Note: Descriptions are shown in the official language in which they were submitted.
CA 03088518 2020-07-14
WO 2019/143620
PCT/US2019/013671
A BIOASSAY FOR THE NON-INVASIVE DETECTION OF DRUG USE AND
PHYSIOLOGIC CONDITIONS
CROSS-REFERENCE TO RELATED APPLICATIONS
[0001] The present application claims priority to U.S. Provisional Application
No.
62/619,434, filed January 19, 2018, the teaching of which is hereby
incorporated by reference
in its entirety for all purposes. This application is further related to U.S.
Patent Application
No. 2015/0116665, filed September 19, 2014, U.S. Patent Application No.
2017/0100061,
filed October 11, 2016, and U.S. Patent No. 9,326,725, filed March 30, 2011,
the contents of
which are incorporated herein by reference.
BACKGROUND
FIELD OF THE DISCLOSURE
[0002] The present disclosure is related to drug use and/or physiologic
impairments and their
impact on pupillary hippus. Specifically, the present disclosure describes the
utilization
pupillometry for the detection of the drug use and/or physiologic impairments.
DESCRIPTION OF THE RELATED ART
[0003] Pupillary control requires a complex physiology involving numerous
neuronal
pathways. Pupillary behavior, therefore, provides a window to the integrity
and functionality
of these neuronal pathways. Furthermore, pupillary behavior, as indicated by
contraction and
dilation of the iris by the sphincter and dilator muscles, can reflect
alterations or
abnormalities in the metabolism or the structure of the central nervous
system. This
connection to the central nervous system makes the determination and
identification of
pathologies critical in clinical and experimental settings, and suggests that
evaluation of
1
CA 03088518 2020-07-14
WO 2019/143620
PCT/US2019/013671
pupillary behavior may provide a mechanism for rapid detection and diagnosis
of
pathologies.
[0004] Pupil assessment, however, while being a routine practice in medical
care and used in
a variety of settings ranging from first responders to intensive care units,
is most commonly
performed using a penlight and visual, subjective observation. This subjective
approach is
hindered by inter-operator variability attributed to operator expertise and,
though an easy
assessment method, fails to provide granular data. For instance, the
information generated by
the penlight approach can be limited to gross pupil features such as the
presence or absence
of light reflex and a rough estimation of pupil size and symmetry. As would be
expected,
.. subtle changes that may be important tools in tracking clinical conditions
such as brain
trauma or viability following cardiac or pulmonary arrest cannot be assessed.
[0005] Even when more resolved methods have been employed, such as
pupillometers,
broad acceptance and deployment has been slow. These methods, though they can
be used to
evaluate pupillary size and reactivity, can be costly and can require stand-
alone equipment
that provides raw data without interpretation, necessitating the introduction
of a trained
professional to evaluate the data, synthesize the information, and provide
proper guidance to
a consumer regarding appropriate interventions.
[0006] Therefore, effective and convenient evaluation of pupillary behavior,
promising to
provide pupillary measurements that can be used to, among other things,
monitor drug use,
drug abuse, drug tolerance, and opioid hyperalgesia, is needed.
[0007] The foregoing "Background" description is for the purpose of generally
presenting
the context of the disclosure. Work of the inventors, to the extent it is
described in this
background section, as well as aspects of the description which may not
otherwise qualify as
prior art at the time of filing, are neither expressly or impliedly admitted
as prior art against
the present invention.
2
CA 03088518 2020-07-14
WO 2019/143620
PCT/US2019/013671
SUMMARY
[0008] According to an embodiment, the present disclosure is related to an
apparatus for
evaluation of a pupillary hippus of a patient.
[0009] In an embodiment, the present disclosure is related to an apparatus for
evaluation of a
pupillary hippus of a patient, comprising a display, and processing circuitry
configured to
transform experimental data of the pupillary hippus of the patient and
reference data via
frequency-based transformation, calculate a first parameter of one or more
selected
parameters based upon the transformed experimental data of the pupillary
hippus of the
patient, calculate, based upon the transformed reference data, a corresponding
first parameter
of the one or more selected parameters, generate a metric from the first
parameter based upon
the experimental data and the corresponding first parameter based upon the
reference data,
the generated metric being a normalization of the first parameter and the
corresponding first
parameter, determine whether the generated metric achieves a predetermined
threshold, the
predetermined threshold being related to a biologically-active target, and
display, on the
display and based upon the determination, the evaluation of the pupillary
hippus of the
patient, wherein the evaluation of the pupillary hippus of the patient is an
identification of an
opioid as the biologically-active target.
[0010] In an embodiment, the present disclosure is further related to an
apparatus for
evaluation of a pupillary hippus of a patient, comprising a display, and
processing circuitry
configured to calculate a first parameter of one or more selected parameters
based upon
experimental data of the pupillary hippus of the patient, calculate, based
upon reference data
of a pupillary hippus, a corresponding first parameter of the one or more
selected parameters,
generate a metric from the first parameter based upon the experimental data
and the
corresponding first parameter based upon the reference data, the generated
metric being a
3
1
CA 03088518 2020-07-14
WO 2019/143620
PCT/US2019/013671
normalization of the first parameter and the corresponding first parameter,
determine whether
the generated metric achieves a predetermined threshold, the predetermined
threshold being
related to a biologically-active target, and display, on the display and based
upon the
determination, the evaluation of the pupillary hippus of the patient.
