Note: Descriptions are shown in the official language in which they were submitted.
CA 03084907 2020-06-05
WO 2019/122520 PCT/F12018/050935
1
VARIANTS OF FUNGAL CELLULASE
FIELD OF THE INVENTION
The present invention relates to polypeptides having cellulase activity, in
particular
the invention relates to variants of cellulolytic enzymes that hydrolyze
cellulose.
The enzymes have been engineered to have improved stability compared to the
parent cellulase. Provided herein are novel polypeptide variants and the uses
thereof.
BACKGROUND OF THE INVENTION
io Cellulases or cellulolytic enzymes are enzymes involved in hydrolysis of
cellulose.
In the textile industry, cellulases are used in denim finishing to create a
fashionable
stone washed appearance in denim cloths in a biostoning process, and they are
also used, for instance, to clean fuzz and prevent formation of pills on the
surface
of cotton garments. In detergent industry cellulases are used to brighten
colors and
to prevent graying, pilling of garments and to improve cleaning. Cellulases
are
further used in food industry and animal feed manufacturing, and they have a
great
potential in biomass hydrolysis and in the pulp and paper industry, for
instance, in
deinking to release ink from fiber surfaces and in improving pulp drainage.
The 20K-cellulase deriving from Melanocarpus albomyces has been used
extensively for years in the textile and detergent industry and it has been a
subject
of several patents (for example EP0857216B1 and EP187492761). This cellulase
has been shown to have good performance especially in antigreying
applications.
The native 20K-cellulase molecule was cloned in the 1990's. Current increased
demand on different washing systems for laundry has substantial consequences
for the composition and amount of detergents needed.
Although cellulolytic enzymes have been used successfully in commercial
applications for many years, a need still exists for new cellulases with
altered
properties, such as improved stability in detergent formulations containing
protease.
BRIEF DESCRIPTION OF THE INVENTION
An object of the present invention is to provide novel variants of the 20K-
cellulase
that show improved performance and stability in detergent, when compared to
the
parental enzyme. The objects of the invention are achieved by a variant
polypeptides and a method for their preparation and its uses which are
characterized by what is stated in the independent claims. The preferred
embodiments of the invention are disclosed in the dependent claims.
CA 03084907 2020-06-05
WO 2019/122520 PCT/F12018/050935
2
The invention discloses a number of amino acid residue positions important for
the
stability of the 20K-cellulase enzyme and thereby for the performance thereof.
The
present invention provides variant cellulase polypeptides basing on
Melanocarpus
albomyces 20K-cellulase core (without CBD). The novel variants have improved
stability compared to the parent cellulase MAO, deriving from the 20K-
cellulase. In
particular, the novel variants have good performance in an antigreying
application
and excellent stability in the presence of a protease in several detergent
compositions. Especially the variants show improved stability in protease
containing detergents even in long-term experiments at elevated temperatures
like
lo 30 C and 37 C. In addition the yield of the variants in fermentation is
comparable
to parental cellulase MAO.
BRIEF DESCRIPTION OF THE DRAWINGS
In the following the invention will be described in greater detail by means of
preferred embodiments with reference to the attached drawings, in which
Figure 1 schematically shows the expression plasmid constructions for the
expression of the MAO variants in Trichoderma reesei. Position of used
promoter
(Pcbh1), terminator (Tcbh1) and selection marker gene (amdS), relevant
restriction
sites and the position of the ampicillin resistance encoding gene (AmpR) in
the
vector are shown. Picture was generated using Geneious version 10.0 created by
Biomatters.
Figure 2 shows the stability of variants MA79 and MA15 compared to parental
cellulase MAO as residual activity in commercial liquid detergent after
incubating
samples at 50 C for 30 minutes in the presence of protease.
Figure 3 shows the antigreying performance of variant MA79 and parental
cellulase MAO in a single wash in the presence of carbon black in a commercial
liquid detergent. Washing conditions: 40 C, 60 min, 16 dH, carbon black
approx.
0.15 g/I, detergent 4.4 g/I.
Figure 4A shows the stability of variant MA79 compared to parental cellulase
MAO
as residual activity in commercial liquid detergent at 37 C in the presence of
protease.
Figure 4B shows the stability of variant MA79 compared to parental cellulase
MAO
as residual activity in commercial liquid detergent at 30 C in the presence of
protease.
Figure 4C shows the stability of variant MA79 compared to parental cellulase
MAO
as residual activity in commercial liquid detergent at room temperature
(approx.
20-22 C) in the presence of protease.
CA 03084907 2020-06-05
WO 2019/122520 PCT/F12018/050935
3
Figure 5 shows the stability of variant MA79 compared to parental cellulase
MAO
as residual activity in commercial protease containing l&I detergent at 37 C.
Figure 6 shows the relative antigreying performance of variant MA79 compared
to
parental cellulase MAO after 88 d and 193 d storage in commercial liquid
detergent
in the presence of protease at 30 C.
Figure 7 shows the stability of variant MA79 compared to commercial cellulase
Celluclean 5000L (Novozymes) as residual activity in commercial liquid
detergent
at 37 C in the presence of protease.
Figure 8A shows the stability of variant MA79 compared to parental cellulase
MAO
and commercial cellulase Celluclean 5000L (Novozymes) as residual activity in
protease containing liquid detergent concentrate at 37 C.
Figure 8B shows the stability of variant MA79 compared to parental cellulase
MAO
and commercial cellulase Celluclean 5000L (Novozymes) as residual activity in
protease containing liquid detergent concentrate at 30 C.
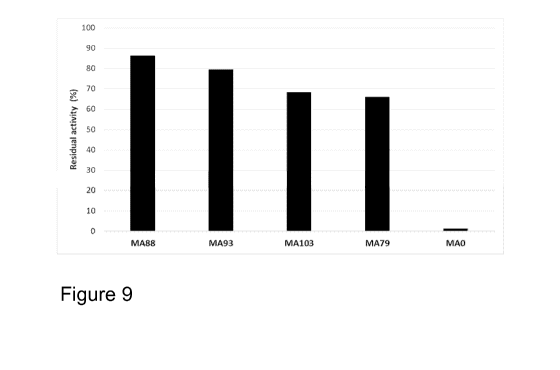
Figure 9 shows the stability of variants MA79, MA88, MA93 and MA103 compared
to parental cellulase MAO as residual activity in commercial liquid detergent
after
incubating samples at 50 C for 24 h minutes in the presence of protease.
DETAILED DESCRIPTION OF THE INVENTION
Cellulolytic enzymes or cellulases are enzymes having cellulolytic activity,
which
means that they are capable of hydrolysing cellulosic substrates or
derivatives
thereof into smaller saccharides. Cellulolytic enzymes thus include both
cellulases
and hemicellulases. Cellulases include (1) endoglucanases (EG, EC 3.2.1.4)
which cut internal beta-1,4-glucosidic bonds; (2) exoglucanases or
cellobiohydrolases (CBH, EC 3.2.1.176, EC 3.2.1.91) that cut the dissaccharide
cellobiose from the reducing or non-reducing end of the cellulose polymer
chain
and (3) beta-1,4-glucosidases (BG, EC 3.2.1.21) which hydrolyze the cellobiose
and other short cello-oligosaccharides to glucose.
The present invention relates in particular to endoglucanases. Specifically,
the
present invention relates to fungal endoglucanases belonging to glycosyl
hydrolase family 45 (Ce145), especially to variants of these endoglucanases.
More
specifically, the invention relates to MAO cellulase variants deriving of
Melanocarpus albomyces ALK04237 20 kDa endoglucanase. "Glycosyl hydrolase
family 45" or "GH45" refers to the glycosyl hydrolase family as defined by
Henrissat
1991, and Henrissat and Bairoch 1993, 1996.
