Note: Descriptions are shown in the official language in which they were submitted.
CA 03070538 2020-01-17
WO 2019/018536 PCT/US2018/042707
COMPOSITIONS CONTAINING CANNABINOID ANALOG CONJUGATES AND
METHODS OF USE
Cross-Reference to Related Application
[0001] This application claims the priority to and the benefit of U.S.
Provisional Application
No. 62/533,894, filed July 18, 2017, the contents of which are incorporated by
reference in its
entirety.
Field
[0002] This disclosure relates to synthetic cannabinoid compositions and
methods generally
directed to using such compositions for diagnostic, therapeutic, and
prognostic applications.
Background
[0003] Medical cannabinoids have not been well developed for theranostic
applications.
Strategic clinical trials still lag behind, due to public stigmatization and
law-imposed
criminalization. However, petitions for therapeutic application of medical
cannabinoids from
healthcare providers, patients and activist advocates have exponentially
increased. The
cannabinoid receptors (CB 1 and CB2), transient receptor potential subfamily V
member 1
(TRPV1), transient receptor potential subfamily A member 1 (TRPA1), and the
orphan receptor
GPR55 are part of the endocannabinoid system (ECS), which is a complex lipid
signaling network
involving different proteins for the control or modulation of numerous
physiological and
pathophysiological processes. Along with these receptors, the ECS includes
arachidonic acid-
derived ligands, anandamide and 2-arachidonoyl glycerol, and the enzymes that
degrade these
endocannabinoids, such as fatty acid amide hydrolase (FAAH) and
monoacylglycerol lipase
(MAGL). While the expression of the CB 1 receptor is ubiquitous, with
predominant presence in
the brain, particularly in the basal ganglia, hippocampus, cerebellum and
cortex, the CB2 receptor
is preferentially located in tissues that comprise the immune system
(especially within the B cell
rich compartments). Consistent with its broad distribution, CBI can be
similarly detected in
peripheral nerves and beyond the neural compartments, i.e., testis, uterus,
vascular endothelium,
eye, spleen, ileum and adipocytes. Because CBI and CB2 receptors share 68%
amino acid sequence
identity at the trans-membrane domain, cannabinoid ligand/receptor pathways
are being
1
CA 03070538 2020-01-17
WO 2019/018536 PCT/US2018/042707
consistently evaluated as rational therapeutic targets for neurodegeneration
in Alzheimer' s,
Parkinson's, Huntington' s diseases; indolent pain, which is common in
glaucoma and multiple
sclerosis; and diverse cardiovascular disorders; and cancer.
Summary
[0004] Several disadvantages were recognized by the inventors and various
embodiments of
this disclosure were developed to address these shortcomings in the art.
Certain embodiments
disclosed and described here include compositions containing a conjugate of a
label, a chelator,
and a cannabinoid analog. The label can be a radionuclide. The label can be
one or more of
Technetium-99, Gallium-68, Copper-60, Copper-64, Iridium-111, Holmium-166,
Rhenium-186,
Rhenium -188, Yttrium-90, Lutetium-177, Radium-223, or Actinium-225. In
certain
embodiments, the label is configured to facilitate contrast-enhanced imaging,
when the
composition is administered to a mammal during use. The cannabinoid analog can
be an immune
check point cannabinoid receptor ligand. The cannabinoid analog can be a
synthetic cannabinoid
receptor agonist or a natural cannabinoid receptor agonist. The cannabinoid
analog can be an
aminoalkylindole. The cannabinoid analog can be a cannabinoid receptor inverse
agonist. The
cannabinoid analog can be a member of the group consisting of anandamide
(AEA), 2-
arachidonoylglycerol (2-AG), noladin ether, virodhamine and N-
arachidonylodopamine (NADA).
The cannabinoid analog can be a diarylopyrazole. The chelator can an aminated
or an acid chelator.
In certain embodiments, the chelator is a cyclam. The cannabinoid analog can
be cyclam-1'-acetyl-
[N-(Piperidin-1-y1)-5-(4-chloropheny1)-1-(2,4-dichloropheny1)-4-methyl-1H-
pyrazole-3-
carboxamidel. The cannabinoid analog can be cyclam-l'-propyl-[N-(Piperidin-l-
y1)-5-(4-
chloropheny1)-1-(2,4-dichloropheny1)-4-methyl-1H-pyrazole-3-carboxamidel .
[0005] Embodiments also include methods for diagnosing a medical condition
in a human
subject, by administering to a human subject in need thereof an effective
amount of any of the
compositions described herein. A method of imaging a plurality of cancer
cells, the method
comprising administering to a human patient an effective amount of any of the
compositions
described herein. Embodiments also include methods for treating a cancer
amenable to treatment
with a cannabinoid, the method comprising administering to a human subject in
need thereof an
effective amount of any of the compositions described herein. Embodiments also
include methods
for treating a neurologic disorder amenable to treatment with a cannabinoid,
the method
comprising administering to a human subject in need thereof an effective
amount of any of the
2
CA 03070538 2020-01-17
WO 2019/018536 PCT/US2018/042707
compositions described herein. Embodiments also include methods for
alleviating pain in a human
subject, the method comprising administering to a human subject in need
thereof an effective
amount of the composition of any of the compositions described herein.
Embodiments also include
kits for imaging cells, comprising a predetermined quantity of a conjugate of
a chelator and a
medical cannabinoid analog; and a predetermined quantity of an imaging agent.
The conjugate of
the chelator and the medical cannabinoid analog can be present in the kit as
precursors that
subsequently interact with the imaging agent, when provided with suitable
reaction conditions.
The kit can include a tin-containing reducing agent. Cells, organs, and
tissues that can be imaged
using the kits include those of the cardiovascular system, precancerous cells
and tissues, cancerous
cells and tissues, and cells and tissues of the nervous system, including the
brain and the spinal
cord.
[0006] Numerous other aspects, features and benefits of the present
disclosure may be made
apparent from the following detailed description taken together with the
drawing figures. The
systems can include less components, more components, or different components
depending on
desired analysis goals.
Brief Description of the Drawings
[0007] While this disclosure is susceptible to various modifications and
alternative forms,
specific embodiments are shown by way of example in the drawings and will be
described in detail
here. The drawings may not be to scale. It should be understood, however, that
the drawings and
the detailed descriptions thereto are not intended to limit the disclosure to
the particular form
disclosed, but, to the contrary, the intention is to cover all modifications,
equivalents, and
alternatives falling within the scope of the present disclosure as defined by
the appended claims.
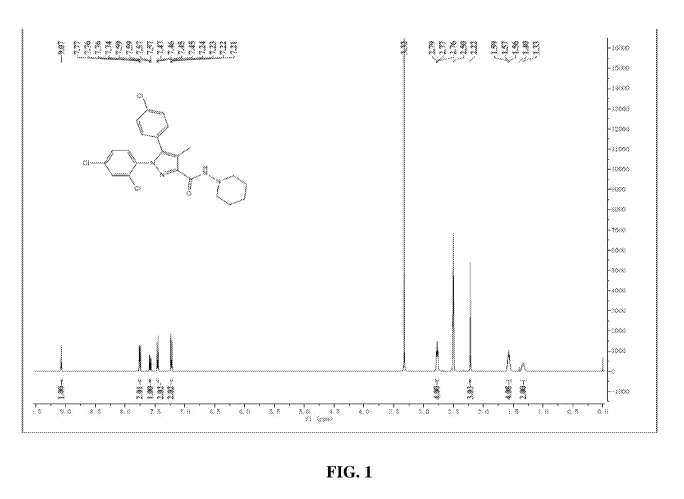
[0008] FIG. 1 is a representative analysis of starting material SR141716
(rimonabant) using
NMR spectroscopy, according to an embodiment.
[0009] FIG. 2 is a representative analysis of starting material SR141716
using high-
performance liquid chromatography (HPLC) analysis, according to an embodiment.
[0010] FIG. 3 is a representative analysis of VYR206 using NMR, according
to an
embodiment.
[0011] FIG. 4 is a representative analysis of VYR206 using mass
spectrometry, according to
an embodiment.
3
CA 03070538 2020-01-17
WO 2019/018536 PCT/US2018/042707
[0012] FIG. 5 is a representative analysis of VYR206 using HPLC analysis,
according to an
embodiment.
[0013] FIGS. 6A - 6C show the protein expression of the CBI receptor using
Western blot
analysis. FIG. 6A panel shows expression of the CBI receptor in cell lysate
from various DLBCL.
FIG. 6B shows expression of 3-actin as the control for sample loading. FIG. 6C
is a graphical
representation of the mRNA expression analysis of CBI and CB2 in various DLBCL
cancer cell
lines.
[0014] FIG. 7 is a graphical representation of the efficacy of cannabinoid
receptor ligand
SR141716 (rimonabant) on diffuse large B-cell lymphoma (DLBCL) cell lines'
viability.
[0015] FIG. 8 is a graphical representation of the efficacy of cannabinoid
receptor ligand
CP945,598 (Otenabant) on DLBCL cell lines' viability.
[0016] FIG. 9 is a graphical representation of the efficacy of cannabinoid
receptor ligand
AM1241 on DLBCL cell lines' viability.
[0017] FIG. 10 is a graphical representation of the efficacy of cannabinoid
receptor ligand
AM251 on DLBCL cell lines' viability.
[0018] FIGS. 11A - 11D are graphical representations of the inhibition
efficacy of two CBI
inverse agonist SR141716 and VYR206 using DLBCL (FIGS. 11, A and B) and mantle
cell
lymphoma (MCL) cell lines (FIGS. 11, Panels C and D).
[0019] FIGS. 12A - 12D are graphical representations of the inhibition
efficacy response of
two selected CBI inverse agonist 5R141716 and VYR206 and N4 using cell
viability assays on
DLBCL cancer cells: LR, MS, RC and DOHH2.
[0020] FIGS. 13A - 13D are graphical representations of the inhibition
efficacy response of
two selected CBI inverse agonist 5R141716 and VYR206 and N4 using cell
viability on MCL
cancer cells: Jeko, Mino, Rec-1 and JMPl.
[0021] FIGS. 14A - 14C are graphical representations of the high through
put screening
assessment of cell viability in 16 DLBCL (FIGS. 14 A and B) and 8 MCL (FIG.
14C) cancer cell
lines using cannabidiol, which was obtained as CBD in a coconut oil extract.
[0022] FIGS. 15A ¨ 15D show the expression of CBI and CB2 RNA in MCL and DLBCL
cancer cell lines treated with VYR206 and CBD using immunoblot analysis. FIG.
15A panel
shows expression of CBI with treatment with VYR206. FIG. 15B panel shows
expression of CB2
4
CA 03070538 2020-01-17
WO 2019/018536 PCT/US2018/042707
with treatment with VYR206. FIG. 15C panel shows expression of CB 2 with
treatment with CBD.
FIG. 15D panel shows expression of 3-actin as the control for sample loading.
[0023] FIGS. 16A ¨ 16B are graphical representations of the efficacy of a
cannabidiol (CBD-
99) on MCL and DLBCL cell lines' viability. FIG. 16A is a graphical
representation of the efficacy
of CBD on MCL cell lines. FIG. 16B is a graphical representation of the
efficacy of CBD on
DLBCL cell lines.
[0024] FIG. 17 is a graphical representation of IC50 of rimonabant
(identified as Rimo) versus
Bruton' s tyrosine kinase inhibitor ¨ ibrutinib (IBN) on various DLBCL cell
lines.
[0025] FIGS. 18A ¨ 18D are graphical representations of the effect of the
cannabinoid receptor
ligand ¨ rimonabant (identified as Rimo) and a Bruton' s tyrosine kinase
inhibitor ¨ ibrutinib
(identified as IBN) on the viability of MCL cell lines (Jeko, Jeko-R, Mino,
Mino-R, PF-4 and PF-
5) cell lines' viability. FIG. 18A is a graphical representation of the effect
of 25 [I,M of rimonabant
and ibrutinib on Jeko and Jeko-R cell lines. FIG. 18B is a graphical
representation of the effect of
25 i.t.M of rimonabant and ibrutinib on Mino and Mino-R. FIG. 18C is a
graphical representation
of efficacy of ibrutinib on PF-4 and PF-5 cell lines. FIG. 18D is a graphical
representation of the
effect of 25 i.t.M of rimonabant and ibrutinib on PF-4 and PF-5.
