Note: Descriptions are shown in the official language in which they were submitted.
CA 03085492 2020-06-10
WO 2019/126120 PCT/US2018/066157
TITLE OF THE INVENTION
Methods and Apparatus for the Delivery of Hepatitis B Virus (HBV) Vaccines
CROSS REFERENCE TO RELATED APPLICATIONS
This application claims priority to International Patent Application No.
PCT/US2017/067269, filed December 19, 2017, and U.S. Provisional Patent
Application No.
62/607,430, filed December 19, 2017, the disclosures of which are incorporated
herein by
reference in their entireties
REFERENCE TO SEQUENCE LISTING SUBMITTED ELECTRONICALLY
This application contains a sequence listing, which is submitted
electronically via EFS-
Web as an ASCII formatted sequence listing with a file name "688097-405
Sequence Listing,"
creation date of December 10, 2018, and having a size of 46.6 KB. The sequence
listing
submitted via EFS-Web is part of the specification and is herein incorporated
by reference in its
entirety.
FIELD
The disclosure is directed to the administration of prophylactic and/or
therapeutic
Hepatitis B virus (HBV) vaccines to subjects in need thereof, and, more
particularly, to the
reproducible, consistent, and efficacious delivery of prophylactic and/or
therapeutic HBV
vaccines, such as nucleic acids encoding HBV antigens, to defined regions in
selected tissue site
of interest, facilitated by the local application of electrical fields, in a
safe, effective, and
consistent fashion across heterogeneous recipient populations with minimal
user training.
BACKGROUND
Hepatitis B virus (HBV) is a small 3.2-kb hepatotropic DNA virus that encodes
four open
reading frames and seven proteins. About two billion people are infected with
HBV, and
approximately 240 million people have chronic hepatitis B infection (chronic
HBV),
characterized by persistent virus and subvirus particles in the blood for more
than 6 months
(Cohen et al. I Viral Hepat. (2011) 18(6), 377-83). Persistent HBV infection
leads to T-cell
exhaustion in circulating and intrahepatic HBV-specific CD4+ and CD8+ T-cells
through
chronic stimulation of HBV-specific T-cell receptors with viral peptides and
circulating antigens.
1
CA 03085492 2020-06-10
WO 2019/126120 PCT/US2018/066157
As a result, T-cell polyfunctionality is decreased (i.e., decreased levels of
IL-2, tumor necrosis
factor (TNF)-a, IFN-y, lack of proliferation, and degranulation). Therapeutic
vaccination has the
potential to eliminate HBV from chronically infected subjects (Michel et al. I
Hepatol. (2011)
54(6), 1286-1296). Immunogenic compositions containing one or more nucleic
acid molecules
encoding one or more HBV antigens can be used to provide therapeutic immunity
to a subject in
need thereof, such as a human having chronic HBV infection.
While naked DNA can be delivered to cells in vivo and result in gene
expression, the
efficiency of gene transfer is relatively low and variable. The local
application of electrical
signals has been shown to enhance the distribution and uptake of
macromolecules in living
tissue. Application of such electrical signals in tissue in association with
the administration of a
prophylactic or therapeutic HBV vaccine can have desirable effects on the
tissue and/or the agent
to be delivered. Specifically, techniques such as electroporation and
iontophoresis have been
utilized to significantly improve the distribution and/or uptake of nucleic
acids, including both
DNA and RNA sequences. Potential clinical applications of such techniques
include the delivery
of nucleic acid sequences for prophylactic and therapeutic immunization, and
the delivery of
nucleic acid sequences encoding therapeutic proteins or peptides.
The application procedures comprise the administration of an agent of interest
to a target
tissue site in conjunction with the application of electrical fields of
sufficient magnitude and
duration to induce the desired effects on the delivery, distribution, and/or
potency of the agent.
The electrical fields are propagated via two or more electrodes in
electrically conductive
communication with the tissue. Electrode configurations suitable for use with
these techniques
include tissue penetrating electrodes, surface contact electrodes, and air gap
electrodes. Specific
electrode configurations include, but are not limited to, elongate needle or
rod electrodes, point
electrodes, meander electrodes (i.e., shaped wire), planar electrodes, and
combinations thereof
The specific type and arrangement of electrodes is selected based on the
target tissue type and
the objectives of the procedure. The desired outcome is best achieved when the
electrical fields
are propagated within the target tissue in the presence of the agent of
interest.
A broad range of methods and devices have been described for the application
of
electrical fields in tissue in the presence of an agent of interest for the
purpose of enhancing
agent delivery in skin and muscle tissue. The devices include the use of both
surface and tissue
penetrating electrode systems as well as combinations thereof. In spite of the
promise associated
2
CA 03085492 2020-06-10
WO 2019/126120 PCT/US2018/066157
with electrically mediated agent delivery and the potential clinical
applications of these
techniques, there is still a need for reliable and consistent deivery of
nucleic acids of interest to
subjects. Significant sources of this variability are due to differences in
the technique and skill
level of the operator. Other sources of variability that are not addressed by
current systems
include differences in the physiologic characteristics between subjects that
can affect the
application of the procedure. Other considerations for the development of
suitable devices
include their ease of use and the implementation of designs that reduce the
frequency and
significance of potential user errors.
Given that safe, reliable, accurate, and consistent application of clinical
therapies is
highly desirable, the development of improved application systems is well
warranted. Such
development should include a means for minimizing operator-associated
variability while
providing a means to accommodate the differences in subject characteristics
likely to be
encountered during widespread clinical application of electrically mediated
agent delivery. In
other words, specific areas for refinement include the ability to maintain
consistent performance
across heterogeneous recipient populations and a reduction in the level of
training and skill
required for effective use by the user. In addition, the device, system or
method should be
designed to facilitate avoidance of user or device errors and minimize their
impact when they
occur.
This Background is provided to introduce a brief context for the Summary and
Detailed
Description that follow. This Background is not intended to be an aid in
determining the scope of
the claimed subject matter nor be viewed as limiting the claimed subject
matter to
implementations that solve any or all of the disadvantages or problems
presented above.
SUMMARY OF THE DISCLOSURE
The disclosure provides apparatus, kits and methods for the reproducible,
consistent, and
efficacious delivery of an HBV vaccine comprising a nucleic acid molecule
encoding an HBV
antigen, to a subject in need thereof, utilizing Electrically Mediated
Therapeutic Agent Delivery
(EMTAD). In embodiments described herein, the therapeutic agent is an HBV
vaccine.
In an aspect of the disclosure, provided is an apparatus for the delivery of
an HBV
vaccine to a predetermined site within a subject comprising an assembly for
controlled
3
CA 03085492 2020-06-10
WO 2019/126120 PCT/US2018/066157
administration of the HBV vaccine to the subject comprising a reservoir
containing the HBV
vaccine, at least one orifice through which the agent is administered, and a
controlled source of
energy sufficient to transfer a predetermined amount of the HBV vaccine at a
predetermined rate
from the reservoir through the orifice to the predetermined site within the
subject. In addition,
the apparatus can comprise a plurality of penetrating electrodes arranged with
a predetermined
spatial relationship relative to the orifice, and an electrical signal
generator operatively connected
to the electrodes.
In another aspect of the disclosure, provided is a kit for the delivery of an
HBV vaccine
to a predetermined site within a subject, comprising the HBV vacine and an
apparatus
comprising an assembly for controlled administration of the HBV vaccine to the
subject, wherein
the apparatus comprises a reservoir for the HBV vaccine, at least one orifice
through which the
HBV vaccine is administered, and a controlled source of energy sufficient to
transfer a
predetermined amount of the HBV vaccine at a predetermined rate from the
reservoir through the
orifice to the predetermined site within the subject. In addition, the
apparatus can comprise a
plurality of penetrating electrodes arranged with a predetermined spatial
relationship relative to
the orifice, and electrical signal generator operatively connected to the
electrodes.
An HBV vaccine included in an apparatus or a kit of the present application
can
comprise:
a first nucleic acid molecule comprising a first polynucleotide encoding an
HBV
polymerase antigen having an amino acid sequence that is at least 98%
identical to SEQ ID NO:
4, wherein the HBV polymerase antigen does not have reverse transcriptase
activity and RNase
H activity;
a second nucleic acid molecule comprising a second polynucleotide encoding a
truncated HBV core antigen consisting of the amino acid sequence of SEQ ID NO:
2 or SEQ ID
NO: 14; and
a pharmaceutically acceptable carrier,
wherein the first nucleic acid molecule and the second nucleic acid molecule
are
present in the same nucleic acid molecule or in two different nucleic acid
molecules.
Other aspects of the disclosure include methods comprising HBV vaccine
administration
in controlled spatial and temporal relation with Electric Signal
Administration (ESA).
4
CA 03085492 2020-06-10
WO 2019/126120 PCT/US2018/066157
Benefits and advantages to certain implementations according to present
principles are
manifold. Some implementations allow the selection of depth of needle and
electrode insertion,
allowing insertion into various types of desired tissue, (e.g., dermis,
muscle, etc.) across
heterogeneous populations of varying body mass and body composition. These
implementations
also facilitate adaptation of the methods for use in specific target
populations, for instance, but
not restricted to, human patients having chronic HBV infection and/or an HBV-
induced disease.
Provided herein are systems and methods comprising design features that render
the systems and
methods resistant to accidental discharge or potential misuse, e.g., due to
dropping, jarring,
and/or falling. In some embodiments are provided devices configured for
multiple injection
depths. Systems and methods according to present principles allow numerous
safety interlocks to
reduce the frequency and/or impact of user errors. These include features to
facilitate proper
preparation and configuration of the dose to be administered, ensuring that
the device is applied
with requisite force to the tissue of the recipient, ensuring that a safety
cap has been removed,
and so on. Systems, apparatus, kits and methods according to present
principles can allow for a
highly consistent therapy to be delivered irrespective of administrator or the
type of recipient.
Systems, apparatus, kits and methods described herein can allow for, e.g., a
consistent force
profile to be obtained prior to and during delivery of the therapy, so that
recipients with varying
skin and muscular characteristics can be dosed consistently.
This Summary is provided to introduce a selection of concepts in a simplified
form. The
concepts are further described in the Detailed Description section. Elements
or steps other than
those described in this Summary are possible, and no element or step is
necessarily required.
This Summary is not intended to identify key features or essential features of
the claimed subject
matter, nor is it intended for use as an aid in determining the scope of the
claimed subject matter.
The claimed subject matter is not limited to implementations that solve any or
all disadvantages
noted in any part of this disclosure.
Other aspects, features and advantages of the invention will be apparent from
the
following disclosure, including the detailed description of the invention and
its preferred
embodiments and the appended claims.
BRIEF DESCRIPTION OF THE DRAWINGS
CA 03085492 2020-06-10
WO 2019/126120 PCT/US2018/066157
The features of the disclosure are set forth with particularity in the
appended claims. A
better understanding of the features and advantages of the disclosure will be
obtained by
reference to the following detailed description that sets forth illustrative
embodiments, in which
the principles of the disclosure are utilized, and the accompanying drawings
of which:
FIG. 1 illustrates potential sources of spatial variability associated with
conventional
needle syringe injection.
FIG. 2 is an overview of a system according to present principles, including a
cartridge
assembly 100, an applicator 400, and a controller system 700.
FIGS. 3A-3B show views of aspects of a device described herein. FIG. 3A shows
a
lateral view of a cartridge assembly 100 according to present principles. FIG.
3B shows a lateral
view of a reservoir for HBV vaccine 101 embodied as a syringe, according to
present principles.
FIG. 4 shows various exemplary components of a cartridge assembly 100
according to
present principles.
FIGS. 5A-5E show views of aspects of an inner cartridge and cartridge breech
in a
device described herein. FIG. 5A illustrates a top view of an inner cartridge
103 according to
present principles. FIG. 5B illustrates a bottom view of an inner cartridge
103 and cartridge
breech according to present principles. FIG. 5C illustrates a detail side
perspective view of an
inner cartridge 103 according to present principles. FIG. 5D shows a lateral
view of a cartridge
breech 112 according to present principles. FIG. 5E illustrates reservoir
interlock 120 with
improved locking features to prevent the cartridge breech 112 from
inadvertently moving
forward.
FIG. 6 illustrates details of a cartridge assembly showing a rack 154, a
initiating flag 172,
and a continuing flag 174 according to present principles.
FIGS. 7A-7B show views of aspects of electrodes and/or electrode contacts in a
device
described herein. FIG. 7A shows details of electrode contacts 130 and various
elecrode contact
portions according to present principles. FIG. 7B shows details of electrodes
122 and various
electrode contacts portions according to present principles.
FIGS. 8A-8B show views of aspects of a force contact interlock system in a
device
described herein. FIG. 8A shows a top view of a force contact interlock system
according to the
present principle. FIG. 8B illustrates details of a force contact interlock
system according to
present principles.
6
CA 03085492 2020-06-10
WO 2019/126120 PCT/US2018/066157
FIGS. 9A-9D show views of aspects of a device described herein. FIG. 9A shows
a
needle 105 and distal inner cartridge electrodes 137 for tissue insertion
according to present
principles. FIG. 9B illustrates details of a stick shield 134 according to
present principles. FIG.
9C illustrates an alignment guide 108 and splay shield 168 according to
present principles. FIG.
9D illustrates stick shield supports intergral to outer cartridge cap 106.
FIGS. 10A-10D show views of aspects of exterior cartridge cap in a device
described
herein. FIG. 10A shows an exterior cartridge cap 110 according to present
principles. FIG. 10B
shows a side view of an exterior cartridge cap 110 according to present
principles. FIG. 10C
shows an exterior cartridge cap 110 in use in an alignment guide 108 and splay
shield according
to present principles. FIG. 10D shows an exterior cartridge cap 110 with
extension members
designed to hold the inner cartridge 103 in place during handlling and
loading.
FIGS. 11A-11C show details of stick shields in aspects of a device described
herein.
FIG. 11A shows a stick shield retaining hook 182 of a stick shield 134
according to present
principles. FIG. 11B shows details of a stick shield 134 according to present
principles. FIG.
11C shows stick shield supports 132 keeping a stick shield 134 in place
according to present
principles.
FIG. 12 shows details of an electrode support 124 according to present
principles.
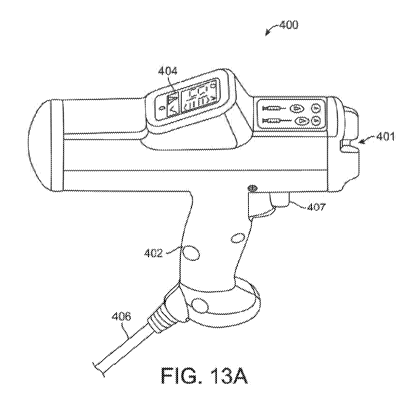
FIGS. 13A-13B show views of an applicator in a device described herein. FIG.
13A
shows a side view of an applicator 400 according to present principles. FIG.
13B shows top
views of an applicator 400 according to present principles.
FIG. 14 is an exploded view of an applicator 400 according to present
principles.
FIG. 15 shows details of a side housing and electroporation electrode
connection 496 of
an applicator 400 according to present principles.
FIG. 16 is an exploded view of an applicator according to present principles,
showing a
cartridge loading subassembly 456.
FIGS. 17A-17B show views of an applicator in a device described herein. FIG.
17A is
an exploded view of an applicator according to present principles, showing a
loading drive
subassembly 454. FIG. 17B shows a rack 154 in a loading drive subassembly 454
according to
present principles.
FIGS. 18A-18C show views of aspects of an applicator in a device described
herein.
FIG. 18A shows details of a cartridge loading subassembly 456 according to
present principles,
7
CA 03085492 2020-06-10
WO 2019/126120 PCT/US2018/066157
showing where the insertion/injection drive assembly of the applicator mates
with the cartridge
assembly. FIG. 18B shows a cross-sectional view of a cartridge assembly
according to present
principles, showing where the insertion/injection drive assembly of the
applicator mates with the
cartridge assembly. FIG. 18C shows details of cartridge loading, electrode
insertion, and
injection subassemblies 452 of an applicator 400 according to present
principles.
FIG. 19 shows various components of a controller system according to present
principles.
FIGS. 20A-20D show views of a device described herein.FIG. 20A shows various
components of a controller system according to present principles. FIG. 20B
shows details of an
applicator connector port 708 and a tray 710 of a controller system according
to present
principles. FIG. 20C shows details of a stimulator display screen of a
controller system
according to present principles. FIG. 20D shows details of a rear view of a
controller system
according to the present principles.
FIG. 21 is a flowchart showing a method of operation according to present
principles.
FIG. 22 illustrates TriGrid electrode array (cross-section) for intramuscular
(IM)
delivery, which is comprised of four electrodes arranged in two equilateral
triangles to form a
diamond shape surrounding a central injection needle.
FIGS. 23A-23B depict the TDS-IM v1.0 TriGrid devices adapted for use in the
mouse
model (FIG. 23A) and the non-human primate (NHP) model (FIG. 23B).
FIG. 24 depicts the TDS-IM v2.0 TriGrid device adapted for use in the non-
human
primate (NHP) model.
FIGS. 25A-251I show the design and optimization of expression cassettes and
DNA
plasmids encoding HBV pol and core antigens as described in Example 1; FIG.
25A is a
schematic representation of an expression strategy in which coding sequences
of the HBV core
and pol antigens are fused in frame; FIG. 25B is a schematic representation of
an expression
strategy in which coding sequences of both the core and pol antigens are
expressed from a single
plasmid by means of the ribosomal FA2 slippage site; FIG. 25C is a schematic
representation of
an expression strategy in which the core and pol antigens are expressed from
two separate
plasmids; FIG. 25D is a Western blot of core antigen expression in HEK293T
cells transfected
with a plasmid expressing core with and without the post-transcriptional
regulatory element
WPRE; expression was tested in cell lysate (left) and supernatant (sup; right)
using an a-core
8
CA 03085492 2020-06-10
WO 2019/126120 PCT/US2018/066157
antibody; FIG. 25E is a Western blot analysis showing a comparison of core
expression in
HEK293T cells transfected with a core expressing plasmid including the
intron/exon sequence
derived from human apolipoprotein Al precursor ("AI intron"), untranslated R-
U5 domain of the
human T-cell leukemia virus type 1 (HTLV-1) long terminal repeat (LTR) ("HTLV
R"), or
triple enhancer composite sequence of the HTLV-1 LTR, synthetic rabbit P-
globin intron, and a
splicing enhancer ("triple"); the unlabeled lane is purified core protein as a
size marker;
expression was tested in both lysate (left) and supernatant (sup; right); core
antigen expression
was highest with the triple enhancer composite sequence; FIG. 25F is a Western
blot analysis of
core antigen secretion using different signal peptides fused to the N-terminus
of the HBV core
antigen; the most efficient protein secretion was observed with the Cystatin S
signal peptide;
FIG. 25G is a schematic representation of optimized HBV core/pol antigen
expression cassettes
for each of the three expression strategies illustrated in FIGS. 25A-25C;
CMVpr: human CMV-
IE promoter; TRE: triple enhancer sequence; SP: cystatin S signal peptide;
FA2: FMDV
ribosomal slippage site; pA: BGH polyadenylation signal; FIG. 2511 is a
Western blot analysis
of HBV core and pol antigen expression of pDK vectors containing each of the
expression
cassettes shown in FIG. 25G; lanes 1 and 2: pDK-core; lanes 3 and 4: pDK-pol;
lanes 5 and 6:
pDK-coreFA2Pol; lanes 7 and 8: pDK-core-pol fusion: the most consistent
expression profile for
cellular and secreted core and pol antigens was observed when the antigens
were encoded by
separate vectors;
FIGS. 26A-26B show schematic representations of DNA plasmids according to
embodiments of the application; FIG. 26A shows a DNA plasmid encoding an HBV
polymerase (pol) antigen according to an embodiment of the application; FIG.
26B shows a
DNA plasmid encoding an HBV core antigen according to an embodiment of the
application;
the HBV core and pol antigens are expressed under control of a CMV promoter
with an N-
terminal cystatin S signal peptide that is cleaved from the expressed antigen
upon secretion from
the cell; transcriptional regulatory elements of the plasmid include an
enhancer sequence located
between the CMV promoter and the polynucleotide sequence encoding the HBV
antigen and a
bGH polyadenylation sequence located downstream of the polynucleotide sequence
encoding the
HBV antigen; a second expression cassette is included in the plasmid in
reverse orientation
including a kanamycin resistance gene under control of an Amp' (bla) promoter;
an origin of
replication (pUC) is also included in reverse orientation;
9
CA 03085492 2020-06-10
WO 2019/126120 PCT/US2018/066157
Like reference numerals refer to like elements throughout. Elements are not to
scale
unless otherwise noted.
DETAILED DESCRIPTION
The following description and examples illustrate embodiments of the invention
in detail.
It is to be understood that this invention is not limited to the particular
embodiments described
herein and as such can vary. Those of skill in the art will recognize that
there are numerous
variations and modifications of this invention, which are encompassed within
its scope.
All terms are intended to be understood as they would be understood by a
person skilled
in the art. Unless defined otherwise, all technical and scientific terms used
herein have the same
meaning as commonly understood by one of ordinary skill in the art to which
the disclosure
pertains.
The section headings used herein are for organizational purposes only and are
not to be
construed as limiting the subject matter described.
Although various features of the invention can be described in the context of
a single
embodiment, the features may also be provided separately or in any suitable
combination.
Conversely, although the invention can be described herein in the context of
separate
embodiments for clarity, the invention may also be implemented in a single
embodiment.
In an attempt to help the reader of the application, the description has been
separated in
various paragraphs or sections, or is directed to various embodiments of the
application. These
separations should not be considered as disconnecting the substance of a
paragraph or section or
embodiments from the substance of another paragraph or section or embodiments.
To the
contrary, one skilled in the art will understand that the description has
broad application and
encompasses all the combinations of the various sections, paragraphs and
sentences that can be
contemplated. The discussion of any embodiment is meant only to be exemplary
and is not
intended to suggest that the scope of the disclosure, including the claims, is
limited to these
examples. For example, while embodiments of HBV vectors that can be used in
the application
(e.g., plasmid DNA or viral vectors) described herein may contain particular
components,
including, but not limited to, certain promoter sequences, enhancer or
regulatory sequences,
signal peptides, coding sequence of an HBV antigen, polyadenylation signal
sequences, etc.
arranged in a particular order, those having ordinary skill in the art will
appreciate that the
CA 03085492 2020-06-10
WO 2019/126120 PCT/US2018/066157
concepts disclosed herein may equally apply to other components arranged in
other orders that
can be used in HBV vectors useful for the application. The application
contemplates use of any
of the applicable components in any combination having any sequence that can
be used in HBV
vectors useful for the application, whether or not a particular combination is
expressly described.
The following definitions supplement those in the art and are directed to the
current
application and are not to be imputed to any related or unrelated case, e.g.,
to any commonly
owned patent or application. Although any methods and materials similar or
equivalent to those
described herein can be used in the practice for testing of the disclosure,
the preferred materials
and methods are described herein. Accordingly, the terminology used herein is
for the purpose
of describing particular embodiments only, and is not intended to be limiting.
In this application, the use of the singular includes the plural unless
specifically stated
otherwise. It must be noted that, as used in the specification, the singular
forms "a," "an" and
"the" include plural references unless the context clearly dictates otherwise.
In this application,
the use of "or" means "and/or" unless stated otherwise. Furthermore, use of
the term "including"
as well as other forms, such as "include", "includes," and "included," is not
limiting.
Reference in the specification to "some embodiments," "an embodiment," "an
embodiment" or "other embodiments" means that a particular feature, structure,
or characteristic
described in connection with the embodiments is included in at least some
embodiments,
although not necessarily all embodiments, of the inventions.
As used in this specification and claim(s), the words "comprising" (and any
form of
comprising, such as "comprise" and "comprises"), "having" (and any form of
having, such as
"have" and "has"), "including" (and any form of including, such as "includes"
and "include") or
"containing" (and any form of containing, such as "contains" and "contain")
are inclusive or
open-ended and do not exclude additional, unrecited elements or method steps.
It is
contemplated that any embodiment discussed in this specification can be
implemented with
respect to any method or composition of the invention, and vice versa.
Furthermore,
compositions of the invention can be used to achieve methods of the invention.
The term "about" in relation to a reference numerical value and its
grammatical
equivalents as used herein can include the numerical value itself and a range
of values plus or
minus 10% from that numerical value. For example, the amount "about 10"
includes 10 and any
amounts from 9 to 11. For example, the term "about" in relation to a reference
numerical value
11
CA 03085492 2020-06-10
WO 2019/126120 PCT/US2018/066157
can also include a range of values plus or minus 10%, 9%, 8%, 7%, 6%, 5%, 4%,
3%, 2%, or 1%
from that value.
The disclosure provides improved system, kits, methods and apparatus for the
reproducible, consistent, and efficacious delivery of HBV vaccines, such as
nucleic acids
encoding HBV antigens and combinations thereof with Electrically Mediated
Therapeutic Agent
(e.g., HBV vaccine) Delivery (EMTAD).
In an aspect, the disclosure provides an apparatus for the delivery of an HBV
vaccine to a
predetermined site within a subject, comprising a cartridge assembly
comprising an outer
cartridge, an inner cartridge, a reservoir containing the HBV vaccine, wherein
a reservoir
containment volume is contained within the outer cartridge and configured to
receive the
reservoir; an applicator comprising a cartridge assembly receiving volume, a
needle hub, and an
insertion detector, wherein the insertion detector senses loading of the
reservoir in the reservoir
containment volume; at least one interlock, wherein the interlock facilitates
proper execution of
the HBV vaccine administration procedure; at least one injection orifice
through which the HBV
vaccine is administered; a plurality of penetrating electrodes arranged with a
predetermined
spatial relationship relative to the orifice; an electrical field generator
for generating an electrical
signal operatively connected to the electrodes; and a controlled source of
energy sufficient to
transfer a predetermined amount of the HBV vaccine at a predetermined rate
from the reservoir
through the orifice to the predetermined site within the subject.
