Note: Descriptions are shown in the official language in which they were submitted.
METHOD FOR IMPROVING QUALITY OF AQUACULTURE POND WATER USING A
NUTRIENT GERMINANT COMPOSITION AND SPORE INCUBATION METHOD
BACKGROUND OF THE INVENTION
1. Field of the Invention
[0001] This invention relates to the treatment of aquaculture pond water with
bacteria germinated in a nutrient germinant composition and using a point-of-
use spore
incubation method to reduce organic waste, ammonia, and disease pressure in a
water
livestock application and to provide probiotics to aquaculture species.
2. Description of Related Art
[0002] Aquaculture refers to the raising of aquatic species that are used as a
human or
animal food source. The technique applies some types of control to the natural
environment of
the raised species to improve overall harvests. This can include the
artificial hatching of species
to increase the commercial harvest of animals in the wild, hatching and
raising of the species in
endosed ponds, and the hatching and raising of species in tidally drained
enclosed areas
adjacent to the shoreline. Problems associated with this process include:
pollution that is
discharged from the raising facility and will deteriorate the water quality
around; loss of product
due to deteriorated water quality in the raising facility; and increased
disease pressures
associated with pathogenic microorganisms in the raising facility. Such
problems may
be identified through testing or monitoring a variety of parameters, including
pH, conductivity,
ammonia, nitrate, phosphate and alkalinity. Conductivity is an indicator of
salt content,
1
Date Regue/Date Received 2020-11-06
CA 03088139 2020-07-08
WO 2019/168627
PCT/1JS2019/016020
amounts greater than 1200 ppm is no longer considered fresh water; an ideal
amount is
700 ppm and range of 300 ¨ 1200 ppm. Ammonia levels measure the amount of
available oxygen for fish. High levels of ammonia block oxygen transfer in
fish from gills
to the blood; however it is also a product of their metabolic waste. While
ammonia from
fish waste is often not concentrated enough to be toxic itself, fish farmers
must closely
monitor ammonia levels due to the high concentration of fish per pond. Oxygen
is
consumed by nitrifying bacteria in the pond which break down the toxic ammonia
to a
non-toxic form; however, this massive use of oxygen reduces the oxygen
available for
uptake by fish. Ammonia levels >1 ppm are considered toxic for fish life.
Additionally,
nitrate levels are examined to determine the amount of plant fertilizer in the
water.
Nitrate is highly leachable from the surrounding soil and can be harmful to
small
children and pregnant women. Nitrate becomes nitrite in the GI tract and
interacts with
the blood's ability to carry oxygen. Max contamination level for nitrate is 10
ppm.
Alkalinity is the measure of a pond's or lake's ability to neutralize acid
without a change
in pH. Alkalinity will decrease over time due to bacteria; however an ideal
level is 100
ppm with acceptable range of 50-200 ppm. Phosphate found in ponds and lakes is
largely from human and animal waste. Fertilizer run-off is a major source of
phosphate
found in golf course and decorative ponds. Elevated levels cause an increased
rate of
eutrophication which in turn increases sludge production. Moderate levels of
phosphate
can stimulate plant growth causing an increase in algae production; levels of
>0.1 ppm
is an indication of accelerated plant growth and is considered outside
acceptable levels.
[0003] Current technologies to address these problems include bioremediation,
antibiotics, and chemical additives. Typical bioremediation technologies
include the
application of supplemental bacteria to the water to enhance the
microbiological
activities to improve the water quality. It is also known to use nitrifiers to
enhance the
nitrification process to convert the toxic ammonia into non-toxic nitrate.
Chemical
additives are added to improve the water quality and aid the microbiological
activities by
providing extra nutrients and alkalinity. Antibiotics are added to inhibit the
growth of the
pathogenic microorganisms. Problems associated with the current technologies
include
high cost and poor water quality improvement performance with the inactive
2
supplemental bacteria, low nitrification activities due to the existence of
organic waste
and lack of nitrifier growing sites, and bioaccumulation of antibiotics in the
raised
aquatic species.
[0004] According to preferred methods disclosed in U.S. Application Serial No.
14/720,088, active bacteria may be generated on-site using a biogenerator to
grow the
bacteria to a useful population from a solid bacteria starter material. The
active bacteria
may then be discharged into an aquaculture application from one or more
biogenerators. Such biogenerators and their methods of use are disclosed, for
example,
in United States Patent Nos. 6,335,191; 7,081,361; 7,635,587; 8,093,040; and
8,551,762. However, it would be useful to have an alternate method of
generating
active bacteria from spores at the point of use in an aquaculture application.
[0005] Spore germination is a multistep, causative process wherein spores
effectively wake-up or are revived from a dormant state to a vegetative growth
state.
The first step is one by which spores are activated and are induced to
germinate,
typically by an environmental signal called a germinant. This signal can be a
nutrient
such as an L-amino acid. Nutrient germinants bind to receptors in the inner-
membrane
of the spore to initiate germination. Additionally, sugars have been shown to
increase
the binding affinity of L-amino acids for their cognate receptors.
[0006] The germinant signal then initiates a cascade that causes the release
of
Dipicolinic Acid (DPA), which is stored in a 1:1 ratio with Ca2+ (CaDPA) in
the core of
the spore. The release of CaDPA is a fast process and is typically >90%
complete in 2
min. CaDPA release represents a point of no return for spores in which they
are
committed to the germination process. Those knowledgeable in the art refer to
this step
as the "commitment" step.
[0007] After CaDPA release, the spore is partially hydrated and the core pH
rises to approx. 8Ø The core of the spore then expands and the cortex
(composed
mostly of peptidoglycan) is degraded by core lytic enzymes. The spore absorbs
water
and consequently loses its refractivity. This loss of refractivity towards the
end of the
3
Date Regue/Date Received 2020-11-06
CA 03088139 2020-07-08
WO 2019/168627
PCT/1JS2019/016020
germination process allows spore germination to be monitored via phase-
contrast
microscopy.
[0008] The second phase of germination is an outgrowth step in which the
spore's metabolic, biosynthetic, and DNA replication/repair pathways initiate.
The
outgrowth period has several phases. The first is known as a ripening period
in which
no morphological changes (such as cell growth) occur, but the spore's
molecular
machinery (e.g. transcription factors, translation machinery, biosynthesis
machinery,
etc.) is activated. This period can vary in length based on the initial
resources that are
packaged with the spore during the process of sporulation. For instance, the
preferred
carbon source of several Bacillus species (including subtilis) is malate and
Bacillus
spores typically contain a large pool of malate that is used during the
revival process.
Interestingly, deletion mutants that cannot utilize the malate pool display an
extended
ripening period compared to wild-type spores indicating that the spore malate
pool is
sufficient to energize the initial outgrowth process. Additionally, spores
store small, acid-
soluble proteins that are degraded within the first several minutes of revival
that serve
as an immediate source of amino acids for protein synthesis. After the
outgrowth step,
spore revival is complete and cells are considered to be vegetatively growing.
[0009] It is known that spores can be induced to germinate via heat-
activation.
Spores of various Bacillus species have been heat-activated at strain-specific
temperatures. For example, B. subtilis spores have been heat-activated at 75 C
for 30
minutes while B. licheniformis spores have been heat-activated at 65 C for 20
minutes.
The heat-activation has been shown to cause a transient, reversible unfolding
of spore
coat proteins. Heat-activated spores can then be germinated for additional
time in
germination buffers containing nutrient germ inants, such as L-alanine. If no
nutrient
germinant is present, however, spores will return to their pre-heated, non-
germinated
state.
[0010] It is also known that germination can occur at ambient temperatures
(near typical room temperature) without heat-activation and with a germination
buffer
containing nutrients, but the process usually takes longer than with heat-
activation. For
example, B. licheniformis and B. subtilis spores will germinate at 35 C or 37
C,
4
CA 03088139 2020-07-08
WO 2019/168627
PCT/1JS2019/016020
respectively, but it takes a longer period of time (e.g. 2 hours) in a
germination buffer
containing nutrient germinants. Additionally, non-heat-activated spores of B.
subtilis
have been known to have been germinated in non-nutrient germinant conditions
(e.g.
CaCl2 + Na2DPA) for an extended period of time.
[0011] It is also known to combine the use of heat activation and a nutrient
germinant to germinate spores in a two-step process in laboratory settings.
The spores
are first heat activated by incubating for a period of time (e.g. 30 minutes)
at a
temperature in the range of 65-75 C (this specific temperature is species
dependent).
Then, the spores are transferred into a buffer solution that contains a
nutrient
germinant, such as L-alanine. It is also known to grow bacteria in a growth
chamber
located near a use site by feeding pelletized nutrient material (containing
sugar, yeast
extract, and other nutrients that are not direct spore germinants), bacteria
starter, and
water into a growth chamber at a controlled temperature range of 16-40 C, and
more
preferably between 29-32 C, for a growth period of around 24 hours as
disclosed in
U.S. Patent No. 7,081,361.
[0012] There is a need for a rapid spore incubation and activation method that
will allow generation of active bacteria, such as Bacillus species, in a
single step at a
point-of-use location where the bacteria will be discharged into an
aquaculture
application. Accordingly, this invention describes a simple method for
spore
germination using a nutrient germ inant concentrate combined with a spore
composition,
or using a nutrient spore composition, simultaneously with heat incubation in
a single
step for use in aquaculture applications.
CA 03088139 2020-07-08
WO 2019/168627
PCT/1JS2019/016020
SUMMARY OF THE INVENTION
[0013] The method of the invention provides a cost-effective approach to
delivering active bacteria to pond water (or a growing pond) in an aquaculture
facility to
degrade the organic waste and inhibit the growth of pathogenic microorganisms
without
bioaccumulation. The method of the invention reduces disease pressure in the
water
livestock, resulting in improved harvests of the species raised in the
aquaculture
operation. The addition of a nitrification enhancement agent as disclosed here
as part
of the method provides a steady alkalinity source and extra growing sites for
the nitrifier
to promote the nitrification activity and ammonia reduction.
[0014] The method of the invention desirably includes the delivery of active
bacteria, optionally including a probiotic bacteria, most preferably generated
from an on-
site incubator using a liquid nutrient germinant concentrate and bacteria in
spore form,
into an aquaculture application. A nutrient-germinant composition according to
one
preferred embodiment of the invention comprises one or a combination of many L-
amino acids, optionally D-glucose (which increases the binding affinity of L-
amino acids
for their cognate receptors in the spore coat), and a neutral buffer such as a
phosphate
buffer, and an industrial preservative, such as the commercially available
Kathon/Lingaurd CG (which has active ingredients comprising methyl chloro
isothiazolinone and methyl isothiazolinone).
A nutrient-germ inant composition
according to another preferred embodiment of the invention comprises one or a
combination of two or more L-amino acids, optionally D-glucose (which
increases the
binding affinity of L-amino acids for their cognate receptors in the spore
coat), HEPES
sodium salt (a biological buffer to provide the proper pH for spore
germination), and an
industrial preservative, such as a combination of propylparaben and
methylparaben or
other U.S. federal GRAS (Generally Regarded As Safe) preservatives. According
to
another preferred embodiment, the spore composition also comprises a source of
potassium ions, such as potassium chloride or monopotassium phosphate or
dipotassium phosphate. According to another preferred embodiment, the spore
composition includes both D-glucose and D-fructose.
6
CA 03088139 2020-07-08
WO 2019/168627
PCT/1JS2019/016020
[0015] According to another preferred embodiment, a nutrient gemiinant
composition also comprises spores of one or more bacteria species, preferably
Bacillus
species but other bacteria may also be used, and includes a germination
inhibitor, such
as NaCI, industrial preservatives, or D-alanine, in combination with any of
the previously
described spore composition ingredients. The germination inhibitor prevents
the spores
from germinating prematurely in the nutrient-germinant composition. The
germination
inhibitor may include chemicals that prevent spore germination such as NaCI,
industrial
preservatives, or D-alanine.
[0016] Alternatively, bacterial spores may be separately provided and added to
a nutrient-germinant composition according to the invention at the point-of-
use and
incubation. When separately added, it is preferred to provide a stable spore
suspension
spore composition comprising one or more bacteria species, preferably Bacillus
species. According to one preferred embodiment, a spore composition comprises
bacteria spores, about 0.00005 to 3.0 % by weight surfactant, about 0.002 to
5.0 % by
weight thickener, and optionally about 0.01 to 2.0% by weight of acidifiers,
acids, or
salts of acids (including those used as a preservative or stabilizer), with
the balance
being water. According to another preferred embodiment, a spore composition
comprises bacterial spores, about 0.1 to 5.0 % by weight thickener, about 0.05
to 0.5%
by weight acids or salts of acids, optionally about 0.1-20% by weight water
activity
reducers, and optionally about 0.1% to 20% additional acidifier (acids or
salts of acids),
with the balance being water.
[0017] Most preferably, the bacterial spores in both preferred spore
composition
embodiments are in a dry, powder blend of 40-60% salt (table salt) and 60-40%
bacteria
spores (prior to adding to the spore composition) that combined make up about
0.1 to
% by weight of the spore composition. The spore compositions preferably
comprise
around 1.0 X 108 to around 3.0 X 10 8 cfu/ml of the spore composition (spore
suspension), which when diluted with drinking water (for animal watering
applications)
provide around 104 to 108 cfu/ml bacterial strains in the drinking water. Most
preferably,
the thickener in both preferred embodiments is one that also acts as a
prebiotic, such as
xanthan gum, to provide additional benefits. Although other commercially
available
7
spore products may be used, preferred spore compositions for use with the
invention
are as disclosed in U.S. Application Serial No. 14/524,858 filed on October
27, 2014.
