Note: Descriptions are shown in the official language in which they were submitted.
CA 03080435 2020-04-27
WO 2019/086921 PCT/IB2017/001479
Thermal Therapy with Dynamic Anatomical Boundaries Using MRI-Based
Temperature Uncertainty Maps
Technical Field
[0001] This invention relates to thermal therapy delivered by a treatment
apparatus to a target tissue within an anatomical boundary based on dynamic
thermal uncertainty maps derived from MRI thermometry systems and data.
Background
[0002]The use of magnetic resonance imaging (MRI) to obtain
temperature related data in a tissue ablation procedure is discussed e.g., in
Chopra (US Pat. No. 7,771,418), which is hereby incorporated by reference. MRI
thermometry, the resulting temperature measurements and temperature
uncertainty maps thereof, and related considerations are discussed by the
present applicant, e.g., in published application U52015/0038883A1,
incorporated herein by reference as well.
[0003]Generally, temperature measurements using MRI methods are
subject to errors from a variety of sources known to those skilled in the art.
When
temperature measurements are used as part of a feedback system for thermal
energy delivery, these errors contribute to unintended heating or lack of
heating
of the target region. Errors in temperature measurements during treatment
using
MRI methods include transient motion, such as bulk patient motion, localized
prostate motion (e.g., due to heating of muscles or nerves), and/or rectum
1
CA 03080435 2020-04-27
WO 2019/086921 PCT/IB2017/001479
displacement. For example, transient motion can cause significant errors in
temperature measurement, which are currently addressed by waiting (e.g., for
20
minutes) for the measured body temperature to return to an approximately
constant value. This results in less than optimal treatment sessions from a
patient
comfort perspective, as well as reduced patient throughput or less economical
use of the MRI-thermal therapy treatment facility, personnel and equipment.
Summary
[0004] The method described here calculates and displays the regions
where the temperature can be reliably measured. The clinician then can make an
informed decision to treat these regions or plan a treatment to avoid them
based
on the sensitivity of surrounding structures to unintended heating.
[0005] An aspect of the invention is directed to a method for dynamically
delivering thermal therapy to a target volume within a patient's body. The
method comprises determining an anatomical boundary corresponding to the
target volume for delivery of thermal therapy thereto; using a thermal therapy
applicator comprising an ultrasound transducer array, delivering a thermal
therapy dose to said target volume; in a computer, receiving N sets of
temperature data for pixels corresponding to a portion of a patient's body,
each
set of temperature data corresponding to a respective capture time of phase
images captured using a magnetic resonance imaging (MRI) device, wherein N is
greater than or equal to M, and M is a rolling capture time window; in the
computer, for each of the past M capture times, determining a corrected
temperature at each pixel; in the computer, for each pixel, calculating a
temperature uncertainty based on said corrected temperature at each of the
past
2
CA 03080435 2020-04-27
WO 2019/086921 PCT/IB2017/001479
M capture times; and in the computer, modifying a portion of the anatomical
boundary only when the temperature uncertainty for the portion of the
anatomical boundary is below a threshold temperature uncertainty.
[0006] In one or more embodiments, the temperature uncertainty
corresponds to a standard deviation of said corrected temperature at each
pixel
across the past M capture times. In one or more embodiments, the method
further comprises pausing the delivery of the thermal therapy dose before
modifying the portion of the anatomical boundary. In one or more embodiments,
the method further comprises modifying a location of a thermal therapy
applicator center.
[0007] In one or more embodiments, the method further comprises, in the
computer, validating the anatomical boundary to confirm that the temperature
uncertainty for the portion of the anatomical boundary is below the threshold
temperature uncertainty. In one or more embodiments, the method further
comprises, in the computer, generating an alert when the temperature
uncertainty for the portion of the anatomical boundary is greater than the
threshold temperature uncertainty.
[0008] In one or more embodiments, the method further comprises, in the
computer, calculating a standard deviation at each point along the anatomical
boundary across the past M capture times. In one or more embodiments, the
method further comprises, in the computer, generating a temperature
uncertainty
map, the temperature uncertainty map including the temperature uncertainty for
each pixel. In one or more embodiments, the method further comprises
displaying the temperature uncertainty map on a display coupled to the
computer.
