Note: Descriptions are shown in the official language in which they were submitted.
CA 03087508 2020-06-30
WO 2019/164693
PCT/1JS2019/017491
METHOD AND DEVICE FOR THE MANAGEMENT OF BODY FLUIDS
LEAKING FROM A SURGICAL DRAIN TUBE INCISION
FIELD OF THE INVENTION
100011 The invention relates to methods and devices for the management of body
fluids leaking from a surgical drainage incision in a patient.
BACKGROUND OF THE INVENTION
[00021 Surgical drains are tubes placed near surgical incisions in the post-
operative
patient, to remove pus, blood or other fluid (herein collectively referred to
as "fluid"),
preventing it from accumulating in the body. The type of drainage system
inserted is
based on the needs of patient, type of surgery, type of wound, how much
drainage is
expected and surgeon preference. Millions of surgical drains are placed daily
in
various body cavities and spaces. Placement of surgical drain typically
involves
making a skin incision matching the size of the drain and subsequently
tunneling the
drain trough the incision, placement of the drain in the appropriate space
according to
the application and securing the drain to the skin with sutnres. Other methods
of
securing the drain in place include taping or coiling of the drain inside the
cavity.
Regardless of the way the drain is placed it is impossible to consistently
match the
size of the incision to the drain size. In addition, the capacity of the human
skin to
stretch contributes to size mismatch between the incision size and the drain
caliber.
The result is a small skin opening around the drain that causes fluid leaks.
[00031 Fluid leaks around surgical drain incisions are a consistent problem in
surgical units around the world. Leaked fluids have a significant impact on
increased
use of disposable surgical dressings leading to increased supply cost,
increased
hospital laundry turnover, significant impact on personnel engagement
requiring
increased staff presence and occupation in surgical units. Moreover, the
leaked fluids
may lead to skin irritation and maceration resulting in skin infections that
could be
extremely serious in some settings. In addition, an open communication with
the
cavity may lead to infection of subcutaneous tissues and the cavity itself.
This
CA 03087508 2020-06-30
WO 2019/164693
PCT/US2019/017491
requires the continuous use of various skin barriers and protective dressings
that need
to be changed frequently, thus leading to increasing cost.
100041 Openly leaking fluids challenge the sterility of the surgical site. In
addition,
leaking fluids increase risk of infection. Both of these problems
significantly impact
the ability to record proper outputs of the drain placement sites thus
influencing
surgical decisions and outcomes. From a hospital's perspective in the era of
Value
Based Purchasing (VBP) this problem turns out to be extremely costly to the
hospital.
Leaking drains cause surgical/drain site infections., skin infections and
irritations lead
to readmissions. Patients staying in beds with soaked sheets and gowns report
lower
level of hospital overall experience and care on surveys decreasing hospital
scores
and ultimately reimbursement. Patient's and family members experience
increased
stress and anxiety observing a surgical drain leaking unfamiliar fluids. This
leads to
perception of poor quality of care, mistrust and tension with physicians and
personnel.
[00051 Any wound management cost is dependent on three major factors such as
cost of supplies, nursing time and extra time patient spends in the hospital.
The .
fourth factor is VIIIr s patient and family experience and overall hospital
score
impacting reimbursement,
100061 It is estimated that one gauze dressing change costs $6.36 for the
material,
$9.14 for nursing service totaling $15.54. It is not uncommon to have
dressings
changed every hour on a patient with an active leaking drain site.
[00071 Accordingly, there is a need for methods and devices that minimize or
eliminate the problem of fluids leaking from a surgical drainage incision
thereby
eliminating the need for frequent dressing changes.
SUMMARY OF THE INVENTION
[00081 The foregoing problems are addressed by the method and device for the
management of body fluids leaking around a surgical drain in accordance with
the
invention.
-2,
86774836
[0009] According
to an aspect of the present invention, there is provided a fluid
collection system comprising: a baseplate having an adhesive backing
configured to couple
the baseplate to a patient's skin and defining a wafer having an approximately
centrally
positioned opening, and a ring shaped, radially-extending wafer connector
coupled to said
wafer and disposed around said opening configured to receive a first end of a
surgical drain
tube; and an appliance including a leaked fluid remover, said leaked fluid
remover having a
fluid remover connector operable to detachably couple with the wafer
connector, the leaked
fluid remover including a first housing component including the fluid remover
connector a
second housing component received by said first housing component, and a
spacer having
an upper surface and a lower surface, the first housing component including an
outer septum
having a lower surface positioned on or coupled to the upper surface of the
spacer, and the
second housing component including an inner septum positioned on or coupled to
the lower
surface of the spacer; and a leaked fluid collector having an outer film and
an inner film, the
outer film coupled to an outer portion of the first housing component and the
inner film
coupled to an inner portion of the first housing component thereby coupling an
interior of
the leaked fluid collector to the fluid remover connector, wherein coupling of
the fluid
remover connector to the wafer connector forms a fluid flow chamber
therebetween through
which leaked fluid from an incision flows through the fluid flow chamber and
into the leaked
fluid collector.
[0009a] According
to another aspect of the present invention, there is provided a
leaked fluid collection system comprising: a baseplate having an adhesive
backing
configured to couple the baseplate to a patient's skin, the baseplate defining
a wafer having
an approximately centrally positioned opening, and a ring shaped, radially-
extending wafer
connector coupled to said wafer and disposed around said opening; an appliance
including
a leaked fluid remover, said leaked fluid remover having a fluid remover
connector operable
to detachably couple with the wafer connector, the leaked fluid remover
including a first
housing component including the fluid remover connector and a second housing
component
received by said first housing component, the first housing component and
second housing
component defining, when coupled, a septum space therewithin; a septum having
a septum
rim, the septum rim housed between the first housing component and the second
housing
component, when coupled, said septum including an orifice for receiving a
surgical drain
tube from a front side of the septum or a backside of the septum, said septum
radially, axially
- 3 -
Date Recue/Date Received 2021-07-05
86774836
and/or pivotally moveable within the septum space to alleviate side load
tension on the
septum as the surgical drain tube is displaced; a leaked fluid collector
having an outer film
and an inner film, the outer film coupled to an outer portion of the first
housing component
and the inner film coupled to an inner portion of the first housing component
thereby
coupling an interior of the leaked fluid collector to the fluid remover
connector, wherein
coupling of the fluid remover connector to the wafer connector forms a fluid
flow chamber
therebetween through which leaked fluid from an incision flows through the
fluid flow
chamber and into the leaked fluid collector.