[0018] According to another preferred embodiment, a nutrient germinant
composition according to the invention is in concentrated form and is diluted
to 0.01% to
10% strength in water or another diluent at the point-of-use. The use of a
concentrated
formula reduces shipping, storage, and packaging costs and makes dosing of the
spore
composition at the point-of-use easier.
Most preferably, the concentrated spore
composition is in a liquid form, which is easier and faster to mix with
diluent at the point-of-
use, but solid forms such as pellets or hicks or powder may also be used. The
inclusion of
a general, industrial preservative in the spore composition aids in long-term
storage and/or
germination inhibition, which is particularly useful when the spore
composition is in the
preferred concentrated form.
[0019] In another preferred embodiment, the present invention comprises a
method of germinating spores of Bacillus species using a nutrient germinant
composition combined with a spore composition or using a nutrient spore
composition
at an elevated temperature; preferably in a range of 35-60 C, more preferably
in the
range of 38-50 C, and most preferably in the range of 41 C to 44 C for a
period of time
(an incubation period). The incubation period preferably ranges from 2-60
minutes, or
longer, depending on the application.
Most preferably, a nutrient-germinant
composition or nutrient spore composition in concentrated form according to
preferred
embodiments of the invention are used in the incubation/germination methods of
the
invention, but other nutrient-germinant compositions and spore compositions
may also
be used. Preferably, the incubation method is carried out at or near the point-
of-use -
the aquaculture site or near the aquaculture site where the germinated spores
will be
used or consumed and further comprises dispensing the germinated spores to the
point-of-use/consumption. Preferred methods according to the invention may be
carried
out in any incubation device that has a reservoir capable of holding a volume
of spores
(if separately added), liquid (typically water as a diluent), nutrient-
germinant composition
and that is capable of heating the mixture during an incubation period. Most
preferably,
8
Date Regue/Date Received 2020-11-06
CA 03088139 2020-07-08
WO 2019/168627
PCT/1JS2019/016020
the methods are carried out in a device that is also capable of mixing those
ingredients,
automatically shutting-off heating at the end of the incubation period, and
automatically
dispensing an incubated bacteria solution comprising the bacteria to an
aquaculture
point-of-use/consumption. Preferred methods may also be carried out as a batch
process or as a continuous process. Although spore compositions according to
the
invention are preferably used, any variety of spore forms or products, such as
dried
powder form, a liquid suspension, or a reconstituted aqueous mixture, may be
used with
the method of the invention.
[0020] The preferred embodiments of the invention allow for rapid germination
of
spores of Bacillus species at an aquaculture point-of-use. The active,
vegetative
bacteria solution discharged from the incubator can be supplied directly to
growing
ponds or can be accumulated and diluted with pond water or another similarly
suitable
diluent, such as water from a municipal water system, prior to discharging it
into growing
ponds. Alternatively, the incubator may be configured to heat a nutrient
germinant
composition and spores, or a nutrient spore composition, for an incubation
period and
temperature range that will produce bacteria in a metastable state, between
dormant
spores and vegetative bacteria. The metastable bacteria solution is then
discharged
into the growing pond where the bacteria are able to become active, vegetative
bacteria. Dilution may aid in delivery of the treatment solution flowing from
the
incubator to the growing pond, if the incubator is located some distance from
the
growing pond. The active bacteria will degrade the organic waste and inhibit
the growth
of the pathogenic microorganisms in the water in the aquaculture facilities,
without
requiring the addition of (or reducing the amount of) chemical treatments and
antibiotics
used in the growing pond.
[0021] The invention also desirably includes contemporaneous application of at
least one nitrification enhancement agent to the growing ponds.
Nitrification
enhancement agents increase the activity of nitrifying bacteria naturally
found in the
water to decrease the ammonia level. These nitrification agents comprise
alkalinity
enhancement agents that increasing the alkalinity of the water, which is
necessary for
nitrification (7 parts alkalinity to 1 part ammonia) and/or surface area
modifying agents
9
CA 03088139 2020-07-08
WO 2019/168627
PCT/1JS2019/016020
to add surfaces for nitrifying bacteria to grow, since nitrifying bacteria
grow as biofilms
and need surfaces to which they can attach. The alkalinity enhancement agents
can
include, for example, calcium carbonate, calcified seaweed or other similarly
effective
additives. These agents can be added at a higher-than-dissolution amount to
provide a
continuing source of alkalinity as they slowly dissolve. Certain nitrification
agents, such
as calcified seaweed act as both an alkalinity enhancement agent and a surface
area
modifying agent by providing both alkalinity and high surface area, providing
a support
surface for biofilms of nitrifiers to grow. Calcified seaweed also acts as a
source of
micronutrients for the bacteria. Other nitrification enhancement agents only
act as
surface area modifying agents, such as plastic or metal pieces, or other
similarly
effective materials to increase the surface area over which favorable
reactions and
interactions can occur. One or more agents that act only as surface area
modifiers (and
not alkalinity enhancers) may also be added to the growing pond, either alone
or
preferably in combination with one or more alkalinity enhancement agents;
however, an
agent that acts only as a surface area enhancer would not degrade in the
growing pond
and would not be added with each batch of bacteria solution. Such agents that
act only
as surface area modifiers would preferably only be added to a growing pond
once.
Agents that act as alkalinity enhancement agents would be added to the growing
pond
contemporaneously with a batch of bacteria on a periodic basis, such as
seasonally
(once per season or once every summer, twice per year, etc.) or as needed. As
used
herein, the term "contemporaneous" is intended to mean "at or about" the time
that a
batch of vegetative bacteria and other components or agents are added to the
growing
pond or other growing medium in which aquatic species are grown at an
aquaculture
facility.
[0021a] In one particular embodiment, there is provided a method of adding
bacteria to water used in an aquaculture application, the method comprising:
providing
a volume of nutrient germinant composition and a volume of a bacteria
comprising at
least one species in spores form, which may be premixed together as a nutrient
spore
composition or separate; optionally mixing a portion of the nutrient germinant
composition and a portion of the bacteria spores if separate to form the
nutrient spore
composition; heating a portion of the nutrient spore composition to a
temperature in a
first range of 35 C to 60 C at or near a site of the aquaculture application;
maintaining
the temperature in the first range for an incubation period of 2 minutes to 6
hours to
form a batch of incubated bacteria solution comprising metastable state
bacteria,
vegetative state bacteria, or a combination thereof; periodically repeating
the heating
and maintaining steps to form additional batches of incubated bacteria
solution over the
course of a treatment cycle; dispersing each batch of incubated bacteria
solution into
the water used in the aquaculture application; providing a nitrification
enhancement
agent that increases alkalinity of the water or provides a surface for
nitrifying bacteria to
grow or both; and dispersing the nitrification enhancement agent in the water
contemporaneously with at least one of the batches of incubated bacteria
solution;
wherein each batch of incubated bacteria solution formed after the incubation
period
comprises bacteria that have at least begun germination; and wherein the
bacteria are
useful for remediating the water by degrading organic waste and inhibiting the
growth of
pathogenic bacteria or the bacteria are probiotic for a species in the
aquaculture
application; and wherein the nutrient germinant composition comprises: an L-
amino
acid; one or more buffers comprising a phosphate buffer, HEPES, Tris base, or
a
combination thereof; and an industrial preservative.
10a
Date Regue/Date Received 2022-06-15
CA 03088139 2020-07-08
WO 2019/168627
PCT/1JS2019/016020
BRIEF DESCRIPTION OF THE DRAWINGS
[0022] The system and method of the invention are further described and
explained in relation to the following drawings
FIG. 1 is a flow diagram for an incubation system and method according to a
preferred embodiment of the invention;
FIG. 2 is a flow diagram for an incubation system and method according to
another preferred embodiment of the invention;
FIG. 3 is a flow diagram for an incubation system and method according to
another preferred embodiment of the invention;
FIG. 4 is a graph of nitrate levels in a laboratory study;
FIG. 5 is a graph of ortho-phosphate levels in a laboratory study;
FIG. 6 is a graph of turbidity in a laboratory study
FIG. 7 shows photographs of bacteria slides using a spore composition and
method according to a preferred embodiment of the invention compared to
control
slides;
FIG. 8 is a graph showing p02 test data to demonstrate germination levels
using
a spore composition and method according to a preferred embodiment of the
invention
compared to control tests;
FIG. 9 is a graph showing p02 test data to demonstrate germination levels
using
a spore composition and varied methods according to preferred embodiments of
the
invention compared to control tests; and
FIG. 10 shows an image of three aquariums, each under control (left), calcium
carbonate only treatment (middle), or treatment with an activated nutrient-
spore
formulation (right).
11
CA 03088139 2020-07-08
WO 2019/168627
PCT/1JS2019/016020
DESCRIPTION OF THE PREFERRED EMBODIMENTS
[0023] Aquaculture Treatment Methods
[0024] According to one preferred embodiment, active bacteria are generated
on site from a nutrient germinant composition combined with a spore
composition or
from a premixed nutrient spore composition, preferably using an incubator
system and a
preferred germination method as described below, and the active bacteria are
periodically fed into a growing pond in an aquaculture application. One or
more
nitrification enhancement agents are also contemporaneously added to the
growing
pond with the active bacteria.
[0025] A satisfactory bacteria growing and delivery device for use in the
method
of the invention will include an on-site incubator system, such as an air
incubator, a
water incubator, or any other chamber or similar device that provides even,
constant
heat at a given temperature range as needed to germinate the spores for
discharge into
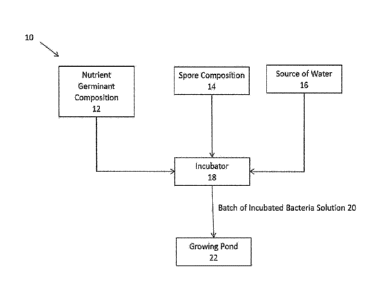
the aquaculture application. Referring to FIG. 1, preferably, the on-site
incubator
system 10 contains one or more tanks or holding containers for holding an
initial volume
of nutrient germinant composition 12 and an initial volume of bacteria spore
solution 14
(if the bacterial spores are not included in the nutrient germinant
composition). These
spore compositions may also arrive at the site of use in containers that are
connectable
in fluid communication with the incubator, in which case separate tanks or
containers
are not needed. A source of water 16 at or near the aquaculture site is also
optionally,
but preferably, connectable in fluid communication with the incubator system.
A well,
source of municipal water supply, or the growing pond may provide water 16 to
the
incubator 18. An incubator system 10 also preferably comprises a chamber or
container 18 configured to receive a portion of the nutrient germinant
composition and
spores and allow them to be heated to germinate the spores; a heater; valves,
tubing,
and pumps (as needed, if gravity flow is not sufficient) to allow the nutrient
germinant
composition 12, bacteria spore solution 14, and optionally water 16 to flow
from their
storage containers/source into a heating chamber or container 18 and to
discharge an
incubated bacteria (or activated bacteria) solution 20 from the heating
chamber or
container 18 and deliver it to the growing pond 22; an optional mixer within
the heating
12
CA 03088139 2020-07-08
WO 2019/168627
PCT/1JS2019/016020
chamber or container 18, and a controller or timer to activate the valves,
pumps,
optional mixer, and heater to control influx of the nutrient and spore
compositions into
the heating chamber, incubation time and temperature, and discharge to the
growing
pond. Most preferably, the on-site incubator system 10 uses a nutrient
germinate
composition combined with a bacteria spore composition (as described below) or
uses a
nutrient spore composition (a nutrient germinant composition pre-mixed with
bacteria
spores, also as described below) (either are also referred to herein as
"starter
material"), to generate an incubated bacteria solution 20 to be discharged
into the
growing pond 22.
[0026] Alternatively, according to another preferred embodiment as shown in
FIG. 2, incubator system 110 uses concentrated nutrient germinant composition
24 and
concentrated spore composition 30, which are diluted with a diluent or water
from
container/source 26 to form a working nutrient germ inant composition 28 and a
working
spore composition 32, a portion of each being fed into incubator 18 to
generate a batch
of activated bacteria 20. Water from source 16 may also be used as a source of
diluent
in place of or in addition to source 26. Additionally, only one of the
nutrient germinant
composition 24 or spore composition 30 may be in concentrated form and require
dilution prior to feeding into incubator 18. According to another preferred
embodiment,
when only one is concentrated, the non-concentrated composition may be used as
a
diluent for the concentrated composition in addition to or in place of
water/diluent from
source 26 and/or source 16. According to another preferred embodiment, a
concentrated nutrient spore composition 34 is used with system 210 as shown in
FIG. 3.
The concentrated nutrient spore composition is diluted with water/diluent from
source 26
and/or source 16 to make a working nutrient spore composition 36 prior to
feeding into
incubator 18 to generate a batch of activated bacteria 20. Alternatively, a
nutrient spore
composition may not be in concentrated form and not require any diluent prior
to
feeding into incubator 18 (similar to direct feeding of nutrient germ inant
composition 12
in FIG. 1), in which case water/diluent source 26 is not needed. Water from
source 16
may still be optionally fed into incubator 18 as needed in this alternate
embodiment.
With any of the incubator system embodiments, diluent may be fed into
incubator 18 to
13
CA 03088139 2020-07-08
WO 2019/168627
PCT/1JS2019/016020
dilute a concentrated composition within the incubator rather than prior to
feeding the
incubator. Any combination of elements from systems 10, 110, and 210 may be
used
together, as will be understood by those of ordinary skill in the art.
[0027] Preferably, bacteria spores are germinated in incubator 18 or other
suitable heating device according to preferred germination methods described
herein.