[0026] FIGS. 19A ¨ 19H are graphical representations of the efficacy of
rimonabant and its
cyclam conjugated form, VYR206 on the viability of DLCBL cell lines (LR, MS,
RC and DOHH2)
and MCL cell lines (Jeko, Rec-1, Mino and JMP-1). FIG. 19A is a graphical
representation of the
efficacy of rimonabant and VYR206 on the viability of LR cell line with cyclam
as a control. FIG.
19B is a graphical representation of the efficacy of rimonabant and VYR206 on
the viability of
MS cell line with cyclam as a control. FIG. 19C is a graphical representation
of the efficacy of
rimonabant and VYR206 on the viability of RC cell line with cyclam as a
control. FIG. 19D is a
graphical representation of the efficacy of rimonabant and VYR206 on the
viability of DOHH2
cell line with cyclam as a control. FIG. 19E is a graphical representation of
the efficacy of
rimonabant and VYR206 on the viability of Jeko cell line with cyclam as a
control. FIG. 19F is a
graphical representation of the efficacy of rimonabant and VYR206 on the
viability of Rec-1 cell
line with cyclam as a control. FIG. 19G is a graphical representation of the
efficacy of rimonabant
and VYR206 on the viability of Mino cell line with cyclam as a control. FIG.
19H is a graphical
representation of the efficacy of rimonabant and VYR206 on the viability of
JMP-1 cell line with
cyclam as a control.
CA 03070538 2020-01-17
WO 2019/018536 PCT/US2018/042707
[0027] FIGS. 20A ¨ 20D are graphical representations of the effect of a
cannabidiol (CBD-99)
on the viability of DLBCL (RC) and MCL (Mino) cell lines, individually and in
combination with
four chemotherapeutics: Bruton's tyrosine kinase inhibitors ¨ ibrutinib (IBN)
and zanubrutinib
(BGB); proteasome inhibitor - carfilzomib (CFZ); and chemotherapeutic agent ¨
tumorex (TMX).
FIG. 20A is a graphical representation of the effect of 25 [I,M of CBD-99 and
IBN, individually
and in combination, on the viability of Mino and RC cells. FIG. 20B is a
graphical representation
of the effect of 6 [I,M of CBD-99 and 10 nM of CFZ, individually and in
combination, on the
viability of Mino and RC cells. FIG. 20C is a graphical representation of the
effect of 25 [I,M of
CBD and BGB, individually and in combination, on the viability of Mino and RC
cells. FIG. 20D
is a graphical representation of the effect of 12.5 [I,M of CBD and 250 nM of
TMX, individually
and in combination, on the viability of Mino and RC cells.
[0028] FIGS. 21A ¨ 21E show the expression of cleaved PARP (cPARP) and cleaved
caspase-
3 expression with increasing treatment of cannabidiol and VYR206 to
demonstrate activation of
apoptosis using immunoblot analysis. FIG. 21A panel shows expression of cPARP
with increasing
levels of cannabidiol and VYR206. FIG. 21B panel shows expression of 3-actin
as the control for
sample loading. FIG. 21C panel shows expression of cPARP with increasing
concentration of
cannabidiol. FIG. 21D panel shows expression of cleaved caspase-3 with
increasing concentration
of cannabidiol. FIG. 21E panel shows expression of 3-actin as the control for
sample loading.
[0029] FIGS. 22A ¨ 22B are graphical representations of the efficacy of
cannabidiol, VYR206
(identified as N4-Rimo) and the combination of cannabidiol and VYR206 on the
viability of
DLCBL cell lines. FIG. 22A is a graphical representations of the efficacy of
cannabidiol,
VYR206, and the combination of cannabidiol and VYR206 on the viability of
DLCBL cell line -
LY19. FIG. 22B is graphical representations of the efficacy of cannabidiol,
VYR206 (identified
as N4-Rimo) and the combination of cannabidiol and VYR206 on the viability of
DLCBL cell line
- LR.
[0030] FIG. 23 is a heat map of the changes in gene expression in RC cell
line following
treatment with rimonabant.
6
CA 03070538 2020-01-17
WO 2019/018536 PCT/US2018/042707
Detailed Description
[0031] In the following description, numerous specific details are set
forth in order to provide
a thorough understanding of the various embodiments. In other instances, well-
known processes
and methods may not be described in particular detail in order not to
unnecessarily obscure the
embodiments described here. Additionally, illustrations of embodiments here
may omit certain
features or details in order to not obscure the embodiments described here.
[0032] In the following, reference is made to the accompanying drawings
that form a part of
the specification. Other embodiments may be utilized, and logical changes may
be made without
departing from the scope of the disclosure. Therefore, the following detailed
description is not to
be taken in a limiting sense.
[0033] The description may use the phrases "in some embodiments," "in
various
embodiments," "in certain embodiment," or "in embodiments," which may each
refer to one or
more of the same or different embodiments. Furthermore, the terms
"comprising," "including,"
"having," and the like, as used with respect to embodiments of the present
disclosure, are
synonymous.
[0034] Embodiments include theranostics compositions containing label-
chelator-medical
cannabinoid analog conjugates. The labels can be radionuclides that are used
to label a medical
cannabinoid analog through a chelator. Certain embodiments include cyclam (N4)
as the chelator.
[0035] As used herein, the term "theranostic" refers to agents or
applications that can function
in both diagnostic and therapeutic modalities.
[0036] As used herein, the term "chelators" refer to compounds that form
coordination
complexes upon binding with metal ions or other substrates. The structure of
chelating ligands and
the metals that are chelated to them may be varied depending on the desired
use. Many ligands
that bind to radionuclide metals are tetradentate and contain a combination of
four nitrogen and/
or sulfur metal coordinating atoms (i.e. N4, N3S, NZS2 and the like). Example
of chelators that
can be used here includes cyclam compounds (N4), diethylentriamine pentaacetic
acid (DTPA),
tetraaZacyclododecane-N,N',N", N"'-tetraacetic acid (DOTA),
ethylenediaminetetraacetic acid
(EDTA); dimercaptosuccinic acid (DMSA), sulfur colloid, and N2S2 systems such
as MAMA
(monoamidemonoaminedithiols), DADS (NZS diaminedithiols), CODADS and the like.
These
chelator systems and a variety of others are described in Liu and Edwards,
Chem Rev. 1999, 99
(9), 2235-2268; N252 is also described in US. Pat. Nos. 4,897,225; 5,164,176;
or 5,120,526.
7
CA 03070538 2020-01-17
WO 2019/018536 PCT/US2018/042707
Method for synthesis of certain N4 compounds is described in U.S. Pat. No.
5,880,281 but they
can also be obtained from commercial sources such as Sigma Aldrich Chemical
(Milwaukee, Wis.)
and, TCI America (Portland, OR). Certain N4 compounds which can be used as
chelators may
include but not limited to, 1,4,7,10-tetraazacyclododecane (cyclen), 1,4,7,10-
tetraazacyclotridecane (Cyclam 13), 1,4,7,11-tetraazacyclotetradecane
(Isocyclam), 1,5,9,13-
tetraazacyclohexadecane, 1,5,9,13-tetraazacycloheptaadecane, 1,5,9,14-
tetraazacyclooctadecane,
1,5,10,14-tetraazacyclooctadecane, 1,5,10,15-tetraazacyclodononadecane, are
described in U.S.
Pat. No. 8,758,723, US 2012/0276005, 6,093,382; 5,608,110; 5,665,329; 5,688,
487.
PCT/GB2005/002807. Other examples of chelator moieties includes but not
limited to,
tetraazacyclododec ane-N,N',N" ,N"
acid, monoamide (DOTA-MA); 10-(2-
hydroxypropy1)-1,4,7, 10-tetraa7acy clododecane-1,4,7-triacetic acid (HP-
DO3A). N4 is
conjugated to a medical cannabinoid compound and further chelated to a metal.
N4 has a closed-
ring structure that helps stabilize the radionuclides. Chelators with higher
lipophilicity, such as N4,
also confer decreased renal and hepatic toxicity because they have shown
decreased accumulation
in these organs, resulting from greater uptake by the targeted cells.
Conjugation of DOTA to highly
selective CI32 receptor inverse agonist 5R144528 following by chelation of
Gallium (Ga),
Technetium (Tc), Copper (Cu) or with lanthanide series such as Gadolinium
(Gd), Europium (Er),
Terbium (Tb) is described in U.S. Patent No. 8,367,714. Imaging C131 receptor
using various
radiotracers is described in PCT/US2009/043491. Radioligands with high
affinity and selectivity
for CBi receptors such as 3,4-diarylpyrazoline derivatives were labeled a
radioisotope selected
from the group consisting of 2H,14C,13N, 18F, 75 Br, 76Br,123I for imaging
with PET or SPECT. U.S.
Patent Nos. U520050070596, 8,840,865, 9,617,215, 8,323,621 and W02007130361
describe
imaging of cannabinoid system for medical and therapeutic purposed to treat
for instance
inflammatory diseases, cancer, neurological disorders therapeutics and medical
imaging.
[0037]
As used herein, the term "label" refers to an atom, a molecule, or a compound
that is
used to identify the location of the composition to which the label is
attached. Labels can have one
or more of fluorescent, phosphorescent, luminescent, electroluminescent,
chemiluminescent or
other spectroscopic properties. These properties enable the detection and
identification of the label-
chelator-medical cannabinoid analog conjugates using any technique capable of
detecting and
identifying the label, including visible light, ultraviolet and infrared
spectroscopy, Raman
8
CA 03070538 2020-01-17
WO 2019/018536 PCT/US2018/042707
spectroscopy, nuclear magnetic resonance, positron emission tomography, and
other methods
known in the art.
[0038] As used herein, the term "imaging" refers to all tissue
visualization processes using
electromagnetic wave technologies for which the instant compositions can be
used, including but
not limited to cells of the nervous system, blood cells, cancerous cells, and
precancerous cells.
Provided here are kits for imaging neurologic cells. In an embodiment, the kit
contains a
predetermined quantity of a conjugate of a chelator and a medical cannabinoid
analog; and a
predetermined quantity of an imaging agent. The conjugate of the chelator and
the medical
cannabinoid analog can be present in the kit as precursors that subsequently
interact with the
imaging agent, when provided with suitable reaction conditions. The kit can
also include a tin-
containing reducing agent. Also provided here are kits for genomic or other
omic assays that
contain a predetermined quantity of a conjugate of a chelator and a medical
cannabinoid analog;
and a predetermined quantity of an imaging agent.
[0039] As used herein, the terms "radionuclide," "radioactive nuclide,"
"radioisotope," or
"radioactive isotope" are synonymous. One or more different radioisotopes can
be used as labels.
The non-limiting examples of radionuclides include 99mTc, 117msn, 177Lu,
188Re, 186Re, 153sm,
166H0, 90y, 89sr, 67Ga, 68Ga, 1111n, 183Gd, 59Fe, 225Ac, 212Bi, 211At, 451i,
60cu, 61cu, 67L,--lu,
64Cu and
62Cu. In other aspects, the metal ion is a non-radioactive metal such as
187Re, 69Ga, 193Pt.