In another aspect, the disclosure provides a kit for the delivery of an HBV
vaccine to a
predetermined site within a subject, comprising the HBV vaccine, and an
apparatus comprising a
cartridge assembly comprising an outer cartridge, an inner cartridge, a
reservoir for the HBV
vaccine, wherein a reservoir containment volume is contained within the outer
cartridge and
configured to receive the reservoir; an applicator comprising a cartridge
assembly receiving
volume, a needle hub, and an insertion detector, wherein the insertion
detector senses loading of
the reservoir in the reservoir containment volume; at least one interlock,
wherein the interlock
facilitates proper execution of the HBV vaccine administration procedure; at
least one injection
orifice through which the HBV vaccine is administered; a plurality of
penetrating electrodes
arranged with a predetermined spatial relationship relative to the orifice; an
electrical field
generator for generating an electrical signal operatively connected to the
electrodes; and a
controlled source of energy sufficient to transfer a predetermined amount of
the HBV vaccine at
12
CA 03085492 2020-06-10
WO 2019/126120 PCT/US2018/066157
a predetermined rate from the reservoir through the orifice to the
predetermined site within the
subject.
An HBV vaccine included in an apparatus or a kit of the present application
can
comprise:
a first nucleic acid molecule comprising a first polynucleotide encoding an
HBV
polymerase antigen having an amino acid sequence that is at least 98%
identical to SEQ ID NO:
4, wherein the HBV polymerase antigen does not have reverse transcriptase
activity and RNase
H activity;
a second nucleic acid molecule comprising a second polynucleotide encoding a
truncated HBV core antigen consisting of the amino acid sequence of SEQ ID NO:
2 or SEQ ID
NO: 14; and
a pharmaceutically acceptable carrier,
wherein the first nucleic acid molecule and the second nucleic acid molecule
are
present in the same nucleic acid molecule or in two different nucleic acid
molecules.
In certain aspects of the disclosure, EMTAD can be refered to as the
administration of an
HBV vaccine to a biological tissue of interest and the earlier, concurrent or
subsequent
application of electrical signals to biological tissue for the purpose of
enhancing movement
and/or uptake of the HBV vaccine in said tissue. The process of EMTAD is
comprised of two
elements: 1) Therapeutic Agent (i.e., HBV vaccine) Administration (TAA), and
2) an Electrical
Signal Application (ESA) sufficient to induce the desired EMTAD effect. In the
disclosure, TAA
can be accomplished, for instance, in a controllable fashion, termed
Controlled Therapeutic
Agent (e.g., HBV vaccine) Administration (CTAA). The term CTAA used herein can
refer to
methods or apparatus that provide spatial and/or temporal control over
administration of an HBV
vaccine relative to the induction of an EMTAD effect. Controllable
administration techniques
can utilize variations on the conventional needle-syringe (e.g. automatic
injection device) and/or
various needleless methodologies (e.g. jet injector,
transdermal/transcutaneous patch, oral, gel,
cream, or inhaled administration). The term ESA used herein can refer to to
the application of
electrical signals to facilitate or enhance the delivery of active agents,
e.g., HBV vaccines, by
improving movement and/or uptake of said agents within tissue, thus inducing
an EMTAD
effect. When used to facilitate or enhance delivery of an HBV vaccine, ESA
processes such as
electroporation, iontophoresis, electroosmosis, electropermeabilization,
electrostimulation,
13
CA 03085492 2020-06-10
WO 2019/126120 PCT/US2018/066157
electromigration, and electroconvection all represent various modes of EMTAD,
one or more of
which can be included in the methods described herein.
Specific applications for apparatus, kits and systems described herein
include, but are not
limited to, the delivery of HBV vaccines containing one or more than one
nucleic acid
molecules. Traditionally with such applications, EMTAD is initiated by HBV
vaccine injection
using a conventional needle-syringe. After the agent has been administered, a
device suitable for
ESA is applied to the subject at a designated location. Finally, an
appropriate ESA protocol is
utilized to provide the desired facilitation or enhancement to HBV vaccine
delivery. With
traditional EMTAD, however, the desired spatial and temporal relationship
between agent
administration and ESA may not be realized.
Spatial Parameters
In some embodiments of the systems, kits, methods and apparatus described
herein, HBV
vaccine administration is performed using a conventional needle syringe. The
need to deliver
certain agents with EMTAD brings an additional level of complexity to the
issue of TAA. As
depicted in FIG. 1, in any conventional needle-syringe injection, as the
needle 5 is inserted into
the tissue, the depth 1 and the angle 2 of insertion relative to the surface
of the tissue 3 can be
difficult to control. Additionally, the point of needle penetration 4 at the
tissue surface 3 may not
be representative of the location of the orifice 6 and the region of agent
administration 7 within
the target tissue. As an illustrative example a transcutaneous intramuscular
injection may not
correspond to the site of insertion on the skin since the two tissues can
often move in relation to
one another.
While this conventional approach is generally adequate for the delivery of
many different
therapeutics that do not require EMTAD, these variables lead to a distribution
of the HBV
vaccine following injection that is often inconsistent and/or indeterminate
and can hamper
effective EMTAD. In certain embodiments described herein, the most effective
use of EMTAD
utilizes a predefined relationship between the HBV vaccine and ESA within the
subject. As a
result, in the absence of spatial control over TAA in a target tissue, using a
conventional needle
syringe can result in reduced effectiveness of the EMTAD application, as
compared to an
apparatus, method or system that provides spatial and temporal control. One
illustrative example
of this concept is the use of electroporation to facilitate the delivery of an
HBV vaccine.
Electroporation is typically most effective in enhancing HBV vaccine delivery
when TAA and
14
CA 03085492 2020-06-10
WO 2019/126120 PCT/US2018/066157
ESA are co-localized within the target region of tissue. In many cases, if the
agent to be
delivered and the induced electroporation effect are not co-localized within
the target region of
tissue, the delivery of said agent is suboptimal.
Another example of the need for adequate spatial control of TAA in EMTAD is
iontophoresis. This mode of EMTAD uses electrical fields to cause movement of
charged
molecules. In order to achieve the desired movement of the agent, the proper
spatial relationship
between the electrodes and the HBV vaccine must be realized. If a negatively
charged agent
were placed in close proximity to the location of a positive electrode, little
or no movement of
the agent through the tissue would be observed. In contrast, localization of
the said negatively
charged agent near the negative electrode would result in significant movement
of the agent
through the tissue in the direction of the positive electrode.
As illustrated by the preceding examples, it is important to control the
precise location of
TAA relative to the application of ESA to achieve the desired effect. As such,
embodiments of
the apparatus and methods described herein provide control of the precise
location of TAA
relative to the application of ESA, and are useful to achieve reproducible,
consistent, and well-
characterized distribution of one or more HBV vaccines.
Temporal Parameters
In the case of conventional needle-syringe injection TAA is that the rate of
injection may
vary from one operator to another, thereby causing inconsistent agent
distribution in the tissue.
Additional temporal variability is introduced when multiple device placements
are required to
complete the EMTAD process. For example, one application of EMTAD calls for
the
administration of plasmid DNA encoding for a therapeutic protein, followed by
generation of an
electroporation-inducing electrical field. Using the traditional method of
EMTAD, the HBV
vaccine is injected with a needle-syringe, followed by placement and
activation of the
electroporation device. By requiring two separate device placements (the
initial needle syringe
followed by the ESA device), this procedure is susceptible to inter-subject
variability arising
from inconsistent temporal application of each device by the operator.
Additionally, the use of
two separate device placements leads to an unavoidable time interval in
between the clinician's
placement and activation of each device. This is compounded in the case where
multiple
application sites are necessary to achieve adequate delivery of the agent to a
specifiable region
within the target tissue.
CA 03085492 2020-06-10
WO 2019/126120 PCT/US2018/066157
These issues are especially critical for agents, such as nucleic acids, that
can be degraded
or inactivated in the extracellular environment. The degradation of nucleic
acids in the HBV
vaccine can lead to a reduction in efficacy and consistency in the application
of the therapy.
Also, the inter-subject rate of degradation of nucleic acids in the HBV
vaccine is not constant,
thus contributing to the overall therapeutic inconsistency of conventional
needle-syringe
injection combined with ESA, and more specifically with electroporation
therapy.
Due to the inherent difficulty of spatial and temporal variability with
conventional
needle-syringe injection used in conjunction with ESA, the precise location
and timing of TAA
relative to ESA is often unknown. As a result, the effective administration
and dosing of HBV
vaccines with EMTAD can be inconsistent and irreproducible. Though
conventional needle-
syringe injection is sometimes adequate for HBV vaccine administration,
reproducible and
consistent delivery of HBV vaccines is significantly enhanced by controlling
the spatial and
temporal relationship between administration of the HBV vaccine and induction
of the desired
EMTAD effect.
Thus, while the traditional EMTAD procedure can be adequate for certain
applications,
temporal and spatial control is highly desirable for clinical applications
that typically require a
high degree of consistency and reproducibility. In contrast to the
conventional EMTAD
approach, embodiments of methods, systems and apparatus described herein
facilitate CTAA and
ESA to provide more advantageous methods and apparatus for the clinical
application of
EMTAD. The disclosure utilizes various aspects of CTAA in conjunction with ESA
to provide
reproducible, consistent, and efficacious HBV vaccine delivery. The disclosure
describes
methods and apparatus to provide spatial and temporal control over
administration of an HBV
vaccine relative to the application of electrical signals, thereby improving
the movement and/or
uptake of said vaccine in the target tissue.
In some embodiments are provided methods and apparatus wherein there exists a
controllable spatial relationship for the administration of the HBV vaccine
relative to the
application of electrical signals. Prior to treatment, the optimal location
for TAA relative to ESA
is determined. This spatial relationship between TAA and ESA is dictated by
treatment
parameters, including the nature of the agent being administered and the
properties of the target
tissue to which the agent is administered. In an exemplary embodiment,
electrical signals are
preferentially applied distal to the site of HBV vaccine administration. In
certain other
16
CA 03085492 2020-06-10
WO 2019/126120 PCT/US2018/066157
embodiments, spatial relationship is to apply the EMTAD-inducing electrical
signals proximal to
the site of agent administration. In certain cases, co-localization between
TAA and ESA is
preferable. This is often the case when electroporation and/or iontophoresis
are utilized for
induction of the desired EMTAD effect.
In another aspect of the disclosure, an apparatus described herein provides a
controllable
temporal relationship for the sequence and timing of TAA relative to ESA.
Prior to treatment, the
optimal sequence and timing for a combination of TAA and ESA is determined. As
with the
spatial relationship, the desired temporal relationship between TAA and ESA is
dictated by
parameters such as the nature of the agent being administered and the
properties of the target
tissue to which the agent is administered. In certain applications, exposure
to the electrical fields
associated with ESA may adversely affect the HBV vaccine. In the practice of
such applications,
generation of such electrical fields is followed by CTAA. However, the typical
temporal
relationship is CTAA followed by ESA.
The disclosure provides improved methods and apparatus for the reproducible,
consistent,
and efficacious delivery of HBV vaccines comprising nucleic acid based
constructs with
EMTAD. This objective is accomplished by controlling the spatial and temporal
administration
of an HBV vaccine relative to application of electrical signals. In a certain
embodiment, EMTAD
is initiated by HBV vaccine injection using a conventional needle-syringe.
After the agent has
been administered, a device suitable for ESA is applied to the subject at a
designated location.
An appropriate ESA protocol is utilized to provide the desired facilitation or
enhancement to
HBV vaccine delivery. An exemplary ESA method that has proven to be effective
in virtually all
cell types is electroporation. Other exemplary methods of electrically
mediated delivery include,
but are not limited to, iontophoresis, electroosmosis,
electropermeabilization, electrostimulation,
electromigration, and electroconvection. These terms are used for illustrative
purposes only and
should not be construed as limitations in the disclosure.
The technique of electroporation utilizes the application of electric fields
to induce a
transient increase in cell membrane permeability and to move charged
particles. By
permeabilizing the cell membranes within the target tissue, electroporation
dramatically
improves the intracellular uptake of exogenous substances that have been
administered to the
target tissue. The increase in cell membrane permeability and molecular
movement due to
electroporation offers a method for overcoming the cell membrane as a barrier
to HBV vaccine
17
CA 03085492 2020-06-10
WO 2019/126120 PCT/US2018/066157
delivery. The application of electroporation as a technique for inducing EMTAD
is advantageous
in that the physical nature of the technique allows electroporation to be
applied in virtually all
tissue types. Accordingly, various aspects and embodiments of the disclosure
discuss, but are not
limited to, electroporation as a technique for inducing EMTAD.
HBV vaccines
The term "HBV vaccine" is used herein in its broadest sense to include any
agent capable
of providing a desired or beneficial immune response, therapeutic and/or
prophylactic effect
against an HBV on a living tissue. Thus, the term includes both prophylactic
and therapeutic
HBV vaccines, as well as any other category of agent having such desired
effects. The scope of
the disclosure is sufficiently broad to include the controlled delivery of any
HBV vaccines,
however categorized. HBV vaccines include, but are not limited to
pharmaceutical drugs and
vaccines, and nucleic acid sequences (such as supercoiled, relaxed, and linear
plasmid DNA,
RNA, antisense constructs, artificial chromosomes, or any other nucleic acid-
based therapeutic),
and any formulations thereof Such agent formulations include, but are not
limited to, cationic
lipids, cationic and/or nonionic polymers, liposomes, saline, nuclease
inhibitors, anesthetics,
poloxamers, preservatives, sodium phosphate solutions, or other compounds that
can improve the
administration, stability, and/or effect of the HBV vaccine. Additional
formulations include
agents and additives conferring the ability to control viscosity and
electrical impedance of the
administered agent.
In a preferred aspect of the application, an HBV vaccine to be used in the
invention can
comprise:
a first nucleic acid molecule, preferably a first plasmid DNA vector,
comprising a first
polynucleotide encoding an HBV polymerase antigen, preferably the HBV
polymerase antigen
has an amino acid sequence that is at least 98% identical to SEQ ID NO: 4,
such as at least 98%,
98.5%, 99%, 99.1%, 99.2%, 99.3%, 99.4%, 99.5%, 99.6%, 99.7%, 99.8%, 99.9% or
100%
identical to SEQ ID NO: 4, wherein the HBV polymerase antigen does not have
reverse
transcriptase activity and RNase H activity;
a second nucleic acid molecule, preferably a second plasmid DNA vector,
comprising a
second polynucleotide encoding a truncated HBV core antigen, preferably the
truncated HBV
core antigen consists of the amino acid sequence of SEQ ID NO: 2 or SEQ ID NO:
14; and
a pharmaceutically acceptable carrier, and
18
CA 03085492 2020-06-10
WO 2019/126120 PCT/US2018/066157
wherein the first nucleic acid molecule and the second nucleic acid molecule
are present in the
same nucleic acid molecule, such as the same plasmid DNA vector, or in two
different nucleic
acid molecules, such as two separate plasmid DNA vectors.
As used herein, each of the terms "HBV core antigen," "HBcAg" and "core
antigen"
refers to an HBV antigen capable of inducing an immune response, e.g., a
humoral and/or
cellular mediated response, against an HBV core protein in a subject. Each of
the terms "core,"
"core polypeptide," and "core protein" refers to the HBV viral core protein.
Full-length core
antigen is typically 183 amino acids in length and includes an assembly domain
(amino acids 1
to 149) and a nucleic acid binding domain (amino acids 150 to 183). The 34-
residue nucleic acid
binding domain is required for pre-genomic RNA encapsidation. This domain also
functions as
a nuclear import signal. It comprises 17 arginine residues and is highly
basic, consistent with its
function. HBV core protein is dimeric in solution, with the dimers self-
assembling into
icosahedral capsids. Each dimer of core protein has four a-helix bundles
flanked by an a-helix
domain on either side. Truncated HBV core proteins lacking the nucleic acid
binding domain are
also capable of forming capsids.
In an aspect of the application, an HBV antigen to be used in the invention is
a truncated
HBV core antigen. As used herein, a "truncated HBV core antigen," refers to an
HBV antigen
that does not contain the entire length of an HBV core protein but is capable
of inducing an
immune response against the HBV core protein in a subject. For example, an HBV
core antigen
can be modified to delete one or more amino acids of the highly positively
charged (arginine
rich) C-terminal nucleic acid binding domain of the core antigen, which
typically contains
seventeen arginine (R) residues. A truncated HBV core antigen of the
application is preferably a
C-terminally truncated HBV core protein which does not comprise the HBV core
nuclear import
signal and/or a truncated HBV core protein from which the C-terminal HBV core
nuclear import
signal has been deleted. In an embodiment, a truncated HBV core antigen
comprises a deletion
in the C-terminal nucleic acid binding domain, such as a deletion of 1 to 34
amino acid residues
of the C-terminal nucleic acid binding domain, e.g., 1, 2, 3, 4, 5, 6, 7, 8,
9, 10, 11, 12, 13, 14, 15,
16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, or 34
amino acid residues,
preferably a deletion of all 34 amino acid residues. In a preferred
embodiment, a truncated HBV
core antigen comprises a deletion in the C-terminal nucleic acid binding
domain, preferably of
all 34 amino acid residues.
19
CA 03085492 2020-06-10
WO 2019/126120 PCT/US2018/066157
An HBV core antigen useful in the application can be a consensus sequence
derived from
multiple HBV genotypes (e.g., genotypes A, B, C, D, E, F, G, and H). As used
herein,
"consensus sequence" means an artificial sequence of amino acids based on an
alignment of
amino acid sequences of homologous proteins, e.g., as determined by an
alignment (e.g., using
Clustal Omega) of amino acid sequences of homologous proteins. It can be the
calculated order
of most frequent amino acid residues, found at each position in a sequence
alignment, based
upon sequences of HBV antigens (e.g., core, pol, etc.) from at least 100
natural HBV isolates. A
consensus sequence can be non-naturally occurring and different from the
native viral sequences.
Consensus sequences can be designed by aligning multiple HBV antigen sequences
from
different sources using a multiple sequence alignment tool, and at variable
alignment positions,
selecting the most frequent amino acid. Preferably, a consensus sequence of an
HBV antigen is
derived from HBV genotypes B, C, and D. The term "consensus antigen" is used
to refer to an
antigen having a consensus sequence.
An exemplary truncated HBV core antigen useful for the application lacks the
nucleic
acid binding function, and can be capable of inducing an immune response in a
mammal against
at least two HBV genotypes. Preferably a truncated HBV core antigen is capable
of inducing a T
cell response in a mammal against at least HBV genotypes B, C and D. More
preferably, a
truncated HBV core antigen is capable of inducing a CD8 T cell response in a
human subject
against at least HBV genotypes A, B, C and D.
Preferably, an HBV core antigen useful for the application is a consensus
antigen,
preferably a consensus antigen derived from HBV genotypes B, C, and D, more
preferably a
truncated consensus antigen derived from HBV genotypes B, C, and D. An
exemplary truncated
HBV core consensus antigen according to the application consists of an amino
acid sequence that
is at least 90% identical to SEQ ID NO: 2 or SEQ ID NO: 14, such as at least
90%, 91%, 92%,
93%, 94%, 95%, 95.5%, 96%, 96.5%, 97%, 97.5%, 98%, 98.5%, 99%, 99.1%, 99.2%,
99.3%,
99.4%, 99.5%, 99.6%, 99.7%, 99.8%, 99.9%, or 100% identical to SEQ ID NO: 2 or
SEQ ID
NO: 14. SEQ ID NO: 2 and SEQ ID NO: 4 are core consensus antigens derived from
HBV
genotypes B, C, and D. SEQ ID NO: 2 and SEQ ID NO :14 contain a 34-amino acid
C-terminal
deletion of the highly positively charged (arginine rich) nucleic acid binding
domain of the
native core antigen.
CA 03085492 2020-06-10
WO 2019/126120 PCT/US2018/066157
In a particular embodiment of the application, an HBV core antigen is a
truncated HBV
antigen consisting of the amino acid sequence of SEQ ID NO: 2. In another
particular
embodiment, an HBV core antigen is a truncated HBV antigen consisting of the
amino acid
sequence of SEQ ID NO: 14.
Examples of polynucleotide sequences encoding a truncated HBV core antigen
consisting
of the amino acid sequence of SEQ ID NO: 2 include, but are not limited to, a
polynucleotide
sequence at least 90% identical to SEQ ID NO: 1, such as or at least 90%, at
least 91%, at least
92%, at least 93%, at least 94%, at least 95%, at least 95.5%, at least 96%,
at least 96.5%, at least
97%, at least 97.5%, at least 98%, at least 98.5%, at least 99%, at least
99.1%, at least 99.2%, at
least 99.3%, at least 99.4%, at least 99.5%, at least 99.6%, at least 99.7%,
at least 99.8%, at least
99.9% or 100% identical to SEQ ID NO: 1, preferably about 98%, about 99% or
100% identical
to SEQ ID NO: 1.
Examples of polynucleotide sequences encoding a truncated HBV core antigen
consisting
of the amino acid sequence of SEQ ID NO: 14 include, but are not limited to, a
polynucleotide
sequence at least 90% identical to SEQ ID NO: 15, such as or at least 90%, at
least 91%, at least
92%, at least 93%, at least 94%, at least 95%, at least 95.5%, at least 96%,
at least 96.5%, at least
97%, at least 97.5%, at least 98%, at least 98.5%, at least 99%, at least
99.1%, at least 99.2%, at
least 99.3%, at least 99.4%, at least 99.5%, at least 99.6%, at least 99.7%,
at least 99.8%, at least
99.9% or 100% identical to SEQ ID NO: 15, preferably about 98%, about 99% or
100% identical
to SEQ ID NO: 15.
In particular embodiments of the application, a HBV vaccine to be used in the
invention
comprises a non-naturally occurring nucleic acid molecule encoding a truncated
HBV core
antigen, and the non-naturally occurring nucleic acid molecule comprises the
polynucleotide
sequence of SEQ ID NO: 1 or SEQ ID NO: 15.
As used herein, the term "HBV polymerase antigen," "HBV Pol antigen" or "HBV
pol
antigen" refers to an HBV antigen capable of inducing an immune response,
e.g., a humoral
and/or cellular mediated response, against an HBV polymerase in a subject.
Each of the terms
"polymerase," "polymerase polypeptide," "Pol" and "pol" refers to the HBV
viral DNA
polymerase. The HBV viral DNA polymerase has four domains, including, from the
N terminus
to the C terminus, a terminal protein (TP) domain, which acts as a primer for
minus-strand DNA
21
CA 03085492 2020-06-10
WO 2019/126120 PCT/US2018/066157
synthesis; a spacer that is nonessential for the polymerase functions; a
reverse transcriptase (RT)
domain for transcription; and a RNase H domain.
In an embodiment of the application, an HBV antigen comprises an HBV Pol
antigen, or
any immunogenic fragment or combination thereof An HBV Pol antigen can contain
further
modifications to improve immunogenicity of the antigen, such as by introducing
mutations into
the active sites of the polymerase and/or RNase H domains to decrease or
substantially eliminate
certain enzymatic activities.
Preferably, an HBV Pol antigen useful in the application does not have reverse
transcriptase activity and RNase H activity, and is capable of inducing an
immune response in a
mammal against at least two HBV genotypes. Preferably, an HBV Pol antigen is
capable of
inducing a T cell response in a mammal against at least HBV genotypes B, C and
D. More
preferably, a HBV Pol antigen is capable of inducing a CD8 T cell response in
a human subject
against at least HBV genotypes A, B, C and D.
Thus, in some embodiments, an HBV Pol antigen useful in the application is an
inactivated Pol antigen. In an embodiment, an inactivated HBV Pol antigen
comprises one or
more amino acid mutations in the active site of the polymerase domain. In
another embodiment,
an inactivated HBV Pol antigen comprises one or more amino acid mutations in
the active site of
the RNaseH domain. In a preferred embodiment, an inactivated HBV pol antigen
comprises one
or more amino acid mutations in the active site of both the polymerase domain
and the RNaseH
domain. For example, the "YXDD" motif in the polymerase domain of an HBV pol
antigen that
can be required for nucleotide/metal ion binding can be mutated, e.g., by
replacing one or more
of the aspartate residues (D) with asparagine residues (N), eliminating or
reducing metal
coordination function, thereby decreasing or substantially eliminating reverse
transcriptase
function. Alternatively, or in addition to mutation of the "YXDD" motif, the
"DEDD" motif in
the RNaseH domain of an HBV pol antigen required for Mg2+ coordination can be
mutated, e.g.,
by replacing one or more aspartate residues (D) with asparagine residues (N)
and/or replacing the
glutamate residue (E) with glutamine (Q), thereby decreasing or substantially
eliminating
RNaseH function. In a particular embodiment, an HBV pol antigen is modified by
(1) mutating
the aspartate residues (D) to asparagine residues (N) in the "YXDD" motif of
the polymerase
domain; and (2) mutating the first aspartate residue (D) to an asparagine
residue (N) and the first
glutamate residue (E) to a glutamine residue (N) in the "DEDD" motif of the
RNaseH domain,
22
CA 03085492 2020-06-10
WO 2019/126120 PCT/US2018/066157
thereby decreasing or substantially eliminating both the reverse transcriptase
and RNaseH
functions of the pol antigen.
