Note: Descriptions are shown in the official language in which they were submitted.
CA 03075454 2020-03-10
WO 2019/077385
PCT/1B2017/001506
PROCESSING SYSTEM AND DYNAMIC CORRECTION METHOD
FOR THERMAL THERAPY
Technical Field
[0001] The present application relates to thermal therapy and/or other
systems
and methods that use temperature measurements derived from magnetic resonance
imaging (MRI).
Background
[0002] The use of
magnetic resonance imaging (MRI) to obtain temperature
related data in a tissue ablation procedure is discussed, e.g., in US Patent.
No.
7,771,418, which is hereby incorporated by reference in its entirety. One
application
for such therapies is in treating a diseased male prostate.
[0003] Temperature
measurements derived from MRI methods are subject to
errors or potential errors from a variety of sources. These errors or
potential errors can
create temperature measurement uncertainty and/or significantly reduce the
accuracy
of measuring temperature changes.
[0004] When temperature measurements are used as part of a feedback system
for thermal energy delivery, temperature measurement uncertainty and/or
reduced
accuracy can make it more difficult to determine whether there has been a lack
of
heating in a target region and/or unintended heating of any other regions.
Lack of
heating in a target region can result in an incomplete thermal therapy
session.
Unintended heating of other regions may require that thermal therapy be
halted, at
1
CA 03075454 2020-03-10
WO 2019/077385 PCT/IB2017/001506
least temporarily, in order to allow such regions to cool. This can result in
a less than
optimal thermal therapy session from a patient comfort perspective, as well as
less
economical use of the MRI-thermal therapy facility, personnel and equipment.
[0005] Methods to address temperature measurement uncertainties are
disclosed in U.S. Patent Application Publication No. 2015/0038883, filed on
August 4,
2014, entitled "Treatment Planning and Delivery Using Temperature Uncertainty
Maps", which is hereby incorporated by reference in its entirety. One method
disclosed therein reduces the magnitude of temperature measurement uncertainty
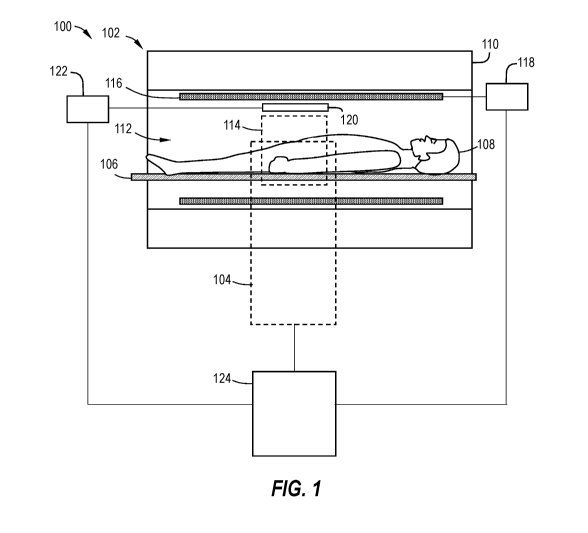
by
detecting drift in temperature measurements, and adjusting all temperatures
measurements based on the detected drift.
Summary
[0006] It has been determined that it is possible to further reduce the
effects of
errors and/or potential errors in systems and methods that use temperature
measurements derived from magnetic resonance imaging (MRI).
[0007] At least some aspects disclosed herein have the ability to address
noise
from various sources, including: magnetic resonance (MR) artifacts, frequency
drift, low
SNR regions, non-uniform tissue structures and/or others.
[0008] Accordingly, improved accuracy and/or efficiency of delivery of MRI-
guided thermal therapies and/or other systems and methods is made possible.
[0009] At least some aspects disclosed herein employ one or more dynamic
correction methodologies during thermal treatment or other procedure, since
noise
levels can change over time.
2
CA 03075454 2020-03-10
WO 2019/077385 PCT/IB2017/001506
[0010] In one aspect, a method comprises: receiving data indicative of at
least
one phase image captured using a magnetic resonance imaging (MRI) device
during
delivery of thermal therapy by a thermal therapy applicator to a target volume
within a
patient's body; and processing said at least one phase image; wherein said
processing
said at least one phase image comprises: applying a first mask; applying phase
unwrap;
and applying a second mask.
[0011] In at least some embodiments, the first mask and the second mask
each
provide diminishment and/or enhancement of one or more pixels in a phase (or
other)
image relative to one or more other pixels in the phase (or other) image,
sometimes
referred to herein as subjugation of one or more pixels in a phase (or other)
image.
[0012] In at least some embodiments, the first mask and the second mask can
each be categorized as either: (1) a static mask, which may be defined before
treatment begins, based on user-defined landmarks or otherwise, and is not
expected
to change during treatment or (2) a dynamic mask, which may be computed or
otherwise determined for every dynamic (or otherwise) during treatment, and
may
change during treatment.
[0013] In at least some embodiments, the method further comprises
determining
a treatment plan based at least in part on the processed at least one phase
image; and
delivering thermal therapy to the target volume within the patient's body
based at
least in part on said treatment plan using a thermal therapy applicator.
[0014] In at least some embodiments, said applying phase unwrap comprises:
applying said phase unwrap between said applying a first mask and said
applying a
second mask.
3
CA 03075454 2020-03-10
WO 2019/077385 PCT/IB2017/001506
[0015] In at least some embodiments, said thermal therapy comprises
ultrasound
thermal therapy; and said thermal therapy applicator comprises an ultrasound
thermal
therapy applicator.
In at least some embodiments, said first mask is a static mask and said second
mask is a dynamic mask.
In at least some embodiments, said first mask and/or said second mask
comprises a therapy applicator mask.
In at least some embodiments, said therapy applicator mask is an ultrasonic
applicator mask
In at least some embodiments, said first mask and/or said second mask
comprises a target region mask.
In at least some embodiments, said target region mask is a prostate mask.
In at least some embodiments, said first mask and/or said second mask
comprises a restricted region mask.
In at least some embodiments, said restricted region mask is a rectum mask.
In at least some embodiments, said first mask and/or said second mask
comprises a dynamic mask and wherein said dynamic mask comprises a sector
mask.
In at least some embodiments, said first mask and/or said second mask
comprises a dynamic mask and wherein said dynamic mask comprises a noise mask.
In another aspect, a system comprises at least one computer hardware
processor configured to perform the method.
In another aspect, at least one non-transitory computer readable storage
medium stores processor-executable instructions that, when executed by at
least one
processor, result in the method.
4
CA 03075454 2020-03-10
WO 2019/077385 PCT/1B2017/001506
[0016] This Summary is intended to provide an overview of at least some of
the
subject matter of the present patent application. It is not intended to
provide an
exclusive or exhaustive explanation of the invention or embodiments thereof.
Further
limitations and disadvantages of conventional and traditional approaches will
become
apparent to one of skill in the art, through comparison of such systems with
some
aspects of the present invention as set forth in the remainder of the present
application
with reference to the drawings.
[0017] However, while various features and/or advantages are described in
this
Summary and/or will become apparent in view of the following detailed
description
and accompanying drawings, it should be understood that such features and/or
advantages are not required in all aspects and embodiments.
[0018] Moreover, this Summary is not exhaustive of the scope of the present
aspects and embodiments. Thus, while certain aspects and embodiments have been
presented and/or outlined in this Summary, it should be understood that the
present
aspects and embodiments are not limited to the aspects and embodiments in this
Summary. Indeed, other aspects and embodiments, which may be similar to and/or
different from, the aspects and embodiments presented in this Summary, will be
apparent from the description, illustrations and/or claims, which follow.
[0019] Any aspects and/or embodiments that are described in this Summary
and
do not appear in the claims that follow are preserved for later presentation
in this
application or in one or more continuation patent applications. Any aspects
and/or
embodiments that are not described in this Summary and do not appear in the
claims
that follow are also preserved for later presentation or in one or more
continuation
patent applications.
CA 03075454 2020-03-10
WO 2019/077385 PCT/1132017/001506
In the Drawings
[0020] Reference is made to the following detailed description in
connection
with the accompanying drawings, in which:
[0021] Fig. 1 is a diagram of one type of system in which at least some of
the
methods disclosed herein are employed, in accordance with at least some
embodiments;
[0022] Fig. 2A is a diagram of an image-guided thermal therapy system,
which
may be used in the medical system of Fig. 1, in accordance with at least some
embodiments;
[0023] Fig. 2B is a schematic diagram of a portion of the image-guided
thermal
therapy system in one possible operating mode, in accordance with at least
some
embodiments;
[0024] Fig. 3 illustrates a cross section of a prostate and a therapy
applicator
inserted therein to allow thermal therapy, in accordance with at least some
embodiments;
[0025] Fig. 4 is a representation of MRI image data that may be captured,
in
accordance with at least some embodiments;
[0026] Fig. 5A is a visualization of an image, in accordance with at least
some
embodiments;
[0027] Fig. 5B is a visualization of an image, in accordance with at least
some
embodiments;
[0028] Fig. 6 is a representation of pixel values defining a portion of a
pixel
array, in accordance with some embodiments;
6
CA 03075454 2020-03-10
WO 2019/077385 PCT/I132017/001506
[0029] Fig. 7 is a representation of a mask, in accordance with some
embodiments;
[0030] Fig. 8 is a representation of an application of a portion of a mask
to a
portion of an image, in accordance with some embodiments;
[0031] Fig. 9 is a representation of an AND operation performed on two
masks;
in accordance with some embodiments;
[0032] Fig. 10. is a table that identifies three different types of
structural masks,
in accordance with some embodiments;
[0033] Figs. 11A-11C are representations of masks shown in Fig. 10, in
accordance with at least some embodiments;
[0034] Fig. 12. is a table that identifies three different types of dynamic
masks, in
accordance with at least some embodiments;
[0035] Figs. 13A-13C are enlarged representations of masks shown in Fig.
