Note: Descriptions are shown in the official language in which they were submitted.
CA 03071314 2020-01-28
WO 2019/025980 PCT/IB2018/055745
NOVEL BATTERY SYSTEMS BASED ON LITHIUM DIFLUOROPHOSPHATE
BACKGROUND
TECHNICAL FIELD
[0001] The present disclosure relates to rechargeable battery systems, and
more
specifically to the chemistry of such systems, including operative electrolyte
additives and
electrodes for improving the properties of the rechargeable lithium-ion-
battery systems.
DESCRIPTION OF RELATED ART
[0002] Rechargeable batteries are an integral component of energy-storage
systems for
electric vehicles and for grid storage (for example, for backup power during a
power outage,
as part of a microgrid, etc.). Li-ion-based batteries are a common type of
rechargeable battery.
[0003] Electrolyte additives have been shown to be operative and increase
the lifetime and
performance of Li-ion-based batteries. For example, in J. C. Burns et al.,
Journal of the
Electrochemical Society, 160, A1451 (2013), five proprietary, undisclosed
electrolyte
additives were shown to increase cycle life compared to an electrolyte system
with no or only
one additive. Other studies have focused on performance gains from electrolyte
systems
containing three or four additives as described in U.S. 2017/0025706. However,
researchers
typically do not understand the interaction between different additives that
allow them to work
together synergistically with the electrolyte and specific positive and
negative electrodes. Thus,
the composition of additive blends of certain systems is often based on trial
and error and
cannot be predicted beforehand.
[0004] Prior studies have not identified two-additive electrolyte systems
that can be
combined into a lithium-ion battery system to yield a robust system with
sufficient properties
for grid or automobile applications. As discussed in U.S. 2017/0025706, two-
additive systems
studied (for example, 2% VC + 1% allyl methanesulfonate and 2% PES + 1% TTSPi)
typically
performed worse than the three- and four-additive electrolyte systems. (See,
e.g., U.S.
2017/0025706 at Tables 1 and 2.)U520170025706 discloses that a third compound,
often tris(-
trimethly-sily1)-phosphate (TTSP) or tris(-trimethyl-sily1)-phosphite (TTSPi),
was necessary
in concentrations of between 0.25-3 wt% to produce a robust lithium-ion-
battery system. (See,
e.g., U.S. 2017/0025706 at 72.) However, because additives can be expensive
and difficult to
include within Li-ion batteries on a manufacture scale, simpler, yet effective
battery systems
are needed, including those with fewer additives.
1
CA 03071314 2020-01-28
WO 2019/025980 PCT/IB2018/055745
SUMMARY
[0005] This disclosure covers novel battery systems with fewer operative,
electrolyte
additives that may be used in different energy storage applications, for
example, in vehicle and
grid-storage. More specifically, this disclosure includes two-additive
electrolyte systems that
enhance performance and lifetime of Li-ion batteries, while reducing costs
from other systems
that rely on more additives. This disclosure also discloses effective positive
electrodes and
negative electrodes that work with the disclosed two-additive electrolyte
systems to provide
further systematic enhancements.
[0006] Two-operative, additive electrolyte systems disclosed include 1)
vinylene carbonate
(VC) combined with 1,3,2-dioxathiolane-2,2-dioxide (DTD, also known as
ethylene sulfate) or
another sulfur-containing additive (such as methylene methane disulfonate,
trimethylene
sulfate, 3-hydroxypropanesulfonic acid y-sultone, glycol sulfite, or another
sulfur-containing
additive), 2) fluor ethylene carbonate (FEC) combined with DTD or another
sulfur-containing
additive, and 3) prop-1-ene-1,3-sultone (PES) combined with DTD or another
sulfur-
containing additive. Further, because VC and FEC provide similar improvements
(and are
believed to function similarly), a mixture of VC and FEC may be considered as
only a single
operative electrolyte. That is, another disclosed two-operative, additive
electrolyte system
includes a mixture of VC and FEC combined with DTD or another sulfur-
containing additive.
When used as part of a greater battery system (which includes the electrolyte,
electrolyte
solvent, positive electrode, and negative electrode), these two-operative,
additive electrolyte
systems produce desirable properties for energy storage applications,
including in vehicle and
grid applications.
[0007] More specifically, lithium nickel manganese cobalt oxide (NMC)
positive
electrodes, a graphite negative electrodes, a lithium salt dissolved in an
organic or non-aqueous
solvent, which may include methyl acetate (MA), and two additives to form a
battery system
with desirable properties for different applications. The electrolyte solvent
may be the
following solvents alone or in combination: ethylene carbonate (EC), ethyl
methyl carbonate
(EMC), methyl acetate, propylene carbonate, dimethyl carbonate, diethyl
carbonate, another
carbonate solvent (cyclic or acyclic), another organic solvent, and/or another
non-aqueous
solvent. Solvents are present in concentrations greater than the additives,
typically greater than
6% by weight. The solvent may be combined with the disclosed two-additive
pairs (such as
VC with DTD, FEC with DTD, a mixture of VC and FEC with DTD, or another
combination)
to form a battery system with desirable properties for different applications.
The positive
electrode may be coated with a material such as aluminum oxide (A1203),
titanium dioxide
2
CA 03071314 2020-01-28
WO 2019/025980 PCT/IB2018/055745
(TiO2), or another coating. Further, as a cost savings, the negative electrode
may be formed
from natural graphite, however depending on the pricing structure, in certain
instances artificial
graphite is cheaper than natural graphite.
[0008] The disclosure herein is supported by experimental data that shows
the symbiotic
nature of the two-additive electrolyte systems and selected electrodes.
Exemplary battery
systems include two additives (for example, FEC, VC, or PES and DTD or another
sulfur-
based additive), a graphite negative electrode (either naturally occurring
graphite or an artificial,
synthetic graphite), an NMC positive electrode, a lithium electrolyte (formed
from, for example,
a lithium salt such as lithium hexafluorophosphate with chemical composition
LiPF6), and an
organic or non-aqueous solvent. A lithium-ion battery may include a negative
electrode, a
positive electrode comprising NMC with micrometer-scale grains, and a
nonaqueous
electrolyte comprising lithium ions dissolved in a first nonaqueous solvent,
and an additive
mixture having a first operative additive of either fluoro ethylene carbonate
or vinylene
carbonate and a second operative additive of either 1,3,2-dioxathiolane-2,2-
dioxide, another
sulfur-containing additive, or lithium difluorophosphate.
BRIEF DESCRIPTION OF THE DRAWINGS
[0009] FIG. 1 is a schematic diagram of a vehicle containing a battery
storage system.
[0010] FIG. 2 is a schematic diagram of an exemplary battery storage
system.
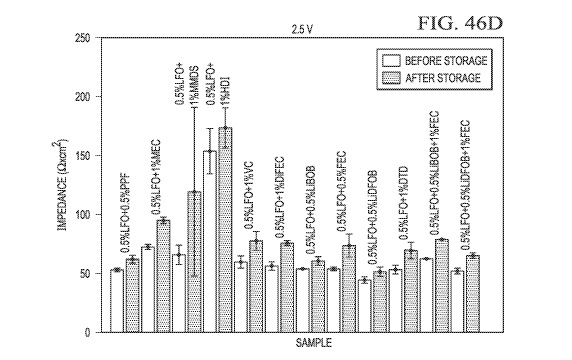
[0011] FIG. 3 is a schematic diagram of a lithium-ion, battery-cell system.
[0012] FIGs. 4A-J illustrate typical experimental data collected during
ultra-high-
precision-charging experiments of battery systems with different electrolyte
compositions.
[0013] FIG. 4A illustrates time normalized coulombic inefficiency per hour
(CIE/h) versus
cycle number for electrolyte systems including 1% DTD, 2% VC, and 2% VC + 1%
DTD.
[0014] FIG. 4B illustrates coulombic efficiency (CE) versus cycle number
for electrolyte
systems including 1% DTD, 2% VC, and 2% VC + 1% DTD.
[0015] FIG. 4C illustrates the capacity of the charge endpoint plotted
versus cycle number
for number for electrolyte systems including 1% DTD, 2% VC, and 2% VC + 1%
DTD.
[0016] FIG. 4D illustrates the discharge capacity verses cycle number for
electrolyte
systems including 1% DTD, 2% VC, and 2% VC + 1% DTD.
[0017] FIG. 4E illustrates the change in open circuit voltage versus cycle
number for
electrolyte systems including 1% DTD, 2% VC, and 2% VC + 1% DTD.
[0018] FIG. 4F illustrates time normalized coulombic inefficiency per hour
(CIE/h) versus
cycle number for electrolyte systems including 1% DTD, 2% FEC, and 2% FEC + 1%
DTD.
3
CA 03071314 2020-01-28
WO 2019/025980 PCT/IB2018/055745
[0019] FIG. 4G illustrates coulombic efficiency (CE) versus cycle number
for electrolyte
systems including 1% DTD, 2% FEC, and 2% FEC + 1% DTD.
[0020] FIG. 411 illustrates the capacity of the charge endpoint plotted
versus cycle number
for electrolyte systems including 1% DTD, 2% FEC, and 2% FEC + 1% DTD.
[0021] FIG. 41 illustrates the discharge capacity verses cycle number for
electrolyte
systems including 1% DTD, 2% FEC, and 2% FEC + 1% DTD.
[0022] FIG. 4J illustrates the difference between the average charge
voltage and the
average charge voltage (Delta V) versus cycle number for electrolyte systems
including 1%
DTD, 2% FEC, and 2% FEC + 1% DTD.
[0023] FIGs. 5A-C illustrates the average of the last three cycles of data
shown in FIG. 4,
and shows lower coulombic inefficiency per hour and lower fractional slippage
per hour for
the combinations of FEC + DTD and VC + DTD compared to any single additive of
FEC, VC,
or DTD.
[0024] FIG. 5A illustrates the average coulombic inefficiency per hour for
the last three
cycles of data generated during the experiments shown in FIG. 4.
[0025] FIG. 5B illustrates the average fractional slippage for the last
three cycles of data
generated during the experiments shown in FIG. 4.
[0026] FIG. 5C illustrates the average fractional fade for the last three
cycles of data
generated during the experiments shown in FIG. 4.
[0027] FIGs. 6A-F illustrate typical experimental data studying long term
cycling at 40 C,
C/3 CCCV showing the advantage of including DTD as an additive to an
electrolyte system
containing VC or FEC.
[0028] FIG. 6A illustrates capacity versus cycle number for electrolyte
systems including
1% DTD, 2% FEC, 2% FEC + 1% DTD, 2% VC, and 2% VC + 1% DTD, cycling between
3.0
V and 4.2 V.
[0029] FIG. 6B illustrates normalized capacity versus cycle number for
electrolyte systems
including 1% DTD, 2% FEC, 2% FEC + 1% DTD, 2% VC, and 2% VC + 1% DTD, cycling
between 3.0 V and 4.2 V.
[0030] FIG. 6C illustrates voltage hysteresis (difference between the
average charge
voltage and the average charge voltage) versus cycle number for electrolyte
systems including
1% DTD, 2% FEC, 2% FEC + 1% DTD, 2% VC, and 2% VC + 1% DTD, cycling between
3.0
V and 4.2 V.
4
CA 03071314 2020-01-28
WO 2019/025980 PCT/IB2018/055745
[0031] FIG. 6D illustrates capacity versus cycle number for electrolyte
systems including
1% DTD, 2% FEC, 2% FEC + 1% DTD, 2% VC, and 2% VC + 1% DTD, cycling between
3.0
V and 4.2 V.
[0032] FIG. 6E illustrates normalized capacity versus cycle number for
electrolyte systems
including 1% DTD, 2% FEC, 2% FEC + 1% DTD, 2% VC, and 2% VC + 1% DTD, cycling
between 3.0 V and 4.2 V.
[0033] FIG. 6F illustrates voltage hysteresis (difference between the
average charge
voltage and the average charge voltage) versus cycle number for electrolyte
systems including
1% DTD, 2% FEC, 2% FEC + 1% DTD, 2% VC, and 2% VC + 1% DTD, cycling between
3.0
V and 4.2 V.
[0034] FIGs. 7A-F illustrate typical experimental data studying long term
cycling at 20 C,
C/3 CCCV showing the advantage of including DTD as an additive to an
electrolyte system
containing VC or FEC.
[0035] FIG. 7A illustrates capacity versus cycle number for electrolyte
systems including
1% DTD, 2% FEC, 2% FEC + 1% DTD, 2% VC, and 2% VC + 1% DTD, cycling between
3.0
V and 4.3 V.
[0036] FIG. 7B illustrates normalized capacity versus cycle number for
electrolyte systems
including 1% DTD, 2% FEC, 2% FEC + 1% DTD, 2% VC, and 2% VC + 1% DTD, cycling
between 3.0 V and 4.3 V.
[0037] FIG. 7C illustrates voltage hysteresis (difference between the
average charge
voltage and the average charge voltage) versus cycle number for electrolyte
systems including
1% DTD, 2% FEC, 2% FEC + 1% DTD, 2% VC, and 2% VC + 1% DTD, cycling between
3.0
V and 4.3 V.
[0038] FIG. 7D illustrates capacity versus cycle number for electrolyte
systems including
1% DTD, 2% FEC, 2% FEC + 1% DTD, 2% VC, and 2% VC + 1% DTD, cycling between
3.0
V and 4.3 V.
[0039] FIG. 7E illustrates normalized capacity versus cycle number for
electrolyte systems
including 1% DTD, 2% FEC, 2% FEC + 1% DTD, 2% VC, and 2% VC + 1% DTD, cycling
between 3.0 V and 4.3 V.
[0040] FIG. 7F illustrates voltage hysteresis (difference between the
average charge
voltage and the average charge voltage) versus cycle number for electrolyte
systems including
1% DTD, 2% FEC, 2% FEC + 1% DTD, 2% VC, and 2% VC + 1% DTD, cycling between
3.0
V and 4.3 V.
CA 03071314 2020-01-28
WO 2019/025980 PCT/IB2018/055745
[0041] FIGs. 8A-I illustrate typical experiential data collected during
cycling experiments
for electrolyte compositions according to certain embodiments of the present
disclosure.
[0042] FIG. 8A illustrates peak capacity versus cycle number for
electrolyte systems
including 2% FEC, 1% FEC + 1% DTD, 2% FEC + 1% DTD, 1% FEC + 1% MMDS, and 2%
FEC + 1% MMDS, cycling between 3.0 V and 4.3 V at 40 C in a base electrolyte
of 1.2M
LiPF6 in 30% ethylene carbonate and 70% ethyl methyl carbonate.
[0043] FIG. 8B illustrates normalized capacity versus cycle number for
electrolyte systems
including 2% FEC, 1% FEC + 1% DTD, 2% FEC + 1% DTD, 1% FEC + 1% MMDS, and 2%
FEC + 1% MMDS, cycling between 3.0 V and 4.3 V at 40 C in a base electrolyte
of 1.2M
LiPF6 in 30% ethylene carbonate and 70% ethyl methyl carbonate.
[0044] FIG. 8C illustrates voltage hysteresis (difference between the
average charge
voltage and the average charge voltage) for electrolyte systems including 2%
FEC, 1% FEC +
1% DTD, 2% FEC + 1% DTD, 1% FEC + 1% MMDS, and 2% FEC + 1% MMDS, cycling
between 3.0 V and 4.3 V at 40 C in abase electrolyte of 1.2M LiPF6 in 30%
ethylene carbonate
and 70% ethyl methyl carbonate.
[0045] FIG. 8D illustrates peak capacity versus cycle number for
electrolyte systems
including 2% VC, 1% VC + 1% DTD, 2% VC + 1% DTD, 1% VC + 1% MMDS, and 2% VC
+ 1% MMDS, cycling between 3.0 V and 4.3 V at 40 C in a base electrolyte of
1.2M LiPF6 in
30% ethylene carbonate and 70% ethyl methyl carbonate.
[0046] FIG. 8E illustrates normalized capacity versus cycle number for
electrolyte systems
including 2% VC, 1% VC + 1% DTD, 2% VC + 1% DTD, 1% VC + 1% MMDS, and 2% VC
+ 1% MMDS, cycling between 3.0 V and 4.3 V at 40 C in a base electrolyte of
1.2M LiPF6 in
30% ethylene carbonate and 70% ethyl methyl carbonate.
[0047] FIG. 8F illustrates voltage hysteresis (difference between the
average charge
voltage and the average charge voltage) for electrolyte systems including 2%
FEC, 1% VC +
1% DTD, 2% VC + 1% DTD, 1% VC + 1% MMDS, and 2% VC + 1% MMDS, cycling
between 3.0 V and 4.3 V at 40 C in abase electrolyte of 1.2M LiPF6 in 30%
ethylene carbonate
and 70% ethyl methyl carbonate by weight.
[0048] FIG. 8G illustrates peak capacity versus cycle number for
electrolyte systems
including 2% PES, 1% PES + 1% DTD, 2% PES + 1% DTD, 1% PES + 1% MMDS, and 2%
PES + 1% MMDS, cycling between 3.0 V and 4.3 V at 40 C in a base electrolyte
of 1.2M
LiPF6 in 30% ethylene carbonate and 70% ethyl methyl carbonate.