[0040] As used herein, the term "cannabinoid analog" refers to a compound
capable of either
interacting with cannabinoid receptors in a subject or sharing chemical
similarity with
cannabinoids or both. Cannabinoid analogs include synthetic or natural
cannabinoid compounds
that can function as agonists or antagonists. Embodiments of cannabinoid
analogs include, but are
not limited to, cannabidiol (CBD), cannabidiolic acid (CBDA), cannabidiol
monomethylester
(CBDM), cannabidiol-C4 (CBD-C4), cannabidivarinic acid (CBDA), cannabidivarin
(CBV),
cannabidiorcol (CBD-C1), tetrahydrocannabinol (THC), N-
arachidonoylethanolamine (AEA) or
anandamide, 2-arachidonoylglycerol (2-AG), rimonabant, AM6538, taranabant,
otenabant,
cannabigerolic acid monomethylether (CB GAM), cannabigerol monomethylether (CB
GM),
cannabigerovarin (CBGV), cannabigerovarinic acid (CBGVA), cannabichromenic
acid (CBCA),
cannabichromene (CBC), cannabichromevarinic acid CBCVA), cannabichromevarin
(CBCV), A9-
tetrahydrocannabinolic acid A (THCA-A), A9-tetrahydrocannabinolic acid B (THCA-
B), A9-
9
CA 03070538 2020-01-17
WO 2019/018536 PCT/US2018/042707
tetrahydrocannabidiol (THC), A9-tetrahydrocannabinolic acid-C4 (THC-C4), A9-
tetrahydrocannabivarinic acid (THCVA), A9-tetrahydrocannabivarin (THCV), A9-
tetrahydrocannabiorcolic acid (THCA-C1), A9-tetrahydrocannabiorcol (THC-C1),
A7-cis-
isotetrahydrocannabivarin (THCV), A8-tetrahydrocannabinolic acid (A8-THCA),
A8_
tetrahydrocannabidiol (A8-THC), cannabicyclolic acid (CBLA), cannabicyclol
(CBL),
cannabicyclovarin (CBLV), cannabielsoic acid A (CBEA-A), cannabielsoic acid B
(CBEA-A),
cannabielsoin (CBEA-A), cannabinolic acid (CBNA), cannabinol (CBN), cannabinol
methylether
(CBNM), cannabinol-C4 (CBN-C4), cannabinol-C2 (CBN-C2), cannabiorcol (CBN-C1),
cannabinodiol (CBND), cannabinodivarin (CBVD), cannabitriol (CBT), 10-ethoxy-9-
hydroxy-
A6a-tetrahydrocannabinol, 8,9-Dihydroxy-A6a-tetrahydrocannabino1,
cannabitriolvarin (CBTV),
and ethoxy-cannabitriolvarin (CBTVE).
[0041] This disclosure provides for the generation and use of novel agents
for precision
medicine. Embodiments include compositions that provide a theranostics
approach targeting the
endocannabinoid system (ECS) implicated in the pathogenesis of neurologic
disorders and cancer.
This individualized medicine platform allows to deliver personalized medicine
designed on the
basis of individual genetic make-up, biochemistry, molecular imaging,
molecular blueprint, and
clinical observations and measurements associated to each patient's disease.
[0042] Embodiments described here include novel compositions and methods of
using these
compositions for imaging or treatment of diseased cells. Certain embodiments
include
compositions containing a cannabinoid analog and a chemotherapeutic agent.
Certain
embodiments include compositions containing a chemotherapeutic agent and a
cannabinoid analog
conjugated to a chelator. In certain embodiments, the chelator is cyclam. In
certain embodiments,
the cannabinoid analog is a cannabidiol. Certain embodiments include
compositions containing a
combination of a chemotherapeutic agent and a cannabinoid analog conjugated to
a chelator and a
label. In certain embodiments, the chemotherapeutic agent is Bruton' s
tyrosine kinase inhibitors,
such as ibrutinib (IBN) and zanubrutinib (B GB). In certain embodiments, the
chemotherapeutic
agent is a proteasome inhibitor, such as carfilzomib (CFZ). In certain
embodiments, the
chemotherapeutic agent is tumorex (TMX).
[0043] Embodiments described here include novel compositions and methods of
using these
compositions for imaging diseased cells. These methods can be applied in the
diagnosis,
assessment, and treatment of any medical disorder with pharmaceuticals,
medical cannabinoids,
CA 03070538 2020-01-17
WO 2019/018536 PCT/US2018/042707
and combinations thereof. The diseases may include various forms of neurologic
disorders and
cancer. In particular, cancer can include one or more carcinoid,
neuroendocrine cancer, breast
cancer, lung cancer, prostate cancer, ovarian cancer, brain cancer, liver
cancer, cervical cancer,
colon cancer, renal cancer, skin cancer, head and neck cancer, bone cancer,
esophageal cancer,
bladder cancer, uterine cancer, lymphatic cancer, stomach cancer, pancreatic
cancer, testicular
cancer, colorectal cancer, and cancers of hematopoietic origin such as
lymphoma, or leukemia.
The neurologic diseases can include Alzheimer's disease (AD), Parkinson's
disease (PD),
Huntington's disease (HD), amyotrophic lateral sclerosis (ALS), multiple
sclerosis, posttraumatic
stress disorder (PTSD), epilepsy, seizures, Tourette's syndrome,
schizophrenia, anxiety disorders,
autism, depression, dementia, and other diseases and disorders that implicate
the nervous system.
[0044] These methods have benefits to enhance existing imaging modalities
that may include
improvement in sensitivity and specificity, improvement of patient
convenience, and reduction of
adverse effects, time and costs. These methods for imaging diseased cells or a
group of cells at a
site of a diseased tissue or organ of a subject will enable health
professionals to diagnose a subject,
especially when that subject is being treated or under treatment with medical
cannabinoids. Certain
embodiments include methods of imaging at the site of a disease in a given
subject to perform a
pre- or post- treatment evaluation and to be able to monitor that subject for
as long as that subject
is being treated or under treatment with medical cannabinoids. In certain
aspects, the method
comprises detecting a signal generated by an imaging agent-labeled chelator-
medical cannabinoid
analog composition at the site of the disease of a subject, where the diseased
cells, if present,
generate a more intense signal than the cells in the surrounding tissue.
[0045] As used herein, the term "subject" refers to all kinds of animals
including humans,
rodents, other mammals, or avian species. The administration of the imaging
agent-labeled
chelator-medical cannabinoid analog conjugates can serve as a diagnostic
agent, a prognostic
agent, or an agent to alleviate or treat a disease in a subject. The target
site can be any tissue of the
subject, including but not limited to the brain, heart, lung, esophagus,
intestine, breast, uterus,
ovary, prostate, testis, stomach, bladder, or liver. Also, embodiments
provided herein can be used
as agents to target diseases, such as cancer or neurologic, gastrointestinal,
metabolic, and
neuroendocrine disorders. As used herein, the term "administration" refers to
an activity of
introducing a composition described herein to a subject by an appropriate
method, and the
composition may be administered via various routes of intravenous, oral,
intramuscular,
11
CA 03070538 2020-01-17
WO 2019/018536 PCT/US2018/042707
transdermal, intra-peritoneal, topical, sublingual, buccal, inhalation, nasal,
or ophthalmic routes as
long as they can deliver the same to the target tissues. The compositions
described herein can be
delivered as pharmaceutical formulation. A 'pharmaceutical formulation" refers
to a mixture of
one or more of the compounds described herein, or a pharmaceutically
acceptable derivative as
an active ingredient, and at least one pharmaceutically acceptable carrier or
excipient. The
purpose of a pharmaceutical composition is to facilitate administration of a
compound to a
subject. In another aspect, a pharmaceutical composition can contain a
compound of one of the
formulae described herein, or a pharmaceutically acceptable derivative, and a
pharmaceutically
acceptable carrier or excipient. In some embodiments, the pharmaceutical
composition includes
two or more pharmaceutically acceptable salts, acids, esters, excipients,
carriers, diluents, and
combinations thereof.
[0046] The term 'pharmaceutically acceptable derivative" as used herein
refers to and
includes any pharmaceutically acceptable salt, pro-drug, metabolite, ester,
ether, hydrate,
polymorph, solvate, complex, and adduct of a compound described herein which,
upon
administration to a subject, is capable of providing (directly or indirectly)
the active ingredient.
For example, the term "a pharmaceutically acceptable derivative" of compounds
described
herein includes all derivatives of the compounds described herein (such as
salts, pro-drugs,
metabolites, esters, ethers, hydrates, polymorphs, solvates, complexes, and
adducts) which, upon
administration to a subject, are capable of providing (directly or indirectly)
the compounds
described herein.
[0047] As used herein, the term 'pharmaceutically acceptable salt" refers
to those salts, which
retain the biological effectiveness and properties of the parent compound. And
unless otherwise
indicated, a pharmaceutically acceptable salt includes salts of acidic or
basic groups, which
may be present in the compounds of the formulae disclosed herein. The present
disclosure also
provides certain processes, as examples, for the preparation of the above
pharmaceutically
acceptable salts, their derivatives, their analogs, their tautomeric forms,
their stereoisomers,
their polymorphs, and pharmaceutical compositions containing them.
[0048] Certain embodiments relate to pharmaceutically acceptable salts
formed by the
compounds described herein, their derivatives, their analogs, their tautomeric
forms, their
stereoisomers, their polymorphs and pharmaceutically acceptable compositions
containing
them. Typical inorganic acids used to form such salts include hydrochloric,
hydrobromic,
12
CA 03070538 2020-01-17
WO 2019/018536 PCT/US2018/042707
hydroiodic, nitric, sulfuric, phosphoric, hypophosphoric, and the like. Salts
derived from
organic acids, such as aliphatic mono and dicarboxylic acids,
phenylsubstituted alkanoic acids,
hydroxyalkanoic and hydroxyalkandioic acids, aromatic acids, aliphatic and
aromatic sulfonic
acids, may also be used. Such pharmaceutically acceptable salts thus include
acetate,
phenylacetate, trifluoroacetate, acrylate, ascorbate, benzoate,
chlorobenzoate, dinitrobenzoate,
hydroxybenzoate, methoxybenzoate, methylbenzoate, o-acetoxybenzoate,
naphthalene-2-
benzoate, bromide, isobutyrate, phenylbutyrate, beta-hydroxybutyrate,
chloride, cinnamate,
citrate, formate, fumarate, glycolate, heptanoate, lactate, maleate,
hydroxymaleate, malonate,
mesylate, nitrate, oxalate, phthalate, phosphate, monohydro genphosphate,
dihydrogenphosphate, metaphosphate, pyrophosphate, propionate,
phenylpropionate, salicylate,
succinate, sulfate, bisulfate, pyrosulfate, sulfite, bisulfite, sulfonate,
benzenesulfonate, p-
bromophenylsulfonate, chlorobenzenesulfonate, ethanesulfonate, 2-
hydroxyethanesulfonate,
methanesulfonate, naphthalene-1- sulfonate,
naphthalene-2- sulfonate, p-toluenesulfonate,
xylenesulfonate, tartarate, and the like.
[0049]
Certain embodiments include novel methods to synthesize agents that are
conjugates of
a chelator and a targeting ligand. Such agents may be used for imaging,
diagnostic and/or
therapeutic purposes. Accordingly, an embodiment includes a method of
synthesizing a chelator-
targeting ligand conjugate containing N4 and medical cannabinoid analogs. The
method can
further include synthesis with a metal in the form of an organometallic or a
photon source. In some
aspects, the metal ion is a radionuclide as described here.
[0050]
Embodiments also include kits to prepare an imaging probe, a diagnostic agent,
or a
pharmaceutical composition. Certain specific embodiments include methods of
imaging,
diagnosing, or delivering a pharmaceutical composition to treat physiological
disorders with
medical cannabinoids. Accordingly, in an embodiment, any imaging modality can
be used to detect
signals from the one or more labels. Non-limiting examples of imaging methods
used to detect the
signals from the labels include PET, PET/CT, CT, SPECT, SPECT/CT, MRI, near-
infrared (NIR),
optical imaging, optoacoustic imaging, and ultrasound. Methods described here
can be used to
assess the personalized and efficacious dose and dosing regimens based on
accurate evaluations
determined through molecular imaging using medical cannabinoids.
[0051]
Embodiments of the label-chelator-medical cannabinoid analog conjugate can
include
phytocannabinoids that are naturally occurring plant-derived cannabinoids. The
active components
13
CA 03070538 2020-01-17
WO 2019/018536 PCT/US2018/042707
in the medical marijuana strains Cannabis sativa and Cannabis indica are
medical cannabinoids,
which include more than 60 analog types, and two additional classes of
bioactive molecules known
as flavonoids and terpenoids, which are all naturally occurring compounds that
are extracted and
isolated from plants. Embodiments of the compositions that function as
theranostics agents also
include label-chelator-terpenoid analog conjugates, label-chelator-flavonoid
conjugates, and label-
chelator-phytosterol conjugates. Among the natural medical phytocannabinoids,
A9-
tetrahydrocannabinol (A9-THC), cannabidiol (CBD), and cannabinol (CBN) are the
most
abundant, yet other phytocannabinoids can play a very important role in
precision medicine. Based
on structure, binding properties and signaling/function features, medical
cannabinoids are grouped
into distinct classes: (i) the classical medical cannabinoids, which consist
of both natural plant
extracts such as A9-THC and chemically synthesized compounds such as Marinol;
(ii) Nonclassical
medical cannabinoids, mainly exemplified by the synthetic cannabinoid receptor
(CB) agonist CP-
55,940; (iii) Aminoalkylindoles, which consist of chemically produced
cannabinoids like
AM1241; (iv) Diarylopyrazoles, mainly comprise CB inverse agonists (or
antagonists) such as
SR141716A, also known as rimonabant; and (v) endogenous endocannabinoids,
which are
naturally produced by animal and human cells and include N-
arachidonoylethanolamine, (AEA)
or anandamide, 2-arachidonoylglycerol (2-AG), noladin ether, virodhamine and N-
arachidonylodop amine (NADA).
