Note: Descriptions are shown in the official language in which they were submitted.
CA 03074989 2020-03-05
DESCRIPTION
NITROGEN-CONTAINING HETEROARYL COMPOUND AND PHARMACEUTICAL USE
THEREOF
Technical Field
[0001]
The present invention relates to a nitrogen-containing
heteroaryl compound having a GLUT9 inhibitory activity, or a
pharmaceutically acceptable salt thereof, a pharmaceutical
lo composition containing the same, and a pharmaceutical use
thereof.
Background Art
[0002]
Uric acid is a poorly-soluble substance having a
/5 molecular weight of 168 and a dissociation constant (pKa value)
of 5.75 and is present in the form of uric acid or a conjugate
base (urate) thereof, depending on the pH, when it is in the
body fluid. In human and many other primates, due to the
functional absence of urate oxidase (uricase) in the liver,
20 uric acid is the final metabolite in purine metabolism. About
70% of the uric acid in the body resulted from dietary intake
or endogenous production is eliminated through urine via the
kidney, and the remaining 30% is eliminated through stools via
the intestinal tube.
25 [0003]
GLUT9 belongs to the family of glucose transporters
encoded by SLC2A9 (Solute carrier family 2, facilitated glucose
transporter member 9) genes, which was cloned as a molecule
expressed in kidney, liver, placenta and the like in human
30 (Non-Patent Document 1). According to a series of subsequent
reports, the genome-wide association analysis confirmed the
correlation between mutations in this molecule and blood uric
acid levels, and this molecule functioned as a high-affinity,
high-capacity uric acid transporter (Non-Patent Documents 2 and
35 3). It has also been reported that GLUT9 has two isoforms
1
CA 03074989 2020-03-05
>
(GLUT9S and GLUT9L) due to N-terminal intracellular portion
splice, and both has the same uric acid transport activity
(Non-Patent Documents 4 and 5). Moreover, it has become
evident that depressed function of this molecule causes severe
hypouricemia from the analysis of GLUT9 genetic variation
family (Non-Patent Documents 6 and 7).
Uric acid transport kinetics in the kidney have been
studied since early times. Thus, uric acid first passes
through glomerular, and then undergoes two-way transport via
m transporter which is either reabsorption or secretion, and
eventually about 90% of the amount of the uric acid which has
passed through glomerular is reabsorbed. GLUT9 is, from the
information mentioned above, considered to play the main role
in the uric acid reabsorption in the kidney, and thus an
important molecule that controls blood uric acid levels.
Therefore, a GLUT9 inhibitor is expected to reduce blood uric
acid levels and be effective for hyperuricemia and pathological
conditions associated therewith.
[0004]
In Japan, hyperuricemia is defined as a condition wherein
serum uric acid level exceeds 7.0 mg/dL, based on the
concentration of uric acid dissolved in blood (Non-Patent
Document 8). Persistent hyperuricemia causes gouty arthritis
or kidney damage (gouty kidney) resulted from deposition of
urate crystals in tissues. A prolonged disease period in gouty
arthritis cases results in granuloma formation such as gouty
tophus which is primarily due to the urate.
[0005]
Further, in the recent years, hyperuricemia has been
recognized as a lifestyle disease, and there has been many
reports suggesting that hyperuricemia is associated with
various pathological conditions. Remedy of hyperuricemia can
be a potential treatment and prophylaxis for those pathological
condition. Pathological conditions listed below are generally
known to be associated with hyperuricemia and are particularly
2
CA 03074989 2020-03-05
w
suggested to have a relationship with high uric acid.
[0006]
1) Chronic kidney disease (CKD)
Many epidemiological studies have shown that
hyperuricemia is a risk factor for development of terminal
kidney failure or CKD onset (Non-Patent Documents 9, 10 and 11),
and there is a report of intervention trial involving use of
uric acid lowering agents which observed a renoprotective
effect (Non-Patent Document 12). It is also reported that gene
polymorphism of GLUT9 is responsible for CKD onset (Non-Patent
Document 13).
[0007]
2) Hypertension
Many clinical studies have gradually convinced that
/5 hyperuricemia is closely associated with the onset of
hypertension (Non-Patent Documents 14 and 15). Also, there are
clinical results reporting that blood pressure has been
decreased by treating hyperuricemia (Non-Patent Document 16).
[0008]
3) Diabetes
In a meta-analysis which puts together multiple
prospective clinical studies, hyperuricemia is reported as an
independent risk factor for type 2 diabetes (Non-Patent
Document 17). Also studies involving use of mice and cultured
cells show that high concentration uric acid suppresses insulin
secretion and induces insulin resistance (Non-Patent Documents
18 and 19).
[0009]
4) Cardiac disease (cardiovascular disease, cardiac failure,
atrial fibrillation)
The blood uric acid level has been reported as an
independent risk factor or cardiovascular events (Non-Patent
Document 20). Besides the events, a study of correlations
between the characteristics of coronary artery and uric acid
using intravascular ultrasound (IVUS) shows that hyperuricemia
3
CA 03074989 2020-03-05
is associated with plaque volume and calcified lesion (Non-
Patent Document 21). Hyperuricemia is also observed in many
patients with chronic cardiac failure. A Japanese
epidemiological study conducted under the Japanese Cardiac
Registry of Heart Failure in Cardiology (J-CARE-CARD) test
demonstrated that cardiac failure patients with hyperuricemia
had a significantly higher rate of all-cause death and cardiac
death (Non-Patent Document 22). In the recent years, a
complication of hyperuricemia and atrial fibrillation has been
/o attracting attention. It has been reported that prevalence of
atrial fibrillation increases according to the serum uric acid
level, and prevalence of hyperuricemia with 8 mg/dL or greater
is significantly higher than those of 6.9 mg/dL or less (Non-
Patent Document 23).
/5 [0010]
5) Arteriosclerotic disease
Frequency of hyperuricemia in patients with
hypertriglyceridemia is as high as about 30%, which is reported
to be closely related with hyperuricemia and hyperlipemia (Non-
a) Patent Documents 24 and 25).
[0011]
6) Non-alcoholic fatty liver disease (NAFLD) and non-alcoholic
steatohepatitis (NASH)
NAFLD is associated with fatty liver and is a chronic
25 hepatic disease of unknown cause which is commonly diagnosed in
people who do not drink alcohol. Further, a pathological
condition with a more progressed inflammation and fibrosis is
called "NASH" which may cause hepatic cirrhosis and hepatoma.
Many NAFLD patients have a complication with hyperuricemia,
30 where the serum uric acid level is an independent risk factor
for NAFLD. There is a meta-analysis result reporting that 1
mg/dL increase in the serum uric acid level increases the risk
of NAFLD onset by 21% (Non-Patent Document 26).
[0012]
35 7) Psoriasis
4
CA 03074989 2020-03-05
It has been long known that psoriasis patients generally
have a higher uric acid level relative to healthy subjects, as
seen in the report that hyperuricemia was observed in about
half of psoriasis patients (Non-Patent Document 27). In
addition, as it has been reported that risks of cardiovascular
disease and kidney damage are significantly higher in psoriasis
patients relative to healthy subjects, hyperuricemia may be a
factor that plays a role in increasing these risks (Non-Patent
Documents 28 and 29).
[0013]
As described above, a GLUT9 inhibitor is considered to be
an agent for the treatment or prophylaxis of pathological
conditions that involve high blood uric acid levels;
specifically, hyperuricemia, gout (for example, gouty arthritis,
gouty kidney, and gouty tophus) and the like. Further, it is
considered to have potential to be useful as an agent for the
treatment or prophylaxis of pathological conditions which are
generally known to have a complication with hyperuricemia and
are particularly suggested to have association with high uric
acid; specifically, chronic kidney disease (CKD), hypertension,
diabetes, cardiac disease (for example, cardiovascular disease,
cardiac failure, and atrial fibrillation), arteriosclerotic
disease, non-alcoholic fatty liver disease (NAFLD), non-
alcoholic steatohepatitis (NASH), psoriasis and the like.
Document List
Non-Patent Document
[0014]
[Non-Patent Document 1] Genomics. 2000 Jun 1; 66(2):217-20.
[Non-Patent Document 2] PLoS Genet. 2007 Nov; 3(11):e194.
[Non-Patent Document 3] Nat Genet. 2008 Apr; 40(4):437-42.
[Non-Patent Document 4] J Biol Chem. 2008 Oct 3; 283(40):26834-
8.
[Non-Patent Document 5] ADMET & DMPK. 2017; 5(2):59-74.
[Non-Patent Document 6] Am J Hum Genet. 2008 Dec; 83(6):744-51.
[Non-Patent Document 7] Nephrol Dial Transplant. 2012 Mar;
5
CA 03074989 2020-03-05
27(3):1035-41.
[Non-Patent Document 8] Japanese guideline for the management
of hyperuricemia and gout; second edition, edited by the
Guideline Revision Committee, Japanese Society of Gout and
Nucleic Acid Metabolism, Osaka, Medical Review Co., Ltd., 2010
[Non-Patent Document 9] Am J Kidney Dis. 2004 Oct; 44(4):642-50.
[Non-Patent Document 10] J Am Soc Nephrol. 2008 Dec;
19(12):2407-13.
[Non-Patent Document 11] PLoS One. 2014 Jun 24; 9(6):e100801.
/o [Non-Patent Document 12] Am J Kidney Dis. 2015 Dec; 66(6):945-
50.
[Non-Patent Document 13] Clin J Am Soc Nephrol. 2014 Jun 6;
9(6):1059-65.
[Non-Patent Document 14] Hypertension. 2006 Dec; 48(6):1031-6.
[Non-Patent Document 15] Ann Rheum Dis. 2013 Aug; 72(8):1321-7.
[Non-Patent Document 16] JAMA. 2008 Aug 27; 300(8):924-32.
[Non-Patent Document 17] PLoS One. 2013; 8(2):e56864.
[Non-Patent Document 18] Mol Cell Endocrinol. 2013 Aug 15;
375(1-2):89-96.
[Non-Patent Document 19] Biochem Biophys Res Commun. 2014 May
16; 447(4):707-14.
[Non-Patent Document 20] National Health and Nutrition
Examination Survey. JAMA. 2000 May 10; 283(18):2404-10.
[Non-Patent Document 21] Coron Artery Dis. 2014 Jun; 25(4):343-
8.
[Non-Patent Document 22] Int J Cardiol. 2011 Sep 1; 151(2):143-
7.
[Non-Patent Document 23] Hypertens Res. 2014 Aug; 37(8):785-9.
[Non-Patent Document 24] Br J Rheumatol. 1994 Aug; 33(8):731-4.
[Non-Patent Document 25] Ther Res 33:1397-1405, 2012
[Non-Patent Document 26] J Clin Endocrinol Metab. 2015 Nov;
100(11):4198-207.
[Non-Patent Document 27] Am J Dermatopathol. 1981 Winter;
3(4):397-404.
[Non-Patent Document 28] Arch Dermatol. 2008 Nov; 144(11):1518-
6
CA 03074989 2020-03-05
,
9.
[Non-Patent Document 29] BMJ. 2013 Oct 15; 347:f5961.
Summary of the Invention
[0015]
The present invention provides a nitrogen-containing
heteroaryl compound having a GLUT9 inhibitory activity, or a
pharmaceutically acceptable salt thereof, a pharmaceutical
composition containing the same, a pharmaceutical use thereof,
and the like. Accordingly, the present invention encompasses
/o the embodiments exemplified below.
[0016]
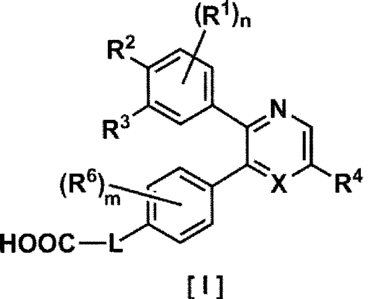
[Item 1]
A compound of Formula [I], or a pharmaceutically
acceptable salt thereof:
[0017]
In
R2
NiR-
(R6)-...._
m --;....., X R4
I
/
HOOC-L
I I l
[0018]
wherein
=X- is =C(R5)- or =N-;
-L-COOH is
(1) -COOH,
(2) -C(R71) (R72)-000H,
(3) -C(R73)(1274)-C(R75)(Rm)-COOH, or
(4) -0-0(R77) (R78)-000H;
n is 0, 1, or 2;
m is 0, 1, 2, or 3;
R1 is each independently halogen or C1-3 alkyl;
7
CA 03074989 2020-03-05
, R2 is
(1) halogen,
(2) hydroxy,
(3) cyano,
(4) C1-6 alkyl optionally substituted with 1 to 3 substituents
independently selected from the group consisting of cyano and
C1-3 alkoxy,
(5) halo C1-6 alkyl,
(6) C1-6 alkoxy,
/o (7) halo C1-6 alkoxy,
(8) -000R21 wherein R21 is hydrogen or 01-3 alkyl,
(9) -CON(R22) (R23) wherein R22 and R23 are each independently
hydrogen or 01-3 alkyl,
(10) C3-6 cycloalkyl or
/5 (11) a 4- to 6-membered saturated heterocyclic group containing
1 or 2 hetero atom as a ring atom in addition to the carbon
atoms, wherein the hetero atom is independently selected from
the group consisting of oxygen, nitrogen and sulfur atoms, and
R3 is
20 (1) hydrogen,
(2) halogen,
(3) cyano,
(4) C1-3 alkyl,
(5) halo 01-3 alkyl,
25 (6) C1-3 alkoxy, or
(7) -00OR31 wherein R31 is hydrogen or 01-3 alkyl or
R2 and R3, together with the carbon atoms that they are
bonded to, form a 4- to 6-membered saturated heterocycle
containing 1 or 2 hetero atom as a ring atom in addition to the
30 carbon atoms, wherein the hetero atom is independently selected
from the group consisting of oxygen, nitrogen and sulfur atoms;
R4 is
(1) 01-8 alkyl optionally substituted with 1 to 3 substituents
independently selected from the following Group A,
35 (2) halo 01-6 alkyl,
8
CA 03074989 2020-03-05
e
(3) -CON(R41 )(R42' ) wherein R41- and R42 are each independently
hydrogen or 01-6 alkyl,
(4) C3-6 cycloalkyl optionally substituted with 1 to 3
substituents independently selected from 01-3 alkoxy, or
(5) a 4- to 6-membered saturated heterocyclic group containing
1 or 2 hetero atom as a ring atom in addition to the carbon
atoms, wherein the hetero atom is independently selected from
the group consisting of oxygen, nitrogen and sulfur atoms, and
wherein the ring atom in the heterocyclic group bonded to
/o [0019]
/ N
I )
\x.s a carbon atom,
[0020]
and
Group A consists of
(a) hydroxy,
(b) 01-3 alkoxy optionally substituted with one hydroxy or one
01-3 alkoxy,
(c) halo 01-3 alkoxy,
(d) 03-6 cycloalkyl optionally substituted with one hydroxy, and
(e) phenyl, and
R5 is hydrogen, halogen or 01-3 alkyl or
R4 and R5, together with the carbon atoms that they are
bonded to, form 03-6 cycloalkane;
R6 are each independently halogen, hydroxy, 01-3 alkyl or
01-3 alkoxy; and
R71, R72, Rm, R74, Rm, Rm, R77, and Rm are each
independently hydrogen or 01-3 alkyl.
[0021]
[Item 2]
The compound according to Item 1 or a pharmaceutically
acceptable salt thereof, wherein =X- is =0(R5)-.
[0022]
9
CA 03074989 2020-03-05
, [Item 3]
The compound according to Item 1 or a pharmaceutically
acceptable salt thereof, wherein =X- is =N-.
[0023]
[Item 4]
The compound according to any one of Items 1 to 3 or a
pharmaceutically acceptable salt thereof, wherein -L-COOH is -
COOH.
[0024]
io [Item 5]
The compound according to any one of Items 1 to 4 or a
pharmaceutically acceptable salt thereof, wherein n is 0 or 1.
[0025]
[Item 6]
The compound according to any one of Items 1 to 4 or a
pharmaceutically acceptable salt thereof, wherein n is 0.
[0026]
[Item 7]
The compound according to any one of Items 1 to 4 or a
pharmaceutically acceptable salt thereof, wherein n is 1.
[0027]
[Item 8]
The compound according to any one of Items 1 to 7 or a
pharmaceutically acceptable salt thereof, wherein m is 0 or 1.
[0028]
[Item 9]
The compound according to any one of Items 1 to 7 or a
pharmaceutically acceptable salt thereof, wherein m is 0.
[0029]
[Item 10]
The compound according to any one of Items 1 to 7 or a
pharmaceutically acceptable salt thereof, wherein m is 1.
[0030]
[Item 11]
The compound according to any one of Items 1 to 10 or a
CA 03074989 2020-03-05
, .
pharmaceutically acceptable salt thereof, wherein R1 is halogen.
[0031]
[Item 12]
The compound according to any one of Items 1 to 10 or a
pharmaceutically acceptable salt thereof, wherein Rl is C1-3
alkyl.
[0032]
[Item 13]
The compound according to any one of Items 1 to 12 or a
pharmaceutically acceptable salt thereof, wherein R3 is
(1) hydrogen, or
(2) halogen.
[0033]
[Item 14]
The compound according to any one of Items 1 to 12 or a
pharmaceutically acceptable salt thereof, wherein R3 is
hydrogen.
[0034]
[Item 15]
The compound according to any one of Items 1 to 12 or a
pharmaceutically acceptable salt thereof, wherein R3 is halogen.
[0035]
[Item 16]
The compound according to any one of Items 1 to 15 or a
pharmaceutically acceptable salt thereof, wherein
R4 is
(1) C1-8 alkyl optionally substituted with 1 to 3 substituents
independently selected from the following Group A,
(2) halo C1-6 alkyl, or
(3) -CON(R41) (R42) wherein R41 and R42 are each independently
hydrogen or C1-6 alkyl, and
Group A consists of
(a) hydroxy,
(b) C1-3 alkoxy optionally substituted with one hydroxy or one
C1-3 alkoxY,
11
CA 03074989 2020-03-05
. ,
(c) halo 01-3 alkoxy,
(d) C3-6 cycloalkyl optionally substituted with one hydroxy, and
(e) phenyl.
[0036]
[Item 17]
The compound according to any one of Items 1 to 15 or a
pharmaceutically acceptable salt thereof, wherein
R4 is 01-8 alkyl optionally substituted with 1 to 3 substituents
independently selected from the following Group A, and
io Group A consists of
(a) hydroxy,
(b) 01-3 alkoxy optionally substituted with one hydroxy or one
01-3 alkoxy,
(c) halo 01-3 alkoxy,
(d) 03-6 cycloalkyl, and
(e) phenyl.
[0037]
[Item 18]
The compound according to any one of Items 1 to 15 or a
pharmaceutically acceptable salt thereof, wherein R4 is -
CON(R41) (R42) wherein R41 and R42 are each independently hydrogen
or 01-6 alkyl.
[0038]
[Item 19]
A compound of the following formula or a pharmaceutically
acceptable salt thereof:
[0039]
12
CA 03074989 2020-03-05
. =
F
H3C'0 I.
N
HO
F
CH3
* N).
C) (Example 21).
[0040]
[Item 20]
A compound of the following formula or a pharmaceutically
acceptable salt thereof:
[0041]
F
#.0
H3C
1411 N
1
F
I
HO (1101 N 0'CH3
C) (Example 35).
[0042]
[Item 21]
/o A compound of the following formula or a pharmaceutically
acceptable salt thereof:
[0043]
F
H3C0' 0
N
I
HO F(1101 4/ 0
0 (Example 67).
13
CA 03074989 2020-03-05
= =
[0044]
[Item 22]
A compound of the following formula or a pharmaceutically
acceptable salt thereof:
[0045]
H3C/0 =
HO 1101 0 NCH3
0
(free form of Example 76).
[0046]
[Item 23]
A compound of the following formula or a pharmaceutically
m acceptable salt thereof:
[0047]
H30
C
=
0
`CH3
HO
C) (Example 84).
[0048]
[Item 24]
A compound of the following formula or a pharmaceutically
acceptable salt thereof:
[0049]
14
CA 03074989 2020-03-05
0
H3C =
CH3
HO 1:101 CH3
0 (Example 98).
[0050]
[Item 25]
A compound of the following formula or a pharmaceutically
acceptable salt thereof:
[0051]
H3C0
=,
0
H3
HO
0 (Example 107).
[0052]
[Item 26]
A compound of the following formula or a pharmaceutically
acceptable salt thereof:
[0053]
,õ0
1411
HO 11101 141-()CH3
0 CH3 (Example 109).
CA 03074989 2020-03-05
, [0054]
[Item 27]
A compound of the following formula or a pharmaceutically
acceptable salt thereof:
[0055]
H3CC)
N
i N*
I
0
`CH3
HO
0 CH3
(Example 116).
[0056]
[Item 28]
A compound of the following formula or a pharmaceutically
io acceptable salt thereof:
[0057]
.,
r-13%.,,C)
Ill N
1 N*.
I
HO 1110 / (3 C H3
C) (Example 118).
[0058]
[Item 29]
A compound of the following formula or a pharmaceutically
acceptable salt thereof:
[0059]
16
CA 03074989 2020-03-05
=
=
H3C0'
N
1 =
I
/ .
0 CH3
HO
0 CH3
(Example 126).
[0060]
[Item 30]
A pharmaceutical composition comprising the compound
according to any one of Items 1 to 29 or a pharmaceutically
acceptable salt thereof, and a pharmaceutically acceptable
carrier.
[0061]
[Item 31]
A GLUT9 inhibitor comprising the compound according to
any one of Items 1 to 29 or a pharmaceutically acceptable salt
thereof.
[0062]
[Item 32]
An agent for the treatment or prophylaxis of a disease
selected from the group consisting of hyperuricemia and gout,
which comprises the compound according to any one of Items 1 to
29 or a pharmaceutically acceptable salt thereof.
[0063]
[Item 33]
A method for the inhibition of GLUT9 in a mammal in need
of such inhibition, which comprises administering a
pharmaceutically effective amount of the compound according to
any one of Items 1 to 29 or a pharmaceutically acceptable salt
thereof to the mammal.
[0064]
[Item 34]
A method for the treatment or prophylaxis of a disease
17
CA 03074989 2020-03-05
,
selected from the group consisting of hyperuricemia and gout in
a mammal in need of such treatment or prophylaxis, which
comprises administering a pharmaceutically effective amount of
the compound according to any one of Items 1 to 29 or a
pharmaceutically acceptable salt thereof to the mammal.
[0065]
[Item 35]
Use of the compound according to any one of Items 1 to 29
or a pharmaceutically acceptable salt thereof for the
/o manufacture of a GLUT9 inhibitor.
[0066]
[Item 36]
Use of the compound according to any one of Items 1 to 29
or a pharmaceutically acceptable salt thereof for the
manufacture of an agent for the treatment or prophylaxis of a
disease selected from the group consisting of hyperuricemia and
gout.
[0067]
[Item 37]
The compound according to any one of Items 1 to 29 or a
pharmaceutically acceptable salt thereof, for use in the
inhibition of GLUT9.
[0068]
[Item 38]
The compound according to any one of Items 1 to 29 or a
pharmaceutically acceptable salt thereof, for use in the
treatment or prophylaxis of a disease selected from the group
consisting of hyperuricemia and gout.
[0069]
[Item 39]
A commercial package comprising the composition according
to Item 30 and a written matter associated therewith, the
written matter stating that the composition can or should be
used for the treatment or prophylaxis of a disease selected
from the group consisting of hyperuricemia and gout.
18
CA 03074989 2020-03-05
[0070]
[Item 40]
A kit comprising the composition according to Item 30 and
a written matter associated therewith, the written matter
stating that the composition can or should be used for the
treatment or prophylaxis of a disease selected from the group
consisting of hyperuricemia and gout.
Embodiments of the Invention
[0071]
lo The definitions of the terms used herein are as follows.
[0072]
The following wavy line:
[0073]
i
/5 [0074]
in the partial structure means an abbreviation of a bonding
partner.
[0075]
Examples of the "halogen" include fluorine, chlorine,
20 bromine and iodine.
[0076]
The "01-3 alkyl" means a straight- or branched-chain
saturated hydrocarbon group having 1 to 3 carbon atoms.
Examples of the "C1-3 alkyl" include methyl, ethyl, n-propyl
25 and isopropyl.
[0077]
The "01-6 alkyl" means a straight- or branched-chain
saturated hydrocarbon group having 1 to 6 carbon atoms.
Examples of the "01-6 alkyl" include methyl, ethyl, n-propyl,
30 isopropyl, n-butyl, isobutyl, sec-butyl, tert-butyl, n-pentyl,
isopentyl and n-hexyl.
[0078]
The "C1-8 alkyl" means a straight- or branched-chain
19
CA 03074989 2020-03-05
saturated hydrocarbon group having 1 to 8 carbon atoms.
Examples of the "C1-8 alkyl" include methyl, ethyl, n-propyl,
isopropyl, n-butyl, isobutyl, sec-butyl, tert-butyl, n-pentyl,
isopentyl, n-hexyl, n-heptyl and n-octyl.
[0079]
The "halo C1-3 alkyl" means the above-mentioned "C1-3
alkyl" substituted with 1 to 5 halogen independently selected
from the group consisting of the above-mentioned "halogen".
Examples of the "halo C1-3 alkyl" include monofluoromethyl,
/o difluoromethyl, trifluoromethyl, 2-fluoroethyl, 2-chloroethyl,
2-bromoethyl, 1,1-difluoroethyl, 2,2,2-trifluoroethyl,
pentafluoroethyl, 3-fluoropropyl, 3-chloropropyl, 1,1-
difluoropropyl and 3,3,3-trifluoropropyl.
[0080]
The "halo C1-6 alkyl" means the above-mentioned "CI-6
alkyl" substituted with 1 to 5 halogen independently selected
from the group consisting of the above-mentioned "halogen".
Examples of the "halo CI-6 alkyl" include monofluoromethyl,
difluoromethyl, trifluoromethyl, 2-fluoroethyl, 2-chloroethyl,
2-bromoethyl, 1,1-difluoroethyl, 2,2,2-trifluoroethyl,
pentafluoroethyl, 3-fluoropropyl, 3-chloropropyl, 1,1-
difluoropropyl, 3,3,3-trifluoropropyl, 4,4,4-trifluorobutyl,
5,5,5-trifluoropentyl and 6,6,6-trifluorohexyl.
[0081]
The "C1-3 alkoxy" means a group wherein the above-
mentioned "C1-3 alkyl" is bonded to an oxygen atom. Examples
of the "C1-3 alkoxy" include methoxy, ethoxy, n-propoxy and
isopropoxy.
[0082]
The "CI-6 alkoxy" means a group wherein the above-
mentioned "CI-6 alkyl" is bonded to an oxygen atom. Examples
of the "CI-6 alkoxy" include methoxy, ethoxy, n-propoxy,
isopropoxy, n-butoxy, isobutoxy, sec-butoxy, tert-butoxy, n-
pentyloxy, isopentyloxy and n-hexyloxy.
[0083]
CA 03074989 2020-03-05
J
The "halo 01-3 alkoxy" means the above-mentioned "01-3
alkoxy" substituted with 1 to 5 halogen independently selected
from the group consisting of the above-mentioned "halogen".
Examples of the "halo 01-3 alkoxy" include monofluoromethoxy,
difluoromethoxy, trifluoromethoxy, 2-fluoroethoxy, 2-
chloroethoxy, 2-bromoethoxy, 1,1-difluoroethoxy, 2,2,2-
trifluoroethoxy, pentafluoroethoxy, 3-fluoropropoxy, 3-
chloropropoxy, 1,1-difluoropropoxy and 3,3,3-trifluoropropoxy.
[0084]
/o The "halo 0I-6 alkoxy" means the above-mentioned "0I-6
alkoxy" substituted with 1 to 5 halogen independently selected
from the group consisting of the above-mentioned "halogen".
Examples of the "halo 0I-6 alkoxy" include monofluoromethoxy,
difluoromethoxy, trifluoromethoxy, 2-fluoroethoxy, 2-
/5 chloroethoxy, 2-bromoethoxy, 1,1-difluoroethoxy, 2,2,2-
trifluoroethoxy, pentafluoroethoxy, 3-fluoropropoxy, 3-
chloropropoxy, 1,1-difluoropropoxy, 3,3,3-trifluoropropoxy,
4,4,4-trifluorobutoxy, 5,5,5-trifluoropentyloxy and 6,6,6-
trifluorohexyloxy.
20 [0085]
The "03-6 cycloalkyl" means a monocyclic saturated
hydrocarbon group having 3 to 6 carbon atoms. Examples of the
"03-6 cycloalkyl" include cyclopropyl, cyclobutyl, cyclopentyl
and cyclohexyl.
25 [0086]
The "03-6 cycloalkane" means a monocyclic saturated
hydrocarbon having 3 to 6 carbon atoms. Examples of the "03-6
cycloalkane" include cyclopropane, cyclobutane, cyclopentane
and cyclohexane. Examples of the "03-6 cycloalkane" formed by
30 R4 and R5, together with the carbon atoms that they are bonded
to, include the following rings:
[0087]
21
CA 03074989 2020-03-05
[0088]
Examples of the "4- to 6-membered saturated heterocyclic
group containing 1 or 2 hetero atom as a ring atom in addition
to the carbon atoms, wherein the hetero atom is independently
selected from the group consisting of oxygen, nitrogen and
sulfur atoms" include oxetanyl, tetrahydrofuranyl,
tetrahydropyranyl, azetidinyl, pyrrolidinyl and piperidyl.
[0089]
/o For example, examples of the "4- to 6-membered saturated
heterocyclic group containing 1 or 2 hetero atom as a ring atom
in addition to the carbon atoms, wherein the hetero atom is
independently selected from the group consisting of oxygen,
nitrogen and sulfur atoms, and wherein the ring atom in the
/5 heterocyclic group bonded to
[0090]
r1.4)
\
X
is a carbon atom"
[0091]
in (5) of R4 include the following groups:
20 [0092]
ssis _I , s s) n AO .siss _I silt n A0
0 0 0 N N .
[0093]
Examples of the "4- to 6-membered saturated heterocycle
containing 1 or 2 hetero atom as a ring atom in addition to the
25 carbon atoms, wherein the hetero atom is independently selected
from the group consisting of oxygen, nitrogen and sulfur atoms"
include oxetane, tetrahydrofuran, tetrahydropyran, 1,4-dioxane,
22
CA 03074989 2020-03-05
pyrrolidine and piperidine.
[0094]
For example, examples of the "4- to 6-membered saturated
heterocycle containing 1 or 2 hetero atom as a ring atom in
addition to the carbon atoms, wherein the hetero atom is
independently selected from the group consisting of oxygen,
nitrogen and sulfur atoms" formed by R2 and R3, together with
the carbon atoms that they are bonded to, include the following
rings:
/o [0095]
(R1)n (R1)n (111)
0* 0 0
(R1)n OR% H (Ft%
0
N *
0
=
[0096]
With regard to the term "substituted", for example, C1-6
alkyl "optionally substituted with 1 to 3 substituents
independently selected from cyano and 01-3 alkoxy" in (4) of R2
means unsubstituted 01-6 alkyl, or 01-6 alkyl substituted with 1
to 3 substituents independently selected from cyano and C1-3
alkoxy at any substitutable position.
[0097]
The "compound of Formula [I]" is hereinafter also
referred to as "Compound [I]".
[0098]
Specific embodiments of each group of Compound [I] are
exemplified below, which should not be construed as limitative.
Compound [I] also encompasses combinations of two or more
embodiments selected appropriately from the specific
embodiments of each group.
[0099]
23
CA 03074989 2020-03-05
,
-L-000H is preferably
(1) -COOH,
(2) -C(R71) (1172)-000H, or
(3) -0(R73)(R74)-C(R75)(Rm)-COOH.
-L-000H is more preferably -COOH.
[0100]
n is preferably 0 or 1.
[0101]
m is preferably 0, 1 or 2.
m is more preferably 0 or 1.
[0102]
R2 is preferably
(1) halogen,
(2) hydroxy,
(3) cyano,
(4) C1-6 alkyl optionally substituted with 1 to 3 substituents
independently selected from the group consisting of cyano and
01-3 alkoxy,
(5) halo 01-6 alkyl,
(6) 01-6 alkoxy,
(7) halo C1-6 alkoxy,
(8) -000R21 wherein R21 is hydrogen or C1-3 alkyl, or
(9) -CON(R22)(R23) wherein R22 and R23 are each independently
hydrogen or C1-3 alkyl.
R2 is more preferably
(2) hydroxy,
(6) 01-6 alkoxy, or
(7) halo 01-6 alkoxy.
[0103]
R3 is preferably
(1) hydrogen,
(2) halogen,
(3) cyano,
(4) 01-3 alkyl,
(5) halo 01-3 alkyl,
24
CA 03074989 2020-03-05
(6) C1-3 alkoxy, or
(7) -000R31 wherein R31 is hydrogen or C1-3 alkyl.