12, in
accordance with at least some embodiments;
[0036] Fig 14. is a table that shows five different types of dynamic
corrections
that may be employed, in accordance with at least some embodiments.
[0037] Fig. 15 is a flowchart of a method, in accordance with at least some
embodiments;
[0038] Fig. 16A is a visualization of an image, in accordance with at least
some
embodiments;
[0039] Fig. 16B is a visualization of an image, in accordance with at least
some
embodiments;
7
CA 03075454 2020-03-10
WO 2019/077385 PCT/IB2017/001506
[0040] Fig. 17 is a graphical representation of one type of phase wrap, in
accordance with at least some embodiments;
[0041] Fig. 18 are representations of a current image and a corresponding
reference image, in accordance with at least some embodiments;
[0042] Fig. 19 is a visualization of an image, in accordance with at least
some
embodiments.
[0043] Fig. 20 is a flowchart of a method, in accordance with at least some
embodiments;
[0044] Fig. 21A-D illustrate flowcharts of a method, in accordance with at
least
some embodiments; and
[0045] Fig. 22 is a block diagram of an architecture, in accordance with at
least
some embodiments.
Detailed Description
[0046] As stated above, it has been determined that it is possible to
further
reduce the effects of errors and/or potential errors in systems and methods
that use
temperature measurements derived from magnetic resonance imaging (MRI).
[0047] At least some aspects disclosed herein have the ability to address
noise
from various sources, including: magnetic resonance (MR) artifacts, frequency
drift, low
SNR regions, non-uniform tissue structures and/or others.
[0048] Accordingly, improved accuracy and/or efficiency of delivery of MRI-
guided thermal therapies and/or other systems and methods is made possible.
8
CA 03075454 2020-03-10
WO 2019/077385 PCT/IB2017/001506
[0049] At least some aspects disclosed herein employ one or more dynamic
correction methodologies during thermal treatment or other procedure, since
noise
levels can change over time.
[0050] Various aspects and embodiments thereof will be discussed below
after a
brief description of one type of system in which at least some of the dynamic
correction
methods disclosed herein are employed.
[0051] Fig. 1 is one type of system 100 in which at least some of the
dynamic
correction methods disclosed herein are employed, in accordance with at least
some
embodiments.
[0052] Referring to Fig. 1, the system 100, which is a medical system,
includes a
patient support 106 (on which a patient 108 is shown), a magnetic resonance
system
102 and an image guided thermal therapy system 104.
[0053] The magnetic resonance system 102 includes a magnet 110 disposed
about an opening 112, an imaging zone 114 in which the magnetic field is
strong and
uniform enough to perform magnetic resonance imaging, a set of magnetic field
gradient coils 116 to acquire magnetic resonance data 114, a magnetic field
gradient
coil power supply 118 that supplies current to the magnetic field gradient
coils 116 and
is controlled as a function of time, a radio-frequency coil 120 to manipulate
the
orientations of magnetic spins within the imaging zone 114, a radio frequency
transceiver 122 connected to the radio frequency coil 120, and a computer 124,
which
performs tasks (by executing instructions and/or otherwise) to facilitate
operation of the
MRI system 102 and is coupled to the radio frequency transceiver 122, the
magnetic
field gradient coil power supply 118, and the mage guided thermal therapy
treatment
system 104.
9
CA 03075454 2020-03-10
WO 2019/077385 PCT/IB2017/001506
[0054] The image guided thermal therapy system 104, which will be further
discussed below, performs image guided thermal therapy and implements one or
more
aspects and/or embodiments disclosed herein (or portion(s)) thereof to reduce
the
effects of errors and/or potential errors (including: magnetic resonance (MR)
artifacts,
frequency drift, low SNR regions, non-uniform tissue structures and/or others)
and/or
otherwise.
[0055] In at least some embodiments, the computer 124 of the MRI system 102
and/or one or more other computing devices (not shown) in and/or coupled to
the
system 100 may also perform one or more tasks (by executing instructions
and/or
otherwise) to implement one or more aspects and/or embodiments disclosed
herein (or
portion(s)) thereof to reduce the effects of errors and/or potential errors
(including:
magnetic resonance (MR) artifacts, frequency drift, low SNR regions, non-
uniform tissue
structures and/or others) and/or otherwise.
[0056] Fig. 2A is a stylized diagram of an implementation of the image
guided
thermal therapy system 104, in accordance with at least some embodiments.
[0057] Referring to Fig. 2A, in accordance with at least some embodiments,
the
image guided thermal therapy system 104 includes a system controller 200, a
therapy
apparatus controller 202 and a therapy apparatus 204. The system controller
200
(which may comprise a portable PC, workstation, or any other type of
processing
device) may performs task (by executing instructions and/or otherwise) to
facilitate
operation of the image guided thermal therapy system 104 and to implement one
or
more aspects and/or embodiments disclosed herein (or portion(s)) thereof to
reduce
the effects of errors and/or potential errors (including: magnetic resonance
(MR)
artifacts, frequency drift, low SNR regions, non-uniform tissue structures
and/or others)
CA 03075454 2020-03-10
WO 2019/077385 PCT/IB2017/001506
and/or otherwise. The system controller 200 may include a display and/or user
interface 210 to facilitate user control of and/or observation of the thermal
therapy
treatment process, and may be coupled to and supply signals to the therapy
apparatus
controller 202 via communication link 220. The therapy apparatus controller
202
(which may be part of the system controller 200) may comprise analog and/or
digital
circuitry to determine and/or provide drive signals to be supplied to the
therapy
apparatus 204, and may be coupled to the therapy apparatus via a power or
other
communication link 240. The therapy apparatus 204 (which may be maneuvered by
a
motor assembly coupled thereto), may comprise an ultrasound or other treatment
apparatus configured to deliver a suitable dose of ultrasound or other energy
to tissue
in a diseased region of a patient's body. In the illustrated embodiment, the
therapy
apparatus 204 comprises an elongated transurethral prostate therapy applicator
having
a portion 255 to be inserted longitudinally into a patient's prostate to
deliver
ultrasound energy to a diseased region of the patient's prostate.
[0058] The computer 124 of the MR system 102 (Fig. 1) may provide real-time
(or other) images of relevant parts of the patient to the system controller
200 and/or
the display and/or graphical user interface 210. The system controller 200 may
use the
images to monitor (in real time or otherwise) the progress or other status of
the thermal
therapy and may generate signals based at least in part thereon to control the
therapy
apparatus controller 202. Information indicative of the progress or other
status may
also be provided to a clinical or other operator, who may provide input (to
the system
controller 200 and/or the therapy apparatus controller 202) to adjust or
otherwise
control the thermal therapy.
[0059] The system 104 may have various operating modes.
11
CA 03075454 2020-03-10
WO 2019/077385 PCT/1B2017/001506
[0060] Fig. 2B is a schematic diagram of a portion of the system 104 in one
possible operating mode, in accordance with at least some embodiments.
[0061] Referring to Fig. 2B, the portion of the system includes an error
amplifier
(which may be in the system controller 200) that receives a signal indicative
of a desired
target temperature and further receives MRI temperature data (that is,
temperature
maps or other temperature data generated based at least in part on MRI data).
An
output from the error amplifier is supplied to the therapy apparatus
controller 202,
which generates drive signals that are based at least in part thereon and
supplied to
the therapy apparatus 204. The therapy apparatus 204 outputs ultrasonic (or
other)
energy based at least in part thereon to one or more regions of a patient
undergoing
thermal treatment. The energy raises temperatures within the region(s), which
are
imaged using MRI techniques. The MRI imaging is mapped to MRI temperature
data,
which is fed back to the error amplifier, which may adjust the output to the
therapy
apparatus controller 202 based at least in part thereon and/or as appropriate
in
subsequent steps of the treatment. This general method may be followed until
treatment's goals are satisfied (e.g., a given temperature is reached in the
treatment
region) or an alarm or other action interrupts the process.
[0062] Fig. 3 illustrates a cross section of a prostate 30 and an elongated
transurethral prostate therapy applicator 304 inserted longitudinally therein
to allow
performance of conformal thermal therapy 308 to the prostate 30 (or a portion
thereof),
shown at a time tO, in accordance with at least some embodiments.
[0063] Referring to Fig. 3, in accordance with at least some embodiments,
the
prostate 30 has an organ boundary 300. To avoid unwanted heating outside the
prostate, a treatment boundary 302 (representing a desired treatment volume),
may be
12
CA 03075454 2020-03-10
WO 2019/077385 PCT/1B2017/001506
defined, for example, in a treatment planning step prior to or during
application of the
thermal therapy treatment.
[0064] As represented in the figure, and according to certain designs of
applicator 304, the thermal therapy 308 may be directionally emitted from an
active
face of applicator 304. In view at least thereof, the location/direction of
the thermal
therapy 308 at any given point in time, and the location of the control point
309 at any
given point in time, may depend on the angular position of applicator 304. The
thermal
therapy 308 is represented in the figure by a flame-shaped profile or zone
(sometimes
referred to herein as a treatment zone lobe) extending from the applicator
304,
however the thermal therapy 308 is not limited and may instead have any
suitable
configuration.
[0065] In at least some embodiments, the thermal therapy applicator 304 may
be rotated about its axis using a computer-controlled motor so as to sweep
through
the treatment volume defined by the treatment boundary 302, as described in
earlier
patents and applications, including: U.S. Patent Nos. 6,589,174; 7,771,418;
U.S. Pubs.
2007/0239062; 2011/0034833; U.S. Patent Application Publication Nos.
12/932,914;
12/932,923; 12/932,920; and 13/065,106, which are all hereby incorporated by
reference.
[0066] The rotation 307 may be performed at any rate(s), which may be
predetermined (e.g., planned) and/or determined dynamically during the therapy
process. In at least some embodiment, applicator 304 rotates in a clockwise
direction
307 as shown, but is not limited to such.
