Note: Descriptions are shown in the official language in which they were submitted.
CA 03068248 2019-12-20
WO 2019/005429
PCT/US2018/035861
IN THE UNITED STATE PATENT AND TRADEMARK OFFICE
COMPOSITION AND METHOD FOR INHIBITING CORROSION AND SCALE
Cross-Reference to Related Application
[0001] This application claims priority to U.S. Application Serial No,
15/634,416 filed on June 27, 2017.
Background of the Invention
1. Field of the Invention
[0002] This invention relates to a treatment composition and method for
inhibiting corrosion or white rust on metal components in low LSI (Langelier
Saturation Index) water systems and for inhibiting scale formation in high LSI
water systems.
2. Description of Related Art
[0003] Various water treatment compositions are used to reduce
corrosion, mineral scale, and white rust formation on metal components in
contact with an aqueous solution in water systems such as open recirculating
systems, closed loop cooling or heating systems, cooling towers and boilers,
and
help protect the metal components of these systems. The metals typically used
in these water systems include ferrous metals, including galvanized steel,
aluminum and its alloys, copper and its alloys, lead, and solder. Many known
corrosion inhibitors contain regulated toxic metals, such as zinc, chromate,
and
molybdate, which are harmful to the environment and increase the costs. Zinc
is
typically used as corrosion inhibitor in water systems with highly corrosive
water
(low LSI). However its usage is undesirable due to toxicity issues and its use
faces regulations in some locations. Tin has also been used as a non-toxic
alternative to zinc, but it is more expensive,
CA 03068248 2019-12-20
WO 2019/005429
PCT/US2018/035861
[00041 The performance of many known corrosion and scale inhibitors is
also negatively impacted by the use of biocides, which are frequently used in
water systems to control the growth of microorganisms. The use of polyaspartic
acid and a single phosphonic acid are disclosed in U.S, Patent No, 5,523,023
as
effective in inhibiting corrosion and scale, even in the presence of a biocide
when
the phosphonic acid is 2-phosphonobutane-1,2,4-tricarboxylic acid (PBTC). The
preferred phosphonic acid in the '023 patent is PBTC, but other phosphonic
acids, including 1-hydroxyethane 1,1-d isphosphonio acid and
hydroxyphosphonoacetic acid (H PA) are also mentioned as suitable. The
corrosion rate results shown in the '023 patent based on the use of
polyaspartic
acid and PBTC are better than other corrosion inhibitors, but there is still a
need
for even greater corrosion inhibition, particularly in the presence of
biocides. The
scale formation results shown in the '023 patent based on the use of
polyaspartic
acid and PBTC are approximately the same as the results obtained by using
PBTC alone, indicating no real improvement in scale inhibition is obtained
with
the two-component formula of the '023 patent.
[00051 Currently utilized solutions for white rust prevention include
passivating the metal surfaces with zinc carbonate and control of water
chemistry
to reduce potential for white rust formation. Known treatments include the use
of
inorganic phosphates, thiocarbamates, organo-phosphorous compounds and
tannins. For example, U.S. Patent Nos. 5,407,597 and 6,468,470 disclose
compositions comprising organaphosphorus compounds (including PBTC), an
alkali metal salt of molybdenum, titanium, tungsten, or vanadium, and either a
carbamate compound or a tannin compound. U.S.
Patent No, 6,183,649
discloses a white-rust treatment composition comprising PBTC, sodium
polyacrylate, sodium tolytriazole, an alkali metal molybdate, and an alkali
metal
bromide for treating circulating water systems. The '649 patent also discloses
the addition of a 1.5% aqueous solution of decyl thioethyletheramine (DTEA) at
a
rate of 251b/1,000 gallons of water/week to the circulating water system prior
to
adding the white rust treatment composition at a rate of 600 ppm per cycle for
ten
cycles of recirculation after addition of the DTEA.
2
CA 03068248 2019-12-20
WO 2019/005429
PCT/US2018/035861
[00061 There is a need for an effective all-in-one treatment composition
and method that can be used to inhibit corrosion, white rust, and scale in a
water
system without the need for separate treatments, which may negatively interact
with each other. There is also a need for an effective all-in-one treatment
that is
more environmentally friendly and capable of adequately performing in
conjunction with biocides
3
CA 03068248 2019-12-20
WO 2019/005429
PCT/US2018/035861
SUMMARY OF THE INVENTION
[0007] According to one preferred embodiment of the invention, an
improved corrosion inhibitor, white rust inhibitor, and scale inhibitor
composition
comprises an amino-acid based polymer (AAP), hydroxyphosphonoacetic acid
(HPA) or its water soluble salt, and another phosphonic acid or its water
soluble
salt. Hydroxyphosphonoacetic acid has the following general structure:
OH
HO _______________ P CH¨ 000H
0008] 0 OH
(0009] Most preferably, the amino-acid based polymer is polyaspartic
acid or its water soluble salt, but other compounds such as polyglycine acid,
polyglutamic acid and their salts may also be used. Most preferably, the amino
acid based polymer has the following formula:
0
I I
[ __________________ (CH )x-C-NHFIR2
[0010]
[0oll] where R1 = H, R2=0H, and R3=COOH and x=1 for polyaspartic
acid. Most preferably, the other phosphonic acid is a phosphonocarboxylic acid
or any organic phosphonate may also be used. Most
preferably, the
phosphonocarboxylic acid is 1-hydroxyethane-1,1-diphosphonic acid (HEDP) or
2-phosphonobutene-1,2,4-tricarboxylic acid (PBTC) or phosphonosuccinic acid.
Preferably the weight ratio of AAP to HPA in the inhibitor composition is
90:10 to
10:90 and the ratio of combined AAP and HPA to other phosphonic acid is in the
range of 90:10 to 6040. More preferably, the weight ratio range of AAP to HPA
in the inhibitor composition is 80:20 to 80:20 and the ratio of combined AAP
and
HPA to other phosphonic acid is 80:20 to 70:30.
4
CA 03068248 2019-12-20
WO 2019/005429
PCT/US2018/035861
[0012] Most preferably, a composition according to a preferred
embodiment of the invention is all organic and does not contain regulated
metals
such as zinc, chromate, and nnolybdate and its performance is not affected by
addition of biocides, Most preferably, a composition according to a preferred
embodiment of the invention does not contain tin,
(0013] It was previously known to use both HPA and AAP, such as
polyaspartic acid, separately as corrosion inhibitors, It was also disclosed
in the
'023 patent that AAP could be used together with phosphonocarboxylic acid to
inhibit corrosion and scale, but it was not previously known to use AAP and
HPA
together along with another phosphonic acid, preferably a phosphonocarboxylic
acid, or an organic phosphonate to inhibit corrosion or scale.