R3 is more preferably
(1) hydrogen, or
(2) halogen.
[0104]
R4 is preferably
(1) C1-8 alkyl optionally substituted with 1 to 3 substituents
independently selected from the following Group A,
/o (2) halo C1-6 alkyl, or
(3) -CON(R41) (R42) wherein R41 and R42 are each independently
hydrogen or C1-6 alkyl.
Group A consists of
(a) hydroxy,
/5 (b) C1-3 alkoxy optionally substituted with one hydroxy or one
C1-3 alkoxy,
(c) halo C1-3 alkoxy,
(d) C3-6 cycloalkyl, and
(e) phenyl.
20 Group A preferably consists of
(a) hydroxy,
(b) C1-3 alkoxy optionally substituted with one hydroxy or one
C1-3 alkoxy, and
(c) halo C1-3 alkoxy.
25 [0105]
R5 is preferably hydrogen.
[0106]
A preferable embodiment is Compound [I] wherein
=X- is =C(R5)- or =N-;
30 -L-COOH is
(1) -COOH,
(2) -C(R11)(R72)-COOH, or
(3) -C(R73 )(R74)-C(R75)(R76)-COOH;
n is 0 or 1;
35 m is 0, 1 or 2;
CA 03074989 2020-03-05
R1 is halogen or 01-3 alkyl;
R2 is
(1) halogen,
(2) hydroxy,
(3) cyano,
(4) 01-6 alkyl optionally substituted with 1 to 3 substituents
independently selected from the group consisting of cyano and
01-3 alkoxy,
(5) halo 01-6 alkyl,
/o (6) 01-6 alkoxy,
(7) halo 01-6 alkoxy,
(8) -000R21 wherein R21 is hydrogen or 01-3 alkyl, or
(9) -CON(R22) (R23) wherein R22 and R23 are each independently
hydrogen or 01-3 alkyl;
R3 is
(1) hydrogen,
(2) halogen,
(3) cyano,
(4) 01-3 alkyl,
(5) halo 01-3 alkyl,
(6) 01-3 alkoxy, or
(7) -000R3' wherein Rn is hydrogen or 01-3 alkyl;
R4 is
(1) 01-8 alkyl optionally substituted with 1 to 3 substituents
independently selected from the following Group A,
(2) halo 01-6 alkyl, or
(3) -CON(R41 )(R42) wherein R41 and R42 are each independently
hydrogen or 01-6 alkyl, and
Group A consists of
(a) hydroxy,
(b) 01-3 alkoxy optionally substituted with one hydroxy or one
01-3 alkoxy,
(c) halo 01-3 alkoxy,
(d) 03-6 cycloalkyl, and
(e) phenyl;
26
CA 03074989 2020-03-05
. .
R5 is hydrogen;
R6 are each independently halogen, hydroxy, C1-3 alkyl or
C1-3 alkoxy; and
R71, R72, R75, R74, R75, Rm, R77, and Rm are each
independently hydrogen or C1-3 alkyl.
[0107]
A more preferable embodiment is Compound [I] wherein
=X- is =C(R5)- or =N-;
-L-COOH is -COOH;
n is 0 or 1;
m is 0 or 1;
R1 is halogen or C1-3 alkyl;
R2 is
(2) hydroxy,
/5 (6) C1-6 alkoxy, or
(7) halo C1-6 alkoxy;
R5 is
(1) hydrogen, or
(2) halogen;
R4 is
(1) C1-8 alkyl optionally substituted with 1 to 3 substituents
independently selected from the following Group A,
(2) halo C1-6 alkyl, or
(3) -CON(R41) (R42) wherein R41 and R42 are each independently
hydrogen or C1-6 alkyl, and
Group A consists of
(a) hydroxy,
(b) C1-3 alkoxy optionally substituted with one hydroxy or one
C1-3 alkoxy,
(c) halo C1-3 alkoxy,
(d) C3-6 cycloalkyl, and
(e) phenyl;
R5 is hydrogen ; and
R6 is halogen, hydroxy, C1-3 alkyl or C1-3 alkoxy.
[0108]
27
CA 03074989 2020-03-05
Another preferable embodiment is a compound of Formula
[II], or a pharmaceutically acceptable salt thereof:
[0109]
(R1)n
R2
R3 N i
I
(R6).'9 R4
HOOC
[ II ]
[0110]
wherein
n is 0 or 1;
m is 0 or 1;
R1 is halogen or 01-3 alkyl;
R2 is hydroxy, 01-6 alkoxy or halo C1-6 alkoxy;
R3 is hydrogen or halogen;
R4 is
(1) 01-6 alkyl optionally substituted with 1 to 3 substituents
independently selected from the following Group A,
(2) halo 01-6 alkyl, or
(3) -CON(R41) (R42) wherein R41 and R42 are each independently
hydrogen or 01-6 alkyl, and
Group A consists of
(a) hydroxy,
(b) 01-3 alkoxy optionally substituted with one hydroxy or one
01-3 alkoxy,
(c) halo 01-3 alkoxy,
(d) 03-6 cycloalkyl, and
(e) phenyl; and
R6 is halogen, hydroxy, 01-3 alkyl or 01-3 alkoxy.
[0111]
Another preferable embodiment is a compound of Formula
28
CA 03074989 2020-03-05
[III], or a pharmaceutically acceptable salt thereof:
[0112]
(R1)n
R2
410
R-
= Ni
(R6)m N R4
HOOC
[ III ]
[0113]
wherein
n is 0 or 1;
m is 0 or 1;
R1 is halogen or 01-3 alkyl;
R2 is hydroxy, 01-6 alkoxy or halo 01-6 alkoxy;
R3 is hydrogen or halogen;
R4 is
(1) C1-8 alkyl optionally substituted with 1 to 3 substituents
independently selected from the following Group A,
(2) halo 01-6 alkyl, or
/5 (3) -CON(R 41)(R42) wherein R41 and R42 are each independently
hydrogen or C1-6 alkyl, and
Group A consists of
(a) hydroxy,
(b) C1-3 alkoxy optionally substituted with one hydroxy or one
C1-3 alkoxy,
(c) halo 01-3 alkoxy,
(d) 03-6 cycloalkyl, and
(e) phenyl; and
R6 is halogen, hydroxy, C1-3 alkyl or 01-3 alkoxy.
[0114]
The "pharmaceutically acceptable salt" may be any salt
known in the art as long as it is not associated with undue
29
CA 03074989 2020-03-05
=
toxicity. Specific examples thereof include salts with
inorganic acid, salts with organic acid, salts with inorganic
base, and salts with organic base. Various forms of
pharmaceutically acceptable salts are well known in the art and,
for example, they are described in the following documents.
(a) Berge et al., J. Pharm. Sci., 66, p 1-19 (1977),
(b) Stahl et al., "Handbook of Pharmaceutical Salt: Properties,
Selection, and Use" (Wiley-VCH, Weinheim, Germany, 2002),
(c) Paulekuhn et al., J. Med. Chem., 50, p 6665-6672 (2007)
io The pharmaceutically acceptable salt of Compound [I] can
be obtained by reacting Compound [I] with an inorganic acid, an
organic acid, an inorganic base or an organic base, according
to a known method.
[0115]
Examples of the salt with inorganic acid include salts
with hydrofluoric acid, hydrochloric acid, hydrobromic acid,
hydriodic acid, nitric acid, phosphoric acid and sulfuric acid.
Examples of the salt with organic acid include salts with
acetic acid, adipic acid, alginic acid, 4-aminosalicylic acid,
anhydromethylenecitric acid, benzoic acid, benzenesulfonic acid,
camphoric acid, camphor-10-sulfonic acid, carbonic acid, citric
acid, edetic acid, ethane-1,2-disulfonic acid, dodecylsulfuric
acid, ethanesulfonic acid, fumaric acid, glucoheptonic acid,
gluconic acid, glucuronic acid, glycollylarsanilic acid,
hydroxynaphthoic acid, 2-hydroxy-1-ethanesulfonic acid, lactic
acid, lactobionic acid, malic acid, maleic acid, mandelic acid,
methanesulfonic acid, methylsulfuric acid, methylnitric acid,
methylenebis(salicylic acid), galactaric acid, naphthalene-2-
sulfonic acid, 2-naphthoic acid, 1,5-naphthalenedisulfonic acid,
oleic acid, oxalic acid, pamoic acid, pantothenic acid, pectic
acid, picric acid, propionic acid, polygalacturonic acid,
salicylic acid, stearic acid, succinic acid, tannic acid,
tartaric acid, teoclic acid, thiocyanic acid, trifluoroacetic
acid, p-toluenesulfonic acid, undecanoic acid, aspartic acid
and glutamic acid.
CA 03074989 2020-03-05
=
[0116]
Examples of the salt with inorganic base include salts
with lithium, sodium, potassium, magnesium, calcium, barium,
aluminium, zinc, bismass and ammonium.
Examples of the salt with organic base include salts with
arecoline, betaine, choline, clemizole, ethylene diamine, N-
methylglucamine, N-benzylphenethylamine,
tris(hydroxymethyl)methylamine, arginine and lysine.
[0117]
Preferable embodiments of the "pharmaceutically
acceptable salt" are as follows.
Examples of the salt with inorganic acid include salts
with hydrochloric acid, nitric acid, sulfuric acid, phosphoric
acid and hydrobromic acid.
Examples of the salt with organic acid include salts with
oxalic acid, maleic acid, citric acid, fumaric acid, lactic
acid, malic acid, succinic acid, tartaric acid, acetic acid,
trifluoroacetic acid, benzoic acid, glucuronic acid, oleic acid,
pamoic acid, methanesulfonic acid, benzenesulfonic acid, p-
a) toluenesulfonic acid and 2-hydroxy-1-ethanesulfonic acid.
Examples of the salt with inorganic base include salts
with sodium, potassium, calcium, magnesium and zinc.
Examples of the salt with organic base include salts with
tris(hydroxymethyl)methylamine, N-methylglucamine and lysine.
[0118]
Compound [I] or a pharmaceutically acceptable salt
thereof may be present as a solvate.
The "solvate" is Compound [I] or a pharmaceutically
acceptable salt thereof which is coordinated with a solvent
molecule, and also encompasses hydrates. The solvate is
preferably a pharmaceutically acceptable solvate, and examples
thereof include a hydrate, an ethanolate and a dimethyl
sulfoxidate of Compound [I] or a pharmaceutically acceptable
salt thereof.
Specific examples include semihydrate, monohydrate,
31
CA 03074989 2020-03-05
'4 dihydrate and monoethanolate of Compound [I], monohydrate of
hydrochloride of Compound [I], and 2/3 ethanolate of
dihydrochloride of Compound [I]. These solvates can be
obtained according to a known method.
[0119]
Compound [I] or a pharmaceutically acceptable salt
thereof may be present as a tautomer. In this case, Compound
[I] or a pharmaceutically acceptable salt thereof can be a
single tautomer or a mixture thereof.
/o [0120]
Compound [I] or a pharmaceutically acceptable salt
thereof may have a carbon-carbon double bond. In this case,
Compound [I] or a pharmaceutically acceptable salt thereof can
be present as an E form, a Z form, or a mixture thereof.
[0121]
Compound [I] or a pharmaceutically acceptable salt
thereof may contain a stereoisomer that should be recognized as
a cis/trans isomer. In this case, Compound [I] or a
pharmaceutically acceptable salt thereof can be present as a
cis form, a trans form, or a mixture thereof.
[0122]
Compound [I] or a pharmaceutically acceptable salt
thereof may contain one or more asymmetric carbons. In this
case, Compound [I] or a pharmaceutically acceptable salt
thereof may be present as a single enantiomer, a single
diastereomer, a mixture of enantiomers or a mixture of
diastereomers.
[0123]
Compound [I] or a pharmaceutically acceptable salt
thereof may be present as an atropisomer. In this case,
Compound [I] or a pharmaceutically acceptable salt thereof may
be present as a single atropisomer or a mixture thereof.
[0124]
Compound [I] or a pharmaceutically acceptable salt
thereof may simultaneously contain plural structural
32
CA 03074989 2020-03-05
N characteristics derived from the above-mentioned isomers.
Moreover, Compound [I] or a pharmaceutically acceptable salt
thereof may contain the above-mentioned isomers at any ratio.
[0125]
The formulae, chemical structures and compound names
indicated herein without specifying the stereochemistry thereof
encompass all the above-mentioned isomers that may be present
unless a particular note to the stereochemistry is made herein.
[0126]
A diastereomeric mixture can be separated into each
diastereomer by conventional methods such as chromatography and
crystallization. Alternatively, each diastereomer can also be
produced by using a stereochemically single starting material,
or by a synthesis method employing a stereoselective reaction.
[0127]
An enantiomeric mixture can be separated into each single
enantiomer by a method well known in the art.
For example, first, a diastereomeric mixture can be
prepared by reacting an enantiomeric mixture with a
substantially pure enantiomer compound known as a chiral
auxiliary. Next, the obtained diastereomeric mixture can be
separated into a single diastereomer having high isomer ratio
or a substantially pure single diastereomer by a conventional
method such as fractional crystallization and chromatography.
Finally, the separated diastereomer can be converted to a
desired enantiomer by removing the added chiral auxiliary by
cleavage.
Moreover, an enantiomeric mixture can also be directly
separated into each enantiomer by a chromatography method using
a chiral solid phase well known in the art. Alternatively, one
of enantiomers can also be obtained by using a substantially
pure optically active starting material or by employing
stereoselective synthesis (asymmetric induction) of a prochiral
intermediate using a chiral auxiliary and an asymmetric
catalyst.
33
CA 03074989 2020-03-05
µ
[0128]
The absolute steric configuration can be determined by
the X-ray crystal analysis of the crystalline product or
intermediate. In this case, a crystalline product or
intermediate derivatized with a reagent having an asymmetric
center with a known steric configuration may be used if
necessary.
[0129]
Compound [I] or a pharmaceutically acceptable salt
/o thereof may be labeled with isotope (e.g., 2H, 3H, 14c, and
35S,).
[0130]
Compound [I] or a pharmaceutically acceptable salt
thereof is preferably substantially pure, more preferably has a
/5 purity of 80% or more.
[0131]
As used herein, the pharmaceutical composition may be
produced according to a method known per se in the art of
pharmaceutical preparations, by mixing Compound [I] or a
20 pharmaceutically acceptable salt thereof with a suitable amount
of at least one type of pharmaceutically acceptable carrier and
the like as appropriate. The content of Compound [I] or a
pharmaceutically acceptable salt thereof in the pharmaceutical
composition varies depending on the dosage form, dose and the
25 like, and is, for example, 0.1 to 100 wt% of the whole
composition.
[0132]
Examples of the dosage foLm of Compound [I] or a
pharmaceutically acceptable salt thereof include oral
30 preparations such as tablet, capsule, granule, powder, troche,
syrup, emulsion, and suspension, and parenteral preparations
such as external preparation, suppository, injection, eye drop,
nasal preparations, and pulmonary preparation.
[0133]
34
CA 03074989 2020-03-05
=
Examples of the "pharmaceutically acceptable carrier"
include various organic or inorganic carrier substances
conventionally used as preparation materials, and specifically
include excipient, disintegrant, binder, glidant, and lubricant
for solid preparations; solvent, solubilizing agent, suspending
agent, isotonicity agent, buffering agent, and soothing agent
for liquid preparations; and base, emulsifier, moistening
agent, stabilizer, stabilizing agent, dispersant, plasticizer,
pH adjuster, absorption enhancer, gelling agent, preservative,
lo filler, solvent, solubilizing agent, and suspending agent for
semi-solid preparations. Where necessary, additives such as
preservative, antioxidant, colorant, and sweetening agent may be
used.
[0134]
Examples of the "excipient" include lactose, sucrose, D-
mannitol, D-sorbitol, corn starch, dextrin, microcrystalline
cellulose, crystalline cellulose, carmellose, carmellose
calcium, sodium carboxymethyl starch, low-substituted
hydroxypropylcellulose and gum arabic.
[0135]
Examples of the "disintegrant" include carmellose,
carmellose calcium, carmellose sodium, sodium carboxymethyl
starch, croscarmellose sodium, crospovidone, low-substituted
hydroxypropylcellulose, hydroxypropylmethylcellulose and
crystalline cellulose.
[0136]
Examples of the "binder" include hydroxypropylcellulose,
hydroxypropylmethylcellulose, povidone, crystalline cellulose,
sucrose, dextrin, starch, gelatin, carmellose sodium and gum
arabic.
[0137]
Examples of the "glidant" include light anhydrous silicic
acid and magnesium stearate.
[0138]
Examples of the "lubricant" include magnesium stearate,
CA 03074989 2020-03-05
calcium stearate and talc.
[0139]
Examples of the "solvent" include purified water, ethanol,
propylene glycol, macrogol, sesame oil, corn oil and olive oil.
[0140]
Examples of the "solubilizing agent" include propylene
glycol, D-mannitol, benzyl benzoate, ethanol, triethanolamine,
sodium carbonate and sodium citrate.
[0141]
Examples of the "suspending agent" include benzalkonium
chloride, carmellose, hydroxypropylcellulose, propylene glycol,
povidone, methylcellulose and glycerol monostearate.
[0142]
Examples of the "isotonic agent" include glucose, D-
sorbitol, sodium chloride and D-mannitol.
[0143]
Examples of the "buffering agent" include sodium
hydrogenphosphate, sodium acetate, sodium carbonate and sodium
citrate.
[0144]
Examples of the "soothing agent" include benzyl alcohol.
[0145]
Examples of the "base" include water, animal and
vegetable oils (e.g., olive oil, corn oil, arachis oil, sesame
oil, and castor oil), lower alcohols (e.g., ethanol, propanol,
propylene glycol, 1,3-butylene glycol, and phenol), higher
fatty acids and esters thereof, wax, higher alcohols,
polyalcohols, hydrocarbons (e.g., white vaseline, liquid
paraffin, and paraffin), hydrophilic vaseline, purified lanolin,
absorptive ointment, hydrous lanolin, hydrophilic ointment,
starch, pullulan, gum arabic, tragacanth gum, gelatin, dextran,
cellulose derivatives (e.g., methyl cellulose, carboxymethyl
cellulose, hydroxyethyl cellulose, and hydroxypropyl cellulose),
synthetic polymers (e.g., carboxyvinyl polymer, sodium
polyacrylate, polyvinyl alcohol, and polyvinyl pyrrolidone),
36
CA 03074989 2020-03-05
propylene glycol, Macrogol (e.g., Macrogol 200 to 600), and
combinations of two or more types thereof.
[0146]
Examples of the "preservative" include ethyl
parahydroxybenzoate, chlorobutanol, benzyl alcohol, sodium
dehydroacetate and sorbic acid.
[0147]
Examples of the "antioxidant" include sodium sulfite and
ascorbic acid.
[0148]
Examples of the "colorant" include food colors (e.g., Food
Color Red No. 2 or 3, and Food Color Yellow No. 4 or 5) and f3-
carotene.
[0149]
Examples of the "sweetening agent" include saccharin
sodium, dipotassium glycyrrhizinate and aspartame.
[0150]
As used herein, the pharmaceutical composition can be
administered orally or parenterally (e.g., topical, rectal,
intravenous, intramuscular, and subcutaneous administration) to
human as well as mammals other than human (e.g., mouse, rat,
hamster, guinea pig, rabbit, cat, dog, swine, bovine, horse,
sheep, and monkey). The dose varies depending on the subject of
administration, disease, symptom, dosage form, administration
route and the like. For example, the daily dose for oral
administration to an adult patient is generally within the range
of about 0.01 mg to 1 g based on the active ingredient (i.e.,
Compound [I]). This amount can be administered in one to
several portions.
[0151]
Since Compound [I] or a pharmaceutically acceptable salt
thereof has a GLUT9 inhibitory action, it is useful as a GLUT9
inhibitor.
[0152]
The expression "have GLUT9 inhibitory action" or "inhibit
37
CA 03074989 2020-03-05
GLUT9" means elimination or attenuation of GLUT9 activity by
inhibiting a GLUT9 function, for example, it means specific
inhibition of GLUT9 function under the below-mentioned
condition of Test Example 1.
The "GLUT9 inhibitor" means a substance which inhibits a
GLUT9 function.
The "GLUT9" is preferably "human GLUT9".
[0153]
In one embodiment, since Compound [I] or a
/o pharmaceutically acceptable salt thereof has a GLUT9 inhibitory
action, it is expected to be effective for diseases that
involve GLUT9.
[0154]
That is, Compound [I] or a pharmaceutically acceptable
/5 salt thereof is expected to be useful for the treatment or
prophylaxis of a disease selected from the group consisting of
hyperuricemia and gout.
Examples of the "gout" include gouty arthritis, gouty
kidney and gouty tophus.
20 [0155]
In another embodiment, Compound [I] or a pharmaceutically
acceptable salt thereof is expected to be useful for the
treatment or prophylaxis of diseases selected from the group
consisting of chronic kidney disease (CKD), hypertension,
25 diabetes, cardiac disease, arteriosclerotic disease, non-
alcoholic fatty liver disease (NAFLD), non-alcoholic
steatohepatitis (NASH) and psoriasis.
Examples of the "cardiac disease" include cardiovascular
disease, cardiac failure and atrial fibrillation.
30 [0156]
As used herein, the "treatment" encompasses improving
symptoms, preventing the aggravation of symptoms, maintaining
the remission of symptoms, preventing the exacerbation of
symptoms, and preventing the relapse of symptoms.
35 As used herein, the "prophylaxis" means suppressing the
38
CA 03074989 2020-03-05
onset of symptoms.
[0157]
Compound [I] or a pharmaceutically acceptable salt
thereof can be used in combination with one or a plurality of
other medicaments (hereinafter to be also referred to as a
concomitant drug) according to a method generally employed in
the medical field (hereinafter to be referred to as combined
use).
[0158]
/o The timing of administering Compound [I] or a
pharmaceutically acceptable salt thereof and the concomitant
drug is not limited, and they may be administered to the subject
as a combination preparation, or the both preparations may be
administered simultaneously or separately at certain intervals.
In addition, the pharmaceutical composition containing Compound
[I] or a pharmaceutically acceptable salt thereof and the
concomitant drug may be used in the form of a kit. The dose of
the concomitant drug is similar to the clinically-employed dose
and can be appropriately selected according to the
administration subject, disease, symptom, dosage form,
administration route, administration time, combination and the
like. The administration form of the concomitant drug is not
particularly limited as long as it is combined with Compound [I]
or a pharmaceutically acceptable salt thereof.
[0159]
Examples of the concomitant drug include
(1) an agent for the treatment and/or prophylaxis of
hyperuricemia, and
(2) an agent for the treatment and/or prophylaxis of gout,
and at least one of these agents can be used in combination
with Compound [I] or a pharmaceutically acceptable salt thereof.
[0160]
As long as the embodiment disclosed herein does not
contradict other embodiments disclosed herein, any combination
of any of two or more such embodiments is intended to be
39
CA 03074989 2020-03-05
,
encompassed by the technical scope of the present invention.
[0161]
The production methods of Compound [I] or a
pharmaceutically acceptable salt thereof are explained in the
following, which should not be construed as limitative. Unless
otherwise referred, the salt of each compound in general
production methods can be selected appropriately from the
above-mentioned "pharmaceutically acceptable salt".
The compound obtained in each step can be, if necessary,
isolated or purified according to a method known per se such as
distillation, recrystallization and column chromatography, or
directly used in the next step without isolation or
purification.
[0162]
[General Production Method]
[0163]
Production Method Al: Production method of Compound [IA] or a
salt thereof
[0164]
CA 03074989 2020-03-05
=
Qn
(R6)m 6%133
H2N N
H2N N Q4100C¨L
I [Al -Q3]
(R6). X )j
(el X Step A1-1
44100C ¨L
[A1-1] [AI-2]
Q"
C122-B,
12411
(R6) X QV , ,
H2N [Al-Q2] H2N
m
(R6)m X Ft"
Step A1-2 Step Al -3
C14100C¨L C14100C¨L
[A1-3] [A1-4]
H2N N Q" N
1
(R6)m X 124A (R6)m
124A
Step A1-4 Step A1-5
CI4100C¨L Omooc-i.
[AI-5] [Al-6]
(R1).
R2
) (R1)
11
4111 co2 (R1 R
R3 2
R2
[Al -QI] 1:1113
R3
R3
Step A1-61 Step A1-7
0164 x R4A
(R.)m 114A
HOOC¨L
Q4100C¨L
[A1-7] [ IA ]
[0165]
wherein
R4a s
(1) C1-3 alkenyl optionally substituted with 1 to 3 substituents
independently selected from Group A,
(2) halo C1-6 alkenyl,
(4) C3-6 cycloalkenyl optionally substituted with 1 to 3
substituents independently selected from C1-3 alkoxy, or
/o (5) a 4- to 6-membered unsaturated heterocyclic group
containing one carbon-carbon double bond, and containing 1 or 2
hetero atom as a ring atom in addition to the carbon atoms,
wherein the hetero atom is independently selected from the
group consisting of oxygen, nitrogen and sulfur atoms, and
41
CA 03074989 2020-03-05
. .
wherein the ring atom in the heterocyclic group of Compound
[A1-Q2] bonded to the boron is a carbon atom, and the ring atom
in the heterocyclic group of Compound [A1-4] bonded to
[0166]
r.scN
I2X
is a carbon atom,
[0167]
R4A is
(1) C1-8 alkyl optionally substituted with 1 to 3 substituents
independently selected from Group A,
/o (2) halo C1-6 alkyl,
(4) C3-6 cycloalkyl optionally substituted with 1 to 3
substituents independently selected from C1-3 alkoxy, or
(5) a 4- to 6-membered saturated heterocyclic group containing
1 or 2 hetero atom as a ring atom in addition to the carbon
atoms, wherein the hetero atom is independently selected from
the group consisting of oxygen, nitrogen and sulfur atoms, and
wherein the ring atom in the heterocyclic group bonded to
[0168]
SN
46(
is a carbon atom,
[0169]
Group A is as defined above,
IQ" and Qn are each independently halogen,
Q31 is a leaving group (e.g., halogen and sulfonyloxy (e.g.,
methanesulfonyloxy, trifluoromethanesulfonyloxy,
benzenesulfonyloxy, and toluenesulfonyloxy)),
412, Qn, 422, Qn, Q.32, and Q33 are hydroxy, or 412 and Q13, 422 and
Qn, and Q32 and Q33, together with the boron atom that they are
bonded to, each independently optionally form a borate,
Q41 is a protecting group for a carboxy group (e.g., methyl,
42
CA 03074989 2020-03-05
benzyl, and tert-butyl), and
the other symbols are as defined above.
[0170]
(Step A1-1)
Compound [A1-2] or a salt thereof can be obtained by
subjecting Compound [A1-1] or a salt thereof and Compound [Al-
Q3] to Suzuki coupling reaction. For example, Compound [A1-2]
or a salt thereof can be obtained by reacting Compound [A1-1]
or a salt thereof with Compound [Al-Q3] under heating in the
_to presence of a base and a palladium catalyst, in a solvent.
Examples of the solvent include ether solvents such as
1,4-dioxane, tetrahydrofuran, and 1,2-dimethoxyethane; alcohol
solvents such as methanol, and ethanol; hydrocarbon solvents
such as benzene, toluene, and xylene; polar solvents such as
N,N-dimethylformamide, dimethyl sulfoxide, and acetonitrile;
mixed solvents thereof, and mixed solvents of the above-
mentioned solvent and water. A preferable solvent is a mixed
solvent of 1,4-dioxane and water, a mixed solvent of
tetrahydrofuran and water, a mixed solvent of 1,2-
dimethoxyethane and water, a mixed solvent of toluene and water,
or a mixed solvent of the above-mentioned mixed solvent and an
alcohol solvent such as ethanol.
Examples of the base include potassium phosphate,
potassium carbonate, sodium carbonate, cesium carbonate and
cesium fluoride. A preferable base is potassium phosphate,
potassium carbonate or sodium carbonate.
Examples of the palladium catalyst include palladium
complexes such as tetrakis(triphenylphosphine)palladium(0),
bis(triphenylphosphine)palladium(II) dichloride, and [1,1'-
bis(diphenylphosphino)-ferrocene]palladium(II) dichloride; and
palladium complexes prepared in reaction system from a
palladium compound (e.g., palladium(II) acetate, and
tris(dibenzylideneacetone)dipalladium(0)), and a phosphine
ligand (e.g., 2-dicyclohexylphosphino-2',6'-dimethoxybiphenyl,
and 2-dicyclohexylphosphino-2',4',6'-triisopropylbipheny1). A
43
CA 03074989 2020-03-05
. .
preferable palladium catalyst is
tetrakis(triphenylphosphine)palladium(0) or [1,1'-
bis(diphenylphosphino)-ferrocene]palladium(II) dichloride.
When Q3' is chlorine, a method using palladium(II) acetate and
2-dicyclohexylphosphino-2',6'-dimethoxybiphenyl is preferably
employed.
The reaction temperature under heating is, for example,
40 C to 140 C, preferably 70 C to 110 C.
Compound [A1-1] or a salt thereof is a commercially
/o available product, or can be obtained by a known method.
Compound [A1-Q3] is a commercially available product, or
can be obtained by a known method.
[0171]
(Step A1-2)
Compound [A1-3] or a salt thereof can be obtained by
subjecting Compound [A1-2] or a salt thereof to a halogenation
reaction. For example, Compound [A1-3] or a salt thereof can
be obtained by reacting Compound [A1-2] or a salt thereof with
a halogenating agent in a solvent.
Examples of the solvent include polar solvents such as
N,N-dimethylformamide, dimethyl sulfoxide, acetonitrile, and
acetic acid; and halogen solvents such as dichloromethane, and
chloroform. A preferable solvent is N,N-dimethylformamide or
acetonitrile.
Examples of the halogenating agent include N-
bromosuccinimide, N-iodosuccinimide, N-chlorosuccinimide and
bromine. A preferable halogenating agent is N-bromosuccinimide
or N-iodosuccinimide.
The reaction temperature is, for example, 0 C to 120 C,
preferably 0 C to room temperature (about 25 C)
[0172]
(Step A1-3)
Compound [A1-4] or a salt thereof can be obtained by
reacting Compound [A1-3] or a salt thereof and Compound [A1-Q2]
according to Step A1-1.
44
CA 03074989 2020-03-05
Compound [A1-Q2] is a commercially available product, or
can be obtained by a known method.
[0173]
(Step A1-4)
Compound [A1-5] or a salt thereof can be obtained by
subjecting Compound [A1-4] or a salt thereof to a hydrogenation
reaction. For example, Compound [A1-5] or a salt thereof can
be obtained by reacting Compound [A1-4] or a salt thereof under
hydrogen gas atmosphere in the presence of a palladium catalyst,
/o in a solvent.
Examples of the solvent include ether solvents such as
1,4-dioxane, tetrahydrofuran, and 1,2-dimethoxyethane; alcohol
solvents such as methanol, and ethanol; ester solvents such as
ethyl acetate, and acetic acid isobutyl, and mixed solvents
thereof. A preferable solvent is methanol, ethyl acetate, or a
mixed solvent of methanol or ethyl acetate and tetrahydrofuran.
Examples of the palladium catalyst include 5% or 10%
palladium on carbon (dry product, wet product). A preferable
palladium catalyst is 10% palladium on carbon (wet product).
The reaction temperature is, for example, room
temperature (about 25 C) to 50 C, preferably room temperature
(about 25 C)
The pressure is, for example, 1 atm to 4 atm, preferably
1 atm.
[0174]
(Step A1-5)
Compound [A1-6] or a salt thereof can be obtained by
subjecting Compound [A1-5] or a salt thereof to Sandmeyer
reaction. For example, Compound [A1-6] or a salt thereof can
be obtained by subjecting Compound [A1-5] or a salt thereof to
a diazotization in a solvent, and then reacting the resulting
compound with a halogenating agent.
Examples of the solvent include dibromomethane,
acetonitrile, tetrahydrofuran, N,N-dimethylformamide, and water.
A preferable solvent is dibromomethane.
ak 03074989 2020-03-05
Examples of the diazotizing agent include nitrites such
as isobutyl nitrite, t-butyl nitrite and isopentyl nitrite, and
sodium nitrite. A preferable diazotizing agent is isobutyl
nitrite, t-butyl nitrite, isopentyl nitrite or sodium nitrite.
Examples of the halogenating agent include
bromotrimethylsilane, and copper(II) bromide. A preferable
halogenating agent is bromotrimethylsilane.
The reaction temperature is, for example, 0 C to 80 C,
preferably 0 C to room temperature (about 25 C)
/o [0175]
(Step A1-6)
Compound [A1-7] or a salt thereof can be obtained by
reacting Compound [A1-6] or a salt thereof with Compound [Al-
Ql] according to Step A1-1.
Compound [Al-Q1] is a commercially available product, or
can be obtained by a known method.
[0176]
(Step A1-7)
Compound [IA] or a salt thereof can be obtained by
subjecting Compound [A1-7] or a salt thereof to a deprotection
reaction to remove Q41. The deprotection reaction can be
carried out in a suitable condition depending on the type of
Q41.
