Note: Descriptions are shown in the official language in which they were submitted.
PROCESS FOR DETERMINING A COMPOSITION OF A HYDROCARBON
SAMPLE OBTAINED FROM A SUBTERRANEAN RESERVOIR
TECHNICAL FIELD
[0001] The present invention relates to determining a composition of
hydrocarbon sample obtained from a subterranean reservoir.
BACKGROUND DISCUSSION
[0002] Hydrocarbon analysis procedures established by the American
Society of Testing and Materials (ASTM) are typically conducted by evolving
all
light hydrocarbons from the liquid phase of a hydrocarbon sample. Such
analysis
procedures are limited in that they provide inaccurate information for total
composition of hydrocarbons, particularly in C5 and lower components, and
provide limited if any information regarding phase behaviour of the sample.
[0003] Improvements to methods for analyzing hydrocarbon emulsions is
desired.
SUMMARY
[0004] The present disclosure provides a method for determining
compositional information of a hydrocarbon sample obtained from a
hydrocarbon-bearing reservoir that is based on the saturation pressure of the
sample at predetermined temperature and pressure conditions. The methods set
forth in the present disclosure facilitate determining the total hydrocarbon
composition of the hydrocarbon sample, the phase behavior of the sample, and
may provide an understanding of liquid and gas phase ratios and compositions
at
a given pressure and temperature condition.
[0005] In conventional analysis procedures, samples may not be
representative of the actual samples hydrocarbon samples because the samples
1
Date Recue/Date Received 2020-05-01
may include water, which may show up as a C30+ component of the sample,
skewing the composition information obtained by the analysis. Further,
conventional analysis procedures may not accurately determine amounts of
hydrocarbon components in solution vs in the gaseous phase because analysis
may not be performed an operating temperature or pressure of interest, which
may provide false analysis. For example, lighter components, e.g., <C5, may be
in gaseous phase during analysis but in liquid phase under the operating
temperature or pressure of interest, which will skew the compositional
information of the gas and liquid components.
[0006] In an aspect of the present disclosure, there is a method for
performing solvent composition analysis in a hydrocarbon sample for a
predetermined condition having a predetermined temperature and a
predetermined pressure, the method includes preparing the sample, determining
a saturation pressure of the sample at the predetermined temperature,
comparing the saturation pressure to the predetermined pressure to determine
the saturation of the sample at the predetermined condition, and performing
composition analysis on the sample based on the determined saturation of the
sample at the predetermined condition.
[0007] In another aspect of the present disclosure, the saturation at
the
predetermined condition is determined to be undersaturated if the saturation
pressure does not exceed the predetermined pressure by more than a
predetermined threshold.
[0008] In another aspect of the present disclosure, when the determined
saturation at the predetermined condition is undersaturated, performing
composition analysis on the sample includes performing a liquid composition
analysis on the sample while maintaining the sample at the predetermined
pressure and predetermined temperature to obtain compositional information of
the sample.
[0009] In another aspect of the present disclosure, when the determined
saturation at the predetermined condition is oversaturated, performing
2
Date Recue/Date Received 2020-05-01
composition analysis on the sample includes performing a first liquid
composition
analysis on the sample while maintaining the sample at a pressure that meets
or
exceeds the saturation pressure to obtain total compositional information for
the
sample, adjusting a temperature of the sample to the predetermined
temperature and the pressure of the sample to the predetermined pressure to
separate the sample into a gas component and a remaining liquid component,
performing a gas composition analysis on the gap component to obtain
compositional information of the gas component, and performing a second liquid
composition analysis on the remaining liquid component to obtain compositional
information on the remaining liquid component.
[0010] In another aspect of the present disclosure, preparing the sample
comprises cleaning the sample.