6
CA 03071314 2020-01-28
WO 2019/025980 PCT/IB2018/055745
[0049] FIG. 811 illustrates normalized capacity versus cycle number for
electrolyte
systems including 2% PES, 1% PES + 1% DTD, 2% PES + 1% DTD, 1% PES + 1% MMDS,
and 2% PES + 1% MMDS, cycling between 3.0 V and 4.3 V at 40 C in a base
electrolyte of
1.2M LiPF6 in 30% ethylene carbonate and 70% ethyl methyl carbonate.
[0050] FIG. 81 illustrates voltage hysteresis (difference between the
average charge
voltage and the average charge voltage) for electrolyte systems including 2%
FEC, 1% PES +
1% DTD, 2% PES + 1% DTD, 1% PES + 1% MMDS, and 2% PES + 1% MMDS, cycling
between 3.0 V and 4.3 V at 40 C in abase electrolyte of 1.2M LiPF6 in 30%
ethylene carbonate
and 70% ethyl methyl carbonate.
[0051] FIGs. 9A-9D and 9F-9I illustrate typical experimental data collected
during some
of the ultra-high-precision-charging experiments that show that methyl acetate
can be added to
electrolyte systems containing VC or FEC with DTD to increase electrolyte
conductivity and
lower viscosity without sacrificing much lifetime. Increasing conductivity and
decreasing
viscosity is important for certain applications requiring a faster rate of
charge.
[0052] FIG. 9A illustrates typical experimental data showing coulombic
efficiency (CE)
versus cycle number for electrolyte systems according to certain embodiments
of the present
disclosure.
[0053] FIG. 9B illustrates typical experimental data showing the capacity
of the charge
endpoint plotted versus cycle number for electrolyte systems according to
certain embodiments
of the present disclosure.
[0054] FIG. 9C illustrates typical experimental data showing the discharge
capacity versus
cycle number for electrolyte systems according to certain embodiments of the
present
disclosure.
[0055] FIG. 9D illustrates typical experimental data showing the difference
between the
average charge voltage and the average charge voltage (Delta V) in open
circuit voltage versus
cycle number for electrolyte systems according to certain embodiments of the
present
disclosure.
[0056] FIG. 9F illustrates typical experimental data of coulombic
efficiency (CE) versus
cycle number for electrolyte systems according to certain embodiments of the
present
disclosure.
[0057] FIG. 9G illustrates typical experimental data of the capacity of the
charge endpoint
plotted versus cycle number for electrolyte systems according to certain
embodiments of the
present disclosure.
7
CA 03071314 2020-01-28
WO 2019/025980 PCT/IB2018/055745
[0058] FIG. 911 illustrates typical experimental data of the discharge
capacity versus cycle
number for electrolyte systems according to certain embodiments of the present
disclosure.
[0059] FIG. 91 illustrates typical experimental data of the difference
between the average
charge voltage and the average charge voltage (Delta V) versus cycle number
for electrolyte
systems according to certain embodiments of the present disclosure.
[0060] FIGs. 10A-C are plots that summarize experimental data and
illustrate that as the
MA content increases, the electrolyte additives VC and FEC, both alone and in
the presence of
DTD, still offer acceptable performance.
[0061] FIG. 10A is a plot that summarizes experimental data of time
normalized CIE as a
function of MA content.
[0062] FIG. 10B is a plot that summarizes experimental data of time
normalized fractional
fade as a function of MA content.
[0063] FIG. 10C is a plot that summarizes experimental data of time
normalized fractional
charge endpoint capacity slippage as a function of MA content.
[0064] FIG. 11 is a plot that summarizes experimental data of parasitic
heat flow and
difference between the parasitic heat flow and that of the cell containing 2%
FEC + 0% MA as
a function of the voltage for different electrolyte compositions that contain
FEC in the voltage
range of 4.0 V to 4.2 V.
[0065] FIG. 12 A-B are plots that summarizes experimental data of parasitic
heat flow and
difference between the parasitic heat flow and that of the cell containing 2%
FEC + 0% MA as
a function of the voltage for different electrolyte compositions that contain
FEC in the voltage
range of 4.0 V to 4.3 V. FIG 12 A shows results for the first cycle to 4.3 V.
Figure 12B shows
results for the second cycle.
[0066] FIG. 13A-B are plots that summarizes experimental data of parasitic
heat flow and
difference between the parasitic heat flow and that of the cell containing 2%
FEC + 0% MA as
a function of the voltage for different electrolyte compositions that contain
FEC in the voltage
range of 4.0 V to 4.4 V. FIG 13A shows results for the first cycle to 4.4 V.
Figure 13B shows
results for the second cycle.
[0067] FIG. 14 is a plot that summarizes experimental parasitic heat flow
data, including
the data shown in FIGs. 11-13.
[0068] FIGs. 15A-F are plots of experimental data taken at 20 C of
capacity, normalized
capacity, and voltage hysteresis (difference between the average charge
voltage and the average
charge voltage) versus cycle number for electrolyte systems that contain FEC.
8
CA 03071314 2020-01-28
WO 2019/025980 PCT/IB2018/055745
[0069] FIG. 15A is a plot of experimental data taken at 20 C of capacity
versus cycle
number for electrolyte systems that contain FEC with cycling up to 4.2 V.
[0070] FIG. 15B is a plot of experimental data taken at 20 C of normalized
capacity versus
cycle number for electrolyte systems that contain FEC with cycling up to 4.2
V.
[0071] FIG. 15C is a plot of experimental data taken at 20 C of voltage
hysteresis
(difference between the average charge voltage and the average charge voltage)
versus cycle
number for electrolyte systems that contain FEC with cycling up to 4.2 V.
[0072] FIG. 15D is a plot of experimental data taken at 20 C of capacity
versus cycle
number for electrolyte systems that contain FEC with cycling up to 4.3 V.
[0073] FIG. 15E is a plot of experimental data taken at 20 C of normalized
capacity versus
cycle number for electrolyte systems that contain FEC with cycling up to 4.3
V.
[0074] FIG. 15F is a plot of experimental data taken at 20 C of voltage
hysteresis
(difference between the average charge voltage and the average charge voltage)
versus cycle
number for electrolyte systems that contain FEC with cycling up to 4.3 V.
[0075] FIGs. 16A-F are plots of experimental data taken at 40 C of
capacity, normalized
capacity, and voltage hysteresis for electrolyte systems that contain FEC.
[0076] FIG. 16A is a plot of experimental data taken at 40 C of capacity
versus cycle
number for electrolyte systems that contain FEC with cycling up to 4.2 V.
[0077] FIG. 16B is a plot of experimental data taken at 40 C of normalized
capacity versus
cycle number for electrolyte systems that contain FEC with cycling up to 4.2
V.
[0078] FIG. 16C is a plot of experimental data taken at 40 C of voltage
hysteresis
(difference between the average charge voltage and the average charge voltage)
versus cycle
number for electrolyte systems that contain FEC with cycling up to 4.2 V.
[0079] FIG. 16D is a plot of experimental data taken at 40 C of capacity
versus cycle
number for electrolyte systems that contain FEC with cycling up to 4.3 V.
[0080] FIG. 16E is a plot of experimental data taken at 40 C of normalized
capacity versus
cycle number for electrolyte systems that contain FEC with cycling up to 4.3
V.
[0081] FIG. 16F is a plot of experimental data taken at 40 C of voltage
hysteresis
(difference between the average charge voltage and the average charge voltage)
versus cycle
number for electrolyte systems that contain FEC with cycling up to 4.3 V.
[0082] FIGs. 17A-F are plots of experimental data of capacity, normalized
capacity, and
voltage hysteresis for electrolyte systems that contain FEC, VC, and/or DTD.
9
CA 03071314 2020-01-28
WO 2019/025980 PCT/IB2018/055745
[0083] FIG. 17A is a plot of experimental data of capacity versus cycle
number for
electrolyte systems that contain FEC and/or DTD with cycling up to 4.3 V.
[0084] FIG. 17B is a plot of experimental data of normalized capacity
versus cycle number
for electrolyte systems that contain FEC and/or DTD with cycling up to 4.3 V.
[0085] FIG. 17C is a plot of experimental data of voltage hysteresis
(difference between
the average charge voltage and the average charge voltage) versus cycle number
for electrolyte
systems that contain FEC and/or DTD with cycling up to 4.3 V.
[0086] FIG. 17D is a plot of experimental data of capacity versus cycle
number for
electrolyte systems that contain VC and/or DTD with cycling up to 4.3 V.
[0087] FIG. 17E is a plot of experimental data of normalized capacity
versus cycle number
for electrolyte systems that contain VC and/or DTD with cycling up to 4.3 V.
[0088] FIG. 17F is a plot of experimental data of voltage hysteresis
(difference between
the average charge voltage and the average charge voltage) versus cycle number
for electrolyte
systems that contain VC and/or DTD with cycling up to 4.3 V.
[0089] FIG. 18A is a plot of experimental data of capacity versus cycle
number for
electrolyte systems that contain FEC with cycling up to 4.3 V.
[0090] FIG. 18B is a plot of experimental data of normalized capacity
versus cycle number
for electrolyte systems that contain FEC with cycling up to 4.3 V.
[0091] FIG. 19 is a plot of experimental data of voltage hysteresis
(difference between the
average charge voltage and the average charge voltage) versus cycle number for
electrolyte
systems that contain FEC with cycling up to 4.3 V.
[0092] FIG. 20 is a plot that summarizes experimental data of the volume of
formation gas
generated during cell formation for different electrolyte systems.
[0093] FIG. 21 is a plot that summarizes experimental data of charge
transfer impedance
for different electrolyte systems.
[0094] FIG. 22 is a plot summarizing experimental data measuring the low
rate capacity
loss for different electrolyte systems after cells were charged at three
different charge rates at
20 C for 30 cycles.
[0095] FIG. 23 is a plot summarizing experimental data summarizing the peak
capacity as
a function of cycle number for different electrolyte systems used in cells
being charged at
different charge rates at 20 C.
[0096] FIG. 24 is a plot summarizing experimental data summarizing the peak
capacity as
a function of cycle number for different electrolyte systems used in cells
being charged at
different charge rates at 20 C.
CA 03071314 2020-01-28
WO 2019/025980 PCT/IB2018/055745
[0097] FIG. 25 is a plot that summarizes experimental data of the volume of
formation gas
for different additives in varying concentrations of MA solvent.
[0098] FIG. 26 is a plot that summarizes experimental data of charge
transfer impedance
for different additives in electrolytes containing varying concentrations of
MA solvent.
[0099] FIG. 27 is a plot that summarizes experimental data of low rate
capacity loss for
different electrolyte compositions and after charging at 1, 1.5, and 2C for 30
cycles at 20 C.
[0100] FIG. 28 is a blowup of certain experimental data shown in FIG. 27.
[0101] FIG. 29 summarizes experimental data of delta V (difference between
the average
charge voltage and the average charge voltage) as a function of cycle number
for electrolyte
systems containing FEC.
[0102] FIG. 30 summarizes experimental data of peak capacity as a function
of cycle
number for electrolyte systems containing FEC.
[0103] FIG. 31 summarizes experimental data of energy hysteresis as a
function of cycle
number for electrolyte systems containing FEC.
[0104] FIG. 32 summarizes experimental data of delta V (difference between
the average
charge voltage and the average charge voltage) as a function of cycle number
for electrolyte
systems containing VC.
[0105] FIG. 33 summarizes experimental data of peak capacity as a function
of cycle
number for electrolyte systems containing VC.
[0106] FIG. 34 summarizes experimental data of energy hysteresis as a
function of cycle
number for electrolyte systems containing VC.
[0107] FIGs. 35A-D summarize experimental data for electrolyte systems with
a positive
electrode of NMC532 and a negative electrode of artificial graphite.
[0108] FIG. 35A summarizes experimental impedance data, plotting the
negative of the
imaginary portion of the impedance against the real portion of the impedance
for different
electrolyte systems, including systems containing LFO.
[0109] FIG. 35B summarizes experimental impedance data, plotting the
negative of the
imaginary portion of the impedance against the real portion of the impedance
for different
electrolyte systems, including containing VC, PES or LFO.
[0110] FIG. 35C summarizes experimental impedance data, plotting the
negative of the
imaginary portion of the impedance against the real portion of the impedance
for different
electrolyte systems, including systems containing FEC, DTD, or LFO.
11
CA 03071314 2020-01-28
WO 2019/025980 PCT/IB2018/055745
[0111] FIG. 35D summarizes experimental impedance data for different
electrolyte
systems containing VC, FEC, DTD, PES, or LFO, a positive electrode of NMC532,
and a
negative electrode of artificial graphite.
[0112] FIG. 36 summarizes experimental data for different electrolyte
systems containing
VC, FEC, DTD, PES, or LFO.
[0113] FIGs. 37A-F summarize experimental storage data for different
electrolyte systems
containing LFO compared to a control without LFO.
[0114] FIG. 37A summarizes voltage-drop data for different electrolyte
systems
containing LFO compared to a control without LFO, after the systems were
stored at 60 C for
500 hours at 4.4V.
[0115] FIG. 37B summarizes voltage-drop data for different electrolyte
systems
containing LFO compared to a control without LFO, after the systems were
stored at 60 C for
500 hours at 2.5V.
[0116] FIG. 37C summarizes volume-change data for different electrolyte
systems
containing LFO compared to a control without LFO, after the systems were
stored at 60 C for
500 hours at 4.4V.
[0117] FIG. 37D summarizes volume-change data for different electrolyte
systems
containing LFO compared to a control without LFO, after the systems were
stored at 60 C for
500 hours at 2.5V.
[0118] FIG. 37E summarizes impedance data for different electrolyte systems
containing
LFO compared to a control without LFO, before and after the systems were
stored at 60 C for
500 hours at 4.4V.
[0119] FIG. 37F summarizes impedance data for different electrolyte systems
containing
LFO compared to a control without LFO, before and after the systems were
stored at 60 C for
500 hours at 2.5V.
[0120] FIGs. 38A-F summarize experimental storage data for different
electrolyte systems
containing LFO compared to a control without LFO.
[0121] FIG. 38A summarizes voltage-drop data for different electrolyte
systems
containing LFO compared to a control without LFO, after the systems were
stored at 60 C for
500 hours at 4.4V.
[0122] FIG. 38B summarizes voltage-drop data for different electrolyte
systems
containing LFO compared to a control without LFO, after the systems were
stored at 60 C for
500 hours at 2.5V.
12
CA 03071314 2020-01-28
WO 2019/025980 PCT/IB2018/055745
[0123] FIG. 38C summarizes volume-change data for different electrolyte
systems
containing LFO compared to a control without LFO, after the systems were
stored at 60 C for
500 hours at 4.4V.
[0124] FIG. 38D summarizes volume-change data for different electrolyte
systems
containing LFO compared to a control without LFO, after the systems were
stored at 60 C for
500 hours at 2.5V.
[0125] FIG. 38E summarizes impedance data for different electrolyte systems
containing
LFO compared to a control without LFO, before and after the systems were
stored at 60 C for
500 hours at 4.4V.
[0126] FIG. 38F summarizes impedance data for different electrolyte systems
containing
LFO compared to a control without LFO, before and after the systems were
stored at 60 C for
500 hours at 2.5V.
[0127] FIGs. 39A-F summarize experimental data for different electrolyte
systems
containing LFO compared to a control without LFO.
[0128] FIG. 39A summarizes data of coulombic efficiency (CE) versus cycle
number for
electrolyte systems, including systems containing LFO, with cycling to 4.1V.
[0129] FIG. 39B summarizes data of the change in voltage versus cycle
number for
different electrolyte systems, including systems containing LFO, with cycling
to 4.1V.
[0130] FIG. 39C summarizes data of the capacity of the charge endpoint
plotted versus
cycle number for different electrolyte systems, including systems containing
LFO, with cycling
to 4.1V.
[0131] FIG. 39D summarizes data of the normalized discharge capacity verses
cycle
number for different electrolyte systems, including systems containing LFO,
with cycling to
4.1V.
[0132] FIG. 39E summarizes data of coulombic efficiency (CE) versus cycle
number for
electrolyte systems, including systems containing LFO, with cycling to 4.2V.
[0133] FIG. 39F summarizes data of the change in voltage versus cycle
number for
different electrolyte systems, including systems containing LFO, with cycling
to 4.2V.
[0134] FIG. 39G summarizes data of the capacity of the charge endpoint
plotted versus
cycle number for different electrolyte systems, including systems containing
LFO, with cycling
to 4.2V.
13
CA 03071314 2020-01-28
WO 2019/025980 PCT/IB2018/055745
[0135] FIG. 3911 summarizes data of the normalized discharge capacity
verses cycle
number for different electrolyte systems, including systems containing LFO,
with cycling to
4.2V.
[0136] FIGs. 40A-F summarize experimental data for different electrolyte
systems
containing LFO compared to a control without LFO.
[0137] FIG. 40A summarizes data of coulombic efficiency (CE) versus cycle
number for
electrolyte systems, including systems containing LFO, with cycling to 4.3V.
[0138] FIG. 40B summarizes data of the change in open circuit voltage
versus cycle
number for different electrolyte systems, including systems containing LFO,
with cycling to
4.3V.
[0139] FIG. 40C summarizes data of the capacity of the charge endpoint
plotted versus
cycle number for different electrolyte systems, including systems containing
LFO, with cycling
to 4.3V.
[0140] FIG. 40D summarizes data of the normalized discharge capacity verses
cycle
number for different electrolyte systems, including systems containing LFO,
with cycling to
4.3V.
