Note: Descriptions are shown in the official language in which they were submitted.
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
C5a receptor modulators
The present invention relates to novel C5a receptor modulators of formula (I)
and their use as pharmaceuticals.
The invention also concerns related aspects including processes for the
preparation of the compounds,
pharmaceutical compositions containing one or more compounds of formula (I),
and their use as C5a receptor
modulators, especially in the treatment of vasculitic diseases or disorders,
inflammatory diseases or disorders
involving intravascular microvesicle release, immune complex (IC) diseases or
disorders, neurodegenerative
diseases or disorders, complement related inflammatory diseases or disorders,
bullous diseases or disorders,
diseases or disorders related to ischemia and/or ischemic reperfusion injury,
inflammatory bowel diseases or
disorders, and autoimmune diseases or disorders; as well as in contact
sensitivity or an inflammation caused by
contact with artificial surfaces; increased leukocyte and platelet activation
(and infiltration to tissues thereof);
pathologic sequelae associated to an intoxication or an injury such as a
trauma, an hemorrhage, a shock, or
surgery including transplantation, such sequeale including multiple organ
failure (MOF), septic shock, shock due to
intoxication, or acute lung inflammatory injury; pathologic sequelae
associated with insulin-dependent diabetes
mellitus; myocardial infarction or thrombosis; edema or an increased capillary
permeability; reduction of coronary
endothelial dysfunction induced by cardiopulmonary bypass and/or cardioplegia;
or cancer.
C5aR1 (CD88) is a seven transmembrane bound G protein coupled receptor (GPCR)
belonging to the rhodopsin
like family, the gene of which is located on chromosome 19. It couples to
pertussis toxin sensitive Gialpha2,
Gialpha3 or pertussis toxin insensitive Galpha16 and initiates several
downstream signaling pathways. C5aR1 is
expressed on a number of immune cell types including monocytes, neutrophils,
mast cells, basophils and
eosinophils. In addition, it is expressed on many other cell types including
hepatocytes, pulmonary and endothelial
cells, microglia, neurons and renal glomerular cells. There are a number of
ligands described which bind to the
C5aR. These include C5a, C5adesArg and C5a +1kDa. C5a is a central effector
molecule of the complement
system which itself is a complex enzymatic cascade evolved to crucially
complement the immune system against
invading pathogens, however, a significant body of evidence shows that
inadvertent complement activation leads to
many acute inflammatory disorders and autoimmune diseases (Ricklin, D., et al.
(2010) "Complement: a key
system for immune surveillance and homeostasis." Nat Immunol 11(9): 785-797)
and specifically C5a has been
shown to be elevated in a number of these inflammatory and autoimmune
disorders. The complement system is
activated through four pathways: The classical pathway, and the mannose
binding lectin (MBL) pathway which is
similar to the classical pathway except for the initial recognition and
activation steps which recognize pathogens or
antibody complexes. The alternative pathway is activated by binding of
spontaneously activated complement C3
protein (C3b fragment) to pathogen surface. These three pathways all lead to
the eventual formation of C3
convertases, which is the point where the 3 pathways converge (Guo, R. F. and
P. A. Ward (2005) Annu Rev
Immunol 23: 821-852). Subsequently C3 convertases lead to the formation of the
anaphalatoxins C3a and C5a,
together with other complement proteins required to produce the membrane
attack complex. A fourth pathway, the
extrinsic pathway involves plasma proteases (eg. elastase, thrombin) which act
directly on C3 or C5 leading to the
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
2
subsequent production of C3a and C5a. The anaphylatoxin C5a leads to the
recruitment and activation of
inflammatory cells of the innate and adaptive system, partly through the
enhancement of cell adhesion molecule
expression, the release of granule-based enzymes, delayed or enhanced
apoptosis, phagocytosis, oxidative burst,
histamine secretion and release and chemotaxis. In addition, it elicits the
release of other pro inflammatory
mediators, such as TNF-a, IL-1, IL-6, IL-8, prostaglandins, and leukotrienes)
(N.S. Merle et al. (2015) "Complement
System Part II: Role in Immunity." Front Immunol 6: 257), activation of
endothelial cells and vascular permeability
which may lead to events in which at the end thrombotic microangiopathy can
occur. Therefore, C5a represents
one of the most potent inflammatory molecules produced during immune responses
and because of its
fundamental biology it is potentially implicated in a very wide range of
pathologies (Janeway's lmmunobiology,
edition (2012), Kenneth Murphy, Garland Science, p. 48-72).
C5a is central to the immune system and as such is important in key aspects of
inflammation and tissue injury. In
addition, there is considerable experimental evidence in the literature that
implicates increased levels of C5a with a
number of diseases and disorders, in particular in autoimmune and inflammatory
diseases and disorders (Ricklin,
D., et al. (2010) Nat Immunol 11(9): 785-797).
There is a large body of evidence about C5a and its receptor C5aR in
contributing to vasculitic diseases, which
demonstrate that C5a levels are elevated and give rise to leukocyte migration
and subsequent inflammation which
then leads to the eventual destruction of vessel walls (Charles J., et al
(2013) Semin Nephrol 33(6): 557-564;
Vasculitis, 2nd Edition (2008), Edited by Ball and Bridges, Oxford University
Press, pp 47-53; Huang, Y. M., et al.
(2015) Arthritis Rheumatol 67(10): 2780-2790; Kallenberg, C. G. and P.
Heeringa (2015) Mol Immunol 68(1): 53-
56). Inhibition of the C5aR with a C5aR antagonist was effective at
ameliorated anti-myeloperoxidase (MPO)-
induced NCGN in mice expressing the human C5a receptor (Xiao, H. et al (2014)
J Am Soc Nephrol 25(2): 225-
231) and was confirmed to be effective in a phase II trial of patients with
anti-neutrophil cytoplasmic antibody
(ANCA) associated vasculitis (ClinicalTrials.gov Identifier NCT02222155).
Therefore, a C5a antagonist may be
useful to treat vasculitic diseases such as ANCA associated vasculitis,
leukoclastic vasculitis, Wegener's
granulomatosis, microscopic polyangiitis, Churg-Strauss syndrome, Henoch-
Schonlein purpura, polyateritis
nodosa, rapidly progressive glomerulonephritis (RPGN), cryoglobulinaemia,
giant cell arteritis (GCA), Behcet's
disease and Takayasu's arteritis (TAK).
C5a is generated when human blood makes contact with artificial surfaces, such
as in cardiopulmonary bypass and
hemodialysis procedures for instance on the artificial surface of the heart -
lung machine in association with
vascular surgery such as coronary artery bypass grafting or heart valve
replacement or on surfaces of a kidney
dialysis machine (Howard, R. J., et al. (1988) Arch Surg 123(12): 1496-1501;
Kirklin, J. K., et al. (1983) J Thorac
Cardiovasc Surg 86(6): 845-857; Craddock, P. R., et al. (1977) J Clin Invest
60(1): 260-264; Craddock, P. R., et al.
(1977) N Engl J Med 296(14): 769-774) or in association with contact with
other artificial vessels or container
surfaces (e.g. ventricular assist devices, artificial heart machines,
transfusion tubing, blood storage bags,
plasmapheresis, plateletpheresis, and the like). As such C5aR antagonists
could prove useful in preventing
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
3
deleterious consequences of contact sensitivity and/or inflammation caused by
contact with artificial surfaces. In
addition, it may be useful in treating inflammatory disorders involving
intravascular microvesicle release such as for
example thrombotic microangiopathy and sickle cell disease (Zecher, D., et al.
(2014) Arterioscler Thromb Vasc
Biol 34(2): 313-320). A C5aR antagonist could also prove useful in certain
hemotological diseases which are
associated with activation of coagulation and fibrinolytic systems,
disseminated intravascular coagulation (DIG),
pernicious anemia, warm and cold autoimmune hemolytic anemia (AIHA), anti-
phospholipid syndrome and its
associated complications, arterial and venous thrombosis, pregnancy
complications such as recurrent miscarriage
and fetal death, preeclampsia, placental insufficiency, fetal growth
restriction, cervical remodeling and preterm
birth, idiopathic thrombocytopenic purpura (ITP), atypical hemolytic uremic
syndrome (aHUS), paroxysmal
nocturnal hemoglobinuria (PNH) and allergic transfusion reactions. The C5-
specific humanized antibody,
eculizumab is approved for paroxysmal nocturnal hemoglobinuria and atypical
haemolytic uraemic syndrome
(aHUS) (Wong EK, Kavanagh D, Transl Res. (2015) 165(2):306-20) and has been
shown to be efficacious in renal
transplant such as acute antibody-mediated kidney allograft rejection and cold
agglutinin disease further supporting
a potential role for C5aR antagonists in these diseases.
In myocardial ischemia-reperfusion injury C5a has been described to have an
important function. Complement
depletion reduced myocardial infarct size in mice (Weisman, H. F., T. et al.
(1990) Science 249(4965): 146-151; De
Hoog, V. C., et al. (2014) Cardiovasc Res 103(4): 521-529) and treatment with
anti-05a antibodies reduced injury
in a rat model of hindlimb ischemia-reperfusion (Bless, N. M., et al. (1999)
Am J Physiol 276(1 Pt 1): L57-63).
Reperfusion injury during myocardial infarction was also markedly reduced in
pigs that were re-treated with a
monoclonal anti-05a IgG (Amsterdam, E. A., et al. (1995) Am J Physiol 268(1 Pt
2): H448-457). A recombinant
human C5aR antagonist reduces infarct size in a porcine model of surgical
revascularization (Riley, R. D., et al.
(2000) J Thorac Cardiovasc Surg 120(2): 350-358) providing evidence for the
utility of a C5aR antagonist in these
diseases. In addition, diseases related to ischemia / reperfusion injury, such
as those resulting from transplants,
including solid organ transplant, where C5a has been shown to play an
important role (Farrar, C. A. and S. H.
Sacks (2014) Curr Opin Organ Transplant 19(1): 8-13), could benefit from a
C5aR antagonist as could related
syndromes such as ischemic reperfusion injury, ischemic colitis and cardiac
ischemia (Mueller, M., et al. (2013)
lmmunobiology 218(9): 1131-1138).
Furthermore, diseases where complement plays a role such as coronary
thrombosis (Distelmaier, K., et al. (2009)
Thromb Haemost 102(3): 564-572), vascular occlusion, post-surgical vascular
reocclusion, atherosclerosis,
traumatic central nervous system injury, arrhythmogenic cardiomyopathy
(Mavroidis, M., et al. (2015) Basic Res
Cardiol 110(3): 27) and Gaucher disease (Pandey et al. (2017) Nature 543: 108-
112) could also benefit from a
C5aR antagonist. Thus, C5aR modulators may be used preventatively in a patient
at risk for myocardial infarction
or thrombosis (i.e. a patient who has one or more recognized risk factors for
myocardial infarction or thrombosis,
such as, but not limited to, obesity, smoking, high blood pressure,
hypercholesterolemia, previous or genetic history
of myocardial infarction or thrombosis) in order reduce the risk of myocardial
infarction or thrombosis.
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
4
C5a causes increased capillary permeability and edema, leukocyte and platelet
activation and infiltration to tissues,
as well as bronchoconstriction (Sarma, J. V. and P. A. Ward (2012) Cell Health
Cytoskelet 4: 73-82; Czermak, B.
J., et al. (1998) J Leukoc Biol 64(1): 40-48). Administration of an anti-05a
monoclonal antibody was shown to
reduce cardiopulmonary bypass and cardioplegia-induced coronary endothelial
dysfunction (Tofukuji, M., et al.
(1998) J Thorac Cardiovasc Surg 116(6): 1060-1068).
C5a and its receptor are also involved in the pathogenesis of acute
respiratory distress syndrome (ARDS)
(Hammerschmidt, D. E., et al. (1980) Lancet 1(8175): 947-949), Chronic
Obstructive Pulmonary Disorder (COPD)
(Marc, M. M., et al. (2004) Am J Respir Cell Mol Biol 31(2): 216-219), and
multiple organ failure (MOF) (Huber-
Lang, M., et al. (2001) "Role of C5a in multiorgan failure during sepsis." J
Immunol 166(2): 1193-1199; Heideman,
M. and T. E. Hugh i (1984) J Trauma 24(12): 1038-1043;). C5a increases
monocyte production of two important
proinflammatory cytokines TNF-a and IL-I which contribute to pathology in
these diseases. C5a has also been
shown to play an important role in the development of tissue injury, and
particularly pulmonary injury, in animal
models of septic shock (Smedegard, G., et al. (1989) Am J Pathol 135(3): 489-
497; Unnewehr, H., et al. (2013) J
Immunol 190(8): 4215-4225). In sepsis models using rats, pigs and non-human
primates, anti-05a antibodies
administered to the animals before treatment with endotoxin or E. coli
resulted in decreased tissue injury, as well as
decreased production of IL-6 (Hopken, U., et al. (1996) Eur J Immunol 26(5):
1103-1109; Stevens, J. H., et al.
(1986) J Chin Invest 77(6): 1812-1816). Inhibition of C5a with anti-05a
polyclonal antibodies has been shown to
significantly improve survival rates in a caecal ligation/puncture model of
sepsis in rats (Czermak, B. J., et al.
(1999) Nat Med 5(7): 788-792). In the same sepsis model, anti-05a antibodies
were shown to inhibit apoptosis of
thymocytes (Guo, R. F., et al. (2000) J Chin Invest 106(10): 1271-1280). Anti-
05a antibodies were also protective in
a cobra venom factor model of lung injury in rats, and in immune complex-
induced lung injury (Mulligan, M. S., et
al. (1996) J Chin Invest 98(2): 503-512). The importance of C5a in immune
complex-mediated lung injury was also
shown in mouse (Bozic, C. R., et al. (1996) Science 273(5282): 1722-1725).
Therefore, a C5aR antagonist could
be of benefit in many inflammatory disorders and related conditions including
neutropenia, sepsis, septic shock,
stroke, inflammation associated with severe burns (Hoesel, L. M., et al.
(2007) J Immunol 178(12): 7902-7910),
osteoarthritis (Yuan, G., et al. (2003) Chin Med J (Engl) 116(9): 1408-1412),
as well as acute (adult) respiratory
distress syndrome (ARDS), chronic pulmonary obstructive disorder (COPD),
bronchial asthma (Pandey, M. K.
(2013) Curr Allergy Asthma Rep 13(6): 596-606), systemic inflammatory response
syndrome (SIRS), tissue graft
rejection, hyperacute rejection of transplanted organs, and the like, and
multiple organ dysfunction syndrome
(MODS). In addition, C5aR antagonists may be beneficial in treating pathologic
sequelae associated with insulin-
dependent diabetes mellitus such as diabetic kidney disease (Li, L., et al.
(2015) Metabolism 64(5): 597-610),
diabetic retinopathy (Cheng, L., et al. (2013). Invest Ophthalmol Vis Sci
54(13): 8191-8198), lupus nephropathy
(Bao, L., et al. (2005) Eur J Immunol 35(8): 2496-2506), Heyman nephritis,
membranous nephritis, and other forms
of glomerulonephritis such as C3 glomerulopathy including dense deposit
disease (DDD) (Zhang et al., Chin J Am
Soc Nephrol (2014) 9: 1876-1882). Furthermore, the compound eculizumab has
been shown to have potential
utility for the treatment of neuromyelitis optica.
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
C5aR antagonists substantially reduced ovalbumin (OVA)-induced total cell
(60%), neutrophil (66%) and eosinophil
(65%) influxes in lavage fluid sampling suggesting that C5aR blockage might
represent a novel therapeutic agent
for reducing asthmatic outcomes (Staab, E. B., et al. (2014) Int
Immunopharmacol 21(2): 293-300).
The complement system and in particular C5a contribute to the development of
many bullous diseases among
5 other things through activation of innate cells including mast cells and
neutrophils (e.g. bullous pemphigoid, bullous
acquisita, pemphigus foliaceus and pemphigus vulgaris). The detachment of
epidermal basal keratinocytes from
the underlying basement membrane is thought to be caused by autoantibodies to
keratinocytes at the cutaneous
basement membrane leading to blisters and a high influx of neutrophils in both
the upper dermal layers and within
the blister cavities. In experimental models a reduction of neutrophils or
absence of complement (total or C5-
selective) can inhibit formation of sub-epidermal blisters (Heimbach, L., et
al. (2011) J Biol Chem 286(17): 15003-
15009; Gammon, W. R. (1989) Immunol Ser 46: 509-525). Recent evidence has
emerged to suggest that inhibition
of C5a may prove beneficial in the treatment of the skin disorder hidradenitis
suppurativa where an antibody
against human C5a was shown to improve patient outcome in an open label phase
II clinical trial. A C5a receptor
antagonist may therefore be useful in bullous diseases.
Complement is believed to be important in inflammatory bowel disease (IBD)
pathology and the C5aR is found to
be expressed in the epithelial cells of the colon. (Cao, Q., et al. (2012) Am
J Physiol Cell Physiol 302(12): C1731-
1740). In addition, pharmacological inhibition of C5a activity by PMX205 a
peptidic C5aR antagonist is efficacious
in preventing DSS-induced colitis, providing further evidence that targeting
CD88 in patients with IBD irritable bowel
syndrome, ulcerative colitis, Crohn's disease, inflammatory bowel disease
(IBD) (Johswich, K., et al. (2009)
Inflamm Bowel Dis 15(12): 1812-1823) could be of therapeutic benefit
(Woodruff, T. M., et al. (2003) J Immunol
171(10): 5514-5520; Jain, U., et al. (2013) Br J Pharmacol 168(2): 488-501).
There is a body of evidence suggesting a role for C5a and its receptor in
pathologies of the CNS. C5aR expression
is upregulated on reactive astrocytes, microglia, and endothelial cells in an
inflamed human central nervous system
(O'Barr, S. A., et al. (2001) J Immunol 166(6): 4154-4162; Gasque, P., et al.
(1997) Am J Pathol 150(1): 31-41) and
C5a has been reported to be involved in the pathogenesis of many
neurodegenerative diseases, such as
amyotrophic lateral sclerosis (ALS) (Mantovani, S., et al. (2014) J
Neuroimmunol 276(1-2): 213-218; Humayun, S.,
et al. (2009) J Neuroimmunol 210(1-2): 52-62; Woodruff, T. M., et al. (2008) J
Immunol 181(12): 8727-8734),
Alzheimer disease (Fonseca, M. I., et al. (2013) J Neuroinflammation 10: 25;
Ager, R. R., et al. (2010) J
Neurochem 113(2): 389-401), Parkinson's disease (Wang, X. J., et al. (2007)
Neurochem Int 50(1): 39-50) and
Huntington's disease (Singhrao et al. (1999) Experimental Neurology 159, 362-
376). Furthermore C5a is found to
be elevated in the CSF of Guillain-Barre syndrome patients (Hartung, H. P., et
al. (1987) Neurology 37(6): 1006-
1009; Wakerley, B. R. and N. Yuki (2015) Expert Rev Neurother 15(8): 847-849)
and an anti C5 antibody was
found to be effective in reducing neuropathy in the mouse (Halstead, S. K., et
al. (2008) Brain 131 (Pt 5): 1197-
1208; Basta, M. and D. R. Branch (2014) Clin Exp Immunol 178 Suppl 1: 87-88).
Also, inhibition of the C5a
receptor alleviates experimental CNS lupus (Zwirner, J., et al. (1999) Mol
Immunol 36(13-14): 877-884; Jacob, A.,
B. Hack, et al. (2010) J Neuroimmunol 221(1-2): 46-52). Therefore, C5aR
antagonists provided herein may be to
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
6
treat ALS, Alzheimer's disease, multiple sclerosis, Guillain-Barre syndrome,
Parkinson's disease, Huntington's
disease and also cognitive function decline associated with cardiopulmonary
bypass surgery and related
procedures in addition to central nervous system involvement in diseases such
as SLE, Sjogren's syndrome and
associated immunological profiles.
In many autoimmune diseases lmmunoglobulin G-containing immune complex (IC)
depositions are found. These
contribute to the pathophysiology of the diseases which frequently manifest in
different organs of the body including
the kidneys, heart, lungs, liver, blood vessels, the nervous system and the
skin. There are numerous such IC
diseases and examples are systemic lupus erthyematosus (SLE),
cryoglobulinemia, rheumatoid arthritis, Sjogren's
syndrome (Lawley, T. J., et al. (1979) J Immunol 123(3): 1382-1387),
Goodpasture syndrome (antiglomerular
basement antibody disease), and hypersensitivity. Immune complexes are known
to induce C5 convertases leading
to C5a production which subsequently contributes to these diseases (Karsten,
C. M. and J. Kohl (2012)
lmmunobiology 217(11): 1067-1079). In animal models reproducing the mechanisms
of IC activation of
complement, C5aR has been shown to play an important role. Studies show that
C5aR deficient mice and the use
of a peptidic C5aR antagonist result in protection from tissue injury induced
by ICs. (Strachan, A. J., et al. (2000) J
Immunol 164(12): 6560-6565; Kohl, J. and J. E. Gessner (1999) Mol Immunol
36(13-14): 893-903; Baumann, U., et
al. (2000) J Immunol 164(2): 1065-1070). Therefore, inhibitors of C5aR could
be useful to treat IC diseases
including the autoimmune diseases rheumatoid arthritis (Jose, P. J., et al.
(1990) Ann Rheum Dis 49(10): 747-752;
Grant, E. P., et al. (2002) J Exp Med 196(11): 1461-1471; Yuan, G., et al.
(2003) Chin Med J (Engl) 116(9): 1408-
1412)), osteoarthritis, systemic lupus erythematosus (Porcel, J. M., et al.
(1995) Clin Immunol lmmunopathol 74(3):
283-288; Pawaria, S., et al. (2014) J Immunol 193(7): 3288-3295), lupus
nephritis (Bao, L., et al. (2005) Eur J
Immunol 35(8): 2496-2506), lupus glomerulonephritis and IgA nephropathy (Liu,
L., et al. (2014) J Clin Immunol
34(2): 224-232), Heyman nephritis, membranous nephritis and other forms of
glomerulonephritis, vasculitis,
dermatomyositis (Fiebiger, E., et al. (1998) J Clin Invest 101(1): 243-251),
pemphigus, systemic sclerosis
(scleroderma) (Sprott, H., et al. (2000) J Rheumatol 27(2): 402-404),
bronchial asthma, autoimmune hemolytic and
thrombocytopenic states, Goodpasture's syndrome (and associated
glomerulonephritis and pulmonary
hemorrhage) (Ma, R., et al. (2013) J Clin Immunol 33(1): 172-178),
immunovasculitis, and complement mediated
thrombotic microangiopathies including atypical haemolytic uremic syndrome
(Song, D., et al. (2015) Am J Reprod
Immunol 74(4): 345-356; Davin, J. C., N. C. van de Kar (2015) Ther Adv Hematol
6(4): 171-185), mixed
cryoglobulinemia, atopic dermatitis (Neuber, K., R. et al. (1991) Immunology
73(1): 83-87; Dang, L., et al. (2015)
Mol Med Rep 11(6): 4183-4189), and chronic urticaria (Kaplan, A. P. (2004) J
Allergy Clin Immunol 114(3): 465-
474; Yan, S., et al. (2014) J Dermatol Sci 76(3): 240-245). Furthermore, the
compound eculizumab has been
shown to have potential utility for the treatment of myasthenia gravis, and
anti-phospholipid syndrome.
C5a is present in psoriatic plaques and C5aR expression has also been reported
in psoriasis where T cells,
neutrophils mast cells and dendritic cells are involved in pathogenesis of the
disease and are chemotactic to C5a
(Diani, M., G. Altomare and E. Reali (2015) Autoimmun Rev 14(4): 286-292).
Neutrophil accumulation under the
stratum corneum is observed in the highly inflamed areas of psoriatic plaques,
and psoriatic lesion (scale) extracts
CA 03087886 2020-07-08
WO 2019/141803 PCT/EP2019/051230
7
contain highly elevated levels of C5a and exhibit potent chemotactic activity
towards neutrophils, an effect that can
be inhibited by addition of a C5a antibody. Furthermore, T cells and
neutrophils are chemo-attracted by C5a under
certain conditions (Nataf, S., et al. (1999) J Immunol 162(7): 4018-4023;
Tsuji, R. F., et al. (2000) J Immunol
165(3): 1588-1598; Werfel, T., et al. (1997) Arch Dermatol Res 289(2): 83-86;
Mrowietz, U., et al. (2001) Exp
.. Dermatol 10(4): 238-245) meaning C5aR antagonists may be of benefit in
treating psoriasis. Furthermore,
complement has been implicated in the pathogenesis of glaucoma (Howell et al.
(2011), J. Clin. Invest. 121(4):
1429-1444). In addition, there is experimental evidence to suggest a
beneficial role of C5aR antagonists in treating
cancer with checkpoint blockers. For example, an antibody against the C5aR
receptor (IPH5401) has been
reported to be efficacious in muring models of cancer (web page Innate Pharma
¨ IPH5401, 2018;
https://www.innate-pharma.com/en/pipeline/iph5401-first-class-anti-c5ar-mab;
Zah H., et al. (2017)
Oncoimmunology 6(10): e1349587; Wang Y., et al., (2016) Cancer Discovery 6(9)
1022-1035).
Thus, C5a and C5aR are believed to be clinically implicated in vasculitic
diseases or disorders, inflammatory
diseases or disorders involving intravascular microvesicle release, immune
complex (IC) diseases or disorders,
neurodegenerative diseases or disorders, complement related inflammatory
diseases or disorders, bullous
diseases or disorders, diseases or disorders related to ischemia and/or
ischemic reperfusion injury, inflammatory
bowel diseases or disorders, and autoimmune diseases or disorders; as well as
in contact sensitivity or an
inflammation caused by contact with artificial surfaces; increased leukocyte
and platelet activation (and infiltration
to tissues thereof); pathologic sequelae associated to an intoxication or an
injury such as a trauma, an hemorrhage,
a shock, or surgery including transplantation, including multiple organ
failure (MOF), septic shock, shock due to
intoxication, or acute lung inflammatory injury; pathologic sequelae
associated with insulin-dependent diabetes
mellitus; myocardial infarction or thrombosis; edema or an increased capillary
permeability; reduction of coronary
endothelial dysfunction induced by cardiopulmonary bypass and/or cardioplegia,
or cancer.
There is therefore a requirement for new small organic molecule modulators of
the C5a receptor (C5aR), especially
antagonists of the C5aR, that could be useful for inhibiting pathogenic events
associated with elevated levels of
C5a and/or with C5aR activation.
Certain benzimidazolone derivatives as chymase inhibitors are disclosed in
W02008/147697. 4-(Benzimidazol-1-
y1)-piperidines as sodium channel inhibitors are disclosed in W02003/037890.
Certain 3-substituted piperidines
comprising urea functionality are disclosed as analgesics in W02001/068604.
Benzimidazolone derivatives as
phosphodiesterase inhibitors are disclosed in W02001/005770. 1-Benzy1-1,3-
dihydro-2H-benzimidazol-2-one
derivatives as vasopressin and/or oxytocin receptor ligands are disclosed in
US 5,661,169. D. R. Owen et al.,
Bioorg. Med. Chem. Lett. 19 (2009) 1702-1706 discloses the compound 3-(1-(4-
aminopyridin-2-yl)piperidin-4-y1)-1-
benzy1-1,3-dihydro-2H-imidazo[4,5-b]pyridin-2-one in a series of 2,4-
diaminopyridine 6-opioid receptor agonists,
which however had pronounced activity in the hERG binding assay.
The present invention provides cyclic urea derivatives of formula (I) which
are modulators of the C5a receptor, and
are useful for the prevention or treatment of diseases which respond to the
C5a receptor.
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
8
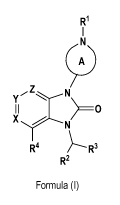
1) A first aspect of the invention relates to compounds of the formula (1)
R1
II
1
cA)
y Z N
> ___________________________________________ 0
X
R4 R3
R2
Formula (1)
wherein
= ring A represents a saturated 4- to 7-membered mono-cyclic carbocyclic
ring containing the ring nitrogen atom
to which R1 is attached, wherein said ring independently is unsubstituted, or
mono-, or di-substituted wherein
the substituents are independently selected from (C1_3)alkyl (especially
methyl), fluoro, or (C1_4)alkoxy-carbonyl
(especially ethoxy-carbonyl) [especially such ring A is azetidin-1,3-diyl,
pyrrolidin-1,3-diyl, 4-methyl-pyrrolidin-
1,3-diyl, piperidin-1,3-diyl, piperidin-1,4-diyl, 3-fluoro-piperidin-1,4-diyl,
2-methyl-piperidin-1,4-diyl, 3-methyl-
piperidin-1,4-diyl, 3-(ethoxycarbonyI)-piperidin-1,4-diyl, 3,3-dimethyl-
piperidin-1,4-diyl, azepan-1,4-diyI]; or
= ring A represents a saturated 7- or 8-membered bridged bi-cyclic
carbocyclic ring containing the ring nitrogen
atom to which R1 is attached, wherein said ring is unsubstituted [especially
such ring is 2-aza-
bicyclo[2.2.1]heptane-2,5-diyl, 3-aza-bicyclo[3.1.1]heptane-3,6-diyI];
X and Z independently represent CR, or N; and Y represents CR5; or, in case
both X and Z represent CH, Y may in
addition represent N; wherein each R5 independently represents hydrogen, or
(C1_3)alkyl (especially methyl)
[notably one of X and Z represents CH or N; the other represents CH; and Y
represents CR,, wherein especially R,
represents hydrogen];
R1 represents
= (Ci4alkyl (especially methyl, isopropyl);
= -CO-R11 wherein R11 represents (C1_4)alkyl; (C1_4)alkoxy; phenyl,
phenoxy; (C3_6)cycloalkyl; or (C3_6)cycloalkoxy
optionally containing one ring oxygen and optionally mono-substituted with
methyl or trifluoromethyl [especially
such group -CO-R11 is tert.-butyl-carbonyl, methoxycarbonyl, ethoxycarbonyl,
isopropoxycarbonyl, n-
butoxycarbonyl, tert.-butoxycarbonyl, oxetan-3-yl-oxy-carbonyl, (3-methyl-
oxetan-3-yI)-oxy-carbonyl, (3-
trifluoromethyl-oxetan-3-yI)-oxy-carbonyl, phenylcarbonyl, phenoxycarbonyl,
cyclopropylcarbonyl];
= benzyl wherein the phenyl ring of said benzyl is optionally mono- or di-
substituted with halogen [especially 2,4-
dichlorobenzyl]; or
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
9
= phenyl, or 5- or 6-membered heteroaryl (especially pyrazolyl,
oxadiazolyl, pyridinyl, pyridazinyl, pyrimidinyl);
wherein said phenyl or 5- or 6-membered heteroaryl independently is
unsubstituted, mono-, di- or tri-
substituted, wherein the substituents are independently selected from
(Ci_4)alkyl (especially methyl, isopropyl);
(Ci_4)alkoxy (especially methoxy, n-butyloxy);
(Ci_3)fluoroalkyl (especially trifluoromethyl);
(C1_3)fluoroalkoxy (especially trifluoromethoxy);
D halogen (especially fluoro, chloro, bromo);
cyano;
D nitro;
D hydroxy;
hydroxy-(C1_3)alkyl (especially 1-hydroxyethyl, hydroxymethyl, 1-hydroxy-1-
methyl-ethyl);
(Ci_4)alkoxy-(Ci_3)alkyl (especially 1-methoxyethyl);
D hydroxy-(C2_3)alkoxy (especially 2-hydroxy-ethoxy);
(C1_4)alkoxy-(C2_3)alkoxy (especially 2-methoxy-ethoxy);
D (C3_6)cycloalkyl-X12-, wherein X12 represents a direct bond, -0-, or -
(C1_3)alkylene-0-, and wherein the (C3_
6)cycloalkyl independently contains one optional ring oxygen [especially such
group (C3_6)cycloalkyl-X12- is
cyclopropyl, cyclopropyl-oxy, cyclobutyl-oxy, oxetan-3-yl-oxy, cyclopropyl-
methoxy, or tetrahydropyran-4-
yl-oxy];
D R13-CO-X13-, wherein X13 represents a direct bond or (C1_3)alkylene, and R13
represents hydrogen or (C1_
4)alkyl (especially such group R13-CO-X13 is formyl, acetyl, 2-oxo-ethyl);
3-(morpholin-4-yI)-prop-1-ynyl;
R14aR14bN_X14_, wherein X14 represents a direct bond or (C1_3)alkylene; and
wherein R14a and R14b
independently represent hydrogen, (C1_4)alkyl, hydroxy-(C2_4)alkyl,
(C1_3)alkoxy-(C24alkyl; or R14a and R14b
together with the nitrogen to which they are attached to form a 4- to 6-
membered saturated ring optionally
containing one additional ring heteroatom selected from oxygen and nitrogen;
and wherein said ring is
optionally mono-substituted with (C1_4)alkyl (especially methyl), hydroxy,
(C1_4)alkoxy (especially methoxy),
or dimethylamino,
[especially such group R14aR14bN_x14_ is amino, methylamino-methyl,
dimethylamino-methyl, [(2-
hydroxyethyp-methylamino]-methyl, [(2-hydroxyethyl)-ethylamino]-methyl, [(2-
methoxy-1-methyl-ethyl)-
amino]-methyl, [(2-methoxyethyp-methylamino]-methyl, [di-(2-hydroxyethyp-
amino]-methyl, (azetidin-1
methyl, (pyrrolidin-1-yI)-methyl, (piperidin-1-yI)-methyl, morpholin-4-yl,
piperazin-1-ylmethyl, (morpholin-4-
y1)-methyl, 2-(morpholin-4-yI)-ethyl, 3-(morpholin-4-yI)-propyl, (3-methoxy-
azetidin-1-y0-methyl, 1-(3-
methoxy-azetidin-1 -yI)-ethyl, (1 -methyl-piperazin-4-yI)-
methyl, (4-hydroxypiperidin-1-yI)-methyl, (4-
methoxypiperidin-1-yI)-methyl, (3-methoxy-pyrrolidin-1-yI)-methyl, (4-
dimethylamino-piperidin-1-yI)-methyl];
or
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
D benzyloxy, wherein the phenylring of benzyloxy is optionally mono- or di-
substituted with halogen or
methyl;
R2 represents phenyl, or 5- or 6-membered heteroaryl (notably 6-membered
heteroaryl; in particular thiazolyl,
pyridinyl, pyrimidinyl, pyrazinyl, pyridazinyl); wherein said phenyl or 5- or
6-membered heteroaryl independently is
5 mono-, di- or tri-substituted (especially mono-substituted, in particular
in ortho position with regard to the point of
attachment of the rest of the molecule), wherein the substituents are
independently selected from
D (Ci_4)alkyl (especially methyl, isopropyl);
(Ci_4)alkoxy (especially methoxy, isopropoxy);
(Ci_3)fluoroalkyl (especially trifluoromethyl);
10 (C1_3)fluoroalkoxy (especially trifluoromethoxy);
D halogen (especially chloro, fluoro); or
(C3_6)cycloalkyl-X21-, wherein X21 represents a direct bond, -0-, or -
(C1_3)alkylene-0-, and wherein the (C3_
6)cycloalkyl independently contains one optional ring oxygen [especially such
group (C3_6)cycloalkyl-X21- is
cyclopropyl, cyclopropyl-oxy, cyclobutyl-oxy, oxetan-3-yl-oxy, cyclopropyl-
methoxy;
R3 represents hydrogen, or (C1_3)alkyl (especially methyl); and
R4 represents
= (Ci4alkyl (especially methyl);
= hydroxy-(C1_3)alkyl (especially hydroxymethyl);
= -0-R41, wherein R41 represents
D hydrogen;
(Ci_4)alkyl (especially methyl);
(C)alkyl which is substituted with one or two hydroxy (especially 2-
hydroxyethyl, 2,3-dihydroxypropyl);
):. R41 aR41bN_(C2_3)alkylene-, wherein R41a and Rob independently
represent hydrogen or (C1_4)alkyl
(especially such group is 2-dimethylamino-ethyl); or
D (C4_7)heterocyclyl-X41-, wherein X41 represents a direct bond or
(C1_3)alkylene, and wherein the
(C4_7)heterocycly1 independently contains one or two ring heteroatoms
independently selected from
nitrogen and oxygen; wherein said (C4_7)heterocycly1 independently is
unsubstituted, or mono-, or di-
substituted, wherein the substituents are independently selected from:
= (Ci4alkyl (especially methyl); and/or
= (Ci4alkoxy-carbonyl attached to a ring nitrogen atom having a free
valency;
[especially such (C4_7)heterocyclyl-X41- group is oxetan-3-yl, 1-methyl-
pyrrolidin-3-yl, 2,2-dimethyl-
dioxolan-4-yl-methyl, 2-morpholin-4-yl-ethyl, 1-tert-butoxy-carbonyl-piperidin-
4-yl, piperidin-4-yI];
. _NR42aR42b wherein R42a and R42b independently represent hydrogen,
(C1_4)alkyl, (C1_3)alkoxy-(C2_3)alkyl,
hydroxy-(C2_3)alkyl, or R42a and R42b together with the nitrogen to which they
are attached form a 4- to 7-
membered saturated ring optionally containing one further ring heteroatom
selected from oxygen and nitrogen,
wherein said ring is unsubstituted or mono-substituted with (C1_3)alkyl
(especially methyl), or (C1_3)alkoxy
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
11
(especially methoxy) [especially such group ¨NR42aR42b is (2-hydroxyethyl)-
amino, (2-hydroxyethyl)-methyl-
amino, (2-methoxyethyl)-amino, morpholin-4-yl, 3-methoxy-azetidinyl, or 4-
methyl-piperazin-1-yI]; or
= -CO-R43 wherein R43 represents (Ci4alkoxy (especially methoxy); or ¨NR
43aR43b wherein R434 and R43b
independently represent hydrogen, (C1_4)alkyl (especially methyl),
(C1_3)alkoxy-(C2_3)alkyl (especially 2-
methoxy-ethyl), or hydroxy-(C2_3)alkyl (especially 2-hydroxy-ethyl);
[especially such group -CO-R43 is
methoxycarbonyl, carbamoyl, methyl-carbamoyl,
dimethylcarbamoyl, hydroxyethylcarbamoyl,
methoxyethylcarbamoyl].
The compounds of formula (I) may contain one or more further stereogenic or
asymmetric centers, such as one or
.. more additional asymmetric carbon atoms. The compounds of formula (I) may
thus be present as mixtures of
stereoisomers or preferably as pure stereoisomers. Mixtures of stereoisomers
may be separated in a manner
known to a person skilled in the art.
In case a particular compound (or generic structure) is designated as (R)- or
(S)-enantiomer / as having an
absolute (R)- or (S)-configuration, such designation is to be understood as
referring to the respective compound (or
generic structure) in enriched, especially essentially pure, enantiomeric
form. Likewise, in case a specific
asymmetric center in a compound is designated as being in (R)- or (S)-
configuration or as being in a certain relative
configuration, such designation is to be understood as referring to the
compound that is in enriched, especially
essentially pure, form with regard to the respective configuration of said
asymmetric center. In analogy, cis- or
trans-designations (or (R*,R*) designations) are to be understood as referring
to the respective stereoisomer of the
respective relative configuration in enriched form, especially in essentially
pure form.
The term "enriched", when used in the context of stereoisomers, is to be
understood in the context of the present
invention to mean that the respective stereoisomer is present in a ratio of at
least 70:30, especially of at least 90:10
(i.e., in a purity of at least 70% by weight, especially of at least 90% by
weight), with regard to the respective other
stereoisomer / the entirety of the respective other stereoisomers.
The term "essentially pure", when used in the context of stereoisomers, is to
be understood in the context of the
present invention to mean that the respective stereoisomer is present in a
purity of at least 95% by weight,
especially of at least 99% by weight, with regard to the respective other
stereoisomer / the entirety of the respective
other stereoisomers.
In some instances, the compounds of formula (I) may contain tautomeric forms.
Such tautomeric forms are
encompassed in the scope of the present invention. For example, in case the
present compounds may contain
heteroaromatic aromatic rings containing unsubstituted ring nitrogen atoms
having a free valency such as
pyrazolyl, such rings may be present in tautomeric forms. For example, the
group pyrazol-3-y1 represents the
tautomeric forms 1H-pyrazol-3-y1 and 2H-pyrazol-3-yl.
The present invention also includes isotopically labelled, especially 2H
(deuterium) labelled compounds of formula
(I), which compounds are identical to the compounds of formula (I) except that
one or more atoms have each been
CA 03087886 2020-07-08
WO 2019/141803 PCT/EP2019/051230
12
replaced by an atom having the same atomic number but an atomic mass different
from the atomic mass usually
found in nature. Isotopically labelled, especially 2H (deuterium) labelled
compounds of formula (I) and salts thereof
are within the scope of the present invention. Substitution of hydrogen with
the heavier isotope 2H (deuterium) may
lead to greater metabolic stability, resulting e.g. in increased in-vivo half-
life and/or reduced dosage requirements,
and/or may lead to a modified metabolism pathway, resulting e.g. in an
improved safety profile. In one embodiment
of the invention, the compounds of formula (I) are not isotopically labelled,
or they are labelled only with one or
more deuterium atoms. In a sub-embodiment, the compounds of formula (I) are
not isotopically labelled at all.
Isotopically labelled compounds of formula (I) may be prepared in analogy to
the methods described hereinafter,
but using the appropriate isotopic variation of suitable reagents or starting
materials.
In this patent application, a bond drawn as a dotted line shows the point of
attachment of the radical drawn. For
example, the radical drawn below
N
is the 2-oxo-2,3-dihydro-1H-benzo[d]imidazol-1,3-diy1 group.
In addition, it is well understood that in certain instances the drawn
structure of the present compounds may
represent several tautomers and such tautomers are comprised in the scope of
the present invention:
For example compounds of formula (I) wherein Z and Y are CH, Xis N, and R4 is
hydroxy: i.e. compounds wherein
the fragment:
Z
y N
> __________________________________ 0
X N
R4 repesents OH
are understood as being one of two possible tautomeric forms. Such compounds,
thus, also comprise the
compounds wherein said fragment represents the corresponding tautomer:
HNJN> _________ 0
0
Where the plural form is used for compounds, salts, pharmaceutical
compositions, diseases and the like, this is
intended to mean also a single compound, salt, or the like.
Any reference to compounds of formula (I) according to embodiments 1) to 25)
is to be understood as referring also
to the salts (and especially the pharmaceutically acceptable salts) of such
compounds, as appropriate and
expedient.
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
13
The term "pharmaceutically acceptable salts" refers to salts that retain the
desired biological activity of the subject
compound and exhibit minimal undesired toxicological effects. Such salts
include inorganic or organic acid and/or
base addition salts depending on the presence of basic and/or acidic groups in
the subject compound. For
reference see for example "Handbook of Phramaceutical Salts. Properties,
Selection and Use.", P. Heinrich Stahl,
Camille G. Wermuth (Eds.), Wiley-VCH, 2008; and "Pharmaceutical Salts and Co-
crystals", Johan Wouters and
Luc QuOrO (Eds.), RSC Publishing, 2012.
Definitions provided herein are intended to apply uniformly to the compounds
of formula (I), as defined in any one
of embodiments 1) to 25), and, mutatis mutandis, throughout the description
and the claims unless an otherwise
expressly set out definition provides a broader or narrower definition. It is
well understood that a definition or
preferred definition of a term defines and may replace the respective term
independently of (and in combination
with) any definition or preferred definition of any or all other terms as
defined herein.
The term "halogen" means fluorine, chlorine, or bromine, preferably fluorine
or chlorine.
The term "alkyl", used alone or in combination, refers to a saturated straight
or branched chain hydrocarbon group
containing one to six carbon atoms. The term '(C)alkyl" (x and y each being an
integer), refers to an alkyl group
as defined before, containing x to y carbon atoms. For example a (C1_6)alkyl
group contains from one to six carbon
atoms. Examples of alkyl groups are methyl, ethyl, propyl, isopropyl, butyl,
isobutyl, tert.-butyl, 3-methyl-butyl, 2,2-
dimethyl-propyl and 3,3-dimethyl-butyl. For example a (C1_4)alkyl group
contains from one to four carbon atoms.
Examples of alkyl groups are methyl, ethyl, propyl, isopropyl, butyl,
isobutyl, sec-butyl and tert.-butyl. For avoidance
of any doubt, in case a group is referred to as e.g. propyl or butyl, it is
meant to be n-propyl, respectively n-butyl.
Preferred are methyl, ethyl and isopropyl. Most preferred is methyl. Examples
of (C1_3)alkyl groups as used for the
substituents at ring A (without R1) is methyl. Examples of (Ci4alkyl groups as
used for R1 are methyl and
isopropyl. An example of R11 in ¨CO-R11 is tert-butyl, an example of a
(C1_4)alkyl substituent at phenyl in R1 is
methyl, an example of (Ci4alkyl of Rua or R14b is methyl and ethyl. Examples
of (C1_4)alkyl groups as used for R2
are methyl and isopropyl. Examples of (C1_3)alkyl groups as used for R3 is
methyl. An example of (C1_4)alkyl groups
as used for R4, R41, R41a, R41b, R43a and R43b is methyl.
The term "-(Cx_y)alkylene-", used alone or in combination, refers to
bivalently bound alkyl group as defined before
containing x to y carbon atoms. Preferably, the points of attachment of a -
(Ci_y)alkylene group are in 1,1-diyl, in 1,2-
diyl, or in 1,3-diy1 arrangement. Preferably, the points of attachment of a -
(C2_y)alkylene group are in 1,2-diy1 or in
1,3-diy1 arrangement. Preferred alkylene groups are methylene, ethylene and
propylene. A -(Co)alkylene- group is
absent and refers to a direct bond.
Alkylene-oxy linker groups -(C1_3)alkylene-0- as used for example in the
substituents (C3_6)cycloalkyl-X12- or (C3_
6)cycloalkyl-X21- are to be read from left to right, i.e. they refer to the
respective (C3_6)cycloalkyl-(C1_3)alkylene-0-
groups. An example for (C3_6)cycloalkyl-(C1_3)alkylene-0- is cyclopropyl-
methoxy.
Alkylene-amino linker groups R14aR14bN_X14_, wherein X14 represents
(C1_3)alkylene, are used for example in the
substituents methylamino-methyl, dimethylamino-methyl, (2-hydroxy-ethyl)-
methyl-amino]-methyl, (2-methoxy-1-
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
14
methyl-ethylamino)-methyl, (2-methoxy-ethyl)-methyl-amino]methyl, [ethyl-(2-
hydroxy-ethyl)-amino]methyl, and
[bis-(2-hydroxy-ethyl)-amino]methyl. Further, alkylene-amino linker groups
R14aR14bN_x14_, wherein X14 represents
(C1_3)alkylene and Rua and Rub together with the nitrogen to which they are
attached to form a 4- to 6-membered
saturated ring optionally containing one additional ring heteroatom selected
from oxygen and nitrogen, are used for
example in the substituents azetidin-1-ylmethyl, pyrrolidin-1-ylmethyl,
piperidin-1-ylmethyl, piperazin-1-ylmethyl,
morpholin-4-ylmethyl, morpholin-4-ykethyl, morpholin-4-yl-propyl, 1-methyl-
piperazin-411)-methyl, 3-methoxy-
azetidin-1-ylmethyl, 4-hydroxy-piperidin-1-ylmethyl, 3-methoxy-pyrrolidin-1-
ylmethyl, 4-methoxy-piperidin-1-
ylmethyl, and 4-dimethylamino-piperidin-1-ylmethyl.
The term "alkoxy", used alone or in combination, refers to an alkyl-0- group
wherein the alkyl group is as defined
before. The term '(C)alkoxy" (x and y each being an integer) refers to an
alkoxy group as defined before
containing x to y carbon atoms. For example a (C1_4)alkoxy group means a group
of the formula (Ci4alky1-0- in
which the term "(Ci4alkyl" has the previously given significance. Examples of
alkoxy groups are methoxy, ethoxy,
n-propoxy, isopropoxy, n-butoxy, isobutoxy, sec.-butoxy and tert.-butoxy.
Preferred are ethoxy and especially
methoxy. Examples of (C1_4)alkoxy groups as used for R11 in ¨CO-R11 are
methoxy, ethoxy,isopropoxy, n-butyloxy
and tert-butoxy. An example of a (Ci4alkoxy substituent at phenyl in R1 is
methoxy and n-butyloxy. Examples of
(C1_4)alkoxy groups as used for R2 are methoxy and isopropoxy. An example of a
(Ci4alkoxy group as used for R43
is methoxy.
The term "hydroxy-(C1_3)alkyl" relates to an alkyl group wherein a hydroxyl
group can be attached to any of the
carbon atoms of the alkyl group, for instance 1-hydroxyethyl, 2-hydroxyethyl,
hydroxymethyl, 1-hydroxy-1-methyl-
ethyl. An example for "hydroxy-(C2_4)alkyl" is 2-hydroxyethyl.
The term "(C1_4)alkoxy-(C1_3)alkyl" relates to an alkyl group wherein an
alkoxy group can be attached to any of the
carbon atoms of the alkyl group, for instance 1-methoxyethyl, 2-methoxy-1-
methyl-ethyl and 2-methoxyethyl.
The term 'hydroxyl-(C23)alkoxy" relates to an alkoxy group wherein a hydroxyl
group can be attached to any of the
carbon atoms of the alkoxy group, for instance 2-hydroxy-ethoxy.
The term "(C1_4)alkoxy-(C2_3)alkoxy" relates to a first alkoxy group wherein a
second alkoxy group can be attached
to any of the carbon atoms of the first alkoxy group, for instance 2-methoxy-
ethoxy.
The term "alkynyl", used alone or in combination, refers to a straight or
branched hydrocarbon chain containing two
to five carbon atoms and one carbon-carbon triple bond. The term "(C)alkynyl "
(x and y each being an integer),
refers to an alkynyl group as defined before containing x to y carbon atoms.
For example a (C2-05)alkynyl group
contains from two to five carbon atoms. An example of an alkynyl group is prop-
2-yn-1-yl. An example for an
alkynyl group as used for the phenyl substituent in R1 is 3-morpholin-4-yl-
prop-1-ynyl.
The term "fluoroalkyl", used alone or in combination, refers to an alkyl group
as defined before containing one to
three carbon atoms in which one or more (and possibly all) hydrogen atoms have
been replaced with fluorine. The
term "(Cx_y)fluoroalkyl" (x and y each being an integer) refers to a
fluoroalkyl group as defined before containing x to
y carbon atoms. For example a (C1_3)fluoroalkyl group contains from one to
three carbon atoms in which one to
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
seven hydrogen atoms have been replaced with fluorine. Representative examples
of fluoroalkyl groups include
trifluoromethyl, 2-fluoroethyl, 2,2-difluoroethyl and 2,2,2-trifluoroethyl.
Preferred are (Ci)fluoroalkyl groups,
especially trifluoromethyl.
The term "fluoroalkoxy", used alone or in combination, refers to an alkoxy
group as defined before containing one
5 to three carbon atoms in which one or more (and possibly all) hydrogen
atoms have been replaced with fluorine.
The term '(C)fluoroalkoxy" (x and y each being an integer) refers to a
fluoroalkoxy group as defined before
containing x to y carbon atoms. For example a (C1_3)fluoroalkoxy group
contains from one to three carbon atoms in
which one to seven hydrogen atoms have been replaced with fluorine.
Representative examples of fluoroalkoxy
groups include trifluoromethoxy, difluoromethoxy, 2-fluoroethoxy, 2,2-
difluoroethoxy and 2,2,2-trifluoroethoxy.
10 Preferred are (Ci)fluoroalkoxy groups, especially trifluoromethoxy.
The term "cyano" refers to a group -CN.
The term "cycloalkyl", used alone or in combination, refers to a saturated
monocyclic hydrocarbon ring containing
three to six carbon atoms. The term "(C)cycloalkyl " (x and y each being an
integer), refers to a cycloalkyl group
as defined before containing x to y carbon atoms. For example a
(C36)cycloalkyl group contains from three to six
15 carbon atoms. Examples of cycloalkyl groups are cyclopropyl, cyclobutyl,
cyclopentyl, cyclohexyl, and cycloheptyl.
Preferred are cyclopropyl, cyclopentyl and cyclohexyl; especially cyclopropyl.
An example of (C36)cycloalkyl
groups as used for the group R11 is cyclopropyl. An example for a
(C36)cycloalkyl group as used for the phenyl
substituent in (C36)cycloalkyl-X12- is cyclopropyl, examples for a
(C36)cycloalkyl group as used for the phenyl or
heteroaryl substituent in (C36)cycloalkyl-X21- are cyclopropyl and cyclobutyl.
The term "(Cx_y)cycloalkyl-(Cx_y)alky1-0-" refers to a (C)cycloalkyl group as
defined before, which is linked through
a (Cx_y)alkylene-0- group as defined before to the rest of the molecule. A
particular example of such groups is
cyclopropyl-methoxy.
The term "(C36)cycloalkyl optionally containing one ring oxygen atom", used
alone or in combination, refers to a
cycloalkyl group as defined before. In addition, one ring carbon atom of said
cycloalkyl may be replaced by an
oxygen atom. Examples of such groups are especially cycloalkyl groups such as
cyclopropyl, cyclobutyl,
cyclopentyl,and cyclohexyl; as well as oxygen containing groups such as
oxetanyl, tetrahydrofuranyl, and
tetrahydro-2H-pyranyl.
The term "heterocyclyl", used alone or in combination, and if not explicitly
defined in a more narrow way, refers to a
saturated monocyclic hydrocarbon ring containing one or two ring heteroatoms
independently selected from
nitrogen and oxygen (especially one nitrogen atom, two nitrogen atoms, one
nitrogen atom and one oxygen atom,
one oxygen atom, or two oxygen atoms; preferably such heterocyclyl contains
one or two ring oxygen atoms, or
one nitrogen atom). The term '(C)heterocyclyl" refers to such a heterocyclyl
group containing x to y ring atoms.
Heterocyclyl groups are unsubstituted or substituted as explicitly defined.
Examples of heterocyclyl groups as used
for the group R41 wherein R41 represents (C47)heterocyclyl-X41- are oxetan-3-
yl, 1-methyl-pyrrolidin-3-yl, piperidin-4-
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
16
yl, 2,2-dimethyl-dioxolan-4-y1 (notably 2,2-dimethyl-dioxolan-4-yl-methyl),
morpholin-4-y1 (notably morpholin-4-yl-
ethyl) and 1-tert-butoxy-carbonyl-piperidin-4-yl.
The term "(Cx_y) heterocycly1-(Cx_y)alkyl" refers to a (Cx_y)heterocycly1
group as defined before, which is linked
through a (Cx_y)alkylene group as defined before to the rest of the molecule.
For the (C4_7)heterocycly1-(C1_3)alkyl
groups as used for R41 examples of -(C1_3)alkylene- groups are methylene, and
ethylene. Examples of heterocyclyl
groups part of such (C4_7)heterocycly1-(C1_3)alkylene groups as used for the
group R41 wherein R41 represents (Ca_
7)heterocyclyl-X41- are 2,2-dimethyl-dioxolan-4-yl-methylene and morpholin-4-
yl-ethylene.
The substituent phenyl of R1 independently is unsubstituted, mono-, di or
trisubstituted. Examples for mono-, di or
trisubstituted phenyl as R1 are 2,6-di-methyl-phenyl, 2-methoxy-phenyl, 2-n-
butyloxy-phenyl, 2,6-di-methoxy-
phenyl, 2-methoxy-ethoxy-phenyl, 2-methoxy-6-methyl-phenyl, 2-bromo-6-fluoro-
phenyl, 2,6-di-fluoro-phenyl, 2-
fluoro-phenyl, 2-fluoro-6-methoxy-phenyl, 2-fluoro-641-methoxy-ethyl)-phenyl,
2-fluoro-6-methyl-phenyl, 2-Fluoro-
6-(3-morpholin-4-yl-prop-1-yny1)-phenyl, 2-fluoro-6-formyl-phenyl,
2-fluoro-6-cyano-phenyl, 2-fluoro-6-
hydroxymethyl-phenyl, 2-fluoro-641-hydroxyethyl)-phenyl, 2-fluoro-641-hydroxy-
1-methyl-ethyl)-phenyl, 2-fluoro-6-
nitro-phenyl, 2-fluoro-6-acetyl-phenyl, 2-amino-6-fluoro-phenyl, 2-fluoro-6-
methylaminomethyl-phenyl, 2-
dimethylaminomethy1-6-fluoro-phenyl, 2-fluoro-6-1[(2-hydroxy-ethyl)-methyl-
amino]-methyl}-phenyl, 2-fluoro-6-[(2-
methoxy-1-methyl-ethylamino)-methy1]-phenyl, 2-Fluoro-6-1[(2-methoxy-ethyl)-
methyl-amino]-methyl}-phenyl, 2-
I[Ethyl-(2-hydroxy-ethyl)-amino]-methyll-6-fluoro-phenyl, 2-
{[bis-(2-hydroxy-ethyl)-amino]-methyl}-6-fluoro-phenyl,
2-azetidin-1-ylmethy1-5-fluoro-phenyl, 2-
fluoro-6-pyrrolidin-1-ylmethyl-phenyl, 2-Fluoro-6-piperidin-1 -ylmethyl-
phenyl, 2-fluoro-6-morpholin-4-yl-phenyl, 2-Fluoro-6-piperazin-1-ylmethyl-
phenyl, 2-Fluoro-6-morpholin-4-ylmethyl-
phenyl, 2-Fluoro-6(2-morpholin-4-yl-ethyl)-phenyl, 2-Fluoro-6-(3-morpholin-4-
yl-propyI)-phenyl, 2-Fluoro-6-(1-
methyl-piperazin-4-y1)-methyl-phenyl, 2-Fluoro-643-methoxy-azetidin-1-
ylmethyl)-phenyl, 2-Fluoro-644-hydroxy-
piperidin-1-ylmethyl)-phenyl, 2-
Fluoro-643-methoxy-pyrrolidin-1-ylmethyl)-phenyl, 2-Fluoro-6-[143-methoxy-
azetidin-111)-ethy1]-phenyl, 2-Fluoro-6(4-methoxy-piperidin-1-ylmethyl)-
phenyl, 244-Dimethylamino-piperidin-1-
ylmethyl)-6-fluoro-phenyl, 1-hydroxy-phenyl, 2-hydroxy-ethoxy-phenyl, oxetan-3-
yl-oxy-phenyl, cyclopropyl-
methoxy-phenyl, cyclobutyl-oxy,-phenyl, 2-(tetrahydro-pyran-4-yloxy)-phenyl, 1-
benzyloxy-phenyl or 1-
trifluoromethoxy-phenyl.
The substituent phenyl of R2 independently is unsubstituted, mono-, di or
trisubstituted, especially mono- or
trisubstituted. Examples for mono or trisubstituted phenyl as R2 are 2-
trifluoromethoxy-phenyl, 2-trifluoromethyl-
phenyl, 2-methoxy-phenyl, 2-isopropoxy-phenyl, 2-cyclopropyl-phenyl, 2-
cyclopropyl-oxy-phenyl, 2-oxetan-3-yl-oxy-
phenyl, 2-cyclopropylmethoxy-phenyl, 2-cyclobutyl-oxy-phenyl or 2,4-difluoro-6-
isopropoxy-phenyl.
The term "heteroaryl", used alone or in combination, means a 5- or 6-membered
monocyclic aromatic ring
containing one to a maximum of three heteroatoms, each independently selected
from oxygen, nitrogen and sulfur.
