and washed
with water
followed by brine. The organic layer was dried over anhydrous sodium sulfate,
filtered and
concentrated under reduced pressure. The residue was purified by silica gel
column
chromatography to yield 52 mg of the desired compound. ESI-MS (m/z) 610
(M+H)+.
Step 3: 1-(3 -(tert-Butyl)-1-(4-cyanopheny1)-1H-pyrazol-5 -y1)-3 -
(443 ,4-dihydro-2H-
pyrido [3 ,2-b] [1,4] oxazin-8-yl)oxy)phenyl)urea
The titled compound was prepared by the reaction of tert-butyl 8-(4-(3-(3-
(tert-buty1)-1-(4-
cyanopheny1)-1H-pyrazol-5-yOureido)phenoxy)-2H-pyrido [3,2-b] [1,4] oxazine-
4(3H)-
carboxylate (step 2 intermediate) (50 mg, 0.08 mmol) with hydrochloric acid in
1,4-dioxane
(3.0 mL) in methanol (0.5 mL) as per the procedure described in step 3 of
Method A to yield
7.0 mg of the product.
Method J
Preparation of 4-chloro-N-(4-((4-ethylpip erazin-l-yl)methyl)-3 -
(trifluoromethyl)pheny1)-3 -
47-oxo-7,8-dihydro-6H-pyrimido [5,4-b] [1,4]oxazin-4-yl)oxy)benzamide
CI al
O WI NNO CFri\j.=
0
k
NNO
Step 1: Methyl 4-chloro-3-48-(4-methoxybenzy1)-7-oxo-7,8-dihydro-6H-pyrimido
[5,4-
b][1,4]oxazin-4-yl)oxy)benzoate
ci
O WI 0,
0
C)
N
N N-0
I;MB
The titled compound was prepared by the reaction of 4-chloro-8-(4-
methoxybenzy1)-6H-
pyrimido[5,4-b][1,4]oxazin-7(8H)-one (Intermediate A2) (330 mg, 1.08 mmol) and
4-chloro-
3-hydroxybenzoate (Intermediate B1) (261 mg, 1.40 mmol) in the presence of
cesium
fluoride (328 mg, 2.16 mmol) in DMSO (2.0 mL) as per the procedure described
in step 2 of
Method G' to yield 210 mg of the compound. 1H NMR (400 MHz, DMSO-d6) 6 3.72
(s, 3H),
3.86 (s, 3H), 5.02 (s, 2H), 5.14 (s, 2H), 6.87 (dd, ./1 = 2.0 Hz, .1-2 = 6.4
Hz, 2H), 7.31 (d, J=
6.8 Hz, 2H), 7.79 (d, J = 8.8 Hz, 1H), 7.88-7.90 (m, 2H), 8.19 (s, 1H).
137
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
Step 2: 4-Chloro-N-(4-((4-ethylpiperazin-1-yl)methyl)-3 -
(trifluoromethyl)pheny1)-3 4(844-
methoxyb enzy1)-7-oxo-7,8-dihydro-6H-pyrimido [5,4-b] [1,4] oxazin-4-yl)oxy)b
enzamide
CI
o N CFrN
0
I ,L
NN 0
PMB
To a solution of methyl 4-chloro-348-(4-methoxybenzy1)-7-oxo-7,8-dihydro-6H-
pyrimido[5,4-b][1,4]oxazin-4-yl)oxy)benzoate (step 1 intermediate) (210 mg,
0.46 mmol)
and 4-((4-ethylpiperazin-1-yl)methyl)-3-(trifluoromethyl)aniline (Intermediate
Cl) (110 mg,
0.38 mmol) in toluene (3.0 mL) was dropwise added trimethyl aluminium solution
(2M in
toluene, 768 L, 1.54 mmol) at RT. The mixture was stirred for 3-5 h at RT.
The mixture was
quenched with aqueous ammonium chloride solution and extracted twice in ethyl
acetate. The
combined organic layers were washed with water followed by brine. The solution
was dried
over anhydrous sodium sulfate, filtered and concentrated under reduced
pressure. The residue
thus obtained was purified by silica gel column chromatography to yield 115 mg
of the
desired product. 1H NMR (400 MHz, DMSO-d6) 6 0.99 (t, J = 7.2 Hz, 3H), 2.40-
2.51 (m,
10H), 3.57 (s, 2H), 3.72 (s, 3H), 5.04 (s, 2H), 5.15 (s, 2H), 6.87 (dd, ./i =
2.0 Hz, .1-2 = 6.4 Hz,
2H), 7.31 (d, J= 8.8 Hz, 2H), 7.72 (d, J= 8.4 Hz, 1H), 7.83 (d, J= 8.4 Hz,
1H), 7.94-8.0 (m,
3H), 8.17 (s, 1H), 8.22 (s, 1H), 10.58 (s, 1H); ESI-MS (m/z) 711 (M+H)+.
Step 3: 4-Chloro-N-(4-((4-ethylpip erazin-l-yl)methyl)-3 -
(trifluoromethyl)pheny1)-3 -47-oxo-
7,8-dihydro-6H-pyrimido [5,4-b] [1,4]oxazin-4-yl)oxy)benzamide
The titled compound was prepared by the reaction of 4-chloro-N-(444-
ethylpiperazin-1-
yl)methyl)-3-(trifluoromethyl)pheny1)-3-48-(4-methoxybenzyl)-7-oxo-7,8-dihydro-
6H-
pyrimido [5,4 -b][1,4]oxazin-4-yl)oxy)benzamide (step 2 intermediate) (100 mg,
0.14 mmol)
with trifluoroacetic acid (3.0 mL) as per the procedure described in step 3 of
Method G to
yield 29 mg of the product.
Method .1'
Preparation of N-(4-((4-ethylpiperazin-1-yl)methyl)-3-(trifluoromethyl)pheny1)-
4-methyl-3-
47-oxo-7,8-dihydro-6H-pyrimido [5,4-b] [1,4]oxazin-4-yl)oxy)benzamide
O NH CFrN
N 0 ir
I
NNO
138
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
Step 1: Methyl 3 -48 -(4-methoxyb enzy1)-7-oxo-7,8-dihydro-6H-pyrimido [5,4-b]
[1,4] oxazin-
4-yl)oxy)-4-methylbenzoate
40 0 ,
0
.,c) 0
N
I
NN -0
PMB
A suspension of 4-chloro-8-(4-methoxybenzy1)-6H-pyrimido[5,4-b][1,4]oxazin-
7(8H)-one
(Intermediate A2) (370 mg, 1.21 mmol), methyl 3-hydroxy-4-methylbenzoate
(Intermediate
B3) (261 mg, 1.57 mmol) and tripotassium phosphate (513 mg, 2.42 mmol) in
toluene (4.0
mL) was degassed and added palladium acetate (54 mg, 0.24 mmol) followed by 2-
di-tert-
butylphosphino-2',4',6'-triisopropylbiphenyl (t-BuXPhos) (77 mg, 0.18 mmol) at
RT. The
mixture was refluxed for 3-5 h. The mixture was cooled to RT, diluted with
diethyl ether and
filtered through celite. The filtrate was concentrated and the residue thus
obtained was
purified by flash column chromatography to yield 309 mg of the desired
product. 1H NMR
(400 MHz, DMSO-d6) 6 2.19 (s, 3H), 3.72 (s, 3H), 3.83 (s, 3H), 5.01 (s, 2H),
5.14 (s, 2H),
6.88 (d, J = 8.4 Hz, 2H), 7.31 (d, J = 8.8 Hz, 2H), 7.50 (d, J = 8.0 Hz, 1H),
7.65 (s, 1H), 7.79
(dd, ./i = 2.0 Hz, .1-2 = 8.0 Hz, 1H), 8.17 (s, 1H).
Step 2: N-(4-
((4-Ethylpip erazin-l-yl)methyl)-3 -(trifluoromethyl)pheny1)-3 4(844-
methoxyb enzy1)-7-oxo-7,8-dihydro-6H-pyrimido [5,4-b] [1,4] oxazin-4-yl)oxy)-4-
methylbenzamide
0N cFn\j..
0 N
I
NN 0
PMB
The titled compound was prepared by the reaction of methyl 3-48-(4-
methoxybenzy1)-7-oxo-
7,8-dihydro-6H-pyrimido[5,4-b] [1,4]oxazin-4-yl)oxy)-4-methylb enzo ate
(step 1
intermediate) (270 mg, 0.62 mmol) with 4-((4-ethylpiperazin-1-yl)methyl)-3-
(trifluoromethyl)aniline (Intermediate Cl) (140 mg, 0.49 mmol) in the presence
of trimethyl
aluminium solution (2M in toluene, 970 L, 1.94 mmol) in toluene (3.0 mL) as
per the
procedure described in step 2 of Method J to yield 210 mg of the compound. 1H
NMR (400
MHz, DMSO-d6) 6 0.98 (t, J= 8.4 Hz, 3H), 2.19 (s, 3H), 2.40-2.51 (m, 10H),
3.56 (br s, 2H),
3.72 (s, 3H), 5.02 (s, 2H), 5.15 (s, 2H), 6.88 (d, J = 8.4 Hz, 2H), 7.32 (d, J
= 8.8 Hz, 2H),
7.52 (d, J = 8.4 Hz, 1H), 7.70 (d, J = 8.8 Hz, 1H), 7.75 (d, J = 1.2 Hz, 1H),
7.86 (d, J= 8.0
Hz, 1H), 8.04 (d, J= 7.2 Hz, 1H), 8.17-8.19 (br s, 2H), 10.44 (s, 1H).
139
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
Step 3: N-(4-((4-ethylpiperazin-1-yl)methyl)-3 -(trifluoromethyl)pheny1)-4-
methy1-3-((7-oxo-
7,8-dihydro-6H-pyrimido [5,4-b] [1,4] oxazin-4-yl)oxy)b enzamide
The titled compound was prepared by the reaction of N-(444-ethylpiperazin-1-
yl)methyl)-3-
(trifluoromethyl)pheny1)-3-48-(4-methoxybenzyl)-7-oxo-7,8-dihydro-6H-pyrimido
[5,4-
.. b] [1,4]oxazin-4-yl)oxy)-4-methylbenzamide (step 2 intermediate) (150 mg,
0.22 mmol) with
trifluoroacetic acid (3.0 mL) as per the procedure described in step 3 of
Method G to yield 18
mg of the product.
Method K
Preparation of 4-chloro -N-(4-((4-(2-cyano ac etyl)pip erazin-
1 -yl)methyl)-3 -
(trifluoromethyl)pheny1)-3 -47, 8-dihydro-6H-pyrimido [5,4-b] [1,4] oxazin-4-
yl)oxy)benzamide
ci oi
H 0
0 WI N 0 CFrN-11.õCN
N o N.,..)
1 )
To a solution of 2-cyanoacetic acid (20 mg, 0.18 mmol) in acetonitrile (5.0
mL) were added
DIPEA (0.1 mL, 0.56 mmol) followed by TBTU (50 mg, 0.18 mmol) at 0 C and the
mixture
was stirred for 30 min. 4-Chloro-3-47,8-dihydro-6H-pyrimido[5,4-b][1,4]oxazin-
4-yl)oxy)-
N-(4-(piperazin-1-ylmethyl)-3-(trifluoromethyl)phenyl)benzamide (dihydro
chloride salt) (80
mg, 0.14 mmol) was added to the mixture and stirred for 3 h at RT. The mixture
was diluted
with water and extracted twice with ethyl acetate. The combined organic layers
were washed
with water followed by brine. The solution was dried over anhydrous sodium
sulfate, filtered
and concentrated under reduced pressure. The residue thus obtained was
purified by silica gel
column chromatography to yield 6.0 mg of the desired product.
Method L
Preparation of 4-chloro-348-methy1-7,8-dihydro-6H-pyrimido[5,4-b][1,4]oxazin-4-
yl)oxy)-
N-(3 -(4-methylpip erazin-1 -y1)-5 -(trifluoromethyl)phenyl)b enz amide
ci al
H
N
0 wi so cF3
N *11:: 0
I j N
N N
I CN)
I
Step 1: Methyl 4-chloro-3-((7,8-dihydro-6H-pyrimido[5,4-b][1,4]oxazin-4-
yl)oxy)benzoate
140
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
CI AI
0 C31
1\jc.,0 0
Nk NJ
H
The titled compound was prepared by the reaction of tert-butyl 4-(2-chloro-5-
(methoxycarbonyl)phenoxy)-6H-pyrimido[5,4-b][1,4]oxazine-8(7H)-carboxylate
(step 1-
Method B) (500 mg, 1.18 mmol) with hydrochloric acid in ethyl acetate (25 mL)
in methanol
(5.0 mL) as per the procedure described in step 3 of Method A to yield 307 mg
of the
product. 1H NMR (400 MHz, DMSO-d6) 6 3.83-3.87 (m, 2H), 4.04 (s, 3H), 4.17-
4.21 (m,
2H), 7.68-7.74 (m, 3H), 7.81 (br s, 2H).
Step 2: Methyl 4-chloro-3-48-methy1-7,8-dihydro-6H-pyrimido [5,4-b]
[1,4] oxazin-4-
yl)oxy)benzoate
ci Ai
0,
0
r\ic.,0, 0
NI.N J
I
To a stirred solution of methyl 4-chloro-3-((7,8-dihydro-6H-pyrimido[5,4-
b][1,4]oxazin-4-
yl)oxy)benzoate (step 1 intermediate) (300 mg, 0.93 mmol) in DMF (5.0 mL) were
added
methyl iodide (132 mg, 0.93 mmol) followed by sodium hydride (60% w/w, 41 mg,
1.03
mmol) at 0 C and the mixture was stirred overnight at RT. The solvent was
removed under
reduced pressure and the residue was dissolved in dichloromethane. The
solution was washed
with water, dried over anhydrous sodium sulfate, filtered and concentrated
under reduced
pressure. The residue was purified by silica gel column chromatography to
yield 105 mg of
the desired product. 1H NMR (400 MHz, DMSO-d6) 6 3.34 (s, 3H), 3.58 (t, J =
4.8 Hz, 2H),
3.85 (s, 3H), 4.27 (t, J = 4.4 Hz, 2H), 7.71-7.78 (m, 2H), 7.80-7.83 (m, 2H).
Step 3: 4-Chloro-3-48-methy1-7,8-dihydro-6H-pyrimido [5,4-b] [1,4] oxazin-4-
yl)oxy)-N-(3 -
(4-methylpip erazin-l-y1)-5 -(trifluoromethyl)phenyl)b enz ami de
The titled compound was prepared by the reaction of methyl 4-chloro-348-methy1-
7,8-
dihydro-6H-pyrimido[5,4-b][1,4]oxazin-4-yl)oxy)benzoate (step 2 intermediate)
(100 mg,
0.30 mmol) and 3-(4-methylpiperazin-1-y1)-5-(trifluoromethyl)aniline
(Intermediate C19)
(78 mg, 0.30 mmol) in the presence of potassium tert-butoxide (1M, 1.9 mL, 1.8
mmol) in
anhydrous THF (5.0 mL) as per the procedure described in step 1 of Method A to
yield 81
mg of the product.
141
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
Method M
Preparation of N-(4-((7,8-dihydro-6H-pyrimido [5,4-b] [1,4] oxazin-4-yl)oxy)-3
-fluoropheny1)-
N-(4-fluorophenyl)cyclopropane-1,1-dicarboxamide
F EAr
0 . 00 110
F
),,0
INC I j
N N
H
Step 1: tert-Butyl 4-(4-amino-2-fluorophenoxy)-6H-pyrimido[5,4-b][1,4]oxazine-
8 (7 H)-
carboxylate
F NH2
0
0
NiC )
N N
eoc
The titled compound was prepared by the reaction of tert-butyl 4-chloro-6H-
pyrimido[5,4-
b.] [1,4]oxazine-8(7H)-carboxylate (Intermediate Al) (500 mg, 1.83 mmol) with
4-amino-2-
fluorophenol (350 mg, 2.76 mmol) in the presence of cesium carbonate (1.2 g,
3.67 mmol) in
DMF (10 mL) as per the procedure described in step 2 of Method A to yield 361
mg of the
compound. 1H NMR (400 MHz, DMSO-d6) 6 1.49 (s, 9H), 3.90 (t, J= 4.4 Hz, 2H),
4.35 (t, J
= 4.0 Hz, 2H), 5.36 (s, 2H), 6.36 (dd, ./i = 2.5 Hz, J2 = 6.8 Hz, 1H), 6.48
(dd, ./i = 2.8 Hz, J2
= 13.2 Hz, 1H), 6.92 (t, J= 8.8 Hz, 1H),7.95 (s, 1H); ESI-MS (m/z) 363 (M+H)+.
Step 2: tert-Butyl 4-
(2-fluoro-4-(1-((4-
fluorophenyl)carbamoyl)cyclopropanecarboxamido)phenoxy)-6H-pyrimido [5,4-
b.] [1,4]oxazine-8(7H)-carboxylate
F 1 NF 1 A r Fr\-1 I
0 0 0 0
F
0
N: )
N N
6oc
To a stirred solution of tert-butyl 4-(4-amino-2-fluorophenoxy)-6H-
pyrimido[5,4-
b][1,4]oxazine-8(7H)-carboxylate (step 1 intermediate) (161 mg, 0.45 mmol) in
dichloromethane (5.0 mL) were added DIPEA (138 L, 0.80 mmol), 1-((4-
fluorophenyl)carbamoyl)cyclopropanecarboxylic acid (Intermediate C68) (100 mg,
0.45
mmol mmol) followed by HATU (305 mg, 0.80 mmol) at RT. The mixture was stirred
at RT
for 18 h before quenching it with water. The layers were separate and the
aqueous layer was
extracted twice with ethyl acetate. The combined organic layers were washed
with brine and
142
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
dried over anhydrous sodium sulfate. The solution was filtered and
concentrated under
reduced pressure to yield 75 mg of the titled compound. . 1H NMR (400 MHz,
DMSO-d6) 6
1.45-1.46 (br s, 4H), 1.49 (s, 9H), 3.92 (t, J= 4.4 Hz, 2H), 4.38 (t, J= 3.6
Hz, 2H), 7.15 (t, J
= 9.2 Hz, 2H), 7.28 (t, J = 7.2 Hz, 1H), 7.41 (d, J = 8.8 Hz, 1H), 7.62- 7.89
(m, 3H), 8.03 (s,
1H), 10.02 (s, 1H), 10.30 (s, 1H).
Step 3: N-(4-((7,8-Dihydro-6H-pyrimido [5,4-b] [1,4] oxazin-4-yl)oxy)-3 -
fluoropheny1)-N-(4-
fluorophenyl)cyclopropane-1,1-dicarboxamide
The titled compound was prepared by the reaction of tert-Butyl 4-(2-fluoro-4-
(1-((4-
fluorophenyl)carbamoyl)cyclopropanecarboxamido)phenoxy)-6H-pyrimido [5,4-
b][1,4]oxazine-8(7H)-carboxylate (step 2 intermediate) (65 mg, 0.11 mmol) with
hydrochloric acid in 1,4-dioxane (8.0 mL) in ethanol (5.0 mL) as per the
procedure described
in step 3 of Method A to yield 20 mg of the product.
Method N
Preparation of 4-chloro-3-43-methy1-2-oxo-1,2,3,4-tetrahydropyrido [2,3 -
d]pyrimidin-5 -
yl)oxy)-N-(3-(4-methylpip erazin-l-y1)-5 -(trifluoromethyl)phenyl)b enz ami de
CI
H
N
0 is u3
I , ONN N
CN)
H
I
Step 1: tert-Butyl (4-(2-chloro-5-((3-(4-
methylpiperazin-1-y1)-5-
(trifluoromethyl)phenyl)carbamoyl)phenoxy)-3-formylpyridin-2-yl)carbamate
ci ai
H
0 0 N so u3
0
Boc. I I ) N
H
NI\r CN)
I
To a solution of 4-chloro-3-hydroxy-N-(3-(4-methylpiperazin-1-y1)-5-
(trifluoromethyl)phenyl)benzamide (Step 1-Method G') (200 mg, 0.48 mmol) in
DMF (3.0
mL) was added cesium carbonate (314 mg, 0.97 mmol) and the mixture was stirred
for 30
min. The solution was cooled to 0 C and added with tert-butyl (3-chloro-2-
formylphenyl)carbamate (CAS# 1260667-07-9) (186 mg, 0.73 mmol) at the same
temperature. The resultant mixture was stirred overnight at RT. The reaction
was quenched
with ice and extracted with ethyl acetate. The organic layer was dried over
anhydrous sodium
143
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
sulfate, filtered and concentrated. The crude material was purified by silica
gel column
chromatography to yield 220 mg of the desired compound. 'H NMR (400 MHz, DMSO-
d6) 6
1.48 (s, 9H), 2.23 (s, 3H), 2.45-2.51 (m, 4H), 3.21 (t, J= 4.8 Hz, 4H), 6.45
(d, J = 6.0 Hz,
1H), 6.98 (s, 1H), 7.59 (s, 1H), 7.62 (s, 1H), 7.91 (d, J= 8.4 Hz, 1H), 8.01
(dd, ./i = 2.0 Hz, .1-2
= 8.4 Hz, 1H), 8.08 (d, J = 2.0 Hz, 1H), 8.37 (d, J = 5.6 Hz, 1H), 10.40 (s,
2H), 10.48 (s,
1H); ESI-MS (m/z) 534 (M+H-B0C)+.
Step 2: 4-chloro-3 -methy1-2-oxo-1,2,3 ,4-tetrahydropyrido [2,3 -
d]pyrimidin-5 -yl)oxy)-N-
(3 -(4-methylpip erazin-l-y1)-5 -(trifluoromethyl)phenyl)b enz amide
To a stirred solution of tert-butyl (4-(2-chloro-5-((3-(4-methylpiperazin-1-
y1)-5-
(trifluoromethyl)phenyl)carbamoyl)phenoxy)-3-formylpyridin-2-yl)carbamate
(step 1
intermediate) (50 mg, 0.08 mmol) in THF (1.0 mL) were added methylamine
solution (33%
in ethanol, 0.1 mL, 0.8 mmol) followed by a drop of acetic acid and the
mixture was stirred
overnight at RT. The precipitated solid was filtered, dried and purified by
silica gel column
chromatography to yield 12 mg of the desired compound.
Method 0
Preparation of 4-chloro-3-((3,4-dihydro-2H-pyrido [3,2-b] [1,4] oxazin-8-
yl)oxy)-N-(3 -(4-
methylpip erazin-l-y1)-5 -(trifluoromethyl)phenyl)b enzamide
ci
0 io
N N CJ
Step 1: 8-Bromo-7-nitro-3,4-dihydro-2H-pyrido[3,2-b][1,4]oxazine (Nitro
adduct)
Br
02N rcj:0
I j
N N
H .NO2
To a stirred solution of 8-bromo-3,4-dihydro-2H-pyrido[3,2-b][1,4]oxazine
(Step 2-
Intermediate A7) (2.0 g, 9.30 mmol) in sulfuric acid (8 mL) was dropwise added
a mixture of
sulfuric acid and nitric acid (1:1, 14 mL) at 0 C over a period of 15 min.
The mixture was
poured on crushed ice and stirred to give yellow solid. An aqueous solution of
sodium
hydroxide was added to the mixture till pH 8-9. The solid was filtered, washed
with water
and dried well. The crude solid was purified by recrystallization from acetone
to yield 1.2 g
of the desired product. 1H NMR (400 MHz, DMSO-d6) 6 4.50-4.52 (m, 2H), 4.56-
4.59 (m,
2H), 8.76 (s, 1H).
144
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
Step 2: 4-Chloro-N-(3 -(4-methylpip erazin-1 -y1)-5 -(trifluoromethyl)pheny1)-
3-47-nitro -3 ,4-
dihydro-2H-pyrido [3,2-b] [1,4] oxazin-8-yl)oxy)b enzamide
ci
0 WI 40 0F3
02N,c,0 0
NN CN)
To a stirred solution of 8-bromo-7-nitro-3,4-dihydro-2H-pyrido[3,2-
b][1,4]oxazine (Nitro
adduct) (step 1 intermediate) (202 mg, 0.66 mmol) and 4-chloro-3-hydroxy-N-(3-
(4-
methylpiperazin-l-y1)-5-(trifluoromethyl)phenyl)benzamide (Step 1-Method G')
(246 mg,
0.59 mmol) in DMF (12 mL) was added potassium carbonate (275 mg, 1.98 mmol)
and the
mixture was stirred at 105 C for 2 h. The mixture was cooled to RT and
quenched with
water. The aqueous mixture was extracted twice with ethyl acetate. The
combined organic
layers were washed with water followed by brine. The solution was dried over
anhydrous
sodium sulfate, filtered and concentrated under reduced pressure. The residue
thus obtained
was purified by silica gel column chromatography to yield 368 mg of the
desired product. 1H
NMR (400 MHz, DMSO-d6) 6 2.32 (s, 3H), 2.51-2.59 (br s, 4H), 3.23 (br s , 4H),
4.40-4.42
(m, 2H), 4.46-4.48 (m, 2H), 6.97 (s, 1H), 7.41 (d, J= 1.6 Hz, 1H), 7.55 (s,
1H), 7.59 (s, 1H),
7.78-7.83 (m, 2H), 8.99 (s, 1H), 10.40 (s, 1H).
Step 3: tert-Butyl
8-(2-chloro-5 -((3 -(4-methylpip erazin-1 -y1)-5 -
(trifluoromethyl)phenyl)c arb amoyl)phenoxy)-7-nitro-2H-pyrido [3,2-b] [1,4]
oxazine-4 (3H)-
carboxylate
CI Ai
0 wi so 0F3
02N0, 0
j
N Neloc CN)
To a solution of 4-chloro-N-(3 -(4-methylpip erazin-1 -y1)-5 -
(trifluoromethyl)p heny1)-3
nitro-3,4-dihydro-2H-pyrido[3,2-b][1,4]oxazin-8-yl)oxy)benzamide (step 2
intermediate)
(361 mg, 0.66 mmol) in dichloromethane (15 mL) were added triethylamine (183
L, 1.31
mmol) followed by di-tert-butyl dicarbonate (227 L, 0.99 mmol) and the
mixture was stirred
for 5 min before the addition of DMAP (8 mg, 0.06 mmol) at RT. The resultant
mixture was
stirred for 30 min at RT. The reaction mixture was diluted with
dichloromethane and washed
with water and brine. The solution was dried over anhydrous sodium sulfate,
filtered and
145
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
concentrated. The residue thus obtained was purified by silica gel column
chromatography to
yield 287 mg of the desired product. 1H NMR (400 MHz, DMSO-d6) 6 1.52 (s, 9H),
2.22 (s,
3H), 2.40-2.51 (m, 4H), 3.16-3.20 (m, 4H), 3.91 (d, J= 4.4 Hz, 2H), 4.28 (d,
J= 4.0 Hz, 2H),
6.94 (s, 1H), 7.31 (d, J = 1.6 Hz, 1H), 7.55 (s, 1H), 7.57 (s, 1H), 7.77-7.81
(m, 2H), 8.80 (s,
.. 1H), 10.36 (s, 1H).
Step 4: tert-Butyl 7-amino-8-(2-chloro-5-((3-(4-
methylpiperazin-1-y1)-5-
(trifluoromethyl)phenyl)carbamoyl)phenoxy)-2H-pyrido [3,2-b] [1,4]oxazine-
4(3H)-
carboxylate
CI Ai
H N
0 wi io 0F3
H2N,c,0 0
, 1 j (1\1
1\11
Boc LN)
I
To a stirred solution of tert-butyl 8-(2-chloro-5-((3-(4-methylpiperazin-l-y1)-
5-
(trifluoromethyl)phenyl)carbamoyl)phenoxy)-7-nitro-2H-pyrido [3,2-b]
[1,4]oxazine-4(3H)
carboxylate (step 3 intermediate) (280 mg, 0.40 mmol) in a mixture of methanol
and water
(3:1, 20 mL) was added ammonium chloride (215 mg, 4.05 mmol) and the mixture
was
heated to 80 C. Zinc dust (135 mg, 2.06 mmol) was added in small portions and
the mixture
was stirred at for 1 h at 80 C. The mixture was cooled to RT and poured in to
aqueous
sodium bicarbonate solution. The aqueous mixture was extracted twice with 5%
mixture of
methanol in chloroform. The organic layer was dried over anhydrous sodium
sulfate, filtered
and concentrated. The residue obtained was purified by column chromatography
to yield 174
mg of the desired compound. 1H NMR (400 MHz, DMSO-d6) 6 1.45 (s, 9H), 2.24 (s,
3H),
2.40 (br s, 4H), 3.21 (br s, 4H), 3.72 (t, J= 4.4 Hz, 2H), 4.08 (t, J= 4.0 Hz,
2H), 5.15 (s, 2H),
6.94 (s, 1H), 7.14 (s, 1H), 7.55-7.75 (m, 5H), 10.38 (s 1H).
Step 5: 4-C hloro-3 -((3 ,4-dihydro-2H-pyrido [3,2-b] [1,4] oxazin-
8-yl)oxy)-N-(3 -(4-
methylpip erazin-l-y1)-5 -(trifluoromethyl)phenyl)b enzamide
To a stirred solution of tert-butyl 7-amino-8-(2-chloro-5-((3-(4-
methylpiperazin-1-y1)-5-
(trifluoromethyl)phenyl)carbamoyl)phenoxy)-2H-pyrido [3,2-b] [1,4]oxazine-
4(3H)-
carboxylate (step 4 intermediate) (119 mg, 0.18 mmol) in dichloromethane (5.0
mL) at -20
C was added BF3-etherate (50%, 135 L, 0.54 mmol) and the mixture was stirred
for 5 min.
Thereafter, a solution of tert-butyl nitrite (43 L, 0.36 mmol) in DCM (5.0
mL) was added
dropwise to the reaction mixture over a period of 10 min. The resultant
mixture was stirred at
-20 C for 30 min. The mixture was allowed to warm up to 10 C and the solvent
was
146
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
removed by blowing nitrogen gas. The crude black residue was dissolved in DMF
(5.0 mL)
and added to a stirred solution of iron sulfate (55 mg, 0.19 mmol) in DMF (5.0
mL) and the
mixture was stirred for 5 min at RT. The poured in to aqueous sodium
bicarbonate solution
and the product was extracted twice in ethyl acetate. The combined organic
layers were dried
over anhydrous sodium sulfate, filtered and concentrated. The residue obtained
was purified
by column chromatography to yield 28 mg of the desired compound. 1H NMR (400
MHz,
DMSO-d6) 6 2.27 (s, 3H), 2.44-2.47 (br s, 4H), 3.19-3.21 (br s, 4H), 3.43 (br
s, 2H), 4.10 (t, J
= 4.0 Hz, 2H), 6.10 (d, J = 5.6 Hz, 1H), 6.96 (s, 2H), 7.53 (d, J= 5.6 Hz,
1H), 7.59 (s, 3H),
7.78-7.85 (m, 2H), 10.38 (s, 1H); ESI-MS (m/z) 548 (M+H)+.
Method P
Preparation of 1-(4-((7-cyano-3,4-dihydro-2H-pyrido [3,2-b] [1,4] oxazin-8-
yl)oxy)pheny1)-3 -
(4-((4-ethylpip erazin-l-yl)methyl)-3 -(trifluoromethyl)phenyl)urea
H H
40
N N so CF3 Yo
0
NCO
N
cj
N N N
H
Step 1: tert-Butyl 8-(4-(((benzyloxy)carbonyl)amino)phenoxy)-7-nitro-2H-pyrido
[3 ,2-
b] [1,4]oxazine-4(3H)-carboxylate
r0 0
40 'o
0
02N Lo
, I )
1\1N
Boc
The titled compound was prepared by the reaction of tert-butyl 8-bromo-7-nitro-
2H-
pyrido[3,2-b][1,4]oxazine-4(3H)-carboxylate (Intermediate A22) (1.5 g, 4.16
mmol) and
benzyl (4-hydroxyphenyl)carbamate (1.11 mg, 4.58 mmol) in the presence of
cesium fluoride
(1.9 g, 12.5 mmol) in DMSO (40 mL) as per the procedure described in step 2 of
Method G'
to yield 1.39 g of the compound. 1H NMR (400 MHz, DMSO-d6) 6 1.51 (s, 9H),
3.89 (t, J =
4.4 Hz, 2H), 4.25 (t, J = 4.0 Hz, 2H), 5.14 (s, 2H), 6.92 (d, J = 9.2 Hz, 2H),
7.34-7.43 (m,
7H), 8.69 (s, 1H), 9.72 (s, 1H).
Step 2: tert-Butyl 7-amino-8-(4-(((benzyloxy)carbonyl)amino)phenoxy)-2H-
pyrido[3,2-
b] [1,4]oxazine-4(3H)-carboxylate
147
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
H
N ((:) 40
wi 8
o
H2N o,
I j. ,
N N
lioc
The titled compound was prepared by the reaction of tert-Butyl 8-(4-
(((benzyloxy)carbonyl)amino)phenoxy)-7-nitro-2H-pyrido [3,2-b] [1,4] oxazine-
4(3H)-
carboxylate (step 1 intermediate) (1.35 g, 2.58 mmol) with zinc dust (844 mg,
12.9 mmol)
and ammonium chloride (1.38 g, 25.8 mmol) in a mixture of methanol and water
(3:1, 40
mL) as per the procedure described in step 4 of Method 0 to yield 921 mg of
the product. 1H
NMR (400 MHz, DMSO-d6) 6 1.45 (s, 9H), 3.73 (t, J= 4.4 Hz, 2H), 4.07 (t, J=
4.0 Hz, 2H),
4.94 (s, 2H), 5.13 (s, 2H), 6.79 (d, J= 9.2 Hz, 2H), 7.34-7.39 (m, 7H), 8.32
(s, 1H), 9.64 (s,
1H); ESI-MS (m/z) 492 (M+H)+.
Step 3: tert-Butyl 8-(4-(((benzyloxy)carbonyl)amino)phenoxy)-7-iodo-2H-pyrido
[3,2-
b] [1,4]oxazine-4(3H)-carboxylate
H
N10 40
o 0 )
N N
6oc
To a stirred suspension of tert-butyl 7-amino-8-(4-
(((benzyloxy)carbonyl)amino)phenoxy)-
2H-pyrido[3,2-b][1,4]oxazine-4(3H)-carboxylate (step 2 intermediate) (200 mg,
0.41 mmol)
in acetonitrile (8.0 mL) was added PTSA (232 mg, 1.22 mmol) and stirred at RT
for 10-15
min. To that mixture a solution of sodium nitrite (56 mg, 0.81 mmol) and
potassium iodide
(169 mg, 1.05 mmol) in water (1.0 mL) was added at 10-15 C. The reaction
mixture was
gradually allowed to attain RT and poured on an aqueous solution of sodium
bicarbonate.
The aqueous mixture was extracted twice with ethyl acetate and the combined
organic
extracts were washed with brine. The organic layer was dried over anhydrous
sodium sulfate,
filtered and concentrated. The residue obtained was purified by column
chromatography to
yield 102 mg of the desired compound. 1H NMR (400 MHz, DMSO-d6) 6 1.48 (s,
9H), 3.83
(t, J= 4.4 Hz, 2H), 4.16 (t, J= 3.6 Hz, 2H), 5.13 (m, 2H), 6.79 (d, J = 8.8
Hz, 2H), 7.34-7.43
(m, 7H), 8.27 (s, 1H),9.70 (s, 1H).
Step 4: tert-Butyl 8-(4-(((benzyloxy)carbonyl)amino)phenoxy)-7-cyano-2H-
pyrido[3,2-
b] [1,4]oxazine-4(3H)-carboxylate
148
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
H
N0 40
wi 8
0
NC j0
I j
N N
iioc
To a solution of tert-butyl 8-(4-(((benzyloxy)carbonyl)amino)phenoxy)-7-iodo-
2H-
pyrido[3,2-b][1,4]oxazine-4(3H)-carboxylate (step 3 intermediate) (52 mg, 0.08
mmol) in
DMF (5.0 mL) were added zinc cyanide (10 mg, 0.09 mmol), [1,1'-
bis(diphenylphosphino)ferrocene]dichloropalladium(II) (10 mg, 0.017 mmol)
followed by
water (10 L) and the mixture was degassed for 5 min before adding
tris(dibenzylideneacetone)dipalladium(0) (Pd2(dba)3) (8.0 mg, 0.008 mmol). The
resultant
mixture was heated to 145 C for 15 min in a microwave reactor. The reaction
mixture cooled
to RT and poured on to water. The mixture was extracted twice with ethyl
acetate. The
combined organic extracts were washed with brine. The organic layer was dried
over
anhydrous sodium sulfate, filtered and concentrated. The residue obtained was
purified by
column chromatography to yield 19 mg of the Boc-deprotected compound. The Boc-
group
was restored by the reaction of the compound with di-tert-butyl dicarbonate
(1.5 eq.) in the
presence of LiHMDS (1.5 eq.) in THF (5.0 vol.) as per the procedure described
in step 3 of
Intermediate A7 to yield the titled compound. 1H NMR (400 MHz, DMSO-d6) 6 6
1.50 (s,
9H), 3.88 (t, J= 3.6 Hz, 2H), 4.21 (t, J= 4.4 Hz, 2H), 5.14 (m, 2H), 6.97 (d,
J= 8.0 Hz, 2H),
7.33-7.44 (m, 7H), 8.41 (s, 1H), 9.78 (s, 1H).
Step 5: tert-Butyl 8-(4-aminophenoxy)-7-cyano-2H-pyrido[3,2-b][1,4]oxazine-4(3
H)-
carboxylate
Ai NH2
0
NC rc(0,
I j
N N
6(:)c
A solution of tert-butyl 8-(4-(((benzyloxy)carbonyl)amino)phenoxy)-7-cyano-2H-
pyrido[3,2-
b] [1,4]oxazine-4(3H)-carboxylate (step 4 intermediate) (120 mg, 0.24 mmol) in
methanol (12
mL) with catalytic amount of 10% palladium on carbon (50% wet, 60 mg) was
hydrogenated
at RT for 1 h. The mixture was filtered through celite and the celite bed was
rinsed with
methanol. The combined filtrate and washings were concentrated and the residue
thus
obtained was purified by silica gel column chromatography to yield 48 mg of
the desired
149
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
product. 1H NMR (400 MHz, DMSO-d6) 6 1.50 (s, 9H), 3.87 (t, J = 4.4 Hz, 2H),
4.22 (t, J =
4.0 Hz, 2H), 6.92 (dd, Ji = 2.0 Hz, .1-2 = 6.8 Hz, 2H), 7.41 (d, J= 8.8 Hz,
2H), 9.34 (s, 1H).
Step 6: tert-Butyl 7-cyano-8-(4-(3-(4-((4-ethylpiperazin-1-
yl)methyl)-3-
(trifluoromethyl)phenyl)ureido)phenoxy)-2H-pyrido [3,2-b] [1,4] oxazine-4(3H)-
carboxylate
H H
N N CF3
40 1r 40
0
NC,x0j N
CN)
N N
Boc
The titled compound was prepared by the reaction of tert-butyl 8-(4-
aminophenoxy)-7-cyano-
2H-pyrido[3,2-b][1,4]oxazine-4(3H)-carboxylate (step 5 intermediate) (45 mg,
0.12 mmol)
with 4-((4-ethylpiperazin-1-yl)methyl)-3-(trifluoromethyl)aniline
(Intermediate Cl) (35 mg,
0.12 mmol) by using triphosgene (13 mg, 0.04 mmol) and triethylamine (71 L,
0.37 mmol)
in THF (10 mL) as per the procedure described in step 2 of Method Ito yield 53
mg of the
compound. ESI-MS (m/z) 682 (M+H)+.