[0011] In another aspect of the present disclosure, the sample is an
emulsion comprising water and hydrocarbons, and wherein cleaning the sample
includes maintaining the sample in a sealed container while heating the sample
to a temperature to cause separation of the hydrocarbon and the water in the
sample, and removing the separated water from the sample, the temperature
not exceeding a density inversion temperature of the emulsion.
[0012] In another aspect of the present disclosure, cleaning the sample
includes adding a separation chemical to the sample.
[0013] In another aspect of the present disclosure, the separation
chemical
comprises an interfacial tension reducing agent, a miscible solvent, or both
an
interfacial tension reducing agent, a miscible solvent.
[0014] In another aspect of the present disclosure, the interfacial
tension
reducing agent comprises a dennulsifier, a surfactant, or both a dennulsifier
and a
surfactant.
[0015] In another aspect of the present disclosure, the miscible solvent
comprises an alcohol, a salt, or both an alcohol and a salt.
3
Date Recue/Date Received 2020-05-01
BRIEF DESCRIPTION OF THE DRAWINGS
[0016] Embodiments of the present application will now be described, by
way
of example only, with reference to the attached Figures, wherein:
[0017] FIG. 1 is a sectional view through a reservoir;
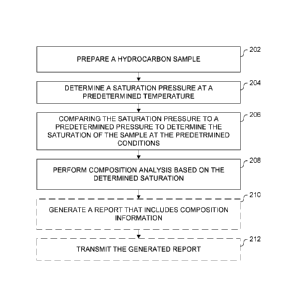
[0018] FIG. 2 is a flow chart of a method of determining solvent
compositional information for a hydrocarbon sample according to an embodiment
of the present disclosure;
[0019] FIG. 3 is a flow chart of a method of determining solvent
compositional information for an undersaturated sample according to an
embodiment of the present disclosure; and
[0020] FIG. 4 is a flow chart of a method of determining solvent
compositional information for an oversaturated sample according to an
embodiment of the present disclosure.
DETAILED DESCRIPTION
[0021] For simplicity and clarity of illustration, reference numerals
may be
repeated among the figures to indicate corresponding or analogous elements.
Numerous details are set forth to provide an understanding of the examples
described herein. The examples may be practiced without these details. In
other instances, well-known methods, procedures, and components are not
described in detail to avoid obscuring the examples described. The description
is
not to be considered as limited to the scope of the examples described herein.
[0022] The disclosure generally relates to a method for determining
compositional information of a hydrocarbon sample obtained from a
hydrocarbon-bearing reservoir that is based on the saturation pressure of the
sample at predetermined conditions. The method includes preparing the sample,
determining a saturation pressure of the sample at the predetermined
temperature, comparing the saturation pressure to the predetermined pressure
4
Date Recue/Date Received 2020-05-01
to determine the saturation of the sample at the predetermined condition, and
performing composition analysis on the sample based on the determined
saturation of the sample at the predetermined condition.
[0023] The hydrocarbons utilized to prepare a sample for analysis
according to the present disclosure are obtained from a production well
drilled
into a subterranean hydrocarbon-bearing formation. One example of a
hydrocarbon production well 100 is illustrated in FIG. 1. The hydrocarbon
production well 100 extends generally vertically from a surface 102 into the
hydrocarbon-bearing reservoir 106. Surface 102 refers to the environment
above the surface of the ground 103. The hydrocarbon production well may
extend near the base or bottom 104 of the hydrocarbon reservoir 106.
[0024] The production well 100 may form part of a steam assisted gravity
drainage (SAGD) production process. In SAGD, the production well 100 may
include a generally horizontal segment (not shown) and an injection well that
also includes a generally horizontal segment (not shown) is disposed generally
parallel to and spaced generally vertically above the horizontal segment of
the
hydrocarbon production well 100. During SAGD, steam is injected into the
injection well to mobilize the hydrocarbons and create a steam chamber in the
reservoir 106, around and above the generally horizontal segment of the
injection well. In a SAGD process, the production well 100 may produce an
emulsion that includes hydrocarbons and water.