[0052] Embodiments of the label-chelator-medical cannabinoid analog
conjugate can include
the medical cannabinoids that have been approved for clinical research
worldwide, as dronabinol,
nabilone, nabiximols, cannador, cannabidiol, cannabinol, cannabigerol,
tetrahydrocannabivarin,
and cannabichromene. Embodiments of the label-chelator-medical cannabinoid
analog conjugate
can include medical cannabinoids such as HU-210, A9-THC, A8-THC and desacetyl-
L-nantradol,
which are recognized as CB i/CB2 receptor agonists, without distinctive
specificity for either
receptor. A9-THC stands out as a C. sativa cannabinoid, which exhibits CB
i/CB2 affinity and the
highest psychotropic effects. By a pentyl substitution on A8-THC side chain,
conversion into the
HU-210 analog occurs, with increased receptor affinity. Other structural
modifications of the THC
backbone lead to new and selective CB2 agonists JWH-133, JWH-139, and HU-308
and L-759633
and L-759656, which display affinities at the nanomolar range.
[0053] Embodiments of the label-chelator-medical cannabinoid analog
conjugate can include
non-classical medical cannabinoids, which are a family of bicyclic (AC) and
tricyclic ACD
14
CA 03070538 2020-01-17
WO 2019/018536 PCT/US2018/042707
medical cannabinoids. They are prominently represented by CP55940, along with
CP55244 and
CP47497 analogs. Of note, CP55940 is the best-known medical cannabinoid
agonist, which
displays a potent in vivo effect via shared CBI and CB2 signaling.
[0054] Embodiments of the label-chelator-medical cannabinoid analog
conjugate can include
aminoalkylindoles. R-(+)-WIN55212 is the prototype of this family with medical
cannabinoid-like
features, which can bind both CBI and CB2 receptors but exhibits higher
specificity for CB2 and
can mimic in vivo THC-mediated effects. Other analogs like JWH-015 and L-
768242 also show
similar CB2 affinity as R-(+)-WIN55212.
[0055] Embodiments of the label-chelator-medical cannabinoid analog
conjugate can include
medical diarylpyrazoles, which are a class of medical cannabinoid analogs
whose distinctive
function is to inhibit CB 1 or CB2-dependent intracellular signaling pathways,
acting as antagonists
(inverse agonist); for example, embodiments can include SR141716A, AM251 and
AM281 that
inhibit CBI receptor mediated effects.
[0056] Embodiments of the label-chelator-medical cannabinoid analog
conjugate can include
certain endocannabinoids. N-arachidonoylethanolamine (AEA), or Anandamide, is
an example of
an eicosanoid that is converted into its active form, via the omega-3 (w-3)
and omega-6 (w-6)
biosynthetic fatty acid pathways, and specifically targets CB receptors in
mammals. Consequently
other eicosanoids that can function in these embodiments include
methanandamide (R and S
isomers), arachidony1-2-chloroethylamide (ACEA), arachidonylcyclopropylamide
(ACPA) and 2-
arachidonoylglycerol (2-AG), which exhibit binding affinity to CBI and CB2.
[0057] Embodiments of the label-chelator-medical cannabinoid analog
conjugate can be used
as theranostic agents targeting serious pathologies, such as cardiovascular,
neurological,
psychiatric, immunological, endocrine and neoplastic disorders. For instance,
specific
formulations of cannabinoid compounds are able to reduce proliferation and
induce tumor cell
death. Moreover, the mechanisms behind the anti-proliferative cell-killing
effects (up to 70%) are
known to result from intracellular signals driven by the binding of the
cannabinoid agents to
specific GPCRs, CB 1 and CB2 of the ECS.
[0058] The ECS facilitates rapid local response to pathologic states or
diseases. For example,
when signals resulting from increased activation of neuronal Ca2+ channels or
cellular stress
cascades are transduced, immediate biosynthetic conversion of membrane
phospholipids ensues,
which leads to production and secretion of anandamide. Then, the
endocannabinoid transmitters
CA 03070538 2020-01-17
WO 2019/018536 PCT/US2018/042707
bind and activate target CB receptors, which in turn modulate adenylyl cyclase
catalytic function
to homeostasis and reduce cyclic adenosine monophosphate (cAMP) levels and
protein kinase A
activity, thus achieving equilibrium of Ca2+ fluxes and simultaneously
correcting potassium (I( )
channels. Upon completion of their regulatory function, Anandamide and or 2-AG
undergo
physiological degradation via the Fatty Acid Acyl Hydrolase (FAAH) activity or
analog enzymatic
pathways. Notably, the ECS also has the capacity to regulate the levels of its
target CB 1 or CB2
receptors, particularly in response to cell stress signaling. This function
could be interpreted as a
positive feedback mechanism to modulate the degree of signal transmission in
certain pathologic
states such as neuropathic pain and multiple sclerosis. The ECS system works
conversely in
disorders, such liver fibrosis or colorectal carcinoma. In the former, CBI
levels are upregulated
and thus signaling could result in deleterious progression toward cirrhosis of
the liver, whereas in
the latter, the low CB 1 expression will impinge upon analgesic benefits. On
the other hand, endo
and exo medical cannabinoids crosstalk with the opioid, serotonin, and N-
methyl-d-aspartate
(NMDA) nociceptive (pain) circuitry networks.
[0059] Embodiments of the label-chelator-medical cannabinoid analog
conjugate can be used
to evaluate the impact of medical cannabinoids in health and disease and to
determine the early
success or failure for specific applications. The label can be used to monitor
the effect of the
specific medical cannabinoid analog in the management of chronic pain. This
can be achieved by
imaging the medullary periaqueductal grey and rostral ventromedial areas to
monitor analgesic
effects. Additionally, one can take advantage of specific medical cannabinoid
analogs targeting
CB2 that are selectively expressed by sensory neurons at the dorsal root
ganglion, the spinal cord,
and brain regions, to study and monitor the mechanisms of medical cannabinoid-
driven analgesia.
[0060] Embodiments of the label-chelator-medical cannabinoid analog
conjugate can be used
to dissect and better understand the ECS and other pathways that modulate
parallel functions aimed
at protecting and maintaining a subject's homeostasis. CB 1 is mainly
expressed in central and
peripheral nervous system but discrete distribution has been observed in other
tissues. Thus,
utilization of the label-chelator-medical cannabinoid analog conjugates
directed to CBI signaling
can result in broad and pleiotropic actions. Although the CB 1 receptor has
been known to primarily
regulate functions associated to cognition, memory, perception, mood, behavior
and psychotropic
activities, there is increasing evidence that it can play a role in analgesia;
cardiovascular,
respiratory, and reproductive functions; as well in the maintenance of overall
homeostasis. CB2
16
CA 03070538 2020-01-17
WO 2019/018536 PCT/US2018/042707
receptors are predominantly expressed in immune competent organs where cells
undergo antigen-
dependent maturation and selection programs, which empowers them to survey and
mount
powerful responses against pathogens and aberrantly developed cells. CB2
exhibits a relatively
limited distribution in the central nervous system (CNS). CB2-dependent
activation involves
regulatory mechanisms that support immune cell migration to the site of
inflammation and the
release cytokines. The expression of CB2 is particularly important for CNS
microglia, as
demonstrated by the capacity of medical cannabinoid agents to reduce cytokine-
mediated neuro-
inflammation. Embodiments of the label-chelator-medical cannabinoid analog
conjugate that
include specific CB2 ligands, such as 0-3223 (a synthetic CB2 specific
agonist), can be used for
anti-inflammatory and anti-nociceptive applications, without apparent CB i-
like mediated effects.
[0061] This disclosure also provides for innovative radiopharmaceutical
imaging analogs that
can be used to diagnose, stage, restage and treat central nervous,
cardiovascular and cancer
diseases. Imaging with radiolabeled physiological agents can be implemented
using nuclear
molecular agents, which provide accurate measurements of molecular pathways,
by integrating
real-time nuclear imaging protocols. This approach is designed to enhance
clinical capability for
patient selection, improve pharmacokinetic assessments, optimize dosage
formulations and predict
therapy outcomes. The overall impact of the comprehensive application of
theranostics
(diagnosis/therapy/prognosis) technology to medical cannabis platforms, stems
from the evolving
versatility of nuclear imaging analogues and the emerging competence of hybrid
instrumentations,
which will provide precision, sensitivity, specificity and real-time data
tailored to individual
patient's diagnosis and therapy.
[0062] Compositions containing the label-chelator-terpenoid conjugates can
be used to study,
monitor, and provide the potent antioxidant, anti-inflammatory, analgesic,
anticancer, antibiotic
and anti-psychiatric (anxiety and depression) benefits of terpenoid compounds.
[0063] Compositions containing the label-chelator-flavonoid conjugates can
be used to study,
monitor, and provide the antioxidant, anti-inflammatory and anticancer
properties of flavonoid
compounds, including polyphenol cannabis flavonoids. The cannabis flavonoids
can provide
significant cardiovascular protection, particularly improving coronary and
peripheral circulation
by maintenance of homeostatic blood pressure, prevention of the formation of
blood clots, and
reduction of atherosclerosis risks. Mechanisms of cannabis flavonoids mediated
antioxidative and
anti-inflammatory effects include apigenin (41,5,7-trihydroxyflavone)-
dependent inhibition of
17
CA 03070538 2020-01-17
WO 2019/018536 PCT/US2018/042707
TNF-a, which has also shown to exhibit therapeutic benefits in multiple
sclerosis and rheumatoid
arthritis.
[0064] Compositions containing the label-chelator-phytosterol conjugates
can be used to study,
monitor, and provide the cardiovascular protection, anti-inflammation, and
anti-systemic edema
properties of phytosterol compounds, including medical cannabis phytosterols.
[0065] Embodiments of the label-chelator-medical cannabinoid conjugate can
utilize tetra-
azacyclic (N4) conjugation-based technology, also known as N4-technology,
which can also be
adapted for serial multi-imaging/therapy of a subject. Here, the hydrophobic
chelator(s)-based N4-
technology coordinates metals to form a complex that may be conjugated to
hydrophobic/hydrophilic molecules, including medical cannabinoid analogs to
produce novel
compounds or to better understand the bio-distribution scope and targeting
capabilities of exiting
compounds. N4 conjugation of novel or existing compounds can be used for
purposes including
imaging, cold therapy, and radiotherapy that targets specific cell receptors,
pathways, tissues or
organs, using medical cannabinoids. Moreover, certain N4 compounds may be
obtained from other
commercial sources, which are noted in U.S. Pat. No. 8,758,723. In addition,
metal isotopes are
either obtained from generators such as 99mTc, 188Re and 68Ga, or from
commercial sources such
as 64Cu and 111In, which are available and cost-effective. N4-conjugates
provide a simple platform
for small molecules, peptides, or antibodies with high purity and efficient
formulation to target
components of the endocannabinoid system. Moreover, radiolabeled N4-conjugates
provide
molecular target assessment in the areas of abnormal cell cycle and epigenetic
microenvironments.
Similarly, N-4 theranostic applications offer post-treatment evaluation and
can be tailored for
internal radio-therapeutics, when the conjugate is labeled with 188Re or 177Lu
(beta and gamma
emitters) or 225Ac, 223Ra (alpha emitters) for simultaneous SEE and TREAT
approaches.
Moreover, the N4 technology provides the flexibility to prepare conjugates
with or without cold
platinum metals, thus enabling the N4 chemistry to increase the cold payload
of medical
cannabinoids to optimal concentrations for cellular and immunotherapy as
theranostics agents. The
radiolabeled N4-conjugates are focused on the imaging of immuno-modulatory
functions and the
therapy of oncological and neurological diseases, via cannabinoid
receptor/transporter-mediated
mechanisms. This medical cannabinoid precision-based technology platform
integrates diagnostic
imaging (molecular imaging) with innovative tools to understand the dynamic
changes in pathway-
activated cell receptors leading to tissue degeneration, inflammatory, and
proliferative disorders
18
CA 03070538 2020-01-17
WO 2019/018536 PCT/US2018/042707
to improve patient diagnosis, therapy and prognosis. This technology allows
for the medical
cannabinoids to be utilized as a therapeutic for treatments amenable to
cannabinoids alone or as
combinations with radiopharmaceuticals, immunopharmaceuticals, and other
compounds.