[0067] In at least some embodiments, a treatment boundary is an intended
boundary within which the energy of the thermal therapy process is
substantially
13
CA 03075454 2020-03-10
WO 2019/077385 PCT3B2017/001506
controlled to a set-point temperature (or thermal dose) ensuring rapid and
sufficient
cell death of diseased cells within the interior of the volume defined by the
treatment
boundary. Heat can be conducted outside the treatment boundary out to the
boundary of an organ (e.g., the prostate), which can be measured and
controlled to
achieve appropriate thermal therapy while reasonably avoiding damage to non-
diseased tissues and organs proximal to said diseased locations. Tissues and
organs
outside the treatment boundary, even if heated, should not exceed lethal
thermal dose
or temperature limits.
[0068] Systems and methods for monitoring and/or controlling thermal
therapy
using ultrasound are described in, for example, U.S. Patent Application
Publication No.
2011/0270366, titled "RF Power Controller for Ultrasound Therapy System," and
U.S.
Patent No. 8,998,889, titled "System and Method for Control and Monitoring of
Conformal Thermal Therapy," which are hereby incorporated by reference.
[0069] Fig. 4 is a representation 400 of MRI image data that may be
captured
before, during and/or after thermal therapy, in accordance with at least some
embodiments.
[0070] Referring to Fig. 4, the MRI image data may be made up of or
otherwise
comprise sets of MRI image data, e.g., sets of MRI image data 4021-402m. Each
set of
MRI image data may include N images, e.g., cross sections (sometimes referred
to as
slices) and may be captured from an MRI device during a respective one of a
plurality
of collection periods, e.g., collection periods 4041-404m. A collection period
for a set of
MRI image data is sometimes referred to herein as a dynamic.
[0071] In at least some embodiments, each set of MR1 image data may
comprise
12 or any other specified number of slices. The amount of time, sometimes
referred to
14
CA 03075454 2020-03-10
WO 2019/077385 PCT/1B2017/001506
herein as a dynamic, needed to capture and/or receive the 12 or other
specified
number of slices in the set may average 6 seconds or other amount of time.
[0072] In at least some embodiments, one or more sets of MRI image data
corresponding to one or more dynamics may be captured prior to start of
therapy and
used in determining a set of reference images. In at least some embodiments,
the set
of reference images will include one reference image for each slice in a set
of MRI
image data. In at least some embodiments, each reference image (phase or
otherwise)
may be generated by taking the mean of five or other number of images (phase
or
otherwise).
[0073] Unless stated otherwise, an "image" is a representation (exact or
otherwise (i.e., non-exact)) of one or more objects (e.g., a body (or
portion(s) thereof) of
a patient, data, or any other type of object(s)) and/or one or more
characteristics
thereof (e.g., temperature(s) and/or other physical characteristic(s)). An
image may have
any form(s). For example, some images may have the form of data that may be
machine readable but need not be visible to a human eye.
[0074] An image may be received from any source(s). An "MRI image" is an
image that is based at least in part on MRI data. A "phase image" is an image
that is
based at least in part on phase data. A "magnitude image" is an image based at
least
in part on magnitude data. The terms "phase image" and "magnitude image" are
not
mutually exclusive. Thus, in at least some embodiments, an image may be both a
"phase image" and a "magnitude image.
[0075] In at least some embodiments, after the start of therapy, an
uncorrected
temperature may be calculated or otherwise determined for each pixel (in any
given
measurement image) as a difference between a phase of the pixel in the
measurement
CA 03075454 2020-03-10
WO 2019/077385 PCT/IB2017/001506
image and a phase of the pixel in the corresponding reference image,
multiplied by a
constant. The phase differences that are determined for the plurality of
pixels in any
given measurement image are sometimes collectively referred to herein as a
phase
difference image (or a phase difference).
[0076] In at least some embodiments, phase images collected during a
dynamic
may be processed to form a temperature map. Each temperature map may be stored
in a buffer that has a width of M temperature maps (corresponding to M
dynamics) and
may be used to hold a rolling window of M temperature maps that may be used to
calculate a temperature uncertainty map.
[0077] In at least some embodiments, the MR image data comprises
measurements (of radio frequency signals emitted by atomic spins) recorded by
the
antenna of a Magnetic resonance apparatus during a magnetic resonance imaging
scan which contains information which may be used for MR thermometry. In at
least
some embodiments, MR thermometry functions by measuring changes in temperature
sensitive parameters. Examples of such parameters are: the proton resonance
frequency shift, the diffusion coefficient, or changes in the Ti and/or 12
relaxation time
may be used to measure the temperature using magnetic resonance. One of the
most
useful of the above measures the proton resonance frequency (PRF) shift of
water
protons. The resonant frequency of the protons is temperature dependent. As
temperature changes in a voxel (an element in an array of volume) the
frequency shifts,
which causes the measured phase of the water protons to change. The
temperature
change between two phase images can therefore be determined. This method of
determining temperature has the advantage that it is relatively fast in
comparison to
the other methods. The PRF method is discussed in greater detail than other
methods
16
CA 03075454 2020-03-10
WO 2019/077385 PCT/IB2017/001506
herein. However, the methods and techniques discussed herein are also
applicable to
the other methods of performing thermometry with magnetic resonance imaging.
[0078] Thus, at least some embodiments may rely on the proton resonant
frequency shift which is known to vary with temperature according to the
formula:
[0079] T= A0*12 *a*Bo*y*TE+BaseTemp
[0080] where T = temperature in degrees, A = phase difference, a = thermal
shift coefficient (ppm/ C), Bo = magnetic field strength (Tesla), y =
gyromagnetic ratio
for H+ nuclei (MHz/Tesla), TE = echo time (sec), BaseTemp = base temperature.
[0081] Since the thermometry formula is based on the PRF-sensitivity of
water
content in tissues, in at least some embodiments, lipid and bone tissues
produces
unreliable temperature measurements which can be excluded from the thermometry
region of interest when making temperature-based decisions.
[0082] Fig. 5A is a visualization 500 of one of the images in one of the
sets of
MRI images, e.g., one image in the set of MRI images 4021, showing temperature
information for a portion of the patient's body in the vicinity of the
treatment volume at
a given point in time during a thermal therapy, in accordance with at least
some
embodiments.
[0083] Referring to Fig. 5A, the visualization 500 employs a grayscale
(i.e.,
different shades of gray) to indicate different temperatures. In the
illustrated
embodiment, the lowest temperatures are shown in black. Increasingly higher
temperatures are shown in increasingly lighter shades. The highest
temperatures are
shown in white.
17
CA 03075454 2020-03-10
WO 2019/077385 PCT/1B2017/001506
[0084] In at least some embodiments, the visualization 500 may be displayed
on
a visual output device such as a computer monitor screen or other display.
[0085] Fig. 5B is a visualization 550 that is similar to the visualization
500 except
that: (i) the temperature information in the visualization 550 has had
thresholding
applied thereto (as a result, each shade in the visualization 550 corresponds
to a wider
temperature range than does each shade in the visualization 500) and pixels
have been
inverted (lowest temperatures are shown in white, increasingly higher
temperatures are
shown in increasingly darker shades, highest temperatures are shown in black)
to assist
in the teaching herein, reproduction and allow use of reference lines to point
to
aspects without a need for color, (ii) a border 560 has been added to identify
the
portion of the visualization 550 that represents a surface of the patient's
body (e.g., the
surface of the patient's abdomen) in the visualization 550, (iii) a border 562
has been
added to identify the portion of the visualization 550 that represents a
treatment
boundary in the patient, and (iv) borders have been added around portions of
the
visualization 550 that are associated with a same temperature range (after
thresholding).
[0086] In at least some embodiments, the visualization 550 may be displayed
on
a visual output device such as a computer monitor screen or other display.
[0087] As known in the art, each image may comprise an array of pixels. The
array may have a plurality of rows and a plurality of columns, e.g., 128 rows
and 128
columns, sometimes referred to herein as a 128 x 128 array configuration. Each
pixel
may define or be defined by, at least in part, a pixel value.
[0088] As used herein, a "pixel" is an element in a picture or any other
type of
image, of any kind, and which may or may not be visible to the human eye.
18
CA 03075454 2020-03-10
WO 2019/077385 PCT/1B2017/001506
[0089] Fig. 6 is a representation 600 of pixel values defining a portion of
a pixel
array, in accordance with some embodiments.
[0090] Referring to Fig. 6, the portion of the pixel array has sixteen
values
arranged in four rows 602-608 and four columns 612-618. For example, the first
row
includes values 50, 52, 54, 60. The first column includes values 50, 20, 40,
41. And so
on. Any given value is sometimes referred to herein as image value,,,, where i
and j
refer to the row in which the value is located and the column in which the
value is
located, respectively.
[0091] As stated above, at least some aspects and embodiments disclosed
herein apply a mask to an image.
[0092] In at least some embodiments, the mask may be defined, at least in
part,
by a plurality of mask values that define, at least in part, a mask array. A
mask array
may have a plurality of rows and a plurality of columns, and in at least some
embodiments, the array will have a configuration that matches the
configuration of an
image to which the array is to be applied, e.g., 128 x 128.
[0093] The phrase ''apply a mask to an image" means to generate a new
(second) image (sometimes referred to herein as a masked image) or other
result based
at least in part on the (first) image and the mask.
[0094] Fig. 7 is a representation 700 of a portion of a mask that may be
applied
to the portion of the image 600 (Fig. 6), in accordance with some embodiments.
[0095] Referring to Fig. 7, the portion of the mask array has sixteen
values
arranged in four rows 702-708 and four columns 712-718. For example, the first
row
includes values 1, 1, 1, 1. The first column includes values 1, 0, 0, 0. And
so on. Any
19
CA 03075454 2020-03-10
WO 2019/077385 PCT/1B2017/001506
given value is sometimes referred to herein as mask value, where i and j refer
to the
row in which the value is associated and the column in which the value is
associated,
respectively.
[0096] Fig. 8 is a representation of an application of a mask to an image,
in
accordance with some embodiments.