In a preferred embodiment of the application, an HBV pol antigen is a
consensus antigen,
preferably a consensus antigen derived from HBV genotypes B, C, and D, more
preferably an
inactivated consensus antigen derived from HBV genotypes B, C, and D. An
exemplary HBV
pol consensus antigen according to the application comprises an amino acid
sequence that is at
least 90% identical to SEQ ID NO: 4, such as at least 90%, 91%, 92%, 93%, 94%,
95%, 95.5%,
96%, 96.5%, 97%, 97.5%, 98%, 98.5%, 99%, 99.1%, 99.2%, 99.3%, 99.4%, 99.5%,
99.6%,
99.7%, 99.8%, 99.9% or 100% identical to SEQ ID NO: 4, preferably at least 98%
identical to
SEQ ID NO: 4, such as at least 98%, 98.5%, 99%, 99.1%, 99.2%, 99.3%, 99.4%,
99.5%, 99.6%,
99.7%, 99.8%, 99.9% or 100% identical to SEQ ID NO: 4. SEQ ID NO: 4 is a pol
consensus
antigen derived from HBV genotypes B, C, and D comprising four mutations
located in the
active sites of the polymerase and RNaseH domains. In particular, the four
mutations include
mutation of the aspartic acid residues (D) to asparagine residues (N) in the
"YXDD" motif of the
polymerase domain; and mutation of the first aspartate residue (D) to an
asparagine residue (N)
and mutation of the glutamate residue (E) to a glutamine residue (Q) in the
"DEDD" motif of the
RNaseH domain.
In a particular embodiment of the application, an HBV pol antigen useful for
the
application comprises the amino acid sequence of SEQ ID NO: 4. In other
embodiments of the
application, an HBV core antigen useful in the application consists of the
amino acid sequence of
SEQ ID NO: 4.
Examples of polynucleotide sequences encoding a HBV pol antigen comprising the
amino acid sequence of SEQ ID NO: 4 include, but are not limited to, a
polynucleotide sequence
at least 90% identical to SEQ ID NO: 3 or SEQ ID NO: 16, such as at least 90%,
91%, 92%,
93%, 94%, 95%, 95.5%, 96%, 96.5%, 97%, 97.5%, 98%, 98.5%, 99%, 99.1%, 99.2%,
99.3%,
99.4%, 99.5%, 99.6%, 99.7%, 99.8%, 99.9% or 100% identical to SEQ ID NO: 3 or
SEQ ID
NO: 16, preferably 98%, 99% or 100% identical to SEQ ID NO: 3 or SEQ ID NO:
16. In
particular embodiments of the application, a HBV vaccine useful for the
application comprises a
non-naturally occurring nucleic acid molecule encoding a HBV pol antigen, and
the non-
naturally occurring nucleic acid molecule comprises the polynucleotide
sequence of SEQ ID
NO: 3 or SEQ ID NO: 16.
23
CA 03085492 2020-06-10
WO 2019/126120 PCT/US2018/066157
As used herein the term "fusion protein" or "fusion" refers to a single
polypeptide chain
having at least two polypeptide domains that are not normally present in a
single, natural
polypeptide.
In an aspect of the application, an HBV antigen to be used in the invention
can comprise
a fusion protein comprising a truncated HBV core antigen operably linked to an
HBV Pol
antigen, or an HBV Pol antigen operably linked to a truncated HBV core
antigen, preferably via
a linker. As used herein, the term "linker" refers to a compound or moiety
that acts as a
molecular bridge to operably link two different molecules, wherein one portion
of the linker is
operably linked to a first molecule, and wherein another portion of the linker
is operably linked
to a second molecule. As used herein, the term "operably linked" refers to a
linkage or a
juxtaposition wherein the components so described are in a relationship
permitting them to
function in their intended manner. For example, a regulatory sequence operably
linked to a
nucleic acid sequence of interest is capable of directing the transcription of
the nucleic acid
sequence of interest, or a signal sequence operably linked to an amino acid
sequence of interest
is capable of secret or translocate the amino acid sequence of interest over a
member.
For example, in a fusion protein containing a first polypeptide and a second
heterologous
polypeptide, a linker serves primarily as a spacer between the first and
second polypeptides. In
an embodiment, a linker is made up of amino acids linked together by peptide
bonds, preferably
from 1 to 20 amino acids linked by peptide bonds, wherein the amino acids are
selected from the
20 naturally occurring amino acids. In an embodiment, the 1 to 20 amino acids
are selected from
glycine, alanine, proline, asparagine, glutamine, and lysine. Preferably, a
linker is made up of a
majority of amino acids that are sterically unhindered, such as glycine and
alanine. Exemplary
linkers are polyglycines, particularly (Gly)5, (Gly)8; poly(Gly-Ala), and
polyalanines. One
exemplary suitable linker is (AlaGly)n, wherein n is an integer of 2 to 5.
Preferably, a fusion protein useful in the application is capable of inducing
an immune
response in a mammal against HBV core and HBV Pol of at least two HBV
genotypes.
Preferably the fusion protein is capable of inducing a T cell response in a
mammal against at
least HBV genotypes B, C and D. More preferably, the fusion protein is capable
of inducing a
CD8 T cell response in a human subject against at least HBV genotypes A, B, C
and D.
In an aspect of the application, a fusion protein useful in the application
can comprise a
truncated HBV core antigen having an amino acid sequence at least 90%, such as
at least 90%,
24
CA 03085492 2020-06-10
WO 2019/126120 PCT/US2018/066157
91%, 92%, 93%, 940, 950, 95.50, 96%, 96.5%, 970, 97.50, 98%, 98.5%, 990,
99.1%,
99.2%, 99.30, 99.40, 99.50, 99.6%, 99.70, 99.8%, 99.9%, or 100 A identical to
SEQ ID NO:
2 or 14, a linker, and a HBV Pol antigen having an amino acid sequence at
least 90%, such as at
least 90%, 91%, 92%, 9300, 9400, 9500, 95.50, 96%, 96.5%, 970, 97.50, 98%,
98.5%, 990
,
99.1%, 99.2%, 99.30, 99.40, 99.50, 99.6%, 99.70, 99.8%, 99.9%, or 1000o,
identical to SEQ
ID NO: 4.
Preferably, a fusion protein useful in the application comprises a truncated
HBV core
antigen consisting of the amino acid sequence of SEQ ID NO: 2 or 14, a linker
comprising
(AlaGly)n, wherein n is an integer of 2 to 5, and a HBV Pol antigen having the
amino acid
sequence of SEQ ID NO: 4. More preferably, a fusion protein useful in the
application
comprises the amino acid sequence of SEQ ID NO: 20.
In some embodiments of the application, a fusion protein useful in the
application further
comprises a signal sequence. Preferably, the signal sequence has the amino
acid sequence of
SEQ ID NO: 6 or SEQ ID NO: 19. More preferably, the fusion protein comprises
the amino acid
sequence of SEQ ID NO: 21.
Examples of polynucleotide sequences encoding a fusion protein useful in the
application
include, but are not limited to, a polynucleotide sequence at least 90 A
identical to SEQ ID NO: 1
or SEQ ID NO: 15, such as at least 90%, 91%, 92%, 93%, 94%, 95%, 95.5%, 96%,
96.5%, 97%,
97.5%, 98%, 98.5%, 99%, 99.1%, 99.2%, 99.3%, 99.4%, 99.5%, 99.6%, 99.7%,
99.8%, 99.9 A
or 100 A identical to SEQ ID NO: 1 or SEQ ID NO: 15, preferably 98%, 99% or
100 A identical
to SEQ ID NO: 1 or SEQ ID NO: 15, operably linked to a linker coding sequence
at least 90 A
identical to SEQ ID NO: 22, such as at least 90%, 91%, 92%, 93%, 94%, 95%,
95.5%, 96%,
96.5%, 97%, 97.5%, 98%, 98.5%, 99%, 99.1%, 99.2%, 99.3%, 99.4%, 99.5%, 99.6%,
99.7%,
99.8%, 99.9% or 100 A identical to SEQ ID NO: 22, preferably 98%, 99% or 100 A
identical to
SEQ ID NO: 22, which is further operably linked a polynucleotide sequence at
least 90 A
identical to SEQ ID NO: 3 or SEQ ID NO: 16, such as at least 90%, 91%, 92%,
93%, 9400, 950
,
95.5%, 96%, 96.5%, 9700, 97.5%, 98%, 98.5%, 990, 99.1%, 99.2%, 99.3%, 99.4%,
99.5%,
99.6%, 99.7%, 99.8%, 99.9% or 100 A identical to SEQ ID NO: 3 or SEQ ID NO:
16, preferably
980o, 99% or 100 A identical to SEQ ID NO: 3 or SEQ ID NO: 16. In particular
embodiments of
the application, a HBV vaccine useful in the application comprises a non-
naturally occurring
nucleic acid molecule encoding a fusion protein, and the non-naturally
occurring nucleic acid
CA 03085492 2020-06-10
WO 2019/126120 PCT/US2018/066157
molecule comprises SEQ ID NO: 1, operably linked to SEQ ID NO: 22, which is
further
operably linked to SEQ ID NO: 3.
In an aspect of the application, in an HBV vaccine to be used in the
application, the first
and second nucleic acid molecules are first and second plasmid DNA vectors,
respectively, and
each of the first and second plasmid DNA vectors comprises an origin of
replication, an
antibiotic resistance gene, and from 5' end to 3' end, a promoter sequence, an
enhancer
sequence, a signal peptide coding sequence, the first polynucleotide sequence
or the second
polynucleotide sequence, and a polyadenylation signal sequence. In a preferred
embodiment of
the application, a DNA plasmid is an expression vector suitable for protein
expression in
mammalian host cells. Expression vectors suitable for protein expression in
mammalian host
cells include, but are not limited to, pcDNATM, pcDNA3TM, pVAX, pVAX-1, etc.
Preferably,
the expression vector is based on pVAX-1, which can be further modified to
optimize protein
expression in mammalian cells. pVAX-1 is a commonly used plasmid in DNA
vaccines, and
contains a strong human intermediate early cytomegalovirus (CMV-IE) promoter
followed by
the bovine growth hormone (bGH)-derived polyadenylation sequence (pA). pVAX-1
further
contains a pUC origin of replication and a kanamycin resistance gene driven by
a small
prokaryotic promoter that allows for bacterial plasmid propagation.
Preferably, the plasmid
DNA vector comprises a codon optimized kanamycin resistance gene having a
polynucleotide
sequence at least 90% identical to SEQ ID NO: 12, preferably 100% identical to
SEQ ID NO:
12.
In a specific embodiment, an HBV vaccine to be used in the invention
comprises:
a) a first plasmid DNA vector comprising, from 3'-end to 5'-end, the
promoter
sequence comprising the polynucleotide sequence of SEQ ID NO: 7, the enhancer
sequence
comprising the polynucleotide sequence of SEQ ID NO: 8, the signal peptide
coding sequence
comprising the polynucleotide sequence of SEQ ID NO: 5, the first
polynucleotide sequence
comprising the polynucleotide sequence of SEQ ID NO: 3, and the
polyadenylation signal
sequence comprising the polynucleotide sequence of SEQ ID NO: 11;
b) a second plasmid DNA vector comprising, from 3'-end to 5'-end, the
promoter
sequence comprising the polynucleotide sequence of SEQ ID NO: 7, the enhancer
sequence
comprising the polynucleotide sequence of SEQ ID NO: 8, the signal peptide
coding sequence
comprising the polynucleotide sequence of SEQ ID NO: 5, the second
polynucleotide sequence
26
CA 03085492 2020-06-10
WO 2019/126120 PCT/US2018/066157
comprising the polynucleotide sequence of SEQ ID NO: 1, and the
polyadenylation signal
sequence comprising the polynucleotide sequence of SEQ ID NO: 11; and
c) a pharmaceutically acceptable carrier,
wherein each of the first plasmid DNA vector and the second plasmid DNA vector
further comprises a kanamycin resistance gene having the polynucleotide
sequence of SEQ ID
NO: 12, and an original of replication having the polynucleotide sequence of
SEQ ID NO: 10,
and
wherein the first plasmid DNA vector and the second plasmid DNA vector are in
the
same composition or two different compositions.
In those embodiments of the application in which an HBV vaccine comprises a
first
vector, such as a first DNA plasmid, and a second vector, such as a second DNA
plasmid, the
amount of each of the first and second vectors is not particularly limited.
For example, the first
DNA plasmid and the second DNA plasmid can be present in a ratio of 10:1 to
1:10, by weight,
such as 10:1, 9:1, 8:1, 7:1, 6:1, 5:1, 4:1, 3:1, 2:1, 1:1, 1:2, 1:3, 1:4, 1:5,
1:6, 1:7, 1:8, 1:9, or 1:10,
by weight. Preferably, the first and second DNA plasmids are present in a
ratio of 1:1, by
weight.
Compositions and immunogenic combinations of the application can comprise
additional
polynucleotides or vectors encoding additional HBV antigens and/or additional
HBV antigens or
immunogenic fragments thereof However, in particular embodiments, the
compositions and
immunogenic combinations of the application do not comprise certain antigens.
In preferred
embodiments, an HBV vaccine to be used in the invention does not contain a
nucleic acid
molecule encoding an HBV antigen selected from the group consisting of a
Hepatitis B surface
antigen (HBsAg), an HBV envelope (Env) antigen, and an HBV L protein antigen,
nor an HBV
antigen selected from the group consisting of a Hepatitis B surface antigen
(HB sAg), an HBV
envelope (Env) antigen, and an HBV L protein antigen.
An HBV vaccine useful in the application can also comprise a pharmaceutically
acceptable carrier. A pharmaceutically acceptable carrier is non-toxic and
should not interfere
with the efficacy of the active ingredient. Pharmaceutically acceptable
carriers can include one
or more, such as water, glycols, sugar, oils, amino acids, alcohols,
preservatives, emollients,
stabilizers, coloring agents and the like.
27
CA 03085492 2020-06-10
WO 2019/126120 PCT/US2018/066157
Other examples of HBV vaccines useful in the application are described in
International
Patent Application entitled "Hepatitis B Virus (HBV) Vaccines and Uses
thereof' filed on the
same day as this application with the Attorney Docket Number 688097-403W01,
the contents of
which are hereby incorporated by reference in their entireties.
Kits/Systems
In a general aspect, the invention relates to a kit or system for the
controlled delivery of a
HBV vaccine to a predetermined tissue site within a subject in need thereof,
comprising the HBV
vaccine and an apparatus for administering the HBV vaccine to the
predetermined tissue site via
electroporation. For example, the kit or system can have a prepacked
container, such as a
syringe, containing a premeasured amount of the HBV vaccine, which can be
loaded to an
apparatus described here for the subsequent administration of the HBV vaccine.
Methods of Inducing an Immune Response
In another general aspect, the invention relates to a method of inducing an
immune
response against hepatitis B virus (HBV) in a subject in need thereof,
comprising administering
to the subject an immunogenically effective amount of an HBV vaccine using an
apparatus, kit
or system of the application. Any of the apparatus, kit or system of the
application described
herein can be used in the methods of the application. As used herein, the term
"infection" refers
to the invasion of a host by a disease causing agent. A disease causing agent
is considered to be
"infectious" when it is capable of invading a host, and replicating or
propagating within the host.
Examples of infectious agents include viruses, e.g., HBV and certain species
of adenovirus,
prions, bacteria, fungi, protozoa and the like. "HBV infection" specifically
refers to invasion of
a host organism, such as cells and tissues of the host organism, by HBV.
As used herein, "inducing an immune response" when used with reference to the
methods
described herein encompasses causing a desired immune response or effect in a
subject in need
thereof against an infection, e.g. HBV infection. "Inducing an immune
response" also
encompasses providing a therapeutic immunity for treating against a pathogenic
agent, e.g.,
HBV. As used herein, the term "therapeutic immunity" or "therapeutic immune
response"
means that the vaccinated subject is able to control an infection with the
pathogenic agent against
which the vaccination was done, for instance immunity against HBV infection
conferred by
vaccination with HBV vaccine. In an embodiment, "inducing an immune response"
means
producing an immunity in a subject in need thereof, e.g., to provide a
therapeutic effect against a
28
CA 03085492 2020-06-10
WO 2019/126120 PCT/US2018/066157
disease, such as HBV infection. In certain embodiments, "inducing an immune
response" refers
to causing or improving cellular immunity, e.g., T cell response, against HBV.
In certain
embodiments, "inducing an immune response" refers to causing or improving a
humoral immune
response against HBV. In certain embodiments, "inducing an immune response"
refers to
causing or improving a cellular and a humoral immune response against HBV.
As used herein, the term "protective immunity" or "protective immune response"
means
that the vaccinated subject is able to control an infection with the
pathogenic agent against which
the vaccination was done. Usually, the subject having developed a "protective
immune response"
develops only mild to moderate clinical symptoms or no symptoms at all.
Usually, a subject
having a "protective immune response" or "protective immunity" against a
certain agent will not
die as a result of the infection with said agent.
Typically, the administration of an HBV vaccine according to embodiments of
the
application will have a therapeutic aim to generate an immune response against
HBV after HBV
infection or development of symptoms characteristic of HIV infection, e.g.,
for therapeutic
vaccination.
As used herein, "an immunogenically effective amount" or "immunologically
effective
amount" means an amount of a composition, polynucleotide, vector, or antigen
sufficient to
induce a desired immune effect or immune response in a subject in need
thereof. In an
embodiment, an immunogenically effective amount means an amount sufficient to
induce an
immune response in a subject in need thereof. In another embodiment, an
immunogenically
effective amount means an amount sufficient to produce immunity in a subject
in need thereof,
e.g., provide a therapeutic effect against a disease such as HBV infection. An
immunogenically
effective amount can vary depending upon a variety of factors, such as the
physical condition of
the subject, age, weight, health, etc.; the particular application, e.g.,
providing protective
immunity or therapeutic immunity; and the particular disease, e.g., viral
infection, for which
immunity is desired. An immunogenically effective amount can readily be
determined by one of
ordinary skill in the art in view of the disclosure.
In particular embodiments of the application, an immunogenically effective
amount
refers to the amount of a composition or immunogenic combination (such as an
HBV vaccine)
which is sufficient to achieve one, two, three, four, or more of the following
effects: (i) reduce or
ameliorate the severity of an HBV infection or a symptom associated therewith;
(ii) reduce the
29
CA 03085492 2020-06-10
WO 2019/126120 PCT/US2018/066157
duration of an HBV infection or symptom associated therewith; (iii) prevent
the progression of
an HBV infection or symptom associated therewith; (iv) cause regression of an
HBV infection or
symptom associated therewith; (v) prevent the development or onset of an HBV
infection, or
symptom associated therewith; (vi) prevent the recurrence of an HBV infection
or symptom
associated therewith; (vii) reduce hospitalization of a subject having an HBV
infection; (viii)
reduce hospitalization length of a subject having an HBV infection; (ix)
increase the survival of a
subject with an HBV infection; (x) eliminate an HBV infection in a subject;
(xi) inhibit or reduce
HBV replication in a subject; and/or (xii) enhance or improve the prophylactic
or therapeutic
effect(s) of another therapy.
In other particular embodiments, an immunogenically effective amount is an
amount
sufficient to reduce HBsAg levels consistent with evolution to clinical
seroconversion; achieve
sustained HBsAg clearance associated with reduction of infected hepatocytes by
a subject's
immune system; induce HBV-antigen specific activated T-cell populations;
and/or achieve
persistent loss of HBsAg within 12 months. Examples of a target index include
lower HBsAg
below a threshold of 500 copies of HBsAg International Unit (IU) and/or higher
CD8 counts.
As general guidance, an immunogenically effective amount when used with
reference to
a DNA plasmid can range from about 0.1 mg/mL to 10 mg/mL of DNA plasmid total,
such as
0.1 mg/mL, 0.25 mg/mL, 0.5 mg/mL. 0.75 mg/mL 1 mg/mL, 1.5 mg/mL, 2 mg/mL, 3
mg/mL, 4
mg/mL, 5 mg/mL, 6 mg/mL, 7 mg/mL, 8 mg/mL, 9 mg/mL, or 10 mg/mL. Preferably,
an
immunogenically effective amount of DNA plasmid is less than 8 mg/mL, more
preferably less
than 6 mg/mL, even more preferably 3-4 mg/mL. An immunogenically effective
amount can be
from one vector or plasmid, or from multiple vectors or plasmids. An
immunogenically effective
amount can be administered in a single composition, or in multiple
compositions, such as 1, 2, 3,
4, 5, 6, 7, 8, 9, or 10 compositions (e.g., tablets, capsules or injectables),
wherein the
administration of the multiple capsules or injections collectively provides a
subject with an
immunogenically effective amount. It is also possible to administer an
immunogenically
effective amount to a subject, and subsequently administer another dose of an
immunogenically
effective amount to the same subject, in a so-called prime-boost regimen. This
general concept of
a prime-boost regimen is well known to the skilled person in the vaccine
field. Further booster
administrations can optionally be added to the regimen, as needed.
CA 03085492 2020-06-10
WO 2019/126120 PCT/US2018/066157
According to embodiments of the application, an immunogenic combination
comprising
two DNA plasmids, e.g., a first DNA plasmid encoding an HBV core antigen and a
second DNA
plasmid encoding an HBV pol antigen can be administered to a subject by mixing
both plasmids
and delivering the mixture to a single anatomic site. Alternatively, two
separate immunizations
each delivering a single expression plasmid can be performed. In such
embodiments, whether
both plasmids are administered in a single immunization as a mixture or in two
separate
immunizations, the first DNA plasmid and the second DNA plasmid can be
administered in a
ratio of 10:1 to 1:10, by weight, such as 10:1, 9:1, 8:1, 7:1, 6:1, 5:1, 4:1,
3:1, 2:1, 1:1, 1:2, 1:3,
1:4, 1:5, 1:6, 1:7, 1:8, 1:9, or 1:10, by weight. Preferably, the first and
second DNA plasmids are
administered in a ratio of 1:1, by weight.
In some embodiments, a subject to be treated according to the methods of the
application
is an HBV-infected subject, particular a subject having chronic HBV infection.
Acute HBV
infection is characterized by an efficient activation of the innate immune
system complemented
with a subsequent broad adaptive response (e.g., HBV-specific T-cells,
neutralizing antibodies),
which usually results in successful suppression of replication or removal of
infected hepatocytes.
In contrast, such responses are impaired or diminished due to high viral and
antigen load, e.g.,
HBV envelope proteins are produced in abundance and can be released in sub-
viral particles in
1,000-fold excess to infectious virus.
Chronic HBV infection is described in phases characterized by viral load,
liver enzyme
levels (necroinflammatory activity), HBeAg, or HBsAg load or presence of
antibodies to these
antigens. cccDNA levels stay relatively constant at approximately 10 to 50
copies per cell, even
though viremia can vary considerably. The persistence of the cccDNA species
leads to
chronicity. More specifically, the phases of chronic HBV infection include:
(i) the immune-
tolerant phase characterized by high viral load and normal or minimally
elevated liver enzymes;
(ii) the immune activation HBeAg-positive phase in which lower or declining
levels of viral
replication with significantly elevated liver enzymes are observed; (iii) the
inactive HBsAg
carrier phase, which is a low replicative state with low viral loads and
normal liver enzyme
levels in the serum that may follow HBeAg seroconversion; and (iv) the HBeAg-
negative phase
in which viral replication occurs periodically (reactivation) with concomitant
fluctuations in liver
enzyme levels, mutations in the pre-core and/or basal core promoter are
common, such that
HBeAg is not produced by the infected cell.
31
CA 03085492 2020-06-10
WO 2019/126120 PCT/US2018/066157
As used herein, "chronic HBV infection" refers to a subject having the
detectable
presence of HBV for more than 6 months. A subject having a chronic HBV
infection can be in
any phase of chronic HBV infection. Chronic HBV infection is understood in
accordance with
its ordinary meaning in the field. Chronic HBV infection can for example be
characterized by
the persistence of HBsAg for 6 months or more after acute HBV infection. For
example, a
chronic HBV infection referred to herein follows the definition published by
the Centers for
Disease Control and Prevention (CDC), according to which a chronic HBV
infection can be
characterized by laboratory criteria such as: (i) negative for IgM antibodies
to hepatitis B core
antigen (IgM anti-HBc) and positive for hepatitis B surface antigen (HBsAg),
hepatitis B e
antigen (HBeAg), or nucleic acid test for hepatitis B virus DNA, or (ii)
positive for HBsAg or
nucleic acid test for HBV DNA, or positive for HBeAg two times at least 6
months apart.
According to particular embodiments, an immunogenically effective amount
refers to the
amount of a composition or immunogenic combination which is sufficient to
treat chronic HBV
infection.
In some embodiments, a subject having chronic HBV infection is undergoing
nucleoside
analog (NUC) treatment, and is NUC-suppressed. As used herein, "NUC-
suppressed" refers to a
subject having an undetectable viral level of HBV and stable alanine
aminotransferase (ALT)
levels for at least six months. Examples of nucleoside/nucleotide analog
treatment include HBV
polymerase inhibitors, such as entacavir and tenofovir. Preferably, a subject
having chronic
HBV infection does not have advanced hepatic fibrosis or cirrhosis. Such
subject would
typically have a METAVIR score of less than 3 for fibrosis and a fibroscan
result of less than 9
kPa. The METAVIR score is a scoring system that is commonly used to assess the
extent of
inflammation and fibrosis by histopathological evaluation in a liver biopsy of
patients with
hepatitis B. The scoring system assigns two standardized numbers: one
reflecting the degree of
inflammation and one reflecting the degree of fibrosis.
It is believed that elimination or reduction of chronic HBV may allow early
disease
interception of severe liver disease, including virus-induced cirrhosis and
hepatocellular
carcinoma. Thus, the methods of the application can also be used as therapy to
treat HBV-
induced diseases. Examples of HBV-induced diseases include, but are not
limited to cirrhosis,
cancer (e.g., hepatocellular carcinoma), and fibrosis, particularly advanced
fibrosis characterized
by a METAVIR score of 3 or higher for fibrosis. In such embodiments, an
immunogenically
32
CA 03085492 2020-06-10
WO 2019/126120 PCT/US2018/066157
effective amount is an amount sufficient to achieve persistent loss of HB sAg
within 12 months
and significant decrease in clinical disease (e.g., cirrhosis, hepatocellular
carcinoma, etc.).