[0011] In an embodiment, the present disclosure is further related to an
apparatus for
evaluation of a pupillary hippus of a patient, comprising processing circuitry
configured to
calculate a first parameter of one or more selected parameters based upon
experimental data
of the pupillary hippus of the patient, calculate, based upon reference data
of a pupillary
hippus, a corresponding first parameter of the one or more selected
parameters, generate a
metric from the first parameter based upon the experimental data and the
corresponding first
parameter based upon the reference data, the generated metric being a
normalization of the
first parameter and the corresponding first parameter, determine whether the
generated metric
achieves a predetermined threshold, the predetermined threshold being related
to a
biologically-active target, and display, on a display and based upon the
determination, the
evaluation of the pupillary hippus of the patient.
BRIEF DESCRIPTION OF THE DRAWINGS
[0012] A more complete appreciation of the disclosure and many of the
attendant advantages
thereof will be readily obtained as the same becomes better understood by
reference to the
following detailed description when considered in connection with the
accompanying
drawings, wherein:
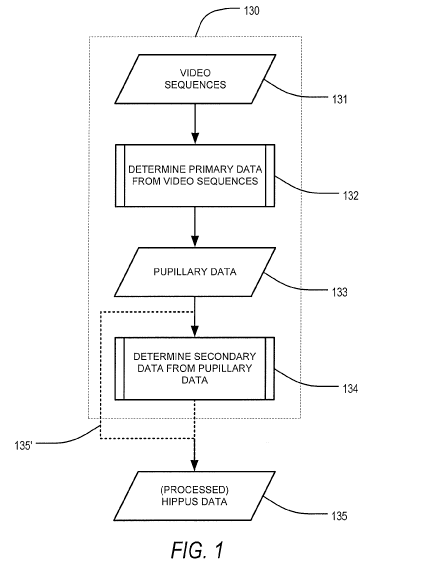
[0013] FIG. 1 is a flow diagram describing processing of acquired data,
according to an
exemplary embodiment of the present disclosure;
[0014] FIG. 2 is a graphical representation of pupillary oscillations as
isolated prior to
spectral analysis, according to an exemplary embodiment of the present
disclosure;
4
CA 03088518 2020-07-14
WO 2019/143620
PCT/US2019/013671
[0015] FIG. 3 is a flow diagram describing evaluation of a spectral analysis,
according to an
exemplary embodiment of the present disclosure;
[0016] FIG. 4 is a graphical representation of evaluation of a pupillary light
reflex after
exposure to opioids, according to an exemplary embodiment of the present
disclosure;
.. [0017] FIG. 5 is a graphical representation of transform data of maximal
drug effect
normalized to baseline for a plurality of drugs, according to an exemplary
embodiment of the
present disclosure; and
[0018] FIG. 6 is a hardware description of an apparatus, according to an
exemplary
embodiment of the present disclosure.
DETAILED DESCRIPTION
[0019] The terms "a" or "an", as used herein, are defined as one or more than
one. The term
"plurality", as used herein, is defined as two or more than two. The term
"another", as used
herein, is defined as at least a second or more. The terms "including" and/or
"having", as
.. used herein, are defined as comprising (i.e., open language). Reference
throughout this
document to "one embodiment", "certain embodiments", "an embodiment", "an
implementation", "an example" or similar terms means that a particular
feature, structure, or
characteristic described in connection with the embodiment is included in at
least one
embodiment of the present disclosure. Thus, the appearances of such phrases or
in various
places throughout this specification are not necessarily all referring to the
same embodiment.
Furthermore, the particular features, structures, or characteristics may be
combined in any
suitable manner in one or more embodiments without limitation.
[0020] According to an embodiment, the present disclosure describes a method
and
apparatus that allows clinicians, health care professionals, and consumers, in
cases, to
.. evaluate, precisely and objectively, the dynamic pupillary oscillations
that, in part, define
5
CA 03088518 2020-07-14
WO 2019/143620
PCT/US2019/013671
pupillary behavior. Moreover, these dynamic pupillary oscillations can be used
in
conjunction with a variety of pathology-specific algorithms, the pathology-
specific
algorithms being specific to different drug signatures and physiologic
conditions, in order to
identify pathologies therefrom. In an embodiment, the pathology-specific
algorithms can be
directed to, among others, alcohol, opioids, cannabinols, alpha-2 agonists,
benzodiazepines,
ketaminemorphine, morphine-3-glucuronide, morphine-6-glucuronide, or a
combination
thereof.
[0021] According to an exemplary embodiment of the present disclosure,
evaluation of
dynamic pupillary oscillations can be performed by an apparatus, or a
pupillometry device,
that combines an imaging apparatus having an imaging sensor, such as an
infrared camera or
CMOS sensor within housing, and a display apparatus which can be a smartphone
or a
dedicated display module. In an embodiment, the imaging apparatus and the
display
apparatus may be both contained within a smartphone or similar mobile
terminal. Connection
with the display will enable a software application to objectively generate
comparative
information of the dynamic pupillary oscillations such that it can facilitate
understanding of
the comparative information. To this end, the above-described apparatus can be
a screening
tool and software applications thereof can be algorithms and methods developed
to
specifically address a variety of clinical situations. These software
applications enable
objective measurement of the dynamic pupillary behavior in, for example, the
clinical setting
and can be stored within a memory of the smartphone or the apparatus.
[0022] According to an embodiment, the above-described apparatus of the
present disclosure
can implement a method in combination with additionally-described hardware.
For example,
such hardware can be a chamber constructed to adapt a smartphone to a
patient's, or a user's,
face. To facilitate data acquisition, the exemplary imaging apparatus, or
infrared camera, can
be adaptable, via the additionally-described hardware, to ergonomically form
to a patient's
6
CA 03088518 2020-07-14
WO 2019/143620
PCT/US2019/013671
face to enable accurate pupil assessment. Moreover, this allows implementation
of the
method in myriad environments, wherein it can be performed by a ubiquitous
device. The
method, in an embodiment, can be performed by processing circuitry configured
to control
the imaging apparatus of the smartphone or other device in order to acquire
video sequences
of an eye of a person. Such video sequences can be acquired, for example, at
100 frames per
second, though it should be appreciated that other frame rates can be used in
order to obtain
the pupillary video sequences.
