Note: Descriptions are shown in the official language in which they were submitted.
DEMANDE OU BREVET VOLUMINEUX
LA PRESENTE PARTIE DE CETTE DEMANDE OU CE BREVET COMPREND
PLUS D'UN TOME.
CECI EST LE TOME 1 DE 2
CONTENANT LES PAGES 1 A 207
NOTE : Pour les tomes additionels, veuillez contacter le Bureau canadien des
brevets
JUMBO APPLICATIONS/PATENTS
THIS SECTION OF THE APPLICATION/PATENT CONTAINS MORE THAN ONE
VOLUME
THIS IS VOLUME 1 OF 2
CONTAINING PAGES 1 TO 207
NOTE: For additional volumes, please contact the Canadian Patent Office
NOM DU FICHIER / FILE NAME:
NOTE POUR LE TOME / VOLUME NOTE:
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
ANTIBODY CONSTRUCT CONJUGATES
PRIORITY
[0001] This application claims the benefit of priority under 35 U.S.C.
119(e) to U.S.
Provisional Application No. 62/516,667, filed June 7, 2017, the disclosure of
which is
incorporated herein by reference in its entirety.
BACKGROUND
[0002] One of the leading causes of death in the United States is cancer. The
conventional
methods of cancer treatment, like chemotherapy, surgery, or radiation therapy,
tend to be either
highly toxic or nonspecific to a cancer, or both, resulting in limited
efficacy and harmful side
effects. However, the immune system has the potential to be a powerful,
specific tool in fighting
cancers. In many cases tumors can specifically express genes whose products
are required for
inducing or maintaining the malignant state. These proteins may serve as
antigen markers for the
development and establishment of more specific anti-cancer immune response.
The immune
response may include the recruitment of immune cells that target tumors
expressing these antigen
markers. Additionally, the immune cells may express genes whose products are
important to
proper immune function and may serve as markers for specific types of immune
cells. The
boosting of this specific immune response has the potential to be a powerful
anti-cancer
treatment that can be more effective than conventional methods of cancer
treatment and can have
fewer side effects.
INCORPORATION BY REFERENCE
[0003] All publications, patents, and patent applications mentioned in this
specification are
herein incorporated by reference to the same extent as if each individual
publication, patent, or
patent application was specifically and individually indicated to be
incorporated by reference.
SUMMARY
[0004] Provided herein are immune-modulatory and immune-stimulatory
conjugates. In certain
embodiments, an immune-modulatory conjugate is provided comprising: (a) an
antibody
construct comprising an antigen binding domain and an Fc domain, wherein the
antigen binding
domain binds to a first antigen; (b) a proteolysis targeting module,
comprising: (i) a protein
targeting moiety that binds to a target protein; (ii) an E3 ubiquitin ligase
binding moiety; and (iii)
a spacer S that is covalently bound to the protein targeting moiety and to the
E3 ubiquitin ligase
binding moiety, wherein the spacer is optionally has 1-25 consecutive non-
hydrogen atoms; and
-1-
CA 03065919 2019-12-02
WO 2018/227023
PCT/US2018/036560
(c) a linker L that is covalently attached to the antibody construct and to
the proteolysis targeting
module. The conjugate can be represented by one of the following formulae:
Ab = t 41401
(VII),
_
7 \ -
(VIII), or
_
/ \ -
Ab . . . =
(IX),
wherein: Ab is the antibody construct; L is the linker; ULM is the E3
ubiquitin ligase binding
moiety; IMC is the protein targeting moiety and comprises an immune-modulatory
compound; S
is the spacer; n is selected from 1 to 20; and z is selected from 1 to 20. In
some embodiments,
the Fc domain of the conjugate is an Fc null.
[0005] In some embodiments, the E3 ubiquitin ligase is selected from the group
consisting of
Von Hippel-Lindaue E3 ubiquitin ligase (VHL), cereblon, mouse double minute 2
homolog
(MDM2), AMFR, APC/Cdc20, APC/Cdhl, C6orf157, Cbl, CBLL1, CHFR, CHIP, DTL
(Cdt2),
E6-AP, HACE1, HECTD1, HECTD2, HECTD3, HECW1, HECW2, HERC2, HERC3, HERC4,
HERC5, HUWEl, HYD, ITCH, LNX1, mahogunin, MARCH-I, MARCH-II, MARCH-III,
MARCH-IV, MARCH-VI, MARCH-VII, MARCH-VIII, MARCH-X, MEKK1, MIB1, MIB2,
MycBP2, NEDD4, NEDD4L, Parkin, PELI1, Pirh2, PJA1, PJA2, RFFL, RFWD2, Rictor,
RNF5,
RNF8, RNF19, RNF190, RNF20, RNF34, RNF40, RNF125, RNF128, RNF138, RNF168,
SCF/?-TrCP, SCF/FBW7, SCF/5kp2, SHPRH, SIAH1, SIAH2, SMURF1, SMURF2, TOPORS,
-2-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
TRAF6, TRAF7, TRIM63, UBE3B, UBE3C, UBR1, UBR2, UHRF2, WWP1, WWP2, and
ZNRF1. In some further embodiments, the E3 ubiquitin ligase binding moiety
binds to VHL,
cereblon or MDM2.
[0006] In some embodiments, the protein targeting moiety binds to a protein
selected from one
of the groups consisting of: a) an aryl hydrocarbon receptor, androgen
receptor, estrogen
receptor, FK506-binding protein 12, fibroblast growth factor receptor
substrate 2,
phosphatidylinosito1-4,5-biphosphate 3-kinase, SMAD family member 3,
bromodomain and
extra-territorial family of proteins (BET), bromodomain-containing protein 4
member of the BET
family, Abelson tyrosine kinase, receptor-interacting serine/threonine-protein
kinase 1, estrogen-
related receptor, and transforming growth factor beta (TGF(3); or b) ALK, Bcr-
Abl, BRAF, BTK,
c-KIT, EGFR, ErbB2, JAK, MEK, MET, PDGFRB, RET, ROS1, Syk, SRC, TNIK, TNKS,
TNKS2, TrkA, TrkB, TrkC, VEGFR1, VEGFR2, VEGFR3, FGFR1, FGFR2, FGFR3, FGFR4,
CSF1R, RON/MST1R, TYR03, MERTK, AXL, PI3K, PI3K, MAP4K1, PERK, and KIT; or c)
TGFPR2, TGFPR1, SMAD2, SMAD3, SMAD4, beta-catenin, CREBB2, Beta catenin/TCF4,
beta catenin/LEF, beta catenin/CREBBP, YAP, TAZ, YAP/TAZ, TNKS1, TNKS2, MST1,
MST2, NRAS, HRAS, KRAS, RASmut12, RASmut13, PERK (EIF2AK3), RON/MST1R,
STAT3, MCT1, MCT2 and MCT4; or d) CSFR1, RON/MST1, PI3Kd, PI3Kg, PARP1, PD-L1,
PP2A, A2ar, TYR03, AXL and MER.
[0007] In some embodiments, the first antigen is a tumor antigen. The tumor
antigen can be
MUC16, CD5, CD19, CD20, CD25, CD37, CD30, CD33, CD45, CAMPATH-1, BCMA, CS-1,
PD-L1, B7-H3, B7-DC, HLD-DR, carcinoembryonic antigen (CEA), TAG-72, EpCAM,
MUC1,
folate-binding protein, A33, G250, prostate-specific membrane antigen (PSMA),
ferritin, GD2,
GD3, GM2, Ley, CA-125, CA19-9, epidermal growth factor, p185HER2, IL-2
receptor,
fibroblast activation protein (FAP), tenascin, a metalloproteinase,
endosialin, vascular endothelial
growth factor, v 3, WT1, LMP2, HPV E6, HPV E7, EGFRvIII, Her-2/neu, MAGE A3,
p53
nonmutant, NY-ESO-1, MelanA/MART1, Ras mutant, gp100, p53 mutant, PR1, bcr-
abl,
tyronsinase, survivin, PSA, hTERT, a Sarcoma translocation breakpoint fusion
protein, EphA2,
PAP, ML-IAP, AFP, ERG, NA17, PAX3, ALK, androgen receptor, cyclin Bl,
polysialic acid,
MYCN, RhoC, TRP-2, fucosyl GM1, mesothelin (MSLN), PSCA, MAGE Al, sLe(animal),
CYP1B1, PLAV1, GM3, BORIS, Tn, GloboH, ETV6-AML, NY-BR-1, RGS5, SART3, STn,
Carbonic anhydrase IX, PAX5, 0Y-TES1, Sperm protein 17, LCK, HMWMAA, AKAP-4,
55X2, XAGE 1, Legumain, Tie 3, Page4, VEGFR2, MAD-CT-1, PDGFR-B, MAD-CT-2,
ROR2, TRAILl, MAGE A4, MAGE C2, GAGE, EGFR, CMET, HER3, MUC15, CA6,
NAPI2B, TROP2, CLDN18.2, RON, LY6E, FRA, DLL3, PTK7, LIV1, ROR1, or Fos-
related
antigen 1.
-3-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
[0008] In some embodiments, the first antigen is an immune cell antigen, and
can be an antigen
present on the surface of an antigen presenting cell such as a dendritic cell
or a macrophage. In
some embodiments, the immune cell antigen is selected from the group
consisting of CD40,
DEC-205, PD-L1, PD-1, CD36 mannose scavenger receptor 1, CLEC9A, DC-SIGN,
CLEC12A,
BDCA-2, OX4OL, 41BBL, CD204, MARCO, CLEC5A, Dectin 1, Dectin 2, CLEC10A,
CD206,
CD64, CD32A, CD16A, HVEM, and CD32B.
[0009] In some embodiments, the protein targeting moiety can be a peptide or a
non-
proteinaceous molecule. In some embodiments, the proteolysis targeting module
is selected from
compounds 1-1, 1-2, 1-3, 1-4, 1-5, 1-6 and 2-1, as disclosed herein. In some
embodiments, the
linker L is a cleavable linker. In some embodiments, the linker L is a non-
cleavable linker. In
some embodiments, the linker L is covalently bound to the antibody construct
at a cysteine
residue, an engineered cysteine residue, a lysine residue, a glutamate
residue, or a glutamine
residue of the antibody construct or is covalently bound to the antibody
construct using a Sortase
linker.
[0010] In some embodiments, the spacer S is an optionally substituted C1-25
alkylene or
optionally substituted C1-25 heteroalkylene, wherein the heteroalkylene is a
C1-24 alkylene chain
interspersed with one or more groups independently selected from: -0-, -S-, -
NH2-, and -
C(0)NH-; and optionally substituted with a reactive group, Rx, that can form a
functional group
selected from an amide bond, an ester bond, an ether bond, a carbonate bond, a
carbamate bond,
or a thioether bond. The spacer can be optionally substituted with C1-C8
alkyl, C2-C8 alkenyl, C2-
C8 alkynyl, -(CH20).1H, -(CH2CH20)n1fl, -(CH20)n1CH3, -C(0)0H or -NH2, wherein
n1 is from
1 to 8. In some embodiments the spacer is substituted with Rx. In some
embodiments, the linker
is unsubstituted.
[0011] In some embodiments, the antibody construct further comprises a second
binding domain.
The second binding domain can specifically bind to an antigen on an immune
cell. The immune
cell antigen is selected from the group consisting of CD40, PD-1, PD-L1, DEC-
205, CD36
mannose scavenger receptor 1, CLEC9A, DC-SIGN, CLEC12A, BDCA-2, OX4OL, 41BBL,
CD204, MARCO, CLEC5A, Dectin 1, Dectin 2, CLEC10A, CD206, CD64, CD32A, CD16A,
HVEM, and CD32B. In some embodiments, the second binding domain is attached to
the
antibody construct at a C-terminal end of the Fc domain.
[0012] In some embodiments, the Fc domain is an Fc domain variant comprising
at least one
amino acid residue change as compared to a wild type sequence of the Fc
domain. In some
embodiments, the Fc domain is an Fc domain variant that decreases binding of
the Fc domain to
an Fc receptor. In some embodiments, the at least one amino acid residue
change in the Fc
domain is: a) F243L, R292P, Y300L, L235V, and P396L, wherein numbering of
amino acid
-4-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
residues in the Fc domain is relative to SEQ ID NO: 898; b) 5239D and 1332E,
wherein
numbering of amino acid residues in the Fc domain is relative to SEQ ID NO:
898; or c) 5298A,
E333A, and K334A, wherein numbering of amino acid residues in the Fc domain is
relative to
SEQ ID NO: 898. In some embodiments, the at least one amino acid residue
change in the Fc
domain is: a) N297A, N297G, N297Q, N297D as in Eu index of Kabat numbering and
relative to
SEQ ID NO: 898; or b) K322A/L234A/L235A N296A as in the EU index of Kabat
numbering
and relative to SEQ ID NO: 898; or c) L234F/L235E/P3315 N296A as in the EU
index of Kabat
numbering and relative to SEQ ID NO: 898; or d) P329G/L234A/L235A as in the EU
index of
Kabat numbering and relative to SEQ ID NO: 898.
[0013] Also provided is a pharmaceutical composition comprising a conjugate as
described
herein along with a pharmaceutically acceptable excipient. In the
pharmaceutical composition,
the average ratio of proteolysis targeting modules to antibody construct in
the conjugate can be
from 2 to 6, from 3 to 5 or 1 to 3. The pharmaceutical composition can be
lyophilized.
[0014] Also provided are methods of treating cancer comprising administering a
conjugate as
described herein, or a pharmaceutical composition of a conjugate as described
herein, to a subject
in need thereof. In some embodiments, the cancer is a solid tumor, such as
breast cancer,
pancreatic cancer, colorectal cancer, renal cell cancer, gastric cancer, or
lung cancer. In some
embodiments, the pharmaceutical composition is administered parenterally. In
some
embodiments, the pharmaceutical composition is administered intravenously.
[0015] In certain aspects, the disclosure also provides immune-stimulatory
conjugates
comprising: (a) an immune-stimulatory compound that binds to a target protein
to stimulate an
immune response by stimulating the activity of the target protein, reducing
immune inhibition by
the target protein or increasing degradation of the target protein; (b) an
antibody construct
comprising an antigen binding domain and an Fc domain, wherein the antigen
binding domain
binds to at least a first antigen and wherein the Fc domain binds to an Fc
receptor; and (c) a
linker, wherein the linker is covalently bound to the antibody construct and
the linker is
covalently bound to the immune-stimulatory compound. In some embodiments, the
dissociation
constant (Kd) for binding of the Fc domain of the conjugate to an Fc receptor
is equal to, or up to
no greater than about 100 times the Kd for binding of a control antibody
construct to the Fc
receptor, wherein the control antibody construct is the unconjugated antibody
construct; and
wherein the Kd for binding of the immune-stimulatory compound of the conjugate
(i.e, attached
to the conjugate) to the target protein is equal to, or up to no greater than
100 times the Kd for
binding of a control compound to the target protein active site or wherein the
EC50 or IC50 of the
immune-stimulatory compound of the conjugate is no greater than 300-fold the
EC50 or IC50 of a
control compound, wherein the control compound is the unbound immune-
stimulatory
-5-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
compound. In some embodiments, the dissociation constant (Kd) for binding of
the Fc domain of
the conjugate to an Fc receptor is greater than about 100 times the Kd for
binding of a control
antibody construct to the Fc receptor, wherein the control antibody construct
is the unconjugated
antibody construct; and wherein the Kd for binding of the immune-stimulatory
compound of the
conjugate (i.e, attached to the conjugate) to the target protein is equal to,
or up to no greater than
100 times the Kd for binding of a control compound to the target protein
active site or wherein
the EC50 or IC50 of the immune-stimulatory compound of the conjugate is no
greater than 300-
fold the EC50 or IC50 of a control compound, wherein the control compound is
the unbound
immune-stimulatory compound. In some embodiments, the Fc domain is an Fc null.
[0016] In certain embodiments, the disclosure provides an immune-stimulatory
conjugate
comprising: (a) an immune-stimulatory compound that binds to a protein active
site to stimulate
an immune response; (b) an antibody construct comprising an antigen binding
domain and an Fc
domain, wherein the antigen binding domain binds to at least a first antigen
and wherein the Fc
domain binds to an Fc receptor; and (c) a linker, wherein the linker is
covalently bound to the
antibody construct and the linker is covalently bound to the immune-
stimulatory compound. In
some embodiments, the dissociation constant (Kd) for binding of the Fc domain
of the conjugate
to an Fc receptor is equal to, or up to no greater than about 100 times the Kd
for binding of a
control antibody construct to the Fc receptor, wherein the control antibody
construct is the
unconjugated antibody construct; and wherein the Kd for binding of immune-
stimulatory
compound of the conjugate to the protein active site is equal to, or up to no
greater than 100
times the Kd for binding of a control compound to the protein active site or
wherein the EC50 or
IC50 of the immune-stimulatory compound of the conjugate (attached to the
conjugate )is no
greater than 300-fold the EC50 or IC50 of a control compound, wherein the
control compound is
the unbound immune-stimulatory compound. In some embodiments, the dissociation
constant
(Kd) for binding of the Fc domain of the conjugate to an Fc receptor is
greater than about 100
times the Kd for binding of a control antibody construct to the Fc receptor,
wherein the control
antibody construct is the unconjugated antibody construct; and wherein the Kd
for binding of
immune-stimulatory compound of the conjugate to the protein active site is
equal to, or up to no
greater than 100 times the Kd for binding of a control compound to the protein
active site or
wherein the EC50 or IC50 of the immune-stimulatory compound of the conjugate
(attached to the
conjugate) is no greater than 300-fold the EC50 or IC50 of a control compound,
wherein the
control compound is the unbound immune-stimulatory compound.
[0017] In certain embodiments, the disclosure provides an immune-stimulatory
conjugate
comprising: (a) an immune-stimulatory compound that binds to a protein active
site of a binding
protein to stimulate an immune response by inhibition of the activity of the
binding protein; (b)
-6-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
an antibody construct comprising an antigen binding domain and an Fc domain,
wherein the
antigen binding domain binds to at least a first antigen and wherein the Fc
domain binds to an Fc
receptor; and (c) a linker, wherein the linker is covalently bound to the
antibody construct and the
linker is covalently bound to the immune-stimulatory compound. In some
embodiments, the
dissociation constant (Kd) for binding of the Fc domain of the conjugate to an
Fc receptor is
equal to, or up to no greater than about 100 times the Kd for binding of a
control antibody
construct to the Fc receptor, wherein the control antibody construct is the
unconjugated antibody
construct; and wherein the Kd for binding of the immune-stimulatory compound
of the conjugate
(attached to the conjugate) to the protein active site is equal to, or up to
no greater than 100 times
the Kd for binding of a control compound to the protein active site or wherein
the IC50 of the
immune-stimulatory compound of the conjugate (attached to the conjugate) is no
greater than
300-fold the IC50 of a control compound, wherein the control compound is the
unbound immune-
stimulatory compound. In some embodiments, the dissociation constant (Kd) for
binding of the
Fc domain of the conjugate to an Fc receptor is greater than about 100 times
the Kd for binding of
a control antibody construct to the Fc receptor, wherein the control antibody
construct is the
unconjugated antibody construct; and wherein the Kd for binding of the immune-
stimulatory
compound of the conjugate (attached to the conjugate) to the protein active
site is equal to, or up
to no greater than 100 times the Kd for binding of a control compound to the
protein active site or
wherein the IC50 of the immune-stimulatory compound of the conjugate (attached
to the
conjugate) is no greater than 300-fold the IC50 of a control compound, wherein
the control
compound is the unbound immune-stimulatory compound. In some embodiments, the
Fc domain
is an Fc null.
[0018] In certain embodiments, the disclosure provides an immune-stimulatory
conjugate
comprising: (a) an immune-stimulatory compound that binds to a protein active
site of a binding
protein to stimulate an immune response by increasing degradation of the
binding protein; (b) an
antibody construct comprising an antigen binding domain and an Fc domain,
wherein the antigen
binding domain binds to at least a first antigen and wherein the Fc domain
binds to an Fc
receptor; and (c) a linker, wherein the linker is covalently bound to the
antibody construct and the
linker is covalently bound to the immune-stimulatory compound. In some
embodiments, the
dissociation constant (Kd) for binding of the Fc domain of the conjugate to an
Fc receptor is
equal to, or up to no greater than about 100 times the Kd for binding of a
control antibody
construct to the Fc receptor, wherein the control antibody construct is the
unconjugated antibody
construct; and wherein the Kd for binding of the immune-stimulatory compound
of the conjugate
(attached to the conjugate) to the protein active site is equal to, or up to
no greater than 100 times
the Kd for binding of a control compound to the protein active site or wherein
the IC50 of the
-7-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
immune-stimulatory compound of the conjugate is no greater than 300-fold the
IC50 of a control
compound, wherein the control compound is the unbound immune-stimulatory
compound. In
some embodiments, the dissociation constant (Kd) for binding of the Fc domain
of the conjugate
to an Fc receptor is greater than about 100 times the Kd for binding of a
control antibody
construct to the Fc receptor, wherein the control antibody construct is the
unconjugated antibody
construct; and wherein the Kd for binding of the immune-stimulatory compound
of the conjugate
(attached to the conjugate) to the protein active site is equal to, or up to
no greater than 100 times
the Kd for binding of a control compound to the protein active site or wherein
the IC50 of the
immune-stimulatory compound of the conjugate is no greater than 300-fold the
IC50 of a control
compound, wherein the control compound is the unbound immune-stimulatory
compound. In
some embodiments, the Fc domain is an Fc null.
[0019] In certain embodiments, the EC50 or IC50 of the immune-stimulatory
compound of the
conjugate is no greater than 100-fold the EC50 or IC50 of a control compound,
wherein the control
compound is the unbound immune-stimulatory compound. The EC50 or IC50 of the
immune-
stimulatory compound of the conjugate may be no greater than 10-fold the EC50
or IC50 of a
control compound, wherein the control compound is the unbound immune-
stimulatory
compound. The EC50 or IC50 of the immune-stimulatory compound of the conjugate
may be
equivalent to or less than the EC50 or IC50 of a control compound, wherein the
control compound
is the unbound immune-stimulatory compound.
[0020] In certain embodiments, the EC50 or IC50 of the immune stimulatory
compound on an
antigen bearing cell is equivalent to or less than the EC50 or IC50 of a
control compound on the
antigen bearing cell and the EC50 or IC50 of the immune-stimulatory conjugate
is 5-fold greater or
more than the EC50 or IC50 of the control compound for a non-antigen bearing
cell.
[0021] The immune-stimulatory compound of the conjugate may have a Kd for
binding to the
protein active site of no greater than 50 times the Kd for binding of a
control compound to the
protein active site, wherein the control compound is the unbound immune-
stimulatory
compound. The immune-stimulatory compound of the conjugate may have a Kd for
binding to
the protein active site of no greater than 10 times the Kd for binding of a
control compound to the
protein active site, wherein the control compound is the unbound immune-
stimulatory
compound. The immune-stimulatory compound of the conjugate may have a Kd for
binding to
the protein active site of equivalent to or less than the Kd for binding of a
control compound to
the protein active site, wherein the control compound is the unbound immune-
stimulatory
compound.
[0022] In certain aspects, the disclosure provides an immune-stimulatory
conjugate comprising:
(a) an immune-stimulatory compound that binds to a protein active site to
stimulate an immune
-8-
CA 03065919 2019-12-02
WO 2018/227023
PCT/US2018/036560
response; (b) an antibody construct comprising an antigen binding domain and
an Fc domain,
wherein the antigen binding domain binds to a first antigen and wherein the Fc
domain binds to
an Fc receptor; and (c) a linker comprising 5-100 consecutive atoms attachment
sites, wherein
one attachment site of the linker is covalently bound to the antibody
construct and another
attachment site of the linker is covalently bound to the immune-stimulatory
compound. In some
embodiments, the dissociation constant (Kd) for binding of the Fc domain of
the conjugate to an
Fc receptor is equal to, or up to no greater than about 100 times the Kd for
binding of a control
antibody construct to the Fc receptor, wherein the control antibody construct
is the unconjugated
antibody construct; and wherein the Kd for binding of the immune-stimulatory
compound when
bound to a the linker to the protein active site is no greater than 100 times
the Kd for binding of a
control compound to the protein active site or wherein the EC50 or IC50 of the
immune-
stimulatory compound of the conjugate is no greater than 300-fold the EC50 or
IC50 of a control
compound, wherein the control compound is the unbound immune-stimulatory
compound. In
some embodiments, the dissociation constant (Kd) for binding of the Fc domain
of the conjugate
to an Fc receptor is greater than about 100 times the Kd for binding of a
control antibody
construct to the Fc receptor, wherein the control antibody construct is the
unconjugated antibody
construct; and wherein the Kd for binding of the immune-stimulatory compound
when bound to a
the linker to the protein active site is no greater than 100 times the Kd for
binding of a control
compound to the protein active site or wherein the EC50 or IC50 of the immune-
stimulatory
compound of the conjugate is no greater than 300-fold the EC50 or IC50 of a
control compound,
wherein the control compound is the unbound immune-stimulatory compound. In
some
embodiments, the Fc domain is an Fc null.
[0023] In certain embodiments, the EC50 or IC50 of the immune-stimulatory
compound when
bound to the 5-100 atom linker is no greater than 100-fold the EC50 or IC50 of
a control
compound, wherein the control compound is the unbound immune-stimulatory
compound. In
certain embodiments, the EC50 or IC50 of the immune-stimulatory compound when
bound to the
5-100 atom linker is no greater than 10-fold the EC50 or IC50 of a control
compound, wherein the
control compound is the unbound immune-stimulatory compound. The EC50 or IC50
of the
immune-stimulatory compound when bound to the 5-100 atom linker may be
equivalent to or
less than the EC50 or IC50 of a control compound, wherein the control compound
is the unbound
immune-stimulatory compound.
[0024] In certain embodiments, the Kd for binding of the immune-stimulatory
compound when
bound to the 5-100 atom linker to the protein active site may be no greater
than 50 times the Kd
for binding of a control compound to the protein active site, wherein the
control compound is the
unbound immune-stimulatory compound. The Kd for binding of the of the immune-
stimulatory
-9-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
compound when bound to a 5-100 atom linker to the protein active site may be
no greater than 10
times the Kd for binding of a control compound to the protein active site,
wherein the control
compound is the unbound immune-stimulatory compound. The Kd for binding of the
immune-
stimulatory compound when bound to a 5-100 atom linker to the protein active
site may be
equivalent to or less than the Kd for binding of a control compound to the
protein active site
wherein the control compound is the unbound immune-stimulatory compound.
[0025] In certain embodiments, the immune-stimulatory conjugate described
herein comprises
moiety that binds to the binding protein and an E3 ubiquitin ligase binding
moiety. The E3
ubiquitin ligase binding moiety may bind to, for example, VHL, cereblon or
MDM2. The E3
ubiquitin ligase binding moiety may be selected from among E3 targeting
compounds. The E3
ubiquitin ligase binding moiety may be attached to the linker or may be part
of the linker. In
certain embodiments, the E3 ubiquitin ligase binding moiety attached to the
linker, wherein the
E3 ubiquitin ligase binding moiety is bound through a first linker having 5-
100 consecutive
atoms between attachment sites to the immune-stimulatory compound and the E3
ubiquitin ligase
binding moiety is bound through a second linker having 5-100 consecutive atoms
between
attachment points to the antibody construct.
[0026] In certain embodiments, the immune-stimulatory compound is a kinase
inhibitor and the
protein active site is a kinase active site. In certain embodiments, the
linker is covalently bound
to the kinase inhibitor at a position on the kinase inhibitor that is at or
near the solvent interface
of the kinase active site as determined by modeling of the kinase inhibitor in
the kinase active
site. In certain embodiments, the linker is covalently bound to the kinase
inhibitor at a position on
the kinase inhibitor such that when the kinase inhibitor is bound to the
active site, the linker
extends out from the kinase active site into the solvent, as determined by
modeling of the kinase
inhibitor in the kinase active site. In certain embodiments, the kinase
inhibitor may be selected
from an inhibitor of ALK, Bcr-Abl, BRAF, BTK, c-KIT, EGFR, ErbB2, JAK, MEK,
MET,
PDGFRB, RET, ROS1, Syk, SRC, TGFPR2, TNIK, TNKS, TNKS2, TrkA, TrkB, TrkC,
VEGF,
VEGFR1, VEGFR2, VEGFR3, FGFR1, FGFR2, FGFR3, FGFR4, CSF1R, RON/MST1R,
TYR03, MERTK, AXL, PI3Kdelta PI3Kgamma, MAP4K1, PERK, and combinations
thereof.
[0027] In certain embodiments, the immune-stimulatory compound is selected
from a toll-like
receptor agonist, STING agonist, or RIG-I agonist. The immune-stimulatory
compound may be a
toll-like receptor (TLR) agonist selected from a TLR1 agonist, a TLR2 agonist,
a TLR3 agonist,
a TLR4 agonist, a TLR5 agonist, a TLR6 agonist, a TLR7 agonist, a TLR8
agonist, a TLR9
agonist, or a TLR10 agonist. The immune-stimulatory compound may be selected
from a
pyrimidine, a purine, a guanine nucleoside, an 8-oxoadenine, an
imidazoquinoline, a
thiazoquinoline, a 2-aminoimidazole, a furo[2,3-c]pyridine, a furo[2,3-
c]quinoline, a 2-
-10-
CA 03065919 2019-12-02
WO 2018/227023
PCT/US2018/036560
aminobenzimidazole, a 2-aminoquinoline, and a 2-aminobenzazepine. The immune-
stimulatory
compound may bind to a G protein-coupled receptor (GCPR), an ion channel, a
membrane
transporter, a phosphatase or an endoplasmic reticulum (ER) protein.
[0028] In certain embodiments, the immune-stimulatory compound is an
antagonist of the GPCR
A2aR, the sphingosine 1-phosphate receptor 1, prostaglandin receptor EP3,
prostanglandin
receptor E2, Frizzled, CXCR4 or an LPA receptor. The immune-stimulatory
compound may be
an ion channel agonist for CRAC, Kv1.3 or KCa3.1. The immune-stimulatory
compound may be
an inhibitor of HSP90 or AAA-ATPase p97.
[0029] In certain embodiments, the immune-stimulatory conjugate has immune-
stimulatory
activity while attached to the conjugate or the linker and without undergoing
cell processing such
as by endosomal or lysosomal degradation, e.g., to release the immune-
stimulatory compound or
a modified form of the compound from the conjugate or linker. In certain
embodiments, the
linker is a non-cleavable linker. The linker may be selected from the group
consisting of
Fleximer linkers. The linker may comprise one or more carbamate or amide
linkages when
attached to an immune-stimulatory compound. The linker may be selected from a
linker is
Rx
0 0
Rx
0-8 30-8
represented by one of the following formula: -- Ra -- , -- Ra -- , 0 -- 0
0 0 Njõ N
NJ= ,ON)=.(4Rx
0 Rx
,
0 F
0
F
0-7
0
F10
0 1
F N peptide ¨Rx
F , or H
, wherein Rx is a
reactive moiety and wherein Ra is selected from the group consisting of
hydrogen, alkyl (e.g., Ci-
C8 alkyl), sulfonate and methyl sulfonate. The linker may be attached to the
antibody construct at
a cysteine or lysine residue of the antibody construct.
[0030] In certain embodiments, the first antigen is a tumor antigen. The first
antigen may be at
least 80% or 100% homologous to CD5, CD19, CD20, CD25, CD37, CD30, CD33, CD45,
CAMPATH-1, BCMA, CS-1, PD-L1, B7-H3, B7-DC (PD-L2), HLD-DR, carcinoembryonic
antigen (CEA), TAG-72, EpCAM, MUC1, MUC15, MUC16, folate-binding protein, A33,
G250,
prostate-specific membrane antigen (PSMA), ferritin, GD2, GD3, GM2, Leg, CA-
125, CA19-9,
epidermal growth factor, p185HER2, IL-2 receptor, EGFRvIII (de2-7 EGFR),
fibroblast
-11-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
activation protein (FAP), tenascin, a metalloproteinase, endosialin, vascular
endothelial growth
factor, avf33, WT1, LMP2, HPV E6 E7, Her-2/neu, p53 nonmutant, NY-ES0-1,
MelanA/MART1, Ras mutant, gp100, p53 mutant, PR1, bcr-abl, tyrosinase,
survivin, PSA,
hTERT, a Sarcoma translocation breakpoint protein, EphA2, PAP, ML-IAP, AFP,
ERG, NA17,
PAX3, ALK, androgen receptor, cyclin Bl, polysialic acid, MYCN, RhoC, TRP-2,
fucosyl GM1,
mesothelin (MSLN), PSCA, MAGE Al, MAGE A3, sLe(animal), CYP1B1, PLAV1, GM3,
BORIS, Tn, GloboH, ETV6-AML, NY-BR-1, RGS5, SART3, STn, Carbonic anhydrase IX,
PAX5, 0Y-TES1, Sperm protein 17, LCK, HMWMAA, AKAP-4, 55X2, XAGE 1, Legumain,
Tie 3, VEGFR2, MAD-CT-1, PDGFR-B, MAD-CT-2, ROR2õ TRAILl, MAGE A4, MAGE
C2, GAGE, EGFR, CMET, HER3, EPCAM, CA6, NAPI2B, TROP2, Claudin-6 (CLDN6),
Claudin-16 (CLDN16), CLDN18.2, RON, LY6E, FRA, DLL3, PTK7, Uroplakin-1B
(UPK1B),
LIV1, ROR1, STRA6, TMPRSS3, TMPRSS4, TMEM238, Clorf186, Fos-related antigen 1,
VEGFR1, endoglin, PD-L1, VTCN1 (B7-H4), VISTA, gpNMB, or any fragment thereof.
[0031] In certain embodiments, the antibody construct further comprises a
second target binding
domain. In certain embodiments, the target binding domain specifically binds
an antigen on an
immune cell. The target binding domain may be attached (e.g., conjugated or
linked) to the
antibody construct at a C-terminal end of the Fc domain. The antigen binding
domain may be
from an antibody or non-antibody scaffold. The antigen binding domain may be
at least 97%
homologous to an antigen binding domain from an antibody or non-antibody
scaffold. The
antibody construct may be a human antibody or a humanized antibody. The Fc
domain may be an
Fc domain variant comprising at least one amino acid residue change as
compared to a wild type
sequence of the Fc domain. The Fc domain may be at least about 80% homologous
to an Fc
domain from an antibody, wherein the Fc domain from an antibody comprises
amino acid
residues 216 to 447 of an IgG1 (within SEQ ID NO: 898), amino acid residues
216 to 443 of an
IgG2 (within SEQ ID NO: 899), or amino acid residues 216 to 444 of an IgG4
(within SEQ ID
NO: 900).
[0032] In certain embodiments, the Fc domain may have at least one amino acid
residue change
as compared to a wildtype Fc domain. In certain embodiments, the Fc domain may
have at least
one amino acid residue change as compared to a wildtype Fc domain, wherein the
Fc domain is
at least 80% homologous to SEQ ID NO: 898. The at least one amino acid residue
change may
be in an IgG Fc domain: a) F243L, R292P, Y300L, L235V, and P396L, wherein
numbering of
amino acid residues in the Fc domain is relative to SEQ ID NO:898; b) 5239D
and 1332E,
wherein numbering of amino acid residues in the Fc domain is relative to SEQ
ID NO: 898; or c)
5298A, E333A, and K334A, wherein numbering of amino acid residues in the Fc
domain is
relative to SEQ ID NO: 296. The Fc domain may have at least one amino acid
residue change as
-12-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
compared to wildtype, wherein the Fc domain is at comprises at least 80%
homologous to SEQ
ID NO: 898, and wherein the at least one amino acid residue change is: (a)
N296A wherein
numbering of amino acid residues in the Fc domain is relative to SEQ ID NO:
898; or (b) N296G
N296A wherein numbering of amino acid residues in the Fc domain is relative to
SEQ ID NO:
898; or (c) K322A/L234A/L235A N296A wherein numbering of amino acid residues
in the Fc
domain is relative to SEQ ID NO: 898; or (d) L234F/L235E/P331S N296A wherein
numbering
of amino acid residues in the Fc domain is relative to SEQ ID NO: 898.
[0033] In certain embodiments, the Kd for binding of the antigen binding
domain to the first
antigen in the presence of the immune-stimulatory compound is less than about
100 nM and is
equal to, or up to no greater than about 10 times the Kd of the binding of the
antigen binding
domain to the first antigen in the absence of the immune-stimulatory compound;
and the Kd for
binding of the Fc domain to the Fc receptor in the presence of the immune-
stimulatory compound
is equal to, or up to no greater than about 10 times the Kd for the binding of
the Fc domain to the
Fc receptor in the absence of the immune-stimulatory compound.
[0034] In certain embodiments, the molar ratio of immune-stimulatory compound
to antibody in
a conjugate is less than 5. In certain embodiments, the average molar ratio of
immune-
stimulatory compound to antibody construct in a composition of conjugates is
less than 5. In
certain embodiments, the average molar ratio of immune-stimulatory compound to
antibody
construct in a composition of conjugates from 1 to 3, 3 to 5, or about 2.
[0035] The linker may be bound to the antibody construct at an amino acid
residue that does not
interfere with the Fc domain binding to the Fc receptor. The linker may not be
attached to an
amino acid residue of an IgG Fc domain selected from a group consisting of:
221, 224, 227, 228,
230, 231, 223, 233, 234, 235, 236, 237, 238, 239, 240, 241, 243, 244, 245,
247, 249, 250, 258,
262, 263, 264, 265, 266, 267, 268, 269, 270, 271, 272, 273, 275, 276, 278,
280, 281, 283, 285,
286, 291, 292, 293, 294, 295, 296, 297, 298, 299, 300, 302, 305, 313, 318,
323, 324, 325, 327,
328, 329, 330, 331, 332, 333, 335, 336, 396, or 428, wherein numbering of
amino acid residues
in the Fc domain is according to the EU index as in Kabat.
[0036] The linker is bound to the antibody construct at an amino acid of an
IgG Fc domain
selected from a group consisting of: 221, 224, 227, 228, 230, 231, 223, 233,
234, 235, 236, 237,
238, 239, 240, 241, 243, 244, 245, 247, 249, 250, 258, 262, 263, 264, 265,
266, 267, 268, 269,
270, 271, 272, 273, 275, 276, 278, 280, 281, 283, 285, 286, 291, 292, 293,
294, 295, 296, 297,
298, 299, 300, 302, 305, 313, 318, 323, 324, 325, 327, 328, 329, 330, 331,
332, 333, 335, 336,
396, or 428, wherein numbering of amino acid residues in the Fc domain is
according to the EU
index as in Kabat.
-13-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
[0037] In certain embodiments, the Fc domain is an IgG Fc domain selected from
a group
consisting of a human IgG1 Fc domain, a human IgG2 Fc domain, a human IgG3 Fc
domain, and
a human IgG4 Fc domain. In certain aspects, the immune-stimulatory conjugate
induces the
secretion of cytokines by an antigen presenting cell.
[0038] In certain aspects, the disclosure provides a pharmaceutical
composition comprising
immune-stimulatory conjugates as described herein and a pharmaceutically
acceptable excipient.
In certain aspects, the disclosure provides a method of treating cancer,
comprising administering
to a subject in need thereof an immune-stimulatory conjugate described herein.
In certain aspects,
the disclosure provides a method of treating cancer, comprising administering
to a subject in
need thereof a pharmaceutical composition comprising immune-stimulatory
conjugates as
described herein.
BRIEF DESCRIPTION OF THE DRAWINGS
[0039] The novel features of the disclosure are set forth with particularity
in the appended
claims. A better understanding of the features and advantages of the present
disclosure will be
obtained by reference to the following detailed description that sets forth
illustrative aspects, in
which the principles of the disclosure are utilized, and the accompanying
drawings of which:
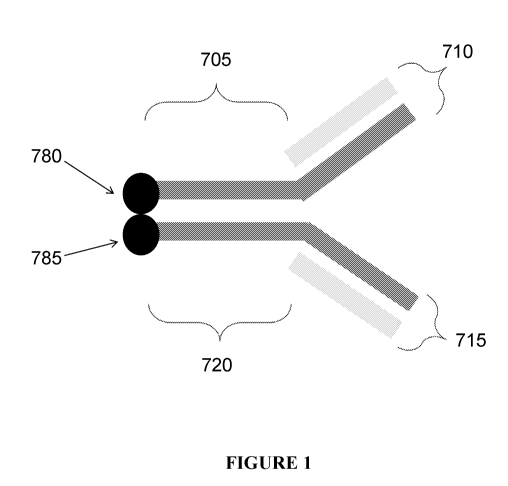
[0040] FIGURE 1 illustrates a schematic of a conjugate comprising an antibody
and a second
binding domain. The antibody contains two heavy chains as shown in gray and
two light chains
as shown in light gray. A portion of the heavy chains contain Fc domains (705
and 720). The
antibody comprises a binding domain comprising two antigen binding sites (710
and 715). The
second binding domain is attached to the antibody (780 and 785), for example,
at the C-terminus
of the heavy chains.
[0041] FIGURE 2 illustrates a schematic of an exemplary conjugate. The
conjugate comprises
an antibody, which contains two heavy chains as shown in gray and two light
chains as shown in
light gray. The antibody comprises a binding domain comprising two antigen
binding sites (910
and 915), and a portion of the heavy chains contain Fc domains (905 and 920).
The immune-
stimulatory compounds (930 and 940) are attached to the antibody by linkers
(960 and 970). A
second binding domain is attached to the antibody (980 and 985), for example,
at the C-terminus
of the heavy chains.
[0042] FIGURE 3 illustrates a schematic of an exemplary conjugate. The
conjugate comprises
the Fc region of an antibody with the heavy chains shown in gray, and two
scaffolds as shown in
light gray. The conjugate comprises a first binding domain comprising two
antigen binding sites
(1110 and 1115) in the scaffolds, and a portion of the heavy chains contain Fc
domains (1105 and
1120). The immune-stimulatory compounds (1130 and 1140) are conjugated to the
Fc domains
-14-
CA 03065919 2019-12-02
WO 2018/227023
PCT/US2018/036560
by linkers (1160 and 1170). A second binding domain is attached to the
conjugate (1180 and
1185), for example, at the C-terminus of the heavy chains.
[0043] FIGURE 4 illustrates a schematic of an exemplary conjugate. The
conjugate comprises
the F(ab')2 region of an antibody with the Fab portions of heavy chains shown
in gray and light
chains shown in light gray, and two scaffolds as shown in dark gray. The
conjugate comprises a
first binding domain comprising two antigen binding sites (1310 and 1315), and
a portion of two
scaffolds contain Fc domains (1340 and 1345). The immune-stimulatory compounds
(1330 and
1340) are attached to the conjugate by linkers (1360 and 1370). A second
binding domain is
attached to the Fc domains (1380 and 1385).
[0044] FIGURE 5 illustrates a schematic of an exemplary conjugate. The
conjugate contains
two scaffolds as shown in light gray and two scaffolds as shown in dark gray.
The conjugate
comprises a first binding domain comprising two antigen binding sites (1510
and 1515), and a
portion of the two dark gray scaffolds contain Fc domains (1540 and 1545). The
immune-
stimulatory compounds (1530 and 1540) are conjugated to the conjugate by
linkers (1560 and
1570). A second binding domain is attached to the conjugate (1580 and 1585).
[0045] FIGURE 6 illustrates a schematic of a conjugate comprising an antibody
and a second
binding domain. The antibody contains two heavy chains as shown in gray and
two light chains
as shown in light gray. A portion of the heavy chains contain Fc domains (1705
and 1720). The
antibody comprises a binding domain comprising two antigen binding sites (1710
and 1715). The
second binding domain is attached to the antibody (1780 and 1785), for
example, at the C-
terminus of the light chains.
[0046] FIGURE 7 illustrates a schematic of an exemplary conjugate. The
conjugate comprises
an antibody, which contains two heavy chains as shown in gray and two light
chains as shown in
light gray. The antibody comprises a binding domain comprising two antigen
binding sites (1910
and 1915), and a portion of the heavy chains contain Fc domains (1905 and
1920). The immune-
stimulatory compounds (1930 and 1940) are conjugated to the antibody by
linkers (1960 and
1970). A second binding domain is attached to the antibody (1980 and 1985),
for example, at the
C-terminus of the light chains.
[0047] FIGURE 8 illustrates a schematic of an exemplary conjugate. The
conjugate comprises
an Fc region of an antibody shown in gray, and two scaffolds as shown in light
gray. The
conjugate comprises a first binding domain comprising two antigen binding
sites (2110 and
2115) in the scaffolds, and a portion containing Fc domains (2105 and 2120).
The immune-
stimulatory compounds (2130 and 2140) are conjugated to the antibody construct
by linkers
(2160 and 2170). A second binding domain is attached to the antibody (2180 and
2185).
-15-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
[0048] FIGURE 9 illustrates a schematic of an exemplary conjugate. The
conjugate comprises
the F(ab')2 region of an antibody with heavy chains shown in gray and light
chains shown in light
gray, and two scaffolds as shown in dark gray. The conjugate comprises a first
binding domain
comprising two antigen binding sites (2310 and 2315), and a portion of two
scaffolds contain Fc
domains (2340 and 2345). The immune-stimulatory compounds (2330 and 2340) are
conjugated
to the antibody by linkers (2360 and 2370). A second binding domain is
attached to the antibody
(2380 and 2385), for example, at the C-terminus of the light chains.
[0049] FIGURE 10 illustrates a schematic of an exemplary conjugate. The
conjugate contains
two scaffolds as shown in light gray and two scaffolds as shown in dark gray.
The conjugate
comprises a first binding domain comprising two antigen binding sites (2510
and 2515), and a
portion of the two dark gray scaffolds contain Fc domains (2540 and 2545). The
immune-
stimulatory compounds (2530 and 2540) are conjugated to the antibody construct
by linkers
(2560 and 2570). A second binding domain is attached to the conjugate (2580
and 2585).
[0050] FIGURE 11 illustrates a schematic of an antibody construct comprising
an antibody. The
antibody contains two heavy chains and two light chains. A portion of the
heavy chains contain
Fc domains (2705 and 2720). The antibody comprises a binding domain comprising
two antigen
binding sites shown in black (2710 and 2715).
[0051] FIGURE 12 illustrates a schematic of an antibody construct comprising
an antibody. The
antibody contains two heavy chains and two light chains. A portion of the
heavy chains contain
Fc domains (2925 and 2930). The antibody comprises a first binding domain
comprising two
antigen binding sites shown in black (2910 and 2915). The antibody comprises a
second binding
domain comprising two single chain variable fragments (2905 and 2920) attached
to a C-
terminus of the light chains. A single chain variable fragment can be attached
to a light chain
chain at a heavy chain variable domain of the single chain variable fragment.
A single chain
variable fragment can be attached to a light chain at a light chain variable
domain of the single
chain variable fragment.
[0052] FIGURE 13 illustrates a schematic of an antibody construct comprising
an antibody. The
antibody contains two heavy chains and two light chains. A portion of the
heavy chains contain
Fc domains (3120 and 3125). The antibody comprises a first binding domain
comprising two
antigen binding sites shown in black (3110 and 3115). The antibody comprises a
second binding
domain comprising two single chain variable fragments (3130 and 3135) attached
to a C-
terminus of the heavy chains. A single chain variable fragment can be attached
to a heavy chain
chain at a heavy chain variable domain of the single chain variable fragment.
A single chain
variable fragment can be attached to a heavy chain at a light chain variable
domain of the single
chain variable fragment.
-16-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
[0053] FIGURE 14 illustrates a schematic of an antibody construct comprising
an antibody. The
antibody contains two heavy chains and two light chains. A portion of the
heavy chains contain
Fc domains (3330 and 3335). The antibody comprises a first binding domain
comprising two
antigen binding sites shown in black (3310 and 3315). The antibody comprises a
second binding
domain comprising two single chain variable fragments (3320 and 3325) attached
to a C-
terminus of the light chains. A single chain variable fragment can be attached
to a light chain
chain at a heavy chain variable domain of the single chain variable fragment.
A single chain
variable fragment can be attached to a light chain at a light chain variable
domain of the single
chain variable fragment. The antibody comprises a third binding domain
comprising two single
chain variable fragments (3340 and 3345) attached to a C-terminus of the heavy
chains. A single
chain variable fragment can be attached to a heavy chain chain at a heavy
chain variable domain
of the single chain variable fragment. A single chain variable fragment can be
attached to a heavy
chain at a light chain variable domain of the single chain variable fragment.
[0054] FIGURE 15A sets forth a structure of an immune-stimulatory compound
(left) and an
immune-stimulatory compound with an amine handle (right top).
[0055] FIGURE 15B shows the x-ray crystal structure and binding orientation
(pdb code 5D7A)
of the immune-stimulatory compound as described in FIGURE 15A in a TNIK (TRAF2
and
NCK-interacting protein kinase) active site. Specific interactions between the
inhibitor and the
TNIK active site are described in Masuda et al., TNIK inhibition abrogates
colorectal cancer
sternness, (2016) Nat Commun 7: 12586-12586.
[0056] FIGURE 15C shows a close up of the binding orientation of the immune-
stimulatory
compound as described in FIGURE 15A in a TNIK active site, where the linker
and antibody
portions are pointing away and sitting outside of the active site.
[0057] FIGURE 16 sets forth a structure of an immune-stimulatory compound
(left) and an
immune-stimulatory compound with an amine handle and linker surrogate (right).
The structure
on the right illustrates that the immune-stimulatory compound is predicted to
sit in the enzyme
active site, whereas the amine handle and linker surrogate are predicted to
sit outside of the
enzyme active site, in the solvent.
[0058] FIGURE 17A sets forth a structure of an immune-stimulatory compound
(left) and an
immune-stimulatory compound with a linker surrogate (left side of molecule on
right).
[0059] FIGURE 17B shows the x-ray crystal structure and binding orientation
(pdb code 5E91)
of the immune-stimulatory compound as described in FIGURE 17A in a TGFPR2
(transforming
growth factor, beta receptor II) active site. Specific interactions between
the inhibitor and the
TGFPRII active site are described in Tebben et al., Crystal structures of apo
and inhibitor-bound
-17-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
TGFbetaR2 kinase domain: insights into TGFbetaR isoform selectivity, (2016)
Acta Crystallogr.,
Sect.D 72: 658-674.
[0060] FIGURE 17C shows a close up of the binding orientation of the immune-
stimulatory
compound as described in FIGURE 17A in a TGFPR2 active site, where the linker
and antibody
construct portions are pointing away and sitting outside of the active site.
[0061] FIGURE 18A sets forth a structure of an immune-stimulatory compound
(left) and an
immune-stimulatory compound with an amine handle (upper left in molecule on
right).
[0062] FIGURE 18B shows the x-ray crystal structure and binding orientation
(pdb code 3KR8)
of the immune-stimulatory compound as described in FIGURE 18A in a TNKS
(tankyrase)
active site.
[0063] FIGURE 18C shows a close up of the binding orientation of the immune-
stimulatory
compound as described in FIGURE 18A in a TNKS active site, where the linker
and antibody
construct portions are pointing away and sitting outside of the active site.
Specific interactions
between the inhibitor and the human tankyrase 2 - catalytic PARP domain active
site are
described in Karlberg et al., Structural basis for the interaction between
tankyrase-2 and a potent
Wnt-signaling inhibitor, (2010) J.Med.Chem. 53: 5352-5355.
[0064] FIGURE 19A shows a structure of an immune-stimulatory compound (left)
and an
immune-stimulatory compound with two amine handles (on right side of molecule
on the right).
[0065] FIGURE 19B shows the x-ray crystal structure and binding orientation
(pdb code 4191)
of the immune-stimulatory compound as described in FIGURE 19A with dual site
binding in the
TNKS (tankyrase) active site. Specific interactions between the inhibitor and
human tankyrase 1
are described in Bregman et al, Discovery of a class of novel tankyrase
inhibitors that bind to
both the nicotinamide pocket and the induced pocket, (2013) J.Med.Chem. 56:
1341-1345.
[0066] FIGURE 19C shows a close up of the binding orientation of the immune-
stimulatory
compound as described in FIGURE 19A in a TNKS active site, where the linker
and antibody
portions are pointing away and sitting outside of the active site.
[0067] FIGURE 20A illustrates a schematic of an exemplary conjugate and its
molecular target.
The conjugate comprises an antibody (3405) attached to a linker (3410) that is
attached to a drug
(3415) at the opposite end of the antibody (3405). The molecular target (3420)
has an active site
(3425) that is complementary to the drug (3415).
[0068] FIGURE 20B illustrates a schematic of an active exemplary conjugate
that is bound to
the the molecular target's active site. The drug (3415) sits within the active
site of the molecular
target (3420). The linker (3410) and antibody (3405) sit outside of the active
site (3430).
[0069] FIGURE 20C illustrates a schematic of an active drug (3415) and linker
(3410) that is
bound to the molecular target (3420).
-18-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
[0070] FIGURE 20D illustrates a schematic of an active drug (3415) that is
bound to the
molecular target (3420).
[0071] FIGURE 21A, FIGURE 21B, and FIGURE 21C show the results of an assay for
degradation of TFGf3R2 by a TGFPR2-VHL PROTAC anti-HER2 antibody conjugate.
[0072] FIGURE 22A and FIGURE 22B show the results of an assay for antigen
targeted
degradation of TGFPR2 by an antibody conjugate with a PROTAC having VHL or
Cereblon E3
binding moieties.
[0073] FIGURE 23A and FIGURE 23B show the results of an assay for cellular
levels of
TGFPR2 and TGFPR1 in the presence of a TGFPR2/TGFPR1-VHL PROTAC with or
without
the addition of a proteasome inhibitor.
DETAILED DESCRIPTION
[0074] Additional aspects and advantages of the present disclosure will become
apparent to those
skilled in this art from the following detailed description, wherein
illustrative aspects of the
present disclosure are shown and described. As will be realized, the present
disclosure is capable
of other and different aspects, and its several details are capable of
modifications in various
respects, all without departing from the disclosure. Accordingly, the drawings
and description are
to be regarded as illustrative in nature, and not as restrictive.
[0075] As used herein, "potency" generally refers to measured bioactivity and
may be quantified
as an EC50 or IC50. Potency refers to the amount of a compound or conjugate
needed to give an
effect. For example, the potency of an immune-stimulatory compound which
requires a lower
amount of the immune-stimulatory compound compared with a different immune-
stimulatory
compound can be considered to have greater potency. Furthermore, the different
immune-
stimulatory compound requires a greater amount of the different immune-
stimulatory compound
to generate a response, and can therefore be considered lower potency.
Potencies of bioactive
compounds or conjugates may be measured over a concentration range and can be
reported as
those molar concentrations required to elicit or inhibit a percentage of the
measured bioresponse.
For example, a concentration required to stimulate 50% of observed maximal
activity in the
assay may be reported as an effective concentration 50 (EC50), to stimulate
90% activity as an
EC90, or to stimulate 10% activity as an EC10. For example, a concentration of
an antagonist
required to give 50% maximal inhibition of a biological activity may be
reported as an inhibitory
concentration 50 (IC50), to inhibit 90% as an IC90, or to inhibit 10% as an
IC10. This may allow
for a comparison of the potencies of bioactive compounds on a molar basis by
comparison of
their EC or IC values for a given bioassay. For example, an immune-stimulatory
conjugate or an
immune-stimulatory compound with an EC50 or IC50 that is greater than 300
times the EC50 or
-19-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
IC50 of a control requires 300-fold higher, or more than 300-fold higher,
concentration compared
to the control to achieve a 50% bioresponse and has a potency weaker than the
control by at least
300-fold. Therefore, an immune-stimulatory compound or conjugate that has an
EC50 or IC50
not greater than about 300 times the EC50 or IC50 of a control compound may
require no more
than a 300-fold higher concentration than the control compound to achieve a
50% maximal
bioresponse, no greater than 100 times the EC50 or IC50 requires no more than
100-fold higher
concentration and no greater than 10 times the EC50 or IC50 requires no more
than 10 times the
concentration of the control. The potency of the immune-stimulatory compound
or conjugate
may be within 300-fold or better, 100-fold or better, or 10-fold or better the
potency of the
control.
[0076] As used herein, "control compound" refers to, in a case of an immune-
stimulatory
compound, the compound before attachment to a linker and antibody construct;
in the case of an
antibody construct, the construct before attachment of the linker and immune-
stimulatory
compound; or, in the case of a conjugate including an E3 ubiquitin ligase
binding moiety, control
compound refers to the immune-stimulatory compound attached to a linker that
is attached to an
E3 ubiquitin ligase binding moiety.
[0077] An "immune-stimulatory compound" as described herein, also referred to
herein as an
immune-stimulatory agent or molecule, refers to a molecule that stimulates the
immune system
by inducing activation or increasing activity of any of its components. For
example, an immune
stimulatory compound may bind directly to a component of the immune system and
activate or
increase an activity of the immune system or a component thereof. An immune
stimulatory
compound may also bind to an inhibitory component of the immune system and
inactivate or
decrease the activity of that component, thereby increasing an activity of the
immune system.
Immune-stimulatory compounds may have or cause antigenic specificity. In
certain embodiments,
an immune-stimulatory compound activates macrophages. In certain embodiments,
an immune-
stimulatory compound induces the secretion of cytokines by an antigen
presenting cell. The
increased activity or activation of the immune system may be evaluated using
methods known in
the field for evaluating an immune response (see, for example, J. Gratama,
Cytometry A. 2008
Nov: 73(11): 971-4, the contents of which are incorporated herein in its
entirety).
[0078] A "protein active site" as described herein, refers to a region of a
protein target where a
molecule (such as a substrate molecule or allosteric effector) binds to the
target protein. The
molecule may trigger a response upon binding to the the binding protein, e.g.,
inhibition of the
activity of the binding protein or degradation of the binding protein. The
protein active site
includes the residues that interact with, e.g., bind covalently or non-
covalently, a molecule such
as an immune-stimulatory compound or a portion thereof. The protein active
site may define a
-20-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
groove or pocket in the target protein wherein the interface between the
groove or pocket and
surrounding solvent is the solvent/active site interface of the protein active
site.
[0079] The phrase "stimulate an immune response" refers to stimulating a
response from
immune cells that increases the activity or responsiveness of the immune
system or a part thereof,
such as, for example, inducing secretion of cytokines and/or chemokines,
activating immune
cells, e.g., T cells, B cells, macrophages, dendritic cells, etc., enhancing
antigen presenting cell
presentation of antigen, inducing antibody-dependent cell-mediated
phagocytosis (ADCP),
inducing antibody-dependent cell-mediated cytotoxicity (ADCC), NK cell or CD8+
T cell
cytolytic activity, and/or blocking immune suppression.
[0080] As used herein, "homologous" or "homology" refer to the similarity or
identity between a
DNA, RNA, nucleotide, amino acid, or protein sequence to another DNA, RNA,
nucleotide,
amino acid, or protein sequence, respectively. Homology can be expressed in
terms of a
percentage of sequence identity of a first sequence to a second sequence.
Percent (%) sequence
identity with respect to a reference DNA sequence is the percentage of DNA
nucleotides in a
candidate sequence that are identical with the DNA nucleotides in the
reference DNA sequence after
aligning the sequences and introducing gaps, as necessary. Percent (%)
sequence identity with respect
to a reference amino acid sequence is the percentage of amino acid residues in
a candidate sequence
that are identical with the amino acid residues in the reference amino acid
sequence after aligning the
sequences and introducing gaps, if necessary, to achieve the maximum percent
sequence identity, and
not considering any conservative substitutions as part of the sequence
identity.
[0081] As used herein, the abbreviations for the natural 1-enantiomeric amino
acids are
conventional and can be as follows: alanine (A, Ala); arginine (R, Arg);
asparagine (N, Asn);
aspartic acid (D, Asp); cysteine (C, Cys); glutamic acid (E, Glu); glutamine
(Q, Gln); glycine (G,
Gly); histidine (H, His); isoleucine (I, Ile); leucine (L, Leu); lysine (K,
Lys); methionine (M,
Met); phenylalanine (F, Phe); proline (P, Pro); serine (S, Ser); threonine (T,
Thr); tryptophan (W,
Trp); tyrosine (Y, Tyr); valine (V, Val). Unless otherwise specified, X can
indicate any amino
acid. In some aspects, X can be asparagine (N), glutamine (Q), histidine (H),
lysine (K), or
arginine (R).
[0082] As used herein, the term "antibody" refers to an immunoglobulin
molecule that
specifically binds to, or is immunologically reactive toward, a specific
antigen. The term
antibody can include, for example, polyclonal, monoclonal, genetically
engineered, and antigen
binding fragments thereof. An antibody can be, for example, murine, chimeric,
humanized,
bispecific, a heteroconjugate, a diabody, a triabody, a tetrabody or a hcAb
(heavy chain
antibody). An antigen binding fragment can include, for example, a Fab',
F(ab')2, Fab, Fv, rIgG,
scFv, a single domain antibody, VHH, VNAR, sdAb, or nanobody.
-21-
CA 03065919 2019-12-02
WO 2018/227023
PCT/US2018/036560
[0083] As used herein, the term "antigen" refers to a molecule that can be
bound by an antibody
or a antibody construct. An antigen may elicit an immune response. An antigen
can be a protein,
polysaccharide, lipid, glycolipid or the like, which can be recognized by an
antibody or an
immune cell, such as a T cell or a B cell. Exposure of immune cells to one or
more of these
antigens can elicit a rapid cell division and differentiation response
resulting in the formation of
clones of the exposed T cells and B cells. B cells can differentiate into
plasma cells which in turn
can produce antibodies which selectively bind to the antigens.
[0084] As used herein, the terms "recognize," "bind," and "specifically bind"
refer to the specific
association or specific binding between an antigen binding domain and a
corresponding antigen,
or an immune stimulatory compound and a protein, as compared to the non-
specific association
or non-specific binding of the antigen binding domain or immune-stimulatory
compound with a
non-target antigen or protein.
[0085] As used herein, a "tumor antigen" is an antigen that can be expressed
by or is present on,
a cancer cell, a neoplastic tumor cell and/or within a tumor microenvironment.
[0086] As used herein, an "antibody construct" refers to a construct that
contains an antigen
binding domain and an Fc domain.
[0087] As used herein, an "antigen binding domain" refers to an antigen
binding domain from an
antibody or from a non-antibody that can specifically bind to the antigen.
Antigen binding
domains can be numbered when there is more than one antigen binding domain in
a given
conjugate or construct (e.g., first binding domain, second antigen binding
domain, third antigen
binding domain, etc.). Different antigen binding domains in the same conjugate
or construct can
target the same antigen or different antigens (e.g., first binding domain that
can bind a tumor
antigen, second antigen binding domain that can bind to a tumor antigen or an
antigen presenting
cell (APC) antigen, and third antigen binding domain that can bind an APC
antigen).
[0088] As used herein, a "Fe domain" is an Fc domain from an antibody or from
a non-antibody
that can bind to an Fc receptor.
[0089] As used herein, an "Fe null" refers to an Fc domain that exhibits weak
to no binding to
any of the Fcgamma receptors. In some embodiments, an Fc null domain or region
exhibits a
reduction in binding affinity (e.g., increase in Kd) to Fc gamma receptors of
at least 1000-fold.
[0090] As used herein, a "target binding domain" refers to a construct that
contains an antigen
binding domain from an antibody or from a non-antibody that can bind to the
antigen.
[0091] The term "salt" or "pharmaceutically acceptable salt" refers to salts
derived from a
variety of organic and inorganic counter ions well known in the art.
Pharmaceutically acceptable
acid addition salts can be formed with inorganic acids and organic acids.
Inorganic acids from
which salts can be derived include, for example, hydrochloric acid,
hydrobromic acid, sulfuric
-22-
CA 03065919 2019-12-02
WO 2018/227023
PCT/US2018/036560
acid, nitric acid, phosphoric acid, and the like. Organic acids from which
salts can be derived
include, for example, acetic acid, propionic acid, glycolic acid, pyruvic
acid, oxalic acid, maleic
acid, malonic acid, succinic acid, fumaric acid, tartaric acid, citric acid,
benzoic acid, cinnamic
acid, mandelic acid, methanesulfonic acid, ethanesulfonic acid, p-
toluenesulfonic acid, salicylic
acid, and the like. Pharmaceutically acceptable base addition salts can be
formed with inorganic
and organic bases. Inorganic bases from which salts can be derived include,
for example, sodium,
potassium, lithium, ammonium, calcium, magnesium, iron, zinc, copper,
manganese, aluminum,
and the like. Organic bases from which salts can be derived include, for
example, primary,
secondary, and tertiary amines, substituted amines including naturally
occurring substituted
amines, cyclic amines, basic ion exchange resins, and the like, specifically
such as
isopropylamine, trimethylamine, diethylamine, triethylamine, tripropylamine,
and ethanolamine.
In some embodiments, the pharmaceutically acceptable base addition salt is
chosen from
ammonium, potassium, sodium, calcium, and magnesium salts.
[0092] As used herein, "agonism" is the binding of a chemical to a receptor to
induce a
biological response. A chemical can be, for example, a small molecule, a
compound, or a protein.
An agonist causes a response, an antagonist can block the action of an
agonist, and an inverse
agonist can cause a response that is opposite to that of the agonist. A
receptor can be activated by
either endogenous or exogenous agonists.
[0093] The term "Cx_y" when used in conjunction with a chemical moiety, such
as alkyl, alkenyl,
or alkynyl is meant to include groups that contain from x to y carbons in the
chain. For example,
the term "Cx_yalkyl" refers to substituted or unsubstituted saturated
hydrocarbon groups,
including straight-chain alkyl and branched-chain alkyl groups that contain
from x to y carbons
in the chain, including haloalkyl groups such as trifluoromethyl and 2,2,2-
trifluoroethyl, etc.
[0094] The terms "Cx_yalkenyl" and "Cx_yalkynyl" refer to substituted or
unsubstituted
unsaturated aliphatic groups analogous in length and possible substitution to
the alkyls described
above, but that contain at least one double or triple bond, respectively.
[0095] The term "carbocycle" as used herein refers to a saturated, unsaturated
or aromatic ring in
which each atom of the ring is carbon. Carbocycle includes 3- to 10-membered
monocyclic rings,
6- to 12-membered bicyclic rings, and 6- to 12-membered bridged rings. Each
ring of a bicyclic
carbocycle may be selected from saturated, unsaturated, and aromatic rings. In
an exemplary
embodiment, an aromatic ring, e.g., phenyl, may be fused to a saturated or
unsaturated ring, e.g.,
cyclohexane, cyclopentane, or cyclohexene. Any combination of saturated,
unsaturated and
aromatic bicyclic rings, as valence permits, is included in the definition of
carbocyclic.
Exemplary carbocycles include cyclopentyl, cyclohexyl, cyclohexenyl,
adamantyl, phenyl,
indanyl, and naphthyl.
-23-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
[0096] The term "heterocycle" as used herein refers to a saturated,
unsaturated or aromatic ring
comprising one or more heteroatoms. Exemplary heteroatoms include N, 0, Si, P,
B, and S
atoms. Heterocycles include 3- to 10-membered monocyclic rings, 6- to 12-
membered bicyclic
rings, and 6- to 12-membered bridged rings. Each ring of a bicyclic
heterocycle may be selected
from saturated, unsaturated, and aromatic rings wherein at least one of the
rings includes a
heteroatom. In an exemplary embodiment, an aromatic ring, e.g., pyridyl, may
be fused to a
saturated or unsaturated ring, e.g., cyclohexane, cyclopentane, morpholine,
piperidine or
cyclohexene. The term "heteroaryl" includes aromatic single ring structures,
preferably 5- to 7-
membered rings, more preferably 5- to 6-membered rings, whose ring structures
include at least
one heteroatom, preferably one to four heteroatoms, more preferably one or two
heteroatoms.
The term "heteroaryl" also include polycyclic ring systems having two or more
cyclic rings in
which two or more carbons are common to two adjoining rings wherein at least
one of the rings
is heteroaromatic, e.g., the other cyclic rings can be aromatic or non-
aromatic carbocyclic, or
heterocyclic. Heteroaryl groups include, for example, pyrrole, furan,
thiophene, imidazole,
oxazole, thiazole, pyrazole, pyridine, pyrazine, pyridazine, and pyrimidine,
and the like.
[0097] The term "substituted" refers to moieties having substituents replacing
a hydrogen on one
or more carbons or substitutable heteroatoms, e.g., NH, of the structure. It
will be understood that
"substitution" or "substituted with" includes the implicit proviso that such
substitution is in
accordance with permitted valence of the substituted atom and the substituent,
and that the
substitution results in a stable compound, i.e., a compound which does not
spontaneously
undergo transformation such as by rearrangement, cyclization, elimination,
etc. In certain
embodiments, substituted refers to moieties having substituents replacing two
hydrogen atoms on
the same carbon atom, such as substituting the two hydrogen atoms on a single
carbon with an
oxo, imino or thioxo group. As used herein, the term "substituted" is
contemplated to include all
permissible substituents of organic compounds. In a broad aspect, the
permissible substituents
include acyclic and cyclic, branched and unbranched, carbocyclic and
heterocyclic, aromatic and
non-aromatic substituents of organic compounds. The permissible substituents
can be one or
more and the same or different for appropriate organic compounds. For purposes
of this
disclosure, the heteroatoms such as nitrogen may have hydrogen substituents
and/or any
permissible substituents of organic compounds described herein which satisfy
the valences of the
heteroatoms.
[0098] In some embodiments, substituents raay include any substituents
described herein, for
example: halogen, hydroxy, oxo (=0), thioxo (=S), cyano (-CN), nitro (-NO2),
imino (=N-H),
oximo (=N-OH), hydrazino (=N-
NH2), -Rb-ORa, -Rb-OC(0)-Ra, -Rb-OC(0)-0Ra, -Rb-OC(0)-N(R1)2, -Rb-N(R1)2, -Rb-
C(0)Ra, -Rb
-24-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
-C(0)0Ra, -Rb-C(0)N(R1)2, -Rb-0-12c-C(0)N(Ra)2, -Rb-N(Ra)C(0)0Ra, -Rb-
N(R1)C(0)Ra, -Rb-N
(Ra)S(0)Ra (where t is 1 or 2), -Rb-S(0)tRa (where t is 1 or 2), -Rb-S(0)tORa
(where t is 1 or 2),
and -Rb-S(0)tN(R1)2 (where t is 1 or 2); and alkyl, alkenyl, alkynyl, aryl,
aralkyl, aralkenyl,
aralkynyl, cycloalkyl, cycloalkylalkyl, heterocyclo alkyl,
heterocycloalkylalkyl, heteroaryl, and
heteroarylalkyl any of which may be optionally substituted by alkyl, alkenyl,
alkynyl, halogen,
haloalkyl, haloalkenyl, haloalkynyl, oxo (=0), thioxo (=S), cyano (-CN), nitro
(-NO2), imino
(=N-H), oximo (=N-OH), hydrazine (=N-
NH2), -Rb-ORa, -Rb-OC(0)-Ra, -Rb-OC(0)-0Ra, -Rb-OC(0)-N(R1)2, -Rb_N(R1)2,
_Rb_c(0)Ra, _Rb
-C(0)0Ra, -Rb-C(0)N(R1)2, -Rb-0-12c-C(0)N(Ra)2, -Rb-N(Ra)C(0)0Ra, -Rb-
N(R1)C(0)Ra, -Rb-N
(Ra)S(0)Ra (where t is 1 or 2), -Rb-S(0)tRa (where t is 1 or 2), -Rb-S(0)tORa
(where t is 1 or 2)
and -Rb-S(0)tN(R1)2 (where t is 1 or 2); wherein each Ra is independently
selected from
hydrogen, alkyl, cycloalkyl, cycloalkylalkyl, aryl, aralkyl, heterocycloalkyl,
heterocycloalkylalkyl, heteroaryl, or heteroarylalkyl, wherein each Ra,
valence permitting, may
be optionally substituted with alkyl, alkenyl, alkynyl, halogen, haloalkyl,
haloalkenyl,
haloalkynyl, oxo (=0), thioxo (=S), cyano (-CN), nitro (-NO2), imino (=N-H),
oximo (=N-OH),
hydrazine (=N-
NH2), -Rb-ORa, -Rb-OC(0)-Ra, -Rb-OC(0)-0Ra, -Rb-OC(0)-N(R1)2, -Rb_N(R1)2,
_Rb_c(0)Ra, _Rb
-C(0)0Ra, -Rb-C(0)N(R1)2, -Rb-0-12c-C(0)N(Ra)2, -Rb-N(Ra)C(0)0Ra, -Rb-
N(R1)C(0)Ra, -Rb-N
(Ra)S(0)Ra (where t is 1 or 2), -Rb-S(0)tRa (where t is 1 or 2), -Rb-S(0)tORa
(where t is 1 or 2)
and -Rb-S(0)tN(R1)2 (where t is 1 or 2); and wherein each Rb is independently
selected from a
direct bond or a straight or branched alkylene, alkenylene, or alkynylene
chain, and each Rc is a
straight or branched alkylene, alkenylene or alkynylene chain.
[0099] It will be understood by those skilled in the art that substituents can
themselves be
substituted, if appropriate. Unless specifically stated as "unsubstituted,"
references to chemical
moieties herein are understood to include substituted variants. For example,
reference to a
"heteroaryl" group or moiety implicitly includes both substituted and
unsubstituted variants.
[0100] Chemical entities having carbon-carbon double bonds or carbon-nitrogen
double bonds
may exist in Z- or E- form (or cis- or trans- form). Furthermore, some
chemical entities may exist
in various tautomeric forms. Unless otherwise specified, chemical entities
described herein are
intended to include all Z-, E- and tautomeric forms as well.
[0101] A "tautomer" refers to a molecule wherein a proton shift from one atom
of a molecule to
another atom of the same molecule is possible. The compounds presented herein,
in certain
embodiments, exist as tautomers. In circumstances where tautomerization is
possible, a chemical
equilibrium of the tautomers will exist. The exact ratio of the tautomers
depends on several
-25-
CA 03065919 2019-12-02
WO 2018/227023
PCT/US2018/036560
factors, including physical state, temperature, solvent, and pH. Some examples
of tautomeric
y H
\\.
\11\r\
H H
\
N H2
N., NH2 \ NH v A )1/2,N
csss.N ciss H rrjS iSSS
ssi\J,N Ns
N N HN N NN'
'
csss N
OH
equilibrium include: N 0
[0102] The compounds, constructs and conjugates disclosed herein, in some
embodiments, are
used in different enriched isotopic forms, e.g., enriched in the content of
2H, 3H, 11,,, '3C and/or
14C. In one particular embodiment, the compound is deuterated in at least one
position. Such
deuterated forms can be made by the procedure described in U.S. Patent Nos.
5,846,514 and
6,334,997, or as otherwise known in the art. As described in U.S. Patent Nos.
5,846,514 and
6,334,997, deuteration can improve the metabolic stability and or efficacy,
thus increasing the
duration of action of drugs.
[0103] Unless otherwise stated, structures depicted herein are intended to
include compounds
which differ only in the presence of one or more isotopically enriched atoms.
For example,
compounds having the present structures except for the replacement of a
hydrogen by a
deuterium or tritium, or the replacement of a carbon by 13C- or 14C-enriched
carbon are within
the scope of the present disclosure.
[0104] The compounds of the present disclosure optionally contain unnatural
proportions of
atomic isotopes at one or more atoms that constitute such compounds. For
example, the
compounds may be labeled with isotopes, such as for example, deuterium (2H),
tritium (3H),
iodine-125 (1251) or carbon-14 (..,) Isotopic substitution with
2H, HC, 13C, 14C, 15C, 12N, 13N,
15N, 16N, 160, 170, 14F, 15F, 16F, 17F, 18F, 33s, 34s, 35s, 36¨,
S 35C1, 37C1, 79Br, 81Br, and 1251 are all
contemplated. All isotopic variations of the compounds of the present
invention, whether
radioactive or not, are encompassed within the scope of the present invention.
[0105] In certain embodiments, the compounds disclosed herein have some or all
of the 1H atoms
replaced with 2H atoms. The methods of synthesis for deuterium-containing
compounds are
-26-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
known in the art and include, by way of non-limiting example only, the
following synthetic
methods.
[0106] Deuterium substituted compounds are synthesized using various methods
such as
described in: Dean, Dennis C.; Editor. Recent Advances in the Synthesis and
Applications of
Radiolabeled Compounds for Drug Discovery and Development. [In: Curr., Pharm.
Des., 2000;
6(10)[ 2000, 110 pp; George W.; Varma, Rajender S. The Synthesis of
Radiolabeled Compounds
via Organometallic Intermediates, Tetrahedron, 1989, 45(21), 6601-21; and
Evans, E. Anthony.
Synthesis of radiolabeled compounds, J. Radioanal. Chem., 1981, 64(1-2), 9-32.
[0107] Deuterated starting materials are readily available and are subjected
to the synthetic
methods described herein or otherwise known to provide for the synthesis of
deuterium-
containing compounds. Large numbers of deuterium-containing reagents and
building blocks are
available commercially from chemical vendors, such as Aldrich Chemical Co.
[0108] Compounds also include crystalline and amorphous forms of those
compounds,
pharmaceutically acceptable salts, and active metabolites of these compounds
having the same
type of activity, including, for example, polymorphs, pseudopolymorphs,
solvates, hydrates,
unsolvated polymorphs (including anhydrates), conformational polymorphs, and
amorphous
forms of the compounds, as well as mixtures thereof.
[0109] The phrases "parenteral administration" and "administered parenterally"
as used herein
means modes of administration other than enteral and topical administration,
usually by injection,
and includes, without limitation, intravenous, intramuscular, intraarterial,
intrathecal,
intracapsular, intraorbital, intracardiac, intradermal, intraperitoneal,
transtracheal, subcutaneous,
subcuticular, intraarticular, subcapsular, subarachnoid, intraspinal and
intrasternal injection and
infusion.
[0110] The phrase "pharmaceutically acceptable" is employed herein to refer to
those
compounds, materials, compositions, and/or dosage forms which are, within the
scope of sound
medical judgment, suitable for use in contact with the tissues of human beings
and animals
without excessive toxicity, irritation, allergic response, or other problem or
complication,
commensurate with a reasonable benefit/risk ratio.
[0111] The phrase "pharmaceutically acceptable excipient" or "pharmaceutically
acceptable
carrier" as used herein means a pharmaceutically acceptable material,
composition or vehicle,
such as a liquid or solid filler, diluent, excipient, solvent or encapsulating
material. Each carrier
must be "acceptable" in the sense of being compatible with the other
ingredients of the
formulation and not injurious to the patient. Some examples of materials which
can serve as
pharmaceutically acceptable carriers include: (1) sugars, such as lactose,
glucose and sucrose; (2)
starches, such as corn starch and potato starch; (3) cellulose, and its
derivatives, such as sodium
-27-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
carboxymethyl cellulose, ethyl cellulose and cellulose acetate; (4) powdered
tragacanth; (5) malt;
(6) gelatin; (7) talc; (8) excipients, such as cocoa butter and suppository
waxes; (9) oils, such as
peanut oil, cottonseed oil, safflower oil, sesame oil, olive oil, corn oil and
soybean oil; (10)
glycols, such as propylene glycol; (11) polyols, such as glycerin, sorbitol,
mannitol and
polyethylene glycol; (12) esters, such as ethyl oleate and ethyl laurate; (13)
agar; (14) buffering
agents, such as magnesium hydroxide and aluminum hydroxide; (15) alginic acid;
(16) pyrogen-
free water; (17) isotonic saline; (18) Ringer's solution; (19) ethyl alcohol;
(20) phosphate buffer
solutions; and (21) other non-toxic compatible substances employed in
pharmaceutical
formulations.
[0112] As used herein, "attached" refers to a covalent bond, between two or
more groups.
Alternatively, attached may refer to the connection of two or more groups via
a linker, e.g., a
linker connecting a second binding domain to an antibody construct. A fusion
may refer to a
nucleic acid sequence of two separate domains being expressed in frame. For
example, a binding
domain can be attached as a fusion or by attachment (e.g., conjugation) via a
linker to an
antibody construct or other portion of a conjugate. For example, an antibody
can be fused with an
additional binding domain to create an antibody construct containing a fusion
of the antibody and
the additional binding domain. The antibody construct can be the result of the
nucleic acid
sequence of the binding domain being expressed in frame with the nucleic acid
sequence of the
antibody construct. The fusion can be the result of an in-frame nucleotide
sequence encoding the
antibody construct with the binding domain. As another example, an additional
binding domain
can be attached to an antibody construct via a linker, wherein the linker is
attached (i.e.,
conjugated) to the binding domain and the linker is attached to the antibody
construct. The
binding domain can be linked to the linker by a chemical conjugation and the
antibody construct
can be linked to the linker by a chemical conjugation. The additional binding
domain can be a
second binding domain and/or a third binding domain as described herein.
Furthermore, a
binding domain can be a first binding domain attached to an Fc domain to
produce the antibody
construct as described herein, which may produce the first binding domain as a
fusion with the
Fc domain or a conjugate wherein the first binding domain can be linked to a
linker and the
linker can be linked to the Fc domain.
Immune-Stimulatory Conjugates
[0113] Disclosed herein are conjugates of antibody constructs, immune-
stimulatory compounds
and linkers, referred to as antibody construct-immune-stimulatory compound
conjugates (also
referred to as immune-stimulatory compound-conjugates, antibody conjugates or
conjugates).
The conjugates may be used in the treatment of various diseases and disorders,
including cancers.
-28-
CA 03065919 2019-12-02
WO 2018/227023
PCT/US2018/036560
Also disclosed pharmaceutical compositions of the conjugates. In certain
embodiments,
immune-stimulatory compounds are attached either directly or through a linker
to an antibody
construct to form antibody construct-immune-stimulatory compound conjugates.
[0114] In some embodiments, the immune-stimulatory compound stimulates the
immune system,
or a component thereof. In some embodiments, the immune-stimulatory compound
has an
immune-modulatory activity. In some embodiments, the immune-stimulatory
compound has an
inhibitory effect on a component of the immune system, thereby stimulating an
immune-
modulatory activity.
[0115] In certain embodiments, conjugates are represented by the following
formula:
_
A
. Dx \
/n
- z
,
wherein A is an antibody construct, L is a linker, D is an immune-stimulatory
compound, x may
be from 1 to 20 (wherein each x denotes a separate compound), n may be from 1
to 20, and z
may be from 1 to 20. In some embodiments, L is a cleavable linker. In some
embodiments, L is
a non-cleavable linker.
[0116] In some embodiments, x is 1, n is 1 and z may be from 1 to 10, such as
from 1 to 9, such
as from 1 to 8, such as from 2 to 8, such as from 1 to 6, such as from 3 to 5
or such as about 2.
[0117] In some embodiments, x is 1, n is 2, and z may be from 1 to 10, such as
from 1 to 9, such
as from 1 to 8, such as from 2 to 8, such as from 1 to 6 or such as from 3 to
5. In certain
embodiments, z is 4.
[0118] In some embodiments, x may be from 1-20, n may be from 1-20, and z may
be from 1 to
20.
[0119] In certain embodiments, conjugates are represented by the following
formula:
_
A
. Dx \
/n
- z
,
wherein A is an antibody construct, L is a linker having the structure -Aa-Ww-
Yy-, where A is a
spacer, a is 0 or 1, W is a cleavable unit, w may be from 0 to 10, Y is a
stretcher, y may be from
0 to 3, D is an immunomodulatory compound, x may be from 1 to 20 (wherein each
x denotes a
distinct compound), n may be from 1 to 20, and z may be from 1 to 20.
-29-
CA 03065919 2019-12-02
WO 2018/227023
PCT/US2018/036560
[0120] In some embodiments, a is 1, w is 0, y is 0, x is 1, n is 1, and z may
be from 1 to 20, 1 to
10, 1 to 9, 1 to 8, 2 to 8, 1 to 6 or 3 to 5. In certain embodiments, z is 4.
[0121] In some embodiments, a isl, w is 1, y is from 1, xis 1, n is 1 and z
may be from 1 to 10, 1
to 9, 1 to 8, 2 to 8, 1 to 6, 3 to 5, 2 or 4.
[0122] In some embodiments, a is 0 or 1, w is from 0 to 10, y is from 0-3,
where at least one of a,
w or y is present, x may be from 1 to 20, n may be from 1-20, and z may be
from 1 to 20.
[0123] In some embodiments, the immune-stimulatory conjugates include a linker
(L) that may
comprise from 5 to 100 linear non-hydrogen atoms that is covalently attached
to an antibody
construct (A) and may be:
i) covalently attached to an immune-stimulatory compound (C1) as in the
following
formula:
A _________________________________
ii) covalently attached to an immune-stimulatory compound (C1) which itself
may be
covalently attached to a spacer (S) comprising from 5 to 100 linear non-
hydrogen atoms
covalently attached to a second immune-stimulatory compound (C2) as in the
following
formula:
A
iii) covalently attached an immune-stimulatory compound (C2) that may be
covalently
attached to a spacer (S) comprising from 5 to 100 linear non-hydrogen atoms
covalently
attached to a first immune-stimulatory compound (C1) as in the following
formula:
A
iv) covalently attached to a spacer (S) comprising from 5 to100 linear non-
hydrogen
atoms that is covalently attached immune-stimulatory compounds (C1 and C2) as
in the
following formula:
A
= = =
= .
-30-
CA 03065919 2019-12-02
WO 2018/227023
PCT/US2018/036560
[0124] In some embodiments, an immune-stimulatory conjugate is provided that
includes a
proteolysis targeting module (PTM; also referred to as a proteolysis-
targetting chimera or
PROTAC) that includes an immune-modulatory compound (IMC) that is covalently
attached to
an E3 ubiquitin ligase binding moiety (ULM) through a spacer (S) and wherein a
linker (L) is
covalently attached to the protein targeting molecule and to the antibody
construct (0), as
represented by the formula <>-(L-PTM), where n is from 1-20 and z is from 1 to
20. In some
embodiments, L is a cleavable linker. In some embodiments, S is a non-
cleavable spacer.
[0125] Referring to the previous formula, in some embodiments, C1 is a target
protein targeting
moiety, such as an immodulatory compound, C2 is an E3 ubiquitin ligase binding
moiety such
that together C1-S-C2 may form a PTM.
[0126] In some embodiments, an immune-modulatory compound or other protein
targeting
moiety (IMC) is covalently attached to an E3 ubiquitin ligase binding moiety
(ULM) through a
spacer (S) and a linker (L) is covalently attached to the spacer, n is from 1-
20 and z is from 1 to
20 as represented by the formula:
= * 40 .
\ -
te
[0127] In some embodiments, L is a cleavable linker. In some embodiments, L is
a non-
cleavable linker. In some embodiments, S is non-cleavable.
[0128] In some embodiments, an immune-modulatory compound or other protein
targeting
moiety (IMC) is covalently attached to an E3 ubiquitin ligase binding moiety
(ULM) through a
spacer (S) and a linker (L) is covalently attached to the IMC, n is from 1-20
and z is from 1 to 20
as represented by the formula:
_
7
A
. . .
[0129] In some embodiments, L is a cleavable linker. In some embodiments, L is
a non-
cleavable linker.
[0130] In some embodiments, an immune-modulatory compound or other protein
targeting
moiety (IMC) is covalently attached to an E3 ubiquitin ligase binding moiety
(ULM) through a
-31-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
spacer (S) and linker L is covalently attached to the ubiquitin E3 ligase
moiety (ULM), n is from
1-20 and z is from 1 to 20 as represented by the formula:
_
\ -
A . 4) 6 .
_
[0131] In some embodiments, L is a cleavable linker. In some embodiments, L is
a non-
cleavable linker. In some embodiments, S is non-cleavable.
Tumor and Immune Cell Antigens
[0132] In cancer, there are several general groups of tumor antigens,
including but not limited to:
(i) viral tumor antigens which can be identical for any viral tumor of this
type, (ii) carcinogenic
tumor antigens which can be specific for patients and for the tumors, (iii)
isoantigens of the
transplantation type or tumor-specific transplantation antigens which can be
different in all
individual types of tumor but can be the same in different tumors caused by
the same inciting
biological event; and (iv) embryonic antigens.