[00141 When added to the water in the water system being treated, a
preferred composition according to the invention for inhibiting corrosion
yields at
least 3 ppm active AAP, at least 3 ppm active HPA, and at least 2 ppm of the
other phosphonic acid. More preferably, when added to the water in the water
system being treated, a preferred composition yields 3 ppm-50 ppm AAP, 3 ppm-
50 ppm HPA, and 2 ppm-20 ppm of the other phosphonic acid and most
preferably between 5ppm-30ppm AAP, 3ppm-20ppm HPA, and 2 ppm-10 ppm of
the other phosphonic acid, Additionally, the combined total of the three
components of a preferred composition yields at least 8 ppm active corrosion
inhibitors when added to the water being treated. These ingredients have the
unexpected synergistic effect of improved corrosion inhibition in low LSI
water
systems (LSI <-0.5) without requiring the use of toxic metals and without
being
adversely impacted by biocides,
[00151 In addition to unexpected and synergistic effect of the inhibitor
composition on ferrous metal corrosion inhibition in low LSI water, the same
composition also has a positive effect on preventing formation of white rust
on
galvanized steel. Galvanized steel consists of a thin coating of zinc fused to
a
steel substrate. White rust is a rapid, localized corrosion attack on zinc
that
usually appears as a voluminous white deposit. This rapid corrosion can
completely remove zinc in a localized area with the resultant reduction in
CA 03068248 2019-12-20
WO 2019/005429
PCT/US2018/035861
equipment life. White rust formation tends to increase with increased
alkalinity
levels in the water. Neither hydroxyphosphonoacetic acid nor amino-acid based
polymers, such as polyaspartic acid, alone or in combination, has been
previously utilized in commercial products for white rust prevention. Without
being bound by theory, it is believed that the compositions according to the
invention may be forming a protective layer on the surface of galvanized steel
and reduce white rust formation. For treating white rust according to the
invention, it is preferred to use hydroxyphosphonoacetic acid, an amino-acid
based polymer, and another phosphonic acid in the amounts indicated above for
inhibiting corrosion (both weight ratios and concentrations when added to the
water in the water system being treated), but it has also been found that the
use
of an amino-acid based polymer without hydroxyphosphonoacetic or the other
phosphonic acid is beneficial at inhibiting white rust. According to another
preferred embodiment, a composition for treating white rust comprises an amino-
acid based polymer and hydroxyphosphonoacetic acid, without another
phosphonic acid. According to yet another preferred embodiment, a composition
for treating white rust comprises an amino-acid based polymer, without any
hydroxyphosphonoacetic acid. The preferred concentrations and ranges for
these components when added to the water being treated for white rust are the
same as for inhibiting corrosion,
(0016] In addition to unexpected and synergistic effect of the inhibitor
composition on white rust and on ferrous metal corrosion inhibition in low LSI
water, the same composition also has a positive effect on preventing formation
of
mineral scale in high LSI water (LSI >1). Mineral scale includes calcium and
magnesium carbonate, calcium phosphate, calcium sulfate, and silica.
Solubility
of calcium carbonate and phosphate decreases when temperature increases,
making calcium carbonate and calcium phosphate more of an issue in water
systems with higher temperatures, such as cooling towers. LSI is determined by
the following formula:
(00171 LSI = pH - pHs, where pHs is pH at CaCO3 saturation point.
6
CA 03068248 2019-12-20
WO 2019/005429
PCT/US2018/035861
[0018] An LSI > 0 indicates scaling, as scale can form and CaCO3
precipitation may occur. An LSI 5 0 indicates nonscaling, as there is no
potential
to scale and the water will dissolve CaCO3, As will be understood by those of
ordinary skill in the art, LSI is an indication of driving force and not
strict
quantitative indication of scale formation, which will depend on the water
characteristics, temperature, and water systems operations. However, without a
scale inhibitor, scale will typically precipitate out of water when the LSI is
greater
than 0.2, Using a treatment composition according to preferred embodiments of
the invention, no scale will form (calcium carbonate will not precipitate out
of the
water) at LSI values of 1-3.
[0019] When added to the water in the water system being treated, a
preferred composition according to the invention for inhibiting scale yields
at least
2 ppm active AAP, at least 2 ppm active HPA, and at least 1.5 ppm of the other
phosphonic acid. More preferably, when added to the water in the water system
being treated, a preferred composition yields 2 ppm-50 ppm AAP, 2 ppm-50 ppm
HPA, and 1.5 ppm-20 ppm of the other phosphonic acid and most preferably
between 3 ppm-30pprn AAP, 2 ppm-20 ppm HPA, and 1.5 ppm-10 ppm of the
other phosphonic acid. Additionally, the combined total of the three
components
of a preferred composition yields at least 6.5 ppm active scale inhibitors
when
added to the water being treated. These ingredients have the unexpected
synergistic effect of improved corrosion inhibition in high LSI water systems
(LSI
>1) without requiring the use of toxic metals and without being adversely
impacted by biocides.
[0020] Treatment compositions according to preferred embodiments of
the invention provide an all-in-one treatment that is able to inhibit
corrosion of
metals such as ferrous metals, aluminum and its alloys, copper and its alloys,
zinc and its alloys, galvanized steel (including white rust), lead, or solder,
and to
prevent mineral scale formation. The treatment compositions are particularly
useful in water systems such as open recirculating systems, closed loop
cooling
or heating systems, and boilers that may experience corrosion, white rust, and
scale formation during different times of the year or under different
operating
7
CA 03068248 2019-12-20
WO 2019/005429
PCT/US2018/035861
conditions, including use in both low LSI (high corrosively water) and high
LSI
(high scale tendency) waters,
[00211 According to other preferred embodiments, compositions for
inhibiting corrosion or white rust or scale also comprise one or more of the
following ingredients: a neutralizing amine, chlorine stabilizer, such as
monoethanol amine (MEA) a secondary scale inhibitor (since the composition
itself also works as a scale inhibitor) and dispersion agent, such as
polycarboxylate polymer and/or carboxylate/sulfonate functional copolymers
(typical examples: polyacryclic acid (PAA), polymetharrylic acid (PMAA),
polymaleic acid (PMA), and copolymers of acrylic acid and 2-acylamido
methylpropane sulfonic acid (AA/AMPS); other scale and corrosion inhibitors,
chelant agents; azole corrosion inhibitors, such as benzotriazole,
alkylbenzotriazole (tolyltriazole); and/or a fluorescent dye tracer, such as
1,3,6,8-
Pyrenetetrasulfonic acid tetrasodium salt (PTSA). The overall composition
preferably comprises around 2%-15% (by weight) of an amino-acid based
polymer (such as polyaspartic acid), around 2% to 10% (by weight) of
hydroxyphosphonoacetic acid, and around 2% to 10% (by weight) of another
phosphonic acid.