Step 7: 1-(4-((7-Cyano-3,4-dihydro-2H-pyrido [3,2-b] [1,4]oxazin-8-
yl)oxy)pheny1)-3 -(4-((4-
ethylpip erazin-l-yl)methyl)-3 -(trifluoromethyl)phenyl)urea
The titled compound was prepared by the reaction of tert-butyl 7-cyano-8-(4-(3-
(4-((4-
ethylpiperazin-l-yl)methyl)-3-(trifluoromethyl)phenyl)ureido)phenoxy)-2H-
pyrido [3,2-
b][1,4]oxazine-4(3H)-carboxylate (step 6 intermediate) (52 mg, 0.07 mmol) with
hydrochloric acid in 1,4-dioxane (2.5 mL) in 1,4-dioxane (2.0 mL) and methanol
(0.5 mL) as
per the procedure described in step 3 of Method A to yield 11 mg of the
product. 1H NMR
(400 MHz, DMSO-d6) 6 1.01 (t, J= 7.2 Hz, 3H), 2.42 (br s, 10H), 3.33 (br s,
2H), 3.54 (br s,
2H), 4.04 (d, J= 8.0 Hz, 2H), 6.91 (d, J= 9.2 Hz, 2H), 7.41 (d, J = 9.2 Hz,
2H), 7.57 (d, J =
8.4 Hz, 1H), 7.62 (d, J = 8.4 Hz, 1H), 7.96 (s, 1H), 8.10 (s, 2H), 8.79 (s,
1H), 9.04 (s, 1H);
ESI-MS (m/z) 582 (M+H)+.
Method Q
Preparation of 1-(3 -(tert-buty1)-1-(3 -cyanopheny1)-1H-pyrazol-5 -y1)-3 -(443
,4-dihydro-2H-
pyrido [3,2-b] [1,4] oxazin-8-yl)oxy)phenyl)urea
* CN
H H
0
N N N x 1 IN
0
eC))
NN
H
150
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
Step 1: Phenyl (3 -(te rt-buty1)-1-(3 -cyanopheny1)-1H-pyrazol-5 -yl)carb
amate
* ON
H
0 0T N N
) 1 IN
To a stirred solution of 3-(5-amino-3-(tert-buty1)-1H-pyrazol-1-
y1)benzonitrile (Intermediate
C92) (150 mg, 0.62 mmol) and pyridine (0.1 mL, 1.25 mmol) in THF (10 mL) was
added
phenyl chloroformate (0.12 mL, 0.94 mmol) at 0 C. The mixture was stirred at
RT for 2 h
and diluted with ethyl acetate. The organic layer was washed with water, brine
and dried over
anhydrous sodium sulfate. The solution of filtered, concentrated and the
residue obtained was
purified by silica gel column chromatography to yield 79 mg of the desired
product ESI-MS
(m/z) 361 (M+H)+.
Step 2: tert-Butyl
84443 -(3 -(tert-buty1)-1-(3 -cyanopheny1)-1H-pyrazol-5 -
yl)ureido)phenoxy)-2H-pyrido [3,2-b] [1,4] oxazine-4(3H)-c arbo xylate
4/0 ON
H H
AI NisN 1 NAT
IN
0
CIC))
N N
Boa
To a cooled (10 C) solution of tert-butyl 8-(4-aminophenoxy)-2H-pyrido[3,2-
b][1,4]oxazine-4(3H)-carboxylate (step 2 of Method I") (60 mg, 0.17 mmol) in
DMSO (5.0
mL) were added triethylamine (75 L, 0.53 mmol) followed by phenyl (3-(tert-
buty1)-1-(3-
cyanopheny1)-1H-pyrazol-5-y1)carbamate (step 1 intermediate) (70 mg, 0.19
mmol) and the
mixture was stirred at RT for 16 h. The mixture was diluted with ethyl acetate
and washed
with water, saturated ammonium chloride solution and brine. The organic layer
was dried
over anhydrous sodium sulfate, filtered, concentrated and the residue obtained
was purified
.. by silica gel column chromatography to yield 69 mg of the desired product.
1H NMR (400
MHz, DMSO-d6) 6 1.30 (s, 9H), 1.47 (s, 9H), 3.86 (t, J= 4.0 Hz, 2H), 4.27 (t,
J = 4.0 Hz,
2H), 6.41 (s, 1H), 6.47 (d, J= 3.2 Hz, 1H), 7.03 (d, J= 8.4 Hz, 2H), 7.45 (d,
J = 8.4 Hz, 2H),
7.71-7.77 (m, 1H), 7.80 (d, J = 5.2 Hz, 1H), 7.87 (d, J = 7.6 Hz, 1H), 7.93
(d, J = 8.4 Hz,
1H), 8.04 (s, 1H), 8.53 (s, 1H), 9.10 (s, 1H); ESI-MS (m/z) 610 (M+H)+.
Step 3: 1-(3 -
(tert-Buty1)-1-(3 -cyanopheny1)-1H-pyrazol-5 -y1)-3 -(443 ,4-dihydro-2H-
pyrido [3 ,2-b] [1,4] oxazin-8-yl)oxy)phenyl)urea
151
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
To a stirred solution of tert-butyl 8-(4-(3-(3-(tert-buty1)-1-(3-cyanopheny1)-
1H-pyrazol-5-
y1)ureido)phenoxy)-2H-pyrido[3,2-b][1,4]oxazine-4(3H)-carboxylate (step 2
intermediate)
(64 mg, 0.11 mmol) in acetonitrile (10 mL) was added PTSA (200 mg, 1.05 mmol)
followed
by few drops of methanol and the mixture was stirred at RT for 16 h. The
mixture was diluted
with ethyl acetate and washed with saturated sodium bicarbonate solution,
water and brine.
The organic layer was dried over anhydrous sodium sulfate, filtered,
concentrated and the
residue obtained was purified by silica gel column chromatography to yield 21
mg of the
desired product.
Method R
Preparation of 1-(3 -(te rt-buty1)-1 -(4-cyanopheny1)-1H-pyrazol-5 -y1)-3 -(2-
fluoro-442,3 ,4,5 -
tetrahydropyrido[3,2-b][1,4]oxazepin-9-yl)oxy)phenyl)urea (Example 228)
ON
F H H 410
Ai NyN 1 NIN
0
0---\
IN N
H
Step 1: tert-Butyl 94443 -(3-(te rt-buty1)-1 -(4-cyanopheny1)-1H-pyrazol-5-
yOureido)-3 -
fluorophenoxy)-3 ,4-dihydropyrido [3,2-b] [1,4] oxazepine-5 (2H)-c arboxylate
ON
F H H 4110
NõN N
NN
BoC
To a solution of 4-(5-amino-3-(tert-buty1)-1H-pyrazol-1-y1)benzonitrile
(Intermediate 91)
(120 mg, 0.34 mmol) in dichloromethane (10 mL) was added 1,1'-
carbonyldiimidazole (CDI)
(55 mg, 0.34 mmol) and the mixture was stirred at 50 C for 1 h followed by
overnight at RT.
To that mixture was added 2-fluoro-4-((2,3,4,5-tetrahydropyrido[3,2-
b][1,4]oxazepin-9-
yl)oxy)aniline (prepared by procedure described in case of step 1 and 2 of
method I") (81 mg,
0.34 mmol) at RT and stirred for 16 h. The reaction was quenched with water
and extracted
twice with dichloromethane. The combined organic extracts were dried over
anhydrous
sodium sulfate, filtered and concentrated. The residue was purified by silica
gel column
152
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
chromatography to yield 60 mg of the titled compound. 41 NMR (400 MHz, DMSO-
d6) 6
1.29 (s, 9H), 1.36 (s, 9H), 1.90-1.93 (m, 2H), 3.60-3.68 (m, 2H), 3.96-4.02
(m, 2H), 6.42 (s,
1H), 6.66 (d, J= 5.6 Hz, 1H), 7.05 (d, J= 8.8 Hz, 2H), 7.48 (d, J = 9.2 Hz,
2H), 7.80 (d, J =
8.4 Hz, 2H), 7.97-8.02 (m, 2H), 8.58 (s, 1H), 9.14 (s, 1H); ESI-MS (m/z) 541
(M+H-Boc)+.
Step 2: 1-(3-(tert-Buty1)-1-(4-cyanopheny1)-1H-pyrazol-5-y1)-3-(2-fluoro-
442,3,4,5-
tetrahydropyrido[3,2-b][1,4]oxazepin-9-y1)oxy)phenyl)urea
The titled compound was prepared by the reaction of tert-butyl 9-(4-(3-(3-
(tert-buty1)-1-(4-
cyanopheny1)-1H-pyrazol-5-yOureido)-3-fluorophenoxy)-3,4-dihydropyrido[3,2-
b][1,4]
oxazepine-5(2H)-carboxylate (step 1 intermediate) (50 mg, 0.08 mmol) with
hydrochloric
acid in 1,4-dioxane (5.0 mL) as per the procedure described in step 3 of
Method A to yield 18
mg of the product.
Table 25: Structure, Chemical name, Method used and analytical data of
Examples
No. Intermed Analytical Data
Structure and Name iates and
Method
1. 0 H
Al, B2, 1I-1 NMR (400 MHz, DMSO-d6)
O N r CFrN
Cl 6 0.98 (t, J = 6.8 Hz, 3H), 2.28-
0 IW NI,)
2.51 (m, 10H), 3.33-3.51 (br s,
I j
Method A 4H), 4.17 (d, J = 4.0 Hz, 2H),
NN
H
7.36-7.37 (m, 1H), 7.56 (d, J =
3-((7,8-Dihydro-6H-pyrimido[5,4-
8.0 Hz, 1H), 7.68-7.82 (m, 5H),
b] [1,4]oxazin-4-yl)oxy)-N-(4-((4-
8.03 (d, J= 2.0 Hz, 1H), 8.20 (s,
ethylpiperazin-l-yl)methyl)-3-
1H), 10.42 (s, 1H); ESI-MS (m/z)
(trifluoromethyl)phenyl)benzamid 543 (M+H)+.
e
2. F
H
Al, B4, 1I-1 NMR (400 MHz, DMSO-d6)
ow No c3
Cl 6 0.98 (t, J = 6.8 Hz, 3H), 2.28-
j,(D cl
2.44 (m, 10H), 3.51-3.56 (m,
1 j
N Method A 4H), 4.20 (t, J = 8.4 Hz, 2H),
NN
C ) 7.52-7.72 (m, 3H), 7.80 (br s,
H
N 1H), 7.91-7.95 (m, 2H), 8.03 (dd,
Ji = 1.6 Hz, J2 = 8.4 Hz, 1H),
3-((7,8-Dihydro-6H-pyrimido[5,4-
8.18 (s, 1H), 10.50 (s, 1H); ESI-
b] [1,4]oxazin-4-yl)oxy)-N-(4-((4- MS (m/z) 561 (M+H)+.
ethylpiperazin-l-yl)methyl)-3-
(trifluoromethyl)pheny1)-4-
fluorobenzamide
3. ci
Al, Bl, 1I-1 NMR (400 MHz, DMSO-d6)
C2
6 2.15-2.38 (m, 11H), 3.51-3.56
O VI Ed cFrNi.
, (br s, 4H), 4.20 (t, J = 4.0
Hz,
N:):()) 0 IW N)
Method A 2H), 7.68-7.71 (br s, 2H), 7.72 (t,
NI N
J= 10.4 Hz, 2H), 7.87-7.89 (br s,
H
2H), 8.02 (d, J = 7.2 Hz, 1H),
4-Chloro-3-((7,8-dihydro-6H-
8.16 (s, 1H), 10.45 (s, 1H); ESI-
153
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
No. Intermed Analytical Data
Structure and Name iates and
Method
pyrimido[5,4-b][1,4]oxazin-4- MS (m/z) 563 (M+H)t
yl)oxy)-N-(4-((4-methylpiperazin-
l-yl)methyl)-3-
(trifluoromethyl)phenyl)benzamid
e
4. a gib
H Al,
Bl, 1I-1 NMR (400 MHz, DMSO-d6)
N cFr...N. C3 6 0.95
(d, J = 6.4 Hz, 6H), 2.38-
0
o IW N,)
Method A 2.62 (m, 9H), 3.51-3.54 (m, 4H),
4.20 (t, J = 4.4 Hz, 2H), 7.69-
1\IN 7.79
(m, 4H), 7.88-7.90 (m, 2H),
H
4-Chloro-3-((7,8-dihydro-6H- 8.02
(dd, Ji = 8.4 Hz, .1-2 = 10.0
pyrimido[5,4-b][1,4]oxazin-4- Hz,
1H), 8.17 (s, 1H), 10.53 (s,
yl)oxy)-N-(4-((4- 1H); ESI-MS (m/z) 591 (M+H)+.
isopropylpiperazin-l-yl)methyl)-
3-
(trifluoromethyl)phenyl)benzamid
e
5. ci H Al,
Bl, 1I-1 NMR (400 MHz, DMSO-d6)
O wi N 0 CF C4
6 0.89 (d, J = 6.4 Hz, 3H), 1.11-
, oj 0 N........õ,- 1.19
(m, 2H), 1.34 (br s, 1H),
It Method
A 1.58 (d, J = 12.0 Hz, 2H), 1.95 (t,
N N J = 11.6 Hz, 2H), 2.74 (d, J =
H
4-Chloro-3-((7,8-dihydro-6H- 11.2
Hz, 2H), 3.52-3.53 (br s,
pyrimido[5,4-b][1,4]oxazin-4- 4H),
4.20 (t, J = 4.0 Hz, 2H),
yl)oxy)-N-(4-((4-methylpiperidin- 7.69-
7.80 (m, 4H), 7.88-7.90 (m,
1-yl)methyl)-3- 2H),
8.02 (d, J = 8.8 Hz, 1H),
(trifluoromethyl)phenyl)benzamid 8.16
(s, 1H), 10.53 (s, 1H); ESI-
e MS (m/z) 562 (M+H)+.
6. a H Al,
Bl, 1I-1 NMR (400 MHz, DMSO-d6)
ow No cF CS
6 2.33-2.45 (m, 4H), 3.28-3.39
, (31 0 N, (m,
2H), 3.41-3.60 (m, 6H), 4.20
NJC Method
A (t, J = 4.4 Hz, 2H), 7.69-7.87
N N (m, 4H), 7.88-7.90 (m, 2H), 8.05
H
4-Chloro-3-((7,8-dihydro-6H- (dd,
J1 = 1.6 Hz, J2 = 8.4 Hz,
pyrimido[5,4-b][1,4]oxazin-4- 1H),
8.18 (s, 1H), 10.55 (s, 1H);
yl)oxy)-N-(4-(morpholinomethyl)- ESI-MS (m/z) 550 (M+H)+.
3-(trifluoromethyl)
phenyl)benzamide
7. a
H Al,
Bl, 1I-1 NMR (400 MHz, DMSO-d6)
ow No cFr...NH C6
6 2.31 (br s, 4H), 2.71 (br s, 4H),
NA, o 0 N,)
3.52 (br s, 4H), 4.20 (br s, 2H),
j
Method A 7.69-7.88 (m, 7H), 8.02 (d, J =
N N 7.2
Hz, 1H), 8.16 (s, 1H), 10.54
H
4-Chloro-3-((7,8-dihydro-6H- (s,
1H); ESI-MS (m/z) 549
pyrimido[5,4-b][1,4]oxazin-4- (M+H)+.
yl)oxy)-N-(4-(piperazin-1-
154
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
No. Intermed Analytical Data
Structure and Name iates and
Method
ylmethyl)-3-
(trifluoromethyl)phenyl)benzamid
e
8. CI
H Al,
Bl, 1I-1 NMR (400 MHz, DMSO-d6)
O VI N la CF C33
6 2.16 (s, 9H), 2.37-2.50 (m,
N(:))0 4H),
3.51-3.59 (m, 4H), 4.20 (br
j(
IW NI' 1\I
I Method
B s, 2H), 7.69-7.99 (m, 4H), 7.88
N N (s, 1H), 8.02 (d, J = 7.2 Hz, 1H),
H
4-Chloro-3-((7,8-dihydro-6H- 8.16
(s, 2H), 10.54 (s, 1H); ESI-
pyrimido[5,4-b][1,4]oxazin-4- MS (m/z) 565 (M+H)+.
yl)oxy)-N-(4-(((2-
(dimethylamino)ethyl)(methyl)am
ino)methyl)-3-
(trifluoromethyl)phenyl)benzamid
e
9. CI Ai
H Al,
Bl, 1I-1 NMR (400 MHz, DMSO-d6)
O N r" CF3 C24
6 2.22 (s, 3H), 2.45-2.51 (br s,
4H), 2.84 (t, J = 4.0 Hz, 4H),
N:,CI CI) N
,N, Method
A 3.51-3.51 (br s, 2H), 4.20 (t, J =
N N H 4.4
Hz, 2H), 7.57 (d, J = 8.8 Hz,
4-Chloro-3-((7,8-dihydro-6H- 1H)
7.74 (s, 1H), 7.87-7.89 (m,
pyrimido[5,4-b][1,4]oxazin-4- 4H),
8.02 (dd, Ji = 2.4 Hz, J2 =
yl)oxy)-N-(4-(4-methylpiperazin- 8.8
Hz, 1H), 8.12 (s, 1H), 10.49
1-y1)-3- (s,
1H); ESI-MS (m/z) 549
(trifluoromethyl)phenyl)benzamid (M+H)+.
e
10. 01 H Al,
Bl, 1I-1 NMR (400 MHz, DMSO-d6)
O W N i& CF3 C27
6 2.75-2.82 (m, 8H), 3.51-3.52
NXI ID) N
(br s, 2H), 4.20 (t, J = 4.0 Hz,
,NH Method
A 2H), 7.53 (d, J = 8.8 Hz, 1H),
N N 7.69-7.80 (m, 3H), 7.87-7.89 (m,
H
4-Chloro-3-((7,8-dihydro-6H- 2H),
8.03 (dd, Ji = 2.4 Hz, J2 =
pyrimido[5,4-b][1,4]oxazin-4- 8.4
Hz, 2H), 8.10 (s, 1H), 10.48
yl)oxy)-N-(4-(pip erazin-1 -y1)-3 - (s,
1H); ESI-MS (m/z) 535
(trifluoromethyl)phenyl)benzamid (M+H)+.
e
11. CI H Al,
Bl, 1I-1 NMR (400 MHz, DMSO-d6)
O WI N CF3 C25
2.83 (t, J = 4.4 Hz, 4H), 3.52 (br
NO N
s, 2H), 3.70 (t, J = 4.0 Hz, 4H),
jc) W
0 Method
A 4.20 (t, J = 4.0 Hz, 2H), 7.61 ( d,
N N J = 8.8 Hz, 1H), 7.69 (s, 1H),
H
4-Chloro-3-((7,8-dihydro-6H- 7.75-
7.80 (m, 2H), 7.88-7.90 (m,
pyrimido[5,4-b][1,4]oxazin-4- 2H),
8.05 ( dd, Ji = 2.4 Hz, J2 =
yl)oxy)-N-(4-morpholino-3- 8.8
Hz, 1H), 8.14 (s, 1H), 10.50
(trifluoromethyl)phenyl)benzamid (s,
1H); ESI-MS (m/z) 536
e (M+H)+.
155
CA 03064975 2019-11-26
WO 2018/215668 PCT/EP2018/063957
No. Intermed Analytical Data
Structure and Name iates and
Method
12. CI a
H Al,
Bl, 'I-1 NMR (400 MHz, DMSO-d6)
O N = r-rir'''. C28
0.97 (t, J = 7.2 Hz, 3H), 2.26-
0 0 N.,9 2.51 (m, 10H),
3.41 (s, 2H), 3.52
Nx )
Method A (d, J = 6.8 Hz, 2H), 4.20 (t, J =
Nr N H 8.4 Hz, 2H), 7.26
( d, J = 8.4 Hz,
4-Chloro-3-((7,8-dihydro-6H- 2H),
7.68-7.79 (m, 5H), 7.85 (s,
pyrimido[5,4-b][1,4]oxazin-4- 2H),
10.28 (s, 1H); ESI-MS (m/z)
yl)oxy)-N-(4-((4-ethylpiperazin-1- 509 (M+H)+.
yl)methyl)phenyl)benzamide
13. CI a
H Al,
Bl, 1I-1 NMR (400 MHz, DMSO-d6)
O N = CI r======.,. C29
0.97 (t, J = 6.8 Hz, 3H), 2.29-
j:c) 0 N 2.51 (m, 10H), 3.51
(s, 4H), 4.20
N )
Method A (t, J = 4.4 Hz, 2H), 7.43 (d, J =
Nr N H 8.8 Hz, 1H), 7.66-
7.74 (m, 2H),
4-Chloro-N-(3-chloro-4((4- 7.76-
7.80 (m, 3H), 7.86-7.93 (m,
ethylpiperazin-1- 2H),
10.41 (s, 1H); ESI-MS (m/z)
yl)methyl)pheny1)-3-47,8- 543 (M+H)+.
dihydro-6H-pyrimido[5,4-
b][1,4]oxazin-4-yl)oxy)benzamide
14. CI a H
Al, Bl, 1I-1 NMR (400 MHz, DMSO-d6)
N C7 6 0.97
(t, J = 7.2 Hz, 3H), 2.28-
0 0 1\1
0 0 N r) 2.35 (m, 13H), 3.32
(br s, 2H),
A
N ' )
Method A 3.52 (br s, 2H), 4.20 (t, J = 4.0
N N H Hz, 2H), 7.16 (d,
J = 8.0 Hz,
4-Chloro-3-((7,8-dihydro-6H- 1H),
7.53-7.79 (m, 4H), 7.85 (s,
pyrimido[5,4-b][1,4]oxazin-4- 1H),
7.86-7.87 (m, 2H), 10.19 (s,
yl)oxy)-N-(4-((4-ethylpiperazin-1- 1H).
yl)methyl)-3-
methylphenyl)benzamide
15. ci a H
Al, Bl, 1I-1 NMR (400 MHz, DMSO-d6)
O N 0 CF3 C19
6 2.22 (s, 3H), 2.46 (t, J = 4.8
ID Hz, 4H), 3.21 (t, J=
4.8 Hz, 4H),
= I ) N
Method A 3.51-3.52 (br s, 2H), 4.20 (t, J =
ON 4.2 Hz, 2H), 6.96 (s, 1H), 7.60 (s,
H C
N) 2H),
7.64 (s, 1H), 7.69-7.77 (m,
I 4-Chloro-3-((7,8-dihydro-6H-
1H), 7.82 (s, 1H), 7.86-7.89 (m,
pyrimido[5,4-b][1,4]oxazin-4-
2H), 10.38 (s, 1H); ESI-MS (m/z)
yl)oxy)-N-(3-(4-methylpiperazin-
549 (M+H)+.
1-y1)-5 -
(trifluoromethyl)phenyl)benzamid
e
156
CA 03064975 2019-11-26
WO 2018/215668 PCT/EP2018/063957
No. Intermed Analytical Data
Structure and Name iates and
Method
16. c1 A
H Al,
Bl, 'I-1 NMR (400 MHz, DMSO-d6)
O N 00
CF3 3-bromo- 6 3.51-3.52 (br s, 2H), 4.21 (t, J
5- = 4.0
Hz, 2H), 7.69 (s, 2H), 7.77-
Nj: C;1 ) Br
(trifluoro 7.80 (m, 2H), 7.78-7.90(m, 2H),
N N H
methyl)an 8.19 (s, 1H), 8.34 (s, 1H), 10.67
N-(3-Bromo-5- iline (s,
1H); ESI-MS (m/z) 531
(trifluoromethyl)pheny1)-4-chloro- (M+2H)+.
3-((7,8-dihydro-6H-pyrimido[5,4- Method A
b][1,4]oxazin-4-yl)oxy)benzamide
17. CI al H Al,
Bl, lli NMR (400 MHz, DMSO-d6)
N CF3 0 C44 6 0.99 (t, J = 7.2 Hz, 3H), 2.22-
0 r1\1
2.50 (m, 6H), 3.09-4.36 (m, 6H),
Method A 4.21 (br s, 2H), 7.45 (d, J = 8.8
0
N N H Hz,
1H), 7.68 (s, 1H), 7.79 (br s,
4-Chloro-3-((7,8-dihydro-6H- 2H),
7.89-7.91 (m, 2H), 8.12 (d,
pyrimido[5,4-b][1,4]oxazin-4- J =
8.0 Hz, 1H), 8.26 (s, 1H),
yl)oxy)-N-(4-(4-ethylpiperazine-1- 10.67
(s, 1H); ESI-MS (m/z) 591
carbonyl)-3- (M)
(trifluoromethyl)phenyl)benzamid
e
18. ci al
H 0 Al,
Bl, lli NMR (400 MHz, DMSO-d6)
N CF3 .1L C8
6 1.99 (s, 3H), 2.33 (s, 2H), 2.39
0 0 r'N
N (s,
2H), 3.33-3.44 (m, 4H), 3.52
er)
Method A (s, 2H), 3.60 (s, 2H), 4.20 (s,
N N H 2H),
7.69-7.88 (m, 4H), 7.90 (s,
N-(4-((4-Acetylpip erazin-1- 2H),
8.05 (d, J = 8.4 Hz, 1H),
yl)methyl)-3- 8.18
(s, 1H), 10.56 (s, 1H); ESI-
(trifluoromethyl)pheny1)-4-chloro- MS (m/z) 591 (M+H)+
3-((7,8-dihydro-6H-pyrimido[5,4-
b][1,4]oxazin-4-yl)oxy)benzamide
19. CI Ai Al,
Bl, lli NMR (400 MHz, DMSO-d6)
H
N
0 Br ...... C30 6 0.98
(t, J = 6.8 Hz, 3H), 2.27-
40 r.N...,
1,1,0, 0 N,) 2.51
(m, 10H), 3.49-4.52 (m,
j Method
A 4H), 4.20 (t, J = 4.4 Hz, 2H),
N N H
7.42 (d, J = 8.4 Hz, 1H), 7.69-
N-(3-Bromo-4-((4-ethylpiperazin- 7.88
(m, 6H), 8.10 (s, 1H), 10.40
1-yl)methyl)pheny1)-4-chloro-3- (s, 1H).
((7,8-dihydro-6H-pyrimido[5,4-
b][1,4]oxazin-4-yl)oxy)benzamide
20. CI al Al,
Bl, 'HNMR (400 MHz, DMSO-d6)
H
CF3
O N
I C16 6 2.23
(s, 6H), 2.65 (t, J = 5.6
Hz, 2H), 3.51 (m, 2H), 4.16-4.20
0 S(21'N Method
B (m, 4H), 7.31 (d, J= 9.6 Hz, 1H),
N N H
7.69 (s, 1H), 7.75 (d, J = 8.4 Hz,
4-Chloro-3-((7,8-dihydro-6H- 1H),
7.81 (s, 1H), 7.86-7.88 (m,
pyrimido[5,4-b][1,4]oxazin-4- 2H),
7.98 (d, J = 9.6 Hz, 1H),
157
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
No. Intermed Analytical Data
Structure and Name iates and
Method
yl)oxy)-N-(4-(2- 8.06
(br s, 1H), 10.40 (s, 1H);
(dimethylamino)ethoxy)-3- ESI-MS (m/z) 538 (M+H)+.
(trifluoromethyl)phenyl)benzamid
e
21. CI Al,
Bl, lli NMR (400 MHz, DMSO-d6)
H
O W N CF12 C43
6 2.18 (s, 6H), 2.35-2.50 (m,
1
0 WI N.,......."... ..-- 2H),
3.29-3.52 (m, 4H), 4.21 (m,
N NJ:CI
I ) 0 I Method
B 2H), 7.51 (d, J = 8.4 Hz, 1H),
N N H
7.69-7.91 (m, 5H), 8.08 (d, J =
4-(4-Chloro-3-((7,8-dihydro-6H- 8.4
Hz, 1H), 8.21 (s, 1H), 8.38
pyrimido[5,4-b][1,4]oxazin-4- (m,
1H), 10.65 (s, 1H); ESI-MS
yl)oxy)benzamido)-N-(2- (m/z) 565 (M+H)+.
(dimethylamino)ethyl)-2-
(trifluoromethyl)benzamide
22. CI Al,
Bl, lli NMR (400 MHz, DMSO-d6)
H
N 3-
6 3.51-3.52 (br s, 2H), 4.20 (t, J
u3
0 WI so
O 0
(trifluoro = 4.0 Hz, 2H), 7.47 (d, J = 7.6
N
I )
methyl)an Hz, 1H), 7.61 (t, J = 8.0 Hz, 1H),
N N iline 7.69 (s, 1H), 7.88-7.99 (m, 4H),
H
4-Chloro-3-((7,8-dihydro-6H- 8.04
(d, J = 8.4 Hz, 1H), 8.21 (s,
pyrimido[5,4-b][1,4]oxazin-4- Method
B 1H), 10.46 (s, 1H); ESI-MS (m/z)
yl)oxy)-N-(3- 451 (M+H)+.
(trifluoromethyl)phenyl)benzamid
e
23. CI Al,
Bl, lli NMR (400 MHz, DMSO-d6)
H
O W N i CFr.N. C17
6 0.97 (t, J = 7.2 Hz, 3H), 1.66-
O 0=
1.68 (m, 2H), 1.90-1.91 (m, 2H),
N:
I ) IW 0 Method
B 2.27-2.35 (m, 4H), 2.50-2.58 (m,
N N H
2H), 3.52 (br s, 2H), 4.20 (t, J =
4-Chloro-3-((7,8-dihydro-6H- 3.6
Hz, 2H), 4.59 (m, 1H), 7.32
pyrimido[5,4-b][1,4]oxazin-4- (d, J
= 6.9 Hz, 1H), 7.69-7.88
yl)oxy)-N-(4-((l-ethylpiperidin-4- (m,
5H), 7.95 (d, J = 9.6 Hz, 1H),
yl)oxy)-3- 8.06
(s, 1H), 10.38 (s, 1H); ESI-
(trifluoromethyl)phenyl)benzamid MS (m/z) 578 (M+H)+.
e
24. CI Al,
Bl, lli NMR (400 MHz, DMSO-d6)
H
N u3 C20
6 1.04 (t, J = 7.2 Hz, 3H), 2.38
0 wi io
O 0
(q, J= 7.2 Hz, 2H), 2.50-2.56 (br
NC
I ) N Method
B s, 4H), 3.21 (t, J = 4.4 Hz, 4H),
N N CN) H
3.51-3.52 (br s, 2H), 4.20 (t, J =
4.0 Hz, 2H), 6.96 (s, 2H), 7.60 (s,
1H), 7.64 (s, 2H), 7.75-7.80 (m,
4-Chloro-3-((7,8-dihydro-6H- 2H),
7.87-7.89 (m, 2H); ESI-MS
pyrimido[5,4-b][1,4]oxazin-4- (m/z) 563 (M+H)+.
yl)oxy)-N-(3-(4-ethylpiperazin-1-
y1)-5-
158
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
No. Intermed Analytical Data
Structure and Name iates and
Method
(trifluoromethyl)phenyl)benzamid
e
25. a H Al,
Bl, 'I-1 NMR (400 MHz, DMSO-d6)
N u3 C18
6 1.0 (s, 6H), 2.50-2.59 (m, 4H),
0 WI 40
, N j : ) 0 3.20
(m, 4H), 3.51 (br s, 3H),
N Method
B 4.20 (s, 2H), 6.94 (s, 1H), 7.59-
N N C )
7.87 (m, 7H), 10.37 (s, 1H); ESI-
H
r) MS (m/z) 577 (M+H)+.
4-Chloro-3-((7,8-dihydro-6H-
pyrimido[5,4-b][1,4]oxazin-4-
yl)oxy)-N-(3-(4-
isopropylpiperazin-l-y1)-5-
(trifluoromethyl)phenyl)benzamid
e
26. ci am
H Al,
Bl, 1I-1 NMR (400 MHz, DMSO-d6)
O WI N 0 cFrN C9
6 0.84 (t, J = 7.2 Hz, 3H), 1.40-
Njo, 0 N) 1.42
(m, 2H), 2.21 (t J = 7.2 Hz,
1 j Method
B 4H), 2.49-2.50 (m, 6H), 3.51-
N N 3.56 (m, 4H), 4.19-4.21 (m, 2H),
H
4-Chloro-3-((7,8-dihydro-6H- 7.69-
7.77 (m, 4H), 7.77-7.87 (m,
pyrimido[5,4-b][1,4]oxazin-4- 2H),
8.07 (d, J = 11.2 Hz, 1H),
yl)oxy)-N-(4-((4-propylpiperazin- 8.17
(s, 1H), 10.54 (s, 1H); ESI-
1-yl)methyl)-3- MS (m/z) 591 (M+H)+.
(trifluoromethyl)phenyl)benzamid
e
27. Ck Al,
Bl, 1I-1 NMR (400 MHz, DMSO-d6)
H
N 40 cF3 C21
6 0.88 (t, J = 7.2 Hz, 3H), 1.45-
1.50 (m, 2H), 2.28 (t, J= 7.6 Hz,
Nts: 1 Oj 0
N
Method B 2H), 3.16-3.22 (m, 8H), 3.51-
N N C ) 3.52
(br s, 2H), 4.20 (t, J = 4.0
H
N Hz,
2H), 6.95 (s, 1H), 7.59 (s,
H 2H),
7.64 (s, 1H), 7.69-7.80 (m,
2H), 7.86-7.89 (m, 2H), 10.37 (s,
4-Chloro-3-((7,8-dihydro-6H- 1H); ESI-MS (m/z) 577 (M+H)+.
pyrimido[5,4-b][1,4]oxazin-4-
yl)oxy)-N-(3-(4-propylpiperazin-
1-y1)-5 -(trifluoromethyl)
phenyl)benzamide
28. ci Ai u3 Al,
Bl, 1I-1 NMR (400 MHz, DMSO-d6)
H
N C22
6 0.084-0.12 (m, 2H), 0.46-0.50
O wi 40
(m, 2H), 0.84-0.88 (m, 1H),
1 j
N Method
B 2.22-2.4 (m, 2H), 2.49-2.58 (m,
NN ( )
4H), 3.21-3.39 (m, 4H), 3.51-
H
3.52 (m, 2H), 4.20 (t, J= 4.4 Hz,
N
2H), 6.96 (s, 1H), 7.59-7.77 (m,
3H), 7.81-7.89 (m, 4H), 10.38 (s,
159
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
No. Intermed Analytical Data
Structure and Name iates and
Method
4-Chloro-N-(3-(4- 1H); ESI-MS (m/z) 589 (M+H)+.
(cyclopropylmethyl)piperazin-l-
y1)-5-(trifluoromethyl)pheny1)-3-
47,8-dihydro-6H-pyrimido[5,4-
b][1,4]oxazin-4-y1)oxy)benzamide
29. CI Al,
Bl, 1I-1 NMR (400 MHz, DMSO-d6)
H
O Wi N 0 0F3 C10
6 0.97 (t, J = 7.2 Hz, 3H), 2.27-
NS(:' 2.51 (m, 10H), 3.34-3.37 (m,
I ) Method
B 1H), 3.39-3.53 (m, 3H), 4.21 (q,
NN N J
= 4.4 Hz, 2H), 7.36 (s, 1H),
H
7.69 (s, 1H), 7.75-7.80 (m, 2H),
4-Chloro-3-((7,8-dihydro-6H- 7.89-
7.91 (m, 2H), 7.98 (s, 1H),
pyrimido[5,4-b][1,4]oxazin-4- 8.16
(s, 1H), 10.57 (s, 1H); ESI-
yl)oxy)-N-(3-((4-ethylpiperazin-1- MS (m/z) 577 (M+H)+.
yl)methyl)-5-
(trifluoromethyl)phenyl)benzamid
e
30. 01 H Al,
Bl, 1I-1 NMR (400 MHz, DMSO-d6)
N C35 6 1.45
(q, J = 2.8 Hz, 2H), 1.85
0 0 u3
0 N: l
rN, (d, J
= 12.0 Hz, 2H), 2.50-2.51
0
Method B (m, 7H), 2.78 (t, J = 11.6 Hz,
N N
24.2(b1r(s, t
23H.7)8, (3m.5, 21-11)3.5,
J2H=)4,.03.11z75-,
H
2H), 6.94 (s, 1H), 7.60 (d, J =
4-Chloro-3-((7,8-dihydro-6H- 10.4
Hz, 2H), 7.69-7.89 (m, 5H),
pyrimido[5,4-b][1,4]oxazin-4- 10.35
(s, 1H); ESI-MS (m/z) 577
yl)oxy)-N-(3-(4- (M+H)+.
(dimethylamino)piperidin-l-y1)-5-
(trifluoromethyl)phenyl)benzamid
e
31. a
H Al,
Bl, 1I-1 NMR (400 MHz, DMSO-d6)
O WI N CF3 C34
6 2.16 (s, 6H), 2.39 (t, J = 6.8
I )
N:
0 0 1W
N Hz,
2H), 2.95 (s, 3H), 3.44-3.51
Method B (m, 4H), 4.20 (t, J = 4.4 Hz, 2H),
1\1
= H
7.39 (s, 1H), 7.47 (s, 1H), 7.69
I (s,
1H), 7.74-7.76 (m, 1H), 7.81
4-Chloro-3-((7,8-dihydro-6H- (s,
1H), 7.87 (s, 1H), 7.88 (s,
pyrimido[5,4-b][1,4]oxazin-4- 2H),
10.34 (s, 1H); ESI-MS (m/z)
yl)oxy)-N-(3-((2- 551 (M+H)+.
(dimethylamino)ethyl)(methyl)am
ino)-5-
(trifluoromethyl)phenyl)benzamid
e
160
CA 03064975 2019-11-26
WO 2018/215668 PCT/EP2018/063957
No. Intermed Analytical Data
Structure and Name iates and
Method
32. ci
Al, Bl, 'I-1 NMR (400 MHz, DMSO-d6)
40 , CN 2-(3- 6 1.69 (s, 6H),
3.51-3.52 (m,
0
aminophe 2H), 4.20 (t, J = 4.4 Hz, 2H),
ny1)-2- 7.25-
7.27 (m, 1H), 7.42 (t, J =
1\1 N methylpro 8.0 Hz,
1H), 7.69-7.82 (m, 4H),
H panenitril 7.88-
7.93 (m, 3H), 10.42 (s, 1H);
4-Chloro-N-(3-(2-cyanopropan-2- e ESI-MS (m/z) 449 (M)+.
yl)pheny1)-3-47,8-dihydro-6H-
pyrimido[5,4-b][1,4]oxazin-4- Method B
yl)oxy)benzamide
33. a Ai Al, Bl, 1I-1 NMR (400 MHz, DMSO-d6)
H
N C36 6 1.47-
1.50 (m, 2H), 1.76-1.79
0
0 NO CN (m,
2H), 3.51-3.52 (br s, 2H),
J, 0
L I j Method
B 4.21(t, J = 4.0 Hz, 2H), 7.01 (dd,
1\1N H ii = 1.2 Hz, J2 =
7.2 Hz, 1H),
4-Chloro-N-(3-(1- 7.57
(s, 1H), 7.75 (s, 1H), 7.76-
cyanocyclopropyl)pheny1)-3- 7.88
(m, 4H), 7.89-7.90 (br s,
((7,8-dihydro-6H-pyrimido[5,4- 2H),
10.44 (s, 1H); ESI-MS (m/z)
b][1,4]oxazin-4-yl)oxy)benzamide 448 (M+H)+.
34. CI a H
Al, Bl, 1I-1 NMR (400 MHz, DMSO-d6)
N io cF3 C39 6 2.84
(t, J= 4.8 Hz, 4H),3.12 (t,
0
J = 4.8 Hz, 4H), 3.52 (br s, 2H),
,C ) N
N O Method
B 4.21 (t, J = 4.0 Hz, 2H), 6.94 (s,
I
N N 1H), 7.59 (s,
2H), 7.63-7.89 (m,
H C ) N 5H), 10.36 (s,
1H); ESI-MS (m/z)
H 535 (M+H)+.
