Note: Descriptions are shown in the official language in which they were submitted.
CA 03067059 2019-12-12
WO 2018/227292
PCT/CA2018/050713
WOUND CLOSURE SYSTEM AND METHOD
Field
[00oi] The disclosure relates to medical devices, and in particular
devices
for the management of open wounds such as a post-surgery wound arising from
abdominal surgery.
Background
[0002] International patent application WO2m6/127256 discloses a
device for management of an open abdomen wound. The device comprises a belt
which extends partway around a patient, with the two ends of the belt being
joined to respective connector members. The connecter members include pads
that contact the patient on opposing sides of the wound. The connecters may be
cinched together using zip ties or similar retractable cords which join
together
the opposing pads across the wound. The cords can be retracted to draw the
pads together across the opposing edges of the wound, to management treatment
of the wound, prevent spreading of the wound margins, and act as a preventive
measure against muscle retraction.
[0003] A problem arising in major surgical operations such as abdominal
surgery that involves incisions of major abdominal muscle groups, is that such
wounds can present difficulties in healing. In particular, normal muscle
contractions, patient breathing and other movements tend to cause the wound
edges to separate rather than draw together to promote healing. It is
desirable
to provide devices which counteract this natural tendency.
Summary
[0004] We disclose herein a wound management system comprising:
1
CA 03067059 2019-12-12
WO 2018/227292
PCT/CA2018/050713
a belt configured to partially wrap around the body of a patient in the area
of a
wound;
first and second base members configured to contact the patient on opposing
sides of the wound, the base members each comprising a lower face configured
for directly or indirectly contacting the patient, and an opposed upper face;
first and second clamp assemblies projecting from the upper faces of the first
and
second base members respectively to grip opposing end regions of the belt,
wherein at least one of the clamp assemblies is configured to releasably
secure
the belt to permit engagement, tensioning and detensioning of the belt
relative to
the base members;
at least one cord assembly for connecting the base members to each other
across
the wound, the cord assembly comprising a cord, a cord anchor configured to
attach a first end of the cord to a first of the base members and a cord
retractor
on the second of the base members for retracting the cord.
[0005] One or both of the clamp assemblies may comprise a rotatable cam
member configured to permit free slippage of the belt when the cam member is
rotated into a disengaged position and to prevent slippage when the cam
member is urged into a closed position by tension on the belt.
[0006] We further disclose herein a method for managing a wound in a
patient comprising the steps of:
-positioning first and second base members to contact the patient on
opposing sides of the wound, the base members each comprising a lower face
configured for directly or indirectly contacting the patient, and an opposed
upper
face;
securing the base members together across the wound by connecting the
base members with at least one cord assembly, the cord assembly comprising a
2
CA 03067059 2019-12-12
WO 2018/227292
PCT/CA2018/050713
cord, a cord anchor configured to attach a first end of the cord to a first of
the
base members and a cord retractor on the second of the base members for
retracting the cord;
retracting the cord of the at least one cord assembly to provide a selected
degree of tension in the cord;
wrapping a belt partially around the body of the patient in the area
opposed to the wound, wherein the belt is unsecured from at least one of the
base
members; and
securing the belt to the at least one base member with a clamp assembly
wherein the clamp assembly projects from the upper face of the at least one
base
member to grip an end region of the belt to releasably secure the belt to
permit
engagement, tensioning and detensioning of the belt relative to the base
members.
[0007] In the present specification and claims, directional references
such
as "horizontal" "upper", "lower" etc. are provided purely for ease of
description
and are not intended to limit the scope of the invention. Unless otherwise
stated,
such terms are in reference to the device as placed on a level surface in
position
or in normal use on a patient, extending across an abdomen wound on the
patient's stomach, with the patient lying horizontally on his back facing
upwardly. Furthermore, dimensions presented herein, as well as specific
materials and the like, are provided merely by way of example.
Brief description of the drawings
[0008] The drawings and description which follow illustrate specific
embodiments of the invention herein and are not intended to limit the scope of
the present invention. The full scope of the present invention is provided in
the
present specification, drawings and claims as a whole. Furthermore, persons
skilled in the art will understand that references herein to any specific
3
CA 03067059 2019-12-12
WO 2018/227292
PCT/CA2018/050713
components, features or elements can include their structural and/or
functional
equivalents.
