Note: Descriptions are shown in the official language in which they were submitted.
Title: Crosslinked highly stable anion-exchange blend membranes with
polyethylene
glycols as the hydrophilic membrane phase
Summary
The invention comprises:
anion-exchange blend membranes from the following blend components:
- A halomethylated polymer (a polymer having ¨(CH2)x-CH2-Hal which is
tertiary or N-alkylated / N-arylated lmidazole, an N-alkylated / N-arylated
benzimidazole or an N-alkylated / N-arylated pyrazole is quaternized to an
anion exchange polymer.
- An inert matrix polymer in which the anion exchange polymer is embedded
and which is optionally covalently crosslinked with the halomethylated
precursor of the anion exchange polymer.
- A polyethylene glycol having epoxide or halomethyl end groups anchored by
reaction with basic N-H groups of the basic matrix polymer covalently
crosslinked
- Optionally an acidic polymer which forms ionic crosslinking
with the anion
exchange polymer (negative fixed acid ions form ionic crosslink sites to the
positive cation of the anion exchange polymer)
- Optionally a sulfinated polymer (polymer having sulfinate
groups ¨502Me,
Me = any cation) which forms covalent crosslinking bridges with the
halomethyl groups of the halomethylated polymer under sulfinate S-
alkylation
- Process for the preparation of these membranes
- Use of these membranes in electrochemical energy conversion processes (eg
redox-flow batteries and other flow batteries, PEM electrolyses, membrane
fuel cells, and in other membrane processes (eg electrodialysis, diffusion
dialysis).
CA 3066028 2019-12-23
Title: Crosslinked highly stable anion-exchange blend membranes with
polyethylene
glycols as the hydrophilic membrane phase
State of the art
Over the past few decades, researchers' interest in anion exchange membranes
(AEMs) for
use in electrochemical conversion processes has greatly increased. Possible
fields of
application of AEMs are alkaline polymer electrolyte fuel cells (APEFCs)
[1,2,3,4,5], alkaline
polymer electrolyte electrolysis (APEE) [6], redox flow batteries (RFBs)
[7,8,9], reverse
electrodialysis (RED) [10,11] and bioelectrochemical systems, including
microbial fuel cells
(MFCs) [12,13] and enzymatic fuel cells [14]. In addition, anion exchange
membranes are
used in electrodialysis (ED) [15,16,17] and in Donnan [18] or diffusion
dialysis [19] (DD). A
major advantage of using AEM in electrochemical conversion processes such as
fuel cells or
electrolysis is that when using AEMs for the electrocatalytic reactions at the
electrodes no
precious metal catalysts consisting of platinum group metals (PGM) are
required, thus
containing AEM Membrane electrode assemblies (MEAs) are significantly less
expensive than
cation exchange membrane (CEM) containing MEAs. AEMs have the following major
drawbacks compared to CEMs:
(1) The ionic conductivity of most AEM types is significantly lower than that
of CEMs of
comparable ion exchange capacity (IEC), in part because most of the AEMs have
a
hydrocarbon backbone that is significantly less hydrophobic than the
perfluorinated one, for
example the polymer backbone of the perfluorinated membranes of the Nafion
type, so
that in the AEM it comes to a lower separation between ionic groups and
polymer backbone,
which leads to lower ionic conductivity because of the then lower local
density of the anion
exchange groups, especially in most AEM types, the solid cations are attached
to the
polymer backbone via a CH2 bridge [20].
(2) In particular, when the AEMs are exchanged with the OH- ion, for example
when used in
APEFC or APEE, their chemical stability is limited, since the OH- counterion
of the anion
exchange group can degrade the positively charged solid ion itself [21] or the
polymer main
chain [22].
CA 3066028 2019-12-23
The global efforts in this research and development segment are aimed at
minimizing these
disadvantages of AEMs and thus improving their properties. As starting
polymers for AEM
often polymers are used which contain aromatic groups, such as polystyrene,
polyphenylene
ethers or other aromatic polyethers such as polyethersulfones, polyether
ketones etc.,
which may be substituted with methyl groups. The first step in the preparation
of AEM is the
synthesis of a polymer with halomethyl side groups. Halomethylation is
achieved by (1)
chloro- or bromomethylation with hydrogen halide, formaldehyde, and a Lewis
acid such as
ZnCl2 or AlC13 (Blanc reaction [23,24]), or (2) bromination of the CH3 pendant
group of
aromatic polymers with N-bromo-succinimide (NBS) by the Wohl-Ziegler
bromination
reaction [25]. The Blanc reaction is associated with the appearance of the
highly carcinogenic
by-product bis (chloromethyl) ether. For this reason, the Wohl-Ziegler
reaction is now
preferably used in the production of halomethylated aromatic polymers.
Literature examples
for the preparation of bromomethylated aromatic polymers by the Wohl-Ziegler
reaction are
the bromomethylation of polyphenylene oxide [26] or the bromomethylation of a
methylated polyethersulfone [27]. Conversion of the CH2Hal group (Hal = Cl,
Br) to an anion
exchange group is achieved by reaction with a tertiary amine such as
trimethylamine [24],
pyridine [28], pentamethylguanidine [29] or an N-alkylated imidazole [30].
One way to increase the conductivity of AEM is to increase the separation
between polymer
backbone and ion group phase in the AEM to obtain a larger local density of
ion-conducting
groups. Phase-segregated AEMs having improved ionic conductivity are
obtainable by the
preparation of linear block copolymers of hydrophobic and ionic blocks [31] or
by graft
copolymers having an anion exchange group-containing graft side chain [32]
(Example:
grafting of vinylbenzyl chloride side chains to e--irradiated ETFE, and
quaternization of the
chloromethylated side chains with trimethylamine [33]).
