Note: Descriptions are shown in the official language in which they were submitted.
CA 03070108 2020-01-15
WO 2019/018807
PCT/US2018/043158
ADJUSTABLE FLOW GLAUCOMA SHUNTS AND
METHODS FOR MAKING AND USING SAME
CROSS-REFERENCE TO RELATED APPLICATION(S)
[0001] This application claims priority to U.S. Provisional Patent
Application
Nos. 62/643,125, filed March 14, 2018; 62/626,615, filed February 5, 2018, and
62/535,125,
filed July 20, 2017, the contents of which are incorporated herein by
reference in their entireties.
TECHNICAL FIELD
[0002] The present technology relates to adjustable flow glaucoma shunts
and methods for
making and using such devices.
BACKGROUND
[0003] Glaucoma, ocular hypertension, is a disease associated with an
increase in pressure
within the eye resultant from an increase in production of aqueous humor
(aqueous) within the
eye and/or a decrease in the rate of outflow of aqueous from within the eye
into the blood stream.
Aqueous is produced in the ciliary body at the boundary of the posterior and
anterior chambers
of the eye. It flows into the anterior chamber and eventually into the
capillary bed in the sclera
of the eye. Glaucoma typically results from a failure in mechanisms that
transport aqueous out
of the eye and into the blood stream.
[0004] Normal aqueous production, for example, is around 2.5uL/min, and if
it is assumed
the lowest pressure that can exist in the capillary bed into which the aqueous
drains is 0 torr,
then maximum outflow resistance in a normal eye at the maximum of normal
pressure is
expected to be approximately 9 torr/(uL/min). Normal pressure within the eye
ranges between
12 torr and 22 torr. As noted above, glaucoma is usually associated with high
pressure inside
the eye that can damage eye tissues and result in vision loss. The condition
where pressures are
significantly below this range is called hypotany or ocular hypotension. In
some patients,
hypotany can be just as damaging (if not more) than glaucoma.
[0005] The early stages of glaucoma are typically treated with drugs. When
drug
treatments no longer suffice, however, surgical approaches are used. Surgical
or minimally
invasive approaches primarily attempt to lower the outflow resistance of
aqueous from the
-1-
CA 03070108 2020-01-15
WO 2019/018807
PCT/US2018/043158
anterior chamber to the blood stream either by the creation of alternative
fluid paths or the
augmentation of the natural paths for aqueous outflow.
[0006] Devices used to lower outflow resistance are generally referred to
as "glaucoma
shunts" or "shunts." FIGS. 1A-1C, for example, illustrate several different
traditional glaucoma
plate shunts 100 (identified individually as 100a-c) configured to provide
constant resistance to
flow. The shunt 100a of FIG. 1A, for example, includes a plate 103a, a
plurality of outflow ports
102a, one or more inflow ports 101, and tie-downs or engagement features 104a.
The shunts
100b and 100c shown in FIGS. 1B and 1C, respectively, include several features
similar to the
features of shunt 100a. For example, these shunts 100b-c include plates 103b-
c, outflow ports
102b-c, and tie-downs or engagement features 104b-c. The shunts 100b-c,
however, include an
inflow tube 105 instead of the inflow ports 101 of the shunt 100a.
[0007] FIGS. 2A and 2B illustrate a human eye E and suitable location(s) in
which shunts
100a-c may be implanted within the eye. More specifically, FIG. 2A is a
simplified front view
of the eye E, and FIG. 2B is an isometric view of the eye capsule of FIG. 2A.
Referring first to
FIG. 2A, the eye E includes a number of muscles to control its movement,
including a superior
rectus SR, inferior rectus IR, lateral rectus LR, medial rectus MR, superior
oblique SO, and
inferior oblique 10. The eye E also includes an iris, pupil, and limbus.
[0008] Referring to FIGS. 2A and 2B together, shunt 100c is positioned such
that inflow
tube 105 is positioned in an anterior chamber of the eye, and outflow ports
102c are positioned
at a different location within the eye. Depending upon the design of the
device, the outflow ports
102c may be place in a number of different suitable outflow locations (e.g.,
between the choroid
and the sclera, between the conjunctiva and the sclera). For purposes of
illustration, only shunt
100c is shown implanted in the eye E. It will be appreciated, however, that
shunts 100a-b may
be similarly implanted within the eye E.
[0009] Outflow resistance typically depends on the outflow location.
Additionally,
outflow resistance changes over time as the outflow location goes through its
healing process
after surgical implantation of the device. Because the outflow resistance
changes over time, in
many procedures the shunt 100a-c is modified at implantation to temporarily
increase its outflow
resistance. After a period of time deemed sufficient to allow for healing of
the tissues and
stabilization of the outflow resistance, the modification to the shunt 100a-c
is reversed, thereby
decreasing the outflow resistance. Such modifications can be invasive, time-
consuming, and
-2-
CA 03070108 2020-01-15
WO 2019/018807
PCT/US2018/043158
expensive for patients. If such a procedure is not followed, however, the
likelihood of creating
hypotany and its resultant problems is high.
BRIEF DESCRIPTION OF THE DRAWINGS
[0010] Many aspects of the present technology can be better understood with
reference to
the following drawings. The components in the drawings are not necessarily
drawn to scale.
Instead, emphasis is placed on illustrating clearly the principles of the
present technology.
Furthermore, components can be shown as transparent in certain views for
clarity of illustration
only and not to indicate that the component is necessarily transparent.
Components may also be
shown schematically.
[0011] FIGS. 1A-1C illustrate traditional glaucoma plate shunts configured
to provide
constant resistance to flow.
[0012] FIG. 2A is simplified front view of an eye E with an implanted
shunt, and FIG. 2B
is an isometric view of the eye capsule of FIG. 2A.
[0013] FIGS. 3A and 3B illustrate an adjustable flow glaucoma shunt
configured in
accordance with an embodiment of the present technology.
[0014] FIG. 3C is a partially schematic illustration of an eye capsule of a
human patient
showing the adjustable flow glaucoma shunt of FIGS. 3A and 3B implanted within
the eye
capsule.
[0015] FIGS. 3D and 3E illustrate inflow regions configured in accordance
with additional
embodiments of the present technology.
[0016] FIGS. 4A-4C illustrate an adjustable flow glaucoma shunt configured
in
accordance with another embodiment of the present technology.
[0017] FIGS. 5A-6B illustrate inflow control assemblies configured in
accordance with
embodiments of the present technology.
[0018] FIGS. 7A-7E illustrate a variable flow shunt configured in
accordance with an
embodiment of the present technology.
[0019] FIGS. 8A-9B illustrate additional embodiments of variable flow
glaucoma shunt
devices configured in accordance with the present technology.
-3-
CA 03070108 2020-01-15
WO 2019/018807
PCT/US2018/043158
[0020] FIG. 10 illustrates a variable flow shunt device including an
actuatable member at
an outflow end of the device in accordance with an embodiment of the present
technology.
[0021] FIGS. 11A-11C illustrate a ribbon or wire composed of shape memory
material
and configured in accordance with an embodiment of the present technology.
[0022] FIGS. 12A and 12B illustrate a fluid control element including
variable fluid
resistors composed of shape memory materials in accordance with an embodiment
of the present
technology.
[0023] FIGS. 13A and 13B are partially schematic, cross-sectional views of
a variable fluid
resistor comprising a dual lumen elastomeric tube configured in accordance
with an embodiment
of the present technology.
[0024] FIGS. 13C-13F illustrate additional embodiments of variable fluid
resistor devices
configured in accordance with the present technology.
[0025] FIGS. 14A and 14B illustrate a fluid control element including
variable fluid
resistors composed of shape memory materials in accordance with additional
embodiments of
the present technology.
[0026] FIGS. 15A-15C illustrate an adjustable flow glaucoma shunt
configured in
accordance with another embodiment of the present technology.
[0027] FIGS. 16A-16E illustrate an adjustable flow glaucoma shunt
configured in
accordance with still another embodiment of the present technology.
DETAILED DESCRIPTION
[0028] The present technology is directed to adjustable flow glaucoma
shunts and methods
for making and using such devices. In many of the embodiments disclosed
herein, the adjustable
flow glaucoma shunts comprise an adjustable fluid resistor ("resistor" within
the context of this
document refers to a fluid resistor), actuator, and/or actuation mechanism.
Additionally, in
certain embodiments, the shunts may also include an adjustable opening
pressure control
mechanism. These mechanisms can be selectively adjusted or modulated to
increase or decrease
the outflow resistance and/or opening pressure of the shunt in response to
changes in any (or any
combination of) intraocular pressure (TOP), aqueous production rate, native
aqueous outflow
resistance, and/or native aqueous outflow rate.
-4-
CA 03070108 2020-01-15
WO 2019/018807
PCT/US2018/043158
[0029] In one embodiment, for example, an adjustable flow shunt for
treating glaucoma in
a human patient comprises an elongated outflow drainage tube having a proximal
inflow region
and a distal outflow region. The proximal inflow region can include one or
more apertures
defining a fluid inlet area positioned to allow fluid to flow therethrough and
into the outflow
drainage tube. The adjustable flow shunt further comprises an inflow control
assembly at the
proximal inflow region. The inflow control assembly can include a control
element sized and
shaped to slidably engage the proximal inflow region and a spring element
operably coupled
between the control element and an anchor element engaged with the proximal
inflow region.
