Note: Descriptions are shown in the official language in which they were submitted.
CA 03082828 2020-05-14
WO 2019/100026 PCT/US2018/061874
HEIN ELECTRO-POLARIZABLE COMPOUND AND CAPACITOR THEREOF
CLAIM OF PRIORITY
This application claims the priority benefit of U.S. Patent Application Number
15/870,504 filed January 12, 2018, the entire contents of which are
incorporated herein by
reference. U.S. Patent Application Number 15/870,504 is a continuation-in-part
of U.S. Patent
Application Number 15/090,509 filed April 4, 2016, U.S. Patent Application
Number
15/163,595 filed May 24, 2016, and U.S. Patent Application Number 15/818,474
filed November
20, 2017, the entire contents of which are incorporated herein by reference.
FIELD OF THE INVENTION
The present disclosure relates generally to passive components of electrical
circuit and
more particularly to an electro-polarizable compound and capacitor based on
this material and
intended for energy storage.
BACKGROUND
A capacitor is a passive electronic component that is used to store energy in
the form of an
electrostatic field, and comprises a pair of electrodes separated by a
dielectric layer. When a
potential difference exists between the two electrodes, an electric field is
present in the dielectric
layer. An ideal capacitor is characterized by a single constant value of
capacitance, which is a
ratio of the electric charge on each electrode to the potential difference
between them. For high
voltage applications, much larger capacitors are necessary.
One important characteristic of a dielectric material is its breakdown field.
The
breakdown field corresponds to the value of electric field strength at which
the material suffers a
catastrophic failure and conducts electricity between the electrodes. For most
capacitor
geometries, the electric field in the dielectric can be approximated by the
voltage between the
two electrodes divided by the spacing between the electrodes, which is usually
the thickness of
the dielectric layer. Since the thickness is usually constant it is more
common to refer to a
breakdown voltage, rather than a breakdown field. There are a number of
factors that can
dramatically reduce the breakdown voltage. In particular, the geometry of the
conductive
electrodes is important factor affecting breakdown voltage for capacitor
applications. In
1
CA 03082828 2020-05-14
WO 2019/100026 PCT/US2018/061874
particular, sharp edges or points hugely increase the electric field strength
locally and can lead to
a local breakdown. Once a local breakdown starts at any point, the breakdown
will quickly
"trace" through the dielectric layer until it reaches the opposite electrode
and causes a short
circuit.
Breakdown of the dielectric layer usually occurs as follows. Intensity of an
electric field
becomes high enough to "pull" electrons from atoms of the dielectric material
and makes them
conduct an electric current from one electrode to another. Presence of
impurities in the dielectric
or imperfections of the crystal structure can result in an avalanche breakdown
as observed in
semiconductor devices.
Another important characteristic of a dielectric material is its dielectric
permittivity.
Different types of dielectric materials are used for capacitors and include
ceramics, polymer film,
paper, and electrolytic capacitors of different kinds. The most widely used
polymer film
materials are polypropylene and polyester. Increasing dielectric permittivity
allows for
increasing volumetric energy density, which makes it an important technical
task.
Second-order nonlinear optical (NLO) effects of organic molecules have been
extensively
investigated for their advantages over inorganic crystals. Properties studied,
for example, include
their large optical non-linearity, ultra-fast response speed, high damage
thresholds and low
absorption loss, etc. Particularly, organic thin films with excellent optical
properties have
tremendous potential in integrated optics such as optical switching, data
manipulation and
information processing. Among organic NLO molecules, azo-dye chromophores have
been a
special interest to many investigators because of their relatively large
molecular hyper-
polarizability (b) due to delocalization of the p-electronic clouds. They were
most frequently
either incorporated as a guest in the polymeric matrix (guest¨host polymers)
or grafted into the
polymeric matrix (functionalized polymers) over the past decade.
Hyper-electronic polarization of organic compounds is described in greater
detail in Roger
D. Hartman and Herbert A. Pohl, "Hyper-electronic Polarization in
Macromolecular Solids",
Journal of Polymer Science: Part A-1 Vol. 6, pp. 1135-1152 (1968). Hyper-
electronic
polarization may be viewed as the electrical polarization external fields due
to the pliant
interaction with the charge pairs of excitons, in which the charges are
molecularly separated and
range over molecularly limited domains. In this article four polyacene quinone
radical polymers
2
CA 03082828 2020-05-14
WO 2019/100026 PCT/US2018/061874
were investigated. These polymers at 100 Hz had dielectric constants of 1800-
2400, decreasing
to about 58-100 at 100,000 Hz. Essential drawback of the described method of
production of
material is use of a high pressure (up to 20 kbars) for forming the samples
intended for
measurement of dielectric constants.
SUMMARY
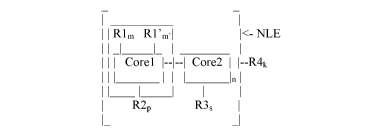
The present disclosure provides an electro-polarizable compound having the
following
general formula (I):
R1 R1'
4.- NLE
,
- m - m
Corel _________________________________ Core2 R4 ____________ (I)
R2 R3
Corel is an aromatic polycyclic conjugated molecule having two-dimensional
flat form and self-
assembles into supramolecular structures. R1 are electron donor groups
connected to the
aromatic polycyclic conjugated molecule (Corel) and R1' are electron acceptor
groups
connected to the aromatic polycyclic conjugated molecule (Corel), m is number
of acceptor
groups R1, m' is a number of donor groups R1', m and m' are equal to 0, 1, 2,
3, 4, 5 or 6,
wherein m and m' are not both equal to 0, R2 is a substituent comprising one
or more ionic
groups from a class of ionic compounds that are used in ionic liquids
connected to the aromatic
polycyclic conjugated molecule (Corel) directly or via a connecting group, p
is number of ionic
groups R2 which is equal to 0, 1, 2, 3 or 4. The fragment marked NLE
containing the Corel
with at least one group R1 and/or R1' has a nonlinear effect of polarization.
Core2 is an electro-conductive oligomer and number n of the electro-conductive
oligomers is
equal to 0, 2, or 4. R3 is a substituent comprising one or more ionic groups
from a class of ionic
3
CA 03082828 2020-05-14
WO 2019/100026 PCT/US2018/061874
compounds that are used in ionic liquids connected to the electro-conductive
oligomer (Core2)
directly or via a connecting group, s is number of the ionic groups R3 which
is equal to 0, 1, 2, 3
or 4.
R4 is a resistive substituent connected to the aromatic polycyclic conjugated
molecule (Corel)
and/or to the electro-conductive oligomer (Core2) directly or via a connecting
group. The
resistive substituent R4 provides solubility of the organic compound in a
solvent and electrically
insulates the supramolecular structures from each other and. The parameter k
is a number of
substituents R4, which is equal to 1, 2, 3, 4, 5, 6, 7 or 8.