[0066] Several synthetic antagonists taranabant, otenabant, and AM6538, as
well as the inverse
agonist/antagonist rimonabant show great promises as therapeutic agents. For
example, in
targeting obesity, rimonabant showed to promote weight loss, weight
maintenance and to prevent
weight regain in patients with a BMI of greater than 27 kg/m2, when
accompanied with at least
one risk factor such as hypertension or type 2 diabetes. However, rimonabant
had increased
frequencies of psychiatric adverse events, including major depression,
dysthymia, seizures and
suicide. These adverse effects discouraged its development as an approved
therapeutic intervention
for other disease states. While the cause of the adverse events has been
speculated to result from
the inverse agonist mechanism of the CB 1 receptor activity, many factors have
not been considered
where this technology platform could resolve other medical conditions.
Likewise, agonists like the
natural medical cannabinoid THC, have demonstrated great promise for
therapeutic intervention.
For example, medical THC has demonstrated benefits with refractory nausea and
vomiting for
patients undergoing chemotherapy along with alleviating neuropathic pain and
spasticity.
However, medical THC is psychoactive, which can also lead to lethargy,
postural hypotension and
decreased motor coordination when overdosing occurs. Conversely, given to the
right patient,
disease, and dose, it is conceivable that the effects against a disease by
pathology biomarker-
guided N4-THC theranostic imaging, can actually result in favorable clinical
outcomes.
[0067] Combinations containing the label-chelator-medical cannabinoid
analog conjugates
combine imaging with the therapeutic intervention and can image in real time
the uptake and
activity of N4 conjugated medical cannabinoids, which is essential to select
the individual patient
with the targeted dysfunctional pathway (right disease) and to assess optimal
dosage (right dose).
This approach allows for visually seeing the composition located at the tissue
site and determining
the actual dose of uptake to that site for that patient. This platform allows
one to evaluate: (a) if
dosing is the cause of the adverse event; (b) if bioavailability is the cause
or (c) if there is a limited
uptake and/or bio-distribution. In keeping with these parameters, the
embodiments serve to dissect
effects that are patient dependent, particularly if one of these effects are
genetic, epigenetic, or
exhibit allelic variations associated to the individual's ECS.
19
CA 03070538 2020-01-17
WO 2019/018536 PCT/US2018/042707
[0068] The effective amount of a compound is determined based on several
factors, such as age
and weight of the patient, severity of the disease, other co-existing factors.
The effective amount
of a compound includes exemplary dosage amounts for an adult human of from
about 0.1 to 100
mg/kg of body weight of active compound per day, which may be administered in
a single dose or
in the form of individual divided doses, such as from 1 to 4 times per day. It
will be understood
that the specific dose level and frequency of dosage for any particular
subject may be varied and
will depend upon a variety of factors including the activity of the specific
compound employed,
the metabolic stability and length of action of that compound, the species,
age, body weight,
general health, sex and diet of the subject, the mode and time of
administration, rate of excretion,
drug combination, and severity of the particular condition.
[0069] Furthermore, some medical cannabinoids have not yet been explored
and this platform
lays a foundation for conjugating several medical cannabinoids for
investigating the effects in
diverse physiological disorders, through a precision medicine approach. The N4
technology can
allow for determination of the effectiveness in targeting, uptake and
distribution; wherein the
conjugates are imaged further allowing for the assessment of efficacy.
Embodiments include the
pharmaceutical formulation, kit preparation, and method of use and
administration of pathway-
directed molecular agents for non-metal/metal labeling and metallic/cold
therapeutics, when such
agents are prepared in aqueous or organic conditions. Synthesis and
purification in organic
solvents requires the use of protecting and de-protecting chemicals to achieve
desired
compositions.
[0070] Embodiments also include kits containing components to prepare an
imaging probe that
has a conjugate of a label, a chelator, and a cannabinoid analog. Embodiments
also include kits
containing components to prepare a diagnostic agent based on a conjugate of a
label, a chelator,
and a cannabinoid analog. Kits can also be constructed to contain components
to deliver effective
amounts of pharmaceutical compositions containing a conjugate of a label, a
chelator, and a
cannabinoid analog. Certain embodiments include kits for diagnostic imaging of
a site of
neurologic disorders and cancers, and delivery of a dual therapeutic
intervention agent to a subject.
The conjugate of the chelator and the cannabinoid analog can be present in the
kit as precursors
that subsequently interact with the imaging agent, when provided with suitable
reaction conditions.
For example, a kit can include one or more sealed containers contain a
predetermined quantity of
a chelator-medical cannabinoid analog conjugate and one or more sealed
containers that contain a
CA 03070538 2020-01-17
WO 2019/018536 PCT/US2018/042707
predetermined quantity of a second imaging agent that can be applied in
imaging the brain of a
subject. In some embodiments, the kit further includes a sealed container
containing a
radionuclide. In certain embodiments, a kit further includes a predetermined
quantity of a chelator-
medical cannabinoid analog conjugate and a sufficient amount of a reducing
agent to label the
conjugate compound with a radionuclide metal ion. In certain embodiments, a
kit further includes
a predetermined quantity of a chelator-medical cannabinoid analog conjugate
and a reducing agent
along with a buffer solution, wherein the reducing agent includes, but not
limited to, tin(II)
chloride. The kit may also contain other components, such as pharmaceutically
acceptable salts,
buffers, antioxidants, and preservatives. In certain embodiments, buffer
solutions are phosphate
buffer solutions to stabilize the chelator-medical cannabinoid analog
conjugates. Phosphate buffer
solutions may contain monosodium phosphate, disodium phosphate, or combination
thereof,
dissolved in water. In certain embodiments, an antioxidant is included in the
chelator-medical
cannabinoid analog conjugate composition to prevent oxidation of the chelator
moiety. In certain
embodiments, the antioxidant is vitamin C (ascorbic acid). Other antioxidants
that can be used to
include tocopherol, pyridoxine, thiamine, butylated hydroxyl toluene, sodium
edetate, or rutin.
In certain embodiments, a stabilizer is included in the chelator-medical
cannabinoid analog
conjugate composition to prevent degradation and to enhance shelf life or
storage life of the
chelator moiety. In certain embodiments, the stabilizer is mannitol. However,
other components,
such as sugars or bulking agents, may also be used, wherein the sugars are
simple sugars, complex
chain sugars, sugar alcohols or salts thereof. Stabilizers may include but are
not limited to glucose,
lactose, maltose, xylose, sorbitol, cellulose, or carboxymethylcellulose
sodium. In certain
embodiments the predetermined quantity of a chelator-medical cannabinoid
analog conjugate may
be present in dosing amounts to treat a neurologic disorder or cancer as a
therapeutic intervention.
The kit may contain the components in liquid, frozen, dry form, or lyophilized
form.
Examples
[0071]
Example 1. Synthesis of 1,4,8, 1 1 -Tetraaz ac yclotetradec ane- 1 '-prop yl-
[N-(Piperidin- 1 -
y1)-5 -(4-chloropheny1)- 1 -(2,4-dichloropheny1)-4-methyl- 1H-pyrazole-3-
carboxamidel (also
known as VYR207)
21
CA 03070538 2020-01-17
WO 2019/018536 PCT/US2018/042707
01
0 ON
(010 N-N
CI CI HN
I-NHII
HN
VYR207
[Formula I]
[0072] VYR207 can be synthesized in several ways. Shown below is Scheme 1
for the synthesis
of 1,4,8-Tris(trifluoroacety1)-1,4,8,11-tetraazacyclotetradecane (N4-TFA3;
TFA3 -c yclam) .
0 0
r.) F3CAO"
F3c1.1..õ1-^1
ENH HN-1 Me0H/NEt3 HN-1
I¨NH HN-1 _________________________________________ I¨N N¨I
RT, 5 h, 93 %
0
0
F3C
TFA3-cyclam
[Scheme 1]
[0073] Cyclam (N4;1,4,8,11-tetraazacyclotetradecane; N4; 7.53 g, 37.58
mmol) was
dissolved in 30 mL of methanol. To this clear solution, NEt3 (5.20 mL, 37.58
mmol) was added in
one portion. Then, ethyl trifluoroacetate (18.0 mL, 150.3 mmol) was added
dropwise over a period
of 5 minutes while stirring. The homogeneous reaction mixture was cooled with
ice-water bath to
control the mild exothermic reaction and stirred at 25 C under the atmosphere
of N2 for 5 h.
Volatiles were then removed under vacuum. The residue was dissolved in minimum
amount of
CH2C12 (2.0 mL) and passed through a short silica gel pad (25 g), eluted with
100% Et0Ac. The
eluent was concentrated to give the product a white semi solid form (17.1 g,
92.5%). The resulting
compound was analyzed by proton nuclear magnetic resonance (1H NMR), carbon-13
nuclear
magnetic resonance (13C NMR), and high-resolution mass spectrometry (HRMS) by
electrospray
ionization (ESI). The 1H NMR data, 13C NMR data, and the HRMS-ESI
determination of mass of
VYR207 are as follows.
22
CA 03070538 2020-01-17
WO 2019/018536 PCT/US2018/042707
[0074] The 1H NMR data (200 MHz, CDC13): 6 3.85 - 3.25 (multiplet, 12 H),
2.80 (broad
singlet, 2 H), 2.74-2.50 (broad singlet, 2H), 2.30-1.90 (multiplet, 2 H), 1.85-
1.63 (multiplet, 2 H),
1.25- 0.60 (multiplet, 1 H), and the 13C NMR data (75.5 MHz, CDC13): 6 158.74-
157.31 (multiplet,
C=0, multiplets due to existence of conformers), 122.84-11.32 (quartet, CF3,
due to C-F coupling,
Jc-F ¨ 264 Hz, further split due to existence of conformers), 51.2 - 46.2
(multiplet, CH2 next to
N), 29.4 - 27.8 (multiplet, CH2); The HRMS-ESI mass calculated for
C16H21F9N403 was C 39.35;
H 4.33; N 11.47; 09.83; and the mass found was: C 39.19; H 4.36; N 11.33; 0
10.04.
[0075] Shown below is Scheme 2: Synthesis of Bromopropyl-[N-(Piperidin-1-
y1)-5-(4-
chloropheny1)-1-(2,4-dichloropheny1)-4-methyl-1H-pyrazole-3-carboxamidel (Step
1 in Scheme
2)
CI CI
0
N/
0 NO
/
0 0
HN N
N¨N HrCH2CH2CH2Br/CH3CN/K2CO3 N¨N
CH3CN/KOH/TFA3 exclarn N¨N
RT. 16h 70% ______________
CI 1 10
CI ________________________________________ RT 1 h%
Br Step 2 EN N
ci step 1 N Nci
F3c0C"...
µscocF3
McOlifIC2CO3
80 C 3 h, 86%
Step 3
ci
0 0
r N/
N¨N
c,=
c,
VYR207
[Scheme 2]
[0076] N-(Piperidin-l-y1)-5-(4-chloropheny1)-1-(2,4-dichloropheny1)-4-
methyl-1H-pyrazole-
3-carboxamide (5R141716, rimonabant; 3.70 g, 7.57 mmol) was added to a
magnetically stirred
anhydrous CH3CN (30 mL). The mixture was stirred at room temperature until a
solution was
obtained (-10 min). To this solution, K2CO3 (1.57 g, 11.43 mmol) and 1, 3-
dibromopropane (2.29
g, 11.35 mmol) were then added. The mixture was refluxed under the atmosphere
of N2 for 16 h.
The progress of the reaction was monitored by TLC (1:1 Et0Ac/Hexane). The
mixture was cooled
to room temperature and filtered through a sintered glass filter to remove
insoluble salt (washed
with 20 mL CH3CN). The solvent was then concentrated to give yellow oil (6.3
g, 70%). Structure
of starting material 5R141716 was determined by NMR (FIG. 1) and HPLC (FIG.
2). As shown
23
CA 03070538 2020-01-17
WO 2019/018536 PCT/US2018/042707
in the Table 1, the peaks and retention times show elution of SR141716 at peak
6 (7.994 minutes)
with significant purity, as evidenced by the area% being greater that 99% for
that data point.