In order to achieve an improvement in the chemical stability of AEM, the
combination of
anion exchange group and polymer main chain must always be investigated, since
the
stability of the anion exchange group always depends on the polymer main
chain. Thus, it
could be shown for polystyrene (P5t) substituted with the solid cation
benzyltrimethylammonium that in alkaline medium (0.6M KOH, 80 C) the solid
cation is
somewhat more stable than if PPO is substituted with the same group and much
more stable
than if pendent to polyphenylene ether sulfone (PES ) [22]. It is not easy to
predict which
polymer backbone is more stable, as can be seen in the above example, since
all three
CA 3066028 2019-12-23
polymers PSt, PPO and PES contain electron-rich aromatic groups linked
together by ether
groups in both PPO and PES.
It has been found, however, that apparently by steric shielding of the anion
exchange groups
of AEM, in particular their alkali stability can be significantly improved,
since then the
nucleophilic attack of the OH- counterions on the quaternary ammonium group is
difficult. In
a study by Holdcroft et al, two different polybenzimidazolium (PB1m+) AEMs
were tested for
their stability in the alkaline medium [34]. One of the PB1m+-AEMs had methyl
groups on the
aromatic adjacent to the dimethylbenzimidazolium cation, the others did not.
While the
sterically hindered PB1m+-AEM showed a very high stability in 2M KOH, the
sterically
unhindered PBIm+-AEM was degraded very rapidly. The very high stability of the
sterically
hindered PB1m+-AEM was explained by the authors of this study as follows: at
the sterically
hindered PB1m+-AEM, the OH- group can not attack the imidazolium ring, while
at the non-
hindered PB1m+-AEM the OH- can attack the imidazolium ring under ring opening
[35].
Herring et al. synthesized sterically highly-hindered PPO-AEM functionalized
with 1,4,5-
trimethy1-2- (2,4,6-trimethoxyphenyl) -imidazolium anion exchange groups,
which were also
characterized by excellent alkaline stability (no decrease in ion exchange
capacity after 25
hours storage in 1M KOH at 80 C) [36]. In contrast, dimethylimidazolium-
modified PPO
showed a large decrease in ion-exchange capacity (approximately 50% decrease
in 2M KOH
at 60 C after 9 days) [37]. The experimental findings can be summarized in
that the steric
shielding of the anion exchange groups is one way to increase the chemical
stability of AEM.
Other strategies for reducing the chemical degradation of AEM are:
(1) Search for alternative solid cations
(2) chemical and / or physical crosslinking
(3) embedding the anion exchange polymer in an inert matrix polymer.
As alternative cations to the most commonly used trialkylammonium groups are
the already
mentioned pentamethylguanidinium groups (PMG) come into consideration.
However, it has
been found that the PMG cations are chemically stable only when they are
resonance-
stabilized (ie, the positive charge of the PMG cation is delocalized), which
is the case when
attached to an aromatic (possibly electron-deficient) moiety, as Kim et al.
could show
[38,39]. Another example of a sterically hindered chemically stabilized
cationic functional
group is the tris (2,4,6-trimethoxyphenyl) phosphonium cation [40], which was
attached to
CA 3066028 2019-12-23
polyvinylbenzyl chloride graft chains and after 75 hours of storage in 1N NaOH
at 60 C had
no degradation. In a work by Zha et al., for example, a positively charged bis
(terpyridine)
ruthenium (II) complex was attached to a norbornene polymer [41]. The AEM thus
prepared
showed excellent stability in an alkaline environment: incorporation of the
polymer in 1N
NaOH at room temperature showed no degradation even after half a year.
Another way of stabilizing AEM is to cross-link them. Thus, in a work by He et
al. PPO-based
AEMs were synthesized, which were cross-linked in a multi-step process with
tertiary
diamines and vinylbenzyl chloride under quaternization, resulting in
mechanically very
robust covalently cross-linked AEMs [42]. In a study by Cheng et al. For
example,
chloromethylated PSU was cross-linked with a novel N-basic difunctional
reagent,
guanimididazole, under quaternization. These new crosslinked polymers showed
better
alkali stability than corresponding AEMs quaternized without crosslinking with
1-
methylimidazole [43].
In our group, bromomethylated PPO embedded in the matrix polymer PVDF was
quaternized
with the diamine DABCO and with 1,4-diiodobutane to mechanically and
chemically
covalently crosslinked AEM. Even after 10 days of incorporation in 1N KOH at
90 C no
degradation of IEC and conductivity was observed. Moreover the membranes
showed good
performance in direct methanol fuel cells (DMFC) (4M Me0H and 5M KOH) [44]. In
another
study, PB100 (manufacturer: Fuma-Tech) methylated by a new non-carcinogenic
reagent
was blended with sulfonated PSU and covalently crosslinked under
quaternization and
alkylation using DABCO and 1,4-diiodobutane [45]. These AEM were tested in a
DMFC using
non-platinum catalysts (anode: 6% Pd / Ce0 2 / C, cathode: 4% FeCo / C) and
gave a good
performance at 80 C (anode feed 4M Me0H + 5M KOH) comparable to a commercial
Tokuyama-AEM (maximum power density 120 mW/cm2). Another study of our work
group
comprises the synthesis of ionically and covalently cross-linked AEM blends of
bromomethylated PPO or a bromomethylated and partially fluorinated arylene
main chain
polymer and a partially fluorinated PBI (F6PBI) as a mechanically and
chemically stable matrix
and a sulfonated polyethersulfone sPPSU [46] added in deficit. The
halomethylated blend
component was quaternized with N-methylmorpholine (NMM) to the anion exchange
group
[47]. The interaction between the sulphonate groups of the sulphonated polymer
and the
basic N-methylmorpholinium cations resulted in the formation of ionic
crosslinks, which led
to an improvement in the mechanical and chemical stability of the AEM blend.
The alkali
CA 3066028 2019-12-23
stability of the membranes was examined in 1M KOH at 90 C over a period of
10 days as
compared to a commercial Tokemama AEM (A201). The most stable of the produced
AEM
blends lost about 40% of their original CI- conductivity while the commercial
A201 only had
21% of the original conductivity after that period. Similar AEM blends were
synthesized in
another work: brominated PPO was blended with PB100 or F6PBI as the matrix
polymer, and
to the blend of brominated PPO and F6PBI, sPPSU was further added as an ionic
crosslinker.