The spring element is configured to be activated by a non-invasive energy and,
upon activation,
slidably move the control element along the proximal inflow region such that
(a) the one or more
apertures are accessible and have a first fluid flow cross-section or (b) the
one or more apertures
are at least partially covered by the control element and have a second fluid-
flow cross-section
less than the first fluid flow cross-section.
[0030] In another embodiment of the present technology, an adjustable flow
shunt for
treatment of glaucoma may comprise an elongated outflow tube having (a) a
proximal inflow
portion configured for placement within an anterior chamber in a region
outside of an optical
field of view of an eye of the patient, and (b) a distal outflow portion at a
different location of
the eye. The adjustable flow shunt also includes an actuator positioned along
the outflow tube
between the inflow portion and the outflow portion. The actuator is
transformable between an
open position that allows fluid to flow through the outflow tube and
resistance positions that
partially obstruct fluid flow through the outflow tube. During operation, the
actuator is movable
between positions in response to non-invasive energy.
[0031] An adjustable flow shunt assembly configured in accordance with
still another
embodiment of the present technology can include an elongated drainage tube
having a proximal
portion and a distal portion. The proximal portion includes an inflow port
configured to be in
fluid communication with a fluid chamber in an eye of the patient. The
adjustable flow shunt
can also include a variable resistor assembly configured to selectively
control flow of fluid into
the inflow port. The variable resistor assembly in this embodiment comprises a
base portion and
an aperture plate carried by the base portion. The aperture plate comprises a
plurality of first
apertures extending therethrough. The variable resistor assembly also
comprises a standoff plate
carried by and extending away from the aperture plate. The standoff plate
comprises a plurality
of second apertures extending therethrough, with the second apertures aligned
with
corresponding first apertures of the aperture plate. The variable resistor
assembly further
-5-
CA 03070108 2020-01-15
WO 2019/018807
PCT/US2018/043158
comprises a membrane disposed on and carried by the standoff plate. The
membrane is
positioned to sealably cover an open end of each of the second apertures.
During operation of
the shunt assembly, a portion of the membrane over one or more second
apertures of the standoff
plate is configured to be selectively targeted and removed via non-invasive
energy, thereby
creating a fluid path from the site of fluid in the patient through the
accessible open ends of the
targeted second apertures, the corresponding first apertures, and into the
drainage tube.
[0032] Specific details of various embodiments of the present technology
are described
below with reference to Figures 3A-16E. Although many of the embodiments are
described
below with respect to adjustable flow glaucoma shunts and associated methods,
other
embodiments are within the scope of the present technology. Additionally,
other embodiments
of the present technology can have different configurations, components,
and/or procedures than
those described herein. For instance, shunts configured in accordance with the
present
technology may include additional elements and features beyond those described
herein, or other
embodiments may not include several of the elements and features shown and
described herein.
[0033] For ease of reference. throughout this disclosure identical
reference numbers are
used to identify similar or analogous components or features, but the use of
the same reference
number does not imply that the parts should be construed to be identical.
Indeed, in many
examples described herein, the identically numbered parts are distinct in
structure and/or
function.
Selected Embodiments of Variable Flow Glaucoma Shunts
[0034] FIGS. 3A-16E illustrate a number of different embodiments for
variable flow
glaucoma shunt devices, along with particular components and features
associated with such
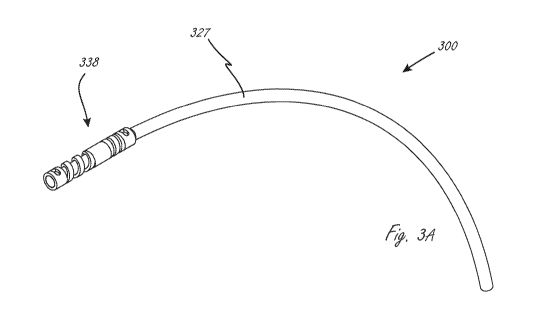
devices. FIG. 3A, for example, illustrates a variable flow glaucoma shunt 300
("shunt 300")
configured in accordance with an embodiment of the present technology. The
shunt 300 includes
an inflow control assembly 338 and an outflow drainage tube or outflow
assembly 327. The
inflow control assembly 338 of the shunt 300 is configured for placement
within an anterior
chamber in a region outside of the optical field of view of the eye, but
within a region visible
through the cornea (as described below with reference to FIG. 3C). The outflow
drainage tube
327 comprises tubing (e.g., a fine bore length of thin walled tubing) sized
and shaped to span the
region between the anterior chamber and a desired outflow location. As
described in greater
detail below, the inflow control assembly 338 comprises a control mechanism
configured to act
as a variable resistor during operation.
-6-
CA 03070108 2020-01-15
WO 2019/018807
PCT/US2018/043158
[0035] FIG. 3B is a partially exploded view of the shunt 300 with a portion
of the inflow
control assembly 338 removed from the outflow drainage tube 327 for purposes
of illustration.
As best seen in FIG. 3B, a proximal end 360 of the outflow drainage tube 327
comprises a
proximal inflow region defined by a core element or core feature 342 extending
therefrom. The
core element 342 may be composed of a relatively stiff material or a
combination of stiff
materials including, but not limited to, polyether ether ketone (PEEK),
acrylic, polycarbonate,
metal, ceramic, quartz, and/or sapphire. The portion of the outflow drainage
tube 327 not
comprised in the core element 342 may be composed of a relatively flexible
material (e.g.,
silicone, urethane, or another suitable material). The core element 342
includes one or more
apertures or openings 341 (only one is shown in the illustrated embodiment)
that define a fluid
inlet area 362. The fluid inlet area 362 is in fluid communication with a
lumen of the outflow
drainage tube 327. In other embodiments, the aperture(s) 341 may have a
different arrangement
and/or there may be a different number of apertures 341. For example, in
another embodiment
the aperture 341 may extend helically about the core element 342. The
aperture(s) 341 are
positioned to allow fluid to flow therethrough during operation of the shunt
300.
[0036] Referring to FIG. 3A and 3B together, for example, the inflow
control assembly
338 in the illustrated embodiment includes a control element 339 configured to
be positioned on
or around an external surface of the core element 342 (as shown by the arrow
in FIG. 3B). During
operation, the control element 339 may be adjusted to cover more or less of
the fluid inlet area
362. For example, in some embodiments, the control element 339 may be adjusted
to increase
or decrease the length of a fluid path between an edge of the control section
339 and the
aperture(s) 341 (FIG. 3B). In some embodiments, a hydrogel coating may be
applied to an inside
surface of the control element 339 to further enhance the ability of the
control element 339 to
slide relative to the core element 342 and enhance sealing of the components
during operation.
In additional embodiments, the hydrogel coating may also be applied to the
core element 342 (in
addition to, or in lieu of, application of the coating on the control element
339). Further details
regarding adjusting/manipulating the control element 339 are described below.
[0037] The inflow control assembly 338 in the illustrated embodiment can
also include an
adjustable spring element (shown as first and second spring elements 340 and
340') arranged on
opposite sides of the control element 339. Each spring element 340 and 340'
may further
comprise a corresponding anchor element 310.
-7-
CA 03070108 2020-01-15
WO 2019/018807
PCT/US2018/043158
[0038] In the embodiment illustrated in FIGS. 3A and 3B, the control
element 339 is
composed of a single material. For example, the control element 339 may be
fabricated from
materials such as (but not limited to) ceramics, alumina oxide, silica oxide,
sapphire, and/or
quartz. Such materials, for example, may be ground to very high
tolerances/precise dimensions.
In other embodiments, however, the control element 339 may have different
portions/regions
composed of different materials. The first and second spring elements 340 and
340' may be
composed of a shape memory material (e.g., nitinol or another suitable shape
memory material)
capable of activation via non-invasive energy, such as light (and or heat).
The anchor elements
310 may be fabricated from similar material(s) or other suitable materials.
[0039] In operation, first and second spring elements 340 and 340' are
configured to be
selectivity activated by non-invasive energy and, upon activation, slidably
move the control
element 339 along the proximal inflow region in a first direction or a second
direction,
respectively, such that (a) the aperture(s) 341 have a first fluid flow cross-
section (e.g.,
completely open and accessible), or (b) the aperture(s) are at least partially
covered by the control
element 339 and have a second fluid-flow cross-section less than the first
fluid flow cross-section
(e.g., partially open/accessible). Further, in some instances the control
element 339 may be
slidably adjusted such that the aperture(s) 341 are fully covered and
inaccessible. One feature
of the arrangement shown in FIGS. 3A and 3B is that the inflow control
assembly 338 can be
selectively adjusted after placement within the eye (e.g., via non-invasive
energy) to provide a
variety of different outflow resistance levels by incrementally adjusting the
control element 339
relative to the aperture(s) 441.