[0025] In other examples, any suitable process for recovering
hydrocarbons from the reservoir via the production well 100 may be any
suitable
hydrocarbon recovery process. The process utilized may depend, at least in
part,
on the geological conditions within the reservoir 106.
[0026] Once the hydrocarbons are pumped to the surface 102 via the
production well, the hydrocarbons may be transported via, for example, a
pipeline (not shown). The pipeline may transport the hydrocarbons to another
facility such as, for example, a storage facility or a refinery.
Date Recue/Date Received 2020-05-01
[0027] In general, compositional information of the hydrocarbons
recovered
from the reservoir 106 is desired. The compositional information may include,
for example, the saturation of the sample under specified pressure and
temperature conditions, the mole or mass fraction of various hydrocarbon
constituents for the total sample, the mole or mass fraction for the various
hydrocarbon constituents of the gas component and the liquid component of the
hydrocarbon sample.
[0028] Determining the compositional information at a particular
predetermined condition, i.e., for a predetermined temperature and a
predetermined pressure may be desired. The predetermined conditions may be,
for example, the conditions within the production well 100, the conditions at
the
surface 102, the conditions within the reservoir 106. The conditions at the
surface may include, for example, conditions within piping, a pipeline, or
downstream of a vessel, or any condition within a hydrocarbon processing
facility.
[0029] The compositional information at a predetermined condition may be
utilized, for example, to verify simulations or predictions for solubility of
solvents
in bitumen or other properties such as viscosity or density dependence of a
particular hydrocarbon component. Further, compositional information
indicating
that the sample is oversaturated under reservoir conditions facilitates
predicting
downhole separation of liquid and gas phases, and provides information
regarding gas flow through liquid pumps. Compositional information at a
predetermined condition corresponding to the surface conditions may be
utilized,
for example, to determine surface equipment requirements based on the
saturation of the sample at the surface conditions, determine surface process
mass balance, or determine fluid properties of streams for surface facility
operations including, for example, heat capacities for exchanger monitoring or
optimization.
[0030] Previous analytic techniques are typically conducted by evolving
all
light hydrocarbons out of the liquid phase of a hydrocarbon sample. Such
6
Date Recue/Date Received 2020-05-01
techniques may provide inaccurate results, particularly for the light
hydrocarbon
component(s) of the sample, and do not provide phase behaviour information of
the sample.
[0031] Referring to FIG. 2, a method determining solvent compositional
information of a hydrocarbon sample is shown.
[0032] At 202 a hydrocarbon sample is prepared from hydrocarbons
recovered from a hydrocarbon reservoir, such as the reservoir 106 shown in
FIG.
1. The hydrocarbons may be recovered utilizing any suitable method for
recovering hydrocarbons from a hydrocarbon-bearing formation.
[0033] The sample may be prepared by placing the hydrocarbons in a
sealable container that facilitates adjusting the temperature and pressure
conditions within the container. The temperature of the sample may be
adjustable by heating the sample through, for example, electrical heating
bands
located on the outside of the container or placing the container in an oven.
The
pressure of the sample may be adjusted by, for example, adjusting a piston
located within a piston-container or injecting an inert gas or a non-soluble
liquid
into the container. The inert gas or non-soluble liquid may be selected such
that
the contribution of the inert gas or non-soluble liquid does not affect the
compositional information obtained through analysis for the hydrocarbon
components of the sample.
[0034] In some examples, preparing the hydrocarbon sample at 202 may
include cleaning the sample. Cleaning the sample may include removing water
from the sample in cases in which the recovered hydrocarbons are an emulsion
comprised of hydrocarbons and water. Emulsions may result, for example, when
water is utilized in the process for recovering the hydrocarbons such as, for
example, when a SAGD process is utilized.