[0097] Referring to Fig. 8, in accordance with at least some embodiments, a
mask, e.g., the mask 700, may be applied to an image, e.g., the image 600, to
produce
a masked image (sometimes referred to herein as a result or output) 800. In at
least
some embodiments, the masked image 800 will have a configuration that is the
same
(i.e., same number of rows and same number of columns) as that of the image,
e.g.,
image 600, prior to application of the mask, e.g., mask 700. In the
illustrated
embodiment, for example, the masked image 801 has four rows 802-808 and four
columns 812-818, which is the same configuration as that of the image 600. Any
given
value in the masked image is sometimes referred to herein as masked image
value,,
where i and j refer to the row in which the value is located and the column in
which the
value is located, respectively.
[0098] In at least some embodiments, the application of the mask to the
image
comprises pixel by pixel multiplication. In other words, the value at each
location in
the masked image is determined as a product (multiplication) of the pixel
value (for the
corresponding location in the input image) and the mask value (for the
corresponding
location in the mask), i.e., masked image value,,, = image value,,,x mask
value,
[0099] In the illustrated embodiment, for example, the value,,,, (50) in
the
masked image is determined as a product (multiplication) of the pixel value,
1, (50) in
the image 600 and mask value, 1, (1) in the mask 700. The va1ue2,,, (0) in the
masked
CA 03075454 2020-03-10
WO 2019/077385 PCT/IB2017/001506
image is determined as a product (multiplication) of the pixel value2,1, (20)
in the image
600 and mask value2,1, (0) in the mask 700. And so on.
[00100] Thus, in at least some embodiments, regions of the mask that have a
value equal to 1 result in regions, within the masked image, where the image
is the
same as that of the input image (i.e., where the masked image shows the input
image).
Regions of the mask that have a value equal to 0 result in regions, within the
masked
image, where the input image is removed (i.e., where the masked image does not
show the original image).
[00101] Although the mask 700 is shown with values of only 1 and 0, in at
least
some embodiments, masks are not limited to such, but rather may have any
suitable
form(s). Moreover, although the mask 700 is applied using pixel by pixel
multiplication,
in at least some embodiments, application of a mask is not limited to such but
rather
may be applied in any suitable manner(s). For example, in at least some
embodiments,
a mask may have values of 1 and 0.0001 (e.g., or other values that are not 0
but are
small relative to the value 1), and may be applied to an image using pixel by
pixel
multiplication followed by thresholding to replace any masked image values
below a
specified threshold (e.g., a threshold near 0) with the value 0.
[00102] Pixels that are subjugated relative to other pixels are sometimes
referred
to herein as excluded pixels. Other pixels are sometimes referred to herein as
included
pixels.
[00103] In at least some embodiments, a plurality of masks may be applied
to an
image. In some embodiments, the masks may be applied one after the other. For
example, one mask may be applied to the image and a second mask may be applied
21
CA 03075454 2020-03-10
WO 2019/077385 PCT/IB2017/001506
to the result thereof. In some embodiments, two or more of the masks may be
combined and the combined mask may be applied to the image.
[00104] Fig. 9 is a representation of one type of BOOLEAN operation that
may be
used to combine masks, in accordance with some embodiments.
[00105] Referring to Fig. 9, in accordance with at least some embodiments,
values
of a first mask, e.g., the mask 700, may be ANDed with values of a second
mask, e.g., a
mask 900, to produce a combined mask 901. In at least some embodiments, the
combined mask 901 will have a configuration that is the same (i.e., same
number of
rows and same number of columns) as that of the first and second masks 700,
900,
respectively. In the illustrated embodiment, for example, the combined masked
901
has four rows 902-908 and four columns 912-918, which is the same
configuration as
that of the first and second masks 700, 900. Any given value in the combined
mask
901 is sometimes referred to herein as combined mask value,, where i and j
refer to
the row with which the value is associated and the column with which the value
is
associated, respectively.
[00106] In at least some embodiments, the combining of the masks comprises
value by value ANDing. In other words, the value at each location in the
combined
masked is determined by ANDing the mask value (for the corresponding location
in the
first mask) and the mask value (for the corresponding location in the second
mask), i.e.,
combined masked value,,, = first mask value,,,AND second mask value.
[00107] In the illustrated embodiment, for example, the valuei,i, (1) in
the
combined mask is determined by ANDing the mask valueti, (1) in the first mask
700
and mask valuel,i, (1) in the second mask 900. The value21, (0) in the
combined mask is
22
CA 03075454 2020-03-10
WO 2019/077385 PCT/1132017/001506
determined by ANDing the mask valuezi, (0) in the first mask 700 and mask
va1ue2,1, (0)
in the second mask 900. And so on.
[00108] The combined mask 901 includes the first mask 700 and the second
mask
900.
[00109] In at least some embodiments, a combined mask may be applied to an
image in a similar manner as described above with respect to the first mask
700. Thus,
in at least some embodiments, regions of a combined mask that have a value
equal to
1 result in regions, within a masked image, where the image is the same as
that of an
input image (i.e., where the masked image shows the input image). Regions of a
combined mask that have a value equal to 0 result in regions, within the
masked
image, where the input image is removed (i.e., where the masked image does not
show the original image).
[00110] In at least some embodiments, the application of the combined mask
901
is in effect application of the first mask 700 and application of the second
mask 900.
[00111] Notwithstanding that a Boolean operation (AND, OR, etc.) may be
used
to combine two or more masks, the combining of masks is not limited to such.
In at
least some other embodiments, multiplication may be used to combine two or
masks.
[00112] As stated above, in at least some embodiments, a mask may be a
static
mask or a dynamic mask. A static mask may be defined before treatment begins,
based on user-defined landmarks or otherwise, and is not expected to change
during
treatment. A dynamic mask is computed or otherwise determined during
treatment,
and may change during treatment.
23
CA 03075454 2020-03-10
WO 2019/077385 PCT/IB2017/(1(11506
[00113] One type of static mask that may be employed is sometimes referred
to
herein as a temperature uncertainty (TU) mask that relates to a MRI
thermometry
method's finite ability to produce a temperature uncertainty map. A
temperature
uncertainty mask stores information identifying pixels that exhibit noise in
excess of a
noise threshold before the start of therapy. In at least some embodiments, a
temperature uncertainty mask may be created by determining, for every pixel, a
standard deviation of its pixel values across a given or other number of
received
images, and for each pixel for which the pixel values exceed a standard
deviation of 2
C or other noise threshold, setting or otherwise providing a flag or other
indication in
the mask to identify the pixel as a noisy pixel. In at least some embodiments,
the mask
value,,, corresponding to each noisy pixel,,, is set to or otherwise defined
as 0, and
mask values corresponding to other pixels are set to or otherwise defined as
1.
[00114] During thermal therapy, each pixel identified as noisy will have
its pixel
values replaced. In at least some embodiments, the pixel value may be replaced
with
an estimated pixel value. In at least some embodiments, the pixel value may be
replaced with an estimated value determined by linear or other interpolation
based at
least in part on the four neighboring pixels (up, down, left, right) of the
pixel.
[00115] Another type of static mask that may be employed is sometimes
referred
to herein as a structural mask. In at least some embodiments, static masks may
be
generated during treatment planning.
[00116] Fig 10. is a table 1000 that identifies three different types of
structural
masks, characteristics thereof and representations of examples thereof
(reduced in size
compared to the size of the visualizations in Fig. 5A-5B), in accordance with
at least
some embodiments.
24
CA 03075454 2020-03-10
WO 2019/077385 PCT/IB2017/001506
[00117] Figs. 11A-11C are full size representations (compared to the size
of the
visualizations in Fig. 5A-5B) illustrating the different regions in each of
the examples in
Fig. 10, in accordance with at least some embodiments.
[00118] Referring to Fig. 10 and Figs. 11A-11C, in accordance with at least
some
embodiments, the three different types of structural masks are: (1) an
ultrasound
applicator (UA) (or other therapy applicator) mask 1100, (2) a prostate (or
other target
region) mask 1102, and (3) a rectum (or other restricted region) mask 1104.
[00119] The ultrasound applicator (UA) (or other therapy applicator) mask
1100
excludes or otherwise subjugates pixels that are within a specified distance
of the
therapy applicator or portion(s) thereof. In the illustrated embodiment, the
therapy
applicator comprises an ultrasound therapy applicator having a center 1106 and
the
mask 1100 excludes or otherwise subjugates pixels in a region 1108 within 40
mm of
the center 1106 of the ultrasound therapy applicator. In at least some
embodiments,
this mask 1100 is slice independent.
[00120] The prostate (or other target region) mask 1102 includes pixels
within a
contour of a prostate (or other target region) or portion(s) thereof and
excludes or
otherwise subjugates others. In the illustrated embodiment, the prostate (or
other
target region) mask 1102 includes all pixels whose center is fully included in
within a
contour 1110 of the prostate (or other target region) and excludes or
otherwise
subjugates others. In at least some embodiments, this mask 1102 is slice
dependent
because the contour of the prostate (or target region) may be different in
each slice of
a dynamic.
[00121] The rectum (or other restricted region) mask 1104 excludes or
otherwise
subjugates pixels within a region having a specified positional relationship
to the
CA 03075454 2020-03-10
WO 2019/077385 PCT/1B2017/001506
rectum (or other restricted region) or portion(s) thereof. In the illustrated
embodiment,
the rectum (or other restricted region) mask 1104 excludes pixels that are
within a
region 1112: (1) below the therapy applicator and (2) laterally within 15mm
(i.e., +/- 15
mm) of a center 1106 of the therapy applicator.
[00122] In accordance with at least some embodiments, including the
illustrated
embodiment, the above structural masks are based at least in part on the
position of
the center of the ultrasound applicator (UA) or other therapy applicator.
Consequently,
if the center of the ultrasound applicator (UA) or other therapy applicator is
modified
during the treatment (either by the user or because of image shift ¨ discussed
below),
these structural masks will have to be generated again.