[0040] FIG. 3C is a partially schematic illustration of an eye capsule of a
human patient
showing the adjustable flow glaucoma shunt 300 of FIGS. 3A and 3B implanted
within the eye
capsule. In particular, a typical surgery for implantation of the shunt 100 in
the eye capsule
comprises the following: (a) a portion of conjunctiva is peeled back; (b) a
portion of sclera is
removed to create a pocket where the plate is to be placed; (c) the inflow
control assembly 338
is routed into the anterior chamber of the eye capsule; (d) the outflow
drainage tube 327 is
extended through the tissue and into a desired pocket; and (e) the outflow
drainage tube 327 and
any other portions of the shunt 300 not otherwise buried in the other tissues
are covered with
conjunctiva. In the embodiment illustrated in FIG. 3C, for example, the shunt
300 is configured
for placement traversing a region in the anterior chamber to a region in a
suprachoroidal location
of the eye. In other embodiments, however, the shunt 300 may be adapted for
placement within
-8-
CA 03070108 2020-01-15
WO 2019/018807
PCT/US2018/043158
different portions of the eye. In one embodiment, for example, shunts
configured in accordance
with the present technology may be positioned at a subconjunctival region
within the eye.
[0041] FIGS.
3D and 3E illustrate core elements configured in accordance with different
embodiments of the present technology. Referring first to FIG. 3D, for
example, core element
342 comprises a plurality of apertures or openings 341' extending therethrough
and defining, at
least in part, a fluid path in communication with a lumen of the corresponding
outflow drainage
tube 327. The
apertures 341' in the illustrated embodiment have a different
arrangement/configuration than the aperture 341 described above with reference
to FIGS. 3A
and 3B. It will be appreciated that while six apertures 341' are shown in FIG.
3D, the core
element 342 may include a different number of apertures 341' in other
embodiments. Moreover,
the apertures 341' may have a different arrangement relative to each other.
FIG. 3E illustrates
yet another embodiment of core element 342 having apertures 341" configured in
accordance
with still yet another arrangement of the present technology. In this
embodiment, the apertures
341" comprise a plurality of elongated slots arranged about the core element
342. In other
embodiments, the apertures 341/341" may have a variety of other suitable
shapes/sizes.
[0042] FIGS.
4A-4C illustrate a variable flow glaucoma shunt 400 ("shunt 400")
configured in accordance with yet another embodiment of the present
technology. The shunt
400 includes an inflow control assembly 438 and an outflow drainage tube or
outflow assembly
427. The inflow control assembly 438 includes several features similar to the
inflow control
assembly 338 of the shunt 300 described above with reference to FIGS. 3A and
3B. For example,
inflow control assembly 438 includes a first or proximal spring element 440'
and a second or
distal spring element 440 arranged adjacent each other. The inflow control
assembly 438 further
includes a core element or feature 442 coupled to an inner portion of the
inflow control assembly
at anchor point 442' (as best seen in FIGS. 4B and 4C) between the spring
elements 440 and
440'. A fixation spine 451 extends between and is operably coupled to the
spring elements 440
and 440'. Although only one fixation spine 451 is shown in the illustrated
embodiment, in other
embodiments the shunt 400 may include one or more additional fixation spines.
In the illustrated
embodiment, the fixation spine 451 and first and second spring elements 440
and 440' are all
integrally formed from the same tube using a laser cutting process. In other
embodiments,
however, the spring elements 440 and 440' and/or fixation spine 451 may be
separate, discrete
components formed from different materials.
-9-
CA 03070108 2020-01-15
WO 2019/018807
PCT/US2018/043158
[0043] In operation, the shunt 400 is configured to operate in an analogous
fashion to the
shunt 300 described above with respect to FIGS. 3A-3C. In particular, the
first and second
spring elements 440 and 440' are configured to be selectivity activated by non-
invasive energy
and, upon activation, slidably move the core element 442 to change the length
of a flow path
through openings or slits 460 of the inflow control assembly 438. Referring to
FIG. 4B, for
example, when the distal spring 440 is expanded/actuated, the core element 442
moves
proximally and the length of the core portion 442 inside an uncut portion of
the shunt 400 (and
the corresponding flow F through openings 460 and along flow path FP in the
inflow control
assembly 438) is at a minimum.
[0044] Referring to FIG. 4C, however, when the distal spring 440 is
compressed and the
proximal spring 440' is expanded/actuated, the length of the core portion 442
inside the uncut
portion (and the corresponding flow F along flow path FP) is maximized. The
disclosed
arrangement is expected to provide an effective and predictable way to
incrementally
increase/decrease flow resistance in a linear fashion via the shunt 400. In
other embodiments,
rather than the incremental adjustments in flow rate provided by the shunt 400
shown in
FIGS. 4A-4C, the shunt 400 may be configured to provide a binary on/off
arrangement via
selective actuation of the first and second spring elements 440 and 440'.
Further, in some
embodiments, the width and/or shape of the openings/slits 460 can be modified
to allow for
further control of the flow resistance of the shunt 400. In yet another
embodiment the core pin
may be affixed to the proximal end of spring element 440' and not extend into
a flow path. In
such an embodiment, the flow path is altered by expanding or compressing the
space between
the elements of the spring 440 and 440'. In other embodiments, the shape of
the pin and or the
inner lumen can be modified to change allow for a nonlinear control of flow as
a function of core
travel.
[0045] FIGS. 5A-6B illustrate inflow control assemblies configured in
accordance with
further embodiments of the present technology. Referring first to FIGS. 5A and
5B, for example,
inflow control assembly 538 is positioned on or around an external surface of
core element 542
at the inflow or inlet region of the drainage tube 527. Inflow control
assembly 538 comprises
control element 539 and spring elements 540 fixed thereto and extending in a
proximal direction
toward the drainage tube 527. The inflow control assembly 538 further includes
an anchor
element 510 operably coupled to the spring elements 540 at a proximal region
of the inflow
control assembly 538. FIG. 5A illustrates the inflow control assembly 538 in a
low or minimum
flow configuration in which control element 539 is positioned entirely over or
approximately
-10-
CA 03070108 2020-01-15
WO 2019/018807
PCT/US2018/043158
entirely over apertures 541 (FIG. 5B) in the core element 542. FIG. 5B
illustrates the inflow
control assembly 538 in a maximum flow configuration in which the spring
elements 540 have
been actuated. In some embodiments, for example, the spring element 540 may be
heated via
non-invasive energy (e.g., laser energy), thereby causing the spring elements
540 to bow
outwardly and slidably move control element 539 in a proximal direction such
that apertures 541
are exposed and fluid can flow therethrough into drainage tube 527.
[0046] FIGS. 6A and 6B illustrate another embodiment of an inflow control
assembly 638
configured in accordance with the present technology. In this embodiment, the
inflow control
assembly 638 includes a control element 639 and first and second spring
elements 640 and 640'
fixed thereto and extending in a proximal direction toward the drainage tube
627. The first and
second spring elements 640 and 640' have a different configuration than spring
elements 540
and 540' described above with reference to FIGS. 5A and 5B. Further, each
spring element 640
and 640' is operably coupled to a corresponding anchor element 610 and 610'.
Because the
individual spring elements 640 and 640' have their own anchor elements 610 and
610',
respectively, the spring elements 640 and 640' can be independently set in an
initial configuration
and independently controlled during operation. As shown in FIG. 6B, for
example, the
individual spring elements 640 and 640' can be actuated (e.g., via heat),
thereby causing the
spring elements 640 and 640' to coil more tightly and slidably move control
element 639 in a
proximal direction along core element 642 and create an open fluid path (to a
lumen of drainage
tube 627) via exposed apertures 641.
[0047] In the embodiments shown in in FIGS. 3A-6B, the inflow ends of the
various
illustrated shunts are sealed. Such shunts may be delivered via a needle (not
shown) traversing
a desired flow path (as described above with reference to FIG. 3C). In other
embodiments,
however, the inflow end of a shunt may be initially open (such that the shunt
can be delivered
over a guide wire) and then sealed after delivery and placement.
Additional Embodiments of Adjustable Flow Glaucoma Shunts
[0048] A collection of additional embodiments of adjustable flow and/or
adjustable
pressure regulated glaucoma shunts comprising plates are described below with
reference to
FIGS. 7A-16E. Such shunts may be implanted as described above and illustrated
in FIG. 3C, or
the shunt(s) may be implanted using other suitable techniques and in other
suitable locations
within the eye. In some of these embodiments, traditional outflow ports are
augmented with
additional tubes to distribute the aqueous over larger regions of tissue.