For example, when (241 is methyl, Compound [IA] or a salt
thereof can be obtained by subjecting Compound [A1-7] or a salt
thereof to a hydrolysis reaction in the presence of a base, in
a solvent.
Examples of the solvent include a mixed solvent of water
and an alcohol solvent such as methanol and ethanol, and a
mixed solvent of an alcohol solvent, water and tetrahydrofuran.
A preferable solvent is a mixed solvent of methanol and water.
Examples of the base include lithium hydroxide, sodium
hydroxide, and potassium hydroxide. A preferable base is
sodium hydroxide.
The reaction temperature is, for example, room
46
CA 03074989 2020-03-05
temperature (about 25 C) to 80 C, preferably room temperature
(about 25 C) to 50 C.
[0177]
Production Method A2: Alternative step of Step A1-3 and Step
A1-4
[0178]
H2N N )1 H2N N , R4AAZnQ24
[A2-Q2]
(R6). X Q21 0164 X R4AA
Step A2-1
Q4100C¨L Q4100C¨L
vo4u pkvu
[0179]
wherein
/o R4RA is
(1) C1-8 alkyl optionally substituted with 1 to 3 substituents
independently selected from Group A, or
(2) halo C1-6 alkyl,
Group A is as defined above,
Q24 is halogen, and
the other symbols are as defined above.
[0180]
(Step A2-1)
Compound [A2-1] or a salt thereof can be obtained by
subjecting Compound [A1-3] or a salt thereof and Compound [A2-
Q2] to Negishi coupling reaction. For example, Compound [A2-1]
or a salt thereof can be obtained by reacting Compound [A1-3]
or a salt thereof with Compound [A2-Q2] in the presence of a
palladium catalyst and a ligand, in a solvent.
Examples of the solvent include ether solvents such as
1,4-dioxane, tetrahydrofuran, and 1,2-dimethoxyethane; and
hydrocarbon solvents such as benzene, toluene, and xylene. A
preferable solvent is tetrahydrofuran or toluene.
Examples of the palladium catalyst include palladium(II)
acetate, and tris(dibenzylideneacetone)dipalladium(0). A
preferable palladium catalyst is palladium(II) acetate.
Examples of the ligand include 2-dicyclohexylphosphino-
47
CA 03074989 2020-03-05
2',6'-dimethoxybiphenyl, 2-dicyclohexylphosphino-2',6'-
diisopropyloxybiphenyl, and 2-dicyclohexylphosphino-2',6'-
bis(N,N-dimethylamino)biphenyl. A preferable ligand is 2-
dicyclohexylphosphino-2',6'-bis(N,N-dimethylamino)biphenyl.
The reaction temperature is, for example, 0 C to 50 C,
preferably 0 C to room temperature (about 25 C)
Compound [A2-Q2] is a commercially available product, or
can be obtained by a known method.
[0181]
Production Method A3: Alternative production method of Compound
[IA] or a salt thereof
[0182]
MD. Q32
IR2D6\
(Ri hi (R6 Q33
6-Q33
\ I n12
11 R2 ,./ R2
"00C¨L
QN 0 [Al -QI] Q13 I N [Al-Q3] --
Ft3 -- .
A 1 ________________ ,.. 113 . 1
0" )( NH2 Step A3-I Step A3-2 (R6)m .
X NH2
Qm X NH2
[A3-1] A3 2] 04100C ¨L
Vaq
Q23 (R1)n
(R1)n I R2
Fe / Q22¨B, ,_
/1
Fe' N
., 1
123 .
OR6
-----õ- N1 -----õ (R6)m X lea
Step A3-3 . )a--.... X Q21 Step A3-4
I 04100C¨L
Q4100C¨L - [A3-5]
[A3-4]
(R1). (R16
R2 / R2
I I
1
Step A3-5 (R6)-- X). 124A Step Al -7 (R6)m X R4A
m -1--
Q4100C¨L - HOOC¨L
[Al -7] [ IA ]
[0183]
wherein each symbol is as defined above, provided that 0 is
preferably a group having reactivity equal to or lower than Q1-1
(for example, when Q11 is chlorine, 0 is chlorine), and
those skilled in the art can easily select such a group.
48
CA 03074989 2020-03-05
[0184]
(Step A3-1)
Compound [A3-2] or a salt thereof can be obtained by
reacting Compound [A3-1] or a salt thereof with Compound [Al-
Ql] according to Step A1-1.
Compound [A3-1] or a salt thereof is a commercially
available product, or can be obtained by a known method.
[0185]
(Step A3-2)
Compound [A3-3] or a salt thereof can be obtained by
reacting Compound [A3-2] or a salt thereof with Compound [Al-
Q3] according to Step A1-1.
[0186]
(Step A3-3)
Compound [A3-4] or a salt thereof can be obtained by
reacting Compound [A3-3] or a salt thereof according to Step
A1-5.
[0187]
(Step A3-4)
Compound [A3-5] or a salt thereof can be obtained by
reacting Compound [A3-4] or a salt thereof with Compound [Al-
Q2] according to Step A1-1.
[0188]
(Step A3-5)
Compound [A1-7] or a salt thereof can be obtained by
reacting Compound [A3-5] or a salt thereof according to Step
A1-4.
[0189]
Production Method A4: Alternative step of Step A3-4 and Step
A3-5
[0190]
49
CA 03074989 2020-03-05
= = , It
(R1)n 01%
R2 R2
410 N R4AAz n Qu 01 N
R3 R3
1
[A2-Q2]
A,
\
(R6)m 0 x Q21
( R6 )m 0 X R4AA
Step A4-1
Q4100C¨L Q4100C¨L
[A3-4] [A4-1]
[0191]
wherein each symbol is as defined above.
[0192]
(Step A4-1)
Compound [A4-1] or a salt thereof can be obtained by
reacting Compound [A3-4] or a salt thereof with Compound [A2-
Q2] according to Step A2-1.
[0193]
/o Production Method A5: Production method of Compound [IAA] or a
salt thereof
[0194]
CA 03074989 2020-03-05
1 0
Qn
(R6) B.33
. 0
H2N N
,
H2N INI Q4100C¨L I
[Al -Q3]
_________________________________________ Ilw (R6)rn R"
Step A5-1
R5A cellooc¨L R"
[A5-1] [A5-2]
4" N
,
----lip- (R6)m I R"
Step A5-2 R5A
Q4100C¨L
[A5-3]
(R1L
R2
OR%
011 Q12 R3 R2
13'
[Al-Q1] (1113 N
R3 ,
....---Ø I
Step A5-3 (R6)m Rax
R5A
Q4100C¨L
[A5-4]
(R1)11
R2
R3 N 1
i
------41..
Step A54 (R6)m R"
R5A
HOOC¨L
[IAA]
[0195]
wherein
R" and R5A, together with the carbon atoms that they are bonded
to, form C3-6 cycloalkane, and
the other symbols are as defined above.
[0196]
(Step A5-1)
Compound [A5-2] or a salt thereof can be obtained by
io reacting Compound [A5-1] or a salt thereof with Compound [Al-
51
CA 03074989 2020-03-05
Q3] according to Step A1-1.
Compound [A5-1] or a salt thereof is a commercially
available product, or can be obtained by a known method.
[0197]
(Step A5-2)
Compound [A5-3] or a salt thereof can be obtained by
reacting Compound [A5-2] or a salt thereof according to Step
A1-5.
[0198]
(Step A5-3)
Compound [A5-4] or a salt thereof can be obtained by
reacting Compound [A5-3] or a salt thereof with Compound [Al-
Ql] according to Step A1-1.
[0199]
/5 (Step A5-4)
Compound [IAA] or a salt thereof can be obtained by
reacting Compound [A5-4] or a salt thereof according to Step
A1-7.
[0200]
Production Method A6: Production method of Compound [IAAA] or a
salt thereof
[0201]
52
CA 03074989 2020-03-05
1 1
032
13
(RA). . W3 WAO N HO N
,
Q4100C¨L 'F ...... )
C133 N C1140 N , il
[At -C13] (W4 atik. N (RA)
1
) D ___________ - _____,
OM N Step A6-1 Qat N Step A6-2 a"
00C¨L Step A6-3 C14100C¨L 411111).frP
[A6-1] [A6-21 [A6.3] [A6-4]
HO N
,
HC.A+194AAA
MAL dik. la [Al-R4a] 4,
H CI 00C¨L
O N 4119-2.P P
: 1
Step A6-5a [A6-6a]
N 021 _______________________________________________________________________
.
Step A64 HO N
Q4100C¨L 41111"P ,
Step A6-5b
[A6-5] ..N)R4AAA > Step A64
H2C4AAA (0). MA
P
04100C¨i 'W
[Al-R4b] [A6-6b] (R÷.
HO N (214 N R2
, L....ty
RA
, AAA , ).,,,(...y 4AAA W Iiiii al2
PR(R')Ai N R 13'
P MAL Ai N
1A14211 c!)13
C24100C¨L
"'-li'. C14100C--1 4W P
..........................
[A6-71 Step A6-7 [A64] Step A64
M.,
M% W
R2
N
R3 1,..1..yR4a,AA
,
,N )..,tyRAAAA (10)., N
(0).
-----p.. P
P Step A6-9 HOOC¨L
Cl4100C¨L
[A6-9] [ IAAA ]
[0202]
wherein
p is 1, 2, 3, 4, 5 or 6,
V is methyl, ethyl or tert-butyl,
Q14 is sulfonyloxy (e.g. , methanesulfonyloxy,
trifluoromethanesulfonyloxy, benzenesulfonyloxy, and
toluenesulfonyloxy)),
RIAAA is selected from Group A, and
lo the other symbols are as defined above.
[0203]
(Step A6-1)
Compound [A6-2] or a salt thereof can be obtained by
reacting Compound [A6-1] or a salt thereof with a metal
/5 alkoxide, in a solvent.
Examples of a combination of the solvent and metal
alkoxide include a combination of methanol and sodium methoxide,
a combination of ethanol and sodium ethoxide, a combination of
benzyl alcohol and sodium benzyloxide, a combination of tert-
53
ak 03074989 2020-03-05
butanol and potassium tert-butoxide, and a combination of
tetrahydrofuran or N,N-dimethylformamide, and sodium methoxide,
sodium ethoxide, sodium benzyloxide or potassium tert-butoxide.
A preferable combination of the solvent and metal alkoxide is a
combination of tetrahydrofuran and potassium tert-butoxide.
The reaction temperature is, for example, -5 C to 30 C,
preferably 0 C to 15 C.
Compound [A6-1] or a salt thereof is a commercially
available product, or can be obtained by a known method.
/o [0204]
(Step A6-2)
Compound [A6-3] or a salt thereof can be obtained by
reacting Compound [A6-2] or a salt thereof with Compound [Al-
Q3] according to Step A1-1.
/5 [0205]
(Step A6-3)
Compound [A6-4] or a salt thereof can be obtained by
subjecting Compound [A6-3] or a salt thereof to a deprotection
reaction to remove Q34. The deprotection reaction can be
20 carried out in a suitable condition depending on the type of
For example, when Q34 is tert-butyl, Compound [A6-4] or a
salt thereof can be obtained by reacting Compound [A6-3] or a
salt thereof with an acid, in a solvent.
25 Examples of the solvent include methanol, ethanol, 2-
propanol, tetrahydrofuran, toluene, and mixed solvent thereof.
A preferable solvent is a mixed solvent of ethanol and
tetrahydrofuran.
Examples of the acid include hydrochloric acid,
30 hydrobromic acid, sulfuric acid, and trifluoroacetic acid. A
preferable acid is hydrochloric acid.
The reaction temperature is, for example, 15 C to 60 C,
preferably 30 C to 40 C.
[0206]
35 (Step A6-4)
54
CA 03074989 2020-03-05
1 .
Compound [A6-5] or a salt thereof can be obtained by
subjecting Compound [A6-4] or a salt thereof to a halogenation
reaction according to Step A1-2.
[0207]
(Step A6-5a)
Compound [A6-6a] or a salt thereof can be obtained by
subjecting Compound [A6-5] or a salt thereof and Compound [Al-
R4a] to Sonogashira coupling reaction. For example, Compound
[A6-6a] or a salt thereof can be obtained by reacting Compound
/o [A6-5] or a salt thereof with Compound [Al-R4a] in the presence
of a base, a palladium catalyst and a copper catalyst, in a
solvent.
Examples of the solvent include N,N-dimethylformamide,
acetonitrile, and tetrahydrofuran. A preferable solvent is
/5 acetonitrile.
Examples of the base include triethylamine,
diisopropylethylamine, and diisopropylamine. A preferable base
is triethylamine.
Examples of the palladium catalyst include
20 tetrakis(triphenylphosphine)palladium(0), and
bis(triphenylphosphine)palladium(II) dichloride. A preferable
palladium catalyst is bis(triphenylphosphine)palladium(II)
dichloride.
Examples of the copper catalyst include copper(I) iodide,
25 and copper(I) bromide. A preferable copper catalyst is
copper(I) iodide.
The reaction temperature is, for example, 15 C to 50 C,
preferably 25 C to 40 C.
[0208]
30 (Step A6-5b)
Compound [A6-6b] or a salt thereof can be obtained by
subjecting Compound [A6-5] or a salt thereof and Compound [Al-
R4b] to Heck reaction. For example, Compound [A6-6b] or a salt
thereof can be obtained by reacting Compound [A6-5] or a salt
35 thereof with Compound [A1-R4b] in the presence of a base and a
ak 03074989 2020-03-05
palladium catalyst, in a solvent.
[0209]
(Step A6-6)
Compound [A6-7] or a salt thereof can be obtained by
subjecting Compound [A6-6a] or a salt thereof or Compound [A6-
6b] or a salt thereof to a hydrogenation reaction according to
Step A1-4.
[0210]
(Step A6-7)
/o Compound [A6-8] or a salt thereof can be obtained by
subjecting Compound [A6-7] or a salt thereof to a sulfonylation
reaction of hydroxy. The sulfonylation reaction can be carried
out in a suitable condition depending on the type of 414.
For example, when Q14 is trifluoromethanesulfonyloxy,
/5 Compound [A6-8] or a salt thereof can be obtained by reacting
Compound [A6-7] or a salt thereof with trifluoromethanesulfonic
anhydride in the presence of a base, in a solvent.
Examples of the solvent include toluene, dichloromethane,
pyridine, and mixed solvents of the above-mentioned solvent and
20 water. A preferable solvent is a mixed solvent of toluene and
water.
Examples of the base include inorganic bases such as
dipotassium hydrogenphosphate, tripotassium phosphate, and
potassium carbonate, and organic bases such as pyridine, 4-
25 dimethylaminopyridine, 2,6-lutidine, triethylamine, and
diisopropylethylamine. A preferable base is dipotassium
hydrogenphosphate.
The reaction temperature is, for example, 0 C to 30 C,
preferably 5 C to 10 C.
30 [0211]
(Step A6-8)
Compound [A6-9] or a salt thereof can be obtained by
reacting Compound [A6-8] or a salt thereof with Compound [Al-
Ql] according to Step A1-6.
35 [0212]
56
CA 03074989 2020-03-05
(Step A6-9)
Compound [IAAA] or a salt thereof can be obtained by
subjecting Compound [A6-9] or a salt thereof to a hydrolysis
reaction according to Step A1-7.
[0213]
Production Method Bl: Production method of Compound [IB] or a
salt thereof
[0214]
(IR%
R2aia,
OR% WI.
V ErQ12 R2 R2
N aril
R3
1
[A1-01] 013 I 411:1 N 4! N
: 24 ¨e.-
H2NI X 0 0 Step B1-1 R3 R3 Step B1-2 4 0-
02
0 H2N ,Xly0-024
Q31 xr
[B1-1] [B1-2] 0 [B1-3] 0
On
(R1).
(R6)
6,Q33 R2
0
Q4100C¨L R3 ).1
[A1-(331 , ritl_c124
Step B1-3 o
ceooc-1_
[B1-4]
(R1). Rm
R2 I R2
HN¨R42
N [B1-R41
------4.- R3 )i, ------4.- R3
OH Step B1-5 '1
Step B1-4
)(1,!:41R42
(R.). x
omooc-4. 0 o4100c-4. 0
[B1-5] [B1 4]
(R1).
R2
N
------D.- R3 Rm
Step B1-6 1)(4_02
mm x
HOOC--L 0
11B]
/o [0215]
wherein each symbol is as defined above.
[0216]
(Step B1-1)
57
CA 03074989 2020-03-05
, ,
Compound [B1-2] or a salt thereof can be obtained by
reacting Compound [B1-1] or a salt thereof with Compound [Al-
Ql] according to Step A1-1.
Compound [B1-1] or a salt thereof is a commercially
available product, or can be obtained by a known method.
[0217]
(Step B1-2)
Compound [B1-3] or a salt thereof can be obtained by
reacting Compound [B1-2] or a salt thereof according to Step
/o A1-5.
[0218]
(Step B1-3)
Compound [B1-4] or a salt thereof can be obtained by
reacting Compound [B1-3] or a salt thereof with Compound [Al-
/5 Q3] according to Step A1-1.
[0219]
(Step B1-4)
Compound [B1-5] or a salt thereof can be obtained by
reacting Compound [B1-4] or a salt thereof at room temperature
20 (about 25 C) according to Step A1-7.
[0220]
(Step B1-5)
Compound [B1-6] or a salt thereof can be obtained by
subjecting Compound [B1-5] or a salt thereof and Compound [B1-
25 R4] or a salt thereof to an amidation reaction. For example,
Compound [B1-6] or a salt thereof can be obtained by reacting
Compound [B1-5] or a salt thereof with Compound [81-R41 or a
salt thereof in the presence of a condensing agent and an
optional base, in a solvent.
30 Examples of the solvent include ether solvents such as
1,4-dioxane, tetrahydrofuran, and 1,2-dimethoxyethane; halogen
solvents such as dichloromethane, and chloroform; and polar
solvents such as N,N-dimethylformamide, and acetonitrile. A
preferable solvent is N,N-dimethylformamide or acetonitrile.
35 Examples of the condensing agent include N,N'-
58
CA 03074989 2020-03-05
a .
dicyclohexylcarbodiimide, 1-(3-dimethylaminopropy1)-3-
ethylcarbodiimide, and HATU [0-(7-azabenzotriazol-1-y1)-
N,N,N',N'-tetramethyluronium hexafluorophosphate] [alias: 2-(7-
aza-1H-benzotriazol-1-y1)-1,1,3,3-tetramethyluronium
hexafluorophosphate]. A preferable condensing agent is HATU.
Examples of the optional base include triethylamine, and
N,N-diisopropylethylamine. A preferable base is triethylamine.
The reaction temperature is, for example, room
temperature (about 25 C) to 60 C, preferably room temperature
io (about 25 C)
Compound [B1-R4] or a salt thereof is a commercially
available product, or can be obtained by a known method.
[0221]
(Step B1-6)
Compound [IB] or a salt thereof can be obtained by
reacting Compound [B1-6] or a salt thereof according to Step
A1-7.
[0222]
Production Method Cl: Production method of Compound [IC] or a
salt thereof
[0223]
59
CA 03074989 2020-03-05
(Rib)
R2 (RiL (RIn
R2 R2 ail
40 r112
R3 B'." 0:125¨R4c
Q" N I 4101 N...i
[Al-Q1] Q13 R4, [C1-Q2] R3
1 1 õ ________________ = --. )-õ, 4C -------40. = a_C
Q31 X L¨OH Step C1-1 e X L cei ..XK L
--OH Step C1-2
o_R4c
[C1-1] [C1-2]
[C1-3]
Q32
MIL
(R6)
6,033 R2
0
N
04100C¨L R3
[Al -Q3] : 4C
____________________________ W (R6). X L ¨0¨R4C
Step C1-3
Q 00C¨L
[C1-4]
MIL
R2
_R3
Step C1-4 N)
4C
(R6). X L-0¨R4c
HOOC¨L
[ IC ]
[0224]
wherein
L4c is CI-B alkylene or C3-6 cycloalkylene,
R4C is C1-3 alkyl optionally substituted with one hydroxy or one
C1-3 alkoxy, or halo C1-3 alkyl,
Q25 is halogen, and
the other symbols are as defined above.
[0225]
/o (Step C1-1)
Compound [C1-2] or a salt thereof can be obtained by
reacting Compound [C1-1] or a salt thereof with Compound [Al-
Ql] according to Step A1-1.
Compound [C1-1] or a salt thereof is a commercially
/5 available product, or can be obtained by a known method.
[0226]
(Step C1-2)
Compound [C1-3] or a salt thereof can be obtained by
CA 03074989 2020-03-05
subjecting Compound [C1-2] or a salt thereof and Compound [C1-
Q2] to an alkylation reaction. For example, Compound [C1-3] or
a salt thereof can be obtained by reacting Compound [C1-2] or a
salt thereof with Compound [C1-Q2] in the presence of a base,
in a solvent.
Examples of the solvent include ether solvents such as
1,4-dioxane, tetrahydrofuran and 1,2-dimethoxyethane, and N,N-
dimethylformamide. A preferable solvent is tetrahydrofuran or
N,N-dimethylformamide.
/o Examples of the base include sodium hydride, potassium t-
butoxide, and sodium t-butoxide. A preferable base is sodium
hydride.
The reaction temperature is, for example, 0 C to 70 C,
preferably 0 C to room temperature (about 25 C)
Compound [C1-Q2] is a commercially available product, or
can be obtained by a known method.
[0227]
(Step C1-3)
Compound [C1-4] or a salt thereof can be obtained by
reacting Compound [C1-3] or a salt thereof with Compound [Al-
Q3] according to Step A1-1.
[0228]
(Step C1-4)
Compound [IC] or a salt thereof can be obtained by
reacting Compound [C1-4] or a salt thereof according to Step
A1-7.
Examples
[0229]
Next, the production method of Compound [I] or a
pharmaceutically acceptable salt thereof is concretely
explained by referring to Examples, which should not be
construed as 1-imitative.
In the following Examples, the following abbreviation is
used.
HATU: 0-(7-azabenzotriazol-1-y1)-N,N,N',W-tetramethyluronium
61
CA 03074989 2020-03-05
hexafluorophosphate [alias: 2-(7-aza-1H-benzotriazol-1-y1)-
1,1,3,3-tetramethyluronium hexafluorophosphate]
[0230]
Production Example 1
Synthesis of 4-(3-(3,5-difluoro-4-methoxypheny1)-6-(3-
methoxypropyl)pyrazin-2-yl)benzoic acid (Example 67)
[0231]
H3C,0
NCH3
HO
0
[0232]
/o Step 1-1: Methyl 4-(3-aminopyrazin-2-yl)benzoate
[0233]
OH
H3C 6
, H2N IN2j
H2NN2J 1111 OH
H3C,0
0 0
[0234]
Under inert gas atmosphere, to a solution of 3-
chloropyrazin-2-amine (3.00 g, 23.2 mmol), (4-
(methoxycarbonyl)phenyl)boric acid (5.00 g, 27.8 mmol) and
[1,1'-bis(diphenylphosphino)-ferrocene]palladium(II) dichloride
dichloromethane adduct (0.378 g, 0.463 mmol) in tetrahydrofuran
(100 mL) was added 2M-aqueous potassium phosphate solution
(23.2 mL, 46.3 mmol), and the mixture was stirred at 70 C for 1
hr. The reaction solution was diluted with water and ethyl
acetate, and separated, and the organic layer was washed
successively with water and saturated brine, and dried over
sodium sulfate. The sodium sulfate was removed by filtration,
and the filtrate was concentrated under reduced pressure. The
residue was purified by silica gel chromatography (hexane:ethyl
62
CA 03074989 2020-03-05
. .
acetate =2:1 to 1:2) to give methyl 4-(3-aminopyrazin-2-
yl)benzoate (2.56 g, yield 48%).
1H-NMR (DMSO-D6) 5: 3.87 (3H, s), 6.27 (2H, br s), 7.84 (2H, dt,
J = 8.4, 1.8 Hz), 7.89 (1H, d, J = 2.5 Hz), 7.97 (1H, d, J =
2.5 Hz), 8.04 (2H, dt, J = 8.6, 1.8 Hz).
[0235]
Step 1-2: Methyl 4-(3-amino-6-bromopyrazin-2-yl)benzoate
[0236]
H2N N
1 :J H2N I N
H3C,0
H3C,0
0 0
/0 [0237]
Under ice cooling, to a suspension of methyl 4-(3-
aminopyrazin-2-yl)benzoate (3.15 g, 13.7 mmol) in acetonitrile
(80 mL) was added N-bromosuccinimide (2.56 g, 14.4 mmol), and
the mixture was stirred for 30 min. To the reaction solution
is was added water (160 mL), and the mixture was stirred at room
temperature for 1 hr. The precipitated solid was collected by
filtration, and dried under reduced pressure to give methyl 4-
(3-amino-6-bromopyrazin-2-yl)benzoate (3.93 g, yield 92%).
1H-NMR (DMSO-D6) 5: 3.88 (3H, s), 6.57 (2H, br s), 7.82 (2H, dt,
20 J = 8.5, 1.8 Hz), 8.05 (2H, dt, J = 8.6, 1.8 Hz), 8.12 (1H, s).
[0238]
Step 1-3: Methyl (E)-4-(3-amino-6-(3-methoxyprop-1-en-l-
y1)pyrazin-2-y1)benzoate
[0239]
H2N N CH3 H2N N .
I H3k.;t. I
N Br +
H3C,0 H3C cy-/30,cH 3
u3%,,0
r-1,
25 0 0
[0240]
Under inert gas atmosphere, to a suspension of methyl 4-
(3-amino-6-bromopyrazin-2-yl)benzoate (2.00 g, 6.49 mmol), (E)-
2-(3-methoxyprop-1-en-1-y1)-4,4,5,5-tetramethyl-1,3,2-
63
CA 03074989 2020-03-05
dioxaborolane (1.54 g, 7.79 mmol) and [1,1f-
bis(diphenylphosphino)-ferrocene]palladium(II) dichloride
dichloromethane adduct (0.106 g, 0.130 mmol) in toluene (20 mL)
was added 2M-aqueous potassium phosphate solution (4.87 mL,
9.74 mmol), and the mixture was stirred at 100 C for 2 hr. The
mixture was allowed to cool to room temperature, and diluted
with ethyl acetate (50 mL), and the insoluble substance was
removed by filtration through Celite. The organic layer was
washed successively with water and saturated brine, and dried
over magnesium sulfate. The magnesium sulfate was removed by
filtration, and the filtrate was concentrated under reduced
pressure. The obtained residue was purified by silica gel
chromatography (hexane:ethyl acetate =2:1 to 1:4) to give
methyl (E)-4-(3-amino-6-(3-methoxyprop-1-en-1-yl)pyrazin-2-
/5 yl)benzoate (1.78 g, yield 91%).
1H-NMR (DMSO-D6) 6: 3.27 (3H, s), 3.88 (3H, s), 4.04 (2H, dd, J
= 5.4, 1.3 Hz), 6.37 (2H, br s), 6.46-6.53 (1H, m), 6.59 (1H,
dt, J = 15.7, 1.2 Hz), 7.86 (2H, dt, J = 8.5, 1.8 Hz), 8.03-
8.06 (3H, m).
[0241]
Step 1-4: Methyl 4-(3-amino-6-(3-methoxypropyl)pyrazin-2-
yl)benzoate
[0242]
H2NN H2N N
I I
N-""C)'CH3
u rs,0 ,0
H3C
0 0
[0243]
To methyl (E)-4-(3-amino-6-(3-methoxyprop-1-en-1-
y1)pyrazin-2-y1)benzoate (777 mg, 2.60 mmol) were added
methanol (13 mL) and 10% palladium on carbon catalyst (50% wet,
155 mg), and the mixture was stirred under hydrogen gas
atmosphere for 24 hr. The palladium on carbon catalyst was
removed from the reaction solution by filtration, and the
filtrate was concentrated under reduced pressure. The residue
64
CA 03074989 2020-03-05
was purified by silica gel chromatography (hexane:ethyl acetate
=1:4) to give methyl 4-(3-amino-6-(3-methoxypropyl)pyrazin-2-
yl)benzoate (772 mg, yield 98%).
1H-NMR (DMSO-D6) 6: 1.80-1.88 (2H, m), 2.64 (2H, t, J = 7.6 Hz),
3.21 (3H, s), 3.34 (2H, t, J = 6.4 Hz), 3.87 (3H, s), 6.02 (2H,
br s), 7.84-7.87 (3H, m), 8.03 (2H, dt, J = 8.6, 1.8 Hz).
[0244]
Step 1-5: Methyl 4-(3-bromo-6-(3-methoxypropyl)pyrazin-2-
yl)benzoate
/o [0245]
H2NN Br N
I
n,0 _0
H3C
0 0
[0246]
A solution of methyl 4-(3-amino-6-(3-
methoxypropyl)pyrazin-2-yl)benzoate (5.00 g, 16.6 mmol) in
dibromomethane (140 mL) was stirred at room temperature, and
isopentyl nitrite (2.40 mL, 18.3 mmol) was added thereto. To
the reaction solution was added dropwise a solution of
bromotrimethylsilane (2.41 mL, 18.3 mmol) in dibromomethane (20
mL) over 10 min, and the reaction solution was stirred at room
temperature for 24 hr. To the reaction solution was added
saturated aqueous sodium hydrogencarbonate solution (50 mL),
and the mixture was extracted with ethyl acetate (100 mL). The
organic layer was washed successively with water and saturated
brine, and dried over sodium sulfate. The sodium sulfate was
removed by filtration, and the filtrate was concentrated under
reduced pressure. The residue was purified by silica gel
chromatography (hexane:ethyl acetate =7:1 to 2:1) to give
methyl 4-(3-bromo-6-(3-methoxypropyl)pyrazin-2-yl)benzoate
(4.31 g, yield 71%).
1H-NMR (DMSO-D6) 6: 1.89-1.96 (2H, m), 2.82-2.87 (2H, m), 3.21
(3H, s), 3.36 (2H, t, J = 6.2 Hz), 3.89 (3H, s), 7.85 (2H, dt,
J = 8.5, 1.8 Hz), 8.08 (2H, dt, J = 8.4, 1.8 Hz), 8.43 (1H, s).
CA 03074989 2020-03-05
[0247]
Step 1-6: Methyl 4-(3-(3,5-difluoro-4-methoxypheny1)-6-(3-
methoxypropyl)pyrazin-2-yl)benzoate
[0248]
Br
I H3C,c) 1110
B4OH I
F
H3C,0 OH
H3C,0
0
0
[0249]
Under inert gas atmosphere, to a solution of methyl 4-(3-
bromo-6-(3-methoxypropyl)pyrazin-2-yl)benzoate (4.30 g, 11.8
mmol) and (3,5-difluoro-4-methoxyphenyl)boric acid (2.66 g,
/o 14.1 mmol) in toluene (44 mL) were added [1,1'-
bis(diphenylphosphino)-ferrocene]palladium(II) dichloride
dichloromethane adduct (0.192 g, 0.235 mmol) and 2M-aqueous
potassium phosphate solution (8.83 mL, 17.7 mmol), and the
mixture was stirred at 100 C for 4 hr. The mixture was allowed
/5 to cool to room temperature, water (100 mL) was added thereto,
and the mixture was extracted with ethyl acetate (200 mL). The
organic layer was washed successively with water and saturated
brine, and dried over sodium sulfate. The sodium sulfate was
removed by filtration, and the filtrate was concentrated under
20 reduced pressure. The obtained residue was purified by silica
gel chromatography (hexane:ethyl acetate =4:1 to 2:1) to give
methyl 4-(3-(3,5-difluoro-4-methoxypheny1)-6-(3-
methoxypropyl)pyrazin-2-yl)benzoate (4.86 g, yield 96%).
1H-NMR (DMSO-D6) 5: 1.94-2.01 (2H, m), 2.90-2.94 (2H, m), 3.23
25 (3H, s), 3.40 (2H, t, J = 6.2 Hz), 3.85 (3H, s), 3.93 (3H, s),
7.05-7.12 (2H, m), 7.56 (2H, dt, J = 8.5, 1.8 Hz), 7.95 (2H, dt,
J = 8.4, 1.8 Hz), 8.65 (1H, s).
[0250]
Step 1-7: 4-(3-(3,5-Difluoro-4-methoxypheny1)-6-(3-
30 methoxypropyl)pyrazin-2-yl)benzoic acid
[0251]
66
= CA 03074989 2020-03-05
,
H3C0 H3Cijl
,
H3C,0 HO
0 0
[0252]
To a solution of methyl 4-(3-(3,5-difluoro-4-
methoxypheny1)-6-(3-methoxypropyl)pyrazin-2-yl)benzoate (1.00 g,
2.33 mmol) in methanol (14 mL) was added 4N-aqueous sodium
hydroxide solution (3.50 mL, 14.0 mmol), and the mixture was
stirred at 50 C for 2 hr. The mixture was allowed to cool to
room temperature, lOwt%-aqueous citric acid solution (10.5 mL)
and water (15 mL) were added thereto, and the mixture was
/o stirred at room temperature for 2 hr. The precipitated solid
was collected by filtration, and dried under reduced pressure
to give 4-(3-(3,5-difluoro-4-methoxypheny1)-6-(3-
methoxypropyl)pyrazin-2-yl)benzoic acid (824 mg, yield 85%).