[00123] As stated above, a mask may also be a dynamic mask, which may be
computed or otherwise determined for every dynamic (or otherwise) during
treatment,
and may change during treatment.
[00124] Fig 12. is a table 1200 that identifies three different types of
dynamic
masks, characteristics thereof and representations of examples thereof
(reduced in size
compared to the size of the visualizations in Fig. 5A-5B), in accordance with
at least
some embodiments.
[00125] Figs. 13A-13C are full size representations (compared to the size
of the
visualizations in Fig. 5A-5B) illustrating the different regions in each of
the examples in
Fig. 12, in accordance with at least some embodiments.
[00126] Referring to Fig. 12 and Figs. 13A-13C, in accordance with at least
some
embodiments, three different types of dynamic masks are: (1) a sector (or
boiling
detection) mask 1300, (2) a signal to noise ratio (SNR) mask 1302, and (3) a
stability
mask 1304.
26
CA 03075454 2020-03-10
WO 2019/077385 PCT/IB2017/001506
[00127] The sector (or boiling detection) mask 1300 includes pixels within
a sector
(or other region) or portion(s) thereof receiving energy from the therapy
applicator at a
current (or other) point in time. In the illustrated embodiment, the sector
(or boiling
detection) mask 1300 includes pixels within a polygon 1308 having four sides
1310,
1312, 1314, 1316. The first side 1310 is defined by a prostate (or other
target region)
boundary 1318. The second side 1312 is defined by a circle 1320 having a
center 1322
at the center 1106 of the therapy applicator and a radius of 6mm (or other
positional
relation to the therapy applicator). The third and fourth sides 1314, 1316 are
defined
by lines that are disposed on opposite sides of a current therapy beam
centerline 1324
(e.g., a line at the center of the thermal therapy beam and extending from the
therapy
applicator 304 in a direction at which the center of the thermal therapy beam
is
emitted a current point in time) and angularly displaced therefrom by angles
1326,
1328 of +15 degrees and - 15 degrees, respectively, or some other angles.
[00128] The signal to noise ratio (SNR) mask 1302 and the stability mask
1304 are
sometimes referred to herein as noise masks. In at least some embodiments,
these
masks are used to filter out pixels that did not appear noisy (did not exceed
a noise
threshold or other criteria for a noisy pixel) prior to the start of the
treatment but
appear noisy during the treatment. In at least some embodiments, each of these
masks is cumulative, meaning that if a pixel is masked at a given dynamic, it
will remain
masked as such throughout the treatment. Thus, in at least some embodiments,
the
mask used in a given dynamic will be based at least in part on the mask used
in the
prior dynamic.
[00129] The stability mask 1304 is used to store information identifying
any pixels
that are outside the target region and/or other heating volume and exhibit
large
27
CA 03075454 2020-03-10
WO 2019/077385 PCT/IB2017/001506
temperature variations or other noise in excess of a noise threshold after
treatment is
started. In at least some embodiments, the stability mask 1304 for a given
dynamic
may be created by determining, for pixels outside the target region or other
heating
volume, a difference between its temperature in that dynamic and its
temperature in a
prior dynamic (e.g., temperature of pixel, , of slicek for dynamic,,ent ¨
temperature of
pixel,,, of slicek for dynamiccu,ew), and for each of such pixels for which
the temperature
difference exceeds 10 C (or other difference threshold) or other noise
criteria, setting
or otherwise providing a flag or other indication in the stability mask to
identify the
pixel as a noisy pixel. In at least some embodiments, the mask value,,,
corresponding
to each noisy pixel,,, is set to or otherwise defined as 0, and mask values
corresponding
to other pixels are set to or otherwise defined as 1. See for example mask
values in a
region 1340 corresponding to noisy pixels and set to 0, and mask values in a
region
1342 corresponding to other pixels and set to 1.
[00130] In at least some embodiments, the pixels outside of the target
region or
other heating volume will be those pixels that are not included in or are
otherwise
subjugated in the prostate (or other target region) mask. Thus, in at least
some
embodiments, the stability mask 1304 will be based at least in part on a
prostate (or
other target region) mask.
[00131] As stated above, in at least some embodiments, the stability mask
1304 is
cumulative, meaning that if a pixel is masked at a given dynamic, it will
remain masked
as such throughout the treatment. Thus, in at least some embodiments, the
stability
mask 1304 used in a given dynamic will be based at least in part on the
stability mask
1304 used in the prior dynamic. In at least some embodiments, a stability mask
1304
for a given dynamic is made cumulative by determining the stability mask 1304
as
28
CA 03075454 2020-03-10
WO 2019/077385 PCT/IB2017/001506
described above and multiplying it by the stability mask 1304 used in the
prior
dynamic.
[00132] During therapy, each pixel identified as a noisy pixel will have
its pixel
values replaced and/or ignored. If it is to be replaced, it may be replaced
with an
estimated pixel value determined by linear or other interpolation based at
least in part
on the values of the four neighboring pixels (up, down, left, right) of the
pixel.
[00133] Accurate temperature measurements require pixels with a high signal
to
noise ratio (SNR). The SNR mask 1302 identifies pixels that have a
satisfactory SNR
(e.g., an SNR that satisfies an SNR criteria). In at least some embodiments,
the SNR
mask 1302 is generated by thresholding magnitude images using Otsu's method.
In at
least some embodiments, the mask value, , corresponding to a pixel,,, having a
satisfactory SNR is set to or otherwise defined as 1, and mask values
corresponding to
other pixels are set to or otherwise defined as 0.
[00134] See for example mask values in a region 1350 corresponding to
pixels
having a satisfactory SNR and set to 1, and mask values in a region 1352
corresponding
to other pixels and set to 0.
[00135] As stated above, in at least some embodiments, the SNR mask 1302 is
cumulative, meaning that if a pixel is masked as having unsatisfactory SNR at
a given
dynamic, it will remain masked as such throughout the treatment. Thus, in at
least
some embodiments, the SNR mask 1302 used in a given dynamic will be based at
least
in part on the SNR mask 1302 used in the prior dynamic. In at least some
embodiments, a SNR mask 1302 fora given dynamic is made cumulative by
determining the SNR mask 1302 as described above and multiplying it by the SNR
mask 1302 used in the prior dynamic.
29
CA 03075454 2020-03-10
WO 2019/077385 PCT/1B2017/001506
[00136] In at least some embodiments, each pixel identified as having
unsatisfactory SNR will have its pixel values replaced and/or ignored. If it
is to be
replaced, it may be replaced with an estimated pixel value determined by
linear or
other interpolation based at least in part on the values of the four
neighboring pixels
(up, down, left, right) of the pixel.
[00137] As stated above, at least some aspects disclosed herein employ one
or
more dynamic correction methodologies during thermal treatment or other
procedure.
[00138] Fig 14. is a table 1400 that shows five different types of dynamic
corrections that may be employed, characteristics thereof and representations
of
examples thereof (reduced in size compared to the size of the visualizations
in Fig. 5A-
5B), in accordance with at least some embodiments.
[00139] Referring to Fig. 14, these five different types of dynamic
corrections are:
drift correction, phase unwrap, temperature corrections, spatial co-
registration and
boing detection shutoff.
[00140] Drift correction is used to compensate, at least in part, for drift
in the
[armor frequency of the MRI scanner, and thereby reduce the effects thereof,
at least
in part. During thermal treatment, the Larmor frequency of the MRI scanner may
drift
over time. As a consequence, the measured phase values, which are expected to
be
constant on unheated regions, may drift over time, which appears as
temperature
increase (sometimes referred to herein as artificial heating).
[00141] Fig. 15 is a flowchart of a method for drift correction to
compensate, at
least in part, for drift in the Larmor frequency of the MRI scanner over time,
and
CA 03075454 2020-03-10
WO 2019/077385 PCT/IB2017/001506
thereby reduce the effects thereof, at least in part, in accordance with at
least some
embodiments.
[00142] Referring to Fig. 15, at 1502, the method includes determining a
drift
correction mask for each slice (e.g., 12 slices) in a current dynamic. In at
least some
embodiments, the mask for each slice may be determined by combining the
following
masks: (i) the thermal (ultrasound or otherwise) applicator mask, (ii) the
rectum (or other
restricted region) mask, (iii) the stability mask for the slice and (iv) the
SNR mask for the
slice as follows:
[00143] combinedMask = UAMaskxRectumMaskxStabilityMaskxSNRMask
[00144] At 1504, the method may further include, for each slice of the
current
dynamic, applying the combined mask for the slice to the phase difference
image for
the slice. In at least some embodiments, the combined mask for a slice may be
applied to the phase difference image for the slice using pixel by pixel
multiplication as
follows:
masked (x, =- 11)(x, y, s)xcombinedMask(x, y, s)
[00145] At 1506, the method may further include, for each slice of the
current
dynamic, determining a geometric fit or other approximation (sometimes
referred to
herein as an estimation or estimate) based at least in part on the masked
phase
difference image for the slice. In at least some embodiments, the geometric
fit will be
a plane fit or a parabola fit, depending on a correction order (sometimes
referred to
herein as an order of correction) that may be needed and/or chosen, which in
at least
some embodiment may be based at least in part on the type of scanner being
used.
For instance, if a second-order correction is to be used, an estimate (T)
based on
masked may be determined as follows:
31
CA 03075454 2020-03-10
WO 2019/077385 PCT/1B2017/001506
(13(X, y, s) = + 132x + 183y + 134xy + le4x2 + 135y2 + c
[00146] where E is the observed error of the model. This forms a linear set
of
equations which may be written as:
(13masked = Xi4
1 .X1
where X= Fi i is the Vandermonde matrix.
1 xr, yn xor,
[00147] The solution of these equations is given by:
(Ex)-ixrc-6
[00148] At 1508, the method may further include determining a corrected
phase
image based at least in part on a difference between the surface and a current
phase
image. In at least some embodiments, this may be performed by subtracting the
surface to the current phase image as follows:
Ocorrected = 4:1) Cilmasked
[00149] Fig. 16A is a full-size visualization 1600 (compared to the size of
the
visualizations in Fig. 5A-5B) of the example of drift correction shown in Fig.