Outflow tube(s) are
-11-
CA 03070108 2020-01-15
WO 2019/018807
PCT/US2018/043158
covered by at least the conjunctiva. Many of the embodiments of the present
technology
additionally comprise an adjustable fluid resistor, some of which may
additionally comprise an
adjustable opening pressure control mechanism. These mechanisms can be
adjusted to increase
or decrease the outflow resistance and/or opening pressure of the shunt in
response to changes
in the following: TOP, aqueous production rate, native aqueous outflow
resistance, native
aqueous outflow rate, and combinations thereof
[0049] FIGS. 7A-7E illustrate another embodiment of a variable flow shunt
700
configured in accordance with the present technology. FIG. 7A, for example, is
a schematic top
view of the shunt 700, which is configured for minimally invasive placement
(like the shunts
described above with reference to FIGS. 3A-6B). The shunt 700 includes an
elongated drainage
tube 702 having a proximal portion with an inflow port 701 and a distal
portion opposite the
proximal portion. The shunt 700 differs from the shunts describe above in that
fluid resistance
of the shunt 700 is selectively controlled by modifying the number of
apertures that allow fluid
to flow through the inflow port 701. In some embodiments, for example, the
shunt 700 can be
configured to allow only for sequential decreases in outflow resistance. In
other embodiments,
however, the shunt 700 may be configured to selectively allow for both finite
decreases and
increases in outflow resistance. Further details regarding the shunt 700 and
its operation are
described below.
[0050] FIG. 7B is an enlarged, partially schematic cross-sectional view of
the shunt 700
taken along line B¨B of FIG. 7A, and FIG. 7C is an enlarged view of the region
C identified in
FIG. 7B. Referring to FIGS. 7B and 7C together, the inflow port 701 of the
shunt 700 further
comprises a variable resistor assembly 720 configured to selectively control
flow of fluid into
the inflow port (and the outflow port 702). The variable resistor assembly 720
comprises a
membrane 745 disposed on and carried by a standoff plate 746. The standoff
plate 746 is
operably coupled to and extends from aperture plate 747. The aperture plate
747 is carried by a
base portion or housing 748 of the shunt 700.
[0051] The aperture plate 747 comprises a plurality of first apertures or
first openings 760
extending therethrough. The first apertures 760 have a first cross-sectional
dimension Di (not
shown). The first apertures 760 can be precisely formed so that each opening
is identical or
nearly identical and all of the first apertures 760 are a predetermined size.
The standoff plate
746 comprises a plurality of second apertures or second openings 741 extending
therethrough.
The second apertures 741 have a second cross-sectional dimension D2 larger
than the first cross-
-12-
CA 03070108 2020-01-15
WO 2019/018807
PCT/US2018/043158
sectional dimension Di. As will be described in greater detail below, the
second apertures 741
do not need to be as precisely formed as the first apertures 760. As shown in
FIG. 7C, the
membrane 745 completely covers one end (an upper or first end) of each of the
second apertures
741. The opposite end of each second aperture 741 (a second or lower end) is
aligned with a
corresponding first aperture or first opening 760 extending through the
aperture plate 747.
[0052] FIG. 7D is a top view of the variable resistor assembly 720. As best
seen in FIG.
7D, the variable resistor assembly 720 further comprises a plurality of target
indicia or markers
713 ("targets 713"). The individual targets 713 correspond to and are aligned
with each first
aperture 741 (FIG. 7B). Referring next to FIG. 7E, after the shunt 700 is
implanted within a
patient and it is desired to reduce the fluid resistance of the shunt 700, non-
invasive energy (e.g.,
a surgical laser) can directed at a selected target 713 on membrane 745. In
embodiments using
laser energy, for example, the laser can be activated or fired to selectively
ablate the targeted
material of the membrane 745, thereby removing such membrane material and
exposing the open
end of the corresponding second aperture 741. Without the membrane blocking
the targeted
second aperture 741, fluid can flow therethrough (as shown by the arrow F),
and subsequently
through the corresponding first aperture 760 and into the outflow drainage
tube 702. If a further
reduction in fluid resistance is desired, one or more additional targets 713
on membrane 745 may
be ablated to expose additional second apertures 741 and allow additional
fluid to flow
therethrough to outflow drainage tube 702.
[0053] In the illustrated embodiment, outflow resistance can only be
lowered as there is
no means of sealing the second apertures 741 of the implanted shunt 700 once
the corresponding
targeted portions of the membrane 745 are removed to open the second
aperture(s) 741 to
aqueous flow. In other embodiments, however, there may be techniques to later
impede or stop
fluid flow by blocking one or more open second apertures 741. For example,
referring to FIGS.
7B and 7C, in some embodiments the membrane 745 and standoff plate 746 may be
composed,
at least in part, from a hydrophobic material (e.g., a low melting point wax)
adapted to be melted
by the surgical laser (not shown) at temperatures that will not cause
particular harm to the
aqueous. In such embodiments, a relatively small, fine beam from the laser
source can be used
to melt the wax material of the target membrane 754 and open the corresponding
second aperture
741. At a later point in time, if it is desired to slow or limit flow of
aqueous, a larger beam from
the laser source can be used to melt the wax material of the standoff plate
746, thereby causing
the material to "puddle" or accumulate over the corresponding second aperture
760 within the
-13-
CA 03070108 2020-01-15
WO 2019/018807
PCT/US2018/043158
previously opened second aperture 741 and close or block fluid flow through
the first aperture
760.
[0054] In the embodiment illustrated in FIGS. 7A-7E, the components of the
variable
resistor assembly 720 are separate, discrete components that are operably
coupled together
before implantation of the shunt 700. The components may be composed of
similar materials,
or one or more different materials. In other embodiments, however, the
membrane 745 and
standoff plate 746 may be fabricated as a single unitary component composed of
the same
material, such as the example described above in which the membrane 745 and
standoff plate
746 comprise a unitary component composed of a hydrophobic material. In other
embodiments,
however, the integral membrane 745/standoff plate 746 may be composed of other
suitable
materials. In still other embodiments, the standoff plate 746 and aperture
plate 747 may be
fabricated of a single unitary component composed of the same material with
the first and second
apertures 741 and 760 formed therein. In yet additional embodiments, the
aperture plate 747
may be integrally formed with the base portion 748 of the shunt 700.
[0055] FIGS. 8A-9B illustrate additional embodiments of variable flow
glaucoma shunt
devices configured in accordance with the present technology. In these
embodiments, the shunts
are configured to be delivered to a target location within an eye capsule of
the patient via a
guidewire, and then transformed between a delivery configuration and a
deployed configuration
upon removal of the guidewire. FIG. 8A, for example, illustrates shunt 800 in
a delivery
configuration on guidewire W. The shunt 800 includes an inflow control
assembly 838 and an
outflow tube or outflow assembly 827. The inflow control assembly 838 can
include several
features generally similar to the shunts described above with reference to
FIGS. 3A-6B. For
example, the shunt 800 includes a control element 839 positioned over one or
more apertures or
openings 841 (shown in broken lines) extending through a body portion 848 of
the inflow control
assembly 838. The aperture(s) 841, when at least partially exposed, are
configured to allow
aqueous to flow therethrough and into the outflow tube 827. The shunt 800 also
comprises a
pair of adjustable spring elements 840 and 840' arranged on opposite sides of
the control element
839. The spring elements 840 and 840' are coupled between the body portion 848
and the control
element 839. In some embodiments, the spring elements 840 and 840' are
composed of a shape
memory material (e.g., nitinol) and adapted to expand/contract when heat is
applied. For
example, applying heat to the first spring element 840 can induce this spring
element to coil
more tightly, thereby moving the control element 839 toward the first spring
element 840 and
stretching or expanding the second spring element 840'. Moving the control
element 839 also at
-14-
CA 03070108 2020-01-15
WO 2019/018807
PCT/US2018/043158
least partially exposes the aperture(s) 841 to allow aqueous to flow
therethrough similar to the
techniques described above with reference to FIGS. 3A-6B.
[0056] In the illustrated embodiment, the inflow control assembly 838 is
composed of a
first material having a first rigidity and the outflow tube 827 is composed of
a second material
having a second rigidity less than the first rigidity. Referring to FIGS. 8A
and 8B together, the
shunt 800 may be preshaped prior to implantation such that the shunt 800
includes one or more
bends along its length. In the illustrated embodiment, for example, the shunt
800 comprises a
generally "L" shaped arrangement and includes a bend or elbow 854 at or near a
distal region of
the outflow tube 827.
[0057] When the shunt 800 is positioned on guidewire W for delivery, the
shunt 800
assumes a generally linear, straight delivery configuration. As shown in FIG.
8B, however,
when the guidewire W is removed, the shunt 800 transforms between its delivery
configuration
and an expanded/deployed configuration in which the shunt 800 assumes its
predetermined "L"
shaped arrangement including elbow 865. This configuration is expected to
allow for rapid and
reliable delivery of the shunt 800 via guidewire W, and enable precise
placement of the inflow
control assembly 838 within the eye capsule of the patient once the guidewire
is removed and
the shunt 800 assumes is predetermined shape.
[0058] FIGS. 9A and 9B illustrate a shunt 900 configured in accordance with
still another
embodiment of the present technology. The shunt 900 includes a number of
features generally
similar to the features of shunt 800. The shunt 900 differs from shunt 800 in
that the shunt 900
is not composed of different materials having different rigidities. Rather,
the shunt 900
comprises an inflow portion or region 938 and an outflow portion or outflow
tube 927 composed
of a single material (e.g., a shape memory material such as nitinol). The
shunt 900, like shunt
800 described above, also includes a preset, generally "L" shaped arrangement
and includes a
bend or elbow 954. In this embodiment, however, removing the guidewire W does
not transform
the shunt 900 between its delivery configuration (FIG. 9A) and its
deployed/expanded
configuration (FIG. 9B). Instead, as best seen in FIG. 9B, once guidewire W is
removed and the
shunt 900 is at a desired location within the patient, a laser source (e.g.,
an ophthalmic laser¨
not shown) can be used to direct a laser beam to selectively heat a portion of
the shunt 900 and
induce the shunt 900 to bend about elbow 954 and return to its preset shape
(the generally "L"
shaped arrangement).
