Note: Descriptions are shown in the official language in which they were submitted.
1
IONIC LIQUIDS FOR UPGRADING OF BITUMEN
TECHNICAL FIELD
[1] The technical field generally relates to the treatment of bitumen, and
more
particularly to the upgrading of bitumen using ionic liquids.
BACKGROUND
[2] Bitumen generally has a high viscosity, irrespective of whether it has
been
recovered by mining operations or by in situ recovery processes. This high
viscosity can
make the pipeline transportation of bitumen difficult. Various methods exist
to decrease
bitumen viscosity and increase suitability for pipeline transportation,
although such
methods have various drawbacks. Bitumen generally also include undesirable
components, such as sulphur, naphthenic acids and heavy metals.
[3] Bitumen upgrader facilities of various designs can upgrade the bitumen
to
produce less viscous products and to remove undesirable components. However,
conventional upgrader facilities have high associated capital and operating
costs. In
addition, in some conventional upgrading methods such as severe thermal
cracking,
hydrogen originally present in the bitumen is lost to the gas phase such that,
in the
absence of added hydrogen, significant yet undesirable olefin production can
occur.
[4] Another option to improve bitumen viscosity is to dilute the bitumen,
for
example with naphtha or natural gas condensate as a diluent. Diluted bitumen
is often
referred to as "dilbit". While bitumen dilution does not have the same capital
cost penalty
as a bitumen upgrader facility, it still has high associated operating costs.
For example,
since dilbit includes a significant volume of diluent (e.g., one third diluent
and two thirds
bitumen per barrel of diluted bitumen), significant pipeline capacity is
therefore taken up
by the diluent for pipelining of the dilbit as well as the return pipelining
of separated
diluent to be reused in bitumen dilution.
[5] Various challenges still exist with regard to bitumen upgrading
processes
and there is a need for enhanced technologies.
CA 3074850 2020-03-05
2
SUMMARY
[6] In accordance with an aspect, there is provided a process for treating
a bitumen
feedstock. The process comprises contacting an acidic ionic liquid catalyst
with the
bitumen feedstock to obtain an ionic liquid-bitumen mixture; subjecting the
ionic liquid-
bitumen mixture to a catalytic cracking treatment, the catalytic cracking
treatment
comprising heating the ionic liquid-bitumen mixture under catalytic cracking
conditions to
obtain an ionic liquid-cracked bitumen mixture; and separating the acidic
ionic liquid
catalyst from the ionic liquid-cracked bitumen mixture to obtain a cracked
bitumen
product and a recovered acidic ionic liquid catalyst.
[7] In some implementations, the bitumen feedstock comprises a diluent-
depleted
bitumen stream from a distillation unit, a diluent stripping unit or a diluent
recovery unit.
[8] In some implementations, the bitumen feedstock comprises a diluent-
depleted
bitumen stream that is obtained from a bitumen froth treatment operation.
[9] In some implementations, the bitumen feedstock comprises a diluent-
depleted
bitumen stream that has not been subjected to fractionation or distillation
prior to being
contacted with the acidic ionic liquid catalyst.
[10] In some implementations, the bitumen feedstock comprises a residuum
stream
from a distillation tower that has been operated to remove light hydrocarbon
components.
[11] In some implementations, the bitumen feedstock comprises a bitumen stream
that is obtained from an in situ recovery operation or from surface mining
operations.
[12] In some implementations, the process further comprises subjecting the
bitumen
feedstock to a pre-treatment prior to the contacting of the bitumen feedstock
with the
acidic ionic liquid catalyst.
[13] In some implementations, the pre-treatment comprises heating the bitumen
feedstock at a pre-treatment temperature.
CA 3074850 2020-03-05
, ,
3
[14] In some implementations, the pre-treatment temperature is sufficient
to reduce
viscosity of the bitumen feedstock.
[15] In some implementations, the pre-treatment comprises adding a diluent
to the
bitumen feedstock.
[16] In some implementations, the diluent comprises at least one of a
naphthenic
solvent, an aromatic hydrocarbon, and a non-deasphalting organic solvent.
[17] In some implementations, the diluent comprises toluene.
[18] In some implementations, the bitumen feedstock comprises bitumen and a
diluent.
[19] In some implementations, the process further comprises recovering the
diluent to
obtain a recovered diluent.
[20] In some implementations, recovering the diluent is performed after the
catalytic
cracking treatment of the ionic liquid-bitumen mixture under catalytic
cracking conditions
and before separating the acidic ionic liquid catalyst from the ionic liquid-
cracked
bitumen mixture.
[21] In some implementations, recovering the diluent is performed after
separating the
acidic ionic liquid catalyst from the ionic liquid-cracked bitumen mixture.
[22] In some implementations, recovering the diluent comprises evaporating
the
diluent from the ionic liquid-cracked bitumen mixture or the cracked bitumen
product.
[23] In some implementations, the process further comprises contacting at
least a
portion of the recovered diluent with the bitumen feedstock.
[24] In some implementations, the recovered acidic ionic liquid catalyst is
reused as
part of the acidic ionic liquid catalyst that contacts the bitumen feedstock.
[25] In some implementations, separating the acidic ionic liquid catalyst
from the ionic
liquid-cracked bitumen mixture comprises a liquid-liquid extraction of the
ionic liquid-
cracked bitumen mixture.
CA 3074850 2020-03-05
4
[26] In some implementations, the liquid-liquid extraction of the ionic
liquid-cracked
bitumen mixture comprises washing the ionic liquid-cracked bitumen mixture
with water.
[27] In some implementations, the acidic ionic liquid catalyst is a Lewis
acidic ionic
liquid catalyst comprising a Lewis acidic anion and a cation selected from the
group
consisting of 1,3-dialkylimidazolium cations, tetraalkylphosphonium cations,
tetraalkylammonium cations, trialkylammonium cations and combinations thereof.
[28] In some implementations, the Lewis acidic ionic liquid catalyst
comprises a Lewis
acidic anion and a 1-alkyl-3-methylimidazolium cation.
[29] In some implementations, the 1-alkyl-3-methylimidazolium cation is
selected from
the group consisting of 1-ethyl-3-methylimidazolium (EMIM) and 1-n-buty1-3-
methylimidazolium (BMIM).
[30] In some implementations, the Lewis acidic ionic liquid catalyst
comprises a Lewis
acidic anion and a trialkylammonium cation.
[31] In some implementations, the trialkylammonium cation comprises
triethylammonium.
[32] In some implementations, the Lewis acidic anion is a chlorometallate
anion.
[33] In some implementations, the chlorometallate anion is selected from
the group
consisting of AlC14-, Al2C17-, FeCI4- and Fe2C17-.
[34] In some implementations, the Lewis acidic ionic liquid is selected
from the group
consisting of [EMIM][AICI4], [BMIM][AICI4], [EMIK[FeC14], [BM1M][FeC14].
[35] In some implementations, the Lewis acidic ionic liquid is
[EMIM][AICI4].
[36] In some implementations, the Lewis acidic ionic liquid comprises a
metal ion-
modified ionic liquid.
[37] In some implementations, the metal ion-modified ionic liquid is
selected from the
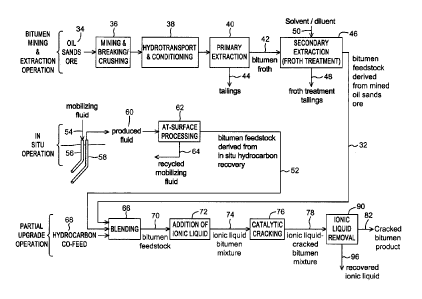
group consisting of [Et3NH][AIC14]-Ni2+,
[EMIM][AIC14]-Ni2+, [BM IM1[AICI4]-Ni2+,
[Et3NH][AIC141-Fe2+, [EMIM][AICI4]-Fe2+, [BM IMHAICI4j-Fe2+ and combinations
thereof.
CA 3074850 2020-03-05
,
[38] In some implementations, the acidic ionic liquid catalyst is a
Bronsted acidic ionic
liquid catalyst.
[39] In some implementations, the heating is performed at a temperature
between
about 50 C and about 250 C.
[40] In some implementations, the concentration of the acidic ionic liquid
catalyst in
the ionic liquid-bitumen mixture is between about 5 wt% and about 50 wt%.
[41] In accordance with another aspect, there is provided a process for
upgrading a
bitumen feedstock. The process comprises contacting the bitumen feedstock with
a
feed-miscible ionic liquid to obtain an ionic liquid-bitumen mixture; and
subjecting the
ionic liquid-bitumen mixture to a non-catalytic treatment, the non-catalytic
treatment
comprising mixing the ionic liquid-bitumen mixture at a mixing temperature
below an
asphaltene aggregation temperature of the ionic liquid-bitumen mixture to
obtain a
treated ionic liquid-bitumen mixture; wherein at least one of a Total Acid
Number (TAN)
of the treated ionic liquid-bitumen mixture, a viscosity of the treated ionic
liquid-bitumen
mixture and an asphaltene content of the treated ionic liquid-bitumen mixture
is reduced
compared to the bitumen feedstock.
[42] In some implementations, the bitumen feedstock comprises diluent-depleted
bitumen stream from a distillation unit, a diluent stripping unit or a diluent
recovery unit.
[43] In some implementations, the bitumen feedstock comprises a diluent-
depleted
bitumen stream that is obtained from a bitumen froth treatment operation.