The design of mutants is based on MAO cellulase 3D structure and sequence
comparisons of Ce145 family enzymes. The amino acid and nucleotide sequences
of Melanocarpus albomyces Ce145 20K-cellulase derived MAO is set forth in SEQ
CA 03084907 2020-06-05
WO 2019/122520
PCT/F12018/050935
4
ID NO: 1 and SEQ ID NO: 2, respectively. The isolation, enzymatic activity and
industrial applications of 20K-cellulase are disclosed for example
inEP1874927B1
and EP0857216B1 (AB Enzymes Oy).
The present invention specifically relates to a cellulase variant polypeptide,
or an
active fragment thereof, wherein the variant polypeptide has cellulase
activity and
comprises an amino acid sequence having at least 90%, but less than 100%
sequence identity to residues 22-235 of SEQ ID NO:1 comprising at least one
substitution or deletion at one or more position selected from: 2, 22, 33, 35,
39, 44,
48, 54, 75, 82, 92, 99, 108, 122, 174, 175, 177, 194, 205, 206, 207, 208, 209,
210,
io 211, 212, 213, and 214 wherein the amino acid positions of said variant
polypeptide or active fragment thereof are numbered by correspondence with the
mature amino acid sequence of SEQ ID NO:3.
The invention further relates to a polypeptide comprising one or more of the
following substitutions or deletions in the cellulase amino acid sequence set
forth
in SEQ ID NO:1: N2W, N2R, G22A, G225, G22V, A33R, F35W, F35M, H39P,
H395, V44R, V44Q, V44W, V44L, E48D, 554M, 554A, 554T, 554L, 554V A75H,
A755, A75R, E825, E82R, E82W, T92I, A99Q, T1081, N122A, N122L, N1225,
N122G, N122T, N122V, N1221, Q174R, N175D, D177Q, D177E, A194Q, G205T,
F206H, A207R, A2075, del(A207-A214), del(V208-A214), del(F209-A214),
del(K210-A214), del(A211-A214), del(P212-A214) and del(5213-A214) wherein
the amino acid positions of said variant polypeptide are numbered by
correspondence with the mature amino acid sequence of SEQ ID NO:3.
The invention further relates to a variant polypeptide, wherein the variant
comprises a substitution at amino acid positions 22 and/or 122, wherein at
position
22 G is preferably substituted to A, S or V, and at position 122 N is
preferably
substituted to A, S, G, T, V or I. In one embodiment the variant comprises a
substitution at positions 22 and 122. It may additionally comprise at least
one
further substitution and/or deletion. The further substitution may be at a
position
selected from the following positions: 2, 35,44, 54, 75, 82, 108, 174, 177,
205, 206
and 207 and the deletion may be selected from the following: del(A207-A214),
del(V208-A214), del(F209- A214), del(K210-A214), del(A211-A214), del(P212-
A214) or del(5213-A214).
In the present invention the variants are derived from a parental molecule MAO
(SEQ ID NO: 2), which is a polynucleotide encoding for the catalytic core
domain
of a cellulase derived from Melanocarpus albomyces ALK04237 20K-cellulase
having no cellulose binding domain (CBD).
The term "variant" as used herein refers to a polypeptide having cellulase
activity
and comprising an experimentally induced mutation, i.e., an insertion,
substitution,
and/or deletion, at one or more positions compared to the parental sequence
MAO
CA 03084907 2020-06-05
WO 2019/122520 PCT/F12018/050935
set forth in SEQ ID NO:1. MAO differs from 20K-cellulase amino acid sequence
by
having a mutation in one specific position. A substitution means a replacement
of
an amino acid occupying a position with a different amino acid; a deletion
means
removal of an amino acid occupying a position; and an insertion means the
addition
5 of one or more amino acids. Deletions are described herein using "del"
before an
indication of the first and last amino acid(s) deleted.
In an embodiment the polypeptide variant is a variant disclosed in Table 3
having
the corresponding mutation as specified in Table 2. This embodiment is
advantageous in obtaining variants that have improved stability in a detergent
lo which contains protease. In a preferred embodiment the variant is a variant
marked
in Table 3 as having at least two "+" signs, even more preferably at least 3,
4 or
even 5 "+" signs, the "+" signs indicating the level of improvement compared
to the
reference.
In a preferable embodiment the variant has at least three substitutions at
positions
22, 54, and 122. More preferably the substitutions comprise substitutions
G22A,
554T, and N122A (MA87).
In a preferable embodiment the variant has at least three substitutions at
positions
22, 108, and 122. More preferably the substitutions comprise substitutions
G22A,
T1081, and N122A (MA93).
In a preferable embodiment the variant has at least three substitutions at
positions
22, 122, 205. More preferably the substitutions comprise substitutions G22A,
N122A, and G205T (MA103). Such variants have improved stability in a liquid
detergent containing protease.
The polypeptide variant preferably comprises a sequence that is at least 90 %,
91%, 92%, 93%, 94%, 95%, 96%, 97%, 98% or at least 99% but less than 100%
identical to SEQ ID NO:1. In some embodiments, the disclosed variant sequence
comprises at least one mutation and the remainder of the sequence is at least
90%
identical to the amino acid residues 22-235 set forth in SEQ ID NO: 1. As used
herein, "identity" means the percentage of exact matches of amino acid
residues
between two aligned sequences over the number of positions where there are
residues present in both sequences. When one sequence has a residue with no
corresponding residue in the other sequence, the alignment program allows a
gap
in the alignment, and that position is not counted in the denominator of the
identity
calculation. Identity is a value determined with the Pairwise Sequence
Alignment
tool EMBOSS Needle at the EMBL-EBI website (www.ebi.ac.ukiToolsipsatem-
boss_needleOwith the following parameters: BLOSUM62, Gap open 10, Gap
extend 0.5.
The variants are generated by deliberately introducing changes in DNA to
produce
mutant gene products i.e. proteins. The changes or modifications of the
parental
CA 03084907 2020-06-05
WO 2019/122520 PCT/F12018/050935
6
nucleotide sequence may be introduced by several methods including e.g. site-
directed and random mutagenesis, recombination or recombinant fusion
engineering. For site-directed mutagenesis and fusion engineering a protein
structure and good understanding of the structure-function relationship is
beneficial. In the absence of such deep understanding, methods based on random
mutagenesis may be used.
A variant may be obtained e.g. by altering hydrogen bond contacts, altering
charge
distribution, introduction of a salt bridge, introduction of metal binding
sites, filling
an internal structural cavity with one or more amino acids with bulkier side
groups
io (in e.g. regions which are structurally mobile), substitution of histidine
residues with
other amino acids, removal of a deamination site, removal of a flexible
region,
truncation of terminal region, shortening of a loop or by helix capping.
Stability of
the protein may be improved by substitution of at least one amino acid with
cysteine residue or insertion of one or more cysteine residues which creates
at
least one disulfide bridge.
The cellulase polypeptide variants of the invention are preferably
recombinantly
produced non-naturally occurring proteins. They are conveniently prepared
using
the generally known recombinant DNA technology. Briefly, the polynucleotide
encoding the endoglucanase is cloned and inserted into an expression vector,
transformed into a host cell and expressed. Preferably, mutations are
introduced
into the coding sequence with codons preferred by the host strain used.
Methods
for protein production by recombinant technology in different host systems are
well
known in the art. Preferably, the polypeptide variants are produced as
extracellular
proteins that are secreted into the culture medium, from which they can easily
be
recovered and isolated.
The polypeptide variants may be designed to comprise in addition to the
catalytic
core domain, which forms the active or functional site of the enzyme, one or
more
"cellulose binding domains" ("CBDs"), also named as carbohydrate binding
domains/modules (CBD/CBM) located either at the N- or C-terminus of the
catalytic domain. CBMs have carbohydrate-binding activity and they mediate the
binding of cellulase to crystalline and amorphous cellulose but have little or
no
effect on hydrolytic activity of the enzyme on soluble substrates.