According to one preferred embodiment, a nutrient germinant composition and
spore
composition (or nutrient spore composition) are heated in incubator 18 to a
temperature
in a range of 35-55 C, more preferably in the range of 38-50 C, and most
preferably in
the range of 41 C to 44 C. The incubation period can vary depending on the end-
use
application, but is preferably between around 20 minutes to 60 minutes to
generate
active bacteria for an aquaculture application and most preferably around 2
minutes to 5
minutes for a probiotic application to generate metastable state bacteria. To
provide
additional growth time for vegetative bacteria, the incubation period may be
around 4 to
6 hours.
[0028] Depending on the desired use of the bacteria in the aquaculture
application, such as use to treat the water or a probiotic for the aquatic
species, different
incubation periods may be used to provide an incubated bacteria solution that
is
primarily still spore form bacteria, primarily metastable state bacteria (in
which the
spores are neither dormant nor in the vegetative growth phase, also referred
to herein
as an activated state), or primarily fully vegetative bacteria. Additionally,
when fully
vegetative bacteria are desired, the bacteria solution may be held within the
incubator
18 or another intermediate container for a period of time after the incubation
period to
allow the bacteria multiply prior to discharging into the aquaculture
application. Most
preferably, the bacteria solution will be maintained at a temperature between
30 to
45 C, more preferably, the vegetative bacteria solution will be heated as
necessary to
maintain the temperature of the solution in the range of 33 to 42 C, and most
preferably
in the range of 36 C to 39 C to facilitate growth during this post incubation
growth
period. When a probiotic application is desired, the bacteria remain primarily
in the
spore state or metastable state when discharged to the aquaculture application
by using
a shorter incubation period, which gives the bacteria a better chance of
surviving
14
CA 03088139 2020-07-08
WO 2019/168627
PCT/1JS2019/016020
through to the aquatic species' intestinal tract where they are most
beneficial as
probiotics. At the end of an incubation period, an incubated bacteria solution
20 is
discharged to the growing pond. An incubated bacteria solution 20 may comprise
fully
vegetative bacteria, metastable state bacteria, spores, or a combination
thereof
depending on the species of bacteria used, incubation temperature, incubation
time,
and content of the nutrients used.
[0029] Each batch of incubated bacteria solution 20 comprises around 1x108 ¨
1 x 1010 cfu/mL of metastable state, vegetative bacteria species, and/or
spores. Once
discharged into growing pond 22, the amount of bacteria in each batch is
diluted based
on the amount of water in the growing pond. Most preferably, sufficient
quantities of
bacteria solution 20 are added to the growing pond 22 to provide an effective
amount of
activated bacteria based on the dilution in the growing pond. In this context,
"effective
amount" can refer to the amount of bacteria and/or nutrient composition that
can be
effective to improve performance of a plant or animal after administration. An
improvement in performance can be measured or evaluated by monitoring one or
more
characteristics, including but not limited to water quality: clarity of water,
ammonia
levels, nitrite levels, nitrate levels, disease incidence, mortality, harvest
weight, meat
quality, individual animal size, premium weights, antibiotic use, and additive
use.
"Effective amount" can also refer to the amount that can reduce the amount of,
competitively exclude, and/or eliminate one or more species of pathogenic
bacteria
(including, but not limited to Escherichia coli and Salmonella) in the
intestines of an
animal. "Effective amount" can also refer to the amount that can reduce NH3
and/or H2S
levels, such as that which can be excreted by an animal into its environment.
[0030] According to one preferred embodiment for use in shrimp aquaculture
applications, the effective amount of the bacteria in the growing pond can be
about 1 to
about 9 x 102 CFU/mL. According to another preferred embodiment, the effective
amount for shrimp aquaculture applications is about 1 to about 9 x 102 to
about 108
CFU/mL. According to another preferred embodiment, the effective amount of the
incubated bacteria in the growing pond can range from about 0.001 % to about
2% Nth/
of the total amount of water in the growing pond and any range or value
therein. As
CA 03088139 2020-07-08
WO 2019/168627
PCT/1JS2019/016020
another example, around 500 mL of incubated bacteria solution comprising
around
1x109 ¨ 1 x 1019 cfu/mL of bacteria species dosed to a growing pond four times
per day
will be sufficient treat a growing pond containing 100,000 gallons of water.
Other
volumes of bacteria solution and dosing intervals may be used to treat growing
ponds,
depending on the size of the pond, based on pond conditions, aquatic species,
temperature of the pond, and other factors to achieve a desired effective
amount of
bacteria in the pond as will be understood by those of ordinary skill in the
art.
[0031] Multiple incubator systems 10, 110, or 210 may be provided to provide
larger quantities of incubated bacteria solution to the growing pond to
achieve the
desired effective amount being added to the pond, to provide different species
of
bacteria to the growing pond or at different times or rates, and/or to space
out the
discharge of incubated bacteria solution around the perimeter of the growing
pond to aid
in dispersing the bacteria through the pond. A pump or other mixing device may
also be
added to the growing pond (if not already in place) to aid in dispersing the
incubated
bacteria solution (and nitrification enhancers or surface area enhancers)
throughout the
growing pond.
[0032] The on-site incubator is preferably configured to incubate multiple
batches of incubated bacteria solution from a container of a nutrient
germinant
composition/spore composition or nutrient spore composition, so that multiple
batches
of bacteria can be discharged at periodic intervals over a prolonged period of
time
before the starter material needs to be replenished. For example, a container
of
nutrient germinant composition 12 may initially hold 0.3 to 3 liters of
nutrient germinant
composition that may be fed to an incubator in incremental amounts of around
10 to 100
mL every 1 to 24 hours. A container of concentrated nutrient germ inant
solution 24 may
initially hold 0.2 to 1 liters of solution, be diluted with water/diluent from
source 26 or 16
to a ratio of around 1:50 to around 1:10 concentrate to water/diluent to feed
an
incubator in incremental amounts of around 0.1 to200 mL of working nutrient
germinant
solution 28 every 1 to 24 hours. A container of bacteria spore solution 14 to
be fed
separately with a nutrient germinant composition may initially hold 0.6 to 6
liters of
solution that may be fed to an incubator in incremental amounts of around 20
to 200 mL
16
CA 03088139 2020-07-08
WO 2019/168627
PCT/1JS2019/016020
every 1 to 24 hours. A container of concentrated bacteria spore solution 30
may initially
hold 0.15 to 6 liters of solution, be diluted with water/diluent from source
26 or 16 to a
ratio of around 1:10 to around 1:3 concentrate to water/diluent to feed an
incubator in
incremental amounts of around 5 to 200 mL of working spore solution 32 every 1
to 24
hours. A container of nutrient spore composition may initially hold 3 to 6
liters of
nutrient germ inant composition that may be fed to an incubator in incremental
amounts
of around 100 to 200 mL every 1 to 24 hours. A container of concentrated
nutrient
spore solution 34 may initially hold 0.3 to 3 liters of solution, be diluted
with water/diluent
from source 26 or 16 to a ratio of around 1:10 to around 1:50 concentrate to
water/diluent to feed an incubator in incremental amounts of around 10 to 100
mL of
working nutrient spore solution 36 every 1 to 24 hours. Each batch of nutrient
germinant composition/bacteria spores or nutrient spore composition is then
incubated
in the incubator, as discussed herein, to form an incubated bacteria solution
that is
discharged to an aquaculture application.
[0033] An incubated bacteria solution 20 is preferably discharged from one or
more incubators 18 to the growing pond 22 once every 4 to 6 hours over the
course of a
treatment cycle. Other dosing intervals may be used depending on the size of
the pond,
conditions of the pond/aquatic species, and type of application. The time
between
doses may be varied as desired by varying the timing of addition of
ingredients to the
incubator and/or incubation time. An incubated bacterial solution may be
discharged
more frequently on a larger pond (e.g. 20 million gallons). For an aquaculture
water
treatment application, it is preferred to discharge an incubated solution
having
vegetative bacteria. To achieve vegetative bacteria, it is preferred to
incubate for at
least 4 to 6 hours before discharging to the growing pond, although longer
incubation
times to allow more time for the bacteria to multiply may also be used. For a
probiotic
application for aquatic species in an aquaculture application, it is preferred
to incubate
for around 2 to 5 minutes. In that application, an incubated bacteria solution
20 may be
discharged multiple times a day, even as frequently as every 4 to 6 minutes,
if needed
for a large pond. The volume of nutrient germinant composition/spore
composition or
nutrient spore composition feeding the incubator is periodically replaced or
replenished
17
CA 03088139 2020-07-08
WO 2019/168627
PCT/1JS2019/016020
as needed. A treatment cycle is preferably continuous with the incubator
running
throughout the year (other than periodic shut-downs for maintenance or
replenishment
of the nutrient germ inant composition).
[0034] Various Bacillus species, as described below, are preferably used with
aquaculture treatment methods according to the invention, but other bacteria
may also
be used. For example, the genera of bacteria suitable for use in the method of
the
invention are believed to include any one or more species in the genera
Bacillus,
Bacteriodes, Bifidobacterium, Lueconostoc, Pediococcus, Enterococcus,
Lactobacillus,
Megasphaera, Pseudomonas and Propionibactetium. Probiotic bacteria that may be
generated on-site include any one or more of the following: Bacillus
amylophilus,
Bacillus licheniformis, Bacillus pumilus, Bacillus subtilis, Bacillus
amyloliquefaciens,
Bacillus coagulans, Bacillus megaterium, Bacteriodes ruminocola, Bacteriodes
ruminocola, Bacterioides suis, Bifidobacterium adolescentis, Bifidobacterium
animalis,
Bifidobacterium bifidum, Bifidobacterium infantis, Bifidobacterium Ion gum,
Bifidobacterium thermophilum, Enterococcus cremoris, Enterococcus
diacetylactis,
Enterococcus faecium, Enterococcus intermedius, Enterococcus lactis,
Enterococcus
thermophiles, Lactobacillus brevis, Lactobacillus buchneri, Lactobacillus
bulgaticus,
Lactobacillus casei, Lactobacillus cellobiosus, Lactobacillus curvatus,
Lactobacillus
delbruekii, Lactobacillus farciminis, Lactobacillus fermentum, Lactobacillus
helveticus,
Lactobacillus lactis, Lactobacillus plantarum, Lactobacillus reuteri,
Leuconostoc
mesenteroides, Megasphaera elsdennii, Pediococcus acidilacticii, Pediococcus
cerevisiae, Pediococcus pentosaceus, Propionibacterium acidipropionici,
Propionibacterium freudenreichii, and Propionibacterium shermanii.
[0035] With at least one dose (or batch) of incubated bacteria solution
discharged to the growing pond, one or more nitrification enhancement agents
are
preferably added contemporaneously. Alkalinity enhancing agents, including
calcium
carbonate or calcified seaweed, may be added periodically, such as seasonally
or as
needed to reduce phosphates, and not with each dose of bacteria. The agents
can be
added at a higher-than-dissolution amount to provide a continuing source of
alkalinity as
they slowly dissolve. Slowly dissolving alkalinity enhancing agents, such as
calcified
18
seaweed, also act as a surface area modifier, providing a support surface for
biofilms of
nitrifying bacteria to grow and they also aid in nutrient delivery.
Additionally, agents that
act only as surface area modifiers (such as pieces of metal or plastic) may be
added to
the growing pond as needed to reduce nitrogen or phosphorous, along with a
batch or
dose of incubated bacteria solution and one or more alkalinity enhancing
agents, but
there are preferably added only once and not with each dose of incubated
bacteria
solution. These surface enhancement agents similarly provide a support surface
for
biofilms of the added bacteria to grow, which aids in faster development of
the
beneficial bacteria.
Most preferably, around 100 pounds of such nitrification
enhancement agents are added per 7.5 million gallons of growing pond, and this
amount may be scaled for other growing pond volumes. Preferred dispersal
methods for
the nitrification enhancement agents can include the use of automated devices
or
manual application to the water in the growing ponds. Automated or manually
operated
devices useful for broadcasting or otherwise dispersing at least one
nitrification
enhancement agent in the form of prills, pellets or granules are commercially
available
and are well known to those of skill in the art. Additionally, these
nitrification enhancing
agents may be dispersed through a pond using the self-dispersing additive
system and
method, which employs effervescent materials along with the treatment agent in
water
soluble packaging, described in U.S. Patent Application Serial No. 14/689,790
filed on
April 17, 2015.
[0036] Suitable applications for the method of the invention include, for
example
and without limitation various types of aquaculture application such as
hatcheries,
ponds, and tidal flow aquaculture. The combined use of germinated or
vegetative
bacteria, preferably grown on-site, and at least one nitrification enhancement
aid such
as calcium carbonate, calcified seaweed or another material that is similarly
effective
for cost-effective treatment of water used in aquaculture applications to
address
organic waste, ammonia, and pathogenic microorganism as well as general water
quality issues. The effectiveness of the subject method for achieving these
objectives is believed to be further enhanced by the addition of calcified
seaweed, or
19
Date Regue/Date Received 2020-11-06
CA 03088139 2020-07-08
WO 2019/168627
PCT/1JS2019/016020
other plastic or metal pieces, particles or fragments that increase the
available surface
area upon which interactions or reactions can occur.