[44] In some implementations, the bitumen feedstock comprises a diluent-
depleted
bitumen stream that has not been subjected to fractionation or distillation
prior to being
contacted with the feed-miscible ionic liquid.
[45] In some implementations, the bitumen feedstock comprises a residuum
stream
from a distillation tower that has been operated to remove light hydrocarbon
components.
[46] In some implementations, the bitumen feedstock comprises a bitumen stream
that is obtained from an in situ recovery operation or from surface mining
operations.
CA 3074850 2020-03-05
6
[47] In some implementations, the process further comprises subjecting the
bitumen
feedstock to a pre-treatment prior to the contacting of the bitumen feedstock
with the
feed-miscible ionic liquid.
[48] In some implementations, the pre-treatment comprises heating the bitumen
feedstock at a pre-treatment temperature.
[49] In some implementations, the pre-treatment temperature is sufficient
to reduce
viscosity of the bitumen feedstock.
[50] In some implementations, the pre-treatment comprises adding a diluent
to the
bitumen feedstock.
[51] In some implementations, the diluent comprises at least one of a
naphthenic
solvent, an aromatic hydrocarbon, and a non-deasphalting organic solvent.
[52] In some implementations, the diluent comprises toluene.
[53] In some implementations, the bitumen feedstock comprises bitumen and a
diluent.
[54] In some implementations, the process further comprises recovering the
diluent to
obtain a recovered diluent.
[55] In some implementations, the process further comprises separating the
feed-
miscible ionic liquid from the treated ionic liquid-bitumen mixture to obtain
a recovered
feed-miscible ionic liquid and a treated bitumen product.
[56] In some implementations, recovering the diluent is performed after the
non-
catalytic treatment of the ionic liquid-bitumen mixture and before separating
the feed-
miscible ionic liquid from the treated ionic liquid-bitumen mixture.
[57] In some implementations, recovering the diluent is performed after
separating the
feed-miscible ionic liquid from the treated ionic liquid-bitumen mixture.
[58] In some implementations, recovering the diluent comprises evaporating
the
diluent from the treated ionic liquid-bitumen mixture or from the treated
bitumen product.
CA 3074850 2020-03-05
, 7
[59] In some implementations, the process further comprises contacting at
least a
portion of the recovered diluent with the bitumen feedstock.
[60] In some implementations, the recovered feed-miscible ionic liquid is
reused as
part of the feed-miscible ionic liquid that contacts the bitumen feedstock.
[61] In some implementations, the feed-miscible ionic liquid comprises a
carbamate
ionic liquid, a phosphonium ionic liquid, and combinations thereof.
[62] In some implementations, the carbamate ionic liquid comprises N, N'-
dipropylammonium, N, N'-dipropyl carbamate (DPCARB) or N, N'-dibenzylammonium,
N,
N'-dibenzyl carbamate (DBCARB), and combinations thereof.
[63] In some implementations, the phosphonium ionic liquid comprises
trihexyl-
tetradecylphosphonium dicyanamide.
[64] In some implementations, the non-catalytic treatment further comprises
heating
the ionic liquid-bitumen mixture at a heating temperature below the mixing
temperature.
[65] In some implementations, the heating temperature is between about 20 C
and
about 120 C.
[66] In some implementations, the heating is performed near atmospheric
pressure.
[67] In some implementations, the concentration of the feed-miscible ionic
liquid in the
ionic liquid-bitumen mixture is between about 5 wt% and about 50 wt%.
[68] In accordance with another aspect, there is provided a process for
upgrading a
bitumen feedstock comprising bitumen and a diluent. The process comprises
contacting
the bitumen feedstock with a feed-immiscible ionic liquid to obtain an ionic
liquid-bitumen
mixture; and subjecting the ionic liquid-bitumen mixture to a non-catalytic
treatment, the
non-catalytic treatment comprising mixing the ionic liquid-bitumen mixture to
obtain a
treated ionic liquid-bitumen mixture; wherein at least one of a Total Acid
Number (TAN)
of the treated ionic liquid-bitumen mixture and a heavy metal content of the
treated ionic
liquid-bitumen mixture is reduced compared to the bitumen feedstock.
CA 3074850 2020-03-05
, ,
8
[69] In some implementations, the bitumen feedstock comprises a residuum
stream
from a distillation tower that has been operated to remove light hydrocarbon
components.
[70] In some implementations, the bitumen feedstock comprises a bitumen stream
that is obtained from an in situ recovery operation or from surface mining
operations.
[71] In some implementations, the diluent is selected from the group
consisting of a
naphthenic diluent, toluene and a mixture thereof.
[72] In some implementations, the process further comprises subjecting the
treated
ionic liquid-bitumen mixture to a liquid-liquid separation to obtain a first
phase comprising
the bitumen and the diluent and a second phase comprising a recovered feed-
immiscible
ionic liquid.
[73] In some implementations, the process further comprises separating the
first
phase to recover the diluent as a recovered diluent, and to obtain a treated
bitumen
product.
[74] In some implementations, recovering the diluent comprises evaporating the
diluent to obtain the treated bitumen product.
[75] In some implementations, the process further comprises contacting at
least a
portion of the recovered diluent with the bitumen feedstock.
[76] In some implementations, the recovered feed-immiscible ionic liquid is
reused as
part of the feed-immiscible ionic liquid that contacts the bitumen feedstock.
[77] In some implementations, the feed-immiscible ionic liquid comprises an
amino
acid based ionic liquid, an imidazolium ionic liquid, a phosphonium ionic
liquid, a
carbamate ionic liquid, and combinations thereof.
[78] In some implementations, the amino acid ionic liquid comprises
tetraethylammonium P-alaninate, 1-ethyl-3-methylimidazolium glycinate, and
combinations thereof.
CA 3074850 2020-03-05
, ,
9
[79] In some implementations, the imidazolium ionic liquid comprises 1-
ethy1-3-
methylimidazolium ethyl sulphate, 1-butyl-3-methylimidazolium
tetrafluoroborate, 1-ethyl-
3-methylimidazolium tetrafluoroborate, and combinations thereof.
[80] In some implementations, the phosphonium ionic liquid comprises
tributyl-
methylphosphonium methyl sulphate.
[81] In some implementations, the carbamate ionic liquid comprises N, N'-
dimethylammonium, N, N'-dimethylcarbamate (DMCARB).
[82] In some implementations, the non-catalytic treatment further comprises
heating
the ionic liquid-bitumen mixture at a heating temperature.
[83] In some implementations, the heating temperature is between about 40 C
and
about 70 C.
[84] In some implementations, the concentration of the feed-immiscible
ionic liquid in
the ionic liquid-bitumen mixture is between about 5 wt% and about 50 wt%.
[85] In some implementations, the proportion of the bitumen relative to the
feed-
immiscible ionic liquid is between about 1:2 w/w and about 1:1 w/w.
[86] In some implementations, the heavy metal content comprises at least one
of a
nickel content, an iron content and a vanadium content.
[87] In accordance with another aspect, there is provided a process for
upgrading a
bitumen feedstock comprising bitumen and a diluent. The process comprises
contacting
the bitumen feedstock with an ionic liquid to obtain an ionic liquid-bitumen
mixture;
mixing the ionic liquid-bitumen mixture at a mixing temperature; and
separating the ionic
liquid-bitumen mixture to obtain a first phase comprising treated bitumen and
the diluent
from a second phase comprising a recovered ionic liquid; wherein the treated
bitumen
has a reduced heavy metal content compared to the bitumen feedstock.
[88] In some implementations, the bitumen feedstock comprises a residuum
stream
from a distillation tower that has been operated to remove light hydrocarbon
components.
CA 3074850 2020-03-05
, 10
[89] In some implementations, the bitumen feedstock comprises a bitumen stream
that is obtained from an in situ recovery operation or from surface mining
operations.
[90] In some implementations, the diluent is selected from the group
consisting of a
naphthenic diluent, toluene and a mixture thereof.
[91] In some implementations, the ionic liquid is a feed-immiscible ionic
liquid, and
wherein the first phase and the second phase are obtained by subjecting the
ionic liquid-
bitumen mixture to a liquid-liquid separation.
[92] In some implementations, the process further comprises separating the
first
phase to recover the diluent as a recovered diluent, and to obtain a treated
bitumen
product.
[93] In some implementations, recovering the diluent comprises evaporating
the
diluent to obtain the treated bitumen product.
[94] In some implementations, the process further comprises contacting at
least a
portion of the recovered diluent with the bitumen feedstock.
[95] In some implementations, the recovered ionic liquid is reused as part
of the ionic
liquid that contacts the bitumen feedstock.
[96] In some implementations, the ionic liquid comprises an amino acid
based ionic
liquid, an imidazolium ionic liquid, a phosphonium ionic liquid, a carbamate
ionic liquid,
and combinations thereof.
[97] In some implementations, the amino acid ionic liquid comprises
tetraethylammonium 13-alaninate, 1-ethy1-3-methylimidazolium glycinate, and
combinations thereof.
[98] In some implementations, the imidazolium ionic liquid comprises 1-
ethy1-3-
methylimidazolium ethyl sulphate, 1-butyl-3-methylimidazolium
tetrafluoroborate, 1-ethyl-
3-methylimidazolium tetrafluoroborate, and combinations thereof.
[99] In some implementations, the phosphonium ionic liquid comprises
tributyl-
methylphosphonium methyl sulphate.
