Note: Descriptions are shown in the official language in which they were submitted.
CA 03077526 2020-03-31
WO 2019/071339 PCT/CA2018/051214
APPARATUS AND METHOD FOR MONITORING AND CONTROLLING THE
FILLING OF A CONTAINER WITH A PHARMACEUTICAL FLUID IN AN ASEPTIC
ENVIRONMENT
BACKGROUND OF THE INVENTION
FIELD OF THE INVENTION
[001] This present invention relates to the medical field as exemplified by
IPC class A61
and more particularly to apparatus and associated methods for sterilization of
and sterile
handling of pharmaceutical materials and containers for pharmaceuticals,
including bringing
pharmaceuticals into form for administration to medical or veterinary
patients. In one aspect,
it relates to the programmed and automatic operation of such apparatus
configured and
arranged for filling pharmaceutical containers with predetermined amounts of
liquid or other
materials.
BACKGROUND ART
[002] The subject of filling pharmaceuticals into pharmaceutical containers is
a major aspect
of the Pharmaceuticals Industry. The subject is heavily controlled by various
governmental
and official bodies in various countries. Technologically, the subject is a
challenge in that the
pharmaceutical products need to be filled into the containers under very
strict aseptic
conditions. Very specific procedures are specified for this task to a degree
that makes the
handling of pharmaceuticals profoundly different from the handling of any
other industrial
product, including specifically semiconductors, which also demand extreme and
consistent
1
CA 03077526 2020-03-31
WO 2019/071339 PCT/CA2018/051214
environmental conditions. Indeed, the parallels between the handling of
semiconductors in
semiconductor "clean laboratories" and the handling of pharmaceuticals in
aseptic isolators are
superficial. They share the use of such "clean laboratories", but there is no
inherent aseptic
requirement associated with semiconductor manufacture.
[003] The filling of pharmaceutical containers with fluid pharmaceuticals
specifically
requires the aseptic handling of both the containers and the fluid
pharmaceutical itself This
leads to complex mechanisms and procedures, many of which may be automated to
one degree
or another. Often, the production equipment for fluid pharmaceutical handling
is bulky and
expensive. This creates a problem for smaller operations, particularly in the
small-scale
production and development environments. As the field has developed, the need
for smaller,
more compact equipment, particularly in the filling and compounding of fluid
pharmaceuticals,
has become evident.
[004] The prior art is typically characterized by the use of vibratory bowls
and escapements.
Many prior art systems also employ gloves for use by the operator to access
the interior of the
chamber.
SUMMARY OF THE INVENTION
[005] In one general aspect, the invention features a method for filling
nested pharmaceutical
containers with a pharmaceutical fluid substance, such as a liquid, solution,
or suspension
having therapeutic properties. The method includes providing a filling system
comprising a
sterilizable chamber capable of maintaining an aseptic condition, with the
chamber comprising
a filling station and a planar rotary stage having a destination fiducial
locating structure
including constraining surfaces. The method also includes transferring into
the chamber at least
one container tub sealed by a container tub cover and containing a container
nest bearing a
plurality of pharmaceutical containers, aseptically sealing the chamber, and
establishing an
aseptic condition within the chamber. The container nest bearing the plurality
of
2
CA 03077526 2020-03-31
WO 2019/071339 PCT/CA2018/051214
pharmaceutical containers is transferred into the destination fiducial
locating structure such
that the container nest is held in place by the constraining surfaces, and the
pharmaceutical
fluid substance is dispensed into at least a portion of the plurality of
pharmaceutical containers
by operating both the rotary stage and the filling station.
[006] In particular embodiments, the operating the filling station may include
rotating the
filling station. The dispensing the pharmaceutical fluid substance may
comprise dispensing the
pharmaceutical fluid substance on an iterative and serial basis into the
containers. Providing a
filling system may comprise providing a filing apparatus comprising at least
one cover removal
station within the chamber, with the transferring into the destination
fiducial locating structure
the container nest comprising removing the container tub cover from the
container tub by
operating both the rotary stage and the at least one cover removal station.
Operating the at least
one cover removal station may comprise rotating the at least one cover removal
station.
Providing the filling system may comprise providing within the chamber at
least one cover
removal station having an engagement tool, transferring into the chamber at
least one container
tub may comprise attaching to the container tub cover a cover removal fixture,
and operating
the at least one cover removal station may comprise engaging the engagement
tool with the
cover removal fixture.
[007] The method may further comprise transferring into the chamber a
container closure tub
sealed by a container closure tub cover and containing at least one container
closure nest
bearing a plurality of pharmaceutical container closures. The method may
further comprise
positioning one of the at least one closure nests to align closures in the at
least one closure nest
with corresponding containers in the container nest, transferring the nests of
aligned closures
and containers to the ramming station by rotating the rotary stage, and
forcing the closures into
the corresponding containers. Positioning one of the at least one closure
nests may comprise
obtaining image information about the one of the at least one closure nest,
and positioning the
one of the at least one closure nests based on the image information.
3
CA 03077526 2020-03-31
WO 2019/071339 PCT/CA2018/051214
[008] Positioning one of the at least one closure nest may comprise applying a
vacuum to
suction cups, lifting the container closure nest with the suction cups, and
operating the rotary
stage. Transferring into the destination fiducial locating opening the
container nest may
comprise applying a vacuum to suction cups, lifting the container nest with
the suction cups,
and operating the rotary stage. Dispensing the pharmaceutical fluid substance
may comprise
simultaneously and/or serially operating the rotary stage and the filling
station, and removing
the container tub cover may comprise simultaneously and/or serially operating
the rotary stage
and the at least one cover removal station.
[009] In another general aspect, the invention features a system for filling
nested
pharmaceutical containers with a pharmaceutical fluid substance comprising a
sterilizable
chamber capable of maintaining an aseptic condition. The chamber includes a
filling station,
and a planar rotary stage having a rotary stage rotation axis and comprising a
destination
fiducial locating structure including constraining surfaces disposed and
shaped to receive and
hold a pharmaceutical container nest bearing a plurality of pharmaceutical
containers.
[0010] In particular embodiments, the filling station may comprise a fluid
product dispenser
head, with the filling station being configured to be rotatable about a
filling station rotation
axis parallel to the rotary stage rotation axis to position in combination
with rotation of the
rotary stage the dispenser head over any one of the plurality of
pharmaceutical containers
held in the container nest in the destination fiducial locating structure. The
chamber may
further comprise at least one cover removal station and the rotary stage may
further comprise
a first source fiducial locating structure including constraining surfaces
disposed and shaped
to receive and hold a pharmaceutical container closure tub sealed by a
container closure tub
cover and containing at least one pharmaceutical container closure nest
bearing a plurality of
pharmaceutical container closures, and at least one second source fiducial
locating opening
disposed and shaped to receive and hold a pharmaceutical container tub sealed
by a container
tub cover and containing a pharmaceutical container nest bearing a plurality
of
pharmaceutical containers.
4
CA 03077526 2020-03-31
WO 2019/071339 PCT/CA2018/051214
[0011] The at least one cover removal station may be disposed and configured
to be rotatable
about a cover removal station rotation axis parallel to the rotary stage
rotation axis to remove
in combination with rotation of the rotary stage the container tub cover from
the at least one
container tub and the container closure tub cover from the container closure
tub. At least one
cover removal station may comprise an engagement tool disposed and configured
to engage
with engagement fixtures pre-attached to the container tub cover and to the
container closure
tub cover.
[0012] The system may further comprise at least one camera disposed to obtain
image
information about at least one of the container nest and the closure nest, and
a controller, with
the chamber further comprising at least one vacuum pickup system comprising
suction cups
disposed to engage with the container nests and the container closure nests,
the at least one
vacuum pickup system being configured in combination with rotation of the
rotary stage to lift
a pharmaceutical container nest from a pharmaceutical container tub held in
one of the at least
one second source fiducial locating openings and to deposit the pharmaceutical
container nest
in the destination fiducial locating opening in combination with rotation of
the rotary stage and
to lift a pharmaceutical container closure nest from a pharmaceutical
container closure tub held
in the first source fiducial locating opening and to deposit the container
closure nest on top of
the pharmaceutical container nest under control of the controller.
[0013] The controller may be operative to instruct the at least one camera to
provide to the
controller the image information and the controller may be operative to
control the rotation of
the rotary stage to place the closures in the closure nest in correspondence
with containers in
the container nest. The system may further comprise a ram system configured
for forcing the
closures into the corresponding containers.
[0014] The system may further comprise at least one rotatable cover removal
station having a
cover removal station rotation axis parallel to the rotary stage rotation
axis, at least one vacuum
pickup system for placing the container closure nest on the container nest
with closures in the
CA 03077526 2020-03-31
WO 2019/071339 PCT/CA2018/051214
closure nest in correspondence with containers in the container nest, and a
ram system for
forcing the closures into the containers, with the filing station being a
rotatable filling station
having a filling station rotation axis parallel to the rotary stage rotation
axis and comprising a
fluid product dispenser head. The system may further comprise at least one
camera for
obtaining image information of at least one of the container nest and the
closure nest, and a
controller comprising a memory and a processor. The controller may be
operative to instruct
the rotary stage to rotate to angular positions that are one of predetermined
and based on the
image information and to control the at least one cover removal station, the
filling station, the
at least one vacuum pickup system, and the ram system to operate in
conjunction with the
rotary stage.
[0015] In a further general aspect, the invention features a system for
filling nested
pharmaceutical containers with a pharmaceutical fluid substance that includes
means for
establishing and maintaining an aseptic condition in a chamber, means for
constraining a
container nest bearing a plurality of pharmaceutical containers in the
chamber, and means for
transferring a container nest to the means for constraining from a container
tub in the chamber.
It also includes means for rotating the means for constraining in the chamber;
and means for
dispensing the pharmaceutical fluid substance into at least a portion of the
plurality of
pharmaceutical containers in the container nest while the container nest is
constrained by the
means for constraining.
[0016] In a further aspect, a system is provided for filling nested
pharmaceutical containers
with a pharmaceutical fluid substance, the system comprising a sterilizable
chamber capable
of maintaining an aseptic condition, the chamber comprising: a planar rotary
stage having a
rotary stage rotation axis, a plurality of locating structures positioned with
respect to the rotary
stage at different positions around the rotary stage rotation axis, for
holding nests of
pharmaceutical container parts at the different positions around the rotary
stage rotation axis,
and a container filling station having a dispensing head for filling the
containers while they are
held in a nest at one of the locating structures. The locating structures may
include surfaces
6
CA 03077526 2020-03-31
WO 2019/071339 PCT/CA2018/051214
associated with a first tub-holding opening in the rotary stage for holding a
first tub containing
at least one nest of containers, surfaces associated with a second tub-holding
opening in the
rotary stage for holding a second tub containing at least one nest of
closures, and surfaces
associated with a destination nest-holding opening in the rotary stage for
holding at least one
nest.
[0017] The chamber may further comprise at least one vacuum pickup system
comprising
suction cups disposed to engage with the container nest and container closure
nest held on the
rotary stage, the at least one vacuum pickup system being configured in
combination with
rotation of the rotary stage to lift a pharmaceutical container nest from a
pharmaceutical
container tub and to deposit the pharmaceutical container nest in the
destination opening in
combination with rotation of the rotary stage and to lift a pharmaceutical
container closure nest
from a pharmaceutical container closure tub and to deposit the container
closure nest on top of
the pharmaceutical container nest.
[0018] At least one of the locating structures may include a reconfigurable
locating structure
with one or more adjustable positioning surfaces to position a tub with
respect to the rotary
stage. The reconfigurable locating structure may include at least one pair of
a reconfigurable
stopping member and a restraining member disposed opposite each other across
an opening in
the rotary stage to precisely position at a first predetermined position a tub
that contains at least
one nest. The stopping member may be adjustable to stop the tub at the first
predetermined
position by a rotary adjustment and the restraining member may be disposed to
restrain the tub
in the first predetermined position.
[0019] At least a first of the reconfigurable locating structures may include
a rotary positioning
element having an axis of rotation parallel to a plane of the rotary stage and
includes a plurality
of different positioning surfaces that are selectable by rotating the rotary
positioning element.
At least one of the reconfigurable locating structures may include a pair of
opposing rotary
positioning elements each having an axis of rotation parallel to a plane of
the rotary stage and
7
CA 03077526 2020-03-31
WO 2019/071339 PCT/CA2018/051214
each may include a plurality of different positioning surfaces that are
selectable by rotating the
rotary positioning elements to accommodate different nest widths.
