Note: Descriptions are shown in the official language in which they were submitted.
CA 03083236 2020-05-21
WO 2019/113050
PCT/US2018/063822
METHODS OF ASSAYING TROPOLONE
This application claims priority to U.S. Application 62/594,863 filed December
5, 2017,
the entire contents of which is incorporated herein by reference.
FIELD OF THE INVENTION
The present disclosure relates to methods of detecting and/or quantifying
tropolone
during the production of a product, e.g., a recombinant protein, e.g., an
antibody.
BACKGROUND
Tropolone (2-hydroxy-2,4,6-cycloheptatrien-1-one) is a small molecule used in
cell
culture media to facilitate uptake of metal ions, essential for growth of
cells such as those used in
biomanufacturing. Because tropolone is a synthetic chemical added to cell
culture during the
manufacturing process of products, regulatory agencies governing biological
products often
require that tropolone clearance be demonstrated.
Therefore, a need exists for methods of separating, detecting, and quantifying
tropolone
in a variety of biopharmaceutical products in a simple, rapid, efficient
manner.
SUMMARY
Methods and compositions described herein provide for quickly and easily
separating a
compound of Formula I, e.g., tropolone, from other sample components and
testing for a
compound of Formula I, e.g., tropolone, levels and clearance. This allows
evaluation of product
purity. Methods and compositions described herein can minimize regulatory
delay and time and
resource expenditure testing for compounds of Formula I, e.g., tropolone.
Accordingly, in one aspect the invention is directed to a method of separating
a
compound of Formula I, e.g., tropolone, from another component of a sample
comprising:
contacting the sample with a partially or fully fluorinated alkyl or aryl,
e.g., a
fluorophenyl, e.g., a pentafluorophenylpropyl, moiety, under conditions
wherein the compound
1
CA 03083236 2020-05-21
WO 2019/113050
PCT/US2018/063822
of Formula I, e.g., tropolone, associates with, e.g., binds to or is retained
by, the moiety to a
greater extent than the component,
thereby separating the compound of Formula I, e.g., tropolone, from the
component, wherein
Formula I is:
X
(R R'
R1
and wherein:
Xis 0 or S;
R1 is hydrogen, Ci-C6 alkyl, Ci-C6 heteroalkyl, OR3, C(0)R5, C(0)0R3,
N(R4a)(R4b),
C(0)N(R4a)(R4b), or N(R4a)C(0)R5;
each R2 is independently Ci-C6 alkyl, Ci-C6 heteroalkyl, N(R4a)(R4b),
c(0)N(R4a)(R4b),
or N(R4a)C(0)R5; or
two R2 are joined to form a heterocyclyl ring optionally substituted with one
or more R6;
or R1 and R2 are joined to form a heterocyclyl ring optionally substituted
with one or more R6;
R3 is hydrogen, Ci-C6 alkyl, or Ci-C6 heteroalkyl;
R4a and R4b are independently hydrogen, Ci-C6 alkyl, or Ci-C6 heteroalkyl;
R5 is Ci-C6 alkyl or Ci-C6 heteroalkyl;
each R6 is independently Ci-C6 alkyl, Ci-C6 heteroalkyl, halo, oxo, or cyano;
and
n is 0, 1, 2, 4, or 5.
In another aspect, the invention is directed to a method of evaluating the
presence, e.g.,
the level, of a compound of Formula I, e.g., tropolone, in a sample comprising
a product,
comprising:
a) i) providing an aliquot of a sample, e.g., a compound of
Formula I (e.g.,
tropolone) depleted phase, e.g., a mobile phase, wherein the compound of
Formula I, e.g.,
tropolone, has been separated from another component of the sample, or
ii) subjecting the sample to conditions wherein the compound of Formula I,
e.g.,
tropolone, is separated from another component of the sample, e.g., to form a
compound of
Formula I, e.g., tropolone, enriched phase or aliquot and a compound of
Formula I, e.g.,
tropolone, depleted phase or aliquot; and
2
CA 03083236 2020-05-21
WO 2019/113050
PCT/US2018/063822
b) evaluating the presence, e.g., the level, of the compound of Formula I,
e.g., tropolone,
e.g., determining a value for the level of the compound of Formula I, e.g.,
tropolone, in the
sample:
i) using tandem mass spectrometry (MS2), or
ii) using ultraviolet (UV) absorption, e.g., UV absorption at about 242 nm or
about 238 nm,
thereby analyzing the sample.
In another aspect, the invention is directed to a reaction mixture comprising
a partially or
fully fluorinated alkyl or aryl, e.g., a fluorophenyl, e.g., a
pentafluorophenylpropyl, moiety, and a
.. sample comprising a compound of Formula I, e.g., tropolone, another
component, and optionally
a product.
In another aspect, the invention is directed to a method of manufacturing a
product, e.g., a
recombinant polypeptide, comprising providing a sample comprising the product
and optionally
a compound of Formula I, e.g., tropolone, wherein:
the sample is analyzed by a method described herein, or
the compound of Formula I, e.g., tropolone, is separated from another
component of the
sample by a method described herein.
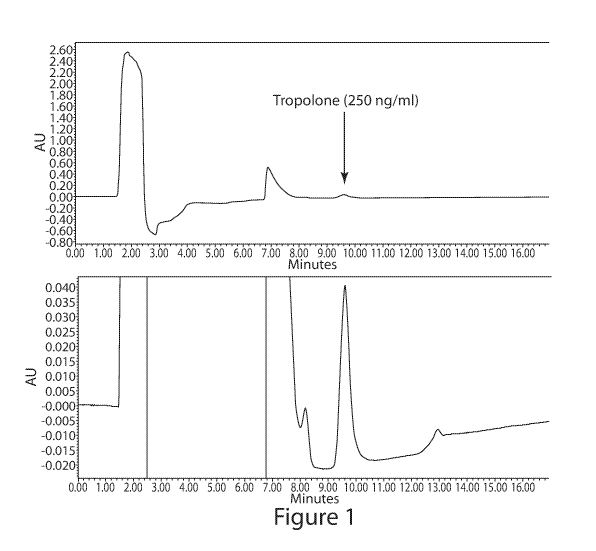
BRIEF DESCRIPTION OF THE DRAWINGS
FIG. 1 shows two views of a chromatogram of tropolone separation by RP-HPLC
using
UV detection; the bottom view is an expanded view of the top view.
FIG. 2 shows total ion current (TIC) traces of the SRM transitions of Table 1
after
separation using the Luna-NH2 column.
FIG. 3 shows TIC traces of the SRM transitions of Table 1 after separation
using the
Discovery HS F5-3 (Supelco) column.
FIG. 4 shows a graph showing the calibration curve plot of tropolone standards
in water
and measuring linear range.
FIG. 5 shows TIC traces of three in process samples, each showing no tropolone
peak.
3
CA 03083236 2020-05-21
WO 2019/113050
PCT/US2018/063822
FIG. 6 shows TIC traces of three in process samples either spiked with
tropolone (top
three) or not spiked with tropolone (bottom three).
FIG. 7 shows two chromatograms detecting tropolone in a tropolone standard
processed
via the chromatography method determined in Example 2, using UV absorption at
242 nm (top)
and 238 nm (bottom).
FIGs. 8A and 8B show detailed parameters of an exemplary LC method of the
disclosure.
DETAILED DESCRIPTION
For recombinant biopharmaceutical proteins to be acceptable for administration
to human
patients, it is important that residual contaminants resulting from the
manufacture and
purification process are removed from the final biological product, e.g.,
recombinant
polypeptide. These process contaminants include compounds added to culture
medium in the
course of culturing cells and purifying biological products.
U.S. and foreign regulations often require removal of such contaminants. For
example,
the U.S. Food and Drug Administration (FDA) requires that biopharmaceuticals
intended for in
vivo human use should be as free as possible of extraneous immunoglobulin and
non-
immunoglobulin impurities, and requires tests for detection and quantitation
of potential
impurities. As well, the International Conference on Harmonization (ICH)
provides guidelines
on test procedures and acceptance criteria for biotechnological/biological
products.
Tropolone (2-hydroxy-2,4,6-cycloheptatrien-1-one) is a 7-membered aromatic
ring. It has
.. several uses, including as an antioxidant in cosmetics and topical
pharmaceutical formulations,
as a UV-absorber in sun-screen, and as a catechol-O-methyl-transferase (COMT)
inhibitor.
Tropolone can be added to cell culture media to facilitate the uptake of metal
ions in cultured
cells. In some embodiments, tropolone is added to cell culture media at a
concentration less than
or equal to 0.1, 0.5, 1, 1.25, 1.5, 1.75, 2, 2.25, 2.5, 2.75, 3, 3.5, 4, 4.5,
5, 6, 7, 8, 9, or 10 mg/ml.
In some embodiments, a compound of Formula I, e.g., tropolone, can be added to
cell
culture media to facilitate the uptake of metal ions in cultured cells. In
some embodiments, a
compound of Formula I, e.g., tropolone, is added to cell culture media at a
concentration less
than or equal to 0.1, 0.5, 1, 1.25, 1.5, 1.75, 2, 2.25, 2.5, 2.75, 3, 3.5, 4,
4.5, 5, 6, 7, 8, 9, or 10
mg/ml.
As a synthetic chemical added to a culture of cells used to produce a
biological product,
many regulatory agencies require demonstration of clearance of compounds of
Formula I, e.g.,
4
CA 03083236 2020-05-21
WO 2019/113050
PCT/US2018/063822
tropolone, from biological products, e.g., before they can be declared safe
for in vivo human use.
Many methods of manufacturing or producing biological products comprise
affinity
chromatography steps, e.g., use columns comprising resins that selectively
retain the desired
biological product, and it is expected that compounds of Formula I, e.g.,
tropolone, would pass
.. through such affinity columns prior to elution of the desired biological
product. Any compound
of Formula I, e.g.,tropolone, remaining could be assayed in samples of the
biological product
using: (i) suitable chromatography steps to separate the possible remaining
compound of
Formula I, e.g.,tropolone, from other components of the biological product,
and (ii) suitable
detection and/or quantification steps to determine the presence and abundance
of compound of
.. Formula I, e.g., tropolone. Suitable chromatography steps and detection
methods are described
herein.
The present disclosure describes, inter alia, methods of analyzing samples
comprising a
product and optionally a compound of Formula I, e.g.,tropolone, to determine a
value for the
level of compound of Formula I, e.g., tropolone, present in the sample,
wherein the method is
.. superior with regard to one or more of linear range, precision, accuracy,
and limits of detection
when compared to previously available methods (e.g., RP-HPLC and
UV/fluorescence
detection). In some embodiments, the methods of the disclosure are unaffected
or not
significantly deleteriously affected (e.g., approximately unaffected) with
regard to one or more
of linear range, precision, accuracy, and limits of detection over a range of
products and/or
.. product formulations when compared to previously available methods (e.g.,
RP-HPLC and
UV/fluorescence detection). For example, a method of the disclosure may
determine a value for
a level of a compound of Formula I, e.g.,tropolone, in samples comprising a
variety of buffer
components with no significant drop in accuracy, whereas previously available
methods may
determine a value for a level of a compound of Formula I, e.g.,tropolone, in
samples comprising
.. one buffer component but exhibit a decrease in accuracy when determining a
value for a level of
a compound of Formula I, e.g.,tropolone, in samples comprising another buffer
component.
In some embodiments, methods of the disclosure have a linear range, with
regard to
determining a value for the level of a compound of Formula I, e.g., tropolone,
present in the
sample, of between about 0.1-10000, 0.2-8000, 0.3-7000, 0.4-6000, 0.5-5000,
0.5-4000, 0.5-
.. 3000, 0.5-2000, or 0.5-1000 i.t.g/ml, e.g., 0.5-1000 .t.g/ml. In some
embodiments, methods of the
disclosure have a lower limit of a linear range, with regard to determining a
value for the level of
5
CA 03083236 2020-05-21
WO 2019/113050
PCT/US2018/063822
a compound of Formula I, e.g.,tropolone, present in the sample, of about 0.01,
0.05, 0.1, 0.2,
0.3, 0.35, 0.4, 0.45, 0.5, 0.6, 0.7, 0.8, 0.9, or 1 i.t.g/ml, e.g., 0.5
t.g/ml. In some embodiments,
methods of the disclosure have an upper limit of a linear range, with regard
to determining a
value for the level of a compound of Formula I, e.g.,tropolone, present in the
sample, of about
500, 600, 700, 800, 900, 1000, 1200, 1400, 1600, 1800, 2000, 3000, 4000, 5000,
6000, 7000,
8000, 9000, or 10,000 i.t.g/ml, e.g., 1000 .t.g/ml.
In some embodiments, methods of the disclosure have a precision, with regard
to
determining a value for the level of a compound of Formula I, e.g.,tropolone,
present in the
sample, represented by the standard deviation between replicate samples. In
the same
embodiments, the precision can be less than or equal to about 50, 40, 30, 25,
20, 19, 18, 17, 16,
15, 14, 13, 12, 11, 10, 9, 8,7, 6, 5,4, 3,2, or 1%, e.g., 17, 16.5, or 16%.
In some embodiments, methods of the disclosure have an accuracy, with regard
to
determining a value for the level of a compound of Formula I, e.g.,tropolone,
present in the
sample, represented by average single point spike recovery in three different
samples. In the
same embodiments, the accuracy can be greater than or equal to about 70, 75,
80, 81, 82, 83, 84,
85, 86, 87, 88, 89, 90, 91, 92, 93, 94, or 95%, e.g., 91%.
In some embodiments, methods of the disclosure have a lower limit of detection
with
regard to determining a value for the level of a compound of Formula I,
e.g.,tropolone, present in
the sample. In the same embodiments, the lower limit of detection can be about
1, 1.5, 2, 2.5, 3,
3.5, 4, 4.5, 5, 5.5, 6, 6.5, 7, 7.5, 8, 8.5, 9, 9.5, or 10 .t.g/ml.
The present disclosure further describes, inter alia, methods of manufacturing
a product,
e.g., a recombinant polypeptide, wherein samples of the product are analyzed
by methods of
analyzing samples described herein for the presence or level of a compound of
Formula I,
e.g.,tropolone.
In some embodiments, the sample is a sample of a cosmetic formulation, e.g.,
comprising
a product for use in a cosmetic formulation.
In some embodiments, the sample is a sample of a topical pharmaceutical
formulation,
e.g., comprising a product for use in a pharmaceutical formulation.
In some embodiments, the sample is a sample of a sun-screen, e.g., comprising
a product
for use in a sun-screen, e.g., a compound of Formula I, e.g.,tropolone, and/or
another product for
use in a sun-screen, e.g., another UV-blocker.
6
CA 03083236 2020-05-21
WO 2019/113050
PCT/US2018/063822
In some embodiments, the sample is a sample of COMT inhibitor, e.g.,
comprising a
product for use as a COMT inhibitor, e.g., a compound of Formula I,
e.g.,tropolone, and/or
another product for use as a COMT inhibitor. In some embodiments, the sample
is a sample
comprising L-DOPA (e.g., levodopa or L-3,4-dihydroxyphenylalanine) and/or an
aromatic L-
amino acid decarboxylase inhibitor (e.g., DOPA decarboxylase inhibitor, DDCI,
or AAADI).
The present disclosure further describes, inter alia, reaction mixtures
comprising a
fluorophenyl moiety, e.g., a pentafluorophenylpropyl group, and a sample,
wherein the sample
comprises a compound of Formula I, e.g.,tropolone, another component, and
optionally a
product. In an embodiment, such reaction mixtures may be useful for separating
a compound of
Formula I, e.g.,tropolone, from the component and/or from the product, and, in
further
embodiments, subsequently for detecting the presence of or determining the
level of a compound
of Formula I, e.g.,tropolone. The moieties of the reaction mixture may be
associated with, e.g.,
bound to, e.g., covalently bound to, a substrate, wherein the substrate
comprises an insoluble
substrate, e.g., a chromatography matrix, resin, gel, or beads, e.g., a
silica, agarose, cellulose,
dextran, polyacrylamide, or latex matrix, resin, gel, or beads.
DEFINITIONS
Unless defined otherwise, all technical and scientific terms used herein have
the same
meaning as commonly understood by one of ordinary skill in the art to which
the invention
pertains. Although any methods and materials similar or equivalent to those
described herein can
be used in the practice of and/or for the testing of the present invention,
the preferred materials
and methods are described herein. In describing and claiming the present
invention, the
following terminology will be used according to how it is defined, where a
definition is
provided.
It is also to be understood that the terminology used herein is for the
purpose of
describing particular embodiments only, and is not intended to be limiting.
The articles "a" and "an" are used herein to refer to one or to more than one
(i.e., to at
least one) of the grammatical object of the article. By way of example, "a
cell" can mean one cell
or more than one cell.
As used herein, "about" and "approximately" shall generally mean an acceptable
degree of
error for the quantity measured given the nature or precision of the
measurements. Exemplary
7
CA 03083236 2020-05-21
WO 2019/113050
PCT/US2018/063822
degrees of error are within 20 percent (%), typically, within 10%, and more
typically, within 5% of a
given value or range of values.
As used herein, the term "semi-quantitative" refers to the comparative
assessment of
different chemical species by mass spectrometry without reference to specific
standards for each
individual species.
As used herein, the term "endogenous" refers to any material from or naturally
produced
inside an organism, cell, tissue or system.
As used herein, the term "exogenous" refers to any material introduced to or
produced
outside of an organism, cell, tissue or system. Accordingly, "exogenous
nucleic acid" refers to a
nucleic acid that is introduced to or produced outside of an organism, cell,
tissue or system. In
an embodiment, sequences of the exogenous nucleic acid are not naturally
produced, or cannot
be naturally found, inside the organism, cell, tissue, or system that the
exogenous nucleic acid is
introduced into. In one embodiment, the sequences of the exogenous nucleic
acids are non-
naturally occurring sequences, or encode non-naturally occurring products.
As used herein, the term "heterologous" refers to any material from one
species, when
introduced to an organism, cell, tissue or system from a different species.
As used herein, the terms "nucleic acid," "polynucleotide," or "nucleic acid
molecule"
are used interchangeably and refers to deoxyribonucleic acid (DNA) or
ribonucleic acid (RNA),
or a combination of a DNA or RNA thereof, and polymers thereof in either
single- or double-
stranded form. The term "nucleic acid" includes, but is not limited to, a
gene, cDNA, or an
mRNA. In one embodiment, the nucleic acid molecule is synthetic (e.g.,
chemically synthesized
or artificial) or recombinant. Unless specifically limited, the term
encompasses molecules
containing analogues or derivatives of natural nucleotides that have similar
binding properties as
the reference nucleic acid and are metabolized in a manner similar to
naturally or non-naturally
occurring nucleotides. Unless otherwise indicated, a particular nucleic acid
sequence also
implicitly encompasses conservatively modified variants thereof (e.g.,
degenerate codon
substitutions), alleles, orthologs, SNPs, and complementary sequences as well
as the sequence
explicitly indicated. Specifically, degenerate codon substitutions may be
achieved by generating
sequences in which the third position of one or more selected (or all) codons
is substituted with
.. mixed-base and/or deoxyinosine residues (B atzer et al., Nucleic Acid Res.
19:5081 (1991);
8
CA 03083236 2020-05-21
WO 2019/113050
PCT/US2018/063822
Ohtsuka et al., J. Biol. Chem. 260:2605-2608 (1985); and Rossolini et al.,
Mol. Cell. Probes
8:91-98 (1994)).
As used herein, the terms "peptide," "polypeptide," and "protein" are used
interchangeably, and refer to a compound comprised of amino acid residues
covalently linked by
peptide bonds, or by means other than peptide bonds. A protein or peptide must
contain at least
two amino acids, and no limitation is placed on the maximum number of amino
acids that can
comprise a protein's or peptide's sequence. In one embodiment, a protein may
comprise of more
than one, e.g., two, three, four, five, or more, polypeptides, in which each
polypeptide is
associated to another by either covalent or non-covalent bonds/interactions.
Polypeptides include
any peptide or protein comprising two or more amino acids joined to each other
by peptide bonds
or by means other than peptide bonds. As used herein, the term refers to both
short chains, which
also commonly are referred to in the art as peptides, oligopeptides and
oligomers, for example,
and to longer chains, which generally are referred to in the art as proteins,
of which there are
many types. "Polypeptides" include, for example, biologically active
fragments, substantially
homologous polypeptides, oligopeptides, homodimers, heterodimers, variants of
polypeptides,
modified polypeptides, derivatives, analogs, fusion proteins, among others.