[0037] A laboratory study was conducted to evaluate the benefits of adding
beneficial bacteria and nitrification enhancement agents to pond water. One
goal of the
study was to evaluate the efficacy of added bacteria (pond blend commercially
available
from EcoBionics) as inhibitory or herbicidal against algae production. This
study
employed the use of six 2 L beakers, each filled with 1.5 L of source water
taken from
an established fish tank with algae present. Each beaker also contained one
gold fish
from a source tank, air stone, light source that alternated 12 hours on, then
12 hours off,
and watch glass cover to reduce loss to evaporation. A pond blend bacteria
solution
was generated in a BIO-AmpTM biogenerator using 37 g of Pond Plus pellets,
rather
than using an incubator and nutrient germinant composition according to
preferred
embodiments of the invention described below. After a 24 hour growth cycle in
the
biogenerator, an aliquot of bacteria solution was obtained and diluted to
maintain a ratio
of 3 L of pond blend: 579024 gallons pond water, however, a preferred ratio to
be
employed in the field is 3L of pond blend:100,000 gallons of pond water. Based
on this
ratio, around 0.4 pL of pond blend bacteria solution was added to specific of
the
beakers having 1.5 L of fish tank water. Calcified seaweed, was added to
specific
beakers according to manufacturer's instructions based on rates for
clarification; this
equated to 0.045g of calcified seaweed per 1.5 L of water. An equal amount of
calcium
carbonate was added to some of the beakers. The additives in each beaker were
as
follows:
[0038] TABLE 1
Beaker 1 0.4 pL of pond blend and 1.5 L of source water only
Beaker 2 0.045 g of calcified seaweed and 1.5 L of source water only
Beaker 3 0.4 pL of pond blend, 0.045 g of calcified seaweed, and 1.5 L
of
source water
Beaker 4 0.045 g of calcium carbonate and 1.5 L of source water only
Beaker 5 0.4 pL of pond blend, 0.045 g of calcium carbonate, and 1.5 L
of
source water
CA 03088139 2020-07-08
WO 2019/168627
PCT/1JS2019/016020
Beaker 6 served as the negative control and contained only 1.5 L of
source
water
[0039] Each test beaker was treated according to one preferred dosing
schedule that would be utilized in the field. Beakers 1, 3 and 5 with pond
blend would
be treated (dosed) once per week with an additional 0.4 pL of pond blend.
Depending
on growing pond conditions, other dosing schedules may be used in the field.
Calcified
seaweed and calcium carbonate were added only once at the beginning of this
study,
however, additional dosing may be used in the field.
[0040] One pre-treatment sample (before the addition of pond blend bacteria
solution, calcified seaweed or calcium carbonate) was taken from each beaker
and
analyzed to obtain a baseline for comparison to the post treatment results.
Chemical
analysis was performed once per week using 200 ¨ 300 mL samples from each
beaker.
These weekly measurements included analysis of pH, conductivity, nitrate,
ortho
phosphate, total alkalinity and ammonia levels. Once per month turbidity was
examined
and photographs were taken to assess changes in algal growth and overall
clarity.
Treatment and analysis of the beakers was continued for a total of three
months; again
to mirror the length of the field study.
[0041] Data analysis was performed using Excel 2003, using a two sampled
two-tailed t-test comparing pre-treatment vs. post-treatment numbers at 95%
confidence
level. The two sample two-tailed t-test tested the null hypothesis of no
difference in the
means of pre and post-treatment with an alternative hypothesis of there is a
difference
in the means.
Ho = p pre-treatment = p post-treatment
HA = p pre-treatment # p post-treatment
[0042] Baseline readings indicated elevated phosphate levels in all beakers,
over 40 times the level indicative of accelerated algal growth. All other
measurements
were within acceptable ranges.
[0043] TABLE 2 ¨ Results from Two Sample T-Test for Nitrate and Phosphate
Beaker Nitrate p- % Reduction Phosphate p- % Reduction
21
CA 03088139 2020-07-08
WO 2019/168627
PCT/1JS2019/016020
value Nitrate value phosphate
1 (pond blend 0.88 20 0.47 38
only)
2 (Calcified 0.01* 79 0.02* 73
Seaweed
only)
3 (pond blend 0.04* 76 0.07 66
calcified
seaweed)
4 (Calcium 0.03* 77 0.05 66
carbonate
only)
(pond lend + 0.24 52 0.02* 72
calcium
carbonate)
6 (control) 0.90 25 increase 0.68 19
* Indicates a significant result
[0044] The test beaker containing only the bacterial blend pellets showed no
statistically significant change over the three month study period. Phosphate
levels
dropped after two weeks but within one month returned to pre-treatment levels.
Nitrate
levels mirrored those of phosphate. Of all the test beakers, only beaker 1 had
nitrate
and phosphate levels rise similar to the negative control. This indicates a
minimal effect
on major chemical indicators of pond health when using bacteria alone.
[0045] The two beakers containing calcified seaweed showed statistically
significant changes in pre vs. post-treatment means. There was a significant
drop in
nitrate levels of 79% and 76% with p-values 0.01 and 0.04 in beaker 2 and 3
respectively. Phosphate levels also dropped significantly in beaker 2 by 74%
with a p-
value of 0.02. The beaker containing calcified seaweed and pellets showed a
phosphate reduction of 66%, however, this value was not significant (see Table
2). It is
important to note that this lack of statistical significance may be subject to
this study's
22
CA 03088139 2020-07-08
WO 2019/168627
PCT/1JS2019/016020
low sample size limitation. This study did not test for changes in pathogenic
bacteria in
the samples, but the addition of the pond blend bacteria solution would be
expected to
reduce those numbers through competition. Additionally, it is believed that
the addition
of a bacteria solution from an incubator using a nutrient germinant
composition into to
an actual growing pond according to a preferred embodiment of the invention
would
achieve better results than in the laboratory study because the bacteria in
the bacteria
solution can act synergistically with the nitrifying bacteria already present
in the growing
pond and the added bacteria in the bacteria solution can aid in consuming
waste in the
water to reduce ammonia levels. Similar to the calcified seaweed beakers, the
two
beakers that contained calcium carbonate showed a significant difference in
pre versus
post treatment means. In beaker 4, the nitrate levels dropped 77% with a p-
value of
0.03. While, beaker 5 which contained calcium carbonate and bacterial pellets
showed
a significant decrease in phosphate levels of 72%, p-value 0.02 (see Table 2).
Calcium
carbonate may be a suitable substitute for calcified seaweed in aquaculture
treatment.
Beakers with calcified seaweed or calcium carbonate out performed those
without.
Beaker 5, which contained bacterial blend pellets and calcium carbonate, had a
significantly lower post-treatment mean of phosphate levels and the least
effect on pH.
However, beaker 2 and 5 had statistically significant drops in phosphate
levels.
[0046] Turbidity examined throughout this study showed a continual decrease
in all the test beakers. When comparing pre-treatment pictures to post, there
is an
increase in the presence of algae in all beakers, however, by the second month
there
was evidence of algal death in two beakers. Beaker 4 containing calcium
carbonate
and Beaker 6 (control) both appeared yellow in color indicating a dying algal
system.
Algal death is a common problem experienced after an initial algal bloom, as
oxygen in
the water is depleted; despite the presence of the air stone. As the algae
died, there
was a marked increase in nitrate levels. This was evident by the increase in
nitrate
levels above pre-treatment in this month, increasing 14% and 38% in beaker 4
and 6
respectively. By the final month, nitrate levels in Beaker 4 recovered and
decreased
though the system still had a dark green, yellow color. Nitrate continued to
increase to
23
CA 03088139 2020-07-08
WO 2019/168627
PCT/1JS2019/016020
6x the contamination level in Beaker 6. FIGS. 4-6 are graphs showing the
results of this
laboratory study.
[0047] A field study focusing on improving general pond health and clarity
while
reducing sludge was also conducted on various ponds. Although this study was
not
aquaculture specific (as the ponds in the study were ornamental or
recreational and not
for raising and harvesting aquatic species) and used a biogenerator rather
than an
incubator and a nutrient germ inant composition according to a preferred
embodiment of
the invention, it provides some useful information on the addition of bacteria
and
nitrification enhancing agents. The study included five ponds in and around
Irving,
Texas; the ponds, identified as Ponds 1-5 ranged from 23,400 ft3 to 720,131
ft3. The
length of this study was seven months. One to two times per week, a surface
water
sample of 200 ¨ 300 mL was taken bank side from each pond. These samples were
analyzed for pH, alkalinity, nitrate, phosphate, ammonia, conductivity,
turbidity and E.
coil spp. concentrations. Phosphate, ammonia and turbidity analysis was
performed
using a Hach DR890 colorimeter. E. coil spp. determination was performed using
specialized media for coliform growth (3M Petrifilm 6404) incubated at 35 C
for 48
hours.
[0048] Once per month each pond was sampled for sludge depth, clarity and
dissolved oxygen (DO). These measurements were taken from a small boat at two
to
four locations, marked by GPS coordinates to obtain representative sampling.
Sludge
depth was measured in inches using a sludge judge; each GPS location was
sampled
three to four times with the average taken. Dissolved oxygen was measured in
ppm
from the bottom layer and again at 18" from the surface using a Hach LDO probe
with a
Hach HQ30d meter. Clarity was determined in %/feet using a Secchi Disk, which
gave
an empirical measurement. Additionally, once per month photographs were taken
at
each pond at two to four locations, again marked by GPS coordinates, to give a
patron
point-of-view of overall surface conditions.
[0049] A BioAMPTm 750 climate controlled biogenerator was installed at each
location for daily on-site dosing of a specialized pond blend of bacteria.
Bacillus spp.
spores were pelletized using a modified FREE-FLOWTM formula; pellets were fed
into
24
CA 03088139 2020-07-08
WO 2019/168627
PCT/1JS2019/016020
the growth vessel where they grew in optimal conditions for 24 hours and were
then
dispersed directly into the pond. Maintenance of the BioAMP 750 had to be
modified
from standard protocol as sodium hypochlorite (bleach) is considered toxic to
surface
water and not allowed by the City of Irving for use. To obtain a similar
whitening effect
as the standard bleach treatment, 155g of sodium bicarbonate (baking soda) was
used
to remove excess biofilm and clean the growth vessels. In addition to monthly
maintenance, the biogenerators were monitored for any malfunctions and ability
to
maintain programmed temperature despite an ambient temperatures exceeding 100
F.
[0050] As a companion to the bacterial treatment, calcified seaweed was also
applied to each pond. The amount of calcified seaweed given was dependent on
volume at a ratio of 100 lbs to 1,000,000 ft3 of water. The calcified seaweed
was dosed
using water soluble packages containing an effervescent couple and the
calcified
seaweed as described in U.S. Patent Application Serial No. 14/689,790.
[0051] Each study pond was given the same amount of bacteria daily (30
trillion
CFUs). A correlation matrix revealed that sludge depth was inversely related
to dose-
rate. Two of the smaller ponds had the greatest observed reduction in sludge
levels and
clarity, as well as, the highest daily dose of bacteria at 2 x107 CFU/L and 7
x 106 CFU/L
respectively. Clarity as observed, show positive effects on all ponds,
regardless of size.
Clarity was approximately 100% in the three smallest ponds of this study.
Conversely
the two largest ponds only achieved clarity of 20% by the end of this study.
[0052] A one-sided 2 sample t-test was used to evaluate if sludge levels
significantly decreased after treatment. A p-value of 0.006 found a
statistically
significant average decrease of 31%. Ho: p pre-treatment = p post-treatment,
HA: p pre-
treatment > p post-treatment. This average reduction observed equates to an
average 3
inch reduction of the sludge layer. Additionally, every pond in this study
experienced a
decrease in sludge level when compared to pre-treatment (see Table 3).
[0053] TABLE 3 - Changes in PO4, NO3 & Sludge by Pond
Pond %A PO4 %A NO3 /0A Sludge
1 -19 -71 -45
2 -77 -100 -18
CA 03088139 2020-07-08
WO 2019/168627
PCT/1JS2019/016020
3 -40 0 -16
4 -70 -100 -37
-48 0 -43
Average -52 -91 -31
[0054] The average observed change in E. coli spp. was a reduction of 59%. It
is important to note the full range included an increase of 145% to a decrease
of 100%.
Such a wide range coupled with a small sample size made it difficult to
determine the
effectiveness of the treatment on E. coil spp. concentration. Three out of the
five study
ponds experienced an increase in E. coil spp. concentrations; however, the
other two
ponds saw a dramatic decrease; however the increase is believed to be the
result of
rainwater runoff into the ponds.
[0055] The overall effect of this treatment on phosphate concentrations was
examined, comparing pre-treatment to post-treatment levels. The data was found
to be
non-parametric and a one-sided Mann-Whitney test was employed to determine if
phosphate concentrations significantly decreased after treatment. Ho: p pre-
treatment =
I-1 Post-treatment, HA: p pre-treatment > p post-treatment. A p-value of
0.0000 was
obtained indicating that the overall 52% decrease in phosphate levels after
treatment
was statistically significant. Detailed examinations of changes in phosphate
level by
pond also revealed meaningful decreases. Each treated pond saw a decrease in
phosphate concentrations ranging from 19% to 77% (see Table 3). The average of
52% reduction was similar to the 57% reduction observed in phase I of this
study. This
indicates that the increase in frequency of bacterial dosing may not be
associated with
the decrease in phosphate concentrations. Furthermore, a marked decrease in
phosphate levels was observed directly following an application of calcified
seaweed.
The first dose was administered in spring with the second given in summer
after
phosphate levels began to rise in June. The non-chemical, eco-friendly nature
of the
powdered product offers promising results for control of phosphate
concentrations.
[0056] Similarly, nitrate levels were examined using a one-sided Mann-Whitney
test to evaluate if nitrate concentrations significantly decreased after
treatment. Ho: p
26
CA 03088139 2020-07-08
WO 2019/168627
PCT/1JS2019/016020
pre-treatment = p post-treatment, HA: p pre-treatment > p post-treatment. With
a p-value
of 0.0000 it was determined that the 91% reduction in concentration was
statistically
significant (see Table 3). To that end, baseline nitrate concentrations were
below
recommended levels so no reductions were anticipated let alone a statistically
significant reduction of 91%. Furthermore, each study pond that had detectable
nitrate
was significantly reduced to below detection limits of 0.01 ppm by month 4 .