[0023] During real-world implementation, the above-described apparatus and
method
thereof, according to an exemplary embodiment, can provide rapid access to
patient data that
can be important tools in a variety of clinical situations. By comprising an
integration-ready
chamber that is adjustable to a patient's face with a dedicated display for
the collected
information, in an embodiment, convenient and mobile acquisition of patient
pupillary data
can be realized and analysis expediently performed. Enhancing the adaptability
of the
approach, specific algorithms can be deployed in order to interpret the
acquired patient
pupillary data, adjustable to different clinical situations, thereby allowing
broad use and
access by a variety of professionals and laypersons, including, but not
limited to, medical
professionals.
[0024] Among multiple applications, the assessment of pupillary oscillations
can be applied
to the identification of drug use. The identification of drug use presents one
of the greatest
opportunities for broader use of pupillometry. Drugs confer specific effects
on the autonomic
nervous system, thereby affecting the pupil, and pupillary oscillations,
directly. Examination
of pupillary oscillations, known as hippus, using spectral analysis, for
example, renders
specific, attributable frequency responses. Drug usage changes the spectral
profile of hippus
in specific, attributable ways. The apparatus and method of the present
disclosure, as
7
CA 03088518 2020-07-14
WO 2019/143620
PCT/US2019/013671
introduced above, may be an important tool in understanding drug usage
correlations and
evaluating patients for drug use status.
[0025] Additionally, and according to an embodiment, the apparatus and method
of the
present disclosure may be employed in the evaluation of the function of the
autonomic
nervous system in the context of a physiologic condition. Pupillary
oscillations are known to
vary due to abnormal activity in the autonomic nervous system, such as the
presence of a
dysautonomia. Therefore, the function of the autonomic nervous system and
abnormal
activities thereof may be evaluated via the apparatus and method of the
present disclosure,
thus render the present disclosure an important tool in evaluating patients
for the presence of
specific physiologic conditions.
[0026] With reference now to the Figures and as described above, the present
disclosure,
according to an embodiment, is related to an apparatus, and a method thereof,
of determining
the presence of a biologically-active compound, a drug, or a physiologic
perturbation in a
patient. Briefly, the method includes, for instance, the steps of: (1)
acquiring a video
sequence of an eye of a patient, the video sequence including a plurality of
video frames, (2)
detecting and measuring pupil dimensions in each of the plurality of video
frames of the
video sequence, wherein the dimensions of the time-based pupil size foal'
pupillary
oscillations of the patient, (3) determine, using local or remote processing
circuitry, based
upon the pupillary oscillations, a frequency spectrum of the detected and
measured pupil
dimensions over time, and (4) determining, using the processing circuitry and
based upon a
band power of the frequency spectrum (i.e. area under the curve), the presence
of a drug or a
physiologic condition of the patient.
[0027] Referring now to FIG. 1, and with additional details as to the above,
the method can
comprise data processing 130 that includes first, as outlined in FIG. 1, the
acquisition of a
video sequence 131 of an eye of a patient, the video including a plurality of
video frames.
8
CA 03088518 2020-07-14
WO 2019/143620
PCT/US2019/013671
Following acquisition 131, primary data such as, for instance, pupillary
dimensions and
pupillary oscillations therefrom, can be determined for each of the plurality
of video frames
in the video sequence of the eye of the patient 132.
[0028] According to an embodiment, FIG. 2 is a graphical illustration of
pupillary data of an
isolated hippus isolated prior to spectral analysis. Specifically, amplitude
pupillary
oscillations over a 5-second period of a pupillary light reflex of are shown.
[0029] Returning now to FIG. 1, the data 133 that defines the pupillary
oscillations can then
be mined, via processing circuitry either local or remote, to determine, for
instance,
secondary data 134 that can include a frequency spectrum of the pupillary
oscillations over
time. The frequency spectrum determined to be secondary data 134 of the
pupillary data 133
can then be provided as processed hippus data 135 to a method of the present
disclosure for
evaluating the newly processed data. Alternatively, or in combination with,
the pupillary data
can forgo additional data manipulation 135' and can immediately define
processed hippus
data 134.
[0030] With regard to implementation of the method of the present disclosure,
the processed
hippus data 134 can be accessible during run time of the method, wherein the
processed
hippus data 134 from an experimental hippus and processed hippus data 134 from
a reference
hippus can be used to determine the presence of, among others, a biologically-
active
compound, a drug, or other physiologic perturbation of the patient. For
instance, this can be a
determination of the presence and/or level of alcohol-induced impairment based
on a band
power calculated from the frequency spectrum.
[0031] Different applications, such as detection of drug use or detection of a
medical
condition or physical perturbation, can take into account different
pupillometric measures and
different amounts of weight or different ways of processing the pupillometric
measures.
9
CA 03088518 2020-07-14
WO 2019/143620
PCT/US2019/013671
[0032] The method of FIG. 1 can be initiated by, for example, (1) during the
initial
processing of the video sequences 132, localizing, in a first frame among the
plurality of
frames, a center of the pupil and two points on a boundary of the pupil and
the iris, (2)
generating, using the processing circuitry, a mask image corresponding to an
expected
location of the iris based on said localizing, said mask image including a
plurality of pixels,
and (3) determining the pupillary dimensions (i.e. primary data), and
pupillary oscillations
therefrom, based on the generated mask image.
[0033] The acquired video sequence can be processed, as above, by a processor
in an
attachable device such as, among others, a smartphone or cloud based
processing. Although a
smartphone, in context of the processing circuitry above, is described herein
and has been
described previously, as evidenced by US 2015/0116665 Al and incorporated
herein by
reference, it can be appreciated that any processor, including an external
processor or cloud-
based processing circuitry, can be used to process the acquired video
sequence.