[0009b] According
to another aspect of the present invention, there is provided a fluid
collection system comprising: an appliance structured to be used with or
without a surgical
drain tube, the appliance including a leaked fluid remover, said leaked fluid
remover having
a fluid remover connector operable to detachably couple with an adhesive
system that is
configured to be affixed onto a skin of a patient, the leaked fluid remover
including a first
housing component including the fluid remover connector and a second housing
component
received by said first housing component, the first housing component and
second housing
component defining a septum space therewithin; a septum having a septum rim,
the septum
housed within the septum space, said septum including an orifice for receiving
a second end
of the surgical drain tube, said septum radially, axially and/or pivotally
moveable within the
septum space to alleviate side load tension-on the septum caused by
displacement of the
surgical drain tube; and a leaked fluid collector having an outer film and an
inner film, the
outer film coupled to an outer portion of the first housing component and the
inner film
coupled to an inner portion of the first housing component thereby coupling an
interior of
the leaked fluid collector to the fluid remover connector, wherein coupling of
the fluid
remover connector to the skin of the patient creates a fluid flow chamber
through which
leaked fluid from an incision in the patient flows through the fluid flow
chamber and into
the leaked fluid collector.
[00010] In one
aspect the device comprises a fluid collection system. The fluid collection
system broadly includes a leaked fluid remover, an adhesive backed wafer for
securing the
leaked fluid remover to the skin of the patient and a leakage collection pouch
for capturing
fluid leaked from the surgical drain incision. The leaked fluid remover is
positioned over an
opening in the wafer. In some aspects, the leaked fluid remover may include a
connector or
- 3a -
Date Recue/Date Received 2021-07-05
86774836
connecting assembly configured to sealably couple onto a mating connector on
the adhesive
wafer assembly.
[0010a] In another aspect, the leaked fluid remover may include a housing
having a
central opening to receive surgical drain tubing, entering from a surgical
incision, and a
spaced apart coaxial second opening through which the surgical drain tubing
passes to exit
the leaked fluid remover housing. The second opening may be configured with a
fluid-tight
elastomeric septum for receiving and sealing around a range of variously sized
surgical drain
tubing
[0011] Fluids
leaked from the surgical incision that have not passed through the surgical
drain tube are captured by a leaked fluid remover and diverted to a collector
pouch that is in
fluid communication with the leaked fluid remover.
BRIEF DESCRIPTION OF THE DRAWINGS
[0012] For a
better understanding of the invention, and to show how the same may be
carried into effect, reference will now be made, by way of example, to the
accompanying
drawings, in which:
[0013] FIG. 1 is
a perspective view of the fluid collection system in accordance with
an aspect of the invention.
[0014] FIG. 2a
is a perspective view of the fluid collection system including a drainage
collection container in accordance with an aspect of the invention.
[0015] FIG. 2b
is a perspective view of the fluid collection system in accordance with
an aspect of the invention showing alternative ways for emptying leaked fluid
for the leaked
fluid collector.
- 3b -
Date Recue/Date Received 2021-07-05
CA 03087508 2020-06-30
WO 2019/164693
PCT/US2019/017491
100161 FIG. 3 is an exploded perspective view of the fluid collection system
in
accordance with an aspect of the invention illustrating the liquid tight seals
throughout the system.
100171 FIGS. 4a and 4b are cross-sectional views of the appliance of the fluid
collection system in accordance with an aspect of the invention.
100181 FIGS. 5a and 5b are perspective views illustrating the dynamic radial
movement of the elastomeric septum of the appliance in accordance with an
aspect of
the invention.
[00191 FIG. 6 is a cross sectional view illustrating another aspect of the
fluid
collection system in accordance with the invention.
[00201 FIGS. 7a and 7b are cross sectional views of the fluid collection
system in
accordance with an aspect of the invention illustrating a static state and
dynamic
state, respectively, of the elastomer pressure seal.
100211 FIG. 8 is a perspective view of one aspect of a leaked fluid remover in
accordance with the invention showing a septum orifice plug.
[00221 FIG. 9a is a cross-sectional view of an appliance including a leaked
fluid
remover with an alternative configuration.
[00231 FIG. 9b is a perspective view of the appliance of FIG. 9a affixed onto
a
patient's skin.
100241 FIGS, 10a and 10b are perspective views of the fluid collection system
in
accordance with the invention illustrating other ways to couple the appliance
to a
patient.
[00251 FIG. 1 la is a cross-sectional view of an alternative appliance or
leaked fluid
remover used with the fluid collection system in accordance with the invention
in a
static position.
[0026] FIG. Ilb is a cross-section view of the appliance/leaked fluid remover
of
FIG. 11A depicting fluid flow through the front side of the appliance and into
the
patient,
-4-
86774836
[0027] FIG. 11c is a cross-sectional view of the appliance of FIG. ha
depicting fluid
flow through the back side (or patient side) of the appliance exiting out to a
collection bag.
[0028] FIG. 11d is a cross-section view of the device showing surgical
tubing and fluid
flow exiting through the patient side of the appliance showing the septum
capable of moving
about radially, axially and/or pivotally to alleviate side load tension on the
septum caused
by displacement of the surgical drain tube.
[0029] FIGS. lie and 11 f are partial perspective views of an alternative
embodiment
with spaced apart inner septum with slit and outer septum with opening, shown
respectively
without and with a passed through drain tube.
[0030] MS, 11g and 11h are partial sectional views of the alternative inner
septum
embodiment of FIGS. lie and 1 if, shown respectively with a closed slit in the
absence of a
drain tube and a slit penetrated by a drain tube.