1H-NMR (DMSO-D6) 5: 1.92-2.02 (2H, m), 2.91 (2H, t, J = 7.7 Hz),
/5 3.24 (3H, s), 3.40 (2H, t, J = 6.4 Hz), 3.93 (3H, s), 7.09 (2H,
d, J = 9.5 Hz), 7.53 (2H, d, J = 8.6 Hz), 7.92 (2H, d, J = 8.6
Hz), 8.64 (1H, s), 13.10 (1H, s).
[0253]
Step 1-8: Crystals of 4-(3-(3,5-difluoro-4-methoxypheny1)-6-(3-
20 methoxypropyl)pyrazin-2-yl)benzoic acid
To 4-(3-(3,5-difluoro-4-methoxypheny1)-6-(3-
methoxypropyl)pyrazin-2-yl)benzoic acid (50 mg) were added 2-
propanol (0.075 mL) and n-heptane (0.025 mL), and the mixture
was stirred at 100 C to give a solution. The stirring was
25 stopped, and the mixture was allowed to cool to room
temperature. The precipitated solid was collected by
filtration, and dried under reduced pressure to give crystals
of 4-(3-(3,5-difluoro-4-methoxypheny1)-6-(3-
methoxypropyl)pyrazin-2-yl)benzoic acid (31 mg, yield 62%).
30 [0254]
67
CA 03074989 2020-03-05
Production Example 2
Synthesis of 4-(5-(butylcarbamoy1)-2-(4-methoxyphenyl)pyridin-
3-yl)benzoic acid hydrochloride (Example 76)
[0255]
,,0
H31,
HO 0
HCI
0
[0256]
Step 2-1: Methyl 5-amino-6-(4-methoxyphenyl)nicotinate
[0257]
m 0
Br N n3u
,0
I
H2NrCI'CH3 H3C 1110
BOH / 0,,
u H2N H3
0
OH 0
/0 [0258]
To a mixture of methyl 5-amino-6-bromonicotinate (0.500 g,
2.16 mmol), (4-methoxyphenyl)boric acid (0.660 g, 4.34 mmol)
and potassium phosphate (1.43 g, 6.73 mmol) were added 1,2-
dimethoxyethane (12 mL) and water (4 mL). Under inert gas
atmosphere, [1,1'-bis(diphenylphosphino)-
ferrocene]palladium(II) dichloride dichloromethane adduct
(0.180 g, 0.220 mmol) was added thereto, and the mixture was
stirred at 100 C for 3.5 hr. The reaction solution was diluted
with water and ethyl acetate, and the insoluble substance was
removed by filtration. The organic layer was washed with
saturated brine, and dried over sodium sulfate. The sodium
sulfate was removed by filtration, and the filtrate was
concentrated under reduced pressure. The residue was dissolved
in ethyl acetate (10 mL), and 4N-hydrogen chloride ethyl
acetate solution (2 mL) was added thereto. The precipitated
solid was collected by filtration, and dissolved in water (20
mL). To this solution was added saturated aqueous sodium
hydrogencarbonate solution, and the resulting solid was
68
CA 03074989 2020-03-05
, .
collected by filtration, and dried under reduced pressure to
give methyl 5-amino-6-(4-methoxyphenyl)nicotinate (0.448 g,
yield 80%).
1H-NMR (DMSO-D6) 6: 3.80 (3H, s), 3.84 (3H, s), 5.36 (2H, br s),
7.02 (2H, dt, J = 9.5, 2.4 Hz), 7.63-7.68 (3H, m), 8.37 (1H, d,
J = 1.8 Hz).
[0259]
Step 2-2: Methyl 5-bromo-6-(4-methoxyphenyl)nicotinate
[0260]
,0 ,
H3C
H3C0
N N
I
______,
1
/ 0,,,,, / 0,,
I-12N un3 Br , t....113
0 0
[0261]
Under inert gas atmosphere, a solution of methyl 5-amino-
6-(4-methoxyphenyl)nicotinate (0.448 g, 1.74 mmol) in
dibromomethane was stirred at room temperature, and isopentyl
nitrite (0.256 mL, 1.91 mmol) was added thereto. To the
reaction solution was added dropwise a solution of
bromotrimethylsilane (0.249 mL, 1.91 mmol) in dibromomethane,
and the mixture was stirred at room temperature for 2 hr. To
the reaction solution was added saturated aqueous sodium
hydrogencarbonate solution (10 mL), and the mixture was
extracted with chloroform. The organic layer was washed with
saturated brine, and dried over sodium sulfate. The sodium
sulfate was removed by filtration, and the filtrate was
concentrated under reduced pressure. The residue was purified
by silica gel chromatography (hexane:ethyl acetate =6:1 to 4:1)
to give methyl 5-bromo-6-(4-methoxyphenyl)nicotinate (0.308 g,
yield 55%).
[0262]
Step 2-3: Methyl 5-(4-(tert-butoxycarbonyl)pheny1)-6-(4-
methoxyphenyl)nicotinate
[0263]
69
CA 03074989 2020-03-05
,0
OH H3C
H3C-0
OH I
H3C0 = k
k.n3
H3C1
Br CH3 0 0
0 H3C1
CH3 0
[0264]
To a mixture of methyl 5-bromo-6-(4-
methoxyphenyl)nicotinate (0.308 g, 0.956 mmol), (4-(tert-
butoxycarbonyl)phenyl)boric acid (0.425 g, 1.91 mmol) and
potassium phosphate (0.609 g, 2.87 mmol) were added toluene
(4.5 mL) and water (1.5 mL). Under inert gas atmosphere,
[1,1'-bis(diphenylphosphino)-ferrocene]palladium (II) dichloride
dichloromethane adduct (0.078 g, 0.096 mmol) was added thereto,
io and the mixture was stirred at 100 C for 4 hr. The reaction
solution was diluted with water and ethyl acetate, and the
insoluble substance was removed by filtration. The organic
layer was washed with saturated brine, and dried over sodium
sulfate. The sodium sulfate was removed by filtration, and the
15 filtrate was concentrated under reduced pressure. The residue
was purified by silica gel chromatography (hexane:ethyl acetate
=5:1 to 3:1), ethyl acetate (1 mL) and hexane (10 mL) were
added thereto, and the obtained suspension was stirred. The
insoluble substance was collected by filtration, and dried
20 under reduced pressure to give methyl 5-(4-(tert-
butoxycarbonyl)pheny1)-6-(4-methoxyphenyl)nicotinate (0.149 mg,
yield 37%).
1H-NMR (DMSO-D6) 6: 1.53 (9H, s), 3.73 (3H, s), 3.91 (3H, s),
6.85 (2H, dt, J = 9.4, 2.5 Hz), 7.29 (2H, dt, J = 9.4, 2.5 Hz),
25 7.37 (2H, dt, J = 8.4, 1.8 Hz), 7.86 (2H, dt, J = 8.3, 1.8 Hz),
8.17 (1H, d, J = 2.1 Hz), 9.14 (1H, d, J = 2.1 Hz).
[0265]
Step 2-4: 5-(4-(tert-Butoxycarbonyl)pheny1)-6-(4-
methoxyphenyl)nicotinic acid
30 [0266]
CA 03074989 2020-03-05
u
ri3L. H3k,
LN
I OH
0 H3C>(O1J 0
3C
CH3 0 CH3 0
[0267]
Methyl 5-(4-(tert-butoxycarbonyl)pheny1)-6-(4-
methoxyphenyl)nicotinate (137 mg, 0.327 mmol) was dissolved in
methanol (2 mL) and tetrahydrofuran (2 mL). To this solution
was added 4M-aqueous lithium hydroxide solution (0.50 mL, 2.00
mmol), and the mixture was stirred at room temperature for 16
hr. To the reaction solution was added 1M-hydrochloric acid
(2.0 mL), the methanol and tetrahydrofuran were evaporated
under reduced pressure, and the residue was diluted with water
(1 mL). The precipitated solid was collected by filtration,
and dried under reduced pressure to give 5-(4-(tert-
butoxycarbonyl)pheny1)-6-(4-methoxyphenyl)nicotinic acid (56
mg).
/5 [0268]
Step 2-5: tert-Butyl 4-(5-(butylcarbamoy1)-2-(4-
methoxyphenyl)pyridin-3-yl)benzoate
[0269]
H3C rjL
H3C,0
,-' OH + H2N CH3 N CH3
0 0
H3C' I H3C.-- I
CH3 0 CH3 0
[0270]
To a solution of 5-(4-(tert-butoxycarbonyl)pheny1)-6-(4-
methoxyphenyl)nicotinic acid (56 mg, 0.138 mmol) obtained in
the previous step in N,N-dimethylformamide were added
successively butan-l-amine (0.050 mL, 0.51 mmol), triethylamine
(0.060 mL, 0.43 mmol) and HATU (80 mg, 0.21 mmol), and the
mixture was stirred at room temperature for 23 hr. The
reaction solution was diluted with saturated aqueous sodium
71
CA 03074989 2020-03-05
hydrogencarbonate solution and water, and extracted with ethyl
acetate. The organic layer was washed successively with water
and saturated brine, and dried over sodium sulfate. The sodium
sulfate was removed by filtration, and the filtrate was
concentrated under reduced pressure. The residue was purified
by silica gel chromatography (hexane:ethyl acetate =3:1 to 1:1)
to give tert-butyl 4-(5-(butylcarbamoy1)-2-(4-
methoxyphenyl)pyridin-3-yl)benzoate (19 mg, yield 29%).
[0271]
/o Step 2-6: 4-(5-(Butylcarbamoy1)-2-(4-methoxyphenyl)pyridin-3-
yl)benzoic acid hydrochloride
[0272]
H3C,o
H3C,0
N N
I H
I
H
H3C,,,0 0 HO 0
H3C--I HCI
CH3 0 0
[0273]
tert-Butyl 4-(5-(butylcarbamoy1)-2-(4-
methoxyphenyl)pyridin-3-yl)benzoate (18 mg, 0.039 mmol) was
dissolved in trifluoroacetic acid (1.0 mL), and the solution
was stirred at room temperature for 1.5 hr. The reaction
solution was concentrated under reduced pressure, and to the
obtained residue was added 4N-hydrogen chloride ethyl acetate
solution. The resulting precipitate was collected by
filtration, and dried under reduced pressure to give 4-(5-
(butylcarbamoy1)-2-(4-methoxyphenyl)pyridin-3-yl)benzoic acid
hydrochloride (7.9 mg, yield 45%).
1H-NMR (DMSO-D6) 5: 0.91 (3H, t, J = 7.4 Hz), 1.32-1.40 (2H, m),
1.50-1.57 (2H, m), 3.31 (2H, dd, J = 12.8, 6.8 Hz), 3.74 (3H, d,
J = 0.7 Hz), 6.86 (2H, dd, J = 8.9, 2.9 Hz), 7.28 (2H, dd, J =
8.8, 1.6 Hz), 7.39 (2H, d, J = 8.1 Hz), 7.91 (2H, d, J = 8.1
Hz), 8.25 (1H, br s), 8.74 (1H, br s), 9.07 (1H, s).
[0274]
Production Example 3
72
CA 03074989 2020-03-05
Synthesis of 4-(2-(4-methoxypheny1)-5-(3-methoxypropyl)pyridin-
3-yl)benzoic acid (Example 84)
[0275]
H3C,0
0,nu
HO
0
[0276]
Step 3-1: 3-(Benzyloxy)-5-bromo-2-chloropyridine
[0277]
CI N
CI N
I 00 Br
HOBr
1111 OBr
[0278]
io To a solution of 5-bromo-2-chloropyridin-3-ol (50.2 g,
241 mmol) in N,N-dimethylformamide (200 mL) were added
successively benzyl bromide (33.0 mL, 278 mmol) and potassium
carbonate (48.6 g, 352 mmol), and the mixture was stirred at
room temperature for 4 hr. To the reaction solution was added
water (600 mL), and the mixture was stirred for 2 hr. The
precipitate was collected by filtration, and dried under
reduced pressure to give 3-(benzyloxy)-5-bromo-2-chloropyridine
(69.7 g, yield 96%).
1H-NMR (DMSO-D6) 6: 5.29 (2H, s), 7.33-7.47 (5H, m), 7.98 (1H,
d, J = 2.1 Hz), 8.14 (1H, d, J = 1.8 Hz).
[0279]
Step 3-2: (E)-3-(Benzyloxy)-2-chloro-5-(3-methoxyprop-1-en-l-
y1)pyridine
[0280]
CI-N H3C CH3 CIN
,
I H3C ? I
-1"
OW`.'o,CH3
(10 OBr H3C I36 'CH3
[0281]
73
. . CA 03074989 2020-03-05
To a suspension of 3-(benzyloxy)-5-bromo-2-chloropyridine
(25.7 mg, 86.0 mmol) and potassium phosphate (54.9 g, 259 mmol)
in tetrahydrofuran (180 mL) were added successively water (130
mL), (E)-2-(3-methoxyprop-1-en-1-y1)-4,4,5,5-tetramethy1-1,3,2-
dioxaborolane (20.1 mL, 95.0 mmol) and [1,1'-
bis(diphenylphosphino)-ferrocene]palladium(II) dichloride
dichloromethane adduct (3.54 g, 4.33 mmol), and the mixture was
stirred at room temperature for 2 hr. [1,1'-
Bis(diphenylphosphino)-ferrocene]palladium(II) dichloride
dichloromethane adduct (1.70 g, 2.04 mmol) was added again
thereto, and the mixture was stirred at room temperature for 3
hr, warmed to 33 C, and stirred for 1 hr. The reaction
solution was diluted with ethyl acetate (180 mL), and the
insoluble substance was removed by filtration. The organic
/5 layer of the filtrate was washed with water and saturated brine,
silica gel (50 g) was added thereto, and the mixture was
stirred at room temperature for 1 hr. The silica gel was
removed by filtration, and the filtrate was concentrated under
reduced pressure. The residue was purified by silica gel
chromatography (hexane:ethyl acetate =15:1 to 4:3) to give (E)-
3-(benzyloxy)-2-chloro-5-(3-methoxyprop-1-en-1-yl)pyridine
(20.9 g, yield 83%).
1H-NMR (DMSO-D6) 6: 3.29 (3H, s), 4.06-4.07 (2H, m), 5.29 (2H,
s), 6.59-6.60 (2H, m), 7.32-7.49 (5H, m), 7.81 (IH, d, J = 1.8
Hz), 8.02 (1H, d, J = 2.1 Hz).
[0282]
Step 3-3: (E)-3-(Benzyloxy)-2-(4-methoxypheny1)-5-(3-
methoxyprop-1-en-1-yl)pyridine
[0283]
H3C,0
I CI N
+ H3C,o 101 1 N.
. 0 0,
CH3 B_ON
110 0
[0284]
To a solution of (E)-3-(benzyloxy)-2-chloro-5-(3-
74
CA 03074989 2020-03-05
, methoxyprop-1-en-1-y1)pyridine (17.8 g, 61.4 mmol), 4-
methoxyphenylboric acid (11.2 g, 73.7 mmol), palladium(II)
acetate (0.276 g, 1.13 mmol) and 2-dicyclohexylphosphino-2',6'-
dimethoxybiphenyl (1.01 g, 2.46 mmol) in 1,2-dimethoxyethane
(138 mL) was added 2M-aqueous potassium phosphate solution
(46.1 mL, 92.2 mmol), and the mixture was stirred at 50 C for 5
hr. The reaction solution was allowed to cool to room
temperature, water (100 mL) was added thereto, and the mixture
was extracted with ethyl acetate (200 mL). The organic layer
/o was washed successively with water and saturated brine, and
dried over sodium sulfate. The sodium sulfate was removed by
filtration, and the filtrate was concentrated under reduced
pressure. The residue was purified by silica gel
chromatography (hexane:ethyl acetate =3:1 to 1:1) to give (E)-
/5 3-(benzyloxy)-2-(4-methoxypheny1)-5-(3-methoxyprop-1-en-1-
y1)pyridine (23.1 g, yield 104%).
1H-NMR (DMSO-D6) 5: 3.30 (3H, s), 3.77 (3H, s), 4.07-4.09 (2H,
m), 5.26 (2H, s), 6.52-6.66 (2H, m), 6.95 (2H, dt, J = 9.6, 2.5
Hz), 7.30-7.46 (5H, m), 7.72 (1H, d, J = 1.6 Hz), 7.93 (2H, dt,
20 J = 9.6, 2.5 Hz), 8.27 (1H, d, J = 1.6 Hz).
[0285]
Step 3-4: 2-(4-Methoxypheny1)-5-(3-methoxypropyl)pyridin-3-ol
[0286]
u r, 0
I 13v.-- H3C,0
N
, N
I
CH3
25 [0287]
To (E)-3-(benzyloxy)-2-(4-methoxypheny1)-5-(3-
methoxyprop-1-en-1-y1)pyridine (23.1 g) obtained in the
previous step were added methanol (230 mL) and 10% palladium on
carbon catalyst (50% wet, 4.62g), and the mixture was stirred
30 under hydrogen gas atmosphere for 24 hr. The palladium on
carbon catalyst was removed from the reaction solution by
filtration, and the filtrate was concentrated under reduced
CA 03074989 2020-03-05
pressure. To the obtained crude crystals was added ethyl
acetate (50 mL), the mixture was stirred at 80 C for 20 min,
and hexane (150 mL) was added thereto. The mixture was stirred
for additional 2 hr while allowed to cool to room temperature.
The precipitate was collected by filtration, and dried under
reduced pressure to give 2-(4-methoxypheny1)-5-(3-
methoxypropyl)pyridin-3-ol (13.6 g, yield in two step 81%).
1H-NMR (DMSO-D6) 5: 1.74-1.81 (2H, m), 2.54-2.58 (2H, m), 3.23
(3H, s), 3.32 (2H, t, J = 6.4 Hz), 3.77 (3H, s), 6.95 (2H, dt,
/o J = 9.5, 2.5 Hz), 7.09 (1H, d, J = 1.8 Hz), 7.95-7.99 (3H, m),
9.94 (1H, br s).
[0288]
Step 3-5: 2-(4-Methoxypheny1)-5-(3-methoxypropyl)pyridin-3-y1
trifluoromethanesulfonate
[0289]
rs,0
u rt,0 1-13%,
I 13Le
N
N i
i I
_______________________________________ r
/ 0,rsu 0 CH3
F =C)
F>r 'No
F
[0290]
To a solution of 2-(4-methoxypheny1)-5-(3-
methoxypropyl)pyridin-3-ol (13.7 g, 50.0 mmol) in N,N-
dimethylformamide (100 mL) was added potassium carbonate (15.2
g, 110 mmol). The reaction solution was ice-cooled, N-(5-
chloropyridin-2-y1)-1,1,1-trifluoro-N-
((trifluoromethyl)sulfonyl)methanesulfonamide (21.6 g, 55.0
mmol) was added thereto, and the mixture was stirred at room
temperature for 2 hr. The reaction solution was diluted with
water, and extracted with ethyl acetate. The organic layer was
washed successively with water and saturated brine, and dried
over sodium sulfate. The sodium sulfate was removed by
filtration, and the filtrate was concentrated under reduced
pressure. The residue was purified by silica gel
chromatography (hexane:ethyl acetate =4:1 to 1:1) to give 2-(4-
76
, CA 03074989 2020-03-05
.
methoxypheny1)-5-(3-methoxypropyl)pyridin-3-y1
trifluoromethanesulfonate (18.9 g, yield 93%).
1H-NMR (DMSO-D6) 5: 1.82-1.89 (2H, m), 2.73-2.77 (2H, m), 3.23
(3H, s), 3.34 (2H, t, J = 6.4 Hz), 3.81 (3H, s), 7.06 (2H, dt,
J = 9.5, 2.5 Hz), 7.68 (2H, dt, J = 9.6, 2.5 Hz), 7.90 (1H, d,
J = 1.6 Hz), 8.59 (1H, d, J = 1.6 Hz).
[0291]
Step 3-6: Methyl 4-(2-(4-methoxypheny1)-5-(3-
methoxypropyl)pyridin-3-yl)benzoate
/o [0292]
H3C,0
H3µ...
OH
, N 1
1 B, i
OH
õO
0 H3C,0
F 0
[0293]
To a solution of 2-(4-methoxypheny1)-5-(3-
methoxypropyl)pyridin-3-y1 trifluoromethanesulfonate (18.9 g,
/5 46.7 mmol) and (4-(methoxycarbonyl)phenyl)boric acid (10.1 g,
56.1 mmol) in 1,2-dimethoxyethane (105 mL) were added
successively 2M-aqueous potassium phosphate solution (35.0 mL,
70.0 mmol) and [1,1'-bis(diphenylphosphino)-
ferrocene]palladium(II) dichloride dichloromethane adduct
20 (0.763 g, 0.934 mmol), and the mixture was stirred at 80 C for
1 hr. The reaction solution was allowed to cool to room
temperature, and water was added thereto. The mixture was
extracted with ethyl acetate, and the organic layer was dried
over sodium sulfate. The sodium sulfate was removed by
25 filtration, and the filtrate was concentrated under reduced
pressure. To the obtained residue were added ethyl acetate (50
mL) and hexane (150 mL), the mixture was stirred at room
temperature for 30 min, and the insoluble substance was removed
by filtration. The filtrate was concentrated under reduced
30 pressure, and the obtained residue was purified by silica gel
chromatography (hexane:ethyl acetate =3:1 to 1:1) to give
77
CA 03074989 2020-03-05
methyl 4-(2-(4-methoxypheny1)-5-(3-methoxypropyl)pyridin-3-
yl)benzoate (16.9 g, 43.4 mmol).
1H-NMR (DMSO-D6) 6: 1.83-1.90 (2H, m), 2.68-2.73 (2H, m), 3.24
(3H, s), 3.36 (2H, t, J = 6.4 Hz), 3.71 (3H, s), 3.83 (3H, s),
6.79 (2H, dt, J = 9.4, 2.5 Hz), 7.18 (2H, dt, J = 9.4, 2.5 Hz),
7.33 (2H, dt, J = 8.4, 1.8 Hz), 7.64 (1H, d, J = 2.1 Hz), 7.88
(2H, dt, J = 8.5, 1.9 Hz), 8.51 (1H, d, J = 2.1 Hz).
[0294]
Step 3-7: 4-(2-(4-Methoxypheny1)-5-(3-methoxypropyl)pyridin-3-
/0 yl)benzoic acid
[0295]
H3O_0 ,0
H3C
rq
I
0, 0,
CH3 CH3
,0 HO
H3C
0 0
[0296]
To a solution of methyl 4-(2-(4-methoxypheny1)-5-(3-
/5 methoxypropyl)pyridin-3-yl)benzoate (2.00 g, 5.11 mmol) in
methanol (30.6 mL) was added 4N-aqueous sodium hydroxide
solution (7.66 mL, 30.6 mmol), and the mixture was stirred at
50 C for 2 hr. The mixture was allowed to cool to room
temperature, lOwt%-aqueous citric acid solution (23.0 mL) and
20 water (46 mL) were added thereto, and the mixture was stirred
at room temperature for 2 hr. The precipitated solid was
collected by filtration, and dried under reduced pressure to
give 4-(2-(4-methoxypheny1)-5-(3-methoxypropyl)pyridin-3-
yl)benzoic acid (1.66 g, yield 86%).
25 1H-NMR (DMSO-D6) 6: 1.84-1.93 (2H, m), 2.72 (2H, t, J = 7.8 Hz),
3.25 (3H, s), 3.38 (2H, t, J = 6.4 Hz), 3.72 (3H, s), 6.81 (2H,
d, J = 8.8 Hz), 7.20 (2H, d, J = 8.8 Hz), 7.32 (2H, d, J = 8.3
Hz), 7.65 (1H, d, J = 2.2 Hz), 7.87 (2H, d, J = 8.6 Hz), 8.52
(1H, d, J = 2.2 Hz), 12.99 (1H, br s).
30 [0297]
78
A CA 03074989 2020-03-05
,
Step 3-8: Crystals (Form II) of 4-(2-(4-methoxypheny1)-5-(3-
methoxypropyl)pyridin-3-yl)benzoic acid
To 4-(2-(4-methoxypheny1)-5-(3-methoxypropyl)pyridin-3-
yl)benzoic acid (300 mg) was added methyl t-butyl ether (3 mL),
and the mixture was stirred at 70 C to give a solution. While
stirring, the mixture was allowed to cool to room temperature,
and then for 3 days. The precipitated solid was collected by
filtration, and dried under reduced pressure to give crystals
(Form II) of 4-(2-(4-methoxypheny1)-5-(3-methoxypropyl)pyridin-
3-yl)benzoic acid (191 mg, yield 64%).
[0298]
Step 3-9: Crystals (Form X) of 4-(2-(4-methoxypheny1)-5-(3-
methoxypropyl)pyridin-3-yl)benzoic acid
4-(2-(4-Methoxypheny1)-5-(3-methoxypropyl)pyridin-3-
yl)benzoic acid (20 mg) was suspended in methanol (0.12 mL),
and the suspension was stirred at room temperature for 2 weeks.
The resulting solid was collected by filtration, and dried
under reduced pressure to give crystals (Form X) of 4-(2-(4-
methoxypheny1)-5-(3-methoxypropyl)pyridin-3-yl)benzoic acid
(8.3 mg, yield 42%).
[0299]
Step 3-10: Crystals (Form VIII) of 4-(2-(4-methoxypheny1)-5-(3-
methoxypropyl)pyridin-3-yl)benzoic acid
To 4-(2-(4-methoxypheny1)-5-(3-methoxypropyl)pyridin-3-
yl)benzoic acid (600 mg) was added methanol (3.6 mL), and the
mixture was stirred at room temperature for 4 days. To this
mixture was added a trace amount of Form X, and the mixture was
stirred for additional 3 days. The resulting solid was
collected by filtration, and dried under reduced pressure to
give crystals (Form VIII) of 4-(2-(4-methoxypheny1)-5-(3-
methoxypropyl)pyridin-3-yl)benzoic acid.
[0300]
Step 3-11: Crystals (Form XV) of 4-(2-(4-methoxypheny1)-5-(3-
methoxypropyl)pyridin-3-yl)benzoic acid
Form II (15 mg) and Form VIII (15 mg) of 4-(2-(4-
79
CA 03074989 2020-03-05
. .
methoxypheny1)-5-(3-methoxypropyl)pyridin-3-yl)benzoic acid
were suspended in a mixed solvent of 1-propanol (0.24 mL) and
water (0.24 mL), and the suspension was stirred at room
temperature for 11 days. The resulting solid was collected by
filtration, and dried under reduced pressure to give crystals
(Form XV) of 4-(2-(4-methoxypheny1)-5-(3-methoxypropyl)pyridin-
3-yl)benzoic acid.
[0301]
Production Example 4
/o Synthesis of 4-(2-(4-methoxypheny1)-5-(3-methoxypropyl)pyridin-
3-y1)-2-methylbenzoic acid (Example 116)
[0302]
H3C_0
N
I
1/4,113
HO
0
[0303]
Step 4-1: Methyl 4-(2-(4-methoxypheny1)-5-(3-
methoxypropyl)pyridin-3-y1)-2-methylbenzoate
[0304]
0 ,0
H30' H30
N N
1 1
H3C
F ===-0 H3C,0
H3C,0
F>r b
F 0 0
[0305]
To a solution of 2-(4-methoxypheny1)-5-(3-
methoxypropyl)pyridin-3-y1 trifluoromethanesulfonate (2.00 g,
4.93 mmol), which was synthesized by a method similar to that
of Step 3-5 in Production Example 3, and (4-(methoxycarbony1)-
3-methylphenyl)boric acid (1.05 g, 5.43 mmol) in 1,2-
dimethoxyethane (11.1 mL) were added successively 2M-aqueous
potassium phosphate solution (3.70 mL, 7.40 mmol) and [1,1'-
bis(diphenylphosphino)-ferrocene]palladium(II) dichloride
CA 03074989 2020-03-05
A dichloromethane adduct (0.081 g, 0.099 mmol), and the mixture
was stirred at 80 C for 2 hr. The reaction solution was
allowed to cool to room temperature, and water was added
thereto. The mixture was extracted with ethyl acetate, and the
organic layer was dried over sodium sulfate. The sodium
sulfate was removed by filtration, and the filtrate was
concentrated under reduced pressure. The residue was purified
by silica gel chromatography (hexane:ethyl acetate =3:1 to 1:1)
to give methyl 4-(2-(4-methoxypheny1)-5-(3-
methoxypropyl)pyridin-3-y1)-2-methylbenzoate (1.85 g, yield
92%).
1H-NMR (DMSO-D6) 6: 1.83-1.90 (2H, m), 2.47 (3H, s), 2.68-2.72
(2H, m), 3.24 (3H, s), 3.36 (2H, t, J = 6.4 Hz), 3.71 (3H, s),
3.80 (3H, s), 6.80 (2H, dt, J = 9.4, 2.5 Hz), 7.01 (1H, dd, J =
/5 8.1, 1.4 Hz), 7.20 (2H, dt, J = 9.4, 2.5 Hz), 7.26 (1H, d, J =
1.4 Hz), 7.63 (1H, d, J = 2.1 Hz), 7.69 (1H, d, J = 8.1 Hz),
8.49 (1H, d, J = 2.1 Hz).
[0306]
Step 4-2: 4-(2-(4-Methoxypheny1)-5-(3-methoxypropyl)pyridin-3-
y1)-2-methylbenzoic acid
[0307]
_0 ,0
H3C H3C
N N
I I
_____________________________________________ ,
1,113
L.H3
,0 HO
H3C
0 0
[0308]
To a solution of methyl 4-(2-(4-methoxypheny1)-5-(3-
methoxypropyl)pyridin-3-y1)-2-methylbenzoate (2.30 g, 5.67
mmol) in methanol (34 mL) was added 4N-aqueous sodium hydroxide
solution (8.51 mL, 34.0 mmol), and the mixture was stirred at
50 C for 2 hr. The mixture was allowed to cool to room
temperature, lOwt%-aqueous citric acid solution (25.5 mL) and
water (55 mL) were added thereto, and the mixture was stirred
81
ak 03074989 2020-03-05
at room temperature for 1 hr. The precipitated solid was
collected by filtration, and dried under reduced pressure to
give 4-(2-(4-methoxypheny1)-5-(3-methoxypropyl)pyridin-3-y1)-2-
methylbenzoic acid (2.05 g, yield 92%).
1H-NMR (DMSO-D6) 5: 1.81-1.91 (2H, m), 2.47 (3H, s), 2.70 (2H,
t, J = 7.7 Hz), 3.24 (3H, s), 3.36 (2H, t, J = 6.2 Hz), 3.71
(3H, s), 6.81 (2H, d, J = 8.8 Hz), 6.98 (1H, dd, J = 8.1, 1.4
Hz), 7.18-7.24 (3H, m), 7.62 (1H, d, J = 2.3 Hz), 7.69 (1H, d,
J = 8.1 Hz), 8.49 (1H, d, J = 2.3 Hz), 12.81 (1H, br s).
[0309]
Step 4-3: Crystals of 4-(2-(4-methoxypheny1)-5-(3-
methoxypropyl)pyridin-3-y1)-2-methylbenzoic acid
To 4-(2-(4-methoxypheny1)-5-(3-methoxypropyl)pyridin-3-
y1)-2-methylbenzoic acid (50 mg) was added 2-propanol (0.300
mL), and the mixture was stirred at 100 C to give a solution.
The stirring was stopped, and the mixture was allowed to cool
to room temperature. The precipitated solid was collected by
filtration, and dried under reduced pressure to give crystals
of 4-(2-(4-methoxypheny1)-5-(3-methoxypropyl)pyridin-3-y1)-2-
methylbenzoic acid (36 mg, yield 72%).
[0310]
Production Example 5
Synthesis of 4-(2-(4-methoxypheny1)-5-(propoxymethyl)pyridin-3-
yl)benzoic acid (Example 118)
[0311]
u rs,0
I
(CH3
HO
0
[0312]
Step 5-1: (5-Chloro-6-(4-methoxyphenyl)pyridin-3-yl)methanol
[0313]
82
CA 03074989 2020-03-05
=
CI
N o H3C'0
H3C
B
:11;1,0 H ,OH
CI / OH
CI
OH
[0314]
To a mixture of (5,6-dichloropyridin-3-yl)methanol (200
mg, 1.12 mmol) and (4-methoxyphenyl)boric acid (188 mg, 1.24
mmol) was added toluene (5 mL). Under inert gas atmosphere,
[1,1'-bis(diphenylphosphino)-ferrocene]palladium(II) dichloride
dichloromethane adduct (45.9 mg, 0.056 mmol) and 2M-aqueous
potassium phosphate solution (1.12 mL, 2.24 mmol) were added
successively thereto, and the mixture was stirred at 70 C for 1
hr. The mixture was allowed to cool to room temperature, and
the reaction solution was diluted with water and ethyl acetate,
and separated. The organic layer was washed successively with
water and saturated brine, and dried over sodium sulfate. The
sodium sulfate was removed by filtration, and the filtrate was
/5 concentrated under reduced pressure. The residue was purified
by silica gel chromatography (hexane:ethyl acetate =2:1 to 1:2)
to give (5-chloro-6-(4-methoxyphenyl)pyridin-3-yl)methanol (250
mg, yield 89%).