[0133] As a result of the discovery of tumor antigens, tumor antigens have
become important in
the development of new cancer treatments that can specifically target the
cancer. This has led to
the development of antibodies directed against these tumor antigens. Such
tumor antigens
include the following: CD5, CD19, CD20, CD25, CD37, CD30, CD33, CD45, CAMPATH-
1,
BCMA, CS-1, PD-L1, B7-H3, B7-DC (PD-L2), HLD-DR, carcinoembryonic antigen
(CEA),
TAG-72, EpCAM, MUC1, MUC15, MUC16, folate-binding protein, A33, G250, prostate-
specific membrane antigen (PSMA), ferritin, GD2, GD3, GM2, Leg, CA-125, CA19-
9, epidermal
growth factor, p185HER2, IL-2 receptor, EGFRvIII (de2-7 EGFR), fibroblast
activation protein
(FAP), tenascin, a metalloproteinase, endosialin, vascular endothelial growth
factor, avf33, WT1,
LMP2, HPV E6, HPV E7, Her-2/neu, p53 nonmutant, NY-ES0-1, MelanA/MART1, Ras
mutant,
gp100, p53 mutant, PR1, bcr-abl, tyrosinase, survivin, PSA, hTERT, a Sarcoma
translocation
breakpoint protein, EphA2, PAP, ML-IAP, AFP, ERG, NA17, PAX3, ALK, androgen
receptor,
cyclin Bl, polysialic acid, MYCN, RhoC, TRP-2, fucosyl GM1, mesothelin (MSLN),
PSCA,
MAGE Al, MAGE A3, sLe(animal), CYP1B1, PLAV1, GM3, BORIS, Tn, GloboH, ETV6-
AML, NY-BR-1, RGS5, SART3, STn, Carbonic anhydrase IX, PAX5, 0Y-TES1, Sperm
protein
17, LCK, HMWMAA, AKAP-4, 55X2, XAGE 1, Legumain, Tie 3, Page 4, VEGFR2, MAD-
-32-
CA 03065919 2019-12-02
WO 2018/227023
PCT/US2018/036560
CT-1, PDGFR-B, MAD-CT-2, ROR2, TRAILl, MAGE A4, MAGE C2, GAGE, EGFR, CMET,
HER3, EPCAM, CA6, NAPI2B, TROP2, Claudin-6 (CLDN6), Claudin-16 (CLDN16),
CLDN18.2, RON, LY6E, FRA, DLL3, PTK7, Uroplakin-1B (UPK1B), LIV1, ROR1, STRA6,
TMPRSS3, TMPRSS4, TMEM238, Clorf186, Fos-related antigen 1, VEGFR1, endoglin,
VTCN1 (B7-H4), VISTA, and gpNMB.
[0134] In addition to the development of antibodies against tumor antigens for
cancer treatment,
antibodies that target immune cells to boost the immune response have also
been developed,
including the following: an anti-CD40 antibody, an anti-CD47 antibody, anti-
TNFR2 antibody,
an anti-DEC205 antibody, an anti-CD36 mannose scavenger receptor 1 antibody,
an anti-
CLEC9A antibody, an anti-CLEC12A antibody, an anti-DC-SIGN antibody, an anti-
BDCA-2
antibody, an anti-OX4OL antibody, an anti-41BBL antibody, an anti-CD204
antibody, an anti-
MARCO antibody, an anti-CLEC5A antibody, an anti-Dectin 1 antibody, and anti-
Dectin 2
antibody, an anti-CLEC10A antibody, an anti-CD206 antibody, an anti-CD64
antibody, an anti-
CD32A antibody, an anti-CD16A antibody, an anti-HVEM antibody, an anti-CD38
antibody, an
anti-PD-Li antibody, an anti-TREM2 antibody, an anti-CSF1R antibody, or an
anti-CD32B
antibody can be used to target, respectively, cell surface CD40, CD47, TNFR2,
DEC-205, CD36
mannose scavenger receptor 1, CLEC9A, CLEC12A, DC-SIGN, BDCA-2, OX4OL, 41BBL,
CD204, MARCO, CLEC5A, Dectin 1, Dectin 2, CLEC10A, CD206, CD64, CD32A, CD16A,
HVEM, CD38, PD-L1, TREM2, CSF1R, or CD32B molecules expressed by antigen
presenting
cells.
[0135] Cluster of Differentiation 40 (CD40) is a member of the Tumor Necrosis
Factor Receptor
(TNF-R) family. CD40 can be a 50 kDa cell surface glycoprotein that can be
constitutively
expressed in normal cells, such as monocytes, macrophages, B lymphocytes,
dendritic cells,
endothelial cells, smooth muscle cells, fibroblasts and epithelium, and in
tumor cells, including
B-cell lymphomas and many types of solid tumors. Expression of CD40 can be
increased in
antigen presenting cells in response to IL-13p, IFN-y, GM-CSF, and LPS induced
signaling
events.
[0136] Examples of agonistic CD40 monoclonal antibodies include CP-870,893,
dacetuzumab,
Chi Lob 7/4, SEA-CD40, ADC-1013, 3C3, or 3G5.
[0137] Cluster of Differentiation 205 (CD205 or DEC-205) is a member of the C-
type
multilectin family of endocytic receptors, which can include the macrophage
mannose receptor
(MMR) and the phospholipase A2 receptor (PLA2R). DEC-205 can be a 205 kDa
endocytic
receptor highly expressed in cortical thymic epithelial cells, thymic
medullary dendritic cells
(CD11c+ CD8+), subpopulations of peripheral dendritic cells (CD11c+ CD8+). The
DEC-205+
CD11c+ CD8+ dendritic cells (DCs) can function in cross-presentation of
antigens derived from
-33-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
apoptotic cells. Additionally, DEC-205 can be significantly upregulated during
DC maturation.
DEC-205 can also be expressed at moderate levels in B cells and low levels in
macrophages and
T cells.
[0138] After antigen binding to DEC-205, the receptor-antigen complex can be
internalized
whereupon the antigen can be processed and be presented on the DC surface by a
major
histocompatibility complex class II (MHC II) or MHC class I. DEC-205 can
deliver antigen to
DCs for antigen presentation on MHC class II and cross-presentation on MHC
class I. DEC-205
mediated antigen delivery for antigen presentation in DCs without an
inflammatory stimulus can
result in tolerance. Conversely, DEC-205 mediated antigen delivery in DCs in
the presence of a
maturational stimulus (e.g., a CD40 agonist) can result in long-term immunity
via activation of
antigen-specific CD4+ and CD8+ T cells.
[0139] CD36 mannose scavenger receptor 1 is an oxidized LDL receptor with two
transmembrane domains located in the caveolae of the plasma membrane. It can
be classified as a
Class B scavenger receptor, which can be characterized by involvement in the
removal of foreign
substances and waste materials. This receptor can also be involved in cell
adhesion, phagocytosis
of apoptotic cells, and metabolism of long-chain fatty acids.
[0140] TNFR2 (tumor necrosis factor receptor 2), also known as TNFRSF1B (tumor
necrosis
factor receptor super family 1B) and CD120b, is a single-pass type I membrane
protein and the
member of TNFR superfamily containing 4 cysteine-rich domains (CRD) repeats.
In addition to
the full length membrane-anchored form, soluble TNFR2 can be generated via two
distinct
mechanisms: (1) shedding via proteolytic processing of the full membrane
anchored from, and
(2) translation from an alternatively spliced message encoding the
extracellular domains of
TNFR2. TNFR2 is the receptor with high affinity for TNF-alpha and
approximately 5-fold lower
affinity for homotrimeric lymphotoxin-alpha. TNFR2 (Tumor Necrosis Factor
Receptor Type II)
and TNF-receptor 1 form a heterocomplex that mediates the recruitment of two
anti-apoptotic
proteins, c-IAP1 and c-IAP2, which possess E3 ubiquitin ligase activity. c-
IAP1 can potentiate
TNF-induced apoptosis by the ubiquitination and degradation of TNF-receptor-
associated factor
2, which mediates anti-apoptotic signals. Knockout studies in mice suggest a
role of TNFR2 in
protecting neurons from apoptosis by stimulating antioxidative pathways.
[0141] CLEC9A is a group V C-type lectin receptor. This receptor can be
expressed as on
myeloid lineage cells, and can be characterized as an activation receptor.
[0142] CLEC12A is a member of the C-type lectin/C-type lectin like domain
super family that
can be a negative regulator of granulocyte and monocyte function. It can also
be involved in cell
adhesion, cell-cell signaling, and glycoprotein turnover, and can play a role
in the inflammatory
response.
-34-
CA 03065919 2019-12-02
WO 2018/227023
PCT/US2018/036560
[0143] Dendritic cell-specific inter cellular adhesion molecule-3-grabbing non-
integrin (DC-
SIGN) or CD209, is a C-type lectin receptor that can be expressed on the
surface of macrophages
and dendritic cells. This receptor can recognize and bind to mannose type
carbohydrates and be
involved in activating phagocytosis, can mediate dendritic cell rolling, and
can be involved in
CD4+ T cell activation.
[0144] BDCA-2 is a C-type lectin that is a membrane protein of plasmacytoid
dendritic cells. It
can be involved in plasmacytoid dendritic cell function, such as ligand
internalization and
presentation.
[0145] OX4OL, which is also referred to as CD252, is the ligand for CD134 that
can be
expressed on dendritic cells. It can be involved in T cell activation.
[0146] 41BBL, which is also referred to as CD137L, is a member of the TNF
superfamily, and
can be expressed on B cells, dendritic cells, activated T cells, and
macrophages. It can provide
co-stimulatory signal for T cell activation and expansion.
[0147] CD204, which is also referred to as macrophage scavenger receptor 1, is
a macrophage
scavenger receptor receptor. The gene for CD204 can encode three different
class A macrophage
scavenger receptor isoforms. The type 1 and type 2 isoforms can be involved in
binding,
internalizing, and processing negatively charged macromolecules, such as low
density
lipoproteins. The type 3 isoform can undergo altered intracellular processing
in which it can be
retained within the endoplasmic reticulum, and has been shown to have a
dominant negative
effect on the type 1 and type 2 isoforms.
[0148] Macrophage receptor with collagenous structure (MARCO), which is also
referred to as
SCARA2, is a class A scavenger receptor with collagen-like and cysteine-rich
domains. It can be
expressed in macrophages, and can bind to modified low density lipoproteins.
It can be involved
in the removal of foreign substances and waste materials.
[0149] C-type lectin domain family 5 member A (CLEC5A) is a C-type lectin. It
can be involved
in the myeloid lineage activating pathway.
[0150] Dendritic cell-associated c-type lectin-1 (Dectin 1), which is also
referred to as CLEC7A,
is member of the C-type lectin/C-type lectin-like super family. It can be
expressed by myeloid
dendritic cells, monocytes, macrophages, and B cells, and can be involved in
antifungal
immunity.
[0151] Dendritic cell-associated c-type lectin-2 (Dectin 2), which is also
referred to as CLEC6A,
is member of the C-type lectin/C-type lectin-like super family. It can be
expressed by dendritic
cells, macrophages, monocytes and neutrophils. It can be involved in
antifungal immunity.
-35-
CA 03065919 2019-12-02
WO 2018/227023
PCT/US2018/036560
[0152] CLEC10A, which is also referred to as CD301, is member of the C-type
lectin/C-type
lectin-like super family. It can be expressed by dendritic cells, monocytes,
and CD33+ myeloid
cells, and can be involved in macrophage adhesion and migration.
[0153] CD206, which is also referred to as macrophage mannose receptor, is a C-
type lectin type
I membrane glycoprotein. It can be expressed on dendritic cells, macrophages
and endothelial
cells, and can act as a pattern recognition receptor and bind high-mannose
structures of viruses,
bacteria, and fungi.
[0154] CD64, which is also referred to as FcyRI, is a high affinity Fc
receptor for IgG. It can be
expressed by monocytes and macrophages. It can be involved in mediating
phagocytosis, antigen
capture, and antibody dependent cell-mediated cytoxicity.
[0155] CD32A, which is also referred to as FcyRIIa, is a low affinity Fc
receptor. It can be
expressed on monocytes, granulocytes, B cells, and eosinophils. It can be
involved in
phagocytosis, antigen capture, and antibody dependent cell-mediated
cytoxicity.
[0156] CD16A, which is also referred to as FcyRIIIa, is low affinity Fc
receptor. It can be
expressed on NK cells, and can be involved in phagocytosis and antibody
dependent cell-
mediated cytotoxicity.
[0157] Herpesvirus entry mediator (HVEM), which is also referred to as CD270,
is a member of
the TNF-receptor superfamily. It can be expressed on B cells, dendritic cells,
T cells, NK cells,
CD33+ myeloid cells, and monocytes. It can be involved in activating the
immune response.
[0158] CD32B, which is also referred to as FcyRIIb, is a low affinity Fc
receptor. It can be
expressed on B cells and myeloid dendritic cells. It can be involved in
inhibiting maturation and
cell activation of dendritic cells.
[0159] The HER2/neu (human epidermal growth factor receptor 2/receptor
tyrosine-protein
kinase erbB-2) is part of the human epidermal growth factor family.
Overexpression of this
protein can be shown to play an important role in the progression of cancer,
for example, breast
cancer. The HER2/neu protein can function as a receptor tyrosine kinase and
autophosphorylates
upon dimerization with binding partners. HER2/neu can activate several
signaling pathways
including, for example, mitogen-activated protein kinase, phosphoinositide 3-
kinase,
phospholipase Cy, protein kinase C, and signal transducer and activator of
transcription (STAT).
Examples of antibodies that can target and inhibit HER2/neu can include
trastuzumab and
pertuzumab.
[0160] EGFR (epidermal growth factor receptor) encodes a member of the human
epidermal
growth factor family. Mutations that can lead to EGFR overexpression or over
activity can be
associated with a number of cancers, including squamous cell carcinoma and
glioblastomas.
EGFR can function as a receptor tyrosine kinase and ligand binding can trigger
dimerization with
-36-
CA 03065919 2019-12-02
WO 2018/227023
PCT/US2018/036560
binding partners and autophosphorylation. The phosphorylated EGFR can then
activate several
downstream signaling pathways including mitogen-activated protein kinase,
phosphoinositide 3-
kinase, phospholipase Cy, protein kinase C, and signal transducer and
activator of transcription
(STAT). Examples of antibodies that can target and inhibit EGFR can include
cetuximab,
panutumumab, nimotuzumab, and zalutumumab. One mutant variant of EGFR is
EGFRvIII
(epidermal growth factor receptor variant III, also referred to as de2-7EGFR).
EGFRvIII is the
result of an EGFR gene rearrangement in which exons 2-7 of the extracellular
domain are
deleted. This mutation can result in a mutant receptor incapable of binding to
any known ligand.
The resulting receptor can engage in a constitutive low-level signaling and
can be implicated in
tumor progression. Examples of antibodies that can target EGFRvIII can include
AMG595 and
ABT806.
[0161] C-Met (hepatocyte growth factor receptor) encodes a member of the
receptor tyrosine
kinase family of proteins. C-Met overexpression and over activity can be
implicated in various
cancers including lung adenocarcinomas, and high c-Met levels can be
associated with poor
patient outcome. Binding of hepatocyte growth factor can induce dimerization
and
autophosphorylation of c-Met. The c-Met receptor can activate various
downstream signaling
pathways including mitogen-activated protein kinase, phosphoinositide 3-
kinase, and protein
kinase C pathways. The antibody onartuzumab can target and inhibit c-Met.
[0162] HER3 (human epidermal growth factor receptor 3) encodes a member of the
human
epidermal growth factor receptor family. Ligand binding can induce
dimerization and
autophosphorylation of cytoplasmic tyrosine residues that then can recruit
signaling proteins for
downstream signaling pathway activation including mitogen-activated protein
kinase and
phosphoinoside 3-kinase pathways. HER3 can play an active role in cell
proliferation and
survival, and can be overexpressed, overactive, and/or mutated in various
cancers. For example,
HER3 can be overexpressed in breast, ovarian, prostate, colon, pancreas,
stomach, oral, and lung
cancers. The antibody patritumab can target and inhibit HER3.
[0163] MUC1 (mucin 1, cell surface associated) encodes a member of the mucin
family of
glycosylated proteins that can play an important role in cell adhesion and
forming protective
mucosal layers on epithelial surfaces. MUC1 can be proteolytically cleaved
into alpha and beta
subunits that form a heterodimeric complex with the N-terminal alpha subunit
providing cell-
adhesion functionality and the C-terminal beta subunit modulating cell
signaling pathways
including the mitogen activated map kinase pathway. MUC1 can play a role in
cancer
progression, for example, by regulating TP53-mediated transcription. MUC1
overexpression,
aberrant intracellular localization, and glycosylation changes can all be
associated with
carcinomas including pancreatic cancer cells. The antibody clivatuzumab can
target MUC1.
-37-
CA 03065919 2019-12-02
WO 2018/227023
PCT/US2018/036560
[0164] MUC16 (mucin 16, cell surface associated) encodes the largest member of
the mucin
family of glycosylated proteins that can play an important role in cell
adhesion and forming
protective mucosal layers on epithelial surfaces. MUC16 can be a highly
glycosylated 2.5MDa
transmembrane protein that can provide a hydrophilic lubricating barrier on
epithelial cells. The
cytoplasmic tail of MUC16 can be involved with various signaling pathways
including the
JAK2-STAT3 and Src kinase pathways. A peptide epitope of MUC16 can be used as
biomarker
for detecting ovarian cancer. Elevated expression of MUC16 can be present in
advanced ovarian
cancers and pancreatic cancers. The antibody sofituzumab can target MUC16.
[0165] EPCAM (epithelial cell adhesion molecule) encodes a transmembrane
glycoprotein that
can be frequently and highly expressed in carcinomas and tumor-initiating
cells. EPCAM can
also be a pluripotent stem cell marker. EPCAM can modulate a variety of
pathways including
cell-cell adhesion, cellular proliferation, migration, invasion, maintenance
of a pluripotent state,
and differentiation in the context of tumor cells. The antibodies edrecolomab
and adecatumumab
can target EPCAM.
[0166] MSLN (mesothelin) encodes a 40 kDa cell GPI-anchored membrane surface
protein
believed to function in cell adhesion. MSLN is overexpressed in mesothelioma
and certain types
of pancreatic, lung, and ovarian cancers. MSLN-related peptides that circulate
in serum of
patients suffering from pleural mesothelioma are used as biomarkers for
monitoring the disease.
MSLN may promote metastasis by inducing matrix metalloproteinase 7 and 9
expression. The
monoclonal antibody anetumab has been developed to target MSLN.
[0167] CA6 (carbonic anhydrase VI) encodes one of several isozymes of carbonic
anhydrase.
CA6 is found in salivary glands and may play a role in the reversible
hydration of carbon
dioxide. CA6 is expressed in human serous ovarian adenocarcinomas. The
monoclonal antibody
huDS6 has been developed to target CA6.
[0168] NAPI2B (sodium/phosphate cotransporter 2B) encodes a type II sodium-
phosphate
cotransporter. NAPI2B is highly expressed on the tumor surface in lung,
ovarian, and thyroid
cancers as well as in normal lung pneumocytes. The monoclonal antibody
lifastuzumab has been
developed to target NAPI2B.
[0169] TROP2 (trophoblast antigen 2) encodes a transmembrane glycoprotein that
acts as an
intracellular calcium signal transducer. TROP2 binds to multiple factors such
as IGF-1, claudin-
1, claudin-7, cyclin D1, and PKC. TROP2 including intracellular calcium
signaling and the
mitogen activated protein kinase pathway. TROP 2 plays a role in cell self-
renewal, proliferation,
invasion, and survival. Discovered first in trophoblast cells that have the
ability to invade uterine
decidua during placental implantation, TROP2 overexpression has been shown to
be capable of
stimulating cancer growth. TROP2 overexpression has been observed in breast,
cervix,
-38-
CA 03065919 2019-12-02
WO 2018/227023
PCT/US2018/036560
colorectal, esophagus, lung, non-Hodgkin's lymphoma, chronic lymphocytic
lymphoma, Raji
Burkitt lymphoma, oral squamous cell, ovarian, pancreatic, prostate, stomach,
thyroid, urinary
bladder, and uterine carcinomas. The monoclonal antibody sacituzumab has been
developed to
target TROP2.
[0170] CEA (carcinoembryonic antigen; also referred to as CEACAM5) is a member
of a family
of related glycoproteins involved in cell adhesion. CEA is a biomarker for
gastrointestinal
cancers and may promote tumor development by means of its cell adhesion
function. CEA levels
have been found to be elevated in serum of individuals with colorectal
carcinoma. CEA levels
have also been found to be elevated in gastric carcinoma, pancreatic
carcinoma, lung carcinoma,
breast carcinoma, and medullary thyroid carcinoma. The monoclonal antibodies
PR1A3 and
Ab2-3 have been developed to target CEA.
[0171] CLDN18.2 (claudin 18) encodes a member of the claudin family of
integral membrane
proteins. CLDN18.2 is a component of tight junctions that create a physical
barrier to prevent
diffusion of solutes and water through the paracellular space between
epithelial cells. CLDN18.2
is overexpressed in infiltrating ductal adenocarcinomas, but is reduced in
some gastric
carcinomas. The monoclonal antibody claudiximab has been developed to target
CLDN18.2.
[0172] FAP (fibroblast activation protein, alpha) encodes a homodimeric
integral membrane
protein from a family of serine proteases. FAP is believed to play a role in
many processes
including tissue remodeling, fibrosis, wound healing, inflammation, and tumor
growth. FAP
enhances tumor growth and invasion by promoting angiogenesis, collagen fiber
degradation and
apoptosis, and by downregulating the immune response. FAP is selectively
expressed on
fibroblasts within the tumor stroma. The monoclonal antibody sibrotuzumab has
been developed
to target FAP.
[0173] EphA2 (EPH Receptor A2) encodes a member of the ephrin receptor
subfamily of the
protein-tyrosine kinase family. EphA2 binds to ephrin-A ligands. Activation of
EphA2 receptor
upon ligand binding can result in modulation of migration, integrin-mediated
adhesion,
proliferation, and differentiation. EphA2 is overexpressed in various cancers
including breast,
prostate, urinary bladder, skin, lung, ovarian, and brain cancers. High EphA2
expression is also
correlated with poor prognosis. The monoclonal antibodies DS-8895a optl, DS-
8895 opt2, and
the 1C1 antibody in MEDI-547 have been developed to target EphA2.
[0174] RON (macrophage stimulating 1 receptor) encodes a cell surface receptor
for macrophage
stimulating protein (MSP) with tyrosine kinase activity and belongs to the MET
proto-oncogene
family. RON has significant structural similarity and sequence identity with
the cancer-related
gene C-MET. RON plays a significant role in KRAS oncogene addiction and has
also been
shown to be overexpressed in pancreatic cancers. Altered Ron expression and
activation has been
-39-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
associated with decreased survival and cancer progression in various cancers
including gastric,
colon, breast, bladder, renal cell, ovarian, and hepatocellular cancers. The
monoclonal antibody
narnatumab has been developed to target RON.
[0175] LY6E (lymphocyte antigen 6 complex, locus E) encodes an interferon
alpha-inducible
GPI-anchored cell membrane protein. LY6E is overexpressed in numerous cancers
including
lung, gastric, ovarian, breast, kidney, pancreatic, and head and neck
carcinomas. The monoclonal
antibody in RG7841 has been developed to target LY6E.
[0176] FRA (folate receptor alpha) encodes a GPI-anchored cell surface
glycoprotein. FRA binds
folic acid, a molecule needed for cell growth and DNA synthesis, and mediates
its internalization
via receptor-mediated endocytosis. FRA is overexpressed in various cancers
including prostate,
breast, ovarian, pancreatic, mesothelioma, non-small cell lung carcinoma, and
head and neck
cancer. FRA expression has also been found to enhance tumor cell
proliferation. The monoclonal
antibodies farletuzumab and mirvetuximab have been developed to target FRA.
[0177] PSMA (prostate specific membrane antigen) is a type II transmembrane
glycoprotein
belonging to the M28 peptidase family that is expressed in all types of
prostate tissues. PSMA is
upregulated in cancer cells within the prostate and is used as a marker for
prostate cancer. PSMA
expression may also serve as a predictor of disease recurrence in prostate
cancer patients. The
monoclonal antibodies J591 variant 1 and J591 variant 2 have been developed to
target PSMA.
[0178] DLL3 (delta-like 3) encodes a ligand in the Notch signaling pathway
that is associated
with neuroendocrine cancer. DLL3 is most highly expressed in the fetal brain
and is involved in
somitogenesis in the paraxial mesoderm. DLL3 is expressed on tumor cell
surfaces but not in
normal tissues. The monoclonal antibody rovalpituzumab has been developed to
target DLL3.
[0179] PTK7 (tyrosine protein kinase-like 7) encodes a receptor tyrosine
kinase that lacks
catalytic tyrosine kinase activity but is nevertheless capable of signal
transduction. PTK7
interacts with the WNT signaling pathway, which itself has important roles in
epithelial
mesenchymal transition and various cancers such as breast cancer. PTK7
overexpression has
been associated with patient prognosis depending on the cancer type. The
monoclonal antibodies
in PF-06647020 and the anti-PTK7 antibody described by SEQ ID NOs: 440 and 445
have been
developed to target PTK7.
[0180] LIV1 (LIV-1 protein, estrogen regulated) encodes a member of the LIV-1
subfamily of
ZIP (Zrt-, Irt-like proteins) zinc transporters. LIV1 is an estrogen regulated
protein that transports
zinc and/or other ions across the cell membrane. Elevated levels of LIV1 have
been shown in
estrogen receptor positive breast cancers, and LIV1 is used as a marker of ER-
positive cancers.
LIV1 has also been implicated as a downstream target of the STAT3
transcription factor and as
playing an essential role in the nuclear localization of the Snail
transcription factor that
-40-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
modulates epithelial-to-mesenchymal transition. The monoclonal antibody in SGN-
LIV1A has
been developed to target LIV1.
[0181] ROR1 (receptor tyrosine kinase-like orphan receptor 1) encodes a member
of the ROR
family of orphan receptors. ROR1 has been found to bind Wnt5a, a non-canonical
Wnt via a
Frizzled domain (FZD), and plays an important role in skeletal,
cardiorespiratory, and
neurological development. ROR1 expression is predominantly restricted to
embryonic
development and is absent in most mature tissues. In contrast, ROR1 expression
is upregulated in
B-Cell chronic lymphocytic leukemia, acute lymphocytic leukemia, non-Hodgkin
lymphoma,
and myeloid malignancies. The monoclonal antibody cirmtuzumab has been
developed to target
ROR1.
[0182] MAGE-A3 (melanoma-associated antigen 3) encodes a member of the
melanoma-
associated antigen gene family. The function of MAGE-A3 is not known, but its
elevated
expression has been observed in various cancers including melanoma, non-small
cell lung cancer,
and in putative cancer stem cell populations in bladder cancer. The monoclonal
antibody
described by SEQ ID NOs: 479 and 484 has been developed to target MAGE-A3.
[0183] NY-ESO-1 (New York esophageal squamous cell carcinoma 1) encodes a
member of the
cancer-testis family of proteins. Cancer-testis antigen expression is normally
restricted to
testicular germ cells in adult tissues, but has been found to be aberrantly
expressed in various
tumors including soft tissue sarcomas, melanoma, epithelial cancers, and
myxoid and round cell
liposarcomas. The monoclonal antibody described by SEQ ID Nos: 492 and 497 has
been
developed to target NY-ESO-1.
Binding Domain
[0184] The binding domain of an antibody construct is selected to recognize an
antigen or
molecule. For example, an antigen can be a cell surface marker on target cells
associated with a
disease or condition. An antigen can be a peptide or fragment thereof. An
antigen can be
expressed on an immune cell. An antigen can be expressed on an antigen-
presenting cell. An
antigen can be expressed on a dendritic cell, a macrophage, or a B cell. An
antigen on an antigen
presenting cell can be a cell lineage marker or a cell surface protein
expressed preferentially on
antigen presenting cells or a subset of antigen presenting cells. An antigen
can be a peptide
presented in a major histocompatibility complex by cell. As another example, a
cell surface
marker recognized by the antigen binding domain can include macromolecules
associated with
viral and bacterial diseases or infections, autoimmune diseases and cancerous
diseases. An
antigen can be a tumor antigen or fragment thereof. A tumor antigen can be any
antigen listed on
tumor antigen databases, such as TANTIGEN, or peptide databases for T cell-
defined tumor
-41-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
antigens, such as the Cancer Immunity Peptide database. A tumor antigen can
also be any antigen
listed in the review by Chen (Chen, Cancer Immun 2004 [updated 2004 Mar 10;
cited 2004 Apr
1]). An antigen can be or can be at least 80% homologous CD5, CD19, CD20,
CD25, CD37,
CD30, CD33, CD45, CAMPATH-1, BCMA, CS-1, PD-L1, B7-H3, B7-DC (PD-L2), HLD-DR,
carcinoembryonic antigen (CEA), TAG-72, EpCAM, MUC1, MUC15, MUC16, folate-
binding
protein, A33, G250, prostate-specific membrane antigen (PSMA), ferritin, GD2,
GD3, GM2, Leg,
CA-125, CA19-9, epidermal growth factor, p185HER2, IL-2 receptor, EGFRvIII
(de2-7 EGFR),
fibroblast activation protein (FAP), tenascin, a metalloproteinase,
endosialin, vascular endothelial
growth factor, avf33, WT1, LMP2, HPV E6, HPV E7, Her-2/neu, p53 nonmutant, NY-
ES0-1,
MelanA/MART1, Ras mutant, gp100, p53 mutant, PR1, bcr-abl, tyrosinase,
survivin, PSA,
hTERT, a Sarcoma translocation breakpoint protein, EphA2, PAP, ML-IAP, AFP,
ERG, NA17,
PAX3, ALK, androgen receptor, cyclin Bl, polysialic acid, MYCN, RhoC, TRP-2,
fucosyl GM1,
mesothelin (MSLN), PSCA, MAGE Al, MAGE A3, sLe(animal), CYP1B1, PLAV1, GM3,
BORIS, Tn, GloboH, ETV6-AML, NY-BR-1, RGS5, SART3, STn, Carbonic anhydrase IX,
PAX5, 0Y-TES1, Sperm protein 17, LCK, HMWMAA, AKAP-4, 55X2, XAGE 1, Legumain,
Tie 3, Page 4, VEGFR2, MAD-CT-1, PDGFR-B, MAD-CT-2, ROR2õ TRAILl, MAGE A4,
MAGE C2, GAGE, EGFR, CMET, HER3, EPCAM, CA6, NAPI2B, TROP2, Claudin-6
(CLDN6), Claudin-16 (CLDN16), CLDN18.2, RON, LY6E, FRA, DLL3, PTK7, Uroplakin-
1B
(UPK1B), LIV1, ROR1, STRA6, TMPRSS3, TMPRSS4, TMEM238, Clorf186, Fos-related
antigen 1, VEGFR1, endoglin, VTCN1 (B7-H4), VISTA, or gpNMB.
[0185] In some embodiments, an antigen binding domain recognizes a single
antigen. In some
embodiments, an antigen binding domain recognizes two or more different
antigens.
[0186] An antibody construct can include one or more binding domains. For
example, an
antibody construct can comprise a first binding domain. An antibody construct
can also comprise
a second binding domain. A binding domain can specifically bind to an antigen
on a cell surface.
A binding domain can specifically bind to an antigen on a cell surface, for
example, of a tumor or
cancer cell, an immune cell, or of an antigen presenting cell, such as a
dendritic cell or
macrophage. A binding domain can be a cell surface receptor agonist. A binding
domain can be
an antigen binding domain. An antigen binding domain can be a cell surface
receptor agonist. An
antigen binding domain can be a domain that can specifically bind to an
antigen. An antigen
binding domain can specifically bind to a tumor antigen. An antigen binding
domain can be an
antigen-binding portion of an antibody or an antigen binding fragment thereof.
A binding domain
can recognize a single antigen.
[0187] An antibody construct can include, for example, one, two, three, four,
five, six, seven,
eight, nine, ten, or more antigen binding domains. An antibody construct, such
as an antibody,
-42-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
can include two antigen binding domains in which each antigen binding domain
can recognize
the same antigen. An antibody construct, such as a bi-specific antibody, can
include two antigen
binding domains in which each antigen binding domain can recognize different
antigens. An
antibody construct can include three antigen binding domains in which each
antigen binding
domain can recognize different antigens. An antibody construct can include
three antigen binding
domains in which two of the antigen binding domains can recognize the same
antigen. An
antigen binding domain can be in a scaffold, in which a scaffold is a
supporting framework for
the antigen binding domain. An antigen binding domain can be in a non-antibody
scaffold. An
antigen binding domain can be in an antibody scaffold. An antibody construct
can comprise an
antigen binding domain in a scaffold. The antibody construct can further
comprise an Fc fusion
protein product. In some embodiments, the antibody construct is an Fc fusion
protein.
[0188] In some embodiments, an antibody construct can comprise an antigen
binding domain
that can specifically bind to a tumor antigen. A tumor antigen, also referred
to as a tumor
associated antigen, is an antigen that can be expressed by a cancer cell, a
neoplastic tumor cell
and/or within a tumor microenvironment. It is preferably not expressed or
expressed at low
levels on normal (non-cancerous) cells. For example, a tumor antigen can be an
antigen
expressed on a cell associated within a tumor, such as a neoplastic cell, or a
tumor associated cell
such as a stromal cell, endothelial cell, fibroblast, or tumor-infiltrating
immune cell. For example,
the tumor antigen Her2/Neu can be overexpressed by certain types of breast and
ovarian cancer.
A tumor antigen can also be ectopically expressed by a tumor and contribute to
deregulation of
the cell cycle, reduced apoptosis, metastasis, or escape from immune
surveillance. Tumor
antigens can generally be proteins or polypeptides derived therefrom, but also
can be glycans,
lipids, or other small organic molecules. Additionally, a tumor antigen can
arise through
increases or decreases in post-translational processing exhibited by a cancer
cell compared to a
normal cell, for example, protein glycosylation, protein lipidation, protein
phosphorylation, or
protein acetylation.
[0189] In certain embodiments, a binding domain specifically can bind to a
tumor associated
antigen, such as CD5, CD19, CD20, CD25, CD37, CD30, CD33, CD45, CAMPATH-1
antigen,
BCMA, CS-1, PD-L1, B7-H3, B7-DC (PD-L2), HLD-DR, carcinoembryonic antigen
(CEA),
TAG-72, EpCAM, MUC1, MUC15, MUC16, folate-binding protein, A33, G250, prostate-
specific membrane antigen (PSMA), ferritin, GD2, GD3, GM2, Leg, CA-125, CA19-
9, epidermal
growth factor, p185HER2, IL-2 receptor, EGFRvIII (de2-7 EGFR), fibroblast
activation protein
(FAP), tenascin, a metalloproteinase, endosialin, vascular endothelial growth
factor, avf33, WT1,
LMP2, HPV E6, HPV E7, Her-2/neu, p53 nonmutant, NY-ESO-1, MelanA/MART1, Ras
mutant,
gp100, p53 mutant, PR1, bcr-abl, tyrosinase, survivin, PSA, hTERT, a Sarcoma
translocation
-43-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
breakpoint protein, EphA2, PAP, ML-IAP, AFP, ERG, NA17, PAX3, ALK, androgen
receptor,
cyclin Bl, polysialic acid, MYCN, RhoC, TRP-2, fucosyl GM1, mesothelin (MSLN),
PSCA,
MAGE Al, MAGE A3, sLe(animal), CYP1B1, PLAV1, GM3, BORIS, Tn, GloboH, ETV6-
AML, NY-BR-1, RGS5, SART3, STn, Carbonic anhydrase IX, PAX5, 0Y-TES1, Sperm
protein
17, LCK, HMWMAA, AKAP-4, 55X2, XAGE 1, Legumain, Tie 3, Page4, VEGFR2, MAD-CT-
1, PDGFR-B, MAD-CT-2, ROR2, TRAILl, MAGE A4, MAGE C2, GAGE, EGFR, CMET,
HER3, EPCAM, CA6, NAPI2B, TROP2, Claudin-6 (CLDN6), Claudin-16 (CLDN16),
CLDN18.2, RON, LY6E, FRA, DLL3, PTK7, Uroplakin-1B (UPK1B), LIV1, ROR1, STRA6,
TMPRSS3, TMPRSS4, TMEM238, Clorf186, Fos-related antigen 1, VEGFR1, endoglin,
VTCN1 (B7-H4), VISTA, or gpNMB.
[0190] In some embodiments, a binding domain specifically can bind to a tumor
associated
antigen having at least 80%, 90%, 95%, 97%, 98%, 99% or 100% sequence identity
to CD5,
CD19, CD20, CD25, CD37, CD30, CD33, CD45, CAMPATH-1 antigen, BCMA, CS-1, PD-
L1,
B7-H3, B7-DC (PD-L2), HLD-DR, carcinoembryonic antigen (CEA), TAG-72, EpCAM,
MUC1,
MUC15, MUC16, folate-binding protein, A33, G250, prostate-specific membrane
antigen
(PSMA), ferritin, CA-125, CA19-9, epidermal growth factor, p185HER2, IL-2
receptor,
EGFRvIII (de2-7 EGFR), fibroblast activation protein (FAP), tenascin, a
metalloproteinase,
endosialin, vascular endothelial growth factor, avf33, WT1, LMP2, HPV E6, HPV
E7, Her-2/neu,
p53 nonmutant, NY-ESO-1, MelanA/MART1, Ras mutant, gp100, p53 mutant, PR1, bcr-
abl,
tyrosinase, survivin, PSA, hTERT, a Sarcoma translocation breakpoint protein,
EphA2, PAP,
ML-IAP, AFP, ERG, NA17, PAX3, ALK, androgen receptor, cyclin Bl, MYCN, RhoC,
TRP-2,
mesothelin (MSLN), PSCA, MAGE Al, MAGE A3, CYP1B1, PLAV1, BORIS, GloboH, ETV6-
AML, NY-BR-1, RGS5, SART3, Carbonic anhydrase IX, PAX5, 0Y-TES1, Sperm protein
17,
LCK, HMWMAA, AKAP-4, 55X2, XAGE 1, Legumain, Tie 3, Page4, VEGFR2, MAD-CT-1,
PDGFR-B, MAD-CT-2, ROR2, TRAILl, MAGE A4, MAGE C2, GAGE, EGFR, CMET, HER3,
EPCAM, CA6, NAPI2B, TROP2, Claudin-6 (CLDN6), Claudin-16 (CLDN16), CLDN18.2,
RON, LY6E, FRA, DLL3, PTK7, Uroplakin-1B (UPK1B), LIV1, ROR1, STRA6, TMPRSS3,
TMPRSS4, TMEM238, Clorf186, Fos-related antigen 1, VEGFR1, endoglin, VTCN1 (B7-
H4),
VISTA, or gpNMB.
[0191] In certain embodiments, a binding domain specifically binds to an
antigen selected from
the group consisting of comprising Her2/Neu (CD340), EGFR, CMET, HER3, MUC1,
MUC16,
EPCAM, MSLN, CA6, NAPI2B, TROP2, CEA, CLDN18.2, EGFRvIII, FAP, EphA2, RON,
LY6E, FRA, PSMA, DLL3, PTK7, LIV1, ROR1, MAGE-A3, and NY-ESO-1.
[0192] A binding domain of an antibody construct can be selected from any
domain that
specifically binds to an antigen, including but not limited to, from a
monoclonal antibody, a
-44-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
polyclonal antibody, a recombinant antibody, or a functional fragment thereof,
for example, a
heavy chain variable domain (VH) and a light chain variable domain (VL), or
from a non-antibody
such as a DARPin, an affimer, an avimer, a knottin, a monobody, an affinity
clamp, an
ectodomain, a receptor ectodomain, a receptor, a cytokine, a ligand, an
immunocytokine, a T-cell
receptor, a VNAR, an anticalin, or a recombinant T-cell receptor. In some
embodiments, a
binding domain of an antibody construct is from a monoclonal antibody, a
polyclonal antibody, a
recombinant antibody, or a functional fragment thereof, for example, a heavy
chain variable
domain (VH) and a light chain variable domain (VL).
[0193] The antigen binding domain of an antibody construct can be at least
80%, at least 85%, at
least 90%, at least 95%, or at least 99% homologous to an antigen binding
domain selected from,
but not limited to, a monoclonal antibody, a polyclonal antibody, a
recombinant antibody, or a
functional fragment thereof, for example, a heavy chain variable domain (VH)
and a light chain
variable domain (VL), or from a non-antibody, such as a DARPin, an affimer, an
avimer, a
knottin, a monobody, an affinity clamp, an ectodomain, a receptor ectodomain,
a receptor, a
cytokine, a ligand, an immunocytokine, a T-cell receptor, a VNAR, an
anticalin, or a
recombinant T-cell receptor.
[0194] A binding domain, for example an antigen binding domain from a
monoclonal antibody,
can comprise a light chain and a heavy chain. In one aspect, the monoclonal
antibody binds to an
antigen present on the surface of an antigen presenting cell (APC antigen) and
comprises the
light chain of an anti-APC antigen antibody and the heavy chain of an anti-APC
antigen
antibody, which bind an APC antigen. In another aspect, the monoclonal
antibody binds to a
tumor antigen comprises the light chain of a tumor antigen (anti-tumor)
antibody and the heavy
chain of a tumor antigen (anti-tumor) antibody, which bind to the tumor
antigen.
[0195] An antibody construct can be an antibody. An antibody molecule can
include of two
identical light protein chains (light chains) and two identical heavy protein
chains (heavy chains),
all held together covalently by interchain disulfide linkages. Structurally,
various functions of an
antibody can be confined to discrete protein regions or domains. The sites
that can recognize and
can bind to antigen consist of three complementarity determining regions
(CDRs; also referred to
as hyper-variable regions) that lie within the variable heavy chain regions
and within the variable
light chain regions at the N-terminal ends of the two heavy and two light
chains. The constant
domains can provide the general framework of the antibody and may not be
involved directly in
binding the antibody to an antigen, but can be involved in various effector
functions, such as
participation of the antibody in antibody-dependent cellular cytotoxicity
(ADCC).
[0196] The variable domains of natural light and heavy chains can have the
same general
structures, and each domain can comprise four framework regions, whose
sequences can be
-45-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
somewhat conserved, connected by three CDRs. The four framework regions can
largely adopt a
(3-sheet conformation and the CDRs can form loops connecting, and in some
aspects forming part
of, the (3 -sheet structure. The CDRs in each chain can be held in close
proximity by the
framework regions and, with the CDRs from the other chain, can contribute to
the formation of
the antigen binding site.
[0197] An antibody can include an antibody of any type, which can be assigned
to different
classes of immunoglobins, e.g., IgA, IgD, IgE, IgG, and IgM. Several different
classes can be
further divided into isotypes, e.g., IgGl, IgG2, IgG3, IgG4, IgAl, and IgA2.
Exemplary heavy
chain sequences of reference antibodies can be used to identify residue
variants and mutants.
[0198] An exemplary heavy chain sequence for human IgG1 heavy chain is that of
the human
IgG1 antibody, and can comprise:
ASTKGPSVFPLAPSSKSTSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSS
GLYSLSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGG
PSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPREEQY
NSTYRVVSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSR
EEMTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTVDK
SRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGK (SEQ ID NO: 898).
[0199] An exemplary heavy chain reference sequence for human IgG2 heavy chain
can
comprise:
ASTKGPSVFPLAPCSRSTSESTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSS
GLYSLSSVVTVPSSNFGTQTYTCNVDHKPSNTKVDKTVERKCCVECPPCPAPPVAGPSVF
LFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVQFNWYVDGVEVHNAKTKPREEQFNSTF
RVVSVLTVVHQDWLNGKEYKCKVSNKGLPAPIEKTISKTKGQPREPQVYTLPPSREEMT
KNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPMLDSDGSFFLYSKLTVDKSRWQ
QGNVFSCSVMHEALHNHYTQKSLSLSPGK (SEQ ID NO: 899).
[0200] An exemplary heavy chain reference sequence for human IgG4 heavy chain
can
comprise:
ASTKGPSVFPLAPCSRSTSESTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSS
GLYSLSSVVTVPSSSLGTKTYTCNVDHKPSNTKVDKRVESKYGPPCPSCPAPEFLGGPSV
FLFPPKPKDTLMISRTPEVTCVVVDVSQEDPEVQFNWYVDGVEVHNAKTKPREEQFNST
YRVVSVLTVLHQDWLNGKEYKCKVSNKGLPSSIEKTISKAKGQPREPQVYTLPPSQEEM
TKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSRLTVDKSRW
QEG NVFSCSVMHEALHNHYTQKSLSLSLGK (SEQ ID NO: 900).
[0201] The heavy-chain constant regions (Fc) that corresponds to the different
classes of
immunoglobulins can be a, 6, , y, and p.. The light chains can be one of
either kappa or lc and
-46-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
lambda or k, based on the amino acid sequences of the constant domains. The Fc
region can
contain an Fc domain. An Fc receptor can bind an Fc domain. An Fc domain can
comprise amino
acid residues 216 to 447 of an IgGl, which are part of SEQ ID NO: 898. An Fc
domain can
comprise amino acid residues 216 to 442 of an IgG2, which are part of SEQ ID
NO: 899. An Fc
domain can comprise amino acid residues 216 to 444 of an IgG4, which are part
of SEQ ID NO:
900. Antibody constructs can also include any fragment or recombinant forms
thereof (e.g.,
scFVs and domain antibodies). Antibody constructs can also include any
fragment or
recombinant forms thereof (e.g., scFVs and domain antibodies) of non-antibody
scaffolds,
including but not limited to anti-calins, affibodies, affilins, atrimers,
avimers, bicyclic peptides,
centyrins, Cys-knots, Darpins, fibronections, Kunitz domains, 0-bodies, or
peptibodies.
[0202] An antibody construct can comprise an antigen binding domain of an
antibody. An
antigen binding domain of an antibody can comprise one or more light chain
CDRs (LCDRs) and
one or more heavy chain CDRs (HCDRs), or one or more LCDRs or one or more
HCDRs. For
example, an antibody binding domain of an antibody construct can comprise one
or more of the
following: a light chain complementary determining region 1 (LCDR1), a light
chain
complementary determining region 2 (LCDR2), or a light chain complementary
determining
region 3 (LCDR3). For another example, an antibody binding domain can comprise
one or more
of the following: a heavy chain complementary determining region 1 (HCDR1), a
heavy chain
complementary determining region 2 (HCDR2), or a heavy chain complementary
determining
region 3 (HCDR3). In some embodiments, an antigen binding domain comprises
LCDR1,
LCDR2, LCDR3, HCDR1, HCDR2 and HCDR3. Unless stated otherwise, the CDRs
described
herein can be defined according to the IMGT (the international ImMunoGeneTics
information
system).
[0203] An antigen binding domain can comprise only the heavy chain of an
antibody. An antigen
binding domain can comprise only the variable domain of the heavy chain of an
antibody.
Alternatively, an antigen binding domain can comprise only the light chain of
an antibody. An
antigen binding domain can comprise only the variable light chain of an
antibody.
[0204] An antibody construct can comprise an antibody fragment. An antibody
fragment can
include (i) a Fab fragment, a monovalent fragment consisting of the VL, VH, CL
and CHi domains;
(ii) a F(ab')2 fragment, a bivalent fragment comprising two Fab fragments
linked by a disulfide
bridge at the hinge region; and (iii) a Fv fragment consisting of the VL and
VH domains of a
single arm of an antibody. Although the two domains of the Fv fragment, VL and
VH, can be
coded for by separate genes, they can be linked by a synthetic linker to be
made as a single
protein chain in which the VL and VH regions pair to form monovalent
molecules. F(ab')2 and
Fab' moieties can be produced recombinantly.
-47-
CA 03065919 2019-12-02
WO 2018/227023
PCT/US2018/036560
[0205] An Fv can be the minimum antibody fragment which contains a complete
antigen-
recognition and antigen-binding site. This region can consist of a dimer of
one heavy chain and
one light chain variable domain in tight, non-covalent association. In this
configuration the three
hypervariable regions of each variable domain can interact to define an
antigen-binding site on
the surface of the VH-VL dimer. A single variable domain (or half of an Fv
comprising only
CDRs specific for an antigen) can recognize and bind antigen, although at a
lower affinity than
the entire binding site.
[0206] An antibody used herein can be chimeric or "humanized." Chimeric and
humanized
forms of non-human (e.g., murine) antibodies can be chimeric immunoglobulins,
immunoglobulin chains or fragments thereof (such as Fv, Fab, Fab', F(ab')2 or
other target-
binding subdomains of antibodies), which can contain minimal sequences derived
from non-
human immunoglobulin. In general, the humanized antibody can comprise
substantially all of at
least one, and typically two, variable domains, in which all or substantially
all of the CDR
regions correspond to those of a non-human immunoglobulin and all or
substantially all of the
frame work regions (FR) are those of a human immunoglobulin sequence. The
humanized
antibody can also comprise at least a portion of an immunoglobulin constant
region (Fc),
typically that of a human immunoglobulin consensus sequence.
[0207] An antibody described herein can be a human antibody. As used herein,
"human
antibodies" can include antibodies having, for example, the amino acid
sequence of a human
immunoglobulin and include antibodies isolated from human immunoglobulin
libraries or from
animals transgenic for one or more human immunoglobulins that do not express
endogenous
immunoglobulins. Human antibodies can be produced using transgenic mice which
are incapable
of expressing functional endogenous immunoglobulins, but which can express
human
immunoglobulin genes. Completely human antibodies that recognize a selected
epitope can be
generated using guided selection. In this approach, a selected non-human
monoclonal antibody,
e.g., a mouse antibody, is used to guide the selection of a completely human
antibody
recognizing the same epitope
[0208] An antibody described herein can be a bispecific antibody or a dual
variable domain
antibody (DVD). Bispecific and DVD antibodies are monoclonal, often human or
humanized,
antibodies that have binding specificities for at least two different
antigens.
[0209] An antibody described herein can be a derivatized antibody. For
example, derivatized
antibodies can be modified by glycosylation, deglycosylation, defucosylation,
acetylation,
pegylation, phosphorylation, amidation, derivatization by known
protecting/blocking groups,
proteolytic cleavage, or linkage to a cellular ligand or other protein.
-48-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
[0210] An antibody described herein can have a sequence that has been modified
to alter at least
one constant region-mediated biological effector function relative to the
corresponding wild type
sequence. For example, in some embodiments, the antibody can be modified to
reduce at least
one constant region-mediated biological effector function relative to an
unmodified antibody, e.g.,
reduced binding or increased binding to the Fc receptor (FcR). FcR binding can
be reduced or
increased by, for example, mutating the immunoglobulin constant region segment
of the antibody
at particular regions necessary for FcR interactions.
[0211] An antibody described herein can be modified to acquire or improve at
least one constant
region-mediated biological effector function relative to an unmodified
antibody, e.g., to enhance
FcyR interactions. For example, an antibody with a constant region that binds
FcyRIIA, FcyRIIB
and/or FcyRIIIA with greater affinity than the corresponding wild type
constant region can be
produced according to the methods described herein.
[0212] An antibody described herein can bind to tumor cells, such as an
antibody against a cell
surface receptor or a tumor antigen.
[0213] An antibody construct can comprise a first binding domain. An antibody
construct can
comprise a first binding domain that specifically binds an antigen. An
antibody construct can
comprise a first binding domain that specifically binds a tumor antigen. A
first binding domain
can specifically bind a tumor antigen, wherein the tumor antigen is selected
from the group
consisting CD5, CD19, CD20, CD25, CD37, CD30, CD33, CD45, CAMPATH-1, BCMA, CS-
1,
PD-L1, B7-H3, B7-DC (PD-L2), HLD-DR, carcinoembryonic antigen (CEA), TAG-72,
EpCAM,
MUC1, MUC15, MUC16, folate-binding protein, A33, G250, prostate-specific
membrane
antigen (PSMA), ferritin, GD2, GD3, GM2, Leg, CA-125, CA19-9, epidermal growth
factor,
p185HER2, IL-2 receptor, EGFRvIII (de2-7 EGFR), fibroblast activation protein
(FAP),
tenascin, a metalloproteinase, endosialin, vascular endothelial growth factor,
avf33, WT1, LMP2,
HPV E6, HPV E7, Her-2/neu, p53 nonmutant, NY-ESO-1, MelanA/MART1, Ras mutant,
gp100,
p53 mutant, PR1, bcr-abl, tyrosinase, survivin, PSA, hTERT, a Sarcoma
translocation breakpoint
protein, EphA2, PAP, ML-IAP, AFP, ERG, NA17, PAX3, ALK, androgen receptor,
cyclin Bl,
polysialic acid, MYCN, RhoC, TRP-2, fucosyl GM1, mesothelin (MSLN), PSCA, MAGE
Al,
MAGE A3, sLe(animal), CYP1B1, PLAV1, GM3, BORIS, Tn, GloboH, ETV6-AML, NY-BR-
1,
RGS5, SART3, STn, Carbonic anhydrase IX, PAX5, 0Y-TES1, Sperm protein 17, LCK,
HMWMAA, AKAP-4, 55X2, XAGE 1, Legumain, Tie 3, Page4, VEGFR2, MAD-CT-1,
PDGFR-B, MAD-CT-2, ROR2, TRAILl, MAGE A4, MAGE C2, GAGE, EGFR, CMET,
HER3, EPCAM, CA6, NAPI2B, TROP2, Claudin-6 (CLDN6), Claudin-16 (CLDN16),
CLDN18.2, RON, LY6E, FRA, DLL3, PTK7, Uroplakin-1B (UPK1B), LIV1, ROR1, STRA6,
TMPRSS3, TMPRSS4, TMEM238, Clorf186, Fos-related antigen 1, VEGFR1, endoglin,
-49-
CA 03065919 2019-12-02
WO 2018/227023
PCT/US2018/036560
VTCN1 (B7-H4), VISTA, gpNMB, or any fragment thereof. An antibody construct
can
comprise a first binding domain that specifically binds a tumor antigen on a
tumor cell, a tumor
fragment, an immune cell or an antigen presenting cell.
[0214] An antibody construct can comprise a first binding domain that
specifically binds a tumor
antigen. The construct can comprise a first binding domain comprising one or
more CDRs,
typically a set of six CDRs. A first binding domain can comprise at least 80%
sequence identity
to any sequence in TABLE 1. An antibody construct can comprise a first binding
domain that
binds a tumor antigen, wherein the first binding domain comprises at least 80%
sequence identity
to: a) HCDR1 comprising an amino acid sequence of SEQ ID NO: 13, HCDR2
comprising an
amino acid sequence of SEQ ID NO: 14, HCDR3 comprising an amino acid sequence
of SEQ ID
NO: 15, LCDR1 comprising an amino acid sequence of SEQ ID NO: 18, LCDR2
comprising an
amino acid sequence of SEQ ID NO: 19, and LCDR3 comprising an amino acid
sequence of
SEQ ID NO: 20; b) HCDR1 comprising an amino acid sequence of SEQ ID NO: 26,
HCDR2
comprising an amino acid sequence of SEQ ID NO: 27, HCDR3 comprising an amino
acid
sequence of SEQ ID NO: 28, LCDR1 comprising an amino acid sequence of SEQ ID
NO: 31,
LCDR2 comprising an amino acid sequence of SEQ ID NO: 32, and LCDR3 comprising
an
amino acid sequence of SEQ ID NO: 33; c) HCDR1 comprising an amino acid
sequence of SEQ
ID NO: 39, HCDR2 comprising an amino acid sequence of SEQ ID NO: 40, HCDR3
comprising
an amino acid sequence of SEQ ID NO: 41, LCDR1 comprising an amino acid
sequence of SEQ
ID NO: 44, LCDR2 comprising an amino acid sequence of SEQ ID NO: 45, and LCDR3
comprising an amino acid sequence of SEQ ID NO: 46; d) HCDR1 comprising an
amino acid
sequence of SEQ ID NO: 52, HCDR2 comprising an amino acid sequence of SEQ ID
NO: 53,
HCDR3 comprising an amino acid sequence of SEQ ID NO: 54, LCDR1 comprising an
amino
acid sequence of SEQ ID NO: 57, LCDR2 comprising an amino acid sequence of SEQ
ID NO:
58, and LCDR3 comprising an amino acid sequence of SEQ ID NO: 59; e) HCDR1
comprising
an amino acid sequence of SEQ ID NO: 65, HCDR2 comprising an amino acid
sequence of SEQ
ID NO: 66, HCDR3 comprising an amino acid sequence of SEQ ID NO: 67, LCDR1
comprising
an amino acid sequence of SEQ ID NO: 70, LCDR2 comprising an amino acid
sequence of SEQ
ID NO: 71, and LCDR3 comprising an amino acid sequence of SEQ ID NO: 72; f)
HCDR1
comprising an amino acid sequence of SEQ ID NO: 78, HCDR2 comprising an amino
acid
sequence of SEQ ID NO: 79, HCDR3 comprising an amino acid sequence of SEQ ID
NO: 80,
LCDR1 comprising an amino acid sequence of SEQ ID NO: 83, LCDR2 comprising an
amino
acid sequence of SEQ ID NO: 84, and LCDR3 comprising an amino acid sequence of
SEQ ID
NO: 85; g) HCDR1 comprising an amino acid sequence of SEQ ID NO: 91, HCDR2
comprising
an amino acid sequence of SEQ ID NO: 92, HCDR3 comprising an amino acid
sequence of SEQ
-50-
CA 03065919 2019-12-02
WO 2018/227023
PCT/US2018/036560
ID NO: 93, LCDR1 comprising an amino acid sequence of SEQ ID NO: 96, LCDR2
comprising
an amino acid sequence of SEQ ID NO: 97, and LCDR3 comprising an amino acid
sequence of
SEQ ID NO: 98; h) HCDR1 comprising an amino acid sequence of SEQ ID NO: 104,
HCDR2
comprising an amino acid sequence of SEQ ID NO: 105, HCDR3 comprising an amino
acid
sequence of SEQ ID NO: 106, LCDR1 comprising an amino acid sequence of SEQ ID
NO: 109,
LCDR2 comprising an amino acid sequence of SEQ ID NO: 110, and LCDR3
comprising an
amino acid sequence of SEQ ID NO: 111; i) HCDR1 comprising an amino acid
sequence of SEQ
ID NO: 117, HCDR2 comprising an amino acid sequence of SEQ ID NO: 118, HCDR3
comprising an amino acid sequence of SEQ ID NO: 119, LCDR1 comprising an amino
acid
sequence of SEQ ID NO: 122, LCDR2 comprising an amino acid sequence of SEQ ID
NO: 123,
and LCDR3 comprising an amino acid sequence of SEQ ID NO: 124; j) HCDR1
comprising an
amino acid sequence of SEQ ID NO: 130, HCDR2 comprising an amino acid sequence
of SEQ
ID NO: 131, HCDR3 comprising an amino acid sequence of SEQ ID NO: 132, LCDR1
comprising an amino acid sequence of SEQ ID NO: 135, LCDR2 comprising an amino
acid
sequence of SEQ ID NO: 136, and LCDR3 comprising an amino acid sequence of SEQ
ID NO:
137; k) HCDR1 comprising an amino acid sequence of SEQ ID NO: 143, HCDR2
comprising an
amino acid sequence of SEQ ID NO: 144, HCDR3 comprising an amino acid sequence
of SEQ
ID NO: 145, LCDR1 comprising an amino acid sequence of SEQ ID NO: 148, LCDR2
comprising an amino acid sequence of SEQ ID NO: 149, and LCDR3 comprising an
amino acid
sequence of SEQ ID NO: 150;1) HCDR1 comprising an amino acid sequence of SEQ
ID NO:
156, HCDR2 comprising an amino acid sequence of SEQ ID NO: 157, HCDR3
comprising an
amino acid sequence of SEQ ID NO: 158, LCDR1 comprising an amino acid sequence
of SEQID
NO: 161, LCDR2 comprising an amino acid sequence of SEQ ID NO: 162, and LCDR3
comprising an amino acid sequence of SEQ ID NO: 163; m) HCDR1 comprising an
amino acid
sequence of SEQ ID NO: 169, HCDR2 comprising an amino acid sequence of SEQ ID
NO: 170,
HCDR3 comprising an amino acid sequence of SEQ ID NO: 171, LCDR1 comprising an
amino
acid sequence of SEQ ID NO: 174, LCDR2 comprising an amino acid sequence of
SEQ ID NO:
175, and LCDR3 comprising an amino acid sequence of SEQ ID NO: 176; n) HCDR1
comprising an amino acid sequence of SEQ ID NO: 182, HCDR2 comprising an amino
acid
sequence of SEQ ID NO: 183, HCDR3 comprising an amino acid sequence of SEQ ID
NO: 184,
LCDR1 comprising an amino acid sequence of SEQ ID NO: 187, LCDR2 comprising an
amino
acid sequence of SEQ ID NO: 188, and LCDR3 comprising an amino acid sequence
of SEQ ID
NO: 189; o) HCDR1 comprising an amino acid sequence of SEQ ID NO: 195, HCDR2
comprising an amino acid sequence of SEQ ID NO: 196, HCDR3 comprising an amino
acid
sequence of SEQ ID NO: 197, LCDR1 comprising an amino acid sequence of SEQ ID
NO: 200,
-51-
CA 03065919 2019-12-02
WO 2018/227023
PCT/US2018/036560
LCDR2 comprising an amino acid sequence of SEQ ID NO: 201, and LCDR3
comprising an
amino acid sequence of SEQ ID NO: 202; p) HCDR1 comprising an amino acid
sequence of
SEQ ID NO: 208, HCDR2 comprising an amino acid sequence of SEQ ID NO: 209,
HCDR3
comprising an amino acid sequence of SEQ ID NO: 210, LCDR1 comprising an amino
acid
sequence of SEQ ID NO: 213, LCDR2 comprising an amino acid sequence of SEQ ID
NO: 214,
and LCDR3 comprising an amino acid sequence of SEQ ID NO: 215; q) HCDR1
comprising an
amino acid sequence of SEQ ID NO: 805, HCDR2 comprising an amino acid sequence
of SEQ
ID NO: 806, HCDR3 comprising an amino acid sequence of SEQ ID NO: 807, LCDR1
comprising an amino acid sequence of SEQ ID NO: 808, LCDR2 comprising an amino
acid
sequence of SEQ ID NO: 809, and LCDR3 comprising an amino acid sequence of SEQ
ID NO:
810; r) HCDR1 comprising an amino acid sequence of SEQ ID NO: 823, HCDR2
comprising an
amino acid sequence of SEQ ID NO: 824, HCDR3 comprising an amino acid sequence
of SEQ
ID NO: 825, LCDR1 comprising an amino acid sequence of SEQ ID NO: 826, LCDR2
comprising an amino acid sequence of SEQ ID NO: 827, and LCDR3 comprising an
amino acid
sequence of SEQ ID NO: 828; s) HCDR1 comprising an amino acid sequence of SEQ
ID NO:
221, HCDR2 comprising an amino acid sequence of SEQ ID NO: 222, HCDR3
comprising an
amino acid sequence of SEQ ID NO: 223, LCDR1 comprising an amino acid sequence
of SEQ
ID NO: 226, LCDR2 comprising an amino acid sequence of SEQ ID NO: 227, and
LCDR3
comprising an amino acid sequence of SEQ ID NO: 228; t) HCDR1 comprising an
amino acid
sequence of SEQ ID NO: 260, HCDR2 comprising an amino acid sequence of SEQ ID
NO: 261,
HCDR3 comprising an amino acid sequence of SEQ ID NO: 262, LCDR1 comprising an
amino
acid sequence of SEQ ID NO: 265, LCDR2 comprising an amino acid sequence of
SEQ ID NO:
266, and LCDR3 comprising an amino acid sequence of SEQ ID NO: 267; u) HCDR1
comprising an amino acid sequence of SEQ ID NO: 273, HCDR2 comprising an amino
acid
sequence of SEQ ID NO: 274, HCDR3 comprising an amino acid sequence of SEQ ID
NO: 275,
LCDR1 comprising an amino acid sequence of SEQ ID NO: 278, LCDR2 comprising an
amino
acid sequence of SEQ ID NO: 279, and LCDR3 comprising an amino acid sequence
of SEQ ID
NO: 280; v) HCDR1 comprising an amino acid sequence of SEQ ID NO: 286, HCDR2
comprising an amino acid sequence of SEQ ID NO: 287, HCDR3 comprising an amino
acid
sequence of SEQ ID NO: 288, LCDR1 comprising an amino acid sequence of SEQ ID
NO: 291,
LCDR2 comprising an amino acid sequence of SEQ ID NO: 292, and LCDR3
comprising an
amino acid sequence of SEQ ID NO: 293; w) HCDR1 comprising an amino acid
sequence of
SEQ ID NO: 299, HCDR2 comprising an amino acid sequence of SEQ ID NO: 300,
HCDR3
comprising an amino acid sequence of SEQ ID NO: 301, LCDR1 comprising an amino
acid
sequence of SEQ ID NO: 304, LCDR2 comprising an amino acid sequence of SEQ ID
NO: 305,
-52-
CA 03065919 2019-12-02
WO 2018/227023
PCT/US2018/036560
and LCDR3 comprising an amino acid sequence of SEQ ID NO: 306; x) HCDR1
comprising an
amino acid sequence of SEQ ID NO: 312, HCDR2 comprising an amino acid sequence
of SEQ
ID NO: 313, HCDR3 comprising an amino acid sequence of SEQ ID NO: 314, LCDR1
comprising an amino acid sequence of SEQ ID NO: 317, LCDR2 comprising an amino
acid
sequence of SEQ ID NO: 318, and LCDR3 comprising an amino acid sequence of SEQ
ID NO:
319; y) HCDR1 comprising an amino acid sequence of SEQ ID NO: 325, HCDR2
comprising an
amino acid sequence of SEQ ID NO: 326, HCDR3 comprising an amino acid sequence
of SEQ
ID NO: 327, LCDR1 comprising an amino acid sequence of SEQ ID NO: 330, LCDR2
comprising an amino acid sequence of SEQ ID NO: 331, and LCDR3 comprising an
amino acid
sequence of SEQ ID NO: 332; z) HCDR1 comprising an amino acid sequence of SEQ
ID NO:
338, HCDR2 comprising an amino acid sequence of SEQ ID NO: 339, HCDR3
comprising an
amino acid sequence of SEQ ID NO: 340, LCDR1 comprising an amino acid sequence
of SEQ
ID NO: 343, LCDR2 comprising an amino acid sequence of SEQ ID NO: 344, and
LCDR3
comprising an amino acid sequence of SEQ ID NO: 345; aa) HCDR1 comprising an
amino acid
sequence of SEQ ID NO: 351, HCDR2 comprising an amino acid sequence of SEQ ID
NO: 352,
HCDR3 comprising an amino acid sequence of SEQ ID NO: 353, LCDR1 comprising an
amino
acid sequence of SEQ ID NO: 356, LCDR2 comprising an amino acid sequence of
SEQ ID NO:
357, and LCDR3 comprising an amino acid sequence of SEQ ID NO: 358; bb) HCDR1
comprising an amino acid sequence of SEQ ID NO: 364, HCDR2 comprising an amino
acid
sequence of SEQ ID NO: 365, HCDR3 comprising an amino acid sequence of SEQ ID
NO: 366,
LCDR1 comprising an amino acid sequence of SEQ ID NO: 369, LCDR2 comprising an
amino
acid sequence of SEQ ID NO: 370, and LCDR3 comprising an amino acid sequence
of SEQ ID
NO: 371; cc) HCDR1 comprising an amino acid sequence of SEQ ID NO: 377, HCDR2
comprising an amino acid sequence of SEQ ID NO: 378, HCDR3 comprising an amino
acid
sequence of SEQ ID NO: 379, LCDR1 comprising an amino acid sequence of SEQ ID
NO: 382,
LCDR2 comprising an amino acid sequence of SEQ ID NO: 383, and LCDR3
comprising an
amino acid sequence of SEQ ID NO: 384; dd) HCDR1 comprising an amino acid
sequence of
SEQ ID NO: 390, HCDR2 comprising an amino acid sequence of SEQ ID NO: 391,
HCDR3
comprising an amino acid sequence of SEQ ID NO: 392, LCDR1 comprising an amino
acid
sequence of SEQ ID NO: 395, LCDR2 comprising an amino acid sequence of SEQ ID
NO: 396,
and LCDR3 comprising an amino acid sequence of SEQ ID NO: 397; ee) HCDR1
comprising an
amino acid sequence of SEQ ID NO: 403, HCDR2 comprising an amino acid sequence
of SEQ
ID NO: 404, HCDR3 comprising an amino acid sequence of SEQ ID NO: 405, LCDR1
comprising an amino acid sequence of SEQ ID NO: 408, LCDR2 comprising an amino
acid
sequence of SEQ ID NO: 409, and LCDR3 comprising an amino acid sequence of SEQ
ID NO:
-53-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
410; ff) HCDR1 comprising an amino acid sequence of SEQ ID NO: 416, HCDR2
comprising an
amino acid sequence of SEQ ID NO: 417, HCDR3 comprising an amino acid sequence
of SEQ
ID NO: 418, LCDR1 comprising an amino acid sequence of SEQ ID NO: 421, LCDR2
comprising an amino acid sequence of SEQ ID NO: 422, and LCDR3 comprising an
amino acid
sequence of SEQ ID NO: 423; gg) HCDR1 comprising an amino acid sequence of SEQ
ID NO:
429, HCDR2 comprising an amino acid sequence of SEQ ID NO: 430, HCDR3
comprising an
amino acid sequence of SEQ ID NO: 431, LCDR1 comprising an amino acid sequence
of SEQ
ID NO: 434, LCDR2 comprising an amino acid sequence of SEQ ID NO: 435, and
LCDR3
comprising an amino acid sequence of SEQ ID NO: 436; hh) HCDR1 comprising an
amino acid
sequence of SEQ ID NO: 442, HCDR2 comprising an amino acid sequence of SEQ ID
NO: 443,
HCDR3 comprising an amino acid sequence of SEQ ID NO: 444, LCDR1 comprising an
amino
acid sequence of SEQ ID NO: 447, LCDR2 comprising an amino acid sequence of
SEQ ID NO:
448, and LCDR3 comprising an amino acid sequence of SEQ ID NO: 449; ii) HCDR1
comprising an amino acid sequence of SEQ ID NO: 455, HCDR2 comprising an amino
acid
sequence of SEQ ID NO: 456, HCDR3 comprising an amino acid sequence of SEQ ID
NO: 457,
LCDR1 comprising an amino acid sequence of SEQ ID NO: 460, LCDR2 comprising an
amino
acid sequence of SEQ ID NO: 461, and LCDR3 comprising an amino acid sequence
of SEQ ID
NO: 462; jj) HCDR1 comprising an amino acid sequence of SEQ ID NO: 468, HCDR2
comprising an amino acid sequence of SEQ ID NO: 469, HCDR3 comprising an amino
acid
sequence of SEQ ID NO: 470, LCDR1 comprising an amino acid sequence of SEQ ID
NO: 473,
LCDR2 comprising an amino acid sequence of SEQ ID NO: 474, and LCDR3
comprising an
amino acid sequence of SEQ ID NO: 475; kk) HCDR1 comprising an amino acid
sequence of
SEQ ID NO: 481, HCDR2 comprising an amino acid sequence of SEQ ID NO: 482,
HCDR3
comprising an amino acid sequence of SEQ ID NO: 483, LCDR1 comprising an amino
acid
sequence of SEQ ID NO: 486, LCDR2 comprising an amino acid sequence of SEQ ID
NO: 487,
and LCDR3 comprising an amino acid sequence of SEQ ID NO: 488; 11) HCDR1
comprising an
amino acid sequence of SEQ ID NO: 494, HCDR2 comprising an amino acid sequence
of SEQ
ID NO: 495, HCDR3 comprising an amino acid sequence of SEQ ID NO: 496, LCDR1
comprising an amino acid sequence of SEQ ID NO: 499, LCDR2 comprising an amino
acid
sequence of SEQ ID NO: 500, and LCDR3 comprising an amino acid sequence of SEQ
ID NO:
501; mm) HCDR1 comprising an amino acid sequence of SEQ ID NO: 673, HCDR2
comprising
an amino acid sequence of SEQ ID NO: 674, HCDR3 comprising an amino acid
sequence of
SEQ ID NO: 675, LCDR1 comprising an amino acid sequence of SEQ ID NO: 676,
LCDR2
comprising an amino acid sequence of SEQ ID NO: 677, and LCDR3 comprising an
amino acid
sequence of SEQ ID NO: 678; nn) HCDR1 comprising an amino acid sequence of SEQ
ID NO:
-54-
CA 03065919 2019-12-02
WO 2018/227023
PCT/US2018/036560
850, HCDR2 comprising an amino acid sequence of SEQ ID NO: 851, HCDR3
comprising an
amino acid sequence of SEQ ID NO: 852, LCDR1 comprising an amino acid sequence
of SEQ
ID NO: 853, LCDR2 comprising an amino acid sequence of SEQ ID NO: 854, and
LCDR3
comprising an amino acid sequence of SEQ ID NO: 855; oo) HCDR1 comprising an
amino acid
sequence of SEQ ID NO: 856, HCDR2 comprising an amino acid sequence of SEQ ID
NO: 857,
HCDR3 comprising an amino acid sequence of SEQ ID NO: 858, LCDR1 comprising an
amino
acid sequence of SEQ ID NO: 859, LCDR2 comprising an amino acid sequence of
SEQ ID NO:
860, and LCDR3 comprising an amino acid sequence of SEQ ID NO: 861; pp) HCDR1
comprising an amino acid sequence of SEQ ID NO: 862, HCDR2 comprising an amino
acid
sequence of SEQ ID NO: 863, HCDR3 comprising an amino acid sequence of SEQ ID
NO: 864,
LCDR1 comprising an amino acid sequence of SEQ ID NO: 865, LCDR2 comprising an
amino
acid sequence of SEQ ID NO: 866, and LCDR3 comprising an amino acid sequence
of SEQ ID
NO: 867; qq) HCDR1 comprising an amino acid sequence of SEQ ID NO: 868, HCDR2
comprising an amino acid sequence of SEQ ID NO: 869, HCDR3 comprising an amino
acid
sequence of SEQ ID NO: 870, LCDR1 comprising an amino acid sequence of SEQ ID
NO: 871,
LCDR2 comprising an amino acid sequence of SEQ ID NO: 872, and LCDR3
comprising an
amino acid sequence of SEQ ID NO: 873; rr) HCDR1 comprising an amino acid
sequence of
SEQ ID NO: 874, HCDR2 comprising an amino acid sequence of SEQ ID NO: 875,
HCDR3
comprising an amino acid sequence of SEQ ID NO: 876, LCDR1 comprising an amino
acid
sequence of SEQ ID NO: 877, LCDR2 comprising an amino acid sequence of SEQ ID
NO: 878,
and LCDR3 comprising an amino acid sequence of SEQ ID NO: 879; or ss) HCDR1
comprising
an amino acid sequence of SEQ ID NO: 880, HCDR2 comprising an amino acid
sequence of
SEQ ID NO: 881, HCDR3 comprising an amino acid sequence of SEQ ID NO: 882,
LCDR1
comprising an amino acid sequence of SEQ ID NO: 883, LCDR2 comprising an amino
acid
sequence of SEQ ID NO: 884, and LCDR3 comprising an amino acid sequence of SEQ
ID NO:
885.
[0215] An antibody construct can comprise a first binding domain that
specifically binds a tumor
antigen. An antibody construct can comprise a first binding domain comprising
one or more
variable domains. An antibody construct can comprise a first binding domain
comprising a light
chain variable domain (VL domain). A first binding domain can have at least
80% or 100%
sequence identity to any VL sequence in TABLE 2. An antibody construct can
comprise a first
binding domain comprising a heavy chain variable domain (VH domain). A first
binding domain
can comprise at least 80% or 100% sequence identity to any VH sequence in
TABLE 2. A first
binding domain can comprise at least 80% sequence identity to any sequence in
TABLE 2. A
-55-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
first binding domain can have a pair of VH and VL regions, having sequence
selected from the
pairs in TABLE 2.
[0216] An antibody construct can comprise a first binding domain that
specifically binds a tumor
antigen, wherein the first binding domain comprises: a) a VH sequence having
at least 80% or
100% sequence identity to an amino acid sequence of SEQ ID NO: 12, and a VL
sequence having
at least 80% sequence identity to an amino acid sequence of SEQ ID NO: 17; b)
a VH sequence
having at least 80% sequence identity to an amino acid sequence of SEQ ID NO:
25, and a VL
sequence having at least 80% sequence identity to an amino acid sequence of
SEQ ID NO: 30; c)
a VH sequence having at least 80% sequence identity to an amino acid sequence
of SEQ ID NO:
38, and a VL sequence having at least 80% sequence identity to an amino acid
sequence of SEQ
ID NO: 43; d) a VH sequence having at least 80% sequence identity to an amino
acid sequence of
SEQ ID NO: 51, and a VL sequence having at least 80% sequence identity to an
amino acid
sequence of SEQ ID NO: 56; e) a VH sequence having at least 80% sequence
identity to an amino
acid sequence of SEQ ID NO: 64, and a VL sequence having at least 80% sequence
identity to an
amino acid sequence of SEQ ID NO: 69; f) a VH sequence having at least 80%
sequence identity
to an amino acid sequence of SEQ ID NO: 77, and a VL sequence having at least
80% sequence
identity to an amino acid sequence of SEQ ID NO: 82; g) a VH sequence having
at least 80%
sequence identity to an amino acid sequence of SEQ ID NO: 90, and a VL
sequence having at
least 80% sequence identity to an amino acid sequence of SEQ ID NO: 95; h) a
VH sequence
having at least 80% sequence identity to an amino acid sequence of SEQ ID NO:
103, and a VL
sequence having at least 80% sequence identity to an amino acid sequence of
SEQ ID NO: 108; i)
a VH sequence having at least 80% sequence identity to an amino acid sequence
of SEQ ID NO:
116, and a VL sequence having at least 80% sequence identity to an amino acid
sequence of SEQ
ID NO: 121; j) a VH sequence having at least 80% sequence identity to an amino
acid sequence
of SEQ ID NO: 129, and a VL sequence having at least 80% sequence identity to
an amino acid
sequence of SEQ ID NO: 134; k) a VH sequence having at least 80% sequence
identity to an
amino acid sequence of SEQ ID NO: 142, and a VL sequence having at least 80%
sequence
identity to an amino acid sequence of SEQ ID NO: 147; 1) a VH sequence having
at least 80%
sequence identity to an amino acid sequence of SEQ ID NO: 155, and a VL
sequence having at
least 80% sequence identity to an amino acid sequence of SEQ ID NO: 160; m) a
VH sequence
having at least 80% sequence identity to an amino acid sequence of SEQ ID NO:
168, and a VL
sequence having at least 80% sequence identity to an amino acid sequence of
SEQ ID NO: 173; n)
a VH sequence having at least 80% sequence identity to an amino acid sequence
of SEQ ID NO:
181, and a VL sequence having at least 80% sequence identity to an amino acid
sequence of SEQ
ID NO: 186; o) a VH sequence having at least 80% sequence identity to an amino
acid sequence
-56-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
of SEQ ID NO: 194, and a VL sequence having at least 80% sequence identity to
an amino acid
sequence of SEQ ID NO: 199; p) a VH sequence having at least 80% sequence
identity to an
amino acid sequence of SEQ ID NO: 207, and a VL sequence having at least 80%
sequence
identity to an amino acid sequence of SEQ ID NO: 212; q) a VH sequence having
at least 80%
sequence identity to an amino acid sequence of SEQ ID NO: 811, and a VL
sequence having at
least 80% sequence identity to an amino acid sequence of SEQ ID NO: 812; r) a
VH sequence
having at least 80% sequence identity to an amino acid sequence of SEQ ID NO:
829, and a VL
sequence having at least 80% sequence identity to an amino acid sequence of
SEQ ID NO: 830; s)
a VH sequence having at least 80% sequence identity to an amino acid sequence
of SEQ ID NO:
220, and a VL sequence having at least 80% sequence identity to an amino acid
sequence of SEQ
ID NO: 225; t) a VH sequence having at least 80% sequence identity to an amino
acid sequence
of SEQ ID NO: 259, and a VL sequence having at least 80% sequence identity to
an amino acid
sequence of SEQ ID NO: 264; u) a VH sequence having at least 80% sequence
identity to an
amino acid sequence of SEQ ID NO: 272, and a VL sequence having at least 80%
sequence
identity to an amino acid sequence of SEQ ID NO: 277; v) a VH sequence having
at least 80%
sequence identity to an amino acid sequence of SEQ ID NO: 285, and a VL
sequence having at
least 80% sequence identity to an amino acid sequence of SEQ ID NO: 290; w) a
VH sequence
having at least 80% sequence identity to an amino acid sequence of SEQ ID NO:
298, and a VL
sequence having at least 80% sequence identity to an amino acid sequence of
SEQ ID NO: 303; x)
a VH sequence having at least 80% sequence identity to an amino acid sequence
of SEQ ID NO:
311, and a VL sequence having at least 80% sequence identity to an amino acid
sequence of SEQ
ID NO: 316; y) a VH sequence having at least 80% sequence identity to an amino
acid sequence
of SEQ ID NO: 324, and a VL sequence having at least 80% sequence identity to
an amino acid
sequence of SEQ ID NO: 328; z) a VH sequence having at least 80% sequence
identity to an
amino acid sequence of SEQ ID NO: 337, and a VL sequence having at least 80%
sequence
identity to an amino acid sequence of SEQ ID NO: 342; aa) a VH sequence having
at least 80%
sequence identity to an amino acid sequence of SEQ ID NO: 350, and a VL
sequence having at
least 80% sequence identity to an amino acid sequence of SEQ ID NO: 355; bb) a
VH sequence
having at least 80% sequence identity to an amino acid sequence of SEQ ID NO:
363, and a VL
sequence having at least 80% sequence identity to an amino acid sequence of
SEQ ID NO: 368;
cc) a VH sequence having at least 80% sequence identity to an amino acid
sequence of SEQ ID
NO: 376, and a VL sequence having at least 80% sequence identity to an amino
acid sequence of
SEQ ID NO: 381; dd) a VH sequence having at least 80% sequence identity to an
amino acid
sequence of SEQ ID NO: 389, and a VL sequence having at least 80% sequence
identity to an
amino acid sequence of SEQ ID NO: 394; ee) a VH sequence having at least 80%
sequence
-57-
CA 03065919 2019-12-02
WO 2018/227023
PCT/US2018/036560
identity to an amino acid sequence of SEQ ID NO: 402, and a VL sequence having
at least 80%
sequence identity to an amino acid sequence of SEQ ID NO: 407; ff) a VH
sequence having at
least 80% sequence identity to an amino acid sequence of SEQ ID NO: 415, and a
VL sequence
having at least 80% sequence identity to an amino acid sequence of SEQ ID NO:
420; gg) a VH
sequence having at least 80% sequence identity to an amino acid sequence of
SEQ ID NO: 428,
and a VL sequence having at least 80% sequence identity to an amino acid
sequence of SEQ ID
NO: 433; hh) a VH sequence having at least 80% sequence identity to an amino
acid sequence of
SEQ ID NO: 441, and a VL sequence having at least 80% sequence identity to an
amino acid
sequence of SEQ ID NO: 446; ii) a VH sequence having at least 80% sequence
identity to an
amino acid sequence of SEQ ID NO: 454, and a VL sequence having at least 80%
sequence
identity to an amino acid sequence of SEQ ID NO: 459; jj) a VH sequence having
at least 80%
sequence identity to an amino acid sequence of SEQ ID NO: 467, and a VL
sequence having at
least 80% sequence identity to an amino acid sequence of SEQ ID NO: 472; kk) a
VH sequence
having at least 80% sequence identity to an amino acid sequence of SEQ ID NO:
480, and a VL
sequence having at least 80% sequence identity to an amino acid sequence of
SEQ ID NO: 485;
11) a VH sequence having at least 80% sequence identity to an amino acid
sequence of SEQ ID
NO: 493, and a VL sequence having at least 80% sequence identity to an amino
acid sequence of
SEQ ID NO: 498; mm) a VH sequence having at least 80% sequence identity to an
amino acid
sequence of SEQ ID NO: 679, and a VL sequence having at least 80% sequence
identity to an
amino acid sequence of SEQ ID NO: 680; nn) a VH sequence having at least 80%
sequence
identity to an amino acid sequence of SEQ ID NO: 886, and a VL sequence having
at least 80%
sequence identity to an amino acid sequence of SEQ ID NO: 887; oo) a VH
sequence having at
least 80% sequence identity to an amino acid sequence of SEQ ID NO: 888, and a
VL sequence
having at least 80% sequence identity to an amino acid sequence of SEQ ID NO:
889; pp) a VH
sequence having at least 80% sequence identity to an amino acid sequence of
SEQ ID NO: 890,
and a VL sequence having at least 80% sequence identity to an amino acid
sequence of SEQ ID
NO: 891; qq) a VH sequence having at least 80% sequence identity to an amino
acid sequence of
SEQ ID NO: 892, and a VL sequence having at least 80% sequence identity to an
amino acid
sequence of SEQ ID NO: 893; rr) a VH sequence having at least 80% sequence
identity to an
amino acid sequence of SEQ ID NO: 894, and a VL sequence having at least 80%
sequence
identity to an amino acid sequence of SEQ ID NO:895; ss) a VH sequence having
at least 80%
sequence identity to an amino acid sequence of SEQ ID NO: 896, and a VL
sequence having at
least 80% sequence identity to an amino acid sequence of SEQ ID NO: 897.
[0217] An antibody construct can comprise a first binding domain and an Fc
domain, wherein
the first binding domain and the Fc domain comprise an antibody. A first
binding domain can
-58-
CA 03065919 2019-12-02
WO 2018/227023
PCT/US2018/036560
specifically bind a tumor antigen. An antibody construct can comprise an
antibody light chain
wherein the antibody construct specifically binds to an antigen. An antibody
construct can
comprise a light chain comprising at least 80% or 100% sequence identity to a
light chain
sequence in TABLE 3, wherein the antibody construct specifically binds to an
antigen. An
antibody construct can comprise an antibody heavy chain, wherein the antibody
construct
specifically binds to an antigen. An antibody construct can comprise a heavy
chain comprising at
least 80% or 100% sequence identity to a heavy chain sequence in TABLE 3,
wherein the
antibody construct specifically binds to an antigen. An antibody construct can
comprise at least
80% sequence identity to any sequence in TABLE 3, wherein the antibody
construct specifically
binds to an antigen. An antibody construct can have a pair of heavy and light
chains having
sequences selected from the pairs of sequences in TABLE 3, wherein the
antibody construct
specifically binds to an antigen.