[0035] Removing water from the emulsion sample includes heating the
sample to a temperature that does not exceed a water-hydrocarbon density
inversion temperature of the emulsion sample in order to cause the water and
7
Date Recue/Date Received 2020-05-01
hydrocarbons to separate, while the emulsion sample is sealed in a container.
In
addition to heating the sample, a separation chemical may optionally be added
to
the emulsion sample to facilitate the separation of the water from the
hydrocarbons.
[0036] In an example, the separation chemical may include an interfacial
tension reducing agent to improve separation of oleic and aqueous phases. Any
suitable interfacial tension reducing agent may be utilized including, for
example
a dennulsifier such as a flocculant, a solvent that is miscible in both water
and
hydrocarbon phases, or a sulphonate dennulsifier.
[0037] In another example, the separation chemical may include one or
both of an alcohol and a salt to increase the density differential between the
oleic
and aqueous phases to facilitate separation of the water from the
hydrocarbons.
Any suitable alcohol may be utilized including, for example, alcohols heavier
than
C4 alcohols such as butanol. In general, the soluability of an alcohol in oil
increases with increased length of the carbon chain of the alcohol. Any
suitable
salt may be utilized including, for example, sodium chloride and anhydrous
sodium sulphate.
[0038] At 204, a saturation pressure, which may also be referred to as
the
"bubble point" of the sample, is determined at the predetermined temperature.
The determining at 204 may include adjusting the temperature of the sample to
the predetermined temperature. The determining at 204 may be performed by
any suitable process for determining a saturation pressure of a sample that
includes a liquid and a gas.
[0039] For example, the determining at 204 may include raising a
pressure
of the sample within a container to a pressure at which all of the gas within
the
sample is forced into the liquid. This may be performed by reducing the volume
the container holding the sample to increase the pressure until an inflection
point
in the pressure readings, observed as a more rapid increase in the measured
pressure with decreased volume. The inflection point indicates that the gas
component has been forced into the liquid and which inflection point is the
8
Date Recue/Date Received 2020-05-01
saturation pressure or bubble point for the sample at the predetermined
temperature. Alternatively, if the gas component is dissolved in liquid
component at the outset of the determining at 204, then the volume of the
container may be increased until an inflection point in the pressure readings
is
observed, observed as a more rapid drop in pressure with increased volume.
[0040] At 206, the saturation pressure determined at 204 is compared to
the predetermined pressure to determine the saturation of the sample at the
predetermined conditions. The saturation of the sample may be determined to
be oversaturated at the predetermined conditions if the determined saturation
pressure exceeds the predetermined pressure and may be determined to be
undersaturated at the predetermined conditions if the determined saturation
pressure does not exceed, or is less than, the predetermined pressure. In an
example, the saturation may be determined to be undersaturated when the
determined saturation pressure does not exceed the predetermined pressure by
more than a threshold amount. The threshold amount may be, for example, a
percentage of the determined saturation pressure. In an example, the threshold
is 5% of the determined saturation pressure.
[0041] At 208, composition analysis is performed on the sample based on
the saturation determination at 206 to determine compositional information for
the sample. For example, the process for performing the composition analysis
at
208 will depend on whether the saturation is determined at 206 to be
oversaturated or undersaturated. Examples of processes based on the
determined saturation that may be utilized are described in more detail below
with reference to FIGS. 3 and 4.
[0042] The compositional information that is determined at 208 may
depend on the composition analysis that is performed, but generally may
include
any or all of a composition of complete sample, a composition of a gas
component of the sample, a composition of the liquid component of the sample.
The compositional information may include mole and mass percent of the
hydrocarbon components of the sample or relevant portion of the sample.
9
Date Recue/Date Received 2020-05-01
[0043] Optionally at 210, a report is generated that includes the
compositional information determined at 208. Additionally, the report
optionally
generated at 210 may include other information in addition to the
compositional
information including, for example, any of the determined saturation pressure,
the predetermined temperature and pressure of the predetermined conditions,
and a gas component to liquid component mass ratio.