10022] According to one preferred method of preventing corrosion of
metal components, white rust on galvanized steel components, and/or scale in a
water system, a treatment composition according to the preferred embodiments
of invention as described above is added to the water system. For a
composition
combining one or more of the AAP, HPA, and another phosphonic acid as
described above, a preferred method for corrosion and white rust inhibition
comprises feeding the composition into the water at an effective feed rate of
20ppm - 600 ppm, or more preferably 100 ¨ 300ppm, of treatment composition,
depending on the treated water chemistry and the amount of optional
components in the treatment composition. Preferably, a sufficient amount of
treatment composition is added to the water system to provide effective active
amounts of one or more of the three treatment components (depending on
whether white rust is being treated or both corrosion and white rust) of at
least 3
8
CA 03068248 2019-12-20
WO 2019/005429
PCT/US2018/035861
ppm AAP, at least 3 ppm HPA, and at least 2 ppm of another phosphonic acid,
each as concentrations when added to the volume of water in the water system
being treated. More preferably, the treatment composition is added in a
sufficient
amount to provide effective active amounts one or more of the components of
between 3 ppm ¨ 50 ppm AAP, between 3pm ¨ 50 ppm HPA, and between 2
ppm - 20 ppm of another phosphonic acid when added to the water in the water
system. Most preferably, these effective active amounts are 5pprn ¨ 30 ppm
AAP, 3 ppm ¨ 20 ppm HPA, and 2 ppm - 10 ppm other phosphonic acid when
added to the water in the water system.
[0023] For a composition combining one or more of the AAP, HPA, and
another phosphonic acid as described above, a preferred method for scale
inhibition comprises feeding the composition into the water at an effective
feed
rate of 20ppm - 600 ppm, or more preferably 50 ¨ 300ppm, of treatment
composition, depending on the treated water chemistry and the amount of
optional components in the treatment composition. Preferably, a sufficient
amount of treatment composition is added to the water system to provide
effective active amounts of one or more of the three treatment components of
at
least 2 ppm AAP, at least 2 ppm HPA, and at least 1.5 ppm of another
phosphonic acid, each as concentrations when added to the volume of water in
the water system being treated. More preferably, the treatment composition is
added in a sufficient amount to provide effective active amounts of the three
treatment components of 2 ppm - 50 ppm AAP, 2 ppm - 50 ppm HPA, and 1.5
ppm -20 ppm of another phosphonic acid, each as concentrations when added to
the volume of water in the water system being treated. Most preferably, the
treatment composition is added in a sufficient amount to provide effective
active
amounts of the three components of between 3 ppm ¨ 30 ppm AAP, between
2pm ¨20 ppm HPA, and between 1.5 ppm - 10 ppm of another phosphonic acid
when added to the water in the water system.
9
CA 03068248 2019-12-20
WO 2019/005429
PCT/US2018/035861
BRIEF DESCRIPTION OF THE DRAWINGS
[0024] The composition and method of the invention are further described
and explained in relation to the following figures wherein:
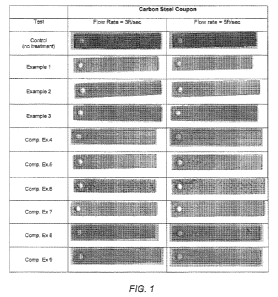
FIG. 1 contains photographs showing corrosion levels on steel coupons
after spinner tests at flow rates of 3ft/sec and 5ft/sec;
FIG. 2 contains photographs showing corrosion levels on steel coupons
after spinner tests run in presence of biocide at flow rates of 3ft/sec and 5
ft/sec;
FIG. 3 contains photographs showing corrosion levels on steel coupons
after spinner tests at a flow rate of 3ft/sec; and
FIG. 4 contains photographs showing white rust levels on galvanized
coupons after spinner tests.
CA 03068248 2019-12-20
WO 2019/005429
PCT/US2018/035861
DESCRIPTION OF PREFERRED EMBODIMENTS
(0025] Several lab tests were run to test the effectiveness of various
compositions according to the invention.
Compositions according to the
invention were evaluated using spinner tests to simulate flowing water over
metal
components in a water system. Each spinner test set-up comprises a stainless
steel container of water with four metal coupons (mild steel coupons (C1010)
and
copper coupons (CDA 11) were used) suspended in the water in each container
from holders hanging from a rotating shaft. The shaft rotates the coupons in
the
water in the stainless steel container at 147 rotations/min, representing a
flow
rate of 3-5 Ws, depending on coupon distance from center of the rotating
shaft.
The initial volume of water used in each spinner test was characteristic of
corrosive, low hardness water typically found in water systems. The water used
had the characteristics shown in Table 1 below.
[0026] Table 1. Low hardness, corrosive water used in Spinner test
experiments
Characteristic Value Unit
pH 8 to 8.5
Conductivity 220 cP
Ca Hardness 30 ppm, (as CaCO3)
Mg Hardness 10 ppm, (as CaCO3)
Chlorides, Total 25 ppm Cl
M Alkalinity 30 ppm, (as CaCO3)
Sulfate, Total 28 ppm, as SO4
[0027] During each spinner test the water is aerated and maintained at
constant temperature of 120F and constant volume (any evaporation is
compensated with automatic addition of deionized water when water level drops
below sensor level). Standard test duration is 48 hours.