-15-
CA 03070108 2020-01-15
WO 2019/018807
PCT/US2018/043158
[0059] FIG. 10 illustrates a variable flow shunt device 1000 configured in
accordance with
yet another embodiment of the present technology. The shunt 1000 comprises an
inflow
assembly 1001 and an outflow drainage tube 1027 with an outflow port 1002. The
shunt 1000
further comprises an actuatable member 1049 at the outflow end of the outflow
port 1002
(opposite the inflow assembly 1001). The actuatable member 1049 comprises one
or more tissue
disruption members 1050 (e.g., barbs or other suitable types of devices) to
disrupt/disturb tissue
at or proximate the outflow end of the outflow port 1001 after the shunt 1000
is implanted within
the patient. In one embodiment, the barbs 1050 of the actuatable member 1049
can be moved
and actuated by an operator via an externally applied magnetic field to
disrupt target tissue
adjacent the outflow end of the shunt 1000. In other embodiments, however, the
barbs 1050
may be moved/actuated using other suitable techniques, such as thermally
induced shape
changes. Further, it will be appreciated that a different number of barbs 1050
may be used and/or
the barbs 1050 may have a different arrangement relative to each other and the
actuatable
member 1049.
[0060] Many of the embodiments disclosed herein make use of a shape memory
material
(SMM), such as nitinol, shape memory polymers, and the like, as a control in
an adjustable fluid
resistor. As noted previously, such fluid resistors allow controlled flow of
aqueous from within
the anterior chamber of the eye to a location into which the aqueous can
defuse. One such
location is within or on top of the sclera posterior to the cornea. In
general, SMM elements
utilized in the various devices disclosed herein can be repeatedly activated
in one direction to
increase fluid resistance and in another direction to decrease fluid
resistance. In some
embodiments, for example, each of multiple activations on targets in one
section of the actuation
element will incrementally increase the resistance, while multiple activations
on targets in
another section of the actuation element will incrementally decrease the
resistance. When a
target is heated above its transition temperature, such as by heating via non-
invasive laser energy,
the SMM shifts from its larger volume, lower stiffness, low temperature
martensite (Mar) form
to its high temperature, smaller volume, stiffer austenite form (Aus).
Aus (austenite) 75-83 GPa, smaller volume, high temperature
Mar (martensite) 28-40 GPa, larger volume, low temperature
[0061] One such configuration is illustrated in FIGS. 11A-11C, which
represents a side
view of a ribbon or wire configured in accordance with embodiments of the
present technology.
-16-
CA 03070108 2020-01-15
WO 2019/018807
PCT/US2018/043158
Referring first to FIG. 11A, the ribbon has been shape-set in a form
comprising multiple uniform
folds. As illustrated, there are six folds, but it will be appreciated that in
other embodiments
ribbons with more or less folds can be used depending on the desired amount of
resolution and
displacement. Referring next to FIG. 11B, the ribbon can then be mounted
between two anchors
such that the constrained length is larger than the heat set length. Referring
now to FIG. 11C,
applying heat to the fold(s) in the portion of SMM heated above its Aus,
shifts it from its less
stiff, higher volume Mar form to its stiffer and lower volume Aus form. In the
illustrated
embodiment, the entire SMM component is not allowed to return to its heat set
shape even if the
entire portion of SMM is heated above the transformation temperature. The
unheated portion
can expand further to compensate. In addition, heating previously unheated
sections is expected
to stretch previously unheated and heated sections reversing the mechanism.
[0062] FIGS. 12A and 12B illustrate a fluid control element 1201 configured
in accordance
with another embodiment of the present disclosure. The fluid control element
1201 may be used
with any of the variable flow shunts described herein or other suitable
shunts. In this
embodiment, the fluid control element 1201 comprises variable fluid resistors
actuated by SMM
elements (like those described above with reference to FIGS. 11A-11C).
Referring first to
FIG. 12A, fluid control element 1201 comprises a base 1211 and a flow-through
drainage tube
1212 carried by and operably coupled to the base 121. For example, the flow-
through tube 1212
can be fixed to the base 1211 via flow-through anchors 1209. In other
embodiments, however,
other suitable techniques may be used to secure the flow-through tube 1212 to
the base 1211.
The flow-through tube 1212 is also operably engaged with an actuator 1218. In
the illustrated
embodiment, the actuator 1218 comprises a ribbon or wire composed of SMM and
including a
plurality of folds. The actuator 1218 has a fixed length and each end of the
actuator 1218 is
anchored to the base 1211.
[0063] The actuator 1218 may be actuated using techniques similar to those
described
above with reference to FIGS. 11A-11C. During operation, for example, the tops
of the folds
along the actuator 518 may be used as target regions to be selectively heated
via non-invasive
energy (e.g., laser energy) to locally heat such regions along the actuator
518. As discussed
previously with respect to FIGS. 11A-11C, heating folds on one side relative
to the other side
will allow incremental shifting of resistance (up or down) to modify the state
of the actuator
1218, and thereby change fluid resistance through the flow-through tube 1212.
FIG. 12A, for
example, illustrates a low-resistance state of the fluid control element 1201
in which the actuator
1218 is fairly uniform along its length and provides minimal resistance or
interference with fluid
-17-
CA 03070108 2020-01-15
WO 2019/018807
PCT/US2018/043158
flow through the flow-through tube 1212. FIG. 12B illustrates a high
resistance state of the of
the fluid control element 1201. The high resistance state or high resistance
position is a result,
for example, of multiple actuations via the actuation element 1218 to the flow-
through tube 1212.
In particular, heating each of the folds of the actuation element 1218 on the
left side of the flow-
through tube 1212 above the actuation temperature causes the actuation element
1218 in this
region to shrink, thereby "pinching" and compressing the flow-through tube
1212 in this
direction and increasing fluid resistance therethrough. When desired, fluid
control element 1201
can be transformed again to additional resistance positions or orientations
than that shown in
FIG. 12B (e.g., back to the state shown in FIG. 12A or a different state) via
further
manipulation/modulation (e.g., heating selected regions) of actuation element
1218.
[0064] FIGS. 13A and 13B are partially schematic, cross-sectional views of
a variable fluid
resistor comprising a dual lumen elastomeric tube 1312 configured in
accordance with still
another embodiment of the present technology. More specifically, FIG. 13A
illustrates the
elastomeric tube 1312 in an initial or low-resistance state before modulation.
The elastomeric
tube 1312 comprises a first lumen or a fluid flow-through lumen 1316 having an
initial cross-
sectional shape (e.g., a "D" shaped lumen). The elastomeric tube 1312 further
comprises a
second lumen or a control lumen 1336 adjacent the first lumen 1316 and a
diaphragm
therebetween. The control lumen 1336 contains one or more actuation elements
1318. In the
illustrated embodiment, for example, the actuation element 1318 is composed of
SMM and
includes a first or expansion portion 1314 and a second or shrinkage portion
1315. Although
only a single actuation element 1318 is shown in the cross-sectional views of
FIGS. 13A and
13B, it will be appreciated that in further embodiments multiple actuation
elements 618 can be
arrayed serially along a length of the elastomeric tube 1312.
[0065] FIG. 13B illustrates the elastomeric tube 1312 in an increased or
higher resistance
state after activation of the actuation element 1318. More specifically, non-
invasive energy (e.g.,
heating via laser energy) has been used on expansion portion 1314 of the
actuation element 1318,
thereby causing the actuation element 1318 to expand. Such expansion pushes
the diaphragm
toward the flow-through lumen 1316 and decreases the cross-sectional dimension
of the flow-
through lumen 1316. This decrease in size of the flow-through lumen 1316
accordingly
increases the fluid resistance through the lumen 1316. The cross-sectional
dimension of the
flow-through lumen 1316 can be further modified via additional modulation of
the actuation
element 1318. For example, fluid resistance through the flow-through lumen
1316 can be further
-18-
CA 03070108 2020-01-15
WO 2019/018807
PCT/US2018/043158
decreased by additional heating of the expansion portion 1314 or returned to a
lower resistance
state via heating of the shrinkage portion 1315.
[0066] In some FIG. 13C illustrates another embodiment of an inflow mounted
variable
resistor 1320 in accordance with the present technology. In this embodiment,
multiple actuation
elements 618 can be arrayed serially along a length of control lumen 636 (FIG.
13A). As the
expansion portion 1314 of each target actuation element 1318 is actuated, the
length of the
restricted area is increased thereby increasing the fluid resistance linearly.