DETAILED DESCRIPTION OF EMBODIMENTS OF THE INVENTION
[0031] As used herein, leaked fluid means the fluid that leaks around a
surgical incision
after surgery that is not captured by the surgical drain tubing that is
inserted into the incision
to aid in removing fluid. Correspondingly, surgical fluid or drained surgical
fluid means the
fluid that is captured by the surgical drain tubing.
[0032] Like elements of the fluid collection system 100 in accordance with
the
invention are labeled with like reference numerals in the FIGS. and throughout
the
disclosure.
[0033] Referring generally to FIGS. I - 3, the fluid collection system 100
in accordance
with an aspect of the invention is illustrated. Fluid collection system 100
broadly includes
baseplate 120 and appliance 140. Fluid collection system 100 may be used in
conjunction
with other surgical products known to those of skill in the art, including
various types of
surgical drains and surgical drain containers.
[0034] Baseplate 120 includes wafer 121, adhesive backing 122 and a
generally
centered wafer opening 124. Wafer 121 includes wafer opening 124. In some
aspects, a
connector 132 may be affixed and generally centered upon the wafer
- 5 -
Date recue / Date received 2021-12-02
CA 03087508 2020-06-30
WO 2019/164693
PCT/US2019/017491
opening 124. The adhesive backing 122 may be constructed of materials that are
non-allergenic relative to skin contact, be sufficiently tenacious to remain
adhered to
skin for several days, and be able to be removed without pain. Such materials
may
include silicone gel, acrylic, hydrocolloid and other like adhesive materials
known to
those of skill in the art. Wafer 121 may be constructed of a resilient
materials so as to
easily deform and flex when adhered to a patient's skin 105. The outer
perimeter
shape of wafer 121 may be round, square, rectangular, rhombus and other like
shapes.
In use, the baseplate 120 may be adhesively affixed onto a patient's skin 105
with the
wafer opening 124 generally centered upon a surgical drain incision 104 for
receiving
a surgical drain tubing 111, in this manner, the surgical drain tubing ill may
be
anchored with sutures to the skin 105 surrounding the incision 104, as
accessible
through with the wafer opening 124. Further, the drain tubing 111 may be
anchored
with tape to the adjacent skin 105 and/or to a portion of wafer surrounding
the
opening 124 and within the wafer collar 132.
100351 As
configured, the fluid collection system 100, enables the wafer opening
124 in a baseplate 120 to be positioned over a previously placed surgical
drain tubing
111. Alternatively, if baseplate 120 has been previously adhered to the
patient's skin
105 surrounding a surgical incision 104, the drain tubing 111 may be placed
through
the wafer opening 124 in the baseplate 120. A first end of the surgical drain
tubing
111 may be inserted through the surgical incision 104 or wound in the
patient's skin
105. The surgical drain tubing 111 may pass through the wafer 121 and,
additionally
pass through the leaked fluid remover 141,
100361 Appliance 140 includes leaked fluid remover 141. Leaked fluid remover
141 may include a rear facing fluid remover connector 134. The appliance 140
may
further be sealably connected to leaked fluid collector 160. Leaked fluid
collector
160 may include a forward facing outer film 163 and a rear facing inner film
162.
The film material may include any thermoplastic material known to those of
skill in
the art such as polyethylenes and polyvinylchlorides, which easily adhere and
seal to
itself and to other thermoplastic injection molded materials, for example by
radiofrequency, ultrasonic and/or heat sealing processes. The forward facing
outer
film 163 may be transparent to facilitate visualization of the color and other
-6-
CA 03087508 2020-06-30
WO 2019/164693
PCT/US2019/017491
characteristics of the leaked fluids by health care professionals while the
rear facing
inner film 162 may be opaque to assist in visualization of the leaked fluids.
[00371 The leaked fluid collector 160 may be generally configured as a pouch.
Leaked fluid collector 160 is depicted as having an elongated form so as to
more
easily visualize the volume of collected fluid within. However, those of skill
in the
art will appreciate that the leaked fluid collector 160 may have any shape
such as
square, rectangular, round, conical, cylindrical and the like and such shapes
are within
the scope of the invention. A graphic scale 166 may be applied, for example by
a
pad printing process, onto the forward facing outer film 163 to enable a
health care
professional to discern the relative volume of collected fluids. The scale 166
may, for
example, be marked in 10 ml increments up to 100 ml or may comprise any other
appropriate scale known to those of skill in the art.
(0038) Appliance 140 may be mechanically and fluidly connected onto the
baseplate 120, by sealingly coupling the fluid remover connector 134 onto the
wafer
connector 132, for example, in a snap-fit, quarter turn, bayonet and other
types of
connectors known to those of skill in the art. Upon coupling, fluids leaked
from the
surgical incision 104 may pass through the wafer opening 124 via flow path F
(best
seen in FIG. 4b), and into the leaked fluid remover 141, and then further on
into the
leaked fluid collector 160.
(0039) Generally surgical drain tubing 111 is positioned through the rear of
the
appliance 140, and then passed through elastomeric septum 151 positioned
within
leaked fluid remover 141. Surgical drain tubing 111 then exits from the
opposing
front side of appliance 140. Surgical drain tubing 111 may be positioned
through
septum 151 from either direction. In this manner, appliance 140 may be coupled
onto
baseplate 120 after the surgical drain tubing I 1 1 has been placed in the
surgical
Incision 104 or alternatively before the surgical drain tubing 111 is placed
in the
surgical incision. Advantageously, therefore, appliance 140 may be removed
from
the surgical drain tubing 111, as necessary, for example, for maintenance of
the
surgical incision 104 or of the skin 105 surrounding the incision 104 or to
replace
-7-
CA 03087508 2020-06-30
WO 2019/164693
PCT/US2019/017491
baseplate 120, or to replace the appliance 140 or any of the component parts
without
disturbing the surgical drain tubing 111.
100401 The fluid collection system 100 thereby enables uninterrupted draining
of
detritus from internal organs from a surgical incision 104, through surgical
drain
tubing 111 that passes axially through leaked fluid remover 141, while the
leaked
fluid remover 141 simultaneously removes leaked fluid away from surgical
incision
104, and diverts it to be captured and collected into leaked fluid collector
160.
100411 Leaked fluid collector 160 may include port 175 at the proximal end
thereof.