Examples of such heteroaryl groups are furanyl, oxazolyl, isoxazolyl,
oxadiazolyl, thiophenyl, thiazolyl, isothiazolyl,
thiadiazolyl, pyrrolyl, imidazolyl, pyrazolyl, triazolyl, pyridinyl,
pyrimidinyl, pyridazinyl, and pyrazinyl. Preferred 5- or
6-membered heteroaryl groups are pyrazolyl, oxodiazolyl, thiazolyl, pyridinyl,
pyridazinyl, pyrimidinyl and pyrazinyl.
The above-mentioned heteroaryl groups are unsubstituted or substituted as
explicitly defined. In case 5- or 6-
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
17
membered heteroaryl group is substituted in ortho-position with regard to the
point of attachment of the rest of the
molecule, it is understood that such substituent is attached in direct
neighbourhood with regard to the point of
attachment of the rest of the molecule, i.e. in a relative 1,2-arrangement. In
case R1 represents "5- or 6-membered
heteroaryl", the term means the above-mentioned 5- or 6-membered groups,
especially pyrazolyl, oxodiazolyl,
pyridinyl, pyrimidinyl or pyridazinyl. For the substituent R1, such 5-or 6-
membered heteroaryl group is unsubstituted
or mono-, di- or tri-substituted (especially mono-, or di-substituted, in
particular di-substituted) wherein the
substituents are independently selected from (C1_4)alkyl, (C1_4)alkoxy, cyano,
or halogen (especially fluoro or chloro,
in particular fluoro). Examples for the 5-membered heteroaryl containing at
least one nitrogen atom and optionally
one or two further heteroatoms selected from nitrogen or oxygen are 2,5-
dimethy1-2H-pyrazol-3-yl, 4-chloro-2,5-
dimethy1-2H-pyrazol-3-yl, 2,4,5-trimethy1-2H-pyrazol-3-yl, 5-cyclopropy1-2-
methyl-2H-pyrazol-3-yl, 4-formy1-2,5-
dimethy1-2H-pyrazol-3-yl, 4-cyano-2,5-dimethy1-2H-pyrazol-3-yl, 4-chloro-5-
cyclopropy1-2-methyl-2H-pyrazol-3-yl, 5-
cyclopropy1-2,4-dimethy1-2H-pyrazol-3-yl, 5-
cyclopropy1-4-formy1-2-methyl-2H-pyrazol-3-yl, 5-isopropyl-
[1,3,4]oxodiazol-2-yl, 5-trifluoromethyl-[1,3,4]oxodiazol-2-yl. Examples for
the 6-membered heteroaryl group
containing one or two nitrogen atoms are 3-methoxy-pyridin-2-yl, 3,5-dimethoxy-
pyridin-4-yl, 2,4-dimetehoxy-
pyridin-3-yl, 3-methyl-pyridin-2-yl, 4-methyl-2-methoxy-pyridin-3-yl, 2-methyl-
4-methoxy-pyridin-3-yl, 2-cyano-4-
methyl-pyridin-3-yl, 2,4-dimethyl-pyridin-3-yl, 3,5-dimethyl-pyridin-4-yl, 3-
fluoro-pyridin-2-yl, 2-fluoro-pyridin-3-yl, 4-
fluoro-pyridin-3-yl, 3,5-difluoro-pyridin-4-yl, 3,5-dichloro-pyridazin-4-yl,
3,4-dichloro-pyridazin-2-yl, 4,6-dimethoxy-
pyrimidin-5-y1 or 4-methyl-6-methoxy-pyrimidin-5-yl.
For the substituent R2, such 5- or 6-membered heteroaryl group is
unsubstituted or mono-, di- or tri-substituted
(especially mono-substituted) pyridinyl, pyridazinyl, pyrimidinyl, or
pyrazinyl, wherein the substituents are
independently selected from (C1_3)fluoroalkyl (especially trifluoromethyl),
(C1_3)fluoroalkoxy (especially
trifluoromethoxy), (C1_4)alkyl (especially methyl and isopropyl), (C1_4)alkoxy
(especially methoxy and isopropoxy),
halogen (especially chloro). Examples for the 5-membered heteroaryl containing
at least one nitrogen atom and
optionally one or two further heteroatoms selected from nitrogen or oxygen are
2-trifluoromethyl-thiazol-4-y1 and 2-
methyl-4-trifluoromethyl-thiazol-5-yl. Examples for the 6-membered heteroaryl
group containing one or two nitrogen
atoms are 3-methoxy-pyridin-2-yl, 3-isopropoxy-pyridin-2-yl, 4-trifluoromethyl-
pyridin-3-yl, 2-trifluoro-pyridin-3-yl, 4-
isopropyl-pyridin-3-yl, 3-trifluoromethyl-pyridin-4-yl, 4-methoxy-pyridazin-3-
yl, 4-isopropoxy-pyridazin-3-yl, 4-methyl-
pyridazin-3-yl, 6-chloro-4-methoxy-pyridazin-3-yl, 6-chloro-4-isopropoxy-
pyridazin-3-yl, 4-methoxy-pyrimidin-5-yl, 4-
trifluoromethyl-pyrimidin-5-yl, 3-trifluoromethyl-pyrazin-2-yl, 3-methoxy-
pyrazin-2-yl, 3-isopropoxy-pyrazin-2-y1 or 3-
isopropyl-pyrazin-2-yl.
Further embodiments of the invention are presented hereinafter:
2) A second embodiment relates to compounds according to embodiment 1),
wherein
= ring A represents a saturated 4- to 7-membered mono-cyclic carbocyclic
ring containing the ring nitrogen atom
to which R1 is attached, wherein said ring independently is unsubstituted, or
mono-substituted with (C1_3)alkyl
(especially methyl), fluoro, or (Ci4alkoxy-carbonyl (especially ethoxy-
carbonyl), or di-substituted wherein the
substituents are two (C1_3)alkyl (especially methyl) substituents, or two
fluoro substituents [especially such ring
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
18
A is azetidin-1,3-diyl, pyrrolidin-1,3-diyl, 4-methyl-pyrolidin-1,3-diyl,
piperidin-1,3-diyl, piperidin-1,4-diyl, 3-
fluoro-piperidin-1,4-diyl, 2-methyl-piperidin-1,4-diyl, 3-methyl-piperidin-1,4-
diyl, 3-(ethoxycarbonyI)-piperidin-
1,4-diyl, 3,3-dimethyl-piperidin-1,4-diyl, azepan-1,4-diyI]; or
= ring A represents a saturated 7- or 8-membered bridged bi-cyclic
carbocyclic ring containing the ring nitrogen
atom to which R1 is attached, wherein said ring is unsubstituted [especially
such ring is 2-aza-
bicyclo[2.2.1]heptane-2,5-diyl, 3-aza-bicyclo[3.1.1]heptane-3,6-diy1].
3) Another embodiment relates to compounds according to embodiment 1), wherein
ring A represents a saturated
4- to 7-membered mono-cyclic carbocyclic ring containing the ring nitrogen
atom to which R1 is attached, wherein
said ring independently is unsubstituted, or mono-substituted with (C1_3)alkyl
(especially methyl), fluoro, or
(C1_4)alkoxy-carbonyl (especially ethoxy-carbonyl), or di-substituted wherein
the substituents are two (C1_3)alkyl
(especially methyl) substituents, or two fluoro substituents [especially such
ring A is azetidin-1,3-diyl, pyrrolidin-1,3-
diyl, 4-methyl-pyrolidin-1,3-diyl, piperidin-1,3-diyl, piperidin-1,4-diyl, 3-
fluoro-piperidin-1,4-diyl, 2-methyl-piperidin-
1,4-diyl, 3-methyl-piperidin-1,4-diyl, 3-(ethoxycarbonyI)-piperidin-1,4-diyl,
3,3-dimethyl-piperidin-1,4-diyl, azepan-
1,4-diy1].
4) Another embodiment relates to compounds according to embodiment 1), wherein
ring A represents
unsubstitueted, mono- or di-substituted azetidin-1,3-diyl, pyrrolidin-1,3-
diyl, piperidin-1,3-diyl, piperidin-1,4-diyl,
azepan-1,4-diyl, 2-aza-bicyclo[2.2.1]heptane-2,5-diyl, 3-aza-
bicyclo[3.1.1]heptane-3,6-diyl, wherein the substituents
are selected from the group of (C1_3)alkyl (especially methyl), fluoro, or
(C14)alkoxy-carbonyl (especially ethoxy-
carbonyl) [especially such ring A is azetidin-1,3-diyl, pyrrolidin-1,3-diyl, 4-
methyl-pyrolidin-1,3-diyl, piperidin-1,3-diyl,
piperidin-1,4-diyl, 3-fluoro-piperidin-1,4-diyl, 2-methyl-piperidin-
1,4-diyl, 3-methyl-piperidin-1,4-diyl, 3-
(ethoxycarbonyI)-piperidin-1,4-diyl, 3,3-dimethyl-piperidin-1,4-diyl, azepan-
1,4-diyl, 2-aza-bicyclo[2.2.1]heptane-2,5-
diyl, 3-aza-bicyclo[3.1.1]heptane-3,6-diy1].
5) Another embodiment relates to compounds according to embodiment 1), wherein
ring A represents
= azetidin-1,3-diyl, pyrrolidin-1,3-diyl, 4-methyl-pyrolidin-1,3-diyl,
piperidin-1,3-diyl, piperidin-1,4-diyl, 3-
fluoro-piperidin-1,4-diyl, 2-methyl-piperidin-1,4-diyl, 3-methyl-piperidin-1,4-
diyl, 3-(ethoxycarbonyI)-
piperidin-1,4-diyl, 3,3-dimethyl-piperidin-1,4-diyl, or azepan-1,4-diy1; or
= 2-aza-bicyclo[2.2.1]heptane-2,5-diyl, 3-aza-bicyclo[3.1.1]heptane-3,6-
diy1;
wherein in a sub-embodiment ring A represents pyrrolidin-1,3-diyl, or
piperidin-1,4-diy1 (especially piperidin-1,4-
diy1).
6) Another embodiment relates to compounds according to any one of embodiments
1) to 5), wherein
= X represents CR5; Z represents CR5; and Y represents CR5; wherein each R5
independently represents
hydrogen, or (C1_3)alkyl (especially methyl); wherein preferably at maximum
one R5 is different from
hydrogen [especially all R5 represent hydrogen];
= X represents CH; Z represents N; and Y represents CR5; wherein each R5
independently represents
hydrogen, or (C1_3)alkyl (especially methyl); [especially R5 represents
hydrogen];
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
19
= X represents N; Z represents CR5; and Y represents CR5; wherein each R5
independently represents
hydrogen, or (C1_3)alkyl (especially methyl); wherein preferably at maximum
one R5 is different from
hydrogen; [especially all R5 represent hydrogen];
= X represents N; Z represents N; and Y represents CR5; wherein R5
represents hydrogen, or (C1_3)alkyl
(especially methyl); [especially R5 represents hydrogen].
7) Another embodiment relates to compounds according to any one of embodiments
1) to 5), wherein one of X and
Z represents CH or N; the other represents CH; and Y represents CR5, wherein
R5 represents hydrogen or
(C1_3)alkyl (especially R5 represents hydrogen).
8) Another embodiment relates to compounds according to any one of embodiments
1) to 7), wherein
R1 represents
= (Ci4alkyl (especially methyl, isopropyl);
= -CO-R11 wherein R11 represents (Ci4alkyl (especially tert-butyl);
(Ci4alkoxy (especially methoxy, ethoxy,
isopropoxy, n-butoxy, tert-butoxy); phenyl, phenoxy; (C3_6)cycloalkyl
(especially cyclopropyl); or (C3_
6)cycloalkoxy optionally containing one ring oxygen (especially oxetan-3-
yloxy) and optionally mono-substituted
with methyl or trifluoromethyl (especially 3-methyl-oxetan-3-yloxy, 3-
trifluoromethyl-oxetan-3-yloxy); [especially
such group -CO-R11 is tert.-butyl-carbonyl, methoxycarbonyl, ethoxycarbonyl,
isopropoxycarbonyl, n-
butoxycarbonyl, tert.-butoxycarbonyl, oxetan-3-yl-oxy-carbonyl, (3-methyl-
oxetan-3-yI)-oxy-carbonyl, (3-
trifluoromethyl-oxetan-3-yI)-oxy-carbonyl, phenylcarbonyl, phenoxycarbonyl,
cyclopropylcarbonyl];
= benzyl wherein the phenyl ring of said benzyl is optionally mono- or di-
substituted with halogen [especially 2,4-
dichlorobenzyl];
= phenyl which is unsubstituted, mono-, di- or tri-substituted (notably
mono- or di-substituted, wherein at least
one substituent is attached in ortho position with regard to the point of
attachment of the rest of the molecule;
especially di-substituted in ortho position), wherein the substituents are
independently selected from
D (Ci_4)alkyl (especially methyl, isopropyl);
(C1_4)alkoxy (especially methoxy, n-butyloxy);
(Ci_3)fluoroalkyl (especially trifluoromethyl);
(C1_3)fluoroalkoxy (especially trifluoromethoxy);
D halogen (especially fluoro, bromo);
cyano;
D nitro;
D hydroxy;
D hydroxy-(C1_3)alkyl (especially 1-hydroxyethyl, hydroxymethyl, 1-hydroxy-1-
methyl-ethyl);
D (Ci_4)alkoxy-(Ci_3)alkyl (especially 1-methoxyethyl);
hydroxy-(C2_3)alkoxy(especially 2-hydroxy-ethoxy);
D (C1_4)alkoxy-(C2_3)alkoxy (especially 2-methoxy-ethoxy);
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
D (C3_6)cycloalkyl-X12-, wherein X12 represents a direct bond, -0-, or -
(C1_3)alkylene-0-, and wherein the (C3_
6)cycloalkyl independently contains one optional ring oxygen [especially such
group (C3_6)cycloalkyl-X12- is
cyclopropyl, cyclopropyl-oxy, cyclobutyl-oxy, oxetan-3-yl-oxy, cyclopropyl-
methoxy or tetrahydropyran-4-yl-
oxy];
5 D R13-CO-X13-, wherein X13 represents a direct bond or (C1_3)alkylene,
and R13 represents hydrogen or (C1_
4)alkyl (especially such group R13-CO-X13 is formyl, acetyl, 2-oxo-ethyl);
3-(morpholin-4-yI)-prop-1-ynyl;
R14aR14bN_X14_, wherein X14 represents a direct bond or (C1_3)alkylene; and
wherein R14a and R14b
independently represent hydrogen, (C1_4)alkyl, hydroxy-(C2_4)alkyl,
(C1_3)alkoxy-(C24alkyl; or R14a and R14b
10 together with the nitrogen to which they are attached to form a 4- to 6-
membered saturated ring optionally
containing one additional ring heteroatom selected from oxygen and nitrogen;
and wherein said ring is
optionally mono-substituted with (C1_4)alkyl (especially methyl), hydroxy,
(C1_4)alkoxy (especially methoxy),
or dimethylamino;
[especially such group R14aR14bN_x14_ is amino, methylamino-methyl,
dimethylamino-methyl, [(2-
15 hydroxyethyl)-methylamino]-methyl, [(2-hydroxyethyl)-ethylamino]-methyl,
[(2-methoxy-1-methyl-ethyl)-
amino]-methyl, [(2-methoxyethyp-methylamino]-methyl, [di-(2-hydroxyethyp-
amino]-methyl, (azetidin-1
methyl, (pyrrolidin-1-yI)-methyl, (piperidin-1-yI)-methyl, morpholin-4-yl,
piperazin-1-ylmethyl (morpholin-4-
y1)-methyl, 2-(morpholin-4-yI)-ethyl, 3-(morpholin-4-yI)-propyl, (3-methoxy-
azetidin-1-y0-methyl, 1-(3-
methoxy-azetidin-1 (1-methyl-piperazin-4-yI)-methyl, (4-
hydroxypiperidin-1-yI)-methyl, (4-
20 methoxypiperidin-1-yI)-methyl, (3-methoxy-pyrrolidin-1-yI)-methyl, (4-
dimethylamino-piperidin-1-yI)-methyl];
or
D benzyloxy; or
= 5- or 6-membered heteroaryl (especially pyrazolyl, oxadiazolyl,
pyridinyl, pyridazinyl, pyrimidinyl); wherein said
5- or 6-membered heteroaryl independently is mono-, di- or tri-substituted
(notably mono-, di-, or tri-substituted
wherein at least one substituent is attached in ortho position with regard to
the point of attachment of the rest
of the molecule; especially di-substituted in ortho position), wherein the
substituents are independently
selected from
D (Ci_4)alkyl (especially methyl, isopropyl);
D (Ci_4)alkoxy (especially methoxy);
(C1_3)fluoroalkyl (especially trifluoromethyl);
D (C1_3)fluoroalkoxy (especially trifluoromethoxy);
D halogen (especially fluoro, chloro);
cyano;
(C3_6)cycloalkyl-X12-, wherein X12 represents a direct bond, -0-, or -
(C1_3)alkylene-0-; [especially such
group (C3_6)cycloalkyl-X12- is cyclopropyl, ]; or
D R13-CO-X13-, wherein X13 represents a direct bond or (C1_3)alkylene, and R13
represents hydrogen or (C1_
4)alkyl (especially such group R13-CO-X13 is formy1).
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
21
wherein in a sub-embodiment R1 represents phenyl, or 5- or 6-membered
heteroaryl (especially pyrazolyl,
oxadiazolyl, pyridinyl, pyridazinyl, pyrimidinyl); wherein said phenyl or 5-
or 6-membered heteroaryl are substituted
as defined herein before.
9) Another embodiment relates to compounds according to any one of embodiments
1) to 7), wherein
R1 represents
= -CO-R11 wherein R11 represents (Ci4alkyl (especially tert-butyl); or
(C1_4)alkoxy (especially methoxy, ethoxy,
isopropoxy, n-butoxy, tert-butoxy); [especially such group -CO-R11 is tert.-
butyl-carbonyl, methoxycarbonyl,
ethoxycarbonyl, isopropoxycarbonyl, n-butoxycarbonyl, tert.-butoxycarbonyl];
= phenyl which is mono-, or di- or tri-substituted (notably mono- or di-
substituted wherein at least one substituent
is attached in ortho position with regard to the point of attachment of the
rest of the molecule; especially di-
substituted in ortho position), wherein the substituents are independently
selected from
D (Ci_4)alkyl (especially methyl, isopropyl);
D (Ci_4)alkoxy (especially methoxy);
(Ci_3)fluoroalkyl (especially trifluoromethyl);
(C1_3)fluoroalkoxy (especially trifluoromethoxy);
D halogen (especially fluoro, bromo);
cyano;
D nitro;
D hydroxy-(C1_3)alkyl (especially 1-hydroxyethyl, hydroxymethyl, 1-hydroxy-1-
methyl-ethyl);
D (C1_4)alkoxy-(Ci_3)alkyl (especially 1-methoxyethyl);
hydroxy-(C2_3)alkoxy (especially 2-hydroxy-ethoxy);
(C1_4)alkoxy-(C2_3)alkoxy (especially 2-methoxy-ethoxy);
D (C3_6)cycloalkyl-X12-, wherein X12 represents a direct bond, -0-, or -
(C1_3)alkylene-0-; [especially such
group (C3_6)cycloalkyl-X12- is cyclobutyl-oxy, or cyclopropyl-methoxy]; or
> R14aR14bN_X14_, wherein X14 represents a direct bond or (C1_3)alkylene; and
wherein R14a and R14b
independently represent hydrogen, (C1_4)alkyl, hydroxy-(C2_4)alkyl,
(C1_3)alkoxy-(C24alkyl; or R14a and R14b
together with the nitrogen to which they are attached to form a 4- to 6-
membered saturated ring optionally
containing one additional ring heteroatom selected from oxygen and nitrogen;
and wherein said ring is
optionally mono-substituted with (C1_4)alkyl (especially methyl), hydroxy,
(C1_4)alkoxy (especially methoxy),
or dimethylamino;
[especially such group R14aR14bN_x14_ is amino, methylamino-methyl,
dimethylamino-methyl, [(2-
hydroxyethyp-methylamino]-methyl, [(2-hydroxyethyl)-ethylamino]-methyl, [(2-
methoxy-1-methyl-ethyl)-
amino]-methyl, [(2-methoxyethyp-methylamino]-methyl, [di-(2-hydroxyethyp-
amino]-methyl, (azetidin-1
methyl, (pyrrolidin-1-yI)-methyl, (piperidin-1-yI)-methyl, morpholin-4-yl,
(morpholin-4-yI)-methyl, piperazin-
1-ylmethyl, 2-(morpholin-4-yI)-ethyl, 3-(morpholin-4-yI)-propyl, (3-methoxy-
azetidin-1-yI)-methyl, 1-(3-
methoxy-azetidin-1 -yI)-ethyl, (1 -methyl-piperazin-4-yI)-
methyl, (4-hydroxypiperidin-1-yI)-methyl, (4-
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
22
methoxypiperidin-1-y1)-methyl, (3-methoxy-pyrrolidin-1-y1)-methyl, (4-
dimethylamino-piperidin-1-y1)-
methyl]; or
= 5- or 6-membered heteroaryl (especially pyrazolyl, oxadiazolyl,
pyridinyl, pyridazinyl, pyrimidinyl); wherein said
5- or 6-membered heteroaryl independently is mono-, di- or tri-substituted
(notably mono-, di-, or tri-substituted
wherein at least one substituent is attached in ortho position with regard to
the point of attachment of the rest
of the molecule; especially di-substituted in ortho position), wherein the
substituents are independently
selected from
D (Ci_4)alkyl (especially methyl, isopropyl);
(Ci_4)alkoxy (especially methoxy);
D (C1_3)fluoroalkyl (especially trifluoromethyl);
D halogen (especially fluoro, chloro);
cyano; or
D (C3_6)cycloalkyl-X12-, wherein X12 represents a direct bond, [especially
such group (C3_6)cycloalkyl-X12- is
cyclopropyl].
wherein in a sub-embodiment R1 represents phenyl, or 5- or 6-membered
heteroaryl (especially pyrazolyl,
oxadiazolyl, pyridinyl, pyridazinyl, pyrimidinyl); wherein said phenyl or 5-
or 6-membered heteroaryl are substituted
as defined herein before.
10) Another embodiment relates to compounds according to any one of
embodiments 1) to 7), wherein R1
represents
= -CO-R11 wherein R11 represents (Ci4alkyl (especially tert-butyl); or
(C1_4)alkoxy (especially methoxy, ethoxy,
isopropoxy, n-butoxy, tert-butoxy); [especially such group -CO-R11 is tert.-
butyl-carbonyl, methoxycarbonyl,
ethoxycarbonyl, isopropoxycarbonyl, n-butoxycarbonyl, tert.-butoxycarbonyl];
= phenyl which is mono-, or di- or tri-substituted (notably mono- or di-
substituted); wherein one substituent is (C1_
4)alkyl (especially methyl), (C1_4)alkoxy (especially methoxy), or halogen
(especially fluoro) [especially such
substituent is methyl, methoxy or fluoro; in particular methyl]; wherein said
substituent is attached in ortho
position with regard to the point of attachment of the rest of the molecule;
and, if present, the remaining
substituent(s) are independently selected from:
D (Ci_4)alkyl (especially methyl, isopropyl);
(Ci_4)alkoxy (especially methoxy);
(C1_3)fluoroalkyl (especially trifluoromethyl);
(C1_3)fluoroalkoxy (especially trifluoromethoxy);
D halogen (especially fluoro, bromo);
D cyano;
D hydroxy-(C1_3)alkyl (especially 1-hydroxyethyl, hydroxymethyl, 1-hydroxy-1-
methyl-ethyl);
D (Ci_4)alkoxy-(Ci_3)alkyl (especially 1-methoxyethyl);
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
23
(C1_4)alkoxy-(C2_3)alkoxy (especially 2-methoxy-ethoxy);
D (C3_6)cycloalkyl-X12-, wherein X12 represents a direct bond, -0-, or -
(C1_3)alkylene-0-; [especially such
group (C3_6)cycloalkyl-X12- is , cyclobutyl-oxy or cyclopropyl-methoxy]; or
R14aR14bN_X14_, wherein X14 represents a direct bond or (C1_3)alkylene; and
wherein R14a and R14b
independently represent hydrogen, (C1_4)alkyl, hydroxy-(C2_4)alkyl,
(C1_3)alkoxy-(C24alkyl; or R14a and R14b
together with the nitrogen to which they are attached to form a 4- to 6-
membered saturated ring optionally
containing one additional ring heteroatom selected from oxygen and nitrogen;
and wherein said ring is
optionally mono-substituted with (C1_4)alkyl (especially methyl), hydroxy,
(C1_4)alkoxy (especially methoxy),
or dimethylamino;
[especially such group R14aR14bN_x14_ is amino, methylamino-methyl,
dimethylamino-methyl, [(2-
hydroxyethyl)-methylamino]-methyl, [(2-hydroxyethyl)-ethylamino]-methyl, [(2-
methoxy-1-methyl-ethyl)-
amino]-methyl, [(2-methoxyethyl)-methylamino]-methyl, [di-(2-hydroxyethyl)-
amino]-methyl, (azetidin-1 -yI)-
methyl, (pyrrolidin-1-yI)-methyl, (piperidin-1-yI)-methyl, morpholin-4-yl,
(morpholin-4-yI)-methyl, piperazin-
1-ylmethyl, 2-(morpholin-4-yI)-ethyl, 3-(morpholin-4-yI)-propyl, (3-methoxy-
azetidin-1-yI)-methyl, 1-(3-
methoxy-azetidin-1 -yI)-ethyl, (1 -methyl-piperazin-4-yI)-methyl,
(4-hydroxypiperidin-1-yI)-methyl, (4-
methoxypiperidin-1 -yI)-methyl, (3-methoxy-pyrrolidin-1-yI)-methyl, (4-
dimethylamino-piperidin-1-yI)-
methyl]; or
= 5- or 6-membered heteroaryl (especially pyrazolyl, oxadiazolyl,
pyridinyl, pyridazinyl, pyrimidinyl); wherein said
5- or 6-membered heteroaryl independently is mono-, di- or tri-substituted;
wherein one substituent is (C1_
4)alkyl (especially methyl), (C1_4)alkoxy (especially methoxy), or halogen
(especially fluoro); wherein said
substituent is attached in ortho position with regard to the point of
attachment of the rest of the molecule; and,
if present, the remaining substituent(s) are independently selected from
D (Ci_4)alkyl (especially methyl, isopropyl);
(Ci_4)alkoxy (especially methoxy);
D (C1_3)fluoroalkyl (especially trifluoromethyl);
D halogen (especially fluoro, chloro);
cyano; or
D (C3_6)cycloalkyl-X12-, wherein X12 represents a direct bond; [especially
such group (C3_6)cycloalkyl-X12- is
cyclopropyl].
wherein in a sub-embodiment R1 represents phenyl, or 5- or 6-membered
heteroaryl selected from pyrazolyl,
oxadiazolyl, pyridinyl, pyridazinyl, and pyrimidinyl; wherein said phenyl or 5-
or 6-membered heteroaryl are
substituted as defined herein before.
11) Another embodiment relates to compounds according to any one of
embodiments 1) to 7), wherein R1
represents
= -CO-R11 wherein R11 represents (Ci4alkoxy (especially isopropoxy, tert-
butoxy);
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
24
= phenyl which is mono-, or di- or tri-substituted (notably mono- or di-
substituted); wherein one substituent is (C1_
4)alkyl (especially methyl), (C1_4)alkoxy (especially methoxy), or halogen
(especially fluoro) [especially such
substituent is methyl, methoxy or fluoro; in particular methyl]; wherein said
substituent is attached in ortho
position with regard to the point of attachment of the rest of the molecule;
and, if present, the remaining
substituent(s) are independently selected from:
D (Ci_4)alkyl (especially methyl, isopropyl);
D (Ci_4)alkoxy (especially methoxy);
(Ci_3)fluoroalkyl (especially trifluoromethyl);
(C1_3)fluoroalkoxy (especially trifluoromethoxy);
D halogen (especially fluoro, bromo);
cyano;
hydroxy-(C1_3)alkyl (especially 1-hydroxyethyl, hydroxymethyl, 1-hydroxy-1-
methyl-ethyl);
(Ci_4)alkoxy-(Ci_3)alkyl (especially 1-methoxyethyl);
(C1_4)alkoxy-(C2_3)alkoxy (especially 2-methoxy-ethoxy);
D (C3_6)cycloalkyl-X12-, wherein X12 represents a direct bond, -0-, or -
(C1_3)alkylene-0-; [especially such
group (C3_6)cycloalkyl-X12- is cyclobutyl-oxy, or cyclopropyl-methoxy]; or
R14aR14bN_X14_, wherein X14 represents a direct bond or (C1_3)alkylene; and
wherein Rua represents
hydrogen or (Ci4alkyl, and Rub independently represents hydrogen, (Ci4alkyl,
hydroxy-(C2_4)alkyl, or
(C1_3)alkoxy-(C24alkyl; or Rua and Rub together with the nitrogen to which
they are attached to form a 4-
to 6-membered saturated ring optionally containing one additional ring
heteroatom selected from oxygen
and nitrogen; and wherein said ring is optionally mono-substituted with
(C1_4)alkyl (especially methyl),
hydroxy, (C1_4)alkoxy (especially methoxy), or dimethylamino;
[especially such group R14aR14bN_x14_ is amino, methylamino-methyl,
dimethylamino-methyl, [(2-
hydroxyethyl)-methylamino]-methyl, [(2-hydroxyethyl)-ethylamino]-methyl, [(2-
methoxy-1-methyl-ethyl)-
amino]-methyl, [(2-methoxyethyp-methylamino]-methyl, [di-(2-hydroxyethyp-
amino]-methyl, (azetidin-111)-
methyl, (pyrrolidin-1-yI)-methyl, (piperidin-1-yI)-methyl, morpholin-4-yl,
piperazin-1-ylmethyl, (morpholin-4-
y1)-methyl, 2-(morpholin-4-yI)-ethyl, 3-(morpholin-4-yI)-propyl, (3-methoxy-
azetidin-1-yI)-methyl, 1-(3-
methoxy-azetidin-111)-ethyl, (1-methyl-piperazin-4-yI)-methyl, (4-
hydroxypiperidin-1-yI)-methyl, (4-
methoxypiperidin-1-yI)-methyl, (3-methoxy-pyrrolidin-1-yI)-methyl, (4-
dimethylamino-piperidin-1-yI)-
methyl.]; or
= 6-membered heteroaryl (especially pyridinyl, pyridazinyl, pyrimidinyl);
wherein said 6-membered heteroaryl
independently is mono-, di- or tri-substituted (notably mono-, or di-
substituted); wherein one substituent is (C1_
4)alkyl (especially methyl), (C1_4)alkoxy (especially methoxy), or halogen
(especially fluoro); wherein said
substituent is attached in ortho position with regard to the point of
attachment of the rest of the molecule; and,
if present, the remaining substituent(s) are independently selected from
D (Ci_4)alkyl (especially methyl, isopropyl);
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
(Ci_4)alkoxy (especially methoxy);
(Ci_3)fluoroalkyl (especially trifluoromethyl);
D halogen (especially fluoro);
cyano; or
5 D (C3_6)cycloalkyl-X12-, wherein X12 represents a direct bond [especially
such group (C3_6)cycloalkyl-X12- is
cyclopropyl];
wherein in a sub-embodiment R1 represents phenyl, or 6-membered heteroaryl
selected from pyridinyl, pyridazinyl,
and pyrimidinyl; wherein said phenyl or 6-membered heteroaryl are substituted
as defined herein before.
12) Another embodiment relates to compounds according to any one of
embodiments 1) to 7), wherein
10 R1 represents phenyl which is mono-, or di- or tri-substituted (notably
mono- or di-substituted); wherein
= one substituent is
(Ci_4)alkyl (especially methyl);
(Ci_4)alkoxy (especially methoxy); or
D halogen (especially fluoro);
15 wherein said substituent is attached in ortho position with regard to
the point of attachment of the rest of the
molecule [especially such substituent is methyl, methoxy or fluoro; in
particular methyl];
= and, if present, the remaining substituent(s) is/are independently
selected from:
D methyl;
D methoxy;
20 D halogen (especially fluoro);
cyano, or
D amino.
13) Another embodiment relates to compounds according to any one of
embodiments 1) to 12), wherein R2
represents phenyl, or 6-membered heteroaryl (in particular pyridinyl,
pyrimidinyl, pyrazinyl, pyridazinyl) [especially
25 R2 represents phenyl]; wherein said phenyl or 6-membered heteroaryl
independently is mono-, or di-substituted
(especially mono-substituted, in particular mono-substituted in ortho position
with regard to the point of attachment
of the rest of the molecule), wherein the substituents are independently
selected from
(Ci_4)alkyl (especially methyl, isopropyl);
(Ci_4)alkoxy (especially methoxy, isopropoxy);
(C1_3)fluoroalkyl (especially trifluoromethyl);
(C1_3)fluoroalkoxy (especially trifluoromethoxy);
D halogen (especially chloro, fluoro); or
(C3_6)cycloalkyl-X21-, wherein X21 represents a direct bond, -0-, or -
(C1_3)alkylene-0-, and wherein the (C3_
6)cycloalkyl independently contains one optional ring oxygen; [especially such
group (C3_6)cycloalkyl-X21- is
cyclopropyl, cyclopropyl-oxy, cyclobutyl-oxy, oxetan-3-yl-oxy, cyclopropyl-
methoxy].
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
26
14) Another embodiment relates to compounds according to any one of
embodiments 1) to 12), wherein R2
represents phenyl, or 6-membered heteroaryl (in particular pyridinyl,
pyrimidinyl, pyrazinyl, pyridazinyl) [especially
R2 represents phenyl]; wherein said phenyl or 6-membered heteroaryl is mono-
substituted in ortho position with
regard to the point of attachment of the rest of the molecule, wherein the
substituent is independently selected from
(Ci_4)alkyl (especially methyl, isopropyl);
= (Ci_4)alkoxy (especially methoxy, isopropoxy);
= (Ci_3)fluoroalkyl (especially trifluoromethyl);
(C1_3)fluoroalkoxy (especially trifluoromethoxy); or
= (C3_6)cycloalkyl-X21-, wherein X21 represents a direct bond, -0-, or -
(C1_3)alkylene-0-, and wherein the (C3_
6)cycloalkyl independently contains one optional ring oxygen; [especially such
group (C3_6)cycloalkyl-X21- is
cyclopropyl, cyclopropyl-oxy, cyclobutyl-oxy, oxetan-3-yl-oxy, cyclopropyl-
methoxy].
15) Another embodiment relates to compounds according to any one of
embodiments 1) to 12), wherein
R2 represents phenyl, which is mono-substituted in ortho position with regard
to the point of attachment of the rest
of the molecule, wherein the substituent is independently selected from
(C1_4)alkyl (especially isopropyl);
(Ci_4)alkoxy (especially methoxy, isopropoxy);
= (Ci_3)fluoroalkyl (especially trifluoromethyl);
(C1_3)fluoroalkoxy (especially trifluoromethoxy); or
D cyclopropyl-oxy, cyclopropyl-methoxy, cyclobutyl-oxy, oxetan-3-yl-oxy;
or R2 represents 6-membered heteroaryl (in particular pyridinyl, pyrimidinyl,
pyrazinyl, pyridazinyl); wherein said 6-
membered heteroaryl is mono-substituted in ortho position with regard to the
point of attachment of the rest of the
molecule, wherein the substituent is independently selected from
D (Ci_4)alkyl (especially isopropyl);
= (Ci_4)alkoxy (especially methoxy, isopropoxy);
> (C1_3)fluoroalkyl (especially trifluoromethyl); or
= (C1_3)fluoroalkoxy (especially trifluoromethoxy).
16) Another embodiment relates to compounds according to any one of
embodiments 1) to 15), wherein R3
represents hydrogen, or methyl (especially hydrogen). In a sub-embodiment R3
represents hydrogen.
17) Another embodiment relates to compounds according to any one of
embodiments 1) to 16),
R4 represents
= (Ci4alkyl (especially methyl);
= hydroxy-(C1_3)alkyl (especially hydroxymethyl);
= -0-R41, wherein R41 represents
D. hydrogen;
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
27
= (Ci_4)alkyl (especially methyl);
= (C)alkyl which is substituted with one or two hydroxy (especially 2-
hydroxyethyl, 2,3-dihydroxypropyl);
R41 a R41 bN¨(C2_3)alkylene-, wherein R41a and Rob independently represent
hydrogen or (C1_4)alkyl
(especially such group is 2-dimethylamino-ethyl); or
D (C4_7)heterocyclyl-X41-, wherein X41 represents a direct bond or
(C1_3)alkylene, and wherein the
(C4_7)heterocycly1 independently contains one or two ring heteroatoms
independently selected from
nitrogen and oxygen; wherein the oxygen atom of such group (C4_7)heterocyclyl-
X41-0- is separated by at
least two (ring and/or chain) carbon atoms from such ring heteroatom; wherein
said (C4_7)heterocycly1
independently is unsubstituted, or mono-, or di-substituted, wherein the
substituents are independently
selected from:
= (Ci4alkyl (especially methyl); and/or
= (Ci4alkoxy-carbonyl attached to a ring nitrogen atom having a free
valency;
[especially such (C4_7)heterocyclyl-X41- group is oxetan-3-yl, 1-methyl-
pyrrolidin-3-yl, 2,2-dimethyl-
dioxolan-4-yl-methyl, 2-morpholin-4-yl-ethyl, 1-tert-butoxy-carbonyl-piperidin-
4-yl, piperidin-4-yI];
. _N R42a R42 b wherein R42a represents hydrogen or (C1_4)alkyl, and R42b
independently represents hydrogen,
(C1_3)alkoxy-(C2_3)alkyl, hydroxy-(C2_3)alkyl, or R42a and R42b together with
the nitrogen to which they
are attached form a 4- to 7-membered saturated ring optionally containing one
further ring heteroatom selected
from oxygen and nitrogen, wherein said ring is unsubstituted or mono-
substituted with (C1_3)alkyl (especially
methyl), or (C1_3)alkoxy (especially methoxy) [especially such group ¨N
R42aR42b is (2-hydroxyethyl)-amino, (2-
hydroxyethyl)-methyl-amino, (2-methoxyethyl)-amino, morpholin-4-yl, 3-methoxy-
azetidinyl, or 4-methyl-
piperazin-1-yI]; or
= -CO-R43 wherein R43
represents (Ci4alkoxy (especially methoxy); or _NR43aR43b wherein R43a
represents
hydrogen or (C1_4)alkyl (especially methyl), and R43b independently represents
hydrogen, (C1_4)alkyl (especially
methyl), (Ci_3)alkoxy-(C2_3)alkyl (especially 2-methoxy-ethyl), or hydroxy-
(C2_3)alkyl (especially 2-hydroxy-ethyl);
[especially such group -CO-R43 is methoxycarbonyl, carbamoyl, methyl-
carbamoyl, dimethylcarbamoyl,
hydroxyethylcarbamoyl, methoxyethylcarbamoyl];
wherein in a sub-embodiment R4 represents -0-R41 as defined herein above;
especially R4 represents (Ci4alkoxy
(in particular methoxy).
18) Another embodiment relates to compounds according to any one of
embodiments 1) to 16), wherein
R4 represents
= (Ci4alkyl (especially methyl);
= hydroxy-(C1_3)alkyl (especially hydroxymethyl);
= -0-R41, wherein R41 represents
D hydrogen;
(Ci_4)alkyl (especially methyl);
= (C)alkyl which is substituted with one or two hydroxy (especially 2-
hydroxyethyl, 2,3-dihydroxypropyl);
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
28
R4laR41bN¨(C2_3)alkylene-, wherein R41a and Rob independently represent
hydrogen or (C1_4)alkyl
(especially such group is 2-dimethylamino-ethyl); or
D (C4_7)heterocyclyl-X41-, wherein X41 represents a direct bond or -CH2-, and
wherein the (C4_7)heterocycly1
independently contains one or two ring heteroatoms independently selected from
nitrogen and oxygen;
wherein the oxygen atom of such group (C4_7)heterocyclyl-X41-0- is separated
by at least two (ring and/or
chain) carbon atoms from such ring heteroatom; wherein said (C4_7)heterocycly1
independently is
unsubstituted, or mono-substituted with (C1_4)alkyl (especially methyl) at a
ring nitrogen atom having a free
valency, or di-substituted with methyl;
[especially such (C4_7)heterocyclyl-X41- group is oxetan-3-yl, 1-methyl-
pyrrolidin-3-yl, 2,2-dimethyl-
dioxolan-4-yl-methyl, 2-morpholin-4-ykethyl];
. _NR42aR42b wherein R42a represents hydrogen or (Ci4alkyl (especially
methyl), and R42b independently
represents (C1_4)alkyl, (C1_3)alkoxy-(C2_3)alkyl, hydroxy-(C2_3)alkyl; or R42a
and R42b together with the nitrogen to
which they are attached form a 4- to 7-membered saturated ring optionally
containing one further ring
heteroatom selected from oxygen and nitrogen, wherein said ring is
unsubstituted, or mono-substituted with
(C1_3)alkyl (especially methyl) attached to a ring nitrogen atom having a free
valency, or (C1_3)alkoxy (especially
methoxy) attached to a ring carbon atom [especially such group ¨NR42aR42b is
(2-hydroxyethyl)-amino,
hydroxyethyl)-methykamino, (2-methoxyethyl)-amino, morpholin-4-yl, 3-methoxy-
azetidinyl, or 4-methyl-
piperazin-1-yI]; or
= -CO-R43 wherein R43
represents (Ci4alkoxy (especially methoxy); or _NR43aR43b wherein R43a
represents
hydrogen or (C1_4)alkyl (especially methyl), and R43b independently represents
hydrogen, (C1_4)alkyl (especially
methyl), (Ci_3)alkoxy-(C2_3)alkyl (especially 2-methoxy-ethyl), or hydroxy-
(C2_3)alkyl (especially 2-hydroxy-ethyl);
[especially such group -CO-R43 is methoxycarbonyl, carbamoyl, methyl-
carbamoyl, dimethylcarbamoyl,
hydroxyethylcarbamoyl, methoxyethylcarbamoyl] ;
wherein in a sub-embodiment R4 represents -0-R41 as defined herein above;
especially R4 represents (Ci4alkoxy
(in particular methoxy).
19) Another embodiment relates to compounds according to any one of
embodiments 1) to 16), wherein
R4 represents
= (Ci4alkyl (especially methyl);
= hydroxy-(C1_3)alkyl (especially hydroxymethyl);
= -0-R41, wherein R41 represents
D hydrogen;
= (Ci_4)alkyl (especially methyl);
= (C)alkyl which is substituted with one or two hydroxy (especially 2-
hydroxyethyl, 2,3-dihydroxypropyl);
R41aR41bN_(C2_3)alkylene-, wherein R41a represent hydrogen or (Ci4alkyl, and
R41b independently
represents (C1_4)alkyl (especially such group is 2-dimethylamino-ethyl); or
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
29
(C4_7)heterocyclyl-X41-, wherein X41 represents a direct bond, -CH2-, or -CH2-
CH2- , and wherein the
(C4_7)heterocycly1 independently contains one or two ring heteroatoms
independently selected from
nitrogen and oxygen; wherein the oxygen atom of such group (C4_7)heterocyclyl-
X41-0- is separated by at
least two (ring and/or chain) carbon atoms from such ring heteroatom; wherein
said (C4_7)heterocycly1
independently is unsubstituted, or mono-substituted with (Ci4alkyl (especially
methyl) at a ring nitrogen
atom having a free valency, or di-substituted with methyl;
[especially such (C4_7)heterocyclyl-X41- group is oxetan-3-yl, 1-methyl-
pyrrolidin-3-yl, 2,2-dimethyl-
dioxolan-4-yl-methyl, 2-morpholin-4-yl-ethyl];
= _NR42aR42b wherein R42a represents hydrogen or (Ci4alkyl (especially
methyl), and R42b independently
represents (C1_4)alkyl, (C1_3)alkoxy-(C2_3)alkyl, hydroxy-(C2_3)alkyl; or R42a
and R42b together with the nitrogen to
which they are attached form a 4- to 7-membered saturated ring optionally
containing one further ring
heteroatom selected from oxygen and nitrogen, wherein said ring is
unsubstituted, or mono-substituted with
(C1_3)alkyl (especially methyl) attached to a ring nitrogen atom having a free
valency, or mono-substituted with
(C1_3)alkoxy (especially methoxy) attached to a ring carbon atom [especially
such group ¨NR42aR42b is (2-
hydroxyethyl)-amino, (2-hydroxyethyl)-methyl-amino, (2-methoxyethyl)-amino,
morpholin-4-yl, 3-methoxy-
azetidinyl, or 4-methyl-piperazin-1-yI]; or
= -CO-R43 wherein R43
represents (Ci4alkoxy (especially methoxy); or _NR43aR43b wherein R43a
represents
hydrogen or (C1_4)alkyl (especially methyl), and R43b independently represents
hydrogen, (C1_4)alkyl (especially
methyl), (Ci_3)alkoxy-(C2_3)alkyl (especially 2-methoxy-ethyl), or hydroxy-
(C2_3)alkyl (especially 2-hydroxy-ethyl);
[especially such group -CO-R43 is methoxycarbonyl, carbamoyl, methyl-
carbamoyl, dimethylcarbamoyl,
hydroxyethylcarbamoyl, methoxyethylcarbamoyl] ;
wherein in a sub-embodiment R4 represents -0-R41 as defined herein above;
especially R4 represents (Ci4alkoxy
(in particular methoxy).
20) Another embodiment relates to compounds according to any one of
embodiments 1) to 16), wherein
R4 represents
= hydroxy;
= (Ci4alkoxy (especially methoxy);
= 2-hydroxy-ethoxy, or 2,3-dihydroxy-propoxy;
= (oxetan-311)-oxy-, (2,2-dimethyl-dioxolan-4-yI)-methoxy, or (2-morpholin-
4-yI)-ethyl;
= (2-hydroxyethyl)-amino, (2-hydroxyethyl)-methyl-amino, or (2-
methoxyethyl)-amino; or
= morpholin-4-yl, or 3-methoxy-azetidinyl;
wherein in a sub-embodiment R4 represents (Ci4alkoxy (especially methoxy) or 2-
hydroxy-ethoxy.
21) Another embodiment relates to compounds according to any one of
embodiments 1) to 16), wherein
R4 represents
= hydroxy;
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
= (Ci4alkoxy (especially methoxy);
= 2-hydroxy-ethoxy;
= 2,3-dihydroxy-propoxy;
= (oxetan-311)-oxy-;
5 = (2,2-dimethyl-dioxolan-4-yI)-methoxy;
= (2-hydroxyethyl)-methyl-amino; or
= morpholin-4-y1; or
= 3-methoxy-azetidinyl;
wherein in a sub-embodiment R4 represents (Ci4alkoxy (especially methoxy).
10 22) The invention, thus, relates to compounds of the formula (I) as
defined in embodiment 1), or to such
compounds further limited by the characteristics of any one of embodiments 2)
to 21), under consideration of their
respective dependencies; to pharmaceutically acceptable salts thereof; and to
the use of such compounds as
medicaments especially in the treatment of disorders relating to diseases and
disorders related to pathogenic
events associated with elevated levels of C5a and/or with C5aR activation. For
avoidance of any doubt, especially
15 the following embodiments relating to the compounds of formula (I) are
thus possible and intended and herewith
specifically disclosed in individualized form:
1, 2+1, 3+1, 5+1, 6+1, 6+2+1, 6+3+1, 7+1, 7+5+1, 8+1, 8+2+1, 8+3+1, 8+6+1,
8+6+2+1, 8+6+3+1, 9+1, 9+2+1,
9+3+1, 9+6+1, 9+6+2+1, 9+6+3+1, 11+1, 11+5+1, 11+7+1, 11+7+5+1, 12+1, 12+5+1,
12+7+1, 12+7+5+1, 13+1,
13+2+1, 13+3+1, 13+6+1, 13+6+2+1, 13+6+3+1, 13+8+1, 13+8+2+1, 13+8+3+1,
13+8+6+1, 13+8+6+2+1,
20 13+8+6+3+1, 13+9+1, 13+9+2+1, 13+9+3+1, 13+9+6+1, 13+9+6+2+1,
13+9+6+3+1, 14+1, 14+5+1, 14+7+1,
14+7+5+1, 14+11+1, 14+11+5+1, 14+11+7+1, 14+11+7+5+1, 14+12+1, 14+12+5+1,
14+12+7+1, 14+12+7+5+1,
15+1, 15+5+1, 15+7+1, 15+7+5+1, 15+11+1, 15+11+5+1, 15+11+7+1, 15+11+7+5+1,
15+12+1, 15+12+5+1,
15+12+7+1, 15+12+7+5+1, 16+1, 16+2+1, 16+3+1, 16+5+1, 16+6+1, 16+6+2+1,
16+6+3+1, 16+7+1, 16+7+5+1,
16+8+1, 16+8+2+1, 16+8+3+1, 16+8+6+1, 16+8+6+2+1, 16+8+6+3+1, 16+9+1,
16+9+2+1, 16+9+3+1, 16+9+6+1,
25 16+9+6+2+1, 16+9+6+3+1, 16+11+1, 16+11+5+1, 16+11+7+1, 16+11+7+5+1,
16+12+1, 16+12+5+1, 16+12+7+1,
16+12+7+5+1, 16+13+1, 16+13+2+1, 16+13+3+1, 16+13+6+1, 16+13+6+2+1,
16+13+6+3+1, 16+13+8+1,
16+13+8+2+1, 16+13+8+3+1, 16+13+8+6+1, 16+13+8+6+2+1, 16+13+8+6+3+1,
16+13+9+1, 16+13+9+2+1,
16+13+9+3+1, 16+13+9+6+1, 16+13+9+6+2+1, 16+13+9+6+3+1, 16+14+1, 16+14+5+1,
16+14+7+1,
16+14+7+5+1, 16+14+11+1, 16+14+11+5+1, 16+14+11+7+1, 16+14+11+7+5+1,
16+14+12+1, 16+14+12+5+1,
30 16+14+12+7+1, 16+14+12+7+5+1, 16+15+1, 16+15+5+1, 16+15+7+1, 16+15+7+5+1,
16+15+11+1,
16+15+11+5+1, 16+15+11+7+1, 16+15+11+7+5+1,
16+15+12+1, 16+15+12+5+1, 16+15+12+7+1,
16+15+12+7+5+1, 17+1, 17+2+1, 17+3+1, 17+6+1, 17+6+2+1, 17+6+3+1, 17+8+1,
17+8+2+1, 17+8+3+1,
17+8+6+1, 17+8+6+2+1, 17+8+6+3+1, 17+9+1, 17+9+2+1, 17+9+3+1, 17+9+6+1,
17+9+6+2+1, 17+9+6+3+1,
17+13+1, 17+13+2+1, 17+13+3+1, 17+13+6+1, 17+13+6+2+1, 17+13+6+3+1, 17+13+8+1,
17+13+8+2+1,
17+13+8+3+1, 17+13+8+6+1, 17+13+8+6+2+1, 17+13+8+6+3+1, 17+13+9+1,
17+13+9+2+1, 17+13+9+3+1,
17+13+9+6+1, 17+13+9+6+2+1, 17+13+9+6+3+1, 17+16+1, 17+16+2+1, 17+16+3+1,
17+16+5+1, 17+16+6+1,
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
31
17+16+6+2+1, 17+16+6+3+1, 17+16+7+1, 17+16+7+5+1, 17+16+8+1, 17+16+8+2+1,
17+16+8+3+1,
17+16+8+6+1, 17+16+8+6+2+1, 17+16+8+6+3+1, 17+16+9+1, 17+16+9+2+1,
17+16+9+3+1, 17+16+9+6+1,
17+16+9+6+2+1, 17+16+9+6+3+1, 17+16+11+1, 17+16+11+5+1, 17+16+11+7+1,
17+16+11+7+5+1,
17+16+12+1, 17+16+12+5+1, 17+16+12+7+1, 17+16+12+7+5+1, 17+16+13+1,
17+16+13+2+1, 17+16+13+3+1,
17+16+13+6+1, 17+16+13+6+2+1, 17+16+13+6+3+1, 17+16+13+8+1, 17+16+13+8+2+1,
17+16+13+8+3+1,
17+16+13+8+6+1, 17+16+13+8+6+2+1, 17+16+13+8+6+3+1,
17+16+13+9+1, 17+16+13+9+2+1,
17+16+13+9+3+1, 17+16+13+9+6+1, 17+16+13+9+6+2+1, 17+16+13+9+6+3+1,
17+16+14+1, 17+16+14+5+1,
17+16+14+7+1, 17+16+14+7+5+1, 17+16+14+11+1,
17+16+14+11+5+1, 17+16+14+11+7+1,
17+16+14+11+7+5+1, 17+16+14+12+1, 17+16+14+12+5+1, 17+16+14+12+7+1,
17+16+14+12+7+5+1,
17+16+15+1, 17+16+15+5+1, 17+16+15+7+1, 17+16+15+7+5+1, 17+16+15+11+1,
17+16+15+11+5+1,
17+16+15+11+7+1, 17+16+15+11+7+5+1, 17+16+15+12+1,
17+16+15+12+5+1, 17+16+15+12+7+1,
17+16+15+12+7+5+1, 19+1, 19+5+1, 19+7+1, 19+7+5+1, 19+11+1, 19+11+5+1,
19+11+7+1, 19+11+7+5+1,
19+12+1, 19+12+5+1, 19+12+7+1, 19+12+7+5+1, 19+14+1, 19+14+5+1, 19+14+7+1,
19+14+7+5+1,
19+14+11+1, 19+14+11+5+1, 19+14+11+7+1, 19+14+11+7+5+1, 19+14+12+1,
19+14+12+5+1, 19+14+12+7+1,
19+14+12+7+5+1, 19+15+1, 19+15+5+1, 19+15+7+1, 19+15+7+5+1, 19+15+11+1,
19+15+11+5+1,
19+15+11+7+1, 19+15+11+7+5+1, 19+15+12+1, 19+15+12+5+1, 19+15+12+7+1,
19+15+12+7+5+1, 19+16+1,
19+16+2+1, 19+16+3+1, 19+16+5+1, 19+16+6+1, 19+16+6+2+1, 19+16+6+3+1,
19+16+7+1, 19+16+7+5+1,
19+16+8+1, 19+16+8+2+1, 19+16+8+3+1, 19+16+8+6+1, 19+16+8+6+2+1,
19+16+8+6+3+1, 19+16+9+1,
19+16+9+2+1, 19+16+9+3+1, 19+16+9+6+1, 19+16+9+6+2+1, 19+16+9+6+3+1,
19+16+11+1, 19+16+11+5+1,
19+16+11+7+1, 19+16+11+7+5+1, 19+16+12+1, 19+16+12+5+1, 19+16+12+7+1,
19+16+12+7+5+1,
19+16+13+1, 19+16+13+2+1, 19+16+13+3+1, 19+16+13+6+1, 19+16+13+6+2+1,
19+16+13+6+3+1,
19+16+13+8+1, 19+16+13+8+2+1, 19+16+13+8+3+1,
19+16+13+8+6+1, 19+16+13+8+6+2+1,
19+16+13+8+6+3+1, 19+16+13+9+1, 19+16+13+9+2+1,
19+16+13+9+3+1, 19+16+13+9+6+1,
19+16+13+9+6+2+1, 19+16+13+9+6+3+1, 19+16+14+1, 19+16+14+5+1, 19+16+14+7+1,
19+16+14+7+5+1,
19+16+14+11+1, 19+16+14+11+5+1, 19+16+14+11+7+1,
19+16+14+11+7+5+1, 19+16+14+12+1,
19+16+14+12+5+1, 19+16+14+12+7+1, 19+16+14+12+7+5+1, 19+16+15+1, 19+16+15+5+1,
19+16+15+7+1,
19+16+15+7+5+1, 19+16+15+11+1, 19+16+15+11+5+1,
19+16+15+11+7+1, 19+16+15+11+7+5+1,
19+16+15+12+1, 19+16+15+12+5+1, 19+16+15+12+7+1, 19+16+15+12+7+5+1, 20+1,
20+5+1, 20+7+1,
20+7+5+1, 20+11+1, 20+11+5+1, 20+11+7+1, 20+11+7+5+1, 20+12+1, 20+12+5+1,
20+12+7+1, 20+12+7+5+1,
20+14+1, 20+14+5+1, 20+14+7+1, 20+14+7+5+1, 20+14+11+1, 20+14+11+5+1,
20+14+11+7+1,
20+14+11+7+5+1, 20+14+12+1, 20+14+12+5+1, 20+14+12+7+1, 20+14+12+7+5+1,
20+15+1, 20+15+5+1,
20+15+7+1, 20+15+7+5+1, 20+15+11+1, 20+15+11+5+1, 20+15+11+7+1,
20+15+11+7+5+1, 20+15+12+1,
20+15+12+5+1, 20+15+12+7+1, 20+15+12+7+5+1, 20+16+1, 20+16+2+1, 20+16+3+1,
20+16+5+1, 20+16+6+1,
20+16+6+2+1, 20+16+6+3+1, 20+16+7+1, 20+16+7+5+1, 20+16+8+1, 20+16+8+2+1,
20+16+8+3+1,
20+16+8+6+1, 20+16+8+6+2+1, 20+16+8+6+3+1, 20+16+9+1, 20+16+9+2+1,
20+16+9+3+1, 20+16+9+6+1,
20+16+9+6+2+1, 20+16+9+6+3+1, 20+16+11+1, 20+16+11+5+1, 20+16+11+7+1,
20+16+11+7+5+1,
20+16+12+1, 20+16+12+5+1, 20+16+12+7+1, 20+16+12+7+5+1, 20+16+13+1,
20+16+13+2+1, 20+16+13+3+1,
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
32
20+16+13+6+1, 20+16+13+6+2+1, 20+16+13+6+3+1, 20+16+13+8+1, 20+16+13+8+2+1,
20+16+13+8+3+1,
20+16+13+8+6+1, 20+16+13+8+6+2+1, 20+16+13+8+6+3+1,
20+16+13+9+1, 20+16+13+9+2+1,
20+16+13+9+3+1, 20+16+13+9+6+1, 20+16+13+9+6+2+1, 20+16+13+9+6+3+1,
20+16+14+1, 20+16+14+5+1,
20+16+14+7+1, 20+16+14+7+5+1, 20+16+14+11+1,
20+16+14+11+5+1, 20+16+14+11+7+1,
20+16+14+11+7+5+1, 20+16+14+12+1, 20+16+14+12+5+1, 20+16+14+12+7+1,
20+16+14+12+7+5+1,
20+16+15+1, 20+16+15+5+1, 20+16+15+7+1, 20+16+15+7+5+1, 20+16+15+11+1,
20+16+15+11+5+1,
20+16+15+11+7+1, 20+16+15+11+7+5+1, 20+16+15+12+1, 20+16+15+12+5+1,
20+16+15+12+7+1,
20+16+15+12+7+5+1, 21+1, 21+5+1, 21+7+1, 21+7+5+1, 21+11+1, 21+11+5+1,
21+11+7+1, 21+11+7+5+1,
21+12+1, 21+12+5+1, 21+12+7+1, 21+12+7+5+1, 21+14+1, 21+14+5+1, 21+14+7+1,
21+14+7+5+1,
21+14+11+1, 21+14+11+5+1, 21+14+11+7+1, 21+14+11+7+5+1, 21+14+12+1,
21+14+12+5+1, 21+14+12+7+1,
21+14+12+7+5+1, 21+15+1, 21+15+5+1, 21+15+7+1, 21+15+7+5+1, 21+15+11+1,
21+15+11+5+1,
21+15+11+7+1, 21+15+11+7+5+1, 21+15+12+1, 21+15+12+5+1, 21+15+12+7+1,
21+15+12+7+5+1, 21+16+1,
21+16+2+1, 21+16+3+1, 21+16+5+1, 21+16+6+1, 21+16+6+2+1, 21+16+6+3+1,
21+16+7+1, 21+16+7+5+1,
21+16+8+1, 21+16+8+2+1, 21+16+8+3+1, 21+16+8+6+1, 21+16+8+6+2+1,
21+16+8+6+3+1, 21+16+9+1,
21+16+9+2+1, 21+16+9+3+1, 21+16+9+6+1, 21+16+9+6+2+1, 21+16+9+6+3+1,
21+16+11+1, 21+16+11+5+1,
21+16+11+7+1, 21+16+11+7+5+1, 21+16+12+1, 21+16+12+5+1, 21+16+12+7+1,
21+16+12+7+5+1,
21+16+13+1, 21+16+13+2+1, 21+16+13+3+1, 21+16+13+6+1, 21+16+13+6+2+1,
21+16+13+6+3+1,
21+16+13+8+1, 21+16+13+8+2+1, 21+16+13+8+3+1,
21+16+13+8+6+1, 21+16+13+8+6+2+1,
21+16+13+8+6+3+1, 21+16+13+9+1, 21+16+13+9+2+1,
21+16+13+9+3+1, 21+16+13+9+6+1,
21+16+13+9+6+2+1, 21+16+13+9+6+3+1, 21+16+14+1, 21+16+14+5+1, 21+16+14+7+1,
21+16+14+7+5+1,
21+16+14+11+1, 21+16+14+11+5+1, 21+16+14+11+7+1,
21+16+14+11+7+5+1, 21+16+14+12+1,
21+16+14+12+5+1, 21+16+14+12+7+1, 21+16+14+12+7+5+1, 21+16+15+1, 21+16+15+5+1,
21+16+15+7+1,
21+16+15+7+5+1, 21+16+15+11+1, 21+16+15+11+5+1,
21+16+15+11+7+1, 21+16+15+11+7+5+1,
21+16+15+12+1, 21+16+15+12+5+1, 21+16+15+12+7+1, 21+16+15+12+7+5+1.