The quaternization of the bromomethylated PPO to generate the anion exchange
groups
was carried out with 1-methylimidazole or 1-ethyl-3-methylimidazole [41.
Examination of the
alkali stability (1M KOH, 90 C, 10 days) revealed a conductivity of 69% of
the original
conductivity for the blend membrane of 1-methylimidazole-quaternized PPO,
F6PBI and
sPPSU as ionic crosslinker after the stability test, while the blends from PPO
quaternized
with the two imidazoles and PB100 had a residual ionic conductivity between 31
and 43% of
the original value.
In addition to chemical stability, the achievement of the highest possible
selectivities for
certain anions is an important research and development topic of AEM, when
used in
electrodialysis or diffusion dialysis. Sata et al. investigated the dependence
of the
permeation of different anions on the hydrophobicity of the AEM functional
groups. The
hydrophobicity of the AEM functional groups has been systematically increased
by
increasing the length of the quaternary ammonium ion-bonded alkyl chains of
trimethylbenzylammonium, triethylbenzylammonium, tri-n-propylbenzylammoniurn,
tri-n-
butylbenzylammonium, and tri-n-pentylbenzylammonium. It has been found that as
the
hydrophobicity of the ammonium group increases, the relative transport of
large hydrate
shell anions, such as sulfate or fluoride ions, to anions with smaller
hydration shells, such as
chloride or nitrate, significantly decreases [49,50]. In another study, in
which AEMs were
hydrophilized by impregnation with ethylene glycols of different molecular
masses, a
marked increase in membrane permselectivity was observed for anions with large
hydration
shells, such as sulfate or fluoride [51]. In a work by Hickner et al, AEMs
were synthesized
consisting of rigid / flexible semi-interpenetrating networks of triethylamine-
quaternized
PPO and a polyethylene glycol network. It was found that this AEM has a high
ionic
conductivity (crow- up to 80 mS / cm) and a high alkali stability (degradation
of ionic
conductivity between 25 and 30% within 30 days of storage in 1M NaOH at 80
C) [52]. In
another work, polyethylene glycols were grafted onto chloromethylated SEBS
polymers, and
CA 3066028 2019-12-23
the resulting copolymers were then quaternized with trimethylamine. The
resulting AEMs
showed very high mechanical and chemical stabilities in 2.5M KOH at 60 C
(increasing the
ionic conductivity during storage in the KOH from 20 to 24 mS / cm) and high
ionic
conductivities (a0H- up to 52 mS / cm) [53].
The above-mentioned own studies have shown that covalent or ionic crosslinking
and / or
embedding of the anion exchange polymer in a chemically stable polymer matrix
is a viable
way to obtain chemically and mechanically stable AEMs. This work and work from
the
scientific community on AEMs with sterically hindered cationic groups as well
as AEMs with
additional hydrophilic phase are the starting point for the novel anion-
exchange blend
membranes described in this invention.
CA 3066028 2019-12-23
Description of the invention
Surprisingly, it has been found that in anion-exchange blend membranes
composed of the
following blend components:
A halomethylated polymer quaternized with a sterically hindered tertiary
nitrogen
compound (a polymer having ¨(CH2).-CH2-Hal groups, Hal = F, Cl, Br, I; x = 0-
12, for
example chloromethylated polystyrene or bromomethylated polyphenylene oxide;
Examples of sterically hindered tertiary nitrogen compounds are:
N
N r
N r N N
'
1 ,2,4,7-tetramethy1-1 H- 1 ,2,4,5,6,7-hexamethyl- 1 ,2,5,7-tetramethy1-1 H-
benzo[d]imidazole 1H-benzo[d]imidazole benzo[d]imidazole
quinuclidin-3-ol
quinuclidine OH N
NNA
1 ,2-dimethy1-1 H-
1 ,4-diazabicyclo[2.2.2]octane benzo[d]imidazole
N-
1\1¨
1 ,2,4,5-tetramethyl- 1 ,3,4,5-tetramethyl- 1 ,3,5-trimethy1-1 H-pyrazole
1 H-imidazole 1H-pyrazole
N N'
1 ,2-dimethy1-4,5-dipheny1-1 H-imidazole
CA 3066028 2019-12-23
Examples for halomethylated polymers are:
Br
CH2
0 C I
CH2
HC.2
,
Br/
CI Poly(epichlorohydrin)
Poly(vinyl- halomethylated
benzylchloride) Polyphenylenether
Br
H2C/
CH3
0
AAA,0 = II
S = 0
0
H2C CH2
bromomethylated Poly(ethersulfon) \B/
Br
F F F H2C CH3
0
F F F H2C\ /CH2
Br Br
bromomethylated partially fluorinated aromatic Polyether I
Br Br\cH2
F F F H2C
0 O¨
F F F F H2C\
Br/CH2
Br
bromomethylated partially fluorinated aromatic Polyether II
a matrix polymer, for example a basic polybenzimidazole; Examples of basic
matrix
polymers are:
CA 3066028 2019-12-23
H H
\ F6PBI /
1CF3
N-.......,.--, N .
JVNAP _________ ( 1
I / =
N----\.% .\-----NI CF3
H H
\ S02PBI / 0
NI N 11 II =
I / S
I I
N----- \------N1 0
H H
\ /
0 N
JVW __________ (NI 101 1p / = 0 =
N PB100 N
H H
\ / PBIO
1=1,....______/\_-. N .
1 / 0 .
14----\% \-----N1
H H
\ PBI Celazol / P4VP
N--____., .-N
sfvv-v- ______ ( 1
I , / I
1µ1"--\% \----1=1 N
H H H
1 para-PBI / AB-PBI /
1 \ 1 __ ( __ ) ¨ N
../VV V' ______ ( 1 -r1/4Ar sn ) ____ JVVV`
NI----\% \------N N
R R
0
I I
rv-tAr ________ 0 S
0
N¨)
R= \/) ____________________________ (N N)
\ /
\___ __
______________________________ OH ________________________ OH __
1
N
------;----N ----"'-'-i, .%-"--- -.õ,
I I
N N
0 0 0 0
- optionally a sulfonated aryl polymer as an ionic macromolecular
crosslinker (ionic
crosslinking with the basic functional groups of the matrix polymer and with
the
anion exchange groups of the quaternized halomethylated polymer.