CA 03068248 2019-12-20
WO 2019/005429
PCT/US2018/035861
[0028] Using the spinner test set-up, compositions according to preferred
embodiments of the invention (Example Nos. 1-3 including AAP, HPA, and
another phosphonic acid - HEDP) without any added zinc or tin (as shown in
Table 2) were compared to compositions using only zinc (Comp. Ex. 4), only tin
(Comp, Ex. 5), only AAP (Comp. Ex. 6), only HPA (Comp. Ex. 7), HPA combined
with tin (Comp. Ex. 8), and AAP combined with tin (Comp. Ex. 9) (all as shown
in
Table 3) as the primary inhibitor(s). The ppm concentrations of the various
treatments are concentrations when added to the volume of water in the spinner
test container. The compositions with zinc or tin were for comparison to those
without. Zinc is typically used as corrosion inhibitor in water systems with
highly
corrosive water (low LSI). However its usage is undesirable due to toxicity
issues and its use face regulations in some locations. Tin has been promoted
and patented as a non-toxic alternative to zinc, but it is more expensive. In
addition to the primary corrosion inhibitor components listed in Tables 2 and
3, all
of the tests were carried out in the presence 4 ppm active AA/AMPS copolymer
and 4 ppm active TTA. These ingredients were added to the water in each
spinner test set-up to provide those concentration levels, The corrosion and
pitting level for mild steel coupons after spinner tests in presence of
different
inhibitors are presented in Figure 1.
12
CA 03068248 2019-12-20
WO 2019/005429
PCT/US2018/035861
(0029] Table 2. Corrosion inhibitor compositions according to the
invention
Inhibitor Unit
Example 1 Example 2 Example 3
AAP (amino acid based ppm T5 5.2 5,2
polymer ¨ such as a active*
commercially available
water solution containing
about 40% of salt)
HPA ppm 2.5 5.0 5.0
(hydroxyphosphonoacetic active
acid)
HEDP ppm 3 3 3
active
MEA ppm 0.25 1.0
Zn (zinc) ppm N/A N/A N/A
active
Sn (tin) pprn N/A N/a N/A
active
*ppm active refers to the amount of active raw material, in contrast to ppm
which refers to the weight of raw material in mg/L. For example, HPA is
commercially available as a 50% water solution, so adding 10 ppm raw
material will provide 5 ppm active HPA.
13
CA 03068248 2019-12-20
WO 2019/005429
PCT/US2018/035861
[0030] Table 3. Corrosion inhibitor compositions ¨ Comparative
Examples
Inhibitor Unit Comp. Comp. Comp. Comp I
Comp. Comp.
Ex 4 Ex 5 Ex 6 Ex 7 Ex 8 Ex 9
AAP ppm active 15 7.5
HPA ppm active 5 5
HEDP ppm active 3 3 3 3 3 3
MEA ppm
Zn ppm active
Sn ppm active 1 1 0.5
[0031] Spinner tests were run with each composition at a flow rate
equivalent to around 3ft/second and at a flow rate equivalent to around
5ft/second. A control test, without any treatment was also carried out for
comparison. FIG. 1 shows photographs of a representative mild steel coupon
after each spinner test with the control and with Example Composition Nos. 1-
9.
The amount of corrosion and pitting on the coupons is shown in the
photographs.
As can be seen, the control coupons show extensive corrosion (dark areas on
photographs). The coupons used with compositions according to preferred
embodiments of the invention (Ex. Nos. 2-3) show little, if any, corrosion or
pitting
(very few dark areas on photographs). The coupons used with Ex. No. 1, which
contains all three components according to a preferred embodiment of the
invention for corrosion inhibition, but only contains 2.5 ppm HPA (less than
the
more preferred amount of at least 3 ppm), shows improved results over the
control and the comparative examples (Comp. Nos. 4-9), but shows slightly more
corrosion than Ex. Nos. 2-3, where 5 ppm of HPA was used. The coupons used
with the comparative compositions (Comp. Nos. 4-9) are significantly better
than
14
CA 03068248 2019-12-20
WO 2019/005429
PCT/US2018/035861
the control, but do show evidence of corrosion and pitting that is greater
than with
Ex. Nos. 1-3, Based on the results, it appears that the combination of AAP,
HPA,
and another phosphonic acid (in these examples, HEDP) interact synergistically
to provide improved corrosion control, without requiring the use of zinc, tin
or
other regulated metals.
[00321 Some prior art water treatment corrosion inhibition compositions
do not provide effective protection when oxidizing biocides are used in the
same
system to prevent biological growth. The most widely used oxidizing biocides
are
chlorine and stabilized bromine. Additional spinner corrosion tests were
carried
out using Example compositions Nos, 2 and 3 compared to comparative
Example compositions Nos. 4 (zinc only) and 7 (HPA only) in the presence of a
stabilized bromine biocide composition (commercially available as Chem-Aqua
42171). Example compositions 4 and 7 were selected because they showed the
best results in the spinner tests of the comparative examples, Both Comp. Ex..
Nos. 4 and 7 perform fairly well in low LSI water, but as discussed below,
significantly worse when biocide is added. Also, Comp. Ex, No. 4 is based on
zinc, which is undesirable to use due to toxicity concerns. As with the prior
tests,
these tests were carried out in presence 4 ppm active ANAMPS copolymer and
4 ppm active TTA. A slug dose of 4Oppm of biocide was added at the beginning
of each spinner test (after the corrosion inhibition composition was added and
the
test started) to yield about 1ppm FHR (free halogen residue),
0033) FIG. 2 shows photographs of a representative mild steel coupon
after each spinner test with the Example Compositions in the presence of
biocide. As can be seen, the coupons used with compositions according to
preferred embodiments of the invention (Ex. Nos. 2-3) show little, if any,
corrosion or pitting, indicating that the functionality of preferred
compositions
according to the invention is not negatively affected by a biocide, The
coupons
used with the comparative compositions (Comp, Ex. Nos. 4 and 7) show
substantially more corrosion than with Ex. Nos. 2-3, It is noted that Comp.
No, 7
was the use of HPA and HEDP, without any AAP, which showed good results
without biocide, but significantly more corrosion occurred when a biocide was
CA 03068248 2019-12-20
WO 2019/005429
PCT/US2018/035861
added. The comparative composition having AAP and HEDP, without any HPA,
(Comp. Ex. No. 6) did so poorly without biocide (Fig. I above) that it was not
tested with biocide because the results would be expected to be even worse
than
in FIG. I. Based on the results, it appears that the combination of MP, HPA,
and another phosphonic acid together interact synergistically to provide
improved
corrosion control even in the presence of a biocide and show improved results
over the use of HPA alone.
[0034] Corrosion rates for the mild steel coupons were also measured
and calculated from weight loss of the coupons. The results of both the
spinner
tests without added biocide and with added biocide are summarized in Table 4.
Information on corrosion mode, particularly the presence of pitting (which is
important in many applications and some corrosion inhibitors, including HPA
used alone, are known to be poor protectors against pitting), is also included
in
Table 4. Most preferably, corrosion inhibitor compositions according to the
embodiments of the invention achieve corrosion rates of 3 MPY or less for
corrosion, even in the presence of a biocide.