[0020] At least one of the reconfigurable locating structures may include at
least a first pair of
opposing positioning elements that define positioning surfaces that oppose
each other along a
first positioning axis that is at least generally parallel to a plane of the
rotary stage and at least
a second pair of opposing positioning elements that define positioning
surfaces that oppose
each other along a second positioning axis that is at least generally parallel
to a plane of the
rotary stage and at least generally perpendicular to the first positioning
axis. The at least one
of the positioning elements in each of the first and second pairs of
positioning elements may
include a rotary positioning element having an axis of rotation parallel to a
plane of the rotary
stage and including a plurality of different positioning surfaces.
[0021] The system may further include a reconfigurable vacuum pickup system
comprising: a
first set of suction cups arranged in a first pattern, a second set of suction
cups arranged in a
second pattern different from the first pattern, and a selection mechanism
operative to position
either the first set of suction cups or the second set of suction cups to
engage with the at least
a first of the nests of pharmaceutical container parts while it is held by one
of the plurality of
locating structures. The selection mechanism of the reconfigurable vacuum
pickup system may
include a rotary mechanism operative to position the first or second sets of
suction cups in an
engagement position.
[0022] The system may further include at least one cover removal station
positioned to remove
covers from tubs containing at least one nest of pharmaceutical packaging
materials held in
one of the locating structures. The at least one cover removal station may be
rotatable about a
cover removal station rotation axis parallel to the rotary stage rotation axis
to remove the tub
covers in combination with rotation of the rotary stage. The at least one
cover removal station
may comprise an engagement tool disposed and configured to engage with a cover
removal
fixture on the tub cover.
8
CA 03077526 2020-03-31
WO 2019/071339 PCT/CA2018/051214
[0023] The filling station may be configured to be rotatable about a filling
station rotation axis
parallel to the rotary stage rotation axis to position in combination with
rotation of the rotary
stage the dispenser head over any one of the plurality of pharmaceutical
containers held by one
of the one of the locating structures.
[0024] The system may further comprise at least one camera disposed to obtain
image
information about at least one of the nests of pharmaceutical container parts.
The system may
further comprise a ram system configured for forcing nested closures into
corresponding nested
containers.
[0025] The system may further comprise at least one rotatable cover removal
station having a
cover removal station rotation axis parallel to the rotary stage rotation
axis; at least one vacuum
pickup system for placing a container closure nest on a container nest with
closures in the
closure nest in correspondence with containers in the container nest; a ram
system for forcing
the closures into the containers; and wherein the filing station is a
rotatable filling station
having a filling station rotation axis parallel to the rotary stage rotation
axis and comprising a
fluid product dispenser head.
[0026] The system may further comprise at least one camera for obtaining image
information
of at least one of the container nest and the closure nest, a controller
comprising a memory and
a processor, and wherein the controller is operative to instruct the rotary
stage to rotate to
angular positions that are one of predetermined and based on the image
information and to
control the at least one cover removal station, the filling station, the at
least one vacuum pickup
system, and the ram system to operate in conjunction with the rotary stage.
[0027] In another aspect, a system is provided for filling nested
pharmaceutical containers with
a pharmaceutical fluid substance, comprising: means for establishing and
maintaining an
aseptic condition in a chamber; means for constraining a container nest
bearing a plurality of
pharmaceutical containers in the chamber; means for transferring to the means
for constraining
9
CA 03077526 2020-03-31
WO 2019/071339 PCT/CA2018/051214
a container nest from a container tub in the chamber; means for rotating the
means for
constraining in the chamber; and means for dispensing the pharmaceutical fluid
substance into
at least a portion of the plurality of pharmaceutical containers in the
container nest while the
container nest is constrained by the means for constraining.
[0028] In a further aspect, a method is provided for filling nested
pharmaceutical containers
with a pharmaceutical fluid substance, the method comprising: providing a
filling system
comprising a sterilizable chamber capable of maintaining an aseptic condition,
the chamber
comprising a filling station and a planar rotary stage having a destination
locating structure;
transferring into the chamber at least one container tub sealed by a container
tub cover and
containing a container nest bearing a plurality of pharmaceutical containers;
aseptically sealing
the chamber; establishing an aseptic condition within the chamber;
transferring into the
destination locating structure the container nest bearing the plurality of
pharmaceutical
containers such that the container nest is held in place; and dispensing the
pharmaceutical fluid
substance into at least a portion of the plurality of pharmaceutical
containers by operating both
the rotary stage and the filling station. The operating the filling station
may include rotating
the filling station. The dispensing the pharmaceutical fluid substance may
comprise dispensing
the pharmaceutical fluid substance on an iterative and serial basis into the
containers.
[0029] The providing a filling system may comprise providing a filing
apparatus comprising
at least one cover removal station within the chamber and wherein the
transferring into the
destination locating structure the container tub comprises removing the
container tub cover
from the container tub by operating both the rotary stage and the at least one
cover removal
station. The operating the at least one cover removal station may comprise
rotating the at least
one cover removal station. The providing the filling system may comprise
providing within
the chamber at least one cover removal station having an engagement tool, the
transferring into
the chamber at least one container tub may comprise attaching to the container
tub cover a
cover removal fixture; and wherein the operating the at least one cover
removal station
comprises engaging the engagement tool with the cover removal fixture.
CA 03077526 2020-03-31
WO 2019/071339 PCT/CA2018/051214
[0030] The method may further comprise transferring into the chamber a
container closure tub
sealed by a container closure tub cover and containing at least one container
closure nest
bearing a plurality of pharmaceutical container closures. The method may
further comprise
positioning one of the at least one closure nests to align closures in the at
least one closure nest
with corresponding containers in the container nest; transferring the nests of
aligned closures
and containers to a ramming station by rotating the rotary stage; and forcing
the closures into
the corresponding containers. The method may further include adjusting a tub
locating
structure to accommodate a size of the closure nest tub. The positioning one
of the at least one
closure nest may comprise: obtaining image information about the one of the at
least one
closure nests; and positioning the one of the at least one closure nests based
on the image
information. The positioning one of the at least one closure nest may
comprise: applying a
vacuum to suction cups; lifting the container closure nest with the suction
cups; and operating
the rotary stage.
[0031] The transferring into the destination locating opening the container
nest may comprise:
applying a vacuum to suction cups; lifting the container nest with the suction
cups; and
operating the rotary stage. The method may further include selecting one of a
plurality of sets
of suction cups and wherein the applying a vacuum to suction cups is performed
for the selected
set of suction cups. The selecting may include rotating one of the plurality
of sets of suction
cups into position. The method may further include the destination locating
structure to
accommodate a size of the container nest. The adjusting may be performed in
two at least
generally orthogonal directions. The method may further include adjusting a
tub locating
structure to accommodate a size of the container nest tub.
[0032] In another general aspect, the invention features a container assembly
for holding
nested pharmaceutical container parts. It includes a container comprising a
bottom, a top lip
that provides a horizontal top sealing surface that has a peripheral outline,
and sidewalls located
between the bottom and the top lip. It also includes a peelable container
cover consisting of a
11
CA 03077526 2020-03-31
WO 2019/071339 PCT/CA2018/051214
sheet of flexible material sealed to the sealing surface of the top lip of the
rectangular container
to seal the contents of the container, and a cover removal fixture on the
container cover.
[0033] The sealed peelable container cover may include a portion that extends
outside of the
peripheral outline of the top sealing surface of the container, and the cover
removal fixture
may be on the portion of the peelable container cover that extends outside of
the peripheral
outline of the top sealing surface of the container. The container may be
rectangular and
includes four sidewalls. The cover removal fixture may include an appendage to
allow it to be
engaged by an engagement tool. The cover removal fixture may include a ball-
shaped
appendage to allow it to be engaged by an engagement tool. The peelable
container cover may
be heat sealed to the sealing surface of the top lip of the rectangular
container to seal the
contents of the container against decontamination. The peelable container
cover may be sealed
to the sealing surface of the top lip of the rectangular container to seal the
contents of the
container against decontamination using a chemical agent. The peelable
container cover may
sealed to the sealing surface of the top lip of the rectangular container to
seal the contents of
the container against decontamination using a radiation. The peelable
container cover may be
sealed to the sealing surface of the top lip of the rectangular container to
seal the contents of
the container against decontamination using plasma. The peelable cover may be
made of a
plastic material. The peelable cover may be made of an impermeable laminated
foil. The
peelable cover may be made of a polymeric membrane. The cover removal fixture
may be
clipped to a portion of the peelable container cover that extends outside of
the peripheral
outline of the top sealing surface of the container. The sealed container may
hold sterilized
pharmaceutical containers or closures.
[0034] In a further aspect, a method is provided for removing within a
controlled environment
enclosure a container cover from a sealed container, the sealed container
being sealed by the
container cover, the method comprising: providing the container in the
controlled environment
enclosure with the cover sealed to a sealing surface of a lip of the container
to seal the contents
of the container against decontamination, the cover having a cover removal
fixture,
12
CA 03077526 2020-03-31
WO 2019/071339 PCT/CA2018/051214
decontaminating the sealed container in the controlled environment enclosure,
engaging the
cover removal fixture with an engagement tool, and removing the cover from the
container
using the engagement tool. The engaging may engage the cover removal fixture
with a fork-
shaped engagement tool. The engaging may engage a ball-shaped appendage on the
cover
removal fixture.
[0035] The providing may include providing sterilized pharmaceutical
containers or closures
in the sealed container before the decontaminating. The attaching may take
place before the
container is in the controlled environment enclosure. The decontaminating the
sealed container
in the controlled environment enclosure may take place before the removing the
cover. The
removing the cover may include moving the engagement tool relative to the
container. The
removing the cover may include moving both the container and the engagement
tool. The
method may further comprise attaching the cover removal fixture to the cover
before providing
the container in the controlled environment enclosure.
[0036] In a further aspect, a method is provided for aseptically dispensing a
pharmaceutical
fluid into a container, the method comprising: providing a sterilizable
chamber capable of
maintaining an aseptic condition, the chamber comprising a pharmaceutical
fluid dispensing
head configured for producing droplets of the pharmaceutical fluid and a
droplet monitoring
system comprising a digital imager; establishing within the sterilizable
chamber an aseptic
condition; providing within the sterilizable chamber an aseptic pharmaceutical
container;
moving at least one of the dispensing head and the container to position an
opening of the
container under the dispensing head to receive the droplets along a droplet
path; dispensing a
plurality of droplets of the fluid from the dispensing head along a droplet
path into the
container; obtaining from the imager a plurality of images of at least one of
the plurality of
droplets along the droplet path; and determining from the plurality of images
a volume of fluid
dispensed into the container. The method may further comprise ceasing the
dispensing of the
fluid based on the volume of fluid dispensed into the container.
13
CA 03077526 2020-03-31
WO 2019/071339 PCT/CA2018/051214
[0037] The determining from the plurality of images a volume of fluid
dispensed into the
container may comprise determining a volume of at least one of the plurality
of droplets. The
determining the volume of the at least one of the plurality of droplets may
comprise: identifying
first and second total portions of the at least one droplet appearing
respectively to the left and
to the right of the droplet path in at least one image of the at least one
droplet; calculating first
and second volumes of the at least one of the plurality of droplets by
separately mathematically
rotating respectively the first and second total portions of the droplet
through 27( about the
droplet path; and equating the volume of the at least one of the plurality of
droplets to the
average of the first and second volumes.
[0038] The obtaining from the imager a plurality of images of at least one of
the plurality of
droplets along the droplet path may comprise obtaining the plurality of images
over a
predetermined portion of the droplet path. Alternatively, the obtaining from
the imager a
plurality of images of at least one of the plurality of droplets along the
droplet path may
comprise: determining from the plurality of images a portion of the droplet
path where droplets
have a stable shape; and selecting the at least one image of the at least one
droplet to be from
among images of the droplet taken when the droplet is in the portion of the
droplet path where
droplets have a stable shape.
[0039] The determining from the plurality of images a volume of fluid
dispensed into the
container may comprise determining a volume of each droplet dispensed into the
container.