The variants of the invention may optionally also contain a linker connecting
the
CBM and catalytic domain via a flexible and usually highly glycosylated
region. By
the term "linker" or "spacer" is meant a polypeptide comprising at least two
amino
acids which may be present between the domains of a multidomain protein, for
example an enzyme comprising an enzyme core and a binding domain such as a
carbohydrate binding module (CBM) or any other enzyme hybrid, or between two
proteins or polypeptides produced as a fusion polypeptide, for example a
fusion
protein comprising two core enzymes. For example, the fusion protein of an
CA 03084907 2020-06-05
WO 2019/122520 PCT/F12018/050935
7
enzyme core with a CBM is provided by fusing a DNA sequence encoding the
enzyme core, a DNA sequence encoding the linker and a DNA sequence encoding
the CBM sequentially into one open reading frame and expressing this
construct.
The modular structure of cellulases containing carbohydrate binding module
and/or the linker region is well known in the art. The carbohydrate binding
module
and the linker region may be heterologous or homologous. "Heterologous" as
used
in the present context means that the CBM and/or the possible linker part of
the
variant polypeptides are obtained from a different organism than the
cellulolytically
active core domain. "Homologous" as used herein means that the CBM and/or the
lo possible linker part of the variant are from the same organism as the
cellulolytically
active core. The invention discloses that any linker or CBM may be used in the
variant polypeptide.
The variants of the invention may optionally also contain a signal sequence.
The
term "signal sequence" denotes a DNA sequence that encodes a polypeptide (a
"secretory peptide") that, as a component of a larger polypeptide, directs the
larger
polypeptide through a secretory pathway of a host cell in which it is
produced. The
secretory signal sequence can be native or it can be replaced with secretory
signal
sequence or carrier sequence from another source. Depending on the host cell,
the larger peptide may be cleaved to remove the secretory peptide during
transit
through the secretory pathway.
"Enzymatically active fragment" refers to the part of a specific amino acid
sequence
that is long enough to have the desired enzymatic activity. In other words a
fragment may be e.g. only the mature part of the polypeptide or even a
subsequence of the mature part. It may or may not contain a linker and CBM
domain. The enzymatic activity refers herein to cellulolytic activity meaning
catalytic ability of the polypeptide to hydrolyse cellulose or derivatives
thereof.
The present invention relates further to novel polynucleotides which encode
the
cellulase variant polypeptide of the invention. The polynucleotide comprises a
nucleotide sequence having SEQ ID NO: 2 or fragments thereof long enough to
encode enzymatically active cellulase variants, or a sequence encoding a novel
polypeptide variant as defined above, including complementary strands thereof.
The polynucleotides of the invention are recombinant molecules containing
genetically engineered non-naturally occurring sequences. "Polynucleotide" as
used herein refers to both RNA and DNA, and it may be single stranded or
double
stranded. It may also be complementary DNA (cDNA). With cDNA is meant a DNA
molecule synthesized from a messenger RNA template obtained from a eukaryotic
or prokaryotic organism. Further, the polynucleotide may be degenerate as a
result
of the genetic code to any one of the sequences as defined above. This means
that different codons may code for the same amino acid.
CA 03084907 2020-06-05
WO 2019/122520 PCT/F12018/050935
8
The present invention relates to a recombinant expression "vector" comprising
a
polynucleotide encoding the polypeptide variants as characterized above,
operably
linked to regulatory sequences, which are capable of directing the expression
of a
gene encoding said polypeptide variants in a suitable host. Said regulatory
sequences may originate from the host organism or from another organism. The
expression vector may further comprise marker genes for selection of the
transformant strains or the selection marker may be introduced to the host in
another vector construct by co-transformation.
Still the present invention relates to a production "host", which can be any
organism
lo capable of expressing the desired polypeptide. As used herein, "host cell"
means
any cell type that is susceptible to transformation, transfection,
transduction,
mating, crossing or the like with a nucleic acid construct or expression
vector
comprising a polynucleotide. The term "host cell" encompasses any progeny that
is not identical due to mutations that occur during replication. Non-limiting
examples of a host cell are fungal cells, filamentous fungal cells from
Division
Ascomycota, Subdivision Pezizomycotina; preferably from the group consisting
of
members of the Class Sordariomycetes, Subclass Hypocreomycetidae, Orders
Hypocrea/es and Microascales and Aspergillus, Chrysosporium, Myceliophthora
and Humicola; more preferably from the group consisting of Families
Hypocreacea, Nectriaceae, Clavicipitaceae, Microascaceae, and Genera
Trichoderma (anamorph of Hypocrea), Fusarium, Gibberella, Nectria,
Stachybotrys, Claviceps, Metarhizium, Villosiclava, Ophiocordyceps,
Cephalosporium, and Scedosporium; more preferably from the group consisting of
Trichoderma reesei (Hypocrea jecorina), T. citrinoviridae, T. longibrachiatum,
T.
virens, T. harzianum, T. asperellum, T. atroviridae, T. parareesei, Fusarium
oxysporum, F. gramineanum, F. pseudo graminearum, F. venenatum, Gibberella
fujikuroi, G. moniliformis, G. zeaea, Nectria (Haematonectria) haematococca,
Stachybotrys chartarum, S. chlorohalonata, Claviceps purpurea, Metarhizium
acridum, M. anisopliae, Villosiclava virens, Ophiocordyceps sinensis,
Acremonium
(Cephalosporium) chrysogenum, and Scedosporium apiospermum, and
Aspergillus niger, Aspergillus awamori, Aspergillus oryzae, Chrysosporium
lucknowense, Myceli- ophthora thermophila, Humicola insolens, and Humicola
grisea, most preferably Trichoderma reesei. Non-limiting examples of a host
cell
are yeasts (e.g. Saccharomyces cerevisiae, Pichia pastoris, Yarrowia
lipolytica)
and bacterial cells, preferably gram positive Bacilli (e.g. Bacillus subtilis,
B.
licheniformis, B. megaterium, B. amyloliquefaciens, B. pumilus), gram-negative
bacteria (e.g. Escherichia coli) and actinomycetales (e.g. Streptomyces sp.).
In an embodiment the host cell is a fungal cell, preferably a filamentous
fungal cell,
such as Trichoderma or Trichoderma reesei. In an embodiment the host cell is a
bacterial cell, preferably a gram positive Bacillus cell, such as B. subtilis,
B.
licheniformis, B. megaterium, B. amyloliquefaciens or B. pumilus.
CA 03084907 2020-06-05
WO 2019/122520 PCT/F12018/050935
9
A "recombinant cell" or "recombinant host cell" refers to a cell or host cell,
which
has been genetically modified or altered to comprise a nucleic acid sequence
which is not native to said cell or host cell. In an embodiment the genetic
modification comprises integrating the polynucleotide in the genome of the
host
cell. In another embodiment the polynucleotide is exogenous in the host cell.
The present invention relates also to a method for producing variant
polypeptides
of the invention, said method comprising the steps of transforming a host cell
with
an expression vector encoding said polypeptide, and culturing said host cell
under
conditions enabling production of said polypeptide, and optionally recovering
and
lo purifying said polypeptide. The production medium may be a medium suitable
for
growing the host organism and optionally containing inducers for efficient
gene
expression.
The present invention relates to an enzyme composition comprising the variant
cellulase polypeptides of the invention. As used in the present context the
"enzyme
composition" refers to any enzyme product, preparation or composition, which
comprises at least one of the novel variant cellulase polypeptides described
herein.