16
CA 03068248 2019-12-20
WO 2019/005429 PCT/US2018/035861
[0035] Table 4. Corrosion Rates form spinner test experiments
Mild Steel Coupon Corrosion Rate, MPY [mil/yr]
Low Hardness Water +
Low Hardness Water
Biocide
Corrosion
Test 3ft/sec 5ftisec Pitting 3ft/sec 5ftisec Pitting
Scale
Control 370 243 N/A
Example 1 2.7 2.5 None
Example 2 2.9 2.4 None 2.2 2.0 1 None
Example 3 2.5 2.5 None 2,7 2.4 None
Sever
Comp, Ex 4 2.7 2.7 Limited 8.0 11
pitting
Comp. Ex 5 4.0 4.6 Pitting
Severe
Comp. Ex 6 13,6 8.2
pitting
Severe
Comp. Ex 7 2.6 3.2 Limited 6.4 5.7
pitting
Comp. Ex 8 3.9 5,2 Pitting
Sever
Comp, Ex 9 3.8 3,2
pitting
Pitting scale description:
None = no pitting observed
Limited = few (1-5) pitts per coupon, usually very shallow
Pitting = significant number of pits on coupons (5-50)
Sever pitting = a large number of pits (> 50), usually dipper and larger
[0036] Compositions according to preferred embodiments of the invention
contain organic phosphate from the HPA and from the other phosphonic acid
used in these examples (HEDP). In the presence of a biocide, the organic
17
CA 03068248 2019-12-20
WO 2019/005429
PCT/US2018/035861
phosphate is often reverted to orthophosphate, which is not as good in
preventing corrosion or scale and also may cause issues with forming calcium
phosphate scale. When the combination of AAP,HPA, and HEDP (or another
phosphonic acid) is used as a corrosion inhibitor according to a preferred
embodiment of the invention, virtually no reversion of organic phosphate to
orthophosphate was detected. Samples from composition Example Nos. 2 and
3 and comparative Example No, 7 were tested for the presence of
orthophosphates upon mixing of the composition and again after 48 hours, The
results are listed below in Table 5. Example Nos. 2 and 3, which use AAP,
HPA, and HEDP (and contain AA/AMPS and 1 ______________________________ IA as
noted above), showed very
little orthophosphate increase over the 48 hour period, but comparative
Example
No. 7 which contains HPA and HEDP (and contains AA/AMPS and TTA as
noted above), but no AAP, showed a substantial increase.
[0037] Table 5. Orthophosphate levels in low hardness test water in
presence of biocide during the spinner corrosion test
Orthophosphate (ppm PO4)
Test Initial 48hr (End of Test) I
Example 2 0.4 0.5
Example 3 0.2 0.4
Comp. Ex -7 0.3 1.6
. , __
[0038] According to another preferred embodiment, a water treatment
composition as listed in Table 6 (which is the same as Ex. 2 tested above) is
effective at inhibiting corrosion and scale in a water system over a broad
range of
LSI values (-2.5 to >3) and in the presence of a biocide.
18
CA 03068248 2019-12-20
WO 2019/005429
PCT/US2018/035861
[0039] Table 6.
Active %* in
Component Wt %
Composition
Sodium polyasparte (AAP) 13.0 5.2% as
AAP
Hydroxy phosphonoacetic Acid (HPA) 10.0 5.0% as
HPA
1-Hydroxyethylidene 1,1-
diphosphonic acid (HEDP)
1,2-3.0% as
or 5.25-6.4
PO4
2-phosphonobutane-1,2,4,tricarboxylic acid
(PBTC)
Monoethanolamine (MEA) (optional) 1.0 0.99%
Copolymer of acrylic acid and sulfonated as
8.78
monomer (AA/AMPS) AA/AMPS
Tolyltriazole (TTA) 9,40 4,0% as
TTA
1,3,6,8-Pyrenetetrasulfonic acid tetrasodium salt
1.00 1% as PTSA
(PTSA)
15.00-
NaOH or KOH N/A
16.25
35.17-
Deionized water N/A
36.57
*Active % refers to active weight percent. Wt% is raw material weight percent.
Most of the raw materials are aqueous solutions and contain only a certain
amount of solids that is the actual chemical component. The amount of active
(Active %) is calculated based on raw material weight percent and the amount
of
the chemical in the solution per the information provided by the supplier. For
example, a commercially available source of AAP may be a 40% solution of AAP
in water, so if 13% of that product is used, the active amount of AAP equals:
0.13*0.40*100% 5.2% of AAP (active) in the formula
19
CA 03068248 2019-12-20
WO 2019/005429
PCT/US2018/035861
[0040] NaOH and/or KOH is preferably also added to the composition
according to an embodiment of the invention. These ingredients are typically
added to water treatment formulations in order to neutralize acid and to bring
the
pH of the final composition to the desired level, Most of the compositions
will
have pH > 8, some will have pH > 12. In compositions where TTA is used (as
with a preferred embodiment of a composition according to the invention) it is
desirable to have higher pH (> 11) for the composition in order to ensure
solubility of TTA, which has very poor solubility at lower pH.
[0041] Additional spinner tests in low LSI water were carried out in order
to test the effectiveness of various concentrations of treatment compositions
for
inhibiting corrosion according to preferred embodiments of the invention. The
same spinner test parameters and low LSI water (Table 1) described above were
used for these tests. The concentrations of the ingredients when added to the
spinner test water and the results of these tests are shown below in Table 7,
Figure 3 shows photographs of the test coupons (tested at a flow rate of 3
ft/sec)
for each composition after the test was completed.
0042] Table 7 ¨ Additional Spinner Test Compositions & Results
Comp. Comp. Comp, Comp.
inhibitor Unit Ex, 11 Ex, 12 Ex, 14 Ex, 16
Ex. 10 Ex. 13 Ex. 15 Ex. 17
ppm
AAP 2.6 5.2 7.8 5.0 10 10 5.0 6,0
active
ppm
HPA 2.6 5.0 7.5 2.5 5 2.5 5.0 6,0
active
MP: H PA
51:49 51:49 51:49 67:33 67:33 80:20 51:49 51;49
Ratio
1.6 4.7
3.26 3.26 326 3.26
ppm (1.5 ' (4.4
HEDP (3 ppm (3 ppm(3 ppm (3 ppm
active ppm ppm
PO4) PO4) PO4) PO4)
PO4) PO4)
ppm 2.6 I
PBTC
active (0.95
CA 03068248 2019-12-20
WO 2019/005429 PCT/US2018/035861
Comp. Comp. Comp. Comp.