[0315]
Step 5-2: 3-Chloro-2-(4-methoxypheny1)-5-
(propoxymethyl)pyridine
[0316]
H3L.
,,0
H3C,0
, ,
+ B H3
OHCH3
CI
[0317]
Under inert gas atmosphere, to a suspension of sodium
hydride (44.0 mg, 1.10 mmol) in tetrahydrofuran (5 mL) were
added successively 1-bromopropane (0.455 mL, 5.01 mmol) and a
solution of (5-chloro-6-(4-methoxyphenyl)pyridin-3-yl)methanol
(250 mg, 1.00 mmol) in tetrahydrofuran (3 mL), and the mixture
was stirred at 100 C for 24 hr. The reaction solution was
83
CA 03074989 2020-03-05
. .
allowed to cool to room temperature, diluted with water (20 mL),
and extracted with ethyl acetate (20 mL). The organic layer
was washed successively with water and saturated brine, and
dried over sodium sulfate. The sodium sulfate was removed by
filtration, and the filtrate was concentrated under reduced
pressure. The residue was purified by silica gel
chromatography (hexane:ethyl acetate =3:1 to 1:1) to give 3-
chloro-2-(4-methoxypheny1)-5-(propoxymethyl)pyridine (123 mg,
yield 42%).
/o [0318]
Step 5-3: Methyl 4-(2-(4-methoxypheny1)-5-
(propoxymethyl)pyridin-3-yl)benzoate
[0319]
H3C,0 OH H3C
BI,
N INI OH N
,
, 1 ,
1 H3C + -
....-
0..õCH3
CI %,n3 0
H3C,0
0
/5 [0320]
Under inert gas atmosphere, to a mixture of 3-chloro-2-
(4-methoxypheny1)-5-(propoxymethyl)pyridine (50.0 mg, 0.171
mmol), palladium(II) acetate (3.9 mg, 0.017 mmol), 2-
dicyclohexylphosphino-2',6'-dimethoxybiphenyl (14.1 mg, 0.034
20 mmol) and (4-(methoxycarbonyl)phenyl)boric acid (93.0 mg, 0.514
mmol) were added successively toluene (2 mL) and 2M-aqueous
potassium phosphate solution (0.343 mL, 0.685 mmol), and the
mixture was stirred at 100 C for 2 hr. The reaction solution
was allowed to cool to room temperature, diluted with water (10
25 mL), and extracted with ethyl acetate (50 mL). The organic
layer was washed successively with water and saturated brine,
and dried over sodium sulfate. The sodium sulfate was removed
by filtration, and the filtrate was concentrated under reduced
pressure. The residue was purified by silica gel
30 chromatography (hexane:ethyl acetate =2:1 to 1:2) to give
methyl 4-(2-(4-methoxypheny1)-5-(propoxymethyl)pyridin-3-
84
CA 03074989 2020-03-05
yl)benzoate (47.4 mg, yield 70%).
[0321]
Step 5-4: 4-(2-(4-Methoxypheny1)-5-(propoxymethyl)pyridin-3-
yl)benzoic acid
[0322]
Hr,,0 H3s, ,õ0
31/4,
CH3
u ,0 HO
0 0
[0323]
To a solution of methyl 4-(2-(4-methoxypheny1)-5-
(propoxymethyl)pyridin-3-yl)benzoate (47.4 mg, 0.121 mmol) in
/0 methanol (0.90 mL) was added 4N-aqueous sodium hydroxide
solution (0.182 mL, 0.726 mmol), and the mixture was stirred at
50 C for 2 hr. The reaction solution was allowed to cool to
room temperature, lOwt%-aqueous citric acid solution (0.546 mL)
and water (2 mL) were added thereto, and the mixture was
/5 stirred. The precipitated solid was collected by filtration,
and dried under reduced pressure to give 4-(2-(4-
methoxypheny1)-5-(propoxymethyl)pyridin-3-yl)benzoic acid (43.5
mg, yield 95%).
1H-NMR (DMSO-D6) 6: 0.88 (3H, t, J = 7.4 Hz), 1.51-1.62 (2H, m),
20 3.45 (2H, t, J = 6.6 Hz), 3.71 (3H, s), 4.57 (2H, s), 6.81 (2H,
d, J = 8.8 Hz), 7.21 (2H, d, J = 8.8 Hz), 7.30 (2H, d, J = 8.3
Hz), 7.71 (1H, d, J = 2.1 Hz), 7.86 (2H, d, J = 8.6 Hz), 8.60
(1H, d, J = 2.1 Hz), 13.01 (1H, br s).
[0324]
25 Production Example 6
Alternative production method of 4-(3-(3,5-difluoro-4-
methoxypheny1)-6-(3-methoxypropyl)pyrazin-2-yl)benzoic acid
(Example 67)
[0325]
CA 03074989 2020-03-05
,0
H3C
I
NC)I'CH3
HO
0
[0326]
Step 6-1: 2-(tert-Butoxy)-3-chloropyrazine
[0327]
CH3
CI, _NI
2)
H3 _C I CH 3
0 N
CKMµi
[0328]
Under nitrogen atmosphere, 2,3-dichloropyrazine (150.0 g,
1007 mmol) was dissolved in tetrahydrofuran (450 mL), and a
solution of potassium tert-butoxide (129.9 g, 1158 mmol) in
/o tetrahydrofuran (600 mL) was added dropwise thereto under ice-
cooling from a dropping funnel. The dropping funnel was washed
with tetrahydrofuran (150 mL), and the wash solution was added
dropwise to the reaction mixture. The reaction mixture was
stirred under ice-cooling for 1 hr, water (450 mL) was added
/5 thereto, and the mixture was separated. The organic layer was
washed with 10% brine to give a solution of 2-(tert-butoxy)-3-
chloropyrazine in tetrahydrofuran. The obtained solution of 2-
(tert-butoxy)-3-chloropyrazine in tetrahydrofuran was used in
the next step, regarded as yield 100%.
20 The solution of 2-(tert-butoxy)-3-chloropyrazine in
tetrahydrofuran was synthesized by the same production method,
and concentrated, and the NMR was measured.
1H-NMR (DMSO-D6) 5: 1.60 (9H, s), 8.01 (1H, d, J = 2.8 Hz),
8.19 (1H, d, J = 2.8 Hz).
25 [0329]
Step 6-2: Ethyl 4-(3-(tert-butoxy)pyrazin-2-yl)benzoate
[0330]
86
CA 03074989 2020-03-05
CH3
H
CH3 OH 3C+CH3
H3C CH3 0 N)
'OH ______________________________________________________ ,
0 N
CIN 0 H3C 0
yLJ
[0331]
Under nitrogen atmosphere, to 2-(tert-butoxy)-3-
chloropyrazine in tetrahydrofuran solution (corresponding to
1007 mmol) were added (4-(ethoxycarbonyl)phenyl)boric acid
(195.3 g, 1007 mmol) and tetrahydrofuran (150 mL). Then, 2-
dicyclohexylphosphino-2',6'-dimethoxybiphenyl (8.27 g, 20.1
mmol) and palladium(II) acetate (2.26 g, 10.1 mmol) were added
thereto. The reaction system was degassed under reduced
lo pressure, and replaced with nitrogen. The procedure was
repeated three times in total. To this mixture was added
dropwise a solution of tripotassium phosphate (363.3 g, 1712
mmol) in water (600 mL) over about 30 min at 40 C. The
reaction mixture was stirred at the same temperature for about
1 hr, allowed to cool, and separated. The organic layer was
washed twice with 10% brine (600 mL). To the organic layer was
added activated carbon (15.00 g), and the mixture was stirred
at room temperature for 2 hr. The activated carbon was removed
by filtration, and washed with tetrahydrofuran (450 mL). The
combined filtrate was concentrated under reduced pressure until
the volume became 400 mL to give a solution of ethyl 4-(3-
(tert-butoxy)pyrazin-2-yl)benzoate in tetrahydrofuran. The
obtained solution of ethyl 4-(3-(tert-butoxy)pyrazin-2-
yl)benzoate in tetrahydrofuran was used in the next step,
regarded as yield 100%.
The solution of ethyl 4-(3-(tert-butoxy)pyrazin-2-
yl)benzoate in tetrahydrofuran was synthesized by the same
production method, and concentrated to dryness, and the solid
was collected by filtration with a mixed solvent of
ethanol/water (2/1), and the NMR was measured.
1H-NMR (DMSO-D6) 5: 1.35 (3H, t, J = 7.1 Hz), 1.62 (9H, s),
87
CA 03074989 2020-03-05
. .
4.35 (2H, q, J = 7.1 Hz), 8.05 (2H, dt, J = 6.8, 2.0 Hz), 8.14
(2H, dt, J = 6.8, 2.0 Hz), 8.22 (1H, d, J = 2.5 Hz), 8.31 (1H,
d, J = 2.5 Hz).
[0332]
Step 6-3: Ethyl 4-(3-hydroxypyrazin-2-yl)benzoate
[0333]
CH3
H3C,CH3 HO N
0 N I )
N H3C0
H3C 0 0
0
[0334]
Under nitrogen atmosphere, to ethyl 4-(3-(tert-
/0 butoxy)pyrazin-2-yl)benzoate in tetrahydrofuran solution
(corresponding to 1007 mmol) was added ethanol (300 mL), and
then 4N hydrochloric acid (300 mL, 1200 mmol) was added
dropwise thereto at room temperature, and the mixture was
stirred for about 1 hr. To the reaction suspension was added
/5 water (750 mL), and the mixture was stirred at room temperature
for 1 hr. Water (750 mL) was added again thereto, and the
mixture was stirred at room temperature for 2 hr. The
precipitated solid was collected by filtration, and the
obtained solid was washed twice with a mixed solvent of
20 water/ethanol (4/1, 300 mL), and dried under reduced pressure
at 60 C to give ethyl 4-(3-hydroxypyrazin-2-yl)benzoate (232.1
g, 950.6 mmol, yield 94.4% from 2,3-dichloropyrazine).
1H-NMR (DMSO-D6) 5: 1.34 (3H, t, J = 7.1 Hz), 4.34 (2H, q, J =
7.1 Hz), 7.53 (1H, d, J = 3.7 Hz), 7.55 (1H, d, J = 3.7 Hz),
25 8.02 (2H, dt, J = 8.6, 1.8 Hz), 8.46 (2H, dt, J = 8.6, 1.8 Hz),
12.67 (1H, s).
[0335]
Step 6-4: Ethyl 4-(3-hydroxy-6-iodopyrazin-2-yl)benzoate
[0336]
88
CA 03074989 2020-03-05
. a
HO N HO N
I ) ,
I
N ___________ y N I
H3C0 H3C 0
yLJ
0 0
[0337]
Under nitrogen atmosphere, to ethyl 4-(3-hydroxypyrazin-
2-yl)benzoate (100 g, 409mmo1) was added acetonitrile (500 mL),
and then, 1,8-diazabicyclo[5.4.0]undec-7-ene (31.2 g, 205 mmol)
was added thereto. To this mixture was added dropwise a
solution of N-iodosuccinimide (101 g, 450 mmol) in acetonitrile
(750 mL) over about 1 hr at room temperature from a dropping
funnel. The dropping funnel was washed with acetonitrile (50
/o mL), the wash solution was added dropwise to the reaction
mixture, and the mixture was stirred at room temperature for
about 2 hr. To the reaction suspension was added dropwise a
solution of sodium sulfite (12.4 g, 123 mmol) in water (600 mL),
and the mixture was stirred for 20 min. Then, a solution of
/5 conc. hydrochloric acid (21.3 g, 205 mmol) in water (600 mL)
was added dropwise thereto, and the mixture was stirred at 45
to 55 C for 30 min, and then at room temperature for about 30
min. The precipitated solid was collected by filtration, and
the obtained solid was washed twice with a mixed solvent of
20 acetonitrile/water (1/2, 300 mL), and dried under reduced
pressure at 50 C to give ethyl 4-(3-hydroxy-6-iodopyrazin-2-
yl)benzoate (135 g, yield 89.3%).
1H-NMR (DMSO-D6) 5: 1.34 (3H, t, J = 7.1 Hz), 4.35 (2H, q, J =
7.1 Hz), 7.98 (1H, hr s), 8.03 (2H, d, J = 8.6 Hz), 8.35 (2H, d,
25 J = 8.6 Hz), 12.85 (1H, s).
[0338]
Step 6-5: Ethyl 4-(3-hydroxy-6-(3-methoxyprop-1-yn-1-
y1)pyrazin-2-y1)benzoate
[0339]
89
CA 03074989 2020-03-05
HO N HO N
N I _______
H3C 0 H3C 0 CH3
0 0
[0340]
Under nitrogen atmosphere, to ethyl 4-(3-hydroxy-6-
iodopyrazin-2-yl)benzoate (200 g, 540 mmol) was added
acetonitrile (1200 mL), and triethylamine (164 g, 1621 mmol)
was added thereto, and then, copper(I) iodide (4.12 g, 21.6
mmol), triphenylphosphine (2.83 g, 10.8mmol) and
bis(triphenylphosphine)palladium(II) dichloride (3.79 g, 5.40
mmol) were added thereto. The reaction system was degassed
/o under reduced pressure, and replaced with nitrogen. The
procedure was repeated three times in total. To this mixture
was added dropwise a solution of methylpropargyl ether (56.8 g,
810 mmol) in acetonitrile (200 mL) over about 1 hr at 40 C, and
the mixture was stirred at the same temperature for about 2 hr.
/5 To the reaction mixture was added acetonitrile (600 mL), and
the mixture was concentrated under reduced pressure until the
volume became 1000 mL. To the residue was added dropwise
acetic acid (64.89 g, 1081 mmol) at 40 C, and the mixture was
stirred at the same temperature for 1 hr, and then at room
20 temperature for an additional 1 hr. The precipitated solid was
collected by filtration, and washed with acetonitrile (400 mL).
The obtained solid was suspended in acetonitrile (1600 mL), and
the suspension was stirred at 70 C for 1 hr, and then at room
temperature for 10 hr. The resulting solid was collected by
25 filtration, washed twice with acetonitrile (400 mL), and dried
under reduced pressure at 50 C to give ethyl 4-(3-hydroxy-6-(3-
methoxyprop-1-yn-1-y1)pyrazin-2-y1)benzoate (121 g, yield
71.7%).
1H-NMR (DMSO-D6) 5: 1.34 (3H, t, J = 7.2 Hz), 3.34 (3H, s),
30 4.35 (4H, q, J = 7.2 Hz), 7.85 (1H, s), 8.03 (2H, d, J = 8.6
Hz), 8.40 (2H, d, J = 8.6 Hz), 12.96 (1H, s).
[0341]
= CA 03074989 2020-03-05
Step 6-6: Ethyl 4-(3-hydroxy-6-(3-methoxypropyl)pyrazin-2-
yl)benzoate
[0342]
HO rsi, HO N
,
H3CO N0
I ,
CH3 H3C0
0 0
[0343]
Under nitrogen atmosphere, to ethyl 4-(3-hydroxy-6-(3-
methoxyprop-1-yn-1-y1)pyrazin-2-y1)benzoate (30.0 g, 96.1 mmol)
was added tetrahydrofuran (360 mL), and 5% palladium on carbon
catalyst (50% wet, 1.50 g) was added thereto. The reaction
/o system was replaced with hydrogen, and the mixture was stirred
under 0.2 MPa of hydrogen pressure for 4 hr. The 5% palladium
on carbon catalyst was removed by filtration, and washed with
tetrahydrofuran (120 mL), and the combined filtrate was
concentrated under reduced pressure until the volume became 150
/5 mL. To the residue was added dropwise heptane (120 mL) at 40 C,
and the mixture was stirred at the same temperature for 10 min.
Heptane (480 mL) was added dropwise thereto at 45 C, and the
mixture was stirred at the same temperature for 30 min, and
then at room temperature for 30 min. The precipitated solid
20 was collected by filtration, and the obtained solid was washed
with heptane (150 mL), and dried under reduced pressure at 50 C
to give ethyl 4-(3-hydroxy-6-(3-methoxypropyl)pyrazin-2-
yl)benzoate (28.0 g, yield 92.1%).
1H-NMR (DMSO-D6) 5: 1.34 (3H, t, J = 6.9 Hz), 1.84-1.91 (2H, m),
25 2.61 (2H, t, J = 7.6 Hz), 3.24 (3H, s), 3.38 (2H, t, J = 6.4
Hz), 4.34 (2H, q, J = 6.9 Hz), 7.37 (1H, s), 8.02 (2H, d, J =
8.6 Hz), 8.47 (2H, d, J = 8.6 Hz), 12.49 (1H, s).
[0344]
Step 6-7: Ethyl 4-(6-(3-methoxypropy1)-3-
30 (((trifluoromethyl)sulfonyl)oxy)pyrazin-2-yl)benzoate
[0345]
91
= CA 03074989 2020-03-05
HO N F*9_0
F S'
N
H3C
0
0
[0346]
Under nitrogen atmosphere, to a solution of dipotassium
hydrogenphosphate (42.1 g, 242 mmol) in water (85 mL) was added
toluene (153 mL), and ethyl 4-(3-hydroxy-6-(3-
methoxypropyl)pyrazin-2-yl)benzoate (17.0 g, 53.7 mmol) was
added thereto. To this mixture was added dropwise
trifluoromethanesulfonic anhydride (22.7 g, 80.5 mmol) over I
hr at 5 to 10 C, and the mixture was stirred at the same
lo temperature for 1 hr. The reaction mixture was separated, and
the organic layer was washed with 20% brine (68 g) to give a
solution of ethyl 4-(6-(3-methoxypropy1)-3-
(((trifluoromethyl)sulfonyl)oxy)pyrazin-2-yl)benzoate in
toluene. The obtained solution of ethyl 4-(6-(3-
/5 methoxypropy1)-3-(((trifluoromethyl)sulfonyl)oxy)pyrazin-2-
yl)benzoate in toluene was used in the next step, regarded as
yield 100%.
The solution of ethyl 4-(6-(3-methoxypropy1)-3-
(((trifluoromethyl)sulfonyl)oxy)pyrazin-2-yl)benzoate in
20 toluene was synthesized by the same production method, and
concentrated to dryness, and the residue was purified by silica
gel chromatography (hexane:ethyl acetate =4:1), and the NMR was
measured.
1H-NMR (DMSO-D6) 5: 1.36 (3H, t, J = 7.2 Hz), 1.97-2.04 (2H, m),
25 3.00 (2H, t, J = 7.7 Hz), 3.23 (3H, s), 3.41 (2H, t, J = 6.2
Hz), 4.37 (2H, q, J = 7.2 Hz), 8.00 (2H, dt, J = 8.6, 1.8 Hz),
8.15 (2H, dt, J = 8.6, 1.8 Hz), 8.53 (IH, s).
[0347]
Step 6-8: Ethyl 4-(3-(3,5-difluoro-4-methoxypheny1)-6-(3-
30 methoxypropyl)pyrazin-2-yl)benzoate
92
= CA 03074989 2020-03-05
[0348]
F>LO.,0
H3C-0
F
0 N
N CH : H3C0=
H3C0 irOH
OH H3C 0o,cF13
0 0
[0349]
Under nitrogen atmosphere, to a solution of ethyl 4-(6-
(3-methoxypropy1)-3-(((trifluoromethyl)sulfonyl)oxy)pyrazin-2-
yl)benzoate in toluene (corresponding to 53.7 mmol) was added
tetrahydrofuran (34 mL), and (3,5-difluoro-4-
methoxyphenyl)boric acid (12.1 g, 64.5 mmol) was added thereto,
and the used container was washed with tetrahydrofuran (17 mL).
/o Bis(triphenylphosphine)palladium(II) dichloride (0.377 g, 0.537
mmol) was added thereto, and the reaction system was degassed
under reduced pressure, and replaced with nitrogen. To this
mixture was added dropwise a solution of tripotassium phosphate
(13.7 g, 64.5 mmol) in water (65 mL) over 2 hr at 70 C, and the
mixture was stirred at the same temperature for 2 hr. The
reaction mixture was separated, and the organic layer was
washed twice with 20% brine (68 g), and concentrated under
reduced pressure until the volume became 68 mL. To the residue
was added toluene until the volume became 136 mL, activated
carbon (3.4 g) and metal scavenger (Fuji Silysia Chemical Ltd,
SCAVENGER SH SILICA, 1.0 g) were added thereto, and the mixture
was stirred at room temperature for 2 hr. The activated carbon
and metal scavenger were removed by filtration, and washed with
toluene (51 mL). The combined filtrate was concentrated under
reduced pressure, to the residue was added 2-propanol (102 mL),
and the mixture was concentrated under reduced pressure. The
procedure was repeated twice in total. To the residue was
added 2-propanol until the volume became 85 mL, and the mixture
was stirred at room temperature for 1 hr, and then under ice-
cooling for 2 hr. The precipitated solid was collected by
filtration, washed with cooled 2-propanol (51 mL), and dried
under reduced pressure at 50 C to give ethyl 4-(3-(3,5-
93
CA 03074989 2020-03-05
difluoro-4-methoxypheny1)-6-(3-methoxypropyl)pyrazin-2-
yl)benzoate (21.9 g, yield 92.1%).
1H-NMR (DMSO-D6) 5: 1.33 (3H, t, J = 7.1 Hz), 1.96-2.03 (2H, m),
2.91-2.95 (2H, m), 3.25 (3H, s), 3.42 (2H, t, J = 6.4 Hz), 3.95
(3H, s), 4.33 (2H, q, J = 7.1 Hz), 7.08-7.14 (2H, m), 7.57 (2H,
dt, J = 8.5, 1.8 Hz), 7.96 (2H, dt, J = 8.5, 1.8 Hz), 8.66 (1H,
s).
[0350]
Step 6-9: 4-(3-(3,5-Difluoro-4-methoxypheny1)-6-(3-
/0 methoxypropyl)pyrazin-2-yl)benzoic acid
[0351]
u
,
N(:)CH3 I
H3C 0 HO
0 0
[0352]
Under nitrogen atmosphere, to ethyl 4-(3-(3,5-difluoro-4-
methoxypheny1)-6-(3-methoxypropyl)pyrazin-2-yl)benzoate (5.0 g,
11.3 mmol) was added ethanol (15 mL), and 2N aqueous sodium
hydroxide solution (7.5 mL, 15.0 mmol) was added thereto, and
the mixture was stirred at 40 C for 1.5 hr. The reaction
mixture was filtered through 0.45 pM membrane filter, and
washed with a mixed solvent of ethanol/water (3/1.4, 22 mL).
To the combined filtrate was added dropwise 3N hydrochloric
acid (5.5 mL, 16.5 mmol) at room temperature, and the mixture
was stirred at room temperature for 0.5 hr. To this mixture
was added dropwise water (10 mL) at 35 C, and the mixture was
stirred at the same temperature for 30 min, and then at room
temperature for about 2 hr. The precipitated solid was
collected by filtration, and the obtained solid was washed
successively with a mixed solvent of ethanol/water (1/2, 22.5
mL) and water (30 mL), and dried under reduced pressure at 50 C
to give 4-(3-(3,5-difluoro-4-methoxypheny1)-6-(3-
94
. .1 CA 03074989 2020-03-05
methoxypropyl)pyrazin-2-yl)benzoic acid (4.45 g, yield 95.1%).
1H-NMR (DMSO-D6) 6: 1.96-2.03 (2H, m), 2.93 (2H, t, J = 7.7 Hz),
3.25 (3H, s), 3.42 (2H, t, J = 6.4 Hz), 3.95 (3H, s), 7.07-7.14
(2H, m), 7.54 (2H, d, J = 8.3 Hz), 7.94 (2H, d, J = 8.3 Hz),
8.66 (1H, s), 13.11 (1H, s).
[0353]
The compounds of the other Examples were obtained
according to the above-mentioned general production methods or
by a method similar to that of the Production Example, using
lo the other known methods as necessary. The structural formulas
and property data of the compounds of Examples 1 to 153 are
shown in the following Table 1-1 to Table 1-20. The MS value
marked with "-COOH" in the tables means a value of fragment
after decarboxylation.
95
a CA 03074989 2020-03-05
[0354]
Table 1-1
MS MS
Ex. Structure NMR
(M+H) (M-H)
H3C-0
1H-NMR (DMS0-06) 8: 0.92 (31-1, t, J = 7.4 Hz), 1.38 (2H, td, J
= 14.8,7.5 Hz), 1.68-1.77 (2H, m), 2.65(211, J = 7.7 Hz), 3.74
1N1\//N,/^U
(3H, s), 6.87 (2H, dt, J = 9.4,2.5 Hz), 7.29 (211, dl. J = 9.4,2.5 363
361
Hz), 7.49 (2H, dl, J = 8.4,1.8 Hz), 7.88 (2H, dt, J = 8.5, 1.8 Hz),
HO
8.59 (1H, t, J = 4.9 Hz), 13.01 (1H, br s).
0
H3c-0 CH3
1H-NMR (DMSO-D6) 8:0.93 (3H, t, J = 7.3 Hz), 1.35-1.44
(2H, m), 1.71-1.79(211, m), 1.92(311, s), 2.88 (2H, t, J = 7.7
2 cii, Hz), 3.73 (3H, s), 6.72 (1H, dd, J =
8.3, 2.5 Hz), 6.76(111, d, J = 377 375
2.5 Hz), 7.04(111, d, J = 8.6 Hz), 7.42 (2H, dd, J = 6.7, 1.8 Hz),
Ho
7.81 (211, dd, J = 6.7, 1.8 Hz), 8.61 (1H, s), 13.00 (1H, br s).
0
LJL.0
N 1H-NMR (DMSO-D6) 8: 0.93 (31-I, t, J =
7.3 Hz), 1.34-1.43
N
I (2H, m), 1.70-1.78 (211, m), 2.88
(211, t, .1= 7.7 Hz), 3.76 (3H,
3 CH3 s), 6.71 (111, dd, J = 12.3,2.5 Hz),
6.87 (1H, dd, J = 8.7,2.4 381 379
HO,y,LsjJ Hz), 7.43-7.51 (3H, m), 7.84 (2H, dd,
J = 6.7, 1.8 Hz), 8.64 (111,
s), 13.04(111, br s).
0
H3c-0
1H-NMR (DMSO-D6) 8:0.90 (3H, t, J = 7.3 Hz), 1.35 (211, td, J
= 14.9, 7.4 Hz), 1.70 (2H, dl, J = 15.8, 7.0 Hz), 1.98 (3H, s),
4 NCH 3 2.84 (2H, t, J = 7.6 Hz), 3.70 (3H,
s), 6.78-6.84 (2H, m), 7.21- 377 375
7.29 (3H, m), 7.74 (1H, dd, J = 7.9, 1.2 Hz), 7.78 (1H, s), 8.62
Ho
CH3 (1H, s), 12.97(111, br s).
0
H3C'0
1H-NMR (DMSO-D6) 5: 0.91 (3H, t, J = 7.4 Hz), 1.31-1.43
(2H, m), 1.65-1.76 (2H, m), 2.85 (2H, t, J = 7.7 Hz), 3.72 (3H,
NN CH3 s), 6.85(211, d, J = 8.6 Hz), 7.29(2H, d, J = 8.6 Hz), 7.56
(1H, 381 379
dd, J = 10.4, 1.4 Hz), 7.68(111, t, J = 7.6 Hz), 7.85(111, dd, J =
HO
7.9, 1.2 Hz), 6.66(111, s), 13.37(111, br s).
0
H3C
1H-NMR (DMSO-D6) 8:0.93 (311, t, J = 7.3 Hz), 1.32-1.45
(2H, m), 1.67-1.79 (2H, m), 2.85 (2H, t., J = 7.6 Hz), 3.58 (3H,
C3
6 H s), 3.74 (3H, s), 6.90 (2H, dl, J =
9.4, 2.5 Hz), 7.00-7.06 (2H, 393 391
HO m), 7.32(211, dl, J = 9.3,2.5 Hz),
7.57(11-I, d, J = 7.9 Hz), 8.58
(1H, s), 12.66 (1H, br s).
0 0,
CH,
0
HO
1H-NMR (DMSO-D6) 8:0.93 (3H, t, J = 7.4 Hz), 1.34-1.45
7 HO (2H, m), 1.69-1.80 (2H, m), 2.89 (2H,
t, J = 7.6 Hz), 7.45-7.50 377 375
CH3
(4H, m), 7.84-7.89 (4H, m), 8.67(111, s), 13.03(2H, br s).
0
H3C-0
1H-NMR (DMSO-D6) 8:0.92 (3H, t, J = 7.4 Hz), 1.32-1.43
H3C (2H, m), 1.67-1.77 (2H, m), 2.07 (3H,
s), 2.84 (2H, t, J = 7.7
8 NNC H3 Hz), 3.75 (3H, s), 6.81 (11-I, d, J =
8.6 Hz), 6.99-7.07 (111, m), 377 375
7.28 (1H, dd, J = 2.3, 0.7 Hz), 7.49 (2H, dl, J = 8.4, 1.8 Hz),
HO
7.88 (2H, dl, J = 8.4, 1.7 Hz), 8.57 (1H, s), 13.02 (1H, br s).
0
96
CA 03074989 2020-03-05
[0355]
Table 1-2
MS MS
Ex Structure NMR
(M+H) (M-K)
N
1H-NMR (DMSO-06) 6:0.92 (3H, t, J = 7.4 Hz), 1.33-1.45
9 (2H, m), 1.69-1.79 (2H, m), 2.89 (21-1, t, J = 7.7
Hz), 7.48 (2H, d,
358 356
J = 8.6 Hz), 7.54 (2H, d, J = 8.1 Hz), 7.80 (21i, d, J = 8.11-14
HoJL7.88 (2H, d, J = 8.6 Hz), 8.69 (1H, s), 13.08 (1H, s).
0
1H-NMR (DMSO-D6) 6:0.92 (3H, J = 7.3 Hz), 1.33-1.44
(2H, m), 1.68-1.78 (2H, m), 2.87 (2H, t, J = 7.7 Hz), 7.16 (2H, t,
CH3
J = 8.9 Hz), 7.39(2K, dd, J = 8.8, 5.5 Hz), 7.48 (2H, d, J = 8.6 351 349
HO Hz), 7.88 (2H, d, J = 8.6 Hz), 8.63 (1H, s), 13.05 (1H, br s).
0
H3C'0
1H-NMR (DMSO-06) 6:0.92 (3H, t, J = 7.3 Hz), 1.31-1.44
(2H, m), 1.66-1.78 (2H, m), 2.86 (2H, t, J = 7.7 Hz), 3.82 (3H,
11 cH3 s), 7.03-7.12(2K, m), 7.19-7.26(1K, m), 7.51 (2H,
d, J = 8.6 381 379
HO Hz), 7.90 (2H, d, J = 8.6 Hz), 8.61 (1H, s), 13.05
(1H, br s).
0
0
H3C' 1H-NMR (DMS0-06) 6: 1.61-1.68 (2H, m), 1.69-1.76
(2H, m),
N, 2.31 (2H, t, J = 6.2 Hz), 2.80 (2H, t, J = 6.1 Hz),
3.66 (3H, s),
12 6.70 (2H, dl, J = 9.4, 2.5 Hz), 7.09 (2H, dt, J =
9.5, 2.5 Hz), 7.21 360 358
(2H, dl, J = 8.2, 1.8 Hz), 7.86 (21-I, dl, J = 8.3, 1.8 Hz), 8.36 (1H,
HO s), 12.97 (1H, br s).
1H-NMR (DMSO-D6) 6: 0.93 (3H, t, J = 7.3 Hz), 1.34-1.45
(2H, m), 1.69-1.80 (2H, m), 2.89 (2H, t, J = 7.7 Hz), 7.49 (2H, d,
13 401 399
NN cH3 J = 8.6 Hz), 7.58 (2H, d, J = 8.1 Hz), 7.69 (21-1,
d, J = 8.8 Hz),
7.89 (2H, d, J = 8.6 Hz), 8.69 (1H, s), 13.09 (1H, br s).
Ho
0
H3c-
, 1H-NMR (DMS0-06) 6:0.92 (3H, t, J = 7.3 Hz), 1.32-
1.44
14 (2H, m), 1.67-1.77 (2H, m), 2.85 (2H, t, J = 7.6
Hz), 3.75 (3H,
381 379
s), 6.90(2K, d, J = 8.8 Hz), 7.22-7.35 (4H, m), 7.78(1K, t, J =
HO 7.9 Hz), 8.61 (1H, s), 13.32 (1H, br s).