Such an enzyme composition may be a spent culture medium or filtrate
containing
one or more variant cellulase polypeptides, or one or more variant cellulase
polypeptides and one or more other enzymes. Spent culture medium means the
culture medium of the host comprising the produced enzymes. Preferably the
host
cells are separated from said medium after the production. The enzyme
composition may be a "whole culture broth" optionally after inactivating the
production host(s) or microorganism(s) without any biomass separation, down-
stream processing or purification of the desired cellulolytic enzyme(s),
because the
variant polypeptides can be secreted into the culture medium, and they display
activity in the ambient conditions of the spent culture medium.
The enzyme composition may contain the enzymes in at least partially purified
and
isolated form. It may even essentially consist of the desired enzyme or
enzymes.
If desired, the enzyme compositions may be dried, spray-dried or lyophilized,
granulated or the enzymatic activity may be otherwise concentrated and/or
stabilized for storage. If required, a desired enzyme may be crystallized or
isolated
or purified in accordance with conventional methods, such as filtration,
extraction,
precipitation, chromatography, affinity chromatography, electrophoresis, or
the
like.
In one embodiment of the present invention the enzyme composition further
comprises one or more additional enzymes selected from the group consisting of
protease, lipase, cutinase, amylase, carbohydrase, cellulase, pectinase,
pectatelyase, pectinolytic enzyme, esterase, phytase, mannanase, arabinase,
galactanase, xylanase, oxidase, xanthanase, xyloglucanase, DNAse, laccase,
CA 03084907 2020-06-05
WO 2019/122520
PCT/F12018/050935
and/or peroxidase, preferably selected from the group consisting of proteases,
amylases, cellulases and lipases.
The present enzyme composition comprising cellulase and an additional enzyme
is advantageous in providing synergistic effects. Such additional enzymes are
5 desired when the present enzyme composition comprising cellulase is used in
detergents e.g. when washing stains. Particularly advantageous synergistic
enzymes that work with cellulases are amylases, proteases and mannanases, or
a combination thereof, such as a composition comprising cellulase, amylase and
protease. The perfect combination of enzymes allows maximal performance.
io In general the properties of the selected enzyme(s) should be compatible
with the
selected detergent, (i.e., pH-optimum, compatibility with other enzymatic and
non-
enzymatic ingredients, etc.), and the enzyme(s) should be present in effective
amounts. In an embodiment the present enzyme composition further comprises:
a. at least one preservative selected from organic acids, e.g. benzoic acid,
citric
acid, ascorbic acid, sorbic acid, and salts thereof, sodium benzoate,
hydroxybenzoate, benzisothiazolinone (BIT) or a combination thereof;
b. optionally at least one polyol selected from propylene glycol, glycerol, a
sugar,
sugar alcohol, sorbitol, hexylene glycol
c. optionally at least one inhibitor selected from formic acid, lactic acid,
boric
acid, boric acid derivative, aromatic borate ester, phenyl boronic acid
derivative,
peptide, other reversible subtilisin inhibitors or a combination thereof;
d. optionally at least one enzyme selected from proteases, amylases,
cellulases,
lipases, xylanases, mannanases, cutinases, esterases, phytases, DNAses,
pectinases, pectinolytic enzymes, pectate lyases, carbohydrases, arabinases,
galactanases, xanthanases, xyloglucanase, laccases, peroxidases and
oxidases with or without a mediator, or a combination thereof;
e. optionally at least one salt selected from sodium chloride, potassium
chloride,
potassium (hydrogen)phosphate, sodium (hydrogen)phosphate, ammonium
sulfate, potassium sulfate, or a combination thereof; and
f. optionally at least one filler or carrier selected from maltodextrin,
flour, sodium
chloride, sulfate, sodium sulfate, sodium acid pyrophosphate, tetra-sodium
pyrophosphate, polyethylene glycol, or a combination thereof.
The additional components a-f provide improved properties for the present
enzyme
composition. The enzyme composition is compatible with the additional
components and improves applicability of the enzyme composition in various
uses.
Salts, such as sodium chloride and sodium sulfate function as drying aids.
CA 03084907 2020-06-05
WO 2019/122520 PCT/F12018/050935
11
In an embodiment the present enzyme composition is in the form of a liquid
composition or a solid composition such as solution, dispersion, paste,
powder,
granule, granulate, coated granulate, tablet, cake, crystal, crystal slurry,
gel or
pellet.
The enzyme composition can be used in cleaning agents or boosters that are
added on top of the detergent during or before the wash and that are for
example
in the form of liquid, gel, powder, granules or tablets. The enzyme
composition and
detergent components may also be soaked in a carrier like textiles.
In an embodiment the enzyme composition is used in textile and detergent
io industry, biomass processing and biomass hydrolysis, preferably in biofuel,
starch,
pulp and paper, food, baking, feed or beverage industries.
The present invention relates further to a detergent composition comprising at
least
one of the novel variant cellulase polypeptides or an enzyme composition
thereof.
The invention relates also to a use of the variant cellulase polypeptides of
the
invention in detergent applications. The terms "detergent composition" and
"detergent" include, unless otherwise indicated, all washing agents in any
form
such as solid, granular or powder-form, liquid, gel or paste-form, and any
combination thereof. The detergent composition may be in the form of a sachet,
pouch, tablet or bar, including multi-compartment products. The detergent
composition can be a free-flowing powder or a liquid. The terms include,
unless
otherwise stated, all-purpose or heavy-duty washing agents, especially
cleaning
detergents; liquid fine-fabric, specialty or low-duty detergents; hand
dishwashing
agents or light duty dishwashing agents, especially those of the high-foaming
type;
machine dishwashing agents, including the various tablet, granular, liquid and
rinse- aid types for household and institutional use; liquid cleaning and
disinfecting
agents, car or carpet shampoos, bathroom cleaners; metal cleaners; as well as
cleaning auxiliaries such as bleach additives and "stain-stick" or pre-treat
types,
and laundry aids.
The terms "detergent", "detergent composition" and "detergent formulation" are
used in reference to mixtures, which are intended for use in a wash medium for
the cleaning of soiled objects. In some embodiments, the term is used in
reference
to laundering fabrics and/or garments (e.g., "laundry detergents"). In
alternative
embodiments, the term refers to other detergents, such as those used to clean
dishes, cutlery, etc. (e.g., "dishwashing detergents"). It is not intended
that the
present invention be limited to any particular detergent formulation or
composition.
It is intended that in addition to the cellulase variants according to the
invention,
the term encompasses detergents that may contain e.g., surfactants, builders,
chelators or chelating agents, bleach system or bleach components, polymers,
fabric conditioners, foam boosters, suds suppressors, dyes, perfume, tannish
inhibitors, optical brighteners, bactericides, fungicides, soil suspending
agents,
CA 03084907 2020-06-05
WO 2019/122520 PCT/F12018/050935
12
anticorrosion agents, hydrotropes, fabric hueing agents, dispersants, dye
transfer
inhibiting agents, fluorescent whitening agents, soil release polymers, anti-
redepositions agents, anti-shrink agents, anti-wrinkling agents, bactericides,
binders, carriers, dyes, enzyme stabilizers, fabric softeners, fillers, foam
regulators, perfumes, pigments, buffers, preservatives, sod suppressors,
solvents,
and structurants for liquid detergents, structure elasticizing agents, enzyme
inhibitors or stabilizers, enzyme activators, transferase(s), hydrolytic
enzymes,
oxido reductases, bluing agents and fluorescent dyes, antioxidants, and
solubilizers.
io A composition for use in solid laundry detergent, for example, may include
0.000001% - 5%, such as 0.000005-2%, such as 0.00001%-1%, such as
0.00001%-0.1`)/0 of variant cellulase polypeptide by weight of the
composition.