Inhibitor Unit Ex. 11 Ex. 12 Ex. 14 Ex 16
Ex. 10 Ex. 13 Ex. 15 Ex 17
PPm
PO4)
MEA PPm 0.5 1 0.5
TTA ppm TTA 4 4 4 4 4 444
ANA M PS ppm
4 4 4 4 4 4 4 4
Copolymer active
Corrosion Results from Spinner Test (low LSI water), mild steel (C1010)
coupons at
3ft/sec flow rate
Corrosion MPY
5.2 2.3 1.5 3.1 2.2 3.5 2.1 3.3
Rate* (n-iil/yr)
Pitting Pitting none none none none none none None
* Average for 2 coupons from the same spinner test pot at 3 ft/sec
0043] Comparative Examples 10, 13, and 15 use AAP, HPA, and HEDP
but in amounts less than the preferred concentrations. These examples show
increased corrosion (and Comp. Ex. 10 showed moderate pitting) at low levels
of
the inhibitors. Example Nos, 11-12, 14, and 16 according to preferred
embodiments of the invention show good performance (low corrosion rate and no
pitting) for different optional components and varying concentrations and
ratios of
AAP to HPA. The examples also show that the change from HEDP to PBTC (Ex,
16) and reduction of secondary chelates does not affect the corrosion
inhibition
performance of compositions according to preferred embodiments of the
invention. Example No. 17 used AAP and HPA, without a second phosphonic
acid, similar to the composition described in the '023 patent. It shows
improved
results in controlling corrosion in low LSI water, but the results are not as
good as
in the examples according to preferred embodiments of the invention,
(0044] Additional spinner tests were conducted to compare compositions
using AAP and PBTC as disclosed in the '023 patent with compositions
according to preferred embodiments of the invention. The test set-up was the
21
CA 03068248 2019-12-20
WO 2019/005429
PCT/US2018/035861
same as described above using low LSI water, mild steel (C1010) coupons, and
a flow rate of 3 ft/sec. The results are shown in Table 8 below.
[0045] Table 8 ¨ Comparing Compositons Using One Phosphonic Acid to
Compositions Using Two Phosphonic Acids
Comp, Comp.
Example 12
Example 18 Example 19
Inhibitor Unit Example 20 Example 21 (same
as in
80:20 40:60
Table 7)
PBTC/AAP PBTC/AAP
PPm
PETC 16 8 4.8 8
active
___________ --------------------
Pprii
HEDP 4,7
active
PPm
AAP 4 12 7,8 4 7,8
active
PPm
HPA 7.5 8 7.5
active
PPm
TTA 4 4 4 4 4
TTA
AA/AM PS ppm
4 4 4 4 4
Copolymer active
Corrosion MPY
3.1 3.1 1.9 1.7 1.5
Rate* (mil/yr)
Pitting none none none none None
*Average for 2 coupons from the same pot at 3 ft/sec
[0046] As can be seen, the examples according to preferred
embodiments of the invention (Example Nos. 20, 21, and 12) with AAP, HPA,
and a second phosphonic acid (HEDP or PBTC) show much beter corrosion
inhibition results than the comparatve examples using only AAP and PBTC
(without any HPA). It is also noted that Comp. Ex. Nos, 18-19 resulted in
corrosion rates greater than 3 MPY even when using 20 ppm total inhibitor (AAP
and PBTC), which is higher than the corrosion rate achievable with preferred
CA 03068248 2019-12-20
WO 2019/005429
PCT/US2018/035861
compositions according to the invention using substantially less total
inhibitor,
such as Example No. 11, which had a corrosion rate of 2.3 MPY using only 13.5
ppm total inhibitors (AAP, HPA, HEDP), and Example No, 16, which had a
corrosion rate of 2,1 MPY using only 12.6 ppm total inhibitors (AAP, HPA,
PBTC). Additionally, the corrosion rates of Comp, Ex, Nos. 18-19 are
comparable to those in Comp. Ex, Nos, 13 and 15, which use AAP, HPA, and a
second phosphonic acid, but the total amount of inhibitor needed to achieve
the
results in Comp, Ex. Nos, 18-19 (20 ppm total) is much higher than that needed
in Nos. 13 and 15 (10,76 and 15,76 ppm total, respectively). The results of
these
experiments show that the addition of a second phosphonic acid, in combination
with AAP and HPA, provides an unexpected synergistic effect that improves
corrosion inhibition even when less total inhibitor is used and even in the
presence of a biocide.
[0047] Those of ordinary skill in the art will understand that other sutiable
or equivalent chemical compounds and other treatment compounds, including
other corrosion inhibitors, may be substituted for any of the above
ingredients or
added to any of the above ingredients within the scope of this invention.
Compositions according to the embodiments of the invention are effective in
inhibiting corrosion on metal components in water systems over a broad range
of
LSi values, including LSI <0, and without requiring the use of regulated toxic
metals. These compositions are also effective at higher pH values (7-9)
typically
found in water systems, such as cooling towers and boilers, whereas some prior
art inhibitors are ineffective or their effectiveness is reduced at such pH
levels
(for example, a polyaspartic acid/stannous salt treatment is effective only at
pH
5-7). These compositions according to the invention also prevent reversion of
organic phosphate to orthophosphate to maintain effectiveness in the presence
of a biocide.
[0048] Other experiments using an electrochemical method were
conducted to test compositions according to the invention for white rust
prevention. The results in Table 9 below show synergistic effect of combining
HPA and AAP (without another phosphonic acid) in reducing white rust formation
23
CA 03068248 2019-12-20
WO 2019/005429
PCT/US2018/035861
as compared to use of each individual component (HPA alone and PAP alone).
The cyclic voltammetry test was conducted in 0.1M sodium carbonate solution
using zinc electrode. The measure of oxidation is the area under the oxidation
curve peak observed; the lower the area the less oxidation occurs, meaning
lower corrosion rate. The results are the averages of 6-10 experiments with
standard deviation.