[0141] FIG. 40E summarizes data of coulombic efficiency (CE) versus cycle
number for
electrolyte systems, including systems containing LFO, with cycling to 4.4V.
[0142] FIG. 40F summarizes data of the change in open circuit voltage
versus cycle
number for different electrolyte systems, including systems containing LFO,
with cycling to
4.4V.
[0143] FIG. 40G summarizes data of the capacity of the charge endpoint
plotted versus
cycle number for different electrolyte systems, including systems containing
LFO, with cycling
to 4.4V.
[0144] FIG. 4011 summarizes data of the normalized discharge capacity
verses cycle
number for different electrolyte systems, including systems containing LFO,
with cycling to
4.4V.
[0145] FIG. 41A summarizes data of coulombic inefficiency verses upper cut-
off voltage
for different electrolyte systems, including systems containing LFO.
[0146] FIG. 41B summarizes data of fractional fade verses upper cut-off
voltage for
different electrolyte systems, including systems containing LFO.
[0147] FIG. 41C summarizes data of charge-end-point capacity slippage
verses upper cut-
off voltage for different electrolyte systems, including systems containing
LFO.
14
CA 03071314 2020-01-28
WO 2019/025980 PCT/IB2018/055745
[0148] FIG. 42A illustrates an expanded view of FIG. 41A and summarizes
data of
coulombic inefficiency verses upper cut-off voltage for different electrolyte
systems, including
systems containing LFO.
[0149] FIG. 42B illustrates an expanded view of FIG. 41B and summarizes
data of
fractional fade verses upper cut-off voltage for different electrolyte
systems, including systems
containing LFO.
[0150] FIG. 42C illustrates an expanded view of FIG. 41C and summarizes
data of charge-
end-point capacity slippage verses upper cut-off voltage for different
electrolyte systems,
including systems containing LFO.
[0151] FIGs. 43A-D summarize long-term-cycling data for different
electrolyte systems,
including systems containing LFO.
[0152] FIG. 43A summarizes normalized-discharge-capacity data for different
electrolyte
systems, including systems containing LFO, with cycling occurring at 40 C.
[0153] FIG. 43B summarizes average-charge-voltage data for different
electrolyte
systems, including systems containing LFO, with cycling occurring at 40 C.
[0154] FIG. 43C summarizes normalized-discharge-capacity data for different
electrolyte
systems, including systems containing LFO, with cycling occurring at 20 C.
[0155] FIG. 43D summarizes average-charge-voltage data for different
electrolyte
systems, including systems containing LFO, with cycling occurring at 20 C.
[0156] FIGs. 44A-D summarize long-term-cycling data under high-rate
charging for
different electrolyte systems, including systems containing LFO.
[0157] FIG. 44A summarizes normalized-discharge-capacity data for different
electrolyte
systems, including systems containing LFO, with cycling occurring at 20 C
during a first
experiment.
[0158] FIG. 44B summarizes average-charge-voltage data for different
electrolyte
systems, including systems containing LFO, with cycling occurring at 20 C
during a first
experiment.
[0159] FIG. 44C summarizes normalized-discharge-capacity data for different
electrolyte
systems, including systems containing LFO, with cycling occurring at 20 C
during a second
experiment.
[0160] FIG. 44D summarizes average-charge-voltage data for different
electrolyte
systems, including systems containing LFO, with cycling occurring at 20 C
during a second
experiment.
CA 03071314 2020-01-28
WO 2019/025980 PCT/IB2018/055745
[0161] FIG. 45A summarizes voltage data for different electrolyte systems,
including
systems containing LFO, as cells were held at 40 C.
[0162] FIG. 45B summarizes volume-change data for different electrolyte
systems,
including systems containing LFO, as cells were held at 40 C.
[0163] FIGs. 46A-D summarize voltage-drop and impedance data generated
during
storage experiments.
[0164] FIG. 46A summarizes voltage-drop data for different electrolyte
systems, including
systems containing LFO, after cells were held at 60 C for 500 hours at 4.4V.
[0165] FIG. 46B summarizes impedance data for different electrolyte
systems, including
systems containing LFO, before and after cells were held at 60 C for 500 hours
at 4.4V.
[0166] FIG. 46C summarizes voltage-drop data for different electrolyte
systems, including
systems containing LFO, after cells were held at 60 C for 500 hours at 2.5V.
[0167] FIG. 46D summarizes impedance data for different electrolyte
systems, including
systems containing LFO, before and after cells were held at 60 C for 500 hours
at 2.5V.
[0168] FIG. 47 illustrates exemplary data during certain charge and
discharge situations.
[0169] FIGs. 48A-F summarize experimental heat-flow data verses voltage.
FIGs. 48A, C,
and E show results for the first cycle to 4.4 V. FIGs. 48B, D, and F show
results for the second
cycle to 4.4 V.
[0170] FIG. 48A summarizes experimental parasitic-heat-flow data and
difference
between the parasitic heat flow and that of a cell containing 2% VC + 1% DTD
as a function
of the voltage for different electrolyte systems, including systems containing
DTD, in the
voltage range of 4.0 V to 4.4 V during a first cycle.
[0171] FIG. 48B summarizes experimental parasitic-heat-flow data and
difference
between the parasitic heat flow and that of a cell containing 2% VC + 1% DTD
as a function
of the voltage for different electrolyte systems, including systems containing
DTD, in the
voltage range of 4.0 V to 4.4 V during a second cycle.
[0172] FIG. 48C summarizes experimental parasitic-heat-flow data and
difference
between the parasitic heat flow and that of a cell containing 2% VC + 1% DTD
as a function
of the voltage for different electrolyte systems, including systems containing
LFO, in the
voltage range of 4.0 V to 4.4 V during a first cycle.
[0173] FIG. 48D summarizes experimental parasitic-heat-flow data and
difference
between the parasitic heat flow and that of a cell containing 2% VC + 1% DTD
as a function
16
CA 03071314 2020-01-28
WO 2019/025980 PCT/IB2018/055745
of the voltage for different electrolyte systems, including systems containing
LFO, in the
voltage range of 4.0 V to 4.4 V during a second cycle.
[0174] FIG. 48E summarizes experimental parasitic-heat-flow data and
difference
between the parasitic heat flow and that of a cell containing 2% VC + 1% DTD
as a function
of the voltage for different electrolyte systems, including systems containing
LFO, in the
voltage range of 4.0 V to 4.4 V during a first cycle.
[0175] FIG. 48F summarizes experimental parasitic-heat-flow data and
difference
between the parasitic heat flow and that of a cell containing 2% VC + 1% DTD
as a function
of the voltage for different electrolyte systems, including systems containing
LFO, in the
voltage range of 4.0 V to 4.4 V during a second cycle.
[0176] FIGs. 50A-C summarize experimental mean-parasitic-heat-flow data as
a function
of the cycle number for different electrolyte systems.
[0177] FIG. 50A summarizes experimental mean-parasitic-heat-flow data as a
function of
the cycle number for electrolyte systems, including 2% VC + 1% DTD and 2% FEC
+ 1%
DTD.
[0178] FIG. 50B summarizes experimental mean-parasitic-heat-flow data as a
function of
the cycle number for electrolyte systems, including 0.5% LFO, 1% LFO, 1.5%
LFO, 0.5% LFO
+ 1% VC + 1% FEC, 1.0% LFO + 1% VC + 1% FEC, and 1.5% LFO + 1% VC + 1% FEC.
[0179] FIG. 50C summarizes experimental mean-parasitic-heat-flow data as a
function of
the cycle number for electrolyte systems, including 1% LFO, 1% LFO + 1% VC, 1%
LFO +
1% FEC, and 1% LFO + 1% VC + 1% FEC.
[0180] FIG. 51 summarizes experimental data from FIGs. 50A-C, illustrating
the best
performing cells from the parasitic-heat-flow experiments.
[0181] FIGs. 52A-D summarize experimental data for different electrolyte
systems
containing LFO compared to a control without LFO, with cycling to 4.2 V.
[0182] FIG. 52A summarizes data of coulombic efficiency versus cycle number
for
electrolyte systems, including systems containing LFO, with cycling to 4.2 V.
[0183] FIG. 52B summarizes data of the capacity of the charge endpoint
plotted versus
cycle number for different electrolyte systems, including systems containing
LFO, with cycling
to 4.2 V.
[0184] FIG. 52C summarizes data of the change in voltage verses cycle
number for
different electrolyte systems, including systems containing LFO, with cycling
to 4.2 V.
17
CA 03071314 2020-01-28
WO 2019/025980 PCT/IB2018/055745
[0185] FIG. 52D summarizes data of the normalized discharge capacity verses
cycle
number for different electrolyte systems, including systems containing LFO,
with cycling to
4.2 V.
[0186] FIGs. 53A-D summarize experimental data for different electrolyte
systems
containing LFO compared to a control without LFO, with cycling to 4.3 V.
[0187] FIG. 53A summarizes data of coulombic efficiency versus cycle number
for
electrolyte systems, including systems containing LFO, with cycling to 4.3 V.
[0188] FIG. 53B summarizes data of the capacity of the charge endpoint
plotted versus
cycle number for different electrolyte systems, including systems containing
LFO, with cycling
to 4.3 V.
[0189] FIG. 53C summarizes data of the change in voltage verses cycle
number for
different electrolyte systems, including systems containing LFO, with cycling
to 4.3 V.
[0190] FIG. 53D summarizes data of the normalized discharge capacity verses
cycle
number for different electrolyte systems, including systems containing LFO,
with cycling to
4.3 V.
[0191] FIGs. 54A-D summarize experimental data for different electrolyte
systems
containing LFO compared to a control without LFO, with cycling to 4.4 V.
[0192] FIG. 54A summarizes experimental data of coulombic efficiency versus
cycle
number for electrolyte systems, including systems containing LFO, with cycling
to 4.4 V.
[0193] FIG. 54B summarizes experimental data of the capacity of the charge
endpoint
plotted versus cycle number for different electrolyte systems, including
systems containing
LFO, with cycling to 4.4 V.
[0194] FIG. 54C summarizes experimental data of the change in voltage
verses cycle
number for different electrolyte systems, including systems containing LFO,
with cycling to
4.4 V.
[0195] FIG. 54D summarizes experimental data of the normalized discharge
capacity
verses cycle number for different electrolyte systems, including systems
containing LFO, with
cycling to 4.4 V.
[0196] FIG. 55A summarizes data of coulombic inefficiency verses upper cut-
off voltage
for different electrolyte systems, including systems containing LFO.
[0197] FIG. 55B summarizes data of fractional fade verses upper cut-off
voltage for
different electrolyte systems, including systems containing LFO.
[0198] FIG. 55C summarizes data of charge-end-point capacity slippage
verses upper cut-
off voltage for different electrolyte systems, including systems containing
LFO.
18
CA 03071314 2020-01-28
WO 2019/025980 PCT/IB2018/055745
[0199] FIG. 56A illustrates an expanded view of FIG. 55A and summarizes
data of
coulombic inefficiency verses upper cut-off voltage for different electrolyte
systems, including
systems containing LFO.
[0200] FIG. 56B illustrates an expanded view of FIG. 55B and summarizes
data of
fractional fade verses upper cut-off voltage for different electrolyte
systems, including systems
containing LFO.
[0201] FIG. 56C illustrates an expanded view of FIG. 55C and summarizes
data of charge-
end-point capacity slippage verses upper cut-off voltage for different
electrolyte systems,
including systems containing LFO.
[0202] FIG. 57 impedance data generated during and after ultrahigh-
precision-cycling
experiments.
[0203] FIGs. 58A-D summarize experimental data for electrolyte systems
containing LFO
with a positive electrode made from NMC 622 with two different coatings.
[0204] FIG. 58A summarizes experimental data of voltage drop after storage
at 60 C for
500 of various electrolyte systems at 4.4V.
[0205] FIG. 58B summarizes experimental data of impedance before storage
and after
storage at 60 C for 500 of various electrolyte systems at 4.4V.
[0206] FIG. 58C summarizes experimental data of voltage drop after storage
at 60 C for
500 of various electrolyte systems at 2.5V.
[0207] FIG. 58D summarizes experimental data of impedance before storage
and after
storage at 60 C for 500 of various electrolyte systems at 2.5V.
[0208] FIG. 60 summarizes experimental data of mass change from air
exposure over time
for LFO from Guangzhou Tinci Materials Technology Co., Ltd. and Shenzhen
Capchem
Technology Co., Ltd.
[0209] FIG. 61 summarizes experimental data of thermal gravimetric analysis
for LFO
from Guangzhou Tinci Materials Technology Co., Ltd. and Shenzhen Capchem
Technology
Co., Ltd.
DETAILED DESCRIPTION OF THE DISCLOSURE
[0210] FIG. 1 illustrates the basic components of a battery powered
electric vehicle
(electric vehicle) 100. The electric vehicle 100 includes at least one drive
motor (traction
motor) 102A and/or 102B, at least one gear box 104A and/or 104B coupled to a
corresponding
drive motor 102A and/or 102B, battery cells 106 and electronics 108.
Generally, the battery
cells 106 provide electricity to power electronics of the electric vehicle 100
and to propel the
19
CA 03071314 2020-01-28
WO 2019/025980 PCT/IB2018/055745
electric vehicle 100 using the drive motor 102A and/or 102B. The electric
vehicle 100 includes
a large number of other components that are not described herein but known to
one or ordinary
skill. While the construct of the electric vehicle 100 of FIG. 1 is shown to
have four wheels,
differing electric vehicles may have fewer or more than four wheels. Further,
differing types
of electric vehicles 100 may incorporate the inventive concepts described
herein, including
motor cycles, aircraft, trucks, boats, train engines, among other types of
vehicles. Certain parts
created using embodiments of the present disclosure may be used in vehicle
100.
[0211] FIG. 2 illustrates a schematic view of an exemplary energy storage
system 200
showing various components. The energy storage system 200 typically includes a
modular
housing with at least a base 202 and four side walls 204 (only two shown in
the figure). The
module housing is generally electrically isolated from the housed battery
cells 206. This may
occur through physical separation, through an electrically insulating layer,
through the choice
of an insulating material as the module housing, any combination thereof, or
another through
another method. The base 202 may be an electrically insulating layer on top of
a metal sheet
or a nonconductive/electrically insulating material, such as polypropylene,
polyurethane,
polyvinyl chlorine, another plastic, a nonconductive composite, or an
insulated carbon fiber.
Side walls 204 may also contain an insulating layer or be formed out of a
nonconductive or
electrically insulating material, such as polypropylene, polyurethane,
polyvinyl chlorine,
another plastic, a nonconductive composite, or an insulated carbon fiber. One
or more
interconnect layers 230 may be positioned above the battery cells 206, with a
top plate 210
positioned over the interconnect layer 230. The top plate 210 may either be a
single plate or be
formed from multiple plates.
[0212] Individual battery cells 106 and 206 often are lithium-ion battery
cells, with an
electrolyte containing lithium ions and positive and negative electrodes. FIG.
3 illustrates a
schematic of a lithium ion cell 300. Lithium ions 350 are dispersed throughout
electrolyte 320,
within container 360. Container 360 may be part of a battery cell. The lithium
ions 350 migrate
between positive electrode 330 and negative electrode 340. Separator 370
separates the
negative electrode and positive electrode. Circuitry 310 connects the negative
electrode and
positive electrode.
[0213] New studies by the inventors have identified novel electrolyte and
battery systems
for use in grid and electric vehicle applications. These systems are based on
two-additive
electrolyte systems combined with solvents and electrodes, including 1)
vinylene carbonate
(VC) combined with 1,3,2-Dioxathiolane-2,2-dioxide (DTD, also known as
ethylene sulfate)
or another sulfur-containing additive, 2) fluoro ethylene carbonate (FEC)
combined with DTD
CA 03071314 2020-01-28
WO 2019/025980 PCT/IB2018/055745
or another sulfur-containing additive, and 3) prop-1-ene-1,3-sultone (PES)
combined with
DTD or another sulfur-containing additive. These two-additive electrolyte
systems are paired
with a positive electrode made from lithium nickel manganese cobalt oxide with
the
composition LNiMnyCo,02 (abbreviated NMC generally or NMCxyz where the x, y,
and z are
the molar ratios of nickel, manganese and cobalt respectively). In certain
embodiments, the
positive electrode is formed from NMC111, NMC532, NMC811, or NMC622. In
certain
embodiments, NMC 532 positive electrodes formed from single-crystal,
micrometer-side
particles, which resulted in an electrode with micrometer-size areas of
continuous crystal lattice
(or grains), have been shown to be particularly robust, in part because the
materials and
processing conditions result in larger grain sizes than using conventional
materials and
processing conditions.
[0214] Typical processing conditions lead to NMC electrodes with nanometer-
sized
particles packed into larger micrometer-sized agglomerates, creating grain
boundaries on the
nanometer scale. Grain boundaries are defects that tend to reduce desirable
properties (for
example, electrical properties), so it is typically desirable to reduce the
number of grains and
increase the grain size. Processing can create larger domains, on the
micrometer size scale,
thereby reducing the number of grain boundaries in the NMC electrodes,
increasing electrical
properties. The increase in properties is results in more robust battery
systems. In certain
embodiments, other NMC electrodes may be processed to create larger domain
sizes (on the
micrometer-size scale or larger), for example, NMC111, NMC811, NMC622, or
another NMC
compound to create more robust systems.