This was a
vast improvement over the reduction observed in Phase I (-69%) and indicates
that the
increased frequency of bacterial dosing had a direct effect on these
concentrations.
Overall this study demonstrated that enhanced treatment with bacteria and
calcified
seaweed increased pond health as measured by chemical proxies and a decrease
in
sludge level.
[0057] Nutrient Germ inant compositions
[0058] A nutrient germinant composition according to one preferred embodiment
of the invention comprises one or more L-amino acids, D-glucose (which
increases the
binding affinity of L-amino acids for their cognate receptors in the spore
coat and is
optional), D-Fructose (optional, depending on bacteria species), a biological
buffer to
provide the proper pH for spore germination (such as HEPES sodium salt, a
phosphate
buffer, or a Tris buffer), an optional source of potassium ions (such as KCl),
and an
industrial preservative. In another preferred embodiment, a nutrient germ
inant
composition further comprises both D-glucose and D-fructose. It is most
preferred to
include a source of potassium ions, such as KCI, when both D-glucose and D-
fructose
are used. The use of D-fructose, a combination of D-glucose and D-fructose,
and a
potassium ion source are dependent on the species of bacteria as will be
understood by
those of ordinary skill in the art. It is preferred to use a preservative that
is pH
compatible with the spore composition, which has a relatively neutral pH.
According to
another preferred embodiment, the nutrient spore composition also comprises
spores of
one or more Bacillus species and preferably one or more germination
inhibitors. A
nutrient germinant composition comprising spores is referred to herein as a
nutrient-
spore composition, formula, or solution. Alternatively, spores may be
separately added
to the nutrient-germinant composition according to the invention at the point-
of-use.
27
CA 03088139 2020-07-08
WO 2019/168627
PCT/1JS2019/016020
When separately added, the spores are preferably part of a spore composition
or spore
formulation described herein, but other commercially available spore products
may also
be used. According to another preferred embodiment, the nutrient germinant or
nutrient
spore composition is in a concentrated form, most preferably as a concentrated
liquid,
and is diluted at the point-of-use.
[0059] Preferred L-amino acids include one or more of L-alanine, L-asparagine,
L-valine, or L-cysteine. The L-amino acids can be provided in the form of any
suitable
source, such as their pure forms and/or a hydrolysate of soy protein. In a
further
embodiment of the concentrate nutrient germinant composition, L-amino acids
can be
provided as a hydrolysate of soy protein. When in a concentrated form, the
spore
composition preferably comprises a solution of one or more of the above
mentioned L-
amino acids in the weight range of about 8.9 to about 133.5 g/L, more
preferably about
13.2 to about 111.25 g/L, and most preferably about 17.8 to about 89 g/L each;
D-
glucose (optional) and/or D-fructose (optional) in the weight range of about
18 to about
54 g/L each, more preferably about 27 to about 45 g/L each, and most
preferably about
30 to about 40 g/L each; KCI (optional, as a source of potassium ions) in the
weight
range of about 7.4 to about 22.2 g/L, more preferably about 11.1 to about 18.5
g/L, and
most preferably about 14 to about 16 g/L; a biological buffer, such as
monosodium
phosphate in a weight range of about 10 to about 36 g/L, more preferably about
15 to
about 30 g/L, and most preferably about 20 to about 24 g/L and/or disodium
phosphate
in a weight range of about 30 to about 90 g/L, more preferably about 21.3 to
about 75
g/L, and most preferably about 28.4 to about 60 g/L. One or more biological
buffers aid
in maintaining the nutrient germinant composition at the proper pH for spore
germination, around pH 6-8. In addition to or in place of the
monosodium/disodium
phosphate buffer, the spore composition may comprise other phosphate
buffer(s), Tris
base in a weight range of about 15 to about 61 g/L, more preferably about 24
to about
43 g/L, and most preferably about 27 to about 33 g/L; or HEPES buffer in a
weight
range of about 32.5 to about 97.5g/L, more preferably about 48.75 to about
81.25 g/L,
and most preferably about 60 to about 70 g/L. Optionally, monopotassium
phosphate
may also be used as a source of potassium ions, preferably in a weight range
of about
28
CA 03088139 2020-07-08
WO 2019/168627
PCT/1JS2019/016020
13.6 to about 40.8 giL, more preferably about 20.4 to about 34 g/L, and most
preferably
about 26 to about 29 g/L. Optionally, dipotassium phosphate may also be used
as a
source of potassium ions, preferably in a weight range of about 8.7 to about
26.1 g/L,
more preferably about 13 to about 21.75 g/L, and most preferably about 16 to
about 19
g/L. According to another preferred embodiment, the amounts of KCI, monosodium
phosphate, and/or disodium phosphate can be adjusted such that the pH in the
nutrient
germ inant solution and/or nutrient-spore solution can be about 6, about 7, or
about 8.
[0060] In another preferred embodiment, the nutrient germinant composition
further comprises one or more industrial preservatives at a final (total)
weight range of
0.8-3.3 g/L, more preferably 1.2-2.7 g/L, most preferably 1.6-2.2. The
preservative(s)
can be beneficial for long-term storage. Suitable preservatives include, NaCI,
D-
alanine, potassium sorbate, and chemical preservatives. Chemical preservatives
can
be preservatives with active ingredients of methyl chloro isothiazolinone
(about 1.15%
to about 1.18% v/v) and methyl isothiazolinone (about 0.35-0.4% v/v);
preservatives
with the active ingredients of diazolidinyl urea (about 30%), methylparaben
(about 11%),
and propylparaben (about 3%); and preservatives with only the active
ingredient of
methylparaben; and other preservatives with the methyl paraben, propylparaben,
and
diazolidinyl urea). Non-limiting examples of chemical preservatives with
methyl chloro
isothiazolinone and methyl isothiazolinone as active ingredients are Linguard
ICP and
KATHON Tm CG (which has active ingredients comprising methyl chloro
isothiazolinone,
around 1.15-1.18% and methyl isothiazolinone, around 0.35-0.4%). A non-
limiting
example of a chemical preservative with diazolidinyl urea, polyparaben, and
methylparaben as active ingredients includes Germaben II. Where the active
ingredients of the chemical preservative are methyl chloro isothiazolinone and
methyl
isothiazolinone, the chemical preservative can be included in a concentrated
nutrient
solution at about 0.8 to about 3.3 g/L, more preferably from about 1.2 to
about 2.7 g/L,
and most preferably from about 1.6 to about 2.2 g/L. Where the active
ingredient(s) of
the chemical preservative is diazolidinyl urea, methylparaben, and/or
propylparaben, the
chemical preservative can be included in a concentrated nutrient solution at
about 0.3 to
about 1% (wt/wt). In some aspects, the amount of a chemical preservative
having
29
CA 03088139 2020-07-08
WO 2019/168627
PCT/1JS2019/016020
diazolidinyl urea, methylparaben, and propylparaben can be included in the
nutrient
formulation at about 10 g/L. In the case of methylparaben, the preservative
can be
included in a concentrated nutrient solution at about 0.27 to about 1.89 g/L,
more
preferably from about 0.81 to about 1.35 g/L, and most preferably from about
1.0 to
about 1.18 g/L. According to another preferred embodiment, where the nutrient
formulation can be used to generate a nutrient-spore formulation effective for
aquaculture applications involving shrimp, or other shellfish, the
preservative can
include an amount of methylparaben and potassium sorbate. According to another
preferred embodiment, a nutrient germinant solution can be used to generate a
nutrient-
spore formulation effect for plants and/or waste water, the nutrient-spore
formulation
can include an amount of Linguard ICP or KATHON TM CG.
[0061] According to yet another preferred embodiment, a nutrient germinant
composition may further optionally comprise an osmoprotectant compound.
Ectoine, a
natural osmoprotectant produced by some species of bacteria, may be included
in one
preferred embodiment. The amount of ectoine (optional) in a concentrated
nutrient
germinant composition can range from about 0.625 to about 4.375 g/L, more
preferably
from about 1.875-3.125 g/L, and most preferably in an amount around 2-3 g/L.
According to another preferred embodiment, a nutrient germinant composition
may
further comprise other standard ingredients including, but not limited to,
surfactants that
aid in the dispersal of active ingredients, additional preservatives ensure
the shelf-life of
the spore composition, buffers, diluents, and/or other ingredients that are
typically
included in a nutrient formulation and/or spore formulation.
[0062] The amounts of the above ingredients are important aspects of the
invention because higher concentrations would render some ingredients
insoluble and
lower concentrations would be ineffective at germinating spores.
[0063] According to another preferred embodiment, a nutrient-germinant
concentrate composition according to embodiments of the invention is in
concentrated
form and is diluted to a working solution in water, a spore composition, or
any other
appropriate diluent, or a combination thereof prior to germination at a point-
of-use as
described further below. According to various preferred embodiments, a working
CA 03088139 2020-07-08
WO 2019/168627
PCT/1JS2019/016020
nutrient germ inant solution may be made by diluting a concentrated nutrient
germ inant
composition according to a preferred embodiment herein with water or other
suitable
diluent in a ratio between 0.01% to 50% (v/v) concentrated nutrient germinant
composition to diluent, but other amounts may also be used. The concentrated
nutrient
germinant compositions according to the invention may diluted anywhere from 2
to 1 X
106 fold or any range or value therein to produce a working nutrient germinant
solution.
Most preferably, dilution is in a range from about 0.1 to about 10% of the
concentrate
and the balance water or other suitable diluent. The amounts of the above
described
ingredients present in a working nutrient solution (a diluted solution from a
concentrated
formula) may be calculated based on the dilution factor and the concentrated
amounts
described above.
[0064] The use of a concentrated nutrient germinant composition reduces
shipping, storage, and packaging costs and makes dosing of the spore
composition at
the point-of-use easier. Most preferably, the concentrated nutrient
germinant
composition is in a liquid form, which is easier and faster to mix with
diluent at the point-
of-use, but solid forms such as pellets or bricks or powder may also be used.
The
inclusion of a general, industrial preservative in the nutrient germ inant
composition aids
in long-term storage.
[0065] Most preferably, all ingredients in nutrient germ inant compositions
according to the invention or used with methods of the invention meet U.S.
federal
GRAS standards.
[0066] Nutrient Spore compositions
[0067] According to another preferred embodiment, the composition is a
nutrient-spore composition comprising ingredients described above for a
nutrient
germinant composition and spores pre-mixed together. According to one
preferred
embodiment, a nutrient spore composition preferably comprises 10% to 90% by
weight
of one or more Bacillus spores or a spore blend (comprising 40-60% spore
powder with
one or more Bacillus species and 60-40% salt). According to another preferred
embodiment, a nutrient spore composition comprises around 5% by weight of one
or
more Bacillus spores or a spore blend. The total concentration of spores in
the nutrient
31
CA 03088139 2020-07-08
WO 2019/168627
PCT/1JS2019/016020
spore composition can range from about 1 x 105 CFU/mL or spores/g to 1 x 1014
CFU/mL or spores/g or any specific concentration or range therein.
[0068] A nutrient-spore composition preferably also comprises one or more
germination inhibitors and/or preservatives.
Preferred germination inhibitors or
preservatives for a concentrated nutrient spore composition include NaCI at a
relatively
high concentration ranging from about 29 to about 117 g/L, more preferably
from about
43 to about 88 g/L, and most preferably from about 52 to about 71 g/L; and/or
D-alanine
in an amount ranging from about 8 to about 116 g/L, more preferably from about
26 to
about 89 g/L, and most preferably from about 40 to about 50 g/L; and/or
potassium
sorbate in an amount ranging from about 1.25 to about 8.75 g/L, more
preferably from
about 3.75 to about 6.25 g/L, and most preferably from about 4.5 to about 5.5
g. Other
chemical preservatives described above with preferred nutrient germinant
composition
may also be used with nutrient spore compositions according to the invention.
These
germination inhibitors or preservatives maintain the spores in an inactive
state and
prevent premature germination of the spores prior to their dilution and
activation at the
point-of-use. The use of germination inhibitors is particularly preferred when
the spore
composition according to this embodiment is used with the preferred method of
the
invention, where germination occurs at the point-of-use. When spores are
included, the
amounts of other ingredients for the nutrient germinant composition described
above
(such as L-amino acids, biological buffers, etc.) make up the balance of the
spore
composition reduced proportionally to correspond to the preferred ranges
described
above. Preferred nutrient spore compositions are also in concentrated form and
diluted
to a working solution at a point-of-use as described above with respect to
nutrient
germinant composition and further described below.
[0069] The preferred Bacillus spores for use in a nutrient spore composition
according to preferred embodiments of the invention include the following
species:
Bacillus licheniformis, Bacillus subtilis, Bacillus amyloliquiefaciens,
Bacillus polymyxa,
Bacillus thuringiensis, Bacillus megaterium, Bacillus coagulans, Bacillus
lentus, Bacillus
clausii, Bacillus circulans, Bacillus firmus, Bacillus lactis, Bacillus
laterosporus, Bacillus
laevolacticus, Bacillus polymyxa, Bacillus pumilus, Bacillus simplex, and
Bacillus
32
CA 03088139 2020-07-08
WO 2019/168627
PCT/1JS2019/016020
sphaericus. Other Bacillus spore species may also be used as will be
understood by
those of ordinary skill in the art. Preferably, a nutrient spore composition
comprises 1 to
20 or more species of Bacillus, more preferably between 3 to 12 Bacillus
species.