Methods according to embodiments of the application further comprise
administering to
the subject in need thereof another immunogenic agent (such as another HBV
antigen or other
antigen) or another anti-HBV agent (such as a nucleoside analog or other anti-
HBV agent) in
combination with a composition of the application.
The ability to induce or stimulate an anti-HBV immune response upon
administration in
an animal or human organism can be evaluated either in vitro or in vivo using
a variety of assays
which are standard in the art. For a general description of techniques
available to evaluate the
onset and activation of an immune response, see for example Coligan et al.
(1992 and 1994,
Current Protocols in Immunology; ed. J Wiley & Sons Inc., National Institute
of Health).
Measurement of cellular immunity can be performed by measurement of cytokine
profiles
secreted by activated effector cells including those derived from CD4+ and
CD8+ T-cells (e.g.
quantification of IL-10 or IFN gamma-producing cells by ELISPOT), by
determination of the
activation status of immune effector cells (e.g. T cell proliferation assays
by a classical [3H]
thymidine uptake), by assaying for antigen-specific T lymphocytes in a
sensitized subject (e.g.
peptide-specific lysis in a cytotoxicity assay, etc.).
The ability to stimulate a cellular and/or a humoral response can be
determined by
antibody binding and/or competition in binding (see for example Harlow, 1989,
Antibodies, Cold
Spring Harbor Press). For example, titers of antibodies produced in response
to administration
of a composition providing an immunogen can be measured by enzyme-linked
immunosorbent
assay (ELISA). The immune responses can also be measured by neutralizing
antibody assay,
where a neutralization of a virus is defined as the loss of infectivity
through
reaction/inhibition/neutralization of the virus with specific antibody. The
immune response can
further be measured by Antibody-Dependent Cellular Phagocytosis (ADCP) Assay.
Target Tissues
Target tissues well suited for EMTAD by use of methods, apparatus and systems
described herein include both healthy and diseased cells located in, for
instance, the epidermis,
dermis, hypodermis, connective, and muscle tissue. The technique can also be
utilized for
application in healthy or diseased organs that must be accessed via minimally
invasive or other
surgical methods. Such target tissues include the liver, lungs, heart, blood
vessels, lymphatic,
33
CA 03085492 2020-06-10
WO 2019/126120 PCT/US2018/066157
brain, kidneys, pancreas, stomach, intestines, colon, bladder, and
reproductive organs. In some
embodiments, a desired therapeutic effect can be derived by use of a method,
or apparatus
described herein to deliver an amount of agent to cell types normally located
within the target
tissues as well as other cell types abnormally found within said tissues (e.g.
chemotherapeutic
treatment of tumors).
As discussed previously, and depicted in FIG. 1, traditional EMTAD suffers
from a lack
of precision and reproducibility in the spatial and temporal relationship
between the
administration of the HBV vaccine and the electrical signal. In contrast to
the traditional
EMTAD approach, the disclosure describes methods and apparatus for combined
CTAA and
ESA to provide a more advantageous clinical application of EMTAD. The
disclosure utilizes
various aspects of CTAA in conjunction with ESA to provide reproducible,
consistent, and
efficacious HBV vaccine delivery. The methods and apparatus provided herein
provide spatial
and temporal control over administration of an HBV vaccine relative to the
application of
electrical signals, thereby improving the movement and/or uptake of said agent
in the target
tissue.
Methods
In an aspect, the disclosure described herein provides systems, kits and
apparatus for use
in methods for controlled administration of an HBV vaccine to a subject in
need thereof followed
by ESA. As used herein, "subject" means any animal, preferably a mammal, most
preferably a
human, to whom will be or has been treated by a method according to an
embodiment of the
application. The term "mammal" as used herein, encompasses any mammal.
Examples of
mammals include, but are not limited to, cows, horses, sheep, pigs, cats,
dogs, mice, rats, rabbits,
guinea pigs, non-human primates (NHPs) such as monkeys or apes, humans, etc.,
more
preferably a human.
In another aspect, the disclosure described herein provides systems, kits and
apparatus for
use in methods for controlled administration of an HBV vaccine preceded by
ESA. In a further
aspect, the disclosure described herein provides systems and apparatus for use
in methods for
controlled administration of an HBV vaccine accompanied by ESA. These methods
include, but
are not limited in scope or sequential relationship to, the determination of
treatment parameters,
subject preparation procedures, CTAA, ESA, and additional measures.
Determination of Treatment Parameters
34
CA 03085492 2020-06-10
WO 2019/126120 PCT/US2018/066157
In some embodiments, treatment parameters are based on the desired amounts
and/or
duration of dosing of the HBV vaccine. HBV vaccine dosing can depend, for
instance, on the
particular indication or treatment application (such as the type and location
of the target tissue),
as well as various subject parameters (such as age and body mass). Dosing of
the HBV vaccine
can be controlled by parameters pertaining to administration of the HBV
vaccine and ESA.
Exemplary controllable parameters pertaining to CTAA include, but are not
limited to, agent
volume, agent viscosity, and injection rate. Exemplary controllable parameters
pertaining to ESA
include, but are not limited to, the characteristics of the electrical
signals, the tissue volume
exposed to the electrical signals, and the electrode array format. The
relative timing and location
of CTAA and ESA are parameters providing further control over HBV vaccine
dosing.
Subject Preparation
In embodiments described herein, methods described herein can include a
subject
preparation step. The subject preparation can include, but is not limited to,
antiseptic cleansing
and anesthetic administration, including local or regional, nerve block,
spinal block, epidural
block, or general anesthesia. In an exemplary case of intramuscular (IM) ESA,
protocols to
minimize the effects of electrical stimulation of the muscle can be included
in a method
described herein, for instance, including thermal control (e.g. cooling the
muscle), administration
of anesthetics, and/or alternative stimulation patterns sufficient for
mitigation of discomfort. It is
to be understood that the selected subject preparation techniques do not
adversely affect
therapeutic efficacy, if acceptable alternatives exist. For example, it has
been shown that in some
cases, the intramuscular administration of amide based anesthetics can have an
undesirable effect
on intramuscular delivery plasmid DNA-based therapies, putatively due to the
mild myotoxicity
of these agents, which can inhibit the muscle cells ability to express the
protein encoded by the
administered DNA sequence.
CTAA and ESA
In some embodiments described herein, is a method wherein CTAA and ESA are
combined, enabling consistent and reproducible HBV vaccine delivery. In some
cases, are
provided apparatus or kits suitable for CTAA, including for instance apparatus
comprising at
least one of automatic injection devices and jet injectors.
The disclosure provides methods, kits and apparatus enabling the
transcutaneous
deployment of a plurality of elongate electrodes to a target depth in a safe
and consistent manner
CA 03085492 2020-06-10
WO 2019/126120 PCT/US2018/066157
with respect to a target site of agent distribution in recipients with
heterogeneous skin thickness
and composition in order to support the application of electrical fields in
tissue to enhance the
intramuscular, intradermal, and/or subcutaneous administration of therapeutic
or prophylactic
agents such as nucleic acids, pharmaceuticals, antibodies, peptides, proteins,
or combinations
thereof.
Systems and methods according to present principles enable the consistent
transcutaneous
deployment of a plurality of elongate, tissue penetrating electrodes to a pre-
determined target
tissue site in order to propagate electrical fields in the skin, subcutaneous
tissue and/or skeletal
muscle. The disclosure provided herein is designed to enable a user with
minimal training to
consistently deploy the electrodes to a target depth while maintaining the
proper spatial
relationship among the plurality of electrodes, even when the procedure is
applied at sites with
varying skin characteristics. Such variation can be due either to variation in
skin characteristics
at different sites within an individual or among heterogeneous recipient
populations. In other
words, systems and methods according to present principles should allow a
consistent profile
irrespective of the administrator or the recipient. In certain embodiments,
the deployment of the
electrodes is accompanied by the insertion of one or more injection needles
which are configured
for the administration of HBV vaccines to the target region of tissue and
arranged in a pre-
determined spatial relationship with the electrodes to be used for ESA. In an
exemplary
embodiment, the electrodes are arranged such that any electrical signals from
said electrodes are
preferentially applied distal to the site of HBV vaccine administration by the
insertion of one or
more injection needles. In another embodiment, the electrodes are arranged
such that any
electrical signals are preferentially applied proximal to the site of HBV
vaccine administration
by the insertion of one or more injection needles.
Aspects of the disclosure can be used singly or in combination to support the
transcutaneous insertion of electrodes for the in vivo application of
electrical fields to enhance
the intramuscular, intradermal, and/or subcutaneous administration of nucleic
acids, small
molecule drugs, antibodies, peptides, proteins, and combinations thereof. In
some embodiments,
the electrode deployment and subsequent electrical field propagation are
performed in
coordinated fashion with the distribution of the agent of interest to the
target tissue site. In an
exemplary embodiment, the administration of the agent and the application of
one or more
electrical fields are performed in a controlled and monitored fashion such
that the probability of
36
CA 03085492 2020-06-10
WO 2019/126120 PCT/US2018/066157
achieving spatial and temporal co-localization of the distribution of the
agent with the site of
electric field application is maximized.
In general terms, the disclosure provides methods and apparatus for the
transcutaneous
deployment of electrodes to a predetermined site within the skin, subcutaneous
tissue, and/or
skeletal muscle of a recipient in conjunction with the administration of an
agent of interest and
the local application of electrical fields to improve the delivery, uptake,
and/or biological effect
of the agent. In some embodiments, the disclosure has been implemented such
that set up and
usage of the device can be performed effectively and reliably by users with
minimal training. In
another embodiment, the disclosure also comprises the implementation of
numerous interlocks,
sensors, and feedback loops to reduce the frequency and/or potential impact of
potential user
errors committed during the set up and use of the device. Referring to FIG. 2,
an embodiment of
an apparatus described herein comprises a "cartridge assembly" 100 detachably
interfaced to an
"applicator" 400 which is configured to connect to a controller 700 which acts
as source of
electrical energy for electrode activation and subsequent electrical field
generation, as well as
diagnostics and other control routines. The controller 700 further provides a
user interface, tray,
holster for the applicator 400, and various other features are described. As
seen in FIG. 2, a
reservoir 101 of suitable uniform size and general shape can inserted in the
cartridge assembly
100 in a method of use.
Details of a cartridge assembly 100 present in some embodiments of an
apparatus
described herein, are described for instance, in FIGS. 3A-12, along with
corresponding
cooperating portions of the applicator 400, in Figs.FIGS. 13A-18C, followed
by, where relevant,
portions of the controller 700, in FIGS. 19-20D. Remaining portions of the
applicator 400 are
described next, followed by remaining portions of the controller 700.
Referring in addition to FIGS. 3A-4, in some embodiments described herein, the
cartridge assembly 100 can comprise a support structure configured to
interface with the
applicator 400 and accommodating two or more elongate electrodes 122 mounted
on the
structure to form an array. To avoid unwanted propagation of electrical
currents within the
device, the design and materials of the electrode mounting structure should be
specified such that
there is an adequate dielectric barrier between electrodes of opposite
polarity within the device.
The distal region 137 of the elongate electrodes are engaged with the mounting
structure using
37
CA 03085492 2020-06-10
WO 2019/126120 PCT/US2018/066157
standard mechanical features and/or bonding agents appropriate for the
material composition of
the electrode mount structure and the electrodes.
In an exemplary embodiment, the cartridge outer housing structure 102 is
configured to
interface with a fluid reservoir 101 containing the HBV vaccine where the
reservoir 101 and
cartridge housing structure 102 are configured to operatively connect to at
least one injection
orifice (needle 105) through which the HBV vaccine is administered into the
target tissue. In
some embodiments, this configuration facilitates the co-localization of the
distribution of the
agent of interest with the site of electrical field application. In another
embodiment, this
configuration facilitates the implementation of a pre-determined spatial
relationship between the
apparatus for ESA and CTAA. In yet another exemplary embodiment, a syringe 101
is inserted
into a cartridge 100, wherein upon loading of the cartridge into an applicator
400, the syringe
101 moves forward to mate with the needle hub 152 and to connect the cartridge
to the needle.
Certain embodiments of the disclosure can include the use of syringes, vials,
ampoules,
cartridges or equivalent structures for storing one or more HBV vaccines. In
some embodiments,
the reservoirs can comprise at least one of glass and plastic, with the
material selected for
compatibility with the agent of interest. Coatings can be applied to the
reservoir used to provide
desirable lubricious or protective properties. As disclosed above, the
electrodes 122 can be
hollow and in some cases configured with injection orifices that can be
operatively connected to
the fluid reservoirs. Alternatively, the injection orifice can comprise one or
more hypodermic
needles and/or needle free injection ports positioned relative to the
electrodes. Selection of the
type and size of injection orifice can dependent on the desired route of
administration, tissue
distribution, and physical characteristics of the agent of interest. In a
certain embodiment, the
cartridge structure is designed to ensure a pre-determined spatial
relationship between the
injection orifice and electrodes in their deployed state such that the
distribution of the agent of
interest occurs substantially in the tissue bounded by the conductive regions
of the plurality of
electrodes. To minimize the need for handling of sharps by the user, in a
certain embodiment, a
cartridge 100 designed for use with hypodermic needles is configured to allow
the hypodermic
needle to be mated to the cartridge at the time of manufacture rather than the
more common
convention of mating the needle to the syringe at the time of use. In certain
embodiments, an
aspect of the disclosure can incorporate features in the cartridge and/or the
needle to ensure
retention of the needle during manufacture, distribution, handling, and use as
well as features to
38
CA 03085492 2020-06-10
WO 2019/126120 PCT/US2018/066157
ensure that proper mating of the reservoir to the needle prior to the use. In
some embodiments,
such features can minimize the risks of leakage of the agent from the
reservoir or the reservoir
orifice interface due to breakage or improper mating.
In certain embodiments, the cartridge can include a tissue contact interface
located at the
distal end of the subassembly. In certain embodiments, the tissue contact
interface comprises a
substantially planar structure oriented perpendicular to the elongate
orientation of the electrodes
and having one or more apertures configured to allow passage of the electrodes
through the
tissue contact interface. For embodiments incorporating an integrated
reservoir and injection
orifice, the interface also has apertures to accommodate injection orifice or
needle free injection.
To minimize the risk of contamination of the electrodes and injection needle
as well as the
occurrence of unintended sharps exposure to the user or recipient, in a
certain embodiment, the
apertures accommodating passage of the electrodes and injection needle are of
a size suitable to
prevent accidental contact with the electrodes and injection needle. Most
commonly, the tissue
contact interface is comprised of one or more plastics suitable for at least
short term tissue
contact.
To avoid potential cross contamination of biological material between
recipients, the
cartridge assembly 100 can be configured for single use. In some cases, the
cartridge includes
one or more mechanical, electrical, and/or identification elements which
restrict use of the
cartridge to a single administration. Examples of mechanical elements of this
nature include for
instance, but are not restricted to, lockouts and/or detents which secure the
electrode mount
structure and/or the stick shield (see below) in the deployed state after use.
Examples of
electrical elements include for instance, fuses or links configured in series
with one or more
electrodes which are deactivated by the source of electrical energy at the
conclusion of the first
use of the cartridge. Examples of identification elements include, for
instance, serialized radio
frequency identification devices, bar codes, or quick response codes
configured to be read by the
applicator and/or the source of electrical energy. The identification
information for a specific
cartridge can then be used to prevent accidental or intentional re-use of that
cartridge by the
applicator and/or the source of energy. In an embodiment, one or more
redundant features are
incorporated to minimize the potential for re-use of the cartridges.
In an embodiment of an apparatus described herein, as shown in FIG. 2, the
applicator
400 comprises a support structure configured to interface with the cartridge
assembly 100, a user
39
CA 03085492 2020-06-10
WO 2019/126120 PCT/US2018/066157
interface 410-418 (FIG. 13B), electrically conductive electrical connections
configured to
provide operative connection between the conductive contact region located on
the distal region
of the elongate electrodes and the source of electrical energy when the
electrodes are deployed
into the target tissue of the recipient. In some cases, the user interface
comprises a handle, one or
more display features designed to convey information to the user, and one or
more features
capable of accepting user input. In certain embodiments, the display features
are configured to
convey the operating status of the device during its set up and use as well as
relevant warning /
error messages. Such displays can comprise mechanical features, lights,
alphanumeric displays,
and/or electronic display screens. In some cases, the features capable of
accepting user input are
configured to allow the user, at the appropriate stage of the procedure, to de-
activate safety
features within the device preventing accidental discharge, make selections
regarding particular
parameters of the procedure (e.g., the intended depth of injection), and to
initiate procedure
administration and can include buttons, triggers, mechanical slides, and/or
levers.
In certain embodiments, the applicator 400 also includes actuation mechanisms
which
interface with the cartridge assembly and which are configured for
transcutaneous deployment of
the electrodes, positioning of the injection orifice relative to the target
tissues, discharge of the
agent of interest from the reservoir through the orifice and into the target
tissue site, and/or
conveying electrical signals from an electric field generator such as a
controller 700 to the
cartridge 100. The applicator 400 can be configured such that the energy to
actuate the
mechanisms is supplied by the user, or more preferably, the apparatus may
incorporate one or
more inanimate sources of energy operatively connected to the actuation
mechanisms within the
applicator. Such inanimate sources of energy include for instance,
electromechanical devices
(solenoids, motors, lead screws), mechanical components (springs and related
devices), and
compressed gases.
An exemplary implementation of a cartridge assembly 100 is as described in
FIG. 3A, a
cartridge assembly 100 includes a reservoir loading port 140 and a reservoir
containment volume
142 to receive and contain a reservoir 101 of a medicament. A cartridge
assembly 100 is
required because, for a device in which an electrical field is to be generated
and used as part of a
therapy, an electric field generator such as a controller 700 is required to
electrically interface
with the device containing the electrodes configured to contact the target
tissue. As the
controller can be configured for multiple uses, and the reservoir 101 can be
intended for single
CA 03085492 2020-06-10
WO 2019/126120 PCT/US2018/066157
use, the cartridge assembly 100 can be present to hold the electrodes 122 and,
the reservoir 101,
to interface with the re-usable device, and for the cartridge assembly 100 to
be configured for
single use. Thus, the applicator 400 can be a reusable component and the
cartridge assembly 100
can be configured for single use. The cartridge assembly 100 can also be
rendered in a condition
to prevent subsequent use if errors or tampering occur in the insertion of a
reservoir 101, or if
defects are present.
The term reservoir 101 can refer to a syringe, vial, or any other device which
can contain
a medicament or HBV vaccine and which can interface with a device having an
orifice, such as a
needle, shown in FIG. 4 as a needle 105 having a needle hub 152. The reservoir
101, for a given
type of cartridge assembly 100, generally has a common shape and size. Various
components
within the cartridge assembly 100 allow for a leeway in exact sizing and/or
manufacturing
tolerances, but generally a common shape and size are required to reduce the
risk that drugs not
labeled for use with cartridge 100 are erroneously delivered with the device.
If an appropriately
sized reservoir 101 is not provided by the user, one or more interlocks within
the cartridge
assembly 100 can be unable to deactivate, and the system can be rendered
unusable until a
proper-sized reservoir 101 is inserted.
As seen in FIG. 3B, the reservoir 101 generally can be equipped with a plunger
and a
port 156 for drug egress. A removable cap 158 can also be provided to maintain
the sterility and
integrity of the agent until such time as the reservoir is to be inserted and
used. The port for drug
egress can be proximal to the needle hub 152 in use, and the plunger can be
opposite this port for
drug egress. As an alternative to an open port for drug egress, in some
embodiments, the
reservoir can be configured with a septum component which covers and seals the
end of the
container opposite the plunger. The septum can be made of elastomeric
compounds such as
silicone or butyl rubber, with the specific formulation and coating of the
material selected for
stability and compatibility with the agent contained within the reservoir. The
septum component
is typically held in place by a crimp seal or other fastening mechanism. This
septum seal
configuration obviates the need for a removable cap, but requires that needle
105 be equipped
with a suitable piercing member such as a needle, spike, or other features to
access the fluid
contained in the reservoir. Specific implementations for this configuration
include dual sided
needle configurations and spike vial adapters.
41
CA 03085492 2020-06-10
WO 2019/126120 PCT/US2018/066157
The cartridge assembly 100 is not only configured for receiving the reservoir
101 but also
for being received by an applicator 400 in an applicator cartridge assembly
receiving port 401
(FIG. 2). Thus, the cartridge assembly 100 includes a device allowing the
applicator 400 to pull
and retain the same within an interior volume, at least partially. In certain
embodiments, the
device is one or more racks on the surface(s) of the cartridge assembly 100
that engage a
corresponding motorized pinion assembly in the applicator 400. In other
implementations, the
applicator 400 can interface with the cartridge assembly 100 without the need
to pull the same
into an interior volume. In yet other implementations, other techniques can be
employed to
cause the cartridge assembly 100 to engage the applicator 400, e.g., motorized
tracks or brackets
and the like onto which the cartridge assembly 100 can interface.
The applicator 400 is further provided with interface elements allowing the
same to
control certain actions within the cartridge assembly 100. In particular, the
applicator 400 can be
configured to control needle insertion, medicament delivery, electrode
insertion, and electrode
activation using various subsystems. In some cases these steps are linked, so
that a single action
of applicator 400 initiates multiple of these steps. In some implementations,
all of these steps but
the medicament delivery and electrode activation are caused by a single
action, as described in
the exemplary implementation below.
Upon appropriate activation, such as the use of electrical or optical signals
conveyed to
mechanical, electrical, or optical elements of the cartridge assembly 100, the
applicator 400 can
be enabled to test subsystems within the cartridge assembly 100, ensuring that
the same are
operating properly and are properly configured for medicament delivery with
electric field
application. For example, such subsystems include that the applicator 400 can
test to ensure that
the cartridge assembly 100 has not been previously used, that the reservoir
has been properly
placed within the cartridge assembly, that appropriate force applied against
the body of a subject
as applied through an alignment guide / splay shield 108, a test that a depth
has been
affirmatively selected by the user, and that an exterior cartridge cap 110 has
been removed.
Moreover, the applicator 400 can be configured to monitor the status of the
cartridge functions
during execution of the procedure. For example, such subsystems include that
the applicator 400
can test to ensure that that electrodes 122 are properly deployed within a
subject prior to
commencing with administration of the medicament, that the plunger in the
reservoir has been
appropriately actuated prior to application of the electrical fields, that the
user has maintained
42
CA 03085492 2020-06-10
WO 2019/126120 PCT/US2018/066157
appropriate force applied against the body of the subject during the
administration procedure,
and so on.
In addition to the subsystems that are operated by the applicator 400, the
cartridge
assembly 100 can incorporate appropriate subsystems, including those that
interact with the
applicator 400 and those that do not so interact, so as to accomplish the
goals of the medicament
delivery and electric field application therapy. These include a subsystem for
causing needle and
electrode insertion, a subsystem for protecting users from sharps following
therapy
administration, a subsystem for providing different depths of
needle/electrodes insertion, a
subsystem for ensuring that adequate force has been applied against the tissue
of the recipient
prior to allowing initiation of the procedure and subsequently during
application of the
administration procedure and so on. While often described in the context of
deployable needles
and electrodes, it is noted here that such are not strictly required, and that
systems with non-
deployable or fixed needles and electrodes also benefits from systems and
methods according to
present principles, including the subsystems described.
In one exemplary implementation, as shown in FIG. 4, the cartridge assembly
100
includes an outer cartridge 102, in some cases termed a housing. The outer
cartridge 102 is
terminated at a distal end by an outer cartridge cap 106. The outer cartridge
102 includes an
inner cartridge containment volume 150, for receiving an inner cartridge 103,
which is received
and moves in a slidable manner in relationship to the outer cartridge 102. The
inner cartridge
103 includes a reservoir containment volume 142 in which the reservoir 101 can
be situated.
The inner cartridge 103 engages with an inner cartridge cap 104 at a distal
end. The inner
cartridge cap 104 has a number of functions, including to lock electrodes 122
in place (the inner
cartridge 103 itself has seams that the electrodes 122 are placed into) and to
provide a bearing
surface for a stick shield 134. The inner cartridge cap 104 locks onto the
inner cartridge 103.
A cartridge breech 112 is received in a portion of the reservoir containment
volume 142
in the inner cartridge 103, in a portion opposite that of the inner cartridge
cap 104. A reservoir
detection cap 118 engages the cartridge breech 112 through a reservoir
detection spring 116. A
cartridge lock ring 114 locks the system in place, including the cartridge
breech 112 to the inner
cartridge 103. The reservoir detection spring 116 also serves to push the
reservoir 101 into
engagement with the needle hub 152, and also serves to accommodate tolerances
in the size of
reservoir 101.
43
CA 03085492 2020-06-10
WO 2019/126120 PCT/US2018/066157
A reservoir interlock 120 provides a mechanical interlock to prevent
inadvertent or
unwanted actuation of cartridge functions. In particular, the reservoir
interlock 120, also termed
a first reservoir insertion trigger, is placed below the inner cartridge 103
and has fingers that
extend through slots or holes defined in the inner cartridge 103 (see FIG.
5B). The fingers
prevent the cartridge breech 112 from slidably moving relative to the inner
cartridge 103, and in
particular from moving within the inner cartridge 103 towards the inner
cartridge cap 104 before
a reservoir has been inserted into a reservoir containment volume 142.
When a reservoir 101 is properly inserted in the reservoir containment volume
142, the
reservoir interlock 120 is pushed down and the fingers are pushed down, no
longer extending
into the reservoir containment volume 142. This pushing down or depression of
the reservoir
interlock 120 may also be configured to provide an audible, tactile, or haptic
"click" that can
inform the user of proper insertion. Once depressed, the cartridge breech 112,
no longer blocked
by the fingers of reservoir interlock 120, is then permitted to move, and in
particular is permitted
to move in the direction towards the inner cartridge cap 104.