CA 3074850 2020-03-05
11
[100] In some implementations, the carbamate ionic liquid comprises N, N'-
dimethylammonium, N, N'-dimethylcarbamate (DMCARB).
[101] In some implementations, the process further comprises heating the ionic
liquid-
bitumen mixture at a heating temperature.
[102] In some implementations, the heating temperature is between about 40 C
and
about 70 C.
[103] In some implementations, the concentration of the ionic liquid in the
ionic liquid-
bitumen mixture is between about 5 wt% and about 50 wt%.
[104] In some implementations, the proportion of the bitumen relative to the
ionic liquid
is between about 1:2 w/w and about 1:1 w/w.
[105] In some implementations, the heavy metal content comprises at least one
of a
nickel content, an iron content, and a vanadium content.
[106] In accordance with another aspect, there is provided a process for
upgrading a
bitumen feedstock comprising bitumen and a diluent. The process comprises
contacting
the bitumen feedstock with an amino acid ionic liquid to obtain an ionic
liquid-bitumen
mixture; mixing the ionic liquid-bitumen mixture at a mixing temperature; and
separating
the ionic liquid-bitumen mixture to obtain a first phase comprising treated
bitumen and
the diluent from a second phase comprising a recovered amino acid ionic
liquid; wherein
the Total Acid Number (TAN) of the treated bitumen is reduced compared to the
TAN of
the bitumen feedstock.
[107] In some implementations, the bitumen feedstock comprises a residuum
stream
from a distillation tower that has been operated to remove light hydrocarbon
components.
[108] In some implementations, the bitumen feedstock comprises a bitumen
stream
that is obtained from an in situ recovery operation or from surface mining
operations.
[109] In some implementations, the diluent is selected from the group
consisting of a
naphthenic diluent, toluene and a mixture thereof.
CA 3074850 2020-03-05
12
[110] In some implementations, the first phase and the second phase are
obtained by
subjecting the ionic liquid-bitumen mixture to a liquid-liquid separation.
[111] In some implementations, the process further comprises separating the
first
phase to recover the diluent as a recovered diluent, and to obtain a treated
bitumen
product.
[112] In some implementations, recovering the diluent comprises evaporating
the
diluent to obtain the treated bitumen product.
[113] In some implementations, the process further comprises contacting at
least a
portion of the recovered diluent with the bitumen feedstock.
[114] In some implementations, the recovered amino acid ionic liquid is reused
as part
of the amino acid ionic liquid that contacts the bitumen feedstock.
[115] In some implementations, the amino acid ionic liquid comprises
tetraethylammonium 13-alaninate, 1-ethyl-3-methylimidazolium glycinate, and
combinations thereof.
[116] In some implementations, the process further comprises heating the ionic
liquid-
bitumen mixture at a heating temperature.
[117] In some implementations, the heating temperature is between about 40 C
and
about 70 C.
[118] In some implementations, the concentration of the amino acid ionic
liquid in the
ionic liquid-bitumen mixture is between about 5 wt% and about 50 wt%.
[119] In some implementations, the proportion of the bitumen relative to the
amino acid
ionic liquid is between about 1:2 w/w and about 1:1 w/w.
[120] In accordance with another aspect, there is provided a process for
upgrading a
bitumen feedstock. The process comprises contacting a first ionic liquid
catalyst with the
bitumen feedstock to obtain a first ionic liquid-bitumen mixture; subjecting
the first ionic
liquid-bitumen mixture to a catalytic cracking treatment, the catalytic
cracking treatment
comprising heating the first ionic liquid-bitumen mixture under catalytic
cracking
CA 3074850 2020-03-05
, ,
13
conditions to obtain an ionic liquid-cracked bitumen mixture; contacting the
ionic liquid-
cracked bitumen mixture with a second ionic liquid to obtain a second ionic
liquid-
bitumen mixture; and subjecting the second ionic liquid-bitumen mixture to a
non-
catalytic treatment, the non-catalytic treatment comprising mixing the second
ionic liquid-
bitumen mixture to obtain a treated ionic liquid-bitumen mixture.
[121] In some implementations, the process further comprises separating the
first ionic
liquid catalyst from the ionic liquid-cracked bitumen mixture to obtain a
recovered first
ionic liquid catalyst.
[122] In some implementations, the recovered first ionic liquid is reused as
part of the
first ionic liquid that contacts the bitumen feedstock.
[123] In some implementations, the process further comprises separating the
second
ionic liquid from the treated ionic liquid-bitumen mixture to obtain a
recovered second
ionic liquid.
[124] In some implementations, the recovered second ionic liquid is reused as
part of
the second ionic liquid that contacts the ionic liquid-cracked bitumen
mixture.
[125] In some implementations, the process further comprises adding a diluent
to the
bitumen feedstock or to the second ionic liquid-bitumen mixture.
[126] In some implementations, the process further comprises subjecting the
bitumen
feedstock to a pre-treatment prior to the contacting of the bitumen feedstock
with the first
ionic liquid catalyst.
[127] In some implementations, the pre-treatment comprises heating the bitumen
feedstock at a pre-treatment temperature.
[128] In some implementations, the pre-treatment temperature is sufficient to
reduce
viscosity of the bitumen feedstock.
[129] In some implementations, the pre-treatment comprises adding a diluent to
the
bitumen feedstock.
CA 3074850 2020-03-05
,
14
[130] In some implementations, the diluent comprises at least one of a
naphthenic
solvent, an aromatic hydrocarbon, and a non-deasphalting organic solvent.
[131] In some implementations, the diluent comprises toluene.
[132] In some implementations, the bitumen feedstock comprises bitumen and a
diluent.
[133] In some implementations, the process further comprises recovering the
diluent
following the non-catalytic treatment to obtain a recovered diluent.
[134] In some implementations, the process further comprises contacting at
least a
portion of the recovered diluent with the bitumen feedstock or the second
ionic liquid-
bitumen mixture.
[135] In some implementations, the first ionic liquid catalyst comprises an
acidic ionic
liquid catalyst.
[136] In some implementations, the acidic ionic liquid catalyst is a Lewis
acidic ionic
liquid comprising a Lewis acidic anion and a cation selected from the group
consisting of
1,3-dialkylimidazolium cations, tetraalkylphosphonium cations,
tetraalkylammonium
cations, trialkylammonium cations and combinations thereof.
[137] In some implementations, the Lewis acidic ionic liquid catalyst
comprises a Lewis
acidic anion and a 1-alkyl-3-methylimidazolium cation.
[138] In some implementations, the 1-alkyl-3-methylimidazolium cation is
selected from
the group consisting of 1-ethyl-3-methylimidazolium (EMIM) and 1-n-buty1-3-
methylimidazolium (BMIM).
[139] In some implementations, the Lewis acidic ionic liquid catalyst
comprises a Lewis
acidic anion and a trialkylammonium cation.
[140] In some implementations, the trialkylammonium cation comprises
triethylam mon ium.
[141] In some implementations, the Lewis acidic anion is a chlorometallate
anion.
CA 3074850 2020-03-05
,
,
[142] In some implementations, the chlorometallate anion is selected from the
group
consisting of AlC14-, Al2C17-, FeCI4- and Fe2C17-.
[143] In some implementations, the Lewis acidic ionic liquid is selected from
the group
consisting of [EMIMMIC14], [BMIM][AICI4], [EMINI][FeC14], [BMIK[FeCI4].
[144] In some implementations, the Lewis acidic ionic liquid is [EMIM][AIC14].
[145] In some implementations, the Lewis acidic ionic liquid comprises a metal
ion-
modified ionic liquid.
[146] In some implementations, the metal ion-modified ionic liquid is selected
from the
group consisting of [Et3NH][AICI4]-Ni2+, [EMIM][AIC14]-Ni2+, [BMIM][AICI4J-
Ni2+,
[Et3NFI][AlC14]-Fe2+, [EMIM][AICI4]-Fe2+, [BMIM][AIC14-Fe2+ and combinations
thereof.
[147] In some implementations, the second ionic liquid is a feed-miscible
ionic liquid.
[148] In some implementations, the feed-miscible ionic liquid comprises a
carbamate
ionic liquid, a phosphonium ionic liquid, and combinations thereof.
[149] In some implementations, the carbamate ionic liquid comprises N, N'-
dipropylammonium, N, N'-dipropyl carbamate (DPCARB) or N, N'-dibenzylammonium,
N,
N'-dibenzyl carbamate (DBCARB), and combinations thereof.
[150] In some implementations, the phosphonium ionic liquid comprises trihexyl-
tetradecylphosphonium dicyanamide.
[151] In some implementations, the second ionic liquid is a feed-immiscible
ionic liquid.
[152] In some implementations, the feed-immiscible ionic liquid comprises an
amino
acid based ionic liquid, an imidazolium ionic liquid, a phosphonium ionic
liquid, a
carbamate ionic liquid, and combinations thereof.
[153] In some implementations, the amino acid ionic liquid comprises
tetraethylammonium 13-alaninate, 1-ethyl-3-methylimidazolium glycinate, and
combinations thereof.
CA 3074850 2020-03-05
, ,
16
[154] In some implementations, the imidazolium ionic liquid comprises 1-ethy1-
3-
methylimidazolium ethyl sulphate, 1-butyl-3-methylimidazolium
tetrafluoroborate, 1-ethyl-
3-methylimidazolium tetrafluoroborate, and corn binations thereof.
[155] In some implementations, the phosphonium ionic liquid comprises tributyl-
methylphosphonium methyl sulphate.