[0218] An antibody construct can comprise an anti-tumor antibody, wherein the
antibody
comprises: a) a heavy chain sequence having at least 80% sequence identity to
an amino acid
sequence of SEQ ID NO: 11, and a light chain sequence having at least 80%
sequence identity to
an amino acid sequence of SEQ ID NO: 16; b) a heavy chain sequence having at
least 80%
sequence identity to an amino acid sequence of SEQ ID NO: 24, and a light
chain sequence
having at least 80% sequence identity to an amino acid sequence of SEQ ID NO:
29; c) a heavy
chain sequence having at least 80% sequence identity to an amino acid sequence
of SEQ ID NO:
37, and a light chain sequence having at least 80% sequence identity to an
amino acid sequence
of SEQ ID NO: 42; d) a heavy chain sequence having at least 80% sequence
identity to an amino
acid sequence of SEQ ID NO: 50, and a light chain sequence having at least 80%
sequence
identity to an amino acid sequence of SEQ ID NO: 55; e) a heavy chain sequence
having at least
80% sequence identity to an amino acid sequence of SEQ ID NO: 63, and a light
chain sequence
having at least 80% sequence identity to an amino acid sequence of SEQ ID NO:
68; f) a heavy
chain sequence having at least 80% sequence identity to an amino acid sequence
of SEQ ID NO:
76, and a light chain sequence having at least 80% sequence identity to an
amino acid sequence
of SEQ ID NO: 81; g) a heavy chain sequence having at least 80% sequence
identity to an amino
acid sequence of SEQ ID NO: 89, and a light chain sequence having at least 80%
sequence
identity to an amino acid sequence of SEQ ID NO: 94; h) a heavy chain sequence
having at least
80% sequence identity to an amino acid sequence of SEQ ID NO: 102, and a light
chain
sequence having at least 80% sequence identity to an amino acid sequence of
SEQ ID NO: 107;
i) a heavy chain sequence having at least 80% sequence identity to an amino
acid sequence of
SEQ ID NO: 115, and a light chain sequence having at least 80% sequence
identity to an amino
acid sequence of SEQ ID NO: 120; j) a heavy chain sequence having at least 80%
sequence
-59-
CA 03065919 2019-12-02
WO 2018/227023
PCT/US2018/036560
identity to an amino acid sequence of SEQ ID NO: 128, and a light chain
sequence having at
least 80% sequence identity to an amino acid sequence of SEQ ID NO: 133; k) a
heavy chain
sequence having at least 80% sequence identity to an amino acid sequence of
SEQ ID NO: 141,
and a light chain sequence having at least 80% sequence identity to an amino
acid sequence of
SEQ ID NO: 146;1) a heavy chain sequence having at least 80% sequence identity
to an amino
acid sequence of SEQ ID NO: 154, and a light chain sequence having at least
80% sequence
identity to an amino acid sequence of SEQ ID NO: 159; m) a heavy chain
sequence having at
least 80% sequence identity to an amino acid sequence of SEQ ID NO: 167, and a
light chain
sequence having at least 80% sequence identity to an amino acid sequence of
SEQ ID NO: 172;
n) a heavy chain sequence having at least 80% sequence identity to an amino
acid sequence of
SEQ ID NO: 180, and a light chain sequence having at least 80% sequence
identity to an amino
acid sequence of SEQ ID NO: 185; o) a heavy chain sequence having at least 80%
sequence
identity to an amino acid sequence of SEQ ID NO: 193, and a light chain
sequence having at
least 80% sequence identity to an amino acid sequence of SEQ ID NO: 198; p) a
heavy chain
sequence having at least 80% sequence identity to an amino acid sequence of
SEQ ID NO: 206,
and a light chain sequence having at least 80% sequence identity to an amino
acid sequence of
SEQ ID NO: 211; q) a heavy chain sequence having at least 80% sequence
identity to an amino
acid sequence of SEQ ID NO: 813, and a light chain sequence having at least
80% sequence
identity to an amino acid sequence of SEQ ID NO: 814; r) a heavy chain
sequence having at least
80% sequence identity to an amino acid sequence of SEQ ID NO: 831, and a light
chain
sequence having at least 80% sequence identity to an amino acid sequence of
SEQ ID NO: 832;
s) a heavy chain sequence having at least 80% sequence identity to an amino
acid sequence of
SEQ ID NO: 219, and a light chain sequence having at least 80% sequence
identity to an amino
acid sequence of SEQ ID NO: 224; t) a heavy chain sequence having at least 80%
sequence
identity to an amino acid sequence of SEQ ID NO: 258, and a light chain
sequence having at
least 80% sequence identity to an amino acid sequence of SEQ ID NO: 263; u) a
heavy chain
sequence having at least 80% sequence identity to an amino acid sequence of
SEQ ID NO: 271,
and a light chain sequence having at least 80% sequence identity to an amino
acid sequence of
SEQ ID NO: 276; v) a heavy chain sequence having at least 80% sequence
identity to an amino
acid sequence of SEQ ID NO: 284, and a light chain sequence having at least
80% sequence
identity to an amino acid sequence of SEQ ID NO: 289; w) a heavy chain
sequence having at
least 80% sequence identity to an amino acid sequence of SEQ ID NO: 297, and a
light chain
sequence having at least 80% sequence identity to an amino acid sequence of
SEQ ID NO: 302;
x) a heavy chain sequence having at least 80% sequence identity to an amino
acid sequence of
SEQ ID NO: 310, and a light chain sequence having at least 80% sequence
identity to an amino
-60-
CA 03065919 2019-12-02
WO 2018/227023
PCT/US2018/036560
acid sequence of SEQ ID NO: 315; y) a heavy chain sequence having at least 80%
sequence
identity to an amino acid sequence of SEQ ID NO: 323, and a light chain
sequence having at
least 80% sequence identity to an amino acid sequence of SEQ ID NO: 328; z) a
heavy chain
sequence having at least 80% sequence identity to an amino acid sequence of
SEQ ID NO: 336,
and a light chain sequence having at least 80% sequence identity to an amino
acid sequence of
SEQ ID NO: 341; aa) a heavy chain sequence having at least 80% sequence
identity to an amino
acid sequence of SEQ ID NO: 349, and a light chain sequence having at least
80% sequence
identity to an amino acid sequence of SEQ ID NO: 354; bb) a heavy chain
sequence having at
least 80% sequence identity to an amino acid sequence of SEQ ID NO: 362, and a
light chain
sequence having at least 80% sequence identity to an amino acid sequence of
SEQ ID NO: 367;
cc) a heavy chain sequence having at least 80% sequence identity to an amino
acid sequence of
SEQ ID NO: 375, and a light chain sequence having at least 80% sequence
identity to an amino
acid sequence of SEQ ID NO: 380; dd) a heavy chain sequence having at least
80% sequence
identity to an amino acid sequence of SEQ ID NO: 388, and a light chain
sequence having at
least 80% sequence identity to an amino acid sequence of SEQ ID NO: 393; ee) a
heavy chain
sequence having at least 80% sequence identity to an amino acid sequence of
SEQ ID NO: 401,
and a light chain sequence having at least 80% sequence identity to an amino
acid sequence of
SEQ ID NO: 406; ff) a heavy chain sequence having at least 80% sequence
identity to an amino
acid sequence of SEQ ID NO: 414, and a light chain sequence having at least
80% sequence
identity to an amino acid sequence of SEQ ID NO: 419; gg) a heavy chain
sequence having at
least 80% sequence identity to an amino acid sequence of SEQ ID NO: 427, and a
light chain
sequence having at least 80% sequence identity to an amino acid sequence of
SEQ ID NO: 432;
hh) a heavy chain sequence having at least 80% sequence identity to an amino
acid sequence of
SEQ ID NO: 440, and a light chain sequence having at least 80% sequence
identity to an amino
acid sequence of SEQ ID NO: 445; ii) a heavy chain sequence having at least
80% sequence
identity to an amino acid sequence of SEQ ID NO: 453, and a light chain
sequence having at
least 80% sequence identity to an amino acid sequence of SEQ ID NO: 458; jj) a
heavy chain
sequence having at least 80% sequence identity to an amino acid sequence of
SEQ ID NO: 466,
and a light chain sequence having at least 80% sequence identity to an amino
acid sequence of
SEQ ID NO: 471; kk) a heavy chain sequence having at least 80% sequence
identity to an amino
acid sequence of SEQ ID NO: 479, and a light chain sequence having at least
80% sequence
identity to an amino acid sequence of SEQ ID NO: 484; 11) a heavy chain
sequence having at
least 80% sequence identity to an amino acid sequence of SEQ ID NO: 492, and a
light chain
sequence having at least 80% sequence identity to an amino acid sequence of
SEQ ID NO: 497;
or mm) a heavy chain sequence having at least 80% sequence identity to an
amino acid sequence
-61-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
of SEQ ID NO: 681, and a light chain sequence having at least 80% sequence
identity to an
amino acid sequence of SEQ ID NO: 682.
[0219] An antibody construct can comprise a second binding domain that
specifically binds to an
antigen. An antibody construct can comprise a second binding domain that
specifically binds to
an antigen on an antigen presenting cell. An antigen presenting cell can be a
dendritic cell or a
macrophage. A second binding domain can specifically bind to an antigen on an
immune cell
such as an antigen presenting cell, wherein the molecule comprises at least
80% sequence
identity to a group consisting of CD40, CD47, TNFR2, DEC-205, PD-L1, CD36
mannose
scavenger receptor 1, CLEC9A, DC-SIGN, CLEC12A, BDCA-2, OX4OL, 41BBL, CD204,
MARCO, CLEC5A, Dectin 1, Dectin 2, CLEC10A, CD206, CD64, CD32A, CD16A, HVEM,
CD38, TREM2, CSF1R, or CD32B.
[0220] An antibody construct can further comprise an Fc domain. An antibody
construct can
comprise, for example, a first binding domain, a second binding domain, and an
Fc domain,
wherein the first binding domain is attached to the Fc domain. An antibody
construct can
comprise a first binding domain, a second binding domain, and an Fc domain,
wherein the
second binding domain is attached to the Fc domain. A first binding domain can
be attached to an
Fc domain as a fusion protein. A second binding domain can be attached to an
Fc domain as a
fusion protein. A first binding domain can be attached to an Fc domain via a
linker. A second
binding domain can be attached to an Fc domain via a linker.
[0221] An antibody construct can comprise a second binding domain comprising
one or more
CDRs. A second binding domain can comprise at least 80% sequence identity to
any sequence in
TABLE 5. A second binding domain can comprise a set of CDRs having the
sequences set forth
in any sete of sequence in TABLE 5.
[0222] An antibody construct can comprise a second binding domain that
specifically binds to
CD40. An antibody construct can comprise a second binding domain that is a
CD40 agonist. An
antibody construct can comprise a second binding domain that binds to CD40,
wherein the
second binding domain comprises at least 80% sequence identity to: a) HCDR1
comprising an
amino acid sequence of SEQ ID NO: 3, HCDR2 comprising an amino acid sequence
of SEQ ID
NO: 4, HCDR3 comprising an amino acid sequence of SEQ ID NO: 5, LCDR1
comprising an
amino acid sequence of SEQ ID NO: 8, LCDR2 comprising an amino acid sequence
of SEQ ID
NO: 9, and LCDR3 comprising an amino acid sequence of SEQ ID NO: 10; b) HCDR1
comprising an amino acid sequence of SEQ ID NO: 582, HCDR2 comprising an amino
acid
sequence of SEQ ID NO: 583, HCDR3 comprising an amino acid sequence of SEQ ID
NO: 584,
LCDR1 comprising an amino acid sequence of SEQ ID NO: 587, LCDR2 comprising an
amino
acid sequence of SEQ ID NO: 588, and LCDR3 comprising an amino acid sequence
of SEQ ID
-62-
CA 03065919 2019-12-02
WO 2018/227023
PCT/US2018/036560
NO: 589; c) HCDR1 comprising an amino acid sequence of SEQ ID NO: 592, HCDR2
comprising an amino acid sequence of SEQ ID NO: 593, HCDR3 comprising an amino
acid
sequence of SEQ ID NO: 594, LCDR1 comprising an amino acid sequence of SEQ ID
NO: 597,
LCDR2 comprising an amino acid sequence of SEQ ID NO: 598, and LCDR3
comprising an
amino acid sequence of SEQ ID NO: 599; d) HCDR1 comprising an amino acid
sequence of
SEQ ID NO: 602, HCDR2 comprising an amino acid sequence of SEQ ID NO: 603,
HCDR3
comprising an amino acid sequence of SEQ ID NO: 604, LCDR1 comprising an amino
acid
sequence of SEQ ID NO: 607, LCDR2 comprising an amino acid sequence of SEQ ID
NO: 608,
and LCDR3 comprising an amino acid sequence of SEQ ID NO: 609; e) HCDR1
comprising an
amino acid sequence of SEQ ID NO: 612, HCDR2 comprising an amino acid sequence
of SEQ
ID NO: 613, HCDR3 comprising an amino acid sequence of SEQ ID NO: 614, LCDR1
comprising an amino acid sequence of SEQ ID NO: 617, LCDR2 comprising an amino
acid
sequence of SEQ ID NO: 618, and LCDR3 comprising an amino acid sequence of SEQ
ID NO:
619; f) HCDR1 comprising an amino acid sequence of SEQ ID NO: 622, HCDR2
comprising an
amino acid sequence of SEQ ID NO: 623, HCDR3 comprising an amino acid sequence
of SEQ
ID NO: 624, LCDR1 comprising an amino acid sequence of SEQ ID NO: 627, LCDR2
comprising an amino acid sequence of SEQ ID NO: 628, and LCDR3 comprising an
amino acid
sequence of SEQ ID NO: 629; or g) HCDR1 comprising an amino acid sequence of
SEQ ID NO:
632, HCDR2 comprising an amino acid sequence of SEQ ID NO: 633, HCDR3
comprising an
amino acid sequence of SEQ ID NO: 634, LCDR1 comprising an amino acid sequence
of SEQ
ID NO: 637, LCDR2 comprising an amino acid sequence of SEQ ID NO: 638, and
LCDR3
comprising an amino acid sequence of SEQ ID NO: 639.
[0223] An antibody construct can comprise a second binding domain that
specifically binds DC-
SIGN. An antibody construct can comprise a second binding domain that binds DC-
SIGN,
wherein the second binding domain comprises at least 80% sequence identity to:
a) HCDR1
comprising an amino acid sequence of SEQ ID NO: 640, HCDR2 comprising an amino
acid
sequence of SEQ ID NO: 641, HCDR3 comprising an amino acid sequence of SEQ ID
NO: 642,
LCDR1 comprising an amino acid sequence of SEQ ID NO: 643, LCDR2 comprising an
amino
acid sequence of SEQ ID NO: 644, and LCDR3 comprising an amino acid sequence
of SEQ ID
NO: 645; b) HCDR1 comprising an amino acid sequence of SEQ ID NO: 646, HCDR2
comprising an amino acid sequence of SEQ ID NO: 647, HCDR3 comprising an amino
acid
sequence of SEQ ID NO: 648, LCDR1 comprising an amino acid sequence of SEQ ID
NO: 649,
LCDR2 comprising an amino acid sequence of SEQ ID NO: 650, and LCDR3
comprising an
amino acid sequence of SEQ ID NO: 651; or c) HCDR1 comprising an amino acid
sequence of
SEQ ID NO: 652, HCDR2 comprising an amino acid sequence of SEQ ID NO: 653,
HCDR3
-63-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
comprising an amino acid sequence of SEQ ID NO: 654, LCDR1 comprising an amino
acid
sequence of SEQ ID NO: 655, LCDR2 comprising an amino acid sequence of SEQ ID
NO: 656,
and LCDR3 comprising an amino acid sequence of SEQ ID NO: 657.
[0224] An antibody construct can comprise a second binding domain that
specifically binds
DEC-205. An antibody construct comprising a second binding domain that binds
DEC-205 can
comprise at least 80% sequence identity to: a) HCDR1 comprising an amino acid
sequence of
SEQ ID NO: 234, HCDR2 comprising an amino acid sequence of SEQ ID NO: 235,
HCDR3
comprising an amino acid sequence of SEQ ID NO: 236, LCDR1 comprising an amino
acid
sequence of SEQ ID NO: 239, LCDR2 comprising an amino acid sequence of SEQ ID
NO: 240,
and LCDR3 comprising an amino acid sequence of SEQ ID NO: 241; orb) HCDR1
comprising
an amino acid sequence of SEQ ID NO: 247, HCDR2 comprising an amino acid
sequence of
SEQ ID NO: 248, HCDR3 comprising an amino acid sequence of SEQ ID NO: 249,
LCDR1
comprising an amino acid sequence of SEQ ID NO: 252, LCDR2 comprising an amino
acid
sequence of SEQ ID NO: 253, and LCDR3 comprising an amino acid sequence of SEQ
ID NO:
254.
[0225] An antibody construct can comprise a second binding domain comprising
one or more
variable domains. An antibody construct can comprise a second binding domain
comprising a
light chain variable domain (VL domain). A second binding domain can comprise
at least 80%
sequence identity to any VL sequence in TABLE 6. An antibody construct can
comprise a second
binding domain comprising a heavy chain variable domain. A second binding
domain can
comprise at least 80% sequence identity to any VH sequence in TABLE 6. A
second binding
domain can comprise at least 80% sequence identity to any sequence in TABLE 6.
A second
binding domain can comprise at a pair of VH and VL domains having a pair of
sequences in
TABLE 6.
[0226] An antibody construct can comprise a second binding domain that
specifically binds
CD40. An antibody construct can comprise a second binding domain that is a
CD40 agonist. An
antibody construct can comprise a second binding domain that binds CD40,
wherein the second
binding domain comprises: a) a VH sequence having at least 80% sequence
identity to an amino
acid sequence of SEQ ID NO: 2, and a VL sequence having at least 80% sequence
identity to an
amino acid sequence of SEQ ID NO: 7; b) a VH sequence having at least 80%
sequence identity
to an amino acid sequence of SEQ ID NO: 581, and a VL sequence having at least
80% sequence
identity to an amino acid sequence of SEQ ID NO: 586; c) a VH sequence having
at least 80%
sequence identity to an amino acid sequence of SEQ ID NO: 591, and a VL
sequence having at
least 80% sequence identity to an amino acid sequence of SEQ ID NO: 596; d) a
VH sequence
having at least 80% sequence identity to an amino acid sequence of SEQ ID NO:
601, and a VL
-64-
CA 03065919 2019-12-02
WO 2018/227023
PCT/US2018/036560
sequence having at least 80% sequence identity to an amino acid sequence of
SEQ ID NO: 606;
e) a VH sequence having at least 80% sequence identity to an amino acid
sequence of SEQ ID
NO: 611, and a VL sequence having at least 80% sequence identity to an amino
acid sequence of
SEQ ID NO: 616; f) a VH sequence having at least 80% sequence identity to an
amino acid
sequence of SEQ ID NO: 621, and a VL sequence having at least 80% sequence
identity to an
amino acid sequence of SEQ ID NO: 626; g) a VH sequence having at least 80%
sequence
identity to an amino acid sequence of SEQ ID NO: 631, and a VL sequence having
at least 80%
sequence identity to an amino acid sequence of SEQ ID NO: 636.
[0227] An antibody construct can comprise a second binding domain that
specifically binds
DEC-205. An antibody construct can comprise a second binding domain that binds
DEC-205,
wherein the second binding domain comprises: a) a VH sequence having at least
80% sequence
identity to an amino acid sequence of SEQ ID NO: 233, and a VL sequence having
at least 80%
sequence identity to an amino acid sequence of SEQ ID NO: 238; or b) a VH
sequence having at
least 80% sequence identity to an amino acid sequence of SEQ ID NO: 246, and a
VL sequence
having at least 80% sequence identity to an amino acid sequence of SEQ ID NO:
251.
[0228] An antibody construct can comprise a second binding domain that
specifically binds to
CD36 mannose scavenger receptor 1. An antibody construct can comprise a second
binding
domain that binds CD36 mannose scavenger receptor 1, wherein the second
binding domain
comprises a VH sequence having at least 80% sequence identity to an amino acid
sequence of
SEQ ID NO: 658, and a VL sequence having at least 80% sequence identity to an
amino acid
sequence of SEQ ID NO: 659.
[0229] An antibody construct can comprise a second binding domain that
specifically binds to
CLEC9A. An antibody construct can comprise a second binding domain that binds
CLEC9A,
wherein the second binding domain comprises a VH sequence having at least 80%
sequence
identity to an amino acid sequence of SEQ ID NO: 660, and a VL sequence having
at least 80%
sequence identity to an amino acid sequence of SEQ ID NO: 661.
[0230] An antibody construct can comprise a second binding domain that
specifically binds to
PD-Li. An antibody construct can comprise a second binding domain that binds
PD-L1, wherein
the second binding domain comprises a VH sequence having at least 80% sequence
identity to an
amino acid sequence of SEQ ID NO: 901, and a VL sequence having at least 80%
sequence
identity to an amino acid sequence of SEQ ID NO: 902; or wherein the second
binding domain
comprises a VH sequence having at least 80% sequence identity to an amino acid
sequence of
SEQ ID NO: 890, and a VL sequence having at least 80% sequence identity to an
amino acid
sequence of SEQ ID NO: 891; or wherein the second binding domain comprises a
VH sequence
-65-
CA 03065919 2019-12-02
WO 2018/227023
PCT/US2018/036560
having at least 80% sequence identity to an amino acid sequence of SEQ ID NO:
892, and a VL
sequence having at least 80% sequence identity to an amino acid sequence of
SEQ ID NO: 893.
[0231] An antibody construct can comprise a second binding domain and an Fc
domain, wherein
the second binding domain and the Fc domain comprise an antibody. An antibody
construct can
comprise a heavy chain and a light chain that target an antigen expressed by
an antigen
presenting cell. An antibody construct can comprise an antibody light chain.
An antibody
construct can comprise a light chain comprising at least 80% sequence identity
to any light chain
sequence in TABLE 7. An antibody construct can comprise an antibody heavy
chain. An
antibody construct can comprise a heavy chain comprising at least 80% sequence
identity to any
heavy chain sequence in TABLE 7. An antibody construct can comprise at least
80% sequence
identity to any sequence in TABLE 7. An antibody construct can comprise a
heavy and light
chains having a pair of sequences in TABLE 7.
[0232] An antibody construct can comprise a heavy chain and a light chain that
target an antigen
expressed by an antigen presenting cell. An antibody construct can comprise a
first binding
domain and an Fc domain, wherein the first binding domain and the Fc domain
comprise an
antibody. An antibody construct may comprise an anti-CD40 antibody, the
antibody construct
comprising: a) a heavy chain sequence having at least 80% sequence identity to
an amino acid
sequence of SEQ ID NO: 1 and a light chain sequence having at least 80%
sequence identity to
an amino acid sequence of SEQ ID NO: 6; b) a heavy chain sequence having at
least 80%
sequence identity to an amino acid sequence of SEQ ID NO: 577 or SEQ ID NO:
578, and a light
chain sequence having at least 80% sequence identity to an amino acid sequence
of SEQ ID NO:
579; c) a heavy chain sequence having at least 80% sequence identity to an
amino acid sequence
of SEQ ID NO: 580, and a light chain sequence having at least 80% sequence
identity to an
amino acid sequence of SEQ ID NO: 585; d) a heavy chain sequence having at
least 80%
sequence identity to an amino acid sequence of SEQ ID NO: 590, and a light
chain sequence
having at least 80% sequence identity to an amino acid sequence of SEQ ID NO:
595; e) a heavy
chain sequence having at least 80% sequence identity to an amino acid sequence
of SEQ ID NO:
600, and a light chain sequence having at least 80% sequence identity to an
amino acid sequence
of SEQ ID NO: 605; f) a heavy chain sequence having at least 80% sequence
identity to an
amino acid sequence of SEQ ID NO: 610, and a light chain sequence having at
least 80%
sequence identity to an amino acid sequence of SEQ ID NO: 615; g) a heavy
chain sequence
having at least 80% sequence identity to an amino acid sequence of SEQ ID NO:
620, and a light
chain sequence having at least 80% sequence identity to an amino acid sequence
of SEQ ID NO:
625; or h) a heavy chain sequence having at least 80% sequence identity to an
amino acid
-66-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
sequence of SEQ ID NO: 630, and a light chain sequence having at least 80%
sequence identity
to an amino acid sequence of SEQ ID NO: 635.
[0233] An antibody construct can comprise a first binding domain and an Fc
domain, wherein
the first binding domain and the Fc domain comprise an antibody. An antibody
construct may
comprise an anti-DEC-205 antibody, the construct comprising: a) a heavy chain
sequence having
at least 80% sequence identity to an amino acid sequence of SEQ ID NO: 232,
and a light chain
sequence having at least 80% sequence identity to an amino acid sequence of
SEQ ID NO: 237;
or b) a heavy chain sequence having at least 80% sequence identity to an amino
acid sequence of
SEQ ID NO: 245, and a light chain sequence having at least 80% sequence
identity to an amino
acid sequence of SEQ ID NO: 250.
[0234] An antibody construct can comprise a first binding domain and an Fc
domain, wherein
the first binding domain and the Fc domain comprise an antibody. A composition
may comprise
an anti-CLEC12A antibody, the construct comprising: a) a heavy chain sequence
having at least
80% sequence identity to an amino acid sequence of SEQ ID NO: 662, and a light
chain
sequence having at least 80% sequence identity to an amino acid sequence of
SEQ ID NO: 665;
b) a heavy chain sequence having at least 80% sequence identity to an amino
acid sequence of
SEQ ID NO: 663, and a light chain sequence having at least 80% sequence
identity to an amino
acid sequence of SEQ ID NO: 665; or c) a heavy chain sequence having at least
80% sequence
identity to an amino acid sequence of SEQ ID NO: 664, and a light chain
sequence having at
least 80% sequence identity to an amino acid sequence of SEQ ID NO: 665.
[0235] An antibody construct can comprise a first binding domain and an Fc
domain, wherein
the first binding domain and the Fc domain comprise an antibody. An antibody
construct may
comprise an anti-BDCA-2 antibody, the construct comprising: a) a heavy chain
sequence having
at least 80% sequence identity to an amino acid sequence of SEQ ID NO: 666,
and a light chain
sequence having at least 80% sequence identity to an amino acid sequence of
SEQ ID NO: 669;
b) a heavy chain sequence having at least 80% sequence identity to an amino
acid sequence of
SEQ ID NO: 667, and a light chain sequence having at least 80% sequence
identity to an amino
acid sequence of SEQ ID NO: 670; or c) a heavy chain sequence having at least
80% sequence
identity to an amino acid sequence of SEQ ID NO: 668, and a light chain
sequence having at
least 80% sequence identity to an amino acid sequence of SEQ ID NO: 671.
Antibody-ScFv Fusion Protein Products
[0236] An antibody construct can comprise a first binding domain, a second
binding domain, and
an Fc domain, wherein the first binding domain and the second binding domain
are attached to
the Fc domain. The first binding domain and the second binding domain can be
attached to the Fc
-67-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
domain as a fusion protein. The first binding domain can be attached to the Fc
domain at an N-
terminal end of the Fc domain, wherein the second binding domain can be
attached to the Fc
domain at a C-terminal end. The first binding domain can be attached to the Fc
domain at an N-
terminal end of the Fc domain, wherein the second binding domain can be
attached to the Fc
domain at a C-terminal end via a polypeptide linker ranging from 10 to 25
amino acids. In some
embodiments, the polypeptide linker has the sequence [G4S]n where n = 2 to 5.
Alternatively, the
first binding domain can be attached to the Fc domain at a C-terminal end of
the Fc domain,
wherein the second binding domain can be attached to the Fc domain at an N-
terminal end. A
second binding domain and an Fc domain can comprise an antibody and a first
binding domain
can comprise a single chain variable fragment (scFv). A single chain variable
fragment can
comprise a heavy chain variable domain and a light chain variable domain of an
antibody. The
first binding domain of the fusion protein can be attached to the second
binding domain at a
heavy chain variable domain of the single chain variable fragment of the first
binding domain
(HL orientation). Alternatively, the first binding domain of the fusion
protein can be attached to
the second binding domain at a light chain variable domain of the single chain
variable fragment
of the first binding domain (LH orientation). In either orientation, the first
binding domain and
the second binding domain can be attached via a polypeptide linker varying in
length from 15 to
25 amino acids. In some embodiments, the polypeptide linker has the sequence
[G4S]n where n
=3 to 5.
[0237] Alternatively, an antibody construct can comprise an antibody (having
two antigen
binding domains and an Fc domain) and the second binding domain can comprise a
single chain
variable fragment (scFv). The second binding domain of the fusion protein can
be attached to the
first binding domain at a heavy chain variable domain of the single chain
variable fragment of the
first binding domain (HL orientation). Alternatively, the second binding
domain of the fusion
protein can be attached to the first binding domain at a light chain variable
domain of the single
chain variable fragment of the first binding domain (LH orientation).
[0238] An antibody construct can comprise a first binding domain and a second
binding domain,
wherein the second binding domain can be attached to the first binding domain.
The antibody
construct can comprise an antibody comprising a light chain and a heavy chain
or pair of heavy
and light chains. The first binding domain can comprise an Fab fragment of the
light and heavy
chains. The second binding domain can be attached to the light chain at a C-
terminus or C-
terminal end of the light chain as a fusion protein. The second binding domain
can comprise a
single chain variable fragment (scFv). In some embodiments, a first binding
domain can be
specific for any of the tumor antigens, e.g., CEA, HER2, TROP2, Claudin-6
(CLDN6), Claudin-
16 (CLDN16), CLDN18.2, RON, LY6E, FRA, DLL3, PTK7, Uroplakin-1B (UPK1B), LIV1,
-68-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
ROR1, STRA6, TMPRSS3, TMPRSS4, TMEM238, Clorf186, Fos-related antigen 1,
VEGFR1,
endoglin, VTCN1 (B7-H4), VISTA, or gpNMB, and an scFv can be a binding domain
with a
specificity selected from a group consisting of antigens on antigen presenting
cells, such as
CD40, DEC205, and PD-Li.
[0239] An antibody construct can comprise a first binding domain, a second
binding domain, and
an Fc domain, wherein the first binding domain and the second binding domain
are attached to
the Fc domain as a fusion protein. The second binding domain of the fusion
protein can
specifically target an antigen with at least 80% sequence identity to CD40.
The second binding
domain of the fusion protein can be a CD40 agonist. The first binding domain
of the fusion
protein can target a tumor antigen. The construct can comprise a fusion
protein comprising a
heavy chain (HC) attached to a single chain variable fragment. The construct
comprising the
fusion protein can comprise at least 80% sequence identity to any sequence in
TABLE 4. The
construct comprising the fusion protein can comprise at least 80% sequence
identity to any
sequence of a heavy chain CD40 monoclonal antibody (mAb) with tumor ScFv in
TABLE 4.
The construct can comprise a fusion protein comprising at least 80% sequence
identity to any
sequence of a heavy chain CD40 mAb with tumor ScFv in TABLE 4 and a light
chain
comprising at least 80% sequence identity to SEQ ID NO: 6. The construct
comprising the fusion
protein can comprise at least 80% sequence identity to any sequence of a heavy
chain tumor mAb
with CD40 ScFv in TABLE 4. The construct comprising the fusion protein can
comprise at least
80% sequence identity to any sequence of a heavy chain tumor antigen mAb with
CD40 ScFv in
TABLE 4, and at least 80% sequence identity to a light chain mAb for the tumor
antigen in
TABLE 3.
[0240] An antibody construct can comprise a first binding domain and a second
binding domain,
wherein the second binding domain can be attached to the first binding domain.
An antibody
construct can comprise a first binding domain, a second binding domain, and an
Fc domain,
wherein the second binding domain can be attached to the first binding domain.
The second
binding domain can be attached at a C-terminal end of the first binding domain
as a fusion
protein. The first binding domain can comprise a Fab fragment comprising a
light chain, wherein
the second binding domain can be attached at a C-terminal end of the light
chain as a fusion
protein. The second binding domain of the fusion protein can comprise a single
chain variable
fragment (scFv). The second binding domain of the fusion protein can be
attached to the first
binding domain at a heavy chain variable domain of the single chain variable
fragment of the first
binding domain (HL orientation). Alternatively, the second binding domain of
the fusion protein
can be attached to the first binding domain at a light chain variable domain
of the single chain
variable fragment of the first binding domain (LH orientation). For example, a
fusion protein
-69-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
comprising a light chain of an anti-CEA antibody attached to an anti-CD40 scFv
in the LH
orientation can be illustrated by SEQ ID NO: 842. The fusion sequences
comprising an scFv
sequence are in the HL orientation unless indicated otherwise (e.g., sequence
name recites
"(LH)" indicating light heavy orientation).
[0241] The first binding domain of the fusion protein can target a tumor
antigen. The second
binding domain of the fusion protein can target an APC antigen. The second
binding domain of
the fusion protein can target CD40. The first binding domain can comprise a
Fab fragment
comprising a light chain, wherein the second binding domain is attached at a C-
terminal end of
the light chain as a fusion protein. The construct comprising the fusion
protein can comprise at
least 80% sequence identity to any sequence in TABLE 9. The construct
comprising the fusion
protein can comprise at least 80% sequence identity to any sequence of a light
chain CD40 mAb
with tumor ScFv in TABLE 9. The construct can comprise a fusion protein
comprising at least
80% sequence identity to any sequence of a light chain CD40 mAb with tumor
ScFv in TABLE
9 and a heavy chain comprising at least 80% sequence identity to SEQ ID NO: 1.
The construct
comprising the fusion protein can comprise at least 80% sequence identity to
any sequence of a
light chain tumor mAb with CD40 ScFv in TABLE 9. The construct can comprise a
fusion
protein comprising at least 80% sequence identity to any sequence of a light
chain tumor antigen
mAb with CD40 ScFv in TABLE 9, and at least 80% sequence identity to a heavy
chain mAb
for the tumor antigen in TABLE 3.
[0242] An antibody construct can comprise a first binding domain, a second
binding domain, and
an Fc domain, wherein the first binding domain and the second binding domain
are attached to
the Fc domain as a fusion protein. The first binding domain of the fusion
protein can specifically
target an antigen with at least 80% sequence identity to DEC-205. The second
binding domain of
the fusion protein can target a tumor antigen. The construct can comprise a
fusion protein
comprising a heavy chain attached to a single chain variable fragment. The
construct comprising
the fusion protein can comprise at least 80% sequence identity to any sequence
in TABLE 8. The
construct comprising the fusion protein can comprise at least 80% sequence
identity to any
sequence of a heavy chain DEC-205 mAb with tumor ScFv in TABLE 8. The
construct can
comprise a fusion protein comprising at least 80% sequence identity to any
sequence of a heavy
chain DEC-205 mAb with tumor ScFv in TABLE 8 and a peptide comprising at least
80%
sequence identity to SEQ ID NO: 237. The construct comprising the fusion
protein can comprise
at least 80% sequence identity to any sequence of a heavy chain tumor antigen
mAb with CD40
ScFv in TABLE 8. The construct comprising the fusion protein can comprise at
least 80%
sequence identity to any sequence of a heavy chain tumor antigen mAb with CD40
ScFv in
-70-
CA 03065919 2019-12-02
WO 2018/227023
PCT/US2018/036560
TABLE 8, and at least 80% sequence identity to a heavy chain mAb for the tumor
antigen in
TABLE 3.
[0243] The second binding domain of the fusion protein can target DEC-205. The
construct
comprising the fusion protein can comprise at least 80% sequence identity to
any sequence in
TABLE 10. The construct comprising the fusion protein can comprise at least
80% sequence
identity to any sequence of a light chain DEC-205 mAb with tumor ScFv in TABLE
10. The
construct comprising a fusion protein can comprise at least 80% sequence
identity to any
sequence of a light chain DEC-205 mAb with tumor ScFv in TABLE 10 and at least
80%
sequence identity to SEQ ID NO: 237. The construct comprising the fusion
protein can comprise
at least 80% sequence identity to any sequence of a light chain tumor mAb with
DEC-205 ScFv
in TABLE 10. The construct comprising a fusion protein can comprise at least
80% sequence
identity to any sequence of a light chain tumor antigen mAb with DEC-205 ScFv
in TABLE 10,
and at least 80% sequence identity to a heavy chain mAb for the tumor antigen
in TABLE 3.
[0244] The second binding domain of the fusion protein can specifically bind
to an antigen of an
Antigen Presenting Cell (APC) or other immune cell. The second binding domain
of the fusion
protein can specifically bind to an antigen with at least 80% sequence
identity to CD40. The
second binding domain of the fusion protein can be a CD40 agonist. The second
binding domain
of the fusion protein can specifically bind to an antigen with at least 80%
sequence identity to
DEC-205. The second binding domain of the fusion protein can specifically bind
to an antigen
with at least 80% sequence identity to DC-SIGN. The second binding domain of
the fusion
protein can specifically bind to an antigen with at least 80% sequence
identity to CD36 mannose
scavenger receptor. The second binding domain of the fusion protein can
specifically bind to an
antigen with at least 80% sequence identity to CLEC12A. The second binding
domain of the
fusion protein can specifically bind to an antigen with at least 80% sequence
identity to BDCA-2.
The second binding domain of the fusion protein can specifically bind to an
antigen with at least
80% sequence identity to PD-Li. The second binding domain of the fusion
protein can
specifically bind to an antigen with at least 80% sequence identity to CD40,
CD47, TNFR2,
DEC-205, PD-L1, CD36 mannose scavenger receptor 1, CLEC9A, DC-SIGN, CLEC12A,
BDCA-2, OX4OL, 41BBL, CD204, MARCO, CLEC5A, Dectin 1, Dectin 2, CLEC10A,
CD206,
CD64, CD32A, CD16A, HVEM, CD38, TREM2, CSF1R, or CD32B. The first binding
domain
of the fusion protein can specifically bind to a tumor antigen. The first
binding domain of the
fusion protein can specificall bind to an antigen with at least 80% sequence
identity to CD5,
CD19, CD20, CD25, CD37, CD30, CD33, CD45, CAMPATH-1, BCMA, CS-1, PD-L1, B7-H3,
B7-DC (PD-L2), HLD-DR, carcinoembryonic antigen (CEA), TAG-72, EpCAM, MUC1,
MUC15, MUC16, folate-binding protein, A33, G250, prostate-specific membrane
antigen
-71-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
(PSMA), ferritin, CA-125, CA19-9, epidermal growth factor, p185HER2, IL-2
receptor,
EGFRvIII (de2-7 EGFR), fibroblast activation protein (FAP), tenascin, a
metalloproteinase,
endosialin, vascular endothelial growth factor, avf33, WT1, LMP2, HPV E6, HPV
E7, Her-2/neu,
p53 nonmutant, NY-ESO-1, MelanA/MART1, Ras mutant, gp100, p53 mutant, PR1, bcr-
abl,
tyrosinase, survivin, PSA, hTERT, a Sarcoma translocation breakpoint protein,
EphA2, PAP,
ML-IAP, AFP, ERG, NA17, PAX3, ALK, androgen receptor, cyclin Bl, MYCN, RhoC,
TRP-2,
mesothelin (MSLN), PSCA, MAGE Al, MAGE A3, CYP1B1, PLAV1, BORIS, ETV6-AML,
NY-BR-1, RGS5, SART3, Carbonic anhydrase IX, PAX5, 0Y-TES1, Sperm protein 17,
LCK,
HMWMAA, AKAP-4, 55X2, XAGE 1, Legumain, Tie 3, Page4, VEGFR2, MAD-CT-1,
PDGFR-B, MAD-CT-2, ROR2, TRAILl, MAGE A4, MAGE C2, GAGE, EGFR, CMET,
HER3, EPCAM, CA6, NAPI2B, TROP2, Claudin-6 (CLDN6), Claudin-16 (CLDN16),
CLDN18.2, RON, LY6E, FRA, DLL3, PTK7, Uroplakin-1B (UPK1B), LIV1, ROR1, STRA6,
TMPRSS3, TMPRSS4, TMEM238, Clorf186, Fos-related antigen 1, VEGFR1, endoglin,
PD-
L1, VTCN1 (B7-H4), VISTA, gpNMB, or any fragment thereof.
[0245] Alternatively, the second binding domain of the fusion protein can
target a tumor antigen.
The second binding domain of the fusion protein can specifically bind to an
antigen selected
from CD5, CD19, CD20, CD25, CD37, CD30, CD33, CD45, CAMPATH-1, BCMA, CS-1, PD-
L1, B7-H3, B7-DC (PD-L2), HLD-DR, carcinoembryonic antigen (CEA), TAG-72,
EpCAM,
MUC1, MUC15, MUC16, folate-binding protein, A33, G250, prostate-specific
membrane
antigen (PSMA), ferritin, GD2, GD3, GM2, Leg, CA-125, CA19-9, epidermal growth
factor,
p185HER2, IL-2 receptor, EGFRvIII (de2-7 EGFR), fibroblast activation protein
(FAP), tenascin,
a metalloproteinase, endosialin, vascular endothelial growth factor, avf33,
WT1, LMP2, HPV E6
E7, Her-2/neu, p53 nonmutant, NY-ES0-1, MelanA/MART1, Ras mutant, gp100, p53
mutant,
PR1, bcr-abl, tyrosinase, survivin, PSA, hTERT, a Sarcoma translocation
breakpoint protein,
EphA2, PAP, ML-IAP, AFP, ERG, NA17, PAX3, ALK, androgen receptor, cyclin Bl,
polysialic
acid, MYCN, RhoC, TRP-2, fucosyl GM1, mesothelin (MSLN), PSCA, MAGE Al, MAGE
A3,
sLe(animal), CYP1B1, PLAV1, GM3, BORIS, Tn, GloboH, ETV6-AML, NY-BR-1, RGS5,
SART3, STn, Carbonic anhydrase IX, PAX5, 0Y-TES1, Sperm protein 17, LCK,
HMWMAA,
AKAP-4, 55X2, XAGE 1, Legumain, Tie 3, Page4, VEGFR2, MAD-CT-1, PDGFR-B, MAD-
CT-2, ROR2, TRAILl, MAGE A4, MAGE C2, GAGE, EGFR, CMET, HER3, EPCAM, CA6,
NAPI2B, TROP2, Claudin-6 (CLDN6), Claudin-16 (CLDN16), CLDN18.2, RON, LY6E,
FRA,
DLL3, PTK7, Uroplakin-1B (UPK1B), LIV1, ROR1, STRA6, TMPRSS3, TMPRSS4,
TMEM238, Clorf186, Fos-related antigen 1, VEGFR1, endoglin, PD-L1, VTCN1 (B7-
H4),
VISTA, gpNMB, or any fragment thereof. The first binding domain of the fusion
protein can
specifically bind to an antigen with at least 80% sequence identity to CD40.
The first binding
-72-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
domain of the fusion protein can be a CD40 agonist. The first binding domain
of the fusion
protein can specifically bind to an antigen with at least 80% sequence
identity to DEC-205. The
first binding domain of the fusion protein can specifically bind to an antigen
with at least 80%
sequence identity to CEA, HER2, TROP2, Claudin-6 (CLDN6), Claudin-16 (CLDN16),
CLDN18.2, RON, LY6E, FRA, DLL3, PTK7, Uroplakin-1B (UPK1B), LIV1, ROR1, STRA6,
TMPRSS3, TMPRSS4, TMEM238, Clorf186, Fos-related antigen 1, VEGFR1, endoglin,
VTCN1 (B7-H4), VISTA or gpNMB. The first binding domain of the fusion protein
can
specifically bind to an antigen with at least 80% sequence identity to CD40,
CD47, TNFR2,
DEC-205, PD-L1, CD36 mannose scavenger receptor 1, CLEC9A, DC-SIGN, CLEC12A,
BDCA-2, OX4OL, 41BBL, CD204, MARCO, CLEC5A, Dectin 1, Dectin 2, CLEC10A,
CD206,
CD64, CD32A, CD16A, HVEM, CD38, TREM2, CSF1R, or CD32B.
[0246] An antibody construct can comprise a first binding domain, a second
binding domain, and
a third binding domain. An antibody construct can comprise a first binding
domain, a second
binding domain, a third binding domain, and an Fc domain. The first binding
domain and the
second binding domain can be attached to the Fc domain. The first and second
binding domains
are described herein throughout the specification. The first binding domain
can be attached to the
Fc domain at an N-terminal end of the Fc domain. The second binding domain can
be attached at
a C-terminal end of the Fc domain. The second binding domain can be attached
at a C-terminal
end of the Fc domain via a polypeptide linker having a length of 10 to 25
amino acid. In some
embodiments, the polypeptide linker has the sequence [G45]n where n = 2 to 5.
The third binding
domain can be attached to a C-terminal end of the first binding domain. The
third binding
domain can be attached at a C-terminal end of the Fc domain via a polypeptide
linker having a
length of 10 to 25 amino acid. In some embodiments, the polypeptide linker has
the sequence
[G45]n where n = 2 to 5. The third binding domain can be attached to a C-
terminal end of a light
chain of the first binding domain. One or more of the first binding domain,
the second binding
domain, the third binding domain, and the Fc domain can be attached as a
fusion protein. The
first binding domain can comprise a Fab fragment comprising a light chain,
wherein the second
binding domain is attached at a C-terminal end of the light chain as a fusion
protein. The second
binding domain of the fusion protein can comprise a single chain variable
fragment (scFv). The
second binding domain of the fusion protein can be attached to the Fc domain
at a heavy chain
variable domain of the single chain variable fragment of the second binding
domain (HL
orientation). The second binding domain of the fusion protein can be attached
to the Fc domain at
a light chain variable domain of the single chain variable fragment of the
second binding domain
(LH orientation). The third binding domain of the fusion protein can comprise
a single chain
variable fragment (scFv). The antibody construct can comprise a fusion protein
comprising the
-73-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
third binding domain attached to the first binding domain having at least 80%
sequence identity
to any sequence in TABLE 9 or TABLE 10. The third binding domain of the fusion
protein can
be attached to the first binding domain at a heavy chain variable domain of
the single chain
variable fragment of the first binding domain (HL orientation). Alternatively,
the third binding
domain of the fusion protein can be attached to the first binding domain at a
light chain variable
domain of the single chain variable fragment of the first binding domain (LH
orientation). The
third binding domain of the fusion protein can target an antigen of an immune
cell, such as an
antigen presenting cell (APC). The third binding domain of the fusion protein
can specifically
bind to an antigen with at least 80% sequence identity to CD40. The third
binding domain of the
fusion protein can be a CD40 agonist. The third binding domain of the fusion
protein can
specifically bind to an antigen with at least 80% sequence identity to DEC-
205. The third binding
domain of the fusion protein can specifically bind to an antigen with at least
80% sequence
identity to CD40, CD47, TNFR2, DEC-205, PD-L1, CD36 mannose scavenger receptor
1,
CLEC9A, DC-SIGN, CLEC12A, BDCA-2, OX4OL, 41BBL, CD204, MARCO, CLEC5A,
Dectin 1, Dectin 2, CLEC10A, CD206, CD64, CD32A, CD16A, HVEM, CD38, TREM2,
CSF1R, or CD32B.
[0247] Alternatively, the third binding domain can target a tumor antigen. The
third binding
domain of the fusion protein can specifically bind to an antigen with at least
80% sequence
identity to CD5, CD19, CD20, CD25, CD37, CD30, CD33, CD45, CAMPATH-1, BCMA, CS-
1,
PD-L1, B7-H3, B7-DC (PD-L2), HLD-DR, carcinoembryonic antigen (CEA), TAG-72,
EpCAM,
MUC1, MUC15, MUC16, folate-binding protein, A33, G250, prostate-specific
membrane
antigen (PSMA), ferritin, CA-125, CA19-9, epidermal growth factor, p185HER2,
IL-2 receptor,
EGFRvIII (de2-7 EGFR), fibroblast activation protein (FAP), tenascin, a
metalloproteinase,
endosialin, vascular endothelial growth factor, avf33, WT1, LMP2, HPV E6, HPV
E7, Her-2/neu,
p53 nonmutant, NY-ESO-1, MelanA/MART1, Ras mutant, gp100, p53 mutant, PR1, bcr-
abl,
tyrosinase, survivin, PSA, hTERT, a Sarcoma translocation breakpoint protein,
EphA2, PAP,
ML-IAP, AFP, ERG, NA17, PAX3, ALK, androgen receptor, cyclin Bl, MYCN, RhoC,
TRP-2,
mesothelin (MSLN), PSCA, MAGE Al, MAGE A3, CYP1B1, PLAV1, BORIS, GloboH, ETV6-
AML, NY-BR-1, RGS5, SART3, Carbonic anhydrase IX, PAX5, 0Y-TES1, Sperm protein
17,
LCK, HMWMAA, AKAP-4, 55X2, XAGE 1, Legumain, Tie 3, PAGE4, VEGFR2, MAD-CT-1,
PDGFR-B, MAD-CT-2, ROR2, TRAILl, MAGE A4, MAGE C2, GAGE, EGFR, CMET,
HER3, EPCAM, CA6, NAPI2B, TROP2, Claudin-6 (CLDN6), Claudin-16 (CLDN16),
CLDN18.2, RON, LY6E, FRA, DLL3, PTK7, Uroplakin-1B (UPK1B), LIV1, ROR1, STRA6,
TMPRSS3, TMPRSS4, TMEM238, Clorf186, Fos-related antigen 1, VEGFR1, endoglin,
VTCN1 (B7-H4), VISTA, gpNMB, or any fragment thereof. In additional
embodiments, the
-74-
CA 03065919 2019-12-02
WO 2018/227023
PCT/US2018/036560
third binding domain of the fusion protein can specifically bind to an antigen
selected from CD5,
CD19, CD20, CD25, CD37, CD30, CD33, CD45, CAMPATH-1, BCMA, CS-1, PD-L1, B7-H3,
B7-DC (PD-L2), HLD-DR, carcinoembryonic antigen (CEA), TAG-72, EpCAM, MUC1,
MUC15, MUC16, folate-binding protein, A33, G250, prostate-specific membrane
antigen
(PSMA), ferritin, GD2, GD3, GM2, Leg, CA-125, CA19-9, epidermal growth factor,
p185HER2,
IL-2 receptor, EGFRvIII (de2-7 EGFR), fibroblast activation protein (FAP),
tenascin, a
metalloproteinase, endosialin, vascular endothelial growth factor, avf33, WT1,
LMP2, HPV E6
E7, Her-2/neu, p53 nonmutant, NY-ES0-1, MelanA/MART1, Ras mutant, gp100, p53
mutant,
PR1, bcr-abl, tyrosinase, survivin, PSA, hTERT, a Sarcoma translocation
breakpoint protein,
EphA2, PAP, ML-IAP, AFP, ERG, NA17, PAX3, ALK, androgen receptor, cyclin Bl,
polysialic
acid, MYCN, RhoC, TRP-2, fucosyl GM1, mesothelin (MSLN), PSCA, MAGE Al, MAGE
A3,
sLe(animal), CYP1B1, PLAV1, GM3, BORIS, Tn, GloboH, ETV6-AML, NY-BR-1, RGS5,
SART3, STn, Carbonic anhydrase IX, PAX5, 0Y-TES 1, Sperm protein 17, LCK,
HMWMAA,
AKAP-4, 55X2, XAGE 1, Legumain, Tie 3, PAGE4, VEGFR2, MAD-CT-1, PDGFR-B, MAD-
CT-2, ROR2, TRAILl, MAGE A4, MAGE C2, GAGE, EGFR, CMET, HER3, EPCAM, CA6,
NAPI2B, TROP2, Claudin-6 (CLDN6), Claudin-16 (CLDN16), CLDN18.2, RON, LY6E,
FRA,
DLL3, PTK7, Uroplakin-1B (UPK1B), LIV1, ROR1, STRA6, TMPRSS3, TMPRSS4,
TMEM238, Clorf186, Fos-related antigen 1, VEGFR1, endoglin, VTCN1 (B7-H4),
VISTA,
gpNMB, or any fragment thereof.
[0248] An antibody construct can comprise a first binding domain targeting
CD40 and a second
binding domain targeting DEC-205. Alternatively, an antibody construct can
comprise a first
binding domain targeting DEC-205 and a second binding domain targeting CD40.
An antibody
construct can comprise a first binding domain, a second binding domain, and an
Fc domain. The
first binding domain and the second binding domain can be attached to the Fc
domain. The first
binding domain can be attached to the Fc domain at an N-terminal end of the Fc
domain, wherein
the second binding domain is attached to the Fc domain at a C-terminal end of
the Fc domain.
Alternatively, the second binding domain can be attached to the Fc domain at
an N-terminal end
of the Fc domain, wherein the first binding domain is attached to the Fc
domain at a C-terminal
end of the Fc domain. An antibody construct can comprise a fusion protein
comprising a first
binding domain targeting CD40 and a second binding domain targeting DEC-205.
The fusion
protein can comprise at least 80% sequence identity to any sequence in TABLE
11.
[0249] Additionally, antibody constucts expressing proteins having the
sequences referenced in
Tables 1-11 can have a dissociation constant (Kd) that is less than 10 nM for
the antigen of the
first binding domain. The antibody constructs expressing proteins having the
sequences
referenced in Tables 1-11 can have a dissociation constant (Kd) that is less
than 10 nM for the
-75-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
antigen of the second binding domain. The antibody constructs expressing the
sequences
referenced in Tables 1-11 can have a dissociation constant (Kd) that is less
than 10 nM for the
antigen of the third binding domain. The antibody constructs can have a
dissociation constant
(Kd) for the antigen of the first binding domain that is less than 1 nM, less
than 100 pM, less than
pM, less than 1 pM, or less than 0.1 pM. The antibody constructs can have a
dissociation
constant (Kd) for the antigen of the second binding domain that is less than 1
nM, less than 100
pM, less than 10 pM, less than 1 pM, or less than 0.1 pM. The antibody
constructs can have a
dissociation constant (Kd) for the antigen of the third binding domain that is
less than 1 nM, less
than 100 pM, less than 10 pM, less than 1 pM, or less than 0.1 pM.
[0250] An anti-CD40 light chain can be expressed with an anti-CD40 heavy chain
or fragment
thereof, wherein the light and heavy chains specifically bind to CD40. The
anti-CD40 light chain
can also be expressed with an anti-CD40 heavy chain or fragment thereof to
form an anti-CD40
antibody or fragment thereof, wherein the antibody or antibody fragment
specifically binds to
CD40. The anti-CD40 antibody or fragment thereof can be purified, and can be
combined with a
pharmaceutically acceptable carrier.
[0251] An anti-DEC-205 light chain can be expressed with any anti-DEC-205
heavy chain or
fragment thereof, wherein the light and heavy chains specifically bind to DEC-
205. The anti-
DEC-205 light chain can also be expressed with an anti-DEC-205 heavy chain or
fragment
thereof to form an anti-DEC-205 antibody or fragment thereof, wherein the
antibody or antibody
fragment specifically binds to DEC-205. The anti-DEC-205 antibody or fragment
thereof can be
purified, and can be combined with a pharmaceutically acceptable carrier.
[0252] An anti-PD-Li light chain can be expressed with any anti-PD-Li heavy
chain or fragment
thereof, wherein the light and heavy chains specifically bind to PD-Li. The
anti-PD-Li light
chain can also be expressed with an anti-PD-Li heavy chain or fragment thereof
to form an anti-
PD-Li antibody or fragment thereof, wherein the antibody or antibody fragment
specifically
binds to PD-Li. The anti-PD-Li antibody or fragment thereof can be purified,
and can be
combined with a pharmaceutically acceptable carrier.
[0253] An anti-tumor antigen light chain can be expressed with any anti-tumor
antigen heavy
chain or fragment thereof, wherein the antibody or antibody fragment
specifically binds to tumor
antigen. The anti-tumor antigen light chain can also expressed with any anti-
tumor antigen heavy
chain or fragment thereof to form an anti-tumor antigen antibody or fragment
thereof. The anti-
tumor antibody or fragment thereof can be purified, and can be combined with a
pharmaceutically acceptable carrier.
[0254] An antibody construct can comprise an antibody heavy chain. A heavy
chain can be a
heavy chain of an anti-CD40 antibody which can bind a CD40 antigen. A heavy
chain of an anti-
-76-
CA 03065919 2019-12-02
WO 2018/227023
PCT/US2018/036560
CD40 antibody can be an IgG1 isotype. A heavy chain of an anti-CD40 antibody
can be
dacetuzumab.
[0255] An antibody construct can comprise an antibody light chain. A light
chain can be a light
chain of an anti-CD40 antibody which can bind a CD40 antigen. A light chain of
an anti-CD40
antibody can be dacetuzumab.
[0256] An antibody construct can comprise an antibody heavy chain. A heavy
chain can be a
heavy chain of an anti-CD40 antibody which can bind a CD40 antigen. A heavy
chain of an anti-
CD40 antibody can be an IgG4 isotype. A heavy chain of an anti-CD40 antibody
can be
bleselumab.
[0257] An antibody construct can comprise an antibody light chain. A light
chain can be a light
chain of an anti-CD40 antibody which can bind a CD40 antigen. A light chain of
an anti-CD40
antibody can be bleselumab.
[0258] An antibody constructcan comprise an antibody heavy chain. A heavy
chain can be a
heavy chain of an anti-CD40 antibody which can bind a CD40 antigen. A heavy
chain of an anti-
CD40 antibody can be an IgG1 isotype. A heavy chain of an anti-CD40 antibody
can be
lucatumumab.
[0259] An antibody construct can comprise an antibody light chain. A light
chain can be a light
chain of an anti-CD40 antibody which can bind a CD40 antigen. A light chain of
an anti-CD40
antibody can be lucatumumab.
[0260] An antibody construct can comprise an antibody heavy chain. A heavy
chain can be a
heavy chain of an anti-CD40 antibody which can bind a CD40 antigen. A heavy
chain of an anti-
CD40 antibody can be an IgG1 isotype. A heavy chain of an anti-CD40 antibody
can be ADC-
1013.
[0261] An antibody construct can comprise an antibody light chain. A light
chain can be a light
chain of an anti-CD40 antibody which can bind a CD40 antigen. A light chain of
an anti-CD40
antibody can be ADC-1013.
[0262] An antibody construct can comprise an antibody heavy chain. A heavy
chain can be a
heavy chain of an anti-CD40 antibody which can bind a CD40 antigen. A heavy
chain of an anti-
CD40 antibody can be the humanized rabbit antibody APX005.
[0263] An antibody construct can comprise an antibody light chain. A light
chain can be a light
chain of an anti-CD40 antibody which can bind a CD40 antigen. A light chain of
an anti-CD40
antibody can be the humanized rabbit antibody APX005.
[0264] An antibody construct can comprise an antibody heavy chain. A heavy
chain can be a
heavy chain of an anti-CD40 antibody which can bind a CD40 antigen. A heavy
chain of an anti-
CD40 antibody can be Chi Lob 7/4.
-77-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
[0265] An antibody construct can comprise an antibody light chain. A light
chain can be a light
chain of an anti-CD40 antibody which can bind a CD40 antigen. A light chain of
an anti-CD40
antibody can be Chi Lob 7/4.
[0266] An antibody construct can comprise an antibody heavy chain. A heavy
chain can be a
heavy chain of an anti-CD40 antibody which can bind a CD40 antigen. A heavy
chain of an anti-
CD40 antibody can be an IgG1 isotype. A heavy chain of an anti-CD40 antibody
can be SBT-
040-G1WT.
[0267] An antibody construct can comprise an antibody heavy chain. A heavy
chain can be a
heavy chain of an anti-CD40 antibody which can bind a CD40 antigen. A heavy
chain of an anti-
CD40 antibody can be an IgG1 isotype. A heavy chain of an anti-CD40 antibody
can be SBT-
040 VH-hIgG1 wt.
[0268] A heavy chain of an anti-CD40 antibody can be an IgG2 isotype. A heavy
chain of an
anti-CD40 antibody can be SBT-040-G2.
[0269] An antibody construct can comprise an antibody with modifications
occurring at least at
one amino acid residue. Modifications can be substitutions, additions,
mutations, deletions, or the
like. An antibody modification can be an insertion of an unnatural amino acid.
[0270] An antibody construct can comprise a light chain of an amino acid
sequence having at
least one, two, three, four, five, six, seven, eight, nine, or ten
modifications but not more than 40,
35, 30, 25, 20, 15, or 10 modifications of the amino acid sequence relative to
the natural or
original amino acid sequence. An antibody construct can comprise a heavy chain
with an amino
acid sequence having at least one, two, three, four, five, six, seven, eight,
nine or ten
modifications but not more than 40, 35, 30, 25, 20, 15, or 10 modifications of
the amino acid
sequence relative to the natural or original amino acid sequence.
[0271] An antibody construct can have an Fc domain of an IgG1 isotype. An
antibody construct
can have an Fc domain of an IgG2 isotype. An antibody construct can have an Fc
domain of an
IgG3 isotype. An antibody construct can have an Fc domainof an IgG4 isotype.
An antibody
construct can have an Fc domain of a hybrid isotype comprising constant
regions from two or
more isotypes. An antibody construct can be an anti-CD40 antibody, in which
the anti-CD40
antibody can be a monoclonal human antibody comprising a wild-type sequence of
an IgG1
isoform, in particular, at an Fc region of the antibody.
[0272] An antibody constructs disclosed herein can be non-natural, designed,
and/or engineered.
Antibody constructs disclosed herein can be non-natural, designed, and/or
engineered scaffolds
comprising an antigen binding domain. Antibody constructs disclosed herein can
be non-natural,
designed, and/or engineered antibodies. Antibody constructs can be monoclonal
antibodies.
Antibody constructs can be human antibodies. Antibody constructs can be
humanized antibodies.
-78-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody constructs can be monoclonal humanized antibodies. Antibody construct
can be
recombinant antibodies.
[0273] The Kd for binding of the Fc domain to an Fc receptor of an antibody
construct as
described herein can increase when the tumor antigen binding domain is bound
to its tumor
antigen as compared to the Kd for binding of the Fc domain to an Fc receptor
of a construct as
described herein when the tumor antigen binding domain is not bound to its
tumor antigen. For
example, an antibody construct as described herein can have a Kd for binding
of the Fc domain to
an Fc receptor in the presence of the binding domain that binds to an antigen
on an immune cell,
such as an antigen presenting cell, and the tumor target binding domain when
the tumor target
binding domain is bound to its tumor antigen that can be greater than or
greater than about 100
nM. The Kd for binding of the Fc domain to an Fc receptor in the presence of
the binding domain
that binds to an antigen on an immune cell, such as an antigen presenting
cell, and the tumor
target binding domain when the tumor target binding domain is bound to its
tumor antigen can be
or can be about 100 nM, 200 nM, 300 nM, 400 nM, 500 nM, or 1000 nM. The Kd for
binding of
the Fc domain to an Fc receptor in the presence of the binding domain that
binds to an antigen on
an immune cell, such as an antigen presenting cell, and the tumor target
binding domain when the
tumor target binding domain is bound to its tumor antigen can be from 100 nM
to 200 nM, 100
nM to 300 nM, 100 nM to 400 nM, 100 nM to 500 nM, or 100 nM to 1000 nM.
Additionally, the
antibody construct as described herein can have a Kd for binding of the Fc
domain to an Fc
receptor in the presence of the binding domain that binds to an antigen on an
immune cell, such
as an antigen presenting cell, and a tumor antigen binding domain when the
tumor antigen
binding domain is not bound to the tumor antigen is no greater than about 100
nM and is no
greater than about 100 times a Kd for binding of the Fc domain to the Fc
receptor in an absence
of the binding domain that binds to an antigen on an immune cell and a tumor
antigen binding
domain.
[0274] The Kd for binding of the binding domain of an antibody construct as
described herein
that binds to an antigen on an immune cell, such as an antigen presenting
cell, can increase when
the tumor antigen binding domain is bound to its tumor antigen as compared to
the Kd for binding
of the binding domain that binds to an antigen on an immune cell when the
tumor antigen
binding domain is not bound to its tumor antigen. For example, an antibody
construct can
comprise a Kd for binding of the binding domain that binds to an antigen on an
antigen
presenting cell when the tumor antigen binding domain is bound to its tumor
antigen can be
greater than or greater than about 100nM. The Kd for binding of the binding
domain that binds to
an antigen on an immune cell, such as an antigen presenting cell, when the
tumor antigen binding
domain is bound to its tumor antigen can be or can be about 100 nM, 200 nM,
300 nM, 400 nM,
-79-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
500 nM, or 1000 nM. Kd for binding of the binding domain that binds to an
antigen on an
immune cell when the tumor antigen binding domain is bound to its tumor
antigen can be from
100 nM to 200 nM, 100 nM to 300 nM, 100 nM to 400 nM, 100 nM to 500 nM, or 100
nM to
1000 nM.
[0275] The effect of the tumor antigen binding domain and the binding domain
that binds to an
antigen on the immune cell together can be to cluster the antibody constructs
or conjugates on
cells expressing tumor antigen, and thus clustering immune cells such as
antigen presenting cells
around cancerous cells and at tumor sites resulting in activation of the
antigen presenting cell
effector functions. This can include the activation of the antigen on the
antigen presenting cell
when a an antibody construct is bound to its antigen, such as activation of
CD40, CD47, TNFR2,
DEC-205, PD-L1, CD36 mannose scavenger receptor 1, DC-SIGN, CLEC9A, CLEC12A,
BDCA-2, OX4OL, 41BBL, CD204, MARCO, CLEC5A, Dectin 1, Dectin 2, CLEC10A,
CD206,
CD64, CD32A, CD16A, HVEM, CD38, TREM2, CSF1R, or CD32B. In some embodiments,
this
activation of the antigen on the antigen presenting cell only occurs when the
tumor targeting
antibody construct is bound to its tumor antigen. An antigen presenting cell
effector function can
include antibody dependent cellular cytotoxicity (ADCC) of the tumor antigen
expressing cell,
which can occur when a bispecific tumor targeting antibody construct is bound
to its tumor
antigen. In some embodiments, ADCC of the tumor antigen expressing cell only
occurs with a
bispecific tumor targeting antibody construct is bound to its tumor antigen.
In certain
embodiments, a bispecific tumor targeting antibody construct density of
greater than 1000, 2000,
3000, 4000, 5000, 6000, 7000, 8000, 9000, 10,000 or more per cell, resulting
from the bispecific
tumor targeting antibody construct binding to the tumor antigen, induces
signaling in the antigen
presenting cell. Signaling can suitably be measured in vitro using a cell line
expressing the tumor
antigen bound by the target antigen binding domain, and primary antigen
presenting cells isolated
from a human subject. Signaling can be assessed as cytokine release, chemokine
release, or
increased expression of cell surface markers. In certain embodiments, a
bispecific tumor
targeting antibody construct density of greater than 1000, 2000, 3000, 4000,
5000, 6000, 7000,
8000, 9000, 10,000 or more per cell, resulting from the bispecific tumor
targeting antibody
construct binding to the tumor antigen, induces ADCC of the cells expressing
tumor antigen.
ADCC can suitably be measured in vitro using a cell line expressing the tumor
antigen bound by
the target antigen binding domain, and cells such as NK cells and/or
macrophages isolated from a
human subject. ADCC can be determined by the frequency of remaining tumor
antigen
expressing cells in the co-culture.
[0276] In some embodiments, the antibody constructs as described herein can
specifically bind to
a tumor antigen in a cluster of constructs or conjugates, and this clustering
can induce a signal in
-80-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
an antigen presenting cell. The constructs as described herein can
specifically bind to a tumor
antigen in a cluster of constructs, and this clustering can induce antibody
dependent cellular
cytotoxicity. The constructs as described herein can specifically bind to a
tumor antigen in a
cluster of constructs and this clustering can result in an increased avidity
for an antigen on an
antigen presenting cell. The antibody constructs as described herein can
specifically bind to a
tumor antigen in a cluster of constructs and this clustering can result in an
increased avidity of
the Fc domain for an Fc receptor.
[0277] Sequences that can be used to produce antibodies for antibody
constructs can include
leader sequences. Leader sequences can include signal sequences. Leader
sequences useful with
the antibody construct and methods described herein can include, but are not
limited to, an amino
acid sequence comprising MRLPAQLLGLLLLWFPGSRC (SEQ ID NO: 847),
MDWTWRILFLVAAATGAHS (SEQ ID NO: 848), and MRAWIFFLLCLAGRALA (SEQ ID
NO: 849).
[0278] An antibody construct can comprise an Fc region with an Fc domain. An
Fc domain is a
structure that can bind to Fc receptors. An antibody construct can comprise an
Fc domain. Fc
domains can be bound by Fc receptors (FcRs). Fc domains can be from
antibodies. An Fc domain
can be at least 80% homologous to an Fc domain from an antibody. An Fc region
can be in a
scaffold. An Fc region with an Fc domain can be in an antibody scaffold. An Fc
region with an
Fc domain can be in a non-antibody scaffold. An antibody construct can
comprise an Fc region
with an Fc domain in an antibody scaffold. An antibody construct can comprise
an Fc region
with an Fc domain in a non-antibody scaffold. An Fc domain can be in a
scaffold. An Fc domain
can be in an antibody scaffold. An Fc domain can be in a non-antibody
scaffold. An antibody
construct can comprise an Fc domain in an antibody scaffold. An antibody
construct can
comprise an Fc domain in a non-antibody scaffold. Fc domains of antibodies,
including those of
the present disclosure, can be bound by Fc receptors (FcRs). An Fc domain can
be a portion of
the Fc region of an antibody. FcRs can bind to an Fc domain of an antibody.
FcRs can bind to an
Fc domain of an antibody bound to an antigen. FcRs are organized into classes
(e.g., gamma (y),
alpha (a) and epsilon (6)) based on the class of antibody that the FcR
recognizes. The FcaR class
can bind to IgA and includes several isoforms, FcaRI (CD89) and Fcai.t.R. The
FcyR class can
bind to IgG and includes several isoforms, FcyRI (CD64), FcyRIIA (CD32a),
FcyRIIB (CD32b),
FcyRIIIA (CD16a), and FcyRIIIB (CD16b). An FcyRIIIA (CD16a) can be an FcyRIIIA
(CD16a)
F158 variant. An FcyRIIIA (CD16a) can be an FcyRIIIA (CD16a) V158 variant.
Each FcyR
isoform can differ in affinity to the Fc region of the IgG antibody. For
example, FcyRI can bind
to IgG with greater affinity than FcyRII or FcyRIII. The affinity of a
particular FcyR isoform to
IgG can be controlled, in part, by a glycan (e.g., oligosacccharide) at
position CH284.4 of the
-81-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
IgG antibody. For example, fucose containing CH284.4 glycans can reduce IgG
affinity for
FcyRIIIA. In addition, GO glucans can have increased affinity for FcyRIIIA due
to the lack of
galactose and terminal GlcNAc moiety.
[0279] Binding of an Fc domain to an FcR can enhance an immune response. FcR-
mediated
signaling that can result from an Fc region binding to an FcR can lead to the
maturation of
immune cells. FcR-mediated signaling that can result from an Fc domain binding
to an FcR can
lead to the maturation of dendritic cells. FcR-mediated signaling that can
result from an Fc
domain binding to an FcR can lead to antibody dependent cellular cytotoxicity.
FcR-mediated
signaling that can result from an Fc domain binding to an FcR can lead to more
efficient immune
cell antigen uptake and processing. FcR-mediated signaling that can result
from an Fc region
binding to an FcR can lead to more efficient dendritic cell antigen uptake and
processing. FcR-
mediated signaling that can result from an Fc region binding to an FcR can
increase antigen
presentation. FcR-mediated signaling that can result from an Fc region binding
to an FcR can
increase antigen presentation by immune cells. FcR-mediated signaling that can
result from an Fc
region binding to an FcR can increase antigen presentation by antigen
presenting cells. FcR-
mediated signaling that can result from an Fc domain binding to an FcR can
increase antigen
presentation by dendritic cells. FcR-mediated signaling that can result from
an Fc domain
binding to an FcR can promote the expansion and activation of T cells. FcR-
mediated signaling
that can result from an Fc domain binding to an FcR can promote the expansion
and activation of
CD8+ T cells. FcR-mediated signaling that can result from an Fc domain binding
to an FcR can
influence immune cell regulation of T cell responses. FcR-mediated signaling
that can result
from an Fc domain binding to an FcR can influence immune cell regulation of T
cell responses.
FcR-mediated signaling that can result from an Fc domain binding to an FcR can
influence
dendritic cell regulation of T cell responses. FcR-mediated signaling that can
result from an Fc
domain binding to an FcR can influence functional polarization of T cells
(e.g., polarization can
be toward a TH1 cell response).
[0280] The profile of FcRs on a dendritic cell (DC) can impact the ability of
the DC to respond
upon stimulation. For example, most DC can express both CD32A and CD32B, which
can have
opposing effects on IgG-mediated maturation and function of DCs: binding of
IgG to CD32A can
mature and activate DCs in contrast with CD32B, which can mediate inhibition
due to
phosphorylation of immunoreceptor tyrosine-based inhibition motif (ITIM),
after CD32B binding
of IgG. Therefore, the activity of these two receptors can establish a
threshold of DC activation.
Furthermore, the difference in functional avidity of these receptors for IgG
can shift their
functional balance. Hence, altering the Fc domain binding to FcRs can also
shift their functional
-82-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
balance, allowing for manipulation (either enhanced activity or enhanced
inhibition) of the DC
immune response.
[0281] A modification in the amino acid sequence of the Fc domain of an
antibody construct can
alter the recognition of an FcR for the Fc domain. However, such modifications
can still allow
for FcR-mediated signaling. A modification can be a substitution of an amino
acid at a residue
(e.g., wildtype) for a different amino acid at that residue. A modification
can permit binding of an
FcR to a site on the Fc domain or region that the FcR may not otherwise bind
to. A modification
can increase the binding affinity of an FcR to the Fc domain that the FcR may
have reduced
binding affinity for. A modification can decrease binding affinity of an FcR
to a site on the Fc
domain that the FcR may have increased binding affinity for. A modification
can increase the
subsequent FcR-mediated signaling after Fc binding to an FcR.
[0282] An antibody construct can comprise an Fc region with at least one amino
acid change as
compared to the sequence of the wild-type Fc region. An antibody construct can
comprise an Fc
domain with at least one amino acid change as compared to the sequence of the
wild-type Fc
domain. An amino acid change in an Fc region can allow the construct to bind
to at least one Fc
receptor with greater affinity compared to a wild-type Fc region. An amino
acid change in an Fc
domain can allow the antibody construct to bind to at least one Fc receptor
with greater affinity
compared to a wild-type Fc domain. An Fc region can comprise an amino acid
sequence having
at least one, two, three, four, five, six, seven, eight, nine or ten
modifications but not more than
40, 35, 30, 25, 20, 15, or 10 modifications of the amino acid sequence
relative to the natural or
original amino acid sequence. An Fc domain can comprise an amino acid sequence
having at
least one, two, three, four, five, six, seven, eight, nine or ten
modifications but not more than 40,
35, 30, 25, 20, 15, or 10 modifications of the amino acid sequence relative to
the natural or
original amino acid sequence. An Fc region can be an Fc region of an IgG1
antibody. An Fc
region can contain an Fc domain. An Fc region can be an Fc domain.
[0283] An antibody construct can be an antibody comprising a sequence of the
IgG1 isoform that
has been modified from the wildtype IgG1 sequence. A modification can comprise
a substitution
at more than one amino acid residue such as at 5 different amino acid residues
including
L235V/F243L/R292P/Y300L/P396L (IgG1VLPLL). The numbering of amino acids
residues is
according to the EU index of Kabat. The 5 amino acid residues can be located
in a portion of an
antibody sequence which can encode an Fc region of the antibody and in
particular, can be
located in portions of the Fc region that can bind to Fc receptors (i.e., the
Fc domain). A
modification can comprise a substitution at more than one amino acid residue
such as at 3
different amino acid residues including S298A/E333A/K334A (IgGlAAA), according
to the EU
index of Kabat. The 3 amino acid residues can be located in a portion of an
antibody sequence
-83-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
which can encode an Fc region of the antibody and in particular, can be
located in portions of the
Fc region that can bind Fc receptors (i.e., the Fc domain).
[0284] In some embodiments, the Fc domain or region can comprise a sequence of
an IgG
isoform that has been modified from the wild-type IgG sequence. In some
embodiments, the Fc
domain or region can comprise a sequence of the IgG1 isoform that has been
modified from the
wild-type IgG1 sequence. In some embodiments, the modification comprises
substitution of one
or more amino acids that reduce binding affinity of an IgG Fc domain or region
to all Fey
receptors. A modification can be substitution of E233, L234 and L235, such as
E233P/L234V/L235A or E233P/L234V/L235A/AG236, according to the EU index of
Kabat. A
modification can be substitution of L235, F243, R292, Y300 and P396, such as
L235V/F243L/R292P/Y300L/P396L (IgG1VLPLL) according to the EU index of Kabat.
A
modification can be a substitution of P238, such as P238A, according to the EU
index of Kabat.
A modification can be a substitution of D265, such as D265A, according to the
EU index of
Kabat. A modification can be a substitution of N297, such as N297A, according
to the EU index
of Kabat. A modification can be a substitution of A327, such as A327Q,
according to the EU
index of Kabat. A modification can be a substitution of P329, such as P239A,
according to the
EU index of Kabat.
[0285] In some embodiments, an IgG Fc domain or region comprises at least one
amino acid
substitution that reduces its binding affinity to FcyR1, as compared to a wild-
type or reference
IgG Fc domain. A modification can comprise a substitution at F241, such as
F241A, according
to the EU index of Kabat. A modification can comprise a substitution at F243,
such as F243A,
according to the EU index of Kabat. A modification can comprise a substitution
at V264, such as
V264A, according to the EU index of Kabat. A modification can comprise a
substitution at
D265, such as D265A according to the EU index of Kabat.
[0286] In some embodiments, an IgG Fc domain or region comprises at least one
amino acid
substitution that increases its binding affinity to FcyR1, as compared to a
wild-type or reference
IgG Fc domain. A modification can comprise a substitution at A327 and P329,
such as
A327Q/P329A, according to the EU index of Kabat.
[0287] In some embodiments, the modification comprises substitution of one or
more amino
acids that reduce binding affinity of an IgG Fc domain or region to FcyRII and
FcyRIIIA
receptors. A modification can be a substitution of D270, such as D270A,
according to the EU
index of Kabat. A modification can be a substitution of Q295, such as Q295A,
according to the
EU index of Kabat. A modification can be a substitution of A327, such as
A237S, according to
the EU index of Kabat.
-84-
CA 03065919 2019-12-02
WO 2018/227023
PCT/US2018/036560
[0288] In some embodiments, the modification comprises substitution of one or
more amino
acids that increases binding affinity of an IgG Fc domain or region to FcyRII
and FcyRIIIA
receptors. A modification can be a substitution of T256, such as T256A,
according to the EU
index of Kabat. A modification can be a substitution of K290, such as K290A,
according to the
EU index of Kabat.
[0289] In some embodiments, the modification comprises substitution of one or
more amino
acids that increases binding affinity of an IgG Fc domain or region to FcyRII
receptor. A
modification can be a substitution of R255, such as R255A, according to the EU
index of Kabat.
A modification can be a substitution of E258, such as E258A, according to the
EU index of
Kabat. A modification can be a substitution of S267, such as S267A, according
to the EU index
of Kabat. A modification can be a substitution of E272, such as E272A,
according to the EU
index of Kabat. A modification can be a substitution of N276, such as N276A,
according to the
EU index of Kabat. A modification can be a substitution of D280, such as
D280A, according to
the EU index of Kabat. A modification can be a substitution of H285, such as
H285A, according
to the EU index of Kabat. A modification can be a substitution of N286, such
as N286A,
according to the EU index of Kabat. A modification can be a substitution of
T307, such as
T307A, according to the EU index of Kabat. A modification can be a
substitution of L309, such
as L309A, according to the EU index of Kabat. A modification can be a
substitution of N315,
such as N315A, according to the EU index of Kabat. A modification can be a
substitution of
K326, such as K326A, according to the EU index of Kabat. A modification can be
a substitution
of P331, such as P331A, according to the EU index of Kabat. A modification can
be a
substitution of S337, such as S337A, according to the EU index of Kabat. A
modification can be
a substitution of A378, such as A378A, according to the EU index of Kabat. A
modification can
be a substitution of E430, such as E430, according to the EU index of Kabat.
[0290] In some embodiments, the modification comprises substitution of one or
more amino
acids that increases binding affinity of an IgG Fc domain or region to FcyRII
receptor and
reduces the binding affinity to FcyRIIIA receptor. A modification can be a
substitution of H268,
such as H268A, according to the EU index of Kabat. A modification can be a
substitution of
R301, such as R301A, according to the EU index of Kabat. A modification can be
a substitution
of K322, such as K322A, according to the EU index of Kabat.
[0291] In some embodiments, the modification comprises substitution of one or
more amino
acids that decreases binding affinity of an IgG Fc domain or region to FcyRII
receptor but does
not affect the binding affinity to FcyRIIIA receptor. A modification can be a
substitution of
R292, such as R292A, according to the EU index of Kabat. A modification can be
a substitution
of K414, such as K414A, according to the EU index of Kabat.
-85-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
[0292] In some embodiments, the modification comprises substitution of one or
more amino
acids that decreases binding affinity of an IgG Fc domain or region to FcyRII
receptor and
increases the binding affinity to FcyRIIIA receptor. A modification can be a
substitution of
S298, such as S298A, according to the EU index of Kabat. A modification can be
substitution of
S239, 1332 and A330, such as S239D/1332E/A330L. A modification can be
substitution of S239
and 1332, such as S239D/I332E.
[0293] In some embodiments, the modification comprises substitution of one or
more amino
acids that decreases binding affinity of an IgG Fc domain or region to
FcyRIIIA receptor and
does not affect the binding affinity to FcyRII receptor. A modification can be
a substitution of
S239, such as S239A, according to the EU index of Kabat. A modification can be
a substitution
of E269, such as E269A, according to the EU index of Kabat. A modification can
be a
substitution of E293, such as E293A, according to the EU index of Kabat. A
modification can be
a substitution of Y296, such as Y296F, according to the EU index of Kabat. A
modification can
be a substitution of V303, such as V303A, according to the EU index of Kabat.
A modification
can be a substitution of A327, such as A327G, according to the EU index of
Kabat. A
modification can be a substitution of K338, such as K338A, according to the EU
index of Kabat.
A modification can be a substitution of D376, such as D376A, according to the
EU index of
Kabat.
[0294] In some embodiments, the modification comprises substitution of one or
more amino
acids that increases binding affinity of an IgG Fc domain or region to
FcyRIIIA receptor and does
not affect the binding affinity to FcyRII receptor. A modification can be a
substitution of E333,
such as E333A, according to the EU index of Kabat. A modification can be a
substitution of
K334, such as K334A, according to the EU index of Kabat. A modification can be
a substitution
of A339, such as A339T, according to the EU index of Kabat. A modification can
be substitution
of S239 and 1332, such as S239D/I332E.
[0295] In some embodiments, an IgG Fc domain or region comprises at least one
amino acid
substitution that reduces the binding affinity to FcRn, as compared to a wild-
type or reference
IgG Fc domain. A modification can comprise a substitution at H435, such as
H435A according
to the EU index of Kabat. A modification can comprise a substitution at 1253,
such as I253A
according to the EU index of Kabat. A modification can comprise a substitution
at H310, such as
H310A according to the EU index of Kabat. A modification can comprise
substitutions at 1253,
H310 and H435, such as 1253A/H310A/H435A according to the EU index of Kabat.
[0296] A modification can comprise a substitution of one amino acid residue
that increases the
binding affinity of an IgG Fc domain for FcRn, relative to a wildtype or
reference IgG Fc
domain. A modification can comprise a substitution at V308, such as V308P
according to the
-86-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
EU index of Kabat. A modification can comprise a substitution at M428, such as
M428L
according to the EU index of Kabat. A modification can comprise a substitution
at N434, such as
N434A according to the EU index of Kabat or N434H according to the EU index of
Kabat. A
modification can comprise substitutions at T250 and M428, such as T250Q and
M428L
according to the EU index of Kabat. A modification can comprise substitutions
at M428 and
N434, such as M428L and N434S, N434A or N434H according to the EU index of
Kabat. A
modification can comprise substitutions at M252, S254 and T256, such as
M252Y/S254T/T256E
according to the EU index of Kabat. A modification can be a substitution of
one or more amino
acids selected from P257L, P257N, P257I, V279E, V279Q, V279Y, A281S, E283F,
V284E,
L306Y, T307V, V308F, Q311V, D376V, and N434H. Other substitutions in an IgG Fc
domain
that affect its interaction with FcRn are disclosed in U.S. Patent No.
9,803,023 (the disclosure of
which is incorporated by reference herein).
[0297] An antibody construct can be a monoclonal anti-CD40 human antibody
comprising a
sequence of the IgG1 isoform that has been modified from the wildtype IgG1
sequence. A
modification can comprise a substitution at more than one amino acid residue
such as at 5
different amino acid residues including L235V/F243L/R292P/Y300L/P396L (SBT-040-
G1VLPLL). The numbering of amino acids residues is according to the EU index.
The 5 amino
acid residues can be located in a portion of an antibody sequence which can
encode an Fc region
of the antibody and in particular, can be located in portions of the Fc region
that can bind to Fc
receptors (i.e., the Fc domain). A modification can comprise a substitution at
more than one
amino acid residue such as at 3 different amino acid residues including
5298A/E333A/K334A
(SBT-040-G1AAA). The 3 amino acid residues can be located in a portion of an
antibody
sequence which can encode an Fc region of the antibody and in particular, can
be located in
portions of the Fc region that can bind Fc receptors (i.e., the Fc domain).
[0298] Binding of Fc receptors to an Fc region can be affected by amino acid
substitutions. For
example, SBT-040-VLPLL is an antibody with an amino acid sequence of a heavy
chain of
human anti-CD40 monoclonal antibody with modifications to a wild-type IgG1 Fc
domain
(L235V/F243L/R292P/Y300L/P396L). Binding of some Fc receptors to the Fc region
of SBT-
040-VLPLL can be enhanced compared to wild-type by as result of the
L235V/F243L/R292P/Y300L/P396L amino acid modifications. However, binding of
other Fc
receptors to the Fc region of SBT-040-VLPLL can be reduced compared to wild-
type by the
L235V/F243L/R292P/Y300L/P396L amino acid modifications. For example, the
binding
affinities of SBT-040-VLPLLto FcyRIIIA and to FcyRIIA can be enhanced compared
to wild-
type whereas the binding affinity of SBT-040-VLPLL to FcyRIIB can be reduced
compared to
wild-type. Binding of Fc receptors to an Fc region of are affected by amino
acid substitutions.
-87-
CA 03065919 2019-12-02
WO 2018/227023
PCT/US2018/036560
SBT-040-G1AAA antibody is an antibody with an amino acid sequence of a heavy
chain of a
human anti-CD40 monoclonal antibody with modifications to a wild-type IgG1 Fc
domain
(S298A/E333A/K334A). Binding of Fc receptors to an Fc region of SBT-040-G1AAA
can be
enhanced compared to wild-type as a result of the S298A/E333A/K334A amino acid
modification. However, binding of some Fc receptors to the Fc region of SBT-
040-G1AAA can
be reduced compared to wild-type by S298A/E333A/K334A amino acid modification.
Binding
affinities of SBT-040-G1AAA to FcyRIIIA can be enhanced compared to wild-type
whereas the
binding affinity of SBT-040-G1AAA to FcyRIIB can be reduced compared to
wildtype.
[0299] In some embodiments, the heavy chain of a human IgG2 antibody can be
mutated at
cysteines as positions 127, 232, or 233. In some embodiments, the light chain
of a human IgG2
antibody can be mutated at a cysteine at position 214. The mutations in the
heavy and light
chains of the human IgG2 antibody can be from a cysteine residue to a serine
residue.
[0300] While an antibody construct of the present disclosure can comprise a
first binding domain
and a second binding domain (or, in some cases, a third binding domain) with
wild-type or
modified amino acid sequences encoding the Fc region or Fc domain, the
modifications of the Fc
region or the Fc domain from the wild-type sequence may not significantly
alter binding and/or
affinity of the binding domains. For example, binding and/or affinity of an
antibody
constructcomprising a first binding domain and a second binding domain (or, in
some cases, a
third binding domain) and having the Fc domain modifications of SBT-040-G1WT,
SBT-040-
G1VLPLL, or SBT-040-G1AAA may not be significantly altered by modification of
an Fc
region or Fc domain amino acid sequence compared to a wild-type sequence.
Modifications of an
Fc region or Fc domain from a wild-type sequence may not alter binding and/or
affinity of a first
binding domain that binds, for example, to CD40 or DEC-205. Additionally, the
binding and/or
affinity of the binding domains described herein, for example, a first binding
domain, a second
binding domain (or, in some cases, a third binding domain), and an Fc domain
modification
selected from SBT-040-G1WT, SBT-040-G1VLPLL, and SBT-040-G1AAA, may be
comparable to the binding and/or affinity of wild-type antibodies.
[0301] In some embodiments, the binding profile of the Fc domain for Fey
receptors can be
retained in the antibody construct or conjugate, which can allow for delivery
of the antibody
construct or conjugate into immune cell types comprising the Fey receptors and
can further
immune activation by Fcy receptor signaling. In some embodiments, APCs can be
activated by
an antibody construct or conjugate as described herein when the antibody
construct or conjugate
is bound to a tumor cell, undergoes Fey receptor mediated uptake, or undergoes
Fey receptor
mediated Antibody Dependent Cellular Phagocytosis (ADCP). In some embodiments,
the
antibody construct or conjugate can retain weak or no Fey receptor binding to
allow for maximal
-88-
CA 03065919 2019-12-02
WO 2018/227023
PCT/US2018/036560
immune activation or decreased toxicity of the immune-stimulatory compound due
to the primary
delivery of the antibody construct or conjugate into tumor cells by antibody
antigen mediated
endocytosis.
[0302] In some embodiments, the antibody construct or conjugate can comprise
an IgG1 Fc
domain variant comprising N297A, N297G, K322A/L234A/L235A, or
L234F/L235E/P331S,
and lacks binding to an Fey receptor but can retain binding to FcRN in the
presence of the
immune-modulatory compound to allow for lower delivery of the conjugate into
tumor cells or
immune cells.