In the list above the numbers refer to the embodiments according to their
numbering provided hereinabove
whereas "+" indicates the dependency from another embodiment. The different
individualized embodiments are
separated by commas. In other words, "20+15+12+1" for example refers to
embodiment 20) depending on
embodiment 15), depending on embodiment 12), depending on embodiment 1), i.e.
embodiment "20+15+12+1"
corresponds to the compounds of formula (I) according to embodiment 1) further
limited by all the features of the
embodiments 12), 15), and 20).
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
33
23) A second aspect of the invention relates to compounds of the formula (1)
according to embodiment 1) which are
also compounds of the formula (II)
R1
1
cf)
Z N
I > __ 0
X====-, N
R4 R3
R2
Formula (II)
wherein X is CH and Z is CH, Xis N and Z is CH, or X is CH and Z is N;
= ring A represents a saturated 4- to 7-membered mono-cyclic carbocyclic
ring containing the ring nitrogen atom
to which R1 is attached, wherein said ring independently is unsubstituted, or
mono-, or di-substituted wherein
the substituents are independently selected from (C1_3)alkyl (especially
methyl), fluoro, or (C1_4)alkoxy-carbonyl
(especially ethoxy-carbonyl) [especially such ring A is azetidin-1,3-diyl,
pyrrolidin-1,3-diyl, 4-methyl-pyrrolidin-
1,3-diyl, piperidin-1,3-diyl, piperidin-1,4-diyl, 3-fluoro-piperidin-1,4-diyl,
2-methyl-piperidin-1,4-diyl, 3-methyl-
piperidin-1,4-diyl, 3-(ethoxycarbonyI)-piperidin-1,4-diyl, 3,3-dimethyl-
piperidin-1,4-diyl, azepan-1,4-diyI]; or
= ring A represents a saturated 7- or 8-membered bridged bi-cyclic
carbocyclic ring containing the ring nitrogen
atom to which R1 is attached, wherein said ring is unsubstituted [especially
such ring is 2-aza-
bicyclo[2.2.1]heptane-2,5-diyl, 3-aza-bicyclo[3.1.1]heptane-3,6-diyI];
R1 represents
= (Ci4alkyl (especially methyl, isopropyl);
= -CO-R11 wherein R11 represents (C1_4)alkyl; (C1_4)alkoxy; phenyl,
phenoxy; (C3_6)cycloalkyl; or (C3_6)cycloalkoxy
optionally containing one ring oxygen and optionally mono-substituted with
methyl or trifluoromethyl [especially
such group -CO-R11 is tert.-butyl-carbonyl, methoxycarbonyl, ethoxycarbonyl,
isopropoxycarbonyl, n-
butoxycarbonyl, tert.-butoxycarbonyl, oxetan-3-yl-oxy-carbonyl, (3-methyl-
oxetan-3-yI)-oxy-carbonyl, (3-
trifluoromethyl-oxetan-3-yI)-oxy-carbonyl, phenylcarbonyl, phenoxycarbonyl,
cyclopropylcarbonyl];
= benzyl wherein the phenyl ring of said benzyl is optionally mono- or di-
substituted with halogen [especially 2,4-
dichlorobenzyl]; or
= phenyl, or 5- or 6-membered heteroaryl (especially pyrazolyl,
oxadiazolyl, pyridinyl, pyridazinyl, pyrimidinyl);
wherein said phenyl or 5- or 6-membered heteroaryl independently is
unsubstituted, mono-, di- or tri-
substituted, wherein the substituents are independently selected from
(Ci_4)alkyl (especially methyl, isopropyl);
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
34
(Ci_4)alkoxy (especially methoxy, n-butyloxy);
(Ci_3)fluoroalkyl (especially trifluoromethyl);
(C1_3)fluoroalkoxy (especially trifluoromethoxy);
D halogen (especially fluoro, chloro, bromo);
cyano;
D nitro;
D hydroxy;
D hydroxy-(C1_3)alkyl (especially 1-hydroxyethyl, hydroxymethyl, 1-hydroxy-1-
methyl-ethyl);
(Ci_4)alkoxy-(Ci_3)alkyl (especially 1-methoxyethyl);
D hydroxy-(C2_3)alkoxy (especially 2-hydroxy-ethoxy);
(C1_4)alkoxy-(C2_3)alkoxy (especially 2-methoxy-ethoxy);
D (C3_6)cycloalkyl-X12-, wherein X12 represents a direct bond, -0-, or -
(C1_3)alkylene-0-, and wherein the (C3_
6)cycloalkyl independently contains one optional ring oxygen [especially such
group (C3_6)cycloalkyl-X12- is
cyclopropyl, cyclopropyl-oxy, cyclobutyl-oxy, oxetan-3-yl-oxy, cyclopropyl-
methoxy, or tetrahydropyran-4-
yl-oxy];
D R13-CO-X13-, wherein X13 represents a direct bond or (C1_3)alkylene, and R13
represents hydrogen or (C1_
4)alkyl (especially such group R13-CO-X13 is formyl, acetyl, 2-oxo-ethyl);
3-(morpholin-4-yI)-prop-1-ynyl;
R14aR14bN_X14_, wherein X14 represents a direct bond or (C1_3)alkylene; and
wherein R14a and R14b
independently represent hydrogen, (C1_4)alkyl, hydroxy-(C2_4)alkyl,
(C1_3)alkoxy-(C24alkyl; or R14a and R14b
together with the nitrogen to which they are attached to form a 4- to 6-
membered saturated ring optionally
containing one additional ring heteroatom selected from oxygen and nitrogen;
and wherein said ring is
optionally mono-substituted with (C1_4)alkyl (especially methyl), hydroxy,
(C1_4)alkoxy (especially methoxy),
or dimethylamino,
[especially such group R14aR14bN_x14_ is amino, methylamino-methyl,
dimethylamino-methyl, [(2-
hydroxyethyp-methylamino]-methyl, [(2-hydroxyethyl)-ethylamino]-methyl, [(2-
methoxy-1-methyl-ethyl)-
amino]-methyl, [(2-methoxyethyp-methylamino]-methyl, [di-(2-hydroxyethyp-
amino]-methyl, (azetidin-1
methyl, (pyrrolidin-1-yI)-methyl, (piperidin-1-yI)-methyl, morpholin-4-yl,
piperazin-1-ylmethyl, (morpholin-4-
y1)-methyl, 2-(morpholin-4-yI)-ethyl, 3-(morpholin-4-yI)-propyl, (3-methoxy-
azetidin-1-y0-methyl, 1-(3-
methoxy-azetidin-1 (1-methyl-piperazin-4-yI)-methyl, (4-hydroxypiperidin-1-
yI)-methyl, (4-
methoxypiperidin-1-yI)-methyl, (3-methoxy-pyrrolidin-1-yI)-methyl, (4-
dimethylamino-piperidin-1-yI)-methyl];
or
D benzyloxy, wherein the phenylring of benzyloxy is optionally mono- or di-
substituted with halogen or
methyl);
wherein in a sub-embodiment R1 represents phenyl, or 5- or 6-membered
heteroaryl (especially pyrazolyl,
oxadiazolyl, pyridinyl, pyridazinyl, pyrimidinyl); wherein said phenyl or 5-
or 6-membered heteroaryl are substituted
as defined herein before;
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
R2 represents phenyl, or 5- or 6-membered heteroaryl (notably 6-membered
heteroaryl; in particular thiazolyl,
pyridinyl, pyrimidinyl, pyrazinyl, pyridazinyl); wherein said phenyl or 5- or
6-membered heteroaryl independently is
mono-, di- or tri-substituted (especially mono-substituted, in particular in
ortho position with regard to the point of
attachment of the rest of the molecule), wherein the substituents are
independently selected from
5 D (Ci_4)alkyl (especially methyl, isopropyl);
(Ci_4)alkoxy (especially methoxy, isopropoxy);
(Ci_3)fluoroalkyl (especially trifluoromethyl);
(C1_3)fluoroalkoxy (especially trifluoromethoxy);
D halogen (especially chloro, fluoro); or
10 (C3_6)cycloalkyl-X21-, wherein X21 represents a direct bond, -0-, or -
(C1_3)alkylene-0-, and wherein the (C3_
6)cycloalkyl independently contains one optional ring oxygen [especially such
group (C3_6)cycloalkyl-X21- is
cyclopropyl, cyclopropyl-oxy, cyclobutyl-oxy, oxetan-3-yl-oxy, cyclopropyl-
methoxy];
R3 represents hydrogen, or (C1_3)alkyl (especially methyl); and
R4 represents
15 = (Ci4alkyl (especially methyl);
= hydroxy-(C1_3)alkyl (especially hydroxymethyl);
= -0-R41, wherein R41 represents
D hydrogen;
(Ci_4)alkyl (especially methyl);
20 (C24)alkyl which is substituted with one or two hydroxy (especially 2-
hydroxyethyl, 2,3-dihydroxypropyl);
), R41 a R41 bN¨(C2_3)alkylene-, wherein R41a and Rob independently
represent hydrogen or (C1_4)alkyl
(especially such group is 2-dimethylamino-ethyl); or
D (C4_7)heterocyclyl-X41-, wherein X41 represents a direct bond or
(C1_3)alkylene, and wherein the
(C4_7)heterocycly1 independently contains one or two ring heteroatoms
independently selected from
25 nitrogen and oxygen; wherein said (C4_7)heterocycly1 independently is
unsubstituted, or mono-, or di-
substituted, wherein the substituents are independently selected from:
= (Ci4alkyl (especially methyl); and/or
= (Ci4alkoxy-carbonyl attached to a ring nitrogen atom having a free
valency;
[especially such (C4_7)heterocyclyl-X41- group is oxetan-3-yl, 1-methyl-
pyrrolidin-3-yl, 2,2-dimethyl-
30 dioxolan-4-yl-methyl, 2-morpholin-4-yl-ethyl, 1-tert-butoxy-carbonyl-
piperidin-4-yl, piperidin-4-y1];
. _NR42aR42b wherein R42a and R42b independently represent hydrogen,
(C1_4)alkyl, (C1_3)alkoxy-(C2_3)alkyl,
hydroxy-(C2_3)alkyl, or R42a and R42b together with the nitrogen to which they
are attached form a 4- to 7-
membered saturated ring optionally containing one further ring heteroatom
selected from oxygen and nitrogen,
wherein said ring is unsubstituted or mono-substituted with (C1_3)alkyl
(especially methyl), or (C1_3)alkoxy
35 (especially methoxy) [especially such group ¨N R42aR42b is (2-
hydroxyethyl)-amino, (2-hydroxyethyl)-methyl-
amino, (2-methoxyethyl)-amino, morpholin-4-yl, 3-methoxy-azetidinyl, or 4-
methyl-piperazin-1-y1]; or
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
36
= -CO-R43 wherein R43 represents (Ci4alkoxy (especially methoxy); or -NR
43a R43b wherein R43a and R43b
independently represent hydrogen, (C1_4)alkyl (especially methyl),
(C1_3)alkoxy-(C2_3)alkyl (especially 2-
methoxy-ethyl), or hydroxy-(C2_3)alkyl (especially 2-hydroxy-ethyl);
[especially such group -CO-R43 is
methoxycarbonyl, carbamoyl, methyl-carbamoyl,
dimethylcarbamoyl, hydroxyethylcarbamoyl,
methoxyethylcarbamoyl].
wherein the characteristics disclosed in embodiments 2) to 22) are intended to
apply mutatis mutandis also to the
compounds formula (II) according to embodiment 23); wherein especially the
following embodiments are thus
possible and intended and herewith specifically disclosed in individualized
form:
23+2, 23+3,23+5,23+8+2,23+8+3,23+8+6, 8,23+9+2,23+9+3,23+9+6,
9,23+11+5,23+11,23+12+5, 23+12,
23+13+2, 23+13+3, 23+13+8+2, 23+13+8+3, 23+13+8+6, 13+8, 23+13+9+2, 23+13+9+3,
23+13+9+6, 13+9,
23+13, 23+14+5, 23+14+11+5, 23+14+11, 23+14+12+5, 23+14+12, 23+14, 23+15+5,
23+15+11+5, 23+15+11,
23+15+12+5, 23+15+12, 23+15, 23+16+2, 23+16+3, 23+16+5, 23+16+8+2, 23+16+8+3,
23+16+8+6, 16+8,
23+16+9+2, 23+16+9+3, 23+16+9+6, 16+9, 23+16+11+5, 23+16+11, 23+16+12+5,
23+16+12, 23+16+13+2,
23+16+13+3, 23+16+13+8+2, 23+16+13+8+3, 23+16+13+8+6, 16+13+8, 23+16+13+9+2,
23+16+13+9+3,
23+16+13+9+6, 16+13+9, 23+16+13, 23+16+14+5, 23+16+14+11+5, 23+16+14+11,
23+16+14+12+5,
23+16+14+12, 23+16+14, 23+16+15+5, 23+16+15+11+5, 23+16+15+11, 23+16+15+12+5,
23+16+15+12,
23+16+15, 23+16, 23+17+2, 23+17+3, 23+17+8+2, 23+17+8+3, 23+17+8+6, 17+8,
23+17+9+2, 23+17+9+3,
23+17+9+6, 17+9, 23+17+13+2, 23+17+13+3, 23+17+13+8+2, 23+17+13+8+3,
23+17+13+8+6, 17+13+8,
23+17+13+9+2, 23+17+13+9+3, 23+17+13+9+6, 17+13+9, 23+17+13, 23+17+16+2,
23+17+16+3, 23+17+16+5,
23+17+16+8+2, 23+17+16+8+3, 23+17+16+8+6, 17+16+8, 23+17+16+9+2, 23+17+16+9+3,
23+17+16+9+6,
17+16+9, 23+17+16+11+5, 23+17+16+11, 23+17+16+12+5, 23+17+16+12,
23+17+16+13+2, 23+17+16+13+3,
23+17+16+13+8+2, 23+17+16+13+8+3,23+17+16+13+8+6,17+16+13+8,
23+17+16+13+9+2,23+17+16+13+9+3,
23+17+16+13+9+6, 17+16+13+9, 23+17+16+13, 23+17+16+14+5, 23+17+16+14+11+5,
23+17+16+14+11,
23+17+16+14+12+5, 23+17+16+14+12, 23+17+16+14, 23+17+16+15+5,
23+17+16+15+11+5,23+17+16+15+11,
23+17+16+15+12+5, 23+17+16+15+12, 23+17+16+15, 23+17+16, 23+17, 23+19+5,
23+19+11+5, 23+19+11,
23+19+12+5, 23+19+12, 23+19+14+5, 23+19+14+11+5, 23+19+14+11, 23+19+14+12+5,
23+19+14+12,
23+19+14, 23+19+15+5, 23+19+15+11+5, 23+19+15+11, 23+19+15+12+5, 23+19+15+12,
23+19+15,
23+19+16+2, 23+19+16+3, 23+19+16+5, 23+19+16+8+2, 23+19+16+8+3, 23+19+16+8+6,
19+16+8,
23+19+16+9+2, 23+19+16+9+3, 23+19+16+9+6, 19+16+9, 23+19+16+11+5, 23+19+16+11,
23+19+16+12+5,
23+19+16+12, 23+19+16+13+2, 23+19+16+13+3, 23+19+16+13+8+2, 23+19+16+13+8+3,
23+19+16+13+8+6,
19+16+13+8, 23+19+16+13+9+2, 23+19+16+13+9+3, 23+19+16+13+9+6, 19+16+13+9,
23+19+16+13,
23+19+16+14+5, 23+19+16+14+11+5, 23+19+16+14+11, 23+19+16+14+12+5,
23+19+16+14+12, 23+19+16+14,
23+19+16+15+5, 23+19+16+15+11+5, 23+19+16+15+11, 23+19+16+15+12+5,
23+19+16+15+12, 23+19+16+15,
23+19+16, 23+19, 23+20+5, 23+20+11+5, 23+20+11, 23+20+12+5, 23+20+12,
23+20+14+5, 23+20+14+11+5,
23+20+14+11, 23+20+14+12+5, 23+20+14+12, 23+20+14, 23+20+15+5, 23+20+15+11+5,
23+20+15+11,
23+20+15+12+5, 23+20+15+12, 23+20+15, 23+20+16+2, 23+20+16+3, 23+20+16+5,
23+20+16+8+2,
23+20+16+8+3, 23+20+16+8+6, 20+16+8, 23+20+16+9+2, 23+20+16+9+3, 23+20+16+9+6,
20+16+9,
23+20+16+11+5, 23+20+16+11, 23+20+16+12+5, 23+20+16+12, 23+20+16+13+2,
23+20+16+13+3,
23+20+16+13+8+2, 23+20+16+13+8+3,23+20+16+13+8+6,20+16+13+8,
23+20+16+13+9+2,23+20+16+13+9+3,
23+20+16+13+9+6, 20+16+13+9, 23+20+16+13, 23+20+16+14+5, 23+20+16+14+11+5,
23+20+16+14+11,
23+20+16+14+12+5, 23+20+16+14+12, 23+20+16+14, 23+20+16+15+5,
23+20+16+15+11+5,23+20+16+15+11,
23+20+16+15+12+5, 23+20+16+15+12, 23+20+16+15, 23+20+16, 23+20, 23+21+5,
23+21+11+5, 23+21+11,
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
37
23+21+12+5, 23+21+12, 23+21+14+5, 23+21+14+11+5, 23+21+14+11, 23+21+14+12+5,
23+21+14+12,
23+21+14, 23+21+15+5, 23+21+15+11+5, 23+21+15+11, 23+21+15+12+5, 23+21+15+12,
23+21+15,
23+21+16+2, 23+21+16+3, 23+21+16+5, 23+21+16+8+2, 23+21+16+8+3, 23+21+16+8+6,
21+16+8,
23+21+16+9+2, 23+21+16+9+3, 23+21+16+9+6, 21+16+9, 23+21+16+11+5, 23+21+16+11,
23+21+16+12+5,
23+21+16+12, 23+21+16+13+2, 23+21+16+13+3, 23+21+16+13+8+2, 23+21+16+13+8+3,
23+21+16+13+8+6,
21+16+13+8, 23+21+16+13+9+2, 23+21+16+13+9+3, 23+21+16+13+9+6, 21+16+13+9,
23+21+16+13,
23+21+16+14+5, 23+21+16+14+11+5,23+21+16+14+11,23+21+16+14+12+5,
23+21+16+14+12, 23+21+16+14,
23+21+16+15+5, 23+21+16+15+11+5,23+21+16+15+11,23+21+16+15+12+5,
23+21+16+15+12, 23+21+16+15,
23+21+16,23+21.
In the list above the numbers refer to the embodiments according to their
numbering provided hereinabove
whereas "+" indicates the limitations as outlined above.
24) A third aspect of the invention relates to compounds of the formula (I)
according to embodiment 1) which are
also compounds of the formula (III)
R1
cA)
Z N
> __ 0
X N
R4 R3
R2
Formula (III)
wherein X is CH and Z is CH, Xis N and Z is CH, or X is CH and Z is N;
= ring A represents a saturated 4- to 7-membered mono-cyclic carbocyclic
ring containing the ring nitrogen atom
to which R1 is attached, wherein said ring independently is unsubstituted, or
mono-, or di-substituted wherein
the substituents are independently selected from (C1_3)alkyl (especially
methyl), fluoro, or (C1_4)alkoxy-carbonyl
(especially ethoxy-carbonyl) [especially such ring A is azetidin-1,3-diyl,
pyrrolidin-1,3-diyl, 4-methyl-pyrrolidin-
1,3-diyl, piperidin-1,3-diyl, piperidin-1,4-diyl, 3-fluoro-piperidin-1,4-diyl,
2-methyl-piperidin-1,4-diyl, 3-methyl-
piperidin-1,4-diyl, 3-(ethoxycarbonyI)-piperidin-1,4-diyl, 3,3-dimethyl-
piperidin-1,4-diyl, azepan-1,4-diyI]; or
= ring A represents a saturated 7- or 8-membered bridged bi-cyclic
carbocyclic ring containing the ring nitrogen
atom to which R1 is attached, wherein said ring is unsubstituted [especially
such ring is 2-aza-
bicyclo[2.2.1]heptane-2,5-diyl, 3-aza-bicyclo[3.1.1]heptane-3,6-diyI];
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
38
R1 represents
= (Ci4alkyl (especially methyl, isopropyl);
= -CO-R11 wherein R11 represents (C1_4)alkyl; (C1_4)alkoxy; phenyl,
phenoxy; (C3_6)cycloalkyl; or (C3_6)cycloalkoxy
optionally containing one ring oxygen and optionally mono-substituted with
methyl or trifluoromethyl [especially
such group -CO-R11 is tert.-butyl-carbonyl, methoxycarbonyl, ethoxycarbonyl,
isopropoxycarbonyl, n-
butoxycarbonyl, tert.-butoxycarbonyl, oxetan-3-yl-oxy-carbonyl, (3-methyl-
oxetan-3-yI)-oxy-carbonyl, (3-
trifluoromethyl-oxetan-3-y0-oxy-carbonyl, phenylcarbonyl, phenoxycarbonyl,
cyclopropylcarbonyl];
= benzyl wherein the phenyl ring of said benzyl is optionally mono- or di-
substituted with halogen [especially 2,4-
dichlorobenzyl]; or
= phenyl, or 5- or 6-membered heteroaryl (especially pyrazolyl,
oxadiazolyl, pyridinyl, pyridazinyl, pyrimidinyl);
wherein said phenyl or 5- or 6-membered heteroaryl independently is
unsubstituted, mono-, di- or tri-
substituted, wherein the substituents are independently selected from
> (Ci_4)alkyl (especially methyl, isopropyl);
(Ci_4)alkoxy (especially methoxy, n-butyloxy);
(C1_3)fluoroalkyl (especially trifluoromethyl);
> (C1_3)fluoroalkoxy (especially trifluoromethoxy);
> halogen (especially fluoro, chloro, bromo);
= cyano;
> nitro;
> hydroxy;
hydroxy-(C1_3)alkyl (especially 1-hydroxyethyl, hydroxymethyl, 1-hydroxy-1-
methyl-ethyl);
(Ci_4)alkoxy-(Ci_3)alkyl (especially 1-methoxyethyl);
hydroxy-(C2_3)alkoxy (especially 2-hydroxy-ethoxy);
(C1_4)alkoxy-(C2_3)alkoxy (especially 2-methoxy-ethoxy);
> (C3_6)cycloalkyl-X12-, wherein X12 represents a direct bond, -0-, or -
(C1_3)alkylene-0-, and wherein the (C3_
6)cycloalkyl independently contains one optional ring oxygen [especially such
group (C3_6)cycloalkyl-X12- is
cyclopropyl, cyclopropyl-oxy, cyclobutyl-oxy, oxetan-3-yl-oxy, cyclopropyl-
methoxy, or tetrahydropyran-4-
yl-oxy];
> R13-CO-X13-, wherein X13 represents a direct bond or (C1_3)alkylene, and
R13 represents hydrogen or (C1_
4)alkyl (especially such group R13-CO-X13 is formyl, acetyl, 2-oxo-ethyl);
> 3-(morpholin-4-yI)-prop-1-ynyl;
R14aR14bN_X14_, wherein X14 represents a direct bond or (C1_3)alkylene; and
wherein R14a and R14b
independently represent hydrogen, (C1_4)alkyl, hydroxy-(C2_4)alkyl,
(C1_3)alkoxy-(C24alkyl; or R14a and R14b
together with the nitrogen to which they are attached to form a 4- to 6-
membered saturated ring optionally
containing one additional ring heteroatom selected from oxygen and nitrogen;
and wherein said ring is
optionally mono-substituted with (C1_4)alkyl (especially methyl), hydroxy,
(C1_4)alkoxy (especially methoxy),
or dimethylamino,
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
39
[especially such group R14aR14bN_x14_ is amino, methylamino-methyl,
dimethylamino-methyl, [(2-
hydroxyethyl)-methylamino]-methyl, [(2-hydroxyethyl)-ethylaminoFmethyl, [(2-
methoxy-1-methykethyl)-
aminoFmethyl, [(2-methoxyethyp-methylaminoFmethyl, [di-(2-hydroxyethyp-
amino]methyl, (azetidin-111)-
methyl, (pyrrolidin-1-yI)-methyl, (piperidin-1-yI)-methyl, morpholin-4-yl,
piperazin-1-ylmethyl, (morpholin-4-
yI)-methyl, 2-(morpholin-4-yI)-ethyl, 3-(morpholin-4-yI)-propyl, (3-methoxy-
azetidin-1-yI)-methyl, 143-
methoxy-azetidin-1 11)-ethyl, (1-methyl-piperazin-4-yI)-methyl, .. (4-
hydroxypiperidin-1-yI)-methyl,
methoxypiperidin-1-yI)-methyl, (3-methoxy-pyrrolidin-1-yI)-methyl, (4-
dimethylamino-piperidin-1-yI)-methyl];
or
D benzyloxy, wherein the phenylring of benzyloxy is optionally mono- or di-
substituted with halogen or
methyl);
wherein in a sub-embodiment R1 represents phenyl, or 5- or 6-membered
heteroaryl (especially pyrazolyl,
oxadiazolyl, pyridinyl, pyridazinyl, pyrimidinyl); wherein said phenyl or 5-
or 6-membered heteroaryl are substituted
as defined herein before;
R2 represents phenyl, or 6-membered heteroaryl ( especially pyridinyl,
pyrimidinyl, pyrazinyl, pyridazinyl); wherein
said phenyl or 6-membered heteroaryl independently is mono-substituted in
ortho position with regard to the point
of attachment of the rest of the molecule, wherein the substituents is
independently selected from
D (Ci_4)alkyl (especially methyl, isopropyl);
(Ci_4)alkoxy (especially methoxy, isopropoxy);
(Ci_3)fluoroalkyl (especially trifluoromethyl);
(C1_3)fluoroalkoxy (especially trifluoromethoxy);
(C3_6)cycloalkyl-X21-, wherein X21 represents a direct bond, -0-, or -
(C1_3)alkylene-0-, and wherein the (C3_
6)cycloalkyl independently contains one optional ring oxygen [especially such
group (C3_6)cycloalkyl-X21- is
cyclopropyl, cyclopropyl-oxy, cyclobutyl-oxy, oxetan-3-yl-oxy, cyclopropyl-
methoxy];
R3 represents hydrogen, or (C1_3)alkyl (especially methyl) [R3 represents
hydrogen]; and
R4 represents
= -0-R41, wherein R41 represents
D hydrogen;
(Ci_4)alkyl (especially methyl);
(C)alkyl which is substituted with one or two hydroxy (especially 2-
hydroxyethyl, 2,3-dihydroxypropyl);
oxetan-3-y1;
D 2,2-dimethyl-dioxolan-4-yl-methyl;or
= (2-hydroxyethyl)-methyl-amino, morpholin-4-yl, or 3-methoxy-azetidinyl;
wherein in a sub-embodiment R4 represents -0-R41 as defined herein above;
especially R4 represents (Ci4alkoxy
(in particular methoxy).
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
wherein the characteristics disclosed in embodiments 2) to 22) are intended to
apply mutatis mutandis also to the
compounds formula (111) according to embodiment 24); wherein especially the
following embodiments are thus
possible and intended and herewith specifically disclosed in individualized
form:
24, 24+2, 24+3, 24+5, 24+8+2, 24+8+3, 24+8, 24+9+2, 24+9+3, 24+9, 24+11+5,
24+11, 24+12+5, 24+12,
5 24+15+5, 24+15+11+5, 24+15+11, 24+15+12+5, 24+15+12, 24+15, 24+16+2,
24+16+3, 24+16+5, 24+16+8+2,
24+16+8+3, 24+16+8, 24+16+9+2, 24+16+9+3, 24+16+9, 24+16+11+5, 24+16+11,
24+16+12+5, 24+16+12,
24+16+15+5, 24+16+15+11+5, 24+16+15+11, 24+16+15+12+5, 24+16+15+12, 24+16+15,
24+16, 24+21+5,
24+21+11+5, 24+21+11, 24+21+12+5, 24+21+12, 24+21+15+5, 24+21+15+11+5,
24+21+15+11, 24+21+15+12+5,
24+21+15+12, 24+21+15, 24+21+16+2, 24+21+16+3, 24+21+16+5, 24+21+16+8+2,
24+21+16+8+3, 24+21+16+8,
10 24+21+16+9+2, 24+21+16+9+3, 24+21+16+9, 24+21+16+11+5, 24+21+16+11,
24+21+16+12+5, 24+21+16+12,
24+21+16+15+5, 24+21+16+15+11+5, 24+21+16+15+11, 24+21+16+15+12+5,
24+21+16+15+12, 24+21+16+15,
24+21+16, 24+21.
In the list above the numbers refer to the embodiments according to their
numbering provided hereinabove
whereas "+" indicates the limitations as outlined above.
15 25) Another embodiment relates to compounds according to embodiment 1)
which are selected from the following
compounds:
4-[4-Methoxy-2-oxo-3-(2-trifluoromethyl-benzy1)-2,3-dihydro-benzoimidazol-1-
y1]-piperidine-1-carboxylic acid tert-
butyl ester;
1-[1-(2,6-Dimethyl-pheny1)-piperidin-4-y1]-4-methoxy-3-(2-trifluoromethyl-
benzy1)-1,3-dihydro-benzoimidazol-2-one;
20 1-(1-(2,6-dimethylphenyl)piperidin-4-y1)-4-methoxy-3-(2-
(trifluoromethyObenzyl)-1,3-dihydro-benzoimidazol-2-one-7-
d;
4-Methoxy-1-(1-phenyl-piperidin-4-y1)-3-(2-trifluoromethyl-benzy1)-1,3-dihydro-
benzoimidazol-2-one;
1-0-(2,4-Dichloro-benzy1)-piperidin-4-y1]-4-methoxy-3-(2-trifluoromethyl-
benzy1)-1,3-dihydro-benzoimidazol-2-one;
(R)-3-[4-Methoxy-2-oxo-3-(2-trifluoromethyl-benzy1)-2,3-dihydro-benzoimidazol-
1-y1]-pyrrolidine-1-carboxylic acid
25 .. tert-butyl ester;
1-[(R)-1-(2,6-Difluoro-pheny1)-pyrrolidin-3-y1]-4-methoxy-3-(2-trifluoromethyl-
benzy1)-1,3-dihydro-benzoimidazol-2-
one;
1-[(R)-1-(2,6-Dimethyl-pheny1)-pyrrolidin-3-y1]-4-methoxy-3-(2-trifluoromethyl-
benzy1)-1,3-dihydro-benzoimidazol-2-
one;
30 1-[1-(2,6-Difluoro-pheny1)-piperidin-4-y1]-4-methoxy-3-(2-
trifluoromethyl-benzy1)-1,3-dihydro-benzoimidazol-2-one;
1-[1-(2,6-Dimethoxy-pheny1)-piperidin-4-y1]-4-methoxy-3-(2-trifluoromethyl-
benzy1)-1,3-dihydro-benzoimidazol-2-
one;
1-[1-(2-Fluoro-6-methoxy-pheny1)-piperidin-4-y1]-4-methoxy-3-(2-
trifluoromethyl-benzy1)-1,3-dihydro-benzoimidazol-
2-one;
35 4-Methoxy-1-[1-(2-methoxy-6-methyl-pheny1)-piperidin-4-y1]-3-(2-
trifluoromethyl-benzy1)-1,3-dihydro-benzoimidazol-
2-one;
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
41
1-[1-(2-Fluoro-6-methyl-pheny1)-piperidin-4-y1]-4-methoxy-3-(2-trifluoromethyl-
benzy1)-1,3-dihydro-benzoimidazol-2-
one;
3-Cyclopropy1-5-14-[4-methoxy-2-oxo-3-(2-trifluoromethyl-benzy1)-2,3-dihydro-
benzoimidazol-1-y1]-piperidin-1-y11-1-
methyl-1H-pyrazole-4-carbaldehyde;
1-[(R)-1-(2-Fluoro-6-methyl-pheny1)-pyrrolidin-3-y1]-4-methoxy-3-(2-
trifluoromethyl-benzy1)-1,3-dihydro-
benzoimidazol-2-one;
4-Methoxy-1-[(R)-1-(2-methoxy-pheny1)-pyrrolidin-3-y1]-3-(2-trifluoromethyl-
benzy1)-1,3-dihydro-benzoimidazol-2-
one;
1-[(R)-1-(2,6-Dimethoxy-pheny1)-pyrrolidin-3-y1]-4-methoxy-3-(2-
trifluoromethyl-benzy1)-1,3-dihydro-benzoimidazol-
2-one;
4-Methoxy-1-[(R)-1-(2-methoxy-6-methyl-pheny1)-pyrrolidin-3-y1]-3-(2-
trifluoromethyl-benzy1)-1,3-dihydro-
benzoimidazol-2-one;
1-[(R)-1-(2-Fluoro-6-methoxy-pheny1)-pyrrolidin-3-y1]-4-methoxy-3-(2-
trifluoromethyl-benzy1)-1,3-dihydro-
benzoimidazol-2-one;
(R)-3-[4-Methoxy-2-oxo-3-(2-trifluoromethoxy-benzy1)-2,3-dihydro-benzoimidazol-
1-y1]-pyrrolidine-1-carboxylic acid
tert-butyl ester;
1-[1-(5-Cyclopropy1-2-methyl-2H-pyrazol-3-y1)-piperidin-4-y1]-4-methoxy-3-(2-
trifluoromethyl-benzy1)-1,3-dihydro-
benzoimidazol-2-one;
1-[(R)-1-(2,6-Dimethyl-pheny1)-pyrrolidin-3-y1]-4-methoxy-3-(2-
trifluoromethoxy-benzy1)-1,3-dihydro-benzoimidazol-
2-one;
1-[(R)-1-(2,6-Difluoro-pheny1)-pyrrolidin-3-y1]-4-methoxy-3-(2-
trifluoromethoxy-benzy1)-1,3-dihydro-benzoimidazol-2-
one;
1-[1-(4-Chloro-5-cyclopropy1-2-methy1-2H-pyrazol-3-y1)-piperidin-4-y1]-4-
methoxy-3-(2-trifluoromethyl-benzy1)-1,3-
dihydro-benzoimidazol-2-one;
1-[1-(5-Cyclopropy1-2,4-dimethy1-2H-pyrazol-3-y1)-piperidin-4-y1]-4-methoxy-3-
(2-trifluoromethyl-benzy1)-1,3-dihydro-
benzoimidazol-2-one;
(R)-3-[4-Methoxy-2-oxo-3-(2-trifluoromethyl-benzy1)-2,3-dihydro-benzoimidazol-
1-y1]-pyrrolidine-1-carboxylic acid
methyl ester;
14(R)-1-Benzoyl-pyrrolidin-311)-4-methoxy-3-(2-trifluoromethyl-benzy1)-1,3-
dihydro-benzoimidazol-2-one;
1-[1-(2,2-Dimethyl-propiony1)-piperidin-4-y1]-4-methoxy-3-(2-trifluoromethyl-
benzy1)-1 ,3-dihydro-benzoimidazol-2-
one;
4-[4-Methoxy-2-oxo-3-(2-trifluoromethyl-benzy1)-2,3-dihydro-benzoimidazol-1-
y1]-piperidine-1-carboxylic acid methyl
ester;
1-(1-Benzoyl-piperidin-4-y1)-4-methoxy-3-(2-trifluoromethyl-benzy1)-1,3-
dihydro-benzoimidazol-2-one;
4-Methoxy-14(R)-1-methyl-pyrrolidin-311)-3-(2-trifluoromethyl-benzy1)-1,3-
dihydro-benzoimidazol-2-one;
14(R)-1-Isopropyl-pyrrolidin-3-y1)-4-methoxy-3-(2-trifluoromethyl-benzy1)-1,3-
dihydro-benzoimidazol-2-one;
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
42
4-Methoxy-1-[(R)-1-(2-methoxy-4-methyl-pyridin-3-y1)-pyrrolidin-3-y1]-3-(2-
trifluoromethyl-benzy1)-1,3-dihydro-
benzoimidazol-2-one;
1-[(R)-1-(3,5-Difluoro-pyridin-411)-pyrrolidin-3-y1]-4-methoxy-3-(2-
trifluoromethyl-benzy1)-1,3-dihydro-
benzoimidazol-2-one;
1-(1-lsopropyl-piperidin-411)-4-methoxy-3-(2-trifluoromethyl-benzy1)-1,3-
dihydro-benzoimidazol-2-one;
1-[(R)-1-(3,5-Dimethyl-pyridin-411)-pyrrolidin-3-y1]-4-methoxy-3-(2-
trifluoromethyl-benzy1)-1,3-dihydro-
benzoimidazol-2-one;
1-[(R)-1-(2-Fluoro-4-methyl-pyridin-3-y1)-pyrrolidin-3-y1]-4-methoxy-3-(2-
trifluoromethyl-benzy1)-1,3-dihydro-
benzoimidazol-2-one;
1-[(R)-1-(3,5-Dimethoxy-pyridin-4-y1)-pyrrolidin-3-y1]-4-methoxy-3-(2-
trifluoromethyl-benzy1)-1,3-dihydro-
benzoimidazol-2-one;
(R)-3-K-Methoxy-2-oxo-3-(2-trifluoromethyl-benzy1)-2,3-dihydro-imidazo[4,5-
c]pyridin-1-y1]-pyrrolidine-1-carboxylic
acid tert-butyl ester;
1-[(R)-1-(2-Fluoro-6-methyl-pheny1)-pyrrolidin-3-y1]-4-hydroxy-3-(2-
trifluoromethyl-benzy1)-1,3-dihydro-
benzoimidazol-2-one;
1-[(R)-1-(2,6-Dimethyl-pheny1)-pyrrolidin-3-y1]-4-methoxy-3-(2-trifluoromethyl-
benzy1)-1,3-dihydro-imidazo[4,5-
c]pyridin-2-one;
1-[(R)-1-(2,6-Difluoro-pheny1)-pyrrolidin-3-y1]-4-methoxy-3-(2-trifluoromethyl-
benzy1)-1,3-dihydro-imidazo[4,5-
c]pyridin-2-one;
(R)-3-[7-Methoxy-2-oxo-1-(2-trifluoromethyl-benzy1)-1,2-dihydro-imidazo[4,5-
b]pyridin-3-y1]-pyrrolidine-1-carboxylic
acid tert-butyl ester;
1-[(R)-1-(2,6-Dimethyl-pheny1)-pyrrolidin-3-y1]-4-hydroxy-3-(2-trifluoromethyl-
benzy1)-1,3-dihydro-benzoimidazol-2-
one;
3-[(R)-1-(2,6-Dimethyl-pheny1)-pyrrolidin-3-y1]-7-methoxy-1-(2-trifluoromethyl-
benzy1)-1,3-dihydro-imidazo[4,5-
b]pyridin-2-one;
3-[(R)-1-(2,6-Difluoro-pheny1)-pyrrolidin-3-y1]-7-methoxy-1-(2-trifluoromethyl-
benzy1)-1,3-dihydro-imidazo[4,5-
b]pyridin-2-one;
4-Methoxy-1-[(R)-1-(2-methoxy-4-methyl-pyridin-3-y1)-pyrrolidin-3-y1]-3-(2-
trifluoromethyl-benzy1)-1,3-dihydro-
imidazo[4,5-c]pyridin-2-one;
1-[(R)-1-(2-Fluoro-4-methyl-pyridin-3-y1)-pyrrolidin-3-y1]-4-methoxy-3-(2-
trifluoromethyl-benzy1)-1,3-dihydro-
imidazo[4,5-c]pyridin-2-one;
1-[(R)-1-(2-Fluoro-6-methoxy-pheny1)-pyrrolidin-3-y1]-4-methoxy-3-(2-
trifluoromethyl-benzy1)-1,3-dihydro-
imidazo[4,5-c]pyridin-2-one;
1-[(R)-1-(2-Fluoro-6-methyl-pheny1)-pyrrolidin-3-y1]-4-methoxy-3-(2-
trifluoromethyl-benzy1)-1,3-dihydro-imidazo[4,5-
c]pyridin-2-one;
4-Methoxy-1-[(R)-1-(2-methoxy-6-methyl-pheny1)-pyrrolidin-3-y1]-3-(2-
trifluoromethyl-benzy1)-1,3-dihydro-
imidazo[4,5-c]pyridin-2-one;
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
43
1-[(R)-1-(2,6-Dimethyl-pheny1)-pyrrolidin-3-y1]-4-(2-hydroxy-ethoxy)-3-(2-
trifluoromethyl-benzy1)-1,3-dihydro-
benzoimidazol-2-one;
4-[4-Methoxy-2-oxo-3-(4-trifluoromethyl-pyridin-3-ylmethyl)-2,3-dihydro-
benzoimidazol-1-y1]-piperidine-1-carboxylic
acid tert-butyl ester;
1-[1-(2,6-Dimethyl-pheny1)-piperidin-4-y1]-4-methoxy-3-(2-trifluoromethyl-
pyridin-3-ylmethyl)-1,3-dihydro-
benzoimidazol-2-one;
1-[1-(2,6-Difluoro-pheny1)-piperidin-4-y1]-4-methoxy-3-(2-trifluoromethyl-
pyridin-3-ylmethyl)-1,3-dihydro-
benzoimidazol-2-one;
1-[(R)-1-(2-Fluoro-6-methoxy-pheny1)-pyrrolidin-3-y1]-3-(2-trifluoromethyl-
benzy1)-3,5-dihydro-1H-imidazo[4,5-
c]pyridine-2,4-dione;
1-[(R)-1-(2-Fluoro-6-methyl-pheny1)-pyrrolidin-3-y1]-3-(2-trifluoromethyl-
benzy1)-3,5-dihydro-1H-imidazo[4,5-
c]pyridine-2,4-dione;
1-[1-(2,6-Dimethyl-pheny1)-piperidin-4-y1]-4-methoxy-3-(4-trifluoromethyl-
pyridin-3-ylmethyl)-1,3-dihydro-
benzoimidazol-2-one;
1-[1-(2,6-Difluoro-pheny1)-piperidin-4-y1]-4-methoxy-3-(4-trifluoromethyl-
pyridin-3-ylmethyl)-1,3-dihydro-
benzoimidazol-2-one;
(R)-3-[4-Methoxy-2-oxo-3-(2-trifluoromethyl-benzy1)-2,3-dihydro-benzoimidazol-
1-y1]-pyrrolidine-1-carboxylic acid
ethyl ester;
(R)-3-[4-Methoxy-2-oxo-3-(2-trifluoromethyl-benzy1)-2,3-dihydro-benzoimidazol-
1-y1]-pyrrolidine-1-carboxylic acid
isopropyl ester;
(R)-3-[4-Methoxy-2-oxo-3-(2-trifluoromethyl-benzy1)-2,3-dihydro-benzoimidazol-
1-y1]-pyrrolidine-1-carboxylic acid
butyl ester;
(R)-3-[4-Methoxy-2-oxo-3-(2-trifluoromethyl-benzy1)-2,3-dihydro-benzoimidazol-
1-y1]-pyrrolidine-1-carboxylic acid
phenyl ester;
4-[4-Methoxy-2-oxo-3-(3-trifluoromethyl-pyridin-2-ylmethyl)-2,3-dihydro-
benzoimidazol-1-y1]-piperidine-1-carboxylic
acid tert-butyl ester;
(R)-3-[4-Methoxy-3-(2-methoxy-benzy1)-2-oxo-2,3-dihydro-benzoimidazol-1-y1]-
pyrrolidine-1-carboxylic acid tert-
butyl ester;
1-[(R)-1-(2,4-Dimethyl-pyridin-311)-pyrrolidin-3-y1]-4-methoxy-3-(2-
trifluoromethyl-benzy1)-1,3-dihydro-
benzoimidazol-2-one;
1-[(R)-1-(2,6-Dimethyl-pheny1)-pyrrolidin-3-y1]-4-methoxy-3-(2-methoxy-benzy1)-
1,3-dihydro-benzoimidazol-2-one;
1-[(R)-1-(2,6-Difluoro-pheny1)-pyrrolidin-3-y1]-4-methoxy-3-(2-methoxy-benzy1)-
1,3-dihydro-benzoimidazol-2-one;
1-[(R)-1-(5-lsopropyl-[1,3,4]oxadiazol-2-y1)-pyrrolidin-3-y1]-4-methoxy-3-(2-
trifluoromethyl-benzy1)-1,3-dihydro-
benzoimidazol-2-one;
(R)-3-[4-Methoxy-2-oxo-3-(2-trifluoromethyl-benzy1)-2,3-dihydro-benzoimidazol-
1-y1]-pyrrolidine-1-carboxylic acid 3-
methyl-oxetan-3-y1 ester;
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
44
5-1(R)-3-[4-Methoxy-2-oxo-3-(2-trifluoromethyl-benzy1)-2,3-dihydro-
benzoimidazol-111]-pyrrolidin-1-y11-1,3-dimethy1-
1H-pyrazole-4-carbaldehyde;
(R)-3-[4-Methoxy-2-oxo-3-(2-trifluoromethyl-benzy1)-2,3-dihydro-benzoimidazol-
1-y1]-pyrrolidine-1-carboxylic acid
oxetan-3-y1 ester;
1-[1-(2,6-Dimethyl-pheny1)-piperidin-4-y1]-4-methoxy-3-(3-trifluoromethyl-
pyridin-4-ylmethyl)-1,3-dihydro-
benzoimidazol-2-one;
1-[1-(2,6-Difluoro-pheny1)-piperidin-4-y1]-4-methoxy-3-(3-trifluoromethyl-
pyridin-4-ylmethyl)-1,3-dihydro-
benzoimidazol-2-one;
1-[1-(2,6-Dimethyl-pheny1)-piperidin-4-y1]-4-methoxy-3-(3-trifluoromethyl-
pyridin-2-ylmethyl)-1,3-dihydro-
benzoimidazol-2-one;
1-[1-(2,6-Difluoro-pheny1)-piperidin-4-y1]-4-methoxy-3-(3-trifluoromethyl-
pyridin-2-ylmethyl)-1,3-dihydro-
benzoimidazol-2-one;
1-[(R)-1-(2,6-Dimethyl-pheny1)-pyrrolidin-3-y1]-4-methoxy-3-(2-methoxy-benzy1)-
1,3-dihydro-imidazo[4,5-c]pyridin-2-
one;
1-[(R)-1-(2-Fluoro-6-methyl-pheny1)-pyrrolidin-3-y1]-4-methoxy-3-(2-methoxy-
benzy1)-1,3-dihydro-imidazo[4,5-
c]pyridin-2-one;
1-[(R)-1-(2,5-Dimethy1-2H-pyrazol-3-y1)-pyrrolidin-3-y1]-4-methoxy-3-(2-
trifluoromethyl-benzy1)-1,3-dihydro-
benzoimidazol-2-one;
1-[(R)-1-(4-Chloro-2,5-dimethy1-2H-pyrazol-3-y1)-pyrrolidin-3-y1]-4-methoxy-3-
(2-trifluoromethyl-benzy1)-1,3-dihydro-
benzoimidazol-2-one;
1-[(R)-1-(2-Fluoro-6-methyl-pheny1)-pyrrolidin-3-y1]-3-(2-methoxy-benzy1)-3,5-
dihydro-1H-imidazo[4,5-c]pyridine-
2,4-dione;
3-Fluoro-2-1(R)-3-[4-methoxy-2-oxo-3-(2-trifluoromethyl-benzy1)-2,3-dihydro-
benzoimidazol-111]-pyrrolidin-1-yll-
benzonitrile;
3-Fluoro-2-1(R)-3-[4-methoxy-2-oxo-3-(2-trifluoromethyl-benzy1)-2,3-dihydro-
benzoimidazol-111]-pyrrolidin-1-yll-
benzaldehyde;
4-Methoxy-3-(2-trifluoromethyl-benzy1)-1-[(R)-1-(5-trifluoromethyl-
[1,3,4]oxadiazol-2-y1)-pyrrolidin-3-y1]-1,3-dihydro-
benzoimidazol-2-one;
4-Methoxy-3-(2-trifluoromethyl-benzy1)-1-[(R)-1-(2,4,5-tri methy1-2H-pyrazol-
311)-pyrrolidin-3-y1]-1,3-dihydro-
benzoimidazol-2-one;
(R)-3-[4-Methoxy-2-oxo-3-(2-trifluoromethyl-benzy1)-2,3-dihydro-benzoimidazol-
1-y1]-pyrrolidine-1-carboxylic acid 3-
trifluoromethyl-oxetan-3-y1 ester;
5-1(R)-3-[4-Methoxy-2-oxo-3-(2-trifluoromethyl-benzy1)-2,3-dihydro-
benzoimidazol-111]-pyrrolidin-1-y11-1,3-dimethy1-
1H-pyrazole-4-carbonitrile;
1-[(R)-1-(2-Fluoro-6-hydroxymethyl-pheny1)-pyrrolidin-3-y1]-4-methoxy-3-(2-
trifluoromethyl-benzy1)-1,3-dihydro-
benzoimidazol-2-one;
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
1-1(R)-1-[2-Fluoro-6-(1-hydroxy-ethyl)-phenyl]-pyrrolidin-3-y11-4-methoxy-3-(2-
trifluoromethyl-benzy1)-1,3-dihydro-
benzoimidazol-2-one;
1-[(R)-1-(2-Acety1-6-fluoro-pheny1)-pyrrolidin-3-y1]-4-methoxy-3-(2-
trifluoromethyl-benzy1)-1,3-dihydro-
benzoimidazol-2-one;
5 1-1(R)-1-[2-Fluoro-6-(1-hydroxy-1-methyl-ethyl)-phenyl]-pyrrolidin-3-y11-
4-methoxy-3-(2-trifluoromethyl-benzy1)-1,3-
dihydro-benzoimidazol-2-one;
3-(6-Chloro-4-methoxy-pyridazin-3-ylmethyl)-1-[(R)-1-(2-fluoro-6-methyl-
pheny1)-pyrrolidin-3-y1]-4-methoxy-1,3-
dihydro-benzoimidazol-2-one;
1-[(R)-1-(2,6-Dimethyl-pheny1)-pyrrolidin-3-y1]-3-(2-trifluoromethyl-benzy1)-
3,5-dihydro-1H-imidazo[4,5-c]pyridine-
10 2,4-dione;
1-[(R)-1-(2-Methoxy-6-methyl-pheny1)-pyrrolidin-3-y1]-3-(2-trifluoromethyl-
benzy1)-3,5-dihydro-1H-imidazo[4,5-
c]pyridine-2,4-dione;
1-[(R)-1-(2-Fluoro-6-methyl-pheny1)-pyrrolidin-3-y1]-4-methoxy-3-(4-methoxy-
pyridazin-3-ylmethyl)-1,3-dihydro-
benzoimidazol-2-one;
15 3-Fluoro-2-14-[4-methoxy-2-oxo-3-(3-trifluoromethyl-pyrazin-2-ylmethyl)-
2,3-dihydro-benzoimidazol-1-y1]-piperidin-
1-yll-benzonitrile;
3-Fluoro-2-14-[4-methoxy-3-(3-methoxy-pyrazin-2-ylmethyl)-2-oxo-2,3-dihydro-
benzoimidazol-1-y1]-piperidin-1-yll-
benzonitrile;
1-[(R)-1-(2-Cyano-6-fluoro-pheny1)-pyrrolidin-3-y1]-2-oxo-3-(2-trifluoromethyl-
benzy1)-2,3-dihydro-1H-
20 benzoimidazole-4-carboxylic acid methyl ester;
1-[(R)-1-(2-Cyano-6-fluoro-pheny1)-pyrrolidin-3-y1]-2-oxo-3-(2-trifluoromethyl-
benzy1)-2,3-dihydro-1H-
benzoimidazole-4-carboxylic acid methylamide;
1-[(R)-1-(2-Cyano-6-fluoro-pheny1)-pyrrolidin-3-y1]-2-oxo-3-(2-trifluoromethyl-
benzy1)-2,3-dihydro-1H-
benzoimidazole-4-carboxylic acid amide;
25 1-[(R)-1-(2-Cyano-6-fluoro-pheny1)-pyrrolidin-3-y1]-2-oxo-3-(2-
trifluoromethyl-benzy1)-2,3-dihydro-1H-
benzoimidazole-4-carboxylic acid dimethylamide;
3-Fluoro-2-1(R)-3-K-hydroxymethyl-2-oxo-3-(2-trifluoromethyl-benzy1)-2,3-
dihydro-benzoimidazol-1-y1]-pyrrolidin-1-
yll-benzonitrile;
1-[(R)-1-(2-Fluoro-6-methylaminomethyl-pheny1)-pyrrolidin-3-y1]-4-methoxy-3-(2-
trifluoromethyl-benzy1)-1,3-dihydro-
30 benzoimidazol-2-one;
3-Fluoro-2-1(R)-3-[6-methoxy-8-oxo-7-(2-trifluoromethyl-benzy1)-7,8-dihydro-
purin-911]-pyrrolidin-1-yll-benzonitrile;
3-Fluoro-2-1(R)-3-[6-methoxy-7-(2-methoxy-benzy1)-8-oxo-7,8-dihydro-purin-911]-
pyrrolidin-1-yll-benzonitrile;
1-[(R)-1-(2-Dimethylaminomethy1-6-fluoro-pheny1)-pyrrolidin-3-y1]-4-methoxy-3-
(2-trifluoromethyl-benzy1)-1,3-
dihydro-benzoimidazol-2-one;
35 1-[(R)-1-(2-Fluoro-6-1[(2-hydroxy-ethyl)-methyl-amino]-methyll-pheny1)-
pyrrolidin-3-y1]-4-methoxy-3-(2-
trifluoromethyl-benzy1)-1,3-dihydro-benzoimidazol-2-one;
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
46
1-[(R)-1-(2-Azetidin-1-ylmethy1-6-fluoro-pheny1)-pyrrolidin-3-y1]-4-methoxy-3-
(2-trifluoromethyl-benzy1)-1,3-dihydro-
benzoimidazol-2-one;
3-Fluoro-2-1(R)-3-[4-methoxy-3-(2-methoxy-benzy1)-2-oxo-2,3-dihydro-
benzoimidazol-111]-pyrrolidin-1-yll-
benzonitrile;
1-[(R)-1-(2-Fluoro-6-methyl-pheny1)-pyrrolidin-3-y1]-4-methoxy-3-(2-methoxy-
benzy1)-1,3-dihydro-benzoimidazol-2-
one;
3-Fluoro-2-1(R)-3-[4-methoxy-2-oxo-3-(3-trifluoromethyl-pyrazin-2-ylmethyl)-
2,3-dihydro-benzoimidazol-111]-
pyrrolidin-1-yll-benzonitrile;
3-Fluoro-2-1(R)-3-[4-methoxy-3-(3-methoxy-pyrazin-2-ylmethyl)-2-oxo-2,3-
dihydro-benzoimidazol-111]-pyrrolidin-1-
yll-benzonitrile;
3-Fluoro-2-14-[4-methoxy-3-(4-methoxy-pyrimidin-5-ylmethyl)-2-oxo-2,3-dihydro-
benzoimidazol-1-y1]-piperidin-1-yll-
benzonitrile;
3-Fluoro-2-1(R)-3-K-methoxy-3-(4-methoxy-pyrimidin-5-ylmethyl)-2-oxo-2,3-
dihydro-benzoimidazol-1-y1]-pyrrolidin-
1-yll-benzonitrile;
1-[(R)-1-(2-Fluoro-6-morpholin-4-ylmethyl-pheny1)-pyrrolidin-3-y1]-4-methoxy-3-
(2-trifluoromethyl-benzy1)-1,3-
dihydro-benzoimidazol-2-one;
1-[(R)-1-(2-Fluoro-6-pyrrolidin-1-ylmethyl-pheny1)-pyrrolidin-3-y1]-4-methoxy-
3-(2-trifluoromethyl-benzy1)-1,3-
dihydro-benzoimidazol-2-one;
3-Fluoro-2-14-[4-methoxy-2-oxo-3-(4-trifluoromethyl-pyrimidin-5-ylmethyl)-2,3-
dihydro-benzoimidazol-1-y1]-piperidin-
1-yll-benzonitrile;
1-[(R)-1-(2,6-Dimethyl-pheny1)-pyrrolidin-3-y1]-4-(2-hydroxy-ethylamino)-3-(2-
trifluoromethyl-benzy1)-1,3-dihydro-
imidazo[4,5-c]pyridin-2-one;
1-[(R)-1-(2,6-Dimethyl-pheny1)-pyrrolidin-3-y1]-4-(2-hydroxy-ethoxy)-3-(2-
trifluoromethyl-benzy1)-1,3-dihydro-
imidazo[4,5-c]pyridin-2-one;
4-(2-Dimethylamino-ethoxy)-1-[(R)-1-(2-fluoro-6-methyl-pheny1)-pyrrolidin-3-
y1]-3-(2-trifluoromethyl-benzy1)-1,3-
dihydro-imidazo[4,5-c]pyridin-2-one;
1-[(R)-1-(2-Fluoro-6-methyl-pheny1)-pyrrolidin-3-y1]-4-(2-morpholin-4-yl-
ethoxy)-3-(2-trifluoromethyl-benzy1)-1,3-
dihydro-imidazo[4,5-c]pyridin-2-one;
4-[1-[(R)-1-(2-Fluoro-6-methyl-pheny1)-pyrrolidin-3-y1]-2-oxo-3-(2-
trifluoromethyl-benzy1)-2,3-dihydro-1 H-
imidazo[4,5-c]pyridin-4-yloxy]-piperidine-1-carboxylic acid tert-butyl ester;
1-[(R)-1-(2-Fluoro-6-methyl-pheny1)-pyrrolidin-3-y1]-4-[(2-hydroxy-ethyl)-
methyl-amino]-3-(2-trifluoromethyl-benzyl)-
1,3-dihydro-imidazo[4,5-c]pyridin-2-one;
1-[(R)-1-(2-Fluoro-6-methyl-pheny1)-pyrrolidin-3-y1]-4-(piperidin-4-yloxy)-3-
(2-trifluoromethyl-benzy1)-1,3-dihydro-
imidazo[4,5-c]pyridin-2-one;
4-(2,2-Dimethyl-[1,3]dioxolan-4-ylmethoxy)-1-[(R)-1-(2-fluoro-6-methyl-pheny1)-
pyrrolidin-3-y1]-3-(2-trifluoromethyl-
benzy1)-1,3-dihydro-imidazo[4,5-c]pyridin-2-one;
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
47
(3-Fluoro-2-1(R)-3-[4-methoxy-2-oxo-3-(2-trifluoromethyl-benzy1)-2,3-dihydro-
benzoimidazol-111]-pyrrolidin-1-yll-
pheny1)-acetaldehyde;
1-[(R)-1-(2-Fluoro-6-methyl-pheny1)-pyrrolidin-3-y1]-4-(oxetan-3-yloxy)-3-(2-
trifluoromethyl-benzy1)-1,3-dihydro-
imidazo[4,5-c]pyridin-2-one;
4-(2,3-Dihydroxy-propoxy)-1-[(R)-1-(2-fluoro-6-methyl-pheny1)-pyrrolidin-3-y1]-
3-(2-trifluoromethyl-benzy1)-1,3-
dihydro-imidazo[4,5-c]pyridin-2-one;
1-[(R)-1-(2-Fluoro-6-methyl-pheny1)-pyrrolidin-3-y1]-4-morpholin-4-y1-3-(2-
trifluoromethyl-benzy1)-1,3-dihydro-
imidazo[4,5-c]pyridin-2-one;
1-[(R)-1-(2-Fluoro-6-methyl-pheny1)-pyrrolidin-3-y1]-4-(1-methyl-pyrrolidin-3-
yloxy)-3-(2-trifluoromethyl-benzy1)-1,3-
dihydro-imidazo[4,5-c]pyridin-2-one;
1-1(R)-1-[2-Fluoro-6-(2-morpholin-4-yl-ethyl)-phenyl]-pyrrolidin-3-y11-4-
methoxy-3-(2-trifluoromethyl-benzy1)-1,3-
dihydro-benzoimidazol-2-one;
1-1(R)-1-[2-Fluoro-6-(4-methyl-piperazin-1-ylmethyl)-phenyl]-pyrrolidin-3-y11-
4-methoxy-3-(2-trifluoromethyl-benzyl)-
1,3-dihydro-benzoimidazol-2-one;
1-[(R)-1-(2-Fluoro-6-pipendin-1-ylmethyl-pheny1)-pyrrolidin-3-y1]-4-methoxy-3-
(2-trifluoromethyl-benzy1)-1,3-dihydro-
benzoimidazol-2-one;
1-1(R)-1-[2-Fluoro-6-(4-hydroxy-piperidin-1-ylmethyl)-phenyl]-pyrrolidin-3-y11-
4-methoxy-3-(2-trifluoromethyl-benzy1)-
1,3-dihydro-benzoimidazol-2-one;
1-[(R)-1-(2-Fluoro-6-piperazin-1-ylmethyl-pheny1)-pyrrolidin-3-y1]-4-methoxy-3-
(2-trifluoromethyl-benzyl)-1,3-
dihydro-benzoimidazol-2-one;
1-1(R)-1-[2-(4-Dimethylamino-pipendin-1-ylmethyl)-6-fluoro-phenylFpyrrolidin-3-
y11-4-methoxy-3-(2-trifluoromethyl-
benzy1)-1,3-dihydro-benzoimidazol-2-one;
1-1(R)-1-[2-Fluoro-6-(4-methoxy-piperidin-1-ylmethyl)-phenyl]-pyrrolidin-3-y11-
4-methoxy-3-(2-trifluoromethyl-
benzy1)-1,3-dihydro-benzoimidazol-2-one;
1-1(R)-1-[2-Fluoro-6-(3-methoxy-pyrrolidin-1-ylmethyl)-phenyl]-pyrrolidin-3-
y11-4-methoxy-3-(2-trifluoromethyl-
benzy1)-1,3-dihydro-benzoimidazol-2-one;
1-[(R)-1-(2-Fluoro-6-1[(2-methoxy-ethyl)-methyl-amino]-methyll-pheny1)-
pyrrolidin-3-y1]-4-methoxy-3-(2-
trifluoromethyl-benzyl)-1,3-dihydro-benzoimidazol-2-one;
1-[(R)-1-(2-1[Ethyl-(2-hydroxy-ethyl)-amino]-methyll-6-fluoro-pheny1)-
pyrrolidin-3-y1]-4-methoxy-3-(2-trifluoromethyl-
benzyI)-1,3-dihydro-benzoimidazol-2-one;
1-[(R)-1-(2-1[Bis-(2-hydroxy-ethyl)-amino]-methyll-6-fluoro-pheny1)-pyrrolidin-
3-y1]-4-methoxy-3-(2-trifluoromethyl-
benzy1)-1,3-dihydro-benzoimidazol-2-one;
14(R)-1-12-Fluoro-6-[(2-methoxy-1-methyl-ethylamino)-methy1]-phenyll-
pyrrolidin-3-y1)-4-methoxy-3-(2-
trifluoromethyl-benzyl)-1,3-dihydro-benzoimidazol-2-one;
1-1(R)-1-[2-Fluoro-6-(3-methoxy-azetidin-1-ylmethyl)-phenyl]-pyrrolidin-3-y11-
4-methoxy-3-(2-trifluoromethyl-benzyl)-
1,3-dihydro-benzoimidazol-2-one;
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
48
4-[4-Methoxy-2-oxo-3-(2-trifluoromethyl-benzy1)-2,3-dihydro-benzoimidazol-1-
y1]-2-methyl-piperidine-1-carboxylic
acid tert-butyl ester;
3-Fluoro-2-14-[4-methoxy-2-oxo-3-(2-trifluoromethyl-benzy1)-2,3-dihydro-
benzoimidazol-1-y1]-2-methyl-piperidin-1-
yll-benzaldehyde;
4-[4-Methoxy-2-oxo-3-(2-trifluoromethyl-benzy1)-2,3-dihydro-benzoimidazol-1-
y1]-3-methyl-piperidine-1-carboxylic
acid tert-butyl ester;
1-[1-(2-Fluoro-6-methyl-pheny1)-3-methyl-piperidin-4-y1]-4-methoxy-3-(2-
trifluoromethyl-benzy1)-1,3-dihydro-
benzoimidazol-2-one;
1-1(R)-1-[2-Fluoro-6-(1-methoxy-ethyl)-phenyl]-pyrrolidin-3-y11-4-methoxy-3-(2-
trifluoromethyl-benzy1)-1,3-dihydro-
benzoimidazol-2-one;
1-[(R)-1-(2-Fluoro-6-methyl-pheny1)-pyrrolidin-3-y1]-4-(oxetan-3-yloxy)-3-(2-
trifluoromethyl-benzy1)-1,3-dihydro-
benzoimidazol-2-one;
1-[(R)-1-(2-Fluoro-6-methyl-pheny1)-pyrrolidin-3-y1]-4-(2-hydroxy-ethoxy)-3-(2-
trifluoromethyl-benzy1)-1,3-dihydro-
benzoimidazol-2-one;
4-(2,2-Dimethyl-[1,3]dioxolan-4-ylmethoxy)-1-[(R)-1-(2-fluoro-6-methyl-pheny1)-