In one aspect, the present disclosure provides a solution comprising an
organic solvent and
at least one disclosed electro-polarizable compound.
In another aspect, the present disclosure provides a crystal metadielectric
layer comprising a
mixture of the electro-polarizable compounds as disclosed above. The
nonlinearly polarizable
fragments comprising an aromatic polycyclic conjugated molecule with at least
one group R1 are
placed into the resistive dielectric envelope formed by resistive substituents
R4 providing
solubility of the organic compound in a solvent and electrically insulating
the supramolecular
structures, such as supramolecular columns, from each other.
In still another aspect, the present disclosure provides a meta-capacitor
comprising two
metal electrodes positioned parallel to each other and which can be rolled or
flat and planar with
said metadielectric layer between said electrodes, wherein the metadielectric
layer comprises one
or more types of the disclosed electro-polarizable. The nonlinearly
polarizable fragments
comprising an aromatic polycyclic conjugated molecule with at least one group
R1, the electro-
conductive oligomers and the ionic groups which have electronic and/or ionic
type of
polarizability are placed into the resistive dielectric envelope formed by
resistive substituents
providing solubility of the organic compound in a solvent and electrically
insulating the
supramolecular structures from each other.
INCORPORATION BY REFERENCE
4
CA 03082828 2020-05-14
WO 2019/100026 PCT/US2018/061874
All publications, patents, and patent applications mentioned in this
specification are
herein incorporated by reference to the same extent as if each individual
publication, patent, or
patent application was specifically and individually indicated to be
incorporated by reference.
BRIEF DESCRIPTION OF THE DRAWING
Figure 1A schematically shows a capacitor with flat and planar electrodes in
accordance with an
aspect of the present disclosure.
Figure 1B schematically shows a capacitor with rolled (circular) electrodes in
accordance with
another aspect of the present disclosure.
Figure 2 shows a chemical formula that illustrates possible variations on a
structure referred to as
a rylene fragment that may be included in a Hein Electro-Polarizable compound
in accordance
with aspects of the present disclosure.
DETAILED DESCRIPTION
While various embodiments of the invention have been shown and described
herein, it will
be obvious to those skilled in the art that such embodiments are provided by
way of example
only. Numerous variations, changes, and substitutions may occur to those
skilled in the art
without departing from the invention. It should be understood that various
alternatives to the
embodiments of the invention described herein may be employed.
The present disclosure provides an electro-polarizable compound. The existence
of the
electrophilic groups (acceptors) and the nucleophilic groups (donors) in an
aromatic polycyclic
conjugated molecule (Corel) promotes non-uniform distribution of electronic
density in the
conjugated molecule: surplus of electrons in one place (in a donor zone) and a
shortage of
electrons in other place (in an acceptor zone). The influence of external
electric field onto non-
uniform distribution of electronic density along the conjugated molecule leads
to the induced
polarization Pind. In the general case the induced polarization is nonlinear
function of intensity of
local electric field Eloc. In the assumption of weak nonlinearity when it is
possible to be limited
to several members of decomposition of an induced polarization into a series
on degrees of
intensity of a local electric field, the induced polarization of the
environment (of molecule) can
be written down in the following form:
CA 03082828 2020-05-14
WO 2019/100026
PCT/US2018/061874
-E, 2
Pind = Eloc I3= E,i _Loc = = = ,
where Gt- linear polarizability, 13 _ square polarizability. Though the
assumption of a smallness of
electric field is not always right, nevertheless parameters a and f3 can be
used for qualitative
analysis of polarizability of the disclosed compounds. In the present
disclosure the main attention
is paid to ways of increase in the induced polarization of the disclosed
compounds and therefore
onto ways of increase of the linear polarizability a and square polarizability
ft Such attention is
caused by that the constant dipole and quadrupole electrical moments are
mutually neutralized at
self-assembly of such conjugated molecules. Analysis shows that linear
polarizability depends on
the size of the average electronic density in the molecule, and nonlinear
polarizability depends
on the size of heterogeneity of electronic density. It is also shown that a
non-centrosymmetric
arrangement of the electron donor and acceptor groups can lead to a strong
nonlinear response of
the compound's electronic polarization in the presence of an electric field.
Influence of chemical
structure on linear polarizability a and square polarizability 0 is shown in
Table 1 below.
Tablet
chemical structure a 13
(au.)
(a.u.)
0 0
N N 945
0.041
0 0
N?SJ 0
1348
0.165
\
6
CA 03082828 2020-05-14
WO 2019/100026
PCT/US2018/061874
0 N /
1537
N N 862
NO2
/
02 N N 0
H2N
0 /NI io
1252
N 21107
0 1\1/
NO2
N 0
0 N
/
H2N 1908
N 40221
0 NI/
NO2
N 0
0
/N 0 NH2
H2N 1431
N 35189
NI
NO2
02N0 N/ 0
H2N
02N 0 N NH2
/
4604
N N 1002570
/
N 0 NH2
02N NO2
7
CA 03082828 2020-05-14
WO 2019/100026 PCT/US2018/061874
An essential feature of the present disclosure is use of rigid non-conjugated
limit carbon
structures as resistive substituents. Such structures distinguish from the
dielectric structures
formed by "fat" tails (such as alkyl, aryl, substituted alkyl, substituted
aryl, fluorinated alkyl,
chlorinated alkyl, branched and complex alkyl) which can be bent (curved) and
lead to stochastic
distribution of electronic density in the dielectric structure that leads to
its electric breakdown.
Thus, as resistive substituent R4 is preferably a non-conjugated compound that
minimizes or
does not contain voids/empty space; that have dense packing of 5P3 carbon with
H and F
substitutes. Otherwise use of fat tails may lead to formation of a friable
dielectric structure (film,
layer, and envelope). It is possible that in friable structure that there will
be a local area ("hole")
in which electronic density is equal to zero and which can be occupied with a
free electron (that
leads to electric breakdown). It is possible to enter a concept of a molecular
hole when one
molecule "is taken out" from the ordered structure (from a crystal lattice).
In this case the
quantum object (a quantum hole, a quantum point) is formed in which there are
empty (non-
occupied) energy levels. Set of such objects creates a condition for
conductivity of electrons and
for electric breakdown of dielectric structure.
Resistive substituents are preferentially selected from single and branched
chains between 5
and 13 carbon-carbons in length in one direction and non-conjugated fused
carbo-cyclic chains
greater than 3 rings in length in one direction.