[156] In some implementations, the carbamate ionic liquid comprises N, N'-
dimethylammonium, N, N'-dimethylcarbamate (DMCARB).
[157] In some implementations, at least one of a Total Acid Number (TAN), a
viscosity,
a heavy metal content, and an asphaltene content of the treated ionic liquid-
bitumen
mixture is reduced compared to the bitumen feedstock.
[158] In some implementations, the heavy metal content comprises at least one
of a
nickel content, an iron content, and a vanadium content.
BRIEF DESCRIPTION OF THE DRAWINGS
[159] Figure 1 is a flowchart of a general flow diagram for treating a
bitumen
feedstock including a catalytic cracking treatment followed by an ionic liquid
separation,
wherein various bitumen recovery processes are shown.
[160] Figure 2 is a flowchart of a general flow diagram for treating a
bitumen
feedstock including a non-catalytic treatment followed by an ionic liquid
separation,
wherein various bitumen recovery processes are shown.
[161] Figure 3 is a flowchart of a process for treating a bitumen
feedstock,
including a catalytic cracking treatment, in accordance with an
implementation.
[162] Figure 4 is a flowchart of a process for treating a bitumen
feedstock,
including a blending/mixing step, a catalytic cracking treatment, and a
separation step to
separate a diluent and an ionic liquid from an ionic liquid-cracked bitumen
mixture, in
accordance with an implementation.
CA 3074850 2020-03-05
, ,
17
[163] Figure 5 is a flowchart of a process for treating a bitumen
feedstock,
including a blending/mixing step, a catalytic cracking treatment, and a
separation step to
separate a diluent and an ionic liquid from an ionic liquid-cracked bitumen
mixture, in
accordance with another implementation.
[164] Figure 6 is a flowchart of a process for treating a bitumen
feedstock,
including a blending/mixing step, a catalytic cracking treatment, and a
separation step to
separate a diluent and an ionic liquid from an ionic liquid-cracked bitumen
mixture, in
accordance with yet another implementation.
[165] Figure 7 is a flowchart of a process for treating a bitumen
feedstock,
including a catalytic cracking treatment, and a separation step to separate a
diluent and
an ionic liquid from an ionic liquid-cracked bitumen mixture, in accordance
with an
implementation.
[166] Figure 8 is a flowchart of a process for treating a bitumen
feedstock,
including a non-catalytic treatment, and a separation step to separate an
ionic liquid from
a treated ionic liquid-bitumen mixture, in accordance with an implementation.
[167] Figure 9 is a flowchart of a process for treating a bitumen
feedstock,
including a non-catalytic treatment, and a separation step to separate a
diluent and an
ionic liquid from a treated ionic liquid-bitumen mixture, in accordance with
an
implementation.
[168] Figure 10 is a flowchart of a process for treating a bitumen
feedstock,
including a catalytic treatment followed by a non-catalytic treatment, in
accordance with
an implementation.
DETAILED DESCRIPTION
[169] Techniques described herein relate to the treatment of a bitumen
feedstock, and
can also be referred to as "upgrading" or "partial upgrading". The upgrading,
or partial
upgrading, of the bitumen feedstock can include subjecting the bitumen
feedstock to a
catalytic cracking treatment in the presence of an ionic liquid or subjecting
the bitumen
feedstock to a non-catalytic treatment in the presence of an ionic liquid at a
given
CA 3074850 2020-03-05
18
temperature, each performed as a standalone step. The upgrading, or partial
upgrading,
of the bitumen feedstock can also include a combination of the catalytic
cracking
treatment in presence of a first ionic liquid and the non-catalytic treatment
in presence of
a second ionic liquid performed according to a given sequence. The upgrading
techniques described herein can facilitate viscosity and/or density reduction
of the
bitumen feedstock, and improve its chemical composition for instance by
reducing
asphaltene content and/or removing impurities such as sulphur and heavy
metals. The
viscosity and/or density reduction can, in turn, help reduce or eliminate
diluent
requirements for the bitumen product to be pipelinable. The upgrading
techniques can
also facilitate avoiding the need for the addition of an external source of
hydrogen (i.e.,
hydroprocessing steps) in order to produce a higher quality bitumen product,
the higher
quality bitumen product having for instance a reduced sulphur content, a
reduced metal
content, a reduced total acid number, and/or a reduced viscosity compared to
an
untreated bitumen feedstock. It should be understood that as used herein, the
expression "a method/process/system for upgrading a bitumen feedstock" may
refer to
an upgrading of the bitumen feedstock (e.g., a treatment of the bitumen
feedstock that
makes the bitumen feedstock pipelinable) or to a partial upgrading of the
bitumen
feedstock (e.g., a treatment of the bitumen feedstock that takes the bitumen
feedstock
closer to being pipelinable). In some implementations, the bitumen feedstock
can be
subjected to a pre-treatment step prior to the addition of the ionic liquid
for the
subsequent catalytic cracking treatment or non-catalytic treatment. For
instance, the pre-
treatment can include the addition of a diluent and/or a heating step. The pre-
treatment
can contribute to achieve desired properties of the bitumen feedstock, for
instance with
regard to its viscosity, to facilitate the subsequent interaction with the
ionic liquid.
Bitumen feedstock and viscosity characteristics and overview of general
process
[170]
As mentioned above, techniques are described herein to facilitate the
reduction in viscosity of bitumen feedstocks and/or improve its chemical
composition of
the bitumen feedstocks. The term "bitumen" as used herein refers to
hydrocarbon
material extracted from bituminous formations, such as oil sands formations,
the density
of which is typically around 1000 kg/m3, and the viscosity of which is
typically between
about 1 million cP to about 100 million cP when measured at 20 C. The bitumen
feedstock can include bitumen that was extracted from oil sands ore using a
surface
CA 3074850 2020-03-05
19
mining process, or using an in situ recovery process (e.g., a thermal energy-
based
recovery method such as steam assisted gravity drainage (SAGD) or cyclic steam
stimulation (CSS), a solvent-based recovery method such as in situ solvent or
solvent-
steam extraction, an in situ combustion recovery method, a cold production
process, an
electromagnetic energy assisted process, or a concurrent or sequential
combination
thereof). The bitumen included in the bitumen feedstock can also come from any
other
suitable source, such as bitumen obtained from a non-aqueous extraction
process or
bitumen obtained from a paraffinic froth treatment.
[171] The bitumen feedstock refers to the bitumen material that is subjected
to the
upgrading techniques in presence of an ionic liquid. In some implementations,
the
bitumen feedstock includes both heavy and light hydrocarbon fractions and has
very low
or substantially zero water content and mineral solids content (e.g., below
2.5% water, or
below 0.5% water and below 1.0% solids). The bitumen feedstock can include
various
non-hydrocarbon compounds (e.g., sulfur, heavy metals, etc.) that are often
found in
bitumen and may be associated with certain fractions or solubility classes of
bitumen,
such as asphaltenes.
[172] It should also be noted that the bitumen feedstock can in some cases be
a blend
of different hydrocarbon streams, e.g., one or more heavy hydrocarbon streams
can be
blended with one or more light hydrocarbon streams to form a blended bitumen
feedstock that has desired properties to be subjected to upgrading techniques
in the
presence of an ionic liquid as described herein.
[173] Referring to Figures 1 and 2, the bitumen feedstock can include bitumen
32
extracted using surface mining operations. In such operations, the oil sands
ore 34 is
extracted through mining, followed by breaking down and crushing of the ore
36, which
produces a looser material that can be mixed with warm or hot water to obtain
a slurry
preparation suitable for hydrotransport 38. At this stage, the slurry can also
be subjected
to various forms of conditioning to improve its properties. The hydrotransport
38 provides
a pipeline connection between mining operations 36 and primary extraction
operations
40. The primary extraction 40 is performed to separate the hydrotransported
slurry into
bitumen froth 42 and tailings 44. The bitumen froth 42 is then subjected to
secondary
extraction 46, or froth treatment, to separate the bitumen 32 from froth
treatment tailings
CA 3074850 2020-03-05
, 20
48 using a solvent or diluent 50, thereby producing a bitumen feedstock 70.
Optionally,
the bitumen feedstock 32 can be further processed in a diluent recovery unit
that has
received the diluted bitumen feedstock from the secondary extraction 46 to
recover the
solvent or diluent 50. The bitumen feedstock 70 can thus also be a diluent-
depleted
bitumen produced by a diluent recovery unit that recovers paraffinic solvent
from a
solvent diluted bitumen overflow stream that is part of a paraffinic froth
treatment
operation.
[174] Still referring to Figuresl and 2, the bitumen feedstock can also
include bitumen
52 extracted using in situ recovery operations. In situ recovery operations
comprise
injecting a pre-heated mobilizing fluid 54 via an injection well 56 overlying
a production
well 58. A produced fluid 60 is extracted from the production well 58 and
subjected to at-
surface processing 62 to separate a stream of recycled mobilizing fluid 64
from a
bitumen feedstock 70 suitable for the upgrading techniques described herein.
In
implementations where the mobilizing fluid 64 comprises a solvent or a
diluent, the
bitumen feedstock 70 can optionally be further processed in a diluent recovery
unit that
has received the bitumen feedstock from an in situ recovery facility to
recover the solvent
or diluent.