4-Chloro-3-((7,8-dihydro-6H-
pyrimido[5,4-b][1,4]oxazin-4-
yl)oxy)-N-(3-(piperazin-l-y1)-5-
(trifluoromethyl)phenyl)benzamid
e
35. a Al
H Al,
Bl, 1I-1 NMR (400 MHz, DMSO-d6)
0 N * CF3 C23 6 0.93
(t, J = 6.4 Hz, 3H), 1.216-
txc) o 1.26
(m, 2H), 1.55-1.57 (br s,
)
Method B 1H), 1.70 (d, J = 11.2 Hz, 2H),
H 2.75 (dt, J1 =
2.0 Hz, J2 = 12.4
Hz, 2H), 3.51-3.52 (m, 2H), 7.73
4-Chloro-3-((7,8-dihydro-6H- (d, J
= 12.4 Hz, 2H), 4.21 (t, J =
pyrimido[5,4-b][1,4]oxazin-4- 4.0
Hz, 2H), 6.93 (s, 1H), 7.59-
yl)oxy)-N-(3-(4-methylpiperidin- 7.61
(m, 2H), 7.76 (s, 1H), 7.81-
1-y1)-5 - 7.77
(m, 1H), 7.87 (s, 1H), 7.88-
(trifluoromethyl)phenyl)benzamid 7.89
(m, 2H), 10.34 (s, 1H); ESI-
e MS (m/z) 548 (M+H)+.
161
CA 03064975 2019-11-26
WO 2018/215668 PCT/EP2018/063957
No. Intermed Analytical Data
Structure and Name iates and
Method
36. CI al Al,
Bl, 1I-1 NMR (400 MHz, DMSO-d6)
H
N io cF3 C40
6 1.47-1.56 (m, 2H), 1.72 (d, J =
0 WI
11.2 Hz, 2H), 2.50-2.72 (m, 3H),
N ,CO Method
B 3.03 (d, J = 11.6 Hz, 2H), 3.52
I )
N N (br s,
2H), 4.21 (t, J = 4.0 Hz,
H
N 2H),
7.31 (s, 1H), 7.69-7.94 (m,
H 6H),
8.07 (s, 1H), 10.52 (s, 1H);
4-Chloro-3-((7,8-dihydro-6H- ESI-MS (m/z) 534 (M+H)+.
pyrimido[5,4-b][1,4]oxazin-4-
yl)oxy)-N-(3-(piperidin-4-y1)-5-
(trifluoromethyl)phenyl)benzamid
e
37. a
H Al,
Bl, 1I-1 NMR (400 MHz, DMSO-d6)
0 WI N iio CF3 C41 6 1.34-
1.67 (m, 2H), 1.77-1.80
0 (m, 2H), 1.98 (t, J
= 10.0 Hz,
Method B 2H), 2.20 (s, 3H), 2.50-2.58 (m,
N N
H 1H), 2.87 (d, J =
11.2 Hz, 2H),
N 3.51-
3.52 (br s, 2H), 4.21 (t, J =
1
4-Chloro-3-((7,8-dihydro-6H- 4.0
Hz, 2H), 7.33 (s, 1H), 7.69-
pyrimido[5,4-b][1,4]oxazin-4- 7.93
(m, 6H), 8.08 (s, 1H), 10.52
yl)oxy)-N-(3-(1-methylpiperidin- (s,
1H); ESI-MS (m/z) 548
(M+H)+.
(trifluoromethyl)phenyl)benzamid
e
38. a Ai Al, Bl, 1I-1 NMR (400 MHz, DMSO-d6)
H
N 0 gip io c3 C37 6 2.13-2.29
(m, 1H), 3.33-3.66
(m, 7H), 4.21 (t, J = 4.0 Hz, 2H),
N N Method B 5.41-5.54 (m, 1H), 6.56 (s, 1H),
NI ) c )1\1
H 7.28 (s, 1H), 7.51 (s, 1H), 7.77
\--F (s, 1H), 7.87-7.90 (m, 4H), 10.37
(S)-4-chloro-3-((7,8-dihydro-6H- (s,
1H); ESI-MS (m/z) 538
pyrimido[5,4-b][1,4]oxazin-4- (M+H)+.
yl)oxy)-N-(3-(3-fluoropyrrolidin-
1-y1)-5 -
(trifluoromethyl)phenyl)benzamid
e
39. CI al Al,
Bl, 1I-1 NMR (400 MHz, DMSO-d6)
H
N cF3 C42
6 3.18-3.19 (br s, 4H), 3.51-3.52
0 WI so
(m, 2H), 3.84 (br s, 4H), 4.21 (t,
N ICI 0
Method B J = 4.0 Hz, 2H), 7.10 (s, 1H),
1 j N
N N H ( ) 7.62 (s, 1H),
7.69-7.89 (m, 6H),
s, 10.41
(s, 1H); ESI-MS (m/z) 584
dro (M+H)+.
4-Chloro-3-((7,8-dihydro-6H-
pyrimido[5,4-b][1,4]oxazin-4-
yl)oxy)-N-(3-(1,1-
dioxidothiomorpholino)-5-
162
CA 03064975 2019-11-26
WO 2018/215668 PCT/EP2018/063957
No. Intermed Analytical Data
Structure and Name iates and
Method
(trifluoromethyl)phenyl)benzamid
e
40. a Al,
Bl, '1-1 NMR (400 MHz, DMSO-d6)
H
N 0 gip 0 c3 C11 6 2.18 (s,
6H), 3.47-3.51 (m,
,C
O 0
4H), 4.21 (t, J = 4.4 Hz, 2H),
N
I ) Method
B 7.36 (s, 1H), 7.69 (s, 1H), 7.75-
N N 1\1 H I
7.80 (m, 2H), 7.89-7.91 (m, 2H),
4-Chloro-3-((7,8-dihydro-6H- 8.01
(s, 1H), 8.15 (s, 1H), 10.56
pyrimido[5,4-b][1,4]oxazin-4- (s,
1H); ESI-MS (m/z) 508
yl)oxy)-N-(3- (M+H)+.
((dimethylamino)methyl)-5-
(trifluoromethyl)phenyl)benzamid
e
41. CI Al,
Bl, 11-1 NMR (400 MHz, DMSO-d6)
H
N 2-(3- 6 3.51-
3.52 (m, 2H), 4.07 (s,
0 w 0 CN
O 0
aminophe 2H), 4.21 (br s, 2H), 7.09 (d, J =
N
I )
nyl)aceto 7.6 Hz, 1H), 7.38 (t, J = 8.0 Hz,
N N nitrile 1H), 7.69-7.89 (m, 5H), 7.90 (s,
H
4-Chloro-N-(3- 2H),
10.40 (s, 1H); ESI-MS (m/z)
(cyanomethyl)pheny1)-347,8- Method D 422 (M+H)+.
dihydro-6H-pyrimido[5,4-
b][1,4]oxazin-4-yl)oxy)benzamide
42. CI F F Al,
Bl, 11-1 NMR (400 MHz, DMSO-d6)
H
N C45 6 3.51
(br s, 2H), 4.20-4.21 (br s,
0 W 101 ON
O 0
2H), 7.55 (d, J = 7.6 Hz, 1H),
N l
I ) Method
E 7.67 (d, J = 10.8 Hz, 2H), 7.79-
N
H N 7.80 (br s, 2H),
7.89-7.91 (br s,
4-Chloro-N-(3- 2H),
8.10 (d, J = 8.8 Hz, 1H),
(cyanodifluoromethyl)pheny1)-3- 8.29
(s, 1H), 10.52 (s, 1H); ESI-
((7,8-dihydro-6H-pyrimido[5,4- MS (m/z) 458 (M+H)+.
b][1,4]oxazin-4-yl)oxy)benzamide
43. a Al,
Bl, 11-1 NMR (400 MHz, DMSO-d6)
H
N
cF3 C38 6 2.20-
2.30 (m, 1H), 3.34-3.63
0 w 0
O 0
(m, 7H), 4.21 (t, J = 3.6 Hz, 2H),
N l
Method B 5.40-5.54 (br s, 1H), 6.56 (s, 1H),
N N
H \__/ 7.28 (s, 1H),
7.51 (s, 1H), 7.81
'F (s,
1H), 7.87-7.89 (s, 4H), 10.37
(R)-4-Chloro-3-((7,8-dihydro-6H- (s,
1H); ESI-MS (m/z) 538
pyrimido[5,4-b][1,4]oxazin-4- (M+H)+.
yl)oxy)-N-(3-(3-fluoropyrrolidin-
1-y1)-5 -
(trifluoromethyl)phenyl)benzamid
e
163
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
No. Intermed Analytical Data
Structure and Name iates and
Method
44. CI Ai H Al,
Bl, 'I-1 NMR (400 MHz, DMSO-d6)
O N io c3 C12
6 1.27-1.29 (m, 1H), 1.75-1.84
N (:) (m, 1H), 1.87-1.91 (m, 1H), 2.08
I ) N Method
B (s, 6H), 2.33-2.75 (m, 4H), 3.50
H c
NN (br s, 2H), 3.70 (q, J = 6.8 Hz,
N¨ 2H),
4.20 (t, J = 4.0 Hz, 2H),
/ 7.68-7.70 (m, 2H), 7.75-7.90
(m,
4-Chloro-3-((7,8-dihydro-6H- 2H),
8.01 (br s, 2H), 8.03 (d, J =
pyrimido[5,4-b][1,4]oxazin-4- 6.6
Hz, 1H), 8.16 (s, 1H), 10.54
yl)oxy)-N-(4-((3- (s,
1H); ESI-MS (m/z) 577
(dimethylamino)pyrrolidin-1- (M+H)+.
yl)methyl)-3-
(trifluoromethyl)phenyl)benzamid
e
45. c1 Ai H Al,
Bl, 1I-1 NMR (400 MHz, DMSO-d6)
N u3 C13 6 1.25-1.29 (m, 1H),
1.62-1.65
0
N-'(-) 40
(m, 1H), 1.84-1.88 (m, 1H), 2.08
I ) N Method
B (s, 6H), 2.33 (t, J = 6.8 Hz, 1H),
NN
H c ) 2.45-
2.76 (m, 3H), 3.52 (br s,
2H), 3.66 (q, J = 14.4 Hz, 2H),
\ 4.20
(d, J = 4.0 Hz, 2H), 7.69-
(S)-4-Chloro-3-((7,8-dihydro-6H- 7.75
(m, 3H), 7.77 (s, 1H), 7.88-
pyrimido[5,4-b][1,4]oxazin-4- 7.90
(m, 2H), 8.03 (d, J = 8.4 Hz,
yl)oxy)-N-(4-((3- 1H),
8.16 (s, 1H), 10.54 (s, 1H);
(dimethylamino)pyrrolidin-1- ESI-MS (m/z) 577 (M+H)+.
yl)methyl)-3-
(trifluoromethyl)phenyl)benzamid
e
46. CI A H Al,
Bl, 1I-1 NMR (400 MHz, DMSO-d6)
O WI N 0 CF C14
6 1.25-1.29 (m, 1H), 1.63-1.66
NCI
(m, 1H), 1.76-1.89 (m, 1H), 2.10
1 ) N Method
B (s, 6H), 2.35 (t, J = 6.8 Hz, 1H),
NN 2.46-2.76 (m, 3H), 3.37 (br s,
H c )
.1 R) N¨
2H), 3.66 (q, J = 14.4 Hz, 2H),
/ 4.20 (t, J = 10.4 Hz, 2H),
7.69-
(R)-4-Chloro-3-((7,8-dihydro-6H- 7.80
(m, 4H), 7.88-7.90 (br s,
pyrimido[5,4-b][1,4]oxazin-4- 2H),
8.03 (d, J = 8.0 Hz, 1H),
yl)oxy)-N-(4-((3- 8.16
(s, 1H), 10.54 (s, 1H); ESI-
(dimethylamino)pyrrolidin-1- MS (m/z) 577 (M+H)+.
yl)methyl)-3-
(trifluoromethyl)phenyl)benzamid
e
164
CA 03064975 2019-11-26
WO 2018/215668 PCT/EP2018/063957
No. Intermed Analytical Data
Structure and Name iates and
Method
47. CI Ai H Al,
Bl, 'I-1 NMR (400 MHz, DMSO-d6)
O WI N is u3 C31
6 1.46-1.52 (m, 1H),1.92-1.99
N- (m, 1H), 2.14-2.25 (m, 4H), 2.51
d
1 ) N (s,
3H), 2.67-2.71 (m, 1H), 3.01-
H C
N N Method
3.09 (m, 1H), 3.63-3.67 (br s,
)
.1 R) B' 2H),
3.68-3.72 (br s, 2H), 4.20 (t,
NH
/ J = 4.0 Hz, 2H), 7.69-7.90 (m,
(R)-4-Chloro-3-((7,8-dihydro-6H- 7H),
8.02 (q, J = 8.4 Hz, 1H),
pyrimido[5,4-b][1,4]oxazin-4- 8.16
(s, 1H), 10.54 (s, 1H); ESI-
yl)oxy)-N-(4-((3- MS (m/z) 563 (M)+.
(methylamino)pyrrolidin-l-
yl)methyl)-3-
(trifluoromethyl)phenyl)benzamid
e
48. a A
H Al, Bl, 1I-1 NMR (400
MHz, DMSO-d6)
O Wj N i& CF3 C32
6 1.54-1.55 (m, 1H),1.98-2.01
Nj: ) 0 (m,
1H), 2.26 (s, 3H), 2.36-2.46
N N N
(m, 1H), 2.46-2.58 (m, 3H), 2.69
H (s)
Method (t, J=
9.6 Hz, 1H), 3.36-3.39 (br
'ClH B' s, 1H), 3.52 (s, 2H), 3.69 (s,
2H),
/
(S)-4-Chloro-3-((7,8-dihydro-6H- 4.20
(t, J = 4.0 Hz, 2H), 7.69 (s,
pyrimido[5,4-b][1,4]oxazin-4- 1H),
7.73-7.90 (m, 5H), 8.04 (d,
yl)oxy)-N-(4-((3- J =
8.4 Hz, 1H), 8.16 (s, 1H),
(methylamino)pyrrolidin-1- 10.55
(s, 1H); ESI-MS (m/z) 563
yl)methyl)-3- (M)t
(trifluoromethyl)phenyl)benzamid
e
49. a Ai
H F Al, Bl, 1I-1 NMR (400
MHz, DMSO-d6)
N 2-fluoro- 6 3.51 (br s, 2H), 4.20 (t, J = 4.0
0 WI so u3
NJõ0, 0 3- Hz,
2H), 7.45 (t, J = 8.0 Hz, 1H),
1 j
(trifluoro 7.66-7.94 (m, 7H), 10.48 (s, 1H);
N N H methyl)an ESI-MS (m/z) 469 (M+H)+.
4-Chloro-3-((7,8-dihydro-6H- iline
pyrimido[5,4-b][1,4]oxazin-4-
yl)oxy)-N-(2-fluoro-3- Method B
(trifluoromethyl)phenyl)benzamid
e
50. ci Ai Al,
Bl, 1I-1 NMR (400 MHz, DMSO-d6)
H
N 4-amino- 6 3.51-3.52 (br s, 2H), 4.20 (t, J
0 WI Ail CF3
0 0 2- = 4.4
Hz, 2H), 7.69 (s, 1H), 7.80-
ON (trifluoro 7.91 (m, 4H), 8.26
(d, J = 1.6 Hz,
N N H
methyl)be 1H), 8.28 (d, J = 2.0 Hz, 1H),
4-Chloro-N-(4-cyano-3-
nzonitrile 8.42 (s, 1H), 10.54 (s, 1H); ESI-
(trifluoromethyl)pheny1)-347,8- MS (m/z) 476 (M+H)+.
dihydro-6H-pyrimido[5,4- Method B
b][1,4]oxazin-4-yl)oxy)benzamide
165
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
No. Intermed Analytical Data
Structure and Name iates and
Method
51. CI H Al,
Bl, lli NMR (400 MHz, DMSO-d6)
N 4-
6 3.51 (br s, 2H), 4.20 (t, J = 4.0
0 WI
IW
(trifluoro Hz, 2H), 7.69-7.79 (m, 5H),
r s-e 1 3
methyl)an 7.88-7.90 (br s, 2H), 7.99 (d, J =
N N iline 8.4 Hz, 2H), 10.62 (s, 1H); ESI-
H
4-Chloro-3-((7,8-dihydro-6H- MS (m/z) 451 (M+H)+.
pyrimido[5,4-b][1,4]oxazin-4- Method B
yl)oxy)-N-(4-
(trifluoromethyl)phenyl)benzamid
e
52. 01 H Al,
Bl, lli NMR (400 MHz, DMSO-d6)
O W N,,CF3 C51
6 2.61 (d, J = 1.2 Hz, 3H), 3.51-
J c(: 1 3.52 (br s, 2H), 4.20 (t, J = 4.4 ,) 0 Ni*-
1
Hz, 2H), 7.69 (s, 1H), 7.77-7.91
N N Method B (m, 4H), 8.52 (d, J = 2.4 Hz, 1H),
H
4-Chloro-3-((7,8-dihydro-6H- 9.06
(d, J = 2.0 Hz, 1H), 10.74
pyrimido[5,4-b][1,4]oxazin-4- (s,
1H); ESI-MS (m/z) 466
yl)oxy)-N-(6-methyl-5- (M+H)+.
(trifluoromethyl)pyridin-3-
yl)benzamide
53. a A Al,
Bl, lli NMR (400 MHz, DMSO-d6)
H
N r CF3
3-amino- 6 3.51 (br s, 2H), 4.20 (t, J = 4.0
O WI
1W 5- Hz,
2H), 7.69 (s, 1H), 7.88-7.90
0
N 1,)
NN) CN (trifluoro (m,
4H), 8.09 (s, 1H), 8.43 (s,
methyl)be 1H), 8.48 (s, 1H), 10.84 (s, 1H);
H nzonitrile ESI-MS (m/z) 476 (M+H)+.
4-Chloro-N-(3-cyano-5-
(trifluoromethyl)pheny1)-347,8- Method B
dihydro-6H-pyrimido[5,4-
b][1,4]oxazin-4-yl)oxy)benzamide
54. a al Al,
Bl, lli NMR (400 MHz, DMSO-d6)
H
N 401 CF3 3-
6 3.51 (br s, 2H), 3.83 (s, 3H),
O WI
methoxy- 4.20 (t, J = 4.0 Hz, 2H), 7.00 (s,
5- 1H),
7.69-7.90 (m, 7H), 10.56 (s,
= I ) OMe (trifluoro 1H); ESI-MS (m/z) 481
(M+H)+.
NN
H methyl)an
4-Chloro-3-((7,8-dihydro-6H- iline
pyrimido[5,4-b][1,4]oxazin-4-
yl)oxy)-N-(3-methoxy-5-
(trifluoromethyl)phenyl)benzamid Method B
e
55. a 0 H Al,
Bl, 'HNMR (400 MHz, DMSO-d6)
3-fluoro- 6 3.51-3.52 (br s, 2H), 4.20 (t, J
O N is c3
5- = 4.0
Hz, 2H), 7.43 (d, J = 8.8
0
(trifluoro Hz, 1H), 7.69 (s, 1H), 7.77-7.90
= I ) F
methyl)an (m, 4H), 8.0-8.02 (br s, 2H),
NN
H iline 10.75
(s, 1H); ESI-MS (m/z) 469
166
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
No. Intermed Analytical Data
Structure and Name iates and
Method
4-Chloro-3-((7,8-dihydro-6H- (M+H)+.
pyrimido[5,4-b][1,4]oxazin-4- Method B
yl)oxy)-N-(3-fluoro-5-
(trifluoromethyl)phenyl)benzamid
e
56. CI Ai H Al,
Bl, 1I-1 NMR (400 MHz, DMSO-d6)
N 3- 6 3.51
(br s, 2H), 4.20 (br s, 2H),
O w is u3
methoxy- 6.79 (s, 1H), 7.60 (br s, 2H),
NC) 5-
7.69-7.88 (m, 5H), 10.31 (s, 1H),
1 ) OH
(trifluoro 10.45 (s, 1H); ESI-MS (m/z) 467
NN
H methyl)an (M+H)+.
4-Chloro-3-((7,8-dihydro-6H- iline
pyrimido[5,4-b][1,4]oxazin-4-
yl)oxy)-N-(3-hydroxy-5- Method F
(trifluoromethyl)phenyl)benzamid
e
57. CI Ai Al,
Bl, 1I-1 NMR (400 MHz, DMSO-d6)
H
N 2- 6 3.51
(br s, 2H), 3.91 (s, 3H),
O WI dli CF3
methoxy- 4.20 (t, J = 4.0 Hz, 2H), 7.29 (d,
5- J =
8.4 Hz, 1H), 7.59 (dd, Ji =
= 1 ) I
NN
(trifluoro 1.6 Hz, .1-2 = 8.4 Hz, 1H), 7.70-
H
methyl)an 7.87 (m, 5H), 8.06 (s, 1H), 9.86
4-Chloro-3-((7,8-dihydro-6H- iline (s,
1H); ESI-MS (m/z) 548
pyrimido[5,4-b][1,4]oxazin-4- (M+H)+.
yl)oxy)-N-(2-methoxy-5- Method B
(trifluoromethyl)phenyl)benzamid
e
58. ci Al,
Bl, 1I-1 NMR (400 MHz, DMSO-d6)
H
O WI N 0 u3 C26
6 3.18 (t, J = 4.4 Hz, 4H), 3.51-
0 0 3.52
(br s, 2H), 3.75 (t, J = 4.4
N: l
I ) N Method
B Hz, 4H), 4.20 (t, J = 4.0 Hz, 2H),
N N H ( D
6.99 (s, 1H), 7.62 (s, 2H), 7.67-
0 7.89
(m, 6H), 10.48 (s, 1H); ESI-
4-Chloro-3-((7,8-dihydro-6H- MS (m/z) 536 (M+H)t
pyrimido[5,4-b][1,4]oxazin-4-
yl)oxy)-N-(3-Morpholino-5-
(trifluoromethyl)phenyl)benzamid
e
59, a Ai Al,
Bl, 1I-1 NMR (400 MHz, DMSO-d6)
H
O WI N io u3 C60
6 1.83-1.85 (m, 1H), 2.21 (s,
0 6H),
2.49-2.51 (m, 1H), 3.06 (m,
1 j
N
H C Method
B 1H), 3.28-3.52 (m, 6H), 4.20 (t, J
NN = 4.4 Hz, 2H), 6.53 (s, 1H), 7.22
IR) N-
(s, 1H), 7.48 (s, 1H), 7.69-7.90
/ (m,
5H), 10.34 (s, 1H); ESI-MS
(R)-4-Chloro-3-((7,8-dihydro-6H- (m/z) 563 (M+H)+.
pyrimido[5,4-b][1,4]oxazin-4-
167
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
No. Intermed Analytical Data
Structure and Name iates and
Method
yl)oxy)-N-(3-(3-
(dimethylamino)pyrrolidin-l-y1)-
5-
(trifluoromethyl)phenyl)benzamid
e
Al, Bl,
60. a 0
H
o N CF C61
1I-1 NMR (400 MHz, DMSO-d6)
1.80-1.82 (m, 1H), 2.20 (s,
I\J N
N(s) Method B 6H), 2.79-2.82 (m,
1H), 3.06 (t, J
H c = 8.4
Hz, 1H), 3.26-3.51 (m,
N¨ 6H), 4.20 (br s, 2H), 6.53
(s, 1H),
/
(S)-4-Chloro-3-((7,8-dihydro-6H- 7.22
(s, 1H), 7.48 (s, 1H), 7.69
pyrimido[5,4-b][1,4]oxazin-4- (s,
1H), 7.74-7.88 (m, 4H), 10.33
yl)oxy)-N-(3-(3- (s,
1H); ESI-MS (m/z) 563
(dimethylamino)pyrrolidin-l-y1)- (M+H)+.
5-
(trifluoromethyl)phenyl)benzamid
e
61. CI 0 Al,
Bl, 1I-1 NMR (400 MHz, DMSO-d6)
H
0
N% N r& CF3 C46 6 2.02-
2.11 (m, 4H), 3.43-3.51
INI
(m, 4H), 3.52 (br s, 2H), 4.20 (t,
C IW
1 ) Method
B J = 4.0 Hz, 2H), 7.05 (s, 1H),
NN 7.65-7.75 (m, 3H), 7.75-7.80 (m,
H
2H), 7.87-7.89 (br s, 2H), 10.39
F F (s,
1H); ESI-MS (m/z) 570
4-Chloro-N-(3-(4,4- (M+H)+.
difluoropiperidin-l-y1)-5-
(trifluoromethyl)pheny1)-347,8-
dihydro-6H-pyrimido[5,4-
b][1,4]oxazin-4-y1)oxy)benzamide
62. CI 0 Al,
Bl, 1I-1 NMR (400 MHz, DMSO-d6)
H
O N CF3 C49
6 3.17 (d, J = 5.2 Hz, 3H), 3.33-
Nc,o, o r 3.51
(m, 4H), 4.09 (d, J = 10.4
1 j N Method
B Hz, 1H), 4.20 (s, 2H), 4.42 (m,
NN
H ( ? 1H),
5.0 (s, 1H), 6.48 (s, 1H),
,f R) OH 7.22 (s, 1H), 7.46 (s, 1H),
7.69-
(R)-4-Chloro-3-((7,8-dihydro-6H- 7.88
(s, 3H), 7.89 (s, 2H), 10.33
pyrimido[5,4-b][1,4]oxazin-4- (s,
1H); ESI-MS (m/z) 536
yl)oxy)-N-(3-(3- (M+H)+.
hydroxypyrrolidin-l-y1)-5-
(trifluoromethyl)phenyl)benzamid
e
168
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
No. Intermed Analytical Data
Structure and Name iates and
Method
63. ci & Al,
Bl, 'I-1 NMR (400 MHz, DMSO-d6)
H
O N s CF3 C52
6 1.73 (br s, 1H), 2.12 (m, 2H),
0 0 3.11
(br s, 2H), 3.25 (br s, 2H),
Nxi j
N 11 rN)
Method 3.54-3.60 (m, 2H),3.76 (br s,
B' 2H),
4.21 (s, 3H), 6.78 (s, 1H),
\-NH 7.49
(s, 1H), 7.58 (s, 1H), 7.73-
HCI 7.77
(br s, 2H), 7.91-8.0 (br s,
N-(3-(1,4-Diazepan-l-y1)-5-
2H), 9.21 (br s, 2H), 10.33 (s,
(trifluoromethyl)pheny1)-4-chloro-
1H) ESI-MS (m/z) 549 (M+H)+.
3-((7,8-dihydro-6H-pyrimido[5,4-
b][1,4]oxazin-4-yl)oxy)benzamide
hydrochloride
64. CI 40
H
Example 1I-1 NMR (400 MHz, DMSO-d6)
N 0 CF N JCUCN 7 6 2.36-
2.41 (m, 4H), 3.46-3.51
o n
N,.)
(dihydroc (m, 6H), 3.61 (br s, 2H), 4.04 (s,
NCI
L I j hloride 2H), 4.20 (t, J =
4.0 Hz, 2H),
'1\IN
H salt) 7.69
(s, 1H), 7.74-7.81 (m, 3H),
4-Chloro-N-(4-((4-(2-
7.88-7.90 (br s, 2H), 8.05 (d, J =
cyanoacetyl)piperazin-1-
8.0 Hz, 1H), 8.18 (s, 1H), 10.57
yl)methyl)-3- Method
K (s, 1H); ESI-MS (m/z) 616
(trifluoromethyl)pheny1)-347,8- (M+H)+.
dihydro-6H-pyrimido[5,4-
b][1,4]oxazin-4-yl)oxy)benzamide
65. a a Al, Bl, 1I-1 NMR (400 MHz, DMSO-d6)
H
O N s CF3 C55
6 1.96 (d, J = 10.4 Hz, 1H), 2.15
(d, J = 10.0 Hz, 1H), 3.17-3.25
NC)l
I ) N Method (m,
2H), 3.37-3.39 (m, 1H), 3.42
NN (5)rõ B'
(br s, 2H), 3.62 (d, J = 10.4 Hz,
H
Ie(S) 1H),
4.0-4.21 (m, 3H), 4.46 (s,
H HCI 1H),
4.67 (s, 1H), 6.71 (s, 1H),
N-(3-41S,4S)-2,5- 7.53
(s, 1H), 7.56 (s, 1H), 7.13-
diazabicyclo[2.2.1]heptan-2-y1)-5- 7.92
(m, 5H), 8.89-8.98 (m, 1H),
(trifluoromethyl)pheny1)-4-chloro- 10.48
(s, 1H); ESI-MS (m/z) 616
3-((7,8-dihydro-6H-pyrimido[5,4- (M+H)+.
b][1,4]oxazin-4-yl)oxy)benzamide
hydrochloride
66. ci &
H Al,
Bl, 1I-1 NMR (400 MHz, DMSO-d6)
O N 0 CF3 C47
6 0.36 (br s, 2H), 0.44-0.45 (m,
N 2H), 1.66 (br s, 1H), 2.68 (br s,
0
I-31 j N Method
B 4H), 3.18 (br s, 4H), 3.52 (br s,
NN H C )
2H), 4.21 (t, J = 8.4 Hz, 2H),
N 6.55
(s, 1H), 7.59-7.88 (m, 7H),
A 10.37
(s, 1H); ESI-MS (m/z) 575
4-Chloro-N-(3-(4- (M+H)+.
cyclopropylpiperazin-l-y1)-5-
(trifluoromethyl)pheny1)-347,8-
dihydro-6H-pyrimido[5,4-
b][1,4]oxazin-4-y1)oxy)benzamide
169
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
No. Intermed Analytical Data
Structure and Name iates and
Method
67. CI AI Al,
Bl, 'I-1 NMR (400 MHz, DMSO-d6)
H
N CF3 C50
6 3.52 (br s, 2H), 4.21 (d, J= 8.4
0
IW
c,0 0 Hz,
2H), 5.82 (d, J = 11.2 Hz,
N 1 )
NN) 0NH Method
B 1H), 6.29-6.33 (m, 1H), 6.43-
6.49 (m, 1H), 7.70 (s, 1H), 7.61-
H % 7.63
(m, 6H), 8.45 (s, 1H), 10.53
N-(3-Acrylamido-5- (s,
1H), 10.62 (s, 1H); ESI-MS
(trifluoromethyl)pheny1)-4-chloro- (m/z) 520 (M+H)+.
3-((7,8-dihydro-6H-pyrimido[5,4-
b][1,4]oxazin-4-yl)oxy)benzamide
68. CI Ai Al,
Bl, 1I-1 NMR (400 MHz, DMSO-d6)
H
O N io u3 C59
6 2.42-2.51 (m, 4H), 3.25 (d, J =
N Method B 4.4 Hz, 4H), 3.33-3.52 (m, 3H),
IC;1 ) N 4.21
(t, J = 4.0 Hz, 2H), 4.48 (t, J
NN = 6.0 Hz, 2H), 4.58 (t, J = 6.4
H C
N) Hz,
2H), 6.97 (s, 1H), 7.60 (s,
6 1H),
7.65 (s, 1H), 7.69-7.89 (m,
0 5H),
10.38 (s, 1H); ESI-MS (m/z)
4-Chloro-3-((7,8-dihydro-6H- 591 (M+H)t
pyrimido[5,4-b][1,4]oxazin-4-
yl)oxy)-N-(3-(4-(oxetan-3-
yl)piperazin-l-y1)-5-
(trifluoromethyl)phenyl)benzamid
e
69. CI Ai Al,
Bl, 1I-1 NMR (400 MHz, DMSO-d6)
H
O N 40 c3 C56
6 1.92-1.94 (m, 1H), 1.95-2.04
= ,c) 0
(m, 1H), 3.11 (d, J = 10.4 Hz,
1 )
N Method
B 2H), 3.17-3.52 (m, 4H), 4.21 (t, J
NN = 4.4 Hz, 2H), 4.42 (s, 1H), 5.0
H ( (S)
OH (s,
1H), 6.49 (s, 1H), 7.22 (s,
(S)-4-Chloro-3-((7,8-dihydro-6H- 1H),
7.46 (s, 1H), 7.74-7.89 (m,
pyrimido[5,4-b][1,4]oxazin-4- 5H),
10.33 (s, 1H); ESI-MS (m/z)
yl)oxy)-N-(3-(3- 536 (M+H)+.
hydroxypyrrolidin-l-y1)-5-
(trifluoromethyl)phenyl)benzamid
e
70. Cl Ai Al,
Bl, 1I-1 NMR (400 MHz, DMSO-d6)
H
N 40 u3 C62
6 1.17 (s, 6H), 3.03-3.06 (m,
0 W
N-1:3
1H), 3.52 (br s, 2H), 4.01 (br s,
L.
I ) Method 2H),
4.21 (br s, 2H), 7.60 (s, 1H),
NN NH B'
7.69 (s, 1H), 7.77-7.81 (m, 2H),
H 7.92
(br s, 2H), 8.11-8.17 (m,
4-Chloro-3-((7,8-dihydro-6H- 2H),
10.65 (s, 1H); ESI-MS (m/z)
pyrimido[5,4-b][1,4]oxazin-4- 522 (M+H)+.
yl)oxy)-N-(3-
((isopropylamino)methyl)-5-
(trifluoromethyl)phenyl)benzamid
170
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
No. Intermed Analytical Data
Structure and Name iates and
Method
e
71. CI
H Al,
Bl, 1I-1 NMR (400 MHz, DMSO-d6)
O WI N 0 CF3 C53
6 1.32 (d, J = 5.2 Hz, 3H), 2.90
0 (t, J
= 11.2 Hz, 1H), 3.10-3.16
N Method
(m, 2H), 3.36-3.40 (m, 3H),
N N
H 4..õ B' 3.54-3.57 (m,
2H), 3.77-3.92 (m,
H Hci 1H),
4.23 (br s, 2H), 7.09 (s, 1H),
(S)-4-Chloro-3-((7,8-dihydro-6H- 7.71-
7.77 (m, 4H), 7.92-7.95 (m,
pyrimido[5,4-b][1,4]oxazin-4- 3H),
9.42-9.47 (br s, 1H), 9.66-
yl)oxy)-N-(3-(3-methylpiperazin- 9.67
(br s, 1H), 10.58 (s, 1H);
1-y1)-5- ESI-MS (m/z) 549 (M+H)+.
(trifluoromethyl)phenyl)benzamid
e hydrochloride
72. a
H Al,
Bl, 1I-1 NMR (400 MHz, DMSO-d6)
O WI N = CF3 C54
6 1.31 (d, J= 6.0 Hz, 3H), 2.83-
2.89 (m, 1H), 3.08-3.10 (m, 2H),
NC)) 0
Method 3.36-
3.40 (m, 3H), 3.51-3.54 (m,
,
N N N
H 4R B' 3H), 3.78-3.88
(m, 1H), 4.21 (t, J
N
H HCI = 4.0
Hz, 2H), 7.70-7.78 (m,
(R)-4-Chloro-3-((7,8-dihydro-6H- 4H),
7.90-7.93 (m, 3H), 9.17-
pyrimido[5,4-b][1,4]oxazin-4- 9.19
(m, 1H), 9.43-9.46 (m, 1H),
yl)oxy)-N-(3-(3-methylpiperazin- 10.51
(s, 1H); ESI-MS (m/z) 549
1-y1)-5- (M+H)+..
(trifluoromethyl)phenyl)benzamid
e hydrochloride
73. CI al Al, Bl, 1I-1 NMR (400 MHz, DMSO-d6)
H
N CF3 C58 6 2.61
(m, 4H), 3.20-3.24 (m,
0 WI
ir 7H),
3.52 (br s, 2H), 4.20 (t, J =
Method B 4.4 Hz, 2H), 6.98 (s, 1H), 7.60 (s,
)\J N ( D
H 1H), 7.65 (s,
2H), 7.69-7.80 (m,
N 2H),
7.87-7.89 (br s, 2H), 10.38
(s, 1H); ESI-MS (m/z) 573
4-Chloro-3-((7,8-dihydro-6H- (M+H)+.
pyrimido[5,4-b][1,4]oxazin-4-
yl)oxy)-N-(3-(4-(prop-2-yn-1-
y1)piperazin-l-y1)-5-
(trifluoromethyl)phenyl)benzamid
e
74. ci
Al, Bl, 1I-1 NMR (400 MHz, DMSO-d6)
W FN1 1" CF3 C48 6 1.79-
1.92 (m, 2H), 1.99-2.04
0
(m, 2H), 3.22-3.26 (m, 2H),
I ) N Method
B 3.34-3.43 (m, 2H), 3.51-3.52 (m,
NN ,,
2H), 4.20 (t, J = 8.4 Hz, 2H),
H
Y 4.80-
4.96 (m, 1H), 7.00 (s, 1H),
F 7.63
(d, J = 8.4 Hz, 2H), 7.69-
4-Chloro-3-((7,8-dihydro-6H- 7.89
(m, 5H), 10.37 (s, 1H); ESI-
171
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
No. Intermed Analytical Data
Structure and Name iates and
Method
pyrimido[5,4-b][1,4]oxazin-4- MS (m/z) 552 (M+H)t
yl)oxy)-N-(3-(4-fluoropiperidin-1-
y1)-5-
(trifluoromethyl)phenyl)benzamid
e
75. a a Al,
Bl, 1H NMR (400 MHz, DMSO-d6)
H
O WI N r cF3 C57
6 1.90-1.99 (m, 2H), 2.26 (s,
.,(:) 0 r 3H),
2.45 (t, J = 5.2 Hz, 2H),
y 1 1 method
B 2.62 (t, J= 4.4 Hz, 2H), 3.46 (t, J
NI12 rN
¨N ) = 6.0 Hz, 2H), 3.52-3.55
(m,
4H), 4.20 (t, J = 3.6 Hz, 2H),
\
\ 6.67 (s, 1H), 7.38 (s,
1H), 7.47
4-Chloro-3-((7,8-dihydro-6H- (s,
1H), 7.69 (s, 1H), 7.75 (d, J =
pyrimido[5,4-b][1,4]oxazin-4- 8.8
Hz, 1H), 7.81 (s, 1H), 7.87-
yl)oxy)-N-(3-(4-methy1-1,4- 7.88
(m, 2H), 10.31 (s, 1H); ESI-
diazepan-l-y1)-5- MS (m/z) 563 (M+H)+..