14, in
accordance with at least some embodiments.
[00150] Figs. 16B is a visualization 1650 that is similar to the
visualization 1600
except that: (i) the temperature information in the visualization 1650 has had
thresholding applied thereto (as a result, each shade in the visualization
1650
corresponds to a wider temperature range than does each shade in the
visualization
1600) and pixels have been inverted (lowest temperatures are shown in white,
increasingly higher temperatures are shown in increasingly darker shades,
highest
32
CA 03075454 2020-03-10
WO 2019/077385 PCT/IB2017/001506
temperatures are shown in black) to assist in the teaching herein,
reproduction and
allow use of reference lines to point to aspects without a need for color,
(ii) a border
1660 has been added to identify the portion of the visualization 1650 that
represents a
surface of the patient's body (e.g., the surface of the patient's abdomen) in
the
visualization 1650, and (iii) borders have been added around portions of the
visualization 1650 that are associated with a same temperature range (after
thresholding).
[00151] In at least some embodiments, the visualization 1600 and/or the
visualization 1650 may be displayed on a visual output device such as a
computer
monitor screen or other display.
[00152] Referring again to Fig. 14, a second type of dynamic correction
that may
be employed is sometimes referred to herein as phase unwrap. Phase values are
bound to a range between ¨7 (-180 degrees) and +rc (+180 degrees). As a
result, a
change in temperature can result in what is sometimes referred to as "phase
wrap".
[00153] Fig. 17 is a graphical representation 1700 of one type of a phase
wrap, in
accordance with at least some embodiments. In the representation, a first
phase
measurement 1702 has a first phase of -175 degrees representing a first
temperature
1704. Because of phase wrap 1706, a second phase measurement 1708,
representing
a second temperature 1710 (which is relatively close to the first temperature
1704), has
a second phase of +175 degrees (which is disproportionately greater than the
first
phase of -175). In at least some embodiments, it is desirable to detect and
compensate
for phase wrap. The process of determining whether phase unwrap has occurred,
and if
it has, compensating for such is sometimes referred to herein as "phase
unwrap".
33
CA 03075454 2020-03-10
WO 2019/077385 PC T/IB2017/001506
[00154] In at least some embodiments, the occurrence of phase wrap is
detected
based at least in part on the change that a pixel phase value has undergone
between
two successive dynamics. In some embodiments, a change of more than T1 (in
either
direction) in a pixel phase value is used as an indication that phase wrap has
occurred,
and if phase wrap has occurred, it is compensated for by applying an offset of
+2n or -
2n (depending on the direction of the change) in accordance with the
following:
4:13 (d) ¨ (1:0(d ¨ 1) > 7T, CD(C1)=-11)(d- 1) - 2m
If
(13(d) ¨ (13(d ¨ 1) < (1)(d) = (I)(d ¨ 1) +
where d is the dynamic number.
[00155] Referring still to Fig. 14, a third type of dynamic correction that
may be
employed is sometimes referred to herein as temperature corrections. In at
least some
embodiments, it may happen that due to noisy measurements, a pixel phase value
will
be incorrectly unwrapped, and as a result may take very high or very low
temperature
values, beyond normal physiological values or another expected range. To
address the
above, in at least some embodiments, temperature correction may be carried out
as
follows. For every pixel in the phase image, if phase unwrap causes the
temperature
value of the pixel to fall outside the range [0;120] C (i.e., outside the
normal
physiological range), or outside some other expected range, the phase is not
unwrapped. For the range of [0;120] C, this may be implemented as follows:
{(DO) > 012o., 0(d) = 0(d) ¨
(D(d) < (I) 0., c13.(d) = cP(d) + 27
where 012Tc and (Doc are the phase values corresponding to the temperatures of
120 C and 0 C respectively.
34
CA 03075454 2020-03-10
WO 2019/077385 PC T/IB2017/001506
[00156] Effectively, the above ensures that all pixels in the temperature
maps are
bound within the range [0;120] C. In at least some embodiments, a range other
than
0 to 120 C may be employed.
[00157] Referring still to Fig. 14, a fourth type of dynamic correction
that may be
employed is sometimes referred to herein as spatial co-registration (or image
shift
correction). If the amount of MRI scanner drift is significant over the course
of the
treatment, it is necessary (or at least desirable) to perform spatial co-
registration
between the received dynamics and the reference image, taken at the beginning
of the
treatment.
[00158] Fig. 18 is a representation 1800 showing a current image 1802 and a
corresponding reference image 1804 prior to any spatial drift, and an
alternative
current image 1812 as a result of spatial drift 1814, in accordance with some
embodiments.
[00159] Therefore, in at least some embodiments, the amount of spatial
drift
between the current dynamic and the reference image is determined every Y
dynamics
(or if not periodically, at least from time to time).
[00160] The amount of spatial drift may be determined by calculating or
otherwise determining a measure of similarity between the current dynamic and
the
reference image, which may be determined by calculating or otherwise
determining a
cross-correlation between a Fourier-transform of the current dynamic and a
Fourier-
transform of the reference image. In at least some embodiments, the result of
the
cross-correlation is an image that contains a peak, the location of which is
equal to or
otherwise defines the amount of spatial shift between the two images. If the
amount of
the spatial shift is greater than 0.1 pixel (or other chosen threshold), it is
necessary (or
CA 03075454 2020-03-10
WO 2019/077385 PCT/IB2017/001506
at least desirable) to spatially register the current dynamic and the
reference image. If
the amount of spatial shift is less than 1 pixel, spatially registering the
current dynamic
and the reference image will require interpolation.
[00161] In accordance with at least some embodiments, two approaches are
possible: either align the reference image onto the current image, or align
the current
image onto the reference image. The former solution will require shifting the
UA center
too, and therefore the structural masks will have to be generated again.
[00162] Fig. 18 shows (a) a compensated reference image 1824 after
compensation 1826 thereto and a compensated current image 1822 as a result of
the
compensation to the reference image, corresponding to the first approach, and
(b) a
compensated current image 1832 as a result of compensation 1836 thereto
without
any change to the reference image 1804, corresponding to the second approach,
in
accordance with some embodiments.
[00163] Fig. 19 is a full-size visualization 1900 (compared to the size of
the
visualizations in Fig. 5A-5B) of the example of boiling detection shutoff
shown in Fig.
14, in accordance with at least some embodiments.
[00164] Referring again to Fig. 14, a fifth type of dynamic correction that
may be
employed is sometimes referred to herein as boiling detection shutoff. In at
least some
embodiments, it is critical or at least desirable to correctly detect
temperature
approaching 100 C in tissue. To address the above, at least some embodiments
employ a boiling detection shutoff method and/or mechanism to reduce the risk
of
tissue boiling. In at least some embodiments, the method and/or mechanism are
based at least in part on the sector mask discussed above, which as discussed
above,
the positioning of which may be primarily (or at least in part) a function of
the current
36
CA 03075454 2020-03-10
WO 2019/077385 PCT/IB2017/001506
beam angle. In at least some embodiments, the method and/or mechanism shut off
at
least a portion of (or otherwise reduce) the power to the therapy applicator
if at any
time, any one or more pixels within the sector mask has a temperature that is
greater
than a threshold or satisfies other criteria. Fig. 19 shows an example of
pixels within an
example of a sector mask (i.e., an example of pixels within the polygon 1308
(Fig. 13A).
In at least some embodiments, the threshold is selected as an indication that
a boiling
temperature is approached. In at least some embodiments, the threshold is a
temperature chosen from a range of from 86 C to 90 C. In at least some
embodiments, the threshold is 86 C. In at least some embodiments, the
threshold is
adjustable during therapy. In at least some embodiments, the adjustable
threshold is
adjustable to any temperature in the range of from 86 C to 90 C. In at least
some
embodiments, at least a portion the at least one element that is shut off
includes one
or more elements supplying energy to a region of the patient associated with
the one
or more pixels that are within the sector mask and have a temperature that is
greater
than the threshold or satisfies the other criteria. In at least some
embodiments, power
to the therapy applicator is reduced by shutting off power to one, some or all
elements
of the therapy applicator that supply energy to a region of the patient
associated with
the one or more pixels that are within the sector mask and have a temperature
that is
greater than the threshold or satisfies the other criteria.
[00165] Thus, at least some aspects disclosed herein further reduce the
effects of
errors and/or potential errors in systems and methods that use temperature
measurements derived from MRI.
[00166] Accordingly, improved accuracy and/or efficiency of delivery of MRI-
guided thermal therapies and/or other systems and methods is made possible.
37
CA 03075454 2020-03-10
WO 2019/077385 PC T/IB2017/001506
[00167] In at least some embodiments, one or more portions of any method
(or
system) disclosed herein may be used without one or more other portions of
such
method (or system).
[00168] In accordance with at least some embodiments, any one or more of
the
embodiments (or feature(s) thereof) disclosed herein may be used in
association with
any other embodiment(s) (or feature(s)) disclosed herein.
[00169] Fig. 20 is a flowchart of a method 2000 that employs a plurality of
the
methods disclosed herein or portion(s) thereof and may be employed in delivery
of
therapy, in accordance with at least some embodiments.
[00170] In at least some embodiments, one or more portions of the method
may
be used in performing dynamic correction(s). In at least some embodiments, the
dynamic correction(s) may improve accuracy and/or reduce uncertainty.
[00171] The method is not limited to the order shown, but rather may be
performed in any practicable order. For that matter, any method disclosed
herein is
not limited to any particular order but rather may be performed in any
practicable
order.
[00172] In at least some embodiments, the method (or one or more portion(s)
thereof) may be performed using one or more portions of one or more other
methods
disclosed herein. For that matter, in at least some embodiments, any method
(or one
or more portions thereof) disclosed herein may be performed using one or more
portions of one or more other methods disclosed herein.