Likewise, actuating
shrinkage portion(s) 1315 of target actuation element(s) 1318 can decease
fluid resistance. As
shown in FIG. 13C, such fluid controls can be incorporated into a shunt plate
1303, inflow tube
1305, an outflow mounted variable resistor 1321, and/or the outflow tube (not
shown).
[0067] FIG. 13D illustrates a variable fluid resistor configured in
accordance with still
another embodiment of the present technology. The embodiment shown in FIG. 13D
can include
a number of features similar to those of the variable fluid resistors
described above with reference
to FIGS. 13A and 13B. In this embodiment, however, the elastomeric tube 1312
comprises a
single fluid flow-through lumen 1316, while an actuation assembly 1322
positioned along the
elastomeric tube 1312 comprises a dual-lumen arrangement similar to that
described above. In
particular, the actuation assembly 1322 comprises a first lumen 1316' having a
predetermined
cross-sectional shape (e.g., a "D" shaped lumen). The elastomeric tube 1312 is
positioned within
and extends through the first lumen 1316' of the actuation assembly 1322. The
actuation
assembly 1322 further comprises a second lumen or a control lumen 1336'
adjacent the first
lumen 1316'. The control lumen 1336' contains one or more actuation elements
1318 similar to
the actuation elements described previously. In this embodiment, for example,
the actuation
element 1318 is composed of SMM and includes a first or expansion portion 1314
and a second
or shrinkage portion 1315.
[0068] Selectively heating the expansion portion 1314 can cause the
actuation element
1318 to expand. Like the arrangement described above with reference to FIGS.
13A and 13B,
such expansion decreases the cross-sectional dimension of the elastomeric tube
1312 by driving
the elastomeric tube 1312 away from the control lumen 1336' and toward fixed
inner walls of
the first lumen 1316'. By decreasing the cross-sectional dimension of the
elastomeric tube 1312,
fluid resistance through the tube 1312 is accordingly increased. The fluid
resistance through
elastomeric tube 1312 can be further decreased by additional heating of the
expansion portion
1314, or the elastomeric tube 1312 can be returned to a lower resistance state
via heating of the
-19-
CA 03070108 2020-01-15
WO 2019/018807
PCT/US2018/043158
shrinkage portion 1315 of actuation element 1318. Although only a single
actuation assembly
1322 is shown, it will be appreciated that in further embodiments multiple
actuation assemblies
1322 can be positioned along a length of elastomeric tube 1312.
[0069] FIGS. 13E and 13F are partially schematic, cross-sectional views of
a fluid resistor
comprising a dual lumen elastomeric tube 1312' configured in accordance with
yet another
embodiment of the present technology. The fluid resistor in the embodiment
illustrated in FIGS.
13E and 13F operates using a similar principle to that described above with
reference to FIGS.
13A and 13B. For example, FIG. 13E illustrates the elastomeric tube 1312' in
an initial or low-
resistance state before modulation. The elastomeric tube 1312' comprises a
first lumen or a fluid
flow-through lumen 1316' having an initial cross-sectional shape (e.g., a "D"
shaped lumen).
The elastomeric tube 1312' further comprises a second lumen or a control lumen
1336' adjacent
the first lumen 1316. The control lumen 1336' is filled with a control fluid.
Referring next to
FIG. 13F, when a volume of control fluid is increased, the cross-sectional
dimension of the flow-
through lumen 1316 is decreased as an elastomeric diaphragm 1337 expands into
the flow-
through lumen 1316', thereby increasing fluid resistance and decreasing flow
through the lumen
1316'. Likewise, when control fluid is removed from the control lumen 1336',
the elastomeric
diaphragm 1337 retracts and the cross-sectional dimension of the flow-through
channel 1316' is
increased, thereby reducing fluid resistance and increasing outflow through
the lumen 1316'.
Control fluid can be removed or added to the control lumen 1336', for example,
using a syringe.
In some embodiments, one or more reservoirs (not shown) may be fluidly
interfaced with the
control lumen 1336' and fluid volume of the control lumen 1336' can be
adjusted by adding or
removing fluid from the reservoir(s). Further, it will be appreciated that in
some embodiments
fluid control systems configured in accordance with the present technology may
comprise
multiple fluid control sealed lumens serially distributed along the length of
the control system.
[0070] FIGS. 14A and 14B illustrate yet another embodiment of a SMM-based
actuator
1418 configured in accordance with the present technology and adapted for use
in an adjustable
flow glaucoma shunt. In this embodiment, the actuator 1418 comprises one or
more coils 1424
arranged about a periphery of clamping arm 1423. The coil(s) 1424 and clamping
arm 1423 may
both be composed of SMM. Anchors 1410 are positioned to fixedly hold the
actuator 1418 in
position on base 1411 such that the clamping arm 1423 is pressed against
elastomeric flow-
through tube 1412. The elastomeric flow-through tube 1412 can have a stiffness
that maintains
the outer coils 1424 in a state comparable to the mounted state for the
ribbon/wire actuators 1318
described above with reference to FIGS. 12A-13B.
-20-
CA 03070108 2020-01-15
WO 2019/018807
PCT/US2018/043158
[0071] In operation, sections of the coil(s) 1424 can be selectively
actuated to adjust the
clamping pressure of the clamping arm 1423 against flow-through tube 1412, and
thereby the
fluid resistance. Referring to FIG. 14B, for example, coils 1424 on one side
(e.g., the right side)
of clamping arm 1423 can be heated via laser energy applied at target site
1413. Such heating
actuates the selected coils 1424 and causes them to coil more tightly, thereby
actuating the
clamping arm 1423 to increase pressure and increase resistance on the flow-
through tube 1412.
Actuation of the coils 1424 on the other side of the clamping arm 1423 (the
left side coils) relaxes
the clamping arm 1423 and thereby decreases pressure and resistance on the
flow-through tube
1412.
[0072] In alternate embodiments, the actuator 1418 can be set in a rest or
initial position
such that the clamping arm 1423 completely occludes the flow-through 1412 and
the coils 1424
can be selectively adjusted to increase or decrease the tension of the
clamping arm 1423 against
the base1411. The base 1411 accordingly acts as an anvil as the clamping arm
1423 drives the
flow-through tube 1412 against it during operation. In some embodiments, such
an arrangement
may be used to operate an adjustable opening pressure valve (not shown), which
is set to
selectively control the desired control Intraocular Pressure (TOP). In other
embodiments,
however, the actuator 1418 may have a different arrangement and/or include
different features.
[0073] FIGS. 15A-15C illustrate an adjustable glaucoma shunt 1500
configured in
accordance with another embodiment of the present technology and include fluid
resistor
elements such as those described above with reference to FIGS. 14A and 14B.
FIG. 15A, for
example, is an exploded view of the shunt 1500, and FIG. 15B is a top view of
the assembled
shunt 800. Referring to FIGS. 15A and 15B together, the shunt 1500 comprises
an elastomeric
flow-through tube 1512 carried by and operably coupled with control assembly
1519. The flow-
through tube 1512 comprises an inflow region or inflow portion 1505 at one end
of the flow-
through tube 1512, and an outflow assembly 1527 including one or more outflow
ports 1502 at
or near an opposite end of the flow-through tube 1512.
[0074] The shunt 1500 also includes an actuator 1518 carried by and
operably coupled to
control assembly 1519. The actuator 1518 can be similar to the actuator 1418
described above
with reference to FIGS. 14A and 14B. In the illustrated embodiment, for
example, actuator 1518
includes a clamping arm 1523 operably coupled to and positioned between a
plurality of coils
1524. The coils 1524 (like the coils 1424 described above) can be composed of
SMM and
-21-
CA 03070108 2020-01-15
WO 2019/018807
PCT/US2018/043158
adapted to selectively modulate the flow-through tube 1512 to
increase/decrease pressure
therethrough as described previously.
[0075] In the illustrated embodiment, the shunt 1500 includes a pressure
port 1528 and
corresponding pressure transducer 1529 configured to be positioned within a
pressure transducer
housing 1530 on the control assembly 1519. The pressure port 1528/pressure
transducer 1529
are configured to provide pressure information to a clinician/operator during
operation of the
shunt 1500. In other embodiments, the pressure port and/or pressure transducer
1529 may have
a different arrangement relative to each other and the other components of the
shunt 1500.
Further, the pressure port 1528/pressure transducer 1529 are optional
components that may not
be included in some embodiments. In some embodiments, the shunt 1500 may also
optionally
include a differential port 1526 in the control assembly 1519.
[0076] The shunt 1500 can further include a plate 1503 configured to be
positioned over
at least a portion of the control assembly 1518, flow-through tube 1512, and
actuator 1518. The
plate 1503 can include a window 1531 such that when the shunt 1500 is
assembled (as shown in
FIG. 15B), the window 1531 provides access to the actuator 1518 and other
components carried
by the control assembly 1519.
[0077] FIG. 15C illustrates an implant tool 1534 configured to deliver and
position shunt
1500 within an eye capsule of a patient (not shown) in accordance with an
embodiment of the
present technology. The implant tool 1534 can include, for example, a guide
needle 1532
configured to carry the shunt 1500 for delivery, and a guide needle release
1533 that an operator
can actuate to release the shunt 1500 once at a desired position/orientation
within the patient. In
other embodiments, however, the implant tool 1534 may have a different
configuration and/or
the shunt 1500 may be delivered using other suitable devices/techniques.