3
CA 03080435 2020-04-27
WO 2019/086921 PCT/IB2017/001479
[0009] In one or more embodiments, the method further comprises
detrending the corrected temperature at each pixel across the past M capture
times to form detrended temperature data. In one or more embodiments, the
method further comprises performing a linear regression of the corrected
temperature at each pixel across the past M capture times. In one or more
embodiments, the method further comprises calculating the standard deviation
of
the detrended temperature data at each pixel. In one or more embodiments, the
method further comprises determining the temperature uncertainty based on the
standard deviation of the detrended temperature data at each pixel.
[0010] In one or more embodiments, the method further comprises, in the
computer, receiving a new set of temperature data for pixels corresponding to
the portion of a patient's body; and calculating an updated temperature
uncertainty based on the past M capture times, the past M capture times
including the new set of temperature data.
Brief Description of the Drawings
[0011] Fora fuller understanding of the nature and advantages of the
present invention, reference is made to the following detailed description of
preferred embodiments and in connection with the accompanying drawings, in
which:
[0012] Fig. 1 illustrates a representation of a cross section of a MRI
temperature uncertainty map and showing the prostate boundary and target
boundary;
[0013] Figs. 2 illustrates an exemplary treatment workflow process;
4
CA 03080435 2020-04-27
WO 2019/086921 PCT/IB2017/001479
[0014] Fig. 3 illustrates an exemplary process for calculating a temperature
uncertainty map;
[0015] Fig. 4 is a flow chart for dynamically calculating a temperature
uncertainty map of temperatures in a target volume;
[0016] Figs. 5A, 5B, and 5C illustrate examples of temperature uncertainty
maps that may be produced according to the flow chart of Fig. 4;
[0017] Fig. 6 is a flow chart of a method for updating the prostate
boundary;
[0018] Figs. 7, 8, 9, and 10 illustrate a flow chart for dynamically
calculating
a temperature uncertainty map of temperatures in a target volume;
[0019] Fig. 11 is a graph that illustrates the effect of detrending
temperature data; and
[0020] Fig. 12 illustrates an example of a coordinate system used in some
embodiments.
Detailed Description
[0021] The present disclosure provides systems and methods for
overcoming the effects of and avoiding errors due to such temperature
measurement uncertainties. Accordingly, improved accuracy and efficiency of
delivery of MRI-guided thermal therapies is made possible. One application for
such therapies is in treating the diseased male prostate.
[0022] Embodiments of the invention relates to dynamically changing and
validating the prostate contour and ultrasound applicator center during
treatment. The prostate contour and/or applicator center may need to be
adjusted (manually or automatically) during treatment due to transient motion,
CA 03080435 2020-04-27
WO 2019/086921 PCT/IB2017/001479
which can cause the baseline treatment parameters (e.g., prostate boundary and
ultrasound applicator center) to be invalid. Examples of transient motion
include
bulk patient motion, localized prostate motion (e.g., due to heating of
muscles or
nerves), and/or rectum displacement. The prostate contour may also need to be
adjusted if noise corrupts some sections of the boundaries. For example, there
may be a low signal region due to gas in the rectum or due to transient
motion.
Further, the prostate contour may need to be adjusted to avoid treatment of a
region (e.g., a section was treated once and retreatment is not desired). The
ultrasound applicator center may need to be adjusted because alignment of the
ultrasound applicator center was incorrect in treatment planning or due to
transient motion.
[0023] To account for transient motion, the temperature and temporal
temperature uncertainty at each pixel are calculated retrospectively at a
given
data capture time over a rolling time window during treatment.
[0024] Fig. 1 illustrates a cross sectional view taken using an imaging
modality such as MRI imaging of a portion of a patient's body in the vicinity
of a
treatment target volume. The scene shown includes for example a visual output
device such as a computer monitor screen 10 or application window of a
computer application program for displaying an image 12. The surface of the
patient's body (e.g., the surface of his abdomen) is shown at 110 while
various
zones 102 in the patient's body are shown by a visual representation of their
temperatures and/or temperature uncertainties within image 12. The zones 102
can be displayed on screen 10 as colored contours, contour plots, gray scale
intensities or other visual representations of the temperature uncertainty.
The
values plotted and represented are determined as described below.
6
CA 03080435 2020-04-27
WO 2019/086921 PCT/IB2017/001479
[0025] The image 12 shows a boundary of a target volume such as a male
prostate or portion thereof 120. This is an outline on image 12, which can be
computer-drawn or drawn with the assistance of an operator on the screen 10. A
treatment target boundary 100 is further shown on the image 12, which can be a
contour of another color, a dashed contour, or other representation. The
target
boundary 100 is the intended boundary within which the energy of the thermal
treatment process is substantially controlled to a set-point temperature (or
thermal dose) ensuring rapid and sufficient cell death of diseased cells
within the
interior of the volume defined by the target boundary 100. Heat can be
conducted outside the target boundary 100 out to the boundary of the prostate
120, which can be measured and controlled to achieve appropriate thermal
therapy while reasonably avoiding damage to non-diseased tissues and organs
proximal to said diseased locations. Tissues and organs outside the target
boundary, even if heated, will not exceed lethal thermal dose or temperature
limits.
[0026] Methods for determining and controlling the intensity of the
thermal therapy treatment as a function of the temperature or desired
temperature at such a boundary 100 are described by the present inventors in
publications and patent applications available to the public, which are hereby
incorporated by reference.
[0027] In all, Fig. 1 thus shows a temperature uncertainty map. Three-
dimensional representations of the same can be constructed from additional
layers, slices or cross-sectional views like that shown in Fig. 1. The methods
described herein can therefore be generalized to three dimensional space by
7
CA 03080435 2020-04-27
WO 2019/086921 PCT/IB2017/001479
stacking slices such as shown in Fig. 1 side by side to form a 3D volume
without
loss of generality.
[0028] Fig. 2 illustrates an exemplary process 20 enabling thermal
treatment in a MRI-guided environment and accounting for temperature
uncertainty in the MRI thermometry portion of the process. The process starts
at
200 and an automated or operator-driven positioning of the thermal therapy
device in or on the patient is done at step 202. In an example, an ultrasound
(u/s)
thermal therapy applicator is inserted trans-urethrally into a diseased male
prostate organ and positioned so as to deliver thermal therapy to the diseased
organ. In another aspect, the patient is placed in a MRI imaging volume or
machine bore and temperature scans using MRI thermometry are obtained, slice
by slice, through a target region to generate thermal imagery and/or
temperature
uncertainty maps of the target region.
[0029] Anatomical images of the patient or portion of the patient in the
vicinity of the target region are obtained at step 204. The system can
automatically or semi-automatically determine whether the thermal therapy
applicator is in the correct position to deliver the desired thermal therapy
to the
target region at 206. If not, the process returns to position the thermal
therapy
applicator at 202.
[0030] Once the thermal therapy applicator device is in the correct
position, temperature uncertainty images like those depicted in Fig. 1 are
collected at 208. A memory or digital storage apparatus can be used to store
the
data so collected for analysis or other purposes.
[0031] The system next calculates and displays the temperature
uncertainty maps as depicted above at step 210. These are preferably output to
a
8
CA 03080435 2020-04-27
WO 2019/086921 PCT/IB2017/001479
computer output or display device such as a computer workstation monitor
connected to the imaging and therapy device in an overall thermal therapy
control system.
[0032] Using the temperature data and temperature uncertainty maps, a
thermal therapy treatment plan is determined and target points or regions are
identified at step 212.
[0033] The thermal therapy itself is delivered from a thermal therapy
applicator, e.g., an ultrasound transducer array device in or proximal to the
desired target region at step 214. During thermal therapy, additional
temperature
uncertainty images are gathered and displayed, as discussed below.
[0034] Once the thermal therapy procedure is complete, the system or
operator terminates the process 20 at 216.
[0035] Fig. 3 illustrates another set of steps in an exemplary computer-
implemented method 30 for gathering images in the context of image-guided
thermal therapy, making appropriate corrections and generating outputs for use
in that context.
[0036] The process starts at 300 and one or more phase images are
gathered from a nuclear magnetic resonance or MRI device in which a patient is
placed. In an embodiment, several (e.g., three to ten) phase images are
gathered
at step 302 and stored in a machine-readable storage device such as a computer
memory device. The MRI device can be configured, arranged, programmed and
operated so as to run a sequence to output the magnitude and phase images in
real time. The output images are output through a signal connection or network
connection as desired, for example to another computer device, coupled to the
9
CA 03080435 2020-04-27
WO 2019/086921 PCT/IB2017/001479
MRI device, where subsequent computations and processing of the MRI data can
be carried out.
[0037] In an example, an EPI sequence is used to gather the channel
uncombined phase images. Other sequences can be used as would be
understood by those skilled in the art, for example a GRE sequence.
[0038] In some thermal therapies using an ultrasound transducer system,
multiple ultrasound transducer elements are deployed in an ultrasonic array
placed within the diseased tissue volume. For multi-transducer ultrasound
therapy
systems, multiple image slices can be taken such that one image slice is taken
per
ultrasound transducer per therapy applicator system. In yet another aspect, a
monitoring slice image can be taken at either end of the imaging slices for
full
monitoring. The sequence is set in an embodiment to automatically repeat so
that stacks of phase images are generated continuously throughout the thermal
therapy treatment.
[0039] A reference phase image is created at step 304 using data from the
gathered phase images in the previous step. This reference phase image is the
phase image prior to initiating heating from the thermal therapy procedure. To
increase signal to noise, the reference phase image is calculated as the
average
phase over several (e.g., 5) reference images for each pixel in the image.
[0040] A measurement image is collected at step 306 prior to and/or
during the thermal therapy procedure. The system then calculates uncorrected
temperatures at step 308. In an example, a weighted sum of the phase
differences across all channels is calculated and scaled so as to determine
temperatures. In an aspect, an MRI device can be programmed to output the
combined phase for all coils. In this case the system only requires to
calculate the
CA 03080435 2020-04-27
WO 2019/086921 PCT/IB2017/001479
phase difference from the reference image to be scaled to output the
temperature in a region of interest.
[0041] At step 310 the system corrects for drift. As mentioned before, the
drift could be due to temporal changes or drift in the main BO magnetic field
of
the MRI machine. The drift could result in erroneous (typically lower)
temperature
measurements if not corrected for. Therefore, according to a present aspect,
we
correct for such drift effects at one or more areas of the image. The
temperature
at these areas is assumed to be that of the patient's body's core temperature,
which substantially does not change throughout a therapy treatment. A two-
dimensional linear interpolation of the drift is calculated for each
measurement
slice image and added to the temperature at each pixel in the image to
generate
a drift-corrected temperature image.
[0042] In step 312, a visual temperature map is displayed on a display
coupled to the computer.
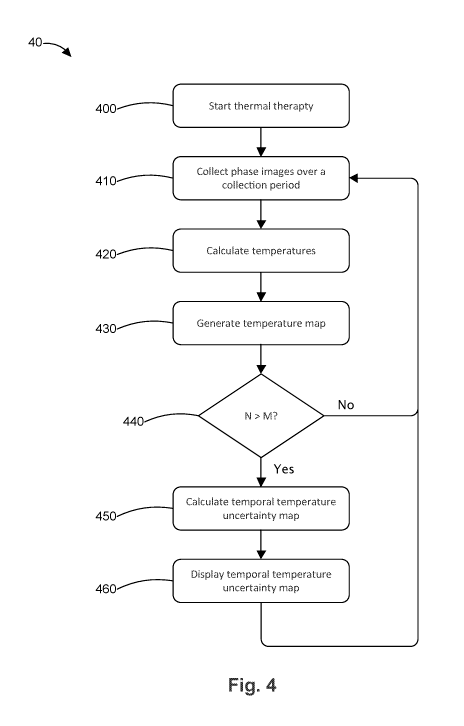
[0043] Fig. 4 is a flow chart 40 for dynamically calculating a temperature
uncertainty map of temperatures in a target volume. In step 400, thermal
therapy
is delivered from a thermal therapy applicator (e.g., an ultrasound transducer
array device in or proximal to the desired target region), as discussed above.
The
thermal therapy can be delivered with a treatment plan, for example as
discussed
above with respect to Fig. 2. In step 410, MRI phase images are collected from
a
MRI device during a collection period (e.g., a dynamic). The dynamic or
collection
period can be based on time (e.g., 3 to 5 seconds) and/or on the number of
phase images collected (e.g., 25 to 50 phase images). In step 420, the
corrected
temperature at each pixel is determined by calculating the phase difference
between (a) the average phase over the phase image collection period (the
11
CA 03080435 2020-04-27
WO 2019/086921 PCT/IB2017/001479
average measurement phase) and (b) the average phase over several (e.g., 5)
references images for each pixel in the image (e.g., as discussed above) and
then
correcting for drift, similar to the manner described in Fig. 3. In step 430,
a
temperature map is generated and optionally displayed to the user, for example
as discussed above with respect to Fig. 3.
[0044] In step 440, the computer determines the number of temperature
maps that are stored in memory. If the number of temperature maps (N) is less
than M, the flow chart returns to step 410 to collect additional MRI phase
images
during another collection period (and generate corresponding temperature
maps). This process repeats until N is greater than or equal to M, where M is
a
rolling window of temperature maps used to calculate a temperature uncertainty
map, as discussed below. Thus, M is an integer greater than or equal to 2, and
preferably is at least 5.
[0045] When N is greater than or equal to M, the flow chart 40 proceeds to
step 450 where the temporal temperature uncertainty map is calculated. The
temporal temperature uncertainty map is formed by calculating the standard
deviation of the temperature at each pixel across the last M temperature maps.
For example, if there are 10 temperature maps (N=10) and the rolling window of
temperature maps is 5 (M=5), only the last 5 temperature maps are used to
calculate the temporal temperature uncertainty map. Alternatively, each of the
past temperature maps is used based on a weighted average, with the more
recent temperature maps having a higher weight than the older temperature
maps.
[0046]In step 460, the temporal temperature uncertainty map is displayed
visually on a display coupled to the computer. The temporal temperature
12
CA 03080435 2020-04-27
WO 2019/086921 PCT/IB2017/001479
uncertainty map can be color-coded according to different temperature
uncertainty ranges. For example, shades of blue can be assigned to temperature
uncertainties below a first threshold value (e.g., less than 2 C), shades of
yellow
and red for temperature uncertainties between the first threshold and a second
threshold (e.g., between 2-4 C), and shades of purple for temperature
uncertainties greater than the second threshold (e.g., greater than 4 C).\
[0047]After step 460, the flow chart 40 returns to step 410 to collect
additional MRI phase images during the next collection period. In the next
iteration through the flow chart 40, a new temperature map (N+1) is generated
and the temperature uncertainty map is calculated based on the temperature
maps in the current rolling window of temperature maps M. In other words, in
the
next iteration, the current rolling window of temperature maps M includes the
latest temperature map (N+1) but does not include the oldest temperature map
used in the last iteration. Alternatively, all temperature maps are used based
on a
weighted average, as discussed above.
[0048] In some embodiments, a linear regression is performed on the
temperature at each pixel across the rolling window M, which can reduce the
impact of heating (or cooling) on the temperature uncertainty map. The
temperature uncertainty map is then calculated in step 450 using the de-
trended
data.
[0049] The rolling window M can reduce the impact of transient motion on
the temperature uncertainty map. For example, transient motion may cause a
shift in the temperatures in a given temperature map because, for example, the
ultrasound applicator center has moved with respect to the baseline image.
However, the impact of such a shift can be reduced over time by comparing the
13
CA 03080435 2020-04-27
WO 2019/086921 PCT/IB2017/001479
shifted temperature map with subsequent temperature maps which may also
have a shift in temperature.
[0050] Examples of temperature uncertainty maps that may be produced
according to flow chart 40 are illustrated in Figs. 5A-5C. Fig. 5A illustrates
a first
temperature uncertainty map 50A corresponding to a first time collection
period
(e.g., time period 10). In temperature uncertainty map 50A, there are few
regions
of high temperature uncertainty 500. The remainder of the temperature
uncertainty map 50A has low temperature uncertainty. The regions of high
temperature uncertainty 500 are disposed outside of the prostate boundary 510
and inside the prostate boundary 510 at flame 520, which corresponds to the
thermal therapy generated by applicator 530.
[0051] Fig. 5B illustrates a temperature uncertainty map 50B corresponding
to a second time collection period (e.g., time period 20), which occurs after
transient motion. As can be seen the regions of high temperature uncertainty
500
are larger in temperature uncertainty map 50B than in temperature uncertainty
map 50A. In addition, the regions of high temperature uncertainty 500 are
disposed adjacent to the prostate boundary 510. The system or operator can
modify any location of prostate boundary 510 or applicator center 530 subject
to
computer validation that the modified locations are below a threshold value
(e.g.,
2 C).
[0052] Fig. 5C illustrates a temperature uncertainty map 50C
corresponding to a third time collection period (e.g., time period 30). As can
be
seen, the regions of high temperature uncertainty 500 are reduced in
temperature uncertainty map 50C after a time period due to the rolling time
window M discussed herein.
14
CA 03080435 2020-04-27
WO 2019/086921 PCT/IB2017/001479
[0053] Fig. 6 is a flow chart 60 of a method for updating the prostate
boundary. In step 600, the temporal temperature uncertainty map is displayed
on
a display coupled to the computer. In optional step 610, the operator manually
or
the computer automatically pauses treatment. Treatment can be paused, for
example, to provide time for additional time collection periods to reduce the
temperature uncertainty (e.g., as discussed above). In step 620, the operator
manually or the computer automatically modifies the prostate boundary and/or
the position of the ultrasound applicator center (e.g., to compensate for
transient
motion). In optional step 630, the operator manually or the computer
automatically resumes treatment. In step 640, the computer validates the new
prostate boundary to confirm that the prostate boundary has not been modified
at a location of high temperature uncertainty.
[0054] Fig. 7 is a flow chart 70 for dynamically calculating a temperature
uncertainty map of temperatures in a target volume. In step 700, thermal
therapy
is delivered from a thermal therapy applicator (e.g., an ultrasound transducer
array device in or proximal to the desired target region), as discussed above.
The
thermal therapy can be delivered with a treatment plan, for example as
discussed
above with respect to Fig. 2. In step 702, MRI phase images are collected from
a
MRI device during a collection period (e.g., a dynamic). The dynamic or
collection
period can be based on time (e.g., 3 to 5 seconds) and/or on the number of
phase images collected (e.g., 25 to 50 phase images). In step 704, the phase
images collected during the dynamic are processed to form a temperature map
(e.g., as described above with respect to Fig. 4). In step 706, the
temperature
map is stored in a buffer having a width of M temperature maps (corresponding
to M dynamics), where M is a rolling window of temperature maps or dynamics
CA 03080435 2020-04-27
WO 2019/086921 PCT/IB2017/001479
used to calculate a temperature uncertainty map. Thus, M is an integer greater
than or equal to 2, and preferably is at least 5.
[0055] If the number of temperature maps or dynamics (N) is less than or
equal to M, the flow chart returns to step 702 to receive another dynamic and
to
process a corresponding temperature map in step 704, which is then added to
the buffer in step 706. This process repeats until N is greater than M in step
708.
[0056] When N is greater than M, the flow chart 70 proceeds to step 710
where the oldest temperature map (corresponding to the oldest dynamic) is
discarded from the buffer. Thus, the buffer only contains the last M
temperature
maps or dynamics. After step 710, the flow chart 70 proceeds to placeholder A,
which also appears in Fig. 8. It is noted that the acquisition and processing
of new
dynamics occurs throughout flow chart 70, and thus the temperature uncertainty
maps can be updated dynamically during any step of flow chart 70.
[0057] Starting at placeholder A on Fig. 8, the flow chart 70 proceeds to
step 712 to perform a linear regression (e.g., a first order linear
regression) for
each pixel, slice, and dynamic in the buffer stack. The first order linear
regression
can be calculated using the formula T
- estimate (X , )1 , Z) = cd(x, y, z)t + bl(x,y, z) + E,
which estimates the temperature increase for each pixel across the last M
dynamics as a linear trend. In this equation, x and y refer to the coordinates
of the
pixel, z refers to the coordinate (e.g., slice number) across the volume of a
dynamic constituted of N slices, al corresponds to the slope of the first
order
regression, bl corresponds to the intercept of the first order regression, and
corresponds to the noise of the data. The coordinates x, y, and z are also
illustrated in Fig. 12.
16
CA 03080435 2020-04-27
WO 2019/086921 PCT/IB2017/001479
[0058] In step 714, the data is detrended according to the formula
Tdetrended (X , Y , Z) = T(x,y,z) ¨ T
- estimate (X , )1 , Z) , where T(x,y,z) is the temperature
measured by MRI thermometry and T- estimate(X , y,z) is calculated in step
712. An
example of a graph that illustrates the effect of detrending temperature data
is
illustrated in Fig. 11, where line 1110 represents the measured heated data of
a
pixel, line 1120 represents a first order fit of line 1110, and line 1130
represents
the detrended temperature data from the pixel. As can be seen, line 1130 does
not include the heating component of line 1110 thus improving the standard
deviation calculation.
[0059] In step 716, the standard deviation of the detrended data is
calculated for each pixel across the last M dynamics. The standard deviation
of
each pixel is then displayed as a temperature uncertainty map in step 718.
[0060] In step 720, the computer determines whether the user has
attempted to modify the prostate boundary or the ultrasound applicator center
location. In some embodiments, the prostate boundary can be modified
regardless of the temperature uncertainty at a given point or pixel. If yes,
the flow
chart 70 proceeds to placeholder B, which also appears in Fig. 9. If not, the
flow
chart 70 proceeds to step 724 to determine if there's any indication that the
prostate boundary may be too uncertain (e.g., due to motion or noise). If yes,
the
flow chart 70 proceeds to placeholder B. In addition, the system may trigger
an
alarm or pause the treatment if it determines that there's any indication that
the
prostate boundary may be too uncertain in step 724. If not, the flow chart 70
proceeds to placeholder C, which also appears in Fig. 10.
[0061] Starting at placeholder B on Fig. 9, the flow chart 70 proceeds to
step 728 to compute the standard deviation of each point of the prostate
17
CA 03080435 2020-04-27
WO 2019/086921 PCT/IB2017/001479
boundary, similar to the manner described above. In step 730, the computer
displays (e.g., on a color-coded map) the prostate boundary sections with high
temperature uncertainty (e.g., greater than 2 C).
[0062] In step 732, the user is allowed to modify any point on the prostate
boundary and/or to move the ultrasound applicator center. In step 734, the
modified sections of the prostate boundary and/or the new location of the
ultrasound applicator are displayed.
[0063] In step 736, the user is asked to confirm the changes made in step
732 (i.e., the modifications to the prostate boundary and/or the ultrasound
applicator center). If the user does not confirm the changes, the flow chart
70
returns to step 702 to receive a new dynamic. If the user confirms the
changes,
the flow chart 70 proceeds to step 738 where the standard deviation of the
temperature in the modified sections of the prostate boundary is calculated.
After
step 738, the flow chart 70 proceeds to placeholder D, which appears in Fig.
10.
[0064] Starting at placeholder Don Fig. 10, the flow chart 70 proceeds to
step 740 to determine if the standard deviation of each pixel is less than 2
C. If
yes, the controller is updated to use the new prostate boundary and/or the new
ultrasound applicator center, which were modified in step 732. If the standard
deviation of any pixel is greater than or equal to 2 C in step 740, the flow
chart
70 determines whether the user has confirmed and acknowledged this large
standard deviation at step 742. If the user has confirmed and acknowledged the
large standard deviation, the flow chart 70 proceeds to step 744 to update the
controller with the new prostate boundary and/or the new ultrasound applicator
center, as discussed above. If the user has not confirmed and acknowledged the
large standard deviation in step 742, the flow chart 70 returns to placeholder
B in
18
CA 03080435 2020-04-27
WO 2019/086921 PCT/IB2017/001479
Fig. 9, at which point the standard deviation of each point of the prostate
boundary is calculated in step 728. If sections of the modified prostate
boundary
are too uncertain (i.e., greater than 2 C), the user can either (a) wait for
the
ultrasound applicator beam to pass if the user has modified a section
currently
being heated; (b) pause the treatment and wait for the temperature uncertainty
map to stabilize; (c) re-draw the prostate boundary to a different location
(e.g., to
avoid the high temperature uncertainty region); (d) acknowledge and confirm
that
at least some sections of the prostate boundary have a high temperature
uncertainty; or (d) discard the changes to the prostate boundary and continue
with the original prostate boundary.
[0065]After the controller is updated in step 744, the flow chart 70
proceeds to step 746 for the controller to perform thermal therapy treatment
based on the new boundary and/or new UA center (if coming from step 744) or
based on the existing boundary and/or UA center (if coming from placeholder
C).
Flow chart 70 also proceeds to step 746 from placeholder C, which is reached
after step 724, as discussed above.
[0066]The present invention should not be considered limited to the
particular embodiments described above. Various modifications, equivalent
processes, as well as numerous structures to which the present invention may
be
applicable, will be readily apparent to those skilled in the art to which the
present
invention is directed upon review of the present disclosure.
[0067] What is claimed is:
19