The ceasing the dispensing of the fluid based on the volume of fluid dispensed
into the
container may comprise ceasing the dispensing of the fluid when a total amount
of fluid
dispensed into the container equals a predetermined volume. The obtaining from
the imager a
plurality of images of at least one of the plurality of droplets along the
droplet path may
comprise obtaining the plurality of images employing light reflected to the
imager by a
retroreflector. The obtaining from the imager a plurality of images of at
least one of the
plurality of droplets along the droplet path may comprise obtaining the
plurality of images by
means of a telecentric lens. The providing within the sterilizable chamber an
aseptic
14
CA 03077526 2020-03-31
WO 2019/071339 PCT/CA2018/051214
pharmaceutical container comprises providing the aseptic pharmaceutical
container within a
container nest.
[0040] The method may further comprise moving at least one of the dispensing
head and the
container to position an opening of the container under the dispensing head to
receive the
droplets along a droplet path. The moving the container may comprise operating
a robotic arm.
Operating the robotic arm may comprise operating an articulated robotic arm.
Moving the
dispensing head may comprise operating a robotic arm, which arm may be an
articulated
robotic arm.
[0041] In a further aspect, a system is provided for aseptically dispensing a
pharmaceutical
fluid into a container, the system comprising: a sealable and sterilizable
chamber capable of
maintaining an aseptic condition; in the chamber a pharmaceutical fluid
dispensing head
configured for producing droplets of the pharmaceutical fluid; in the chamber
a droplet
monitoring system comprising a digital imager disposed to obtain images of
droplets dispensed
by the fluid dispensing head; a controller comprising a memory and a
processor, the controller
in communication with the fluid dispensing head and the digital imager; and
software
configured for controlling dispensing of the pharmaceutical fluid droplets by
the fluid
dispensing head and for collection of images of the pharmaceutical fluid
droplets along a
droplet path when the software is loaded in the memory and executed by the
processor.
[0042] The system may further comprise in communication with the controller at
least one of
a fluid dispensing head positioning system and a container positioning system,
the software
further configured for controlling the at least one of a fluid dispensing head
positioning system
and a container positioning system. The fluid dispensing head positioning
system may
comprise a robotic arm that may be an articulated robotic arm. The articulated
robotic arm may
be hermetically sealed to the chamber. The container positioning system may
comprise a
robotic arm. The robotic arm used in the container positioning system may
comprise an end
effector arranged for holding a container nest. The robotic arm used in the
container positioning
CA 03077526 2020-03-31
WO 2019/071339 PCT/CA2018/051214
system may comprise an articulated robotic arm which may, in some embodiments,
be
hermetically sealed to the chamber. The droplet monitoring system may comprise
a
retroreflector disposed to reflect light through the droplets to the digital
imager. The digital
imager may comprise a telecentric lens.
[0043] Systems and methods according to the invention need not employ either
vibratory
bowls or escapements. Nor do such systems or method require gloves. Systems
and methods
according to the invention may therefore address needs for compact, small-
scale filling and
compounding of fluid pharmaceuticals.
BRIEF DESCRIPTION OF THE DRAWINGS
[0044] The above-mentioned and other features and objects of this invention,
and the manner
of attaining them, will become more apparent and the invention itself will be
better understood
by reference to the following description of an embodiment of the invention
taken in
conjunction with the accompanying drawings, wherein:
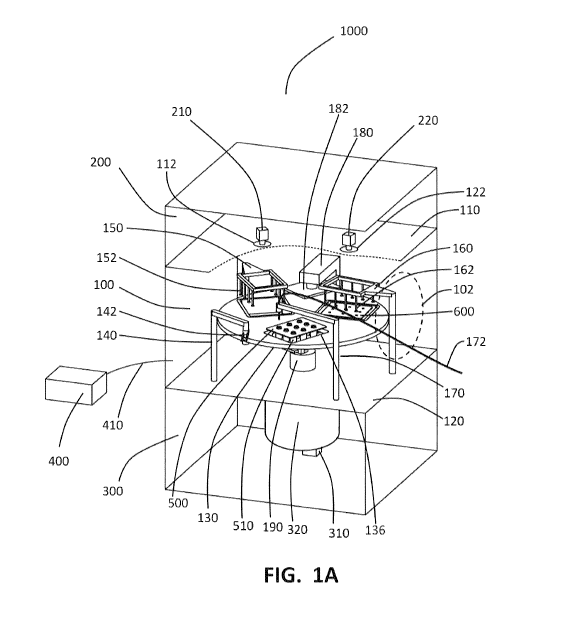
[0045] FIG. 1A is a drawing of an apparatus for filling pharmaceutical
containers with a
pharmaceutical fluid product. For the sake of clarity some surfaces are shown
in cutaway form
and others are shown as transparent.
[0046] FIG. 1B is a plan view of one chamber of the apparatus of FIG. 1A.
[0047] FIG. 1C shows a rotary stage of the apparatus of FIG. 1A and FIG. 1B.
[0048] FIG. 1D shows a side view of a portion of the apparatus of FIG. 1A and
FIG. 1B.
16
CA 03077526 2020-03-31
WO 2019/071339 PCT/CA2018/051214
[0049] FIG. 1E shows a pharmaceutical container tub cover seated in the rotary
stage of FIG.
1A to FIG. 1D being removed.
[0050] FIG. 1F shows pharmaceutical containers being filled with a
pharmaceutical fluid
substance in the apparatus of FIG. 1A to FIG. 1E.
[0051] FIG. 1G provides a more detailed view of the cover removal components
of the
apparatus of FIG. 1A, FIG. 1B and FIG. 1E.
[0052] FIG. 2A and FIG. 2B jointly form a drawing of a flow chart for a method
of aseptically
filling pharmaceutical containers with a pharmaceutical fluid substance in a
spatially
constrained environment.
[0053] FIG. 3A is a drawing of subsystems of another embodiment of an
apparatus for filling
pharmaceutical containers with a pharmaceutical fluid product.
[0054] FIG. 3B shows a portion of FIG. 3A in more detail.
[0055] FIG. 4A is a drawing of subsystems of a further embodiment of an
apparatus for filling
pharmaceutical containers with a pharmaceutical fluid product.
[0056] FIG. 4B shows a portion of FIG. 4A in more detail.
[0057] FIG. 5A is a drawing of subsystems of yet a further embodiment of an
apparatus for
filling pharmaceutical containers with a pharmaceutical fluid product.
[0058] FIG. 5B shows a portion of FIG. 5A in more detail.
17
CA 03077526 2020-03-31
WO 2019/071339 PCT/CA2018/051214
[0059] FIG. 6 shows a flow chart of a further method for filling nested
pharmaceutical
containers with a pharmaceutical fluid substance.
[0060] FIG. 7A is a drawing of subsystems of another embodiment of an
apparatus for filling
pharmaceutical containers with a pharmaceutical fluid product based on the
system of FIG.
5A and FIG. 5B.
[0061] FIG. 7B is a drawing of a droplet monitoring system.
[0062] FIG. 8 is a drawing of subsystems of another embodiment of an apparatus
for filling
pharmaceutical containers with a pharmaceutical fluid product.
[0063] FIG. 9 is a drawing of subsystems of a further embodiment of an
apparatus for filling
pharmaceutical containers with a pharmaceutical fluid product.
[0064] FIG. 10 is a drawing of subsystems of yet another embodiment of an
apparatus for
filling pharmaceutical containers with a pharmaceutical fluid product.
[0065] FIG. 11 is a drawing of a flow chart for a method for aseptically
dispensing a
pharmaceutical fluid into a container.
[0066] Corresponding reference characters indicate corresponding parts
throughout the several
views. Although the drawings represent embodiments of the present invention,
the drawings
are not necessarily to scale and certain features may be exaggerated in order
to better illustrate
and explain the present invention. The flow charts are also representative in
nature, and actual
embodiments of the invention may include further features or steps not shown
in the drawings.
The exemplifications set out herein illustrate embodiments of the invention,
in one or more
18
CA 03077526 2020-03-31
WO 2019/071339 PCT/CA2018/051214
forms, and such exemplifications are not to be construed as limiting the scope
of the invention
in any manner.
DETAILED DESCRIPTION
[0067] The embodiments disclosed below are illustrative and not intended to be
exhaustive or
limit the invention to the precise form disclosed in the following detailed
description. Rather,
the embodiments are chosen and described so that others skilled in the art may
utilize their
teachings.
[0068] The present invention relates to an apparatus and method for filing
pharmaceutical
containers with a pharmaceutical fluid substance in a spatially constrained
environment. In
FIG.1A, filling system 1000 comprises sealable chamber 100 in communication
with an
ambient environment, sealable chamber 100 being capable of having an aseptic
environment
established within its interior and capable of maintaining that aseptic
environment within its
interior. The interior of sealable chamber 100 may be rendered aseptic by any
one or more of
treatments, including but not limited to treatment with a sterilant, such as
steam, hydrogen
peroxide vapor, ozone, nitrogen dioxide, and ethylene oxide. The structures
and mechanisms
to perform such sterilization steps are well known in the art and are not
shown in FIG. 1A.
[0069] Chambers 200 and 300 are separated from chamber 100 by upper wall 110
and lower
wall 120 respectively and are not required to be capable of maintaining
aseptic environments
within their interiors. The communication of chamber 100 with the ambient
environment may
be via suitable aseptically sealable access door 102, schematically shown in
broken outline in
FIG. 1A. Suitable sealable doors and ports are well known in the art and will
not be dwelt
upon further in this specification. The ambient environment may be, for
example, a clean room
adapted for the handling of pharmaceuticals during production. Since space is
at a premium in
such spatially constrained clean environments, there is much merit in reducing
the so-called
"footprint" of equipment to be housed in the clean environment.
19
CA 03077526 2020-03-31
WO 2019/071339 PCT/CA2018/051214
[0070] The terms "aseptic" and "sterilize" and their derivatives are to be
understood as follows
for the purposes of the present specification. Establishing an aseptic
condition in the interior
of a chamber shall be understood to mean establishing that condition
throughout the internal
atmosphere of the chamber as well as on substantially all exposed interior
surfaces of the
chamber. This shall include the surfaces of all items, containers, subsystems
and the like
exposed to the interior atmosphere of the chamber. To the extent that
extremely tight crevices
or microscopic crevices may exist in the interior of the chamber such that a
sterilizing gas or
vapor may not perfectly penetrate into such tight regions, for example, the
degree of
sterilization in practical cases may not be total. This is acknowledged in
both the industry and
in the standards set for the industry. The action of establishing an aseptic
condition within the
interior of the chamber and "sterilizing the interior of the chamber" shall
have the same
meaning in this specification.
[0071] Introducing into the interior of a chamber with an aseptic condition an
item of which
the surfaces are not suitably sterilized destroys the existing aseptic
condition within the
chamber. Conversely, introducing an aseptic or sterilized item into an
interior of a chamber
that does not have an aseptic condition within that interior does not render
that interior aseptic.
In fact, all it does is to destroy the aseptic condition of the surface of the
item so introduced.
Similarly, introducing filtered air, even with all biological entities
filtered out, into an
unsterilized chamber does not in any way sterilize the chamber or render it
aseptic to a degree
acceptable in the pharmaceutical industry. The reason is that the interior
surfaces of the
chamber are not sterilized by the introduction of such air. All that is
achieved is to contaminate
the filtered air with active biological species resident on the interior
surfaces of the unsterilized
chamber.
[0072] In the interest of clarity and completeness, it should also be recorded
that in the art the
term "aseptic" is also sometimes used in association with the introduction of
pharmaceutical
fluids along aseptic tubes into bodies within controlled chambers. In such
cases the term in the
art refers to the condition inside the tube or to the fact that the
pharmaceutical fluid may be
CA 03077526 2020-03-31
WO 2019/071339 PCT/CA2018/051214
filtered to a suitable degree. This in no way sterilizes or renders aseptic
the interior of the
chamber in question. The aseptic condition in such cases is confined to the
interior of the tube
bearing the pharmaceutical stream. Such streams are often filtered to a high
degree, but such
filtering affects only the interior of the particular tube and does not in any
way sterilize the
interior of the chamber.
[0073] In some prior art systems, containers introduced into a chamber for the
purposes of
being filled with a pharmaceutical are routed through sterilizing subsystems.
This kills
biological species on the containers. When such sterilized containers are
introduced into the
chamber when the chamber itself is not aseptic the containers lose their
aseptic condition as
biological species contained within the chamber will deposit on the previously
aseptic
containers.