[0034] Further to the above, the acquired video sequence can include pupillary
reaction to,
for instance, a flash of light. In order to create this reaction, or pupillary
light reflex, a flash of
light, according to standardized lighting conditions, can be provided by the
flashlight of the
aforementioned smartphone or similar mobile device.
[0035] Pupillary oscillations and/or reactions to light, as described above,
can reflect the
activity of the autonomic nervous system. For instance, in exhibiting the
pupillary light reflex
and reflecting the integrity of the autonomic nervous system, constriction, or
miosis, occurs
in response to the flash of light as a result of increased parasympathetic
tone while dilation,
or mydriasis, reflects increased sympathetic tone. The pupillary light reflex
can be evaluated
via the method, and apparatus thereof, of the present disclosure, wherein
higher frequency
activation occurs with increased sympathetic tone and lower frequency
activation occurs from
increased parasympathetic tone. Applied in the real world, pupillary
oscillations may be
CA 03088518 2020-07-14
WO 2019/143620
PCT/US2019/013671
impacted by the activity of certain biologically-active compounds, drugs, of
physiological
conditions that interact with receptors of the autonomic nervous system,
impacting either
sympathetic or parasympathetic responses.
[0036] According to an embodiment, a variety of pupillometric measures can be
evaluated
from pupillary data following initial video sequence processing 132 such that,
in combination
with secondary data 134 including frequency spectra, patient response profiles
can be better
characterized. There are at least six pupillometric measures used in the
generation of
algorithms that can aid in the determination of a physiological characteristic
such as, for
example, usage of drugs or a medical condition. At least two of the
pupillometric measures
are static measures and can include baseline pupil size and maximally
constricted size. These
measures can be used to generate, for example, constriction amplitude. As
introduced above,
the baseline pupil size can be found before the flash of light and the
maximally constricted
size can be determined after the flash of light. At least four of the
pupillometric measures can
be dynamic measures and can be dynamic responses to the flash of light,
including velocity
of constriction (average constriction velocity and maximum constriction
velocity), latency of
constriction, and velocity of re-dilation. As related to the detection and
identification of drug
use or pathologic condition, the various parameters of the pupillary light
reflex are impacted
in a predictable way by various drugs and medical conditions. Any of the at
least six
pupillometric measures can be suitable metrics according to the application of
the
.. measurement. As the application changes, such as the detection of specific
drug use or
detection of a specific medical condition, different pupillometric measures
and different
amounts of weight or different ways of processing in pupillometric measures
can be
considered, as appropriate.
[0037] According to an embodiment, the above-described pupillometric measures,
or
parameters, can include at least one of a plurality of additional parameters
including a
11
CA 03088518 2020-07-14
WO 2019/143620
PCT/US2019/013671
maximum pupil size, a maximum change in size of the pupil, a maximum velocity
of re-
dilation of the pupil, a mean velocity of re-dilation of the pupil, a maximum
area of the pupil,
a minimum area of the pupil, a mean area of the pupil, the time to 75%
recovery of pupil size,
the time to 100% recovery of pupil size, and the area under the curve of the
pupillary light
reflex.
[0038] According to an embodiment, the secondary data 134 can include, for
instance, a
frequency spectrum. The frequency spectrum can be derived from the pupillary
data via
frequency-based transform methods. Such frequency-based transform methods may
be a fast
Fourier transform, Hilbert Huang transform, and the like, as would be
understood by one of
.. ordinary skill in the art. From the frequency spectrum, parameters such as
an amplitude at a
specific frequency or a band power across a range of frequencies, wherein the
specific
frequency or range of frequencies are correlated with a level of activity of a
pathology, can be
determined. Moreover, the frequency spectrum may be evaluated write large,
wherein a
mathematical model of the frequency spectrum is correlated with a level of
activity of a
.. pathology. To this end, heuristic models can be used in the development of
algorithms.
[0039] During implementation of the above-described methods, and referring now
to FIG. 3,
selected parameters can be detelinined for experimental and reference data and
compared
such that the presence and/or quantity of a substance, drug, or physiologic
substance can be
determined.
.. [0040] To this end, first, reference hippus data 335" can be acquired from
a reference
database 340 and experimental hippus data 335' can be acquired, for example,
from a current
patient. This hippus data is analogous to the processed hippus data of FIG. 1,
wherein the
method of FIG. 1 has been applied to an acquired video sequence.
[0041] Having acquired appropriate hippus data, a first parameter, or
experimental parameter
.. 336', can be determined from the experimental hippus data 335' of a
pupillary hippus of the
12
CA 03088518 2020-07-14
WO 2019/143620
PCT/US2019/013671
patient. The experimental parameter 336' can be, but is not limited to,
amplitude, frequency,
band power, and a mathematical model of the waveform, as described above.
Additionally,
the experimental parameter 336' can be, among others, baseline pupil size,
maximum pupil
size, minimum pupil size, velocity of constriction (average constriction
velocity and
maximum constriction velocity), latency of constriction, velocity of re-
dilation, maximum
change in size of the pupil, maximum velocity of re-dilation of the pupil,
mean velocity of re-
dilation of the pupil, maximum area of the pupil, minimum area of the pupil,
mean area of the
pupil, time to 75% recovery of pupil size, time to 100% recovery of pupil
size, and area under
the curve of the pupillary light reflex.