(trifluoromethyl)phenyl)benzamid
e
76. a gam A2 1H NMR
(400 MHz, DMSO-d6)
H
N 0 CF3 , Bl,
C19 6 2.23 (br s, 3H), 2.40-2.51 (br s,
0 WI
NO 0 4H),
3.18-3.21 (br s, 4H), 4.85 (s,
I N Method 2H),
6.97 (s, 1H), 7.60-7.97 (m,
NN 0 H G' 5H), 8.10 (s, 1H), 10.41 (s, 1H),
C
N) 11.80
(s, 1H); ESI-MS (m/z) 563
I
4-Chloro-N-(3-(4- (M+H)+.
methylpiperazin-l-y1)-5-
(trifluoromethyl)pheny1)-347-
oxo-7,8-dihydro-6H-
pyrimido[5,4-b][1,4]oxazin-4-
yl)oxy)benzamide
77. a abh A2,
Bl, 1H NMR (400 MHz, DMSO-d6)
H
O VI N 0 CFp.N Cl
6 0.98 (t, J = 7.2 Hz, 3H), 2.28-
0 N) 2.50 (m, 10H), 3.56
(s, 2H), 4.84
NN 0 Method J (s, 2H), 7.72 (d, J = 8.4 Hz, 1H),
¨
H 7.82
(d, J = 8.0 Hz, 1H), 7.94-
4-Chloro-N-(4-((4-ethylpiperazin- 8.04
(m, 3H), 8.09 (s, 1H), 8.18
1-yl)methyl)-3- (s,
1H), 10.59 (s, 1H), 11.80 (s,
(trifluoromethyl)pheny1)-347- 1H); ESI-MS (m/z) 591 (M+H)+.
oxo-7,8-dihydro-6H-
pyrimido[5,4-b][1,4]oxazin-4-
yl)oxy)benzamide
78. ci al
H A3, 1H NMR
(400 MHz, DMSO-d6)
O N 40, cFril. Bl, Cl
6 0.98 (t, J = 7.2 Hz, 3H), 1.35
N%y N, (d, J
= 6.0 Hz, 3H), 2.28-2.33
.).7s) Method B (m,
10H), 3.16-3.34 (m, 1H),
N N H 3.56
(br s, 3H), 4.17 (t, J = 6.0
172
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
No. Intermed Analytical Data
Structure and Name iates and
Method
(S)-4-Chloro-N-(4-((4- Hz,
1H), 7.69-7.87 (m, 4H),
ethylpiperazin-1-yl)methyl)-3- 7.87-
7.90 (m, 2H), 8.03 (d, J =
(trifluoromethyl)pheny1)-3-46- 8.8
Hz, 1H), 8.17 (s, 1H), 10.54
methyl-7,8-dihydro-6H- (s, 1H).
pyrimido[5,4-b][1,4]oxazin-4-
yl)oxy)benzamide
79, ci a
H A3, 'H NMR
(400 MHz, DMSO-d6)
0 W N = CF3 Bl,
C19 6 1.35 (d, J = 6.0 Hz, 3H), 2.23
0 (s, 3H), 2.45-2.50
(m, 4H), 3.15-
Nc0),õts) N
Method B 3.22 (m, 5H), 3.53-3.57 (m, 1H),
N N CNI) 4.17
(t, J = 6.0 Hz, 1H), 6.96 (s,
H
1H), 7.60-7.69 (m, 3H), 7.75-
(S)-4-Chloro-3-46-methyl-7,8- 7.86
(m, 2H), 7.87-7.89 (m, 2H),
dihydro-6H-pyrimido[5,4- 10.36
(s, 1H); ESI-MS (m/z) 563
b] [1,4]oxazin-4-yl)oxy)-N-(3-(4- (M+H)+.
methylpiperazin-l-y1)-5-
(trifluoromethyl)phenyl)benzamid
e
80. ci am
H AS,
Bl, 1I-1 NMR (400 MHz, DMSO-d6)
N Cl
6 0.98 (t, J = 7.2 Hz, 3H), 1.19
0 0 N,) (d, J
= 6.4 Hz, 3H), 2.33-2.51
NILX (Rlip Method
A (m, 10H), 3.56 (s, 2H), 3.69-3.78
H (m,
2H), 4.25-4.28 (m, 1H),
(R)-4-Chloro-N-(4-((4- 7.71-
7.77 (m, 3H), 7.88-7.89 (br
ethylpiperazin-1-yl)methyl)-3- s,
3H), 8.03 (d, J = 8.8 Hz, 1H),
(trifluoromethyl)pheny1)-3-47- 8.17
(s, 1H), 10.54 (s, 1H); ESI-
methy1-7,8-dihydro-6H- MS (m/z) 591 (M+H)+.
pyrimido[5,4-b][1,4]oxazin-4-
yl)oxy)benzamide
81. CI H A6,
Bl, 1I-1 NMR (400 MHz, DMSO-d6)
N Cl
6 0.98 (t, J = 7.2 Hz, 3H), 1.19
NOj 0
N I ''', N...-I , (d, J
= 6.4 Hz, 3H), 2.31-2.51
L :NS) Method
A (m, 10H), 3.56 (s, 2H), 3.69-3.78
H (m,
2H), 4.26 (dd, Ji = 2.4 Hz, .1-2
(S)-4-Chloro-N-(4-((4- = 10.4
Hz, 1H), 7.71-7.77 (m,
ethylpiperazin-1-yl)methyl)-3- 3H),
7.88-7.89 (br s, 3H), 8.03
(trifluoromethyl)pheny1)-3-47- (d, J
= 8.8 Hz, 1H), 8.17 (s, 1H),
methyl-7,8-dihydro-6H- 10.55
(s, 1H); ESI-MS (m/z) 591
pyrimido[5,4-b][1,4]oxazin-4- (M+H)+.
yl)oxy)benzamide
82. ci H A4,
Bl, 1I-1 NMR (400 MHz, DMSO-d6)
N cF N.--, Cl
6 0.98 (t, J = 6.8 Hz, 3H), 1.24
0 r
0 VII' N....) (s, 6H), 2.27-2.51
(m, 10H), 3.56
= I
Method A (s, 2H), 3.89 (s, 2H), 7.72 (s,
N'N' 1H),
7.77 (d, J = 8.0 Hz, 2H),
H
4-Chloro-3-47,7-dimethy1-7,8- 7.88-
7.99 (m, 3H), 8.03 (dd, Ji =
173
CA 03064975 2019-11-26
WO 2018/215668 PCT/EP2018/063957
No. Intermed Analytical Data
Structure and Name iates and
Method
dihydro-6H-pyrimido[5,4- 2.0
Hz, J2 = 8.8 Hz, 1H), 8.17 (s,
b][1,4]oxazin-4-yl)oxy)-N-(4-44- 1H),
10.55 (s, 1H); ESI-MS (m/z)
ethylpiperazin-l-yl)methyl)-3- 605 (M+H)+.
(trifluoromethyl)phenyl)benzamid
e
83. CI H A4,
Bl, 1I-1 NMR (400 MHz, DMSO-d6)
o
N io u3 C19 6 1.24
(s, 6H), 2.22 (s, 3H), 2.45-
,II0 0 2.51
(m, 4H), 3.21 (t, J= 4.8 Hz,
N
N Method A 4H), 3.89 (s, 2H), 6.96 (s, 1H),
N r\ C j 7.60
(s, 1H), 7.64 (s, 1H), 7.76
N
I (d, J
= 8.4 Hz, 2H), 7.87-7.90
4-Chloro-3-47,7-dimethy1-7,8- (m,
3H), 10.38 (s, 1H); ESI-MS
dihydro-6H-pyrimido[5,4- (m/z) 577 (M)+.
b][1,4]oxazin-4-yl)oxy)-N-(3-(4-
methylpiperazin-l-y1)-5-
(trifluoromethyl)phenyl)benzamid
e
84. I. Al,
B3, 1I-1 NMR (400 MHz, DMSO-d6)
O CFr.N C2 ..
6 2.15 (s, 3H), 2.18 (s, 3H), 2.49-
Ni.L,0 0 1W N,.) 2.51
(m, 8H), 3.33 (br s, 4H),
Method A 4.20 (t, J = 4.0 Hz, 2H), 7.46 (d,
N1\1)
H J =
8.0 Hz, 1H), 7.67-7.71 (m,
3-((7,8-Dihydro-6H-pyrimido[5,4- 4H),
7.80 (dd, ./i = 1.6 Hz, J2 =
b][1,4]oxazin-4-yl)oxy)-4-methyl- 7.6
Hz, 1H), 8.04 (dd, ./i = 2.0
N-(4-((4-methylpiperazin-1- Hz, J2
= 8.4 Hz, 1H), 8.18 (s,
yl)methyl)-3- 1H),
10.41 (s, 1H); ESI-MS (m/z)
(trifluoromethyl)phenyl)benzamid 543 (M+H)+.
e
85. 140 H Al,
B3, 1I-1 NMR (400 MHz, DMSO-d6)
O N io c3 C20 ..
6 1.03 (t, J = 7.2 Hz, 3H), 2.18
Nj1:3 0 (s,
3H), 2.37 (q, J = 7.2 Hz, 2H),
I j N 2.50-
2.55 (br s 4H), 3.20-3.25 (br
NN
H C ) Method
B s,4H), 3.51-3.52 (br s, 2H), 4.20
N (t, J = 4.0 Hz, 2H), 6.93 (s, 1H),
7.46 (d, J = 8.0 Hz, 1H), 7.61-
3-((7,8-Dihydro-6H-pyrimido[5,4- 7.71
(m, 5H), 7.79 (d, J= 8.0 Hz,
b][1,4]oxazin-4-yl)oxy)-N-(3-(4- 1H),
10.25 (s, 1H); ESI-MS (m/z)
ethylpiperazin-l-y1)-5- 543 (M+H)+.
(trifluoromethyl)pheny1)-4-
methylbenzamide
86. Al,
B3, 1I-1 NMR (400 MHz, DMSO-d6)
H
J''
0 N io ur N C3 6 0.95
(d, J= 6.8 Hz, 6H), 2.18
0
N,) (s, 3H), 2.38-2.50 (m, 9H), 3.50-
N ='(:) 0 Method
A 3.54 (m, 4H), 4.19 (t, J = 4.0 Hz,
NI\J) 2H),
7.46 (d, J = 8.4 Hz, 1H),
H 7.67-
7.78 (m, 4H), 7.79 (d, J =
3-((7,8-Dihydro-6H-pyrimido[5,4-
6.0 Hz, 1H), 8.03 (d, J = 8.8 Hz,
174
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
No. Intermed Analytical Data
Structure and Name iates and
Method
b] [1,4]oxazin-4-yl)oxy)-N-(4-44- 1H),
8.18 (s, 1H), 10.41 (s, 1H);
isopropylpiperazin-l-yl)methyl)- ESI-MS (m/z) 571 (M+H)+.
3-(trifluoromethyl)pheny1)-4-
methylbenzamide
87. 1. Al,
B3, 1H NMR (400 MHz, DMSO-d6)
O 401 cF3 C19
6 2.22 (s, 6H), 2.46 (t, J = 4.8
0 Hz,
4H), 3.21 (t, J = 4.8 Hz, 4H),
N Method
A 3.50-3.51 (br s, 2H), 4.20 (t, J =
NN CN)
H 4.0
Hz, 2H), 6.94 (s, 1H),7.46 (d,
I J =
8.0 Hz, 1H), 7.62 (s, 1H),
3-((7,8-Dihydro-6H-pyrimido[5,4- 7.67-
7.77 (m, 3H), 7.78 (s, 1H),
b][1,4]oxazin-4-yl)oxy)-4-methyl- 7.80
(d, J = 2.0 Hz, 1H), 10.24
N-(3 -(4-methylpiperazin-l-y1)-5 - (s,
1H); ESI-MS (m/z) 529
(trifluoromethyl)phenyl)benzamid (M+H)+.
e
88. 40 cF3 Al,
B3, 1H NMR (400 MHz, DMSO-d6)
O 0 C18
6 1.01 (d, J = 6.8 Hz, 5H), 2.18
N CCI (s,
3H), 2.49-2.58 (m, 4H), 2.67-
I ,( ) N Method
B 2.70 (m, 1H), 3.19 (t, J = 4.8 Hz,
N N C )
4H), 3.50-3.51 (br s, 2H), 4.20 (t,
H
N J = 4.0 Hz, 2H), 6.92 (s,
1H),7.46 (d, J = 8.0 Hz, 1H),
3-((7,8-Dihydro-6H-pyrimido[5,4- 7.61-
7.78 (m, 6H), 7.80 (d, J =
b] [1,4]oxazin-4-yl)oxy)-N-(3-(4- 2.0
Hz, 1H), 10.24 (s, 1H); ESI-
isopropylpiperazin-l-y1)-5- MS (m/z) 557 (M+H)+.
(trifluoromethyl)pheny1)-4-
methylbenzamide
89. 0 H Al,
B3, 1H NMR (400 MHz, DMSO-d6)
O N 0 cF3 C21
6 0.88 (t, J = 7.6 Hz, 3H), 1.45-
0 0 1.50
(m, 2H), 2.18 (s, 3H), 2.28
N ' j
N (t, J = 7.6 Hz, 2H), 3.19-3.20 (br
N N H C )
s, 4H), 2.50 (br s, 4H), 3.50-3.51
N Method B (br s, 2H), 4.20 (t, J = 4.0 Hz,
H 2H),
6.93 (s, 1H),7.46 (d, J = 8.0
3-((7,8-Dihydro-6H-pyrimido[5,4- Hz,
1H), 7.61 (s, 1H), 7.68 (s,
b][1,4]oxazin-4-yl)oxy)-4-methyl- 2H),
7.78-7.80 (m, 3H), 10.25 (s,
N-(3-(4-propylpiperazin-l-y1)-5- 1H); ESI-MS (m/z) 557 (M+H)+.
(trifluoromethyl)phenyl)benzamid
e
90. 0 H Al,
B3, 1H NMR (400 MHz, DMSO-d6)
O N 0 cF3 C22
6 0.10-0.12 (m, 2H), 0.46-0.51
0 (m,
2H), 0.87 (m, 1H), 2.18 (s,
L 1 j N Method
B 3H), 2.23 (d, J = 6.4 Hz, 2H),
1\1N
H C ) 2.49-
2.59 (m, 4H), 3.21-3.28 (m,
N 4H), 3.50-3.51 (m, 2H), 4.20 (t, J
= 4.4 Hz, 2H), 6.94 (s, 1H), 7.46
175
CA 03064975 2019-11-26
WO 2018/215668 PCT/EP2018/063957
No. Intermed Analytical Data
Structure and Name iates and
Method
N-(3-(4- (d, J=
8.0 Hz, 1H), 7.61 (s, 1H),
(Cyclopropylmethyl)piperazin-1- 7.67-
7.71 (m, 4H), 7.78 (dd, J1 =
y1)-5-(trifluoromethyl)pheny1)-3- 1.6
Hz, .12 = 7.6 Hz, 1H), 10.25
((7,8-dihydro-6H-pyrimido[5,4- (s,
1H); ESI-MS (m/z) 569
b] [1,4]oxazin-4-yl)oxy)-4- (M+H)+.
methylbenzamide
91. e Al, B3, 1I-1 NMR (400 MHz, DMSO-d6) l ki cF3
C10 6 0.97 (t, J = 7.2 Hz, 3H), 2.18
0
NC) o IW (s, 3H), 2.27-2.51 (m, 10H),
1 ) Method
B 3.50-3.53 (m, 4H), 4.20 (t, J =
NN N
4.4 Hz, 2H), 7.34 (s, 1H), 7.46
H N
(d, J = 8.0 Hz, 1H), 7.73 (s, 3H),
3-((7,8-Dihydro-6H-pyrimido[5,4- 7.80
(d, J = 6.4 Hz, 1H), 8.00 (s,
b] [1,4]oxazin-4-yl)oxy)-N-(3-44- 1H),
8.17 (s, 1H), 10.46 (s, 1H);
ethylpiperazin-l-yl)methyl)-5- ESI-MS (m/z) 557 (M+H)+.
(trifluoromethyl)pheny1)-4-
methylbenzamide
92. 40 a Al,
B3, 1I-1 NMR (400 MHz, DMSO-d6)
O 4-
6 2.19 (s, 3H), 3.50-3.51 (br s,
NC) cF3
(trifluoro 2H), 4.20 (t, J = 4.0 Hz, 2H),
1 )
methyl)an 7.47 (d, J= 8.0 Hz, 1H), 7.79 (d,
NN iline
J = 2.0 Hz, 3H), 7.81 (d, J = 2.0
H
3-((7,8-Dihydro-6H-pyrimido[5,4- Hz,
3H), 8.00 (d, J = 8.4 Hz,
b][1,4]oxazin-4-yl)oxy)-4-methyl- Method B 2H), 10.49 (s, 1H); ESI-MS (m/z)
N-(4- 431 (M+H)+.
(trifluoromethyl)phenyl)benzamid
e
93. 0Al, B3, 1I-1 NMR (400 MHz, DMSO-d6)
IR"
o cF3 C26 6 2.51
(s, 3H), 3.18 (t, J = 4.8
Hz, 4H), 3.51 (br s, 2H), 3.75 (t,
N Method
B J = 4.4 Hz, 4H), 4.20 (t, J = 4.0
N N Co)
H Hz,
2H), 6.97 (s, 1H), 7.46 (d, J
3-((7,8-Dihydro-6H-pyrimido[5,4- = 8.0
Hz, 1H), 7.63-7.78 (m,
b][1,4]oxazin-4-yl)oxy)-4-methyl- 5H),
7.79 (dd, J1 = 1.6 Hz, .12 =
N-(3-morpholino-5- 8.0
Hz, 1H), 10.28 (s, 1H); ESI-
(trifluoromethyl)phenyl)benzamid MS (m/z) 516 (M+H)+.
e
94. Al,
B3, 1I-1 NMR (400 MHz, DMSO-d6)
40 CF3 C60 6 1.79-1.85 (m,
1H), 2.13 (s,
0 0
NICI 0 9H), 2.78-2.82 (m, 1H), 3.06 (t, J
I j N Method
B = 8.4 Hz, 1H), 3.24-3.51 (m,
H C .f R)
1\1"NI 6H),
4.20 (t, J = 4.4 Hz, 2H),
N¨ 6.51
(s, 1H), 7.24 (s, 1H), 7.45
/ (d, J
= 8.0 Hz, 1H), 7.50 (s, 1H),
(R)-3-((7,8-Dihydro-6H-
7.72 (s, 3H), 7.79 (dd, J1 = 1.6
pyrimido[5,4-b][1,4]oxazin-4-
Hz, J2 = 8.0 Hz, 1H), 10.21 (s,
yl)oxy)-N-(3-(3-
1H); ESI-MS (m/z) 543 (M+H)+.
176
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
No. Intermed Analytical Data
Structure and Name iates and
Method
(dimethylamino)pyrrolidin-l-y1)-
5-(trifluoromethyl)pheny1)-4-
methylbenzamide
95. I. H A2,
B3, 1H NMR (400 MHz, DMSO-d6)
O N CFrN Cl
1.13 (br s, 3H), 2.19 (s, 3H),
i\jj,0, 0 IW N,) 2.50-
2.80 (m, 10H), 3.63 (br s,
Method J' 2H), 4.84 (s, 2H),7.52 (d, J = 8.0
NN-0 Hz, 1H), 7.72 (d, J = 8.4 Hz,
H
N-(4-((4-Ethylpiperazin-1- 1H),
7.76 (s, 1H), 7.85 (d, J= 1.6
yl)methyl)-3- Hz,
1H), 8.07-8.08 (br s, 2H),
(trifluoromethyl)pheny1)-4- 8.20
(s, 1H), 10.48 (s, 1H), 11.75
methyl-3-47-oxo-7,8-dihydro-6H- (s,
1H); ESI-MS (m/z) 571
pyrimido[5,4-b][1,4]oxazin-4- (M+H)+.
yl)oxy)benzamide
96. 0 A3, B3, 1H NMR (400 MHz, DMSO-d6) , oFrN,
Cl 0.98 (t, J = 6.8 Hz, 3H), 1.35 (t, J
0
0 0 r N,) = 6.4
Hz, 3H), 2.18 (s, 3H), 2.23-
L= XI (S) Method
B 2.55 (m, 10H), 3.15-3.20 (m,
N N 1H), 3.52-3.56 (m, 3H), 4.17 (t, J
H
(S)-N-(4-((4-Ethylpiperazin-1- = 6.4
Hz, 1H), 7.46 (d, J = 8.0
yl)methyl)-3- Hz,
1H), 7.67-7.71 (m, 4H), 7.79
(trifluoromethyl)pheny1)-4- (d, J
= 8.4 Hz, 1H), 8.04 (d, J =
methyl-3-46-methyl-7,8-dihydro- 8.4
Hz, 1H), 8.18 (s, 1H), 10.41
6H-pyrimido[5,4-b][1,4]oxazin-4- (s,
1H); ESI-MS (m/z) 571
yl)oxy)benzamide (M+H)+.
97. ci & H Al, 4-
1H NMR (400 MHz, DMSO-d6)
HN N 0 cFrN chloro-
3- 6 0.98 (t, J = 7.2 Hz, 3H), 2.30-
o N,)
nitrobenz 2.34 (m, 10H), 3.48 (s, 2H), 3.56
= I ) oic
acid, (s, 2H), 4.23 (t, J = 8.0 Hz, 2H),
NN Cl
7.31 (s, 1H), 7.65-7.72 (m, 4H),
H
4-Chloro-3-((7,8-dihydro-6H- 7.81
(s, 1H), 8.02 (d, J = 8.4 Hz,
pyrimido[5,4-b][1,4]oxazin-4- Method
C 1H), 8.20 (s, H), 8.80 (s, 1H),
yl)amino)-N-(4-((4- 10.46
(s, 1H); ESI-MS (m/z) 576
ethylpiperazin-l-yl)methyl)-3- (M+H)+.
(trifluoromethyl)phenyl)benzamid
e
98. el H Al,
B14, 1H NMR (400 MHz, DMSO-d6)
S N 0 oFrN, Cl
0.98 (t, J= 7.2 Hz, 3H), 2.31 (s,
NO 0 N1,)
3H), 2.45-2.51 (m, 10H), 3.56 (s,
I ) Method
B 3H), 3.60 (s, 2H), 4.23 (t, J = 4.0
NN Hz, 2H), 7.54 (d, J = 8.0 Hz,
H
3-((7,8-Dihydro-6H-pyrimido[5,4- 1H),
7.69-7.74 (m, 3H), 7.98 (dd,
b][1,4]oxazin-4-yl)thio)-N-(4-((4- Ji =
2.0 Hz, .12 = 8.0 Hz, 1H),
ethylpiperazin-l-yl)methyl)-3- 8.05
(dd, Ji = 1.6 Hz, .12 = 8.8
(trifluoromethyl)pheny1)-4- Hz,
1H), 8.13 (s, 1H), 8.19 (s,
methylbenzamide 1H),
10.49 (s, 1H); ESI-MS (m/z)
177
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
No. Intermed Analytical Data
Structure and Name iates and
Method
573 (M+H)+.
99. A7, B3, 1H NMR (400 MHz, DMSO-d6)
S
H Cl 0.99
(t, J = 6.8 Hz, 3H), 2.32 (s,
O N t CFn\j..
3H), 2.40-2.51 (m, 10H), 3.39
0 0 IW N,)
I j Method
B (m, 2H), 3.56 (s, 2H), 4.12 (t, J=
1\1N 4.0 Hz, 2H),
5.96 (d, J = 5.6 Hz,
H 1H), 6.84 (s,
1H), 7.47-7.50 (m,
3-((3,4-Dihydro-2H-pyrido[3,2-
3H), 7.69 (d, J = 8.8 Hz, 1H),
b] [1,4]oxazin-8-yl)oxy)-N-(4-44-
7.77 (dd, ./i = 2.0 Hz, .12 = 7.6
ethylpiperazin-1-yl)methyl)-3-
Hz, 1H), 8.01 (d, J = 8.4 Hz,
(trifluoromethyl)pheny1)-4- 1H),
8.17 (s, 1H), 10.43 (s, 1H);
methylbenzamide
ESI-MS (m/z) 556 (M+H)+.
100. A7, B3, 1H NMR (400 MHz, DMSO-d6)
cF3 C10 0.98 (t, J = 7.2 Hz, 3H),
2.32 (s,
0
3H), 2.45-2.51 (m, 10H), 3.38-
3.43 (m, 2H), 3.53 (s, 2H), 4.12
N N N' Method
B (t, J = 4.0 Hz, 2H), 5.97 (d, J =
H .,N,
5.6 Hz, 1H), 6.83 (s, 1H), 7.34 (s,
3-((3,4-Dihydro-2H-pyrido[3,2- 1H),
7.47-7.52 (m, 3H), 7.78 (dd,
b] [1,4]oxazin-8-yl)oxy)-N-(3-((4-
./i = 1.6 Hz, .12 = 7.6 Hz, 1H),
ethylpiperazin-1-yl)methyl)-5-
7.97 (s, 1H), 8.14 (s, 1H), 10.45
(trifluoromethyl)pheny1)-4-
(s, 1H); ESI-MS (m/z) 556
methylbenzamide (M+H)+.
101. 0 H
A7, B3, 1H NMR (400 MHz, DMSO-d6)
O N 0 CF3
C20 1.04
(t, J= 7.2 Hz, 3H), 2.27 (s,
a:0) 0 3H),
2.45-2.57 (m, 10H), 3.20-
N Method
B 3.29 (br s, 2H), 4.12 (t, J = 4.0
N N c)H Hz, 2H),
5.97 (d, J = 2.0 Hz,
K 1H),
6.84 (s, 2H), 7.47-7.49 (m,
3-((3,4-Dihydro-2H-pyrido[3,2- 3H),
7.62 (d, J = 9.6 Hz, 2H),
b] [1,4]oxazin-8-yl)oxy)-N-(3-(4- 7.76
(dd, ./i = 2.0 Hz, .12 = 8.0
ethylpiperazin-1-y1)-5- Hz,
1H), 10.26 (s, 1H); ESI-MS
(trifluoromethyl)pheny1)-4- (m/z) 542 (M+H)+.
methylbenzamide
102. A7,
B3, 1H NMR (400 MHz, DMSO-d6)
C17 0.99
(t, J = 7.2 Hz, 3H), 1.65-
140 H CF 1.67
(m, 2H), 1.89-1.93 (m, 2H),
O N la
ny' Method B 2.31 (s, 3H), 2.45-2.58 (m, 6H),
on 0 iw (:).
3.43 (br s, 2H), 4.12 (t, J = 4.0
N N Hz,
2H), 4.58 (m, 1H), 5.96 (d, J
H
3-((3,4-Dihydro-2H-pyrido[3,2- = 9.6
Hz, 1H), 6.84 (s, 1H), 7.30
b] [1,4]oxazin-8-yl)oxy)-N-(4-41- (d, J
= 9.6 Hz, 1H), 7.47-7.48
ethylpiperidin-4-yl)oxy)-3- (m,
3H), 7.75 (dd, ./i = 1.6 Hz, .12
(trifluoromethyl)pheny1)-4-
= 8.0 Hz, 1H), 7.93 (dd, ./1 = 2.0
methylbenzamide
Hz, J2 = 8.8 Hzõ 1H), 8.05 (s,
1H), 10.27 (s, 1H); ESI-MS (m/z)
557 (M+H)+.
178
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
No. Intermed Analytical Data
Structure and Name iates and
Method
103. a H
A7, B3, 1I-1 NMR (400 MHz, DMSO-d6)
0 N 0 cF3 C19 2.22
(s, 3H), 2.49-2.51 (m, 8H),
1 0 3.20
(t, J = 4.8 Hz, 3H), 3.42-
10)
N Method
B 3.43 (m, 2H), 4.12 (t, J = 4.0 Hz,
N
H CN) 2H),
5.97 (d, J = 5.6 Hz, 1H),
I 6.83
(s, 1H), 6.93 (s, 1H), 7.47-
3-((3,4-Dihydro-2H-pyrido[3,2- 7.49
(m, 3H), 7.62 (s, 2H), 7.76
b][1,4]oxazin-8-yl)oxy)-4-methyl- (dd,
Ji = 1.6 Hz, .12 = 8.0 Hz,
N-(3-(4-methylpiperazin-1-y1)-5- 1H),
10.25 (s, 1H); ESI-MS (m/z)
(trifluoromethyl)phenyl)benzamid 528 (M+H)+.
e
104. a H
A8, 1I-1 NMR (400 MHz, DMSO-d6)
0 N cFrN B3, Cl 0.98
(t, J = 7.2 Hz, 3H), 2.26 (s,
cp, 0 r 11,) 3H),
2.35-2.51 (m, 10H), 3.56 (s,
I Method
J' 2H), 4.70 (s, 2H), 6.38 (d, J = 5.6
il 'i\l'ip
H Hz,
1H), 7.55 (t, J = 8.0 Hz, 1H),
N-(4-((4-Ethylpiperazin-1- 7.64-
7.85 (m, 4H), 8.01 (dd, ./1 =
yl)methyl)-3- 1.6
Hz, .12 = 8.0 Hz, 1H), 8.17 (s,
(trifluoromethyl)pheny1)-4- 1H),
10.45 (s, 1H), 11.36 (s, 1H);
methyl-3-43-oxo-3,4-dihydro-2H- ESI-MS (m/z) 570 (M+H)+.
pyrido[3,2-b][1,4]oxazin-8-
yl)oxy)benzamide
105. CI H
A9, Bl, 1I-1 NMR (400 MHz, DMSO-d6)
0 7 N CFrN Cl 6 0.97
(t, J = 6.8 Hz, 3H), 2.29-
Ntn0 IW 1\1) 2.65
(m, 12H), 3.56 (br s, 4H),
I Method
G 7.71 (d, J = 8.4 Hz, 1H), 7.77 (t,
N N J =
8.8 Hz, 1H), 7.90-8.07 (m,
H
4-Chloro-N-(4-((4-ethylpiperazin-
4H), 8.17 (s, 1H), 8.62 (s, 1H),
1-yl)methyl)-3-
10.57 (s, 1H); ESI-MS (m/z) 589
+
(trifluoromethyl)pheny1)-34 (M+H).
5-
oxo-5,6,7,8-tetrahydropyrido[2,3 -
d] pyrimidin-4-yl)oxy)benzamide
106. CI a H
Example 1I-1 NMR (400 MHz, DMSO-d6)
N cFr.N 105-
Step 6 1.09 (t, J = 6.8 Hz, 3H), 1.83-
0
N 0 IW N.) 2 1.86
(m, 2H), 2.42-2.55 (m,
I 10H),
2.71 (t, J = 6.0 Hz, 2H),
N1\1 Method
H 3.34-3.39 (m, 2H), 3.58 (s,
H
4-Chloro-N-(4-((4-ethylpiperazin-
2H),7.51 (s, 1H), 7.70-7.91 (m,
1-yl)methyl)-3- 5H),
8.04 (d, J = 8.0 Hz, 1H),
(trifluoromethyl)pheny1)-3- 8.18 (s, 1H), 10.56 (s, 1H).
((5,6,7,8-tetrahydropyrido[2,3 -
d] pyrimidin-4-yl)oxy)benzamide
179
CA 03064975 2019-11-26
WO 2018/215668 PCT/EP2018/063957
No. Intermed Analytical Data
Structure and Name iates and
Method
107. ci 0
H
Example 'I-1 NMR (400 MHz, DMSO-d6)
OH 0
N 0 cFN 105 6 1.01
(t, J = 6.8 Hz, 3H), 2.41-
/N 0 N,) 2.55
(m, 10H), 3.30-3.40 (m,
1 Step 1- 4H),
3.58 (br s, 2H), 4.98 (d, J =
IVN Method H 4.4 Hz,
1H), 5.20 (s, 1H), 7.71-
H
4-Chloro-N-(4-((4-ethylpiperazin- 7.77
(m, 3H), 7.89-7.94 (m, 3H),
1-yl)methyl)-3- 8.03
(d, J= 2.0 Hz, 1H), 8.17 (s,
(trifluoromethyl)pheny1)-345- 1H),
10.61 (s, 1H); ESI-MS (m/z)
hydroxy-5,6,7,8- 591 (M+H)+.
tetrahydropyrido[2,3-d]pyrimidin-
4-yl)oxy)benzamide
108. 0 H
Example 1I-1 NMR (400 MHz, DMSO-d6)
O N io
CF/..N, 105-Step 6 1.23 (m, 3H), 1.84-1.86 (m,
Nr' 0 2
2H), 2.15 (s, 3H), 2.45-2.55 (m,
N N
10H), 2.71 (t, J = 6.0 Hz, 3H),
H Method G 3.58
(br s, 3H), 7.42-7.71 (m,
N-(4-((4-Ethylpiperazin-1- and 4H),
7.79 (d, J = 7.6 Hz, 2H),
yl)methyl)-3- Method
H 8.06 (d, J= 8.4 Hz, 1H), 8.19 (s,
(trifluoromethyl)pheny1)-4- 1H),
10.44 (s, 1H); ESI-MS (m/z)
methyl-34(5,6,7,8- 555 (M+H)+.
tetrahydropyrido[2,3-d]pyrimidin-
4-yl)oxy)benzamide
109. I Al,
B9, 1I-1 NMR (400 MHz, DMSO-d6)
0
0 cFv Cl 6 0.98 (t, J = 7.2
Hz, 3H), 2.29-
0 0 .NI 2.39 (m,
10H), 3.50 (br s, 4H),
0 ri,) Method
B 3.81 (s, 3H), 4.18 (t, J= 4.0 Hz,
= 1 j
2H), 7.27 (d, J = 8.8 Hz, 1H),
NN
H 7.64 (s, 3H),
7.69 (d, J = 8.8 Hz,
3-((7,8-Dihydro-6H-pyrimido[5,4- 1H),
7.98 (dd, J1 = 2.0 Hz, .1-2 =
b] [1,4]oxazin-4-yl)oxy)-N-(4-44- 8.4
Hz, 1H), 7.89 (dd, J1 = 2.0
ethylpiperazin-l-yl)methyl)-3- Hz, J2
= 8.4 Hz, 1H), 8.19 (s,
(trifluoromethyl)pheny1)-4- 1H),
10.33 (s, 1H); ESI-MS (m/z)
methoxybenzamide 573 (M+H)+.
110. cF3 Al,
B10, 1I-1 NMR (400 MHz, DMSO-d6)
0 H Cl 6 1.01 (br s, 3H),
2.45-2.51 (m,
0 N 401 CFK=i\(= 10H), 3.33-
3.59 (m, 4H), 4.19 (t,
o ri,) Method
B J = 4.4 Hz, 2H), 7.73-7.81 (m,
= 1 )
4H), 8.02-8.06 (m, 2H), 8.16-
V.N 8.17 (m, 2H),
10.70 (s, 1H); ESI-
H
3-((7,8-Dihydro-6H-pyrimido[5,4- MS (m/z) 611 (M+H)+.
b] [1,4]oxazin-4-yl)oxy)-N-(4-((4-
ethylpiperazin-1-yl)methyl)-3-
(trifluoromethyl)pheny1)-5-
(trifluoromethyl)benzamide
180
CA 03064975 2019-11-26
WO 2018/215668 PCT/EP2018/063957
No. Intermed Analytical Data
Structure and Name iates and
Method
111. Al,
B6, 1I-1 NMR (400 MHz, DMSO-d6)
011 Cl 6 1.09 (t, J = 7.2
Hz, 3H),1.23-
O 0Fri,i,
1.30 (m, 3H), 2.14 (s, 3H), 2.45-
,orINI 0 LW N,.)
I )
N Nr Method
B 2.51 (m, 7H), 3.50 (br s, 2H),
3.56 (s, 2H), 4.19 (t, J = 4.0 Hz,
H
3-((7,8-Dihydro-6H-pyrimido[5,4- 2H),
7.18 (dd, Ji = 3.2 Hz, J2 =
b] [1,4]oxazin-4-yl)oxy)-N-(4-44- 6.0
Hz, 1H), 7.33-7.35 (m, 2H),
ethylpiperazin-l-yl)methyl)-3-
7.67-7.71 (m, 3H), 7.95 (d, J =
(trifluoromethyl)pheny1)-2-
8.0 Hz, 1H), 8.20 (s, 1H), 10.73
methylbenzamide (s,
1H); ESI-MS (m/z) 557
(M+H)+.
112. F3C 0
H Al,
B13, 1I-1 NMR (400 MHz, DMSO-d6)
O N 0 CFrN\ Cl
6 0.98 (t, J = 6.8 Hz, 3H), 2.30-
),0 2.51
(m, 10H), 3.28-3.57 (m,
N' i ) Method
B 4H), 4.19 (t, J = 10.4 Hz, 2H),
NN H 7.73 (s, 2H), 7.84-7.87 (m, 2H),
3-((7,8-Dihydro-6H-pyrimido[5,4- 7.98-
9\8.03 (m, 3H), 8.16 (s,
b] [1,4]oxazin-4-yl)oxy)-N-(4-44- 1H),
10.67 (s, 1H); ESI-MS (m/z)
ethylpiperazin-l-yl)methyl)-3- 611 (M+H)+.
(trifluoromethyl)pheny1)-4-
(trifluoromethyl)benzamide
113. ci Al,
B8, 1I-1 NMR (400 MHz, DMSO-d6)
Sii 0F Cl 6 0.98
( t, J = 7.2 Hz, 3H), 1.0-
O 0 ,
1.23 (m, 2H), 2.29-2.49 (m, 8H),
NO N,) Method
B 3.48-3.49 (br s, 2H), 3.56 (s, 2H),
I )
NN 4.16 (t, J = 4.4 Hz, 2H), 7.27
H (dd,
J1 = 4.0 Hz, J2 = 8.4 Hz,
2-Chloro-5-((7,8-dihydro-6H-
1H), 7.38 (d, J = 2.8 Hz, 1H),
pyrimido[5,4-b][1,4]oxazin-4-
7.71 (d, J = 8.4 Hz, 1H), 7.88 (d,
yl)oxy)-N-(4-((4-ethylpiperazin-1-
J = 2.0 Hz, 2H), 7.91 (s, 1H),
yl)methyl)-3-
8.15 (d, J= 2.0 Hz, 1H), 9.05 (s,
(trifluoromethyl)phenyl)benzamid
1H), 10.86 (s, 1H); ESI-MS (m/z)
e
577 (M+H)+.
114. a
H Al,
B7, 1I-1 NMR (400 MHz, DMSO-d6)
O N CF Cl
6 0.98 ( t, J = 6.8 Hz, 3H), 1.23
OLI\i 0 =NO (br s,
2H), 2.16 (s, 3H), 2.31-2.51
L I j
NI\r Method
B (m, 8H), 3.56-3.57 (br s, 4H),
H 4.21
(t, J = 4.0 Hz, 2H), 7.43 (d,
4-Chloro-3-((7,8-dihydro-6H- J =
8.0 Hz, 1H), 7.53 (d, J = 8.0
pyrimido[5,4-b][1,4]oxazin-4- Hz,
1H), 7.70-7.74 (m, 3H), 7.94
yl)oxy)-N-(4-((4-ethylpiperazin-1- (d, J
= 8.4 Hz, 1H), 8.18 (s, 1H),
yl)methyl)-3- 10.77
(s, 1H); ESI-MS (m/z) 591
(trifluoromethyl)pheny1)-2- (M+H)+.
methylbenzamide
181
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
No. Intermed Analytical Data
Structure and Name iates and
Method
115. F H Al, B11, NMR
(400 MHz, DMSO-d6)
O N Cl ..
6 0.99 ( t, J = 7.2 Hz, 3H), 2.40-
2.51 (m, 9H), 3.33-3.39 (m, 1H),
NO
Method B 3.47-3.50 (m, 2H), 3.57 (s, 2H),
NN 4.17 (t, J = 4.4 Hz, 2H), 7.34-
H
5-((7,8-Dihydro-6H-pyrimido[5,4- 7.42
(m, 3H), 7.70-7.75 (m, 3H),
b] [1,4]oxazin-4-yl)oxy)-N-(4-((4- 7.92
(t, J = 8.8 Hz, 1H), 8.15 (s,
ethylpiperazin-l-yl)methyl)-3- 1H),
10.75 (s, 1H); ESI-MS (m/z)
(trifluoromethyl)pheny1)-2- 561 (M+H)+.
fluorobenzamide
116. CI Al, B5, NMR
(400 MHz, DMSO-d6)
H Cl 6 0.98
( t, J = 5.1 Hz, 3H), 3.29-
O N
CFnr\j.. 3.39 (m, 10H), 3.50-3.56 (m,
0 4H),
4.17 (br s, 2H), 7.54 (s, 1H),
Method B 7-71-8.01 (s, 6H), 8.17 (s, 1H),
NN 10.63(s, 1H); ESI-MS (m/z) 577
3-Chloro-5-((7,8-dihydro-6H- (M+H)+.
pyrimido[5,4-b][1,4]oxazin-4-
yl)oxy)-N-(4-((4-ethylpiperazin-1-
y1)methyl)-3-
(trifluoromethyl)phenyl)benzamid
117. ci 0
Al, 5- NMR
(400 MHz, DMSO-d6)
VI 0 N AN 1. amino-
2- 6 0.98 ( t, J= 7.2 Hz, 3H), 2.31-
NX k.,F3
0) H H
chlorophe 2.51 (m, 10H), 3.50-3.52 (m,
nol, Cl 4H),
4.19 (t, J = 4.4 Hz, 2H),
N N
7.25 (dd, J, = 2.4 Hz, .1-2 = 8.8
1-(4-Chloro-3-((7,8-dihydro-6H- Method
I Hz, 1H), 7.42 (d, J = 8.8 Hz,
pyrimido[5,4-b][1,4]oxazin-4- 2H),
7.48-7.93 (m, 4H), 7.93 (s,
yl)oxy)pheny1)-3-(4((4- 1H),
9.02 (s, 1H), 9.08 (s, 1H);
ethylpiperazin-l-yl)methyl)-3- ESI-MS (m/z) 592 (M)+.