The presence of the electro-conductive oligomers leads to increasing
polarizability of the
disclosed electro-polarizable compound because of electronic super
conductivity of the electro-
conductive oligomers. Ionic groups increase an ionic component of polarization
of the disclosed
electro-polarizable compound. The nonlinearly polarizable fragments comprising
an aromatic
polycyclic conjugated molecule with at least one dopant group, the electro-
conductive oligomers
and the ionic groups are placed into the resistive dielectric envelope formed
by resistive
substituents providing solubility of the organic compound in a solvent and
electrically insulating
the supramolecular structures from each other. The resistive substituents
increase the electric
strength of these electro-polarizable compounds and breakdown voltage of the
dielectric layers
made on their basis.
In some implementations, among others, the aromatic polycyclic conjugated
molecule
(Corel) may comprise rylene fragments, which may be in conjugation with phenyl
amides,
naphthalene amides, and/or anthracene amides. In another embodiment of the
disclosed electro-
8
CA 03082828 2020-05-14
WO 2019/100026 PCT/US2018/061874
polarizable compound, the rylene fragments are selected from structures from 1
to 17 as given in
Table 2.
Table 2. Examples of the aromatic polycyclic conjugated molecule comprising
rylene fragments
NN 0
1
\N
0
2
0 0
0
3
0
0 0
N N 4
0
0 0
0
6
0
9
CA 03082828 2020-05-14
WO 2019/100026
PCT/US2018/061874
N N
\ /
N N 7
o 0
n
0 0
0 N/ NH 8
N 0
n
O 0
HN NH 9
O 0
n
O 0
II. N N * 10
O 0
n
O 0
4i N NH 11
O 0
n
N N
\ /
N N 12
O 0
n
N 0
\
N N 13
\
0 N
n
CA 03082828 2020-05-14
WO 2019/100026 PCT/US2018/061874
14
0 0
0
0
0
16
0
17
0 0
By way of example and not by way of limitation, the electron donor and
acceptor groups
(R1) may be selected from nucleophilic groups (donors) and electrophilic
groups (acceptors) and
the set (variety) of groups (R1). containing of m elements comprises donors
(R1') and/or
acceptors (R1). The electrophilic groups (acceptors) are selected from - NO2, -
NH3+ and -NR3+
(quaternary nitrogen salts), counterion Cl- or Br-, -CHO (aldehyde), -CRO
(keto group), -S03H
(sulfonic acids), -SO3R (sulfonates), -SO2NH2 (sulfonamides), -COOH
(carboxylic acid), -
COOR (esters, from carboxylic acid side), -00C1 (carboxylic acid chlorides), -
CONH2 (amides,
from carboxylic acid side), - CF3, -CC13,-CN, -C(CN)2 wherein R is radical
selected from the list
11
CA 03082828 2020-05-14
WO 2019/100026 PCT/US2018/061874
comprising alkyl (methyl, ethyl, iso-propyl, tert-butyl, neopentyl, cyclohexyl
etc.), allyl (-CH2-
CH=CH2), benzyl (-CH2C6H5) groups, phenyl (+substituted phenyl) and other aryl
(aromatic)
groups. The nucleophilic groups (donors) are selected from -0- (phenoxides,
like -0Na or -OK),
-NH2, -NHR, -NR2, -OH, -OR (ethers), -NHCOR (amides, from amine side), -OCOR
(esters,
from alcohol side), alkyls, - C6H5, vinyls, wherein R is radical selected from
the list comprising
alkyl (methyl, ethyl, isopropyl, tert-butyl, neopentyl, cyclohexyl etc.),
allyl (-CH2-CH=CH2),
benzyl (-CH2C6H5) groups, phenyl (+substituted phenyl) and other aryl
(aromatic) groups.
In another embodiment, the polycyclic aromatic Cores may be similarly expanded
in the
lateral dimension, herein defined as the direction that is in plane and
perpendicular to the length
wise dimension which is variable in compounds 1-17, which is herein defined as
the longitudinal
dimension. Such expansion is demonstrated in Figure 2. Lateral expansion makes
the Cores
more planar and results in greater surface area for pi-pi interactions to
enhance stacking effect.
Lateral expansion also prevents the molecules from warping due to steric
influences from
neighboring molecules or substituents. These embodiments may still possess the
electron donor
and acceptor groups as described for compounds 1-17 and still possess the
essential rigid non-
conjugated limit carbon structures as resistive substituents. Some non-
limiting examples of
lateral expansion are shown below.
Table 3. Lateral Expansion Examples
0 N 0
eel
00****0
NO2 36
0 NI 0
12
CA 03082828 2020-05-14
WO 2019/100026 PCT/US2018/061874
NO2 NH2
411 N\ N
/ N \ N
H25C120 ilb . ill 0C12H25
37
0 0
0C12H25 H25C120
R1
R2 N
\ 0
R6
N N 38
R3
\
0 N R5
R4
R 1
R2 N
\ 0
R6
N N 39
R3
\
0 N R5
R4
R1 R4
R2 )-N\ \ /N
R5
N N 40
R3 R6
0 0
13
CA 03082828 2020-05-14
WO 2019/100026 PCT/US2018/061874
Ri R4
R2 R5
41
R3 R6
0 0
Ri R4
R2
/N
R5
42
R3 R6
0 0
Wherein R1, R2, R3, R4, R5, R6 are each independently selected from hydrogen,
electrophilic
groups, nucleophilic groups, and resistive groups. In some embodiments, the
resistive groups are
connected via at least one connecting group.
In some implementations, the electro-polarizable compound may take the form of
the
following structure
Ra Rb
0
Rc
wherein Ra is a nucleophile with or without alkyl resistive groups, and Rb and
It, are electrophilic
groups. A non-limiting example of such a structure includes the structure
14
CA 03082828 2020-05-14
WO 2019/100026 PCT/US2018/061874
NC
R'RN ON
101 I 11 I
411
0 CN
NC
wherein R and R' are independently selected from hydrogen, and alkyl groups
ranging between
Cl-C18.
In still another embodiment of the disclosed electro-polarizable compound, at
least one
connecting group is selected from the list comprising structures 18-35 given
in Table 4, where X
is hydrogen (H) or an alkyl group.
Table 4. Examples of the connecting group
¨0
¨0
18 19
0
0
-N
20 21
N-
X 0
0
0
22
N- 23
0-
X
CA 03082828 2020-05-14
WO 2019/100026 PCT/US2018/061874
24 25
0
%
¨S 26 ¨S 27
0¨
x
X
28 29
30 31
0
32 33
Si
34 35
In one embodiment of the present disclosure, the at least one connecting group
is selected from
the group of CH2, CF2, SiR20, CH2CH20, wherein R is selected from the list
comprising H,
alkyl, and fluorine.