F
H3C'0
HO 1H-NMR (DMSO-D6) 6:0.92 (3H, t, J = 7.4 Hz), 1.33-
1.44
(2H, m), 1.67-1.78 (2H, m), 2.86 (2H, t, J = 7.7 Hz), 3.79 (3H,
0 NN cH3 s), 7.02 (1H, d, J = 9.0 Hz), 7.37(1K, dd, J = 8.7,2.4
Hz), 7.51 407 405
HO d, J = 8.6 Hz), 7.78 (1H, d, J = 2.5 Hz),
7.89(2K, d, J = 8.6
Hz), 8.61 (1H, s), 12.81 (1H, br s).
0
,0
H,C
1H-NMR (DMSO-D6) 6: 0.92 (3H, t, J = 7.4 Hz), 1.31-1.45
CI (2H, m), 1.66-1.78 (2H, m), 2.86(2K, t J = 7.7 Hz),
3.83 (3H,
16 cH3 s), 7.05 (1H, d, J = 8.8 Hz), 7.19 (1H, dd, J =
8.6,2.1 Hz), 7.45- 397 395
Ho 7.54 (3H, m), 7.90 (2H, d, J = 8.6 Hz), 8.61 (1H,
s), 13.09 (1H,
br s).
0
97
J CA 03074989 2020-03-05
[0356]
Table 1-3
MS MS
Ex Structure NMR
(M+H) (M-I-
1)
H3C'0
1H-NMR (DMSO-D6) 8:0.92 (3H, t, J = 7.4 Hz), 1.33-1.44
(2H, m), 1.68-1.77 (2H, m), 2.87 (2H, t, J = 7.7 Hz), 3.90 (3H,
N
17 CH3 s), 7.18 (1H, d, J = 9.0 Hz), 7.50 (2H, d,
J = 8.6 Hz), 7.56 (1H, 388 386
dd, J = 8.8, 2.3 Hz), 7.73 (1H, d, J = 2.1 Hz), 7.91 (2H, d, J =
HOJL
6.7 Hz), 8.63 (1H, s), 13.09 (1H, br s).
0
H3C_0
1H-NMR (DMS0-06) 8:0.91 (3H, t, J = 7.3 Hz), 1.30-1.40
N,
(2H, m), 1.58-1.66 (2H, m), 2.67 (2H, t, J = 7.7 Hz), 3.71 (3H,
18 CH3 s), 6.79 (2H, dt, J = 9.4,2.5 Hz), 7.19
(2H, dt, J = 9.5, 2.5 Hz), 362 360
7.30(2K, dt, J = 8.4, 1.8 Hz), 7.62 (1H, d, J = 2.3 Hz), 7.85 (2H,
HO
dt, J = 8.6, 1.8 Hz), 8.50 (1H, d, J =2.1 Hz), 12.93 (11-I, br s).
0
H3C'0
1H-NMR (DMSO-D6) 8:0.92 (3H, t, J = 7.3 Hz), 1.32-1.44
(2H, m), 1.66-1.79 (2H, m), 2.87 (2H, t, J = 7.6 Hz), 3.87 (3H,
19 CH3 431 429
s), 7.18 (1H, d, J = 8.8 Hz), 7.48-7.53 (3H, m), 7.66 (1H, d, J =
O
2.3 Hz), 7.91 (2H, d, J = 8.6 Hz), 8.63(1K, s), 13.06 (1H, br s).
OH
H3c'0
1H-NMR (DMSO-D6) 8:1.56-1.63 (2H, m), 1.75-1.83(2H, m),
2.87 (2H, J = 7.6 Hz), 3.22 (3H, s), 3.36 (2H, t, J = 6.3 Hz),
20 cy-CH3 3.75 (3H,
s), 6.88 (2H, d, J = 8.7 Hz), 7.31 (2H, d, J = 8.7 Hz), 393 391
HO 7.51 (2H, d, J = 7.8 Hz), 7.90 (2H, d, J =
8.1 Hz), 8.60 (1H, s),
13.05 (1H, br s).
0
113C0
1H-NMR (DMSO-D6) 8:0.92 (3H, t, J = 7.4 Hz), 1.31-1.44
(2H, m), 1.66-1.78 (2H, m), 2.87 (2H, t, J = 7.7 Hz), 3.93 (3H,
21 399 397
CH, s), 7.08 (2H, d, J = 9.2 Hz), 7.51 (2H, d, J = 8.3 Hz), 7.92 (2H,
HO d, J =8.3 Hz), 8.64 (1H, s), 13.11 (1H, br
s).
Hs%
6H3 1H-NMR (DMSO-D6) 8:0.93 (3H, t, J = 7.4 Hz),
1.33-1.45
(2H, m), 1.68-1.79 (2H, m), 2.80-3.00 (8H, m), 7.33 (2H, d, J =
22CH, 404 402
8.3 Hz), 7.41 (2H, d, J = 8.3 Hz), 7.49 (2H, d, J = 8.3 Hz), 7.87
HO (2H, d, J 8.3 Hz), 8.66 (1H, s), 13.04 (1H,
br s).
FF
1H-NMR (DMS0-1206) 8:0.93 (3H, t, J = 7.4 Hz), 1.32-1.46
(2H, m), 1.68-1.80 (2H, m), 2.90 (2H, t, J = 7.6 Hz), 3.67 (3H,
23 CH 1õ,,õCH3 s), 7.03
(1H, d, J = 8.1 Hz), 7.24 (1H, s), 7.50 (2H, d, J = 8.3 431 429
Hz), 7.54 (1H, d, J = 8.1 Hz), 7.90 (21-i, d, J = 8.3 Hz), 8.69(1H,
HO s),13.11 (1H, br s).
FF
1H-NMR (DMSO-D6) 6: 0.93 (3H, t, J = 7.4 Hz), 1.33-1.45
(2H, m), 1.68-1.80 (2H, m), 2.90 (2H, t, J = 7.6 Hz), 7.31 (1H, d,
24 419 417
cH3 J = 8.1 Hz), 7.47-7.57 (3H, m), 7.72 (1H, t, J = 7.9 Hz), 7.91
HO (2H, d, J = 8.3 Hz), 8.70 (1H, s), 13.08 (1H, br s).
0
98
= CA 03074989 2020-03-05
[0357]
Table 1-4
nis MS
Ex Structure NMR
(M+H) (M-K)
H3C'0
1H-NMR (DMSO-D6) 8:0.91 (3H, t, J = 7.3 Hz), 1.31-1.39
N,
(2H, m), 1.57-1.64 (2H, m), 2.68 (2H, t, J = 7.6 Hz), 3.69 (3H,
25 CH3 s), 6.76-6.79 (2H, m), 7.16 (2H, dt, J =
9.4,2.5 Hz), 7.32 (2H, d, 378 380
J = 8.1 Hz), 7.89 (2H, d, J = 8.1 Hz), 8.57 (11-I, d, J = 9.9 Hz),
HO F13.03 (1H, br s).
0
F F
1H-NMR (DMSO-D6) 8:0.93 (3H, t, J = 7.3 Hz), 1.33-1.44
(2H, m), 1.69-1.79(2H, m), 2.38 (3H, s), 2.89 (2H, t, J = 7.7
H3C
26 Hz), 7.23 (1H, d, J = 8.3 Hz), 7.50 (2H,
d, J = 8.3 Hz), 7.56 (2H, 415 413
d, J = 8.3 Hz), 7.89 (2H, d, J = 8.3 Hz), 8.68 (1H, s), 13.07 (1H,
HO br s).
0
1H-NMR (DMSO-D6) 6: 0.93 (3H, t, J = 7.4 Hz), 1.33-1.44
(2H, m), 1.69-1.80 (2H, m), 2.90 (2H, t, J = 7.6 Hz), 7.42 (1H, d,
ci
27 J = 8.3 Hz), 7.51 (2H, d, J = 8.3 Hz),
7.75 (1H, s), 7.78(1K, d, J 435 433
= 8.3 Hz), 7.91 (2H, d, J = 8.1 Hz), 8.70 (1H, s), 13.13 (1H, br
HO s).
0
FF
1H-NMR (DMSO-D6) 6: 0.93 (3H, t, J = 7.4 Hz), 1.34-1.46
(2H, m), 1.69-1.80 (2H, m), 2.92 (2H, t, J = 7.7 Hz), 7.53 (2H, d,
28 469
467
'N CH3 J = 8.6 Hz), 7.83(1K, d, J = 8.6 Hz),
7.92(2K, d, J = 8.6 Hz),
HO 7.96-8.03 (2H, m), 8.74 (1H, s), 13.09
(1H, br s).
0
FF
1H-NMR (DMSO-D6) 8:1.56-1.63 (2H, m), 1.76-1.84(2K, m),
2.91 (2H, t, J = 7.6 Hz), 3.20 (3H, s), 3.35 (2H, t, J = 6.4 Hz),
29 I 7.50(2K, dd, J = 6.7, 1.8 Hz), 7.58 (2H,
d, J = 8.1 Hz), 7.70 429 431
N'
(2H, d, J = 8.1 Hz), 7.89 (2H, dd, J = 6.7, 1.8 Hz), 8.69 (1H, s),
HO 13.07 (1H, br s).
H,C'0
1H-NMR (DMSO-D6) 8:0.91 (3H, t, J = 7.4 Hz), 1.30-1.40
(2H, m), 1.58-1.66 (2H, m), 2.67 (2H, t, J = 7.7 Hz), 3.79 (3H,
s), 6.93-6.96 (1H, m), 7.01 (1H, t, J = 8.7 Hz), 7.11 (1H, dd, J =
30 CH,
12.7,2.1 Hz), 7.32 (21-1, dt, J = 8.4, 1.8 Hz), 7.65 d, J = 2.3
378 380
HO Hz), 7.88 (21-I, dt, J = 8.5, 1.8 Hz),
8.51 (1H, d, J = 2.1 Hz),
13.00 (1H, br s).
0
CI
1H-NMR (DMSO-D6) 6: 0.94 (3H, t, J = 7.4 Hz), 1.35-1.45
(2H, m), 1.79-1.71 (2H, m), 2.89(2K, t, J = 7.7 Hz), 7.37-7.42
31 CH, (4H, m), 7.50 (2H, dt, J = 8.4, 1.8
Hz), 7.91 (2H, dt, J = 8.4, 1.8 367 365
HO Hz), 8.66 (1H, s), 13.07 (1H, br s).
0
0
JJLN
1H-NMR (DMS0-06) 8:0.93 (3H, t, J = 7.3 Hz), 1.35-1.44
(2H, m), 1.77-1.70 (2H, m), 2.85 (2H, t, J = 7.7 Hz), 3.13 (2H, t,
J = 8.7 Hz), 4.54 (21-I, t, J = 8.7 Hz), 6.65 d, J = 8.3 Hz),
32 CH, 375
373
6.99 (11-1, dd, J = 8.3,2.0 Hz), 7.35(1K, d, J = 1.4 Hz), 7.52
HO (211, dt, J = 8.4,1.8 Hz), 7.90 (21-I,
dt, J = 8.5, 1.8 Hz), 8.58 (1H,
s), 13.02 (1H, s).
0
99
CA 03074989 2020-03-05
4 = ,
[0358]
Table 1-5
Ms
MS
Ex. Structure NMR
(M+H) (M-H)
HO
LJLN1H-NMR (DMSO-D6) 6:0.93 (3H, t, J = 7.4 Hz), 1.34-1.44
(2H, m), 1.69-1.77 (2H, m), 2.85 (2H, t, J = 7.6 Hz), 6.69 (2H,
ICH, 33 dt, J = 9.2,2.4 Hz), 7.19 (2H, d J = 9.2, 2.4 Hz),
7.50 (2H, dt, J 349 347
= 8.4, 1.7 Hz), 7.89 (2H, dt, J = 8.4, 1.7 Hz), 8.57 (1H, s), 9.69
HO (1H, s), 13.03 (1H, br s).
0
1H-NMR (DMSO-D6) 6:0.66-0.69 (2H, m), 0.92-0.98 (5H, in),
1.44-1.35 (2H, m), 1.70-1.78 (2H, m), 1.87-1.92 (1H, m), 2.87
34 (2H, t, J = 7.6 Hz), 7.02 (2H, dt, J = 8.5, 1.8 Hz),
7.25 (2H, dt, J 373 371
cH,
= 8.4, 1.8 Hz), 7.50 (2H, dt, J = 8.4, 1.8 Hz), 7.89 (2H, dt, J =
HO 8.5, 1.8 Hz), 8.62 (1H, s), 13.04 (1H,
s).
0
H3C'0
1H-NMR (DMSO-D6) 6:1.54-1.63 (2H, m), 1.72-1.83 (2H, m),
2.88 (2H, t, J = 7.6 Hz), 3.20 (3H, s), 3.34 (2H, J = 6.5 Hz),
35 I :L.õ../" 429 427
N 0'CH, 3.93 (3H, s), 7.09 (2H, d, J =
9.5 Hz), 7.52 (2H, d, J = 8.6 Hz),
HO 7.92 (2H, d, J = 8.6 Hz), 8.64 (1H, s),
13.10 (1H, br s).
0
FF
1H-NMR (DMSO-D6) 6: 0.98 (3H, t, J = 7.4 Hz), 1.73-1.85
(2H, m), 2.87 (2H, t, J = 7.5 Hz), 7.49 (2H, d, J = 8.6 Hz), 7.58
36 I 387 385
N cH, (2H, d, J = 8.1 Hz), 7.70 (2H,
d, J = 8.3 Hz), 7.89 (2H, d, J = 8.6
HoJ!J Hz), 8.69 (1H, s), 13.08 (1H, br s).
H,c,0
1H-NMR (DMS0-06) 6:0.96 (3H, t, J = 7.3 Hz), 1.70-1.82
37
(2H, m), 2.82 (2H, t, J = 7.6 Hz), 3.74 (3H, s), 6.87 (2H, d, J =
349
347
CH3 8.8 Hz), 7.30 (2H, d, J = 8.8 Hz), 7.49
(2H, d, J = 8.6 Hz), 7.88
HO (2H, d, J = 8.8 Hz), 8.58 (1H, s),
13.04 (1H, br s).
0
HA'0
1H-NMR (DMSO-D6) 6: 0.93 (3H, t, J = 7.2 Hz), 1.34-1.44
I (2H, m), 1.70-1.77 (2H, m), 2.84 (2H,
t, J = 7.5 Hz), 3.58 (2H, 331
38 CH,
s), 3.75 (3H, s), 6.88 (2H, d, J = 8.7 Hz), 7.22 (2H, d, J = 7.8 377
(-COOH)
Hz), 7.31-7.36 (4H, m), 8.54 (1H, s), 12.35 (1H, s).
Ho o
H,C'0
1H-NMR (DMSO-D6) 6:0.95 (3H, t, J = 7.3 Hz), 1.36-1.46
(2H, m), 1.53-1.61 (2H, m), 2.02 (3H, s), 2.68 (2H, t, J = 7.6
39 CH, Hz), 3.68 (3H, s), 6.71 (2H, d, J = 8.7 Hz), 7.09
(2H, d, J = 8.7 376 374
Hz), 7.23 (2H, d, J = 8.1 Hz), 7.88 (2H, d, J = 8.1 Hz), 8.41 (1H,
Ho cH, s), 12.98 (1H, br s).
0
H3C
LOJLN
1H-NMR (DMSO-06) 6:0.94 (3H, t, J = 7.4 Hz), 1.35-1.44
ICH, (2H, m), 1.70-1.78 (2H, m), 2.30 (3H,
s), 2.87 (2H, t, J = 7.7
40 Hz), 7.13 (2H, d, J = 8.1 Hz), 7.26 (2H, d, J = 8.1
Hz), 7.49 (2H, 347 345
d, J = 8.6 Hz), 7.88 (2H, dt, J = 8.6, 1.7 Hz), 8.63 (1H, s), 13.04
HOAJ
(1H, s).
0
100
CA 03074989 2020-03-05
= If
[0359]
Table 1-6
Ms MS
Ex Structure NMR
(M+H) (M-H)
-0
1H-NMR (DMS0-136) 5:0.93 (3H, t, J = 7.3 Hz), 1.61-1.71
N,
(2H, m), 2.64(2K, t, J = 9.7 Hz), 3.71 (3H, s), 6.79 (2H, dd, J =
CH
41 6.7,2.1 Hz), 7.19 (2H, dd, J = 6.8,2.2
Hz), 7.30 (2H, dd, J = 346 348
H3C
6.7, 1.8 Hz), 7.62 (1H, d, J = 2.3 Hz), 7.85 (2H, dd, J = 6.7, 1.8
HO
Hz), 8.50 (1H, d, J = 2.3 Hz), 12.97 (1H, br s).
0
H3c'0
1H-NMR (DMS0-06) 5:0.86-1.00 (2H, m), 1.08-1.35 (4H, m),
, 1.47-1.81 (7H, m), 2.67 (2H, t, J = 8.0
Hz), 3.71 (3H, s), 6.79
42 (2H, d, J = 9.0 Hz), 7.18(2K, d, J = 8.8
Hz), 7.29 (2H, d, J = 8.6 416 414
Hz), 7.62 (1H, d, J = 2.1 Hz), 7.85 (2H, d, J = 8.6 Hz), 8.49(1K,
HO d, J = 2.1 Hz), 13.00 (1H, br s).
0
H3C'0
1H-NMR (DMSO-D6) 5:1.81-1.92 (2H, m), 2.72 (2H, t, J = 7.6
N,
Hz), 3.36-3.53 (6H, m), 3.71 (3H, s), 4.56 (1H, t, J = 5.3 Hz),
43 ON./\OH
6.80 (2H, d, J = 9.0 Hz), 7.19 (2H, d, J = 8.8 Hz), 7.30 (2H, d, J 408
406
HO = 8.3 Hz), 7.64(1K, d, J = 2.1 Hz),
7.85(2K, d, J = 8.6 Hz),
8.51 (1H, d, J =2.1 Hz), 12.97 (1H, br s).
0
H3C'0
1H-NMR (DMSO-D6) 6: 1.32-1.47 (1H, m), 1.49-1.74 (5H, m),
N,
1.89 (4H, dd, J = 8.8,6.5 Hz), 2.68 (2H, t, J = 7.5 Hz), 3.71 (3H,
44 s), 4.75 (1H, s), 6.80 (2H, d, J = 9.0
Hz), 7.19(2K, d, J = 8.8 418 416
OH
Hz), 7.30(2K, d, J = 8.6 Hz), 7.63(1K, d, J = 2.3 Hz), 7.85 (2H,
HO
d, J = 8.6 Hz), 8.51 (1H, d, J = 2.1 Hz), 12.96 (1H, br s).
0
0
H3C- 1H-NMR (DMSO-D6) 5:0.87 (3H, t, J = 6.9
Hz), 1.28-1.36
(4H, m), 1.60-1.68 (2H, m), 2.66 (2H, t, J =7.7 Hz), 3.71 (3H,
s), 6.79 (2H, dt, J = 9.4, 2.5 Hz), 7.19 (21-I, dt, J = 9.3, 2.5 Fiz),
45 374 --
376
CH 3 7.30 (2H, dd, J = 6.6, 1.7 Hz), 7.62
(1H, d, J = 2.1 Hz), 7.85
HO (21-1, dd, J = 6.7, 1.8 Hz), 8.50(1H, d,
J=2.1 Hz), 12.97(1K, br
s).
0
0
H3c'
N
1H-NMR (DMSO-D6) 6: 1.17-1.58 (5H, m), 1.65-1.90(5K, m), ,
2.58-2.70 (1H, m), 3.71 (3H, s), 6.79(2K, d, J = 8.8 Hz), 7.18
46 (2H, d, J 8.8 Hz), 7.30 (2H, d, J = 8.6 Hz), 7.62 (1H, d, J = 2.3
388 386
HO
Hz), 7.85 d, J = 8.6 Hz), 8.53(1K, d, J =
2.1 Hz), 13.00
(1H, s).
0
H3C-0
1H-NMR (DMSO-D6) 5:0.92-122 (5H, m), 1.55-1.67 (6H, m),
2.55 (2H, d, J = 6.9 Hz), 3.71 (3H, s), 6.79(2K, dt, J = 9.5,2.4
47 Hz), 7.19 (2H, dt, J = 9.4,2.5 Hz), 7.26
(2H, d, J = 8.3 Hz), 7.57 400 402
(1H, d, 2.1 Hz),
7.84 (2H, d, J = 8.3 Hz), 8.44 (1H, d, J = 2.1
HO
Hz), 13.13(1K, br s).
0
H3C'0
1H-NMR (DMSO-D6) 5:0.93 (3H, t, J = 7.9 Hz), 1.34-1.44
ICH3
(2H, m), 1.47 (6H, s), 1.69-1.77 (2H, m), 2.83 (2H, t, J = 7.6
Hz), 3.76 (3H, s), 6.88 (2H, d, J = 9.0 Hz), 7.30-7.38 (6H, m), 405
48 H3C 359
(-COOK)
8.53 (1H, s), 12.36 (1H, s).
H3C
Ho o
101
CA 03074989 2020-03-05
[0360]
Table 1-7
Ms MS
Ex Structure NMR
(M+H) (M-K)
0
1H-NMR (DMSO-D6) 5:0.93 (3H, t, J = 7.5 Hz), 1.34-1.44
(2H, m), 1.69-1.77 (2H, m), 1.86-1.93 (2H, m), 2.66 (2H, t, J =
ICH, 6.3 Hz), 2.85 (2H, t, J = 7.6 Hz), 4.14 (2H, t, J =
4.9 Hz), 6.60
49 389 387
(1H, d, J = 8.4 Hz), 6.92 (1H, dd, J = 8.5, 1.9 Hz), 7.23 (1H, d,
HO = 1.9 Hz), 7.52 (2H, d, J = 8.6 Hz), 7.91 (2H, d, J
= 8.6 Hz),
8.58 (1H, s), 13.05 (1H, br s).
0
(0
1H-NMR (DMSO-D6) 5:0.93 (3H, t, J = 7.3 Hz), 1.34-1.44
(2H, m), 1.69-1.77 (2H, m), 2.85 (2H, t, J = 7.8 Hz), 4.19-4.26
50 CH, 391 389
(4H, m), 6.74-6.79 (2H, m), 6.92 (1H, br s), 7.52 (2H, d, J = 8.1
HO Hz), 7.91 (2H, d, J = 8.1 Hz), 8.59 (1H, s), 13.07
(1H, br s).
0
H,C'0
1H-NMR (DMSO-D6) 5:1.42-1.53 (2H, m), 1.60-1.72(2K, m),
2.67 (2H, t, J = 7.6 Hz), 3.38-3.46 (2H, m), 3.71 (3H, s), 4.38
51 (1H, br s), 6.79 (2H, d, J = 8.8 Hz), 7.19 (2H, d,
J = 8.8 F14, 378 376
OH 7.30 (2H, d, J = 8.6 Hz), 7.63 (1H, d, J = 2.1 Hz), 7.85(2H, d, J
HO = 8.6 Hz), 8.50 (1H, d, J = 2.1 Hz), 12.97 (1H, br
s).
0
H,C
11-1-NMR (DMSO-D6) 5:0.03-0.11 (2H, m), 0.37-0.45 (2H, m),
0.66-0.80 (1H, m), 1.54 (2H, dd, J = 15.4, 7.1 Hz), 2.74 (2H,
dd, J = 14.4, 6.6 Hz), 3.71 (3H, s), 6.79 (2H, d, J = 8.8 Hz),
52 374 372
7.18 (2H, d, J = 8.8 Hz), 7.29 (2H, d, J = 8.6 Hz), 7.64 (1H, d, J
Ho = 2.1 Hz), 7.85 (2H, d, J = 8.6 Hz), 8.51 (1K, d, J
= 2.3 F14,
0 12.99 (1H, br s).
H,c'0
1H-NMR (DMSO-D6) 5:1.50-1.57 (2H, m), 1.61-1.67 (2H, m),
1.71-1.82(2K, m), 1.95-2.02(2K, m), 2.67-2.76(1K, m), 3.25
53
(3H, s), 3.49 (1H, br s), 3.72 (3H, s), 6.81 (2H, d, J = 8.4 Hz),
7.20 (2H, d, J =8.4 Hz), 7.32 (2H, d, J =8.1 Hz), 7.58 (1H, d, J 418 416
HO 0'CH3 = 1.7 Hz), 7.87 (2H, d, J = 8.1 Hz), 8.54
(1H, d, J = 1.7 Hz),
13.00 (1H, br s).
0
H3C'0
1H-NMR (DMSO-D6) 5:1.20-1.32 (2H, m), 1.55-1.67 (2H, m),
N, 1.87-1.95 (2H, m), 2.09-2.16 (2H, m), 2.61-2.70
(1H, m), 3.15-
o
3.25 (1H, m), 3.27 (3H, s), 3.72 (3H, s), 6.81 (2H, d, J = 8.4
54 418 416
Hz), 7.20 (2H, d, J = 8.4 Hz), 7.31 (2H, d, J = 8.0 Hz), 7.65 (1H,
HO CH, d, J = 1.5 Hz), 7.86 (2H, d, J = 8.0 Hz), 8.56
d, J = 1.5 Hz),
13.00 (1H, br s).
0
H.0 CH3
1H-NMR (DMSO-D6) 5: 0.92 (3H, t, J = 7.3 Hz), 1.25 (9H, s),
1.33-1.43 (2H, m), 1.68-1.77 (2H, m), 2.86 (2H, t, J = 7.7 Hz),
55 ICH, 7.30 (2H, d, J = 8.8 Hz), 7.33(2K, d, J = 8.8 Hz),
7.49 (2H, d, J 389 387
= 8.3 Hz), 7.87 (2H, d, J = 8.3 Hz), 8.62(1K, s), 13.08(1K, br
HO 3).
0
HA'0
N, 1H-NMR (DMSO-D6) 5:1.26 (3H, t, J = 7.7 Hz), 2.71
(2H, q, J
56 CH, = 7.7 Hz), 3.72(3K, s), 6.81 (2H, d, J = 9.0 Hz),
7.20(2K, d, J =
334 332
9.0 Hz), 7.32 (2H, d, J = 8.6 Hz), 7.66(1K, d, J = 2.1 Hz), 7.87
HO (2H, d, J = 8.6 Hz), 8.54(1K, d, J = 2.1 Hz), 12.99(1K, br
s).
0
102
CA 03074989 2020-03-05
[0361]
Table 1-8
MS MS
Ex Structure NMR
(M4+) (M-N)
H3C"
1H-NMR (DMSO-D6) 6:1.30 (6H, d, J = 6.9 Hz), 2.99-3.10
(1H, m), 3.72 (3H, s), 6.81 (2H, d, J = 8.9 Hz), 7.20 (2H, d, J =
,
57 CH3 348 346
8.9 Hz), 7.33 (2H, d, J = 8.1 Hz), 7.67 (1H, d, J = 2.1 Hz), 7.87
HO CH3 (2H, d, J 8.1 Hz), 8.58 (1H, d, J = 2.1 Hz), 12.99
(1H, br s).
0
0
H3C-
1H-NMR (DMSO-D6) 6: 0.93 (6H, d, J = 6.6 Hz), 1.87-1.99
CH3 (1H, m), 2.56 (2H, d, J = 7.2 Hz), 3.72 (3H, s),
6.81 (2H, d, J =
58 8.9 Hz), 7.21 (2H, d, J = 8.9 Hz), 7.32 (2H, d, J =
8.7 Hz), 7.61 362 360
CH3 (1H, d, J 2.1 Hz), 7.87 (21i, d, J = 8.7 Hz), 8.48
(1H, d, J = 2.1
HO Hz), 12.99 (1H, br s).
0
H3C'0
1H-NMR (DMSO-D6) 6: 1.58-1.87 (6H, m), 2.04-2.15 (2H, m),
N.õ
3.04-3.15 (1H, m), 3.72 (3H, s), 6.81 (2H, d, J = 9.0 Hz), 7.20
59 (2H, d, J = 9.0 Hz), 7.32 (2H, d, J = 8.6 Hz), 7.65
(1H, d, J = 2.1 374 372
Hz), 7.87 (2H, d, J = 8.6 Hz), 8.57(1K, d, J = 2.1 Hz), 12.99
HO
(1H, br s).
0
H3C'0
1H-NMR (DMSO-06) 6: 1.74-1.83 (4H, m), 2.88-2.99(1K, m),
3.41-3.52(2K, m), 3.72 (3H, s), 3.94-4.01 (2H, m), 6.81 (2H, d,
60 J = 9.0 Hz), 7.20(2K, d, J = 9.0 Hz), 7.33 (2H, d,
J = 8.2 Hz), 390 388
HO 0 7.67 (1H, br s), 7.87 (2H, d, J = 8.2 Hz), 8.59
(1H, br s), 12.91
(1H, br s).
0
_0
H3C
1H-NMR (DMSO-06) 6: 1.75-1.84(2K, m), 2.68-2.75(2K, m),
3.42-3.49 (2H, m), 3.72 (3H, s), 4.51-4.56 (1H, m), 6.81 (2H, d,
61 OH J = 9.0 Hz), 7.20 (2H, d, J = 9.0 Hz), 7.32 (2H, d,
.1= 8.1 Hz), 364 362
7.64 (1H, br s), 7.87 (2H, d, J = 8.1 Hz), 8.52 (1H, br s), 12.99
HO (1H, br s).
0
_0
H3c
N,
1H-NMR pmso-06) 6: 2.66-2.79 (2H, m), 2.93 (2H, dd, J =
9.8, 6.4 Hz), 3.71 (3H, s), 6.80(2N, d, J = 8.8 Hz), 7.19 (2H, d,
62 J = 8.8 Hz), 7.31 (2H, d, J = 8.6 Hz), 7.79 (1H, d,
J = 2.1 Hz), 402 400
7.86 (21-1, d, J = 8.3 Hz), 8.59 (1H, d, J = 2.3 Hz), 13.01(1K, br
Ho FF
s).
0
H3C-
0
1H-NMR (DMS0-136) 8:1.06-122 (2H, m), 1.40-1.70 (6H, m),
1.70-1.85 (3H, m), 2.67 (2H, t, J = 7.9 Hz), 3.71 (3H, s), 6.79
63 (2H, d, J = 9.0 Hz), 7.18 (2H, d, J = 9.0 Hz), 7.29
(2H, d, J = 8.6 402 400
Hz), 7.63 (1H, d, J = 2.1 Hz), 7.85 (2H, d, J = 8.3 Hz), 8.50 (1H,
HO
d, J = 2.1 Hz), 13.01 (1H, br s).
0
Cl
H3C'0 1H-NMR (DMSO-D6) 6:0.92 (3H, t, J = 7.3 Hz), 1.32-
1.44
(2H, m), 1.68-1.78 (2H, m), 2.87 (2H, t, J = 7.7 Hz), 3.90(3K, d,
J = 1.6 Hz), 7.21 (1H, dd, J = 11.9, 2.0 Hz), 7.29 (1H, J = 1.7 415 413
64
Hz), 7.53 (2H, d, J = 8.3 Hz), 7.93 (2H, d, J = 8.6 Hz), 8.65 (1H,
HO s), 13.09 (1H, br s).
0
103
CA 03074989 2020-03-05
=
[0362]
Table 1-9
MS MS
Ex Structure NMR
(M+H) (M-H)
CI
H3C' 1H-NMR (DMSO-D6) 6: 0.92 (3H, t, J =7.4
Hz), 1.32-1.44
ci (2H, m), 1.68-1.78 (2H, m), 2.88 (2H, t,
J = 7.7 Hz), 3.82 (3H,
65H3 431 429
s), 7.42 (2H, s), 7.53 (2H, d, J = 8.6 Hz), 7.93 (2H, d, J = 8.6
HO Hz), 8.65 (1H, s), 13.13 (1H, br s).
0
H3C'0
1H-NMR (DMSO-D6) 6:0.96 (3H, t J = 7.4 Hz), 1.71-1.83
F_T(2H, m), 2.85 (2H, t, J = 7.6 Hz), 3.93 (3H, s), 7.09 (2H, d, J =
9.5 Hz), 7.52 (2H, d, J = 8.6 Hz), 7.92 (2H, d, J = 8.6 Hz) 64
66 I 385
383
N CH3 , 8.
HO (1H, s), 13.11 (1H, br s).
0
1.43C'C'
1H-NMR (DMSO-D6) 6: 1.92-2.02 (2H, m), 2.91 (2H, t, J = 7.7
67 I Hz), 3.24 (3H, s), 3.40 (2H, t, J = 6.4
Hz), 3.93 (3H, s), 7.09
cLce, (2H, d, J 9.5 Hz), 7.53 (2H, d, J = 8.6
Hz), 7.92 (2H, d, J = 8.6 415 413
HO Hz), 8.64 (1H, s), 13.10 (1H, s).