A composition for use in laundry liquid, for example, may include 0.000001%-
3%,
such as 0.000005%-1%, such as 0.00001%-0.1% of variant cellulase polypeptide
by weight of the composition.
A composition for use in automatic dishwash, for example, may include
0.000001%-5%, such as 0.000005%-2%, such as 0.00001%-1%, such as
0.00001%-0.1`)/0 of variant cellulase polypeptide by weight of the
composition.
The detergent composition may be in the form of a bar, a homogenous tablet, a
tablet having two or more layers, a pouch having one or more compartments, a
regular or compact powder, a granule, a paste, a gel, or a regular, compact or
concentrated liquid. In one embodiment the detergent composition can be a
laundry detergent composition, preferably a liquid or solid laundry detergent
composition. There are a number of detergent formulation forms such as layers
(same or different phases), pouches, as well as forms for machine dosing unit.
The variant cellulase polypeptides of the invention and enzyme compositions or
detergent compositions containing them when used in detergent and textile
industries have excellent performance and stability.
The term "stability" includes storage stability and stability during use, e.g.
during a
wash process (in wash stability). The stability of the cellulase according to
the
invention is described as a function of time, e.g. how much activity is
retained when
the cellulase is kept in solution, in particular in a detergent solution.
The stability is influenced by many factors, e.g. pH, temperature, detergent
composition e.g. proteases, stabilizers, builders, surfactants etc.
In the present invention the term "improved detergent stability" means that
the
cellulase variant retains its activity and/or performance in detergent
solution, during
storage and/or washing better than the parental enzyme. The stability may be
CA 03084907 2020-06-05
WO 2019/122520 PCT/F12018/050935
13
assayed by determining the residual activity of the enzyme added to detergent
after incubation at certain conditions for e.g. after 1 or several weeks
incubation at
37 C or lower temperatures or after shorter time at 50 C, like described in
Examples 2, 4 and 5. The residual activity of cellulase may be determined
using
the method described in Example 1 or any other method disclosed in the
literature.
The stability may be measured also as wash performance after storage, e.g. as
antigreying performance like described in Example 5.
The variant cellulase polypeptides and the enzyme compositions thereof may be
used for treating any cellulosic material. In the present context, "cellulosic
material"
io refers to any material comprising cellulose or derivatives thereof as a
significant
component. Such a material may be textile material, plants or material of
plant
origin used in food or animal feed, plant material for oil extraction, or wood-
derived
mechanical or chemical pulp or secondary fiber.
The term "textile" means any textile material including yarns, yarn
intermediates,
fibers, non-woven materials, natural materials, synthetic materials, and any
other
textile material, fabrics made of these materials and products made from
fabrics
(e.g., garments, linen and other articles). The textile or fabric may be in
the form of
knits, wovens, denims, non-wovens, felts, yarns, and towelling. The textile
may be
cellulose based, such as natural cellulosics including cotton, flax/linen,
jute, ramie,
sisal or coir or manmade cellulosics (e.g. originating from wood pulp)
including
viscose/rayon, ramie, cellulose acetate fibers (tricell), lyocell or blends
thereof. The
textile or fabric may also be non-cellulose based such as natural polyamides
including wool, camel, cashmere, mohair, rabbit and silk or synthetic polymer
such
as nylon, aramid, polyester, acrylic, polypropylen and spandex/elastane, or
blends
thereof as well as blend of cellulose based and non-cellulose based fibers.
Examples of blends are blends of cotton and/or rayon/viscose with one or more
companion material such as wool, synthetic fibers (e.g. polyamide fibers,
acrylic
fibers, polyester fibers, polyvinyl alcohol fibers, polyvinyl chloride fibers,
polyurethane fibers, polyurea fibers, aramid fibers), and cellulose-containing
fibers
(e.g. rayon/viscose, ramie, flax/linen, jute, cellulose acetate fibers,
lyocell). Fabric
may be conventional washable laundry, for example stained household laundry.
When the term fabric or garment is used it is intended to include the broader
term
textiles as well.
The cellulosic material is reacted with the variant polypeptides of the
invention or
the enzyme composition comprising said variant polypeptides under suitable
conditions, such as appropriate pH, and temperature, and the reaction is
allowed
to continue for a time sufficient for the enzymatic reaction to take place,
whereby
at least partially hydrolyzed cellulosic material is obtained. The enzymes are
added
in an enzymatically effective amount either simultaneously e.g. in the form of
an
enzyme mixture, or sequentially.
CA 03084907 2020-06-05
WO 2019/122520
PCT/F12018/050935
14
The variant cellulase polypeptides and the enzyme compositions containing them
may be added to detergent compositions to improve textile cleaning, for
instance
by removal of pigment dirt and byantiredeposition and antigreying. The variant
cellulases may also improve fiber and color care properties by prevention and
removal of fuzz and pills resulting in brightening or freshening of colors and
softening. The terms "depilling" (removal of pilling) and "color revival" are
typically
used to describe the cellulase effects on old, used cotton textiles. The terms
"antipilling" (prevention of pilling), "color maintenance" or "color care" are
typically
used to describe cellulase effects on new garments. The effect of fiber and
color
lo care properties can be detected by visible and measurable decrease of
lightness
(i.e. increase of darkness) or change in color of colored cotton textiles
exposed to
repeated washing cycles. Standardized tests monitors of prepilled and
unpilled,
new fabrics are commercially available.
The variant endoglucanase polypeptides and the enzyme compositions containing
them are especially useful in detergent applications as an anti-greying agent.
As
used herein, the term "antigreying performance" or "antigreying effects" mean
antiredepositioning and pigment removal properties. With increasing number of
wash cycles, pigments, particles and soluble soils, salts and other material
can
adhere on the textile fibers, most likely in areas with damaged cotton fibers.
This
can cause a greying effect and a darkening or yellowing of the cotton textile.
By
theory, cellulases hydrolyze the cellulose chains in these areas at random
positions with amorphous structure, leading to the removal of fibers with
attached
particles or higher accessibility for surfactants, and therefore showing a
whitening
or anti-greying effect. In addition, traces of cellulosic material in the
washing liquor
may be digested, preventing the adhesion of such fibers at the cotton surface
of
the garment. These effects are called antigreying or antiredeposition and can
be
evaluated using optical measurements. Suitable test methods are generally
known
in the art and are typically based on using artificial ballast soil systems
with
standard white test fabrics in repeated washing cycles in washing machines.
The
antigreying effect can be tested also by a single wash as stressed test using
redeposition liquid based on carbon black.
The variant cellulase polypeptides may also be added into detergent
compositions
to improve textile cleaning or stain removal by removal of pigmented dirt or
by
having synergistic effects on some stains with other enzymes typically used in
detergents, like proteases, amylases, lipases, pectinases, and mannanases.
Stain
removal effect can be measured as increased lightness or change of color of
stained material, e.g. in artificially soiled swatches or test cloths by
optical
measurements and is visually detectable as fading of stains.
The variant cellulase polypeptides and the enzyme compositions containing them
may also be useful in finishing processes of the textile industry, such as
biofinishing
of fabrics, garments or yarn. As used in the present context, the expression
CA 03084907 2020-06-05
WO 2019/122520 PCT/F12018/050935
"biofinishing" (also called depilling, defuzzing, dehairing or biopolishing)
refers to
the use of the variant enzymes in a controlled hydrolysis of cellulosic fibers
in order
to modify the fabric or yarn surface in a manner that permanently prevents the
tendency for pilling, improves fabric handle like softness and smoothness,
clears
5 the surface structure by reducing fuzzing. Biofinishing results in
clarification of
colors, improves the drapability of the fabric and improves moisture
absorbability,
which may further improve also the dyeability. Biofinishing may be performed
before, after or at the same time as dyeing.