[0077] Table I
Rank Retention Time Area (mAUs) Area %
(minutes)
1 5.570 6.55957 0.0804
2 7.331 5.72351 0.0702
3 7.588 3.46697 0.0425
4 7.682 9.81644 0.1204
7.824 5.12544 0.0628
6 7.994 8113.12109 99.4709
7 8.491 2.70567 0.0332
8 8.690 9.76111 0.1197
[0078] Conditions for HPLC were as set forth below. An Athena C18 column
with particle
size of 3 micrometer (pm) and physical dimensions of 2.1 millimeter (mm)
diameter and 100 mm
length. The two solvents used were 0.1 % phosphoric acid in 100% acetonitrile
(Solvent A) and
0.1 % phosphoric acid in 100% acetonitrile (Solvent B). The two solvents were
utilized to create
a gradient as shown in Table 2. The HPLC system was operated at a flowrate of
0.5 milliliters per
minute (mL/min) and detection at 210 nanometers (nm).
[0079] Table 2
Time Solvent A Solvent B
(in minutes) (Percent) (Percent)
0 0 100
2 0 100
7 95 5
95 5
10 Stop Stop
[0080] This compound 5R141716 was then subject to conjugation and
deprotection to yield
1,4,8,11-Tetraazacyclotetradecane-1'-propyl-[N-(Piperidin-1-y1)-5-(4-
chloropheny1)-1-(2,4-
dichloro phenyl) - 4-methy1-1H-pyrazole-3-carboxamidel (VYR207) (Steps 2-3 in
Scheme 2).
24
CA 03070538 2020-01-17
WO 2019/018536 PCT/US2018/042707
[0081] A mixture of bromopropyl- [N-(piperidin-l-y1)-5-(4-
chloropheny1)-1-(2,4-
dichloropheny1)-4-methyl-1H-pyrazole-3-carboxamide] (4.4 g, 7.57 mmol) in
anhydrous CH3CN
(20 mL) was magnetically stirred at room temperature. To the reaction mixture,
KOH powder (g,
11.35 mmol) and TFA3-cyclam (11.35 mmol) were added and the mixture was
stirred at room
temperature for 1 hour. The solvent was evaporated on a rotary evaporator
under reduced pressure.
The residue was purified by flash chromatography using Hexane:Et0Ac (6:4) as
an eluent to afford
TFA3-cyclam-conjugate as a white solid (16 g, 45 %). To a solution of the TFA3-
cyclam-
conjugate (3.0 g, 3.05 mmol) in Me0H (6.0 mL) K2CO3 (1.26 g, 9.1 mmol) was
added in one
portion. The suspension was heated under reflux for 3 h. Toluene (30 mL) was
then added to the
cooled mixture. Me0H was removed by forming an azeotrope with toluene. After
Me0H solvent
was completely removed, the hot toluene suspension with inorganic salt was
filtered and
concentrated to give a desired free base (6.2 g, 86 %) as a white solid.
[0082] Example 2 - Synthesis of 1,4,8,11-tetraazac yclotetradec ane- l'-
acetyl- [N-(Piperidin-1-
y1)-5 -(4-chloropheny1)- 1-(2,4-dichloropheny1)-4-methyl-1H-pyrazole-3 -c
arboxamide]
(VYR206).
[0083] The structure of target compound VYR206 (aliphatic chain with
carbonyl group) is
shown as Formula II and complete synthesis of VYR206 is shown in Scheme 3.
/ ______________________________
s'>
s'
A Ic
N.k?
\ 7
¨ .14
-'==r=-= . 0
cr.-A hH ..
V'Y R20(5
[Formula II]
[0084] VYR206 can be synthesized in several ways. Shown below is a
representative scheme,
Scheme 3.
CA 03070538 2020-01-17
WO 2019/018536 PCT/US2018/042707
ci
o NO
a
V HN/ 0 NO ci
/ / 0 N¨N Z
0
/
BrCH2CO2Et/K2C01/CH3CN
01N¨Ni \CH, ¨0O2Et NaOH (1 NFThoxane NO
70 C, 48 h, 55 %
500C, 4 h, 95 % I. Z
N-11 N \CH2
¨COON
CI 1110 CI
Step 1 Step 2
1110 a
a
ci
CI TrfrBOC Cyclam/DEA 0 0 a
0 0
EDC/HOBECH2C12 r / / TFA/CH2C12
/
ii
Step 3 401 Boc N¨N ss, n Step 4 o
a a EN NN
CI a ENNHHEINN
Bo/ c/JµBoc
VYR206 V
[Scheme 3]
[0085] Step 1 in Scheme 3: Synthesis of 1'-Ethylacetyl-[N-(Piperidiny-1-y1)-5-
(4-
chloropheny1)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide]
[0086] N-(Piperidiny- 1- y1)-5-(4-chloropheny1)- 1-(2,4-dichloropheny1)-4-
methyl-1H-p yrazole-
3-carboxamide ; 5R141716; 200 mg, 0.43 mmol) was added to a magnetically
stirred anhydrous
CH3CN (10 mL). The mixture was stirred at room temperature until a solution
was obtained (10
min). To this solution, K2CO3 (200 mg, 1.44 mmol) and bromo ethylacetate (400
mg, 2.4 mmol)
were then added. The mixture was refluxed under the atmosphere of N2 for 48 h.
TLC
(CH2C12/Me0H, 100:7) was used to monitor the reaction progress, which was
completed after 4 ¨
8 hours. The mixture was cooled to room temperature and filtered through a
sintered glass filter to
remove insoluble salt (washed twice with 20 mL CH3CN). The residue was then
isolated by
column chromatography and eluted with methylene chloride followed by methylene
chloride/methanol (100:6) to yield the desire compound as a white powder (428
mg, 55 %).
[0087] Step 2 in Scheme 3: Synthesis of 1'-Acetic acid4N-(Piperidin-1-y1)-5-(4-
chloropheny1)-1-(2,4-dichloropheny1)-4-methyl-1H-pyrazole-3-carboxamide]
[0088] The deesterification of ethylacetyled compound from step 1 was
carried out as follows:
1' -ethylacetyl- [N-(Piperidin-l-y1)-5-(4-chloropheny1)- 1-(2,4-
dichloropheny1)-4 -methyl- 1H-
pyrazole-3-carboxamide (200 mg, 0.36 mmol) was dissolved in dioxane (9 mL). To
the reaction
mixture, NaOH (250 i.tt, 10 N) was added along with 3 mL of water. The
reaction mixture was
heated at 50 C for 4 h. The dioxane in the reaction mixture was evaporated
and then water was
26
CA 03070538 2020-01-17
WO 2019/018536 PCT/US2018/042707
added. HC1 (5 N) was slowly added to the mixture to reach pH at 4-5. Reaction
mixture was then
extracted with CH2C12 to afford the product as a white solid (197 mg, 95%).
[0089] Steps 3-4 in Scheme 3: Conjugation and Deprotection of 1,4,8,11-
Tetraazac yclotetradec ane-l'- acetyl- [N-(Piperidin-l-y1)-5-(4-chloropheny1)-
1-(2,4-dichloro
phenyl)-4-methyl- 1H-p yrazole-3 -c arbox amide] (VYR206):
[0090] To a solution of 1'-Acetic acid4N-(Piperidiny-1-y1)-5-(4-chloropheny1)-
1-(2,4-
dichlorophenyl)-4-methyl-lH-pyrazole-3-carboxamidel (170 mg, 0.32 mmol) in
anhydrous
CH2C12 (25 mL), 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) (91 mg,
0.48 mmol),
hydroxybenzotriazole (HOBt) (65 mg, 0.45 mmol), diethylamine (DEA) (300 t.L)
and 1,4,8-Tri-
Boc-1,4,8,11-tetraazacyclotetradecane (B0C3-cyclam; 163 mg, 0.32 mmol) were
added. The
mixture was stirred at room temperature for 48 h. The protected BOC3-cyclam-
conjugate was
isolated by flash column chromatograph using gradient CH2C12/Me0H (100:1 to
100:10) to yield
(300 mg, 95 %). To de-protect the cyclam conjugate, the BOC3-protected
conjugate (175 mg, 0.17
mmol) was dissolved in TFA/CH2C12 (8 mL; 2:1). The reaction mixture was
stirred at room
temperature for 6 h. The solvent was then concentrated and the desired product
was purified by
flash chromatography eluted with CH2C12/Me0H (100:1 to 100:25) to afford the
final compound
as a white powder (150 mg, 81 %). The structure of VYR206 was determined by
NMR, MS, and
HPLC techniques (FIGS. 3, 4, and 5 respectively).
[0091] Example 3
H
0
LIN HN-1
NH HN ____________________________ I
C.)
N4-Cannabidiol (N4-CBD)
VYC203
[Formula III]
27
CA 03070538 2020-01-17
WO 2019/018536 PCT/US2018/042707
[0092] According to the similar N4-strategy approach as described for
VYR207 compound in
Example 1, two compositions based on natural cannabinoid compounds N4-
Cannabidiol
(VYC203) and N4-Tetrahydrocannabidiol (VYT205) were synthesized.
C H3
FN HN-1
H
NH HN
H 3C
0
H 3C CH3
N4-Tetrahydrocannabinol (N4-THC)
VYT205
[Formula IV]
[0093] VYR203 and VYR205 can be synthesized in several ways. Shown below is a
representative scheme, Scheme 4.
CH3
H3
HO C0
H3C CH3
Cannabidiol (CBD) Tetrahydrocannabinol (THC)
1. CH3CN/KOH
(-) ,CH,(CH2)2Br
F,C0C, RT, 1 h
N, NN
F3C0Cr 'COCF3 2. Me0H/K2CO3
80 C, 3 h
0\µµH OH
CH3
HN
0 ss.µ I¨NH HN¨I
/\()
EN HN
EN
HN¨I-1 H3C
0
H3C CH3
N4-Cannabidiol (N4-CBD) N4-Tetrahydrocannabinol (N4-THC)
VYC203 VYT205
[Scheme 4]
28
CA 03070538 2020-01-17
WO 2019/018536 PCT/US2018/042707
[0094] Briefly, Tetrahydrocannabinol (THC) (350 mg, 1.11 mmol) was added to
a magnetically
stirred anhydrous CH3CN (12 mL). The mixture was stirred at room temperature
until a solution
was obtained (-10 min). To this solution, bromopropy141,4,8-
Tris(trifluoroacety1)-1,4,8,11-
tetraazacyclotetradecanel (bromopropyl-N4-TFA3; bromopropyl-TFA3; 280 mg, 0.3
mmol) and
KOH (157 mg, 2.8 mmol) were then added. The mixture was stirred at room
temperature for 1 h.
The progress of the reaction was monitored by TLC (3:1 Et0Ac/Hexane). The
residue was purified
by flash chromatography using Hexane:Et0Ac (6:4) as an eluent to afford TFA3-
cyclam-THC as
a white solid (510 mg, 51 %). To a solution of TFA3-cyclam-THC conjugate in
Me0H (4.0 mL)
K2CO3 (420 mg, 7.2 mmol) was added in one portion. Suspension was heated under
reflux for 3
h. Toluene (20 mL) was then added to the cooled mixture. Me0H was removed by
forming an
azeotrope with toluene. After Me0H solvent was completely removed, the hot
toluene suspension
with inorganic salt was filtered and concentrated to give a desired free base
(575 mg, 76 %) as a
white solid VYT205. The same synthetic route is applied to VYC203 compound.
[0095] Example 4
[0096] Immunoblot analysis of cell lysates from various DLBCL cell lines
was performed to
evaluate expression of the CBI receptor (FIG. 6A) with expression of 3-actin
as the control for
sample loading (FIG. 6B), with each cell line identified by respective
initials. Western blotting
showed very high protein expression levels of CBI in the RC cell line in
comparison to Tole, TMD,
EJ, LP, LY3, and CJ cell lines, while no expression was detectable in FN, HF,
DOHH2, and DBR
cell lines.