[0042] Similarly to the above, a first parameter, or reference parameter 336",
can be
determined from reference hippus data 335" of a pupillary hippus of a
reference patient or a
representative pupillary hippus of a population of patients. The reference
parameter 336" can
be, but is not limited to, amplitude, frequency, band power, and a
mathematical model of the
waveform, as described above. Additionally, the experimental parameter 336'
can be, among
.. others, baseline pupil size, maximum pupil size, minimum pupil size,
velocity of constriction
(average constriction velocity and maximum constriction velocity), latency of
constriction,
velocity of re-dilation, maximum change in size of the pupil, maximum velocity
of re-dilation
of the pupil, mean velocity of re-dilation of the pupil, maximum area of the
pupil, minimum
area of the pupil, mean area of the pupil, time to 75% recovery of pupil size,
time to 100%
recovery of pupil size, and area under the curve of the pupillary light
reflex.
[0043] In an exemplary embodiment, a second parameter, or comparative metric
337, can be
determined as a computation based upon the experimental parameter 337' and the
reference
parameter 337" determined from the pupillary hippus of the patient and the
pupillary hippus
of the reference patient, for example, respectively. The comparative metric
can include,
among others, delta band power, or the difference between the band power of
the
13
CA 03088518 2020-07-14
WO 2019/143620
PCT/US2019/013671
experimental data and a corresponding band power of the reference data, %
delta band power,
no' __ inalized delta band power, and a similarity ratio between mathematical
models of the
experimental data and the reference data.
[0044] In an embodiment, the comparative metric 337 can be a correlation of an
.. experimental waveform and a reference waveform, wherein a lack of
correlation of the
respective waveforms can be indicative or not of a physiologic condition.
[0045] Following determination of the comparative metric 337, according to an
embodiment,
the comparative metric 337 can be evaluated 338 with respect to a pre-
determined threshold
to determine the presence or absence of a biologically-active substance, a
drug, or a
physiologic perturbation. The biologically-active substance, the drug, or the
physiologic
perturbation, as defined by the comparative metric evaluated, can be indicated
via a display.
[0046] For example, a patient may be suspected of recreational use of opioids
or, in
particular, methadone. If delta band power is the comparative metric and, over
a frequency
range associated with methadone users, is determined to be significantly large
when
comparing the patient's data with reference data of a comparable patient, it
can be determined
that the patient has had an acute exposure to methadone. In another example, a
patient may be
suspected of over use of a prescribed opioid such as hydrocodone. If delta
band power, over a
frequency range associated with hydrocodone use, is determined to be
significantly large
when comparing the patient's data with reference data from an expected
hydrocodone band
power user, it can be determined that the patient has had an acute
overexposure to
hydrocodone.
[0047] According to an embodiment, following the evaluation of the comparative
metric
with respect to a selected criterion 338, the outcome or, physiologic
condition, can be
displayed 339 via a display of the device described with reference to FIG. 7
such that a user
can be alerted of the patient's condition, normal or otherwise.
14
CA 03088518 2020-07-14
WO 2019/143620
PCT/US2019/013671
[0048] Evaluation of the comparative metric relative to a criterion may
reflect analysis of
patterns and correlations of quantified frequency spectra that may be
predictive of particular
scenarios. The patterns and correlations may further predictive of
interactions of drugs and
their impact on pupillary hippus. According to an embodiment, these patterns
and
correlations can be identified by comparison against a library of frequency
spectra associated
with specific biologically-active compounds or drugs, a panel of specific
biologically-active
compounds or drugs, or multiple, interacting biologically-active compounds or
drugs.
[0049] As discussed with respect to FIG. 3, comparisons of unknown, or
experimental data,
and reference data can be conducted by evaluating, for example, amplitudes at
one or more,
or a set of, specific frequencies along the frequency domain.
[0050] Accordingly, FIG. 4 provides a graphical representation of a spectral
evaluation of
experimental hippus data and reference hippus data, as would be performed
during the
generation of secondary data in FIG. 1. As shown, experimental hippus data,
captured at a
time period of 'maximum opioid effect' is illustrated alongside reference data
displayed as a
'baseline'. The impact of opioid use can be observed at varying frequencies
across a
spectrum for a single patient and attendant analysis of parasympathetic and
sympathetic
actions can be inferred therefrom. As observed in FIG. 4, for instance, opioid
use modifies
pupillary oscillations between 8 Hz and 11 Hz, as compared with baseline, and
high
frequency pupillary oscillations between 12 Hz and 14 Hz. Such modifications
may be
indicative, in the case of high frequency pupillary oscillations, increased
sympathetic tone in
response to opioid exposure. In an example, the identification of physiologic
perturbations
could be performed by evaluation of a correlation between mathematical models
of the
plotted data.
[0051] The specificity suggested in FIG. 4 is displayed in FIG. 5 with regards
to a plurality
of drugs, wherein fast Fourier transform data of the hippus of a patient using
opioids and a
CA 03088518 2020-07-14
WO 2019/143620
PCT/US2019/013671
patient using cannabis normalized to baseline is presented. As can be
observed, the change in
amplitude of each patient is varied relative to baseline over a frequency
range of 8 Hz to 11
Hz, where cannabis use may increase sympathetic tone, for example, and opioid
use may
decrease sympathetic tone, relative to baseline.
[0052] According to an embodiment, experimental hippus data containing unknown
frequency spectra may be analyzed, or filtered, with respect to a specific
target biologically-
active compound. This analysis, or filtering, can be based upon prior
investigations of the
biologically-active target compound. Filtering may include removal of data
above, below, or
within a predetermined frequency, and the removal of data above, below, or at
predetermined
amplitude, for example, wherein the predetermined frequency and the
predetermined
amplitude are correlated with the specific target biologically-active
compound. For instance,
it may be known that an opioid may have increased amplitude oscillations
between 12 Hz and
14 Hz along the frequency domain, as shown in FIG. 4. Through determination of
the area
under the curve between these two frequencies, the area under the curve being
referred to as a
band power, the unknown frequency spectra data may be compared to reference
frequency
spectra data of a known entity to determine a delta band power. The delta band
power, as
discussed with respect to FIG. 3, can be a comparative metric or second
parameter and, if
present, the delta band power may be above a pre-determined threshold
according to the
sensitivity of the data acquisition equipment.