[0044] The report that is optionally generated at 210 may be transmitted
at
212. The transmitting at 212 may include automatically transmitting the report
electronically in any suitable manner including, for example, utilizing a file
transfer protocol (FTP) to transmit the report to a server or other computer.
Additionally, or alternatively, the transmitting at 212 may include
automatically
transmitting the report via email or text message.
[0045] The compositional information generated at 208 may be utilized to
verify simulations or models of hydrocarbon characteristics, to determine
downhole separation, or to determine downhole liquid and gas separation, and
to
determine surface equipment and processes to utilize, as described previously.
[0046] Referring now to FIG. 3, an example process for performing the
composition analysis at 208 in the example method described above with
reference to FIG. 2 is shown. The example process for performing composition
analysis shown in FIG. 3 may be utilized when the saturation of the sample is
determined, such as at the determining 206 described above, to be
undersaturated.
[0047] At 302, the sample is determined to be undersaturated. As
described above, the sample may be determined to be undersaturated if the
determined saturation pressure does not exceed, or is less than, the
predetermined pressure. The determination at 302 may be made when, for
example, the determined saturation pressure does not exceed the predetermined
pressure by more than a threshold amount. The threshold amount may be, for
example, a percentage of the determined saturation pressure. In an example
the threshold is 5% of the determined saturation pressure.
Date Recue/Date Received 2020-05-01
[0048] At 304, liquid composition analysis is performed on the sample
while
the sample is maintained at the predetermined conditions, i.e., at the
predetermined temperature and the predetermined pressure. A determination at
302 that the sample is undersaturated at the predetermined conditions means
that the gas in the sample is dissolved in the liquid component. Thus, the
liquid
composition analysis performed at 304 will provide total composition analysis
for
the total sample.
[0049] The liquid composition analysis may be performed utilizing any
suitable process or technique for determining the composition of a liquid
hydrocarbon sample. The liquid composition analysis may be an analysis process
that provides the mole or mass fraction of each of hydrocarbon present in the
sample. For example, the liquid composition analysis may be a C36+
composition analysis in which the mole or mass fractions of hydrocarbons Cl to
C36 are determined as well as the mole or mass fraction of all of the
hydrocarbons heavier than C36. Techniques that may be utilized for the liquid
composition analysis may include, for example, gas chromatography or liquid
chromatography (LC) which may utilize infrared spectroscopy (IR), mass
spectroscopy (MS) or modulation ratio (MR).
[0050] Referring to FIG. 4, another example process for performing the
composition analysis at 208 in the example method described above with
reference to FIG. 2 is shown. The example process for performing composition
analysis shown in FIG. 4 may be utilized when the saturation of the sample is
determined, such as at the determining 206 described above, to be
oversaturated.
[0051] At 402, the sample is determined to be oversaturated. As described
above, the sample may be determined to be oversaturated if the determined
saturation pressure exceeds the predetermined pressure. The determination at
402 may be made when, for example, the determined saturation pressure
exceeds the predetermined pressure by more than a threshold amount. The
threshold amount may be, for example, a percentage of the determined
11
Date Recue/Date Received 2020-05-01
saturation pressure. In an example the threshold is 5% of the determined
saturation pressure.
[0052] Because the sample being analyzed in the example process set out
in FIG. 4 is oversaturated, the sample will include a gas component and a
liquid
component at the predetermined conditions. Thus, three separate analyses may
be performed to determine the total compositional information for the total
sample, as well as the compositional information of each of the gas component
and the liquid component of the sample that are present at the predetermined
conditions.
[0053] At 404, a first liquid composition analysis is performed while
maintaining the sample at a pressure that meets or exceeds a saturation
pressure to obtain the total compositional information. The saturation
pressure
that is met or exceeded will depend on the temperature of the sample while the
first liquid composition analysis is performed. Maintaining the sample at a
pressure that meets or exceeds the saturation facilitates most, if not all, of
the
gas component being dissolved in the sample. The first liquid composition
analysis may be performed utilizing any suitable process or technique, similar
to
the liquid composition processes and techniques described above with reference
to step 304 in FIG. 3.
[0054] At 406, the pressure, and the temperature if necessary, of the
sample is adjusted to the predetermined pressure and the predetermined
temperature, respectively. Adjusting the pressure and temperature of the
sample to the predetermined pressure and predetermined temperature will cause
an oversaturated sample to separate into a gas component and a remaining,
fully
saturated, liquid component.
[0055] At 408, a gas composition analysis is performed on the gas
component of the sample to obtain gas compositional information for the gas
component.
12
Date Recue/Date Received 2020-05-01
[0056] The gas composition analysis may be performed utilizing any
suitable process or technique for determining the composition of a gas
hydrocarbon sample. The gas composition analysis may be an analysis that
provides the mole or mass fraction of each of hydrocarbon present in a gas
sample. For example, the gas composition analysis may be a C11+ composition
analysis in which the mole or mass fractions of hydrocarbons Cl to C11 are
determined as well as the mole or mass fraction of all of the hydrocarbons
heavier than C11. Generally, lighter hydrocarbons will more easily evolve out
of
the sample into a gas phase, while heavier hydrocarbons will be more likely to
remain in the liquid phase in an oversaturated sample. Thus, separate mole or
mass fractions for heavier hydrocarbons are less important in the gas
composition analysis than in the liquid composition analysis described above.
Techniques that may be utilized for the gas composition analysis may include,
for
example, absorption spectroscopy or gas chromatography (GC) which may utilize
mass spectroscopy (MS), infrared spectroscopy (IR) or modulation ratio (MR).
[0057] At 410, a second liquid composition analysis may be performed on
the remaining liquid component to obtain compositional information for the
remaining liquid component. The second liquid composition analysis may be
performed utilizing the same process or technique as the first liquid
composition
analysis performed at 404, or may utilize a different process or technique.
The
second liquid composition analysis may be performed utilizing any suitable
process or technique, similar to the liquid composition processes and
techniques
described above with reference to step 304 in FIG. 3.
[0058] The present disclosure describes methods for performing
composition analysis of a hydrocarbon sample based on a determined saturation
of the hydrocarbon sample at predetermined conditions. By performing
composition analysis based on the saturation of the hydrocarbon sample
provides
more accurate compositional information at the predetermined conditions by,
for
example, removing water from the sample prior to analysis and performing
analysis at predetermined conditions. Further, additional information
regarding
13
Date Recue/Date Received 2020-05-01
the hydrocarbons under the predetermined conditions may be provided by
performing composition analysis based on the determined saturation including,
for example, the phase behaviour and the compositional information of the gas
component and liquid component, which is not provided by conventional analysis
processes.
[0059] In an example, the accuracy of mass balance determinations may
be improved utilizing the compositional information determined in the present
disclosure. Conventionally, mass balance determinations may include errors
when a stream includes a multiphase flow by assuming that the flow is single
phase flow. By knowing it is oversaturated and two phase flow, the properties
of
the fluid (i.e. instead of pure liquid density, knows it's a mixed flow with a
ratio
of gas-liquid of X, density Y and viscosity Z) may be input to the flownneter
to
obtain a more accurate reading. Oversaturation information may also be
utilized
to select the type of sensor technology to be used used. For example, instead
of
differential pressure (dP), switch to a mass and dP meter in series to more
accurately determine gas phase flow.
[0060] The above-described embodiments are intended to be examples
only. Alterations, modifications and variations can be effected to the
particular
embodiments by those of skill in the art. The scope of the claims should not
be
limited by the embodiments set forth in the examples, but should be given the
broadest interpretation consistent with the description as a whole.
14
Date Recue/Date Received 2020-05-01