[0097] Cell lysates from 24 DLBCL cell lines were analyzed for relative
expression of CBI and
CB2 mRNAs. In certain cell lines, such as TJ, LY-19, CJ, and BJAB, CBI mRNA
was expressed
about 10-15 % more than CB2 mRNA. In certain cell lines, such as SF, MZ,
Toledo, DBr, SUDHL-
4, and MCA, CBI mRNA was expressed about 100-200 % more than CB2 mRNA. In
certain cell
lines, such as SF, MZ, Toledo, DBr, SUDHL-4, and MCA, CBI mRNA was expressed
about 100-
200 % more than CB2 mRNA; and in cell lines, such as U2392 and EJ, CBI mRNA
was expressed
about 300-600 % more than CB2 mRNA. In certain cell lines, such as LY-3 and
Pheiffer, CB2
mRNA was expressed about 2-10 % more than CBI mRNA. These results show CBI is
a viable
target in malignant immune cells, along with CB2 that is known to be primarily
associated.
29
CA 03070538 2020-01-17
WO 2019/018536 PCT/US2018/042707
Furthermore, CBI may play a pivotal role in lymphoblastic cells, including
determination of
therapeutic sensitivity or resistance.
[0098] Example 5
[0099] Cell viability was assessed using MTS assays, a colorimetric method
for the sensitive
quantification of viable cells based on the reduction of the MTS tetrazolium
compound to a colored
formazan dye by NAD(P)H-dependent dehydrogenase enzymes in metabolically
active cells. The
formazan dye is quantified by measuring the absorbance at 490-500 nm. Cell
viability assays
(MTS) were performed to determine the viability of four DLBCL cancer cell
lines¨EJ, U2932,
CJ and LY19 in vitro (72 h incubation) in the presence of four CB i/CB2
cannabinoid receptor
ligands: CB 1-selective antagonist CP945,598 (Otenabant), CB 1-selective
inverse agonists¨
SR141716 (rimonabant) and AM251, and CB2-specific agonist AM1241. The effect
of
cannabinoid receptor ligand SR141716 on the viability of the DLBCL cancer cell
lines¨EJ,
U2932, CJ and LY19 is shown in FIG. 7. The effect of cannabinoid receptor
ligand CP945,598
(Otenabant) on the viability of the DLBCL cancer cell lines¨EJ, U2932, CJ and
LY19 is shown
in FIG. 8. The effect of cannabinoid receptor ligand AM1241 on the viability
of the DLBCL cancer
cell lines¨EJ, U2932, CJ and LY19 is shown in FIG. 9. The effect of
cannabinoid receptor ligand
AM251 on the viability of the DLBCL cancer cell lines¨EJ, U2932, CJ and LY19
is shown in
FIG. 10. All compounds depicted in FIGS. 7-10 correspond to non-conjugated (N4
chelator-free)
agents. As expected, CB2 agonist AM1241 demonstrated a significant effect,
however CB 1-
selective antagonist and inverse agonists (CP945,598, SR141716 and AM251) also
had significant
results depending on the specific cell line. For example, 5R141716 and AM251
appear to have a
more potent effect on LY19 where AM251 appears to have the greatest potency
for U2932.
Overall, the compounds demonstrate that CBI as a target for therapeutic
intervention. CB2 is
previously known to be associated with immune cells.
[00100] Example 6
[00101] In Examples 6-8, N4-conjugated compounds were analyzed. The effects of
CBI inverse
agonist 5R141716 and its conjugated form-VYR206 were analyzed using diffuse
large B-cell
lymphoma (DLBCL; 16 cancer cell lines; FIGS. 11A and 11B) and mantle cell
lymphoma (MCL;
8 cell lines; FIGS. 11C and 11D) cancer cells. Exposure of 5R141716 to DLBCL
(FIG. 11A)
CA 03070538 2020-01-17
WO 2019/018536 PCT/US2018/042707
markedly decreased viability than VYR206 (FIG. 11B). Diffuse large B-cell
lymphoma cancer
cells were resistant to lower VYR206 concentration (< 50 t.M) (FIG. 11B). The
lower-dose of
SR141716 (10-40 t.M) drug compared to VYR206 indicated the reduction of cell
viability
(relatively steep initial slopes especially in Rec-1 and SP-53) in MCL cell
lines (FIG. 11C). The
results showed that exposure of MCL cancer cells to VYR206 (FIG. 11D) led to a
substantial
decrease in cell viability when compared with the all DLBCL cell lines (FIG.
11B). Exposure to
50 i.t.M VYR206 drug had a significant effect on the induction of cell death
(90 %) in MCL cell
line Jeko and Mino (FIG. 11D). The less effective VYR206 may prove to be
beneficial for
controlling the dosing. Rimonabant by itself has severe adverse effects in
patients with high or
chronic dosing. Curtailing or modifying the potency may help to reduce adverse
effects while still
providing a therapeutic effect against cancerous cells.
[00102] Example 7
[00103] The effects of SR141716 and VYR206 were analyzed. MTS assays for cell
viability (72
h incubation) were performed on diffuse large B-cell lymphoma (DLBCL)
cells¨LR, MS, RC
and DOHH2 (FIGS. 12A - 12D) and mantle cell lymphoma (MCL) cells¨Jeko, Mino,
Rec-1 and
JMP1 (FIGS. 13A - 13D). Preliminary data shows DLBCL cancer cell lines MS,
DOHH2 and
mantle cell lymphoma cell line REC-1 are more resistant to drug VYR206 with
comparison to
other cell lines from FIGS. 12B, 12C, and 13B. The results show that exposure
of DLBCL to
SR141716 led to induction of cell death (< 90 %) at drug concentration 50 [I,M
in LR, MS and RC
cell lines (FIGS. 12A, 12B, 12D; blue lines) with comparison to DOHH2 cell
line, inhibition was
>50 % 5R141716 was more effective to DLBCL than VYR206. Complete DLBCL
inhibition with
VYR206 was reached at drug concentrations: 200 [I,M for LR, MS and DOHH2
(FIGS. 12A, 12B,
12C) and 100 [I,M for RC (FIG. 12D). Mantle cell lymphoma cell lines JEKO, JMP-
1 and Mino
(FIGS. 13A, 13B, 13D, red lines) were less resistant to VYR206 than DLBCL. It
was also
observed from FIGS. 12 and 13 that N4 (cyclam) has been proven to have no
effect on cell viability
in all tested lymphoma cell lines.
[00104] Example 8
[00105] High throughput screening (THS) assessment of cell viability in 16
DLBCL (FIGS. 14
A and 14B) and 8 MCL (FIG. 14C) cell lines were assessed following treatment a
72-hour
incubation of the cell lines with cannabidiol, which was obtained as CBD in a
coconut oil extract.
31
CA 03070538 2020-01-17
WO 2019/018536 PCT/US2018/042707
Cell viability, 90% of the all cells tested, remained viable in a
concentration range of 0-5 tM CBD.
Complete cell death was obtained at 25 tM CBD concentration only for two
cancer cell lines, HT
and EJ (FIG. 14B); 50 tM CBD concentration gave a complete cell death
viability in seven cell
lines MZ, HF, HB, CJ, MS, Toledo and EJ (FIGS. 14 A and 14B). Cancer cell line
DOHH2 was
less susceptible to lower CBD concentration (0-25 t.M) (FIG. 14B). After
exposure to CBD
concentration range 15-20 i.t.M 50 % cell viability was reached in 8 cell
lines (MZ, HB, CJ, LP,
MCA, MT, EJ) (FIGS. 14A and 14B). All screened mantle cell lymphoma cancer
cells (Mino,
Jeko, SP53, Maver-1Z138, JMP-1, Rec-1 and PF-1) were less sensitive at CBD
concentration
range from 15 i.t.M to 25 i.t.M (FIG. 14C).
[00106] Example 9
CI
ci
_ON N
Route A 68GaC13/0 1N HCl/NaHCO3 0 N
N
70 _________________________________ C, 15 min
40 N Route B 99mTc04 N¨N-/SnC12/saline
A,ro
70 C, 10 math
ci CI ENH N-1
I¨NH HN¨I CI [N. N1
I¨N N¨I
VYR206 M=Ga, Te Ls)
[Scheme 5]
[00107] 68Ga-N4-Compound labeling process (Route A of Schemes 5, 6, and 7) was
generally
initiated by the addition of 68Ga chloride (20 mCi in 0.1 N HC1) in vials. The
reaction was heated
at 70 C for 15 min and the pH of the product was adjusted to 5-6 with sodium
bicarbonate. The
tracer product was validated using two analytical tools (reverse phase HPLC
and radio-TLC) to
determine the active pharmaceutical ingredient (API) purity, API yield,
radiochemical purity and
yield and specific 68Ga-N4-compound activity. In certain compounds, there was
a need to use C-
18 column to remove unreacted 68Ga chloride or 68Ga oxide, followed by elution
with ethanol.
99mTc-N4-compound labeling process (Route B of Schemes 5, 6, and 7) was
generally initiated by
the addition of tin (II) chloride (0.1g) and 99mTc pertechnetate (20 mCi in
0.1 mL saline) in vials.
The reaction was immediately chelated and the pH of the product was adjusted
to 5-6 with saline.
The tracer product was validated using two analytical tools (reverse phase
HPLC and radio-TLC)
to determine the active pharmaceutical ingredient (API) purity, API yield,
radiochemical purity
and yield and specific 99mTc-N4-compound activity. Radiochemical purity of
99mTc-N4-
compounds was assessed by high-performance liquid chromatography (HPLC),
equipped with NaI
32
CA 03070538 2020-01-17
WO 2019/018536 PCT/US2018/042707
and UV detector (274 nm), and was performed using a C-18 reverse column with a
mobile phase
of acetonitrile:water (7:3) at a flow rate of 0.5 mL/min.
[00108] Radiochemical purity was also determined by radio-TLC (ITLC SG, Gelman
Sciences,
Ann Arbor, MI) eluted with ammonium acetate (1M in water): methanol (6:1).
High-performance
liquid chromatography (HPLC), equipped with a NaI detector and UV detector
(254 nm), was
performed on a gel permeation column (Biosep SEC-53000, 7.8 300 mm,
Phenomenex, Torrance,
CA) using a flow rate of 1.0 mL/min. The eluant was 0.1% LiBr in phosphate
buffered saline
(PBS) (10 mM, pH 7.4).
OH
.\\H
Route A: 68GaCI3/0. IN HCl/NaHCO3
70 C, 15 min
/\() Route B: 99mTc047SnC12/saline
EN NN1 70 C, 10 min
/\()
NH HN 1N M
N4-Cannabidiol (N4-CBD)
VYC203
[Scheme 6]
CH,
or-/N HN
\H
NH HN 0
,s= Route A: "GaC13/0.1N HC1/NaHCO3
70 C, 15 min
H3C Route B: 99mTe04-/SnC12/saline
0 H3C
H3C CH3 70 C, I 0 min 0
H3c cH3
N4-Tetrahydrocannabinol (N4-THC)
VYT205
[Scheme 7]
[00109] Example II
The effect of different amounts of combinations of VYR206 and CBD on various
DLBCL and
MCL cell lines were evaluated by immunoblot analysis. Immunoblot analysis of
cell lysates from
various DLBCL and MCL cell lines was performed to show effects on expression
of CBI and CB2
receptors with the expression of 3-actin as the control for sample loading
(FIGS. 15A-15D), with
33
CA 03070538 2020-01-17
WO 2019/018536 PCT/US2018/042707
each cell line identified by respective initials. Northern blotting showed
decreasing RNA
expression levels of CBI with increased amounts of conjugated CBI inverse
agonist VYR206 with
predominately no change in CB2 expression. Moreover, FIGS. 15B and 15C shows a
modulation
in CB2 mRNA expression levels with VYR206 and CBD administration.
[00110] Example 12
[00111] Cell viability assays (MTS) were performed to determine the viability
of MCL (Mino,
Jeko, Sp-53, Mayer, Z-138, JMP-1, Rec-1 and PF-1) and DLBCL (CJ, LP, RC, TMD-
8, WP, LY-
3, LY-19 and MZ) cancer cell lines in vitro (72 h incubation) in the presence
of a cannabinoid
receptor ligand, cannabidiol CBD-99, which is a hemp derived cannabidiol from
Isodiol
International Inc., headquartered in Vancouver, Canada, and has 99% purity.
The efficacy of the
cannabidiol CBD-99 on the viability of various MCL and DLBCL cell lines is
shown in FIGS.
16A and 16B, respectively. The cannabidiol compound CBD-99 used in Example 12
is a non-
conjugated (N4 chelator-free) agent.