pyrrolidin-3-y1]-3-(2-trifluoromethyl-
benzy1)-1,3-dihydro-benzoimidazol-2-one;
4-(2,3-Dihydroxy-propoxy)-1-[(R)-1-(2-fluoro-6-methyl-pheny1)-pyrrolidin-3-y1]-
3-(2-trifluoromethyl-benzy1)-1,3-
dihydro-benzoimidazol-2-one;
3-Fluoro-2-1(R)-3-[4-methoxy-2-oxo-3-(4-trifluoromethyl-pyrimidin-5-ylmethyl)-
2,3-dihydro-benzoimidazol-111]-
.. pyrrolidin-1-yll-benzonitrile;
1-[(R)-1-(2-Fluoro-6-nitro-pheny1)-pyrrolidin-3-y1]-4-methoxy-3-(2-
trifluoromethyl-benzy1)-1,3-dihydro-benzoimidazol-
2-one;
1-[(R)-1-(2-Amino-6-fluoro-pheny1)-pyrrolidin-3-y1]-4-methoxy-3-(2-
trifluoromethyl-benzy1)-1,3-dihydro-
benzoimidazol-2-one;
.. 1-[(R)-1-(2-Bromo-6-fluoro-pheny1)-pyrrolidin-3-y1]-4-methoxy-3-(2-
trifluoromethyl-benzy1)-1,3-dihydro-
benzoimidazol-2-one;
1-[(R)-1-(4,5-Dichloro-pyridazin-3-y1)-pyrrolidin-3-y1]-4-methoxy-3-(2-
trifluoromethyl-benzy1)-1,3-dihydro-
benzoimidazol-2-one;
1-[(R)-1-(3,5-Dichloro-pyridazin-4-y1)-pyrrolidin-3-y1]-4-methoxy-3-(2-
trifluoromethyl-benzy1)-1,3-dihydro-
benzoimidazol-2-one;
1-1(R)-1-[2-Fluoro-6-(3-morpholin-4-yl-prop-1-yny1)-phenyl]-pyrrolidin-3-y11-4-
methoxy-3-(2-trifluoromethyl-benzy1)-
1,3-dihydro-benzoimidazol-2-one;
1-[(R)-1-(2-Fluoro-pheny1)-pyrrolidin-3-y1]-4-methoxy-3-(2-trifluoromethyl-
benzy1)-1,3-dihydro-benzoimidazol-2-one;
1-[(R)-1-(2-Fluoro-6-morpholin-4-yl-pheny1)-pyrrolidin-3-y1]-4-methoxy-3-(2-
trifluoromethyl-benzy1)-1,3-dihydro-
benzoimidazol-2-one;
1-1(R)-1-[2-Fluoro-6-(3-morpholin-4-yl-propy1)-phenyl]-pyrrolidin-3-y11-4-
methoxy-3-(2-trifluoromethyl-benzy1)-1,3-
dihydro-benzoimidazol-2-one;
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
49
14(R)-1-12-Fluoro-6-[1-(3-methoxy-azetidin-1-y1)-ethy1]-phenyll-pyrrolidin-3-
y1)-4-methoxy-3-(2-trifluoromethyl-
benzy1)-1,3-dihydro-benzoimidazol-2-one;
4-[4-Methoxy-2-oxo-3-(2-trifluoromethyl-benzy1)-2,3-dihydro-benzoimidazol-1-
y1]-piperidine-1,3-dicarboxylic acid 1-
tert-butyl ester 3-ethyl ester;
1-[(R)-1-(2-Fluoro-6-methyl-pheny1)-pyrrolidin-3-y1]-3-(2-isopropoxy-benzy1)-4-
methoxy-1,3-dihydro-benzoimidazol-
2-one;
1-[1-(2-Fluoro-6-methyl-pheny1)-piperidin-4-y1]-3-(2-isopropoxy-benzy1)-4-
methoxy-1,3-dihydro-benzoimidazol-2-
one;
1-[(R)-1-(2-Fluoro-6-methyl-pheny1)-pyrrolidin-3-y1]-4-methoxy-3-(3-
trifluoromethyl-pyrazin-2-ylmethyl)-1,3-dihydro-
benzoimidazol-2-one;
1-[1-(2-Fluoro-6-methyl-pheny1)-piperidin-4-y1]-4-methoxy-3-(3-trifluoromethyl-
pyrazin-2-ylmethyl)-1,3-dihydro-
benzoimidazol-2-one;
1-[(R)-1-(2-Fluoro-6-methyl-pheny1)-pyrrolidin-3-y1]-4-methoxy-3-(3-methoxy-
pyrazin-2-ylmethyl)-1,3-dihydro-
benzoimidazol-2-one;
1-[1-(2-Fluoro-6-methyl-pheny1)-piperidin-4-y1]-4-methoxy-3-(3-methoxy-pyrazin-
2-ylmethyl)-1,3-dihydro-
benzoimidazol-2-one;
3-[(R)-1-(2-Fluoro-6-methyl-pheny1)-pyrrolidin-3-y1]-7-methoxy-5-methy1-1-(2-
trifluoromethyl-benzy1)-1,3-dihydro-
imidazo[4,5-b]pyridin-2-one;
3-(6-Chloro-4-isopropoxy-pyridazin-3-ylmethyl)-1-[(R)-1-(2-fluoro-6-methyl-
pheny1)-pyrrolidin-3-y1]-4-methoxy-1,3-
dihydro-benzoimidazol-2-one;
1-[(R)-1-(2-Fluoro-6-methyl-pheny1)-pyrrolidin-3-y1]-3-(4-isopropoxy-pyridazin-
3-ylmethyl)-4-methoxy-1,3-dihydro-
benzoimidazol-2-one;
1-[(R)-1-(2-Fluoro-6-methyl-pheny1)-pyrrolidin-3-y1]-4-methoxy-3-(4-methyl-
pyridazin-3-ylmethyl)-1,3-dihydro-
benzoimidazol-2-one;
1-[(R)-1-(2-Fluoro-6-methyl-pheny1)-pyrrolidin-3-y1]-4-methoxy-3-(4-methoxy-
pyrimidin-5-ylmethyl)-1,3-dihydro-
benzoimidazol-2-one;
1-[(R)-1-(2-Fluoro-6-methyl-pheny1)-pyrrolidin-3-y1]-4-methoxy-3-(4-
trifluoromethyl-pyrimidin-5-ylmethyl)-1,3-
dihydro-benzoimidazol-2-one;
1-[(R)-1-(2-Fluoro-6-methyl-pheny1)-pyrrolidin-3-y1]-3-(3-isopropoxy-pyrazin-2-
ylmethyl)-4-methoxy-1,3-dihydro-
benzoimidazol-2-one;
1-[1-(2-Fluoro-6-methyl-pheny1)-piperidin-4-y1]-3-(3-isopropoxy-pyrazin-2-
ylmethyl)-4-methoxy-1,3-dihydro-
benzoimidazol-2-one;
4-[4-Methoxy-2-oxo-3-(2-trifluoromethyl-benzy1)-2,3-dihydro-benzoimidazol-1-
y1Fazepane-1-carboxylic acid tert-
butyl ester;
1-[(R)-1-(2-Fluoro-6-methyl-pheny1)-pyrrolidin-3-y1]-3-(4-isopropyl-pyridin-3-
ylmethyl)-4-methoxy-1,3-dihydro-
benzoimidazol-2-one;
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
1-[1-(2-Fluoro-6-methyl-pheny1)-piperidin-4-y1]-3-(4-isopropyl-pyridin-3-
ylmethyl)-4-methoxy-1,3-dihydro-
benzoimidazol-2-one;
1-[(R)-1-(2-Fluoro-6-methyl-pheny1)-pyrrolidin-3-y1]-3-(3-isopropoxy-pyridin-2-
ylmethyl)-4-methoxy-1,3-dihydro-
benzoimidazol-2-one;
5 1-[1-(2-Fluoro-6-methyl-pheny1)-piperidin-4-y1]-3-(3-isopropoxy-pyridin-2-
ylmethyl)-4-methoxy-1,3-dihydro-
benzoimidazol-2-one;
1-[1-(2-Fluoro-6-methyl-pheny1)-azepan-4-y1]-4-methoxy-3-(2-trifluoromethyl-
benzy1)-1,3-dihydro-benzoimidazol-2-
one;
1-[1-(2,6-Dimethyl-pheny1)-azepan-4-y1]-4-methoxy-3-(2-trifluoromethyl-benzy1)-
1,3-dihydro-benzoimidazol-2-one;
10 1-[(R)-1-(2-Fluoro-6-methyl-pheny1)-pyrrolidin-3-y1]-3-(4-isopropyl-
pyrimidin-5-ylmethyl)-4-methoxy-1,3-dihydro-
benzoimidazol-2-one;
1-[1-(2-Fluoro-6-methyl-pheny1)-piperidin-4-y1]-3-(3-isopropyl-pyrazin-2-
ylmethyl)-4-methoxy-1,3-dihydro-
benzoimidazol-2-one;
5-[4-Methoxy-2-oxo-3-(2-trifluoromethyl-benzy1)-2,3-dihydro-benzoimidazol-1-
y1]-2-aza-bicyclo[2.2.1]heptane-2-
15 carboxylic acid tert-butyl ester;
1-[(R)-1-(2-Fluoro-6-methyl-pheny1)-pyrrolidin-3-y1]-3-(3-isopropyl-pyrazin-2-
ylmethyl)-4-methoxy-1,3-dihydro-
benzoimidazol-2-one;
1-[2-(2-Fluoro-6-methyl-pheny1)-2-aza-bicyclo[2.2.1Thept-5-y1]-4-methoxy-3-(2-
trifluoromethyl-benzy1)-1,3-dihydro-
benzoimidazol-2-one;
20 6-[4-Methoxy-2-oxo-3-(2-trifluoromethyl-benzy1)-2,3-dihydro-
benzoimidazol-1-y1]-3-aza-bicyclo[3.1.1]heptane-3-
carboxylic acid tert-butyl ester;
1-[1-(2-Fluoro-6-methyl-pheny1)-piperidin-4-y1]-3-(4-isopropyl-pyrimidin-5-
ylmethyl)-4-methoxy-1,3-dihydro-
benzoimidazol-2-one;
1-[(R)-1-(2-Cyano-6-fluoro-pheny1)-pyrrolidin-3-y1]-2-oxo-3-(2-trifluoromethyl-
benzy1)-2,3-dihydro-1H-
25 benzoimidazole-4-carboxylic acid (2-hydroxy-ethyl)-amide;
1-[(R)-1-(2-Cyano-6-fluoro-pheny1)-pyrrolidin-3-y1]-2-oxo-3-(2-trifluoromethyl-
benzy1)-2,3-dihydro-1H-
benzoimidazole-4-carboxylic acid (2-methoxy-ethyl)-amide;
1-[(R)-1-(2-Fluoro-6-methyl-pheny1)-pyrrolidin-3-y1]-4-methoxy-3-(2-
trifluoromethyl-pyridin-3-ylmethyl)-1,3-dihydro-
benzoimidazol-2-one;
30 (3R*,4S*)-3-K-Methoxy-2-oxo-3-(2-trifluoromethyl-benzy1)-2,3-dihydro-
benzoimidazol-1-y1]-4-methyl-pyrrolidine-1-
carboxylic acid tert-butyl ester;
1-[3-(2-Fluoro-6-methyl-pheny1)-3-aza-bicyclo[3.1.1]hept-6-y1]-4-methoxy-3-(2-
trifluoromethyl-benzy1)-1,3-dihydro-
benzoimidazol-2-one;
1-[(3R*,4S*)-1-(2-Fluoro-6-methyl-pheny1)-4-methyl-pyrrolidin-3-y1]-4-methoxy-
3-(2-trifluoromethyl-benzy1)-1,3-
35 dihydro-benzoimidazol-2-one;
(3R*,4R*)-3-[4-Methoxy-2-oxo-3-(2-trifluoromethyl-benzy1)-2,3-dihydro-
benzoimidazol-1-y1]-4-methyl-pyrrolidine-1-
carboxylic acid tert-butyl ester;
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
51
1-R3R*,4R*)-1-(2-Fluoro-6-methyl-pheny1)-4-methyl-pyrrolidin-3-y1]-4-methoxy-3-
(2-trifluoromethyl-benzy1)-1,3-
dihydro-benzoimidazol-2-one;
4-Methoxy-1-[(R)-1-(2-trifluoromethoxy-pheny1)-pyrrolidin-3-y1]-3-(2-
trifluoromethyl-benzy1)-1,3-dihydro-
benzoimidazol-2-one;
1-[(R)-1-(2-Fluoro-6-methyl-pheny1)-pyrrolidin-3-y1]-4-methoxy-3-(3-
trifluoromethyl-pyridin-2-ylmethyl)-1,3-dihydro-
benzoimidazol-2-one;
1-[(R)-1-(2-Fluoro-6-methyl-pheny1)-pyrrolidin-3-y1]-4-methoxy-3-(4-
trifluoromethyl-pyridin-3-ylmethyl)-1,3-dihydro-
benzoimidazol-2-one;
4-Methoxy-1-1(R)-1-[2-(2-methoxy-ethoxy)-pheny1]-pyrrolidin-3-y11-3-(2-
trifluoromethyl-benzy1)-1,3-dihydro-
benzoimidazol-2-one;
1-[(R)-1-(2-Fluoro-6-methyl-pheny1)-pyrrolidin-3-y1]-4-(3-methoxy-azetidin-1-
y1)-3-(2-trifluoromethyl-benzy1)-1,3-
dihydro-imidazo[4,5-c]pyridin-2-one;
1-[(R)-1-(2-Fluoro-6-methyl-pheny1)-pyrrolidin-3-y1]-4-(4-methyl-piperazin-1-
y1)-3-(2-trifluoromethyl-benzy1)-1,3-
dihydro-imidazo[4,5-c]pyridin-2-one;
1-[(R)-1-(2-Fluoro-6-methyl-pheny1)-pyrrolidin-3-y1]-4-(2-methoxy-ethylamino)-
3-(2-trifluoromethyl-benzy1)-1,3-
dihydro-imidazo[4,5-c]pyridin-2-one;
3-(2-Cyclopropoxy-benzy1)-1-[(R)-1-(2-fluoro-6-methyl-pheny1)-pyrrolidin-3-y1]-
4-methoxy-1,3-dihydro-
benzoimidazol-2-one;
3-(2-Cyclopropoxy-benzy1)-1-[1-(2-fluoro-6-methyl-pheny1)-piperidin-4-y1]-4-
methoxy-1,3-dihydro-benzoimidazol-2-
one;
3-Fluoro-4-[4-methoxy-2-oxo-3-(2-trifluoromethyl-benzy1)-2,3-dihydro-
benzoimidazol-1-y1]-piperidine-1-carboxylic
acid tert-butyl ester;
1-[3-Fluoro-1-(2-fluoro-6-methyl-pheny1)-piperidin-4-y1]-4-methoxy-3-(2-
trifluoromethyl-benzy1)-1,3-dihydro-
benzoimidazol-2-one;
1-[1-(2-Fluoro-6-methyl-pheny1)-piperidin-3-y1]-4-methoxy-3-(2-trifluoromethyl-
benzy1)-1,3-dihydro-benzoimidazol-2-
one;
3-[4-Methoxy-2-oxo-3-(2-trifluoromethyl-benzy1)-2,3-dihydro-benzoimidazol-1-
y1Fazetidine-1-carboxylic acid tert-
butyl ester;
3-[4-Methoxy-2-oxo-3-(2-trifluoromethyl-benzy1)-2,3-dihydro-benzoimidazol-1-
y1]-piperidine-1-carboxylic acid tert-
butyl ester;
1-[1-(2-Fluoro-6-methyl-pheny1)-azetidin-3-y1]-4-methoxy-3-(2-trifluoromethyl-
benzy1)-1,3-dihydro-benzoimidazol-2-
one;
1-[1-(2-Fluoro-6-methyl-pheny1)-piperidin-4-y1]-4-methoxy-3-(3-trifluoromethyl-
pyridin-2-ylmethyl)-1,3-dihydro-
benzoimidazol-2-one;
1-[1-(2,6-Dimethyl-pheny1)-piperidin-3-y1]-4-methoxy-3-(2-trifluoromethyl-
benzy1)-1,3-dihydro-benzoimidazol-2-one;
1-[(R)-1-(2-Fluoro-6-methyl-pheny1)-pyrrolidin-3-y1]-4-methoxy-3-(2-
trifluoromethyl-thiazol-4-ylmethyl)-1,3-dihydro-
benzoimidazol-2-one;
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
52
1-[1-(2-Fluoro-6-methyl-pheny1)-pyrrolidin-3-y1]-4-methoxy-3-[(S)-1-(2-
trifluoromethyl-pheny1)-ethyl]-1,3-dihydro-
benzoimidazol-2-one;
3-(2,4-Difluoro-6-isopropoxy-benzy1)-1-[(R)-1-(2-fluoro-6-methyl-pheny1)-
pyrrolidin-3-y1]-4-methoxy-1,3-dihydro-
benzoimidazol-2-one;
1-[(R)-1-(2-Fluoro-6-methyl-pheny1)-pyrrolidin-3-y1]-4-methoxy-3-(2-methy1-4-
trifluoromethyl-thiazol-5-ylmethyl)-1,3-
dihydro-benzoimidazol-2-one;
1-(1-Cyclopropanecarbonyl-piperidin-4-y1)-4-methoxy-3-(2-trifluoromethyl-
benzy1)-1,3-dihydro-benzoimidazol-2-one;
3-(2-Cyclopropyl-benzy1)-1-[1-(2-fluoro-6-methyl-pheny1)-piperidin-4-y1]-4-
methoxy-1,3-dihydro-benzoimidazol-2-
one;
3-(2-Cyclopropylmethoxy-benzy1)-1-[1-(2-fluoro-6-methyl-pheny1)-piperidin-4-
y1]-4-methoxy-1,3-dihydro-
benzoimidazol-2-one;
1-[1-(2-Fluoro-6-methyl-pheny1)-piperidin-4-y1]-4-methoxy-3-[2-(oxetan-3-
yloxy)-benzy1]-1,3-dihydro-benzoimidazol-
2-one;
3-(2-Cyclobutoxy-benzy1)-1-[1-(2-fluoro-6-methyl-pheny1)-piperidin-4-y1]-4-
methoxy-1,3-dihydro-benzoimidazol-2-
one;
1-[1-(2-Fluoro-6-methyl-pheny1)-3,3-dimethyl-piperidin-4-y1]-4-methoxy-3-(2-
trifluoromethyl-benzy1)-1,3-dihydro-
benzoimidazol-2-one;
4-[4-Methoxy-2-oxo-3-(2-trifluoromethyl-benzy1)-2,3-dihydro-benzoimidazol-1-
y1]-3,3-dimethyl-piperidine-1-
carboxylic acid tert-butyl ester;
3-[1-(2-Fluoro-6-methyl-pheny1)-piperidin-4-y1]-7-methoxy-1-(2-trifluoromethyl-
benzy1)-1,3-dihydro-imidazo[4,5-
c]pyridin-2-one;
1-[1-(2-Hydroxy-pheny1)-piperidin-4-y1]-4-methoxy-3-(2-trifluoromethyl-benzy1)-
1,3-dihydro-benzoimidazol-2-one;
1-[1-(2-Benzyloxy-pheny1)-piperidin-4-y1]-4-methoxy-3-(2-trifluoromethyl-
benzy1)-1,3-dihydro-benzoimidazol-2-one;
4-Methoxy-1-(3'-methy1-3,4,5,6-tetrahydro-2H-[1,2]bipyridinyl-4-y1)-3-(2-
trifluoromethyl-benzyl)-1,3-dihydro-
benzoimidazol-2-one;
4-Methoxy-1-(3'-methoxy-3,4,5,6-tetrahydro-2H-[1,2]bipyridinyl-4-y1)-3-(2-
trifluoromethyl-benzy1)-1,3-dihydro-
benzoimidazol-2-one;
4-Methoxy-1-(2'-methoxy-4'-methy1-3,4,5,6-tetrahydro-2H-[1,3]bipyridiny1-4-y1)-
3-(2-trifluoromethyl-benzy1)-1,3-
dihydro-benzoimidazol-2-one;
1-(2'-Fluoro-4'-methy1-3,4,5,6-tetrahydro-2H-[1,3]bipyridinyl-4-y1)-4-methoxy-
3-(2-trifluoromethyl-benzy1)-1,3-
dihydro-benzoimidazol-2-one;
1-(3'-Fluoro-3,4,5,6-tetrahydro-2H-0,21bipyridinyl-4-y1)-4-methoxy-3-(2-
trifluoromethyl-benzyl)-1,3-dihydro-
benzoimidazol-2-one;
1-(2',4'-Dimethy1-3,4,5,6-tetrahydro-2H-[1,3]bipyridinyl-4-y1)-4-methoxy-3-(2-
trifluoromethyl-benzy1)-1,3-dihydro-
benzoimidazol-2-one;
1-11 -[2-(2-Hydroxy-ethoxy)-pheny1]-piperidin-4-y11-4-methoxy-3-(2-
trifluoromethyl-benzy1)-1,3-dihydro-
benzoimidazol-2-one;
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
53
4-Methoxy-1-11 -[2-(tetrahydro-pyran-4-yloxy)-pheny1]-piperidin-4-y11-3-(2-
trifluoromethyl-benzy1)-1,3-dihydro-
benzoimidazol-2-one;
1-[1-(2-Cyclopropylmethoxy-pheny1)-piperidin-4-y1]-4-methoxy-3-(2-
trifluoromethyl-benzy1)-1,3-dihydro-
benzoimidazol-2-one;
1-[1-(2-Cyclobutoxy-pheny1)-piperidin-4-y1]-4-methoxy-3-(2-trifluoromethyl-
benzy1)-1,3-dihydro-benzoimidazol-2-
one;
4-Methoxy-1-11 -[2-(oxetan-3-yloxy)-pheny1]-piperidin-4-y11-3-(2-
trifluoromethyl-benzy1)-1,3-dihydro-benzoimidazol-2-
one;
1-[1-(2-Butoxy-pheny1)-piperidin-4-y1]-4-methoxy-3-(2-trifluoromethyl-benzy1)-
1,3-dihydro-benzoimidazol-2-one;
1-(4'-Fluoro-2'-methyl-3,4 ,5,6-tetrahydro-2H-[1,3']bipyridiny1-4-y1)-4-
methoxy-3-(2-trifluoromethyl-benzyI)-1,3-
dihydro-benzoimidazol-2-one;
1-[1-(2-Fluoro-6-methyl-pheny1)-piperidin-4-y1]-4-methy1-3-(2-trifluoromethyl-
benzy1)-1,3-dihydro-benzoimidazol-2-
one;
4-[4-Methoxy-2-oxo-3-(2-trifluoromethyl-benzy1)-2,3-dihydro-benzoimidazol-1-
y1]-4'-methy1-3,4,5,6-tetrahydro-2H-
[1,3']bipyridinyl-2-carbonitrile;
4-Methoxy-1-(4'-methoxy-2'-methy1-3,4,5,6-tetrahydro-2H-[1,3']bipyridinyl-411)-
3-(2-trifluoromethyl-benzy1)-1,3-
dihydro-benzoimidazol-2-one;
1-(2',4'-Dimethoxy-3,4,5,6-tetrahydro-2H-[1,3]bipyridinyl-411)-4-methoxy-3-(2-
trifluoromethyl-benzyl)-1,3-dihydro-
benzoimidazol-2-one;
4-Methoxy-1-0-(4-methoxy-6-methyl-pyrimidin-5-y1)-piperidin-4-y1]-3-(2-
trifluoromethyl-benzy1)-1,3-dihydro-
benzoimidazol-2-one; or
1-[1-(4,6-Dimethoxy-pyrimidin-5-y1)-piperidin-4-y1]-4-methoxy-3-(2-
trifluoromethyl-benzy1)-1,3-dihydro-
benzoimidazol-2-one ;
or a pharmaceutically acceptable salt thereof.
.. The compounds of formula (I), (II) and (111) according to embodiments 1) to
25) and their pharmaceutically
acceptable salts can be used as medicaments, e.g. in the form of
pharmaceutical compositions for enteral (such
especially oral) or parenteral administration (including topical application
or inhalation).
The production of the pharmaceutical compositions can be effected in a manner
which will be familiar to any person
skilled in the art (see for example Remington, The Science and Practice of
Pharmacy, 21st Edition (2005), Part 5,
.. "Pharmaceutical Manufacturing" [published by Lippincott Williams &
Wilkins]) by bringing the described compounds
of formula (I), (II) or (111), or their pharmaceutically acceptable salts,
optionally in combination with other
therapeutically valuable substances, into a galenical administration form
together with suitable, non-toxic, inert,
therapeutically compatible solid or liquid carrier materials and, if desired,
usual pharmaceutical adjuvants.
The present invention also relates to a method for the prevention or treatment
of a disease or disorder mentioned
herein comprising administering to a subject a pharmaceutically active amount
of a compound of formula (I), (II) or
(111) as defined in any one of embodiments 1) to 25).
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
54
In a preferred embodiment of the invention, the administered amount is
comprised between 1 mg and 1000 mg per
day, particularly between 5 mg and 500 mg per day, more particularly between
25 mg and 400 mg per day,
especially between 50 mg and 200 mg per day.
Whenever the word "between" is used to describe a numerical range, it is to be
understood that the end points of
the indicated range are explicitly included in the range. For example: if a
temperature range is described to be
between 40 C and 80 C, this means that the end points 40 C and 80 C are
included in the range; or if a variable
is defined as being an integer between 1 and 4, this means that the variable
is the integer 1, 2, 3, or 4.
Unless used regarding temperatures, the term "about" placed before a numerical
value "X" refers in the current
application to an interval extending from X minus 10% of X to X plus 10% of X,
and preferably to an interval
extending from X minus 5% of X to X plus 5% of X. In the particular case of
temperatures, the term "about" placed
before a temperature "Y" refers in the current application to an interval
extending from the temperature Y minus
10 C to Y plus 10 C, and preferably to an interval extending from Y minus 5 C
to Y plus 5 C.
For avoidance of any doubt, if compounds are described as useful for the
prevention or treatment of certain
diseases, such compounds are likewise suitable for use in the preparation of a
medicament for the prevention or
treatment of said diseases.
The compounds of formula (I), (II) and (III) as defined in any one of
embodiments 1) to 25) are useful for the
prevention / prophylaxis or treatment of diseases and disorders related to
pathogenic events associated with
elevated levels of C5a and/or with C5aR activation.
Such diseases and disorders related to pathogenic events associated with
elevated levels of C5a and/or with C5aR
activation are especially:
= vasculitic diseases or disorders,
= inflammatory diseases or disorders involving intravascular microvesicle
release,
= immune complex (IC) diseases or disorders,
= neurodegenerative diseases or disorders,
= complement related inflammatory diseases or disorders,
= bullous diseases or disorders,
= diseases or disorders related to ischemia and/or ischemic reperfusion
injury,
= inflammatory bowel diseases or disorders,
= autoimmune diseases or disorders, or, in addition to the above listed;
= cancer.
In addition to the above-listed diseases and disorders, further diseases and
disorders related to pathogenic events
associated with elevated levels of C5a and/or with C5aR activation are:
= further inflammatory diseases or disorders associated with elevated
levels of C5a and/or with C5aR
activation such as especially neutropenia, sepsis, septic shock, stroke,
inflammation associated with
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
severe burns, osteoarthritis, acute (adult) respiratory distress syndrome
(ARDS), chronic pulmonary
obstructive disorder (COPD), asthma (especially bronchial asthma), systemic
inflammatory response
syndrome (SIRS), tissue graft rejection, hyperacute rejection of transplanted
organs, multiple organ
dysfunction syndrome (MODS), diabetic retinopathy, neuromyelitis optica, and
glomerulonephritis
5 including Heyman nephritis / membranous glomerulonephritis, Berger's
disease (IgA nephropathy), and
other forms of glomerulonephritis including C3 glomerulopathy;
as well as
= hemotological diseases which are associated with activation of
coagulation and fibrinolytic systems,
disseminated intravascular coagulation (DIG), pernicious anemia, warm and cold
autoimmune hemolytic
10 anemia (AIHA), anti-phospholipid syndrome and its associated
complications, arterial or venous
thrombosis, pregnancy complications such as recurrent miscarriage and fetal
death, preeclampsia,
placental insufficiency, fetal growth restriction, cervical remodeling and
preterm birth, idiopathic
thrombocytopenic purpura (ITP), atypical hemolytic uremic syndrome (aHUS),
paroxysmal nocturnal
hemoglobinuria (PNH), allergic transfusion reactions, acute antibody-mediated
kidney allograft rejection,
15 cold agglutinin disease and glaucoma.
The present compounds may in addition be useful for
= the prevention or treatment of deleterious consequences of contact
sensitivity and inflammation caused by
contact with artificial surfaces;
= the prevention or treatment of increased leukocyte and platelet
activation (and infiltration to tissues thereof);
20 = the prevention or treatment of pathologic sequelae (such as especially
prevention or treatment of the
development of tissue injury, especially of pulmonary tissue injury)
associated to an intoxication or an injury
such as a trauma, an hemorrhage, a shock, or surgery including
transplantation, including multiple organ
failure (MOF), septic shock, shock due to intoxication (such as shock due to
snake venom), or acute lung
inflammatory injury;
25 = the prevention or treatment of pathologic sequelae associated with
insulin-dependent diabetes mellitus;
= the prevention of / the reduction of the risk of myocardial infarction or
thrombosis; prevention or treatment of
edema or increased capillary permeability;
= the prevention of / the reduction of coronary endothelial dysfunction
induced by cardiopulmonary bypass
and/or cardioplegia.
30 Vasculitic diseases or disorders include especially vasculitis, ANCA
associated vasculitis and glomerulonephritis
(GN, especially rapidly progressive GN) associated with ANCA associated
vasculitis, leukoclastic vasculitis,
granulomatosis with polyangiitis (GPA, also referred to as Wegener's
granulomatosis), microscopic polyangiitis,
Churg-Strauss syndrome, Henoch-Schonlein purpura, polyateritis nodosa,
cryoglobulinaemia, giant cell arteritis
(GCA), Behcet's disease, and Takayasu's arteritis (TAK).
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
56
Inflammatory diseases or disorders involving intravascular microvesicle
release include especially thrombotic
microangiopathy, and sickle cell disease.
Immune complex (IC) diseases or disorders include especially cryoglobulinemia,
Sjogren's syndrome (and
associated immunological profiles), Goodpasture syndrome (antiglomerular
basement antibody disease) and
glomerulonephritis (GN, especially rapidly progressive GN) or pulmonary
hemorrhage associated with Goodpasture
syndrome, and hypersensitivity;
Neurodegenerative diseases and disorders include especially amyotrophic
lateral sclerosis (ALS), Alzheimer's
disease, Parkinson's disease, Guillain-Barre syndrome, neuropathyõ and
cognitive function decline associated
with cardiopulmonary bypass surgery and related procedures.
Complement related inflammatory diseases or disorders include especially
coronary thrombosis, vascular
occlusion, post-surgical vascular reocclusion, atherosclerosis, traumatic
central nervous system injury,
arrhythmogenic cardiomyopathy, bronchoconstriction, acute respiratory distress
syndrome (ARDS), Chronic
Obstructive Pulmonary Disorder (COPD), complement mediated thrombotic
microangiopathies including atypical
haemolytic uremic syndrome, and Gaucher disease.
Bullous diseases or disorders include especially bullous pemphigoid, bullous
acquisita, pemphigus foliaceus,
pemphigus vulgaris, and sub-epidermal blisters.
Diseases or disorders related to ischemia and/or ischemic reperfusion injury
include especially ischemic
reperfusion injury (including myocardial ischemia-reperfusion injury, and
ischemic / reperfusion injury resulting from
transplantation, including solid organ transplant), ischemic colitis, and
cardiac ischemia.
Inflammatory bowel diseases or disorders include especially irritable bowel
syndrome, ulcerative colitis, Crohn's
disease, and inflammatory bowel disease (IBD).
Autoimmune diseases or disorders include especially rheumatoid arthritis,
osteoarthritis, systemic lupus
erythematosus (SLE) and glomerulonephritis (GN, especially rapidly progressive
GN) associated with lupus
erythematosus (lupus nephritis), central nervous system (CNS) lupus,
dermatomyositis, pemphigus, systemic
.. sclerosis (scleroderma), autoimmune hemolytic and thrombocytopenic states,
immunovasculitis, mixed
cryoglobulinemia, atopic dermatitis, chronic urticaria, psoriasis, myasthenia
gravis, and anti-phospholipid
syndrome.
Further inflammatory diseases or disorders associated with elevated levels of
C5a and/or with C5aR activation
include especially neutropenia, sepsis, septic shock, stroke, inflammation
associated with severe burns,
osteoarthritis, acute (adult) respiratory distress syndrome (ARDS), chronic
pulmonary obstructive disorder (COPD),
asthma, especially bronchial asthma, systemic inflammatory response syndrome
(SIRS), tissue graft rejection,
hyperacute rejection of transplanted organs, multiple organ dysfunction
syndrome (MODS), diabetic retinopathy,
neuromyelitis optica, and glomerulonephritis including Heyman nephritis /
membranous glomerulonephritis,
Berger's disease (IgA nephropathy), and other forms of glomerulonephritis
including C3 glomerulopathy.
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
57
The term "cancer" notably refers to skin cancer including melanoma including
metastatic melanoma; lung cancer
including non-small cell lung cancer; bladder cancer including urinary bladder
cancer, urothelial cell carcinoma;
renal carcinomas including renal cell carcinoma, metastatic renal cell
carcinoma, metastatic renal clear cell
carcinoma; gastro-intestinal cancers including colorectal cancer, metastatic
colorectal cancer, familial adenomatous
polyposis (FAP), oesophageal cancer, gastric cancer, gallbladder cancer,
cholangiocarcinoma, hepatocellular
carcinoma, and pancreatic cancer such as pancreatic adenocarcinoma or
pancreatic ductal carcinoma; endometrial
cancer; ovarian cancer; cervical cancer; neuroblastoma; prostate cancer
including castrate-resistant prostate
cancer; brain tumors including brain metastases, malignant gliomas,
glioblastoma multiforme, medulloblastoma,
meningiomas; breast cancer including triple negative breast carcinoma; oral
tumors; nasopharyngeal tumors;
thoracic cancer; head and neck cancer; leukemias including acute myeloid
leukemia, adult T-cell leukemia;
carcinomas; adenocarcinomas; thyroid carcinoma including papillary thyroid
carcinoma; choriocarcinoma; Ewing's
sarcoma; osteosarcoma; rhabdomyosarcoma; Kaposi's sarcoma; lymphoma including
Burkitt's lymphoma,
Hodgkin's lymphoma, MALT lymphoma; multiple myelomas; or virally induced
tumors.
When used for the prevention / prophylaxis or treatment of a cancer, such use
includes use of the present
compounds as single therapeutic agents and their use in combination with one
or more chemotherapy agents and /
or radiotherapy and / or targeted therapy (especially in combination with
targeted therapy).
The terms "radiotherapy or "radiation therapy' or "radiation oncology', refer
to the medical use of ionizing radiation
in the prevention / prophylaxis (adjuvant therapy) and / or treatment of
cancer; including external and internal
radiotherapy.
The term "targeted therapy' refers to the prevention / prophylaxis (adjuvant
therapy) and / or treatment of cancer
with one or more anti-neoplastic agents such as small molecules or antibodies
which act on specific types of
cancer cells or stromal cells. Some targeted therapies block the action of
certain enzymes, proteins, or other
molecules involved in the growth and spread of cancer cells. Other types of
targeted therapies help the immune
system kill cancer cells (immunotherapies); or inhibit angiogenesis, the
growth and formation of new blood vessels
in the tumor; or deliver toxic substances directly to cancer cells and kill
them. An example of a targeted therapy
which is in particular suitable to be combined with the compounds of the
present invention is immunotherapy,
especially immunotherapy targeting the progammed cell death receptor 1 (PD-1
receptor) or its ligand PD-L1.
When used in combination with the present compounds, the term "targeted
therapy' especially refers to agents
such as:
a) Epidermal growth factor receptor (EGFR) inhibitors or blocking antibodies
(for example Gefitinib, Erlotinib,
Afatinib, lcotinib, Lapatinib, Panitumumab, Zalutumumab, Nimotuzumab,
Matuzumab and Cetuximab);
b) RAS/RAF/MEK pathway inhibitors (for example Vemurafenib, Sorafenib,
Dabrafenib,GDC-0879, PLX-
4720, LGX818, RG7304, Trametinib (GSK1120212), Cobimetinib (GDC-0973/XL518),
Binimetinib
(MEK162, ARRY-162), Selumetinib (AZD6244));
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
58
c) Aromatase inhibitors (for example Exemestane, Letrozole, Anastrozole,
Vorozole, Formestane,
Fadrozole);
d) Angiogenesis inhibitors, especially VEGF signalling inhibitors such as
Bevacuzimab (Avastin),
Ramucirumab, Sorafenib or Axitinib;
e) Immune Checkpoint inhibitors (for example: anti-PD1 antibodies such as
Pembrolizumab (Lambrolizumab,
MK-3475), Nivolumab, Pidilizumab (CT-011), AMP-514/MED10680, PDR001, SHR-1210;
REGN2810,
BGBA317; fusion proteins targeting PD-1 such as AMP-224; small molecule anti-
PD1 agents such as for
example compounds disclosed in W02015/033299, W02015/044900 and W02015/034820;
anti-PD1L
antibodies, such as BMS-936559, atezolizumab (MPDL3280A, RG7446), MEDI4736,
avelumab
(MS60010718C), durvalumab (MEDI4736); anti-PDL2 antibodies, such as AMP224;
anti-CTLA-4
antibodies, such as ipilimumab, tremilmumab; anti-Lymphocyte-activation gene 3
(LAG-3) antibodies,
such as BMS-986016, IMP701, MK-4280, ImmuFact IMP321; anti T cell
immunoglobulin mucin-3 (TIM-3)
antibodies, such as MBG453; anti-CD137/4-166 antibodies, such as BMS-663513 /
urelumab, PF-
05082566; anti T cell immunoreceptor with Ig and ITIM domains (TIGIT)
antibodies, such as RG6058
(anti-TIGIT, MTIG7192A);
f) Vaccination approaches (for example dendritic cell vaccination, peptide or
protein vaccination (for
example with gp100 peptide or MAGE-A3 peptide);
g) Re-introduction of patient derived or allogenic (non-self) cancer cells
genetically modified to secrete
immunomodulatory factors such as granulocyte monocyte colony stimulating
factor (GMCSF) gene-
transfected tumor cell vaccine (GVAX) or Fms-related tyrosine kinase 3 (Flt-3)
ligand gene-transfected
tumor cell vaccine (FVAX),or Toll like receptor enhanced GM-CSF tumor based
vaccine (TEGVAX);
h) T-cell based adoptive immunotherapies, including chimeric antigen receptor
(CAR) engineered T-cells (for
example CTL019);
i) Cytokine or immunocytokine based therapy (for example Interferon alpha,
interferon beta, interferon
gamma, interleukin 2, interleukin 15);
j) Toll-like receptor (TLR) agonists (for example resiquimod, imiquimod,
glucopyranosyl lipid A, CpG
oligodesoxynucleotides);
k) Thalidomide analogues (for example Lenalidomide, Pomalidomide);
1) Indoleamin-2,3-Dioxgenase (IDO) and/or Tryptophane-2,3-Dioxygenase
(TDO) inhibitors (for example
RG6078 / NLG919 / GDC-0919; lndoximod / 1MT (1-methyltryptophan), INC6024360 /
Epacadostat, PF-
06840003 (E0S200271), F001287);
m) Activators of T-cell co-stimulatory receptors (for example anti-OX40/CD134
(Tumor necrosis factor
receptor superfamily, member 4, such as RG7888 (M0XR0916), 9612; MEDI6469,
GSK3174998,
MED10562), anti 0X40-Ligand/CD252; anti-glucocorticoid-induced TNFR family
related gene (GITR) (such
as TRX518, MEDI1873, MK-4166, BMS-986156), anti-CD40 (TNF receptor superfamily
member 5)
antibodies (such as Dacetuzumab (SGN-40), HCD122, CP-870,893, RG7876, ADC-
1013, APX005M,
SEA-CD40); anti-CD4O-Ligand antibodies (such as BG9588); anti-CD27 antibodies
such as Varlilumab);
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
59
n)
Molecules binding a tumor specific antigen as well as a 1-cell surface marker
such as bispecific antibodies
(for example RG7802 targeting CEA and CD3) or antibody fragments, antibody
mimetic proteins such as
designed ankyrin repeat proteins (DARPINS), bispecific 1-cell engager (BITE,
for example AMG103,
AMG330);
o) Antibodies or small molecular weight inhibitors targeting colony-
stimulating factor-1 receptor (CSF-1R) (for
example Emactuzumab (RG7155), Cabiralizumab (FPA-008), PLX3397);
p) Agents targeting immune cell check points on natural killer cells such as
antibodies against Killer-cell
immunoglobulin-like receptors (KIR) for example Lirilumab (IPH2102/BMS-
986015);
q) Agents targeting the Adenosine receptors or the ectonucleases CD39 and CD73
that convert ATP to
Adenosine, such as MEDI9447 (anti-CD73 antibody), PBF-509; CPI-444 (Adenosine
A2a receptor
antagonist).
When used in combination with the present compounds, immune checkpoint
inhibitors, and especially those
targeting the PD-1 receptor or its ligand PD-L1, are preferred.
The invention further relates to a method of modulating (especially
downregulating) the consequences of the
complement activation (especially by activating innate cells) in a subject in
need thereof [especially in a subject
having a disease or disorder related to pathogenic events associated with
elevated levels of C5a and/or with C5aR
activation; in particular in a subject having a vasculitic disease or
disorder, an inflammatory disease or disorder
involving intravascular microvesicle release, an immune complex (IC) disease
or disorder, a neurodegenerative
disease or disorder, a complement related inflammatory disease or disorder, a
bullous disease or disorder, a
disease or disorder related to ischemia and/or ischemic reperfusion injury, an
inflammatory bowel disease or
disorder, or an autoimmune disease or disorder; or in a subject having a
contact sensitivity or an inflammation
caused by contact with artificial surfaces; an increased leukocyte and
platelet activation (and infiltration to tissues
thereof); a pathologic sequelae associated to an intoxication or an injury
such as a trauma, an hemorrhage, a
shock, or surgery including transplantation, including multiple organ failure
(MOF), septic shock, shock due to
intoxication (such as shock due to snake venom), or acute lung inflammatory
injury; a pathologic sequelae
associated with insulin-dependent diabetes mellitus; a myocardial infarction
or thrombosis; an edema or an
increased capillary permeability; or a reduction of coronary endothelial
dysfunction induced by cardiopulmonary
bypass and/or cardioplegia], comprising administering to said subject a
pharmaceutically active amount of a
compound of formula (I), (II) and (III) as defined in any one of embodiments
1) to 25). For avoidance of doubt, the
term "modulating the complement activation" is to be understood as
downregulating / reducing the amplification of
the immune response and downregulating / reducing the activation of the cell-
killing membrane attack complex,
especially by activating innate cells.
Preparation of compounds of formula (I):
The compounds of formula (I), (II) and (III) can be prepared by the methods
given below, by the methods given in
the experimental part below or by analogous methods. Optimum reaction
conditions may vary with the particular
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
reactants or solvents used, but such conditions can be determined by a person
skilled in the art by routine
optimisation procedures. In the schemes below, the generic groups Ring A, R15
R1a5 R14a, R141o5 X145 R25 R2a5 X215 R35
R45 R415 R41a, R411o5 X415 R42a, R421o5 R43a, R43b are as defined for the
compounds of formula (I), (II) and (III). In some
instances, the generic groups Ring A, R15 R1a5 R14a, R141o5 X145 R25 R2a5 X215
R35 R45 R415 R41a, R411o5 X415 R42a, R421o5
5 R43a, R43b may be incompatible with the assembly illustrated in the
schemes, or will require the use of protecting
groups (PG). The use of protecting groups is well known in the art (see for
example "Protective Groups in Organic
Synthesis", T.W. Greene, P.G.M. Wuts, Wiley-lnterscience, 1999). For the
purposes of this discussion, it will be
assumed that such protecting groups as necessary are in place. In some cases,
the final product may be further
modified, for example, by manipulation of substituents to give a new final
product. These manipulations may
10 include, but are not limited to, reduction, oxidation, alkylation,
acylation, and hydrolysis reactions which are
commonly known to those skilled in the art. The compounds obtained may also be
converted into salts, especially
pharmaceutically acceptable salts in a manner known per se.
Compounds of structure la can be prepared according to the synthetic route
given in scheme A below.
Compounds of structure A-1 can be prepared by aromatic nucleophilic
substitution of amine of structure BB-1 on
15 halides of structure BB-2 wherein X represents fluorine or chlorine in
the presence of a suitable base such as
DIPEA and heating in a suitable solvent such as DMSO at temperatures between
80 C and 100 C (Scheme A,
step a).
Diamino compounds of structure A-2 can be prepared by reduction of the nitro
group in compounds of structure A-
1 using standard conditions such as catalytic hydrogenation with a suitable
catalyst such as Pd/C and in the
20 presence of a suitable solvent such as Et0Ac and under a hydrogen
atmosphere. Alternatively, reduction of the
nitro group in compounds of structure A-1 may be performed by treatment with
zinc dust and ammonium formate in
solvents such as Me0H at temperatures around 0 C (Scheme A, step b).
Cyclic ureas of structure A-3 can be prepared by cyclisation of diamines of
structure A-2 by treatment with a
suitable carbonyl transfer agent such as CDI in the presence of a suitable non
protic solvent such as MeCN at
25 temperatures between RT and 45 C (Scheme A, step c).
Alkylation of the nitrogen atom having a free valency in compounds of
structure A-3 with a suitable halide of
structure BB-3 wherein X represents chlorine or bromine, in the presence of a
suitable base such as NaH or K2CO3
and in solvents such as THF, DMF or a mixture of both at temperatures between
0 C and 50 C may afford
compounds of structure la (Scheme A, step d).
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
61
Scheme A
,Z X
Y- I ,Boc Boc
,Boc
X
NO2
,Boc R4
BB-2 yõZ NH ,Z NH
I
H2N a NO2 Xb X.NH2
BB-1 R4 R4 R4
A-1 A-2 A-3
R3 ,Boc
41130
X R-
BB-3 y
X
R4 LR2
R3
la
Alternatively, compounds of structure la can be prepared according to the
synthetic route given in scheme B below.
Compounds of structure B-1 can be prepared by aromatic nucleophilic
substitution of amine of structure BB-5 on
halides of structure BB-6 wherein X represents fluorine or chlorine in the
presence of a suitable base such as
DIPEA and heating at temperatures between 150 C and 180 C (Scheme B, step a).
Reduction of the nitro group in compounds of structure B-1 using standard
conditions such as treatment by zinc
dust and ammonium formate in solvents such as Me0H at temperatures around 0 C
may afford diamines of
structure B-2. Alternatively, reduction of the nitro group in compounds of
structure B-1 may be performed by
catalytic hydrogenation with a suitable catalyst such as Pd/C in the presence
of a suitable solvent such as Et0Ac
and under a hydrogen atmosphere (Scheme B, step b).
Cyclisation of diamines of structure B-2 to form cyclic urea of structure B-3
(Scheme B, step c) can be performed
following conditions as those previously described for the synthesis of
compounds of structure A-3 (Scheme A,
step c).
Alkylation of the nitrogen atom having a free valency in compounds of
structure B-3 can be achieved using
Mitsunobu conditions by treatment with a suitable alcohol of structure BB-7
and for instance a
(cyanomethylene)trialkylphosphorane reagent in the presence of a suitable
solvent such as toluene at
temperatures around 110 C (Scheme B, step d).
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
62
Scheme 13
Z NO2
xx
R2 R4 BB-6 y N 02 y ,Z NH2
,Z N
y
FI2N-K I I I I I I
C.,
R3 a X )NH X X N
Ra LR2 R4 R2 R4
R2
BB-5 R3 R3 R3
B-1 B-2 B-3
,Boc
o
0 Boc
HO
BB-7
I
X y'N
R4 )--R2
R3
la
Compounds of structure lb, lc, Ig, lh, Ii, lq and lag can be prepared
according to the synthetic route given in
scheme C below.
Cleavage of the Boc protecting group in compounds of formula la can be
performed using a suitable acid such as
HCI or TFA in the presence of a suitable solvent such as dioxane, Me0H or DCM
at temperatures around RI
(Scheme C, step a).
Compounds of structure lb wherein R1 represents an unsubstituted, mono-, di-
or tri-substituted phenyl or 5- or 6-
membered heteroaryl can be prepared by Buchwald-Hartwig cross coupling of
halides of structure R1-X wherein X
represents iodine, bromine or chloride and R1 represents an unsubstituted,
mono-, di- or tri-substituted phenyl or 5-
or 6-membered heteroaryl with an amine of structure C-1 in the presence of a
suitable palladium catalyst such as
Pd2(dba)3 and a ligand such as BINAP, in the presence of a suitable base such
as sodium tert-butoxide and
heating in a suitable solvent such as toluene at temperatures between 100 C
and 110 C (Scheme C, step b).
Compounds of structure lb wherein R1 represents an unsubstituted, mono- or di-
substituted benzyl can be
prepared by reductive amination of benzaldehyde of structure Rla-CHO wherein
Rla represents a mono- or di-
substituted phenyl with an amine of structure C-1 using standard conditions
such as treatment with NaBH(OAc)3 in
the presence of AcOH and a suitable solvent such as DCM or THF at temperatures
around RI (Scheme C, step b).
Compounds of structure lc wherein R1 represents an unsubstituted, mono-, di-
or tri-substituted phenyl or 5- or 6-
membered heteroaryl can be prepared by aromatic nucleophilic substitution of
amines of structure C-1 on activated
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
63
halides of structure R1-X wherein X represents fluorine or chlorine and R1
represents a suitable unsubstituted,
mono-, di- or tri-substituted phenyl or 5- or 6- membered heteroaryl in the
presence of a suitable base such as CsF
or K2CO3 and heating in a suitable solvent such as DMSO at temperatures
between 60 C and 110 C (Scheme C,
step b).
Alternatively, compounds of structure lc wherein R1 represents a 5- or 6-
membered heteroaryl can be prepared by
aromatic nucleophilic substitution of amines of structure C-1 on alcohol of
structure R1-X wherein X represents
hydroxy and R1 represents a suitable 5- or 6- membered heteroaryl by
activation of the corresponding alcohol with
PyBOP in the presence of a suitable base such as DIPEA in solvents such as DMF
at temperatures around RI
(Scheme C, step b).
.. Compounds of structure Ig wherein R1 represents a (Ci4alkoxy-carbonyl,
phenoxy-carbonyl or (C3_6)cycloalkoxy-
carbonyl can be prepared by treatment of amines of structure C-1 with
chloroformate (or
pentafluorophenylcarbonate, respectively) of structure R1-X wherein R1
represents (Ci4alkoxy-carbonyl, phenoxy-
carbonyl or (C3_6)cycloalkoxy-carbonyl and X represents chlorine (or X
represents pentafluorophenoxy,
respectively) in the presence of a suitable base such as TEA and in a suitable
solvent such as DCM or DMF at
temperatures between RI and 110 C (Scheme C, step b).