In yet another embodiment of the present disclosure, the resistive substituent
R4 is selected
from the group of alkyl, aryl, substituted alkyl, substituted aryl,
fluorinated alkyl, chlorinated
alkyl, branched and complex alkyl, branched and complex fluorinated alkyl,
branched and
complex chlorinated alkyl groups, and any combination thereof, and wherein the
alkyl group is
16
CA 03082828 2020-05-14
WO 2019/100026 PCT/US2018/061874
selected from methyl, ethyl, propyl, n-butyl, iso-butyl and tert-butyl groups,
and the aryl group is
selected from phenyl, benzyl and naphthyl groups or siloxane, and/or
polyethylene glycol as
linear or branched chains. In still another embodiment of the present
disclosure, the resistive
substituent R4 is CxQ2x+i, where X > 1 and Q is hydrogen (H), fluorine (F), or
chlorine (Cl).
In one embodiment of the electro-polarizable compound, the aromatic polycyclic
conjugated
molecule (Corel) and the groups (R1) form a non-centrosymmetric molecular
structure. In
another embodiment of the electro-polarizable compound, the aromatic
polycyclic conjugated
molecule (Corel), the groups (R1) and the resistive substituents (R4) form a
non-
centrosymmetric molecular structure. In one embodiment of the present
disclosure, the electro-
polarizable compound has the following general formula (II):
- NIE
RI, R1'] *-- .
(II)
Corel
,
,
----------- R4 1
....................................................
In general formula II, Corel is the aromatic polycyclic conjugated molecule,
as discussed above,
the resistive substituent R4 is a non-conjugated part of disclosed compound,
which may be
saturated and fused cyclo-hydrocarbons or saturated and fused cyclo-
halocarbons with rigid
spatial structure including, but not limited to cyclohexane, cyclopentane,
polycyclic
perflourohexyls, polycyclic perflouropentyls, and structures that are built
from tiles of cyclic
carbon molecules. The tiles of cyclic carbon molecules may have dense packing
of SP3 carbon
saturated with H, F, Cl, or Br. In one particular implementation, parameters n
= p = s =0. In
another embodiment of the electro-polarizable compound, a length of the non-
conjugated part is
selected such that its resistivity is greater than 1015 ohm cm. In yet another
embodiment of the
electro-polarizable compound, the resistive substituent R4 is selected from
benzyl groups, benzyl
alkoxy groups, benzyl halo-alkoxy groups, alkoxy groups, benzyl alkyl groups,
benzyl halo-alkyl
groups, alkyl groups, halo-alkoxy groups, halo-alkyl groups, benzyl aryl
groups, and benzyl
halo-aryl groups, wherein in the R4 substituents are connected to the apex of
Corel on which the
nucleophilic groups (donor) R1 are connected, or the apex of Corel on which
the electrophilic
group (acceptor) R1' is connected, but not both. In still another embodiment
of the electro-
17
CA 03082828 2020-05-14
WO 2019/100026 PCT/US2018/061874
polarizable compound, the resistive substituent R4 is resistive polycyclic
substituents selected
from the list comprising long C25H34 and C25H35 or C25F34 and C25F35 and
located on the apex
phenyl rings of Corel. In one embodiment of the present disclosure, the
electro-polarizable
compound has the following general formula (III):
R1
R4 Corel ______________________________________ R4
V
N LE
(III)
In general formula III, the parameters m and m' are equal to 1, R1' is an
acceptor group, R1 is a
donor group, k and k' indicate R4 resistive groups are on either end of the
molecule. In another
embodiment of the electro-polarizable compound, the Corel is rylene fragment
having the
following structural formula where repetition parameter t is an integer
varying from 0 to 5:
N
0 0
wherein the set of the electron donor and acceptor groups comprises one donor
group -NEt2 and
one acceptor groups -NO2 (m and m' are both equal to 1) located on rylene
phenyl rings and/or
apex phenyl ring positions of the Corel, so that the fragment having a
nonlinear effect of
polarization (NILE) is represented by the following chemical structure (when t
= 0):
ON N Et2
0 0
18
CA 03082828 2020-05-14
WO 2019/100026
PCT/US2018/061874
wherein the resistive substituents (R4) are benzyl alkoxy groups and in some
instances are
attached via a benzyl group such as:
H2n+1CnO
H2n1+1C1110
wherein n and n' range from 4-25. This leads to the following structural
formula (IV):
Et2N
H2n+1On-0 C)2N
0
\c,
0 0õ
\r, 1_4 0 0
L'n'H2n'+1
µ-'11'112n'+1
(IV).
In some embodiments resistive substituents (R4) are branched alkyl or alkoxy
groups attached
via an alkyne connecting group, for example:
H2n+1Cn
H2n1+1Cn.
wherein n and n' range from 4-25. This leads to the following structural
formula (V):
19
CA 03082828 2020-05-14
WO 2019/100026
PCT/US2018/061874
Et2N
02N
N N
0 0 0 CnH2n+i
0
H2n+1Cnr. I-1
¨n'-2n'+1 Lin'n2n'+1
(V).
In another embodiment of the present disclosure, the electro-polarizable
compound has the
following general formula (VI):
R1' R1
R4 ____________________
____________________________________________________ R4
Corel
R1'
\
R is
(VI)
In general formula VI, Corel is the above-described aromatic polycyclic
conjugated molecule, m
is equal to 6, R1' is donor group, R1 is acceptor group, k is equal to 2. In
yet another
embodiment of the electro-polarizable compound, the Corel is rylene fragment
having the
following structural formula where repetition parameter t varies from 1 to 5:
0 N
* N N *
* N 0
CA 03082828 2020-05-14
WO 2019/100026 PCT/US2018/061874
wherein the set of the electron donor and acceptor groups comprises three
donor groups -NH2
and three acceptor groups -NO2 (m is equal to 6) are located on rylene phenyl
rings and/or apex
phenyl ring positions of the Corel, so that the fragment having a nonlinear
effect of polarization
(NILE) is represented by following chemical structure (when t = 1):
NH2
02N
N NH2
= N N
02N 11 0 NH2
02N
wherein the resistive substituent (R4) is an amine structure of the following
type:
¨N
leading to the following structural formula (VII):
NH2
02N 0 N
= N N31818 N
8:81EN
0 NH2
02N
(VII)
21
CA 03082828 2020-05-14
WO 2019/100026 PCT/US2018/061874
wherein the resistive substituents are connected via a connecting group.
Non-limiting examples of the electro-polarizable cores includes at least two
regioisomers
selected from the below structures:
Ri Ri R4
R2 40 N 0
N
\ R2 401 /N ill R5
R6 \
N N
N N 111-
R3
R3 R6
0 n-1 N iiiii R5
0 n-1 0
R4
ANTI SYN
wherein R1, R2, R3, R4, R5, R6 are each independently selected from hydrogen,
electrophilic
groups, nucleophilic groups, and resistive groups; and n is an integer greater
than or equal to 1.
Non-limiting examples of such combinations of substituents are listed in Table
5.