The cartridge breech 112 is caused to move such by the action of the spring
cap /
cartridge interface 470 when the cartridge assembly 100 is inserted in the
applicator 400 in a
fashion described below. When the cartridge breech 112 moves far enough
forward, it locks in
place, securing the reservoir 101 in the reservoir containment volume 142 and
ensuring that it is
properly positioned relative to the needle hub 152 to ensure an intact fluid
pathway from the
reservoir 101 to the orifice of needle 105.
For embodiments of the device where the injection needle is incorporated into
the
cartridge 100, the use of standard "off the shelf' single use hypodermic
injection needles can be
employed within the device. However, the operational and reliability
characteristics of the device
can be improved through the incorporation of customized design elements that
are not present in
hypodermic needles intended for conventional parenteral administration
procedures. Specific
aspects of the needle hub 152 can include the material from which it is
comprised, the inclusion
of retention features to prevent the needle hub 152 from becoming dislodged
from inner cartridge
103 during distribution and use, and the orientation of any bevel features in
the needle relative to
the hub.
Conventional single use disposable injection needles are commonly comprised of
injection molded polypropylene thermoplastic. However, for many applications,
the impact
44
CA 03085492 2020-06-10
WO 2019/126120 PCT/US2018/066157
strength, tensile strength, and flexural strength of polypropylene may not be
adequate to ensure
integrity of the hub when subjected to the forces characteristic of needle
deployment and
injection with this device. Specific failures of concern include failure in
the hub wall due to
impact or injection forces as well as failure of the hub needle joint due to
same. While
adjustments in the design of the hub, including its geometry and wall
thickness can be utilized to
address prevent these failures, it is not always feasible to modify the design
sufficiently to
prevent hub failure while ensuring that the hub retains the dimensional
properties required for
proper mating to a conical male luer slip connectors as described in the
relevant standard
published by the International Standards Organization (ISO) ISO 80369-7:2016
Small-bore
connectors for liquids and gases in healthcare applications -- Part 7:
Connectors for intravascular
or hypodermic application. Specifically, given the forces that the syringe
needle and hub are
subjected to during deployment, in certain embodiments, a material with
improved impact
strength, tensile strength, and flexural strength is used. One example is the
use of injection
molded polycarbonate plastics (such as ZELUX GS, Makrolon, or Lexan) or
copolyesters
(such as Eastman TritanTm Copolyester MX731, MX711, and MX 730). When assessed
according to ISO 180:2000 Plastics ¨ Determination of Izod impact strength, a
notched impact
strength of at least 70 kJ/m2 is considered suitable for this application.
When assessed according
to the ISO 527-1:2012 Plastics -- Determination of tensile properties -- Part
1: General
principles, a tensile strength of at least 30 MPa is considered suitable for
this application. When
assessed according to ISO 178:2010 Plastics -- Determination of flexural
properties, a flexural
strength of at least 50 MPa is considered suitable for this application. In
some embodiments, the
specific resin selected exhibits compatibility with the intended method of
sterilization (e.g.,
gamma radiation) without exhibiting detrimental changes in its physical
properties that could
compromise its function.
For embodiments where a custom injection needle is utilized, one or more
mechanical
features are included which are not ordinarily present on conventional syringe
hubs that enable
the device to be inserted into inner cartridge 103. Such features can include
tabs, snaps, or ridges
with corresponding mechanical features located on inner cartridge 103. In some
cases, the
features are implemented such that the hub mates with the inner cartridge 103
in a consistent
orientation. Combined with a needle manufacturing process that is capable of
consistently
orienting the bevel or other needle orifice feature, this insures that biases
in injection location or
CA 03085492 2020-06-10
WO 2019/126120 PCT/US2018/066157
medicament distribution due to the location and design of the orifice can be
accounted for in the
design of the device. For example, needles with asymmetrical penetrating tip
features (e.g., a
bevel cut) can exhibit a directional bias during deployment into tissue due to
interaction between
the tissue and the asymmetrical penetration feature on the needle. If the
electrodes have a
symmetrical penetrating tip feature (e.g., a trocar tip) then the electrodes
would not exhibit a
corresponding bias in their deployment characteristics. Therefore, mounting
feature for needle
hub 152 on inner cartridge 103 can include an offset in the position of the
injection orifice on
needle 105 relative the electrodes 122 prior to deployment to account for the
expected
deployment characteristics of the asymmetrical bevel of the needle. The
precise dimension of the
offset can depend on the nature of the target tissue and the expected range of
penetration depths,
but in certain embodiments the needle is offset by 0.5-1 mm for each 10 mm of
penetration
depth. When using electrodes and injection needles with differing tip profiles
or where the tip
profiles must be consistently oriented with one another, such features are
advantageous for
insuring co-localization of the medicament distribution with the application
of the electrical
fields.
The incorporation of a syringe detection cap 118 mounted to a syringe
detection spring
116 ensures that the cartridge assembly 100 can accept and properly position
the syringe 101
relative to needle hub 152 across the range of manufacturing tolerances
expected for syringe 101.
The applicator 400 causes the cartridge breech 112 to move forward during the
loading
procedure when the spring cap / cartridge interface 470 within the applicator
400 moves distally,
relative to the cartridge assembly 100. This action occurs when the cartridge
assembly 100 is
loaded into the applicator 400 and the cartridge assembly 100 is pulled into
the applicator 400,
e.g., by the action of a loading mechanism, e.g., a rack-and-pinion mechanism
described below.
The movement of the cartridge breech 112 can act as a second interlock. In
particular, in
one implementation, as seen in FIGS. 5C-5D, a line of sight is visible and
detectable, by an
appropriately configured sensor, within the cartridge assembly receiving
volume 403, through a
set of reservoir locking holes 144' (FIG. 5D). This line of sight is visible
when the cartridge
assembly 100 is loaded into the applicator 400. The visible light of sight, or
occlusion of the
same, can act as part of a second interlock that must be deactivated for the
controller 700 to
allow activation and triggering of the device, including needle and electrode
insertion,
medicament delivery, and electrode activation.
46
CA 03085492 2020-06-10
WO 2019/126120 PCT/US2018/066157
For example, in one implementation, the reservoir locking holes 144' (FIG. 5D)
must be
occluded for the device to operate. If a visible line of sight is present,
e.g., as detected by an IR
or visible light emitter and detector paired within the cartridge assembly
receiving volume 403 of
the applicator, the device can be rendered inoperable and generate and display
an error message
on the applicator display 404 and/or controller 700, including the controller
display 712, to notify
the user of the state of the device as well as the recommended steps to be
performed to address
the error.
Thus, in this implementation, one error state can be that no reservoir 101 was
loaded into
the cartridge 100 or that the syringe was not properly seated into the inner
cartridge 103. In this
case, the reservoir interlock 120 cannot be depressed as there is no reservoir
101 to perform this
action. In this case, the cartridge breech 112 cannot be moved forward, in the
distal direction,
towards the inner cartridge cap 104. The construction of these components can
be such that an
open breech state results in an open line of sight 146 through first reservoir
locking holes 144'
(FIG. 5D). An ancillary check is that the cartridge breech 112 cannot be
closed, and this can
manifest itself as an inability of the cartridge to move the required distance
backward or distally
into the cartridge assembly receiving volume 403. As these conditions are
defined by the system
to be an error state, the same can be identified and used in the generation of
an error message,
e.g., with an appropriate message to the user on a user interface on the
applicator display 404
and/or controller 700, including the controller display 712. A similar error
state may occur if the
reservoir 101 is improperly loaded, or if the reservoir interlock 120 is
damaged. Generally in
this case, an appropriate error message can be accompanied by instructions to
the user to remove
the cartridge, reinstall a new reservoir, and attempt to reintroduce the
cartridge assembly 100 into
the applicator 400.
Another error state can be that the cartridge breech 112 is moved forward
manually by
the user without a reservoir 101 present, such occurring by the user manually
pushing the
reservoir interlock 120 out of the reservoir containment volume 142. This
situation can also be
defined as an error state, and the same can be detected because another
(second) set of reservoir
locking holes 144 (FIG. 5C) are placed on a portion of the outer cartridge
102. If no reservoir is
in place, but the cartridge breech 112 is moved forward by the action of the
reservoir interlock
120 being depressed, then the reservoir locking holes 144' (FIG. 5D) align
with the reservoir
locking holes 144 (FIG. 5C), again creating an open line of sight 146 and a
subsequent error
47
CA 03085492 2020-06-10
WO 2019/126120 PCT/US2018/066157
state. This error state may also occur if the reservoir interlock 120 is
damaged and its fingers are
no longer within the reservoir containment volume 142. In a certain
embodiment, this error state
need not be not remediable by attempting to reinstall a reservoir, as a
reservoir may not fit in the
reservoir containment volume 142 with the cartridge breech 112 locked. In a
certain
embodiemnt, a new cartridge assembly 100 is required.
In contrast, if an appropriately sized reservoir 101 is positioned properly in
place, then
the reservoir detection cap 118 is pushed back against the reservoir detection
spring 116, and the
movement of the reservoir detection cap 118 occludes the reservoir locking
holes 144 (FIG. 5C)
and the reservoir locking holes 144' (FIG. 5D). In this case there is no error
state, allowing the
device to operate. The occlusion, and detection of the occlusion, occurs
within the body of the
applicator 400, after the cartridge assembly 100 is inserted, and thus is
insusceptible to user
attempts to defeat this interlock, whether intentional, accidental, or caused
by a defect. It is
noted that this "no error" state still occurs even if the user intentionally
or inadvertently closes
the cartridge breech 112 themselves during handling of the cartridge 100.
Depending on which error state occurs, the cartridge assembly 100 can remain
usable or
not. If the cartridge breech 112 has been locked into place, the cartridge
assembly 100 is
rendered unusable. If, however, the cartridge breech 112 has not been locked
into place, then the
cartridge assembly 100 can be removed from the applicator 400 and a new
reservoir 101
inserted.
While the above-noted set of two interlocks (one mechanical using reservoir
interlock
120 and one using a light emitter and collector and reservoir lockout holes
144 and 144') have
been found particularly useful in some implementations, it is to be understood
that other types of
interlocks can also be employed (FIGS. 5C-5D). For example, instead of having
an error state
occur when the reservoir locking holes 144 are not occluded, the error state
can be configured to
occur (via a change in reservoir locking hole location and program logic) when
the reservoir
locking holes 144 are occluded (and the clear line of sight 146 then
corresponding to the non-
error state). The reservoir interlock 120 can incorporate additional
mechanical flag features that
are recessed in the cartridge when the interlock is active, but become visible
to an appropriately
configured sensor when the syringe 101 has been properly inserted and the
reservoir interlock
120 is depressed into its released position. In other variations, other ways
can be employed to
determine if a line of sight is present, e.g., optical, acoustic, electrical,
or the like, so long as a
48
CA 03085492 2020-06-10
WO 2019/126120 PCT/US2018/066157
suitable emitter and collector can be positioned within the applicator 400.
Other ways may also
be employed to determine if the reservoir 101 is properly loaded, e.g.,
mechanical techniques, as
it is to be understood by one of ordinary skill in the art given this
teaching. Depending on
implementation, if an error state is detected, the applicator 400 can be
prevented from operating
either with the cartridge assembly 100 in place, or the applicator 400 can be
prevented from even
accepting the cartridge assembly 100 in the first place. For example, the
cartridge can be
designed such that the reservoir interlock 120 includes mechanical tab or
locking features which
extend from one or more of the cartridge surfaces until a syringe 101 is
properly inserted into the
cartridge 100. The mechanical tabs are designed to interact with a
corresponding detent feature
located in the applicator 400 such that loading of the cartridge 100 into the
applicator 400 is
physically blocked unless the reservoir interlock 120 is released. This
mechanical interaction
would provide feedback to the user or the system that an error condition must
be resolved before
proceeding with the loading of the cartridge 100 into applicator 400. In other
variations, more or
less than two interlocks can be provided, although the same can be
correspondingly associated
with a different safety profile.
In certain implementations, other features can also be employed in the above
determinations, or to enhance the above determinations. For example, where a
motor is
employed to pull the cartridge assembly 100 into the applicator 400, sensors
can be employed as
described below to detect the spatial position of the cartridge assembly 100
during the insertion
process. Put another way, the applicator 400 may detect where the cartridge
assembly 100 is
within the cartridge assembly receiving volume 403. In some cases, such may
allow
determination of additional error states either directly, or by prompting the
activation of
additional sensors to assess the state of the device. For example, in a used
cartridge, the
cartridge breech 112 is locked into position. If the used cartridge assembly
100 is attempted to
be re-used, the optical detector detects the cartridge assembly 100 at a
different point than it
would for an unused cartridge assembly 100. The use of an electrical motor for
one or more
system functions also provides the opportunity to monitor its operational
status including the
voltage and current levels supplied to the motor as well as the number of
revolutions that the
motor has performed during a specific operation. Measurement of these
quantities during system
operation can be used as a primary or secondary method for detecting potential
or actual fault
conditions. For example, the use of mechanical interlocks designed to block
the loading
49
CA 03085492 2020-06-10
WO 2019/126120 PCT/US2018/066157
procedure when the cartridge 100 is not properly configured can be coupled
with sensors and
logical circuits monitoring the motor to ensure proper loading of the
cartridge 100 into applicator
400. For example, the interaction of the mechanical features described above
that are designed to
prevent the loading of cartridge 100 without a properly inserted reservoir 102
would result in
increased load on the motor drive mechanism, resulting in a higher current
draw. Detection of
the elevated current draw by the motor would prompt the loading procedure to
be halted and a
fault condition displayed to the user, for example on stimulator display 712
and/or applicator
display 404.
Since it is possible that medicaments not intended for administration by this
method
could be contained within reservoirs of similar size and configuration as that
intended for use by
this delivery method. Therefore, an additional aspect of the system is the
incorporation of one or
more methods to ensure that the reservoir 101 inserted into the cartridge 100
by the user is
specifically, intended for use with the device. The implementation of such
features would reduce
the risk that an incorrect medicament is administered to a given subject.
Customarily, specific
information in the user instructions and labeling of the medicament includes
the route and
method of administration. However, to further reduce the potential for user
errors, the
incorporation of mechanical, optical, and/or electrical features within the
reservoir and device
can be desirable. In an embodiment, the syringe can be designed to incorporate
one or more
unique mechanical features that are not present in other reservoirs which can
be similar to those
designed for use with this delivery method. For example, the reservoir can be
specified to
incorporate a rib or other elongate feature on the flange or barrel of the
reservoir. In this
embodiment, a corresponding mating feature would be included on the reservoir
interlock 120
such that the reservoir interlock would be deactivated only if a reservoir
with the appropriate
mating feature were properly inserted into the device. In the event that it is
not feasible to
directly implement the feature in the design of the reservoir, an alternative
embodiment would
include the placement of a secondary mechanical component on the reservoir
that would be
unique to reservoirs intended for use with the device. For example, a ring or
other appropriately
configured feature designed to slide over the barrel of the reservoir can be
used to "key" the
reservoir for use with the device by mating with a corresponding feature in
the outer cartridge
102, inner cartridge 103, reservoir interlock 120, reservoir detection cap
118, or other suitable
feature within the cartridge 100. An additional embodiment a custom label of
suitable size, color,
CA 03085492 2020-06-10
WO 2019/126120 PCT/US2018/066157
and/or electrical conductivity applied to a pre-determined location on the
outer surface of
reservoirs that are intended for use with the device. Corresponding optical or
electrical sensors
in applicator 400 would be configured to assess the presence or absence of the
label on the
surface of the reservoir in order to verify that the medicament inserted into
the cartridge is
intended for use with the device. The detection method would comprise the use
of optical or
electrical signals applied to the surface of the label in order to assess its
presence or absence. In
this way, reservoirs containing medicaments not intended for use with the
device (and therefore
missing the relevant label) could be detected and excluded from potential
misuse.
A configuration of sensors is now described to perform the cartridge loading
and syringe
detection determination described above, such sensors further forming a
portion of a cartridge
loading subassembly within the application 400. In more detail, and referring
in addition to
FIGS. 6, 17B, and 18C, an exemplary way of detecting where the cartridge
assembly 100 is
located is by use of a cartridge loading sensor 436 and a cartridge loaded
sensor 438, which
forms a portion of a loading drive subassembly 454, the subassembly 454
further including
cartridge guide rails 442 and a loading motor 444 which has a connection to a
pinion gear
assembly 448 that pulls the cartridge assembly 100 into the cartridge assembly
receiving volume
403 via racks 154 on the base of the outer cartridge 102. In more detail, when
the cartridge
loading sensor 436 detects an initiating flag 172 on the cartridge assembly
100 (see FIG. 6), the
motor can be caused to initiate loading. When the cartridge loaded sensor 438
detects the same
flag, loading can be caused to cease. A continuing flag 174 can be employed
that is required to
be present for loading to continue.
The first "teeth" of the rack 154 can be configured to provide a tactile
sensation (or
audible or haptic) for the user when they are inserting the cartridge assembly
100 into the
cartridge assembly receiving volume 403. Such configuration may include the
shape and/or size
of the rack teeth 154 as well as the amount of flexion permitted by their
positioning in the outer
cartridge. By adapting the rack teeth implementation, the desired degree of
tactile feedback can
be achieved while ensuring that it does not provide a significant force
against the loading motor
444 from receiving and loading the cartridge assembly 100.
Referring in addition to FIG. 17A, and as noted above, the cartridge assembly
100 is
inserted into a cartridge assembly receiving volume 403 within the applicator
400. While
various ways can be employed to perform this insertion, one way that has been
found particularly
51
CA 03085492 2020-06-10
WO 2019/126120 PCT/US2018/066157
useful is by way of a pinion gear assembly 448 engaging racks 154 on the outer
cartridge 102.
The use of more than one rack provides additional stability, particularly
torsional stability during
the loading phase. Referring also to the insertion/injection drive assembly
456 of FIG. 18A, in
addition to drawing the cartridge assembly 100 within the cartridge assembly
receiving volume
403, the insertion action further compresses an electrode / needle insertion
spring 472 through a
spring cap / cartridge interface 470. The electrode / needle insertion spring
472 is used as the
primary driving force for the needle and electrode insertion during medicament
delivery.
This hybrid motor/spring action provides numerous benefits. The motor drive is
beneficial as it is highly controllable and allows the cartridge 100 to be
loaded into the applicator
400 in a semi-automated fashion with minimal input of mechanical force
required by the user. As
described above, the implementation of motor drive based mechanisms provides
monitoring of
the operational status of the system. For instance, conveying the current draw
and revolution
count to the logical and control circuitry in the system provides a
supplementary method for
detection and diagnosis of potential fault conditions. Despite these
advantages, in certain cases
electric motors can be poorly adapted to exerting the necessary linear force
over sufficiently brief
time scales that is most desirable for effective transcutaneous deployment of
arrays comprising a
plurality of elongate electrodes and, in selected embodiments, hypodermic
injection needles. In
particular, the penetration of dermal tissues is most consistently achieved by
the application of a
large linear force over a brief time scale. In some embodiments, the most
favorable insertion
characteristics are achieved when the penetrating electrodes, and when
present, injection needle
contact the skin at higher velocity. This is because sharps travelling at
increased velocity at the
point of skin contact result in less tissue deformation as they cut or
penetrate the tissue.
Therefore, in some embodiments, rapidly accelerating the sharps prior to
contact with the skin is
desired. In some embodiments, multiple electrodes and an injection needle are
frequently
utilized. In another embodiment, the velocity of the electrodes is at least 50
mm/second prior to
contact with the skin. In yet another embodiment, the velocity of the eletrode
is at least 500
mm/second prior to contact with the skin. This deployment approach minimizes
the discomfort
perceived by the subject during the electrode penetration and is most
favorable for maintaining a
consistent spatial relationship between the plurality of electrodes. In
contrast to
electromechanical motors, spring driven mechanisms exhibit a more favorable
discharge profile
that is capable of imparting the rapid impulse force to the electrodes and
injection needle that is
52
CA 03085492 2020-06-10
WO 2019/126120 PCT/US2018/066157
desirable for transcutaneous electrode implantation. In particular, the force
exerted by a
compression spring is at its peak at initial discharge. This is favorable for
transcutaneous
deployment where a high velocity at the point of skin contact is favorable
and, due to the
viscoelasticity of skin tissue, the greatest force is required for penetration
of the skin, particularly
when contacting the skin with a plurality of electrodes and/or injection
needles. In addition, a
spring based mechanism is capable of generating this force from a simple,
durable, and compact
form factor that can be readily integrated into a handheld device format.
However, a
disadvantage of spring based mechanisms is that they typically require the
input of substantial
mechanical force by the user in order to prime them for operation, especially
for springs with
high force constants and/or large displacements. The use of a hybrid motor and
spring
mechanism, as described in the disclosure, achieves the desired deployment
force characteristics
while being simple for the user to operate. While the hybrid motor and spring
mechanism is a
preferred embodiment, depending on implementation, other hybrid mechanisms
incorporating
two or more drive mechanisms wherein one is capable of generating a rapid
impulse force and
the other is capable of priming the impulse force mechanism, e.g., a pump
capable of
compressing gas into a chamber and then discharging the compressed gas in
order to apply an
impulse force for deployment of the electrodes and, where applicable,
hypodermic needle(s) can
also be used.
In any case, once loaded, the desired depth electrode deployment and/or agent
administration is affirmatively selected by the physician or other medicament
administrator and
the same transmitted to the applicator 400. Referring in addition to FIG. 13B,
the depth can be
selected by depth selection buttons 409 (or other equivalent interface such as
a toggle switch or
sliding switch) and the result displayed on injection depth selection
indicators 408 (or, again,
other equivalent interface). The available injection depths are conveyed to
the user by
appropriate labeling of the applicator 400 and/or the cartridge 100. In some
embodiments, any
labeling regarding the injection depth is located on the cartridge 100 and
remains visible to the
user following installation in the applicator 400. For example, the available
injection depths can
be labeled on the upper surface of the alignment guide / splay shield 108. In
order to avoid
circumstances in which the user forgets or neglects to select a depth of
injection, it is preferable
that the device does not allow the user to proceed with the administration
procedure until such
affirmative selection is made. This can be accomplished by the implementation
of appropriate
53
CA 03085492 2020-06-10
WO 2019/126120 PCT/US2018/066157
control logic within the system such that subsequent elements of the device
set up or usage are
not accessible until a valid depth selection has been entered by the user. In
certain embodiments,
when the user is prompted to affirmatively select the injection depth, the
controller display may
convey to the user information regarding the proper methods for assessing the
subject and
determining the appropriate injection depth for the selected administration
site.
Referring in addition to FIGS. 18A-18B, upon proper cartridge assembly
insertion, the
spring cap / cartridge interface 470 engages and also presses against the
spring cover hole 471
and tabs 491. While a large spring force presses against the inner cartridge
103, the same is
prevented from moving forward by engagement of a set of retaining posts 488
against walls 494
of the outer cartridge 102. However, the force exerted by 470 splays apart
tabs 491(which
prevent inadvertent rotation of the lock ring during handling) thereby
allowing rotation of the
cartridge lock ring 114 by the motor drive mechanism. The rotation of the
cartridge lock ring 114
causes rotation of the retaining posts 488. The retaining posts 488 can be
rotated into either the
channels for first depth 490 or the channels for second depth 492. The length
of the channels for
first depth 490 correspond to one of the choices of depths, and the length of
the channels for
second depth 492 corresponds to the other, with one or the other depths being
selected by the
user using buttons 409. For example, the length of channels 490 can be in the
range of 20-30
mm and the length of the channels 492 can be in the range of 12-20 mm. The
requirement of a
user to affirmatively select a depth provides yet another interlock. Without
an affirmative
selection, the applicator may not allow activation / needle insertion. The
rotation of the cartridge
lock ring 114 is thus caused by a clockwise or a counterclockwise rotation
directed by the
applicator 400 according to the dictates of the user. Requiring rotational
motion of a set of posts
into such channels to achieve deployment of the electrodes and, if present,
injection needle
greatly reduces the chances for accidental discharge, even upon violent
jarring or falling.
In more detail, the rotation of the cartridge lock ring 114 is transmitted to
the cartridge
assembly 100 by the retaining posts 488, which are disposed upon proper
cartridge assembly 100
insertion into slots on an insertion mechanism gear drive ring 478. In FIG.
18A, the slots on the
insertion mechanism gear drive ring 478 are disposed at 3 o'clock and 9
o'clock positions. The
insertion mechanism gear drive ring 478 is mounted to a partial insertion gear
ring 479, which is
driven by an insertion mechanism drive motor 482. Driving the partial
insertion gear ring 479
causes the insertion mechanism gear drive ring 478 to rotate either clockwise
or counter-
54
CA 03085492 2020-06-10
WO 2019/126120 PCT/US2018/066157
clockwise. A flag 481 on a ring 480 and accompanying insertion mechanism
position sensor 483
are employed to determine the position of the insertion mechanism gear drive
ring 478, and is
further used to accurately return the same to the 3 o'clock and 9 o'clock
positions when the
applicator 400 is to be re-used with another cartridge.
The above implementation provides various advantages. For example, the user
has to
actively perform a step of selecting the depth before proceeding with the
administration
procedure. In so doing, the user has to assess the proper depth of injection
for the selected
injection site and prepare the site as noted in a guide or instructions for
use document. As noted,
the insertion mechanism, requiring rotational motion to deploy, is
significantly hardened against
accidental deployment due to falling, dropping, jarring, and so on.
Variations are to be understood by a person skilled in the art. For example,
while two
channels and two retaining posts are employed for each depth, one channel and
one retaining
post may also be used. Various types of motors and mechanisms can be employed
for conveying
the rotational motion necessary to rotate the posts into the channels. Other
variations are also to
be understood, including the use of solenoids and the like. In lieu of the use
of channels, motor
driven deployment can be used to provide variable depths, which depths can be
controlled
simply by how far the motor is controlled to drive the deployment. Preferably,
in this context, the
hybrid drive mechanism described would be configured such that the impulse
discharge
mechanism (e.g., the spring) is used for initial deployment through the dermis
and then the motor
drive mechanism is used to advance the electrodes to their desired depth.