Compounds of structure lh wherein R1 represents a (C1_4)alkyl-carbonyl, phenyl-
carbonyl or (C3_6)cycloalkyl-
carbonyl optionally containing one ring oxygen and optionally mono-substituted
with methyl or trifluoromethyl can
be prepared by treatment of amines of structure C-1 with acid chlorides of
structure R1-X wherein R1 represents
(C1_4)alkyl-carbonyl, phenyl-carbonyl or (C3_6)cycloalkyl-carbonyl optionally
containing one ring oxygen and
optionally mono-substituted with methyl or trifluoromethyl and X represents
chlorine in the presence of a suitable
base such as TEA and in a suitable solvent such as DCM at temperatures between
0 C and RI (Scheme C, step
b).
Compounds of structure Ii wherein R1 represents (C1_4)alkyl can be prepared by
reductive amination of a suitable
aldehyde of structure Rla-CHO wherein Rla represents hydrogen or (C1_3)alkyl,
acetone or butanone with amines of
structure C-1 using standard conditions such as treatment with NaBH(OAc)3 in
the presence of AcOH and a
suitable solvent such as DCM, Me0H, THF or mixture thereof at temperatures
around RI (Scheme C, step b).
Alternatively, triethylsilane in solvents such as Me0H in the presence of
catalytic amount of indium chloride (III) at
temperatures around RI can be used as reductive conditions. Alternatively,
alkylation of amines of structure C-1
with halides of structure R1-X wherein X represents chlorine, bromine or
iodine in the presence of a suitable base
such as K2CO3 and a suitable solvent such as MeCN at temperatures between RI
and 65 C may afford
compounds of structure Ii (Scheme C, step b).
Compounds of structure C-2 can be prepared by alkylation of carbamates of
structure BB-10 by treatment with an
appropriate halide of formula BB-3 wherein X represents chlorine or bromine in
the presence of a suitable base
such as K2CO3 and carrying out the rxn in a suitable solvent such as MeCN at
temperatures around 80 C (Scheme
C, step c).
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
64
Compounds of formula lag can be prepared by Buchwald-Hartwig cross coupling of
halides of formula C-2 with
amines of formula BB-4 by treatment with a suitable palladium catalyst such as
BrettPhos precatalyst and a
suitable ligand such as BrettPhos, in the presence of a suitable base such as
sodium tert-butoxide and heating in a
suitable solvent such as dioxane at temperatures around 80 C. Using those
conditions, the consecutive cyclisation
to form the cyclic urea occurred and afforded the compounds of formula lag
(Scheme C, step d).
Compounds of structure C-3 can be prepared by aromatic nucleophilic
substitution of amine of structure BB-4 on
halides of structure BB-2 wherein X represents fluorine or chlorine in the
presence of a suitable base such as
DIPEA and heating in a suitable solvent such as DMSO at temperatures between
50 C and 105 C (Scheme C,
step e).
Diamino compounds of structure C-4 can be prepared by reduction of the nitro
group in compounds of structure C-
3 using standard conditions such as catalytic hydrogenation with a suitable
catalyst such as Pd/C and in the
presence of a suitable solvent such as Et0Ac and under a hydrogen atmosphere.
Alternatively, the reduction of the
nitro group can be performed by treatment with ammonium formate and zinc dust
in the presence of a suitable
solvent such as Me0H at temperatures around RI (Scheme C, step f).
Cyclic urea of structure C-6 can be prepared by cyclisation of diamines of
structure C-4 by treatment with a suitable
carbonyl transfer agent such as CD I in the presence of a suitable non protic
solvent such as MeCN at temperatures
between RI and 45 C (Scheme C, step g).
Alternatively, compounds of structure C-6 can be prepared by aromatic
nucleophilic substitution of amines of
structure C-5 on halides of structure BB-8 wherein X represents fluorine or
chlorine in the presence of a suitable
base such as K2CO3 and heating in a suitable solvent such as DMSO at
temperatures around 100 C (Scheme C,
step i). Compounds of structure C-5 can be prepared by cleavage of the Boc
protecting group in compounds of
formula A-3 using a suitable acid such as HCI or TFA in the presence of a
suitable solvent such as dioxane, Me0H
or DCM at temperatures around RI (Scheme C, step h).
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
Scheme C
,Boc
0 OH
I I>=
Xy-N a X y-N
R1-X
.4
" or
R1 a-CHO
la C-1
b BB-8
R2 ,R1 ,R1
X--(
0 GO
R3
,Z Br^ ,ZBro
y- %., H2N
' I A
Xy-N OMe BB-3 __ õ NI 1 A
OMe _______________________________________________________ I
...- ..- BB-4
^
..- I 0
H c d
D Xy-N
R4 4 )---.D2
" R3 " R4 )--- R2
R3
BB-10 C-2
lb, c, g, h, i, q, ag
R2
k jX
0 0 I x--
-(R3
R1
.-.1 No2 i
CIO BB-2
R4 ,Z NH .Z NH
y- ,
X Y
- k j BB-3
H2N e T No2 f NH2
BB-4
R4 C-3 R4 ,RI
C-4
\ CIO
i I 0
R1-X y "14
,Boc 1 H
0 OH BB-8/ R4
C-6
,Z N
Yj o - ;,- I o
x. N h ,, y----N
H ,, H
R4 R-
A-3 C-5
Compounds of structure Id to If, lj to 1p, lu to lz and lae can be prepared
according to the synthetic route given in
scheme D below.
5 Alkylation of the nitrogen atom having a free valency in compounds of
structure C-6 with a suitable halide of
structure BB-3 wherein X represents chlorine or bromine, in the presence of a
suitable base such as NaH or K2CO3
and in solvents such as THF, DMF or a mixture of both at temperatures between
0 C and 50 C may afford
compounds of structure lq (Scheme C, step j). Alternatively, alkylation of the
nitrogen atom having a free valency in
compounds of structure C-6 can be achieved using Mitsunobu conditions by
treatment with a suitable alcohol of
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
66
structure BB-3 wherein X represents hydroxy and for instance a
(cyanomethylene)trialkylphosphorane reagent in
the presence of a suitable solvent such as toluene at temperatures around 110
C (Scheme C, step j).
Compounds of structure Id wherein R1 represents a 5- or 6- membered heteroaryl
with at least one unsubstituted
position can be prepared by decarbonylation of compounds of formula lb or lc
wherein R1 represents a 5- or 6-
membered heteroaryl which is substituted by a formyl group by treatment with a
suitable acid such as toluene-4-
sulfonic acid and in the presence of a suitable solvent such as Me0H under
microwave conditions at temperatures
around 120 C (Scheme D, step a).
Compounds of structure If wherein R1 represents a 5- or 6- membered heteroaryl
with at least one methyl
substituent can be prepared by Wolff-Kishner type reduction of compounds of
formula lb or lc wherein R1
represents a 5- or 6- membered heteroaryl which is substituted by one formyl
group. The treatment with 4-toluene-
sulfonyl hydrazide in the presence of toluene-4-sulfonic acid monohydrate with
a suitable solvent such as a mixture
of DMF and tetramethylene sulfone at temperatures around 100 C affords the
hydrazone intermediate which is
consecutively reduced by treatment with sodium cyanoborohydride at
temperatures around 100 C (Scheme D,
step a).
Compounds of structure lj wherein R4 represents hydroxy can be prepared by
treatment of suitable compounds of
formula lb and lc wherein R4 represents methoxy with a suitable Lewis acid
such as boron tribromide in the
presence of a suitable solvent such as DCM at temperatures between -10 C and
RT (Scheme D, step a).
Compounds of structure Im wherein R1 represents a hydroxymethyl substituted
phenyl or 5- or 6- membered
heteroaryl can be prepared by treatment of compounds of formula lb and lc
wherein R1 represents a formyl
substituted phenyl or 5- or 6- membered heteroaryl by treatment with a
suitable reducing reagent such as NaBHa in
the presence of a suitable solvent such as Me0H or THF at temperatures between
0 C and RT (Scheme D, step
a).
Compounds of structure In wherein R1 represents a 1-hydroxy-(C2-C3)-alkyl
substituted phenyl or 5- or 6-
membered heteroaryl can be prepared by treatment of compounds of formula lb
and lc wherein R1 represents a
formyl substituted phenyl or 5- or 6- membered heteroaryl by treatment with a
suitable Grignard reagent in the
presence of a suitable solvent such as THF at temperatures between 0 C and RT
(Scheme D, step a).
Compounds of structure lu wherein R1 represents a R14aR14bN_x14_ substituted
phenyl, 5- or 6-membered
heterocycle wherein X14 represents a methylene and Rua and Rub independently
represent hydrogen, (C1_4)alkyl,
hydroxy-(C24alkyl, (C1_3)alkoxy-(C2_4)alkyl; or R14a and Rub together with the
nitrogen to which they are attached to
form a 4- to 6-membered saturated ring optionally containing one additional
ring heteroatom selected from oxygen
and nitrogen; and wherein said ring is optionally mono-substituted with
(C1_4)alkyl can be prepared by reductive
amination of compounds of formula lb or lc wherein R1 represents a formyl
substituted phenyl or 5- or 6-
membered heteroaryl using standard conditions such as treatment with
NaBH(OAc)3 in the optional presence of
AcOH and a suitable solvent such as DCM or THF at temperatures around RT
(Scheme D, step a).
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
67
Compounds of structure le wherein R1 represents a chlorine substituted 5- or 6-
membered heteroaryl can be
prepared by chlorination of compounds of formula Id wherein R1 represents a 5-
or 6- membered heteroaryl with
one unsubstituted position by treatment with a chlorinating reagent such as
NCS in the presence of a suitable
solvent such as THF at temperatures around RI (Scheme D, step b).
Compounds of structure lk wherein X and Z represents CH and R4 represents ¨0-
R41 wherein R41 represents
(C1_4)alkyl, (C24)alkyl which is substituted with one or two hydroxyl,
R4laR4MN¨(C2_3)alkylene-, wherein R41a and R41b
independently represent hydrogen or (C1_4)alkyl; or (C4_7)heterocyclyl-X41-,
wherein X41 represents a direct bond or
(C1_3)alkylene, and wherein the (C4_7)heterocycly1 independently contains one
or two ring heteroatoms
independently selected from nitrogen and oxygen; wherein said
(C4_7)heterocycly1 independently is unsubstituted,
or mono-, or di-substituted, wherein the substituents are independently
selected from (Ci4alkyl or (C1_4)alkoxy-
carbonyl attached to a ring nitrogen atom having a free valency can be
prepared by alkylation of compounds of
formula lj wherein X and Z represents CH and R4 represents hydroxyl by
treatment with an appropriate halide such
as R41-W wherein W represents chlorine or bromine in the presence of a
suitable base such as K2CO3 and carrying
out the rxn in a suitable solvent such as DMSO at temperatures between 50 and
105 C (Scheme D, step b).
Compounds of structure lo wherein R1 represents a 1-oxo-(C2-05)-alkyl
substituted phenyl or 5- or 6- membered
heteroaryl can be prepared by oxidation of compounds of formula In wherein R1
represents a (Ci-C4)-alkyl-
hydroxymethyl substituted phenyl or 5- or 6- membered heteroaryl by treatment
with an oxidizing reagent such as
DMP in the presence of a suitable solvent such as DCM at temperatures around
RI (Scheme D, step b).
Compounds of structure le can be prepared by Suzuki cross coupling of
chlorides of structure le wherein R1
represents a chlorine substituted 5- or 6-membered heteroaryl with a (Ci-C4)-
alkyl boronic acid or boroxine in the
presence of a suitable palladium catalyst such as PEPPSI-IPr, in the presence
of a suitable base such as K2CO3
and heating in a suitable solvent such as dioxane at temperatures between 115
C (Scheme D, step c).
Compounds of structure 1p wherein R1 represents a 1-hydroxy-1-methyl-ethyl
substituted phenyl or 5- or 6-
membered heteroaryl can be prepared by treatment of compounds of formula lo
wherein R1 represents an acetyl
.. substituted phenyl or 5- or 6- membered heteroaryl by treatment with a
suitable Grignard reagent in the presence of
a suitable solvent such as THF at temperatures between 0 C and RI (Scheme D,
step c).
Compounds of structure Ix wherein X and Z represents CH and R4 represents a
diol containing substituent
(respectively a substituent containing a nitrogen atom having a free valency)
can be prepared by acidic
deprotection of compounds of structure lk wherein R4 represents a substituent
which contains a ketal protected diol
(respectively a substituent containing a Boc protected nitrogen) in the
presence of a suitable acid such as aqueous
HCI (respectively TFA) and a suitable solvent such as THF (respectively DCM)
at temperatures between 0 C and
RI (Scheme D, step c).
Alkylation of the free hydroxyl group in compounds of structure Im wherein R1
represents a hydroxymethyl
substituted phenyl or 5- or 6- membered heteroaryl or in compounds of
structure In wherein R1 represents a 1-
hydroxy-(C2-C3)-alkyl substituted phenyl or 5- or 6- membered heteroaryl with
a suitable (Ci-C4)-alkyl halide, in the
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
68
presence of a suitable base such as NaH and in solvents such as THF, DMF or a
mixture of both at temperatures
between 0 C and 50 C may afford compounds of structure lz (Scheme D, step d).
Compounds of structure lae wherein RI represents a RuaRubN_cH (mo,
R14aR14bN_CH(Et)-, or RuaRi4bN_c(me)2_
substituted phenyl or 5- or 6- membered heteroaryl wherein Rua and Rub
independently represent hydrogen,
(Ci_4)alkyl, hydroxy-(C24alkyl, (C1_3)alkoxy-(C2_4)alkyl; or Rua and Rub
together with the nitrogen to which they are
attached to form a 4- to 6-membered saturated ring optionally containing one
additional ring heteroatom selected
from oxygen and nitrogen; and wherein said ring is optionally mono-substituted
with (Ci4alkyl (especially methyl),
hydroxy, (Ci_4)alkoxy (especially methoxy), or dimethylamino can be prepared
following a two-step procedure: (i)
chlorination by treatment of compounds of formula In wherein R1 represents a 1-
hydroxy-(C2-C3)-alkyl substituted
phenyl or 5- or 6- membered heteroaryl with a chlorinating reagent such as
50Cl2 in the presence of a suitable
solvent such as DCM at temperatures around RI and (ii) consecutive
nucleophilic substitution with amines of
formula RuaRubNH wherein Rua and Rub independently represent hydrogen,
(C1_4)alkyl, hydroxy-(C2_4)alkyl, (C1-
3)alkoxy-(C2_4)alkyl; or Rua and Rub together with the nitrogen to which they
are attached to form a 4- to 6-
membered saturated ring optionally containing one additional ring heteroatom
selected from oxygen and nitrogen;
and wherein said ring is optionally mono-substituted with (Ci4alkyl
(especially methyl), hydroxy, (C1_4)alkoxy
(especially methoxy), or dimethylamino in the presence of a base such as TEA
and a suitable solvent such as DCM
at temperatures between 0 C and RI (Scheme D, step e).
Compounds of structure D-1 can be prepared by chlorination of compounds of
structure lj wherein R4 represents
hydroxyl, Y represents CR5 wherein R5 represents hydrogen or (Ci-C3)alkyl and
one of X and Z or both represents
N and the possible other one represents CR5 wherein R' represents hydrogen or
(Ci-C3)alkyl, using a neat
chlorinating reagent such as P0CI3 and heating at temperatures around 85 C
(Scheme D, step f).
Compounds of structure Iv wherein R5 represents hydrogen or (Ci-C3)alkyl, one
of X and Z or both represents N
and the possible other one represents CR5 wherein R' represents hydrogen or
(Ci-C3)alkyl and R4 represents ¨
NR42aR42b wherein R42a and R42b independently represent hydrogen, (Ci4alkyl,
(C1_3)alkoxy-(C2_3)alkyl, hydroxy-
(C23)alkyl, or R42 and R42b together with the nitrogen to which they are
attached form a 4- to 7-membered saturated
ring optionally containing one further ring heteroatom selected from oxygen
and nitrogen, wherein said ring is
unsubstituted or mono-substituted with (C1_3)alkyl (especially methyl), or
(C1_3)alkoxy (especially methoxy) can be
prepared by aromatic nucleophilic substitution of neat amines of structure
FINR42aR42b wherein R42a and R42b are
defined above on compounds of structure D-1 at temperatures between 100 and
140 C. Alternatively, Buchwald-
Hartwig cross coupling conditions can be used by treatment with a suitable
palladium catalyst such as RuPhos
precatalyst and a suitable ligand such as RuPhos, in the presence of a
suitable base such as Cs2CO3 and heating
in a suitable solvent such as t-BuOH at temperatures around 110 C (Scheme D,
step g).
Compounds of structure Iv wherein R5 represents hydrogen or (Ci-C3)alkyl, one
of X and Z or both represents N
and the possible other one represents CR5 wherein R5 represents hydrogen or
(Ci-C3)alkyl and R4 represents -0-
R41 wherein R41 represents (Ci4alkyl (especially methyl); (C24)alkyl which is
substituted with one or two hydroxy
(especially 2-hydroxyethyl, 2,3-dihydroxypropyl); R4laR4lbN¨(C2_3)alkylene-,
wherein R4la and Rob independently
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
69
represent hydrogen or (Ci4alkyl (especially such group is 2-dimethylamino-
ethyl); or (C4_7)heterocyclyl-X41-,
wherein X41 represents a direct bond or (C1_3)alkylene, and wherein the
(C4_7)heterocycly1 independently contains
one or two ring heteroatoms independently selected from nitrogen and oxygen;
wherein said (C4_7)heterocycly1
independently is unsubstituted, or mono-, or di-substituted, wherein the
substituents are independently selected
from (Ci4alkyl (especially methyl); and/or (C1_4)alkoxy-carbonyl attached to a
ring nitrogen atom having a free
valency can be prepared by aromatic nucleophilic substitution of alcohol of
structure H0R41 wherein R41 is defined
above on compounds of structure D-1 in the presence of a suitable base such as
KOH and heating in a suitable
solvent such as DMSO at temperatures around 50 (Scheme D, step g).
Compounds of structure Ix wherein R5 represents hydrogen or (Ci-C3)alkyl, one
of X and Z or both represents N
and the possible other one represents CR5 wherein R5 represents hydrogen or
(Ci-C3)alkyl and R4 represents a diol
containing substituent (respectively a substituent containing a nitrogen atom
having a free valency) can be
prepared by acidic deprotection of compounds of structure Iv wherein R4
represents a substituent which contains a
ketal protected diol (respectively a substituent containing a Boc protected
nitrogen) in the presence of a suitable
acid such as aqueous HCI (respectively TFA) and a suitable solvent such as THF
(respectively DCM) at
temperatures between 0 C and RI (Scheme D, step h).
Compounds of structure D-2 can be prepared by Wittig type rxn between
compounds of formula lc wherein R1
represents a formyl substituted phenyl and (methoxymethyl)triphenylphosphonium
chloride by treatment with a
strong base such as n-butyllithium in the presence of a suitable solvent such
as THF at temperatures around -78 C
(Scheme D, step i).
Compounds of structure lw can be prepared by acidic hydrolysis of enol ether
of structure D-2 in the presence of a
suitable acid such as aqueous HCI and heating in a suitable solvent such as
THF at temperatures around 70 C
(Scheme D, step j).
Compounds of structure ly wherein R1 represents a R14aR14bN_x14_ substituted
phenyl, wherein X14 represents a
ethylene; and wherein Rua and R14b independently represent hydrogen,
(C1_4)alkyl, hydroxy-(C24)alkyl, (C1_3)alkoxy-
(C24)alkyl; or Rua and R14b together with the nitrogen to which they are
attached to form a 4- to 6-membered
saturated ring optionally containing one additional ring heteroatom selected
from oxygen and nitrogen; and wherein
said ring is optionally mono-substituted with (Ci4alkyl (especially methyl),
hydroxy, (C1_4)alkoxy (especially
methoxy), or dimethylamino can be prepared by reductive amination of compounds
of structure lw with amines of
structure R14aR14bNH wherein R14a and R14b are defined above using standard
conditions such as treatment with
NaBH(OAc)3 in the presence of AcOH and a suitable solvent such as DCM or THF
at temperatures around RI
(Scheme D, step k).
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
Scheme D
,RI
0
,
Y -Z ---1%1
, I
y 0
- y---N
R4 R3)--.R2
lb, c, g, h, i
a/ \
,R1 R1
,
C110 0 Me0
_am
IV
, b
i I 0 i
y y
-N ,..;;.,..c-N f
R4 )--.R2 R4 )--...R2 0 0
R3 R3 R5 Z N
Y I>=0 I I 0
le, k, o Id, f, j, m, n, u X y-N X y-N
CI R3 R4
)-R2 R3)----R2
c I D-2
D-1
,R1
0
N
d/ \ R5
g I i I 0
\
,RI
4
yi I 0
0
-y----
0
Z N
R4 kR2
,R1 ,RI x -y-N
I I>=o
II, p, x
0 0 Rit R3)--R2
X y-N
Iv R4 )--R2
Y
R3
I I>=0 i I 0
Xy-N - y----N 1W
X
D4 R3" L042 R4 R3 L R2 hi
" ,R1
lz lae 0 k I
R14a
R5 Z
X.. N
R14b_Ni
1 0
_,,,,N
R4 ---.R2 .
R3
Ix 0
,Z N
1 j
N ly
R4 LR2
R3
Compounds of structure Ir to It and laf can be prepared according to the
synthetic route given in scheme E below.
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
71
The chlorine or bromine atom on either R1 or R2 which represent a phenyl or 5-
or 6-membered heteroaryl or when
X, Y or Z represents C-CI or C-Br in compounds of structure lq can be replaced
by one hydrogen atom
(respectively by one deuterium atom) by catalytic hydrogenation (respectively
deuteration) using a suitable catalyst
such as Pd/C in the presence of a suitable solvent such as Et0Ac, Me0H or a
mixture of both and under a
hydrogen (respectively a deuterium) atmosphere at temperatures around RT. That
transformation provides
compounds of structure Ir. Catalytic transfer hydrogenation conditions using
for instance ammonium formate can
be an alternative procedure (Scheme E, step a).
Compounds of structure lq wherein R4 represents methoxycarbonyl can be
transformed to compounds of formula
E-1 by hydrolysis with a suitable base such as Li0H, NaOH or KOH in the
presence of water and a suitable organic
solvent such as THF, Me0H or Et0H or a mixture of them at temperatures between
RT and 50 C (Scheme E, step
b).
Compounds of structure Is can be prepared by amide coupling of carboxylic
acids of structure E-1 with amines of
structure R43aR43bNH wherein R43a and R43b independently represents hydrogen,
(Ci_4)alkyl, (Ci_3)alkoxy-(C2_3)alkyl,
or hydroxy-(C2_3)alkyl using standard amide coupling reagent such as
EDC.HCl/HOBt, HATU or PyBOP in the
.. presence of a suitable base such as DIPEA or Et3N and in a suitable solvent
such as DCM or DMF at temperatures
around RT (Scheme E, step c).
Compounds of structure It can be prepared by reduction of compounds of
structure lq wherein R4 represents
methoxycarbonyl by treatment with a suitable reducing reagent such as CaBH4
(formed in situ from NaBHa and
CaCl2) in the presence of a suitable solvent such as Et0H at temperatures
between 0 C and RT (Scheme E, step
d).
Compounds of structure lq wherein R2 represents an acetoxy substituted phenyl
or 5- or 6-membered heteroaryl
can be transformed to compounds of formula E-2 wherein R2 represents an
hydroxy substituted phenyl or 5- or 6-
membered heteroaryl by hydrolysis with a suitable base such as Li0H, NaOH or
KOH in the presence of water and
a suitable organic solvent such as THF, Me0H or Et0H or a mixture of them at
temperatures around RT (Scheme
E, step e).
Compounds of structure laf wherein R2 represents a (Ci4alkoxy or
(C3_6)cycloalkyl-X21- substituted phenyl, wherein
)(21 represents a -0-, or -(C1_3)alkylene-0-, and wherein the (C3_6)cycloalkyl
independently contains one optional
ring oxygen can be prepared by 0-alkylation of compounds of structure E-2
wherein R2 represents an hydroxy
substituted phenyl with halogenide of formula R2a-X wherein X represents
chlorine or bromine and R2a represents
(C1_4)alkyl, (C3_6)cycloalkyl or (C3_6)cycloalkyl-(C1_3)alkylene-, in the
presence of a suitable base such as NaH or
K2CO3 and in solvents such as THF, DMF or a mixture of both at temperatures
between 0 C and 100 C (Scheme
E, step f).
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
72
Scheme E
,R1
,R1 ,
aR.1
0 ,Z N
T"
-...õ- ,Z N 1 o
. 0 0
.,_ 1 I 0
e
XjNC)
X y----N
RR 3R
R3 IN R3 IN
lq E-2
Ir
R.1
R4=COOMe
b
d R4=COOMe
f 1
R1
, ,R1 ,R.1
0
0
k N
j'
Ix( -(:NO xVI N XjN
Ra L R2
R43a L. , Lri2 HO
0R3 )---R2 R3
¨N 0 rx
HO R3 IR` '..... R3
I R43b It laf
E-1
Is
Compounds of structure laa, lab, lac, lad, lah, lai and laj can be prepared
according to the synthetic route given in
scheme F below.
Compounds of structure lah wherein R1 represents a hydroxy substituted phenyl
or 5- or 6-membered heteroaryl
can be prepared by hydrolysis of the acetyl protecting group (respectively by
cleavage of the benzyl protecting
group) on compounds of formula lc wherein R1 represents a suitable acetoxy
(respectively benzyloxy) substituted
phenyl or 5- or 6-membered heteroaryl with a suitable base such as Li0H, NaOH
or KOH in the presence of water
and a suitable organic solvent such as THF, Me0H or Et0H or a mixture of them
(respectively by catalytic
hydrogenation using a suitable catalyst such as palladium hydroxide on carbon
under a hydrogen atmosphere in
the presence of a suitable solvent such as Et0Ac) at temperatures around RI
(Scheme F, step a).
Alkylation of the free hydroxyl group in compounds of structure lah wherein R1
represents a hydroxy substituted
phenyl or 5- or 6-membered heteroaryl with a suitable halide of structure R15-
X wherein X represents bromine or
chlorine and R15 represents (Ci-C4)-alkyl, hydroxy-(C2_3)alkyl, (C1_4)alkoxy-
(C2_3)alkyl, (C3_6)cycloalkyl, or (C3_
6)cycloalkyl-(Ci_3)alkyl in the presence of a suitable base such as NaH or
K2CO3 and in solvents such as THF, DMF
or a mixture of both at temperatures between 0 C and 80 C may afford compounds
of structure lai wherein R1
represents a (Ci4alkoxy, hydroxy-(C2_3)alkoxy, or (C3_6)cycloalkyl-X12-
substituted phenyl or 5- or 6-membered
heteroaryl, wherein X12 represents a -0-, or -(C1_3)alkylene-0-, and wherein
the (C3_6)cycloalkyl independently
contains one optional ring oxygen (Scheme F, step b). Alternatively,
alkylation of the free hydroxyl group in
compounds of structure lah wherein R1 represents a hydroxy substituted phenyl
or 5- or 6-membered heteroaryl
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
73
can be achieved using Mitsunobu conditions by treatment with a suitable
alcohol of structure R15-0H wherein R15
represents (Ci-C4)-alkyl, hydroxy-(C2_3)alkyl, (Ci_4)alkoxy-(C2_3)alkyl,
(C3_6)cycloalkyl, or (C3_6)cycloalkyl-(C1_3)alkyl
and for instance a (cyanomethylene)trialkyl phosphorane reagent in the
presence of a suitable solvent such as
toluene at temperatures around 110 C (Scheme F, step b). A possible protective
group on the substituent R15 can
be consecutively cleaved by standard deprotection conditions to provide
compounds of structure laj. Treatment for
instance by TBAF in THF at temperatures around RT can lead to deprotection of
TBDMS protected alcohol.
Compounds of structure laa wherein R1 represents an amino substituted phenyl
can be prepared by reduction of
compounds of formula lc wherein R1 represents a nitro substituted phenyl by
catalytic hydrogenation for instance
using a suitable catalyst such as Pd/C under a hydrogen atmosphere in the
presence of a suitable solvent such as
Et0Ac at temperatures around RT (Scheme F, step c).
Compounds of structure lab wherein R1 represents a bromine substituted phenyl
can be prepared by a Sandmeyer
type rxn of compounds of formula laa wherein R1 represents an amino
substituted phenyl using standard conditions
such as treatment with NaNO2 in aq. HBr at temperatures around -20 C and
consecutive treatment with CuBr at
temperatures around 60 C (Scheme F, step d).
Compounds of structure lac wherein R1 represents a R14aR14bN_X14_ substituted
phenyl wherein X14 represents 1,3-
prop-1-ynylene; and wherein R14a and Rub independently represent (C1_4)alkyl,
(C1_3)alkoxy-(C2_4)alkyl; or R14a and
Rub together with the nitrogen to which they are attached to form a 4- to 6-
membered saturated ring optionally
containing one additional ring heteroatom selected from oxygen and nitrogen;
and wherein said ring is optionally
mono-substituted with (C1_4)alkyl (especially methyl), (C1_4)alkoxy
(especially methoxy), or dimethylamino can be
prepared by Sonogashira type cross-coupling of bromides of formula lab wherein
R1 represents a bromine
substituted phenyl with R14aR14bN¨CH2-CCH wherein R14a and Rub are defined
above, in the presence of a
suitable palladium catalyst such as bis(tri-tert-butylphosphine)palladium(0),
in the presence of a suitable copper
catalyst such as copper iodide, in the presence of a suitable base such as DBU
and heating in a suitable solvent
such as THF at temperatures around 70 C (Scheme F, step e).
Compounds of structure lad wherein R1 represents a R14aR14bN_X14_ substituted
phenyl wherein X14 represents 1,3-
propylene; and wherein R14a and Rub independently represent (Ci4alkyl,
(C1_3)alkoxy-(C2_4)alkyl; or R14a and Rub
together with the nitrogen to which they are attached to form a 4- to 6-
membered saturated ring optionally
containing one additional ring heteroatom selected from oxygen and nitrogen;
and wherein said ring is optionally
mono-substituted with (C1_4)alkyl (especially methyl), (C1_4)alkoxy
(especially methoxy), or dimethylamino can be
prepared by reduction of the acethylenic group in compounds of formula lac
wherein R1 represents a R14aR14bN_
X14- substituted phenyl wherein X14 represents 1,3-prop-1-ynylene; and wherein
R14a and Rub are defined above
using standard conditions such as catalytic hydrogenation with a suitable
catalyst such as Pd/C and in the
presence of a suitable solvent such as Et0Ac and under a hydrogen atmosphere
(Scheme F, step f).
CA 03087886 2020-07-08
WO 2019/141803 PCT/EP2019/051230
74
Scheme F
,R1 ,R1¨OH R15
a
0 0 4:10
, Z N
y , .....- ,Z N b ,Z N
y, .....-
()jN1
r---N X r---N
R4 )--...R2 Rtt )---..R2 -- R4 -- -====. R2
R3 R3 R3
lc lah lai, laj
c I
,R1...--N H2 .R1,-- Br RiiN,R14a
=
0 GO 0 1
R14b
d ,Z N
y , ,......e., e v 2 N
Y - 1
v' I 0 ' ,' ,., I
,...õ1õ-----N
R4 )--..R2 R4 )--- R2 .. 4 .. )-- R2
R3 R3 RR3
laa lab lac
R14a
N/
0 \
R14b
f 2 N
_,.. 1 jN
I 0
R 4 .-- ) R2
R3
lad
If not commercially available, amines of structure BB-4 can be prepared
according to the synthetic route given in
scheme G below.
Compounds of structure G-2 wherein R1 represents an unsubstituted, mono-, di-
or tri-substituted phenyl or 5- or 6-
membered heteroaryl can be prepared by Buchwald-Hartwig cross coupling of
halides of structure R1-X wherein X
represents iodine, bromine or chloride and R1 represents an unsubstituted,
mono-, di- or tri-substituted phenyl or 5-
or 6-membered heteroaryl with an amine of structure G-1 in the presence of a
suitable palladium catalyst such as
Pd2(dba)3 and a ligand such as BINAP, in the presence of a suitable base such
as sodium tert-butoxide and
heating in a suitable solvent such as toluene at temperatures between 100 C
and 110 C (Scheme G, step a).
Alternatively, compounds of structure G-2 wherein R1 represents an
unsubstituted, mono-, di- or tri-substituted
phenyl or 5- or 6-membered heteroaryl can be prepared by aromatic nucleophilic
substitution of amines of structure
G-1 on activated halides of structure R1-X wherein X represents fluorine or
chlorine and R1 represents a suitable
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
mono-, di- or tri-substituted phenyl or 5- or 6- membered heteroaryl in the
presence of a suitable base such as
K2CO3 and heating in a suitable solvent such as DMSO at temperatures between
60 C and 110 C (Scheme G,
step a).
Cleavage of the ketal protecting group in compounds of structure G-2 by acidic
hydrolysis in the presence of a
5 suitable acid such as aq. HCI and heating in a suitable solvent such as
THF at temperatures around 70 C may
afford ketones of structure G-3 (Scheme G, step b).
Transformation of ketones of structure G-3 to amines of structure BB-4 can be
achieved by reductive amination
with for instance aq. ammonia under catalytic hydrogenation conditions using a
suitable catalyst such as Pd/C in
the presence of a suitable solvent such as dioxane under a hydrogen atmosphere
at temperatures around RT
10 (Scheme G, step c).
Compounds of structure G-5 wherein R1 represents an unsubstituted, mono-, di-
or tri-substituted phenyl or 5- or 6-
membered heteroaryl can be prepared by aromatic nucleophilic substitution of
amines of structure G-4 on activated
halides of structure R1-X wherein X represents fluorine or chlorine and R1
represents a suitable mono-, di- or tri-
substituted phenyl or 5- or 6- membered heteroaryl in the presence of a
suitable base such as K2CO3 and heating
15 in a suitable solvent such as DMSO at temperatures between 60 C and 110
C (Scheme G, step d).
Compounds of structure G-5 wherein R1 represents a mono-, di- or tri-
substituted phenyl which is substituted by
one methyl group can be prepared following a four-step procedure: (i) aromatic
nucleophilic substitution of amines
of structure G-4 on halides of structure R1-X wherein X represents fluorine or
chlorine and R1 represents a suitable
mono-, di- or tri-substituted phenyl which is substituted by one formyl group
in the presence of a suitable base such
20 .. as K2CO3 and heating in a suitable solvent such as DMSO at temperatures
between 60 C and 110 C and (ii)
consecutive reduction of the benzaldehyde derivative by treatment with a
suitable reducing reagent such as NaBHa
in the presence of a suitable solvent such as Me0H at temperatures between 0 C
and RT and (iii) consecutive
acetylation of the resulting benzyl alcohol by treatment with acetyl chloride
in the presence of a suitable base such
as TEA and in a suitable solvent such as DCM at temperatures between 0 C and
RT and (iv) final catalytic
25 hydrogenation of the resulting benzyl ester with a suitable catalyst
such as Pd/C in the presence of a suitable
solvent such as Et0Ac, Me0H or mixture thereof and under a hydrogen atmosphere
at temperatures around RT
(Scheme G, step d).
Cleavage of the Boc protecting group in compounds of structure G-5 can be
performed using a suitable acid such
as HCI or TFA in the presence of a suitable solvent such as dioxane, Me0H or
DCM at temperatures around RT to
30 afford amines of structure BB-4 (Scheme G, step e).
CA 03087886 2020-07-08
WO 2019/141803 PCT/EP2019/051230
76
Scheme G
R1
,R1
õ. 0 0
0 0 R1- X
cr0 a (O G2 0
G-1 G-3
Cl
R1 R1
Rtx Co.
NH Boc d NH Boc e H2N
G-4 G-5 BB-4
Experimental Part
Chemistry
All temperatures are stated in C. Commercially available starting materials
were used as received without further
purification. If not explicitly indicated as having a specific absolute
configuration, example compounds or
intermediates that are chiral are in generalprepared as a racemic mixture of
two enantiomers, even if the
corresponding example compound's name or precursor's name is not preceded with
the mention rac.Relative
configuration is indicated for example as (R*,R*) meaning that the two
respective absolute configurations are either
(R,R) or (S,S); likewise, (R*,S*) indicates a relative configuration of either
(R,S) or (S,R).
Characterization of compounds
Compounds described in the invention are characterized by LC-MS data
(retention time tR is given in min) and/or
NMR using the conditions described below.
Analytical LC-MS:
Dionex Ultimate 3000 system with Dionex HPG-3200RS binary pump, Thermo MSQ
Plus MS detector and Dionex
DAD-3000R5 PDA detector.
Eluents (acidic conditions): A: H20 + 0.04% TFA; B: MeCN; gradient: 5% B
95% B; runtime: 1.5 min ; flow: 4.5
mL/min ; detection: UVNis + MS
Column Agilent Zorbax SB-aq, 4.6 x 50 mm, 3.51..tm
NMR spectroscopy:
Bruker Avance HD spectrometer equipped with a 500MHz UltrashieldTM Magnet and
a 5 mm DCH cryoprobe or
Bruker Avance II spectrometer equipped with a 400 MHz UltrashieldTM Magnet and
a BBO 5mm probehead.
Chemical shifts (6) are reported in parts per million (ppm) relative to proton
resonances resulting from incomplete
deuteration of the NMR solvent, e.g. for dimethylsulfoxide 6(H) 2.49 ppm, for
chloroform 6(H) 7.24 ppm. The
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
77
abbreviations s, d, t, q and m refer to singlet, doublet, triplet, quartet,
multiplet, respectively and br to broad.
Coupling constants J are reported in Hz.
Purification of compounds
The compounds were purified by either column chromatography on silica-gel
and/or prep. LC-MS using the
conditions described below.
Column chromatography
Column chromatography (CC) was performed using prepacked cartridges (SNAP
UltraTM, SNAP KPSlLTM, SNAP
KPNHTM, lsoluteTM Silica II or lsoluteTM NH2) from Biotage.
Preparative LC-MS:
Gilson 333/334 Prep-Scale HPLC pump equipped with Gilson LH215 autosampler,
Dionex SRD-3200 degasser,
Dionex ISO-3100A make-up pump, Dionex DAD-3000 DAD detector and Thermo MSQ
Plus Single Quadrupole MS
detector. Flow: 75 mL/min. Detection: UVNis and/or MS.
Additional informations for the purification are summerized in the table below
using following explanations:
XBridge: column Waters XBridge C18, 10 m, 30x75 mm
Acidic: eluant: A = H20 with 0.5% HCOOH, B = MeCN
Basic: eluant: A = H20 with 0.125% NH4OH, B = MeCN
Very lipophilic gradient: 50% B 95% B over 4 min then 95%6
over 2 min
Lipophilic gradient: 30% B 95% B over 4 min then 95%6 over 2 min
Normal gradient: 20% B 95% B over 4 min then 95%6 over 2 min
Polar gradient: 10% B 95% B over 4 min then 95%6 over 2 min
Very polar gradient: 5% B 50% B over 3 min then 50% B 95%
B over 1 min and finally 95%6 over 2 min
XBridge
acidic basic
Very lipophilic gradient Method 1 Method 5
Lipophilic gradient Method 2 Method 6
Normal gradient Method 3
Polar gradient Method 4
Abbreviations (as used hereinbefore or hereinafter):
Ac acetyl
AcOH acetic acid
AIBN azobisisobutyronitrile
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
78
anh. anhydrous
aq. aqueous
BINAP racemic 2,2'-bis(diphenylphosphino)-1,1'-binaphthyl
Boc tert.-butyloxycarbonyl
BrettPhos 2-(dicyclohexylphosphino)-3,6-dimethoxy-2,4',6'-triisopropy1-
1,1'-biphenyl
BrettPhos precatalyst chloro[2-(dicyclohexylphosphino)-3,6-dimethoxy-Z4-6'-
triisopropy1-1,1'-biphenyl][2-(2-
aminoethyl)phenyl]palladium(II)
CC column chromatography
CDI carbonyl diimidazole
CPhos 2-dicyclohexylphosphino-2,6'-bis(N,N-dimethylamino)biphenyl
DBU 1,8-diazabicyclo[5.4.0]undec-7-ene
DCM dichloromethane
dioxane 1,4-dioxane
DIPEA diisopropyl-ethylamine, Hunig's base, ethyl-
diisopropylamine
DMA dimethylacetamide
DMF dimethylformamide
DMP Dess Martin periodinane
DMSO dimethylsulfoxide
eq equivalent(s)
Et0Ac ethyl acetate
Et0H ethanol
Et20 diethylether
gram(s)
hour(s)
HATU 2-(7-aza-1H-benzotriazole-1-yI)-1,1,3,3-tetramethyluronium
hexafluorophosphate
Hept heptane
HPLC high performance liquid chromatography
LC-MS liquid chromatography ¨ mass spectrometry
MeCN acetonitrile
Mel methyl iodide
Me0H methanol
mg milligram(s)
mL milliliter(s)
min minute(s)
mmol millimole(s)
MS mass spectroscopy
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
79
NaBH(OAc)3 sodium triacetoxyborohydride
Na0Me sodium methoxide
NBS N-bromosuccinimide
n-BuOH n-butanol
NCS N-chlorosuccinimide
N MR nuclear magnetic resonance spectroscopy
OAc acetate
org. organic
ON overnight
PBS phosphate buffer saline
PEPPSI-IPr [1 ,3-bis(2,6-Diisopropylphenyimidazol-2-ylidene](3-
chloropyridypalladium(l I) dichloride
Pd2(dba)3 tris(dibenzylideneacetone)dipalladium(0)
prep. preparative
rac racemic
RI room temperature
RuPhos 2-dicyclohexylphosphino-2 ,6'-diisopropoxybiphenyl
RuPhos precatalyst chloro(2-dicyclohexylphosphino-2',6'-diisopropoxy-1,1'-
bipheny0[2-(2'-amino-
1,1-biphenylApalladium(11)
rxn reaction
sat. saturated
SEM [2-(trimethylsilypethoxy]methyl
soln. solution
TBAF tetrabutylammonium fluoride
TBDMS tert-butyldimethylsilyl
t-BuOH tert-butanol
TEA triethylamine
TFA trifluoroacetic acid
THF tetrahydrofuran
tR retention time
Synthesis of building blocks BB-2
Synthesis of 4-chloro-2-methoxy-3-nitropyridine (BB-2-1)
To a suspension of 4-chloro-3-nitro-2-pyridone (1.0 g, 5.7 mmol) and silver
carbonate (1.9 g, 6.9 mmol) in hexane
(17 mL) was added Mel (0.72 mL, 11.5 mmol). The rxn mixture was heated to 80 C
for 1h and concentrated in
vacuo. The crude was purified by CC using Hept/Et0Ac to give the title
compound as white solid.
LC-MS: tR=0.82 min
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
1H NMR (400 MHz, DMSO-d6) 6: 8.41 (d, J= 5.6 Hz, 1 H), 7.50 (d, J= 5.6 Hz, 1
H), 4.02 (s, 3 H)
Synthesis of 4-chloro-1-methy1-3-nitro-1H-pyridin-2-one (BB-2-2)
To a suspension of 4-chloro-3-nitro-2-pyridone (1.0 g, 5.7 mmol) and K2CO3
(1.6 g, 11.5 mmol) in DMA (5 mL) was
added Mel (0.72 mL, 11.5 mmol). The rxn mixture was heated to 45 C and stirred
for 1h. It was quenched with
5 H20, acidified to pH 5 with a 25% aq. soln. of HCI and extracted with
Et0Ac (3x). The combined org. phases were
washed with brine, dried over MgSO4 and concentrated in vacuo.
LC-MS: tR=0.60 min ; [M+H]: 189.15
1H NMR (400 MHz, DMSO-d6) 6: 8.10 (d, J= 7.4 Hz, 1 H), 6.70 (d, J= 7.4 Hz, 1
H), 3.55 (s, 3 H)
Synthesis of 4-chloro-6-methoxy-5-nitro-pyrimidine (BB-2-3)
10 A suspension of 4,6-dichloro-5-nitropyrimidine (2.0 g, 10.2 mmol) in
anh. Me0H (37 mL) was cooled to 0 C and
Na0Me (579 mg, 10.2 mmol) was portionwise added over 10 min. The rxn mixture
was stirred for 30 min and
filtered. The filtrate was concentrated in vacuo and the crude was purified by
CC using Hept/Et0Ac.
LC-MS: tR=0.75 min
1H NMR (400 MHz, DMSO-d6) 6: 8.93 (s, 1 H), 4.14 (s, 3 H)
15 Synthesis of building blocks BB-3
Synthesis of 3-bromomethy1-2-trifluoromethyl-pyridine (BB-3-1) and 2-
bromomethy1-3-trifluoromethyl-
pyrazine (BB-3-5)
A suspension of methyl-heteroarene (1 eq) in chlorobenzene (4 mL/mmol) was
heated to 50 C and NBS (1.3 eq)
was dropwise added at 50 C. The flask was purged with argon and AIBN (0.1 eq)
was added in one portion. The
20 rxn mixture was heated to 80 C and stirred for 5h (see Table 1). After
cooling to RT, the mixture was diluted with
Et20 and washed with a 1M aq. soln. of HCI (3x). The combined org. phases were
washed with brine, dried over
MgSO4 and concentrated in vacuo. The crude was purified by CC using
Hept/Et0Ac.
Table 1
1H NMR (400 MHz,
BB-3 Name Methyl-heteroarene tR [min]
DMSO-d6) 5:
8.72 (d, J=4.6 Hz, 1 H), 8.23
3-Bromomethy1-2- 3-Methyl-2- (d, J=7.8 Hz, 1 H), 7.79
(dd,
BB-3-1 0.80
trifluoromethyl-pyridine (trifluoromethyl) pyridine Ji = 4.6 Hz, J2 =
7.9 Hz, 1 H),
4.84 (d, J= 0.7 Hz, 2 H)
9.03 (d, J= 2.2 Hz, 1 H), 8.84
2-Bromomethy1-3- 2-Methyl-3-
BB-3-5 0.76 (d, J= 2.3 Hz, 1 H),
4.84 (d, J
trifluoromethyl-pyrazine (trifluoromethyl) pyrazine
= 0.9 Hz, 2 H)
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
81
Synthesis of 3-chloromethy1-4-trifluoromethyl-pyridine (BB-3-2)
To a soln. of 4-trifluoromethyl-pyridin-3-yl-methanol (419 mg, 2.3 mmol) in
DCM (7 mL) was added S0Cl2 (0.84 mL,
11.6 mmol). The rxn mixture was stirred for 1h at RT, quenched with a 10% aq.
soln. of Na2CO3 and extracted with
DCM (3x). The combined org. phases were washed with brine, dried over MgSO4
and concentrated in vacuo. The
crude was purified by CC using Hept/Et0Ac.
LC-MS: tR=0.79 min, [M+MeCN+H]: 237.05
1H NMR (400 MHz, DMSO-d6) 6: 9.00 (s, 1 H), 8.86 (dd, Ji = 0.5 Hz, J2 = 5.1
Hz, 1 H), 7.81 (d, J = 5.1 Hz, 1 H),
4.95 (s, 2 H)
Synthesis of 4-bromomethy1-3-trifluoromethyl-pyridine (BB-3-3) and 2-
bromomethy1-3-trifluoromethyl-
pyridine (BB-3-4)
The appropriate methylpyridine (1 eq) was dissolved in a 1M soln. of bromine
in acetic acid (1 mL/mmol) and the
solution was stirred ON at RT and for 6h at 80 C (see Table 2). The mixture
was basified with a 1M aq. soln. of
NaOH and extracted with Et0Ac (3x). The combined org. phases were washed with
brine, dried over MgSO4 and
concentrated in vacuo. The crude dibromo product was dissolved in THF (4
mL/mmol) and DIPEA (1.05 eq) and
diethyl phosphite (1.05 eq) were sequencially added at 0 C. The mixture was
stirred for 10 min at 0 C and ON at
RT. It was quenched with water and extracted with Et0Ac (3x). The combined
org. phases were washed with a sat.
aq. soln. of NH4CI and brine, dried over MgSO4 and concentrated in vacuo. The
crude was purified by CC using
Hept/Et0Ac.
Table 2
1H NMR (400 MHz,
BB-3 Name Methyl-pyridine tR [min]
DMSO-d6) 5:
8.96 (s, 1 H), 8.91 (d, J =
4-Bromomethy1-3- 4-Methyl-3-
BB-3-3 0.80 5.1 Hz, 1 H), 7.78
(d, J =
trifluoromethyl-pyridine (trifluoromethyl)pyridine
5.1 Hz, 1 H), 4.77 (s, 2 H)
8.88 (d, J = 4.7 Hz, 1 H),
2-Bromomethy1-3- 2-Methyl-3-
BB-3-4 0.79 8.24 (d, J = 8.0
Hz, 1 H),
trifluoromethyl-pyridine (trifluoromethyl)pyridine
7.63 (m, 1 H), 4.76 (s, 2 H)
Synthesis of (4-methoxy-pyrimidin-5-y1)-methanol (BB-3-6) and (4-
trifluoromethyl-pyrimidin-5-y1)-methanol
(BB-3-8)
To a suspension of carboxylic acid (1 eq) in anh. THF (5 mL/mmol) was added at
-10 C 4-methylmorpholine (1.8
eq) and ethyl chloroformate (1.5 eq). The mixture was stirred for 30 min at -
10 C and NaBF14 (3 eq) was added in
one portion. It was warmed to 0 C and Me0H (3 mL/mmol) was added dropwise (see
Table 3). The rxn mixture
was stirred for 1h at 0 C and for 1h at RT. It was quenched with water,
acidified with a 1M aq. soln. of HCI until pH
CA 03087886 2020-07-08
WO 2019/141803 PCT/EP2019/051230
82
4 and extracted with n-BuOH (3x). The combined org. phases were dried and
concentrated in vacuo. The crude
was purified by CC using Hept/Et0Ac/Me0H.
Table 3
MS-data m/z
BB-3 Name Carboxylic acid tR [min]
[M+H]4
4-Methoxypyrimidine-5-
BB-3-6 (4-Methoxy-pyrimidin-5-yI)-methanol 0.29 141.19
carboxylic acid
220.08
(4-Trifluoromethyl-pyrimidin-5-yI)- 4-(Trifluoromethyl)
BB-3-8 0.47 [M+MeCN+H
methanol pyrimidine-5-
carboxylic acid
+1
Synthesis of 5-(chloromethyl)-4-methoxypyrimidine (BB-3-7) and 5-
(chloromethyl)-4-(trifluoromethyl)
pyrimidine (BB-3-9)
To a soln. of alcohol (1 eq) in DCM (5 mL/mmol) was added S0Cl2 (1.5 to 2.5
eq) at 0 C. The rxn mixture was
stirred for 2h at RT (see Table 4) and partitioned between Et0Ac and a sat.
aq. soln. of NaHCO3. The org. phase
was washed with a sat. aq. soln. of NaHCO3 (1x) and brine (1x), dried over
MgSO4 and concentrated in vacuo.
Table 4
MS-data m/z 1H NMR (400 MHz,
BB-3 Name Alcohol tR [min]
[M+H]4 DMSO-d6) 5:
(4-Methoxy-
5-(Chloromethyl)-4-
BB-3-7 pyrimidin-5-yI)- 0.61 159.25
methoxypyrimidine
methanol
5-(Chloromethyl)-4- (4-Trifluoromethyl- 9.46
(s, 1 H), 9.33 (s,
BB-3-9 (trifluoromethyl) pyrimidin-5-yI)- 0.72 1 H),
4.99 (d, J=0.9
pyrimidine methanol Hz, 2 H)
Synthesis of 6-chloro-3-chloromethy1-4-methoxy-pyridazine (BB-3-10)
Step A: 6-Chloro-4-methoxy-pyridazine-3-carboxylic acid methyl ester
To a soln. of methyl 4,6-dichloropyridazine-3-carboxylate (550 mg, 2.66 mmol)
in anh. THF (13 mL) was added
dropwise at 0 C a 25% soln. of Na0Me in Me0H (0.638 mL, 2.79 mmol). The rxn
mixture was stirred ON at RT
and poured onto a 1M aq. soln. of HCI. The soln. was neutralized with a sat.
aq. soln. of NaHCO3 and extracted
with Et0Ac (3x). The combined org. phases were washed with brine, dried over
MgSO4 and concentrated in vacuo.
The crude was purified by CC using Hept/Et0Ac.
LC-MS: tR=0.54 min, [M+H]: 203.14
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
83
Step 8: (6-Chloro-4-methoxy-pyridazin-3-yI)-methanol
To a soln. of 6-chloro-4-methoxy-pyridazine-3-carboxylic acid methyl ester
(120 mg, 0.592 mmol) in anh. Et0H (4.7
mL) was added CaCl2 (20 mg, 0.178 mmol) and the rxn mixture was cooled to -10
C. NaBHa (56 mg, 1.48 mmol)
was added portionwise and the mixture was stirred for 1.5 h at -10 C and for
30 min at RT. It was quenched at 0 C
with water and Et0H was evaporated off. The residue was partitioned between
Et0Ac and water and the aq. phase
was further extracted with Et0Ac (2x). The combined org. phases were washed
with brine (1x), dried over MgSO4
and concentrated in vacuo.
LC-MS: tR=0.40 min, [M+H]: 175.20
Step C: 6-Chloro-3-chloromethy1-4-methoxy-pyridazine (88-3-10)
To a soln. of (6-Chloro-4-methoxy-pyridazin-3-yI)-methanol (73 mg, 0.393 mmol)
in DCM (1.97 mL) was added
S0Cl2 (0.043 mL, 0.59 mmol) at 0 C. The rxn mixture was stirred for 2h at RT
and partitioned between Et0Ac and
a sat. aq. soln. of NaHCO3. The org. phase was washed with a sat. aq. soln. of
NaHCO3 (1x) and brine (1x), dried
over MgSO4 and concentrated in vacuo.
LC-MS: tR=0.63 min, [M+H]: 193.13
Synthesis of (6-chloro-4-isopropoxy-pyridazin-3-yI)-methanol (BB-3-11)
Step A: 6-Chloro-4-isopropoxy-pyridazine-3-carboxylic acid isopropyl ester
To a soln. of 4,6-dichloropyridazine-3-carboxylic acid methyl ester (500 mg,
2.42 mmol) in anh. THF (12 mL) was
added dropwise at 0 C a 2M soln. of lithium isopropoxide in THF (1.27 mL, 2.54
mmol). The rxn mixture was stirred
for 30 min at 0 C and poured into a 1M aq. soln. of HCI. The aq. soln. was
neutralized with a sat. aq. soln. of
NaHCO3 and extracted with Et0Ac (3x). The combined org. phases were washed
with brine, dried over MgSO4 and
concentrated in vacuo. The crude was purified by CC using Hept/Et0Ac.
LC-MS: tR=0.82 min, [M+H]: 259.12
Step 8: (6-Chloro-4-isopropoxy-pyridazin-3-yI)-methanol (88-3-11)
To a soln. of 6-chloro-4-isopropoxy-pyridazine-3-carboxylic acid isopropyl
ester (80 mg, 0.309 mmol) in anh. Et0H
(4.6 mL) was added CaCl2 (10 mg, 0.093 mmol) and the rxn mixture was cooled to
-10 C. NaBHa (29 mg, 0.773
mmol) was added portionwise and the mixture was stirred for 30 min at -10 C
and for 1h min at RT. It was
quenched at 0 C with water and Et0H was evaporated off. The residue was
partitioned between Et0Ac and water
and the aq. phase was further extracted with Et0Ac (2x). The combined org.
phases were washed with brine (1x),
dried over MgSO4 and concentrated in vacuo.
LC-MS: tR=0.55 min, [M+H]t 203.14
Synthesis of (BB-3-12), (BB-3-13), (BB-3-14), (BB-3-15), (BB-3-16), (BB-3-17)
Step A: Esterification
To a soln. of carboxylic acid (1 eq) in anh. Me0H (4 mL/mmol) was added AcCI
(3 eq) and the rxn mixture was
stirred for 2.5 h at 80 C (see Table 5). Me0H was evaporated off and the
residue was partitioned between a sat.
CA 03087886 2020-07-08
WO 2019/141803 PCT/EP2019/051230
84
aq. soln. of NaHCO3 and Et0Ac. The org. phase was washed with a 10% aq. soln.
of Na2CO3 (1x) and with brine
(1x), dried over MgSO4 and concentrated in vacuo.
Table 5
MS-data
Carboxylic acid
BB-3A Name tR [min] m/z
reactant
[M+H]4
BB-3- 4-Methylpyridazine-3-carboxylic acid 4-methylpyridazine-3-
0.47 153.43
12A methyl ester carboxylic acid
BB-3- 3-lsopropoxy-pyrazine-2-carboxylic acid 3-lsopropoxy-pyrazine-
2-
0.70 197.17
13A methyl ester carboxylic acid
BB-3- 3-lsopropoxy-pyridine-2-carboxylic acid 3-lsopropoxy-pyridine-
2-
0.64 196.21
15A methyl ester carboxylic acid
Step A (Negishi): synthesis of 3-isopropyl-pyrazine-2-carboxylic acid ethyl
ester (BB-3-17A)
An oven dried flask was charged with 3-chloropyrazine-2-carboxylic acid ethyl
ester (500 mg, 2.63 mmol), PEPPSI-
IPr (18 mg, 0.026 mmol) and CPhos (12 mg, 0.026 mmol). The flask was evacuated
and refilled with argon (3x)
and toluene (5 mL) was added. The rxn mixture was cooled to 0 C and a 0.5M
soln. of 2-propyl zinc bromide in
THF (6.83 mL, 3.41 mmol) was added dropwise. The rxn mixture was stirred for
72h at RT and partitioned between
half sat. brine and DCM. The org. phase was dried over MgSO4 and concentrated
in vacuo. The crude was purified
by CC using Hept/Et0Ac.
LC-MS: tR=0.74 min, [M+H]: 195.18
Step A (0-alkylation): synthesis of 2,4-difluoro-6-isopropoxybenzoic acid
methyl ester (BB-3-19A)
To a soln. of methyl 2,4-difluoro-6-hydroxybenzoic acid methyl ester (200 mg,
1.01 mmol) and 2-propanol (0.116
mL, 1.51 mmol) in toluene (1.5 mL) was added a 1M soln. of
(tributylphosphoranylidene)acetonitrile in toluene (2.02
mL, 2.02 mmol) under argon. The rxn mixture was heated to 110 C and stirred
for 2h. It was quenched with water
and extracted with DCM (3x). The combined org. phases were washed with brine,
dried over MgSO4 and
concentrated in vacuo. The crude was purified by CC using Hept/Et0Ac.
LC-MS: tR=0.89 min, [M+H]: 231.10
Step B: Methyl/ethyl ester reduction using CaCl2/NaBH4 (method A)
To a soln. of methyl ester (BB-3A) (1 eq) in anh. Et0H (15 mL/mmol) was added
CaCl2 (0.3 eq) and the rxn mixture
was cooled to -10 C. NaBHa (2.5 eq) was added portionwise and the mixture was
stirred for 30 min at -10 C and
for a given time at a given temperature (see Table 6). It was quenched at 0 C
with water and Et0H was
evaporated off. The residue was partitioned between Et0Ac and water and the
aq. phase was further extracted
with Et0Ac (2x). The combined org. phases were washed with brine (1x), dried
over MgSO4 and concentrated in
vacuo.