[0215] The positive electrode may be coated with a material such as
aluminum oxide
(A1203), titanium dioxide (TiO2), or another coating. Coating the positive
electrode is
advantageous because it can help reduce interfacial phenomena at the positive
electrode, such
as parasitic reactions, thermal abuse, or another phenomenon, that can
deteriorate the system.
The negative electrode may be made from natural graphite, artificial graphite,
or another
material.
[0216] The electrolyte may be a lithium salt dissolved (such as LiPF6) in a
combination of
organic or non-aqueous solvents, including ethylene carbonate, ethyl methyl
carbonate, methyl
acetate, propylene carbonate, dimethyl carbonate, diethyl carbonate, another
carbonate solvent
(cyclic or acyclic), another organic solvent, and/or another non-aqueous
solvent. Solvents are
present in concentrations greater than the additives, typically greater than
6% by weight. While
the experimental data was generated using an electrolyte solvent that included
EC and EMC
(with or without MA), these solvents are merely exemplary of other carbonate
solvents in
21
CA 03071314 2020-01-28
WO 2019/025980 PCT/IB2018/055745
particular and to other non-aqueous solvents. EC and EMC solvents were used in
the
experiments to control the systems tested in order to understand the effects
of the additives,
electrodes, and addition of MA as a solvent. Electrolyte systems may therefore
may use other
carbonate solvents and/or other non-carbonate solvents, including propylene
carbonate,
ethylene carbonate, dimethyl carbonate, ethyl methyl carbonate, diethyl
carbonate, another
carbonate solvent (cyclic or acyclic), another organic solvent, and/or another
non-aqueous
solvent. Solvents are present in concentrations greater than the additives,
typically greater than
6% by weight.
[0217] In the two-additive mixture FEC and DTD, the concentration of FEC is
preferentially between 0.5 to 6% by weight and the concentration of the DTD is
preferentially
between 0.25 to 5% by weight. In the two-additive mixture VC and DTD, the
concentration of
VC is preferentially between 0.5 to 6% by weight and the concentration of the
DTD is
preferentially between 0.25 to 5% by weight.
[0218] Certain of these new battery systems may be used in energy-storage
applications
and also automobile application (including energy storage within an electric
vehicle) in which
charge and discharge speeds, and lifetime when charging and discharging
quickly are
important. Specifically, MA may be used as an electrolyte solvent to provide
longer lifetime
when charging and discharging at higher current rates.
Pre-Experimental Setup
Although the battery systems themselves may be packaged differently according
to the present
disclosure, the experimental setup typically used machine made "pouch cells"
to systematically
evaluate the battery systems using a common setup, including the two-additive
electrolyte
systems and the specific materials for use the positive and negative
electrodes. All percentages
mentioned within this disclosure are weight percentages unless otherwise
specified. A person
of skill in the art will appreciate that the type of additive to be used and
the concentration to be
employed will depend on the characteristics which are most desirably improved
and the other
components and design used in the lithium ion batteries to be made and will be
apartment from
this disclosure.
Pouch Cells
[0219] The pouch cells used in the experimental setup contain 1 M LiPF6 in
the solvent to
which additives were added. Depending on the concentration of methyl acetate
(0, 20, or 40%),
the electrolyte consisted of 1 M LiPF6 in (1) 1.2M LiPF6 in 30% ethylene
carbonate and 70%
ethyl methyl carbonate, (2) 1.2M LiPF6 in 24% ethylene carbonate, 56% ethyl
methyl
carbonate, and 20% methyl acetate; or (3) 1.2M LiPF6 in 18% ethylene
carbonate, 42% ethyl
22
CA 03071314 2020-01-28
WO 2019/025980 PCT/IB2018/055745
methyl carbonate, and 40% methyl acetate. To this electrolyte, the additive
components were
added at specified weight percentages.
[0220] The pouch cells used a positive electrode made of NMC532 with
micrometer-sized
grains (sometimes referred to as single-crystal NMC532), and a negative
electrode made of
artificial graphite, unless otherwise specified. To test certain battery
systems, other positive,
including standard NMC532 (with smaller grains than the NMC with micrometer-
sized grains)
and NMC622, and negative electrodes (including natural graphite) were used.
[0221] Before electrolyte filling, the pouch cells were cut open below the
heat seal and
dried at 100 C under vacuum for 12 h to remove any residual water. Then the
cells were
transferred immediately to an argon-filled glove box for filling and vacuum
sealing and then
were filled with electrolyte. After filling, cells were vacuum-sealed.
[0222] After sealing, the pouch cells were placed in a temperature box at
40.0 +/- 0.1 C
and held at 1.5 V for 24 hours to allow for the completion of wetting. Then,
pouch cells were
subjected to the formation process. Unless specified otherwise, the formation
process consisted
of charging the pouch cells at 11 mA (C/20) to 4.2 V and discharging to 3.8 V.
C/x indicates
the that the time to charge or discharge the cell at the current selected is x
hours when the cell
has its initial capacity. For example, C/20 indicates that a charge or
discharge would take 20
hours. After formation, cells were transferred and moved into the glove box,
cut open to release
any generated gas and then vacuum sealed again and the appropriate experiments
were
performed.
Electrochemical Impedance Spectroscopy
[0223] Electrochemical impedance spectroscopy (EIS) measurements were
conducted on
pouch cells after storage and formation. Cells were charged or discharged to
3.8 V then
transferred to a temperature box set to 10.0 0.1 C. AC impedance spectra were
collected with
ten points per decade from 100 kHz to 10 mHz with a signal amplitude of 10 mV
at 10.0 0.1
C.
[0224] LFO Impact On Impedance: In certain embodiments, LFO is included in
two or
three electrolyte-additive systems, in part to decrease the impedance of the
system. FIGs. 35A-
D show that LiP02F2 (LFO, or lithium difluorophosphate) reduces cells
impedance after
formation in most cases. However, when LFO is included with the 2% PES + 1%
DTD + 1%
TTSPi (collectively, PES211), an increased impedance is observed. The positive
electrode is
single-crystal NMC 532 and the negative electrode is artificial graphite.
[0225] FIGs. 35A-D summarize experimental data for electrolyte systems with
a positive
electrode of NMC111 and a negative electrode of artificial graphite. After
formation, the pouch
23
CA 03071314 2020-01-28
WO 2019/025980 PCT/IB2018/055745
cells were measured at 10 C and 3.8 V. The control electrolyte in FIGs. 35A-D
is 1.0 M LiPF6
in 30% ethylene carbonate and 70% ethyl methyl carbonate. FIG. 35A summarizes
experimental impedance data, plotting the negative of the imaginary portion of
the impedance
against the real portion of the impedance for 1.0 M LiPF6 in 30% ethylene
carbonate and 70%
ethyl methyl carbonate (control electrolyte); 1.2 M LiPF6 in 30% ethylene
carbonate and 70%
ethyl methyl carbonate; control electrolyte + 1% LiP02F2; control electrolyte
+ 2% LiP02F2;
and 20% methyl acetate + 1% LiP02F2. FIG. 35B summarizes experimental
impedance data,
plotting the negative of the imaginary portion of the impedance against the
real portion of the
impedance for the control electrolyte (same control as for FIG. 35A), 2% VC,
2% VC + 1%
LiP02F2; 20% MA + 1% LiP02F2 +2% VC; PES211; and PES211 + 1% LiP02F2. FIG. 35C
summarizes experimental impedance data, plotting the negative of the imaginary
portion of the
impedance against the real portion of the impedance for the control
electrolyte (same control
as for FIG. 35A), 2% FEC, 2% FEC + 1% LiP02F2; 1% DTD; and 1% DTD + 1%
LiP02F2.
FIG. 35D summarizes experimental impedance data for the control electrolyte
(same control
as for FIG. 35A); 1.2 M LiPF6 in 30% ethylene carbonate and 70% ethyl methyl
carbonate; 1%
LiP02F2; 2% LiP02F2; 20% MA + 1% LiP02F2; 2% VC; 2% VC + 1% LiP02F2; 20% MA +
1% LiP02F2 + 2% VC; PES211; PES211 + 1% LiP02F2; 2% FEC, 2% FEC + 1% LiP02F2;
1% DTD; and 1% DTD + 1% LiP02F2.
[0226] As can be seen from FIGs. 35A-D, the addition of LFO to most systems
reduces
impedance. However, in the presence of PES211, the addition of LFO increases
the
impedance.
[0227] FIG. 36 summarizes experimental EIS data for electrolyte systems
containing
additives of 2% VC; 1% LiP02F2 + 2% VC; 1% LiP02F2 + 2% FEC; and 1% LiP02F2 +
1%
VC + 1% FEC in an electrolyte solution of 1.2 M LiPF6 in 30% ethylene
carbonate and 70%
ethyl methyl carbonate. The EIS measurements were taken after formation, at
3.8 V, and at
C. The positive electrode is single-crystal NMC532 and the negative electrode
is artificial
graphite.
[0228] The LFO did not reduce the impedance in the tested systems with an
NMC532
positive electrode and an artificial graphite negative electrode. The failure
to reduce impedance
may be due to a larger cathode or anode surface. However, the LFO did not
increase the
impedance either. Thus, the addition of LFO either decreases the impedance or
is neutral.
Ultrahigh Precision Cycling and Storage Experiments
24
CA 03071314 2020-01-28
WO 2019/025980 PCT/IB2018/055745
[0229] To study the effectiveness of the battery systems of the present
disclosure, including
the operative electrolyte additives and electrodes, ultrahigh precision
cycling (UHPC) was
performed. The standard UHPC procedure consisted of cycling cells between 2.8
and 4.3 V at
40 C using a current corresponding to C/20 for 15 cycles to produce the data.
UPHC is
employed to measure the coulombic efficiency, charge endpoint capacity
slippage and other
parameters to an accuracy of 30 ppm, in the case of the coulombic efficiency.
Details of the
UHPC procedure are described in in T. M. Bond, J. C. Burns, D. A. Stevens, H.
M. Dahn, and
J. R. Dahn, Journal of the Electrochemical Society, 160, A521 (2013), which is
incorporated
herein in its entirety.
[0230] Metrics measured and/or determined from the UHPC measurements of
particular
interest include the following: coulombic efficiency, normalized coulombic
inefficiency,
normalized charge endpoint capacity slippage, normalized discharge capacity
(or fade rate),
and delta V. Coulombic efficiency is the discharge capacity (Qd) divided by
charge capacity
(Qc) of the previous cycle. It tracks the parasitic reactions happening at the
in the Li-ion cell
and includes contributions from both the positive and negative electrodes. A
higher CE value
indicates less electrolyte degradation in the cell. Coulombic inefficiency per
hour (CIE/h) is a
normalized (per hour) coulombic inefficiency where the coulombic inefficiency
is defined as
1-CE. It is calculated by taking 1-CE and dividing by the time of the cycle
for which the CE
was measured. Charge endpoint capacity motion (or slippage) tracks the
parasitic reactions
occurring at the positive electrode as well as the positive material mass
loss, if any. Less motion
is better and relates to less electrolyte oxidation. Normalized discharge
capacity, or fade rate,
is another important metric, with a lower fade rate desirable and normally
indicative of a battery
system with a longer lifetime. Delta V is calculated as the difference between
the average
charge voltage and average discharge voltage. Delta V change relates closely
to polarization
growth with lower Delta V change as cycling occurs is preferable. UHPC
measurements are
particularly appropriate for comparing electrolyte compositions because it
allows for the
tracking of metrics with a higher accuracy and precision and allows for the
evaluation of
various degradation mechanisms in a relatively rapid fashion.
[0231] Two-Electrolyte Systems With FEC Or VC As Additive: In certain
embodiments, two-additive electrolyte systems, the concentration of each
additive about 0.25-
6%, form part of the battery system. The battery systems may also include
positive electrodes
made from NMC111, NMC532, NMC811, NMC622, or another NMC composition
(NMCxyz). In certain embodiments, positive electrodes made from NMC532 with
micrometer-
CA 03071314 2020-01-28
WO 2019/025980 PCT/IB2018/055745
scale grains have been shown to be particularly robust, in part because
processing conditions
created larger the grain sizes than typically processing conditions create.
[0232] Typical processing conditions lead to NMC electrodes with nanometer-
sized
particles packed into larger micrometer-sized agglomerates, creating grain
boundaries on the
nanometer scale. Grain boundaries are defects that tend to reduce desirable
properties (for
example, electrical properties), so it is typically desirable to reduce the
number of grains and
increase the grain size. Current processing can create larger domains, on the
micrometer size
scale, thereby reducing the number of grain boundaries in the NMC electrodes,
increasing
electrical properties. The increase in properties is results in more robust
battery systems. In
certain embodiments, other NMC electrodes may be processed to create larger
domain sizes
(on the micrometer-size scale or larger), for example, NMC111, NMC811, NMC622,
or
another NMC compound to create more robust systems.
[0233] The positive electrode may be coated with a material such as
aluminum oxide
(A1203), titanium dioxide (TiO2), or another coating. FIGs. 4A-J illustrate
typical experimental
data of two-additive systems of the present disclosure collected during UHPC
experiments
comparing single-additive electrolyte systems with the novel two-additive
electrolyte systems
(VC + DTD and FEC + DTD) in a base electrolyte system containing 1.2M LiPF6 in
30%
ethylene carbonate and 70% ethyl methyl carbonate, using a positive electrode
consisting of
single-crystal NMC532 and a negative electrode consisting of artificial
graphite. FIGs. 4A-J
illustrate benefits of two-additive systems of the present disclosure,
specifically adding DTD
to an electrolyte system containing VC or FEC.
[0234] FIG. 4A illustrates time normalized coulombic inefficiency per hour
(CIE/h)
versus cycle number for electrolyte systems including 1% DTD, 2% VC, and 2% VC
+ 1%
DTD. FIG. 4B illustrates coulombic efficiency (CE) versus cycle number for
electrolyte
systems including 1% DTD, 2% VC, and 2% VC + 1% DTD. FIG. 4C illustrates the
capacity
of the charge endpoint plotted versus cycle number for electrolyte systems
including 1% DTD,
2% VC, and 2% VC + 1% DTD. FIG. 4D illustrates the discharge capacity versus
cycle number
for electrolyte systems including 1% DTD, 2% VC, and 2% VC + 1% DTD. FIG. 4E
illustrates
the difference between average charge voltage and average discharge voltage
versus cycle
number for electrolyte systems including 1% DTD, 2% VC, and 2% VC + 1% DTD.
FIG. 4F
illustrates time normalized coulombic inefficiency per hour (CIE/h) versus
cycle number for
electrolyte systems including 1% DTD, 2% FEC, and 2% FEC + 1% DTD. FIG. 4G
illustrates
coulombic efficiency (CE) versus cycle number for electrolyte systems
including 1% DTD,
2% FEC, and 2% FEC + 1% DTD. FIG. 411 illustrates the capacity of the charge
endpoint
26
CA 03071314 2020-01-28
WO 2019/025980 PCT/IB2018/055745
plotted versus cycle number for electrolyte systems including 1% DTD, 2% FEC,
and 2% FEC
+ 1% DTD. FIG. 41 illustrates the discharge capacity verses cycle number for
electrolyte
systems including 1% DTD, 2% FEC, and 2% FEC + 1% DTD. FIG. 4J illustrates the
difference between average charge voltage and average discharge voltage versus
cycle number
for electrolyte systems including 1% DTD, 2% FEC, and 2% FEC + 1% DTD.
[0235] FIGs. 4A-J show the benefits of electrolytes with two
additives¨specifically VC
+ DTD and FEC + DTD. The experimental data shows that adding DTD to an
electrolyte
system containing VC or FEC in a base electrolyte system containing 1.2M LiPF6
in 30%
ethylene carbonate and 70% ethyl methyl carbonate by weight increases the
performance of
electrolyte systems that contain only VC or FEC as additives. Specifically,
FIGs. 4A-J
illustrate that two-additive systems containing (VC + DTD and FEC + DTD) have
a higher CE
(lower electrolyte degradation in the cell) and lower charge endpoint motion
(lower electrolyte
degradation at the positive electrode) compared to systems without the
additives, or with only
one additive. Further, FIGs. 4A-J also show a desirable lower fade rate (Qa).
Thus, an
electrolyte system with two additives (VC + DTD and/or FEC + DTD) performs
better (in
terms of CIE/h, CE, charge end point slippage) than an electrolyte system that
only contains a
single additive of DTD, VC, or FEC.
[0236] FIG. 5 summarizes the last three cycles of data generated during the
experiments
shown in FIGs. 4A-J. FIG. 5A shows a summary of the last three cycles of the
time normalized
coulombic inefficiency per hour (CIE/h) for electrolyte systems including 1%
DTD, 2% FEC,
2% FEC + 1% DTD, 2% VC, and 2% VC + 1% DTD. FIG. 5B shows a summary of the
last
three cycles of the fractional slippage per hour for electrolyte systems
including 1% DTD, 2%
FEC, 2% FEC + 1% DTD, 2% VC, and 2% VC + 1% DTD. FIG. 5C shows a summary of
the
last three cycles of the fractional fade per hour for 1% DTD, 2% FEC, 2% FEC +
1% DTD,
2% VC, and 2% VC + 1% DTD.