Enzymatic depilling can be carried out at any stage during textile wet
processing,
10 preferably after optional desizing and/or bleaching, and similar conditions
as in
biostoning can be used. Textiles in garment form can be also treated.
The variant cellulase polypeptides and enzyme compositions containing them may
be used in biostoning of denim. As used in the present context, the expression
"biostoning" of fabric or garment means the use of enzymes in place of, or in
15 addition to, pumice stones for the treatment of fabric or garment,
especially denim
to obtain an aged or worn look. The term "aged or worn look" means that as a
result of uneven dye removal, there are contrasts between dyed areas and areas
from which dye has been removed.
The liquor ratio (the ratio of the volume of liquid per weight of fabric) in
both
biostoning and biofinishing may range from about 3:1 to 20:1, preferably 5:1
to
10:1. The treatment time can vary between 15 min to 90 min and preferably
between 30 min to 60 min. It should be emphasized that the enzyme dosage
greatly depends on the type of the fabrics, machinery, process conditions (pH,
temperature, liquor ratio, treatment time, denim load, process scale) and type
of
the enzyme preparation or composition. Typical process parameters for e.g.
industrial biofinishing are pH 4.5 - 8 at temperature of 40 - 65 C. A person
skilled
in art is capable in defining suitable dosages and conditions.
SEQUENCE LISTING
SEQ ID NO:1 The full-length amino acid sequence of MAO cellulase deriving from
the Melanocarpus albomyces ALK04237 GH45 20K-cellulase including amino
acids from Met1 to Ala235.
SEQ ID NO:2 The nucleotide sequence of the full-length MAO cellulase deriving
from Melanocarpus albomyces ALK04237 ce145 20K-cellulase.
SEQ ID NO:3 The amino acid sequence of the mature MAO cellulase deriving from
the Melanocarpus albomyces ALK04237 GH45 20K-cellulase including amino
acids from Alai to Ala214.
DEPOSITS
CA 03084907 2020-06-05
WO 2019/122520
PCT/F12018/050935
16
Melanocarpus albomyces ALK04237 was deposited at the Centraalbureau Voor
Schimmelcultures at Upsalalaan 8, 3584 CT, Utrecht, The Netherlands on 2 March
2012 and assigned accession number CBS132099.
EXAMPLES
Standard molecular biology methods were used in the isolation and enzyme
treatments of DNA (e.g. isolation of plasmid DNA, digestion of DNA to produce
DNA fragments), in E. coli transformations, sequencing etc. The basic
laboratory
methods used were either as described by the enzyme, reagent or kit
manufacturer
or as described in the standard molecular biology handbooks, e.g. Sambrook and
Russell (2001) or as described in the following examples.
Example 1. Production of Melanocarpus albomyces MAO variants in
Trichoderma reesei
Melanocarpus albomyces ce/45 20K-cellulase variants were derived from a
parental molecule, designated here as MAO (nucleic acid sequence SEQ ID NO:
2, corresponding to amino acid sequence SEQ ID NO: 1). Expression plasmids
were constructed for production of recombinant ce/45 MAO variants (Figure 1).
The
constructs contain T. reesei cei7A promoter and terminator and the amdS marker
gene as described in Paloheimo et al. 2003. The variant genes contain a signal
sequence at position 1-63 nt and intron sequences at positions 84-154 nt and
404-
473 nt. Synthetic genes, including mutations introduced in the core region of
the
parental molecule (Table 1), were exactly fused as SacII-BamH1 fragments to
the
T. reesei ce/7A promoter by ligation. For construction of the expression
plasmid for
MA79 variant a 328 bp Sall - BamHI fragment of pALK4472 was isolated and
ligated into a 8523 bp Sall - BamHI fragment of pALK4458. Expression plasm ids
are listed in Table 1.
Table 1. The synthetic genes used in construction of the expression cassettes
for
production of MAO cellulase variants in T. reesei. Amino acid positions are
numbered by correspondence with the mature amino acid sequence without signal
sequence (SEQ ID NO: 3).
Gene/ variant Expression
Mutation
designation plasmid
MA1 G22A pALK4458
MA2 P23R pALK4459
MA3 F35W pALK4460
MA4 F35D pALK4461
MA5 H39S pALK4462
MA6 H39P pALK4463
MA7 A43S pALK4464
MA8 V44R, E48D pALK4465
CA 03084907 2020-06-05
WO 2019/122520
PCT/F12018/050935
17
MA9 V44Q pALK4466
MA10 F53Y pALK4467
MA11 A62S pALK4468
MA13 E82S pALK4470
MA14 A99Q pALK4471
MA15 N122A pALK4472
MA16 N122L pALK4473
MA17 L130G pALK4474
MA18 P136S pALK4475
MA19 S151D pALK4476
MA20 Q153S pALK4477
MA21 D156S pALK4478
MA22 P161S pALK4479
MA24 V193T pALK4481
MA25 V193D pALK4482
MA26 A194Q pALK4483
MA27 F206Y pALK4484
MA28 S54M, N122A pALK4485
MA29 G22A, E82S pALK4486
MA30 F35W, H39P pALK4487
MA31 H39S, V44Q pALK4488
MA32 A43S, F53Y pALK4489
MA35 F53T, P136S pALK4492
MA37 V44R, E48D, G22A, E82S pALK4494
MA40 A19D pALK4497
MA41 K21E pALK4498
MA42 K21Q pALK4499
MA43 G2OR pALK4209
MA44 S3OL pALK4210
MA45 S3OP pALK4211
MA46 A33R pALK4212
MA47 F35M pALK4213
MA48 Q36R pALK4214
MA49 V44 L pALK4215
MA50 E481 pALK4216
MA52 A75S pALK4218
MA53 A75H pALK4219
MA54 T92I pALK4220
MA55 T94 R pALK4221
MA56 T1081 pALK4222
MA57 F131I pALK4223
MA59 Q174H pALK4225
MA60 N175D pALK4226
MA61 D177Q pALK4227
MA62 Vi 931 pALK4228
MA63 A194S pALK4229
MA64 G205R pALK4230
MA65 A207R pALK4231
MA66 A207S pALK4232
CA 03084907 2020-06-05
WO 2019/122520
PCT/F12018/050935
18
MA67 A33L pALK4233
MA68 Q36H pALK4234
MA69 V44L, E481 pALK4235
MA70 A33L, F35M, Q36R pALK4236
MA71 N175D, D177Q pALK4237
MA72 T92I, T94R pALK4238
MA73 G205R, A207S pALK4239
MA74 P1240, G1650 pALK4240
MA75 S5C, F119C, V1051 pALK4241
MA76 F350, A560 pALK4242
MA77 S680, M1030, V1051 pALK4243
MA78 V1860, A2070 pALK4244
MA79 G22A, N122A pALK4621
MA80 N122S pALK4718
MA81 N122G pALK4719
MA82 N122T pALK4720
MA83 N122V pALK4721
MA84 N122I pALK4722
MA85 G22S pALK4723
MA86 G22V pALK4724
MA87 G22A, S54A, N122A pALK4725
MA88 G22A, S54T, N122A pALK4726
MA89 G22A, S54L, N122A pALK4727
MA90 G22A, S54V, N122A pALK4728
MA91 G22A, E82R, N122A pALK4729
MA92 G22A, E82W, N122A pALK4730
MA93 G22A, T1081, N122A pALK4731
MA94 G22A, V44R, N122A pALK4732
MA95 G22A, V44W, N122A pALK4733
MA96 G22A, V44L, N122A pALK4734
MA97 G22A, A75S, N122A pALK4735
MA98 G22A, A75R, N122A pALK4736
MA99 G22A, F35M, N122A pALK4737
MA100 N2W, G22A, N122A pALK4738
MA101 N2R, G22A, N122A pALK4739
MA102 G22A, N122A, Q174R pALK4740
MA103 G22A, N122A, G205T pALK4741
MA104 G22A, F35W, A75H, T1081, N122A, A207S pALK4742
MA107 G22A, N122A, Q174R, D177E pALK4745
MA109 G22A, N122A, F206H pALK4748
MA110 G22A, N122A, del(A207-A214) pALK4749
MA111 G22A, N122A, del(V208-A214) pALK4750
MA112 G22A, N122A, del(F209-A214) pALK4751
MA113 G22A, N122A, del(K210-A214) pALK4752
MA114 G22A, N122A, del(A211-A214) pALK4753
MA115 G22A, N122A, del(P212-A214) pALK4754
MA116 G22A, N122A, del(S213-A214) pALK4755
CA 03084907 2020-06-05
WO 2019/122520 PCT/F12018/050935
19
The linear expression cassette was isolated from the vector backbone by EcoRI
digestion or the circular plasmid was used directly in T. reesei protoplast
transformation. The transformants were selected with acetamide as the sole
nitrogen source. The host strain lacks the four major endogenous cellulases:
CBHI/Cel7A, CBHII/Cel6A, EGI/Cel7B and EGII/Cel5A. The transformations were
performed according to Penttila et al, 1987, with the modifications described
in
Karhunen et al., 1993. The transformants were purified on selection plates
through
single conidia prior to sporulating them on potato dextrose agar.