[0074] It should also be pointed out that pharmaceutical or semiconductor
clean rooms of any
quality level, including "Class 100", "Class 10" or "Class 1", even when
employing laminar
flow hoods and the like or any quality of HEPA (High Efficiency Particulate
Air) filters or
ULPA (Ultra Low Particulate Air) filters, cannot constitute an aseptic chamber
because they
do not have an assurable means to render the surfaces of the room sterile or
aseptic. Standards
for clean rooms exist from both the United States Federal Government and ISO
(International
Standards Organization). These specify in great detail to different standards
the allowed
particulate content of a cubic volume of air in such a clean room facility.
None of these
standards address the matter of biological species present on surfaces in the
room. This serves
to make the point that a chamber cannot be rendered aseptic by the management
of its
atmosphere or airflow only. Nor, conversely, can the chamber be rendered
aseptic by the
sterilization of only the surfaces of its interior.
[0075] The text "Guideline for Disinfection and Sterilization in healthcare
Facilities, 2008" by
Rutala et al from the Center for Disease Control lists a compendium of
mechanisms and
methods for sterilization. Our concern in this specification is specifically
with those
21
CA 03077526 2020-03-31
WO 2019/071339 PCT/CA2018/051214
mechanisms for sterilizing the interior of a chamber; that is, sterilizing
both the interior
surfaces and the atmosphere within the chamber. Given the requirements, vapor
base methods
are most appropriate to the task. These include, but are not limited to,
treatment with heated
water vapor, hydrogen peroxide vapor, ozone, nitrogen dioxide, ethylene oxide,
glutaraldehyde
vapor or other suitable sterilizing gases and vapors. In one suitable method
appropriate to the
present invention, the sterilization is by means of hydrogen peroxide vapor
which is then
flushed using ozone before the chamber is employed in the filling of
pharmaceutical
containers.
[0076] The subsystems of apparatus 1000 contained with sealable chamber 100
will now be
described at the hand of FIG. 1A to FIG. 1G. Due to the compactness and
density of
components and subsystems of apparatus 1000, certain components and subsystems
are
omitted from the drawings of FIG. 1B to FIG. 1G in the interest of clarity and
the focus is
placed on components and subsystems most relevant to the supporting text in
this specification.
Planar rotary stage 130 is fully rotatable through 3600 (degrees) in a
horizontal plane parallel
to lower wall 120 about rotary stage rotation axis 131 and may be raised and
lowered by means
of bellows feed-through 190. The use of bellows feed-through 190 allows
chamber 100 to
retain its aseptic condition during the motion of rotary stage 130. A suitable
engine and gearing
system 320 may be housed within chamber 300. Engines, for example stepper
motors, as well
as gearing systems suitable for rotating rotary stage 130 with suitable
angular precision and
repeatability are well known in the art and are not further discussed in this
specification.
[0077] As shown in FIG. 1C, at least three fiducial locating openings 132,
134, and 136 are
provided in rotary stage 130. Fiducial locating opening 132 is employed for
receiving container
tubs 530 holding sterilized pharmaceutical containers 510 pre-packed in a
predetermined
pattern in container nests 500. Container tubs 530 are typically substantially
rectangular and
are sealed with peelable covers 520. Suppliers of pharmaceutical containers
provide their
product in this format to users of the apparatus of the present specification.
Fiducial locating
opening 134 is employed for receiving container closure tubs 630 holding
sterilized
22
CA 03077526 2020-03-31
WO 2019/071339 PCT/CA2018/051214
pharmaceutical containers closures 610 pre-packed in a predetermined pattern
in container
closure nests 600. Container closure tubs 630 are typically substantially
rectangular and are
sealed with peelable tub covers not shown in FIG. lA to FIG. 1G. The peelable
covers of tubs
630 are functionally identical to peelable covers 520. Suppliers of
pharmaceutical containers
provide their product in this format to users of the apparatus of the present
specification. In the
interest of the compactness of system 1000, the rectangular axes of locating
openings 132, 134,
and 136 may be oriented at an angle with respect to the radial direction of
rotary stage 130 in
order to ensure a suitably small radius for rotary stage 130.
[0078] Suitable container nests 500 and container closure nests 600; container
tubs 530 and
container closure tubs 630; and peelable tub covers 520 are described in
United States Patent
Publication No. 2016/0200461, published July 14, 2016, the disclosures of
which is hereby
incorporated in full. Alternative cover gripping arrangements for the removal
of tub covers
from tubs are also described in United States Patent Publication No.
2016/0251206, published
September 1, 2016, the disclosures of which is hereby incorporated in full.
The removal of tub
covers may be controlled and monitored by the subsystem and method described
in PCT
International Publication No. WO 2018/049516 Al, published March 22, 2018, the
disclosures
of which is hereby incorporated in full.
[0079] In the interest of clarity, FIG. lA to FIG. 1G show, and the associated
text to follow
below will describe, the use of single tub 530 of pharmaceutical containers
510 along with
single tub 630 of container closures 610. In practice, container closures 610
are provided as
multiple nests 600 per container closure tub 630. To this end rotary stage 130
may contain
more than one fiducial locating opening 132 to each receive container tub 530
holding
sterilized pharmaceutical containers 510 pre-packed in one container nest 500.
In yet other
implementations, more than one nest 500 of containers 510 may be present in a
single
pharmaceutical container tub 530.
23
CA 03077526 2020-03-31
WO 2019/071339 PCT/CA2018/051214
[0080] Fiducial locating opening 136 is specifically arranged to receive
container nests 500
bearing pharmaceutical containers 510. Whereas tubs 530 and 630 naturally
locate in fiducial
locating openings 132 and 134 and are suspended by their own rims once in
opening 132 and
134, containers 510 are correctly located in opening 136 and retained in
position by some other
mechanism. To this end, fiducial locating opening 136 comprises four fiducial
retaining guides
137. Baseplate 138 is located within fiducial locating opening 136 as a loose
component of
system 1000, and rests on the horizontal portions at the bottoms of each of
the four fiducial
retaining guides 137 (see FIG. 1C and FIG. 1D). This arrangement allows
baseplate 138 to
move freely, guided by fiducial retaining guides 137. We shall return to this
arrangement when
discussing the closing of containers with container closures.
[0081] FIG.1E shows fiducial locating opening 136 as empty, while cover 520 is
being peeled
from container tub 530 in fiducial locating opening 132 (not visible) to
expose nest 500 bearing
pharmaceutical containers 510. At this point in the operation of system 1000,
a cover similar
to cover 520 has already been pealed from tub 630 in fiducial locating opening
134 (not visible)
to expose nest 600 bearing container closures 610. FIG. 1G shows a close-up
detailed view
of the peeling of cover 520. Cover removal station 140 is rotatable about
cover removal station
rotation axis 144 parallel to rotary stage rotation axis 131 and comprises
engagement tool 142,
which, in this particular embodiment, is fork-shaped in order to engage with
cover removal
fixture 540 attached to cover 520. Cover removal fixture 540 is pre-attached
to cover 520
before tub 530 is transferred into system 1000 via door 102 (See FIG.1A). In
the embodiment
shown in FIG. 1E and FIG. 1G, cover removal fixture 540 is clipped to cover
520 and has a
ball-shaped appendage to allow it to be engaged by engagement tool 142. Other
combinations
of cover removal fixtures and engagement tools are contemplated and system
1000 is not
limited to the particular combination of cover removal fixture and engagement
tool shown in
FIG. 1A, FIG. 1E and FIG. 1G. Cover removal fixture 540, for example, may be
manufactured as an integral part of cover 520 for use in filling systems such
as filling system
1000. Or it may be clipped to cover 520 during the placement into tub 530 of
nests 500 bearing
24
CA 03077526 2020-03-31
WO 2019/071339 PCT/CA2018/051214
containers 530 and during the placement into tub 630 of nests 600 bearing
container closures
610.
[0082] Rotary stage 130 may be lowered to assist in obtaining a less acute
angle between cover
520 and tub 530. Too acute an angle may lead to the tearing of cover 520.
Cover removal
station 140 may be rotated while rotary stage 130 rotates so that the combined
motions of cover
removal station 140 and rotary stage 130 provide a low stress path for the
removal of cover
520, thereby limiting the chances of tearing of cover 520. In particular,
cover removal station
140 may be rotated to ensure that engagement tool 142 is not present above
fiducial locating
opening 132 when container tub 530 is placed in or removed from fiducial
locating opening
132.
[0083] In some embodiments, system 1000 comprises single cover removal station
140 for
sequentially removing covers from tubs 520 and 620. In other embodiments,
system 1000 may
be equipped with two or more cover removal stations 140 for dedicated removal
of covers from
tubs 520 and 620 and other additional tubs. In some embodiments covers are
simultaneously
removed from tubs 520 and 620 and from other tubs, all the removal processes
benefiting from
a single rotary motion of rotary stage 130.
[0084] In FIG. 1A, FIG. 1B, and FIG. 1F filling station 170 for filling
pharmaceutical
containers 510 with pharmaceutical fluid product comprises pharmaceutical
fluid product feed
line 172 supplying pharmaceutical fluid product to pharmaceutical fluid
product dispenser
head 174 (See FIG. 1F). Filling station 170 is rotatable about filling station
rotation axis 176
parallel to rotary stage rotation axis 131. Filling station 170 and rotary
stage 130 may
simultaneously or sequentially rotate to place dispenser head 174 over an
opening of any
selected container 510 in nest 500 when nest 500 is seated in fiducial
locating opening 136.
This allows every container 510 in nest 500 to be filled with pharmaceutical
fluid product by
product dispenser head 174. When not engaged in filling containers 510,
filling station 170
may be rotated to swing dispenser head 174 completely away from fiducial
locating opening
CA 03077526 2020-03-31
WO 2019/071339 PCT/CA2018/051214
136, thereby allowing nests 600 bearing container closures 610 to be placed on
top of nest 500
with closure 610 directly on top of an opening of every container 510 residing
in fiducial
locating opening 136.
[0085] Another term employed to describe dispenser head 174 is "filling
needle". Suitable
filling needles and protective sheathing arrangements for such filling needles
are described in
co-pending United States Patent Publications Nos. 2016/0346777 and
2017/0248626,
published December 1, 2016, and August 31, 2017, respectively, the disclosures
of which are
hereby incorporated in full.
[0086] FIG. 1A and FIG. 1B show two vacuum pickup systems 150 and 160, each
respectively comprising a plurality of suction cups 152 and 162 (See FIG. 1B).
Vacuum pickup
system 150 is arranged to pick up nests 500 of containers 510 by means of
suction cups 152,
and vacuum pickup system 160 is arranged to pick up nests 600 of containers
610 by means of
suction cups 162. Vacuum pickup system 160 may be raised and lowered in order
to allow
suction cups 162 to engage with different nests 600 of container closures 610
contained at
differing depths inside tub 630. To this end, vacuum pickup system 160 may
comprise a
bellows feed-through allowing vertical motion whilst maintaining the aseptic
integrity of
chamber 100. Suitable vacuum pumps, or vacuum lines from a vacuum source
external to
system 1000, may be connected to vacuum pickup systems 150 and 160, and ensure
suitable
vacuum at suction cups 152 and 162.
[0087] Cameras 210 and 220 are disposed to view and record the positioning of
suction cups
152 and 162 on nests 500 and 600 respectively. In the embodiment shown in FIG.
1A, cameras
210 and 220 are disposed within chamber 200 and view nests 500 and 600 through
sealed
windows 112 and 122 respectively. In other embodiments, cameras 210 and 220
may be
disposed within chamber 100 and view nests directly from within chamber 100.
26
CA 03077526 2020-03-31
WO 2019/071339 PCT/CA2018/051214
[0088] Container closing ram system 180, shown in FIG. 1A, FIG. 1B, and FIG.
1D,
comprises upper ram plate 182 disposed within chamber 100 above rotary stage
130, lower
ram plate 184 disposed within chamber 100 below rotary stage 130, and ram
drive 310 within
chamber 300. Ram drive 310 is disposed for driving lower ram plate 184
vertically toward
upper ram plate 182 via bellows feed-through 186. Loose base plate 138 of
fiducial locating
opening 136, when located above lower ram plate 184 by suitably rotating
rotary stage 130, is
pushed upward by ram plate 184 and is guided in the process by fiducial
retaining guides 137
(See FIG. 1D). When closures 610 in closure nest 600 are ultimately pushed
against upper ram
plate 182, they are forced into the openings of containers 510 in nest 500.