Conf. n R1 R2 R3 R4 R5 R6
ANTI 2 H NH2 H H NO2 H
ANTI 2 H NH2 NH2 H NO2 NO2
ANTI 2 H NO2 NH2 H NH2 NO2
ANTI 2 NH2 H DB NO2 H DB
SYN 2 NH2 H DB NO2 H DB
ANTI 3 NH2 H DB NO2 H DB
SYN 3 NH2 H DB NO2 H DB
ANTI 4 NH2 H DB NO2 H DB
SYN 4 NH2 H DB NO2 H DB
ANTI 2 NRR' H DB NO2 H DB
SYN 2 NRR' H DB NO2 H DB
Wherein R and R' can be the same or independently selected from alkyl, alkene,
and substituted
alkyl groups; and wherein DB is 3,5-dimethoxyphenyl. The electro-polarizable
compounds may
be further modified to include resistive substituents connected to the core
via DB groups, or
connecting groups listed in Table 4.
In some embodiments, a dielectric layer of electro-polarizable compounds is
comprised of
more than one regioisomer. In some embodiments, a dielectric layer comprised
of electro-
polarizable compounds includes a mixture of electro-polarizable compounds.
22
CA 03082828 2020-05-14
WO 2019/100026 PCT/US2018/061874
In one embodiment of the present disclosure, the induced polarization Pind of
the electro-
polarizable compound may be written in the form of decomposition into a series
on degrees of
intensity of a local electric field Eloc:
2 Pind = _L
Ot=Eloc p= = = = ,
where a represents linear polarizability, 0 represents square polarizability.
In an aspect, the present disclosure provides the organic solvent comprising
the disclosed
electro-polarizable compound. In one embodiment, the solution comprises a
mixture of different
electro-polarizable compounds. In another embodiment of the disclosed organic
solvent, the
mixture of the electro-polarizable compounds comprises the rylene fragments of
different length.
In still another embodiment, the organic solvent is selected from the list
comprising ketones,
carboxylic acids, hydrocarbons, cyclic hydrocarbons, chlorohydrocarbons,
alcohols, ethers,
esters, and any combination thereof In yet another, the organic solvent is
selected from the list
comprising acetone, xylene, toluene, ethanol, methylcyclohexane, ethyl
acetate, diethyl ether,
octane, chloroform, methylene chloride, dichloroethane, trichloroethene,
tetrachloroethene,
carbon tetrachloride, 1,4-dioxane, tetrahydrofuran, pyridine, triethylamine,
nitromethane,
acetonitrile, dimethylformamide, dimethyl sulfoxide, and any combination
thereof In yet another
embodiment of disclose, the solution is a lyotropic liquid crystal solution.
In another aspect, aspects of the present disclosure provide a crystal
metadielectric layer
comprising at least one type of the disclosed electro-polarizable compounds.
The crystal
metadielectric layers are produced from the disclosed organic compound by
Cascade
Crystallization; a method of thin crystal film (or thin crystal layer)
manufacturing known as the
Optiva-Process. See U.S. Pat. Nos. 5,739,296 and 6,049,428, and P. Lazarev et
al., "X-ray
Diffraction by Large Area Organic Crystalline Nano-films", Molecular
Materials, 14 (4), 303-
311 (2001), and Bobrov, "Spectral Properties of Thin Crystal Film Polarizers",
Molecular
Materials, 14 (3), 191-203 (2001).
Cascade Crystallization process involves a chemical modification step and four
steps of
ordering during the crystal metadielectric layer formation. The chemical
modification step
introduces hydrophilic groups on the periphery of the molecule of the
disclosed organic
compound in order to impart amphiphilic properties to the molecule.
Amphiphilic molecules
stack together into supramolecular structures, which is the first step of
ordering. At certain
23
CA 03082828 2020-05-14
WO 2019/100026 PCT/US2018/061874
concentration, supramolecular structures are converted into a liquid-
crystalline state to form a
lyotropic liquid crystal, which is the second step of ordering. The lyotropic
liquid crystal is
deposited under the action of a shear force (or meniscus force) onto a
substrate based on a Mayer
Rod shearing technique, so that shear force (or the meniscus) direction
determines the crystal
axis direction in the resulting solid crystal layer. The external alignment
upon the lyotropic liquid
crystal can be produced using any other means, for example by applying an
external electric field
at normal or elevated temperature, with or without additional illumination,
magnetic field, or
optical field (e.g., coherent photovoltaic effect); the degree of the external
alignment should be
sufficient to impart necessary orientation to the supramolecular structures of
the lyotropic liquid
crystal and form a structure, which serves as a base of the crystal lattice of
the dielectric layer.
This directional deposition is third step of ordering, representing the global
ordering of the
crystalline or polycrystalline structure on the substrate surface. The last
fourth step of the
Cascade Crystallization process is drying/crystallization, which converts the
lyotropic liquid
crystal into a solid crystal dielectric layer. The term Cascade
Crystallization process is used to
refer to the chemical modification and four ordering steps as a combination
process.
The Cascade Crystallization process is used for production of thin crystalline
metadielectric
layers. The dielectric layer produced by the Cascade Crystallization process
has a global order
which means that a direction of the crystallographic axis of the layer over
the entire substrate
surface is controlled by the deposition process. Molecules of the deposited
material are packed
into supramolecular structures with a limited freedom of diffusion or motion.
The thin crystalline
dielectric layer is characterized by an interplanar spacing of 3.4 0.3
Angstroms (A) in the
direction of one of the optical axes.
In one embodiment of the present disclosure, the crystal metadielectric layer
comprises
supramolecular structures such as columns, needles, etc., formed by the
electro-polarizable
compounds comprising the rylene fragments of different length. The variety of
the rylene
fragment lengths increases the randomness of the stack. In one embodiment
according to aspects
of the present disclosure, the layer's relative permittivity is greater than
or equal to 1000. In one
embodiment, the real part of the relative permittivity (e) of the crystal
metadielectric layer
comprises first-order (cm) and second-order (c(2)) permittivities according to
follow formula:
6. = 26(2) Vo
24
CA 03082828 2020-05-14
WO 2019/100026 PCT/US2018/061874
where Vo is the DC-voltage which is applied to the crystal metadielectric
layer, d is the
layer thickness. In another embodiment of the present invention, the layer's
resistivity is greater
than or equal to 101' ohm/cm.
The present disclosure provides the metacapacitor comprising two metal
electrodes
positioned parallel to each other and which can be rolled or flat and planar
and metadielectric
layer between said electrodes. The layer comprises the electro-polarizable
compounds as
disclosed above.
The metacapacitor comprises a first electrode 1, a second electrode 2, and a
metadielectric
layer 3 disposed between said first and second electrodes as shown in Figure
1A. The electrodes
1 and 2 may be made of a metal, such as copper, zinc, or aluminum or other
conductive material
such as graphite or carbon nanomaterials and are generally planar in shape.
The electrodes 1, 2 may be flat and planar and positioned parallel to each
other.