The cellulase production of the transformants was analyzed from the culture
supernatants of shake flask cultivations (50 ml). The transformants were grown
for
7 days at 3000, 250 rpm in a complex cellulase-inducing medium (Joutsjoki et
al.,
1993) buffered with 5% KH2PO4 at pH 5.5. The enzyme activity of the
recombinant
protein was measured from the culture supernatant as the release of reducing
sugars from carboxymethylcellulose (3% CMC) at 50 C in 50 mM HEPES buffer
pH 7.0 essentially as described by Bailey and Nevalainen, 1981; Haakana, et
al,
2004 (NCU activity). Production of the recombinant protein was also detected
from
the culture supernatant by sodium dodecyl sulfate polyacrylamide gel
electrophoresis (SDS-PAGE).
Chosen transformants and the reference strain producing the parental MAO
cellulase were cultivated in shake flasks or bioreactors in complex cellulase-
inducing medium to obtain material for the application tests (Examples 2 to
6).
Example 2. Screening of MAO variants MAl-MA79 based on their stability in
commercial liquid detergent at 50 C
MAO variants produced in Trichoderma as described in Example 1 were screened
based on detergent stability at 50 C by a rapid test in the presence of
protease.
Parental cellulase MAO was used as reference. A 0.8% w/wamount of protease,
Savinase 16L (Novozymes, Denmark), was added to a commercial liquid detergent
containing no enzymes. The composition of detergent is described in Table 2.
Culture supernatants of cellulases at 5% w/w were added to the detergent, and
samples in plastic tubes with caps were incubated in a water bath at 50 C for
30
minutes. The enzyme activity (NCU) was measured after incubation by the
activity
assay described in Example 1, except using longer reaction time of 30 minutes.
Results were calculated as residual activity CYO, which was obtained by
dividing
the activity of a sample after incubation at 50 C by the initial activity of
the sample.
CA 03084907 2020-06-05
WO 2019/122520
PCT/F12018/050935
Table 2. Composition of commercial liquid detergent
Ingredient %
Anionic surfactants 15 ¨ 30
Nonionic surfactants, soap 5¨ 15
Phosphonate, Soap <5
Boric acid
Other ingredients: e.g. optical brighteners, perfumes,
pH 8.2-8.6
Several variants showed improved stability of enzyme activity in commercial
liquid
detergent at 50 C compared to the parental MAO cellulase. Results
5 are shown in Table 3. Best stability was obtained with variants MA15, MA28
and
especially MA79. Stability of variants MA15 and MA79 compared to MAO is shown
in Figure 2 as residual activity. Both variants showed remarkably better
stability
than the parental cellulase.
io Table 3. Stability of MAO variants in detergent in the presence of protease
(50 C,
min). Symbol "+" indicates improved stability (measured as residual activity)
compared to parental MAO cellulase, "+++++" indicates the best stability
achieved
in the test set.
Variant Stability
MA1 ++
MA3 ++
MA9 ++
MA14 +
MA15 ++++
MA16 +++
MA26 +
MA28 ++++
MA29 +++
MA30 +
MA31 +
MA37 ++
MA46 +
MA53 +
CA 03084907 2020-06-05
WO 2019/122520
PCT/F12018/050935
21
MA54 +
MA56 +
MA61 ++
MA66 +++
MA71 ++
MA79 +++++
Example 3. Testing the antigreying performance of MAO variants in Launder-
Ometer
The most stable variant MA79 from Example 2 was tested for antigreying
performance by a single wash method (40 C, 60 min, 16 dH) using carbon black
(approx. 0.15 g/l) in a wash solution in addition to detergent (4.4 g/l).
Parental
cellulase MAO was used as reference.
Cotton interlock double jersey with optical brighteners (CN-42) supplied from
CFT
(Center for Testmaterials NV, the Netherlands) was used as test fabric. The
fabric
io was first prewashed in a washing machine (15 min 50 C and 60min at 60 C)
and
tumble dried, then cutted to swatches of approx. 14-14.5 cm (total weight of 4
swatches 25 g).
As a source of carbon black RD-liq 01 from CFT containing 7 g of carbon black
liquid (about 33 (:)/0 carbon black) in plastic bottles, that are normally
intended for
full scale washes in washing machine (one bottle per one single wash with test
fabrics), were used. In this Example the method was adapted to small scale
using
approximately similar ratio of carbon black and water that would be in full
scale.
First a stock solution carbon black was prepared by placing an opened bottle
of
RD- liq 01 (i.e. about 2.3 g carbon black) in a decanter flask containing 1
liter of
deionized water. The solution was stirred with a magnetic stirrer for
overnight until
the contents of the bottle were totally released. After that 65 g of stock
solution
mixed with 935 ml of synthetic tap water with hardness of 17.1 dH ending up to
diluted carbon black solution having hardness of 16 dH and carbon black
content
approximately 0.15 g/I (or 0.45 g RD-liq 01).
For synthetic tap water with hardness of 17.1 dH the following stock solutions
were
prepared in deionized water (Milli-Q or equivalent):
Stock solution with 1000 d Calcium-hardness: CaCl2 x 2 H20
(1.02382.1000, Merck KGaA, Germany) 26.22 g/I
Stock solution with 200 d Magnesium-hardness: MgSO4 x 7 H20
(1.05886.1000, Merck KGaA, Germany) 8.79 g/I H20
CA 03084907 2020-06-05
WO 2019/122520 PCT/F12018/050935
22
NaHCO3 stock solution: NaHCO3 (1.06329.1000 Merck KGaA, Germany) 29.6 g/I.
made NaHCO3 solution were added in volumetric flask in the given order, made
up to 1 liter with deionized water and mixed. The hardness of water was
determined by complexometric titration and found correct.
Antigreying tests were performed in Atlas LP-2 Launder-Ometer as follows.
Launder-Ometer was first preheated to 40 C. 60 g of steel balls (diameter 0.6
cm),
1.1 g of commercial liquid detergent described in Example 2, 250 ml of diluted
carbon black liquor and diluted enzyme (<1.0 ml) were added into 1.2 liter
containers. After that, 4 swatches of prewashed test fabric CN-42 were added
and
lo the Launder-Ometer was run at 40 C for 60 min with a rotation speed of 42
rpm.
Enzymes were dosed 0, 0.25, 0.05, 0.1 and 0.2 activity units (NCU) per liter.