CA 3066028 2019-12-23
Examples of sulfonated aryl polymers are:
sulfonated partially fluorinated aromatic polyether I (designation SFS001)
F F F F
CF3
F3
HO3 SO3H F F F F
sulfonated partially fluorinated aromatic polyether II
F3C F
CF3
_________________________ 0 0
CF3
H03 03H F F3
sulfonated Poly(phenyleneethersulfone), statistic Copolymer (designation
SAC098)
¨0 = 0 =
Ho3 03H Ho3 03H
sulfonated partially fluorinated aromatic polyethersulfone sulfonated
partially fluorinated
F F F F aromatic Poly(sulfone)
0 CF3
^^^-0 = ---s
0 cF3
Ho3 03H F F F F HO3S SO3H
sulfonated aromatic Poly(phenylphosphinoxide) I
0 0
HO3S 0 3 H it
SO3H
sulfonated aromatic Poly(phenylphosphinoxide) II
011
0
HO3S S 03H
SO3 H
optionally a sulfonated polymer as a covalent macromolecular crosslinker whose
sulfinate groups undergo covalent crosslinking via the sulfinate-S-alkylation
with the
halomethyl groups of the halomethylated polymer. As an example, the covalent
CA 3066028 2019-12-23
crosslinking reaction between a sulfonated and a halomethylated polymer is
shown:
Li CI 0
0
c/)--0 = 0 ____ avvv
130-150 C
DMSO
-LiCI
ÃfS
0-0
The addition of a hydrophilic linear polyethylene glycol bearing functional
groups on
both chain ends which can undergo nucleophilic substitutions with the basic
functional groups of the matrix polymer (examples: epoxide groups, halomethyl
groups) and thereby covalently anchored in the blend membrane which leads to
the
following property enhancements of the anion exchange blend membranes:
To a significant increase in the anion conductivity towards the previously
measured with best for anion exchange membranes conductivity values
- to a significant improvement in the chemical stability in strongly
alkaline
solutions even at elevated temperatures (for example, 1 molar aqueous KOH
solution at 90 C.)
- covalent crosslinking by the epoxide-terminated polyethylene glycols,
which
leads to a reduction in the swelling and thus to an improvement in the
mechanical stability.
The crosslinking reaction of the polyethylene glycols with the basic groups of
the matrix
polymers is schematically illustrated below for the reaction of an epoxide
group-terminated
polyethylene glycol with the imidazole group moieties of a polybenzimidazole:
CA 3066028 2019-12-23
'NAP _____ N N)__( ____________ ) CF3e )_,õ
N N ¨ CF3 \¨
H I-I
\ n 0
0
H H
N N CF3 ¨
-v-v¨(
)-----0 ( __ >---1."
N N i CF3
heat 1
>130 C
N3 ___________________________________________________
N\>_____<¨ CF ) CF ( )__,AA,
rlf Vs _______ N
N 3 ¨
121
HOr____,
0
z"-----4,
kJ
----OH
H
N N/>---(___) ____
N N
Surprisingly, it has furthermore been found that the membrane properties such
as
conductivity and thermal and chemical stability, in particular stability in
strongly alkaline
solutions such as aqueous potassium hydroxide solution or sodium hydroxide
solution can
be further improved by a sulfinated polymer optionally added to the blend
mixture. In
particular, it has surprisingly been found that the sulfinate groups of the
sulfinated polymer
are capable of reaction with epoxy or halomethyl end groups of the
polyethylene glycol,
presumably under sulfinate S-alkylation of the sulfinate groups by the epoxide
or halomethyl
CA 3066028 2019-12-23
groups. The reaction of the sulfinate groups of the sulfinated polymer with
the epoxide end
groups of the polyethylene glycol are shown below:
0
Sil 0-4vvy
_____________________________________ 0 __
O¨S
0
0
0
\O
Li 0
0¨S =
0
II =0-0 = Si, 0¨=^^^,
0
130-150 C
DMSO
0
______________________ )-0 Sil
S.,
OH
0
n
0 j0
r \0H
0 *
0
The anion-exchange blend membranes (AEBM) according to the invention can be
obtained
by means of three process routes:
1) The polymeric blend components (halomethylated polymer, matrix
polymer (eg
polybenzimidazole), polyethylene glycol with epoxide or halomethyl end groups,
optionally sulfonated polymer and / or sulfinated polymer) are co-agitated in
a
dipolar aprotic solvent or in a mixture of different dipolar aprotic solvents
(examples:
CA 3066028 2019-12-23
N, N-dimethylacetamide, N-methylpyrrolidinone, N-ethylpyrrolidinone,
dimethylsulfoxide, sulfolane). Thereafter, the polymer solutions are doctored
or cast
on a support (glass plate, metal plate, plastic film, etc.), and the solvent
is evaporated
in a circulating air dryer or a vacuum oven at temperatures between room
temperature and 150 C. Thereafter, the polymer film formed is removed from
the
backing and aftertreated as follows: 1) in a 10-50% solution of the tertiary
amine or
N-monoalkylated (benz) imidazole or N-monoalkylated pyrazole in an alcohol
(preferably ethanol or 2-propanol ) or in water or a water / alcohol mixture
at
temperatures from room temperature to the boiling point of the solvent for a
period
of 24-72 hours; 2) demineralized water at T = room temperature to T = 90 C
for a
period of 24-72 hours; 3) 10% aqueous NaCl solution at T = room temperature to
T =
90 C for a period of 24-72 hours; 4) DI water at T = room temperature to T =
90 C
for a period of 24-72 hours.