This creates a
sandwiched nest of closed containers 510, each closed by a corresponding
closure 610. As
shown in FIG. 1D, nests 500 and 600 are forced together in the process to
create a compound
nest 500/600.
[0089] Controller 400, shown in FIG. 1A and FIG. 1B, may communicate with the
rest of
system 1000 via control communications line 410, or may be contained
physically within
system 1000, for example, within chamber 200. Controller 400 may have suitable
memory and
a processor containing suitable software programming instructions which, when
loaded in the
memory executed by the processor, control the motions of ram system 180,
vertical motion
and rotating action of rotary stage 130, the application of vacuum to vacuum
pickup systems
150 and 160, the imaging by cameras 210 and 220, the vertical motion of vacuum
pickup
system 160, any rotational or vertical motions required from cover removal
stations 140 and
filling station 170, as well as the on-and-off valving of pharmaceutical fluid
product supply to
dispenser head 174. Suitable valves and pumps, typically peristaltic pumps,
required for
pharmaceutical fluid product supply to dispenser head 174 are well known in
the art and may
be housed in chamber 200 or may be located outside system 1000. The various
mechanical
drives for the subsystems described above are well-known in the art, will not
be discussed here
in detail. These may typically be housed in chamber 200 of system 1000. The
software, when
executed by the processor, instructs the rotary stage to rotate to angular
positions that are either
predetermined or based on image information from the cameras and controls the
cover removal
27
CA 03077526 2020-03-31
WO 2019/071339 PCT/CA2018/051214
stations, the filling station, the vacuum pickup systems, and the ram system
to operate
specifically in conjunction with the rotary stage.
[0090] A method based on system 1000 for filling nested pharmaceutical
containers with a
pharmaceutical fluid product will now be described at the hand of the flow
chart given in FIG.
2A, and which is continued in FIG. 2B. The method comprises providing [2010]
filling
apparatus 1000 comprising sterilizable chamber 100 capable of maintaining an
aseptic
condition, the chamber comprising rotary stage 130 with destination fiducial
locating opening
136 and at least two source fiducial locating openings (132 and 134); filling
station 170; at
least one cover removal station 140; vertically oriented container ramming
system 180; and at
least one vacuum pickup system (for example 150 and/or 160). The method
further comprises
transferring [2020] into at least a first of the at least two source fiducial
locating openings (132
and 134) at least one container tub 530 sealed by container tub cover 520 and
containing
container nest 500 bearing a plurality of pharmaceutical containers 510; and
transferring
[2025] into a second of the at least two source fiducial locating openings
(134 and 132)
container closure tub 630 sealed by a closure tub cover and containing at
least one container
closure nest 600 bearing a plurality of pharmaceutical container closures 610.
[0091] The method further comprises aseptically sealing [2030] chamber 100 and
establishing
[2035] an aseptic condition within chamber 100. Establishing [2035] an aseptic
condition
within chamber 100 may comprise treating the interior of chamber 100 with any
one or more
of steam, hydrogen peroxide vapor, ozone, nitrogen dioxide, and ethylene
oxide.
[0092] The method further comprises operating [2040] the at least one cover
removal station
140 and rotating rotary stage 130 to remove container tub cover 520 from the
at least one
container tub 530 and remove the closure tub cover from closure tub 630;
operating [2050]
rotary stage 130 and one of the at least one vacuum pickup systems (for
example 150 and/or
160) to transfer to destination fiducial locating opening 136 container nest
500 bearing the
plurality of pharmaceutical containers 510; and dispensing [2060] on an
iterative and serial
28
CA 03077526 2020-03-31
WO 2019/071339 PCT/CA2018/051214
basis a pharmaceutical fluid substance into at least a portion of the
plurality of pharmaceutical
containers 510 by operating rotary stage 130 and filling station 170. The
phrase "iterative and
serial" is employed in this specification to describe the fact that the same
operational steps are
repeatedly used to fill the various containers and the fact that the
containers are filled one after
another, as opposed to simultaneously. In some embodiments multiple containers
may be
simultaneously filled using a filling station with multiple dispenser heads.
[0093] Steps [2040], [2050], and [2060] each involves rotating rotary stage
130 and operating
another device, being respectively cover removal station 140, one of the at
least one vacuum
pickup systems (for example 150 and/or 160), and filling station 170. The
motions involved
may be simultaneous in some cases or embodiments, and serial in other cases or
embodiments.
In some embodiments some of the motions may be simultaneous and others may be
serial.
[0094] Operating [2040] the at least one cover removal station 140 may
comprise engaging an
engagement tool (for example tool 142) with a cover removal fixture (for
example fixture 540)
pre-attached to the cover being removed. Operating [2050] one of the at least
one vacuum
pickup systems may comprise contacting container nest 500 with a plurality of
suction cups
152 while applying a vacuum to suction cups 152. Dispensing [2060] a
pharmaceutical fluid
substance into at least a portion of the plurality of pharmaceutical
containers may comprise
disposing on an iterative and serial basis fluid product dispenser head 174 of
filling station 170
over the openings of the at least a portion of the plurality of pharmaceutical
containers 510.
Operating [2050] rotary stage 130 and one of the at least one vacuum pickup
systems may
comprise operating camera 210 to obtain image information of container nest
500 bearing the
plurality of pharmaceutical containers 510 and to position the one of the at
least one vacuum
pickup systems over container nest 500.
[0095] The method further comprises operating [2070] one of the at least one
vacuum pickup
systems (for example 150 and/or 160) and rotary stage 130 to transfer to
destination fiducial
locating opening 136 one of the at least one container closure nests 600
bearing the plurality
29
CA 03077526 2020-03-31
WO 2019/071339 PCT/CA2018/051214
of pharmaceutical container closures 610 and positioning the at least one
closure nest 600 to
align closures 610 with containers 510; operating [2080] rotary stage 130 to
jointly position
aligned container nest 500 and closure nest 600 in ramming system 180; and
operating [2090]
ramming system 180 to force the plurality of container closures 610 into the
plurality of
containers 510.
[0096] Operating [2070] one of the at least one vacuum pickup systems may
comprise
contacting container closure nest 600 with a plurality of suction cups 162
while applying a
vacuum to suction cups 162. Operating [2090] ramming system 180 may comprise
driving the
plurality of pharmaceutical containers 510 toward upper ram plate 182 of
ramming system
180.
[0097] The operating [2070] rotary stage 130 and one of the at least one
vacuum pickup
systems may comprise operating camera 220 to obtain image information of the
one of the at
least one container closure nests 600 bearing the plurality of pharmaceutical
container closures
610 and to position the one of the at least one vacuum pickup systems over the
one of the at
least one container closure nests 600.
[0098] Providing [2010] a filling apparatus may comprise providing a filling
apparatus further
comprising controller 400 and a software program executable by controller 400.
Any one or
more of the aseptically sealing [2030] chamber 100; establishing [2035] an
aseptic condition
within chamber 100; operating rotary stage 130; operating the at least one
cover removal
station 140; operating [2070] one of the at least one vacuum pickup systems
(150 and/or 160);
operating filling station 170; and operating [2090] ramming system 180 may be
done
automatically by executing the software program in controller 400.
[0099] In the embodiment described at the hand of FIGS. 1A to 1F, each of
steps [2040],
[2050], [2060], [2070], and [2080] comprises rotating a rotary stage, for
example rotary stage
130, bearing the container nests and container closure nests.
CA 03077526 2020-03-31
WO 2019/071339 PCT/CA2018/051214
[00100] In other embodiments, a plurality of the steps of removing a container
tub cover from
at least one container tub 530; removing a container tub cover from at least
one container
closure tub 630; transferring to destination fiducial locating opening 136
container nest 500;
dispensing a pharmaceutical fluid substance into pharmaceutical containers
510; transferring
to destination fiducial locating opening 136 one of the at least one container
closure nests 600;
and positioning aligned container nest 500 and closure nest 600 in ramming
system 180
comprises rotating a rotary stage bearing the container nests and container
closure nests.
[00101] In a general embodiment, at least one of the steps of removing a
container tub cover
from at least one container tub 530; removing a container tub cover from at
least one container
closure tub 630; transferring to destination fiducial locating opening 136
container nest 500;
dispensing a pharmaceutical fluid substance into pharmaceutical containers
510; transferring
to destination fiducial locating opening 136 one of the at least one container
closure nests 600;
and positioning aligned container nest 500 and closure nest 600 in ramming
system 180
comprises rotating a rotary stage bearing the container nests and container
closure nests.
[00102] It is to be noted that neither filling system 1000, nor the associated
method, needs to
employ the vibratory bowls or escapements that are typical of the prior art.
Unlike many prior
art systems, filling system 1000 also does not require the use of gloves for
use by an operator
to access the interior of the chamber.
[00103] The system above has been described as employing a controller that
runs stored
software running on a general-purpose computer platform, but it could also be
implemented in
whole or in part using special-purpose hardware.
[00104] The system described above also employs fiducial openings defined in
the rotary stage
to hold tubs and nests, but it could also employ other types of fiducial
structures that include
other configurations of constraining surfaces sufficient to hold tubs and
nests in place. Notched
posts mounted on the rotary stage may hold tubs and/or nests above the rotary
stage, for
31
CA 03077526 2020-03-31
WO 2019/071339 PCT/CA2018/051214
example. Further fiducial locating structures for holding tubs of nests for
containers or
container closures are described below at the hand of FIGS.3A, 3B, 4A, and 5A.
[00105] Another embodiment of a filling system according to the invention may
be in all
respects identical to the embodiments described above at the hand of Figures
1A and 1B, with
the exception of vacuum pickup system(s) 150 or 160. FIGS. 3A and 3B show a
portion of a
filling system as described above. FIG. 3B, in particular, focuses on the
general area of one of
the vacuum pickup systems, by way of example, vacuum pickup system 150. In
this alternative
embodiment, vacuum pickup system 150 is replaced by reconfigurable vacuum
pickup system
150'. Vacuum pickup system 160 of FIGS. 1A and 1B may similarly be replaced by
reconfigurable vacuum pickup system 160' of the same arrangement as vacuum
pickup system
150'. In the interest of clarity, vacuum pickup system 160' is not shown in
FIG. 3A or 3B. In
other embodiments, single reconfigurable vacuum pickup system 150' may be
employed to
pick up both container nests and container closure nests. Vacuum pickup system
150' may
access the container nests and container closure nests by rotation of rotary
stage 130.
[00106] Vacuum pickup system 150' comprises two rotary arms 154a' and 154b',
in their turn
respectively comprising pluralities of suction cups 152a' and 152b'. Vacuum
pickup system
150' is arranged to pick up nests 500 of containers 510 by means of suction
cups 152a' and
152b'. Vacuum pickup system 150' may also be arranged to pick up nests 600 of
container
closures 610 by means of suction cups 152a' and 152b'. As with vacuum pickup
system 150,
vacuum pickup system 150' may be raised and lowered in order to allow suction
cups 152a'
and 152b' to engage with different nests 600 of container closures 610
contained at differing
depths inside tub 630.
[00107] Suction cups 152a' and 152b' are arranged on rotary arms 154a' and
154b' as
pluralities of sets of linearly arranged suction cups 152a' and 152b', each
set of linearly
arranged suction cups 152a' and 152b' being arranged at a different angle
perpendicular to the
longitudinal axes of rotary arms 154a' and 154b'. This arrangement allows
rotary arms 154a'
32
CA 03077526 2020-03-31
WO 2019/071339 PCT/CA2018/051214
and 154b' to be rotated about their longitudinal axes in order to orient
different sets of linearly
arranged suction cups 152a' and 152b' to engage with different nests 500 of
containers 510.
This allows the sets of suction cups 152a' and 152b' to be individually
selectable for use.
Rotation of rotary arms 154a' and 154b' may be performed manually. In other
embodiments,
rotation of rotary arms 154a' and 154b' may be by means of a suitable
motorized drive
incorporated in vacuum pickup system 150' and controlled by controller 400
shown in FIG.
1A.
[00108] By selecting different sets of linearly arranged suction cups 152a'
and 152b' via the
rotation of rotary arms 154a' and 154b', the sets of suction cups 152a' and
152b' may be
disposed to engage with different container nests 500 bearing containers 510,
or container
closure nests 600 bearing container closures 610.