[175] It is to be understood that as used herein, the expression "bitumen
feedstock"
can thus refer to either one of a diluted bitumen feedstock, i.e., a bitumen
feedstock that
still includes a solvent or a diluent, or to a bitumen feedstock from which
solvent or
diluent has been removed therefrom, in both cases when the bitumen feedstock
has
been obtained from surface mining operations or when the bitumen feedstock is
obtained from in situ recovery operations.
[176] In some implementations, the bitumen feedstock is a diluent-depleted
bitumen
stream that has not been subjected to certain conventional separation steps,
such as
fractionation or distillation, prior to the upgrading, and therefore still has
many if not
substantially all of the heavy and light hydrocarbon components of the native
bitumen. In
other implementations, the bitumen feedstock can be a residuum stream from a
distillation tower (e.g., vacuum distillation tower) that has been operated to
remove light
hydrocarbon components (e.g., gas oils and other hydrocarbons that boil below
about
525 C).
CA 3074850 2020-03-05
21
[177] In some implementations, the bitumen feedstock subjected to upgrading in
the
presence of an ionic liquid can include bitumen extracted from various
sources, and can
be combined in a blending step 66 prior to being subjected to the upgrading. A
hydrocarbon co-feed 68 can also be added to the blending step 66.
[178] Referring to Figure 1, an ionic liquid is then added 72 to the bitumen
feedstock 70
to obtain an ionic liquid-bitumen mixture 74, which is then subjected to a
catalytic
cracking treatment 76 under conditions to produce an ionic liquid-cracked
bitumen
mixture 78. Further details regarding the expression "catalytic cracking
treatment" as
used herein and the conditions at which the catalytic cracking treatment is
performed are
provided below. The ionic liquid-cracked bitumen mixture 78 can optionally be
separated
80 to recover at least a portion of the ionic liquid as a recovered ionic
liquid 96. A
cracked bitumen product 82 is then obtained. In some implementations, the
cracked
bitumen product 82 can be a partially upgraded product that can be subjected
to further
upgrading treatment(s) if deemed necessary. In other implementations, the
cracked
bitumen product 82 can be sufficiently upgraded such that pipeline
specifications are
met, or further processed if needed.
[179] Referring to Figure 2, an ionic liquid is added 72 to the bitumen
feedstock 70 to
obtain an ionic liquid-bitumen mixture 74, which is then subjected to a non-
catalytic
treatment 84 to produce a treated ionic liquid-bitumen mixture 86. The treated
ionic
liquid-bitumen mixture 86 can optionally be separated 88 to recover at least a
portion of
the ionic liquid as a recovered ionic liquid. A bitumen product 90 is then
obtained.
Similarly to what is mentioned above regarding the cracked bitumen product 82,
the
bitumen product 90 can be a partially upgraded product that can be subjected
to further
upgrading treatment(s) if deemed necessary, or the bitumen product 90 can be
sufficiently upgraded such that pipeline specifications are met, for instance.
[180] In some implementations, the bitumen feedstock 70 can be subjected to a
pre-
treatment prior to the addition of the ionic liquid to the bitumen feedstock
70 for the
subsequent catalytic cracking treatment 76 or non-catalytic treatment 84. The
pre-
treatment can include various steps depending on the results that are desired
to be
achieved, and depending on the characteristics of the bitumen feedstock 70.
The pre-
treatment can include adding a diluent, or additive agent, to the bitumen
feedstock. The
CA 3074850 2020-03-05
= ,
22
addition of the diluent to the bitumen feedstock 70 can contribute to dilute
the bitumen
feedstock 70 and reduce its viscosity. In some scenarios, the reduced
viscosity of the
bitumen feedstock 70 can facilitate the blending of the mixture of bitumen
feedstock/diluent with the ionic liquid. Examples of suitable diluents can
include for
instance aromatic hydrocarbons, non-deasphalting organic solvents, and the
like. In
some scenarios, the diluent can be for instance toluene or xylene.
Furthermore, in some
implementations, the pre-treatment can include a heating step, whether or not
a diluent
has been added to the bitumen feedstock 70 during the pre-treatment step, and
if a
diluent has been added, prior to or after the addition of the diluent. The
heating step can
be performed prior to or during the addition of the ionic liquid to the
bitumen feedstock
70. In some implementations, the heating step can be performed at a
temperature
sufficient to decrease the viscosity of the bitumen feedstock 70, which can
facilitate the
subsequent blending of the bitumen feedstock 70 with the ionic liquid. In some
implementations and as will be discussed in further detail below, the addition
of the
diluent, or additive agent, can also be performed simultaneously with the
addition of the
ionic liquid, in a blending/mixing step. It is to be noted that while Figures
4-6 and 9
illustrate a mixing/blending step where the bitumen feedstock 70 is
blended/mixed with
the ionic liquid and optionally a diluent, the addition of the diluent can be
done prior to
the addition of the ionic liquid, i.e., not necessarily simultaneously.
[181] The upgrading techniques in presence of an ionic liquid, including the
catalytic
cracking treatment 76 and the non-catalytic treatment 84, will now be
described in further
detail.
Catalytic cracking using ionic liquids for upgrading bitumen
[182] Conventional upgrading techniques can include cracking treatments such
as
thermal cracking and catalytic cracking to convert heavy hydrocarbon
fractions, such as
bitumen, to lighter fractions that are considered more valuable, such as
gasoline and
distillate, by breaking the chemical bonds of long-chain hydrocarbons into
smaller-chain
hydrocarbons. These conventional upgrading techniques generally involve
treating
heavy hydrocarbons at high temperature and/or high pressure, and can lead to
the
formation of coke as an undesirable by-product. In the case of catalytic
cracking, coke
can in turn deactivate the catalyst when depositing thereon, and so can heavy
metals,
CA 3074850 2020-03-05
,
23
nitrogen and sulfur. In the context of the present description and in contrast
with
conventional cracking techniques, the expression "catalytic cracking
treatment" in the
presence of an ionic liquid and the conditions at which the catalytic cracking
treatment is
performed (which can also be referred to as "catalytic cracking conditions" in
the context
of the present description) refer to a treatment operated at a temperature
and/or a
pressure that is lower compared to the temperature and pressure conditions at
which are
conducted conventional cracking processes, as will be described in more detail
below.
[183] With reference to Figure 3, an implementation of a bitumen feedstock 70
that is
subjected to a catalytic cracking treatment 76 in presence of an ionic liquid
used as a
catalyst is shown. In some implementations, the conditions at which the
catalytic
cracking treatment is performed can include operating the catalytic cracking
treatment 76
at temperatures between about 50 C and about 250 C. In some scenarios and
without
being limiting, the conditions at which the catalytic cracking treatment is
performed can
include operating the catalytic cracking treatment 76 near atmospheric
pressure.
[184] In some implementations, the ionic liquid catalyst is a Lewis acidic
ionic liquid
catalyst and comprises a complex anion (e.g., AlC14- formed from complexation
between
Lewis acid A1C13 and anion CI-) and a cation (e.g., an imidazolium cation or
an
ammonium cation). Lewis acids have the ability to act as an electron pair
acceptor.
Lewis acids are formed by reacting a metal halide with an organic halide salt,
at various
molar ratios. Several factors can contribute to make Lewis acidic ionic
liquids
advantageous for catalytic application. In some implementations, the ionic
liquid catalyst
is a Bronsted acidic ionic liquid catalyst. Bronsted acidic ionic liquids can
catalyse
reactions due to a labile proton which is present in their structure (in the
cation or in the
anion). For example, Bronsted acidic ionic liquids can include acidic
functional groups
such as SO3H or COOH, attached to the cation, or can include an acidic
hydrogen on a
nitrogen or oxygen atom in the cation. Bronsted acidic ionic liquids can be
prepared by
proton transfer between a Bronsted acid and a Bronsted base. The most common
Bronsted acidic ionic liquids include a protonated imidazolium cation, a
protonated
pyridinium cation, a guanidinium cation, a protonated ammonium cation, a COOH-
bearing imidazolium cation or a SO3H-bearing imidazolium cation. For instance,
their
acidity can be modulated to provide the opportunity to design materials that
are tailor
made for certain applications, their ability to dissolve a wide range of
materials can
CA 3074850 2020-03-05
24
eliminate the need to add solvent, and they can act as both catalyst and
solvent at the
same time. In what follows and unless otherwise specified, the term "acidic
ionic liquid
catalyst" is meant to refer to a Bronsted acidic ionic liquid catalyst, a
Lewis acidic ionic
liquid catalyst, or a combination thereof.
Examples of Bronsted acidic ionic liquid cations:
R.._
NH+
N+
\_/
R R N R2 NR2
N+ SO3H
N+HR
[185] In some implementations, the cation can include for instance 1,3-
dialkylimidazolium cations, tetraalkylphosphonium cations, tetraalkylammonium
cations,
trialkylammonium cations, and combinations thereof. In some implementations,
the
Lewis acidic ionic liquid catalyst comprises a complex anion formed from
complexation
between a Lewis acid and Lewis base anion, and a 1-alkyl-3-methylimidazolium
cation.
In some scenarios, the 1-alkyl-3-methylimidazolium cation can be for instance
1-ethy1-3-
methylimidazolium (EMIM) or 1-n-butyl-3-methylimidazolium (BMIM). When the
Lewis
acidic ionic liquid catalyst comprises a complex anion and a trialkylammonium
cation, the
trialkylammonium cation can be for instance triethylammonium. In some
implementations, the complex anion is a chlorometallate anion, and can be for
instance
AlC14-, Al2C17-, FeC14- and Fe2CI7-. In some implementations, the Lewis acidic
ionic liquid
catalyst can be for instance [EMIM][AICI4], [BMIM][AIC14], [EMIM][FeCI4],
[BMIM][FeCI4].