Alternatively, the electrodes may be planar and parallel, but not necessarily
flat, they may be
coiled, rolled, bent, folded, or otherwise shaped to reduce the overall form
factor of the capacitor.
It is also possible for the electrodes to be non-flat, non-planar, or non-
parallel or some
combination of two or more of these. By way of example and not by way of
limitation, a
spacing d between the electrodes 1 and 2 may range from about 100 nm to about
10,000 pm. The
maximum voltage Vbd between the electrodes 1 and 2 is approximately the
product of the
breakdown field Ebd and the electrode spacing d. If Ebd = 0.1 V/nm and the
spacing d between
the electrodes 1 and 2 is 10,000 microns (100,000 nm), the maximum voltage Vbd
would be
100,000 volts.
The electrodes land 2 may have the same shape as each other, the same
dimensions, and
the same area A. By way of example, and not by way of limitation, the area A
of each electrode
1 and 2 may range from about 0.01 m2 to about 1000 m2. By way of example and
not by way of
limitation for rolled capacitors, electrodes up to, e.g., 1000 m long and 1 m
wide.
These ranges are non-limiting. Other ranges of the electrode spacing d and
area A are
within the scope of the aspects of the present disclosure.
If the spacing d is small compared to the characteristic linear dimensions of
electrodes
(e.g., length and/or width), the capacitance C of the capacitor may be
approximated by the
formula:
C = ccoA/d, (V)
CA 03082828 2020-05-14
WO 2019/100026 PCT/US2018/061874
where 60 is the permittivity of free space (8.85X10-12
Coulombs2/(Newton=meter2)) and c is the
dielectric constant of the dielectric layer. The energy storage capacity U of
the capacitor may be
approximated as:
U =1/2 ccoAEbd2d (VI)
The energy storage capacity U is determined by the dielectric constant , the
area A, and
the breakdown field Ebd. By appropriate engineering, a capacitor or capacitor
bank may be
designed to have any desired energy storage capacity U. By way of example, and
not by way of
limitation, given the above ranges for the dielectric constant , electrode
area A, and breakdown
field Ebd a capacitor in accordance with aspects of the present disclosure may
have an energy
storage capacity U ranging from about 500 Joules to about 2.1016 Joules.
For a dielectric constant c ranging, e.g., from about 100 to about 1,000,000
and constant
breakdown field Ebd between, e.g., about 0.1 and 0.5 V/nm, a capacitor of the
type described
herein may have a specific energy capacity per unit mass ranging from about 10
W=h/kg up to
about 100,000 W=h/kg, though implementations are not so limited.
The present disclosure includes metacapacitors that are coiled, e.g., as
depicted in Figure
1B. In this example, a metacapacitor 20 comprises a first electrode 21, a
second electrode 22,
and a metadielectric material layer 23 of the type described hereinabove
disposed between said
first and second electrodes. The electrodes 21 and 22 may be made of a metal,
such as copper,
zinc, or aluminum or other conductive material such as graphite or carbon
nanomaterials and are
generally planar in shape. In one implementation, the electrodes and
metadielectric material
layer 23 are in the form of long strips of material that are sandwiched
together and wound into a
coil along with an insulating material, e.g., a plastic film such as
polypropylene or polyester to
prevent electrical shorting between the electrodes 21 and 22.
In order that the invention may be more readily understood, reference is made
to the
following examples, which are intended to be illustrative of the invention,
but are not intended to
limit its scope.
Example 1:
26
CA 03082828 2020-05-14
WO 2019/100026 PCT/US2018/061874
H2N NO2
=Ci2H250
N N
=
0 0 OCi2H25
C12H250 C12H250
Br BBr3 Br
r-N (101 r-N
CH2Cl2 HO(101 OH
Synthesis of 3,5-dihydroxybromobenzene: To a reaction flask oven dried
overnight at 90 C,
3,5-dimethoxybromobenzene (1 eq.) was dissolved in anhydrous CH2C12 and placed
in an ice
water bath to cool for 10 minutes. To this chilled solution, BBr3 (1M in
CH2C12, 2.2 eq.) was
slowly added over 5 minutes. Once this addition was complete, the reaction was
removed from
the ice water bath and allowed to warm in in air to room temperature and
allowed to stir
overnight. The reaction was confirmed to be completed after 18 hours by SiO2
TLC using 1:1
Hexanes : Et0Ac. The reaction was placed back on an ice water bath to cool for
10 minutes
before 1 mL of methanol was added to quench any unreacted BBr3 still present.
This reaction
mixture was washed with aqueous HC1 (2 M) and extracted with Et0Ac (3x). The
organic
fractions were collected and dried with Na2S03 before being filtered. The
crude reaction mixture
was concentrated under vacuum and precipitated into hexanes to yield 3,5-
dihydroxy-
bromobenzene.
BrC12H25 Br
Br
1101 K2CO3
0 101
0
HO OH DMF
C12H25 C12H25
A
27
CA 03082828 2020-05-14
WO 2019/100026 PCT/US2018/061874
Synthesis of A: To reaction flask oven dried overnight at 90 C, 3,5-
dihydroxybromobenzene (1
eq.) and K2CO3 (3 eq.) was dissolved in anhydrous DMF and stirred at room
temperature for 10
minutes. To this mixture, bromododecane (3 eq.) was added and the reaction was
placed in a
preheated 100 C oil bath and stirred overnight. The reaction was confirmed to
be completed after
18 hours by SiO2 TLC using 1:1 Hexanes : Et0Ac. The reaction removed from the
oil bath and
allowed to cool in air to room temperature. Excess K2CO3 was quenched with
aqueous HC1 (2
M) and the reaction was extracted with Et0Ac. The organic fractions were
collected, washed
with deionized water and dried with Na2S03 before being filtered. The solvent
was removed
under vacuum and the product was purified by silica gel chromatography (100%
Hexanes to 10%
Et0Ac : 90 % Hexanes) and isolated as a colorless oil that slowly solidified
into a white solid.
0 0
Br Pd(dppf)Cl2 / AcOK
110
1101 Dioxane / 90 C / 30 min C12H250 0C12H25
C12H250 0C12H25
A
Synthesis of B: A (1 eq.), bis(pinacolato)diboron (1.6 eq.), potassium acetate
(3 eq.),
Pd(dppf)C12 (0.03 eq.) were evacuated inside a 100 mL round bottom flask and
backfilled with
N2. In a separate flask, dioxane was sparged under a N2 flow for 15 min before
being added to
the reaction flask via syringe. This reaction solution was placed in a
preheated oil bath set to 90
C and monitored by TLC (9:1 Hexanes: Et0Ac). When the reaction was complete,
the reaction
mixture was washed with 2M HC1 and extracted with ethyl acetate. The organic
fractions were
collected and dried using Na2SO4 and filtered before removing the solvent
under reduced
pressure. The crude material was re-dissolved in hexanes and filtered using a
silica plug using
hexanes as the eluent. Hexane was removed under reduced pressure to isolate a
viscous oil. This
crude mixture stirred for 1 h in methanol to give a white solid precipitate
that was collected by
vacuum filtration. B was isolated as a white solid.