[00109] FIGS. 3A and 3B show vacuum pickup system 150' as comprising two
rotary arms,
being rotary arms 154a' and 154b'. In other embodiments, one or more arms may
be employed,
all embodiments sharing the concept of a selectable configuration of suction
cups. Whereas
the selection of suction cup configurations in FIG. 3A and FIG. 3B is by means
of rotation of
arms 154a' and 154b' bearing suction cups 152a' and 152b', the selecting in
other
embodiments may be on a different basis of configuration, including, for
example without
limitation, lateral translation of suction-cup-bearing arms in a plane
parallel to the rotation
plane of rotary stage 130 in order to engage different sets of suction cups
with container nests
or container closure nests. In FIGS. 3A and 3B suction cups are arranged in
linear sets. In
other embodiments non-linear arrangements of suction cups may be employed.
[00110] Turning now to FIG. 3B specifically, we consider members 149 and 139
in more
detail. In one embodiment, reconfigurable stopping member 149 is shown as
having two
different ends of which a first end may be selected for use by suitable
rotation of reconfigurable
stopping member 149 about stopping member rotation axis 141 to a predetermined
set position.
In the set position, reconfigurable stopping member 149 provides a hard stop
for a proximal
33
CA 03077526 2020-03-31
WO 2019/071339 PCT/CA2018/051214
end of container 530 against the selected end of reconfigurable stopping
member 149 along a
direction parallel to the longitudinal axes of rotary arms 154a' and 154b'. In
this embodiment,
reconfigurable stopping member 149 may be rotated through 180 (degrees) to
dispose the
second end of reconfigurable stopping member 149 to stop container 530. The
second end of
reconfigurable stopping member 149 may be configured to stop the proximal end
of container
530 at a different point than where the first end of reconfigurable stopping
member 149 stops
the proximal end of container 530.
[00111] Restraining member 139 is configured to push against a distal end of
container 530.
While different mechanisms are contemplated to ensure the pushing action of
restraining
member 139, one particular suitable mechanism involves providing restraining
member 139
with suitable spring loading to rotate about axis 143. By the above operation,
reconfigurable
stopping member 149 and restraining member 139 together allow container 530 to
be
positioned at an exact location parallel to the longitudinal axes of rotary
arms 154a' and 154b'.
The particular exact location is selectable by selecting the appropriate end
of reconfigurable
stopping member 149 to stop container 530. This arrangement allows containers
530 of
different dimensions parallel to the longitudinal axes of rotary arms 154a'
and 154b' to be
located at exact predetermined locations with respect to sets of suction cups
152a' and 152b'.
[00112] A particular set of suction cups 152a' and 152b' may be selected to
match the selection
of the particular end of reconfigurable stopping member 149. In this way,
vacuum pickup
system 150' may be set to a configuration that ensures that a selected size of
container 530 is
precisely positioned to allow container nests 500 within container 530 to be
engaged by
specific sets of suction cups 152a' and 152b'. Vacuum pickup system 150' is
thereby
reconfigurable to engage with nests of different sizes within containers of
different sizes.
[00113] In the interest of clarity, the description above, as well as FIGS. 3A
and 3B, show an
arrangement that allows for the exact positioning of containers 530 along only
one dimension
in the rotation plane of rotary stage 130, the dimension of the containers
perpendicular to the
34
CA 03077526 2020-03-31
WO 2019/071339 PCT/CA2018/051214
one dimension being assumed to be identical. In such an arrangement, fiducial
locating
openings 132 and 134 are sized to constrain containers 530 in the
perpendicular dimension in
the rotation plane of rotary stage 130.
[00114] In another embodiment, a further reconfigurable stopping member and
restraining
member may be added to the arrangement of FIG. 3A and FIG. 3B in order to
address the
positioning of container 530 in the perpendicular direction within the
rotation plane of rotary
stage 130. To allow the positioning of container 530 in this perpendicular
direction, fiducial
locating openings 132 and 134 are not sized to constrain containers in any
direction within the
rotation plane of rotary stage 130.
[00115] In the embodiments described above, reconfigurable stopping member 149
has been
described as having two ends of which one is selected for use at any one time
by rotating
reconfigurable stopping member 149 about stopping member rotation axis 141. In
other
embodiments, reconfigurable stopping member 149 may be shaped or configured to
have more
than two stopping ends, the ends being selectable by suitable rotation of
reconfigurable
stopping member 149 about stopping member rotation axis 141. In one
embodiment, in which
the reconfigurable stopping member has a very large number of stopping ends,
the
reconfigurable stopping member may assume the shape of a cam, representing a
large plurality
of possible stopping ends that may be selected via rotation of the
reconfigurable stopping
member about a suitable stopping member rotation axis.
[00116] In general, the system described at the hand of FIGS. 3A and 3B
comprises a
reconfigurable fiducial nest positioning system. The reconfigurable fiducial
nest positioning
system comprises a movable platform comprising fiducial locating opening 132,
reconfigurable stopping member 149, and restraining member 139. In the case of
the system
of FIGS. 3A and 3B, the movable platform is rotary stage 130. As explained
later, other
movable platforms are also contemplated. To the extent that, for example, tub
530 positionally
CA 03077526 2020-03-31
WO 2019/071339 PCT/CA2018/051214
constrains and locates nest 500 inside tub 530, any system that fiducially
locates tub 530
inherently also fiducially locates nest 500.
[00117] The various embodiments contemplated all comprise a reconfigurable
vacuum pickup
system that may be configured to engage its suction cups with corresponding
areas on a
pharmaceutical container nest. The containers in the container nest may be
closed by
corresponding container closures suspended in a container closure nest. The
planar surface of
the container closure nest may have an outline that leaves pass-throughs on
its perimeter for
the suction cups to pass through to engage with the container nest. By way of
example, in FIG.
3a pass-throughs 602 are shown on the perimeter of closure nest 600.
Alternatively or
additionally, the container closure nest may have suitable openings in its
planar interior to
serve as pass-throughs for the suction cups to pass through to engage with the
container nest.
The vacuum pickup systems contemplated are further configured and disposed to
pick up the
combination of nested containers and their closures by the container nest, as
opposed to by the
closure nest.
[00118] In a general embodiment, a nest handling subsystem comprises a
reconfigurable
vacuum pickup system for picking up container nests and/or container closure
nests may
comprise one or more arms bearing a plurality of sets of suction cups. By
reconfiguration of
the vacuum pickup system a set of suction cups may be selected from among the
plurality of
sets of suction cups, the selected set of suction cups being pre-arranged to
engage with a
particular container nest or container closure nest. The selection may be on
the basis of one or
both of the size and the shape of the nest. The nest handling system may
further comprise at
least one pair of a reconfigurable stopping member 149 and a restraining
member 139 disposed
proximate opposing ends of a fiducial locating opening 132 for holding a tub
530 containing
container nests 500 bearing containers 510 in order to engage with opposing
ends of tub 530.
The stopping and restraining members are disposed to position tub 530 in a
predetermined
position that ensures that the selected set of suction cups may engage with
the container nests
and/or container closure nests.
36
CA 03077526 2020-03-31
WO 2019/071339 PCT/CA2018/051214
[00119] As is the case with opening 132, opening 134 of FIG. 3A may also be
served by at
least one set of a reconfigurable stopping member, being member 145 in this
case, and a
restraining member, being member 135 in this case. Reconfigurable stopping
member 145 and
restraining member 135 function with respect any tub in opening 134 in the
same way as
reconfigurable stopping member 149 and restraining member 139 function with
respect any
tub in opening 132.
[00120] The various embodiments above have been described in terms of FIGS. 1A
to 1E and
FIG. 3A, and FIG. 3B in which the vacuum pickup system 150, 160 is described
as part of a
pharmaceutical filling system 1000. However, vacuum pickup system 150', 160'
may also be
employed in its own right other apparatus not limited to the filling system of
FIGS. 1A to 1E,
or, in fact, to filling systems in general. Some other example applications
include, without
limitation, lyophilizing systems. It may be applied to suitable nests of any
objects arranged in
a predetermined pattern. Furthermore, while system 1000 of FIGS. 1A to FIG. 1E
employs
rotary stage 130, reconfigurable vacuum pickup system 150' may employ any
suitable movable
platform comprising suitable fiducial locating openings.
[00121] The method described above at the hand of FIGS. 2A and 2B may now also
be
described in more detail with reference to FIG. 3A and FIG. 3B. Providing at
least one vacuum
pickup system as part of the providing a filling apparatus step [2010] may
comprise providing
at least one reconfigurable vacuum pickup system 150', the at least one
reconfigurable vacuum
pickup system 150' comprising a plurality of sets of suction cups 152a' and
152b'.
[00122] Providing a filling apparatus step [2010] may comprise providing
rotary stage 130
with destination fiducial locating opening 136 and at least two source
fiducial locating
openings 132, 134, each source fiducial opening having at least one pair of
reconfigurable
stopping member 149 and restraining member 139.
37
CA 03077526 2020-03-31
WO 2019/071339 PCT/CA2018/051214
[00123] Transferring step [2020] may comprise operating at least a first
reconfigurable
stopping member 149 to stop container tub 530 at a predetermined container tub
position and
operating at least first restraining member 139 to restrain container tub 530
at the
predetermined container tub position.
[00124] Transferring step [2025] may comprise operating at least a second
reconfigurable
stopping member 145 to stop container closure tub 630 at a predetermined
closure tub position
and operating at least second restraining member 135 to restrain container tub
630 at the
predetermined closure tub position.
[00125] Operating [2050] the at least one vacuum pickup system 150', 160' may
comprise
configuring the at least one reconfigurable vacuum pickup system 150', 160' to
select a first
predetermined set of suction cups disposed to engage with container nest 500.
[00126] Operating [2070] of one of the at least one vacuum pickup system 150',
160' may
comprise configuring the at least one reconfigurable vacuum pickup system
150', 160' to select
a second predetermined set of suction cups disposed for engaging with
container closure nest
600.
[00127] The method may further comprise operating [2095] the at least one
vacuum pickup
system 150', 160' with the first predetermined set of suction cups selected to
engage with
container nest 500 and jointly remove container nest 500 and container closure
nest 600 from
ramming system 180.
[00128] We have considered in FIG. 3A and FIG. 3B alternative embodiments of
the
arrangements of vacuum pickup systems 150 and 160 of FIG. 1A in the form of
vacuum pickup
systems 150' and 160'; and the positioning arrangements associated with source
openings 132
and 134 in the form of elements 135, 145, 139, and 149. We now turn our
attention to
38
CA 03077526 2020-03-31
WO 2019/071339 PCT/CA2018/051214
alternative embodiments for the arrangements around destination opening 136 of
FIG. 1A and
FIG. 3A. FIG. 4A and its close up view in FIG. 4B show the system of FIG. 3A
with a
different embodiment of the arrangement around destination opening 136. While
cameras 210
and 220 of FIG. 1A may be employed in conjunction with controller 400 and
rotation of rotary
stage 130 to position nest 500 at opening 136, and to position nest 600 over
nest 500 at opening
136, the adjustable destination fiducial positioning system of FIG. 4A and
FIG. 4B comprising
rotary positioning elements 164a and 164b may be alternatively or additionally
employed to
accurately position nests 600 and 500.
[00129] Typical industrial container nests are not manufactured to a
dimensional standard,
and, as a result, any system for filling and closing nested containers 510
should have a
mechanism to accurately position differently sized nests 500 bearing
containers 510. To this
end, rotary positioning elements 164a and 164b may have different sets of
paired positioning
surfaces 167a, 167b and 163a, 163b allowing nests 500 of specific dimensions
to be accurately
fitted between such paired positioning surfaces. In Fig. 4B, nest 500 fits
such that its two
opposing ends in a first dimension touch mutually facing surfaces 167a and
167b of rotary
positioning elements 164a and 164b respectively. By mutually counter-rotating
elements 164a
and 164b about respectively axes 166a and 166b, surfaces 167a and 167b may be
made to face
each other and may thereby allow the precise positioning between them of a
nest of different
length in the first dimension.
[00130] As is evident from FIG. 4B, when surfaces 167a and 167b face each
other, the nest
positioned snugly between them may be retained in a precise and predetermined
vertical
position by resting on surfaces 165a and 165b of rotary positioning elements
164a and 164b
respectively. When surfaces 163a and 163b face each other, the alternative
nest positioned
snugly between them may retained in a precise and predetermined vertical
position by resting
on surfaces 161a and 161b of rotary positioning elements 164a and 164b
respectively.