(trifluoromethyl)phenyl)urea
118. CI 40 0 Al, 5- NMR
(400 MHz, DMSO-d6)
O N
amino-2- 6 1.09 ( t, J = 5.6 Hz, 3H), 2.50-
H chlorophe 2.77 (m, 10H), 3.50 (br s, 2H), )
nol, C15 3.82
(br s, 2H), 4.19 (t, J = 8.4
NN Hz, 2H), 7.55 (t, J = 8.8 Hz, 1H),
N-(4-Chloro-3-((7,8-dihydro-6H- Method
B 7.65-7.95 (m, 5H), 8.15-8.27 (m,
pyrimido[5,4-b][1,4]oxazin-4- 2H),
10.64 (s, 1H); ESI-MS (m/z)
yl)oxy)pheny1)-444- 577 (M+H)+.
ethylpiperazin-l-yl)methyl)-3-
(trifluoromethyl)benzamide
182
CA 03064975 2019-11-26
WO 2018/215668 PCT/EP2018/063957
No. Intermed Analytical Data
Structure and Name iates and
Method
119. CI al
H Al,
Bl, 1I-1 NMR (400 MHz, DMSO-d6)
N CF3 C63
6 1.17 (d, J = 6.4 Hz, 6H), 2.33
0 13
c, 0 0 (t, J
= 11.6 Hz, 2H), 3.62-3.65
1 )
N Method J (br s, 2H), 3.68-
3.72 (m, 4H),
Nil (R?. J(S) (Step
1 4.20 (t, J = 4.0 Hz, 2H), 7.02 (s,
and 2) 1H), 7.57 (s, 1H), 7.68-7.90 (m,
4-Chloro-3-((7,8-dihydro-6H- 6H),
10.39 (s, 1H); ESI-MS (m/z)
pyrimido[5,4-b][1,4]oxazin-4- 564 (M+H)+.
yl)oxy)-N-(3-((2R,6S)-2,6-
dimethylmorpholino)-5-
(trifluoromethyl)phenyl)benzamid
e
120. CI al A6, 1H NMR (400 MHz, DMSO-d6)
H
N 0 cF3
Bl, C19 6 1.18 (d, J = 6.4 Hz, 3H), 2.27
0
(s, 3H), 2.47-2.51 (m, 4H), 3.22
N Method A (br s, 4H), 3.69-
3.78 (m, 2H),
(s)j,,
N El ", C j
4.25-4.28 (m, 1H), 6.97 (s, 1H),
7.61 (s, 1H), 7.64 (s, 1H), 7.71-
N
I 7.77 (m, 2H), 7.87-7.89
(m, 3H),
(S)-4-Chloro-3-((7-methyl-7,8- 10.38
(s, 1H); ESI-MS (m/z) 563
dihydro-6H-pyrimido[5,4- (M+H)+.
b] [1,4]oxazin-4-yl)oxy)-N-(344-
methylpiperazin-l-y1)-5-
(trifluoromethyl)phenyl)benzamid
e
121. CI Ai AS, 1H NMR (400 MHz, DMSO-d6)
H
O N 40
cF3 Bl, C19 6 1.18 (d, J = 6.8 Hz, 3H), 2.24
0 0 (s,
3H), 2.47-2.51 (m, 4H), 3.21-
NIX(R....,
N Method
A 3.22 (m, 4H), 3.69-3.78 (m, 2H),
H CN) 4.24-
4.28 (m, 1H), 6.97 (s, 1H),
I 7.60
(s, 1H), 7.64 (s, 1H), 7.71-
(R)-4-Chloro-3-((7-methyl-7,8- 7.77
(m, 2H), 7.87-7.89 (m, 3H),
dihydro-6H-pyrimido[5,4- 10.38
(s, 1H); ESI-MS (m/z) 563
b][1 ,4]o x a zin- 4 -y 1)o xy)-N - (3 - (4 - (M+H)+.
methylpiperazin-l-y1)-5-
(trifluoromethyl)phenyl)benzamid
e
122. CI a, H A10 1H NMR
(400 MHz, DMSO-d6)
N CF 3
Bl, C19 6 1.35 (d, J = 6.4 Hz, 3H), 2.23
0
mo .P r (s,
2H), 2.46-2.51 (m, 4H), 3.18-
IL I (R))
N Method B 3.21 (m, 5H),
3.53-3.57 (m, 2H),
NN CN)
4.15-4.19 (m, 1H), 6.96 (s, 1H),
H
1
7.60 (s, 1H), 7.64 (s, 1H), 7.75-
(R)-4-Chloro-3-((6-methyl-7,8- 7.79
(m, 2H), 7.86-7.89 (m, 3H),
dihydro-6H-pyrimido[5,4- 10.37
(s, 1H); ESI-MS (m/z) 563
b] [1,4]oxazin-4-yl)oxy)-N-(3-(4- (M+H)+.
methylpiperazin-l-y1)-5-
183
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
No. Intermed Analytical Data
Structure and Name iates and
Method
(trifluoromethyl)phenyl)benzamid
e
123. Ci al A10, Bl, 1I-1 NMR (400 MHz, DMSO-d6)
H
N 40 cF3,,,,,....... Cl
6 0.98 (t, J = 7.2 Hz, 3H), 1.35
0 0 Ij f\ I
(d, J = 6.4 Hz, 3H), 2.28-2.50 (m
N:ci ((pia` Method
B , 9H), 3.16-3.21 (m, 2H), 3.34-
N N 3.56
(m, 3H), 4.17 (t, J = 6.0 Hz,
H
(R)-4-Chloro-N-(4-((4-
1H), 7.69-7.79 (m, 4H), 7.88-
ethylpiperazin-1-yl)methyl)-3-
7.90 (m, 2H), 8.03 (d, J= 8.0 Hz,
(trifluoromethyl)pheny1)-3-46-
1H), 8.77 (s, 1H), 10.54 (s, 1H);
methy1-7,8-dihydro-6H-
ESI-MS (m/z) 591 (M+H)+.
pyrimido[5,4-b][1,4]oxazin-4-
yl)oxy)benzamide
124. a Al, Bl, 1I-1 NMR (400 MHz, DMSO-d6)
H
N 0 CF3 C69
6 1.04 (d, J = 6.4 Hz, 3H), 1.24
0
(s, 1H), 2.65-3.0 (m, 3H), 3.22-
NJC N Method
3.34 (m, 3H), 3.52 (br s, 2H),
N N
H C (Id. B ' 3.93
(m, 1H), 4.20 (br s, 2H),
N H 6.87
(s, 1H), 7.54 (s, 1H), 7.59
(S)-4-Chloro-3-((7,8-dihydro-6H- (s,
1H), 7.69 (s, 1H), 7.76 (d, J
pyrimido[5,4-b][1,4]oxazin-4- = 8.8
Hz, 1H), 7.81 (s, 1H), 7.87
yl)oxy)-N-(3-(2-methylpiperazin- (s,
2H),10.35 (s, 1H); ESI-MS
1-y1)-5- (m/z) 549 (M+H)+.
(trifluoromethyl)phenyl)benzamid
e
125. a Al, Bl, 1I-1 NMR (400 MHz, DMSO-d6)
H
N 0 CF3 C70
6 1.04 (d, J= 6.4 Hz, 3H), 2.50-
0 WI
3.17 (m, 5H), 3.17-3.25 (m, 2H),
Method 3.52
(br s, 2H), 3.93 (br s, 1H),
N N C
H (I) B' 4.20
(t, J = 4.4 Hz, 2H), 6.87 (s,
N H 1H),
7.53 (s, 1H),7.59 (s, 1H),
(R)-4-Chloro-3-((7,8-dihydro-6H- 7.51-
7.76 (br s, 1H), 7.69 (s, 1H),
pyrimido[5,4-b][1,4]oxazin-4- 7.77
(s, 1H), 7.81-7.88 (m, 2H),
yl)oxy)-N-(3-(2-methylpiperazin- 10.35
(s, 1H); ESI-MS (m/z) 549
1-y1)-5- (M+H)+.
(trifluoromethyl)phenyl)benzamid
e
126. CI a H Al,
Bl, 1I-1 NMR (400 MHz, DMSO-d6)
N u3 C74
6 3.51-3.52 (m, 2H), 3.80-3.82
0 so
(m, 2H), 3.99-4.01 (m, 2H), 4.20
r\X 0 N (m,
1H), 4.25 (s, 3H), 7.60 (s,
N X ) Method
J 1H), 7.69 (s, 1H), 7.77-7.82 (m,
0 (Step 1 2H),
7.89-7.91 (m, 2H), 8.12 (d,
4-Chloro-3-((7,8-dihydro-6H- and 2) J= 9.6 Hz, 2H), 10.67 (s, 1H);
pyrimido[5,4-b][1,4]oxazin-4-
yl)oxy)-N-(3-(3-oxomorpholino)-
184
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
No. Intermed Analytical Data
Structure and Name iates and
Method
5-
(trifluoromethyl)phenyl)benzamid
e
127. cl a H Al,
Bl, 1H NMR (400 MHz, DMSO-d6)
N 0 c3 C71
6 1.78 (d, J = 9.2 Hz, 1H), 1.88
0
0 (d, J
= 9.6 Hz, 1H), 2.25 (s, 3H),
t'Cj' (0)
s () N Method B 2.42-2.51 (m,
1H), 2.82 (d, J =
N
N C'
H 9.6 Hz, 2H), 3.18
(d, J= 8.8 Hz,
N (s)
2H), 3.51-3.52 (m, 2H), 4.20 (t, J
1
4-Chloro-3-((7,8-dihydro-6H- = 4.0
Hz, 2H), 4.32 (s, 1H), 6.57
pyrimido[5,4-b][1,4]oxazin-4- (s,
1H), 7.23 (s, 1H), 7.46 (s,
yl)oxy)-N-(3-((1S,4S)-5-methyl- 1H),
7.69 (s, 1H), 7.86-7.88 (m,
2,5-diazabicyclo[2.2.1]heptan-2- 4H),
10.33 (s, 1H); ESI-MS (m/z)
561 (M+H)+.
(trifluoromethyl)phenyl)benzamid
e
128. CI a H Al,
Bl, 1H NMR (400 MHz, DMSO-d6)
N 0 CF3 C75
6 1.08 (d, J = 5.6 Hz, 3H), 1.25
0
(br s, 3H), 2.25 (br s, 3H), 2.84-
NCJCO) a
N Method B 2.86 (m, 2H), 3.51-3.60 (m, 4H),
N N
H C (R144. 4.20 (t, J = 3.6
Hz, 2H), 6.99 (s,
N 1H), 7.58 (s, 1H), 7.65-7.81 (m,
I
(R)-4-Chloro-3-((7,8-dihydro-6H- 2H),
7.87-7.89 (m, 4H), 10.38 (s,
pyrimido[5,4-b][1,4]oxazin-4- 1H);
ESI-MS (m/z) 563 (M+H)+.
yl)oxy)-N-(3-(3,4-
dimethylpiperazin-l-y1)-5-
(trifluoromethyl)phenyl)benzamid
e
129. cl a H Al,
Bl, 1H NMR (400 MHz, DMSO-d6)
N 0 CF3 C76
6 1.06 (d, J = 6.0 Hz, 3H), 1.24
0 Wj
, N j: oj 0 (s,
3H), 2.22 (s, 3H), 2.82-2.85
N Method B (m, 2H), 3.51-
3.60 (m, 4H), 4.21
N N
H C (s) (t, J = 4.0 Hz,
2H), 6.98 (s, 1H),
7.57 (s, 1H), 7.57-7.77 (m, 2H),
I
(S)-4-Chloro-3-((7,8-dihydro-6H- 7.87-
7.89 (m, 4H), 10.37 (s, 1H);
pyrimido[5,4-b][1,4]oxazin-4- ESI-MS (m/z) 563 (M+H)+.
yl)oxy)-N-(3-(3,4-
dimethylpiperazin-l-y1)-5-
(trifluoromethyl)phenyl)benzamid
e
130. a
H A7,
B3, 1H NMR (400 MHz, DMSO-d6)
0 N I* CF3 C26 6 2.27
(s, 3H), 3.16-3.18 (m,
4H), 3.42-3.44 (m, 2H), 3.74-
CY)) N Method
B 3.76 (m, 4H), 4.12 (t, J = 4.0 Hz,
N N ( ) 2H),
5.97 (d, J = 5.6 Hz, 1H),
H
0 6.85 (s, 1H), 6.96 (s, 1H), 7.48-
185
CA 03064975 2019-11-26
WO 2018/215668 PCT/EP2018/063957
No. Intermed Analytical Data
Structure and Name iates and
Method
3-((3,4-Dihydro-2H-pyrido[3,2- 7.50
(m, 3H), 7.64 (d, J = 9.6 Hz,
b][1,4]oxazin-8-yl)oxy)-4-methyl- 2H),
7.77 (dd, Ji = 1.6 Hz, J2 =
N-(3-morpholino-5- 7.6
Hz, 1H), 10.29 (s, 1H); ESI-
(trifluoromethyl)phenyl)benzamid MS (m/z) 515 (M+H)+.
e
131. a H
A7, B3, 1H NMR (400 MHz, DMSO-d6)
0 N 0 CF3 C61 6 1.81-
2.27 (m, 9H), 2.84 (m,
,exNoN) 0
I
(N 1H),
3.07 (t, J = 8.4 Hz, 1H),
Method B 3.23-3.50 (m, 7H), 4.12(t, J= 4.0
H \ i(S) Hz,
2H), 5.96 (d, J = 5.6 Hz,
N¨ 1H),
6.51 (s, 1H), 6.84 (s, 1H),
/
(S)-3-((3,4-Dihydro-2H- 7.23
(s, 1H), 7.47-7.50 (m, 4H),
pyrido[3,2-b][1,4]oxazin-8- 7.77
(dd, Ji = 1.6 Hz, J2 = 8.0
yl)oxy)-N-(3-(3- Hz,
1H), 10.22 (s, 1H); ESI-MS
(dimethylamino)pyrrolidin-l-y1)- (m/z) 542 (M+H)+.
5-(trifluoromethyl)pheny1)-4-
methylbenzamide
132. CF3
Al, 5- 1H NMR (400 MHz, DMSO-d6)
ci a 0 6
amino-2- 6 1.76 (m, 3H), 2.48-2.51 (m,
O NN I\1
chlorophe 4H), 3.149 (br s, 4H), 3.50 (br s,
N o H H LN, nol,
C19 2H), 4.19 (t, J = 4.0 Hz, 2H),
NkN) 6.83
(s, 1H), 7.21-7.26 (m, 3H),
H Method
I 7.42 (t, J = 8.4 Hz, 1H), 7.49 (d,
1-(4-Chloro-3-((7,8-dihydro-6H- J =
2.4 Hz, 1H), 7.68 (s, 1H),
pyrimido[5,4-b][1,4]oxazin-4- 7.73
(s, 1H), 8.99 (s, 1H), 9.04
yl)oxy)pheny1)-3-(3-(4- (s,
1H); ESI-MS (m/z) 564
methylpiperazin-l-y1)-5- (M+H)+.
(trifluoromethyl)phenyl)urea
133. a Ai H Al,
Bl, 1H NMR (400 MHz, DMSO-d6)
N 0 CF3 C72
6 1.07 (t, J = 6.0 Hz, 3H), 2.02
0
0 0 (m,
1H), 2.21 (br s, 3H), 2.68-
NO ',CI j
N Method B 3.03 (m, 4H), 3.52 (br s, 3H),
N N
H C (s 4.05-
4.07 (m, 1H), 4.20 (t, J =
N1 4.4
Hz, 2H), 6.90 (s, 1H), 7.56-
(S)-4-Chloro-3-((7,8-dihydro-6H- 7.81
(m, 3H), 7.87-7.89 (m, 4H),
pyrimido[5,4-b][1,4]oxazin-4- 10.36
(s, 1H); ESI-MS (m/z) 563
yl)oxy)-N-(3-(2,4- (M+H)t
dimethylpiperazin-l-y1)-5-
(trifluoromethyl)phenyl)benzamid
e
134. CI a H Al,
Bl, 1H NMR (400 MHz, DMSO-d6)
N 0 CF3 C73
6 1.07 (t, J= 6.8 Hz, 3H), 2.02 (t,
0
0 0 J =
8.8 Hz, 1H), 2.20 (s, 3H),
NO ',CI )
Method B 2.70 (d, J = 11.6 Hz, 2H), 2.84
N N C (RD
H (d, J
= 11.2 Hz, 1H), 2.99 (t, J=
N 8.4 Hz, 1H), 3.51-3.52 (m, 2H),
I
186
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
No. Intermed Analytical Data
Structure and Name iates and
Method
(R)-4-Chloro-3-((7,8-dihydro-6H- 4.05
(m, 1H), 4.20 (t, J = 4.8 Hz,
pyrimido[5,4-b][1,4]oxazin-4- 2H),
6.89 (s, 1H), 7.558 (s, 2H),
yl)oxy)-N-(3-(2,4- 7.60
(s, 1H), 7.69-7.81 (m, 1H),
dimethylpiperazin-l-y1)-5- 7.86-
7.89 (m, 4H), 10.35 (s, 1H);
(trifluoromethyl)phenyl)benzamid ESI-MS (m/z) 563 (M+H)+.
e
135. CI H Al,
Bl, 1H NMR (400 MHz, DMSO-d6)
N cF3 C66
6 2.29 (s, 3H), 3.01 (t, J = 5.6
0 0
0 0 Hz,
2H), 3.51-3.52 (br s, 2H),
N tC
L I )
1\1 N 0,,,..n 3.75
(t, J = 6.0 Hz, 2H), 4.21 (t, J
H \-- .N1 , = 4.0
Hz, 2H), 4.80-4.86 (m,
4-Chloro-3-((7,8-dihydro-6H-
Method B 1H), 6.88 (s, 1H), 7.60 (s, 1H),
pyrimido[5,4-b][1,4]oxazin-4-
7.69 (s, 1H), 7.76-7.89 (m, 5H),
yl)oxy)-N-(3-((1-methylazetidin-
10.53 (s, 1H).
3-yl)oxy)-5-
(trifluoromethyl)phenyl)benzamid
e
136. al H AS, 1H NMR
(400 MHz, DMSO-d6)
N u
0 40 3 B3,
C19 6 1.18 (d, J = 6.0 Hz, 3H), 2.18
0 0 (s,
3H), 2.23 (s, 3H), 2.46-2.51
NA N N (R
N Method
A (m, 4H), 3.21 (t, J= 4.4 Hz, 4H),
A=0'
H C ) 3.73-
3.77 (m, 2H), 4.25 (dd, J1 =
NI 2.4
Hz, J2 = 10.4 Hz, 1H), 6.94
(R)-4-Methyl-3-((7-methyl-7,8- (s,
1H), 7.46 (d, J = 8.4 Hz, 1H),
dihydro-6H-pyrimido[5,4- 7.62
(s, 1H), 7.67-7.69 (m, 3H),
b] [1,4]oxazin-4-yl)oxy)-N-(3-(4- 7.78
(dd, J1 = 4.0 Hz, .1-2 = 10.0
methylpiperazin-l-y1)-5- Hz,
2H), 10.25 (s, 1H); ESI-MS
(trifluoromethyl)phenyl)benzamid (m/z) 543 (M+H)+.
e
137. al H A4,
B3, 1H NMR (400 MHz, DMSO-d6)
N u
0 so 3 C19 6 1.24
(s, 6H), 2.19 (s, 3H), 2.22
N:0 0 (s,
3H), 2.45-2.51 (m, 4H), 3.21
NX N
N Method
A (t, J = 4.4 Hz, 4H), 3.88 (s, 2H),
H CN) 6.94
(s, 1H), 7.46 (d, J = 8.0 Hz,
1H), 7.63 (s, 1H), 7.67-7.70 (m,
I
3((7,7-Dimethy1-7,8-dihydro-6H- 3H),
7.78-7.80 (m, 2H), 10.25 (s,
pyrimido[5,4-b][1,4]oxazin-4- 1H); ESI-MS (m/z) 557 (M+H)+.
yl)oxy)-4-methyl-N-(3-(4-
methylpiperazin-l-y1)-5-
(trifluoromethyl)phenyl)benzamid
e
187
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
No. Intermed Analytical Data
Structure and Name iates and
Method
138. ci
H Al,
Bl, 'I-1 NMR (400 MHz, DMSO-d6)
N C64 6 3.50-3.52 (br s, 2H), 3.89 (s,
cF
0 0 3
0 3H), 4.21 (t, J = 4.0
Hz, 2H),
Method B 7.66 (s, 1H), 7.70 (s, 1H), 7.71-
N
H N-N N v / 7.82 (m,
2H), 7.90-7.92 (br s,
/ 2H),
8.01 (s, 1H), 8.20 (s, 1H),
4-Chloro-3-((7,8-dihydro-6H- 8.27
(s, 2H), 10.58 (s, 1H); ESI-
pyrimido[5,4-b][1,4]oxazin-4- MS (m/z) 531 (M+H)+.
yl)oxy)-N-(3-(1-methy1-1H-
pyrazol-4-y1)-5-
(trifluoromethyl)phenyl)benzamid
e
139. H H Al, 4- 1I-1 NMR (400 MHz, DMSO-d6)
Ai NN 0 CF3
aminophe 6 2.24 (s, 3H), 2.47-2.51 (m,
0
0 nol,
C19 4H), 3.19-3.20 (m, 4H), 3.47-
CN 3.48 (br s, 2H), 4.16 (t, J = 4.4
N ) Method I Hz, 2H), 6.82 (s, 1H), 7.02 (d, J
N N
H I = 8.8 Hz, 2H),
7.22 (s, 1H), 7.28
1-(4-((7,8-Dihydro-6H- (s,
1H), 7.44 (t, J = 8.8 Hz, 2H),
pyrimido[5,4-b][1,4]oxazin-4- 7.64
(s, 1H), 7.69 (s, 1H), 8.75
yl)oxy)pheny1)-3-(3-(4- (s,
1H), 8.88 (s, 1H); ESI-MS
methylpiperazin-l-y1)-5- (m/z) 530 (M+H)+.
(trifluoromethyl)phenyl)urea
140. CI a H Al,
Bl, 1I-1 NMR (400 MHz, DMSO-d6)
N CF3 C77 6 1.63-
1.64 (m, 1H), 1.89-1.99
0
onIr (m, 1H), 2.09 (s, 6H), 2.30-2.33 o /
Method B (m, 1H), 2.46-2.74 (m, 4H),
1\1 N 1;1--,,N
H 1...j \ 3.68-3.72 (m,
3H), 4.01 (t, J =
(R)-4-Chloro-3-((7,8-dihydro-6H- 7.2
Hz, 1H), 4.21 (t, J = 4.4Hz,
pyrimido[5,4-b][1,4]oxazin-4- 2H),
7.37 (s, 1H), 7.69 (s, 1H),
yl)oxy)-N-(3-((3- 7.75-
7.91 (m, 4H), 7.99 (s, 1H),
(dimethylamino)pyrrolidin-1- 8.14 (s, 1H), 10.57 (s, 1H).
yl)methyl)-5-
(trifluoromethyl)phenyl)benzamid
e
141. CI 0 H Al,
Bl, 1I-1 NMR (400 MHz, DMSO-d6)
0 N CF3 C78 6 1.87-
1.89 (m, 1H), 1.91-1.99
0 I. (m,
1H), 2.11 (s, 6H), 2.31 (t, J=
Method B 6.8 Hz, 1H), 2.56-2.76 (m, 4H),
N N H NILyiNi
\ 3.52-
3.69 (m, 3H), 3.6-3.72 (m,
(S)-4-Chloro-3-((7,8-dihydro-6H- 1H),
4.21 (t, J = 4.0 Hz, 2H),
pyrimido[5,4-b][1,4]oxazin-4- 7.37
(s, 1H), 7.69 (s, 1H), 7.75-
yl)oxy)-N-(3-((3- 7.90
(m, 4H), 7.99 (s, 1H), 8.14
(dimethylamino)pyrrolidin-1- (s, 1H), 10.56 (s, 1H).
yl)methyl)-5-
(trifluoromethyl)phenyl)benzamid
e
188
CA 03064975 2019-11-26
WO 2018/215668 PCT/EP2018/063957
No. Intermed Analytical Data
Structure and Name iates and
Method
142. CI H A15,
'I-1 NMR (400 MHz, DMSO-d6)
N o CF3 Bl,
C19 6 1.55 (d, J = 6.8 Hz, 3H), 2.50-
0 0 IW 2.51
(m, 3H), 2.76 (m, 4H), 3.33
N:INX y N
(m, 4H), 5.02 (q, J = 6.8 Hz, 1H),
H j Method 7.08
(s, 1H), 7.84-8.0 (m, 4H),
N G' 8.09
(d, J= 2.0 Hz, 1H), 8.01 (s,
1
(R)-4-Chloro-3-46-methyl-7-oxo- 1H),
8.11 (s, 1H), 11.79 (s, 1H);
7,8-dihydro-6H-pyrimido[5,4- ESI-MS (m/z) 577 (M+H)+.
b][1,4]oxazin-4-yl)oxy)-N-(3-(4-
methylpiperazin-l-y1)-5-
(trifluoromethyl)phenyl)benzamid
e
143. CI H All, Bl, 1I-1 NMR (400 MHz, DMSO-d6)
N
cF3 C19 6 0.98
(t, J = 7.6 Hz, 3H), 1.55-
o
0 0 1.59
(m, 2H), 2.23 (s, 3H), 2.46
N (t, J
= 4.8 Hz, 4H), 3.21 (t, J =
1\1 N H C ) Method
A 4.4 Hz, 4H), 3.50 (br s, 1H),
N
I 3.94-
3.96 (m, 1H), 4.21 (t, J =
(R)-4-Chloro-3-47-ethyl-7,8- 8.0
Hz, 1H), 6.96 (s, 1H), 7.60 (s,
dihydro-6H-pyrimido[5,4- 1H),
7.64 (s, 1H), 7.71 (s, 1H),
b] [1,4]oxazin-4-yl)oxy)-N-(3-(4- 7.76
(d, J = 9.2 Hz, 1H), 7.95 (m,
methylpiperazin-l-y1)-5- 2H),
8.0 (s, 1H), 10.37 (s, 1H);
(trifluoromethyl)phenyl)benzamid ESI-MS (m/z) 577 (M+H)+.
e
144. All, B3, 1I-1 NMR (400 MHz, DMSO-d6)
0 H CF3 C19 6 0.97
(t, J = 7.6 Hz, 3H), 1.53-
o N 0
1.61 (m, 2H), 2.18 (s, 3H), 2.23
0 o
NIL (R),,,... N
(s, 3H), 2.46 (t, J = 4.8 Hz, 4H),
NN N H CN) Method A 3.21
(t, J = 4.4 Hz, 4H), 3.48-
3.49 (br s, 1H), 3.91-3.95 (m,
I 1H),
4.20 (dd, Ji = 2.8 Hz, .12 =
(R)-3-47-Ethy1-7,8-dihydro-6H-
10.8 Hz, 1H), 6.94 (s, 1H), 7.46
pyrimido[5,4-b][1,4]oxazin-4-
(d, J = 8.0 Hz, 1H), 7.62 (s, 1H),
yl)oxy)-4-methyl-N-(3-(4-
7.67-7.69 (br s, 3H), 7.79 (dd, Ji
methylpiperazin-l-y1)-5-
= 2.0 Hz, .12 = 8.0 Hz, 1H), 7.87
(trifluoromethyl)phenyl)benzamid
(s, 1H), 10.25 (s, 1H); ESI-MS
e
(m/z) 557 (M+H)+.
145. Cl Al2, 1I-1 NMR (400
MHz, DMSO-d6)
H
o
N 0 c3 Bl,
C19 6 0.98 (t, J = 7.2 Hz, 3H), 1.54-
o 0
1.61 (m, 2H), 2.24 (s, 3H), 2.48-
N)1' (s
N ." )
N 2.51
(m, 4H), 3.21-3.22 (br s,
C
H
N )
Method A 4H), 3.49-3.50 (m, 1H), 3.92-
I N 3.96
(m, 1H), 4.20-4.23 (m, 1H),
(S)-4-Chloro-3((7-ethy1-7,8- 6.97
(s, 1H), 7.60 (s, 1H), 7.64
dihydro-6H-pyrimido[5,4- (s,
1H), 7.71-7.77 (m, 2H), 7.86-
b] [1,4]oxazin-4-yl)oxy)-N-(3-(4- 7.94
(m, 2H), 7.95 (s, 1H), 10.37
189
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
No. Intermed Analytical Data
Structure and Name iates and
Method
methylpiperazin-l-y1)-5- (s, 1H); ESI-MS (m/z) 577 (M)+.
(trifluoromethyl)phenyl)benzamid
e
146. a H Al2, lli
NMR (400 MHz, DMSO-d6)
N 0 CF3
B3, C19 6 0.98 (t, J = 7.2 Hz, 3H), 1.51-
e 10
0 0 1.63
(m, 2H), 2.18 (s, 3H), 2.23
( s)
(s, 3H), 2.47-2.51 (m, 4H), 3.21
H
CN)
Method A (t, J= 4.4 Hz, 4H), 3.48-3.49 (m,
N 1H),
3.91-3.95(m, 1H), 4.21 (dd,
I
(S)-3((7-Ethy1-7,8-dihydro-6H- Ji =
2.8 Hz, .12 = 10.8 Hz, 1H),
pyrimido[5,4-b][1,4]oxazin-4- 6.94
(s, 1H), 7.46 (d, J = 8.4 Hz,
yl)oxy)-4-methyl-N-(3-(4- 1H),
7.62-7.78 (m, 4H), 7.80 (d,
methylpiperazin-1-y1)-5- J =
1.6 Hz, 1H), 7.87 (s, 1H),
(trifluoromethyl)phenyl)benzamid 10.25
(s, 1H); ESI-MS (m/z) 557
e (M+H)+.
147. F VrI-1
N
VI oo Ir Al, 4-
'HNMR (400 MHz, DMSO-d6)
AI
amino-2- 6 1.45 ( br s, 4H), 3.49 (br s, 2H),
0 F
fluorophe 4.17 (t, J = 4.0 Hz, 2H), 7.12-
NIIX ) nol,
C68 7.23 (m, 3H), 7.37 (d, J = 8.8 Hz,
NI N 1H),
7.62-7.75 (m, 5H), 10.03 (s,
H
N-(4-((7,8-Dihydro-6H-
Method 1H),
10.25 (s, 1H); ESI-MS (m/z)
+
pyrimido[5,4-b][1,4]oxazin-4-
M 468 (M+H).
yl)oxy)-3-fluoropheny1)-N-(4-
fluorophenyl)cyclopropane-1,1-
dicarboxamide
148. a H Al,
Bl, lli NMR (400 MHz, DMSO-d6)
N r CF3 C67
6 1.70-1.72 (m, 2H), 1.87-1.90
0 WI
0o IW (m,
2H), 2.08 (s, 3H), 2.19 (t, J =
1\11 \-- N) a
8.0 Hz, 2H), 2.46-2.51 (m, 2H),
H Method
B 3.51 (br s, 2H), 4.20 (t, J = 4.0
NI Hz, 2H), 4.650 (br s,
1H), 7.33
4-Chloro-3-((7,8-dihydro-6H- (d, J
= 8.8 Hz, 1H), 7.53 (d, J =
pyrimido[5,4-b][1,4]oxazin-4- 7.2
Hz, 1H), 7.70 (s, 1H),7.78-
yl)oxy)-N-(2-((l-methylpiperidin- 7.85
(m, 4H), 8.09 (s, 1H), 9.71
4-yl)oxy)-5- (s,
1H); ESI-MS (m/z) 564
(trifluoromethyl)phenyl)benzamid (M+H)+.
e
149. a H A16, lli
NMR (400 MHz, DMSO-d6)
N 0 CF3
Bl, C19 6 1.45 (d, J = 6.4 Hz, 3H), 2.22
0 W
- 0 0 (s,
3H), 2.46-2.51 (m, 4H), 3.21
NCX (sjr 4, J=
4.4 Hz, 4H), 4.74 (m, 1H),
N INIO CN) 6.96
(s, 1H), 7.60 (s, 1H), 7.65
N Method (s,
1H), 7.89 (d, J = 8.4 Hz, 1H),
1
(S)-4-Chloro-3-46-methyl-7-oxo- G' 7.90
(dd, Ji = 2.0 Hz, .12 = 8.4
7,8-dihydro-6H-pyrimido[5,4- Hz,
1H), 7.92-7.96 (m, 3H),
b] [1,4]oxazin-4-yl)oxy)-N-(3-(4- 10.48 (s, 1H).
190
CA 03064975 2019-11-26
WO 2018/215668 PCT/EP2018/063957
No. Intermed Analytical Data
Structure and Name iates and
Method
methylpiperazin-l-y1)-5-
(trifluoromethyl)phenyl)benzamid
150. a Ai A17,
NMR (400 MHz, DMSO-d6)
N CF3 Bl, C19 6 1.54
(s, 6H), 2.29 (br s, 4H),
0 WI
N:iC 2.50-
2.51 (brs , 3H), 3.24 (br s,
C:t9
4H), 6.99 (s, 1H), 7.62 (s, 1H),
N 0 C
7.65 (s, 1H), 7.82 (d, J = 8.4 Hz,
Method 1H),
7.94 (dd, J, = 2.0 Hz, J2 =
4-Chloro-3((6,6-dimethy1-7-oxo- G' 8.4
Hz, 1H), 8.01 (s, 1H), 8.13
7,8-dihydro-6H-pyrimido[5,4- (s,
1H), 10.43 (s, 1H), 11.79 (s,
b] [1,4]oxazin-4-yl)oxy)-N-(3-(4- 1H); ESI-MS (m/z)591(M+H)+.
methylpiperazin-l-y1)-5-
(trifluoromethyl)phenyl)benzamid
151. H H
Al, 4- 1I-1 NMR (400 MHz, DMSO-d6)
NN cFrNJ
aminophe 6 0.98 (t, J = 7.2 Hz, 3H), 2.29-
0 1W N,)
0 WI nol, Cl 2.51
(m, 10H), 3.48-3.52 (m ,
4H), 4.16 (t, J = 4.0 Hz, 2H),
N:Cin
N N Method
I 7.02 (d, J = 8.8 Hz, 2H), 7.46 (d,
J = 8.8 Hz, 2H), 7.45-7.64 (m,
1-(4-((7,8-Dihydro-6H- 3H),
7.69 (s, 1H), 7.8 (s, 1H),
pyrimido[5,4-b][1,4]oxazin-4- 9.08
(br s, 1H), 9.32 (br s, 1H);
yl)oxy)pheny1)-3-(444- ESI-MS (m/z)558(M+H)+.
ethylpiperazin-l-yl)methyl)-3-
(trifluoromethyl)phenyl)urea
152. cF3
Al, B12, NMR (400 MHz, DMSO-d6)
0
40 C19 6 2.24
(s, 3H), 2.48-2.51 ( br s,
CI Ai
4H), 3.22 (t, J = 4.8 Hz, 4H),
3.51 (t, J = 4.0 Hz, 2H), 4.20 (t, J
WI
NLC0) Method
B = 4.0 Hz, 2H), 6.97 (s, 1H), 7.43
N (d, J = 8.4 Hz, 1H), 7.62-7.66
(m, 3H), 7.83 (br s, 1H), 7.93
3-Chloro-4-((7,8-dihydro-6H- (dd,
J, = 2.0 Hz, J2 = 8.4 Hz,
pyrimido[5,4-b][1,4]oxazin-4- 1H), 8.15 (s, 1H), 10.42 (s, 1H).
yl)oxy)-N-(3-(4-methylpiperazin-
1-y1)-5
(trifluoromethyl)phenyl)benzamid
153. Al,
B12, NMR (400 MHz, DMSO-d6)
0 N
CI N CF
Cl 6 0.99
(t, J = 6.8 Hz, 3H), 1.99-
Ai
_ 3 2.33 (m, 2H), 2.40-2.51 (m,
7H),
0 WI Method
B 3.51 (t, J = 4.0 Hz, 3H), 3.58 (s,
2H), 4.20 (t, J = 4.0 Hz, 2H),
1 ) 7.43
(d, J = 8.4 Hz, 1H), 7.70 (s,
NN
2H), 7.72-8.05 (m, 2H), 8.16-
3-Chloro-4-((7,8-dihydro-6H- 8.20 (m, 3H), 10.59 (s, 1H).
191
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
No. Intermed Analytical Data
Structure and Name iates and
Method
pyrimido[5,4-b][1,4]oxazin-4-
yl)oxy)-N-(4-((4-ethylpiperazin-1-
y1)methyl)-3-
(trifluoromethyl)phenyl)benzamid
e
154. H H
N N j Al, 4- 1H NMR (400
MHz, DMSO-d6)
Al isr
amino-2- 6 0.98 (t, J = 6.8 Hz, 3H), 2.06
o WI tW CF N kl,)
methylph (s, 3H), 2.28-2.51 (m, 9H), 3.48-
j' enol, Cl 3.52
(m, 5H), 4.17 (t, J= 4.0 Hz,
NIC
N N H 2H),
6.92 (d, J = 8.8 Hz, 1H),
1-(4-((7,8-Dihydro-6H- Method
I 7.24 (dd, ./i = 2.4 Hz, J2 = 8.4
pyrimido[5,4-b][1,4]oxazin-4- Hz,
1H), 7.37 (d, J = 2.4 Hz,
yl)oxy)-3-methylpheny1)-3-(4-44- 1H),
7.54-7.64 (m, 4H), 7.99 (s,
ethylpiperazin-l-yl)methyl)-3- 1H),
8.74 (s, 1H), 9.04 (s, 1H);
(trifluoromethyl)phenyl)urea ESI-MS (m/z) 572 (M+H)+.
155. a H
A7, B3, 1H NMR (400 MHz, DMSO-d6)
0 N I. CF3 C59 6 2.27
(s, 3H), 2.42-2.51 (m,
0
I j N 3H),
3.16-3.23 (m, 4H), 3.45-
3.47 (m, 4H), 4.11-4.12 (m, 2H),
*1\IN
H (N) Method
B 4.80 (d, J = 6.0 Hz, 2H), 4.57 (d,
J = 6.4 Hz, 2H), 5.96 (d, J = 5.6
6 Hz,
1H), 6.84 (s, 1H), 6.94 (s,
0 1H),
7.48 (d, J = 5.6 Hz, 3H),
3-((3,4-Dihydro-2H-pyrido[3,2- 7.62
(d, J = 10.0 Hz, 2H), 7.76
b][1,4]oxazin-8-yl)oxy)-4-methyl- (dd,
./1 = 1.2 Hz, J2 = 7.6 Hz,
N-(3 -(4-(oxetan-3 -yl)piperazin-1- 1H),
10.27 (s, 1H); ESI-MS (m/z)
570 (M+H)+.