[00173] In at least some embodiments, the method (or one or more portion(s)
thereof) may be performed in performance of one or more portions of one or
more
38
CA 03075454 2020-03-10
WO 2019/077385 PCT/1B2017/001506
other methods disclosed herein. For that matter, in at least some embodiments,
any
method (or one or more portions thereof) disclosed herein may be performed in
performance of one or more portions of one or more other methods disclosed
herein.
[00174] In at least some embodiments, the method (or one or more portion(s)
thereof) may be performed by system controller 200 (Figs. 1-2).
[00175] Referring to Fig. 20, at 2002, the method may include receiving
data
indicative of at least one phase image captured using a magnetic resonance
imaging
(MRI) device during delivery of thermal therapy by a thermal therapy
applicator to a
target volume within a patient's body.
[00176] In at least some embodiments, the data may have any form(s) and may
be
received from any source(s) (internal and/or external).
[00177] In at least some embodiments the thermal therapy comprises
ultrasound
thermal therapy and the thermal therapy applicator comprises an ultrasound
thermal
therapy applicator.
[00178] At 2004, the method may further include applying a first mask.
[00179] In at least some embodiments, the first mask may comprise any mask,
or
any combination of masks, disclosed below or otherwise herein.
[00180] At 2006, the method may further include applying phase unwrap.
[00181] At 2008, the method may further include applying a second mask.
[00182] In at least some embodiments, the second mask may comprise any
mask,
or any combination of masks, disclosed below or otherwise herein.
39
CA 03075454 2020-03-10
WO 2019/077385 PCT/IB2017/001506
[00183] In some embodiments, the first mask and the second mask may be
combined with one another into a combined mask that is subsequently applied
such
that the first mask and second mask are applied at the same time as one
another.
[00184] In accordance with at least some embodiments, the first mask, phase
unwrap and the second mask may be applied in any suitable order. Thus, in at
least
some embodiments, the applying of the phase unwrap may be between the applying
of the first mask and the applying of the second mask. In at least some
embodiments,
this may comprise applying the first mask to a first phase image to generate a
first
result image, applying the phase unwrap to the first result image to generate
a second
result image, and applying the second mask to the second result image to
generate a
third result image. In at least some embodiments, intermediate processing need
not
be excluded. Thus, in at least some embodiments, the applying of the phase
unwrap
between the applying of the first mask and the applying of the second mask may
comprise: applying the first mask to a phase image to generate a first result
image,
applying the phase unwrap to a phase image that is based at least in part on
the first
result image to generate a second result image, and applying the second mask
to a
phase image that is based at least in part on the second result to generate a
third
result image.
[00185] In at least some embodiments, the applying of the phase unwrap may
be
prior to the applying of the first mask and the applying of the second mask.
In at least
some embodiments, the applying of the phase unwrap may be after the applying
of
the first mask and the applying of the second mask. As indicated above, in at
least
some embodiments, intermediate processing need not be excluded.
CA 03075454 2020-03-10
WO 2019/077385 PCT/IB2017/001506
[00186] The method may further include determining a treatment plan after
processing the at least one phase image.
[00187] The method may further include delivering thermal therapy to the
target
volume within the patient's body based at least in part on said treatment plan
using a
thermal therapy applicator.
[00188] Figs. 21A-21D are a flowchart 2100 of another method that employs a
plurality of the methods disclosed herein or portion(s) thereof and may be
employed in
delivery of therapy, in accordance with at least some embodiments.
[00189] In at least some embodiments, the method reduces the effects of
errors
and/or potential errors in MRI guided thermal therapy.
[00190] In at least some embodiments, one or more portions of the method
may
be used in performing dynamic correction(s). In at least some embodiments, the
dynamic correction(s) may improve accuracy and/or reduce uncertainty.
[00191] Accordingly, improved accuracy and/or efficiency of delivery of MRI-
guided thermal therapies is made possible.
[00192] As stated above, in at least some embodiments, one or more portions
of
any method (or system) disclosed herein may be used without one or more other
portions of such method (or system).
[00193] In at least some embodiments, references below to static masks,
dynamic
masks, temperature uncertainty masks, structural masks, ultrasound applicator
masks,
rectum masks, prostate masks, sector masks, noise masks, SNR mask, stability
mask,
drift correction, phase unwrap, temperature corrections, spatial co-
registration (image
shift correction), boiling detection and/or boiling detection shutoff (and so
on) refer to
41
CA 03075454 2020-03-10
WO 2019/077385 PCT/1B2017/001506
the static masks, dynamic masks, temperature uncertainty masks, structural
masks,
ultrasound applicator masks, rectum masks, prostate masks, sector masks, noise
masks,
SNR mask, stability mask, drift correction, phase unwrap, temperature
corrections,
spatial co-registration (image shift correction), boiling detection and/or
boiling
detection shutoff (and so on), respectively, described above with respect to
Figs. 1-20.
[00194] In at least some embodiments, the method (or one or more portion(s)
thereof) may be performed by system controller 200 (Figs. 1-2).
[00195] Referring now to Figs. 21A-21D, at 2102, the method may include
receiving information associated with a new patient 2102, calculating a pre-
treatment
TU map 2104, identifying any pixels that are within the prostate (or other
region) and
have a standard deviation greater than 200 (or other threshold or other
criteria) 2106,
and storing a TU mask generated based at least in part on the results thereof
2108.
[00196] The method further includes starting treatment 2110, calculating
structural masks (i.e., UA mask, rectum mask and prostate mask and/or other
structure
mask(s))) 2112, storing the structural masks 2114, receiving a new dynamic
2116 and
determining whether more than 5 dynamics have been received 2118.
[00197] If at 2118 it is determined that more than 5 dynamics have not been
received, the method returns to 2116. Otherwise, the method proceeds to
calculate a
reference phase image 2120, store the reference phase image as a reference
phase
2122 and determine whether a current dynamic is a multiple of 100 at 2124.
[00198] If at 2124 it is determined that the current dynamic is not a
multiple of
100, the method proceeds to calculate phase difference between a current phase
image and the reference plane 2126. Otherwise, the method stores the next 5
42
CA 03075454 2020-03-10
WO 2019/077385 PCT/1B2017/001506
dynamics 2128, evaluate image shift 2130 and determine if the image shift is
more
than 0.1 pixel 2132.
[00199] If at 2132 it is determined that the image shift is more than 0.1
pixel, the
method returns to 2124. Otherwise, the method proceeds to apply special co-
registration 2134, recalculate structural masks 2136, and calculate phase
difference
between a current phase image and the reference plane 2126.
[00200] After calculating the phase difference between a current phase
image
and the reference plane at 2126, the method proceeds to apply phase unwrap
2138,
identify pixels with low magnitude SNR 2140, and storing a SNR mask generated
based at least in part on the results thereof 2142.
[00201] The method further includes identifying pixels whose temperature
changed by greater than 10 C (or other threshold or other criteria) between
two
dynamics, storing a stability mask generated based at least in part on the
results
thereof 2146, multiplying current SNR and stability masks with previous SNR
and
stability masks 2148, applying drift correction to masked phase images (UA &
rectum &
SNR and stability) 2150, applying phase unwrap 2152, applying temperature
correction
2154, converting phase to temperature 2156, calculating sector mask based on
current
beam angle 2158, and storing a sector mask generated based at least in part on
the
results thereof 2160.
[00202] The method further includes determining whether any pixels are
within
the sector mask and above a threshold indicating that the temperature is
approaching
a boing temperature (or other temperature reference) 2162. If at 2162 it is
determined
that no pixels are within the sector mask and above a threshold indicating
that the
temperature is approaching a boing temperature (or other temperature
reference), the
43
CA 03075454 2020-03-10
WO 2019/077385 PCT/IB2017/001506
method proceeds to apply controller decisions 2164. Otherwise, the method
shuts off
the element 2166 and then proceeds to apply controller decisions at 2164.
Execution
may then return to 2116.
[00203] Having thus described several aspects and embodiments of the
technology of this application, it is to be appreciated that various
alterations,
modifications, and improvements will readily occur to those of ordinary skill
in the art.
Such alterations, modifications, and improvements are intended to be within
the spirit
and scope of the technology described in the application. For example, those
of
ordinary skill in the art will readily envision a variety of other means
and/or structures
for performing the function and/or obtaining the results and/or one or more of
the
advantages described herein, and each of such variations and/or modifications
is
deemed to be within the scope of the embodiments described herein.
[00204] Those skilled in the art will recognize, or be able to ascertain
using no
more than routine experimentation, many equivalents to the specific
embodiments
described herein. It is, therefore, to be understood that the foregoing
embodiments
are presented by way of example only and that, within the scope of the
appended
claims and equivalents thereto, inventive embodiments may be practiced
otherwise
than as specifically described. In addition, any combination of two or more
features,
systems, articles, materials, kits, and/or methods described herein, if such
features,
systems, articles, materials, kits, and/or methods are not mutually
inconsistent, is
included within the scope of the present disclosure.
[00205] The above-described embodiments may be implemented in any of
numerous ways. One or more aspects and embodiments of the present application
involving the performance of processes or methods may utilize program
instructions
44
CA 03075454 2020-03-10
WO 2019/077385 PCT/IB2017/001506
executable by a device (e.g., a computer, a processor, or other device) to
perform, or
control performance of, the processes or methods. In this respect, various
inventive
concepts may be embodied as a computer readable storage medium (or multiple
computer readable storage media) (e.g., a computer memory, one or more floppy
discs, compact discs, optical discs, magnetic tapes, flash memories, circuit
configurations in Field Programmable Gate Arrays or other semiconductor
devices, or
other tangible computer storage medium) encoded with one or more programs
that,
when executed on one or more computers or other processors, perform methods
that
implement one or more of the various embodiments described above.
[00206] The computer readable medium or media may be transportable, such
that the program or programs stored thereon may be loaded onto one or more
different computers or other processors to implement various ones of the
aspects
described above. In some embodiments, computer readable media may be non-
transitory media.