2) The polymeric blend components (halomethylated polymer, matrix
polymer (eg
polybenzimidazole), polyethylene glycol with epoxide or halomethyl end groups,
optionally sulfonated polymer and / or sulfinated polymer) are co-mixed in a
dipolar
aprotic solvent or in a mixture of different dipolar aprotic solvents
(examples: N, N-
dimethylacetamide, N-methylpyrrolidinone, N-ethylpyrrolidinone,
dimethylsulfoxide,
sulfolane). Thereafter, the tertiary amine or the N-monoalkylated (benz)
imidazole or
N-monoalkylated pyrazole is added either in bulk or dissolved in a dipolar
aprotic
solvent in a molar excess of 50-200%, based on the concentration of halomethyl
groups, to the solution , Thereafter, the polymer solutions are doctored or
cast on a
support (glass plate, metal plate, plastic film, etc.), and the solvent is
evaporated in a
circulating air dryer or a vacuum oven at temperatures between room
temperature
and 150 C. Thereafter, the polymer film formed is removed from the support
and
aftertreated as follows: 1) optionally in a 10-50% solution of the tertiary
amine or N-
monoalkylated (benz) imidazole or N-monoalkylated pyrazole in an alcohol
(preferably ethanol or 2- Propanol) or in water or a water / alcohol mixture
at
temperatures from room temperature to the boiling point of the solvent for a
period
of 24-72 hours; 2) demineralized water at T = room temperature to T = 90 " C
for a
period of 24-72 hours; 3) 10% aqueous NaCI solution at T = room temperature to
T =
CA 3066028 2019-12-23
90 C for a period of 24-72 hours; 4) DI water at T = room temperature to T =
90 C
for a period of 24-72 hours.
3) All
components of the polymer blend are separately dissolved in a dipolar aprotic
solvent or a mixture of different dipolar aprotic solvents. Thereafter, the
various
solutions are combined in the desired mass ratio, and then continue with the
resulting blend solution after homogenization as in the items 1) or 2).
CA 3066028 2019-12-23
Figure description
Figure 1 shows the chloride conductivities of the membranes 2175 and 2176 in
the temperature
range between 30 and 90 0 C with a constant relative humidity of 90%.
Figure 2 shows the chloride conductivity of the membrane 2176 before and after
10, 20 and 30 days
incorporation in 1M KOH in a temperature range of 30 to 90 C and a relative
humidity of 90%.
Figure 3 shows the TGA curves of membranes 2175 and 2176 before and after 10
days treatment in
1M KOH at 900 C
Figure 4 shows the TGA curves of membrane 2176 before and after 10, 20 and 30
days treatment in
1M KOH at 90 C
Figure 5 shows the chloride conductivity of the membrane 2190A before and
after 10 days storage in
1M KOH in the temperature range 30-90 C at a relative humidity of 90%
Figure 6 shows the TGA curves of membrane 2190A before and after 10 days
storage in 1M KOH at
90 0 C
Figure 7 shows the chloride conductivity of the membrane 2215 before and after
10 days storage in
1M KOH in the temperature range 30-90 C at a relative humidity of 90%
Figure 8 shows the TGA curves of membrane 2215 before and after 10 days
storage in 1M KOH at 90
C
Figure 9 shows the chloride conductivity of the membrane 2179B before and
after 10 days storage in
1M KOH in the temperature range 30-90 C at a relative humidity of 90%
Figure 10 shows the chloride conductivity of the membrane 2216 before and
after 10 days storage in
1M KOH in the temperature range 30-90 C at a relative humidity of 90%
Figure 11 shows the chloride conductivity of the commercial anion exchange
membrane Tokuyama
A201 in the temperature range 30-80 C at a relative humidity of 90%
CA 3066028 2019-12-23
Application Examples
Example 1: AEM blends of PVBCI, PB100, a sulfonated polyethersulfone (SAC098,
see
description) Tetramethylimidazole for quaternization of PVBC1 and an epoxide-
terminated polyethylene glycol (membranes MJK2175 and MJK2176)
Membrane production and aftertreatment:
12 g of a 10 % by weight solution of polyvinylbenzyl chloride (ALDRICH product
no. 182532,
structure see FIG. 2) in N,N-dimethylacetamide (DMAc) are mixed with 6 g of a
33.3% by
weight solution of 1,2,4,5-tetramethy1-1H-imidazole (TCI Product No. T0971,
see Figure 1 for
structure), 6.7 g of a 10% by weight solution of PB100 (manufacturer FumaTech,
structure
see Figure 3) and 2.67 g of a 10% by weight solution of a sulfonated
polyethersulfone
(SAC098, IEC = 1.8 meq SO3H / g, see description) mixed in DMAc. In the case
of membrane
2175, 0.25 g of epoxide-terminated polyethylene glycol (molecular mass 500
daltons,
ALDRICH product no. 475696) are added to this mixture after homogenization, in
the case of
membrane 2176 0.25 g of epoxide-terminated polyethylene glycol ( Molecular
mass 6000
daltons, ALDRICH product no. 731803). After homogenization, the polymer
solutions are
doctored on a glass plate. Thereafter, the solvent is evaporated in a
convection oven at 130 *
C for a period of 2 hours. The polymer films are then removed under water and
after-treated
as follows:
- At 60 0 C for 24 hours in a 10% by weight solution of
tetramethylimidazole in ethanol
- At 90 0 C for 48 hours in a 10 wt% solution of NaCI in water
- At 60 C for 48 hours in deionised water
- Parts of the membranes are placed in an aqueous 1M KOH solution for a
period of 10 days
at a temperature of 90 0 C *
Membrane characterization:
Membrane 2175:
- ion exchange capacity before / after KOH treatment * [meq OH- / g membrane]:
2.92 / 2.96
- Conductivity before / after KOH treatment * (Cl- form, measured in 0.5N
NaCI at room
temperature) [S / cm]: 29.3 / 72.7
- Water uptake at 25 0 C before / after KOH treatment * [%]: 367/324
Gel content after extraction in DMAc at 90 C before / after KOH treatment *
[%]: 97.6 / 100
CA 3066028 2019-12-23
Membrane 2176:
- ion exchange capacity before / after KOH treatment * [meq OH- / g membrane]:
2.79 / 2.84
- Conductivity before / after KOH treatment * (Cl- form, measured in 0.5N NaCI
at room
temperature) [5 / cm]: 21.6 / 69.9
- Water uptake at 25 C before / after KOH treatment * [%]: 370/313
Gel content after extraction in DMAc at 90 C before / after KOH treatment *