(trifluoromethyl)phenyl)benzamid
e
156. H H
Al, 4- 1H NMR (400 MHz, DMSO-d6)
N N CF3
Y 1.1 amino-
2- 6 2.05 (s, 3H), 2.22 (s, 3H), 2.45
methylph (br s, 4H), 3.19 (br s, 4H), 3.49
CN enol,
C19 (br s, 2H), 4.17 (t, J = 4.0 Hz,
N) 2H),6.81 (s, 1H), 6.92 (d, J = 8.4
N N
H I Hz,
1H), 7.21-7.28 (m, 3H), 7.37
1-(4-((7,8-Dihydro-6H- Method
I (s, 1H), 7.60 (s, 2H), 8.69 (s,
pyrimido[5,4-b][1,4]oxazin-4- 1H),
8.89 (s, 1H); ESI-MS (m/z)
yl)oxy)-3-methylpheny1)-3-(3-(4- 544 (M+H)+.
methylpiperazin-l-y1)-5-
(trifluoromethyl)phenyl)urea
157. H H
CF J Al, 4-
1H NMR (400 MHz, DMSO-d6)
CI N N An y Allik r"..N
0 ir N,) amino-2- 6 0.98 (t, J
= 7.2 Hz, 3H), 2.28-
W
0
chlorophe 2.51 (m, 10H), 3.49-3.53 (m,
0 11: j nol, Cl 4H),
4.18 (br s, 2H), 7.18 (d, J =
1\1
8.8 Hz, 1H), 7.32 (d, J = 8.8 Hz,
N N
H Method
I 1H), 7.58-7.69 (m, 4H), 7.78 (s,
1-(3-Chloro-4-((7,8-dihydro-6H- 1H),
7.97 (s, 1H), 9.02 (s, 1H),
192
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
No. Intermed Analytical Data
Structure and Name iates and
Method
pyrimido[5,4-b][1,4]oxazin-4- 9.16
(s, 1H); ESI-MS (m/z) 592
yl)oxy)pheny1)-3-(444- (M+H)+.
ethylpiperazin-l-yl)methyl)-3-
(trifluoromethyl)phenyl)urea
158. H H Al, 4- 11-1
NMR (400 MHz, DMSO-d6)
ci N N u3
40 'or 40 amino-
2- 6 2.22 (s, 3H), 2.45 (t, J = 4.4
O chlorophe Hz, 4H), 3.19 (t, J = 4.4 Hz, 4H),
N:X1 a) CN) nol,
C19 3.49-3.50 (m, 2H), 4.18 (t, J =
N N N 4.0 Hz, 2H), 6.83
(s, 1H), 7.16-
H I 7.32 (m, 4H),
7.65-7.69 (m, 2H),
1-(3-Chloro-4-((7,8-dihydro-6H- Method
I 7.78 (s, 1H), 9.05 (br s, 2H);
pyrimido[5,4-b][1,4]oxazin-4- ESI-MS (m/z) 564 (M+H)+.
yl)oxy)pheny1)-3-(3-(4-
methylpiperazin-l-y1)-5-
(trifluoromethyl)phenyl)urea
159. CI al Al,
Bl, 11-1 NMR (400 MHz, DMSO-d6)
H
N
CF C19 6 2.4
(s, 3H), 2.50-2.51 (m, 4H),
O W is
3.12 (s, 3H), 3.20-3.23 (br s, 4H),
3.59 (t, J = 4.4 Hz, 2H), 4.28 (t, J
I ) N
N N Method L =
4.4 Hz, 2H), 6.97 (s, 1H), 7.63
I C ) N (d, J = 13.6 Hz,
2H), 7.75-7.79
I (m,
2H), 7.86-7.89 (m, 2H),
4-Chloro-3-((8-methyl-7,8- 10.38
(s, 1H); ESI-MS (m/z) 563
dihydro-6H-pyrimido[5,4- (M+H)+.
b] [1,4]oxazin-4-yl)oxy)-N-(3-(4-
methylpiperazin-l-y1)-5-
(trifluoromethyl)phenyl)benzamid
e
160. CI al Al,
Bl, 11-1 NMR (400 MHz, DMSO-d6)
H
N 0 c3 C65 6 3.51-
3.52 (br, 2H), 4.21 (t, J =
0 WI
4.0 Hz, 2H), 7.70 (s, 2H), 7.71-
N:1 c j Method
7.93 (m, 5H), 8.04 (s, 2H), 8.20
N N N B' (s, 1H),
10.58 (s, 1H), 13.13(br s,
H %
N-NH 1H) ; ESI-MS (m/z)517(M+H)+.
N-(3-(1H-Pyrazol-4-y1)-5-
(trifluoromethyl)pheny1)-4-chloro-
3-((7,8-dihydro-6H-pyrimido[5,4-
b][1,4]oxazin-4-yl)oxy)benzamide
161. CI A13, 11-1 NMR (400 MHz, DMSO-d6)
H
O WI N 0 CF3 Bl,
6 (t, J = 6.8 Hz, 6H),
r\i,(0, 0 C19 1.90
(m, 1H), 2.24 (s, 3H), 2.50-
N
1 (R) 2.51 (m, 4H),
3.22 (br s, 4H),
N N-y CN)
H 4.12 (br s, 3H),
6.97 (s, 1H), 7.61
I Method
A (s, 1H), 7.64 (s, 1H), 7.71 (s,
(R)-4-Chloro-3-((7-isopropyl-7,8- 1H),
7.75-7.77 (m, 1H), 7.87-
dihydro-6H-pyrimido[5,4- 7.88
(m, 2H), 8.0 (s, 1H), 10.38
b] [1,4]oxazin-4-yl)oxy)-N-(3-(4- (s, 1H); ESI-MS (m/z) 591 (M)+.
193
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
No. Intermed Analytical Data
Structure and Name iates and
Method
methylpiperazin-l-y1)-5-
(trifluoromethyl)phenyl)benzamid
e
162. CI A14, 1H NMR
(400 MHz, DMSO-d6)
H
O WI N 0 CF3 Bl,
6 (t, J = 7.2 Hz, 6H),
Nj,, 0(s ). 0 C19 1.85-
1.87 (m, 1H), 2.23 (s, 3H),
2.46-2.50 (m, 4H), 3.21 (t, J =
N H õr cN)
4.8 Hz, 4H), 4.12 (br s, 3H), 6.97
N Method
A (s, 2H), 7.60 (s, 2H), 7.64 (s,
1
(S)-4-Chloro-3-((7-isopropyl-7,8- 1H),
7.75 (s, 1H), 7.87-7.88 (m,
dihydro-6H-pyrimido[5,4- 1H),
8.0 (br s, 1H), 10.38 (s, 1H);
b] [1,4]oxazin-4-yl)oxy)-N-(3-(4- APCI-MS (m/z) 591 (M)t
methylpiperazin-l-y1)-5-
(trifluoromethyl)phenyl)benzamid
e
163. CI ai H AS, 1H NMR
(400 MHz, DMSO-d6)
O W N io
c3 Bl, C81 6 1.06-1.09 (m, 3H), 1.18 (d, J =
N,,, 0 0 6.0 Hz, 3H), 1.26 (d, J = 6.0 Hz,
(R),,. N Method
A 3H), 2.34 (m, 2H), 2.46-2.67 (m,
N N CN)
8H), 3.34-3.56 (m, 2H), 3.70-
H
3.78 (m, 1H), 4.27(t, J = 9.6 Hz,
1H), 7.71 (s, 1H), 7.76-7.79 (m,
4-Chloro-N-(4-(1-(4- 1H),
7.89 (br s, 4H), 8.07 (d, J =
ethylpiperazin-1-yl)ethyl)-3- 11.2
Hz, 1H), 8.51 (s, 1H), 10.57
(trifluoromethyl)pheny1)-3 - (((R)- (s,
1H); ESI-MS (m/z) 605
7-methyl-7,8-dihydro-6H- (M+H)+.
pyrimido[5,4-b][1,4]oxazin-4-
yl)oxy)benzamide
164. CI ai Al,
Bl, 1H NMR (400 MHz, DMSO-d6)
H
N CF3 C79
6 3.52 (br s, 2H), 4.21 (t, J = 4.4
0 W
Hz, 2H), 7.70 (s, 1H), 7.79-7.88
NC)
I ) (m,
3H), 7.91-8.01 (m, 6H), 8.30
N N Method B (s, 1H), 8.41 (s, 1H), 10.71 (s,
H
1H); ESI-MS (m/z) 552 (M+H)+.
CN
4-Chloro-N-(4'-cyano-5-
(trifluoromethy1)41,1'-bipheny1]-
3-y1)-347,8-dihydro-6H-
pyrimido[5,4-b][1,4]oxazin-4-
yl)oxy)benzamide
165. CI al Al,
Bl, 1H NMR (400 MHz, DMSO-d6)
H
N CF3 C80
6 3.52 (br s, 2H), 4.21 (br s, 2H),
0 W
7.70-7.93 (m, 8H), 8.08 (d, J =
NC;ll
L I ) Method
B 8.0 Hz, 1H), 8.28 (d, J = 11.2 Hz,
N N
H 2H),
8.38 (s, 1H), 10.69 (s, 1H);
CN ESI-MS (m/z) 552 (M+H)+.
4-Chloro-N-(3'-cyano-5-
194
CA 03064975 2019-11-26
WO 2018/215668 PCT/EP2018/063957
No. Intermed Analytical Data
Structure and Name iates and
Method
(trifluoromethyl)-[1,1'-bipheny1]-
3-y1)-347,8-dihydro-6H-
pyrimido[5,4-b][1,4]oxazin-4-
y1)oxy)benzamide
166. a a
H te rt-
Butyl 1H NMR (400 MHz, DMSO-d6)
N
cF3 (3-
chloro- 6 2.23 (s, 3H), 2.46 (br s, 4H),
0 is
2- 2.93
(s, 3H), 3.21 (br s, 4H), 4.57
0.N.-tN N
formylphe (s, 2H), 6.15 (d, J= 5.6 Hz, 1H),
H CN)
nyl)carba 6.97 (s, 1H), 7.61 (d, J = 12.0
mate, Hz, 2H), 7.88-7.89 (m, 1H),
I Bl, C19 7.96-
7.98 (m, 3H), 9.8 (s, 1H),
4-Chloro-3-43-methy1-2-oxo-
10.41 (s, 1H); ESI-MS (m/z) 575
1,2,3,4-tetrahydropyrido[2,3-
(M+H)+.
d]pyrimidin-5 -yl)oxy)-N-(3 - (4-
Method N
methylpiperazin-l-y1)-5-
(trifluoromethyl)phenyl)benzamid
e
167. CI 0 All, 1H NMR
(400 MHz, DMSO-d6)
H
N
Bl, Cl 6 0.96 (t, J=
O CFrNi.
NO 0 I, N,) 6.0
Hz, 6H), 1.54-1.61 (m, 2H),
Method A 2.28-2.39 (m, 10H), 3.49-3.50
1 (R)
NN (m, 1H), 3.56 (s, 2H), 3.92-3.96
H
(R)-4-Chloro-3-47-ethyl-7,8-
(m, 1H), 4.22 (dd, J1 = 2.8 Hz,
= 10.8 Hz, 1H), 7.71 (s, 1H),
dihydro-6H-pyrimido[5,4-
7.72-7.96 (m, 5H), 8.03 (dd, ./1 =
b] [1,4]oxazin-4-yl)oxy)-N-(4-((4-
1.6 Hz, J2 = 8.4 Hz, 1H), 8.17
(trifluoromethyl)phenyl)benzamid
ethylpiperazin-l-yl)methyl)-3-
(d, J = 2.0 Hz, 1H), 10.54 (s,
1H); ESI-MS (m/z) 605 (M+H)+.
e
168. CI al Al, Bl, 1H NMR (400 MHz, DMSO-d6)
H
O N io cF3 C82
6 1.09 (t, J = 7.6 Hz, 3H), 2.36
(q, J= 7.2 Hz, 2H), 3.51-3.52 (br
õ,j,o, 0
NO
1 j HNO Method
B s, 2H), 4.21 (t, J = 4.4 Hz, 2H),
NN 7.69 (s, 1H), 7.75-7.91 (m, 6H),
H
8.37 (s, 1H), 10.27 (s, 1H), 10.95
4-Chloro-3-((7,8-dihydro-6H- (s,
1H); ESI-MS (m/z) 522
pyrimido[5,4-b][1,4]oxazin-4- (M+H)t
yl)oxy)-N-(3-propionamido-5-
(trifluoromethyl)phenyl)benzamid
e
169. a
H Al,
B3, 1H NMR (400 MHz, DMSO-d6)
O N s CF3 3-(4-
6 2.16 (s, 3H), 2.18 (s, 3H), 3.49
i\i O methyl- (s,
2H), 4.18 (m, 2H), 7.43-7.51
j-'N
1 j 1H- (m,
2H), 7.63-7.74 (m, 4H), 7.80
NN
H N?
imidazol- (dd, J= 7.9, 1.8 Hz, 1H), 8.12 (s,
N-
1-y1)-5- 1H),
8.19 (s, 1H), ), 8.26 (d, J =
3((7,8-Dihydro-6H-pyrimido[5,4- (trifluoro 2.1 Hz, 1H), 10.58 (s, 1H);
b][1,4]oxazin-4-yl)oxy)-4-methyl- methyl)an LCMS: ESI 511 (M+H)+.
195
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
No. Intermed Analytical Data
Structure and Name iates and
Method
N-(3-(4-methy1-1H-imidazol-1- iline
y1)-5-
(trifluoromethyl)phenyl)benzamid Method A
e or B
170. CI Al,
Bl, 'I-1 NMR (400 MHz, DMSO-d6)
H 3-(4- 6 2.15
(s, 3H), 3.49 (s, 2H), 4.18
O W N 5 CF3
methyl- (t, J
= 4.4 Hz, 2H), 7.43 (s, 2H),
1 ) 1H- 7.65
(m, 2H), 7.75 (s, 1H), 7.83¨
N
H N
imidazol- 7.94 (m, 2H), 8.05 (m, 2H),
N
N¨\ 1-y1)-
5- 8.13 (s, 1H), 10.74 (br, 1H);
4-Chloro-3-((7,8-dihydro-6H- (trifluoro LCMS: ESI (M+H)+ 531, 533.
pyrimido[5,4-b][1,4]oxazin-4- methyl)an
yl)oxy)-N-(3-(4-methyl-1H- iline
imidazol-1-y1)-5-
(trifluoromethyl)phenyl)benzamid Method A
or B
e
171. a H Al,
Bl, 1I-1 NMR (400 MHz, DMSO-d6)
N r CF3 2-
fluoro- 6 2.17 (s, 3H), 10.29 (s, 1H),
0
5- 3.48
(m, 2H), 4.17 (m, 2H), 4.17
NC:1 0 F W
1 j (trifluoro (m, 2H), 7.45 (d, J = 7.9
N N H
methyl)an Hz, 1H), 7.54 (t, J= 9.4 Hz, 1H),
3-((7,8-Dihydro-6H-pyrimido[5,4- iline 7.60-
7.71 (m, 4H), 7.77 (dd, J =
b][1,4]oxazin-4-yl)oxy)-N-(2- 7.9,
1.8 Hz, 1H), 7.96-8.06 (m,
fluoro-5-(trifluoromethyl)pheny1)- Method A 1H); LCMS: ESI (M+H)+ 459.
4-methylbenzamide or B
172. a Al, B3, 1I-1 NMR (400 MHz, DMSO-d6)
H
N 0 WO ..õ1. CF3 2-fluoro- 6 3.49 (s, 2H);
5- 4.18
(t, J = 4.3 Hz, 2H), 7.52 (t, J
i\jc,o, 0 F IW
I j
(trifluoro = 9.4 Hz, 1H), 7.62 (s, 1H), 7.67
N N H
methyl)an (s, 1H), 7.73 (d, J = 8.2 Hz, 1H),
4-Chloro-3-((7,8-dihydro-6H- iline 7.77
(s, 1H), 7.82-7.91 (m, 2H),
pyrimido[5,4-b][1,4]oxazin-4- 8.07
(d, J = 6.8 Hz, 1H), 10.44
yl)oxy)-N-(2-fluoro-5- Method A (s, 1H).
(trifluoromethyl)phenyl)benzamid or B
e
173. Al, B3, 1H NMR (400 MHz,
0
0 0 CFrN,I.- Cl D20) 6 1.34 (t, J = 7.4 Hz,
1\j*c,0 0 N 3H),
2.22 (s, 3H), 3.29 (q, J = 7.4
Method A Hz, 2H), 3.10-3.80 (m, 8H), 3.68
1 j
N N or B
(br s, 2H), 4.28 (br s, 2H), 4.35
H (br s,
2H), 7.52 (d, J = 8.0 Hz,
3-((7,8-Dihydro-6H-pyrimido[5,4- 1H),
7.61 (s, 1H), 7.74 (d, J = 6.3
b][1,4]oxazin-4-yl)oxy)-N-(4-44-
Hz, 2H), 7.75 (d, J = 8.0 Hz,
ethylpiperazin-l-yl)methyl)-3-
1H), 7.87 (d, J = 8.0 Hz, 1H),
(trifluoromethyl)pheny1)-4-
8.01 (s, 1H); LCMS: ESI
methylbenzamide (M+H)+ 557.
196
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
No. Intermed Analytical Data
Structure and Name iates and
Method
174. CI A, Al,
Bl, lliNMR (400 MHz, D20) 6 1.34
C1 (t, J
= 7.5 Hz, 3H), 3.33 (q, J =
O WI N CFrN,
0 7.5
Hz, 2H), 3.40-3.80 (m, 8H),
Method A 3.66 (t, J= 4.1 Hz, 2H), 4.32 (t, J
)
NN = 4.1 Hz, 2H), 4.55 (s, 2H), 7.70
(d, J = 6.5 Hz, 2H), 7.75-7.82
4-Chloro-3-((7,8-dihydro-6H- (m,
4H), 7.84 (d, J = 7.8 Hz, 1H),
pyrimido[5,4-b][1,4]oxazin-4- 8.02
(s, 1H); LCMS: ESI
yl)oxy)-N-(4-((4-ethylpiperazin-1- (M+H)+ 577.
yl)methyl)-3-
(trifluoromethyl)phenyl)benzamid
175. CI Step 2- 1I-1
NMR (400 MHz, DMSO-d6)
O WI N cF3 A7,
6 2.41 (t, J = 4.4 Hz, 4H), 3.23 (t,
0= Bl, C59 J = 4.4 Hz, 4H),
3.43-3.47 (m,
3H), 4.10 (t, J = 3.6 Hz, 2H),
NN (N)
Method 0 4.47 (t, J = 6.0 Hz, 2H), 4.57 (t, J
= 6.8 Hz, 2H), 6.10 (d, J = 5.6
Hz, 1H), 6.96 (s, 2H), 7.53 (d, J
0 = 5.6
Hz, 1H), 7.60 ( br s, 3H),
4-Chloro-3-((3,4-dihydro-2H- 7.77-
7.84 (m, 2H), 10.39 (s, 1H);
pyrido[3,2-b][1,4]oxazin-8- ESI-MS (m/z) 590 (M+H)+.
yl)oxy)-N-(3-(4-(oxetan-3-
yl)piperazin-l-y1)-5-
(trifluoromethyl)phenyl)benzamid
176. CI Ai Step 1- NMR
(400 MHz, DMSO-d6)
O WI N cF3 A7,
6 2.27 (s, 3H), 2.44-2.47 (br s,
Bl, C19 4H),
3.19-3.21 (br s, 4H), 3.43
C ) (br s,
2H), 4.10 (t, J = 4.0 Hz,
N N (N) Method
0 2H), 6.10 (d, J = 5.6 Hz, 1H),
6.96 (s, 2H), 7.53 (d, J = 5.6 Hz,
4-Chloro-3-((3,4-dihydro-2H-
1H), 7.59 (s, 3H), 7.78-7.85 (m,
pyrido[3,2-b][1,4]oxazin-8-
2H), 10.38 (s, 1H); ESI-MS (m/z)
548 (M+H)+.
yl)oxy)-N-(3-(4-methylpiperazin-
1-y1)-5 -
(trifluoromethyl)phenyl)benzamid
177. H H Al, 4-
1I-1 NMR (400 MHz, DMSO-d6)
ci NN c3
= amino-2- 6 3.49 (br s, 2H), 4.18 (br s, 2H),
0
0 WI
chlorophe 7.19 (d, J= 8.8 Hz, 1H), 7.32 (d,
N'
nol, 3- J =
8.4 Hz, 2H), 7.52 (t, J = 8.0
(trifluoro Hz, 1H), 7.60 (d, J = 8.0 Hz,
N N
methyl)an 1H), 7.66-7.68 (m, 2H), 7.78 (s,
1-(3-Chloro-4-((7,8-dihydro-6H- iline 1H),
8.01 (s, 1H), 9.0 (s, 1H),
pyrimido[5,4-b][1,4]oxazin-4- 9.15
(s, 1H); ESI-MS (m/z) 466
yl)oxy)pheny1)-3-(3- Method I (M+H)+.
197
CA 03064975 2019-11-26
WO 2018/215668 PCT/EP2018/063957
No. Intermed Analytical Data
Structure and Name iates and
Method
(trifluoromethyl)phenyl)urea
178. H H Al, 3- lli NMR (400 MHz, DMSO-d6)
A NN 401 CF3
(trifluoro 6 3.48 (t, J= 4.4 Hz, 2H), 4.16 (t,
0
0 Wj methyl)an J = 4.4
Hz, 2H), 7.02-7.04 (m,
NA' iline, 4- 2H), 7.31 (d, J = 8.8 Hz, 1H),
) aminophe 7.44-7.46 (m, 2H), 7.51 (t, J =
N N
H nylbenzyl 7.6 Hz, 1H), 7.58 (d, J= 8.8 Hz,
1-(4-((7,8-Dihydro-6H-
carbonate 1H), 7.64 (s, 1H), 7.69 (s, 1H),
pyrimido[5,4-b][1,4]oxazin-4- 8.32
(s, 1H), 8.18 (s, 1H), 9.05
yl)oxy)pheny1)-3-(3- Method
I' (s, 1H); ESI-MS (m/z) 432
(trifluoromethyl)phenyl)urea (M+H)+.
179. H H
Al, 4- lli NMR (400 MHz, DMSO-d6)
0 NN 40, cF3
aminophe 6 3.47-3.48 (br s, 2H), 4.16 (t, J
O ON nol, 4-
= 8.8 Hz, 2H), 7.05 (d, J = 8.8
N:CXI0j amino-
2- Hz, 2H), 7.47 (d, J = 9.2 Hz,
(trifluoro 2H), 7.66 (s, 2H), 7.78 (d, J = 7.2
N N
H
methyl)be Hz, 1H), 8.04 (d, J = 8.4 Hz,
1-(4-Cyano-3-
nzonitrile 1H), 8.22 (s, 1H), 9.08 (s, 1H),
(trifluoromethyl)pheny1)-3-(4- 9.63
(s, 1H); ESI-MS (m/z) 457
((7,8-dihydro-6H-pyrimido[5,4- Method I (M+H)+.
b] [1,4]oxazin-4-
yl)oxy)phenyl)urea
180. CI Ai Al, Bl, 'H NMR
(400 MHz, DMSO-d6)
H
N C83 6 1.81 (s, 6H), 3.52
(br s, 2H),
O WI so cF3
4.21 (t, J = 4.0 Hz, 2H), 7.69 (s,
NAC)) 0 ON Method
D 1H), 7.78-7.89 (m, 3H), 7.90-
N
H N 7.92
(m, 2H), 8.13 (dd, ./1 = 2.0
4-Chloro-N-(4-(2-cyanopropan-2-
Hz, J2 = 8.8 Hz, 1H), 8.31 (s,
1H), 10.67 (s, 1H); ESI-MS (m/z)
y1)-3-(trifluoromethyl)pheny1)-3-
518 (M+H)+.
((7,8-dihydro-6H-pyrimido[5,4-
b][1,4]oxazin-4-yl)oxy)benzamide
181. ci A18,
Bl, 'HNMR (400 MHz, DMSO-d6)
H
oW NOCF3 3-
6 2.42-2.44 (m, 6H), 3.59-3.61
N,10
(trifluoro (m, 4H), 3.81-3.83 (m, 1H),
c 0
N J. N
methyl)an 4.09-4.19 (m, 2H), 7.48 (d, J =
iline 7.6
Hz, 1H), 7.61 (t, J = 8.0 Hz,
H N 1H),
7.72-7.91 (m, 5H), 8.04 (d,
( ) Method A J= 8.0 Hz,
Hz, 1H), 8.21 (s, 1H),
0
(R)-4-Chloro-3-((7- 10.60
(s, 1H); ESI-MS (m/z) 550
(morpholinomethyl)-7,8-dihydro- (M+H)+.
6H-pyrimido[5,4-b][1,4]oxazin-4-
yl)oxy)-N-(3-
(trifluoromethyl)phenyl)benzamid
e
198
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
No. Intermed Analytical Data
Structure and Name iates and
Method
182. H H
Al, 4- 'll NMR (400 MHz, DMSO-d6)
N N CN
40 X 40 aminophe 6 3.4 (br s, 2H),
4.16 (br s, 2H),
0 nol, 3- 7.01 (d, J =
8.0 Hz, 2H), 7.51 (d,
N:N CX10) CF3 amino-
5- J = 8.8 Hz, 2H), 7.69 (s, 2H),
(trifluoro 7.78 (s, 1H), 8.12 (s, 1H), 8.23
N
H
methyl)be (s, 1H), 10.27 (br s, 2H); ESI-MS
1-(3 -Cyano-5 - nzonitrile (m/z) 457 (M+H)+.
(trifluoromethyl)pheny1)-3-(4-
((7,8-dihydro-6H-pyrimido[5,4- Method I
b] [1,4]oxazin-4-
yl)oxy)phenyl)urea
183. H H
AS, lli NMR (400 MHz, DMSO-d6)
ci N N CF
4-amino- 6 0.85 (t, J = 6.4 Hz, 1H), 0.97 (t,
WI Yo 01
0 2- J = 6.8 Hz,
3H), 1.17-1.28 (m,
0 N
chlorophe 3H), 2.27-2.38 (m, 8H), 3.52 (br
(N) nol, Cl s,
2H), 3.66-3.74 (m, 3H), 4.23
N N
H
(d, J = 9.2 Hz, 1H), 7.18 (t, J =
(R)-1-(3-Chloro-4-47-methyl-7,8- Method I 8.4 Hz, 1H), 7.31 (t, J = 9.2 Hz,
dihydro-6H-pyrimido[5,4- 1H),
7.57-7.67 (m, 3H), 7.77 (s,
b][1,4]oxazin-4-yl)oxy)pheny1)-3- 2H),
7.96 (s, 1H), 9.04 (s, 1H),
(4-((4-ethylpip erazin-1- 9.18
(s, 1H); ESI-MS (m/z) 606
yl)methyl)-3- (M+H)+.
(trifluoromethyl)phenyl)urea
184. F H H
Al, 4- lli NMR (400 MHz, DMSO-d6)
N N CF3 amino-
3- 6 2.23 (s, 3H), 2.47 (br s, 4H),
0
W Yo 0 fluorophe 3.16-3.20 (m, 4H),
3.48 (br s,
N nol,
C19 2H), 4.10-4.17 (m, 2H), 6.84 (s,
CN) 1H), 6.91 (d, J = 9.2 Hz,
1H),
N N
H I Method
I 7.12-7.18 (m, 2H), 7.27 (s, 1H),
1-(4-((7,8-Dihydro-6H- 7.72
(s, 2H), 7.99 (t, J = 9.2 Hz,
pyrimido[5,4-b][1,4]oxazin-4- 1H),
8.55 (s, 1H), 9.26 (s, 1H);
yl)oxy)-2-fluoropheny1)-3-(3-(4- ESI-MS (m/z) 548 (M+H)+.
methylpiperazin-l-y1)-5-
(trifluoromethyl)phenyl)urea
185. CF3
Al, lli NMR (400 MHz, DMSO-d6)
0 0 methyl
4- 6 2.30 (s, 3H), 2.50-25.51 (br s,
hydroxyb 4H), 3.25 (br s, 4H), 3.50 (d, J =
1.1 N N
c.,N,
enzoate, 2.8 Hz, 2H), 4.17 (t, J = 4.4 Hz,
0
C19 2H),
6.96 (s, 1H), 7.25 (d, J = 8.8
N:CiCo) Hz,
2H), 7.67 (d, J = 9.6 Hz,
N N H 2H), 7.81 (s,
2H), 7.99 (d, J= 8.8
4-((7,8-Dihydro-6H-pyrimido[5,4- Method B Hz, 2H),10.33 (s, 1H); ESI-MS
b] [1,4]oxazin-4-yl)oxy)-N-(3-(4- (m/z) 515 (M+H)t
methylpiperazin-l-y1)-5-
(trifluoromethyl)phenyl)benzamid
e
199
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
No. Intermed Analytical Data
Structure and Name iates and
Method
186. cF3 Al, 1t1
NMR (400 MHz, DMSO-d6)
0 0 N
methyl 4- 6 1.0 (t, J = 6.8 Hz, 3H), 2.41-
hydroxyb 2.51 (m, 10H), 3.49-3.50 (br s,
OP ri
enzoate, 4H), 4.17 (t, J = 4.4 Hz, 2H),
0
NOCl 7.25
(d, J = 8.8 Hz, 2H), 7.21 (d,
I ) J =
8.4 Hz, 3H), 7.99 (d, J = 4.8
N N Method B Hz, 3H), 8.22 (s, 1H), 10.49 (s,
H
4-((7,8-Dihydro-6H-pyrimido[5,4- 1H); ESI-MS (m/z) 543 (M)+.
b] [1,4]oxazin-4-yl)oxy)-N-(4-((4-
ethylpiperazin-l-yl)methyl)-3-
(trifluoromethyl)phenyl)b enzamid
e
187. H H A7, 1H NMR (400 MHz, DMSO-d6)
Al NN I. CF3
4- 6 2.23
(s, 3H), 2.44-2.49 (m,
0
o
aminophe 2H), 3.17-3.21 (m, 4H), 3.39-
nol, C19 3.43 (m, 4H), 4.08-4.12 (m, 2H),
1 ) (NJ 6.01
(d, J = 5.6 Hz, 1H), 6.76-
N N
H I Method
I 6.83 (m, 2H), 6.97 (d, J = 8.8 Hz,
1-(4-((3,4-Dihydro-2H- 2H),
7.20-7.29 (m, 2H), 7.42-
pyrido[3,2-b][1,4]oxazin-8- 7.49
(m, 3H), 8.76 (s, 1H), 8.87
yl)oxy)pheny1)-3-(3-(4- (s,
1H); ESI-MS (m/z) 529
methylpiperazin-l-y1)-5- (M+H)+.
(trifluoromethyl)phenyl)urea
188. H H A7,
1H NMR (400 MHz, DMSO-d6)
Al Ny N 0 CF3 4- 6 0.98
(t, J = 7.2 Hz, 3H), 2.30-
0
aminophe 2.40 (m, 10H), 3.17 (d, J = 5.2
0
c,lo
I j N
) nol, Cl Hz,
1H), 3.39-3.44 (m, 2H), 3.53
C
(s, 2H), 4.09-4.11 (m, 2H), 6.01
1\1 N N Method
I (d, J = 4.4 Hz, 1H), 6.76 (s, 1H),
H
6.97 (d, J = 8.8 Hz, 2H), 7.46
1-(4-((3,4-Dihydro-2H-
(dd, Ji =1.6 Hz, ./i = 7.2 Hz, 2H),
pyrido[3,2-b][1,4]oxazin-8- 7.56
(d, J = 8.4 Hz, 1H), 7.62 (d,
yl)oxy)pheny1)-3-(4((4- J =
8.4 Hz, 1H), 7.97 (d, J = 2.0
ethylpiperazin-l-yl)methyl)-3- Hz,
1H), 8.77 (s, 1H), 8.98 (s,
(trifluoromethyl)phenyl)urea 1H); ESI-MS (m/z) 557 (M+H)+.
189. CI Ai Al, Bl, 1H NMR (400 MHz, DMSO-d6)
H
O N 0 cF3 C84
6 2.28 (s, 6H), 3.11 (s, 2H), 3.51-
3.52 (m, 2H), 4.20 (t, J= 4.4 Hz,
N c,1:2 0
I ) HN,0 2H),
7.76 (d, J = 8.8 Hz, 1H),
N N
H L Method
B 7.81-7.83 (m, 3H), 7.88-7.90 (m,
1\1
3H), 8.46 (s, 1H), 10.15 (s, 1H),
I 10.60
(s, 1H); ESI-MS (m/z) 551
4-Chloro-3-((7,8-dihydro-6H-
(M+H)+.
pyrimido[5,4-b][1,4]oxazin-4-
yl)oxy)-N-(3-(2-
(dimethylamino)acetamido)-5-
(trifluoromethyl)phenyl)benzamid
200
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
No. Intermed Analytical Data
Structure and Name iates and
Method
e
190. CI Al, Bl, 1I-1 NMR (400 MHz, DMSO-d6)
H
O N 0 CF3 C85
6 0.84 (d, J = 6.0 Hz, 6H), 2.23-
0 0 2.26
(m, 1H), 3.11-3.40 (m, 5H),
)):
N ' j
HN TO 3.51-
3.52 (br s, 2H), 4.21 (t, J =
N N
6 4.0
Hz, 2H), 7.69-7.90 (m, 6H),
8.40 (s, 1H), 10.30 (s, 1H), 10.59
H
Method B
N (s,
1H); ESI-MS (m/z) 591
(M+H)+.
N-(3-(4-Chloro-3-((7,8-dihydro-
6H-pyrimido[5,4-b][1,4]oxazin-4-
yl)oxy)benzamido)-5-
(trifluoromethyl)pheny1)-1-
isopropylazetidine-3-carboxamide
191. a A19, Bl, 1I-1 NMR (400 MHz, DMSO-d6)
H
O N 0 CF3 Cl
6 0.98 (t, J = 7.2 Hz, 3H), 2.30-
Nj):0 0 2.51 (m, 10H), 3.33-3.60 (m,
I N)=''OH (N) Method
A 5H), 4.12-4.21 (m, 2H), 5.06-
N
5.08 (m, 1H), 7.71-7.90 (m, 6H),
H
) 8.01-
8.04 (m, 1H), 8.17 (s, 1H),
10.54 (s, 1H); ESI-MS (m/z) 607
(S)-4-Chloro-N-(4-((4- (M+H)+.
ethylpiperazin-l-yl)methyl)-3-
(trifluoromethyl)pheny1)-3-47-
(hydroxymethyl)-7,8-dihydro-6H-
pyrimido[5,4-b][1,4]oxazin-4-
yl)oxy)benzamide
192. a H Al,
B2, 1I-1 NMR (400 MHz, DMSO-d6)
O N 0 CF3 C19
6 2.24 (s, 3H), 2.50 (br s, 4H),
3.21 (br s, 4H), 3.50 (br s, 2H),
I ) N Method
B 4.18 (t, J = 4.0 Hz, 2H), 6.96 (s,
N N
H CN) 1H),
7.36 (dd, ./i =1.6 Hz, J1 =
I 7.2 Hz, 1H), 7.56 (d, J =
8.0 Hz,
3-((7,8-Dihydro-6H-pyrimido[5,4- 1H),
7.63-7.74 (m, 4H), 7.78-
b] [1,4]oxazin-4-yl)oxy)-N-(3-(4- 7.82
(m, 2H), 10.34 (s, 1H);
methylpiperazin-l-y1)-5- ESI-MS (m/z) 515 (M+H)+.
(trifluoromethyl)phenyl)benzamid
e
193. CI Al, Bl, 1I-1 NMR (400 MHz, DMSO-d6)
H
O N I* CF3 C88
6 3.52 (br s, 2H), 4.21 (t, J = 4.4
Hz, 2H), 7.59-7.60 (m, 2H),
NO
I ) Method
B 7.78-7.80 (m, 3H), 7.89-7.91 (m,
N N I I
3H), 8.24 (s, 1H), 8.35 (s, 1H),
H
8.67 (d, J = 5.6 Hz, 2H), 10.70
I (s,
1H); ESI-MS (m/z) 552
N (M+H)+.
4-Chloro-3-((7,8-dihydro-6H-
201
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
No. Intermed Analytical Data
Structure and Name iates and
Method
pyrimido[5,4-b][1,4]oxazin-4-
yl)oxy)-N-(3-(pyridin-4-
ylethyny1)-5-
(trifluoromethyl)phenyl)benzamid
e
194. F H H
Al, 4- 1I-1 NMR (400 MHz, DMSO-d6)
N N CF3 amino-
3- 6 0.98 (t, J = 7.2 Hz, 3H), 2.28-
0 WI Yo 110
fluorophe 2.33 (m, 10H), 3.48 (br s, 4H),
N ' Cll N nol, Cl 4.16
(t, J = 4.0 Hz, 2H), 6.91 (d,
)): ) ( ) J =
9.2 Hz, 1H), 7.14 (dd, Ji =2.8
N N N Method
I Hz, J, = 12.0 Hz, 1H), 7.54 (d, J
H
= 8.8 Hz, 1H), 7.64 (d, J = 8.4
1-(4-((7,8-Dihydro-6H-
Hz, 1H), 7.72 (s, 2H), 7.98-8.03
pyrimido[5,4-b][1,4]oxazin-4-
(m, 2H), 8.56 (s, 1H), 9.33 (s,
yl)oxy)-2-fluoropheny1)-3-(444-
1H); ESI-MS (m/z) 576 (M+H)+.
ethylpiperazin-l-yl)methyl)-3-
(trifluoromethyl)phenyOurea
195. CI al A, Bl, 1I-1 NMR (400 MHz, DMSO-d6)
H
0 WI N 0 CF3 C86 6 2.11
(s, 6H), 3.16-3.22 (m,
NO 1H),
3.51-3.52 (br s, 2H), 3.61 (t,
c, 0
I j N J =
5.2 Hz, 2H), 3.96 (t, J = 7.2
N N H Hz,
2H), 4.20 (t, J = 4.4 Hz, 2H),
? Method
B 6.43 (s, 1H), 7.16 (s, 1H), 7.48
(s, 1H), 7.69 (s, 1H), 7.75 (d, J =
4-Chloro-3-((7,8-dihydro-6H-
8.8 Hz, 1H), 7.86 (s, 1H), 7.88-
pyrimido[5,4-b][1,4]oxazin-4-
7.93 (m, 2H), 10.34 (s, 1H);
yl)oxy)-N-(3-(3-
ESI-MS (m/z) 549 (M+H)+.
(dimethylamino)azetidin-l-y1)-5-
(trifluoromethyl)phenyl)benzamid
e
196. CI H Step 2-
1I-1 NMR (400 MHz, DMSO-d6)
N 0 CF3 A7, 6 0.98
(t, J = 6.8 Hz, 3H), 2.27-
0 WI
Bl, Cl 2.38
(m, 10H), 3.43 (br s, 2H),
0 0
I ) N 3.55
(s, 2H), 4.12 (d, J = 4.8 Hz,
I\JN
H C ) Method
0 2H), 6.10 (d, J = 5.2 Hz, 1H),
N 6.95
(s, 1H), 7.53 (d, J = 5.6 Hz,
2H), 7.61 (s, 1H), 7.70 (d, J = 8.4
4-Chloro-3-((3,4-dihydro-2H- Hz,
1H), 7.82-7.85 (m, 1H), 7.99
pyrido[3,2-b][1,4]oxazin-8- (d, J=
8.8 Hz, 1H), 8.15 (s, 1H),
yl)oxy)-N-(4-((4-ethylpiperazin-1- 10.55
(s, 1H) ;ESI-MS (m/z) 576
yl)methyl)-3- (M+H)+.