Elements 164a and 164b may be rotated manually about axes 166a and 166b
respectively. In
some embodiments, the rotation of elements 164a and 164b may be done
automatically, for
39
CA 03077526 2020-03-31
WO 2019/071339 PCT/CA2018/051214
example, by motorized drives controlled by controller 400 and suitable control
software. That
control may be based on predetermined dimensional data relating to the nest
being positioned
between the surfaces of elements 164a and 164b. It may also be based,
independently or in
combination, on input data derived from imaging data obtained from cameras 210
and/or 220.
Further, the rotation may take place as nest 500 is lowered into position so
that the particular
surfaces of elements 164a and 164b destined to engage with the opposing ends
of nest 500
along the first dimension may serve as closing horizontal grip on nest 500 as
the surfaces rotate
toward the position in which they face each other. In this embodiment, the
horizontal
positioning and vertical positioning of a nest between elements 164a and 164b
are not mutually
independent.
[00131] Another arrangement as shown in FIG. 4A and FIG. 4B for the first
dimension of
nest 500, may also be established for the second planar dimension of nest 500
perpendicular
to the first dimension. This allows any nest 500 placed at opening 136 to be
accurately located
in a location predetermined by the choice of setting of rotary positioning
elements 164a and
164b.
[00132] Another embodiment of rotary positioning elements is shown in FIG. 5A
and FIG.
5B. In contrast with the embodiment of FIG. 4A and FIG. 4B described
immediately above,
the horizontal positioning and vertical positioning of a nest between two
mutually counter-
rotatable elements 164a' and 164b' in FIG. 5A and FIG. 5B are mutually
independent
positioning actions. This is achieved by employing, in each of the two
mutually perpendicular
planar dimensions addressed in the embodiment immediately above, a pair of
fixed opposing
planar tabs 165a' and 165b' to position nest 500 in the vertical dimension,
and a pair of rotary
positioning elements 164a' and 164b' to position nest 500 in the first
horizontal dimension. In
this embodiment, each of elements 164a' and 164b' comprises two rotatable
elements ganged
on axles 166a' and 166b' respectively to rotate in unison and mutual alignment
either side of
planar tabs 165a' and 165b' within bosses 169a' and 169b' respectively. The
sets of rotary
elements 164a' and 164b', beyond each being divided in to two ganged elements,
serve to
CA 03077526 2020-03-31
WO 2019/071339 PCT/CA2018/051214
confine nest 500 in the horizontal dimension in the same fashion as rotary
elements 164a and
164b in the embodiment of FIG. 4A and FIG. 4B described immediately above.
[00133] While elements 164a' and 164b' may be designed to be of more complex
shape, we
show in FIG. 5A and FIG. 5B a very simple implementation in which surfaces
167a' of rotary
elements 164a' and surfaces 167b' of rotary elements 164b' serve to position
nest 500 in the
first horizontal dimension. By rotating elements 164a' joined by axle 166a'
counter-clockwise
within boss 169a' and rotating elements 164b' joined by axle 166b' clockwise
within boss
169b', surfaces 163a' and 163b' may be made to face each other and thereby a
nest of different
length in the first horizontal dimension may be positioned and accurately
located between
elements 164a' and 164b'.
[00134] Ganged elements 164a' and 164b' may be rotated manually about the axes
of axles
166a' and 166b' respectively inside bosses 169a' and 169b' respectively. In
some
embodiments, the rotation of elements 164a' and 164b' may be done
automatically by
motorized drives controlled by controller 400 and suitable control software.
That control may
be based on predetermined dimensional data relating to the nest being
positioned between the
surfaces of elements 164a' and 164b'. It may also be based, independently or
in combination,
on input data derived from imaging data obtained from cameras 210 and/or 220.
Further, the
rotation may take place as nest 500 is lowered into position so that the
particular surfaces of
elements 164a' and 164b' destined to engage with the opposing ends of nest 500
along the first
dimension may serve as closing horizontal grip on nest 500 as the surfaces
rotate toward the
position in which they face each other.
[00135] FIG. 5A and FIG. 5B show a further set of paired mutually counter-
rotatable rotary
positioning elements, not numbered for the sake of clarity, ganged similarly
to rotary elements
164a' and 164b', and disposed to accurately locate nest 500 independently in
the vertical
dimension and in a second planar dimension of nest 500 perpendicular to the
first dimension.
41
CA 03077526 2020-03-31
WO 2019/071339 PCT/CA2018/051214
[00136] In a further aspect, described at the hand of FIG. 6, a method is
provided for filling
nested pharmaceutical containers 510 with a pharmaceutical fluid substance,
the method
comprising: providing [6010] filling system 1000 comprising sterilizable
chamber 100 capable
of maintaining an aseptic condition, chamber 100 comprising filling station
170 and planar
rotary stage 130 having destination locating structure 136, 164a, 164b, 164a',
164b';
transferring [6020] into chamber 100 at least one container tub 530 sealed by
container tub
cover 520 and containing container nest 500 bearing a plurality of
pharmaceutical containers
510; aseptically sealing [6040] chamber 100; establishing [6050] an aseptic
condition within
chamber 100; transferring [6060] into destination locating structure 136,
164a, 164b, 164a',
164b' container nest 500 bearing the plurality of pharmaceutical containers
510 such that
container nest 500 is held in place; and dispensing [6070] the pharmaceutical
fluid substance
into at least a portion of the plurality of pharmaceutical containers 510 by
operating both rotary
stage 130 and filling station 170. Operating filling station 170 may include
rotating filling
station 170. Dispensing the pharmaceutical fluid substance may comprise
dispensing the
pharmaceutical fluid substance on an iterative and serial basis into
containers 510.
[00137] Providing [6010] filling system 1000 may comprise providing a filing
apparatus
comprising at least one cover removal station 140 within chamber 100 and
wherein transferring
into the destination locating structure container tub 530 comprises removing
container tub
cover 520 from container tub 530 by operating both rotary stage 130 and the at
least one cover
removal station 140. Operating the at least one cover removal station 140 may
comprise
rotating the at least one cover removal station 140. Providing [6010] filling
system 1000 may
comprise providing within chamber 100 at least one cover removal station 140
having
engagement tool 142, transferring [6020] into chamber 100 at least one
container tub 530 may
comprise attaching to container tub 520 cover removal fixture 540; and wherein
operating the
at least one cover removal station 140 comprises engaging engagement tool 142
with cover
removal fixture 540.
42
CA 03077526 2020-03-31
WO 2019/071339 PCT/CA2018/051214
[00138] The method may further comprise transferring [6030] into chamber 100
container
closure tub 630 sealed by a container closure tub cover and containing at
least one container
closure nest 600 bearing a plurality of pharmaceutical container closures 610.
The method may
further comprise positioning [6080] one of the at least one closure nests 600
to align closures
610 in the at least one closure nest 600 with corresponding containers 530 in
container nest
500; transferring [6090] nests 500, 600 of aligned closures 610 and containers
510 to a
ramming station by rotating rotary stage 130; and forcing [6100] closures 610
into
corresponding containers 510. The method may further include adjusting tub
locating structure
135, 145 to accommodate a size of closure nest tub 630. Positioning [6080] one
of the at least
one closure nest 600 may comprise: obtaining image information about the one
of the at least
one closure nests 600; and positioning the one of the at least one closure
nests 600 based on
the image information. Positioning [6080] one of the at least one closure nest
600 may
comprise: applying a vacuum to suction cups 162, 152a, 152b, 152a', 152b';
lifting container
closure nest 600 with the suction cups; and operating rotary stage 130.
[00139] Transferring [6020] into the destination locating opening container
nest 500 may
comprise: applying a vacuum to the suction cups; lifting container nest 500
with the suction
cups; and operating rotary stage 130. The method may further include selecting
one of a
plurality of sets of suction cups and wherein the applying a vacuum to suction
cups is
performed for the selected set of suction cups. The selecting may include
rotating one of the
plurality of sets of suction cups into position. The method may further
include adjusting
destination locating structure 136, 164a, 164b, 164a', 164b' to accommodate a
size of
container nest 500. The adjusting may be performed in two at least generally
orthogonal
directions. The method may further include adjusting tub locating structure
139, 149 to
accommodate a size of container nest tub 530.
[00140] In a further aspect, a method is provided (see FIG. 1G) for removing
within a
controlled environment enclosure a container cover from a sealed container,
for example tub
530 or tub 630, the sealed container being sealed by the container cover, for
example cover
43
CA 03077526 2020-03-31
WO 2019/071339 PCT/CA2018/051214
520, the method comprising: providing the container in controlled environment
enclosure 100
with cover 520 sealed to a sealing surface of a lip of the container to seal
the contents of the
container against decontamination, cover 520 having cover removal fixture 540,
decontaminating the sealed container in controlled environment enclosure 100,
engaging cover
removal fixture 540 with engagement tool 142, and removing the cover from the
container
using engagement tool 142. Engaging may involve engaging cover removal fixture
540 with
fork-shaped engagement tool 142. Engaging may involve engaging a ball-shaped
appendage
on cover removal fixture 540.
[00141] Providing may include providing sterilized pharmaceutical containers
510 or closures
610 in the sealed container, for example tub 530 or 630, before the
decontaminating. Attaching
may take place before the container is in controlled environment enclosure
100.
Decontaminating the sealed container in controlled environment enclosure 100
may take place
before removing cover 520. Removing cover 520 may include moving engagement
tool 142
relative to container 530. Removing cover 520 may include moving both
container 530 and
engagement tool 142. The method may further comprise attaching cover removal
fixture 540
to cover 520 before providing container 530 in the controlled environment
enclosure.
[00142] FIG. 7A shows a drawing of subsystems of a further embodiment of an
apparatus for
filling pharmaceutical containers with a pharmaceutical fluid product, based
on the subsystems
shown in FIG. 1A, FIG. 1C, FIG. 1F, FIG. 5A and FIG. 5B. For the sake of
clarity, several
subsystems have been omitted in order to show only aseptic sealable chamber
100 of FIG. 1A;
rotary stage 130 of FIG. 1A and FIG. 1C; openings 132, 134, and 136 of FIG.
1C; with
container nest 500 bearing pharmaceutical containers 510, nest 500 held in
position by the
arrangement shown in FIG. 5B. In FIG. 7A, fill arm 170 of FIG. 1A is replaced
by articulated
robotic fill arm 170'. Any alternative fiducial arrangement for holding nest
500 may be
employed as long as it allows the opening of each container 510 to be known
with suitable
accuracy and precision for reliably dispensing droplets of pharmaceutical
fluid into containers
510.
44
CA 03077526 2020-03-31
WO 2019/071339 PCT/CA2018/051214
[00143] To the aforementioned elements in FIG. 7A is added a droplet
monitoring subsystem
250, shown separately in FIG. 7B, comprising illuminating imager system 252,
mirror 254,
and retroreflector 256. Droplet monitoring subsystem 250 may be controlled by
controller 400,
to which end controller 400 is in communication with droplet monitoring
subsystem 250.
Controller 400 may comprise a memory and a processor. As in the case of fill
arm 170 of FIG.
1A and FIG. 1F, articulated robotic fill arm 170' is supplied with
pharmaceutical fluid via a
pharmaceutical fluid product feed line 172. In FIG. 7A, fill arm 170' is
equipped with a
pharmaceutical fluid product dispenser head 174'. Dispenser head 174' is
arranged and
configured to produce droplets of pharmaceutical fluid of consistent volume
and within a
limited range of droplet shapes to travel down along droplet path 710. To this
end, dispenser
head 174' may be equipped with a suitable nozzle. Controller 400 may control
the dispensing
action of dispenser head 174', to which end controller 400 may be in
communication with
dispenser head 174' or a pump supplying dispenser head 174' with
pharmaceutical fluid.
Imager system 252 may comprise a telecentric lens, thereby to render imager
system 252
capable of making consistent size measurements of droplets produced by
dispenser head 174'.