[0009] Figure 1 is a perspective view of an embodiment of the abdominal
closure system, in which the belt is unlocked to permit adjustment.
[oclio] Figure 2 is a perspective view as in figure 1, showing the belt
in the
locked position against slippage relative to the base members.
[oon] Figure 3 is a perspective view of base member of the system,
showing the unlocked position.
[0012] Figure 4 is partial cross-sectional view, in perspective, along
line 4-
4 of Figure 3 showing components of the system and showing the cord assembly
removed to show internal structures.
[0013] Figure 5 is a partial cross-sectional view similar to Figure 4,
showing the cord assembly installed.
[0014] Figure 6 is a further cross-sectional perspective view similar to
Figure 5, showing the clamp assembly in the fully disengaged position.
[0015] Figure 7 is a side elevation showing of the base member.
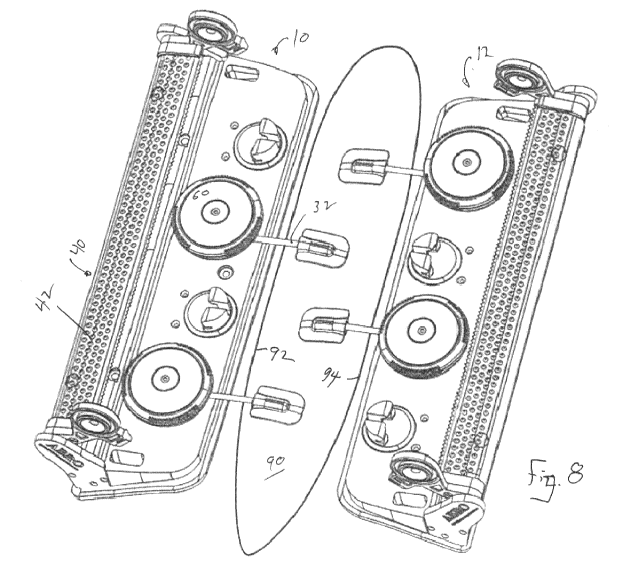
[0016] Figure 8 is a perspective view of the device positioned on a
patient.
[0017] Figure 9A a shows a portion of the system, consisting of opposing
base members which are positioned across a surgical wound of a patient.
[oca8] Figure 9B is a perspective view as in figure 9A, showing
retraction
of the device across a wound.
[0019] Figure 10 is a side view of a portion of the system, showing the
device being retracted across a surgical wound.
4
CA 03067059 2019-12-12
WO 2018/227292
PCT/CA2018/050713
[0020] Figure 11 is a side view, showing the device in use on a patient.
[434321] Figure 12 is a sectional view showing the device in use on a
patient.
Detailed description
[0022] Referring first to Figs. 1 - 7, the present device 1 includes a
pair of
rigid base members 10 and 12, respectively, which are secured around a patient
in the area of a wound, with a combination of a belt 30 and cords 32,
described
below. Base members 10 and 12 are identical to each other and interchangeable.
Members 10 and 12 comprise rectangular plates which can be fabricated from a
rigid moulded or machined plastic such as ABS, Nylon, PVC, acetal and other
polymers displaying similar physical properties. Base members 10 and 12 each
comprise an upper face 14, an opposing lower face 16, opposing lateral edges
18a
and 18b and opposing end edges 20a and 20b. Base members 10 and 12 may be
flat or curved to match the curvature of the midriff area of a typical
patient. For
reference herein, each base member has a longitudinal axis a extending between
end edges 20 and a lateral axis b extending between lateral edges 18.
[434323] Typical dimensions for base members 10 and 12 are between 150
and 25omm in length (axis a) and 30-100mm in width (axis b). In one
example, the length/width dimensions are about 225 mm x 65mm. A device
having these dimensions is intended for use in treating normal adult patients
undergoing major abdomen surgery. It will be readily apparent that different
dimensions may be provided, depending on the application.
[434324] For reference and convenience of description, device 1 will be
described herein with members 10 and 12 located in a horizontal position
wherein the upper faces 14 are horizontal and face upwardly. When base
members 10 and 12 are secured to a patient for managing a wound, as discussed
below, lateral edges 18a of opposing members 10 and 12 face each other across
a
wound 24.