0
H3C'0
1H-NMR (DMSO-D6) 6:0.91 (3H, t, J = 7.4 Hz), 1.29-1.39
(2H, m), 1.58-1.66 (2H, m), 2.65-2.71 (2H, m), 3.90 (3H, s),
68 I 6.95 (2H, d, J = 9.7 Hz), 7.34 (2H, dd,
J = 6.7, 1.8 Hz), 7.70 396 398
CH3
(1H, d, J = 2.3 Hz), 7.90 (2H, dd, J = 6.5, 1.8 Hz), 8.54 (1H, d, J
HO =2.1 Hz), 13.04 (1H, br s).
H3O-0
1H-NMR (DMSO-D6) 5: 0.94 (3H, t, J = 7.4 Hz), 1.35-1.44
69
(2H, m), 1.70-1.78 (2H, m), 2.87 (2H, t, J = 7.7 Hz), 3.78 (3H,
353
399
s), 6.95 (2H, dt, J = 9.4,2.5 Hz), 7.18 (2H, d, J = 9.0 Hz), 7.36
(-COOH)
HO (2H, dt, J = 9.4,2.5 Hz), 8.65 (1H, s),
14.03 (1H, s).
o F
H3c"o
1H-NMR (DMSO-06) 6:0.93 (3H, t, J = 7.4 Hz), 1.34-1.43
(2H, m), 1.69-1.77 (2H, m), 2.82 (2H, t, J = 7.6 Hz), 3.76 (3H, 393
391
70 N CH3
s), 4.69 (2H, s), 6.86-6.91 (4H, m), 7.35-7.31 (4H, m), 8.49
HO_
11 (1H, s), 13.02 (1H, s).
0
H3c'0
1H-NMR (DMSO-D6) 6:0.93 (3H, t, J = 7.3 Hz), 1.33-1.43
(2H, m), 1.68-1.76 (2H, m), 2.23 (2H, t J = 7.7 Hz), 2.76 (2H, t,
71 CH3 Ho J = 7.7
Hz), 2.82 (2H, t, J = 7.7 Hz), 3.75 (3H, s), 6.87 (2H, dt, J 391 389
= 9.5, 2.5 Hz), 7.15 (2H, d, J = 8.1 Hz), 7.27(2K, d, J = 8.1 Hz),
7.31 (2H, dt, J = 9.4, 2.5 Hz), 8.50 (1H, s).
0
H3C'0
1H-NMR (DMSO-06) 6:0.93 (3H, t, J = 7.4 Hz), 1.34-1.42
(2H, m), 1.70-1.77 (2H, m), 2.84 (2H, t, J = 7.6 Hz), 3.22 (2H, br
349
72 s), 3.76 (3H, s), 6.91 (2H, dt, J = 9.4,
2.5 Hz), 7.03-7.10 (2H, 395
0 (-COOE)
m), 7.20 (1H, 1, J = 7.7 Hz), 7.35(2K, dt, J = 9.4, 2.5 Hz), 8.53
HO (1H, s).
104
CA 03074989 2020-03-05
[0363]
Table 1-10
MS MS
Ex Structure NMR
(M+H) (M-H)
0
H3c"
1H-NMR (DMSO-D6) 6: 1.10 (3H, t, J = 7.0 Hz), 2.91 (2H, t, J
= 6.7 Hz), 3.46 (2H, q, J = 7.0 Hz), 3.66 (2H, t, J = 6.7 Hz), 3.72
73 (3H, s), 6.81 (2H, d, J = 8.8 Hz), 7.20 (2H, d, J =
8.8 Hz), 7.31 378 376
OCE13 (2H, d, J = 8.5 Hz), 7.70 (1H, d, J = 2.2 Hz), 7.87 (2H, d, J = 8.5
HO
Hz), 8.54 (1H, d, J = 2.2 Hz), 12.99 (1H, br s).
0
H3C
1H-NMR (DMSO-D6) 6:0.94 (3H, t, J = 7.3 Hz), 1.17 (3H, t J
ICH3 = 7.6 Hz), 1.33-1.46 (2H, m), 1.69-1.79 (2H, m), 2.60 (2H, q, J
74 = 7.6 Hz), 2.87 (2H, t, J = 7.8 Hz), 7.17 (2H, d, J
= 8.1 Hz), 7.29 361 359
(2H, d, J = 8.1 Hz), 7.50 (2H, d, J = 8.1 Hz), 7.88(2K, d, J = 8.1
Ho
Hz), 8.63 (1H, s), 13.09 (1H, br s).
0
F,0
1H-NMR (DMS0-06) 6:0.94 (3H, t, J = 7.3 Hz), 1.34-1.46
(2H, m), 1.69-1.81 (2H, m), 2.90 (2H, t, J = 7.6 Hz), 7.34 (2H, d,
75 CH3 417 415
J = 8.4 Hz), 7.46-7.53 (4H, m), 7.90 (2H, d, J = 8.1 Hz), 8.67
Ho (1H, s), 13.11 (1H, brs).
0
HCI
H3C_0
1H-NMR (DMSO-D6) 6:0.91 (3H, t, J = 7.4 Hz), 1.32-1.40
(2H, m), 1.50-1.57 (2H, m), 3.31 (2H, dd, J = 12.8,6.8 Hz),
N
76 cH3 3.74 (3H, d, J = 0.7 Hz), 6.86 (2H, dd, J = 8.9,
2.9 Hz), 7.28 405 403
(2H, dd, J = 8.8, 1.6 Hz), 7.39(2K, d, J = 8.1 Hz), 7.91 (2H, d, J
HO 0 = 8.1 Hz), 8.25(1K, br s), 8.74 (1H, br s), 9.07
(1H, s).
0
H3C,0
1H-NMR (DMSO-D6) 5:1.11 (3H, t, J = 7.0 Hz), 1.83-1.92
N,
(2H, m), 2.70-2.76 (2H, m), 3.38-3.46 (4H, m), 3.72 (3H, s),
77 0 CH3 6.81 (2H, d, J = 8.8
Hz), 7.20 (2H, d, J = 8.8 Hz), 7.32 (2H, d, J 392 390
= 8.6 Hz), 7.65 (111, d, J = 2.2 Hz), 7.87 (2H, d, J = 8.6 Hz),
HO
8.52 (1H, d, J = 2.2 Hz), 12.98 (1H, br s).
0
H3C'0
1H-NMR (DMSO-D6) 6:3.36 (3H, s), 3.73(3K, s), 4.55 (2H,
s), 6.82 (2H, d, J = 8.8 Hz), 7.23 (2H, d, J = 8.8 Hz), 7.33 (2H,
78 O'CH3 350 348
d, J = 8.6 Hz), 7.74 (1H, d, J = 2.1 Hz), 7.88 (2H, d, J = 8.6 Hz),
HO 8.62 (1H, d, J = 2.1 Hz), 13.01 (1H, br s).
0
H3C
1H-NMR (DMSO-D6) 6:0.86 (3H, t, J = 7.3 Hz), 0.92(3K, t, J
= 7.3 Hz), 1.33-1.43 (2H, m), 1.50-1.62 (2H, m), 1.68-1.78 (2H,
79 CH3 m), 2.53 (2H, t, J = 7.6 Hz), 2.86 (2H, t, J =
7.7 Hz), 7.13 (2H, d, 375 373
J = 8.3 Hz), 7.26(2K, d, J = 8.1 Hz), 7.47(2K, d, J = 8.6 Hz),
HO 7.85 (2H, d, J = 8.3 Hz), 8.61 (1H, s), 13.04 (1H,
s).
0
H3C-0
1H-NMR (DMSO-D6) 6: 1.16-1.26 (2H, m), 1.45-1.55(2K, m),
N, 1.58-1.73(4K, m), 2.10-2.17(1K, m), 2.67 (2H, d, J
= 7.4 Hz),
3.71 (3H, s), 6.79 (2H, dt, J = 9.5, 2.5 Hz), 7.19 (2H, dt, J = 9.4,
BO 386 388
2.5 Hz), 7.30 (21-I, cit. J = 8.4, 1.8 Hz), 7.62 (1H, d, J = 2.1 Hz),
HO 7.85 (2H, dt, J = 8.5, 1.8 Hz), 8.49(1K, d, J = 2.1
Hz), 12.97
(1H, br s).
0
105
CA 03074989 2020-03-05
=
[0364]
Table 1-11
Nis MS
Ex Structure NMR
(M+H) (M-K)
H3C-0
1H-NMR (DMSO-D6) 8:1.70-1.86 (4H, m), 1.96-2.05 (2H, m),
, 2.57-2.66 (1H, m), 2.76 (2H, d, J = 7.4
Hz), 3.71 (3H, s), 6.79
81 (2H, dt, J = 9.5,2.4 Hz), 7.18 (2H, dt, J
= 9.4,2.5 Hz), 7.29 (2H, 372 374
dt, J = 8.4,1.7 Hz), 7.58 (1 d, J = 2.1 Hz), 7.85 (2H, dt, J =
HO 8.4, 1.8 Hz), 8.47 (1H, d, J = 2.1 Hz),
12.97 (1H, br s).
1H-NMR (DMSO-D6) 8:0.92 (3H, t, J = 7.4 Hz), 1.29 (3H, t, J
H3c
,
82 = 7.1 Hz), 1.32-1.43 (2H, m), 1.66-1.77 (2H, m), 2.84 (2H, J =
N
7.6 Hz), 4.00 (2H, q, J = 6.9 Hz), 6.85 (2H, d, J = 8.8 Hz), 7.28 377
375
HO (2H, d, J = 8.8 Hz), 7.47 (2H, d, J = 8.6
Hz), 7.87 (2H, d, J = 8.6
Hz), 8.58 (1H, s), 13.13 (1H, br s).
0
H3c'0
1H-NMR (DMSO-D6) 6:0.94 (3H, t, J = 7.4 Hz), 1.35-1.42
(2H, m), 1.69-1.77 (2H, m), 2.48 (3H, s), 2.85 (2H, t, J = 7.7
83 rrNI Lõ,.,CH3 Hz), 3.75 (3H, s), 6.89(2K, dt, J =
9.4,2.5 Hz), 7.18 (1H, dd, J 377 375
= 8.1, 1.6 Hz), 7.32 (21-I, dt, J = 9.4, 2.5 Hz), 7.41 (1H, d, J = 1.6
HO
Hz), 7.72 (1H, d, J = 8.1 Hz), 8.59(1K, s), 12.92 (1H, br s).
0 cH3
H3C,0
1H-NMR (DMSO-D6) 6: 1.84-1.93(2K, m), 2.72 (2H, t, J = 7.8
N,
Hz), 3.25 (3H, s), 3.38(2K, t, J = 6.4 Hz), 3.72(3K, s), 6.81
84 0'CH3 (2H, d, J = 8.8 Hz), 7.20 (2H, d, J =
8.8 Hz), 7.32 (2H, d, J = 8.3 378 376
HO Hz), 7.65 (1H, d, J = 2.2 Hz), 7.87(2K,
d, J = 8.6 Hz), 8.52 (1 Ft,
d, J = 2.2 Hz), 12.99 (1H, br s).
0
H3C'0
1H-NMR (DMSO-D6) 8:0.94 (6H, d, J = 6.4 Hz), 1.50-1.65
(3H, m), 2.68 (2H, t, J = 7.8 Hz), 3.72 (3H, s), 6.81 (2H, d, J =
85 CH3 8.9 Hz), 7.20 (2H, d, J = 8.9 Hz), 7.31
(2H, d, J = 8.3 Hz), 7.65 376 374
HO CH3 (1H, d, J = 2.1 Hz), 7.87 (2H, d, J = 8.3
Hz), 8.52 (11-I, d, J = 2.1
Hz), 12.99 (1H, br s).
0
H3C'0
1H-NMR (DMS0-136) 6:0.92 (3H, t, J = 7.3 Hz), 1.32-1.44
86 I (2H, m), 1.68-1.79 (2H, m), 2.87 (2H, t,
J = 7.7 Hz), 3.27 (3H,
s), 4.39 (2H, s), 7.25 (2H, d, J = 8.6 Hz), 7.33 (2H, d, J = 8.3 377
375
Hz), 7.47(2K, d, J = 8.6 Hz), 7.86 (2H, d, J = 8.6 Hz), 8.63 (1H,
HO
s), 13.02 (1H, br s).
0
N
1H-NMR (DMSO-D6) 6: 0.92 (3H, t, J = 7.4 Hz), 1.33-1.45
(2H, m), 1.67-1.79(2K, m), 2.87 (2H, t, J = 7.7 Hz), 4.04 (2H,
87 CH3 br s).
s), 7.29 (2H, d, J = 8.6 Hz), 7.38(2K, d, J = 8.6 Hz), 7.48(2K, 372
370
d, J = 8.6 Hz), 7.87 (21-1, d, J = 8.6 Hz), 8.64(1K, s), 13.06(1K,
HO
0
cH,
rr,c 1H-NMR (DMS0-06) 6:0.92 (3H, t, J = 7.4
Hz), 1.17 (6H, d, J
= 6.9 Hz), 1.32-1.44 (2H, m), 1.67-1.78 (2H, m), 2.81-2.91 (3H,
88 ICH, m), 7.18 (2H, d, J = 8.1 Hz), 7.28 (2H,
d, J = 8.3 Hz), 7.49 (2H, 375 373
d, J = 8.6 Hz), 7.87 (2H, d, J = 8.6 Hz), 8.61 (1H, s), 13.04(1K,
Ho br s).
106
CA 03074989 2020-03-05
[0365]
Table 1-12
MS MS
Ex Structure NMR
(M+H) (M-H)
0
1H-NMR (DMS0-06) 5:0.92 (3H, t, J = 7.3 Hz), 1.33-1.44
(2H, m), 1.67-1.80 (2H, m), 2.87 (2H, t, J = 7.6 Hz), 4.19-4.28
(1H, m), 4.59 (2H, t, J = 6.4 Hz), 4.90 (2H, dd, J = 8.4, 5.9 Fiz),
89
cH, 7.34 (2H, d, J = 8.8 Hz), 7.37 (2H, d, J = 8.6 Hz),
7.49 (2H, d, J 389 387
HO = 8.6 Hz), 7.87 (2H, d, J = 8.6 Hz), 8.63 (1H, s),
13.05 (1H, br
s).
FyO
1H-NMR (DMSO-D6) 5:0.94 (3H, t, J = 7.4 Hz), 1.35-1.45
FLN
(2H, m), 1.71-1.78 (2H, m), 2.88 (2H, t, J = 7.7 Hz), 7.10-7.15
90 CH3 (2H, m), 7.29 (1H, t, J = 73.5 Hz), 7.42 (2H, dt, J =
9.2,2.4 Hz), 399 397
7.51 (2H, dl, J = 8.4, 1.8 Hz), 7.90 (2H, dl, J = 8.4, 1.8 Hz), 8.65
HO (1H, s), 13.07 (1H, s).
0
H3C'0
1H-NMR (DMSO-D6) 5:0.92 (3H, t, J = 7.3 Hz), 1.31-1.44
91 CH3 (2H, m), 1.66-1.78 (2H, m), 2.84(2K, 1, J = 7.6 Hz),
3.74 (3H, d,
379 377
J = 3.7 Hz), 6.83-6.92 (3H, m), 6.97 (1H, d, J = 1.6 Hz), 7.33
HO (2H, d, J = 9.0 Hz), 7.70 (1H, d, J = 8.1 Hz),
8.58(1K, s).
0 OH
HsC'0
1H-NMR (DMS0-06) 5:0.92 (3H, t, J = 7.4 Hz), 1.33-1.44
92 CH3 (2H, m), 1.67-1.77 (2H, m), 2.85 (2H, t, J = 7.6 Hz),
3.75 (3H,
397 395
s), 6.91 (2H, d, J = 8.8 Hz), 7.29-7.37 (3H, m), 7.57 (1H, d, J =
HO 1.6 Hz), 7,70 (1H, d, J = 7.9 Hz), 8.61 (1H, s),
13.47(1K, br s).
0 Cl
H3C'0
1H-NMR (DMSO-D6) 5:0.92 (3H, t, J = 7.3 Hz), 1.31-1.44
(2H, m), 1.66-1.77(2K, m), 2.49-2.54(2K, m), 2.82(4K, t, J =
93 cH3 7.6 Hz), 3.75 (3H, s), 6.88 (2H, d, J = 8.8 Hz),
7.09(1K, dd, J = 409 407
7.9, 1.6 Hz), 7.15 (11-1, dd, J = 11.1, 1.6 Hz), 7.25(1K, J = 7.9
HO
Hz), 7.31 (2H, d, J = 9.0 Hz), 8.55 (1H, s), 12.23 (1H, br s).
0
H3c'0
1H-NMR (DMSO-D6) 6: 1.83-1.90 (2H, m), 2.51-2.55 (2H, m),
1 2.69 (2H, t, J = 7.6 Hz), 2.81 (2H, t, J = 7.6 Hz),
3.25(3K, s),
94 CL 3 37 (2H, t, J = 6.3 Hz), 3.72 (3H, s), 6.79 (2H, d,
J = 8.4 Hz), 406 404
CH3 =
HO 7.10 (2H, d, J = 7.8 Hz), 7.17-7.20(4K, m), 7.57
(1H, d, J = 1.8
Hz), 8.45 (1H, d, J = 1.5 Hz), 12.12 (1H, br s).
0
H30"0 1H-NMR (DMSO-D6) 6: 1.83-1.92 (2H, m), 2.50-2.57
(2H, m),
2.72(2K, t J = 7.7 Hz), 2.84 (2H, t J = 7.7 Hz), 3.25 (3H, s),
95 I 3.37 (2H, t, J = 6.3 Hz), 3.91 (3H, s), 6.91-6.99(2K,
m), 7.14 442 440
o,CH, d, J = 8.0 Hz), 7.24 (211, d, J = 8.0 Hz),
7.65(1K, d, J = 1.8
HO Hz), 8.50 (11-1, d, J = 1.8 Hz), 12.13 (11-1, s).
H3C'0
1H-NMR (DMS0-06) 6: 0.24-0.30 (2H, m), 0.47-0.54 (2H, m),
N,
1.00-1.11(1K, m), 2.60 (2H, d, J = 6.9 Hz), 3.73 (3H, s), 6.81
96 (2H, d, J = 8.7 Hz), 7.21 (2H, d, J = 8.4 Hz), 7.32
(2H, d, J = 8.1 360 358
Hz), 7.71 OH, d, J = 1.5 Hz), 7.88 (2H, d, J =8.1 Hz), 8.57 (1H,
HO d, J = 1.8 Hz), 12.99 (11-1, br s).
0
107
CA 03074989 2020-03-05
=
[0366]
Table 1-13
MS MS
Ex Structure NMR
(M+H) (M-H)
H3C'0 1H-NMR (DMSO-D6) 6: 1.95-2.02 (2H, m), 2.54
(2H, t, J = 7.7
Hz), 2.85 (2H, t, J = 7.6 Hz), 2.90 (2H, J = 7.6 Hz), 3.25 (3H,
97 s), 3.41 (2H, t, J = 6.2 Hz), 3.94 (3H, s),
7.09 (2H, d, J = 9.9 443 441
I
N 'CH, Hz), 7.25 (2H, d, J = 8.1 Hz), 7.34 (2H, d, J = 8.1 Hz), 8.58
(1H,
HO s), 12.14 (1H, s).
if
H3C".0
1H-NMR (DMSO-D6) 8:0.94 (6H, d, J = 6.7 Hz), 2.06-2.19
, CH
(1H, m), 2.75 (2H, d, J = 7.2 Hz), 3.93 (3H, s), 7.10 (2H, d, J =
98 399 397
9.5 Hz), 7.52(2K, d, J = 8.3 Hz), 7.92 (2H, d, J = 8.3 Hz), 8.61
HO(IH, s), 13.07 (1H, br s).
H3C'0
1H-NMR (DMSO-D6) 8:0.91 (6H, t, J = 5.4 Hz), 1.86-1.99
, N CH3 (1H, m), 2.57(2K, d, J = 7.2 Hz), 3.90 (3H,
s), 6.96 (2H, d, J =
99
9.7 Hz), 7.34 (21-I, d, J = 8.6 Hz), 7.67 (1H, d, J = 2.3 Hz), 7.90 398
396
HO cH, (2H, d, J 8.6 Hz), 8.51 (1H, d, J = 2.1 Hz),
12.97(1K, br s).
H,C'0
1H-NMR (DMSO-D6) 6: 0.85 (3H, t, J = 7.1 Hz), 1.19-1.41
N,
(6H, m), 1.56-1.69 (2H, m), 2.66 (2H, t, J = 7.7 Hz), 3.71 (3H,
100 CH, s), 6.79 (2H, d, J = 9.0 Hz), 7.19 (2H, d, J =
8.8 Hz), 7.27(2K, 390 388
d, J = 8.3 Hz), 7.62(1K, d, J = 2.1 Hz), 7.84 (21-4, d, J = 8.6 Hz),
HO
8.49 (1H, d, J = 2.1 Hz).
0
H,C'0
N, 1H-NMR (DMSO-D6)6: 3.70(3K, s), 4.04(2K, s), 6.79(2K, d,
J = 8.8 Hz), 7.14-7.23 (3H, m), 7.23-7.36 (6H, m), 7.65 (1H, d,
101 396 394
J = 2.1 Hz), 7.84 (2H, d, J = 8.6 Hz), 8 57 d, J = 2.3 Hz),
HO 13.01 (11-1, br s).
0
0
H3C-
N
1H-NMR (DMSO-D6) 6: 2.91 (2H, t, J = 6.6 Hz), 3.27 (3H, s), ,
3.63 (2H, t, J = 6.6 Hz), 3.72 (3H, s), 6.81 (2H, d, J = 8.8 Hz),
102 7.20(2K, d, J = 8.8 Hz), 7.31 (2H, d, J = 8.3
Hz), 7.69(1K, d, J 364 362
HO = 2.2 Hz), 7.87(2K, d, J = 8.3 Hz), 8.54(1K,
d, J = 2.2 Hz),
12.99 (1H, br s).
0
H3C".0 1H-NMR (DMSO-D6) 8:0.93 (3H, t, J = 7.3 Hz),
1.61-1.71
(2H, m), 2.66 (2H, t, J = 7.5 Hz), 3.90 (3H, s), 6.95 (2H, d, J =
103 9.7 Hz), 7.34 (2H, dt, J = 8.5,1.8 Hz), 7.70
(1H, d, J = 2.1 Hz), 382 384
HO CH3 7.90 (2H, dt, J = 8.3, 1.8 Hz), 8.54 (1H, d, J
= 2.1 Hz), 13.04
(1H, br s).
H,C'0
1H-NMR (DMSO-D6) 8:1.88-2.00 (2H, m), 2.73(2K, t, J = 7.6
N,
F Hz), 3.64 (2H, t, J = 6.3 Hz), 3.72 (3H, s),
4.06 (2H, q, J = 9.5
104 01<" Hz), 6.81 (2H, d, J = 8.7 Hz), 7.20(2K, d, J =
8.4 Hz), 7.32(2K, 446 444
d, J = 8.1 Hz), 7.67(1K, d, J = 1.8 Hz), 7.87(2K, d, J = 8.1 Hz),
0 8.53(1K, d, J = 1.8 Hz), 13.04(1K, br s).
OH
108
CA 03074989 2020-03-05
[0367]
Table 1-14
MS MS
Ex Structure NMR
(M+H) (M-H)
o
H,c-
õ N,
1H-NMR (DMS0-06) 6:2.98 (4H, s), 3.72 (3H, s), 6.81 (2H, d,
105 J = 8.7 Hz), 7.17-7.23 (3H, m), 7.25-7.34 (6H, m),
7.66 (1H, s), 410 408
7.67(2K, d, J = 8.1 Hz), 8.52 (1H, s), 13.05 (1H, br s).
Ho
0
11,C'0 1H-NMR (DMSO-D6) 6:1.83-1.94 (2H, m), 2.73 (2H, t,
J = 7.8
Hz), 3.26 (3H, s), 3.38 (2H, t, J = 6.3 Hz), 3.80 (3H, s), 6.93-
106 7.07 (2H, m), 7.13 (1H, d, J = 12.9 Hz), 7.34 (2H,
d, J = 8.1 Hz), 396 394
0'CH3 7.68 (1H, s), 7.89 (2H, d, J = 8.1 Hz), 8.54 (1H,
s), 13.04 (1H,
HO br s).
0
H,C.0
1H-NMR (DMSO-D6) 6:1.83-1.94 (2H, m), 2.74 (2H, t, J = 7.8
Hz), 3.25 (3H, s), 3.38 (2H, t, J = 6.3 Hz), 3.91 (3H, s), 6.96
107 I 0,CH d, J = 9.6 Hz), 7.35 (2H, d, J = 8.4 Hz), 7.72
(1H, d, J = 1.8 414 412
,
Hz), 7.91 (2H, d, J = 8.4 Hz), 8.56 (1H, d, J = 1.8 Hz), 13.09
HO (1H, br s).
H3C= '0
1H-NMR (DMSO-D6) 6:1.82-1.92 (2H, m), 2.51-2.56(2K, m),
,
2.70 (2H, t, J = 7.7 Hz), 2.83 (2H, t, J = 7.6 Hz), 3.25 (3H, s),
108 0
3.37 (2H, t, J = 6.2 Hz), 3.81 (3H, s), 7.00-7.14 (5H, m), 7.20 424 .. 422
HO (2H, d, J = 8.1 Hz), 7.60 (1H, d, J = 2.2 Hz), 8.47
(1H, d, J = 2.2
Hz), 12.13(1H, br s).
0
HA'0
1H-NMR (DMSO-D6) 6: 1.95-2.02 (2H, m), 2.51 (3H, s), 2.92
(2H, t, J = 8.4 Hz), 3.25(3K, s), 3.42 (2H, t, J = 6.2 Hz), 3.95
109 (3H, s), 7.12 (2H, d, J = 9.7 Hz), 7.23 (1H, dd, J
= 8.1, 1.4 Hz), 429 427
N *-CH3 7.43 (1H, d, J = 1.4 Hz), 7.77 (1H, d, J
=8.1 Hz), 8.64 (1H, s),
HOJLJ
12.96 (1H, s).
O cH,
0
H3c-
, 1H-NMR (DMSO-D6) 6:0.91 (3H, t, J = 7.4 Hz), 1.31-
1.42
(2H, m), 1.65-1.76 (2H, m), 2.14 (6H, s), 2.81 (2H, t, J = 7.7
110 H3c cH, 391 389
Hz), 3.74 (3H, s), 6.88(2K, d, J = 9.0 Hz), 7.03 (2H, s), 7.33
HOyJ
(2H, d, J = 8.8 Hz), 8.52 (1H, s).
O cH3
H3Co
1H-NMR (DMSO-D6) 6:0.92 (3H, t, J = 7.4 Hz), 0.97 (3H, t, J
= 7.5 Hz), 1.32-1.44 (2H, m), 1.67-1.77 (2H, m), 2.78 (2H, q, J
111 CH, = 7.5 Hz), 2.84 (2H, t, J = 7.6 Hz), 3.73 (3H, s),
6.88 (2H, d, J =
391 389
9.0 Hz), 7.23 (1H, d, J = 1.6 Hz), 7.29 (2H, d, J = 9.0 Hz), 7.35
Ho
= cH, (1H, dd, J = 8.1, 1.8 Hz), 7.73 (1H, d, J =
8.1 Hz), 8.57 (1H, s),
12.92 (1H, br s).
H3C0
1H-NMR (DMS0-06) 6:1.93-2.03 (2H, m), 2.92(2K, t, J = 7.7
Hz), 3.24 (3H, s), 3.41 (2H, t, J = 6.2 Hz), 3.94(3K, s), 7.13
112 I (2H, d, J 9.5 Hz), 7.26 (1H, dd, J = 8.1, 1.6 Hz),
7.37 (1H, dd, 433 431
N CH J = 11.7, 1.5 Hz), 7.82 (1H, t, J = 7.9 Hz),
8.67 (11-I, s), 13.38
HO (1H, br s).
O F
109
CA 03074989 2020-03-05
[0368]
Table 1-15
Nis MS
Ex Structure NMR
(M+H) (M-H)
H,C'e0
1H-NMR (DMSO-D6) 5:1.93-2.02 (2H, m), 2.90 (2H, t, J = 7.7
: Hz), 3.24 (3H, s), 3.40 (2H, t, J = 6.4 Hz), 3.83
(3H, s), 7.07-
113 I = ].õõõ 415 413
7.15 (2H, m), 7.24-7.28 (2H, m), 7.32 (1H, dt, J = 15.0, 5.5 Hz),
N 'CH,
HO 7.80 (1H, t, J = 7.9 Hz), 8.63 (1H, s), 13.37 (1H,
br s).
O F
0
1H-NMR (DMSO-D6) 6:0.93 (3H, t, J = 7.3 Hz), 1.31-1.42
I (2H, m), 1.58-1.68(2K, m), 2.49 (3H, s), 2.67 (2H,
t, J = 7.6
114 H3C cH3 Hz), 3.73 (3H, s), 6.82 (2H, d, J = 9.0 Hz), 7.00
(1H, dd, J = 8.0, 376 374
1.5 Hz), 7.21-7.23 (3H, m), 7.62 (1H, d, J = 2.0 Hz), 7.70 (1H,
HO
d, J = 8.0 Hz), 8.50 (1H, d, J = 2.0 Hz), 12.84 (1H, br s).
0
113C'C) 1H-NMR (DMSO-D6) 6:1.82-1.91 (2H, m), 2.49 (3H, s),
2.72
(2H, t, J = 7.7 Hz), 3.24 (3H, s), 3.36 (2H, t, J = 6.2 Hz), 3.90
115 I (3H, s), 6.97 (2H, d, J = 9.5 Hz), 7.04 (1H, dd, J
= 8.1, 1.6 Hz), 428 426
7.23 (1H, s), 7.69 (1H, d, J = 2.1 Hz), 7.74 (1H, d, J = 8.1 Hz),
HO 8.53(1K, d, J = 2.3 Hz), 12.90 (1H, br s).
O CH,
H,C'0
1H-NMR (Dmso-D6) 5: 1.81-1.91(2K, m), 2.47 (3H, s), 2.70
, (2H, t, J = 7.7 Hz), 3.24 (3H, s), 3.36 (2H, t, J =
6.2 Hz), 3.71
116 (3H, s), 6.81 (2H, d, J = 8.8 Hz), 6.98 (1H, dd, J
= 8.1, 1.4 Hz), 392 390
7.18-7.24 (3H, m), 7.62 (1H, d, J = 2.3 Hz), 7.69 (1H, d, J = 8.1
HO
Hz), 8.49 (1H, d, J = 2.3 Hz), 12.81 (1H, br s).
O CH3
H3C'0
1H-NMR (DMS0-136) 5: 1.08 (3H, t, J = 7.1 Hz), 3.11(2K, t, J
= 6.5 Hz), 3.46 (2H, q, J = 7.0 Hz), 3.80 (2H, t, J = 6.5 Hz), 3.93
117 I ) 415 413
NCH, (3H, s), 7.10 (2H, d, J = 9.7 Hz), 7.52 (2H, d, J = 8.6 Hz), 7.92
HO (2H, d, J = 8.6 Hz), 8.65 (1H, s), 13.10 (1H, s).
0
H3C'0
1H-NMR (DMS0-06) 5:0.88 (3H, t, J = 7.4 Hz), 1.51-1.62
N,
I (2H, m), 3.45 (2H, t, J = 6.6 Hz), 3.71 (3H, s),
4.57 (2H, s), 6.81
118 (2H, d, J 8.8 Hz), 7.21 (2H, d, J = 8.8 Hz), 7.30
(2H, d, J = 8.3 378 376
H3
HO Hz), 7.71 (1H, d, J = 2.1 Hz), 7.86 (2H, d, J = 8.6
Hz), 8.60 (1H,
d, J =2.1 Hz), 13.01 (1H, br s).
0
H,C'0
1H-NMR (DMSO-D6) 5: 1.86-1.97 (2H, m), 2.83 (2H, t, J = 7.6
Hz), 3.21 (3H, s), 3.39-3.51 (6H, m), 3.75 (3H, s), 6.91 (2H, d, J
119 = 9.0 Hz), 7.26 (2H, d, J = 9.0 Hz), 7.34 (2H, d, J
= 8.6 Hz), 422 420
7.89 (2H, d, J = 8.6 Hz), 8.15 br s), 8.68 (1H, d, J = 1.8
HO
HCI Hz), 13.04 (1H, br s).
H3C_0
N,
1H-NMR (DMSO-D6) 5: 1.82-1.92 (2H, m), 2.70 (2H, t, J = 7.7
Hz), 3.24 (3H, s), 3.36(2K, t, J = 6.4 Hz), 3.72 (3H, s), 6.84
120 (2H, d, J = 8.8 Hz), 7.14 (1H, dd, J =8.1, 1.6 Hz),
7.22 (2H, d, J 412 410
= 8.8 Hz), 7.42(1K, d, J = 1.6 Hz), 7.64-7.70(2K, m), 8.51 (1H,
HO
d, J =2.1 Hz), 13.39 (1H, br s).