[00144] Illuminating imager system 252 is arranged and disposed to illuminate
retroreflector
256 and to obtain high speed images of droplets 700 dispensed by dispenser
head 174' to travel
along droplet path 710 into any container 510. The line a-a' in FIG. 7A and
FIG. 7B indicates
the light beam path. Since rotary stage 130 moves every container 510 along a
circular path
around the rotation axis of rotary stage 130, articulated robotic fill arm
170' is operated to
move dispenser head 174' along a linear trajectory following the imaging path
a-a' of droplet
monitoring subsystem 250. In this implementation, therefore, both rotary stage
130 and
articulated robotic fill arm 170' are operated to position any container 510
for filling by
dispenser head 174'. Any operating of fill arm 170' may, in addition to the
operating of rotary
stage 130, be controlled via controller 400. To this end, controller 400 is in
communication
with both fill arm 170' and rotary stage 130, allowing controller 400 to
coordinate the motion
of fill arm 170' and rotary stage 130.
CA 03077526 2020-03-31
WO 2019/071339 PCT/CA2018/051214
[00145] Software may be supplied for loading into the memory of controller 400
and
configured, when executed by the processor, for controlling dispensing of the
pharmaceutical
fluid droplets 700 by fluid dispensing head 174', and for collection of images
of pharmaceutical
fluid droplets 700 along droplet path 710. The software may also allow
controller 400' to
control robotic fill arm 170' and rotary stage 130.
[00146] An alternative embodiment, shown in FIG. 8, shows another articulated
robotic fill
arm 170" into which alternative droplet monitoring subsystem 250' has been
integrated. This
particular embodiment employs two mirrors 254' and 258' along with
illuminating imager
system 252' and retroreflector 256'. We retain the same numbering, namely
174', for dispenser
head and 172 for pharmaceutical fluid product feed line. Illuminating imager
system 252' is
arranged and disposed to illuminate retroreflector 256' and to obtain via
mirrors 254' and 258'
high speed images of droplets 700 dispensed by dispenser head 174' to travel
along droplet
path 710 into any container 510. In this particular implementation, only
articulated robotic fill
arm 170" needs to be operated in order to position any container 510 held in
nest 500 for filling
by dispenser head 174' and rotary stage 130 may be held stationary during the
positioning of
filling of all containers 510 held in nest 500. In a more general case, both
rotary stage 130 and
articulated robotic fill arm 170" may be operated to position any container
510 for filling by
dispenser head 174'. Any operating of fill arm 170" may, in addition to the
operating of rotary
stage 130, be controlled via controller 400. To this end, controller 400 is in
communication
with both fill arm 170" and rotary stage 130. Imager system 252' may comprise
a telecentric
lens, thereby to render imager system 252' capable of making consistent size
measurements of
droplets produced by dispenser head 174'.
[00147] The use of droplet monitoring subsystems of the present invention is
not limited to
the rotary stage pharmaceutical filling systems of FIG. 1A to FIG. 8. They may
also be
employed in any system in which any fluid is dropwise dispensed into
containers, whether
nested or not. One group of filling systems suitable for filling
pharmaceutical containers with
a pharmaceutical fluid in an aseptic chamber using the droplet monitoring
system of the present
46
CA 03077526 2020-03-31
WO 2019/071339 PCT/CA2018/051214
invention employs robotic arms to hold containers by means of a suitable end
effector. The
robotic arms may be articulated robotic arms and may be hermetically sealed to
chamber 100.
Suitable examples of such systems are provided in United States Patent
Publication No.
2017/121046 Al, United States Patent Publication No. 2016/0200461A1, United
States Patent
Publication No. 2016/0184986 Al, United States Patent Publication No.
2016/0346777 Al,
and United States Patent Publication No. 2014/0196411 Al, the disclosures of
which are all
wholly incorporated herein by reference. We describe below embodiments of the
droplet
monitoring subsystem of the present invention used in conjunction with an
articulated arm of
the type described in more detail in these four listed publications.
[00148] FIG. 9 shows droplet monitoring system 250 of FIG. 7A and FIG. 7B
implemented
in a pharmaceutical container filling system having aseptic sealable chamber
100' in which
container nest 500 bearing pharmaceutical containers 510 is held by end
effector 810 of
articulated arm 800. Articulated arm 800 may be a robotic articulated arm. In
some
embodiments, articulated robotic arm 800 may be controlled by suitable
controller 400'. To
this end, as shown in FIG. 9, controller 400' is in communication with robotic
arm 800.
Robotic arm 800 may be of the type described in detail in the publications
listed above and
incorporated by reference. Controller 400' may be, for example without
limitation, controller
440 used by the filling system described at the hand of FIG. 1 of United
States Patent
Publication No. 2016/0346777 Al or controller 13 of FIG. 1 of United States
Patent
Publication No. 2017/121046 Al. Articulated arm 800 may be, for example
without limitation,
articulated arm 200 of FIG. 2 of United States Patent Publication No.
2016/0184986 Al,
articulated arm 22 of FIG. 1 of United States Patent Publication No.
2016/0200461 Al, or
articulated arm 30 of FIG. 2 of United States Patent Publication No.
2017/121046 Al.
Controller 400' may also be used to control droplet monitoring system 250, to
which end it is
in communication with droplet monitoring system 250.
[00149] FIG. 10 shows the droplet monitoring system 250' of FIG. 8 employed in
the same
pharmaceutical container filling system as described at the hand of FIG. 9.
Controller 400'
47
CA 03077526 2020-03-31
WO 2019/071339 PCT/CA2018/051214
may also be used to control droplet monitoring system 250', to which end it is
in
communication with droplet monitoring system 250'.
[00150] In further embodiments of the system, both dispensing head 174' and
container(s) 510
may be moved by robotic arms, being robotic arms 170', 170" on the one hand
and 800 on the
other. Either or both of the robotic arms may be articulated robotic arms of
the types described
in the incorporated United States Patent Publications listed above. In yet
further embodiments,
both dispensing head 174' and container 510 may be in fixed positions, these
particular
embodiments pertaining, for example, to the filling of single container 510 at
a time.
[00151] The embodiments shown in Figures 7A, 7B, 8, 9 and 10 all employ a
retroreflector
256, 256' illuminated by a light source housed in the illuminating digital
imager system 252,
252'. In other embodiments, droplets 700 may be backlit, or illuminated from
any other angle.
In such embodiments, the imager systems do not require an integrated
illuminator and the
illuminator may be disposed elsewhere separate from the imager.
[00152] We now turn to a method, described at the hand of the flowchart in
FIG. 11, for
aseptically dispensing a pharmaceutical fluid into pharmaceutical container
510, the method
comprising: providing [3010] sterilizable chamber 100, 100' capable of
maintaining an aseptic
condition, the chamber comprising pharmaceutical fluid dispensing head 174'
configured for
producing droplets 700 of the pharmaceutical fluid and droplet monitoring
system 250, 250'
comprising digital imager 252, 252'; establishing [3020] within sterilizable
chamber 100,100'
an aseptic condition; providing [3030] within sterilizable chamber 100, 100'
aseptic
pharmaceutical container 510; dispensing [3040] a plurality of droplets 500 of
the fluid from
dispensing head 174' along droplet path 710 into container 510; obtaining
[3050] from imager
252,252' a plurality of images of at least one of the plurality of droplets
700 along droplet path
710; and determining [3060] from the plurality of images a volume of fluid
dispensed into
container 510.
48
CA 03077526 2020-03-31
WO 2019/071339 PCT/CA2018/051214
[00153] The method may, in some embodiments, further comprise ceasing [3070]
dispensing
of the fluid based on the volume of fluid dispensed into container 510. In
other embodiments,
ceasing may be based on the length of time of dispensing of the pharmaceutical
fluid into
container 510 or on weighing of the amount of pharmaceutical fluid dispensed
into container
510. The droplet information from the imager may therefore be used either in
merely
monitoring the pharmaceutical fluid dispensing process, or as a way of
controlling the fluid
dispensing process, as in when it forms the basis of the ceasing [3070].
[00154] Determining [3060] from the plurality of images a volume of fluid
dispensed into
container 510 may comprise determining a volume of at least one of the
plurality of droplets
700. Determining the volume of the at least one of the plurality of droplets
700 may comprise:
identifying first and second total portions of the at least one droplet 700
appearing respectively
to the left and to the right of droplet path 710 in at least one image of the
at least one droplet
700; calculating first and second volumes of the at least one of the plurality
of droplets 700 by
separately mathematically rotating respectively the first and second total
portions of droplet
700 through 27E about droplet path 710; and equating the volume of the at
least one of the
plurality of droplets 700 to the average of the first and second volumes. The
term "total
portion" is used in this specification to describe all of the side-on planar
view of the droplet to
either the left or the right side of droplet path 710. The two total portions
of the droplet will
not in general be quite equal. The two planar total portions, or approximate
"halves", are then
taken and separately rotated in software about droplet path 710 to obtain two
"droplet
volumes", which are then averaged to obtain the assumed volume of the droplet.
[00155] Obtaining [3050] from imager 252, 252' a plurality of images of at
least one of the
plurality of droplets 700 along the droplet path may comprise obtaining the
plurality of images
over a predetermined portion of the droplet path over which droplets 700 have
a stable shape.
In this specification, the shape of droplets may be considered "stable" when
the droplets have
distinctly detached from the dispensing head 174' and have assumed a shape
confined to a
49
CA 03077526 2020-03-31
WO 2019/071339 PCT/CA2018/051214
predetermined perimeter as viewed by the imager, the shape being allowed to
vary within that
predetermined perimeter.
[00156] Determining [3060] from the plurality of images a volume of fluid
dispensed into
container 510 may comprise determining a volume of each droplet 700 dispensed
into
container 510. Ceasing dispensing of the fluid based on the volume of fluid
dispensed into
container 510 may comprise ceasing dispensing of the fluid when a total amount
of fluid
dispensed into container 510 equals a predetermined volume. The predetermined
volume may
be, for example without limitation, a single adult human dosage volume of the
pharmaceutical
fluid. Other predetermined volumes may be integer multiples of dosages or
volumes specified
by a health authority, regulatory body, or MSDS sheet of the pharmaceutical
fluid.
[00157] In other embodiments, determining [3060] from the plurality of images
a volume of
fluid dispensed into container 510 may comprise determining a representative
volume of
droplet 700, counting the total number of droplets dispensed into container
510, and then
multiplying the representative droplet volume with the number of droplets.
Determining a
representative volume of droplet 700 may comprise measuring only a first
droplet and
assuming it to be representative. In other embodiments, determining a
representative volume
of droplet 700 may comprise measuring a plurality of droplets and calculating
an average
droplet volume across the plurality of droplets.
[00158] Obtaining [3050] from imager 252, 252' a plurality of images of at
least one of the
plurality of droplets 700 along droplet path 710 may comprise obtaining the
plurality of images
employing light reflected to the imager by retroreflector 256,256'. Obtaining
from imager 252,
252' a plurality of images of at least one of the plurality of droplets 700
along droplet path 710
may comprise obtaining the plurality of images by using a telecentric lens.
The telecentric lens
may be incorporated within imager 252, 252'. Providing within sterilizable
chamber 100, 100'
aseptic pharmaceutical container 510 may comprise providing aseptic
pharmaceutical
container 510 within container nest 500.
CA 03077526 2020-03-31
WO 2019/071339 PCT/CA2018/051214
[00159] The method may further comprise moving at least one of dispensing head
174' and
container 510 to position [3035] an opening of container 510 under dispensing
head 174' to
receive droplets 700 along droplet path 710. Moving the container may comprise
operating
robotic arm 800. Moving container 510 may comprise moving container nest 500
holding
container 510. Operating robotic arm 800 may comprise operating an articulated
robotic arm.
Moving dispensing head 174' may comprise operating robotic arm 170', 170".
Moving
dispensing head 174' may comprise operating articulated robotic arm 170',
170".
[00160] In the embodiments of FIG. 7A, 7B, 8, 9 and 10, controller 400, 400'
is also in
communication with dispensing head 174', or the pump supplying it with
pharmaceutical fluid,
allowing thereby controller 400, 400' to regulate and turn on or off the flow
of droplets via
dispensing head 174'. For the sake of clarity this communication line is not
shown in FIG. 7A,
7B, 8, 9 and 10.
[00161] While this invention has been described as having an exemplary design,
the present
invention may be further modified within the spirit and scope of this
disclosure. This
application is therefore intended to cover any variations, uses, or
adaptations of the invention
using its general principles. Further, this application is intended to cover
such departures from
the present disclosure as come within known or customary practice in the art
to which this
invention pertains.
51