Activity was measured as described in Example 1.
After the cellulase treatment in Launder-Ometer, the swatches were first
quickly
rinsed separately under running tap water (ca. 20 C) to remove the steel
balls,
then rinsed separately under running water in specific cups containing holes
for 3
times and finally dipped in a bucket containing water. After that the swatches
were
extracted in a washing machine and let to dry on a grid at room temperature.
Enzyme treated fabrics and controls without enzyme were rinsed and extracted
separately to avoid contamination.
Antigreying performance of cellulase was evaluated by measuring reflectance of
test fabrics by Konica Minolta CM3610A spectrophotometer as Y-value
(illuminant
D65/10 , 420 nm cut). Cellulase performance was calculated as AY (delta Y),
which means value Y of enzyme treated fabric minus value Y of fabric treated
with
carbon black and detergent containing washing liquor without enzyme (enzyme
blank, control). Values were the average of 4 swatches. The higher the Y or AY
value, the better the antigreying effect and whiteness of the fabric.
Results in Figure 3 show that the most stable variant MA79 had excellent
antigreying properties similar to parental MAO cellulase in liquid detergent.
Also several other variants from Example 2, like MA1, MA3, MA15, MA29, had as
good antigreying performance as MAO (data not shown).
Example 4. Long term stability of variant MA79 in liquid detergent measured
as enzyme activity
A cultivation sample of variant MA79 was tested for long term stability in a
liquid
detergent at 37 C, 30 C and room temperature (approx. 20 ¨ 22 C), using
parental
cellulase MAO as a reference. Tests were carried out with commercial liquid
detergent described in Example 2 in the presence of added protease (0.8% w/w
Savinase 16L). Cellulase preparations at 1(Yow/w were added to the detergent
and
CA 03084907 2020-06-05
WO 2019/122520 PCT/F12018/050935
23
samples in plastic tubes with caps were incubated at each temperature for
several
weeks or months. The enzyme activity was measured and results were calculated
as residual activity as described in Example 2.
Results of tests obtained in commercial liquid detergent with protease are
shown
in Figures 4A-C. The long-term stability of variant MA79 was considerably
improved compared to MAO at all temperatures, especially at 37 C.
Stability tests with variant MA79 and MAO were carried out also in a
commercial
l&I (Industrial & Institutional) detergent formulation containing protease and
other
enzymes except cellulase. A 0.5% w/w amount of cellulase preparation was added
lo to the detergent and samples were incubated at 37 C for 6 weeks. Variant
MA79
showed remarkably better stability than MAO also in this l&I detergent
(Figure. 5).
Example 5. Long term stability of variant MA79 in liquid detergent measured
as antigreying performance
The stability of variant MA79 compared to the parental MAO cellulase was
evaluated also as antigreying performance. Antigreying tests similar to that
described in Example 3 were carried out with detergent samples stored for 88
and
193 days at 30 C. Cellulases were initially added to the detergent in such
amount
that dosage would be 0.2 activity units (NCU) per liter of wash solution.
Performance of stored samples was compared to washes, in which the enzymes
had been added fresh into the washing liquor containing detergent and carbon
black and results were calculated as relative performance indicating the
residual
performance level.
Results in Figure 6 show that variant MA79 had still excellent antigreying
performance after storage of over 6 months at elevated temperature like 30 C.
The
performance of MAO was considerably reduced compared to MA79 already after
12 weeks.
Example 6. Stability of variant MA79 in liquid detergents compared to
commercial cellulases
A production like sample was prepared from pilot scale fermentation sample of
MA79 and retested for stability in liquid detergents at various temperatures
for 6
weeks, using preparation of parental cellulase MAO and/or commercial cellulase
Celluclean 5000L (Novozymes, Denmark) for comparison.
Tests were carried out in commercial liquid detergent (described in
Table2/Example 2) in the presence of added protease like described in Example
4. Also a concentrated liquid detergent containing protease and other enzymes
except cellulase was tested. Cellulases at 0.5% w/w were added to the
detergents.
CA 03084907 2020-06-05
WO 2019/122520
PCT/F12018/050935
24
Based on results shown in Figure 7 variant MA79 had considerably better
stability
compared to Celluclean 5000L in commercial liquid detergent at 37 C. Results
obtained with another detergent, liquid detergent concentrate show that
variant
MA79 had significantly better stability than both MAO and Celluclean 5000L at
37 C
(Figure 8A). In this detergent the stability of MA79 was better than
Celluclean
5000L also at 30 C (Figure 8B) and at room temperature approx. 20-22 C (data
not shown).
Example 7. Screening of MAO variants MA80-MA116 based on their stability
in commercial liquid detergent at 50 C
Stability of MAO variants MA80 - MA116 produced in Trichoderma as described in
Example 1 was tested in a commercial liquid detergent in the presence of
protease
at 50 C as described in Example 2 Parental cellulase MAO and stable variant
MA79
were used as references.
Several variants showed improved stability in commercial liquid detergent
compared to the parental MAO cellulase, when incubation time of 30 min at 50 C
was used. For differentiating variants having higher stability than the most
stable
variant MA79 from Example 2, the incubation time had to be increased to 24
hours.
Thermal incubator was used instead of water bath. Residual activities of the
best
variants after 24 h incubation at 50 C are shown in Figure 9. Especially
variants
MA88 and MA93 had improved stability compared to MA79. Parental molecule
MAO had practically no activity left after 24 h. Variants MA88, MA93 and MA103
showed also excellent antigreying properties, at least as good as parental
molecule MO, when tested like described in Example 3 (data not shown).
REFERENCES
Bailey M and Nevalainen H. 1981. Induction, isolation and testing of stable
Trichoderma
reesei mutants with improved production of solubilizing cellulase. Enzyme
Microb.
Technol. 3:153-157.
Haakana H, Miettinen-Oinonen A, Joutsjoki V, Mantyla A, Suominen P and
Vehmaanpera
J. 2004. Cloning of cellulase genes from Melanocarpus albomyces and their
efficient
expression in Trichoderma reesei. Enzyme Microb. Technol. 34:159-167.
Henrissat B. 1991. A classification of glycosyl hydrolases based on amino acid
sequence
similarities. Biochem. J. 280:309-316.
Henrissat B and Bairoch A. 1993. New families in the classification of
glycosyl hydrolases
based on amino acid sequence similarities. Biochem. J. 293:781-788.
Henrissat B and Bairoch A. 1996. Updating the sequence-based classification of
glycosyl
hydrolases. Biochem. J. 316:695-696.
CA 03084907 2020-06-05
WO 2019/122520
PCT/F12018/050935
Joutsjoki VV, TK Torkkeli and KMH Nevalainen. 1993. Transformation of
Trichoderma
reesei with the Hormoconis resinae glucoamylase P (gamP) gene: production of a
heterologous glucoamylase by Trichoderma reesei. Curr. Genet. 24:223-228.
Karhunen T, A Mantyla, KMH Nevalainen and PL Suominen. 1993. High frequency
one-
5 step gene replacement in Trichoderma reesei. I. Endoglucanase I
overproduction. Mol.
Gen. Genet. 241:515-522.
Paloheimo M, A Mantyla, J Kallio, and P Suominen. 2003. High-yield production
of a
bacterial xylanase in the filamentous fungus Trichoderma reesei requires a
carrier
polypeptide with an intact domain structure. Appl. Env. Microbiol. 69:7073-
7082.
10 Penttila M, H Nevalainen, M Rano, E Salminen and J Knowles. 1987. A
versatile
transformation system for the cellulolytic filamentous fungus Trichoderma
reesei. Gene
61:155-164.
Sambrook J and DW Russell. 2001. Molecular cloning, a laboratory manual. Cold
Spring
Harbor Laboratory, New York, US.