In yet other implementations, Lewis acidic ionic liquid catalyst can include a
metal ion-
modified ionic liquid, which can be for instance [Et3NH][AICI4]-Ni2+,
[EMIM][AIC14]-Ni2+,
[BMIM][AIC14]-Ni2+, [Et3NH][AIC14]-Fe2+, [EMIM][AICI4]-Fe2+, [BM IM][AIC14]-
Fe2+, and
combinations thereof. It should be understood that as used herein, the term
"complex
anion" can be referred to as a "Lewis acidic anion".
CA 3074850 2020-03-05
25
[186] In some implementations, the concentration of the acidic ionic liquid
catalyst in
the ionic liquid-bitumen mixture can be between about 5 wt% and about 50 wt%.
In
other implementations, the concentration of the acidic ionic liquid catalyst
in the
bitumen/diluent mixture 210 can be below 5 wt%, or above 50 wt%. The
concentration of
the acidic ionic liquid catalyst in the ionic liquid-bitumen mixture can
depend for instance
of the characteristics of the bitumen feedstock, and/or the characteristics of
the acidic
ionic liquid catalyst.
[187]
The catalytic cracking treatment 76 can be performed in any suitable vessel,
or reactor, that can be operated at conditions as described herein and that
enables the
obtention of an ionic liquid-cracked bitumen mixture 78. In some
implementations, the
vessel can be a vessel designed such that the ionic liquid-cracked bitumen
mixture 78
can be retrieved as an overflow stream and an undesirable products stream such
as
coke can be retrieved as an underflow stream. In other implementations, the
ionic liquid-
cracked bitumen mixture 78 can be retrieved from the vessel as a single stream
that
includes all of the hydrocarbon components but that has improved physical
and/or
chemical properties. As mentioned above, the conditions enabling the catalytic
cracking
of the ionic liquid-bitumen mixture 74 generally include a temperature and a
pressure at
which the catalytic cracking treatment 76 is conducted, for a given duration.
The
conditions at which the catalytic cracking treatment is performed can vary
depending on
the characteristics of the bitumen feedstock 70. For instance, for a bitumen
feedstock 70
having a high proportion of heavy hydrocarbon, the severity of the catalytic
cracking can
be increased, while for a bitumen feedstock having a low proportion of heavy
hydrocarbon, the severity of the catalytic cracking can be decreased.
"Severity" as used
herein refers to the severity of the conditions of temperature and residence
time at which
the bitumen feedstock is treated. The severity can be expressed in terms of an
equivalent reaction time (ERT) in seconds of residence time when a reactor is
operating
at 427 C (800 F). The ERT corresponds to the residence time that would achieve
the
same conversion of heavy material at a given temperature as if the reaction
was
conducted at 427 C (800 F).
[188] Still referring to Figure 3, optionally, the ionic liquid-cracked
bitumen mixture 78
can be subjected to a separation step 92 to separate the acidic ionic liquid
catalyst 98
and/or to separate diluent 94 that was initially part of the bitumen
feedstock, if
CA 3074850 2020-03-05
26
applicable, from the ionic liquid-cracked bitumen mixture 78. A cracked
bitumen product
90 is then obtained. The cracked bitumen product 90 can be subjected to
separation to
be separated into various streams according to their boiling points, for
instance in a
fractionator or distillation column.
[189] Referring to Figures 4 to 6, a bitumen feedstock 70 and an acidic ionic
liquid
catalyst are blended or mixed 100 together to obtain an ionic liquid-bitumen
mixture 74.
Optionally, a diluent 102 can also be added to the ionic liquid-bitumen
mixture 74 during
the mixing step 100, for instance to improve characteristic(s) of the ionic
liquid-bitumen
mixture 74 or to facilitate the obtention of an at least partially homogenized
ionic liquid-
bitumen mixture 74 by further reducing its viscosity. As mentioned above, in
some
scenarios, the bitumen feedstock 70 can already include a diluent, such that
it may not
be advantageous to add additional diluent during the mixing step 100. In other
scenarios, even if bitumen feedstock 70 already includes a diluent, there may
still
advantages to add additional diluent during the mixing step 100, for instance
to obtain
given characteristics of the ionic liquid-bitumen mixture 74. Examples of
suitable diluents
that can be used include natural gas condensates, hexane or cyclohexane,
naphthenic
diluent, or any other suitable diluent.
[190] Still with reference to Figures 4 to 6, the ionic liquid-bitumen mixture
74 is then
subjected to the catalytic cracking treatment 76 to produce the ionic liquid-
cracked
bitumen mixture 78. The ionic liquid-cracked bitumen mixture 78 is then
subjected to one
or two separation steps, to recover at least a portion of diluent 102 if
present, and at
least a portion of the acidic ionic liquid catalyst 72. The ionic liquid-
cracked bitumen
mixture 78 can be subjected to a single separation step 104 configured to
separate the
diluent 102 and the acidic ionic liquid catalyst 72 from the ionic liquid-
cracked bitumen
mixture 78 in a single step, as shown in Figure 4. In some implementations,
the acidic
ionic liquid catalyst 72 and the diluent 102 can be separated from the cracked
bitumen
product 90 as a single stream, and optionally separated from each other
thereafter.
When the ionic liquid-cracked bitumen mixture 78 is subjected to two
separation steps
106,108, the two separation steps 106,108 can be performed in one sequence or
the
other. In some implementations, the separation step 104, 106, 108 can be
performed for
instance in a gravity settler, a diluent recovery unit, or a solvent recovery
unit. Figure 5
illustrates an implementation where the ionic liquid-cracked bitumen mixture
78 is
CA 3074850 2020-03-05
, ,
27
subjected to a first separation step 106 to recover a recovered diluent 94 and
produce a
diluent-depleted cracked bitumen mixture 110, which is then subjected to a
second
separation step 108 to recover a recovered acidic ionic liquid catalyst 98 and
produce
the cracked bitumen product 90. Figure 6 illustrates another implementation
where the
ionic liquid-cracked bitumen mixture 78 is subjected to a first separation
step 108 to
recover a recovered acidic ionic liquid catalyst 98 and produce an ionic
liquid-depleted
cracked bitumen mixture 112 which is then subjected to a second separation
step 106 to
recover a recovered diluent 94 and produce the cracked bitumen product 90.
[191] The separation step 106 is configured to recover the diluent 102 from
the ionic
liquid-cracked bitumen mixture 78 or from the ionic liquid-depleted cracked
bitumen
mixture 112. In some implementations, the separation step 106 can include
evaporating
the diluent 102 from the liquid-cracked bitumen mixture 78 or from the ionic
liquid-
depleted cracked bitumen mixture 112 to obtain the recovered diluent 94. At
least a
portion of the recovered diluent 94 can be recycled to be used as the diluent
102 that
can be optionally added to the bitumen feedstock 70 and the acidic ionic
liquid catalyst
72 for the mixing step 100.
[192] The separation step 108 is configured to recover the acidic ionic liquid
catalyst 98
from the ionic liquid-cracked bitumen mixture 78 or the diluent-depleted
cracked bitumen
mixture 110. In some implementations, the separation step 108 includes a
liquid-liquid
extraction of the ionic liquid-cracked bitumen mixture 78 or the diluent-
depleted cracked
bitumen mixture 110. In implementations where the acidic ionic liquid catalyst
is water
soluble, the liquid-liquid extraction of the ionic liquid-cracked bitumen
mixture 78 or the
diluent-depleted cracked bitumen mixture 110 can include washing the ionic
liquid-
cracked bitumen mixture 78 or the diluent-depleted cracked bitumen mixture 110
with
water. At least a portion of the recovered acidic ionic liquid catalyst 98 can
be recycled to
be reused as the acidic ionic liquid catalyst 72 to be combined with the
bitumen
feedstock 70.
[193] Referring now to Figure 7, an embodiment of the implementations
described
above is presented. In the embodiment shown, a bitumen feedstock 200 is
blended/mixed 208 with a diluent 206 to obtain a bitumen/diluent mixture 210.
The
diluent 206 can be chosen for instance to arrive at certain characteristics of
the
CA 3074850 2020-03-05
, ,
28
bitumen/diluent mixture 210, for example in terms of viscosity. The diluent
206 can
include for instance natural gas condensates, hexane or cyclohexane,
naphthenic
diluent, or any other suitable diluent. In some implementations, the
blending/mixing step
208 can include heating the bitumen feedstock 200 for a given period of time.
The
bitumen/diluent mixture 210 is then subjected to catalytic cracking 212 in
presence of an
acidic ionic liquid catalyst 214, the bitumen/diluent mixture 210 and the
acidic ionic liquid
catalyst 214 forming an ionic liquid-bitumen mixture. In some implementations,
the
concentration of the acidic ionic liquid catalyst in the ionic liquid-bitumen
mixture can be
between about 5 wt% and about 50 wt%. In other implementations, the
concentration of
the acidic ionic liquid catalyst in the ionic liquid-bitumen mixture can be
below 5 wt%, or
above 50 wt%. The catalytic cracking 212 is performed under conditions that
can include
for instance heating the ionic liquid-bitumen mixture at temperatures between
50 C and
250 C. In some scenarios and without being !imitative, the catalytic cracking
can be
performed at pressures near normal atmospheric pressure. It is to be
understood that
other conditions can be implemented for the catalytic cracking treatment, and
can be
determined or adjusted depending on the bitumen/diluent mixture 210 physical
and
chemical properties, among other factors. Following the catalytic cracking
212, a cracked
reaction mixture 216 is subjected to diluent removal 218 to recover at least a
portion of
the diluent as recovered diluent 220 and obtain a diluent-depleted cracked
bitumen
mixture 222. The diluent removal 218 step can be done by evaporation of the
diluent
206. In some implementations, a system including a diluent recovery unit, a
solvent
recovery unit or a flash drum can be used to recover the diluent 206.