As used herein, "product" refers to a molecule, nucleic acid, polypeptide, or
any hybrid
thereof, that is produced, e.g., expressed, by a cell which has been modified
or engineered to
produce the product. In one embodiment, the product is a naturally occurring
product or a non-
naturally occurring product, e.g., a synthetic product. In one embodiment, a
portion of the
product is naturally occurring, while another portion of the product is non-
naturally occurring.
In one embodiment, the product is a polypeptide, e.g., a recombinant
polypeptide. In one
embodiment, the product is suitable for diagnostic or pre-clinical use. In
another embodiment,
the product is suitable for therapeutic use, e.g., for treatment of a disease.
In one embodiment,
the product is selected from Table 1, Table 2, Table 3, or Table 4. In one
embodiment, the
modified or engineered cells comprise an exogenous nucleic acid that controls
expression or
encodes the product. In other embodiments, the modified or engineered cells
comprise other
molecules, e.g., that are not nucleic acids, that controls the expression or
construction of the
product in the cell.
In one embodiment, the modification of the cell comprises the introduction of
an
exogenous nucleic acid comprising a nucleic acid sequence that controls or
alters, e.g., increases,
9
CA 03083236 2020-05-21
WO 2019/113050
PCT/US2018/063822
the expression of an endogenous nucleic acid sequence, e.g., endogenous gene.
In such
embodiments, the modified cell produces an endogenous polypeptide product that
is naturally or
endogenously expressed by the cell, but the modification increases the
production of the product
and/or the quality of the product as compared to an unmodified cell, e.g., as
compared to
endogenous production or quality of the polypeptide.
In another embodiment, the modification of the cell comprises the introduction
of an
exogenous nucleic acid encoding a recombinant polypeptide as described herein.
In such
embodiments, the modified cell produces a recombinant polypeptide product that
can be
naturally occurring or non-naturally occurring. In such embodiments, the
modified cell produces
.. a recombinant polypeptide product that can also be endogenously expressed
by the cell or not.
In embodiments where the recombinant polypeptide product is also endogenously
expressed by
the cell, the modification increases the production of the product and/or the
quality of the product
as compared to an unmodified cell, e.g., as compared to endogenous production
or quality of the
polypeptide.
As used herein, "recombinant polypeptide" or "recombinant protein" refers to a
polypeptide that can be produced by a cell described herein. A recombinant
polypeptide is one
for which at least one nucleotide of the sequence encoding the polypeptide, or
at least one
nucleotide of a sequence which controls the expression of the polypeptide, was
formed by
genetic engineering (of the cell or of a precursor cell). E.g., at least one
nucleotide was altered,
e.g., it was introduced into the cell or it is the product of a genetically
engineered rearrangement.
In an embodiment, the sequence of a recombinant polypeptide does not differ
from a naturally
occurring isoform of the polypeptide or protein. In an embodiment, the amino
acid sequence of
the recombinant polypeptide differs from the sequence of a naturally occurring
isoform of the
polypeptide or protein. In an embodiment, the recombinant polypeptide and the
cell are from the
same species. In an embodiment, the recombinant polypeptide is endogenous to
the cell, in other
words, the cell is from a first species and the recombinant polypeptide is
native to that first
species. In an embodiment, the amino acid sequence of the recombinant
polypeptide is the same
as or is substantially the same as, or differs by no more than 1%, 2%, 3%, 4%,
5%, 10%, 15%,
20%, 25%, 30%, 35%, 40%, 45%, 50%, 55%, 60%, 65%, 70%, 75%, 80%, 85%, 90%,
95%, or
99% from, a polypeptide encoded by the endogenous genome of the cell. In an
embodiment, the
recombinant polypeptide and the cell are from different species, e.g., the
recombinant
CA 03083236 2020-05-21
WO 2019/113050
PCT/US2018/063822
polypeptide is a human polypeptide and the cell is a non-human, e.g., a
rodent, e.g., a CHO, or
an insect cell. In an embodiment, the recombinant polypeptide is exogenous to
the cell, in other
words, the cell is from a first species and the recombinant polypeptide is
from a second species.
In one embodiment, the polypeptide is a synthetic polypeptide. In one
embodiment, the
.. polypeptide is derived from a non-naturally occurring source. In an
embodiment, the
recombinant polypeptide is a human polypeptide or protein which does not
differ in amino acid
sequence from a naturally occurring isoform of the human polypeptide or
protein. In an
embodiment, the recombinant polypeptide differs from a naturally occurring
isoform of the
human polypeptide or protein at no more than 1, 2, 3, 4, 5, 10, 15 or 20 amino
acid residues. In
an embodiment, the recombinant polypeptide differs from a naturally occurring
isoform of the
human polypeptide by no more than 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, or 15% of its
amino acid residues.
"Acquire" or "acquiring" as the terms are used herein, refer to obtaining
possession of a
physical entity, or a value, e.g., a numerical value, by "directly acquiring"
or "indirectly
acquiring" the physical entity or value. "Directly acquiring" means performing
a process (e.g.,
.. performing a synthetic or analytical method) to obtain the physical entity
or value. "Indirectly
acquiring" refers to receiving the physical entity or value from another party
or source (e.g., a
third party laboratory that directly acquired the physical entity or value).
Directly acquiring a
physical entity includes performing a process that includes a physical change
in a physical
substance, e.g., a starting material. Exemplary changes include making a
physical entity from
two or more starting materials, shearing or fragmenting a substance,
separating or purifying a
substance, combining two or more separate entities into a mixture, performing
a chemical
reaction that includes breaking or forming a covalent or non-covalent bond.
Directly acquiring a
value includes performing a process that includes a physical change in a
sample or another
substance, e.g., performing an analytical process which includes a physical
change in a
substance, e.g., a sample, analyte, or reagent (sometimes referred to herein
as "physical
analysis"), performing an analytical method, e.g., a method which includes one
or more of the
following: separating or purifying a substance, e.g., an analyte, or a
fragment or other derivative
thereof, from another substance; combining an analyte, or fragment or other
derivative thereof,
with another substance, e.g., a buffer, solvent, or reactant; or changing the
structure of an
analyte, or a fragment or other derivative thereof, e.g., by breaking or
forming a covalent or non-
covalent bond, between a first and a second atom of the analyte; or by
changing the structure of a
11
CA 03083236 2020-05-21
WO 2019/113050
PCT/US2018/063822
reagent, or a fragment or other derivative thereof, e.g., by breaking or
forming a covalent or non-
covalent bond, between a first and a second atom of the reagent.
As used herein, a "method of manufacturing" and a "method of production" are
used
interchangeably, and are a series of one or more operations and/or conditions
that produces a
sample comprising a product, e.g., a recombinant polypeptide or a therapeutic
product.
As used herein, MS1 means mass spectrometry.
As used herein, MS2 means tandem mass spectrometry.
The disclosures of each and every patent, patent application, and publication
cited herein
are hereby incorporated herein by reference in their entirety. While this
invention has been
disclosed with reference to specific aspects, it is apparent that other
aspects and variations of this
invention may be devised by others skilled in the art without departing from
the true spirit and
scope of the invention. The appended claims are intended to be construed to
include all such
aspects and equivalent variations.
SAMPLE PREPARATION
Samples for use in the methods of the disclosure can be generated by many
steps of
methods of manufacturing and production of a product, e.g., a recombinant
polypeptide. In some
embodiments, a sample comprises one or more of culture supernatant, cell
lysate, a product
purification intermediate (e.g., a product partially purified from cellular
proteins or other
contaminants), a purified product, and a final formulated product (e.g.,
formulated for in vivo
human use). The product comprised within a sample or generated by a method of
manufacturing
and production may be any product described herein, or known in the art.
CHROMATOGRAPHY
Methods of chromatography suitable for use in the methods described herein are
known
to one of skill in the art and include, e.g., affinity chromatography, gel
filtration chromatography,
ion exchange chromatography, reversed phase chromatography, hydrophobic
interaction
chromatography. In some embodiments, the chromatography method is HPLC
reversed phase
chromatography. Chromatography can include high performance liquid
chromatography
(HPLC), gas chromatography (GC), capillary electrophoresis, ion mobility. See
also, e.g.,
12
CA 03083236 2020-05-21
WO 2019/113050
PCT/US2018/063822
Process Scale Purification of Antibodies, Uwe Gottschalk 2011 John Wiley &
Sons ISBN:
1118210743; Antibodies Vol 1 Production and Purification, G. Subramanian 2013
Springer
Science & Business Media; Basic Methods in Antibody Production and
Characterization, Gary
C. Howard 2000 CRC Press.
Additional exemplary chromatographic methods include, but are not limited to,
Strong
Anion Exchange chromatography (SAX), liquid chromatography (LC), high
performance liquid
chromatography (HPLC), ultra performance liquid chromatography (UPLC), thin
layer
chromatography (TLC), amide column chromatography, and combinations thereof.
In some embodiments, methods of the disclosure employ LC comprising one or
more
(e.g., one, two, or more) mobile phases and a stationary phase. In some
embodiments, the LC
comprises using one mobile phase. In some embodiments, the LC comprises using
two mobile
phases (e.g., a first mobile phase and a second mobile phase). In some
embodiments, the mobile
phase (e.g., a first and/or second mobile phase) comprises formic acid in
water, e.g., about
0.01%, 0.05%, 0.06%, 0.07%, 0.08%, 0.09%, 0.1%, 0.11%, 0.12%, 0.13%, 0.14%,
0.15%,
0.16%, 0.17%, 0.18%, 0.19%, 0.2%, 0.3%, 0.4%, 0.5%, 0.6%, 0.7%, 0.8%, 0.9%, or
1% formic
acid in water. In some embodiments, the mobile phase (e.g., a first and/or
second mobile phase)
comprises formic acid in acetonitrile, e.g., about 0.01%, 0.05%, 0.06%, 0.07%,
0.08%, 0.09%,
0.1%, 0.11%, 0.12%, 0.13%, 0.14%, 0.15%, 0.16%, 0.17%, 0.18%, 0.19%, 0.2%,
0.3%, 0.4%,
0.5%, 0.6%, 0.7%, 0.8%, 0.9%, or 1% formic acid in acetonitrile, e.g., 0.1%
formic acid in
acetonitrile. In some embodiments, "in acetonitrile" refers to a solution,
e.g., mobile phase,
wherein at least about 50, 55, 60, 65,70, 75, 80, 85, 90, 95, or 100% of the
solution, e.g., solvent,
is acetonitrile, e.g., about 100% of the solvent is acetonitrile. In some
embodiments, the
stationary phase comprises a partially or fully fluorinated alkyl or aryl
group, e.g., a fluorophenyl
group, e.g., a pentafluorophenylpropyl group. In some embodiments, the
stationary phase
comprises a silica gel particle attached to a partially or fully fluorinated
alkyl or aryl group, e.g.,
a fluorophenyl group, e.g., a pentafluorophenylpropyl group. In some
embodiments, the
stationary phase pore size is about 100, 110, 120, 130, 140, or 150 A (e.g.,
120 A). In some
embodiments, the LC comprises using a Discovery HS F5 stationary phase, e.g.,
a Discovery HS
F5 column.
Without wishing to be bound by theory, it is thought that the partially or
fully fluorinated
alkyl or aryl group, e.g., a fluorophenyl group, e.g., pentafluorophenyl,
coating of the column
13
CA 03083236 2020-05-21
WO 2019/113050
PCT/US2018/063822
resin changes how tropolone is retained by the column. Whereas more
traditional reverse phase
columns were not sufficiently separating tropolone from interfering
components, the partially or
fully fluorinated alkyl or aryl group, e.g., a fluorophenyl group, e.g.,
pentafluorophenyl, resin
coating is thought to retain hydrophobic groups more readily, and hydrophilic
moieties elute
more readily as a consequence.
MASS SPECTROMETRY
Mass spectrometry methods suitable for use in the methods described herein are
known
to one of skill in the art and include, e.g., electrospray ionization MS,
matrix-assisted laser
desportion/ionization MS (MALDI-MS), time of flight MS, fourier-transform ion
cyclotron
resonance MS, quadrupole time of flight MS, linear quadrupole, quadrupole ion
trap MS,
orbitrap, cylindrical ion trap, three dimensional ion trap, quadruple mass
filter, tandem mass
spectrometry, LC-MS, LC-MS/MS, Fourier transform mass spectrometry (FTMS), ion
mobility
separation with mass spectrometry (IMS-MS), electron transfer dissociation
(ETD-MS), and
combinations thereof. In some embodiments, the mass spectrometry is tandem
mass
spectrometry (M52). See also, e.g., Protein Mass Spectrometry, Julian
Whitelegge 2008,
Elsevier; Protein Sequencing and Identification Using Tandem Mass
Spectrometry, Michael
Kinter 2005, John Wiley & Sons; Characterization of Protein Therapeutics using
Mass
Spectrometry, Guodong Chen 2014, Springer Science & Business Media.
In some embodiments, mass spectrometry suitable for use in the methods
described
herein comprises selected reaction monitoring (SRM), e.g., monitoring a
selected precursor and
product ion pair, e.g., transition. In some embodiments, mass spectrometry
suitable for use in the
methods described herein comprises multiple reaction monitoring (MRM), e.g.,
monitoring a
plurality of product ions derived from one or more precursor ions, e.g., a
plurality of transitions.
In some embodiments, mass spectrometry suitable for use in the methods
described herein
comprises parallel reaction monitoring (PRM), e.g., monitoring a plurality of
transitions in a
single analysis step, e.g., using a high resolution mass spectrometer. In some
embodiments, mass
spectrometry suitable for use in the methods described herein comprises
monitoring a transition
recited in Table 1, e.g., under conditions recited in Table 1.
TROPOLONE AND COMPOUNDS USEFUL IN BIOMANUFACTURING
14
CA 03083236 2020-05-21
WO 2019/113050
PCT/US2018/063822
In some embodiments, a compound may be added to a cell culture medium to
enhance
cell growth. For example, the compound may be used to facilitate the uptake of
metal ions in
cultured cells. In some embodiments, compound added to a cell culture medium
is a compound
of Formula (I):
X
(R2)n Ilk
R1
(I)
or a pharmaceutically acceptable salt, stereoisomer, racemate, or solvate
thereof, wherein:
Xis 0 or S;
R1 is hydrogen, Ci-C6 alkyl, Ci-C6 heteroalkyl, OR3, C(0)R5, C(0)0R3,
N(R4a)(R4b),
C(0)N(R4a)(R4b), or N(R4a)C(0)R5;
each R2 is independently Ci-C6 alkyl, Ci-C6 heteroalkyl, N(R4a)(R4b),
c(0)N(R4a)(R4b),
or N(R4a)C(0)R5; or
two R2 are joined to form a heterocyclyl ring optionally substituted with one
or more R6;
or R1 and R2 are joined to form a heterocyclyl ring optionally substituted
with one or more R6;
R3 is hydrogen, Ci-C6 alkyl, or Ci-C6 heteroalkyl;
R4a and R4b are independently hydrogen, Ci-C6 alkyl, or Ci-C6 heteroalkyl;
R5 is Ci-C6 alkyl or Ci-C6 heteroalkyl;
each R6 is independently Ci-C6 alkyl, Ci-C6 heteroalkyl, halo, oxo, or cyano;
and
n is 0, 1, 2, 4, or 5.
In some embodiments, X is 0. In some embodiments, R1 is OR3 (e.g., OH). In
some
embodiments, n is 0. In some embodiments, the compound of Formula (I) is
tropolone (i.e., 2-
hydroxy-2,4,6-cycloheptatrien-1-one) . In some embodiments, the compound of
Formula (I) is
0
14111k OH
or a pharmaceutically acceptable salt thereof.
In some embodiments, X is 0. In some embodiments, R1 is OR3 (e.g., OH). In
some
embodiments, R2 is OR3 or C(0)0R3 (e.g., OH or C(0)0H). In some embodiments, n
is 3. In
some embodiments, n is 3 and R2 is OH, OH, and C(0)0H. In some embodiments,
the
compound of Formula (I) is puberulic acid (i.e., 4,5,6-trihydroxy-3-
oxocyclohepta-1,4,6-triene-
CA 03083236 2020-05-21
WO 2019/113050
PCT/US2018/063822
0 0
HO
OH
OH
1-carboxylic acid). In some embodiments, the compound of Formula (I) is
HO ,
or a pharmaceutically acceptable salt thereof.
In some embodiments, X is 0. In some embodiments, R1 is hydrogen. In some
embodiments, R2 is OR3 or C(0)0R3 (e.g., OH or C(0)0H). In some embodiments, n
is 3. In
some embodiments, n is 3 and 2 R2 are OH and 1 R2 is C(0)0H. In some
embodiments, the
compound of Formula (I) is stipitatic acid (i.e., 5,6-dihydroxy-3-
oxocyclohepta-1,4,6-triene-1-
0 0
HO
IkT
OH
carboxylic acid). In some embodiments, the compound of Formula (I) is HO
or a
pharmaceutically acceptable salt thereof.
In some embodiments, X is 0. In some embodiments, R1 is OR3 (e.g., OH). In
some
embodiments, R2 is OR3, C(0)R5, or C(0)0R3 (e.g., OH or C(0)0H). In some
embodiments, n
is 3. In some embodiments, n is 3 and 1 R2 is OH. In some embodiments, 2 R2
are joined to
form a heterocylyl ring (e.g., a 5-membered heterocylyl ring, e.g., maleic
anhydride). In some
embodiments, the compound of Formula (I) is stipitatonic acid (i.e., 4,7-
dihydroxy-1H-
cyclohepta[c]furan-1,3,6-trione). In some embodiments, the compound of Formula
(I) is
0
HO
OH
0
0
0 or a pharmaceutically acceptable salt thereof.
In some embodiments, X is 0. In some embodiments, R1 is OR3 (e.g., OH). In
some
embodiments, R2 is OR3, C(0)R5, or C(0)0R3 (e.g., OH or C(0)0H). In some
embodiments, n
is 3. In some embodiments, n is 4 and 2 R2 are OH. In some embodiments, 2 R2
are joined to
form a heterocylyl ring (e.g., a 5-membered heterocylyl ring, e.g., succinic
anhydride). In some
embodiments, the compound of Formula (I) is puberulonic acid (i.e., 6,7,8-
trihydroxy-1H-
16
CA 03083236 2020-05-21
WO 2019/113050
PCT/US2018/063822
cyclohepta[c]furan-1,3,5-trione). In some embodiments, the compound of Formula
(I) is
0 0
0 OH
0 OH
HO or a pharmaceutically acceptable salt thereof.
In some embodiments, X is 0. In some embodiments, R1 is OR3 (e.g., OH). In
some
embodiments, R2 is Ci-C6 alkyl, Ci-C6 heteroalkyl, or OR3 (e.g., OH). In some
embodiments, n
is 3. In some embodiments, n is 3 and 1 R2 is OH. In some embodiments, 2 R2
are joined to
form a heterocylyl ring (e.g., a 6-membered heterocylyl ring, e.g., pyranyl
ring) optionally
substituted with one or more R6. In some embodiments, R6 is OR3 (e.g., OH) or
Ci-C6 alkyl
(e.g., CH3). In some embodiments, the compound of Formula (I) is sepedonin
(i.e., 3,7,9-
trihydroxy-3-methy1-3,4-dihydrocyclohepta[c]pyran-6(1H)-one). In some
embodiments, the
CH3 0
HO
OH
0
compound of Formula (I) is HO or a pharmaceutically
acceptable salt thereof.
In some embodiments, the compound of Formula (I) is a compound disclosed in
U.S.
Patent No. 3,135,768, which is incorporated herein by reference in its
entirety.
Selected Chemical Definitions
Definitions of specific functional groups and chemical terms are described in
more detail
below. The chemical elements are identified in accordance with the Periodic
Table of the
Elements, CAS version, Handbook of Chemistry and Physics, 75th Ed., inside
cover, and specific
functional groups are generally defined as described therein. Additionally,
general principles of
organic chemistry, as well as specific functional moieties and reactivity, are
described in Thomas
Sorrell, Organic Chemistry, University Science Books, Sausalito, 1999; Smith
and March,
March's Advanced Organic Chemistry, 5th Edition, John Wiley & Sons, Inc., New
York, 2001;
Larock, Comprehensive Organic Transformations, VCH Publishers, Inc., New York,
1989; and
Carruthers, Some Modern Methods of Organic Synthesis, 3rd Edition, Cambridge
University
Press, Cambridge, 1987.