CA 03067059 2019-12-12
WO 2018/227292
PCT/CA2018/050713
[0025] Referring to figures 6 and 7, the upper face 14 of base members
10
and 12 has a flat region adjacent to the inner lateral edge 18a (facing the
opposing base member across the wound when in use), which is generally
horizontal when in use. An outer portion of surface 14, adjacent to outer
lateral
edge 1813, has a ridge that provides a sloping surface 15.
[0026] The lower faces 16 of members 10 and 12 are slightly curved to
provide a trough-shaped, concave surface 23, in which the trough 23 extends
along axis a. Lower face 16 has an arcuate cross sectional profile. The
curvature
of lower face 16 is configured to match the curvature of a typical human
abdomen. The thickness of base members 10 and 12 (dimension c, seen in Figure
7) increases from first lateral edge 18a, which faces the opposing base member
across the wound when in use, to the second lateral edge 1813, which faces
outwardly from the wound. As seen in Figure 7, base members 10 and 12 are
generally tapered between lateral edges 18a and b, with the thin portion of
the
taper being located at the sides of the base members 10 and 12 that face each
other across the wound. In use, this provides a generally horizontal upper
surface 14 when the device is positioned on a patient.
[0027] Lower faces 16 are further characterized by a ridge 21 adjacent
to
outer later edge 1813. Ridge 21 defines an inflection line between the
concavely-
curved region 23 of lower surface 16, and a flat, horizontally-projecting
strip 25
that is adjacent to outer edge 1813. Ridge 21 provides additional traction
against
the patient's skin when the device is tensioned, to assist in drawing together
the
opposing edges of the wound 90.
[0028] The lower faces 16 of members 10 and 12 are each lined with a pad
28, comprising a soft, elastomeric and somewhat grippy material such as
elastomeric silicon, having properties which make it suitable for contacting
the
patient's skin during a medical procedure. Preferably, pad 28 protrudes
slightly
beyond the edges 18 and 20 of members 10 and 12, to prevent direct contact
between the patient and the rigid plastic material of members 10 and 12.
6
CA 03067059 2019-12-12
WO 2018/227292
PCT/CA2018/050713
[0029] A flexible belt 30 is provided to secure base members 10 and 12
to
the patient (see Figs. 1 and 2). Belt 30 is configured to wrap partially
around the
patient to secure members 10 and 12 to the patient, in cooperation with cords
32
described below. As such, the combination of member 10 and 12, belt 30 and
cords 32 fully encircle the patient, typically around the midriff region, when
device 1 is engaged on a patient. Belt 30 may be tightened or loosened
relative
to members 10 and 12 to fit the device snugly to the patient. Typically, belt
30
will be adjusted on members lo and 12 whereby members 10 and 12 are
positioned on opposing sides of the wound, close to the margins of the wound,
with belt 30 providing a snug fit around the patient. The method by which this
may be performed is discussed below.
[0030] Bell 30 is fabricated from a flexible material, such as mesh,
woven
fabric, flexible film or the like. Typical materials include such as spandex,
neoprene containing latex rubber and similar fibers. Preferably, belt 30 is
stretchable and elastic in at least its elongate dimension. The elasticity of
belt 30
permits the device to retain a substantially consistent tension and a snug fit
during patient muscle contractions, breathing and other movements by the
patient. The degree of elasticity should be provided which provides a
sufficiently
tight fit to permit the device to accomplish the wound management results
discussed below, while at the same time permitting a reasonably consistent
tension during normal expansion and contraction of the patient's muscles and
abdomen. For example, belt 30 may be configured to provide a maximum of
tensile force in the range of 10-20lb5 linear force when secured around an
average patient. Bell 30 is configured to wrap partially around a portion of
the
patient's body (such as the torso) out of contact with the wound area, to
secure
members 10 and 12 to the patient. At the wound area itself, members 10 and 12
are joined together with a retractable cord assembly 34 comprised of multiple
retractable cords 32, as discussed in more detail below. Device 1 in its fully
assembled form thus fully encircles the patient's torso. In particular, the
patient
is encircled with a combination of members 10 and 12, belt 30 and cords 32, as
may be seen for example in figure 9.
7
CA 03067059 2019-12-12
WO 2018/227292
PCT/CA2018/050713
[0031] At least one of base members 10 and 12, and optionally both of
members 10 and 12, includes a clamp assembly 35 to releasably and adjustably
secure belt 30 to members 10 and 12. Clamp assembly 35 engages belt 30 to the
respective base member 10/12 and also permits belt 30 to be released from base
members 10 and 12 and also adjusted in effective length relative to one or
both of
base members 10 and 12.