[0237] FIGs. SA-C show that electrolyte systems that contain VC + DTD and
FEC + DTD
exhibit lower time normalize coulombic inefficiency (CIE/h), and lower
fractional slippage per
hour (meaning that these electrolyte systems have longer lifetimes) compared
to systems that
only contained one additional additive-2% FEC, 2% VC, or 1% DTD. FIGs. 5A and
5B show
that 1% DTD without another additive shows the highest CIE/h and fractional
slippage.
However, when DTD is combined with VC or FEC, the two additives form a
previously
unexpected, synergistic effect causing the CIE/h and fractional slippage to be
less in the two-
additive electrolyte system compared to either single additive. FIG. 5C shows
that the presence
of 1% DTD decreases the fractional fade per hour, either as a single additive
or as part of a
27
CA 03071314 2020-01-28
WO 2019/025980 PCT/IB2018/055745
two-additive electrolyte system with VC or FEC. This indicates that DTD is an
important
additive for increasing lifetime of the battery systems of the current
invention. In addition to
DTD, other sulfur-containing compounds can function in a similar manner and
increase battery
lifetime.
[0238] Methyl Acetate As Electrolyte Solvent: In certain embodiments,
methyl acetate
is used as a solvent (in concentrations of up to 60%) to improve battery-
system lifetime when
higher charging and discharging rates are expected as well as other
properties. This is
particularly important for vehicle and other applications. FIGs. 9A-I
illustrate typical data
collected during some of the ultra-high-precision-charging experiments that
show that methyl
acetate can be added to electrolyte systems containing VC or FEC with DTD to
increase
electrolyte conductivity and lower viscosity without sacrificing much
lifetime. Increasing
conductivity and decreasing viscosity is important for certain applications
requiring a faster
rate of charge.
[0239] FIG. 9A illustrates coulombic efficiency (CE) versus cycle number
for electrolyte
systems including 2% FEC in a base electrolyte of 1.2M LiPF6 in 30% ethylene
carbonate and
70% ethyl methyl carbonate; 2% FEC + 1% DTD in a base electrolyte of 1.2M
LiPF6 in 30%
ethylene carbonate and 70% ethyl methyl carbonate; 2% FEC in a base
electrolyte of 1.2M
LiPF6 in 24% ethylene carbonate, 56% ethyl methyl carbonate, and 20% methyl
acetate; 2%
FEC + 1% DTD in a base electrolyte of 1.2M LiPF6 in 24% ethylene carbonate,
56% ethyl
methyl carbonate, and 20% methyl acetate; 2% FEC in a base electrolyte of 1.2M
LiPF6 in 18%
ethylene carbonate, 42% ethyl methyl carbonate, and 40% methyl acetate; and 2%
FEC + 1%
DTD in a base electrolyte of 1.2M LiPF6 in 18% ethylene carbonate, 42% ethyl
methyl
carbonate, and 40% methyl acetate.
[0240] FIG. 9B illustrates the capacity of the charge endpoint plotted
versus cycle number
for electrolyte systems including 2% FEC in a base electrolyte of 1.2M LiPF6
in 30% ethylene
carbonate and 70% ethyl methyl carbonate; 2% FEC + 1% DTD in a base
electrolyte of 1.2M
LiPF6 in 30% ethylene carbonate and 70% ethyl methyl carbonate; 2% FEC in a
base
electrolyte of 1.2M LiPF6 in 24% ethylene carbonate, 56% ethyl methyl
carbonate, and 20%
methyl acetate; 2% FEC + 1% DTD in a base electrolyte of 1.2M LiPF6 in 24%
ethylene
carbonate, 56% ethyl methyl carbonate, and 20% methyl acetate; 2% FEC in a
base electrolyte
of 1.2M LiPF6 in 18% ethylene carbonate, 42% ethyl methyl carbonate, and 40%
methyl
acetate; and 2% FEC + 1% DTD in a base electrolyte of 1.2M LiPF6 in 18%
ethylene carbonate,
42% ethyl methyl carbonate, and 40% methyl acetate.
28
CA 03071314 2020-01-28
WO 2019/025980 PCT/IB2018/055745
[0241] FIG. 9C illustrates the discharge capacity versus cycle number for
electrolyte
systems including 2% FEC in a base electrolyte of 1.2M LiPF6 in 30% ethylene
carbonate and
70% ethyl methyl carbonate; 2% FEC + 1% DTD in a base electrolyte of 1.2M
LiPF6 in 30%
ethylene carbonate and 70% ethyl methyl carbonate; 2% FEC in a base
electrolyte of 1.2M
LiPF6 in 24% ethylene carbonate, 56% ethyl methyl carbonate, and 20% methyl
acetate; 2%
FEC + 1% DTD in a base electrolyte of 1.2M LiPF6 in 24% ethylene carbonate,
56% ethyl
methyl carbonate, and 20% methyl acetate; 2% FEC in a base electrolyte of 1.2M
LiPF6 in 18%
ethylene carbonate, 42% ethyl methyl carbonate, and 40% methyl acetate; and 2%
FEC + 1%
DTD in a base electrolyte of 1.2M LiPF6 in 18% ethylene carbonate, 42% ethyl
methyl
carbonate, and 40% methyl acetate.
[0242] FIG. 9D illustrates the difference between average charge voltage
and average
discharge voltage versus cycle number for electrolyte systems including 2% FEC
in a base
electrolyte of 1.2M LiPF6 in 30% ethylene carbonate and 70% ethyl methyl
carbonate; 2% FEC
+ 1% DTD in a base electrolyte of 1.2M LiPF6 in 30% ethylene carbonate and 70%
ethyl
methyl carbonate; 2% FEC in a base electrolyte of 1.2M LiPF6 in 24% ethylene
carbonate,
56% ethyl methyl carbonate, and 20% methyl acetate; 2% FEC + 1% DTD in a base
electrolyte
of 1.2M LiPF6 in 24% ethylene carbonate, 56% ethyl methyl carbonate, and 20%
methyl
acetate; 2% FEC in a base electrolyte of 1.2M LiPF6 in 18% ethylene carbonate,
42% ethyl
methyl carbonate, and 40% methyl acetate; and 2% FEC + 1% DTD in a base
electrolyte of
1.2M LiPF6 in 18% ethylene carbonate, 42% ethyl methyl carbonate, and 40%
methyl acetate.
[0243] FIG. 9F illustrates coulombic efficiency (CE) versus cycle number
for electrolyte
systems including 2% VC in a base electrolyte of 1.2M LiPF6 in 30% ethylene
carbonate and
70% ethyl methyl carbonate; 2% VC + 1% DTD in a base electrolyte of 1.2M LiPF6
in 30%
ethylene carbonate and 70% ethyl methyl carbonate; 2% VC in a base electrolyte
of 1.2M LiPF6
in 24% ethylene carbonate, 56% ethyl methyl carbonate, and 20% methyl acetate;
2% VC +
1% DTD in a base electrolyte of 1.2M LiPF6 in 24% ethylene carbonate, 56%
ethyl methyl
carbonate, and 20% methyl acetate; 2% VC in a base electrolyte of 1.2M LiPF6
in 18% ethylene
carbonate, 42% ethyl methyl carbonate, and 40% methyl acetate; and 2% VC + 1%
DTD in a
base electrolyte of 1.2M LiPF6 in 18% ethylene carbonate, 42% ethyl methyl
carbonate, and
40% methyl acetate.
[0244] FIG. 9G illustrates the capacity of the charge endpoint plotted
versus cycle number
for electrolyte systems including 2% VC in a base electrolyte of 1.2M LiPF6 in
30% ethylene
carbonate and 70% ethyl methyl carbonate; 2% VC + 1% DTD in a base electrolyte
of 1.2M
LiPF6 in 30% ethylene carbonate and 70% ethyl methyl carbonate; 2% VC in a
base electrolyte
29
CA 03071314 2020-01-28
WO 2019/025980 PCT/IB2018/055745
of 1.2M LiPF6 in 24% ethylene carbonate, 56% ethyl methyl carbonate, and 20%
methyl
acetate; 2% VC + 1% DTD in a base electrolyte of 1.2M LiPF6 in 24% ethylene
carbonate,
56% ethyl methyl carbonate, and 20% methyl acetate; 2% VC in a base
electrolyte of 1.2M
LiPF6 in 18% ethylene carbonate, 42% ethyl methyl carbonate, and 40% methyl
acetate; and
2% VC + 1% DTD in a base electrolyte of 1.2M LiPF6 in 18% ethylene carbonate,
42% ethyl
methyl carbonate, and 40% methyl acetate.
[0245] FIG. 911 illustrates the discharge capacity versus cycle number for
electrolyte
systems including 2% VC in a base electrolyte of 1.2M LiPF6 in 30% ethylene
carbonate and
70% ethyl methyl carbonate; 2% VC + 1% DTD in a base electrolyte of 1.2M LiPF6
in 30%
ethylene carbonate and 70% ethyl methyl carbonate; 2% VC in a base electrolyte
of 1.2M LiPF6
in 24% ethylene carbonate, 56% ethyl methyl carbonate, and 20% methyl acetate;
2% VC +
1% DTD in a base electrolyte of 1.2M LiPF6 in 24% ethylene carbonate, 56%
ethyl methyl
carbonate, and 20% methyl acetate; 2% VC in a base electrolyte of 1.2M LiPF6
in 18% ethylene
carbonate, 42% ethyl methyl carbonate, and 40% methyl acetate; and 2% VC + 1%
DTD in a
base electrolyte of 1.2M LiPF6 in 18% ethylene carbonate, 42% ethyl methyl
carbonate, and
40% methyl acetate.
[0246] FIG. 91 illustrates the difference between average charge voltage
and average
discharge voltage versus cycle number for electrolyte systems including 2% VC
in a base
electrolyte of 1.2M LiPF6 in 30% ethylene carbonate and 70% ethyl methyl
carbonate; 2% VC
+ 1% DTD in a base electrolyte of 1.2M LiPF6 in 30% ethylene carbonate and 70%
ethyl
methyl carbonate; 2% VC in a base electrolyte of 1.2M LiPF6 in 24% ethylene
carbonate, 56%
ethyl methyl carbonate, and 20% methyl acetate; 2% VC + 1% DTD in a base
electrolyte of
1.2M LiPF6 in 24% ethylene carbonate, 56% ethyl methyl carbonate, and 20%
methyl acetate;
2% VC in a base electrolyte of 1.2M LiPF6 in 18% ethylene carbonate, 42% ethyl
methyl
carbonate, and 40% methyl acetate; and 2% VC + 1% DTD in a base electrolyte of
1.2M LiPF6
in 18% ethylene carbonate, 42% ethyl methyl carbonate, and 40% methyl acetate.
[0247] FIGs. 9A-I show that in systems that include both VC and FEC, the
addition of MA
as an electrolyte solvent does not significantly sacrifice the overall
performance of the battery
system and as the long-term-cycling and plating experiments to be described
later will show,
it increases lifetime under higher charging rates. In particular, the
performance of the two-
additive electrolyte systems of the present disclosure are not sacrificed with
the addition of MA
as a solvent. FIGs. 10A-C show the average of three last cycles of data
generated during the
experiments shown in FIGs. 9A-I. FIGs. 10A-C confirm that the addition of MA
as an
CA 03071314 2020-01-28
WO 2019/025980 PCT/IB2018/055745
electrolyte solvent does not significantly sacrifice the overall performance
of the battery
systems of the present disclosure that include two-additive electrolyte
systems.
[0248] LFO As An Additive: FIGs. 39A-H and 40A-H summarize results of UHPC
experiments that show that LFO generally performs well in electrolyte systems
such that the
properties of the systems perform well compared to the control electrolyte.
[0249] FIGs. 37A-F and FIGs. 38A-F summarize experimental storage data for
different
electrolyte systems containing LFO compared to a control without LFO.
[0250] FIGs. 37A-F show that in the absence of other additives, LFO
dramatically
improves storage. Voltage drop is dramatically reduced, gassing is
dramatically reduced and
impedance is dramatically reduced after storage when LFO is added. LFO is
effective in the
presence of MA as well. FIGs. 38A-F show similar results in when LFO is added
in electrolytes
based on EC/DMC. When a good additive package like 1% FEC + 1% DTD is used,
then the
additional benefits brought by LFO are small. However, DTD-based electrolyte
systems may
be removed in the future because they often will change color over time when
mixed and stored
in a glove box.
[0251] FIGs. 46A-D shows the results of storage experiments for cells with
more
complicated electrolytes show ability to match 2% FEC + 1% DTD in storage
performance.
[0252] FIGs. 52A-D, 53A-D, 54A-D and 55A-C show the results of additional
electrolyte
systems containing LFO and also 2% VC + 1% DTD for comparison purposes. As can
be
observed certain electrolyte systems with LFO perform as well or slightly
better than the 2%
VC + 1% DTD system. FIGs. 56A-C shows the results of additional experiments
for CIE,
fractional fade, and fractional slippage. The electrolyte system of 2% VC + 1%
DTD performs
very well. Electrolyte systems of 1% LFO + 2% VC and 1% LFO + 1% VC + 1% FEC
also
perform well (although not quite as good as the 2% VC + 1% DTD system). This
experimental
data agrees with the TAM experimental data.
[0253] FIG. 57 shows the impact of UHPC cycling on the impedance. LFO
systems
generally perform well. FIGs. 58A-D summarize experimental data for
electrolyte systems
containing LFO with a positive electrode made from NMC 622 with two different
coatings,
indicated as A and B. For the different electrolyte systems studied, LFO has
the impact of
decreasing the voltage drop and also the impedance of the system.
[0254] LFO may be acquired by multiple suppliers, including Guangzhou Tinci
Materials
Technology Co., Ltd. and Shenzhen Capchem Technology Co., Ltd. FIG. 60 shows
that
independent of supplier, the reaction rate is similar in the presence of air
for time periods of at
least 50 minutes or less. FIG. 61 shows that the mass loss is also similar
through thermal
31
CA 03071314 2020-01-28
WO 2019/025980 PCT/IB2018/055745
gravimetric analysis ("TGA") experiments performed in an argon environment
with a
temperature ramp of 5 C/min.
Long Term Cycling
[0255] Lifetime of a battery system is an important property of a battery
system. Charging
and discharging rates can affect lifetime. Long term cycling experiments help
determine how
resilient battery systems are over time under anticipated operation
conditions. It is important
to select battery systems that have sufficient lifetimes for the desired
application.
[0256] Embodiments of the present disclosure exhibit desirable long term
cycling for
different applications, including grid and vehicle storage. Specifically, two-
additive electrolyte
systems of VC + DTD and FEC + DTD in which MA, in concentrations of up to 60%,
is used
as a solvent are particularly relevant for automobile applications (especially
energy storage
within an electric vehicle) in which charging and discharging rates are
typically higher than for
grid-storage applications.
[0257] In the long-term-cycling experiments, single-crystal NMC532 was
typically used
as the positive electrode (unless otherwise specified) and artificial graphite
was used as the
negative electrode (unless otherwise specified). Before the long term cycling
experiments,
pouch cells were subjected to the formation process. The cells were first
charged at 11 mA
(C/20) to 4.2 V and discharged to 3.8 V. Cells were transferred and moved into
the glove box,
cut open to release gas generated and then vacuum sealed again. After
formation, cells were
cycled on a Neware charging systems. Cells were housed in a temperature
controlled box at
40 C +/- 0.2 C or 20 C +/- 0.2 C. The cells were cycled between 3.0 V and the
top of charge
(4.2 V or 4.3 V) with a current of C/3 (half cycle of 3h) and a constant
voltage step at the top
of charge until the current dropped below C/20. Every 50 cycles, cells
underwent one full cycle
at C/20.
[0258] Two-Electrolyte Systems With FEC Or VC As Additive: In certain
embodiments, two-additive electrolyte systems, the concentration of each
additive about 0.25-
6%, form part of the battery system. FIGs. 6A-F illustrate typical
experimental data studying
long term cycling at 40 C, and a C/3 constant charge, constant voltage (CCCV)
charging rate.
FIGs. 6A-F illustrate the advantage of two-additive electrolyte systems of the
present
disclosure, specifically, electrolytes that include DTD with VC or FEC. FIG.
6A shows
experimental data of capacity versus cycle number for electrolyte systems
including 1% DTD,
2% FEC, 2% FEC + 1% DTD, 2% VC, and 2% VC + 1% DTD, cycling between 3.0 V and
4.2
V. FIG. 6B shows experimental data of normalized capacity versus cycle number
for
32
CA 03071314 2020-01-28
WO 2019/025980 PCT/IB2018/055745
electrolyte systems including 1% DTD, 2% FEC, 2% FEC + 1% DTD, 2% VC, and 2%
VC +
1% DTD, cycling between 3.0 V and 4.2 V. FIG. 6C shows experimental data of
voltage
hysteresis (difference between average charge voltage and average discharge
voltage) versus
cycle number for electrolyte systems including 1% DTD, 2% FEC, 2% FEC + 1%
DTD, 2%
VC, and 2% VC + 1% DTD, cycling between 3.0 V and 4.2 V. FIG. 6D shows
experimental
data of capacity versus cycle number for electrolyte systems including 1% DTD,
2% FEC, 2%
FEC + 1% DTD, 2% VC, and 2% VC + 1% DTD, cycling between 3.0 V and 4.2 V. FIG.