Unless otherwise stated, structures depicted herein are also meant to include
all isomeric
(e.g., enantiomeric, diastereomeric, and geometric (or conformational)) forms
of the structure;
17
CA 03083236 2020-05-21
WO 2019/113050
PCT/US2018/063822
for example, the R and S configurations for each asymmetric center, Z and E
double bond
isomers, and Z and E conformational isomers. Therefore, single stereochemical
isomers as well
as enantiomeric, diastereomeric, and geometric (or conformational) mixtures of
the present
compounds are within the scope of the invention. Unless otherwise stated, all
tautomeric forms
of the compounds of the invention are within the scope of the invention.
Additionally, unless
otherwise stated, structures depicted herein are also meant to include
compounds that differ only
in the presence of one or more isotopically enriched atoms. For example,
compounds having the
present structures including the replacement of hydrogen by deuterium or
tritium, or the
replacement of a carbon by a 13C- or 14C-enriched carbon are within the scope
of this invention.
Such compounds are useful, for example, as analytical tools, as probes in
biological assays, or as
therapeutic agents in accordance with the present invention.
Where a particular enantiomer is preferred, it may, in some embodiments be
provided
substantially free of the corresponding enantiomer, and may also be referred
to as "optically
enriched." "Optically-enriched," as used herein, means that the compound is
made up of a
significantly greater proportion of one enantiomer. In certain embodiments the
compound is
made up of at least about 90% by weight of a preferred enantiomer. In other
embodiments the
compound is made up of at least about 95%, 98%, or 99% by weight of a
preferred enantiomer.
Preferred enantiomers may be isolated from racemic mixtures by any method
known to those
skilled in the art, including chiral high pressure liquid chromatography
(HPLC) and the
formation and crystallization of chiral salts or prepared by asymmetric
syntheses. See, for
example, Jacques et al., Enantiomers, Racemates and Resolutions (Wiley
Interscience, New
York, 1981); Wilen, et al., Tetrahedron 33:2725 (1977); Eliel, E.L.
Stereochemistry of Carbon
Compounds (McGraw-Hill, NY, 1962); Wilen, S.H. Tables of Resolving Agents and
Optical
Resolutions p. 268 (E.L. Eliel, Ed., Univ. of Notre Dame Press, Notre Dame, IN
1972).
The term "alkyl," as used herein, refers to a monovalent saturated, straight-
or
branched-chain hydrocarbon such as a straight or branched group of 1-12, 1-10,
or 1-6 carbon
atoms, referred to herein as C i-C 12 alkyl, Ci-Cio alkyl, and C1-C6 alkyl,
respectively. Examples
of alkyl groups include, but are not limited to, methyl, ethyl, n-propyl,
isopropyl, n-butyl,
iso-butyl, sec-butyl, sec-pentyl, iso-pentyl, tert-butyl, n-pentyl, neopentyl,
n-hexyl, sec-hexyl,
and the like.
18
CA 03083236 2020-05-21
WO 2019/113050
PCT/US2018/063822
The term "heterocycly1" refers to a monocyclic, or fused, spiro-fused, and/or
bridged
bicyclic and polycyclic ring system where at least one ring is saturated or
partially unsaturated
(but not aromatic) and comprises a heteroatom. A heterocyclyl can be attached
to its pendant
group at any heteroatom or carbon atom that results in a stable structure and
any of the ring
atoms can be optionally substituted. Representative heterocyclyls include ring
systems in which
(i) every ring is non-aromatic and at least one ring comprises a heteroatom,
e.g.,
tetrahydrofuranyl, tetrahydrothienyl, pyrrolidinyl, pyrrolidonyl, piperidinyl,
pyrrolinyl,
decahydroquinolinyl, oxazolidinyl, piperazinyl, dioxanyl, dioxolanyl,
diazepinyl, oxazepinyl,
thiazepinyl, morpholinyl, and quinuclidinyl; (ii) at least one ring is non-
aromatic and comprises a
heteroatom and at least one other ring is an aromatic carbon ring, e.g.,
1,2,3,4-tetrahydroquinolinyl, 1,2,3,4-tetrahydroisoquinolinyl; and (iii) at
least one ring is
non-aromatic and comprises a heteroatom and at least one other ring is
aromatic and comprises a
heteroatom, e.g., 3,4-dihydro-1H-pyrano[4,3-c]pyridine, and
1,2,3,4-tetrahydro-2,6-naphthyridine
As described herein, compounds of the invention may contain "optionally
substituted"
moieties. In general, the term "substituted", whether preceded by the term
"optionally" or not,
means that one or more hydrogens of the designated moiety are replaced with a
suitable
substituent. Unless otherwise indicated, an "optionally substituted" group may
have a suitable
substituent at each substitutable position of the group, and when more than
one position in any
given structure may be substituted with more than one substituent selected
from a specified
group, the substituent may be either the same or different at each position.
Combinations of
substituents envisioned under this invention are preferably those that result
in the formation of
stable or chemically feasible compounds. The term "stable", as used herein,
refers to compounds
that are not substantially altered when subjected to conditions to allow for
their production,
detection, and, in certain embodiments, their recovery, purification, and use
for one or more of
the purposes disclosed herein.
As used herein, the term "pharmaceutically acceptable salt" refers to those
salts which
are, within the scope of sound medical judgment, suitable for use in contact
with the tissues of
humans and lower animals without undue toxicity, irritation, allergic response
and the like, and
are commensurate with a reasonable benefit/risk ratio. Pharmaceutically
acceptable salts are
well known in the art. For example, Berge et al., describe pharmaceutically
acceptable salts in
19
CA 03083236 2020-05-21
WO 2019/113050
PCT/US2018/063822
detail in J. Pharmaceutical Sciences, 1977, 66, 1-19, incorporated herein by
reference.
Pharmaceutically acceptable salts of the compounds of this invention include
those derived from
suitable inorganic and organic acids and bases. Examples of pharmaceutically
acceptable, salts
are salts of an amino group formed with inorganic acids such as hydrochloric
acid, hydrobromic
acid, phosphoric acid, sulfuric acid, and perchloric acid or with organic
acids such as acetic acid,
oxalic acid, maleic acid, tartaric acid, citric acid, succinic acid, or
malonic acid or by using other
methods known in the art such as ion exchange. Other pharmaceutically
acceptable salts include
adipate, alginate, ascorbate, aspartate, benzenesulfonate, benzoate,
bisulfate, borate, butyrate,
camphorate, camphorsulfonate, citrate, cyclopentanepropionate, digluconate,
dodecylsulfate,
ethanesulfonate, formate, fumarate, glucoheptonate, glycerophosphate,
gluconate, hemisulfate,
heptanoate, hexanoate, hydroiodide, 2¨hydroxy¨ethanesulfonate, lactobionate,
lactate, laurate,
lauryl sulfate, malate, maleate, malonate, methanesulfonate,
2¨naphthalenesulfonate, nicotinate,
nitrate, oleate, oxalate, palmitate, pamoate, pectinate, persulfate,
3¨phenylpropionate, phosphate,
picrate, pivalate, propionate, stearate, succinate, sulfate, tartrate,
thiocyanate, p-toluenesulfonate,
undecanoate, valerate salts, and the like. Salts derived from appropriate
bases include alkali
metal, alkaline earth metal, ammonium and N (C 1_4 alky1)4- salts.
Representative alkali or
alkaline earth metal salts include sodium, lithium, potassium, calcium,
magnesium, and the like.
Further pharmaceutically acceptable salts include, when appropriate, nontoxic
ammonium,
quaternary ammonium, and amine cations formed using counterions such as
halide, hydroxide,
carboxylate, sulfate, phosphate, nitrate, lower alkyl sulfonate, and aryl
sulfonate.
The term "solvate" refers to forms of the compound that are associated with a
solvent,
usually by a solvolysis reaction. This physical association may include
hydrogen bonding.
Conventional solvents include water, methanol, ethanol, acetic acid, DMS 0,
THF, diethyl ether,
and the like. The compounds of Formula (I) may be prepared, e.g., in
crystalline form, and may
be solvated. Suitable solvates include pharmaceutically acceptable solvates
and further include
both stoichiometric solvates and non-stoichiometric solvates. In certain
instances, the solvate
will be capable of isolation, for example, when one or more solvent molecules
are incorporated
in the crystal lattice of a crystalline solid. "Solvate" encompasses both
solution-phase and
isolable solvates. Representative solvates include hydrates, ethanolates, and
methanolates.
It is also to be understood that compounds that have the same molecular
formula but
differ in the nature or sequence of bonding of their atoms or the arrangement
of their atoms in
CA 03083236 2020-05-21
WO 2019/113050
PCT/US2018/063822
space are termed "isomers". Isomers that differ in the arrangement of their
atoms in space are
termed "stereoisomers". Stereoisomers that are not mirror images of one
another are termed
"diastereomers" and those that are non-superimposable mirror images of each
other are termed
"enantiomers". When a compound has an asymmetric center, for example, it is
bonded to four
different groups, a pair of enantiomers is possible. An enantiomer can be
characterized by the
absolute configuration of its asymmetric center and is described by the R- and
S-sequencing
rules of Cahn and Prelog, or by the manner in which the molecule rotates the
plane of polarized
light and designated as dextrorotatory or levorotatory (i.e., as (+) or (-)-
isomers respectively). A
chiral compound can exist as either individual enantiomer or as a mixture
thereof. A mixture
containing equal proportions of the enantiomers is called a "racemic mixture".
PRODUCTION PARAMETERS
The methods described herein can be used to analyze samples generated by
methods of
manufacturing and production, e.g., of recombinant polypeptides. The methods
of
.. manufacturing and production may be characterized by a variety of
production parameters.
A production parameter as used herein is a parameter or element in a
production process.
Production parameters that can be selected include, e.g., the cell or cell
line used to produce the
glycoprotein preparation, the culture medium, culture process or bioreactor
variables (e.g., batch,
fed-batch, or perfusion), purification process and formulation of a
glycoprotein preparation.
Primary production parameters include: 1) the types of host; 2) genetics of
the host; 3)
media type; 4) fermentation platform; 5) purification steps; and 6)
formulation. Secondary
production parameter, as used herein, is a production parameter that is
adjustable or variable
within each of the primary production parameters. Examples include: selection
of host subclones
based on desired glycan properties; regulation of host gene levels
constitutive or inducible;
introduction of novel genes or promoter elements; media additives (e.g.
partial list on Table IV);
physiochemical growth properties; growth vessel type (e.g. bioreactor type, T
flask); cell density;
cell cycle; enrichment of product with a desired glycan type (e.g. by lectin
or antibody-mediated
enrichment, ion-exchange chromatography, CE, or similar method); or similar
secondary
production parameters clear to someone skilled in the art.
Media
21
CA 03083236 2020-05-21
WO 2019/113050
PCT/US2018/063822
The methods of manufacturing and production described herein can include
determining
and/or selecting a media component and/or the concentration of a media
component that has a
positive correlation to a desired glycan property or properties. A media
component can be added
in or administered over the course of glycoprotein production or when there is
a change media,
depending on culture conditions. Media components include components added
directly to
culture as well as components that are a byproduct of cell culture.
Media components include, e.g., buffer, amino acid content, vitamin content,
salt content,
mineral content, serum content, carbon source content, lipid content, nucleic
acid content,
hormone content, trace element content, ammonia content, co-factor content,
indicator content,
small molecule content, hydrolysate content and enzyme modulator content.
Examples of various media components are provided below:
amino acids sugar precursors
Vitamins Indicators
Carbon source (natural and Nucleosides or nucleotides
unnatural)
Salts butyrate or organics
Sugars DMSO
Sera Animal derived products
Plant derived hydrolysates Gene inducers
sodium pyruvate Non natural sugars
Surfactants Regulators of intracellular pH
Ammonia Betaine or osmoprotectant
Lipids Trace elements
Hormones or growth factors minerals
Buffers Non natural amino acids
Non natural amino acids Non natural vitamins
Exemplary buffers include Tris, Tricine, HEPES, MOPS, PIPES, TAPS, bicine,
BES,
TES, cacodylate, MES, acetate, MKP, ADA, ACES, glycinamide and
acetamidoglycine. The
media can be serum free or can include animal derived products such as, e.g.,
fetal bovine serum
(FBS), fetal calf serum (FCS), horse serum (HS), human serum, animal derived
serum substitutes
(e.g., Ultroser G, SF and HY; non-fat dry milk; Bovine EX-CYTE), fetuin,
bovine serum
albumin (BSA), serum albumin, and transferrin. When serum free media is
selected lipids such
as, e.g., palmitic acid and/or steric acid, can be included.
Lipids components include oils, saturated fatty acids, unsaturated fatty
acids, glycerides,
steroids, phospholipids, sphingolipids and lipoproteins. Exemplary amino acid
that can be
22
CA 03083236 2020-05-21
WO 2019/113050
PCT/US2018/063822
included or eliminated from the media include alanine, arginine, asparagine,
aspartic acid,
cysteine, glutamic acid, glutamine, glycine, histidine, proline, isoleucine,
leucine, lysine,
methionine, phenylalanine, proline, serine, threonine, tryptophan, tyrosine
and valine. Examples
of vitamins that can be present in the media or eliminated from the media
include vitamin A
(retinoid), vitamin B1 (thiamine), vitamin B2 (riboflavin), vitamin B3
(niacin), vitamin B5
(pantothenic acid), vitamin B6 (pyroxidone), vitamin B7 (biotin), vitamin B9
(folic acid),
vitamin. B12 (cyanocobalamin), vitamin C (ascorbic acid), vitamin D, vitamin
E, and vitamin K.
Minerals that can be present in the media or eliminated from the media include
bismuth,
boron, calcium, chlorine, chromium, cobalt, copper, fluorine, iodine, iron,
magnesium,
manganese, molybdenum, nickel, phosphorus, potassium, rubidium, selenium,
silicon, sodium,
strontium, sulfur, tellurium, titanium, tungsten, vanadium, and zinc.
Exemplary salts and
minerals include CaCl2 (anhydrous), CuSO4 5H20, Fe(NO3).9H20, KC1, KNO3,
KH2PO4,
MgSO4 (anhydrous), NaCl, NaH2PO4H20, NaHCO3, Na2SE3 (anhydrous), ZnSO4.7H20;
linoleic acid, lipoic acid, D-glucose, hypoxanthine 2Na, phenol red,
putrescine 2HC1, sodium
pyruvate, thymidine, pyruvic acid, sodium succinate, succinic acid, succinic
acid.Na.hexahydrate, glutathione (reduced), para-aminobenzoic acid (PABA),
methyl linoleate,
bacto peptone G, adenosine, cytidine, guanosine, 2'-deoxyadenosine HC1, 2'-
deoxycytidine HC1,
2'-deoxyguanosine and uridine. When the desired glycan characteristic is
decreased fucosylation,
the production parameters can include culturing a cell, e.g., CHO cell, e.g.,
dhfr deficient CHO
cell, in the presence of manganese, e.g., manganese present at a concentration
of about 0.111M to
50 p.M. Decreased fucosylation can also be obtained, e.g., by culturing a cell
(e.g., a CHO cell,
e.g., a dhfr deficient CHO cell) at an osmolality of about 350 to 500 mOsm.
Osmolality can be
adjusted by adding salt to the media or having salt be produced as a byproduct
as evaporation
occurs during production.
Hormones include, for example, somatostatin, growth hormone-releasing factor
(GRF),
insulin, prolactin, human growth hormone (hGH), somatotropin, estradiol, and
progesterone.
Growth factors include, for example, bone morphogenic protein (BMP), epidermal
growth factor
(EGF), basic fibroblast growth factor (bFGF), nerve growth factor (NGF), bone
derived growth
factor (BDGF), transforming growth factor-betal (TGF-betal), [Growth factors
from U.S. Pat.
No. 6,838,284 B2], hemin and NAD. Examples of surfactants that can be present
or eliminated
23
CA 03083236 2020-05-21
WO 2019/113050
PCT/US2018/063822
from the media include Tween-80 and pluronic F-68. Small molecules can
include, e.g., butyrate,
ammonia, non natural sugars, non natural amino acids, chloroquine, and
betaine.
Physiochemical Parameters
Production parameters can also include physiochemical parameters. Such
conditions can
include temperature, pH, osmolality, shear force or agitation rate, oxidation,
spurge rate, growth
vessel, tangential flow, DO, CO2, nitrogen, fed batch, redox, cell density and
feed strategy.
Examples of physiochemical parameters that can be selected include, e.g., pH,
osmolality, shear
force or agitation rate, oxidation, spurge rate, growth vessel, tangential
flow, batch dissolved 02,
CO2, nitrogen, fed batch, redox, cell density, perfusion culture, feed
strategy, temperature and
time of culture.
Additional production parameters are known to one of skill in the art, see
e.g., Antibody
Expression and Production (2011) Ed. Mohamed Al-Rubeai; Springer Publishing.
PRODUCTS AND NUCLEIC ACIDS ENCODING THEM
Provided herein are methods of analyzing samples, e.g., samples produced by
methods of
manufacturing and production, e.g., of recombinant polypeptides. The methods
of
manufacturing and production may comprise identifying, selecting, or making a
cell or cell line
capable of producing a product, e.g., cells and products as recited herein.
The products
encompassed by the present disclosure include, but are not limited to,
molecules, nucleic acids,
polypeptides (e.g., recombinant polypeptides, e.g., antibodies, bispecific
antibodies,
multispecific antibodies), or hybrids thereof, that can be produced by, e.g.,
expressed in, a cell.
In some embodiments, the cells are engineered or modified to produce the
product. Such
modifications include the introducing molecules that control or result in
production of the
product. For example, a cell is modified by introducing an exogenous nucleic
acid that encodes
a polypeptide, e.g., a recombinant polypeptide, and the cell is cultured under
conditions suitable
for production, e.g., expression and secretion, of the polypeptide, e.g.,
recombinant polypeptide.
In embodiments, the cultured cells are used to produce proteins e.g.,
antibodies, e.g.,
monoclonal antibodies, and/or recombinant proteins, for therapeutic use. In
embodiments, the
cultured cells produce peptides, amino acids, fatty acids or other useful
biochemical
intermediates or metabolites. For example, in embodiments, molecules having a
molecular
24
CA 03083236 2020-05-21
WO 2019/113050
PCT/US2018/063822
weight of about 4000 daltons to greater than about 140,000 daltons can be
produced. In
embodiments, these molecules can have a range of complexity and can include
posttranslational
modifications including glycosylation.