(trifluoromethyl)phenyl)benzamid
e
202
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
No. Intermed Analytical Data
Structure and Name iates and
Method
197. H H Al, 4- 1t1
NMR (400 MHz, DMSO-d6)
N N
40 'or 40 aminophe 6 1.24
(t, J = 6.4 Hz, 3H), 3.17-
O nylbenzyl 3.41 (m, 6H), 3.51 (m, 2H), 3.57-
N
carbonate, 3.69 (m, 4H), 4.18-4.19 (br s,
2 HCI
r )
) L N ) C28 2H), 4.33 (br s,
2H), 7.04 (d, J =
N N
H
7.2 Hz, 2H), 7.46 (d, J = 8.0 Hz,
1-(4-((7,8-Dihydro-6H- Method
I' 2H), 7.51-7.56 (m, 4H), 7.82 (m,
pyrimido[5,4-b][1,4]oxazin-4- 2H),
9.29-9.51 (m, 2H); ESI-MS
yl)oxy)pheny1)-3-(444- (m/z) 490 (M+H)+.
ethylpiperazin-l-
yl)methyl)phenyl)urea
198. CI H H Al, 4- 1H NMR
(400 MHz, DMSO-d6)
Ai NN
0 CF
amino-3- 6 0.98 (t, J = 7.2 Hz, 3H), 2.29-
0
chlorophe 2.39 (m, 10H), 3.49 (br s, 4H),
0
0 N nol, Cl 4.16
(t, J = 4.4 Hz, 2H), 7.08 (dd,
1\1CX
I ) (N) Ji =
2.40 Hz, .1-2 = 8.8 Hz, 1H),
N N
Method I 7.31 (s, 1H), 7.56 (d, J= 8.4 Hz,
H
C
1H),7.64 (d, J = 8.4 Hz, 1H),
1-(2-Chloro-4-((7,8-dihydro-6H-
7.73 (s, 2H), 7.99 (s, 1H), 8.05
pyrimido[5,4-b][1,4]oxazin-4-
(dd, J = 8.8 Hz, 1H), 8.40 (s,
yl)oxy)pheny1)-3-(444-
1H), 9.70 (s, 1H); ESI-MS (m/z)
ethylpiperazin-l-yl)methyl)-3-
592 (M+H)+.
(trifluoromethyl)phenyl)urea
199. H H
A7, 1H NMR (400 MHz, DMSO-d6)
NN 40 is 0 4- 6 0.98
(t, J = 7.2 Hz, 3H), 2.30-
0
aminophe 2.40 (m, 10H), 3.35-3.45 (m,
X ,0
N
CN ) nol, C28 5H), 4.10 (s, 2H),
6.00 (d, J = 5.6
Hz, 1H), 6.75 (s, 1H), 6.97 (d, J
N N
H
C = 8.4 Hz, 2H), 7.18 (d, J = 8.0
1-(4-((3,4-Dihydro-2H- Hz,
2H), 7.39 (d, J= 8.0 Hz, 2H),
pyrido[3,2-b][1,4]oxazin-8- 7.43-
7.47 (m, 2H), 8.65 (s, 1H),
yl)oxy)pheny1)-3-(4((4- 8.69
(s, 1H); ESI-MS (m/z) 489
ethylpiperazin-1- (M+H)+.
yl)methyl)phenyl)urea
200. a H
Al, B3, 1H NMR (400 MHz, DMSO-d6)
O N 0 CF3 C87
6 2.19 (s, 3H), 3.581 (br s, 2H),
0 4.20
(t, J = 4.0 Hz, 2H), 6.72 (d,
Method B J = 16.0 Hz, 1H), 7.23 (s, 1H),
NN /
H 7.45-
7.49 (m, 2H), 7.69-7.72 (m,
H2N 0 5H),
7.82 (d, J = 2.0 Hz, 1H),
(E)-N-(3 -(3 -Amino-3 -oxoprop-1- 8.16
(s, 1H), 8.34 (s, 1H), 10.54
en-1-y1)-5- (s,
1H); ESI-MS (m/z) 500
(trifluoromethyl)pheny1)-347,8- (M+H)+.
dihydro-6H-pyrimido[5,4-
b][1,4]oxazin-4-yl)oxy)-4-
methylbenzamide
203
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
No. Intermed Analytical Data
Structure and Name iates and
Method
201. a H Al,
B3, lli NMR (400 MHz, DMSO-d6)
O N 0 CF3 C89
6 2.19 (s, 3H), 3.51 (s, 2H), 4.20
N:CXI0j 0 (s,
2H), 7.49 (d, J = 6.8 Hz, 1H),
Method B 7.64-7.73 (m, 4H), 7.80-7.81 (m,
N N 11
H 2H), 8.26 (s,
2H), 8.34 (s, 1H),
0 NH2 10.59
(s, 1H); ESI-MS (m/z) 498
N-(3 -(3 -Amino-3 -oxoprop-1-yn-1- (M+H)+.
y1)-5-(trifluoromethyl)pheny1)-3-
47,8-dihydro-6H-pyrimido[5,4-
b] [1,4]oxazin-4-yl)oxy)-4-
methylbenzamide
202. H H
A20, 4- lli NMR (400 MHz, DMSO-d6)
N N CF3
aminophe 6 1.02 (m, 3H), 1.24 (br s, 2H),
WI 110 ISI
0 nol, Cl 1.90
(br s, 2H), 2.50 (br s, 5H),
rL re/C1) CN N
) 3.34-3.41 (m, 5H), 3.54 (s,
2H),
Method I 3.99 (t, J = 5.2 Hz, 2H), 6.16 (d,
NI\I
H
) 1-(4-((4- J = 5.6 Hz, 2H), 6.98 (d, J
= 8.8
Hz, 2H), 7.47 (d, J = 8.8 Hz,
Ethylpiperazin-l-yl)methyl)-3-
(trifluoromethyl)pheny1)-3-(4-
2H), 7.59-7.63 (m, 3H), 7.97 (s,
1H), 8.84 (s, 1H), 9.05 (s, 1H);
((2,3,4,5-tetrahydropyrido[3,2-
ESI-MS (m/z) 571 (M+H)+.
b][1,4]oxazepin-9-
yl)oxy)phenyl)urea
203. H H
A21, lli NMR (400 MHz, DMSO-d6)
NCn14N N CF3
11 C)r 1.1 benzyl
(4- 6 1.01 (t, J = 7.2 Hz, 3H), 2.42
0 hydroxyp (br s, 10H), 3.33 (br
s, 2H), 3.54
O N
henyl)car (br s, 2H), 4.04 (d, J = 8.0 Hz,
1 ) (N) bamate, 2H), 6.91 (d, J =
9.2 Hz, 2H),
N N
H
Cl 7.41
(d, J= 9.2 Hz, 2H), 7.57 (d,
1-(4-((7-Cyano-3,4-dihydro-2H- J =
8.4 Hz, 1H), 7.62 (d, J = 8.4
pyrido[3,2-b][1,4]oxazin-8- Method
P Hz, 1H), 7.96 (s, 1H), 8.10 (s,
yl)oxy)pheny1)-3-(4((4- 2H),
8.79 (s, 1H), 9.04 (s, 1H);
ethylpiperazin-l-yl)methyl)-3- ESI-MS (m/z) 582 (M+H)+.
(trifluoromethyl)phenyl)urea
204. a H
Al, B3, lli NMR (400 MHz, DMSO-d6)
O N 0 CF3 C90
6 2.19 (s, 3H), 3.51 (br s, 2H),
NOc, 0 4.20
(t, J = 4.0 Hz, 2H), 6.60 (d,
1 j Method
B J = 16.8 Hz, 1H), 7.48 (d, J = 8.0
N N /
H CN Hz, 1H), 7.68-
7.83 (m, 6H), 8.25
(E)-N-(3-(2-Cyanoviny1)-5- (d, J
= 14.8 Hz, 2H), 10.59 (s,
(trifluoromethyl)pheny1)-347,8- 1H); ESI-MS (m/z) 482 (M+H)+.
dihydro-6H-pyrimido[5,4-
b] [1,4]oxazin-4-yl)oxy)-4-
methylbenzamide
204
CA 03064975 2019-11-26
WO 2018/215668 PCT/EP2018/063957
No. Intermed Analytical Data
Structure and Name iates and
Method
205. H H
Al, 4- 'I-1 NMR (400 MHz, DMSO-d6)
N N CF3
aminophe 6 1.25 (t, J = 7.2 Hz, 3H), 3.17-
VI Yo 1101
0
nylbenzyl 3.68 (m, 13H), 4.18 (t, J = 4.0
N' C:Il HCI N
carbonate, Hz, 2H), 4.45 (m, 2H), 7.05 (d, J
) C10 = 8.8
Hz, 2H), 7.47 (d, J = 9.2
N N
H Hz,
2H), 7.81 (d, J = 6.4 Hz,
1-(4-((7,8-Dihydro-6H- Method
I' 2H), 8.09 (s, 2H), 9.61 (s, 1H),
pyrimido[5,4-b][1,4]oxazin-4- 9.82
(s, 1H), 11.50 (br s, 1H);
yl)oxy)pheny1)-3-(344- ESI-MS (m/z) 558 (M+H)+.
ethylpiperazin-l-yl)methyl)-5-
(trifluoromethyl)phenyl)urea
hydrochloride
206. AS, 4- 1I-1 NMR (400 MHz, DMSO-d6)
H H
N N CF3
aminophe 6 1.06 (s, 3H), 1.17 (d, J = 6.4
VI Yo 40
nylbenzyl Hz, 6H), 2.50-2.51 (m, 8H), 3.56
O carbonate (br s, 2H), 3.65-3.73 (m, 2H),
NO N
I ) ,Cl 4.22
(dd, J1 = 2.4 Hz, J2 = 7.2
CN
NN Hz, 2H), 7.45 (dd, J1 = 2.4 Hz,
H
Method I' J2 = 6.8 Hz, 2H), 7.60-7.63 (m,
(R) - 1-(4-((4-Ethylpip erazin-1- 2H),
7.72 (d, J = 6.0 Hz, 2H),
yl)methyl)-3- 7.07
(s, 1H), 8.90 (s, 1H), 9.15
(trifluoromethyl)pheny1)-3-(4-47- (s,
1H); ESI-MS (m/z) 572
methyl-7,8-dihydro-6H- (M+H)+.
pyrimido [5 ,4-b] [1,4]oxazin-4-
yl)oxy)phenyl)urea
207. a
Example 1I-1 NMR (400 MHz, DMSO-d6)
H
ow Ns cF3 163
6 0.85-1.24 (m, 9H), 2.28-2.34
No 0 cH3 (br s,
6H), 3.51 (br s, 3H), 3.70-
1 N Chiral 3.76
(m, 3H), 4.27 (d, J = 10.8
NN ( )
separation Hz, 2H), 7.71 (d, J = 3.6 Hz,
H
N 3H),
7.78 (t, J = 3.6 Hz, 2H),
) 7.89
(br s, 1H), 8.06 (d, J = 9.2
4-Chloro-N-(4-(1-(4- Hz,
1H), 8.15 (s, 1H), 10.55 (s,
ethylpiperazin-l-yl)ethyl)-3- 1H);
ESI-MS (m/z) 605 (M)+;
(trifluoromethyl)pheny1)-3 -(((R)- chiral purity: 98.45%.
7-methy1-7,8-dihydro-6H-
pyrimido [5 ,4-b] [1,4]oxazin-4-
yl)oxy)benzamide (Isomer I)
205
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
No. Intermed Analytical Data
Structure and Name iates and
Method
208. Example 11-1 NMR (400 MHz, DMSO-d6)
ci Ai
H 163 6 1.18-
1.30 (m, 9H), 2.30-2.50
N I* CF3 (m,
6H), 3.51-3.65 (m, 3H),
0
0 cH3 Chiral 3.69-
3.78 (m, 3H), 4.26 (d, J =
I L 1 N
separation 8.0 Hz, 2H), 7.71-7.79 (m, 3H),
N N H CN) 7.89-
7.90 (m, 3H), 8.08 (d, J =
8.0 Hz, 1H), 8.15 (s, 1H), 10.57
) (s,
1H); ESI-MS (m/z) 605 (M)+;
4-Chloro-N-(4-(1-(4- chiral purity: 99.48%.
ethylpip erazin-l-yl)ethyl)-3-
(trifluoromethyl)pheny1)-3 -(((R)-
7-methy1-7,8-dihydro-6H-
pyrimido [5 ,4-b] [1,4]oxazin-4-
yl)oxy)benzamide (Isomer II)
209. ON
A7, 1H NMR (400 MHz, DMSO-d6)
* benzyl
(4- 6 1.29 (s, 9H), 3.40 (br s, 2H),
hydroxyp 4.09 (t, J = 4.4 Hz, 2H), 6.00 (d,
H H
N N N
henyl)car J = 5.6 Hz, 1H), 6.41 (s, 1H),
0 lr 1 IN
bamate, 6.76 (s, 1H), 6.95 (d, J = 9.2 Hz,
0
C91 2H),
7.40 (d, J = 9.2 Hz, 1H),
1 ) 7.46
(d, J = 5.6 Hz, 2H), 7.80 (d,
N N Method
I" J = 8.8 Hz, 2H), 7.99 (d, J = 8.8
H
1 -(3-(tert-Buty1)-1-(4-
Hz, 2H), 8.55 (s, 1H), 9.06 (s,
+
cyanopheny1)-1H-pyrazol-5 -y1)-3 - 1H); ESI-MS (m/z) 510 (M+H).
(4-43,4-dihydro-2H-pyrido[3,2-
b] [1,4]oxazin-8-
yl)oxy)phenyl)urea
210. H H AS, 4- 1H NMR (400 MHz, DMSO-d6)
ci
a NYN -(----- amino-
2- 6 1.17 (d, J = 6.4 Hz, 3H), 1.13
N-0
0
0 chlorophe (s, 9H), 3.67-6.74 (m, 2H), 4.25
0
R
N Cj( nol, 5- (dd,
Ji = 2.4 Hz, J2 = 10.0 Hz,
(tert- 1H), 6.51 (s, 1H), 7.20 (d, J =
N N
H
butyl)isox 8.8 Hz, 1H), 7.29 (dd, J1 = 2.4
(R)-1-(5-(tert-Butypisoxazol-3- azol-3- Hz, J2
= 8.8 Hz, 1H), 7.67 (s,
y1)-3-(3-chloro-44(7-methyl-7,8- amine 1H),
7.79 (s, 2H), 9.02 (s, 1H),
dihydro-6H-pyrimido[5,4- Method
I 9.63 (s, 1H); ESI-MS (m/z) 459
b] [1,4]oxazin-4- (M+H)+.
yl)oxy)phenyl)urea
211. H H
A7, 1H NMR (400 MHz, DMSO-d6)
laNYN ----(-- benzyl
(4- 6 1.29 (s, 9H), 3.39-3.42 (m,
0 N-0
0
hydroxyp 2H), 4.10 (t, J = 4.0 Hz, 2H),
C,0 henyl)car 6.03 (d, J = 5.6 Hz, 1H),
6.49 (s,
1 j bamate, 1H),
6.78 (s, 1H), 6.96-6.99 (m,
N N
H 5-(tert- 2H),
7.43-7.47 (m, 3H), 8.81 (s,
1-(5-(tert-Butyl)isoxazol-3-y1)-3-
butyl)isox 1H), 9.49 (s, 1H); ESI-MS (m/z)
(4-((3 ,4-dihydro-2H-pyrido [3,2- azol-3- 410 (M+H)+.
206
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
No. Intermed Analytical Data
Structure and Name iates and
Method
b] [1,4]oxazin-8- amine
yl)oxy)phenyl)urea
Method I"
212. H H A26, 1H NMR
(400 MHz, DMSO-d6)
NN 0 CF3
benzyl (4- 6 1.17 (t, J = 7.2 Hz, 3H), 3.13-
O W
hydroxyp 4.0 (m, 16H), 4.26 (t, J = 4.0 Hz,
N henyl)car 2H), 6.89 (m, 1H),
7.0 (d, J = 8.8
cj)
N bamate, Hz,
2H), 7.42 (s, 1H), 7.45-7.47
. HCI
HN) Cl (m, 2H), 7.65
(d, J= 9.6 Hz, 1H),
, >
1-(4-((3,4-Dihydro-2H- 7.87
(m, 1H), 8.03 (br s, 1H),
pyrido[3,2-b][1,4]oxazin-7- 9.57-
9.61 (m, 1H), 10.03 (br s,
yl)oxy)pheny1)-3-(4((4- Method I" 1H); ESI-MS (m/z) 557 (M+H)+.
ethylpiperazin-l-yl)methyl)-3-
(trifluoromethyl)phenyl)urea
213. 0 H
A7, B3, 1H NMR (400 MHz, DMSO-d6)
0 N,ci...¶_ 5-(tert- 6 1.31
(s, 9H), 2.27 (s, 3H), 3.42
),o 0 N-0
I )
butyl)isox (br s, 2H), 4.10 (t, J = 4.0 Hz,
azol-3- 2H),
6.02 (d, J = 5.6 Hz, 1H),
*NI N
H amine 6.69 (s, 1H),
6.84 (s, 1H), 7.45
N-(5-(tert-Butyl)isoxazol-3-y1)-3- (d, J
= 8.0 Hz, 1H), 7.50 (d, J =
((3,4-dihydro-2H-pyrido[3,2- Method
B 5.6 Hz, 2H), 7.78 (dd, J1 = 1.6
b] [1,4]oxazin-8-yl)oxy)-4- Hz, J2
= 7.6 Hz, 1H), 11.32 (s,
methylbenzamide 1H); ESI-MS (m/z) 409 (M+H)+.
214. CI a A22, Bl, 1H NMR (400 MHz, DMSO-d6)
H
O N CF3 Cl
6 0.86 (t, J = 6.4 Hz, 3H), 2.28-
N j10) 0
1 1\1).'() N 2.51
(m, 10H), 3.33 (d, J = 6.8
Method A Hz, 3H), 3.40-3.47 (m, 2H), 3.56
H ci) (s, 2H), 3.79-3.81 (br s, 1H), 4.14
) (t, J= 3.6 Hz, 2H), 7.70-7.78 (m,
3H), 7.88-8.02 (m, 2H), 8.08 (d,
(R)-4-Chloro-N-(4-((4- J =
11.6 Hz, 2H), 8.17 (s, 1H),
ethylpiperazin-1-yl)methyl)-3- 10.55
(s, 1H); ESI-MS (m/z) 621
(trifluoromethyl)pheny1)-3-47- (M+H)+.
(methoxymethyl)-7,8-dihydro-6H-
pyrimido[5,4-b][1,4]oxazin-4-
yl)oxy)benzamide
215. CI Ai A23,
Bl, 1H NMR (400 MHz, DMSO-d6)
H
O WI N 0 CF3 Cl
6 0.98 (t, J = 7.2 Hz, 3H), 2.22
(s, 6H), 2.27-2.51 (m, 12 H),
Method A 3.56 (s, 2H), 3.74-3.75 (br s, 1H),
I
NN'I\I (NJ
4.08 (dd, J1 = 4.8 Hz, J2 = 11.2
H
N Hz,
1H), 4.14-4.18 (m, 1H),
) 7.70-
7.78 (m, 4H), 7.88-7.90 (m,
(R)-4-Chloro-3-((7- 2H),
8.02 (dd, J1 = 1.6 Hz, .1-2 =
((dimethylamino)methyl)-7,8- 12.0
Hz, 1H), 8.17 (s, 1H), 10.55
dihydro-6H-pyrimido[5,4- (s,
1H); ESI-MS (m/z) 634
b] [1,4]oxazin-4-yl)oxy)-N-(4-((4- (M+H)+.
207
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
No. Intermed Analytical Data
Structure and Name iates and
Method
ethylpiperazin-l-yl)methyl)-3-
(trifluoromethyl)phenyl)benzamid
e
216. H H
A5 , 4- 1H NMR (400 MHz, DMSO-d6)
y
amino-2- 6 1.17 (d, J = 6.0 Hz, 3H), 1.24
F Ai NN 0 CF3
0
0
fluorophe (br s, 3H), 2.50-2.61 (m, 10H),
0 N nol, Cl 3.56-3.75 (m,
4H), 4.23 (d, J =
N'CX,
1 (R C ) 8.0 Hz, 1H), 7.12-
7.22 (m, 2H),
N N N
H Method I 7.57-
7.68 (m, 4H), 7.77 (s, 1H),
(R)-1-(4-((4-Ethylpiperazin-1- 7.95
(s, 1H), 9.42 (s, 1H), 9.50
yl)methyl)-3- (s,
1H); ESI-MS (m/z) 590
(trifluoromethyl)pheny1)-3-(3- (M+H)+.
fluoro-447-methy1-7,8-dihydro-
6H-pyrimido[5,4-b][1,4]oxazin-4-
yl)oxy)phenyl)urea
217. F H H
A20, 1H NMR (400 MHz, DMSO-d6)
w
benzy1 (2- 6 0.98 (t, J = 7.2 Hz, 3H), 1.90
AI NN s CF3
0 fluoro-
4- (br s, 2H), 2.30-2.38 (m, 10H),
((C)) N
c) hydroxyp 3.23 (s, 2H), 3.53
(s, 2H), 3.97 (t,
henyl)car J = 5.6 Hz, 2H), 6.22 (s, 1H),
N N
H > bamate, 6.27 (d, J = 5.2
Hz, 1H), 6.82 (d,
1-(4-((4-Ethylpiperazin-1- Cl J =
9.2 Hz, 1H),7.05 (dd, J1= 2.8
yl)methyl)-3- Hz, J2
= 12.0 Hz, 1H), 7.53 (d, J
(trifluoromethyl)pheny1)-3-(2- Method
I" = 8.0 Hz, 1H), 7.63-7.65 (m,
fluoro-4-((2,3,4,5- 2H),
7.98-8.04 (m, 2H), 8.53 (s,
tetrahydropyrido[3,2- 1H),
9.30 (s, 1H); ESI-MS (m/z)
b] [1,4]oxazepin-9- 589 (M+H)+.
yl)oxy)phenyl)urea
218. jib ON
A7, B3, 1H NMR (400 MHz, DMSO-d6)
C92 6 1.30
(s, 9H), 2.26 (s, 3H), 3.40
140N N (br s,
2H), 4.08 (br s, 2H), 6.0 (d,
0 1 11) 0 ,N
Method D J = 5.6 Hz, 1H), 6.44 (s, 1H),
c
N N
6.86 (s, 1H), 7.32 (s, 1H), 7.44-
H 7.49 (m, 2H),
7.63-7.65 (m, 2H),
N-(3-(tert-Butyl)-1-(3- 7.78
(d, J = 6.8 Hz, 1H), 7.84 (d,
cyanopheny1)-1H-pyrazol-5-y1)-3- J =
7.6 Hz, 1H), 7.95 (s, 1H),
((3,4-dihydro-2H-pyrido[3,2- 10.39
(s, 1H); ESI-MS (m/z) 509
b] [1,4]oxazin-8-yl)oxy)-4- (M+H)+.
methylbenzamide
219. ON
A7, B3, 1H NMR (400 MHz, DMSO-d6)
410 C91 6 1.30
(s, 9H), 2.51 (s, 3H), 3.41
Method D (br s, 2H), 4.09 (br s, 2H), 6.02
0 N (d, J = 4.8 Hz,
1H), 6.47 (s, 1H),
0
0 1 iN 6.86
(s, 1H), 7.34 (s, 1H), 7.46
1 j (d, J
= 8.0 Hz, 1H), 7.51 (d, J =
N'N H 5.6 Hz, 1H), 7.64 (d, J = 6.4 Hz,
208
CA 03064975 2019-11-26
WO 2018/215668 PCT/EP2018/063957
No. Intermed Analytical Data
Structure and Name iates and
Method
N-(3-(tert-Butyl)-1-(4- 1H),
7.72 (d, J = 8.0 Hz, 2H),
cyanopheny1)-1H-pyrazo1-5-y1)-3- 7.91
(d, J = 8.4 Hz, 2H), 10.45
((3,4-dihydro-2H-pyrido[3,2- (s,
1H); ESI-MS (m/z) 509
b][1,4]oxazin-8-yl)oxy)-4- (M+H)+.
methylbenzamide
220. CI H
A5 , Bl, 1I-1 NMR (400 MHz, DMSO-d6)
N 3-(4- 6 1.18
(d, J = 6.4 Hz, 3H), 2.22
0
Ntc0 0 IW methylpip (s, 3H), 2.45 (br s, 4H), 3.11 (br
I N erazin-
1- s, 4H), 3.69-3.77 (m, 2H), 4.25
N N CN) yl)aniline (d,
J= 10.0 Hz, 1H), 6.71 (d, J =
H
8.4 Hz, 1H), 7.16 (t, J = 8.0 Hz,
I
1H), 7.24 (d, J = 7.6 Hz, 1H),
(R)-4-Chloro-347-methy1-7,8-
Method B 7.35 (s, 1H), 7.70-7.74 (m, 2H),
dihydro-6H-pyrimido[5,4-
b][1,4]oxazin-4-yl)oxy)-N-(3-(4-
7.86 (d, J = 8.4 Hz, 3H), 10.12
methylpiperazin-1-
(s, 1H); ESI-MS (m/z) 495
+
yl)phenyl)benzamide (M+H).
221. I
AS, Bl, 1I-1 NMR (400 MHz, DMSO-d6)
rN
CI L ) C99 6 1.18 (d, J =
6.0 Hz, 3H), 2.15
la H N (s,
3H), 2.40 (br s, 4H), 2.92 (br
N
0 Method B s,
4H), 3.69-3.77 (m, 2H), 4.26
N 0 VI (d, J
= 8.4 Hz, 1H), 7.37 (d, J =
L I rp
VI 3 8.4 Hz, 1H), 7.51 (d, J=
8.8 Hz,
1\1N
H 1H),
7.73-7.85 (m, 4H), 7.91 (s,
(R)-4-Chloro-3((7-methy1-7,8- 1H),
8.22 (s, 1H), 9.71 (s, 1H);
dihydro-6H-pyrimido[5,4- ESI-MS (m/z) 563 (M+H)+.
b][1,4]oxazin-4-yl)oxy)-N-(2-(4-
methylpiperazin-l-y1)-5-
(trifluoromethyl)phenyl)benzamid
e
222. a H
Al, B3, 1I-1 NMR (400 MHz, DMSO-d6)
O N 40 cF3 C96
6 2.19 (s, 3H), 3.51-3.52 (br s,
i\ijp0 2H), 4.10-4.12 (m, 2H), 4.21 (t, J
1 ) Method D = 4.0 Hz, 2H), 5.43 (t, J = 6.0
N N 11
H Hz,
1H), 7.48 (d, J = 8.4 Hz,
HO 2H),
7.7.3-7.79 (m, 3H), 7.81 (d,
3-((7,8-Dihydro-6H-pyrimido[5,4- J =
1.6 Hz, 1H), 8.19 (d, J = 8.0
b][1,4]oxazin-4-yl)oxy)-N-(3-(3- Hz,
2H), 10.51 (s, 1H); ESI-MS
hydroxyprop-1-yn-l-y1)-5- (m/z) 485 (M+H)+.
(trifluoromethyl)pheny1)-4-
methylbenzamide
223. CI H H AS, 4- 1I-1
NMR (400 MHz, DMSO-d6)
NNv amino-3- 6 0.41-0.43 (m,
2H), 0.64-Ø68
O chlorophe (m, 2H), 1.17 (d, J= 6.4 Hz, 3H),
I
0
nylbenzyl 2.51-2.59 (m, 1H), 3.66-3.74 (m,
I\&(Rcarbonate, 2H), 4.21 (dd, J1 = 2.8 Hz, J2 =
N N
H cycloprop 9.2 Hz, 1H), 7.03 (dd, J1 = 2.8
209
CA 03064975 2019-11-26
WO 2018/215668 PCT/EP2018/063957
No. Intermed Analytical Data
Structure and Name iates and
Method
(R)-1-(2-Chloro-4-47-methyl-7,8-
anamine Hz, J2 = 9.2 Hz, 1H), 7.11 (d, J
dihydro-6H-pyrimido[5,4- = 2.8
Hz, 1H), 7.24 (d, J = 2.8
b][1,4]oxazin-4-yl)oxy)pheny1)-3- Hz,
1H), 7.73 (s, 1H), 7.79 (s,
cyclopropylurea Method
I' 1H), 7.87 (s, 1H), 8.07 (d, J = 8.8
Hz, 1H); ESI-MS (m/z) 376
(M+H)+.
224. CI
A24 , Bl, 1H NMR (400 MHz, DMSO-d6)
=N CF3 Cl
6 0.99 (t, J = 6.4 Hz, 3H), 2.33-
0 IW 2.34
(m, 10H), 4.10 (br s, 4H),
N N 4.21
(t, J= 4.40 Hz, 2H), 7.73 (d,
CI
CrI) Method A J = 8.4 Hz, 1H), 7.80
(d, J = 8.8
Hz, 1H), 7.93 (s, 2H), 8.03 (d, J
4-Chloro-3-((2-chloro-7,8- = 8.4
Hz, 1H), 8.18 (s, 1H), 8.35
dihydro-6H-pyrimido[5,4- (s,
1H), 10.59 (s, 1H); ESI-MS
b][1,4]oxazin-4-yl)oxy)-N-(4-((4- (m/z) 611 (M+H)+.
ethylpiperazin-l-yl)methyl)-3-
(trifluoromethyl)phenyl)benzamid
225. H H A4, 4-
1H NMR (400 MHz, DMSO-d6)
N N CF3
aminophe 6 1.10 (t, J = 7.2 Hz, 3H), 1.23
W To 10
nylbenzy1 (s, 6H), 2.37-2.41 (m, 8H), 3.54
carbonate, (m, 4H), 3.84 (s, 2H), 7.05 (d, J
CNJ Cl = 8.8 Hz, 2H), 7.46 (d, J =
8.8
N
Hz, 2H), 7.59-7.64 (m, 2H),
1-(4((7,7-Dimethyl-7,8-dihydro- Method
I' 7.72-7.74 (m, 2H), 7.98 (m, 1H),
6H-pyrimido[5,4-b][1,4]oxazin-4- 8.82
(s, 1H), 9.06 (s 1H); ESI-
yl)oxy)pheny1)-3-(444- MS (m/z) 586 (M+H)+.
ethylpiperazin-l-yl)methyl)-3-
(trifluoromethyl)phenyl)urea
226. ON
A20, 1H NMR (400 MHz, DMSO-d6)
H H 41Ik benzyl
(2- 6 1.30 (s, 9H), 1.87-1.93 (m,
fluoro-4- 2H), 3.17-3.25 (m, 2H), 3.98 (t, J
N N N
hydroxyp = 8.8 Hz, 2H), 6.15 (d, J = 5.6
=Is iN
henyl)car Hz, 2H), 6.40 (s, 1H), 6.96 (d, J
bamate, = 8.8 Hz, 2H), 7.42 (d, J = 9.2
C91 Hz,
2H), 7.64 (d, J = 9.2 Hz,
N 2H),
8.02 (d, J = 8.8 Hz, 2H),
Method R 8.55 (s, 1H), 9.13 (s, 1H); ESI-
1 -(3-(tert-Buty1)-1-(4-
+
cyanopheny1)-1H-pyrazol-5 -y1)-3 MS (m/z) 542 (M+H).
(2-fluoro-442,3,4,5-
tetrahydropyrido[3,2-
b] [1,4]oxazepin-9-
yl)oxy)phenyl)urea
210
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
No. Intermed Analytical Data
Structure and Name iates and
Method
227. ,ON A4,
Bl, '1-1 NMR (400 MHz, DMSO-d6)
CI Ai
H C92 6 1.22
(s, 6H), 1.30 (s, 9H), 3.86
N N (s,
2H), 6.46 (s, 1H), 7.65-7.75
o 1 ,N
NICI o Method
A (m, 6H), 7.82 (d, J= 5.2 Hz, 2H),
1 7.99
(s, 1H), 10.56 (s, 1H); ESI-
N N\-
MS (m/z) 558 (M+H) H +.
N-(3-(tert-Buty1)-1-(3-
cyanopheny1)-1H-pyrazol-5-y1)-4-
chloro-3-47,7-dimethy1-7,8-
dihydro-6H-pyrimido[5,4-
b.] [1,4]oxazin-4-yl)oxy)
benzamide
228. H H A4, 4- 11-1
NMR (400 MHz, DMSO-d6)
F N N CF3
amino-2- 6 1.10 (t, J = 7.2 Hz, 3H), 1.23
VI Yo 40
o fluorophe (s, 6H), 2.50-2.52 (m, 10H), 3.58
N nol, Cl (s,
2H), 3.86 (s, 2H), 7.14-7.24
NCX:j
I CN) (m,
2H), 7.58-7.72 (m, 4H), 7.79
N N
H
(s, 1H), 7.96 (s, 1H), 9.18 (s,
1-(4-47,7-Dimethy1-7,8-dihydro- Method
I' 1H), 9.29 (s, 1H); ESI-MS (m/z)
6H-pyrimido[5,4-b][1,4]oxazin-4- 604 (M+H)+.
yl)oxy)-3-fluoropheny1)-3-(444-
ethylpiperazin-1-y1)methyl)-3-
(trifluoromethyl)phenyl)urea
229. H H
A4, 4- 11-1 NMR (400 MHz, DMSO-d6)
ci
a NYN -(--- amino-
2- 6 1.23 (s, 6H), 1.31 (s, 9H), 3.87
-0
0 N
0 '
chlorophe (s, 2H), 6.52 (s, 1H), 7.22 (d, J =
I
N,C nol, 5- 8.8
Hz, 1H), 7.30 (dd, Ji = 2.8
(tert- Hz, .1-
2 = 9.2 Hz, 1H), 7.68 (s,
N N\-
H butyl)isox 1H),
7.79 (s, 2H), 8.99 (s, 1H),
1-(5-(tert-Butyl)isoxazol-3-y1)-3- azol-3- 9.63
(s, 1H); ESI-MS (m/z) 473
(3-chloro-447,7-dimethy1-7,8- amine (M+H)+.
dihydro-6H-pyrimido[5,4-
b.] [1,4]oxazin-4- Method I
yl)oxy)phenyl)urea
230. CN
A4, 11-1 NMR (400 MHz, DMSO-d6)
Nit C91 6 1.22
(s, 6H), 1.29 (s, 9H), 3.84
H H Method
Q (s, 2H), 6.43 (s, 1H), 7.15 (d,
CI N N N5 1H),
7.30 (d, 1H), 7.66-7.80 (m,
140 Y '17
0 , 5H),
7.99 (d, J = 7.2 Hz, 2H),
0
0 8.65 (s, 1H),
9.25 (s, 1H); ESI-
NCN):N_
MS (m/z) 573 (M+H)+.
H
1 -(3 -(t e rt-Buty1)-1-(4-
cyanopheny1)-1H-pyrazol-5-y1)-3-
(3-chloro-4-47,7-dimethy1-7,8-
dihydro-6H-pyrimido[5,4-
b.] [1,4]oxazin-4-
211
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
No. Intermed Analytical Data
Structure and Name iates and
Method
yl)oxy)phenyl)urea
231. H H A4, 4- 1H NMR
(400 MHz, DMSO-d6)
CI N N CF3
140 X 1.1 amino-
2- 6 0.99 (t, J = 7.2 Hz, 3H), 1.23
O chlorophe (s, 6H), 2.50-2.52 (m, 10H), 3.53
.j,,, o N
N
N CN) nol, Cl (s,
2H), 3.87 (s, 2H), 7.21 (d, J=
N \- 8.8 Hz, 1H), 7.31-7.34 (m, 1H),
H
) Method
I 7.60-7.78 (m, 3H), 7.79 (s, 2H),
1-(3-Chloro-4-47,7-dimethy1-7,8- 7.97
(s, 1H), 8.98 (s, 1H), 9.12
dihydro-6H-pyrimido [5,4- (s,
1H); ESI-MS (m/z) 620
b][1,4]oxazin-4-yl)oxy)pheny1)-3- (M+H)+.
(4-((4-ethylpiperazin-1-
yl)methyl)-3-
(trifluoromethyl)phenyl)urea
232. H H
A4, 1H NMR (400 MHz, DMSO-d6)
N N CF3
4-amino- 6 0.87 (s, 3H), 1.27 (s, 6H), 2.05
VI Yo 1101
0 2- (s,
3H), 2.39 (m, 10H), 3.53 (s,
N
methylph 2H), 3.84 (s, 2H), 6.94 (d, J = 6.8
N:C)
I CN) enyl Hz,
1H), 7.36 (d, J = 2.0 Hz,
N N
H
benzylcar 1H), 7.57 (s, 1H), 7.60-7.67 (m,
1-(4-47,7-Dimethy1-7,8-dihydro- bonate , 4H),
7.97 (s, 1H), 8.71 (s, 1H),
6H-pyrimido[5,4-b][1,4]oxazin-4- Cl 9.02
(s, 1H); ESI-MS (m/z) 600
yl)oxy)-3-methylpheny1)-3-(4-44- (M+H)+.
ethylpiperazin-l-yl)methyl)-3-
(trifluoromethyl)phenyl)urea Method I'
233. * ON
A4 , C92 1H NMR (400 MHz, DMSO-d6)
H H 6 1.10
(s, 6H), 1.25 (s, 9H), 3.84
oi 00NN N Method Q (s, 2H), 6.42 (s, 1H), 7.18-7.23
11 I...5Nv..
0 ' (m,
2H), 7.50-7.80 (m, 6H), 7.76
0
NO (s,
1H), 8.61 (s, 1H), 9.22 (s,
1 1H); ESI-MS (m/z) 573 (M+H)+.
NV7
H
1-(3-(tert-Buty1)-1-(3-
cyanopheny1)-1H-pyrazol-5 -y1)-3 -
(3-chloro-4-47,7-dimethy1-7,8-
dihydro-6H-pyrimido [5,4-
b.] [1,4]oxazin-4-
yl)oxy)phenyl)urea
234. a H Al,
B3, 1H NMR (400 MHz, DMSO-d6)
N s SO2Me
0 C94 6 2.18
(s, 3H), 3.30 (s, 3H), 3.50
(br s, 2H), 4.19 (t, J = 3.6 Hz,
1 j Method
B 2H), 7.46 (d, J = 6.4 Hz, 1H),
NN
H 7.63-
7.70 (m, 5H), 7.80 (s, 1H),
3 -((7,8-Dihydro-6H-pyrimido [5,4- 8.13
(s, 1H), 8.39 (s, 1H), 10.52
b][1,4]oxazin-4-yl)oxy)-4-methyl- (s,
1H); ESI-MS (m/z) 441
N-(3- (M+H)+.
(methylsulfonyl)phenyl)benzamid
212
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
No. Intermed Analytical Data
Structure and Name iates and
Method
e
235. a H A4 ,
B3, 1I-1 NMR (400 MHz, DMSO-d6)
O N 0 cF3 Cl
6 0.97 (d, J= 6.0 Hz, 3H), 1.24
,,,cx0v (s,
6H), 2.18-2.32 (m, 11H), 3.34
IL I N Method
A (s, 2H), 3.55 (m, 2H). 3.88 (s,
N N H CN)
2H), 7.46 (d, J = 6.8 Hz, 2H),
7.68-8.04 (m, 5H), 8.18 (s, 1H),
) 10.42
(s, 1H); ESI-MS (m/z) 585
347,7-Dimethy1-7,8-dihydro-6H-
(M+H)+.
pyrimido[5,4-b][1,4]oxazin-4-
yl)oxy)-N-(4-((4-ethylpiperazin-1-
y1)methyl)-3-
(trifluoromethyl)pheny1)-4-
methylbenzamide
236. * CN
A7, C92 1I-1 NMR (400 MHz, DMSO-d6)
H H 6 1.29
(s, 9H), 3.41 (br s, 2H),
N N N Method
Q 4.10 (t, J = 4.0 Hz, 2H), 6.01 (d,
(:
00 )r) , ,N
J = 6.0 Hz, 1H), 6.40 (s, 1H),
0
CY) 6.85
(s, 1H), 6.95 (d, J = 8.8 Hz,
) 2H), 7.40 (d, J = 8.8 Hz, 2H),
IV N 7.46
(d, J = 6.0 Hz, 1H), 7.73 (t,
H
1 - (3 - (t e rt -Buty1)-1-(3- J =
8.0 Hz, 1H), 7.86 (d, J = 8.0
cyanopheny1)-1H-pyrazol-5-y1)-3- Hz,
1H), 7.91 (d, J = 8.4 Hz,
(4-((3,4-dihydro-2H-pyrido[3,2- 1H),
8.03 (s, 1H), 8.52 (s, 1H),
b] [1,4]oxazin-8- 9.06
(s, 1H); ESI-MS (m/z) 510
yl)oxy)phenyl)urea (M+H)+.