According to another preferred embodiment, a nutrient spore composition
comprises 3
strains of Bacillus bacteria, most preferably 2 strains of the Bacillus
bacteria can each
be a strain of the species Bacillus licheniformis and the third strain is a
species of
Bacillus subtilis. According to another preferred embodiment, the spores in a
spore
blend comprise about 80% Bacillus licheniformis (40% of each strain) and 20%
Bacillus
subtilis.
[0070] In another preferred embodiment, a nutrient-spore composition for use
as
a probiotic comprises one or more Bacillus strains that are probiotic in
nature in that
they aid in the breakdown of nutrients in the digestive tract of the consumer.
The
strains preferably produce one or more of the following enzymes: proteases to
hydrolyze proteins, amylases to hydrolyze starches and other carbohydrates,
lipases to
hydrolyze fats, glycosidases to assist in the hydrolysis of glycosidic bonds
in complex
sugars and to assist in degradation of cellulose, cellulases to degrade
cellulose to
glucose, esterase which is a lipase-like enzyme, and xylanases that degrade
xylan, a
polysaccharide found in plant cell walls. Bacillus strains that produce these
enzymes
are well known in the art.
[0071] According to another preferred embodiment, a nutrient spore composition
is in a concentrated form and is diluted with to a working solution in water
or any other
appropriate diluent, or a combination thereof, prior to germination at a point-
of-use as
described further below. According to various preferred embodiments, a working
nutrient spore solution may be made by diluting a concentrated nutrient spore
composition according to a preferred embodiment herein with water or other
suitable
diluent in a ratio between 0.01% to 50% (v/v) concentrated nutrient germinant
composition to diluent, but other amounts may also be used. The concentrated
nutrient
spore compositions according to the invention may diluted anywhere from 2 to 1
X 1013
fold or any range or value therein to produce a working nutrient germinant
solution.
Most preferably, dilution is in a range from about 0.1 to about 10% of the
concentrate
33
CA 03088139 2020-07-08
WO 2019/168627
PCT/1JS2019/016020
and the balance water or other suitable diluent. The amounts of the above
described
ingredients (such as L-amino acids and germination inhibitors) present in a
working
nutrient solution (a diluted solution from a concentrated formula) may be
calculated
based on the dilution factor and the concentrated amounts described above
[0072] Most preferably, all ingredients in nutrient spore compositions
according
to the invention or used with methods of the invention meet U.S. federal GRAS
standards.
[0073] Spore compositions
[0074] A probiotic spore composition according to one preferred embodiment of
the invention comprises one or more bacterial species, an optional surfactant,
a
thickener, and optionally one or more acidifiers, acids or salts or acids to
act as a
preservative. According to another preferred embodiment, a spore composition
further
comprises one or more prebiotics, to the extent the thickener is not also a
prebiotic, or
in addition to any thickener that is a prebiotic. According to another
preferred
embodiment, a spore composition further comprises one or more water activity
reducers. Most preferably, the spore compositions according to the invention
comprise
various species of suspended probiotic spores, as described in more detail
below. The
use of these species in spore form increases the stability of the probiotics
in the harsh
environmental conditions that may be found near aquaculture application sites.
The
total concentration of spores in the spore composition can range from about 1
x 105
CFU/mL or spores/g to 1 x 1014 CFU/mL or spores/g or any specific
concentration or
range therein.
[0075] A suitable thickener is included in the spore composition according to
preferred embodiments. The thickener is preferably one that does not separate
or
degrade at varying temperatures typically found in non-climate controlled
aquaculture
environments. The thickener aids in stabilizing the suspension so the
bacterial mixture
remains homogenous and dispersed through a volume of the spore composition and
does not settle out of the suspension. When used with an incubation system and
aquaculture treatment methods according to preferred embodiments of the
invention
described herein, this ensures that the concentration of probiotic materials
is evenly
34
CA 03088139 2020-07-08
WO 2019/168627
PCT/1JS2019/016020
distributed throughout the container so that the dosage of spores delivered to
an
incubator remains consistent or relatively consistent (depending on the
specific delivery
method and control mechanism used) throughout a treatment cycle.
[0076] The most preferred thickener is xanthan gum, which is a polysaccharide
composed of pentasaccharide repeat units of glucose, mannose, and glurcuronic
acid
and a known prebiotic. Unlike some other gums, xanthan gum is very stable
under a
wide range of temperatures and pH. Another preferred thickener is acacia gum,
which
is also a known prebiotic. Other preferred thickeners include locust bean gum,
guar gum
and gum arabic, which are also believed to be prebiotics. In addition to
prebiotic
benefits, these fibers do not bind to minerals and vitamins, and therefore, do
not restrict
or interfere with their absorption and may even improve absorption of certain
minerals,
such as calcium, by aquatic species. Other thickeners that are not considered
prebiotics may also be used.
[0077] Preferred embodiments may optionally include one or more prebiotics,
which are preferably used if the thickener used is not a prebiotic but may
also be used
in addition to a prebiotic thickener.
Prebiotics are classified as disaccharides,
oligosaccharides and polysaccharides, and can include lnulin, Oligofructose,
Fructo-
oligosaccharides (FOS), Galacto-oligosaccharide (GOS), trans-Glacto-
Oligosaccharides
(TOS) and Short-Chain Fructo-oligosaccharides (scF0S), soy Fructo-
oligosaccharide
(soyFOS), Gluco-oligosaccharides, Glyco-oligosaccharides, Lactitol, MaIto-
oligosaccharides, Xylo-oligosaccharides, Stachyose, Lactulose, Raffinose.
Mannan-
oligosaccharide (MOS) are prebiotics may not enrich probiotic bacterial
populations, but
will bind with and remove pathogens from the intestinal tract and are believed
to
stimulate the immune system.
[0078] Preferred embodiments also preferably include one or more acidifiers,
acids, or salts of acids to act as a preservative or to acidify the spore
composition.
Preferred preservatives are acetic acid, citric acid, fumaric acid, propionic
acid, sodium
propionate, calcium propionate, formic acid, sodium formate, benzoic acid,
sodium
benzoate, sorbic acid, potassium sorbate, and calcium sorbate.
Other known
preservatives, preferably generally regarded as safe (GRAS) food
preservatives, may
CA 03088139 2020-07-08
WO 2019/168627
PCT/1JS2019/016020
also be used. Preferably, the pH of the spore composition is between about 4.0
and
7Ø More preferably it is between about 4.0 and 5.5 and most preferably
around 4.5 to
prevent premature germination of the spores prior to use or addition to an
incubator as
described below. Reducing the pH of the spore composition may also have
antimicrobial activity with respect to yeast, molds, and pathogenic bacteria.
[0079] One or more water activity reducers, such as sodium chloride, potassium
chloride, or corn syrup (a 70% solution of corn syrup), are optionally
included in the
spore composition according either preferred embodiment. The water activity
reducer
aids in inhibiting microorganism growth, so that the bacterial spores do not
prematurely
germinate while the spore composition is being stored prior to the time it is
discharged
to the point of consumption by the animals or plants to be treated or point of
use in
discharging to a growing pond. They also aid in inhibiting growth of
contamination
microorganisms
[0080] The optional surfactant is preferably one that is safe for ingestion by
animals, although other surfactants may be used with other applications, such
as
delivery to plants. Most preferably, the surfactant is Polysorbate 80.
Although any
GRAS or AAFC0 approved surfactants or emulsifiers may be used with either
embodiment, there are concerns that some animals may not tolerate all approved
surfactants well. Because the benefits of the surfactant in stabilizing the
suspension so
the bacterial mixture remains homogenous and does not settle out may also be
achieved by the use of the thickener, it is not necessary to add the
surfactant. If a
surfactant is used in the spore composition according to this second
embodiment, it is
preferably used in about the same weight percentage range as in the first
embodiment.
[0081] Preferred bacteria for use with a spore composition according to the
invention are the same as those described above for a preferred nutrient spore
composition. Most preferably, the bacterial species used in a spore
composition are
one or more species from the Bacillus genus. The most preferred species for
the
probiotic bacteria include the following: Bacillus pumilus, Bacillus
licheniformis, Bacillus
amylophilus, Bacillus subtilis, Bacillus amyloliquefaciens, Bacillus
coagulans, Bacillus
clausii, Bacillus firmus, Bacillus megaterium, Bacillus mesentericus, Bacillus
subtilis var.
36
CA 03088139 2020-07-08
WO 2019/168627
PCT/1JS2019/016020
natto, or Bacillus toyonensis, but any Bacillus species approved as a
probiotic in the
country of use may also be used. It is preferred that the bacteria are in
spore form, as
the spore form is more stable to environmental fluctuations, such as ambient
temperature changes. Most preferably, the spores used in the spore
compositions
according to the invention are a dry powder blend that comprises around 40-60%
salt
(table salt) and 60-40% bacterial spores. The spores are preferably spray-
dried from a
liquid fermentation concentrate. Salt is used to dilute the pure spray-dried
spore
powder to a standard spore count in the final spore powder blend. During
production
fermentation, different Bacillus strains will grow at different rates,
resulting in varying
final count numbers for the fermentation batch liquor. The fermentation liquor
is
centrifuged to concentrate the spores in the liquor. Then, the concentrated
liquor is
spray-dried which results in a powder containing only Bacillus spores. The
addition of
salt to the spray-dried Bacillus spore powder aids in standardizing the spore
blend count
per gram from batch to batch. Other forms of bacterial spores or spore blends
may also
be used. Most preferably, the dry spore blend is pre-mixed with a portion of
the water
used in the spore composition, around 3-30% of the total water, and the
resulting
bacteria spore solution is added to the other ingredients, including the
remaining water.
This aids in dispersing the bacteria spores throughout the spore composition.
[0082] A probiotic spore composition according to a first preferred embodiment
of the invention preferably comprises bacterial spores that provide 108 cfu/ml
of the
spore suspension (most preferably around 1.0 X 108 to around 3.0 X 108 cfu/ml
of spore
composition, which, when diluted in growing pond water provides approximately
101 to
104 cfu/ml pond water), 0.00005 to 3.0 % surfactant, and 0.002 to 5.0%
thickener, and
optionally the about 0.01 to 2.0 % of one or more acids or salts of acids as a
preservative, all percentages by weight of the spore composition. A probiotic
spore
composition according to another preferred embodiment of the invention
comprises
bacterial spores that provide 109 cfu/ml of the spore suspension (which, when
diluted in
pond water provides approximately 101 to 104 cfu/ml pond water), about 0.1 to
5.0%
thickener (preferably one that also acts as a prebiotic), about 0.05-0.5% of
one or more
preservatives, optionally about 0.1-20% of one or more water activity
reducers, and
37
CA 03088139 2020-07-08
WO 2019/168627
PCT/1JS2019/016020
optionally 0.1-20% of one or more acidifiers, all percentages by weight of the
composition. The balance of the spore composition in both preferred
embodiments is
water and the percentages herein are by weight. It is preferred to use
deionized or
distilled water, to remove salts or outside bacteria, but tap water or other
sources of
water may also be used.
[0083] According to another preferred embodiment, a spore composition
comprises around 1% to 10% of a bacteria spore blend containing salt and one
or more
of Bacillus pumilus, Bacillus licheniformis, Bacillus amylophilus, Bacillus
subtilis,
Bacillus clausii, Bacillus coagulans, Bacillus firmus, Bacillus megaterium,
Bacillus
mesentericus, Bacillus subtilis var. natto, or Bacillus toyonensis in spore
form; around
0.3% to 1% total of one or more acids or salts of acids; around 0.2% to 0.5%
of a
thickener; around 0.1-0.3% sodium chloride, potassium chloride, or a
combination
thereof; around 0.00005% to 3.0% of a surfactant; and 86.2% to 98.4% water.
According to another preferred embodiment, a spore composition comprises
around
0.01% to 10% of the bacteria spore blend; around 0.1-0.33% sorbic acid, its
salt, or a
combination thereof; around 0.1-0.34% citric acid, its salt, or a combination
thereof;
around 0.1-0.33% benzoic acid, its salt, or a combination thereof; around 0.2-
0.5%
xanthan gum; around 0.00005% to 3.0% of a surfactant; and around 0.1-0.3%
sodium
chloride, potassium chloride, or a combination thereof, all percentages by
weight of the
composition. According to yet another preferred embodiment, a spore
composition
comprises around 5% bacteria spores or a spore blend; around 0.25% thickener;
around 0.3% total of one or more acids or salts of acids; around 0.1%
surfactant;
around 0.2% sodium chloride, potassium chloride, or a combination thereof (in
addition
to any salt in the spore blend); and water. According to another preferred
embodiment,
the acids or salts of acids are one or more of potassium sorbate, sodium
benzoate, and
citric acid anhydrous.
[0084] Several examples of probiotic spore compositions according preferred
embodiments of the invention were made and tested for different parameters.
These
spore compositions are set forth in Table 4 below.
[0085] TABLE 4
38
CA 03088139 2020-07-08
WO 2019/168627
PCT/1JS2019/016020
Ingredient/ 1 2 3 4 5 6 7 8
Formula No.