One or more interlocks can be in place which must be deactivated before the
insertion
mechanism gear drive ring 478 is caused to rotate, rotating retaining posts
488 into the channels.
First, a force detection interlock subsystem can be in place that requires the
device to be
applied to the subject at a force of greater than a predetermined amount prior
to allowing the
administration procedure to be initiated. This force can be measured by an
appropriate
mechanical or electromechanical system and the result fed back into the
controller 700 and used
as an interlock to prevent activation of the device where insufficient force
is provided. In some
embodiments, the controller 700 is capable of conveying the state of the force
detection interlock
to the user through visual, haptic, or auditory signals so that a state of
inadequate force can be
corrected and the user may proceed with administration. In the event that the
user attempts to
proceed with the administration in a state of inadequate force contact (e.g.,
by depressing a
CA 03085492 2020-06-10
WO 2019/126120 PCT/US2018/066157
trigger 407 or other activation button), additional visual, haptic, or
auditory signals can be
provided by the applicator 400 or controller 700 to notify the user that the
interlock must be
deactivated prior to proceeding with administration.
The detection of the force applied to the subject can be accomplished in a
number of
ways. Referring to the particular implementation of FIGS. 4 and 8A-8B, the
alignment guide /
splay shield 108 is equipped with a force contact pickup 128. The alignment
guide / splay shield
108 can be mechanically biased in a distal direction (towards the subject)
using one or more
force contact springs 126, of which four are shown in FIG. 4. The force
contact pickup 128
changes its position by virtue of the force applied to the alignment guide /
splay shield 108. In so
doing, it also changes the state of an electrical circuit formed by the force
contact pickup 128, a
set of first pads 162, a set of second pads 164, and a flexible circuit 160.
In particular, by testing
for continuity between one or more pads 162 and one or more respective pads
164, it can be
determined how far backward or proximal the alignment guide / splay shield 108
has been
moved by applied force, and thus if sufficient force exists for proper
delivery. The state of the
circuit is read by the applicator 400 using sensor contacts 434 (see FIG. 16).
If sufficient force
is indicated, the force contact interlock is deactivated, allowing the user to
operate the device.
In one implementation, at the first contact point, the system may not register
that any
particular force has been applied. At the second contact point, the system may
register that it is
at partial (but not sufficient) pressure. At the third contact point, the
system may register that the
prescribed level of pressure required to proceed with procedure administration
has been
achieved, and the interlock may deactivate. Preferably, the status of the
force contact circuit is
provided to the user via the applicator display 404.
Variations are to be understood by a skilled person in the art. For example,
the force
contact interlock may form an electrical lock that is deactivated either
within the controller 700
or within the applicator 400 itself. In another variation the force contact
circuit can be
configured to provide information regarding the status of the device
throughout the procedure
administration. In particular, the system may include a feedback loop between
the force contact
circuit and the controller 700 wherein a reduction in the force applied by the
user precipitates the
generation of a visual, haptic, or auditory signal by the controller 700
and/or applicator 400 so
that a state of reduced force can be corrected. In a certain embodiment, a
feedback loop exists
between the force contact circuit and the controller 700 such that the
detection of a change in the
56
CA 03085492 2020-06-10
WO 2019/126120 PCT/US2018/066157
applied force prompts the system to initiate a check as to whether the
electrodes remain properly
deployed into the tissue of the subject, e.g., via an impedance or resistance
check between the
electrodes. If the check is passed, then the procedure proceeds normally. In
the event that the
position of the electrodes is no longer acceptable, then the procedure can be
aborted and the user
notified of the state of the device through the generation of a visual,
haptic, or auditory signal by
the controller 700 and/or applicator 400. This feedback loop is of particular
significance during
the injection of the medicament. By monitoring the position of the device and
the state of the
electrodes, the feedback loop between the force contact circuit and electrode
resistance/impedance monitor, the system may detect if the electrodes (and
therefore the
injection needle) are no longer in the subject, allowing system halt operation
of the injection
drive mechanism 456 to cease depressing the reservoir plunger 484 and
terminating the injection
of the medicament. While this embodiment is most readily implemented with a
motor driven
injection drive 456, other variations can be implemented in the case of
manually operated or
spring drive mechanisms, wherein the activation of mechanical interlocks can
be used to halt
actuation of the reservoir plunger following detection of a fault condition.
This feature can be
particularly useful in stopping the HBV vaccine from inadvertently spraying
out into the
environment when the applicator 400 is removed from the tissue of the subject
prior to
completion of a medicament delivery. For medicaments or therapeutc agents that
are potentially
hazardous for exposure to users or the environment, such a design may avoid
inadvertent
discharges / exposures.
Since the quantity of the dose delivered to the subject in a partial dose
situation can be
critical to inform the decision making of the clinician regarding further
treatment, it is preferable
that the injection drive mechanism 456 include appropriate sensor and control
features to
determine the position of the injection drive plunger 484 at the time the
injection stroke was
halted due to the detection of a fault condition or other circumstance in
which halting the
injection stroke is necessary. Preferably this is achieved through monitoring
of the revolution
count of the motor used to drive the injection drive plunger 484, but other
methods for
monitoring the position of the injection drive plunger 484 including optical
or electrical sensors
can be employed. Based on the known dimensions of the injection drive plunger
484, the depth
of insertion, and the reservoir 101, the use of appropriate logic and control
circuits can translate
the position of the injection drive plunger into an estimate of the volume of
medicament
57
CA 03085492 2020-06-10
WO 2019/126120 PCT/US2018/066157
remaining in the syringe at the point at which the injection stroke was
terminated. Such
information can be conveyed to the user via the display 712.
In addition to providing a termination feature to terminate the injection
stroke in the event
that a fault condition is detected, the use of a motor injection drive 456 can
also provide a
supplementary detector for detection of a fault condition or other operational
issue. Specifically,
the incorporation of appropriate measurement and logic circuits to monitor the
circuit drawn by
the motor during the injection stroke can be used to confirm that the
injection has been
administered within defined specifications. Expected ranges for the current
drawn by the motor
can be established for the various stages of the injection stroke including
the initial run up of the
injection drive plunger 484 before it contacts the plunger stopper 159, the
initial interface
between the injection drive plunger 484 and the plunger stopper 159, actuation
of plunger
stopper 159 forward in the barrel of the reservoir 101, and conclusion of the
injection stroke as
the plunger stopper 159 contacts the end of the barrel of reservoir 101. By
correlating the
position of the injection drive plunger 484 with the measured current drawn by
the motor to the
expected values during each phase of the injection of the agent, the system is
capable of
identifying potential fault conditions and conveying them to the user. For
example, if the user
inadvertently inserted a reservoir 101 in which the plunger stopper 159 had
been partially
actuated (and therefore did not contain the full intended dose of medicament)
into cartridge 100,
the system could detect that the expected increase in current drawn by the
motor injection drive
456 did not occur when the injection drive plunger 484 reached the outer
tolerance for plunger
positioning. Under this circumstance the system could terminate the
administration procedure
and notify the user of the detected fault. Additional fault conditions
including (but not limited to)
faulty components in the applicator 400, breakage of the reservoir 101, and/or
a defective
cartridge 100 would be potentially detectable via this method.
Another 'interlock' to facilitate proper execution of the administration
procedure is
provided by the alignment guide / splay shield 108, which is illustrated in
FIG. 9C. In this
figure, the alignment guide / splay shield 108 is shown with splay features
168 and a hole 170
defined therein for slidable movement of a stick shield 134. The stick shield
134 is illustrated in
FIG. 9B, and electrode holes 167 are also illustrated. For transcutaneous
insertion of electrodes
and, where relevant, injection needles, having a consistent skin interface
facilitates deployment
of electrodes into the target tissue while maintaining the desired spatial
relationship between the
58
CA 03085492 2020-06-10
WO 2019/126120 PCT/US2018/066157
members of the plurality. In particular, proper deployment is most
consistently achieved when
the skin is positioned perpendicularly to the direction of deployment. In
addition, misalignment
of the electrodes and injection needle are reduced when the skin is placed
into tension in the
orientation perpendicular to the direction of deployment. As can be seen, the
splay shield 108
includes mechanical features including ribs and edges to engage with the skin
and place it into
tension perpendicular to the direction of deployment. Combined with the force
contact circuit
pick up system described above which ensures a consistent force applied to the
skin, the
alignment guide / splay shield 108 ensures that the skin is oriented and
placed under tension in
the direction perpendicular to the direction of electrode deployment. While
this embodiment
utilizes mechanical rib features to engage the skin at the device interface,
numerous other
designs and features could be utilized for engagement including the use of
alternative materials
with a high coefficient of friction when placed in contact with skin (e.g.,
rubber insets, adhesive
patches, and the like) or alternative mechanical features including molded
textures, cut outs, and
sawtooth features capable of placing the skin into tension.
As can be seen, the alignment guide / splay shield 108 has a preferential
direction
defined. As described above, spatial and temporal co-localization of the
medicament distribution
and electric field application are desirable. Notably, the inherent structural
properties of skeletal
muscle lead to a characteristic ellipsoid distribution pattern of
intramuscular injections where the
major axis of the ellipsoid aligns with the striations of the muscle fibers.
For applications
involving intramuscular injections, it is therefore favorable to arrange the
electrodes to generate
an ellipsoid electric field profile. In order to ensure that the electrode
array and resultant electric
field profile are properly oriented relative to the striations of the target
skeletal muscle, the use of
an alignment guiding feature is of particular utility for intramuscular
administration. The
objective of the alignment guiding feature is to facilitate placement of the
device such that the
orientation of the electrode array is most favorable relative to the muscle
striations and the
resulting medicament distribution following injection. For an arm injection,
the alignment guide /
splay shield 108 would desirably wrap around the arm horizontally like an arm
band. A similar
orientation would be desired for the leg with the splay shield wrapping around
the leg
horizontally. It has been found that having the alignment guide / splay shield
108 configured in
this manner results in >98% accurate medicament deliveries by users even in
the absence of
verbal instructions. The preferred direction shown and resulting skin
placement is particularly
59
CA 03085492 2020-06-10
WO 2019/126120 PCT/US2018/066157
useful for intramuscular injections, because the diamond shaped array of
electrodes (see the array
of distal ends of electrodes 137 in FIG. 9A, which roughly matches the shape
of the stick shield
134 and its associated holes 167) is then oriented properly to deliver a
medicament and to cause
electroporation of the medicament along the preferred muscular striation
direction. A primary
feature is that, when aligned properly, the skin should be flush with the skin
interface where the
orifice is located, e.g., where the needle emerges. In this way, a consistent
interface to the skin is
obtained.
Where the alignment relative to the target muscle is improper, the arc
generally causes a
visually evident gap, e.g., 2-5 mm, to occur between the alignment guide /
splay shield 108 and
the skin. The visually evident gap can be employed as a reminder to the user
to reorient the
applicator 400. The applicator 400 can be less stable against the skin of the
subject. Alignment
guide features wherein the distance between edges of the "wings" of the
feature are at least 1.3
times the vertical height of the feature are preferable for facilitating
proper placement. In
addition, in some cases the design of the feature is such that the tissue
interface can be placed
flush against the skin in the desired orientation while exhibiting at least a
2 mm air gap when
placed at a 90 degree angle relative to the desired orientation.
Variations on the design of the alignment guide are to be understood by a
skilled person
in the art. For example, instead of an arc shaped alignment guide / splay
shield 108, a "V"
shaped one can be used. Other variations are also to be understood with the
key characteristic
being that the device can be placed directly against the skin in the desired
orientation whereas a
visually apparent gap between the tissue interface and the skin of the
recipient of 2 mm or
greater is present when the device is misaligned relative to the striations of
the target muscle.
As noted above, the orientation of the alignment guide / splay shield 108 is
related in
some implementations to the shape of the electrode array, because the diamond
symmetry of the
electrode array has a certain distribution associated with it, and this
distribution should bear a
certain predetermined relationship to the shape of the alignment guide / splay
shield 108.
Referring to FIGS. 9A-9B, the electrodes, and more particularly the distal
portions 137, are
shown, which are four in number. The electrodes are positioned in a diamond
shaped array,
which can mean they have second order symmetry, i.e., are twofold symmetric,
in that they can
be rotated and at two different positions appear the same. The electrodes
extend in this array
from holes 167 defined in the stick shield 134. The use of a diamond shaped
array is particularly
CA 03085492 2020-06-10
WO 2019/126120 PCT/US2018/066157
useful because it is generally desired for intramuscular injections as
discussed above. To
reiterate, the second order symmetric array leads to a second order symmetric
applied electric
field. This type of applied electric field can have a preferred direction
along the striations of the
muscles if the alignment guide / splay shield 108 is oriented properly. Thus
the alignment guide
/ splay shield 108 and the second order electric field orientation work
together in a synergistic
fashion to accomplish medicament propagation along the direction of muscle
striation.
Broadly speaking, in a diamond shape, or other second-order symmetric shape,
there is
generally a major (long) axis and a minor (short) axis. The axes can bear a
predetermined
relationship with a preferred axis of the alignment guide / splay shield 108.
For example, if the
alignment guide / splay shield 108 is thought of as having wings, with the
arcuate shape of the
wings encircling the arm, then a line segment connecting the center of the
wings can be
perpendicular to the major axis of the diamond shaped array.
Variations are to be understood by a skilled person in the art. For example,
while a
diamond is one possible shape for an electrode array, another exemplary shape
is a rectangle,
which is also second-order symmetric. The electrodes can be placed into the
appropriate
positions but can be moved to within a predetermined or desired tolerance,
e.g., within 5%, 10%,
and so on. Electrodes can be in various other shapes so long as they create a
second order
rotationally symmetrical electric field.
In another variation, for non-intramuscular injections, other order symmetries
can be
used. For example, for skin, there is generally no preferred direction of
medicament propagation
and so even a circular array of electrodes can be employed for intradermal
injections.
Other considerations of electrode arrays are as follows. The simplest array
configuration
comprises two electrodes connected to the opposite poles of the electrical
energy source. As
disclosed in U.S. patents 5,873,549 and 6,041,252 (incorporated herein by
reference in their
entirety), utilization of three or more simultaneously active electrodes
arranged in a multi-
element array can be used to increase target volumes of tissue and improve the
uniformity in
electrical field propagation within the target tissue. A wide range of
geometrical electrode
arrangements and activation patterns have been developed for electric field
application in tissue.
These include electrodes arranged in linear, rectangular, circular, or
triangular configurations
capable of propagating electrical fields in a volume of tissue of roughly
ellipsoid, cuboid,
cylindroid, or spheroid shape. Most commonly, the electrodes are arranged
parallel to one
61
CA 03085492 2020-06-10
WO 2019/126120 PCT/US2018/066157
another and configured for transcutaneous insertion in an orientation
substantially perpendicular
to the skin surface. In order to ensure that the target volume and shape of
tissue is affected by the
application of electrical field, it is desirable that the intended spatial
relationship between each of
the electrodes within the array is achieved following transcutaneous
insertion. Specifically,
unintended changes in the inter-electrode spacing should be avoided as they
can cause changes in
the magnitude of the electrical fields propagated within the target tissue,
potentially leading to
negative consequences for the safety and/or efficacy of the procedure.
Another interlock involves use of an exterior cartridge cap 110. In
particular, the
alignment guide / splay shield 108 is covered while stored with an exterior
cartridge cap 110.
The exterior cartridge cap 110 is configured to not just generally protect the
distal end of the
device until use but also to serve as an interlock feature itself. While
protection of the distal end
of the cartridge assembly 100 serves the purpose of protecting the needle and
electrodes from the
environment, a commonly-encountered problem is that users often forget to
remove such caps.
One solution is to make the cap a bright color that is different from the
color(s) of the other
components in cartridge 100, so as to notify the user of its presence and thus
remind the user to
remove it. Another part of this solution is to include an extension or
reminder tab 190, as shown
by the arcuate section adjacent a rectangular section, the arcuate section
visible even when the
cartridge assembly 100 is placed up against a subject. As this reminder tab
190 is visible, it can
serve as a reminder to remove the exterior cartridge cap 110 even when the
remainder of the
exterior cartridge cap 110 is not visible.
Referring in more detail to FIGS. 10A-10B, the exterior cartridge cap 110
includes an
outer surface facing distally, towards the subject, and an inner surface
facing the alignment guide
/ splay shield 108. On the inner surface are provided a number of hooks 176
which engage a
corresponding wall 180 defined on the alignment guide / splay shield 108. The
hooks hold the
exterior cartridge cap 110 in place.
However, if the exterior cartridge cap 110 were inadvertently left in place,
and the
applicator 400 pressed up against an insertion site with force, the force of
the alignment guide /
splay shield 108 against the exterior cartridge cap 110 may tend to cause the
exterior cartridge
cap 110 to "pop off' by being pushed away from its hooked position by the
alignment guide /
splay shield 108. However, chamfered surfaces 178 are provided on the hooks
176 which tend to
act against the stick shield 134 when pressure is applied, causing the hooks
176 to splay outward,
62
CA 03085492 2020-06-10
WO 2019/126120 PCT/US2018/066157
increasing their retaining force against the alignment guide / splay shield
108, and preventing the
exterior cartridge cap 110 from popping off. In addition, the chamfered
surface acts further
against the stick shield, preventing the alignment guide / splay shield 108
from moving relative
to the stick shield 134. If the alignment guide / splay shield 108 cannot move
relative to the stick
shield 134, the force detection interlock cannot be deactivated, as the force
contact pickup 128
cannot move to the third force contact point discussed above, where full
pressure is detected (or
for that matter even to the second force contact point). The controller can
provide a report when
this happens to the controller. For example, a user interface indication such
as "You need to
remove outer cartridge cap." can be displayed, the same caused by detection of
a trigger pull
performed in the absence of adequate force.
While certain interlocks have been described above, more or less interlocks
can be
provided in any given implementation. For example, other ways can be employed
for force
detection and use as triggering signals for interlocks. Other types of
interlocks may also be
employed, including trigger locks, safety switches, and the like. Yet other
types are to be
understood by one of ordinary skill in the art, given this teaching.
Once a depth of insertion is affirmatively selected by the user, and the
exterior cartridge
cap 110 is removed, and the proper force is detected by the force interlock,
activation of the
trigger causes clockwise or counterclockwise rotation of the cartridge lock
ring 114, with the
direction of rotation dependent on the depth of insertion selected by the
user. The rotation
causes the retaining posts 488 to move into the channels of selected depth,
which in turn causes
the needle and electrodes to deploy. In particular, the inner cartridge 103,
cartridge breech 112,
reservoir 101, inner cartridge cap 104, needle hub 152, needle 105, electrodes
122, and
associated elements to move forward under the influence of the electrode /
needle insertion
spring 472. In the present embodiment, these elements are rigidly connected to
each other and
thus move forward as a unit.
To further minimize the risk of sharps injuries to the user, it is preferable
that the
cartridge assembly also incorporates a mechanism for sheathing the electrodes
and any injection
needles following their removal from the recipients' tissue. This can be
accomplished through
the incorporation of a stick shield feature that houses the electrodes and
injection needle (if
present) prior to their use and then extends over the electrodes and injection
needle (if present)
following their deployment and removal from the tissue of the subject.
Commonly, the tissue
63
CA 03085492 2020-06-10
WO 2019/126120 PCT/US2018/066157
contact interface comprises the distal surface of the shield feature. Although
the use of manually
operated shield features can be considered, preferably, the device
incorporates a mechanism to
automatically extend the shield feature over the electrodes and further to
engage a locking
feature to maintain the shield feature in the extended state once it has been
removed from the
tissue of the recipient. Examples include a shield feature slidably engaged
with the cartridge
outer housing and operatively connected to a source of stored energy, such as
a spring, which
slides the shield forward upon withdrawal of the electrodes from the
recipients' tissue.
Alternatively, the mechanism can be contained within the applicator and
configured to reverse
the electrode deployment step, thereby retracting the electrodes and any
associated injection
needles behind the tissue contact interface. This can be accomplished using
simple
electromechanical mechanisms for linear motion such as motors or solenoids.
In a particular implementation, and referring back to FIG. 4 as well as to
FIGS. 11A and
11C, the stick shield 134 is configured to stay in position while the
medicament delivery
happens, but the movement forward of the inner cartridge 103 and other
associated components
during medicament delivery causes compression of a stick shield spring 138.
This compressed
stick shield spring 138 then relaxes as the applicator is removed from the
subject, as the stick
shield 134 is caused to move forward when the applicator 400 is removed from
the subject,
covering the needle and electrodes, and protecting the subject and others
against the uncovered
sharps. Notably, this feature also prevents the visualization of the sharps
features during
withdrawal, which can facilitate acceptance from subjects experiencing anxiety
related to
needles.
While the relaxed spring would otherwise be capable of recompressing, and in
particular
if the user moved the stick shield 134 proximally, it is prevented from doing
so by the action of
stick shield support arm 132, which are mounted to the interior of the outer
cartridge 102 and
which serve a ratcheting function as the stick shield 134 moves forward. In
particular, and
referring to FIGS. 11A and 11C, stick shield support arms 132 move over
various sequential
retaining walls, and can do so easily when the stick shield 134 is moving
distally. However, in
the proximal direction, the stick shield 134 is prevented from moving because
the stick shield
support arms 132 abut the retaining walls in this direction, in a ratcheting
fashion, and do not
allow passage.
64
CA 03085492 2020-06-10
WO 2019/126120 PCT/US2018/066157
An initial set of retaining walls 188 prevent proximal movement of the stick
shield 134
prior to use of the cartridge assembly 100. A set of first depth retaining
walls 184 prevent
proximal movement of the stick shield 134 after discharge at a first selected
depth. A set of
second depth retaining walls 186 prevent proximal movement of the stick shield
134 after
discharge at a second selected depth. The stick shield 134 is prevented from
complete removal
from the cartridge assembly 100 by the action of the stick shield retaining
hooks 182 acting
against the inner cartridge 103.
Variations are to be understood by a skilled person in the art. For example,
in one
implementation the stick shield support arms 132 can be provided by stamped
metal support
arms that interface with the outer cartridge 102. In some embodiments, the
support arm features
are directly integrated as injection molded plastic features in the outer
cartridge cap 106 and/or
alignment guide / splay shield 108. The specific material should be selected
to achieve sufficient
rigidity to provide support to the elongate electrodes while retaining
sufficient elasticity to
prevent fracture of the component under load. Material selection should also
consider the
intended method of sterilization for the electrode array (e.g., gamma
radiation, steam
sterilization, ethylene oxide, or e-beam) to ensure that the features retain
adequate material
properties following sterilization. In an exemplary embodiment, the features,
as implemented,
should be capable of holding the stick shield 134 against proximal movement
when at least 5 N,
but more preferably at least 15 N of force applied. Other ways of providing a
ratcheted function
to prevent proximal movement of the stick shield may also be employed
including a gear rack
implemented on stick shield 134 and a corresponding ratchet feature
implemented in outer
cartridge cap 106..
Referring to FIG. 4, each elongate electrode 122 within the array comprises a
distal
portion 137 and a proximal portion 135 that are in conductive communication.
The distal portion
is configured for tissue penetration and electric field propagation in tissue
and the proximal
portion is configured with a conductive contact region capable of reliably
achieving conductive
communication with the electrical connections contained within the applicator
with a suitable
source of electrical energy. The source of electrical energy can, by temporal
variation in the
power applied to individual electrodes, cause a spatially and temporally
varying electric field
within the body of the subject, generally confined to a volume around the
region of medicament
distribution, and the same can be used to advantage in electroporation
therapy.
CA 03085492 2020-06-10
WO 2019/126120 PCT/US2018/066157
The relevant characteristics of the electrodes include shape, diameter, tip
profile, length,
material composition, and conductivity, the specifics of which are selected
based on the intended
application. Most commonly, the electrodes comprise electrically conductive
elongate rods with
a curvilinear cross section with diameter 0.1 - 1.5 mm. The electrodes can be
solid core or
hollow. Most commonly hollow electrodes incorporate one or more orifices and
an operative
connection to a fluid reservoir, thereby allowing for the administration of
the agent of interest or
other associated medicaments including anesthetics, surfactants, proteins,
adjuvants, or
enhancers from the electrode itself Depending on the application, appropriate
metallic electrode
materials include, but are not limited to titanium, gold, silver, aluminum,
copper, tantalum,
tungsten, molybdenum, tungsten, stainless steel, MP35N and alloys thereof
Electrodes may also
be comprised of electrically conductive ceramics or plastics. To minimize
unwanted
electrochemical reactions at the tissue electrode interface, it is often
desirable that one or more of
the electrodes are coated in a conductive material providing improved
electrochemical stability
compared to the electrode material itself. Such coating materials include, but
are not limited to,
platinum, iridium, palladium, osmium, gold, silver, titanium, and alloys
thereof.
As described above, the tip of the distal portion is configured for tissue
penetration.
Common tip profiles include, but are not limited to, trocar, bevel, cone,
blade, lance, and taper.
For transcutaneous electrode deployment, tip profiles with one or more cutting
edges are
preferred. For certain applications where the penetrating tip profile can be
undesirable for
generating the required electrical fields, the tip can be comprised of a non-
conductive material
fixed to the distal tip of the electrode or the penetrating tip can be covered
in an adherent coating
or tubing comprised of a biocompatible electrically insulating material such
aspoly(p-xylylene)
polymer, polyolefin, polyvinyl chloride, polyurethane, polyester, polyimide,
silicone rubber,
thermoplastic elastomer/rubber, ethylene tetrafluoroethylene, fluorinated
ethylene propylene,
and/or perfluoroalkoxy plastic.