In embodiments, the polypeptide is, e.g., BOTOX, Myobloc, Neurobloc, Dysport
(or
other serotypes of botulinum neurotoxins), alglucosidase alpha, daptomycin, YH-
16,
choriogonadotropin alpha, filgrastim, cetrorelix, interleukin-2, aldesleukin,
teceleulin, denileukin
diftitox, interferon alpha-n3 (injection), interferon alpha-nl, DL-8234,
interferon, Suntory
(gamma-la), interferon gamma, thymosin alpha 1, tasonermin, DigiFab,
ViperaTAb, EchiTAb,
CroFab, nesiritide, abatacept, alefacept, Rebif, eptoterminalfa, teriparatide,
calcitonin,
etanercept, hemoglobin glutamer 250 (bovine), drotrecogin alpha, collagenase,
carperitide,
recombinant human epidermal growth factor, DWP401, darbepoetin alpha, epoetin
omega,
epoetin beta, epoetin alpha, desirudin, lepirudin, bivalirudin, nonacog alpha,
Mononine, eptacog
alpha (activated), recombinant Factor VIII+VWF, Recombinate, recombinant
Factor VIII, Factor
VIII (recombinant), Alphnmate, octocog alpha, Factor VIII,
palifermin,Indikinase, tenecteplase,
alteplase, pamiteplase, reteplase, nateplase, monteplase, follitropin alpha,
rFSH, hpFSH,
micafungin, pegfilgrastim, lenograstim, nartograstim, sermorelin, glucagon,
exenatide,
pramlintide, iniglucerase, galsulfase, Leucotropin, molgramostirn, triptorelin
acetate, histrelin
(Hydron), deslorelin, histrelin, nafarelin, leuprolide (ATRIGEL), leuprolide
(DUROS),
goserelin, Eutropin, somatropin, mecasermin, enlfavirtide, Org-33408, insulin
glargine, insulin
glulisine, insulin (inhaled), insulin lispro, insulin deternir, insulin
(RapidMist), mecasermin
rinfabate, anakinra, celmoleukin, 99 mTc-apcitide, myelopid, Betaseron,
glatiramer acetate,
Gepon, sargramostim, oprelvekin, human leukocyte-derived alpha interferons,
Bilive, insulin
(recombinant), recombinant human insulin, insulin aspart, mecasenin, Roferon-
A, interferon-
alpha 2, Alfaferone, interferon alfacon-1, interferon alpha, Avonex'
recombinant human
luteinizing hormone, dornase alpha, trafermin, ziconotide, taltirelin,
diboterminalfa, atosiban,
becaplermin, eptifibatide, Zemaira, CTC-111, Shanvac-B, octreotide,
lanreotide, ancestirn,
agalsidase beta, agalsidase alpha, laronidase, prezatide copper acetate,
rasburicase, ranibizumab,
Actimmune, PEG-Intron, Tricomin, recombinant human parathyroid hormone (PTH) 1-
84,
epoetin delta, transgenic antithrombin III, Granditropin, Vitrase, recombinant
insulin, interferon-
alpha, GEM-21S, vapreotide, idursulfase, omnapatrilat, recombinant serum
albumin,
certolizumab pegol, glucarpidase, human recombinant Cl esterase inhibitor,
lanoteplase,
CA 03083236 2020-05-21
WO 2019/113050
PCT/US2018/063822
recombinant human growth hormone, enfuvirtide, VGV-1, interferon (alpha),
lucinactant,
aviptadil, icatibant, ecallantide, omiganan, Aurograb, pexigananacetate, ADI-
PEG-20, LDI-200,
degarelix, cintredelinbesudotox, Favld, MDX-1379, ISAtx-247, liraglutide,
teriparatide,
tifacogin, AA4500, T4N5 liposome lotion, catumaxomab, DWP413, ART-123,
Chrysalin,
desmoteplase, amediplase, corifollitropinalpha, TH-9507, teduglutide, Diamyd,
DWP-412,
growth hormone, recombinant G-CSF, insulin, insulin (Technosphere), insulin
(AERx), RGN-
303, DiaPep277, interferon beta, interferon alpha-n3, belatacept, transdermal
insulin patches,
AMG-531, MBP-8298, Xerecept, opebacan, AIDS VAX, GV-1001, LymphoScan,
ranpirnase,
Lipoxysan, lusupultide, MP52, sipuleucel-T, CTP-37, Insegia, vitespen, human
thrombin,
thrombin, TransMID, alfimeprase, Puricase, terlipressin, EUR-1008M,
recombinant FGF-I,
BDM-E, rotigaptide, ETC-216, P-113, MBI-594AN, duramycin, SCV-07, OPI-45,
Endostatin,
Angiostatin, ABT-510, Bowman Birk Inhibitor, XMP-629, 99 mTc-Hynic-Annexin V,
kahalalide F, CTCE-9908, teverelix, ozarelix, rornidepsin, BAY-504798,
interleukin4, PRX-321,
Pepscan, iboctadekin, rhlactoferrin, TRU-015, IL-21, ATN-161, cilengitide,
Albuferon,
Biphasix, IRX-2, omega interferon, PCK-3145, CAP-232, pasireotide, huN901-DMI,
SB-
249553, Oncovax-CL, OncoVax-P, BLP-25, CerVax-16, MART-1, gp100, tyrosinase,
nemifitide, rAAT, CGRP, pegsunercept, thymosinbeta4, plitidepsin, GTP-200,
ramoplanin,
GRASPA, OBI-1, AC-100, salmon calcitonin (eligen), examorelin, capromorelin,
Cardeva,
velafermin, 131I-TM-601, KK-220, T-10, ularitide, depelestat, hematide,
Chrysalin, rNAPc2,
recombinant Factor V111 (PEGylated liposomal), bFGF, PEGylated recombinant
staphylokinase
variant, V-10153, SonoLysis Prolyse, NeuroVax, CZEN-002, rGLP-1, BIM-51077, LY-
548806,
exenatide (controlled release, Medisorb), AVE-0010, GA-GCB, avorelin, ACM-
9604, linaclotid
eacetate, CETi-1, Hemospan, VAL, fast-acting insulin (injectable, Viadel),
insulin (eligen),
recombinant methionyl human leptin, pitrakinra, Multikine, RG-1068, MM-093,
NBI-6024, AT-
001, PI-0824, Org-39141, Cpn10, talactoferrin, rEV-131, rEV-131, recombinant
human insulin,
RPI-78M, oprelvekin, CYT-99007 CTLA4-Ig, DTY-001, valategrast, interferon
alpha-n3, IRX-
3, RDP-58, Tauferon, bile salt stimulated lipase, Merispase, alaline
phosphatase, EP-2104R,
Melanotan-II, bremelanotide, ATL-104, recombinant human microplasmin, AX-200,
SEMAX,
ACV-1, Xen-2174, CJC-1008, dynorphin A, SI-6603, LAB GHRH, AER-002, BGC-728,
ALTU-135, recombinant neuraminidase, Vacc-5q, Vacc-4x, Tat Toxoid, YSPSL, CHS-
13340,
PTH(1-34) (Novasome), Ostabolin-C, PTH analog , MBRI-93.02, MTB72F, MVA-Ag85A,
26
CA 03083236 2020-05-21
WO 2019/113050
PCT/US2018/063822
FARA04, BA-210, recombinant plague FIV, AG-702, OxSODrol, rBetV1, Der-pl/Der-
p2/Der-
p'7, PR1 peptide antigen, mutant ras vaccine, HPV-16 E7 lipopeptide vaccine,
labyrinthin, WT1-
peptide, IDD-5, CDX-110, Pentrys, Norelin, CytoFab, P-9808, VT-111,
icrocaptide, telbermin,
rupintrivir, reticulose, rGRF, HA, alpha-galactosidase A, ACE-011, ALTU-140,
CGX-1160,
angiotensin, D-4F, ETC-642, APP-018, rhMBL, SCV-07, DRF-7295, ABT-828, ErbB2-
specific
immunotoxin, DT3SSIL-3, TST-10088, PRO-1762, Combotox, cholecystokinin-
B/gastrin-
receptor binding peptides, 111In-hEGF, AE-37, trasnizumab-DM1, Antagonist G,
IL-12, PM-
02734, IMP-321, rhIGF-BP3, BLX-883, CUV-1647, L-19 based ra, Re-188-P-2045,
AMG-386,
DC/1540/KLH, VX-001, AVE-9633, AC-9301, NY-ES0-1 (peptides), NA17.A2 peptides,
CBP-
501, recombinant human lactoferrin, FX-06, AP-214, WAP-8294A, ACP-HIP, SUN-
11031,
peptide YY [3-36], FGLL, atacicept, BR3-Fc, BN-003, BA-058, human parathyroid
hormone 1-
34, F-18-CCR1, AT-1100, JPD-003, PTH(7-34) (Novasome), duramycin, CAB-2, CTCE-
0214,
GlycoPEGylated erythropoietin, EPO-Fc, CNTO-528, AMG-114, JR-013, Factor XIII,
aminocandin, PN-951, 716155, SUN-E7001, TH-0318, BAY-73-7977, teverelix, EP-
51216,
hGH, OGP-I, sifuvirtide, TV4710, ALG-889, Org-41259, rhCC10, F-991,
thymopentin,
r(m)CRP, hepatoselective insulin, subalin, L19-IL-2 fusion protein, elafin,
NMK-150, ALTU-
139, EN-122004, rhTPO, thrombopoietin receptor agonist, AL-108, AL-208, nerve
growth factor
antagonists, SLV-317, CGX-1007, INNO-105, teriparatide (eligen), GEM-0S1, AC-
162352,
PRX-302, LFn-p24 fusion, EP-1043, gpEl, gpE2, MF-59, hPTH(1-34) , 768974, SYN-
101,
PGN-0052, aviscumnine, BIM-23190, multi-epitope tyrosinase peptide, enkastim,
APC-8024,
GI-5005, ACC-001, TTS-CD3, vascular-targeted TNF, desmopressin, onercept, and
TP-9201.
In some embodiments, the polypeptide is adalimumab (HUMIRA), infliximab
(REMICADETm), rituximab (RITUXANTm/MAB THERATm) etanercept (ENBRELTm),
bevacizumab (AVASTINTm), trastuzumab (HERCEPT1NTm), pegrilgrastim
(NEULASTATm), or
any other suitable polypeptide including biosimilars and biobetters.
Other suitable polypeptides are those listed below and in Table 1 of
US2016/0097074:
27
CA 03083236 2020-05-21
WO 2019/113050 PCT/US2018/063822
Table
Protein Product Reference Listed Drug
interferon a III ITIa- lb _________________ Actimmune
ate .lase. tissue .lasmino=-n activator Actwase /C.athflo
Recombinant antihemophilic factor Advate_ ......
human albumin Albuteine
Laronldase __________________________________ Aldurazyme0
Interferon alfa-N3, human ieulcoate derived __ Alferon N
human antihemoohilic factor Alptianate0
virus-filtered human cokagation factor IX _Alp.haNine0SO
6.1422g2t-Lrecornb1nant, dimeric fusion_protein LFA3-1q. Amevivee
Bivalirudin Angiomax
darbe.oetin alfa Aranesf"
Beyacizumab Avastinm
interferon beta-lai recombinant Avonea ........
coaplation factor IX BeneFIx'"
interferon beta-lb Betaseronds)
Tositumomab BEXXARO
anthem() I hilic racWr Bloclate"
human crowth hormone BloTro.g.nr"
botulinum toxin type A j__BOTCX
______________________________________________________________________ 1
Alemtuzurnab Campathrti
acritumomab; technetium-99 labeled CEA-Scan
aclucerase; modified form of beta;sthisocerebrosidase CeredaseQ
aniclucerase; recombinant form of beta-alucocerebrosidase Cerezyme
crotalidae polyvalent immune Fab., ovine CroFab'" 1
28
CA 03083236 2020-05-21
WO 2019/113050 PCT/US2018/063822
Protein Product Reference Listed Drug
dicioxin immune fab (ovine] DigiFabnil
Rasburicase Elitekr,Fiz)
Etanercept ENBRELO
epoletin alfa Epogengi
Cetuximab Erbitux''
_f_l_gasiOase beta Fabrazyme
Urofailitrobin Fertinex'm ________________
failitropin beta
Teriparatide FORTE0(k)
human sornatropin _____________________________ GenoTropin(A)
Glucager GlucaGen
follitrooin ails Gonal-F
antiherrophiilc factor Helixate(F)
Antihemophilic Factor,,Factor XIII FiEMOF1L
adefovir dipivoxil Hepsera
Trastuzurnab 1-ierceptiWg
Insulin Humalog __
_antihemophilic factor/von Willebrand factor complex-human Humate-P
Sornatotropin FlumatrobeR
Adalimumab 1-11.1MIRA'm
human insulin Hun-luting
recombinant human tiyaluronldase Hylenex''
interferon alfacon-1 Inferclen(Pi)
eptifibatide Intedrilin"
alpha-interferon Intron
Petite-1min Kepivance
Anakinra Kineret'''
antihemophik factor Kolenato FS
insulin glarbine Lantus
granulocyte macrophage colony-stimulating factor Leukine /Leuidne Liquid
lutroin aifa for Injection Luveris
OspA lipoprotein LYMErIx'
Ranibizumab LUCENTIS ______________________________________
gerrtuzumab ozogamicin Mylptarcin^
GalsulFase Naolazypeol4
Nesiritide Natrecor
PegfilorastIm Neulasta`"
Opreivekin Neurne.ga
Fii rastim Neupocen
Fanolesomab NeutroSpec.",
(forrnerly_LeuTechK___
somatruin ilorditropin /Nordilrppin
Nordiflex
ffitLrt. ...................................... Novantrone
insulini zinc supsnsion; Novolin L
I insulin; j .2phane s.uspens-lon Novolln N
insulin., regular Novolin R
Insulin Novolin
coagulation factor VITa NovoSeven
Somatro In Nutropin
immunoqlobulin intravenous Octagon, i --
PEG-Liaspar,aglilase Oncaspar
abatacept, fully human soluable fusion protein Orencia fr'
rnuremornab-CD3 Orthoclone OKT3
rinh-molecular weIght hyaiuronan Orthovisc
hurman chorionic onadotropin Ovidrelqi)
I live attenuated Bacillus Calmette-Guerin .... Pads
CA 03083236 2020-05-21
WO 2019/113050 PCT/US2018/063822
abatacept, fully human soluable fusion Rrotel n
1.,
muromornab-CD3
_______________________________________________________ orthocione OKT36.3)
22Lgh-molecular weight hyaluronan
human chorlooic lonaotr.2 oo12 Orencia"'
Orthovisc
Oyidrel(R)
live attenuated Bacillus Calmette-Guerin Pads
P rotei n Product Reference Lasted
Drug
peqinterferon alfa-2a
peoyEated version of Interferon alfa-2b PEG-IntronT"
____________________________
Aberelix (injectable suspension); gonadotropin-releasing hormone Plenaxis''
antagonist
.1
,--
1 enoietin alfe _____________________ Procrit
=
Aldesieukin proieukin, IL-20
SOillatrem Protropin
dornase AKA Puimozymefik)_
Efalizurnab- selective; reversible T-cell blocker RAPTIVA'm
combination of ribavirin and alpha interferon RebetroriT''
Interferon beta la Rebif(qi
antihemophilic factor , Recorrhinatee rAHF/
antinemophilic factor ReFactoQ-P,
Legirudin Refludar
Infilxirnab i __ REMICADE ..
_
....
Abciximab ReoPro'"
Retepiase Retavase''
._
Rituxima Rituxan'" ..
interferon alta-25 Roferon-A(6, __
Somatrp ,in _ Saizen
s mthetic oorcine secretin Se.creFlom __
Basiliximab Sirnulect
______________
Ecuurnab SOURIS (R)
PeL visomant SONAVERT
Paiiyrguiriabi recombiriantiy produced, humanized mAb S nagis'm
_thyi9tr,2pin alfa ihyrogena
Tenectepiase TNKase'''
ilatalizumab ri'SABRTS
................._,
human immune g I obu I in intravenous; 5% and 10% solutions
Venoglobulici:SCA)_
inte.rferon alfa-rilLlymptioblastoid .................. itv'ellferon(k
, dretrecccin alfa =Eqris ''"
,,Dmaiizurnab; recombinant DNA-derived humanized monoclonal Xolair
antibody targeting immunoglobillin-E
Daclizumab Zena ax
ibriturflOrnab tluxetan Zevalinu4
Somatotropin Zorbtivem
j5erostime)
In embodiments, the polypeptide is a hormone, blood clotting/coagulation
factor,
cytokine/growth factor, antibody molecule, fusion protein, protein vaccine, or
peptide as shown
in Table 2.
CA 03083236 2020-05-21
WO 2019/113050
PCT/US2018/063822
Table 2. Exemplary Products
Therapeutic Product Trade Name
Product type
Hormone Erythropoietin, Epoein-a Epogen, Procrit
Darbepoetin¨a Aranesp
Growth hormone (GH), Genotropin , Humatrope,
Norditropin,
somatotropin NovIVitropin, Nutropin, Omnitrope,
Protropin, Siazen, Serostim, Valtropin
Gonal-F, Follistim
Human follicle-stimulating
hormone (FSH)
Human chorionic Ovidrel
gonadotropin Luveris
Lutropin-a GlcaGen
Glucagon Geref
Growth hormone releasing ChiRhoStim (human peptide),
SecreFlo
hormone (GHRH) (porcine peptide)
Secretin Thyrogen
Thyroid stimulating
hormone (TSH), thyrotropin
Blood Factor VIIa NovoSeven
Clotting/Coagulation Factor VIII Bioclate, Helixate, Kogenate,
Factors Recombinate, ReFacto
Factor IX
Antithrombin III (AT-III) Benefix
Protein C concentrate Thrombate III
Ceprotin
31
CA 03083236 2020-05-21
WO 2019/113050
PCT/US2018/063822
Cytokine/Growth Type I alpha-interferon Infergen
factor Interferon-an3 (IFNan3) Alferon N
Interferon-31a (rIFN- r3) Avonex, Rebif
Interferon-31b (rIFN- r3) Betaseron
Interferon-ylb (IFN 7) Actimmune
Aldesleukin (interleukin Proleukin
2(IL2), epidermal
theymocyte activating
factor; ETAF
Kepivance
Palifermin (keratinocyte
Regranex
growth factor; KGF)
Becaplemin (platelet-
Anril, Kineret
derived growth factor;
PDGF)
Anakinra (recombinant IL1
antagonist)
Antibody molecules Bevacizumab (VEGFA Avastin
mAb) Erbitux
Cetuximab (EGFR mAb) Vectibix
Panitumumab (EGFR mAb) Campath
Alemtuzumab (CD52 mAb) Rituxan
Rituximab (CD20 chimeric Herceptin
Ab)
Orencia
Trastuzumab (HER2/Neu
Humira
mAb)
Enbrel
Abatacept (CTLA Ab/Fc
32
CA 03083236 2020-05-21
WO 2019/113050
PCT/US2018/063822
fusion) Remicade
Adalimumab (TNFa mAb) Amevive
Etanercept (TNF Raptiva
receptor/Fc fusion) Tysabri
Infliximab (TNFa chimeric Soliris
mAb) Orthoclone, OKT3
Alefacept (CD2 fusion
protein)
Efalizumab (CD1 la mAb)
Natalizumab (integrin a4
subunit mAb)
Eculizumab (C5mAb)
Muromonab-CD3
Other: Insulin Humulin, Novolin
Fusion Hepatitis B surface antigen Engerix, Recombivax HB
proteins/Protein (HBsAg)
vaccines/Peptides HPV vaccine Gardasil
OspA LYMErix
Anti-Rhesus(Rh) Rhophylac
immunoglobulin G
Fuzeon
Enfuvirtide
Spider silk, e.g., fibrion
QMONOS
In embodiments, the protein is a multispecific protein, e.g., a bispecific
antibody as
shown in Table 3.