6E
shows experimental data of normalized capacity versus cycle number for
electrolyte systems
including 1% DTD, 2% FEC, 2% FEC + 1% DTD, 2% VC, and 2% VC + 1% DTD, cycling
between 3.0 V and 4.2 V. FIG. 6F shows experimental data of voltage hysteresis
versus cycle
number for electrolyte systems including 1% DTD, 2% FEC, 2% FEC + 1% DTD, 2%
VC,
and 2% VC + 1% DTD, cycling between 3.0 V and 4.2 V. The experimental data
shows that
the two-additive electrolyte systems (DTD + FEC and DTD + VC) experience less
capacity
loss when cycling to 4.2 or 4.3 V and also lower polarization growth compared
to the single
additive electrolyte systems of VC or FEC.
[0259] FIGs. 7A-F illustrate typical experimental data studying long term
cycling at 20 C,
C/3 CCCV charging rate. Similar to FIGs. 6A-F, FIGs. 7A-F illustrate the
advantage of
including DTD as an additive to an electrolyte system containing VC or FEC.
FIGs. 7A-F
confirm that the advantages seen at 40 C are still present at lower
temperatures, 20 C in this
case. FIG. 7A shows experimental data of capacity versus cycle number for
electrolyte systems
including 1% DTD, 2% FEC, 2% FEC + 1% DTD, 2% VC, and 2% VC + 1% DTD, cycling
between 3.0 V and 4.3 V. FIG. 7B shows experimental data of normalized
capacity versus
cycle number for electrolyte systems including 1% DTD, 2% FEC, 2% FEC + 1%
DTD, 2%
VC, and 2% VC + 1% DTD, cycling between 3.0 V and 4.3 V. FIG. 7C shows
experimental
data of voltage hysteresis (difference between average charge voltage and
average discharge
voltage) versus cycle number for electrolyte systems including 1% DTD, 2% FEC,
2% FEC +
1% DTD, 2% VC, and 2% VC + 1% DTD, cycling between 3.0 V and 4.3 V. FIG. 7D
shows
experimental data of capacity versus cycle number for electrolyte systems
including 1% DTD,
2% FEC, 2% FEC + 1% DTD, 2% VC, and 2% VC + 1% DTD, cycling between 3.0 V and
4.3
V. FIG. 7E shows experimental data of normalized capacity versus cycle number
for
electrolyte systems including 1% DTD, 2% FEC, 2% FEC + 1% DTD, 2% VC, and 2%
VC +
1% DTD, cycling between 3.0 V and 4.3 V. FIG. 7F shows experimental data of
voltage
hysteresis (difference between average charge voltage and average discharge
voltage) versus
33
CA 03071314 2020-01-28
WO 2019/025980 PCT/IB2018/055745
cycle number for electrolyte systems including 1% DTD, 2% FEC, 2% FEC + 1%
DTD, 2%
VC, and 2% VC + 1% DTD, cycling between 3.0V and 4.3V. FIGs. 7A-F confirm the
advantages of including DTD in the electrolyte with VC or FEC, especially when
cycling
occurs up to 4.3 V.
[0260] FIGs. 6A-F illustrate the benefits of two-additive electrolyte
systems consisting of
DTD + VC or DTD + FEC. FIGs. 6A-F show that the inclusion of DTD with VC or
FEC as
part of a two-additive electrolyte system and cycling at 40 C, leads to less
capacity loss at 4.2
and 4.3 V and lowers polarization growth. Similarly, FIGs. 7A-F shows the
benefits of DTD
when long-term cycling at 20 C. FIGs. 7A-F show that the inclusion of DTD with
VC or FEC
as part of a two-additive electrolyte system and cycling at 20 C, leads to
less capacity loss at
4.2 V (slightly) and 4.3 V (more significantly) and lowers polarization
growth. Thus, at either
20 C or 40 C, two-additive systems including DTD with VC or FEC improves the
battery
system by reducing the capacity loss and lowering the polarization growth.
[0261] In certain embodiments, the positive electrode is formed from
NMC111, NMC532,
NMC822, NMC622, and/or NMCxyz. In particular, positive electrodes made from
single-
crystal NMC532 have been shown to be particularly robust, in part because the
grain size of
NMC532 is larger than the grain size of other standard NMC materials that are
more
polycrystalline, having smaller grain sizes. FIGs. 8A-I shows typical
experiential data
collected during cycling experiments for electrolyte compositions according to
certain
embodiments of the present disclosure that include a positive electrode formed
from single-
crystal NMC532. FIG. 8A shows experimental data of peak capacity versus cycle
number for
electrolyte systems including 2% FEC, 1% FEC + 1% DTD, 2% FEC + 1% DTD, 1% FEC
+
1% MMDS, and 2% FEC + 1% MMDS, cycling between 3.0 V and 4.3 V at 40 C in a
base
electrolyte of 1.2M LiPF6 in 30% ethylene carbonate and 70% ethyl methyl
carbonate. FIG.
8B shows experimental data of normalized capacity versus cycle number for
electrolyte
systems including 2% FEC, 1% FEC + 1% DTD, 2% FEC + 1% DTD, 1% FEC + 1% MMDS,
and 2% FEC + 1% MMDS, cycling between 3.0 V and 4.3 V at 40 C in a base
electrolyte of
1.2M LiPF6 in 30% ethylene carbonate and 70% ethyl methyl carbonate. FIG. 8C
shows
experimental data of voltage hysteresis (difference between average charge
voltage and
average discharge voltage) versus cycle number for electrolyte systems
including 2% FEC, 1%
FEC + 1% DTD, 2% FEC + 1% DTD, 1% FEC + 1% MMDS, and 2% FEC + 1% MMDS,
cycling between 3.0 V and 4.3 V at 40 C in a base electrolyte of 1.2M LiPF6 in
30% ethylene
carbonate and 70% ethyl methyl carbonate. FIG. 8D shows experimental data of
peak capacity
34
CA 03071314 2020-01-28
WO 2019/025980 PCT/IB2018/055745
versus cycle number for electrolyte systems including 2% VC, 1% VC + 1% DTD,
2% VC +
1% DTD, 1% VC + 1% MMDS, and 2% VC + 1% MMDS, cycling between 3.0 V and 4.3 V
at 40 C in a base electrolyte of 1.2M LiPF6 in 30% ethylene carbonate and 70%
ethyl methyl
carbonate. FIG. 8E shows experimental data of normalized capacity versus cycle
number for
electrolyte systems including 2% VC, 1% VC + 1% DTD, 2% VC + 1% DTD, 1% VC +
1%
MMDS, and 2% VC + 1% MMDS, cycling between 3.0 V and 4.3 V at 40 C in a base
electrolyte of 1.2M LiPF6 in 30% ethylene carbonate and 70% ethyl methyl
carbonate. FIG.
8F shows experimental data of voltage hysteresis (difference between average
charge voltage
and average discharge voltage) versus cycle number for electrolyte systems
including 2% FEC,
1% VC + 1% DTD, 2% VC + 1% DTD, 1% VC + 1% MMDS, and 2% VC + 1% MMDS,
cycling between 3.0 V and 4.3 V at 40 C in a base electrolyte of 1.2M LiPF6 in
30% ethylene
carbonate and 70% ethyl methyl carbonate by weight. FIG. 8G shows experimental
data of
peak capacity versus cycle number for electrolyte systems including 2% PES, 1%
PES + 1%
DTD, 2% PES + 1% DTD, 1% PES + 1% MMDS, and 2% PES + 1% MMDS, cycling between
3.0 V and 4.3 V at 40 C in a base electrolyte of 1.2M LiPF6 in 30% ethylene
carbonate and
70% ethyl methyl carbonate. FIG. 811 shows experimental data of normalized
capacity versus
cycle number for electrolyte systems including 2% PES, 1% PES + 1% DTD, 2% PES
+ 1%
DTD, 1% PES + 1% MMDS, and 2% PES + 1% MMDS, cycling between 3.0 V and 4.3 V
at
40 C in a base electrolyte of 1.2M LiPF6 in 30% ethylene carbonate and 70%
ethyl methyl
carbonate. FIG. 81 shows experimental data of voltage hysteresis (difference
between average
charge voltage and average discharge voltage) for electrolyte systems
including 2% FEC, 1%
PES + 1% DTD, 2% PES + 1% DTD, 1% PES + 1% MMDS, and 2% PES + 1% MMDS,
cycling between 3.0 V and 4.3 V at 40 C in a base electrolyte of 1.2M LiPF6 in
30% ethylene
carbonate and 70% ethyl methyl carbonate. FIGs. 8A-I illustrate that NMC532
performs well
with two-additive electrolyte systems of 1% DTD with 1% VC, 2% VC, 1% FEC, or
2 FEC.
DTD performed better as an additive with VC or FEC than did MMDS.
[0262] Methyl Acetate As Electrolyte Solvent: In certain embodiments,
methyl acetate
is used as an electrolyte solvent, in concentrations up to 60% (by weight) and
generally in
combination with ethylene carbonate and/or ethyl methyl carbonate. FIGs. 15A-F
and 16A-F
shows the results of experiments run at 20 C and 40 C, respectively. Cells
with DTD performed
better than cells without DTD in cells containing MA as a solvent.
[0263] FIG. 15A is a plot of experimental data taken at 20 C of capacity
versus cycle
number for electrolyte systems including 2% FEC in a base electrolyte of 1.2M
LiPF6 in 30%
CA 03071314 2020-01-28
WO 2019/025980 PCT/IB2018/055745
ethylene carbonate and 70% ethyl methyl carbonate; 1% FEC + 1% DTD in a base
electrolyte
of 1.2M LiPF6 in 30% ethylene carbonate and 70% ethyl methyl carbonate; 2% FEC
in a base
electrolyte of 1.2M LiPF6 in 24% ethylene carbonate, 56% ethyl methyl
carbonate, and 20%
methyl acetate; 2% FEC + 1% DTD in a base electrolyte of 1.2M LiPF6 in 24%
ethylene
carbonate, 56% ethyl methyl carbonate, and 20% methyl acetate; 2% FEC in a
base electrolyte
of 1.2M LiPF6 in 18% ethylene carbonate, 42% ethyl methyl carbonate, and 40%
methyl
acetate; and 2% FEC + 1% DTD in a base electrolyte of 1.2M LiPF6 in 18%
ethylene carbonate,
42% ethyl methyl carbonate, and 40% methyl acetate with cycling up to 4.2 V.
[0264] FIG. 15B is a plot of experimental data taken at 20 C of normalized
capacity versus
cycle number for electrolyte systems including 2% FEC in a base electrolyte of
1.2M LiPF6 in
30% ethylene carbonate and 70% ethyl methyl carbonate; 1% FEC + 1% DTD in a
base
electrolyte of 1.2M LiPF6 in 30% ethylene carbonate and 70% ethyl methyl
carbonate; 2% FEC
in a base electrolyte of 1.2M LiPF6 in 24% ethylene carbonate, 56% ethyl
methyl carbonate,
and 20% methyl acetate; 2% FEC + 1% DTD in a base electrolyte of 1.2M LiPF6 in
24%
ethylene carbonate, 56% ethyl methyl carbonate, and 20% methyl acetate; 2% FEC
in a base
electrolyte of 1.2M LiPF6 in 18% ethylene carbonate, 42% ethyl methyl
carbonate, and 40%
methyl acetate; and 2% FEC + 1% DTD in a base electrolyte of 1.2M LiPF6 in 18%
ethylene
carbonate, 42% ethyl methyl carbonate, and 40% methyl acetate with cycling up
to 4.2 V.
[0265] FIG. 15C is a plot of experimental data taken at 20 C of voltage
hysteresis
(difference between average charge voltage and average discharge voltage)
versus cycle
number for electrolyte systems including 2% FEC in a base electrolyte of 1.2M
LiPF6 in 30%
ethylene carbonate and 70% ethyl methyl carbonate; 1% FEC + 1% DTD in a base
electrolyte
of 1.2M LiPF6 in 30% ethylene carbonate and 70% ethyl methyl carbonate; 2% FEC
in a base
electrolyte of 1.2M LiPF6 in 24% ethylene carbonate, 56% ethyl methyl
carbonate, and 20%
methyl acetate; 2% FEC + 1% DTD in a base electrolyte of 1.2M LiPF6 in 24%
ethylene
carbonate, 56% ethyl methyl carbonate, and 20% methyl acetate; 2% FEC in a
base electrolyte
of 1.2M LiPF6 in 18% ethylene carbonate, 42% ethyl methyl carbonate, and 40%
methyl
acetate; and 2% FEC + 1% DTD in a base electrolyte of 1.2M LiPF6 in 18%
ethylene carbonate,
42% ethyl methyl carbonate, and 40% methyl acetate with cycling up to 4.2 V.
[0266] FIG. 15D is a plot of experimental data taken at 20 C of capacity
versus cycle
number for electrolyte systems including 2% FEC in a base electrolyte of 1.2M
LiPF6 in 30%
ethylene carbonate and 70% ethyl methyl carbonate; 1% FEC + 1% DTD in a base
electrolyte
of 1.2M LiPF6 in 30% ethylene carbonate and 70% ethyl methyl carbonate; 2% FEC
in a base
36
CA 03071314 2020-01-28
WO 2019/025980 PCT/IB2018/055745
electrolyte of 1.2M LiPF6 in 24% ethylene carbonate, 56% ethyl methyl
carbonate, and 20%
methyl acetate; 2% FEC + 1% DTD in a base electrolyte of 1.2M LiPF6 in 24%
ethylene
carbonate, 56% ethyl methyl carbonate, and 20% methyl acetate; 2% FEC in a
base electrolyte
of 1.2M LiPF6 in 18% ethylene carbonate, 42% ethyl methyl carbonate, and 40%
methyl
acetate; and 2% FEC + 1% DTD in a base electrolyte of 1.2M LiPF6 in 18%
ethylene carbonate,
42% ethyl methyl carbonate, and 40% methyl acetate with cycling up to 4.3 V.
[0267] FIG. 15E is a plot of experimental data taken at 20 C of normalized
capacity versus
cycle number for electrolyte systems including 2% FEC in a base electrolyte of
1.2M LiPF6 in
30% ethylene carbonate and 70% ethyl methyl carbonate; 1% FEC + 1% DTD in a
base
electrolyte of 1.2M LiPF6 in 30% ethylene carbonate and 70% ethyl methyl
carbonate; 2% FEC
in a base electrolyte of 1.2M LiPF6 in 24% ethylene carbonate, 56% ethyl
methyl carbonate,
and 20% methyl acetate; 2% FEC + 1% DTD in a base electrolyte of 1.2M LiPF6 in
24%
ethylene carbonate, 56% ethyl methyl carbonate, and 20% methyl acetate; 2% FEC
in a base
electrolyte of 1.2M LiPF6 in 18% ethylene carbonate, 42% ethyl methyl
carbonate, and 40%
methyl acetate; and 2% FEC + 1% DTD in a base electrolyte of 1.2M LiPF6 in 18%
ethylene
carbonate, 42% ethyl methyl carbonate, and 40% methyl acetate with cycling up
to 4.3 V.
[0268] FIG. 15F is a plot of experimental data taken at 20 C of voltage
hysteresis
(difference between average charge voltage and average discharge voltage)
versus cycle
number for electrolyte systems including 2% FEC in a base electrolyte of 1.2M
LiPF6 in 30%
ethylene carbonate and 70% ethyl methyl carbonate; 1% FEC + 1% DTD in a base
electrolyte
of 1.2M LiPF6 in 30% ethylene carbonate and 70% ethyl methyl carbonate; 2% FEC
in a base
electrolyte of 1.2M LiPF6 in 24% ethylene carbonate, 56% ethyl methyl
carbonate, and 20%
methyl acetate; 2% FEC + 1% DTD in a base electrolyte of 1.2M LiPF6 in 24%
ethylene
carbonate, 56% ethyl methyl carbonate, and 20% methyl acetate; 2% FEC in a
base electrolyte
of 1.2M LiPF6 in 18% ethylene carbonate, 42% ethyl methyl carbonate, and 40%
methyl
acetate; and 2% FEC + 1% DTD in a base electrolyte of 1.2M LiPF6 in 18%
ethylene carbonate,
42% ethyl methyl carbonate, and 40% methyl acetate with cycling up to 4.3 V.
[0269] FIGs. 15A-F illustrates the importance of DTD in systems containing
FEC and
systems that use MA as a solvent at 20 C. Cells with DTD performed better than
cells without
DTD, especially in the cells that contained MA. In particular, 2% FEC + 1% DTD
with 20%
MA solvent shows very stable capacity retention at 4.3 V.
[00100] FIGs. 16A-F shows the results of experiments run at 40 C. Cells with
DTD
performed better than cells without DTD in cells containing MA. FIG. 16A is a
plot of
37
CA 03071314 2020-01-28
WO 2019/025980 PCT/IB2018/055745
experimental data taken at 40 C of capacity versus cycle number for
electrolyte systems
including 2% FEC in a base electrolyte of 1.2M LiPF6 in 30% ethylene carbonate
and 70%
ethyl methyl carbonate; 1% FEC + 1% DTD in a base electrolyte of 1.2M LiPF6 in
30%
ethylene carbonate and 70% ethyl methyl carbonate; 2% FEC in a base
electrolyte of 1.2M
LiPF6 in 24% ethylene carbonate, 56% ethyl methyl carbonate, and 20% methyl
acetate; 2%
FEC + 1% DTD in a base electrolyte of 1.2M LiPF6 in 24% ethylene carbonate,
56% ethyl
methyl carbonate, and 20% methyl acetate; 2% FEC in a base electrolyte of 1.2M
LiPF6 in 18%
ethylene carbonate, 42% ethyl methyl carbonate, and 40% methyl acetate; and 2%
FEC + 1%
DTD in a base electrolyte of 1.2M LiPF6 in 18% ethylene carbonate, 42% ethyl
methyl
carbonate, and 40% methyl acetate with cycling up to 4.2 V.