237. so H
Al, B3, 1I-1 NMR (400 MHz, DMSO-d6)
0 0 C93 6 1.48
(s, 6H), 2.18 (s, 3H), 3.51
0 0 (br s,
2H), 4.20 (t, J = 4.0 Hz,
N OH
N)X j
Method D 2H), 5.58 (s, 1H), 7.41 (s, 1H),
N N I I
H 7.48
(d, J = 8.0 Hz, 1H), 7.68 (s,
OH 2H),
7.73 (s, 1H), 7.80 (dd, ./1 =
3-((7,8-Dihydro-6H-pyrimido[5,4- 1.6
Hz, .1-2 = 8.0 Hz, 1H), 8.17 (s,
b] [1,4]oxazin-4-yl)oxy)-N-(3-(3- 2H),
10.48 (s, 1H); ESI-MS (m/z)
hydroxy-3-methylbut-l-yn-l-y1)- 513 (M+H)+.
5-(trifluoromethyl)pheny1)-4-
methylbenzamide
238. 0 H Al,
B3, 1I-1 NMR (400 MHz, DMSO-d6)
N CHF2 C97 6 2.18 (s, 3H), 3.50
(br s, 2H),
0
N (3' 4.19
(t, J = 3.6 Hz, 2H), 6.95-
I ) Method
B 7.10 (m, 1H), 7.45 (d, J = 6.0 Hz,
NN
H 3H),
7.49-7.67 (m, 2H), 7.71-
N-(3-(Difluoromethyl)pheny1)-3- 7.78
(m, 2H), 7.79 (d, J= 5.2 Hz,
((7,8-dihydro-6H-pyrimido[5,4- 1H),
8.05 (s, 1H), 10.36 (s, 1H);
b] [1,4]oxazin-4-yl)oxy)-4- ESI-MS (m/z) 413 (M+H)+.
methylbenzamide
213
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
No. Intermed Analytical Data
Structure and Name iates and
Method
239. NH2
A20, NMR (400 MHz, DMSO-d6)
0 benzyl
(2- 6 1.28 (s, 9H), 1.87-1.90 (br s,
F H H fluoro-
4- 2H), 3.21-3.23 (br s, 2H), 3.21-
N N N
IN
hydroxyp 3.23 (br s, 2H), 3.95 (t, J = 4.8
0
henyl)car Hz, 2H), 6.19-6.25 (m, 2H), 6.40
,0 bamate, (s,
2H), 6.80 (s, 1H), 7.0 (d, J =
C92 10.0
Hz, 1H), 7.62-7.63 (m, 4H),
1\1 N
7.90-7.95 (br s, 1H), 8.0-8.01 (m,
3-(3-(tert-Butyl)-5-(3-(2-fluoro-4- 1H),
8.85 (s, 1H); ESI-MS (m/z)
42,3,4,5-tetrahydropyrido[3,2- Method R 560 (M+H)+.
b][1,4]oxazepin-9-
yl)oxy)phenyOureido)-1H-
pyrazol-1-y1)benzamide
240. CI &.1 AS ,
Bl, NMR (400 MHz, DMSO-d6)
5-(tert- 6 1.18
(d, J = 6.0 Hz, 3H), 1.32
0
0 0 N butyl)isox (s, 9H), 3.69-3.77 (m, 2H), 4.26
N-0
azol-3- (d, J=
8.4 Hz, 1H), 6.71 (s, 1H),
N N amine
7.71-7.75 (br s, 2H), 7.91 (s, 3H),
(R)-N-(5-(tert-Butyl)isoxazol-3- 11.45
(s, 1H); ESI-MS (m/z) 444
y1)-4-chloro-3-47-methyl-7,8- (M+H)+.
Method B
dihydro-6H-pyrimido[5,4-
b][1,4]oxazin-4-yl)oxy)benzamide
241. Al, B3, NMR
(400 MHz, DMSO-d6)
N SO2Et C98 6 1.12
(t, J = 7.6 Hz, 3H), 2.19
0
) Method B (s, 3H),
3.28 (q, J = 7.2 Hz, 2H),
3.58 (s, 2H), 4.20 (s, 2H), 7.47
N N
(d, J = 8.0 Hz, 1H), 7.59-7.72
3-((7,8-Dihydro-6H-pyrimido[5,4- (m,
5H), 7.82 (d, J = 7.6 Hz, 1H),
b][1,4]oxazin-4-yl)oxy)-N-(3- 8.15
(d, J = 7.6 Hz, 1H), 8.36 (s,
(ethylsulfonyl)pheny1)-4- 1H),
10.54 (s, 1H); ESI-MS (m/z)
methylbenzamide 455 (M+H)+.
242. a Al, B3, NMR
(400 MHz, DMSO-d6)
O N cF3 C95
6 2.51 (s, 3H), 3.36 (s, 3H), 3.51
Nk,0, 0 (s,
2H), 4.20 (t, J = 3.6 Hz, 2H),
1 j Method D 4.43 (s,
2H), 7.48 (d, J = 8.0 Hz,
N N
1H), 7.54 (s, 1H), 7.72 (s, 3H),
'0 7.80
(d, J= 8.0 Hz, 1H), 8.21 (s,
3-((7,8-Dihydro-6H-pyrimido[5,4- 2H),
10.52 (s, 1H); ESI-MS (m/z)
b][1,4]oxazin-4-yl)oxy)-N-(3-(3- 499 (M+H)+.
methoxyprop-1-yn-l-y1)-5-
(trifluoromethyl)pheny1)-4-
methylbenzamide
214
CA 03064975 2019-11-26
WO 2018/215668 PCT/EP2018/063957
No. Intermed Analytical Data
Structure and Name iates and
Method
243. H H
A7, C94 1I-1 NMR (400 MHz, DMSO-d6)
N N SO2Me
6 3.20 (s, 3H), 3.39 (s, 2H), 4.13
0 Yo I I 0 I
0 Method Q (s,
2H), 6.06 (d, J = 5.2 Hz, 1H),
?IC') (Boc 7.01
(d, J = 8.0 Hz, 3H), 7.48-
deprotecti 7.58 (m, 5H), 7.67 (d, J= 7.2 Hz,
N N
H on done 1H),
8.16 (s, 1H), 8.91 (s, 1H),
1-(4-((3,4-Dihydro-2H- using 9.20
(s, 1H); ESI-MS (m/z) 441
pyrido[3,2-b][1,4]oxazin-8- hydrochlo (M+H)+.
yl)oxy)pheny1)-3-(3- ric acid)
(methylsulfonyl)phenyl)urea
244. a H
F F Al, B3, 1I-1 NMR (400 MHz, DMSO-d6)
O 1 N 0 OH C100
6 2.19 (s, 3H), 3.51 (br s, 2H),
N (3' 0 3.79-
3.88 (m, 2H), 4.20 (s, 2H),
I ) Method
D 5.65 (t, J = 5.6 Hz, 1H), 7.24 (d,
1\1N1
H J =
7.6 Hz, 1H), 7.45-7.46 (m,
N-(3-(1,1-Difluoro-2- 2H),
7.68-7.71 (m, 3H), 7.80 (d,
hydroxyethyl)pheny1)-3-47,8- J =
8.0 Hz, 1H), 7.93 (d, J = 8.0
dihydro-6H-pyrimido[5,4- Hz,
1H), 7.97 (s, 1H), 10.33 (s,
b] [1,4]oxazin-4-yl)oxy)-4- 1H);
ESI-MS (m/z) 443 (M+H)+.
methylbenzamide
245. a H Al,
B3, 1I-1 NMR (400 MHz, DMSO-d6)
O N 0 cF3 C101
6 2.19 (s, 3H), 3.83 (br s, 2H),
Ni 0 0 4.20
(br s, 2H), 7.49 (d, J = 8.0
l
Hz, 1H), 7.68-7.74 (m, 2H), 7.80
N N ) 11
H Method
D (d, J = 7.6 Hz, 2H), 7.99 (s, 1H),
ON 8.38
(s, 1H), 8.49 (s, 1H), 10.68
N-(3-(Cyanoethyny1)-5-
(s, 1H); ESI-MS (m/z) 480
(trifluoromethyl)pheny1)-3#7,8- (M+H)+.
dihydro-6H-pyrimido[5,4-
b] [1,4]oxazin-4-yl)oxy)-4-
methylbenzamide
246. H H
A7, C98 1I-1 NMR (400 MHz, DMSO-d6)
N N SO2Et
6 1.09-1.13 (m, 3H), 3.25-3.44
VI Yo 1101
0 Method Q (m,
4H), 4.13 (s, 2H), 6.07 (d, J
) , 0 (Boc = 3.2
Hz, 1H), 6.99 (d, J = 7.2
deprotecti Hz, 3H), 7.48-7.58 (m, 5H), 7.67
N N
H on done (d, J
= 7.2 Hz, 1H), 8.13 (s, 1H),
1-(4-((3,4-Dihydro-2H- using 8.91
(d, J = 5.2 Hz, 1H), 9.22 (d,
pyrido[3,2-b][1,4]oxazin-8-
hydrochlo J = 6.0 Hz, 1H); ESI-MS (m/z)
yl)oxy)pheny1)-3-(3- ric acid) 455 (M+H)+.
(ethylsulfonyl)phenyl)urea
247. CI Al, Bl, 1I-1 NMR (400 MHz, DMSO-d6)
H
O WI N 0 c3 C102
6 1.40 (d, J = 6.8 Hz, 3H), 3.51-
N j(31 0 3.52
(br s, 2H), 4.20 (t, J = 4.4
L I j Method
D Hz, 2H), 4.61-4.64 (m, 1H), 5.56
N N I I
(d, J = 5.2 Hz, 1H), 7.46 (s, 1H),
H
HO 7.69
(s, 1H), 7.78-7.90 (m, 4H),
215
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
No. Intermed Analytical Data
Structure and Name iates and
Method
4-Chloro-3-((7,8-dihydro-6H- 8.16
(s, 2H), 10.62 (s, 1H); ESI-
pyrimido[5,4-b][1,4]oxazin-4- MS (m/z) 519 (M+H)+.
yl)oxy)-N-(3-(3-hydroxybut-1-yn-
1-y1)-5-
(trifluoromethyl)phenyl)benzamid
e
248. A7, 3- 1H NMR (400 MHz, DMSO-d6)
H H
aminoben 6 3.46 (br s, 2H), 4.15 (br s, 2H),
N N
WI To 0 so2NH2
zenesulfo 6.08 (d, J= 5.2 Hz, 1H), 7.02 (d,
0 namide J =
6.8 Hz, 2H), 7.16 (br s, 1H),
X0 Method
Q 7.41-7.57 (m, 8H), 8.07 (s, 1H),
j N N (Boc 8.91 (s, 1H),
9.14 (s, 1H); ESI-
H deprotecti MS (m/z) 442 (M+H)+.
3-(3-(4-((3,4-Dihydro-2H- on done
pyrido[3,2-b][1,4]oxazin-8- using
yl)oxy)phenyl)ureido)benzenesulf hydrochlo
onamide ric acid)
249. ci A4 ,
Bl, 1H NMR (400 MHz, DMSO-d6)
H
WI N, 5-(tert- 6 1.23
(s, 6H), 1.31 (s, 9H), 3.88
0
N N -0
butyl)isox (s, 2H), 6.70 (s, 1H), 7.0-7.50 (br
I )7 azol-3- s,
1H), 7.74-7.74 (m, 2H), 7.88-
N N amine 7.91 (m, 3H);
ESI-MS (m/z) 458
H
N-(5-(tert-Butyl)isoxazol-3-y1)-4- Method B (M+H)+.
chloro-3-47,7-dimethy1-7,8-
dihydro-6H-pyrimido[5,4-
b][1,4]oxazin-4-yl)oxy)benzamide
250. A4, 1H NMR (400 MHz, DMSO-d6)
7
ci 0
N N N 3-(tert- 6 1.22
(m, 15H), 3.61 (s, 3H),
I. A I N buty0-
1- 3.86 (s, 2H), 6.06 (s, 1H), 7.19
0
N ,H H aH3 methyl- (br s,
1H), 7.31 (br s, 1H), 7.68
*L 1H- (s,
1H), 7.80-7.90 (m, 3H), 7.06-
N N pyrazol-5- 7.10
(m, 1H); ESI-MS (m/z) 486
H
1-(3-(tert-Buty1)-1-methy1-1H- amine (M+H)+.
pyrazol-5-y1)-3-(4-chloro-3-47,7- Method Q
dimethy1-7,8-dihydro-6H-
pyrimido[5,4-b][1,4]oxazin-4-
yl)oxy)phenyl)urea
251. ci Al,
Bl, 1H NMR (400 MHz, DMSO-d6)
=H
N 0 SO2Me C104 6 0.98
(t, J = 6.8 Hz, 3H), 2.33-
0
2.50 (m, 10H), 3.47 (s, 3H), 3.51
Method B (br s, 2H), 3.81 (s, 2H), 4.21 (br
N
N N C N)
H t, 2H), 7.52 (d, J = 8.4 Hz, 1H),
7.69 (s, 1H), 7.75-7.80 (m, 2H),
7.90-7.91 (m, 2H), 8.13 (dd, ./1 =
4-Chloro-3-((7,8-dihydro-6H- 1.6
Hz, .1-2 = 8.0 Hz, 1H), 8.44 (s,
216
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
No. Intermed Analytical Data
Structure and Name iates and
Method
pyrimido[5,4-b][1,4]oxazin-4- 1H),
10.66 (s, 1H); ESI-MS (m/z)
yl)oxy)-N-(4-((4-ethylpiperazin-1- 587 (M+H)+.
yl)methyl)-3-
(methylsulfonyl)phenyl)benzamid
252. CN
A20, 4- 1I-1 NMR (400 MHz, DMSO-d6)
amino-3- 6 1.28 (s, 9H), 1.87-1.90 (m,
H
N N
fluorophe 2H), 3.21-3.25 (m, 2H), 3.95 (t, J
ytt
0 nol,
C92 = 5.6 Hz, 2H), 6.21 (t, J = 3.6
0 F
Hz, 1H), 6.26 (d, J = 5.2 Hz,
e(c)) 1H),
6.42 (s, 1H), 6.80 (dd, J, =
N N Method
I 1.6 Hz, .1-2 = 8.8 Hz, 1H), 7.02
1-(3-(tert-Butyl)-1-(3-
(dd, J, = 2.8 Hz, J2 = 12.0 Hz,
cyanopheny1)-1H-pyrazol-5-y1)-3-
1H), 7.64 (d, J = 5.2 Hz, 1H),
(2-fluoro-442,3,4,5- 7.74
(t, J = 8.0 Hz, 1H), 7.87 (d,
tetrahydropyrido[3,2-
J = 8.0 Hz, 1H), 7.91-7.96 (m,
b][1,4]oxazepin-9- 2H),
8.05 (s, 1H), 8.89-8.93 (m,
yl)oxy)phenyl)urea 2H); ESI-MS (m/z) 542 (M+H)+.
253. A4,
NMR (400 MHz, DMSO-d6)
O N CF3
B3, C103 6 1.24 (s, 6H), 1.38 (d, J = 6.4
NI*1,0 0 Hz,
3H), 2.19 (s, 3H), 3.88 (s,
2H), 4.60-4.63 (m, 1H), 5.52 (d,
NN
OH Method
D J = 5.2 Hz, 1H), 7.48 (d, J = 8.4
3((7,7-Dimethy1-7,8-dihydro-6H- Hz,
1H), 7.63 (d, J = 8.8 Hz,
pyrimido[5,4-b][1,4]oxazin-4- 1H),
7.68-7.70 (m, 2H), 7.78-
yl)oxy)-N-(4-(3-hydroxybut-1-yn- 7.81
(m, 2H), 8.09 (d, J = 3.0 Hz,
1-y1)-3-(trifluoromethyl)pheny1)- 1H),
8.25 (s, 1H), 10.56 (s, 1H);
4-methylbenzamide ESI-MS (m/z)527(M+H)+.
254. * ON A4, NMR
(400 MHz, DMSO-d6)
H H C92 6 1.21
(s, 6H), 1.29 (s, 9H), 3.82
N N N (s,
2H), 6.41 (s, 1H), 7.62 (d, J =
x IN
Method Q 6.8 Hz, 2H), 7.38 (d, J = 8.8 Hz,
0
2H), 7.70-7.76 (m, 3H), 7.90-
7.93 (m, 2H), 8.04 (t, J= 1.6 Hz,
NT 1H), 8.52 (s, 1H), 9.04 (s, 1H);
1-(3-(tert-Butyl)-1-(3- ESI-MS (m/z) 539 (M+H)+.
cyanopheny1)-1H-pyrazol-5-y1)-3-
(4-47,7-dimethyl-7,8-dihydro-6H-
pyrimido[5,4-b][1,4]oxazin-4-
yl)oxy)phenyl)urea
255. A20, B3, NMR
(400 MHz, DMSO-d6)
0 N cF3 C19 6 1.23-
1.35 (m, 5H), 1.91-1.95
c,0 (m,
2H), 2.21-2.27 (m, 4H), 2.46
Method B (s, 3H), 3.20 (s, 3H), 3.31-3.65
1\1 N
C (m,
2H), 4.00-4.02 (m, 2H), 6.66
(d, J= 5.6 Hz, 1H), 6.94 (s, 1H),
217
CA 03064975 2019-11-26
WO 2018/215668
PCT/EP2018/063957
No. Intermed Analytical Data
Structure and Name iates and
Method
4-Methyl-N-(3-(4- 7.55
(d, J = 8.0 Hz, 2H), 7.60-
methylpiperazin-1-y1)-5- 7.65
(m, 2H), 7.84 (d, J = 7.2 Hz,
(trifluoromethyl)pheny1)-3- 1H),
8.04 (d, J = 5.2 Hz, 1H),
((2,3,4,5-tetrahydropyrido[3,2- 10.28
(s, 1H); ESI-MS (m/z) 542
b] [1,4]oxazepin-9- (M+H)+.
yl)oxy)benzamide
256. A4,
B3, 1H NMR (400 MHz, DMSO-d6)
1401 id cF3 C105 6 1.24
(s, 6H), 2.19 (s, 3H), 2.59
O (t, J = 6.8 Hz, 2H), 3.59 (q, J =
N'Cx ` IW
I
Method D 6.8 Hz, 2H), 3.88 (s, 2H), 4.91 (t,
OH
N N7 J = 5.6 Hz, 1H), 7.47 (d, J = 8.0
H
Hz, 1H), 7.62 (d, J = 8.4 Hz,
347,7-Dimethy1-7,8-dihydro-6H-
1H), 7.69 (d, J = 4.8 Hz, 2H),
pyrimido[5,4-b][1,4]oxazin-4-
7.76-7.82 (m, 2H), 8.07 (d, J =
yl)oxy)-N-(4-(4-hydroxybut-l-yn-
8.8 Hz, 1H), 88.23 (d, J = 1.6 Hz,
1-y1)-3 -(trifluoromethyl)pheny1)-
4-methylbenzamide 1H),
10.53 (s, 1H); ESI-MS
(m/z) 527 (M+H)+.
257. a H A4, 1H NMR
(400 MHz, DMSO-d6)
O N so
SO2Me B3, C106 6 1.24 (s, 6H), 2.19 (s, 3H), 2.24
N0 0 (s,
3H), 2.48-2.52 (m, 4H), 3.17-
I N Method
D 2.25 (m, 7H), 3.88 (s, 2H), 7.14
N N CN)
H (s,
1H), 7.46 (d, J = 8.4 Hz, 1H),
7.69-7.73 (m, 3H), 7.78-7.84 (m,
I
3((7,7-Dimethy1-7,8-dihydro-6H- 3H),
10.31 (s, 1H); ESI-MS (m/z)
pyrimido[5,4-b][1,4]oxazin-4- 567 (M+H)+.
yl)oxy)-4-methyl-N-(3-(4-
methylpiperazin-l-y1)-5-
(methylsulfonyl)phenyl)benzamid
e
258. H H
A7, 1H NMR (400 MHz, DMSO-d6)
=4-y I*
6 2.23 (s, 3H), 2.44-2.52 (m,
N N SO2Me
0
0
aminophe 4H), 3.16-3.23 (m, 6H), 3.37-
X _0
j N
CN) nol,
C106 3.42 (m, 5H), 4.10 (t, J = 4.8 Hz,
N N
1H), 6.01 (d, J = 5.6 Hz, 1H),
H I Method
I 6.76 (s, 1H), 6.95-7.03 (m, 3H),
1-(4-((3,4-Dihydro-2H- 7.32
(s, 1H), 7.41-7.48 (m, 3H),
pyrido[3,2-b][1,4]oxazin-8- 8.74
(s, 1H), 8.93 (s, 1H); ESI-
yl)oxy)pheny1)-3-(3-(4- MS (m/z) 539 (M+H)+.
methylpiperazin-l-y1)-5-
(methylsulfonyl)phenyl)urea
259. H H A7, 4-
1H NMR (400 MHz, DMSO-d6)
Ai NyN to SO2Me
aminophe 6 0.99 (t, J = 7.2 Hz, 3H), 2.22-
0 nol,
C104 2.49 (m, 10H), 3.37 (q, J = 7.2
0
N
C N) Hz,
2H), 3.46 (s, 3H), 3.78 (s,
Method I 2H), 4.10 (t, J = 4.0 Hz, 2H),
N N H 6.02
(d, J= 5.6 Hz, 1H), 6.76 (s,
) 1H), 6.98 (d, J = 9.2 Hz, 2H),
218
CA 03064975 2019-11-26
WO 2018/215668 PCT/EP2018/063957
No. Intermed Analytical Data
Structure and Name iates and
Method
1-(4-((3,4-Dihydro-2H- 7.40-
7.49 (m, 4H), 7.70 (dd, J1 =
pyrido[3,2-b][1,4]oxazin-8- 2.4,
J2= 8.4 Hz, 1H), 8.17 (d, J =
yl)oxy)pheny1)-3-(4((4- 2.0
Hz, 1H), 8.75 (s, 1H), 9.14 (s,
ethylpiperazin-1-yl)methyl)-3- 1H); ESI-MS (m/z) 567 (M+H)+.
(methylsulfonyl)phenyl)urea
260. CI A4, Bl, 1I-1 =
NMR (400 MHz, DMSO-d6)
H
N C81 6 0.98
(t, J = 6.8 Hz, 3H), 1.24
N):0
0 WI 0 cF3
, 0 cH3 (s,
6H), 2.27-2.51 (m, 10H), 3.56
)
N N N
(N) Method
A (s, 2H), 3.89 (s, 2H), 7.72 (s,
Chiral 1H), 7.77 (d, J = 8.0 Hz, 2H),
H
separation 7.88-7.99 (m, 3H), 8.03 (dd, J1 =
) 2.0
Hz, .12 = 8.8 Hz, 1H), 8.17 (s,
4-Chloro-3-47,7-dimethy1-7,8- 1H),
10.55 (s, 1H); ESI-MS (m/z)
dihydro-6H-pyrimido[5,4- 605 (M+H)+.
b][1,4]oxazin-4-yl)oxy)-N-(4-(1-
(4-ethylpiperazin-l-y1)ethyl)-3-
(trifluoromethyl)phenyl)benzamid
e
261. CI A4, Bl, 1I-1 =
NMR (400 MHz, DMSO-d6)
H
OWI N.CF3 C81
6 0.98 (t, J = 6.8 Hz, 3H), 1.24
INIc,o, 0 cH3 (s,
6H), 2.27-2.51 (m, 10H), 3.56
1 j< N Method
A (s, 2H), 3.89 (s, 2H), 7.72 (s,
NN CN) Chiral
1H), 7.77 (d, J = 8.0 Hz, 2H),
H
separation 7.88-7.99 (m, 3H), 8.03 (dd, J1 =
) 2.0
Hz, .12 = 8.8 Hz, 1H), 8.17 (s,
4-Chloro-3-47,7-dimethy1-7,8- 1H),
10.55 (s, 1H); ESI-MS (m/z)
dihydro-6H-pyrimido[5,4- 605 (M+H)t
b][1,4]oxazin-4-yl)oxy)-N-(4-(1-
(4-ethylpiperazin-l-y1)ethyl)-3-
(trifluoromethyl)phenyl)benzamid
e
262. A4,
Bl, 1I-1 NMR (400 MHz, DMSO-d6)
ci
0 H
O N CF3 C81
d 0.96 (t, J = 7.2 Hz, 3H), 1.22-
1.25 (m, 9H), 2.20-2.34 (m, 8H),
N c), 0 ScH3 Method
A 3.46-3.51 (m, 3H), 3.89 (s, 2H),
1 k N 7.71
(s, 1H), 7.74-7.80 (m, 2H),
NN H CN )
7.86-7.91 (m, 3H), 8.02-8.06 (m,
1H), 8.14 (d, J = 2.4 Hz, 1H),
)
10.54 (s, 1H); ESI-MS (m/z) 619
4-Chloro-3-47,7-dimethy1-7,8-
(M+H)+.
dihydro-6H-pyrimido[5,4-
b] [1,4]oxazin-4-yl)oxy)-N-(4-(1-
(4-ethylpiperazin-1-yl)ethyl)-3-
(trifluoromethyl)phenyl)benzamid
e (Racemic)
219
CA 03064975 2019-11-26
WO 2018/215668 PCT/EP2018/063957
PHARMACOLOGICAL ACTIVITY
This is a one step binding assay based on the binding and displacement of the
labeled
tracer where compound addition is followed by addition of the anti-GST tagged
europium
(Eu) as the donor and Alexa Fluor labelled tracer as the acceptor.
Simultaneous binding of
both the tracer and GST-antibody to the kinase domain of HPK1 results in a
high degree of
FRET (fluorescence resonance energy transfer) from the anti-GST tagged
europium (Eu)
fluorophore to the Alexa Fluor 647 fluorophore on the kinase tracer and this
signal is
reduced in presence of the inhibitor that can be measured.
Test compounds or reference compounds such as Sunitinib were dissolved in
dimethylsulfoxide (DMSO) to prepare 10.0 mM stock solutions and diluted to the
desired
concentration. The final concentration of DMSO in the reaction was 3% (v/v).
The assay
mixture was prepared by mixing 6nM of the Eu-Anti-GST Antibody and 15nM MAP4K-
1
enzyme in the Kinase buffer containing 50mM HEPES (pH 7.5), 10 mM MgCl2, 1 mM
EGTA, 0.01% Brij-35 with or without the desired concentration of the compound.
The
reaction was incubated on ice for 15mins. The pre-incubation step was
terminated by addition
of the 30nM Kinase Tracer 222 into the reaction mixture. After shaking for 5
min the reaction
was further incubated for 1 hour at room temperature and this was kept and
read at 4 C on an
ARTEMIS reader as per the kit instructions (Thermo). The inhibition of test
compound was
calculated based on the FRET ratio of 665 / 615. The activity was calculated
as a percent of
control reaction. IC50 values were calculated from dose response curve by
nonlinear
regression analysis using GraphPad Prism software.
The compounds prepared were tested using the above assay procedure and the
results
obtained are given in Table 26. Percentage inhibition at concentrations of 1.0
ILLM and 10.0
ILLM are given in the table along with IC50 (nM) details for selected
examples.
The ICso (nM) values are set forth in Table 26 wherein "A" refers to an ICso
value of
less than 50 nM, "B" refers to ICso value in range of 50.01 to 100.0 nM, "C"
refers to ICso
values more than 100.01 to 500 nM and "D" refers to ICso values more than 500
nM.
Table 26:
% Inhibition at IC50 value
Sr. no. Compound No.
1 uM 10 MM (nM)
1. Example 1 53.13 70.95
2. Example 2 6L76 78.75
3. Example 3 68.47
67
4. Example 4 72.67 78.09
C
220
CA 03064975 2019-11-26
WO 2018/215668 PCT/EP2018/063957
% Inhibition at IC50 value
Sr. no. Compound No.
1 pM 10 pM (nM)
5. Example 5 33.21 47.41 -
6. Example 6 33.33 57.59 -
7. Example 7 66.26 72.1 D
8. Example 8 18.8 66.8 D
9. Example 9 36.71 58.38 -
10. Example 10 28.23 62.99 -
11. Example 11 60.05 59.56 D
12. Example 12 14.1 4.4 -
13. Example 13 35.6 67.1 D
14. Example 14 17.8 42.4 D
15. Example 15 69.5 78.7 C
16. Example 16 21.2 20.6 -
17. Example 17 21.8 70 D
18. Example 18 14.5 40.5 -
19. Example 19 38.53 68.87 D
20. Example 20 2.7 30.2 -
21. Example 21 16.9 55.27 D
22. Example 22 23.53 64.92 D
23. Example 23 56 70.1 D
24. Example 24 57.24 84.42 D
25. Example 25 40.01 81.32 D
26. Example 26 70.66 87.25 C
27. Example 27 49.07 77.88 D
28. Example 28 37.96 64.08 D
29. Example 29 33.41 69.51 D
30. Example 30 35.3 74.9 D
31. Example 31 41.66 73.12 D
32. Example 32 17.47 71.32 D
33. Example 33 8.97 65.23 D
34. Example 34 46.96 77.43 D
35. Example 35 5.34 10.79 -
36. Example 36 36.69 66.31 D
37. Example 37 36.87 65.59 D
38. Example 38 10.94 10.37 -
39. Example 39 37.42 68.78 D
40. Example 40 24.77 65.08 D
41. Example 41 9.31 40.69 -
42. Example 42 9.08 2.28 -
221
CA 03064975 2019-11-26
WO 2018/215668 PCT/EP2018/063957
% Inhibition at IC50 value
Sr. no. Compound No.
1 pM 10 pM (nM)
43. Example 43 32.96 5.15 -
44. Example 44 48.2 77.9 D
45. Example 45 34.1 75.9 D
46. Example 46 56.68 85.26 D
47. Example 47 43.65 72.8 D
48. Example 48 37.25 53.68 D
49. Example 49 15A4 34.98 -
50. Example 50 9.5 3.9 -
51. Example 51 0 19.84 -
52. Example 52 17.81 67.33 D
53. Example 53 54.92 52.04 D
54. Example 54 24.44 38.07 D
55. Example 55 21.99 67.76 D
56. Example 56 31.8 83.85 D
57. Example 57 10.61 30.02 -
58. Example 58 53.52 21.58 D
59. Example 59 24.08 66.02 D
60. Example 60 47.98 75.2 D
61. Example 61 35.07 42.95 D
62. Example 62 24.71 67.35 D
63. Example 63 44.7 79.5 D
64. Example 64 41.76 71.6 D
65. Example 65 51.9 76.53 D
66. Example 66 48.78 63.97 D
67. Example 67 62.28 84.47 D
68. Example 68 62.5 84.42 D
69. Example 69 41.19 76.6 D
70. Example 70 43.79 79.6 D
71. Example 71 32.37 73.02 D
72. Example 72 40.12 73.38 D
73. Example 73 42.94 14.64 D
74. Example 74 32.11 17.20 D
75. Example 75 49.16 75.14 D
76. Example 76 53.25 77.82 D
77. Example 77 69.82 82.63 D
78. Example 78 76.4 86.3 D
79. Example 79 55.2 80.8 D
80. Example 80 81.39 84.78 A
222
CA 03064975 2019-11-26
WO 2018/215668 PCT/EP2018/063957
% Inhibition at IC50 value
Sr. no. Compound No.
1 pM 10 pM (nM)
81. Example 81 77.83 77.31 B
82. Example 82 84.2 85.2 A
83. Example 83 63.73 29.77 C
84. Example 84 58.9 78.1 D
85. Example 85 49.65 76.31 D
86. Example 86 49.8 78 D
87. Example 87 65 80 C
88. Example 88 20.34 69.98 D
89. Example 89 34.91 70.3 D
90. Example 90 31.48 71.86 D
91. Example 91 8.08 40.76 -
92. Example 92 0 31.48 -
93. Example 93 50.82 18.14 D
94. Example 94 20.34 51.33 D
95. Example 95 58.57 80.37 D
96. Example 96 68.63 88.18 C
97. Example 97 5.6 76 D
98. Example 98 34.4 72.5 D
99. Example 99 75.94 80.67 C
100. Example 100 35.47 69.93 D
101. Example 101 47.52 75.18 D
102. Example 102 73.73 85.52 C
103. Example 103 61 79.9 D
104. Example 104 68.32 82.95 C
105. Example 105 44.1 77.8
D
106. Example 106 68.28 79.05 C
107. Example 107 19.96 61.1 D
108. Example 108 62.83 68.35 D
109. Example 109 11.61 48.97 D
110. Example 110 1.82 38.89 -
111. Example 111 4.24 26.9 -
112. Example 112 35.08 76.48 D
113. Example 113 7.32 18.27 -
114. Example 114 24.76 63.45 D
115. Example 115 30.79 63.74 D
116. Example 116 24.82 55.72 D
117. Example 117 68.8 86.6 D
118. Example 118 5.56 21.19 -
223
CA 03064975 2019-11-26
WO 2018/215668 PCT/EP2018/063957
% Inhibition at IC50 value
Sr. no. Compound No.
1 pM 10 pM (nM)
119. Example 119 16.96 64.21 D
120. Example 120 69.88 33.44 C
121. Example 121 72.32 47.1 C
122. Example 122 68.5 84.28 C
123. Example 123 77.94 88.52 C
124. Example 124 37.6 77.54 D
125. Example 125 13.1 58.05 D
126. Example 126 19.14 56.79 D
127. Example 127 51.5 81.31 D
128. Example 128 35.59 75.33 D
129. Example 129 22.62 75.02 D
130. Example 130 58.41 77.74 D
131. Example 131 59.69 77.59 D
132. Example 132 2.76 19.96 -
133. Example 133 42.99 74.42 D
134. Example 134 4.57 60.59 D
135. Example 135 23.26 70.69 D
136. Example 136 58.43 79.18 D
137. Example 137 52.62 ppt D
138. Example 138 49.09 81.44 D
139. Example 139 55.4 78.93 D
140. Example 140 34.55 68.28 D
141. Example 141 38.67 74.26 D
142. Example 142 45.46 ppt D
143. Example 143 55.9 81.2 D
144. Example 144 52.6 70.5 D
145. Example 145 65.8 82.6 C
146. Example 146 59.9 81.8 D
147. Example 147 2.9 29.2 D
148. Example 148 16.1 23.9 -
149. Example 149 50.9 78.4 D
150. Example 150 39.7 66.1 D
151. Example 151 81.6 88.4 C
152. Example 152 2.6 25.3 D
153. Example 153 21.9 45.5 D
154. Example 154 87.3 88.7 C
155. Example 155 71.2 ppt C
156. Example 156 29.57 81.52 D
224
CA 03064975 2019-11-26
WO 2018/215668 PCT/EP2018/063957
% Inhibition at IC50 value
Sr. no. Compound No.
1 pM 10 pM (nM)
157. Example 157 81.5 83.04 B
158. Example 158 27.89 82.15 D
159. Example 159 9.12 31.9 -
160. Example 160 33.12 ppt D
161. Example 161 36.3 83.12 D
162. Example 162 53.39 84.28 D
163. Example 163 80.21 85.83 B
164. Example 164 40.25 ppt -
165. Example 165 0 ppt -
166. Example 166 36.05 ppt -
167. Example 167 85.06 88.47 B
168. Example 168 36.73 80.81 D
169. Example 169 12.51 27.14 -
170. Example 170 20.06 32.35 -
171. Example 171 9.71 9.18 -
172. Example 172 13.92 19.34 -
173. Example 173 64.87 74.86 D
174. Example 174 71.14 72.8 C
175. Example 175 61.3 43.34 D
176. Example 176 58.99 82.66 D
177. Example 177 29.34 76.34 D
178. Example 178 22.6 69.27 D
179. Example 179 38.74 83.38 D
180. Example 180 31.59 68.17 D
181. Example 181 12.17 45.63 D
182. Example 182 23.13 78.33 D
183. Example 183 89.24 86.1 A
184. Example 184 34.25 77.07 D
185. Example 185 0.23 1.68 -
186. Example 186 3.18 0 -
187. Example 187 42.7 78.2 D
188. Example 188 83.9 81.4 A
189. Example 189 28.04 64.65 D
190. Example 190 15.19 59.03 D
191. Example 191 74.76 84.18 C
192. Example 192 2.06 16.41 D
193. Example 193 0.05 6.15 -
194. Example 194 84.23 85.32 B
225
CA 03064975 2019-11-26
WO 2018/215668 PCT/EP2018/063957
% Inhibition at IC50 value
Sr. no. Compound No.
1 pM 10 pM (nM)
195. Example 195 55.56 83.59 D
196. Example 196 80.62 85.73 C
197. Example 197 0 34.89 D
198. Example 198 80.17 83.16 B
199. Example 199 2.95 61.89 D
200. Example 200 35.42 83.32 D
201. Example 201 29.54 66.36 D
202. Example 202 87.83 87.57 A
203. Example 203 32.34 76.3 D
204. Example 204 30.75 54.15 D
205. Example 205 0 38.18 D
206. Example 206 75.93 74.6 B
207. Example 207 88.2 86.32 A
208. Example 208 11.88 74.06 D
209. Example 209 78.23 87.05 C
210. Example 210 76.66 90.18 C
211. Example 211 71.03 90.78 C
212. Example 212 63.57 89.35 C
213. Example 213 71.56 80.6 C
214. Example 214 85.85 93.25 B
215. Example 215 46.68 84.15 D
216. Example 216 86.76 89.77 B
217. Example 217 86.87 88.31 A
218. Example 218 62.7 85.64 D
219. Example 219 32.66 75.29 D
220. Example 220 0 15.85 D
221. Example 221 30.35 71.36 D
222. Example 222 52.09 ppt D
223. Example 223 0 29.98 -
224. Example 224 1.14 54.61 D
225. Example 225 83.78 88.31 A
226. Example 226 85.77 88.27 A
227. Example 227 64.83 82.36 C
228. Example 228 84.54 87.6 A
229. Example 229 85.18 84.82 C
230. Example 230 85 87.16 B
231. Example 231 88.34 81.52 A
232. Example 232 84.18 86.82 A
226
CA 03064975 2019-11-26
WO 2018/215668 PCT/EP2018/063957
% Inhibition at IC50 value
Sr. no. Compound No.
1 pM 10 pM (nM)
233. Example 233 79.07 83.61
A
234. Example 234 19.03 51.95
D
235. Example 235 79 86.5
C
236. Example 236 86.73 88.02
B
237. Example 237 42.78 77.72
D
238. Example 238 0 33.03
D
239. Example 239 87.31 88.65
A
240. Example 240 78.57 86.3
C
241. Example 241 4 13.4 -
242. Example 242 31.88 42.35
-
243. Example 243 43.09 73.18
D
244. Example 244 0.623
17.31 -
245. Example 245 22.94
16.97 -
246. Example 246 45.39 77.93
D
247. Example 247
44.20 , 94.21 D
248. Example 248 33.96 81.92
D
249. Example 249 47.38 62.97
D
250. Example 250 71.89 79.55
C
251. Example 251 3.24 39.98
D
252. Example 252 86.49 85.32
A
253. Example 253
49.74 D
254. Example 254 74.61 80.32
C
255. Example 255 40.22 53.24
D
256. Example 256 48.07 -
D
257. Example 257 22.12 53.42
D
258. Example 258 46.90 79.11
D
259. Example 259 55.00 84.44
D
(-): Not determined.
Although the invention herein has been described with reference to particular
embodiments, it is to be understood that these embodiments are merely
illustrative of the
principles and applications of the present invention. It is therefore to be
understood that
numerous modifications may be made to the illustrative embodiments and that
other
arrangements may be devised without departing from the spirit and scope of the
present
invention as described above.
227
CA 03064975 2019-11-26
WO 2018/215668 PCT/EP2018/063957
All publications and patent applications cited in this application are herein
incorporated by reference to the same extent as if each individual publication
or patent
application was specifically and individually indicated to be incorporated
herein by reference.
228