[0303] In some embodiments, an antibody construct or conjugate can comprise an
Fc domain
with higher affinity to one or more Fey receptors, which can result in greater
immune activation
than for an antibody construct or conjugate with an Fc domain that can bind to
one or more Fey
receptors with lower affinity.
TABLE 1. Tumor Antibody CDRs
Antibody Region SEQ ID Sequence
NO:
Pertuzumab HCDR1 13 GFTFTDYT
HCDR2 14 VNPNSGGS
HCDR3 15 ARNLGPSFYFDY
LCDR1 18 QDVSIG
LCDR2 19 SAS
LCDR3 20 QQYYIYPYT
Cetuximab HCDR1 26 GFSLTNYG
HCDR2 27 IWSGGNT
HCDR3 28 ARALTYYDYEFAY
LCDR1 31 QSIGTN
LCDR2 32 YAS
LCDR3 33 QQNNNWPTT
Panitumumab HCDR1 39 GGSVSSGDYY
HCDR2 40 IYYSGNT
HCDR3 41 VRDRVTGAFDI
LCDR1 44 QDISNY
LCDR2 45 DAS
LCDR3 46 QHFDHLPLA
Nimotuzumab HCDR1 52 GYTFTNYY
HCDR2 53 INPTSGGS
HCDR3 54 ARQGLWFDSDGRGFDF
LCDR1 57 QNIVHSNGNTY
LCDR2 58 KVS
LCDR3 59 FQYSHVPWT
Zalutumumab HCDR1 65 GFTFSTYG
HCDR2 66 IWDDGSYK
HCDR3 67 ARDGITMVRGVMKDYFDY
LCDR1 70 QDISSA
LCDR2 71 DAS
LCDR3 72 QQFNSYPLT
Onartuzumab HCDR1 78 GYTFTSYW
HCDR2 79 IDPSNSDT
HCDR3 80 ATYRSYVTPLDY
LCDR1 83 QSLLYTSSQKNY
LCDR2 84 WAS
-89-
CA 03065919 2019-12-02
WO 2018/227023
PCT/US2018/036560
Antibody Region SEQ ID Sequence
NO:
LCDR3 85 QQYYAYPWT
Patritumab HCDR1 91 GGSFSGYY
HCDR2 92 INHSGST
HCDR3 93 ARDKWTWYFDL
LCDR1 96 QSVLYSSSNRNY
LCDR2 97 WAS
LCDR3 98 QQYYSTPRT
Clivatuzumab HCDR1 104 GYTFPSYV
HCDR2 105 INPYNDGT
HCDR3 106 ARGFGGSYGFAY
LCDR1 109 SSVSSSY
LCDR2 110 STS
LCDR3 111 HQWNRYPYT
Sofituzumab HCDR1 117 GYSITNDYA
HCDR2 118 ISYSGYT
HCDR3 119 ARWT SGLDY
LCDR1 122 DLIHNW
LCDR2 123 GAT
LCDR3 124 QQWTTPFT
Edrecolomab HCDR1 130 GYAFTNYL
HCDR2 131 INPGSGGT
HCDR3 132 ARDGPWFAY
LCDR1 135 ENVVTY
LCDR2 136 GAS
LCDR3 137 GQGYSYPYT
Adecatumumab HCDR1 143 GFTFSSYG
HCDR2 144 ISYDGSNK
HCDR3 145 AKDMGWGSGWRPYYYYGMDV
LCDR1 148 QSISSY
LCDR2 149 WAS
LCDR3 150 QQSYDIPYT
Anetumab HCDR1 156 GYSFTSYW
HCDR2 157 IDPGDSRT
HCDR3 158 ARGQLYGGTYMDG
LCDR1 161 SSDIGGYNS
LCDR2 162 GVN
LCDR3 163 SSYDIESATPV
huDS6 HCDR1 169 GYTFTSYN
HCDR2 170 IYPGNGAT
HCDR3 171 ARGDSVPFAY
LCDR1 174 SSVSF
LCDR2 175 STS
LCDR3 176 QQRSSFPLT
Lifastuzumab HCDR1 182 GFSFSDFA
HCDR2 183 IGRVAFHT
HCDR3 184 ARHRGFDVGHFDF
LCDR1 187 ETLV HSSGNTY
LCDR2 188 RVS
LCDR3 189 FQGSFNPLT
Sacituzumab HCDR1 195 GYTFTNYG
HCDR2 196 INTYTGEP
HCDR3 197 ARGGFGSSWYFDV
LCDR1 200 QDVSIA
LCDR2 201 SAS
LCDR3 202 QQHYITPLT
PR1A3 HCDR1 208 GYTFTEFG
HCDR2 209 INTKTGEA
HCDR3 210 ARWDFYDYVEAMDY
-90-
CA 03065919 2019-12-02
WO 2018/227023
PCT/US2018/036560
Antibody Region SEQ ID Sequence
NO:
LCDR1 213 QNVGTN
LCDR2 214 SAS
LCDR3 215 HQYYTYPLFT
Humanized PR1A3 HCDR1 805 GYTFTEFG
HCDR2 806 INTKTGEA
HCDR3 807 ARWDFAYYVEAMDY
LCDR1 808 AAVGTY
LCDR2 809 SAS
LCDR3 810 HQYYTYPLFT
Humanized Ab2-3 HCDR1 823 GFVFSSYD
HCDR2 824 YISSGGGIT
HCDR3 825 AAHYFGSSGPFAY
LCDR1 826 ENIFSY
LCDR2 827 NTR
LCDR3 828 QHHYGTPFT
IMAB362, HCDR1 221 GYTFTSYW
CLAUDIXIMAB HCDR2 222 IYPSDSYT
HCDR3 223 TRSWRGNSFDY
LCDR1 226 QSLLNSGNQKNY
LCDR2 227 WAS
LCDR3 228 QNDYSYPFT
AMG595 HCDR1 260 GFTFRNYG
HCDR2 261 IWYDGSDK
HCDR3 262 ARDGYDILTGNPRDFDY
LCDR1 265 QSLVHSDGNTY
LCDR2 266 RIS
LCDR3 267 MQSTHVPRT
ABT806 HCDR1 273 GYSISRDFA
HCDR2 274 ISYNGNT
HCDR3 275 VTASRGFPY
LCDR1 278 QDINSN
LCDR2 279 HGT
LCDR3 280 VQYAQFPWT
Sibrotuzumab HCDR1 286 RYTFTEYT
HCDR2 287 INPNNGIP
HCDR3 288 ARRRIAYGYDEGHAMDY
LCDR1 291 QSLLYSRNQKNY
LCDR2 292 WAS
LCDR3 293 QQYFSYPLT
DS-8895a variant 1 HCDR1 299 GYTFIDYS
HCDR2 300 INTYTGEP
HCDR3 301 ATYYRYERDFDY
LCDR1 304 QSIVHSSGITY
LCDR2 305 KVS
LCDR3 306 FQGSHVPYT
DS-8895a variant 2 HCDR1 312 GYTFIDYS
HCDR2 313 INTYTGEP
HCDR3 314 ATYYRYERDFDY
LCDR1 317 QSIVHSSGITY
LCDR2 318 KVS
LCDR3 319 FQGSHVPYT
MEDI-547 HCDR1 325 GFTFSHYM
HCDR2 326 IGPSGGPT
HCDR3 327 AGYDSGYDYVAVAGPAEYFQH
LCDR1 330 QSISTW
LCDR2 331 KAS
LCDR3 332 QQYNSYSRT
Narnatumab HCDR1 338 GFTFSSYL
-91-
CA 03065919 2019-12-02
WO 2018/227023
PCT/US2018/036560
Antibody Region SEQ ID Sequence
NO:
HCDR2 339 IKQDGSEK
HCDR3 340 TRDGYSSGRHYGMDV
LCDR1 343 QSVSRY
LCDR2 344 DAS
LCDR3 345 QQRSNWPRT
RG7841 HCDR1 351 GFSLTGYS
HCDR2 352 IWGDGST
HCDR3 353 ARDYYFNYASWFAY
LCDR1 356 QGISNYL
LCDR2 357 YTS
LCDR3 358 QQYSELPWT
Farletuzumab HCDR1 364 GFTFSGYG
HCDR2 365 ISSGGSYT
HCDR3 366 ARHGDDPAWFAY
LCDR1 369 SSISSNN
LCDR2 370 GTS
LCDR3 371 QQWSSYPYMYT
Mirvetuximab HCDR1 377 GYTFTGYF
HCDR2 378 IHPYDGDT
HCDR3 379 TRYDGSRAMDY
LCDR1 382 QSVSFAGTSL
LCDR2 383 RAS
LCDR3 384 QQSREYPYT
J591 variant 1 HCDR1 390 GYTFTEYT
HCDR2 391 INPNNGGT
HCDR3 392 AAGWNFDY
LCDR1 395 QDVGTA
LCDR2 396 WAS
LCDR3 397 QQYNSYPLT
J591 variant 2 HCDR1 403 GYTFTEYT
HCDR2 404 INPNNGGT
HCDR3 405 AAGWNFDY
LCDR1 408 ENVVTY
LCDR2 409 GAS
LCDR3 410 GQGYSYPYT
Rovalpituzumab HCDR1 416 GYTFTNYG
HCDR2 417 INTYTGEP
HCDR3 418 ARIGDSSPSDY
LCDR1 421 QSVSND
LCDR2 422 YAS
LCDR3 423 QQDYTSPWT
PF-06647020 HCDR1 429 GYTFTDYA
HCDR2 430 ISTYNDYT
HCDR3 431 ARGNSYFYALDY
LCDR1 434 ESVDSYGKSF
LCDR2 435 RAS
LCDR3 436 QQSNEDPWT
Antibody to PTK7 HCDR1 442 GFTFSSYA
HCDR2 443 ISYDGSIK
HCDR3 444 ARTYYFDY
LCDR1 447 QSIGSS
LCDR2 448 YAS
LCDR3 449 HQSSSLPIT
Ladiratuzumab HCDR1 455 GLTIEDYY
HCDR2 456 IDPENGDT
HCDR3 457 AVHNAHYGTWFAY
LCDR1 460 QSLLHSSGNTY
LCDR2 461 KIS
-92-
CA 03065919 2019-12-02
WO 2018/227023
PCT/US2018/036560
Antibody Region SEQ ID Sequence
NO:
LCDR3 462 FQGSHVPYT
Cirmtuzumab HCDR1 468 GYAFTAYN
HCDR2 469 FDPYDGGS
HCDR3 470 ARGWYYFDY
LCDR1 473 KSISKY
LCDR2 474 SGS
LCDR3 475 QQHDESPYT
Antibody to HCDR1 481 GFRFRSHG
MAGE-A3 HCDR2 482 SYDGNNK
HCDR3 483 ASPYTSDWQYFQY
LCDR1 486 QNISTT
LCDR2 487 DTS
LCDR3 488 QQSNSWPLT
Antibody to NY- HCDR1 494 GFSFIDYG
ESO-1 HCDR2 495 MNWSGDKK
HCDR3 496 ARGEYSNRFDP
LCDR1 499 QSLVFTDGNTY
LCDR2 500 KVS
LCDR3 501 MQGTHWPPI
Trastuzumab HCDR1 673 GFNIKDTY
HCDR2 674 IYPTNGYT
HCDR3 675 SRWGGDGFYAMDY
LCDR1 676 QDVNTA
LCDR2 677 SAS
LCDR3 678 QQHYTTPPT
Ipilumumab HCDR1 850 GFTFSSYT
HCDR2 851 ISYDGNNK
HCDR3 852 ARTGWLGPFDY
LCDR1 853 QSVGSSY
LCDR2 854 SSY
LCDR3 855 QQYGSSPWT
Vonlerolizumab HCDR1 856 GYTFTDSY
HCDR2 857 MYPDNGDS
HCDR3 858 VLAPRWYFSV
LCDR1 859 QDISNY
LCDR2 860 YTS
LCDR3 861 QQGHTLPPT
Anti-CD27 HCDR1 862 GFTFSSYD
Antibody HCDR2 863 IWYDGSNK
HCDR3 864 ARGSGNWGFFDY
LCDR1 865 QGISRW
LCDR2 866 AAS
LCDR3 867 QQYNTYPRT
Atezolizumab HCDR1 868 GFTFSDSW
HCDR2 869 ISPYGGST
HCDR3 870 ARRHWPGGFDY
LCDR1 871 QDVSTA
LCDR2 872 SAS
LCDR3 873 QQYLYHPAT
Durvalumab HCDR1 874 GFTFSRYW
HCDR2 875 IKQDGSEK
HCDR3 876 AREGGWFGELAFDY
LCDR1 877 QRVSSSY
LCDR2 878 DAS
LCDR3 879 QQYGSLPWT
MDX-1106 HCDR1 880 GDTFSTYA
HCDR2 881 IIPIFGKA
HCDR3 882 ARKFHFVSGSPFGMDV
-93-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ ID Sequence
NO:
LCDR1 883 QSVSSY
LCDR2 884 DAS
LCDR3 885 QQRSNWPT
TABLE 2. Tumor Antibody VH sequences and VL sequences
Antibody Region SEQ Sequence
ID
NO:
Pertuzumab VH 12 EVQLVESGGGLVQPGGSLRLSCAASGFTFTDYTMDWVRQAPGKGLEWVA
DVNPNSGGSIYNQRFKGRFTLSVDRSKNTLYLQMNSLRAEDTAVYYCARN
LGPSFYFDYWGQGTLVTVSS
VL 17 DIQMTQSPSSLSASVGDRVTITCKASQDVSIGVAWYQQKPGKAPKLLIYSAS
YRYTGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCQQYYIYPYTFGQGTKV
EIK
Cetuximab VH 25 QVQLKQSGPGLVQPSQSLSITCTVSGFSLTNYGVHWVRQSPGKGLEWLGVI
WSGGNTDYNTPFTSRLSINKDNSKSQVFFKMNSLQSNDTAIYYCARALTYY
DYEFAWGQGTLVTVSA
VL 30 DILLTQSPVILSVSPGERVSFSCRASQSIGTNIHWYQQRTNGSPRLLIKYASES
ISGIPSRFSGSGSGTDFTLSINSVESEDIADYYCQQNNNWPTTFGAGTKLELK
Panitumumab VH 38 QVQLQESGPGLVKPSETLSLTCTVSGGSVSSGDYWTWIRQSPGKGLEWIG
HIYYSGNTNYNPSLKSRLTISIDTSKTQFSLKLSSVTAADTAIYYCVRDRVTG
AFDIWGQGTMVTVSS
VL 43 DIQMTQSPSSLSASVGDRVTITCQASQDISNYLNWYQQKPGKAPKWYDA
SNLETGVPSRFSGSGSGTDFTFTISSLQPEDIATYFCQHFDHLPLAFGGGTKV
EIK
Nimotuzumab VH 51 QVQLQQSGAEVKKPGS SVKVSCKASGYTFTNYYIWVRQAPGQGLEWIG
GINPTSGGSNFNEKFKTRVTITVDESTNTAYMELSSLRSEDTAFYFCARQGL
WFDSDGRGFDFWGQGSTVTVSS
VL 56 DIQMTQSPSSLSASVGDRVTITCRSSQNIVHSNGNTYLDWYQQTPGKAPKL
LIYKVSNRFSGVPSRFSGSGSGTDFTFTISSLQPEDIATYYCFQYSHVPWTFG
QGTKLEIK
Zalutumumab VH 64 QVQLVESGGGVVQPGRSLRLSCAASGFTFSTYGMHWVRQAPGKGLEWVA
VIWDDGSYKYYGDSVKGRFTISRDNSKNTLYLQMNSLRAEDTAVYYCAR
DGITMVRGVMKDYFDYWGQGTLVTVSS
VL 69 AIQLTQSPSSLSASVGDRVTITCRASQDISSALVWYQQKPGKAPKLLIYDAS
SLESGVPSRFSGSESGTDFTLTISSLQPEDFATYYCQQFNSYPLTFGGGTKVE
IK
Onartuzumab VH 77 EVQLVESGGGLVQPGGSLRLSCAASGYTFTSWLHWVRQAPGKGLEWVG
MIDPSNSDTRFNPNFKDRFTISADTSKNTAYLQMNSLRAEDTAVYYCATYR
SYVTPLDWGQGTLVTVSS
VL 82 DIQMTQSPSSLSASVGDRVTITCKSSQSLLYTSSQKNYLAWYQQKPGKAPK
LLIWASTRESGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCQQYYAYPWT
FGQGTKVEIK
Patritumab VH 90 QVQLQQWGAGLLKPSETLSLTCAVYGGSFSGYWSWIRQPPGKGLEWIGE
INHSGSTNYNPSLKSRVTISVETSKNQFSLKLS SVTAADTAVYYCARDKWT
WYFDLWGRGTLVTVSS
-94-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
VL 95 DIEMTQSPDSLAVSLGERATINCRS SQSVLYSSSNRNYLAWYQQNPGQPPK
LLIWASTRESGVPDRFSGSGSGTDFTLTISSLQAEDVAVYYCQQYYSTPRT
FGQGTKVEIK
Clivatuzumab VH 103 QVQLQQSGAEVKKFGAS VKVSCEASGYTFPSYVLHWVKQAPGQGLEWIG
YINPYNDGTQTNKKFKGKATLTRDTSINTAYMELSRLRSDDTAVYYCARG
FGGSYGFAYNGQGTLVTVSS
VL 108 DIQLTQSPSSLSASVGDRVTMTCSASSSVSSSYLYWYQQKPGKAPKLWIYS
TSNLASGVPARFSGSGSGTDFTLTISSLQPEDSASYFCHQWNRYPYTFGGGT
RLEIK
Sofituzumab VH 116 EVQLVESGGGLVQPGGSLRLSCAASGYSITNDYAWNWVRQAPGKGLEWV
GYISYSGYTTYNPSLKSRFTISRDTSKNTLYLQMNSLRAEDTAVYYCARWT
SGLDWGQGTLVTVSS
VL 121 DIQMTQSPSSLSASVGDRVTITCKASDLIHNWLAWYQQKPGKAPKWYGA
TSLETGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCQQWTTPFTFGQGTK
VEIK
Edrecolomab VH 129 QVQLQQSGAELVRPGTSVKVSCKASGYAFTNYLIEWVKQRPGQGLEWIGV
INPGSGGTNYNEKFKGKATLTADKSSSTAYMQLSSLTSDDSAVYFCARDGP
WFAWGQGTLVTVSA
VL 134 NIVMTQSPKSMSMSVGERVTLTCKASENVVTYVSWYQQKPEQSPKLLIYG
ASNRYTGVPDRFTGSGSATDFTLTISSVQAEDLADYHCGQGYSYPYTFGGG
TKLEIK
Adecatumum VH 142 EVQLLESGGGVVQPGRSLRLSCAASGFTFSSYGMHWVRQAPGKGLEWVA
ab VISYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAEDTAVYYCAKD
MGWGSGWRPYYYYGMDVWGQGTTVTVSS
VL 147 ELQMTQSPSSLSASVGDRVTITCRTSQSISSYLNWYQQKPGQPPKLLIWAS
TRESGVPDRFSGSGSGTDFTLTISSLQPEDSATYYCQQSYDIPYTFGQGTKLE
IK
Anetumab VH 155 QVELVQSGAEVKKPGESLKISCKGSGYSFTSWIGWVRQAPGKGLEWMGII
DPGDSRTRYSPSFQGQVTISADKSISTAYLQWSSLKASDTAMYYCARGQLY
GGTYMDGWGQGTLVTVSS
VL 160 DIALTQPASVSGSPGQSITISCTGTSSDIGGYNSVSWYQQHPGKAPKLMIYG
VNNRPSGVSNRFSGSKSGNTASLTISGLQAEDEADYYCSSYDIESATPVFGG
GTKLTVL
huDS6 VH 168 QAQLVQSGAEVVKPGAS VKMSCKASGYTFTSYNMHWVKQTPGQGLEWIG
YIYPGNGATNYNQKFQGKATLTADPSSSTAYMQISSLTSEDSAVYFCARGD
SVPFAWGQGTLVTVSA
VL 173 EIVLTQSPATMSASPGERVTITCSAHSSVSFMHWFQQKPGTSPKLWIYSTSS
LASGVPARFGGSGSGTSYSLTISSMEAEDAATYYCQQRSSFPLTFGAGTKLE
LK
Lifastuzumab VH 181 EVQLVESGGGLVQPGGSLRLSCAASGFSFSDFAMSWVRQAPGKGLEWVAT
IGRVAFHTYYPDSMKGRFTISRDNSKNTLYLQMNSLRAEDTAVYYCARHR
GFDVGHFDFWGQGTLVTVSS
VL 186 DIQMTQSPSSLSASVGDRVTITCRSSETLVHSSGNTYLEWYQQKPGKAPKL
-95-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
LIYRVSNRFSGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCFQGSFNPLTFG
QGTKVEIK
Sacituzumab VH
194 QVQLQQSGSELKKPGASVKVSCKASGYTFTNYGMNWVKQAPGQGLKWM
GWINTYTGEPTYTDDFKGRFAFSLDTSVSTAYLQISSLKADDTAVYFCARG
GFGSSWYFDVWGQGSLVTVSS
VL 199 DIQLTQSPSSLSASVGDRVSITCKASQDVSIAVAWYQQKPGKAPKLLIYSAS
YRYTGVPDRFSGSGSGTDFTLTISSLQPEDFAVYYCQQHYITPLTFGAGTKV
EIK
PR1A3 VH 207 QVKLQQSGPELKKPGETVKISCKASGYTFTEFGMNWVKQAPGKGLKWMG
WINTKTGEATYVEEFKGRFAFSLETSATTAYLQINNLKNEDTAKYFCARW
DFYDYVEAMDWGQGTTVTVSS
VL 212 DIVMTQSQRFMSTSVGDRVSVTCKASQNVGTNVAWYQQKPGQSPKALIYS
ASYRYSGVPDRFTGSGSGTDFTLTISNVQSEDLAEYFCHQYYTYPLFTFGSG
TKLEMK
Humanized VH 811 QVQLVQSGAEVKKPGASVKVSCKASGYTFTEFGMNWVRQAPGQGLEWM
PR1A3 GWINTKTGEATYVEEFKGRVTFTTDTSTSTAYMELRSLRSDDTAVYYCAR
WDFAYYVEAMDWGQGTTVTVSS
VL 812 DIQMTQSPSSLSASVGDRVTITCKASAAVGTYVAWYQQKPGKAPKLLIYSA
SYRKRGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCHQYYTYPLFTFGQGT
KLEIK
Humanized VH
829 EVQLQESGPGLVKPGGSLSLSCAASGFVFSS YDMSWVRQTPERGLEWVAYI
Ab2-3
SSGGGITYAPSTVKGRFTVSRDNAKNTLYLQMNSLTSEDTAVYYCAAHYF
GSSGPFAWGQGTLVTVSS
VL 830 DIQMTQSPASLSASVGDRVTITCRASENIFSYLAWYQQKPGKSPKLLVYNT
RTLAEGVPSRFSGSGSGTDFSLTISSLQPEDFATYYCQHHYGTPFTFGSGTKL
EIK
IMAB362, VH
220 QVQLQQPGAELVRPGAS VKLSCKASGYTFTSWINWVKQRPGQGLEWIGN
CLAUDIXIM
IYPSDSYTNYNQKFKDKATLTVDKSSSTAYMQLS SPTSEDSAVYYCTRSWR
AB GNSFDWGQGTTLTVSS
VL 225 DIVMTQSPSSLTVTAGEKVTMSCKSSQSLLNSGNQKNYLTWYQQKPGQPP
KLLIWASTRESGVPDRFTGSGSGTDFTLTISSVQAEDLAVYYCQNDYSYPF
TFGSGTKLEIK
AMG595 VH 259 QVQLVESGGGVVQSGRSLRLSCAASGFTFRNYGMHWVRQAPGKGLEWVA
VIWYDGSDKYYADSVRGRFTISRDNSKNTLYLQMNSLRAEDTAVYYCARD
GYDILTGNPRDFDWGQGTLVTVSS
VL 264 DTVMTQTPLSSHVTLGQPASISCRSSQSLVHSDGNTYLSWLQQRPGQPPRL
LIYRISRRFSGVPDRFSGSGAGTDFTLEISRVEAEDVGVYYCMQSTHVPRTF
GQGTKVEIK
ABT806 VH 272 EVQLQESGPGLVKPSQTLSLTCTVSGYSISRDFAWNWIRQPPGKGLEWMG
YISYNGNTRYQPSLKSRITISRDTSKNQFFLKLNSVTAADTATYYCVTASRG
FPWGQGTLVTVSS
VL 277 DIQMTQSPSSMSVSVGDRVTITCHSSQDINSNIGWLQQKPGKSFKGLIYHGT
NLDDGVPSRFSGSGSGTDYTLTISSLQPEDFATYYCVQYAQFPWTFGGGTK
LEIK
Sibrotuzumab VH
285 QVQLVQSGAEVKKPGAS VKVSCKTSRYTFTEYTIHWVRQAPGQRLEWIGG
INPNNGIPNYNQKFKGRVTITVDTSASTAYMELSSLRSEDTAVYYCARRRIA
YGYDEGHAMDYWGQGTLVTVSS
VL 290 DIVMTQSPDSLAVSLGERATINCKSSQSLLYSRNQKNYLAWYQQKPGQPPK
LLIFWASTRESGVPDRFSGSGFGTDFTLTISSLQAEDVAVYYCQQYFSYPLT
-96-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
FGQGTKVEIK
DS-8895a VH 298 QVQLVQSGAEVKKPGASVKVSCKASGYTFIDYSMHWVRQAPGQGLEWM
variant 1 GWINTYTGEPTYSDDFKGRVTITADTSTSTAYLELSSLRSEDTAVYYCATY
YRYERDFDWGQGTLVTVSS
VL 303 DIVMTQSPLSLPVTPGEPASISCRSSQSIVHSSGITYLEWYLQKPGQSPQLLIY
KVSNRFSGVPDRFSGSGSGTDFTLKISRVEAEDVGVYYCFQGSHVPYTFGQ
GTKVEIK
DS-8895a VH 311 QIQLVQSGAEVKKPGASVKVSCKASGYTFIDYSMHWVRQAPGQGLKWMG
variant 2 WINTYTGEPTYSDDFKGRFAFSLDTSTSTAYLELSSLRSEDTAVYYCATYY
RYERDFDWGQGTLVTVSS
VL 316 DVLMTQSPLSLPVTPGEPASISCRSSQSIVHSSGITYLEWYLQKPGQSPQLLI
YKVSNRFSGVPDRFSGSGSGTDFTLKISRVEAEDVGVYYCFQGSHVPYTFG
QGTKVEIK
MEDI-547 VH 324 EVQLLESGGGLVQPGGSLRLSCAASGFTFSHYMMAWVRQAPGKGLEWVS
RIGPSGGPTHYADSVKGRFTISRDNSKNTLYLQMNSLRAEDTAVYYCAGY
DSGYDYVAVAGPAEYFQHWGQGTLVTVSS
VL 329 DIQMTQSPSSLSASVGDRVTITCRASQSISTWLAWYQQKPGKAPKLLIYKAS
NLHTGVPSRFSGSGSGTEFSLTISGLQPDDFATYYCQQYNSYSRTFGQGTKV
EIK
Narnatumab VH 337 EVQLVESGGGLVQPGGSLRLSCAASGFTFSSYLMTWVRQAPGKGLEWVA
NIKQDGSEKYYVDSVKGRFTISRDNAKNSLNLQMNSLRAEDTAVYYCTRD
GYSSGRHYGMDVWGQGTTVIVSS
VL 342 EIVLTQSPATLSLSPGERATLSCRASQSVSRYLAWYQQKPGQAPRLLIYDAS
NRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRSNWPRTFGQGTKV
EIK
RG7841 VH 350 EVQLVESGPALVKPTQTLTLTCTVSGFSLTGYSVNWIRQPPGKALEWLGMI
WGDGSTDYNSALKSRLTISKDTSKNQVVLTMTNMDPVDTATYYCARDYY
FNYASWFAYWGQGTLVTVSS
VL 355 DIQMTQSPSSLSASVGDRVTITCSASQGISNYLNWYQQKPGKTVKLLIYYTS
NLHSGVPSRFSGSGSGTDYTLTISSLQPEDFATYYCQQYSELPWTFGQGTKV
EIK
Farletuzumab VH
363 EVQLVESGGGVVQPGRSLRLSCSASGFTFSGYGLSWVRQAPGKGLEWVA
MISSGGSYTYYADSVKGRFAISRDNAKNTLFLQMDSLRPEDTGVYFCARH
GDDPAWFAWGQGTPVTVSS
VL 368 DIQLTQSPSSLSASVGDRVTITCSVSSSISSNNLHWYQQKPGKAPKPWIYGTS
NLASGVPSRFSGSGSGTDYTFTISSLQPEDIATYYCQQWSSYPYMYTFGQGT
KVEIK
Mirvetuximab VH 376 QVQLVQSGAEVVKPGASVKISCKASGYTFTGYFMNWVKQSPGQSLEWIGR
IHPYDGDTFYNQKFQGKATLTVDKSSNTAHMELLSLTSEDFAVYYCTRYD
GSRAMDYWGQGTTVTVSS
VL 381 DIVLTQSPLSLAVSLGQPAIISCKASQSVSFAGTSLMHWYHQKPGQQPRLLI
YRASNLEAGVPDRFSGSGSKTDFTLTISPVEAEDAATYYCQQSREYPYTFG
GGTKLEIK
-97-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
J591 variant 1 VH 389
EVQLQQSGPELKKPGTSVRISCKTSGYTFTEYTIHWVKQSHGKSLEWIGNIN
PNNGGTTYNQKFEDKATLTVDKSSSTAYMELRSLTSEDSAVYYCAAGWNF
DWGQGTTLTVSS
VL 394 DIVMTQSHKFMSTSVGDRVSIICKASQDVGTAVDWYQQKPGQSPKWYW
ASTRHTGVPDRFTGSGSGTDFTLTITNVQSEDLADYFCQQYNSYPLTFGAG
TMLDLK
J591 variant 2 VH
402 EVQLQQSGPELVKPGTSVRISCKTSGYTFTEYTIHWVKQSHGKSLEWIGNIN
PNNGGTTYNQKFEDKATLTVDKSSSTAYMELRSLTSEDSAVYYCAAGWNF
DWGQGTTLTVSS
VL 407 NIVMTQSPKSMSMSVGERVTLTCKASENVVTYVSWYQQKPEQSPKLLIYG
ASNRYTGVPDRFTGSGSATDFTLTISSVQAEDLADYHCGQGYSYPYTFGGG
TKLEIK
Rovalpituzum VH
415 QVQLVQSGAEVKKPGASVKVSCKASGYTFTNYGMNWVRQAPGQGLEWM
ab
GWINTYTGEPTYADDFKGRVTMTTDTSTSTAYMELRSLRSDDTAVYYCAR
IGDSSPSDWGQGTLVTVSS
VL 420 EIVMTQSPATLSVSPGERATLSCKASQSVSNDVVWYQQKPGQAPRLLIYYA
SNRYTGIPARFSGSGSGTEFTLTISSLQSEDFAVYYCQQDYTSPWTFGQGTK
LEIK
PF-06647020 VH
428 QVQLVQSGPEVKKPGASVKVSCKASGYTFTDYAVHWVRQAPGKRLEWIG
VISTYNDYTYNNQDFKGRVTMTRDTSASTAYMELSRLRSEDTAVYYCARG
NSYFYALDYWGQGTSVTVSS
VL 433 EIVLTQSPATLSLSPGERATLSCRASESVDSYGKSFMHWYQQKPGQAPRLLI
YRASNLESGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQSNEDPWTFGG
GTKLEIK
Antibody to VH
441 QVQLVESGGGVVQPGRSLRLSCAASGFTFSSYAFHWVRQAPGKGLEWVA
PTK7 VISYDGSIKYYADSVKGRFTISRDNSKNTLYLQMNSLRAEDTAVYYCARTY
YFDWGQGTLVTVSS
VL 446 EIVLTQSPDFQSVTPKEKVTITCRASQSIGSSLHWYQQKPDQSPKLLIKYASQ
SFSGVPSRFSGSGSGTDFTLTINSLEAEDAAAYYCHQS SSLPITFGQGTRLEI
K
Ladiratuxuma VH
454 QVQLVQSGAEVKKPGASVKVSCKASGLTIEDYYMHWVRQAPGQGLEWM
b GWIDPENGDTEYGPKFQGRVTMTRDTSINTAYMELSRLRSDDTAVYYCAV
HNAHYGTWFAWGQGTLVTVSS
VL
459 DVVMTQSPLSLPVTLGQPASISCRSSQSLLHS SGNTYLEYFQQRPGQSPRPLI
YKISTRFSGVPDRFSGSGSGTDFTLKISRVEAEDVGVYYCFQGSHVPYTFGG
GTKVEIK
Cirmtuzumab VH
467 QVQLQESGPGLVKPSQTLSLTCTVSGYAFTAYNIHWVRQAPGQGLEWMGS
FDPYDGGSSYNQKFKDRLTISKDTSKNQVVLTMTNMDPVDTATYYCARG
WYYFDWGHGTLVTVSS
VL 472 DIVMTQTPLSLPVTPGEPASISCRASKSISKYLAWYQQKPGQAPRLLIYSGST
LQSGIPPRFSGSGYGTDFTLTINNIESEDAAYYFCQQHDESPYTFGEGTKVEI
K
Antibody to VH
480 QVQLVESGGGVVQPGRSLRLSCTASGFRFRSHGMHWVRQAPGKGLEWVA
MAGE-A3 VISYDGNNKLYADSVKGRITISRDNSKNTLFLQMNNVRAEDTAVYYCASP
YTSDWQYFQWGQGTLVIVSS
-98-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
VL 485 EIVMTQSPATLSVSPGERATFSCRASQNISTTLAWYQQKPGQAPRLLIYDTS
TRATGIPARFSGSGSGTEFTLTISSLQSEDLAVYYCQQSNSWPLTFGGGTKV
EIK
Antibody to VH
493 QVQLVQSGGGVVRPGGSLRLSCAASGFSFIDYGMSWVRQVPGKGLEWVA
NY-ESO-1
GMNWSGDKKGHAESVKGRFIISRDNAKNTLYLEMSSLRVEDTALYFCARG
EYSNRFDPRGRGTLVTVSS
VL
498 DIVMTQTPLSLPVTLGQPASLSCRS SQSLVFTDGNTYLNWFQQRPGQSPRRL
IYKVSSRDPGVPDRFSGTGSGTDFTLEISRVEAEDIGVYYCMQGTHWPPIFG
QGTKVEIK
Trastuzumab VH
679 EVQLVESGGGLVQPGGSLRLSCAASGFNIKDTYIHWVRQAPGKGLEWVAR
IYPTNGYTRYADSVKGRFTISADTSKNTAYLQMNSLRAEDTAVYYCSRWG
GDGFYAMDWGQGTLVTVSS
VL 680 DIQMTQSPSSLSASVGDRVTITCRASQDVNTAVAWYQQKPGKAPKLLIYSA
SFLYSGVPSRFSGSRSGTDFTLTISSLQPEDFATYYCQQHYTTPPTFGQGTKV
EIK
Vonlerolizum VH
886 EVQLVQSGAEVKKPGASVKVSCKASGYTFTDSYMSWVRQAPGQGLEWIG
ab
DMYPDNGDSSYNQKFRERVTITRDTSTSTAYLELSSLRSEDTAVYYCVLAP
RWYFSVWGQGTLVTVSS
VL 887 DIQMTQSPSSLSASVGDRVTITCRASQDISNYLNWYQQKPGKAPKLLIYYTS
RLRSGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCQQGHTLPPTFGQGTKV
EIK
Varlilumab VH 888 QVQLVESGGGVVQPGRSLRLSCAASGFTFSSYDMHWVRQAPGKGLEWVA
VIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAEDTAVYYCAR
GSGNWGFFDYWGQGTLVTVSS
VL 889 DIQMTQSPSSLSASVGDRVTITCRASQGISRWLAWYQQKPEKAPKSLIYAAS
SLQSGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCQQYNTYPRTFGQGTKV
EIK
Atezolizumab VH 890 EVQLVESGGGLVQPGGSLRLSCAASGFTFSDSWIHWVRQAPGKGLEWVA
WISPYGGSTYYADSVKGRFTISADTSKNTAYLQMNSLRAEDTAVYYCARR
HWPGGFDWGQGTLVTVSS
VL 891 DIQMTQSPSSLSASVGDRVTITCRASQDVSTAVAWYQQKPGKAPKLLIYSA
SFLYSGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCQQYLYHPATFGQGTK
VEIK
Durvalumab VH 892 EVQLVESGGGLVQPGGSLRLSCAASGFTFSRWMSWVRQAPGKGLEWVA
NIKQDGSEKYYVDSVKGRFTISRDNAKNSLYLQMNSLRAEDTAVYYCARE
GGWFGELAFDWGQGTLVTVSS
VL 893 EIVLTQSPGTLSLSPGERATLSCRASQRVSSSYLAWYQQKPGQAPRLLIYDA
SSRATGIPDRFSGSGSGTDFTLTISRLEPEDFAVYYCQQYGSLPWTFGQGTK
VEIK
MDX-1106 VH 894 QVQLVQSGAEVKKPGS SVKVSCKTSGDTFSTYAISWVRQAPGQGLEWMG
GIIPIFGKAHYAQKFQGRVTITADESTSTAYMELSSLRSEDTAVYFCARKFH
FVSGSPFGMDVWGQGTTVTVSS
VL 895 EIVLTQSPATLSLSPGERATLSCRASQSVS SYLAWYQQKPGQAPRLLIYDAS
NRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRSNWPTFGQGTKVE
IK
Ipilumumab VH 896 QVQLVESGGGVVQPGRSLRLSCAASGFTFSSYTMHWVRQAPGKGLEWVT
FISYDGNNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAEDTAIYYCARTG
WLGPFDYWGQGTLVTVSS
VL 897 EIVLTQSPGTLSLSPGERATLSCRASQSVGSSYLAWYQQKPGQAPRLLIYGA
FSRATGIPDRFSGSGSGTDFTLTISRLEPEDFAVYYCQQYGSSPWTFGQGTK
VEIK
MDX-1105 VH 901 QVQLVQSGAEVKKPGS SVKVSCKTSGDTFSTYAISWVRQAPGQGLEWMG
GIIPIFGKAHYAQKFQGRVTITADESTSTAYMELSSLRSEDTAVYFCARKFH
-99-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
FVSGSPFGMDVWGQGTTVTVSS
VL 902 EIVLTQSPATLSLSPGERATLSCRASQSVS SYLAWYQQKPGQAPRLLIYDAS
NRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRSNWPTFGQGTKVE
IK
TABLE 3. Tumor Antibody Heavy Chain and Light Chain sequences
Antibody Region SEQ Sequence
ID
NO:
Pertuzumab Heavy 11 EVQLVESGGGLVQPGGSLRLSCAASGFTFTDYTMDWVRQAPGKGLEWVA
Chain DVNPNSGGSIYNQRFKGRFTLSVDRSKNTLYLQMNSLRAEDTAVYYCARN
LGPSFYFDYWGQGTLVTVSSASTKGPSVFPLAPSSKSTSGGTAALGCLVKD
YFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGTQTYIC
NVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPSVFLFPPKPKDTL
MISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPREEQYNSTYR
VVSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLP
PSREEMTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGS
FFLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPG
Light 16 DIQMTQSPSSLSASVGDRVTITCKASQDVSIGVAWYQQKPGKAPKLLIYSAS
Chain YRYTGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCQQYYIYPYTFGQGTKV
EIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREAKVQWKVDNALQ
SGNSQESVTEQDSKDSTYSLSSTLTLSKADYEKHKVYACEVTHQGLSSPVT
KSFNRGEC
Cetuximab Heavy 24 QVQLKQSGPGLVQPSQSLSITCTVSGFSLTNYGVHWVRQSPGKGLEWLGVI
Chain WSGGNTDYNTPFTSRLSINKDNSKSQVFFKMNSLQSNDTAIYYCARALTYY
DYEFAWGQGTLVTVSAASTKGPSVFPLAPSSKSTSGGTAALGCLVKDYFP
EPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGTQTYICNVN
HKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPSVFLFPPKPKDTLMISR
TPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPREEQYNSTYRVVS
VLTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSR
DELTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFL
YSKLTVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGK
Light 29 DILLTQSPVILSVSPGERVSFSCRASQSIGTNIHWYQQRTNGSPRLLIKYASES
Chain ISGIPSRFSGSGSGTDFTLSINSVESEDIADYYCQQNNNWPTTFGAGTKLELK
RTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREAKVQWKVDNALQSGN
SQESVTEQDSKDSTYSLSSTLTLSKADYEKHKVYACEVTHQGLS SPVTKSF
NRGEC
Panitumumab Heavy 37 QVQLQESGPGLVKPSETLSLTCTVSGGSVSSGDYWTWIRQSPGKGLEWIG
Chain HIYYSGNTNYNPSLKSRLTISIDTSKTQFSLKLSSVTAADTAIYYCVRDRVTG
AFDIWGQGTMVTVSSASTKGPSVFPLAPCSRSTSESTAALGCLVKDYFPEPV
TVSWNSGALTSGVHTFPAVLQS SGLYSLSSVVTVPSSNFGTQTYTCNVDHK
PSNTKVDKTVERKCCVECPPCPAPPVAGPSVFLFPPKPKDTLMISRTPEVTC
VVVDVSHEDPEVQFNWYVDGVEVHNAKTKPREEQFNSTFRVVSVLTVVH
QDWLNGKEYKCKVSNKGLPAPIEKTISKTKGQPREPQVYTLPPSREEMTKN
QVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPMLDSDGSFFLYSKLTV
DKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGK
Light 42 DIQMTQSPSSLSASVGDRVTITCQASQDISNYLNWYQQKPGKAPKWYDA
Chain SNLETGVPSRFSGSGSGTDFTFTISSLQPEDIATYFCQHFDHLPLAFGGGTKV
EIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREAKVQWKVDNALQ
SGNSQESVTEQDSKDSTYSLSSTLTLSKADYEKHKVYACEVTHQGLSSPVT
KSFNRGEC
-100-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
Nimotuzumab Heavy 50 QVQLQQSGAEVKKPGS SVKVSCKASGYTFTNYYIWVRQAPGQGLEWIG
Chain GINPTSGGSNFNEKFKTRVTITVDESTNTAYMELSSLRSEDTAFYFCARQGL
WFDSDGRGFDFWGQGSTVTVSSASTKGPSVFPLAPSSKSTSGGTAALGCLV
KDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGTQTY
ICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPSVFLFPPKPKDT
LMISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPREEQYNSTY
RVVSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKAKGQPREPQVYT
LPPSREEMTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSD
GSFFLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGK
Light 55 DIQMTQSPSSLSASVGDRVTITCRSSQNIVHSNGNTYLDWYQQTPGKAPKL
Chain LIYKVSNRFSGVPSRFSGSGSGTDFTFTISSLQPEDIATYYCFQYSHVPWTFG
QGTKLEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREAKVQWKV
DNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEKHKVYACEVTHQG
LSSPVTKSFNRGEC
Zalutumumab Heavy 63 QVQLVESGGGVVQPGRSLRLSCAASGFTFSTYGMHWVRQAPGKGLEWVA
Chain VIWDDGSYKYYGDSVKGRFTISRDNSKNTLYLQMNSLRAEDTAVYYCAR
DGITMVRGVMKDYFDYWGQGTLVTVSSASTKGPSVFPLAPSSKSTSGGTA
ALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSS
SLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPSVFLF
PPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPRE
EQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKAKGQP
REPQVYTLPPSREEMTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTT
PPVLDSDGSFFLYSKLTVDKSRWQQGNVFSC SVMHEALHNHYTQKSLSLSP
GK
Light 68 AIQLTQSPSSLSASVGDRVTITCRASQDISSALVWYQQKPGKAPKLLIYDAS
Chain SLESGVPSRFSGSESGTDFTLTISSLQPEDFATYYCQQFNSYPLTFGGGTKVE
IKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREAKVQWKVDNALQS
GNSQESVTEQDSKDSTYSLSSTLTLSKADYEKHKVYACEVTHQGLSSPVTK
SFNRGEC
Onartuzumab Heavy 76 EVQLVESGGGLVQPGGSLRLSCAASGYTFTSWLHWVRQAPGKGLEWVG
Chain MIDPSNSDTRFNPNFKDRFTISADTSKNTAYLQMNSLRAEDTAVYYCATYR
SYVTPLDWGQGTLVTVSSASTKGPSVFPLAPSSKSTSGGTAALGCLVKDY
FPEPVTVSWNSGALTSGVHTFPAVLQS SGLYSLSSVVTVPSSSLGTQTYICN
VNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPSVFLFPPKPKDTLMI
SRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPREEQYNSTYRV
VSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPP
SREEMTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSF
FLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGK
Light 81 DIQMTQSPSSLSASVGDRVTITCKSSQSLLYTSSQKNYLAWYQQKPGKAPK
Chain LLIWASTRESGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCQQYYAYPWT
FGQGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVC LLNNFYPREAKVQW
KVDNALQSGNS QESVTEQDSKDSTYSLSSTLTLSKADYEKHKVYACEVTH
QGLSSPVTKSFNRGEC
Patritumab Heavy 89 QVQLQQWGAGLLKPSETLSLTCAVYGGSFSGYWSWIRQPPGKGLEWIGE
Chain INHSGSTNYNPSLKSRVTISVETSKNQFSLKLS SVTAADTAVYYCARDKWT
WYFDLWGRGTLVTVSSASTKGPSVFPLAPSSKSTSGGTAALGCLVKDYFPE
PVTVSWNSGALTSGVHTFPAVLQSSGLYSLSS VVTVPSSSLGTQTYICNVNH
KPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPS VFLFPPKPKDTLMISRT
PEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPREEQYNSTYRVVSV
LTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSRE
EMTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLY
SKLTVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGK
Light 94 DIEMTQSPDSLAVSLGERATINCRS SQSVLYSSSNRNYLAWYQQNPGQPPK
Chain LLIWASTRESGVPDRFSGSGSGTDFTLTISSLQAEDVAVYYCQQYYSTPRT
FGQGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVC LLNNFYPREAKVQW
KVDNALQSGNS QESVTEQDSKDSTYSLSSTLTLSKADYEKHKVYACEVTH
QGLSSPVTKSFNRGEC
Clivatuzumab Heavy 102 QVQLQQSGAEVKKPGAS VKVSCEASGYTFPSYVLHWVKQAPGQGLEWIG
- 101-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
Chain YINPYNDGTQYNEKFKGKATLTRDTSINTAYMELSRLRSDDTAVYYCARG
FGGSYGFAWGQGTLVTVSSASTKGPSVFPLAPSSKSTSGGTAALGCLVKD
YFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGTQTYIC
NVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPSVFLFPPKPKDTL
MISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPREEQYNSTYR
VVSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLP
PSREEMTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGS
FFLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGK
Light 107 DIQLTQSPSSLSASVGDRVTMTCSASSSVSSSYLYWYQQKPGKAPKLWIYS
Chain TSNLASGVPARFSGSGSGTDFTLTISSLQPEDSASYFCHQWNRYPYTFGGGT
RLEIKRTVAAPS VFIFPPSDEQLKSGTASVVCLLNNFYPREAKVQWKVDNA
LQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEKHKVYACEVTHQGLSSP
VTKSFNRGEC
Sofituzumab Heavy 115 EVQLVESGGGLVQPGGSLRLSCAASGYSITNDYAWNWVRQAPGKGLEWV
Chain GYISYSGYTTYNPSLKSRFTISRDTSKNTLYLQMNSLRAEDTAVYYCARWT
SGLDWGQGTLVTVSSASTKGPSVFPLAPSSKSTSGGTAALGCLVKDYFPE
PVTVSWNSGALTSGVHTFPAVLQSSGLYSLSS VVTVPSSSLGTQTYICNVNH
KPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPS VFLFPPKPKDTLMISRT
PEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPREEQYNSTYRVVSV
LTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSRE
EMTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLY
SKLTVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGK
Light 120 DIQMTQSPSSLSASVGDRVTITCKASDLIHNWLAWYQQKPGKAPKWYGA
Chain TSLETGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCQQWTTPFTFGQGTK
VEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREAKVQWKVDNAL
QSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEKHKVYACEVTHQGLSSPV
TKSFNRGEC
Edrecolomab Heavy 128 QVQLQQSGAELVRPGTSVKVSCKASGYAFTNYLIEWVKQRPGQGLEWIGV
Chain INPGSGGTNYNEKFKGKATLTADKSSSTAYMQLSSLTSDDSAVYFCARDGP
WFAWGQGTLVTVSAASTKGPSVFPLAPSSKSTSGGTAALGCLVKDYFPEP
VTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPS SSLGTQTYICNVNH
KPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPS VFLFPPKPKDTLMISRT
PEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPREEQYNSTYRVVSV
LTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSRE
EMTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLY
SKLTVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGK
Light 133 NIVMTQSPKSMSMSVGERVTLTCKASENVVTYVSWYQQKPEQSPKLLIYG
Chain ASNRYTGVPDRFTGSGSATDFTLTISSVQAEDLADYHCGQGYSYPYTFGGG
TKLEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREAKVQWKVDN
ALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEKHKVYACEVTHQGLSS
PVTKSFNRGEC
Adecatumum Heavy 141 EVQLLESGGGVVQPGRSLRLSCAASGFTFSSYGMHWVRQAPGKGLEWVA
ab Chain VISYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAEDTAVYYCAKD
MGWGSGWRPYYYYGMDVWGQGTTVTVSSASTKGPSVFPLAPSSKSTSGG
TAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVP
SSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPSVF
LFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKP
REEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKAKG
QPREPQVYTLPPSRDELTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYK
TTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYTQKSLS
LSPGK
Light 146 ELQMTQSPSS LSASVGDRVT ITCRTSQSIS SYLNWYQQKP GQPPKLLIYW
Chain ASTRESGVPD RFSGSGSGTD FTLTISSLQP EDSATYYCQQ SYDIPYTFGQ
GTKLEIKRTV AAPSVFIFPP SDEQLKSGTA SVVCLLNNFY PREAKVQWKV
DNALQSGNSQ ESVTEQDSKD STYSLSSTLT LSKADYEKHK
VYACEVTHQG LSSPVTKSFN RGEC
Anetumab Heavy 154 QVELVQSGAEVKKPGESLKISCKGSGYSFTSWIGWVRQAPGKGLEWMGII
-102-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
Chain DPGDSRTRYSPSFQGQVTISADKSISTAYLQWSSLKASDTAMYYCARGQLY
GGTYMDGWGQGTLVTVSSASTKGPSVFPLAPSSKSTSGGTAALGCLVKDY
FPEPVTVSWNSGALTSGVHTFPAVLQS SGLYSLSSVVTVPSSSLGTQTYICN
VNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPSVFLFPPKPKDTLMI
SRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPREEQYNSTYRV
VSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPP
SRDELTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSF
FLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGK
Light 159 DIALTQPASVSGSPGQSITISCTGTSSDIGGYNSVSWYQQHPGKAPKLMIYG
Chain VNNRPSGVSNRFSGSKSGNTASLTISGLQAEDEADYYCSSYDIESATPVFGG
GTKLTVLGQPKAAPSVTLFPPSSEELQANKATLVCLISDFYPGAVTVAWKG
DSSPVKAGVETTTPSKQSNNKYAASSYLSLTPEQWKSHRSYSCQVTHEGST
VEKT VAPTECS
huDS6 Heavy 167 QAQLVQSGAEVVKPGAS VKMSCKASGYTFTSYNMHWVKQTPGQGLEWIG
Chain YIYPGNGATNYNQKFQGKATLTADPSSSTAYMQISSLTSEDSAVYFCARGD
SVPFAWGQGTLVTVSAASTKGPSVFPLAPSSKSTSGGTAALGCLVKDYFP
EPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGTQTYICNVN
HKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPSVFLFPPKPKDTLMISR
TPEVTCWVDVSHEDPEVKFNWYVDGVEVHNAKTKPREEQYNSTYRVVSV
LTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSRD
ELTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLY
SKLTVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGK
Light 172 EIVLTQSPATMSASPGERVTITCSAHSSVSFMHWFQQKPGTSPKLWIYSTSS
Chain LASGVPARFGGSGSGTSYSLTISSMEAEDAATYYCQQRSSFPLTFGAGTKLE
LKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREAKVQWKVDNALQS
GNSQESVTEQDSKDSTYSLSSTLTLSKADYEKHKVYACEVTHQGLSSPVTK
SFNRGEC
Lifastuzumab Heavy 180 EVQLVESGGGLVQPGGSLRLSCAASGFSFSDFAMSWVRQAPGKGLEWVAT
Chain IGRVAFHTYYPDSMKGRFTISRDNSKNTLYLQMNSLRAEDTAVYYCARHR
GFDVGHFDFWGQGTLVTVSSASTKGPSVFPLAPSSKSTSGGTAALGCLVKD
YFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGTQTYIC
NVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPSVFLFPPKPKDTL
MISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPREEQYNSTYR
VVSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLP
PSREEMTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGS
FFLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGK
Light 185 DIQMTQSPSSLSASVGDRVTITCRSSETLVHSSGNTYLEWYQQKPGKAPKL
Chain LIYRVSNRFSGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCFQGSFNPLTFG
QGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREAKVQWKV
DNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEKHKVYACEVTHQG
LSSPVTKSFNRGEC
Sacituzumab Heavy 193 QVQLQQSGSELKKPGASVKVSCKASGYTFTNYGMNWVKQAPGQGLKWM
Chain GWINTYTGEPTYTDDFKGRFAFSLDTSVSTAYLQISSLKADDTAVYFCARG
GFGSSWYFDVWGQGSLVTVSSASTKGPSVFPLAPSSKSTSGGTAALGCLV
KDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGTQTY
ICNVNHKPSNTKVDKRVEPKSCDKTHTCPPCPAPELLGGPSVFLFPPKPKDT
LMISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPREEQYNSTY
RVVSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKAKGQPREPQVYT
LPPSREEMTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSD
GSFFLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGK
Light 198 DIQLTQSPSS LSASVGDRVS ITCKASQDVS IAVAWYQQKP GKAPKLLIYS
Chain ASYRYTGVPD RFSGSGSGTD FTLTISSLQP EDFAVYYCQQ HYITPLTFGA
GTKVEIKRTV AAPSVFIFPP SDEQLKSGTA SVVCLLNNFY PREAKVQWKV
DNALQSGNSQ ESVTEQDSKD STYSLSSTLT LSKADYEKHK
VYACEVTHQG LSSPVTKSFN RGEC
PR1A3 Heavy 206 QVKLQQSGPELKKPGETVKISCKASGYTFTEFGMNWVKQAPGKGLKWMG
Chain WINTKTGEATYVEEFKGRFAFSLETSATTAYLQINNLKNEDTAKYFCARW
DFYDYVEAMDWGQGTTVTVSSASTKGPSVFPLAPS SKSTSGGTAALGCL
-103-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
VKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGTQT
YICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPSVFLFPPKPKD
TLMISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPREEQYNST
YRVVSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKAKGQPREPQVY
TLPPSRDELTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDS
DGSFFLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGK
Light 211 DIVMTQSQRFMSTSVGDRVSVTCKASQNVGTNVAWYQQKPGQSPKALIYS
Chain ASYRYSGVPDRFTGSGSGTDFTLTISNVQSEDLAEYFCHQYYTYPLFTFGSG
TKLEMKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREAKVQWKVD
NALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEKHKVYACEVTHQGLS
SPVTKSFNRGEC
Humanized Heavy 813 QVQLVQSGAEVKKPGASVKVSCKASGYTFTEFGMNWVRQAPGQGLEWM
PR1A3 Chain GWINTKTGEATYVEEFKGRVTFTTDTSTSTAYMELRSLRSDDTAVYYCAR
WDFAYYVEAMDWGQGTTVTVSSASTKGPSVFPLAPSSKSTSGGTAALGC
LVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGTQ
TYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPSVFLFPPKPK
DTLMISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPREEQYNS
TYRVVSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKAKGQPREPQV
YTLPPSREEMTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLD
SDGSFFLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGK
Light 814 DIQMTQSPSSLSASVGDRVTITCKASAAVGTYVAWYQQKPGKAPKLLIYSA
Chain SYRKRGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCHQYYTYPLFTFGQGT
KLEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREAKVQWKVDNA
LQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEKHKVYACEVTHQGLSSP
VTKSFNRGEC
Humanized Heavy 831 EVQLQESGPGLVKPGGSLSLSCAASGFVFSS YDMSWVRQTPERGLEWVAYI
Ab2-3 Chain SSGGGITYAPSTVKGRFTVSRDNAKNTLYLQMNSLTSEDTAVYYCAAHYF
GSSGPFAWGQGTLVTVSSASTKGPSVFPLAPSSKSTSGGTAALGCLVKDY
FPEPVTVSWNSGALTSGVHTFPAVLQS SGLYSLSSVVTVPSSSLGTQTYICN
VNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPSVFLFPPKPKDTLMI
SRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPREEQYNSTYRV
VSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPP
SREEMTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSF
FLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGK
Light 832 DIQMTQSPASLSASVGDRVTITCRASENIFSYLAWYQQKPGKSPKLLVYNT
Chain RTLAEGVPSRFSGSGSGTDFSLTISSLQPEDFATYYCQHHYGTPFTFGSGTKL
EIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREAKVQWKVDNALQ
SGNSQESVTEQDSKDSTYSLSSTLTLSKADYEKHKVYACEVTHQGLSSPVT
KSFNRGEC
IMAB362, Heavy 219 QVQLQQPGAELVRPGAS VKLSCKASGYTFTSWINWVKQRPGQGLEWIGN
CLAUDIXIM Chain IYPSDSYTNYNQKFKDKATLTVDKSSSTAYMQLS SPTSEDSAVYYCTRSWR
AB GNSFDWGQGTTLTVSSASTKGPSVFPLAPSSKSTSGGTAALGCLVKDYFP
EPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGTQTYICNVN
HKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPSVFLFPPKPKDTLMISR
TPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPREEQYNSTYRVVS
VLTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSR
EEMTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFL
YSKLTVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGK
Light 224 DIVMTQSPSSLTVTAGEKVTMSCKSSQSLLNSGNQKNYLTWYQQKPGQPP
Chain KLLIWASTRESGVPDRFTGSGSGTDFTLTISSVQAEDLAVYYCQNDYSYPF
TFGSGTKLEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREAKVQW
KVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEKHKVYACEVTH
QGLSSPVTKSFNRGEC
AMG595 Heavy 258 QVQLVESGGGVVQSGRSLRLSCAASGFTFRNYGMHWVRQAPGKGLEWVA
Chain VIWYDGSDKYYADSVRGRFTISRDNSKNTLYLQMNSLRAEDTAVYYCARD
GYDILTGNPRDFDWGQGTLVTVSSASTKGPSVFPLAPSSKSTSGGTAALG
CLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGT
QTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPSVFLFPPKP
KDTLMISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPREEQY
-104-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
NSTYRVVSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKAKGQPREP
QVYTLPPSREEMTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPV
LDSDGSFFLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGK
Light 263 DTVMTQTPLSSHVTLGQPASISCRSSQSLVHSDGNTYLSWLQQRPGQPPRL
Chain LIYRISRRFSGVPDRFSGSGAGTDFTLEISRVEAEDVGVYYCMQSTHVPRTF
GQGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREAKVQWK
VDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEKHKVYACEVTHQ
GLSSPVTKSFNRGEC
ABT806 Heavy 271 EVQLQESGPGLVKPSQTLSLTCTVSGYSISRDFAWNWIRQPPGKGLEWMG
Chain YISYNGNTRYQPSLKSRITISRDTSKNQFFLKLNSVTAADTATYYCVTASRG
FPWGQGTLVTVSSASTKGPSVFPLAPS SKSTSGGTAALGCLVKDYFPEPV
TVSWNSGALTSGVHTFPAVLQS SGLYSLSSVVTVPSS SLGTQTYICNVNHKP
SNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPSVFLFPPKPKDTLMISRTPE
VTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPREEQYNSTYRVVSVLT
VLHQDWLNGKEYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREEM
TKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSK
LTVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGK
Light 276 DIQMTQSPSSMSVSVGDRVTITCHSSQDINSNIGWLQQKPGKSFKGLIYHGT
Chain NLDDGVPSRFSGSGSGTDYTLTISSLQPEDFATYYCVQYAQFPWTFGGGTK
LEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREAKVQWKVDNAL
QSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEKHKVYACEVTHQGLSSPV
TKSFNRGEC
Sibrotuzumab Heavy 284 QVQLVQSGAEVKKPGAS VKVSCKTSRYTFTEYTIHWVRQAPGQRLEWIGG
Chain INPNNGIPNYNQKFKGRVTITVDTSASTAYMELSSLRSEDTAVYYCARRRIA
YGYDEGHAMDYWGQGTLVTVSSASTKGPSVFPLAPSSKSTSGGTAALGCL
VKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGTQT
YICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPSVFLFPPKPKD
TLMISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPREEQYNST
YRVVSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKAKGQPREPQVY
TLPPSREEMTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDS
DGSFFLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGK
Light 289 DIVMTQSPDSLAVSLGERATINCKSSQSLLYSRNQKNYLAWYQQKPGQPPK
Chain LLIFWASTRESGVPDRFSGSGFGTDFTLTISSLQAEDVAVYYCQQYFSYPLT
FGQGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREAKVQW
KVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEKHKVYACEVTH
QGLSSPVTKSFNRGEC
DS-8895a Heavy 297 QVQLVQSGAEVKKPGASVKVSCKASGYTFIDYSMHWVRQAPGQGLEWM
variant 1 Chain GWINTYTGEPTYSDDFKGRVTITADTSTSTAYLELSSLRSEDTAVYYCATY
YRYERDFDWGQGTLVTVSSASTKGPSVFPLAPSSKSTSGGTAALGCLVKD
YFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGTQTYIC
NVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPSVFLFPPKPKDTL
MISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPREEQYNSTYR
VVSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLP
PSREEMTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGS
FFLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGK
Light 302 DIVMTQSPLSLPVTPGEPASISCRSSQSIVHSSGITYLEWYLQKPGQSPQLLIY
Chain KVSNRFSGVPDRFSGSGSGTDFTLKISRVEAEDVGVYYCFQGSHVPYTFGQ
GTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREAKVQWKVD
NALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEKHKVYACEVTHQGLS
SPVTKSFNRGEC
DS-8895a Heavy 310 QIQLVQSGAEVKKPGASVKVSCKASGYTFIDYSMHWVRQAPGQGLKWMG
variant 2 Chain WINTYTGEPTYSDDFKGRFAFSLDTSTSTAYLELSSLRSEDTAVYYCATYY
RYERDFDWGQGTLVTVSSASTKGPSVFPLAPS SKSTSGGTAALGCLVKDY
FPEPVTVSWNSGALTSGVHTFPAVLQS SGLYSLSSVVTVPSSSLGTQTYICN
VNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPSVFLFPPKPKDTLMI
SRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPREEQYNSTYRV
VSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPP
SREEMTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSF
FLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGK
-105-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
Light 315 DVLMTQSPLSLPVTPGEPASISCRSSQSIVHSSGITYLEWYLQKPGQSPQLLI
Chain YKVSNRFSGVPDRFSGSGSGTDFTLKISRVEAEDVGVYYCFQGSHVPYTFG
QGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREAKVQWKV
DNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEKHKVYACEVTHQG
LSSPVTKSFNRGEC
MEDI-547 Heavy 323 EVQLLESGGGLVQPGGSLRLSCAASGFTFSHYMMAWVRQAPGKGLEWVS
Chain RIGPSGGPTHYADSVKGRFTISRDNSKNTLYLQMNSLRAEDTAVYYCAGY
DSGYDYVAVAGPAEYFQHWGQGTLVTVSSASTKGPS VFPLAPSSKSTSGG
TAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVP
SSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPSVF
LFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKP
REEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKAKG
QPREPQVYTLPPSREEMTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYK
TTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYTQKSLS
LSPGK
Light 328 DIQMTQSPSSLSASVGDRVTITCRASQSISTWLAWYQQKPGKAPKLLIYKAS
Chain NLHTGVPSRFSGSGSGTEFSLTISGLQPDDFATYYCQQYNSYSRTFGQGTKV
EIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREAKVQWKVDNALQ
SGNSQESVTEQDSKDSTYSLSSTLTLSKADYEKHKVYACEVTHQGLSSPVT
KSFNRGEC
Narnatumab Heavy 336 EVQLVESGGGLVQPGGSLRLSCAASGFTFSSYLMTWVRQAPGKGLEWVA
Chain NIKQDGSEKYYVDSVKGRFTISRDNAKNSLNLQMNSLRAEDTAVYYCTRD
GYSSGRHYGMDVWGQGTTVIVSSASTKGPSVFPLAPS SKSTSGGTAALGCL
VKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGTQT
YICNVNHKPSNTKVDKRVEPKSCDKTHTCPPCPAPELLGGPSVFLFPPKPKD
TLMISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPREEQYNST
YRVVSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKAKGQPREPQVY
TLPPSREEMTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDS
DGSFFLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGK
Light 341 EIVLTQSPATLSLSPGERATLSCRASQSVSRYLAWYQQKPGQAPRLLIYDAS
Chain NRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRSNWPRTFGQGTKV
EIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREAKVQWKVDNALQ
SGNSQESVTEQDSKDSTYSLSSTLTLSKADYEKHKVYACEVTHQGLSSPVT
KSFNRGEC
RG7841 Heavy 349 EVQLVESGPALVKPTQTLTLTCTVSGFSLTGYSVNWIRQPPGKALEWLGMI
Chain WGDGSTDYNSALKSRLTISKDTSKNQVVLTMTNMDPVDTATYYCARDYY
FNYASWFAYWGQGTLVTVSSASTKGPSVFPLAPSSKSTSGGTAALGCLVK
DYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSS VVTVPSSSLGTQTYI
CNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPSVFLFPPKPKDT
LMISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPREEQYNSTY
RVVSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKAKGQPREPQVYT
LPPSREEMTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSD
GSFFLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGK
Light 354 DIQMTQSPSSLSASVGDRVTITCSASQGISNYLNWYQQKPGKTVKLLIYYTS
Chain NLHSGVPSRFSGSGSGTDYTLTISSLQPEDFATYYCQQYSELPWTFGQGTKV
EIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREAKVQWKVDNALQ
SGNSQESVTEQDSKDSTYSLSSTLTLSKADYEKHKVYACEVTHQGLSSPVT
KSFNRGEC
Farletuzumab Heavy 362 EVQLVESGGGVVQPGRSLRLSCSASGFTFSGYGLSWVRQAPGKGLEWVA
Chain MISSGGSYTYYADSVKGRFAISRDNAKNTLFLQMDSLRPEDTGVYFCARH
GDDPAWFAWGQGTPVTVSSASTKGPSVFPLAPSSKS TSGGTAALGCLVK
DYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSS VVTVPSSSLGTQTYI
CNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPSVFLFPPKPKDT
LMISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPREEQYNSTY
RVVSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKAKGQPREPQVYT
LPPSRDELTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSD
GSFFLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGK
Light 367 DIQLTQSPSSLSASVGDRVTITCSVSSSISSNNLHWYQQKPGKAPKPWIYGTS
-106-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
Chain NLASGVPSRFSGSGSGTDYTFTISSLQPEDIATYYCQQWSSYPYMYTFGQGT
KVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREAKVQWKVDNA
LQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEKHKVYACEVTHQGLSSP
VTKSFNRGEC
Mirvetuximab Heavy 375 QVQLVQSGAEVVKPGAS VKISCKASGYTFTGYFMNWVKQSPGQSLEWIGR
Chain IHPYDGDTFY
NQKFQGKATLTVDKSSNTAHMELLSLTSEDFAVYYCTRYDGSRAMDWG
QGTTVTVSSAS
TKGPSVFPLAPSSKSTSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTF
PAVLQSSGL
YSLSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPA
PELLGGPS
VFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKT
KPREEQYNST
YRVVSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKAKGQPREPQVY
TLPPSRDELT
KNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKL
TVDKSRWQQ
GNVFSCSVMHEALHNHYTQKSLSLSPG
Light 380 DIVLTQSPLSLAVSLGQPAIISCKASQSVSFAGTSLMHWYHQKPGQQPRLLI
Chain YRASNLEAGVPDRFSGSGSKTDFTLTISPVEAEDAATYYCQQSREYPYTFG
GGTKLEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREAKVQWKV
DNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEKHKVYACEVTHQG
LSSPVTKSFNRGEC
J591 variant 1 Heavy 388 EVQLQQSGPELKKPGTSVRISCKTSGYTFTEYTIHWVKQSHGKSLEWIGNIN
Chain PNNGGTTYNQKFEDKATLTVDKSSSTAYMELRSLTSEDSAVYYCAAGWNF
DWGQGTTLTVSSASTKGPSVFPLAPS SKSTSGGTAALGCLVKDYFPEPVT
VSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGTQTYICNVNHKPS
NTKVDKKVEPKSCDKTHTCPPCPAPELLGGPSVFLFPPKPKDTLMISRTPEV
TCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTV
LHQDWLNGKEYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREEMT
KNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKL
TVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGK
Light 393 DIVMTQSHKFMSTSVGDRVSIICKASQDVGTAVDWYQQKPGQSPKWYW
Chain ASTRHTGVPDRFTGSGSGTDFTLTITNVQSEDLADYFCQQYNSYPLTFGAG
TMLDLKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREAKVQWKVD
NALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEKHKVYACEVTHQGLS
SPVTKSFNRGEC
J591 variant 2 Heavy 401
EVQLQQSGPELVKPGTSVRISCKTSGYTFTEYTIHWVKQSHGKSLEWIGNIN
Chain PNNGGTTYNQKFEDKATLTVDKSSSTAYMELRSLTSEDSAVYYCAAGWNF
DWGQGTTLTVSSASTKGPSVFPLAPS SKSTSGGTAALGCLVKDYFPEPVT
VSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGTQTYICNVNHKPS
NTKVDKKVEPKSCDKTHTCPPCPAPELLGGPSVFLFPPKPKDTLMISRTPEV
TCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTV
LHQDWLNGKEYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREEMT
KNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKL
TVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGK
Light 406 NIVMTQSPKSMSMSVGERVTLTCKASENVVTYVSWYQQKPEQSPKLLIYG
Chain ASNRYTGVPDRFTGSGSATDFTLTISSVQAEDLADYHCGQGYSYPYTFGGG
TKLEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREAKVQWKVDN
ALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEKHKVYACEVTHQGLSS
PVTKSFNRGEC
Rovalpituzum Heavy 414 QVQLVQSGAEVKKPGASVKVSCKASGYTFTNYGMNWVRQAPGQGLEWM
ab Chain GWINTYTGEPTYADDFKGRVTMTTDTSTSTAYMELRSLRSDDTAVYYCAR
IGDSSPSDWGQGTLVTVSSASTKGPSVFPLAPSSKSTSGGTAALGCLVKD
YFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGTQTYIC
NVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPSVFLFPPKPKDTL
-107-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
MISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPREEQYNSTYR
VVSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLP
PSRDELTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGS
FFLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPG
Light 419 EIVMTQSPATLSVSPGERATLSCKASQSVSNDVVWYQQKPGQAPRLLIYYA
Chain SNRYTGIPARFSGSGSGTEFTLTISSLQSEDFAVYYCQQDYTSPWTFGQGTK
LEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREAKVQWKVDNAL
QSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEKHKVYACEVTHQGLSSPV
TKSFNRGEC
PF-06647020 Heavy 427 QVQLVQSGPEVKKPGASVKVSCKASGYTFTDYAVHWVRQAPGKRLEWIG
Chain VISTYNDYTYNNQDFKGRVTMTRDTSASTAYMELSRLRSEDTAVYYCARG
NSYFYALDYWGQGTSVTVSSASTKGPSVFPLAPSSKSTSGGTAALGCLVKD
YFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGTQTYIC
NVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPSVFLFPPKPKDTL
MISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPREEQYNSTYR
VVSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLP
PSREEMTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGS
FFLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGK
Light 432 EIVLTQSPATLSLSPGERATLSCRASESVDSYGKSFMHWYQQKPGQAPRLLI
Chain YRASNLESGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQSNEDPWTFGG
GTKLEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREAKVQWKVD
NALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEKHKVYACEVTHQGLS
SPVTKSFNRGEC
Antibody to Heavy 440 QVQLVESGGGVVQPGRSLRLSCAASGFTFSSYAFHWVRQAPGKGLEWVA
PTK7 Chain VISYDGSIKYYADSVKGRFTISRDNSKNTLYLQMNSLRAEDTAVYYCARTY
YFDWGQGTLVTVSSASTKGPSVFPLAPS SKSTSGGTAALGCLVKDYFPEP
VTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPS SSLGTQTYICNVNH
KPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPS VFLFPPKPKDTLMISRT
PEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPREEQYNSTYRVVSV
LTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSRE
EMTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLY
SKLTVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGK
Light 445 EIVLTQSPDFQSVTPKEKVTITCRASQSIGSSLHWYQQKPDQSPKLLIKYASQ
Chain SFSGVPSRFSGSGSGTDFTLTINSLEAEDAAAYYCHQS SSLPITFGQGTRLEI
KRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREAKVQWKVDNALQSG
NSQESVTEQDSKDSTYSLSSTLTLSKADYEKHKVYACEVTHQGLSSPVTKS
FNRGEC
Ladiratuzuma Heavy 453 QVQLVQSGAEVKKPGASVKVSCKASGLTIEDYYMHWVRQAPGQGLEWM
b Chain GWIDPENGDTEYGPKFQGRVTMTRDTSINTAYMELSRLRSDDTAVYYCAV
HNAHYGTWFAWGQGTLVTVSSASTKGPSVFPLAPS SKSTSGGTAALGCL
VKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGTQT
YICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPSVFLFPPKPKD
TLMISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPREEQYNST
YRVVSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKAKGQPREPQVY
TLPPSREEMTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDS
DGSFFLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGK
Light 458 DVVMTQSPLSLPVTLGQPASISCRSSQSLLHS SGNTYLEYFQQRPGQSPRPLI
Chain YKISTRFSGVPDRFSGSGSGTDFTLKISRVEAEDVGVYYCFQGSHVPYTFGG
GTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREAKVQWKVD
NALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEKHKVYACEVTHQGLS
SPVTKSFNRGEC
Cirmtuzumab Heavy 466 QVQLQESGPGLVKPSQTLSLTCTVSGYAFTAYNIHWVRQAPGQGLEWMGS
Chain FDPYDGGSSYNQKFKDRLTISKDTSKNQVVLTMTNMDPVDTATYYCARG
WYYFDWGHGTLVTVSSASTKGPSVFPLAPSSKSTSGGTAALGCLVKDYF
PEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGTQTYICNV
NHKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPS VFLFPPKPKDTLMIS
RTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPREEQYNSTYRVV
SVLTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPS
REEMTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFF
-108-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
LYSKLTVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGK
Light 471 DIVMTQTPLSLPVTPGEPASISCRASKSISKYLAWYQQKPGQAPRLLIYSGST
Chain LQSGIPPRFSGSGYGTDFTLTINNIESEDAAYYFCQQHDESPYTFGEGTKVEI
KRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREAKVQWKVDNALQSG
NSQESVTEQDSKDSTYSLSSTLTLSKADYEKHKVYACEVTHQGLSSPVTKS
FNRGEC
Antibody to Heavy 479 QVQLVESGGGVVQPGRSLRLSCTASGFRFRSHGMHWVRQAPGKGLEWVA
MAGE-A3 Chain VISYDGNNKLYADSVKGRITISRDNSKNTLFLQMNNVRAEDTAVYYCASP
YTSDWQYFQWGQGTLVIVSSASTKGPSVFPLAPSSKSTSGGTAALGCLVK
DYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSS VVTVPSSSLGTQTYI
CNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPSVFLFPPKPKDT
LMISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPREEQYNSTY
RVVSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKAKGQPREPQVYT
LPPSREEMTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSD
GSFFLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGK
Light 484 EIVMTQSPATLSVSPGERATFSCRASQNISTTLAWYQQKPGQAPRLLIYDTS
Chain TRATGIPARFSGSGSGTEFTLTISSLQSEDLAVYYCQQSNSWPLTFGGGTKV
EIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREAKVQWKVDNALQ
SGNSQESVTEQDSKDSTYSLSSTLTLSKADYEKHKVYACEVTHQGLSSPVT
KSFNRGEC
Antibody to Heavy 492 QVQLVQSGGGVVRPGGSLRLSCAASGFSFIDYGMSWVRQVPGKGLEWVA
NY-ESO-1 Chain GMNWSGDKKGHAESVKGRFIISRDNAKNTLYLEMSSLRVEDTALYFCARG
EYSNRFDPRGRGTLVTVSSASTKGPSVFPLAPSSKSTSGGTAALGCLVKDYF
PEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGTQTYICNV
NHKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPS VFLFPPKPKDTLMIS
RTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPREEQYNSTYRVV
SVLTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPS
REEMTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFF
LYSKLTVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGK
Light
497 DIVMTQTPLSLPVTLGQPASLSCRS SQSLVFTDGNTYLNWFQQRPGQSPRRL
Chain
IYKVSSRDPGVPDRFSGTGSGTDFTLEISRVEAEDIGVYYCMQGTHWPPIFG
QGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREAKVQWKV
DNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEKHKVYACEVTHQG
LSSPVTKSFNRGEC
Trastuzumab Heavy 681 EVQLVESGGGLVQPGGSLRLSCAASGFNIKDTYIHWVRQAPGKGLEWVAR
Chain IYPTNGYTRYADSVKGRFTISADTSKNTAYLQMNSLRAEDTAVYYCSRWG
GDGFYAMDWGQGTLVTVSSASTKGPS VFPLAPSSKSTSGGTAALGCLVK
DYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSS VVTVPSSSLGTQTYI
CNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPSVFLFPPKPKDT
LMISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPREEQYNSTY
RVVSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKAKGQPREPQVYT
LPPSREEMTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSD
GSFFLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGK
Light 682 DIQMTQSPSSLSASVGDRVTITCRASQDVNTAVAWYQQKPGKAPKLLIYSA
Chain SFLYSGVPSRFSGSRSGTDFTLTISSLQPEDFATYYCQQHYTTPPTFGQGTKV
EIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREAKVQWKVDNALQ
SGNSQESVTEQDSKDSTYSLSSTLTLSKADYEKHKVYACEVTHQGLSSPVT
KSFNRGEC
TABLE 4. Fusion Sequences ¨ CD40 fusions via the heavy chain
Antibody Region SEQ Sequence
ID
NO:
Pertuzumab HC CD40 21
QVQLVQSGAEVKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQGLE
mAb with
WMGWINPDSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSDDTAV
tumor mAb
YYCARDQPLGYCTNGVCSYFDWGQGTLVTVSSASTKGPSVFPLAPSS
ScFv
KSTSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYS
-109-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
LSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCP
APELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYV
DGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNK
ALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLTCLVKGFYPSD
IAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFS
CSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSEVQLVESGGGLVQPGG
SLRLSCAASGFTFTDYTMDWVRQAPGKGLEWVADVNPNSGGSIYNQRF
KGRFTLSVDRSKNTLYLQMNSLRAEDTAVYYCARNLGPSFYFDWGQ
GTLVTVSSGGGGSGGGGSGGGGSGGGGSDIQMTQSPS SLSASVGDRVTI
TCKASQDVSIGVAWYQQKPGKAPKLLIYS ASYRYTGVPSRFSGSGSGTD
FTLTISSLQPEDFATYYCQQYYIYPYTFGQGTKVEIK
HC tumor
22 EVQLVESGGGLVQPGGSLRLSCAASGFTFTDYTMDWVRQAPGKGLEW
mAb with
VADVNPNSGGSIYNQRFKGRFTLSVDRSKNTLYLQMNSLRAEDTAVYY
CD40 mAb
CARNLGPSFYFDYWGQGTLVTVSSASTKGPSVFPLAPSSKSTSGGTAAL
ScFv
GCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSS
SLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPSV
FLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAK
TKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKTIS
KAKGQPREPQVYTLPPSREEMTKNQVSLTCLVKGFYPSDIAVEWESNG
QPENNYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFSCSVMHEALH
NHYTQKSLSLSPGGGGGSGGGGSQVQLVQSGAEVKKPGASVKVSCKAS
GYTFTGYYMHWVRQAPGQGLEWMGWINPDSGGTNYAQKFQGRVTMT
RDTSISTAYMELNRLRSDDTAVYYCARDQPLGYCTNGVCSYFDWGQ
GTLVTVSSGGGGSGGGGSGGGGSGGGGSDIQMTQSPS SVSASVGDRVTI
TCRASQGIYSWLAWYQQKPGKAPNLLIYTASTLQSGVPSRFSGSGSGTD
FTLTISSLQPEDFATYYCQQANIFPLTFGGGTKVEIK
HC tumor 845 EVQLVESGGGLVQPGGSLRLSCAASGFTFTDYTMDWVRQAPGKGLEW
mAb with
VADVNPNSGGSIYNQRFKGRFTLSVDRSKNTLYLQMNSLRAEDTAVYY
CD40 mAb
CARNLGPSFYFDYWGQGTLVTVSSASTKGPSVFPLAPSSKSTSGGTAAL
scFv (LH)
GCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSS
SLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPSV
FLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAK
TKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKTIS
KAKGQPREPQVYTLPPSREEMTKNQVSLTCLVKGFYPSDIAVEWESNG
QPENNYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFSCSVMHEALH
NHYTQKSLSLSPGGGGGSGGGGSDIQMTQSPSSVSAS VGDRVTITCRAS
QGIYSWLAWYQQKPGKAPNLLIYTASTLQSGVPSRFSGSGSGTDFTLTIS
SLQPEDFATYYCQQANIFPLTFGGGTKVEIKGGGGSGGGGSGGGGSGGG
GSQVQLVQSGAEVKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQG
LEWMGWINPDSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSDDT
AVYYCARDQPLGYCTNGVCSYFDWGQGTLVTVSS
Cetuximab HC CD40 34
QVQLVQSGAEVKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQGLE
mAb with
WMGWINPDSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSDDTAV
tumor mAb
YYCARDQPLGYCTNGVCSYFDWGQGTLVTVSSASTKGPSVFPLAPSS
ScFv
KSTSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYS
LSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCP
APELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYV
DGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNK
ALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLTCLVKGFYPSD
IAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFS
CSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSQVQLKQSGPGLVQPSQ
SLSITCTVSGFSLTNYGVHWVRQSPGKGLEWLGVIWSGGNTDYNTPFTS
RLSINKDNSKSQVFFKMNSLQSNDTAIYYCARALTYYDYEFAWGQGT
LVTVSAGGGGSGGGGSGGGGSGGGGSDILLTQSPVILSVSPGERVSFSCR
ASQSIGTNIHWYQQRTNGSPRLLIKYASESISGIPSRFSGSGSGTDFTLSIN
SVESEDIADYYCQQNNNWPTTFGAGTKLELK
HC tumor 35
QVQLKQSGPGLVQPSQSLSITCTVSGFSLTNYGVHWVRQSPGKGLEWL
mAb with
GVIWSGGNTDYNTPFTSRLSINKDNSKSQVFFKMNSLQSNDTAIYYCAR
CD40 mAb
ALTYYDYEFAWGQGTLVTVSAASTKGPSVFPLAPSSKSTSGGTAALG
-110-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
ScFv CLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSL
GTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPSVFL
FPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTK
PREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKA
KGQPREPQVYTLPPSREEMTKNQVSLTCLVKGFYPSDIAVEWESNGQPE
NNYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFSC SVMHEALHNHY
TQKSLSLSPGGGGGSGGGGSQVQLVQSGAEVKKPGASVKVSCKASGYT
FTGYYMHWVRQAPGQGLEWMGWINPDSGGTNYAQKFQGRVTMTRDT
SISTAYMELNRLRSDDTAVYYCARDQPLGYCTNGVCSYFDWGQGTL
VTVSSGGGGSGGGGSGGGGSGGGGSDIQMTQSPSSVS ASVGDRVTITCR
ASQGIYSWLAWYQQKPGKAPNLLIYTASTLQSGVPSRFSGSGSGTDFTL
TISSLQPEDFATYYCQQANIFPLTFGGGTKVEIK
Panitumuma HC CD40 47 QVQLVQSGAEVKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQGLE
b mAb with WMGWINPDSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSDDTAV
tumor mAb YYCARDQPLGYCTNGVCSYFDWGQGTLVTVSSASTKGPSVFPLAPSS
ScFv KSTSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYS
LSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCP
APELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYV
DGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNK
ALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLTCLVKGFYPSD
IAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFS
CSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSQVQLQESGPGLVKPSE
TLSLTCTVSGGSVSSGDYWTWIRQSPGKGLEWIGHIYYSGNTNYNPSL
KSRLTISIDTSKTQFSLKLSSVTAADTAIYYCVRDRVTGAFDIWGQGTMV
TVSSGGGGSGGGGSGGGGSGGGGSDIQMTQSPSSLSASVGDRVTITCQA
SQDISNYLNWYQQKPGKAPKWYDASNLETGVPSRFSGSGSGTDFTFTI
SSLQPEDIATYFCQHFDHLPLAFGGGTKVEIK
HC tumor 48 QVQLQESGPGLVKPSETLSLTCTVSGGSVSSGDYWTWIRQSPGKGLE
mAb with WIGHIYYSGNTNYNPSLKSRLTISIDTSKTQFSLKLSSVTAADTAIYYCVR
CD40 mAb DRVTGAFDIWGQGTMVTVSSASTKGPSVFPLAPSSKS TSGGTAALGCLV
ScFv KDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGTQ
TYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPSVFLFPPK
PKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPREE
QYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKAKGQ
PREPQVYTLPPSREEMTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNY
KTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYTQK
SLSLSPGGGGGSGGGGSQVQLVQSGAEVKKPGASVKVSCKASGYTFTG
YYMHWVRQAPGQGLEWMGWINPDSGGTNYAQKFQGRVTMTRDTSIS
TAYMELNRLRSDDTAVYYCARDQPLGYCTNGVCSYFDWGQGTLVTV
SSGGGGSGGGGSGGGGSGGGGSDIQMTQSPSSVSASVGDRVTITCRASQ
GIYSWLAWYQQKPGKAPNLLIYTASTLQSGVPSRFSGSGSGTDFTLTISS
LQPEDFATYYCQQANIFPLTFGGGTKVEIK
Nimotuzuma HC CD40 60 QVQLVQSGAEVKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQGLE
b mAb with WMGWINPDSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSDDTAV
tumor mAb YYCARDQPLGYCTNGVCSYFDWGQGTLVTVSSASTKGPSVFPLAPSS
ScFv KSTSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYS
LSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCP
APELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYV
DGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNK
ALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLTCLVKGFYPSD
IAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFS
CSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSQVQLQQSGAEVKKPG
SSVKVSCKASGYTFTNYYIWVRQAPGQGLEWIGGINPTSGGSNFNEKF
KTRVTITVDESTNTAYMELSSLRSEDTAFYFCARQGLWFDSDGRGFDF
WGQGSTVTVSSGGGGSGGGGSGGGGSGGGGSDIQMTQSPSSLSASVGD
RVTITCRSSQNIVHSNGNTYLDWYQQTPGKAPKLLIYKVSNRFSGVPSRF
SGSGSGTDFTFTISSLQPEDIATYYCFQYSHVPWTFGQGTKLEIK
HC tumor 61 QVQLQQSGAEVKKPGS SVKVSCKASGYTFTNYYIWVRQAPGQGLEWI
mAb with GGINPTSGGSNFNEKFKTRVTITVDESTNTAYMELSSLRSEDTAFYFCAR
-111-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
CD40 mAb QGLWFDSDGRGFDFWGQGSTVTVSSASTKGPSVFPLAPSSKSTSGGTAA
ScFv LGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPS
SSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPS
VFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNA
KTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKT
ISKAKGQPREPQVYTLPPSREEMTKNQVSLTCLVKGFYPSDIAVEWESN
GQPENNYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFSCSVMHEAL
HNHYTQKSLSLSPGGGGGSGGGGSQVQLVQSGAEVKKPGASVKVSCK
ASGYTFTGYYMHWVRQAPGQGLEWMGWINPDSGGTNYAQKFQGRVT
MTRDTSISTAYMELNRLRSDDTAVYYCARDQPLGYCTNGVCSYFDYW
GQGTLVTVSSGGGGSGGGGSGGGGSGGGGSDIQMTQSPSSVSASVGDR
VTITCRASQGIYSWLAWYQQKPGKAPNLLIYTASTLQSGVPSRFSGSGS
GTDFTLTISSLQPEDFATYYCQQANIFPLTFGGGTKVEIK
Zalutumuma HC CD40 73 QVQLVQSGAEVKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQGLE
b mAb with WMGWINPDSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSDDTAV
tumor mAb YYCARDQPLGYCTNGVCSYFDWGQGTLVTVSSASTKGPSVFPLAPSS
ScFv KSTSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYS
LSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCP
APELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYV
DGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNK
ALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLTCLVKGFYPSD
IAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFS
CSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSQVQLVESGGGVVQPG
RSLRLSCAASGFTFSTYGMHWVRQAPGKGLEWVAVIWDDGSYKYYGD
SVKGRFTISRDNSKNTLYLQMNSLRAEDTAVYYCARDGITMVRGVMK
DYFDWGQGTLVTVSSGGGGSGGGGSGGGGSGGGGSAIQLTQSPSSLS
ASVGDRVTITCRASQDISSALVWYQQKPGKAPKLLIYDASSLESGVPSRF
SGSESGTDFTLTISSLQPEDFATYYCQQFNSYPLTFGGGTKVEIK
HC tumor 74 QVQLVESGGGVVQPGRSLRLSCAASGFTFSTYGMHWVRQAPGKGLEW
mAb with VAVIWDDGSYKYYGDSVKGRFTISRDNSKNTLYLQMNSLRAEDTAVY
CD40 mAb YCARDGITMVRGVMKDYFDWGQGTLVTVSSASTKGPSVFPLAPSSKS
ScFv TSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLS
SVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAP
ELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYVDG
VEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNKAL
PAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLTCLVKGFYPSDIA
VEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFSCS
VMHEALHNHYTQKSLSLSPGGGGGSGGGGSQVQLVQSGAEVKKPGAS
VKVSCKASGYTFTGYYMHWVRQAPGQGLEWMGWINPDSGGTNYAQK
FQGRVTMTRDTSISTAYMELNRLRSDDTAVYYCARDQPLGYCTNGVCS
YFDWGQGTLVTVSSGGGGSGGGGSGGGGSGGGGSDIQMTQSPSSVSA
SVGDRVTITCRASQGIYSWLAWYQQKPGKAPNLLIYTASTLQSGVPSRF
SGSGSGTDFTLTISSLQPEDFATYYCQQANIFPLTFGGGTKVEIK
Onartuzuma HC CD40 86 QVQLVQSGAEVKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQGLE
b mAb with WMGWINPDSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSDDTAV
tumor mAb YYCARDQPLGYCTNGVCSYFDWGQGTLVTVSSASTKGPSVFPLAPSS
ScFv KSTSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYS
LSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCP
APELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYV
DGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNK
ALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLTCLVKGFYPSD
IAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFS
CSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSEVQLVESGGGLVQPGG
SLRLSCAASGYTFTSWLHWVRQAPGKGLEWVGMIDPSNSDTRFNPNF
KDRFTISADTSKNTAYLQMNSLRAEDTAVYYCATYRS YVTPLDWGQ
GTLVTVSSGGGGSGGGGSGGGGSGGGGSDIQMTQSPS SLSASVGDRVTI
TCKSSQSLLYTSSQKNYLAWYQQKPGKAPKLLIWASTRESGVPSRFSG
SGSGTDFTLTISSLQPEDFATYYCQQYYAYPWTFGQGTKVEIK
HC tumor 87 EVQLVESGGGLVQPGGSLRLSCAASGYTFTSWLHWVRQAPGKGLEW
-112-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
mAb with VGMIDPSNSDTRFNPNFKDRFTISADTSKNTAYLQMNSLRAEDTAVYYC
CD40 mAb ATYRSYVTPLDWGQGTLVTVSSASTKGPSVFPLAPS SKSTSGGTAALG
ScFv CLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSL
GTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPSVFL
FPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTK
PREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKA
KGQPREPQVYTLPPSREEMTKNQVSLTCLVKGFYPSDIAVEWESNGQPE
NNYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFSC SVMHEALHNHY
TQKSLSLSPGGGGGSGGGGSQVQLVQSGAEVKKPGASVKVSCKASGYT
FTGYYMHWVRQAPGQGLEWMGWINPDSGGTNYAQKFQGRVTMTRDT
SISTAYMELNRLRSDDTAVYYCARDQPLGYCTNGVCSYFDWGQGTL
VTVSSGGGGSGGGGSGGGGSGGGGSDIQMTQSPSSVS ASVGDRVTITCR
ASQGIYSWLAWYQQKPGKAPNLLIYTASTLQSGVPSRFSGSGSGTDFTL
TISSLQPEDFATYYCQQANIFPLTFGGGTKVEIK
Patritumab HC CD40 99 QVQLVQSGAEVKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQGLE
mAb with WMGWINPDSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSDDTAV
tumor mAb YYCARDQPLGYCTNGVCSYFDWGQGTLVTVSSASTKGPSVFPLAPSS
ScFv KSTSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYS
LSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCP
APELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYV
DGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNK
ALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLTCLVKGFYPSD
IAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFS
CSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSQVQLQQWGAGLLKPS
ETLSLTCAVYGGSFSGYWSWIRQPPGKGLEWIGEINHSGSTNYNPSLK
SRVTISVETSKNQFSLKLSSVTAADTAVYYCARDKWTWYFDLWGRGTL
VTVSSGGGGSGGGGSGGGGSGGGGSDIEMTQSPDSLAVSLGERATINCR
SSQSVLYS SSNRNYLAWYQQNPGQPPKLLIWASTRESGVPDRFSGSGS
GTDFTLTISSLQAEDVAVYYCQQYYSTPRTFGQGTKVEIK
HC tumor 100 QVQLQQWGAGLLKPSETLSLTCAVYGGSFSGYWSWIRQPPGKGLEWI
mAb with GEINHSGSTNYNPSLKSRVTISVETSKNQFSLKLSSVTAADTAVYYCARD
CD40 mAb KWTWYFDLWGRGTLVTVSSASTKGPSVFPLAPSSKSTSGGTAALGCLV
ScFv KDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGTQ
TYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPSVFLFPPK
PKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPREE
QYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKAKGQ
PREPQVYTLPPSREEMTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNY
KTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYTQK
SLSLSPGGGGGSGGGGSQVQLVQSGAEVKKPGASVKVSCKASGYTFTG
YYMHWVRQAPGQGLEWMGWINPDSGGTNYAQKFQGRVTMTRDTSIS
TAYMELNRLRSDDTAVYYCARDQPLGYCTNGVCSYFDWGQGTLVTV
SSGGGGSGGGGSGGGGSGGGGSDIQMTQSPSSVSASVGDRVTITCRASQ
GIYSWLAWYQQKPGKAPNLLIYTASTLQSGVPSRFSGSGSGTDFTLTISS
LQPEDFATYYCQQANIFPLTFGGGTKVEIK
Clivatuzuma HC CD40 112 QVQLVQSGAEVKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQGLE
b mAb with WMGWINPDSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSDDTAV
tumor mAb YYCARDQPLGYCTNGVCSYFDWGQGTLVTVSSASTKGPSVFPLAPSS
ScFv KSTSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYS
LSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCP
APELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYV
DGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNK
ALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLTCLVKGFYPSD
IAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFS
CSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSQVQLQQSGAEVKKPG
ASVKVSCEASGYTFPSYVLHWVKQAPGQGLEWIGYINPYNDGTQYNEK
FKGKATLTRDTSINTAYMELSRLRSDDTAVYYCARGFGGSYGFAWGQ
GTLVTVSSGGGGSGGGGSGGGGSGGGGSDIQLTQSPSSLSASVGDRVT
MTCSASSSVSSSYLYWYQQKPGKAPKLWIYSTSNLASGVPARFSGSGSG
TDFTLTISSLQPEDSASYFCHQWNRYPYTFGGGTRLEIK
-113-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
HC tumor 113 QVQLQQSGAEVKKPGASVKVSCEASGYTFPSYVLHWVKQAPGQGLEW
mAb with IGYINPYNDGTQYNEKFKGKATLTRDTSINTAYMELSRLRSDDTAVYYC
CD40 mAb ARGFGGS YGFAWGQGTLVTVSSASTKGPSVFPLAPS SKSTSGGTAALG
ScFv CLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSL
GTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPSVFL
FPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTK
PREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKA
KGQPREPQVYTLPPSREEMTKNQVSLTCLVKGFYPSDIAVEWESNGQPE
NNYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFSC SVMHEALHNHY
TQKSLSLSPGGGGGSGGGGSQVQLVQSGAEVKKPGASVKVSCKASGYT
FTGYYMHWVRQAPGQGLEWMGWINPDSGGTNYAQKFQGRVTMTRDT
SISTAYMELNRLRSDDTAVYYCARDQPLGYCTNGVCSYFDWGQGTL
VTVSSGGGGSGGGGSGGGGSGGGGSDIQMTQSPSSVS ASVGDRVTITCR
ASQGIYSWLAWYQQKPGKAPNLLIYTASTLQSGVPSRFSGSGSGTDFTL
TISSLQPEDFATYYCQQANIFPLTFGGGTKVEIK
Sofituzumab HC CD40 125 QVQLVQSGAEVKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQGLE
mAb with WMGWINPDSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSDDTAV
tumor mAb YYCARDQPLGYCTNGVCSYFDWGQGTLVTVSSASTKGPSVFPLAPSS
ScFv KSTSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYS
LSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCP
APELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYV
DGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNK
ALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLTCLVKGFYPSD
IAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFS
CSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSEVQLVESGGGLVQPGG
SLRLSCAASGYSITNDYAWNWVRQAPGKGLEWVGYISYSGYTTYNPSL
KSRFTISRDTSKNTLYLQMNSLRAEDTAVYYCARWTSGLDWGQGTLV
TVSSGGGGSGGGGSGGGGSGGGGSDIQMTQSPSSLSASVGDRVTITCKA
SDLII-INWLAWYQQKPGKAPKWYGATSLETGVPSRFSGSGSGTDFTLTI
SSLQPEDFATYYCQQWTTPFTFGQGTKVEIK
HC tumor 126 EVQLVESGGGLVQPGGSLRLSCAASGYSITNDYAWNWVRQAPGKGLE
mAb with WVGYISYSGYTTYNPSLKSRFTISRDTSKNTLYLQMNSLRAEDTAVYYC
CD40 mAb ARWTSGLDWGQGTLVTVSSASTKGPSVFPLAPS SKS TSGGTAALGCL
ScFv VKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGT
QTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPSVFLFPP
KPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPRE
EQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKAKG
QPREPQVYTLPPSREEMTKNQVSLTCLVKGFYPSDIAVEWESNGQPENN
YKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYTQ
KSLSLSPGGGGGSGGGGSQVQLVQSGAEVKKPGASVKVSCKASGYTFT
GYYMHWVRQAPGQGLEWMGWINPDSGGTNYAQKFQGRVTMTRDTSI
STAYMELNRLRSDDTAVYYCARDQPLGYCTNGVCSYFDWGQGTLVT
VSSGGGGSGGGGSGGGGSGGGGSDIQMTQSPSSVSAS VGDRVTITCRAS
QGIYSWLAWYQQKPGKAPNLLIYTASTLQSGVPSRFSGSGSGTDFTLTIS
SLQPEDFATYYCQQANIFPLTFGGGTKVEIK
Edrecoloma HC CD40 138 QVQLVQSGAEVKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQGLE
b mAb with WMGWINPDSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSDDTAV
tumor mAb YYCARDQPLGYCTNGVCSYFDWGQGTLVTVSSASTKGPSVFPLAPSS
ScFv KSTSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYS
LSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCP
APELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYV
DGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNK
ALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLTCLVKGFYPSD
IAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFS
CSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSQVQLQQSGAELVRPGT
SVKVSCKASGYAFTNYLIEWVKQRPGQGLEWIGVINPGSGGTNYNEKF
KGKATLTADKSSSTAYMQLSSLTSDDSAVYFCARDGPWFAWGQGTL
VTVSAGGGGSGGGGSGGGGSGGGGSNIVMTQSPKSMSMSVGERVTLT
CKASENVVTYVSWYQQKPEQSPKLLIYGASNRYTGVPDRFTGSGSATD
-114-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
FTLTISSVQAEDLADYHCGQGYSYPYTFGGGTKLEIK
HC tumor 139 QVQLQQSGAELVRPGTSVKVSCKASGYAFTNYLIEWVKQRPGQGLEWI
mAb with GVINPGSGGTNYNEKFKGKATLTADKSSSTAYMQLSSLTSDDSAVYFCA
CD40 mAb RDGPWFAWGQGTLVTVSAASTKGPSVFPLAPSSKSTSGGTAALGCLV
ScFv KDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGTQ
TYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPSVFLFPPK
PKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPREE
QYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKAKGQ
PREPQVYTLPPSREEMTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNY
KTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYTQK
SLSLSPGGGGGSGGGGSQVQLVQSGAEVKKPGASVKVSCKASGYTFTG
YYMHWVRQAPGQGLEWMGWINPDSGGTNYAQKFQGRVTMTRDTSIS
TAYMELNRLRSDDTAVYYCARDQPLGYCTNGVCSYFDWGQGTLVTV
SSGGGGSGGGGSGGGGSGGGGSDIQMTQSPSSVSASVGDRVTITCRASQ
GIYSWLAWYQQKPGKAPNLLIYTASTLQSGVPSRFSGSGSGTDFTLTISS
LQPEDFATYYCQQANIFPLTFGGGTKVEIK
Adecatumu HC CD40 151 QVQLVQSGAEVKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQGLE
mab mAb with WMGWINPDSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSDDTAV
tumor mAb YYCARDQPLGYCTNGVCSYFDWGQGTLVTVSSASTKGPSVFPLAPSS
ScFv KSTSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYS
LSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCP
APELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYV
DGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNK
ALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLTCLVKGFYPSD
IAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFS
CSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSEVQLLESGGGVVQPGR
SLRLSCAASGFTFSSYGMHWVRQAPGKGLEWVAVISYDGSNKYYADS