O CI
110
CA 03074989 2020-03-05
[0369]
Table 1-16
MS MS
Ex Structure NMR
(M+H) (M-H)
1.13C 1H-NMR (DMSO-D6) 6: 1.96-2.03 (2H, m), 2.93 (2H, t,
J = 7.7
CH Hz), 3.25 (3H, s), 3.42 (2H, t, J = 6.4 Hz), 3.96 (3H, s), 7.17
121 449 447
(2H, d, J = 9.2 Hz), 7.36 (1H, dd, J = 8.1,1.8 Hz), 7.64 (1H, d, J
HO,,,,1.JLJ= 1.6 Hz), 7.75 (1H, d, J = 8.1 Hz), 8.68 (1H, s), 13.52 (1H, s).
O CI
H3C,0
1H-NMR (DMSO-D6) 6: 1.82-1.92 (2H, m), 2.72 (2H, t, J = 7.7
Hz), 3.24 (3H, s), 3.36 (2H, t, J = 6.2 Hz), 3.91 (3H, s), 7.00
1 N
122 (2H, d, J = 9.7 Hz), 7.18 (1H, dd, J = 8.0,1.7 Hz),
7.47 (1H, d, J 446 448
0 'C H3 = 1.6 Hz), 7.71 (1H, d, J =7.9 Hz), 7.76 (1H, d, J =2.1 Hz),
HO 8.55 (1H, d, J = 2.1 Hz), 13.42(1H, br s).
O ci
0
H3C' 1H-NMR (DMS0-06) 6: 1.51-1.74 (4H, m), 2.69 (2H, t,
J = 7.5
N, Hz), 3.22 (3H, s), 3.35 (2H, t, J = 6.3 Hz), 3.72
(3H, s), 6.81
123 (2H, d, J = 8.9 Hz), 7.20 (2H, d, J = 8.9 Hz), 7.32
(2H, d, J = 8.1 392 390
_CH3
0 Hz), 7.64 (1H, d, J = 1.5 Hz), 7.87 (2H, d, J = 8.1
Hz), 8.52 (1H,
HO d, J = 1.5 Hz), 12.99 (11-I, br s).
0_
H3C0 1H-NMR (DMSO-D6) 6:1.82-1.91 (2H, m), 2.72 (2H, t,
J = 7.7
Hz), 3.24 (3H, s), 3.36 (2H, t, J = 6.2 Hz), 3.91 (3H, s), 6.70
124 (1H, dd, J = 8.1, 1.6 Hz), 6.87 (1H, d, J = 1.6
Hz), 7.00 (2H, d, J 430 428
'cH, = 9.7 Hz), 7.70 (1H, d, J = 2.1 Hz), 7.71 (1H, d, J = 8.1 Hz),
HO 8.54(1H, d, J =2.1 Hz), 11.37(1H, br s), 14.00 (1H,
brs).
O OH
H3C'0
1H-NMR (DMSO-D6) 6:1.50-1.59 (2H, m), 1.62-1.72 (2H, m),
N,
2.67 (2H, t, J = 7.6 Hz), 3.20 (3H, s), 3.34 (2H, t, J = 6.4 Hz),
125 0õCH3 3.72 (3H, s), 6.84 (2H, d, J = 9.0 Hz), 7.14 (1H,
dd, J = 8.0, 1.7 426 424
Hz), 7.22 (2H, d, J = 8.8 Hz), 7.41 (1H, d, J = 1.8 Hz), 7.65-7.69
HO
(2H, m), 8.51 (1H, d, J = 2.1 Hz), 13.39 (1H, br s).
O CI
H3C'0
1H-NMR (DMSO-D6) 6: 1.50-1.59 (2H, m), 1.62-1.71 (2H, m),
N,
2.47 (3H, s), 2.67 (2H, t, J = 7.5 Hz), 3.20(3K, s), 3.33 (2H, t, J
126 0-CH3 = 6.4 Hz), 3.71 (3H, s), 6.81 (2H, d, J = 9.0 Hz),
6.98 (1H, dd, J 406 404
= 8.0, 1.5 Hz), 7.19-7.23 (3H, m), 7.61 (1H, d, J = 2.1 Hz), 7.68
HO
(1H, d, J = 8.1 Hz), 8.48 (1H, d, J =2.1 Hz), 12.81 (1H, br s).
o cH3
H3C"0
1H-NMR (pmso-06) 6:1.49-1.58 (2H, m), 1.60-1.71 (2H, m),
N, 2.47-2.53 (2H, m), 2.65 (2H, t, J = 7.5 Hz), 2.80
(2H, t, J = 7.6
127 c+13 Hz), 3.20 (3H, s), 3.33(2K, t, J = 6.4 Hz), 3.71
(3H, s), 6.77 420 418
0' (2H, d, J = 9.0 Hz), 7.08 (2H, d, J = 8.3 Hz), 7.16
(2H, d, J = 8.3
Ho Hz), 7.19 (2H, d, J = 8.8 Hz), 7.55 (1H, d, J = 2.3
Hz), 8.44 (1H,
d,J = 2.1 Hz), 12.15 (1H, brs).
0
= 0 1H-NMR (DMSO-D6) 6:1.08 (3H, t, J = 7.1 Hz),
2.49-2.55
(2H, m), 2.82 (2H, t, J = 7.6 Hz), 2.89 (2H, t, J = 6.6 Hz), 3.44
(2H, q, J = 6.9 Hz), 3.64 (2H, t, J = 6.7 Hz), 3.90 (3H, s), 6.94
128 442 420
(2H, d, -1= 9.7 Hz), 7.11 (2H, d, J = 8.3 Hz), 7.22 (2H, d, J = 8.3
HO Hz), 7.67 (1H, d, J = 2.1 Hz), 8.51 (1H, d, J =2.1
Hz), 12.15
(1H, br s).
111
CA 03074989 2020-03-05
[0370]
Table 1-17
MS MS
Ex Structure NMR
(M+H) (M-H)
H3C,0 1H-NMR (DMS0-06) 8:1.52-1.72 (4H, m), 2.71 (2H, t,
J = 7.6
Hz), 3.21 (3H, s), 3.35 (2H, t, J = 6.3 Hz), 3.91 (3H, s), 6.94-
129 õCH, 6.99 (2H, m), 7.36 (2H, d, J = 8.1 Hz), 7.71
(1H, d, J = 1.8 Hz), 428 426
7.92 (2H, d, J =8.1 Hz), 8.56 (1H, d, J = 1.8 Hz), 13.06(1H, br
HO s).
0
1H-NMR (DMSO-D6) 6:1.50-1.73 (4H, m), 2.51 (3H, s), 2.71
(2H, t, J = 7.3 Hz), 3.22 (3H, s), 3.35 (2H, t, J = 6.4 Hz), 3.91
130 I (3H, s), 6.94-7.01 (2H, m), 7.06 (1H, d, J = 8.1
Hz), 7.25 (1H, br 442 440
esc
0...CH,
s), 7.70 (1H, br s), 7.76 (1H, d, J = 8.1 Hz), 8.54 (1H, br s),
HO 12.90 (1H, br s).
0
0
1H-NMR (DMS0-06) 6:1.50-1.75 (4H, m), 2.71 (2H, t, J = 7.6
Hz), 3.22 (3H, s), 3.32-3.38 (2H, m), 3.93 (3H, s), 6.97-7.06
131 CH,462 460
(2H, m), 7.20 (1H, d, J = 8.1 Hz), 7.48 (1H, Drs), 7.71-7.77 (2H,
'
HO m), 8.56 (1H, Drs), 13.46 (1H, br s).
0
H3C'0 1H-NMR (DMSO-D6) 6:1.50-1.73 (4H, m), 2.50-2.56
(2H, m),
2.69(2K, t, J = 7.5 Hz), 2.84 (2H, t, J = 7.6 Hz), 3.21 (3H, s),
NN
132 3.31-3.37 (2H, m), 3.91 (3H, s), 6.91-7.00 (2H, m),
7.13 (2H, d, 456 454
0" J = 8.0 Hz), 7.24 (2H, d, J = 8.0 Hz), 7.64 (1H, d, J = 1.7 Hz),
HO 8.50 (1H, d, J = 1.7 Hz), 12.13 (1H, br s).
11,C 1H-NMR (DMSO-D6) 6:1.08 (3H, t, J = 7.1 Hz), 2.49
(3H, s),
3.10(2K, t, J = 6.5 Hz), 3.46(2K, q, J = 7.0 Hz), 3.79 (21-1, t, J =
133 6.5 Hz), 3.93 (3H, s), 7.11 (2H, d, J = 9.5 Hz),
7.21 (1H, dd, J = 429 427
N 8.1,1.4 Hz), 7.41 (1H, d, J = 1.6 Hz), 7.75 (11-1, d, J = 8.1 Hz),
HO 8.64 (1H, s), 12.96 (1H, br s).
O CH,
H3C'0 1H-NMR (DMSO-D6) 6:1.08 (3H, t, J = 7.1 Hz), 2.53
(2H, t, J
= 7.6 Hz), 2.83 (2H, t, J = 7.5 Hz), 3.08(2K, t, J = 6.6 Hz), 3.45
134 I (2H, q, J = 7.0 Hz), 3.79(2K, t, J = 6.5 Hz), 3.93
(3H, s), 7.08 443 441
(2H, d, J = 9.7 Hz), 7.24(2K, d, J = 8.3 Hz), 7.32 (2H, d, J = 8.3
HO Hz), 8.58 (1H, s), 12.07 (1H, Drs).
0
1H-NMR (DMSO-D6) 6: 1.56-1.63 (2H, m), 1.75-1.82(2K, m),
2.51 (3H, s), 2.89 (2H, t, J = 7.6 Hz), 3.22 (3H, s), 3.36 (2H, t, J
135 I = 6.5 Hz), 3.94(3K, s), 7.11(2K, d, J = 9.2 Hz),
7.23(1K, dd, J 443 441
NCH,
= 8.1, 1.4 Hz), 7.42 (1 d, J = 1.41-1z), 7.77 (1H, d, J = 8.1
Hz),
HOyJJ8.64(1K, s), 12.96 (1H, s).
O CH,
H,C***0
1H-NMR (Dniso-D6) 6:1.56-1.63 (2H, m), 1.75-1.83 (2H, m),
2.89(2K, t, J = 7.6 Hz), 3.22 (3H, s), 3.36(2K, t, J = 6.5 Hz),
136 I,CH3 3.94 (3H, s), 7.11 (2H, d, J = 9.2 Hz), 7.23 (1H,
dd, J = 8.1,1.5 463 461
Hz), 7.42 (1H, d, J = 1.5 Hz), 7.77 (1H, d, J = 8.1 Fiz), 8.64 (1H,
HOJJs), 12.96 (1H, s).
O CI
112
CA 03074989 2020-03-05
[0371]
Table 1-18
nois MS
Ex Structure NMR
(M+H) (M-H)
H3C)3 1H-NMR (DMSO-D6) 6:1.11 (3H, t, J = 7.0 Hz), 2.49
(3H, s),
2.90 (2H, t, J = 6.7 Hz), 3.46 (2H, q, J = 7.0 Hz), 3.65 (2H, t, J =
137 H3C 6.7 Hz), 3.73 (3H, s), 6.83 (2H, d, J = 8.7 Hz),
7.00 (1H, d, J =
OcN3 8.1 Hz), 7.23 (2H, d, J = 8.7 Hz), 7.23 (1H, brs),
7.68 (1H, d, J = 392 390
HO 1.8 Hz), 7.71 (1H, d, J = 8.1 Hz), 8.53 (1H, d, J =
1.8 Hz), 12.84
(1H, s).
0
H3C-0 1H-NMR (DMSO-D6) 6: 1.11 (3H, t, J = 7.0 Hz), 2.91
(2H, t, J
= 6.7 Hz), 3.47 (2H, q, J = 7.0 Hz), 3.66 (2H, t, J = 6.7 Hz), 3.74
N,
(3H, s), 6.86 (2H, d, J = 9.0 Hz), 7.16 (1H, dd, J = 8.1, 1.5 Hz),
138 7.23 (2H, d, J = 9.0 Hz), 7A3 (1H, d, J = 1.5
H4,7.71 (1H, d, J 412 410
= 8.1 Hz), 7.74 (1H, d, J = 2.1 Hz), 8.55 (1H, d, J = 1.8 Hz),
HO
13.41 (1H, s).
0
H3C-0 1H-NMR (DMSO-D6) 6:1.10 (3H, t, J = 7.0 Hz), 2.51
(2H, t, J
= 7.6 Hz), 2.81 (2H, t, J = 7.6 Hz), 2.88 (2H, t, J = 6.7 Hz), 3.46
(2H, q, J = 7.0 Hz), 3.65 (2H, J = 6.7 Hz), 3.73 (3H, s), 6.79
139
0CH3 (2H, d, J =9.0 Hz), 7.09(2H, d, J =8.1 Hz), 7.18 (2H, d, J = 8.1 406
404
Hz), 7.21 (2H, d, J = 9.0 Hz), 7.62 (1H, d, J = 1.8 Hz), 8.48 (1H,
HO
d, J = 1.8 Hz), 12.18 (1H, s).
0
H3C'0 1H-NMR (DMSO-D6) 6:1.10 (3H, t, J = 7.0 Hz), 2.93
(2H, t, J =
6.6 Hz), 3.46 (2H, q, J = 7.1 Hz), 3.66 (2H, t, J = 6.6 Hz), 3.91
N,
(3H, s), 6.97 (2H, d, J = 9.3 Hz), 7.35 (2H, d, J = 8.1 Hz), 7.76
140 414 412
0CH, (1H, d, J =1 .8 Hz), 7.92 (2H, d, J = 8.1 Hz), 8.59
(1H, d, J = 2.1
HO Hz), 13.06 (1H, s).
0
H,C".0
1H-NMR (DMSO-D6) 6:1.10 (3H, t, J = 7.0 Hz), 2.51 (3H, s),
. 2.92 (2H, J = 6.4 Hz), 3.43-3.49 (2H, m), 3.66 (2H,
t, J = 6.7
141 428 426
HacCH3 Hz), 3.92 (3H, s), 6.96-7.07 (3H, m), 7.22-7.25
(1H, m), 7.73-
7.77 (2H, m), 8.57 (1H, d, J = 1.8 Hz), 12.91 (1H, br s).
HO
0
1H-NMR (DMS0-136) 6:1.10 (3H, t, J = 6.9 Hz), 2.93 (2H, t, J =
6.6 Hz), 3.46 (2H, q, J = 6.9 Hz), 3.66 (2H, t, J = 6.6 Hz), 3.93
142 I (3H, s), 7.02 (2H, d, J = 9.3 Hz), 7.19 (1H, d, J =
7.8 Hz), 7.47 448 446
ci
cm3 (1H, s), 7.73 (1H, d, J = 7.8 Hz), 7.81 (1H, s),
8.59 (1H, s),
No 13.47 (1H, s).
H3C
1H-NMR (DMS0-136) 6:0.88 (3H, t, J 7.5 Hz), 1.50-1.61
N,
(2H, m), 2.47 (3H, s), 3.45 (2H, t, J = 6.6 Hz), 3.72 (3H, s), 4.56
143
(2H, s), 6.82 (2H, d, J = 8.8 Hz), 6.99 (1H, dd, J = 8.0,1.5 Hz), 392
390
HO H3 7.20-7.26 (3H, m), 7.70 (2H, dd, J = 5.1,3.0 Hz),
8.59 (1H, d, J
=2.1 Hz), 12.82(1H, br s).
0 CH3
H3C,0 1H-NMR (DMSO-D6) 6:0.88 (3H, t, J = 7.4 Hz), 1.50-
1.61
N, (2H, m), 2.48-2.54 (2H, m), 2.80 (21-I, t, J = 7.5
Hz), 3.44 (2H, t,
J = 6.6 Hz), 3.71 (31-I, s), 4.55 (2H, s), 6.79 (2H, d, J = 9.0 Hz),
144 0_ 406 404
'CH3 7.08 (2H, d, J = 8.1 Hz), 7.17 (2H, d, J = 8.3 Hz), 7.21 (21-I, d, J
HO = 9.0 Hz), 7.64 (1H, d, J = 2.3 Hz), 8.54 (1H, d, J
= 2.1 Hz),
12.09 (1H, br s).
0
113
CA 03074989 2020-03-05
[0372]
Table 1-19
Ms MS
Ex Structure NMR
(M+H) (M-1-1)
H3C".0 1H-NMR (DMSO-D6) .5: 0.88 (3H, t, J = 7.4 Hz), 1.51-
1.62
(2H, m), 3.46 (2H, J = 6.6 Hz), 3.90 (3H, s), 4.59 (2H, s), 6.97
,
145 d, J = 9.7 Hz), 7.35 (2H, d, J = 8.3 Hz), 7.78
(1H, d, J = 2.1 414 412
Hz), 7.91 (2H, d, J = 8.6 Hz), 8.65 (1H, d, J = 2.1 Hz), 12.99
Ho (1H, Drs).
0
H3C,0 1H-NMR (DMSO-D6) 8: 0.88 (3H, t, J = 7.4 Hz), 1.51-
1.62
(2H, m), 2.50-2.55(2K, m), 2.83 (2H, t, J = 7.6 Hz), 3.45 (2H, t,
146 J = 6.6 Hz), 3.90 (3H, s), 4.57 (2H, s), 6.96 (2H,
d, J = 9.7 Hz), 442 440
0"--"CH3 7.12 (2H, d, J = 8.3 Hz), 7.23(2K, d, J = 8.3 Hz),
7.71 (1H, d, J
Ho =2.1 Hz), 8.59 (1H, d, J = 1.8 Hz), 12.12 (1H, br
s).
0
H3C,0
1H-NMR (DMSO-D6)5: 0.88 (3H, 1, J = 7.4 Hz), 1.51-1.62
(21-1, m), 2.49 (3H, s), 3.45 (2H, J = 6.6 Hz), 3.91 (3H, s), 4.59
147 I (2H, s), 6.99 (2H, d, J = 9.7 Hz), 7.05 (1H, dd, J
= 8.0, 1.5 Hz), 428 426
7.23 (1H, d, J = 1.4 Hz), 7.72-7.78(2K, m), 8.63 (1H, d, J = 2.1
HO Hz), 12.89 (1H, br s).
O CH3
H3C0
1H-NMR (DMSO-D6) 5:1.56-1.63 (2H, m), 1.74-1.82 (2H, m),
2.54 (2H, t, J = 7.6 Hz), 2.83-2.89 (4H, m), 3.21 (3H, s), 3.36
148 I cH (2H, t, J 6.5 Hz), 3.94 (3H, s), 7.09 (2H, d, J =
9.5 Hz), 7.25 457 455
(2H, d, J = 8.3 Hz), 7.33 (2H, d, J =8.3 Hz), 8.58 (1H, s), 12.13
HO (1H, s).
0
H3C,..0 1H-NMR (DMSO-D6) 5: 0.94 (6H, d, J = 6.7 Hz), 2.07-
2.18
(1H, m), 2.49 (3H, s), 2.75 (2H, d, J = 7.2 Hz), 3.93 (3H, s),
149 5: = 3
7.10 (2H, d, J = 9.5 Hz), 7.22 (1H, dd, J = 8.1, 1.4 Fiz), 7.40 413 411
= CH3 (1H, d, J = 1.6 Hz), 7.76 (1H, d, J =8.1
Hz), 8.59 (1H, s), 12.91
HO (1H, br s).
O CH3
H3C'0
1H-NMR (DMSO-D6) 5:0.94 (6H, d, J = 6.7 Hz), 2.06-2.17
(1H, m), 2.52 (2H, t, J = 7.6 Hz), 2.72 (2H, d, J = 7.2 Hz), 2.83
150 H3
(211, t, J = 7.5 Hz), 3.93 (3H, s), 7.08 (2H, d, J = 9.5 Hz), 7.23 427
425
(2H, d, J = 8.3 Hz), 7.32 (21-I, d, J = 8.3 Hz), 8.53 (11-1, s), 12.13
HO (1H, br s).
0
HAI)
11-1-NMR (DMSO-D6) 5: 0.94 (6H, d, J = 6.5 Hz), 2.07-2.19
(1H, m), 2.76 (2H, d, J = 7.2 Hz), 3.94 (3H, s), 7.16(2K, d, J =
151 433 431
9.5 Hz), 7.35 (1H, dd, J = 8.1, 1.8 Hz), 7.61 (1H, d, J = 1.6 Hz),
CH3
HO 7.74 (1H, d, J = 8.1 Hz), 8.62 (1H, s), 13.50 (1H,
br s).
O CI
H3C".0
1H-NMR (DMSO-D6) 5: 0.93 (3H, t, J = 7.4 Hz), 1.35-1.44
(2H, m), 1.70-1.77 (2H, m), 2.51 (3H, s), 2.88 (2H, t, J = 7.6
152 CH3 Hz), 3.95 (3H, s), 7.11(2K, d, J = 9.5 Hz),
7.23(1K, dd, J = 8.1, 413 411
1.5 Hz), 7.42 (11-1, d, J= 1.5 Hz), 7.77 (1H, d, J = 8.1 Hz), 8.64
Ho
(1H, s), 12.95 (1H, s).
O CH3
114
CA 03074989 2020-03-05
[0373]
Table 1-20
ms MS
Ex Structure NMR
(M+H) (M-P0
1H-NMR (DMSO-D6) 8:0.93 (3H, t, J = 7.4 Hz), 1.34-1.43
(2H, m), 1.69-1.77 (2H, m), 2.54 (2H, t, J = 7.6 Hz), 2.83-2.88
153 I CF13 427 425
(4H, m), 3.94 (3H, s), 7.09 (2H, d, J = 9.2 Hz), 7.25 (2H, d, J =
HO 8.3 Hz), 7.33 (2H, d, J = 8.3 Hz), 8.58 (1H, s),
12.12 (1H, s).
[0374]
The formulation examples of the present invention include
the following formulations. However, the present invention is
not limited by such formulation examples.
[0375]
Formulation Example 1 (Production of capsule)
/o 1) Compound of Example 1 30 mg
2) Microcrystalline cellulose 10 mg
3) Lactose 19 mg
4) Magnesium stearate 1 mg
1), 2), 3) and 4) are mixed and filled in a gelatin
/5 capsule.
[0376]
Formulation Example 2 (Production of tablet)
1) Compound of Example 1 10 g
2) Lactose 50 g
20 3) Corn starch 15 g
4) Carmellose calcium 44 g
5) Magnesium stearate 1 g
The total amount of 1), 2), 3) and 30 g of 4) are kneaded
with water, vacuum dried and sieved. The sieved powder is mixed
25 with 14 g of 4) and 1 g of 5), and the mixture is tableted by a
tableting machine. In this way, 1000 tablets containing 10 mg of
the compound of Example 1 per tablet are obtained.
[0377]
Test Example 1: Evaluation of human GLUT9 inhibitory activity
30 A method for measuring GLUT9 inhibitory activity using
human GLUT9 expressing cells transduced with Uricase/Hyper is
115
CA 03074989 2020-03-05
shown hereinafter. GLUT9 inhibitory activity (IC50 value) of
the test compound was calculated based on the intracellular
uptake amount of labeled uric acid (['4C] uric acid) transported
by GLUT9.
[0378]
1. Preparation of human GLUT9 expression plasmid
Human GLUT9S coding region (NM 001001290.1) was amplified
by PCR method using PrimeSTAR MAX DNA Polymerase (Takara Bio).
The obtained PCR product was subjected to agarose gel
lo electrophoresis, and the desired size of FOR product was
purified by QIAquick Gel Extraction Kit (QIAGEN). Next,
pEBMulti-Bsd vector (Wako Pure Chemical Industries, Ltd.) was
subjected to digestion using restriction enzymes Sail and EcoR
V, and linked to the PCR product containing GLUT9S by In-Fusion
].5 reaction using In-Fusion HD Cloning Kit (Takara Bio). XL10-
Gold Competent Cells (Agilent Technologies) were transformed in
In-Fusion reaction solution, and the obtained transfectants
were cultured with shaking overnight in LB medium to which
Blasticidin S HC1 (Thermo Fisher Scientific) was added. Human
20 GLUT9 expression plasmid DNA was prepared from the collected
transfectants using EndoFree Plasmid Kit (Takara Bio).
[0379]
2. Establish of human GLUT9 stably expressing cell
HEK293T cells (American Type Culture Collection, DS
25 Pharma Biomedical) were seeded in a 6 well plate at 1x106
cells/well. Next day, human GLUT9 expression plasmid was
transfected by using Lipofectamine 3000 Reagent (Thermo Fisher
Scientific) as follows. Lipofectamine 3000 Reagent (5 pL) was
diluted with Opti-MEM (125 pL, Thermo Fisher Scientific).
30 Separately, plasmid DNA (2.5 pg) was diluted with Opti-MEM (125
pL), and P3000 Reagent (5 pL) was added thereto. The obtained
solution was mixed with the previously obtained diluted
solution, and the mixture was left stand at room temperature
for 5 min, and added to the cells. Next day, the transfected
35 cells were diluted and reseeded in a 6 well plate at 4x105
116
ak 03074989 2020-03-05
=
cells/well, and drug selection was performed in the presence of
30 pg/mL of Blasticidin S HC1, and thereby, Blasticidin-
resistant human GLUT9 stably expressing cell line was
established. Mock cells were prepared by introducing pEBMulti-
Bsd vector into HEK293T cells in a similar manner.
[0380]
3. Preparation of Uricase/Hyper expression plasmid
Uricase/Hyper expression plasmid was prepared by linking
the coding regions of Aspergillus Flavus-derived uricase gene
lo and pHyPer-Cyto vector (Evrogen) HyPer gene to the pEBMulti-Hyg
vector (Wako Pure Chemical Industries, Ltd.) and then
introducing it. The preparation method was performed by
reference to Gout and Nucleic Acid Metabolism 2013, 37(2), 93-
101, and seven repeated linker sequence of glycine-glycine-
15 glycine-serine was prepared herein.
[0381]
4. Preparation of human GLUT9-Uricase/Hyper coexpressing cell
Human GLUT9 stably expressing cells were seeded at
3.6x107 cells per 225cm2 flask. Next day, Uricase/Hyper
20 expression plasmid was transfected by using Lipofectamine 3000
Reagent as follows. Lipofectamine 3000 Reagent (40 pL) was
diluted with Opti-MEM (750 uL). Separately, plasmid DNA (20
fig) was diluted with Opti-MEM (750 pL), and P3000 Reagent (40
pL) was added thereto. The obtained solution was mixed with
25 the previously obtained diluted solution, and the mixture was
left stand at room temperature for 5 min, and added to the
cells. Next day, transfected cells were collected to prepare
human GLUT9-Uricase/Hyper coexpressing cells.
[0382]
39 5. Evaluation of GLUT9 inhibitory activity
Uricase/Hyper transiently-transfected human GLUT9 stably
expressing cells or mock cells (blank) were seeded in a 96 well
plate (Corning) at 1.6x105 cells/well, and cultured overnight
at 37 C, 5% CO2. D-MEM/high glucose (Wako Pure Chemical
33 Industries, Ltd.) containing 10% Fetal Bovine Serum
117
CA 03074989 2020-03-05
(Lifetechnology) and 100 units/ml penicillin/100 pg/ml
streptomycin (GIBCO) was used as a medium. High K4- buffer
(129.8 mM KC1, 1.2 mM KH2PO4, 1.2 mM MgSO4=7H20, 1.3 mM CaC12'
2H20, 25 mM HEPES, pH 7.4 with 1 M Tris) and the medium were
mixed in equal amount to prepare Assay Buffer. The medium in
each well was removed, and the test compound solution (final 1%
DMSO) diluted with Assay Buffer was added thereto at 50 p1/well,
and the mixture was left stand at room temperature for 30 to 60
min. For the solvent control and blank, Assay Buffer
containing DMSO alone was added at 50 ill/well, and the mixture
was left stand at room temperature for 30 to 60 min. In
addition, uric acid solution (containing ['4C]uric acid as a
tracer) diluted with Assay Buffer was added to each well at 15
p1/well (final 300 pM uric acid), and the uptake reaction was
/5 performed at room temperature for 6 min. After the completion
of the reaction, the cells were washed three times with ice-
cooled Wash Buffer (Hank's Balanced Salt Solution containing
0.01% Bovine Serum Albumin) at 150 p1/well, and 0.1N aqueous
NaOH solution was added thereto at 25 p1/well to dissolve the
cell. MicroScint-20 (Perkin-Elmer) was added thereto at 150
p1/well, the plate was shaked, and CPM of pc,
J was measured by
TopCount NXT (Perkin-Elmer).
Data was obtained by deducting average of CPM in blank
well from average of CPM in each treated well. The inhibitory
rate of the test compound in each concentration was calculated
from the following formula: [(A-B)/A]x100, A is data of solvent
control, B is data of test compound treatment. IC50 value (50%
inhibition concentration) of the test compound was obtained by
applying the inhibitory rate of the test compound in each
concentration to logistic curve.
[0383]
The results are shown in Table 2-1 to Table 2-6. With
regard to Examples 7, 15, 22, 33, 47, 54, 60 and 76 in tables,
the GLUT9 inhibitory rate at 10 uM of compound are shown
therein.
118
CA 03074989 2020-03-05
[0384]
Table 2-1
humans GLUT9 inhibitory activity
Example
(IC50 value (1.1M))
1 0.0807
2 0.9461
3 0.1341
4 0.6124
0.0626
6 0.1409
7 9% inhibition at 10 uM
8 0.1504
9 0.4831
1.7769
11 0.0659
12 5.0637
13 0.2188
14 0.0914
5% inhibition at 10 TIM
16 0.0819
17 0.6922
18 0.0942
19 3.0737
0.1198
21 0.0607
22 9% inhibition at 10 uM
23 0.9916
24 0.1342
0.0837
26 0.1543
27 0.2548
28 4.2515
29 0.1578
0.0892
119
CA 03074989 2020-03-05
[0385]
Table 2-2
humans GLUT9 inhibitory activity
Example
(1050 value (pM))
31 0.1336
32 0.0888
33 28% inhibition at 10 uM
34 0.1241
35 0.1193
36 0.1891
37 0.1106
38 0.8652
39 1.4456
40 0.3405
41 0.0890
42 6.5049
43 7.1322
44 1.6522
45 0.0506
46 2.7530
47 47% inhibition at 10 jiM
48 7.1178
49 0.2005
50 0.1333
51 0.9618
52 0.1186
53 0.4600
54 43% inhibition at 10 uM
55 3.9895
56 0.1798
57 0.3443
58 0.0520
59 0.1315
60 45% inhibition at 10 uM
120
CA 03074989 2020-03-05
*
[0386]
Table 2-3
humans GLUT9 inhibitory activity
Example
(IC50 value (pM))
61 5.5905
62 0.1267
63 0.6586
64 0.0987
65 0.0946
66 0.0770
67 0.1024
68 0.0578
69 0.8563
70 1.6718
71 0.1318
72 0.5258
73 0.1044
74 0.1410
75 0.0844
76 10% inhibition at 10 pM
77 0.0585
78 2.2102
79 0.3686
80 0.8421
81 0.1924
82 0.2450
83 0.0662
84 0.1167
85 0.8527
86 1.8180
87 1.7851
88 0.9282
89 1.7982
90 0.1397
121
ak 03074989 2020-03-05
[0387]
Table 2-4
humans GLUT9 inhibitory activity
Example
(IC50 value (I'M))
91 0.2470
92 0.1810
93 0.1837
94 0.0918
95 0.0576
96 0.0887
97 0.0795
98 0.0605
99 0.0514
100 0.0820
101 0.1642
102 0.7899
103 0.0720
104 0.1388
105 0.4533
106 0.1007
107 0.0627
108 0.0712
109 0.0799
110 0.2649
111 0.0826
112 0.5607
113 1.0001
114 0.0604
115 0.0497
116 0.0684
117 0.5432
118 0.1078
119 0.5012
120 0.5391
122
CA 03074989 2020-03-05
. ,
[0388]
Table 2-5
humans GLUT9 inhibitory activity
Example
(IC50 value (TIM))
121 0.5007
122 0.2579
123 0.0589
124 0.1826
125 0.2287
126 0.0497
127 0.0688
128 0.0732
129 0.0569
130 0.0521
131 0.1552
132 0.0519
133 0.3134
134 0.5248
135 0.0613
136 0.2502
137 0.0917
138 0.4993
139 0.1672
140 0.0713
141 0.0639
142 0.2263
143 0.1253
144 0.3270
145 0.1065
146 0.1387
147 0.0868
148 0.0807
149 0.0574
150 0.1018
123
CA 03074989 2020-03-05
[0389]
Table 2-6
humans GLUT9 inhibitory activity
Example
(I050 value (11M))
151 0.1064
152 0.0590
153 0.1023
Industrial Applicability
[0390]
Since Compound [I] or a pharmaceutically acceptable salt
thereof has a GLUT9 inhibitory activity, it may be useful for
the treatment or prophylaxis of a disease selected from the
group consisting of hyperuricemia and gout.
124