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
Step B: Methyl/ethyl ester reduction using LiAIH4 (method B)
To a soln. of methyl or ethyl ester (1 eq) in anh. THF (4.5 to 7 mL/mmol) was
added dropwise at 0 C a 2.4 M soln.
of LiAIH4 in THF (1 eq). The rxn mixture was stirred for 1.5 h at 0 C (see
Table 6), quenched with a sat. aq. soln. of
NH4CI and extracted with Et0Ac (3x). The combined org. phases were dried over
MgSO4 and concentrated in
5 vacuo. When necessary the crude was purified by CC using Et0Ac.
Table 6
MS-
Methyl/ethyl Method 1H NMR
data
BB-3 Name ester T [ C] tR [min] (400 MHz,
m/z
reactant time [h] DMSO-d6) 5:
[M+H]4
A
BB-3- (4-Methyl-pyridazin-3-
BB-3-12A RT 0.18 125.44
12 yI)-methanol
0.5
A
BB-3- (3-lsopropoxy-pyrazin-
BB-3-13A RT 0.58 169.11
13 2-yI)-methanol
0.5
4-Isopropyl
A
BB-3- (4-lsopropylpyridin-3- pyridine-3-
RT 0.34 152.45
14 yl)methanol carboxylic acid
48
ethyl ester
A
BB-3- (3-lsopropoxy-pyridin-
BB-3-15A RT 0.40 168.47
15 2-yI)-methanol
24
4-Isopropyl
A
BB-3- (4-Isopropyl-pyrimidin- pyrimidine-5-
70 0.46 153.47
16 5-yI)-methanol carboxylic acid
1
ethyl ester
A
BB-3- (3-lsopropyl-pyrazin-2-
BB-3-17A 0 0.48 153.45
17 yI)-methanol
2.5
CA 03087886 2020-07-08
WO 2019/141803 PCT/EP2019/051230
86
Mixture of 5-
(trifluoromethyl)
Mixture of (5- thiazole-4-
(trifluoromethyl)thiazol- carboxylic acid
BB-3-
18 4-yl)methanol and (2- ethyl ester and 0 0.52
184.05
(trifluoromethyl)thiazol- 2-(trifluoromethyl) 1.5
4-yl)methanol thiazole-4-
carboxylic acid
ethyl ester
6.41-6.47 (m,
BB (2,4-Difluoro-6- no 2 H),
4.70 (d, J
-3-
isopropoxy-phenyI)- BB-3-19A 0 0.78 ionisati = 1.5
Hz, 2 H),
19 4.58
(m, 1 H),
methanol 1.5 on 1.40-
1.45 (m,
6H)
Synthesis of building blocks BB-4 (via G-5)
Step A: Aromatic nucleophilic substitution
To a solution of the appropriate amine (1 eq) and the appropriate fluoroarene
(1.1 eq) in DMSO (0.9 mL/mmol) was
added K2CO3 (2 eq) and the mixture was heated to 105 C and stirred for 18h
(see Table 7). It was quenched with
water and extracted with DCM. The org. phase was washed with water (5x) and
brine (1x), dried over MgSO4 and
concentrated in vacuo. The crude was purified by CC using DCM/Me0H.
Table 7
MS-data
Reactant Reactant
BB-4A Name tR [min] m/z
amine fluoroarene
[M+H]4
[(R)-1-(2-Fluoro-6-formyl-
BB-4- (R)-(+)-3-(Boc- 2,3-Difluoro
pheny1)-pyrrolidin-3-y1]-carbamic 0.90 309.10
1A amino)pyrrolidine benzaldehyde
acid tert-butyl ester
[1-(2-Fluoro-6-formyl-phenyI)-
BB-4- 4-(N-Boc-amino) 2,3-Difluoro
piperidin-4-yI]-carbamic acid 0.93 323.20
2A piperidine benzaldehyde
tert-butyl ester
[(R)-1-(2-Cyano-6-fluoro-
BB-4- (R)-(+)-3-(Boc- 2,3-Difluoro
pheny1)-pyrrolidin-3-y1]-carbamic 0.91 306.03
3A amino)pyrrolidine benzonitrile
acid tert-butyl ester
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
87
Step B: Reduction
A suspension of intermediate BB-4A (1 eq) in anh. Me0H (2 mL/mmol) was cooled
to 0 C and NaBHa (1.3- to 1.5
eq) was added portionwise at 0 C (see Table 8). The rxn mixture was stirred
for 1h at 0 C to reach completion. It
was carefully quenched by dropwise addition of water at 0 C and extracted with
Et0Ac. The org. phase was
washed with water and brine, dried over MgSO4 and concentrated in vacuo.
Table 8
Reactant MS-data m/z
BB-4B Name tR [min]
BB-4A [M+H]4
[(R)-1-(2-Fluoro-6-hydroxymethyl-phenyI)-
BB-4-1B BB-4-1A 0.77 311.11
pyrrolidin-3-yI]-carbamic acid tert-butyl ester
[1-(2-Fluoro-6-hydroxymethyl-phenyI)-
BB-4-2B BB-4-2A 0.82 325.24
piperidin-4-yI]-carbamic acid tert-butyl ester
Step C: Acetylation
A solution of intermediate BB-4B (1 eq) and TEA (1.5 eq) in DCM (0.5 mL/mmol)
was cooled to 0 C and AcCI (1.5
eq) was added dropwise at 0 C (see Table 9). The rxn mixture was stirred for
lh at 0 C to reach completion. It was
diluted with DCM and washed with a 10% solution of citric acid (2x), with a
sat. solution of NaHCO3 (2x) and with
brine (1x). The org. phase was dried over MgSO4 and concentrated in vacuo. The
crude was purified by CC using
Hept/Et0Ac.
Table 9
Reactant MS-data m/z
BB-4C Name tR [min]
BB-4B [M+H]4
Acetic acid 2-((R)-3-tert-
BB-4-1C butoxycarbonylamino-pyrrolidin-111)-3- BB-4-1B 0.94
353.13
fluoro-benzyl ester
Acetic acid 2-(4-tert-butoxycarbonylamino-
BB-4-2C BB-4-2B 0.97 367.25
piperidin-111)-3-fluoro-benzyl ester
Step D: Hydrogenation
Intermediate BB-4C (1 eq) was dissolved in a mixture of Me0H (6 mL/mmol) and
Et0Ac (2 mL/mmol) and the flask
was evacuated three times and refilled with nitrogen. Wet Pd/C (0.08 eq) was
added and the flask was evacuated
and refilled three times with hydrogen. The suspension was stirred under a
hydrogen atmosphere for 3h (see Table
10) and filtered over a pad of Celite. The cake was washed with Et0Ac and Me0H
and the filtrate was
concentrated in vacuo. The crude was purified by CC using Hept/Et0Ac.
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
88
Table 10
BB-4D Reactant MS-data m/z
Name tR [min]
(G-5) BB-4C [M+H]4
[(R)-1-(2-Fluoro-6-methyl-phenyI)-
BB-4-1D pyrrolidin-3-yI]-carbamic acid tert-butyl BB-4-1C 0.91
295.14
ester
[1-(2-Fluoro-6-methyl-phenyI)-piperidin-
BB-4-2D BB-4-2C 1.00 309.16
4-yI]-carbamic acid tert-butyl ester
Final step: Boc cleavage
To a solution of intermediate BB-4D or BB-4A (1 eq) in DCM (4 mL/mmol) was
added dropwise TFA (1 mL/mmol)
and the rxn mixture was stirred for 1h to 18h at RT (see Table 11). It was
basified with a 1M solution of NaOH until
pH 12-13 and extracted with DCM (3x). The combined org. phases were dried over
MgSO4 and concentrated in
vacuo.
Table 11
Reactant
MS-data m/z
BB-4 Name BB-4D (G-5) or tR [min]
[M+H]4
BB-4A
(R)-1-(2-Fluoro-6-methyl-phenyI)-
BB-4-1 BB-4-1D 0.57 195.29
pyrrolidin-3-ylamine
1-(2-Fluoro-6-methyl-phenyI)-piperidin-4-
BB-4-2 BB-4-2D 0.60 209.28
ylamine
24(R)-3-Amino-pyrrolidin-111)-3-fluoro-
BB-4-3 BB-4-3A 0.49 205.89
benzonitrile
Synthesis of building blocks BB-4 (via G-2 and G-3)
Step A: Buchwald Hartwig
To a mixture of the appropriate amine G-1 (1 eq), the appropriate halide (1.2
eq) and sodium tert-butoxide (2 eq) in
toluene (3 mL/mmol) under N2, was added BINAP (0.2 eq) and Pd2(dba)3 (0.1 eq)
(see Table 12). The rxn mixture
was flushed with N2, heated to 100 C in a sealed vial and stirred for 24h. It
was partitioned between water and
Et0Ac and the org. phase was washed with brine, dried over MgSO4 and
concentrated in vacuo. The crude was
purified by CC using Hept/Et0Ac.
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
89
Table 12
MS-data
Reactant Reactant
G-2 Name tR [min] m/z
amine (G-1) halide
[M+Fl]4
1,4-Dioxa-8-
8-(2,6-Dimethyl-phenyI)-1,4-dioxa- 2-Bromo-1,3-
G-2-1 azaspiro[4.5] 0.92 248.24
8-aza-spiro[4.5]decane dimethylbenzene
decane
Step B: Ketal cleavage
To a soln. of ketal intermediate G-2 (1 eq) in anh. THF (3 mL/mmol) was added
a 1M aq. soln. of HCI (2 mL/mmol)
at RT (see Table 13). The rxn mixture was heated to 70 C and stirred for 24h.
It was quenched with a sat. aq. soln.
of NaHCO3 and extracted with Et0Ac (3x). The combined org. phases were washed
with brine, dried over MgSO4
and concentrated in vacuo. The crude was purified by CC using Hept/Et0Ac.
Table 13
MS-data
Reactant
G-3 Name tR [min] m/z
G-2
[M+Fl]4
1-(2,6-Dimethyl-phenyI)-
G-3-1 G-2-1 0.88 204.22
piperidin-4-one
Final step: Reductive amination
To a soln. of ketone intermediate G-3 (1 eq) in dioxane (9.1 mL/mmol) was
added a 25% aq. soln. of NR4OH (38
eq) and H20 (0.35 mL/mmol). The flask was evacuated three times and refilled
with nitrogen. Wet Pd/C (0.06 eq)
was added and the flask was evacuated and refilled three times with hydrogen.
The suspension was stirred under a
hydrogen atmosphere for 24h (see Table 14) and filtered over a pad of Celite.
The cake was washed with dioxane
and Me0H and the filtrate was concentrated in vacuo. The crude was purified by
CC using DCM/Me0H.
Table 14
MS-data
Reactant
BB-4 Name tR [min] m/z
G-3
[WE]4
1-(2,6-Dimethyl-phenyI)-
BB-4-4 G-3-1 0.63 205.33
piperidin-4-ylamine
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
Synthesis of building block BB-8
Synthesis of carbonic acid 3-methyl-oxetan-3-y1 ester pentafluorophenyl ester
(BB-8-1), carbonic acid
oxetan-3-y1 ester pentafluorophenyl ester (BB-8-2) and carbonic acid
pentafluorophenyl ester 3-
trifluoromethyl-oxetan-3-y1 ester (BB-8-3)
5 A soln. of the appropriate alcohol (1 eq) and
bis(pentafluorophenyl)carbonate (1.2 eq) in MeCN (0.55 mL/mmol)
was cooled to 0 C and Et3N (3.2 eq) was added dropwise. The rxn mixture was
stirred ON allowing the
temperature to reach RT (see Table 15). The mixture was concentrated in vacuo
and the residue was purified by
CC using DCM/Me0H.
Table 15
Reactant 1H NMR (400 MHz,
BB-8 Name tR [min]
alcohol DMSO-d6) 5:
4.77 (d, J = 7.7 Hz, 2 H),
Carbonic acid 3-methyl-oxetan-3-y1 3-Methyl
BB-8-1 0.90 4.54 (d, J = 8.2
Hz, 2 H),
ester pentafluorophenyl ester oxetan-3-ol
1.76 (s, 3 H)
5.65 (m, 1 H), 4.87 (m, 2
Carbonic acid oxetan-3-ylester 3-Hydroxy H), 4.65 (ddd, Ji =
0.9
BB-8-2 0.86
pentafluorophenyl ester oxetane Hz, J2 = 4.7 Hz, th =
8.1
Hz, 2 H)
The carbonic acid pentafluorophenyl ester 3-trifluoromethyl-oxetan-3-y1 ester
(BB-8-3) was prepared according to
Med. Chem. Commun., 2013, 4, 95.
Synthesis of 4-(2-bromo-3-fluoro-phenyl)-morpholine (BB-8-4)
To a soln. of 2-fluoro-6-morpholinoaniline (100 mg, 0.51 mmol) in anh. MeCN
was added tert-butyl nitrite (0.121
mL, 1.02 mmol) and copper (II) bromide (137 mg, 0.612 mmol). The rxn mixture
was stirred for 30 min at RT and
partitioned between water and Et0Ac. The aq. phase was washed with Et0Ac (2x)
and the combined org. phases
were washed with brine (1x), dried over MgSO4 and concentrated in vacuo. The
crude was purified by CC using
Hept/Et0Ac and additionally by prep. LC-MS using method 2.
LC-MS: tR=0.97 min, [M+H]: 260.05
Synthesis of 5-isopropy1-3H41,3,4]oxadiazol-2-one (BB-8-5)
CDI (1228 mg, 7.34 mmol) was added to a soln. of isobutyric acid hydrazide
(500 mg, 4.9 mmol) in anh. dioxane
(21 mL) and the rxn mixture was heated at 85 C and stirred ON. Dioxane was
evaporated off and the residue was
partitioned between water and Et0Ac. The org. phase was washed with brine
(1x), dried over MgSO4 and
concentrated in vacuo. The crude was purified by CC using Hept/Et0Ac.
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
91
LC-MS: tR=0.49 min, [M+MeCN+H]: 170.27
1H NMR (400 MHz, DMSO-d6) 6: 12.05 (m, 1 H), 2.86 (m, 1 H), 1.19 (d, J = 6.9
Hz, 6 H)
Synthesis of 3-chloro-4-methoxy-2-methyl-pyridine (BB-8-6)
To a soln. of 3,4-dichloro-2-methylpyridine (228 mg, 1.3 mmol) in Me0H (6.7
mL) was added a 25% soln. of
Na0Me in Me0H (3.5 mL, 15.3 mmol) and the soln. was heated to 65 C for 18h. It
was partitioned between DCM
and water and the org. phase was washed with brine (1x), dried over MgSO4 and
concentrated in vacuo to give the
title compound as white solid.
LC-MS: tR=0.38 min, [M+H]: 158.43
1H NMR (500 MHz, DMSO) 6: 8.28 (d, J = 5.6 Hz, 1 H), 7.07 (d, J = 5.7 Hz, 1
H), 3.92 (s, 3 H), 2.51 (s, 3 H)
.. Synthesis of (3-bromo-5-methoxy-pyridin-4-yI)-carbamic acid methyl ester
(BB-10-1)
Step A (bromination): 3-bromo-5-methoxypyridin-4-amine
To a soln. of 4-amino-3-methoxypyridine (905 mg, 7 mmol) in MeCN (20 mL) was
added N-bromosuccinimide
(1384 mg, 7.7 mmol) and the soln. was stirred for 1h at RT. It was quenched
with a sat. aq. soln. of Na2S203 and
extracted with Et0Ac. The org. phase was washed with a sat. aq. soln. of
NaHCO3 (5x) and brine (1x), dried over
MgSO4 and concentrated in vacuo to give the title compound as brown oil.
LC-MS: tR=0.38 min, [M+H]: 203.17
1H NMR (500 MHz, DMSO) 6: 7.98 (s, 1 H), 7.89 (s, 1 H), 5.87 (s, 2 H), 3.86
(s, 4 H)
Step B (carbamate formation): (3-bromo-5-methoxy-pyridin-4-yI)-carbamic acid
methyl ester (8 8-1 0-1)
To a soln. of 3-bromo-5-methoxy-pyridin-4-ylamine (1140 mg, 5.61 mmol) and 2,6-
lutidine (1.33 mL, 11.20 mmol) in
DCM (40 mL) was added dropwise a soln. of methyl chloroformate (0.482 mL, 6.18
mmol) in DCM (5 mL) at 0 C.
The rxn mixture was allowed to warm to RT and stirred for 18h. Additional
amount of 2,6-lutidine (2.67 mL, 22.5
mmol) and methylchloroformate (0.876 mL, 11.2 mmol) was added at 0 C and the
rxn mixture stirred for lh at RT.
It was quenched with a sat. aq. soln. of NaHCO3 and extracted with DCM (2x).
The combined org. phases were
dried over MgSO4 and concentrated in vacuo. The crude was purified by CC using
Hept/Et0Ac.
LC-MS: tR=0.62 min, [M+H]: 261.14
1H NMR (400 MHz, DMSO) 6: 9.23 (s, 1 H), 8.40 (s, 1 H), 8.39 (s, 1 H), 3.91
(s, 3 H), 3.63 (s, 3 H)
Synthesis of intermediates of formula A-1
To a soln. of 1-fluoro/chloro-2-nitro-(hetero)arene (BB-2, 1 eq) and Boc-
protected diamine (BB-1, 1 to 1.2 eq) in
DMSO (1.5 mL/mmol) was added DIPEA (2 eq) and the soln. was heated at a given
temperature for a given time
(see Table 16). The rxn mixture was partitioned between Et0Ac and water. The
org. phase was washed with water
(4x) and with brine, dried over MgSO4 and concentrated in vacuo. The crude was
purified by CC using Hept/Et0Ac
and/or DCM/Me0H.
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
92
Table 16
T [ C] MS-data
Reactant
A-1 Name Reactant BB-1 time tR [min]
m/z
BB-2
[h] [M+Fl]4
4-(3-Methoxy-2-nitro-
3-Fluoro-2- 1-N-Boc-4- 100
A-1-1 phenylamino)-piperidine-1- 0.96 352.16
nitroanisole aminopiperidine 24
carboxylic acid tert-butyl ester
(R)-3-(3-Methoxy-2-nitro- (R)-(+)-1-Boc-3-
3-Fluoro-2- 100
A-1-2 phenylamino)-pyrrolidine-1- aminopyrrolidin 0.92 338.74
nitroanisole 24
carboxylic acid tert-butyl ester
(R)-3-(2-Methoxy-3-nitro-
(R)-(+)-1-Boc-3-
pyridin-4-ylamino)-pyrrolidine- 80
A-1-3 BB-2-1 aminopyrrolidin 0.88 339.11
1-carboxylic acid tert-butyl 24
ester
(R)-3-(4-Methoxy-3-nitro- 2-Chloro-4-
(R)-(+)-1-Boc-3-
pyridin-2-ylamino)-pyrrolidine- methoxy-3- 85
A-1-4 aminopyrrolidin 0.87 339.13
1-carboxylic acid tert-butyl nitro- 24
ester pyridine
4-(3-Methoxy-2-nitro- 1-N-Boc-4-
phenylamino)-2-methyl- 3-Fluoro-2- amino-2- 100
A-1-5 0.98 366.22
piperidine-1-carboxylic acid nitroanisole methyl- 24
tert-butyl ester piperidine
4-(3-Methoxy-2-nitro- 1-N-Boc-4-
phenylamino)-3-methyl- 3-Fluoro-2- amino-3- 100
A-1-6 0.98 366.39
piperidine-1-carboxylic acid nitroanisole methyl- 24
tert-butyl ester piperidine
4-(3-Methoxy-2-nitro- 1-N-Boc-4-
phenylamino)-piperidine-1,3- 3-Fluoro-2- aminopiperidine 90
A-1-7 0.97 424.14
dicarboxylic acid 1-tert-butyl nitroanisole -3-carboxylic 24
ester 3-ethyl ester acid ethyl ester
4-(3-Methyl-2-nitro-
3-Fluoro-2- 1-N-Boc-4- 100
A-1-8 phenylamino)-piperidine-1- 1.14 336.32
nitrotoluene aminopiperidine 18
carboxylic acid tert-butyl ester
CA 03087886 2020-07-08
WO 2019/141803 PCT/EP2019/051230
93
Synthesis of intermediates of formula A-2
To a soln. of intermediate A-1 (1 eq) in Et0Ac (3 to 3.3 mL/mmol) was added
10% Pd/C moistened with ¨50%
water (0.01 to 0.05 eq) and the rxn mixture was hydrogenated under atmospheric
pressure for a given time (see
Table 17). It was filtered over a pad of celite and the filtrate was
concentrated in vacuo. The crude was purified by
CC using Hept/Et0Ac or DCM/Me0H.
Table 17
MS-data
Reactant
A-2 Name time [h] tR [min]
m/z
A-1
[M+Fl]4
4-(2-Amino-3-methoxy-phenylamino)-
A-2-1 piperidine-1-carboxylic acid tert-butyl A-1-1 24
0.68 322.00
ester
(R)-3-(2-Amino-3-methoxy-
A-2-2 phenylamino)-pyrrolidine-1-carboxylic A-1-2 10
0.67 308.13
acid tert-butyl ester
(R)-3-(3-Amino-2-methoxy-pyridin-4-
A-2-3 ylamino)-pyrrolidine-1-carboxylic acid A-1-3 4,5
0.59 309.11
tert-butyl ester
(R)-3-(3-Amino-4-methoxy-pyridin-2-
A-2-4 ylamino)-pyrrolidine-1-carboxylic acid A-1-4 3 0.58
309.18
tert-butyl ester
4-(2-Amino-3-methoxy-phenylamino)-
A-2-5 2-methyl-piperidine-1-carboxylic acid A-1-5 3.5
0.71 336.32
tert-butyl ester
4-(2-Amino-3-methoxy-phenylamino)-
A-2-6 3-methyl-piperidine-1-carboxylic acid A-1-6 1
0.72/0.73 336.23
tert-butyl ester
4-(2-Amino-3-methoxy-phenylamino)-
A-2-7 piperidine-1,3-dicarboxylic acid 1-tert- A-1-7 1 0.76
394.09
butyl ester 3-ethyl ester
4-(2-Amino-3-methyl-phenylamino)-
A-2-8 piperidine-1-carboxylic acid tert-butyl A-1-8 18
0.76 306.26
ester
CA 03087886 2020-07-08
WO 2019/141803 PCT/EP2019/051230
94
Synthesis of intermediates of formula A-3
To a soln. of intermediate A-2 (1 eq) in MeCN (3.7 mL/mmol) was added CDI (1.2
eq) and the rxn mixture was
stirred at a given temperature for a given time (see Table 18). The solvent
was evaporated off and the residue was
partitioned between Et0Ac or DCM and water. The org. phase was washed with
brine, dried over MgSO4 and
concentrated in vacuo. The crude was purified by CC using Hept/Et0Ac or
DCM/Me0H.
Table 18
Reactant T [ C] MS-data m/z
A-3 Name tR [min]
A-2 time [h] [M+Fly
4-(4-Methoxy-2-oxo-2,3-dihydro-
RT
A-3-1 benzoimidazol-1-y1)-piperidine-1-carboxylic A-2-1 2 0.82
348.14
acid tert-butyl ester
(R)-3-(4-Methoxy-2-oxo-2,3-dihydro-
RT
A-3-2 benzoimidazol-1-y1)-pyrrolidine-1-carboxylic A-2-2 0.2
0.80 334.15
acid tert-butyl ester
(R)-3-(4-Methoxy-2-oxo-2,3-dihydro-
RT
A-3-3 imidazo[4,5-c]pyridine-1-yI)-pyrrolidine-1- A-2-3 2 0.72
335.12
carboxylic acid tert-butyl ester
(R)-3-(7-Methoxy-2-oxo-1,2-dihydro-
45 C
A-3-4 imidazo[4,5-b]pyridine-3-yI)-pyrrolidine-1- A-2-4 0.2
0.74 335.34
carboxylic acid tert-butyl ester
4-(4-Methoxy-2-oxo-2,3-dihydro-
RT
A-3-5 benzoimidazol-111)-2-methyl-piperidine-1- A-2-5 0.25
0.86 362.21
carboxylic acid tert-butyl ester
4-(4-Methoxy-2-oxo-2,3-dihydro-
RT
A-3-6 benzoimidazol-111)-3-methyl-piperidine-1- A-2-6 0 0.85
362.21
.75
carboxylic acid tert-butyl ester
4-(4-Methoxy-2-oxo-2,3-dihydro-
benzoimidazol-1-y1)-piperidine-1,3- RT
A-3-7 A-2-7 0.84 420.13
dicarboxylic acid 1-tert-butyl ester 3-ethyl 0.75
ester
4-(4-Methy1-2-oxo-2,3-dihydro-
RT
A-3-8 benzoimidazol-1-y1)-piperidine-1-carboxylic A-2-8 21 0.97
332.31
acid tert-butyl ester
CA 03087886 2020-07-08
WO 2019/141803 PCT/EP2019/051230
Synthesis of compounds of formula la (from compounds of formula A-3)
Method A (K2CO3/ DMF)
To a soln. of intermediate A-3 (1 eq) in anh. DMF (3.5 to 6 mL/mmol) was added
K2CO3 (3 eq) at RT then reactant
BB-3 (1.1 to 2 eq) at 0 C. The rxn mixture was allowed to reach RT and stirred
at a given temperature for a given
5 time (see Table 19). It was quenched with water and extracted with Et0Ac
(3x). The combined org. phases were
washed with brine, dried over MgSO4 and concentrated in vacuo. The crude was
purified by CC using Hept/Et0Ac
or DCM/Me0H or by trituration in a mixture of Hept/Et0Ac 1/1 and filtration.
Method B (NaH / THF)
To a soln. of intermediate A-3 (1 eq) in anh. THF (3 mL/mmol) was added NaH (2
eq, as a 60% dispersion in
10 mineral oil) at RT followed by BB-3 (1.2 eq). When necessary in term of
solubility, anh. DMF (0.1 to 2 mL/mmol)
could be added. The rxn mixture was stirred at a given temperature for a given
time (see Table 19), quenched with
a sat. soln. of NaHCO3 and extracted with Et0Ac (3x). The combined org. phases
were washed with brine, dried
over MgSO4 and concentrated in vacuo. The crude was purified by CC using
Hept/Et0Ac or DCM/Me0H
Table 19
Method MS-
data
Reactant Reactant
la Name T [ C] tR [min] m/z
A-3 BB-3
time [h]
[M+Fl]4
4-[4-Methoxy-2-oxo-3-(2-
trifluoromethyl-benzyI)-2,3-
2- A
dihydro-benzoimidazol-1-y1]-
la-1 A-3-1 (Trifluoromethyl) RT 1.04 505.98
piperidine-1-carboxylic acid
benzyl bromide 24
tert-butyl ester
(Example 1)
(R)-3-[4-Methoxy-2-oxo-3-(2-
trifluoromethyl-benzy1)-2,3-
2- A
dihydro-benzoimidazol-1-y1]-
la-2 A-3-2 (Trifluoromethyl) RT 1.02 492.10
pyrrolidine-1-carboxylic acid
benzyl bromide 24
tert-butyl ester
(Example 6)
(R)-3-[4-Methoxy-2-oxo-3-(2-
trifluoromethoxy-benzy1)-2,3- 2-
dihydro-benzoimidazol-1-y1]- (Trifluoromethox
la-3 A-3-2 50 C 1.03
507.95
pyrrolidine-1-carboxylic acid y) benzyl
24
tert-butyl ester bromide
(Example 20)
CA 03087886 2020-07-08
WO 2019/141803 PCT/EP2019/051230
96
(R)-3-[4-Methoxy-2-oxo-3-(2-
trifluoromethyl-benzy1)-2,3-
2- A
dihydro-imidazo[4,5-
la-4 A-3-3 (Trifluoromethyl) RI 1.01 493.15
c]pyridin-1-yI]-pyrrolidine-1-
benzyl bromide 1.5
carboxylic acid tert-butyl
ester (Example 39)
(R)-3-[7-Methoxy-2-oxo-1-(2-
trifluoromethyl-benzy1)-1,2-
2-
dihydro-imidazo[4,5-
la-5 A-3-4 (Trifluoromethyl) 50 C 1.00 493.13
b]pyridine-3-yI]-pyrrolidine-1-
benzyl bromide 5
carboxylic acid tert-butyl
ester (Example 43)
4-[4-Methoxy-2-oxo-3-(2-
trifluoromethyl-pyridin-3-
A
ylmethyl)-2,3-dihydro-
la-6 A-3-1 BB-3-1 RI 0.99 507.13
benzoimidazol-1-y1]-
24
piperidine-1-carboxylic acid
tert-butyl ester
4-[4-Methoxy-2-oxo-3-(4-
trifluoromethyl-pyridin-3-
A
ylmethyl)-2,3-dihydro-
la-7 A-3-1 BB-3-2 RI 1.01 507.13
benzoimidazol-1-y1]-
3
piperidine-1-carboxylic acid
tert-butyl ester (Example 53)
(R)-3-[4-Methoxy-3-(2-
methoxy-benzyI)-2-oxo-2,3- RT
2-
dihydro-benzoimidazol-1-y1]- 18
la-8 A-3-2 Methoxybenzyl 0.98 454.19
pyrrolidine-1-carboxylic acid then
chloride
tert-butyl ester 50 C
(Example 65) 4
4-[4-Methoxy-2-oxo-3-(3-
trifluoromethyl-pyridin-4-
A
ylmethyl)-2,3-dihydro-
la-9 A-3-1 BB-3-3 RI 1.02 507.14
benzoimidazol-1-y1]-
24
piperidine-1-carboxylic acid
tert-butyl ester
CA 03087886 2020-07-08
WO 2019/141803 PCT/EP2019/051230
97
4-[4-Methoxy-2-oxo-3-(3-
trifluoromethyl-pyridin-2-
A
ylmethyl)-2,3-dihydro-
la-10 A-3-1 BB-3-4 RI 0.99 507.12
benzoimidazol-1-y1]-
24
piperidine-1-carboxylic acid
tert-butyl ester (Example 64)
(R)-3-[4-Methoxy-3-(2-
methoxy-benzyI)-2-oxo-2,3-
2- A
dihydro-imidazo[4,5-
la-11 A-3-3 Methoxybenzyl RI 0.95 455.21
c]pyridine-1-yI]-pyrrolidine-1-
chloride 24
carboxylic acid tert-butyl
ester
4-[4-Methoxy-2-oxo-3-(2-
trifluoromethyl-benzyI)-2,3-
2-
dihydro-benzoimidazol-1-y1]-
la-12 A-3-5 (Trifluoromethyl) RI 1.06 520.24
2-methyl-piperidine-1-
benzyl bromide 24
carboxylic acid tert-butyl
ester (Example 144)
4-[4-Methoxy-2-oxo-3-(2- A
trifluoromethyl-benzyI)-2,3- RT
2-
dihydro-benzoimidazol-1-y1]- 1
la-13 A-3-6 (Trifluoromethyl) 1.06 520.13
3-methyl-piperidine-1- then
benzyl bromide
carboxylic acid tert-butyl 50 C
ester (Example 146) 18
4-[4-Methoxy-2-oxo-3-(2-
trifluoromethyl-benzyI)-2,3-
2- A
dihydro-benzoimidazol-1-y1]-
la-14 A-3-7 (Trifluoromethyl) RI 1.04 578.05
piperidine-1,3-dicarboxylic
benzyl bromide 8.5
acid 1-tert-butyl ester 3-ethyl
ester (Example 164)
4-[4-Methyl-2-oxo-3-(2-
trifluoromethyl-benzyI)-2,3- 2- A
la-24 dihydro-benzoimidazol-1-y1]- A-3-8 (Trifluoromethyl) RI 1.21
490.29
piperidine-1-carboxylic acid benzyl bromide 18
tert-butyl ester
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
98
Synthesis of intermediates of formula B-1
A mixture of BB-6 (1 eq) and BB-5 (8.3 eq) in DIPEA (2 eq) was heated to 180 C
and stirred for a given
temperature (see Table 20). It was partitioned between DCM and water and the
org. phase was washed with brine,
dried over MgSO4 and concentrated in vacuo. The crude was purified by CC using
Hept/Et0Ac.
Table 20
MS-data
Reactant Reactant time
B-1 Name tR [min] m/z
BB-6 BB-5 [h]
[M+Fl]4
(2-Methoxy-6-nitro-phenyI)- 2-
2-Chloro-3-
B-1-1 (2-trifluoromethyl-benzyI)-
(Trifluoromethyl) 3.5 0.99 327.04
nitroanisole
amine benzylamine
Synthesis of intermediates of formula B-2
To a suspension of intermediate B-1 (1 eq) and zinc dust (10 eq) in Me0H (10
mL/mmol) was added ammonium
formate (10 eq) at 0 C. The rxn mixture was stirred at a given temperature for
a given time (see Table 21), filtered
over a pad of celite and the filtrate was concentrated in vacuo. The crude was
purified by CC using DCM/Me0H.
Table 21
MS-data
Reactant T [ C]
B-2 Name tR [min] m/z
B-1 time [h]
[M+Fl]4
3-Methoxy-N2-(2-trifluoromethyl-benzyI)- 0
B-2-1 B-1-1 0.74 297.07
benzene-1,2-diamine 0.5
Synthesis of intermediates of formula B-3
Intermediates B-3 were prepared using a similar protocol as for the synthesis
of intermediates A-3 replacing
intermediates A-2 by intermediates B-2 (see Table 22).
Table 22
MS-data
Reactant T [ C]
B-3 Name tR [min] m/z
B-2 time [h]
[M+Fl]4
7-Methoxy-1-(2-trifluoromethyl-benzyI)-1,3- RT
B-3-1 B-2-1 0.87 323.06
dihydro-benzoimidazol-2-one 2
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
99
Synthesis of compounds of formula la (from compounds of formula B-3)
To a soln. of intermediate B-3 (1 eq) and BB-7 (1.5 eq) in toluene (6.8
mL/mmol) was added a 1M soln. of
(tributylphosphoranylidene)acetonitrile in toluene (2 eq) under argon. The rxn
mixture was heated to 110 C and
stirred for a given time (see Table 23). It was quenched with water and
extracted with Et0Ac (3x). The combined
org. phases were washed with brine, dried over MgSO4 and concentrated in
vacuo. The crude was purified by CC
using Hept/Et0Ac and/or DCM/Me0H. When necessary, an additional purification
by prep. LC-MS Method 1 can
be performed.
Table 23
MS-data
Reactant time
la Name Reactant BB-7 tR [min] m/z
B-3 [h]
[WE]4
4-[4-Methoxy-2-oxo-3-(2-
4-Hydroxy
trifluoromethyl-benzyI)-2,3-
azepane-1-
la-15 dihydro-benzoimidazol-1-y1]- B-3-1 2 1.04
520.12
carboxylic acid
azepane-1-carboxylic acid tert-
tert-butyl ester
butyl ester (Example 179)
5-[4-Methoxy-2-oxo-3-(2-
5-hydroxy-2-
trifluoromethyl-benzyI)-2,3-
azabicyclo[2.2.1
dihydro-benzoimidazol-1-y1]-2-
la-16 B-3-1 ] heptane-2- 8 1.04
518.10
aza-bicyclo[2.2.1]heptane-2-
carboxylic acid
carboxylic acid tert-butyl ester
tert-butyl ester
(Example 188)
6-[4-Methoxy-2-oxo-3-(2-
6-hydroxy-3-
trifluoromethyl-benzyI)-2,3-
azabicyclo[3.1.1
dihydro-benzoimidazol-1-y1]-3-
la-17 B-3-1 ] heptane-3- 18 1.04
518.11
aza-bicyclo[3.1.1]heptane-3-
carboxylic acid
carboxylic acid tert-butyl ester
tert-butyl ester
(Example 191)
(3R*,4S*)-3-[4-Methoxy-2-oxo-3-
(2-trifluoromethyl-benzy1)-2,3-
cis-1-Boc-4-
dihydro-benzoimidazol-1-y1]-4-
la-18 B-3-1 methylpyrrolidin- 18
1.03 506.13
methyl-pyrrolidine-1-carboxylic
3-ol
acid tert-butyl ester
(Example 196)
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
100
(3R*,4R*)-3-[4-Methoxy-2-oxo-3-
(2-trifluoromethyl-benzyI)-2,3-
trans-1-Boc-4-
dihydro-benzoimidazol-1-y1]-4-
la-19 B-3-1 methylpyrrolidin- 18 1.03
506.12
methyl-pyrrolidine-1-carboxylic
3-ol
acid tert-butyl ester
(Example 199)
3-Fluoro-4-[4-methoxy-2-oxo-3- 3-Fluoro-4-
(2-trifluoromethyl-benzyI)-2,3- hydroxypiperidin
la-20 dihydro-benzoimidazol-1-y1]- B-3-1 e-1-
carboxylic 18 1.03 524.04
piperidine-1-carboxylic acid tert- acid tert-butyl
butyl ester (Example 210) ester
3-[4-Methoxy-2-oxo-3-(2-
trifluoromethyl-benzy1)-2,3- 1-Boc-3-
la-21 dihydro-benzoimidazol-1-y1]- B-3-1
hydroxypiperidin 18 1.05 506.08
piperidine-1-carboxylic acid tert-
butyl ester (Example 214)
3-[4-Methoxy-2-oxo-3-(2-
trifluoromethyl-benzy1)-2,3- 1-Boc-3-
la-22 dihydro-benzoimidazol-1-y1]- B-3-1
hydroxyazetidin 16 1.01 478.03
azetidine-1-carboxylic acid tert-
butyl ester (Example 213)
4-[4-Methoxy-2-oxo-3-(2-
trifluoromethyl-benzy1)-2,3- 1-Boc-4-
dihydro-benzoimidazol-1-y1]-3,3- hydroxy-3,3-
la-23 B-3-1 18 1.07 534.22
dimethyl-piperidine-1-carboxylic dimethylpiperidi
acid tert-butyl ester ne
(Example 228)
Synthesis of intermediates of formula C-1
To a soln. of intermediate la (1 eq) in DCM (2 to 10 mL/mmol) was added TFA
(1.5 to 5 mL/mmol) and the rxn
mixture was stirred at RT for a given time (see Table 24). It was quenched
with a 1M soln. of NaOH until pH
reached 12 to 13 and extracted with DCM (3x). The combined org. phases were
washed with brine, dried over
MgSO4 and concentrated in vacuo. The crude was purified by CC using DCM/Me0H.
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
101
Table 24
MS-data
Reactant time
C-1 Name tR [min] m/z
la [h]
[M+Fl]4
4-Methoxy-1-piperidin-4-y1-3-(2-trifluoromethyl-benzy1)-
C-1-1 la-1 2.5 0.72 406.13
1,3-dihydro-benzoimidazol-2-one
4-Methoxy-1-(R)-pyrrolidin-3-y1-3-(2-trifluoromethyl-
C-1-2 la-2 0.5 0.73 392.17
benzy1)-1,3-dihydro-benzoimidazol-2-one
4-Methoxy-1-(R)-pyrrolidin-3-y1-3-(2-trifluoromethoxy-
C-1-3 la-3 2 0.73 407.93
benzy1)-1,3-dihydro-benzoimidazol-2-one
4-Methoxy-1-(R)-pyrrolidin-3-y1-3-(2-trifluoromethyl-
C-1-4 la-4 1 0.67 393.09
benzy1)-1,3-dihydro-imidazo[4,5-c]pyridine-2-one
7-Methoxy-3-(R)-pyrrolidin-3-y1-1-(2-trifluoromethyl-
C-1-5 la-5 1.5 0.68 393.23
benzy1)-1,3-dihydro-imidazo[4,5-b]pyridine-2-one
4-Methoxy-1-piperidin-4-y1-3-(2-trifluoromethyl-pyridin-
C-1-6 la-6 1.5 0.65 407.20
3-ylmethyl)-1,3-dihydro-benzoimidazol-2-one
4-Methoxy-1-piperidin-4-y1-3-(4-trifluoromethyl-pyridin-
C-1-7 la-7 0.7 0.65 407.19
3-ylmethyl)-1,3-dihydro-benzoimidazol-2-one
4-Methoxy-3-(2-methoxy-benzy1)-1-(R)-pyrrolidin-3-yl-
C-1-8 la-8 2.5 0.66 354.10
1,3-dihydro-benzoimidazol-2-one
4-Methoxy-1-piperidin-4-y1-3-(3-trifluoromethyl-pyridin-
C-1-9 la-9 0.5 0.66 407.19
4-ylmethyl)-1,3-dihydro-benzoimidazol-2-one
4-Methoxy-1-piperidin-4-y1-3-(3-trifluoromethyl-pyridin-
C-1-10 la-10 2 0.66 407.17
2-ylmethyl)-1,3-dihydro-benzoimidazol-2-one
4-Methoxy-3-(2-methoxy-benzy1)-1-(R)-pyrrolidin-3-yl-
C-1-11 la-11 2 0.62 355.14
1,3-dihydro-imidazo[4,5-c]pyridine-2-one
4-Methoxy-1-(2-methyl-piperidin-4-y1)-3-(2-
C-1-12 trifluoromethyl-benzy1)-1,3-
dihydro-benzoimidazol-2- la-12 18 0.73 420.15
one
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
102
4-Methoxy-1-(3-methyl-piperidin-4-yI)-3-(2-
C-1-13 trifluoromethyl-benzy1)-1,3-dihydro-benzoimidazol-2- la-13
1.5 0.73 420.14
one
1-Azepan-4-y1-4-methoxy-3-(2-trifluoromethyl-benzy1)-
C-1-14 la-15 0.5 0.74 420.11
1,3-dihydro-benzoimidazol-2-one
2-Aza-bicyclo[2.2.1]hept-5-y1-4-methoxy-3-(2-
C-1-15 trifluoromethyl-benzy1)-1,3-dihydro-benzoimidazol-2- la-16
1 0.76 418.09
one
1-(3-Aza-bicyclo[3.1.1]hept-6-y1)-4-methoxy-3-(2-
C-1-16 trifluoromethyl-benzy1)-1,3-dihydro-benzoimidazol-2- la-17
0.5 0.75 418.10
one
trans-4-Methoxy-1-(4-methyl-pyrrolidin-3-yI)-3-(2-
C-1-17 trifluoromethyl-benzy1)-1,3-dihydro-benzoimidazol-2- la-18
1 0.74 406.18
one
cis-4-Methoxy-1-(4-methyl-pyrrolidin-311)-3-(2-
C-1-18 trifluoromethyl-benzy1)-1,3-dihydro-benzoimidazol-2- la-19
1 0.74 406.11
one
1-(3-Fluoro-piperidin-411)-4-methoxy-3-(2-
0.73/0.
C-1-19 trifluoromethyl-benzy1)-1,3-dihydro-benzoimidazol-2- la-20
0.5 424.07
74
one
4-Methoxy-1-piperidin-3-y1-3-(2-trifluoromethyl-benzy1)-
C-1-20 la-21 2 0.73 406.15
1,3-dihydro-benzoimidazol-2-one
1-Azetidin-3-y1-4-methoxy-3-(2-trifluoromethyl-benzy1)-
C-1-21 la-22 0.75 0.70 378.11
1,3-dihydro-benzoimidazol-2-one
1-(3,3-Dimethyl-piperidin-411)-4-methoxy-3-(2-
C-1-22 trifluoromethyl-benzy1)-1,3-dihydro-benzoimidazol-2- la-23
0.5 0.75 434.05
one
4-Methyl-1-piperidin-4-y1-3-(2-trifluoromethyl-benzy-
C-1-23 la-24 1 0.82 390.25
1,3-dihydro-benzoimidazol-2-one
Synthesis of compounds of formula lb
Method A (Buchwald-Hartwiq)
To a mixture of C-1 (1 eq), BB-8 (1.3 eq) and sodium tert-butoxide (2 eq) in
toluene (3 to 10 mL/mmol) under N2,
was added BINAP (0.2 eq) and Pd2(dba)3 (0.1 eq). The rxn mixture was flushed
with N2, heated at a given
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
103
temperature and stirred for a given time (see Table 25). It was partitioned
between water and Et0Ac and the org.
phase was washed with brine, dried over MgSO4 and concentrated in vacuo. The
crude was purified by CC using
Hept/Et0Ac. When necessary an additional purification by prep. LC-MS was
performed.
Method B (Reductive amination)
To a soln. of C-1 (1 eq) in THF (10 mL/mmol) was added BB-8 (3 eq) and the rxn
mixture was stirred for 1h. AcOH
(1.1 eq) and NaBH(OAc)3 (3 eq) were added and the rxn mixture was stirred at
RT for a given time (see Table 25).
It was quenched with a 1M aq. soln. of NaOH and extracted with DCM (3x). The
combined org. phases were
washed with brine, dried over MgSO4 and concentrated in vacuo. The crude was
purified by CC using Hept/Et0Ac.
Table 25
Method MS-
data
Reactant Reactant
lb Name T [ C] tR [min] m/z
C-1 BB-8
time [h] [WE]4
i-[1-(2,6-Dimethyl-pheny-
pipericlin-4-y1]-4-methoxy-3-(2- A
2-Bromo-1,3-
lb-1 trifluoromethyl-benzyI)-1,3- C-1-1 . 100 1.15 --
510.12
dimethylbenzene
dihydro-benzoimidazol-2-one 18
(Example 2)
4-Methoxy-1-(1-phenyl-piperidin-
4-yI)-3-(2-trifluoromethyl- A
lb-2 benzyI)-1,3-dihydro- C-1-1 Bromobenzene 100 --
0.91 -- 481.94
benzoimidazol-2-one 18
(Example 4)
1-[1-(2,4-Dichloro-benzyI)-
piperidin-4-yI]-4-methoxy-3-(2-
2,4-Dichloro
lb-3 trifluoromethyl-benzyI)-1,3- C-1-1 RT 0.85
563.96
benzaldehyde
dihydro-benzoimidazol-2-one 4
(Example 5)
1-[(R)-1-(2,6-Difluoro-pheny-
pyrrolidin-3-yI]-4-methoxy-3-(2- A
2,6-Difluoro
lb-4 trifluoromethyl-benzyI)-1,3- C-1-2 100 1.09
504.07
bromobenzene
dihydro-benzoimidazol-2-one 18
(Example 7)
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
104
1-[(R)-1-(2,6-Dimethyl-pheny1)-
pyrrolidin-3-yI]-4-methoxy-3-(2- A
2-Bromo-1,3-
lb-5 trifluoromethyl-benzyI)-1,3- C-1-2 100 1.00
496.12
dimethylbenzene
dihydro-benzoimidazol-2-one 18
(Example 8)
1-[1-(2,6-Difluoro-phenyI)-
piperidin-4-yI]-4-methoxy-3-(2- A
2,6-Difluoro
lb-6 trifluoromethyl-benzyI)-1,3- C-1-1 100 1.11
518.10
bromobenzene
dihydro-benzoimidazol-2-one 18
(Example 9)
1-[1-(2,6-Dimethoxy-pheny1)-
piperidin-4-y1]-4-methoxy-3-(2- 2-Bromo-1,3- A
lb-7 trifluoromethyl-benzyI)-1,3- C-1-1 dimethoxy 100 0.87
542.08
dihydro-benzoimidazol-2-one benzene 18
(Example 10)
1-[1-(2-Fluoro-6-methoxy-
pheny1)-piperidin-4-y1]-4-
A
methoxy-3-(2-trifluoromethyl- 2-Bromo-3-
lb-8 C-1-1 100 1.04 529.98
benzyI)-1,3-dihydro- fluoroanisole
18
benzoimidazol-2-one
(Example 11)
4-Methoxy-1-[1-(2-methoxy-6-
methyl-pheny1)-piperidin-4-y1]-3- 2-Bromo-1- A
lb-9 (2-trifluoromethyl-benzyI)-1,3- C-1-1 methoxy-3- 100 1.03
526.09
dihydro-benzoimidazol-2-one methylbenzene 24
(Example 12)
i-[1-(2-Fluoro-6-methyl-pheny-
piperidin-4-yI]-4-methoxy-3-(2- A
2-Bromo-3-
lb-10 trifluoromethyl-benzyI)-1,3- C-1-1 100 1.14
514.04
fluorotoluene
dihydro-benzoimidazol-2-one 18
(Example 13)
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
105
1-[(R)-1-(2-Fluoro-6-methyl-
pheny1)-pyrrolidin-3-y1]-4-
A
methoxy-3-(2-trifluoromethyl- 2-Bromo-3-
lb-11 C-1-2 100 1.09 500.25
benzyI)-1,3-dihydro- fluorotoluene
18
benzoimidazol-2-one
(Example 15)
4-Methoxy-1-[(R)-1-(2-methoxy-
pheny1)-pyrrolidin-3-y1]-3-(2- A
lb-12 trifluoromethyl-benzyI)-1,3- C-1-2 2-Bromoanisole 100
0.90 497.99
dihydro-benzoimidazol-2-one 18
(Example 16)
1-[(R)-1-(2,6-Dimethoxy-pheny1)-
pyrrolidin-3-yI]-4-methoxy-3-(2- 2-Bromo-1,3- A
lb-13 trifluoromethyl-benzyI)-1,3- C-1-2 dimethoxy 100 0.87
527.94
dihydro-benzoimidazol-2-one benzene 18
(Example 17)
4-Methoxy-1-[(R)-1-(2-methoxy-
6-methyl-pheny1)-pyrrolidin-3-y1]- 2-Bromo-1- A
lb-14 3-(2-trifluoromethyl-benzyI)-1,3- C-1-2 methoxy-3- 100
0.90 511.98
dihydro-benzoimidazol-2-one methylbenzene 18
(Example 18)
1-[(R)-1-(2-Fluoro-6-methoxy-
pheny1)-pyrrolidin-3-y1]-4-
A
methoxy-3-(2-trifluoromethyl- 2-Bromo-3-
lb-15 C-1-2 100 0.98 515.95
benzyI)-1,3-dihydro- fluoroanisole
18
benzoimidazol-2-one
(Example 19)
1-[(R)-1-(2,6-Dimethyl-pheny1)-
pyrrolidin-3-yI]-4-methoxy-3-(2- A
2-Bromo-1,3-
lb-16 trifluoromethoxy-benzyI)-1,3- C-1-3 100 0.99
511.95
dimethylbenzene
dihydro-benzoimidazol-2-one 18
(Example 22)
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
106
1-[(R)-1-(2,6-Difluoro-pheny1)-
pyrrolidin-3-yI]-4-methoxy-3-(2- A
2,6-Difluoro
lb-17 trifluoromethoxy-benzyI)-1,3- C-1-3 100 1.09
519.90
bromobenzene
dihydro-benzoimidazol-2-one 18
(Example 23)
4-Methoxy-1-[(R)-1-(2-methoxy-
4-methyl-pyridin-3-y1)-pyrrolidin- 3-Bromo-2- A
lb-18 3-y1]-3-(2-trifluoromethyl-benzy1)- C-1-2 methoxy-4- 100 1.00
513.12
1,3-dihydro-benzoimidazol-2- methylpyridine 18
one (Example 33)
1-[(R)-1-(3,5-Difluoro-pyridin-4-
y-pyrrolidin-3-y1]-4-methoxy-3- A
4-Bromo-3,5-
lb-19 (2-trifluoromethyl-benzyI)-1,3- C-1-2 100 0.83
505.08
difluoropyridine
dihydro-benzoimidazol-2-one 18
(Example 34)
1-[(R)-1-(3,5-Dimethyl-pyridin-4-
y-pyrrolidin-3-y1]-4-methoxy-3- 4-Bromo-3,5- A
lb-20 (2-trifluoromethyl-benzyI)-1,3- C-1-2 dimethylpyridine .. 100
.. 0.81 .. 497.13
dihydro-benzoimidazol-2-one hydrochloride 18
(Example 36)
1-[(R)-1-(2-Fluoro-4-methyl-
pyridin-3-y-pyrrolidin-3-y1]-4-
3-Bromo-2- A
methoxy-3-(2-trifl uoromethyl-
lb-21 C-1-2 fluoro-4- 100 1.07 501.11
benzyI)-1,3-dihydro-
methylpyridine 18
benzoimidazol-2-one
(Example 37)
1-[(R)-1-(3,5-Dimethoxy-pyridin-
4-y-pyrrolidin-3-y1]-4-methoxy- 4-Bromo-3,5- A
lb-22 3-(2-trifluoromethyl-benzyI)-1,3- C-1-2 dimethoxypyridin
100 0.82 529.11
dihydro-benzoimidazol-2-one e 18
(Example 38)
1-[(R)-1-(2,6-Dimethyl-pheny-
pyrrolidin-3-yI]-4-methoxy-3-(2- A
2-Bromo-1,3-
lb-23 trifluoromethyl-benzyI)-1,3- C-1-4 100 1.03
497.12
dimethylbenzene
dihydro-imidazo[4,5-c]pyridin-2- 18
one (Example 41)
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
107
1-[(R)-1-(2,6-Difluoro-pheny1)-
pyrrolidin-3-yI]-4-methoxy-3-(2- A
2,6-Difluoro
lb-24 trifluoromethyl-benzyI)-1,3- C-1-4 100
1.08 505.06
bromobenzene
dihydro-imidazo[4,5-c]pyridin-2- 18
one (Example 42)
3-[(R)-1-(2,6-Dimethyl-pheny1)-
pyrrolidin-3-yI]-7-methoxy-1-(2- A
2-Bromo-1,3-
lb-25 trifluoromethyl-benzyI)-1,3- C-1-5 100
0.86 497.14
dimethylbenzene
dihydro-imidazo[4,5-b]pyridin-2- 18
one (Example 45)
3-[(R)-1-(2,6-Difluoro-pheny1)-
pyrrolidin-3-yI]-7-methoxy-1-(2- A
2,6-Difluoro
lb-26 trifluoromethyl-benzyI)-1,3- C-1-5 100
1.04 505.11
bromobenzene
dihydro-imidazo[4,5-b]pyridin-2- 18
one (Example 46)
4-Methoxy-1-[(R)-1-(2-methoxy-
4-methyl-pyridin-3-y1)-pyrrolidin-
3-Bromo-2- A
3-y1]-3-(2-trifluoromethyl-benzy1)-
lb-27 C-1-4 methoxy-4- 100 1.05 514.13
1,3-dihydro-imidazo[4,5-
methylpyridine 18
c]pyridin-2-one
(Example 47)
1-[(R)-1-(2-Fluoro-4-methyl-
pyridin-3-y-pyrrolidin-3-y1]-4- 3-Bromo-2- A
lb-28 methoxy-3-(2-trifluoromethyl- C-1-4 fluoro-4- 100 1.05
502.11
benzyI)-1,3-dihydro-imidazo[4,5- methylpyridine 18
c]pyridin-2-one (Example 48)
1-[(R)-1-(2-Fluoro-6-methoxy-
pheny-pyrrolidin-3-y1]-4- A
2-Bromo-3-
lb-29 methoxy-3-(2-trifluoromethyl- C-1-4 100 1.03
517.13
fluoroanisole
benzyI)-1,3-dihydro-imidazo[4,5- 18
c]pyridin-2-one (Example 49)
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
108
1-[(R)-1-(2-Fluoro-6-methyl-
pheny1)-pyrrolidin-3-y1]-4-
A
methoxy-3-(2-trifluoromethyl- 2-Bromo-3-
lb-30 C-1-4 100 1.10 501.12
benzyI)-1,3-dihydro-imidazo[4,5- fluorotoluene
18
c]pyridin-2-one
(Example 50)
4-Methoxy-1-[(R)-1-(2-methoxy-
6-methyl-pheny1)-pyrrolidin-3-y1]- 2-Bromo-1- A
lb-31 3-(2-trifluoromethyl-benzyI)-1,3- C-1-4 methoxy-3- 100
0.88 513.05
dihydro-imidazo[4,5-c]pyridin-2- methylbenzene 18
one (Example 51)
1-[1-(2,6-Dimethyl-pheny1)-
piperidin-4-y1]-4-methoxy-3-(2-
A
trifluoromethyl-pyridin-3- 2-Bromo-1,3-
lb-32 C-1-6 100 1.11 511.15
ylmethyl)-1,3-dihydro- dimethylbenzene
18
benzoimidazol-2-one
(Example 54)
1-[1-(2,6-Difluoro-phenyI)-
piperidin-4-yI]-4-methoxy-3-(2-
A
trifluoromethyl-pyridin-3- 2,6-Difluoro
lb-33 C-1-6 100 1.07 519.11
ylmethyl)-1,3-dihydro- bromobenzene
18
benzoimidazol-2-one
(Example 55)
1-[1-(2,6-Dimethyl-pheny1)-
piperidin-4-y1]-4-methoxy-3-(4-
A
trifluoromethyl-pyridin-3- 2-Bromo-1,3-
lb-34 C-1-7 100 1.14 511.19
ylmethyl)-1,3-dihydro- dimethylbenzene
18
benzoimidazol-2-one
(Example 58)
1-[1-(2,6-Difluoro-phenyI)-
piperidin-4-yI]-4-methoxy-3-(4-
A
trifluoromethyl-pyridin-3- 2,6-Difluoro
lb-35 C-1-7 100 1.09 519.15
ylmethyl)-1,3-dihydro- bromobenzene
24
benzoimidazol-2-one
(Example 59)
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
109
1-[(R)-1-(2,4-Dimethyl-pyridin-3-
yI)-pyrrolidin-3-y1]-4-methoxy-3- A
3-Bromo-2,4-
lb-36 (2-trifluoromethyl-benzyI)-1,3- C-1-2 105 0.81
497.12
dimethylpyridine
dihydro-benzoimidazol-2-one 18
(Example 66)
1-[(R)-1-(2,6-Dimethyl-pheny1)-
pyrrolidin-3-yI]-4-methoxy-3-(2- A
2-Bromo-1,3-
lb-37 methoxy-benzyI)-1,3-dihydro- C-1-8 105 0.94
458.17
dimethylbenzene
benzoimidazol-2-one 18
(Example 67)
1-[(R)-1-(2,6-Difluoro-pheny1)-
pyrrolidin-3-yI]-4-methoxy-3-(2- A
2,6-Difluoro
lb-38 methoxy-benzyI)-1,3-dihydro- C-1-8 105 1.05
466.49
bromobenzene
benzoimidazol-2-one 18
(Example 68)
1-[1-(2,6-Dimethyl-pheny1)-
piperidin-4-y1]-4-methoxy-3-(3-
A
trifluoromethyl-pyridin-4- 2-Bromo-1,3-
lb-39 C-1-9 100 1.16 511.17
ylmethyl)-1,3-dihydro- dimethylbenzene
18
benzoimidazol-2-one
(Example 73)
1-[1-(2,6-Difluoro-phenyI)-
piperidin-4-yI]-4-methoxy-3-(3-
A
trifluoromethyl-pyridin-4- 2,6-Difluoro
lb-40 C-1-9 100 1.11 519.11
ylmethyl)-1,3-dihydro- bromobenzene
18
benzoimidazol-2-one
(Example 74)
1-[1-(2,6-Dimethyl-pheny1)-
piperidin-4-y1]-4-methoxy-3-(3-
A
trifluoromethyl-pyridin-2- 2-Bromo-1,3-
lb-41 C-1-10 100 1.10 511.20
ylmethyl)-1,3-dihydro- dimethylbenzene
18
benzoimidazol-2-one
(Example 75)
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
110
1-[1-(2,6-Difluoro-phenyI)-
piperidin-4-yI]-4-methoxy-3-(3-
A
trifluoromethyl-pyridin-2- 2,6-Difluoro
lb-42 C-1-10 100 1.06 519.11
ylmethyl)-1,3-dihydro- bromobenzene
18
benzoimidazol-2-one
(Example 76)
1-[(R)-1-(2,6-Dimethyl-pheny1)-
pyrrolidin-3-yI]-4-methoxy-3-(2- A
2-Bromo-1,3-
lb-43 methoxy-benzyI)-1,3-dihydro- C-1-11 100 0.98
459.16
dimethylbenzene
imidazo[4,5-c]pyridin-2-one 18
(Example 77)
1-[(R)-1-(2-Fluoro-6-methyl-
pheny1)-pyrrolidin-3-y1]-4- A
2-Bromo-3-
lb-44 methoxy-3-(2-methoxy-benzyI)- C-1-11 100 1.05
463.12
fluorotoluene
1,3-dihydro-imidazo[4,5- 18
c]pyridin-2-one (Example 78)
1-[(R)-1-(2-Fluoro-6-methyl-
pheny1)-pyrrolidin-3-y1]-4- A
2-Bromo-3-
lb-45 methoxy-3-(2-methoxy-benzyI)- C-1-8 100 1.04 462.24
fluorotoluene
1,3-dihydro-benzoimidazol-2- 18
one (Example 110)
1-[1-(2-Fluoro-6-methyl-phenyI)-
3-methyl-piperidin-4-yI]-4-
A
methoxy-3-(2-trifl uoromethyl- 2-Bromo-3-
lb-46 C-1-13 105 1.14 528.16
benzyI)-1,3-dihydro- fluorotoluene
18
benzoimidazol-2-one
(Example 147)
1-[(R)-1-(2-Fluoro-pheny1)-
pyrrolidin-3-y1]-4-methoxy-3-(2- A
1-Bromo-2-
lb-47 trifluoromethyl-benzyI)-1,3- C-1-2 105 1.07
486.07
fluorobenzene
dihydro-benzoimidazol-2-one 18
(Example 160)
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
111
1-[(R)-1-(2-Fluoro-6-morpholin-
4-yl-pheny1)-pyrrolidin-3-y1]-4-
A
methoxy-3-(2-trifluoromethyl-
lb-48 C-1-2 BB-8-4 105 0.92 571.03
benzyI)-1,3-dihydro-
18
benzoimidazol-2-one
(Example 161)
1-[1-(2-Fluoro-6-methyl-phenyI)-
azepan-4-yI]-4-methoxy-3-(2- A
2-Bromo-3-
lb-49 trifluoromethyl-benzyI)-1,3- C-1-14 110 1.13
528.09
fluorotoluene
dihydro-benzoimidazol-2-one 18
(Example 184)
1-[1-(2,6-Dimethyl-pheny1)-
azepan-4-y1]-4-methoxy-3-(2- A
2-Bromo-1,3-
lb-50 trifluoromethyl-benzyI)-1,3- C-1-14 110 1.14
524.13
dimethylbenzene
dihydro-benzoimidazol-2-one 18
(Example 185)
1-[2-(2-Fluoro-6-methyl-phenyI)-
2-aza-bicyclo[2.2.1]hept-5-yI]-4-
A
methoxy-3-(2-trifluoromethyl- 2-Bromo-3-
lb-51 C-1-15 110 0.99 526.06
benzyI)-1,3-dihydro- fluorotoluene
18
benzoimidazol-2-one
(Example 190)
1-[3-(2-Fluoro-6-methyl-phenyI)-
3-aza-bicyclo[3.1.1]hept-6-yI]-4-
A
methoxy-3-(2-trifluoromethyl- 2-Bromo-3-
lb-52 C-1-16 110 1.12 526.14
benzyI)-1,3-dihydro- fluorotoluene
2.5
benzoimidazol-2-one
(Example 197)
1-[(3R*,4S*)-1-(2-Fluoro-6-
methyl-phenyI)-4-methyl-
A
pyrrolidin-3-yI]-4-methoxy-3-(2- 2-Bromo-3-
lb-53 C-1-17 110 1.12 514.12
trifluoromethyl-benzyI)-1,3- fluorotoluene
2
dihydro-benzoimidazol-2-one
(Example 198)
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
112
1
methyl-phenyI)-4-methyl-
A
pyrrolidin-3-yI]-4-methoxy-3-(2- 2-Bromo-3-
lb-54 C-1-18 110 1.05 514.10
trifluoromethyl-benzyI)-1,3- fluorotoluene
18
dihydro-benzoimidazol-2-one
(Example 200)
4-Methoxy-1-[(R)-1-(2-
trifluoromethoxy-pheny1)-
2- A
pyrrolidin-3-yI]-3-(2-
lb-55 C-1-2 (Trifluoromethox 110 1.10 552.02
trifluoromethyl-benzyI)-1,3-
y)bromobenzene 20
dihydro-benzoimidazol-2-one
(Example 201)
4-Methoxy-1-1(R)-1-[2-(2-
methoxy-ethoxy)-phenyl]-
1-Bromo-2-(2- A
pyrrolidin-3-y11-3-(2-
lb-56 C-1-2 methoxyethoxy)b 110 0.91 542.05
trifluoromethyl-benzyI)-1,3-
enzene 20
dihydro-benzoimidazol-2-one
(Example 204)
1-[3-Fluoro-1-(2-fluoro-6-methyl-
pheny1)-piperidin-4-y1]-4-
A
methoxy-3-(2-trifluoromethyl- 2-Bromo-3-
lb-57 C-1-19 110 1.09/1.11 532.02
benzyI)-1,3-dihydro- fluorotoluene
2
benzoimidazol-2-one
(Example 211)
1-[1-(2-Fluoro-6-methyl-phenyI)-
piperidin-3-yI]-4-methoxy-3-(2- A
2-Bromo-3-
lb-58 trifluoromethyl-benzyI)-1,3- C-1-20 110 1.11
514.05
fluorotoluene
dihydro-benzoimidazol-2-one 18
(Example 212)
1-[1-(2-Fluoro-6-methyl-phenyI)-
azetidin-3-yI]-4-methoxy-3-(2- A
2-Bromo-3-
lb-59 trifluoromethyl-benzyI)-1,3- C-1-21 110 1.07
486.04
fluorotoluene
dihydro-benzoimidazol-2-one 2
(Example 215)
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
113
1-[1-(2,6-Dimethyl-pheny1)-
piperidin-3-y1]-4-methoxy-3-(2- A
2-Bromo-1,3-
lb-60 trifluoromethyl-benzyI)-1,3- C-1-20 110 1.11
510.09
dimethylbenzene
dihydro-benzoimidazol-2-one 20
(Example 217)
1-[1-(2-Fluoro-6-methyl-phenyI)-
3,3-dimethyl-piperidin-4-yI]-4-
A
methoxy-3-(2-trifluoromethyl- 2-Bromo-3-
lb-61 C-1-22 110 1.16 542.18
benzyI)-1,3-dihydro- fluorotoluene
18
benzoimidazol-2-one
(Example 227)
1-[1-(2-Benzyloxy-pheny1)-
piperidin-4-y1]-4-methoxy-3-(2- A
2-Benzyloxy
lb-62 trifluoromethyl-benzyI)-1,3- C-1-1 100 0.96
588.15
bromobenzene
dihydro-benzoimidazol-2-one 5
(Example 231)
4-Methoxy-1-(3'-methyl-3,4,5,6-
tetrahydro-2H-[1,2]bipyridiny1-4- A
2-Bromo-3-
lb-63 yI)-3-(2-trifluoromethyl-benzy1)- C-1-1 110
0.81 497.06
methylpyridine
1,3-dihydro-benzoimidazol-2- 18
one (Example 232)
4-Methoxy-1-(3-methoxy-
3,4,5,6-tetrahydro-2H-
A
[1,Z]bipyridinyl-4-y1)-3-(2- 2-Bromo-3-
lb-64 C-1-1 110 0.82 513.06
trifluoromethyl-benzyI)-1,3- methoxypyridine
18
dihydro-benzoimidazol-2-one
(Example 233)
4-Methoxy-1-(2'-methoxy-4'-
methyl-3,4,5,6-tetrahydro-2H-
3-Bromo-2- A
[1,3]bipyridiny1-4-y1)-3-(2-
lb-65 C-1-1 methoxy-4- 110 1.10 527.10
trifluoromethyl-benzyI)-1,3-
methylpyridine 18
dihydro-benzoimidazol-2-one
(Example 234)
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
114
1-(Z-Fluoro-4'-methyl-3,4,5,6-
tetrahydro-2H-[1,3']bipyridiny1-4-
3-Bromo-2- A
yI)-4-methoxy-3-(2-
lb-66 C-1-1 fluoro-4- 110 1.08 515.09
trifluoromethyl-benzyI)-1,3-
methylpyridine 18
dihydro-benzoimidazol-2-one
(Example 235)
1-(3'-Fluoro-3,4,5,6-tetrahydro-
2H-[1,2]bipyridiny1-4-y1)-4-
A
methoxy-3-(2-trifl uoromethyl- 2-Bromo-3-
lb-67 C-1-1 110 1.00 501.12
benzyI)-1,3-dihydro- fluoropyridine
18
benzoimidazol-2-one
(Example 236)
tetrahydro-2H-[1,3']bipyridiny1-4-
A
yI)-4-methoxy-3-(2- 3-Bromo-2,4-
lb-68 C-1-1 110 0.81 511.18
trifluoromethyl-benzyI)-1,3- dimethylpyridine
18
dihydro-benzoimidazol-2-one
(Example 237)
tetrahydro-2H-[1,3']bipyridiny1-4-
3-Bromo-4- A
yI)-4-methoxy-3-(2-
lb-69 C-1-1 fluoro-2- 110 0.84 515.16
trifluoromethyl-benzyI)-1,3-
methylpyridine 6
dihydro-benzoimidazol-2-one
(Example 244)
i-[1-(2-Fluoro-6-methyl-pheny-
piperidin-4-yI]-4-methyl-3-(2- A
2-Bromo-3-
lb-70 trifluoromethyl-benzyI)-1,3- C-1-23 100 1.32
498.17
fluorotoluene
dihydro-benzoimidazol-2-one 2
(Example 245)
4-[4-Methoxy-2-oxo-3-(2-
trifluoromethyl-benzy1)-2,3-
3-Bromo-4- A
dihydro-benzoimidazol-1-y1]-4'-
lb-71 C-1-1 methylpicolinonit 110 1.06 522.18
methyl-3,4,5,6-tetrahydro-2H-
rile 18
[1,3']bipyridiny1-2'-carbonitrile
(Example 246)
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
115
4-Methoxy-1-(4'-methoxy-2-
methyl-3,4,5,6-tetrahydro-2H-
A
[1,3']bipyridiny1-4-y1)-3-(2-
lb-72 C-1-1 BB-8-6 110 0.83 527.18
trifluoromethyl-benzyI)-1,3-
18
dihydro-benzoimidazol-2-one
(Example 247)
1-(2',4'-Dimethoxy-3,4,5,6-
tetrahydro-2H-[1,3']bipyridiny1-4-
3-Bromo-2,4- A
yI)-4-methoxy-3-(2-
lb-73 C-1-1 dimethoxypyridin 110 0.87 543.06
trifluoromethyl-benzyI)-1,3-
18
dihydro-benzoimidazol-2-one
(Example 248)
4-Methoxy-1-[1-(4-methoxy-6-
methyl-pyrimidin-5-y1)-piperidin- 5-Bromo-4- A
lb-74 4-y1]-3-(2-trifluoromethyl-benzy1)- C-1-1 methoxy-6- 110
0.93 528.12
1,3-dihydro-benzoimidazol-2- methylpyrimidine 18
one (Example 249)
1-[1-(4,6-Dimethoxy-pyrimidin-5-
yI)-piperidin-4-y1]-4-methoxy-3- 5-Bromo-4,6- A
lb-75 (2-trifluoromethyl-benzyI)-1,3- C-1-1
dimethoxypyrimi 110 1.04 544.05
dihydro-benzoimidazol-2-one dine 4
(Example 250)
Synthesis of compounds of formula lc
Method A (standard SNAr/ CsF)
To a soln. of C-1 (1 eq) and BB-8 (1 to 1.1 eq) in DMSO (3 mL/mmol) was added
CsF (2 eq). The rxn mixture was
heated at a given temperature for a given time (see Table 26) and was
partitioned between Et0Ac and water. The
org. phase was washed with water (3x) and with brine, dried over MgSO4 and
concentrated in vacuo. The crude
was purified by CC using Hept/Et0Ac.