[0078] FIGS. 16A-16E illustrate various features of an adjustable glaucoma
shunt 1600
configured in accordance with yet another embodiment of the present
technology. The shunt
1600 can include a number of features similar to the shunt 1500 described
above with reference
to FIGS. 15A-15C. For example, as best seen in FIG. 16A, the shunt 1600
comprises a flow-
through tube 1612 having an inflow port or inflow region 1601 at one end, and
an outflow port
1602 at an opposite end of the flow-through tube 1612. The shunt 1600 further
comprises a
control assembly 1619 configured to modulate flow through the flow-through
tube 1612. The
flow-through tube 1612, control assembly 1619, and a number of other
components of the shunt
are carried by plate 1603.
-22-
CA 03070108 2020-01-15
WO 2019/018807
PCT/US2018/043158
[0079] The shunt 1600 differs from the shunt 1500, however, in that the
shunt 1600
includes a different system for modulating fluid flow along the flow-through
tube 1612. In
particular, rather than the actuator 1518 including the clamping arm
1523/coils 1524 described
previously, the shunt 1600 in the present embodiment comprises an arrangement
similar to that
described above with reference to FIGS. 13E and 13F. Referring to FIGS. 16B-
16D, for
example, the control assembly 1629 of shunt 1600 comprises a control fluid
1644 contained
within a control fluid chamber 1636 comprising an annular region around a thin
walled tubular
flow-through channel of tube 1612. The control fluid chamber 1636 is fluidly
isolated from the
flow-through channel. A reservoir 1643 is interfaced with and in fluid
communication with the
control fluid chamber. The reservoir 1643 is configured to provide a larger
target for
conveniently injecting or removing control fluid 1636 from the system. In
operation, control
fluid 1644 may be added/removed from the control fluid chamber 1636 to
increase/decrease a
fluid cross-sectional dimension of an aqueous flow path 1616 through flow-
through channel
1612, thereby decreasing/increasing the corresponding fluid flow therethrough.
[0080] In some embodiments, a solid core may optionally be introduced into
flow path
1616 to initially reduce the fluid cross-sectional dimension even further and
thereby make the
flow path more sensitive to small changes in the diameter of flow-through
channel 1612. In
FIG. 16E, for example, optional solid core pin or element 1637 has been
introduced into the
flow-through channel 1612 and flow path 1616 now has an annular cross-
sectional profile.
[0081] In the illustrated embodiment, the shunt 1600 further comprises a
pressure
transducer 1629. The pressure transducer 1629 is an optional component that
may not be
included in some embodiments. Further, it will be appreciated that shunt 1600
may include
features other than those described herein and/or the features of shunt 1600
may have a different
arrangement relative to each other.
[0082] In many of the embodiments described herein, the actuators or fluid
resistors are
configured to compress or "pinch" the drainage tube during operation. In this
way, the
actuators/fluid resistors can incrementally or continuously change the flow
resistance through
the drainage tube to selectively regulate pressure/flow. The actuators and
fluid resistors
configured in accordance with the present technology can accordingly adjust
the level of
resistance or compression between a number of different positions, and
accommodate a
multitude of variables (e.g., IOP, aqueous production rate, native aqueous
outflow resistance,
and/or native aqueous outflow rate) to precisely regulate flow rate through
the drainage tube.
-23-
CA 03070108 2020-01-15
WO 2019/018807
PCT/US2018/043158
[0083] The disclosed actuators and fluid resistors can all be operated
using non-invasive
energy. This feature allows such devices to be implanted in the patient and
then
modified/adjusted over time without further invasive surgeries or procedures
for the patient.
Further, because the devices disclosed herein may be actuated via non-invasive
energy, such
devices do not require any additional power to maintain a desired orientation
or position. Rather,
the actuators/fluid resistors disclosed herein can maintain a desired
position/orientation without
power. This can significantly increase the usable lifetime of such devices and
enable such
devices to be effective long after the initial implantation procedure.
Examples
[0084] Several aspects of the present technology are set forth in the
following examples.
1. An adjustable flow shunt for treating glaucoma in a human patient,
the shunt
comprising:
an elongated outflow drainage tube having a proximal inflow region and a
distal outflow
region; and
an inflow control assembly at the proximal inflow region, wherein the inflow
control
assembly compri s es¨
a control element sized and shaped to slidably engage the proximal inflow
region;
and
a spring element operably coupled between the control element and an anchor
element engaged with the proximal inflow region;
wherein the proximal inflow region comprises one or more apertures defining a
fluid
inlet area positioned to allow fluid to flow therethrough and into the outflow
drainage tube,
wherein the spring element is configured to be activated by a non-invasive
energy and,
upon activation, slidably actuate the control element along the proximal
inflow
region such that (a) the one or more apertures are accessible and have a first
fluid
flow cross-section or (b) the one or more apertures are at least partially
covered
by the control element and have a second fluid-flow cross-section less than
the
first fluid flow cross-section.
-24-
CA 03070108 2020-01-15
WO 2019/018807
PCT/US2018/043158
2. The adjustable flow shunt of example 1 wherein the proximal inflow
region
comprises a core element operably coupled to and extending from a proximal end
of the outflow
drainage tube, and wherein the one or more apertures extend through a sidewall
of the core
element to define the fluid inlet area.
3. The adjustable flow shunt of example 2 wherein the core element is
composed of
a different material than the outflow drainage tube.
4. The adjustable flow shunt of example 2 wherein the core element is
composed of
a first material having a first rigidity, and wherein the outflow drainage
tube is composed of a
second material having a second rigidity less than the first rigidity.
5. The adjustable flow shunt of example 2 wherein the core element is
composed of
polyether ether ketone (PEEK), acrylic, polycarbonate, metal, ceramic, quartz,
and/or sapphire.
6. The adjustable flow shunt of any one of examples 1-5 wherein the
elongated
outflow drainage tube is composed of silicone and/or urethane.
7. The adjustable flow shunt of any one of examples 1-6 wherein the spring
element
is composed of a shape memory material.
8. The adjustable flow shunt of any one of examples 1-6 wherein the spring
element
is composed of nitinol.
9. The adjustable flow shunt of any one of examples 1-8 wherein the inflow
control
assembly is configured for placement within an anterior chamber in a region
outside of the
optical field of view of the eye.
10. The adjustable flow shunt of example 9 wherein the outflow drainage
tube is sized
and shaped to traverse a region between the anterior chamber to a region in a
suprachoroidal
location of the eye.
-25-
CA 03070108 2020-01-15
WO 2019/018807
PCT/US2018/043158
11. The adjustable flow shunt of example 9 wherein the outflow drainage
tube is
sized and shaped to traverse a region between the anterior chamber to a region
in a
subconjunctival location of the eye.
12. The adjustable flow shunt of any one of examples 1-11 wherein the one
or more
apertures comprises a single elongated slot extending axially along the
proximal inflow region.
13. The adjustable flow shunt of any one of examples 1-11 wherein the one
or more
apertures comprises a plurality of apertures extending radially about the
proximal inflow region.
14. The adjustable flow shunt of any one of examples 1-11 wherein the one
or more
apertures comprises a plurality of apertures extending helically about the
proximal inflow region.
15. The adjustable flow shunt of any one of examples 1-14 wherein the
spring
element is configured to be activated via laser energy.
16. The adjustable flow shunt of any one of examples 1-15 wherein the
spring
element comprises a first spring and the anchor comprises a first anchor, and
wherein the first
spring and first anchor are positioned on a first side of the control element,
and wherein the
inflow control assembly further comprises:
a second spring and a corresponding second anchor on a second, opposite side
of the
control element;
wherein the first and second spring elements are configured to be selectivity
activated by
non-invasive energy and, upon activation, slidably move the control element
along the proximal inflow region in a first direction or a second direction,
respectively, such that (a) the one or more apertures have the first fluid
flow
cross-section, or (b) the one or more apertures are at least partially covered
by the
control element and have the second fluid-flow cross-section less than the
first
fluid flow cross-section.
17. The adjustable flow shunt of example 16 wherein the first and second
spring
elements are configured such that, upon activation, the control element
slidably moves the
-26-
CA 03070108 2020-01-15
WO 2019/018807
PCT/US2018/043158
control element along the proximal inflow region such that the one or more
apertures are fully
covered and inaccessible.
18. The adjustable flow shunt of any one of examples 1-15 wherein the
spring
element and corresponding anchor element are positioned on a proximal end of
the control
element between the control element and the outflow drainage tube.
19. The adjustable flow shunt of any one of examples 1-15 wherein the
spring
element comprises one or more coil springs extending about the proximal inflow
region.
20. The adjustable flow shunt of any one of examples 1-15 wherein the
spring
element comprises one or more elongated bow springs extending between the
control element
and the anchor element.