Optionally, the
recovered diluent 220 can be recycled to be reused to blend/mix with the
bitumen
feedstock 200. The diluent-depleted cracked bitumen mixture 222 is then
subjected to
another separation step 224 to separate at least a portion of the acidic ionic
liquid
catalyst 214 from the diluent-depleted cracked bitumen mixture 222 as a
recovered
acidic ionic liquid catalyst 226 and to produce a cracked bitumen product 228.
In some
scenarios, when the acidic ionic liquid catalyst 214 is water soluble, the
second
separation step 224 can be performed as a water washing step. Although Figure
7
shows the diluent separation step 218 performed prior to the acidic ionic
liquid catalyst
separation step 224, it is to be understood that the order of these steps can
be
interchanged such that the acidic ionic liquid catalyst separation step 224
can be
performed prior to the diluent separation step 218. In some implementations
and as
CA 3074850 2020-03-05
29
mentioned above, the sequence of the separation steps 218, 224 can be
determined
according to the characteristics of the diluent 206 and the acidic ionic
liquid catalyst 214
and the corresponding separation techniques that have to be put in place to
recover
each of them.
Non-catalytic treatment using ionic liquids for upgrading bitumen
[194] As mentioned above, reducing the viscosity of a bitumen feedstock can be
advantageous to improve its pipelinability. There can also be advantages in
reducing the
total acid number (TAN) of the bitumen feedstock, which can in turn contribute
to reduce
corrosive properties of the bitumen feedstock. It can be also advantageous to
remove
certain contaminants such as sulphur and heavy metals, and/or to decrease the
asphaltene content of the bitumen feedstock. The techniques described below
are aimed
at improving such characteristics of the bitumen feedstock by subjecting the
bitumen
feedstock to a non-catalytic treatment in presence of an ionic liquid.
[195] With reference to Figure 8, in an implementation of a non-catalytic
treatment, a
bitumen feedstock 300 is combined with an ionic liquid 304 in a
blending/mixing step 306
to form an ionic liquid-bitumen mixture 308. The bitumen feedstock 300 can be
of
various sources and have various characteristics as previously described
above. The
bitumen feedstock 300 can optionally include a diluent. If the bitumen
feedstock 300
incudes a diluent, the diluent can be separated from the bitumen feedstock 300
prior to
the non-catalytic treatment, or the bitumen feedstock 300 can remain as a
mixture of
bitumen and diluent. In some implementations, the ionic liquid 304 can be
added to the
bitumen feedstock 300 to reach a concentration of the ionic liquid 304 ranging
from
about 5 wt% to about 50 wt%. In other implementations, the concentration of
the acidic
ionic liquid catalyst in the ionic liquid-bitumen mixture can be below 5 wt%,
or above 50
wt%.
[196] The ionic liquid-bitumen mixture 308 is then subjected to a non-
catalytic
treatment 310 in a suitable vessel. In the context of the present description,
the non-
catalytic treatment 310 is performed under non-catalytic cracking conditions,
and could
thus be considered as a mild thermal treatment that is performed at low
severity
conditions. In some implementations, the non-catalytic cracking conditions
include
CA 3074850 2020-03-05
30
performing the non-catalytic treatment 310 at a temperature at which
asphaltene
aggregation within the ionic liquid-bitumen mixture 308 is avoided. For
instance, in some
embodiments, the temperature at which is conducted the non-catalytic treatment
310
can be between about 20 C and about 120 C. In some implementations, the
temperature at which is conducted the non-catalytic treatment 310 can be a
temperature
that enables proper mixing of the bitumen feedstock 300 with the ionic liquid
304 during
the blending/mixing step 306, i.e., a temperature at which the respective
viscosity of the
bitumen feedstock 300 and the ionic liquid 304 is low enough to allow
sufficient contact
between the bitumen feedstock 300 and the ionic liquid 304. In some
implementations,
the non-catalytic treatment 310 can be performed at room temperature. The
temperature
at which the non-catalytic treatment 310 is conducted and the duration of the
non-
catalytic treatment 310 can depend on the endpoint that is desired to be
achieved, on
the initial viscosity of the bitumen feedstock 300 or of the ionic liquid 304,
and/or on the
properties of the ionic liquid used, for example.
[197] In the implementation shown in Figure 8, the ionic liquid 304 that is
combined
with the bitumen feedstock 300 for the non-catalytic treatment 310 includes
ionic liquids
that are miscible in bitumen or a mixture of bitumen and diluent, L e., that
are feed-
miscible. Examples of ionic liquids that are miscible in a mixture of bitumen
and toluene
can include for instance some carbamate ionic liquids such as N, N'-
dipropylammonium,
N, N'-dipropyl carbamate (DPCARB) and N, N'-dibenzylammonium, N, N'-dibenzyl
carbamate (DBCARB), phosphonium ionic liquids such as Trihexyl-
tetradecylphosphonium dicyanamide (Cyphos IL 105), among others. In some
implementations, the ionic liquid 304 used for the non-catalytic treatment 310
can be
chosen according to its likelihood to not have cracking functionality,
although the solvent
properties of anionic liquid used for catalytic cracking may still favor its
use under non-
catalytic conditions.
[198] Following the non-catalytic treatment 310, a treated ionic liquid-
bitumen mixture
312 is obtained, which then may optionally be subjected to a separation step
314 to
separate the ionic liquid 304 from the treated ionic liquid-bitumen mixture
312 and
produce a recovered ionic liquid 316 and a treated bitumen product 318. When
no
separation step is performed, the treated ionic liquid-bitumen mixture 312 can
be
considered to correspond to the treated bitumen product 318. In some
implementations,
CA 3074850 2020-03-05
31
such a non-catalytic treatment 310 of the ionic liquid-bitumen mixture 306 can
contribute
to improve physical properties of the bitumen feedstock 300 such as viscosity
reduction
and asphaltene content reduction, as well as TAN reduction. In some
implementations,
the alkalinity of the ionic liquid 304 can be correlated with the TAN
reduction that is
achieved: the stronger the alkalinity of the ionic liquid 304, the greater the
TAN reduction
in the treated ionic liquid-bitumen mixture 312 or the treated bitumen product
90. In
some implementations, subjecting the ionic liquid-bitumen mixture 308 to the
non-
catalytic treatment 310 as described herein can facilitate achieving viscosity
reduction
ranging from 40% to 90% and/or TAN reduction ranging from 15% to 55%, when
ionic
liquids such as DPCARB, DBCARB and Cyphos IL 105 are used.
[199] Referring now to Figure 9, in another implementation of a non-catalytic
treatment,
a bitumen feedstock 300, a diluent 302 and an ionic liquid 304 are combined in
a
blending/mixing step 306 to produce an ionic liquid-bitumen mixture 308. The
diluent 302
can be for instance a naphthenic diluent. In implementations where the bitumen
feedstock 300 already includes a diluent, the diluent 302 addition can be
omitted.
[200] In the implementation shown in Figure 9, the ionic liquid 304 that is
combined
with the bitumen feedstock 300 for the non-catalytic treatment 311 include
ionic liquids
that are immiscible in a mixture of bitumen and toluene, e., that are feed-
immiscible.
Ionic liquids that are immiscible in a bitumen and toluene mixture can include
for
instance amino acid based ionic liquids such as tetraethylammonium 6-alaninate
and 1-
ethy1-3-methylimidazolium glycinate, imidazolium ionic liquids such as 1-ethy1-
3-
methylimidazolium ethyl sulphate and 1-butyl-3-methylimidazolium
tetrafluoroborate,
phosphonium ionic liquids such as Tributyl-methylphosphonium methyl sulphate,
and
carbamate ionic liquids such as N, N'-dimethylammonium, N, N'-
dimethylcarbamate
(DMCARB).
[201] In some implementations, the ionic liquid 304 can be added to the
bitumen
feedstock 300 to reach a concentration of the ionic liquid 304 ranging from
about 5 wt%
to about 50 wt%. In some implementations, the proportion of the bitumen
relative to the
feed-immiscible ionic liquid is between about 1:2 w/w and about 1:1 w/w.
CA 3074850 2020-03-05
32
[202] The ionic liquid-bitumen mixture 309 is then subjected to the non-
catalytic
treatment 311 to produce a treated ionic liquid-bitumen mixture 312. The non-
catalytic
treatment 311 shown in Figure 9 can include heating the ionic liquid-bitumen
mixture
309, for instance at temperatures ranging from about 40 C to 70 C, which can
facilitate
efficient mixing of the three components of the ionic liquid-bitumen mixture
309. The
non-catalytic treatment 311 can also include mixing, which can facilitate the
transfer of at
least a portion of contaminants contained in the bitumen feedstock 300 from
the bitumen
feedstock 300 to the mixture of diluent 302 and ionic liquid 304. In some
implementations, the non-catalytic treatment 311 can include heating the ionic
liquid-
bitumen mixture 308 at a first temperature for a first duration as part of a
first thermal
treatment, and then heating the ionic liquid-bitumen mixture 309 at a second
temperature for a second duration as part of a second thermal treatment and so
on, to
ensure that proper mixing and contact between the bitumen feedstock 300 and
the ionic
liquid 304 can be achieved.