[00207] Additionally, it should be appreciated that according to one
aspect, one
or more computer programs that when executed perform methods of the present
application need not reside on a single computer or processor, but may be
distributed
in a modular fashion among a number of different computers or processors to
implement various aspects of the present application.
[00208] Computer-executable instructions may be in many forms, such as
program modules, executed by one or more computers or other devices.
Generally,
program modules include routines, programs, objects, components, data
structures,
etc. that perform particular tasks or implement particular abstract data
types. Typically,
CA 03075454 2020-03-10
WO 2019/077385 PCT/1B2017/001506
the functionality of the program modules may be combined or distributed as
desired in
various embodiments.
[00209] Further, it should be appreciated that a computer may be embodied
in
any of a number of forms, such as a rack-mounted computer, a desktop computer,
a
laptop computer, or a tablet computer, as non-limiting examples. Additionally,
a
computer may be embedded in a device not generally regarded as a computer but
with suitable processing capabilities, including a Personal Digital Assistant
(FDA), a
smart phone or any other suitable portable or fixed electronic device.
[00210] Fig. 22 is a block diagram of a computer architecture 2200
according to
some embodiments. In some embodiments, one or more of the systems (or
portion(s)
thereof), apparatus (or portion(s) thereof) and/or devices (or portion(s)
thereof)
disclosed herein may have an architecture that is the same as and/or similar
to one or
more portions of the architecture 2200.
[00211] In some embodiments, one or more of the methods (or portion(s)
thereof)
disclosed herein may be performed by a system, apparatus and/or device having
an
architecture that is the same as or similar to the architecture 2200 (or
portion(s)
thereof). The architecture may be implemented as a distributed architecture or
a non-
distributed architecture.
[00212] Referring to Fig. 22, in accordance with at least some embodiments,
the
architecture 2200 may include one or more processors 2210 and one or more
articles
of manufacture that comprise non-transitory computer-readable storage media
(e.g.,
memory 2220 and one or more non-volatile storage media 2230). The processor
2210
may control writing data to and reading data from the memory 2220 and the non-
volatile storage device 2230 in any suitable manner, as the aspects of the
disclosure
46
CA 03075454 2020-03-10
WO 2019/077385 PCT/1B2017/001506
provided herein are not limited in this respect. The storage media may store
one or
more programs and/or other information for operation of the architecture 2200.
In at
least some embodiments, the one or more programs include one or more
instructions
to be executed by the processor 2210 to provide one or more portions of one or
more
tasks and/or one or more portions of one or more methods disclosed herein. In
some
embodiments, other information includes data for one or more portions of one
or more
tasks and/or one or more portions of one or more methods disclosed herein. To
perform any of the functionality described herein, the processor 2210 may
execute one
or more processor-executable instructions stored in one or more non-transitory
computer-readable storage media (e.g., the memory 2220), which may serve as
non-
transitory computer-readable storage media storing processor-executable
instructions
for execution by the processor 2210.
[00213] The terms "program" or "software" are used herein in a generic
sense to
refer to any type of computer code or set of computer-executable instructions
that may
be employed to program a computer or other processor to implement various
aspects
as described above. Additionally, it should be appreciated that according to
one
aspect, one or more computer programs that when executed perform methods of
the
present application need not reside on a single computer or processor, but may
be
distributed in a modular fashion among a number of different computers or
processors
to implement various aspects of the present application.
[00214] Computer-executable instructions may be in many forms, such as
program modules, executed by one or more computers or other devices.
Generally,
program modules include routines, programs, objects, components, data
structures,
etc. that perform particular tasks or implement particular abstract data
types. Typically,
47
CA 03075454 2020-03-10
WO 2019/077385 PCT/IB2017/001506
the functionality of the program modules may be combined or distributed as
desired in
various embodiments.
[00215] Also, data structures may be stored in computer-readable media in
any
suitable form. For simplicity of illustration, data structures may be shown to
have fields
that are related through location in the data structure. Such relationships
may likewise
be achieved by assigning storage for the fields with locations in a computer-
readable
medium that convey relationship between the fields. However, any suitable
mechanism
may be used to establish a relationship between information in fields of a
data
structure, including through the use of pointers, tags or other mechanisms
that
establish relationship between data elements.
[00216] When implemented in software, the software code may be executed on
any suitable processor or collection of processors, whether provided in a
single
computer or distributed among multiple computers.
[00217] Further, it should be appreciated that a computer may be embodied
in
any of a number of forms, such as a rack-mounted computer, a desktop computer,
a
laptop computer, or a tablet computer, as non-limiting examples. Additionally,
a
computer may be embedded in a device not generally regarded as a computer but
with suitable processing capabilities, including a Personal Digital Assistant
(PDA), a
smart phone or any other suitable portable or fixed electronic device.
[00218] Also, a computer may have one or more communication devices 2240,
which may be used to interconnect the computer to one or more other devices
and/or
systems, such as, for example, one or more networks in any suitable form,
including a
local area network or a wide area network, such as an enterprise network, and
intelligent network (IN) or the Internet. Such networks may be based on any
suitable
48
CA 03075454 2020-03-10
WO 2019/077385 PCT/1B2017/001506
technology and may operate according to any suitable protocol and may include
wireless networks or wired networks.
[00219] Also, a computer may have one or more input devices 2250 and/or one
or more output devices 2260. These devices can be used, among other things, to
present a user interface. Examples of output devices that may be used to
provide a
user interface include printers or display screens for visual presentation of
output and
speakers or other sound generating devices for audible presentation of output.
Examples of input devices that may be used for a user interface include
keyboards, and
pointing devices, such as mice, touch pads, and digitizing tablets. As another
example,
a computer may receive input information through speech recognition or in
other
audible formats.
[00220] Also, as described, some aspects may be embodied as one or more
methods. The acts performed as part of the method may be ordered in any
suitable
way. Accordingly, embodiments may be constructed in which acts are performed
in an
order different than illustrated, which may include performing some acts
simultaneously, even though shown as sequential acts in illustrative
embodiments.
[00221] It should be understood that the features disclosed herein can be
used in
any combination or configuration. Thus, for example, in some embodiments, any
one
or more of the features disclosed herein may be used without any one or more
other
feature disclosed herein.
[00222] Unless stated otherwise, a computing device is any type of device
that
includes at least one processor.
[00223] Unless stated otherwise, a processing device is any type of device
that
includes at least one processor.
49
CA 03075454 2020-03-10
WO 2019/077385 PCT/IB2017/001506
[00224] Unless stated otherwise, a processing system is any type of system
that
includes at least one processor.
[00225] Unless stated otherwise, a mobile (or portable) computing device
includes, but is not limited to, any computing device that may be carried in
one or two
hands and/or worn.
[00226] Unless stated otherwise, a processor may comprise any type of
processor. For example, a processor may be programmable or non-programmable,
general purpose or special purpose, dedicated or non-dedicated, distributed or
non-
distributed, shared or not shared, and/or any combination thereof. A processor
may
include, but is not limited to, hardware, software (e.g., low-level language
code, high-
level language code, microcode), firmware, and/or any combination thereof.
[00227] Unless stated otherwise, a program may include, but is not limited
to,
instructions in a high-level language, low-level language, machine language
and/or
other type of language or combination thereof.
[00228] Unless stated otherwise, a "communication link" may comprise any
type(s) of communication link(s), for example, but not limited to, wired links
(e.g.,
conductors, fiber optic cables) or wireless links (e.g., acoustic links, radio
links,
microwave links, satellite links, infrared links or other electromagnetic
links) or any
combination thereof, each of which may be public and/or private, dedicated
and/or
shared. In some embodiments, a communication link may employ a protocol or
combination of protocols including, for example, but not limited to the
Internet
Protocol.
[00229] Unless stated otherwise, information may include data and/or any
other
type of information.
CA 03075454 2020-03-10
WO 2019/077385 PCT/IB2017/001506
[00230] Unless stated otherwise, terms such as, for example, "in response
to" and
"based on'' mean "in response (directly and/or indirectly) at least to" and
"based
(directly and/or indirectly) at least on", respectively, so as not to preclude
intermediates and being responsive to and/or based on, more than one thing.
[00231] Unless stated otherwise, the term "represents" means "directly
represents" and/or "indirectly represents."
[00232] Unless stated otherwise, terms such as, for example, "comprises,"
"has,"
"includes," and all forms thereof, are considered open-ended, so as not to
preclude
additional elements and/or features.
[00233] Also, unless stated otherwise, terms such as, for example, "a,"
"one,"
"first," are considered open-ended, and do not mean "only a", "only one" or
"only
a first", respectively. Also, unless stated otherwise, the term "first" does
not, by itself,
require that there also be a "second."
[00234] Also, unless stated otherwise, the phrase "and/or," as used herein
in the
specification and in the claims, should be understood to mean "either or both"
of the
elements so conjoined, i.e., elements that are conjunctively present in some
cases and
disjunctively present in other cases. Multiple elements listed with "and/or"
should be
construed in the same fashion, i.e., "one or more" of the elements so
conjoined.
Elements other than those specifically identified by the "and/or" clause may
optionally
be present, whether related or unrelated to those elements specifically
identified. Thus,
as a non-limiting example, a reference to "A and/or B", when used in
conjunction with
open-ended language such as "comprising" may refer, in one embodiment, to A
only
(optionally including elements other than B); in another embodiment, to B only
51
CA 03075454 2020-03-10
WO 2019/077385 PCT/IB2017/001506
(optionally including elements other than A); in yet another embodiment, to
both A
and B (optionally including other elements); etc.
[00235] The present invention should therefore not be considered limited to
the
particular embodiments described above. Various modifications, equivalent
processes,
as well as numerous structures to which the present invention may be
applicable, will
be readily apparent to those skilled in the art to which the present invention
is directed
upon review of the present disclosure.
[00236] What is claimed is:
52