21. An adjustable flow shunt assembly for treatment of glaucoma, the shunt
assembly
comprising:
an elongated drainage tube having a proximal portion and a distal portion,
wherein the
proximal portion includes an inflow port configured to be in fluid
communication
with a fluid chamber in an eye of the patient;
a variable resistor assembly configured to selectively control flow of fluid
into the inflow
port, wherein the variable resistor assembly comprises¨
a base portion;
an aperture plate carried by the base portion, wherein the aperture plate
comprises
a plurality of first apertures extending therethrough;
a standoff plate carried by and extending away from the aperture plate,
wherein
the standoff plate comprises a plurality of second apertures extending
therethrough, and wherein the second apertures are aligned with
corresponding first apertures of the aperture plate; and
a membrane disposed on a carried by the standoff plate, wherein the membrane
is positioned to sealably cover an open end of each of the second
apertures;
wherein, during operation, a portion of the membrane over one or more second
apertures
of the standoff plate is configured to be selectively targeted and removed via
non-
-27-
CA 03070108 2020-01-15
WO 2019/018807
PCT/US2018/043158
invasive energy, thereby creating a fluid path from the site of fluid in the
patient
through the accessible open ends of the targeted second apertures, the
corresponding first apertures, and into the drainage tube.
22. The adjustable flow shunt assembly of example 21 wherein:
the first apertures have a first cross-sectional dimension; and
the second apertures have a second cross-sectional dimension greater than the
first cross-
sectional dimension.
23. The adjustable flow shunt assembly of example 21 wherein the first
apertures
have identical cross-sectional dimensions.
24. The adjustable flow shunt assembly of any one of examples 21-23 wherein
the
standoff plate is composed, at least in part, of a hydrophobic material
configured to be at least
partially melted via non-invasive energy.
25. The adjustable flow shunt assembly of any one of examples 21-23 wherein
the
standoff plate is composed, at least in part, of a wax material configured to
be at least partially
melted via non-invasive energy.
26. The adjustable flow shunt assembly of any one of examples 21-23 wherein
the
base portion, aperture plate, and standoff plate of the variable resistor
assembly are separate,
discrete components operably coupled together.
27. The adjustable flow shunt assembly of any one of examples 21-23 wherein
the
standoff plate and membrane are fabricated as a single, unitary component
composed of the same
material.
28. The adjustable flow shunt assembly of any one of examples 21-23 wherein
the
aperture plate and standoff plate are fabricated as a single, unitary
component composed of the
same material.
-28-
CA 03070108 2020-01-15
WO 2019/018807
PCT/US2018/043158
29. The adjustable flow shunt assembly of any one of examples 21-28
wherein:
the membrane further comprises a plurality of target indicia aligned with and
corresponding with individual second apertures; and
during operation, the non-invasive energy is delivered to corresponding target
indicia of
the membrane to selectively remove membrane material at the targeted location.
30. An adjustable flow shunt for treatment of glaucoma in a human patient,
the
adjustable flow shunt comprising:
an elongated outflow tube having (a) a proximal inflow portion configured for
placement
within an anterior chamber in a region outside of an optical field of view of
an
eye of the patient, and (b) a distal outflow portion at a different location
of the
eye; and
an actuator positioned along the outflow tube between the inflow portion and
the outflow
portion, wherein the actuator is transformable between an open position that
allows fluid to flow through the outflow tube and resistance positions that
partially obstruct fluid flow through the outflow tube,
wherein during operation, the actuator is movable between positions in
response to non-
invasive energy.
31. The adjustable flow shunt of example 30 wherein the actuator is
configured to
partially obstruct fluid flow through the outflow tube in the resistance
positions by engaging the
outflow tube and changing a diameter and/or a cross-sectional shape of the
outflow tube.
32. The adjustable flow shunt of example 30 or example 31 wherein the
actuator is
movable between positions in response to laser energy.
33. The adjustable flow shunt of example 30 wherein:
the outflow tube comprises a dual lumen tube having a first lumen for carrying
fluid
therethrough and a second lumen adjacent to the first lumen and separated by
the
first lumen by a diaphragm;
the actuator is positioned within the second lumen, and wherein the actuator
comprises
one or more actuation elements configured to transform between and expanded
state and an initial state in response to the non-invasive energy,
-29-
CA 03070108 2020-01-15
WO 2019/018807
PCT/US2018/043158
in the expanded state, actuation elements engage and push the diaphragm toward
the first
lumen and decrease a cross-sectional dimension thereof
34. The adjustable flow shunt of any one of examples 30-33 wherein the
actuator is
configured to hold the open position or one of the resistance positions
without power.
35. An adjustable flow shunt, comprising:
an elongated outflow tube having a proximal inflow portion configured for
placement at
a first location within an eye of the patient, and a distal outflow portion at
a second
location of the eye spaced apart from the first location,
wherein the outflow tube comprises a dual lumen tube having a first lumen for
carrying
fluid therethrough and a second lumen adjacent to the first lumen and fluidly
isolated from the first lumen; and
a control fluid disposed within the second lumen,
and wherein, during operation¨
increasing a volume of control fluid within the second lumen decreases a cross-
sectional dimension of the first lumen, thereby partially obstructing fluid
flow through the first lumen, and
decreasing a volume of control fluid within the second lumen increases a cross-
sectional dimension of the first lumen, thereby increasing fluid flow
through the first lumen.
36. The adjustable flow shunt of example 35 wherein the elongated outflow
tube
comprises an elastomeric tube.
37. The adjustable flow shunt of example 35 or example 36, further
comprising a
reservoir in fluid communication with the second lumen, and wherein the volume
of control fluid
within the second lumen is changed by transferring control fluid to and/or
from the reservoir.
38. The adjustable flow shunt of any one of examples 35-37 wherein the
volume of
control fluid within the second lumen is changed by transferring control fluid
to and/or from the
second lumen via a syringe.
-30-
CA 03070108 2020-01-15
WO 2019/018807
PCT/US2018/043158
39. The adjustable flow shunt of any one of examples 35-38 wherein the
first lumen
is separated from the second lumen by a diaphragm, and wherein:
increasing a volume of control fluid within the second lumen moves the
diaphragm
toward the first lumen and decreases a cross-sectional dimension thereof; and
decreasing a volume of control fluid within the second lumen moves the
diaphragm away
from the first lumen and increases a cross-sectional dimension thereof
40. A shunt for treatment of glaucoma in a human patient, the shunt
comprising:
an elongated outflow drainage tube having a proximal inflow region and a
distal outflow
region;
an inflow control assembly at the proximal inflow region; and
a transition region along the outflow tube between the inflow region and the
outflow
region, wherein, during operation, the transition region is transformable
between
a first generally linear delivery shape and a second shape different than the
first
shape to anchor the shunt at a desired location of the eye.
41. The shunt of example 40 wherein the outflow drainage tube is configured
to be
delivered via guidewire, and wherein the transition region is configured to
transform between
the first delivery shape and the second shape upon removal of the guidewire.
42. The shunt of example 40 or example 41 wherein the transition region is
configured to transform between the first delivery shape and the second shape
upon application
of non-invasive energy to one or more selected areas of the transition region.
43. The shunt of example 40 or example 41 wherein the transition region is
configured to transform between the first delivery shape and the second shape
in response to
application of non-invasive laser energy to one or more selected areas of the
transition region.
44. The shunt of any one of examples 40-43 wherein the second shape
comprises a
generally "L" shaped configuration.
-31-
CA 03070108 2020-01-15
WO 2019/018807
PCT/US2018/043158
Conclusion
[0085] The above detailed description of embodiments of the technology are
not intended
to be exhaustive or to limit the technology to the precise form disclosed
above. Although specific
embodiments of, and examples for, the technology are described above for
illustrative purposes,
various equivalent modifications are possible within the scope of the
technology as those skilled
in the relevant art will recognize. For example, any of the features of the
variable flow shunts
described herein may be combined with any of the features of the other
variable flow shunts
described herein and vice versa. Moreover, although steps are presented in a
given order,
alternative embodiments may perform steps in a different order. The various
embodiments
described herein may also be combined to provide further embodiments.
[0086] From the foregoing, it will be appreciated that specific embodiments
of the
technology have been described herein for purposes of illustration, but well-
known structures
and functions associated with variable flow shunts have not been shown or
described in detail to
avoid unnecessarily obscuring the description of the embodiments of the
technology. Where the
context permits, singular or plural terms may also include the plural or
singular term,
respectively.
[0087] Moreover, unless the word "or" is expressly limited to mean only a
single item
exclusive from the other items in reference to a list of two or more items,
then the use of "or" in
such a list is to be interpreted as including (a) any single item in the list,
(b) all of the items in
the list, or (c) any combination of the items in the list. Additionally, the
term "comprising" is
used throughout to mean including at least the recited feature(s) such that
any greater number of
the same feature and/or additional types of other features are not precluded.
It will also be
appreciated that specific embodiments have been described herein for purposes
of illustration,
but that various modifications may be made without deviating from the
technology. Further,
while advantages associated with some embodiments of the technology have been
described in
the context of those embodiments, other embodiments may also exhibit such
advantages, and
not all embodiments need necessarily exhibit such advantages to fall within
the scope of the
technology. Accordingly, the disclosure and associated technology can
encompass other
embodiments not expressly shown or described herein.
-32-