Port 175 is configured to allow a user to drain the leaked fluid from the
leaked fluid
collector 160. The port 175 may include an openableiclosable outlet valve 171
with a
valve actuator 173, for example, a lever, collar, !mob or paddle. The port 175
may
also optionally include a removable cap 176 to prevent dripping of any
residual
voided matter. Cap 176 may optionally include a tether 177 so as to be affixed
adjacent to port 175 for ease of use and accessibility.
100421 Referring now to FIG. 2a a perspective view of the fluid collection
system
100 in use is depicted. Fluid collection system 100 is depicted as a closed
system
used in conjunction with drainage collection container 114a. Appliance 140 is
shown
coupled to baseplate 120 with a wafer 121 adhesively attached to a patient's
skin 105.
A length of surgical drain tubing 111 passes through the leaked fluid remover
141 and
exits the appliance 140 through the elastomeric septum 151. A second end 112
of the
surgical drain tubing 1.1.1 is shown connected to a remotely located surgical
drainage
collection container 114a and is configured to collect drained (non-leaked)
surgical
fluid.. Surgical drain bags are known and are generally positioned away from
and
below a bed-ridden patient, often, for example, to a bed frame to facilitate
optimal
passive gravity flow through the surgical drain tubing and into a drainage
collection
container 114a.
[00431 Referring now to FIG. 2b the overall system configuration of FIG. 2a is
depicted and shows in dashed lines alternative ways 178a, I 78b, 178c leaked
fluid
may be emptied from the leaked fluid collector 160. Leaked fluid may be
emptied
from the leaked fluid collector through valve 171 to port 175 where it may be
directed
-8-
CA 03087508 2020-06-30
WO 2019/164693
PCT/US2019/017491
into a selected waste container of choice for disposal. A first end 109 of a
leaked
fluid drain conduit 178a may be connected onto the leaked fluid collector port
175
and a second end 113 routed to a waste container or receptacle of choice (not
shown).
Alternatively the second end 113 of the leaked fluid drain conduit 178b may be
connected onto a secondary drainage collection container, as may be desired to
further monitor overall total leaked fluid volume over an extended period of
time.. Or
the second end of the leaked fluid drain conduit 178c may be connected onto a
connector 179, inserted downstream within the length of surgical drain tubing,
such
that the leaked fluid may be added to and collected together, along with
accumulated
surgical drainage.
100441 Referring now to FIG. 3,, the locations of basic liquid tight seals
throughout
the fluid collection system 100 are depicted. Appliance 140 and wafer 120 may
be
easily assembled using custom fixtures in conjunction with conventional types
of
ultrasonic, radio frequency or heat sealing methods to achieve liquid tight
bonds. As
such, components may be manually assembled for low pilot production
quantities.
Alternatively, the assembly may be automated with web fed film inputs and
automated component placements.
100451 Flange 131 is positioned on wafer opening 124 and circumferentially
coupled along a peripheral edge to wafer 121 at bond 133. Bond 133. may
comprise
welding or other methods known to those of skill in the art. Housing 142
and/or the
fully assembled leaked fluid remover 141, may also be bonded 165 about its
peripheral edge, onto the inner film 162, positioned on center with respect to
the inner
film opening 167. The sub-assembled valve 171 may be bonded 172 onto the lower
extremity of the outer film 163, positioned on center with respect to the
valve opening
174. Finally, the outer film 163 may be bonded 165 onto the housing 142,
centering
the outer film opening 168 about the central axis 144 of the housing, and the
outer
film 163 may be bonded 164 to the inner film 162, creating a sealed peripheral
edge
about the leaked fluid collection chamber 169.
[00461 Referring now to FIGS. 4 ¨ 7, cross sectional views of the appliance
140 and
fluid collection system 100 are depicted. Each of the components depicted,
with
-9-
CA 03087508 2020-06-30
WO 2019/164693
PCT/US2019/017491
exception of the wafer 121 and leaked fluid collector 160, are generally
circular in
form and radially structured about the central axis 144. Therefore, the
sectional
views, when showing components in static state, are shown symmetrical left to
right,
aside from particular features on the circular forms.
100471 Referring to FIG. 4a, an exploded view of baseplate 120 is shown below
appliance 140. In various aspects, the basep late 120 includes a
circumferential wafer
connector 132 with a radially disposed wiper seal 135 around its inner
surface, both
integrally molded upon a flange 131. In various aspects, the flange 131 may
bonded
or heat sealed to flexible wafer 121 and centered on a wafer opening 124.
100481 Appliance 140 broadly includes leaked fluid remover 141 and septum 151
positioned within housing 142. Those of skill in the art will appreciate that
housing
142 may be injection molded and may comprise a single part or two or more
parts. A
two part housing 142 includes housing component 142.1 and a housing component
142.2, configured to house a septum rim 151.2 therewithin. Housing components
142.1 and 142.2 may be joined, for example, with mating snap fit structure,
ultrasonic
welding, or adhesive bonding. In other aspects a housing 142 provides a
structure
used to interconnect adjacent spaces and components in functional
relationships. In
other aspects, the housing includes a pair of spaced apart circular platform
surfaces
for film to housing bond 165, one for sealably bonding the outer film 163 and
the
other for the inner film 162.
100491 Housing 142 is depicted as a structural body comprised here, for
example, of
two injection molded thermoplastic housing components 142.1 and 142.2. One of
ordinary skill in the art will appreciate that such a structural body with
such particular
functions may be configured in a variety of different ways and still fall
within the
scope of the invention. For example, in some aspects, looking closely at the
cross
hatching of housing components 142.1 and 142,2, housing component 142.1
includes
a fluid remover connector 134, a leaked fluid remover opening 145a (above the
septum) and a film to housing bond 165 for both the inner film 162 and for the
outer
film 163. Mating component 142.2 includes a leaked fluid remover opening 145b
(below the septum) and captures the septum rim 151.2 from below.. In other
aspects,
-10..