[0053] Furthermore, comparisons of complete, longitudinal pupillary responses,
in the
frequency domain, can be compared to a library of frequency spectra via
pattern recognition
techniques employed in machine learning for determining irregularities in
data. This
approach can identify, for example, one or more amplitude inflection points in
the frequency
domain that correlate to one or more known biologically-active compounds,
drugs, or
physiologic conditions.
16
CA 03088518 2020-07-14
WO 2019/143620
PCT/US2019/013671
[0054] Complementary to the above approaches, and as suggested, each unknown
frequency
spectra can be analyzed with respect to the effects of multiple, interacting
biologically-active
compounds, providing context to the impact of drug-drug interactions on the
nervous system.
For example, an unknown frequency spectra data may be filtered in the targeted
context of
the pupillary effects of the interaction of alcohol and opioids in order to
isolate said
compounds.
[0055] Moreover, in an embodiment, each unknown frequency spectra can be
compared
against a library of reference hippus data and it may be determined that one
or more drug-
drug interactions can be correlated with physical perturbations of the
pupillary light reflex.
[0056] For instance, the method can be applied such that, separately, alcohol
use is evaluated
in one instance and opioid use is evaluated in a second instance. In a third
instance, the
impact of combined alcohol use and opioid use can be evaluated. Clinically-
significant
frequency ranges, or bands, such as 0.3 Hz ¨ 3.0 Hz or 3.1 Hz - 5.0 Hz, for
example, can be
evaluated to probe for specific biologically-active compounds, drugs, and the
like, wherein
one indicates alcohol presence and the other indicates opioid use. In the case
wherein the
combination of alcohol and opioids modifies the impact that either would
impart separately, a
filter can be applied to eliminate one from the frequency spectra such that
the other may be
detected and quantified. This can be a common occurrence in real world
applications,
wherein a first compound of a group of compounds may substantially outcompete
the group
for access to a specific receptor, thereby subduing the effect of competing
compounds,
masking the presence of other compounds of the group, and modifying the
pupillary light
reflex writ large.
[0057] Moreover, the band power determined at each of these frequency bands
can indicate,
when calibrated, a concentration of a biologically-active substance or drug,
thereby providing
a potentially powerful, non-invasive tool for drug usage detection and
monitoring.
17
CA 03088518 2020-07-14
WO 2019/143620
PCT/US2019/013671
[0058] According to an embodiment, experimental hippus data containing unknown
frequency spectra may be compared each every entry of the reference hippus
data of the
reference database in order to identify the unknown affecter of the frequency
spectra. For
example, one or more drugs may have an influence on a frequency spectrum of
data from an
.. experimental hippus. This frequency spectrum can be compared against a
reference database,
containing frequency spectra being impacted by a plurality of drugs, such that
the presence
and, if possible, identity of the one or more drugs of the experimental hippus
can be
determined. Importantly, this approach, while more computationally intensive,
does not
require a user to have a prediction of the one or more drugs of the frequency
spectrum of the
.. experimental hippus and, instead, allows for comparison of the unknown
spectrum against a
panel of possible drug candidates.
[0059] According to an embodiment, unknown and quantified frequency spectra
data can be
evaluated ad hoc to detect the presence of a biologically-active compound, as
compared to a
baseline. This approach may be useful when merely the presence of a specific
biologically-
active compound is in question. In an embodiment, the baseline can be
established from a
library a reference data of a variety of control patients, a prior control
dataset of the same
patient, or a combination thereof.
[0060] Further to the above, according to an embodiment, the present method
can be used to
detect dysautonomias, which include a variety of conditions including diabetic
neuropathy
and postural orthostatic tachycardia syndrome.
[0061] The method of the present embodiments can also be used for management
of drug use
and monitoring thereof. Currently, drug dose management is subjective
according to clinician
judgment. The approach of the present disclosure can be applied to long-term
or repeated
drug monitoring, including the detection of biologically-active compounds and
respective,
subsequent metabolites. Drug use and impairment with time, including dose
response effects,
18
CA 03088518 2020-07-14
WO 2019/143620
PCT/US2019/013671
can be observed per this method. Metrics determined therein can be used
clinically for
objective analyses.
[0062] Moreover, the method can be developed to work as triage test in drivers
suspected to
be under the influence of alcohol or controlled substances. If there are any
spectra unique to
illegal substances discovered during the test, the driver will be submitted to
other tests.
[0063] In addition to the above, the method of the present embodiments can be
further
implemented for the monitoring of post-surgery sedation of surgical patients.
[0064] According to an embodiment, the method of the present embodiments can
also be
used to discriminate between direct drug effects on the pupil vs. analgesic
impact, (i.e., the
method allows for the discrimination of drug vs. system-dependent parameters
by using
elements of static or dynamic pupil parameters as analogues of
pharmacokinetics and area
under the curve of the pupillary reflex dilation as the analogue of analgesic
pharmacodynamics. The fast Fourier transfomi-derived "signature" of the
present disclosure
provides a non-invasive approach for further informing this paradigm by
indicating the
presence of a substance.
[0065] In an embodiment, the method of the present disclosure can be used in
the context of
analgesic response or other drug effects when combined with other features of
the pupillary
response including, but not limited to, the pupillary light reflex and the
neurospecific
neurostimulus-induced pupillary light reflex. This approach allows for
isolation of drug-
induced hyperalgesia, or a state of exposure-mediated nociceptive
sensitization, from
increased pain sensitivity resulting from injury or disease progression.