[0049] Table 9
Inhibitor Concentration Measure of Oxidation
[ppm active] [Coulombel 0-3]
AAP 50 1.2 0.2
HPA 50 1.0 O.1
AAP/HPA (1:1 ratio) 25 : 25 0,8 0.1
[0050] Additional spinner corrosion tests were carried out in stainless
steel containers in high alkalinity water known to form white rust on
galvanized
surfaces to test the effectiveness of compositions according to preferred
embodiments of the invention for the prevention of white rust formation. The
water chemistry, characteristic of high alkalinity synthetic water, in these
tests is
detailed in Table 10 below. Four Hot Dip Galvanized steel coupons (HDG G70)
with dimensions 1.0x4,0x0.02in were installed in each container on the holders
hanging from a rotating shaft that rotates at 147 rotations/min that
represents
flow rate of 3-5 ft/s, depending on coupon distance from center of the
rotating
shaft, During the tests the water was aerated and maintained at constant
temperature of 120F and constant volume (any evaporation was compensated
with automatic addition of DI water when the water level dropped below a
sensor
level). Standard test duration was 48 hours. The active ingredients used in
two
comparative examples and three examples of preferred compositions according
to the invention, along with corrosion rates, are listed in Table 11.
[0051] Table 10- High alkalinity/no hardness water used in Spinner test
experiments for white rust prevention
24
CA 03068248 2019-12-20
WO 2019/005429
PCT/US2018/035861
Characteristic Value Unit
pH 8.7-8.9
Conductivity 2300 cP
Ca Hardness 0 ppm, (as CaCO3)
Mg Hardness 0 ppm, (as CaCO3)
Chlorides, Total 250 ppm Cl
M Alkalinity 200 ppm, (as CaCO3)
Sulfate, Total 500 ppm, as SO4
[0052] Table 11 - Active Ingredients Composition and Galvanized
Coupon Corrosion Rate
Inhibitor Unit Comp. Ex, Comp, Ex, 24 Ex, 25 Ex. 26
22 No Ex. 23
Inhibitor
AAP ppm 15 7,5 15
active
HPA ppm 7.5 7.5 2.5
active
HEDP ppm 3,26 3.26 3.26 3.26
active (3 ppm (3 ppm
(3 ppm (3 ppm
PO4) PO4) PO4) PO4)
TTA ppm TTA 4 4 4 4
AA/AMPS ppm 4 4 4 4
Copoiym active
er
Corrosion Results- Galvanized Coupons (HDG G70)
Corrosion MPY
53.7 24.3 9.9 14.0 10,7
Rate* (mil/yr)
*Average for 4 coupons from the same pot (two at 3 ft/sec and two at 5 ft/sec
flow rate)
CA 03068248 2019-12-20
WO 2019/005429
PCT/US2018/035861
[0053] In order to calculate the corrosion rate using the weight loss
method, the galvanized coupons from these tests were cleaned according to
standard procedure by immersing coupons in concentrated ammonium acetate
and rinsing. FIG. 4 shows photographs of the galvanized coupons after the
spinner tests with the compositions described in Table 12, both before and
after
cleaning. The white deposit visible on the coupons before cleaning is white
rust.
The damage of the galvanized layer due to corrosion, shown as dark spots, is
visible on the coupons after cleaning. The blank (Comp. Ex. 22 ¨ No Treatment)
coupon was completely covered in white deposit and after cleaning most of the
galvanized layer was removed with visible mild steel corrosion. The coupon
treated with HPA and HEDP without an amino-acid based polymer (Comp. Ex.
23) showed substantial white rust formation, but was still a great improvement
over the control (Comp. Ex. 22). Significantly better results were obtained
with
compositions in Examples 24-26. The best results were achieved with Ex. 24
using AAP, HPA at greater than 3 ppm, and a second phosphonic acid (HEDP).
Although the use of HPA is important in inhibiting mild steel corrosion, its
use is
optional for white rust treatment. As can be seen from Example 26, the results
of
using AAP and HEDP without HPA were almost as good as the three combined.
Accordingly, a preferred composition for treating white rust according to the
invention comprises 2-15% amino-acid based polymer, 0-10% HPA, and 0-10%
of a second phosphonic acid. Preferably, the amount of active amino-acid based
polymer in a treatment composition according to the invention is at least
3ppm,
more preferably 3 ppm ¨ 50 ppm, and most preferably 5 ppm ¨ 30 ppm, all as
concentrations when added to the volume of water in the water system being
treated. More preferably, the AAP is used in conjunction with HPA in an amount
of at least 3 ppm, more preferably from 3 ppm - 50 ppm, and most preferably
from about 3 ppm - 20 ppm and/or another phosphonic acid in an amount of at
least 2 ppm more preferably from 2 ppm- 20 ppm, and most preferably from
about 2 ppm - 10 ppm.
[0054] For treating white rust according to the invention, it is preferred to
use both hydroxyphosphonoacetic acid and an amino-acid based polymer, and
26
CA 03068248 2019-12-20
WO 2019/005429
PCT/US2018/035861
more preferably in conjunction with a second phosphortic acid, in the weight
range amounts indicated above, but it has also been found that the use of an
amino-acid based polymer or hydroxyphosphonoacetic without the other is
beneficial at inhibiting white rust.
(0055] A pilot cooling tower scale test using a composition according to a
preferred embodiment of the invention was also conducted to test the ability
to
inhibit scale formation in high LSI water (LSI >1). The objective of the
cooling
tower scale test was to determine the number of cycles at which the tower can
operate without scaling and the LSI limit of treatment in typical water with
scaling
characteristics as it cycles up. The cooling tower pilot test used 4 heat
transfer
surface rods and a DATS (Deposit Accumulation Testing System) operating at
800 Watts. The number of cycles of concentration (COC) is calculated as the
ratio of concentration of any ions in the cooling tower water to the
concentration
of the same ion in makeup (starting) water. Conductivity ratio can also be
used
to calculate COC. It is desirable to operate at as high COC as possible to
reduce
water usage. Typically, the COC in a cooling tower is maintained at a certain
level by measuring water conductivity, bleeding the system when conductivity
increases over a set limit and adding more makeup water. The initial volume of
water used in the cooling tower pilot test was characteristic of high LSI
water
having 100 ppm alkalinity as CaCO3 and 100 ppm calcium hardness as CaCO3
typically found in cooling tower water systems. The water used had the
characteristics shown in Table 12 below.