CA 03087508 2020-06-30
WO 2019/164693
PCT/US2019/017491
and as best seen in FIG. 6 an alternative structural embodiment for housing
142 is
shown. Component 142.1 includes a fluid remover connector 134 and a film to
housing bond 165 for the inner film 162. However, the film to housing bond 165
for
the outer film 163, as well as the leaked fluid remover opening 145a (above
the
septum), are now both instead positioned on mating component 142.2. Further,
leaked fluid remover opening 145a (above the septum) and the film to housing
bond
165 for the outer film 163 are now part of the mating component 142.2. These
and
other design variations may be conceived, for example, to optimize molding,
manufacturing, heat sealing and/or assembly sequences.
[00501 Referring again to FIG. 4b, baseplate 120 and appliance 140 are shown
matingly coupled by wafer connector 132 and fluid remover connector 134. Fluid
remover connector 134 is inset as a mating circular channel into the lower
portion of
housing 142. Circular ring-shaped wafer connector 132 extends radially upward
from flange 131 and couples to fluid remover connector 134 in a snap-fit
arrangement Those of skill in the art will appreciate, however, that couplings
other
than snap fit arrangements may be used. Mating mechanical interlocks 136
engage
the connector components in assembly. Hoop stress, inherent in mated circular
connector components 132 and 134, which are injection molded with selected
thermoplastic materials, facilitate a secure yet releasable attachment, as
well as an
intimate fluid tight interference fit upon wiper seal 135.. Baseplate 120 and
appliance
140, when coupled together in this manner, create a fluid flow chamber 143 for
fluid
leaked from a surgical incision to flow into and through a leaked fluid
remover 141
and onward to be captured and collected within leaked fluid collector 160
through
flow path F.
[00511 Elastorneric septum 151 housed within housing 142 is molded, for
example,
in highly elastic silicone or thermoplastic elastomer, such as 20 to 30 Shore
A.
Septum orifice 151.1 is generally centered on septum 151 and may also be
generally
centered upon the central axis 144 of housing 142 when in a static state. The
septum
orifice 151.1 may be generally round, cylindrical or frustoconical through its
length
and may be sized to a internal diameter that is smaller than the surgical
drain tubing
111 for which it is intended to be used. Those of skill in the art will
appreciate that
-11..
CA 03087508 2020-06-30
WO 2019/164693
PCT/US2019/017491
various septum 151 sizes may be provided depending on commercially available
outer diameters of surgical tubing. It is contemplated, therefore, that a
range of
appliance 140 products may be made available, each with variously sized
septums
151.
100521 Alternatively, a single appliance 140 may include a small quantity of
easily
interchangeable alternately sized septums 151, each intended for use in
conjunction
with various types of surgical drain tubing 111 or for specific types of
procedures.
For example, one septum orifice 151.1, with a diameter of approximately
2.3mm/.09", may be useful to achieve a positive interference fit around
surgical drain
tubing 111 intended for use to drain abscesses, for which surgical drain
tubing 111
typically ranges in diameter from 2.7mm/8 French up to 4.7mm/14Fr in diameter.
Septum orifices 151.1, of other sizes may be intended for other specific
surgical
applications, for example, a septum orifice of approximately 8mm diameter, may
be
useful for chest/bronchial procedures, for which surgical drain tubing
typically ranges
from approximately 9.3imn/28 Fr up to approximately 11.3mm/34 Fr.
Alternatively,
as another example, fluid collection devices 100 may be offered with two or
more
alternately sized septums 151, with differently sized orifices 151.1 - ranging
from a
smaller size of approximately 2.3mm diameter to a larger of approximately 5mm
diameter, so as together, a sealed fit may be achieved upon surgical drain
tubing 111
needed for small abscesses up to those needed for larger chest/bronchial
procedures -
typically ranging up to approximately 34 Fr/11.3mm diameter.
100531 Septum 151 may include integrally molded thick and thin sections and
alternative feature geometries to achieve specific functions. In other
aspects, a
generally circular elastomeric septum 151 may be captured, about a
circumferential
rim 151.2, between mating injection molded housing 142 components to achieve a
fluid seal closure of a leaked thud remover aperture 147a. In other aspects,
the
septum 151 includes a highly elastic septum orifice 151.1, with ability to
stretch to
receive and yet remain sealed around a range of surgical drain sizes. The
septum
orifice 151.1 is centered within a stiffer septum body 151.3. A highly
flexible septum
diaphragm 151.4 surrounds the stiffer septum body 151.3, enabling the stiffer
septum
body 151.3 to move about radially, axially and/or pivotally, so as to
alleviate side
-12-
CA 03087508 2020-06-30
WO 2019/164693
PCT/US2019/017491
load tension upon the septum orifice 151.1 - caused, for example, by pulling
upon a
surgical drain passing through the septum orifice 151.1 - as may otherwise
induce a
leaking situation should the septum orifice 151.1 become elongated. In other
aspects,
the septum diaphragm 151.4 and septum body 151.3 are enclosed within a loosely
fitting septum space 152, as may be used to controllably limit axial and
radial and/or
pivotal movements of the septum body 151.3.
[0054] Appliance 140 is presented in FIGS. 4a and 4b in static state and in
FIGS. 5a
and 5b in a dynamic state to illustrate and describe dynamic inner functions
of the
fluid collection system 100, which have only been generally described through
earlier
FIGS. With reference to both FIG. 5a and FIG. 5b, a fluid collection system
100 is
illustrated in situ, with wafer 121 affixed by adhesive backing 122 to a
patient's skin
105. The septum orifice 151.1 is shown stretching to accommodate variously
sized
surgical drain tubing 111. The septum body 151.3 is shown as it may move about
axially, radially and/or pivotally within the confines of a septum space 152.
I0055] Referring to FIG. 5a, appliance 140 may be installed or replaced over
surgical drain tubing 111 which has previously been placed through a surgical
incision 104. Similarly, appliance 140 may be uncoupled from a base-plate 120
and
further removed over the second end of the surgical drain tubing Ill, without
replacing or disturbing the surgical drain tubing 111 - as may be needed, for
example,
to clean or treat the surgical incision 104 and/or the adjacent area of
patient's skin
105. Additionally, appliance 140 may be removed as necessary to ease
replacement
of a surgical drain tubing 111, without need to remove the base-plate 120 from
the
patient's skin 105.