[203] The treated ionic liquid-bitumen mixture 312 is then subjected to a
liquid-liquid
extraction 320, during which a first phase 324 that includes the bitumen
feedstock 300
and the diluent 302 (or the bitumen feedstock 300 if the bitumen feedstock 300
already
includes a diluent) is separated from a second phase that includes the ionic
liquid 304.
The liquid-liquid extraction 320 can facilitate at least partial removal of
undesirable
components from the bitumen feedstock 300, such as sulphur, naphthenic acids
and
heavy metals, by enabling transfer of the undesirable components to the ionic
liquid 304.
The liquid-liquid extraction 320 can be performed in any suitable vessel,
which can be for
instance a liquid-liquid separation unit or a decanter. The first phase that
includes the
bitumen and the diluent can be withdrawn as a bitumen-diluent mixture stream
324. In
some implementations, the bitumen-diluent mixture stream 324 can be subjected
to
additional separation step(s) 326 to separate the diluent from the bitumen and
obtain a
recovered diluent 330, which can optionally be recycled for reuse in the
blending/mixing
step 306, and a treated bitumen product 328. In other implementations, it can
also be
advantageous to keep the diluent and the bitumen combined together, for
instance such
that the viscosity of the diluent-bitumen mixture is within a given range. The
second
phase that includes the ionic liquid 304 can be withdrawn as a recovered ionic
liquid
stream 322, which can optionally be recycled for reuse in the blending/mixing
step 306.
CA 3074850 2020-03-05
33
The non-catalytic treatment 311 thus produces the bitumen-diluent mixture
stream 324,
or the treated bitumen product 328, if diluent has been separated therefrom.
[204] As mentioned above, different ionic liquids that are immiscible in a
mixture of
bitumen and toluene can be used, or ionic liquids that are feed-immiscible,
the choice of
which can depend for instance of the characteristic(s) of the bitumen
feedstock that is
desired to be improved. For instance, subjecting the ionic liquid-bitumen
mixture 309 to
the non-catalytic treatment 311 as described herein in the presence of amino
acid based
ionic liquids can facilitate TAN reduction, which in some implementations, can
be up to
100%. Similarly to the non-catalytic treatment 310 in presence of an ionic
liquid that is
miscible in a mixture of bitumen and toluene, the alkalinity of the ionic
liquid 304 that is
immiscible in a mixture of bitumen and toluene can be correlated with the TAN
reduction
achieved when the non-catalytic treatment 311 is performed. In some
implementations,
the concentration of metals such as nickel and vanadium can also be reduced
when the
ionic liquid-bitumen mixture 309 is subjected to the non-catalytic treatment
311. For
instance, the reduction of nickel can be up to about 60%, and the reduction in
vanadium
can be up to 30%, depending on the ionic liquid used.
Catalytic cracking treatment and non-catalytic treatment in sequence
[205] The techniques described herein to upgrade a bitumen feedstock in
presence of
an ionic liquid can also include a combination of a catalytic cracking
treatment and a
non-catalytic treatment performed sequentially to take advantage of the
respective
improvements on the bitumen properties that can be achieved by performing each
of
these two treatments. The choice of the ionic liquid used for the catalytic
cracking
treatment and the non-catalytic treatment and the sequence of the treatments,
L e., which
ones of the catalytic cracking treatment and a non-catalytic treatment is
performed first,
can depend for instance on the characteristics of the bitumen feedstock, on
the desired
characteristics of the resulting partially upgraded bitumen product and/or on
the overall
process configuration and design.
[206] Referring to Figure 10, an implementation of an upgrading technique that
includes a catalytic cracking treatment of a mixture of bitumen and an ionic
liquid
followed by a non-catalytic treatment of the resulting cracked mixture of
bitumen and an
CA 3074850 2020-03-05
,
34
ionic liquid is shown. In this implementation, a bitumen feedstock 400 and a
first ionic
liquid 402 are combined in a first blending/mixing step 406 to produce a first
ionic liquid-
bitumen mixture 408. In certain embodiments, the first ionic liquid 402
comprises an
acidic ionic liquid catalyst as described above. The first ionic liquid-
bitumen mixture 408
is then subjected to a catalytic cracking treatment 410 under conditions as
detailed
above, to convert heavy hydrocarbons to lighter hydrocarbons. The catalytic
cracking
treatment 410 produces an ionic liquid-cracked bitumen mixture 412. In some
implementations, the ionic liquid-cracked bitumen mixture 412 can be separated
to
remove the diluent, if present in the bitumen feedstock 400, and/or the ionic
liquid 402,
to obtain a cracked bitumen product 416. The separation step 414 can thus be
configured to separate a recovered ionic liquid 418 and optionally a recovered
diluent
420. Various separation methods can be implemented to separate the first ionic
liquid
402 from the ionic liquid-cracked bitumen mixture 412, depending on the
properties of
the first ionic liquid 402. For instance, in some implementations, the first
ionic liquid 402
can be miscible in water and thus can be removed by a water washing step. Each
one of
the recovered ionic liquid 418 and the recovered diluent 420 can be recycled
to be
reused in other parts of the process.
[207] Following the separation step 414, the cracked bitumen product 416 is
then
combined with a second ionic liquid 422 in a second blending/mixing step 424.
The
blending/mixing step 424 can also include the addition of a diluent, for
instance if the
bitumen feedstock 400 does not initially include such diluent and depending on
the
choice of the second ionic liquid 422 and the choice of non-catalytic
treatment that
follows. For instance, when the second ionic liquid 422 is an ionic liquid
that is miscible
in a mixture of bitumen and toluene as described above, the presence of a
diluent can
be optional. When the second ionic liquid 422 is an ionic liquid that is
immiscible in a
mixture of bitumen and toluene as described above, it can be advantageous to
add a
diluent if the bitumen feedstock 400 does not initially include such diluent.
[208] Following the second blending/mixing step 424, a second ionic liquid-
bitumen
mixture 426 is obtained. The second ionic liquid-bitumen mixture 426 is
subjected to a
non-catalytic treatment 428 to obtain a treated ionic liquid-bitumen mixture
430. The
configuration of the non-catalytic treatment 428 can be chosen according to
the
CA 3074850 2020-03-05
, 35
endpoint(s) that are desired to be achieved, which in turn can contribute to
determine the
type of ionic liquid that is used as the second ionic liquid 422.
[209] In some implementations, when a reduction in viscosity or a reduction of
TAN in
the cracked bitumen product 416 is desired, the second ionic liquid 422 can be
chosen
to be an ionic liquid that is miscible in a mixture of bitumen and diluent,
and the non-
catalytic treatment 428 generally includes mixing the second ionic liquid-
bitumen mixture
426 at a given temperature for a given duration. Examples of suitable second
ionic
liquids 422 in such implementations include carbamate ionic liquids such as
DPCARB,
DBCARB, and phosphonium ionic liquids such as Cyphos IL 105. In some
implementations, the temperature at which is performed the non-catalytic
treatment 428
can range for instance from room temperature to about 100 C. In some
implementations, the duration of the non-catalytic treatment 428 can be for
instance from
24 hours to 96 hours. It is to be understood that the temperature and the
duration of the
non-catalytic treatment 428 can vary according to numerous factors including
the
characteristics of the cracked bitumen product 416 and the second ionic liquid
422
chosen. Optionally, the second ionic liquid 422 can be separated 432 from the
treated
ionic liquid-bitumen mixture 430 to obtain a treated bitumen product 434.
[210] In other implementations, when a reduction in contaminants such as
sulphur and
heavy metals, a TAN reduction, and/or a decrease the asphaltene content of the
cracked
bitumen product 416 is desired, the second ionic liquid 422 can be chosen to
be an ionic
liquid that is immiscible in a mixture of bitumen and diluent. Examples of
suitable second
ionic liquids 422 in such implementations include amino acid based ionic
liquids,
phosphonium ionic liquids and carbamate ionic liquids such as DMCARB. In these
implementations, the non-catalytic treatment 428 can include mixing the second
ionic
liquid-bitumen mixture 426 at a given temperature for a given duration, and
can be
followed by a separation 432 that can be a liquid-liquid separation. As
mentioned above,
the mixing of the second ionic liquid-bitumen mixture 426 can facilitate the
transfer of at
least a portion of contaminants from the bitumen to the mixture of diluent and
the second
ionic liquid 422. The liquid-liquid extraction can then be performed to
separate a first
phase that includes bitumen and diluent of the second ionic liquid-bitumen
mixture 426
from a second phase that includes the second ionic liquid 422. The first phase
can be
considered to correspond to the treated bitumen product 434, or if the diluent
can then
CA 3074850 2020-03-05
, ,
36
be separated from the first phase, the resulting bitumen can correspond to the
treated
bitumen product 434.
[211] It should to be understood that although Figure 10 illustrates an
implementation
that includes a catalytic cracking treatment followed by a non-catalytic
treatment, in other
implementations, the process for upgrading a bitumen feedstock can be in the
reverse
sequence and include the non-catalytic treatment followed by the catalytic
cracking
treatment.
CA 3074850 2020-03-05