Method B (PvB0P activated SNAd
C-1 (1 eq), BB-8 (1.5 eq) and DIPEA (3 eq) were dissolved in anh. DMF (5
mL/mmol) and the mixture was stirred
for 5 min at RT. PyBOP (1.8 eq) was added portionwise and the rxn mixture was
further stirred at RT for a given
time (see Table 26). It was partitioned between Et0Ac and a 5% aq. soln. of
KHSO4 and the org. phase was
washed with a sat. aq. soln. of NaHCO3 (2x) and brine, dried over MgSO4 and
concentrated in vacuo. The crude
was purified by CC using Hept/Et0Ac.
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
116
Method C (standard SNAr/ K2CO2)
The procedure is similar to method A replacing CsF by K2CO3 (2 eq).
Method D (PvB0P activated SNAr + cyclodehydration)
The procedure is similar to method B but the resulting open form product is
additionally submitted to a
cyclodehydration as follows:
The open form product (1 eq) was dissolved in anh. THF and Burgess'reagent
(2.5 eq) was added portionwise at
RT (see Table 26). The rxn mixture was stirred at 150 C for 30 min under
microwave condition and partitioned
between Et0Ac and H20. The org. phase was washed with brine, dried over MgSO4
and concentrated in vacuo.
The crude was purified by CC using Hept/Et0Ac.
Table 26
Method MS-
data
Reactant Reactant
lc Name T [ C] tR [min] m/z
C-1 BB-8
time [h] [M+Fl]4
3-Cyclopropy1-5-14-[4-methoxy-
2-oxo-3-(2-trifluoromethyl- 5-Chloro-3-
benzyI)-2,3-dihydro- cyclopropyl-1- A
lc-1 benzoimidazol-1-y1]-piperidin-1- C-1-1 methyl -1H-
100 1.04 554.09
y11-1 -methyl-1H-pyrazole-4- pyrazole-4- 96
carbaldehyde carbaldehyde
(Example 14)
1-[(R)-1-(5-lsopropyl-
[1,3,4]oxadiazol-211)-pyrrolidin-
3-yI]-4-methoxy-3-(2-
lc-2 C-1-2 BB-8-5 RT 0.98 502.14
trifluoromethyl-benzyI)-1,3-
18
dihydro-benzoimidazol-2-one
(Example 69)
5-1(R)-3-[4-Methoxy-2-oxo-3-(2-
trifluoromethyl-benzyI)-2,3- 5-chloro-1,3-
A
dihydro-benzoimidazol-1-y1]- dimethyl-1H-
lc-3 C-1-2 100 0.99 514.16
pyrrolidin-1-y11-1,3-dimethy1-1H- pyrazole-4-
18
pyrazole-4-carbaldehyde carbaldehyde
(Example 71)
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
117
3-Fluoro-2-1(R)-3-[4-methoxy-2-
oxo-3-(2-trifluoromethyl-benzyI)-
2,3-Difluoro
lc-4 2,3-dihydro-benzoimidazol-1-y1]- C-1-2 80 1.06
511.13
benzonitrile
pyrrolidin-1-yll-benzonitrile 18
(Example 82)
3-Fluoro-2-1(R)-3-[4-methoxy-2-
oxo-3-(2-trifluoromethyl-benzyI)-
2,3-Difluoro
lc-5 2,3-dihydro-benzoimidazol-1-y1]- C-1-2 100 1.06
514.16
benzaldehyde
pyrrolidin-1-yll-benzaldehyde 18
(Example 83)
4-Methoxy-3-(2-trifluoromethyl-
benzy1)-1-[(R)-1-(5- 5-
trifluoromethyl-[1,3,4]oxadiazol- (Trifluorometh
lc-6 C-1-2 RI 1.00 528.10
211)-pyrrolidin-3-y1]-1,3-dihydro- yI)-1,3,4-
24
benzoimidazol-2-one oxadiazol-2-ol
(Example 84)
5-1(R)-3-[4-Methoxy-2-oxo-3-(2-
trifluoromethyl-benzyI)-2,3- 5-chloro-1,3-
A
dihydro-benzoimidazol-1-y1]- dimethyl-1H-
lc-7 C-1-2 80 1.00 511.34
pyrrolidin-1-y11-1,3-dimethy1-1H- pyrazole-4-
24
pyrazole-4-carbonitrile carbonitrile
(Example 87)
3-Fluoro-2-1(R)-3-[4-methoxy-3-
(2-methoxy-benzyI)-2-oxo-2,3-
2,3-Difluoro
lc-8 dihydro-benzoimidazol-1-y1]- C-1-8 105
1.02 473.21
benzonitrile
pyrrolidin-1-yll-benzonitrile 3
(Example 109)
3-Fluoro-2-14-[4-methoxy-2-oxo-
3-(2-trifluoromethyl-benzyI)-2,3-
dihydro-benzoimidazol-1-y1]-2- 2,3-Difluoro
lc-9 C-1-13 105 1.10 542.13
benzaldehyde
72
benzaldehyde
(Example 145)
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
118
1-[(R)-1-(2-Fluoro-6-nitro-
pheny1)-pyrrolidin-3-y1]-4-
methoxy-3-(2-trifluoromethyl- 2,3-Difluoro
Ic-10 C-1-2 100 1.07
530.98
benzyI)-1,3-dihydro- nitrobenzene
18
benzoimidazol-2-one
(Example 154)
1-[(R)-1-(4,5-Dichloro-pyridazin-
RT
3-y1)-pyrrolidin-3-y1]-4-methoxy-
3,4,5-Trichloro 48
Ic-11 3-(2-trifluoromethyl-benzyI)-1,3- C-1-2 1.04
537.92
pyridazine then
dihydro-benzoimidazol-2-one
(Example 157)
24
1-[(R)-1-(3,5-Dichloro-pyridazin-
RT
4-y1)-pyrrolidin-3-y1]-4-methoxy-
3,4,5-Trichloro 48
1c-12 3-(2-trifluoromethyl-benzyI)-1,3- C-1-2 1.03
538.00
pyridazine then
dihydro-benzoimidazol-2-one
(Example 158)
24
Synthesis of compounds of formula Id
To a soln. of lc (1 eq) in Me0H (8 mL/mmol) was added toluene-4-sulfonic acid
monohydrate (0.2 eq) and the rxn
mixture was heated at 120 C under microwave condition for a given time (see
Table 27). It was concentrated in
5 vacuo and partitioned between Et0Ac and a sat. aq. soln. of NaHCO3. The
org. phase was washed with brine,
dried over MgSO4 and concentrated in vacuo. The crude was purified by CC using
Hept/Et0Ac.
Table 27
MS-data
Reactant T [ C]
Id Name tR [min]
m/z
lc time [h]
[M+Fl]4
1-[1-(5-Cyclopropy1-2-methy1-2H-pyrazol-3-y1)-
120
1d-1 piperidin-4-y1]-4-methoxy-3-(2-trifluoromethyl-benzy1)- lc-1
0.25 0.94 525.97
1,3-dihydro-benzoimidazol-2-one (Example 21)
1-[(R)-1-(2,5-Dimethy1-2H-pyrazol-3-y1)-pyrrolidin-3-
120
Id-2 yI]-4-methoxy-3-(2-trifluoromethyl-benzy1)-1,3- lc-3 0
0.84 486.33
.35
dihydro-benzoimidazol-2-one (Example 79)
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
119
Synthesis of compounds of formula le
To a soln. of Id (1 eq) in THF (4.5 mL/mmol) was added NCS (1.4 eq) and the
rxn mixture was stirred at RT for a
given time (see Table 28). It was partitioned between Et0Ac and water and the
org. phase was washed with brine,
dried over MgSO4 and concentrated in vacuo. The crude was purified by CC using
Hept/Et0Ac.
Table 28
MS-data
Reactant
le Name time [h] tR [min]
m/z
Id
[M+Fl]4
1-[1-(4-Chloro-5-cyclopropy1-2-methyl-2H-pyrazol-3-
y1)-piperidin-4-y1]-4-methoxy-3-(2-trifluoromethyl-
le-1 Id-1 1 1.11 560.11
benzy1)-1,3-dihydro-benzoimidazol-2-one
(Example 24)
1-[(R)-1-(4-Chloro-2,5-dimethy1-2H-pyrazol-3-y-
pyrrolidin-3-y1]-4-methoxy-3-(2-trifluoromethyl-
le-2 Id-2 0.25 1.07 520.12
benzy1)-1,3-dihydro-benzoimidazol-2-one
(Example 80)
Synthesis of compounds of formula If
To a stirred soln. of lc (1 eq) in a mixture of DMF (0.5 mL) was added toluene-
4-sulfonic acid monohydrate (0.13
eq) and 4-toluenesulfonylhydrazide (1.3 eq) followed by sulfolane (0.5 mL)
(see Table 29). The mixture was stirred
at 100 C for 1h and cooled to RT. Sodium cyanoborohydride (4 eq) was added
portionwise and the mixture was
stirred at 100 C for 24h. It was quenched with a sat. aq. soln. of NaHCO3 and
extracted 3x with Et0Ac. The
combined org. phases were washed with brine, dried over MgSO4 and concentrated
in vacuo. The crude was
purified by prep. LC-MS using method 3.
Table 29
MS-data
Reactant
If Name tR [min] m/z
lc
[M+Fl]4
1-[1-(5-Cyclopropy1-2,4-dimethy1-2H-pyrazol-311)-piperidin-4-y1]-
If-1 4-methoxy-3-(2-trifluoromethyl-benzyI)-1,3-dihydro- lc-1
0.95 540.15
benzoimidazol-2-one (Example 25)
Synthesis of compounds of formula Ig
To a soln. of C-1 (1 eq) and TEA (3 eq) in a given solvent (5 mL/mmol) (see )
was added at 0 C alkyl choroformate
or pentafluorophenylcarbonate BB-8 (1.2 eq). The rxn mixture was allowed to
warm to RT and stirred at a given
temperature for a given time (see Table 30). It was partitioned between DCM
and a sat. aq. soln. of NaHCO3. The
org. phase was washed with brine, dried over MgSO4 and concentrated in vauo.
The crude was purified by CC
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
120
using DCM/Me0H or Hept/Et0Ac. If necessary a second purification by prep. LC-
MS using method 2, 3 or 6 can be
performed.
Table 30
Solvent MS-data
Reactant Reactant
Ig Name T [ C] tR [min]
m/z
C-1 BB-8
time [h] [M+Fl]4
(R)-3-[4-Methoxy-2-oxo-3-(2-
trifluoromethyl-benzyI)-2,3-dihydro- DCM
Methyl
Ig-1 benzoimidazol-1-y1]-pyrrolidine-1- C-1-2 RI 0.95
450.04
chloroformate
carboxylic acid methyl ester 2
(Example 26)
4-[4-Methoxy-2-oxo-3-(2-
trifluoromethyl-benzyI)-2,3-dihydro- DCM
Methyl
Ig-2 benzoimidazol-1-y1]-piperidine-1- C-1-1 RI 0.97
464.05
chloroformate
carboxylic acid methyl ester 1
(Example 29)
(R)-3-[4-Methoxy-2-oxo-3-(2-
trifluoromethyl-benzyI)-2,3-dihydro- DCM
Ethyl
Ig-3 benzoimidazol-1-y1]-pyrrolidine-1- C-1-2 RI 0.97
464.06
chloroformate
carboxylic acid ethyl ester 18
(Example 60)
(R)-3-[4-Methoxy-2-oxo-3-(2-
trifluoromethyl-benzyI)-2,3-dihydro- DCM
lsopropylchlor
Ig-4 benzoimidazol-1-y1]-pyrrolidine-1- C-1-2 RI 1.00
478.09
oformate
carboxylic acid isopropyl ester 18
(Example 61)
(R)-3-[4-Methoxy-2-oxo-3-(2-
trifluoromethyl-benzyI)-2,3-dihydro- DCM
n-Butyl
Ig-5 benzoimidazol-1-y1]-pyrrolidine-1- C-1-2 RI 1.03
492.11
chloroformate
carboxylic acid butyl ester 18
(Example 62)
(R)-3-[4-Methoxy-2-oxo-3-(2-
trifluoromethyl-benzyI)-2,3-dihydro- DCM
Phenyl
Ig-6 benzoimidazol-1-y1]-pyrrolidine-1- C-1-2 RI 1.01
512.10
chloroformate
carboxylic acid phenyl ester 18
(Example 63)
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
121
(R)-3-[4-Methoxy-2-oxo-3-(2-
trifluoromethyl-benzyI)-2,3-dihydro- DMF
Ig-7 benzoimidazol-1-y1]-pyrrolidine-1- C-1-2 BB-8-1 110 C --
0.95 -- 506.13
carboxylic acid 3-methyl-oxetan-3- 0.25
yl ester (Example 70)
(R)-3-[4-Methoxy-2-oxo-3-(2-
trifluoromethyl-benzyI)-2,3-dihydro- DMF
Ig-8 benzoimidazol-1-y1]-pyrrolidine-1- C-1-2 BB-8-2 110 C
0.93 492.09
carboxylic acid oxetan-3-ylester 0.25
(Example 72)
(R)-3-[4-Methoxy-2-oxo-3-(2-
trifluoromethyl-benzyI)-2,3-dihydro- DMF
Ig-9 benzoimidazol-1-y1]-pyrrolidine-1- C-1-2 BB-8-3 110 C --
1.00 -- 560.13
carboxylic acid 3-trifluoromethyl- 0.5
oxetan-3-y1 ester (Example 86)
Synthesis of compounds of formula lh
To a soln. of C-1 (1 eq) and TEA (3 eq) in DCM (5 mL/mmol) was added at 0 C
acid chloride BB-8 (1 to 1.2 eq).
The rxn mixture was stirred at RT for a given time (see Table 31) and
partitioned between DCM and a sat. aq. soln.
of NaHCO3. The org. phase was washed with brine, dried over MgSO4 and
concentrated in vacuo. The crude was
purified by CC using Hept/Et0Ac.
Table 31
MS-data
Reactant Reactant
lh Name time [h] tR [min]
m/z
C-1 BB-8
[M+Fl]4
1-((R)-1-Benzoyl-pyrrolidin-3-yI)-4-
methoxy-3-(2-trifluoromethyl- Benzoyl
lh-1 C-1-2 1.5 0.97 496.00
benzyI)-1,3-dihydro-benzoimidazol- chloride
2-one (Example 27)
1-[1-(2,2-Dimethyl-propionyI)-
piperidin-4-yI]-4-methoxy-3-(2-
Pivaloyl
lh-2 trifluoromethyl-benzyI)-1,3-dihydro- C-1-1 1 1.00
490.12
chloride
benzoimidazol-2-one
(Example 28)
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
122
1-(1-Benzoyl-piperidin-4-yI)-4-
methoxy-3-(2-trifluoromethyl- Benzoyl
Ih-3 C-1-1 1 0.98 510.10
benzyI)-1,3-dihydro-benzoimidazol- chloride
2-one (Example 30)
1-(1-Cyclopropanecarbonyl-
piperidin-4-yI)-4-methoxy-3-(2- Cyclopropane
Ih-4 trifluoromethyl-benzyI)-1,3-dihydro- C-1-1 carbonyl
1.5 0.95 474.07
benzoimidazol-2-one chloride
(Example 222)
Synthesis of compounds of formula Ii
Method A (sodium triacetoxyborohydride)
To a soln. of C-1 (1 eq) in a mixture of DCM (25 mL/mmol) and Me0H (50
mL/mmol) were added AcOH (1.2 eq)
and aldehyde BB-8 (1.3 eq) followed by NaBH(OAc)3 (1.4 eq) portionwise. The
rxn mixture was stirred at RT for a
given time (see Table 32). It was quenched with a sat. aq. soln. of NaHCO3 and
extracted with DCM (3x). The
combined org. phases were dried over MgSO4 and concentrated in vacuo. The
crude was purified by CC using
DCM/Me0H.
Method B (indium(III) chlorideltriethylsilane)
To a soln. of C-1 (1 eq) in Me0H (5 mL/mmol) was added BB-8 (1.1 eq) and the
rxn mixture was stirred for 15 min.
Triethylsilane (2 eq) and indium(III) chloride (0.3 eq) were added and the rxn
mixture was stirred at RT for a given
time (see Table 32). It was quenched with a sat. aq. soln. of K2CO3 and
extracted with DCM (3x). The combined
org. phases were washed with brine, dried over MgSO4 and concentrated in
vacuo. The crude was purified by prep.
LC-MS using method 6.
Method C (Alkylation)
To a soln. of C-1 (1 eq) in MeCN (5 mL/mmol) was added K2CO3 (4 eq) and BB-8
(5 eq) and the rxn mixture was
stirred at 65 C for a given time (see Table 32). It was partitioned between
Et0Ac and water and the org. phase was
washed with brine, dried over MgSO4 and concentrated in vacuo. The crude was
purified by CC using DCM/Me0H.
Table 32
Reactant Reactant Method MS-
data m/z
Ii Name tR [min]
C-1 BB-8 time [h] [WE]4
4-Methoxy-1-((R)-1-methyl-
pyrrolidin-3-yI)-3-(2-trifluoromethyl- Formaldehyde A
li-1 C-1-2 0.75 406.15
benzyI)-1,3-dihydro-benzoimidazol- (37% in H20) 18
2-one (Example 31)
CA 03087886 2020-07-08
WO 2019/141803 PCT/EP2019/051230
123
14(R)-1-Isopropyl-pyrrolidin-3-y1)-
4-methoxy-3-(2-trifluoromethyl-
li-2 C-1-2 Acetone 0.76 434.13
benzyI)-1,3-dihydro-benzoimidazol- 18
2-one (Example 32)
1-(1-lsopropyl-piperidin-4-y1)-4-
methoxy-3-(2-trifluoromethyl-
li-3 C-1-1 2-lodopropane 0.76
448.13
benzyI)-1,3-dihydro-benzoimidazol- 2
2-one (Example 35)
Synthesis of compounds of formula lj
Method A (methyl ether cleavage with BBr2)
To a soln. of lb (1 eq) in DCM (4 mL/mmol) was added dropwise at 0 C a 1M
soln. of BBr3 in DCM (3 eq). The rxn
mixture was allowed to warm to RT and stirred for a given time (see Table 33).
The mixture was quenched with
chilled H20 and extracted with DCM (3x). The combined org. phases were dried
over MgSO4 and concentrated in
vacuo. The crude was purified by CC using Hept/Et0Ac.
Method B (methoxypyridine cleavage with BBr3)
To a soln. of lb (1 eq) in DCM (20 to 35 mL/mmol) was added dropwise at -10 C
a 1M soln. of BBr3 in DCM (2 eq).
The rxn mixture was stirred at -10 C for 30 min, allowed to warm to RT and
stirred for a given time (see Table 33).
The mixture was quenched with chilled H20, basified with a sat. aq. soln. of
NaHCO3 and extracted with Et0Ac
(3x). The combined org. phases were washed with brine, dried over MgSO4 and
concentrated in vacuo. The crude
was purified by CC using Hept/Et0Ac or DCM/Me0H.
Table 33
MS-data
Method
lj Name Reactant tR [min] m/z
time [h]
[M+Fl]4
1-[(R)-1-(2-Fluoro-6-methyl-pheny1)-pyrrolidin-3-
A
Ij-1 yI]-4-hydroxy-3-(2-trifluoromethyl-benzy1)-1,3-
lb-11 18 0.99 486.11
dihydro-benzoimidazol-2-one (Example 40)
1-[(R)-1-(2,6-Dimethyl-pheny1)-pyrrolidin-3-y1]-4-
A
lj-2 hydroxy-3-(2-trifluoromethyl-benzyI)-1,3- lb-5 0.91 482.15
3
dihydro-benzoimidazol-2-one (Example 44)
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
124
1-[(R)-1-(2-Fluoro-6-methoxy-pheny1)-pyrrolidin-
3-y1]-4-hydroxy-3-(2-trifluoromethyl-benzy1)-1,3-
dihydro-2H-imidazo[4,5-c]pyriclin-2-one
lj-3 (tautomeric form: 1-[(R)-1-(2-Fluoro-6-methoxy- lb-29 18 0.85
503.10
pheny1)-pyrrolidin-3-y1]-3-(2-trifluoromethyl-
benzy1)-3,5-dihydro-1H-imidazo[4,5-c]pyricline-
2,4-dione) (Example 56)
1-[(R)-1-(2-Fluoro-6-methyl-pheny1)-pyrrolidin-3-
yI]-4-hydroxy-3-(2-trifluoromethyl-benzy1)-1,3-
dihydro-2H-imidazo[4,5-c]pyriclin-2-one
lj-4 (tautomeric form 1-[(R)-1-(2-Fluoro-6-methyl- lb-30 42 0.94
487.22
pheny1)-pyrrolidin-3-y1]-3-(2-trifluoromethyl-
benzy1)-3,5-dihydro-1H-imidazo[4,5-c]pyricline-
2,4-dione) (Example 57)
1-[(R)-1-(2-Fluoro-6-methyl-pheny1)-pyrrolidin-3-
yI]-4-hydroxy-3-(2-methoxy-benzy1)-1,3-dihydro-
imidazo[4,5-c]pyriclin-2-one (tautomeric form 1-
lj-5 lb-44 0.88 449.14
[(R)-1-(2-Fluoro-6-methyl-phenyI)-pyrrolidin-3- 24
yI]-3-(2-methoxy-benzy1)-3,5-dihydro-1H-
imidazo[4,5-c]pyridine-2,4-dione) (Example 81)
1-[(R)-1-(2,6-Dimethyl-pheny1)-pyrrolidin-3-y1]-4-
hydroxy-3-(2-trifluoromethyl-benzy1)-1,3-
dihydro-2H-imidazo[4,5-c]pyridin-2-one
lj-6 (tautomeric form 1-[(R)-1-(2,6-Dimethyl-pheny1)- lb-23 36
0.85 483.15
pyrrolidin-3-y1]-3-(2-trifluoromethyl-benzy1)-3,5-
dihydro-1H-imidazo[4,5-c]pyridine-2,4-dione)
(Example 93)
4-Hydroxy-1-[(R)-1-(2-methoxy-6-methyl-
pheny1)-pyrrolidin-3-y1)-3-(2-trifluoromethyl-
benzyI)-1,3-dihydro-2H-imidazo[4,5-c]pyridin-2-
one (tautomeric form 1-[(R)-1-(2-Methoxy-6-
lj-7 lb-31 0.75 499.15
methyl-pheny1)-pyrrolidin-3-y1]-3-(2- 36
trifluoromethyl-benzyI)-3,5-dihydro-1H-
imidazo[4,5-c]pyridine-2,4-dione)
(Example 94)
CA 03087886 2020-07-08
WO 2019/141803 PCT/EP2019/051230
125
Synthesis of compounds of formula lk
To a stirred soln. of lj (1 eq) in DMSO (10 mL/mmol) was added K2CO3 (2 eq)
followed by the corresponding halide
(1.1 eq). The rxn mixture was stirred at a given temperature for a given time
(see Table 34). It was partitioned
between Et0Ac and H20. The org. phase was washed with water (3x) and brine,
dried over MgSO4 and
concentrated in vacuo. The crude was purified by CC using Hept/Et0Ac or by
prep. LC-MS using method 3.
Table 34
MS-data
Reactant Reactant T [ C]
lk Name tR [min] m/z
lj halide time [h]
[M+Fl]4
1-[(R)-1-(2,6-Dimethyl-phenyI)-
pyrrolidin-3-yI]-4-(2-hydroxy-
2-
ethoxy)-3-(2-trifluoromethyl- 50
1k-1 lj-2 Bromoethan 0.92 526.15
benzyI)-1,3-dihydro- 72
ol
benzoimidazol-2-one
(Example 52)
1-[(R)-1-(2-Fluoro-6-methyl-
105
pheny1)-pyrrolidin-3-y1]-4-(oxetan-
3- 2
3-yloxy)-3-(2-trifluoromethyl-
lk-2 lj-1 Bromoxetan then 1.06
542.10
benzyI)-1,3-dihydro-
benzoimidazol-2-one
72
(Example 149)
1-[(R)-1-(2-Fluoro-6-methyl-
100
pheny1)-pyrrolidin-3-y1]-4-(2-
2- 1.5
hydroxy-ethoxy)-3-(2-
lk-3 lj-1 Bromoethan then 1.00 529.99
trifluoromethyl-benzyI)-1,3-
ol 80
dihydro-benzoimidazol-2-one
18
(Example 150)
4-(2,2-Dimethyl-[1,3]dioxolan-4-
4- 100
ylmethoxy)-1-[(R)-1-(2-fluoro-6-
(Bromometh 1.5
methyl-pheny1)-pyrrolidin-3-y1]-3-
lk-4 lj-1 yI)-2,2- then 1.10 600.05
(2-trifluoromethyl-benzyI)-1,3-
dimethyl-1,3- 80
dihydro-benzoimidazol-2-one
dioxolane 18
(Example 151)
Synthesis of compounds of formula le
To a mixture of le (1 eq), boron species (3 eq) and K2CO3 (5 eq) in dioxane (4
mL/mmol) under N2, was added
10 PEPPSI-IPr (0.1 eq). The rxn mixture was flushed with N2, heated at a
given temperature and stirred for a given
CA 03087886 2020-07-08
WO 2019/141803 PCT/EP2019/051230
126
time (see Table 35). It was partitioned between water and Et0Ac and the org.
phase was washed with brine, dried
over MgSO4 and concentrated in vacuo. The crude was purified by CC using
Hept/Et0Ac. When necessary an
additional purification by prep. LC-MS was performed.
Table 35
MS-data
Reactant Boron T [ C]
le Name tR [min] m/z
le species time [h]
[M+Fl]4
4-Methoxy-3-(2-trifluoromethyl-
benzy1)-1-[(R)-1-(2,4,5-trimethy1-2H-
Trimethyl 115
le-1 pyrazol-3-y1)-pyrrolidin-3-y1]-1,3- le-2 0.90
500.98
boroxine 5.5
dihydro-benzoimidazol-2-one
(Example 85)
Synthesis of compounds of formula Im
To a stirred soln. of lc (1 eq) in Me0H (4 mL/mmol) was added portionwise at 0
C NaBHa (2 eq). The rxn mixture
was stirred at RT for a given time (see Table 36) and quenched with H20. It
was extracted with DCM (3x) and the
combined org. phases were washed with brine, dried over MgSO4 and concentrated
in vacuo. The crude was
purified by CC using Hept/Et0Ac.
Table 36
MS-data
Reactant
Im Name time [h] tR [min]
m/z
lc
[M+Fl]4
1-[(R)-1-(2-Fluoro-6-hydroxymethyl-pheny1)-
pyrrolidin-3-y1]-4-methoxy-3-(2-trifluoromethyl-
1m-1 lc-5 1 0.99 516.15
benzy1)-1,3-dihydro-benzoimidazol-2-one
(Example 88)
Synthesis of compounds of formula In
To a stirred soln. of lc (1 eq) in THF (20 mL/mmol) under argon was added
dropwise at 0 C a 3M soln. of MeMgBr
in Et20 (2 eq). The rxn mixture was stirred for a given time (see Table 37)
allowing temperature to reach RT. The
mixture was cooled to 0 C, quenched with a sat. aq. soln. of NR4C1 and
extracted with Et0Ac (3x). The combined
org. phases were washed with brine, dried over MgSO4 and concentrated in
vacuo. The crude was purified by CC
using Hept/Et0Ac.
CA 03087886 2020-07-08
WO 2019/141803 PCT/EP2019/051230
127
Table 37
MS-data
Reactant
In Name time [h] tR [min]
m/z
lc
[M+Fl]4
1-1(R)-1-[2-Fluoro-6-(1-hydroxy-ethyl)-phenyl]-
pyrrolidin-3-y11-4-methoxy-3-(2-trifluoromethyl-
In-1 lc-5 0.25 1.00 530.05
benzy1)-1,3-dihydro-benzoimidazol-2-one
(Example 89)
Synthesis of compounds of formula lo
To a stirred soln. of In (1 eq) in DCM (3 mL/mmol) was added a 15% soln. of
DMP in DCM (1.5 eq). The rxn
mixture was stirred at RT for a given time (see Table 38). The mixture was
quenched with a sat. aq. soln. of
NaHCO3 and extracted with DCM (3x). The combined org. phases were washed with
brine, dried over MgSO4 and
concentrated in vacuo. The crude was purified by CC using Hept/Et0Ac.
Table 38
MS-data
Reactant
lo Name time [h] tR [min]
m/z
lc
[M+Fl]4
1-[(R)-1-(2-Acetyl-6-fluoro-pheny1)-pyrrolidin-3-y1]-
lo-1 4-methoxy-3-(2-trifluoromethyl-benzyI)-1,3-dihydro- In-1 4.5
1.04 528.15
benzoimidazol-2-one (Example 90)
.. Synthesis of compounds of formula 1p
The procedure was similar to the one synthesizing compounds of formula In
except that reactant lc were replaced
by reactant lo (see Table 39).
Table 39
MS-data
Reactant
1p Name time [h] tR [min]
m/z
In
[M+Fl]4
1-1(R)-1-[2-Fluoro-6-(1-hydroxy-1-methyl-ethyl)-
phenyl]-pyrrolidin-3-y11-4-methoxy-3-(2-
lp-1 lo-1 0.4 0.96 544.17
trifluoromethyl-benzyI)-1,3-dihydro-benzoimidazol-
2-one (Example 91)
Synthesis of intermediates of formula C-3
Intermediates C-3 were prepared using a similar protocol as for the synthesis
of intermediates A-1 replacing amine
building blocks BB-1 by amine building blocks BB-4 (see Table 40).
CA 03087886 2020-07-08
WO 2019/141803 PCT/EP2019/051230
128
Table 40
MS-data
Reactant Reactant T [ C]
C-3 Name tR [min] m/z
BB-2 BB-4 time [h]
[M+Fl]4
[(R)-1-(2-Fluoro-6-methyl-
3-Fluoro-2- 105
C-3-1 pheny1)-pyrrolidin-3-y1]-(3- BB-4-1 1.03 346.08
nitroanisole 24
methoxy-2-nitro-phenyI)-amine
3-[(R)-1-(2-Cyano-6-fluoro- Methyl 3-
C-3-2 phenyI)-pyrrolidin-3-ylamino]-2- Fluoro-2- BB-4-3 24
0.96 385.16
nitro-benzoic acid methyl ester nitrobenzoate
3-Fluoro-2-[(R)-3-(6-methoxy-5-
105
C-3-3 nitro-pyrimidin-4-ylamino)- BB-2-3 BB-4-3 0.95
359.13
3
pyrrolidin-1-yI]-benzonitrile
[1-(2-Fluoro-6-methyl-phenyI)-
3-Fluoro-2- 105
C-3-4 piperidin-4-yI]-(3-methoxy-2- BB-4-2 1.07 360.22
nitroanisole 24
nitro-phenyI)-amine
[(R)-1-(2-Fluoro-6-methyl- 2-Chloro-4-
pheny1)-pyrrolidin-3-y1]-(4- methoxy-6- 50
C-3-5 BB-4-1 1.01 361.05
methoxy-6-methyl-3-nitro- methyl-3- 48
pyridin-211)-amine nitropyridine
(6-Bromo-3-methoxy-2-nitro- 4-Bromo-3-
100
C-3-6 phenyI)-[1-(2,6-dimethyl-pheny1)- fluoro-2- BB-4-4 18 1.11
433.93
piperidin-4-y1Famine nitroanisole
Synthesis of intermediates of formula C-4
Intermediates C-4 were prepared using a similar protocol (method A) as for the
synthesis of intermediates A-2
5 replacing intermediates A-1 by intermediates C-3 (see Table 41).
Alternatively, intermediates C-4 were prepared using the protocol described
below (method B).
Method B (ammonium formate / Zn dust)
To a suspension of intermediate C-3 (1 eq) in Me0H (18.7 mL/mmol) was added Zn
dust (10 eq) and the rxn
mixture was cooled to 0 C. Ammonium formate (10 eq) was added and the rxn
mixture was stirred at RT for a
10 given time (see Table 41). It was filtered over a pad of celite and the
filtrate was concentrated in vacuo. The residue
was diluted with Et0Ac and washed with a sat. aq. soln. of NaHCO3. The org.
phase was washed with brine, dried
over MgSO4 and concentrated in vacuo. The crude was purified by CC using
Hept/Et0Ac.
CA 03087886 2020-07-08
WO 2019/141803 PCT/EP2019/051230
129
Table 41
MS-data
Reactant Method
C-4 Name tR [min] m/z
C-3 time [h]
[M+Fl]4
N1-[(R)-1-(2-Fluoro-6-methyl-pheny1)-pyrrolidin- A
C-4-1 C-3-1 0.75 316.13
3-yI]-3-methoxy-benzene-1,2-diamine 24
2-Amino-3-[(R)-1-(2-cyano-6-fluoro-pheny1)- A
C-4-2 C-3-2 0.97 355.14
pyrrolidin-3-ylaminoppenzoic acid methyl ester 2.5
2-[(R)-3-(5-Amino-6-methoxy-pyrimidin-4- A
C-4-3 C-3-3 0.75 329.11
ylamino)-pyrrolidin-1-yI]-3-fluoro-benzonitrile 1
N1-[1-(2-Fluoro-6-methyl-pheny1)-piperidin-4-y1]- A
C-4-4 C-3-4 0.78 330.11
3-methoxy-benzene-1,2-diamine 3
N2-[(R)-1-(2-Fluoro-6-methyl-pheny-pyrrolidin- A 0.74
C-4-5 C-3-5 331.08
3-yI]-4-methoxy-6-methyl-pyridine-2,3-diamine 2 A
3-Bromo-N2-0 -(2,6-dimethyl-phenyI)-piperidin-
C-4-6 C-3-6 1.03 404.15
4-yI]-6-methoxy-benzene-1,2-diamine 0.3
Synthesis of intermediates of formula C-6 (from intermediates of formula C-4)
Intermediates C-6 were prepared using a similar protocol as for the synthesis
of intermediates A-3 replacing
intermediates A-2 by intermediates C-4 (see Table 42).
Table 42
MS-data
Reactant T [ C]
C-6 Name tR [min] m/z
C-4 time [h]
[M+Fl]4
1-[(R)-1-(2-Fluoro-6-methyl-pheny-pyrrolidin-3- RT
C-6-1 C-4-1 0.88 342.15
y1]-4-methoxy-1,3-dihydro-benzoimidazol-2-one 1
1-[(R)-1-(2-Cyano-6-fluoro-phenyI)-pyrrolidin-3-
C-6-2 yI]-2-oxo-2,3-dihydro-1H-benzoimidazole-4-
C-4-2 0.91 381.11
3
carboxylic acid methyl ester
CA 03087886 2020-07-08
WO 2019/141803 PCT/EP2019/051230
130
3-Fluoro-2-[(R)-3-(6-methoxy-8-oxo-7,8-dihydro- .. RI
C-6-3 C-4-3 0.82 355.13
purin-9-y1)-pyrrolidin-1-y1]-benzonitrile 24
1-[1-(2-Fluoro-6-methyl-pheny1)-piperidin-4-y1]-4- .. RI
C-6-4 C-4-4 0.96 356.06
methoxy-1,3-dihydro-benzoimidazol-2-one .. 4.5
3-[(R)-1-(2-Fluoro-6-methyl-pheny1)-pyrrolidin-3-
RT
C-6-5 y1]-7-methoxy-5-methy1-1,3-dihydro-
imidazo[4,5- C-4-5 2.5 0.73 357.05
b]pyridine-2-one
7-Bromo-1-[1-(2,6-dimethyl-phenyI)-piperidin-4- .. up to 80
C-6-6 C-4-6 1.04 430.06
y1]-4-methoxy-1,3-dihydro-benzoimidazol-2-one 48
Synthesis of intermediates of formula C-5
Intermediates C-5 were prepared using a similar protocol as for the synthesis
of intermediates C-1 replacing
intermediates la by intermediates A-3 (see Table 43).
Table 43
Reactant MS-
data m/z
C-5 Name time [h] tR [min]
A-3
[M+Fl]4
4-Methoxy-1-piperidin-4-y1-1,3-dihydro-
C-5-1 A-3-1 0.25 0.47 248.20
benzoimidazol-2-one
4-Methoxy-1-(R)-pyrrolidin-3-y1-1,3-dihydro-
C-5-2 A-3-2 1 0.46 234.15
benzoimidazol-2-one
Synthesis of intermediate of formula C-6 (from C-5)
To a soln. of C-5 (1 eq) in DMSO (1.5 mL/mmol) was added K2CO3 (2 eq) and BB-8
(1.2 eq). The rxn mixture was
heated at a given temperature for a given time (see Table 44) and was
partitioned between DCM and water. The
org. phase was washed with water (3x) and with brine, dried over MgSO4 and
concentrated in vacuo. The crude
was purified by CC using Et0Ac/Me0H.
Table 44
MS-data
Reactant Reactant T [ C]
C-6 Name tR [min] m/z
C-5 BB-8 time [h]
[M+Fl]4
3-Fluoro-2-[4-(4-methoxy-2-oxo-2,3- 2,3-
100
C-6-7 dihydro-benzoimidazol-1-y1)-piperidin- C-5-1 Difluorobe 0.90
367.12
3
1-yI]-benzonitrile nzonitrile
CA 03087886 2020-07-08
WO 2019/141803 PCT/EP2019/051230
131
3-Fluoro-2-[(R)-3-(4-methoxy-2-oxo- 2,3-
100
C-6-8 2,3-dihydro-benzoimidazol-111)- C-5-2 Difluorobe 18
0.87 353.17
pyrrolidin-1-yI]-benzonitrile nzonitrile
Synthesis of compounds of formula lq
Compounds of formula lq were prepared using similar protocols (method A or B)
as for the synthesis of
intermediates or examples la replacing intermediates A-3 by intermediates C-6
(see Table 45).
Alternatively, intermediates or examples lq were prepared using the protocol
described below (method C).
Method C (Mitsunobu)
To a soln. of intermediate C-6 (1 eq) and BB-3 (1 to 1.5 eq) in toluene (3.4
to 6.8 mL/mmol) was added a 1M soln.
of (tributylphosphoranylidene)acetonitrile in toluene (2 eq) under argon. The
rxn mixture was heated to 110 C and
stirred for a given time (see Table 45). It was quenched with water and
extracted with Et0Ac (3x). The combined
org. phases were washed with brine, dried over MgSO4 and concentrated in
vacuo. The crude was purified by CC
using Hept/Et0Ac and/or DCM/Me0H. When necessary, an additional purification
by prep. LC-MS using method 1
or 2 can be performed.
Table 45
Method
Reactant Reactant MS-
data m/z
lq Name T [ C] tR [min]
C-6 BB-3 [M+Fl]4
time [h]
3-(6-Chloro-4-methoxy-
pyridazin-3-ylmethyl)-1-[(R)- A
1-(2-fluoro-6-methyl-phenyI)- RT to
lq-1 C-6-1 BB-3-10 0.96 498.14
pyrrolidin-3-yI]-4-methoxy- 45 C
1,3-dihydro-benzoimidazol-2- 40
one (Example 92)
1-[(R)-1-(2-Cyano-6-fluoro-
pheny1)-pyrrolidin-3-y1]-2-
oxo-3-(2-trifluoromethyl- A
2-(Trifluoromethyl)
lq-2 benzyI)-2,3-dihydro-1H- C-6-2 RT 1.05
539.24
benzyl bromide
benzoimidazole-4-carboxylic 24
acid methyl ester
(Example 98)
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
132
3-Fluoro-2-1(R)-3-[6-
methoxy-8-oxo-7-(2-
A
trifluoromethyl-benzyI)-7,8- 2-(Trifluoromethyl)
lq-3 C-6-3 RI 1.05 513.19
dihydro-purin-9-yI]-pyrrolidin- benzyl bromide
3
1-yll-benzonitrile
(Example 104)
3-Fluoro-2-1(R)-3-[6-
methoxy-7-(2-methoxy- A
2-Methoxybenzyl
lq-4 benzyI)-8-oxo-7,8-dihydro- C-6-3 RI 1.01
475.11
chloride
4
benzonitrile (Example 105)
1-[1-(2-Fluoro-6-methyl-
pheny1)-piperidin-4-y1]-3-(2- A
1-(Chloromethyl)-
isopropoxy-benzyI)-4- RI to
lq-5 C-6-4 2-(propan-2- 1.13 504.11
methoxy-1,3-dihydro- 60 C
yloxy)benzene
benzoimidazol-2-one 24
(Example 166)
3-[(R)-1-(2-Fluoro-6-methyl-
pheny1)-pyrrolidin-3-y1]-7-
methoxy-5-methy1-1-(2- A
2-(Trifluoromethyl)
lq-6 trifluoromethyl-benzyI)-1,3- C-6-5 RI 1.03
515.03
benzyl bromide
dihydro-imidazo[4,5- 1.5
b]pyridin-2-one
(Example 171)
3-Fluoro-2-14-[4-methoxy-2-
oxo-3-(3-trifluoromethyl-
A
pyrazin-2-ylmethyl)-2,3-
lq-7 C-6-7 BB-3-5 RI 1.03 527.11
dihydro-benzoimidazol-1-y1]-
24
piperidin-1-yll-benzonitrile
(Example 96)
3-Fluoro-2-14-[4-methoxy-3-
(3-methoxy-pyrazin-2-
A
ylmethyl)-2-oxo-2,3-dihydro- 2-(Bromomethyl)-
lq-8 C-6-7 RI 0.99 489.11
benzoimidazol-1-y1]- 3-methoxypyrazine
24
piperidin-1-yll-benzonitrile
(Example 97)
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
133
3-Fluoro-2-1(R)-3-[4-
methoxy-2-oxo-3-(3-
trifluoromethyl-pyrazin-2- A
lq-9 ylmethyl)-2,3-dihydro- C-6-8 BB-3-5 RI 1.01
513.01
benzoimidazol-1-y1]- 24
pyrrolidin-1-yll-benzonitrile
(Example 111)
3-Fluoro-2-1(R)-3-[4-
methoxy-3-(3-methoxy-
pyrazin-2-ylmethyl)-2-oxo- 2-(Bromomethyl)- A
lq-10 2,3-dihydro-benzoimidazol-1- C-6-8 3-methoxy RI 0.97
475.22
pyrazine
hydrochloride 24
benzonitrile
(Example 112)
3-Fluoro-2-14-[4-methoxy-3-
(4-methoxy-pyrimidin-5-
A
ylmethyl)-2-oxo-2,3-dihydro-
lq-11 C-6-7 BB-3-7 RI 0.99 489.19
benzoimidazol-1-y1]-
24
piperidin-1-yll-benzonitrile
(Example 113)
3-Fluoro-2-1(R)-3-[4-
methoxy-3-(4-methoxy-
pyrimidin-5-ylmethyl)-2-oxo- A
lq-12 2,3-dihydro-benzoimidazol-1- C-6-8 BB-3-7 RI 0.95
475.12
24
benzonitrile
(Example 114)
3-Fluoro-2-14-[4-methoxy-2-
oxo-3-(4-trifluoromethyl-
A
pyrimidin-5-ylmethyl)-2,3-
lq-13 C-6-7 BB-3-9 RI 1.05 527.11
dihydro-benzoimidazol-1-y1]-
24
piperidin-1-yll-benzonitrile
(Example 117)
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
134
3-Fluoro-2-1(R)-3-[4-
methoxy-2-oxo-3-(4-
trifluoromethyl-pyrimidin-5- A
lq-14 ylmethyl)-2,3-dihydro- C-6-8 BB-3-9 RI 1.01
512.96
benzoimidazol-1-y1]- 48
pyrrolidin-1-yll-benzonitrile
(Example 153)
1-[(R)-1-(2-Fluoro-6-methyl- A
pheny1)-pyrrolidin-3-y1]-3-(2- RI
isopropoxy-benzyI)-4- 1-(Chloromethyl)- 24
lq-15 C-6-1 2-(propan-2- 1.08 490.11
methoxy-1,3-dihydro- then
yloxy)benzene
benzoimidazol-2-one 60
(Example 165) 2
1-[(R)-1-(2-Fluoro-6-methyl-
pheny1)-pyrrolidin-3-y1]-4-
A
methoxy-3-(3-trifluoromethyl-
lq-16 C-6-1 BB-3-5 60 1.03 502.04
pyrazin-2-ylmethyl)-1,3-
24
dihydro-benzoimidazol-2-one
(Example 167)
1-[1-(2-Fluoro-6-methyl-
pheny1)-piperidin-4-y1]-4-
A
methoxy-3-(3-trifluoromethyl-
lq-17 C-6-4 BB-3-5 60 1.08 516.01
pyrazin-2-ylmethyl)-1,3-
24
dihydro-benzoimidazol-2-one
(Example 168)
1-[(R)-1-(2-Fluoro-6-methyl- A
pheny1)-pyrrolidin-3-y1]-4-
2-(Bromomethyl)- 60
methoxy-3-(3-methoxy-
C-6-1 3-methoxypyrazine 24
lq-18 0.98 464.11
pyrazin-2-ylmethyl)-1,3-
hydrochloride then
dihydro-benzoimidazol-2-one 100
(Example 169) 18
CA 03087886 2020-07-08
PCT/EP2019/051230
WO 2019/141803
135
1-[1-(2-Fluoro-6-methyl- A
pheny1)-piperidin-4-y1]-4-
2-(Bromomethyl)- 60
24
methoxy-3-(3-methoxy-
C-6-4 3-methoxypyrazine 1.04 478.24
lq-19
pyrazin-2-ylmethyl)-1,3-
hydrochloride then
dihydro-benzoimidazol-2-one 100
(Example 170) 18
3-(6-Chloro-4-isopropoxy-
pyridazin-3-ylmethyl)-1-[(R)-
1-(2-fluoro-6-methyl-phenyI)-
C-6-1 BB-3-11 110 1.02 526.12
lq-20
pyrrolidin-3-yI]-4-methoxy-
3
1,3-dihydro-benzoimidazol-2-
one (Example 172)
1-[(R)-1-(2-Fluoro-6-methyl-
pheny1)-pyrrolidin-3-y1]-4-
methoxy-3-(4-methyl-
C-6-1 BB-3-12 110 0.88 448.14
lq-21
pyridazin-3-ylmethyl)-1,3-
1.5
dihydro-benzoimidazol-2-one
(Example 174)
1-[(R)-1-(2-Fluoro-6-methyl-
pheny1)-pyrrolidin-3-y1]-4-
methoxy-3-(4-methoxy-
C-6-1 BB-3-6 110 0.99 464.15
lq-22
pyrimidin-5-ylmethyl)-1,3-
1.5
dihydro-benzoimidazol-2-one
(Example 175)
1-[(R)-1-(2-Fluoro-6-methyl-
pheny1)-pyrrolidin-3-y1]-4-
methoxy-3-(4-trifluoromethyl-
C-6-1 BB-3-8 110 1.04 502.06
lq-23
pyrimidin-5-ylmethyl)-1,3-
4
dihydro-benzoimidazol-2-one
(Example 176)
1-[(R)-1-(2-Fluoro-6-methyl-
pheny1)-pyrrolidin-3-y1]-3-(3-
isopropoxy-pyrazin-2-
C-6-1 BB-3-13 110 1.05 492.17
lq-24
ylmethyl)-4-methoxy-1,3-
2
dihydro-benzoimidazol-2-one
(Example 177)
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
136
1-[1-(2-Fluoro-6-methyl-
pheny1)-piperidin-4-y1]-3-(3-
isopropoxy-pyrazin-2-
1.10 506.19 lq-25 C-6-4 BB-3-13
ylmethyl)-4-methoxy-1,3- 2
dihydro-benzoimidazol-2-one
(Example 178)
1-[(R)-1-(2-Fluoro-6-methyl-
pheny1)-pyrrolidin-3-y1]-3-(4-
isopropyl-pyridin-3-ylmethyl)-
lq-26 C-6-1 BB-3-14 110 0.85 475.17
4-methoxy-1,3-dihydro-
1.5
benzoimidazol-2-one
(Example 180)
1-[1-(2-Fluoro-6-methyl-
pheny1)-piperidin-4-y1]-3-(4-
isopropyl-pyridin-3-ylmethyl)-
lq-27 C-6-4 BB-3-14 110 0.89 489.20
4-methoxy-1,3-dihydro-
1.5
benzoimidazol-2-one
(Example 181)
1-[(R)-1-(2-Fluoro-6-methyl-
pheny1)-pyrrolidin-3-y1]-3-(3-
isopropoxy-pyridin-2-
lq-28 C-6-1 BB-3-15 110 0.90 491.16
ylmethyl)-4-methoxy-1,3-
1.5
dihydro-benzoimidazol-2-one
(Example 182)
1-[1-(2-Fluoro-6-methyl-
pheny1)-piperidin-4-y1]-3-(3-
isopropoxy-pyridin-2-
lq-29 C-6-4 BB-3-15 110 0.94 505.13
ylmethyl)-4-methoxy-1,3-
1.5
dihydro-benzoimidazol-2-one
(Example 183)
1-[(R)-1-(2-Fluoro-6-methyl-
pheny1)-pyrrolidin-3-y1]-3-(4-
isopropyl-pyrimidin-5-
lq-30 C-6-1 BB-3-16 110 1.06 476.09
ylmethyl)-4-methoxy-1,3-
3
dihydro-benzoimidazol-2-one
(Example 186)
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
137
1-[1-(2-Fluoro-6-methyl-
pheny1)-piperidin-4-y1]-3-(3-
isopropyl-pyrazin-2-
lq-31 C-6-4 BB-3-17 110 1.07 490.16
ylmethyl)-4-methoxy-1,3-
1.5
dihydro-benzoimidazol-2-one
(Example 187)
1-[(R)-1-(2-Fluoro-6-methyl-
pheny1)-pyrrolidin-3-y1]-3-(3-
isopropyl-pyrazin-2-
lq-32 C-6-1 BB-3-17 110 1.01 476.10
ylmethyl)-4-methoxy-1,3-
1.5
dihydro-benzoimidazol-2-one
(Example 189)
1-[1-(2-Fluoro-6-methyl-
pheny1)-piperidin-4-y1]-3-(4-
isopropyl-pyrimidin-5-
lq-33 C-6-4 BB-3-16 110 1.13 490.17
ylmethyl)-4-methoxy-1,3-
24
dihydro-benzoimidazol-2-one
(Example 192)
1-[(R)-1-(2-Fluoro-6-methyl-
pheny1)-pyrrolidin-3-y1]-4-
(2-(TrifluoromethYl) C
methoxy-3-(2-trifluoromethyl-
C-6-1
lq-34 pyridin-3-y1) 110 1.05 501.12
pyridin-3-ylmethyl)-1,3-
methanol 2
dihydro-benzoimidazol-2-one
(Example 195)
1-[(R)-1-(2-Fluoro-6-methyl-
pheny1)-pyrrolidin-3-y1]-4- (3-(TrifluoromethYl) C
methoxy-3-(3-trifluoromethyl-
C-6-1
lq-35 pyridin-2-y1) 110 1.03 501.05
pyridin-2-ylmethyl)-1,3-
methanol 18
dihydro-benzoimidazol-2-one
(Example 202)
1-[(R)-1-(2-Fluoro-6-methyl-
pheny1)-pyrrolidin-3-y1]-4- (4-(TrifluoromethYl) .. C
methoxy-3-(4-trifluoromethyl-
C-6-1
lq-36 pyridin-3-y1) 110 1.08 501.09
pyridin-3-ylmethyl)-1,3-
methanol 2
dihydro-benzoimidazol-2-one
(Example 203)
CA 03087886 2020-07-08
PCT/EP2019/051230
WO 2019/141803
138
3-(2-Cyclopropoxy-benzyI)-1-
[(R)-1-(2-fluoro-6-methyl-
1-(Bromomethyl)- A
pheny1)-pyrrolidin-3-y1]-4-
C-6-1 2-cyclopropyloxY RI 1.06
488.12
lq-37
methoxy-1,3-dihydro-
benzene 6
benzoimidazol-2-one
(Example 208)
A
3-(2-Cyclopropoxy-benzyI)-1-
RT
[1-(2-fluoro-6-methyl-
1-(Bromomethyl)-
pheny C-6-4 2-cyclopropyloxy 1)-piperidin-4-y1]-4-
18
1.13 502.15
lq-38
then
methoxy-1,3-dihydro-
benzene
benzoimidazol-2-one
(Example 209) 3.5
1-[1-(2-Fluoro-6-methyl-
pheny1)-piperidin-4-y1]-4-
[3-(trifluoromethYI) C
methoxy-3-(3-trifluoromethyl-
C-6-4 pyridin-2-yl] 110 1.09 515.10
lq-39
pyridin-2-ylmethyl)-1,3-
methanol 24
dihydro-benzoimidazol-2-one
(Example 216)
1-[(R)-1-(2-Fluoro-6-methyl-
pheny1)-pyrrolidin-3-y1]-4-
methoxy-3-(2-trifluoromethyl-
C-6-1 BB-3-18 110 1.04 507.00
lq-40
thiazol-4-ylmethyl)-1,3-
18
dihydro-benzoimidazol-2-one
(Example 218)
1-[1-(2-Fluoro-6-methyl-
pheny1)-pyrrolidin-3-y1]-4-
1-(2-
methoxy-3-[(S)-1-(2-
C-6-1 (trifluoromethYI) 110 1.07
514.02
lq-41
trifluoromethyl-pheny1)-ethyl]-
phenyl)ethan-1-ol 2.5
1,3-dihydro-benzoimidazol-2-
one (Example 219)
3-(2,4-Difluoro-6-isopropoxy-
benzyI)-1-[(R)-1-(2-fluoro-6-
methyl-phenyI)-pyrrolidin-3-
C-6-1 BB-3-19 110 1.09 526.04
lq-42
yI]-4-methoxy-1,3-dihydro-
2.5
benzoimidazol-2-one
(Example 220)
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
139
1-[(R)-1-(2-Fluoro-6-methyl-
pheny1)-pyrrolidin-3-y1]-4-
[2-methyl-4-
methoxy-3-(2-methyl-4-
(trifluoromethyl)-
lq-43 trifluoromethyl-thiazol-5- C-6-1 110 1.06
521.01
1,3-thiazol-5-
ylmethyl)-1,3-dihydro- 4
yl]methanol
benzoimidazol-2-one
(Example 221)
3-(2-Cyclopropyl-benzyI)-1-
[1-(2-fluoro-6-methyl-
(2-
pheny1)-piperidin-4-y1]-4- C-6-4 CyclopropylphenYI) 110
lq-44 1.14 486.13
methoxy-1,3-dihydro-
methanol 2
benzoimidazol-2-one
(Example 223)
2-13-[1-(2-fluoro-6-methyl-
pheny1)-piperidin-4-y1]-7- 2- A
lq-45 methoxy-2-oxo-2,3-dihydro- C-6-4 (Chloromethyl)phe 70 1.08 504.16
benzoimidazol-1-ylmethyll- nyl acetate 10
phenyl acetate
4-Bromo-3-[1-(2,6-dimethyl-
pheny1)-piperidin-4-y1]-7- A
2-(Trifluoromethyl)
lq-46 methoxy-1-(2-trifluoromethyl- C-6-6
benzyl bromide RI 1.20 588.03
benzyI)-1,3-dihydro- 2
benzoimidazol-2-one
Synthesis of compounds of formula Ir
Method A
To a soln. of intermediate lq (1 eq) in Et0H (5 to 12 mL/mmol) was added
ammonium formate (2 eq) and 10%
Pd/C moistened with ¨50% water (0.05 eq) and the rxn mixture was heated to 65
C for a given time (see Table
46). It was filtered over a pad of celite and the filtrate was concentrated in
vacuo. The crude was purified by CC
using Et0Ac/Me0H or DCM/Me0H.