[00561 As previously noted, surgical drain tubing 111 may be inserted through
appliance 140 from either direction, i.e. from the top of the appliance 140 or
from the
bottom. The housing 142 therefore may include two leaked fluid remover
openings
145, namely an outer facing opening 145a, above the septum 151 and an inner
facing
opening 145b, below the septum 151. Both openings 145a, 145b may be of
approximately the same size and to clear the largest size surgical drain
tubing 111 for
which the appliance 140 may be intended. 34Fr/11.3mm surgical drain tubing I 1
1
-13-
CA 03087508 2020-06-30
WO 2019/164693
PCT/US2019/017491
for chest/bronchial procedures are typically the largest used. Therefore, such
openings 145 may range in size up to about 12 or 14 ram diameter. Whereas the
septum orifice 151.1, through which a user needs to insert surgical drain
tubing is
visible to the user through an outward facing opening 145a, the inner facing
opening
145 b and associated septum orifice 151.1 are essentially hidden below.
Therefore,
an inner facing opening 145b may include a bi-directional inner funneled
passage
147a and outer funneled passage 147b to assist in guiding surgical drain
tubing
through a septum orifice.
100571 Referring again to both FIG. 5a and 5b, those of skill in the art will
appreciate that it is important that pulling on the surgical drain tubing 111
does not
induce a leak from the appliance 140 by elongating the septum orifice 151.1
through
which it passes, particularly in regard to smaller diameters of drain tubing
111
passing through a larger outer facing opening 145.1. Therefore, the sizing of
the
leaked fluid remover openings 145 may be coordinated relative to the size of
the
smallest intended surgical drain tubing 111 and the radial movement of the
septum
orifice 151.1 as controllable within the septum space 152. That is to say, if
a pulling
force be applied upon the surgical drain tubing 111, relative to the fixed
position of
housing 142, the force will be generally transmitted through the septum
orifice
151.1. If the outer perimeter of a septum orifice 151.1 were to be fixed in
position -
for example to a housing 142, or for example within a septum body 151.3 that
may be
fixed in position to a housing 142 - then a radially applied pulling force
upon the
surgical drain tube 111 could cause the elastomerie septum orifice 151.1
through
which it passes to stretch, distort and elongate. A deformed and elongated
septum
orifice 151,1 may become sufficiently enlarged as to enable fluid to escape
outward
from the fluid flow chamber 143, flowing between the surgical drain tubing ill
and
the inner perimeter of the distorted septum orifice 151.1.
10058] To alleviate such a potential leakage problem, a leaked fluid remover
132
may include a particularly configured septum 151, An elastomeric septum
orifice
151.1 may be generally centered within an outer facing opening 145a and also
generally centered upon and contained within a septum body 151.3, which in
turn
may be generally centered within a flexible elastomeric diaphragm, which in
turn
-14-
CA 03087508 2020-06-30
WO 2019/164693
PCT/US2019/017491
may be affixed within a housing 142, for example with a septum rim 151.2
clamped
between housing components 142.1 and 141.2 of a housing 142.
100591 As generally shown in FIGS. 5a and 5b, an elastomeric septum diaphragm
151.4, may enable a septum body 151.3 and an accompanying septum orifice
151..1
to move about together within a septum space 152 - in the direction of a
pulling force
upon surgical drain tubing 111 passing through the septum orifice 151.1.
[0060] If a leaked fluid remover 141 is configured for use with a
defined/limited
range of variously sized surgical drain tubing 111, the smallest sized tubing
111 will
inherently move about radially further than larger surgical drain tubing 111,
within
the outer facing opening 145a of the leaked fluid remover 141.
[0061] Therefore, the amount of radial movement of the septum body 151.3
enabled within the septum space 152 should be greater than the amount of
radial
movement of the smallest diameter surgical drain tubing 111 enabled within the
outer
facing opening 145a. In such manner, when pulled upon radially, the surgical
tubing
111 will become supported against the peripheral edge of the outer facing
opening
145a prior to the septum body being supported against the peripheral edge of
the
septum space - so as to limit pulling of the surgical drain tubing 111 upon
the septum
orifice and thereby abate elongation of septum orifice 151.1 and consequential
leakage from the fluid flow chamber 143,
[00621 In at least one aspect, the septum orifice 151.1, the septum body
151.3, the
septum diaphragm and septum rim may be a single integrally molded component.
In
other aspects the septum features may be produced as an assembly. In yet
another
aspect, the elastomeric septum orifice 151.1 and the elastomeric septum
diaphragm
151.4 may be injection over-molded or two-shot molded onto a plastic septum
body
151.3.
[00631 Referring now to FIG. 6, the fluid collection system 100 is shown
oriented
vertically to more clearly depict how leaked fluid will passively flow with
gravity
from a surgical incision 104, through a fluid flow channel 143 and into a
leaked fluid
collection chamber 169. Cross sectional exploded view depicts appliance 140
spaced
apart from baseplate 120, which is affixed on a patient's skin 105 by the
adhesive
-15-
CA 03087508 2020-06-30
WO 2019/164693
PCT/US2019/017491
backing 122 of wafer 121. This baseplate 120 is alternatively configured to
include,
what is commonly referred to in ostomy products as, an accordion 125.
Accordion
125 broadly includes accordion flange 127 and accordion flange bond 128.
Accordion 125 in this application is typically circular, formed of flexible
film that
includes a center opening corresponding with a wafer opening 124 and is heat
seal
bonded 133 about an outer peripheral edge of wafer 121 and along an inner
peripheral
edge to the peripheral edge of wafer opening 124. In this manner, the
peripheral
outer edge of wafer 121 may he spaced away from the wafer so as to create a
finger
clearance space 129 below the accordion flange 127 with which to more easily
engage wafer connector 132 on the fluid remover connector 134. As can be seen,
the
fluid flow path F of the leaked fluid exits the patient's skin through the
wafer opening
124 and flows into the fluid flow chamber 143 and out to the leaked fluid
collector
chamber 169 and eventually to the leaked fluid collector 160.
[0064] Referring now to FIG. 7 another aspect of the invention is shown.