Proximal to the penetrating tip are one or more conductive regions configured
for the
propagation of electrical fields in tissue. In order to confine the
propagation of electrical fields to
the target region of tissue, commonly, especially for subcutaneous and
intramuscular
administration, at least a portion of the electrode length configured for
penetration in the tissue is
covered in an adherent coating or tubing comprised of an biocompatible
electrically insulating
material such aspoly(p-xylylene) polymer, polyolefin, polyvinyl chloride,
polyurethane,
66
CA 03085492 2020-06-10
WO 2019/126120 PCT/US2018/066157
polyester, polyimide, silicone rubber, thermoplastic elastomer/rubber,
ethylene
tetrafluoroethylene, fluorinated ethylene propylene, and/or perfluoroalkoxy
plastic. In this way,
electrodes can be configured to only activate at a particular depth within the
tissue.
Collectively, the number of electrodes and their inter-electrode distance as
well as the
penetration depth of the electrodes and the length of their conductive region
define the volume of
tissue in which the electric fields is applied. These parameters are selected
based on the specific
objectives for the administration procedure, including target tissue site as
well as the volume,
dose, and viscosity of the agent to be delivered as well as the variation in
skin and subcutaneous
tissue thickness in the intended recipient population. Generally, for
intradermal administration in
human recipients, arrays comprise 2-16 electrodes of diameter 0.2 - 0.7 mm, an
inter-electrode
distance of 2-8 mm, a depth of electrode penetration of 0.5 - 4 mm, and a
conductive length of
0.5-4 mm. Generally, for subcutaneous administration in human recipients,
arrays comprise 2-8
electrodes of diameter 0.3-0.8 mm, an inter-electrode distance of 4-10 mm, a
depth of electrode
penetration of 5-15 mm, and a conductive length of 2-8 mm. Generally for
intramuscular
administration in human recipients, arrays comprise 2-8 electrodes of diameter
0.3 - 1.2 mm,
with an inter-electrode distance of 4-12 mm, a depth of electrode penetration
of 10-60 mm, and a
conductive length of 2 - 20 mm.
In order to avoid undesirable visualization and exposure to the electrodes
during the
usage of the device, the cartridge assembly 100 is preferably configured such
that the electrodes
122 are recessed within the device prior to their insertion into the tissue of
the recipient. Most
commonly, this is accomplished by providing the outer housing structure 102
slidably engaged
with the electrode mount support, which in one implementation comprises an
inner cartridge 103
having seems formed therein in which the electrodes are disposed. Prior to
use, the electrodes are
recessed within the outer housing 102. During use, the sliding engagement of
the electrode
mount structure, e.g., the inner cartridge 103, with the outer housing allows
the electrodes 122 to
slide forward relative to the distal tip of the outer housing 102, thereby
deploying the electrodes
122 from the outer housing 102. The length of the sliding engagement between
the electrode
mount structure and the outer housing corresponds to the maximum desired depth
of electrode
penetration for the given application.
As can be seen in FIG. 4, and the electrodes 122 have a proximal portion 135
and a distal
portion 137. The proximal portion is separated from the distal portion by a
shoulder or bend 139.
67
CA 03085492 2020-06-10
WO 2019/126120 PCT/US2018/066157
The shoulder or bend 139 is secured by and between the inner cartridge cap 104
and the inner
cartridge 103. The shoulder or bend 139 provides a number of functions. First,
while the
electrodes at the distal end of the cartridge assembly 100 are required to
form an array of a
certain size and shape as described above, putting the electrodes 122 in the
desired array size and
shape at the proximal end of the cartridge assembly 100 is impractical due to
the presence of the
reservoir 101. In other words, the electrodes 122 have to bend "out-of-the-
way" to make room
for the reservoir 101. In addition, having the bend, particularly when the
bend is locked in place
frictionally between the inner cartridge 103 and the inner cartridge cap 104,
provides a surface
for the electrodes 122 to abut against when the force of the needle and
electrodes insertion
recoils in the distal direction as the electrodes 122 interact with the tissue
during their
deployment. The bend, can resistant to axial rotations, which is beneficial so
that the electrodes
do not exhibit significant torsional movement and rotate away from their
electrical contact pads
130 (described below). In addition, in contrast to prior art techniques in
which multiple
electrodes interfaced with axially separated coaxial rings, requiring a
different configuration for
each electrode, the present system allows a common electrode type to be used
for all four
electrodes.
Systems and methods according to present principles provide for the
propagation of an
electrical field in the skin, subcutaneous tissue, and/or skeletal muscle of a
recipient which
facilitates the intracellular delivery of an HBV vaccine within said
recipient's tissue. In this
aspect, the apparatus includes two or more elongate electrodes 122 arranged in
a predetermined
spatial relationship and configured for deployment into the target tissue in
which the agent of
interest has been or is to be distributed. The desired enhancement in agent
activity is contingent
on achieving co-localization of the site of agent distribution with the
propagation of electrical
fields of sufficient magnitude to induce the desired physiological effects.
Therefore it is desirable
that the apparatus consistently achieve electrode deployment to the target
depth and with
specified intraelectrode spacing. Specifically, this ensures that the
electrical fields are propagated
at the proper tissue site, and, since the magnitude of the electrical fields
propagated within the
tissue is a function of the intraelectrode spacing, a safe and effective
electric field intensity.
However, the variation in the thickness, density, and composition of skin and
subcutaneous
tissue between sites in a given recipient and across recipient populations can
lead to significant
variation in electrode deployment characteristics. For example, recipients
with increased
68
CA 03085492 2020-06-10
WO 2019/126120 PCT/US2018/066157
thickness of skin are at increased susceptibility for electrode distortion
and/or insufficient depth
of penetration which can affect co-localization with the site of agent
distribution.
Systems and methods according to present principles further facilitate
consistent
transcutaneous insertion of electrode arrays comprising 2-16 electrodes of
diameter 0.2 - 1.3 mm
with an inter-electrode distance of 2-12 mm, and a depth of electrode
penetration of 0.5 - 60 mm
in recipients with skin of heterogeneous thickness, composition, and
condition. In order to permit
transcutaneous insertion, tissue penetrating electrodes are most commonly
elongate and
configured with a tissue penetrating tip and a tissue contact region in the
distal portion and an
electrical contact region in the proximal portion. Given their elongate
configuration, they are
susceptible to bowing, bending, and/or buckling (collectively termed
distortion) when subjected
to the compression forces generated during transcutaneous insertion into the
recipient. Such
distortions during electrode insertion are undesirable as they can lead to
improper electrode
insertion characterized by insufficient depth of penetration and/or excessive
changes in
intraelectrode spacing. Of note, humans and animals exhibit significant intra-
and inter-species
differences in the thickness, composition, and condition of skin, any of which
can have
significant impact on the forces which the electrodes are subjected to during
transcutaneous
insertion. In some embodiments, devices for transcutaneous insertion of
elongate electrodes are
designed to withstand the forces present under the most stringent conditions
of electrode
insertion that is to be encountered in the target population of recipients.
The occurrence of
electrode distortion can be partially mitigated by electrode material
selection as well as reducing
the length and increasing the diameter of the electrodes within the array.
However, material
selection can be constrained by issues of performance, cost,
manufacturability, and
biocompatibility. In addition, electrodes of reduced length may preclude
adequate coverage of a
target population with heterogeneous tissue thickness while larger diameter
electrodes are
associated with increased discomfort and tissue trauma. Thus, the disclosure
is designed to
provide methods and apparatus to facilitate the transcutaneous insertion of
electrodes
independent of these variables.
In a first embodiment of the disclosure the subassembly cartridge housing the
electrode
array incorporates one or more support dynamic support members in physical
contact with one
of the electrodes during tissue insertion and configured to constrain movement
of the electrodes
perpendicular to the direction of insertion and maintain the desired spatial
relationship between
69
CA 03085492 2020-06-10
WO 2019/126120 PCT/US2018/066157
the electrodes. For example, and referring to FIG. 12, an electrode support
feature 124 is
illustrated in which holes 192 are provided for electrodes and a hole 194 is
provided for passage
of the needle. The tendency of the elongate electrodes to distort or buckle
under load is
exacerbated by any bending, eccentricity, or bowing of the electrode that may
have been
introduced during manufacture or assembly. Thus, it is advantageous to employ
a device such as
the electrode support feature 124 to constrain the electrodes to prevent, or
correct, any bending,
eccentricity, or bowing they might exhibit at rest, and to prevent unwanted
perpendicular motion
of the electrode as a result of the loading that occurs during deployment into
tissue.
In the context of the disclosure, a dynamic support member is defined as a
structural
element which provides sliding engagement with the elongate electrodes during
insertion into the
tissue of the recipient and which is configured to undergo a change in
position, size, and/or
conformation during electrode insertion in order to maintain the desired
spatial relationship of
the electrodes as they are deployed to the target tissue depth. Since the
electrodes are subjected
to the largest loading forces at the initial contact with the skin, the
engagement of the dynamic
support member with the electrodes comprising the array is preferably affected
prior to initial
contact with the tissue of the recipient and to continue to provide support as
the electrodes
deploy to the full depth of penetration. It is also favorable that the dynamic
support member be
designed to provide support to the electrodes and injection needle while
minimizing losses in
electrode penetration force due to friction.
While the primary function of the dynamic support member is to maintain the
desired
spatial relationship of the plurality of the electrodes within the array
relative to one another and
to the injection needle, it is also desirable for the design of the dynamic
support member to
include features capable of stabilizing the array as a whole. This is
preferably accomplished by
providing additional structural support features which constrain lateral
movement of the support
member. In a certain embodiment, these support features are integrated into a
sharps protection
shield, which also serves to protect the user against accidental exposure to
the electrodes and/or
injection needle. Disclosed below are various embodiments of dynamic support
members
consistent with the disclosure.
An embodiment of the disclosure, described above as electrode support feature
124,
involves the use of a planar support structure 124 positioned perpendicularly
relative to the
elongate orientation of the electrodes. The planar support structure is
configured with one or
CA 03085492 2020-06-10
WO 2019/126120 PCT/US2018/066157
more apertures 192 which correspond to the positions of said electrodes in
their specified spatial
relationship. The size, shape, and position of the apertures are configured to
allow the support
structure to slide smoothly along the elongate length of the electrodes within
the array while
constraining unwanted motion perpendicular to the direction of electrode
deployment.
Commonly, the apertures can be configured as holes or slots 192 in the planar
structure with
adequate clearance for the electrode (at least 10% larger than the largest
cross section of the
electrode, including any coatings or other adherent materials). However, if
more substantial
support is required for specific electrodes, one or more of the apertures may
comprise tubular
structures 196 arranged perpendicularly to the planar support structure.
Apertures comprising
such tubular structures increase the surface area contacting the electrode and
thereby increase the
support provided to the elongate electrodes. The planar support structure can
be made from any
material with appropriate structural characteristics including metal, polymer,
ceramic, or
composite materials and can be formed, machined, molded, or produced with
other methods. To
avoid unwanted electrical interactions with the electrodes, it is preferable
that the interface
between the electrodes and the planar support structure is not electrically
conductive. The
material and manufacturing method should also be selected to minimize the
amount of friction at
the interface between the electrodes and the dynamic support member. Due to a
number of
factors including their low cost, ease of manufacturability, and favorable
electrical properties, the
electrode support structure is commonly made of a thermoplastic such as
polycarbonate,
polystyrene, polypropylene, acrylic, or polyethylene. The specific material
should be selected to
achieve sufficient rigidity to provide support to the elongate electrodes
while retaining sufficient
elasticity to prevent fracture of the component under load. Material selection
should also
consider the intended method of sterilization for the electrode array (e.g.,
gamma radiation,
steam sterilization, ethylene oxide, or e-beam) to ensure that the dynamic
support member is
compatible. The specific dimensions and design of the support structure depend
on the
properties of the selected material. However, it is desirable to minimize the
dimensions of the
support structure so that it does not excessively limit the distance that the
electrodes can be
deployed or to interfere with other functional properties of the device. Rigid
planar structures of
0.5 mm ¨ 2 mm thickness are typically sufficient
Another embodiment of a dynamic support member is the use of a compression
spring
with apertures accommodating the electrodes and constraining their lateral
movement. The
71
CA 03085492 2020-06-10
WO 2019/126120 PCT/US2018/066157
compression spring can be made from metal, polymer, or elastomeric materials,
and can be
formed, machined, molded, or produced with other methods. At rest, the spring
is uncompressed
or minimally compressed with the electrodes inserted into the apertures along
the spring's length.
As the electrodes are deployed, the spring compresses in the direction of
deployment, with the
apertures accommodating the sliding movement of the electrodes perpendicular
to the spring
coils. This embodiment is of particular utility when combined with a shield or
sheath used to
house the penetrating electrodes / needles following removal from the tissue
site. In these
embodiments, the force imparted to the spring by the forward deployment of the
electrodes can
be used to deploy a sheath or shield over the electrodes as the device is
removed from the tissue.
In the implementation of FIG. 4, the stick shield spring 138 can be used to
partially
support the electrode support 124, and in particular the radius of the spring
can be configured to
match (or be just slightly greater than) the radius of the wall 198 of the
electrode support 124. In
this way, the electrode support 124 can be inserted into the middle of the
stick shield spring 138
during use. The electrode support 124 may then slide within the interior of
the stick shield 134.
By being placed in the center of the spring, the electrode support 124
naturally maintains a
position in the center of the spring, which provides a desired "halfway point"
for support of the
electrodes, roughly halfway between their point of support at the inner
cartridge cap 104 and
their point of penetration at the tissue interface.
Other spring-based electrode supports are contemplated. For example, a formed
compression spring is positioned in the region of the electrodes, to provide
adaptive electrode
support. Formed compression springs can be shaped and proportioned to conform
closely to the
relative positions of the electrodes, in order to restrict their lateral
motion. An optional biasing
element can be positioned in conjunction with a formed compression spring, and
may serve to
bias the electrodes outward against formed compression spring. Formed
compression springs can
be made from metal, polymer, or elastomer materials, and can be formed,
machined, molded, or
produced with other methods. The biasing element can be made from metal,
polymer, or
elastomer materials, and can be formed, machined, molded, or produced with
other methods.
Electrode supports based on other mechanisms are also contemplated, for
example
telescoping tubes. Telescoping tubes serve to support electrodes during
insertion into the subject.
The telescoping tubes can be sized to move freely relative to each other, or
can be sized to move
only when an axial force is applied to them.
72
CA 03085492 2020-06-10
WO 2019/126120 PCT/US2018/066157
Other support structures are contemplated, for example support structures
based on
movable, flexible, or pivoting support members. Lateral support members can
attach to the
electrodes at optional hinge features. Lateral support members can be formed
integrally with the
structure housing the electrodes or can be separate components attached by
conventional
methods (snaps, welding, adhesives, fasteners, etc.).
Another embodiment is the use of a compressible matrix material in which the
electrodes
are embedded. As the electrodes are deployed, the material compresses in the
direction of
deployment, providing lateral support along the direction of travel. Examples
of compressible
matrix materials include cellulose, foamed plastic or rubber polymers such as
microcellular
plastics, foamed silicone or foamed polychloroprene, or carbon foam matrices.
Since the
materials are designed to contact the electrodes and/or injection needle (if
applicable), the
materials should be selected to be compatible with indirect tissue contact.
The above described structures thus support transcutaneous deployment of a
plurality of
elongate electrodes at tissue depths of up to 60 mm while maintaining the
desired spatial
relationship among the plurality of electrodes and, in specific embodiments,
the orifice of a
hypodermic injection needle. Such support members engage the plurality of
elongate electrodes
during transcutaneous insertion, and constraining deflection of the electrodes
in one or more
directions perpendicular to their elongate orientation. Another aspect of the
disclosure provides
methods and apparatus for utilizing the application of biocompatible
lubricious compounds to the
surfaces of the plurality of electrodes in order to reduce the applied force
required to achieve
consistent transcutaneous deployment of a plurality of elongate electrodes to
depths of up to 60
mm. In an exemplary embodiment, biocompatible silicone compounds such as Dow
Corning 360
Medical Fluid or Dow Corning MDX4-4159 can be applied by conventional spray or
dip coating
to the plurality of the electrodes comprising the array in order to improve
the insertion
characteristics of the electrodes. The specific selection of the coating and
application conditions,
such as coating method and thickness depends on the number, size, composition,
and tip
configuration of the electrodes as well as the target tissue in which the
electrodes are deployed.
Referring to FIGS. 4 and 7A-7B, the proximal portions 135 of the electrodes
122 can be
positioned on the exterior of the inner cartridge 103 of the cartridge
assembly 100 (FIG. 7B). In
this way, when the inner cartridge 103 is slidably disposed within the outer
cartridge 102 (FIG.
7A), the proximal portions 135 contact corresponding electrode contacts 130,
and in particular
73
CA 03085492 2020-06-10
WO 2019/126120 PCT/US2018/066157
outer cartridge interior portions 133 of the electrode contacts 130, these
interior portions 133
configured for power communication with the proximal portions 135. Due to the
length of the
proximal portions 135, and the length of the outer cartridge interior portions
133, the electrical
communication can be made at a number of locations along their continuous
interface, no matter
the longitudinal position of the inner cartridge with respect to the outer
cartridge. The electrode
contacts 130 further include outer cartridge exterior contacts 131, configured
for power
communication with corresponding connections 496 on the applicator 400 (see
FIG. 18C).The
electrode contacts 130 are comprised of a conductive material sufficient to
convey an electrical
signal. In certain embodiments, the design and material selection ensure that
the contact provide
for adequate engagement to ensure an electrically conductive interface with
the corresponding
electrode over the range of expected manufacturing variation in electrode and
contact position
while not inhibiting or interfering with the forward travel of the electrodes
122 mounted on the
inner cartridge 103. The design must also permit this engagement to persist
when exposed to the
expected storage conditions over the labeled shelf life of the product. In a
certain embodiment,
electrical contacts 130 are made of stamped or formed metal with appropriate
temper to faciliate
engagement with the electrode and may include coatings such as gold or copper
to ensure the
integrity of the electrical contacts and avoid corrosion. In addition to
ensuring that electrical
contact can be maintained with the electrodes at a number of locations along
their continuous
interface, the incorporation of the electrode contacts into outer cartrdige
102 in this configuration
ensures that any wear due to the sliding interaction between the electrodes
122 and the electrode
contacts 130 occurs within the cartridge 100 designed for single use, thereby
allowing for a static
interface between the outer cartridge contacts 131 and applicator electrical
contacts 496 which
minimizes potential mechanical wear on the electrode connections 496 of the
applicator designed
for multiple uses. This configuration has the benefit of extending the useful
functional life of the
multi-use applicator.
Remaining portions of the applicator 400 are now described. These portions are
generally
those that are independent of operation with the cartridge assembly 100.
Referring first to FIG.
13A, the applicator 400 includes a handle 402 and a multi-conductor cable 406
designed to carry
power and control signals as well as the electrical signals to be applied to
the tissue. The cable
406 is generally terminated in a connector with a corresponding connector
interface in the
controller 700. The applicator 400 further includes a user interface 404, in
which aspects of the
74
CA 03085492 2020-06-10
WO 2019/126120 PCT/US2018/066157
procedure can be viewed by the user, and in which the user can direct the
applicator to perform
various functions, in particular, depth selection. The applicator 400 further
includes a procedure
activation trigger 407, which is used by the subject to initiate the
procedure.
Referring in addition to FIG. 13B, the applicator 400 includes a procedure
countdown
timer 410, which informs the user of the remaining duration of the procedure,
and further
impliedly indicates to the user that they should not remove the applicator 400
from the subject
until such time as the countdown trigger has counted down to zero. A power
indicator 418 is
provided to indicate a satisfactory and powered connection with the controller
700. A procedure
fault indicator 414 is provided to indicate to the user if a fault has
occurred, e.g., one of the
interlocks described above has not been deactivated. An application placement
indicator 412 is
provided to inform the user if a proper pressure has been obtained against the
tissue of the
subject, allowing the procedure to commence.
FIG. 14 indicates a number of structural components of the applicator 400,
including a
connector 426 (not to scale) for connection to the controller 700, a top
housing 420, side
housings 422 and 424, and an inner protective shell 432. A front cap 430 is
provided, along with
an end cap 428. Various electromechanical subassemblies 450 are also provided,
several of
which have been described above.
FIG. 15 illustrates a more detailed view of the applicator 400, indicating
cartridge
pressure sensor contacts 434 and subassemblies 452 corresponding to the
cartridge loading,
electrode insertion, and injection functions as well as the associated
sensors.
FIG. 16 illustrates a more detailed view of the group of subassemblies 452,
including the
loading drives subassemblies 454 and the cartridge loading subassembly 456.
The operation of
the subassemblies has been described above.
FIG. 17A illustrates a more detailed view of the loading drive subassembly
454, the
general to operation of which has been described above. Here it is noted that
the loading is
triggered by a flag on the cartridge assembly 100, and that detection of the
flag leads to the
system being triggered at switch 464. The loading drives subassembly is
mounted to the
applicator housing using brackets 466 and 468. The action of the motor 444 is
transmitted to the
pinion gear assembly 448 by the motor drive shaft 462.
FIG. 17B illustrates a more detailed view of the insertion/injection drive
assembly 456.
Many of these components have been described above. Here it is noted that the
operative
CA 03085492 2020-06-10
WO 2019/126120 PCT/US2018/066157
components are mounted to the applicator housing by a mounting bracket 476.
Following
insertion of the electrodes 122 and the needle 105, the plunger of the needle
105 is depressed by
the injection drive plunger 484, whose action ejects the medicament from the
orifice of the
needle. The injection drive plunger is driven by an injection drive motor and
gear assembly 486.
Generally the injection drive plunger, driven by the injection drive assembly
486, moves
forward until such point at which it can no longer move forward, i.e.,
distally, indicating that it
has reached the end of each stroke. This indication is generally given by the
current used in the
injection drive motor 486 rising substantially, indicating that the reservoir
plunger has reached
the end of the reservoir and that the injection has been completed. However,
in some cases, the
number of turns of the motor can be employed to determine how much medicament
has been
delivered. Such a feature could be used to support metered dosing of
medicament or in order to
notify the user in cases where the volume of delivery was not within the
expected total, e.g.,
where the full volume of reservoir 101 was expected to be injection, but based
on the position of
the plunger rod, it was not emptied. These situations may arise where the user
failed to hold the
applicator 400 against the body of the subject until the procedure was
completed, e.g., until the
countdown timer had counted down to zero. As noted above, in a mid-procedure
timeframe,
where the force against the subject is no longer being detected, an impedance
check can be
performed to determine if the electrodes are still within the subject. If they
are not, then it can be
presumed that the applicator was prematurely removed, and a signal can be sent
to the injection
drive motor 486 to cease injection, limiting the amount of medicament ejected
outside of the
subj ect.
Referring to FIG. 19, the controller system/assembly 700 can be seen including
an
electrical field controller / generator 750 and a handle 702 and an applicator
cradle 706. The
controller can be configured for both table top as well as cart mounted use.
In the cart mounted
configuration it is the inclusion of a storage bin 704 is favorable. Details
of the controller in the
cart mounted configuration are shown in FIGS. 20A-20D, including wheel locks
to secure the
controller assembly against movement in an operational setting, a tray 710 for
placing supplies
including subject preparation supplies, reservoirs/vials/syringes, and so on.
An applicator
connector port 708 is illustrated to connect the applicator 400 to the
controller assembly 700. A
display 712 displays status of an administration procedure, particularly with
regard to the IFU
attached.
76
CA 03085492 2020-06-10
WO 2019/126120 PCT/US2018/066157
A cartridge eject button 714 is provided to cause ejection of the cartridge
assembly 100
from the applicator 400. Menu navigation buttons 716 allow navigation and
manipulation of
components as seen on the display 712. A mute button 718 is provided to mute
alerts or other
audible indicators if desired. A power button 722 is provided to power the
unit, and the same is
activatable if the main power switch 726 (FIG. 20D) has been turned on.
The display also includes a battery indicator 720, which provides an
indication of battery
level where a battery backup system is provided. Such a battery backup system
can be included
in the controller/generator 750 to accommodate situations in which power loss
prevents a main
power source from powering the unit. Such may also be employed as a backup
where a
procedure has been started under main power, but where a main power loss has
been
encountered. In this case, logic and control circuitry is implemented to
provide for essentially
seamless transition from mains to battery power so that the procedure can be
finished using the
battery backup. It is favorable for the controller to include battery
monitoring circuitry that is
capable of monitoring whether the battery has sufficient charge to complete
the procedure
following loss of mains power. In some embodiments, the controller also
includes display to
notify the user in the event that the current charge status of the battery is
not sufficient to
complete the procedure in the event of mains power loss.
Referring to FIG. 20D, in which a rear view of the controller 750 is
illustrated, the same
can be seen to include a USB port 724, a main power switch 726, and a main
power input 728.
Referring to FIG. 21, in one method of use, as illustrated by the flowchart
800, the
controller/generator 750 is powered and its program automatically started
(step 802). The
applicator is connected (step 804), and an indication or instruction to the
user to perform this
action can be displayed on the display screen 712, if the applicator has not
already been
connected. The system may perform a self test (step 806), the self test not
only ensuring proper
operation of the controller/generator 750 but also ensuring correct connection
of the applicator
400 to the controller/generator 750.
The program may cause the display screen to provide instructions to the user
on
preparation of the site of administration (step 808). This step may include
ensuring that the
correct medicament agent is being delivered, that the same is not expired,
that contraindications
have been reviewed, and that warnings/precautions have been followed.
77
CA 03085492 2020-06-10
WO 2019/126120 PCT/US2018/066157
The user then removes the reservoir cap and inserts the reservoir 101 in the
cartridge
assembly 100 (step 810). In some embodiments, the user experiences an audible,
tactile, or
haptic click (step 811) indicating proper placement of the reservoir in the
cartridge assembly.