[0066] Next, a hardware description of an apparatus, or device, according to
exemplary
embodiments is described with reference to Figure 6. In Figure 6, the device
includes a CPU
600 which performs the processes described above. The process data and
instructions may be
stored in memory 602. These processes and instructions may also be stored on a
storage
19
CA 03088518 2020-07-14
WO 2019/143620
PCT/US2019/013671
medium disk 604 such as a hard drive (HDD) or portable storage medium or may
be stored
remotely. Further, the claimed advancements are not limited by the form of the
computer-
readable media on which the instructions of the inventive process are stored.
For example,
the instructions may be stored on CDs, DVDs, in FLASH memory, RAM, ROM, PROM,
EPROM, EEPROM, hard disk or any other information processing device with which
the
device communicates, such as a server or computer.
[0067] Further, the claimed advancements may be provided as a utility
application,
background daemon, or component of an operating system, or combination
thereof, executing
in conjunction with CPU 600 and an operating system such as Microsoft Windows
7, UNIX,
.. Solaris, LINUX, Apple MAC-OS and other systems known to those skilled in
the art.
[0068] The hardware elements in order to achieve the device may be realized by
various
circuitry elements, known to those skilled in the art. For example, CPU 600
may be a Xenon
or Core processor from Intel of America or an Opteron processor from AMD of
America, or
may be other processor types that would be recognized by one of ordinary skill
in the art.
Alternatively, the CPU 600 may be implemented on an FPGA, ASIC, PLD or using
discrete
logic circuits, as one of ordinary skill in the art would recognize. Further,
CPU 600 may be
implemented as multiple processors cooperatively working in parallel to
perform the
instructions of the inventive processes described above.
[0069] The device in Figure 6 also includes a network controller 606, such as
an Intel
Ethernet PRO network interface card from Intel Corporation of America, for
interfacing with
network 650. As can be appreciated, the network 650 can be a public network,
such as the
Internet, or a private network such as an LAN or WAN network, or any
combination thereof
and can also include PSTN or ISDN sub-networks. The network 650 can also be
wired, such
as an Ethernet network, or can be wireless such as a cellular network
including EDGE, 3G
CA 03088518 2020-07-14
WO 2019/143620
PCT/US2019/013671
and 4G wireless cellular systems. The wireless network can also be WiFi,
Bluetooth, or any
other wireless form of communication that is known.
[0070] The device further includes a display controller 608, such as a NVIDIA
GeForce
GTX or Quadro graphics adaptor from NVIDIA Corporation of America for
interfacing with
display 610, such as a Hewlett Packard HPL2445w LCD monitor. A general purpose
I/O
interface 612 interfaces with a keyboard and/or mouse 614 as well as a touch
screen panel
616 on or separate from display 610. General purpose I/O interface also
connects to a variety
of peripherals 618 including printers and scanners, such as an OfficeJet or
DeskJet from
Hewlett Packard.
[0071] A sound controller 620 is also provided in the device, such as Sound
Blaster X-Fi
Titanium from Creative, to interface with speakers/microphone 622 thereby
providing sounds
and/or music.
[0072] The general purpose storage controller 624 connects the storage medium
disk 604
with communication bus 626, which may be an ISA, EISA, VESA, PCI, or similar,
for
interconnecting all of the components of the device. A description of the
general features and
functionality of the display 610, keyboard and/or mouse 614, as well as the
display controller
608, storage controller 624, network controller 606, sound controller 620, and
general
purpose I/O interface 612 is omitted herein for brevity as these features are
known.
[0073] Embodiments of the present disclosure may also be as set forth in the
following
parentheticals.
[0074] (1) An apparatus for evaluation of a pupillary hippus of a patient,
comprising a
display, and processing circuitry configured to transform experimental data of
the pupillary
hippus of the patient and reference data via frequency-based transformation,
calculate a first
parameter of one or more selected parameters based upon the transformed
experimental data
of the pupillary hippus of the patient, calculate, based upon the transformed
reference data, a
21
CA 03088518 2020-07-14
WO 2019/143620
PCT/US2019/013671
corresponding first parameter of the one or more selected parameters, generate
a metric from
the first parameter based upon the experimental data and the corresponding
first parameter
based upon the reference data, the generated metric being a normalization of
the first
parameter and the corresponding first parameter, determine whether the
generated metric
achieves a predetermined threshold, the predetermined threshold being related
to a
biologically-active target, and display, on the display and based upon the
determination, the
evaluation of the pupillary hippus of the patient, wherein the evaluation of
the pupillary
hippus of the patient is an identification of an opioid as the biologically-
active target.
[0075] (2) The apparatus according to (1), wherein the processing circuitry is
further
configured to determine whether the generated metric achieves the
predetermined threshold
based upon a correlation between the first parameter of the experimental data
and the
corresponding first parameter of the reference data.
[0076] (3) The apparatus according to either (1) or (2), wherein the first
parameter based
upon the experimental data is an amplitude at a predetermined frequency.
.. [0077] (4) The apparatus according to any of (1) to (3), wherein the first
parameter based
upon the experimental data is band power.
[0078] (5) The apparatus according to any of (1) to (4), wherein the generated
metric is a
difference between a band power of the experimental data and a band power of
the reference
data.
[0079] (6) The apparatus according to any of (1) to (5), wherein the first
parameter based
upon the experimental data is a mathematical model of the experimental data.
[0080] (7) The apparatus according to any of (1) to (6), wherein the first
parameter based
upon the experimental data is a mathematical model of a frequency spectrum of
the
experimental data.
22
CA 03088518 2020-07-14
WO 2019/143620
PCT/US2019/013671
[0081] (8) The apparatus according to any of (1) to (7), wherein the generated
metric is a
similarity ratio of mathematical models of a frequency spectrum of the
experimental data and
of the reference data.