Table 3: Bispecific Formats
33
CA 03083236 2020-05-21
WO 2019/113050 PCT/US2018/063822
Name (other
Proposed Diseases (or
names, BsAb Development
Targets mechanisms of healthy
sponsoring format stages
action volunteers)
organizations)
Catumaxomab
Retargeting of T
(Removab , Malignant
ascites
BsIgG: CD3, cells to tumor, Fc Approved in
Fresenius Biotech, in EpCAM
Triomab EpCAM mediated effector EU
Trion Pharma, positive tumors
functions
Neopharm)
Ertumaxomab
BsIgG: Retargeting of T Advanced solid
(Neovii Biotech, CD3, HER2 Phase I/II
Triomab cells to tumor tumors
Fresenius Biotech)
Approved in
Blinatumomab Precursor B-
cell
USA
(Blincyto , AMG ALL
Retargeting of T Phase II and
103, MT 103, BiTE CD3, CD19 ALL
cells to tumor III
MEDI 538, DLBCL
Phase II
Amgen) NHL
Phase I
REGN1979
BsAb CD3, CD20
(Regeneron)
Solitomab (AMG
CD3, Retargeting of T
110, MT110, BiTE Phase I Solid tumors
EpCAM cells to tumor
Amgen)
MEDI 565 (AMG
Retargeting of T
Gastrointestinal
211, MedImmune, BiTE CD3, CEA Phase I
cells to tumor adenocancinoma
Amgen)
R06958688
BsAb CD3, CEA
(Roche)
BAY2010112 BiTE CD3, PSMA Retargeting of T Phase I Prostate cancer
34
CA 03083236 2020-05-21
WO 2019/113050 PCT/US2018/063822
Name (other
Proposed Diseases (or
names, BsAb Development
Targets mechanisms of healthy
sponsoring format stages
action volunteers)
organizations)
(AMG 212, Bayer; cells to tumor
Amgen)
MGD006 Retargeting of T
DART CD3, CD123 Phase I AML
(Macrogenics) cells to tumor
MGD007 Retargeting of T
DART CD3, gpA33 Phase I Colorectal
cancer
(Macrogenics) cells to tumor
MGD011
DART CD19, CD3
(Macrogenics)
SCORPION
(Emergent Retargeting of T
BsAb CD3, CD19
Biosolutions, cells to tumor
Trubion)
AFM11 (Affimed Retargeting of T
TandAb CD3, CD19 Phase I NHL and ALL
Therapeutics) cells to tumor
Retargeting of NK
AFM12 (Affimed
TandAb CD19, CD16 cells to tumor
Therapeutics)
cells
Retargeting of NK
AFM13 (Affimed CD30, Hodgkin's
TandAb cells to tumor Phase II
Therapeutics) CD16A Lymphoma
cells
GD2 (Barbara Ann T cells Neuroblastoma
Retargeting of T
Karmanos Cancer preloaded CD3, GD2 Phase I/II and
cells to tumor
Institute) with BsAb osteosarcoma
pGD2 (Barbara T cells Retargeting of T Metastatic
breast
CD3, Her2 Phase II
Ann Karmanos preloaded cells to tumor cancer
CA 03083236 2020-05-21
WO 2019/113050 PCT/US2018/063822
Name (other
Proposed Diseases (or
names, BsAb Development
Targets mechanisms of healthy
sponsoring format stages
action volunteers)
organizations)
Cancer Institute) with BsAb
EGFRBi-armed
Autologous
autologous T cells
activated T cells Lung and other
activated T cells preloaded CD3, EGFR Phase I
to EGFR-positive solid tumors
(Roger Williams with BsAb
tumor
Medical Center)
Anti-EGFR-armed
Autologous
activated T-cells T cells Colon and
activated T cells
(Barbara Ann preloaded CD3, EGFR Phase I pancreatic
to EGFR-positive
Karmanos Cancer with BsAb cancers
tumor
Institute)
rM28 (University Tandem CD28, Retargeting of T Metastatic
Phase II
Hospital Tubingen) scFv MAPG cells to tumor melanoma
IMCgp100 CD3, peptide Retargeting of T Metastatic
ImmTAC Phase I/II
(Immunocore) MHC cells to tumor melanoma
2 scFv
DT2219ARL Targeting of
linked to B cell leukemia
(NCI, University of CD19, CD22 protein toxin to Phase I
diphtheria or lymphoma
Minnesota) tumor
toxin
XmAb5871 CD19,
BsAb
(Xencor) CD32b
NI-1701
BsAb CD47, CD19
(NovImmune)
MM-111 ErbB2,
BsAb
(Merrimack) ErbB3
36
CA 03083236 2020-05-21
WO 2019/113050 PCT/US2018/063822
Name (other
Proposed Diseases (or
names, BsAb Development
Targets mechanisms of healthy
sponsoring format stages
action volunteers)
organizations)
MM-141 IGF-1R,
BsAb
(Merrimack) ErbB3
HER2,
NA (Merus) BsAb
HER3
CD3,
NA (Merus) BsAb
CLEC12A
EGFR,
NA (Merus) BsAb
HER3
PD1,
NA (Merus) BsAb
undisclosed
CD3,
NA (Merus) BsAb
undisclosed
Duligotuzumab Head and neck
EGFR, Blockade of 2 Phase I and II
(MEHD7945A, DAF cancer
HER3 receptors, ADCC Phase II
Genentech, Roche) Colorectal
cancer
LY3164530 (Eli Not Blockade of 2 Advanced or
EGFR, MET Phase I
Lily) disclosed receptors metastatic
cancer
Gastric and
MM-111
HER2, Blockade of 2 Phase II esophageal
(Merrimack HSA body
HER3 receptors Phase I cancers
Pharmaceuticals)
Breast cancer
MM-141,
IGF-1R, Blockade of 2 Advanced solid
(Merrimack IgG-scFv Phase I
HER3 receptors tumors
Pharmaceuticals)
37
CA 03083236 2020-05-21
WO 2019/113050 PCT/US2018/063822
Name (other
Proposed Diseases (or
names, BsAb Development
Targets mechanisms of healthy
sponsoring format stages
action volunteers)
organizations)
RG7221
Ang2, VEGF Blockade of 2
(R05520985, CrossMab Phase I Solid tumors
A proangiogenics
Roche)
Ang2, VEGF Blockade of 2
RG7716 (Roche) CrossMab Phase I Wet AMD
A proangiogenics
OMP-305B83
BsAb DLL4/VEGF
(OncoMed)
Pretargeting Colorectal,
TF2 Dock and
CEA, HSG tumor for PET or Phase II breast and lung
(Immunomedics) lock
radioimaging cancers
Blockade of 2
ABT-981
DVD-Ig IL-la, IL-10 proinflammatory Phase II
Osteoarthritis
(AbbVie)
cytokines
Blockade of 2
ABT-122 Rheumatoid
DVD-Ig TNF, IL-17A proinflammatory Phase II
(AbbVie) arthritis
cytokines
Blockade of 2
IgG-
COVA322 TNF, IL17A proinflammatory Phase I/II Plaque
psoriasis
fynomer
cytokines
Tetravalent Blockade of 2 Idiopathic
SAR156597
bispecific IL-13, IL-4 proinflammatory Phase I pulmonary
(Sanofi)
tandem IgG cytokines fibrosis
Dual- Blockade of 2
G5K2434735 (Healthy
targeting IL-13, IL-4 proinflammatory Phase I
(GSK) volunteers)
domain cytokines
38
CA 03083236 2020-05-21
WO 2019/113050
PCT/US2018/063822
Name (other
Proposed
Diseases (or
names, BsAb Development
Targets mechanisms of healthy
sponsoring format stages
action volunteers)
organizations)
Blockade of
proinflammatory
Ozoralizumab Rheumatoid
Nanobody TNF, HSA cytokine, binds to Phase II
(ATN103, Ablynx) arthritis
HSA to increase
half-life
Blockade of 2
proinflammatory
ALX-0761 (Merck IL-17A/F, (Healthy
Nanobody cytokines, binds Phase I
Serono, Ablynx) HSA volunteers)
to HSA to
increase half-life
Blockade of
proinflammatory
ALX-0061 Rheumatoid
Nanobody IL-6R, HSA cytokine, binds to Phase I/II
(AbbVie, Ablynx; arthritis
HSA to increase
half-life
Blockade of bone
ALX-0141
RANKL, resorption, binds Postmenopausal
(Ablynx, Nanobody Phase I
HSA to HSA to bone loss
Eddingpharm)
increase half-life
RG6013/ACE910 Factor IXa, Plasma
ART-Ig Phase II Hemophilia
(Chugai, Roche) factor X coagulation
39
CA 03083236 2020-05-21
WO 2019/113050
PCT/US2018/063822
Table 4
Protein Product Reference Listed Drug
interferon gamma- lb Actimmune @
alteplase; tissue plasminogen activator Activase @/Cathflo @
Recombinant antihemophilic factor Advate
human albumin Albutein @
Laronidase Aldurazyme @
Interferon alfa-N3, human leukocyte derived Alferon N @
human antihemophilic factor Alphanate @
virus-filtered human coagulation factor IX AlphaNine @ SD
Alefacept; recombinant, dimeric fusion
Amevive @
protein LFA3-Ig
Bivalirudin Angiomax @
darbepoetin alfa Aranesp TM
Bevacizumab Avastin TM
interferon beta-1a; recombinant Avonex @
coagulation factor IX BeneFix TM
Interferon beta- lb Betaseron 0
Tositumomab BEXXAR @
antihemophilic factor Bioclate TM
human growth hormone BioTropin TM
botulinum toxin type A BOTOX @
Alemtuzumab Campath @
acritumomab; technetium-99 labeled CEA-Scan @
alglucerase; modified form of beta- Ceredase @
CA 03083236 2020-05-21
WO 2019/113050
PCT/US2018/063822
Table 4
Protein Product Reference Listed Drug
glucocerebrosidase
imiglucerase; recombinant form of beta-
Cerezyme @
glucocerebrosidase
crotalidae polyvalent immune Fab, ovine CroFab TM
digoxin immune fab [ovine] DigiFab TM
Rasburicase Elitek 0
Etanercept ENBREL 0
epoietin alfa Epogen 0
Cetuximab Erbitux TM
algasidase beta Fabrazyme @
Urofollitropin Fertinex TM
follitropin beta Follistim TM
Teriparatide FORTEO 0
human somatropin GenoTropin @
Glucagon GlucaGen @
follitropin alfa Gonal-F @
antihemophilic factor Helixate @
Antihemophilic Factor; Factor XIII HEMOFIL
adefovir dipivoxil Hepsera TM
Trastuzumab Herceptin @
Insulin Humalog @
antihemophilic factor/von Willebrand factor
Humate-P @
complex-human
Somatotropin Humatrope @
41
CA 03083236 2020-05-21
WO 2019/113050 PCT/US2018/063822
Table 4
Protein Product Reference Listed Drug
Adalimumab HUMIRA TM
human insulin Humulin @
recombinant human hyaluronidase Hylenex TM
interferon alfacon-1 Infergen 0
Eptifibatide Integrilin TM
alpha-interferon Intron A 0
Palifermin Kepivance
Anakinra Kineret TM
antihemophilic factor Kogenate @ FS
insulin glargine Lantus @
granulocyte macrophage colony-stimulating Leukine @/Leukine @
factor Liquid
lutropin alfa for injection Luveris
OspA lipoprotein LYMErix TM
Ranibizumab LUCENTIS @
gemtuzumab ozogamicin Mylotarg TM
Galsulfase Naglazyme TM
Nesiritide Natrecor 0
Pegfilgrastim Neulasta TM
Oprelvekin Neumega @
Filgrastim Neupogen @
NeutroSpec TM (formerly
Fanolesomab
LeuTech @)
somatropin [rDNA] Norditropin @/Norditropin
42
CA 03083236 2020-05-21
WO 2019/113050
PCT/US2018/063822
Table 4
Protein Product Reference Listed Drug
Nordiflex @
Mitoxantrone Novantrone @
insulin; zinc suspension; Novolin L @
insulin; isophane suspension Novolin N @
insulin, regular; Novolin R @
Insulin Novolin @
coagulation factor VIIa NovoSeven @
Somatropin Nutropin @
immunoglobulin intravenous Octagam @
PEG-L-asparaginase Oncaspar @
abatacept, fully human soluable fusion
Orencia TM
protein
muromomab-CD3 Orthoclone OKT3 @
high-molecular weight hyaluronan Orthovisc @
human chorionic gonadotropin Ovidrel @
live attenuated Bacillus Calmette-Guerin Pacis @
peginterferon alfa-2a Pegasys @
pegylated version of interferon alfa-2b PEG-Intron TM
Abarelix (injectable suspension);
Plenaxis TM
gonadotropin-releasing hormone
Antagonist
epoietin alfa Procrit @
Aldesleukin Proleukin, IL-2 @
Somatrem Protropin @
43
CA 03083236 2020-05-21
WO 2019/113050
PCT/US2018/063822
Table 4
Protein Product Reference Listed Drug
dornase alfa Pulmozyme
Efalizumab; selective, reversible T-cell
RAPTIVA TM
blocker
combination of ribavirin and alpha interferon Rebetron TM
Interferon beta la Rebif 0
antihemophilic factor Recombinate rAHF/
antihemophilic factor ReFacto
Lepirudin Refludan
Infliximab REMICADE
Abciximab ReoPro TM
Reteplase Retavase TM
Rituxima Rituxan TM
interferon alfa-2a Roferon-A 0
Somatropin Saizen
synthetic porcine secretin SecreFlo TM
Basiliximab Simulect
Eculizumab SOLIRIS (R)
Pegvisomant SOMAVERT
Palivizumab; recombinantly produced,
Synagis TM
humanized mAb
thyrotropin alfa Thyrogen
Tenecteplase TNKase TM
Natalizumab TYSABRI
human immune globulin intravenous 5% and Venoglobulin-S
44
CA 03083236 2020-05-21
WO 2019/113050
PCT/US2018/063822
Table 4
Protein Product Reference Listed
Drug
10% solutions
interferon alfa-nl, lymphoblastoid Wellferon
drotrecogin alfa Xigris TM
Omalizumab; recombinant DNA-derived
Xolair
humanized monoclonal
antibody targeting immunoglobulin-E
Daclizumab Zenapax
ibritumomab tiuxetan Zevalin TM
Somatotropin Zorbtive TM
(Serostim C),)
In some embodiments, the polypeptide is an antigen expressed by a cancer cell.
In some
embodiments the recombinant or therapeutic polypeptide is a tumor-associated
antigen or a
tumor-specific antigen. In some embodiments, the recombinant or therapeutic
polypeptide is
selected from HER2, CD20, 9-0-acetyl-GD3, (3hCG, A33 antigen, CA19-9 marker,
CA-125
marker, calreticulin, carboanhydrase IX (MN/CA IX), CCR5, CCR8, CD19, CD22,
CD25,
CD27, CD30, CD33, CD38, CD44v6, CD63, CD70, CC123, CD138, carcinoma embryonic
antigen (CEA; CD66e), desmoglein 4, E-cadherin neoepitope, endosialin, ephrin
A2 (EphA2),
epidermal growth factor receptor (EGFR), epithelial cell adhesion molecule
(EpCAM), ErbB2,
fetal acetylcholine receptor, fibroblast activation antigen (FAP), fucosyl
GM1, GD2, GD3, GM2,
ganglioside GD3, Globo H, glycoprotein 100, HER2/neu, HER3, HER4, insulin-like
growth
factor receptor 1, Lewis-Y, LG, Ly-6, melanoma-specific chondroitin-sulfate
proteoglycan
(MCSCP), mesothelin, MUC1, MUC2, MUC3, MUC4,
MUC5Ac, MUC5B, MUC7, MUC16, Mullerian inhibitory substance (MIS) receptor type
II,
plasma cell antigen, poly SA, PSCA, PSMA, sonic hedgehog (SHH), SAS, STEAP,
sTn
antigen, TNF-alpha precursor, and combinations thereof.
In some embodiments, the polypeptide is an activating receptor and is selected
from 2B4
(CD244), a4(31 integrin, (32 integrins, CD2, CD16, CD27, CD38, CD96, CD100,
CD160, CD137,
CA 03083236 2020-05-21
WO 2019/113050
PCT/US2018/063822
CEACAM1(CD66), CRTAM, CS1 (CD319), DNAM-1 (CD226), GITR (TNFRSF18), activating
forms of KIR, NKG2C, NKG2D, NKG2E, one or more natural cytotoxicity receptors,
NTB-A,
PEN-5, and combinations thereof, optionally wherein the (32 integrins comprise
CD1la-CD 18,
CD11 b-CD 18, or CD11c-CD 18, optionally wherein the activating forms of KIR
comprise
K1R2DS1, KIR2DS4, or KIR-S, and optionally wherein the natural cytotoxicity
receptors
comprise NKp30, NKp44, NKp46, or NKp80.
In some embodiments, the polypeptide is an inhibitory receptor and is selected
from KIR,
ILT2/LIR-1/CD85j, inhibitory forms of KIR, KLRG1, LAIR-1, NKG2A, NKR-P1A,
Siglec-3,
Siglec-7, Siglec-9, and combinations thereof, optionally wherein the
inhibitory forms of KIR
comprise KIR2DL1, KIR2DL2, KIR2DL3, KIR3DL1, KIR3DL2, or KIR-L.
In some embodiments, the polypeptide is an activating receptor and is selected
from
CD3, CD2 (LFA2, 0X34), CD5, CD27 (TNFRSF7), CD28, CD30 (TNFRSF8), CD4OL, CD84
(SLAMF5), CD137 (4-1BB), CD226, CD229 (Ly9, SLAMF3), CD244 (2B4, SLAMF4),
CD319
(CRACC, BLAME), CD352 (Ly108, NTBA, SLAMF6), CRTAM (CD355), DR3 (TNFRSF25),
GITR (CD357), HVEM (CD270), ICOS, LIGHT, LT(3R (TNFRSF3), 0X40 (CD134), NKG2D,
SLAM (CD150, SLAMF1), TCRa, TCR(3, TCR6y, TIM1 (HAVCR, KIM1), and combinations
thereof.
In some embodiments, the polypeptide is an inhibitory receptor and is selected
from PD-1
(CD279), 2B4 (CD244, SLAMF4), B71 (CD80), B7H1 (CD274, PD-L1), BTLA (CD272),
CD160 (BY55, NK28), CD352 (Ly108, NTBA, SLAMF6), CD358 (DR6), CTLA-4 (CD152),
LAG3, LAIR1, PD-1H (VISTA), TIGIT (VSIG9, VSTM3), TIM2 (TIMD2), TIM3 (HAVCR2,
KIM3), and combinations thereof.
Other exemplary proteins include, but are not limited to any protein described
in Tables
1-10 of Leader et al., "Protein therapeutics: a summary and pharmacological
classification",
Nature Reviews Drug Discovery, 2008, 7:21-39 (incorporated herein by
reference); or any
conjugate, variant, analog, or functional fragment of the recombinant
polypeptides described
herein.
Other recombinant protein products include non-antibody scaffolds or
alternative protein
scaffolds, such as, but not limited to: DARPins, affibodies and adnectins.
Such non-antibody
scaffolds or alternative protein scaffolds can be engineered to recognize or
bind to one or two, or
more, e.g., 1, 2, 3, 4, or 5 or more, different targets or antigens.
46
CA 03083236 2020-05-21
WO 2019/113050
PCT/US2018/063822
Also provided herein are nucleic acids, e.g., exogenous nucleic acids that
encode the
products, e.g., polypeptides, e.g., recombinant polypeptides described herein.
The nucleic acid
sequences coding for the desired recombinant polypeptides can be obtained
using recombinant
methods known in the art, such as, for example by screening libraries from
cells expressing the
desired nucleic acid sequence, e.g., gene, by deriving the nucleic acid
sequence from a vector
known to include the same, or by isolating directly from cells and tissues
containing the same,
using standard techniques. Alternatively, the nucleic acid encoding the
recombinant polypeptide
can be produced synthetically, rather than cloned. Recombinant DNA techniques
and
technology are highly advanced and well established in the art. Accordingly,
the ordinarily
skilled artisan having the knowledge of the amino acid sequence of a
recombinant polypeptide
described herein can readily envision or generate the nucleic acid sequence
that would encode
the recombinant polypeptide.
In some embodiments, the exogenous nucleic acid controls the expression of a
product
that is endogenously expressed by the host cell. In such embodiments, the
exogenous nucleic
acid comprises one or more nucleic acid sequences that increase the expression
of the
endogenous product (also referred to herein as "endogenous product
transactivation sequence").
For example, the nucleic acid sequence that increases the expression of an
endogenous product
comprises a constitutively active promoter or a promoter that is stronger,
e.g., increases
transcription at the desired site, e.g., increases expression of the desired
endogenous gene
product. After introduction of the exogenous nucleic acid comprising the
endogenous product
transactivation sequence, said exogenous nucleic acid is integrated into the
chromosomal
genome of the cell, e.g., at a preselected location proximal to the genomic
sequence encoding the
endogenous product, such that the endogenous product transactivation sequence
increases the
transactivation or expression of the desired endogenous product. Other methods
for modifying a
cell, e.g., introducing an exogenous nucleic acid, for increasing expression
of an endogenous
product is described, e.g., in U.S. Patent No. 5,272,071; hereby incorporated
by reference in its
entirety.
The expression of a product described herein is typically achieved by operably
linking a
nucleic acid encoding the recombinant polypeptide or portions thereof to a
promoter, and
incorporating the construct into an expression vector. The vectors can be
suitable for replication
47
CA 03083236 2020-05-21
WO 2019/113050
PCT/US2018/063822
and integration eukaryotes or prokaryotes. Typical cloning vectors contain
other regulatory
elements, such as transcription and translation terminators, initiation
sequences, and promoters
useful for regulation of the expression of the desired nucleic acid sequence.
The nucleic acid sequences described herein encoding a product, e.g., a
recombinant
.. polypeptide, or comprising a nucleic acid sequence that can control the
expression of an
endogenous product, can be cloned into a number of types of vectors. For
example, the nucleic
acid can be cloned into a vector including, but not limited to a plasmid, a
phagemid, a phage
derivative, an animal virus, and a cosmid. Vectors of particular interest
include expression
vectors, replication vectors, probe generation vectors, and sequencing
vectors. In embodiments,
the expression vector may be provided to a cell in the form of a viral vector.
Viral vector
technology is well known in the art and is described, for example, in Sambrook
et al., 2012,
MOLECULAR CLONING: A LABORATORY MANUAL, volumes 1 -4, Cold Spring Harbor
Press, NY), and in other virology and molecular biology manuals. Viruses,
which are useful as
vectors include, but are not limited to, retroviruses, adenoviruses, adeno-
associated viruses,
herpes viruses, and lentiviruses. In general, a suitable vector contains an
origin of replication
functional in at least one organism, a promoter sequence, convenient
restriction endonuclease
sites, and one or more selectable markers, (e.g., WO 01/96584; WO 01/29058;
and U.S. Pat. No.
6,326,193). Vectors derived from viruses are suitable tools to achieve long-
term gene transfer
since they allow long-term, stable integration of a transgene and its
propagation in daughter cells.
A vector may also include, e.g., a signal sequence to facilitate secretion, a
polyadenylation signal and transcription terminator (e.g., from Bovine Growth
Hormone (BGH)
gene), an element allowing episomal replication and replication in prokaryotes
(e.g. 5V40 origin
and ColE1 or others known in the art) and/or elements to allow selection,
e.g., a selection marker
or a reporter gene.