The user then inserts the cartridge assembly 100 into the applicator 400 (step
816). An
error status is then tested for (step 818), e.g., for proper reservoir
placement, and if one is
detected, the procedure is stopped, an error message is displayed, and the
user is instructed to
take remedial action. If the error state can be corrected, e.g., the user has
inserted the reservoir
improperly but not engaged or closed the cartridge breech, then the user can
be instructed to
remove the cartridge and reinsert the reservoir properly (state 820). In some
cases, the cartridge
is automatically ejected, and in other cases the user may have to push the
"cartridge eject" button
to accomplish the same. In other error states, e.g., where the cartridge
breech has been closed, the
user can be instructed to use a new cartridge.
In any case, once the device set up is completed and a "no error" state has
been achieved,
the user can proceed to administration of the medicament and electroporation
therapy (step 822).
The primary function of the controller is to generate the electrical fields
required to
achieve the desired delivery of the medicament, to control the operation of
the system during set
up and use, to monitor the state of the system during set up and use, and to
convey the state of
the system to the user during set up and use. In some embodiments, the
controller is capable of
providing recommendations and instructions for use of the device both in the
context of user
training as well as during resolution of fault conditions during ordinary
usage.
The controller system and controller method of operation can be fully
implemented
utilizing any number of computing devices including microprocessors,
microcontrollers,
programmable logic controllers. Typically, instructions are laid out on
computer readable media,
generally non-transitory, and these instructions are sufficient to allow a
processor in the
computing device to implement the method of the disclosure. The computer
readable medium
can be a hard drive or solid state storage having instructions that, when run,
are loaded into
random access memory. Inputs to the application, e.g., from the plurality of
users or from any
one user, can be by any number of appropriate computer input devices. For
example, users may
employ a keypad, keyboard, mouse, touchscreen, joystick, trackpad, other
pointing device, or
any other such computer input device to input data relevant to the
calculations. Data may also
be input by way of an inserted memory chip, hard drive, flash drives, flash
memory, optical
78
CA 03085492 2020-06-10
WO 2019/126120 PCT/US2018/066157
media, magnetic media, or any other type of file ¨ storing medium. The outputs
can be delivered
to a user by way of a video graphics card or integrated graphics chipset
coupled to a display that
maybe seen by a user. Alternatively, the system may output one or more formats
of electronic
document or a printer can be employed to output hard copies of the results.
Given this teaching,
any number of other tangible outputs is also be understood to be contemplated
by the disclosure.
For example, outputs can be stored on a memory chip, hard drive, flash drives,
flash memory,
optical media, magnetic media, or any other type of output. It should also be
noted that the
disclosure can be implemented on any number of different types of computing
devices, e.g.,
personal computers, laptop computers, notebook computers, net book computers,
handheld
computers, personal digital assistants, mobile phones, smart phones, tablet
computers, and also
on devices specifically designed for these purpose. In one implementation, a
user of a smart
phone or wi-fl ¨ connected device downloads a copy of the application to their
device from a
server using a wireless Internet connection. An appropriate authentication
procedure and secure
transaction process may provide for payment to be made to the seller. The
application may
download over the mobile connection, or over the WiFi or other wireless
network connection.
The application may then be run by the user. Such a networked system may
provide a suitable
computing environment for an implementation in which a plurality of users
provide separate
inputs to the system and method. In the below system where control of an
applicator is
contemplated, the plural inputs may allow plural users to input relevant data
at the same time.
TABLE OF ELEMENTS
REF # PART
100 Cartridge assembly
101 Reservoir
102 Outer cartridge, aka housing
103 Inner cartridge
104 Inner cartridge cap
105 Needle
106 Outer cartridge cap
108 Alignment guide / splay feature
110 Exterior (safety) cartridge cap
112 Cartridge breech
114 Cartridge lock ring
116 Reservoir detection spring
118 Reservoir detection cap
120 Reservoir interlock, aka reservoir insertion trigger
79
CA 03085492 2020-06-10
WO 2019/126120
PCT/US2018/066157
122 Electrodes
124 Electrodes support
126 Force contact springs
128 Force contact pickup
130 Electrode contacts
131 Exterior Outer cartridge electrode portions for coupling to applicator
132 Stick shield supports
133 Interior Outer cartridge electrode portions for coupling to inner
cartridge
134 Stick shield
135 Proximal Inner cartridge electrode portions for coupling to outer
cartridge
137 Distal Inner cartridge electrode portions for tissue insertion
138 Force contact flexible circuit
139 Electrode shoulder or bend
140 Reservoir loading port
142 Reservoir containment volume
144 & Reservoir lockout holes (14 or 2nd set)
144'
146 Optical line of sight
148 Insertion detector, e.g., emitter/collector IR sensor within applicator
150 Inner cartridge containment volume
152 Needle hub
154 Rack
156 Egress port
158 Reservoir cap
159 Plunger stopper
160 Flexible circuit
162 First set of pads
164 second set of pads
166 Stick Shield nubs
167 Stick shield holes
168 Splay feature
170 Alignment guide hole for stick shield
172 Initiating flag
174 Continuing flag
176 Exterior cartridge cap hooks
178 Exterior cartridge cap chamfer surfaces
180 Hook engaging wall of alignment guide / splay shield 108
182 Stick shield retaining hooks
184 First depth retaining wall
186 Second depth retaining wall
188 Initial or rest retaining wall
190 Reminder tab
192 Electrode support feature electrode holes
194 Electrode support feature needle hole
196 Electrode support feature electrode hole support structure
CA 03085492 2020-06-10
WO 2019/126120
PCT/US2018/066157
198 Electrode support feature wall
400 Applicator
401 Applicator cartridge assembly receiving port
402 Handle
403 Cartridge assembly receiving volume, which is defined by a housing
404 User interface
406 Multi-conductor cable
407 Trigger
408 Injection depth selection indicator(s)
409 Injection depth selection button(s) (could be toggle or other forms in
another
implementation)
410 Procedure countdown timer
412 Application placement indicator
414 Procedure fault indicator
416 Procedure complete indicator
418 Power indicator
420 top housing
422 First side housing
424 Second side housing
426 Electrical connector
428 End cap
430 Front cap
432 Inner protective shell
433 Electrical contacts for motor drive 444
434 Cartridge force sensor contacts
435 Connectors for switch
436 Cartridge loading sensor
438 Cartridge loaded sensor
440 Guide or track
442 Cartridge guide rails
444 Loading drive motor
446 Motor trigger connector
448 Pinion gear assembly
450 Electromechanical subassemblies
452 Cartridge loading, electrode insertion, and injection subassemblies
454 Loading drive subassembly
456 Cartridge loading subassembly
462 Motor drive shaft
464 System trigger switch
466 gear cover bracket
468 Mounting bracket
470 Spring cover / cartridge interface
471 Spring cover hole
472 Electrodes/needle insertion spring
474 Insertion gear bushing
81
CA 03085492 2020-06-10
WO 2019/126120 PCT/US2018/066157
476 Mounting bracket
478 Insertion mechanism gear drive ring
479 Insertion gear ring
480 Flag holder
481 Insertion mechanism flag
482 Insertion mechanism drive motor
483 Insertion mechanism position sensor
484 Injection drive plunger
486 Injection drive motor and gearing
488 Retaining posts (retaining feature)
490 Channels for first depth
491 Lock tabs
492 Channels for second depth
494 Abutment wall
496 Applicator electroporation electrode contacts
700 Controller Assembly
702 Handle
704 Storage bin
706 Applicator cradle
708 Applicator connector port
710 Tray
712 Display screen
714 Eject cartridge button
716 Menu navigation buttons
718 Mute button
720 Battery indicator
722 Power button
724 USB port
726 Main power switch
728 Main power port
750 Electrical Field Generator
EXAMPLES
The following examples of the invention are to further illustrate the nature
of the
invention. It should be understood that the following examples do not limit
the invention and the
scope of the invention is to be determined by the appended claims.
Example 1: Generation of HBV Core and Pot Antigen Sequences and Plasmid
Optimization
82
CA 03085492 2020-06-10
WO 2019/126120 PCT/US2018/066157
T-cell epitopes on the hepatitis core protein are considered important for
elimination of
hepatitis B infection and hepatitis B viral proteins, such as polymerase, may
serve to improve the
breadth of the response. Thus, hepatitis B core and polymerase proteins were
selected as
antigens for the design of a therapeutic hepatitis B virus (HBV) vaccine.
Derivation of HBV Core and Polymerase Antigen Consensus Sequences
HBV pol and core antigen consensus sequences were generated based on HBV
genotypes
B, C, and D. Different HBV sequences were obtained from different sources and
aligned
separately for core and polymerase proteins. Original sequence alignments for
all subtypes (A to
H) were subsequently limited to HBV genotypes, B, C, and D. Consensus
sequences were
defined for each protein alignment in each subtype separately and in all joint
BCD sequences. In
variable alignment positions, the most frequent amino acid was used in the
consensus sequence.
Optimization of HBV Core Antigen
The HBV core antigen consensus sequence was optimized by a deletion in the
native
viral protein. In particular, a deletion of thirty-four amino acids
corresponding to the C-terminal
highly positively charged segment was made, which is required for pre-genomic
RNA
encapsidation.
Optimization of the HBV Pol Antigen
The HBV pol antigen consensus sequence was optimized by changing four residues
to
remove reverse transcriptase and RNAseH enzymatic activities. In particular,
the asparate
residues (D) were changed to asparagine residues (N) in the "YXDD" motif of
the reverse
transcriptase domain to eliminate any coordination function, and thus
nucleotide/metal ion
binding. Additionally, the first aspartate residue (D) was changed to an
asparagine residue (N)
and the first glutamate residue (E) was changed to a glutamine residue (A) in
the "DEDD" motif
of the RNAseH domain to eliminate Mg2+ coordination. Additionally, the
sequence of the HBV
pol antigen was codon optimized to scrambe the internal open reading frames
for the envelope
proteins, including the S protein and versions of the S protein with the N-
terminal extensions
pre-S1 and pre-S2. As a result, open reading frames for the envelope proteins
(pre-S1, pre-S2,
and S protein) and the X protein were removed.
Optimization of HBV Core and Pot Antigen Expression Strategies
Three different strategies were tested to obtain maximum and equal expression
of both
core and pol antigens from plasmid vectors: (1) fusion of HBV core and pol
antigens in frame
83
CA 03085492 2020-06-10
WO 2019/126120 PCT/US2018/066157
with a small AGAG inserted between the coding sequences to produce a single
Core-Pol fusion
protein (FIG. 25A); (2) expression of both core and pol antigens from one
plasmid by means of a
ribosomal slippage site, particularly the FA2 slippage site from foot-and-
mouth disease (FMDV)
to produce a biscistronic expression vector expressing individual core and pol
proteins from a
single mRNA (FIG. 25B); and (3) two separate plasmids encoding for HBV core
and pol
antigens, respectively (FIG. 25C).
In vitro Expression Analysis
The coding sequences of consensus HBV core and pol antigens according to each
of the
above three expression strategies were cloned into the commercially available
expression
plasmid pcDNA3.1. HEK-293T cells were transfected with the vectors and protein
expression
was evaluated by Western blot using a HBV core-specific antigen.
Optimization of Post-Transcriptional Regulatory Elements
Four different post-transcriptional regulatory elements were evaluated for
enhancement
of protein expression by stabilizing the primary transcript, facilitating its
nuclear export, and/or
improving transcriptional-translational coupling: (1) Woodchuck HBV post-
transcriptional
regulatory element (WPRE) (GenBank: J04514.1); (2) intron/exon sequence
derived from human
apolipoprotein Al precursor (GenBank: X01038.1); (3) untranslated R-U5 domain
of the human
T-cell leukemia virus type 1 (HTLV-1) long terminal repeat (LTR) (GenBank:
KM023768.1);
and (4) composite sequence of the HTLV-1 LTR, synthetic rabbit P-globin intron
(GenBank:
V00882.1), and a splicing enhancer (triple composite sequence). The enhancer
sequences were
introduced between a CMV promoter and the HBV antigen coding sequences in the
plasmids.
No significant difference was observed by Western blot in the expression of
the core antigen
when expressed from a plasmid in the presence and absence of the WPRE element
(FIG. 25D).
However, when core antigen expression in HEK293T transfected cells from
plasmids having the
other three post-transcriptional regulatory sequences was evaluated by Western
blot, the triple
enhancer sequence resulted in the strongest core antigen expression (FIG.
25E).
Selection of Signal Peptide for Efficient Protein Secretion
Three different signal peptides introduced in frame at the N-terminus of the
HBV core
antigen were evaluated: (1) Ig heavy chain gamma signal peptide SPIgG
(BAA75024.1); (2) the
Ig heavy chain epsilon signal peptide SPIgE (AAB59424.1); and (3) the Cystatin
S precursor
signal peptide SPCS (NP 0018901.1). Signal peptide cleavage sites were
optimized in silico for
84
CA 03085492 2020-06-10
WO 2019/126120 PCT/US2018/066157
core fusion using the Signal P prediction program. Secretion efficiency was
determined by
analyzing core protein levels in the supernatant. Western blot analysis of
core antigen secretion
using the three different signal peptides fused at the N-terminus demonstrated
that the Cystatin S
signal peptide resulted in the most efficient protein secretion (FIG. 25F).
DNA Vaccine Vector Optimization
The optimized expression cassettes containing the triple composite enhancer
sequence
upstream of the HBV antigen coding sequence with an N-terminal Cystatin S
signal peptide
sequence were cloned in the DNA vaccine vector pVax-1 (Life Technologies,
Thermo Fisher
Scientific, Waltham, MA). The expression cassette in pVax-1 contains a human
CMV-IE
promoter followed by the bovine growth hormone (BGH)-derived polyadenylation
sequence
(pA). Bacterial propagation is driven by the pUC on replicon and kanamycin
resistance gene
(Kan R) driven by a small eukaryotic promoter. The pUC on replication, KanR
expression
cassette, and expression cassette driven by the CMV-IE promoter are all in the
same orientation
within the plasmid backbone. However, a marked reduction in core antigen
expression was
observed in the pVax-1 vector as compared to the expression level observed in
the pcDNA3.1
vector.
Several strategies were employed to improve protein expression: (1) reversal
of the entire
pUCori-KanR cassette into counterclockwise orientation (pVD-core); and (2)
changing the
codon usage of the KanR gene along with replacement of the Kan promoter with
the Amp
promoter from the pcDNA3.1 vector (pDK-core). Both strategies restore core
antigen
expression, but core antigen expression was highest with the pDK vector, which
contained the
codon-adjusted Kan R gene, AmpR promotor (instead of KanR promoter), and
inverse
orientation of the pUCori-KanR cassette.
The four different HBV core/pol antigen optimized expression cassettes as
shown in FIG.
25G were introduced into the pDK plasmid backbone to test each of the three
expression
strategies illustrated in FIGS. 25A-25C. The plasmids were tested in vitro for
core and pol
antigen expression by Western blot analysis using core and pol specific
antibodies. The most
consistent expression profile for cellular and secreted core and pol antigens
was achieved when
the core and pol antigens were encoded by separate vectors, namely the
individual DNA vectors
pDK-core and pDK-pol (FIG. 25H). A schematic representation of the pDK-pol and
pDK-core
vectors is shown in FIGS. 26A and 26B, respectively.
CA 03085492 2020-06-10
WO 2019/126120 PCT/US2018/066157
Example 2. Electroporation mediated intramuscular administration of nucleic
acid
based biopharmaceuticals with TriGrid Delivery System (TDS-IM) device
The intracellular delivery of nucleic acid sequences in the skeletal muscle of
the upper or
lower limb in a subject can be enhanced with the use of an exemplary device,
e.g. TriGrid
Delivery System (TDS-IM) model II, as provided herein. In some embodiments,
the TDS-IM
device is used in conjunction with agents approved for investigational use
with the TDS-IM
device. In an exemplary embodiment, the approved agent is nucleic acids, i.e.,
DNA or RNA. In
some embodiments, the use of TDS-IM device is restricted to a subject in need
thereof. In some
embodiments, the use of TDS-IM device is restricted to a subject enrolled in
an open clinical
trial of electroporation mediated intramuscular nucleic acid delivery.
To start up the system for administration, the main power of the device is
connected to
the stimulator and the system battery is adequately charged for use. The main
power switch is
turned on and the front panel power button is depressed. The applicator is
connected to the
applicator connector. Proper connection of the applicator is confirmed by the
illumination of the
applicator power indicator. The start screen appears once the system completes
all self-checks.
The OK button is pressed to proceed with the procedure administration.
To insert a syringe into a TDS cartridge, the syringe cap is removed from the
syringe, and
the syringe flange is aligned with the TDS cartridge syringe loading port. The
syringe should
snap into place and be fully seated in the TDS cartridge. Once the syringe is
loaded, the OK
button is pressed on the pulse stimulator to continue. The cartridge cap
should remain affixed to
the cartridge until the cartridge is loaded into the applicator.
To insert the syringe loaded cartridge into the applicator, the cartridge is
aligned with the
applicator with the cartridge syringe loading port facing upward. When the
cartridge is inserted
into the applicator, and the cartridge is automatically drawn into its fully
loaded position in the
applicator. Successful loading of the cartridge is indicated in the
stimulator. Once the cartridge
is loaded, the applicator is returned to its cradle, and an appropriate
injection site on the subject
is selected. In some embodiments, the injection site for intramuscular nucleic
acid delivery is
medial deltoid muscle at approximately three finger widths below the edge of
acromion process
(shoulder bone). In an exemplary embodiment, the injection depth at medial
deltoid is about
0.75"-1.25" (19-30 mm). In some embodiments, the injection site for
intramuscular nucleic
acid delivery is vastus lateralis muscle (outer thigh) at approximately the
midpoint between the
86
CA 03085492 2020-06-10
WO 2019/126120 PCT/US2018/066157
hip and the knee. In an exemplary embodiment, the injection depth at vastus
lateralis is about
1.0"-1.5" (25-38 mm). Once the injection site is selected, the applicator
depth selection button
followed by the injection depth selection button corresponding to the
injection site/depth are
pressed. In some embodiments, the injection depth selection indicator turns to
solid illumination,
confirming the selected injection depth. In some embodiments, the depth
selection button on the
right side corresponds to a deeper injection depth. In an embodiment, wherein
the initially
selected injection site is to be changed, the selection button corresponding
to the other injection
depth is pressed, and the other injection depth is selected when the the
select injection depth
screen returns.
To start administration of an approved agent via TDS-IM device to the subject,
the
cartridge cap is removed and discarded. The device is aligned with and firmly
pressed against
the target injection site. When the device is firmly pressed against the
target injection site, all
four bars of the applicator placement indicator illuminates, and the procedure
countdown timer
illuminates with "8" seconds, indicating the time remaining in the
administration procedure. The
applicator trigger is depressed for the agent to be administered while the
pressure is consistently
maintained. When the procedure countdown timer reaches "0," the electrical
stimulation is
delivered. Once the administration procedure is completed, the procedure
complete indicator
illuminates, and the device can be withdrawn from the injection site. The
device may not be
withdrawn from the injection site until the procedure is completed or a
procedure fault indicator
illuminates. In some embodiments, wherein the device detects a problem during
the
administration procedure, the device aborts the administration procedure and
illuminates the
procedure fault indicator. In an exemplary embodiment, wherein the device
aborts the
administration procedure and the device promptly is removed from the subject,
the stimulator
display of the device provides further instructions.
To eject the cartridge from the applicator after completion of the agent
administration to
the subject in need thereof, the eject button located on the stimulator is
depressed. The
applicator automatically advances the cartridge to the position where it can
be manually removed
from the applicator. Once the cartridge stops moving, the sides of the
cartridge as indicated by
arrows can be grasped for pulling the cartridge out of the applicator. After
the cartridge is
removed, completion of the full injection can be verified by inspecting the
syringe plunger
87
CA 03085492 2020-06-10
WO 2019/126120 PCT/US2018/066157
position. To turn off the device, the applicator is placed in the holster, and
the front panel power
button is pressed for 5 seconds.
Example 3. TDS-IM Electroporation to Mouse and Non-human Primate
TriGrid Delivery System ¨ Intramuscular (TDS-IM) electroporation technology is
used to
enhance the delivery of DNA-based constructs in muscle tissue. The TriGrid
electrode array for
intramuscular (IM) delivery is comprised of four electrodes arranged in two
equilateral triangles
to form a diamond shape surrounding a central injection needle (FIG. 22).
Integration of the
agent delivery and the electric field propagation into a single device assures
that induction of the
electroporation effect occurs at the site of agent distribution and allows
injection of the plasmid
construct and application of the electric pulses in a single step. In this
manner, IM delivery of
plasmid DNA is achieved in an effective and reproducible manner.
The TriGrid electrode arrays for each animal model are scaled to efficiently
deliver the
DNA plasmids, such as those made in Example 1, to the selected muscle
regardless of size and
overlying tissue characteristics. The primary electroporation device
parameters that are scaled
for use in each animal model are the TriGrid size (i.e., electrode spacing),
applied voltage (250
V/cm where cm is the TriGrid size), electrode diameter, and the electrode
penetration depth.
Secondary adaptations to the device include variation in syringe volume,
plasmid DNA volume,
and hypodermic needle gauge/length.
The mouse studies were performed with a TDS-IM v1.0 device using an electrode
array
with a 2.5 mm spacing between the electrodes. This adjustment in electrode
array size is to
accommodate the smaller size of the mouse muscle. FIG. 23A depicts a TDS-IM
v1.0 TriGrid
version adapted for use in the mouse model. The non-GLP monkey studies were
also performed
with a TDS-IM v1.0 device, but with a 6 mm electrode array spacing. FIG. 23B
depicts a TDS-
IM v1.0 TriGrid version adapted for use in the non-human primate (NHP) model.
The device used in the GLP monkey study was a TDS-IM v2.0 device, which is
also
intended for human clinical study. This device has a 6 mm electrode spacing
and the same
activation conditions as the TDS-IM v1.0 device. The electrode materials of
the TDS-IM v2.0
device are identical with the TDS-IM v1.0 device used in the NHP study
described above, but
the Cartridge support structure and syringe used for administration are
different. Also, the
insertion depth for humans and non-human primates is different. Therefore, the
TDS-IM v2.0
device was fitted with a spacer in order to adapt the TDS-IM v2.0 device for
use in the much
88
CA 03085492 2020-06-10
WO 2019/126120 PCT/US2018/066157
smaller NHP muscles. Figure 24 depicts the TDS-IM v2.0 TriGrid version adapted
for use in the
non-human primate (NHP) model.
In each animal study, the animals were anesthetized to immobilize them prior
to the
electroporation procedure administration. The administration site was located
using regional
bony anatomical landmarks as reference points and the hair was removed over
the selected site
with an electric hair clipper followed by an aseptic swab. The TDS-IM array
was inserted
percutaneously into the selected muscle with the major axis of the diamond
configuration
oriented in parallel with the muscle fibers. Following electrode insertion,
the injection was
initiated to distribute the DNA in the muscle. Following completion of the IM
injection, a 250
V/cm electrical field was locally applied for a total duration of 400 ms at a
10% duty cycle (i.e.,
voltage is actively applied for a total of 40 ms of the 400 ms duration). Once
the electroporation
procedure was completed, the TriGridTM array was removed and the animals were
recovered.
The variable components for each TDS-IM version and/or animal model are as
follows in Table
1 and Table 2.
Table 1: TDS-IM version and TriGrid details
TDS-IM Animal TriGrid Electrode Conductive Effective DNA Syringe
Injection Injection
Version Model size diameter length (mm) Penetration Injection Volume needle
-- method
(mm) (in) depth (mm) Volume
(cc)(cc) gauge
TDS-IM mouse 2.5 0.030 3.2 3.2 0.020
3/10 30 manual
v1.0 cc
TDS-IM NHP 6.0 0.021 5.0 15.5 1.0 1.0 cc 22
automated
v1.0
TDS-IM NHP 6.0 0.023 5.0 9.0* 1.0 1.0 cc 22
automated
v2.0
*NHP body weight and muscle size required a shorter penetration depth; a
10nnnn depth limiter was used to
shorten the penetration depth to 9nnnn.
Table 2: TDS-IM version and Pulse Stimulator details
TDS-IM Animal Applied Applied Applied Applied Total Active Maximum
Version Model electric field Voltage Current
current limit pulse stimulation sequence
(V/cm) range (V) limits (A) (A/sec) number
duration (ms) duration (ms)
TDS-IM mouse 250 59.4- N/A* - 4 0.16 6 40.8 369.3
v1.0 65.6
TDS-IM NHP 250 142.4- 0.6 - 4 0.16 6 40.8
369.3
v1.0 157.6
TDS-IM NHP 250 142.4- 0.6 - 4 0.16 6 40.8
369.3
v2.0 157.6
*low current limit disabled to accommodate mouse model characteristics
89
CA 03085492 2020-06-10
WO 2019/126120 PCT/US2018/066157
Example 4. TDS-IM Electroporation to human
A TDS-IM v2.0 device is used in the clinical study. The device which is
essential the
same as that used for the GLP monkey. The insertion depth for humans and non-
human primates
is different. Therefore, the TDS-IM v2.0 device was fitted with a spacer in
order to adapt the
TDS-IM v2.0 device for use in the much smaller NHP muscles. The stimulation
conditions were
essentially the same as those used for GLP monkeys, with the exception that
the penetration
depth about 19 mm, instead of 9 mm as in the GLP monkeys.
While preferred embodiments of the disclosure have been shown and described
herein, it
will be obvious to those skilled in the art that such embodiments are provided
by way of example
only. Numerous variations, changes, and substitutions will now occur to those
skilled in the art
without departing from the disclosure. It should be understood that various
alternatives to the
embodiments described herein, or combinations of one or more of these
embodiments or aspects
described therein can be employed in practicing the disclosure. It is intended
that the following
claims define the scope of the disclosure and that methods and structures
within the scope of
these claims and their equivalents be covered thereby.