VKGRFTISRDNSKNTLYLQMNSLRAEDTAVYYCAKDMGWGSGWRPYY
YYGMDVWGQGTTVTVSSGGGGSGGGGSGGGGSGGGGSELQMTQSPSS
LSASVGDRVTITCRTSQSISSYLNWYQQKPGQPPKLLIWASTRESGVPD
RFSGSGSGTDFTLTISSLQPEDSATYYCQQSYDIPYTFGQGTKLEIK
HC tumor 152 EVQLLESGGGVVQPGRSLRLSCAASGFTFSSYGMHWVRQAPGKGLEW
mAb with VAVISYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAEDTAVYY
CD40 mAb CAKDMGWGSGWRPYYYYGMDVWGQGTTVTVSSASTKGPSVFPLAPSS
ScFv KSTSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYS
LSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCP
APELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYV
DGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNK
ALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLTCLVKGFYPSD
IAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFS
CSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSQVQLVQSGAEVKKPG
ASVKVSCKASGYTFTGYYMHWVRQAPGQGLEWMGWINPDSGGTNYA
QKFQGRVTMTRDTSISTAYMELNRLRSDDTAVYYCARDQPLGYCTNGV
CSYFDWGQGTLVTVSSGGGGSGGGGSGGGGSGGGGSDIQMTQSPSSV
SASVGDRVTITCRASQGIYSWLAWYQQKPGKAPNLLIYTASTLQSGVPS
RFSGSGSGTDFTLTISSLQPEDFATYYCQQANIFPLTFGGGTKVEIK
Anetumab HC CD40 164 QVQLVQSGAEVKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQGLE
mAb with WMGWINPDSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSDDTAV
tumor mAb YYCARDQPLGYCTNGVCSYFDWGQGTLVTVSSASTKGPSVFPLAPSS
ScFv KSTSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYS
LSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCP
APELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYV
DGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNK
ALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLTCLVKGFYPSD
IAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFS
CSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSQVELVQSGAEVKKPGE
SLKISCKGSGYSFTSWIGWVRQAPGKGLEWMGIIDPGDSRTRYSPSFQ
GQVTISADKSISTAYLQWSSLKASDTAMYYCARGQLYGGTYMDGWGQ
GTLVTVSSGGGGSGGGGSGGGGSGGGGSDIALTQPASVSGSPGQSITISC
-115-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
TGTSSDIGGYNSVSWYQQHPGKAPKLMIYGVNNRPSGVSNRFSGSKSG
NTASLTISGLQAEDEADYYCSSYDIESATPVFGGGTKLTVL
HC tumor 165 QVELVQSGAEVKKPGESLKISCKGSGYSFTSWIGWVRQAPGKGLEWM
mAb with GIIDPGDSRTRYSPSFQGQVTISADKSISTAYLQWSSLKASDTAMYYCAR
CD40 mAb GQLYGGTYMDGWGQGTLVTVSSASTKGPSVFPLAPSSKSTSGGTAALG
ScFv CLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSL
GTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPSVFL
FPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTK
PREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKA
KGQPREPQVYTLPPSREEMTKNQVSLTCLVKGFYPSDIAVEWESNGQPE
NNYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFSC SVMHEALHNHY
TQKSLSLSPGGGGGSGGGGSQVQLVQSGAEVKKPGASVKVSCKASGYT
FTGYYMHWVRQAPGQGLEWMGWINPDSGGTNYAQKFQGRVTMTRDT
SISTAYMELNRLRSDDTAVYYCARDQPLGYCTNGVCSYFDWGQGTL
VTVSSGGGGSGGGGSGGGGSGGGGSDIQMTQSPSSVS ASVGDRVTITCR
ASQGIYSWLAWYQQKPGKAPNLLIYTASTLQSGVPSRFSGSGSGTDFTL
TISSLQPEDFATYYCQQANIFPLTFGGGTKVEIK
huDS6 HC CD40 177 QVQLVQSGAEVKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQGLE
mAb with WMGWINPDSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSDDTAV
tumor mAb YYCARDQPLGYCTNGVCSYFDWGQGTLVTVSSASTKGPSVFPLAPSS
ScFv KSTSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYS
LSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCP
APELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYV
DGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNK
ALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLTCLVKGFYPSD
IAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFS
CSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSQAQLVQSGAEVVKPG
ASVKMSCKASGYTFTSYNMHWVKQTPGQGLEWIGYIYPGNGATNYNQ
KFQGKATLTADPSSSTAYMQISSLTSEDSAVYFCARGDSVPFAWGQGT
LVTVSAGGGGSGGGGSGGGGSGGGGSEIVLTQSPATMSASPGERVTITC
SAHSSVSFMHWFQQKPGTSPKLWIYSTSSLASGVPARFGGSGSGTSYSL
TISSMEAEDAATYYCQQRSSFPLTFGAGTKLELK
HC tumor 178 QAQLVQSGAEVVKPGASVKMSCKASGYTFTSYNMHWVKQTPGQGLE
mAb with WIGYIYPGNGATNYNQKFQGKATLTADPSSSTAYMQISSLTSEDSAVYF
CD40 mAb CARGDSVPFAWGQGTLVTVSAASTKGPSVFPLAPS SKSTSGGTAALGC
ScFv LVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLG
TQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPSVFLFP
PKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPR
EEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKAK
GQPREPQVYTLPPSREEMTKNQVSLTCLVKGFYPSDIAVEWESNGQPEN
NYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFSCS VMHEALHNHYT
QKSLSLSPGGGGGSGGGGSQVQLVQSGAEVKKPGASVKVSCKASGYTF
TGYYMHWVRQAPGQGLEWMGWINPDSGGTNYAQKFQGRVTMTRDTS
ISTAYMELNRLRSDDTAVYYCARDQPLGYCTNGVCS YFDWGQGTLV
TVSSGGGGSGGGGSGGGGSGGGGSDIQMTQSPSSVS ASVGDRVTITCRA
SQGIYSWLAWYQQKPGKAPNLLIYTASTLQSGVPSRFSGSGSGTDFTLTI
SSLQPEDFATYYCQQANIFPLTFGGGTKVEIK
Lifastuzuma HC CD40 190 QVQLVQSGAEVKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQGLE
b mAb with WMGWINPDSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSDDTAV
tumor mAb YYCARDQPLGYCTNGVCSYFDWGQGTLVTVSSASTKGPSVFPLAPSS
ScFv KSTSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYS
LSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCP
APELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYV
DGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNK
ALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLTCLVKGFYPSD
IAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFS
CSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSEVQLVESGGGLVQPGG
SLRLSCAASGFSFSDFAMSWVRQAPGKGLEWVATIGRVAFHTYYPDSM
KGRFTISRDNSKNTLYLQMNSLRAEDTAVYYCARHRGFDVGHFDFWG
-116-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
QGTLVTVSSGGGGSGGGGSGGGGSGGGGSDIQMTQSPSSLSASVGDRV
TITCRSSETLVHSSGNTYLEWYQQKPGKAPKLLIYRVSNRFSGVPSRFSG
SGSGTDFTLTISSLQPEDFATYYCFQGSFNPLTFGQGTKVEIK
HC tumor 191 EVQLVESGGGLVQPGGSLRLSCAASGFSFSDFAMSWVRQAPGKGLEWV
mAb with ATIGRVAFHTYYPDSMKGRFTISRDNSKNTLYLQMNSLRAEDTAVYYC
CD40 mAb ARHRGFDVGHFDFWGQGTLVTVSSASTKGPSVFPLAPSSKSTSGGTAAL
ScFv GCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSS
SLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPSV
FLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAK
TKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKTIS
KAKGQPREPQVYTLPPSREEMTKNQVSLTCLVKGFYPSDIAVEWESNG
QPENNYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFSCSVMHEALH
NHYTQKSLSLSPGGGGGSGGGGSQVQLVQSGAEVKKPGASVKVSCKAS
GYTFTGYYMHWVRQAPGQGLEWMGWINPDSGGTNYAQKFQGRVTMT
RDTSISTAYMELNRLRSDDTAVYYCARDQPLGYCTNGVCSYFDWGQ
GTLVTVSSGGGGSGGGGSGGGGSGGGGSDIQMTQSPS SVSASVGDRVTI
TCRASQGIYSWLAWYQQKPGKAPNLLIYTASTLQSGVPSRFSGSGSGTD
FTLTISSLQPEDFATYYCQQANIFPLTFGGGTKVEIK
Sacituzumab HC CD40 203 QVQLVQSGAEVKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQGLE
mAb with WMGWINPDSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSDDTAV
tumor mAb YYCARDQPLGYCTNGVCSYFDWGQGTLVTVSSASTKGPSVFPLAPSS
ScFv KSTSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYS
LSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCP
APELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYV
DGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNK
ALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLTCLVKGFYPSD
IAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFS
CSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSQVQLQQSGSELKKPGA
SVKVSCKASGYTFTNYGMNWVKQAPGQGLKWMGWINTYTGEPTYTD
DFKGRFAFSLDTSVSTAYLQIS SLKADDTAVYFCARGGFGSSWYFDV
WGQGSLVTVSSGGGGSGGGGSGGGGSGGGGSDIQLTQSPSSLSASVGD
RVSITCKASQDVSIAVAWYQQKPGKAPKLLIYS ASYRYTGVPDRFSGSG
SGTDFTLTISSLQPEDFAVYYCQQHYITPLTFGAGTKVEIK
HC tumor 204 QVQLQQSGSELKKPGASVKVSCKASGYTFTNYGMNWVKQAPGQGLK
mAb with WMGWINTYTGEPTYTDDFKGRFAFSLDTSVSTAYLQISSLKADDTAVYF
CD40 mAb CARGGFGSSWYFDVWGQGSLVTVSSASTKGPSVFPLAPSSKSTSGGTA
ScFv ALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVP
SSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPS
VFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNA
KTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKT
ISKAKGQPREPQVYTLPPSREEMTKNQVSLTCLVKGFYPSDIAVEWESN
GQPENNYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFSCSVMHEAL
HNHYTQKSLSLSPGGGGGSGGGGSQVQLVQSGAEVKKPGASVKVSCK
ASGYTFTGYYMHWVRQAPGQGLEWMGWINPDSGGTNYAQKFQGRVT
MTRDTSISTAYMELNRLRSDDTAVYYCARDQPLGYCTNGVCSYFDYW
GQGTLVTVSSGGGGSGGGGSGGGGSGGGGSDIQMTQSPSSVSASVGDR
VTITCRASQGIYSWLAWYQQKPGKAPNLLIYTASTLQSGVPSRFSGSGS
GTDFTLTISSLQPEDFATYYCQQANIFPLTFGGGTKVEIK
PR1A3 HC CD40 216 QVQLVQSGAEVKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQGLE
mAb with WMGWINPDSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSDDTAV
tumor mAb YYCARDQPLGYCTNGVCSYFDWGQGTLVTVSSASTKGPSVFPLAPSS
ScFv KSTSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYS
LSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCP
APELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYV
DGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNK
ALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLTCLVKGFYPSD
IAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFS
CSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSQVKLQQSGPELKKPGE
TVKISCKASGYTFTEFGMNWVKQAPGKGLKWMGWINTKTGEATYVEE
-117-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
FKGRFAFSLETSATTAYLQINNLKNEDTAKYFCARWDFYDYVEAMDY
WGQGTTVTVSSGGGGSGGGGSGGGGSGGGGSDIVMTQSQRFMSTSVG
DRVSVTCKASQNVGTNVAWYQQKPGQSPKALIYSASYRYSGVPDRFTG
SGSGTDFTLTISNVQSEDLAEYFCHQYYTYPLFTFGSGTKLEMK
HC tumor 217 QVKLQQSGPELKKPGETVKISCKASGYTFTEFGMNWVKQAPGKGLKW
mAb with MGWINTKTGEATYVEEFKGRFAFSLETSATTAYLQINNLKNEDTAKYFC
CD40 mAb ARWDFYDYVEAMDWGQGTTVTVSSASTKGPSVFPLAPSSKSTSGGTA
ScFv ALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVP
SSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPS
VFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNA
KTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKT
ISKAKGQPREPQVYTLPPSREEMTKNQVSLTCLVKGFYPSDIAVEWESN
GQPENNYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFSCSVMHEAL
HNHYTQKSLSLSPGGGGGSGGGGSQVQLVQSGAEVKKPGASVKVSCK
ASGYTFTGYYMHWVRQAPGQGLEWMGWINPDSGGTNYAQKFQGRVT
MTRDTSISTAYMELNRLRSDDTAVYYCARDQPLGYCTNGVCSYFDYW
GQGTLVTVSSGGGGSGGGGSGGGGSGGGGSDIQMTQSPSSVSASVGDR
VTITCRASQGIYSWLAWYQQKPGKAPNLLIYTASTLQSGVPSRFSGSGS
GTDFTLTISSLQPEDFATYYCQQANIFPLTFGGGTKVEIK
Humanized HC CD40 815 QVQLVQSGAEVKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQGLE
PR1A3 mAb with WMGWINPDSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSDDTAV
tumor mAb YYCARDQPLGYCTNGVCSYFDWGQGTLVTVSSASTKGPSVFPLAPSS
ScFv KSTSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYS
LSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCP
APELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYV
DGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNK
ALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLTCLVKGFYPSD
IAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFS
CSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSQVQLVQSGAEVKKPG
ASVKVSCKASGYTFTEFGMNWVRQAPGQGLEWMGWINTKTGEATYV
EEFKGRVTFTTDTSTSTAYMELRSLRSDDTAVYYCARWDFAYYVEAM
DWGQGTTVTVSSGGGGSGGGGSGGGGSGGGGSDIQMTQSPSSLSASV
GDRVTITCKASAAVGTYVAWYQQKPGKAPKWYSASYRKRGVPSRFS
GSGSGTDFTLTISSLQPEDFATYYCHQYYTYPLFTFGQGTKLEIK
HC tumor 816 QVQLVQSGAEVKKPGASVKVSCKASGYTFTEFGMNWVRQAPGQGLE
mAb with WMGWINTKTGEATYVEEFKGRVTFTTDTSTSTAYMELRSLRSDDTAVY
CD40 mAb YCARWDFAYYVEAMDWGQGTTVTVSSASTKGPSVFPLAPSSKSTSGG
ScFv TAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVT
VPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLG
GPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVH
NAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNKALPAPIE
KTISKAKGQPREPQVYTLPPSREEMTKNQVSLTCLVKGFYPSDIAVEWE
SNGQPENNYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFSCSVMHE
ALHNHYTQKSLSLSPGGGGGSGGGGSQVQLVQSGAEVKKPGASVKVSC
KASGYTFTGYYMHWVRQAPGQGLEWMGWINPDSGGTNYAQKFQGRV
TMTRDTSISTAYMELNRLRSDDTAVYYCARDQPLGYCTNGVCSYFDY
WGQGTLVTVSSGGGGSGGGGSGGGGSGGGGSDIQMTQSPSSVSASVGD
RVTITCRASQGIYSWLAWYQQKPGKAPNLLIYTASTLQSGVPSRFSGSGS
GTDFTLTISSLQPEDFATYYCQQANIFPLTFGGGTKVEIK
HC tumor 843 QVQLVQSGAEVKKPGASVKVSCKASGYTFTEFGMNWVRQAPGQGLE
mAb with WMGWINTKTGEATYVEEFKGRVTFTTDTSTSTAYMELRSLRSDDTAVY
CD40 mAb YCARWDFAYYVEAMDWGQGTTVTVSSASTKGPSVFPLAPSSKSTSGG
scFv (LH) TAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVT
VPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLG
GPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVH
NAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNKALPAPIE
KTISKAKGQPREPQVYTLPPSREEMTKNQVSLTCLVKGFYPSDIAVEWE
SNGQPENNYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFSCSVMHE
ALHNHYTQKSLSLSPGGGGGSGGGGSDIQMTQSPSS VSASVGDRVTITC
-118-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
RASQGIYSWLAWYQQKPGKAPNLLIYTASTLQSGVPSRFSGSGSGTDFT
LTISSLQPEDFATYYCQQANIFPLTFGGGTKVEIKGGGGSGGGGSGGGGS
GGGGSQVQLVQSGAEVKKPGASVKVSCKASGYTFTGYYMHWVRQAP
GQGLEWMGWINPDSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRS
DDTAVYYCARDQPLGYCTNGVCSYFDWGQGTLVTVSS
Humanized HC CD40 833 QVQLVQSGAEVKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQGLE
Ab2-3 mAb with WMGWINPDSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSDDTAV
tumor mAb YYCARDQPLGYCTNGVCSYFDWGQGTLVTVSSASTKGPSVFPLAPSS
ScFv KSTSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYS
LSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCP
APELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYV
DGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNK
ALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLTCLVKGFYPSD
IAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFS
CSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSEVQLQESGPGLVKPGG
SLSLSCAASGFVFSSYDMSWVRQTPERGLEWVAYIS SGGGITYAPSTVK
GRFTVSRDNAKNTLYLQMNSLTSEDTAVYYCAAHYFGSSGPFAWGQ
GTLVTVSSGGGGSGGGGSGGGGSGGGGSDIQMTQSPASLSASVGDRVTI
TCRASENIFSYLAWYQQKPGKSPKLLVYNTRTLAEGVPSRFSGSGSGTD
FSLTISSLQPEDFATYYCQHHYGTPFTFGSGTKLEIK
HC tumor 834 EVQLQESGPGLVKPGGSLSLSCAASGFVFSSYDMSWVRQTPERGLEWV
mAb with AYISSGGGITYAPSTVKGRFTVSRDNAKNTLYLQMNSLTSEDTAVYYCA
CD40 mAb AHYFGSSGPFAYWGQGTLVTVSSASTKGPSVFPLAPSSKSTSGGTAALG
ScFv CLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSL
GTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPSVFL
FPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTK
PREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKA
KGQPREPQVYTLPPSREEMTKNQVSLTCLVKGFYPSDIAVEWESNGQPE
NNYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFSC SVMHEALHNHY
TQKSLSLSPGGGGGSGGGGSQVQLVQSGAEVKKPGASVKVSCKASGYT
FTGYYMHWVRQAPGQGLEWMGWINPDSGGTNYAQKFQGRVTMTRDT
SISTAYMELNRLRSDDTAVYYCARDQPLGYCTNGVCSYFDWGQGTL
VTVSSGGGGSGGGGSGGGGSGGGGSDIQMTQSPSSVS ASVGDRVTITCR
ASQGIYSWLAWYQQKPGKAPNLLIYTASTLQSGVPSRFSGSGSGTDFTL
TISSLQPEDFATYYCQQANIFPLTFGGGTKVEIK
HC tumor 841 EVQLQESGPGLVKPGGSLSLSCAASGFVFSSYDMSWVRQTPERGLEWV
mAb with AYISSGGGITYAPSTVKGRFTVSRDNAKNTLYLQMNSLTSEDTAVYYCA
CD40 mAb AHYFGSSGPFAYWGQGTLVTVSSASTKGPSVFPLAPSSKSTSGGTAALG
scFv (LH) CLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSL
GTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPSVFL
FPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTK
PREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKA
KGQPREPQVYTLPPSREEMTKNQVSLTCLVKGFYPSDIAVEWESNGQPE
NNYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFSC SVMHEALHNHY
TQKSLSLSPGGGGGSGGGGSDIQMTQSPS SVSASVGDRVTITCRASQGIY
SWLAWYQQKPGKAPNLLIYTASTLQSGVPSRFSGSGSGTDFTLTISSLQP
EDFATYYCQQANIFPLTFGGGTKVEIKGGGGSGGGGSGGGGSGGGGSQ
VQLVQSGAEVKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQGLEW
MGWINPDSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSDDTAVY
YCARDQPLGYCTNGVCSYFDWGQGTLVTVSS
IMAB362, HC CD40 229 QVQLVQSGAEVKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQGLE
CLAUDIXI mAb with WMGWINPDSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSDDTAV
MAB tumor mAb YYCARDQPLGYCTNGVCSYFDWGQGTLVTVSSASTKGPSVFPLAPSS
ScFv KSTSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYS
LSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCP
APELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYV
DGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNK
ALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLTCLVKGFYPSD
IAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFS
-119-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
CSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSQVQLQQPGAELVRPG
ASVKLSCKASGYTFTSWINWVKQRPGQGLEWIGNIYPSDSYTNYNQK
FKDKATLTVDKSSSTAYMQLSSPTSEDSAVYYCTRSWRGNSFDYWGQG
TTLTVSSGGGGSGGGGSGGGGSGGGGSDIVMTQSPSSLTVTAGEKVTM
SCKSSQSLLNSGNQKNYLTWYQQKPGQPPKLLIWAS TRESGVPDRFTG
SGSGTDFTLTISSVQAEDLAVYYCQNDYSYPFTFGSGTKLEIK
HC tumor 230 QVQLQQPGAELVRPGAS VKLSCKASGYTFTSWINWVKQRPGQGLEWI
mAb with GNIYPSDSYTNYNQKFKDKATLTVDKSSSTAYM
CD40 mAb QLSSPTSEDSAVYYCTRSWRGNSFDWGQGTTLTVSSASTKGPSVFPLA
ScFv PSSKSTSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSG
LYSLSS VVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPP
CPAPELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNW
YVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVS
NKALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLTCLVKGFYP
SDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNV
FSCSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSQVQLVQSGAEVKKP
GAS VKVSCKASGYTFTGYYMHWVRQAPGQGLEWMGWINPDSGGTNY
AQKFQGRVTMTRDTSISTAYMELNRLRSDDTAVYYCARDQPLGYCTNG
VCSYFDWGQGTLVTVSSGGGGSGGGGSGGGGSGGGGSDIQMTQSPSS
VSASVGDRVTITCRASQGIYSWLAWYQQKPGKAPNLLIYTASTLQSGVP
SRFSGSGSGTDFTLTISSLQPEDFATYYCQQANIFPLTFGGGTKVEIK
AMG595 HC CD40 268 QVQLVQSGAEVKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQGLE
mAb with WMGWINPDSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSDDTAV
tumor mAb YYCARDQPLGYCTNGVCSYFDWGQGTLVTVSSASTKGPSVFPLAPSS
ScFv KSTSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYS
LSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCP
APELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYV
DGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNK
ALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLTCLVKGFYPSD
IAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFS
CSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSQVQLVESGGGVVQSG
RSLRLSCAASGFTFRNYGMHWVRQAPGKGLEWVAVIWYDGSDKYYA
DSVRGRFTISRDNSKNTLYLQMNSLRAEDTAVYYCARDGYDILTGNPR
DFDWGQGTLVTVSSGGGGSGGGGSGGGGSGGGGSDTVMTQTPLSSH
VTLGQPASISCRS SQSLVHSDGNTYLSWLQQRPGQPPRLLIYRISRRFSGV
PDRFSGSGAGTDFTLEISRVEAEDVGVYYCMQSTHVPRTFGQGTKVEIK
HC tumor 269 QVQLVESGGGVVQSGRSLRLSCAASGFTFRNYGMHWVRQAPGKGLEW
mAb with VAVIWYDGSDKYYADSVRGRFTISRDNSKNTLYLQMNSLRAEDTAVYY
CD40 mAb CARDGYDILTGNPRDFDYWGQGTLVTVSSASTKGPSVFPLAPSSKSTSG
ScFv GTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVV
TVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLG
GPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVH
NAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNKALPAPIE
KTISKAKGQPREPQVYTLPPSREEMTKNQVSLTCLVKGFYPSDIAVEWE
SNGQPENNYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFSCSVMHE
ALHNHYTQKSLSLSPGGGGGSGGGGSQVQLVQSGAEVKKPGASVKVSC
KASGYTFTGYYMHWVRQAPGQGLEWMGWINPDSGGTNYAQKFQGRV
TMTRDTSISTAYMELNRLRSDDTAVYYCARDQPLGYCTNGVCSYFDY
WGQGTLVTVSSGGGGSGGGGSGGGGSGGGGSDIQMTQSPSSVSASVGD
RVTITCRASQGIYSWLAWYQQKPGKAPNLLIYTASTLQSGVPSRFSGSGS
GTDFTLTISSLQPEDFATYYCQQANIFPLTFGGGTKVEIK
ABT806 HC CD40 281 QVQLVQSGAEVKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQGLE
mAb with WMGWINPDSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSDDTAV
tumor mAb YYCARDQPLGYCTNGVCSYFDWGQGTLVTVSSASTKGPSVFPLAPSS
ScFv KSTSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYS
LSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCP
APELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYV
DGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNK
ALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLTCLVKGFYPSD
-120-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
IAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFS
CSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSEVQLQESGPGLVKPSQ
TLSLTCTVSGYSISRDFAWNWIRQPPGKGLEWMGYISYNGNTRYQPSLK
SRITISRDTSKNQFFLKLNSVTAADTATYYCVTASRGFPWGQGTLVTV
SSGGGGSGGGGSGGGGSGGGGSDIQMTQSPSSMSVSVGDRVTITCHSSQ
DINSNIGWLQQKPGKSFKGLIYHGTNLDDGVPSRFSGSGSGTDYTLTISS
LQPEDFATYYCVQYAQFPWTFGGGTKLEIK
HC tumor 282 EVQLQESGPGLVKPSQTLSLTCTVSGYSISRDFAWNWIRQPPGKGLEWM
mAb with GYISYNGNTRYQPSLKSRITISRDTSKNQFFLKLNS VTAADTATYYCVTA
CD40 mAb SRGFPYWGQGTLVTVSSASTKGPSVFPLAPSSKSTSGGTAALGCLVKDY
ScFv FPEPVTVSWNSGALTSGVHTFPAVLQS SGLYSLSSVVTVPSSSLGTQTYI
CNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPSVFLFPPKPK
DTLMISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPREEQY
NSTYRVVSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKAKGQPR
EPQVYTLPPSREEMTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKT
TPPVLDSDGSFFLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYTQKSL
SLSPGGGGGSGGGGSQVQLVQSGAEVKKPGASVKVSCKASGYTFTGYY
MHWVRQAPGQGLEWMGWINPDSGGTNYAQKFQGRVTMTRDTSISTA
YMELNRLRSDDTAVYYCARDQPLGYCTNGVCSYFDYWGQGTLVTVSS
GGGGSGGGGSGGGGSGGGGSDIQMTQSPSSVSASVGDRVTITCRASQGI
YSWLAWYQQKPGKAPNLLIYTASTLQSGVPSRFSGSGSGTDFTLTISSLQ
PEDFATYYCQQANIFPLTFGGGTKVEIK
Sibrotuzuma HC CD40 294 QVQLVQSGAEVKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQGLE
b mAb with WMGWINPDSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSDDTAV
tumor mAb YYCARDQPLGYCTNGVCSYFDWGQGTLVTVSSASTKGPSVFPLAPSS
ScFv KSTSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYS
LSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCP
APELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYV
DGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNK
ALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLTCLVKGFYPSD
IAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFS
CSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSQVQLVQSGAEVKKPG
ASVKVSCKTSRYTFTEYTIHWVRQAPGQRLEWIGGINPNNGIPNYNQKF
KGRVTITVDTSASTAYMELSSLRSEDTAVYYCARRRIAYGYDEGHAMD
YWGQGTLVTVSSGGGGSGGGGSGGGGSGGGGSDIVMTQSPDSLAVSL
GERATINCKSSQSLLYSRNQKNYLAWYQQKPGQPPKLLIFWASTRESGV
PDRFSGSGFGTDFTLTISSLQAEDVAVYYCQQYFSYPLTFGQGTKVEIK
HC tumor 295 QVQLVQSGAEVKKPGASVKVSCKTSRYTFTEYTIHWVRQAPGQRLEWI
mAb with GGINPNNGIPNYNQKFKGRVTITVDTSASTAYMELSSLRSEDTAVYYCA
CD40 mAb RRRIAYGYDEGHAMDWGQGTLVTVSSASTKGPSVFPLAPSSKSTSGG
ScFv TAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVT
VPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLG
GPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVH
NAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNKALPAPIE
KTISKAKGQPREPQVYTLPPSREEMTKNQVSLTCLVKGFYPSDIAVEWE
SNGQPENNYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFSCSVMHE
ALHNHYTQKSLSLSPGGGGGSGGGGSQVQLVQSGAEVKKPGASVKVSC
KASGYTFTGYYMHWVRQAPGQGLEWMGWINPDSGGTNYAQKFQGRV
TMTRDTSISTAYMELNRLRSDDTAVYYCARDQPLGYCTNGVCSYFDY
WGQGTLVTVSSGGGGSGGGGSGGGGSGGGGSDIQMTQSPSSVSASVGD
RVTITCRASQGIYSWLAWYQQKPGKAPNLLIYTASTLQSGVPSRFSGSGS
GTDFTLTISSLQPEDFATYYCQQANIFPLTFGGGTKVEIK
DS-8895a HC CD40 307 QVQLVQSGAEVKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQGLE
variant 1 mAb with WMGWINPDSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSDDTAV
tumor mAb YYCARDQPLGYCTNGVCSYFDWGQGTLVTVSSASTKGPSVFPLAPSS
ScFv KSTSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYS
LSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCP
APELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYV
DGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNK
-121-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
ALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLTCLVKGFYPSD
IAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFS
CSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSQVQLVQSGAEVKKPG
ASVKVSCKASGYTFIDYSMHWVRQAPGQGLEWMGWINTYTGEPTYSD
DFKGRVTITADTSTSTAYLELSSLRSEDTAVYYCATYYRYERDFDWG
QGTLVTVSSGGGGSGGGGSGGGGSGGGGSDIVMTQSPLSLPVTPGEPAS
ISCRSSQSIVHS SGITYLEWYLQKPGQSPQLLIYKVSNRFSGVPDRFSGSG
SGTDFTLKISRVEAEDVGVYYCFQGSHVPYTFGQGTKVEIK
HC tumor 308 QVQLVQSGAEVKKPGASVKVSCKASGYTFIDYSMHWVRQAPGQGLEW
mAb with MGWINTYTGEPTYSDDFKGRVTITADTSTSTAYLELSSLRSEDTAVYYC
CD40 mAb ATYYRYERDFDYWGQGTLVTVSSASTKGPSVFPLAPSSKSTSGGTAALG
ScFv CLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSL
GTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPSVFL
FPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTK
PREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKA
KGQPREPQVYTLPPSREEMTKNQVSLTCLVKGFYPSDIAVEWESNGQPE
NNYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFSC SVMHEALHNHY
TQKSLSLSPGGGGGSGGGGSQVQLVQSGAEVKKPGASVKVSCKASGYT
FTGYYMHWVRQAPGQGLEWMGWINPDSGGTNYAQKFQGRVTMTRDT
SISTAYMELNRLRSDDTAVYYCARDQPLGYCTNGVCSYFDWGQGTL
VTVSSGGGGSGGGGSGGGGSGGGGSDIQMTQSPSSVS ASVGDRVTITCR
ASQGIYSWLAWYQQKPGKAPNLLIYTASTLQSGVPSRFSGSGSGTDFTL
TISSLQPEDFATYYCQQANIFPLTFGGGTKVEIK
DS-8895a HC CD40 320 QVQLVQSGAEVKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQGLE
variant 2 mAb with WMGWINPDSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSDDTAV
tumor mAb YYCARDQPLGYCTNGVCSYFDWGQGTLVTVSSASTKGPSVFPLAPSS
ScFv KSTSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYS
LSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCP
APELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYV
DGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNK
ALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLTCLVKGFYPSD
IAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFS
CSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSQIQLVQSGAEVKKPGA
SVKVSCKASGYTFIDYSMHWVRQAPGQGLKWMGWINTYTGEPTYSDD
FKGRFAFSLDTSTSTAYLELSSLRSEDTAVYYCATYYRYERDFDWGQ
GTLVTVSSGGGGSGGGGSGGGSGGGGSDVLMTQSPLSLPVTPGEPASIS
CRSSQSIVHS SGITYLEWYLQKPGQSPQLLIYKVSNRFSGVPDRFSGSGS
GTDFTLKISRVEAEDVGVYYCFQGSHVPYTFGQGTKVEIK
HC tumor 321 QIQLVQSGAEVKKPGASVKVSCKASGYTFIDYSMHWVRQAPGQGLKW
mAb with MGWINTYTGEPTYSDDFKGRFAFSLDTSTSTAYLELSSLRSEDTAVYYC
CD40 mAb ATYYRYERDFDYWGQGTLVTVSSASTKGPSVFPLAPSSKSTSGGTAALG
ScFv CLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSL
GTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPSVFL
FPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTK
PREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKA
KGQPREPQVYTLPPSREEMTKNQVSLTCLVKGFYPSDIAVEWESNGQPE
NNYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFSC SVMHEALHNHY
TQKSLSLSPGGGGGSGGGGSQVQLVQSGAEVKKPGASVKVSCKASGYT
FTGYYMHWVRQAPGQGLEWMGWINPDSGGTNYAQKFQGRVTMTRDT
SISTAYMELNRLRSDDTAVYYCARDQPLGYCTNGVCSYFDWGQGTL
VTVSSGGGGSGGGGSGGGGSGGGGSDIQMTQSPSSVS ASVGDRVTITCR
ASQGIYSWLAWYQQKPGKAPNLLIYTASTLQSGVPSRFSGSGSGTDFTL
TISSLQPEDFATYYCQQANIFPLTFGGGTKVEIK
MEDI-547 HC CD40 333 QVQLVQSGAEVKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQGLE
mAb with WMGWINPDSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSDDTAV
tumor mAb YYCARDQPLGYCTNGVCSYFDWGQGTLVTVSSASTKGPSVFPLAPSS
ScFv KSTSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYS
LSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCP
APELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYV
-122-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
DGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNK
ALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLTCLVKGFYPSD
IAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFS
CSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSEVQLLESGGGLVQPGG
SLRLSCAASGFTFSHYMMAWVRQAPGKGLEWVSRIGPSGGPTHYADSV
KGRFTISRDNSKNTLYLQMNSLRAEDTAVYYCAGYDSGYDYVAVAGP
AEYFQHWGQGTLVTVSSGGGGSGGGGSGGGGSGGGGSDIQMTQSPSSL
SASVGDRVTITCRASQSISTWLAWYQQKPGKAPKLLIYKASNLHTGVPS
RFSGSGSGTEFSLTISGLQPDDFATYYCQQYNSYSRTFGQGTKVEIK
HC tumor 334 EVQLLESGGGLVQPGGSLRLSCAASGFTFSHYMMAWVRQAPGKGLEW
mAb with VSRIGPSGGPTHYADSVKGRFTISRDNSKNTLYLQMNSLRAEDTAVYYC
CD40 mAb AGYDSGYDYVAVAGPAEYFQHWGQGTLVTVSSASTKGPSVFPLAPSSK
ScFv STSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSL
SSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAP
ELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYVDG
VEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNKAL
PAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLTCLVKGFYPSDIA
VEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFSCS
VMHEALHNHYTQKSLSLSPGGGGGSGGGGSQVQLVQSGAEVKKPGAS
VKVSCKASGYTFTGYYMHWVRQAPGQGLEWMGWINPDSGGTNYAQK
FQGRVTMTRDTSISTAYMELNRLRSDDTAVYYCARDQPLGYCTNGVCS
YFDWGQGTLVTVSSGGGGSGGGGSGGGGSGGGGSDIQMTQSPSSVSA
SVGDRVTITCRASQGIYSWLAWYQQKPGKAPNLLIYTASTLQSGVPSRF
SGSGSGTDFTLTISSLQPEDFATYYCQQANIFPLTFGGGTKVEIK
Narnatumab HC CD40 346 QVQLVQSGAEVKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQGLE
mAb with WMGWINPDSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSDDTAV
tumor mAb YYCARDQPLGYCTNGVCSYFDWGQGTLVTVSSASTKGPSVFPLAPSS
ScFv KSTSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYS
LSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCP
APELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYV
DGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNK
ALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLTCLVKGFYPSD
IAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFS
CSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSEVQLVESGGGLVQPGG
SLRLSCAASGFTFSSYLMTWVRQAPGKGLEWVANIKQDGSEKYYVDSV
KGRFTISRDNAKNSLNLQMNSLRAEDTAVYYCTRDGYSSGRHYGMDV
WGQGTTVIVSSGGGGSGGGGSGGGGSGGGGSEIVLTQSPATLSLSPGER
ATLSCRASQSVSRYLAWYQQKPGQAPRLLIYDASNRATGIPARFSGSGS
GTDFTLTISSLEPEDFAVYYCQQRSNWPRTFGQGTKVEIK
HC tumor 347 EVQLVESGGGLVQPGGSLRLSCAASGFTFSSYLMTWVRQAPGKGLEWV
mAb with ANIKQDGSEKYYVDSVKGRFTISRDNAKNSLNLQMNSLRAEDTAVYYC
CD40 mAb TRDGYSSGRHYGMDVWGQGTTVIVSSASTKGPSVFPLAPSSKSTSGGTA
ScFv ALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVP
SSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPS
VFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNA
KTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKT
ISKAKGQPREPQVYTLPPSREEMTKNQVSLTCLVKGFYPSDIAVEWESN
GQPENNYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFSCSVMHEAL
HNHYTQKSLSLSPGGGGGSGGGGSQVQLVQSGAEVKKPGASVKVSCK
ASGYTFTGYYMHWVRQAPGQGLEWMGWINPDSGGTNYAQKFQGRVT
MTRDTSISTAYMELNRLRSDDTAVYYCARDQPLGYCTNGVCSYFDYW
GQGTLVTVSSGGGGSGGGGSGGGGSGGGGSDIQMTQSPSSVSASVGDR
VTITCRASQGIYSWLAWYQQKPGKAPNLLIYTASTLQSGVPSRFSGSGS
GTDFTLTISSLQPEDFATYYCQQANIFPLTFGGGTKVEIK
RG7841 HC CD40 359 QVQLVQSGAEVKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQGLE
mAb with WMGWINPDSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSDDTAV
tumor mAb YYCARDQPLGYCTNGVCSYFDWGQGTLVTVSSASTKGPSVFPLAPSS
ScFv KSTSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYS
LSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCP
-123-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
APELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYV
DGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNK
ALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLTCLVKGFYPSD
IAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFS
CSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSEVQLVESGPALVKPTQ
TLTLTCTVSGFSLTGYSVNWIRQPPGKALEWLGMIWGDGSTDYNSALK
SRLTISKDTSKNQVVLTMTNMDPVDTATYYCARDYYFNYASWFAYWG
QGTLVTVSSGGGGSGGGGSGGGGSGGGGSDIQMTQSPSSLSASVGDRV
TITCSASQGISNYLNWYQQKPGKTVKLLIYYTSNLHSGVPSRFSGSGSGT
DYTLTISSLQPEDFATYYCQQYSELPWTFGQGTKVEIK
HC tumor 360 EVQLVESGPALVKPTQTLTLTCTVSGFSLTGYSVNWIRQPPGKALEWLG
mAb with MIWGDGSTDYNSALKSRLTISKDTSKNQVVLTMTNMDPVDTATYYCA
CD40 mAb RDYYFNYASWFAYWGQGTLVTVSSASTKGPSVFPLAPSSKSTSGGTAA
ScFv LGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPS
SSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPS
VFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNA
KTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKT
ISKAKGQPREPQVYTLPPSREEMTKNQVSLTCLVKGFYPSDIAVEWESN
GQPENNYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFSCSVMHEAL
HNHYTQKSLSLSPGGGGGSGGGGSQVQLVQSGAEVKKPGASVKVSCK
ASGYTFTGYYMHWVRQAPGQGLEWMGWINPDSGGTNYAQKFQGRVT
MTRDTSISTAYMELNRLRSDDTAVYYCARDQPLGYCTNGVCSYFDYW
GQGTLVTVSSGGGGSGGGGSGGGGSGGGGSDIQMTQSPSSVSASVGDR
VTITCRASQGIYSWLAWYQQKPGKAPNLLIYTASTLQSGVPSRFSGSGS
GTDFTLTISSLQPEDFATYYCQQANIFPLTFGGGTKVEIK
Farletuzuma HC CD40 372 QVQLVQSGAEVKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQGLE
b mAb with WMGWINPDSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSDDTAV
tumor mAb YYCARDQPLGYCTNGVCSYFDWGQGTLVTVSSASTKGPSVFPLAPSS
ScFv KSTSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYS
LSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCP
APELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYV
DGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNK
ALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLTCLVKGFYPSD
IAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFS
CSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSEVQLVESGGGVVQPG
RSLRLSCSASGFTFSGYGLSWVRQAPGKGLEWVAMISSGGSYTYYADS
VKGRFAISRDNAKNTLFLQMDSLRPEDTGVYFCARHGDDPAWFAWG
QGTPVTVSSGGGGSGGGGSGGGGSGGGGSDIQLTQSPSSLSASVGDRVT
ITCSVSSSISSNNLHWYQQKPGKAPKPWIYGTSNLASGVPSRFSGSGSGT
DYTFTISSLQPEDIATYYCQQWSSYPYMYTFGQGTKVEIK
HC tumor 373 EVQLVESGGGVVQPGRSLRLSCSASGFTFSGYGLSWVRQAPGKGLEWV
mAb with AMISSGGSYTYYADSVKGRFAISRDNAKNTLFLQMDSLRPEDTGVYFCA
CD40 mAb RHGDDPAWFAWGQGTPVTVSSASTKGPSVFPLAPSSKSTSGGTAALG
ScFv CLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSL
GTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPSVFL
FPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTK
PREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKA
KGQPREPQVYTLPPSREEMTKNQVSLTCLVKGFYPSDIAVEWESNGQPE
NNYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFSC SVMHEALHNHY
TQKSLSLSPGGGGGSGGGGSQVQLVQSGAEVKKPGASVKVSCKASGYT
FTGYYMHWVRQAPGQGLEWMGWINPDSGGTNYAQKFQGRVTMTRDT
SISTAYMELNRLRSDDTAVYYCARDQPLGYCTNGVCSYFDWGQGTL
VTVSSGGGGSGGGGSGGGGSGGGGSDIQMTQSPSSVSASVGDRVTITCR
ASQGIYSWLAWYQQKPGKAPNLLIYTASTLQSGVPSRFSGSGSGTDFTL
TISSLQPEDFATYYCQQANIFPLTFGGGTKVEIK
Mirvetuxima HC CD40 385 QVQLVQSGAEVKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQGLE
b mAb with WMGWINPDSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSDDTAV
tumor mAb YYCARDQPLGYCTNGVCSYFDWGQGTLVTVSSASTKGPSVFPLAPSS
ScFv KSTSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYS
-124-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
LSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCP
APELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYV
DGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNK
ALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLTCLVKGFYPSD
IAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFS
CSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSQVQLVQSGAEVVKPG
ASVKISCKASGYTFTGYFMNWVKQSPGQSLEWIGRIHPYDGDTFYNQK
FQGKATLTVDKSSNTAHMELLSLTSEDFAVYYCTRYDGSRAMDYWGQ
GTTVTVSSGGGGSGGGGSGGGGSGGGGSDIVLTQSPLSLAVSLGQPAIIS
CKASQSVSFAGTSLMHWYHQKPGQQPRLLIYRASNLEAGVPDRFSGSG
SKTDFTLTISPVEAEDAATYYCQQSREYPYTFGGGTKLEIK
HC tumor 386 QVQLVQSGAEVVKPGASVKISCKASGYTFTGYFMNWVKQSPGQSLEWI
mAb with GRIHPYDGDTFYNQKFQGKATLTVDKSSNTAHMELLSLTSEDFAVYYC
CD40 mAb TRYDGSRAMDWGQGTTVTVSSASTKGPSVFPLAPSSKSTSGGTAALG
ScFv CLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSL
GTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPSVFL
FPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTK
PREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKA
KGQPREPQVYTLPPSREEMTKNQVSLTCLVKGFYPSDIAVEWESNGQPE
NNYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFSC SVMHEALHNHY
TQKSLSLSPGGGGGSGGGGSQVQLVQSGAEVKKPGASVKVSCKASGYT
FTGYYMHWVRQAPGQGLEWMGWINPDSGGTNYAQKFQGRVTMTRDT
SISTAYMELNRLRSDDTAVYYCARDQPLGYCTNGVCSYFDWGQGTL
VTVSSGGGGSGGGGSGGGGSGGGGSDIQMTQSPSSVS ASVGDRVTITCR
ASQGIYSWLAWYQQKPGKAPNLLIYTASTLQSGVPSRFSGSGSGTDFTL
TISSLQPEDFATYYCQQANIFPLTFGGGTKVEIK
J591 HC CD40 398 QVQLVQSGAEVKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQGLE
variantl mAb with WMGWINPDSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSDDTAV
tumor mAb YYCARDQPLGYCTNGVCSYFDWGQGTLVTVSSASTKGPSVFPLAPSS
ScFv KSTSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYS
LSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCP
APELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYV
DGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNK
ALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLTCLVKGFYPSD
IAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFS
CSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSEVQLQQSGPELKKPGT
SVRISCKTSGYTFTEYTIHWVKQSHGKSLEWIGNINPNNGGTTYNQKFE
DKATLTVDKSSSTAYMELRSLTSEDSAVYYCAAGWNFDWGQGTTLT
VSSGGGGSGGGGSGGGGSGGGGSDIVMTQSHKFMSTSVGDRVSIICKAS
QDVGTAVDWYQQKPGQSPKLLIYWASTRHTGVPDRFTGSGSGTDFTLT
ITNVQSEDLADYFCQQYNSYPLTFGAGTMLDLK
HC tumor 399 EVQLQQSGPELKKPGTSVRISCKTSGYTFTEYTIHWVKQSHGKSLEWIG
mAb with NINPNNGGTTYNQKFEDKATLTVDKSSSTAYMELRSLTSEDSAVYYCA
CD40 mAb AGWNFDWGQGTTLTVSSASTKGPSVFPLAPSSKSTSGGTAALGCLVK
ScFv DYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGTQT
YICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPSVFLFPPKP
KDTLMISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPREEQ
YNSTYRVVSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKAKGQP
REPQVYTLPPSREEMTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYK
TTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYTQKS
LSLSPGGGGGSGGGGSQVQLVQSGAEVKKPGASVKVSCKASGYTFTGY
YMHWVRQAPGQGLEWMGWINPDSGGTNYAQKFQGRVTMTRDTSIST
AYMELNRLRSDDTAVYYCARDQPLGYCTNGVCSYFDWGQGTLVTVS
SGGGGSGGGGSGGGGSGGGGSDIQMTQSPS SVSASVGDRVTITCRASQG
IYSWLAWYQQKPGKAPNLLIYTASTLQSGVPSRFSGSGSGTDFTLTISSL
QPEDFATYYCQQANIFPLTFGGGTKVEIK
J591 variant HC CD40 411 QVQLVQSGAEVKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQGLE
2 mAb with WMGWINPDSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSDDTAV
tumor mAb YYCARDQPLGYCTNGVCSYFDWGQGTLVTVSSASTKGPSVFPLAPSS
-125-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
ScFv KSTSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYS
LSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCP
APELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYV
DGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNK
ALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLTCLVKGFYPSD
IAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFS
CSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSEVQLQQSGPELVKPGT
SVRISCKTSGYTFTEYTIHWVKQSHGKSLEWIGNINPNNGGTTYNQKFE
DKATLTVDKSSSTAYMELRSLTSEDSAVYYCAAGWNFDWGQGTTLT
VSSGGGGSGGGGSGGGGSGGGGSNIVMTQSPKSMSMSVGERVTLTCKA
SENVVTYVSWYQQKPEQSPKLLIYGASNRYTGVPDRFTGSGSATDFTLT
ISSVQAEDLADYHCGQGYSYPYTFGGGTKLEIK
HC tumor 412 EVQLQQSGPELVKPGTSVRISCKTSGYTFTEYTIHWVKQSHGKSLEWIG
mAb with NINPNNGGTTYNQKFEDKATLTVDKSSSTAYMELRSLTSEDSAVYYCA
CD40 mAb AGWNFDWGQGTTLTVSSASTKGPSVFPLAPSSKSTSGGTAALGCLVK
ScFv DYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGTQT
YICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPSVFLFPPKP
KDTLMISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPREEQ
YNSTYRVVSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKAKGQP
REPQVYTLPPSREEMTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYK
TTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYTQKS
LSLSPGGGGGSGGGGSQVQLVQSGAEVKKPGASVKVSCKASGYTFTGY
YMHWVRQAPGQGLEWMGWINPDSGGTNYAQKFQGRVTMTRDTSIST
AYMELNRLRSDDTAVYYCARDQPLGYCTNGVCSYFDWGQGTLVTVS
SGGGGSGGGGSGGGGSGGGGSDIQMTQSPS SVSASVGDRVTITCRASQG
IYSWLAWYQQKPGKAPNLLIYTASTLQSGVPSRFSGSGSGTDFTLTISSL
QPEDFATYYCQQANIFPLTFGGGTKVEIK
Rovalpituzu HC CD40 424 QVQLVQSGAEVKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQGLE
mab mAb with WMGWINPDSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSDDTAV
tumor mAb YYCARDQPLGYCTNGVCSYFDWGQGTLVTVSSASTKGPSVFPLAPSS
ScFv KSTSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYS
LSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCP
APELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYV
DGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNK
ALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLTCLVKGFYPSD
IAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFS
CSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSQVQLVQSGAEVKKPG
ASVKVSCKASGYTFTNYGMNWVRQAPGQGLEWMGWINTYTGEPTYA
DDFKGRVTMTTDTSTSTAYMELRSLRSDDTAVYYCARIGDSSPSDWG
QGTLVTVSSGGGGSGGGGSGGGGSGGGGSEIVMTQSPATLSVSPGERAT
LSCKASQSVSNDVVWYQQKPGQAPRLLIYYASNRYTGIPARFSGSGSGT
EFTLTISSLQSEDFAVYYCQQDYTSPWTFGQGTKLEIK
HC tumor 425 QVQLVQSGAEVKKPGASVKVSCKASGYTFTNYGMNWVRQAPGQGLE
mAb with WMGWINTYTGEPTYADDFKGRVTMTTDTSTSTAYMELRSLRSDDTAV
CD40 mAb YYCARIGDSSPSDWGQGTLVTVSSASTKGPSVFPLAPSSKSTSGGTAAL
ScFv GCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSS
SLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPSV
FLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAK
TKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKTIS
KAKGQPREPQVYTLPPSREEMTKNQVSLTCLVKGFYPSDIAVEWESNG
QPENNYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFSCSVMHEALH
NHYTQKSLSLSPGGGGGSGGGGSQVQLVQSGAEVKKPGASVKVSCKAS
GYTFTGYYMHWVRQAPGQGLEWMGWINPDSGGTNYAQKFQGRVTMT
RDTSISTAYMELNRLRSDDTAVYYCARDQPLGYCTNGVCSYFDWGQ
GTLVTVSSGGGGSGGGGSGGGGSGGGGSDIQMTQSPS SVSASVGDRVTI
TCRASQGIYSWLAWYQQKPGKAPNLLIYTASTLQSGVPSRFSGSGSGTD
FTLTISSLQPEDFATYYCQQANIFPLTFGGGTKVEIK
PF- HC CD40 437 QVQLVQSGAEVKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQGLE
06647020 mAb with WMGWINPDSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSDDTAV
-126-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
tumor mAb YYCARDQPLGYCTNGVCSYFDWGQGTLVTVSSASTKGPSVFPLAPSS
ScFv KSTSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYS
LSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCP
APELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYV
DGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNK
ALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLTCLVKGFYPSD
IAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFS
CSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSQVQLVQSGPEVKKPG
ASVKVSCKASGYTFTDYAVHWVRQAPGKRLEWIGVISTYNDYTYNNQ
DFKGRVTMTRDTSASTAYMELSRLRSEDTAVYYCARGNSYFYALDYW
GQGTSVTVSSGGGGSGGGGSGGGGSGGGGSEIVLTQSPATLSLSPGERA
TLSCRASESVDSYGKSFMHWYQQKPGQAPRLLIYRASNLESGIPARFSG
SGSGTDFTLTISSLEPEDFAVYYCQQSNEDPWTFGGGTKLEIK
HC tumor 438 QVQLVQSGPEVKKPGASVKVSCKASGYTFTDYAVHWVRQAPGKRLEW
mAb with IGVISTYNDYTYNNQDFKGRVTMTRDTSASTAYMELSRLRSEDTAVYY
CD40 mAb CARGNSYFYALDWGQGTSVTVSSASTKGPSVFPLAPSSKSTSGGTAAL
ScFv GCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSS
SLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPSV
FLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAK
TKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKTIS
KAKGQPREPQVYTLPPSREEMTKNQVSLTCLVKGFYPSDIAVEWESNG
QPENNYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFSCSVMHEALH
NHYTQKSLSLSPGGGGGSGGGGSQVQLVQSGAEVKKPGASVKVSCKAS
GYTFTGYYMHWVRQAPGQGLEWMGWINPDSGGTNYAQKFQGRVTMT
RDTSISTAYMELNRLRSDDTAVYYCARDQPLGYCTNGVCSYFDWGQ
GTLVTVSSGGGGSGGGGSGGGGSGGGGSDIQMTQSPS SVSASVGDRVTI
TCRASQGIYSWLAWYQQKPGKAPNLLIYTASTLQSGVPSRFSGSGSGTD
FTLTISSLQPEDFATYYCQQANIFPLTFGGGTKVEIK
Antibody to HC CD40 450 QVQLVQSGAEVKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQGLE
PTK7 mAb with WMGWINPDSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSDDTAV
tumor mAb YYCARDQPLGYCTNGVCSYFDWGQGTLVTVSSASTKGPSVFPLAPSS
ScFv KSTSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYS
LSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCP
APELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYV
DGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNK
ALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLTCLVKGFYPSD
IAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFS
CSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSQVQLVESGGGVVQPG
RSLRLSCAASGFTFSSYAFHWVRQAPGKGLEWVAVISYDGSIKYYADSV
KGRFTISRDNSKNTLYLQMNSLRAEDTAVYYCARTYYFDWGQGTLV
TVSSGGGGSGGGGSGGGGSGGGGSEIVLTQSPDFQSVTPKEKVTITCRA
SQSIGSSLHWYQQKPDQSPKLLIKYASQSFSGVPSRFSGSGSGTDFTLTIN
SLEAEDAAAYYCHQSSSLPITFGQGTRLEIK
HC tumor 451 QVQLVESGGGVVQPGRSLRLSCAASGFTFSSYAFHWVRQAPGKGLEWV
mAb with AVISYDGSIKYYADSVKGRFTISRDNSKNTLYLQMNSLRAEDTAVYYCA
CD40 mAb RTYYFDWGQGTLVTVSSASTKGPSVFPLAPSSKSTSGGTAALGCLVKD
ScFv YFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGTQTY
ICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPSVFLFPPKPK
DTLMISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPREEQY
NSTYRVVSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKAKGQPR
EPQVYTLPPSREEMTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKT
TPPVLDSDGSFFLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYTQKSL
SLSPGGGGGSGGGGSQVQLVQSGAEVKKPGASVKVSCKASGYTFTGYY
MHWVRQAPGQGLEWMGWINPDSGGTNYAQKFQGRVTMTRDTSISTA
YMELNRLRSDDTAVYYCARDQPLGYCTNGVCSYFDYWGQGTLVTVSS
GGGGSGGGGSGGGGSGGGGSDIQMTQSPSSVSASVGDRVTITCRASQGI
YSWLAWYQQKPGKAPNLLIYTASTLQSGVPSRFSGSGSGTDFTLTISSLQ
PEDFATYYCQQANIFPLTFGGGTKVEIK
SGN-LIV1A HC CD40 463 QVQLVQSGAEVKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQGLE
-127-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
mAb with WMGWINPDSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSDDTAV
tumor mAb YYCARDQPLGYCTNGVCSYFDWGQGTLVTVSSASTKGPSVFPLAPSS
ScFv KSTSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYS
LSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCP
APELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYV
DGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNK
ALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLTCLVKGFYPSD
IAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFS
CSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSQVQLVQSGAEVKKPG
ASVKVSCKASGLTIEDYYMHWVRQAPGQGLEWMGWIDPENGDTEYGP
KFQGRVTMTRDTSINTAYMELSRLRSDDTAVYYCAVHNAHYGTWFAY
WGQGTLVTVSSGGGGSGGGGSGGGGSGGGGSDVVMTQSPLSLPVTLG
QPASISCRSSQSLLHSSGNTYLEYFQQRPGQSPRPLIYKISTRFSGVPDRFS
GSGSGTDFTLKISRVEAEDVGVYYCFQGSHVPYTFGGGTKVEIK
HC tumor 464 QVQLVQSGAEVKKPGASVKVSCKASGLTIEDYYMHWVRQAPGQGLEW
mAb with MGWIDPENGDTEYGPKFQGRVTMTRDTSINTAYMELSRLRSDDTAVYY
CD40 mAb CAVHNAHYGTWFAWGQGTLVTVSSASTKGPSVFPLAPSSKSTSGGTA
ScFv ALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVP
SSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPS
VFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNA
KTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKT
ISKAKGQPREPQVYTLPPSREEMTKNQVSLTCLVKGFYPSDIAVEWESN
GQPENNYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFSCSVMHEAL
HNHYTQKSLSLSPGGGGGSGGGGSQVQLVQSGAEVKKPGASVKVSCK
ASGYTFTGYYMHWVRQAPGQGLEWMGWINPDSGGTNYAQKFQGRVT
MTRDTSISTAYMELNRLRSDDTAVYYCARDQPLGYCTNGVCSYFDYW
GQGTLVTVSSGGGGSGGGGSGGGGSGGGGSDIQMTQSPSSVSASVGDR
VTITCRASQGIYSWLAWYQQKPGKAPNLLIYTASTLQSGVPSRFSGSGS
GTDFTLTISSLQPEDFATYYCQQANIFPLTFGGGTKVEIK
Cirmtuzuma HC CD40 476 QVQLVQSGAEVKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQGLE
b mAb with WMGWINPDSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSDDTAV
tumor mAb YYCARDQPLGYCTNGVCSYFDWGQGTLVTVSSASTKGPSVFPLAPSS
ScFv KSTSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYS
LSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCP
APELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYV
DGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNK
ALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLTCLVKGFYPSD
IAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFS
CSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSQVQLQESGPGLVKPSQ
TLSLTCTVSGYAFTAYNIHWVRQAPGQGLEWMGSFDPYDGGSSYNQKF
KDRLTISKDTSKNQVVLTMTNMDPVDTATYYCARGWYYFDWGHGT
LVTVSSGGGGSGGGGSGGGGSGGGGSDIVMTQTPLSLPVTPGEPASISC
RASKSISKYLAWYQQKPGQAPRLLIYSGSTLQSGIPPRFSGSGYGTDFTL
TINNIESEDAAYYFCQQHDESPY TFGEGTKVEIK
HC tumor 477 QVQLQESGPGLVKPSQTLSLTCTVSGYAFTAYNIHWVRQAPGQGLEWM
mAb with GSFDPYDGGSSYNQKFKDRLTISKDTSKNQVVLTMTNMDPVDTATYYC
CD40 mAb ARGWYYFDWGHGTLVTVSSASTKGPS VFPLAPSSKS TSGGTAALGCL
ScFv VKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGT
QTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPSVFLFPP
KPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPRE
EQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKAKG
QPREPQVYTLPPSREEMTKNQVSLTCLVKGFYPSDIAVEWESNGQPENN
YKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYTQ
KSLSLSPGGGGGSGGGGSQVQLVQSGAEVKKPGASVKVSCKASGYTFT
GYYMHWVRQAPGQGLEWMGWINPDSGGTNYAQKFQGRVTMTRDTSI
STAYMELNRLRSDDTAVYYCARDQPLGYCTNGVCSYFDWGQGTLVT
VSSGGGGSGGGGSGGGGSGGGGSDIQMTQSPSSVSAS VGDRVTITCRAS
QGIYSWLAWYQQKPGKAPNLLIYTASTLQSGVPSRFSGSGSGTDFTLTIS
SLQPEDFATYYCQQANIFPLTFGGGTKVEIK
-128-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
Antibody to HC CD40 489 QVQLVQSGAEVKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQGLE
MAGE-A3 mAb with WMGWINPDSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSDDTAV
tumor mAb YYCARDQPLGYCTNGVCSYFDWGQGTLVTVSSASTKGPSVFPLAPSS
ScFv KSTSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYS
LSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCP
APELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYV
DGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNK
ALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLTCLVKGFYPSD
IAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFS
CSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSQVQLVESGGGVVQPG
RSLRLSCTASGFRFRSHGMHWVRQAPGKGLEWVAVISYDGNNKLYAD
SVKGRITISRDNSKNTLFLQMNNVRAEDTAVYYCASPYTSDWQYFQYW
GQGTLVIVSSGGGGSGGGGSGGGGSGGGGSEIVMTQSPATLSVSPGERA
TFSCRASQNISTTLAWYQQKPGQAPRLLIYDTSTRATGIPARFSGSGSGT
EFTLTISSLQSEDLAVYYCQQSNSWPLTFGGGTKVEIK
HC tumor 490 QVQLVESGGGVVQPGRSLRLSCTASGFRFRSHGMHWVRQAPGKGLEW
mAb with VAVISYDGNNKLYADSVKGRITISRDNSKNTLFLQMNNVRAEDTAVYY
CD40 mAb CASPYTSDWQYFQWGQGTLVIVSSASTKGPSVFPLAPSSKSTSGGTAA
ScFv LGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPS
SSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPS
VFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNA
KTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKT
ISKAKGQPREPQVYTLPPSREEMTKNQVSLTCLVKGFYPSDIAVEWESN
GQPENNYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFSCSVMHEAL
HNHYTQKSLSLSPGGGGGSGGGGSQVQLVQSGAEVKKPGASVKVSCK
ASGYTFTGYYMHWVRQAPGQGLEWMGWINPDSGGTNYAQKFQGRVT
MTRDTSISTAYMELNRLRSDDTAVYYCARDQPLGYCTNGVCSYFDYW
GQGTLVTVSSGGGGSGGGGSGGGGSGGGGSDIQMTQSPSSVSASVGDR
VTITCRASQGIYSWLAWYQQKPGKAPNLLIYTASTLQSGVPSRFSGSGS
GTDFTLTISSLQPEDFATYYCQQANIFPLTFGGGTKVEIK
Antibody to HC CD40 502 QVQLVQSGAEVKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQGLE
NY-ESO-1 mAb with WMGWINPDSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSDDTAV
tumor mAb YYCARDQPLGYCTNGVCSYFDWGQGTLVTVSSASTKGPSVFPLAPSS
ScFv KSTSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYS
LSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCP
APELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYV
DGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNK
ALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLTCLVKGFYPSD
IAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFS
CSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSQVQLVQSGGGVVRPG
GSLRLSCAASGFSFIDYGMSWVRQVPGKGLEWVAGMNWSGDKKGHA
ESVKGRFIISRDNAKNTLYLEMSSLRVEDTALYFCARGEYSNRFDPRGR
GTLVTVSSGGGGSGGGGSGGGGSGGGGSDIVMTQTPLSLPVTLGQPASL
SCRSSQSLVFTDGNTYLNWFQQRPGQSPRRLIYKVSSRDPGVPDRFSGT
GSGTDFTLEISRVEAEDIGVYYCMQGTHWPPIFGQGTKVEIK
HC tumor 503 QVQLVQSGGGVVRPGGSLRLSCAASGFSFIDYGMSWVRQVPGKGLEW
mAb with VAGMNWSGDKKGHAESVKGRFIISRDNAKNTLYLEMSSLRVEDTALYF
CD40 mAb CARGEYSNRFDPRGRGTLVTVSSASTKGPSVFPLAPSSKSTSGGTAALGC
ScFv LVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLG
TQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPSVFLFP
PKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPR
EEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKAK
GQPREPQVYTLPPSREEMTKNQVSLTCLVKGFYPSDIAVEWESNGQPEN
NYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFSCS VMHEALHNHYT
QKSLSLSPGGGGGSGGGGSQVQLVQSGAEVKKPGASVKVSCKASGYTF
TGYYMHWVRQAPGQGLEWMGWINPDSGGTNYAQKFQGRVTMTRDTS
ISTAYMELNRLRSDDTAVYYCARDQPLGYCTNGVCSYFDWGQGTLV
TVSSGGGGSGGGGSGGGGSGGGGSDIQMTQSPSSVSASVGDRVTITCRA
SQGIYSWLAWYQQKPGKAPNLLIYTASTLQSGVPSRFSGSGSGTDFTLTI
-129-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
SSLQPEDFATYYCQQANIFPLTFGGGTKVEIK
Trastuzumab HC CD40 683 QVQLVQSGAEVKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQGLE
mAb with WMGWINPDSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSDDTAV
tumor mAb YYCARDQPLGYCTNGVCSYFDWGQGTLVTVSSASTKGPSVFPLAPSS
ScFv KSTSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYS
LSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCP
APELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYV
DGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNK
ALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLTCLVKGFYPSD
IAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFS
CSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSEVQLVESGGGLVQPGG
SLRLSCAASGFNIKDTYIHWVRQAPGKGLEWVARIYPTNGYTRYADSV
KGRFTISADTSKNTAYLQMNSLRAEDTAVYYCSRWGGDGFYAMDYW
GQGTLVTVSSGGGGSGGGGSGGGGSGGGGSDIQMTQSPSSLSASVGDR
VTITCRASQDVNTAVAWYQQKPGKAPKLLIYSASFLYSGVPSRFSGSRS
GTDFTLTISSLQPEDFATYYCQQHYTTPPTFGQGTKVEIK
HC tumor 684 EVQLVESGGGLVQPGGSLRLSCAASGFNIKDTYIHWVRQAPGKGLEWV
mAb with ARIYPTNGYTRYADSVKGRFTISADTSKNTAYLQMNSLRAEDTAVYYC
CD40 mAb SRWGGDGFYAMDWGQGTLVTVSSASTKGPSVFPLAPSSKSTSGGTAA
ScFv LGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPS
SSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPS
VFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNA
KTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKT
ISKAKGQPREPQVYTLPPSREEMTKNQVSLTCLVKGFYPSDIAVEWESN
GQPENNYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFSCSVMHEAL
HNHYTQKSLSLSPGGGGGSGGGGSQVQLVQSGAEVKKPGASVKVSCK
ASGYTFTGYYMHWVRQAPGQGLEWMGWINPDSGGTNYAQKFQGRVT
MTRDTSISTAYMELNRLRSDDTAVYYCARDQPLGYCTNGVCSYFDYW
GQGTLVTVSSGGGGSGGGGSGGGGSGGGGSDIQMTQSPSSVSASVGDR
VTITCRASQGIYSWLAWYQQKPGKAPNLLIYTASTLQSGVPSRFSGSGS
GTDFTLTISSLQPEDFATYYCQQANIFPLTFGGGTKVEIK
HC CD40 796 QVQLVQSGAEVKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQGLE
mAb with WMGWINPDSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSDDTAV
tumor mAb YYCARDQPLGYCTNGVCSYFDWGQGTLVTVSSASTKGPSVFPLAPSS
ScFv KSTSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYS
(LH,25mer) LSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCP
APELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYV
DGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNK
ALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLTCLVKGFYPSD
IAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFS
CSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSDIQMTQSPSSLSASVG
DRVTITCRASQDVNTAVAWYQQKPGKAPKLLIYSASFLYSGVPSRFSGS
RSGTDFTLTISSLQPEDFATYYCQQHYTTPPTFGQGTKVEIKGGGGSGGG
GSGGGGSGGGGSGGGGSEVQLVESGGGLVQPGGSLRLSCAASGFNIKD
TYIHWVRQAPGKGLEWVARIYPTNGYTRYADSVKGRFTISADTSKNTA
YLQMNSLRAEDTAVYYCSRWGGDGFYAMDWGQGTLVTVSS
TABLE 5. APC Antibody CDRs
Antibody Region SEQ ID NO: Sequence
CP-8709893 HCDR1 3 GYTFTGYY
HCDR2 4 INPDSGGT
HCDR3 5 ARDQPLGYCTNGVCSYFDY
LCDR1 8 QGIYSW
LCDR2 9 TAS
LCDR3 10 QQANIFPLT
SBT-040 (G1/G2) HCDR1 3 GYTFTGYY
HCDR2 4 INPDSGGT
-130-
CA 03065919 2019-12-02
WO 2018/227023
PCT/US2018/036560
Antibody Region SEQ ID NO: Sequence
HCDR3 5 ARDQPLGYCTNGVCSYFDY
LCDR1 8 QGIYSW
LCDR2 9 TAS
LCDR3 10 QQANIFPLT
Dacetuzumab HCDR1 582 GYSFTGYY
HCDR2 583 VIPNAGGT
HCDR3 584 AREGIYW
LCDR1 587 QSLVHSNGNTF
LCDR2 588 TVS
LCDR3 589 SQTTHVPWT
Bleselumab HCDR1 592 GGSISSPGYY
HCDR2 593 IYKSGST
HCDR3 594 TRPVVRYFGWFDP
LCDR1 597 QGISSA
LCDR2 598 DAS
LCDR3 599 QQFNSYPT
lucatumumab HCDR1 602 GFTFSSYG
HCDR2 603 ISYEESNR
HCDR3 604 ARDGGIAAPGPDY
LCDR1 607 QSLLYSNGYNY
LCDR2 608 LGS
LCDR3 609 MQARQTPFT
ADC-1013 HCDR1 612 GFTFSTYG
HCDR2 613 ISGGSSYI
HCDR3 614 ARILRGGSGMDL
LCDR1 617 SSNIGAGYN
LCDR2 618 GNI
LCDR3 619 AAWDKSISGLV
APX005 HCDR1 622 GFSFSSTY
HCDR2 623 IYTGDGTN
HCDR3 624 ARPDITYGFAINFW
LCDR1 627 QSISSR
LCDR2 628 RAS
LCDR3 629 QCTGYGISWP
Chi Lob 7/4 HCDR1 632 GYTFTEYI
HCDR2 633 IIPNNGGT
HCDR3 634 TRREVYGRNYYALDY
LCDR1 637 QGINNY
LCDR2 638 YTS
LCDR3 639 QQYSNLPYT
DEC-205 variant 1 HCDR1 234 GFTFSNYG
HCDR2 235 IWYDGSNK
HCDR3 236 ARDLWGWYFDY
LCDR1 239 QSVSSY
LCDR2 240 DAS
LCDR3 241 QQRRNWPLT
DEC-205 variant 2 HCDR1 247 GDSFTTYW
HCDR2 248 IYPGDSDT
HCDR3 249 TRGDRGVDY
LCDR1 252 QGISRW
LCDR2 253 AAS
LCDR3 254 QQYNSYPRT
DC-SIGN variant 1 HCDR1 640 QHFWNTPWT
HCDR2 641 QQGHTLPYT
HCDR3 642 SNDGYYS
LCDR1 643 RYYLGVD
LCDR2 644 DDSGRFP
LCDR3 645 YGYAVDY
DC-SIGN variant 2 HCDR1 646 YYGIYVDY
-131-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ ID NO: Sequence
HCDR2 647 FLVY
HCDR3 648 NFGILGY
LCDR1 649 YPNALDY
LCDR2 650 GLKSFYAMDH
LCDR3 651 QQGKTLPWT
DC-SIGN variant 3 HCDR1 652 QQGNTLPPT
HCDR2 653 QQHYITPLT
HCDR3 654 QQYGNLPYT
LCDR1 655 QQYYSTPRT
LCDR2 656 GQSYNYPPT
LCDR3 657 WQDTHFPHV
TABLE 6. APC Antibody VH sequences and VL sequences
Antibody Region SEQ Sequence
ID
NO:
CP-8709893 VH 2 QVQLVQSGAEVKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQGLEWM
GWINPDSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSDDTAVYYCAR
DQPLGYCTNGVCSYFDWGQGTLVTVSS
VL 7 DIQMTQSPSSVSASVGDRVTITCRASQGIYSWLAWYQQKPGKAPNLLIYTA
STLQSGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCQQANIFPLTFGGGTKV
EIK
SBT-040 VH 2 QVQLVQSGAEVKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQGLEWM
GWINPDSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSDDTAVYYCAR
DQPLGYCTNGVCSYFDWGQGTLVTVSS
VL 7 DIQMTQSPSSVSASVGDRVTITCRASQGIYSWLAWYQQKPGKAPNLLIYTA
STLQSGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCQQANIFPLTFGGGTKV
EIK
Dacetuzumab VH 581 EVQLVESGGGLVQPGGSLRLSCAASGYSFTGYYTHWVRQAPGKGLEWVAR
VIPNAGGTSYNQKFKGRFTLSVDNSKNTAYLQMNSLRAEDTAVYYCAREG
IYWWGQGTLVTVSS
VL 586 DIQMTQSPSSLSASVGDRVTITCRSSQSLVHSNGNTFLHWYQQKPGKAPKL
LIYTVSNRFSGVPSRFSGSGSGTDFTLTISSLQPEDFATYFCSQTTHVPWTFG
QGTKVEIK
Bleselumab VH 591 QLQLQESGPGLLKPSETLSLTCTVSGGSISSPGYYGGWIRQPPGKGLEWIGSI
YKSGSTYHNPSLKSRVTISVDTSKNQFSLKLSSVTAADTAVYYCTRPVVRY
FGWFDPWGQGTLVTVSS
VL 596 AIQLTQSPSSLSASVGDRVTITCRASQGISSALAWYQQKPGKAPKLLIYDAS
NLESGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCQQFNSYPTFGQGTKVEI
K
Lucatumuma VH 601 QVQLVESGGGVVQPGRSLRLSCAASGFTFSSYGMHWVRQAPGKGLEWVA
b VISYEESNRYHADSVKGRFTISRDNSKITLYLQMNSLRTEDTAVYYCARDG
GIAAPGPDWGQGTLVTVSS
VL 606 DIVMTQSPLSLTVTPGEPASISCRSSQSLLYSNGYNYLDWYLQKPGQSPQVL
ISLGSNRASGVPDRFSGSGSGTDFTLKISRVEAEDVGVYYCMQARQTPFTFG
PGTKVDIR
ADC-1013 VH 611 EVQLLESGGGLVQPGGSLRLSCAASGFTFSTYGMHWVRQAPGKGLEWLSY
ISGGSSYIFYADSVRGRFTISRDNSENALYLQMNSLRAEDTAVYYCARILRG
GSGMDLWGQGTLVTVSS
VL 616 QSVLTQPPSASGTPGQRVTISCTGSSSNIGAGYNVWYQQLPGTAPKWYG
NINRPSGVPDRFSGSKSGTSASLAISGLRSEDEADYYCAAWDKSISGLVFGG
GTKLTVL
APX005 VH 621 QVQLVESGGGVVQPGRSLRLSCAASGFSFSSTYVCWVRQAPGKGLEWIACI
YTGDGTNYSASWAKGRFTISKDSSKNTVYLQMNSLRAEDTAVYFCARPDI
TYGFAINFWGPGTLVTVSS
VL 626 DIQMTQSPSSLSASVGDRVTIKCQASQSISSRLAWYQQKPGKPPKLLIYRAS
TLASGVPSRFSGSGSGTDFTLTISSLQPEDVATYYCQCTGYGISWPIGGGTK
-132-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
VEIK
Chi Lob 7/4 VH 631 EVQLQQSGPDLVKPGASVKISCKTSGYTFTEYIMHWVKQSHGKSLEWIGGI
IPNNGGTSYNQKFKDKATMTVDKSSSTGYMELRSLTSEDSAVYYCTRREV
YGRNYYALDWGQGTLVTVSS
VL 636 DIQMTQTTSSLSASLGDRVTITCSASQGINNYLNWYQQKPDGTVKLLIYYTS
SLHSGVPSRFSGSGSGTDYSLTISNLEPEDIATYYCQQYSNLPYTFGGGTKLE
IK
VH 233 QVQLVESGGGVVQPGRSLRLSCAASGFTFSNYGMWVRQAPGKGLEWVA
DEC-205 VIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAEDTAVYYCAR
variant 1 DLWGWYFDWGQGTLVTVSS
VL 238 EIVLTQSPATLSLSPGERATLSCRASQSVS SYLAWYQQKPGQAPRLLIYDAS
NRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRRNWPLTFGGGTKV
EIK
VH 246 EVQLVQSGAEVKKPGESLRISCKGSGDSFTTWIGWVRQMPGKGLEWMGI
DEC-205 IYPGDSDTIYSPSFQGQVTISADKSISTAYLQWSSLKASDTAMYYCTRGDRG
variant 2 VDWGQGTLVTVSS
VL 251 DIQMTQSPSSLSASVGDRVTITCRASQGISRWLAWYQQKPEKAPKSLIYAAS
SLQSGVPSRFSGSGSGTDFTLTISGLQPEDFATYYCQQYNSYPRTFGQGTKV
EIK
VH 658 DIQMTQSPSSLSASVGDRVTITCRASQGISRWLAWYQQKPEKAPKSLIYAAS
CD36 SLQSGVPSRFSGSGSGTDFTLTISGLQPEDFATYYCQQYNSYPRTFGQGTKV
mannose EIK
Scavenger VL 659 EVQLVQSGAEVKKPGESLRISCKGSGDSFTTWIGWVRQMPGKGLEWMGI
Receptor IYPGDSDTIYSPSFQGQVTISADKSISTAYLQWSSLKASDTAMYYCTRGDRG
VDWGQGTLVTVSS
VH 660 QIVESGGGLVQPKESLKISCTASGFTFSNAAIWVRQTPGKGLEWVGRIRTR
CLEC9A PSKYATDYADSVRGRFTISRDDSKSMVYLQMDNLRTEDTAMYYCTPRATE
DVPFWGQGVMVTVSS
VL 661 DIVMTQTPSSQAVSAGEKVTMNCKSSQSVLYDENKKNYLAWYQQKSGQS
PKLLIWASTGESGVPDRFIGSGSGTDFTLTISSVQAEDLAVYYCQQYYDFP
PTFGGGTK
TABLE 7. APC Antibody Heavy Chain and Light Chain sequences
Antibody Region SEQ Sequence
ID
NO:
CP-8709893 Heavy 1 QVQLVQSGAEVKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQGLEWM
Chain GWINPDSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSDDTAVYYCAR
DQPLGYCTNGVCSYFDWGQGTLVTVSSASTKGPSVFPLAPCSRSTSESTA
ALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSS
NFGTQTYTCNVDHKPSNTKVDKTVERKCCVECPPCPAPPVAGPSVFLFPPK
PKDTLMISRTPEVTCVVVDVSHEDPEVQFNWYVDGVEVHNAKTKPREEQF
NSTFRVVSVLTVVHQDWLNGKEYKCKVSNKGLPAPIEKTISKTKGQPREPQ
VYTLPPSREEMTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPML
DSDGSFFLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGK
Light 6 DIQMTQSPSSVSASVGDRVTITCRASQGIYSWLAWYQQKPGKAPNLLIYTA
Chain STLQSGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCQQANIFPLTFGGGTKV
EIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREAKVQWKVDNALQ
SGNSQESVTEQDSKDSTYSLSSTLTLSKADYEKHKVYACEVTHQGLSSPVT
KSFNRGEC
SBT-040 Heavy 577 MDWTWRILFLVAAATGAHSQVQLVQSGAEVKKPGASVKVSCKASGYTFT
Chain GYYMHWVRQAPGQGLEWMGWINPDSGGTNYAQKFQGRVTMTRDTSIST
(IgG1) AYMELNRLRSDDTAVYYCARDQPLGYCTNGVCSYFDWGQGTLVTVSSA
STKGPSVFPLAPCSRSTSESTAALGCLVKDYFPEPVTVSWNSGALTSGVHTF
PAVLQSSGLYSLSSVVTVPSSNFGTQTYTCNVDHKPSNTKVDKTVEPKSCD
KTHTCPPCPAPELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEV
KFNWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKC
-133-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
KVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLTCLVKGFY
PSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFS
CSVMHEALHNHYTQKSLSLSPGK
Heavy 578 MDWTWRILFLVAAATGAHSQVQLVQSGAEVKKPGASVKVSCKASGYTFT
Chain GYYMHWVRQAPGQGLEWMGWINPDSGGTNYAQKFQGRVTMTRDTSIST
(IgG2) AYMELNRLRSDDTAVYYCARDQPLGYCTNGVCSYFDWGQGTLVTVSSA
STKGPSVFPLAPCSRSTSESTAALGCLVKDYFPEPVTVSWNSGALTSGVHTF
PAVLQSSGLYSLSSVVTVPSSNFGTQTYTCNVDHKPSNTKVDKTVERKCCV
ECPPCPAPPVAGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVQFNW
YVDGVEVHNAKTKPREEQFNSTFRVVSVLTVVHQDWLNGKEYKCKVSNK
GLPAPIEKTISKTKGQPREPQVYTLPPSREEMTKNQVSLTCLVKGFYPSDIA
VEWESNGQPENNYKTTPPMLDSDGSFFLYSKLTVDKSRWQQGNVFSCSVM
HEALHNHYTQKSLSLSPGK
Light 579 DIQMTQSPSSVSASVGDRVTITCRASQGIYSWLAWYQQKPGKAPNLLIYTA
Chain STLQSGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCQQANIFPLTFGGGTKV
EIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREAKVQWKVDNALQ
SGNSQESVTEQDSKDSTYSLSSTLTLSKADYEKHKVYACEVTHQGLSSPVT
KSFNRGEC
Dacetuzumab Heavy 580 EVQLVESGGGLVQPGGSLRLSCAASGYSFTGYYTHWVRQAPGKGLEWVAR
Chain VIPNAGGTSYNQKFKGRFTLSVDNSKNTAYLQMNSLRAEDTAVYYCAREG
WWWGQGTLVTVSSASTKGPSVFPLAPSSKSTSGGTAALGCLVKDYFPEPV
TVSWNSGALTSGVHTFPAVLQS SGLYSLSSVVTVPSS SLGTQTYICNVNHKP
SNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPSVFLFPPKPKDTLMISRTPE
VTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPREEQYNSTYRVVSVLT
VLHQDWLNGKEYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREEM
TKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSK
LTVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGK
Light 585 DIQMTQSPSSLSASVGDRVTITCRSSQSLVHSNGNTFLHWYQQKPGKAPKL
Chain LIYTVSNRFSGVPSRFSGSGSGTDFTLTISSLQPEDFATYFCSQTTHVPWTFG
QGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREAKVQWKV
DNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEKHKVYACEVTHQG
LSSPVTKSFNRGEC
Bleselumab Heavy 590 QLQLQESGPGLLKPSETLSLTCTVSGGSISSPGYYGGWIRQPPGKGLEWIGSI
Chain YKSGSTYHNPSLKSRVTISVDTSKNQFSLKLSSVTAADTAVYYCTRPVVRY
FGWFDPWGQGTLVTVSSASTKGPSVFPLAPCSRSTSESTAALGCLVKDYFP
EPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGTKTYTCNV
DHKPSNTKVDKRVESKYGPPCPPCPAPEFEGGPSVFLFPPKPKDTLMISRTP
EVTCVVVDVSQEDPEVQFNWYVDGVEVHNAKTKPREEQFNSTYRVVSVL
TVLHQDWLNGKEYKCKVSNKGLPSSIEKTISKAKGQPREPQVYTLPPSQEE
MTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYS
RLTVDKSRWQEGNVFSCSVMHEALHNHYTQKSLSLSLGK
Light 595 AIQLTQSPSSLSASVGDRVTITCRASQGISSALAWYQQKPGKAPKLLIYDAS
Chain NLESGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCQQFNSYPTFGQGTKVEI
KRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREAKVQWKVDNALQSG
NSQESVTEQDSKDSTYSLSSTLTLSKADYEKHKVYACEVTHQGLSSPVTKS
FNRGEC
Lucatumuma Heavy 600 QVQLVESGGGVVQPGRSLRLSCAASGFTFSSYGMHWVRQAPGKGLEWVA
b Chain VISYEESNRYHADSVKGRFTISRDNSKITLYLQMNSLRTEDTAVYYCARDG
GIAAPGPDWGQGTLVTVSSASTKGPSVFPLAPASKS TSGGTAALGCLVKD
YFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGTQTYIC
NVNHKPSNTKVDKRVEPKSCDKTHTCPPCPAPELLGGPSVFLFPPKPKDTL
MISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPREEQYNSTYR
VVSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLP
PSREEMTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGS
FFLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGK
Light 605 DIVMTQSPLSLTVTPGEPASISCRSSQSLLYSNGYNYLDWYLQKPGQSPQVL
Chain ISLGSNRASGVPDRFSGSGSGTDFTLKISRVEAEDVGVYYCMQARQTPFTFG
PGTKVDIRRTVAAPS VFIFPPSDEQLKSGTASVVCLLNNFYPREAKVQWKV
-134-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
DNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEKHKVYACEVTHQG
LSSPVTKSFNRGEC
ADC-1013 Heavy 610 EVQLLESGGGLVQPGGSLRLSCAASGFTFSTYGMHWVRQAPGKGLEWLSY
Chain ISGGSSYIFYADSVRGRFTISRDNSENALYLQMNSLRAEDTAVYYCARILRG
GSGMDLWGQGTLVTVSSASTKGPSVFPLAPSSKSTSGGTAALGCLVKDYFP
EPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSWTVPSSSLGTQTYICNVN
HKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPSVFLFPPKPKDTLMISR
TPEVTCNAVDVSHEDPEVKFNWYVDGVEVHNAKTKPREEQYNSTYRWSV
LTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSRD
ELTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLY
SKLTVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGK
Light 615 QSVLTQPPSASGTPGQRVTISCTGSSSNIGAGYNVWYQQLPGTAPKWYG
Chain NINRPSGVPDRFSGSKSGTSASLAISGLRSEDEADYYCAAWDKSISGLVFGG
GTKLTVLGQPKAAPSVTLFPPSSEELQANKATLVCLISDFYPGAVTVAWKA
DSSPVKAGVETTTPSKQSNNKYAASSYLSLTPEQWKSHRSYSCQVTHEGST
VEKTVAPTECS
APX005 Heavy 620 QVQLVESGGGVVQPGRSLRLSCAASGFSFSSTYVCWVRQAPGKGLEWIACI
Chain YTGDGTNYSASWAKGRFTISKDSSKNTVYLQMNSLRAEDTAVYFCARPDI
TYGFAINFWGPGTLVTVSSASTKGPSVFPLAPSSKSTSGGTAALGCLVKDYF
PEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGTQTYICNV
NHKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPS VFLFPPKPKDTLMIS
RTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPREEQYNSTYRVV
SVLTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPS
REEMTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFF
LYSKLTVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGK
Light 625 DIQMTQSPSSLSASVGDRVTIKCQASQSISSRLAWYQQKPGKPPKLLIYRAS
Chain TLASGVPSRFSGSGSGTDFTLTISSLQPEDVATYYCQCTGYGISWPIGGGTK
VEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREAKVQWKVDNAL
QSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEKHKVYACEVTHQGLSSPV
TKSFNRGEC
Chi Lob 7/4 Heavy 630 EVQLQQSGPDLVKPGASVKISCKTSGYTFTEYIMHWVKQSHGKSLEWIGGI
Chain IPNNGGTSYNQKFKDKATMTVDKSSSTGYMELRSLTSEDSAVYYCTRREV
YGRNYYALDWGQGTLVTVSSASTKGPSVFPLAPSSKSTSGGTAALGCLV
KDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGTQTY
ICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPSVFLFPPKPKDT
LMISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPREEQYNSTY
RVVSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKAKGQPREPQVYT
LPPSREEMTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSD
GSFFLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGK
Light 635 DIQMTQTTSSLSASLGDRVTITCSASQGINNYLNWYQQKPDGTVKLLIYYTS
Chain SLHSGVPSRFSGSGSGTDYSLTISNLEPEDIATYYCQQYSNLPYTFGGGTKLE
IKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREAKVQWKVDNALQS
GNSQESVTEQDSKDSTYSLSSTLTLSKADYEKHKVYACEVTHQGLSSPVTK
SFNRGEC
DEC-205 Heavy 232 QVQLVESGGGVVQPGRSLRLSCAASGFTFSNYGMWVRQAPGKGLEWVA
(variant 1) Chain VIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAEDTAVYYCAR
DLWGWYFDWGQGTLVTVSSASTKGPSVFPLAPSSKSTSGGTAALGCLVK
DYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSS VVTVPSSSLGTQTYI
CNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPSVFLFPPKPKDT
LMISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPREEQYNSTY
RVVSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKAKGQPREPQVYT
LPPSREEMTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSD
GSFFLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGK
Light 237 EIVLTQSPATLSLSPGERATLSCRASQSVS SYLAWYQQKPGQAPRLLIYDAS
Chain NRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRRNWPLTFGGGTKV
EIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREAKVQWKVDNALQ
SGNSQESVTEQDSKDSTYSLSSTLTLSKADYEKHKVYACEVTHQGLSSPVT
-135-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
KSFNRGEC
DEC-205 Heavy 245 EVQLVQSGAEVKKPGESLRISCKGSGDSFTTWIGWVRQMPGKGLEWMGI
(variant 2) Chain IYPGDSDTIYSPSFQGQVTISADKSISTAYLQWSSLKASDTAMYYCTRGDRG
VDWGQGTLVTVSSASTKGPSVFPLAPSSKSTSGGTAALGCLVKDYFPEPV
TVSWNSGALTSGVHTFPAVLQS SGLYSLSSVVTVPSS SLGTQTYICNVNHKP
SNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPSVFLFPPKPKDTLMISRTPE
VTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPREEQYNSTYRVVSVLT
VLHQDWLNGKEYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREEM
TKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSK
LTVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGK
Light 250 DIQMTQSPSSLSASVGDRVTITCRASQGISRWLAWYQQKPEKAPKSLIYAAS
Chain SLQSGVPSRFSGSGSGTDFTLTISGLQPEDFATYYCQQYNSYPRTFGQGTKV
EIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREAKVQWKVDNALQ
SGNSQESVTEQDSKDSTYSLSSTLTLSKADYEKHKVYACEVTHQGLSSPVT
KSFNRGEC
CLEC12A Heavy 662 QVQLQESGPGLVKPSETLSLTCVVSGGSISSSNWWSWVRQPPGKGLEWIGE
Chain IYHSGSPDYNPSLKSRVTISVDKSRNQFSLKLSSVTAADTAVYYCAKVSTG
variant GFFDWGQGTLVTVSSASTKGPSVFPLAPSSKSTSGGTAALGCLVKDYFPE
1 PVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGTQTYICNVNH
KPSNTKVDKRVEPKSCDKTHTCPPCPAPELLGGPSVFLFPPKPKDTLMISRT
PEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPREEQYNSTYRVVSV
LTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSRE
EMTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLY
SKLTVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGK
Heavy 663 QVQLQESGPGLVKPSETLSLTCVVSGGSISSSNWWSWVRQPPGKGLEWIGE
Chain IYHSGSPNYNPSLKSRVTISVDKSKNQFSLKLS SVTAADTAVYYCARSSSGG
variant FFDWGQGTLVTVSSASTKGPSVFPLAPS SKSTSGGTAALGCLVKDYFPEP
2 VTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPS SSLGTQTYICNVNH
KPSNTKVDKRVEPKSCDKTHTCPPCPAPELLGGPSVFLFPPKPKDTLMISRT
PEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPREEQYNSTYRVVSV
LTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSRE
EMTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLY
SKLTVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGK
Heavy 664 QVQLQESGPGLVKPSETLSLTCVVSGGSISSSNWWSWVRQPPGKGLEWIGE
Chain IYHSGSPNYNPSLKSRVTISVDKSKNQFSLKLS SVTAADTAVYYCARQTTA
variant GSFDWGQGTLVTVSSASTKGPSVFPLAPSSKSTSGGTAALGCLVKDYFPE
3 PVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGTQTYICNVNH
KPSNTKVDKRVEPKSCDKTHTCPPCPAPELLGGPSVFLFPPKPKDTLMISRT
PEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPREEQYNSTYRVVSV
LTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSRE
EMTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLY
SKLTVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGK
Light 665 DIQMTQSPSSLSASVGDRVTITCRASQSISSYLNWYQQKPGKAPKLLIYAAS
Chain SLQSGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCQQSYSTPPTFGQGTKVE
IKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREAKVQWKVDNALQS
GNSQESVTEQDSKDSTYSLSSTLTLSKADYEKHKVYACEVTHQGLSSPVTK
SFNRGEC
BDCA-2 Heavy 666 QVQLVESGGGVVQPGRSLRLSCAASGFTLS SYGMHWVRQAPGKGLEWVA
Variant 1 Chain VIWYDGNDKYYADSVKGRFTISRDNSKNTLYLQVNSLRAEDTAVYYCAR
GTGTPWYFDLWGRGTLVTVSSASTKGPSVFPLAPSSKSTSGGTAALGCLV
KDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGTQTY
ICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPSVFLFPPKPKDT
LMISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPREEQYNSTY
RVVSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKAKGQPREPQVYT
LPPSRDELTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSD
GSFFLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGK
Light 669 EIVLTQSPATLSLSPGERATLSCRASQSVNNYLAWYQQKPGQAPRLLIYDAS
Chain NRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRSTWPPYTFGQGTK
LEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREAKVQWKVDNAL
-136-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
QSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEKHKVYACEVTHQGLSSPV
TKSFNRGEC
BDCA-2 Heavy 667 EVQLVESGGGLVQPGGSLRLSCAASGFTFSNYLMNWVRQAPGKGLEWVA
Variant 2 Chain NIEQDGSEKYYVDSVKGRFTISRDNAKNSLYLQMNSLRAEDTAVYFCARD
GDTAMITFDFWGQGTLVTVSSASTKGPSVFPLAPS SKSTSGGTAALGCLVK
DYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSS VVTVPSSSLGTQTYI
CNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPSVFLFPPKPKDT
LMISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPREEQYNSTY
RVVSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKAKGQPREPQVYT
LPPSRDELTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSD
GSFFLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGK
Light 670 DIQMTQSPSSVSASVGDRVTITCRASQGIRRWLAWYQQKPGKAPKLLIYAA
Chain SSLQRGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCQQANSFPWTFGQGTK
VEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREAKVQWKVDNAL
QSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEKHKVYACEVTHQGLSSPV
TKSFNRGEC
BDCA-2 Heavy 668 QVQLQESGPGLVKPSQTLSLTCTVSGGSISSGGYWNWIRQHPGKGLEWIG
Variant 3 Chain YIYYSGNTYYNPSLKSRVTISVDTSKNQFSLKLSSVTAADAAVYHCARGYG
DYGGGYFDWGQGTLVTVSSASTKGPSVFPLAPSSKSTSGGTAALGCLVK
DYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSS VVTVPSSSLGTQTYI
CNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPSVFLFPPKPKDT
LMISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPREEQYNSTY
RVVSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKAKGQPREPQVYT
LPPSRDELTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSD
GSFFLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGK
Light 671 DIQMTQSPSSLSASVGDRVTITCQASQDISNYLNWYQQKPGKAPKFLIYDVS
Chain NLETGVPSRFSGSGSGTDFTFTISSLQPEDIATYYCQQYDNLPYTFGQGTKL
EIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREAKVQWKVDNALQ
SGNSQESVTEQDSKDSTYSLSSTLTLSKADYEKHKVYACEVTHQGLSSPVT
KSFNRGEC
TABLE 8. Fusion Sequences ¨ DEC-205 fusions via the heavy chain
Antibody Region SEQ Sequence
ID
NO:
Pertuzumab HC DEC-205 505 QVQLVESGGGVVQPGRSLRLSCAASGFTFSNYGMWVRQAPGKGL
mAb with EWVAVIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAED
tumor mAb TAVYYCARDLWGWYFDWGQGTLVTVSSASTKGPS VFPLAPSSKS
ScFv TSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLY
SLSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCP
PCPAPELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKF
NWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEY
KCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLT
CLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTV
DKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSE
VQLVESGGGLVQPGGSLRLSCAASGFTFTDYTMDWVRQAPGKGLE
WVADVNPNSGGSIYNQRFKGRFTLSVDRSKNTLYLQMNSLRAEDT
AVYYCARNLGPSFYFDWGQGTLVTVSSGGGGSGGGGSGGGGSG
GGGSDIQMTQSPSSLSASVGDRVTITCKASQDVSIGVAWYQQKPGK
APKLLIYSASYRYTGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCQQ
YYIYPYTFGQGTKVEIK
HC tumor 506 EVQLVESGGGLVQPGGSLRLSCAASGFTFTDYTMDWVRQAPGKGL
mAb with EWVADVNPNSGGSIYNQRFKGRFTLSVDRSKNTLYLQMNSLRAED
DEC-205 mAb TAVYYCARNLGPSFYFDWGQGTLVTVSSASTKGPS VFPLAPSSKS
ScFv TSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLY
SLSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCP
-137-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
PCPAPELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKF
NWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEY
KCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLT
CLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTV
DKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSQ
VQLVESGGGVVQPGRSLRLSCAASGFTFSNYGMYWVRQAPGKGLE
WVAVIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAEDT
AVYYCARDLWGWYFDWGQGTLVTVSSGGGGSGGGGSGGGGSG
GGGSEIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQA
PRLLIYDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQR
RNWPLTFGGGTKVEIK
LC tumor 846 DIQMTQSPSSLSASVGDRVTITCKASQDVSIGVAWYQQKPGKAPKL
mAb LIYSASYRYTGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCQQYYIY
containing PYTFGQGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPR
CD40 mab EAKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYE
scFv (LH) KHKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSDIQMTQSPS
SVSASVGDRVTITCRASQGIYSWLAWYQQKPGKAPNLLIYTASTLQ
SGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCQQANIFPLTFGGGTK
VEIKGGGGSGGGGSGGGGSGGGGSQVQLVQSGAEVKKPGASVKVS
CKASGYTFTGYYMHWVRQAPGQGLEWMGWINPDSGGTNYAQKF
QGRVTMTRDTSISTAYMELNRLRSDDTAVYYCARDQPLGYCTNGV
CSYFDWGQGTLVTVSS
Cetuximab HC DEC-205 507 QVQLVESGGGVVQPGRSLRLSCAASGFTFSNYGMWVRQAPGKGL
mAb with EWVAVIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAED
tumor mAb TAVYYCARDLWGWYFDWGQGTLVTVSSASTKGPS VFPLAPSSKS
ScFv TSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLY
SLSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCP
PCPAPELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKF
NWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEY
KCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLT
CLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTV
DKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSQ
VQLKQSGPGLVQPSQSLSITCTVSGFSLTNYGVHWVRQSPGKGLEW
LGVIWSGGNTDYNTPFTSRLSINKDNSKSQVFFKMNSLQSNDTAIYY
CARALTYYDYEFAWGQGTLVTVSAGGGGSGGGGSGGGGSGGGG
SDILLTQSPVILS VSPGERVSFSCRASQSIGTNIHWYQQRTNGSPRLLI
KYASESISGIPSRFSGSGSGTDFTLSINSVESEDIADYYCQQNNNWPT
TFGAGTKLELK
HC tumor 508 QVQLKQSGPGLVQPSQSLSITCTVSGFSLTNYGVHWVRQSPGKGLE
mAb with WLGVIWSGGNTDYNTPFTSRLSINKDNSKSQVFFKMNSLQSNDTAI
DEC-205 mAb YYCARALTYYDYEFAWGQGTLVTVSAASTKGPSVFPLAPSSKSTS
ScFv GGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSL
SSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPC
PAPELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFN
WYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYK
CKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLTC
LVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTVD
KSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSQV
QLVESGGGVVQPGRSLRLSCAASGFTFSNYGMYWVRQAPGKGLE
WVAVIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAEDT
AVYYCARDLWGWYFDWGQGTLVTVSSGGGGSGGGGSGGGGSG
GGGSEIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQA
PRLLIYDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQR
RNWPLTFGGGTKVEIK
Panitumumab HC DEC-205 509 QVQLVESGGGVVQPGRSLRLSCAASGFTFSNYGMWVRQAPGKGL
mAb with EWVAVIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAED
tumor mAb TAVYYCARDLWGWYFDWGQGTLVTVSSASTKGPS VFPLAPSSKS
ScFv TSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLY
SLSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCP
-138-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
PCPAPELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKF
NWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEY
KCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLT
CLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTV
DKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSQ
VQLQESGPGLVKPSETLSLTCTVSGGSVSSGDYWTWIRQSPGKGL
EWIGHIYYSGNTNYNPSLKSRLTISIDTSKTQFSLKLS S VTAADTAIY
YCVRDRVTGAFDIWGQGTMVTVSSGGGGSGGGGSGGGGSGGGGS
DIQMTQSPSSLSASVGDRVTITCQASQDISNYLNWYQQKPGKAPKL
LIYDASNLETGVPSRFSGSGSGTDFTFTISSLQPEDIATYFCQHFDHLP
LAFGGGTKVEIK
HC tumor 510 QVQLQESGPGLVKPSETLSLTCTVSGGSVSSGDYWTWIRQSPGKG
mAb with LEWIGHIYYSGNTNYNPSLKSRLTISIDTSKTQFSLKLS
SVTAADTAI
DEC-205 mAb YYCVRDRVTGAFDIWGQGTMVTVSSASTKGPSVFPLAPSSKSTSGG
ScFv TAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQ S SGLYS LS S
VVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCP
APELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNW
YVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKC
KVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLTCL
VKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTVDK
SRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSQVQ
LVESGGGVVQPGRSLRLSCAASGFTFSNYGMYWVRQAPGKGLEW
VAVIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAEDTA
VYYCARDLWGWYFDWGQGTLVTVSSGGGGSGGGGSGGGGSGG
GGSEIVLTQSPATLSLSPGERATLSCRASQ SVSSYLAWYQQKPGQAP
RLLIYDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRR
NWPLTFGGGTKVEIK
Nimotuzumab HC DEC-205 511 QVQLVESGGGVVQPGRSLRLSCAASGFTFSNYGMWVRQAPGKGL
mAb with EWVAVIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAED
tumor mAb TAVYYCARDLWGWYFDWGQGTLVTVSSASTKGPS VFPLAPS S KS
ScFv TSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLY
SLSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCP
PCPAPELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKF
NWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEY
KCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLT
CLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTV
DKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSQ
VQLQQSGAEVKKPGSS VKVSCKASGYTFTNYYIWVRQAPGQGLE
WIGGINPTSGGSNFNEKFKTRVTITVDESTNTAYMELSSLRSEDTAF
YFCARQGLWFDSDGRGFDFWGQGSTVTVSSGGGGSGGGGSGGGG
SGGGGSDIQMTQSPSSLSASVGDRVTITCRSSQNIVHSNGNTYLDWY
QQTPGKAPKLLIYKVSNRFSGVPSRFSGSGSGTDFTFTISSLQPEDIAT
YYCFQYSHVPWTFGQGTKLEIK
HC tumor 512 QVQLQQSGAEVKKPGS SVKVSCKASGYTFTNYYIWVRQAPGQGL
mAb with EWIGGINPTSGGSNFNEKFKTRVTITVDESTNTAYMELSSLRSEDTAF
DEC-205 mAb YFCARQGLWFDSDGRGFDFWGQGSTVTVSSASTKGPSVFPLAPS SK
ScFv STSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGL
YSLSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTC
PPCPAPELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVK
FNWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKE
YKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSL
TCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTV
DKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSQ
VQLVESGGGVVQPGRSLRLSCAASGFTFSNYGMYWVRQAPGKGLE
WVAVIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAEDT
AVYYCARDLWGWYFDWGQGTLVTVSSGGGGSGGGGSGGGGSG
GGGSEIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQA
PRLLIYDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQR
RNWPLTFGGGTKVEIK
-139-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
Zalutumumab HC DEC-205 513 QVQLVESGGGVVQPGRSLRLSCAASGFTFSNYGMWVRQAPGKGL
mAb with EWVAVIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAED
tumor mAb TAVYYCARDLWGWYFDWGQGTLVTVSSASTKGPS VFPLAPSSKS
ScFv TSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLY
SLSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCP
PCPAPELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKF
NWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEY
KCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLT
CLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTV
DKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSQ
VQLVESGGGVVQPGRSLRLSCAASGFTFSTYGMHWVRQAPGKGLE
WVAVIWDDGSYKYYGDSVKGRFTISRDNSKNTLYLQMNSLRAEDT
AVYYCARDGITMVRGVMKDYFDYWGQGTLVTVSSGGGGSGGGGS
GGGGSGGGGSAIQLTQSPSSLSASVGDRVTITCRASQDISSALVWYQ
QKPGKAPKLLIYDASSLESGVPSRFSGSESGTDFTLTISSLQPEDFATY
YCQQFNSYPLTFGGGTKVEIK
HC tumor 514 QVQLVESGGGVVQPGRSLRLSCAASGFTFSTYGMHWVRQAPGKGL
mAb with EWVAVIWDDGSYKYYGDSVKGRFTISRDNSKNTLYLQMNSLRAED
DEC-205 mAb TAVYYCARDGITMVRGVMKDYFDWGQGTLVTVSS ASTKGPSVFP
ScFv LAPS SKSTSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAV
LQSSGLYSLSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSC
DKTHTCPPCPAPELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVS
HEDPEVKFNWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQ
DWLNGKEYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREE
MTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGS
FFLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGGG
GGSGGGGSQVQLVESGGGVVQPGRSLRLSCAASGFTFSNYGMWV
RQAPGKGLEWVAVIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQ
MNSLRAEDTAVYYCARDLWGWYFDYWGQGTLVTVSSGGGGSGG
GGSGGGGSGGGGSEIVLTQSPATLSLSPGERATLSCRASQSVSSYLA
WYQQKPGQAPRLLIYDASNRATGIPARFSGSGSGTDFTLTISSLEPED
FAVYYCQQRRNWPLTFGGGTKVEIK
Onartuzumab HC DEC-205 515 QVQLVESGGGVVQPGRSLRLSCAASGFTFSNYGMWVRQAPGKGL
mAb with EWVAVIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAED
tumor mAb TAVYYCARDLWGWYFDWGQGTLVTVSSASTKGPS VFPLAPSSKS
ScFv TSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLY
SLSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCP
PCPAPELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKF
NWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEY
KCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLT
CLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTV
DKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSE
VQLVESGGGLVQPGGSLRLSCAASGYTFTSWLHWVRQAPGKGLE
WVGMIDPSNSDTRFNPNFKDRFTISADTSKNTAYLQMNSLRAEDTA
VYYCATYRSYVTPLDWGQGTLVTVSSGGGGSGGGGSGGGGSGG
GGSDIQMTQSPSSLSASVGDRVTITCKSSQSLLYTSSQKNYLAWYQQ
KPGKAPKLLIWASTRESGVPSRFSGSGSGTDFTLTISSLQPEDFATY
YCQQYYAYPWTFGQGTKVEIK
HC tumor 516 EVQLVESGGGLVQPGGSLRLSCAASGYTFTSWLHWVRQAPGKGL
mAb with EWVGMIDPSNSDTRFNPNFKDRFTISADTSKNTAYLQMNSLRAEDT
DEC-205 mAb AVYYCATYRSYVTPLDWGQGTLVTVSSASTKGPSVFPLAPSSKST
ScFv SGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYS
LSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPP
CPAPELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKF
NWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEY
KCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLT
CLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTV
DKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSQ
VQLVESGGGVVQPGRSLRLSCAASGFTFSNYGMYWVRQAPGKGLE
-140-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
WVAVIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAEDT
AVYYCARDLWGWYFDWGQGTLVTVSSGGGGSGGGGSGGGGSG
GGGSEIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQA
PRLLIYDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQR
RNWPLTFGGGTKVEIK
Patritumab HC DEC-205 517 QVQLVESGGGVVQPGRSLRLSCAASGFTFSNYGMWVRQAPGKGL
mAb with EWVAVIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAED
tumor mAb TAVYYCARDLWGWYFDWGQGTLVTVSSASTKGPS VFPLAPSSKS
ScFv TSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLY
SLSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCP
PCPAPELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKF
NWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEY
KCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLT
CLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTV
DKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSQ
VQLQQWGAGLLKPSETLSLTCAVYGGSFSGYWSWIRQPPGKGLE
WIGEINHSGSTNYNPSLKSRVTISVETSKNQFSLKLSSVTAADTAVY
YCARDKWTWYFDLWGRGTLVTVSSGGGGSGGGGSGGGGSGGGGS
DIEMTQSPDSLAVSLGERATINCRSSQSVLYSSSNRNYLAWYQQNP
GQPPKLLIYWASTRESGVPDRFSGSGSGTDFTLTISSLQAEDVAVYY
CQQYYSTPRTFGQGTKVEIK
HC tumor 518 QVQLQQWGAGLLKPSETLSLTCAVYGGSFSGYWSWIRQPPGKGL
mAb with EWIGEINHSGSTNYNPSLKSRVTISVETSKNQFSLKLSSVTAADTAV
DEC-205 mAb YYCARDKWTWYFDLWGRGTLVTVSSASTKGPSVFPLAPSSKSTSG
ScFv GTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLS
SVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCP
APELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNW
YVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKC
KVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLTCL
VKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTVDK
SRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSQVQ
LVESGGGVVQPGRSLRLSCAASGFTFSNYGMYWVRQAPGKGLEW
VAVIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAEDTA
VYYCARDLWGWYFDWGQGTLVTVSSGGGGSGGGGSGGGGSGG
GGSEIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAP
RLLIYDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRR
NWPLTFGGGTKVEIK
Clivatuzumab HC DEC-205 519 QVQLVESGGGVVQPGRSLRLSCAASGFTFSNYGMWVRQAPGKGL
mAb with EWVAVIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAED
tumor mAb TAVYYCARDLWGWYFDWGQGTLVTVSSASTKGPS VFPLAPSSKS
ScFv TSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLY
SLSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCP
PCPAPELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKF
NWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEY
KCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLT
CLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTV
DKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSQ
VQLQQSGAEVKKPGASVKVSCEASGYTFPSYVLHWVKQAPGQGLE
WIGYINPYNDGTQYNEKFKGKATLTRDTSINTAYMELSRLRSDDTA
VYYCARGFGGSYGFAWGQGTLVTVSSGGGGSGGGGSGGGGSGG
GGSDIQLTQSPSSLSASVGDRVTMTCSASSSVSSSYLYWYQQKPGK
APKLWIYSTSNLASGVPARFSGSGSGTDFTLTISSLQPEDSASYFCHQ
WNRYPYTFGGGTRLEIK
HC tumor 520 QVQLQQSGAEVKKPGASVKVSCEASGYTFPSYVLHWVKQAPGQGL
mAb with EWIGYINPYNDGTQYNEKFKGKATLTRDTSINTAYMELSRLRSDDT
DEC-205 mAb AVYYCARGFGGSYGFAWGQGTLVTVSSASTKGPSVFPLAPSSKST
ScFv SGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYS
LSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPP
CPAPELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKF
-141-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
NWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEY
KCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLT
CLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTV
DKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSQ
VQLVESGGGVVQPGRSLRLSCAASGFTFSNYGMYWVRQAPGKGLE
WVAVIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAEDT
AVYYCARDLWGWYFDWGQGTLVTVSSGGGGSGGGGSGGGGSG
GGGSEIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQA
PRLLIYDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQR
RNWPLTFGGGTKVEIK
Sofituzumab HC DEC-205 521 QVQLVESGGGVVQPGRSLRLSCAASGFTFSNYGMWVRQAPGKGL
mAb with EWVAVIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAED
tumor mAb TAVYYCARDLWGWYFDWGQGTLVTVSSASTKGPS VFPLAPSSKS
ScFv TSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLY
SLSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCP
PCPAPELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKF
NWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEY
KCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLT
CLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTV
DKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSE
VQLVESGGGLVQPGGSLRLSCAASGYSITNDYAWNWVRQAPGKGL
EWVGYISYSGYTTYNPSLKSRFTISRDTSKNTLYLQMNSLRAEDTAV
YYCARWTSGLDYWGQGTLVTVSSGGGGSGGGGSGGGGSGGGGSD
IQMTQSPSSLSASVGDRVTITCKASDLII-INWLAWYQQKPGKAPKLLI
YGATSLETGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCQQWTTP
FTFGQGTKVEIK
HC tumor 522 EVQLVESGGGLVQPGGSLRLSCAASGYSITNDYAWNWVRQAPGKG
mAb with LEWVGYISYSGYTTYNPSLKSRFTISRDTSKNTLYLQMNSLRAEDTA
DEC-205 mAb VYYCARWTSGLDWGQGTLVTVSSASTKGPSVFPLAPSSKSTSGGT
ScFv AALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSV
VTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAP
ELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWY
VDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKCK
VSNKALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLTCLV
KGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTVDKSR
WQQGNVFSCSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSQVQL
VESGGGVVQPGRSLRLSCAASGFTFSNYGMWVRQAPGKGLEWV
AVIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAEDTAV
YYCARDLWGWYFDWGQGTLVTVSSGGGGSGGGGSGGGGSGGG
GSEIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAPR
LLIYDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRRN
WPLTFGGGTKVEIK
Edrecolomab HC DEC-205 523 QVQLVESGGGVVQPGRSLRLSCAASGFTFSNYGMWVRQAPGKGL
mAb with EWVAVIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAED
tumor mAb TAVYYCARDLWGWYFDWGQGTLVTVSSASTKGPS VFPLAPSSKS
ScFv TSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLY
SLSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCP
PCPAPELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKF
NWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEY
KCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLT
CLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTV
DKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSQ
VQLQQSGAELVRPGTSVKVSCKASGYAFTNYLIEWVKQRPGQGLE
WIGVINPGSGGTNYNEKFKGKATLTADKSSSTAYMQLSSLTSDDSA
VYFCARDGPWFAWGQGTLVTVSAGGGGSGGGGSGGGGSGGGGS
NIVMTQSPKSMSMSVGERVTLTCKASENVVTYVSWYQQKPEQSPK
LLIYGASNRYTGVPDRFTGSGSATDFTLTISSVQAEDLADYHCGQGY
SYPYTFGGGTKLEIK
HC tumor 524 QVQLQQSGAELVRPGTSVKVSCKASGYAFTNYLIEWVKQRPGQGL
-142-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
mAb with EWIGVINPGSGGTNYNEKFKGKATLTADKSSSTAYMQLSSLTSDDS
DEC-205 mAb AVYFCARDGPWFAWGQGTLVTVSAASTKGPSVFPLAPSSKSTSGG
ScFv TAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSS
VVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCP
APELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNW
YVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKC
KVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLTCL
VKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTVDK
SRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSQVQ
LVESGGGVVQPGRSLRLSCAASGFTFSNYGMYWVRQAPGKGLEW
VAVIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAEDTA
VYYCARDLWGWYFDWGQGTLVTVSSGGGGSGGGGSGGGGSGG
GGSEIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAP
RLLIYDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRR
NWPLTFGGGTKVEIK
Adecatumum HC DEC-205 525 QVQLVESGGGVVQPGRSLRLSCAASGFTFSNYGMWVRQAPGKGL
ab mAb with EWVAVIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAED
tumor mAb TAVYYCARDLWGWYFDWGQGTLVTVSSASTKGPS VFPLAPSSKS
ScFv TSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLY
SLSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCP
PCPAPELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKF
NWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEY
KCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLT
CLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTV
DKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSE
VQLLESGGGVVQPGRSLRLSCAASGFTFSSYGMHWVRQAPGKGLE
WVAVISYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAEDT
AVYYCAKDMGWGSGWRPYYYYGMDVWGQGTTVTVSSGGGGSG
GGGSGGGGSGGGGSELQMTQSPSSLSASVGDRVTITCRTSQSISSYL
NWYQQKPGQPPKWYWASTRESGVPDRFSGSGSGTDFTLTISSLQP
EDSATYYCQQSYDIPYTFGQGTKLEIK
HC tumor 526 EVQLLESGGGVVQPGRSLRLSCAASGFTFSSYGMHWVRQAPGKGL
mAb with EWVAVISYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAED
DEC-205 mAb TAVYYCAKDMGWGSGWRPYYYYGMDVWGQGTTVTVSSASTKGP
ScFv SVFPLAPSSKSTSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTF
PAVLQSSGLYSLSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEP
KSCDKTHTCPPCPAPELLGGPSVFLFPPKPKDTLMISRTPEVTCVVV
DVSHEDPEVKFNWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVL
HQDWLNGKEYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSR
EEMTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSD
GSFFLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPG
GGGGSGGGGSQVQLVESGGGVVQPGRSLRLSCAASGFTFSNYGMY
WVRQAPGKGLEWVAVIWYDGSNKYYADSVKGRFTISRDNSKNTL
YLQMNSLRAEDTAVYYCARDLWGWYFDWGQGTLVTVSSGGGG
SGGGGSGGGGSGGGGSEIVLTQSPATLSLSPGERATLSCRASQSVSS
YLAWYQQKPGQAPRLLIYDASNRATGIPARFSGSGSGTDFTLTISSL
EPEDFAVYYCQQRRNWPLTFGGGTKVEIK
Anetumab HC DEC-205 527 QVQLVESGGGVVQPGRSLRLSCAASGFTFSNYGMWVRQAPGKGL
mAb with EWVAVIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAED
tumor mAb TAVYYCARDLWGWYFDWGQGTLVTVSSASTKGPS VFPLAPSSKS
ScFv TSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLY
SLSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCP
PCPAPELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKF
NWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEY
KCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLT
CLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTV
DKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSQ
VELVQSGAEVKKPGESLKISCKGSGYSFTSWIGWVRQAPGKGLE
WMGIIDPGDSRTRYSPSFQGQVTISADKSISTAYLQWSSLKASDTAM
-143-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
YYCARGQLYGGTYMDGWGQGTLVTVSSGGGGSGGGGSGGGGSG
GGGSDIALTQPASVSGSPGQSITISCTGTSSDIGGYNSVSWYQQHPGK
APKLMIYGVNNRPSGVSNRFSGSKSGNTASLTISGLQAEDEADYYCS
SYDIESATPVFGGGTKLTVL
HC tumor 528 QVELVQSGAEVKKPGESLKISCKGSGYSFTSWIGWVRQAPGKGLE
mAb with WMGIIDPGDSRTRYSPSFQGQVTISADKSISTAYLQWSSLKASDTAM
DEC-205 mAb YYCARGQLYGGTYMDGWGQGTLVTVSSASTKGPSVFPLAPSSKST
ScFv SGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYS
LSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPP
CPAPELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKF
NWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEY
KCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLT
CLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTV
DKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSQ
VQLVESGGGVVQPGRSLRLSCAASGFTFSNYGMYWVRQAPGKGLE
WVAVIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAEDT
AVYYCARDLWGWYFDWGQGTLVTVSSGGGGSGGGGSGGGGSG
GGGSEIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQA
PRLLIYDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQR
RNWPLTFGGGTKVEIK
huDS6 HC DEC-205 529 QVQLVESGGGVVQPGRSLRLSCAASGFTFSNYGMWVRQAPGKGL
mAb with EWVAVIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAED
tumor mAb TAVYYCARDLWGWYFDWGQGTLVTVSSASTKGPS VFPLAPSSKS
ScFv TSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLY
SLSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCP
PCPAPELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKF
NWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEY
KCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLT
CLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTV
DKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSQ
AQLVQSGAEVVKPGASVKMSCKASGYTFTSYNMHWVKQTPGQGL
EWIGYIYPGNGATNYNQKFQGKATLTADPSSSTAYMQISSLTSEDSA
VYFCARGDSVPFAWGQGTLVTVSAGGGGSGGGGSGGGGSGGGG
SEIVLTQSPATMSASPGERVTITCSAHSSVSFMHWFQQKPGTSPKLW
IYSTSSLASGVPARFGGSGSGTSYSLTISSMEAEDAATYYCQQRSSFP
LTFGAGTKLELK
HC tumor 530 QAQLVQSGAEVVKPGASVKMSCKASGYTFTSYNMHWVKQTPGQG
mAb with LEWIGYIYPGNGATNYNQKFQGKATLTADPSSSTAYMQISSLTSEDS
DEC-205 mAb AVYFCARGDS VPFAWGQGTLVTVSAASTKGPSVFPLAPSSKSTSG
ScFv GTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLS
SVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCP
APELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNW
YVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKC
KVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLTCL
VKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTVDK
SRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSQVQ
LVESGGGVVQPGRSLRLSCAASGFTFSNYGMYWVRQAPGKGLEW
VAVIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAEDTA
VYYCARDLWGWYFDWGQGTLVTVSSGGGGSGGGGSGGGGSGG
GGSEIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAP
RLLIYDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRR
NWPLTFGGGTKVEIK
Lifastuzumab HC DEC-205 531 QVQLVESGGGVVQPGRSLRLSCAASGFTFSNYGMWVRQAPGKGL
mAb with EWVAVIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAED
tumor mAb TAVYYCARDLWGWYFDWGQGTLVTVSSASTKGPS VFPLAPSSKS
ScFv TSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLY
SLSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCP
PCPAPELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKF
NWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEY
-144-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
KCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLT
CLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTV
DKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSE
VQLVESGGGLVQPGGSLRLSCAASGFSFSDFAMSWVRQAPGKGLE
WVATIGRVAFHTYYPDSMKGRFTISRDNSKNTLYLQMNSLRAEDT
AVYYCARHRGFDVGHFDFWGQGTLVTVSSGGGGSGGGGSGGGGS
GGGGSDIQMTQSPSSLSASVGDRVTITCRSSETLVHSSGNTYLEWYQ
QKPGKAPKLLIYRVSNRFSGVPSRFSGSGSGTDFTLTISSLQPEDFAT
YYCFQGSFNPLTFGQGTKVEIK
HC tumor 532 EVQLVESGGGLVQPGGSLRLSCAASGFSFSDFAMSWVRQAPGKGLE
mAb with WVATIGRVAFHTYYPDSMKGRFTISRDNSKNTLYLQMNSLRAEDT
DEC-205 mAb AVYYCARHRGFDVGHFDFWGQGTLVTVSSASTKGPSVFPLAPSSKS
ScFv TSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLY
SLSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCP
PCPAPELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKF
NWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEY
KCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLT
CLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTV
DKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSQ
VQLVESGGGVVQPGRSLRLSCAASGFTFSNYGMYWVRQAPGKGLE
WVAVIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAEDT
AVYYCARDLWGWYFDWGQGTLVTVSSGGGGSGGGGSGGGGSG
GGGSEIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQA
PRLLIYDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQR
RNWPLTFGGGTKVEIK
Sacituzumab HC DEC-205 533 QVQLVESGGGVVQPGRSLRLSCAASGFTFSNYGMWVRQAPGKGL
mAb with EWVAVIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAED
tumor mAb TAVYYCARDLWGWYFDWGQGTLVTVSSASTKGPS VFPLAPSSKS
ScFv TSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLY
SLSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCP
PCPAPELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKF
NWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEY
KCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLT
CLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTV
DKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSQ
VQLQQSGSELKKPGASVKVSCKASGYTFTNYGMNWVKQAPGQGL
KWMGWINTYTGEPTYTDDFKGRFAFSLDTSVSTAYLQISSLKADDT
AVYFCARGGFGSSWYFDVWGQGSLVTVSSGGGGSGGGGSGGGG
SGGGGSDIQLTQSPSSLSASVGDRVSITCKASQDVSIAVAWYQQKPG
KAPKLLIYSASYRYTGVPDRFSGSGSGTDFTLTISSLQPEDFAVYYCQ
QHYITPLTFGAGTKVEIK
HC tumor 534 QVQLQQSGSELKKPGASVKVSCKASGYTFTNYGMNWVKQAPGQG
mAb with LKWMGWINTYTGEPTYTDDFKGRFAFSLDTSVSTAYLQISSLKADD
DEC-205 mAb TAVYFCARGGFGSSYWYFDVWGQGSLVTVSSASTKGPSVFPLAPSS
ScFv KSTSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSG
LYSLSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHT
CPPCPAPELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEV
KFNWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGK
EYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVS
LTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLT
VDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGGGGGSGGGGS
QVQLVESGGGVVQPGRSLRLSCAASGFTFSNYGMWVRQAPGKGL
EWVAVIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAED
TAVYYCARDLWGWYFDWGQGTLVTVSSGGGGSGGGGSGGGGS
GGGGSEIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQ
APRLLIYDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQ
RRNWPLTFGGGTKVEIK
PR1A3 HC DEC-205 535 QVQLVESGGGVVQPGRSLRLSCAASGFTFSNYGMWVRQAPGKGL
mAb with EWVAVIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAED
-145-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
tumor mAb TAVYYCARDLWGWYFDWGQGTLVTVSSASTKGPS VFPLAPSSKS
ScFv TSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLY
SLSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCP
PCPAPELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKF
NWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEY
KCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLT
CLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTV
DKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSQ
VKLQQSGPELKKPGETVKISCKASGYTFTEFGMNWVKQAPGKGLK
WMGWINTKTGEATYVEEFKGRFAFSLETSATTAYLQINNLKNEDTA
KYFCARWDFYDYVEAMDWGQGTTVTVSSGGGGSGGGGSGGGG
SGGGGSDIVMTQSQRFMSTSVGDRVSVTCKASQNVGTNVAWYQQ
KPGQSPKALIYSASYRYSGVPDRFTGSGSGTDFTLTISNVQSEDLAE
YFCHQYYTYPLFTFGSGTKLEMK
HC tumor 536 QVKLQQSGPELKKPGETVKISCKASGYTFTEFGMNWVKQAPGKGL
mAb with KWMGWINTKTGEATYVEEFKGRFAFSLETSATTAYLQINNLKNEDT
DEC-205 mAb AKYFCARWDFYDYVEAMDWGQGTTVTVSSASTKGPSVFPLAPSS
ScFv KSTSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSG
LYSLSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHT
CPPCPAPELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEV
KFNWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGK
EYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVS
LTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLT
VDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGGGGGSGGGGS
QVQLVESGGGVVQPGRSLRLSCAASGFTFSNYGMWVRQAPGKGL
EWVAVIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAED
TAVYYCARDLWGWYFDWGQGTLVTVSSGGGGSGGGGSGGGGS
GGGGSEIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQ
APRLLIYDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQ
RRNWPLTFGGGTKVEIK
Humanized HC DEC-205 818 QVQLVESGGGVVQPGRSLRLSCAASGFTFSNYGMWVRQAPGKGL
PR1A3 mAb with EWVAVIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAED
tumor mAb TAVYYCARDLWGWYFDWGQGTLVTVSSASTKGPS VFPLAPSSKS
ScFv TSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLY
SLSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCP
PCPAPELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVK
FNWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKE
YKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSL
TCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTV
DKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGGGGGSGGGGS
QVQLVQSGAEVKKPGASVKVSCKASGYTFTEFGMNWVRQAPGQG
LEWMGWINTKTGEATYVEEFKGRVTFTTDTSTSTAYMELRSLRSD
DTAVYYCARWDFAYYVEAMDYWGQGTTVTVSSGGGGSGGGGSG
GGGSGGGGSDIQMTQSPSSLSASVGDRVTITCKASAAVGTYVAWY
QQKPGKAPKWYSASYRKRGVPSRFSGSGSGTDFTLTISSLQPEDFA
TYYCHQYYTYPLFTFGQGTKLEIK
HC tumor 819 QVQLVQSGAEVKKPGASVKVSCKASGYTFTEFGMNWVRQAPGQG
mAb with LEWMGWINTKTGEATYVEEFKGRVTFTTDTSTSTAYMELRSLRSD
DEC-205 mAb DTAVYYCARWDFAYYVEAMDYWGQGTTVTVSSASTKGPSVFPLA
ScFv PSSKSTSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQ
SSGLYSLSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDK
THTCPPCPAPELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHE
DPEVKFNWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDW
LNGKEYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREEMTK
NQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFL
YSKLTVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGGGGGS
GGGGSQVQLVESGGGVVQPGRSLRLSCAASGFTFSNYGMWVRQ
APGKGLEWVAVIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQM
NSLRAEDTAVYYCARDLWGWYFDYWGQGTLVTVSSGGGGSGGG
-146-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
GSGGGGSGGGGSEIVLTQSPATLSLSPGERATLSCRASQSVSSYLAW
YQQKPGQAPRLLIYDASNRATGIPARFSGSGSGTDFTLTISSLEPEDF
AVYYCQQRRNWPLTFGGGTKVEIK
Humanized HC DEC-205 836 QVQLVESGGGVVQPGRSLRLSCAASGFTFSNYGMWVRQAPGKGL
Ab2-3 mAb with EWVAVIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAED
tumor mAb TAVYYCARDLWGWYFDWGQGTLVTVSSASTKGPS VFPLAPSSKS
ScFv TSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLY
SLSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCP
PCPAPELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVK
FNWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKE
YKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSL
TCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTV
DKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSE
VQLQESGPGLVKPGGSLSLSCAASGFVFSSYDMSWVRQTPERGLEW
VAYISSGGGITYAPSTVKGRFTVSRDNAKNTLYLQMNSLTSEDTAV
YYCAAHYFGSSGPFAYWGQGTLVTVSSGGGGSGGGGSGGGGSGG
GGSDIQMTQSPASLSASVGDRVTITCRASENIFSYLAWYQQKPGKSP
KLLVYNTRTLAEGVPSRFSGSGSGTDFSLTISSLQPEDFATYYCQHH
YGTPFTFGSGTKLEIK
HC tumor 837 EVQLQESGPGLVKPGGSLSLSCAASGFVFSSYDMSWVRQTPERGLE
mAb with WVAYISSGGGITYAPSTVKGRFTVSRDNAKNTLYLQMNSLTSEDTA
DEC-205 mAb VYYCAAHYFGSSGPFAWGQGTLVTVSSASTKGPSVFPLAPSSKST
ScFv SGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYS
LSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPP
CPAPELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKF
NWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEY
KCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLT
CLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTV
DKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGGGGGSGGGGS
QVQLVESGGGVVQPGRSLRLSCAASGFTFSNYGMWVRQAPGKGL
EWVAVIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAED
TAVYYCARDLWGWYFDWGQGTLVTVSSGGGGSGGGGSGGGGS
GGGGSEIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQ
APRLLIYDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQ
RRNWPLTFGGGTKVEIK
IMAB362, HC DEC-205 537 QVQLVESGGGVVQPGRSLRLSCAASGFTFSNYGMWVRQAPGKGL
CLAUDIXIM mAb with EWVAVIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAED
AB tumor mAb TAVYYCARDLWGWYFDWGQGTLVTVSSASTKGPS VFPLAPSSKS
ScFv TSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLY
SLSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCP
PCPAPELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKF
NWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEY
KCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLT
CLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTV
DKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSQ
VQLQQPGAELVRPGASVKLSCKASGYTFTSWINWVKQRPGQGLE
WIGNIYPSDSYTNYNQKFKDKATLTVDKSSSTAYMQLSSPTSEDSA
VYYCTRSWRGNSFDYWGQGTTLTVSSGGGGSGGGGSGGGGSGGG
GSDIVMTQSPSSLTVTAGEKVTMSCKSSQSLLNSGNQKNYLTWYQQ
KPGQPPKLLIWASTRESGVPDRFTGSGSGTDFTLTISSVQAEDLAV
YYCQNDYSYPFTFGSGTKLEIK
HC tumor 538 QVQLQQPGAELVRPGAS VKLSCKASGYTFTSWINWVKQRPGQGL
mAb with EWIGNIYPSDSYTNYNQKFKDKATLTVDKSSSTAYMQLSSPTSEDSA
DEC-205 mAb VYYCTRSWRGNSFDYWGQGTTLTVSSASTKGPSVFPLAPSSKSTSG
ScFv GTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLS
SVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCP
APELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNW
YVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKC
KVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLTCL
-147-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
VKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTVDK
SRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSQVQ
LVESGGGVVQPGRSLRLSCAASGFTFSNYGMYWVRQAPGKGLEW
VAVIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAEDTA
VYYCARDLWGWYFDWGQGTLVTVSSGGGGSGGGGSGGGGSGG
GGSEIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAP
RLLIYDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRR
NWPLTFGGGTKVEIK
AMG595 HC DEC-205 539 QVQLVESGGGVVQPGRSLRLSCAASGFTFSNYGMWVRQAPGKGL
mAb with EWVAVIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAED
tumor mAb TAVYYCARDLWGWYFDWGQGTLVTVSSASTKGPS VFPLAPSSKS
ScFv TSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLY
SLSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCP
PCPAPELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKF
NWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEY
KCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLT
CLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTV
DKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSQ
VQLVESGGGVVQSGRSLRLSCAASGFTFRNYGMHWVRQAPGKGLE
WVAVIWYDGSDKYYADSVRGRFTISRDNSKNTLYLQMNSLRAEDT
AVYYCARDGYDILTGNPRDFDWGQGTLVTVSSGGGGSGGGGSG
GGGSGGGGSDTVMTQTPLSSHVTLGQPASISCRSSQSLVHSDGNTY
LSWLQQRPGQPPRLLIYRISRRFSGVPDRFSGSGAGTDFTLEISRVEA
EDVGVYYCMQSTHVPRTFGQGTKVEIK
HC tumor 540 QVQLVESGGGVVQSGRSLRLSCAASGFTFRNYGMHWVRQAPGKG
mAb with LEWVAVIWYDGSDKYYADSVRGRFTISRDNSKNTLYLQMNSLRAE
DEC-205 mAb DTAVYYCARDGYDILTGNPRDFDYWGQGTLVTVSSASTKGPSVFPL
ScFv APSSKSTSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVL
QSSGLYSLSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCD
KTHTCPPCPAPELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHE
DPEVKFNWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDW
LNGKEYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREEMTK
NQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFL
YSKLTVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGGGGGS
GGGGSQVQLVESGGGVVQPGRSLRLSCAASGFTFSNYGMWVRQ
APGKGLEWVAVIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMN
SLRAEDTAVYYCARDLWGWYFDWGQGTLVTVSSGGGGSGGGGS
GGGGSGGGGSEIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQ
QKPGQAPRLLIYDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAV
YYCQQRRNWPLTFGGGTKVEIK
ABT806 HC DEC-205 541 QVQLVESGGGVVQPGRSLRLSCAASGFTFSNYGMWVRQAPGKGL
mAb with EWVAVIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAED
tumor mAb TAVYYCARDLWGWYFDWGQGTLVTVSSASTKGPS VFPLAPSSKS
ScFv TSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLY
SLSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCP
PCPAPELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKF
NWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEY
KCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLT
CLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTV
DKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSE
VQLQESGPGLVKPSQTLSLTCTVSGYSISRDFAWNWIRQPPGKGLE
WMGYISYNGNTRYQPSLKSRITISRDTSKNQFFLKLNSVTAADTATY
YCVTASRGFPWGQGTLVTVSSGGGGSGGGGSGGGGSGGGGSDIQ
MTQSPSSMSVSVGDRVTITCHSSQDINSNIGWLQQKPGKSFKGLIYH
GTNLDDGVPSRFSGSGSGTDYTLTISSLQPEDFATYYCVQYAQFPWT
FGGGTKLEIK
HC tumor 542 EVQLQESGPGLVKPSQTLSLTCTVSGYSISRDFAWNWIRQPPGKGLE
mAb with WMGYISYNGNTRYQPSLKSRITISRDTSKNQFFLKLNSVTAADTATY
DEC-205 mAb YCVTASRGFPWGQGTLVTVSSASTKGPSVFPLAPSSKSTSGGTAAL
-148-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
ScFv GCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTV
PSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPELL
GGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYVDG
VEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSN
KALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLTCLVKGF
YPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTVDKSRWQ
QGNVFSCSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSQVQLVES
GGGVVQPGRSLRLSCAASGFTFSNYGMWVRQAPGKGLEWVAVI
WYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAEDTAVYYC
ARDLWGWYFDWGQGTLVTVSSGGGGSGGGGSGGGGSGGGGSEI
VLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAPRLLIY
DASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRRNWPL
TFGGGTKVEIK
Sibrotuzumab HC DEC-205 543 QVQLVESGGGVVQPGRSLRLSCAASGFTFSNYGMWVRQAPGKGL
mAb with EWVAVIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAED
tumor mAb TAVYYCARDLWGWYFDWGQGTLVTVSSASTKGPS VFPLAPSSKS
ScFv TSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLY
SLSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCP
PCPAPELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKF
NWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEY
KCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLT
CLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTV
DKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSQ
VQLVQSGAEVKKPGASVKVSCKTSRYTFTEYTIHWVRQAPGQRLE
WIGGINPNNGIPNYNQKFKGRVTITVDTSASTAYMELSSLRSEDTAV
YYCARRRIAYGYDEGHAMDWGQGTLVTVSSGGGGSGGGGSGGG
GSGGGGSDIVMTQSPDSLAVSLGERATINCKSSQSLLYSRNQKNYL
AWYQQKPGQPPKLLIFWASTRESGVPDRFSGSGFGTDFTLTISSLQA
EDVAVYYCQQYFSYPLTFGQGTKVEIK
HC tumor 544 QVQLVQSGAEVKKPGASVKVSCKTSRYTFTEYTIHWVRQAPGQRL
mAb with EWIGGINPNNGIPNYNQKFKGRVTITVDTSASTAYMELSSLRSEDTA
DEC-205 mAb VYYCARRRIAYGYDEGHAMDWGQGTLVTVSSASTKGPSVFPLAP
ScFv SSKSTSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQS
SGLYSLSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKT
HTCPPCPAPELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDP
EVKFNWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLN
GKEYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQ
VSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSK
LTVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGGGGGSGGG
GSQVQLVESGGGVVQPGRSLRLSCAASGFTFSNYGMWVRQAPGK
GLEWVAVIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRA
EDTAVYYCARDLWGWYFDWGQGTLVTVSSGGGGSGGGGSGGG
GSGGGGSEIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKP
GQAPRLLIYDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYC
QQRRNWPLTFGGGTKVEIK
DS-8895a HC DEC-205 545 QVQLVESGGGVVQPGRSLRLSCAASGFTFSNYGMWVRQAPGKGL
variant 1 mAb with EWVAVIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAED
tumor mAb TAVYYCARDLWGWYFDWGQGTLVTVSSASTKGPS VFPLAPSSKS
ScFv TSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLY
SLSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCP
PCPAPELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKF
NWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEY
KCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLT
CLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTV
DKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSQ
VQLVQSGAEVKKPGASVKVSCKASGYTFIDYSMHWVRQAPGQGLE
WMGWINTYTGEPTYSDDFKGRVTITADTSTSTAYLELSSLRSEDTA
VYYCATYYRYERDFDYWGQGTLVTVSSGGGGSGGGGSGGGGSGG
GGSDIVMTQSPLSLPVTPGEPASISCRSSQSIVHSSGITYLEWYLQKP
-149-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
GQSPQLLIYKVSNRFSGVPDRFSGSGSGTDFTLKISRVEAEDVGVYY
CFQGSHVPYTFGQGTKVEIK
HC tumor 546 QVQLVQSGAEVKKPGASVKVSCKASGYTFIDYSMHWVRQAPGQG
mAb with LEWMGWINTYTGEPTYSDDFKGRVTITADTSTSTAYLELSSLRSEDT
DEC-205 mAb AVYYCATYYRYERDFDYWGQGTLVTVSSASTKGPSVFPLAPSSKST
ScFv SGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYS
LSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPP
CPAPELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKF
NWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEY
KCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLT
CLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTV
DKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSQ
VQLVESGGGVVQPGRSLRLSCAASGFTFSNYGMYWVRQAPGKGLE
WVAVIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAEDT
AVYYCARDLWGWYFDWGQGTLVTVSSGGGGSGGGGSGGGGSG
GGGSEIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQA
PRLLIYDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQR
RNWPLTFGGGTKVEIK
DS-8895a HC DEC-205 547 QVQLVESGGGVVQPGRSLRLSCAASGFTFSNYGMWVRQAPGKGL
variant 2 mAb with EWVAVIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAED
tumor mAb TAVYYCARDLWGWYFDWGQGTLVTVSSASTKGPS VFPLAPSSKS
ScFv TSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLY
SLSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCP
PCPAPELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKF
NWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEY
KCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLT
CLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTV
DKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSQ
IQLVQSGAEVKKPGASVKVSCKASGYTFIDYSMHWVRQAPGQGLK
WMGWINTYTGEPTYSDDFKGRFAFSLDTSTSTAYLELSSLRSEDTA
VYYCATYYRYERDFDYWGQGTLVTVSSGGGGSGGGGSGGGSGGG
GSDVLMTQSPLSLPVTPGEPASISCRSSQSIVHSSGITYLEWYLQKPG
QSPQLLIYKVSNRFSGVPDRFSGSGSGTDFTLKISRVEAEDVGVYYC
FQGSHVPYTFGQGTKVEIK
HC tumor 548 QIQLVQSGAEVKKPGASVKVSCKASGYTFIDYSMHWVRQAPGQGL
mAb with KWMGWINTYTGEPTYSDDFKGRFAFSLDTSTSTAYLELSSLRSEDT
DEC-205 mAb AVYYCATYYRYERDFDYWGQGTLVTVSSASTKGPSVFPLAPSSKST
ScFv SGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYS
LSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPP
CPAPELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKF
NWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEY
KCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLT
CLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTV
DKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSQ
VQLVESGGGVVQPGRSLRLSCAASGFTFSNYGMYWVRQAPGKGLE
WVAVIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAEDT
AVYYCARDLWGWYFDWGQGTLVTVSSGGGGGSGGGGSGGGSG
GGGSEIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQA
PRLLIYDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQR
RNWPLTFGGGTKVEIK
MEDI-547 HC DEC-205 549 QVQLVESGGGVVQPGRSLRLSCAASGFTFSNYGMWVRQAPGKGL
mAb with EWVAVIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAED
tumor mAb TAVYYCARDLWGWYFDWGQGTLVTVSSASTKGPS VFPLAPSSKS
ScFv TSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLY
SLSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCP
PCPAPELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKF
NWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEY
KCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLT
CLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTV
-150-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
DKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSE
VQLLESGGGLVQPGGSLRLSCAASGFTFSHYMMAWVRQAPGKGLE
WVSRIGPSGGPTHYADSVKGRFTISRDNSKNTLYLQMNSLRAEDTA
VYYCAGYDSGYDYVAVAGPAEYFQHWGQGTLVTVSSGGGGSGGG
GSGGGGSGGGGSDIQMTQSPSSLSASVGDRVTITCRASQSISTWLAW
YQQKPGKAPKLLIYKASNLHTGVPSRFSGSGSGTEFSLTISGLQPDDF
ATYYCQQYNSYSRTFGQGTKVEIK
HC tumor 550 EVQLLESGGGLVQPGGSLRLSCAASGFTFSHYMMAWVRQAPGKGL
mAb with EWVSRIGPSGGPTHYADSVKGRFTISRDNSKNTLYLQMNSLRAEDT
DEC-205 mAb AVYYCAGYDSGYDYVAVAGPAEYFQHWGQGTLVTVSSASTKGPS
ScFv VFPLAPSSKSTSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFP
AVLQSSGLYSLSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPK
SCDKTHTCPPCPAPELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVD
VSHEDPEVKFNWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLH
QDWLNGKEYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSRE
EMTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDG
SFFLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGG
GGGSGGGGSQVQLVESGGGVVQPGRSLRLSCAASGFTFSNYGMYW
VRQAPGKGLEWVAVIWYDGSNKYYADSVKGRFTISRDNSKNTLYL
QMNSLRAEDTAVYYCARDLWGWYFDYWGQGTLVTVSSGGGGSG
GGGSGGGGSGGGGSEIVLTQSPATLSLSPGERATLSCRASQSVSSYL
AWYQQKPGQAPRLLIYDASNRATGIPARFSGSGSGTDFTLTISSLEPE
DFAVYYCQQRRNWPLTFGGGTKVEIK
Narnatumab HC DEC-205 551 QVQLVESGGGVVQPGRSLRLSCAASGFTFSNYGMWVRQAPGKGL
mAb with EWVAVIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAED
tumor mAb TAVYYCARDLWGWYFDWGQGTLVTVSSASTKGPS VFPLAPSSKS
ScFv TSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLY
SLSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCP
PCPAPELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKF
NWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEY
KCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLT
CLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTV
DKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSE
VQLVESGGGLVQPGGSLRLSCAASGFTFSSYLMTWVRQAPGKGLE
WVANIKQDGSEKYYVDSVKGRFTISRDNAKNSLNLQMNSLRAEDT
AVYYCTRDGYSSGRHYGMDVWGQGTTVIVSSGGGGSGGGGSGGG
GSGGGGSEIVLTQSPATLSLSPGERATLSCRASQSVSRYLAWYQQKP
GQAPRLLIYDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYC
QQRSNWPRTFGQGTKVEIK
HC tumor 552 EVQLVESGGGLVQPGGSLRLSCAASGFTFSSYLMTWVRQAPGKGLE
mAb with WVANIKQDGSEKYYVDSVKGRFTISRDNAKNSLNLQMNSLRAEDT
DEC-205 mAb AVYYCTRDGYSSGRHYGMDVWGQGTTVIVSSASTKGPSVFPLAPSS
ScFv KSTSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSG
LYSLSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHT
CPPCPAPELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEV
KFNWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGK
EYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVS
LTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLT
VDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGGGGGSGGGGS
QVQLVESGGGVVQPGRSLRLSCAASGFTFSNYGMWVRQAPGKGL
EWVAVIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAED
TAVYYCARDLWGWYFDWGQGTLVTVSSGGGGSGGGGSGGGGS
GGGGSEIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQ
APRLLIYDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQ
RRNWPLTFGGGTKVEIK
RG7841 HC DEC-205 553 QVQLVESGGGVVQPGRSLRLSCAASGFTFSNYGMWVRQAPGKGL
mAb with EWVAVIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAED
tumor mAb TAVYYCARDLWGWYFDWGQGTLVTVSSASTKGPS VFPLAPSSKS
ScFv TSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLY
-151-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
SLSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCP
PCPAPELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKF
NWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEY
KCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLT
CLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTV
DKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSE
VQLVESGPALVKPTQTLTLTCTVSGFSLTGYSVNWIRQPPGKALEW
LGMIWGDGSTDYNSALKSRLTISKDTSKNQVVLTMTNMDPVDTAT
YYCARDYYFNYASWFAYWGQGTLVTVSSGGGGSGGGGSGGGGSG
GGGSDIQMTQSPSSLSASVGDRVTITCSASQGISNYLNWYQQKPGKT
VKLLIYYTSNLHSGVPSRFSGSGSGTDYTLTISSLQPEDFATYYCQQY
SELPWTFGQGTKVEIK
HC tumor 554 EVQLVESGPALVKPTQTLTLTCTVSGFSLTGYSVNWIRQPPGKALE
mAb with WLGMIWGDGSTDYNSALKSRLTISKDTSKNQVVLTMTNMDPVDTA
DEC-205 mAb TYYCARDYYFNYASWFAWGQGTLVTVSSASTKGPS VFPLAPS S KS
ScFv TSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLY
SLSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCP
PCPAPELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKF
NWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEY
KCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLT
CLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTV
DKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSQ
VQLVESGGGVVQPGRSLRLSCAASGFTFSNYGMYWVRQAPGKGLE
WVAVIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAEDT
AVYYCARDLWGWYFDWGQGTLVTVSSGGGGSGGGGSGGGGSG
GGGSEIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQA
PRLLIYDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQR
RNWPLTFGGGTKVEIK
Farletuzumab HC DEC-205 555 QVQLVESGGGVVQPGRSLRLSCAASGFTFSNYGMWVRQAPGKGL
mAb with EWVAVIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAED
tumor mAb TAVYYCARDLWGWYFDWGQGTLVTVSSASTKGPS VFPLAPS S KS
ScFv TSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLY
SLSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCP
PCPAPELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKF
NWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEY
KCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLT
CLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTV
DKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSE
VQLVESGGGVVQPGRSLRLSCSASGFTFSGYGLSWVRQAPGKGLE
WVAMISSGGSYTYYADSVKGRFAISRDNAKNTLFLQMDSLRPEDTG
VYFCARHGDDPAWFAYWGQGTPVTVSSGGGGSGGGGSGGGGSGG
GGSDIQLTQSPSSLSASVGDRVTITCSVSSSISSNNLHWYQQKPGKAP
KPWIYGTSNLASGVPSRFSGSGSGTDYTFTISSLQPEDIATYYCQQW
SSYPYMYTFGQGTKVEIK
HC tumor 556 EVQLVESGGGVVQPGRSLRLSCSASGFTFSGYGLSWVRQAPGKGLE
mAb with WVAMISSGGSYTYYADSVKGRFAISRDNAKNTLFLQMDSLRPEDTG
DEC-205 mAb VYFCARHGDDPAWFAYWGQGTPVTVSSASTKGPSVFPLAPSSKSTS
ScFv GGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSL
S SVVTVPS S SLGTQTYICNVNHKPSNTKVDKKVEPKS CDKTHTCPPC
PAPELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFN
WYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYK
CKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLTC
LVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTVD
KSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSQV
QLVESGGGVVQPGRSLRLSCAASGFTFSNYGMYWVRQAPGKGLE
WVAVIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAEDT
AVYYCARDLWGWYFDWGQGTLVTVSSGGGGSGGGGSGGGGSG
GGGSEIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQA
PRLLIYDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQR
-152-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
RNWPLTFGGGTKVEIK
Mirvetuximab HC DEC-205 557 QVQLVESGGGVVQPGRSLRLSCAASGFTFSNYGMWVRQAPGKGL
mAb with EWVAVIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAED
tumor mAb TAVYYCARDLWGWYFDWGQGTLVTVSSASTKGPS VFPLAPSSKS
ScFv TSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLY
SLSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCP
PCPAPELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKF
NWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEY
KCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLT
CLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTV
DKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSQ
VQLVQSGAEVVKPGASVKISCKASGYTFTGYFMNWVKQSPGQSLE
WIGRIHPYDGDTFYNQKFQGKATLTVDKSSNTAHMELLSLTSEDFA
VYYCTRYDGSRAMDWGQGTTVTVSSGGGGSGGGGSGGGGSGGG
GSDIVLTQSPLSLAVSLGQPAIISCKASQSVSFAGTSLMHWYHQKPG
QQPRLLIYRASNLEAGVPDRFSGSGSKTDFTLTISPVEAEDAATYYC
QQSREYPYTFGGGTKLEIK
HC tumor 558 QVQLVQSGAEVVKPGASVKISCKASGYTFTGYFMNWVKQSPGQSL
mAb with EWIGRIHPYDGDTFYNQKFQGKATLTVDKSSNTAHMELLSLTSEDF
DEC-205 mAb AVYYCTRYDGSRAMDWGQGTTVTVSSASTKGPSVFPLAPSSKSTS
ScFv GGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSL
SSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPC
PAPELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFN
WYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYK
CKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLTC
LVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTVD
KSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSQV
QLVESGGGVVQPGRSLRLSCAASGFTFSNYGMYWVRQAPGKGLE
WVAVIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAEDT
AVYYCARDLWGWYFDWGQGTLVTVSSGGGGSGGGGSGGGGSG
GGGSEIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQA
PRLLIYDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQR
RNWPLTFGGGTKVEIK
J591 variant 1 HC DEC-205 559 QVQLVESGGGVVQPGRSLRLSCAASGFTFSNYGMWVRQAPGKGL
mAb with EWVAVIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAED
tumor mAb TAVYYCARDLWGWYFDWGQGTLVTVSSASTKGPS VFPLAPSSKS
ScFv TSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLY
SLSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCP
PCPAPELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKF
NWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEY
KCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLT
CLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTV
DKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSE
VQLQQSGPELKKPGTSVRISCKTSGYTFTEYTIHWVKQSHGKSLEWI
GNINPNNGGTTYNQKFEDKATLTVDKSSSTAYMELRSLTSEDSAVY
YCAAGWNFDWGQGTTLTVSSGGGGSGGGGSGGGGSGGGGSDIV
MTQSHKFMSTSVGDRVSIICKASQDVGTAVDWYQQKPGQSPKLLIY
WASTRHTGVPDRFTGSGSGTDFTLTITNVQSEDLADYFCQQYNSYP
LTFGAGTMLDLK
HC tumor 560 EVQLQQSGPELKKPGTSVRISCKTSGYTFTEYTIHWVKQSHGKSLE
mAb with WIGNINPNNGGTTYNQKFEDKATLTVDKSSSTAYMELRSLTSEDSA
DEC-205 mAb VYYCAAGWNFDWGQGTTLTVSSASTKGPSVFPLAPSSKSTSGGTA
ScFv ALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVV
TVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPE
LLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYV
DGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKV
SNKALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLTCLVK
GFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTVDKSR
WQQGNVFSCSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSQVQL
-153-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
VESGGGVVQPGRSLRLSCAASGFTFSNYGMWVRQAPGKGLEWV
AVIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAEDTAV
YYCARDLWGWYFDWGQGTLVTVSSGGGGSGGGGSGGGGSGGG
GSEIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAPR
LLIYDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRRN
WPLTFGGGTKVEIK
J591 variant 2 HC DEC-205 561 QVQLVESGGGVVQPGRSLRLSCAASGFTFSNYGMWVRQAPGKGL
mAb with EWVAVIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAED
tumor mAb TAVYYCARDLWGWYFDWGQGTLVTVSSASTKGPS VFPLAPSSKS
ScFv TSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLY
SLSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCP
PCPAPELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKF
NWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEY
KCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLT
CLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTV
DKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSE
VQLQQSGPELVKPGTSVRISCKTSGYTFTEYTIHWVKQSHGKSLEWI
GNINPNNGGTTYNQKFEDKATLTVDKSSSTAYMELRSLTSEDSAVY
YCAAGWNFDWGQGTTLTVSSGGGGSGGGGSGGGGSGGGGSNIV
MTQSPKSMSMSVGERVTLTCKASENVVTYVSWYQQKPEQSPKLLI
YGASNRYTGVPDRFTGSGSATDFTLTISSVQAEDLADYHCGQGYSY
PYTFGGGTKLEIK
HC tumor 562 EVQLQQSGPELVKPGTSVRISCKTSGYTFTEYTIHWVKQSHGKSLE
mAb with WIGNINPNNGGTTYNQKFEDKATLTVDKSSSTAYMELRSLTSEDSA
DEC-205 mAb VYYCAAGWNFDWGQGTTLTVSSASTKGPSVFPLAPSSKSTSGGTA
ScFv ALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVV
TVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPE
LLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYV
DGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKV
SNKALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLTCLVK
GFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTVDKSR
WQQGNVFSCSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSQVQL
VESGGGVVQPGRSLRLSCAASGFTFSNYGMWVRQAPGKGLEWV
AVIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAEDTAV
YYCARDLWGWYFDWGQGTLVTVSSGGGGSGGGGSGGGGSGGG
GSEIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAPR
LLIYDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRRN
WPLTFGGGTKVEIK
Rovalpituzum HC DEC-205 563 QVQLVESGGGVVQPGRSLRLSCAASGFTFSNYGMWVRQAPGKGL
ab mAb with EWVAVIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAED
tumor mAb TAVYYCARDLWGWYFDWGQGTLVTVSSASTKGPS VFPLAPSSKS
ScFv TSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLY
SLSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCP
PCPAPELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKF
NWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEY
KCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLT
CLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTV
DKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSQ
VQLVQSGAEVKKPGASVKVSCKASGYTFTNYGMNWVRQAPGQGL
EWMGWINTYTGEPTYADDFKGRVTMTTDTSTSTAYMELRSLRSDD
TAVYYCARIGDSSPSDYWGQGTLVTVSSGGGGSGGGGSGGGGSGG
GGSEIVMTQSPATLSVSPGERATLSCKASQSVSNDVVWYQQKPGQA
PRLLIYYASNRYTGIPARFSGSGSGTEFTLTISSLQSEDFAVYYCQQD
YTSPWTFGQGTKLEIK
HC tumor 564 QVQLVQSGAEVKKPGASVKVSCKASGYTFTNYGMNWVRQAPGQG
mAb with LEWMGWINTYTGEPTYADDFKGRVTMTTDTSTSTAYMELRSLRSD
DEC-205 mAb DTAVYYCARIGDSSPSDYWGQGTLVTVSSASTKGPSVFPLAPSSKST
ScFv SGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYS
LSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPP
-154-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
CPAPELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKF
NWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEY
KCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLT
CLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTV
DKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSQ
VQLVESGGGVVQPGRSLRLSCAASGFTFSNYGMYWVRQAPGKGLE
WVAVIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAEDT
AVYYCARDLWGWYFDWGQGTLVTVSSGGGGSGGGGSGGGGSG
GGGSEIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQA
PRLLIYDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQR
RNWPLTFGGGTKVEIK
PF-06647020 HC DEC-205 565 QVQLVESGGGVVQPGRSLRLSCAASGFTFSNYGMWVRQAPGKGL
mAb with EWVAVIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAED
tumor mAb TAVYYCARDLWGWYFDWGQGTLVTVSSASTKGPS VFPLAPS S KS
ScFv TSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLY
SLSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCP
PCPAPELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKF
NWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEY
KCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLT
CLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTV
DKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSQ
VQLVQSGPEVKKPGAS VKVSCKASGYTFTDYAVHWVRQAPGKRLE
WIGVISTYNDYTYNNQDFKGRVTMTRDTSASTAYMELSRLRSEDTA
VYYCARGNSYFYALDYWGQGTSVTVSSGGGGSGGGGSGGGGSGG
GGSEIVLTQSPATLSLSPGERATLSCRASESVDSYGKSFMHWYQQKP
GQAPRLLIYRASNLESGIPARFSGSGSGTDFTLTISSLEPEDFAVYYC
QQSNEDPWTFGGGTKLEIK
HC tumor 566 QVQLVQSGPEVKKPGASVKVSCKASGYTFTDYAVHWVRQAPGKR
mAb with LEWIGVISTYNDYTYNNQDFKGRVTMTRDTSASTAYMELSRLRSED
DEC-205 mAb TAVYYCARGNSYFYALDWGQGTSVTVSSASTKGPS VFPLAPS S KS
ScFv TSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLY
SLSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCP
PCPAPELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKF
NWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEY
KCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLT
CLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTV
DKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSQ
VQLVESGGGVVQPGRSLRLSCAASGFTFSNYGMYWVRQAPGKGLE
WVAVIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAEDT
AVYYCARDLWGWYFDWGQGTLVTVSSGGGGSGGGGSGGGGSG
GGGSEIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQA
PRLLIYDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQR
RNWPLTFGGGTKVEIK
Antibody to HC DEC-205 567 QVQLVESGGGVVQPGRSLRLSCAASGFTFSNYGMWVRQAPGKGL
PTK7 mAb with EWVAVIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAED
tumor mAb TAVYYCARDLWGWYFDWGQGTLVTVSSASTKGPS VFPLAPS S KS
ScFv TSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLY
SLSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCP
PCPAPELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKF
NWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEY
KCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLT
CLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTV
DKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSQ
VQLVESGGGVVQPGRSLRLSCAASGFTFSSYAFHWVRQAPGKGLE
WVAVISYDGSIKYYADSVKGRFTISRDNSKNTLYLQMNSLRAEDTA
VYYCARTYYFDWGQGTLVTVSSGGGGSGGGGSGGGGSGGGGSEI
VLTQSPDFQSVTPKEKVTITCRASQSIGSSLHWYQQKPDQSPKLLIK
YASQSFSGVPSRFSGSGSGTDFTLTINSLEAEDAAAYYCHQSSSLPIT
FGQGTRLEIK
-155-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
HC tumor 568 QVQLVESGGGVVQPGRSLRLSCAASGFTFSSYAFHWVRQAPGKGL
mAb with EWVAVISYDGSIKYYADSVKGRFTISRDNSKNTLYLQMNSLRAEDT
DEC-205 mAb AVYYCARTYYFDWGQGTLVTVSSASTKGPSVFPLAPSSKSTSGGT
ScFv AALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSV
VTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAP
ELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWY
VDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKCK
VSNKALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLTCLV
KGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTVDKSR
WQQGNVFSCSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSQVQL
VESGGGVVQPGRSLRLSCAASGFTFSNYGMWVRQAPGKGLEWV
AVIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAEDTAV
YYCARDLWGWYFDWGQGTLVTVSSGGGGSGGGGSGGGGSGGG
GSEIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAPR
LLIYDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRRN
WPLTFGGGTKVEIK
Ladiratuzuma HC DEC-205 569 QVQLVESGGGVVQPGRSLRLSCAASGFTFSNYGMWVRQAPGKGL
b mAb with EWVAVIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAED
tumor mAb TAVYYCARDLWGWYFDWGQGTLVTVSSASTKGPS VFPLAPSSKS
ScFv TSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLY
SLSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCP
PCPAPELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKF
NWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEY
KCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLT
CLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTV
DKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSQ
VQLVQSGAEVKKPGASVKVSCKASGLTIEDYYMHWVRQAPGQGL
EWMGWIDPENGDTEYGPKFQGRVTMTRDTSINTAYMELSRLRSDD
TAVYYCAVHNAHYGTWFAYWGQGTLVTVSSGGGGSGGGGSGGG
GSGGGGSDVVMTQSPLSLPVTLGQPASISCRSSQSLLHSSGNTYLEY
FQQRPGQSPRPLIYKISTRFSGVPDRFSGSGSGTDFTLKISRVEAEDV
GVYYCFQGSHVPYTFGGGTKVEIK
HC tumor 570 QVQLVQSGAEVKKPGASVKVSCKASGLTIEDYYMHWVRQAPGQG
mAb with LEWMGWIDPENGDTEYGPKFQGRVTMTRDTSINTAYMELSRLRSD
DEC-205 mAb DTAVYYCAVHNAHYGTWFAYWGQGTLVTVSSASTKGPSVFPLAPS
ScFv SKSTSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSS
GLYSLSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKT
HTCPPCPAPELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDP
EVKFNWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLN
GKEYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQ
VSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSK
LTVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGGGGGSGGG
GSQVQLVESGGGVVQPGRSLRLSCAASGFTFSNYGMWVRQAPGK
GLEWVAVIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRA
EDTAVYYCARDLWGWYFDWGQGTLVTVSSGGGGSGGGGSGGG
GSGGGGSEIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKP
GQAPRLLIYDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYC
QQRRNWPLTFGGGTKVEIK
Cirmtuzumab HC DEC-205 571 QVQLVESGGGVVQPGRSLRLSCAASGFTFSNYGMWVRQAPGKGL
mAb with EWVAVIWYDGSNKYYADSVKGRFTISRDNSKNTLY
tumor mAb LQMNSLRAEDTAVYYCARDLWGWYFDWGQGTLVTVSSASTKGP
ScFv SVFPLAPSSKSTSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTF
PAVLQSSGLYSLSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEP
KSCDKTHTCPPCPAPELLGGPSVFLFPPKPKDTLMISRTPEVTCVVV
DVSHEDPEVKFNWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVL
HQDWLNGKEYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSR
EEMTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSD
GSFFLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPG
GGGGSGGGGSQVQLQESGPGLVKPSQTLSLTCTVSGYAFTAYNIHW
-156-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
VRQAPGQGLEWMGSFDPYDGGSSYNQKFKDRLTISKDTSKNQVVL
TMTNMDPVDTATYYCARGWYYFDYWGHGTLVTVSSGGGGSGGG
GSGGGGSGGGGSDIVMTQTPLSLPVTPGEPASISCRASKSISKYLAW
YQQKPGQAPRLLIYSGSTLQSGIPPRFSGSGYGTDFTLTINNIESEDA
AYYFCQQHDESPY TFGEGTKVEIK
HC tumor 572 QVQLQESGPGLVKPSQTLSLTCTVSGYAFTAYNIHWVRQAPGQGLE
mAb with WMGSFDPYDGGSSYNQKFKDRLTISKDTSKNQVVLTMTNMDPVDT
DEC-205 mAb ATYYCARGWYYFDYWGHGTLVTVSSASTKGPSVFPLAPSSKSTSG
ScFv GTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLS
SVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCP
APELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNW
YVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKC
KVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLTCL
VKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTVDK
SRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSQVQ
LVESGGGVVQPGRSLRLSCAASGFTFSNYGMYWVRQAPGKGLEW
VAVIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAEDTA
VYYCARDLWGWYFDWGQGTLVTVSSGGGGSGGGGSGGGGSGG
GGSEIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAP
RLLIYDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRR
NWPLTFGGGTKVEIK
Antibody to HC DEC-205 573 QVQLVESGGGVVQPGRSLRLSCAASGFTFSNYGMWVRQAPGKGL
MAGE-A3 mAb with EWVAVIWYDGSNKYYADSVKGRFTISRDNSKNTLY
tumor mAb LQMNSLRAEDTAVYYCARDLWGWYFDWGQGTLVTVSSASTKGP
ScFv SVFPLAPSSKSTSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTF
PAVLQSSGLYSLSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEP
KSCDKTHTCPPCPAPELLGGPSVFLFPPKPKDTLMISRTPEVTCVVV
DVSHEDPEVKFNWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVL
HQDWLNGKEYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSR
EEMTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSD
GSFFLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPG
GGGGSGGGGSQVQLVESGGGVVQPGRSLRLSCTASGFRFRSHGMH
WVRQAPGKGLEWVAVISYDGNNKLYADSVKGRITISRDNSKNTLFL
QMNNVRAEDTAVYYCASPYTSDWQYFQYWGQGTLVIVSSGGGGS
GGGGSGGGGSGGGGSEIVMTQSPATLSVSPGERATFSCRASQNISTT
LAWYQQKPGQAPRLLIYDTSTRATGIPARFSGSGSGTEFTLTISSLQS
EDLAVYYCQQSNSWPLTFGGGTKVEIK
HC tumor 574 QVQLVESGGGVVQPGRSLRLSCTASGFRFRSHGMHWVRQAPGKGL
mAb with EWVAVISYDGNNKLYADSVKGRITISRDNSKNTLFLQMNNVRAEDT
DEC-205 mAb AVYYCASPYTSDWQYFQWGQGTLVIVSSASTKGPS VFPLAPSSKS
ScFv TSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLY
SLSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCP
PCPAPELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKF
NWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEY
KCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLT
CLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTV
DKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSQ
VQLVESGGGVVQPGRSLRLSCAASGFTFSNYGMYWVRQAPGKGLE
WVAVIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAEDT
AVYYCARDLWGWYFDWGQGTLVTVSSGGGGSGGGGSGGGGSG
GGGSEIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQA
PRLLIYDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQR
RNWPLTFGGGTKVEIK
Antibody to HC DEC-205 575 QVQLVESGGGVVQPGRSLRLSCAASGFTFSNYGMWVRQAPGKGL
NY-ESO-1 mAb with EWVAVIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAED
tumor mAb TAVYYCARDLWGWYFDWGQGTLVTVSSASTKGPS VFPLAPSSKS
ScFv TSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLY
SLSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCP
PCPAPELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKF
-157-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
NWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEY
KCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLT
CLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTV
DKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSQ
VQLVQSGGGVVRPGGSLRLSCAASGFSFIDYGMSWVRQVPGKGLE
WVAGMNWSGDKKGHAESVKGRFIISRDNAKNTLYLEMSSLRVEDT
ALYFCARGEYSNRFDPRGRGTLVTVSSGGGGSGGGGSGGGGSGGG
GSDIVMTQTPLSLPVTLGQPASLSCRSSQSLVFTDGNTYLNWFQQRP
GQSPRRLIYKVSSRDPGVPDRFSGTGSGTDFTLEISRVEAEDIGVYYC
MQGTHWPPIFGQGTKVEIK
HC tumor 576 QVQLVQSGGGVVRPGGSLRLSCAASGFSFIDYGMSWVRQVPGKGL
mAb with EWVAGMNWSGDKKGHAESVKGRFIISRDNAKNTLYLEMSSLRVED
DEC-205 mAb TALYFCARGEYSNRFDPRGRGTLVTVSSASTKGPSVFPLAPSSKSTS
ScFv GGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSL
SSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPC
PAPELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFN
WYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYK
CKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLTC
LVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTVD
KSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSQV
QLVESGGGVVQPGRSLRLSCAASGFTFSNYGMYWVRQAPGKGLE
WVAVIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAEDT
AVYYCARDLWGWYFDWGQGTLVTVSSGGGGSGGGGSGGGGSG
GGGSEIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQA
PRLLIYDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQR
RNWPLTFGGGTKVEIK
Trastuzumab HC DEC-205 686 QVQLVESGGGVVQPGRSLRLSCAASGFTFSNYGMWVRQAPGKGL
mAb with EWVAVIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAED
tumor mAb TAVYYCARDLWGWYFDWGQGTLVTVSSASTKGPS VFPLAPSSKS
ScFv TSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLY
SLSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCP
PCPAPELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKF
NWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEY
KCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLT
CLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTV
DKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSE
VQLVESGGGLVQPGGSLRLSCAASGFNIKDTYIHWVRQAPGKGLE
WVARIYPTNGYTRYADSVKGRFTISADTSKNTAYLQMNSLRAEDT
AVYYCSRWGGDGFYAMDYWGQGTLVTVSSGGGGSGGGGSGGGG
SGGGGSDIQMTQSPSSLSASVGDRVTITCRASQDVNTAVAWYQQKP
GKAPKLLIYSASFLYSGVPSRFSGSRSGTDFTLTISSLQPEDFATYYC
QQHYTTPPTFGQGTKVEIK
HC tumor 687 EVQLVESGGGLVQPGGSLRLSCAASGFNIKDTYIHWVRQAPGKGLE
mAb with WVARIYPTNGYTRYADSVKGRFTISADTSKNTAYLQMNSLRAEDT
DEC-205 mAb AVYYCSRWGGDGFYAMDYWGQGTLVTVSSASTKGPSVFPLAPSSK
ScFv STSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGL
YSLSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTC
PPCPAPELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVK
FNWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKE
YKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSL
TCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTV
DKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSQ
VQLVESGGGVVQPGRSLRLSCAASGFTFSNYGMYWVRQAPGKGLE
WVAVIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAEDT
AVYYCARDLWGWYFDWGQGTLVTVSSGGGGSGGGGSGGGGSG
GGGSEIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQA
PRLLIYDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQR
RNWPLTFGGGTKVEIK
HC DEC205 797 QVQLVESGGGVVQPGRSLRLSCAASGFTFSNYGMWVRQAPGKGL
-158-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
mAb with EWVAVIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAED
tumor mAb TAVYYCARDLWGWYFDWGQGTLVTVSSASTKGPSVFPLAPSSKS
ScFv TSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLY
(LH,25mer) SLSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCP
PCPAPELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKF
NWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEY
KCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLT
CLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTV
DKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSD
IQMTQSPSSLSASVGDRVTITCRASQDVNTAVAWYQQKPGKAPKLL
IYSASFLYSGVPSRFSGSRSGTDFTLTISSLQPEDFATYYCQQHYTTPP
TFGQGTKVEIKGGGGSGGGGSGGGGSGGGGSGGGGSEVQLVESGG
GLVQPGGSLRLSCAASGFNIKDTYIHWVRQAPGKGLEWVARIYPTN
GYTRYADSVKGRFTISADTSKNTAYLQMNSLRAEDTAVYYCSRWG
GDGFYAMDWGQGTLVTVSS
TABLE 9. Fusion Sequences ¨ CD40 fusions via the light chain
Antibody Region SEQ Sequence
ID
NO:
Pertuzumab LC CD40 766 DIQMTQSPSSVSASVGDRVTITCRASQGIYSWLAWYQQKPGKAPNL
mAb LIYTASTLQSGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCQQANIFP
containing LTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
tumor mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
ScFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSEVQLVESGG
GLVQPGGSLRLSCAASGFTFTDYTMDWVRQAPGKGLEWVADVNP
NSGGSIYNQRFKGRFTLSVDRSKNTLYLQMNSLRAEDTAVYYCAR
NLGPSFYFDWGQGTLVTVSSGGGGSGGGGSGGGGSGGGGSDIQM
TQSPSSLSASVGDRVTITCKASQDVSIGVAWYQQKPGKAPKLLIYSA
SYRYTGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCQQYYIYPYTFG
QGTKVEIK
LC tumor 23 DIQMTQSPSSLSASVGDRVTITCKASQDVSIGVAWYQQKPGKAPKL
mAb LIYSASYRYTGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCQQYYIY
containing PYTFGQGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYP
CD40 mAb REAKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADY
ScFv EKHKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLVQS
GAEVKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQGLEWMGW
INPDSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSDDTAVYYC
ARDQPLGYCTNGVCSYFDWGQGTLVTVSSGGGGSGGGGSGGGG
SGGGGSDIQMTQSPSSVSASVGDRVTITCRASQGIYSWLAWYQQKP
GKAPNLLIYTASTLQSGVPSRFSGSGSGTDFTLTISSLQPEDFATYYC
QQANIFPLTFGGGTKVEIK
Cetuximab LC CD40 703 DIQMTQSPSSVSASVGDRVTITCRASQGIYSWLAWYQQKPGKAPNL
mAb LIYTASTLQSGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCQQANIFP
containing LTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
tumor mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
ScFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLKQSGP
GLVQPSQSLSITCTVSGFSLTNYGVHWVRQSPGKGLEWLGVIWSGG
NTDYNTPFTSRLSINKDNSKSQVFFKMNSLQSNDTAIYYCARALTY
YDYEFAWGQGTLVTVSAGGGGSGGGGSGGGGSGGGGSDILLTQS
PVILSVSPGERVSFSCRASQSIGTNIHWYQQRTNGSPRLLIKYASESIS
GIPSRFSGSGSGTDFTLSINSVESEDIADYYCQQNNNWPTTFGAGTK
LELK
LC tumor 36
DILLTQSPVILSVSPGERVSFSCRASQSIGTNIHWYQQRTNGSPRLLIK
mAb YASESISGIPSRFSGSGSGTDFTLSINSVESEDIADYYCQQNNNWPTT
containing FGAGTKLELKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREA
CD40 mAb KVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEKH
ScFv KVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLVQSGAE
-159-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
VKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQGLEWMGWINP
DSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSDDTAVYYCAR
DQPLGYCTNGVCSYFDWGQGTLVTVSSGGGGSGGGGSGGGGSG
GGGSDIQMTQSPSSVSASVGDRVTITCRASQGIYSWLAWYQQKPGK
APNLLIYTASTLQSGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCQQ
ANIFPLTFGGGTKVEIK
Panitumumab LC CD40 760 DIQMTQSPSSVSASVGDRVTITCRASQGIYSWLAWYQQKPGKAPNL
mAb LIYTASTLQSGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCQQANIFP
containing LTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
tumor mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
ScFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLQESGP
GLVKPSETLSLTCTVSGGSVSSGDYWTWIRQSPGKGLEWIGHIYY
SGNTNYNPSLKSRLTISIDTSKTQFSLKLSSVTAADTAIYYCVRDRVT
GAFDIWGQGTMVTVSSGGGGSGGGGSGGGGSGGGGSDIQMTQSPS
SLSASVGDRVTITCQASQDISNYLNWYQQKPGKAPKLLIYDASNLE
TGVPSRFSGSGSGTDFTFTISSLQPEDIATYFCQHFDHLPLAFGGGTK
VEIK
LC tumor 49 DIQMTQSPSSLSASVGDRVTITCQASQDISNYLNWYQQKPGKAPKL
mAb LIYDASNLETGVPSRFSGSGSGTDFTFTISSLQPEDIATYFCQHFDHLP
containing LAFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPR
CD40 mAb EAKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYE
ScFv KHKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLVQSG
AEVKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQGLEWMGWI
NPDSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSDDTAVYYC
ARDQPLGYCTNGVCSYFDWGQGTLVTVSSGGGGSGGGGSGGGG
SGGGGSDIQMTQSPSSVSASVGDRVTITCRASQGIYSWLAWYQQKP
GKAPNLLIYTASTLQSGVPSRFSGSGSGTDFTLTISSLQPEDFATYYC
QQANIFPLTFGGGTKVEIK
Nimotuzumab LC CD40 751 DIQMTQSPSSVSASVGDRVTITCRASQGIYSWLAWYQQKPGKAPNL
mAb LIYTASTLQSGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCQQANIFP
containing LTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
tumor mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
ScFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLQQSGA
EVKKPGSSVKVSCKASGYTFTNYYIWVRQAPGQGLEWIGGINPTS
GGSNFNEKFKTRVTITVDESTNTAYMELSSLRSEDTAFYFCARQGL
WFDSDGRGFDFWGQGSTVTVSSGGGGSGGGGSGGGGSGGGGSDIQ
MTQSPSSLSASVGDRVTITCRSSQNIVHSNGNTYLDWYQQTPGKAP
KLLIYKVSNRFSGVPSRFSGSGSGTDFTFTISSLQPEDIATYYCFQYSH
VPWTFGQGTKLEIK
LC tumor 62 DIQMTQSPSSLSASVGDRVTITCRSSQNIVHSNGNTYLDWYQQTPG
mAb KAPKLLIYKVSNRFSGVPSRFSGSGSGTDFTFTISSLQPEDIATYYCFQ
containing YSHVPWTFGQGTKLEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLN
CD40 mAb NFYPREAKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSK
ScFv ADYEKHKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQL
VQSGAEVKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQGLEW
MGWINPDSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSDDTA
VYYCARDQPLGYCTNGVCSYFDWGQGTLVTVSSGGGGSGGGGS
GGGGSGGGGSDIQMTQSPSSVSASVGDRVTITCRASQGIYSWLAWY
QQKPGKAPNLLIYTASTLQSGVPSRFSGSGSGTDFTLTISSLQPEDFA
TYYCQQANIFPLTFGGGTKVEIK
Zalutumumab LC CD40 802 DIQMTQSPSSVSASVGDRVTITCRASQGIYSWLAWYQQKPGKAPNL
mAb LIYTASTLQSGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCQQANIFP
containing LTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
tumor mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
ScFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLVESGG
GVVQPGRSLRLSCAASGFTFSTYGMHWVRQAPGKGLEWVAVIWD
DGSYKYYGDSVKGRFTISRDNSKNTLYLQMNSLRAEDTAVYYCAR
DGITMVRGVMKDYFDYWGQGTLVTVSSGGGGSGGGGSGGGGSGG
GGSAIQLTQSPSSLSASVGDRVTITCRASQDISSALVWYQQKPGKAP
-160-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
KLLIYDASSLESGVPSRFSGSESGTDFTLTISSLQPEDFATYYCQQFNS
YPLTFGGGTKVEIK
LC tumor 75 AIQLTQSPSSLSASVGDRVTITCRASQDISSALVWYQQKPGKAPKLLI
mAb YDASSLESGVPSRFSGSESGTDFTLTISSLQPEDFATYYCQQFNSYPL
containing TFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
CD40 mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
ScFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLVQSGA
EVKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQGLEWMGWIN
PDSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSDDTAVYYCA
RDQPLGYCTNGVCSYFDWGQGTLVTVSSGGGGSGGGGSGGGGS
GGGGSDIQMTQSPSSVSASVGDRVTITCRASQGIYSWLAWYQQKPG
KAPNLLIYTASTLQSGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCQ
QANIFPLTFGGGTKVEIK
Onartuzumab LC CD40 757 DIQMTQSPSSVSASVGDRVTITCRASQGIYSWLAWYQQKPGKAPNL
mAb LIYTASTLQSGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCQQANIFP
containing LTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
tumor mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
ScFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSEVQLVESGG
GLVQPGGSLRLSCAASGYTFTSWLHWVRQAPGKGLEWVGMIDPS
NSDTRFNPNFKDRFTISADTSKNTAYLQMNSLRAEDTAVYYCATYR
SYVTPLDWGQGTLVTVSSGGGGSGGGGSGGGGSGGGGSDIQMTQ
SPSSLSASVGDRVTITCKSSQSLLYTSSQKNYLAWYQQKPGKAPKLL
IWASTRESGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCQQYYAY
PWTFGQGTKVEIK
LC tumor 88 DIQMTQSPSSLSASVGDRVTITCKSSQSLLYTSSQKNYLAWYQQKP
mAb GKAPKLLIYWASTRESGVPSRFSGSGSGTDFTLTISSLQPEDFATYYC
containing QQYYAYPWTFGQGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVC
CD40 mAb LLNNFYPREAKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLT
ScFv LSKADYEKHKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQ
VQLVQSGAEVKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQGL
EWMGWINPDSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSDD
TAVYYCARDQPLGYCTNGVCSYFDYWGQGTLVTVSSGGGGSGGG
GSGGGGSGGGGSDIQMTQSPSSVSASVGDRVTITCRASQGIYSWLA
WYQQKPGKAPNLLIYTASTLQSGVPSRFSGSGSGTDFTLTISSLQPED
FATYYCQQANIFPLTFGGGTKVEIK
Patritumab LC CD40 763 DIQMTQSPSSVSASVGDRVTITCRASQGIYSWLAWYQQKPGKAPNL
mAb LIYTASTLQSGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCQQANIFP
containing LTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
tumor mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
ScFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLQQWG
AGLLKPSETLSLTCAVYGGSFSGYWSWIRQPPGKGLEWIGEINHS
GSTNYNPSLKSRVTISVETSKNQFSLKLSSVTAADTAVYYCARDKW
TWYFDLWGRGTLVTVSSGGGGSGGGGSGGGGSGGGGSDIEMTQSP
DSLAVSLGERATINCRSSQSVLYSSSNRNYLAWYQQNPGQPPKLLIY
WASTRESGVPDRFSGSGSGTDFTLTISSLQAEDVAVYYCQQYYSTP
RTFGQGTKVEIK
LC tumor 101 DIEMTQSPDSLAVSLGERATINCRSSQSVLYSSSNRNYLAWYQQNP
mAb GQPPKLLIYWASTRESGVPDRFSGSGSGTDFTLTISSLQAEDVAVYY
containing CQQYYSTPRTFGQGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVC
CD40 mAb LLNNFYPREAKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLT
ScFv LSKADYEKHKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQ
VQLVQSGAEVKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQGL
EWMGWINPDSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSDD
TAVYYCARDQPLGYCTNGVCSYFDYWGQGTLVTVSSGGGGSGGG
GSGGGGSGGGGSDIQMTQSPSSVSASVGDRVTITCRASQGIYSWLA
WYQQKPGKAPNLLIYTASTLQSGVPSRFSGSGSGTDFTLTISSLQPED
FATYYCQQANIFPLTFGGGTKVEIK
Clivatuzumab LC CD40 709 DIQMTQSPSSVSASVGDRVTITCRASQGIYSWLAWYQQKPGKAPNL
mAb LIYTASTLQSGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCQQANIFP
-161-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
containing LTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
tumor mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
ScFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLQQSGA
EVKKPGASVKVSCEASGYTFPSYVLHWVKQAPGQGLEWIGYINPY
NDGTQYNEKFKGKATLTRDTSINTAYMELSRLRSDDTAVYYCARG
FGGSYGFAWGQGTLVTVSSGGGGSGGGGSGGGGSGGGGSDIQLT
QSPSSLSASVGDRVTMTCSASSSVSSSYLYWYQQKPGKAPKLWIYS
TSNLASGVPARFSGSGSGTDFTLTISSLQPEDSASYFCHQWNRYPYT
FGGGTRLEIK
LC tumor 114 DIQLTQSPSSLSASVGDRVTMTCSASSSVSSSYLYWYQQKPGKAPK
mAb LWIYSTSNLASGVPARFSGSGSGTDFTLTISSLQPEDSASYFCHQWN
containing RYPYTFGGGTRLEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNF
CD40 mAb YPREAKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKAD
ScFv YEKHKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLVQ
SGAEVKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQGLEWMG
WINPDSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSDDTAVY
YCARDQPLGYCTNGVCSYFDWGQGTLVTVSSGGGGSGGGGSGG
GGSGGGGSDIQMTQSPSSVSASVGDRVTITCRASQGIYSWLAWYQQ
KPGKAPNLLIYTASTLQSGVPSRFSGSGSGTDFTLTISSLQPEDFATY
YCQQANIFPLTFGGGTKVEIK
Sofituzumab LC CD40 790 DIQMTQSPSSVSASVGDRVTITCRASQGIYSWLAWYQQKPGKAPNL
mAb LIYTASTLQSGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCQQANIFP
containing LTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
tumor mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
ScFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSEVQLVESGG
GLVQPGGSLRLSCAASGYSITNDYAWNWVRQAPGKGLEWVGYISY
SGYTTYNPSLKSRFTISRDTSKNTLYLQMNSLRAEDTAVYYCARWT
SGLDWGQGTLVTVSSGGGGSGGGGSGGGGSGGGGSDIQMTQSPS
SLSASVGDRVTITCKASDLII-INWLAWYQQKPGKAPKWYGATSLE
TGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCQQWTTPFTFGQGT
KVEIK
LC tumor 127 DIQMTQSPSSLSASVGDRVTITCKASDLII-INWLAWYQQKPGKAPKL
mAb LIYGATSLETGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCQQWTT
containing PFTFGQGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPR
CD40 mAb EAKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYE
ScFv KHKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLVQSG
AEVKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQGLEWMGWI
NPDSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSDDTAVYYC
ARDQPLGYCTNGVCSYFDWGQGTLVTVSSGGGGSGGGGSGGGG
SGGGGSDIQMTQSPSSVSASVGDRVTITCRASQGIYSWLAWYQQKP
GKAPNLLIYTASTLQSGVPSRFSGSGSGTDFTLTISSLQPEDFATYYC
QQANIFPLTFGGGTKVEIK
Edrecolomab LC CD40 718 DIQMTQSPSSVSASVGDRVTITCRASQGIYSWLAWYQQKPGKAPNL
mAb LIYTASTLQSGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCQQANIFP
containing LTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
tumor mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
ScFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLQQSGA
ELVRPGTSVKVSCKASGYAFTNYLIEWVKQRPGQGLEWIGVINPGS
GGTNYNEKFKGKATLTADKSSSTAYMQLSSLTSDDSAVYFCARDG
PWFAWGQGTLVTVSAGGGGSGGGGSGGGGSGGGGSNIVMTQSP
KSMSMSVGERVTLTCKASENVVTYVSWYQQKPEQSPKLLIYGASN
RYTGVPDRFTGSGSATDFTLTISSVQAEDLADYHCGQGYSYPYTFG
GGTKLEIK
LC tumor 140 NIVMTQSPKSMSMSVGERVTLTCKASENVVTYVSWYQQKPEQSPK
mAb LLIYGASNRYTGVPDRFTGSGSATDFTLTISSVQAEDLADYHCGQG
containing YSYPYTFGGGTKLEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNF
CD40 mAb YPREAKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKAD
ScFv YEKHKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLVQ
SGAEVKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQGLEWMG
-162-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
WINPDSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSDDTAVY
YCARDQPLGYCTNGVCSYFDWGQGTLVTVSSGGGGSGGGGSGG
GGSGGGGSDIQMTQSPSSVSASVGDRVTITCRASQGIYSWLAWYQQ
KPGKAPNLLIYTASTLQSGVPSRFSGSGSGTDFTLTISSLQPEDFATY
YCQQANIFPLTFGGGTKVEIK
Adecatumum LC CD40 694 DIQMTQSPSSVSASVGDRVTITCRASQGIYSWLAWYQQKPGKAPNL
ab mAb
LIYTASTLQSGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCQQANIFP
containing LTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
tumor mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
ScFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSEVQLLESGG
GVVQPGRSLRLSCAASGFTFSSYGMHWVRQAPGKGLEWVAVISYD
GSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAEDTAVYYCAKD
MGWGSGWRPYYYYGMDVWGQGTTVTVSSGGGGSGGGGSGGGGS
GGGGSELQMTQSPSSLSASVGDRVTITCRTSQSISSYLNWYQQKPGQ
PPKLLIYWASTRESGVPDRFSGSGSGTDFTLTISSLQPEDSATYYCQQ
SYDIPYTFGQGTKLEIK
LC tumor 153 ELQMTQSPSSLSASVGDRVTITCRTSQSISSYLNWYQQKPGQPPKLLI
mAb WASTRESGVPDRFSGSGSGTDFTLTISSLQPEDSATYYCQQSYDIP
containing YTFGQGTKLEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
CD40 mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
ScFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLVQSGA
EVKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQGLEWMGWIN
PDSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSDDTAVYYCA
RDQPLGYCTNGVCSYFDWGQGTLVTVSSGGGGSGGGGSGGGGS
GGGGSDIQMTQSPSSVSASVGDRVTITCRASQGIYSWLAWYQQKPG
KAPNLLIYTASTLQSGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCQ
QANIFPLTFGGGTKVEIK
Anetumab LC CD40 700 DIQMTQSPSSVSASVGDRVTITCRASQGIYSWLAWYQQKPGKAPNL
mAb LIYTASTLQSGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCQQANIFP
containing LTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
tumor mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
ScFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVELVQSGA
EVKKPGESLKISCKGSGYSFTSWIGWVRQAPGKGLEWMGIIDPGD
SRTRYSPSFQGQVTISADKSISTAYLQWSSLKASDTAMYYCARGQL
YGGTYMDGWGQGTLVTVSSGGGGSGGGGSGGGGSGGGGSDIALT
QPASVSGSPGQSITISCTGTSSDIGGYNSVSWYQQHPGKAPKLMIYG
VNNRPSGVSNRFSGSKSGNTASLTISGLQAEDEADYYCSSYDIESAT
PVFGGGTKLTVL
LC tumor 166 DIALTQPASVSGSPGQSITISCTGTSSDIGGYNSVSWYQQHPGKAPKL
mAb MIYGVNNRPSGVSNRFSGSKSGNTASLTISGLQAEDEADYYCSSYDI
containing ESATPVFGGGTKLTVLGQPKAAPSVTLFPPSSEELQANKATLVCLIS
CD40 mAb DFYPGAVTVAWKGDSSPVKAGVETTTPSKQSNNKYAASSYLSLTPE
ScFv QWKSHRSYSCQVTHEGSTVEKTVAPTECSGGGGSGGGGSQVQLVQ
SGAEVKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQGLEWMG
WINPDSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSDDTAVY
YCARDQPLGYCTNGVCSYFDWGQGTLVTVSSGGGGSGGGGSGG
GGSGGGGSDIQMTQSPSSVSASVGDRVTITCRASQGIYSWLAWYQQ
KPGKAPNLLIYTASTLQSGVPSRFSGSGSGTDFTLTISSLQPEDFATY
YCQQANIFPLTFGGGTKVEIK
huDS6 LC CD40 724 DIQMTQSPSSVSASVGDRVTITCRASQGIYSWLAWYQQKPGKAPNL
mAb LIYTASTLQSGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCQQANIFP
containing LTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
tumor mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
ScFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQAQLVQSGA
EVVKPGASVKMSCKASGYTFTSYNMHWVKQTPGQGLEWIGYIYPG
NGATNYNQKFQGKATLTADPSSSTAYMQISSLTSEDSAVYFCARGD
SVPFAWGQGTLVTVSAGGGGSGGGGSGGGGSGGGGSEIVLTQSP
ATMSASPGERVTITCSAHSSVSFMHWFQQKPGTSPKLWIYSTSSLAS
GVPARFGGSGSGTSYSLTISSMEAEDAATYYCQQRSSFPLTFGAGTK
-163-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
LELK
LC tumor 179 EIVLTQSPATMSASPGERVTITCSAHSSVSFMHWFQQKPGTSPKLWI
mAb YSTSSLASGVPARFGGSGSGTSYSLTISSMEAEDAATYYCQQRSSFP
containing LTFGAGTKLELKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPR
CD40 mAb EAKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYE
ScFv KHKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLVQSG
AEVKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQGLEWMGWI
NPDSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSDDTAVYYC
ARDQPLGYCTNGVCSYFDWGQGTLVTVSSGGGGSGGGGSGGGG
SGGGGSDIQMTQSPSSVSASVGDRVTITCRASQGIYSWLAWYQQKP
GKAPNLLIYTASTLQSGVPSRFSGSGSGTDFTLTISSLQPEDFATYYC
QQANIFPLTFGGGTKVEIK
Lifastuzumab LC CD40 736 DIQMTQSPSSVSASVGDRVTITCRASQGIYSWLAWYQQKPGKAPNL
mAb LIYTASTLQSGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCQQANIFP
containing LTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
tumor mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
ScFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSEVQLVESGG
GLVQPGGSLRLSCAASGFSFSDFAMSWVRQAPGKGLEWVATIGRV
AFHTYYPDSMKGRFTISRDNSKNTLYLQMNSLRAEDTAVYYCARH
RGFDVGHFDFWGQGTLVTVSSGGGGSGGGGSGGGGSGGGGSDIQ
MTQSPSSLSASVGDRVTITCRSSETLVHSSGNTYLEWYQQKPGKAP
KLLIYRVSNRFSGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCFQGS
FNPLTFGQGTKVEIK
LC tumor 192 DIQMTQSPSSLSASVGDRVTITCRSSETLVHSSGNTYLEWYQQKPGK
mAb APKLLIYRVSNRFSGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCFQ
containing GSFNPLTFGQGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNN
CD40 mAb FYPREAKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKA
ScFv DYEKHKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLV
QSGAEVKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQGLEWM
GWINPDSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSDDTAV
YYCARDQPLGYCTNGVCSYFDWGQGTLVTVSSGGGGSGGGGSG
GGGSGGGGSDIQMTQSPSSVSASVGDRVTITCRASQGIYSWLAWYQ
QKPGKAPNLLIYTASTLQSGVPSRFSGSGSGTDFTLTISSLQPEDFAT
YYCQQANIFPLTFGGGTKVEIK
Sacituzumab LC CD40 781 DIQMTQSPSSVSASVGDRVTITCRASQGIYSWLAWYQQKPGKAPNL
mAb LIYTASTLQSGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCQQANIFP
containing LTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
tumor mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
ScFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLQQSGS
ELKKPGASVKVSCKASGYTFTNYGMNWVKQAPGQGLKWMGWIN
TYTGEPTYTDDFKGRFAFSLDTSVSTAYLQISSLKADDTAVYFCARG
GFGSSWYFDVWGQGSLVTVSSGGGGSGGGGSGGGGSGGGGSDIQ
LTQSPSSLSASVGDRVSITCKASQDVSIAVAWYQQKPGKAPKLLIYS
ASYRYTGVPDRFSGSGSGTDFTLTISSLQPEDFAVYYCQQHYITPLTF
GAGTKVEIK
LC tumor 205 DIQLTQSPSSLSASVGDRVSITCKASQDVSIAVAWYQQKPGKAPKLL
mAb IYSASYRYTGVPDRFSGSGSGTDFTLTISSLQPEDFAVYYCQQHYITP
containing LTFGAGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
CD40 mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
ScFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLVQSGA
EVKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQGLEWMGWIN
PDSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSDDTAVYYCA
RDQPLGYCTNGVCSYFDWGQGTLVTVSSGGGGSGGGGSGGGGS
GGGGSDIQMTQSPSSVSASVGDRVTITCRASQGIYSWLAWYQQKPG
KAPNLLIYTASTLQSGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCQ
QANIFPLTFGGGTKVEIK
PR1A3 LC CD40 772 DIQMTQSPSSVSASVGDRVTITCRASQGIYSWLAWYQQKPGKAPNL
mAb LIYTASTLQSGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCQQANIFP
containing LTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
-164-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
tumor mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
ScFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVKLQQSGP
ELKKPGETVKISCKASGYTFTEFGMNWVKQAPGKGLKWMGWINT
KTGEATYVEEFKGRFAFSLETSATTAYLQINNLKNEDTAKYFCARW
DFYDYVEAMDWGQGTTVTVSSGGGGSGGGGSGGGGSGGGGSDI
VMTQSQRFMSTSVGDRVSVTCKASQNVGTNVAWYQQKPGQSPKA
LIYSASYRYSGVPDRFTGSGSGTDFTLTISNVQSEDLAEYFCHQYYT
YPLFTFGSGTKLEMK
LC tumor 218 DIVMTQSQRFMSTSVGDRVSVTCKASQNVGTNVAWYQQKPGQSP
mAb KALIYSASYRYSGVPDRFTGSGSGTDFTLTISNVQSEDLAEYFCHQY
containing YTYPLFTFGSGTKLEMKRTVAAPSVFIFPPSDEQLKSGTASVVCLLN
CD40 mAb NFYPREAKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSK
ScFv ADYEKHKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQL
VQSGAEVKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQGLEW
MGWINPDSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSDDTA
VYYCARDQPLGYCTNGVCSYFDWGQGTLVTVSSGGGGSGGGGS
GGGGSGGGGSDIQMTQSPSSVSASVGDRVTITCRASQGIYSWLAWY
QQKPGKAPNLLIYTASTLQSGVPSRFSGSGSGTDFTLTISSLQPEDFA
TYYCQQANIFPLTFGGGTKVEIK
Humanized LC CD40 820 DIQMTQSPSSVSASVGDRVTITCRASQGIYSWLAWYQQKPGKAPNL
PR1A3 mAb
LIYTASTLQSGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCQQANIFP
containing LTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
tumor mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
ScFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLVQSGA
EVKKPGASVKVSCKASGYTFTEFGMNWVRQAPGQGLEWMGWINT
KTGEATYVEEFKGRVTFTTDTSTSTAYMELRSLRSDDTAVYYCAR
WDFAYYVEAMDWGQGTTVTVSSGGGGSGGGGSGGGGSGGGGS
DIQMTQSPSSLSASVGDRVTITCKASAAVGTYVAWYQQKPGKAPK
LLIYSASYRKRGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCHQYYT
YPLFTFGQGTKLEIK
LC tumor 817 DIQMTQSPSSLSASVGDRVTITCKASAAVGTYVAWYQQKPGKAPK
mAb LLIYSASYRKRGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCHQYYT
containing YPLFTFGQGTKLEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFY
CD40 mAb PREAKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADY
ScFv EKHKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLVQS
GAEVKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQGLEWMGW
INPDSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSDDTAVYYC
ARDQPLGYCTNGVCSYFDWGQGTLVTVSSGGGGSGGGGSGGGG
SGGGGSDIQMTQSPSSVSASVGDRVTITCRASQGIYSWLAWYQQKP
GKAPNLLIYTASTLQSGVPSRFSGSGSGTDFTLTISSLQPEDFATYYC
QQANIFPLTFGGGTKVEIK
LC tumor 844 DIQMTQSPSSLSASVGDRVTITCKASAAVGTYVAWYQQKPGKAPK
mAb LLIYSASYRKRGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCHQYYT
containing YPLFTFGQGTKLEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFY
CD40 mab PREAKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADY
scFv (LH) EKHKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSDIQMTQS
PSSVSASVGDRVTITCRASQGIYSWLAWYQQKPGKAPNLLIYTASTL
QSGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCQQANIFPLTFGGGT
KVEIKGGGGSGGGGSGGGGSGGGGSQVQLVQSGAEVKKPGASVK
VSCKASGYTFTGYYMHWVRQAPGQGLEWMGWINPDSGGTNYAQ
KFQGRVTMTRDTSISTAYMELNRLRSDDTAVYYCARDQPLGYCTN
GVCSYFDWGQGTLVTVSS
Humanized LC CD40 838 DIQMTQSPSSVSASVGDRVTITCRASQGIYSWLAWYQQKPGKAPNL
Ab2-3 mAb
LIYTASTLQSGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCQQANIFP
containing LTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
tumor mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
ScFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSEVQLQESGP
GLVKPGGSLSLSCAASGFVFSSYDMSWVRQTPERGLEWVAYISSGG
GITYAPSTVKGRFTVSRDNAKNTLYLQMNSLTSEDTAVYYCAAHY
-165-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
FGSSGPFAYWGQGTLVTVSSGGGGSGGGGSGGGGSGGGGSDIQMT
QSPASLSASVGDRVTITCRASENIFSYLAWYQQKPGKSPKLLVYNTR
TLAEGVPSRFSGSGSGTDFSLTISSLQPEDFATYYCQHHYGTPFTFGS
GTKLEIK
LC tumor 835 DIQMTQSPASLSASVGDRVTITCRASENIFSYLAWYQQKPGKSPKLL
mAb VYNTRTLAEGVPSRFSGSGSGTDFSLTISSLQPEDFATYYCQHHYGT
containing PFTFGSGTKLEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPR
CD40 mAb EAKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYE
ScFv KHKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLVQSG
AEVKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQGLEWMGWI
NPDSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSDDTAVYYC
ARDQPLGYCTNGVCSYFDWGQGTLVTVSSGGGGSGGGGSGGGG
SGGGGSDIQMTQSPSSVSASVGDRVTITCRASQGIYSWLAWYQQKP
GKAPNLLIYTASTLQSGVPSRFSGSGSGTDFTLTISSLQPEDFATYYC
QQANIFPLTFGGGTKVEIK
LC tumor 842 DIQMTQSPASLSASVGDRVTITCRASENIFSYLAWYQQKPGKSPKLL
mAb VYNTRTLAEGVPSRFSGSGSGTDFSLTISSLQPEDFATYYCQHHYGT
containing PFTFGSGTKLEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPR
CD40 mab EAKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYE
scFv (LH) KHKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSDIQMTQSPS
SVSASVGDRVTITCRASQGIYSWLAWYQQKPGKAPNLLIYTASTLQ
SGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCQQANIFPLTFGGGTK
VEIKGGGGSGGGGSGGGGSGGGGSQVQLVQSGAEVKKPGASVKVS
CKASGYTFTGYYMHWVRQAPGQGLEWMGWINPDSGGTNYAQKF
QGRVTMTRDTSISTAYMELNRLRSDDTAVYYCARDQPLGYCTNGV
CSYFDWGQGTLVTVSS
IMAB362, LC CD40 727 DIQMTQSPSSVSASVGDRVTITCRASQGIYSWLAWYQQKPGKAPNL
CLAUDIXIM mAb
LIYTASTLQSGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCQQANIFP
AB containing LTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
tumor mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
ScFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLQQPGA
ELVRPGASVKLSCKASGYTFTSWINWVKQRPGQGLEWIGNIYPSD
SYTNYNQKFKDKATLTVDKSSSTAYMQLSSPTSEDSAVYYCTRSW
RGNSFDYWGQGTTLTVSSGGGGSGGGGSGGGGSGGGGSDIVMTQS
PSSLTVTAGEKVTMSCKSSQSLLNSGNQKNYLTWYQQKPGQPPKLL
IWASTRESGVPDRFTGSGSGTDFTLTISSVQAEDLAVYYCQNDYS
YPFTFGSGTKLEIK
LC tumor 231 DIVMTQSPSSLTVTAGEKVTMSCKSSQSLLNSGNQKNYLTWYQQK
mAb PGQPPKLLIWASTRESGVPDRFTGSGSGTDFTLTISSVQAEDLAVY
containing YCQNDYSYPFTFGSGTKLEIKRTVAAPSVFIFPPSDEQLKSGTASVVC
CD40 mAb LLNNFYPREAKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLT
ScFv LSKADYEKHKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQ
VQLVQSGAEVKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQGL
EWMGWINPDSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSDD
TAVYYCARDQPLGYCTNGVCSYFDYWGQGTLVTVSSGGGGSGGG
GSGGGGSGGGGSDIQMTQSPSSVSASVGDRVTITCRASQGIYSWLA
WYQQKPGKAPNLLIYTASTLQSGVPSRFSGSGSGTDFTLTISSLQPED
FATYYCQQANIFPLTFGGGTKVEIK
AMG595 LC CD40 697 DIQMTQSPSSVSASVGDRVTITCRASQGIYSWLAWYQQKPGKAPNL
mAb LIYTASTLQSGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCQQANIFP
containing LTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
tumor mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
ScFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLVESGG
GVVQSGRSLRLSCAASGFTFRNYGMHWVRQAPGKGLEWVAVIWY
DGSDKYYADSVRGRFTISRDNSKNTLYLQMNSLRAEDTAVYYCAR
DGYDILTGNPRDFDWGQGTLVTVSSGGGGSGGGGSGGGGSGGG
GSDTVMTQTPLSSHVTLGQPASISCRSSQSLVHSDGNTYLSWLQQRP
GQPPRLLIYRISRRFSGVPDRFSGSGAGTDFTLEISRVEAEDVGVYYC
MQSTHVPRTFGQGTKVEIK
-166-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
LC tumor 270 DTVMTQTPLSSHVTLGQPASISCRSSQSLVHSDGNTYLSWLQQRPG
mAb QPPRLLIYRISRRFSGVPDRFSGSGAGTDFTLEISRVEAEDVGVYYC
containing MQSTHVPRTFGQGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCL
CD40 mAb LNNFYPREAKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTL
ScFv SKADYEKHKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQV
QLVQSGAEVKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQGLE
WMGWINPDSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSDDT
AVYYCARDQPLGYCTNGVCSYFDWGQGTLVTVSSGGGGSGGGG
SGGGGSGGGGSDIQMTQSPSSVSASVGDRVTITCRASQGIYSWLAW
YQQKPGKAPNLLIYTASTLQSGVPSRFSGSGSGTDFTLTISSLQPEDF
ATYYCQQANIFPLTFGGGTKVEIK
ABT806 LC CD40 691 DIQMTQSPSSVSASVGDRVTITCRASQGIYSWLAWYQQKPGKAPNL
mAb LIYTASTLQSGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCQQANIFP
containing LTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
tumor mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
ScFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSEVQLQESGP
GLVKPSQTLSLTCTVSGYSISRDFAWNWIRQPPGKGLEWMGYISYN
GNTRYQPSLKSRITISRDTSKNQFFLKLNSVTAADTATYYCVTASRG
FPWGQGTLVTVSSGGGGSGGGGSGGGGSGGGGSDIQMTQSPSSM
SVSVGDRVTITCHSSQDINSNIGWLQQKPGKSFKGLIYHGTNLDDGV
PSRFSGSGSGTDYTLTISSLQPEDFATYYCVQYAQFPWTFGGGTKLE
IK
LC tumor 283 DIQMTQSPSSMSVSVGDRVTITCHSSQDINSNIGWLQQKPGKSFKGL
mAb IYHGTNLDDGVPSRFSGSGSGTDYTLTISSLQPEDFATYYCVQYAQF
containing PWTFGGGTKLEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYP
CD40 mAb REAKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADY
ScFv EKHKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLVQS
GAEVKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQGLEWMGW
INPDSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSDDTAVYYC
ARDQPLGYCTNGVCSYFDWGQGTLVTVSSGGGGSGGGGSGGGG
SGGGGSDIQMTQSPSSVSASVGDRVTITCRASQGIYSWLAWYQQKP
GKAPNLLIYTASTLQSGVPSRFSGSGSGTDFTLTISSLQPEDFATYYC
QQANIFPLTFGGGTKVEIK
Sibrotuzumab LC CD40 787 DIQMTQSPSSVSASVGDRVTITCRASQGIYSWLAWYQQKPGKAPNL
mAb LIYTASTLQSGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCQQANIFP
containing LTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
tumor mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
ScFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLVQSGA
EVKKPGASVKVSCKTSRYTFTEYTIHWVRQAPGQRLEWIGGINPNN
GIPNYNQKFKGRVTITVDTSASTAYMELSSLRSEDTAVYYCARRRIA
YGYDEGHAMDYWGQGTLVTVSSGGGGSGGGGSGGGGSGGGGSDI
VMTQSPDSLAVSLGERATINCKSSQSLLYSRNQKNYLAWYQQKPG
QPPKLLIFWASTRESGVPDRFSGSGFGTDFTLTISSLQAEDVAVYYC
QQYFSYPLTFGQGTKVEIK
LC tumor 296 DIVMTQSPDSLAVSLGERATINCKSSQSLLYSRNQKNYLAWYQQKP
mAb GQPPKLLIFWASTRESGVPDRFSGSGFGTDFTLTISSLQAEDVAVYY
containing CQQYFSYPLTFGQGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVC
CD40 mAb LLNNFYPREAKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLT
ScFv LSKADYEKHKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQ
VQLVQSGAEVKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQGL
EWMGWINPDSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSDD
TAVYYCARDQPLGYCTNGVCSYFDYWGQGTLVTVSSGGGGSGGG
GSGGGGSGGGGSDIQMTQSPSSVSASVGDRVTITCRASQGIYSWLA
WYQQKPGKAPNLLIYTASTLQSGVPSRFSGSGSGTDFTLTISSLQPED
FATYYCQQANIFPLTFGGGTKVEIK
DS-8895a LC CD40 712 DIQMTQSPSSVSASVGDRVTITCRASQGIYSWLAWYQQKPGKAPNL
variant 1 mAb
LIYTASTLQSGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCQQANIFP
containing LTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
tumor mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
-167-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
ScFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLVQSGA
EVKKPGASVKVSCKASGYTFIDYSMHWVRQAPGQGLEWMGWINT
YTGEPTYSDDFKGRVTITADTSTSTAYLELSSLRSEDTAVYYCATYY
RYERDFDWGQGTLVTVSSGGGGSGGGGSGGGGSGGGGSDIVMT
QSPLSLPVTPGEPASISCRSSQSIVHSSGITYLEWYLQKPGQSPQLLIY
KVSNRFSGVPDRFSGSGSGTDFTLKISRVEAEDVGVYYCFQGSHVP
YTFGQGTKVEIK
LC tumor 309
DIVMTQSPLSLPVTPGEPASISCRSSQSIVHSSGITYLEWYLQKPGQSP
mAb QLLIYKVSNRFSGVPDRFSGSGSGTDFTLKISRVEAEDVGVYYCFQG
containing SHVPYTFGQGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNN
CD40 mAb FYPREAKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKA
ScFv DYEKHKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLV
QSGAEVKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQGLEWM
GWINPDSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSDDTAV
YYCARDQPLGYCTNGVCSYFDWGQGTLVTVSSGGGGSGGGGSG
GGGSGGGGSDIQMTQSPSSVSASVGDRVTITCRASQGIYSWLAWYQ
QKPGKAPNLLIYTASTLQSGVPSRFSGSGSGTDFTLTISSLQPEDFAT
YYCQQANIFPLTFGGGTKVEIK
DS-8895a LC CD40 715 DIQMTQSPSSVSASVGDRVTITCRASQGIYSWLAWYQQKPGKAPNL
variant 2 mAb
LIYTASTLQSGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCQQANIFP
containing LTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
tumor mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
ScFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQIQLVQSGA
EVKKPGASVKVSCKASGYTFIDYSMHWVRQAPGQGLKWMGWINT
YTGEPTYSDDFKGRFAFSLDTSTSTAYLELSSLRSEDTAVYYCATYY
RYERDFDWGQGTLVTVSSGGGGSGGGGSGGGSGGGGSDVLMTQ
SPLSLPVTPGEPASISCRSSQSIVHSSGITYLEWYLQKPGQSPQLLIYK
VSNRFSGVPDRFSGSGSGTDFTLKISRVEAEDVGVYYCFQGSHVPYT
FGQGTKVEIK
LC tumor 322 DVLMTQSPLSLPVTPGEPASISCRSSQSIVHSSGITYLEWYLQKPGQS
mAb PQLLIYKVSNRFSGVPDRFSGSGSGTDFTLKISRVEAEDVGVYYCFQ
containing GSHVPYTFGQGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLN
CD40 mAb NFYPREAKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSK
ScFv ADYEKHKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQL
VQSGAEVKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQGLEW
MGWINPDSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSDDTA
VYYCARDQPLGYCTNGVCSYFDWGQGTLVTVSSGGGGSGGGGS
GGGGSGGGGSDIQMTQSPSSVSASVGDRVTITCRASQGIYSWLAWY
QQKPGKAPNLLIYTASTLQSGVPSRFSGSGSGTDFTLTISSLQPEDFA
TYYCQQANIFPLTFGGGTKVEIK
MEDI-547 LC CD40 742 DIQMTQSPSSVSASVGDRVTITCRASQGIYSWLAWYQQKPGKAPNL
mAb LIYTASTLQSGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCQQANIFP
containing LTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
tumor mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
ScFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSEVQLLESGG
GLVQPGGSLRLSCAASGFTFSHYMMAWVRQAPGKGLEWVSRIGPS
GGPTHYADSVKGRFTISRDNSKNTLYLQMNSLRAEDTAVYYCAGY
DSGYDYVAVAGPAEYFQHWGQGTLVTVSSGGGGSGGGGSGGGGS
GGGGSDIQMTQSPSSLSASVGDRVTITCRASQSISTWLAWYQQKPG
KAPKLLIYKASNLHTGVPSRFSGSGSGTEFSLTISGLQPDDFATYYCQ
QYNSYSRTFGQGTKVEIK
LC tumor 335 DIQMTQSPSSLSASVGDRVTITCRASQSISTWLAWYQQKPGKAPKLL
mAb IYKASNLHTGVPSRFSGSGSGTEFSLTISGLQPDDFATYYCQQYNSY
containing SRTFGQGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPR
CD40 mAb EAKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYE
ScFv KHKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLVQSG
AEVKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQGLEWMGWI
NPDSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSDDTAVYYC
ARDQPLGYCTNGVCSYFDWGQGTLVTVSSGGGGSGGGGSGGGG
-168-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
SGGGGSDIQMTQSPSSVSASVGDRVTITCRASQGIYSWLAWYQQKP
GKAPNLLIYTASTLQSGVPSRFSGSGSGTDFTLTISSLQPEDFATYYC
QQANIFPLTFGGGTKVEIK
Narnatumab LC CD40 748 DIQMTQSPSSVSASVGDRVTITCRASQGIYSWLAWYQQKPGKAPNL
mAb LIYTASTLQSGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCQQANIFP
containing LTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
tumor mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
ScFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSEVQLVESGG
GLVQPGGSLRLSCAASGFTFSSYLMTWVRQAPGKGLEWVANIKQD
GSEKYYVDSVKGRFTISRDNAKNSLNLQMNSLRAEDTAVYYCTRD
GYSSGRHYGMDVWGQGTTVIVSSGGGGSGGGGSGGGGSGGGGSEI
VLTQSPATLSLSPGERATLSCRASQSVSRYLAWYQQKPGQAPRLLIY
DASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRSNWPR
TFGQGTKVEIK
LC tumor 348 EIVLTQSPATLSLSPGERATLSCRASQSVSRYLAWYQQKPGQAPRLL
mAb IYDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRSNWP
containing RTFGQGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPR
CD40 mAb EAKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYE
ScFv KHKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLVQSG
AEVKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQGLEWMGWI
NPDSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSDDTAVYYC
ARDQPLGYCTNGVCSYFDWGQGTLVTVSSGGGGSGGGGSGGGG
SGGGGSDIQMTQSPSSVSASVGDRVTITCRASQGIYSWLAWYQQKP
GKAPNLLIYTASTLQSGVPSRFSGSGSGTDFTLTISSLQPEDFATYYC
QQANIFPLTFGGGTKVEIK
RG7841 LC CD40 775 DIQMTQSPSSVSASVGDRVTITCRASQGIYSWLAWYQQKPGKAPNL
mAb LIYTASTLQSGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCQQANIFP
containing LTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
tumor mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
ScFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSEVQLVESGP
ALVKPTQTLTLTCTVSGFSLTGYSVNWIRQPPGKALEWLGMIWGD
GSTDYNSALKSRLTISKDTSKNQVVLTMTNMDPVDTATYYCARDY
YFNYASWFAYWGQGTLVTVSSGGGGSGGGGSGGGGSGGGGSDIQ
MTQSPSSLSASVGDRVTITCSASQGISNYLNWYQQKPGKTVKLLIYY
TSNLHSGVPSRFSGSGSGTDYTLTISSLQPEDFATYYCQQYSELPWT
FGQGTKVEIK
LC tumor 361 DIQMTQSPSSLSASVGDRVTITCSASQGISNYLNWYQQKPGKTVKLL
mAb IYYTSNLHSGVPSRFSGSGSGTDYTLTISSLQPEDFATYYCQQYSELP
containing WTFGQGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPR
CD40 mAb EAKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYE
ScFv KHKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLVQSG
AEVKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQGLEWMGWI
NPDSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSDDTAVYYC
ARDQPLGYCTNGVCSYFDWGQGTLVTVSSGGGGSGGGGSGGGG
SGGGGSDIQMTQSPSSVSASVGDRVTITCRASQGIYSWLAWYQQKP
GKAPNLLIYTASTLQSGVPSRFSGSGSGTDFTLTISSLQPEDFATYYC
QQANIFPLTFGGGTKVEIK
Farletuzumab LC CD40 721 DIQMTQSPSSVSASVGDRVTITCRASQGIYSWLAWYQQKPGKAPNL
mAb LIYTASTLQSGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCQQANIFP
containing LTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
tumor mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
ScFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSEVQLVESGG
GVVQPGRSLRLSCSASGFTFSGYGLSWVRQAPGKGLEWVAMISSG
GSYTYYADSVKGRFAISRDNAKNTLFLQMDSLRPEDTGVYFCARH
GDDPAWFAWGQGTPVTVSSGGGGSGGGGSGGGGSGGGGSDIQL
TQSPSSLSASVGDRVTITCSVSSSISSNNLHWYQQKPGKAPKPWIYG
TSNLASGVPSRFSGSGSGTDYTFTISSLQPEDIATYYCQQWSSYPYM
YTFGQGTKVEIK
LC tumor 374 DIQLTQSPSSLSASVGDRVTITCSVSSSISSNNLHWYQQKPGKAPKP
-169-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
mAb WIYGTSNLASGVPSRFSGSGSGTDYTFTISSLQPEDIATYYCQQWSS
containing YPYMYTFGQGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNN
CD40 mAb FYPREAKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKA
ScFv DYEKHKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLV
QSGAEVKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQGLEWM
GWINPDSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSDDTAV
YYCARDQPLGYCTNGVCSYFDWGQGTLVTVSSGGGGSGGGGSG
GGGSGGGGSDIQMTQSPSSVSASVGDRVTITCRASQGIYSWLAWYQ
QKPGKAPNLLIYTASTLQSGVPSRFSGSGSGTDFTLTISSLQPEDFAT
YYCQQANIFPLTFGGGTKVEIK
Mirvetuximab LC CD40 745 DIQMTQSPSSVSASVGDRVTITCRASQGIYSWLAWYQQKPGKAPNL
mAb LIYTASTLQSGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCQQANIFP
containing LTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
tumor mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
ScFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLVQSGA
EVVKPGASVKISCKASGYTFTGYFMNWVKQSPGQSLEWIGRIHPYD
GDTFYNQKFQGKATLTVDKSSNTAHMELLSLTSEDFAVYYCTRYD
GSRAMDYWGQGTTVTVSSGGGGSGGGGSGGGGSGGGGSDIVLTQ
SPLSLAVSLGQPAIISCKASQSVSFAGTSLMHWYHQKPGQQPRLLIY
RASNLEAGVPDRFSGSGSKTDFTLTISPVEAEDAATYYCQQSREYPY
TFGGGTKLEIK
LC tumor 387 DIVLTQSPLSLAVSLGQPAIISCKASQSVSFAGTSLMHWYHQKPGQQ
mAb PRLLIYRASNLEAGVPDRFSGSGSKTDFTLTISPVEAEDAATYYCQQ
containing SREYPYTFGGGTKLEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNN
CD40 mAb FYPREAKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKA
ScFv DYEKHKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLV
QSGAEVKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQGLEWM
GWINPDSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSDDTAV
YYCARDQPLGYCTNGVCSYFDWGQGTLVTVSSGGGGSGGGGSG
GGGSGGGGSDIQMTQSPSSVSASVGDRVTITCRASQGIYSWLAWYQ
QKPGKAPNLLIYTASTLQSGVPSRFSGSGSGTDFTLTISSLQPEDFAT
YYCQQANIFPLTFGGGTKVEIK
J591 variant 1 LC CD40 730
DIQMTQSPSSVSASVGDRVTITCRASQGIYSWLAWYQQKPGKAPNL
mAb LIYTASTLQSGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCQQANIFP
containing LTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
tumor mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
ScFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSEVQLQQSGP
ELKKPGTSVRISCKTSGYTFTEYTIHWVKQSHGKSLEWIGNINPNNG
GTTYNQKFEDKATLTVDKSSSTAYMELRSLTSEDSAVYYCAAGWN
FDWGQGTTLTVSSGGGGSGGGGSGGGGSGGGGSDIVMTQSHKF
MSTSVGDRVSIICKASQDVGTAVDWYQQKPGQSPKLLIWASTRHT
GVPDRFTGSGSGTDFTLTITNVQSEDLADYFCQQYNSYPLTFGAGT
MLDLK
LC tumor 400 DIVMTQSHKFMSTSVGDRVSIICKASQDVGTAVDWYQQKPGQSPK
mAb LLIWASTRHTGVPDRFTGSGSGTDFTLTITNVQSEDLADYFCQQY
containing NSYPLTFGAGTMLDLKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNN
CD40 mAb FYPREAKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKA
ScFv DYEKHKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLV
QSGAEVKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQGLEWM
GWINPDSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSDDTAV
YYCARDQPLGYCTNGVCSYFDWGQGTLVTVSSGGGGSGGGGSG
GGGSGGGGSDIQMTQSPSSVSASVGDRVTITCRASQGIYSWLAWYQ
QKPGKAPNLLIYTASTLQSGVPSRFSGSGSGTDFTLTISSLQPEDFAT
YYCQQANIFPLTFGGGTKVEIK
J591 variant 2 LC CD40 733
DIQMTQSPSSVSASVGDRVTITCRASQGIYSWLAWYQQKPGKAPNL
mAb LIYTASTLQSGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCQQANIFP
containing LTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
tumor mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
ScFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSEVQLQQSGP
-170-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
ELVKPGTSVRISCKTSGYTFTEYTIHWVKQSHGKSLEWIGNINPNNG
GTTYNQKFEDKATLTVDKSSSTAYMELRSLTSEDSAVYYCAAGWN
FDWGQGTTLTVSSGGGGSGGGGSGGGGSGGGGSNIVMTQSPKSM
SMSVGERVTLTCKASENVVTYVSWYQQKPEQSPKLLIYGASNRYT
GVPDRFTGSGSATDFTLTISSVQAEDLADYHCGQGYSYPYTFGGGT
KLEIK
LC tumor 413 NIVMTQSPKSMSMSVGERVTLTCKASENVVTYVSWYQQKPEQSPK
mAb LLIYGASNRYTGVPDRFTGSGSATDFTLTISSVQAEDLADYHCGQG
containing YSYPYTFGGGTKLEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNF
CD40 mAb YPREAKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKAD
ScFv YEKHKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLVQ
SGAEVKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQGLEWMG
WINPDSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSDDTAVY
YCARDQPLGYCTNGVCSYFDWGQGTLVTVSSGGGGSGGGGSGG
GGSGGGGSDIQMTQSPSSVSASVGDRVTITCRASQGIYSWLAWYQQ
KPGKAPNLLIYTASTLQSGVPSRFSGSGSGTDFTLTISSLQPEDFATY
YCQQANIFPLTFGGGTKVEIK
Rovalpituzum LC CD40 778 DIQMTQSPSSVSASVGDRVTITCRASQGIYSWLAWYQQKPGKAPNL
ab mAb
LIYTASTLQSGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCQQANIFP
containing LTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
tumor mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
ScFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLVQSGA
EVKKPGASVKVSCKASGYTFTNYGMNWVRQAPGQGLEWMGWIN
TYTGEPTYADDFKGRVTMTTDTSTSTAYMELRSLRSDDTAVYYCA
RIGDSSPSDWGQGTLVTVSSGGGGSGGGGSGGGGSGGGGSEIVM
TQSPATLSVSPGERATLSCKASQSVSNDVVWYQQKPGQAPRLLIYY
ASNRYTGIPARFSGSGSGTEFTLTISSLQSEDFAVYYCQQDYTSPWTF
GQGTKLEIK
LC tumor 426 EIVMTQSPATLSVSPGERATLSCKASQSVSNDVVWYQQKPGQAPRL
mAb LIYYASNRYTGIPARFSGSGSGTEFTLTISSLQSEDFAVYYCQQDYTS
containing PWTFGQGTKLEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYP
CD40 mAb REAKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADY
ScFv EKHKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLVQS
GAEVKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQGLEWMGW
INPDSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSDDTAVYYC
ARDQPLGYCTNGVCSYFDWGQGTLVTVSSGGGGSGGGGSGGGG
SGGGGSDIQMTQSPSSVSASVGDRVTITCRASQGIYSWLAWYQQKP
GKAPNLLIYTASTLQSGVPSRFSGSGSGTDFTLTISSLQPEDFATYYC
QQANIFPLTFGGGTKVEIK
PF-06647020 LC CD40 769 DIQMTQSPSSVSASVGDRVTITCRASQGIYSWLAWYQQKPGKAPNL
mAb LIYTASTLQSGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCQQANIFP
containing LTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
tumor mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
ScFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLVQSGP
EVKKPGASVKVSCKASGYTFTDYAVHWVRQAPGKRLEWIGVISTY
NDYTYNNQDFKGRVTMTRDTSASTAYMELSRLRSEDTAVYYCAR
GNSYFYALDYWGQGTSVTVSSGGGGSGGGGSGGGGSGGGGSEIVL
TQSPATLSLSPGERATLSCRASESVDSYGKSFMHWYQQKPGQAPRL
LIYRASNLESGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQSNEDP
WTFGGGTKLEIK
LC tumor 439 EIVLTQSPATLSLSPGERATLSCRASESVDSYGKSFMHWYQQKPGQ
mAb APRLLIYRASNLESGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQS
containing NEDPWTFGGGTKLEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNN
CD40 mAb FYPREAKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKA
ScFv DYEKHKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLV
QSGAEVKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQGLEWM
GWINPDSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSDDTAV
YYCARDQPLGYCTNGVCSYFDWGQGTLVTVSSGGGGSGGGGSG
GGGSGGGGSDIQMTQSPSSVSASVGDRVTITCRASQGIYSWLAWYQ
-171-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
QKPGKAPNLLIYTASTLQSGVPSRFSGSGSGTDFTLTISSLQPEDFAT
YYCQQANIFPLTFGGGTKVEIK
Antibody to LC CD40 688 DIQMTQSPSSVSASVGDRVTITCRASQGIYSWLAWYQQKPGKAPNL
PTK7 mAb
LIYTASTLQSGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCQQANIFP
containing LTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
tumor mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
ScFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLVESGG
GVVQPGRSLRLSCAASGFTFSSYAFHWVRQAPGKGLEWVAVISYD
GSIKYYADSVKGRFTISRDNSKNTLYLQMNSLRAEDTAVYYCARTY
YFDWGQGTLVTVSSGGGGSGGGGSGGGGSGGGGSEIVLTQSPDF
QSVTPKEKVTITCRASQSIGSSLHWYQQKPDQSPKLLIKYASQSFSG
VPSRFSGSGSGTDFTLTINSLEAEDAAAYYCHQSSSLPITFGQGTRLE
IK
LC tumor 452 EIVLTQSPDFQSVTPKEKVTITCRASQSIGSSLHWYQQKPDQSPKLLI
mAb KYASQSFSGVPSRFSGSGSGTDFTLTINSLEAEDAAAYYCHQSSSLPI
containing TFGQGTRLEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREA
CD40 mAb KVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEKH
ScFv KVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLVQSGAE
VKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQGLEWMGWINP
DSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSDDTAVYYCAR
DQPLGYCTNGVCSYFDWGQGTLVTVSSGGGGSGGGGSGGGGSG
GGGSDIQMTQSPSSVSASVGDRVTITCRASQGIYSWLAWYQQKPGK
APNLLIYTASTLQSGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCQQ
ANIFPLTFGGGTKVEIK
Ladiratuzuma LC CD40 784 DIQMTQSPSSVSASVGDRVTITCRASQGIYSWLAWYQQKPGKAPNL
b mAb
LIYTASTLQSGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCQQANIFP
containing LTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
tumor mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
ScFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLVQSGA
EVKKPGASVKVSCKASGLTIEDYYMHWVRQAPGQGLEWMGWIDP
ENGDTEYGPKFQGRVTMTRDTSINTAYMELSRLRSDDTAVYYCAV
HNAHYGTWFAWGQGTLVTVSSGGGGSGGGGSGGGGSGGGGSD
VVMTQSPLSLPVTLGQPASISCRSSQSLLHSSGNTYLEYFQQRPGQSP
RPLIYKISTRFSGVPDRFSGSGSGTDFTLKISRVEAEDVGVYYCFQGS
HVPYTFGGGTKVEIK
LC tumor 465 DVVMTQSPLSLPVTLGQPASISCRSSQSLLHSSGNTYLEYFQQRPGQ
mAb SPRPLIYKISTRFSGVPDRFSGSGSGTDFTLKISRVEAEDVGVYYCFQ
containing GSHVPYTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLN
CD40 mAb NFYPREAKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSK
ScFv ADYEKHKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQL
VQSGAEVKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQGLEW
MGWINPDSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSDDTA
VYYCARDQPLGYCTNGVCSYFDWGQGTLVTVSSGGGGSGGGGS
GGGGSGGGGSDIQMTQSPSSVSASVGDRVTITCRASQGIYSWLAWY
QQKPGKAPNLLIYTASTLQSGVPSRFSGSGSGTDFTLTISSLQPEDFA
TYYCQQANIFPLTFGGGTKVEIK
Cirmtuzumab LC CD40 706 DIQMTQSPSSVSASVGDRVTITCRASQGIYSWLAWYQQKPGKAPNL
mAb LIYTASTLQSGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCQQANIFP
containing LTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
tumor mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
ScFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLQESGP
GLVKPSQTLSLTCTVSGYAFTAYNIHWVRQAPGQGLEWMGSFDPY
DGGSSYNQKFKDRLTISKDTSKNQVVLTMTNMDPVDTATYYCARG
WYYFDWGHGTLVTVSSGGGGSGGGGSGGGGSGGGGSDIVMTQT
PLSLPVTPGEPASISCRASKSISKYLAWYQQKPGQAPRLLIYSGSTLQ
SGIPPRFSGSGYGTDFTLTINNIESEDAAYYFCQQHDESPY
LC tumor 478 DIVMTQTPLSLPVTPGEPASISCRASKSISKYLAWYQQKPGQAPRLLI
mAb YSGSTLQSGIPPRFSGSGYGTDFTLTINNIESEDAAYYFCQQHDESPY
containing TFGEGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREA
-172-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
CD40 mAb KVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEKH
ScFv KVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLVQSGAE
VKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQGLEWMGWINP
DSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSDDTAVYYCAR
DQPLGYCTNGVCSYFDWGQGTLVTVSSGGGGSGGGGSGGGGSG
GGGSDIQMTQSPSSVSASVGDRVTITCRASQGIYSWLAWYQQKPGK
APNLLIYTASTLQSGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCQQ
ANIFPLTFGGGTKVEIK
Antibody to LC CD40 739 DIQMTQSPSSVSASVGDRVTITCRASQGIYSWLAWYQQKPGKAPNL
MAGE-A3 mAb
LIYTASTLQSGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCQQANIFP
containing LTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
tumor mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
ScFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLVESGG
GVVQPGRSLRLSCTASGFRFRSHGMHWVRQAPGKGLEWVAVISYD
GNNKLYADSVKGRITISRDNSKNTLFLQMNNVRAEDTAVYYCASP
YTSDWQYFQWGQGTLVIVSSGGGGSGGGGSGGGGSGGGGSEIV
MTQSPATLSVSPGERATFSCRASQNISTTLAWYQQKPGQAPRLLIYD
TSTRATGIPARFSGSGSGTEFTLTISSLQSEDLAVYYCQQSNSWPLTF
GGGTKVEIK
LC tumor 491 EIVMTQSPATLSVSPGERATFSCRASQNISTTLAWYQQKPGQAPRLL
mAb IYDTSTRATGIPARFSGSGSGTEFTLTISSLQSEDLAVYYCQQSNSWP
containing LTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
CD40 mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
ScFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLVQSGA
EVKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQGLEWMGWIN
PDSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSDDTAVYYCA
RDQPLGYCTNGVCSYFDWGQGTLVTVSSGGGGSGGGGSGGGGS
GGGGSDIQMTQSPSSVSASVGDRVTITCRASQGIYSWLAWYQQKPG
KAPNLLIYTASTLQSGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCQ
QANIFPLTFGGGTKVEIK
Antibody to LC CD40 754 DIQMTQSPSSVSASVGDRVTITCRASQGIYSWLAWYQQKPGKAPNL
NY-ESO-1 mAb
LIYTASTLQSGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCQQANIFP
containing LTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
tumor mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
ScFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLVQSGG
GVVRPGGSLRLSCAASGFSFIDYGMSWVRQVPGKGLEWVAGMNW
SGDKKGHAESVKGRFIISRDNAKNTLYLEMSSLRVEDTALYFCARG
EYSNRFDPRGRGTLVTVSSGGGGSGGGGSGGGGSGGGGSDIVMTQ
TPLSLPVTLGQPASLSCRSSQSLVFTDGNTYLNWFQQRPGQSPRRLI
YKVSSRDPGVPDRFSGTGSGTDFTLEISRVEAEDIGVYYCMQGTHW
PPIFGQGTKVEIK
LC tumor 504 DIVMTQTPLSLPVTLGQPASLSCRSSQSLVFTDGNTYLNWFQQRPG
mAb QSPRRLIYKVSSRDPGVPDRFSGTGSGTDFTLEISRVEAEDIGVYYC
containing MQGTHWPPIFGQGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCL
CD40 mAb LNNFYPREAKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTL
ScFv SKADYEKHKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQV
QLVQSGAEVKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQGLE
WMGWINPDSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSDDT
AVYYCARDQPLGYCTNGVCSYFDWGQGTLVTVSSGGGGSGGGG
SGGGGSGGGGSDIQMTQSPSSVSASVGDRVTITCRASQGIYSWLAW
YQQKPGKAPNLLIYTASTLQSGVPSRFSGSGSGTDFTLTISSLQPEDF
ATYYCQQANIFPLTFGGGTKVEIK
Trastuzumab LC CD40 793 DIQMTQSPSSVSASVGDRVTITCRASQGIYSWLAWYQQKPGKAPNL
mAb LIYTASTLQSGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCQQANIFP
containing LTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
tumor mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
ScFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSEVQLVESGG
GLVQPGGSLRLSCAASGFNIKDTYIHWVRQAPGKGLEWVARIYPTN
GYTRYADSVKGRFTISADTSKNTAYLQMNSLRAEDTAVYYCSRWG
-173-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
GDGFYAMDWGQGTLVTVSSGGGGSGGGGSGGGGSGGGGSDIQM
TQSPSSLSASVGDRVTITCRASQDVNTAVAWYQQKPGKAPKLLIYS
ASFLYSGVPSRFSGSRSGTDFTLTISSLQPEDFATYYCQQHYTTPPTF
GQGTKVEIK
LC tumor 685 DIQMTQSPSSLSASVGDRVTITCRASQDVNTAVAWYQQKPGKAPKL
mAb LIYSASFLYSGVPSRFSGSRSGTDFTLTISSLQPEDFATYYCQQHYTT
containing PPTFGQGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPR
CD40 mAb EAKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYE
ScFv KHKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLVQSG
AEVKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQGLEWMGWI
NPDSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSDDTAVYYC
ARDQPLGYCTNGVCSYFDWGQGTLVTVSSGGGGSGGGGSGGGG
SGGGGSDIQMTQSPSSVSASVGDRVTITCRASQGIYSWLAWYQQKP
GKAPNLLIYTASTLQSGVPSRFSGSGSGTDFTLTISSLQPEDFATYYC
QQANIFPLTFGGGTKVEIK
LC CD40 798 DIQMTQSPSSVSASVGDRVTITCRASQGIYSWLAWYQQKPGKAPNL
mAb LIYTASTLQSGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCQQANIFP
containing LTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
tumor mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
scFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSDIQMTQSPSS
(LH,25mer) LSASVGDRVTITCRASQDVNTAVAWYQQKPGKAPKLLIYSASFLYS
GVPSRFSGSRSGTDFTLTISSLQPEDFATYYCQQHYTTPPTFGQGTK
VEIKGGGGSGGGGSGGGGSGGGGSGGGGSEVQLVESGGGLVQPGG
SLRLSCAASGFNIKDTYIHWVRQAPGKGLEWVARIYPTNGYTRYAD
SVKGRFTISADTSKNTAYLQMNSLRAEDTAVYYCSRWGGDGFYAM
DWGQGTLVTVSS
TABLE 10. Fusion Sequences ¨ DEC-205 fusions via the light chain
Antibody Region SEQ Sequence
ID
NO:
Pertuzumab LC DEC-205 767 EIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAPRLLI
mAb YDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRRNWP
containing LTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
tumor mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
ScFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSEVQLVESGG
GLVQPGGSLRLSCAASGFTFTDYTMDWVRQAPGKGLEWVADVNP
NSGGSIYNQRFKGRFTLSVDRSKNTLYLQMNSLRAEDTAVYYCAR
NLGPSFYFDWGQGTLVTVSSGGGGSGGGGSGGGGSGGGGSDIQM
TQSPSSLSASVGDRVTITCKASQDVSIGVAWYQQKPGKAPKLLIYSA
SYRYTGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCQQYYIYPYTFG
QGTKVEIK
LC tumor 768 DIQMTQSPSSLSASVGDRVTITCKASQDVSIGVAWYQQKPGKAPKL
mAb LIYSASYRYTGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCQQYYIY
containing PYTFGQGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPR
DEC-205 mAb EAKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYE
ScFv KHKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLVESG
GGVVQPGRSLRLSCAASGFTFSNYGMWVRQAPGKGLEWVAVIW
YDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAEDTAVYYCA
RDLWGWYFDYWGQGTLVTVSSGGGGSGGGGSGGGGSGGGGSEIV
LTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAPRLLIYD
ASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRRNWPLTF
GGGTKVEIK
Cetuximab LC DEC-205 704 EIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAPRLLI
mAb YDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRRNWP
containing LTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
tumor mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
ScFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLKQSGP
-174-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
GLVQPSQSLSITCTVSGFSLTNYGVHWVRQSPGKGLEWLGVIWSGG
NTDYNTPFTSRLSINKDNSKSQVFFKMNSLQSNDTAIYYCARALTY
YDYEFAWGQGTLVTVSAGGGGSGGGGSGGGGSGGGGSDILLTQS
PVILSVSPGERVSFSCRASQSIGTNIHWYQQRTNGSPRLLIKYASESIS
GIPSRFSGSGSGTDFTLSINSVESEDIADYYCQQNNNWPTTFGAGTK
LELK
LC tumor 705
DILLTQSPVILSVSPGERVSFSCRASQSIGTNIHWYQQRTNGSPRLLIK
mAb YASESISGIPSRFSGSGSGTDFTLSINSVESEDIADYYCQQNNNWPTTF
containing GAGTKLELKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREAK
DEC-205 mAb VQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEKHK
ScFv VYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLVESGGGV
VQPGRSLRLSCAASGFTFSNYGMWVRQAPGKGLEWVAVIWYDG
SNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAEDTAVYYCARDL
WGWYFDWGQGTLVTVSSGGGGSGGGGSGGGGSGGGGSEIVLTQ
SPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAPRLLIYDASN
RATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRRNWPLTFGG
GTKVEIK
Panitumumab LC DEC-205 761 EIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAPRLLI
mAb YDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRRNWP
containing LTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
tumor mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
ScFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLQESGP
GLVKPSETLSLTCTVSGGSVSSGDYWTWIRQSPGKGLEWIGHIYYS
GNTNYNPSLKSRLTISIDTSKTQFSLKLSSVTAADTAIYYCVRDRVTG
AFDIWGQGTMVTVSSGGGGSGGGGSGGGGSGGGGSDIQMTQSPSS
LSASVGDRVTITCQASQDISNYLNWYQQKPGKAPKLLIYDASNLET
GVPSRFSGSGSGTDFTFTISSLQPEDIATYFCQHFDHLPLAFGGGTKV
EIK
LC tumor 762 DIQMTQSPSSLSASVGDRVTITCQASQDISNYLNWYQQKPGKAPKL
mAb LIYDASNLETGVPSRFSGSGSGTDFTFTISSLQPEDIATYFCQHFDHLP
containing LAFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPR
DEC-205 mAb EAKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYE
ScFv KHKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLVESG
GGVVQPGRSLRLSCAASGFTFSNYGMWVRQAPGKGLEWVAVIW
YDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAEDTAVYYCA
RDLWGWYFDYWGQGTLVTVSSGGGGSGGGGSGGGGSGGGGSEIV
LTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAPRLLIYD
ASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRRNWPLTF
GGGTKVEIK
Nimotuzumab LC DEC-205 752 EIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAPRLLI
mAb YDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRRNWP
containing LTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
tumor mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
ScFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLQQSGA
EVKKPGSSVKVSCKASGYTFTNYYIWVRQAPGQGLEWIGGINPTS
GGSNFNEKFKTRVTITVDESTNTAYMELSSLRSEDTAFYFCARQGL
WFDSDGRGFDFWGQGSTVTVSSGGGGSGGGGSGGGGSGGGGSDIQ
MTQSPSSLSASVGDRVTITCRSSQNIVHSNGNTYLDWYQQTPGKAP
KLLIYKVSNRFSGVPSRFSGSGSGTDFTFTISSLQPEDIATYYCFQYSH
VPWTFGQGTKLEIK
LC tumor 753 DIQMTQSPSSLSASVGDRVTITCRSSQNIVHSNGNTYLDWYQQTPGK
mAb APKLLIYKVSNRFSGVPSRFSGSGSGTDFTFTISSLQPEDIATYYCFQY
containing SHVPWTFGQGTKLEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNN
DEC-205 mAb FYPREAKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKA
ScFv DYEKHKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLV
ESGGGVVQPGRSLRLSCAASGFTFSNYGMWVRQAPGKGLEWVA
VIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAEDTAVY
YCARDLWGWYFDYWGQGTLVTVSSGGGGSGGGGSGGGGSGGGG
SEIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAPRL
-175-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
LIYDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRRN
WPLTFGGGTKVEIK
Zalutumumab LC DEC-205 803 EIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAPRLLI
mAb YDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRRNWP
containing LTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
tumor mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
ScFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLVESGG
GVVQPGRSLRLSCAASGFTFSTYGMHWVRQAPGKGLEWVAVIWD
DGSYKYYGDSVKGRFTISRDNSKNTLYLQMNSLRAEDTAVYYCAR
DGITMVRGVMKDYFDYWGQGTLVTVSSGGGGSGGGGSGGGGSGG
GGSAIQLTQSPSSLSASVGDRVTITCRASQDISSALVWYQQKPGKAP
KLLIYDASSLESGVPSRFSGSESGTDFTLTISSLQPEDFATYYCQQFNS
YPLTFGGGTKVEIK
LC tumor 804 AIQLTQSPSSLSASVGDRVTITCRASQDISSALVWYQQKPGKAPKLLI
mAb YDASSLESGVPSRFSGSESGTDFTLTISSLQPEDFATYYCQQFNSYPL
containing TFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
DEC-205 mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
ScFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLVESGG
GVVQPGRSLRLSCAASGFTFSNYGMWVRQAPGKGLEWVAVIWY
DGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAEDTAVYYCAR
DLWGWYFDWGQGTLVTVSSGGGGSGGGGSGGGGSGGGGSEIVL
TQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAPRLLIYDA
SNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRRNWPLTFG
GGTKVEIK
Onartuzumab LC DEC-205 758 EIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAPRLLI
mAb YDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRRNWP
containing LTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
tumor mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
ScFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSEVQLVESGG
GLVQPGGSLRLSCAASGYTFTSWLHWVRQAPGKGLEWVGMIDPS
NSDTRFNPNFKDRFTISADTSKNTAYLQMNSLRAEDTAVYYCATYR
SYVTPLDWGQGTLVTVSSGGGGSGGGGSGGGGSGGGGSDIQMTQ
SPSSLSASVGDRVTITCKSSQSLLYTSSQKNYLAWYQQKPGKAPKLL
IWASTRESGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCQQYYAY
PWTFGQGTKVEIK
LC tumor 759 DIQMTQSPSSLSASVGDRVTITCKSSQSLLYTSSQKNYLAWYQQKPG
mAb KAPKLLIYWASTRESGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCQ
containing QYYAYPWTFGQGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLL
DEC-205 mAb NNFYPREAKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLS
ScFv KADYEKHKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQ
LVESGGGVVQPGRSLRLSCAASGFTFSNYGMYWVRQAPGKGLEW
VAVIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAEDTA
VYYCARDLWGWYFDWGQGTLVTVSSGGGGSGGGGSGGGGSGG
GGSEIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAP
RLLIYDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRR
NWPLTFGGGTKVEIK
Patritumab LC DEC-205 764
EIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAPRLLI
mAb YDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRRNWP
containing LTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
tumor mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
ScFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLQQWG
AGLLKPSETLSLTCAVYGGSFSGYWSWIRQPPGKGLEWIGEINHSG
STNYNPSLKSRVTISVETSKNQFSLKLSSVTAADTAVYYCARDKWT
WYFDLWGRGTLVTVSSGGGGSGGGGSGGGGSGGGGSDIEMTQSPD
SLAVSLGERATINCRSSQSVLYSSSNRNYLAWYQQNPGQPPKLLIY
WASTRESGVPDRFSGSGSGTDFTLTISSLQAEDVAVYYCQQYYSTPR
TFGQGTKVEIK
LC tumor 765 DIEMTQSPDSLAVSLGERATINCRSSQSVLYSSSNRNYLAWYQQNP
mAb GQPPKLLIYWASTRESGVPDRFSGSGSGTDFTLTISSLQAEDVAVYY
-176-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
containing CQQYYSTPRTFGQGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVC
DEC-205 mAb LLNNFYPREAKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLT
ScFv LSKADYEKHKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQ
VQLVESGGGVVQPGRSLRLSCAASGFTFSNYGMYWVRQAPGKGLE
WVAVIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAEDT
AVYYCARDLWGWYFDWGQGTLVTVSSGGGGSGGGGSGGGGSG
GGGSEIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQA
PRLLIYDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQR
RNWPLTFGGGTKVEIK
Clivatuzumab LC DEC-205 710
EIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAPRLLI
mAb YDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRRNWP
containing LTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
tumor mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
ScFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLQQSGA
EVKKPGASVKVSCEASGYTFPSYVLHWVKQAPGQGLEWIGYINPY
NDGTQYNEKFKGKATLTRDTSINTAYMELSRLRSDDTAVYYCARG
FGGSYGFAWGQGTLVTVSSGGGGSGGGGSGGGGSGGGGSDIQLT
QSPSSLSASVGDRVTMTCSASSSVSSSYLYWYQQKPGKAPKLWIYS
TSNLASGVPARFSGSGSGTDFTLTISSLQPEDSASYFCHQWNRYPYT
FGGGTRLEIK
LC tumor 711 DIQLTQSPSSLSASVGDRVTMTCSASSSVSSSYLYWYQQKPGKAPKL
mAb WIYSTSNLASGVPARFSGSGSGTDFTLTISSLQPEDSASYFCHQWNR
containing YPYTFGGGTRLEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYP
DEC-205 mAb REAKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYE
ScFv KHKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLVESG
GGVVQPGRSLRLSCAASGFTFSNYGMWVRQAPGKGLEWVAVIW
YDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAEDTAVYYCA
RDLWGWYFDYWGQGTLVTVSSGGGGSGGGGSGGGGSGGGGSEIV
LTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAPRLLIYD
ASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRRNWPLTF
GGGTKVEIK
Sofituzumab LC DEC-205 791 EIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAPRLLI
mAb YDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRRNWP
containing LTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
tumor mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
ScFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSEVQLVESGG
GLVQPGGSLRLSCAASGYSITNDYAWNWVRQAPGKGLEWVGYISY
SGYTTYNPSLKSRFTISRDTSKNTLYLQMNSLRAEDTAVYYCARWT
SGLDWGQGTLVTVSSGGGGSGGGGSGGGGSGGGGSDIQMTQSPS
SLSASVGDRVTITCKASDLIHNWLAWYQQKPGKAPKLLIYGATSLE
TGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCQQWTTPFTFGQGT
KVEIK
LC tumor 792 DIQMTQSPSSLSASVGDRVTITCKASDLIHNWLAWYQQKPGKAPKL
mAb LIYGATSLETGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCQQWTT
containing PFTFGQGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPR
DEC-205 mAb EAKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYE
ScFv KHKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLVESG
GGVVQPGRSLRLSCAASGFTFSNYGMWVRQAPGKGLEWVAVIW
YDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAEDTAVYYCA
RDLWGWYFDYWGQGTLVTVSSGGGGSGGGGSGGGGSGGGGSEIV
LTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAPRLLIYD
ASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRRNWPLTF
GGGTKVEIK
Edrecolomab LC DEC-205 719 EIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAPRLLI
mAb YDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRRNWP
containing LTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
tumor mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
ScFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLQQSGA
ELVRPGTSVKVSCKASGYAFTNYLIEWVKQRPGQGLEWIGVINPGS
-177-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
GGTNYNEKFKGKATLTADKSSSTAYMQLSSLTSDDSAVYFCARDG
PWFAWGQGTLVTVSAGGGGSGGGGSGGGGSGGGGSNIVMTQSP
KSMSMSVGERVTLTCKASENVVTYVSWYQQKPEQSPKLLIYGASN
RYTGVPDRFTGSGSATDFTLTISSVQAEDLADYHCGQGYSYPYTFG
GGTKLEIK
LC tumor 720 NIVMTQSPKSMSMSVGERVTLTCKASENVVTYVSWYQQKPEQSPK
mAb LLIYGASNRYTGVPDRFTGSGSATDFTLTISSVQAEDLADYHCGQGY
containing SYPYTFGGGTKLEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFY
DEC-205 mAb PREAKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADY
ScFv EKHKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLVES
GGGVVQPGRSLRLSCAASGFTFSNYGMWVRQAPGKGLEWVAVI
WYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAEDTAVYYC
ARDLWGWYFDWGQGTLVTVSSGGGGSGGGGSGGGGSGGGGSEI
VLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAPRLLIY
DASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRRNWPL
TFGGGTKVEIK
Adecatumum LC DEC-205 695 EIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAPRLLI
ab mAb YDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRRNWP
containing LTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
tumor mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
ScFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSEVQLLESGG
GVVQPGRSLRLSCAASGFTFSSYGMHWVRQAPGKGLEWVAVISYD
GSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAEDTAVYYCAKD
MGWGSGWRPYYYYGMDVWGQGTTVTVSSGGGGSGGGGSGGGGS
GGGGSELQMTQSPSSLSASVGDRVTITCRTSQSISSYLNWYQQKPGQ
PPKLLIYWASTRESGVPDRFSGSGSGTDFTLTISSLQPEDSATYYCQQ
SYDIPYTFGQGTKLEIK
LC tumor 696 ELQMTQSPSSLSASVGDRVTITCRTSQSISSYLNWYQQKPGQPPKLLI
mAb WASTRESGVPDRFSGSGSGTDFTLTISSLQPEDSATYYCQQSYDIP
containing YTFGQGTKLEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
DEC-205 mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
ScFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLVESGG
GVVQPGRSLRLSCAASGFTFSNYGMWVRQAPGKGLEWVAVIWY
DGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAEDTAVYYCAR
DLWGWYFDWGQGTLVTVSSGGGGSGGGGSGGGGSGGGGSEIVL
TQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAPRLLIYDA
SNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRRNWPLTFG
GGTKVEIK
Anetumab LC DEC-205 701 EIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAPRLLI
mAb YDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRRNWP
containing LTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
tumor mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
ScFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVELVQSGA
EVKKPGESLKISCKGSGYSFTSWIGWVRQAPGKGLEWMGIIDPGD
SRTRYSPSFQGQVTISADKSISTAYLQWSSLKASDTAMYYCARGQL
YGGTYMDGWGQGTLVTVSSGGGGSGGGGSGGGGSGGGGSDIALT
QPASVSGSPGQSITISCTGTSSDIGGYNSVSWYQQHPGKAPKLMIYG
VNNRPSGVSNRFSGSKSGNTASLTISGLQAEDEADYYCSSYDIESAT
PVFGGGTKLTVL
LC tumor 702 DIALTQPASVSGSPGQSITISCTGTSSDIGGYNSVSWYQQHPGKAPKL
mAb MIYGVNNRPSGVSNRFSGSKSGNTASLTISGLQAEDEADYYCSSYDI
containing ESATPVFGGGTKLTVLGQPKAAPSVTLFPPSSEELQANKATLVCLIS
DEC-205 mAb DFYPGAVTVAWKGDSSPVKAGVETTTPSKQSNNKYAASSYLSLTPE
ScFv QWKSHRSYSCQVTHEGSTVEKTVAPTECSGGGGSGGGGSQVQLVE
SGGGVVQPGRSLRLSCAASGFTFSNYGMYWVRQAPGKGLEWVAVI
WYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAEDTAVYYC
ARDLWGWYFDWGQGTLVTVSSGGGGSGGGGSGGGGSGGGGSEI
VLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAPRLLIY
DASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRRNWPL
-178-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
TFGGGTKVEIK
huDS6 LC DEC-205 725 EIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAPRLLI
mAb YDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRRNWP
containing LTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
tumor mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
ScFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQAQLVQSGA
EVVKPGASVKMSCKASGYTFTSYNMHWVKQTPGQGLEWIGYIYPG
NGATNYNQKFQGKATLTADPSSSTAYMQISSLTSEDSAVYFCARGD
SVPFAWGQGTLVTVSAGGGGSGGGGSGGGGSGGGGSEIVLTQSP
ATMSASPGERVTITCSAHSSVSFMHWFQQKPGTSPKLWIYSTSSLAS
GVPARFGGSGSGTSYSLTISSMEAEDAATYYCQQRSSFPLTFGAGTK
LELK
LC tumor 726 EIVLTQSPATMSASPGERVTITCSAHSSVSFMHWFQQKPGTSPKLWI
mAb YSTSSLASGVPARFGGSGSGTSYSLTISSMEAEDAATYYCQQRSSFP
containing LTFGAGTKLELKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPR
DEC-205 mAb EAKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYE
ScFv KHKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLVESG
GGVVQPGRSLRLSCAASGFTFSNYGMWVRQAPGKGLEWVAVIW
YDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAEDTAVYYCA
RDLWGWYFDYWGQGTLVTVSSGGGGSGGGGSGGGGSGGGGSEIV
LTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAPRLLIYD
ASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRRNWPLTF
GGGTKVEIK
Lifastuzumab LC DEC-205 737
EIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAPRLLI
mAb YDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRRNWP
containing LTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
tumor mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
ScFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSEVQLVESGG
GLVQPGGSLRLSCAASGFSFSDFAMSWVRQAPGKGLEWVATIGRV
AFHTYYPDSMKGRFTISRDNSKNTLYLQMNSLRAEDTAVYYCARH
RGFDVGHFDFWGQGTLVTVSSGGGGSGGGGSGGGGSGGGGSDIQ
MTQSPSSLSASVGDRVTITCRSSETLVHSSGNTYLEWYQQKPGKAP
KLLIYRVSNRFSGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCFQGSF
NPLTFGQGTKVEIK
LC tumor 738 DIQMTQSPSSLSASVGDRVTITCRSSETLVHSSGNTYLEWYQQKPGK
mAb APKLLIYRVSNRFSGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCFQ
containing GSFNPLTFGQGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNN
DEC-205 mAb FYPREAKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKA
ScFv DYEKHKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLV
ESGGGVVQPGRSLRLSCAASGFTFSNYGMWVRQAPGKGLEWVA
VIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAEDTAVY
YCARDLWGWYFDYWGQGTLVTVSSGGGGSGGGGSGGGGSGGGG
SEIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAPRL
LIYDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRRN
WPLTFGGGTKVEIK
Sacituzumab LC DEC-205 782 EIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAPRLLI
mAb YDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRRNWP
containing LTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
tumor mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
ScFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLQQSGS
ELKKPGASVKVSCKASGYTFTNYGMNWVKQAPGQGLKWMGWIN
TYTGEPTYTDDFKGRFAFSLDTSVSTAYLQISSLKADDTAVYFCARG
GFGSSWYFDVWGQGSLVTVSSGGGGSGGGGSGGGGSGGGGSDIQ
LTQSPSSLSASVGDRVSITCKASQDVSIAVAWYQQKPGKAPKLLIYS
ASYRYTGVPDRFSGSGSGTDFTLTISSLQPEDFAVYYCQQHYITPLTF
GAGTKVEIK
LC tumor 783 DIQLTQSPSSLSASVGDRVSITCKASQDVSIAVAWYQQKPGKAPKLL
mAb IYSASYRYTGVPDRFSGSGSGTDFTLTISSLQPEDFAVYYCQQHYITP
containing LTFGAGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
-179-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
DEC-205 mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
ScFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLVESGG
GVVQPGRSLRLSCAASGFTFSNYGMWVRQAPGKGLEWVAVIWY
DGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAEDTAVYYCAR
DLWGWYFDWGQGTLVTVSSGGGGSGGGGSGGGGSGGGGSEIVL
TQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAPRLLIYDA
SNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRRNWPLTFG
GGTKVEIK
PR1A3 LC DEC-205 773 EIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAPRLLI
mAb YDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRRNWP
containing LTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
tumor mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
ScFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVKLQQSGP
ELKKPGETVKISCKASGYTFTEFGMNWVKQAPGKGLKWMGWINT
KTGEATYVEEFKGRFAFSLETSATTAYLQINNLKNEDTAKYFCARW
DFYDYVEAMDWGQGTTVTVSSGGGGSGGGGSGGGGSGGGGSDI
VMTQSQRFMSTSVGDRVSVTCKASQNVGTNVAWYQQKPGQSPKA
LIYSASYRYSGVPDRFTGSGSGTDFTLTISNVQSEDLAEYFCHQYYT
YPLFTFGSGTKLEMK
LC tumor 774 DIVMTQSQRFMSTSVGDRVSVTCKASQNVGTNVAWYQQKPGQSPK
mAb ALIYSASYRYSGVPDRFTGSGSGTDFTLTISNVQSEDLAEYFCHQYY
containing TYPLFTFGSGTKLEMKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNN
DEC-205 mAb FYPREAKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKA
ScFv DYEKHKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLV
ESGGGVVQPGRSLRLSCAASGFTFSNYGMWVRQAPGKGLEWVA
VIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAEDTAVY
YCARDLWGWYFDYWGQGTLVTVSSGGGGSGGGGSGGGGSGGGG
SEIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAPRL
LIYDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRRN
WPLTFGGGTKVEIK
Humanized LC DEC-205 821 EIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAPRLL
PR1A3 mAb IYDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRRNW
containing PLTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPR
tumor mAb EAKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYE
ScFv KHKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLVQSG
AEVKKPGASVKVSCKASGYTFTEFGMNWVRQAPGQGLEWMGWIN
TKTGEATYVEEFKGRVTFTTDTSTSTAYMELRSLRSDDTAVYYCAR
WDFAYYVEAMDWGQGTTVTVSSGGGGSGGGGSGGGGSGGGGS
DIQMTQSPSSLSASVGDRVTITCKASAAVGTYVAWYQQKPGKAPK
LLIYSASYRKRGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCHQYYT
YPLFTFGQGTKLEIK
LC tumor 822 DIQMTQSPSSLSASVGDRVTITCKASAAVGTYVAWYQQKPGKAPK
mAb LLIYSASYRKRGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCHQYYT
containing YPLFTFGQGTKLEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFY
DEC-205 mAb PREAKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADY
ScFv EKHKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLVES
GGGVVQPGRSLRLSCAASGFTFSNYGMWVRQAPGKGLEWVAVI
WYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAEDTAVYYC
ARDLWGWYFDWGQGTLVTVSSGGGGSGGGGSGGGGSGGGGSEI
VLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAPRLLIY
DASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRRNWPL
TFGGGTKVEIK
Humanized LC DEC-205 839 EIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAPRLL
Ab2-3 mAb IYDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRRNW
containing PLTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPR
tumor mAb EAKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYE
ScFv KHKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSEVQLQESG
PGLVKPGGSLSLSCAASGFVFSSYDMSWVRQTPERGLEWVAYISSG
GGITYAPSTVKGRFTVSRDNAKNTLYLQMNSLTSEDTAVYYCAAH
-180-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
YFGSSGPFAYWGQGTLVTVSSGGGGSGGGGSGGGGSGGGGSDIQM
TQSPASLSASVGDRVTITCRASENIFSYLAWYQQKPGKSPKLLVYNT
RTLAEGVPSRFSGSGSGTDFSLTISSLQPEDFATYYCQHHYGTPFTFG
SGTKLEIK
LC tumor 840 DIQMTQSPASLSASVGDRVTITCRASENIFSYLAWYQQKPGKSPKLL
mAb VYNTRTLAEGVPSRFSGSGSGTDFSLTISSLQPEDFATYYCQHHYGT
containing PFTFGSGTKLEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPR
DEC-205 mAb EAKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYE
ScFv KHKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLVESG
GGVVQPGRSLRLSCAASGFTFSNYGMWVRQAPGKGLEWVAVIW
YDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAEDTAVYYCA
RDLWGWYFDYWGQGTLVTVSSGGGGSGGGGSGGGGSGGGGSEIV
LTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAPRLLIYD
ASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRRNWPLT
FGGGTKVEIK
IMAB362, LC DEC-205 728 EIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAPRLLI
CLAUDIXIM mAb YDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRRNWP
AB containing LTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
tumor mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
ScFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLQQPGA
ELVRPGASVKLSCKASGYTFTSWINWVKQRPGQGLEWIGNIYPSD
SYTNYNQKFKDKATLTVDKSSSTAYMQLSSPTSEDSAVYYCTRSW
RGNSFDYWGQGTTLTVSSGGGGSGGGGSGGGGSGGGGSDIVMTQS
PSSLTVTAGEKVTMSCKSSQSLLNSGNQKNYLTWYQQKPGQPPKLL
IWASTRESGVPDRFTGSGSGTDFTLTISSVQAEDLAVYYCQNDYSY
PFTFGSGTKLEIK
LC tumor 729 DIVMTQSPSSLTVTAGEKVTMSCKSSQSLLNSGNQKNYLTWYQQKP
mAb GQPPKLLIYWASTRESGVPDRFTGSGSGTDFTLTISSVQAEDLAVYY
containing CQNDYSYPFTFGSGTKLEIKRTVAAPSVFIFPPSDEQLKSGTASVVCL
DEC-205 mAb LNNFYPREAKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTL
ScFv SKADYEKHKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQV
QLVESGGGVVQPGRSLRLSCAASGFTFSNYGMYWVRQAPGKGLE
WVAVIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAEDT
AVYYCARDLWGWYFDWGQGTLVTVSSGGGGSGGGGSGGGGSG
GGGSEIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQA
PRLLIYDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQR
RNWPLTFGGGTKVEIK
AMG595 LC DEC-205 698 EIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAPRLLI
mAb YDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRRNWP
containing LTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
tumor mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
ScFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLVESGG
GVVQSGRSLRLSCAASGFTFRNYGMHWVRQAPGKGLEWVAVIWY
DGSDKYYADSVRGRFTISRDNSKNTLYLQMNSLRAEDTAVYYCAR
DGYDILTGNPRDFDWGQGTLVTVSSGGGGSGGGGSGGGGSGGGG
SDTVMTQTPLSSHVTLGQPASISCRSSQSLVHSDGNTYLSWLQQRPG
QPPRLLIYRISRRFSGVPDRFSGSGAGTDFTLEISRVEAEDVGVYYCM
QSTHVPRTFGQGTKVEIK
LC tumor 699 DTVMTQTPLSSHVTLGQPASISCRSSQSLVHSDGNTYLSWLQQRPG
mAb QPPRLLIYRISRRFSGVPDRFSGSGAGTDFTLEISRVEAEDVGVYYCM
containing QSTHVPRTFGQGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLL
DEC-205 mAb NNFYPREAKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLS
ScFv KADYEKHKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQ
LVESGGGVVQPGRSLRLSCAASGFTFSNYGMYWVRQAPGKGLEW
VAVIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAEDTA
VYYCARDLWGWYFDWGQGTLVTVSSGGGGSGGGGSGGGGSGG
GGSEIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAP
RLLIYDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRR
NWPLTFGGGTKVEIK
-181-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
ABT806 LC DEC-205 692
EIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAPRLLI
mAb YDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRRNWP
containing LTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
tumor mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
ScFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSEVQLQESGP
GLVKPSQTLSLTCTVSGYSISRDFAWNWIRQPPGKGLEWMGYISYN
GNTRYQPSLKSRITISRDTSKNQFFLKLNSVTAADTATYYCVTASRG
FPWGQGTLVTVSSGGGGSGGGGSGGGGSGGGGSDIQMTQSPSSM
SVSVGDRVTITCHSSQDINSNIGWLQQKPGKSFKGLIYHGTNLDDGV
PSRFSGSGSGTDYTLTISSLQPEDFATYYCVQYAQFPWTFGGGTKLE
IK
LC tumor 693 DIQMTQSPSSMSVSVGDRVTITCHSSQDINSNIGWLQQKPGKSFKGL
mAb IYHGTNLDDGVPSRFSGSGSGTDYTLTISSLQPEDFATYYCVQYAQF
containing PWTFGGGTKLEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYP
DEC-205 mAb REAKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYE
ScFv KHKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLVESG
GGVVQPGRSLRLSCAASGFTFSNYGMWVRQAPGKGLEWVAVIW
YDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAEDTAVYYCA
RDLWGWYFDYWGQGTLVTVSSGGGGSGGGGSGGGGSGGGGSEIV
LTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAPRLLIYD
ASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRRNWPLTF
GGGTKVEIK
Sibrotuzumab LC DEC-205 788
EIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAPRLLI
mAb YDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRRNWP
containing LTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
tumor mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
ScFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLVQSGA
EVKKPGASVKVSCKTSRYTFTEYTIHWVRQAPGQRLEWIGGINPNN
GIPNYNQKFKGRVTITVDTSASTAYMELSSLRSEDTAVYYCARRRIA
YGYDEGHAMDYWGQGTLVTVSSGGGGSGGGGSGGGGSGGGGSDI
VMTQSPDSLAVSLGERATINCKSSQSLLYSRNQKNYLAWYQQKPG
QPPKLLIFWASTRESGVPDRFSGSGFGTDFTLTISSLQAEDVAVYYC
QQYFSYPLTFGQGTKVEIK
LC tumor 789 DIVMTQSPDSLAVSLGERATINCKSSQSLLYSRNQKNYLAWYQQKP
mAb GQPPKLLIFWASTRESGVPDRFSGSGFGTDFTLTISSLQAEDVAVYY
containing CQQYFSYPLTFGQGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCL
DEC-205 mAb LNNFYPREAKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTL
ScFv SKADYEKHKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQV
QLVESGGGVVQPGRSLRLSCAASGFTFSNYGMYWVRQAPGKGLE
WVAVIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAEDT
AVYYCARDLWGWYFDWGQGTLVTVSSGGGGSGGGGSGGGGSG
GGGSEIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQA
PRLLIYDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQR
RNWPLTFGGGTKVEIK
DS-8895a LC DEC-205 713
EIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAPRLLI
variant 1 mAb YDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRRNWP
containing LTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
tumor mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
ScFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLVQSGA
EVKKPGASVKVSCKASGYTFIDYSMHWVRQAPGQGLEWMGWINT
YTGEPTYSDDFKGRVTITADTSTSTAYLELSSLRSEDTAVYYCATYY
RYERDFDWGQGTLVTVSSGGGGSGGGGSGGGGSGGGGSDIVMT
QSPLSLPVTPGEPASISCRSSQSIVHSSGITYLEWYLQKPGQSPQLLIY
KVSNRFSGVPDRFSGSGSGTDFTLKISRVEAEDVGVYYCFQGSHVP
YTFGQGTKVEIK
LC tumor 714
DIVMTQSPLSLPVTPGEPASISCRSSQSIVHSSGITYLEWYLQKPGQSP
mAb QLLIYKVSNRFSGVPDRFSGSGSGTDFTLKISRVEAEDVGVYYCFQG
containing SHVPYTFGQGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNN
DEC-205 mAb FYPREAKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKA
-182-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
ScFv DYEKHKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLV
ESGGGVVQPGRSLRLSCAASGFTFSNYGMWVRQAPGKGLEWVA
VIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAEDTAVY
YCARDLWGWYFDYWGQGTLVTVSSGGGGSGGGGSGGGGSGGGG
SEIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAPRL
LIYDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRRN
WPLTFGGGTKVEIK
DS-8895a LC DEC-205 716
EIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAPRLLI
variant 2 mAb YDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRRNWP
containing LTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
tumor mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
ScFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQIQLVQSGAE
VKKPGASVKVSCKASGYTFIDYSMHWVRQAPGQGLKWMGWINTY
TGEPTYSDDFKGRFAFSLDTSTSTAYLELSSLRSEDTAVYYCATYYR
YERDFDWGQGTLVTVSSGGGGSGGGGSGGGSGGGGSDVLMTQS
PLSLPVTPGEPASISCRSSQSIVHS SGITYLEWYLQKPGQSPQLLIYKV
SNRFSGVPDRFSGSGSGTDFTLKISRVEAEDVGVYYCFQGSHVPYTF
GQGTKVEIK
LC tumor 717
DVLMTQSPLSLPVTPGEPASISCRSSQSIVHSSGITYLEWYLQKPGQS
mAb PQLLIYKVSNRFSGVPDRFSGSGSGTDFTLKISRVEAEDVGVYYCFQ
containing GSHVPYTFGQGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLN
DEC-205 mAb NFYPREAKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSK
ScFv ADYEKHKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQL
VESGGGVVQPGRSLRLSCAASGFTFSNYGMWVRQAPGKGLEWV
AVIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAEDTAV
YYCARDLWGWYFDWGQGTLVTVSSGGGGGSGGGGSGGGSGGG
GSEIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAPR
LLIYDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRRN
WPLTFGGGTKVEIK
MEDI-547 LC DEC-205 743
EIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAPRLLI
mAb YDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRRNWP
containing LTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
tumor mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
ScFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSEVQLLESGG
GLVQPGGSLRLSCAASGFTFSHYMMAWVRQAPGKGLEWVSRIGPS
GGPTHYADSVKGRFTISRDNSKNTLYLQMNSLRAEDTAVYYCAGY
DSGYDYVAVAGPAEYFQHWGQGTLVTVSSGGGGSGGGGSGGGGS
GGGGSDIQMTQSPSSLSASVGDRVTITCRASQSISTWLAWYQQKPG
KAPKLLIYKASNLHTGVPSRFSGSGSGTEFSLTISGLQPDDFATYYCQ
QYNSYSRTFGQGTKVEIK
LC tumor 744 DIQMTQSPSSLSASVGDRVTITCRASQSISTWLAWYQQKPGKAPKLL
mAb IYKASNLHTGVPSRFSGSGSGTEFSLTISGLQPDDFATYYCQQYNSYS
containing RTFGQGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
DEC-205 mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
ScFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLVESGG
GVVQPGRSLRLSCAASGFTFSNYGMWVRQAPGKGLEWVAVIWY
DGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAEDTAVYYCAR
DLWGWYFDWGQGTLVTVSSGGGGSGGGGSGGGGSGGGGSEIVL
TQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAPRLLIYDA
SNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRRNWPLTFG
GGTKVEIK
Narnatumab LC DEC-205 749
EIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAPRLLI
mAb YDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRRNWP
containing LTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
tumor mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
ScFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSEVQLVESGG
GLVQPGGSLRLSCAASGFTFSSYLMTWVRQAPGKGLEWVANIKQD
GSEKYYVDSVKGRFTISRDNAKNSLNLQMNSLRAEDTAVYYCTRD
GYSSGRHYGMDVWGQGTTVIVSSGGGGSGGGGSGGGGSGGGGSEI
-183-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
VLTQSPATLSLSPGERATLSCRASQSVSRYLAWYQQKPGQAPRLLIY
DASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRSNWPR
TFGQGTKVEIK
LC tumor 750 EIVLTQSPATLSLSPGERATLSCRASQSVSRYLAWYQQKPGQAPRLL
mAb IYDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRSNWP
containing RTFGQGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
DEC-205 mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
ScFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLVESGG
GVVQPGRSLRLSCAASGFTFSNYGMWVRQAPGKGLEWVAVIWY
DGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAEDTAVYYCAR
DLWGWYFDWGQGTLVTVSSGGGGSGGGGSGGGGSGGGGSEIVL
TQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAPRLLIYDA
SNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRRNWPLTFG
GGTKVEIK
RG7841 LC DEC-205 776 EIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAPRLLI
mAb YDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRRNWP
containing LTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
tumor mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
ScFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSEVQLVESGP
ALVKPTQTLTLTCTVSGFSLTGYSVNWIRQPPGKALEWLGMIWGDG
STDYNSALKSRLTISKDTSKNQVVLTMTNMDPVDTATYYCARDYY
FNYASWFAYWGQGTLVTVSSGGGGSGGGGSGGGGSGGGGSDIQM
TQSPSSLSASVGDRVTITCSASQGISNYLNWYQQKPGKTVKLLIYYT
SNLHSGVPSRFSGSGSGTDYTLTISSLQPEDFATYYCQQYSELPWTF
GQGTKVEIK
LC tumor 777 DIQMTQSPSSLSASVGDRVTITCSASQGISNYLNWYQQKPGKTVKLL
mAb IYYTSNLHSGVPSRFSGSGSGTDYTLTISSLQPEDFATYYCQQYSELP
containing WTFGQGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPR
DEC-205 mAb EAKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYE
ScFv KHKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLVESG
GGVVQPGRSLRLSCAASGFTFSNYGMWVRQAPGKGLEWVAVIW
YDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAEDTAVYYCA
RDLWGWYFDYWGQGTLVTVSSGGGGSGGGGSGGGGSGGGGSEIV
LTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAPRLLIYD
ASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRRNWPLTF
GGGTKVEIK
Farletuzumab LC DEC-205 722
EIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAPRLLI
mAb YDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRRNWP
containing LTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
tumor mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
ScFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSEVQLVESGG
GVVQPGRSLRLSCSASGFTFSGYGLSWVRQAPGKGLEWVAMISSGG
SYTYYADSVKGRFAISRDNAKNTLFLQMDSLRPEDTGVYFCARHG
DDPAWFAWGQGTPVTVSSGGGGSGGGGSGGGGSGGGGSDIQLT
QSPSSLSASVGDRVTITCSVSSSISSNNLHWYQQKPGKAPKPWIYGT
SNLASGVPSRFSGSGSGTDYTFTISSLQPEDIATYYCQQWSSYPYMY
TFGQGTKVEIK
LC tumor 723 DIQLTQSPSSLSASVGDRVTITCSVSSSISSNNLHWYQQKPGKAPKP
mAb WIYGTSNLASGVPSRFSGSGSGTDYTFTISSLQPEDIATYYCQQWSS
containing YPYMYTFGQGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNN
DEC-205 mAb FYPREAKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKA
ScFv DYEKHKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLV
ESGGGVVQPGRSLRLSCAASGFTFSNYGMWVRQAPGKGLEWVA
VIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAEDTAVY
YCARDLWGWYFDYWGQGTLVTVSSGGGGSGGGGSGGGGSGGGG
SEIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAPRL
LIYDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRRN
WPLTFGGGTKVEIK
Mirvetuximab LC DEC-205 746
EIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAPRLLI
-184-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
mAb YDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRRNWP
containing LTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
tumor mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
ScFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLVQSGA
EVVKPGASVKISCKASGYTFTGYFMNWVKQSPGQSLEWIGRIHPYD
GDTFYNQKFQGKATLTVDKSSNTAHMELLSLTSEDFAVYYCTRYD
GSRAMDYWGQGTTVTVSSGGGGSGGGGSGGGGSGGGGSDIVLTQS
PLSLAVSLGQPAIISCKASQSVSFAGTSLMHWYHQKPGQQPRLLIYR
ASNLEAGVPDRFSGSGSKTDFTLTISPVEAEDAATYYCQQSREYPYT
FGGGTKLEIK
LC tumor 747 DIVLTQSPLSLAVSLGQPAIISCKASQSVSFAGTSLMHWYHQKPGQQ
mAb PRLLIYRASNLEAGVPDRFSGSGSKTDFTLTISPVEAEDAATYYCQQ
containing SREYPYTFGGGTKLEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNN
DEC-205 mAb FYPREAKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKA
ScFv DYEKHKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLV
ESGGGVVQPGRSLRLSCAASGFTFSNYGMWVRQAPGKGLEWVA
VIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAEDTAVY
YCARDLWGWYFDYWGQGTLVTVSSGGGGSGGGGSGGGGSGGGG
SEIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAPRL
LIYDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRRN
WPLTFGGGTKVEIK
J591 variantl LC DEC-205 731
EIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAPRLLI
mAb YDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRRNWP
containing LTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
tumor mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
ScFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSEVQLQQSGP
ELKKPGTSVRISCKTSGYTFTEYTIHWVKQSHGKSLEWIGNINPNNG
GTTYNQKFEDKATLTVDKSSSTAYMELRSLTSEDSAVYYCAAGWN
FDWGQGTTLTVSSGGGGSGGGGSGGGGSGGGGSDIVMTQSHKF
MSTSVGDRVSIICKASQDVGTAVDWYQQKPGQSPKLLIWASTRHT
GVPDRFTGSGSGTDFTLTITNVQSEDLADYFCQQYNSYPLTFGAGT
MLDLK
LC tumor 732 DIVMTQSHKFMSTSVGDRVSIICKASQDVGTAVDWYQQKPGQSPKL
mAb LIWASTRHTGVPDRFTGSGSGTDFTLTITNVQSEDLADYFCQQYNS
containing YPLTFGAGTMLDLKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFY
DEC-205 mAb PREAKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADY
ScFv EKHKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLVES
GGGVVQPGRSLRLSCAASGFTFSNYGMWVRQAPGKGLEWVAVI
WYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAEDTAVYYC
ARDLWGWYFDWGQGTLVTVSSGGGGSGGGGSGGGGSGGGGSEI
VLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAPRLLIY
DASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRRNWPL
TFGGGTKVEIK
J591 variant 2 LC DEC-205 734
EIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAPRLLI
mAb YDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRRNWP
containing LTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
tumor mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
ScFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSEVQLQQSGP
ELVKPGTSVRISCKTSGYTFTEYTIHWVKQSHGKSLEWIGNINPNNG
GTTYNQKFEDKATLTVDKSSSTAYMELRSLTSEDSAVYYCAAGWN
FDWGQGTTLTVSSGGGGSGGGGSGGGGSGGGGSNIVMTQSPKSM
SMSVGERVTLTCKASENVVTYVSWYQQKPEQSPKLLIYGASNRYT
GVPDRFTGSGSATDFTLTISSVQAEDLADYHCGQGYSYPYTFGGGT
KLEIK
LC tumor 735 NIVMTQSPKSMSMSVGERVTLTCKASENVVTYVSWYQQKPEQSPK
mAb LLIYGASNRYTGVPDRFTGSGSATDFTLTISSVQAEDLADYHCGQGY
containing SYPYTFGGGTKLEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFY
DEC-205 mAb PREAKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADY
ScFv EKHKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLVES
-185-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
GGGVVQPGRSLRLSCAASGFTFSNYGMWVRQAPGKGLEWVAVI
WYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAEDTAVYYC
ARDLWGWYFDWGQGTLVTVSSGGGGSGGGGSGGGGSGGGGSEI
VLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAPRLLIY
DASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRRNWPL
TFGGGTKVEIK
Rovalpituzum LC DEC-205 779
EIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAPRLLI
ab mAb YDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRRNWP
containing LTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
tumor mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
ScFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLVQSGA
EVKKPGASVKVSCKASGYTFTNYGMNWVRQAPGQGLEWMGWIN
TYTGEPTYADDFKGRVTMTTDTSTSTAYMELRSLRSDDTAVYYCA
RIGDSSPSDWGQGTLVTVSSGGGGSGGGGSGGGGSGGGGSEIVMT
QSPATLSVSPGERATLSCKASQSVSNDVVWYQQKPGQAPRLLIYYA
SNRYTGIPARFSGSGSGTEFTLTISSLQSEDFAVYYCQQDYTSPWTFG
QGTKLEIK
LC tumor 780 EIVMTQSPATLSVSPGERATLSCKASQSVSNDVVWYQQKPGQAPRL
mAb LIYYASNRYTGIPARFSGSGSGTEFTLTISSLQSEDFAVYYCQQDYTS
containing PWTFGQGTKLEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYP
DEC-205 mAb REAKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYE
ScFv KHKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLVESG
GGVVQPGRSLRLSCAASGFTFSNYGMWVRQAPGKGLEWVAVIW
YDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAEDTAVYYCA
RDLWGWYFDYWGQGTLVTVSSGGGGSGGGGSGGGGSGGGGSEIV
LTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAPRLLIYD
ASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRRNWPLTF
GGGTKVEIK
PF-06647020 LC DEC-205 770 EIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAPRLLI
mAb YDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRRNWP
containing LTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
tumor mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
ScFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLVQSGP
EVKKPGASVKVSCKASGYTFTDYAVHWVRQAPGKRLEWIGVISTY
NDYTYNNQDFKGRVTMTRDTSASTAYMELSRLRSEDTAVYYCARG
NSYFYALDYWGQGTSVTVSSGGGGSGGGGSGGGGSGGGGSEIVLT
QSPATLSLSPGERATLSCRASESVDSYGKSFMHWYQQKPGQAPRLLI
YRASNLESGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQSNEDPW
TFGGGTKLEIK
LC tumor 771 EIVLTQSPATLSLSPGERATLSCRASESVDSYGKSFMHWYQQKPGQ
mAb APRLLIYRASNLESGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQS
containing NEDPWTFGGGTKLEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNN
DEC-205 mAb FYPREAKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKA
ScFv DYEKHKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLV
ESGGGVVQPGRSLRLSCAASGFTFSNYGMWVRQAPGKGLEWVA
VIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAEDTAVY
YCARDLWGWYFDYWGQGTLVTVSSGGGGSGGGGSGGGGSGGGG
SEIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAPRL
LIYDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRRN
WPLTFGGGTKVEIK
Antibody to LC DEC-205 689
EIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAPRLLI
PTK7 mAb YDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRRNWP
containing LTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
tumor mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
ScFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLVESGG
GVVQPGRSLRLSCAASGFTFSSYAFHWVRQAPGKGLEWVAVISYD
GSIKYYADSVKGRFTISRDNSKNTLYLQMNSLRAEDTAVYYCARTY
YFDWGQGTLVTVSSGGGGSGGGGSGGGGSGGGGSEIVLTQSPDF
QSVTPKEKVTITCRASQSIGSSLHWYQQKPDQSPKLLIKYASQSFSG
-186-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
VPSRFSGSGSGTDFTLTINSLEAEDAAAYYCHQS SSLPITFGQGTRLEI
K
LC tumor 690 EIVLTQSPDFQSVTPKEKVTITCRASQSIGSSLHWYQQKPDQSPKLLI
mAb KYASQSFSGVPSRFSGSGSGTDFTLTINSLEAEDAAAYYCHQSSSLPI
containing TFGQGTRLEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREA
DEC-205 mAb KVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEKH
ScFv KVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLVESGGG
VVQPGRSLRLSCAASGFTFSNYGMWVRQAPGKGLEWVAVIWYD
GSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAEDTAVYYCARD
LWGWYFDWGQGTLVTVSSGGGGSGGGGSGGGGSGGGGSEIVLT
QSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAPRLLIYDAS
NRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRRNWPLTFG
GGTKVEIK
Ladiratuzuma LC DEC-205 785
EIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAPRLLI
b mAb YDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRRNWP
containing LTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
tumor mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
ScFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLVQSGA
EVKKPGASVKVSCKASGLTIEDYYMHWVRQAPGQGLEWMGWIDP
ENGDTEYGPKFQGRVTMTRDTSINTAYMELSRLRSDDTAVYYCAV
HNAHYGTWFAWGQGTLVTVSSGGGGSGGGGSGGGGSGGGGSD
VVMTQSPLSLPVTLGQPASISCRSSQSLLHSSGNTYLEYFQQRPGQSP
RPLIYKISTRFSGVPDRFSGSGSGTDFTLKISRVEAEDVGVYYCFQGS
HVPYTFGGGTKVEIK
LC tumor 786 DVVMTQSPLSLPVTLGQPASISCRSSQSLLHSSGNTYLEYFQQRPGQ
mAb SPRPLIYKISTRFSGVPDRFSGSGSGTDFTLKISRVEAEDVGVYYCFQ
containing GSHVPYTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLN
DEC-205 mAb NFYPREAKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSK
ScFv ADYEKHKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQL
VESGGGVVQPGRSLRLSCAASGFTFSNYGMWVRQAPGKGLEWV
AVIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAEDTAV
YYCARDLWGWYFDWGQGTLVTVSSGGGGSGGGGSGGGGSGGG
GSEIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAPR
LLIYDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRRN
WPLTFGGGTKVEIK
Cirmtuzumab LC DEC-205 707 EIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAPRLLI
mAb YDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRRNWP
containing LTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
tumor mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
ScFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLQESGP
GLVKPSQTLSLTCTVSGYAFTAYNIHWVRQAPGQGLEWMGSFDPY
DGGSSYNQKFKDRLTISKDTSKNQVVLTMTNMDPVDTATYYCARG
WYYFDWGHGTLVTVSSGGGGSGGGGSGGGGSGGGGSDIVMTQT
PLSLPVTPGEPASISCRASKSISKYLAWYQQKPGQAPRLLIYSGSTLQ
SGIPPRFSGSGYGTDFTLTINNIESEDAAYYFCQQHDESPY
LC tumor 708 DIVMTQTPLSLPVTPGEPASISCRASKSISKYLAWYQQKPGQAPRLLI
mAb YSGSTLQSGIPPRFSGSGYGTDFTLTINNIESEDAAYYFCQQHDESPY
containing TFGEGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREA
DEC-205 mAb KVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEKH
ScFv KVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLVESGGG
VVQPGRSLRLSCAASGFTFSNYGMWVRQAPGKGLEWVAVIWYD
GSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAEDTAVYYCARD
LWGWYFDWGQGTLVTVSSGGGGSGGGGSGGGGSGGGGSEIVLT
QSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAPRLLIYDAS
NRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRRNWPLTFG
GGTKVEIK
Antibody to LC DEC-205 740
EIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAPRLLI
MAGE-A3 mAb YDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRRNWP
containing LTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
-187-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
tumor mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
ScFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLVESGG
GVVQPGRSLRLSCTASGFRFRSHGMHWVRQAPGKGLEWVAVISYD
GNNKLYADSVKGRITISRDNSKNTLFLQMNNVRAEDTAVYYCASP
YTSDWQYFQWGQGTLVIVSSGGGGSGGGGSGGGGSGGGGSEIVM
TQSPATLSVSPGERATFSCRASQNISTTLAWYQQKPGQAPRLLIYDT
STRATGIPARFSGSGSGTEFTLTISSLQSEDLAVYYCQQSNSWPLTFG
GGTKVEIK
LC tumor 741 EIVMTQSPATLSVSPGERATFSCRASQNISTTLAWYQQKPGQAPRLL
mAb IYDTSTRATGIPARFSGSGSGTEFTLTISSLQSEDLAVYYCQQSNSWP
containing LTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
DEC-205 mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
ScFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLVESGG
GVVQPGRSLRLSCAASGFTFSNYGMWVRQAPGKGLEWVAVIWY
DGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAEDTAVYYCAR
DLWGWYFDWGQGTLVTVSSGGGGSGGGGSGGGGSGGGGSEIVL
TQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAPRLLIYDA
SNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRRNWPLTFG
GGTKVEIK
Antibody to LC DEC-205 755
EIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAPRLLI
NY-ESO-1 mAb YDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRRNWP
containing LTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
tumor mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
ScFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLVQSGG
GVVRPGGSLRLSCAASGFSFIDYGMSWVRQVPGKGLEWVAGMNW
SGDKKGHAESVKGRFIISRDNAKNTLYLEMSSLRVEDTALYFCARG
EYSNRFDPRGRGTLVTVSSGGGGSGGGGSGGGGSGGGGSDIVMTQ
TPLSLPVTLGQPASLSCRSSQSLVFTDGNTYLNWFQQRPGQSPRRLI
YKVSSRDPGVPDRFSGTGSGTDFTLEISRVEAEDIGVYYCMQGTHW
PPIFGQGTKVEIK
LC tumor 756 DIVMTQTPLSLPVTLGQPASLSCRSSQSLVFTDGNTYLNWFQQRPGQ
mAb SPRRLIYKVSSRDPGVPDRFSGTGSGTDFTLEISRVEAEDIGVYYCM
containing QGTHWPPIFGQGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLL
DEC-205 mAb NNFYPREAKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLS
ScFv KADYEKHKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQ
LVESGGGVVQPGRSLRLSCAASGFTFSNYGMYWVRQAPGKGLEW
VAVIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAEDTA
VYYCARDLWGWYFDWGQGTLVTVSSGGGGSGGGGSGGGGSGG
GGSEIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAP
RLLIYDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRR
NWPLTFGGGTKVEIK
Trastuzumab LC DEC-205 794 EIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAPRLLI
mAb YDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRRNWP
containing LTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
tumor mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
ScFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSEVQLVESGG
GLVQPGGSLRLSCAASGFNIKDTYIHWVRQAPGKGLEWVARIYPTN
GYTRYADSVKGRFTISADTSKNTAYLQMNSLRAEDTAVYYCSRWG
GDGFYAMDWGQGTLVTVSSGGGGSGGGGSGGGGSGGGGSDIQM
TQSPSSLSASVGDRVTITCRASQDVNTAVAWYQQKPGKAPKLLIYS
ASFLYSGVPSRFSGSRSGTDFTLTISSLQPEDFATYYCQQHYTTPPTF
GQGTKVEIK
LC tumor 795 DIQMTQSPSSLSASVGDRVTITCRASQDVNTAVAWYQQKPGKAPKL
mAb LIYSASFLYSGVPSRFSGSRSGTDFTLTISSLQPEDFATYYCQQHYTTP
containing PTFGQGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
DEC-205 mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
ScFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLVESGG
GVVQPGRSLRLSCAASGFTFSNYGMWVRQAPGKGLEWVAVIWY
DGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAEDTAVYYCAR
-188-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
DLWGWYFDWGQGTLVTVSSGGGGSGGGGSGGGGSGGGGSEIVL
TQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAPRLLIYDA
SNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRRNWPLTFG
GGTKVEIK
LC DEC205 799 EIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAPRLLI
mAb YDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRRNWP
containing LTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
tumor mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
scFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSDIQMTQSPSS
(LH,25mer) LSASVGDRVTITCRASQDVNTAVAWYQQKPGKAPKLLIYSASFLYS
GVPSRFSGSRSGTDFTLTISSLQPEDFATYYCQQHYTTPPTFGQGTKV
EIKGGGGSGGGGSGGGGSGGGGSGGGGSEVQLVESGGGLVQPGGS
LRLSCAASGFNIKDTYIHWVRQAPGKGLEWVARIYPTNGYTRYADS
VKGRFTISADTSKNTAYLQMNSLRAEDTAVYYCSRWGGDGFYAM
DWGQGTLVTVSS
TABLE 11. Fusion Sequences ¨ CD40 fusion with DEC205
Antibody Region SEQ Sequence
ID
NO:
DEC205 HC DEC205 243
QVQLVESGGGVVQPGRSLRLSCAASGFTFSNYGMWVRQAPGKGL
variant 1 mAb with EWVAVIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAED
CD40 mAb TAVYYCARDLWGWYFDWGQGTLVTVSSASTKGPS VFPLAPSSKS
ScFv TSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLY
SLSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCP
PCPAPELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVK
FNWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKE
YKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSL
TCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTV
DKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGGGGGSGGGGS
QVQLVQSGAEVKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQG
LEWMGWINPDSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSD
DTAVYYCARDQPLGYCTNGVCSYFDYWGQGTLVTVSSGGGGSGG
GGSGGGGSGGGGSDIQMTQSPSSVSASVGDRVTITCRASQGIYSWL
AWYQQKPGKAPNLLIYTASTLQSGVPSRFSGSGSGTDFTLTISSLQPE
DFATYYCQQANIFPLTFGGGTKVEIK
HC CD40 242 QVQLVQSGAEVKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQG
mAb with LEWMGWINPDSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSD
DEC205 mAb DTAVYYCARDQPLGYCTNGVCSYFDYWGQGTLVTVSSASTKGPSV
ScFv FPLAPSSKSTSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPA
VLQSSGLYSLSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKS
CDKTHTCPPCPAPELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDV
SHEDPEVKFNWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQ
DWLNGKEYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREE
MTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGS
FFLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGGG
GGSGGGGSQVQLVESGGGVVQPGRSLRLSCAASGFTFSNYGMWV
RQAPGKGLEWVAVIWYDGSNKYYADSVKGRFTISRDNSKNTLYLQ
MNSLRAEDTAVYYCARDLWGWYFDYWGQGTLVTVSSGGGGSGG
GGSGGGGSGGGGSEIVLTQSPATLSLSPGERATLSCRASQSVSSYLA
WYQQKPGQAPRLLIYDASNRATGIPARFSGSGSGTDFTLTISSLEPED
FAVYYCQQRRNWPLTFGGGTKVEIK
LC DEC205 244 EIVLTQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAPRLL
mAb IYDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRRNW
containing PLTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPR
CD40 mAb EAKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYE
scFv KHKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLVQSG
AEVKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQGLEWMGWI
-189-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
NPDSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSDDTAVYYC
ARDQPLGYCTNGVCSYFDWGQGTLVTVSSGGGGSGGGGSGGGG
SGGGGSDIQMTQSPSSVSASVGDRVTITCRASQGIYSWLAWYQQKP
GKAPNLLIYTASTLQSGVPSRFSGSGSGTDFTLTISSLQPEDFATYYC
QQANIFPLTFGGGTKVEIK
LC CD40 800 DIQMTQSPSSVSASVGDRVTITCRASQGIYSWLAWYQQKPGKAPNL
mAb LIYTASTLQSGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCQQANIFP
containing LTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
DEC205 mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
scFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLVESGG
GVVQPGRSLRLSCAASGFTFSNYGMWVRQAPGKGLEWVAVIWY
DGSNKYYADSVKGRFTISRDNSKNTLYLQMNSLRAEDTAVYYCAR
DLWGWYFDWGQGTLVTVSSGGGGSGGGGSGGGGSGGGGSEIVL
TQSPATLSLSPGERATLSCRASQSVSSYLAWYQQKPGQAPRLLIYDA
SNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRRNWPLTF
GGGTKVEIK
DEC205 HC DEC205 256 EVQLVQSGAEVKKPGESLRISCKGSGDSFTTWIGWVRQMPGKGL
variant 2 mAb with EWMGIIYPGDSDTIYSPSFQGQVTISADKSISTAYLQWSSLKASDTA
CD40 mAb MYYCTRGDRGVDWGQGTLVTVSSASTKGPSVFPLAPSSKSTSGG
ScFv TAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSS
VVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCP
APELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNW
YVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKC
KVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLTCL
VKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTVDK
SRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGGGGGSGGGGSQV
QLVQSGAEVKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQGLE
WMGWINPDSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSDDT
AVYYCARDQPLGYCTNGVCSYFDWGQGTLVTVSSGGGGSGGGG
SGGGGSGGGGSDIQMTQSPSSVSASVGDRVTITCRASQGIYSWLAW
YQQKPGKAPNLLIYTASTLQSGVPSRFSGSGSGTDFTLTISSLQPEDF
ATYYCQQANIFPLTFGGGTKVEIK
HC CD40 255 QVQLVQSGAEVKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQG
mAb with LEWMGWINPDSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSD
DEC205 mAb DTAVYYCARDQPLGYCTNGVCSYFDYWGQGTLVTVSSASTKGPSV
ScFv FPLAPSSKSTSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPA
VLQSSGLYSLSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKS
CDKTHTCPPCPAPELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDV
SHEDPEVKFNWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQ
DWLNGKEYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREE
MTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGS
FFLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGGG
GGSGGGGSEVQLVQSGAEVKKPGESLRISCKGSGDSFTTWIGWVR
QMPGKGLEWMGIIYPGDSDTIYSPSFQGQVTISADKSISTAYLQWSS
LKASDTAMYYCTRGDRGVDWGQGTLVTVSSGGGGSGGGGSGGG
GSGGGGSDIQMTQSPSSLSASVGDRVTITCRASQGISRWLAWYQQK
PEKAPKSLIYAASSLQSGVPSRFSGSGSGTDFTLTISGLQPEDFATYY
CQQYNSYPRTFGQGTKVEIK
LC DEC205 257 DIQMTQSPSSLSASVGDRVTITCRASQGISRWLAWYQQKPEKAPKS
mAb LIYAASSLQSGVPSRFSGSGSGTDFTLTISGLQPEDFATYYCQQYNSY
containing PRTFGQGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPR
CD40 mAb EAKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYE
scFv KHKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSQVQLVQSG
AEVKKPGASVKVSCKASGYTFTGYYMHWVRQAPGQGLEWMGWI
NPDSGGTNYAQKFQGRVTMTRDTSISTAYMELNRLRSDDTAVYYC
ARDQPLGYCTNGVCSYFDWGQGTLVTVSSGGGGSGGGGSGGGG
SGGGGSDIQMTQSPSSVSASVGDRVTITCRASQGIYSWLAWYQQKP
GKAPNLLIYTASTLQSGVPSRFSGSGSGTDFTLTISSLQPEDFATYYC
QQANIFPLTFGGGTKVEIK
-190-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
Antibody Region SEQ Sequence
ID
NO:
LC CD40 801 DIQMTQSPSSVSASVGDRVTITCRASQGIYSWLAWYQQKPGKAPNL
mAb LIYTASTLQSGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCQQANIFP
containing LTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPRE
DEC205 mAb AKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
scFv HKVYACEVTHQGLSSPVTKSFNRGECGGGGSGGGGSEVQLVQSGA
EVKKPGESLRISCKGSGDSFTTYWIGWVRQMPGKGLEWMGIIYPGD
SDTIYSPSFQGQVTISADKSISTAYLQWSSLKASDTAMYYCTRGDRG
VDYWGQGTLVTVSSGGGGSGGGGSGGGGSGGGGSDIQMTQSPSSL
SASVGDRVTITCRASQGISRWLAWYQQKPEKAPKSLIYAASSLQSG
VPSRFSGSGSGTDFTLTISGLQPEDFATYYCQQYNSYPRTFGQGTKV
EIK
Immune-Stimulatory Compounds
[0304] In some embodiments, the immune-stimulatory conjugates described herein
further
comprise an immune-stimulatory compound. An immune-stimulatory compound can be
a small
molecule, a compound or molecule that binds to a protein target and can
activate the target
protein's function, or a compound that binds to a protein target and can
inhibit the protein
target's function, resulting in immune stimulation or modulation. In certain
embodiments, an
immune-stimulatory compound of a conjugate (i.e., attached to an antibody
construct either
directly or via a linker) can stimulate or activate its protein target with no
or minimal processing
of the conjugate. In certain embodiments, an immune-stimulatory compound of a
conjugate (i.e.,
attached to an antibody construct either directly or via a linker) can inhibit
its protein target with
no or minimal processing of the conjugate. In this context, processing refers
to degradation of
the antibody construct or cleavage of the linker to liberate the immune-
stimulatory compound or
degredation product of the conjugate containing the immune-stimulatory
compound. In certain
embodiments, the protein target of the immune-stimulatory compound is an
extracellular protein
target and is located on the cell surface membrane or cellular compartments in
communication
with the cell surface, such as in the endoplasmic reticulum (ER). In certain
embodiments, the
protein target is intracellular, such as in the cytoplasm.
[0305] In some embodiments, an immune-stimulatory compound can activate immune
cells. In
some embodiments, an immune-stimulatory compound can reduce inhibition of
immune cells. In
some embodiments, an immune-stimulatory compound can stimulate immune
activation by
triggering degradation of a protein target.
[0306] In some embodiments, an immune-stimulatory compound can be a molecule
or
compound whose action on its target can lead to immune stimulation by direct
immune cell
activation. In some embodiments, the immune activation can be indirect by
alteration of the
immune suppressive microenvironment of a tumor (e.g., removing an
immunosuppressive signal
or altering an immunosuppressive state). In some embodiments, the immune-
stimulatory
-191-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
compound's activity can be both direct and indirect. In certain embodiments,
an immune-
stimulatory conjugate can alter the activity of its protein target in cells
having an antigen bound
by the conjugate as compared to activity of the protein target in non-antigen
bearing cells (i.e.,
the immune-stimulatory activity is antigen-specific).
[0307] In certain embodiments, the immune-stimulatory compound can be coupled
to an Fc
domain or other portion of an antibody construct via a linker. In each of the
embodiments
described herein, the immune-stimulatory compound can be coupled to the
antibody construct via
a linker.
[0308] An immune-stimulatory compound can be a Pattern recognition receptor
(PRR) agonist.
Pattern recognition receptors (PRRs) can recognize pathogen-associated
molecular patterns
(PAMPs) and damage-associated molecular patterns (DAMPs). A PRR can be
membrane bound.
A PRR can be cytosolic. A PAMP molecule can be a toll-like receptor agonist. A
PRR can be a
toll-like receptor (TLR). A PRR can be RIG-I-like receptor. A PRR can be a
receptor kinase. A
PRR can be a C-type lectin receptor. A PRR can be a NOD-like receptor. A PRR
can be TLR1,
TLR2, TLR3, TLR4, TLR5, TLR6, TLR7, TLR8, TLR9, TLR10, TLR11, TLR12, or TLR13.
[0309] A PRR agonist can be a damage-associated molecular pattern (DAMP)
molecule. A
DAMP molecule can be an intracellular protein. A DAMP molecule can be a heat-
shock protein.
A DAMP molecule can be an HMGB1 protein. A DAMP molecule can be a protein
derived from
the extracellular matrix that is generated after tissue injury. A DAMP
molecule can be a
hyaluronan fragment. A DAMP molecule can be DNA. A DAMP molecule can be RNA. A
DAMP molecule can be an S100 molecule. A DAMP molecule can be a nucleotide(s).
A DAMP
molecule can be ATP. A DAMP molecule can be a nucleoside(s). A DAMP molecule
can be an
adenosine. A DAMP molecule can be uric acid.
[0310] In some embodiments, the immune-stimulatory compound can be a Toll-like
receptor
agonist, a RIG-I agonist, a STING agonist, a GPCR agonist, an ion channel
agonist, a membrane
transporter agonist, an ER protein agonist, a beta-catenin pathway inhibitor,
a kinase inhibitor, a
TNIK inhibitor, a Tankyrase inhibitor, a GPCR antagonist, an HSP90 inhibitor,
or an AAA-
ATPase p97 inhibitor. In some embodiments, the immune-stimulatory compound can
be a Toll-
like receptor agonist, a RIG-I agonist or a STING agonist. In some
embodiments, the immune-
stimulatory compound can be a beta-catenin pathway inhibitor, such as a TNIK
inhibitor or a
Tankyrase inhibitor.
[0311] In some embodiments, the immune-stimulatory compound is a Toll-like
receptor agonist.
A toll-like receptor agonist can be any molecule that acts as an agonist to at
least one toll-like
receptor. In some embodiments, the Toll-like receptor agonist can be a
molecule selected from a
CpG oligonucleotide, Poly G10, Poly G3, Poly I:C, a lipopolysaccharide, a
zymosan, a bacterial
-192-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
flagella protein (e.g., flagellin), Pam3CSK4, PamCysPamSK4, dsRNA, ssRNA, a
diacylated
lipopeptide, a bacterial lipoprotein, a triacylated lipoprotein, lipoteichoic
acid, or a peptidoglycan
(such as a bacterial peptidoglycan).
[0312] In some embodiments, an immune-stimulatory compound is not a toll-like
receptor
agonist. In some embodiments, a toll-like receptor agonist is not a naturally
occurring molecule,
such as CpG oligonucleotide, a lipopolysaccharide, a zymosan, a bacterial
flagella protein (e.g.,
flagellin), Pam3CSK4, PamCysPamSK4, dsRNA, ssRNA, a diacylated lipopeptide, a
bacterial
lipoprotein, a triacylated lipoprotein, lipoteichoic acid, or a peptidoglycan
(such as a bacterial
peptidoglycan). In some further embodiments, a toll-like receptor agonist is
not a synthetic
nucleic acid, such as Poly G10, Poly G3 or Poly I:C.
[0313] In some embodiments, a toll-like receptor agonist can be a synthetic
small molecule. A
toll-like receptor agonist can be imiquimod. A toll-like receptor agonist can
be CL307. A toll-like
receptor agonist can be S-27609. A toll-like receptor agonist can be
resiquimod. A toll-like
receptor agonist can be UC-IV150. A toll-like receptor agonist can be
gardiquimod. A toll-like
receptor agonist can be motolimod. A toll-like receptor agonist can be a
motolimod analog. A
toll-like receptor agonist can be VTX-1463. A toll-like receptor agonist can
be GS-9620. A toll-
like receptor agonist can be GSK2245035. A toll-like receptor agonist can be
TMX-101. A toll-
like receptor agonist can be TMX-201. A toll-like receptor agonist can be TMX-
202. A toll-like
receptor agonist can be isatoribine. A toll-like receptor agonist can be
AZD8848. A toll-like
receptor agonist can be MEDI9197. A toll-like receptor agonist can be 3M-051.
A toll-like
receptor agonist can be 3M-852. A toll-like receptor agonist can be 3M-052. A
toll-like receptor
agonist can be 3M-854A. A toll-like receptor agonist can be S-34240. A toll-
like receptor agonist
can be CL663. A toll-like receptor agonist can be KU34B.
[0314] A RIG-I agonist can be KIN1148. A RIG-I agonist can be SB-9200. A RIG-I
agonist
comprises a 5'ppp-dsRNA.
[0315] In some embodiments, the immune-stimulatory compound can comprise a non-
naturally
occurring chemotype, such as a substituted pyrimidine, a substituted purine, a
substituted guanine
nucleoside, a substituted 8-oxoadenine, a substituted imidazoquinoline, a
substituted
thiazoquinoline, a substituted 2-aminoimidazole, a substituted furo[2,3-
c]pyridine, a substituted
pyrazine, a substituted furo[2,3-c]quinoline, a substituted 2-
aminobenzimidazole, a substituted 2-
aminoquinoline, or a substituted 2-aminobenzazepine.
[0316] In certain embodiments, the immune-stimulatory compound can comprise S-
27609,
CL307, UC-IV150, imiquimod, gardiquimod, resiquimod, motolimod, VTS-1463, GS-
9620,
GSK2245035, TMX-101, TMX-201, TMX-202, isatoribine, AZD8848, MEDI9197, 3M-051,
-193-
CA 03065919 2019-12-02
WO 2018/227023
PCT/US2018/036560
3M-852, 3M-052, 3M-854A, S-34240, KU34B, SB9200, SB11285, 8-substituted
imidazo[1,5-
a]pyridine, or CL663.
[0317] An immune-stimulatory compound can comprise an inhibitor of TGFbeta,
Beta-Catenin,
PI3K-beta, STAT3, IL-10, IDO or TDO. The immune-stimulatory compound can
comprise
LY2109761, GSK263771, iCRT3, iCRT5, iCRT14, LY2090314, CGX-1321, PRI-724,
BC21,
ZINCO2092166, LGK974, IWP2, LY3022859, LY364947, SB431542, AZD8186, SD-208,
indoximod (NLG8189), F001287, GDC-0919, epacadostat (INCB024360), RG70099, 1-
methyl-
L-tryptophan, methylthiohydantoin tryptophan, brassinin, annulin B, exiguamine
A, PIM, LM10,
8-substituted 2-amino-3H-benzo[b]azepine-4-carboxamide, or INCB023843.
[0318] Additionally, stimulator of interferon genes (STING) can act as a
cytosolic DNA sensor
wherein cytosolic DNA and unique bacterial nucleic acids called cyclic
dinucleotides are
recognized by STING, and therefore STING agonists. In certain embodiments, the
STING
agonist can comprise a cyclic dinucleotide. Other non-limiting examples of
STING agonists
include:
0
0
0 X4
0
H
A6
p0 OX5
0 X2-
[0319] , wherein in some
embodiments, Xi=X2=0; X3=G; X4=G; X5=CO(CH2)12CH3; X6=2 TEAH; in some
embodiments,
X1=X2=S [Rp,Rp]; X3=G; X4=A; X5=H; X6=2 TEAH; in some embodiments, X1=X2=S
[Rp,Rp] ;
X3=A; X4=A; X5=H; X6=2 Na; in some embodiments, X1=X2=S [Rp,Rp] ; X3=A; X4=A;
X5=H;
X6=2 NH4; and in some embodiments, Xi=X2=0 ; X3=G; X4=A; X5=H; X6=2 TEAH;
-194-
CA 03065919 2019-12-02
WO 2018/227023
PCT/US2018/036560
N
0 \i .=S
C)
¨{ . Ck,õ 47 N li
,,JIN,
= i \ = = e 1 s 1" t' '===='''%
0........ ;`,.
41
''''''w ' 3 . = 4.... 0
.N., A, 0., %.
õ..... 0
11
k , ,, i ,. . . .
x.õ...õ:õ...õ;\..4 ..... Ai ..."4=.0 (:$.1r- = r .>=f -
...'"I'f..
s' \ ,i't . t,=.,,z`" N -.
i...1 yk N ltk42,
..tt ...t.k.= 34 . s';') . =
(., N .....,õ..L.,,,z.......0,,, il.t \t" *),,_..,i 5:, 0 tt
. ti.,..- =,,,,*E ,
i
<*.
\\,.....,,,c) 6( rk R t= e.,3 "'-'0.....4.,..0 .C.M.1
= ====,,,,,,,,o ,.. .;
ijii= \ S. = 4=-=,, - *`t..,..,..
0
=-=n===( N
'''''Nct 0
,... ,...,
, . =-...,1
i = ,, µi N 11%.
o = i 0
/1 t=
0.= 0
, wherein Ri=R2=H; Ri=propargyl, R2=H; Ri=H, R2=propargyl; Ri=allyl, R2=H;
Ri=H, R2=ally1;
Ri=methyl, R2=H; Ri=H, R2=methyl; Ri=ethyl, R2=H; Ri=H, R2=ethyl; Ri=propyl,
R2=H; Ri=H,
R2=propyl; Ri=benzyl, R2=H; Ri=H, R2=benzyl; Ri=myristoyl, R2=H; Ri=H,
R2=myristoyl;
Ri=R2=heptanoyl; Ri=R2=hexanoyl; or Ri=R2=pentanoyl;
li % so , \ , N ttt=,zµ
11 k' / \\ ,
(f='*.
Na0=========<:). 1=1 i ' i.:,,N...;:' 34#
i 0 IN. .' ).' '''( _________________ I
N
, ""'"'" 3 . / õ ,..A.....õ... .,. õ
.õ.3õ..õ
õ ---1 -,....... µ,./....., .....s,
, , ,
EV, rf.2isN.,) \ 11 1 NI.k.,õ,,,õ...N,4... 9* 7 , \ I
.......,,../ Nte`'NSH 1 ,. ,4.....,="C.,õ ...a:9
1
4 sk
0 0" o
Nw=-===\
":"p \S ii,::',i=-=-=-=-=\.,.,.,.,.
-s------ ,.........,z, :. f,=..¨ ..õ4.:::=) __________ -
,.... )......H =..,,, ,, µ,),/,:õ. s .õ,..,.... .
0 ..,..õ
1... ,,,),..,........1> tL, y "=,,,k
1
,
[0320] wherein Ri=R2=H; Ri=propargyl, R2=H; Ri=H, R2=propargyl; Ri=allyl,
R2=H; Ri=H,
R2=ally1; Ri=methyl, R2=H; Ri=H, R2=methyl; Ri=ethyl, R2=H; Ri=H, R2=ethyl;
Ri=propyl,
-195-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
R2=H; Ri=H, R2=propyl; Ri=benzyl, R2=H; Ri=H, R2=benzyl; Ri=myristoyl, R2=H;
Ri=H,
R2=myristoyl; Ri=R2=heptanoyl; Ri=R2=hexanoyl; or Ri=R2=pentanoyl;
N34,, WI? NiiA
N N
t4 0.===={1 N
i \\.., 6i34z .. I ''..."( Nlik
,3, ...,L.,:j
""'n Ci,,v,e FIN'^^rAN 1 ========= t --s,õ4,0,p
e ' .-* =.t.z K 11 1 ,!' >, ,,,.. i
o 3c
4 '\=... ,==== \ p I o
0 st=a, 4.--f. '.: T,:k &.=
= t'= C> 3 L!. eA
ss-40.,==== .
t44,, N N
N "
%,.======<rs 1
/ Um\ 14
\ V RI Nii,
WI:
>=11 -= fi s ,1) N, .1,
..7 Ny.=
'''s,ti :.õ(e õ,
, ,
.,,..õ........L.:
ti ti. ce $is.i li ts?. &; ,' 1 i I
N,...=-=1,µ, .13i
t""'*c,ti".1
,
1-.....õ,..... &,,, L4 ' .3
0õ,..0
4V1e NY
,
[0321] wherein Ri=R2=H; Ri=propargyl, R2=H; Ri=H, R2=propargyl; Ri=allyl,
R2=H; Ri=H,
R2=ally1; Ri=methyl, R2=H; Ri=H, R2=methyl; Ri=ethyl, R2=H; Ri=H, R2=ethyl;
Ri=propyl,
R2=H; Ri=H, R2=propyl; Ri=benzyl, R2=H; Ri=H, R2=benzyl; Ri=myristoyl, R2=H;
Ri=H,
R2=myristoyl; Ri=R2=heptanoyl; Ri=R2=hexanoyl; or Ri=R2=pentanoyl;
X
11 R1
1
R4 0
rki 3
1
0 0-1)1,1:-011,
1
, wherein each X is independently 0 or S, and R3
and R4 are each independently H or an optionally substituted straight chain
alkyl of from 1 to 18
carbons and from 0 to 3 heteroatoms, an optionally substituted alkenyl of from
1-9 carbons, an
optionally substituted alkynyl of from 1-9 carbons, or an optionally
substituted aryl, wherein
substitution(s), when present, may be independently selected from the group
consisting of C1,6
alkyl straight or branched chain, benzyl, halogen, trihalomethyl, Ci_6alkoxy,
¨NO2, ¨NH2, ¨
-196-
CA 03065919 2019-12-02
WO 2018/227023
PCT/US2018/036560
OH, ¨0, ¨COOR - where R - is H or lower alkyl, ¨CH2OH, and ¨CONH2, wherein R3
and R4 are not both H;
0
---- ,
..."
9
IA o
k i
\ I
immomm \ i
S \
/ \
\
s
0 0 H
i
Bf .--...- 0-- P ----
,
I
4 , wherein Xi=X2=0; Xi=X2=S; or X1=0 and
X2=S,
N142
T4 i
'`....,'N
\
L
HO I9
f
\ I
/ OH
I \
1
P \
i
N
r:õN ,._.,
:--- N___.----N . 0- ) =:::.=:,-)
NE-t.
,
0
1 !
i
a <7
H ,0--
-0-------. ---\ N=------`,. <-<:;* N.
HO -...,,V
\********* 1
i
..........,µ i
p O. H
-..., ,..--...., -...,
- - -- -.,............- S.S
t
HN,
: 3
i I
O ,
-197-
CA 03065919 2019-12-02
WO 2018/227023
PCT/US2018/036560
N -12
..N.,,.. ....."."¨,=,,,õ
0 .
1 1 0
1 ..-=''' `-,,I
i.
.......µ .
\
I \
0 H
/
,----'
tsi 1 0 \ Rp i 0
.....--;.: .=,..,
i 1
s
' \.........,..,="^,..,i4=.,,
N 1-i a
,
õN.,......,.. .õ...----..õ,
-- NH
\
N------",,,, ...^-:::="'N.N
.1 RP
\ ..",. /
0
HO
1
µ
3-IA N,
-,-...., =,--N
\
¨i
HN
1
CS ,
Ntii.l.
0
1 I
/
'N-------\N --"`-----"' ,
¨ S Pt '----.".8
\ '''"N
H 0
1.,
/
;
H
0 0,,
õ
,õ0õ...,
./----\_
,õ...,........._, ...Ø........,,,......,,,,..
,....õ, ,
/1 .
0
_..-= N
tst H),
,
-198-
CA 03065919 2019-12-02
WO 2018/227023
PCT/US2018/036560
a
H
0- i
H 1
\ i
.-$ ...............
Hp p.--, \ N.----....., ...,5*---....,
\ , , 0, i - N" .'N Hõ
H0 1
0
k
hi \
{
i \
i
OH
-4) iip
N.,¨.*
i
I .4)\ -------ll --s-
6
I
i
0
,
0
"õ =-=-=.õiõ...---. "---õiv ,,..i
S" '''' I = 1"
C3=P-"' -Th, N---- -...õ, ,,
11.SP ..,.....õõo.õ, / N''' N H2
HO
i
1.....k / \
i \
0 H
i=-s. ......--k ;")
/12N,,, 1 .-0- v
=,-,---- N._--N 07-=
R..¨ ..
---$
-- 0
,and
N H2
N
,,,,e2
S-
1 ,......,0--, \ 1
,...---S--" Sp
1
0
HO
o
i \
\
17$ / OH
N i ''' 0 --' \_. Sp/
....--"...; N,
re., ,......_ ¨ som,,,,.......v =._
1\ 8
0
- N
1
i
-199-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
[0322] In some embodiments, an immune-stimulatory compound can be a kinase
inhibitor. An
immune-stimulatory compound can inhibit one or more kinases.
[0323] An immune-stimulatory compound can be an inhibitor of ALK, Bcr-Abl,
BRAF, BTK, c-
KIT, EGFR, ErbB2, JAK, MEK, MET, PDGFRB, RET, ROS1, Syk, SRC, TGF(3122, TNIK,
TNKS, TNKS2, TrkA, TrkB, TrkC, VEGFR1, VEGFR2, VEGFR3, FGFR1, FGFR2, FGFR3,
FGFR4, CSF1R, RON/MST1R, TYR03, MERTK, AXL, PI310, PI3Ky, MAP4K1, PERK, KIT,
or any combination thereof. An immune-stimulatory compound can be an inhibitor
of TGF(3121,
TGF(3122, TNIK, TNKS, PI3K-f3, STAT3, IL-10, IDO, or TDO.
[0324] In various embodiments, the immune-stimulatory compound comprises
LY2109761,
GSK263771, iCRT3, iCRT5, iCRT14, LY2090314, CGX-1321, PRI-724, BC21,
ZINCO2092166, LGK974, IWP2, LY3022859, LY364947, SB431542, AZD8186, SD-208,
indoximod (NLG8189), F001287, GDC-0919, epacadostat (INCB024360), RG70099, 1-
methyl-
L-tryptophan, methylthiohydantoin tryptophan, brassinin, annulin B, exiguamine
A, PIM, LM10,
INCB023843, or 8-substituted imidazo[1,5-a]pyridine.
[0325] An immune-stimulatory compound can be an agonist of a GPCR, an ion
channel, a
membrane transporter, or an ER protein.
[0326] An immune-stimulatory compound can be an antagonist of the GPCR A2aR,
the
sphingosine 1-phosphate receptor 1, prostaglandin receptor EP3, prostanglandin
receptor E2,
Frizzled, CXCR4, or an LPA receptor.
[0327] An immune-stimulatory compound can be an ion channel agonist for CRAC,
Kv1.3, or
KCa3.1.
[0328] An immune-stimulatory compound can be an inhibitor of HSP90 or AAA-
ATPase p97.
Immune-Stimulatory Conjugate Properties
[0329] In certain embodiments, an immune-stimulatory compound of a conjugate
(i.e, attached to
an antibody construct either directly or via a linker) has a biological
potency no less than at least
about 0.33%, about 1%, about 5%, about 10%, about 20%, about 30%, about 40%,
about 50%,
about 60%, about 70%, about 80%, about 85%, about 90%, about 95%, or about
100% of the
potency of the control (free) immune-stimulatory compound (i.e., not attached
to an antibody
construct).
[0330] The specificity of the antigen-binding domain (of a conjugate) for an
antigen can be
influenced by the attachment of an immune-stimulatory compound to an antibody
construct. In
various embodiments, an antigen-binding domain of the conjugate can bind to
its antigen with at
least about 10%, about 20%, about 30%, about 40%, about 50%, about 60%, about
70%, about
-200-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
80%, about 85%, about 90%, about 95%, or about 100% of the specificity of the
antigen-binding
domain for the antigen in the absence of attachment of the immune-stimulatory
compound.
[0331] The specificity of the Fc domain (of a conjugate) for an Fc receptor
can be influenced by
the attachment site of an immune-stimulatory compound (directly or via a
linker). In some
embodiments, the Fc domain of a conjugate retains the specificity of the
unconjugated Fc domain
to bind to an Fc receptor. In specific embodiments, the Fc domain of the
conjugate can bind to
an Fc receptor with at least about 10%, about 20%, about 30%, about 40%, about
50%, about
60%, about 70%, about 80%, about 85%, about 90%, about 95%, or about 100% of
the
specificity of the Fc domain to the Fc receptor in the absence of attachment
of the immune-
stimulatory compound.
[0332] In some embodiments, the Fc domain of a conjugate has an altered
specificity for an Fc
receptor relative to the corresponding unconjugated Fc domain. In specific
embodiments, the Fc
domain of the conjugate can bind to an Fc receptor with at least about 10%,
about 20%, about
30%, about 40%, about 50%, about 60%, about 70%, about 80%, about 85%, about
90%, about
95%, or about 100% loss of specificity of the Fc domain to the Fc receptor as
compared to an Fc
domain of a conjugate not attached to an immune-stimulatory compound (in the
absence of the
immune-stimulatory compound). In some embodiments, the Fc domain is an Fc
null.
[0333] The affinity of the antigen-binding domain of a conjugate to an antigen
can be influenced
by the attachment of an immune-stimulatory compound attached to the antibody
construct. In
some embodiments, the affinity of the antigen-binding domain of the conjugate
for binding to an
antigen is at least about 1%, about 5%, about 10%, about 20%, about 30%, about
40%, about
50%, about 60%, about 70%, about 80%, about 85%, about 90%, about 95%, or
about 100% of
the affinity of the antigen-binding domain to the antigen in the absence of
the immune-
stimulatory compound (not attached to the antibody construct).
[0334] The affinity of the Fc domain to an Fc receptor of a conjugate can be
influenced by
attachment of an immune-stimulatory compound to the antibody construct (either
directly or
indirectly). In some embodiments, the Fc domain of the conjugate can bind to
an Fc receptor with
at least about 1%, about 5%, about 10%, about 20%, about 30%, about 40%, about
50%, about
60%, about 70%, about 80%, about 85%, about 90%, about 95%, or about 100% of
the affinity of
the Fc domain to the Fc receptor in the absence of the immune-stimulatory
compound attached to
the antibody construct. In some embodiments, the Fc domain of the conjugate
can bind to an Fc
receptor with a reduced affinity of at least about 1%, about 5%, about 10%,
about 20%, about
30%, about 40%, about 50%, about 60%, about 70%, about 80%, about 85%, about
90%, about
95%, or about 100% of the affinity of the Fc domain to the Fc receptor in the
absence of
-201-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
attachment the immune-stimulatory compound (directly or via a linker). In some
embodiments,
the Fc domain is an Fc null.
[0335] The Kd for binding of an antigen-binding domain to an antigen when an
immune-
stimulatory compound is attached to the antibody construct can be about 2
times, about 3 times,
about 4 times, about 5 times, about 6 times, about 7 times, about 8 times,
about 9 times, about 10
times, about 15 times, about 20 times, about 25 times, about 30 times, about
35 times, about 40
times, about 45 times, about 50 times, about 60 times, about 70 times, about
80 times, about 90
times, about 100 times, about 110 times, or about 120 times greater than the
Kd for binding of the
antigen binding domain to the antigen in the absence of the immune-stimulatory
compound
attached to the antibody construct.
[0336] In some embodiments, the Kd for binding of an Fc domain to an Fc
receptor when an
immune-stimulatory compound is attached to an antibody construct can be about
2 times, about 3
times, about 4 times, about 5 times, about 6 times, about 7 times, about 8
times, about 9 times,
about 10 times, about 15 times, about 20 times, about 25 times, about 30
times, about 35 times,
about 40 times, about 45 times, about 50 times, about 60 times, about 70
times, about 80 times,
about 90 times, about 100 times, about 110 times, or about 120 times greater
than the Kd for
binding of the Fc domain to the Fc receptor without the immune-stimulatory
compound attached
to the antibody construct.
[0337] In some embodiments, the Kd for binding of an Fc domain to a Fc
receptor when the
immune-stimulatory compound is attached to the antibody construct can be about
2 times, about
3 times, about 4 times, about 5 times, about 6 times, about 7 times, about 8
times, about 9 times,
about 10 times, about 15 times, about 20 times, about 25 times, about 30
times, about 35 times,
about 40 times, about 45 times, about 50 times, about 60 times, about 70
times, about 80 times,
about 90 times, about 100 times, about 110 times, or about 120 times less than
the Kd for binding
of the Fc domain to the Fc receptor when the immune-stimulatory compound is
not attached to
the antibody construct.
[0338] Affinity is the strength of the sum total of noncovalent interactions
between a single
binding site of a molecule, for example, an antibody, and the binding partner
of the molecule, for
example, an antigen. The affinity can also measure the strength of an
interaction between an Fc
domain of an antibody and an Fc receptor. Unless indicated otherwise, as used
herein, "binding
affinity" refers to intrinsic binding affinity which reflects a 1:1
interaction between members of a
binding pair (e.g., antibody and antigen or Fc domain and Fc receptor). The
affinity of a molecule
X for its partner Y can generally be represented by the dissociation constant
(Kd). Affinity can be
measured by common methods known in the art. Specific illustrative and
exemplary
embodiments for measuring binding affinity are described in the following.
-202-
CA 03065919 2019-12-02
WO 2018/227023
PCT/US2018/036560
[0339] In some embodiments, an antibody construct (e.g., an antibody or
antigen-binding
fragment thereof) can have a dissociation constant (Kd) for an antigen or Fc
receptor of about 1
i.t.M, about 100 nM, about 10 nM, about 5 nM, about 2 nM, about 1 nM, about
0.5 nM, about 0.1
nM, about 0.05 nM, about 0.01 nM, or about 0.001 nM or less (e.g., 10-6 or
less, 10-8 M or less,
from 10-8M to 10-13 M, or from 10 M to 10-13 M). An affinity matured antibody
can be an
antibody with one or more alterations in one or more complementarity
determining regions
(CDRs), compared to a parent antibody, which may not possess such alterations,
such alterations
resulting in an improvement in the affinity of the antibody for antigen. These
antibodies can bind
to their antigen with a Kd of about 5x10-9 M, about 2x10-9 M, about 1x10-9 M,
about 5x10-1 M,
-. .-,
about 1x10-10 m about 5x10- 1 1 M, about 1x10- 1 1 M, about 5x10-12 M, about
1x10-12 M, or less.
In some embodiments, the antibody construct (e.g., affinity matured antibody)
can have an
increased affinity of at least 1.5-fold, 2-fold, 2.5-fold, 3-fold, 4-fold, 5-
fold, 10-fold, 20-fold, or
greater as compared to an antibody construct without alterations in one or
more CDRs.
[0340] Kd can be measured by any suitable assay. For example, Kd can be
measured by a
radiolabeled antigen binding assay (RIA). For example, Kd can be measured
using surface
plasmon resonance assays (e.g., using a BIACOREC)-2000 or a BIACOREC)-3000).
[0341] The molar ratio or drug-antibody ratio of a conjugate refers to the
number of immune-
stimulatory compounds attached to an antibody construct in a conjugate or
preparation of
immune-stimulatory conjugates. The molar ratio can refer to the number of
immune-stimulatory
compounds attached (e.g., conjugated) to an antibody construct of a particular
conjugate and is
an integer, such as from 0-8 or 0 to 20. The molar ratio can also refer to the
average number of
immune-stimulatory compounds attached to antibody constructs in a mixture of
conjugates, such
as in a pharmaceutical composition.
[0342] The molar ratio can be determined, for example, by Liquid
Chromatography/Mass
Spectrometry (LC/MS), in which the number of immune-stimulatory compounds
attached to the
antibody construct can be directly determined. Additionally, as non-limiting
examples, the molar
ratio can be determined based on hydrophobic interaction chromatography (HIC)
peak area, by
liquid chromatography coupled to electrospray ionization mass spectrometry (LC-
ESI-MS), by
UV/Vis spectroscopy, by reversed-phase-HPLC (RP-HPLC), or by matrix-assisted
laser
desorption/ionization time of flight mass spectrometry (MALDI-TOF-MS).
[0343] In some embodiments, the molar ratio of immune-stimulatory compound to
antibody
construct can be less than 8 or less than 20. In other embodiments, the molar
ratio of immune-
stimulatory compound to an antibody construct can be 8, 7, 6, 5, 4, 3, 2, or
1. In some
embodiments, the average molar ratio of immune-stimulatory compounds to
antibody constructs
in a composition can be less than 8, such as about 3 to 5 or about 2. In other
embodiments, the
-203-
CA 03065919 2019-12-02
WO 2018/227023
PCT/US2018/036560
average molar ratio of immune-stimulatory compounds to antibody constructs in
a composition
can be 8, 7, 6, 5, 4, 3, 2, or 1 or fractions hereof, or such as about 3.5 or
about 1.8.
[0344] In a conjugate, an antibody construct (e.g., an antibody) can be linked
to an immune-
stimulatory compound in such a way that the antibody construct can still bind
to an antigen and
the Fc domain of the antibody construct can still bind to an FcR. In a
conjugate, an antibody
construct can be linked to an immune-stimulatory compound in such a way that
the linking does
not interfere with the ability of the antigen binding domain of the antibody
construct to bind to its
antigen, the ability of the Fc domain of the antibody construct to bind to an
FcR, or FcR-
mediated signaling resulting from the Fc domain of the antibody construct
binding to an FcR. In
a conjugate, an immune-stimulatory compound can be linked to an antibody
construct in such a
way that the linking does not interfere with the ability of the immune-
stimulatory compound to
bind to its receptor or otherwise can induce a biological effect. In some
embodiments, a
conjugate can produce stronger immune stimulation and a greater therapeutic
window than
components of the conjugate alone. For example, in an anti-tumor or anti-CD40
antibody linked
to a TLR agonist, the combination of CD40 agonism, TLR agonism and an
accessible Fc domain
of the anti-CD40 antibody resulting in FcR-mediated signaling can produce
stronger immune
stimulation and a greater therapeutic window than the CD40 agonism, TLR
agonism, or the FcR-
mediated signaling alone.
[0345] In some embodiments, a conjugate can comprise a first binding domain,
wherein the first
binding domain contributes to immune-stimulatory activity; a first and second
binding domain,
wherein the second binding domain contributes to immune stimulatory activity;
or a first binding
domain and second binding domain, wherein the first binding domain and the
second binding
domain contribute to immune-stimulatory activity. The first binding domain and
the second
binding domain can contribute to the same immune-stimulatory activity. The
first binding
domain and the second binding domain can contribute to different immune-
stimulatory activities.
[0346] In some embodiments, a conjugate in which the first binding domain
contributes to
immune-stimulatory activity can increase the immune-stimulation of the
conjugate.
Immune-Stimulatory Compound Potency and Binding Activity
[0347] In certain embodiments, an immune-stimulatory compound has similar
activity when
bound to the antibody construct as when not bound to the antibody construct.
In certain
embodiments, the immune-stimulatory compound maintains the same level of
potency and/or
binding affinity when bound to an antibody construct as compared to the
unbound immune-
stimulatory compound.
-204-
CA 03065919 2019-12-02
WO 2018/227023
PCT/US2018/036560
[0348] For many known antibody-drug conjugates, the payload/drug of the
conjugate is a
cytotoxic agent that acts on an intracellular target. The antibody generally
targets a certain tumor
marker on the surface of a cancer cell and upon binding of the antibody to the
tumor cell, the
cancer cell then internalizes the antibody-cytotoxic agent conjugate. The
cytotoxic agent is
released within the cell through enzymatic cleavage or other cleavage of the
agent's linkage to
the antibody or through enzymatic degradation of the antibody. The released
cytotoxic agent or
degredation product acts on the intracellular target of the cytotoxic agent to
kill the cancer cell.
Important to the mechanism of action of many antibody-cytotoxic agent
conjugates is: (1) that
the cytotoxic agent is bound to the antibody with a linker, wherein the linker
is not cleaved until
exposed to enzymes or conditions inside a target cell, e.g., a cancer cell;
(2) that the cytotoxic
agent is released from the antibody inside of the cell to perform its
cytotoxic function; and (3)
that the cytotoxic agent is not active or minimally active (i.e., in a prodrug
form) when bound to
the antibody such that the cytotoxic agent does not indiscriminately kill
cells and harm organ
systems distributed thorught the body on the path to the cancer cell.
[0349] In certain aspects, the immune-stimulatory conjugates of the disclosure
operate under a
different paradigm from such antibody-cytotoxic agent conjugates. The immune-
stimulatory
conjugates of the disclosure can be designed in a way that the payload immune-
stimulatory
compound has the same potency, similar potency, or increased potency when
bound to the
antibody construct as compared to the unbound immune-stimulatory compound and
in contrast to
antibody-cytotoxic agent conjugates. The immune-stimulatory conjugates of the
disclosure may
perform, and in some cases preferably perform, the intended function of the
compound, i.e.,
stimulate or modulate the function of immune cells or other target cells,
without prior release of
the compound from the conjugate (i.e., while attached to the antibody
construct). The features of
the immune-stimulatory compound-conjugates and assays that enable these
functions and others
are described further herein.
[0350] The potency of the immune-stimulatory compound of the conjugate may not
be
significantly reduced relative to the potency of the un-attached (free) immune-
stimulatory
compound. In particular, the potency of the immune-stimulatory compound of the
conjugate (i.e.,
as part of the conjugate or covalently bound to the antibody construct) is
preferably no greater
than 500-fold less, no greater than 400-fold less, no greater than 300-fold
less, no greater than
200-fold less, no greater than 100-fold less, no greater than 50-fold less, or
no greater than 10-
fold less than the potency of a control compound, wherein the control compound
is the unbound
immune-stimulatory compound. For example, for an immune-stimulatory conjugate
represented
by the structure: Ab ¨ L ¨ C, wherein A is an antibody construct, L is a
linker, and C is an
immune-stimulatory compound, the immune-stimulatory activity of C of the
conjugate A ¨ L ¨ C
-205-
CA 03065919 2019-12-02
WO 2018/227023 PCT/US2018/036560
is preferably no greater than 500-fold less, no greater than 400-fold less, no
greater than 300-fold
less, no greater than 200-fold less, no greater than 100-fold less, no greater
than 50-fold less, or
no greater than 10-fold less than the potency of the immune-stimulatory
compound, C, unbound
from the immune-stimulatory conjugate, A ¨ L ¨ C, in the absence of processing
of the immune-
stimulatory conjugate in a cell.
[0351] In particular embodiments, the potency of an immune-stimulatory
compound of the
conjugate (i.e., covalently bound to the antibody construct) is near or
equivalent to the potency of
the unbound immune-stimulatory compound, such as within about 10-fold, within
about 8-fold,
within about 5-fold, or within about 2-fold of the potency of the unbound
immune-stimulatory
compound. The potency of the immune-stimulatory compound of the conjugate may
be greater
than the potency of the unbound immune-stimulatory compound, such as about 2-
fold or greater,
5-fold or greater, 10-fold or greater, 100-fold or greater, 200-fold or
greater, 300-fold or greater,
400-fold or greater, or even 500-fold or greater than the potency of the
unbound immune-
stimulatory compound. In certain embodiments, the tolerability of an immune-
stimulatory
compound when part of a construct is greater than the tolerability of the
unbound immune-
stimulatory compound in a subject.
[0352] In certain embodiments, the potency of the immune-stimulatory compound
when bound
to a 5-500 atom linker is the same, similar, or increased as compared to the
potency of the
immune-stimulatory compound not bound to the 5-500 atom linker. In certain
embodiments, the
tolerability of the immune-stimulatory compound in a subject when bound to a 5-
500 atom linker
is the same, similar, or increased as compared to the tolerability of the
immune-stimulatory
compound not bound to the 5-500 atom linker.
[0353] The "5-500 atom linker" referred to herein has 5 to 500 consecutive
atoms from end to
end. When attached to an immune-stimulatory compound and to an antibody
construct, a 5-500
atom linker has 5-500 consecutive atoms between the point of attachment to the
immune-
stimulatory compound and the point of attachment to the antibody construct. A
5-500 atom
linker can have, for example, from about 50 to about 500 atoms, such as about
50 to about 300
atoms or such as about 50 to about 200 atoms. A 5-500 atom linker can have,
for example, from
about 25 to about 500 atoms, such as about 25 to about 300 atoms or such as
about 25 to about
200 atoms. In certain embodiments, the linker includes one or more peptide
bonds. In certain
embodiments, the linker includes one or more ethylene glycol groups. In
certain embodiments,
the linker includes a peptide backbone and one or more side chains. In certain
embodiments, the
linker is not cleaved from the immune-stimulatory compound in the assay
evaluating potency.
[0354] Exemplary 5-500 atom linkers include Fleximer linkers, linkers with one
or more
carbamate or amide linkages and linkers represented by the formula:
-206-
CA 03065919 2019-12-02
WO 2018/227023
PCT/US2018/036560
Rx 0
0 0 0
Rx
0-8 8 0
Ra Ra 0-7 H 0-9
, ,
0
F
0 0 NjOC)(C)
0
F
Nj.LHN 0-7
0
0-9 H F
Rx F
or
0
YLO 0 I
N peptide ¨Rx
H , wherein 12' is a reactive moiety for attachment
to the
antibody construct, 12a is hydrogen, Ci_loalkyl, sulfonate and methyl
sulfonate and the wavy line
indicates an attachment to the rest of the linker or to the immune-stimulatory
compound.
[0355] In some embodiments, the linker is a non-cleavable linker. Examples of
non-cleavable
linkers include the following:
Rx 0
0 0 0
Rx
0-8 8 0
Ra Ra 0-7 H 0-9
, ,
0
F
0 0 NjOC)(C) F
Nj.LHN 0-7
0
0 0-9 H F
Rx F
wherein 12' is a reactive moiety for attachment to the antibody construct, 12a
is hydrogen, Ci_
malkyl, sulfonate and methyl sulfonate and the wavy line indicates an
attachment to the rest of
the linker or to the immune-stimulatory compound.
[0356] Further examples of non-cleavable linkers include:
-207-
DEMANDE OU BREVET VOLUMINEUX
LA PRESENTE PARTIE DE CETTE DEMANDE OU CE BREVET COMPREND
PLUS D'UN TOME.
CECI EST LE TOME 1 DE 2
CONTENANT LES PAGES 1 A 207
NOTE : Pour les tomes additionels, veuillez contacter le Bureau canadien des
brevets
JUMBO APPLICATIONS/PATENTS
THIS SECTION OF THE APPLICATION/PATENT CONTAINS MORE THAN ONE
VOLUME
THIS IS VOLUME 1 OF 2
CONTAINING PAGES 1 TO 207
NOTE: For additional volumes, please contact the Canadian Patent Office
NOM DU FICHIER / FILE NAME:
NOTE POUR LE TOME / VOLUME NOTE: