Note: Descriptions are shown in the official language in which they were submitted.
CA 03080875 2020-04-29
WO 2019/086668 PCT/EP2018/080179
1
ORGANIC MOLECULES
FOR USE IN OPTOELECTRONIC DEVICES
The invention relates to light-emitting organic molecules and their use in
organic light-emitting
diodes (OLEDs) and in other optoelectronic devices.
Description
The object of the present invention is to provide molecules which are suitable
for use in
optoelectronic devices.
This object is achieved by the invention which provides a new class of organic
molecules.
The organic molecules of the invention are purely organic molecules, i.e. they
do not contain
any metal ions in contrast to metal complexes known for use in optoelectronic
devices.
The organic molecules exhibit emission maxima in the blue, sky-blue or green
spectral range.
The photoluminescence quantum yields of the organic molecules according to the
invention
are, in particular, 26 % or more. The molecules of the invention exhibit in
particular thermally
activated delayed fluorescence (TADF). The use of the molecules according to
the invention
in an optoelectronic device, for example, an organic light-emitting diode
(OLED), leads to
higher efficiencies of the device. Corresponding OLEDs have a higher stability
than OLEDs
with known emitter materials and comparable color. In particular, the
molecules can be used
in combination with a fluorescence emitter to enable so-called
hyperfluorescence.
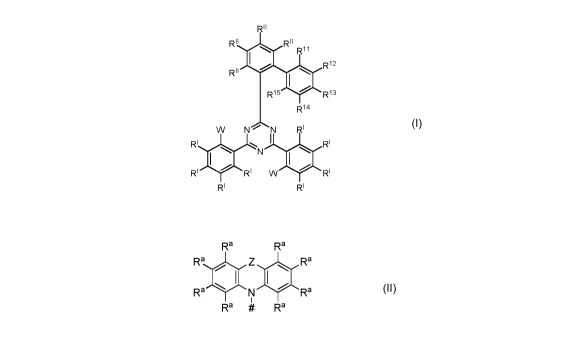
The organic molecules according to the invention comprise or consist of one
first chemical
moiety comprising or consisting of a structure of Formula I,
RH
RH RH
Ri2
RH
R15 R13
Ri4
W N N RI
RI I RI
RI 0 N 0
RI W RI
RI RI
Formula I
CA 03080875 2020-04-29
WO 2019/086668 PCT/EP2018/080179
2
and
- two second chemical moieties comprising or consisting of a structure of
Formula II,
Ra Ra
Ra 0 Z 0 Ra
Ra N Ra
I
Ra # Ra
Formula II
wherein each second chemical moiety is linked to the first chemical moiety via
a single bond.
# represents the binding site of a single bond linking the second chemical
moiety to the first
chemical moiety.
W shows the position of the single bond linking the first chemical moiety to
one of the two
second chemical moieties.
Z is at each occurrence independently from another selected from the group
consisting of: a
direct bond, CR3R4, C=CR3R4, 0=0, C=NR3, NR3, 0, SiR3R4, S, S(0) and S(0)2.
R' is at each occurrence independently from another selected from the group
consisting of:
hydrogen, deuterium,
Ci-05-alkyl,
wherein one or more hydrogen atoms are optionally substituted by deuterium;
02-08-alkenyl,
wherein one or more hydrogen atoms are optionally substituted by deuterium;
02-08-alkynyl,
wherein one or more hydrogen atoms are optionally substituted by deuterium;
and
06-018-aryl.
RI' is at each occurrence independently from another selected from the group
consisting of:
hydrogen, deuterium,
Ci-05-alkyl,
wherein one or more hydrogen atoms are optionally substituted by deuterium;
02-08-alkenyl,
wherein one or more hydrogen atoms are optionally substituted by deuterium;
02-08-alkynyl,
wherein one or more hydrogen atoms are optionally substituted by deuterium;
and
CA 03080875 2020-04-29
WO 2019/086668 PCT/EP2018/080179
3
06-018-aryl.
R11, R12, R13, R14 and rCr,15
is at each occurrence independently from another selected from the
group consisting of:
hydrogen, deuterium, ON, CF3, phenyl,
Ci-Cs-alkyl,
wherein one or more hydrogen atoms are optionally substituted by deuterium;
02-08-alkenyl,
wherein one or more hydrogen atoms are optionally substituted by deuterium;
02-08-alkynyl,
wherein one or more hydrogen atoms are optionally substituted by deuterium;
06-018-aryl,
which is optionally substituted with one or more substituents R6; and
03-017-heteroaryl,
which is optionally substituted with one or more substituents R6.
Ra, R3 and R4 is at each occurrence independently from another selected from
the group
consisting of: hydrogen, deuterium, N(R5)2, OR5, Si(R5)3, B(0R5)2, 0S02R5,
CF3, ON, F, Br, I,
CI-am-alkyl,
which is optionally substituted with one or more substituents R5 and
wherein one or more non-adjacent 0H2-groups are optionally substituted by
R50=0R5,
CEO, Si(R5)2, Ge(R5)2, Sn(R5)2, 0=0, C=S, C=Se, C=NR5, P(=0)(R5), SO, SO2,
NR5,
0, S or CONR5;
C1-040-alkoxy,
which is optionally substituted with one or more substituents R5 and
wherein one or more non-adjacent 0H2-groups are optionally substituted by
R50=0R5,
CEO, Si(R5)2, Ge(R5)2, Sn(R5)2, 0=0, C=S, C=Se, C=NR5, P(=0)(R5), SO, SO2,
NR5,
0, S or CONR5;
C1-040-thioalkoxy,
which is optionally substituted with one or more substituents R5 and
wherein one or more non-adjacent 0H2-groups are optionally substituted by
R50=0R5,
CEO, Si(R5)2, Ge(R5)2, Sn(R5)2, 0=0, C=S, C=Se, C=NR5, P(=0)(R5), SO, SO2,
NR5,
0, S or CONR5;
02-040-alkenyl,
which is optionally substituted with one or more substituents R5 and
CA 03080875 2020-04-29
WO 2019/086668 PCT/EP2018/080179
4
wherein one or more non-adjacent CH2-groups are optionally substituted by
R5C=CR5,
CEO, Si(R5)2, Ge(R5)2, Sn(R5)2, 0=0, C=S, C=Se, C=NR5, P(=0)(R5), SO, SO2,
NR5,
0, S or CONR5;
02-040-alkynyl,
which is optionally substituted with one or more substituents R5 and
wherein one or more non-adjacent CH2-groups are optionally substituted by
R5C=CR5,
CEO, Si(R5)2, Ge(R5)2, Sn(R5)2, 0=0, C=S, C=Se, C=NR5, P(=0)(R5), SO, SO2,
NR5,
0, S or CONR5;
06-060-aryl,
which is optionally substituted with one or more substituents R5; and
03-057-heteroaryl,
which is optionally substituted with one or more substituents R5.
R5 is at each occurrence independently from another selected from the group
consisting of:
hydrogen, deuterium, N(R6)2, OR6, Si(R6)3, B(0R6)2, 0S02R6, CF3, ON, F, Br, I,
CI-am-alkyl,
which is optionally substituted with one or more substituents R6 and
wherein one or more non-adjacent CH2-groups are optionally substituted by
R6C=CR6,
CEO, Si(R6)2, Ge(R6)2, Sn(R6)2, 0=0, C=S, C=Se, C=NR6, P(=0)(R6), SO, SO2,
NR6,
0, S or CONR6;
C1-C40-alkoxy,
which is optionally substituted with one or more substituents R6 and
wherein one or more non-adjacent CH2-groups are optionally substituted by
R6C=CR6,
CEO, Si(R6)2, Ge(R6)2, Sn(R6)2, 0=0, C=S, C=Se, C=NR6, P(=0)(R6), SO, SO2,
NR6,
0, S or CONR6;
C1-C40-thioalkoxy,
which is optionally substituted with one or more substituents R6 and
wherein one or more non-adjacent CH2-groups are optionally substituted by
R6C=CR6,
CEO, Si(R6)2, Ge(R6)2, Sn(R6)2, 0=0, C=S, C=Se, C=NR6, P(=0)(R6), SO, SO2,
NR6,
0, S or CONR6;
02-C40-alkenyl,
which is optionally substituted with one or more substituents R6 and
wherein one or more non-adjacent CH2-groups are optionally substituted by
R6C=CR6,
CEO, Si(R6)2, Ge(R6)2, Sn(R6)2, 0=0, C=S, C=Se, C=NR6, P(=0)(R6), SO, SO2,
NR6,
0, S or CONR6;
02-C40-alkynyl,
which is optionally substituted with one or more substituents R6 and
CA 03080875 2020-04-29
WO 2019/086668 PCT/EP2018/080179
wherein one or more non-adjacent CH2-groups are optionally substituted by
R6C=CR6,
CEO, Si(R6)2, Ge(R6)2, Sn(R6)2, 0=0, C=S, C=Se, C=NR6, P(=0)(R6), SO, SO2,
NR6,
0, S or CONR6;
06-060-aryl,
which is optionally substituted with one or more substituents R6; and
03-057-heteroaryl,
which is optionally substituted with one or more substituents R6.
R6 is at each occurrence independently from another selected from the group
consisting of
hydrogen, deuterium, OPh, CF3, ON, F,
Ci-05-alkyl,
wherein optionally one or more hydrogen atoms are independently from each
other
substituted by deuterium, ON, CF3, or F;
C1-05-alkoxy,
wherein optionally one or more hydrogen atoms are independently from each
other
substituted by deuterium, ON, CF3, or F;
C1-05-thioalkoxy,
wherein optionally one or more hydrogen atoms are independently from each
other
substituted by deuterium, ON, CF3, or F;
02-05-alkenyl,
wherein optionally one or more hydrogen atoms are independently from each
other
substituted by deuterium, ON, CF3, or F;
02-05-alkynyl,
wherein optionally one or more hydrogen atoms are independently from each
other
substituted by deuterium, ON, CF3, or F;
06-018-aryl,
which is optionally substituted with one or more Ci-Cs-alkyl substituents;
03-017-heteroaryl,
which is optionally substituted with one or more Ci-Cs-alkyl substituents;
N(06-018-ary1)2;
N(03-017-heteroary1)2; and
N(03-017-heteroary1)(C6-018-aryl).
The substituents Ra, R3, R4 or R5, independently from each other, optionally
form a mono- or
polycyclic, aliphatic, aromatic and/or benzo-fused ring system with one or
more substituents
Ra, R3, R4 or R5.
CA 03080875 2020-04-29
WO 2019/086668 PCT/EP2018/080179
6
In one embodiment, R", R12, R13, R14 and Kr,15
is independently from each other selected from
the group consisting of H, methyl, ON, CF3 and phenyl.
In one embodiment, R' is at each occurrence independently from each other
selected from the
group consisting of H, methyl and phenyl.
In one embodiment, RI' is at each occurrence independently from each other
selected from the
group consisting of H, methyl and phenyl.
In one embodiment, R11 and R15 is independently from each other at each
occurrence selected
from the group consisting of H, ON, CF3 and phenyl.
In one embodiment, R" is selected from the group consisting of H, ON, CF3 and
phenyl.
In one embodiment, R13 is selected from the group consisting of H, ON, CF3 and
phenyl.
In one embodiment, R15 is selected from the group consisting of H, ON, CF3 and
phenyl.
In one embodiment, R", R12, R13, rc r,14,
and R15 is H.
In one embodiment, R' is H.
In one embodiment, RI' is H.
In one embodiment, R", R12, R13, R14, rc r,15,
R' and RI' is H.
In a further embodiment of the invention, the second chemical moiety comprises
or consists of
a structure of Formula Ila:
Ra Ra
Ra Ra
Ra N Ra
1
Ra # Ra
Formula Ila
wherein # and Ra are defined as above.
CA 03080875 2020-04-29
WO 2019/086668 PCT/EP2018/080179
7
In a further embodiment of the invention, Ra is at each occurrence
independently from another
selected from the group consisting of:
hydrogen,
Me,
'Pr,
Su,
ON,
CF3,
Ph, which is optionally substituted with one or more substituents
independently from each other
selected from the group consisting of Me, 'Pr, Su, ON, CF3, and Ph,
pyridinyl, which is optionally substituted with one or more substituents
independently from each
other selected from the group consisting of Me, 'Pr, Su, ON, CF3, and Ph,
pyrimidinyl, which is optionally substituted with one or more substituents
independently from
each other selected from the group consisting of Me, 'Pr, Su, ON, CF3, and Ph,
carbazolyl, which is optionally substituted with one or more substituents
independently from
each other selected from the group consisting of Me, 'Pr, Su, ON, CF3, and Ph,
triazinyl, which is optionally substituted with one or more substituents
independently from each
other selected from the group consisting of Me, 'Pr, Su, ON, CF3, and Ph,
and N(Ph)2.
In a further embodiment of the invention, Ra is at each occurrence
independently from another
selected from the group consisting of:
hydrogen,
Me,
'Pr,
Su,
ON,
CF3,
Ph, which is optionally substituted with one or more substituents
independently from each other
selected from the group consisting of Me, 'Pr, Su, ON, CF3, and Ph,
pyridinyl, which is optionally substituted with one or more substituents
independently from each
other selected from the group consisting of Me, 'Pr, Su, ON, CF3, and Ph,
pyrimidinyl, which is optionally substituted with one or more substituents
independently from
each other selected from the group consisting of Me, 'Pr, Su, ON, CF3, and Ph,
and
triazinyl, which is optionally substituted with one or more substituents
independently from each
other selected from the group consisting of Me, 'Pr, Su, ON, CF3, and Ph.
CA 03080875 2020-04-29
WO 2019/086668 PCT/EP2018/080179
8
In a further embodiment of the invention, the second chemical moiety comprises
or consists of
a structure of Formula Ilb, a structure of Formula Ilb-2, a structure of
Formula Ilb-3 or a
structure of Formula Ilb-4:
Rb Rb
Rb...........7õ......õ .,,,,,......õ..Rb
I 1
N Rb N Rb N N
# 1
# 1
Rb # Rb #1
Formula Ilb Formula Ilb-2 Formula Ilb-3
Formula Ilb-4
wherein
Rb is at each occurrence independently from another selected from the group
consisting of
deuterium, N(R5)2, OR5, Si(R5)3, B(0R5)2, 0S02R5, CF3, ON, F, Br, I,
C1-040-alkyl,
which is optionally substituted with one or more substituents R5 and
wherein one or more non-adjacent 0H2-groups are optionally substituted by
R50=0R5,
CEO, Si(R5)2, Ge(R5)2, Sn(R5)2, 0=0, C=S, C=Se, C=NR5, P(=0)(R5), SO, SO2,
NR5,
0, S or CONR5;
C1-040-alkoxy,
which is optionally substituted with one or more substituents R5 and
wherein one or more non-adjacent CH2-groups are optionally substituted by
R5C=CR5,
CEO, Si(R5)2, Ge(R5)2, Sn(R5)2, 0=0, C=S, C=Se, C=NR5, P(=0)(R5), SO, SO2,
NR5,
0, S or CONR5;
C1-040-thioalkoxy,
which is optionally substituted with one or more substituents R5 and
wherein one or more non-adjacent CH2-groups are optionally substituted by
R5C=CR5,
CEO, Si(R5)2, Ge(R5)2, Sn(R5)2, 0=0, C=S, C=Se, C=NR5, P(=0)(R5), SO, SO2,
NR5,
0, S or CONR5;
02-040-alkenyl,
which is optionally substituted with one or more substituents R5 and
wherein one or more non-adjacent CH2-groups are optionally substituted by
R5C=CR5,
CEO, Si(R5)2, Ge(R5)2, Sn(R5)2, 0=0, C=S, C=Se, C=NR5, P(=0)(R5), SO, SO2,
NR5,
0, S or CONR5;
02-040-alkynyl,
which is optionally substituted with one or more substituents R5 and
wherein one or more non-adjacent CH2-groups are optionally substituted by
R5C=CR5,
CEO, Si(R5)2, Ge(R5)2, Sn(R5)2, 0=0, C=S, C=Se, C=NR5, P(=0)(R5), SO, SO2,
NR5,
0, 5 or CONR5;
CA 03080875 2020-04-29
WO 2019/086668 PCT/EP2018/080179
9
06-060-aryl,
which is optionally substituted with one or more substituents R5; and
03-057-heteroaryl,
which is optionally substituted with one or more substituents R5.
Apart from that, the aforementioned definitions apply.
In further embodiments of the invention, the second chemical moiety comprises
or consists of
a structure of Formula 11c, a structure of Formula 11c-2, a structure of
Formula 11c-3 or a structure
of Formula 11c-4:
Rb
Rb
Rb
Y Y ,_ Y Y
Formula Ilc Formula 11c-2 Formula 11c-3
Formula 11c-4
wherein the aforementioned definitions apply.
In a further embodiment of the invention, Rb is at each occurrence
independently from another
selected from the group consisting of:
Me, 'Pr, Su, ON, CF3,
Ph, which is optionally substituted with one or more substituents
independently from each other
selected from the group consisting of Me, 'Pr, Su, ON, CF3, and Ph,
pyridinyl, which is optionally substituted with one or more substituents
independently from each
other selected from the group consisting of Me, 'Pr, Su, ON, CF3, and Ph,
carbazolyl, which is optionally substituted with one or more substituents
independently from
each other selected from the group consisting of Me, 'Pr, Su, ON, CF3, and Ph,
triazinyl, which is optionally substituted with one or more substituents
independently from each
other selected from the group consisting of Me, 'Pr, Su, ON, CF3, and Ph,
and N(Ph)2.
In a further embodiment of the invention, Rb is at each occurrence
independently from another
selected from the group consisting of:
Me,
'Pr,
Su,
ON,
CF3,
CA 03080875 2020-04-29
WO 2019/086668 PCT/EP2018/080179
Ph, which is optionally substituted with one or more substituents
independently from each other
selected from the group consisting of Me, 'Pr, '13u, ON, CF3, and Ph,
pyridinyl, which is optionally substituted with one or more substituents
independently from each
other selected from the group consisting of Me, 'Pr, '13u, ON, CF3, and Ph,
pyrimidinyl, which is optionally substituted with one or more substituents
independently from
each other selected from the group consisting of Me, 'Pr, '13u, ON, CF3, and
Ph, and
triazinyl, which is optionally substituted with one or more substituents
independently from each
other selected from the group consisting of Me, 'Pr, '13u, ON, CF3, and Ph.
Below, examples of embodiments of the second chemical moiety are shown:
Ra Ra Ra Ra Ra R3 A Ra Ra R3 Ra
Ra 0 R a Ra 0 S 0 R a Ra Ra Ra N Ra
Ra N Ra Ra N Ra Ra N Ra Ra N Ra
1 1 1
Ra #1 Ra Ra # Ra Ra # Ra Ra # Ra
RaR41 R3 R3Ra Ra N ' Ra Ra 0 Ra
I 1
Ra Ra Ra Ra Ra 0 0 Ra
Ra N Ra Ra N Ra Ra N Ra
1 1 1
Ra # Ra Ra # Ra Ra # Ra
R3 R4 RI3
Ra 0 0 401 Ra Ra 0 s 0 0 Ra Ra Ra R
0 a N Ra 0
N N N N
# # 1
# 1 1
#
R5
R5 Ra 0 Z
Ra Z Ni
N N ¨R5
1
N #
1 Ra 0 Z N ¨R5
#
R5
R5 N
1
#
R5
R5,
R a Z N
Ra 0 Z 0 R5
R a Z
N
N N, # 0 N R5
1 R5 N
# R5 1
#
CA 03080875 2020-04-29
WO 2019/086668 PCT/EP2018/080179
11
Ra Ra Ra
R5 R5
0
R5 R5 R5
Ra
R5
R5
Ra R5 Ra R5 Ra R5
N,
R5
Ra
Ra R5
R15
N ¨
N
R5 R5 R5
R5 R5
R5
Ra Ra R5 Ra
0 R5 R5 R5
R5
R RRa
R5
N ¨R5
Ra Ra Ra
# o R5
R5 R5
Ra # S R5
R5
Ra
N-R5
# N R5
R5 R5
CA 03080875 2020-04-29
WO 2019/086668 PCT/EP2018/080179
12
Ra Ra Ra
Ili
el R5
N N N
#i 0 #i Ala. R5 s
\IIIF
R5 R5 R5
R5 R5 R5
Ra Ra Ra
R5
0 S
R5
N N N
li li li
RRa
0 S
N N
li li
R5 R5
wherein for #, Z, Ra, R3, R4 and R5 the aforementioned definitions apply.
In one embodiment, Ra and R5 is at each occurrence independently from another
selected from
the group consisting of hydrogen (H), methyl (Me), i-propyl (CH(CH3)2) ('Pr),
t-butyl (Su),
phenyl (Ph), ON, CF3, and diphenylamine (NPh2).
In one embodiment of the invention, the organic molecules comprise or consist
of Formula III:
RH
Ril Ril
R11
R12
Ra Ra Ril
Ra
Ra Ra R15 R13
IR14
Ra N Ra
N / N RI
Ra I
RI RI
N
RI RI RI
RI Ra RI
Ra N Ra
Ra
Ra
Ra
Ra Ra
Formula III
wherein the aforementioned definitions apply.
CA 03080875 2020-04-29
WO 2019/086668 PCT/EP2018/080179
13
In a further embodiment of the invention, the organic molecules comprise or
consist of a
structure of Formula IIla:
RH
RH RH
R11
IR
Ri2
* RH
R15 R13
R14
R =?N
N N RI
RI I RI
N
RI RI RI
RI N RI
RC 'RC
Formula Illa
wherein
RC is at each occurrence independently from another selected from the group
consisting of:
Me,
'Pr,
Su,
Ph, which is optionally substituted with one or more substituents
independently from each other
selected from the group consisting of Me, 'Pr, Su, ON, CF3, and Ph,
pyridinyl, which is optionally substituted with one or more substituents
independently from each
other selected from the group consisting of Me, 'Pr, Su, ON, CF3, and Ph,
pyrimidinyl, which is optionally substituted with one or more substituents
independently from
each other selected from the group consisting of Me, 'Pr, Su, ON, CF3, and Ph,
carbazolyl, which is optionally substituted with one or more substituents
independently from
each other selected from the group consisting of Me, 'Pr, Su, ON, CF3, and Ph,
triazinyl, which is optionally substituted with one or more substituents
independently from each
other selected from the group consisting of Me, 'Pr, Su, ON, CF3, and Ph,
and
N(Ph)2,
and wherein R11, R12, R13, R14, r< ^15
and R' are defined as above.
In a further embodiment of the invention, the organic molecule comprise or
consist of a
structure of Formula Illb:
CA 03080875 2020-04-29
WO 2019/086668 PCT/EP2018/080179
14
RH
RH RH
R11
IR
Ri2
* RH
R15 R13
= ia N
N N R RI
RI I RI
RI I.1 RI N RI
RI N RI
Rc
Formula Illb
wherein the aforementioned definitions apply.
In a further embodiment of the invention, the organic molecules comprise or
consist of a
structure of Formula 111c:
RH
RH RH
R11
R0 Ri2
it RH
R15 R13
. i4 N
N N R RI
RI I RI
Rc 0 N
RI RI RI
RI N RI
IR IR
Formula IIIc
wherein the aforementioned definitions apply.
In a further embodiment of the invention, the organic molecules comprise or
consist of a
structure of Formula Illd:
CA 03080875 2020-04-29
WO 2019/086668 PCT/EP2018/080179
RH
RH RH
IR Ri2
RH
R15 R13
R14
N
N N RI
RI I RI
N
RI RI RI
RI N RI
RC
Formula hid
wherein wherein the aforementioned definitions apply.
In a further embodiment of the invention, the organic molecules comprise or
consist of a
structure of Formula IIle:
RH
RH RH
Ri2
RII
IR
R15 R13
N
Ria
N N RI
IR
I
RI RI
RI RI N . RI
RI RI
Rc Rc
N
Formula IIle
wherein the aforementioned definitions apply.
In a further embodiment of the invention, the organic molecules comprise or
consist of a
structure of Formula IIIf:
CA 03080875 2020-04-29
WO 2019/086668 PCT/EP2018/080179
16
RH
RH RH
Ri2
RII
'RC
R15 R13
N
R14
N N RI
RI RI I RI
RI N . RI
RI RI
IR
N
Formula Illf
wherein the aforementioned definitions apply.
In a further embodiment of the invention, the organic molecules comprise or
consist of a
structure of Formula Illg:
RH
RH RH
Ri2
Rc RH
RC
R15 R13
N R14
N N RI
RI I RI
N
RI RI RI
RI RI
N
IR IR
Formula Illg
wherein the aforementioned definitions apply.
In a further embodiment of the invention, the organic molecules comprise or
consist of a
structure of Formula Illh:
CA 03080875 2020-04-29
WO 2019/086668 PCT/EP2018/080179
17
RH
RH 0 R, R11
RH Ri2
Rc
R15 Ile R13
N
R14
N N RI
RI 1 RI
N
RI RI RI
RI RI
N
IR
Formula Illh
wherein the aforementioned definitions apply.
As used above and herein, the terms "aryl" and "aromatic" may be understood in
the broadest
sense as any mono-, bi- or polycyclic aromatic moieties. Accordingly, an aryl
group contains 6
to 60 aromatic ring atoms, and a heteroaryl group contains 5 to 60 aromatic
ring atoms, of
which at least one is a heteroatom. Notwithstanding, throughout the
application the number of
aromatic ring atoms may be given as subscripted number in the definition of
certain
substituents. In particular, the heteroaromatic ring includes one to three
heteroatoms. Again,
the terms "heteroaryl" and "heteroaromatic" may be understood in the broadest
sense as any
mono-, bi- or polycyclic hetero-aromatic moieties that include at least one
heteroatom. The
heteroatoms may at each occurrence be the same or different and be
individually selected
from the group consisting of N, 0 and S. Accordingly, the term "arylene"
refers to a divalent
substituent that bears two binding sites to other molecular structures and
thereby serving as a
linker structure. In case, a group in the exemplary embodiments is defined
differently from the
definitions given here, for example, the number of aromatic ring atoms or
number of
heteroatoms differs from the given definition, the definition in the exemplary
embodiments is
to be applied. According to the invention, a condensed (annulated) aromatic or
heteroaromatic
polycycle is built of two or more single aromatic or heteroaromatic cycles,
which formed the
polycycle via a condensation reaction.
In particular, as used throughout the present application the term aryl group
or heteroaryl group
comprises groups which can be bound via any position of the aromatic or
heteroaromatic
group, derived from benzene, naphthaline, anthracene, phenanthrene, pyrene,
dihydropyrene,
chrysene, perylene, fluoranthene, benzanthracene, benzphenanthrene, tetracene,
pentacene,
CA 03080875 2020-04-29
WO 2019/086668 PCT/EP2018/080179
18
benzpyrene, furan, benzofuran, isobenzofuran, dibenzofuran, thiophene,
benzothiophene,
isobenzothiophene, dibenzothiophene; pyrrole, indole, isoindole, carbazole,
pyridine,
quinoline, isoquinoline, acridine, phenanthridine, benzo-5,6-quinoline, benzo-
6,7-quinoline,
benzo-7,8-quinoline, phenothiazine, phenoxazine, pyrazole, indazole,
imidazole,
benzimidazole, naphthoimidazole, phenanthroimidazole, pyridoimidazole,
pyrazinoimidazole,
quinoxalinoimidazole, oxazole, benzoxazole, napthooxazole, anthroxazol,
phenanthroxazol,
isoxazole, 1,2-thiazole, 1,3-thiazole, benzothiazole, pyridazine,
benzopyridazine, pyrimidine,
benzopyrimidine, 1,3,5-triazine, quinoxaline, pyrazine, phenazine,
naphthyridine, carboline,
benzocarboline, phenanthroline, 1,2,3-triazole, 1,2,4-triazole, benzotriazole,
1,2,3-oxadiazole,
1,2,4-oxadiazole, 1,2,5-oxadiazole, 1,2,3,4-tetrazine, purine, pteridine,
indolizine and
benzothiadiazole or combinations of the abovementioned groups.
As used throughout the present application the term cyclic group may be
understood in the
broadest sense as any mono-, bi- or polycyclic moieties.
As used above and herein, the term alkyl group may be understood in the
broadest sense as
any linear, branched, or cyclic alkyl substituent. In particular, the term
alkyl comprises the
substituents methyl (Me), ethyl (Et), n-propyl (nPr), i-propyl ('Pr),
cyclopropyl, n-butyl (Bu), i-
butyl ('Bu), s-butyl (sBu), t-butyl (Su), cyclobutyl, 2-methylbutyl, n-pentyl,
s-pentyl, t-pentyl, 2-
pentyl, neo-pentyl, cyclopentyl, n-hexyl, s-hexyl, t-hexyl, 2-hexyl, 3-hexyl,
neo-hexyl,
cyclohexyl, 1-methylcyclopentyl, 2-methylpentyl, n-heptyl, 2-heptyl, 3-heptyl,
4-heptyl,
cycloheptyl, 1-methylcyclohexyl, n-octyl, 2-ethylhexyl, cyclooctyl, 1-
bicyclo[2,2,2]octyl, 2-
bicyclo[2,2,2]-octyl, 2-(2,6-dimethyl)octyl, 3-(3,7-dimethyl)octyl, adamantyl,
2,2,2-trifluorethyl,
1 ,1-dimethyl-n-hex-1-yl, 1 ,1 -di methyl-n-hept-1 -yl, 1 ,1-dimethyl-n-oct-1-
yl, 1 ,1 -d imethyl-n-dec-
1 -yl, 1 ,1-dimethyl-n-dodec-1-yl, 1 ,1-dimethyl-n-tetradec-1-yl, 1 ,1-
dimethyl-n-hexadec-1-yl,
1 ,1-dimethyl-n-octadec-1-yl, 1 ,1-diethyl-n-hex-1-yl, 1 ,1-diethyl-n-hept-1-
yl, 1 ,1-diethyl-n-oct-1-
yl, 1 ,1-diethyl-n-dec-1-yl, 1 ,1-diethyl-n-dodec-1-yl, 1 ,1-diethyl-n-
tetradec-1-yl, 1 ,1-diethyln-n-
hexadec-1-yl, 1 ,1-diethyl-n-octadec-1-yl, 1 -(n-propyl)-cyclohex-1 -yl, 1 -(n-
buty1)-cyclohex-1 -yl,
1 -(n-hexyl)-cyclohex-1 -yl, 1 -(n-octyI)-cyclohex-1 -yl and 1 -(n-decyl)-
cyclohex-1 -yl.
As used above and herein, the term alkenyl comprises linear, branched, and
cyclic alkenyl
substituents. The term alkenyl group exemplarily comprises the substituents
ethenyl, propenyl,
butenyl, pentenyl, cyclopentenyl, hexenyl, cyclohexenyl, heptenyl,
cycloheptenyl, octenyl,
cyclooctenyl or cyclooctadienyl.
CA 03080875 2020-04-29
WO 2019/086668 PCT/EP2018/080179
19
As used above and herein, the term alkynyl comprises linear, branched, and
cyclic alkynyl
substituents. The term alkynyl group exemplarily comprises ethynyl, propynyl,
butynyl,
pentynyl, hexynyl, heptynyl or octynyl.
As used above and herein, the term alkoxy comprises linear, branched, and
cyclic alkoxy
substituents. The term alkoxy group exemplarily comprises methoxy, ethoxy, n-
propoxy, i-
propoxy, n-butoxy, i-butoxy, s-butoxy, t-butoxy and 2-methylbutoxy.
As used above and herein, the term thioalkoxy comprises linear, branched, and
cyclic
thioalkoxy substituents, in which the 0 of the exemplarily alkoxy groups is
replaced by S.
As used above and herein, the terms "halogen" and "halo" may be understood in
the broadest
sense as being preferably fluorine, chlorine, bromine or iodine.
Whenever hydrogen (H) is mentioned herein, it could also be replaced by
deuterium at each
occurrence.
It is understood that when a molecular fragment is described as being a
substituent or
otherwise attached to another moiety, its name may be written as if it were a
fragment (e.g.
naphtyl, dibenzofuryl) or as if it were the whole molecule (e.g. naphthalene,
dibenzofuran). As
used herein, these different ways of designating a substituent or attached
fragment are
considered to be equivalent.
In one embodiment, the organic molecules according to the invention have an
excited state
lifetime of not more than 150 ps, of not more than 100 ps, in particular of
not more than 50 ps,
more preferably of not more than 10 ps or not more than 7 ps in a film of
poly(methyl
methacrylate) (PMMA) with 10% by weight of organic molecule at room
temperature.
In one embodiment of the invention, the organic molecules according to the
invention represent
thermally-activated delayed fluorescence (TADF) emitters, which exhibit a
.8.EsT value, which
corresponds to the energy difference between the first excited singlet state
(Si) and the first
excited triplet state (Ti), of less than 5000 cm', preferably less than 3000
cm', more
preferably less than 1500 cm', even more preferably less than 1000 cm' or even
less than
500 cm'.
In a further embodiment of the invention, the organic molecules according to
the invention have
an emission peak in the visible or nearest ultraviolet range, i.e., in the
range of a wavelength
CA 03080875 2020-04-29
WO 2019/086668 PCT/EP2018/080179
of from 380 to 800 nm, with a full width at half maximum of less than 0.50 eV,
preferably less
than 0.48 eV, more preferably less than 0.45 eV, even more preferably less
than 0.43 eV or
even less than 0.40 eV in a film of poly(methyl methacrylate) (PMMA) with 10%
by weight of
organic molecule at room temperature.
In a further embodiment of the invention, the organic molecules according to
the invention have
an emission peak in the visible or nearest ultraviolet range, i.e., in the
range of a wavelength
of from 380 to 800 nm, with a full width at half maximum of less than 0.40 eV
in a film of
poly(methyl methacrylate) (PMMA) with 10% by weight of organic molecule at
room
temperature.
In a further embodiment of the invention, the organic molecules according to
the invention have
a "blue material index" (BMI), calculated by dividing the photoluminescence
quantum yield
(PLQY) in % by the CI Ey color coordinate of the emitted light, of more than
150, in particular
more than 200, preferably more than 250, more preferably of more than 300 or
even more than
500.
Orbital and excited state energies can be determined either by means of
experimental methods
or by calculations employing quantum-chemical methods, in particular density
functional theory
calculations. The energy of the highest occupied molecular orbital E" m is
determined by
methods known to the person skilled in the art from cyclic voltammetry
measurements with an
accuracy of 0.1 eV. The energy of the lowest unoccupied molecular orbital EI-
um is determined
as the onset of the absorption spectrum.
The onset of an absorption spectrum is determined by computing the
intersection of the
tangent to the absorption spectrum with the x-axis. The tangent to the
absorption spectrum is
set at the low-energy side of the absorption band and at the point at half
maximum of the
maximum intensity of the absorption spectrum.
The energy of the first excited triplet state Ti is determined from the onset
of the emission
spectrum at low temperature, typically at 77 K. For host compounds, where the
first excited
singlet state and the lowest triplet state are energetically separated by >
0.4 eV, the
phosphorescence is usually visible in a steady-state spectrum in 2-Me-THF. The
triplet energy
can thus be determined as the onset of the phosphorescence spectrum. For TADF
emitter
molecules, the energy of the first excited triplet state Ti is determined from
the onset of the
delayed emission spectrum at 77 K, if not otherwise stated measured in a film
of PMMA with
10% by weight of emitter. Both for host and emitter compounds, the energy of
the first excited
CA 03080875 2020-04-29
WO 2019/086668 PCT/EP2018/080179
21
singlet state Si is determined from the onset of the emission spectrum, if not
otherwise stated
measured in a film of PMMA with 10% by weight of host or emitter compound.
The onset of an emission spectrum is determined by computing the intersection
of the tangent
to the emission spectrum with the x-axis. The tangent to the emission spectrum
is set at the
high-energy side of the emission band and at the point at half maximum of the
maximum
intensity of the emission spectrum.
A further aspect of the invention relates to a process for preparing the
organic molecules of
the invention (with an optional subsequent reaction), wherein a tetra-R'-
substituted
2-fluoro-benzonitrile is used as reactant:
coci RH
RH Br RH RH
RH 01 RH R" Br
RH
CN F N ' N RI
RI F 1) SbC15, DCM, rt, 4h
RI I RI
RI RI
2) NH3 aq. RI N
VI RI F RI
RI RI RI
RH
0õ0 HO _OH RH I RH
B B Rii
R11 R15 R11 R15 R12
or so RH
R12 R14 R12 R14
R15 R13
R13 R13 Ri4
Pd2(dpa)3, SPhos F N ' N RI
_________________________ VP- RI I I
RI
N
Toluene/water 10:1, K3PO4
RI RI F RI
RI RI
a RH
R
Da Rii Rii
Ra 40 '' Ri 1
Ra Ra R12
Ra 0 Z 0 Ra Z Ra Rii
N
R15 R13
Ra Ra . Ra N Ri4
H
Ra Ra Ra
Ra N 'N RI
Cs2CO3 Ra RI I
_______________________ OP- RI
N
DMF, 150 C
RI RI RI
RI RI
Ra
Ra Ra
Ra * N fik Ra
Z
Ra Ra
Ra
CA 03080875 2020-04-29
WO 2019/086668 PCT/EP2018/080179
22
For the reaction of a nitrogen heterocycle in a nucleophilic aromatic
substitution with an aryl
halide, preferably an aryl fluoride, typical conditions include the use of a
base, such as tribasic
potassium phosphate for example, in an aprotic polar solvent, such as dimethyl
sulfoxide
(DMSO) or N,N-dimethylformamide (DMF), for example.
A further aspect of the invention relates to the use of an organic molecule
according to the
invention as a luminescent emitter or as an absorber, and/or as host material
and/or as electron
transport material, and/or as hole injection material, and/or as hole blocking
material in an
optoelectronic device.
The optoelectronic device may be understood in the broadest sense as any
device based on
organic materials that is suitable for emitting light in the visible or
nearest ultraviolet (UV) range,
i.e., in the range of a wavelength of from 380 to 800 nm. More preferably, the
optoelectronic
device may be able to emit light in the visible range, i.e., of from 400 to
800 nm.
In the context of such use, the optoelectronic device is more particularly
selected from the
group consisting of:
= organic light-emitting diodes (OLEDs),
= light-emitting electrochemical cells,
= OLED sensors, especially in gas and vapor sensors not hermetically
externally
shielded,
= organic diodes,
= organic solar cells,
= organic transistors,
= organic field-effect transistors,
= organic lasers and
= down-conversion elements.
A light-emitting electrochemical cell consists of three layers, namely a
cathode, an anode, and
an active layer, which contains the organic molecule according to the
invention.
In a preferred embodiment in the context of such use, the optoelectronic
device is a device
selected from the group consisting of an organic light emitting diode (OLED),
a light emitting
electrochemical cell (LEO), an organic laser, and a light-emitting transistor.
In one embodiment, the light-emitting layer of an organic light-emitting diode
comprises not
only the organic molecules according to the invention but also a host material
whose triplet
CA 03080875 2020-04-29
WO 2019/086668 PCT/EP2018/080179
23
(Ti) and singlet (Si) energy levels are energetically higher than the triplet
(Ti) and singlet (Si)
energy levels of the organic molecule.
A further aspect of the invention relates to a composition comprising or
consisting of:
(a) the organic molecule of the invention, in particular in the form of an
emitter and/or a
host, and
(b) one or more emitter and/or host materials, which differ from the
organic molecule of the
invention, and
(c) optionally, one or more dyes and/or one or more solvents.
In a further embodiment of the invention, the composition has a
photoluminescence quantum
yield (PLQY) of more than 26 %, preferably more than 40 %, more preferably
more than 60 %,
even more preferably more than 80 % or even more than 90 % at room
temperature.
Compositions with at least one further emitter
One embodiment of the invention relates to a composition comprising or
consisting of:
(i) 1-50 % by weight, preferably 5-40 % by weight, in particular 10-30 % by
weight, of the
organic molecule according to the invention;
(ii) 5-98 % by weight, preferably 30-93.9 % by weight, in particular 40-88%
by weight, of
one host compound H;
(iii) 1-30 % by weight, in particular 1-20 % by weight, preferably 1-5 % by
weight, of at least
one further emitter molecule F with a structure differing from the structure
of the
molecules according to the invention; and
(iv) optionally 0-94 % by weight, preferably 0.1-65 % by weight, in
particular 1-50 % by
weight, of at least one further host compound D with a structure differing
from the
structure of the molecules according to the invention; and
(v) optionally 0-94 % by weight, preferably 0-65 % by weight, in particular
0-50 % by
weight, of a solvent.
The components or the compositions are chosen such that the sum of the weight
of the
components add up to 100 %.
In a further embodiment of the invention, the composition has an emission peak
in the visible
or nearest ultraviolet range, i.e., in the range of a wavelength of from 380
to 800 nm.
In one embodiment of the invention, the at least one further emitter molecule
F is a purely
organic emitter.
CA 03080875 2020-04-29
WO 2019/086668 PCT/EP2018/080179
24
In one embodiment of the invention, the at least one further emitter molecule
F is a purely
organic TADF emitter. Purely organic TADF emitters are known from the state of
the art, e.g.
Wong and Zysman-Colman (õPurely Organic Thermally Activated Delayed
Fluorescence
Materials for Organic Light-Emitting Diodes", Adv. Mater. 2017 Jun;29(22)).
In one embodiment of the invention, the at least one further emitter molecule
F is a
fluorescence emitter, in particular a blue, a green or a red fluorescence
emitter.
In a further embodiment of the invention, the composition, containing the at
least one further
emitter molecule F shows an emission peak in the visible or nearest
ultraviolet range, i.e., in
the range of a wavelength of from 380 to 800 nm, with a full width at half
maximum of less than
0.30 eV, in particular less than 0.25 eV, preferably less than 0.22 eV, more
preferably less
than 0.19 eV or even less than 0.17 eV at room temperature, with a lower limit
of 0.05 eV.
Composition wherein the at least one further emitter molecule F is a blue
fluorescence emitter
In one embodiment of the invention, the at least one further emitter molecule
F is a
fluorescence emitter, in particular a blue fluorescence emitter.
In one embodiment, the at least one further emitter molecule F is a blue
fluorescence emitter
selected from the following group:
CA 03080875 2020-04-29
WO 2019/086668
PCT/EP2018/080179
Q
N "
-
\
N
= Si
- 000
N
N
N 41 Si *
N
N
CA 03080875 2020-04-29
WO 2019/086668 PCT/EP2018/080179
26
___________ CC/ ______________ \
I
[-
\ __ /
\ /
I
, 41 =
4
\/ 111
\ _____
¨
*O / 0 *
.......... ¶ \ / o
i
iiklig
NI w
1
... __ 0
0 _____________________
\ /
/
i \ ip W. = 0
*
. = 0 =
Q. . = wt *
= 0
air
dik- = 0 .16.
0
, \/* 44 *
, * . S
.11- ' = *
'''' .... 0 O
. _ 0 *
. 4 *
= * . .
CA 03080875 2020-04-29
WO 2019/086668
1114 PCT/EP2018/080179
27
0 N I.
ill n C6H13 NLii
C6H13
1401
mn IlL
C6H13 L.,-. u 101 6n13
n = 1, 2, 3, 4 C6H13111
C6H13 C6H13
m = 0, 1 N 0
C6H13 110 C6H13 C6H13C6H13 ,
'
C6H13 II* SI 101
C6Hm =* 40i
n m
t-61-113
* . 101
n . Att 11
0
., c63 I N
*it
ir 4.
, ..= c6,3 C6F1j.
L.6F113
I /
n N S
n
m .'"*- 40 0
N 0 N
t
lik MI
0
O 41,
N II Oi
0,p/, _
0 0 it
II N
O-P
_i 6
N
1 N
P
P-
N 6 o
N N
001 101
N
Os
P,
0- vo ,07
) -P
0' \
0
Q
N-N 4* N ,N
/ \
4111 0
N
1.1 N
0 I. N N ISI
4110 N
le 01
Q
N N
1101 101 I I
* N N *
0 N N 0
CA 03080875 2020-04-29
WO 2019/086668
PCT/EP2018/080179
28
CN
0 *
\N
LjLN . p
N
N
6 N ¨
* N
CN 4/
0
0 N
\
N
N
i N
N
6 N __
1--( __ --E
n
lik . n
C6H13
40 C6H13 0 IktM.. C
C6H13 6H13
n 11110
IWil 1 00 P 1 Pig I 40 I
C6H13
C6H13 ail n
C6Hi3C6H13
N 'C61-113 C61-113C61-113 . W. . n = 2, 3
n = 0, 1, 2, 3 C6H13 N
I 110
C6H13 C6H13
*C6 ik 404. *Ami
H13 --N C6H13 IF
n
n = C6H13
I 410)
C6H13
ir C6H13 c Fip II. n
_NJ
6 =-=6H13 \ /
n
n
CgH17
C8H17
C8N117 \ IN
n N 411 n
0 N
0 C81W17 N\ I
n = 1, 2, 3, 4 N 41 N
0' C81-117C8H17 N
N
it
n C8H17 11i
1111
CgH17
n
N
C8 H17
C8H17 N
* n N
Uo
CA 03080875 2020-04-29
WO 2019/086668
PCT/EP2018/080179
29
P 2
N N N
N N N
N N N
\ / / \
N N N
\ /
N 0 0
\ /
oo
0 0
N
N N
/ \
N N
N N
9 N \
I / N
I
*
N \
N
N N
2
0 N \
N
b b
N N
* 0 N 2
N
0
. YI ii
P *
b b
0
=
CA 03080875 2020-04-29
WO 2019/086668 PCT/EP2018/080179
Q IN * = .
N = N 41
N
0 N
o = 40
Q , __ \
N il--- --N
N / 0
*
* N * N,
' N . * 4N
41
N /
N
N
CS * * .
N ' 0 0 N, 4Ik I( 0 N
il-- -N -N
N C61113 %
C61113
0 C61113
= * C61113
* 41 N * * *
N C61113 a
C61113 *
= * * N N
0 * ill B I.N, ,
N / 0"N
N
*
pl.__ NI NC CN
N \ 0 0 ;NI n
C6 N
110 . 1113
C61113 n
lei C61113C61113
N
= 11 N
4I 4
N
CA 03080875 2020-04-29
WO 2019/086668 PCT/EP2018/080179
31
III
=
c-3
P
2 =
0
0 N 0 IIQ N 0B S
N
40 02H5 02H5 C2H5 02E15 2000
q
N
*
N ----\
N /
/
*
/ B
¨\
B*
* N
/ \
N --
* \ NC
0 /¨ IP
N
¨\ / N Q N
N N
N --
0 N
0
Q g
N
* N is N-
N 0 N * \N
N 6 N S
/ \ I
o ab
N
P0'N N gab
114N.
N S Q
/ N N
N
6 C5
N ________________ N N
N
C5
* C5 *
41/ C6H13
n C H
a 6 13
* n = 1,2, 3
e *
* C6H1306H13 06H13
*O. C6H13 n
n
C6H13 4i
06H13 e c6H13 c6H13
* 0 c6H1306H13
N
0 C6H13 n
06H13 n = 1, 2, 3
n
CA 03080875 2020-04-29
WO 2019/086668 PCT/EP2018/080179
32
In a certain embodiment, the one further emitter molecule F is a blue
fluorescence emitter
selected from the following group:
Q
N "
_
N
N
101
b SOO
101
S
N
-
N
N
N
N
Composition wherein the at least one further emitter molecule F is a triplet-
triplet annihilation
(TTA) fluorescence emitter
In one embodiment of the invention, the at least one further emitter molecule
F is a triplet-triplet
annihilation (TTA) emitter. In one embodiment, F is a blue TTA emitter
selected from the
following group:
CA 03080875 2020-04-29
WO 2019/086668 PCT/EP2018/080179
33
\o CN
/ ___________________________ \
_ 2
w
4 i = i ic
w 6
Cornposition wherein the at least one further emitter molecule F is a green
fluorescence emitter
In a further embodiment of the invention, the at least one further emitter
molecule F is a
fluorescence emitter, in particular a green fluorescence emitter.
In one embodiment, the at least one further emitter molecule F is a
fluorescence emitter
selected from the following group:
cA
N
,¨i
o
oo
o
oo
,¨i
o
el
a,
2
,.,
. 0
a,
/
dP d2=>= z
0 z
*
z zq _z u_
\ ,
z z .
,
= =
u) 0 0 2 0
2
CV
1 41 z
pop.
"
,
CV
0
CV Z Z .1.
d .,z
Cy,
r-
oo
0
o
oo
õ
0
,
= (-
0
u) 0
z
, \z __________________________________________ 0 0.
, z z_
=
=
0
z
==z
* z . z z
* 0
0
0 0 b
oo
-- z
z * 0 I
¨z
oe ¨z 0 0 9 2
c7,
_ e,z co z z
el
Z co ' z¨N
0 0 z¨ 0 z 0 6 * z
0 0 U 0 0* 0
z
CA 03080875 2020-04-29
WO 2019/086668 PCT/EP2018/080179
o
101
N
N = 4111 N
N 1.I
0 0010.1110 0
101 40 0
N
N cfl
. N N-
N
2 II
00 ON N 0
it 101 N - N-C4H9
411 0
. N gi oN lip,
AI
QN N ilk
'.õ
N
S *
4 .1 1 rN
",. \
N i N N 0 0 , ,õ.
N "\
0 Si 1101
N-
N
N N
is N
0LJ
0 0
In a further embodiment of the invention, the composition has an emission peak
in the visible
or nearest ultraviolet range, i.e., in the range of a wavelength of from 380
to 800 nm, in
particular between 485 nm and 590 nm, preferably between 505 nm and 565 nm,
even more
preferably between 515 nm and 545 nm.
Composition wherein the at least one further emitter molecule F is a red
fluorescence emitter
In a further embodiment of the invention, the at least one further emitter
molecule F is a
fluorescence emitter, in particular a red fluorescence emitter.
In one embodiment, the at least one further emitter molecule F is a
fluorescence emitter
selected from the following group:
CA 03080875 2020-04-29
WO 2019/086668 PCT/EP2018/080179
36
NC CN NC CN NC CN
1 1 1
1OFO
1 1 1 1 1 1
0 0 0
\
N N I\1 0
I NC CN
1
1 0
/ S
N N
/
NC CN 0
1 0
/
NC CN 1 1
1 0 /
1 N
N
I\1
1
HNC CN NC CN NC CN
1 1 1
1 1 1 1 1
0 0 0
0
N N
NC CN NNC CN
1 1 NC CN
1 0
1 1
/ 1
0 0 /
0
N N 0
N
0
CA 03080875 2020-04-29
WO 2019/086668 PCT/EP2018/080179
37
NC CN NC CN NC CN NC CN
1 1 1
---' ..-' ---' ----
N
I
1 I C4H9 C4H9
N 0 N 0
N N
C4E19-- 0 'C41-19
0
----- 0
I I
1
1
0
0 NC CN
* NC CN
* N 0 N0 N
0 CN
----*
1 * 0
0
0
NC CN N CN
CN
CN
0 0
0
0
N
N CN
SONS S
1 /
CN
* * N
I N
/
N * /
N 0 N
. --... 0
/ S
N-S
Q * N
/ µ
, N
S N * 04 oN *
I /
*
0
d'
*
N
\ 0 iN
CA 03080875 2020-04-29
WO 2019/086668 PCT/EP2018/080179
38
s ,...
II
0 = N
=
SOO NC CN
1
,I I
40 0
N
ir I 1
0
N N
NC CN
/
1
/ 1 1
\
N 0
40 N
NC CN LI
1
/\
NC ON \ 0 1
1 1
1 1
\ 0
N
In a further embodiment of the invention, the composition has an emission peak
in the visible
or nearest ultraviolet range, i.e., in the range of a wavelength of from 380
to 800 nm, in
particular between 590 nm and 690 nm, preferably between 610 nm and 665 nm,
even more
preferably between 620 nm and 640 nm.
Light-emitting layer EML
In one embodiment, the light-emitting layer EML of an organic light-emitting
diode of the
invention comprises (or essentially consists of) a composition comprising or
consisting of:
(i) 1-50 % by weight, preferably 5-40 % by weight, in particular 10-30 % by
weight, of one
or more organic molecules according to the invention;
(ii) 5-99 % by weight, preferably 30-94.9 % by weight, in particular 40-89%
by weight, of
at least one host compound H; and
(iii) optionally 0-94 % by weight, preferably 0.1-65 % by weight, in
particular 1-50 % by
weight, of at least one further host compound D with a structure differing
from the
structure of the molecules according to the invention; and
(iv) optionally 0-94 % by weight, preferably 0-65 % by weight, in
particular 0-50 % by
weight, of a solvent; and
CA 03080875 2020-04-29
WO 2019/086668 PCT/EP2018/080179
39
(v) optionally 0-30 % by weight, in particular 0-20 % by weight, preferably
0-5 % by weight,
of at least one further emitter molecule F with a structure differing from the
structure of
the molecules according to the invention.
Preferably, energy can be transferred from the host compound H to the one or
more organic
molecules of the invention, in particular transferred from the first excited
triplet state Ti (H) of
the host compound H to the first excited triplet state Ti (E) of the one or
more organic molecules
according to the invention and/ or from the first excited singlet state S1(H)
of the host
compound H to the first excited singlet state S1(E) of the one or more organic
molecules
according to the invention.
In one embodiment, the host compound H has a highest occupied molecular
orbital HOMO(H)
having an energy E" m (H) in the range of from -5 eV to -6.5 eV and one
organic molecule
according to the invention E has a highest occupied molecular orbital HOMO(E)
having an
energy E"'(E), wherein EHomoot > EHomo(E).
In a further embodiment, the host compound H has a lowest unoccupied molecular
orbital
LUMO(H) having an energy ELum (H) and the one organic molecule according to
the invention
E has a lowest unoccupied molecular orbital LUMO(E) having an energy ELum (E),
wherein
ELumoot > ELumo(E).
Light-emitting layer EML comprising at least one further host compound D
In a further embodiment, the light-emitting layer EML of an organic light-
emitting diode of the
invention comprises (or essentially consists of) a composition comprising or
consisting of:
(i) 1-50 % by weight, preferably 5-40 % by weight, in particular 10-30 % by
weight, of one
organic molecule according to the invention;
(ii) 5-99 % by weight, preferably 30-94.9 % by weight, in particular 40-89%
by weight, of
one host compound H; and
(iii) 0-94 % by weight, preferably 0.1-65 % by weight, in particular 1-50 %
by weight, of at
least one further host compound D with a structure differing from the
structure of the
molecules according to the invention; and
(iv) optionally 0-94 % by weight, preferably 0-65 % by weight, in
particular 0-50 % by
weight, of a solvent; and
(v) optionally 0-30 % by weight, in particular 0-20 % by weight, preferably
0-5 % by weight,
of at least one further emitter molecule F with a structure differing from the
structure of
the molecules according to the invention.
CA 03080875 2020-04-29
WO 2019/086668 PCT/EP2018/080179
In one embodiment of the organic light-emitting diode of the invention, the
host compound H
has a highest occupied molecular orbital HOMO(H) having an energy E" m (H) in
the range of
from -5 eV to -6.5 eV and the at least one further host compound D has a
highest occupied
molecular orbital HOMO(D) having an energy E"'(D), wherein EH m (H) >
EHomo(u,¨). The
relation EH m (H) > EHom (D) favors an efficient hole transport.
In a further embodiment, the host compound H has a lowest unoccupied molecular
orbital
LUMO(H) having an energy ELum (H) and the at least one further host compound D
has a
lowest unoccupied molecular orbital LUMO(D) having an energy ELum (D), wherein
ELum (H)
> ELumo(u,¨). The relation ELum (H) > ELum (D) favors an efficient electron
transport.
In one embodiment of the organic light-emitting diode of the invention, the
host compound H
has a highest occupied molecular orbital HOMO(H) having an energy EH m (H) and
a lowest
unoccupied molecular orbital LUMO(H) having an energy ELum (H), and
the at least one further host compound D has a highest occupied molecular
orbital
HOMO(D) having an energy EH m (D) and a lowest unoccupied molecular orbital
LUMO(D)
having an energy ELum (D),
the organic molecule E of the invention has a highest occupied molecular
orbital
HOMO(E) having an energy EH m (E) and a lowest unoccupied molecular orbital
LUMO(E)
having an energy ELum (E),
wherein
EHomo(H) > EHomo(D) and the difference between the energy level of the highest
occupied
molecular orbital HOMO(E) of organic molecule according to the invention (EH m
(E)) and the
energy level of the highest occupied molecular orbital HOMO(H) of the host
compound H
(EHomo(,)) is between -0.5 eV and 0.5 eV, more preferably between -0.3 eV and
0.3 eV, even
more preferably between -0.2 eV and 0.2 eV or even between -0.1 eV and 0.1 eV;
and
ELumo(H) > ELumo(D) and the difference between the energy level of the lowest
unoccupied
molecular orbital LUMO(E) of organic molecule according to the invention (ELum
(E)) and the
lowest unoccupied molecular orbital LUMO(D) of the at least one further host
compound D
(ELum (D)) is between -0.5 eV and 0.5 eV, more preferably between -0.3 eV and
0.3 eV, even
more preferably between -0.2 eV and 0.2 eV or even between -0.1 eV and 0.1 eV.
Light-emitting layer EML comprising at least one further emitter molecule F
In a further embodiment, the light-emitting layer EML comprises (or
(essentially) consists of) a
composition comprising or consisting of:
CA 03080875 2020-04-29
WO 2019/086668 PCT/EP2018/080179
41
(i) 1-50 % by weight, preferably 5-40 % by weight, in particular 10-30 % by
weight, of one
organic molecule according to the invention;
(ii) 5-98 % by weight, preferably 30-93.9 % by weight, in particular 40-88%
by weight, of
one host compound H;
(iii) 1-30 % by weight, in particular 1-20 % by weight, preferably 1-5 % by
weight, of at least
one further emitter molecule F with a structure differing from the structure
of the
molecules according to the invention; and
(iv) optionally 0-94 % by weight, preferably 0.1-65 % by weight, in
particular 1-50 % by
weight, of at least one further host compound D with a structure differing
from the
structure of the molecules according to the invention; and
(v) optionally 0-94 % by weight, preferably 0-65 % by weight, in particular
0-50 % by
weight, of a solvent.
In a further embodiment, the light-emitting layer EML comprises (or
(essentially) consists of) a
composition as described in Compositions with at least one further emitter,
with the at least
one further emitter molecule F as defined in Composition wherein the at least
one further
emitter molecule F is a blue fluorescence emitter.
In a further embodiment, the light-emitting layer EML comprises (or
(essentially) consists of) a
composition as described in Compositions with at least one further emitter,
with the at least
one further emitter molecule F as defined in Composition wherein the at least
one further
emitter molecule F is a triplet-triplet annihilation (TTA) fluorescence
emitter.
In a further embodiment, the light-emitting layer EML comprises (or
(essentially) consists of) a
composition as described in Compositions with at least one further emitter,
with the at least
one further emitter molecule F as defined in Composition wherein the at least
one further
emitter molecule F is a green fluorescence emitter.
In a further embodiment, the light-emitting layer EML comprises (or
(essentially) consists of) a
composition as described in Compositions with at least one further emitter,
with the at least
one further emitter molecule F as defined in Composition wherein the at least
one further
emitter molecule F is a red fluorescence emitter.
In one embodiment of the light-emitting layer EML comprising at least one
further emitter
molecule F, energy can be transferred from the one or more organic molecules
of the invention
E to the at least one further emitter molecule F, in particular transferred
from the first excited
CA 03080875 2020-04-29
WO 2019/086668 PCT/EP2018/080179
42
singlet state S1(E) of one or more organic molecules of the invention E to the
first excited
singlet state Si (F) of the at least one further emitter molecule F.
In one embodiment, the first excited singlet state S1(H) of one host compound
H of the light-
emitting layer is higher in energy than the first excited singlet state Si (E)
of the one or more
organic molecules of the invention E: S1(H) > S1(E), and the first excited
singlet state S1(H)
of one host compound H is higher in energy than the first excited singlet
state Si (F) of the at
least one emitter molecule F: S1(H) > S1(F).
In one embodiment, the first excited triplet state T1(H) of one host compound
H is higher in
energy than the first excited triplet state Ti (E) of the one or more organic
molecules of the
invention E: Ti (H) > Ti (E), and the first excited triplet state Ti (H) of
one host compound H is
higher in energy than the first excited triplet state Ti (F) of the at least
one emitter molecule F:
T1 (H) > T1 (F).
In one embodiment, the first excited singlet state Si (E) of the one or more
organic molecules
of the invention E is higher in energy than the first excited singlet state Si
(F) of the at least
one emitter molecule F: S1(E) > S1(F).
In one embodiment, the first excited triplet state Ti (E) of the one or more
organic molecules E
of the invention is higher in energy than the first excited singlet state Ti
(F) of the at least one
emitter molecule F: Ti (E) > Ti (F).
In one embodiment, the first excited triplet state Ti (E) of the one or more
organic molecules E
of the invention is higher in energy than the first excited singlet state Ti
(F) of the at least one
emitter molecule F: T1 (E) > T1 (F), wherein the absolute value of the energy
difference between
T1(E) and T1(F) is larger than 0.3 eV, preferably larger than 0.4 eV, or even
larger than 0.5 eV.
In one embodiment, the host compound H has a highest occupied molecular
orbital HOMO(H)
having an energy E" m (H) and a lowest unoccupied molecular orbital LUMO(H)
having an
energy Ewm (H), and
the one organic molecule according to the invention E has a highest occupied
molecular orbital HOMO(E) having an energy E" m (E) and a lowest unoccupied
molecular
orbital LUMO(E) having an energy Ewm (E),
the at least one further emitter molecule F has a highest occupied molecular
orbital
HOMO(F) having an energy E" m (F) and a lowest unoccupied molecular orbital
LUMO(E)
having an energy Ewm (F),
CA 03080875 2020-04-29
WO 2019/086668 PCT/EP2018/080179
43
wherein
EHomo(H) > EHomo(E) and the difference between the energy level of the highest
occupied
molecular orbital HOMO(F) of the at least one further emitter molecule (EH m
(F)) and the
energy level of the highest occupied molecular orbital HOMO(H) of the host
compound H
(EHomo(,)) is between -0.5 eV and 0.5 eV, more preferably between -0.3 eV and
0.3 eV, even
more preferably between -0.2 eV and 0.2 eV or even between -0.1 eV and 0.1 eV;
and
ELumo(H) > ELumo(E) and the difference between the energy level of the lowest
unoccupied
molecular orbital LUMO(F) of the at least one further emitter molecule (E'(F))
and the
lowest unoccupied molecular orbital LUMO(E) of the one organic molecule
according to the
invention (E'(E)) is between -0.5 eV and 0.5 eV, more preferably between -0.3
eV and 0.3
eV, even more preferably between -0.2 eV and 0.2 eV or even between -0.1 eV
and 0.1 eV.
Optoelectronic devices
In a further aspect, the invention relates to an optoelectronic device
comprising an organic
molecule or a composition as described herein, more particularly in the form
of a device
selected from the group consisting of organic light-emitting diode (OLED),
light-emitting
electrochemical cell, OLED sensor, more particularly gas and vapour sensors
not hermetically
externally shielded, organic diode, organic solar cell, organic transistor,
organic field-effect
transistor, organic laser and down-conversion element.
In a preferred embodiment, the optoelectronic device is a device selected from
the group
consisting of an organic light emitting diode (OLED), a light emitting
electrochemical cell (LEO),
and a light-emitting transistor.
In one embodiment of the optoelectronic device of the invention, the organic
molecule
according to the invention is used as emission material in a light-emitting
layer EML.
In one embodiment of the optoelectronic device of the invention, the light-
emitting layer EML
consists of the composition according to the invention described herein.
When the optoelectronic device is an OLED, it may, for example, exhibit the
following layer
structure:
1. substrate
2. anode layer A
3. hole injection layer, HIL
4. hole transport layer, HTL
CA 03080875 2020-04-29
WO 2019/086668 PCT/EP2018/080179
44
5. electron blocking layer, EBL
6. emitting layer, EML
7. hole blocking layer, HBL
8. electron transport layer, ETL
9. electron injection layer, EIL
10. cathode layer,
wherein the OLED comprises each layer only optionally, different layers may be
merged and
the OLED may comprise more than one layer of each layer type defined above.
Furthermore, the optoelectronic device may optionally comprise one or more
protective layers
protecting the device from damaging exposure to harmful species in the
environment including,
exemplarily moisture, vapor and/or gases.
In one embodiment of the invention, the optoelectronic device is an OLED,
which exhibits the
following inverted layer structure:
1. substrate
2. cathode layer
3. electron injection layer, EIL
4. electron transport layer, ETL
5. hole blocking layer, HBL
6. emitting layer, B
7. electron blocking layer, EBL
8. hole transport layer, HTL
9. hole injection layer, HIL
10. anode layer A
wherein the OLED with an inverted layer structure comprises each layer only
optionally,
different layers may be merged and the OLED may comprise more than one layer
of each layer
types defined above.
In one embodiment of the invention, the optoelectronic device is an OLED,
which may exhibit
stacked architecture. In this architecture, contrary to the typical
arrangement, where the
OLEDs are placed side by side, the individual units are stacked on top of each
other. Blended
light may be generated with OLEDs exhibiting a stacked architecture, in
particular white light
may be generated by stacking blue, green and red OLEDs. Furthermore, the OLED
exhibiting
a stacked architecture may optionally comprise a charge generation layer
(CGL), which is
CA 03080875 2020-04-29
WO 2019/086668 PCT/EP2018/080179
typically located between two OLED subunits and typically consists of a n-
doped and p-doped
layer with the n-doped layer of one CGL being typically located closer to the
anode layer.
In one embodiment of the invention, the optoelectronic device is an OLED,
which comprises
two or more emission layers between anode and cathode. In particular, this so-
called tandem
OLED comprises three emission layers, wherein one emission layer emits red
light, one
emission layer emits green light and one emission layer emits blue light, and
optionally may
comprise further layers such as charge generation layers, blocking or
transporting layers
between the individual emission layers. In a further embodiment, the emission
layers are
adjacently stacked. In a further embodiment, the tandem OLED comprises a
charge generation
layer between each two emission layers. In addition, adjacent emission layers
or emission
layers separated by a charge generation layer may be merged.
The substrate may be formed by any material or composition of materials. Most
frequently,
glass slides are used as substrates. Alternatively, thin metal layers (e.g.,
copper, gold, silver
or aluminum films) or plastic films or slides may be used. This may allow a
higher degree of
flexibility. The anode layer A is mostly composed of materials allowing to
obtain an (essentially)
transparent film. As at least one of both electrodes should be (essentially)
transparent in order
to allow light emission from the OLED, either the anode layer A or the cathode
layer C is
transparent. Preferably, the anode layer A comprises a large content or even
consists of
transparent conductive oxides (TC0s). Such anode layer A may exemplarily
comprise indium
tin oxide, aluminum zinc oxide, fluorine doped tin oxide, indium zinc oxide,
Pb0, SnO,
zirconium oxide, molybdenum oxide, vanadium oxide, wolfram oxide, graphite,
doped Si,
doped Ge, doped GaAs, doped polyaniline, doped polypyrrol and/or doped
polythiophene.
Preferably, the anode layer A (essentially) consists of indium tin oxide (ITO)
(e.g.,
(In03)0.9(5n02)0.1). The roughness of the anode layer A caused by the
transparent
conductive oxides (TC0s) may be compensated by using a hole injection layer
(HI L). Further,
the HIL may facilitate the injection of quasi charge carriers (i.e., holes) in
that the transport of
the quasi charge carriers from the TOO to the hole transport layer (HTL) is
facilitated. The hole
injection layer (HIL) may comprise poly-3,4-ethylendioxy thiophene (PEDOT),
polystyrene
sulfonate (PSS), Mo02, V205, CuPC or Cul, in particular a mixture of PEDOT and
PSS. The
hole injection layer (HIL) may also prevent the diffusion of metals from the
anode layer A into
the hole transport layer (HTL). The HIL may exemplarily comprise PEDOT:PSS
(poly-3,4-
ethylendioxy thiophene: polystyrene sulfonate), PEDOT (poly-3,4-ethylendioxy
thiophene),
mMTDATA (4,4',4"-tris[phenyl(m-tolyl)amino]triphenylamine),
Spiro-TAD (2,2',7,7'-
tetrakis(n,n-diphenylamino)-9,9'-spirobifluorene), DNTPD (N1,N11-(biphenyl-
4,4'-diy1)bis(N1-
CA 03080875 2020-04-29
WO 2019/086668 PCT/EP2018/080179
46
phenyl-N4,N4-di-m-tolylbenzene-1,4-diamine), NPB (N,N1-nis-(1-naphthaleny1)-
N,N1-bis-
phenyl-(1,11-biphenyl)-4,4'-diamine),
NPNPB (N,N1-diphenyl-N,N1-di-[4-(N,N-diphenyl-
amino)phenyl]benzidine), Me0-TPD (N,N,N',N'-tetrakis(4-
methoxyphenyl)benzidine), HAT-
ON (1,4,5,8,9,11-hexaazatriphenylen-hexacarbonitrile) and/or Spiro-NPD (N,N'-
diphenyl-N,N'-
bis-(1-naphthyl)-9,9'-spirobifluorene-2,7-diamine).
Adjacent to the anode layer A or hole injection layer (HIL) typically a hole
transport layer (HTL)
is located. Herein, any hole transport compound may be used. Exemplarily,
electron-rich
heteroaromatic compounds such as triarylamines and/or carbazoles may be used
as hole
transport compound. The HTL may decrease the energy barrier between the anode
layer A
and the light-emitting layer EML. The hole transport layer (HTL) may also be
an electron
blocking layer (EBL). Preferably, hole transport compounds bear comparably
high energy
levels of their triplet states Ti. Exemplarily the hole transport layer (HTL)
may comprise a star-
shaped heterocycle such as tris(4-carbazoy1-9-ylphenyl)amine (TCTA), poly-TPD
(poly(4-
butylphenyl-diphenyl-amine)), [alpha]-NPD (poly(4-butylphenyl-diphenyl-
amine)), TAPC (4,4'-
cyclohexyliden-bis[N,N-bis(4-methylphenyl)benzenamine]), 2-
TNATA (4,4',4"-tris[2-
naphthyl(phenyl)amino]triphenylamine), Spiro-TAD, DNTPD, NPB, NPNPB, Me0-TPD,
HAT-
ON and/or TrisPcz (9,9'-dipheny1-6-(9-phenyl-9H-carbazol-3-y1)-9H,911-1-3,3'-
bicarbazole). In
addition, the HTL may comprise a p-doped layer, which may be composed of an
inorganic or
organic dopant in an organic hole-transporting matrix. Transition metal oxides
such as
vanadium oxide, molybdenum oxide or tungsten oxide may exemplarily be used as
inorganic
dopant. Tetrafluorotetracyanoquinodimethane (F4-TCNQ), copper-
pentafluorobenzoate
(Cu(l)pFBz) or transition metal complexes may exemplarily be used as organic
dopant.
The EBL may exemplarily comprise mCP (1,3-bis(carbazol-9-yl)benzene), TCTA, 2-
TNATA,
mCBP (3,3-di(9H-carbazol-9-yl)biphenyl), tris-Pcz, CzSi (9-(4-tert-
ButylphenyI)-3,6-
bis(triphenylsily1)-9H-carbazole), and/or DOB (N,N'-dicarbazolyI-1,4-
dimethylbenzene).
Adjacent to the hole transport layer (HTL), typically, the light-emitting
layer EML is located. The
light-emitting layer EML comprises at least one light emitting molecule.
Particular, the EML
comprises at least one light emitting molecule according to the invention.
Typically, the EML
additionally comprises one or more host material. Exemplarily, the host
material is selected
from CBP (4,4'-Bis-(N-carbazolyI)-biphenyl), mCP, mCBP 5if87
(dibenzo[b,d]thiophen-2-
yltriphenylsilane), CzSi, 5if88 (dibenzo[b,d]thiophen-2-yl)diphenylsilane),
DPEPO (bis[2-
(diphenylphosphino)phenyl] ether oxide), 9[3-(dibenzofuran-2-yl)pheny1]-9H-
carbazole, 943-
(d ibenzofuran-2-yl)phenyI]-9 H-carbazole, 943-(d ibenzothiophen-2-yl)phenyI]-
9 H-carbazole,
9[3,5-bis(2-dibenzofuranyl)pheny1]-9H-carbazole, 943,5-bis(2-
dibenzothiophenyl)phenyl]-9H-
CA 03080875 2020-04-29
WO 2019/086668 PCT/EP2018/080179
47
carbazole, T2T (2,4,6-tris(bipheny1-3-y1)-1,3,5-triazine), T3T (2,4,6-
tris(tripheny1-3-y1)-1,3,5-
triazine) and/or TST (2,4,6-tris(9,9'-spirobifluorene-2-yI)-1,3,5-triazine).
The host material
typically should be selected to exhibit first triplet (Ti) and first singlet
(Si) energy levels, which
are energetically higher than the first triplet (Ti) and first singlet (Si)
energy levels of the
organic molecule.
In one embodiment of the invention, the EML comprises a so-called mixed-host
system with
at least one hole-dominant host and one electron-dominant host. In a
particular embodiment,
the EML comprises exactly one light emitting molecule according to the
invention and a mixed-
host system comprising T2T as electron-dominant host and a host selected from
CBP, mCP,
mCBP, 943-(dibenzofuran-2-yl)pheny1]-9H-carbazole, 943-(dibenzofuran-2-
yl)pheny1]-9H-
carbazole, 943-(d ibenzothiophen-2-yl)phenyI]-9 H-carbazole, 9-
[3,5-bis(2-
dibenzofuranyl)pheny1]-9H-carbazole and
-- 943,5-bis(2-dibenzothiophenyl)pheny1]-9H-
carbazole as hole-dominant host. In a further embodiment the EML comprises 50-
80 % by
weight, preferably 60-75 % by weight of a host selected from CBP, mCP, mCBP, 9-
[3-
(dibenzofuran-2-yl)pheny1]-9H-carbazole, 943-(dibenzofuran-2-yl)pheny1]-9H-
carbazole, 943-
(d ibenzoth iophen-2-yl)phenyI]-9 H-carbazole,
943,5-bis(2-dibenzofuranyl)pheny1]-9H-
carbazole and 9[3,5-bis(2-dibenzothiophenyl)pheny1]-9H-carbazole; 10-45 % by
weight,
preferably 15-30 % by weight of T2T and 5-40 % by weight, preferably 10-30 %
by weight of
light emitting molecule according to the invention.
Adjacent to the light-emitting layer EML an electron transport layer (ETL) may
be located.
Herein, any electron transporter may be used. Exemplarily, compounds poor of
electrons such
as, e.g., benzimidazoles, pyridines, triazoles, oxadiazoles (e.g., 1,3,4-
oxadiazole),
phosphinoxides and sulfone, may be used. An electron transporter may also be a
star-shaped
heterocycle such as 1,3,5-tri(1-pheny1-1H-benzo[d]imidazol-2-yl)phenyl (TP6i).
The ETL may
comprise NBphen (2,9-bis(naphthalen-2-y1)-4,7-dipheny1-1,10-phenanthroline),
Alq3
(Aluminum-tris(8-hydroxyquinoline)), TSPO1 (dipheny1-4-triphenylsilylphenyl-
phosphinoxide),
BPyTP2 (2,7-di(2,2'-bipyridin-5-yl)triphenyle), 5if87 (dibenzo[b,d]thiophen-2-
yltriphenylsilane),
5if88 (dibenzo[b,d]thiophen-2-yl)diphenylsilane),
BmPyPhB (1,3-bis[3,5-di(pyridin-3-
yl)phenyl]benzene) and/or BTB (4,4'-bis42-(4,6-dipheny1-1,3,5-triaziny1)]-1,1'-
biphenyl).
Optionally, the ETL may be doped with materials such as Liq. The electron
transport layer
(ETL) may also block holes or a holeblocking layer (HBL) is introduced.
The HBL may, for example, comprise BCP (2,9-dimethy1-4,7-dipheny1-1,10-
phenanthroline =
Bathocuproine), BAlq
(bis(8-hydroxy-2-methylquinoline)-(4-phenylphenoxy)aluminum),
NBphen (2,9-bis(naphthalen-2-y1)-4,7-dipheny1-1,10-phenanthroline), Alq3
(Aluminum-tris(8-
CA 03080875 2020-04-29
WO 2019/086668 PCT/EP2018/080179
48
hydroxyquinoline)), TSPO1 (dipheny1-4-triphenylsilylphenyl-phosphinoxide), T2T
(2,4,6-
tris(bipheny1-3-y1)-1,3,5-triazine), T3T (2,4,6-tris(tripheny1-3-y1)-1,3,5-
triazine), TST (2,4,6-
tris(9,9'-spirobifluorene-2-yI)-1,3,5-triazine), and/or TCB/TCP (1,3,5-tris(N-
carbazolyl)benzol/
1,3,5-tris(carbazol)-9-y1) benzene).
A cathode layer C may be located adjacent to the electron transport layer
(ETL). For example,
the cathode layer C may comprise or may consist of a metal (e.g., Al, Au, Ag,
Pt, Cu, Zn, Ni,
Fe, Pb, LiF, Ca, Ba, Mg, In, W, or Pd) or a metal alloy. For practical
reasons, the cathode layer
may also consist of (essentially) non-transparent metals such as Mg, Ca or Al.
Alternatively or
additionally, the cathode layer C may also comprise graphite and or carbon
nanotubes (CNTs).
Alternatively, the cathode layer C may also consist of nanoscalic silver
wires.
An OLED may further, optionally, comprise a protection layer between the
electron transport
layer (ETL) and the cathode layer C (which may be designated as electron
injection layer
(EIL)). This layer may comprise lithium fluoride, cesium fluoride, silver, Liq
(8-
hydroxyquinolinolatolithium), Li2O, BaF2, MgO and/or NaF.
Optionally, also the electron transport layer (ETL) and/or a hole blocking
layer (HBL) may
comprise one or more host compounds.
In order to modify the emission spectrum and/or the absorption spectrum of the
light-emitting
layer EML further, the light-emitting layer EML may further comprise one or
more further emitter
molecule F. Such an emitter molecule F may be any emitter molecule known in
the art.
Preferably such an emitter molecule F is a molecule with a structure differing
from the structure
of the molecules according to the invention. The emitter molecule F may
optionally be a TADF
emitter. Alternatively, the emitter molecule F may optionally be a fluorescent
and/or
phosphorescent emitter molecule which is able to shift the emission spectrum
and/or the
absorption spectrum of the light-emitting layer EML. Exemplarily, the triplet
and/or singlet
excitons may be transferred from the emitter molecule according to the
invention to the emitter
molecule F before relaxing to the ground state SO by emitting light typically
red-shifted in
comparison to the light emitted by emitter molecule E. Optionally, the emitter
molecule F may
also provoke two-photon effects (i.e., the absorption of two photons of half
the energy of the
absorption maximum).
Optionally, an optoelectronic device (e.g., an OLED) may exemplarily be an
essentially white
optoelectronic device. Exemplarily such white optoelectronic device may
comprise at least one
(deep) blue emitter molecule and one or more emitter molecules emitting green
and/or red
CA 03080875 2020-04-29
WO 2019/086668 PCT/EP2018/080179
49
light. Then, there may also optionally be energy transmittance between two or
more molecules
as described above.
As used herein, if not defined more specifically in the particular context,
the designation of the
colors of emitted and/or absorbed light is as follows:
violet: wavelength range of >380-420 nm;
deep blue: wavelength range of >420-480 nm;
sky blue: wavelength range of >480-500 nm;
green: wavelength range of >500-560 nm;
yellow: wavelength range of >560-580 nm;
orange: wavelength range of >580-620 nm;
red: wavelength range of >620-800 nm.
With respect to emitter molecules, such colors refer to the emission maximum.
Therefore,
exemplarily, a deep blue emitter has an emission maximum in the range of from
>420 to
480 nm, a sky-blue emitter has an emission maximum in the range of from >480
to 500 nm, a
green emitter has an emission maximum in a range of from >500 to 560 nm, a red
emitter has
an emission maximum in a range of from >620 to 800 nm.
A further embodiment of the present invention relates to an OLED, which emits
light with ClEx
and ClEy color coordinates close to the ClEx (= 0.131) and ClEy (= 0.046)
color coordinates
of the primary color blue (ClEx = 0.131 and ClEy = 0.046) as defined by ITU-R
Recommendation BT.2020 (Rec. 2020) and thus is suited for the use in Ultra
High Definition
(UHD) displays, e.g. UHD-TVs. In this context, the term "close to" refers to
the ranges of ClEx
and ClEy coordinates provided at the end of this paragraph. In commercial
applications,
typically top-emitting (top-electrode is transparent) devices are used,
whereas test devices as
described throughout the present application represent bottom-emitting devices
(bottom-
electrode and substrate are transparent). The ClEy color coordinate of a blue
device can be
reduced by up to a factor of two, when changing from a bottom- to a top-
emitting device, while
the ClEx remains nearly unchanged (Okinaka et al. doi:10.1002/sdtp.10480).
Accordingly, a
further embodiment of the present invention relates to an OLED, whose emission
exhibits a
ClEx color coordinate of between 0.02 and 0.30, preferably between 0.03 and
0.25, more
preferably between 0.05 and 0.20 or even more preferably between 0.08 and 0.18
or even
between 0.10 and 0.15 and/ or a ClEy color coordinate of between 0.00 and
0.45, preferably
between 0.01 and 0.30, more preferably between 0.02 and 0.20 or even more
preferably
between 0.03 and 0.15 or even between 0.04 and 0.10.
CA 03080875 2020-04-29
WO 2019/086668 PCT/EP2018/080179
A further embodiment of the present invention relates to an OLED, which emits
light with ClEx
and ClEy color coordinates close to the ClEx (= 0.170) and ClEy (= 0.797)
color coordinates
of the primary color green (ClEx = 0.170 and ClEy = 0.797) as defined by ITU-R
Recommendation BT.2020 (Rec. 2020) and thus is suited for the use in Ultra
High Definition
(UHD) displays, e.g. UHD-TVs. In this context, the term "close to" refers to
the ranges of ClEx
and ClEy coordinates provided at the end of this paragraph. In commercial
applications,
typically top-emitting (top-electrode is transparent) devices are used,
whereas test devices as
used throughout the present application represent bottom-emitting devices
(bottom-electrode
and substrate are transparent). The ClEy color coordinate of a blue device can
be reduced by
up to a factor of two, when changing from a bottom- to a top-emitting device,
while the ClEx
remains nearly unchanged (Okinaka et al. doi:10.1002/sdtp.10480). Accordingly,
a further
aspect of the present invention relates to an OLED, whose emission exhibits a
ClEx color
coordinate of between 0.06 and 0.34, preferably between 0.07 and 0.29, more
preferably
between 0.09 and 0.24 or even more preferably between 0.12 and 0.22 or even
between 0.14
and 0.19 and/ or a ClEy color coordinate of between 0.75 and 1.20, preferably
between 0.76
and 1.05, more preferably between 0.77 and 0.95 or even more preferably
between 0.78 and
0.90 or even between 0.79 and 0.85.
A further embodiment of the present invention relates to an OLED, which emits
light with ClEx
and ClEy color coordinates close to the ClEx (= 0.708) and ClEy (= 0.292)
color coordinates
of the primary color red (ClEx = 0.708 and ClEy = 0.292) as defined by ITU-R
Recommendation BT.2020 (Rec. 2020) and thus is suited for the use in Ultra
High Definition
(UHD) displays, e.g. UHD-TVs. In this context, the term "close to" refers to
the ranges of ClEx
and ClEy coordinates provided at the end of this paragraph. In commercial
applications,
typically top-emitting (top-electrode is transparent) devices are used,
whereas test devices as
used throughout the present application represent bottom-emitting devices
(bottom-electrode
and substrate are transparent). The ClEy color coordinate of a blue device can
be reduced by
up to a factor of two, when changing from a bottom- to a top-emitting device,
while the ClEx
remains nearly unchanged (Okinaka et al. doi:10.1002/sdtp.10480). Accordingly,
a further
aspect of the present invention relates to an OLED, whose emission exhibits a
ClEx color
coordinate of between 0.60 and 0.88, preferably between 0.61 and 0.83, more
preferably
between 0.63 and 0.78 or even more preferably between 0.66 and 0.76 or even
between 0.68
and 0.73 and/ or a ClEy color coordinate of between 0.25 and 0.70, preferably
between 0.26
and 0.55, more preferably between 0.27 and 0.45 or even more preferably
between 0.28 and
0.40 or even between 0.29 and 0.35.
Accordingly, a further aspect of the present invention relates to an OLED,
which exhibits an
external quantum efficiency at 1000 cd/m2 of more than 8%, more preferably of
more than
CA 03080875 2020-04-29
WO 2019/086668 PCT/EP2018/080179
51
10%, more preferably of more than 13%, even more preferably of more than 15%
or even more
than 20% and/or exhibits an emission maximum between 420 nm and 500 nm,
preferably
between 430 nm and 490 nm, more preferably between 440 nm and 480 nm, even
more
preferably between 450 nm and 470 nm and/or exhibits a LT80 value at 500 cd/m2
of more
than 100 h, preferably more than 200 h, more preferably more than 400 h, even
more
preferably more than 750 h or even more than 1000 h.
The optoelectronic device, in particular the OLED according to the present
invention can be
produced by any means of vapor deposition and/ or liquid processing.
Accordingly, at least
one layer is
- prepared by means of a sublimation process,
- prepared by means of an organic vapor phase deposition process,
- prepared by means of a carrier gas sublimation process,
- solution processed or printed.
The methods used to produce the optoelectronic device, in particular the OLED
according to
the present invention are known in the art. The different layers are
individually and successively
deposited on a suitable substrate by means of subsequent deposition processes.
The
individual layers may be deposited using the same or differing deposition
methods.
Vapor deposition processes exemplarily comprise thermal (co)evaporation,
chemical vapor
deposition and physical vapor deposition. For active matrix OLED display, an
AMOLED
backplane is used as substrate. The individual layer may be processed from
solutions or
dispersions employing adequate solvents. Solution deposition process
exemplarily comprise
spin coating, dip coating and jet printing. Liquid processing may optionally
be carried out in an
inert atmosphere (e.g., in a nitrogen atmosphere) and the solvent may
optionally be completely
or partially removed by means known in the state of the art.
CA 03080875 2020-04-29
WO 2019/086668
PCT/EP2018/080179
52
Examples
General synthesis scheme I
coci RH
RH Br RH RH
RH 0 RH RH Br
RH
CN F N ' N RI
RI F 1) SbC15, DCM, rt, 4 h
RI I RI
2) NH3 aq. N
RI RI RI RI F RI
RI RI RI
RH
0õ0 HO _OH RH RH
B B RáCJ
ii
R11 R15 R11 so R15 Ri5 Ri3
Riz
or RI,
R12 R14 R12 R14
R13 R13 R14
Pd2(dpa)3, SPhos F N ' N RI
_________________________ VP- RI I RI
N
Toluene/water 10:1, K3PO4
RI RI F RI
RI RI
RH
Ra
Da Rii Rii
Ri 1
Ra
010 ''
Ra Ra Ri2
Ra 0 Z 0 Ra Z Ra Rii
N R15 R13
Ra N Ra Ra . R14
H
Ra Ra Ra
Ra N ' N RI
Cs2CO3 Ra RI I
N RI
DMF, 150 C
RI RI RI
RI RI
Ra
Ra Ra
Ra * N fik Ra
Z
Ra Ra
Ra
General procedure for synthesis AAV1:
COCI
0 Br
0
CN Br
0 F 1) SbC15, DCM, it, 4 h
F N N
2) NH3 aq.
Ili- I
40 N 0
El F
CA 03080875 2020-04-29
WO 2019/086668 PCT/EP2018/080179
53
2-Fluorobenzonitrile (2.00 equivalents) and 2-bromobenzoyl chloride (1.00
equivalents) are
dissolved in dichloromethane and cooled in an ice-bath. Antimony(V)-chloride
(1.00
equivalents) is added dropwise to the solution and the mixture is stirred at
room temperature
(rt) for 1 hour and subsequently at 45 C for 6 hours. The product is filtered
and washed with
dichloromethane.
The dried solid is added to a cooled 25%-ammonia solution (0 ¨ 5 C) and
stirred overnight
at rt. The mixture is filtered. The collected solid is washed with water. The
solid is added to
DMF and stirred at 155 C for 30 min. The insoluble solid was separated by hot
filtration. Pure
water was added to the hot DMF-solution to precipitate the product. The solid
product was
separated by filtration.
General procedure for synthesis AAV2:
COCI
0 OBI
Br
F N N Pd2(dpa)3, SPhos F -- N -- N
40/ N 40 Toluene/water 10:1, K3PO4 . N 40
El F ZI F
El (1 equivalent), phenylboronic acid (1.5 equivalents), potassium phosphate
tribasic
(4 equivalents) and the catalyst-system are suspended under nitrogen
atmosphere in toluene
/ water (10:1) and stirred at 110 C for 24 h. After chilling to rt the
reaction mixture is poured
into water and extracted with DCM. The organic layer was washed with water,
dried with
Na2SO4 and filtrated. Solvents were removed and the product was purified with
a filtercolumn.
General procedure for synthesis AAV3:
Ra
Ra
Ra Ra 40 Ra
Ra 0 z 0 Ra Z
Ra Ra
Ra N Ra * N
H N \ N
Ra Ra Ra 1 ,
CS2CO3 Ra I.
Ra N- I.
F N N _____________ lb- Ra
1
/
0 N 0
zi F DMF, 150 C Ra a
Ra * N R
. Ra
Z
Ra Ra
Ra
Zi (1 equivalent each), the corresponding donor molecule D-H (2.0 0
equivalents) and cesium
carbonate (6.00 equivalents) are suspended under nitrogen atmosphere in DMF
and stirred at
CA 03080875 2020-04-29
WO 2019/086668 PCT/EP2018/080179
54
150 C (7 days). After chilling to rt the reaction mixture is poured into
water in order to
precipitate the organics. The precipitate is filtered off (fiber glass filter)
and subsequently
washed with water.
In particular, the donor molecule D-H is a 3,6-substituted carbazole (e.g.,
3,6-
dimethylcarbazole, 3,6-diphenylcarbazole, 3,6-di-tert-butylcarbazole), a 2,7-
substituted
carbazole (e.g., 2,7-dimethylcarbazole, 2,7-diphenylcarbazole, 2,7-di-tert-
butylcarbazole), a
1,8-substituted carbazole (e.g., 1,8-dimethylcarbazole, 1,8-diphenylcarbazole,
1,8-di-tert-
butylcarbazole), a 1-substituted carbazole (e.g., 1-methylcarbazole, 1-
phenylcarbazole, 1-tert-
butylcarbazole), a 2-substituted carbazole (e.g., 2-methylcarbazole, 2-
phenylcarbazole, 2-tert-
butylcarbazole), or a 3-substituted carbazole (e.g., 3-methylcarbazole, 3-
phenylcarbazole, 3-
tert-butylcarbazole).
Exemplarily a halogen-substituted carbazole, particularly 3-bromocarbazole,
can be used as
D-H.
In a subsequent reaction a boronic acid ester functional group or boronic acid
functional group
may be exemplarily introduced at the position of the one or more halogen
substituents, which
was introduced via D-H, to yield the corresponding carbazol-3-ylboronic acid
ester or carbazol-
3-ylboronic acid, e.g., via the reaction with bis(pinacolato)diboron (CAS No.
73183-34-3).
Subsequently, one or more substituents Ra may be introduced in place of the
boronic acid
ester group or the boronic acid group via a coupling reaction with the
corresponding
halogenated reactant Ra-Hal, preferably Ra-CI and Ra-Br.
Alternatively, one or more substituents Ra may be introduced at the position
of the one or more
halogen substituents, which was introduced via D-H, via the reaction with a
boronic acid of the
substituent Ra [Ra-B(OH)2] or a corresponding boronic acid ester.
HPLC-MS:
HPLC-MS spectroscopy is performed on a HPLC by Agilent (1100 series) with MS-
detector
(Thermo LTQ XL). A reverse phase column 4,6mm x 150mm, particle size 5,0 pm
from Waters
(without pre-column) is used in the HPLC. The HPLC-MS measurements are
performed at
room temperature (rt) with the solvents acetonitrile, water and THF in the
following
concentrations:
solvent A: H20 (90%) MeCN (10%)
solvent B: H20 (10%) MeCN (90%)
solvent C: THF (100%)
From a solution with a concentration of 0.5mg/m1 an injection volume of 15 pL
is taken for the
measurements. The following gradient is used:
CA 03080875 2020-04-29
WO 2019/086668
PCT/EP2018/080179
Flow rate [ml/min] time [min] A[%] B[%] D[%]
3 0 40 50 10
3 10 10 15 75
3 16 10 15 75
3 16.01 40 50 10
3 20 40 50 10
Ionisation of the probe is performed by APCI (atmospheric pressure chemical
ionization).
Cyclic voltammetty
Cyclic voltammograms are measured from solutions having concentration of 10-3
mo1/1 of the
organic molecules in dichloromethane or a suitable solvent and a suitable
supporting
electrolyte (e.g. 0.1 mo1/1 of tetrabutylammonium hexafluorophosphate). The
measurements
are conducted at room temperature under nitrogen atmosphere with a three-
electrode
assembly (Working and counter electrodes: Pt wire, reference electrode: Pt
wire) and
calibrated using FeCp2/FeCp2+ as internal standard. The HOMO data was
corrected using
ferrocene as internal standard against a saturated calomel electrode (SCE).
Density functional theory calculation
Molecular structures are optimized employing the BP86 functional and the
resolution of identity
approach (RI). Excitation energies are calculated using the (BP86) optimized
structures
employing Time-Dependent DFT (TD-DFT) methods. Orbital and excited state
energies are
calculated with the B3LYP functional. Def2-SVP basis sets (and a m4-grid for
numerical
integration are used. The Turbomole program package is used for all
calculations.
Photophysical measurements
Sample pretreatment: Spin-coating
Apparatus: Spin150, SPS euro.
The sample concentration is 10 mg/ml, dissolved in a suitable solvent.
Program: 1) 3s at 400 U/min; 20s at 1000 U/min at 1000 Upm/s. 3) 10 s at 4000
U/min at
1000 Upm/s. After coating, the films are tried at 70 C for 1 min.
Photoluminescence spectroscopy and TCSPC (Time-correlated single-photon
counting)
Steady-state emission spectroscopy is measured by a Horiba Scientific, Modell
FluoroMax-4
equipped with a 150 W Xenon-Arc lamp, excitation- and emissions monochromators
and a
Hamamatsu R928 photomultiplier and a time-correlated single-photon counting
option.
Emissions and excitation spectra are corrected using standard correction fits.
CA 03080875 2020-04-29
WO 2019/086668 PCT/EP2018/080179
56
Excited state lifetimes are determined employing the same system using the
TCSPC method
with FM-2013 equipment and a Horiba Yvon TCSPC hub.
Excitation sources:
NanoLED 370 (wavelength: 371 nm, puls duration: 1,1 ns)
NanoLED 290 (wavelength: 294 nm, puls duration: <1 ns)
SpectraLED 310 (wavelength: 314 nm)
SpectraLED 355 (wavelength: 355 nm).
Data analysis (exponential fit) is done using the software suite DataStation
and DAS6 analysis
software. The fit is specified using the chi-squared-test.
Photoluminescence quantum yield measurements
For photoluminescence quantum yield (PLQY) measurements an Absolute PL Quantum
Yield
Measurement C9920-03G system (Hamamatsu Photonics) is used. Quantum yields and
CIE
coordinates are determined using the software U6039-05 version 3.6Ø
Emission maxima are given in nm, quantum yields (I) in `)/0 and CIE
coordinates as x,y values.
PLQY is determined using the following protocol:
1) Quality assurance: Anthracene in ethanol (known concentration) is used as
reference
2) Excitation wavelength: the absorption maximum of the organic molecule is
determined
and the molecule is excited using this wavelength
3) Measurement
Quantum yields are measured for sample of solutions or films under nitrogen
atmosphere. The yield is calculated using the equation:
A
"
nphoton, emited f ¨ [int7,t
p
hc e m t el e sample
d (A) ¨ Int aõ07- b ed (A)1dA
OPL =
nphoton, absorbed A
[intreference
nC emitted
) Intreference 1
absorbed (A)idA
wherein nphoton denotes the photon count and Int. the intensity.
Production and characterization of optoelectronic devices
OLED devices comprising organic molecules according to the invention can be
produced via
vacuum-deposition methods. If a layer contains more than one compound, the
weight-
percentage of one or more compounds is given in %. The total weight-percentage
values
amount to 100 %, thus if a value is not given, the fraction of this compound
equals to the
difference between the given values and 100%.
The not fully optimized OLEDs are characterized using standard methods and
measuring
electroluminescence spectra, the external quantum efficiency (in %) in
dependency on the
intensity, calculated using the light detected by the photodiode, and the
current. The OLED
device lifetime is extracted from the change of the luminance during operation
at constant
current density. The LT50 value corresponds to the time, where the measured
luminance
CA 03080875 2020-04-29
WO 2019/086668 PCT/EP2018/080179
57
decreased to 50 % of the initial luminance, analogously LT80 corresponds to
the time point, at
which the measured luminance decreased to 80 % of the initial luminance, LT 95
to the time
point, at which the measured luminance decreased to 95 % of the initial
luminance etc.
Accelerated lifetime measurements are performed (e.g. applying increased
current densities).
Exemplarily LT80 values at 500 cd/m2 are determined using the following
equation:
cd2 ( 1.6
Lo
LT80 (500 ¨2) = LT80(4) _________________________ 2
in cd
\ 500 j
wherein Lo denotes the initial luminance at the applied current density.
The values correspond to the average of several pixels (typically two to
eight), the standard
deviation between these pixels is given.
CA 03080875 2020-04-29
WO 2019/086668 PCT/EP2018/080179
58
Additional Examples of Organic Molecules of the Invention
N 401
-- N
N N "N N
I
N -- N \ N/ .
411111 N
N I N
N .
N
N
N 110 I
N ".. N = i .
N I
N
1 N N
N
N
40 4.
*
N N
/ \
- N NNN
N\ /
N IIP N
N
N
N
N
Ph
N 1N
101\10
ilt
N Ili
N 1411
N
11
N \ i N
N I
N N N
P h
N
N
N ' N
N
CA 03080875 2020-04-29
WO 2019/086668 PCT/EP2018/080179
59
Ph
--- N N NC
N ,- N ' N
N N
1 / . Ph I N N I N N-... N r
Ph
0 0
N
* Ph Ph Ph Ph N
N
Ph
*
NC
CN CN
N
N -- N
' N 1 z *
N I N
Nr N 411 NC N
,-
NC I N N
N-. N * NC CN
N
CF3
CN CN
CF3
N
NC NC
*
N 0
N
NS
,-
N I
* *
N , N N N
1
N / N
" I
Ils,, N N
QN
N
N Ph
110 0 CF3 Ph CF3
N
*
N
N N ==== N
I /
* N
N
N * N
* N
N
CA 03080875 2020-04-29
WO 2019/086668 PCT/EP2018/080179
NN
N\1'
N
0 N
I N N I
,
0 N
N
*
0
0 N N
,
N I
N N
-N
*
N
\ /
N
N
N
0 N NC
NC0
,
N I
,
N I
0
N N
N N
CF3 * N
* CN
N NC
/ \
F3C N
- N
NC CN
N
0 N
N 0 CF3 NC 0 NC CN
I N NC CN
N , N
CF3 0 N
N
* / N
N \
-N
CN
CA 03080875 2020-04-29
WO 2019/086668 PCT/EP2018/080179
61
CN CN
rc * = #
N
N N
\
* CN
*
N
0 N
N 0
N . CN
I
* N
1 N
N N * CN
N*
CN
* *
CN
CN NC N
CN CN
N-
#
N
110 IV N
1 * 1110 CN
NC = N N
* CN
N
0 *
0 CN
NC CN NC
NC
CN NC
* CN
NC N
N
N ' N
1 N
*
N NC
" / N N N . N
N- 0
CN--Si
CN
N /
\
N
CN
N
*
N
NC C
NC
CN
NC N
CN
0 N
0
N CN N ' N
1
1 N
N N N
CN NC
NC N
. )--CN
CN
CA 03080875 2020-04-29
WO 2019/086668 PCT/EP2018/080179
62
0
N ' N
l\r 0
441 \ N
/ N * 0
N N
N
0 N N
0
---
N I
N ... N
*
N._
\ N 40
N /
N =
N
N *
NLN
I
1\r
N ' N
#N
I N
0 0 l\r
NO* *
* *
* * N
N
N * N-
\
= / N N
\ N
N
...- N
F3C N IN N
F3C CF3
N
F3C
CA 03080875 2020-04-29
WO 2019/086668
PCT/EP2018/080179
63
NC Ph
CN )/-N
N \
NC )=1\I
CN
Ph N N \ N
/
NC N * N
N
CN
N ".= N N *
I
N--4
-
Ph -N
(N---- N
CN * N)_N /)-Ph
Ph--
\\ N Ph NC
N /
N Ph
N N
N ----
N Ph \
-N N N N
\r-
N N N II
)\--I\?---- NI\r
Ni ----
N \ _N
N
*
N /
N- \
Ph
* / N
N \
0 ) N N
N \ N * N
Ph I
0 N
N N N
N -- N
Ph N 'Ph
_N
N ---
CF3
*
-N
N N
\ / * N
N N
0
N
CF3. / \ N
N- N
* N
N I
N ,N
F3C F3C
0 N