In one embodiment, the vector comprising a nucleic acid sequence encoding a
polypeptide, e.g., a recombinant polypeptide, further comprises a promoter
sequence responsible
for the recruitment of polymerase to enable transcription initiation for
expression of the
polypeptide, e.g., the recombinant polypeptide. In one embodiment, promoter
sequences
suitable for the methods described herein are usually associated with
enhancers to
drive high amounts of transcription and hence deliver large copies of the
target exogenous
mRNA. In an embodiment, the promoter comprises cytomegalovirus (CMV) major
48
CA 03083236 2020-05-21
WO 2019/113050
PCT/US2018/063822
immediate early promoters (Xia, Bringmann et al. 2006) and the SV40 promoter
(Chernajovsky,
Mory et al. 1984), both derived from their namesake viruses or promoters
derived therefrom.
Several other less common viral promoters have been successfully employed to
drive
transcription upon inclusion in an expression vector including Rous Sarcoma
virus long terminal
repeat (RSV-LTR) and Moloney murine leukaemia virus (MoMLV) LTR (Papadakis,
Nicklin et
al. 2004). In another embodiment,specific endogenous mammalian promoters can
be utilized to
drive constitutive transcription of a gene of interest (Pontiller, Gross et
al. 2008). The CHO
specific Chinese Hamster elongation factor 1-alpha (CHEF1a) promoter has
provided a high
yielding alternative to viral based sequences (Deer, Allison 2004). In
addition to promoters, the
vectors described herein further comprise an enhancer region as described
above; a specific
nucleotide motif region, proximal to the core promoter, which can recruit
transcription factors to
upregulate the rate of transcription (Riethoven 2010). Similar to promoter
sequences, these
regions are often derived from viruses and are encompassed within the promoter
sequence such
as hCMV and 5V40 enhancer sequences, or may be additionally included such as
adenovirus
derived sequences (Gaillet, Gilbert et al. 2007).
In one embodiment, the vector comprising a nucleic acid sequence encoding a
product,
e.g., a polypeptide, e.g, a recombinant polypeptide, described herein further
comprises a nucleic
acid sequence that encodes a selection marker. In one embodiment, the
selectable marker
comprises glutamine synthetase (GS); dihydrofolate reductase (DHFR) e.g., an
enzyme which
confers resistance to methotrexate (MTX); or an antibiotic marker, e.g., an
enzyme that confers
resistance to an antibiotic such as: hygromycin, neomycin (G418), zeocin,
puromycin, or
blasticidin. In another embodiment, the selection marker comprises or is
compatible with the
Selexis selection system (e.g., SUREtechnology Platform TM and Selexis Genetic
ElementsTM,
commercially available from Selexis SA) or the Catalant selection system.
In one embodiment, the vector comprising a nucleic acid sequence encoding a
recombinant product described herein comprises a selection marker that is
useful in identifying a
cell or cells comprise the nucleic acid encoding a recombinant product
described herein. In
another embodiment, the selection marker is useful in identifying a cell or
cells that comprise the
integration of the nucleic acid sequence encoding the recombinant product into
the genome, as
described herein. The identification of a cell or cells that have integrated
the nucleic acid
sequence encoding the recombinant protein can be useful for the selection and
engineering of a
49
CA 03083236 2020-05-21
WO 2019/113050
PCT/US2018/063822
cell or cell line that stably expresses the product.
Suitable vectors for use are commercially available, and include vectors
associated with
the GS Expression SystemTM, GS XceedTM Gene Expression System, or Potelligent
CHOK1SV
technology available from Lonza Biologics, Inc, e.g., vectors as described in
Fan et al., Pharm.
Bioprocess. (2013); 1(5):487-502, which is incorporated herein by reference in
its entirety. GS
expression vectors comprise the GS gene, or a functional fragment thereof
(e.g., a GS mini-
gene), and one or more, e.g., 1, 2, or 3, or more, highly efficient
transcription cassettes for
expression of the gene of interest, e.g., a nucleic acid encoding a
recombinant polypeptide
described herein. A GS mini-gene comprises, e.g., consists of, intron 6 of the
genomic CHO GS
gene. In one embodiment, a GS vector comprises a GS gene operably linked to a
SV4OL
promoter and one or two polyA signals. In another embodiment, a GS vector
comprises a GS
gene operably linked to a SV40E promoter, 5V40 splicing and polyadenylation
signals. In such
embodiments, the transcription cassette, e.g., for expression of the gene of
interest or
recombinant polypeptide described herein, includes the hCMV-MIE promoter and
5'
untranslated sequences from the hCMV-MIE gene including the first intron.
Other vectors can
be constructed based on GS expression vectors, e.g., wherein other selection
markers are
substituted for the GS gene in the expression vectors described herein.
Vectors suitable for use in the methods described herein include, but are not
limited to,
other commercially available vectors, such as, pcDNA3.1/Zeo, pcDNA3.1/CAT,
pcDNA3.3TOPO (Thermo Fisher, previously Invitrogen); pTarget, HaloTag
(Promega); pUC57
(GenScript); pFLAG-CMV (Sigma-Aldrich); pCMV6 (Origene); pEE12 or pEE14 (Lonza
Biologics), or pBK-CMV/ pCMV-3Tag-7/ pCMV-Tag2B (Stratagene).
CELLS AND CELL CULTURE
In embodiments, the cell is a mammalian cell. In other embodiments, the cell
is a cell
other than a mammalian cell. In an embodiment, the cell is a mouse, rat,
Chinese hamster,
Syrian hamster, monkey, ape, dog, horse, ferret, or cat. In embodiments, the
cell is a mammalian
cell, e.g., a human cell or a rodent cell, e.g., a hamster cell, a mouse cell,
or a rat cell. In another
embodiment, the cell is from a duck, parrot, fish, insect, plant, fungus, or
yeast. In one
embodiment, the cell is an Archaebacteria. In an embodiment, the cell is a
species of
Actinobacteria, e.g., Mycobacterium tuberculosis).
CA 03083236 2020-05-21
WO 2019/113050
PCT/US2018/063822
In one embodiment, the cell is a Chinese hamster ovary (CHO) cell. In one
embodiment,
the cell is a CHO-Kl cell, a CHO-Kl SV cell, a DG44 CHO cell, a DUXB11 CHO
cell, a
CHOS, a CHO GS knock-out cell, a CHO FUT8 GS knock-out cell, a CHOZN, or a CHO-
derived cell. The CHO GS knock-out cell (e.g., GSKO cell) is, for example, a
CHO-K1SV GS
knockout cell (Lonza Biologics, Inc.). The CHO FUT8 knockout cell is, for
example, the
Potelligent CHOK1 SV (Lonza Biologics, Inc.).
In another embodiment, the cell is a HeIa, HEK293, HT1080, H9, HepG2, MCF7,
Jurkat,
NIH3T3, PC12, PER.C6, BHK (baby hamster kidney cell), VERO, SP2/0, NSO, YB2/0,
YO,
EB66, C127, L cell, COS, e.g., COS 1 and COS7, QC1-3, CHOK1, CHOK1SV,
Potelligent
CHOK1SV, CHO GS knockout, CHOK1SV GS-KO, CHOS, CHO DG44, CHO DXB11, and
CHOZN, or any cells derived therefrom. In one embodiment, the cell is a stem
cell. In one
embodiment, the cell is a differentiated form of any of the cells described
herein. In one
embodiment, the cell is a cell derived from any primary cell in culture.
In an embodiment, the cell is any one of the cells described herein that
comprises an
exogenous nucleic acid encoding a recombinant polypeptide, e.g., expresses a
recombinant
polypeptide, e.g., a recombinant polypeptide selected from Table 1 or 2.
Large scale production
The methods described herein are of use in analyzing samples, e.g., samples
produced by
devices, facilities and methods of manufacturing and production. The devices,
facilities, and
methods of manufacturing and production described herein are suitable for
culturing any desired
cell line including prokaryotic and/or eukaryotic cell lines. Further, in
embodiments, the devices,
facilities and methods of manufacturing and production are suitable for
culturing suspension
cells or anchorage-dependent (adherent) cells and are suitable for production
operations
configured for production of pharmaceutical and biopharmaceutical
products¨such as
polypeptide products, nucleic acid products (for example DNA or RNA), or cells
and/or viruses
such as those used in cellular and/or viral therapies.
In embodiments, the cells express or produce a product, such as a recombinant
therapeutic or diagnostic product. Examples of products produced by cells
include, but are not
limited to, antibody molecules (e.g., monoclonal antibodies, bispecific
antibodies), antibody
mimetics (polypeptide molecules that bind specifically to antigens but that
are not structurally
51
CA 03083236 2020-05-21
WO 2019/113050
PCT/US2018/063822
related to antibodies such as e.g. DARPins, affibodies, adnectins, or IgNARs),
fusion proteins
(e.g., Fc fusion proteins, chimeric cytokines), other recombinant proteins
(e.g., glycosylated
proteins, enzymes, hormones), viral therapeutics (e.g., anti-cancer oncolytic
viruses, viral vectors
for gene therapy and viral immunotherapy), cell therapeutics (e.g.,
pluripotent stem cells,
mesenchymal stem cells and adult stem cells), vaccines or lipid-encapsulated
particles (e.g.,
exosomes, virus-like particles), RNA (such as e.g. siRNA) or DNA (such as e.g.
plasmid DNA),
antibiotics or amino acids. In embodiments, the devices, facilities and
methods can be used for
producing biosimilars.
As mentioned, in embodiments, methods described herein are of use in analyzing
samples, e.g., samples produced by devices, facilities and methods of
manufacturing and
production. The devices, facilities and methods of manufacturing and
production allow for the
production of eukaryotic cells, e.g., mammalian cells or lower eukaryotic
cells such as for
example yeast cells or filamentous fungi cells, or prokaryotic cells such as
Gram-positive or
Gram-negative cells and/or products of the eukaryotic or prokaryotic cells,
e.g., proteins,
peptides, antibiotics, amino acids, nucleic acids (such as DNA or RNA),
synthesised by the
eukaryotic cells in a large-scale manner. Unless stated otherwise herein, the
devices, facilities,
and methods can include any desired volume or production capacity including
but not limited to
bench-scale, pilot-scale, and full production scale capacities.
In embodiments, devices, facilities, and methods of manufacturing and
production allow
for the production of cells and products of the cells, especially proteins,
peptides (discussed in
detail above), antibiotics or amino acids, synthesized by cells, e.g.,
mammalian cells, in a large-
scale manner.
A wide array of flasks, bottles, reactors, and controllers allow the
production and scale up
of cell culture systems. The system can be chosen based, at least in part,
upon its correlation with
a desired glycan property or properties. Cells can be grown, for example, as
batch, fed-batch,
perfusion, or continuous cultures. Production parameters that can be selected
include, e.g.,
addition or removal of media including when (early, middle or late during
culture time) and how
often media is harvested; increasing or decreasing speed at which cell
cultures are agitated;
increasing or decreasing temperature at which cells are cultured; adding or
removing media such
that culture density is adjusted; selecting a time at which cell cultures are
started or stopped; and
selecting a time at which cell culture parameters are changed. Such parameters
can be selected
52
CA 03083236 2020-05-21
WO 2019/113050
PCT/US2018/063822
for any of the batch, fed-batch, perfusion and continuous culture conditions.
In embodiments, the cultivated cells for large scale production are eukaryotic
cells, e.g.,
animal cells, e.g.,marninalian cells. The mammalian cells can be, for example,
human cell lines,
mouse m),eloma (NS0)- cell lines, Chinese hamster ovary (CH0)-cell lines or
hybri-dorna- cell
lines. Preferably the mammalian cells are CHO-cell lines.
In embodiments, the cultivated cells for large scale production are used to
produce
antibodies discussed in detail above, e.g., monoclonal antibodies, and/or
recombinant proteins,
e.g., recombinant proteins for therapeutic use. In embodiments, the cells
produce peptides, amino
acids, fatty acids or other useful biochemical intermediates or metabolites.
In embodiments, the cells for large scale production are eukaryotic cells,
biochemical
markers, recombinant peptides or nucleotide sequences of interest, proteins,
yeast, insect cells,
stable or viral infected, avian cells or mammalian cells such as CHO cells,
monkey cells, lytic
products and the like for medical, research or commercial purposes.
In embodiments, the cells for large scale production are prokaryotic cells,
strains of
Gram-positive cells such as Bacillus and Streptomyces. In embodiments, the
host cell is of
phylum Firmicutes, e.g., the host cell is Bacillus. BSacillus that can be used
are, e.g. the strains
B.subtilis, B.amyloliquefaciens, B.licheniformis, B.natto, B.megaterium, etc.
In embodiments,
the host cell is B.subtilis, such as B.subtilis 3NA and B.subtilis 168.
Bacillus is obtainable from,
e.g., the Bacillus Genetic Stock Center, Biological Sciences 556, 484 West
12th Avenue,
Columbus OH 43210-1214
In embodiments, the prokaryotic cells for large scale production are Gram
negative cells,
such as Salmonella spp. or E.coli, e.g.,the strains TG1, W3110, DH1, XL1-Blue
and Origami,
which are commercially available.
Suitable host cells are commercially available, for example, from culture
collections such
as the DSMZ (Deutsche Sammlung von Mikroorganismen and Zellkulturen GmbH,
Braunschweig, Germany).
In an embodiment, the cell culture is carried out as a batch culture, fed-
batch culture,
draw and fill culture, or a continuous culture. In an embodiment, the cell
culture is a suspension
culture. In one embodiment, the cell or cell culture is placed in vivo for
expression of the
recombinant polypeptide, e.g., placed in a model organism or a human subject.
53
CA 03083236 2020-05-21
WO 2019/113050
PCT/US2018/063822
In one embodiment, the culture media is free of serum. Serum-free and protein-
free
media are commercially available, e.g., Lonza Biologics.
Suitable media and culture methods for mammalian cell lines are well-known in
the art,
as described in U.S. Pat. No. 5,633,162, for instance. Examples of standard
cell culture media
for laboratory flask or low density cell culture and being adapted to the
needs of particular cell
types are for instance: Roswell Park Memorial Institute (RPMI) 1640 medium
(Morre, G., The
Journal of the American Medical Association, 199, p. 519 f. 1967), L-15 medium
(Leibovitz, A.
et al., Amer. J. of Hygiene, 78, 1p. 173 ff, 1963), Dulbecco's modified
Eagle's medium (DMEM),
Eagle's minimal essential medium (MEM), Ham's F12 medium (Ham, R. et al.,
Proc. Natl. Acad.
Sc.53, p288 ff. 1965) or Iscoves' modified DMEM lacking albumin, transferrin
and lecithin
(Iscoves et al., J. Exp. med. 1, p. 923 ff., 1978). For instance, Ham's F10 or
F12 media were
specially designed for CHO cell culture. Other media specially adapted to CHO
cell culture are
described in EP-481 791. It is known that such culture media can be
supplemented with fetal
bovine serum (FBS, also called fetal calf serum FCS), the latter providing a
natural source of a
plethora of hormones and growth factors. The cell culture of mammalian cells
is nowadays a
routine operation well-described in scientific textbooks and manuals, it is
covered in detail e.g. in
R. Ian Fresney, Culture of Animal cells, a manual, 4th edition, Wiley-
Liss/N.Y., 2000.
Other suitable cultivation methods are known to the skilled artisan and may
depend upon
the recombinant polypeptide product and the host cell utilized. It is within
the skill of an
ordinarily skilled artisan to determine or optimize conditions suitable for
the expression and
production of the recombinant polypeptide to be expressed by the cell.
In one aspect, the cell or cell line for large scale production comprises an
exogenous
nucleic acid that encodes a product, e.g., a recombinant polypeptide. In an
embodiment, the cell
or cell line expresses the product, e.g., a therapeutic or diagnostic product.
Methods for
genetically modifying or engineering a cell to express a desired polypeptide
or protein are well
known in the art, and include, for example, transfection, transduction (e.g.,
viral transduction), or
electroporation.
Physical methods for introducing a nucleic acid, e.g., an exogenous nucleic
acid or vector
described herein, into a host cell include calcium phosphate precipitation,
lipofection, particle
bombardment, microinjection, electroporation, and the like. Methods for
producing cells
comprising vectors and/or exogenous nucleic acids are well-known in the art.
See, for example,
54
CA 03083236 2020-05-21
WO 2019/113050
PCT/US2018/063822
Sambrook et al., 2012, MOLECULAR CLONING: A LABORATORY MANUAL, volumes 1 -
4, Cold Spring Harbor Press, NY).
Chemical means for introducing a nucleic acid, e.g., an exogenous nucleic acid
or vector
described herein, into a host cell include colloidal dispersion systems, such
as macromolecule
complexes, nanocapsules, microspheres, beads, and lipid-based systems
including oil-in-water
emulsions, micelles, mixed micelles, and liposomes. An exemplary colloidal
system for use as a
delivery vehicle in vitro and in vivo is a liposome (e.g., an artificial
membrane vesicle). Other
methods of state-of-the-art targeted delivery of nucleic acids are available,
such as delivery of
polynucleotides with targeted nanoparticles or other suitable sub-micron sized
delivery system.
In embodiments, the integration of the exogenous nucleic acid into a nucleic
acid of the
host cell, e.g., the genome or chromosomal nucleic acid of the host cell is
desired. Methods for
determining whether integration of an exogenous nucleic acid into the genome
of the host cell
has occurred can include a GS/MSX selection method. The GS/MSX selection
method uses
complementation of a glutamine auxotrophy by a recombinant GS gene to select
for high-level
expression of proteins from cells. Briefly, the GS/MSX selection method
comprises inclusion of
a nucleic acid encoding glutamine synthetase on the vector comprising the
exogenous nucleic
acid encoding the recombinant polypeptide product. Administration of
methionine sulfoximine
(MSX) selects cells that have stably integrated into the genome the exogenous
nucleic acid
encoding both the recombinant polypeptide and GS. As GS can be endogenously
expressed by
some host cells, e.g., CHO cells, the concentration and duration of selection
with MSX can be
optimized to identify high producing cells with stable integration of the
exogenous nucleic acid
encoding the recombinant polypeptide product into the host genome. The GS
selection and
systems thereof is further described in Fan et al., Pharm. Bioprocess. (2013);
1(5):487-502,
which is incorporated herein by reference in its entirety.
Other methods for identifying and selecting cells that have stably integrated
the
exogenous nucleic acid into the host cell genome can include, but are not
limited to, inclusion of
a reporter gene on the exogenous nucleic acid and assessment of the presence
of the reporter
gene in the cell, and PCR analysis and detection of the exogenous nucleic
acid.
In one embodiment, the cells selected, identified, or generated using the
methods described
herein are capable of producing higher yields of protein product than cells
that are selected using
only a selection method for the stable expression, e.g., integration of
exogenous nucleic acid
CA 03083236 2020-05-21
WO 2019/113050
PCT/US2018/063822
encoding the recombinant polypeptide. In an embodiment, the cells selected,
identified, or
generated using the methods described herein produce 2-fold, 3-fold, 4-fold, 5-
fold, 6-fold, 7-
fold, 8-fold, 9-fold, or 10-fold or more of the product, e.g., recombinant
polypeptide, as
compared to cells that were not contacted with an inhibitor of protein
degradation, or cells that
were only selected for stable expression, e.g., integration, of the exogenous
nucleic acid
encoding the recombinant polypeptide.
METHODS FOR CELL LINE AND RECOMBINANT POLYPEPTIDE PRODUCTION
Methods for recovering and purification of a product, e.g., a recombinant
polypeptide, are
well established in the art. For recovering the recombinant polypeptide
product, a physical or
chemical or physical-chemical method is used. The physical or chemical or
physical-chemical
method can be a filtering method, a centrifugation method, an
ultracentrifugation method, an
extraction method, a lyophilization method, a precipitation method, a
crystallization method, a
chromatography method or a combination of two or more methods thereof. In an
embodiment,
the chromatography method comprises one or more of size-exclusion
chromatography (or gel
filtration), ion exchange chromatography, e.g., anion or cation exchange
chromatography,
affinity chromatography, hydrophobic interaction chromatography, and/or
multimodal
chromatography.
The methods described herein are suitable for analyzing samples produced by
manufacturing and production methods that culture any desired cell including
prokaryotic cells
and/or eukaryotic cells. The methods of manufacturing and production can be
performed in, e.g.,
a reactor, e.g., a bioreactor. Further, in embodiments, samples and products
can be produced
using devices, facilities and production methods suitable for culturing
suspension cells or
anchorage-dependent (adherent) cells and suitable for production operations
configured for
production of molecular products¨such as polypeptide products - or cells
and/or viruses such as
those used in cellular and/or viral therapies.