Potassium
0.33% 0.33% 0.1% 0.1% 0.1% 0.1% 0.1% 0.1%
Sorbate
Citric Acid
0.34% 0.34% 0.1% 0.1% 5.0% 0.1% 0.1% 0.1%
Sodium
0.33% 0.33% 0.1% 0.1% 0.1% 0.1% 0.1% 0.1%
Benzoate
Benzoic Acid 0.1% - 0.1%
0.1% --
Sorbic Acid 0.1% - 0.1% --
Sodium 10.0% 0.1% -
Propionate
Xanthan Gum 0.2%
0.2% 0.2% 0.3% 0.4% 0.4% 0.5% 0.5%
Sodium Chloride 0.2% 0.2% - 0.2% - 0.2%
0.1% 0.2%
Potassium 0.1%
0.1%
Chloride
Spore Blend 0.1%
0.1% 0.1% 0.1% 0.1% 0.1% 0.1% 0.1%
[0086] The balance of each spore composition is water (around 1 L in these
samples). Deionized water was used in each spore composition, except spore
composition No. 1, which used tap water. The percentages indicated are by
weight.
Each formula was targeted to have a pH between about 4.0 and 5.5, but some
formulas
were found to have actual pH values far less than expected. Formula No. 1 was
targeted to have a pH between 5.0 and 5.5, but its actual pH was around 2.1-
2.3, which
is too low and may be harmful to the spores, create stability issues with
packaging, and
be subject to more restrictive transportation regulations. Formula No. 1 also
exhibited
weak thickening. Formula No. 2 is the same as No. 1, except the source of
water is
different. Formula No. 2 had an actual pH of around 2.2-2.3 and also exhibited
weak
thickening. The amount of acids and salts of acids in Formula No. 3 was
decreased to
raise the pH and to determine if the thickness improved while using the same
amount of
thickener as in Nos. 1 and 2. While Formula No. 3 was an improvement over Nos.
1
and 2, it still exhibited weak thickening and its actual pH was 6.6, over the
target value
range. Additional acids were added to Formula No. 4 to lower the pH and
additional
thickener was added. Formula No. 4 had improved thickening, but further
improvements
in thickening would be beneficial. The amount of acid in Formula No. 5 was
substantially increased, which resulted in an actual pH of around 1Ø The
amount of
acid in Formula No. 6 was decreased and the thickener increased, which
resulted in a
39
CA 03088139 2020-07-08
WO 2019/168627
PCT/1JS2019/016020
spore composition that was too thick to drop. Formula No. 7 increased the
thickener
and amount of water activity reducers, but exhibited issues with mixing of
benzoic acid
and sorbic acid. The benzoic acid and sorbic acid were removed in Formula No.
8.
Formula Nos. 1-7 provided 2 X 1011 cfu/gm and No. 8 provided 1 X 1011 cfu/gm
bacteria
spores. Of these sample formulas, No. 8 is the most preferred as it exhibited
adequate
thickening and had an actual pH of around 4.5 +/- 0.2, and used less spore
blend.
[0087] It is preferred that the spore compositions according the embodiments
of
the invention use around 0.01% to around 0.3% bacteria spore blend and more
preferably between about 0.03% to 0.1% bacteria spore blend. A reduction in
the
amount of spore blend used substantially reduces the costs of the spore
composition.
Depending on the end use application, differing amounts of spore blend may be
used in
the spore compositions according to the invention. For example, smaller
percentages
of spore blend may be used in the spore compositions for use with chickens,
whereas
larger percentages would be used in spore composition for use with pigs.
[0088] A spore composition according to formula No. 8 was tested for shelf-
life
at various temperatures. Samples of Formula No. 8 were sealed in a plastic
bag, such
as one used in a preferred delivery system as described below, and stored for
two
months at temperatures around 4-8 C (39-46 F), 30 C (86 F), and 35 C (95
F) to
simulate typical temperature ranges in which the probiotic spore composition
may be
stored and used in agricultural settings. At the end of the first month of the
storage
period, each sample was observed and tested. All three samples had a pH of
around
4.5 and there was no settling, layering or change of appearance in any of the
three
samples, indicating that the bacteria spores remained suspended and dispersed
throughout the spore composition during the storage period. None of the
samples
contained any fungal contamination or gram-negative bacteria contamination. At
time
count zero (when the samples were initially stored), each sample contained
bacteria
spores of around 2.12 X 108 cfu/mL. At the end of the one month storage
period, the
samples contained bacteria spores of around 2.09 X 108 cfu/mL spore suspension
(lowest temperature sample), 1.99 X 108 cfu/mL (middle temperature sample),
and 2.15
108 cfu/mL (high temperature sample). The bacteria counts are somewhat
variable in
CA 03088139 2020-07-08
WO 2019/168627
PCT/1JS2019/016020
different samples, especially thickened samples; however, these are considered
to be
comparable counts. Each sample was tested again after two months in storage.
The
samples contained bacteria spores of around 2.08 X 108 cfu/ml (lowest
temperature
sample); 2.01 X 108 cfu/ml (middle temperature sample); and 2.0 X 108 cfu/ml
(high
temperature sample). The target shelf life is around 2 X 108 cfu/ml spore
suspension,
so the samples are within the targeted shelf life after two months of storage.
These test
results demonstrate that probiotic spore compositions according to a preferred
embodiment of the invention are stable over a range of temperatures, with the
bacteria
spores remaining viable, suspended, and dispersed throughout the spore
composition.
The spore blend (40-60% spore powder and 60-40% salt) used in each sample
formula
was the same, providing at least around 2 X 1011 spores/gram. The spore
species in the
blend were multiple Bacillus subtilis and Bacillus licheniformis strains. The
spore blend
powder was premixed with 100 mL of water with stirring for 30 minutes prior to
adding to
the other ingredients. Premixing with water aids in mixing the spore blend
with the other
ingredients and dispersing the spores throughout the spore composition.
[0089] Although it is preferred to use probiotic spore compositions comprising
one or more Bacillus species as according to the spore compositions of the
invention,
the methods of the invention may be used with spore compositions comprising
other
bacteria genera and other species. For example, one or more species from the
following genera: Bacillus, Bacteriodes, Bifidobacterium, Pediococcus,
Enterococcus,
Lactobacillus, and Propionibacterium (including Bacillus pumilus, Bacillus
licheniformis,
Bacillus amylophilus, Bacillus subtilis, Bacillus amyloliquefaciens, Bacillus
clausii,
Bacillus coagulans, Bacillus firmus, Bacillus megaterium, Bacillus
mesentericus,
Bacillus subtilis var. natto, or Bacillus toyonensis Bacteriodes ruminocola,
Bacteriodes
ruminocola, Bacterioides suis, Bifidobacterium adolescentis, Bifidobacterium
animalis,
Bifidobacterium bifidum, Bifidobacterium infantis, Bifidobacterium Ion gum,
Bifidobacterium the rmophilum, Pediococcus acidilacticii, Pediococcus
cerevisiae,
Pediococcus pentosaceus, Enterococcus cremoris, Enterococcus diacetylactis,
Enterococcus faecium, Enterococcus intermedius, Enterococcus lactis,
Enterococcus
thermophilus, Lactobacillus delbruekii, Lactobacillus fermentum, Lactobacillus
41
CA 03088139 2020-07-08
WO 2019/168627
PCT/1JS2019/016020
helveticus, Lactobacillus lactis, Lactobacillus plantarum, Lactobacillus
reuteri,
Lactobacillus brevis, Lactobacillus buchneri, Lactobacillus bulgaricus,
Lactobacillus
casei, Lactobacillus farciminis, Lactobacillus cellobiosus, Lactobacillus
curvatus,
Propionibacterium acidipropionici, Propionibacterium freudenreichii,
Propionibacterium
sherrnanii) and/or one or more of the following species: Leuconostoc
mesenteroides,
Megasphaera elsdennii may be used with compositions and method of the
invention.
[0090] Spore compositions may also be in a concentrated form, using less water
with a proportional increase in the amounts of other ingredients as described
above.
Such concentrated spore compositions may be diluted at the point-of-use with a
nutrient
germinant composition, water, other suitable diluent or a combination thereof
prior to
germination. Most preferably, all ingredients in spore compositions according
to the
invention or used with methods of the invention meet U.S. federal GRAS
standards.
[0091] Methods of Germination
[0092] According to one preferred embodiment, a method of germinating spores
at a point-of-use according to the invention comprises providing nutrients and
spores
(preferably providing a nutrient germinant composition and a spore composition
or
providing a nutrient spore composition according to the invention, but other
commercially available products containing spores and nutrients, together or
separately,
may be used) and heating them to an elevated temperature or range of
temperatures
and maintaining them at that temperature or within that range for a period of
time
(incubation period) to allow germination at a point-of-use location near a
point-of-
consumption. Heating during the incubation period takes place in a single step
with
both the nutrients and spores together. The method also preferably comprises
the step
of dispensing the germinated spores to an aquaculture application as
previously
discussed. Preferably, the nutrient germinant composition and spore
composition (or
nutrient spore composition) is heated to a temperature in a range of 35-55 C,
more
preferably in the range of 38-50 C, and most preferably in the range of 41 C
to 44 C.
The incubation period can vary depending on the end-use application. For a
probiotic
application, where the aquatic species with a digestive system (e.g. fish or
eels) will
ingest the bacteria, it is preferred that the incubation period lasts no
longer than 10
42
CA 03088139 2020-07-08
WO 2019/168627
PCT/1JS2019/016020
minutes. Most preferably, in a probiotic application, the incubation period is
between 2-
minutes. In this way, spores are released to the growing pond before the
spores have
fully germinated and stand a better chance of surviving through to aquatic
species'
intestinal tract where they are most beneficial. For treating the water in an
aquaculture
application, such as may be done with a shrimp aquaculture application, the
preferred
incubation time is at least one hour to allow the spores to fully germinate
before
discharging to the water, more preferably 4 to 6 hours, to allow the bacteria
to become
vegetative before discharging to the water. Most preferably, a nutrient
germinant
composition and a spore composition (or a nutrient spore composition),
preferably in
accordance with an embodiment of the invention discussed herein, are added to
an
incubator to incubate the spores at the above preferred temperature ranges and
durations to produce a bacteria solution having bacteria in a vegetative
state. The
incubation may be in an air incubator, a water incubator, or any other chamber
that
provides even, constant heat at the given temperature range. The bacteria
solution is
then discharged to an aquaculture application as previously discussed.
If a
concentrated nutrient germinant composition is used, diluent water is
preferably added
to the incubator with the nutrient germinant composition.
[0093] Various nutrient germ inant compositions according to preferred
embodiments of the invention were tested according to preferred methods of the
invention. The compositions, methods, and results are described below.
[0094] EXAMPLE 1 - To germinate spores, FreeFlow LF-88 Probiotic (spore
liquid formula commercially available from NCH Corporation) was added to 1mL
of tap
water at a final concentration of approx. 1 x 109 CFU/mL, where CFU stands for
colony
forming unit. A nutrient germinant concentrate composition according to a
preferred
embodiment of the invention comprising L-alanine (89 g/L), monosodium
phosphate (20
g/L), disodium phosphate (60 g/L), and Linguard CP (1.6 g/L total) was added
to the
water and bacteria mixture to provide a 4% final concentration of nutrient-
germinant
composition by total weight of the mixture. For comparison, negative control
reactions
were prepared with the same amount of FreeFlow LF-88 Probiotic and water, but
without adding the nutrient germinant concentrate composition.
Both mixtures
43
CA 03088139 2020-07-08
WO 2019/168627
PCT/1JS2019/016020
(germinant and negative control without the nutrient-germinant composition)
were
blended and incubated for 60 minutes in a pre-incubated heat block set to 42 C
or at
ambient room temperature (around 23 C).
[0095] Spores from each reaction were observed using phase contrast
microscopy. Slides were prepared using standard procedures. Spores were viewed
on
an Olympus BX41 microscope (100X oil emersion objective) and imaged using an
Olympus UC30 camera controlled by the cellSens Dimension software package.
[0096] Images were taken and germinated spores were counted as a
percentage of the total spores in the field. A total of 10 representative
images were
analyzed for each condition (test mixture). Germinated spores lose their
refractivity due
to the influx of water and are phase-dark while non-germinated spores are
phase-bright.
[0097] FIG. 7 shows representative images from these tests.
Image A
represents spores that had been germinated using a nutrient-germinant
composition
and heated during the incubation period at 42 C according to a preferred spore
composition and preferred method of the invention. The darker spots show
germinated
spores, the lighter spots show non-germinated spores. Image B represents
spores that
had been germinated using a nutrient-germinant composition according to a
preferred
embodiment of the invention, but were incubated at ambient temperature (23 C).
Images C-D represent control spores that had not been treated with a nutrient
germinant composition according to the invention, one having been incubated at
42 C
and one incubated at ambient temperature (23 C).
[0098] As can be seen in FIG. 7, the "A" image shows significantly more
germinated spores (dark spots) than the other images. Spores incubated with a
nutrient-germinant composition according to a preferred embodiment invention
in
combination with a germination method according to a preferred embodiment of
the
invention show an apparent germination efficiency of 96.8% (Example 1, Figure
7A).
Control spores that had been incubated with a nutrient-germinant composition
according to a preferred embodiment of the invention, but without using a
germination
method according to a preferred embodiment of the invention showed an apparent
germination efficiency of 2.3% (Example 1, Figure 7B). Similarly, spores that
had not
44
CA 03088139 2020-07-08
WO 2019/168627
PCT/1JS2019/016020
been incubated with a nutrient-germinant composition according to the
invention
showed an apparent activation efficiency of 1.2% and 2.6% at 42 C and 23 C,
respectively (Example 1, Figures 7C and 7D). Germinated spores in the samples
not
treated by preferred embodiments of the present method represent the small
percentage of spores already germinated in the FreeFlow LF-88 Probiotic
solution. This
example demonstrates that spore germination is significantly increased when a
nutrient-
germinant composition and incubation method according to preferred embodiments
of
the invention are used together.