28
CA 03082828 2020-05-14
WO 2019/100026 PCT/US2018/061874
1
101 12 / Ag2SO4
I101
02N NO2 rt / 18h 02N NO2
NH2 NH2
Synthesis of C: 2,6-dinitroaniline (1 eq.), Ag2SO4 (1.4 eq.), and I2 (1.4 eq)
were added to a 50
mL round bottom flask at room temperature. To this mixture, ethanol was added
and the reaction
was allowed to stir at room temperature for 18 hours. The next morning a
yellow precipitate had
formed and TLC analysis (1:1 Et0Ac : Hexanes) had shown complete consumption
of the
starting material. This reaction mixture was filtered, and the solid residue
was washed with
Et0Ac until the filtrate ran clear. The solvent was then removed from the
filtrate under vacuum
and the crude solid was re-dissolved in a minimum amount of CH2C12 before
being precipitated
into of hexanes. The mixture was set aside for 30 minutes until no more solid
precipitated and
the solid was isolated via vacuum filtration. C was isolated as an orange
solid.
012E1250 0012H25 i 012E1250 0012H25
401
1101 Pd(PPh3)Cl2
K2CO3
101
1
/B\ 02N NO 101 NO2Toluene /
H20 (9:1)
0 0 NH2 100 C 0 IN
2N 1/4./
NH2
Synthesis of D: C (1 eq.), B (1.1 eq,), Pd(PPh3)2C12 (0.03 eq.), and K2CO3 (2
eq.) were added to
a 25 mL round bottom flask before being evacuated and backfilled with N2 three
times. In a
separate flask, N2 was bubbled through a solution of toluene and H20 for 30
min before adding
this solution to the reaction flask. This solution was then placed in a
preheated oil bath at 100 C
and stirred for overnight. The reaction was monitored by TLC (7:3
Hexanes:Et0Ac). Once the
reaction was complete, it was removed from the oil bath and allowed to cool to
room
temperature in air for 30 min. The mixture was washed distilled water and
excess base was
carefully acidified with the addition of 2M HC1 then extracted with Et0Ac. The
organic fractions
were collected and dried with NaSO4, filtered, and the solvent was removed
under vacuum
29
CA 03082828 2020-05-14
WO 2019/100026 PCT/US2018/061874
distillation. The crude product was dissolved in a minimum amount of CH2C12
and precipitated
into Me0H. The solid was filtered to give D as a yellow solid.
012E1250 1, 0C12H25
C121-1250
OCi2H25
(NH4)2S
__________________________________________ Xi^
101 Butanol
101
02N NO2 80 C 02N NH2
H2N NH2
Synthesis of E: D (1 eq.), was added to a round bottom flask and dissolved
into n-butanol at
80 C. To this solution was added a 20 wt % aqueous solution of (NH4)2S (2
eq.). The reaction
was stirred for 1 hour and was monitored by TLC (7:3 Hexanes/Et0Ac). When the
reaction was
complete, the reaction mixture was washed with 2 M HC1 and extracted with
ethyl acetate. The
organic fractions were collected and dried using Na2SO4 and filtered before
removing the solvent
under reduced pressure. The crude material was re-dissolved in hexanes
purified using SiO2
column chromatography (7:3 Hexanes/Et0Ac) to give E as a viscous red oil.
OCi2H25
C12H250
C12H250 (40 0C12H25
0 0 0 Zn(0Ac)2 2H20 NO2
1101
02N NH2
Quinoline / 170C
1.10
NH2 Br
Br
Synthesis of F: E (1 eq.) and 4-bromonaphthalic anhydride (1.2 eq), and
Zn(0Ac)2 2H20 (0.4
eq.) were added to a round bottom flask before being evacuated and backfilled
with N2. In a
separate flask, quinoline was purged for 15 min under a flow of N2 and added
to the reaction
mixture. This suspension was heated to 170 C and let to stir overnight. When
the reaction is
complete, the hot solution was poured into Me0H and the resulting solid was
washed with 20
CA 03082828 2020-05-14
WO 2019/100026 PCT/US2018/061874
mL of additional Me0H before being collected. Residual Me0H was removed under
reduced
pressure to give F.
0012H25 0012H25
012F1250 410 012H250
ilk NO2 NH2
reducing agent
0 N ,N 0 N ,N
800
010 010
Br Br
Synthesis of G: F (1 eq.) was added to a round bottom flask with butanol (0.3
M). This
suspension was heated to 80 C and a reducing agent (SnC12, (NH4)25, or HNaS;
1 eq.) was
transferred to the hot reaction mixture. The reaction was monitored by TLC
analysis and allowed
to stir overnight. When the reaction was complete, the reaction mixture was
diluted with water
and extracted with ethyl acetate. The organic fractions were collected and
dried using Na2SO4
and filtered before removing the solvent under reduced pressure. The crude
material was re-
dissolved in hexanes purified using 5i02 column chromatography (Hexanes/Et0Ac,
then Et0Ac)
to give G.
OCi2H25
OCi2H25
C121-1250
C121-1250 NO2
NO2 B2Pin2
Pd(dppf)012 0 N N
ONN AcOK
Dioxane / 90 C
B.
0 0
Br
H
Synthesis of H: F (1 eq.), Pd(dppf)C12 (0.05 eq.), AcOK (2 equiv.), and B2Pin2
(1.5 eq.) were
added to 25 mL round bottom flask. This mixture was then evacuated and
backfilled with N2 3
times. In a separate flask, dioxane (0.3 M) was bubbled with N2 for 30
minutes. This degassed
solvent was then added to the reaction flask under an N2 atmosphere and placed
into a preheated
31
CA 03082828 2020-05-14
WO 2019/100026 PCT/US2018/061874
100 C oil bath and allowed to stir overnight. When the reaction was complete,
it was removed
from the oil bath and allowed to cool to room temperature before being washed
with 2M HC1
and extracted using Et0Ac. The organic layers were collected, dried with
Na2SO4, filtered, and
the solvent was removed under reduced pressure. The crude product was purified
by column
chromatography (100% Hexanes ¨ 8:2 Hexanes/Et0Ac). The solvent was removed to
give H.