In embodiments, the cells express or produce a product, such as a recombinant
therapeutic or diagnostic product. As described in more detail below, examples
of products
produced by cells include, but are not limited to, antibody molecules (e.g.,
monoclonal
antibodies, bispecific antibodies), fusion proteins (e.g., Fc fusion proteins,
chimeric cytokines),
other recombinant proteins (e.g., glycosylated proteins, enzymes, hormones),
or lipid-
56
CA 03083236 2020-05-21
WO 2019/113050
PCT/US2018/063822
encapsulated particles (e.g., exosomes, virus-like particles). In embodiments,
the devices,
facilities and methods can be used for producing biosimilars.
In embodiments, devices, facilities and production methods allow for the
production of
eukaryotic cells, e.g., mammalian cells, and/or products of the eukaryotic
cells, e.g., proteins,
peptides, antibiotics or amino acids, synthesized by the eukaryotic cells in a
large-scale manner.
Unless stated otherwise herein, the devices, facilities, and methods can
include any desired
volume or production capacity including but not limited to bench-scale, pilot-
scale, and full
production scale capacities.
Moreover and unless stated otherwise herein, the devices, facilities, and
production
methods can include any suitable reactor(s) including but not limited to
stirred tank, airlift, fiber,
mierofiber, hollow fiber, ceramic matrix, fluidized bed, fixed bed, spouted
bed, and/or stirred
tank bioreactors. For example, in some aspects, an example bioreactor unit can
perform one or
more, or all, of the following: feeding of nutrients and/or carbon sources,
injection of suitable gas
(e.g., oxygen), flow of fermentation or cell culture medium, separation of gas
and liquid phases,
maintenance of temperature, maintenance of pH level, agitation (e.g.,
stirring), and/or
cleaning/sterilizing. Example reactor units, such as a fermentation unit, may
contain 1, 2, 3, 4, 5,
10, 15, 20, 25, 30, 35, 40, 45, 50, 60, 70, 80, 90, or 100, or more
bioreactors. In various
embodiments, the bioreactor can be suitable for batch, semi fed-batch, fed-
batch, perfusion,
and/or continuous fermentation processes. Any suitable reactor diameter can be
used. In
embodiments, the bioreactor can have a volume between about 100 mL and about
50,000 L.
Non-limiting examples include a volume of 100 mL, 250 mL, 500 mL, 750 mL, 1
liter, 2 liters, 3
liters, 4 liters, 5 liters, 6 liters, 7 liters, 8 liters, 9 liters, 10 liters,
15 liters, 20 liters, 25 liters, 30
liters, 40 liters, 50 liters, 60 liters, 70 liters, 80 liters, 90 liters, 100
liters, 150 liters, 200 liters,
250 liters, 300 liters, 350 liters, 400 liters, 450 liters, 500 liters, 550
liters, 600 liters, 650 liters,
700 liters, 750 liters, 800 liters, 850 liters, 900 liters, 950 liters, 1000
liters, 1500 liters, 2000
liters, 2500 liters, 3000 liters, 3500 liters, 4000 liters, 4500 liters, 5000
liters, 6000 liters, 7000
liters, 8000 liters, 9000 liters, 10,000 liters, 15,000 liters, 20,000 liters,
and/or 50,000 liters.
Additionally, suitable reactors can be multi-use, single-use, disposable, or
non-disposable and
can be formed of any suitable material including metal alloys such as
stainless steel (e.g., 316L
or any other suitable stainless steel) and Inconel, plastics, and/or glass. In
some embodiments,
suitable reactors can be round, e.g., cylindrical. In some embodiments,
suitable reactors can be
57
CA 03083236 2020-05-21
WO 2019/113050
PCT/US2018/063822
square, e.g., rectangular. Square reactors may in some cases provide benefits
over round reactors
such as ease of use (e.g., loading and setup by skilled persons), greater
mixing and homogeneity
of reactor contents, and lower floor footprint.
In embodiments and unless stated otherwise herein, the devices, facilities,
and production
methods described herein can also include any suitable unit operation and/or
equipment not
otherwise mentioned, such as operations and/or equipment for separation,
purification, and
isolation of such products. Any suitable facility and environment can be used,
such as traditional
stick-built facilities, modular facilities, or any other suitable
construction, facility, and/or layout.
For example, in some embodiments modular clean-rooms can be used. Additionally
and unless
otherwise stated, the devices, systems, and methods described herein can be
housed and/or
performed in a single location or facility or alternatively be housed and/or
performed at separate
or multiple locations and/or facilities.
By way of non-limiting examples and without limitation, U.S. Publication Nos.
2013/0280797; 2012/0077429; 2011/0280797; 2009/0305626; and U.S. Patent Nos.
8,298,054;
.. 7,629,167; and 5,656,491, which are hereby incorporated by reference in
their entirety, describe
example facilities, equipment, and/or systems that may be suitable.
In embodiments, the cells are eukaryotic cells, e.g., mammalian cells. The
mammalian
cells can be for example human or rodent or bovine cell lines or cell strains.
Examples of such
cells, cell lines or cell strains are e.g. mouse myeloma (NS0)-cell lines,
Chinese hamster ovary
(CHO)-cell lines, HT1080, H9, HepG2, MCF7, MDBK Jurkat, NIH3T3, PC12, BHK
(baby
hamster kidney cell), VERO, 5P2/0, YB2/0, YO, C127, L cell, COS, e.g., COS 1
and C057,
QC1-3,HEK-293, VERO, PER.C6, HeLA, EB1, EB2, EB3, oncolytic or hybridoma-cell
lines.
Preferably the mammalian cells are CHO-cell lines. In one embodiment, the cell
is a CHO cell.
In one embodiment, the cell is a CHO-Kl cell, a CHO-Kl SV cell, a DG44 CHO
cell, a
DUXB11 CHO cell, a CHOS, a CHO GS knock-out cell, a CHO FUT8 GS knock-out
cell, a
CHOZN, or a CHO-derived cell. The CHO GS knock-out cell (e.g., GSKO cell) is,
for example,
a CHO-Kl SV GS knockout cell. The CHO FUT8 knockout cell is, for example, the
Potelligent CHOK1 SV (Lonza Biologics, Inc.). Eukaryotic cells can also be
avian cells, cell
lines or cell strains, such as for example, EBx cells, EB14, EB24, EB26,
EB66, or EBv13.
In one embodiment, the eukaryotic cells are stem cells. The stem cells can be,
for
example, pluripotent stem cells, including embryonic stem cells (ESCs), adult
stem cells,
58
CA 03083236 2020-05-21
WO 2019/113050
PCT/US2018/063822
induced pluripotent stem cells (iPSCs), tissue specific stem cells (e.g.,
hematopoietic stem cells)
and mesenchymal stem cells (MSCs).
In embodiments, the cultivated cells are eukaryotic cells, e.g., mammalian
cells. The
mammalian cells can be for example human cell lines, mouse myeloma (NS0)- cell
lines,
Chinese hamster ovary (CHO)-cell lines or hybridoma-cell lines. Preferably the
mammalian cells
are CHO-cell lines. In one embodiment, the cell is a CHO cell. In one
embodiment, the cell is a
CHO-Kl cell, a CHO-Kl SV cell, a DG44 CHO cell, a DUXB11 CHO cell, a CHOS, a
CHO GS
knock-out cell, a CHO FUT8 GS knock-out cell, a CHOZN, or a CHO-derived cell.
The CHO
GS knock-out cell (e.g., GSKO cell) is, for example, a CHO-Kl SV GS knockout
cell. The CHO
FUT8 knockout cell is, for example, the Potelligent CHOK1 SV (Lonza
Biologics, Inc.).
In embodiments, the cell is a yeast cell (e.g., S. cerevisae, T. reesei), an
insect cell (e.g.,
Sf9), an algae cell (e.g., cyanobacteria), or a plant cell (e.g., tobacco,
alfalfa, Physcomitrella
patens). In one embodiment, the cell is a rodent cell. In another embodiment,
the cell is a HeLa,
HEK293, HT1080, H9, HepG2, MCF7, Jurkat, NIH3T3, PC12, PER.C6, BHK (baby
hamster
kidney cell), VERO, SP2/0, NSO, YB2/0, YO, EB66, C127, L cell, COS, e.g., COS
1 and COS7,
QC1-3, CHO-Kl.
In embodiments, the cell is a stem cell. In one embodiment, the cell is a
differentiated
form of any of the cells described herein. In one embodiment, the cell is a
cell derived from any
primary cell in culture.
In embodiments, the cell is a hepatocyte such as a human hepatocyte, animal
hepatocyte,
or a non-parenchymal cell. For example, the cell can be a plateable metabolism
qualified human
hepatocyte, a plateable induction qualified human hepatocyte, plateable
Qualyst Transporter
Certified Tm human hepatocyte, suspension qualified human hepatocyte
(including 10-donor and
20-donor pooled hepatocytes), human hepatic kupffer cells, human hepatic
stellate cells, dog
hepatocytes (including single and pooled Beagle hepatocytes), mouse
hepatocytes (including
CD-1 and C57BI/6 hepatocytes), rat hepatocytes (including Sprague-Dawley,
Wistar Han, and
Wistar hepatocytes), monkey hepatocytes (including Cynomolgus or Rhesus monkey
hepatocytes), cat hepatocytes (including Domestic Shorthair hepatocytes), and
rabit hepatocytes
(including New Zealand White hepatocytes). Example hepatocytes are
commercially available
from Triangle Research Labs, LLC, 6 Davis Drive Research Triangle Park, North
Carolina, USA
27709.
59
CA 03083236 2020-05-21
WO 2019/113050
PCT/US2018/063822
In one embodiment, the eukaryotic cell is a lower eukaryotic cell such as e.g.
a yeast cell
(e.g., Pichia genus (e.g. Pichia pastoris, Pichia methanolica, Pichia
kluyveri, and Pichia angusta),
Komagataella genus (e.g. Komagataella pastoris, Komagataella pseudopastoris or
Komagataella
phaffii), Saccharomyces genus (e.g. Saccharomyces cerevisae, cerevisiae,
Saccharomyces
kluyveri, Saccharomyces uvarum), Kluyveromyces genus (e.g. Kluyveromyces
lactis,
Kluyveromyces marxianus), the Candida genus (e.g. Candida utilis, Candida
cacaoi, Candida
boidinii,), the Geotrichum genus (e.g. Geotrichum fermentans), Hansenula
polymorpha,
Yarrowia lipolytica, or Schizosaccharomyces pombe, . Preferred is the species
Pichia pastoris.
Examples for Pichia pastoris strains are X33, GS115, KM71, KM71H; and CBS7435.
In one embodiment, the eukaryotic cell is a fungal cell (e.g. Aspergillus
(such as A. niger,
A. fumigatus, A. orzyae, A. nidula), Acremonium (such as A. thermophilum),
Chaetomium (such
as C. thermophilum), Chrysosporium (such as C. thermophile), Cordyceps (such
as C. militaris),
Corynascus, Ctenomyces, Fusarium (such as F. oxysporum), Glomerella (such as
G.
graminicola), Hypocrea (such as H. jecorina), Magnaporthe (such as M. orzyae),
Myceliophthora
(such as M. thermophile), Nectria (such as N. heamatococca), Neurospora (such
as N. crassa),
Penicillium, Sporotrichum (such as S. thermophile), Thielavia (such as T.
terrestris, T.
heterothallica), Trichoderma (such as T. reesei), or Verticillium (such as V.
dahlia)).
In one embodiment, the eukaryotic cell is an insect cell (e.g., Sf9, MimicTM
Sf9, Sf21,
High FiveTm (BT1-TN-5B1-4), or BT1-Ea88 cells), an algae cell (e.g., of the
genus Amphora,
Bacillariophyceae, Dunaliella, Chlorella, Chlamydomonas, Cyanophyta
(cyanobacteria),
Nannochloropsis, Spirulina,or Ochromonas), or a plant cell (e.g., cells from
monocotyledonous
plants (e.g., maize, rice, wheat, or Setaria), or from a dicotyledonous plants
(e.g., cassava, potato,
soybean, tomato, tobacco, alfalfa, Physcomitrella patens or Arabidopsis).
In one embodiment, the cell is a bacterial or prokaryotic cell.
In embodiments, the prokaryotic cell is a Gram-positive cells such as
Bacillus,
Streptomyces Streptococcus, Staphylococcus or Lactobacillus. Bacillus that can
be used is, e.g.
the B.subtilis, B.amyloliquefaciens, B.lichernformis, B.natto, or
B.megaterium. In embodiments,
the cell is B.subtilis, such as B.subtilis 3NA and B.subtilis 168. Bacillus is
obtainable from, e.g.,
the Bacillus Genetic Stock Center, Biological Sciences 556, 484 West 12th
Avenue, Columbus
OH 43210-1214.
CA 03083236 2020-05-21
WO 2019/113050
PCT/US2018/063822
In one embodiment, the prokaryotic cell is a Gram-negative cell, such as
Salmonella spp.
or Escherichia co/i, such as e.g., TG1, TG2, W3110, DH1, DHB4, DH5a, HMS 174,
HM5174
(DE3), NM533, C600, HB101, JM109, MC4100, XL1-Blue and Origami, as well as
those
derived from E.coli B-strains, such as for example BL-21 or BL21 (DE3), all of
which are
commercially available.
Suitable host cells are commercially available, for example, from culture
collections such
as the DSMZ (Deutsche Sammlung von Mikroorganismen and Zellkulturen GmbH,
Braunschweig, Germany) or the American Type Culture Collection (ATCC).
In embodiments, the cultured cells are used to produce proteins e.g.,
antibodies, e.g.,
monoclonal antibodies, and/or recombinant proteins, for therapeutic use. In
embodiments, the
cultured cells produce peptides, amino acids, fatty acids or other useful
biochemical
intermediates or metabolites. For example, in embodiments, molecules having a
molecular
weight of about 4000 daltons to greater than about 140,000 daltons can be
produced. In
embodiments, these molecules can have a range of complexity and can include
posttranslational
modifications including glycosylation.
NUMBERED EMBODIMENTS
1. A method of separating a compound of Formula I, e.g., tropolone,
from another
component of a sample comprising:
contacting the sample with a partially or fully fluorinated alkyl or aryl,
e.g., a
fluorophenyl, e.g., a pentafluorophenylpropyl, moiety, under conditions
wherein the compound
of Formula I, e.g., tropolone, associates with, e.g., binds to or is retained
by, the moiety to a
greater extent than the component,
thereby separating the compound of Formula I, e.g., tropolone, from the
component, wherein
Formula I is:
X
(R2)n 41Ik R1
and wherein:
Xis 0 or S;
61
CA 03083236 2020-05-21
WO 2019/113050
PCT/US2018/063822
R1 is hydrogen, Ci-C6 alkyl, Ci-C6 heteroalkyl, OR3, C(0)R5, C(0)0R3,
N(R4a)(R4b),
C(0)N(R4a)(R4b), or N(R4a)C(0)R5;
each R2 is independently Ci-C6 alkyl, Ci-C6 heteroalkyl, N(R4a)(R4b),
c(0)N(R4a)(R4b),
or N(R4a)C(0)R5; or
two R2 are joined to form a heterocyclyl ring optionally substituted with one
or more R6;
or R1 and R2 are joined to form a heterocyclyl ring optionally substituted
with one or more R6;
R3 is hydrogen, Ci-C6 alkyl, or Ci-C6 heteroalkyl;
R4a and R4b are independently hydrogen, Ci-C6 alkyl, or Ci-C6 heteroalkyl;
R5 is Ci-C6 alkyl or Ci-C6 heteroalkyl;
each R6 is independently Ci-C6 alkyl, Ci-C6 heteroalkyl, halo, oxo, or cyano;
and
n is 0, 1, 2, 4, or 5.
2. The method of paragraph 1, wherein the moiety comprises a
pentafluorophenylpropyl
group.
3. The method of either of paragraphs 1 or 2, wherein the
pentafluorophenylpropyl group is
associated with, e.g., bound to, e.g., covalently bound to, a substrate.
4. The method of paragraph 3, wherein the substrate comprises an insoluble
substrate, e.g., a
chromatography matrix, e.g., a silica gel.
5. The method of any of paragraphs 1-4, comprising contacting the moiety
with one or more
mobile phases (e.g., one or two mobile phases) under conditions wherein the
compound is
preferentially eluted.
6. The method of any of paragraphs 1-5, wherein the method comprises
subjecting the
sample to a liquid chromatography (LC) separation.
62
CA 03083236 2020-05-21
WO 2019/113050
PCT/US2018/063822
7. A method of evaluating the presence, e.g., the level, of a compound
of Formula I, e.g.,
tropolone, in a sample comprising a product, comprising:
a) i) providing an aliquot of a sample, e.g., a compound of
Formula I (e.g.,
tropolone) depleted phase, e.g., a mobile phase, wherein the compound of
Formula I, e.g.,
tropolone, has been separated from another component of the sample, or
ii) subjecting the sample to conditions wherein the compound of Formula I,
e.g.,
tropolone, is separated from another component of the sample, e.g., to form a
compound of
Formula I, e.g., tropolone, enriched phase or aliquot and a compound of
Formula I, e.g.,
tropolone, depleted phase or aliquot; and
b) evaluating the presence, e.g., the level, of the compound of Formula I,
e.g., tropolone,
e.g., determining a value for the level of the compound of Formula I, e.g.,
tropolone, in the
sample:
i) using tandem mass spectrometry (MS2), or
ii) using ultraviolet (UV) absorption, e.g., UV absorption at about 242 nm or
about 238 nm,
thereby analyzing the sample,
wherein Formula I is:
X
(R2),-, ill
R1
and wherein:
X is 0 or S;
R1 is hydrogen, Ci-C6 alkyl, Ci-C6 heteroalkyl, OR3, C(0)R5, C(0)0R3,
N(R4a)(R4b),
C(0)N(R4a)(R4b), or N(R4a)C(0)R5;
each R2 is independently Ci-C6 alkyl, Ci-C6 heteroalkyl, N(R4a)(R4b),
c(0)N(R4a)(R4b),
or N(R4a)C(0)R5; or
two R2 are joined to form a heterocyclyl ring optionally substituted with one
or more R6;
or R1 and R2 are joined to form a heterocyclyl ring optionally substituted
with one or more R6;
R3 is hydrogen, Ci-C6 alkyl, or Ci-C6 heteroalkyl;
R4a and R4b are independently hydrogen, Ci-C6 alkyl, or Ci-C6 heteroalkyl;
63
CA 03083236 2020-05-21
WO 2019/113050
PCT/US2018/063822
R5 is Ci-C6 alkyl or Ci-C6 heteroalkyl;
each R6 is independently C1-C6 alkyl, Ci-C6 heteroalkyl, halo, oxo, or cyano;
and
n is 0, 1, 2, 4, or 5.
8. The method of paragraph 7, wherein a) comprises providing an aliquot of
a sample, e.g.,
a compound of Formula I, e.g., tropolone, depleted phase, e.g., a mobile
phase, wherein the
compound of Formula I, e.g., tropolone, has been separated from another
component of the
sample.
9. The method of paragraph 7, wherein a) comprises subjecting the sample to
conditions
wherein the compound of Formula I, e.g., tropolone, is separated from another
component of the
sample, e.g., to form a compound of Formula I, e.g., tropolone, enriched phase
or aliquot and a
compound of Formula I, e.g., tropolone, depleted phase or aliquot.
10. The method of any of any of paragraphs 7-9, wherein a) comprises
subjecting the sample
to a liquid chromatography (LC) separation.
11. The method of any of paragraphs 7-10, wherein a) comprises contacting
the sample with
a partially or fully fluorinated alkyl or aryl, e.g., a fluorophenyl, e.g., a
pentafluorophenylpropyl,
moiety , under conditions wherein the compound of Formula I, e.g., tropolone,
associates with,
e.g., binds to, or is retained by, the moiety to a greater extent than the
component.
12. The method of paragraph 11, wherein the moiety comprises a
pentafluorophenylpropyl
group.
13. The method of any of paragraphs 7-12, wherein b) comprises comprising
evaluating the
level or presence of the compound of Formula I, e.g., tropolone, e.g.,
determining a value for the
level of the compound of Formula I, e.g., tropolone, in the sample using
tandem mass
spectrometry (MS2).
64
CA 03083236 2020-05-21
WO 2019/113050
PCT/US2018/063822
14. The method of any of paragraphs 7-12, wherein b) comprises evaluating
the level or
presence of the compound of Formula I, e.g., tropolone, e.g., determining a
value for the level of
the compound of Formula I, e.g., tropolone, in the sample using ultraviolet
(UV) absorption, e.g.,
UV absorption at about 242 nm or about 238 nm.