[%]: 97.4 / 97
Comparison of Characterization Results of Membranes 2175 and 2176
Remarkable and surprising in the two membranes 2175 and 2176 of this
application example
was that the conductivity of the membranes after 10 days of KOH treatment was
significantly higher than before the KOH treatment. Because of this surprising
finding, the
chloride conductivities were measured in another impedance measurement stand
as a
function of the temperature in a temperature range between 30 and 90 C at a
constant
relative humidity of 90%. The chloride conductivity vs. temperature curves of
the two
membranes 2175 and 2176 are shown in Figure 1. It shows, that:
1) both membranes have nearly equal conductivity curves;
2) even under these conditions, the conductivities measured after 10 days of
KOH
incorporation were significantly higher than before, although the molecular
masses of the
epoxide-terminated polyethylene glycols (PEG) used in membrane production are
very
different (2175: PEG molecular mass 500 daltons; 2176: PEG molecular mass 6000
daltons)
The gel content of the membranes of almost 100% surprisingly shows a complete
formation
of the network of these anion exchange blend membranes. Due to the excellent
membrane
stabilities, the storage time of membrane 2176 in 1M KOH at 90 C was
extended by a
further 20 days to a total of 30 days, and the membrane chloride conductivity
was
determined experimentally after a total of 20 days and after a total of 30
days in the
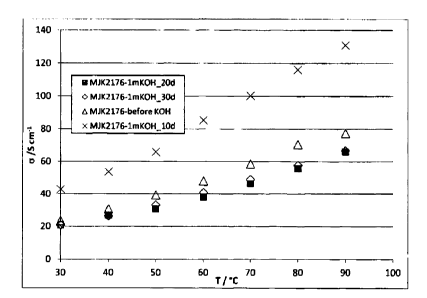
temperature range from 30 to 90 C under a relative humidity of 90%. Figure 2
shows the
chloride conductivities of the membrane 2176 before and after 10, 20 and 30
days
incorporation in 1M KOH in the temperature range from 30 to 90 C. There was
a surprising
development: after 10 days, the conductivity of the membrane was greatly
increased over
before the KOH treatment, and then decreased to a slightly lower level after
20 days
compared to before the KOH treatment. This value then no longer changed in the
time
CA 3066028 2019-12-23
interval between 20 and 30 days storage in KOH. Since the thermogravimetry
(TGA) studies
of the membranes can also give indications of degradation processes in the
membranes, for
the two membranes 2175 and 2176 TGA curves were recorded before and after the
KOH
treatment. Figure 3 shows the TGA curves of membranes 2175 and 2176 before and
after 10
days of treatment in 1M KOH at 90 C. From the TGA curves of both membranes
no
conclusions can be drawn on degradation processes in KOH solution, since the
TGA curves of
both membranes before and after 10 days of KOH treatment are almost congruent.
To determine if in 2176 membrane degradation occurs during the KOH long-term
stability
test of the membrane, TGA curves of the 2176 were recorded before and after
10, 20 and 30
days of incorporation in KOH. These TGA curves are shown in Figure 4. From
Figure 4, it can
be seen that the TGA curves of all 4 samples are nearly congruent up to a
temperature of
about 430 C, from which one can conclude that the 2176 still shows no sign
of significant
degradation even after 30 days of incorporation into KOH which confirms the
results of the
conductivity tests.
Example 2: AEM blend of PVBCI, PB100, a sulfonated polyethersulfone (SAC098,
see
description), tetramethylimidazole for quaternization of the PVBCI and an
epoxide-
terminated polyethylene glycol having a lower AEM content than in Application
Example 1 but the same molar ratio between PB100 and PEG -Diepoxid 6000
(Membrane MJK2190A)
Membrane production and aftertreatment:
12 g of a 10% by weight solution of polyvinylbenzyl chloride (ALDRICH product
no. 182532,
structure as described) in N, N-dimethylacetamide (DMAc) are mixed with 6 g of
a 33.3% by
weight solution of 1,2,4,5 -Tetramethy1-1H-imidazole (TCI product no. T0971,
structure see
description), 10.34 g of a 10 wt% solution of PB100 (manufacturer FumaTech,
structure see
description) and 2.67 g of a 10 wt% solution of a sulfonated polyethersulfone
(SAC098, IEC =
1.8 meq 503H / g, structure see description) mixed in DMAc. After
homogenization, 0.386 g
of epoxide-terminated polyethylene glycol (molecular mass 6000 daltons,
ALDRICH product
no. 731803) are added to this mixture. After homogenization, the polymer
solution is
doctored onto a glass plate. Thereafter, the solvent is evaporated in a
convection oven at
130 C for a period of 2 hours. The polymer film is then removed under water
and after-
treated as follows:
- At 60 C for 24 hours in a 10% strength by weight solution of
tetramethylimidazole in
CA 3066028 2019-12-23
ethanol
- At 90 C for 48 hours in a 10 wt% solution of NaCl in water
- At 60 C for 48 hours in deionised water
Part of the membrane is placed in an aqueous 1M KOH solution for a period of
10 days at a
temperature of 90 C *
Membrane characterization:
- ion exchange capacity before / after KOH treatment * [meq OH- / g
membrane]:
2.1 / 2.7
- Conductivity before / after KOH treatment * (Cl- form, measured in 0.5N NaCl
at room
temperature) [S / cm]: 14.3 / 16.3
- Water absorption at 25 C before / after KOH treatment * [%]: 67 / 90.5
- Gel content after extraction in DMAc at 90 C before KOH treatment [%]:
95.9
As with the membranes 2175 and 2176, the chloride conductivity was also
determined in
this membrane as a function of the temperature between 30 and 900 C at a
relative
humidity of 90%. The conductivity curves are shown in Figure 5. Surprisingly,
the
conductivity of the 2190A membrane also increases during KOH treatment. In
order to
determine the thermal stability of the membrane and possible degradation
processes in the
membrane, TGA curves of the membrane were recorded before and after 10 days of
KOH
treatment. The TGA curves are shown in Figure 6. Also in this membrane, the
TGA curves
before and after 10 days of KOH treatment almost congruent, at least up to a
temperature
of about 350 C, indicating that after 10 days of incorporation in 1M KOH at
90 C still no
significant degradation of the membranes has taken place.