Leaked
fluid remover 141 now includes a septum 151 with a mating elastomeric pressure
seal
155, placed on the same axis as the elastomeric septum 151. This feature
advantageously allows the surgical drainage tube 111 to be removed, for
example in a
variety of patient care settings such as during the patient's hospital stay or
when the
patient returns home. The elastomeric pressure seal 155 prevents leaking of
the
leaked fluid and ensures that it exits into the leaked fluid collector 16 via
flow path F
as hereinafter described,
[00651 A surgical incision 104 may need to remain open after removing the
surgical
drain tubing 111 until leaked fluid ceases to flow. Without a surgical drain
tube 111
passing through the septum orifice 151,1, a non-occluded septum orifice 151.1
could
potentially leak fluid from the appliance 140. Inclusion of an elastomeric
pressure
seal 155, such as a one-way duckbill type valve, can work in tandem with an
elastomeric septum orifice 151,1 to occlude flow in the absence of a surgical
drain
tube 111. While an elastomeric septum type valve may be defeated in the
absence of
an occluding surgical drain tube 111, an elastomeric duck-bill type pressure
seal may
be defeated upon insertion of a surgical drain tube 1 1 1 However, together,
they
assure a secure seal is maintained both with and without surgical drain tube
111.
-16-
CA 03087508 2020-06-30
WO 2019/164693
PCT/US2019/017491
100661 As shown configured, for example, in FIG. 7a in static state and in
FIG. 7b
in dynamic state, elastomeric septum 151, with a septum orifice 151.1 and with
a
septum body 151.3 may be configured to function within a septum space 152 - in
similar manner as previously described - in conjunction with an elastomeric
pressure
seal 155. with a pressure seal aperture 155.1 and with an alternatively
configured
funneled passage 147b, with both positioned along a common central axis 144.
Septum 151 may be sealably captured upon a septum rim 151.2 and the pressure
seal
sealably captured upon a mating pressure seal rim 155.2, both captured
together
between a first housing component 141.1 and a second housing component 141.2.
100671 In other aspects of the invention, other methods may be implemented to
achieve a scaled appliance 140 in the absence of a surgical drain tube 111.
Referring
to FIG. 8, a septum orifice plug 158 is shown. Septum orifice plug 158 is
sized and
configured to press fit into an open septum orifice 151.1. Septum orifice plug
158
may, for example, include a fluid remover cap 157, connected, for example, by
cap
tether 159 onto the housing 142 of appliance 140. Those of skill in the art
will
appreciate that all or portions of these components may be integrally molded.
In such
manner the septum orifice plug 158 may be readily available and appropriately
positioned to be inserted along a central axis 144 into an open septum orifice
151.1 in
the absence of a surgical drain tubing 111.
[00681 Referring now to FIG. 9a, appliance 940 including a leaked fluid
remover
941 is shown alternatively configured with an outlet port 949 in lieu of an
affixed
leaked fluid collector 960, for example without an inner film 962 or outer
film 963.
As such, the leaked fluid remover 941 is comprised, as previously described,
of
housing 942, including a housing component 942.1, sealably connected to a
housing
component 942.2, together sealably capturing a septum 951 about a septum rim
951.2; with the septum 951 functioning similarly in regard to having a septum
orifice
951.1, a septum. body 951.3 and a septum diaphragm 951.4; an outer facing
opening
945a and an inner facing opening 945b; a funneled passage 947a and 947b; a
fluid
flow chamber 943 and a fluid remover connector 934 which couples in like
manner
onto wafer connector 933 on base-plate 920 with lange 931 on a wafer 921 with
adhesive backing 922 and a center opening 924. This appliance 940 differs from
-17-
CA 03087508 2020-06-30
WO 2019/164693
PCT/US2019/017491
previously described embodiments, relative to its housing 942 fully enclosing
a fluid
flow chamber 943 and having an outlet port 949 with which this leaked fluid
remover
941 may be coupled via a leaked fluid collection conduit 978 to, for example,
a
drainage collection container 914,
100691 The embodiment of an appliance 940 as described in FIG, 9a is shown
more
generally in FIG. 9b. As such, this appliance 940 is coupled onto a base-plate
920,
shown with a wafer 921 and affixed by adhesive backing 922 onto a patient's
skin
905.. The appliance has a fluid outlet port 949, onto which a leaked fluid
collection
conduit 978 may be connected to divert removed leaked fluids through, for
example,
a 'Y' connector 979, to be accumulated along with drained surgical fluid
emitted
from surgical drain tubing 911.
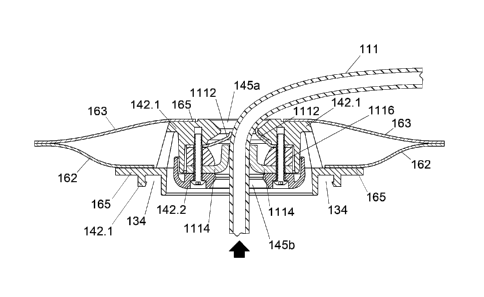
100701 Referring now to FIGS. 10a and 10b, alternative methods of affixing a
fluid
collection system 100 onto a patient's skin 105 are provided. In FIG. 10a, a
one-
piece adhesive affixed system 191 is shown. The one-piece adhesive system
includes
a adhesive backed pad that is applied directly against the patient's skin,
FIG.10b
depicts a two-piece adhesive affixed system 194. Pad 195 includes an adhesive
back
197. Adhesive back 197 is applied to wafer 196 which also includes an adhesive
back 199 that is applied to a patient's skin 105. Wafer 196 may have a smooth
outer
face upon which pad 195 may be adhered. The pad 195 with adhesive back 197 is
applied to the wafer 196 making the two-piece adhesive affixed system 194
easily
removable when necessary.
[00711 Referring now to FIGS. 1 1 a lid an
alternative embodiment of the
appliance 1100 in accordance with the invention is depicted. Like features are
labeled with like reference numerals. The appliance 1100 depicted in FIGS. 11
a ¨
lid does not show the baseplate 120, wafer 121, adhesive backing 122 and
connector
132 coupled thereto. However, the baseplate system 120 is a previously
disclosed
hereinbefore and is best seen in FIG. 3.