[0032] Clamp assembly 35 includes a pair of opposing, spaced apart tabs
36 that project upwardly from the upper faces 14 of base members 10 and 12.
Tabs 36 are aligned with end edges 20 of members 10 and 12. Each of tabs 36
comprises an inwardly-projecting horizontal post 38, whereby the posts 38 on
opposing tabs 36 face each other. Posts 38 are co-axial and are aligned with
elongate axis a. A releasable belt clamp bar 40 is rotatably journalled on
posts
38. Clamp bar 40 is configured to releasably secure belt 30 to respective base
members 10 and 12 by gripping belt 30 between clamp bar 30 and a portion of
base member 10/12. Clamp bar 40 extends substantially the length of base
member 10 or 12 respectively, extending between the opposing end edges 20
thereof. Clamp bar 40 has post-holes (not shown) recessed into its end faces
whereby it may rotate freely on posts 38.
[0033] Clamp bar 40 rotates between a first, locked position, seen in
Fig.
2, in which belt 30 is gripped between clamp bar 40 and sloping portion 15 of
upper surface 14 and thus prevented from slipping, and a second, unlocked
position, seen in Figs. 1 and 3, in which clamp bar 40 is spaced from surface
14
and belt 30 can be adjusted or fully released from base member 10/12.
[0034] As seen in Figures 4-6, clamp bar 40 has an eccentric (non-
circular) shape in cross section, which provides a cam-like function when
rotated
between the first and second positions. For this purpose, the axis of rotation
of
clamp bar 40 is offset from the central axis of the bar. Clamp bar 40 has a
generally wing-shaped cross-sectional profile, characterized by a generally
flat
lower face 41 which is configured to contact the corresponding flat sloping
portion 15 of base member 10/12. Clamp bar 40 and associated clamp assembly
8
CA 03067059 2019-12-12
WO 2018/227292
PCT/CA2018/050713
components are configured whereby lower face 41 of bar 40 is in contact and
flush against upper face 14 of base member 10/12. When the respective faces
are
in contact, belt 30 is gripped between the respective flat surfaces 15 and 41.
Clamp bar 40 further comprises an upper flat face 42, opposed to lower face
41,
which slopes upwardly. Upper face 42 is covered with an array of protrusions
43
or other surface which is adapted to grip the belt 30, using a material such
as a
Velcro TM hook material. This feature permits the free end of belt 30 to be
wrapped around bar 40 and further secured to clamp bar 40, as seen in Figures
1
and 2. Lower face 41 of clamp bar 40 has a grip-plate 45 secured thereto,
having
a serrated edge 54 which engages belt 30 when clamp bar 40 is rotated into the
grip position, as described below.
[0035] When clamp bar 40 is rotated into the open position, as seen in
Figure 6, a gap 44 is exposed between clamp bar 40 and upper face 14 to permit
belt 30 to slide through this gap.
[0036] In use, an end portion of belt 30 is wrapped over clamp bar 40
whereby a portion of belt 30 is engaged to protrusions 43. Clamp bar 40 is
then
rotated into the closed position by gripping and rotating tabs 36. The cam-
like
shape of clamp bar 40 tends to urge clamp bar 40 further into the clamped
position when belt 30 is retracted, thereby resisting slippage of belt 30 when
this
is retracted against base members 10 and 12.
[0037] As seen in detail in Figs. 5 and 7, clamp assembly 35 includes
locking members 46 that lock clamp bar 40 to the corresponding member 10 or
12 into the locked position. Lock members 46 comprise a pair of opposing L-
shaped projections extending from the opposing ends of clamp bar 40. Lock
members 46 are attached to clamp bar 40 to rotate with it. Each of lock
members comprises a resilient tab 49 aligned with end edges 18, engaged at its
proximal end to clamp bar 40. Plates 49 each have a disk-shaped portion at
their
proximal regions that have a slight depression to accommodate a user's finger,
whereby opposing plates 49 may be squeezed together by the user. Plates 49 are
configured to provide a suitable degree of resilience whereby the respective
lock
9
CA 03067059 2019-12-12
WO 2018/227292
PCT/CA2018/050713
members may be squeezed together without too much difficulty, but not so
easily
as to permit inadvertent release during normal use. A flat projection 48
extends
downwardly from the disc-shaped portion of plate 49 and is shaped to fit into
a
corresponding slot 50 within base member 10/12. Slots 50 are located adjacent
to end edges 20. Projections 48 are each provided with a laterally-projecting
protrusion 52 at its distal end, whereby opposing protrusions 52 project away
from each other.