0012H25
012H250
0012H25 0012H25 = NO2
012H250 012H250 = Pd(PPh3)4 0 N
fh, NO2 = NH2 K2003 040
0 N N 0 N N H20 / Toluene / Et0H
1000 400
0 N 'N
NH2
0 0
012F1250 *
0012 H25
Synthesis of!: H (1 eq.), Pd(PPh3)4 (0.05 eq.), K2CO3 (2 eq.), and G (1 eq.)
were added to a
reaction flask. This mixture was then evacuated and backfilled with N2 3
times. In a separate
flask, a mixture of toluene, H20 (2:1) was bubbled with N2 for 10 minutes.
This degassed solvent
was then added to the reaction flask under an N2 atmosphere via syringe and
placed into a
preheated 100 C oil bath and allowed to stir overnight. When completed, the
reaction was
removed from the oil bath and allowed to cool to room temperature before being
washed with
2M HC1 and extracted using Et0Ac. The organic layers were collected, dried
with Na2SO4,
filtered, and the solvent was removed under reduced pressure. The crude solid
was dissolved in a
minimum amount of CH2C12 and precipitated into Me0H. H was isolated by
filtration.
32
CA 03082828 2020-05-14
WO 2019/100026 PCT/US2018/061874
0C12H25 12 25
C12H250 C12H250
NH2 NH2
0 N ,N1 N
411* K2CO3 / N(C2H4OH)3
1. On 01
0 N 'N 0 N 'N
NO2 NO2
C12H250 Cl2H250
0C12H25 0C12H25
I (1 eq.) was dispersed in triethanolamine (0.02 M) and K2CO3 (25 eq.) was
added. The mixture
was stirred at 130 C for 24 hours under argon atmosphere. Upon cooling to
room temperature,
the reaction mixture was diluted with dichloromethane and washed with water.
The organic layer
was dried over anhydrous sodium sulfate and purified by precipitation into
methanol to yield J as
dark purple solid.
Example 2: This Example describes synthesis of the disclosed organic compound
according
following structural scheme:
=
02N Oaf.
N H2 10110,
0
= =
O..
02N 0
Odt. NH2
imur
33
CA 03082828 2020-05-14
WO 2019/100026 PCT/US2018/061874
Procedure:
NO2
Br\N H2N 411
4 H2N N
Pd(PPh3)4
K2CO3 NO2
H2N NH2
02N NO2
B(0iPr)2
14
Bromo-amine 4 (1 eq.), Naphthalene 14 (1 eq.), Pd(PPh3)4 (10 mol %), K2CO3
(1.5 eq.) were
stirred in toluene at 70 C for 18 h. The mixture was filtered through
Diatomaceous earth and the
filtrate was washed with NaHCO3 and brine. The organics were dried over MgSO4,
and the
solvents were removed under reduced pressure to give 15.
NO2
H2N
H2N
02N
NO2
15 imidazole
0
0 0 0 NO2
Br
17
Br
16
34
CA 03082828 2020-05-14
WO 2019/100026 PCT/US2018/061874
Naphthalene anhydride 16 (1 eq.) and naphthalene 15 (1 eq.) were stirred in
imidazole at 130 C
overnight. The mixture was dissolved in THF and washed with water 3 times. The
organics were
combined and dried over MgSO4. The solvent was removed under reduced pressure
to give 17.
02N
=
0 SI 00Ø NH2
=
N 140 N =
0,0.a. =
0
70) N NO2 Pd/C, H2 N 140 N
Br N NH2
17 Br 18
Amidine 17 (1 eq.) and Pd/C (20% wt/wt) were stirred in THF in a three-neck
flask with a H2
balloon attached for 18 h. The mixture was filtered through Diatomaceous earth
and the solvents
were removed under reduced pressure to give 18.
02N
0 Ili NO2
N (10 N
0
NO2 n-BuLi, B(0iP03
Br
17 NO2
(0iPr)2B
19
Amidine 17 (1 eq.) was dissolved in THF and stirred at -80 C. N-butyllithium
(1.2 eq., 2.5 M in
hexanes) was added dropwise. After 1 h, triisopropylborane was added dropwise
and allow to
warm to room temperature overnight. The mixture was washed with NaHCO3 and
brine and
dried over MgSO4. The solvent was removed under reduced pressure to give 19.
CA 03082828 2020-05-14
WO 2019/100026 PCT/US2018/061874
S
.11 NH2
0
H2N
NH2 1114 NH2
0
Br 18 -N
Pd(PPh3)4 44*
K2CO3
02N
N- N
0 0
02N**
NO2N NO2
19 ill
(0iPr)2B
ad)
Wirk
Bromo-amidine 18 (1 eq.), Amidine boronic ester 19 (1 eq.), Pd(PPh3)4 (10 mol
%), K2CO3 (1.5
eq.) were stirred in toluene at 70 C for 18 h. The mixture was filtered
through Diatomaceous
earth and the filtrate was washed with NaHCO3 and brine. The organics were
dried over MgSO4,
and the solvents were removed under reduced pressure to give 20.
36
CA 03082828 2020-05-14
WO 2019/100026 PCT/US2018/061874
=
Ind&
=
H2N fel
14 NH2 H2 N
0
-N 0 4140
NH2
DBU, KOt13U
411111.
*ft ethanolamine
N- 0 Mir
0 20 N
02N b. 02N =
21
NO2
N NO2
A mixture of potassium tert-butoxide (1 eq), diazabicyclo[5.4.0]undec-7-ene
(DBU) (1.2 eq.),
ethanolamine (2.8 eq.) and 20 (1 eq.) was heated to 140 C for 11 hours.
Afterwards, the same
amount of potassium tert-butoxide, DBU and ethanolamine were added and the
mixture was kept
at 140 C for 18 hours. The reaction mixture was cooled to room temperature,
poured into 1M
HC1, filtered, washed until neutral pH and then dried to give the final
product 21.
Aspects of the present disclosure provide compounds characterized by highly
nonlinear electric
polarizability. Such compounds are useful as high dielectric constant
metadielectrics for meta-
capacitors with extremely high capacitance and extremely high energy storage
capacity.
While the above is a complete description of the preferred embodiment of the
present invention,
it is possible to use various alternatives, modifications and equivalents.
Therefore, the scope of
the present invention should be determined not with reference to the above
description but
should, instead, be determined with reference to the appended claims, along
with their full scope
of equivalents. Any feature described herein, whether preferred or not, may be
combined with
any other feature described herein, whether preferred or not. In the claims
that follow, the
indefinite article "A", or "An" refers to a quantity of one or more of the
item following the
article, except where expressly stated otherwise. As used herein, in a listing
of elements in the
37
CA 03082828 2020-05-14
WO 2019/100026 PCT/US2018/061874
alternative, the word "or" is used in the logical inclusive sense, e.g., "X or
Y" covers X alone, Y
alone, or both X and Y together, except where expressly stated otherwise. Two
or more
elements listed as alternatives may be combined together. The appended claims
are not to be
interpreted as including means-plus-function limitations, unless such a
limitation is explicitly
recited in a given claim using the phrase "means for."
38