15. The method of any of paragraphs 7, 11, or 12 comprising: a)i) and b)i).
16. The method of any of paragraphs 7, 11, or 12 comprising: a)i) and
b)ii).
17. The method of any of paragraphs 7, 11, or 12 comprising: a)ii) and
b)i).
18. The method of any of paragraphs 7, 11, or 12 comprising: a)ii) and
b)ii).
19. The method of any of paragraphs 7-18, wherein the linear range of the
method with
regard to determining a value for the level of the compound of Formula I,
e.g., tropolone, present
in the sample is about 0.1-10000, 0.2-8000, 0.3-7000, 0.4-6000, 0.5-5000, 0.5-
4000, 0.5-3000,
0.5-2000, or 0.5-1000 i.t.g/ml, e.g., 0.5-1000 .t.g/ml.
20. The method of any of paragraphs 7-19, wherein the lower limit of the
linear range of the
method with regard to determining a value for the level of the compound of
Formula I, e.g.,
tropolone, in the sample is about 0.01, 0.05, 0.1, 0.2, 0.3, 0.35, 0.4, 0.45,
0.5, 0.6, 0.7, 0.8, 0.9, or
1 i.t.g/ml, e.g., 0.5 .t.g/ml.
21. The method of any of paragraphs 7-20, wherein the upper limit of the
linear range of the
method with regard to determining a value for the level of the compound of
Formula I, e.g.,
tropolone, in the sample is about 500, 600, 700, 800, 900, 1000, 1200, 1400,
1600, 1800, 2000,
3000, 4000, 5000, 6000, 7000, 8000, 9000, or 10,000 i.t.g/ml, e.g., 1000
.t.g/ml.
CA 03083236 2020-05-21
WO 2019/113050
PCT/US2018/063822
22. The method of any of paragraphs 7-21, wherein the precision (e.g.,
represented by the
standard deviation between replicate samples) of the method with regard to
determining a value
for the level of the compound of Formula I, e.g., tropolone, present in the
sample can be less than
or equal to about 50, 40, 30, 25, 20, 19, 18, 17, 16, 15, 14, 13, 12, 11, 10,
9, 8, 7, 6, 5, 4, 3, 2, or
1%, e.g., 17, 16.5, or 16%.
23. The method of any of paragraphs 7-22, wherein the accuracy (e.g.,
represented by
average single point spike recovery in three different samples) of the method
with regard to
determining a value for the level of the compound of Formula I, e.g.,
tropolone, present in the
sample is greater than or equal to about 70, 75, 80, 81, 82, 83, 84, 85, 86,
87, 88, 89, 90, 91, 92,
93, 94, or 95%, e.g., 91%.
24. The method of any of paragraphs 7-23, wherein the lower limit of
detection of the
method with regard to determining a value for the level of the compound of
Formula I, e.g.,
tropolone, present in the sample is about 1, 1.5, 2, 2.5, 3, 3.5, 4, 4.5, 5,
5.5, 6, 6.5, 7, 7.5, 8, 8.5,
9, 9.5, or 10 i.t.g/ml, e.g., 5 .t.g/ml.
25. The method of either paragraph 6 or 10, wherein the LC is reversed
phase
chromatography.
26. The method of either paragraph 6 or 10, wherein the LC is not reversed
phase
chromatography.
27. The method of either paragraph 6 or 10, wherein the LC comprises using
a stationary
phase comprising a partially or fully fluorinated alkyl or aryl, e.g., a
fluorophenyl, e.g., a
pentafluorophenylpropyl, group.
28. The method of paragraph 27, wherein the LC comprises using a stationary
phase
comprising a fluorophenyl group.
66
CA 03083236 2020-05-21
WO 2019/113050
PCT/US2018/063822
29. The method of paragraph 27, wherein the LC comprises using a
stationary phase
comprising a pentafluorophenylpropyl group.
30. The method of any of paragraphs 6, 10, or 25-29, wherein the LC
comprises using a first
mobile phase and a second mobile phase.
31. The method of paragraph 30, wherein the first mobile phase comprises
formic acid in
water, e.g., about 0.01%, 0.05%, 0.06%, 0.07%, 0.08%, 0.09%, 0.1%, 0.11%,
0.12%, 0.13%,
0.14%, 0.15%, 0.16%, 0.17%, 0.18%, 0.19%, 0.2%, 0.3%, 0.4%, 0.5%, 0.6%, 0.7%,
0.8%, 0.9%,
or 1% formic acid in water.
32. The method of paragraph 31, wherein the first mobile phase comprises
about 0.1%
formic acid in water.
33. The method of paragraph 30, wherein the second mobile phase comprises
formic acid in
acetonitrile, e.g., about 0.01%, 0.05%, 0.06%, 0.07%, 0.08%, 0.09%, 0.1%,
0.11%, 0.12%,
0.13%, 0.14%, 0.15%, 0.16%, 0.17%, 0.18%, 0.19%, 0.2%, 0.3%, 0.4%, 0.5%, 0.6%,
0.7%,
0.8%, 0.9%, or 1% formic acid in acetonitrile.
34. The method of paragraph 33, wherein the second mobile phase comprises
about 0.1%
formic acid in acetonitrile.
35. The method of either of paragraphs 33 or 34, wherein the second mobile
phase comprises
at least about 50, 55, 60, 65,70, 75, 80, 85, 90, 95, or 100% acetonitrile,
e.g., about 100%
acetonitrile.
67
CA 03083236 2020-05-21
WO 2019/113050
PCT/US2018/063822
36. The method of any of paragraphs 6, 10, or 25-35, wherein the LC
comprises: using a
stationary phase comprising a pentafluorophenylpropyl group, and using a first
mobile phase and
a second mobile phase, wherein the first mobile phase comprises about 0.1%
formic acid in
water, and wherein the second mobile phase comprises about 0.1% formic acid in
acetonitrile.
37. The method of any of paragraphs 6, 10, or 25-36, wherein the LC
comprises using a
Discovery HS F5-3 column.
38. The method of any of paragraphs 7-13, 15, 17, and 19-37, wherein using
MS2 comprises
selected reaction monitoring (SRM).
39. The method of any of paragraphs 7-13, 15, 17, and 19-37, wherein using
MS2 comprises
multiple reaction monitoring (MRM), e.g., parallel reaction monitoring (PRM).
40. The method of either of paragraphs 38 or 39, wherein SRM or MRM (e.g.,
PRM), is used
to monitor one or more transitions selected from transition i, ii, iii, iv, v,
and vi of Table 1.
41. The method of paragraph 40, wherein SRM or MRM (e.g., PRM), is used to
monitor
transition i.
42. The method of paragraph 40, wherein SRM or MRM (e.g., PRM), is used to
monitor
transition ii.
43. The method of paragraph 40, wherein SRM or MRM (e.g., PRM), is used to
monitor
transition iii.
44. The method of paragraph 40, wherein SRM or MRM (e.g., PRM), is used to
monitor
transition iv.
68
CA 03083236 2020-05-21
WO 2019/113050
PCT/US2018/063822
45. The method of paragraph 40, wherein SRM or MRM (e.g., PRM), is used
to monitor
transition v.
46. The method of paragraph 40, wherein SRM or MRM (e.g., PRM), is used to
monitor
transition vi.
47. A reaction mixture comprising a partially or fully fluorinated alkyl
or aryl, e.g., a
fluorophenyl, e.g., a pentafluorophenylpropyl, moiety, and a sample comprising
a compound of
Formula I, e.g., tropolone, another component, and optionally a product,
wherein Formula I is
given by:
X
(R2)n 41Ik R1
and wherein:
Xis 0 or S;
R1 is hydrogen, Ci-C6 alkyl, Ci-C6 heteroalkyl, OR3, C(0)R5, C(0)0R3,
N(R4a)(R4b),
C(0)N(R4a)(R4b), or N(R4a)C(0)R5;
each R2 is independently Ci-C6 alkyl, Ci-C6 heteroalkyl, N(R4a)(R4b),
c(0)N(R4a)(R4b),
or N(R4a)C(0)R5; or
two R2 are joined to form a heterocyclyl ring optionally substituted with one
or more R6;
or R1 and R2 are joined to form a heterocyclyl ring optionally substituted
with one or more R6;
R3 is hydrogen, Ci-C6 alkyl, or Ci-C6 heteroalkyl;
R4a and R4b are independently hydrogen, Ci-C6 alkyl, or Ci-C6 heteroalkyl;
R5 is Ci-C6 alkyl or Ci-C6 heteroalkyl;
each R6 is independently Ci-C6 alkyl, Ci-C6 heteroalkyl, halo, oxo, or cyano;
and
n is 0, 1, 2, 4, or 5.
69
CA 03083236 2020-05-21
WO 2019/113050
PCT/US2018/063822
48. A method of manufacturing a product, e.g., a recombinant polypeptide,
comprising
providing a sample comprising the product and optionally a compound of Formula
I, e.g.,
tropolone, wherein:
the sample is analyzed by a method of any of paragraphs 7-43, 45, or 46, or
the compound of Formula I, e.g., tropolone, is separated from another
component of the
sample by a method of any of paragraphs 1-6,
wherein Formula I is given by:
X
(R2)n 41Ik R1
and wherein:
X is 0 or S;
R1 is hydrogen, Ci-C6 alkyl, Ci-C6heteroalkyl, OR3, C(0)R5, C(0)0R3,
N(R4a)(R4b),
C(0)N(R4a)(R4b), or N(R4a)C(0)R5;
each R2 is independently Ci-C6 alkyl, Ci-C6heteroalkyl, N(R4a)(R4b),
c(0)N(R4a)(R4b),
or N(R4a)C(0)R5; or
two R2 are joined to form a heterocyclyl ring optionally substituted with one
or more R6;
or R1 and R2 are joined to form a heterocyclyl ring optionally substituted
with one or more R6;
R3 is hydrogen, Ci-C6 alkyl, or Ci-C6heteroalkyl;
R4a and R4b are independently hydrogen, Ci-C6 alkyl, or Ci-C6heteroalkyl;
R5 is Ci-C6 alkyl or Ci-C6heteroalkyl;
each R6 is independently Ci-C6 alkyl, Ci-C6heteroalkyl, halo, oxo, or cyano;
and
n is 0, 1, 2, 4, or 5.
49. The method of paragraph 48, wherein the method of manufacturing
comprises expression
and secretion from a plurality of cells (e.g., a plurality of CHO cell, e.g.,
a plurality of GS-CHO
.. cells).
CA 03083236 2020-05-21
WO 2019/113050
PCT/US2018/063822
50. The method or reaction mixture of any of paragraphs 1-49, wherein the
sample comprises
culture supernatant.
51. The method or reaction mixture of any of paragraphs 1-49, wherein the
sample comprises
cell lysate.
52. The method or reaction mixture of any of paragraphs 1-51, wherein the
sample comprises
culture supernatant and cell lysate.
53. The method or reaction mixture of any of paragraphs 1-52, wherein the
sample was
generated by a method of manufacturing a product, e.g., a recombinant
polypeptide.
54. The method or reaction mixture of any of paragraphs 1-53, wherein the
sample comprises
a final product, e.g., a final product formulated for delivery (e.g.,
administration to a patient).
55. The method or reaction mixture of any of paragraphs 1-54, wherein the
product or
recombinant polypeptide is a homopolymeric or heteropolymeric polypeptide,
e.g., a hormone,
growth factor, receptor, antibody, cytokine, receptor ligand, transcription
factor or enzyme,
preferably an antibody or an antibody fragment, e.g., a human antibody or a
humanized antibody
or fragment thereof, e.g., a humanized antibody or fragment thereof derived
from a mouse, rat,
rabbit, goat, sheep, or cow antibody, typically of rabbit origin.
56. The method or reaction mixture of any of paragraphs 1-55, wherein the
product or
recombinant polypeptide is a therapeutic polypeptide.
57. The method or reaction mixture of any of paragraphs 1-56, wherein the
product or
recombinant polypeptide is one disclosed in Table 1, Table 2, Table 3, or
Table 4.
58. The method or reaction mixture of any of paragraphs 1-57, wherein the
product or
recombinant polypeptide is an antibody.
71
CA 03083236 2020-05-21
WO 2019/113050
PCT/US2018/063822
59. The method or reaction mixture of paragraph 58, wherein the antibody is
a monoclonal
antibody.
60. The method or reaction mixture of either of paragraphs 58 or 59,
wherein the monoclonal
antibody is a therapeutic antibody.
61. The method or reaction mixture of any of paragraphs 49-60, wherein the
cells are
mammalian cells.
62. The method or reaction mixture of paragraph 61, wherein the cell is a
mouse, rat, Chinese
hamster, Syrian hamster, monkey, ape, dog, horse, ferret, or cat.
63. The method or reaction mixture of paragraph 61, wherein the cells are
Chinese hamster
ovary (CHO) cells.
64. The method or reaction mixture of paragraph 63, wherein the CHO cells
are CHO-Kl
cells, CHO-Kl SV cells, DG44 CHO cells, DUXB11 CHO cells, CHOS cells, CHO GS
knock-
out cells, CHO FUT8 GS knock-out cells, CHOZN cells, or CHO-derived cells.
65. The method or reaction mixture of paragraph 61, wherein the cells are
HeIa, HEK293,
HT1080, H9, HepG2, MCF7, Jurkat, NIH3T3, PC12, PER.C6, BHK (baby hamster
kidney cell),
VERO, 5P2/0, NSO, YB2/0, YO, EB66, C127, L cell, COS, e.g., COS 1 and C057,
QC1-3, or
any cells derived therefrom.
72
CA 03083236 2020-05-21
WO 2019/113050
PCT/US2018/063822
EXAMPLES
The invention is further described in detail by reference to the following
experimental
examples. These examples are provided for purposes of illustration only, and
are not intended to
be limiting unless otherwise specified. Thus, the invention should in no way
be construed as
being limited to the following examples, but rather, should be construed to
encompass any and
all variations which become evident as a result of the teaching provided
herein.
Without further description, it is believed that one of ordinary skill in the
art can, using
the preceding description and the following illustrative examples, make and
utilize the
compounds of the present invention and practice the claimed methods. The
following working
examples specifically point out various aspects of the present invention, and
are not to be
construed as limiting in any way the remainder of the disclosure.
Example 1: Equipment and Reagents
The following equipment, reagents, and acronyms are used in Examples 2-6.
Acronyms:
BDS Bulk drug substance
LC-MS/MS Liquid chromatography tandem mass spectrometry
LOD Limit of detection
LLOD Lower limit of detection
SRM Selected reaction monitoring
RSD Relative standard deviation
Equipment:
Phenomenex Luna-NH2 150 x 2 mm, 5 p.m column, part no. 00F-4378-BO, serial no.
H15-
228806 and H15-045780.
Supelco Discovery HS F5 150 x 2.1 mm 3 p.m, product no. 567503-U, column no.
149000-03,
BL: 8129
Waters Acquity UPLC System ID 270419.
Waters Xevo TQ-MS. System ID 270418.
5 3 C Storage: Room G100, Slough.
73
CA 03083236 2020-05-21
WO 2019/113050
PCT/US2018/063822
Software:
Waters MassLynxTM Mass Spectrometry software
Waters TargetLynxTm Application Manager
Materials & Reagents:
Tropolone, Sigma Aldrich, part no. 15702-5G, batch no. BCBR4016V
Eluate 1 ¨ formulation: 10 mM sodium phosphate / 40 mM sodium chloride, pH 7.5
Eluate 2 ¨ formulation: 10 mM sodium phosphate / 40 mM sodium chloride / 400
mM sodium
citrate, pH 6.1
BASM ¨ formulation: 30 mM histidine / histidine HC1, 225 mM sorbitol, pH 6.0
Example 2: Development of the Method
Existing RP-HPLC separation methods and UV detection were used to separate and
detect tropolone in a typical sample with typical formulation components. The
UV
chromatogram shows multiple peaks present, attributable to sample buffer
components, and at
levels that can make quick and accurate identification and quantification of
tropolone difficult
(Figure 1).
Using IntelliStart software, a SRM transition was developed. Tropolone was
dissolved in
50% acetonitrile and infused directly into the mass spectrometer with both
positive and negative
ionisation modes scanned. The results of which are shown in Table 1.
74
CA 03083236 2020-05-21
WO 2019/113050
PCT/US2018/063822
Table 1: InternStart Developed SRM Transitions
Transition Collision Ion Mode
Parent (m/z) Cone voltage Daughter (m/z)
Number Energy (V)
i 123.07 8 123.07 14 +
ii 123.07 8 105.00 14 +
iii 123.07 8 77.00 14 +
iv 123.07 8 51.00 14 +
v 121.07 10 121.07 4 -
vi 121.07 10 65.03 4 -
These SRMs were tested for detection of chromatographic separation using a
Luna-NH2
(Phenomenex) LC column using 40 mM ammonium acetate pH 9.45 / 5% acetonitrile
as mobile
phase A and acetonitrile as mobile phase B. This configuration showed no
analyte retention
(Figure 2), so this column was not used for further experiments.
The LC column was switched to a Discovery HS F5-3 (Supelco) using 0.1% formic
acid
in water as mobile phase A and 0.1% formic acid in acetonitrile as mobile
phase B. Using this
configuration, both SRMs showed a single, sharp peak (eluting at 5.18 minutes)
for an injection
of tropolone dissolved in 50:50 mobile phase A:B (Figure 3).
Example 3: Method Performance ¨ Linear Range, Precision, and Accuracy
The developed LC-MS method of Example 2 was tested for the following method
performance parameters: linear range, accuracy and precision. Linear range was
assessed using a
5-point calibration curve (0.5, 1.0, 100.0, 500.0 and 1000.0 i.t.g/mL),
analysed across 2 days (1
injection followed by triplicate injection). One further calibration point
(0.1 i.t.g/mL) was
analysed but found to be below the LOD of the method and so no peak areas were
plotted
(Figure 4). Across the 0.5¨ 1000.0 i.t.g/mL range, linearity was found to be
R2= 0.9911.
Precision was calculated using the average of the relative standard deviation
(RSD) from
the replicate injections of the standard curve giving a value of 16.56%.
Accuracy was calculated from a single point spike recovery experiment into 3
different
process samples giving an average recovery of 91.4%.
Example 4: Method Performance ¨ Testing In-Process Samples
CA 03083236 2020-05-21
WO 2019/113050
PCT/US2018/063822
The method developed and tested in Examples 2 and 3 was further tested on
three
samples from various stages of purification of a manufactured biological
product. The three in-
process samples tested were from various downstream stages (post Sartobind Q
and Sartobind
Phenyl columns and bulk drug substance (BDS)) of different formulations.
Samples were
analysed as a neat injection and showed no tropolone signal in any of the
samples (Figure 5).
As part of method performance evaluation, tropolone standard was spiked into
these in-
process samples at 0.05 mg/mL (Figure 6). This experiment confirmed the
recovery of tropolone
from in-process samples of various formulations. This also confirmed that
tropolone was not
present in the tested samples at above the lower limit of detection (LLOD) of
the method (5.0
i.t.g/mL).
Example 5: UV Detection and the Method
As seen in Example 2 (Figure 1), previous methods using UV detection at 242 nm
showed interfering peaks detected from the sample matrix (sample buffer
peaks). It was
considered whether using 238 nm absorption might increase the tropolone peak
response. Using
the new chromatography conditions established and tested in Examples 2-4, UV
detection was
tested in place of MS and the issues of interfering buffer peaks appear to
have been resolved
(Figure 7). The top trace shows UV detection at 242 nm and the bottom trace at
238 nm, at both
wavelengths it appears that the buffer peak previously seen at 7.0 minutes
(Figure 1) has moved
retention time and no longer interferes with the tropolone peak (5.28
minutes). With increased
peak resolution, MS detection may not be necessary for a new assay although it
would provide
greater specificity than UV detection.
Example 6: Conclusion
A new assay for the detection of tropolone in process and purified samples was
developed using LC-MS. Without optimisation this improves upon the previously
used RP-
HPLC-UV method by reduction of interfering peaks through altered
chromatography and
increased specificity of detection.
Method performance parameters were assessed for linear range, accuracy and
precision
with the results as follows:
= Linearity: R2 = 0.9911
76
CA 03083236 2020-05-21
WO 2019/113050
PCT/US2018/063822
= Precision = 16.56 % RSD
= Accuracy = 91.4 % recovery
Using the detection wavelength of 238 nm, a small increase in tropolone peak
intensity
was observed when compared to 242 nm. The improved chromatographic performance
obtained
using the Discovery HS-F5 column and associated mobile phases resolves the
issue of
formulation buffer interference previously noted. It may be possible to use
this LC configuration
with UV detection only.
77