[0038] When clamp bar 40 is rotated towards the locked position,
projections 48 of lock members 46 enter into slots 50. As protrusions 52 come
into contact with the edges of slots 50, this urges projections 48 to flex
towards
each other to permit projections 48 to enter into slots 50. Projections 48 can
thus snap-lock into slots 50 to lock clamp bar 40 into the locked position.
Lock
members 46 may afterwards be squeezed together to release lock members 46
from slot 50, thereby permitting rotation of clamp bars 40 into the undamped
position. This in turn disengages belt 30 from clamp assembly 35 to allow
adjustment of belt 30 or its release from base member 10 or 12.
[0039] The serrated edge 54 of grip plate 45 serves to grip the belt 30
when in the clamped position. When clamp bar 40 is rotated away from the
locked position, serrated edge 54 is out of contact with belt 30, thereby
allowing
belt 30 to slide through clamp assembly 35.
[0040] Cord assembly 34 will now be described with specific reference to
Figs. 3-8.
[0041] Cord assembly 34 comprises multiple cord retractors, consisting
of
rotatable cord wind-up reels 60 which are independently and rotatably mounted
to base plates 10 and 12. Reels 60 may comprise a commercially available take
up reel of the type that provides a releasable ratchet mechanism to permit
wind-
up retraction of cord 32 by rotation of reel 60, and release of cord 60 by
pulling
upwardly or other manipulation of reel 60. Reels 60 are each mounted to an
upwardly-projecting mount 66 on base plates 10 and 12. In the present example,
CA 03067059 2019-12-12
WO 2018/227292
PCT/CA2018/050713
each of base members 10 and 12 is provided with two spaced apart reels 60,
each
configured to retract an individual one of cords 32. Reels 60 each comprise a
spool surface 68 for winding up a corresponding cord, and is capped with a
gripper disk 70 to allow the user to rotate the corresponding reel 60.
[0042] Cords 32 may comprise monofilament style, braided and/ or spiral
styled constructions, and may be fabricated from a metal or polymer or a
combination thereof. Examples include stainless steel, nylon, polyesters, pvc,
and other materials.
[0043] Each of cords 32 comprises a free end 72 which is releasably
anchored to a respective base member 10/12 with a cleat 74. A corresponding
reel 60 is mounted to an opposing base member to retract the opposing end of
the cord. Cleat 74 may fixedly or releasably secure free end 72; in the
present
example, cord 32 is releasably secured. Cleat 74 consists of an upwardly-
projecting member, having a vertical slot 76 open to its upper surface and
extending laterally. Anchor 74 also has a horizontal slot 78, open to the rear
face
of cleat 74. The free end of cord 32 is fixed to a flat tab 79, which is
configured
to fit snugly within horizontal slot 78 to retain cord 32 to cleat 74 in a
position in
which cord 32 is inserted within vertical slot. The tension applied to cord 32
when device 1 is secured to a patient retains tab 79 within corresponding slot
78.
In this fashion, the free end of cord is securely anchored to one of base
members
or 12 when in use, with the other end of cord 32 being engaged to reel 60 on
the other one of base members 10 or 12.
[0044] In the illustrated embodiment, four cords 32 are provided, each
associated with a corresponding wind up reel 60 and cleat 74 for independently
anchoring the respective ends of each cord 32. The reels 60 and cleats 74 are
staggered, whereby each of members 10 and 12 is provided with two cleats and
two reels, arranged an alternating fashion. It will be seen that a different
configuration and number of such cords 32 and anchoring cleats may be
provided, depending on the overall size of base members 10 and 12 and other
considerations.
11
CA 03067059 2019-12-12
WO 2018/227292
PCT/CA2018/050713
[0045] Device 1 may be provided within a range of dimensions of its
components. For example base members 10/12 may be approximately 225 x
65mm in length x width to accommodate a typical adult. In other examples, the
base members may be outside of this range in versions scaled to accommodate
above or below-average sized persons. The band may be dimensioned to
accommodate a range of typical adult circumferences of between 75cm and
150cm but may fall outside of that range in versions scaled to accommodate
above or below average sized persons. The cables 32 may be about 45mm in
length, which permits a broad range of extension and wind up inside of the
reel.