[0082] (9) The apparatus according to any of (1) to (8), wherein the
processing circuitry is
further configured to acquire a plurality of video sequences of an eye of the
patient, generate
pupillary data based upon primary data calculated from the plurality of video
sequences, the
primary data including time-based pupillary dimensions, and calculate, from
the generated
pupillary data, secondary data, wherein the secondary data include the
frequency spectrum of
the pupillary hippus.
[0083] (10) The apparatus according to any of (1) to (9), wherein the primary
data are
calculated based upon a mask image, the processing circuitry, in order to
generate the mask
image, being further configured to locate a center of a pupil of the eye, a
boundary of the
pupil of the eye, and an iris of the eye, and generate the mask image, the
mask image
corresponding to an expected location of the iris based upon the location of
the center of the
pupil of the eye, the boundary of the pupil of the eye, and the iris of the
eye.
[0084] (11) An apparatus for evaluation of a pupillary hippus of a patient,
comprising a
display, and processing circuitry configured to calculate a first parameter of
one or more
selected parameters based upon experimental data of the pupillary hippus of
the patient,
calculate, based upon reference data of a pupillary hippus, a corresponding
first parameter of
the one or more selected parameters, generate a metric from the first
parameter based upon
the experimental data and the corresponding first parameter based upon the
reference data,
the generated metric being a normalization of the first parameter and the
corresponding first
parameter, determine whether the generated metric achieves a predetermined
threshold, the
predetermined threshold being related to a biologically-active target, and
display, on the
23
CA 03088518 2020-07-14
WO 2019/143620
PCT/US2019/013671
display and based upon the determination, the evaluation of the pupillary
hippus of the
patient.
[0085] (12) The apparatus according to (11), wherein the processing circuitry
is further
configured to determine whether the generated metric achieves the
predetermined threshold
based upon a correlation between the first parameter of the experimental data
and the
corresponding first parameter of the reference data.
[0086] (13) The apparatus according to either of (11) or (12), wherein the
processing
circuitry is further configured to transform the experimental data of the
pupillary hippus of
the patient and the reference data via frequency-based transfoimation, and the
generated
metric is a difference between a band power of the experimental data and a
band power of the
reference data.
[0087] (14) The apparatus according to any of (11) to (13), wherein the
processing circuitry
is further configured to transform the experimental data of the pupillary
hippus of the patient
and the reference data via frequency-based transformation, and the generated
metric is a
similarity ratio of mathematical models of a frequency spectrum of the
experimental data and
of the reference data.
[0088] (15) The apparatus according to any of (11) to (14), wherein the
processing circuitry
is further configured to acquire a plurality of video sequences of an eye of
the patient,
generate pupillary data based upon primary data calculated from the plurality
of video
sequences, the primary data including time-based pupillary dimensions, and
calculate, from
the generated pupillary data, secondary data, wherein the secondary data
include the
frequency spectrum of the pupillary hippus.
[0089] (16) The apparatus according to any of (11) to (15), wherein the
primary data are
calculated based upon a mask image, the processing circuitry, in order to
generate the mask
image, being further configured to locate a center of a pupil of the eye, a
boundary of the
24
CA 03088518 2020-07-14
WO 2019/143620
PCT/US2019/013671
pupil of the eye, and an iris of the eye, and generate the mask image, the
mask image
corresponding to an expected location of the iris based upon the location of
the center of the
pupil of the eye, the boundary of the pupil of the eye, and the iris of the
eye.
[0090] (17) The apparatus according to any of (11) to (16), wherein the
evaluation of the
pupillary hippus of the patient is an identification of the biologically-
active target, the
biologically-active target being selected from a group including alcohol,
opioids,
cannabinols, alpha-2 agonists, benzodiazepines, ketaminemorphine, morphine-3-
glucuronide,
morphine-6-glucuronide, or a combination thereof.
[0091] (18) The apparatus according to any of (11) to (17), wherein the
processing circuitry
is further configured to transform the experimental data of the pupillary
hippus of the patient
via frequency-based transformation, and remove, from the transformed
experimental data,
data according to a predetermined frequency range.
[0092] (19) The apparatus according to any of (11) to (18), wherein the
evaluation of the
pupillary hippus of the patient can be an identification of a presence of a
dysautonomia, the
dysautonomia being one selected from a group including postural orthostatic
tachycardia
syndrome and diabetic neuropathy.
[0093] (20) An apparatus for evaluation of a pupillary hippus of a patient,
comprising
processing circuitry configured to calculate a first parameter of one or more
selected
parameters based upon experimental data of the pupillary hippus of the
patient, calculate,
based upon reference data of a pupillary hippus, a corresponding first
parameter of the one or
more selected parameters, generate a metric from the first parameter based
upon the
experimental data and the corresponding first parameter based upon the
reference data, the
generated metric being a normalization of the first parameter and the
corresponding first
parameter, determine whether the generated metric achieves a predetermined
threshold, the
CA 03088518 2020-07-14
WO 2019/143620
PCT/US2019/013671
predetermined threshold being related to a biologically-active target, and
display, on a display
and based upon the determination, the evaluation of the pupillary hippus of
the patient.
[0094] Thus, the foregoing discussion discloses and describes merely exemplary
embodiments of the present invention. As will be understood by those skilled
in the art, the
present invention may be embodied in other specific forms without departing
from the spirit
or essential characteristics thereof. Accordingly, the disclosure of the
present invention is
intended to be illustrative, but not limiting of the scope of the invention,
as well as other
claims. The disclosure, including any readily discernible variants of the
teachings herein,
defines, in part, the scope of the foregoing claim terminology such that no
inventive subject
matter is dedicated to the public.
26