[00112] Example 13
[00113] Cell viability assays (MTS) were performed to determine the viability
of DLBCL and
MCL cancer cell lines in vitro (72 h incubation) in the presence of CBi-
selective inverse agonists¨
rimonabant (identified as Rimo) and Bruton' s tyrosine kinase inhibitor-
Ibrutinib (identified as
IBN). The IC50 values of rimonabant and Ibrutinib on the viability of various
DLBCL cell lines
are shown in FIG. 17, and of various MCL cell lines (Jeko, Mino, PF-4 and PF-
5) are shown in
FIGS. 18A-18D. All compounds depicted in FIGS. 17 and 18A-18D correspond to
non-
conjugated (N4 chelator-free) agents. FIGS. 18A ¨ 18D are graphical
representations of the effect
of the rimonabant and ibrutinib on the viability of MCL cell lines (Jeko, Jeko-
R, Mino, Mino-R,
PF-4 and PF-5) cell lines' viability. FIG. 18A is a graphical representation
of the effect of 25 [I,M
of rimonabant and ibrutinib on Jeko and Jeko-R cell lines. FIG. 18B is a
graphical representation
of the effect of 25 i.t.M of rimonabant and ibrutinib on Mino and Mino-R. FIG.
18C is a graphical
representation of efficacy of ibrutinib on PF-4 and PF-5 cell lines. FIG. 18D
is a graphical
representation of the effect of 25 i.t.M of rimonabant and ibrutinib on PF-4
and PF-5.
34
CA 03070538 2020-01-17
WO 2019/018536 PCT/US2018/042707
Table 3
IC50 (uM)
rimonabant ibrutinib
EJ 20.61 30
CJ 13.51 25
LY19 15.19 10
HF 32.68 18
McA 21.79 20
MS 23.67 20
LY-10 25.06 0.5
Ly-3 25.22 20
LP 27.1 6
LR 30.89 20
HB 29.44 20
WP 25.49 1
U2932 26.15 15
Average ICso 24.367 15.80
[00114] Example 14
[00115] Cell viability assays (MTS) were also performed to determine the
viability of DLBCL
and MCL cancer cell lines in vitro (72 h incubation) in the presence of
conjugated CBi-selective
inverse agonists¨VYR206. IC50 values on DLBCL cell line's viability is shown
in Table 3.
[00116] Table 3
Cell line VYR206 ICso (M)
SUDHL-4 63
HF 74
McA 6
MZ 138
FN 69
CJ 100
MS 63
DS 57
Toledo 29
Pfeiffer 25
LY19 8
BJAB 58
RC 5
DB 8
HT 45
SUDHL-10 155
CA 03070538 2020-01-17
WO 2019/018536 PCT/US2018/042707
Cell line VYR206 ICso (M)
LR 8
LY10 7
LY3 6
U2932 88
LP 13
HB L- 1 101
[00117] Example 15
[00118] The efficacy of rimonabant and its cyclam conjugated form, VYR206 on
the viability
of DLCBL cell lines (LR, MS, RC and DOHH2) and MCL cell lines (Jeko, Rec-1,
Mino and JMP-
1) was evaluated with cyclam as the control. FIGS. 19A ¨ 19H are graphical
representations of
the efficacy of rimonabant and its cyclam conjugated form, VYR206 on the
viability of DLCBL
cell lines (LR, MS, RC and DOHH2) and MCL cell lines (Jeko, Rec-1, Mino and
JMP-1). FIG.
19A is a graphical representation of the efficacy of rimonabant and VYR206 on
the viability of
LR cell line with cyclam as a control. FIG. 19B is a graphical representation
of the efficacy of
rimonabant and VYR206 on the viability of MS cell line with cyclam as a
control. FIG. 19C is a
graphical representation of the efficacy of rimonabant and VYR206 on the
viability of RC cell line
with cyclam as a control. FIG. 19D is a graphical representation of the
efficacy of rimonabant and
VYR206 on the viability of DOHH2 cell line with cyclam as a control. FIG. 19E
is a graphical
representation of the efficacy of rimonabant and VYR206 on the viability of
Jeko cell line with
cyclam as a control. FIG. 19F is a graphical representation of the efficacy of
rimonabant and
VYR206 on the viability of Rec-1 cell line with cyclam as a control. FIG. 19G
is a graphical
representation of the efficacy of rimonabant and VYR206 on the viability of
Mino cell line with
cyclam as a control. FIG. 19H is a graphical representation of the efficacy of
rimonabant and
VYR206 on the viability of JMP-1 cell line with cyclam as a control. VYR206
follows the same
dose response pattern as the unconjugated rimonabant but with decreased
potency. This decreased
potency can help decrease the toxicity and other side effects associated with
rimonabant. Most of
the cell lines presented have some sensitivity to VYR206 where Rec-1 appears
to be resistant.
This may prove that VYR206 may be selective to certain cell lines.
[00119] Example 16
[00120] Cell viability assays (MTS) were performed to determine the viability
of DLBCL (RC)
and MCL (Mino) cancer cell lines in vitro (72 hour-incubation) in the presence
of CB2- agonist-
36
CA 03070538 2020-01-17
WO 2019/018536 PCT/US2018/042707
CBD alone and in combination with ibrutinib. Results are shown in FIG. 20A.
Cell viability assays
(MTS) were performed to determine the viability of DLBCL (RC) and MCL (Mino)
cancer cell
lines in vitro (72 h incubation) in the presence of CBD alone and in
combination with zanubrutinib
(BGB). Results are shown in FIG. 20B. Cell viability assays (MTS) were
performed to determine
the viability of DLBCL (RC) and MCL (Mino) cancer cell lines in vitro (72 h
incubation) in the
presence of CBD alone and in combination with carfilzomib (CFZ). Results are
shown in FIG.
20C. Cell viability assays (MTS) were performed to determine the viability of
DLBCL (RC) and
MCL (Mino) cancer cell lines in vitro (72 h incubation) in the presence CBD
alone and in
combination with tumorex (TMX). Results are shown in FIG. 20D. Comparative
reduction in
viability with CBD versus chemotherapeutic agents were demonstrated in FIGS.
20A ¨ 20D where
each combination demonstrates a marked reduction in both cell lines, for what
appears to be
synergistic effect. At the given amounts of CBD and the chosen
chemotherapeutics individually,
there was modest reduction in viability, but the combination in each instance
caused a synergistic
effect on MCL (Mino) and DLBCL (RC) cell lines. This demonstrates combinations
of the
cannabinoid ligands with chemotherapeutics can enhance therapeutic effects of
the
chemotherapeutic compound.
[00121] Example 17
[00122] To determine whether cyclam-conjugated CB2 agonist CBD and cyclam-
conjugated
CBI inverse agonist VYR206 at various concentrations resulted in induction of
apoptosis, RC cells
were treated with increasing concentration of cyclam-conjugated conjugated CBD
or VYR206 for
24 hrs. No activation of apoptosis in untreated cells was observed (control
not shown).
Concentration-dependent increase was detected in cleaved PARP after treatment
with cyclam-
conjugated conjugated CBD and VYR206. At 12.5 1.tM CBD and 50 1.tM VYR206,
there was
significant apoptosis induction in cleaved PARP expression. Immunoblot
analysis showing the
induction of apoptosis through the effects on expression of cPARP are shown in
FIG. 21A with
expression of 3-actin as the control for sample loading in FIG. 21B for cells
treated with cyclam-
conjugated CB2 agonist CBD and cyclam-conjugated CBI inverse agonist VYR206 at
6.25, 12.5,
25, and 50 t.M. Western blotting showed increasing protein expression levels
of cPARP with
increased amounts of cyclam-conjugated conjugated CBD and VYR206.
[00123] To determine whether different CBD concentrations resulted in
induction of apoptosis,
BJAB cells were treated with increasing concentration of CBD for 24 hrs. No
activation of
37
CA 03070538 2020-01-17
WO 2019/018536 PCT/US2018/042707
apoptosis in untreated cells (control not shown). It was observed a
concentration-dependent
increase in cleaved caspases 3 and cleaved PARP after CBD treatment. At 12.5
11M CBD, there
was significant apoptosis induction in cleaved PARP expression. The effect of
CBD on both
cleaved caspase 3 (FIG. 21C) and cleaved PARP expression (FIG. 21D) was
analyzed using
Western analysis. Expression of the 13-actin (FIG. 21E) was used as a control
for equal loading.
[00124] Example 18
[00125] Cell viability assays (MTS) were performed to determine the viability
of DLBCL (LY19
and LR) cancer cell lines in vitro (72 h incubation) in the presence of CB2-
agonist¨CBD in
comparison and in combination with conjugated CBI inverse agonist-VYR206.
Results are shown
in FIGS. 22A and 22B. CBD has mixed affinity for both CBI and CB2 receptors
and had
demonstrated generally the same response as VYR206 alone and in combination.
[00126] Example 19
[00127] Genetic profile analysis of DLBCL (RC) cell line for probing for
potential molecular
marker to develop a target based molecular signature. The expression of genes
in untreated and
rimonabant treated cells showing the range of under expression (green) to over
expression (red)
shown in FIG. 27. Genes with significant change in expression in untreated
versus treated cells
and their functional association are further listed in Table 4.
[00128] Table 4
Function Gene
DNA Damage Histone H3, DH-Histone H3, H2AX, DM-K9-Histone
H3, PMS2, RPA32
Apoptosis Caspase 3, cleaved caspase 7, Bad, bak, Smac
Cell cycle Chkl, Chk2, Aurora B, p27-kip-1, cyclin D1, Rb,
ATM, cyclin B1
Metabolic Glutamate, HIF- la, SLC1A5, LDHA, TIGAR, TFAM
Growth/Survival AKT, PI3K, Src, b-catenin, STAT3, Rictor, S6,
NDRG1, Prex-1, SMAD3, c-Met, Lck
Ubiqutin/proteasome UBAC1, BAP1
Autophagy LC3A
38
CA 03070538 2020-01-17
WO 2019/018536 PCT/US2018/042707
[00129] Proteomic profile analysis of DLBCL (RC) cell line to demonstrate
correlation between
most significant proteins is shown in Table 6. These proteins regulate various
cellular function
ranging from cell adhesion to cell growth and proliferation to apoptosis. For
example, Fox03a,
known to be associated with the trigger of apoptosis, is shown to have a
positive linear relationship
with N-Cadherin and RPA32 which are associated with cell adhesion and
replication respectively.
These correlations provide further insight to potentially understanding the
mechanism of cell
signaling that is occurring in the DLBCL cell line (RC) and where the
cannabinoid receptor
activation may be involved.
[00130] Table 6
Pearson r value, p value
Notchl vs RPA32 -03939 0.0036
tsl-Cadhafin fOttor -0,4318 0.0448
N-Cadherin vs RPA32 0.476 -00251
N-Cadherin vs Fox03a 0.7755 0.0001
RPA32 Fox03a 03947 0.0035
Spearman r value p value
Notchl vs alF4G 4,4376 0.0417
Notchl vs RPA32 -0,5088 0.0156
N-Cadherin vs Rictor -0.5336 0.0105
N-cadherin v. RPA32 05471 0.0084
N-Cactherin vs Fox03a 0.6375 0.0014
RPA32 vs Fox03a 0.6126 0,0024
[00131] Further modifications and alternative embodiments of various aspects
of the
compositions and methods disclosed here will be apparent in view of this
description. Accordingly,
this description is to be construed as illustrative only and is for the
purpose of teaching those skilled
in the art the general manner of carrying out the embodiments. It is to be
understood that the forms
of the embodiments shown and described here are to be taken as examples of
embodiments.
Elements and materials may be substituted for those illustrated and described
here, parts and
processes may be reversed or omitted, and certain features of the embodiments
may be utilized
independently, all as would be apparent after having the benefit of this
description of the
embodiments. Changes may be made in the elements described here without
departing from the
scope of the embodiments as described in the following claims.
39
CA 03070538 2020-01-17
WO 2019/018536 PCT/US2018/042707
[00132] The foregoing descriptions of methods, compositions, and results
obtained using them
are provided merely as illustrative examples. Descriptions of the methods are
not intended to
require or imply that the steps of the various embodiments must be performed
in the order
presented. The steps in the foregoing embodiments may be performed in any
order. Words such
as "then" are not intended to limit the order of the steps; these words are
simply used to guide the
reader through the description of the methods. Many of the operations may be
performed in parallel
or concurrently. In addition, the order of the operations may be re-arranged.
Various modifications
to these embodiments will be readily apparent based on the description
provided here, and the
generic principles defined here may be applied to other embodiments without
departing from the
scope of the disclosure.