[00721 Appliance
1100 broadly includes leaked fluid remover 1110 having an
outer septum 1112 and an inner septum 1114 positioned within housing 142.
Housing 142 is the same as housing 142 of FIGS. 1-10 hereinbefore disclosed.
Those
-18-
CA 03087508 2020-06-30
WO 2019/164693
PCT/US2019/017491
of skill in the art will appreciate that housing 142 may be injection molded
and may
comprise a single part or two or more parts. A two part housing 142 includes
housing
component 142,1 and housing component 142.2. Housing components 142.1 and
142.2 may be joined, for example, with mating snap fit structure, ultrasonic
welding,
or adhesive bonding. As shown two part housing 142.1, 142.2 is matingly joined
by
threaded fasteners 1118. In other aspects housing 142 provides a structure
used to
interconnect adjacent spaces and components in functional relationships. In
other
aspects, the housing includes a pair of spaced apart circular platform
surfaces for film
to housing bond 165, one for sealably bonding the outer film 163 and the other
for the
inner film 162.
[00731 Housing 142 is depicted as a structural body comprised here, for
example, of
two injection molded thermoplastic housing components 142.1 and 142.2. One of
ordinary skill in the art will appreciate that such a structural body with
such particular
functions may he configured in a variety of different ways and still fall
within the
scope of the invention. For example, in some aspects, looking closely at
housing
components 142.1 and 142.2, housing component 142.1 includes a fluid remover
connector 134, a leaked fluid remover opening 145a (above the outer septum
1112)
and a film to housing bond 165 for both the inner film 162 and for the outer
film 163.
Mating component 142.2 includes a leaked fluid remover opening 145b (below the
septum). In other aspects, an alternative structural embodiment for the
housing 142
of appliance 1100 may include a film to housing bond 165 for the outer film
163, as
well as the leaked fluid remover opening 145a (above the septum positioned on
mating component 142.2 similar to that seen in FIG. 6. These and other design
variations may be conceived, for example, to optimize molding, manufacturing,
heat
sealing and/or assembly sequences.
[0074] Appliance 1100 includes spacer 1116, which includes an upper spacer
surface 1120 and a lower spacer surface 1122. The lower surface of outer
septum
1112 is positioned on or integrally formed with upper spacer surface 1120
while the
upper surface of inner septum 1114 is positioned on or integrally formed with
lower
spacer surface 1122. Spacer 1116 is configured to space apart inner and outer
septums 1112, 1114 providing space between the two septums that enable the
septum
-i 9-
CA 03087508 2020-06-30
WO 2019/164693
PCT/US2019/017491
lips 1124, 1126 to splay up and down as the surgical drain tube is inserted
from one
direction or the other, without the flexed lips 1124 of the outer septum 1112
interfering with the flexed lips 1126 of the inner septum 1114 and vice versa
as best
seen in FIGS. 1 1 b ¨ 11d,
100751 Those of skill in the art will appreciate that spacer 1116 may be
constructed
of elastomeric or plastic materials. Spacer 1118 may be molded as a separate
component or may be an integrally formed as part of either or both septums.
100761 Outer septum 1112 and inner septum 1114 may be constructed of
elastomeric materials such as thermoplastic elastomers, silicone and the like.
The
elastomeric inner and outer septum may be compliant, for example having a
durometer of approximately 20 - 40 shore A, so as to adapt to the diameter of
a
passed through surgical drain tube 111.
[00771 Referring to FIGS. 11 e and llf, an outer septum 1112 includes a
generally
centered opening 1128 punched, molded, or cut through the body of outer septum
1112. The opening 1128 may be produced in various diameters, each sized to
stretch
around a defined range of max to min diameter surgical drain tubes Iii. The
opening
1128 through the outer septum 1112 is preferably circular, thin, and compliant
so as
to sealably contain an air and fluid tight seal upon the fluid flow chamber
143 when
the opening 128 is penetrated by a surgical drain tube 111.
[00781 Additionally shown in FIGS. lie and 11F, an inner septum 1114 includes
a
slit 1130 therein, generally along a central portion of its centerline. The
slit 1130
enables a surgical drain tube 111 to be passed through inner septum 1114. The
slit
1130 through inner septum 1114 functions to seal against air and liquid flow
escaping
from the fluid flow chamber 143 in the absence of surgical drain tube 11 1
100791 FIG. I 1g is a cross sectional view of the inner septum 1114 of FIG.
lie,
shown with the slit 1130 sealably closed in its static state. The inner septum
should
preferably be about 3-4 mm thickness of elastomeric material so that it is
thick
enough to prevent air or fluid pressure from forcing it to unseal in the
absence of a
drain tube 111. FIG.. 11h is a cross sectional view of the inner septum
showing the
opposing sides 1131 of slit 1130 as they may flex to resist outward pressure
1132, for
-20-
CA 03087508 2020-06-30
WO 2019/164693
PCT/US2019/017491
example should force be applied upon flexible fluid collection chamber 169 and
transmitted through fluid flow chamber 143, Outward pressure 1132 upon the
inner
septum 1114 will create an interference fit 1133 between the opposing sides
1131 of
the slit 1130, thereby sealing against escaping air or fluid. Whereas a slit
1130
achieves an effective seal through the inner septum 1114 in the absence of a
drain
tube 111, fluids and air may pass through voids 1134 (FIG. 11f) at either end
of the
slit 1130 in the presence of an inserted drain tube 111. Therefore, the outer
septum
1112 provides sealed closure when in use with an inserted surgical drain tube
111.
[00801 While the invention has been described in connection with a plurality
of
different aspects, as illustrated in the various figures and discussed herein,
those of
ordinary skill in the art will appreciate that other similar aspects or
features may be
used and modifications and additions may be made without deviating from the
scope
of the invention. For example, various features may have been described in
particular detail with respect to one aspect of the invention, but such
features may be
incorporated into other aspects described herein without deviating from the
scope of
the invention contemplated by the disclosure. Accordingly, the invention is
not to be
limited by what has been particularly shown and described,
-21 -