[0046] A method of using device 1 to manage a wound 90 is shown in
figures 8-12. Wound 90 may be an abdominal wound, such as a post-surgical
wound as shown in figures 9A, 9B and 10, which extends longitudinally (i.e.
head
to toe direct) along the midline of the patient's abdomen. Wound 90 is defined
by wound margins 92 and 94. Device 1 is normally supplied to the health
practitioner with belt 30 disengaged from one or both of base members 10 and
12, and cords 32 disengaged from cleats 74. One end of belt 30 may then be
engaged with one of base members 10 and 12. Base members 10 and 12 are then
positioned adjacent the margins of the wound, as seen in the figures. In this
example, base members 10 and 12 are positioned on the rectus muscles of the
abdomen. The tensioning effect of device 1 provides a splinting effect that
acts
against the naturally occurring wound ¨ spreading effect.
[0047] Typically, lateral edges 18 will be slightly displaced from the
exposed edges of wound 90 to prevent the risk of contact of the base members
10
and 12 with the open wound edge. The free (unattached) ends of cords 32 are
then anchored to corresponding cleats whereby cords 32 extend across the
wound and base members 10 and 12 are prevented from further separation from
each other. Cords 32 remain spaced above wound 90 at all times during
application, adjustment and use of the device, to avoid contact between wound
90 and cords 32.
12
CA 03067059 2019-12-12
WO 2018/227292
PCT/CA2018/050713
[0048] At this point, belt 30 is placed around the patient's body. For
example, if wound 90 is on the dorsal midriff (belly) side of the patient, as
shown
in the figures, belt 30 will be passed around the back (dorsal) midriff of the
patient. The free ends of belt 30 are then engaged to the clamp assemblies 35
of
the respective base members 10 and 12. Belt 30 is then retracted to provide a
snug fit of device 1 to the patient. Figure 7 schematically shows tightening
of
belt 30 around the patient's midriff, whereby device 1 is snuggly affixed to
the
patient. As discussed above, the elastic properties of band 30 permits base
members 10 and 12 to remain in their original positions without shifting as
the
patient breathes and other muscle movements.
[0049] Cords 32 are periodically retracted to draw the wound edges
together. For example, cord retractions may be performed in a series of
discrete
retractions performed in a staged fashion 2-3 times daily for several days
following surgery, to fully close wound 90. In other examples, discrete
retractions of cords 32 are performed hourly, 12X daily, 6x daily, 3x daily,
2X
daily or daily. The device may be used for treatment of the patient over a
period
of 1/2 day, 1 day, 2 days, 3 days, 4 days, 5 days, 6 days or 7 days. The
device may
secured to the patient for the entire course of treatment. Adjustments of
cords
32 may typically be performed as fine adjustments, for example about 5mm of
retraction per discrete retraction stage. This may range to a gross adjustment
of
about loomm. For example, the gross adjustment of cords 32 may be performed
during the initial accommodation phase and the magnitude of the adjustments
may then decrease over time. Patients are monitored for intra-abdominal
pressure and this characteristic would supersede any linear measurement
targets, whereby the amount of retraction that is performed, as well as the
timing
of stages, is adjusted based on patient response as determined by individual
monitoring of the patient.
[0050] Retraction of the cords 32 to close a wound 90 is shown
schematically in figures 9B and 10. According to one aspect, retraction
prevents
the loss of domain, in that it engages oblique and a transverse muscles of the
abdomen, creating an opposing force to intra- abdominal pressure that would
13
CA 03067059 2019-12-12
WO 2018/227292
PCT/CA2018/050713
otherwise tend to separate the wound edges thereby preventing healing. When
the device is used as described herein, the discrete retractions of the cords
32
delivers gradual traction of the abdominal muscles towards the midline. This
action prevents lateralization of the abdominal wall muscles. Furthermore, use
of the device 1 as described herein serves to engage oblique and transverse
muscles, thereby creating an opposing force against intra-abdominal pressure.
[0051] The description of the device and the accompanying drawings are
intended to provide an example of the present invention, and are not intended
to
limit the scope of the invention in any respect. The full scope of the
invention is
provided in the present specification as a whole, including the claims.
14