Method B
To a soln. of intermediate lq (1 eq) in a mixture of Et0Ac (65 mL/mmol) and
Me0H (89 mL/mmol) was added 10%
Pd/C moistened with ¨50% water (0.1 eq) and the rxn mixture was hydrogenated
under an atmospheric pressure of
deuterium for a given time (see Table 46). It was filtered over a pad of
celite and the filtrate was concentrated in
vacuo. The crude was purified by CC using Hept/Et0Ac.
CA 03087886 2020-07-08
WO 2019/141803 PCT/EP2019/051230
140
Table 46
MS-data
Reactant Method
Ir Name tR [min] m/z
lq time [h]
[M+Fl]4
1-[(R)-1-(2-Fluoro-6-methyl-pheny1)-pyrrolidin-3-
y1]-4-methoxy-3-(4-methoxy-pyridazin-3- A
Ir-1 lq-1 0.81 464.18
ylmethyl)-1,3-dihydro-benzoimidazol-2-one 24
(Example 95)
1-[(R)-1-(2-Fluoro-6-methyl-pheny1)-pyrrolidin-3-
y1]-3-(4-isopropoxy-pyridazin-3-ylmethyl)-4- A
Ir-2 lq-20 0.87 492.16
methoxy-1,3-dihydro-benzoimidazol-2-one 1
(Example 173)
1-(1-(2,6-dimethylphenyl)piperidin-411)-4-
methoxy-3-(2-(trifluoromethyl)benzyI)-1,3-
Ir-3 lq-46 1.14 511.18
dihydro-benzoimidazol-2-one-7-d 4.5
(Example 3)
Synthesis of acid intermediates of formula E-1
To a soln. of intermediates lq (1 eq) in a 1/1/1 mixture of THF/Me0H/water (9
mL/mmol) was added lithium
hydroxide hydrate (4 eq). The rxn mixture was heated to 50 C and stirred for a
given time (see Table 47). It was
diluted with water and extracted with Et20 (3x). The aq. phase was acidified
until pH 2-3 with a 1M soln. of HCI and
extracted with DCM (3x). The combined org. phases were dried over MgSO4 and
concentrated in vacuo.
Table 47
MS-data
Reactant
E-1 Name time [h] tR [min]
m/z
lq
[M+Fl]4
1-[(R)-1-(2-cyano-6-fluoro-pheny1)-pyrrolidin-3-
E-1-1 yI]-2-oxo-3-(2-trifluoromethyl-benzy1)-2,3- lq-2 2
0.97 525.10
dihydro-1H-benzoimidazole-4-carboxylic acid
Synthesis of compounds of formula Is
To a soln. of acids of formula E-1 (1 eq) in DMF (4 mL/mmol) was added DIPEA
(3 eq) and HATU (1 eq). The rxn
mixture was stirred for 5 min at RT and the appropriate amine (1.2 eq) pure or
as soln. was added. The rxn mixture
was further stirred ON at RT (see Table 48) and diluted with DCM. The org.
phase was washed with water and
brine, dried over MgSO4 and concentrated in vacuo. The crude was purified by
CC using Hept/Et0Ac.
CA 03087886 2020-07-08
WO 2019/141803 PCT/EP2019/051230
141
Table 48
MS-data
Reactant
Is Name Amine tR [min] m/z
E-1
[M+Fl]4
1-[(R)-1-(2-Cyano-6-fluoro-pheny1)-pyrrolidin-3-
2M soln. of
yI]-2-oxo-3-(2-trifluoromethyl-benzy1)-2,3-
Is-1 E-1-1 methylamine 0.94
538.21
dihydro-1H-benzoimidazole-4-carboxylic acid
in THF
methylamide (Example 99)
1-[(R)-1-(2-Cyano-6-fluoro-pheny1)-pyrrolidin-3-
0.5 M soln. of
yI]-2-oxo-3-(2-trifluoromethyl-benzy1)-2,3-
Is-2 E-1-1 ammonia in 0.91
524.16
dihydro-1H-benzoimidazole-4-carboxylic acid
dioxane
amide (Example 100)
1-[(R)-1-(2-Cyano-6-fluoro-pheny1)-pyrrolidin-3-
2M soln. of
yI]-2-oxo-3-(2-trifluoromethyl-benzy1)-2,3-
Is-3 E-1-1 dimethylamine 0.98
552.21
dihydro-1H-benzoimidazole-4-carboxylic acid
in THF
dimethylamide (Example 101)
1-[(R)-1-(2-Cyano-6-fluoro-pheny1)-pyrrolidin-3-
y1]-2-oxo-3-(2-trifluoromethyl-benzy1)-2,3-
Is-4 E-1-1 Ethanolamine 0.88 568.04
dihydro-1H-benzoimidazole-4-carboxylic acid
(2-hydroxy-ethyl)-amide (Example 193)
1-[(R)-1-(2-Cyano-6-fluoro-pheny1)-pyrrolidin-3-
y1]-2-oxo-3-(2-trifluoromethyl-benzy1)-2,3- 2-Methoxy
Is-5 E-1-1 0.95 582.1
dihydro-1H-benzoimidazole-4-carboxylic acid ethylamine
(2-methoxy-ethyl)-amide (Example 194)
Synthesis of compounds of formula It
To a soln. of compounds of formula lq (1 eq) and CaCl2 (0.3 eq) in Et0H (15
mL/mmol) was added at 0 C a
suspension of NaBHa (2.5 eq) in Et0H (8 mL/mmol). The rxn mixture was allowed
to warm to RT and stirred at that
temperature for a given time (see Table 49). It was quenched with water and
extracted with DCM (3x). The
combined org. phases were dried over MgSO4 and concentrated in vacuo. The
crude was purified by CC using
Hept/Et0Ac.
CA 03087886 2020-07-08
WO 2019/141803 PCT/EP2019/051230
142
Table 49
MS-data
Reactant
It Name time [h] tR [min] m/z
lq
[M+Fl]4
3-Fluoro-2-1(R)-3-[4-hydroxymethy1-2-oxo-3-(2-
trifluoromethyl-benzyI)-2,3-dihydro-
It-1 lq-2 24 0.97 511.24
benzoimidazol-111]-pyrrolidin-1-yll-benzonitrile
(Example 102)
Synthesis of compounds of formula lu
Method A (Reductive amination I)
To a stirred soln. of lc (1 eq) in a mixture of DCM (25 mL/mmol), Me0H (50
mL/mmol) and AcOH (1.2 eq) was
added the appropriate amine (1.3 eq) followed by NaBH(OAc)3 (1.4 eq). The rxn
mixture was stirred at RT for a
given time (see Table 50) and the volatiles were evaporated in vacuo. The
residue was partitioned between DCM
and a sat. aq. soln. of NaHCO3. The org. phase was dried over MgSO4 and
concentrated in vacuo. The crude was
purified by prep. LC-MS using method 4 and 5.
Method B (Reductive amination II)
To a stirred soln. of aldehyde lc (1 eq) and the appropriate amine (1.1 eq) in
THF (4 mL/mmol) was added
NaBH(OAc)3 (1.5 eq). The rxn mixture was stirred at RT for a given time (see
Table 50) and partitioned between
DCM and a 1M aq. soln. of NaOH. The org. phase was dried over MgSO4 and
concentrated in vacuo. The crude
was purified by prep. LC-MS using method 2.
Table 50
MS-data
Reactant Reactant Method
lu Name tR [min] m/z
lc amine time [h]
[WE]4
1-[(R)-1-(2-Fluoro-6-
methylaminomethyl-phenyI)- 2M soln. of
A
lu-1 pyrrolidin-3-yI]-4-methoxy-3-(2- lc-5 methylami
0.86 529.27
1
trifluoromethyl-benzyI)-1,3-dihydro- ne in THF
benzoimidazol-2-one (Example 103)
1-[(R)-1-(2-Dimethylaminomethy1-6-
2M soln. of
fluoro-pheny1)-pyrrolidin-3-y1]-4-
dimethyla A
lu-2 methoxy-3-(2-trifluoromethyl- lc-5 0.90
543.30
mine in 24
benzyI)-1,3-dihydro-benzoimidazol-
THF
2-one (Example 106)
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
143
1-[(R)-1-(2-Fluoro-6-1[(2-hydroxy-
ethyl)-methyl-amino]-methyll-
2-
pheny1)-pyrrolidin-3-y1]-4-methoxy-3- A
lu-3 lc-5 (Methylami 0.86 573.28
(2-trifluoromethyl-benzyI)-1,3- 24
no)ethanol
dihydro-benzoimidazol-2-one
(Example 107)
1-[(R)-1-(2-Azetidin-1-ylmethy1-6-
fluoro-pheny1)-pyrrolidin-3-y1]-4-
A
lu-4 methoxy-3-(2-trifluoromethyl- lc-5 Azetidine 18 0.91
555.25
benzyI)-1,3-dihydro-benzoimidazol-
2-one (Example 108)
1-[(R)-1-(2-Fluoro-6-morpholin-4-
ylmethyl-pheny1)-pyrrolidin-3-y1]-4-
A
lu-5 methoxy-3-(2-trifluoromethyl- lc-5 Morpholine 24 0.90
585.14
benzyI)-1,3-dihydro-benzoimidazol-
2-one (Example 115)
1-[(R)-1-(2-Fluoro-6-pyrrolidin-1-
ylmethyl-pheny1)-pyrrolidin-3-y1]-4-
A
lu-6 methoxy-3-(2-trifluoromethyl- lc-5 Pyrrolidine 2 0.93
569.16
benzyI)-1,3-dihydro-benzoimidazol-
2-one (Example 116)
1-1(R)-1-[2-Fluoro-6-(4-methyl-
piperazin-1-ylmethyl)-phenyl]-
1-Methyl
lu-7 pyrrolidin-3-y11-4-methoxy-3-(2- lc-5 0.76
598.30
piperazine 1.5
trifluoromethyl-benzyI)-1,3-dihydro-
benzoimidazol-2-one (Example 132)
1-[(R)-1-(2-Fluoro-6-piperidin-1-
ylmethyl-pheny1)-pyrrolidin-3-y1]-4-
lu-8 methoxy-3-(2-trifluoromethyl- lc-5 Piperidine 18 0.94
583.26
benzyI)-1,3-dihydro-benzoimidazol-
2-one (Example 133)
1-1(R)-1-[2-Fluoro-6-(4-hydroxy-
piperidin-1-ylmethyl)-phenyl]-
4-Hydroxy B
lu-9 pyrrolidin-3-y11-4-methoxy-3-(2- lc-5 0.87
599.27
piperidine 18
trifluoromethyl-benzyI)-1,3-dihydro-
benzoimidazol-2-one (Example 134)
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
144
1-[(R)-1-(2-Fluoro-6-piperazin-1-
ylmethyl-pheny1)-pyrrolidin-3-y1]-4-
lu-10 methoxy-3-(2-trifluoromethyl- lc-5 Piperazine 18 0.73
584.24
benzyI)-1,3-dihydro-benzoimidazol-
2-one (Example 135)
1-1(R)-1-[2-(4-Dimethylamino-
piperidin-1-ylmethyl)-6-fluoro-
4-Dimethyl
phenyl]-pyrrolidin-3-y11-4-methoxy-3-
lu-11 lc-5 amino 0.73 626.30
(2-trifluoromethyl-benzyI)-1,3- 18
piperidine
dihydro-benzoimidazol-2-one
(Example 136)
1-1(R)-1-[2-Fluoro-6-(4-methoxy-
piperidin-1-ylmethyl)-phenyl]-
4-Methoxy B
lu-12 pyrrolidin-3-y11-4-methoxy-3-(2- lc-5 0.92
613.08
piperidine 3.5
trifluoromethyl-benzyI)-1,3-dihydro-
benzoimidazol-2-one (Example 137)
1-1(R)-1-[2-Fluoro-6-(3-methoxy-
pyrrolidin-1-ylmethyl)-phenyl]-
3-Methoxy B
lu-13 pyrrolidin-3-y11-4-methoxy-3-(2- lc-5 0.92
599.07
pyrrolidine 3.5
trifluoromethyl-benzyI)-1,3-dihydro-
benzoimidazol-2-one (Example 138)
1-[(R)-1-(2-Fluoro-6-1[(2-methoxy-
N-(2-
ethyI)-methyl-amino]-methyll-
Methoxy
pheny1)-pyrrolidin-3-y1]-4-methoxy-3-
lu-14 lc-5 ethyl) 0.91 587.10
(2-trifluoromethyl-benzyI)-1,3- 3.5
methylami
dihydro-benzoimidazol-2-one
ne
(Example 139)
1-[(R)-1-(2-1[Ethyl-(2-hydroxy-ethy-
amino]-methyll-6-fluoro-pheny- 2-
lu-15 pyrrolidin-3-yI]-4-methoxy-3-(2- lc-5 (Ethylamin
0.87 587.40
3.5
trifluoromethyl-benzyI)-1,3-dihydro- o)ethanol
benzoimidazol-2-one (Example 140)
CA 03087886 2020-07-08
WO 2019/141803 PCT/EP2019/051230
145
1-[(R)-1-(2-1[Bis-(2-hydroxy-ethyl)-
amino]-methyll-6-fluoro-pheny1)-
Diethanol
lu-16 pyrrolidin-3-yI]-4-methoxy-3-(2- lc-5
0.83 603.14
amine 72
trifluoromethyl-benzyI)-1,3-dihydro-
benzoimidazol-2-one (Example 141)
1-((R)-1-12-Fluoro-6-[(2-methoxy-1-
methyl-ethylamino)-methyl]-phenyll- 2-Amino-1-
lu-17 pyrrolidin-3-yI)-4-methoxy-3-(2- lc-5
methoxy 0.90 587.27
3.5
trifluoromethyl-benzyI)-1,3-dihydro- propane
benzoimidazol-2-one (Example 142)
1-1(R)-1-[2-Fluoro-6-(3-methoxy-
azetidin-1-ylmethyl)-phenyl]-
3-Methoxy B
lu-18 pyrrolidin-3-y11-4-methoxy-3-(2- lc-5
0.92 585.20
azetidine 3.5
trifluoromethyl-benzyI)-1,3-dihydro-
benzoimidazol-2-one (Example 143)
Synthesis of intermediates of formula D-1
A suspension of lj (leg) in P0CI3 (2 mL/mmol) was heated to 85 C and stirred
ON (see Table Si). The rxn mixture
was carefully quenched with a ice cold 2M aq. soln. of NaOH and extracted with
Et0Ac (3x). The combined org.
phases were washed with brine, dried over MgSO4 and concentrated in vacuo. The
crude was purified by CC using
Hept/Et0Ac.
Table 51
MS-data
Reactant
D-1 Name tR [min] m/z
lj
[WE]4
4-Chloro-1-[(R)-1-(2,6-dimethyl-pheny1)-pyrrolidin-3-y1]-3-(2-
D-1-1 lj-6 1.06 501.09
trifluoromethyl-benzyI)-1,3-dihydro-imidazo[4,5-c]pyridin-2-one
4-Chloro-1-[(R)-1-(2-fluoro-6-methyl-pheny1)-pyrrolidin-3-y1]-3-
D-1-2 (2-trifluoromethyl-benzyI)-1,3-dihydro-imidazo[4,5-c]pyridin-2-
lj-4 1.09 505.03
one
Synthesis of compounds of formula ly
Method A (amine as solvent)
A soln. of D-1 (1 eq) in the appropriate amine (5 mL/mmol) was heated to a
given temperature and stirred for a
given time (see Table 52). The rxn mixture was diluted with Et0Ac and washed
with a sat. aq. soln. of NaHCO3 (2x)
CA 03087886 2020-07-08
WO 2019/141803 PCT/EP2019/051230
146
and with brine. The org. phase was dried over MgSO4 and concentrated in vacuo.
The crude was purified by CC
using Hept/Et0Ac.
Method B (alcohol/KOH/DMSO)
To a stirred soln. of D-1 (1 eq) in DMSO (8 mL/mmol) was added the appropriate
alcohol (5 to 10 eq) and KOH (2.5
eq). The rxn mixture was heated to a given temperature for a given time (see
Table 52). It was diluted with Et0Ac
and washed with H20 (3x) and brine. The org. phase was dried over MgSO4 and
concentrated in vacuo. The crude
was purified by CC using Hept/Et0Ac or DCM/Me0H.
Method C (Buchwald)
An oven dried flask was charged with intermediate D-1 (1 eq), the appropriate
amine (1.2 eq), Cs2CO3 (2.5 eq)
.. RuPhos precatalyst (0.1 eq) and RuPhos (0.1 eq). The flask was evacuated
and refilled with argon (3x) and
t-BuOH (17 mL/mmol) was added. The rxn mixture was degassed under vacuum,
refilled with argon (3x) and
heated to a given temperature and stirred for a given time (see Table 52). It
was partitioned between water and
Et0Ac and the org. phase was washed with brine, dried over MgSO4 and
concentrated in vacuo. The crude was
purified by prep. LC-MS using method 2 and 5.
Table 52
Reactant Method MS-data
Reactant
lv Name D amine / T [ C] tR [min]
m/z
-1
alcohol time [h] [M+Fl]4
A
1-[(R)-1-(2,6-Dimethyl-phenyI)-
100
pyrrolidin-3-yI]-4-(2-hydroxy-
Ethanol 72
lv-1 ethylamino)-3-(2-trifluoromethyl- D-1-1 0.81
526.28
amine then
benzyI)-1,3-dihydro-imidazo[4,5-
130
c]pyridin-2-one (Example 118)
72
1-[(R)-1-(2,6-Dimethyl-pheny1)-
pyrrolidin-3-y1]-4-(2-hydroxy-ethoxy)-
Ethylene
lv-2 3-(2-trifluoromethyl-benzyI)-1,3- D-1-1 50 0.97
527.17
glycol
dihydro-imidazo[4,5-c]pyridin-2-one 2.5
(Example 119)
4-(2-Dimethylamino-ethoxy)-1-[(R)-
1-(2-fluoro-6-methyl-pheny1)- Dimethyl
lv-3 pyrrolidin-3-yI]-3-(2-trifluoromethyl- D-1-2 amino 50
0.85 558.14
benzyI)-1,3-dihydro-imidazo[4,5- ethanol 1.5
c]pyridin-2-one (Example 120)
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
147
1-[(R)-1-(2-Fluoro-6-methyl-phenyI)- 4-(2-
pyrrolidin-3-yI]-4-(2-morpholin-4-yl- Hydroxy
lv-4 ethoxy)-3-(2-trifluoromethyl-benzyI)- D-1-2 ethyl) 50 ..
0.85 .. 600.11
1,3-dihydro-imidazo[4,5-c]pyridin-2- morpholi 1.5
one (Example 121) ne
tert-Butyl
4-[1-[(R)-1-(2-Fluoro-6-methyl- 4-
pheny1)-pyrrolidin-3-y1]-2-oxo-3-(2- hydroxy-
trifluoromethyl-benzyI)-2,3-dihydro- 1-
lv-5 D-1-2 50 1.15 670.17
1H-imidazo[4,5-c]pyridin-4-yloxy]- piperidin
2
piperidine-1-carboxylic acid tert-
butyl ester (Example 122) carboxyla
te
A
1-[(R)-1-(2-Fluoro-6-methyl-phenyI)-
2- 120
pyrrolidin-3-y1]-4-[(2-hydroxy-ethyl)-
(Methyla 48
lv-6 methyl-amino]-3-(2-trifluoromethyl- D-1-2 0.86
544.22
mino)eth then
benzyI)-1,3-dihydro-imidazo[4,5-
anol 130
c]pyridin-2-one (Example 123)
120
2,2-
4-(2,2-Dimethyl-[1,3]clioxolan-4-
Dimethyl-
ylmethoxy)-1-[(R)-1-(2-fluoro-6-
4-
methyl-pheny1)-pyrrolidin-3-y1]-3-(2-
lv-7 D-1-2 hydroxy 50 1.10 601.34
trifluoromethyl-benzyI)-1,3-dihydro-
methyl- 4
imidazo[4,5-c]pyridin-2-one
1,3-
(Example 125)
dioxolane
1-[(R)-1-(2-Fluoro-6-methyl-phenyI)-
pyrrolidin-3-yI]-4-(oxetan-3-yloxy)-3- 3-
72
lv-8 (2-trifluoromethyl-benzyI)-1,3- D-1-2 Hydroxy
1.08 543.20
then
dihydro-imidazo[4,5-c]pyridin-2-one oxetane
(Example 127)
120
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
148
1-[(R)-1-(2-Fluoro-6-methyl-pheny1)-
pyrrolidin-3-y1]-4-morpholin-4-y1-3- A
Morpholi
lv-9 (2-trifluoromethyl-benzyI)-1,3- D-1-2 100 0.97
556.25
ne
dihydro-imidazo[4,5-c]pyridin-2-one 770
(Example 129)
1-[(R)-1-(2-Fluoro-6-methyl-pheny1)-
pyrrolidin-3-y1]-4-(1-methyl- 1-Methyl-
pyrrolidin-3-yloxy)-3-(2- 3-
lv-10 D-1-2 50 0.85 570.13
trifluoromethyl-benzyI)-1,3-dihydro- pyrrolidin
2
imidazo[4,5-c]pyridin-2-one ol
(Example 130)
1-[(R)-1-(2-Fluoro-6-methyl-phenyI)- 3-
pyrrolidin-3-yI]-4-(3-methoxy- Methoxy
lv-11 azetidin-1-yI)-3-(2-trifluoromethyl- D-1-2 azetidine 110
0.91 556.13
benzyI)-1,3-dihydro-imidazo[4,5- hydrochl 2
c]pyridin-2-one (Example 205) oride
A
1-[(R)-1-(2-Fluoro-6-methyl-phenyI)-
120
pyrrolidin-3-yI]-4-(4-methyl- 1-Methyl
48
lv-12 piperazin-1-yI)-3-(2-trifluoromethyl- D-1-2
piperazin 0.82 569.14
then
benzyI)-1,3-dihydro-imidazo[4,5-
140
c]pyridin-2-one (Example 206)
120
1-[(R)-1-(2-Fluoro-6-methyl-phenyI)-
2-
pyrrolidin-3-yI]-4-(2-methoxy- A
Methoxy
lv-13 ethylamino)-3-(2-trifluoromethyl- D-1-2 140 0.87
544.15
ethylamin
benzyI)-1,3-dihydro-imidazo[4,5- 144
c]pyridin-2-one (Example 207)
Synthesis of intermediates of formula D-2
A suspension of (methoxymethyl)triphenylphosphonium chloride (1.2 eq) in anh.
THF (12.5 mL/mmol) was cooled
to -78 C. A 1.6 M soln. of n-butyllithium in hexanes (1.2 eq) was dropwise
added at -78 C and the mixture was
stirred for 30 min at -78 C. A solution of aldehyde lc (1 eq) in anh. THF
(6.25 mL/mmol) was dropwise added at -
78 C and the rxn mixture was stirred for a given time (see Table 53) at RT. It
was quenched with a sat. aq. soln. of
NH4CI and extracted with DCM (3x). The combined org. phases were washed with
brine, dried over MgSO4 and
concentrated in vacuo. The crude was purified by CC using DCM/Me0H and/or by
prep. LC-MS using method 1.
CA 03087886 2020-07-08
WO 2019/141803 PCT/EP2019/051230
149
Table 53
MS-data
Reactant
D-2 Name time [h] tR [min] m/z
lc
[M+Fl]4
1-1(R)-1-[2-Fluoro-64(E)-2-methoxy-viny1)-
phenyl]-pyrrolidin-3-y11-4-methoxy-3-(2-
D-2-1 lc-5 18 1.08-1.09 542.27
trifluoromethyl-benzyI)-1,3-dihydro-
benzoimidazol-2-one
Synthesis of compounds of formula lw
To a soln. of enol ether D-2 (1 eq) in anh. THF (8.7 mL/mmol) was added a 5M
aq. soln. of HCI (1.8 mL/mmol) and
the rxn mixture was heated to 70 C and stirred for a given time (see Table
54). The mixture was diluted with Et20
and washed with H20 and a sat. aq. soln. of NaHCO3. The org. phase was dried
over MgSO4 and concentrated in
vacuo. The crude was purified by CC using DCM/Me0H.
Table 54
MS-data
Reactant
lw Name time [h] tR [min] m/z
D-2
[M+Fl]4
(3-Fluoro-2-1(R)-3-K-methoxy-2-oxo-3-(2-
trifluoromethyl-benzyI)-2,3-dihydro-
lw-1 D-2-1 0.6 0.89 528.13
benzoimidazol-111]-pyrrolidin-1-yll-pheny1)-
acetaldehyde (Example 126)
Synthesis of compounds of formula lx
Method A (Boc cleavage)
To a solution of intermediate lv (1 eq) in DCM (4 mL/mmol) was added dropwise
TFA (1 mL/mmol) and the rxn
mixture was stirred at RT for a given time (see Table 55). It was basified
with a 1M solution of NaOH until pH 12-13
and extracted with DCM (3x). The combined org. phases were dried over MgSO4
and concentrated in vacuo. The
crude was purified by prep. LC-MS using method 4.
Method B (ketal cleavage)
To a soln. of ketal lv or lk (1 eq) in anh. THF (9 mL/mmol) was added a 25%
aq. soln. of HCI (3 mL/mmol) at 0 C
and the rxn mixture was stirred for a given time (see Table 55) at RT. The rxn
mixture was quenched with a sat. aq.
soln. of NaHCO3 and extracted with Et0Ac (3x). The combined org. phases were
washed with brine, dried over
MgSO4 and concentrated in vacuo. The crude was purified by CC using
Hept/Et0Ac.
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
150
Table 55
MS-data
Reactant Method
lx Name tR [min]
m/z
lv or lk time [h]
[M+Fl]4
1-[(R)-1-(2-Fluoro-6-methyl-pheny1)-pyrrolidin-3-
y1]-4-(piperidin-4-yloxy)-3-(2-trifluoromethyl- A
Ix-1 Iv-5 0.83 570.27
benzyI)-1,3-dihydro-imidazo[4,5-c]pyridin-2-one 1.5
(Example 124)
4-(2,3-Dihydroxy-propoxy)-1-[(R)-1-(2-fluoro-6-
methyl-pheny1)-pyrrolidin-3-y1]-3-(2-
lx-2 lv-7 0.94 561.21
trifluoromethyl-benzyI)-1,3-dihydro-imidazo[4,5- 1
c]pyridin-2-one (Example 128)
4-(2,3-Dihydroxy-propoxy)-1-[(R)-1-(2-fluoro-6-
methyl-pheny1)-pyrrolidin-3-y1]-3-(2-
lx-3 lk-4 0.94 560.10
trifluoromethyl-benzyI)-1,3-dihydro- 18
benzoimidazol-2-one (Example 152)
Synthesis of compounds of formula ly
To a stirred soln. of lw (1 eq) in DCM (4 mL/mmol) was added AcOH (1.5 eq) and
the appropriate amine (1.1 eq)
followed by NaBH(OAc)3 (1.5 eq). The rxn mixture was stirred at RT for a given
time (see Table 56) and partitioned
between DCM and a sat. aq. soln. of NaHCO3. The org. phase was dried over
MgSO4 and concentrated in vacuo.
The crude was purified by CC using Et0Ac/Me0H.
Table 56
Reactant Reactant MS-data m/z
ly Name time [h] tR [min]
lw amine [M+Fl]4
1-1(R)-1-[2-Fluoro-6-(2-
morpholin-4-yl-ethyl)-phenyl]-
pyrrolidin-3-y11-4-methoxy-3-(2-
ly-1 lw-1 morpholine 2 0.89 599.32
trifluoromethyl-benzyI)-1,3-
dihydro-benzoimidazol-2-one
(Example 131)
Synthesis of compounds of formula lz
To a stirred suspension of alcohol In (1 eq) in THF (15 mL/mmol) was added at
0 C NaH (5 eq). The mixture was
stirred for 10 min and the corresponding halide (1.5 eq) was added. The rxn
mixture was allowed to reach RT and
stirred at a given temperature for a given time (see Table 57). It was
quenched with water and extracted with DCM
CA 03087886 2020-07-08
WO 2019/141803 PCT/EP2019/051230
151
(3x). The combined org. phases were dried over MgSO4 and concentrated in
vacuo. The crude was purified by CC
using Hept/Et0Ac or DCM/Me0H.
Table 57
MS-data
Reactant Reactant T [ C]
lz Name tR [min] m/z
In halide time [h]
[M+Fl]4
1-1(R)-1-[2-Fluoro-6-(1-methoxy-
ethyl)-pheny1]-pyrrolidin-3-y11-4-
Methyl RT
lz-1 methoxy-3-(2-trifluoromethyl- In-1 1.09
543.92
iodide 4
benzyI)-1,3-dihydro-benzoimidazol-
2-one (Example 148)
Synthesis of compounds of formula laa
To a soln. of intermediate lc (1 eq) in Et0Ac (3 mL/mmol) was added 10% Pd/C
moistened with ¨50% water (0.01
eq) and the rxn mixture was hydrogenated under atmospheric pressure for a
given time (see Table 58). It was
filtered over a pad of celite and the filtrate was concentrated in vacuo. The
crude was purified by CC using
Hept/Et0Ac.
Table 58
MS-data
Reactant
laa Name time [h] tR [min] m/z
lc
[M+Fl]4
1-[(R)-1-(2-Amino-6-fluoro-pheny1)-pyrrolidin-3-
laa-1 yI]-4-methoxy-3-(2-trifluoromethyl-benzy1)-1,3- Ic-10 1
1.01 501.03
dihydro-benzoimidazol-2-one (Example 155)
Synthesis of compounds of formula lab
A stirred soln. of aniline laa (1 eq) in a 48% aq. soln. of HBr (2.7 mL/mmol)
was cooled to -20 C and a soln. of
NaNO2 (1 eq) in H20 (0.4 mL/mmol) was dropwise added. The mixture was stirred
for 30 min at 0 C and a soln. of
CuBr (0.55 eq) in a 48% aq. soln. of HBr (0.8 mL/mmol) was dropwise added. The
rxn mixture was heated to 60 C
and stirred for a given time (see Table 59). The pH was adjusted to around 7
by the addition of a 2M aq. soln. of
NaOH and the mixture was extracted with Et0Ac (3x). The combined org. phases
were washed with brine, dried
over MgSO4 and concentrated in vacuo. The crude was purified by prep. LC-MS
using method 1.
CA 03087886 2020-07-08
WO 2019/141803 PCT/EP2019/051230
152
Table 59
MS-data
Reactant
lab Name time [h] tR [min] m/z
laa
[M+Fl]4
1-[(R)-1-(2-Bromo-6-fluoro-pheny1)-pyrrolidin-3-
lab-1 yI]-4-methoxy-3-(2-trifluoromethyl-benzy1)-1,3- laa-1 1
1.11 563.96
dihydro-benzoimidazol-2-one (Example 156)
Synthesis of compounds of formula lac
To a degassed mixture of bromide laa (1 eq) and alkyne (1.6 eq) in anh. THF
(24 mL/mmol) was added under
argon Cul (0.06 eq), bis(tri-tert-butylphosphine)palladium(0) (0.05 eq) and
DBU (1.3 eq). The mixture was heated to
70 C and stirred for 2h (see Table 60). The volatiles were evaporated off and
the residue was purified by prep. LC-
MS using method 3.
Table 60
MS-data
Reactant Reactant
lac Name tR [min] m/z
lab alkyne
[WE]4
1-1(R)-1-[2-Fluoro-6-(3-morpholin-4-yl-prop-1-
4-(Prop-2-
yny1)-phenyl]-pyrrolidin-3-y11-4-methoxy-3-(2-
lac-1 lab-1 yn-1-y1) 0.87
609.04
trifluoromethyl-benzyI)-1,3-dihydro-
morpholine
benzoimidazol-2-one (Example 159)
Synthesis of compounds of formula lad
To a soln. of alkyne lac (1 eq) in Et0Ac (3 mL/mmol) was added 10% Pd/C
moistened with ¨50% water (0.01 eq)
and the rxn mixture was hydrogenated under atmospheric pressure for a given
time (see Table 61). It was filtered
over a pad of celite and the filtrate was concentrated in vacuo. The crude was
purified by prep. LC-MS using
method 5.
Table 61
MS-data
Reactant
lad Name time [h] tR [min] m/z
lac
[WE]4
i-1(R)-1-[2-Fluoro-6-(3-morpholin-4-yl-propy-
phenyl]-pyrrolidin-3-y11-4-methoxy-3-(2-
lad-1 lac-1 7 0.87 613.09
trifluoromethyl-benzyI)-1,3-dihydro-
benzoimidazol-2-one (Example 162)
CA 03087886 2020-07-08
WO 2019/141803 PCT/EP2019/051230
153
Synthesis of compounds of formula lae
A soln. of alcohol In (1 eq) in DCM (3 mL/mmol) was cooled to 0 C and thionyl
chloride (1.1 eq) was dropwise
added. The rxn mixture was stirred for 30 min at RI and the volatiles were
evaporated off. The residue was taken
in DCM (5 mL/mmol) and TEA (5 eq) followed by the appropriate amine (1.1 eq)
were added at 0 C. The mixture
was allowed to warm to RI and stirred for a given time (see Table 62). It was
diluted with DCM and washed with a
1M aq. soln. of NaOH. The org. phase was dried over MgSO4 and concentrated in
vacuo. The crude was purified
by prep. LC-MS using method 2 and 5.
Table 62
MS-data
Reactant Reactant time
lae Name tR [min] m/z
In amine [h]
[M+Fl]4
14(R)-1-12-Fluoro-6-[1-(3-methoxy-
azetidin-1-y1)-ethyl]-phenyll-pyrrolidin-3-
3-Methoxy
lae-1 yI)-4-methoxy-3-(2-trifluoromethyl- In-1 4 0.91/0.93 ..
599.04
azetidine
benzy1)-1,3-dihydro-benzoimidazol-2-
one (Example 163)
Synthesis of intermediates of formula E-2
To a soln. of carboxylic ester lq (1 eq) in THF (7.2 mL/mmol) was added a 2M
aq. soln. of NaOH (10 eq) and the
rxn mixture was stirred at RT for a given time (see Table 63). It was
acidified with a 1M aq. soln. of HCI until pH-4-
5 and extracted with Et0Ac (3x). The combined org. phases were washed with
brine, dried over MgSO4 and
concentrated in vacuo. The crude was purified by CC using Hept/Et0Ac.
Table 63
MS-data
Reactant time
E-2 Name tR [min] m/z
lq [h]
[M+Fl]4
i-[1-(2-Fluoro-6-methyl-pheny-
piperidin-4-y1]-3-(2-hydroxy-benzy-4-
E-2-1 lq-45 4 1.09 462.11
methoxy-1,3-dihydro-benzoimidazol-2-
one
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
154
Synthesis of compounds of formula laf
Method A (NaH / THF)
To a stirred suspension of phenol E-2 (1 eq) in THF (15 mL/mmol) was added at
0 C NaH (5 eq). The mixture was
stirred for 10 min and the corresponding halide (1.5 eq) was added. The rxn
mixture was stirred at a given
temperature for a given time (see Table 64). It was quenched with water and
extracted with Et0Ac (3x). The
combined org. phases were dried over MgSO4 and concentrated in vacuo. The
crude was purified by CC using
Hept/Et0Ac.
Method B (K2CO3/ DMF)
To a stirred soln. of phenol E-2 (1 eq) in anh. DMF (8 mL/mmol) was added
K2CO3 (2 eq) and the corresponding
halide (2 eq). The rxn mixture was stirred for a given time and a given
temperature (see Table 64). Additional
amount of halide (3 x 2 eq) were added to bring the rxn to completion. It was
partitioned between Et0Ac and H20
and the org. phase was washed with brine, dried over MgSO4 and concentrated in
vacuo. The crude was purified
by CC using Hept/Et0Ac.
Table 64
Method MS-
data
Reactant Reactant
laf Name T [ C] tR [min] m/z
E-2 halide
time [h]
[M+Fl]4
3-(2-Cyclopropylmethoxy-benzyI)-1-
(Bromo
[1-(2-fluoro-6-methyl-phenyI)- A
methyl)
laf-1 piperidin-4-yI]-4-methoxy-1,3- E-2-1 60 C 1.14
516.20
cyclo
dihydro-benzoimidazol-2-one 18h
propane
(Example 224)
1-[1-(2-Fluoro-6-methyl-phenyI)-
piperidin-4-yI]-4-methoxy-3-[2-
3-Bromo
laf-2 (oxetan-3-yloxy)-benzyI]-1,3- E-2-1 up to 90 C
1.08 518.17
oxetane
dihydro-benzoimidazol-2-one 120
(Example 225)
3-(2-Cyclobutoxy-benzy1)-1-0-(2-
Bromo
fluoro-6-methyl-phenyI)-piperidin-4- up to
laf-3 E-2-1 cyclo 1.15
516.18
yI]-4-methoxy-1,3-dihydro- 100 C
butane
benzoimidazol-2-one (Example 226) 120
Synthesis of intermediates of formula C-2
To a soln. of methyl carbamate BB-10 (1 eq) in MeCN (4.3 mL/mmol) was added
K2CO3 (2.2 eq) and the
corresponding halide (1.05 eq). The rxn mixture was heated to a given
temperature and stirred for a given time
CA 03087886 2020-07-08
WO 2019/141803
PCT/EP2019/051230
155
(see Table 65). It was filtered and the filtrate was concentrated in vacuo.
The crude was purified by CC using
Hept/Et0Ac.
Table 65
MS-data
Reactant Reactant T [ C]
C-2 Name tR [min] m/z
BB-10 halide time [h]
[M+Fl]4
2-
(3-Bromo-5-methoxy-pyridin-4-yI)- (Trifluorom
C-2-1 (2-trifluoromethyl-benzyI)-carbamic BB-10-1
ethyl) 2 1.09 419.05
acid methyl ester benzyl
bromide
5 .. Synthesis of compounds of formula lag
Sodium tert-butoxide (6 eq), BrettPhos precatalyst (0.3 eq) and BrettPhos (0.3
eq) were placed in a sealed cap vial
and a soln. of intermediate C-2 (3 eq) and amine BB-4 (1 eq) in anh. dioxane
were added. The rxn mixture was
flushed with N2, heated at a given temperature and stirred for a given time
(see Table 66). It was quenched with
water and extracted with DCM (3x). The combined org. phases were dried over
MgSO4 and concentrated in vacuo.
10 The crude was purified by prep. LC-MS using method 1.
Table 66
MS-data
Reactant Reactant T [ C]
lag Name tR [min] m/z
C-2 BB-4 time [h]
[M+Fl]4
3-[1-(2-Fluoro-6-methyl-phenyI)-
piperidin-4-yI]-7-methoxy-1-(2-
lag-1 trifluoromethyl-benzyI)-1,3-dihydro- C-2-1
BB-4-2 2 1.01 515.23
imidazo[4,5-c]pyridin-2-one
(Example 229)
Synthesis of compounds of formula lah
To a soln. of intermediate lb (1 eq) in Et0H (20 mL/mmol) was added 20%
palladium hydroxide on carbon (0.1 eq)
15 and the rxn mixture was hydrogenated under atmospheric pressure for a
given time (see Table 67). It was filtered
over a pad of celite and the filtrate was concentrated in vacuo. The crude was
purified by CC using Hept/Et0Ac.
CA 03087886 2020-07-08
WO 2019/141803 PCT/EP2019/051230
156
Table 67
MS-data
Reactant
lah Name lb time [h] tR [min] m/z
[M+Fl]4
1-[1-(2-Hydroxy-phenyI)-piperidin-4-
yI]-4-methoxy-3-(2-trifluoromethyl-
lah-1 lb-62 96 0.83 498.02
benzyI)-1,3-dihydro-benzoimidazol-
2-one (Example 230)
Synthesis of compounds of formula lai
To a soln. of intermediate lah (1 eq) and alcohol R15-0H (1 to 1.5 eq) in
toluene (10 mL/mmol) was added a 1M
soln. of (tributylphosphoranylidene)acetonitrile in toluene (2 eq) under
argon. The rxn mixture was heated to 110 C
and stirred for a given time (see Table 68). It was quenched with water and
extracted with DCM (3x). The combined
org. phases were washed with brine, dried over MgSO4 and concentrated in
vacuo. The crude was purified by CC
using Hept/Et0Ac or DCM/Me0H. When necessary, an additional purification by
prep. LC-MS using method 5 can
be performed.
Table 68
MS-data
Reactant Alcohol
lai Name time [h] tR [min]
m/z
lah R15-0F1
[M+Fl]4
1-(1-12-[2-(tert-Butyl-dimethyl-
silanyloxy)-ethoxy]-phenyll- 2-(t-Butyl
lai-1 piperidin-411)-4-methoxy-3-(2- lah-1 dimethylsiloxy)
4.5 1.00 656.18
trifluoromethyl-benzyI)-1,3-dihydro- ethanol
benzoimidazol-2-one
4-Methoxy-1-11 -[2-(tetrahydro-
pyran-4-yloxy)-phenyl]-piperidin-4-
Tetrahydro-
lai-2 y11-3-(2-trifluoromethyl-benzy1)-1,3- lah-1 4.5 0.89
582.17
2H-pyran-4-ol
dihydro-benzoimidazol-2-one
(Example 239)
1-[1-(2-Cyclopropylmethoxy-pheny1)-
piperidin-4-y1]-4-methoxy-3-(2- Hydroxymethyl
lai-3 lah-1 4.5 0.92 552.20
trifluoromethyl-benzyI)-1,3-dihydro- cyclopropane
benzoimidazol-2-one (Example 240)
CA 03087886 2020-07-08
WO 2019/141803 PCT/EP2019/051230
157
1-[1-(2-Cyclobutoxy-pheny1)-
piperidin-4-y1]-4-methoxy-3-(2-
lai-4 lah-1 Cyclobutanol 4.5 0.91 552.11
trifluoromethyl-benzyI)-1,3-dihydro-
benzoimidazol-2-one (Example 241)
4-Methoxy-1-11-[2-(oxetan-3-yloxy)-
phenyl]-piperidin-4-y11-3-(2-
lai-5 lah-1 Oxetan-3-ol 18 0.88 554.18
trifluoromethyl-benzyI)-1,3-dihydro-
benzoimidazol-2-one (Example 242)
1-[1-(2-Butoxy-phenyI)-piperidin-4-
yI]-4-methoxy-3-(2-trifluoromethyl-
lai-6 lah-1 1-Butanol 18 0.94 554.22
benzyI)-1,3-dihydro-benzoimidazol-
2-one (Example 243)
Synthesis of compounds of formula la]
To a soln. of lai (1 eq) in THF (20 mL/mmol) was added dropwise a 1M soln. of
TBAF in THF (1.2 eq) and the rxn
mixture was stirred at RT for a given time (see Table 69). It was partitioned
between Et0Ac and a sat. aq. soln. of
NaHCO3. The org. phase was dried over MgSO4 and concentrated in vacuo. The
crude was purified by prep. LC-
MS using method 6.
Table 69
MS-data
Reactant
laj Name time [h] tR [min] m/z
lai
[M+Fl]4
1-11 -[2-(2-Hydroxy-ethoxy)-phenyl]-
piperidin-4-y11-4-methoxy-3-(2-
laj-1 lai-1 18 0.82 542.18
trifluoromethyl-benzyI)-1,3-dihydro-
benzoimidazol-2-one (Example 238)
II. Biological Assays
In vitro assay
hC5a DISCO IC50 assay
Adherent cells (CHO-K1 C5AR1 beta-arrestin cell line, DiscoverX, CA USA) are
washed with PBS, detached by
incubation with Dissociation Buffer (Gibco Cat# 13151-014,2 ml per 165 cm2
dish) for 3 minutes, then washed with
10 ml PBS (without Mg++ and Ca++) and counted. 7'500 cells/384-well are seeded
out in 384-well plates (Cell
CA 03087886 2020-07-08
WO 2019/141803 PCT/EP2019/051230
158
culture plate M1P384 white Polystyrene, Corning, Cat# 3570) in 20 111/well
Cell plating medium (F12 HAMs/10%
FCS/1% PIS) and incubated at 37 C / 5% CO2 / 24h.
1..t1 Antagonist at 6-fold end concentration or DMSO control is added to assay
medium and subsequently 5 1..t1 1 -
nM C5a agonist at 6 fold end concentration. Cells are centrifuged for 1 min at
1000 rpm and incubated for 1.5
5 hour in at 37 C. Plates are equilibrated at room temperature for several
minutes before adding 12111/well Detection
Reagent (PathHunter Detection Kit, DiscoverX, Cat# 93-0001). Plates are
centrifuged for 1 min at 1000 rpm and
incubated for 45 minutes at RI before being measured on a Fluostar Optima, BMG
Labtech. IC50 values are
calculated from a serial dilution range of antagonist using inhouse software
and given in nmo1/1.
The calculated IC50 values may fluctuate depending on the daily cellular assay
performance. Fluctuations of this
10 kind are known to those skilled in the art. Average IC50 values from
several measurements are given as geometric
mean values.
Antagonistic activities of exemplified compounds are displayed in Table 70
below.
Table 70: list of examples and their antagonistic activities
Example Compound C5aR 1050 Example Compound C5aR IC50
Example Compound C5aR IC50
Number N (nM) Number N (nM) Number N (nM)
1 la-1 621 85 le-1 712 169 lq-18 17
2 lb-1 18 86 Ig-9 229 170 lq-19 45
3 Ir-3 23 87 lc-7 629 171 lq-6 64
4 lb-2 131 88 1m-1 39 172 lq-20 275
5 lb-3 284 89 1n-1 44 173 Ir-2 107
6 la-2 66 90 lo-1 38 174 lq-21 544
7 lb-4 11 91 1p-1 70 175 lq-22 221
8 lb-5 14 92 lq-1 2024 176 lq-23 48
9 lb-6 32 93 lj-6 149 177 lq-24 24
10 lb-7 23 94 lj-7 313 178 lq-25 49
11 lb-8 29 95 Ir-1 191 179 la-15 122
12 lb-9 10 96 lq-7 231 180 lq-26 9
13 lb-10 16 97 lq-8 1351 181 lq-27 22
14 lc-1 216 98 lq-2 165 182 lq-28 9
lb-11 13 99 1s-1 391 183 lq-29 14
16 lb-12 19 100 Is-2 1428 184 lb-49 36
CA 03087886 2020-07-08
WO 2019/141803 PCT/EP2019/051230
159
17 lb-13 22 101 Is-3 1580 185 lb-50 36
18 lb-14 13 102 It-1 759 186 lq-30 14
19 lb-15 12 103 lu-1 669 187 lq-31 26
20 la-3 36 104 lq-3 863 188 la-16 313
21 Id-1 3442 105 lq-4 4483 189 lq-32 13
22 lb-16 30 106 lu-2 227 190 lb-51 118
23 lb-17 8 107 lu-3 1318 191 la-17 114
24 le-1 330 108 lu-4 360 192 lq-33 42
25 If-1 185 109 lc-8 61 193 Is-4 632
26 Ig-1 459 110 lb-45 6 194 Is-5 1485
27 Ih-1 3344 111 lq-9 264 195 lq-34 20
28 Ih-2 1326 112 lq-10 511 196 la-18 139
29 Ig-2 182 113 lq-11 2240 197 lb-52 74
30 Ih-3 1523 114 lq-12 1543 198 lb-53 15
31 li-1 3454 115 lu-5 21 199 la-19 119
32 li-2 642 116 lu-6 165 200 lb-54 19
33 lb-18 8 117 lq-13 308 201 lb-55 31
34 lb-19 27 118 lv-1 201 202 lq-35 2
35 li-3 4510 119 lv-2 12 203 lq-36 6
36 lb-20 450 120 lv-3 659 204 lb-56 30
37 lb-21 7 121 lv-4 270 205 lv-11 45
38 lb-22 470 122 lv-5 509 206 lv-12 2298
39 la-4 2434 123 lv-6 42 207 lv-13 508
40 Ij-1 6 124 lx-1 5220 208 lq-37 19
41 lb-23 18 125 lv-7 124 209 lq-38 34
42 lb-24 75 126 lw-1 20 210 la-20 2294
43 la-5 393 127 lv-8 17 211 lb-57 27
44 lj-2 13 128 lx-2 207 212 lb-58 2079
45 lb-25 16 129 lv-9 53 213 la-22 469
46 lb-26 39 130 lv-10 3908 214 la-21 205
CA 03087886 2020-07-08
WO 2019/141803 PCT/EP2019/051230
160
47 lb-27 95 131 ly-1 53 215 lb-59 27
48 lb-28 165 132 lu-7 314 216 lq-39 12
49 lb-29 132 133 lu-8 70 217 lb-60 158
50 lb-30 42 134 lu-9 2265 218 lq-40 346
51 lb-31 62 135 lu-10 5764 219 lq-41 34
52 1k-1 30 136 lu-11 1586 220 lq-42 109
53 la-7 1845 137 lu-12 133 221 lq-43 175
54 lb-32 43 138 lu-13 156 222 Ih-4 2651
55 lb-33 178 139 lu-14 227 223 lq-44 130
56 lj-3 717 140 lu-15 1034 224 laf-1 18
57 lj-4 89 141 lu-16 2128 225 laf-2 40
58 lb-34 25 142 lu-17 108 226 laf-3 36
59 lb-35 49 143 lu-18 108 227 lb-61 30
60 Ig-3 84 144 la-12 312 228 la-23 259
61 Ig-4 65 145 lc-9 16 229 lag-1 268
62 Ig-5 99 146 la-13 201 230 lah-1 129
63 Ig-6 729 147 lb-46 20 231 lb-62 66
64 la-10 2916 148 lz-1 28 232 lb-63 82
65 la-8 396 149 lk-2 14 233 lb-64 195
66 lb-36 60 150 lk-3 11 234 lb-65 17
67 lb-37 36 151 lk-4 31 235 lb-66 28
68 lb-38 84 152 lx-3 38 236 lb-67 171
69 lc-2 2819 153 lq-14 215 237 lb-68 90
70 Ig-7 538 154 lc-10 23 238 laj-1 133
71 lc-3 133 155 laa-1 15 239 lai-2 82
72 Ig-8 397 156 lab-1 21 240 lai-3 46
73 lb-39 272 157 Ic-11 630 241 lai-4 36
74 lb-40 1330 158 lc-12 683 242 lai-5 74
75 lb-41 27 159 lac-1 73 243 lai-6 61
76 lb-42 38 160 lb-47 35 244 lb-69 227
CA 03087886 2020-07-08
WO 2019/141803 PCT/EP2019/051230
161
77 lb-43 89 161 lb-48 69 245 lb-70 111
78 lb-44 121 162 lad-1 135 246 lb-71 39
79 Id-2 911 163 lae-1 591 247 lb-72 563
80 le-2 157 164 la-14 179 248 lb-73 182
81 lj-5 2427 165 lq-15 6 249 lb-74 273
82 lc-4 28 166 lq-5 17 250 lb-75 853
83 lc-5 23 167 lq-16 6
84 lc-6 2035 168 lq-17 10