Example 3: AEM blend of PVBCI, F6PBI, a sulfonated partially fluorinated
aromatic
polyether (SF5001, see description), tetramethylimidazole for quaternization
of the
PVBCI and a double-sidedly epoxide-terminated polyethylene glycol having a
molecular mass of 2000 daltons (membrane MJK2215)
Membrane production and aftertreatment:
3 g of a 20% by weight solution of polyvinylbenzyl chloride (ALDRICH product
no. 182532,
structure see Figure 2) in dimethyl sulfoxide (DMSO) are mixed with 3 g of a
33.3% by weight
CA 3066028 2019-12-23
solution of 1,2,4,5-tetramethyl 1H-imidazole (TCI Product No. T0971, see
Figure 1 structure),
10.34 g of a 5% by weight solution of F6PBI (see structure in description) in
DMSO and 1.11 g
of a 10% by weight solution of a sulfonated partially fluorinated aromatic
Polyether (SFS001)
in S03Li form (IEC = 2.39 meq SO3H / g, structure see description) mixed in
DMSO. After
homogenization, 0.193 g of epoxide-terminated polyethylene glycol (molecular
mass 2000
daltons, ALDRICH product no. 731811) are added to this mixture. After
homogenization, the
polymer solution is doctored onto a glass plate. Thereafter, the solvent is
evaporated in a
convection oven at 140 C for a period of 2 hours. The polymer film is then
removed under
water and after-treated as follows:
- At 60 C for 24 hours in a 10% strength by weight solution of
tetramethylimidazole in
ethanol
- At 90 C for 48 hours in a 10 wt% solution of NaCI in water
- At 60 C for 48 hours in deionised water
Part of the membrane is placed in an aqueous 1M KOH solution for a period of
10 days at a
temperature of 90 C *
Membrane characterization:
- ion exchange capacity before / after KOH treatment * [meq OH- / g
membrane]:
2.37 / 2.7
Conductivity before! after KOH treatment * (Cl-form, measured in 0.5N NaCI at
room
temperature) [S / cm]: 37.2 / 29.2
- Water uptake at 25 C before / after KOH treatment * [%]: 56.7 / 68
- Gel content after extraction in DMAc at 90 C before KOH treatment [%]:
92.7
As with the membranes 2175 and 2176 as well as 2190A, the chloride
conductivity was also
determined in this membrane as a function of the temperature between 30 and 90
C at a
relative humidity of 90%. The conductivity curves are shown in Figure 7.
Again, as in the
previous examples, the chloride conductivity after 10d storage in 1M KOH at 90
C is higher
than before. In order to determine the thermal stability of the membrane and
possible
degradation processes in the membrane, TGA curves of the membrane were
recorded
before and after 10 days of KOH treatment. The TGA curves are shown in Figure
8. Also in
this membrane, the TGA curves before and after 10 days of KOH treatment almost
CA 3066028 2019-12-23
congruent, at least up to a temperature of about 350 C, indicating that
after 10 days of
incorporation in 1M KOH at 90 C still no significant degradation of the
membranes has
taken place.
Comparative Example 1: AEM blend of PVBCI, PB100, a sulfonated
polyethersulfone
(SAC098, see description), tetramethylimidazole for quaternization of the
PVBCI with
the same calculated IEC as the membranes MJK2175 and MJK2176, but without PEG
diglycidyl ether (membrane 2179B)
Membrane production and aftertreatment:
6 g of a 10% by weight solution of polyvinylbenzyl chloride (ALDRICH product
no. 182532,
structure see description) in DM50 are mixed with 2.2 g of a 33.3% by weight
solution of
1,2,4,5-tetramethy1-1H- lmidazole (TCI product no. T0971, see structure for
description) in
DMAc, 4.6 g of a 10% strength solution of PB100 (manufacturer FumaTech,
structure see
description) in DMAc and 1.335 g of a 10% by weight solution of a sulfonated
polyethersulfone ( SAC098, IEC = 1.8 meq SO3H / g, structure see description)
mixed in
DMAc. After homogenization, the polymer solutions are doctored on a glass
plate.
Thereafter, the solvent is evaporated in a convection oven at 140 C for a
period of 2 hours.
The polymer films are then removed under water and after-treated as follows:
- At 60 C for 24 hours in a 10% strength by weight solution of
tetramethylimidazole in
ethanol
- At 90 C for 48 hours in a 10 wt% solution of NaCI in water
- At 60 C for 48 hours in deionised water
- Parts of the membranes are placed in an aqueous 1M KOH solution for a period
of 10 days
at a temperature of 90 C *
Membrane characterization:
- ion exchange capacity before / after KOH treatment * [meq OH- / g
membrane]:
2.5 / 2.64
Conductivity before / after KOH treatment * (Cl-form, measured in 0.5N NaCI at
room
temperature) [S / cm]: 10.7 / 15.9
- Water uptake at 25 C before / after KOH treatment * [%]: 63/87
Gel content after extraction in DMAc at 90 C before KOH treatment * [%]:
94.2
CA 3066028 2019-12-23
If these data are compared with those of membranes 2175 and 2176, the
following results:
- The Cl - conductivity is much lower than in the two membranes of the
invention. This
shows what a positive influence the addition of a hydrophilic PEG phase has to
the
membrane
- Water uptake is significantly lower than at 2175 and 2176. This can be
explained by the
lower hydrophilicity of the control membrane.