[0056] Table 12. High LSI water used in Pilot Cooling Tower Scale Test
Characteristic I Value Unit
pH 8
Conductivity 450-520 i_tS
Ca Hardness 100 ppm, (as CaCO3)
Mg Hardness 30 ppm, (as CaCO3)
Chlorides, Total 71 ppm Cl
Total Alkalinity 100 ppm, (as CaCO3)
CA 03068248 2019-12-20
WO 2019/005429
PCT/US2018/035861
Total Hardness 130 ppm, (as CaCO3)
Sulfate, Total 30 ppm, as SO4
LSI at 60 C 1.1
[0057] Scale is indicated when the HTR (Heat Transfer Resistance)
suddenly increases above stable level and exceed 12x10'6 Cm2AN and/or
heater % clean drops below 97% (as determined from the Heater Transfer
coefficient fouled (UF) and clean (UC) values, where UF = 1/HTR Scaled+ UC
and % Cleanliness = UF/UC x 100). The LSI limit (the LSI measurement at
which scale will form) can also be determined by monitoring changes in water
chemistry, water turbidity and visually by observing scale formation. A
composition according to Table 6 at a concentration of 100 ppm (when added to
the water in the pilot cooling tower system) was found to increase the
operational
limit of cooling tower to 6 COC and LSI of 3.2 based on HTR and water
chemistry
data. The pilot cooling tower was operated for 7 days before scale began
forming. The test was started with high scaling water, LSI around 1, and was
cycled up to 6 COC, which increased LSI to 3.2 before scale began to form.
[0058] For comparison, a typical prior art scale treatment, such as Chem-
Aqua 31155 (which contains PBTC, sodium tolytriazole, sodium polyacrylate,
polymaleic acid (sodium salt) and sodium hydroxide), at the same 100 ppm
concentration allows to operate cooling tower only 3 COC that is at LSI limit
of
only 2.6. Even at double the treatment concentration (200 ppm) of Chem-Aqua
31155, the COC in cooling tower can only be increased to 3A, with LSI limit of
2.85, which is well under the COC increase and LSI limit achieved using a
preferred embodiment of the composition of the invention. In another
experiment
using the same treatment composition used in the previous pilot cooling tower
scale test at 50 ppm (when added to the water in the pilot cooling tower
system),
the system reached 4.3 COC and LSI of 2.84 before scale began to form. These
results further indicate that this three component formula is far better at
scale
prevention that prior art formulas containing PBTC, even when the prior art
formulas are used at 2 to 4 times the concentration. With treatment
compositions
28
CA 03068248 2019-12-20
WO 2019/005429
PCT/US2018/035861
according to the invention, water in the water system (such as a cooling
tower)
may be cycled/recirculated more times before scale formation begins compared
to prior art treatments. This provides substantial savings on water, since
there
will be less blovv-down and less make-up water added to the water system.
(0059] According to one preferred method of preventing corrosion of
metal components and/or white rust on galvanized steel components and/or
mineral scale formation in a water system, a treatment composition according
to
the invention as described above is added to the water system at an effective
feed rate. For a composition combining one or more of the AAP, HPA, and
another phosphonic acid as described above, a preferred method for corrosion
and white rust inhibition comprises feeding the composition into the water at
an
effective feed rate of 20pprn - 600 ppm, or more preferably 100 ¨ 300pprn, of
treatment composition, depending on the treated water chemistry and the
amount of optional components in the treatment composition, Preferably, a
sufficient amount of treatment composition is added to the water system to
provide effective active amounts of one or more of the three treatment
components (depending on whether white rust is being treated or both corrosion
and white) rust of at least 3 ppm AAP, at least 3 ppm HPA, and at least 2 ppm
of
another phosphonic acid, each as concentrations when added to the volume of
water in the water system being treated. More preferably, the treatment
composition is added in a sufficient amount to provide effective active
amounts
one or more of the components of between 3 ppm ¨ 50 ppm AAP, between 3prn
50 ppm HPA, and between 2 ppm - 20 ppm of another phosphonic acid when
added to the water in the water system. Most preferably, these effective
active
amounts are 5ppm ¨ 30 ppm AAP, 3 ppm ¨ 20 ppm HPA, and 2 ppm - 10 ppm
other phosphonic acid when added to the water in the water system. For
treating
white rust, the use of HPA is optional, so the treatment composition used in a
preferred method according to the invention may comprise AAP without any HPA
and be added in amounts sufficient to provide these same concentration ranges
of AAP in the water of the water system being treated.
29
CA 03068248 2019-12-20
WO 2019/005429
PCT/US2018/035861
(0060] For a composition combining one or more of the MP, HPA, and
another phosphonic acid as described above, a preferred method for scale
inhibition comprises feeding the composition into the water at an effective
feed
rate of 20ppm - 600 ppm, or more preferably 50 ¨ 300ppm, of treatment
composition, depending on the treated water chemistry and the amount of
optional components in the treatment composition. Preferably, a sufficient
amount of treatment composition is added to the water system to provide
effective active amounts of one or more of the three treatment components of
at
least 2 ppm AAP, at least 2 ppm HPA, and at least 1,5 ppm of another
phosphonic acid, each as concentrations when added to the volume of water in
the water system being treated. More preferably, the treatment composition is
added in a sufficient amount to provide effective active amounts of the three
treatment components of 2 ppm - 50 ppm AAP, 2 ppm - 50 ppm HPA, and 1.5
ppm -20 ppm of another phosphonic acid, each as concentrations when added to
the volume of water in the water system being treated. Most preferably, the
treatment composition is added in a sufficient amount to provide effective
active
amounts of the three components of between 3 ppm ¨ 30 ppm AAP, between
2pm ¨ 20 ppm HPA, and between 1.5 ppm - 10 ppm of another phosphonic acid
when added to the water in the water system
[0061] According to another preferred embodiment, the composition
added to the water system (for treating corrosion, white rust, and/or scale)
comprises a fluorescent tracer so that the level of composition in the water
system can be measured and monitored, Additional treatment composition is
added to the water system as needed, based on the tracer measurements, to
maintain an effective amount of treatment within the water system.
[0062] All ppm concentrations of the various treatments in the example
tests described herein are concentrations when added to the water in the
spinner
test, to correlate to the concentrations when added to the water in the water
system being treated. Unless specifically excluded, all references to acids
herein
and in the claims include water soluble salts of the acid, as will be
understood by
those of ordinary skill in the art. Those of ordinary skill in the art will
also
CA 03068248 2019-12-20
WO 2019/005429
PCT/US2018/035861
appreciate upon reading this specification, including the examples contained
herein, that modifications and alterations to the preferred embodiments of the
composition and method for using the composition to treat water may be made
within the scope of the invention and it is intended that the scope of the
invention
disclosed herein be limited only by the broadest interpretation of the
appended
claims to which the inventor is legally entitled.
3 I