[0099] EXAMPLE 2 ¨Another set of incubation tests were run using the same
test mixture/incubation method (using the same nutrient-germinant composition
and
heated incubation, "Treated Spores, 42 C") and control mixture/incubation
method (no
nutrient-germinant composition and no heat, "Non-treated Spores, 23 C") as
described
above in Example 1 were repeated, but different tests were run to compare the
efficacy
of the test mixture according to preferred embodiments of the invention as
compared to
the control mixture. Additionally, two other mixtures were tested ¨ one in
which the
nutrient-germinant composition of Example 1 was used but without heat
("Treated
Spores, 23 C") and one in which no nutrient-germinant was used but the spores
were
heated ("Non-Treated Spores, 42 C"). Briefly, spores were incubated at 42 C or
23 C
for 1 hour with or without treatment with a preferred nutrient-germinant
composition.
After incubation, the spores from 1mL of each reaction were pelleted at 14K
RPM for 3
min at 23 C and resuspended in 1 mL of Butterfield's buffer. Approx. 6 x 105
CFUs
(0.02mL) were added to 0.980 mL of Davis minimal media (containing 3% glucose
as a
carbon source and trace elements) with an excess of D-alanine. D-alanine is a
potent
inhibitor of L-amino acid-mediated germination.
[00100] Approx. 1.2 x 105 CFUs were added to each of four wells of a PreSens
OxoPlate. PreSens OxoPlates use optical oxygen sensors to fluorescently
measure the
oxygen content of the sample using two filter pairs (excitation: 540nm,
emission: 650nm
and excitation: 540, emission: 590nm). Controls were performed as described by
the
manufacturer and measurements were taken on a BioTek 800FLx fluorescence plate
reader. Time points were taken every 10 minutes for 24 hours at 37 C with
continual
CA 03088139 2020-07-08
WO 2019/168627
PCT/1JS2019/016020
shaking and data was processed to determine the partial pressure of oxygen
(p02)
using the following formula:
[00101] p02 = 1001(K0/1R)-1 (Ko/K100)-1]
[00102] Spores that have germinated and continue to divide and grow as
vegetative cells consume oxygen as part of their metabolic growth. Oxygen
consumption is represented by a drop in p02. Presumably, the growth that is
observed
is due to the outgrowth and vegetative growth of spores germinated by the
present
invention. The p02 levels for these tests are shown in FIG. 8.
[00103] As shown in FIG. 8, incubation with the test mixture and method
according to preferred embodiments of the invention (Treated spores 42 C,
using both
the nutrient-germinant composition and heating) resulted in spores that began
vegetative growth 4 hours faster than the control spore mixtures that had not
been
treated or heated according to preferred embodiments of the invention or had
been
either treated with a nutrient-germ inant composition or heated, but not both
together.
The growth seen in the control experiments presumably represents the approx.
2% of
germinated spores present in FreeFlow LF-88 Probiotic (see EXAMPLE 1). This
example further indicates that spore germination is significantly increased
when a
nutrient-germinant composition and incubation method according to preferred
embodiments of the invention are used.
[00104] EXAMPLE 3 ¨ Another set of incubation tests were run using a similar
test and control mixture and incubation method as described above in Example
1.
Briefly, LF-88 was added to 10mLs of distilled water at a final concentration
of approx.
108 CFU/mL. Samples were incubated at various temperatures to show the
efficacy of
the test method according to preferred embodiments of the invention as
compared to
the control mixture. Reactions were prepared with the nutrient-germ inant
composition
described in Example 1 ("Treated spores" in FIG. 9) and incubated at 23 C
(ambient
temperature, no heating), 32 C, 42 C, or 60 C. A control reaction was
incubated at
ambient room temperature with no nutrient-germinant composition. After one
hour of
incubation, 1mL of each reaction was pelleted at 14K RPM for 3 min at 23 C and
46
CA 03088139 2020-07-08
WO 2019/168627
PCT/1JS2019/016020
resuspended in Butterfield's buffer. Approx. 6 x 105 CFUs (0.02mL) were added
to
0.980 mL of Davis minimal media (containing 3% glucose as a carbon source and
trace
elements) with an excess of D-alanine.
[00105] Approx. 1.2 x 105 CFUs were added to each of four wells of a PreSens
OxoPlate. Controls were performed as described by the manufacturer and
measurements were taken on a BioTek 800FLx fluorescence plate reader using two
filter pairs (excitation: 540nm, emission: 650nm and excitation: 540,
emission: 590nm)..
Time points were taken every 10 minutes for 24 hours at 37 C with continual
shaking
and data was processed to determine the partial pressure of oxygen (p02). The
p02
levels for these tests are shown in FIG. 9.
[00106] As shown in FIG. 9, incubation using a nutrient-germinant composition
and heating according to preferred embodiments of the invention resulted in
spores that
began vegetative growth hours before the control. Spores treated with the
nutrient-
germinant composition but not heated are comparable to the control mixture.
Spores
treated with the nutrient-germinant composition that were incubated at a
temperature
below the preferred range of range of 35-55 C according to one embodiment of
the
invention (represented by the "Treated spores 32 C" curve) begin vegetative
growth
faster than control experiments, but not as fast as spores treated at elevated
temperatures within the preferred ranges according to the invention. Spores
treated
with a nutrient-germinant composition and incubated at a temperature within
the most
preferred range of 41 C to 44 C according to an embodiment of the invention
showed
the best results, being the first to begin vegetative growth and beginning
growth 4 hours
faster than the control. As seen in previous examples, growth seen in the no-
treatment
control experiment presumably represents the approx. 2% of germinated spores
present
in FreeFlow LF-88 Probiotic (see EXAMPLE 1). This example further indicates
that
spore germination is significantly increased when a nutrient-germinant
composition and
incubation method according to preferred embodiments of the invention are
used.
[00107] Aquaculture Study Using Nutrient Germ inant Composition
and Spore Composition.
47
CA 03088139 2020-07-08
WO 2019/168627
PCT/1JS2019/016020
[00108] Another study was conducted to evaluate the use of preferred nutrient
germinant and spore compositions with a preferred germination and aquaculture
treatment method according to the invention. Three aquariums were used for
this study
as representative aquaculture applications. Each held 55 gallons of water and
25
Malaysian prawns to mimic stocking densities of commercial shrimp farms. Each
aquarium contained the same type of netting and substrate composed of PVC pipe
provided for shrimp habitation and resting. All aquariums were lined with
Caribbean live
sand to discourage algal growth, reduce nitrates, help buffer the aquarium
system, and
ensure safer aquarium cycling. Aeration stones were used in all three
aquariums to
improve biological filtration and increase dissolved oxygen content for shrimp
and
beneficial bacteria. All three aquariums used the same type of filter and
filters were
rinsed off, as needed, and reused. All three aquariums were refilled with
deionized (DI)
water as needed. DI water was used to control mineral content of the water.
[00109] When large amounts of water needed to be removed from an
aquarium, the same amount of water was removed from all aquariums and replaced
with the same amount of DI water. Calcium carbonate was used in aquariums 2
and 3
for water replacements to mimic the use of a Pond Powder, such as ECOChargerTM
Pond Powder available from NCH Life Science. When water was replaced in
aquariums 2 or 3, about 0.5 g of calcium carbonate was added to tank water.
About 1
mL of an incubated or activated bacteria solution was applied once daily
Monday-Friday
to aquarium 3. Aquarium 1 was the control aquarium. Briefly, 20 pL of a
starting spore
solution (containing about 1010 CFU/mL) was mixed with 20 pL of a starting
concentrated nutrient solution and 960 pL of water to form a working solution
that
contained about 2 X 108 CFU/mL spores (Table 6). The starting spore solution
contained about 1010 CFU/mL spores from a spore blend. The spore blend
contained 3
strains of Bacillus bacteria: 2 strains were each a strain of the species
Bacillus
licheniformis and the third strain was a species of Bacillus subtilis. About
80% of the
spore blend formulation was Bacillus licheniformis (40% of each strain) spores
and 20%
of the spores in the spore blend formulation were Bacillus subtilis. The spore
48
CA 03088139 2020-07-08
WO 2019/168627
PCT/1JS2019/016020
composition also included water, thickener, and organic salts, according to a
preferred
embodiment described above.
[00110] The combined working solution (containing nutrient germinant
composition, spore composition, and water) was incubated at about 42 C for
about 1
hour to produce an activated bacteria solution. Following this incubation, the
entire
activated bacteria solution (about 1 mL) was added to aquarium 3. Mixing was
accomplished via aeration by mixing stone. Table 5 shows the composition of
the
starting nutrient germinant formulation. Table 6 shows the composition of the
working
solution that was incubated. After mixing the incubated bacteria solution into
55 gallons
of aquarium 3, the concentration of the bacteria was about 9.6 x 102 CFU/mL
and the
final percent of the nutrient germ inant composition in aquarium 3 was about
9.6 x 10-6 %
v/v. The contents of each aquarium after their respective treatments have been
applied
are shown in Table 7. The trial continued for 120 days.
[00111] Table 5 - Components of a Nutrient Formulation.
Component Wt% in Starting g/L in Starting g/L in Working g/L in
Final
Nutrient Nutrient Nutrient-Spore Dilution
Formulation Formulation formulation
Water 82.9 829 996.58 999.97
L-alanine 8.9 89 1.78 8.5 X i05
Disodium 6 60 1.2 5.76 X 10-6
Phosphate
Monosodium 2 20 0.4 1.93 X 10-6
phosphate
Germaben II 0.2 2 0.04 1.92 X 10-'
49
CA 03088139 2020-07-08
WO 2019/168627
PCT/1JS2019/016020
[00112] Table 6 - Working Nutrient-Spore Formulation.
Component Volume Starting Final
Concentration
Concentration
Starting Nutrient 20 pL 100% (v/v) 2% (v/v)
Formulation
Starting Spore 20 pL 100% (v/v) about About 2 X 108
Formulation 1010 CFU/ML CFU/mL
Water 960 pL 100% (v/v) 96% (v/v)
Final Volume 1000 pL NA NA
[00113] Table 7 - Aquarium Contents (Treatment Groups)
Aquarium 1 (Control Aquarium 2 (Pond Powder Aquarium 3 (Nutrient-Spore
Treatment Group) Only Treatment Group) Formulation and Pond
Powder Treatment Group)
No additions 0.5 g of Calcium Carbonate 1 mL of activated
bacteria
added per every water solution (incubated
replacement Working-Nutrient Spore
Formulation) and 0.5 g of
Calcium Carbonate added
per every water
replacement
[00114] Table 8 shows the final weight and body measurements of the
averaged trial groups as well as standard deviations. The control group in
aquarium 1
had the smallest shrimp weight and body measurements compared to treatment
groups
in aquariums 2 and 3. Aquarium 3 had the largest shrimp and had the best
results in
terms of shrimp size compared to the prawns in aquariums 2 and 1. The average
final
weight of shrimp in aquarium 3 was 6.48 g. The average final weight of the
prawns in
CA 03088139 2020-07-08
WO 2019/168627
PCT/1JS2019/016020
aquarium 2 was 4.87 g on average. The average final weight of the prawns in
aquarium
1 (the control) was 3.43 g. The average total length for prawns in aquarium 3
was also
the greatest at 7.98 cm. The average total length for prawns in aquarium 2 was
7.41.
The average total length for prawns in aquarium 1 (the control group) was
6.95. The
average tail length of prawns in aquarium 3 was 4.67 cm. The average tail
length of
prawns in aquarium 2 was 4.26 cm. The average tail length of prawns in
aquarium 1
(control) was the smallest at 3.87 cm.
[00115] Table 8 - Effect of Treatment on Growth Performance.
Control Calcium Carbonate Spores + Calcium
Carbonate
Average final weight 3.43 4.87 6.48
(9)
Average total length 6.95 7.41 7.98
(cm)
Average Tail length 3.87 4.26 4.67
(cm)
Standard Deviation 0.77 4.11 4.76
Average Final
Weight
Standard Deviation 0.42 1.49 1.30
Average Total
length
Standard Deviation 0.27 0.68 0.81
Average Tail length
[00116] FIG. 10 shows an image of the three aquariums that can demonstrate
the water clarity in each group by the end of the 120 day trial. In terms of
water clarity,
aquarium 3 was observed to be clearest. Aquarium 1, the control, was observed
to have
the greatest amount of algal growth covering the aquarium walls as compared to
aquariums 2 and 3. Aquarium 2 was observed to have only moderate algal growth
on
the aquarium walls as compared to the control and aquarium 3.
[00117] During the 120-day trial, all three aquariums started off with little
to no
algae on the sides of the aquariums. As the trial progressed, the control
aquarium
(aquarium 1) accumulated more algal growth on the sides of the aquarium (see
e.g.
51
CA 03088139 2020-07-08
WO 2019/168627
PCT/1JS2019/016020
FIG. 10). Aquarium 2 had less algal growth than aquarium 1. Aquarium 3 had
little to no
algal growth as compared to aquariums 1 and 2.
[00118] Water parameters were consistent throughout the trial. Ammonia levels
were zero for all three aquariums. Nitrite/nitrate were also within safe
ranges for the
duration of the trial. pH also stayed within normal ranges of about 7.5 to 8.5
for all of the
aquariums. There were no water parameter spikes observed that could have
harmed
the prawns as aquarium cycling occurred safely and parameters remained
consistent
for the full length of the 120-day trial.
[00119] Those of ordinary skill in the art will also appreciate upon reading
this
specification and the description of preferred embodiments herein that
modifications
and alterations to the methods and nutrient germinant and spore compositions
may be
made within the scope of the invention and it is intended that the scope of
the invention
disclosed herein be limited only by the broadest interpretation of the
appended claims to
which the inventors are legally entitled.
52