[0270] FIG. 16B is a plot of experimental data taken at 40 C of normalized
capacity versus
cycle number for electrolyte systems including 2% FEC in a base electrolyte of
1.2M LiPF6 in
30% ethylene carbonate and 70% ethyl methyl carbonate; 1% FEC + 1% DTD in a
base
electrolyte of 1.2M LiPF6 in 30% ethylene carbonate and 70% ethyl methyl
carbonate; 2% FEC
in a base electrolyte of 1.2M LiPF6 in 24% ethylene carbonate, 56% ethyl
methyl carbonate,
and 20% methyl acetate; 2% FEC + 1% DTD in a base electrolyte of 1.2M LiPF6 in
24%
ethylene carbonate, 56% ethyl methyl carbonate, and 20% methyl acetate; 2% FEC
in a base
electrolyte of 1.2M LiPF6 in 18% ethylene carbonate, 42% ethyl methyl
carbonate, and 40%
methyl acetate; and 2% FEC + 1% DTD in a base electrolyte of 1.2M LiPF6 in 18%
ethylene
carbonate, 42% ethyl methyl carbonate, and 40% methyl acetate with cycling up
to 4.2 V.
[0271] FIG. 16C is a plot of experimental data taken at 40 C of voltage
hysteresis
(difference between average charge voltage and average discharge voltage)
versus cycle
number for electrolyte systems including 2% FEC in a base electrolyte of 1.2M
LiPF6 in 30%
ethylene carbonate and 70% ethyl methyl carbonate; 1% FEC + 1% DTD in a base
electrolyte
of 1.2M LiPF6 in 30% ethylene carbonate and 70% ethyl methyl carbonate; 2% FEC
in a base
electrolyte of 1.2M LiPF6 in 24% ethylene carbonate, 56% ethyl methyl
carbonate, and 20%
methyl acetate; 2% FEC + 1% DTD in a base electrolyte of 1.2M LiPF6 in 24%
ethylene
carbonate, 56% ethyl methyl carbonate, and 20% methyl acetate; 2% FEC in a
base electrolyte
of 1.2M LiPF6 in 18% ethylene carbonate, 42% ethyl methyl carbonate, and 40%
methyl
acetate; and 2% FEC + 1% DTD in a base electrolyte of 1.2M LiPF6 in 18%
ethylene carbonate,
42% ethyl methyl carbonate, and 40% methyl acetate with cycling up to 4.2 V.
[0272] FIG. 16D is a plot of experimental data taken at 40 C of capacity
versus cycle
number for electrolyte systems including 2% FEC in a base electrolyte of 1.2M
LiPF6 in 30%
38
CA 03071314 2020-01-28
WO 2019/025980 PCT/IB2018/055745
ethylene carbonate and 70% ethyl methyl carbonate; 1% FEC + 1% DTD in a base
electrolyte
of 1.2M LiPF6 in 30% ethylene carbonate and 70% ethyl methyl carbonate; 2% FEC
in a base
electrolyte of 1.2M LiPF6 in 24% ethylene carbonate, 56% ethyl methyl
carbonate, and 20%
methyl acetate; 2% FEC + 1% DTD in a base electrolyte of 1.2M LiPF6 in 24%
ethylene
carbonate, 56% ethyl methyl carbonate, and 20% methyl acetate; 2% FEC in a
base electrolyte
of 1.2M LiPF6 in 18% ethylene carbonate, 42% ethyl methyl carbonate, and 40%
methyl
acetate; and 2% FEC + 1% DTD in a base electrolyte of 1.2M LiPF6 in 18%
ethylene carbonate,
42% ethyl methyl carbonate, and 40% methyl acetate with cycling up to 4.3 V.
[0273] FIG. 16E is a plot of experimental data taken at 40 C of normalized
capacity versus
cycle number for electrolyte systems including 2% FEC in a base electrolyte of
1.2M LiPF6 in
30% ethylene carbonate and 70% ethyl methyl carbonate; 1% FEC + 1% DTD in a
base
electrolyte of 1.2M LiPF6 in 30% ethylene carbonate and 70% ethyl methyl
carbonate; 2% FEC
in a base electrolyte of 1.2M LiPF6 in 24% ethylene carbonate, 56% ethyl
methyl carbonate,
and 20% methyl acetate; 2% FEC + 1% DTD in a base electrolyte of 1.2M LiPF6 in
24%
ethylene carbonate, 56% ethyl methyl carbonate, and 20% methyl acetate; 2% FEC
in a base
electrolyte of 1.2M LiPF6 in 18% ethylene carbonate, 42% ethyl methyl
carbonate, and 40%
methyl acetate; and 2% FEC + 1% DTD in a base electrolyte of 1.2M LiPF6 in 18%
ethylene
carbonate, 42% ethyl methyl carbonate, and 40% methyl acetate with cycling up
to 4.3 V.
[0274] FIG. 16F is a plot of experimental data taken at 40 C of voltage
hysteresis
(difference between average charge voltage and average discharge voltage)
versus cycle
number for electrolyte systems including 2% FEC in a base electrolyte of 1.2M
LiPF6 in 30%
ethylene carbonate and 70% ethyl methyl carbonate; 1% FEC + 1% DTD in a base
electrolyte
of 1.2M LiPF6 in 30% ethylene carbonate and 70% ethyl methyl carbonate; 2% FEC
in a base
electrolyte of 1.2M LiPF6 in 24% ethylene carbonate, 56% ethyl methyl
carbonate, and 20%
methyl acetate; 2% FEC + 1% DTD in a base electrolyte of 1.2M LiPF6 in 24%
ethylene
carbonate, 56% ethyl methyl carbonate, and 20% methyl acetate; 2% FEC in a
base electrolyte
of 1.2M LiPF6 in 18% ethylene carbonate, 42% ethyl methyl carbonate, and 40%
methyl
acetate; and 2% FEC + 1% DTD in a base electrolyte of 1.2M LiPF6 in 18%
ethylene carbonate,
42% ethyl methyl carbonate, and 40% methyl acetate with cycling up to 4.3 V.
[0275] FIGs. 16A-F illustrates the importance of DTD in systems containing
FEC and
systems that use MA as a solvent at 40 C. Generally, cells with DTD performed
better than
cells without DTD, especially in the cells that contained MA. In the two-
additive electrolyte
system with 2% FEC + 1% DTD with 20% MA solvent, the impact of the DTD is a
bit muted
39
CA 03071314 2020-01-28
WO 2019/025980 PCT/IB2018/055745
compared to the same-two additive electrolyte system, but without MA as a
solvent. Further,
the addition of up to 40% MA at 4.3 V reduces the cycle life. That is, DTD and
MA can have
a symbiotic increase of the performance of the two-additive electrolyte
system, but this increase
is muted when operating on cycles up to 4.3 V. Thus, in certain embodiments of
this present
disclosure, the electrolyte system only operates up to 4.2 V. In other
embodiments of the
present disclosure, the electrolyte system operates up to 4.3 V, but with a MA
concentration of
less than 40%.
[0276] NMC622 As Positive Electrode: In certain embodiments, the battery
systems have
a positive electrode made from NMC622. In certain embodiments, the positive
electrode is
coated with a material such as aluminum oxide (A1203), titanium dioxide
(TiO2), or another
coating. FIGs. 17A-F shows experimental data for long term cycling of one- and
two-additive
electrolyte systems with a coated NMC622 as the positive electrode at 40 C,
C/3 CCCV. The
dashed lines are extrapolated from the experimental data.
[0277] More specifically, FIG. 17A is a plot of experimental data of
capacity versus cycle
number for electrolyte systems that contain FEC and/or DTD with cycling up to
4.3 V. FIG.
17B is a plot of experimental data of normalized capacity versus cycle number
for electrolyte
systems that contain FEC and/or DTD with cycling up to 4.3 V. FIG. 17C is a
plot of
experimental data of voltage hysteresis (difference between average charge
voltage and
average discharge voltage) versus cycle number for electrolyte systems that
contain FEC and/or
DTD with cycling up to 4.3 V. FIG. 17D is a plot of experimental data of
capacity versus cycle
number for electrolyte systems that contain VC and/or DTD with cycling up to
4.3 V. FIG.
17E is a plot of experimental data of normalized capacity versus cycle number
for electrolyte
systems that contain VC and/or DTD with cycling up to 4.3 V. FIG. 17F is a
plot of
experimental data of voltage hysteresis (difference between average charge
voltage and
average discharge voltage) versus cycle number for electrolyte systems that
contain VC and/or
DTD with cycling up to 4.3 V.
[0278] FIGs. 17A-F illustrate that even when a different positive electrode
is chosen, the
experimental data shows that an electrolyte system with two additives¨VC + DTD
and FEC
+ DTD¨performs superior to any single additive of VC, FEC, or DTD.
[0279] Natural Graphite As Negative Electrode: In certain embodiments, the
battery
systems have negative electrode made from natural graphite. FIGs. 18A-B and 19
show data
from additional long-term cycling experiments carried out using single-crystal
NMC532 as the
positive electrode and natural graphite as the negative electrode at 40 C, C/3
CCCV. FIG. 18A
shows capacity plotted versus cycle number. FIG. 18B shows normalized capacity
plotted
CA 03071314 2020-01-28
WO 2019/025980 PCT/IB2018/055745
versus cycle number. FIG. 19 shows voltage hysteresis (difference between
average charge
voltage and average discharge voltage) versus cycle number. FIGs. 18A-B and 19
illustrate
that the two-electrolyte additive system, including DTD + FEC improves
performance over an
electrolyte system that only includes FEC as an additive, but comparisons to
FIGs. 6-F in which
an artificial-graphite negative electrode was used, suggests that the
performance of this specific
artificial-graphite negative electrode outperforms this specific natural-
graphite negative
electrode in the two-additive electrolyte systems of the present disclosure.
[0280] In certain embodiments, the battery system has a natural graphite
negative
electrode. Using natural graphite as the negative electrode is important as a
cost savings
measure over artificial graphite, which is typically more expensive. Thus,
when cost is the
primary driver and some performance tradeoffs may be made, natural graphite
may be a good
choice.
[0281] LFO As An Additive: In certain embodiments, LFO is added as an
electrolyte
system. FIG. 41A summarizes data of coulombic inefficiency verses upper cut-
off voltage for
different electrolyte systems, including systems containing LFO. FIG. 41B
summarizes data
of fractional fade verses upper cut-off voltage for different electrolyte
systems, including
systems containing LFO. FIG. 41C summarizes data of charge-end-point capacity
slippage
verses upper cut-off voltage for different electrolyte systems, including
systems containing
LFO. FIG. 42A illustrates an expanded view of FIG. 41A and summarizes data of
coulombic
inefficiency verses upper cut-off voltage for different electrolyte systems,
including systems
containing LFO. FIG. 42B illustrates an expanded view of FIG. 41B and
summarizes data of
fractional fade verses upper cut-off voltage for different electrolyte
systems, including systems
containing LFO. FIG. 42C illustrates an expanded view of FIG. 41C and
summarizes data of
charge-end-point capacity slippage verses upper cut-off voltage for different
electrolyte
systems, including systems containing LFO. Adding LFO to control electrolyte
vastly
improves UHPC results. In the presence of MA, 1% LFO dramatically improves the
situation
compared to 0.5 % LFO. The CIE/h is about 4x10-5 h-1 for 1% LFO in control. By
comparison,
the best electrolyte systems without LFO, like 2% VC + 1% DTD in control are
near 3x10-5 h-
i.
[0282] FIGs. 43A-D summarize long-term-cycling data for different
electrolyte systems,
including systems containing LFO. The long-term cycling results illustrate
that the addition of
LFO dramatically improves impedance growth in the systems tested and confirms
the UHPC
data. In particular, 1% LFO added to the control electrolyte and to the
electrolyte systems
containing 20% MA improves the long-term cycling and impedance.
41
CA 03071314 2020-01-28
WO 2019/025980 PCT/IB2018/055745
Microcalorimetry Measurements
[0283] Microcalorimetry measures heat flow to the cell during operation.
The heat flow to
the cell is a combination of three different effects: (1) ohmic heating, (2)
entropy changes due
to Li intercalating in the electrodes, and (3) parasitic reactions
(electrolyte, including additive,
degradation at either electrode). Because the test cells contain the same
physical design,
different only in the electrolyte, the difference in heat flow is primarily
due to the differences
in parasitic heat flow. Nevertheless, the parasitic heat flow can be extracted
from the total heat
flow using the procedures developed by Downie et al. (Journal of the
Electrochemical Society,
161, A1782-A1787 (2014)) and by Glazier et al. (Journal of the Electrochemical
Society, 164
(4) A567-A573 (2017)). Both of these references are incorporated herein in
their entirety. Cells
that have lower parasitic heat flow during cycling have better lifetimes. The
voltage
dependence of the parasitic reaction rate may be observed by plotting the
measured parasitic
heat flow as a function of cell voltage.
[0284] Microcalorimetry Measurement Procedure: Two cells of each
electrolyte were
connected to a Maccor charger and inserted into a TAMIII Microcalorimeter (TA
Instruments,
stability +/- 0.0001 C, accuracy +/- 1 [LW, precision +/- 1 nW) at 40.0 C. The
baseline drift
over the course of the experiments did not exceed +/- 0.5 [LW. All
specifications and
information regarding microcalorimetry calibration, cell connections, and
operation procedures
can be found in previous literature. (For example, Downie et al, ECS
Electrochemical Letters
2, A106-A109 (2013).) Cells were cycled four times at a C/20 rate between 3.0
V and 4.2 V
to ensure well-formed, stable SEIs and were then charged between 4.0 V and
different upper
cut off limits at 1 mA to investigate the performance and the parasitic heat
flow in different
voltage ranges. Each pair of cells yielded near identical performance, so only
one set of heat
flow data is presented for each electrolyte.
[0285] The 1 mA cycling protocol was:
1. Charge to 4.2 V, discharge to 4.0 V
2. Charge to 4.3 V, discharge to 4.0 V (repeat)
3. Charge to 4.4 V, discharge to 4.0 V (repeat)
6. Charge to 4.2 V, discharge to 4.0 V
Additional experimental detail is described in the Journal of the
Electrochemical Society, 164
(4) A567-A573 (2017), which is incorporated herein by reference in its
entirety.
[0286] In the experimental data shown in FIGs. 11-14, pouch cells with a
positive electrode
made from single-crystal NMC532 and an artificial-graphite negative electrode
were used.
42
CA 03071314 2020-01-28
WO 2019/025980 PCT/IB2018/055745
Excluding additives, the electrolyte was either (1) 1.2M LiPF6 in 30% ethylene
carbonate and
70% ethyl methyl carbonate, (2) 1.2M LiPF6 in 24% ethylene carbonate, 56%
ethyl methyl
carbonate, and 20% methyl acetate; or (3) 1.2M LiPF6 in 18% ethylene
carbonate, 42% ethyl
methyl carbonate, and 40% methyl acetate, depending on the concentration of
methyl acetate
(0, 20, or 40%).
[0287] FIG. 11 shows experimental data of calorimetry experiments
(parasitic heat flow
versus voltage) when charging to 4.2 V. FIGs. 12A and B show experimental data
of
calorimetry experiments (parasitic heat flow versus voltage) when charging to
4.3 V. Since the
charging to 4.3 V was repeated, each plot shows the results of the one
charging. FIGs. 13A
and B show experimental data (parasitic heat flow versus voltage) when
charging to 4.4 V.
Since the charging to 4.4V was repeated, each plot shows the results of the
one charging. The
plot of the difference (lower plot in FIGs 11, 12A, 12B, 13A, and 13B) is
calculated by taking
the heat flow for each electrolyte mixture and subtracting the heat flow
produced by the control
(2% FEC). FIG. 14 shows a summary of the experimental data conveyed in FIGs.
11-13. Table
1 summarizes the data displayed in FIG. 14 in tabular form.
Table 1: Average Parasitic Heat Flow per Cycle (pW) (40. C, 4.0 V to UCV, 1
mA)
Upper Cut
Off 20 MA+ 2
Voltage 2 FEC Error FEC Error 40
MA +2 FEC Error
4.2 70.4 0.2 82 3 94 2
4.3 60.9 0.1 75 3 93 1
4.3 41.00 0.07 54 2 69.6 0.9
4.4 53.7 0.2 73 3 95 1
4.4 38.3 0.3 54 3 74.5 0.7
4.2 27.1 0.6 36 2 46.3 0.5
20 MA +2
2 FEC FEC 40 MA +2 FEC
1 DTD Error 1 DTD Error 1 DTD Error
4.2 63.9 0.2 69.7 0.3 77.3 0.3
4.3 54.7 0.3 64.9 0.7 76.95 0.07
4.3 36.8 0.2 46.5 0.3 58.6 0.1
4.4 48.4 0.5 65.0 0.6 84.7 0.1
4.4 34.6 0.4 49.1 0.6 66.0 0.2
43
CA 03071314 2020-01-28
WO 2019/025980
PCT/IB2018/055745
4.2 24.44 0.04 31.4 0.3 39.3 0.7
[0288] FIGs. 11-14 and Table 1 show that that adding DTD to FEC leads to
lowers
parasitic heat flow (lower parasitic reaction rate). FIGs. 11-14 and Table 1
also show that
adding MA leads to higher parasitic heat flow (higher parasitic reaction
rate), but this increase
may be mitigated by DTD, which helps reduce the increased parasitic reaction
rate from the
addition of the MA.