Since the Cl conductivity of the 21798 was higher in conductivity measurement
at room
temperature and in 0.5N NaCI as at 2175 and 2176 after the KOH treatment, the
impedance
of the 21798 was again measured in dependence of the temperature at a relative
humidity
of 90%. The conductivity curve of the 2179B under these conditions is shown in
Figure 9.
Here, it is found that, as in the impedance measurement at room temperature in
0.5M NaCI,
the chloride conductivity is much lower than that of the 2175 and 2176
containing a PEG
phase and that the impedance after KOH treatment is significantly lower than
before. Since
at 2175 and 2176 the chloride conductivity was higher after 10d KOH treatment
than before,
on the one hand shows the conductivity-increasing effect and on the other
hand, the
stabilizing effect of the presence of a PEG microphase in the blend AEMs.
Comparative Example 2: AEM blend of PVBCI, F6PBI, a sulfonated partially
fluorinated
polyether (SFS001, see description), tetramethylimidazole for quaternization
of PVBCI
with the same calculated IEC as the membrane MJK2215, but without PEG
diglycidyl
ether (membrane 2216)
Membrane production and aftertreatment:
3 g of a 20% by weight solution of polyvinylbenzyl chloride (ALDRICH product
no. 182532,
structure as described) in DMSO are mixed with 3 g of a 33.3% by weight
solution of 1,2,4,5-
tetramethy1-1H-imidazole (ICI Product No. T0971, structure see description) in
DMSO, 14.2
g of a 5 wt% solution of F6PBI (structure see description) in DMSO and 1.1 g
of a 10 wt%
solution of the sulfonated polyether SF5001 (IEC = 2.39 meq SO3H / g,
structure see
description) mixed in DMSO. After homogenization, the polymer solutions are
doctored on a
glass plate. Thereafter, the solvent is evaporated in a convection oven at 140
C for a period
of 2 hours. The polymer film is then removed under water and after-treated as
follows:
- At 60 C for 24 hours in a 10% strength by weight solution of
tetramethylimidazole in
CA 3066028 2019-12-23
ethanol
- At 90 C for 48 hours in a 10 wt% solution of NaCI in water
- At 60 C for 48 hours in deionised water
- Parts of the membranes are placed in an aqueous 1M KOH solution for a period
of 10 days
at a temperature of 90 C.
Membrane characterization:
- ion exchange capacity before / after KOH treatment * [meq OH- / g membrane]:
2.48 / 2.7
- Conductivity before / after KOH treatment (Cl- form, measured in 0.5N NaCI
at room
temperature) [S / cm]: 7.4 / 8.2
- Water absorption at 25 C before / after KOH treatment [%]: 44/33
- Gel content after extraction in DMAc at 90 C before KOH treatment * [%]:
95.7
If these data are compared with those of the membrane 2215, the following
results:
- The Cl - conductivity at room temperature in 0.5N NaCI is significantly
lower than in the
inventive membrane 2215. This shows the positive influence of the addition of
a hydrophilic
PEG phase has to the membrane.
- The water absorption is significantly lower than at 2215. This can be
explained by the lower
hydrophilicity of the control membrane.
Since the Cl conductivity of the 2216 was higher in the conductivity
measurement at room
temperature and in 0.5N NaCI as in 2215 after the KOH treatment, the impedance
of the
2215 was again measured as a function of the temperature at a relative
humidity of 90%.
measured. The conductivity curve of the 2215 under these conditions is shown
in Figure 10.
Here it can be seen that, as in the impedance measurement at room temperature
in 0.5M
NaCI, the chloride conductivity is much lower than in the 2215 containing a
PEG phase, and
that the impedance after the KOH treatment is significantly lower than before.
Comparative
Example 2 shows, as in Comparative Example 1, on the one hand, the
conductivity-increasing
effect and, on the other hand, the stabilizing effect of the presence of a PEG
microphase in
the blend AEMs.
CA 3066028 2019-12-23
Comparative Example 3: Commercial anion exchange membrane A201 (development
code A006) of the manufacturer Tokuyama
The structure of this membrane is company secret. The anion exchange group of
this
membrane is the trimethylammonium group. But it is obviously a cross-linked
membrane
because the extraction of the membrane gave a gel content of 95%.
Membrane characterization:
- Ion exchange capacity [meq OH / g membrane]: 1.7
- Conductivity (Cl- form, measured in 1 N NaCI at room temperature) [S / cm]:
12
- Water absorption at 30 C [%]: 19
- Gel content after extraction in DMAc at 90 C before KOH treatment *
[%]: 95
- Conductivity (Cl- form, measured at 90 C and 90% relative humidity, after
10d
incorporation in 1M KOH at 900 C): 21% of the original conductivity
This commercial membrane is thus much less stable in 1M KOH at 90 C compared
to the
membranes of the invention. In addition, the chloride conductivity of this
membrane is
substantially lower than most of the membranes of this invention listed as
examples in this
chapter. The chloride conductivity of the A201 in the temperature range of 30
to 80 C at
90% relative humidity is shown in Figure 11.
Comparative Example 4: Commercial anion exchange membrane FAB from the
manufacturer Fuma-Tech
The structure of this membrane is company secret. But it is obviously a cross-
linked
membrane, as the extraction of the membrane gave a gel content of 93.3%.
Membrane characterization:
- Ion exchange capacity before / after 10d in 1M KOH at 90 C [meq OH- / g
membrane]:
0.88 / 0.89
- Conductivity before / after 10d in 1M KOH at 90 C (Cl- form, measured in 1
N NaCI at
room temperature) [S / cm]: 4 / 3.2
- Water absorption at room temperature / at 90 C C [%]: 12.1 / 13.2
Gel content after extraction in DMAc at 90 C before / after KOH treatment *
[%]: 93.3 / 97
The chloride conductivity of this membrane is substantially lower than that of
most of the
CA 3066028 2019-12-23
,
membranes of this invention listed as examples, which is also (among others)
because this
membrane is fabric-reinforced.
CA 3066028 2019-12-23