[0289] LFO As An Additive: FIG. 47 shows how the charge heat flow relates
to the
parasitic heat flow, the charge overpotential, and discharge overpotential.
FIGs. 48a-F show
the result of the TAM experiments. The difference plots compare the system to
2% VC + 1%
DTD. FIGs. 48a-F show that 2% VC + 1DTD is better than 2% FEC + 1DTD. They
also
show that when optimizing for LFO within the system, 1% LFO + 1% VC + 1% FEC
is better
than 2% VC + 1% DTD above 4.3 V. When comparing the systems with 1% LFO, 1%
LFO +
1% VC performs about as well as 1% LFO + 1% VC + 1% FEC and is better than 2%
VC +
1% DTD above 4.3 V. As observed in FIGs. 50A-C, the optimum LFO composition is
about
1.0 %. FIG. 51 shows the mean parasitic heat flow as a function of cycles for
the best
performing cells. 0.5% LFO with 1% VC + 1% FEC is the best performing system
after 4.4
V cycles. 2% VC + 1% LFO is very comparable to 2% VC + 1% DTD. Thus a system
with
VC and with or without DTD is possible.
Plating Experiments
[0290] Plating experiments test the ability to charge at a fast rate. Fast
charging is very
important in energy-storage when part of a vehicle, while slower charging
rates may be
acceptable in grid-storage applications. High rate charging is mainly limited
by lithium plating
on the negative electrode which leads to safety issues and diminishes cycle
and calendar life.
Electrolyte systems that allow higher charging rates without plating are thus
advantageous. To
study plating on the negative electrode, plating experiments were performed.
Larger capacity
loss indicates greater lithium plating.
[0291] Plating experiments were performed to test the charging ability of
cells. After EIS
measurements, cells were charged and discharged with constant currents (C-
rates) of 1 C, 1.5
C, and 2.0 C between 2.8 and 4.1 V using a Maccor charger system at 20.0 0.1
C. Pair cells
were tested for every charge rate to ensure reproducibility. In order to
determine the active
lithium loss during cycling, cells were cycled at C/20 one time before and
after the high charge
rate segments. The upper cutoff voltage was set to 4.1 V in order to minimize
electrolyte
44
CA 03071314 2020-01-28
WO 2019/025980 PCT/IB2018/055745
oxidation at the positive electrode and to ensure that the cells were far from
having a fully
loaded negative electrode which would occur at 4.4 V for these cells. All
pouch cells were
cycled with external clamps to eliminate effects of small amounts of gas that
may be produced
during cycling. Cells were stopped after about 350 hours cycling or after the
capacity loss
reached 20%.
[0292] Two-Electrolyte Systems With FEC Or VC As Additive: In certain
embodiments, two-additive electrolyte systems, the concentration of each
additive at about
0.25-6%, form part of the battery system. FIG. 22 shows experimental data of
the plating
experiments for different battery systems at different current charging rates.
FIG. 22 indicates
that addition of DTD does not significantly increase the maximum current at
which plating
occurs. For example, the low rate capacity loss of the electrolyte system
consisting of two
additives-2% FEC + 1% DTD¨is decreased compared to the electrolyte system
consisting
of the single additive 2% FEC at 1C, 1.5C, and 2C. Similarly, FIG. 22 shows
that the low rate
capacity loss of the electrolyte system consisting of two additives-1% FEC +
1% DTD¨is
decreased compared to the electrolyte system consisting of the single additive
2% FEC at 1C
and 1.5C. It is also only slightly higher at 2C.
[0293] FIG. 23 shows experimental data from plating tests, in which after
every 30 cycles,
the charging current was increased. A large capacity loss rate indicates
lithium plating. At a
charging current of 2C, every cell began to plate lithium. However, cells with
DTD lose less
capacity during plating. This indicates that the amount of plating in cells
with DTD is less than
in cells without. In addition to DTD, other sulfur-containing compounds can
function in a
similar manner to decrease plating.
[0294] FIG. 24 shows the results of experimental data of peak capacity as a
function of
cycle number for different electrolyte systems. DTD performed better than MMDS
in retaining
the peak capacity of the two-additive electrolyte system when DTD or MMDS were
combined
with VC.
[0295] Methyl Acetate As Electrolyte Solvent: Methyl acetate, in
concentrations of up to
60% by weight, is used as a solvent to reduce plating according to certain
embodiments. FIGs.
27-34 illustrate the impact of using MA as a solvent in the presence of
different electrolyte
systems. FIG. 27 shows the results of plating experiments to determine the
impact of MA as a
solvent and the presence of DTD as an additive on the cell impedance. The
electrolyte systems
tested include 2% of an additive (VC, FEC, and PES) in an electrolyte with 0%,
20%, and 40%
MA. The remaining electrolyte for 0% MA is 1.2M LiPF6 in 30% ethylene
carbonate and 70%
ethyl methyl carbonate. The remaining electrolyte for 20% MA is 1.2M LiPF6 in
24% ethylene
CA 03071314 2020-01-28
WO 2019/025980 PCT/IB2018/055745
carbonate and 56% ethyl methyl carbonate. The remaining electrolyte for 40% MA
is 1.2M
LiPF6 in 18% ethylene carbonate and 42% ethyl methyl carbonate. FIG. 28 is an
expanded
view of certain data shown in in FIG. 27 that had a small low rate capacity
loss.
[0296] In FIGs. 27 and 28, a larger capacity loss indicates greater lithium
plating. FIGs.
27 and 28 show that the presence of MA decreases the low rate capacity loss,
even at charging
rates of 2C. Thus, electrolyte systems containing 20% or 40% of MA are good
candidates for
use in applications that fast charging, such as energy storage in a vehicle
that may be subjected
to high charging current rates.
[0297] FIGs. 29, 30, and 31 show the results of experimental data of
electrolyte systems
containing FEC as an additive. Different data sets include DTD and/or MA, as
indicated by the
legend in the figures. FIGs. 29, 30, and 31 show that the addition of MA to
one- and two-
additive electrolyte systems with FEC allows for higher charging rates,
including charging up
to 2 C, without significant plating.
[0298] Similarly, FIGs. 32, 33, and 34 show the results of experimental
data of electrolyte
systems containing VC as an additive. Different data sets include DTD and/or
MA, as indicated
by the legend in the figures. FIGs. 32, 33, and 34 show that the addition of
MA to one- and
two-additive electrolyte systems with VC allows for higher charging rates,
including charging
up to 2 C, without significant plating.
[0299] LFO As Electrolyte Additive: FIGs. 44A-D summarize long-term-cycling
data
under high-rate charging for different electrolyte systems, including systems
containing LFO.
As can be seen from the experimental data the presence of MA reduces the
amount of plating.
Further, LFO decreases the likelihood of Li-plating during high-rate charging.
For example,
in FIG. 44A, the electrolyte system with 20%MA + 1% LFO showed significantly
less loss of
normalized discharge capacity than the other systems with either less MA or
LFO. Loss of
normalized discharge capacity is indicative of plating.
Gas Volume Measurements
[0300] The formation process is performed prior to cells being used in
their intended
application, such as grid storage or energy storage in an automobile, such as
an electric vehicle.
During formation, cells are subject to a precisely controlled charge and
discharge cycle, which
is intended to activate the electrodes and electrolyte for use in their
intended application.
During formation, gas is generated. If sufficient amounts of gas are generated
(depending on
the specific tolerances allowed by the cell and cell packaging), the gas may
need to be released
after the formation process and prior to application use. This typically
requires the additional
steps of breaking of a seal followed by a resealing. While these steps are
common for many
46
CA 03071314 2020-01-28
WO 2019/025980 PCT/IB2018/055745
battery systems, it is desirable to remove them if possibly by choosing a
system that produces
less gas.
[0301] Gas volume experiments proceeded as follows: Ex-situ (static) gas
measurements
were used to measure gas evolution during formation and during cycling. The
measurements
were made using Archimedes' principle with cells suspended from a balance
while submerged
in liquid. The changes in the weight of the cell suspended in fluid, before
and after testing are
directly related to the volume changes by the change in the buoyant force. The
change in mass
of a cell, Am, suspended in a fluid of density, p, is related to the change in
cell volume, Av, by
Av = Am/p.
[0302] Two-Electrolyte Systems With FEC Or VC As Additive: In certain
embodiments, two-additive electrolyte systems, the concentration of each
additive about 0.25-
6%, form part of the battery system. FIG. 20 shows the results of gas-
generation experiments,
in which the amount of gas generated was measured according to the procedure
described
above. FIG. 20 shows that systems without DTD typically performed better, for
example a
system containing only 2% FEC as an additive, performs better than 1% FEC + 1%
DTD and
2% FEC + 1% DTD. That is, DTD leads to higher gas volume production during
formation, if
DTD is to be used as an additive because of its desirable properties when
combined with other
additives, for example VC and FEC, then the system must include a mechanism to
safely deal
with the gas produced by the DTD, such as gas release after formation as
discussed above.
FIG. 20 shows that two-additive electrolyte systems that include MMDS and PES
or FEC do
not produce much (if any) additional gas than when 2% PES or FEC is the only
additive.
[0303] Methyl Acetate As Electrolyte Solvent: Methyl acetate, in
concentrations of up to
60% by weight, is used as a solvent to reduce plating according to certain
embodiments. FIG.
25 shows the results of gas-generation experiments to determine the impact of
MA as a solvent
and presence of DTD as an additive on the formation gas generated. The
electrolyte systems
tested include 2% of an additive (VC, FEC, and PES) in an electrolyte with 0%,
20%, and 40%
MA. The remaining electrolyte for 0% MA is 1.2M LiPF6 in 30% ethylene
carbonate and 70%
ethyl methyl carbonate. The remaining electrolyte for 20% MA is 1.2M LiPF6 in
24% ethylene
carbonate and 56% ethyl methyl carbonate. The remaining electrolyte for 40% MA
is 1.2M
LiPF6 in 18% ethylene carbonate and 42% ethyl methyl carbonate.
[0304] FIG. 25 shows that in two-additive electrolyte systems that contain
VC or FEC with
DTD, the increase of the amount of gas with the addition of MA varies less as
the amount of
MA increases. That is, the marginal amount of gas generated is less when DTD
is part of the
47
CA 03071314 2020-01-28
WO 2019/025980 PCT/IB2018/055745
two-additive electrolyte system compared to a one-additive electrolyte system
that has only
VC or FEC.
In-Situ Gas Volume Measurements
[0305] FIGs. 45A and 45B summarize the results from in-situ gas experiments
at 40 C.
Cells with LFO, but no MA, show less gassing during hold segments of these
tests.
Cell Impedance
[0306] The two-additive electrolyte systems and novel battery systems
disclosed herein
have low cell impedance. Minimizing cell impedance is desirable since cell
impedance
decrease the energy efficiency of a cell. Conversely, low impedance leads to a
higher charging
rate and higher energy efficiency.
[0307] Cell impedance was measured using electrochemical impedance
spectroscopy
(EIS). The pouch cells used a single-crystal NMC532 positive electrode and an
artificial
negative electrode unless stated otherwise, with the EIS measurements
performed after
formation. Cells were charged or discharged to 3.80 V before they were moved
to a 10.0 +/-
0.1 C temperature box. AC impedance spectra were collected with ten points
per decade from
100 kHz to 10 mHz with a signal amplitude of 10 mV at 10.0 +/- 0.1 C. From
the measured
AC impedance, the charge transfer resistance (Rct) was calculated and plotted.
[0308] Two-Electrolyte Systems With FEC Or VC As Additive: In certain
embodiments, two-additive electrolyte systems, the concentration of each
additive about 0.25-
6%, form part of the battery system. FIG. 21 shows experimental data of cell
charge transfer
impedance experiments for two-additive electrolyte systems consisting of 1%
DTD with 1%
or 2% of PES, FEC, or VC. FIG. 21 shows that these two-additive electrolyte
systems of 1%
DTD with 1% or 2% of PES, FEC, or VC do not significantly increase the cell
charge transfer
impendence. In particular, systems of 1% DTD with 1% VC, 1% DTD with 2% VC, 1%
DTD
with 1% FEC, and 1% DTD with 2% FEC exhibit cell impedance values similar to
the cell
charge transfer impendence observed for the single-additive systems with DTD
excluded.
Therefore, these novel two-additive electrolyte systems do not sacrifice
significant charge
transfer impedance performance by including DTD.
[0309] Methyl Acetate As Electrolyte Solvent: Methyl acetate, in
concentrations of up to
60% by weight, is used as a solvent to reduce plating according to certain
embodiments. FIG.
26 shows the results of cell-charge transfer impedance experiments on
electrolyte systems
consisting of one- and two-additive systems with MA as one of the solvents.
The additive-
electrolyte systems tested included 2% of additives VC, FEC, and PES without
and without
48
CA 03071314 2020-01-28
WO 2019/025980 PCT/IB2018/055745
1% DTD to show impact of the DTD and MA to the electrolyte system in an
electrolyte solvent
with 0%, 20%, and 40% MA. The electrolyte for 0% MA is 1.2M LiPF6 in 30%
ethylene
carbonate and 70% ethyl methyl carbonate. The remaining electrolyte for 20% MA
is 1.2M
LiPF6 in 24% ethylene carbonate and 56% ethyl methyl carbonate. The remaining
electrolyte
for 40% MA is 1.2M LiPF6 in 18% ethylene carbonate and 42% ethyl methyl
carbonate. FIG.
26 shows that DTD produces only slight increases in charge transfer impedance.
Further, in
two-additive electrolyte systems that contain VC or FEC with DTD, the addition
of MA
decreases cell charge transfer impedance. At 40% MA solvent, the VC + DTD and
FEC + DTD
systems showed reduced charge transfer impedance from the corresponding
systems without
DTD and no MA as a solvent. In the PES + DTD two-additive electrolyte system,
MA also
reduced the charge transfer impedance of the system.
[0310] The foregoing disclosure is not intended to limit the present
disclosure to the precise
forms or particular fields of use disclosed. As such, it is contemplated that
various alternative
embodiments and/or modifications to the present disclosure, whether explicitly
described or
implied herein, are possible in light of the disclosure. Having thus described
embodiments of
the present disclosure, a person of ordinary skill in the art will recognize
that changes may be
made in form and detail without departing from the scope of the present
disclosure. Thus, the
present disclosure is limited only by the claims. Reference to additives in
the specification are
generally to operative additives unless otherwise noted in the specification.
[0311] In the foregoing specification, the disclosure has been described
with reference to
specific embodiments. However, as one skilled in the art will appreciate,
various embodiments
disclosed herein can be modified or otherwise implemented in various other
ways without
departing from the spirit and scope of the disclosure. Accordingly, this
description is to be
considered as illustrative and is for the purpose of teaching those skilled in
the art the manner
of making and using various embodiments of the disclosed battery system. It is
to be
understood that the forms of disclosure herein shown and described are to be
taken as
representative embodiments. Equivalent elements, or materials may be
substituted for those
representatively illustrated and described herein. Moreover, certain features
of the disclosure
may be utilized independently of the use of other features, all as would be
apparent to one
skilled in the art after having the benefit of this description of the
disclosure. Expressions such
as "including", "comprising", "incorporating", "consisting of', "have", "is"
used to describe
and claim the present disclosure are intended to be construed in a non-
exclusive manner,
namely allowing for items, components or elements not explicitly described
also to be present.
Reference to the singular is also to be construed to relate to the plural.
Reference to "about" or
49
CA 03071314 2020-01-28
WO 2019/025980 PCT/IB2018/055745
"approximately" is to be construed to mean plus or minus 10%. Similarly,
reference to any
percentage of an additive is construed to mean plus or minus 10%.
[0312] Further, various embodiments disclosed herein are to be taken in the
illustrative and
explanatory sense, and should in no way be construed as limiting of the
present disclosure. All
joinder references (e.g., attached, affixed, coupled, connected, and the like)
are only used to
aid the reader's understanding of the present disclosure, and may not create
limitations,
particularly as to the position, orientation, or use of the systems and/or
methods disclosed
herein. Therefore, joinder references, if any, are to be construed broadly.
Moreover, such
joinder references do not necessarily infer that two elements are directly
connected to each
other.
[0313] Additionally, all numerical terms, such as, but not limited to,
"first", "second",
"third", "primary", "secondary", "main" or any other ordinary and/or numerical
terms, should
also be taken only as identifiers, to assist the reader's understanding of the
various elements,
embodiments, variations and/ or modifications of the present disclosure, and
may not create
any limitations, particularly as to the order, or preference, of any element,
embodiment,
variation and/or modification relative to, or over, another element,
embodiment, variation
and/or modification.
[0314] It will also be appreciated that one or more of the elements
depicted in the
drawings/figures can also be implemented in a more separated or integrated
manner, or even
removed or rendered as inoperable in certain cases, as is useful in accordance
with a particular
application.